Abstract
Nickel catalysis offers exciting opportunities for addressing unmet challenges in organic synthesis. Herein, we report the first nickel-catalyzed radical migratory cross-coupling reaction for the direct preparation of 2-aryl-2-deoxy glycosides from readily available 1-bromo sugars and aryl boronic acids. The reaction features a broad substrate scope and tolerates a wide range of functional groups and complex molecular architectures. Preliminary experimental and computational studies suggest a concerted 1,2-acyloxy rearrangement via a cyclic five-membered ring transition state followed by nickel-catalyzed carbon-carbon bond formation. The novel reactivity provides an efficient route to valuable C-2 arylated carbohydrates mimics and building blocks, allows for new strategic bond disconnections, and expands the reactivity profile of nickel catalysis.
Graphical Abstract
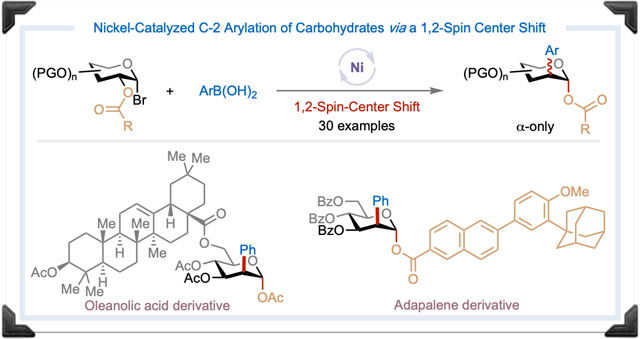
Carbohydrates, the most abundant biomolecules, play vital roles in a wide array of biological processes, including cell-cell recognition, protein folding, neurobiology, inflammation, and infection.1 The modification of carbohydrate structure(s) to enhance or alter the physiological properties of the parent molecule is, therefore, an attractive strategy for the development of novel pharmaceuticals. Indeed, carbohydrates and their mimics are present in a range of commercially available therapeutics and vaccines, and the evolving methods for carbohydrate synthesis and modification continue to influence the drug discovery landscape.2 Over the past few decades, tremendous progress has been made towards C-1 modification of carbohydrates such as the O-glycosylation3 and C-glycosylation.4 Yet, a general catalytic strategy for the preparation of diverse and valuable C-2 functionalized 2-deoxy sugars from readily available sugar precursors remains elusive.5,6 Given that C-2 functionalized 2-deoxy sugars are ubiquitous in nature and are found in medicine, molecular imaging, cell engineering, and catalysis,7 the establishment of a versatile catalytic approach for the preparation of this class of sugars is highly attractive.
Nickel catalysis has advanced as a general technology for chemical synthesis.8 Recently, significant progress has been made in nickel-catalyzed migratory cross-coupling (MCC) reactions9 that enable a range of remote functionalization reactions of alkyl halides (Figure 1A). These include hydroarylation,10 hydroalkylation,11 alkenylation,10d acylation,12 and carboxylation.13 In such reactions, the nickel catalyst typically migrates from the activation site to the cross-coupling site via the 2-electron β-hydrogen elimination/migratory insertion sequences (Figure 1B).9 In contrast, Ni-catalyzed MCC reactions that proceed through a radical migratory pathway such as a 1,2-spin-center shift (SCS)14 are rare.15 Inspired by the seminal work of Surzur and Tanner, who showed that β-(acyloxy)alkyl radical could undergo a 1,2-SCS with concomitant acyloxy migration,16 we hypothesized that such a reactivity could serve as the basis of a nickel-catalyzed radical MCC reaction via a 1,2-SCS pathway (Figure 1C). The success of such a reaction could (i) provide new strategic bond formation that leads to otherwise difficult or unobtainable molecular architecture; (ii) expand the reactivity profile of Ni catalysis; (iii) advance fundamental knowledge in radical chemistry; and (iv) promote new reaction design and development. Herein, we report the establishment and application of such a reaction platform for the preparation of synthetically challenging C-2 arylated carbohydrates from readily available 1-bromo sugars and aryl boronic acids (Figure 1C).17
Figure 1.
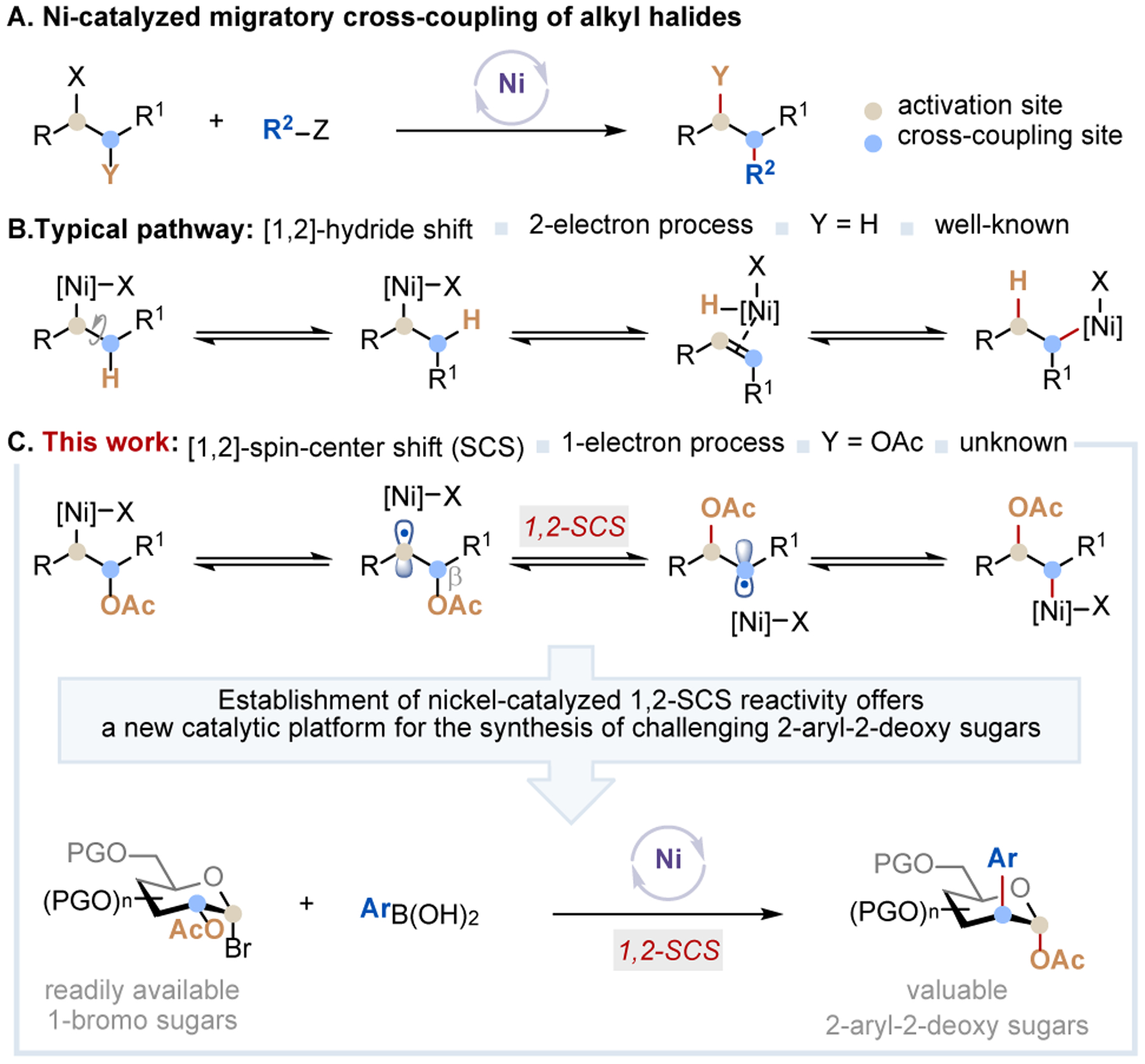
Ni-catalyzed migratory cross-coupling reaction enables the catalytic synthesis of challenging 2-aryl-2-deoxy sugars.
Noteworthily, catalytic C-2 arylation of readily available sugar precursors for the preparation of saturated, fully oxygenated 2-aryl-2-deoxy sugars has not been reported.18 The existing approaches to this class of sugar derivatives involve either the construction of carbon skeletons by homologation of chiral aldehydes using carbonyl ene cyclization strategy19 or epoxide ring-opening of 2,3-epoxy sugars with aryl magnesium iodides or lithium diarylcuprates.20 These methods, however, require the multi-step synthesis of advanced intermediates, involve harsh reaction conditions, and have limited substrate and reaction scopes. Thus, the work described here offers rapid access to novel 2-aryl-2-deoxy sugars and serves as the first example of a nickel-catalyzed radical MCC reaction proceeding through a 1,2-SCS pathway.
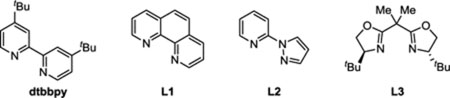
We commenced our investigation by examining the reaction of α-glucosyl bromide (1a) and phenylboronic acid (2a) in the presence of Ni catalysts and found that when a mixture of 1a (1.00 equiv), 2a (2.00 equiv), NiBr2·DME (5.00 mol%), 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbbpy, 10.0 mol%), isopropanol (i-PrOH, 0.75 equiv), and Cs2CO3 (2.00 equiv) in benzene (0.100 M) was heated at 80 °C for 20 h, the desired C-2 arylated 2-deoxyglucoside (3a) was produced in 84% yield with 3.6:1 axial to equatorial selectivity together with a small amount of the C-1 arylated byproduct (Table 1, entry 1).21,22 The nature of the ligand is critical for the success of the reaction because the replacement of dtbbpy with other classes of (N,N)-bidentate ligands such as phenanthroline (L1), pyridine-pyrazole (L2), and bisoxazoline (L3) greatly reduced reaction yields (entries 2–4).23 Removal of i-PrOH, which is known to promote the transmetallation in the nickel-catalyzed Suzuki-Miyaura cross-coupling reaction,24 also diminished the efficiency of the reaction (entry 5). The use of 1,4-dioxane as a solvent formed hydro-debromination side products, lowering the product yield (entry 6). Finally, control experiments showed that NiBr2·DME, Cs2CO3, elevated reaction temperature, and an oxygen-free environment were critical for the success of the reaction (entries 7–10).
Table 1.
Selected optimization experiments.a

| ||
|---|---|---|
| Entry | Deviation from standard conditions | Yield of 3a (%) (ax:eq) |
| 1 | - | 84 (3.6:1) |
| 2 | L1 instead of dtbbpy | 0 |
| 3 | L2 instead of dtbbpy | 10 (2.9:1) |
| 4 | L3 instead of dtbbpy | <1 |
| 5 | Without i-PrOH | 63 (3.5:1) |
| 6 | 1,4-Dioxane as solvent | 40 (3.4:1) |
| 7 | Ni(cod)2 instead of NiBr2·DME | 35 (3.8:1) |
| 8 | DIPEA instead of CS2CO3 | 0 |
| 9 | Room temp instead of 80 °C | <1 |
| 10 | With air | 0 |
| ||
See Supporting Information (SI) for experimental details. Yields of 3a and axial:equatorial (ax:eq) ratios were determined by 1H-NMR using dibromomethane as the internal standard.
Next, we explored the scope of aryl and heteroaryl boronic acids (Table 2A). The reaction tolerates a range of aryl boronic acids with different substituents such as methyl, t-butyl, phenyl, methoxy, diphenylamino, methyl sulfide, and methyl ester, forming the corresponding products (3b-3i) in 46–86% yields with moderate axial/equatorial selectivity. 2-Naphthyl boronic acid and heteroaryl boronic acids, including 9-phenyl-9H-carbazol-3-yl and 2-benzofuranylboronic acids, were viable substrates and gave the desired products (3j-3l) with moderate yields. Examination of the generality of 1-bromo sugars revealed that an array of sugar derivatives bearing different protecting and migratory groups were competent under this protocol (Table 2B).25 D-Galactoside and L-fucoside derivatives reacted smoothly and formed the corresponding products (3m, 3n, 3p) with yields of 40–74%. Noteworthily, these substrates gave the product with the opposite stereoselectivity. Steric interaction between the nickel catalyst and the axial C-4 OAc appears to favor the formation of the equatorial product. Protecting groups such as t-butyldimethylsilyl, benzyl, acetyl, pivaloyl, and benzoyl are well-tolerated. A substrate with a fused ring structure was compatible, producing 3q. We also investigated the effect of structural modification of the migratory ester group on the reaction efficiency and found that C-2 esters substituted with alkyl, aryl, or heteroaryl groups successfully migrated, delivering the corresponding products (3r-3x) in 38–85% yields.
Table 2.
Scope of C-2 arylation of α-glycosyl bromides via Ni-catalyzed 1,2-SCS strategy.a
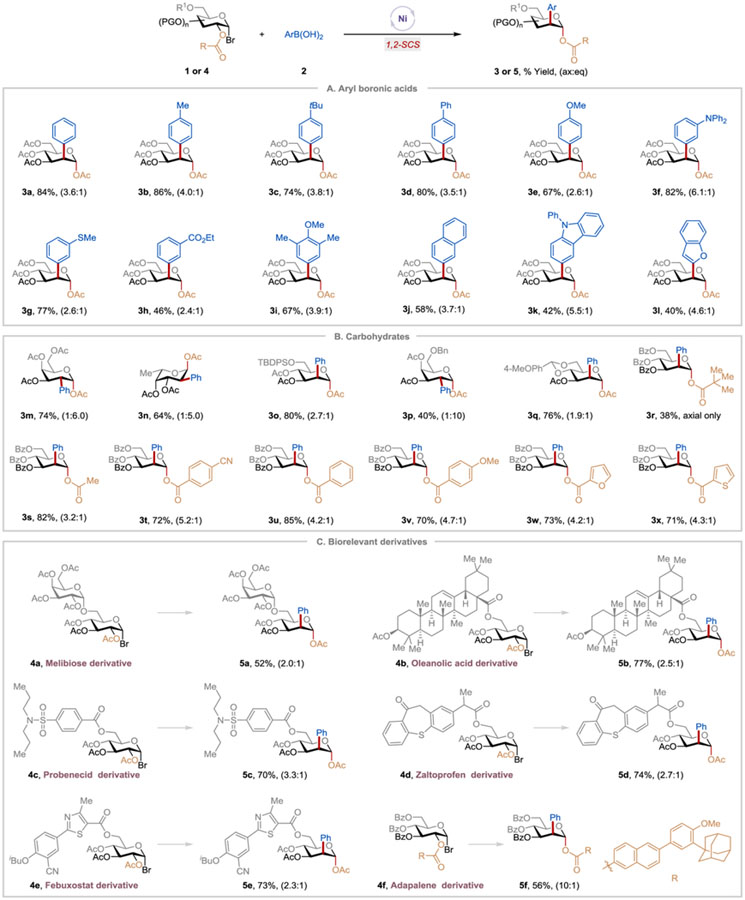
|
See SI for experimental details. Isolated yield and axial:equatorial (ax:eq) ratio are indicated below each entry.
The synthetic utility of the reaction is further highlighted by its amenability to a late-stage modification of functionally dense natural product- and drug-conjugated sugar derivatives (Table 2C). For instance, a melibiose derivative and the oleanolic acid-derived α-glucosyl bromide reacted under the standard conditions, affording the desired products (5a, 5b) in 52% and 77% yields, respectively. 1-Bromo-glucosyl derivatives of the uricosuric agent Probenecid, the anti-inflammatory drug Zaltoprofen, and the anti-hyperuricemic drug Febuxostat all underwent C-2 arylation giving the corresponding products (5c-5e) in good yields, demonstrating that the method can be used in the preparation of pharmaceutically relevant compounds. With the anti-acne agent Adapalene (4f) as a migratory group, the desired product (5f) was obtained in 56% yield with 10:1 axial:equatorial selectivity. This and earlier results, such as the formation of 3r indicated that increasing the size of the migratory group enhances the axial selectivity. It is worth noting that our protocol (i) affords the α-2-aryl-2-deoxy glycosides exclusively, with none of the corresponding β-isomers; (ii) enables access to previously inaccessible C-2 arylated carbohydrate derivatives and building blocks; and (iii) expands chemical and intellectual spaces for drug discovery.
While a detailed understanding of the reaction mechanism awaits further investigation, preliminary mechanistic studies suggested a radical process. The addition of a radical scavenger such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) completely inhibited the reaction (Figure 2A),8b, 15b and when the 1,2-trans- and 1,2-cis 2-iodo-sugars (6a, 6b) were subjected to the reaction conditions, they both formed the desired product (3a) in excellent yields with the same level of stereoselectivity (Figure 2B). This stereoselectivity was similar to that observed in the standard reaction using α-glucosyl bromide (1a) as the substrate, suggesting that these reactions proceed through a common C-2 radical intermediate. Cross-over experiments using substrates 1a and 1u afforded only the non-cross-over products (3a, 3u), indicating that the acyloxy migration likely takes place through a concerted mechanism (Figure 2C).
Figure 2.
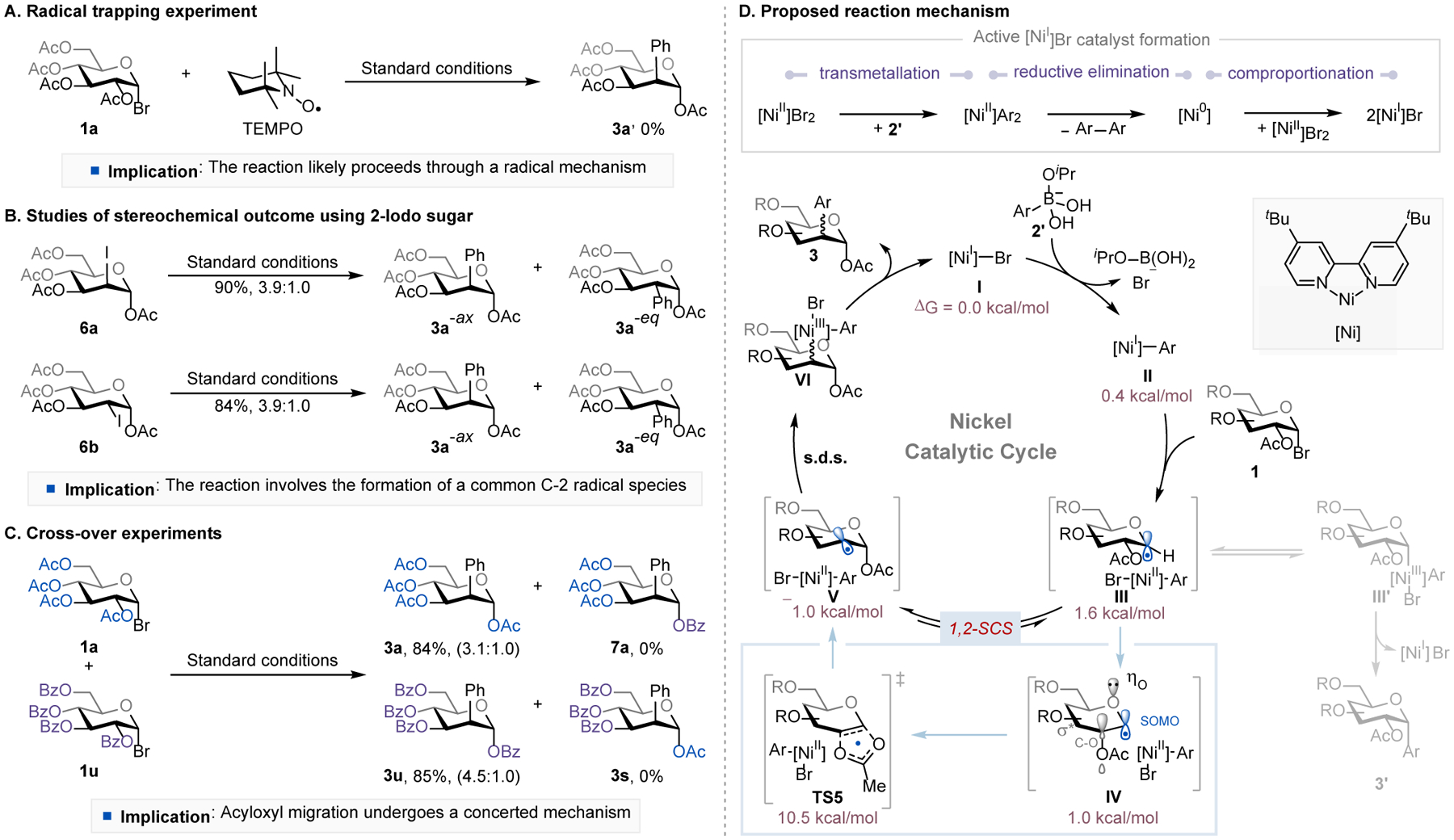
Mechanistic studies and proposed reaction mechanism. See SI for experimental and computational details. s.d.s. = stereoselectivity-determining step.
On the basis of these results, the known acyloxy migration,17, 26 the nickel-catalyzed Suzuki-Miyaura coupling,8b, 8d and DFT calculations (see SI, Figure S4 for the computed reaction energy profiles),27 a plausible catalytic cycle is shown in Figure 2D. The active catalyst [NiI]Br (I)28 is presumably generated under the standard conditions through (i) transmetallation of [NiII]Br2 precatalyst with two equivalents of dihydroxyisopropoxyaryl borate (2’), (ii) reductive elimination of the resulting [NiII]Ar2 complex to liberate diaryl side products and [Ni0] species, and (iii) comproportionation of [Ni0] with [NiII]Br2.29 [NiI]Br could undergo transmetallation with an arylboronate, forming a [NiI]Ar species (II). Bromine atom abstraction of α-glycosyl bromide (1) by complex II generates [NiII](Br)Ar species and chair 1-glycosyl radical (III). This radical intermediate could directly recombine with [NiII](Br)Ar and then reductive eliminate to form C-1 arylated side products (3’).22 However, DFT calculations showed that the conversion of III to its B2,5 boat conformation (IV) followed by a concerted 1,2-acyloxy rearrangement is more favorable under our reaction conditions (see SI, Figures S6 and S7). The 1-glucosyl radical prefers the B2,5 boat conformation (IV) by 0.6 kcal/mol, which stems from the extended anomeric interaction between the lone-pair electron of the endocyclic-O, the singly occupied molecular orbital (SOMO), and the σ*C-O orbital of the C-2 OAc group.30 This interaction weakens the C-2 OAc bond and promotes the 1,2-SCS through a concerted 1,2-acyloxy rearrangement via a cyclic five-membered ring transition state (TS5),26c, 30 affording the deoxypyranosan-2-yl radical (V).31 Although a typical secondary alkyl radical would be less stable than an anomeric radical, in this case, the molecular stability gained from the formation of an anomeric C–O bond in V drives the desired 1,2-SCS32 and makes this step (IV → V) exergonic by 2.0 kcal/mol. DFT calculations suggested that the stereoselectivity-determining step (s.d.s.) is the addition of the [NiII](Br)Ar species to deoxypyranosan-2-yl radical where the axial addition is more favorable than the equatorial addition, because the equatorial addition to square planar Ni complex is hindered by unfavorable steric interactions with the cis C-1 acetoxy group (Figures S4 and S5). These results agree with the experimentally observed preference for the 1,2-trans product. Once intermediate VI is formed, it undergoes reductive elimination, liberating the desired C-2 arylated product (3) and regenerating [NiI]Br catalyst (I). At this stage, we cannot rule out an alternative mechanism involving bromine atom abstraction of α-glycosyl bromide by [NiI]Br, transmetallation of the resulting [NiII]Br2 with aryl borate to form [NiII]Br(Ar), and then recombination of [NiII]Br(Ar) with 2-glycosyl radical followed by reductive elimination to give 2-arylated carbohydrates and regenerate [NiI]Br (see Figure S8 in SI for details).
In conclusion, we have developed the first nickel-catalyzed 1,2-SCS cross-coupling reaction that enables a direct synthesis of saturated, fully oxygenated 2-aryl-2-deoxy glycosides. The reaction features broad substrate scope, is amenable for late-stage functionalization of natural product and drug-conjugated sugar derivatives, and allows for the formation of C-2 arylated glycosides that cannot otherwise be easily accessed. Preliminary mechanistic studies suggest a radical reaction pathway with a concerted acyloxy migration. It is anticipated that this reaction will serve as the basis for the development of Ni-catalyzed radical migratory coupling reactions and a broadly useful C-2 functionalization of carbohydrates. This approach will eventually allow for the preparation of a wide array of novel carbohydrate mimics and building blocks for synthesis, medicinal chemistry, and materials science. A myriad of exciting studies and extensions of this chemistry can be envisaged, including detailed mechanistic studies, the identification of factors that govern the regio- and diastereoselectivities, the introduction of different functional groups at the C-2 position, alternative transition metal catalysts, and reaction development beyond carbohydrate functionalization. These are the subjects of an ongoing investigation.
Supplementary Material
ACKNOWLEDGMENT
The research reported in this publication was supported by the National Institutes of Health (R35-GM119652 to M.-Y.N and R35-GM128779 to P.L.). DFT calculations were performed at the Center for Research Computing at the University of Pittsburgh, the TACC Frontera supercomputer, and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation grant number ACI1548562. The Shimadzu UPLC/MS used for portions of this work were purchased with funds from NIGMS equipment administrative supplement, Shimadzu Scientific Instruments grant, and Office of the Vice President for Research at Stony Brook University. We also thank all the reviewers for their insightful comments and suggestions.
Footnotes
Supporting Information
Experimental details and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This paper is dedicated to Prof. Shunichi Fukuzumi on the occasion of his 70th birthday. The authors declare no competing financial interests.
REFERENCES
- (1).Varki A, Essentials of Glycobiology. 3rd ed.; Cold Spring Harbor Press: Cold Spring Harbor, New York, 2017. [PubMed] [Google Scholar]
- (2).(a) Nicolaou K; Mitchell HJ, Adventures in Carbohydrate Chemistry: New Synthetic Technologies, Chemical Synthesis, Molecular Design, and Chemical Biology. Angew. Chem. Int. Ed 2001, 40, 1576–1624; [PubMed] [Google Scholar]; (b) Seeberger PH; Werz DB, Automated Synthesis of Oligosaccharides as a Basis for Drug Discovery. Nat. Rev. Drug Discovery 2005, 4, 751–763; [DOI] [PubMed] [Google Scholar]; (c) Fernández-Tejada A; Cañada FJ; Jiménez-Barbero J, Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. - Eur. J 2015, 21, 10616–10628; [DOI] [PubMed] [Google Scholar]; (d) Yuan SS; Li ML; Chen JS; Zhou L; Zhou W, Application of Mono-and Disaccharides in Drug Targeting and Efficacy. ChemMedChem 2018, 13, 764–778. [DOI] [PubMed] [Google Scholar]
- (3).(a) Demchenko AV, Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. John Wiley & Sons: 2008; [Google Scholar]; (b) Zhu X; Schmidt RR, New Principles for Glycoside-Bond Formation. Angew. Chem. Int. Ed 2009, 48, 1900–1934; [DOI] [PubMed] [Google Scholar]; (c) Park Y; Harper KC; Kuhl N; Kwan EE; Liu RY; Jacobsen EN, Macrocyclic Bis-Thioureas Catalyze Stereospecific Glycosylation Reactions. Science 2017, 355, 162–166; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu C; Loh CC, A Multistage Halogen Bond Catalyzed Strain-Release Glycosylation Unravels New Hedgehog Signaling Inhibitors. J. Am. Chem. Soc 2019, 141, 5381–5391; [DOI] [PubMed] [Google Scholar]; (e) Li Q; Levi SM; Jacobsen EN, Highly Selective B-Mannosylations and B-Rhamnosylations Catalyzed by Bis-Thiourea. J. Am. Chem. Soc 2020, 142, 11865–11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Zhu F; Rourke MJ; Yang T; Rodriguez J; Walczak MA, Highly Stereospecific Cross-Coupling Reactions of Anomeric Stannanes for the Synthesis of C-Aryl Glycosides. J. Am. Chem. Soc 2016, 138, 12049–12052; [DOI] [PubMed] [Google Scholar]; (b) Yang Y; Yu B, Recent Advances in the Chemical Synthesis of C-Glycosides. Chem. Rev 2017, 117, 12281–12356; [DOI] [PubMed] [Google Scholar]; (c) Zhu F; Rodriguez J; Yang T; Kevlishvili I; Miller E; Yi D; O’Neill S; Rourke MJ; Liu P; Walczak MA, Glycosyl Cross-Coupling of Anomeric Nucleophiles: Scope, Mechanism, and Applications in the Synthesis of Aryl C-Glycosides. J. Am. Chem. Soc 2017, 139, 17908–17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Bennett CS; Galan MC, Methods for 2-Deoxyglycoside Synthesis. Chem. Rev 2018, 118, 7931–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Selected recent reviews and examples of site-selective functionalization of carbohydrates:Wang H-Y; Blaszczyk SA; Xiao G; Tang W, Chiral Reagents in Glycosylation and Modification of Carbohydrates. Chem. Soc. Rev 2018, 47, 681–701; [DOI] [PubMed] [Google Scholar]; (b) Dimakos V; Taylor MS, Site-Selective Functionalization of Hydroxyl Groups in Carbohydrate Derivatives. Chem. Rev 2018, 118, 11457–11517; [DOI] [PubMed] [Google Scholar]; (c) Blaszczyk SA; Homan TC; Tang W, Recent Advances in Site-Selective Functionalization of Carbohydrates Mediated by Organocatalysts. Carbohydr. Res 2019, 471, 64–77; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dimakos V; Su HY; Garrett GE; Taylor MS, Site-Selective and Stereoselective C–H Alkylations of Carbohydrates Via Combined Diarylborinic Acid and Photoredox Catalysis. J. Am. Chem. Soc 2019, 141, 5149–5153. [DOI] [PubMed] [Google Scholar]
- (7).(a) Lu S; Li X; Wang A, A New Chiral Diphosphine Ligand and Its Asymmetric Induction in Catalytic Hydroformylation of Olefins. Catal. Today 2000, 63, 531–536; [Google Scholar]; (b) Hang HC; Bertozzi CR, Ketone Isosteres of 2-N-Acetamidosugars as Substrates for Metabolic Cell Surface Engineering. J. Am. Chem. Soc 2001, 123, 1242–1243; [DOI] [PubMed] [Google Scholar]; (c) De Lederkremer RM; Marino C, Deoxy Sugars: Occurrence and Synthesis. Adv. Carbohydr. Chem. Biochem 2007, 61, 143–216; [DOI] [PubMed] [Google Scholar]; (d) Pajak B; Siwiak E; Sołtyka M; Priebe A; Zieliński R; Fokt I; Ziemniak M; Jaśkiewicz A; Borowski R; Domoradzki T, 2-Deoxy-D-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci 2020, 21, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) For selected reviews, accounts, and perspectives, see:Netherton MR; Fu GC, Nickel-Catalyzed Cross-Couplings of Unactivated Alkyl Halides and Pseudohalides with Organometallic Compounds. Adv. Synth. Catal 2004, 346, 1525–1532; [Google Scholar]; (b) Hu X, Nickel-Catalyzed Cross-Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem. Sci 2011, 2, 1867–1886; [Google Scholar]; (c) Montgomery J, Organonickel Chemistry. In Organometallics in Synthesis, Lipshutz BH, Ed. Wiley: Hoboken, NJ, 2013; pp 319–428; [Google Scholar]; (d) Han F-S, Transition-Metal-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions: A Remarkable Advance from Palladium to Nickel Catalysts. Chem. Soc. Rev 2013, 42, 5270–5298; [DOI] [PubMed] [Google Scholar]; (e) Tasker SZ; Standley EA; Jamison TF, Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tellis JC; Kelly CB; Primer DN; Jouffroy M; Patel NR; Molander GA, Single-Electron Transmetalation Via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for Sp3–Sp2 Cross-Coupling. Acc. Chem. Res 2016, 49, 1429–1439; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Iwasaki T; Kambe N, Ni-Catalyzed C–C Couplings Using Alkyl Electrophiles. In Ni-and Fe-Based Cross-Coupling Reactions, Springer: 2017; pp 1–36; [Google Scholar]; (h) Twilton J; Zhang P; Shaw MH; Evans RW; MacMillan DW, The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem 2017, 1, 1–19; [Google Scholar]; (i) Milligan JA; Phelan JP; Badir SO; Molander GA, Alkyl Carbon–Carbon Bond Formation by Nickel/Photoredox Cross-Coupling. Angew. Chem. Int. Ed 2019, 58, 6152–6163; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ogoshi S, Nickel Catalysis in Organic Synthesis: Methods and Reactions. John Wiley & Sons: 2020; [Google Scholar]; (k) Poremba KE; Dibrell SE; Reisman SE, Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions. ACS Catal. 2020, 10, 8237–8246; [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Diccianni J; Lin Q; Diao T, Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res 2020, 53, 906–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Sommer H; Juliá-Hernández F; Martin R; Marek I, Walking Metals for Remote Functionalization. ACS Cent. Sci 2018, 4, 153–165; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Janssen-Müller D; Sahoo B; Sun SZ; Martin R, Tackling Remote sp3 C–H Functionalization Via Ni-Catalyzed “Chain-Walking” Reactions. Isr. J. Chem 2020, 60, 195–206. [Google Scholar]
- (10).(a) Chen F; Chen K; Zhang Y; He Y; Wang Y-M; Zhu S, Remote Migratory Cross-Electrophile Coupling and Olefin Hydroarylation Reactions Enabled by in Situ Generation of Nih. J. Am. Chem. Soc 2017, 139, 13929–13935; [DOI] [PubMed] [Google Scholar]; (b) Peng L; Li Y; Li Y; Wang W; Pang H; Yin G, Ligand-Controlled Nickel-Catalyzed Reductive Relay Cross-Coupling of Alkyl Bromides and Aryl Bromides. ACS Catal. 2018, 8, 310–313; [Google Scholar]; (c) Peng L; Li Z; Yin G, Photochemical Nickel-Catalyzed Reductive Migratory Cross-Coupling of Alkyl Bromides with Aryl Bromides. Org. Lett 2018, 20, 1880–1883; [DOI] [PubMed] [Google Scholar]; (d) Kumar GS; Peshkov A; Brzozowska A; Nikolaienko P; Zhu C; Rueping M, Nickel-Catalyzed Chain-Walking Cross-Electrophile Coupling of Alkyl and Aryl Halides and Olefin Hydroarylation Enabled by Electrochemical Reduction. Angew. Chem. Int. Ed 2020, 59, 6513–6519. [DOI] [PubMed] [Google Scholar]
- (11).Zhu C; Liu Z-Y; Tang L; Zhang H; Zhang Y-F; Walsh PJ; Feng C, Migratory Functionalization of Unactivated Alkyl Bromides for Construction of All-Carbon Quaternary Centers Via Transposed Tert-C-Radicals. Nat. Commun 2020, 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).He J; Song P; Xu X; Zhu S; Wang Y, Migratory Reductive Acylation between Alkyl Halides or Alkenes and Alkyl Carboxylic Acids by Nickel Catalysis. ACS Catal. 2019, 9, 3253–3259. [Google Scholar]
- (13).(a) Juliá-Hernández F; Moragas T; Cornella J; Martin R, Remote Carboxylation of Halogenated Aliphatic Hydrocarbons with Carbon Dioxide. Nature 2017, 545, 84–88; [DOI] [PubMed] [Google Scholar]; (b) Sahoo B; Bellotti P; Juliá-Hernández F; Meng QY; Crespi S; König B; Martin R, Site-Selective, Remote sp3 C–H Carboxylation Enabled by the Merger of Photoredox and Nickel Catalysis. Chem. - Eur. J 2019, 25, 9001–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Spin-center shift is broadly defined as shifting the position of the radical center to another atom in the course of the reaction.Wessig P; Muehling O, Spin-Center Shift (SCS)–a Versatile Concept in Biological and Synthetic Chemistry. Eur. J. Org. Chem 2007, 2007, 2219–2232; for selected recent examples, see: [Google Scholar]; (b) Jin J; MacMillan DW, Alcohols as Alkylating Agents in Heteroarene C–H Functionalization. Nature 2015, 525, 87–90; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nacsa ED; MacMillan DW, Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols. J. Am. Chem. Soc 2018, 140, 3322–3330; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dimakos V; Gorelik D; Su HY; Garrett GE; Hughes G; Shibayama H; Taylor MS, Site-Selective Redox Isomerizations of Furanosides Using a Combined Arylboronic Acid/Photoredox Catalyst System. Chem. Sci 2020, 11, 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) For selected examples of Ni-catalyzed MCC via 1,6-spin-center shift, see:Powell DA; Maki T; Fu GC, Stille Cross-Couplings of Unactivated Secondary Alkyl Halides Using Monoorganotin Reagents. J. Am. Chem. Soc 2005, 127, 510–511; [DOI] [PubMed] [Google Scholar]; (b) Phapale VB; Buñuel E; García-Iglesias M; Cárdenas DJ, Ni-Catalyzed Cascade Formation of C(sp3)-C(sp3) Bonds by Cyclization and Cross-Coupling Reactions of Iodoalkanes with Alkyl Zinc Halides. Angew. Chem. Int. Ed 2007, 46, 8790–8795. [DOI] [PubMed] [Google Scholar]
- (16).(a) Surzur J; Teissier P, Addition Radicalaire Desters Sur Les Alcools Ethyleniques. C. R. Acad. Sci. Fr. Ser. C 1967, 264, 1981–1984; [Google Scholar]; (b) Tanner DD; Law FC, Free-Radical Acetoxy Group Migration. J. Am. Chem. Soc 1969, 91, 7535–7537. [Google Scholar]
- (17).We recently reported an excited-state Pd-catalyzed C-2 reduction, deuteration, and iodination of 1-halo sugars, but the corresponding C-2 arylation failed under the reported conditions.Zhao G; Yao W; Mauro JN; Ngai M-Y, Excited-State Palladium-Catalyzed 1,2-Spin-Center Shift Enables Selective C-2 Reduction, Deuteration, and Iodination of Carbohydrates. J. Am. Chem. Soc 2021, 143, 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).(a) For the synthesis of unsaturated or partially oxygenated 2-arylsugars, see:Tenaglia A; Karl F, Intramolecular Heck Reaction of Hex-2-Enopyranosides: An Easy Entry to Cis-Fused Furo-or Pyrano [2, 3b] Pyranones. Synlett 1996, 1996, 327–329; [Google Scholar]; (b) Cobo I; Matheu MI; Castillón S; Boutureira O; Davis BG, Phosphine-Free Suzuki–Miyaura Cross-Coupling in Aqueous Media Enables Access to 2-C-Aryl-Glycosides. Org. Lett 2012, 14, 1728–1731; [DOI] [PubMed] [Google Scholar]; (c) Kusunuru AK; Jaladanki CK; Tatina MB; Bharatam PV; Mukherjee D, Tempo-Promoted Domino Heck–Suzuki Arylation: Diastereoselective Cis-Diarylation of Glycals and Pseudoglycals. Org. Lett 2015, 17, 3742–3745; [DOI] [PubMed] [Google Scholar]; (d) Probst N; Grelier G; Dahaoui S; Alami M. d.; Gandon V; Messaoudi S, Palladium (Ii)-Catalyzed Diastereoselective 2, 3-Trans C (Sp3)–H Arylation of Glycosides. ACS Catal. 2018, 8, 7781–7786; [Google Scholar]; (e) Ghouilem J; Franco R; Retailleau P; ALAMI M; Gandon V; Messaoudi S, Regio-and Diastereoselective Pd-Catalyzed Synthesis of C2 Aryl Glycosides. Chem. Commun 2020, 56, 7175–7178. [DOI] [PubMed] [Google Scholar]
- (19).(a) Sugimura H; Osumi K; Koyama T, A Convenient Route for the Synthesis of 2-C-Substituted 2-Deoxyhexoses. Chem. Lett 1991, 20, 1379–1382; [Google Scholar]; (b) Robertson J; Green SP; Hall MJ; Tyrrell AJ; Unsworth WP, Further Studies on Silatropic Carbonyl Ene Cyclisations: B-Crotyl (Diphenyl) Silyloxy Aldehyde Substrates; Synthesis of 2-Deoxy-2-C-Phenylhexoses. Org. Biomol. Chem 2008, 6, 2628–2635. [DOI] [PubMed] [Google Scholar]
- (20).(a) Richards G, The Action of Grignard Reagents on Anhydro-Sugars of Ethylene Oxide Type. Part Iv. The Behaviour of Methyl 2:3-Anhydro-4:6-O-Benzylidene-α-D-Mannoside Towards Diphenylmagnesium. J. Chem. Soc, 1955, 2013–2016; [Google Scholar]; (b) Hladezuk I; Olesker A; Cléophax J; Lukacs G, Synthesis of 2-C- and 3-C-Aryl Pyranosides. J. Carbohydr. Chem 1998, 17, 869–878. [Google Scholar]
- (21).For detailed reaction optimization, please see the Supporting Information.
- (22).(a) Gong H; Gagne MR, Diastereoselective Ni-Catalyzed Negishi Cross-Coupling Approach to Saturated, Fully Oxygenated C-Alkyl and C-Aryl Glycosides. J. Am. Chem. Soc 2008, 130, 12177–12183; [DOI] [PubMed] [Google Scholar]; (b) Liu J; Gong H, Stereoselective Preparation of A-C-Vinyl/Aryl Glycosides Via Nickel-Catalyzed Reductive Coupling of Glycosyl Halides with Vinyl and Aryl Halides. Org. Lett 2018, 20, 7991–7995. [DOI] [PubMed] [Google Scholar]
- (23).Using two equivalents of ligand w.r.t. NiBr2DME is necessary for high reaction efficiency, decreased the ligand:Ni ratio lowered the product yields (Table S10), for similar observation, see, Zhou J; Fu GC, Suzuki Cross-Couplings of Unactivated Secondary Alkyl Bromides and Iodides. J. Am. Chem. Soc 2004, 126, 1340–1341. [DOI] [PubMed] [Google Scholar]
- (24).Saito B; Fu GC, Alkyl–Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2007, 129, 9602–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Mannose and rhamnose derivatives where the B2,5 boat conformation is less favorable failed to afford the desired C-2 arylated product.
- (26).(a) Giese B; Gröninger KS; Witzel T; Korth HG; Sustmann R, Synthesis of 2-Deoxy Sugars. Angew. Chem. Int. Ed 1987, 26, 233–234; [Google Scholar]; (b) Zipse H, [1, 2]-Acyloxy Shifts in Radicals. A Computational Investigation of Substituent and Solvent Effects. J. Am. Chem. Soc 1997, 119, 1087–1093; [Google Scholar]; (c) Beckwith ALJ; Crich D; Duggan PJ; Yao Q, Chemistry of β-(Acyloxy)Alkyl and β-(Phosphatoxy)Alkyl Radicals and Related Species: Radical and Radical Ionic Migrations and Fragmentations of Carbon–Oxygen Bonds. Chem. Rev 1997, 97, 3273–3312. [DOI] [PubMed] [Google Scholar]
- (27).DFT calculations were performed using a simplified model of glucosyl bromide (1y), where the OMe group was used instead of OAc group at C-3, 4, and 6 positions of the sugar backbone, see Figures S4 and S5 in SI for details.
- (28).At this stage, we cannot rule out the possibility of involving a dimeric Ni(I)Br species in the reaction.Mohadjer Beromi M; Brudvig GW; Hazari N; Lant HM; Mercado BQ, Synthesis and Reactivity of Paramagnetic Nickel Polypyridyl Complexes Relevant to C(sp2)–C(sp3) Coupling Reactions. Angew. Chem. Int. Ed 2019, 58, 6094–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).(a) We detected the biaryl side product in the reaction mixture. For examples of formation of Ni(I)-Br from Ni(II)Br2, see:Jones GD; Martin JL; McFarland C; Allen OR; Hall RE; Haley AD; Brandon RJ; Konovalova T; Desrochers PJ; Pulay P, Ligand Redox Effects in the Synthesis, Electronic Structure, and Reactivity of an Alkyl–Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc 2006, 128, 13175–13183; [DOI] [PubMed] [Google Scholar]; (b) Wilsily A; Tramutola F; Owston NA; Fu GC, New Directing Groups for Metal-Catalyzed Asymmetric Carbon–Carbon Bond-Forming Processes: Stereoconvergent Alkyl–Alkyl Suzuki Cross-Couplings of Unactivated Electrophiles. J. Am. Chem. Soc 2012, 134, 5794–5797; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zultanski SL; Fu GC, Nickel-Catalyzed Carbon–Carbon Bond-Forming Reactions of Unactivated Tertiary Alkyl Halides: Suzuki Arylations. J. Am. Chem. Soc 2013, 135, 624–627; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Singh S; Sunoj RB, Mechanism and Origin of Enantioselectivity in Nickel-Catalyzed Alkyl–Alkyl Suzuki Coupling Reaction. J. Phys. Chem. A 2019, 123, 6701–6710. [DOI] [PubMed] [Google Scholar]
- (30).(a) Dupuis J; Giese B; Rüegge D; Fischer H; Korth HG; Sustmann R, Conformation of Glycosyl Radicals: Radical Stabilization by B-Co Bonds. Angew. Chem. Int. Ed 1984, 23, 896–898; [Google Scholar]; (b) Abe H; Shuto S; Matsuda A, Highly α- and β-Selective Radical C-Glycosylation Reactions Using a Controlling Anomeric Effect Based on the Conformational Restriction Strategy. A Study on the Conformation–Anomeric Effect–Stereoselectivity Relationship in Anomeric Radical Reactions. J. Am. Chem. Soc 2001, 123, 11870–11882. [DOI] [PubMed] [Google Scholar]
- (31).Korth H-G; Sustmann R; Gröninger KS; Witzel T; Giese B, Electron Spin Resonance Spectroscopic Investigation of Carbohydrate Radicals. Part 3. Conformation in Deoxypyranosan-2-, -3-, and -4-yl Radicals. J. Chem. Soc., Perkin Trans 21986, 1461–1464. [Google Scholar]
- (32).Korth HG; Sustmann R; Groeninger KS; Leisung M; Giese B, Electron Spin Resonance Spectroscopic Investigation of Carbohydrate Radicals. 4. 1, 2-Acyloxyl Migration in Pyranosyl Radicals. J. Org. Chem 1988, 53, 4364–4369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


