Abstract
Reactive oxygen species (ROS; e.g., superoxide [O2•−] and hydrogen peroxide [H2O2]) and reactive nitrogen species (RNS; e.g., nitric oxide [NO•]) at the physiological level function as signaling molecules that mediate many biological responses, including cell proliferation, migration, differentiation, and gene expression. By contrast, excess ROS/RNS, a consequence of dysregulated redox homeostasis, is a hallmark of cardiovascular disease. Accumulating evidence suggests that both ROS and RNS regulate various metabolic pathways and enzymes. Recent studies indicate that cells have mechanisms that fine-tune ROS/RNS levels by tight regulation of metabolic pathways, such as glycolysis and oxidative phosphorylation. The ROS/RNS-mediated inhibition of glycolytic pathways promotes metabolic reprogramming away from glycolytic flux toward the oxidative pentose phosphate pathway to generate nicotinamide adenine dinucleotide phosphate (NADPH) for antioxidant defense. This review summarizes our current knowledge of the mechanisms by which ROS/RNS regulate metabolic enzymes and cellular metabolism and how cellular metabolism influences redox homeostasis and the pathogenesis of disease. A full understanding of these mechanisms will be important for the development of new therapeutic strategies to treat diseases associated with dysregulated redox homeostasis and metabolism. Antioxid. Redox Signal. 34, 1319–1354.
Keywords: metabolism, oxidative stress, redox signaling, reactive oxygen species, reactive nitrogen species
Introduction
Dysregulated redox homeostasis is a hallmark of cardiovascular disease (CVD) (88, 118, 262). Enzymes that produce reactive oxygen species (ROS; e.g., superoxide [O2•−], hydrogen peroxide [H2O2]) and reactive nitrogen species (RNS; e.g., nitric oxide [NO•]) are regulated by location-dependent changes in metabolic flux (Figs. 2 and 3). Metabolic changes are among the most prominent features of aging and have been identified in numerous disease states (Fig. 6), because metabolism impacts cellular function through various mechanisms (Fig. 1). How these changes serve to influence the redox balance (and vice versa) is poorly understood (Figs. 4–6) (1, 102, 178, 254, 283). The ROS play the role of a double-edged sword in both physiologic and pathologic processes. Ambient levels at any given time reflect the balance between the rate and the magnitude of ROS production versus its elimination (185, 371, 386). At the physiological level, ROS are involved in cellular signaling. However, when present in excess, ROS can drive pathologies associated with aging, cancer, and atherosclerosis (102). Reductive stress, a state in which ROS levels are too low, can also promote and exacerbate a wide spectrum of pathologies ranging from cancer to cardiomyopathy (371).
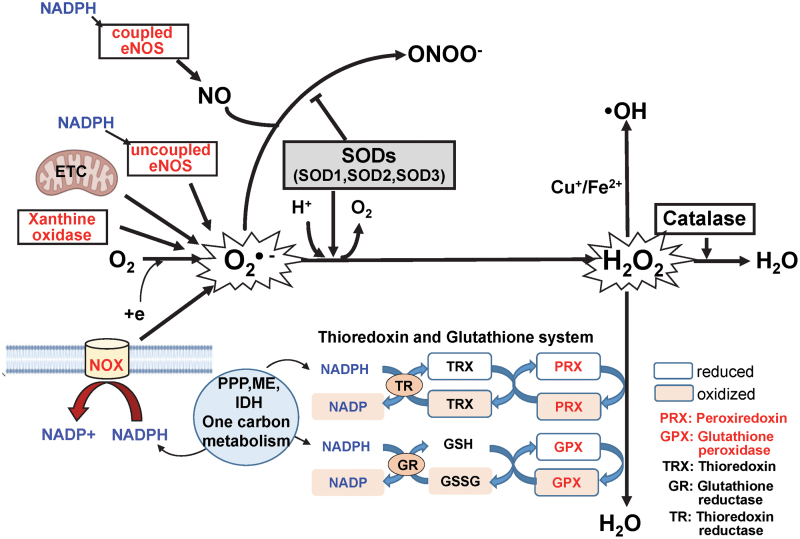
FIG. 2.
Generation and metabolism of ROS/RNS. O2•− is produced by NOXs, the mitochondrial ETC, XO, lipoxygenase, cyclooxygenase, and uncoupled NOS. O2•− is converted by SODs to H2O2, which, in turn, is reduced to water via the actions of catalase, GPXs, and PRXs. The PRX/TRX and GPX/GSH systems are fueled by NADPH, which is generated by the PPP, IDHs, MEs, and 1C metabolism. Of note, NADPH is also a substrate for the ROS-generating NOXs and NOS. In the presence of reduced transition metals (Fe2+ and Cu2+), H2O2 undergoes spontaneous conversion to reactive OH• or related metal-associated reactive species. NO• is produced by coupled NOS. The NOS enzymes utilize NADPH and l-arginine as co-substrates and BH4 (a product of 1C metabolism) as essential co-factors. Although all NOS isoforms generate NO•, they can also generate O2•− at the expense of NO• via a process known as uncoupling. The mechanisms underlying the uncoupling process include the formation of monomers, altered Hsp90 binding, and insufficient levels of BH4 and l-arginine. Importantly, NO• can be rapidly inactivated via a reaction with O2•−, which leads to the formation of the strong oxidant, ONOO−. Thus, SODs are the first line of defense against O2•−-mediated toxicity. The SODs also participate in cell signaling events via their capacity to regulate levels of ROS (e.g., O2•−, H2O2) while preserving available NO•. BH4, tetrahydrobiopterin; GPX, glutathione peroxidase; H2O2, hydrogen peroxide; Hsp, heat-shock protein; NO•, nitric oxide; NOS, nitric oxide synthase; NOX, NADPH oxidase; O2•−, superoxide; OH•, hydroxyl radical; ONOO−, peroxynitrite; PRX, peroxiredoxin; RNS, reactive nitrogen species; SOD, superoxide dismutase; XO, xanthine oxidase. Color images are available online.
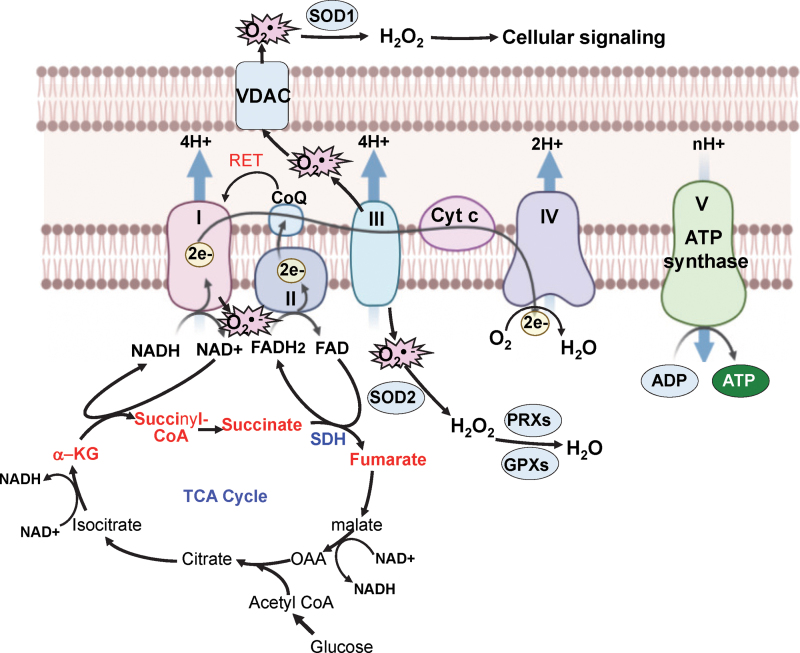
FIG. 3.
Interplay between mitoROS production, the ETC, and the TCA cycle. mitoROS are produced by the ETC at complexes I and III during oxidative phosphorylation. The reducing equivalents NADH and FADH2, which are generated by the TCA cycle in a series of enzymatic reactions, transfer electrons to the ETC to produce ATP. Thus, mitoROS, the ETC, and the TCA cycle are closely connected during oxidative phosphorylation. When the pool of CoQ is reduced, mitoROS are produced by complex I via RET. Further, VDACs control the release of O2•− from the mitochondria to the cytosol. NADH is produced during the conversion of α-α-KG to succinyl CoA to provide electrons for complex I in the ETC. NADH is also produced in the conversion of isocitrate to α-KG and the conversion of malate to OAA in the TCA cycle. FADH2 is produced during the conversion of succinate to fumarate via the actions of SDH, which is an enzyme that participates in both the TCA cycle and the ETC. α-KG, α-ketoglutarate; CoA, coenzyme A; complex I, NADH–ubiquinone oxidoreductase; complex III, ubiquinol–cytochrome c oxidoreductase; coQ, coenzyme Q; Cyt c: cytochrome c; FADH2, reduced flavin adenine dinucleotide; NADH, nicotinamide adenine dinucleotide; OAA, oxaloacetate; RET, reverse electron transport; SDH, succinate dehydrogenase; VDAC, voltage-dependent anion channel. Color images are available online.
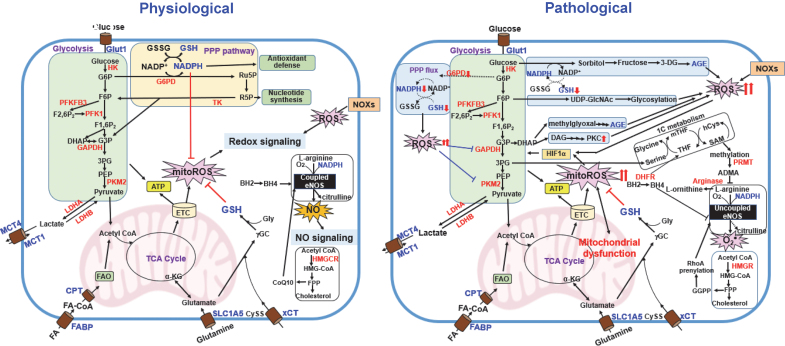
FIG. 6.
The interplay between metabolic pathways and ROS (redox homeostasis) in physiological and pathological states.Left panel, glycolysis plays an essential role in the control of redox homeostasis by generating PPP-derived NADPH that is involved in the antioxidant system. FAO also generates NADPH through metabolic reactions. Glutaminolysis is involved in redox control by increasing GSH synthesis. During mitochondrial metabolism, ROS will be produced via ETC. NO• is produced by NOS by utilizing BH4, NADPH, and molecular O2 to convert l-arginine to l-citrulline (coupled eNOS). NOXs- and mitochondria-derived ROS activate redox signaling. Right panel, in pathological states such as diabetes and atherosclerosis, excess ROS due to eNOS uncoupling and PPP-GSH impairment inhibit glycolytic flux, which diverts glycolytic intermediates into alternative metabolic pathways such as polyol pathway (AGE production) and PKC activation. This, in turn, further increases mitochondria- or NOX-derived ROS production and mitochondrial dysfunction. mitoROS or Nox-derived ROS stabilize HIF-1α, which induces a metabolic shift toward glycolysis, resulting in reduced oxidative phosphorylation and mitoROS production. The ADMA produced by l-arginine methylation via the 1C metabolism as well as arginase inhibit l-arginine binding to NOS, which decreases NO production. The mevalonate pathway also facilitates eNOS uncoupling by RhoAprenylation induced by GGPP, thereby promoting excess ROS production. ADMA, asymmetric dimethylarginine; GGPP, geranylgeranyl pyrophosphate; PKC, protein kinase C. Color images are available online.
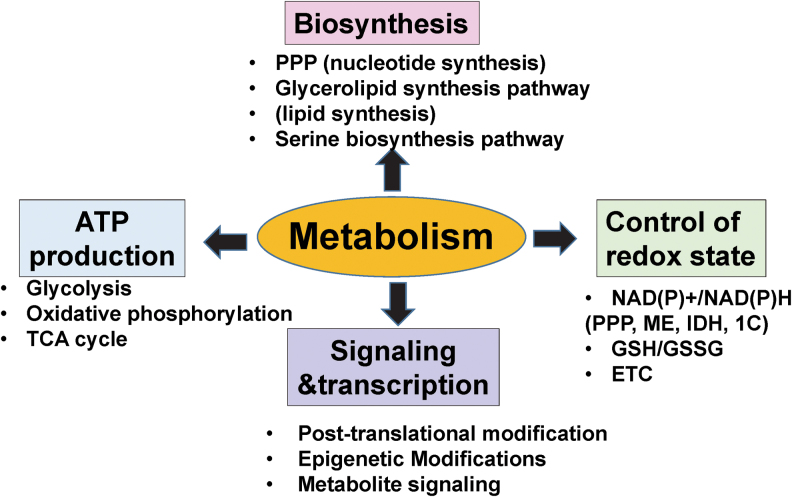
FIG. 1.
Role of metabolism in cellular function. Metabolism regulates cellular function by integrating energy production, biosynthesis, control of redox state, cell signaling, and transcription. The main metabolic task is to produce ATP to meet energetic demands for cellular function via glycolysis, oxidative phosphorylation and the TCA cycle. Metabolism is also necessary for biosynthetic pathways, including the PPP for nucleotide synthesis, as well as the glycerolipid synthesis pathway (lipid synthesis) and serine biosynthesis pathway. Metabolic pathways also regulate the intracellular redox state by controlling NAD(P)+/NAD(P)H pools and GSH to fuel the TRX/GSH antioxidant defense system. NADPH is derived from PPP, IDHs, MEs, and 1C metabolism, whereas GSH is derived from glutaminolysis. Further, metabolism regulates ETC to produce mitoROS. Finally, metabolism influences signaling and transcription by regulating post-translational modification (e.g., glycosylation via the hexosamine biosynthetic pathway), epigenetic modification, and metabolite signaling. 1C, one-carbon; ATP, adenosine triphosphate; ETC, electron transport chain; GSH, glutathione; ME, malic enzyme; mitoROS, mitochondrial ROS; NADPH, nicotinamide adenine dinucleotide phosphate; PPP, pentose phosphate pathway; ROS, reactive oxygen species; TCA, tricarboxylic acid; TRX, thioredoxin. Color images are available online.
FIG. 4.
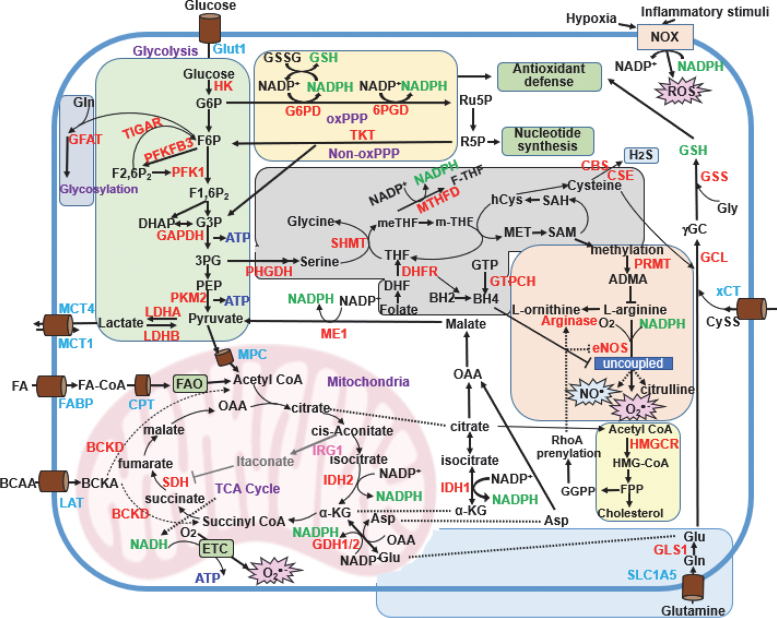
Cellular metabolic pathway involved in redox homeostasis. The major metabolic pathways that regulate redox homeostasis in ECs are as shown. Parts of metabolic pathways that take place in immune cells (e.g., those involving itaconate) are also included. Metabolic pathways that regulate redox homeostasis are limited to those involved in the production of NADPH and GSH (shown in green) and that regulate eNOS activity (shown in red). The ECs primarily utilize glycolysis (shaded in green) to obtain ATP. During this process, ECs generate pyruvate and lactate from glucose, thereby contributing to four additional pathways. The first of these, known as the PPP (shaded in yellow), includes both oxPPP and non-oxPPP pathways that contribute to antioxidant defense and nucleotide synthesis, respectively. Second, 1C metabolism (shaded in gray) contributes to protein and nucleotide methylation. Third, the hexosamine pathway (shaded in blue) uses F6P to promote protein glycosylation and synthesis of the luminal glycocalyx. Finally, after glycolysis, pyruvate can enter the mitochondria where it is converted to acetyl-CoA and can then enter the TCA cycle (shown in purple). NADPH is essential not only for antioxidant defense pathways, including the PRX/TRX and GPX/GSH systems that mitigate ROS-related cellular damage, but it is also necessary for the generation of NO• (a cofactor for NOS) and O2•− (a cofactor of the NOX enzymes). The major metabolic pathways that generate NADPH include oxPPP, ME1, 1C metabolism, IDH1/2, glutamine metabolism, and CPT1-mediated FAO. De novo synthesis of the antioxidant, GSH (shown in green) involves CySS import into the cell via the CySS/glutamate transporter (xCT), cysteine generated from methionine via the transsulfuration pathway, and glutamine metabolism. Cysteine is also involved in the synthesis of the gaseous transmitter, H2S. The primary metabolic pathways contributing to coupled and uncoupled eNOS include the ornithine cycle (shaded in pink), the mevalonate pathway (shaded in pale blue), and 1C metabolism (via BH4). Lastly, itaconate synthesized from aconitate in activated macrophages via the actions of IRG1 inhibits the activity of SDH. This inhibits ROS generation by RET at complex I. CPT1, carnitine palmitoyltransferase-1; CySS, cystine; EC, endothelial cell; eNOS, endothelial NOS; FAO, fatty acid oxidation; H2S, hydrogen sulfide; IRG1, immune-responsive gene 1; LDH, lactate dehydrogenase; oxPPP, oxidative PPP; xCT, the cystine/glutamate antiporter SLC7A11. Color images are available online.
FIG. 5.
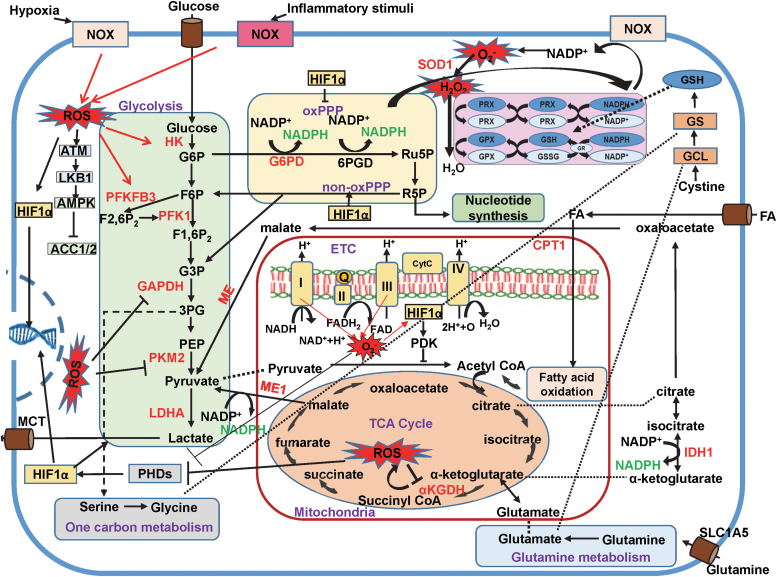
Interplay between ROS and cellular metabolic pathways. Metabolic pathways contribute to redox homeostasis by regulating ROS generation via NOX/NADPH and mitochondrial ETC as well as by their impact on antioxidant systems via production of NADPH and GSH, as outlined in Figure 3. Conversely, cytosolic and mitoROS, which are produced by NOX, mitochondrial respiration, as well as metabolic and other enzymes, regulate metabolic pathways by targeting specific enzymes and transcription factors, including AMPK, glycolytic enzymes, mitochondrial enzymes, and HIFs. HIF-1α promotes a shift in metabolism toward glycolysis, while inhibiting mitochondrial O2 consumption. This leads to decreased production of ATP through oxidative phosphorylation and thus reduced levels of mitoROS. AMPK, 5′ adenosine monophosphate-activated protein kinase; HIF, hypoxia-inducible factor; O2, oxygen. Color images are available online.
Metabolism is profoundly affected by oxidative stress (Figs. 5 and 6) (1, 102, 178, 254, 283). For example, ROS/RNS can inhibit multiple glycolytic enzymes, including glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and pyruvate kinase M2 (PKM2) (4, 259) (Figs. 5 and 6). The inhibition of glycolytic pathways by ROS/RNS promotes metabolic reprogramming away from glycolytic flux toward the oxidative arm of the pentose phosphate pathway (PPP). This shift results in increased production of nicotinamide adenine dinucleotide phosphate (NADPH), which is needed to support antioxidant defense. Cancer cells can promote their own survival via antioxidant defense, specifically via metabolic reprogramming, to prevent cell death due to excessive ROS accumulation (178, 254, 283). Interestingly, cancer-prone adenomatous polyposis coli-deficient cells exhibit increased mitochondrial- and NADPH oxidase (NOX)-mediated ROS production as well as increases in the TP53-induced glycolysis and apoptosis regulator (TIGAR)-mediated antioxidant defense; however, both pathways contribute to cell proliferation (51) (Fig. 4). In this case, NOX enzymes generate ROS that serve to increase cell proliferation whereas TIGAR limits the damaging effects of ROS (Fig. 4). These findings underscore the apparent importance of both temporal and spatial regulation of redox balance. In this review, we will summarize our current knowledge of this field with a focus on the reciprocal regulation of ROS/RNS and metabolic pathways and their contributions to vascular biology and disease.
ROS Homeostasis
This section summarizes our current understanding of the cellular sources of ROS (O2•− and H2O2) with a focus on the roles played by the NOX enzymes, the mitochondrial electron transport chain (ETC), and uncoupled NO• synthases (NOSs) (81) (Figs. 2 and 3). We will also review the role of coupled NOSs as a source of RNS (NO•) (Fig. 4). Other sources of ROS and RNS have been considered extensively in other reviews (321). In this section, we will also consider the roles of antioxidant enzymes, including superoxide dismutases (SODs), catalase, glutathione peroxidases (GPXs), and peroxiredoxins (PRXs), as well as their essential substrates, NADPH and reduced glutathione (GSH) (Figs. 2, 4, and 5). As an example of redox balance, NADPH is essential not only for the functioning of the PRX/thioredoxin (TRX) and GPX/GSH antioxidant systems, but it is also critical for the activities of NOX and NOS, which are the enzymes that generate ROS and RNS, respectively (Fig. 2).
Generation of ROS
NADPH oxidases
The NOXs are flavocytochrome enzymes (32, 294). Both phagocytic and non-phagocytic cells throughout the plant and animal kingdom express functional NOXs, although these enzymes have not been identified in prokaryote species. The NOX proteins produce O2•− through NADPH electron exchange (Fig. 2). NOX-dependent ROS production has an impact on many metabolic processes and disease states (Fig. 5). There are five NOX isoforms known as NOX1, NOX2, NOX3, NOX4, and NOX5. There are also two isoforms of the related dual oxidases (DUOXes). NOX2 is the prototype NOX enzyme; it is also known as gp91phox (nb: phox is an abbreviation for “phagocytic oxidase”) because it was first identified in phagocytic cells. The NOX2 complex includes two membrane catalytic subunits, the aforementioned gp91phox and the regulatory subunit, p22phox, and five cytosolic subunits, including p47phox, p67phox, p40phox, p22phox, and Rac1. Structurally, NOX2 shares 20%–50% sequence similarity with the other NOXs (50). These similarities have created difficulties for those designing targeted therapies (184).
NOX1, NOX2, NOX4, and NOX5 are all expressed in vascular tissues (32, 179, 187). NOX1 and NOX2 are O2•− generating enzymes, whereas NOX4 generates H2O2 (32, 179, 187). NOX5 also produces O2•− in a calcium-dependent manner (107). Results from previous studies suggest that NOX1, NOX2, and NOX5 promote endothelial dysfunction, inflammation, and apoptosis in the vessel wall. By contrast, NOX4 is primarily vasoprotective, as it increases the bioavailability of NO and inhibits apoptotic pathways (32, 102). However, the actions of NOX4 can also be deleterious (15). NOX2 is found in the plasma membrane or in endosomes where it produces O2•−either extracellularly or within the cytosol, respectively. O2•− is rapidly scavenged to generate H2O2 outside the cell by superoxide dismutase 3 (extracellular SOD [ecSOD], SOD3) or within the cytosol by the actions of coper zinc superoxide dismutase (Cu,ZnSOD, SOD1). By contrast, NOX4 is located in focal adhesions, the endoplasmic reticulum, nuclei, and mitochondria and it generates H2O2 at these locales (32, 179, 187). NOX1 is found in various subcellular localizations, including the nuclei and caveolae, whereas NOX5 is localized at the plasma membrane (32, 107, 179, 187). Other recent reviews include more detailed examples of the roles of NOX enzymes in the pathophysiology of CVD (32, 171, 179, 187, 321, 379).
Mitochondria
The primary site of ROS generation in mitochondria is the ETC (Fig. 2). As shown, Nicotinamide adenine dinucleotide (NADH)–ubiquinone oxidoreductase (complex I) accepts electrons from NADH, which are then transferred to complex II (succinate dehydrogenase [SDH]), which oxidizes succinate to fumarate (12). Electrons continue to travel down the electrochemical gradient to complex III (ubiquinol–cytochrome c oxidoreductase) and then to complex IV (cytochrome c oxidase), where they reduce molecular oxygen (O2) to water. Approximately 0.2% of the total O2 undergoes incomplete reduction to become O2•− (12, 124, 310, 313). Complexes I and III are the major sites of electron leakage involved in the premature reduction of O2, thereby resulting in the formation of O2•−, whereas complex II can also contribute to O2•− formation (267). Complexes I and II produce O2•− that is released into the matrix only (194, 394), whereas complex III can produce O2•− on both sides of the inner mitochondrial membrane, thereby resulting in its release into the intermembrane space (IMS) (242). O2•−within the IMS is physiologically more important with respect to signaling capacity, as it has easier access to the cytosol from this site; by contrast, matrix O2•− needs to cross both the inner and the outer mitochondrial membranes to have access to the cytosol. O2•− is a charged species and, thus, it is not capable of diffusing across mitochondrial membranes. Therefore, O2•− generated at IMS exits mitochondria through a voltage-dependent mitochondrial anion channel (VDAC) and enters the cytosol, where it is converted to H2O2 by cytosolic SOD1 (123). Complex III is the major site of ROS production in human endothelial cells (ECs) during the process of hypoxia reoxygenation and after stimulation with the cytokine, tumor necrosis factor α (TNFα) (63, 327), whereas complex II plays a more important role in lysophosphatidylcholine-induced ROS formation in these cells (355). Complexes I and/or III are responsible for ROS production that elicits dilation in response to shear stress in human coronary arteriolar ECs (207). Thus, the generation of O2•− by each complex in the ETC appears to be agonist/stimulant-dependent. Several recent reviews provide additional details on the role of mitochondrial ROS (mitoROS) in the pathogenesis of CVD (13, 71, 82, 171, 321, 379).
Elimination of ROS
Antioxidant enzymes
The SODs are the primary cellular antioxidant enzymes that can eliminate O2•−. The SODs rapidly scavenge O2•− and use it as a substrate to generate H2O2, thereby protecting the cell from the harmful effects of this highly reactive molecule (Fig. 2). The three distinct SODs are found in different cellular locations, including the cytosol (SOD1, or Cu,ZnSOD), the mitochondria (SOD2, or manganese superoxide dismutase [MnSOD]), and the extracellular matrix (ECM; SOD3, or ecSOD) (106). Cellular antioxidant systems capable of scavengingH2O2 include catalase, the PRXs/TRX system, and the GPXs/GSH system, which can degrade H2O2 to water and molecular O2 (135) (Fig. 2). These antioxidant scavenging systems also have different cellular localizations, including the cytosol, mitochondria, endoplasmic reticulum, peroxisomes, and extracellular space (22, 36). In the presence of a reduced transition metal (e.g., Fe2+ or Cu2+), H2O2 can be converted to a hydroxyl radical (OH•), which is extremely reactive. In the presence of iron (Fe2+), OH• can generate lipid peroxides that promote ferroptosis; this pathway can be inhibited by GPX4 (314).
Oxidized and inactivated TRX is reactivated and reduced by the enzyme, TRX reductase (TRXR) via the oxidation of a reducing equivalent, NADPH (Fig. 2). Similar to PRX and TRX, GPX and GSH cooperate with one another to detoxify H2O2 and generate H2O (Fig. 2). This process yields oxidized GSH (GSSG), which is then reduced by glutathione reductase (GSR) and NADPH (26). Thus, both systems are ultimately dependent on cellular NADPH-reducing equivalents for their regeneration. TRXR and GSR use NADPH to reduce oxidized TRX and GSSG, respectively (Fig. 2). This key reducing equivalent is generated by a complex network of metabolic pathways and enzymes, as discussed later (Fig. 4). Several previous reviews have included a more extensive consideration of the role of SODs, TRX, and GRX in the pathogenesis of CVD (22, 36, 85, 106, 135, 171, 321, 379).
Nicotinamide adenine dinucleotide phosphate
NADPH is an essential electron donor that is found in all eukaryotic cells. NADPH is essential not only for use by the PRX/TRX and GPX/GSH antioxidant defense systems that mitigate ROS-related cellular damage but also as a cofactor for NOS to generate NO• and similarly for NOXs to generate O2•− or H2O2 (Fig. 2). NADPH serves as both a substrate for NOX to generate O2•−/H2O2 and a coenzyme for the reductive removal of peroxides (366). NADPH is also required for anabolic biosynthetic reactions that are important for cell growth, such as the synthesis of fatty acids (FAs) and cholesterol, degradation of heme, and metabolism of polyol compounds (160, 372). Approximately 60% of the intracellular NADPH is generated via the oxidative PPP with the remaining 40% generated by one-carbon (1C) metabolism, isocitrate dehydrogenases (IDHs), and malic enzymes (MEs) (37), as discussed later (Fig. 4). Consequently, cancer cells maintain high levels of NADPH that sustain their rapid growth and protect them from the deleterious effects of excessive ROS (160, 372).
The ROS are closely linked to the systems that generate NADPH, thereby serving to induce antioxidant defense (Fig. 2). Oxidative PPP, one of the major sources of NADPH, is a branch of the metabolic process of glycolysis. In response to ROS, several enzymes that regulate glycolysis, including GAPDH, PKM2, and TIGAR, can redirect glycolytic intermediates to the oxidative PPP (4, 259, 352) (Figs. 4 and 5). The ROS-induced S-glutathionylation and inactivation of specific cysteine residues in both GAPDH and PKM2 contribute to this response (4, 259). Further, inactivation of PKM2 can channel glycolytic precursors into the NADPH-generating 1C metabolism pathway. In this setting, phosphoglycerate dehydrogenase (PHGDH) catalyzes the biosynthesis of serine, and serine hydroxymethyltransferase (SHMT) then incorporates 1C from serine into the folate cycle, which also generates NADPH (28, 381) (Fig. 4). During hypoxia in Myc-transformed cells, hypoxia-inducible factor (HIF) and Myc function cooperatively to increase SHMT levels; this results in the generation of NADPH in the mitochondria and reduces the elevated levels of mitoROS (380). Mitochondrial NADPH can also be generated by the oxidation of malate to pyruvate by MEs, a mechanism that plays an important role in insulin secretion (126) (Fig. 4).
Different cell types most likely rely on different metabolic pathways to generate basal levels of NADPH. In mutant KRAS-driven pancreatic ductal adenocarcinoma cells, glutamine-derived malate was used to generate basal NADPH via ME1, rather than via oxidative PPP; decreased levels of glucose-6-phosphate dehydrogenase (G6PD) had no impact on the levels of NADPH in these cells (308). Another metabolic enzyme, 5′ adenosine monophosphate-activated protein kinase (AMPK), also regulates NADPH homeostasis. In response to glucose-deprivation stress conditions in which generation of NADPH cannot proceed via the PPP, activation of AMPK maintains NADPH levels by inhibiting acetyl-coenzyme A (acetyl-CoA) carboxylases ACC1 and/or ACC2 (154) (Fig. 5).
Reduced GSH
GSH is a highly abundant antioxidant tripeptide (1–10 mM) that is produced by most mammalian cells (14, 104, 214) and distributed ubiquitously within the cell, including in the cytosol (90%) as well as in the mitochondria, nucleus, endoplasmic reticulum, and extracellular space (10%) (104, 214). GSH is an antioxidant and detoxifying agent that scavenges ROS/RNS. GSH can be found in the cell in one of three main forms: reduced GSH, oxidized GSSG, and protein-glutathione mixed disulfides (PSSGs) (Figs. 2 and 5). Under physiological conditions, reduced GSH is the predominant form in the cell, where it is 10- to 100 times more abundant than its oxidized form. Thus, together with NADP/NADPH and TRX systems, the relative concentrations of GSH/GSSG determine the redox state at cellular homeostasis (Figs. 2 and 5). Further, GSH is involved in the maintenance of cysteine pools and the detoxification of xenobiotics. In response to oxidative stress, steady-state levels of cellular GSH are regulated by synthesis, recycling of oxidized GSSG, degradation of extracellular GSH, and extrusion of the reduced, oxidized, or conjugated forms (14, 104, 214). GSH is synthesized de novo by adenosine triphosphate (ATP)-dependent glutamate-cysteine ligase (GCL) and GSH synthetase (GSS) (Fig. 4). GCL catalyzes the rate-limiting step of this process, which is a glutamate ligation with cysteine to form a dipeptide. GCL levels, together with levels of the cystine/glutamate transporter, are controlled by the critical transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2) that activates antioxidant responsive genes (152). This dipeptide is then combined with glycine via the actions of GSS to produce GSH.
Cysteine is a rate-limiting substrate for GSH synthesis. Levels of this amino acid are controlled by the cystine/glutamate antiporter SLC7A11 (xCT), which also encodes the cystine/glutamate transporter (65, 304) (Fig. 4). Cysteine can also be generated by the transsulfuration of methionine. In addition to cysteine, glutamine, glutamate, and glycine are also important for GSH synthesis (Fig. 4). The availability of glutamine regulates GSH production in three ways (391) (Fig. 4). First, glutamine is the primary source of glutamate via the actions of glutaminases (GLSs) 1 and 2. GLS activity is tightly regulated to maintain appropriate intracellular GSH concentrations. Glutamine can be transported into cells by various amino acid transporter systems, including solute carrier family 1, member 5 (SLC1A5, also known as alanine/serine/cysteine-preferring transporter 2, or ASCT2), which is among the most commonly overexpressed transporters in cancer cells. SLC1A5 and GLSs regulate intracellular GSH levels by controlling glutamine availability and its conversion to glutamate, respectively. Second, glutamine contributes to the maintenance of GSH levels via the production of NADPH by ME, as described in the previous section. Third, the cystine/glutamate transporter system also regulates the levels of intracellular glutamine (304). Thus, an overall abundance of glutamine and glutamate is crucial to maintaining appropriate levels of intracellular GSH, which can promote tumor initiation and proliferation (125). In addition, GSH is an essential cofactor of GPX4 and can thus prevent ferroptosis; it also regulates the levels of cysteine, which also can trigger ferroptosis (378). Taken together, these findings highlight the importance of GSH-mediated antioxidant pathways in maintaining cell survival and promoting their growth.
Generation of NO• by NOS:NO•/ROS generation
Coupled NOS (NO• generation)
NO• is a free radical gas that is synthesized in humans by three distinct NOS isoforms, neuronal NOS (nNOS, NOS1), inducible NOS (iNOS, NOS2), and endothelial NOS (eNOS, NOS3) (Figs. 2, 4, and 6). NO• has several distinct biological roles that range from mediating antimicrobial immune response, neurotransmission, and endothelium-dependent relaxation; these properties are dictated in an isoform- and cell-specific manner. The nNOS isoform is expressed primarily in the central and peripheral nervous systems, the gastrointestinal tract, and skeletal muscle. At these sites, NO• is synthesized on demand in a calcium-dependent manner to regulate neurotransmission, peristalsis, and penile erection. By contrast, expression of iNOS takes place primarily in activated immune cells, most notably in macrophages and neutrophils that constitutively produce large amounts of NO• and RNS that are used in their microbicidal and immunomodulatory functions. The eNOS isoform is expressed primarily in ECs within the blood vessels where, similar to nNOS, it produces NO• on-demand in a calcium-dependent manner to relax vascular smooth muscle cells (VSMCs), thereby reducing vascular tone and blood pressure. The enzymology underlying NO• biosynthesis by each of the three NOS isoforms is well conserved and involves the NADPH-dependent conversion of l-arginine to NO• with l-citrulline as a byproduct. The three NOS isoforms each contain two domains, including NADPH-binding oxygenase and heme-containing reductase domains that are found in a head-to-tail configuration as parts of a functional homodimer. NADPH binds to a C-terminal binding domain in the reductase domain of one monomer, which delivers electrons via a flavin bridge (i.e., flavin adenine dinucleotide [FAD], flavin mononucleotide [FMN]) to an N-terminal heme moiety that binds O2 at the oxygenase domain of the other monomer. This results in the reduction of molecular O2 and its insertion into the guanidine nitrogen of l-arginine (103). Electron flow to the heme occurs constitutively in iNOS, but it is controlled by calcium-calmodulin-dependent binding in eNOS and nNOS and is further fine-tuned by post-translational modifications.
Uncoupled NOS (ROS generation)
Although all NOS isoforms generate NO•, they can also generate O2•− at the expense of NO• via a process known as uncoupling (Figs. 2, 4, and 6). The mechanisms underlying uncoupling have undergone intensive investigation and various schemes have been proposed ranging from the formation of monomers, NOS phosphorylation at threonine (T)495, altered heat-shock protein (Hsp)90 binding, and insufficient levels of tetrahydrobiopterin (BH4) and l-arginine. Of these mechanisms, low levels of BH4 or low BH4 to dihydrobiopterin (BH2) ratios are the most reproducible findings associated with NOS uncoupling (111). BH4 is an essential cofactor for all NOS isoforms; two molecules of BH4 are bound stoichiometrically to a single NOS dimer. A BH4 binding site can be found near the dimer interface on NOS isoforms. This site also binds to the heme iron and converts it from a low-spin to a high-spin state (21). Reduced BH4 facilitates the oxidation of l-arginine; oxidized biopterin (BH2) can also bind to NOS but it does not facilitate NO• production. Bound BH4 also facilitates arginine binding and is important for isoform-specific dimerization (338). BH4 is a redox-sensitive molecule and is particularly susceptible to degradation by peroxynitrite (ONOO−), which is more potent at oxidizing BH4 than is either O2•− or H2O2 (188). Indeed, high intracellular levels of O2•− are not sufficient to block the eNOS-mediated synthesis of NO•; however, this condition does reduce its bioavailability (392). Under physiological conditions, there is typically a high ratio of BH4 to BH2. By contrast, in disease settings, particularly those associated with increased levels of ROS, BH2 levels are elevated to the point at which they can become dominant. However, the BH4:BH2 ratio is believed to be more important for NOS uncoupling than are the absolute levels of BH4. BH2 and BH4 bind to NOS enzymes with equal affinity, although BH2 does not facilitate the insertion of activated O2 into l-arginine; this leads to O2•−escape (337). In addition to the direct oxidation of BH4, conditions including hyperglycemia and availability of ONOO− can also prevent BH4 synthesis by promoting the ubiquitin-dependent degradation of GTP cyclohydrolase (GTPCH) 1 (373) (Fig. 4). Patients with diabetes, atherosclerosis, and/or hypertension typically have lower levels of BH4 and thus the potential for dysregulated eNOS activity. Supplementation with BH4 or folate and increased expression of GTPCH can all serve to improve endothelial function (340, 373). BH4-deficient macrophages have improved microbicidal activity compared with those that are iNOS-deficient. These findings suggest a functional role for uncoupled NOS enzymes (234). Unexpectedly, reduced levels of BH4 can promote increased production of mitoROS along with the accumulation of the tricarboxylic acid (TCA) cycle metabolites succinate and fumarate (7). The mitoROS have been shown to have an important role in killing bacteria (359). Low levels of BH4 in human macrophages have been proposed as a mechanism to explain their comparatively weak ability to generate NO• when compared with rodent macrophages, although they maintain potent microbicidal activity (193). Loss of BH4 in human macrophages was also linked to impaired activation of Nrf2 (233). To counteract BH4 deficiency, ascorbic acid, folic acid, and overexpression of the rate-limiting enzyme in BH4 synthesis, GTP cyclohydrolase (GCH1, the gene encoding GTPCH), have all been shown to promote NO• production and thus to protect against the development of atherosclerosis (79, 182, 312). In another proof-of-concept study, novel analogs of BH4 have been developed that are resistant to oxidation; administration of these analogs results in improvements in eNOS expression endothelial function and eNOS expression (114). Uncoupled eNOS has been identified as a source of ROS in multiple CVD states. Additional information on this subject can be found in previous reviews (2, 165, 171, 321, 379).
Metabolic Pathways and ROS
Cellular metabolism maintains redox homeostasis by generating ROS via the mitochondrial ETC as well as by the actions of the antioxidant systems via NADPH and GSH (Figs. 2–6). Further, metabolic reprogramming and increased flux through specific pathways play important roles in shaping both inflammatory and immune responses, to which ROS/RNS are also important contributors (10, 102, 108, 177). In this section, we will highlight how specific metabolic pathways regulate ROS production and inflammation.
Glycolysis and ROS
The ROS and glycolysis are closely linked to one another (Figs. 4–6, shaded in green). For example, to minimize the potential damage to DNA that can occur in cells proliferating under oxidative stress, tumor cells can increase their uptake of glucose and shift the metabolism toward glycolysis to release lactate even in the presence of molecular O2 (i.e., aerobic glycolysis, also known as the Warburg effect) (354). This results in reduced ROS levels compared with cells undergoing mitochondrial oxidative phosphorylation (200). Pharmacological inhibition or knockdown of glycolytic enzymes will result in the suppression of tumor growth in a variety of cancers (284, 368), whereas induction of mitochondrial respiration will result in slower growth rates both in vitro and in vivo (48, 290). Similarly, cells with mutant mitochondria with a reduced capacity for oxidative phosphorylation will promote growth through induction of glycolysis (399). The necessity of glycolytic flux in angiogenic ECs (both tip and stalk cells) is supported by observations that include impaired spheroid sprouting, postnatal outgrowth, and branching of murine retinal vasculature in cultured ECs devoid of the glycolytic enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) as well as in vivo (74, 375). PFKFB3 overexpression in zebrafish stimulates tip cells (74). Increased glycolysis in ECs provides energy not only for cell proliferation but also for cell migration; this was clear from the results of experiments that colocalized PFKFB3 or other glycolytic enzymes with F-actin in filopodia and lamellipodia, which are sites at which ATP is produced to support rapid remodeling. A recent report showed that loss of PFKFB3 from ECs impairs ischemic muscle revascularization and regeneration by reducing the extent of lactate-mediated macrophage polarization (390).
The role played by glycolysis in promoting oxidative stress associated with atherosclerosis is complex and not fully understood. Disturbed blood flow at aortic bifurcations promotes atherosclerosis as well as induces glycolysis and reduces mitochondrial respiratory capacity in ECs via the activation of HIF-1α by NOX4-derived ROS (369) (Figs. 5 and 6). Activated HIF-1α induces the expression of glycolytic enzymes and pyruvate dehydrogenase kinase 1 (PDHK1). This results in a reduction in the mitochondrial respiratory capacity, vascular inflammation, and atherosclerosis (369). The metabolite signatures identified in high-risk atherosclerotic plaques showed increased levels of glycolysis, elevated amino acid utilization, and decreased fatty acid oxidation (FAO), compared with those from low-risk atherosclerotic plaques (329). By contrast, another study revealed that adaptive increases in AMPKα1 induced by disturbed blood flow stimulated EC glycolysis and regeneration and was atheroprotective (376). Further, proinflammatory signaling enhances glycolysis in ECs, which can promote nuclear factor-kappa B (NF-κB)-driven vascular inflammation via lactate signaling (393); this will promote a cycle that results in sustained proinflammatory signaling (326). Activated neutrophils responding to oxidative stress shift toward hyperglycolysis via the phosphorylation of phosphofructokinase 2 (PFK2) by NOX2-derived ROS (9). Other reports have highlighted the metabolic dependency of astroglial cells on glucose availability for regeneration of the NADPH (181). Finally, hyperproliferative ECs due to pulmonary hypertension also rely on increased glycolytic flux and reduced O2 consumption, both of which are associated with HIF-1α overexpression (334).
Similar to tumor cells, ECs and brain tissue obtain ATP mainly from glycolysis rather than from oxidative phosphorylation, even in O2-rich environments (16, 74, 200). Eighty percent of the ATP in ECs is generated by glycolysis, even in the presence of O2 (74). As glycolysis produces much less energy than does oxidative phosphorylation, the physiologic mechanism underlying this observation remains unclear, although several potential advantages have been proposed (1, 16, 200). For example, one of the benefits of the metabolic shift toward glycolysis in ECs is to reduce the production of ROS by decreasing oxidative phosphorylation, which is the main source of ROS production (199), as well as by generating NADPH via PPP to counteract ROS that are produced. Glycolysis is also O2-sparing and can generate ATP more rapidly than can be achieved with oxidative phosphorylation. This may be an important adaptive mechanism to promote rapid vascularization of hypoxic tissue.
Therapeutic implications
Given that ECs are both highly glycolytic and angiogenic, therapeutic modulation of glucose metabolism and glucose transporters (GLUTs) are of great interest from a therapeutic perspective (73, 74). However, complete and permanent inhibition of glycolysis with 2-deoxy-D-glucose was associated with unacceptable levels of toxicity and yielded minimal success as monotherapy (324). Interestingly, partial and transient reduction of glycolysis in response to low doses, but not high doses of the PFKFB3 inhibitor, 3PO, reversed excessive vascular growth observed in response to genetic ablation of Notch or vascular endothelial growth factor receptor (VEGFR) 1 in mice with no effect on EC maintenance (287). Other PFKFB3 inhibitors that improve the pharmacokinetic properties and toxicological parameters include PFK-158, an agent that recently entered Phase I clinical trials (57), as well as a phenoxyindole derivative with higher selectivity for PFKFB3 over the other PFKFB isoforms (31). Because glycolysis plays an essential role in controlling redox homeostasis via regulation of PPP-derived NADPH, inhibition of glycolysis by the lactate dehydrogenase A inhibitor, FX11, impaired cancer cell growth by decreasing the intracellular ATP levels and inducing oxidative stress (189). Further, inhibition of glycolysis and the PPP combined with the disruption of the TRX system selectively increased its cytotoxicity in several cancers, but not in normal counterparts (285). Thus, one might speculate that a combined approach that included inhibition of glycolysis and the antioxidant system may prove to be an important therapeutic strategy for the treatment of various vascular diseases that depend on glycolysis.
PPP and ROS
Once glucose enters the cell via a GLUT, it undergoes phosphorylation by hexokinase (HK) to generate glucose-6-phosphate (G6P) and thus becomes a substrate for glycolysis, glycogen formation, and the PPP. The PPP includes both oxidative and non-oxidative pathways (Figs. 4–6 shaded in yellow). The oxidative PPP produces cellular NADPH that is required for antioxidant defense and FA synthesis, whereas the non-oxidative PPP produces pentose (5-carbon) sugars. Both of these pathways produce ribose 5-phosphate, which is a precursor for nucleotide synthesis. Glycolytic flux can supply the oxidative PPP pathway via the actions of G6PD, which is the first committed and rate-limiting step.
The functional significance of oxidative PPP and ROS is revealed by a common human enzyme defect known as X-linked G6PD deficiency, which is an enzyme that protects against oxidative stress. Erythrocytes are very sensitive to oxidative stress and highly dependent on oxidative PPP to maintain adequate levels of NADPH and GSH. One phenotype commonly associated with G6D deficiency is hemolytic anemia after ingestion of agents that can induce oxidative stress (e.g., sulfonamides and fava beans, among others). Under conditions of oxidative stress, glucose utilization is shifted from glycolysis to PPP to produce more NADPH and to generate GSH from GSSG (276). The glycolytic enzyme, GAPDH, has critical cysteine residues in its active site that can be oxidized and thus inactivated by H2O2 (259). PKM2 is another key glycolytic enzyme that can be S-glutathionylated at C358 by H2O2; this compromises its enzymatic activity, thereby reducing the rate of glycolysis and increasing flux into the PPP pathway to increase levels of NADPH (4).
Although NADPH functions to maintain the GSH and TRX levels (Figs. 2 and 5), in some tissues, NADPH may take on a pro-oxidative role via its actions as a cofactor for the enzyme, NOX (32) (Figs. 2 and 4). The enzyme, G6PD, which is a rate-limiting enzyme for PPP (a key source of NADPH in oxidative stress), can play distinct roles depending on the specific cell types and conditions. Under conditions of pathologic oxidative stress, for example, vascular tissue challenged with angiotensin II (228) or macrophages responding to lipopolysaccharide (LPS) (122), G6PD promotes ROS production in VSMCs (228), while also protecting against ROS in ECs (195). G6PD deficiency inhibits oxidant-mediated angiotensin II-induced signaling pathways by limiting the production of NADPH, which, in these situations, serves as a substrate for NOX (228). In the context of atherosclerosis, G6PD-mutant/apolipoprotein E (ApoE)−/− mice display reduced G6PD activity (20%) together with decreased levels of vascular O2•−, inflammatory responses, and atherosclerotic lesions (229). By contrast, G6PD overexpression in ECs reduces TNFα-induced ROS production and increases eNOS activity because NADPH is also a substrate for eNOS (195). Thus, whether or not NADPH-derived from G6PD can function as an antioxidant seems to be dependent on the levels of NOX and peroxidases that are upregulated in disease states associated with oxidative stress.
Glutaminolysis and ROS
Glutamine is the most abundant non-essential amino acid in the human body. Circulating concentrations of glutamine are typically between 400 and 600 μM. Glutamine is a key source of carbon and nitrogen for biosynthetic processes. The enzyme, GLS, converts glutamine to glutamate, which then undergoes decarboxylation to produce α-ketoglutarate (α-KG) (Figs. 4 and 5, shaded in blue). The Krebs cycle intermediate, α-KG, is ultimately used to produce ATP. The importance of glutamine as a carbon source to supply the TCA cycle in ECs was also demonstrated by the enhanced levels of glutamine metabolism observed in association with pulmonary artery hypertension (89). Further, the ongoing metabolism of glutamine is essential to meet the metabolic needs of hyperproliferative vascular cells and proceeds via a mechanism that relies on both mechanotransducer Yes-associated protein (YAP) and the transcriptional coactivator with PDZ binding motif (TAZ) (23). The ECM stiffening results in mechanoactivation of YAP/TAZ, which then stimulates GLS expression. The GLS is critical for proliferation and migration via its role in replenishing the amino acid, aspartate (23). Glutamine metabolism is also essential for EC proliferation. Inhibition of glutamine metabolism in ECs by inhibiting GLS-1 or glutamine deprivation prevents EC proliferation by impairing lipid biosynthesis via reductive carboxylation and complete loss of TCA intermediates. This inhibition of glutamine metabolism also increases oxidative stress by decreasing the rate of GSH synthesis (141, 174). The importance of glutamine in angiogenesis has been demonstrated by observations on the proliferation of aggressive cancer cells and its dependence on glutamine availability (45, 347). As one example, glutamine deprivation induces apoptosis in human breast cancer cells (47). However, the role of glutamine metabolism in EC migration remains controversial (141, 174, 260). Glutamine metabolism also plays a role in injury-induced neointimal formation in arteries by regulating VSMC proliferation (250). The TEA domain transcription factor 1 (TEAD1) promotes VSMC proliferation via transcriptional induction of the glutamine uptake transporter, SLC1A5. This results in the activation of the mammalian target of rapamycin complex 1 (mTORC1) signaling and promotion of neointima formation (250).
Glutamine is involved in ROS homeostasis as a precursor for GSH. To be available to cells, glutamine must be transported into cells by specific transporters, including SLC1A5/ASCT2, and converted to glutamate by GLS. Glutamate directly contributes to GSH synthesis by promoting the uptake of cystine through the cystine/glutamate exchanger, Slc7a11. Thus, the availability of glutamine, glutamate, and cysteine regulates the biosynthesis of cellular GSH. Glutamine can also produce NADPH via the malate system, and it also serves as a precursor for the GSH system. Similarly, TCA cycle intermediates, for example, citrate, can be exported into the cytosol, where ME or IDH1 uses them to generate NADPH (39). Since NADP/NADPH levels control the oxidative state of GSH, tumor cells maintain the GSH pool in a reduced state, thereby supporting the TRX system. Further, the mitochondrial enzyme, glutamate dehydrogenase 1, positively regulates the enzymatic activity of the antioxidant enzyme GPX by controlling intracellular levels of fumarate (157). Thus, glutamine promotes ROS homeostasis by regulating the synthesis of GSH, NADPH, and the mitochondrial antioxidant enzyme, GPX.
Therapeutic implications
Given the importance of glutamine in promoting angiogenesis (141, 174), GLS1 inhibitors have been used in clinical trials for solid tumor and leukemia cells and might ultimately be repurposed for the treatment of pathologic angiogenesis in vascular disease (168). The GLS inhibitors include compound 968, BPTES, and CB-839 (149, 168). Because glutamine also plays an important role in maintaining redox balance, inhibition of glutaminolysis can result in the depletion of the intracellular GSH and subsequent generation of ROS, both of which contribute to impaired cell proliferation (117, 147).
FAO and ROS
The FAO is important for NADPH homeostasis and redox balance (Fig. 4). The FAs are an excellent source of energy, as they can produce twice as much ATP as can be obtained from carbohydrates. They are also a source of NADPH and thus serve as an alternative to the PPP. When NADPH generation by the PPP is impaired under conditions of energy stress, for example, glucose deprivation, the actions of AMPK result in an increase in NADPH via FAO, which will ultimately inhibit cell death (154). Inhibition of FAO results in decreased NADPH and GSH levels and elevated levels of intracellular ROS (261). In macrophages, inhibition of FAO results in the generation of mitoROS; this promotes the recruitment of NOX to the phagosomal membrane to limit the growth of the pathogen, Mycobacterium tuberculosis (Mtb) (41).
The FAO includes a series of cyclic oxidation reactions via which long- and short-chain FAs are degraded, resulting in the generation of NADH, FADH2, and acetyl-CoA. The FAO-derived acetyl-CoA can be introduced into the TCA cycle to generate ATP and aspartate for the synthesis of deoxynucleotide triphosphates (dNTPs) that are required for DNA replication in proliferating ECs. In cancer cells, however, only a fraction of the acetyl-CoA produced completes the TCA cycle to produce ATP; the acetyl-CoA that remains is used to generate citrate. Citrate is then exported into the cytosol, where it ultimately supports the production of large amounts of NADPH with the help of ME and IDH1 (39) (Fig. 4). In ECs, stalk cells depend on FAO for vessel sprout elongation, specifically via its capacity to sustain the synthesis of dNTPs (80, 286, 367). By contrast, tip cells depend on PFKFB3-driven glycolysis for rapid production of ATP for vessel sprouting (74, 375). Interestingly, Notch signaling serves as a molecular switch and promotes the transition from FAO from nucleotide synthesis pathways in proliferating ECs to NADPH regeneration in quiescent ECs. This promotes the protection of the vasculature against oxidative stress-induced cell damage (162). Mice with an EC-selective deletion of the FAO rate-limiting enzyme, carnitine palmitoyltransferase-1 (CPT1) showed endothelial dysfunction, including inflammatory cell recruitment and barrier disruption typically associated with increased oxidative stress (162).
In the heart, FAs are the main source of energy. In normal states, FAO, followed by carbohydrate (glucose and lactate) oxidation from mitochondrial oxidative phosphorylation, are the major sources of ATP production (98, 153). Interestingly, there is a reciprocal relationship between FAs and glucose oxidative metabolism; this is known as the Randle Cycle or the glucose/FA cycle (271). In various heart diseases, including ischemic heart disease and heart failure, the relationship between FAO and glucose oxidation is disrupted; this results in impaired cardiac efficiency and function (167, 209). During ischemia/reperfusion, circulating FAs and cardiac FAO levels are elevated. This results in decreased glucose oxidation through the inhibition of pyruvate dehydrogenase (PDH) activity, which is the rate-limiting enzyme that catalyzes the conversion of pyruvate to acetyl-CoA and NADH in mitochondria (167, 209). It has been proposed that reduced cardiac efficiency in disease states associated with elevated FAO is due to the use of a less efficient energy source than glucose oxidation based on the amount of ATP produced per O2 molecules consumed (97, 209). Consistent with this concept, inhibiting FAO and/or increasing glucose oxidation can result in improved cardiac function in ischemic heart disease, heart failure, and diabetic cardiomyopathy (87, 98, 153, 166).
Therapeutic implications
The introduction of FAO inhibitors has provided clear therapeutic benefits to patients with type II diabetes or myocardial ischemia (39), because inhibition of FAO alleviates both O2 shortage and insulin resistance. The CPT1 inhibitor, perhexiline, has been evaluated as a potential treatment for heart disease. Many other CPT1 inhibitors are undergoing preclinical evaluation (e.g., oxfenicine) or exhibited toxic side effects in clinical trials (e.g., etomoxir) (137). Both trimetazidine (164) and ranolazine (245) inhibit 3-ketoacylthiolase (3-KAT), the enzyme that catalyzes the final step in FAO, and they are in use for the treatment of angina. In macrophages, inhibition of FAO promotes key antimicrobial functions and overcomes the immune evasion mechanisms associated with infection with Mtb (41), as mentioned earlier. The FAO also plays a critical role in tumor growth via its capacity to regulate NADPH homeostasis and oxidative stress (261). In human glioblastoma cells, pharmacological inhibition of CPT1 by etomoxir enhances cell death by promoting decreased levels of NADPH and GSH and by elevating the levels of intracellular ROS (261). Overexpression of CPT1A has been associated with a high tumor grade, unfavorable clinical outcomes in acute myeloid leukemia and ovarian cancer (218). Thus, targeting FAO may be a promising approach toward reducing pathologic angiogenesis and heart failure (80, 166).
1C metabolism and ROS
In proliferating cells, 1C metabolism (in particular, serine-glycine 1C metabolism, or SGOC) is one of the major sources of NADPH (Fig. 4, shaded in gray) other than the oxidative PPP (94, 196, 226). SGOC is mediated by a folate cofactor and is a universal metabolic process that serves to activate and transfer 1C units to support the biosynthesis of purines and thymidine, amino acid homeostasis (glycine, serine, and methionine), epigenetic maintenance, and redox balance (3). Serine and glycine 1C metabolism involves three pathways (Fig. 4, shaded in gray), including the folate cycle, the methionine cycle, and the transsulfuration pathway. PHGDH catalyzes the biosynthesis of serine; SHMT subsequently introduces 1C unit from serine into the folate cycle. Carbon units then enter the methionine cycle, resulting in the generation of S-adenosyl-methionine (SAM), which undergoes further conversion to homocysteine and ultimately to cysteine that can be diverted toward the synthesis of GSH. A tracing study of NAPDH compartmentalization revealed that serine was used predominantly in the mitochondria of mammalian cells to generate NADPH (196) (Fig. 4). During hypoxia, HIF-1α and MYC work cooperatively to increase the expression of SHMT2 (the mitochondrial isoform of SHMT) to promote the production of mitochondrial NADPH and to counteract the elevated levels of mitoROS (380). Upregulation of 1C metabolism is another metabolic shift used to evade ROS-induced cell death.
In addition to reducing ROS production by supplying NADPH, 1C metabolism plays an important role in supporting endothelial function and preventing CVD (Fig. 4, shaded in gray). These activities are mediated via the modulation of eNOS activity and the regulation of its cofactor, BH4 (20), and the methylation of arginine residues in proteins, which will be discussed in the section focused on RNS to follow (Figs. 4 and 6, shaded in orange). Further, 1C metabolism is also involved in the generation of homocysteine. For example, inactivating mutations in the 1C folate metabolism gene encoding methylenetetrahydrofolate reductase (MTHFR) result in hyperhomocysteinemia, which is a prominent risk factor associated with CVD (110) (Fig. 4, shaded in gray).
Therapeutic implications
Anti-folates that target 1C metabolism have been explored as treatments for cancer (84). Further studies will be needed to develop therapeutic strategies that selectively inhibit SHMT2 for the treatment of CVD. Since 1C metabolism is involved in supporting antioxidant defense via the actions of GSH and NADPH in a cell-type and context-dependent manner, it may be possible to achieve greater selectivity than the simple inhibition of DNA synthesis alone. Therapeutic approaches that modulate NOS activity by targeting SGOC in BH4 and arginine metabolism will be discussed in the section focused on RNS.
Branch-chain amino acids and ROS
Branch-chained amino acids (BCAAs), including valine (V), leucine (L), and isoleucine (I), function as critical nitrogen donors in processes involving intracellular nitrogen shuttling. BCAA uptake is facilitated by the large neutral aminoacid transporter (LAT/SLC7a5); once inside the cells, BCAAs are converted to branched-chain ketoacids (BCKAs) by the actions of the enzyme, branched-chain aminotransferase (BCAT). BCKAs undergo further conversion to acetyl-CoA and succinyl-CoA via the actions of the BCKA dehydrogenase (BCKD) complex, which is linked to the TCA cycle (Fig. 3). Although most amino acids are metabolized in the liver, catabolism of BCAAs takes place in several non-hepatic tissues, including cardiac muscle, adipose tissue, brain, and kidney (49, 143, 151, 358). Treatment with BCAAs can be beneficial, but, paradoxically, increased circulating levels of BCAA have also been associated with obesity and diabetes. For example, Tanada et al. (319) showed that supplementation with BCAAs resulted in clinical improvement in a rat model of heart failure. Further, Zhao et al. (395) found that leucine (L) supplementation reduced the size of atherosclerotic lesions in ApoE−/− mice; this finding was associated with an improved plasma lipid profile and reduced levels of systemic inflammation. By contrast, 3-hydroxy-isobutyrate (3HIB), a catabolic intermediate of the BCAA valine (V), activates trans-endothelial FA transport and thus stimulates FA uptake in muscle tissue in vivo and promotes lipid accumulation and insulin resistance (151). Thus, BCAAs may provide a novel therapeutic strategy for atherosclerosis and cardiometabolic disease (387).
Oxidative stress has been closely associated with the pathophysiology of an inherited metabolic genetic disorder of BCAA metabolism known as maple syrup urine disease (MSUD). The MSUD results from a BCKD dehydrogenase deficiency and results in the accumulation of all BCAAs (151, 274). The patients with MSUD show high levels of lipid and protein oxidation in plasma (17) and an inflammatory profile that results from unbalanced ROS production (235). The importance of oxidative stress in the pathophysiology of other inherited metabolic disorders of BCAA metabolism, including methylmalonic acidurias (MMAs) and homocystinuria, has been well characterized. Fibroblasts derived from these patients showed elevated ROS, apoptosis, and phosphorylation of the stress kinases p38MAPK and JNK (274). Interestingly, BCATs have redox-active CXXC motifs. When these enzymes are S-glutathionylated, they support the chaperone role of BCAT and promote appropriate protein folding (60, 90). Therefore, BCAA metabolism also plays an important role in regulating the redox balance (274).
Alpha-ketoglutarate dehydrogenase and ROS
The primary sources of ROS in mitochondria are complex I and III of the ETC. Also, metabolic enzymes, including alpha-ketoglutarate dehydrogenase (α-KGDH) and PDH complexes, produce mitoROS (266, 332) (Fig. 5). The enzyme, α-KGDH catalyzes the conversion of α-KG to succinyl-CoA via the actions of IDH2 or IDH3 and produces NADH that provides electrons for the ETC. Importantly, α-KGDH together with PDH complexes are believed to produce more ROS than complex I, which is regulated by NADH/NAD ratio (266, 332). In addition to generating ROS, KGDH can also be inactivated by oxidative stress (332), which, in turn, limits the supply of NADH to the ETC. In tumors grown under hypoxic conditions or in the presence of a defective ETC, α-KGDH plays an important role in maintaining cell proliferation and lipogenesis (236, 364). During hypoxia, citrate is generated from glutamine-derived α-KG via reductive carboxylation by cytosolic and mitochondrial NADPH-dependent IDH1 and IDH2. The generation of isocitrate from α-KG implies a reduced level of α-KGDH activity and an unbalanced α-KG/citrate ratio. This will lead to a reverse TCA cycle that ultimately promotes FA synthesis and favors tumor growth. α-KGDH can also be inhibited via the degradation of its E3 subunit by HIF-1 (96).
Inflammation, metabolic shifts, and ROS
Chronic low-grade inflammation plays a key role in promoting CVD via the regulation of energy metabolism (10). The master transcription factor, NF-κB, is one of the critical regulators of metabolic reprogramming that promotes aerobic glycolysis in innate immune defense and during acute inflammation (10, 230, 331). By contrast, activation of sirtuin 1 (SIRT1) inhibits NF-κB signaling, enhances oxidative metabolism, and promotes the resolution of inflammation (140). Thus, both innate immunity and energy metabolism can be regulated by antagonistic crosstalk between NF-κB-and SIRT1-mediated signaling pathways (169). Given that ROS regulate the actions of NF-κB in response to inflammatory agonists (227) and that SOD2 overexpression inhibits ROS-induced NF-κB (43), ROS, inflammation, and metabolism appear to be closely linked.
To meet their bioenergetic, biomass, and redox demands, T cell activation and differentiation require coordinated programming of cellular metabolism. However, several studies have revealed that the T cell metabolic program differs depending on the specific cell type (99, 216, 256). For example, activated effector T (Teff) cells generate energy by augmenting aerobic glycolysis, whereas memory T cells (Tm) engage FAO. Moreover, regulatory T cells (Tregs) activate AMPK and depend on lipid oxidation for their energy requirements (237, 257, 303, 351). The results of several studies suggest that mitochondrial dynamics control T cell fate through metabolic reprogramming and by altering the morphology of their cristae (35). Tm cell fusion configures the ETC complex associations to favor oxidative phosphorylation and FAO, whereas fission in Teff cells leads to expansion of the cristae, reduced ETC efficiency, and augmented glycolysis (35). Cell growth, clonal expansion, and the effector functions of Teff cells require enhanced aerobic glycolysis, the PPP, and glutaminolysis (5, 100, 105, 112, 148).
During T cell activation, increased mitochondrial biogenesis results in more mitochondria, an expanded mitochondrial-dependent metabolic flux, and the production of ROS (68). Also, mitoROS-induced by fission contributes to NF-κB-mediated activation in T cells (278). T cell activation, proliferation, differentiation, and immune responses all require ROS-mediated signaling and activation of transcription factors such as NF-κB and activator protein 1 (AP-1) (76, 163, 243). However, excessive ROS production induces apoptosis in T cells via mechanisms that depend on B cell lymphoma 2 (Bcl-2), FAS ligand (FasL), and the mitochondrial membrane potential (130, 131). Thus, a fine-tuned balance between glycolysis, the PPP, and glutaminolysis ensures appropriate levels of intracellular ROS that drive T cell activation, differentiation, and immune responses (296).
Activation of proinflammatory macrophages during inflammation is caused by metabolic reprogramming from oxidative phosphorylation to glycolysis. The mitoROS stabilize and activate hypoxia-inducible factor HIF-1α, which, in turn, increases both glycolytic capacity and expression of the proinflammatory cytokine, interleukin (IL)-1β (66, 238); this pathway also regulates the formation and activation of inflammasomes. Of note, succinate can drive mitoROS production at complex I via reverse electron transport (RET) as part of the pathogenesis of ischemia–reperfusion injury (53, 258, 292) (Fig. 3). The SDH deficiency directs macrophages toward an anti-inflammatory phenotype (i.e., production of IL-10), thereby resulting in RET-induced generation of mitoROS. This, in turn, enhances ATP production via oxidative phosphorylation and reduced mitochondrial membrane potential (158, 238, 398). Interestingly, the endogenous metabolite, itaconate, which is highly induced in activated macrophages, inhibits SDH-mediated succinate oxidation, thereby promoting anti-inflammatory effects (186) (Fig. 4). Thus, mitoROS produced in response to metabolic reprogramming and itaconate-induced succinate oxidation plays a key role in macrophage phenotype switching from M1 (inflammatory) to M2 (anti-inflammatory).
The NLR family pyrin domain containing 3 (NLRP3) inflammasome functions as a sensor of metabolic stress and regulates inflammation via interactions with thioredoxin-interacting protein (TxNIP) (289, 299, 333, 397, 398); TxNIP binds to TRX, thereby reducing its activity (382). In response to glucose stimulation, TxNIP dissociates from TRX and interacts with NLRP3 via an ROS-sensitive mechanism to activate inflammasome and inflammatory cytokine signaling (289, 299, 333, 397, 398). In addition to inflammasome activation, ROS also promote critical efferocytotic activities (i.e., removal of apoptotic cells) of macrophages, which is a critical aspect of the resolution of inflammation (101). Lysophosphatidylcholine released from apoptotic cells reduces mitochondrial membrane potential and ATP production; this results in the generation of mitoROS and activation of AMPK (156). The AMPK activation facilitates metabolic reprogramming toward glycolysis and induces the synthesis of tubulin that is needed to promote macrophage chemokinesis and efferocytosis (156). Thus, ROS play an important role in inflammasome activation by regulating the actions of TXNIP and macrophage-mediated efferocytosis by metabolic reprogramming via AMPK.
Therapeutic implications
The results of the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) trial support the inflammatory hypothesis of atherosclerosis and cancer in humans by demonstrating the beneficial effects of canakinumab, which is an anti-IL-1β neutralizing monoclonal antibody (204, 357). However, limited effects on cardiovascular mortality and the prominence of side effects, including the higher incidence of fatal infections, warrant further investigations directed at new therapeutic strategies. Although promising clinical outcomes have resulted from immunotherapy, including immune checkpoint blockade, these therapies have proven to be ineffective for a significant number of patients (119). Given that metabolic reprogramming of immune cells influences the responses to immunotherapy and that metabolic programs differ among immune cell subsets, inhibitors designed to target specific metabolic pathways may be a promising therapeutic approach for inflammatory CVD. This topic is covered in detail in previous reviews (24, 119, 370).
Itaconate and ROS
Itaconate is one of the most abundant metabolites in activated macrophages (186). Itaconate is synthesized from aconitate, a molecule that would otherwise contribute to the TCA cycle via the actions of aconitate decarboxylase 1 (ACOD1, also known as immune-responsive gene 1 [IRG1]) (Fig. 4). Itaconate inhibits the activity of SDH; inhibition or knockout of SDH suppresses succinate-mediated inflammatory processes (including responses mediated by IL-1β and HIF-1α) (238) and induces the expression of anti-inflammatory Nrf2 factor 3 (ATF3) (248). Interestingly, itaconate formation contributes to the decreased mitochondrial O2 consumption observed in response to LPS (186). Succinate oxidation leads to an elevated mitochondrial membrane potential and ROS production likely via RET at complex I of the ETC (Fig. 3). Elevated levels of mitoROS are responsible for driving the increased inflammatory response (238). Alternatively, elevated levels of succinate can promote the succinylation of susceptible lysine residues. Numerous succinylated substrates have been identified in the cytosol, nucleus, and mitochondria that play major roles in modulating metabolic processes (309). The enzyme, PKM2, plays an important role in promoting glycolysis; elevated levels of succinate can induce succinylation of PKM2 on K498, thereby inhibiting its activity (344). The SDH converts succinate into fumarate, and endogenous fumarate can succinylate and covalently modify cysteine residues of numerous substrates (Fig. 4). One of the best-characterized examples of this process is the succinylation of C152 found within the active site of GAPDH, thereby resulting in reduced levels of glycolysis and inflammation (180). Kelch-like ECH-associated protein 1 (KEAP1) can also undergo succinylation. Both dimethyl fumarate and monomethyl fumarate promote succinylation of KEAP1, which results in the activation of Nrf2 (291). Itaconate can also form covalent attachments via post-translational modification. A cell-permeable form of itaconate can form a covalent linkage with C22 of GAPDH, thereby inhibiting its enzyme activity and glycolytic flux (203).
Metabolism and RNS
Since all NOS activity (i.e., the actions of nNOS, iNOS, and eNOS) depends on the availability of l-arginine and BH4, the metabolic pathways leading to the synthesis of these factors (i.e., 1C metabolism) may have an impact on the availability of NO•(Figs. 2 and 4). In the sections that follow, we review the pathways via which various metabolic processes regulate RNS homeostasis.
l-arginine metabolism and RNS
Cellular metabolism plays an important role in the generation of RNS. As described in the earlier sections, NOS enzymes utilize NADPH and l-arginine as co-substrates, whereas BH4 is an essential co-factor that is not consumed (Figs. 2 and 4). l-arginine levels are typically high in the circulation (∼100 μM) and are even higher within ECs (>400 μM). Depletion of cellular l-arginine has been identified as an important mechanism to prevent full activation of NOS isoforms and eNOS-mediated uncoupling and endothelial dysfunction in CVD. Elevated expression arginase I and arginase II, which are enzymes that consume l-arginine, has been observed in association with CVD and has been proposed as a mechanism that might be used to decrease l-arginine levels to a point at which the NOS enzymes no longer have sufficient fuel to promote catalysis. However, despite many beneficial effects that have been attributed to l-arginine in animal models (62) and humans (365), long-term l-arginine consumption has not been associated with the reduced incidence of myocardial infarction nor has it been implicated in reducing the rates of post-infarction mortality (343). Mechanistically, the Michaelis constant (Km) for l-arginine with respect to the NOS enzymes is ∼2 μM; it is unlikely (and potentially threatening to cell viability) that arginine levels can be reduced to an extent that will severely compromise NO• synthesis. Indeed, it has been shown that high levels of arginase expression can result in decreased eNOS activity, but only by ∼10%. The specific intracellular location of arginase has no impact on this ability (91). High-dose l-arginine can also induce compensatory changes over time, including the upregulation of arginase enzymes. This response may result in the synthesis of deleterious quantities of metabolites such as ornithine and thus contribute to maladaptive vascular remodeling. The metabolite, l-citrulline, is recycled to l-arginine by the actions of argininosuccinate synthase and argininosuccinate lyase (121). This has been proposed as an alternative approach, as supplementation with l-citrulline has been associated with cardiovascular benefits, including reducing systemic blood pressure (170).
Asymmetric dimethylarginine (ADMA) and N-monomethyl l-arginine (l-NMMA) are naturally occurring metabolites that regulate NOS activity. Concentrations of ADMA and l-NMMA are increased in renal failure (336), atherosclerosis (240), and homocysteinemia (316). Although l-NMMA is a potent inhibitor of NOS activity, ADMA is a weak inhibitor. However, ADMA can promote uncoupling of eNOS more effectively than l-NMMA (83) (Fig. 4). High levels of methylated arginines promote endothelial dysfunction (61), which is believed to involve both reduced NOS activity and uncoupled NOS and ROS production (318). ADMA and l-NMMA are metabolized by the enzyme, dimethylarginine dimethylaminohydrolase 1 (DDAH1), to form l-citrulline. The activity of DDAH1 can be compromised by ROS and RNS (191), a response that leads to increased levels of ADMA. Homocysteinemia has been associated with impaired endothelium-dependent relaxation responses (335). Elevated levels of homocysteine and methionine can promote increased protein methylation and thus increased levels of ADMA, thereby compromising endothelial function (27). Administration of folate can restore endothelial function and eNOS uncoupling (340). Similarly, NO• may be capable of regulating homocysteine levels via direct inhibition of methionine synthase (MTR), an enzyme that uses 5-methyltetrahydrofolate to convert homocysteine to methionine (70). This mechanism could limit the effectiveness of high levels of NO• donors on cardiovascular health.
BH4 metabolism and RNS
It is not clear whether NADPH is rate-limiting for eNOS activity, as it also has many important roles in regulating other enzyme systems, including those contributing to both prooxidant and antioxidant pathways. The affinity of eNOS for NADPH (i.e., calmodulin-bound eNOS) is ∼1 μM (231). Cellular levels of NADPH levels have been estimated at ∼100 μM (202) and are tightly regulated (372). NADPH in the cytosol is primarily generated by the PPP, including the actions of G6PD, which converts NADP+ to NADPH. Overexpression of G6PD results in increased eNOS activity (195) although it is not clear whether or not this response is due to increased levels of NADPH. NADPH is also important for the synthesis of BH4 (Fig. 4). Dihydrofolate reductase (DHFR) and dihydropteridine reductase (DHPR, also known as quinoid dihydropteridine reductase [QDPR]) can catalyze the reduction of BH2 to BH4 in an NADPH-dependent manner. Human DHFR has a reduced affinity for BH2. Likewise, conversion to BH4 can be inhibited by folate, which may limit the effectiveness of folic acid for the treatment of endothelial dysfunction (362). Mitochondrial function is also critical for BH4 synthesis. Depletion of CR6-interacting factor 1 (CRIF1), a factor that is important for the assembly of mitochondrial subunits and complexes, leads to loss of GTPCH expression, diminished BH4 levels, and uncoupled eNOS (190). The expression of NOS enzymes can also be regulated by cellular metabolism, most notably by glycolysis. For example, LPS-dependent expression of iNOS can be inhibited by glycolysis (301). C-terminal-binding protein (CtBP) is an NADH-sensitive transcription factor that can regulate transcription of genes via mechanisms that are dependent on cellular metabolism. Suppression of CtBP activity impairs LPS-mediated induction of iNOS, thereby connecting changes in metabolism with gene transcription (301). The expression of eNOS can also be regulated by glycolysis in ECs. Lactate promotes increases and 2-deoxyglucose results in inhibition of the expression of eNOS messenger RNA (mRNA) (134).
1C metabolism and RNS
1C metabolism plays an important role in modulating eNOS activity via the regulation of its cofactor BH4 (20) and methylation of arginine residues in proteins. These actions contribute to endothelial function and CVD (Fig. 4). DHFR, which is a key enzyme in both folate and 1C metabolism, plays an important role in regulating eNOS activity via a salvage pathway in which BH2 is consumed to maintain endothelial BH4 levels, together with de novo biosynthesis via the rate-limiting enzyme, GTPCH (20). Reduced BH4 availability contributes to eNOS uncoupling and results in the production of O2•− instead of NO•, thereby inducing endothelial dysfunction. Similarly, ADMA inhibits the activity of eNOS via competition with its cofactor, l-arginine; this results in increased ROS production and reduced bioavailability of NO• (26, 192). The ADMA is generated by protein arginine methyltransferase (PRMT) in the presence of SAM as part of the methionine cycle of 1C metabolism. The SAM also plays a major role in promoting epigenetic modifications in its role as a universal methyl group donor for methyl transfer reactions (Fig. 3).
Therapeutic implications
Although impaired metabolism can lead to the production of RNS, therapeutic strategies, there are currently few to no metabolic strategies available to counter the resulting endothelial dysfunction. Supplementation with BH4 limits atherosclerosis (127) and sepiapterin, which can result in increased levels of BH4 in vivo, and it improves endothelial function in numerous models (64, 77). However, the impact of folate or BH4 supplementation as a means to prevent CVD in clinical and preclinical settings remains controversial (55, 67, 339). There is a considerable body of evidence in support of the notion that preserving endothelial function has positive effects on metabolism. The loss of endothelial NO• observed in eNOS-deficient mice results in insulin resistance and hyperlipidemia (86, 142) secondary to lower energy expenditures, reduced O2 consumption, and mitochondrial dysfunction with lower rates of beta-oxidation.
Metabolic Enzymes and ROS
The ROS induce a variety of post-translational protein modifications, including cysteine oxidation in the form of sulfenylation (SOH) and S-glutathionylation (136). These modifications can have a direct influence on the activity of susceptible metabolic pathways. In this section, we will highlight how ROS can affect metabolic enzymes, including mitochondrial protein dynamics.
AMPK and ROS
AMPK is a critical metabolic redox sensor in glucose metabolism that promotes angiogenesis in ECs and postnatal neovascularization. AMPK also protects against atherosclerosis (201, 244, 311, 374, 376). Interestingly, ROS can stimulate AMPK activity to regulate metabolic requirements even in the presence of appropriate levels of ATP (38) (Fig. 4). For example, H2O2 can induce GLUT4 translocation via the activation of AMPK in cardiac myocytes. (139). H2O2 directly activates AMPK via oxidative S-glutathionylation of the AMPKα and AMPKβ subunits at C299 and C304 (401). The mitoROS can regulate autophagy (307), efferocytosis (156), and HIF-1α-dependent longevity (132, 144) via activation of AMPK. In response to hypoxic conditions, mitoROS activate AMPK via a mechanism that functions independently of the adenosine monophosphate (AMP)/ATP ratio (92). As an adaptive response, mitoROS-induced AMPK activation induces upregulation of peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α-dependent expression of antioxidant enzymes, which, in turn, limits excess mitoROS production (268). Taken together, AMPK functions not only as a metabolic sensor but also as a redox sensor to regulate cellular metabolism and redox state (38, 401).
AMPK also contributes to vascular health via its capacity to confer potent antioxidant defense. For example, under metabolic stress, AMPK increases NADPH levels and thus decreases levels of H2O2 by induction of FAO (154). AMPK activation also improves endothelial function by reducing oxidative stress associated with atherosclerosis via increasing the expression of uncoupling protein 2 (UCP2) in ECs (349). UCP2 deficiency in mice increases oxidative stress, which amplifies the progression of atherosclerotic plaques (25). Additional evidence supporting AMPK-mediated protection against oxidative stress has emerged from studies that demonstrate its capacity for negative regulation of NOX via reduced phosphorylation of the p47phox NOX subunit. This prevents p47phox translocation from cytosol to membrane, which is required for the activation of NOX (232).
Therapeutic implications
AMPK activators (e.g., metformin) have been used to treat type 2 diabetes and CVD (72, 116, 388). Metformin activates the Reperfusion Injury Salvage Kinase (RISK) pathway (388) as part of its cardioprotective mechanism. Recently, a small molecule activator of AMPK (e.g., A-769662, MIF20) showed efficacy at protecting the heart tissue from the negative sequelae of ischemia–reperfusion injury (172, 346).
PKM2 and ROS
Pyruvate kinase (PK) catalyzes the final rate-limiting step in glycolysis by transferring the phosphate from phosphoenolpyruvate (PEP) to ADP to generate pyruvate and ATP. There are four isoforms of PK, including PKM1, PKM2 (encoded by PKM), PKL, and PKR (encoded by PKLR) (377). PKM1 and PKM2 are alternatively spliced products of the PKM gene (247). Interestingly, PKM2 (but not PKM1) is expressed exclusively in growing and confluent ECs (173). The PKM1 homo-tetramer has high constitutive PK activity, whereas the activity of PKM2 is regulated by post-translational modifications that promote the formation of the less active dimer or the more active tetrameric form (4, 54, 133, 215).
Oxidative stress due to H2O2, diamide, or hypoxia inactivates PKM2 and prevents the formation of the active tetramer via oxidation of Cys358; oxidation can be inhibited in the presence of the reducing agent, dithiothreitol (DTT) (4) (Fig. 4). Inhibition of PKM2 increases the levels of G6P and redirects the glycolytic flux toward the oxidative PPP pathway to generate additional NADPH. Since NADPH is one of the factors required to maintain appropriate levels of reduced GSH, inactivation of PKM2 leads to the reduction of oxidative stress (4). Also, PKM2 has been shown to promote increased expression of genes encoding glycolytic proteins (e.g., SLC2A1 [solute carrier family 2 member 1, Glut1], LDHA, PDHK1) and VEGFA via its interactions with the transcription factors HIF-1α and HIF-2α (212, 213). Therefore, PKM2 not only reduces oxidative stress by generating NADPH but also alleviates ischemia by increasing the expression of vascular endothelial growth factor (VEGF). Further, in the setting of pulmonary arterial hypertension, Guo et al. (120) reported that ROS-induced phosphorylation and inhibition of PKM2 stimulates cell proliferation and survival via increased flux through the PPP pathway. In proliferating human T cells (e.g., acute lymphoblastic leukemia), phosphorylation of PKM2 reduces glycolytic flux and activates the PPP (345). In proliferating ECs, PKM2 maintains cell cycle progression by suppressing p53-mediated signaling (173). By contrast, in quiescent ECs, PKM2 maintains vascular barrier function by suppressing NF-κB/angiopoietin 2 signaling (173, 315) independent of its canonical protein kinase activity. PKM2 activation also promotes angiogenic differentiation that maintains ROS at low levels; this has been associated with enhanced glycolysis and mitochondrial fission (273). The mitoROS promote dimerization of the PKM2 in monocytes and macrophages from atherosclerotic patients. PKM2 dimerization stimulates its nuclear translocation and thereby promotes signal transducer and activator of transcription 3 (STAT3) phosphorylation and production of IL-6 and IL-1β (305). Moreover, activation of PKM2 promotes angiogenesis via its actions on vascular resident endothelial progenitor cells via modulation of glycolysis as well as mitochondrial fission and fusion, which are required for the treatment of diseases and injuries requiring strategies that promote or inhibit angiogenesis (273).
Therapeutic implications
Many studies have reported the clinical relevance and therapeutic potential of agents that target PKM2 in CVD. The results of one study revealed significant increases in PKM2 expression in failing compared with non-failing hearts. Thus, induction of PKM2 is a signature of not only cardiotoxicity but also cardiac stress in general (272). Tetrameric PKM2–stabilizing drugs (e.g., TEPP-46) suppress p53-mediated transcriptional activity and cardiomyocyte apoptosis, thereby preventing anthracycline-induced cardiotoxicity (280). The therapeutic potential of PKM2 was further demonstrated in cardiomyocytes in which PKM2 participates in cell cycle regulatory mechanisms that enhance myocardial regeneration after myocardial infarction (220).
PFKFB3 and ROS
PFKFB is a bifunctional and rate-limiting enzyme in glycolysis that controls the cellular levels of fructose-2, 6-bisphosphate (F-2, 6-BP), which is an allosteric activator of PFK-1 (249, 275). Mammalian PFKFB (also known as PFK2) consists of four isoenzymes, including PFKFB 1, 2, 3, and 4. Among them, PFKFB3 plays the most crucial role in promoting the production of fructose-2, 6-bisphosphate (F-2,6-BP) and glycolysis, as it has the highest kinase to bisphosphatase activity ratio (740:1). PFKFB3 is expressed mainly in leukocytes, vascular cells, and cancer cells. Oxidative stress regulates PFKFB3 activity and protein stability mainly via post-translational modification (217, 277, 297) (Fig. 4). In cancer cells, oxidative stress inactivates PFKFB3, but not other PFKFB isoenzymes, by S-glutathionylation of C206. This results in a shift from glycolysis to the PPP, and thus NADPH production, regeneration of GSH, and ROS detoxification (297). The ROS generated by NOX2 in cells from patients with acute myeloid leukemia promotes glycolysis by increasing the expression of PFKFB3 (277). Further, activation of cSrc promotes cell proliferation and migration via PFKFB3 phosphorylation at Y194; this results in increased flux through the glycolytic and TCA cycle pathways and increasing ROS levels via associated reductions in the oxidative PPP, NADPH, and antioxidant systems (217). Given that ROS are known to activate cSrc, the cSrc-pY-PFKFB3-ROS axis may represent a positive feedback loop that can induce sustained ROS production. Taken together, these findings suggest that persistent glycolysis and redox regulation are closely linked to one another. The therapeutic implications of these observations are discussed in the section focused on glycolysis and ROS.
HIF-1 and ROS
HIF-1 is a key regulator of the developmental and physiological states required for the maintenance of O2 homeostasis. HIF-1 also promotes the adaptive responses to reduced O2 availability by regulating gene expression (161, 222, 295). HIF contributes to the metabolic shift from glucose oxidation to aerobic glycolysis in various CVDs and cancer (222, 295). HIF-1 is a heterodimeric transcription factor that consists of an O2•−-labile α subunit and a stable β subunit. Under conditions of normoxia, two proline residues on HIF-1α are hydroxylated by prolyl hydroxylase domain-containing proteins (PHDs) to become substrates for ubiquitination; they are then degraded by the von Hippel-Lindau (VHL) complex. By contrast, in response to hypoxia, mitoROS derived from respiratory complex III or cytosolic ROS can stabilize both HIF-1αand HIF-2α by inhibiting PHDs (19, 222) (Figs. 5 and 6). The actions of HIF-1α and HIF-2α serve to increase the expression of angiogenic genes such as VEGF and thereby promote angiogenesis associated with embryonic vascular development (270), hindlimb ischemia (29), hypertrophic cardiomyopathy (281), myocardial infarction (129), skin wound healing (219), and retinal neovascularization (155).
HIF-1-mediated regulation of the glycolytic pathway is closely linked to ROS homeostasis (Figs. 5 and 6). Overexpression of HIF-1 increases the uptake of glucose through GLUTs (i.e., GLUT1 and sodium-glucose transporters [SGLTs]) (75). Hypoxia-induced HIF-1α increases the extent of metabolic pathway reprogramming from oxidative phosphorylation to glycolysis to maintain ATP production. This metabolic reprogramming results from the upregulation of GLUTs and glycolytic enzymes (222), downregulation of PDH complexes via activation of PDHK1 (175, 211), or through suppression of mitochondrial respiration by inducing the expression of NADH dehydrogenase 1 alpha subcomplex, 4-like 2 (NDUFA4L2) (322). Loss of SIRT3, another regulator of HIF, results in impaired glycolysis via the reduction of signaling via the HIF-2α-PFKFB3 axis. This will ultimately result in impaired myocardial angiogenesis and cardiac dysfunction (128). Macrophage proinflammatory signaling is also induced in part via HIF-1α, which preferentially promotes ATP production via glycolysis (238). In this case, RET-derived mitoROS generated by mitochondrial hyperpolarization and succinate oxidation serve to stabilize HIF-1α; this leads to increases in glycolytic capacity and IL-1β mRNA and protein expression (320).
PDHK1 activation by HIF-1α shunts pyruvate away from the mitochondria, resulting in decreased flux through the TCA cycle and the ETC and potential attenuation of mitoROS production. The functional significance of this adaptive response has been demonstrated as follows: (i) Chronic hypoxia in HIF-1α-deficient fibroblasts results in cell death due to excessive accumulation of ROS accumulation (175, 295); (ii) hypoxia-induced expression of HIF-1α induces mitophagy via the actions of BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3), which results in a reduction in mitochondrial mass, O2 consumption, and ROS generation (389); and (iii) hypoxia-induced HIF-1 increases the expression of the mitochondrial isoforms of SHMT1 and SHMT2, leading to increased mitochondrial NADPH and decreased mitoROS levels in Myc-transformed cells (380). Thus, the critical adaptive response involving the hypoxia-HIF1 axis reduces the production of mitoROS via decreases in mitochondrial mass, O2 consumption, and increased NADPH, in addition to the impact of metabolic reprogramming.
Therapeutic implications
Given the importance of HIF for both physiological and pathological angiogenesis, several strategies have been developed to target HIF-related pathways for the treatment of ischemic diseases and cancer (222). PHD inhibitors (e.g., FG-2216, FG-4592), HIF activators (HIF-1α adenoviral-based therapy), and HIF inhibitors (EZN-2968, digoxin, anthracyclines) are all under consideration for clinical use (222). Additional details on these issues can be found in several excellent reviews (222).
Mitochondrial dynamics, ROS, and metabolism: mitochondrial dynamics and ROS
Mitochondrial dynamics refers to a multifaceted process that includes both the fission and fusion of the mitochondrial outer and inner membranes. Mitochondrial fission and fusion proteins coordinate this dynamic process. These proteins are closely associated with mitochondrial function and can influence and be regulated by mitoROS production (Figs. 3 and 5). The ROS can regulate mitochondria dynamics and vice versa. The ROS induce post-translational modifications of proteins that regulate mitochondrial dynamics, thereby influencing their expression and/or their activity. These actions serve to regulate mitochondrial dynamics, morphology, and function (221, 282, 353, 363). Increases in ROS generation during conditions of oxidative stress, high glucose concentrations, or elevations in FAs can contribute to mitochondrial fragmentation whereas sublethal amounts of H2O2 can induce hyperfusion.
Mitochondrial fission is mainly mediated by small GTPase, dynamin-related protein 1 (Drp1), and its receptor proteins mitochondrial fission factor (Mff), mitochondrial fission 1 protein (Fis1), mitochondrial dynamics protein of 49 kDa (MiD49), and MiD50. Drp1 is localized in the cytosol during resting states. When activated, Drp1 is recruited to the mitochondria outer membranes where it induces constriction and scission of the mitochondria in a GTP-dependent manner (360). The ROS can induce phosphorylation of S616 of Drp1 and activation of Drp1 GTPase activity. For example, ROS (including mitoROS) induced by ischemia/reperfusion injury promote Drp1 oligomerization and phosphorylation at S616. This leads to excessive mitochondrial fission in target ECs (115). In the setting of neurodegenerative diseases, NO• has been found to induce S-nitrosylation of Drp1, which increases its GTPase activity and mitochondrial fragmentation (52); however, this finding remains controversial (30). Sulfenylation (Cys-OH formation) is a reversible initial step in ROS-mediated oxidation of reactive cysteine residues of proteins that participate in redox signaling (255, 263). We reported that Drp1 sulfenylation at C644 as a result of the loss of protein disulfide isomerase A1 augments Drp1 GTPase activity and mitochondrial fragmentation. These events drive the production of mitoROS, which leads to EC senescence (176). Thus, targeting cysteine oxidation or other modifications of Drp1 may be a potential therapeutic strategy for diseases associated with mitochondrial dysregulation and dysfunction associated with oxidative stress. Several reports document a link between Drp1-mediated mitochondrial fission and ROS. First, hyperglycemia induces mitochondrial fragmentation via Ca2+ and extracellular signal-regulated kinase (ERK)1/2-dependent phosphorylation of Drp1 S616 or increases in Drp1 expression. These responses induce the overproduction of mitoROS (223, 279, 302, 341, 350, 383, 384). Second, H2O2 stimulation of cardiac myocytes promotes increased mitochondrial fragmentation. This response results in mitochondrial membrane depolarization and increased resistance to insulin via the upregulation of Drp1 (356). Thus, mitochondrial fission and ROS are interconnected.
Mitochondrial fusion is mediated by three GTPases, including the dynamin proteins mitofusin 1 (Mfn1), Mfn2, and optic atrophy 1 (Opa1). Mfn1 and Mfn2 are outer membrane proteins that facilitate the fusion of this membrane, whereas Opa1 is associated with the inner membrane and facilitates inner membrane fusion. Increased expression of Mfn2 induced by H2O2 is both necessary and sufficient to induce apoptosis of heart muscle cells in response to oxidative stress (300). In ECs, knockdown of the Mfns disrupts mitochondrial networks and decreases mitochondrial membrane potential, VEGF-induced migration, and capillary network formation (210). Depletion of Mfn2 limits ROS generation and blunts expression of components of the ETC and transcription factors associated with oxidative metabolism, whereas ablation of Mfn1 inhibits VEGF-induced Akt-eNOS signaling (210). GSSG stimulates mitochondrial fusion by inducing disulfide bond-mediated oligomerization of Mfn1 and via the C684 of Mfn2 (306). Taken together, these findings point to the regulation of redox-regulated mitochondrial hyperfusion of the mitochondrial IMS. Importantly, mutation of C684, which is found within a disulfide bridge when cells are in an oxidative state, renders Mfn2 more susceptible to alterations in the redox environment. Thus, the thiol switching of C684 in Mfn2 plays an important role in mediating redox-induced alternations of mitochondrial shape and activity (325). Thus, an understanding of how the mechanisms that define the relationships between redox homeostasis, mitochondrial structure, and mitochondrial dynamics are disrupted in pathological conditions may lead to the development of new therapeutic strategies.
Mitochondrial dynamics and metabolism
Mitochondrial dynamics mediated by fission and fusion and bioenergetics have a reciprocal influence on one another. Dynamic properties of the mitochondria are regulated by cellular signaling events and have a discernible impact on cellular metabolism (239, 342). Changes in mitochondrial morphology are frequently observed in response to alterations in the surrounding cellular milieu (e.g., metabolic flux), which influence cellular bioenergetics. Thus, an understanding of the mechanisms that govern mitochondrial morphology and their emerging role in mitochondrial bioenergetics will be of critical importance (109). Deletion of any of the components that support mitochondrial dynamics will perturb oxidative phosphorylation and glycolysis even at baseline (205). Several reports suggest roles for Mfn and Drp1 in cell metabolism and the pathogenesis of metabolic disorders (239, 288, 342). Mitochondrial fusion is particularly important in cells undergoing cellular respiration, as it facilitates the dissemination of metabolites, enzymes, and mitochondrial gene products throughout the entire mitochondrial compartment. This serves to optimize mitochondrial function and counteracts the effects of mitochondrial mutations that accumulate during the aging process. Muscle-specific gene-deletion of Mfn2 disrupts glucose homeostasis (293). Similarly, Mfn deficiency in ECs inhibits migration, network formation, viability, and mitoROS production via reduced expression of coenzyme Q and transcription factors associated with oxidative metabolism (210). Although Mfn gene-deleted embryos show developmental delays, expression of Mfn2 is increased in tissues under conditions of nutrient deprivation in the nervous system, skeletal muscle, and heart (6, 93). Loss of Mfn2 reduces mitochondrial membrane potential, O2 consumption, and activity of the ETC and the TCA cycle whereas anaerobic respiration increases as a compensatory mechanism (44, 241). Depletion of Mfn2 also results in the decreased expression of complexes that support oxidative phosphorylation. By contrast, Mfn2 overexpression increases both glycolysis and mitochondrial membrane potential (95) as well as the expression of ETC proteins, including subunits that contribute to complexes I, IV, and V (208). Recently, Buck et al. (35) reported that, by remodeling mitochondrial cristae, fusion in Tm configures associations of the ETC complex that favor oxidative phosphorylation and FAO. By contrast, fission in Teff cells leads to expansion of the mitochondrial cristae, thereby reducing ETC efficiency and promoting aerobic glycolysis. Thus, remodeling of the mitochondrial cristae via fusion/fission is a signaling mechanism that directs T cell fate via metabolic programing.
Fragmented mitochondria are frequently found in resting cells. Mitochondrial fission plays an important role in removing damaged organelles by autophagy. Thus, both mitochondrial fusion and fission contribute to the maintenance of mitochondrial function and the optimization of bioenergetic capacity. Multiple signaling pathways regulate the machinery of mitochondrial dynamics so that the shape of the mitochondrial compartment will adapt to specific metabolic conditions within the cell (361). Ablation of Drp1 in liver cells results in reduced adiposity and elevated whole-body energy expenditure, thereby protecting mice from diet-induced obesity (348). Drp1-mediated fission also regulates glycolysis during cell transformation (298). Activation of protein kinase A/A-kinase anchoring protein 1 (AKAP1) results in the phosphorylation of Drp1 at S637. This modification inhibits Drp1 activity and mitochondrial fission, thereby resulting in enhanced mitochondrial tubulation that promotes ATP production (42).
Mitochondrial dynamics as a therapeutic target
Alterations in the proteins that support mitochondrial dynamics can lead to CVD. As an example, reduced expression of Mfn2 led to hyperproliferation of VSMCs and accelerated cardiac hypertrophy and cardiomyopathy in mouse and rat models (46). Another report suggested that lactate accelerated vascular calcification that resulted from excessive Drp-mediated mitochondrial fission and a deficiency in BNIP3-related mitophagy (400). Also, increased mitochondrial fission contributes to impaired endothelial function in patients diagnosed with diabetes mellitus (302) as well as to the hyperproliferation of pulmonary artery smooth muscle cells in patients with pulmonary arterial hypertension (225). Drp1 is also crucial for the O2-induced constriction and closure of the ductus arteriosus at birth in healthy humans and rabbits (138) as well as for arterial constriction (206). Finally, Drp1 is upregulated in response to pathological conditions of the heart; excessive mitochondrial fission appears to be detrimental in this setting (145, 330).
Given that mitochondrial dynamics include responses and adaptations to metabolic demands and are involved in the regulation of mitophagy, compounds that target mitochondrial fission and fusion are currently of great interest. For example, mitochondrial division inhibitor (Mdivi)-1, which can inhibit the GTPase activity of Drp1, protects the heart from ischemia–reperfusion injury via the inhibition of mitochondria outer membrane permeabilization, which will result in mitochondrial-mediated cell death (146). In addition to Mdivi-1, the Drp1 inhibitor P110 can also protect the heart against ischemia–reperfusion injury (330). The antihypertensive drug, cilnidipine, is a small molecule that inhibits the interaction between filamin and Drp1. Administration of cilnidipine to mice after the induction of myocardial infarction limited the extent of mitochondrial fission, cardiomyocyte senescence, and myocardial dysfunction independent of its capacity to block Ca2+ channels (246). P110 also blocks the interaction between Drp1 and Fis1. This drug was shown to be neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) animal model of Parkinson's disease via its capacity to inhibit hyperactivation of Drp1 (265, 317). Similarly, 15-oxospiramilacone (S3) is an anticancer agent that inhibits Wnt/beta-catenin signaling that can also enhance Mfn1/2 activity and induce mitochondrial fusion by targeting the deubiquitinase ubiquitin carboxyl-terminal hydrolase 30 (USP30) in mitochondria. USP30 enhances the irreversible ubiquitination of Mfn1/2 (385). However, administration of S3 can restore mitochondrial fusion and function in cells that are deficient in either Mfn1 or Mfn2, suggesting its potential therapeutic potential as a means to target mitochondrial dynamics in patients with CVD.
Metabolic Enzymes and RNS
NO• and its metabolites have long been associated with maintaining metabolic homeostasis within cells. NO• can inhibit and activate numerous metabolic pathways as described in the sections to follow.
Glycolytic enzymes and NO•
NO• is a regulator of glycolysis. Endothelial NO• promotes glycolysis via activation of HIF-1α (33) and the AMPK-dependent activation of PFK1. NO• also supports glycolysis in dendritic cells (328). NO• derived from eNOS can limit ischemic injury by inhibiting the activity of PKM2, a response that is mediated via direct nitrosylation of a specific cysteine residue. Inhibition of PKM2 leads to the accumulation of glycolytic intermediates, which can then be shunted to other pathways including the PPP for the production of NADPH, a molecule that is necessary for antioxidant activity (396). Alternatively, iNOS can promote the nuclear translocation of PKM2, thereby increasing glycolytic flux (198). The relative concentration of NO• may be one mechanism to account for its different effects on metabolism. For example, low concentrations of NO• can promote glycolysis whereas higher concentrations can be inhibitory.
Mitochondrial enzymes and NO•
Aside from its role in promoting vasodilation, physiologic levels of NO• can support reversible binding and inhibition of the mitochondrial enzyme, complex IV, an action that serves to suppresses mitochondrial respiration (34, 56). Complex IV is the terminal oxidase (i.e., complex IV) of the mitochondrial ETC (Fig. 3). NO•, but not O2 binding to heme results in the accumulation of O2. NO•-mediated inhibition of complex IV is a competitive process that can be reversed by O2. Physiological levels of NO• may repress complex IV, thereby beneficially reducing O2 consumption and ATP formation due to the inhibition of electron flux at this site. This inhibition results in the redistribution of O2 to other sites, most notably under conditions of increased O2 demand (323). By contrast, prolonged exposure to NO• can lead to S-nitrosylation and inhibition of complex I (58), which will also inhibit cellular respiration. In ECs, endogenous NO• can suppress mitochondrial respiration (59).
NO• has been shown to stimulate the biogenesis of mitochondria via activation of peroxisome proliferator-activated receptor-gamma (PPARγ) and PGC1-α (30). NO• can also stimulate mitochondrial fragmentation that results from activation of mitochondrial fission or suppression of fusion, which can lead to mitochondrial dysfunction. In carbon tracing studies and via an analysis of O2 consumption, NO• -mediated inhibition of metabolism was tracked to the TCA cycle, mitochondrial aconitase, and PDH (253). Ultimately, NO• accumulation leads to suppression and loss of mitochondrial ETC complexes. The cytokine IL-10 can alter glycolytic responses by regulating the production of NO• to limit iNOS-mediated suppression of oxidative phosphorylation (18).
The reaction between NO• and O2•− generates ONOO− and occurs at or near-diffusion-limited rates and more rapidly than SOD-mediated O2•− dismutation. ONOO− reacts avidly with proteins and contributes to the nitration of tyrosine residues. Within cells, nitrotyrosine staining is concentrated in the mitochondria (76); ONOO− is a potent inhibitor of the mitochondrial ETC (269). ONOO− generated outside or within the mitochondria can oxidize and inhibit complexes I and II of the ETC, as well as aconitase and manganese (Mn)-dependent SOD, also known as SOD2 (40). Mitochondria are known to emit O2•− and have a dedicated SOD (i.e., SOD2). In pathological states, the amount of O2•− produced increases. Therefore, in the presence of NO•, mitochondria are the major sites of ONOO− production. ONOO− promotes the depolarization and cyclosporine-sensitive calcium efflux from the mitochondria via the mitochondria permeability transition pore or mitochondrial permeability transition pore (251). The formation of ONOO− in mitochondria is largely driven by O2•− flux. Although there have been reports of a mitochondrial NOS isoform that is similar to NOS1 that might contribute to ONOO− formation in the mitochondria (114), this issue remains controversial (183). Forced subcellular targeting of NOS isoforms to the mitochondria revealed that although levels of BH4 and l-arginine are sufficient to support NO• synthesis, only calcium-independent enzymes are active in the mitochondrial IMS (150). This result suggests that the mitochondrial NOS isoform involved in this process must either reside outside the mitochondria or be an isoform of NOS2 (as opposed to NOS1).
Mitochondrial enzymes and NO in inflammation
Macrophages that express NOS2 and generate large amounts of NO• have been found to suppress mitochondrial respiration. NO• is important for metabolic reprogramming as part of the proinflammatory shift toward glycolysis (8). Of note, these findings have been reported in mouse macrophages, but not in human macrophages, which produce comparatively lower levels of NO•. In addition to its ability to suppress the ETC and respiration, under conditions of relative hypoxia, NO• can stimulate the production of mitoROS that promotes proinflammatory signaling (59, 252). Suppression of mitochondrial respiration is not an essential feature of inflammatory polarization, compared with the upregulation of glycolysis. NO• is important for the regulation of TCA metabolism and its intermediates, citrate, and succinate, as well as production of the immunometabolite, itaconate (8). Itaconate production and its ability to shape the immune response are examples of the importance of metabolic reprogramming in support of this phenotypic change.
Therapeutic implications
Pharmacological modification of NO• signaling can have a direct impact on metabolism. NO• donors can promote glycolysis in multiple cell types (78, 224). Further, NO• and elevated levels of cyclic guanosine monophosphate (cGMP) contribute to the browning of adipose tissue in lean animals. However, in obesity, treatment with sildenafil and elevated levels of cGMP does not promote adipose tissue browning and result in compromised glucose disposal (159). In the heart, elevated levels of cGMP can promote glycolysis associated with the accumulation of both malate and α-KG and the increased activity of malate dehydrogenase (113). In pulmonary hypertension, a disorder associated with the upregulation of glycolytic pathways, administration of sildenafil results in the decreased expression of HK 2 (197). Thus, although NO• donors and modulators of cGMP signaling can have an impact on cellular metabolism via the inhibition of phosphodiesterase, these actions can be both dose and context-dependent. As one example of an intriguing connection between glycolysis and NO• signaling, GAPDH has been shown to function as a heme chaperone for soluble guanylate cyclase, an action that is necessary for binding NO• and production of cGMP (69). Similarly, H2O2 can activate PKG-1α to control vasodilation and blood pressure via a mechanism that is independent of the NO•-cGMP pathway (264). The role of crosstalk between ROS and RNS in metabolism warrants future investigation.
Summary and Conclusion
Mitochondrial oxidative phosphorylation, glycolytic metabolism, and redox homeostasis are all closely connected in vascular and inflammatory cells and are important in shaping cell behavior (Figs. 5 and 6). Imbalances in any of these pathways will compromise cellular function and contribute to CVD and cancer. Reciprocal interactions between the pathways that regulate metabolic flux and redox balance are important drivers of both physiological and pathophysiological processes (Figs. 5 and 6). For example, inhibition of glycolysis by ROS/RNS promotes metabolic reprogramming so that the cells shift from glycolytic flux to the oxidative arm of the PPP to generate NADPH. This shift will serve to increase antioxidant defense, which is important for preserving NO• and endothelial function. In macrophages, NO• is important for metabolic reprogramming and contributes to the proinflammatory shift away from mitochondrial oxidation and toward the glycolytic pathways. Thus, these are not merely passive metabolic adaptations; these changes play active roles in shaping physiological and pathological processes.
Given the pivotal role of metabolism in the process of angiogenesis, there is substantial interest in the therapeutic possibilities associated with the manipulation of EC metabolism (Fig. 1). However, strategies that target single pathways have been largely unsuccessful. Combined approaches using both metabolic inhibitors and ROS-modulating agents may offer greater promise, as they can function synergistically to stimulate angiogenesis required for cell repair or eradicate cancer cells via the regulation of intracellular ROS levels and cellular metabolism to achieve the desired outcome. An improved understanding of these mechanisms and how they are integrated into and contribute to various disease settings may lead to new opportunities for intervention and improved therapeutic strategies for CVD. Drugs targeting metabolic pathways are currently in use for the treatment of cancer; the findings obtained may have critical implications for the development of treatments for CVD. For CVD, drugs targeting ATP citrate synthase (ACLY), which links carbohydrate and lipid metabolism, may serve to reduce cholesterol levels and limit atherosclerosis via its actions primarily in the liver. The roles in this process played by other cell types are currently emerging.
Abbreviations Used
- α-KG
α-ketoglutarate
- α-KGDH
alpha-ketoglutarate dehydrogenase
- 1C
one-carbon
- ACC
acetyl-CoA carboxylase
- ADMA
asymmetric dimethylarginine
- AMPK
5′ adenosine monophosphate-activated protein kinase
- ApoE
apolipoprotein E
- ATP
adenosine triphosphate
- BCAA
branch-chained amino acid
- BCAT
branched-chain aminotransferase
- BCKA
branched-chain ketoacids
- BCKD
BCKA dehydrogenase
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- BNIP3
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- cGMP
cyclic guanosine monophosphate
- CoA
coenzyme A
- Complex I
NADH–ubiquinone oxidoreductase
- Complex III
ubiquinol–cytochrome c oxidoreductase
- Complex IV
cytochrome c oxidase
- CoQ
coenzyme Q
- CPT1
carnitine palmitoyltransferase-1
- CtBP
C-terminal-binding protein
- Cu,ZnSOD, SOD1
coper zinc superoxide dismutase
- CVD
cardiovascular disease
- CySS
cystine
- Cyt c
cytochrome c
- DDAH1
dimethylarginine dimethylaminohydrolase 1
- DHFR
dihydrofolate reductase
- dNTPs
deoxynucleotide triphosphates
- Drp1
dynamin-related protein 1
- EC
endothelial cell
- ECM
extracellular matrix
- ecSOD, SOD3
extracellular SOD
- ETC
electron transport chain
- F6P
fructose-6-phosphate
- FA
fatty acid
- FAD
flavin adenine dinucleotide
- FAO
fatty acid oxidation
- Fis1
mitochondrial fission 1 protein
- G6P
glucose-6-phosphate
- G6PD
glucose-6-phosphate dehydrogenase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCL
glutamate-cysteine ligase
- GGPP
geranylgeranyl pyrophosphate
- GLS
glutaminase
- GLUT
glucose transporter
- GPX
glutathione peroxidase
- GSH
glutathione
- GSR
glutathione reductase
- GSS
GSH synthetase
- GSSG
oxidized glutathione
- GTPCH
GTP cyclohydrolase
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HIF
hypoxia-inducible factor
- HK
hexokinase
- Hsp
heat-shock protein
- IDH
isocitrate dehydrogenase
- IL
interleukin
- IMS
intermembrane space
- IRG1/ACOD1
immune-responsive gene 1/aconitate decarboxylase 1
- KEAP1
Kelch-like ECH-associated protein 1
- LDH
lactate dehydrogenase
- l-NMMA
N-monomethyl l-arginine
- LPS
lipopolysaccharide
- Mdivi
mitochondrial division inhibitor
- ME
malic enzyme
- Mfn
mitofusin
- mitoROS
mitochondrial ROS
- MnSOD, SOD2
manganese superoxide dismutase
- mRNA
messenger RNA
- MSUD
maple syrup urine disease
- Mtb
Mycobacterium tuberculosis
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-kappa B
- NLRP3
NLR family pyrin domain containing 3
- NO•
nitric oxide
- NOS
nitric oxide synthase
- NOS1, nNOS
neuronal NOS
- NOS2, iNOS
inducible NOS
- NOS3, eNOS
endothelial NOS
- NOX
NADPH oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- O2
oxygen
- O2•−
superoxide
- OAA
oxaloacetate
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- Opa1
optic atrophy 1
- PDH
pyruvate dehydrogenase
- PDHK1
pyruvate dehydrogenase kinase 1
- PDHK
pyruvate dehydrogenase kinase
- PFK
phosphofructokinase
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- PGC
peroxisome proliferator-activated receptor-gamma coactivator
- PHD
prolyl hydroxylase domain-containing protein
- PHGDH
phosphoglycerate dehydrogenase
- PK
pyruvate kinase
- PKC
protein kinase C
- PKM2
pyruvate kinase M2
- PPP
pentose phosphate pathway
- PRX
peroxiredoxin
- RET
reverse electron transport
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAM
S-adenosyl-methionine
- SDH
succinate dehydrogenase
- SGOC
serine-glycine one-carbon metabolism
- SHMT
serine hydroxymethyltransferase
- SIRT
sirtuin
- SLC1A5/ASCT2
solute carrier family 1, member 5/alanine/serine/cysteine-preferring transporter 2
- TAZ
transcriptional coactivator with PDZ binding motif
- TCA
tricarboxylic acid
- Teff
effector T
- TIGAR
TP53-inducible glycolysis and apoptosis regulator
- Tm
memory T cells
- TNF
tumor necrosis factor
- TRX
thioredoxin
- TRXR
thioredoxin reductase
- TxNIP
thioredoxin-interacting protein
- UCP
uncoupling protein
- USP30
ubiquitin carboxyl-terminal hydrolase 30
- VDAC
voltage-dependent mitochondrial anion channel
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
- xCT
the cystine/glutamate antiporter SLC7A11
- XO
xanthine oxidase
- YAP
yes-associated protein
Authors' Contributions
T.F., M.U.-F., D.A., and S.N. prepared the figures; T.F., M.U.-F., D.A., S.N., and D.J.R.F. drafted the article; T.F., M.U-F., D.F., and E.J.B.C. edited and revised the article; and T.F., M.U.-F., D.A., S.N., D.J.R.F., and E.J.B.C. approved the final version of the article.
Author Disclosure Statement
The authors declare no competing financial interests.
Funding Information
This work was supported by NIHR01HL135584 (to M.U.-F.), NIHR01HL116976, NIHR01HL133613, NIH1R01HL147550 (to T.F., M.U.-F.), Department of Veterans Affairs Merit Review grant 2I01BX001232 (to T.F.).
References
- 1.Alhayaza R, Haque E, Karbasiafshar C, Sellke FW, and Abid MR. The relationship between reactive oxygen species and endothelial cell metabolism. Front Chem 8: 592688, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alp NJ and Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Amelio I, Cutruzzola F, Antonov A, Agostini M, and Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci 39: 191–198, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, and Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334: 1278–1283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angela M, Endo Y, Asou HK, Yamamoto T, Tumes DJ, Tokuyama H, Yokote K, and Nakayama T. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARgamma directs early activation of T cells. Nat Commun 7: 13683, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacín M, Vidal H, Rivera F, Brand M, and Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bailey J, Shaw A, Fischer R, Ryan BJ, Kessler BM, McCullagh J, Wade-Martins R, Channon KM, and Crabtree MJ. A novel role for endothelial tetrahydrobiopterin in mitochondrial redox balance. Free Radic Biol Med 104: 214–225, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, Hale A, Starr A, Nandi M, Stylianou E, McShane H, Davis S, Fischer R, Kessler BM, McCullagh J, Channon KM, and Crabtree MJ. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep 28: 218.e7–230.e7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baillet A, Hograindleur MA, El Benna J, Grichine A, Berthier S, Morel F, and Paclet MH. Unexpected function of the phagocyte NADPH oxidase in supporting hyperglycolysis in stimulated neutrophils: key role of 6-phosphofructo-2-kinase. FASEB J 31: 663–673, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Baker RG, Hayden MS, and Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab 13: 11–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. This reference has been deleted
- 12.Balaban RS, Nemoto S, and Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Ballinger SW.Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38: 1278–1295, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Bansal A and Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 217: 2291–2298, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, and Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barros LF, Ruminot I, San Martin A, Lerchundi R, Fernandez-Moncada I, and Baeza-Lehnert F. Aerobic glycolysis in the brain: Warburg and Crabtree Contra Pasteur. Neurochem Res 46: 15–22, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Barschak AG, Sitta A, Deon M, Barden AT, Dutra-Filho CS, Wajner M, and Vargas CR. Oxidative stress in plasma from maple syrup urine disease patients during treatment. Metab Brain Dis 23: 71–80, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Baseler WA, Davies LC, Quigley L, Ridnour LA, Weiss JM, Hussain SP, Wink DA, and McVicar DW. Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol 10: 12–23, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell EL and Chandel NS. Mitochondrial oxygen sensing: regulation of hypoxia-inducible factor by mitochondrial generated reactive oxygen species. Essays Biochem 43: 17–27, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Bendall JK, Douglas G, McNeill E, Channon KM, and Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal 20: 3040–3077, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson MA, Batchelor H, Chuaiphichai S, Bailey J, Zhu H, Stuehr DJ, Bhattacharya S, Channon KM, and Crabtree MJ. A pivotal role for tryptophan 447 in enzymatic coupling of human endothelial nitric oxide synthase (eNOS): effects on tetrahydrobiopterin-dependent catalysis and eNOS dimerization. J Biol Chem 288: 29836–29845, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berndt C, Lillig CH, and Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol 292: H1227–H1236, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J, 3rd, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, and Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettencourt IA and Powell JD. Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. J Immunol 198: 999–1005, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, and Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation 107: 388–390, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Boger RH.Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 134: 2842S–2847S; discussion 2853S, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, and Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87: 99–105, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Boroughs LK and DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17: 351–359, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, and Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ Res 101: 1310–1318, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, and Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. J Alzheimers Dis 20(Suppl 2): S513–S526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd S, Brookfield JL, Critchlow SE, Cumming IA, Curtis NJ, Debreczeni J, Degorce SL, Donald C, Evans NJ, Groombridge S, Hopcroft P, Jones NP, Kettle JG, Lamont S, Lewis HJ, MacFaull P, McLoughlin SB, Rigoreau LJ, Smith JM, St-Gallay S, Stock JK, Turnbull AP, Wheatley ER, Winter J, and Wingfield J. Structure-based design of potent and selective inhibitors of the metabolic kinase PFKFB3. J Med Chem 58: 3611–3625, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Brandes RP, Weissmann N, and Schroder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76: 208–226, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Brix B, Mesters JR, Pellerin L, and Johren O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1alpha-mediated target gene activation. J Neurosci 32: 9727–9735, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown GC and Borutaite V. Nitric oxide, cytochrome c and mitochondria. Biochem Soc Symp 66: 17–25, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, Chen Q, Huang SC, O'Neill CM, Edelson BT, Pearce EJ, Sesaki H, Huber TB, Rambold AS, and Pearce EL. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166: 63–76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns M, Rizvi SHM, Tsukahara Y, Pimentel DR, Luptak I, Hamburg NM, Matsui R, and Bachschmid MM. Role of glutaredoxin-1 and glutathionylation in cardiovascular diseases. Int J Mol Sci 21: 6803, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cairns RA, Harris I, McCracken S, and Mak TW. Cancer cell metabolism. Cold Spring Harb Symp Quant Biol 76: 299–311, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Cardaci S, Filomeni G, and Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J Cell Sci 125: 2115–2125, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Carracedo A, Cantley LC, and Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 13: 227–232, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro L, Rodriguez M, and Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem 269: 29409–29415, 1994 [PubMed] [Google Scholar]
- 41.Chandra P, He L, Zimmerman M, Yang G, Koster S, Ouimet M, Wang H, Moore KJ, Dartois V, Schilling JD, and Philips JA. Inhibition of fatty acid oxidation promotes macrophage control of Mycobacterium tuberculosis. mBio 11: e01139-20, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang CR and Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Chen CJ, Fu YC, Yu W, and Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem Biophys Res Commun 430: 798–803, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Chomyn A, and Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Chen JQ and Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim Biophys Acta 1826: 370–384, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, and Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6: 872–883, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Chen L and Cui H. Targeting glutamine induces apoptosis: a cancer therapy approach. Int J Mol Sci 16: 22830–22855, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Cairns R, Papandreou I, Koong A, and Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One 4: e7033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Ikeda K, Yoneshiro T, Scaramozza A, Tajima K, Wang Q, Kim K, Shinoda K, Sponton CH, Brown Z, Brack A, and Kajimura S. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565: 180–185, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Cheung EC, Lee P, Ceteci F, Nixon C, Blyth K, Sansom OJ, and Vousden KH. Opposing effects of TIGAR- and RAC1-derived ROS on Wnt-driven proliferation in the mouse intestine. Genes Dev 30: 52–63, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, and Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324: 102–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, and Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christofk HR, Vander Heiden MG, Wu N, Asara JM, and Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452: 181–186, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bonaa KH, Spence JD, Nygard O, Jamison R, Gaziano JM, Guarino P, Bennett D, Mir F, Peto R, and Collins R; B-Vitamin Treatment Trialists' Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med 170: 1622–1631, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, and Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345: 50–54, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Clem BF, O'Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, 2nd, Klarer AC, Redman R, Miller DM, Trent JO, Telang S, and Chesney J. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12: 1461–1470, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clementi E, Brown GC, Feelisch M, and Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A 95: 7631–7636, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clementi E, Brown GC, Foxwell N, and Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc Natl Acad Sci U S A 96: 1559–1562, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conway ME.Emerging moonlighting functions of the branched-chain aminotransferase proteins. Antioxid Redox Signal 34: 1048–1067, 2021 [DOI] [PubMed] [Google Scholar]
- 61.Cooke JP.Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol 20: 2032–2037, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, and Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest 90: 1168–1172, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corda S, Laplace C, Vicaut E, and Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol 24: 762–768, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Crabtree MJ and Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25: 81–88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, Zhang YJ, Keating MJ, Huang P, DiGiovanni J, Georgiou G, and Stone E. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med 23: 120–127, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, and Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cunnington C, Van Assche T, Shirodaria C, Kylintireas I, Lindsay AC, Lee JM, Antoniades C, Margaritis M, Lee R, Cerrato R, Crabtree MJ, Francis JM, Sayeed R, Ratnatunga C, Pillai R, Choudhury RP, Neubauer S, and Channon KM. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 125: 1356–1366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D'Souza AD, Parikh N, Kaech SM, and Shadel GS. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion 7: 374–385, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai Y, Sweeny EA, Schlanger S, Ghosh A, and Stuehr DJ. GAPDH delivers heme to soluble guanylyl cyclase. J Biol Chem 295: 8145–8154, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danishpajooh IO, Gudi T, Chen Y, Kharitonov VG, Sharma VS, and Boss GR. Nitric oxide inhibits methionine synthase activity in vivo and disrupts carbon flow through the folate pathway. J Biol Chem 276: 27296–27303, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Davidson SM and Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100: 1128–1141, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Day EA, Ford RJ, and Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab 28: 545–560, 2017 [DOI] [PubMed] [Google Scholar]
- 73.De Bock K, Georgiadou M, and Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab 18: 634–647, 2013 [DOI] [PubMed] [Google Scholar]
- 74.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, and Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154: 651–663, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Denko NC.Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8: 705–713, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Devadas S, Zaritskaya L, Rhee SG, Oberley L, and Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med 195: 59–70, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhillon B, Badiwala MV, Maitland A, Rao V, Li SH, and Verma S. Tetrahydrobiopterin attenuates homocysteine induced endothelial dysfunction. Mol Cell Biochem 247: 223–227, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Dittmann J, Finsterbusch F, and Ludt H. Sodium nitroprusside (NPS) stimulates the lactate formation and causes intracellular electrolyte shifts in brain slices. Acta Neurochir (Wien) 50: 327–334, 1979 [DOI] [PubMed] [Google Scholar]
- 79.Douglas G, Hale AB, Patel J, Chuaiphichai S, Al Haj Zen A, Rashbrook VS, Trelfa L, Crabtree MJ, McNeill E, and Channon KM. Roles for endothelial cell and macrophage Gch1 and tetrahydrobiopterin in atherosclerosis progression. Cardiovasc Res 114: 1385–1399, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Draoui N, de Zeeuw P, and Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol 7: 170219, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Droge W.Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Dromparis P and Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol 75: 95–126, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Druhan LJ, Forbes SP, Pope AJ, Chen CA, Zweier JL, and Cardounel AJ. Regulation of eNOS-derived superoxide by endogenous methylarginines. Biochemistry 47: 7256–7263, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Ducker GS and Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab 25: 27–42, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunn LL, Buckle AM, Cooke JP, and Ng MK. The emerging role of the thioredoxin system in angiogenesis. Arterioscler Thromb Vasc Biol 30: 2089–2098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, and Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104: 342–345, 2001 [DOI] [PubMed] [Google Scholar]
- 87.Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G, Nadzan AM, and Lopaschuk GD. Malonyl coenzyme A decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res 94: e78–e84, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Eelen G, de Zeeuw P, Simons M, and Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res 116: 1231–1244, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Egnatchik RA, Brittain EL, Shah AT, Fares WH, Ford HJ, Monahan K, Kang CJ, Kocurek EG, Zhu S, Luong T, Nguyen TT, Hysinger E, Austin ED, Skala MC, Young JD, Roberts LJ, 2nd, Hemnes AR, West J, and Fessel JP. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm Circ 7: 186–199, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El Hindy M, Hezwani M, Corry D, Hull J, El Amraoui F, Harris M, Lee C, Forshaw T, Wilson A, Mansbridge A, Hassler M, Patel VB, Kehoe PG, Love S, and Conway ME. The branched-chain aminotransferase proteins: novel redox chaperones for protein disulfide isomerase—implications in Alzheimer's disease. Antioxid Redox Signal 20: 2497–2513, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elms S, Chen F, Wang Y, Qian J, Askari B, Yu Y, Pandey D, Iddings J, Caldwell RB, and Fulton DJ. Insights into the arginine paradox: evidence against the importance of subcellular location of arginase and eNOS. Am J Physiol Heart Circ Physiol 305: H651–H666, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, and Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med 46: 1386–1391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eura Y, Ishihara N, Yokota S, and Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem 134: 333–344, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, and Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510: 298–302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filadi R, Pendin D, and Pizzo P. Mitofusin 2: from functions to disease. Cell Death Dis 9: 330, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Filipp FV, Scott DA, Ronai ZA, Osterman AL, and Smith JW. Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res 25: 375–383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fillmore N and Lopaschuk GD. Targeting mitochondrial oxidative metabolism as an approach to treat heart failure. Biochim Biophys Acta 1833: 857–865, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Fillmore N, Mori J, and Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171: 2080–2090, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Finlay D and Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol 11: 109–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, and Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med 209: 2441–2453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forman HJ and Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med 166: S4–S8, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, and Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122: 877–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forstermann U and Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 837 a–837d, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Franco R, Schoneveld OJ, Pappa A, and Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem 113: 234–258, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, and Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity 16: 769–777, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Fukai T and Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fulton DJ.Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaber T, Strehl C, and Buttgereit F. Metabolic regulation of inflammation. Nat Rev Rheumatol 13: 267–279, 2017 [DOI] [PubMed] [Google Scholar]
- 109.Galloway CA, Lee H, and Yoon Y. Mitochondrial morphology-emerging role in bioenergetics. Free Radic Biol Med 53: 2218–2228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ganguly P and Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J 14: 6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gebhart V, Reiss K, Kollau A, Mayer B, and Gorren ACF. Site and mechanism of uncoupling of nitric-oxide synthase: uncoupling by monomerization and other misconceptions. Nitric Oxide 89: 14–21, 2019 [DOI] [PubMed] [Google Scholar]
- 112.Gerriets VA and Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol 33: 168–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gevi F, Campolo F, Naro F, and Zolla L. The cardioprotective effect of sildenafil is mediated by the activation of malate dehydrogenase and an increase in the malate-aspartate shuttle in cardiomyocytes. Biochem Pharmacol 127: 60–70, 2017 [DOI] [PubMed] [Google Scholar]
- 114.Ghafourifar P and Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26: 190–195, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Giedt RJ, Yang C, Zweier JL, Matzavinos A, and Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic Biol Med 52: 348–356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goodman M, Liu Z, Zhu P, and Li J. AMPK activators as a drug for diabetes, cancer and cardiovascular disease. Pharm Regul Aff 3: 118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goto M, Miwa H, Shikami M, Tsunekawa-Imai N, Suganuma K, Mizuno S, Takahashi M, Mizutani M, Hanamura I, and Nitta M. Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Invest 32: 241–247, 2014 [DOI] [PubMed] [Google Scholar]
- 118.Goveia J, Stapor P, and Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med 6: 1105–1120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guerra L, Bonetti L, and Brenner D. Metabolic modulation of immunity: a new concept in cancer immunotherapy. Cell Rep 32: 107848, 2020 [DOI] [PubMed] [Google Scholar]
- 120.Guo D, Gu J, Jiang H, Ahmed A, Zhang Z, and Gu Y. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to the development of pulmonary arterial hypertension. J Mol Cell Cardiol 91: 179–187, 2016 [DOI] [PubMed] [Google Scholar]
- 121.Haines RJ, Pendleton LC, and Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol 2: 8–23, 2011 [PMC free article] [PubMed] [Google Scholar]
- 122.Ham M, Lee JW, Choi AH, Jang H, Choi G, Park J, Kozuka C, Sears DD, Masuzaki H, and Kim JB. Macrophage glucose-6-phosphate dehydrogenase stimulates proinflammatory responses with oxidative stress. Mol Cell Biol 33: 2425–2435, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han D, Antunes F, Canali R, Rettori D, and Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003 [DOI] [PubMed] [Google Scholar]
- 124.Han D, Williams E, and Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 353: 411–416, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA, Elia A, Berger T, Cescon DW, Adeoye A, Brustle A, Molyneux SD, Mason JM, Li WY, Yamamoto K, Wakeham A, Berman HK, Khokha R, Done SJ, Kavanagh TJ, Lam CW, and Mak TW. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27: 211–222, 2015 [DOI] [PubMed] [Google Scholar]
- 126.Hasan NM, Longacre MJ, Stoker SW, Kendrick MA, and MacDonald MJ. Mitochondrial malic enzyme 3 is important for insulin secretion in pancreatic beta-cells. Mol Endocrinol 29: 396–410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hattori Y, Hattori S, Wang X, Satoh H, Nakanishi N, and Kasai K. Oral administration of tetrahydrobiopterin slows the progression of atherosclerosis in apolipoprotein E-knockout mice. Arterioscler Thromb Vasc Biol 27: 865–870, 2007 [DOI] [PubMed] [Google Scholar]
- 128.He X, Zeng H, Chen ST, Roman RJ, Aschner JL, Didion S, and Chen JX. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J Mol Cell Cardiol 112: 104–113, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heinl-Green A, Radke PW, Munkonge FM, Frass O, Zhu J, Vincent K, Geddes DM, and Alton EW. The efficacy of a ‘master switch gene’ HIF-1alpha in a porcine model of chronic myocardial ischaemia. Eur Heart J 26: 1327–1332, 2005 [DOI] [PubMed] [Google Scholar]
- 130.Hildeman DA.Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med 36: 1496–1504, 2004 [DOI] [PubMed] [Google Scholar]
- 131.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, and Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10: 735–744, 1999 [DOI] [PubMed] [Google Scholar]
- 132.Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, Bright TP, Krieg T, Carling D, and Murphy MP. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J Biol Chem 293: 17208–17217, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, and Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2: ra73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoffmann A, Gloe T, and Pohl U. Hypoxia-induced upregulation of eNOS gene expression is redox-sensitive: a comparison between hypoxia and inhibitors of cell metabolism. J Cell Physiol 188: 33–44, 2001 [DOI] [PubMed] [Google Scholar]
- 135.Holmgren A.Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2: 811–820, 2000 [DOI] [PubMed] [Google Scholar]
- 136.Holmström KM and Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15: 411–421, 2014 [DOI] [PubMed] [Google Scholar]
- 137.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, and Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 113: 205–212, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, Trivedi K, Weir EK, and Archer SL. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res 112: 802–815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Horie T, Ono K, Nagao K, Nishi H, Kinoshita M, Kawamura T, Wada H, Shimatsu A, Kita T, and Hasegawa K. Oxidative stress induces GLUT4 translocation by activation of PI3-K/Akt and dual AMPK kinase in cardiac myocytes. J Cell Physiol 215: 733–742, 2008 [DOI] [PubMed] [Google Scholar]
- 140.Houtkooper RH, Pirinen E, and Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Bruning U, Visnagri A, Yuldasheva N, Goveia J, Cruys B, Brepoels K, Wyns S, Rayport S, Ghesquiere B, Vinckier S, Schoonjans L, Cubbon R, Dewerchin M, Eelen G, and Carmeliet P. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J 36: 2334–2352, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang PL.eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20: 295–302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang Y, Zhou M, Sun H, and Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc Res 90: 220–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hwang AB, Ryu EA, Artan M, Chang HW, Kabir MH, Nam HJ, Lee D, Yang JS, Kim S, Mair WB, Lee C, Lee SS, and Lee SJ. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A 111: E4458–E4467, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ikeda Y, Sciarretta S, Nagarajan N, Rubattu S, Volpe M, Frati G, and Sadoshima J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid Med Cell Longev 2014: 210934, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ishikita A, Matoba T, Ikeda G, Koga J, Mao Y, Nakano K, Takeuchi O, Sadoshima J, and Egashira K. Nanoparticle-mediated delivery of mitochondrial division inhibitor 1 to the myocardium protects the heart from ischemia-reperfusion injury through inhibition of mitochondria outer membrane permeabilization: a new therapeutic modality for acute myocardial infarction. J Am Heart Assoc 5: e003872, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Izaki S, Goto H, and Yokota S. Increased chemosensitivity and elevated reactive oxygen species are mediated by glutathione reduction in glutamine deprived neuroblastoma cells. J Cancer Res Clin Oncol 134: 761–768, 2008 [DOI] [PubMed] [Google Scholar]
- 148.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, and Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol 180: 4476–4486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jacque N, Ronchetti AM, Larrue C, Meunier G, Birsen R, Willems L, Saland E, Decroocq J, Maciel TT, Lambert M, Poulain L, Hospital MA, Sujobert P, Joseph L, Chapuis N, Lacombe C, Moura IC, Demo S, Sarry JE, Recher C, Mayeux P, Tamburini J, and Bouscary D. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 126: 1346–1356, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jagnandan D, Sessa WC, and Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol 289: C1024–C1033, 2005 [DOI] [PubMed] [Google Scholar]
- 151.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, and Arany Z. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22: 421–426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jaramillo MC and Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27: 2179–2191, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jaswal JS, Keung W, Wang W, Ussher JR, and Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation—a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 1813: 1333–1350, 2011 [DOI] [PubMed] [Google Scholar]
- 154.Jeon SM, Chandel NS, and Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485: 661–665, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang J, Xia XB, Xu HZ, Xiong Y, Song WT, Xiong SQ, and Li Y. Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1alpha and VEGF. J Cell Physiol 218: 66–74, 2009 [DOI] [PubMed] [Google Scholar]
- 156.Jiang S, Park DW, Stigler WS, Creighton J, Ravi S, Darley-Usmar V, and Zmijewski JW. Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J Biol Chem 288: 26013–26026, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, Boggon TJ, Jin P, Yi H, Wright ER, Duong D, Seyfried NT, Egnatchik R, DeBerardinis RJ, Magliocca KR, He C, Arellano ML, Khoury HJ, Shin DM, Khuri FR, and Kang S. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 27: 257–270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jo EK, Kim JK, Shin DM, and Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13: 148–159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Johann K, Reis MC, Harder L, Herrmann B, Gachkar S, Mittag J, and Oelkrug R. Effects of sildenafil treatment on thermogenesis and glucose homeostasis in diet-induced obese mice. Nutr Diabetes 8: 9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ju HQ, Lin JF, Tian T, Xie D, and Xu RH. NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Transduct Target Ther 5: 231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kaelin WG Jr.and Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Bruning U, Scheinok S, Treps L, Cantelmo AR, Dubois C, de Zeeuw P, Goveia J, Zecchin A, Taverna F, Morales-Rodriguez F, Brajic A, Conradi LC, Schoors S, Harjes U, Vriens K, Pilz GA, Chen R, Cubbon R, Thienpont B, Cruys B, Wong BW, Ghesquiere B, Dewerchin M, De Bock K, Sagaert X, Jessberger S, Jones EAV, Gallez B, Lambrechts D, Mazzone M, Eelen G, Li X, Fendt SM, and Carmeliet P. Quiescent endothelial cells upregulate fatty acid beta-oxidation for vasculoprotection via redox homeostasis. Cell Metab 28: 881.e13–894.e13, 2018 [DOI] [PubMed] [Google Scholar]
- 163.Kaminski MM, Sauer SW, Kaminski M, Opp S, Ruppert T, Grigaravicius P, Grudnik P, Grone HJ, Krammer PH, and Gulow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2: 1300–1315, 2012 [DOI] [PubMed] [Google Scholar]
- 164.Kantor PF, Lucien A, Kozak R, and Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res 86: 580–588, 2000 [DOI] [PubMed] [Google Scholar]
- 165.Karbach S, Wenzel P, Waisman A, Munzel T, and Daiber A. eNOS uncoupling in cardiovascular diseases—the role of oxidative stress and inflammation. Curr Pharm Des 20: 3579–3594, 2014 [DOI] [PubMed] [Google Scholar]
- 166.Karwi QG, Uddin GM, Ho KL, and Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 5: 68, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, and Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3: 420–430, 2010 [DOI] [PubMed] [Google Scholar]
- 168.Katt WP and Cerione RA. Glutaminase regulation in cancer cells: a druggable chain of events. Drug Discov Today 19: 450–457, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, and Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25: 1939–1948, 2013 [DOI] [PubMed] [Google Scholar]
- 170.Khalaf D, Kruger M, Wehland M, Infanger M, and Grimm D. The effects of oral l-arginine and l-citrulline supplementation on blood pressure. Nutrients 11: 1679, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Khosravi M, Poursaleh A, Ghasempour G, Farhad S, and Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol Chem 400: 711–732, 2019 [DOI] [PubMed] [Google Scholar]
- 172.Kim AS, Miller EJ, Wright TM, Li J, Qi D, Atsina K, Zaha V, Sakamoto K, and Young LH. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol 51: 24–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim B, Jang C, Dharaneeswaran H, Li J, Bhide M, Yang S, Li K, and Arany Z. Endothelial pyruvate kinase M2 maintains vascular integrity. J Clin Invest 128: 4543–4556, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim B, Li J, Jang C, and Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J 36: 2321–2333, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim JW, Tchernyshyov I, Semenza GL, and Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006 [DOI] [PubMed] [Google Scholar]
- 176.Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, Kitajewski J, Rehman J, Yoon Y, Cho J, Fukai T, and Ushio-Fukai M. Redox regulation of mitochondrial fission protein Drp1 by protein disulfide isomerase limits endothelial senescence. Cell Rep 23: 3565–3578, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kominsky DJ, Campbell EL, and Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol 184: 4062–4068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kong H and Chandel NS. Regulation of redox balance in cancer and T cells. J Biol Chem 293: 7499–7507, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Konior A, Schramm A, Czesnikiewicz-Guzik M, and Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal 20: 2794–2814, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kornberg MD, Bhargava P, Kim PM, Putluri V, Snowman AM, Putluri N, Calabresi PA, and Snyder SH. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360: 449–453, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kussmaul L, Hamprecht B, and Dringen R. The detoxification of cumene hydroperoxide by the glutathione system of cultured astroglial cells hinges on hexose availability for the regeneration of NADPH. J Neurochem 73: 1246–1253, 1999 [DOI] [PubMed] [Google Scholar]
- 182.Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003 [DOI] [PubMed] [Google Scholar]
- 183.Lacza Z, Pankotai E, and Busija DW. Mitochondrial nitric oxide synthase: current concepts and controversies. Front Biosci (Landmark Ed) 14: 4436–4443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lambeth JD, Krause KH, and Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol 30: 339–363, 2008 [DOI] [PubMed] [Google Scholar]
- 185.Lambeth JD and Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9: 119–145, 2014 [DOI] [PubMed] [Google Scholar]
- 186.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, and Artyomov MN. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 24: 158–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lassegue B, San Martin A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, and Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 189.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, and Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107: 2037–2042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee I, Kim S, Nagar H, Choi SJ, Jeon BH, Piao S, and Kim CS. CR6-interacting factor 1 deficiency reduces endothelial nitric oxide synthase activity by inhibiting biosynthesis of tetrahydrobiopterin. Sci Rep 10: 842, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Leiper J, Murray-Rust J, McDonald N, and Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci U S A 99: 13527–13532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Leiper J and Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10: 277–291, 2011 [DOI] [PubMed] [Google Scholar]
- 193.Leitner KL, Meyer M, Leimbacher W, Peterbauer A, Hofer S, Heufler C, Muller A, Heller R, Werner ER, Thony B, and Werner-Felmayer G. Low tetrahydrobiopterin biosynthetic capacity of human monocytes is caused by exon skipping in 6-pyruvoyl tetrahydropterin synthase. Biochem J 373: 681–688, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, and Biondi A. Mitochondrial complex I: structural and functional aspects. Biochim Biophys Acta 1757: 1406–1420, 2006 [DOI] [PubMed] [Google Scholar]
- 195.Leopold JA, Zhang YY, Scribner AW, Stanton RC, and Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vasc Biol 23: 411–417, 2003 [DOI] [PubMed] [Google Scholar]
- 196.Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, and Metallo CM. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55: 253–263, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li B, He W, Ye L, Zhu Y, Tian Y, Chen L, Yang J, Miao M, Shi Y, Azevedo HS, Ma Z, and Hao K. Targeted delivery of sildenafil for inhibiting pulmonary vascular remodeling. Hypertension 73: 703–711, 2019 [DOI] [PubMed] [Google Scholar]
- 198.Li L, Zhu L, Hao B, Gao W, Wang Q, Li K, Wang M, Huang M, Liu Z, Yang Q, Li X, Zhong Z, Huang W, Xiao G, Xu Y, Yao K, and Liu Q. iNOS-derived nitric oxide promotes glycolysis by inducing pyruvate kinase M2 nuclear translocation in ovarian cancer. Oncotarget 8: 33047–33063, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, He J, Hunter T, Wang L, and Lu Z. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell 61: 705–719, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li X, Sun X, and Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab 30: 414–433, 2019 [DOI] [PubMed] [Google Scholar]
- 201.Li Y, Sun R, Zou J, Ying Y, and Luo Z. Dual roles of the AMP-activated protein kinase pathway in angiogenesis. Cells 8: 752, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li Y, Zhu H, Kuppusamy P, Zweier JL, and Trush MA. Mitochondrial electron transport chain-derived superoxide exits macrophages: implications for mononuclear cell-mediated pathophysiological processes. React Oxyg Species (Apex) 1: 81–98, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liao ST, Han C, Xu DQ, Fu XW, Wang JS, and Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun 10: 5091, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Libby P and Everett BM. Novel antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol 39: 538–545, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liesa M and Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17: 491–506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu MY, Jin J, Li SL, Yan J, Zhen CL, Gao JL, Zhang YH, Zhang YQ, Shen X, Zhang LS, Wei YY, Zhao Y, Wang CG, Bai YL, and Dong DL. Mitochondrial fission of smooth muscle cells is involved in artery constriction. Hypertension 68: 1245–1254, 2016 [DOI] [PubMed] [Google Scholar]
- 207.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, and Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 208.Loiseau D, Chevrollier A, Verny C, Guillet V, Gueguen N, Pou de Crescenzo MA, Ferré M, Malinge MC, Guichet A, Nicolas G, Amati-Bonneau P, Malthièry Y, Bonneau D, and Reynier P. Mitochondrial coupling defect in Charcot-Marie-Tooth type 2A disease. Ann Neurol 61: 315–323, 2007 [DOI] [PubMed] [Google Scholar]
- 209.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, and Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010 [DOI] [PubMed] [Google Scholar]
- 210.Lugus JJ, Ngoh GA, Bachschmid MM, and Walsh K. Mitofusins are required for angiogenic function and modulate different signaling pathways in cultured endothelial cells. J Mol Cell Cardiol 51: 885–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, and Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev 21: 1037–1049, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, and Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145: 732–744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Luo W and Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget 2: 551–556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lushchak VI.Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012: 736837, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki N, Jiang Y, Zhou X, Li TT, Guan KL, Lei QY, and Xiong Y. Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell 52: 340–352, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ma EH, Poffenberger MC, Wong AH, and Jones RG. The role of AMPK in T cell metabolism and function. Curr Opin Immunol 46: 45–52, 2017 [DOI] [PubMed] [Google Scholar]
- 217.Ma H, Zhang J, Zhou L, Wen S, Tang HY, Jiang B, Zhang F, Suleman M, Sun D, Chen A, Zhao W, Lin F, Tsau MT, Shih LM, Xie C, Li X, Lin D, Hung LM, Cheng ML, and Li Q. c-Src promotes tumorigenesis and tumor progression by activating PFKFB3. Cell Rep 30: 4235.e6–4249.e6, 2020 [DOI] [PubMed] [Google Scholar]
- 218.Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, and Fang X. Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett 435: 92–100, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mace KA, Yu DH, Paydar KZ, Boudreau N, and Young DM. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen 15: 636–645, 2007 [DOI] [PubMed] [Google Scholar]
- 220.Magadum A, Singh N, Kurian AA, Munir I, Mehmood T, Brown K, Sharkar MTK, Chepurko E, Sassi Y, Oh JG, Lee P, Santos CXC, Gaziel-Sovran A, Zhang G, Cai CL, Kho C, Mayr M, Shah AM, Hajjar RJ, and Zangi L. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation 141: 1249–1265, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mailloux RJ, Jin X, and Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol 2: 123–139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Majmundar AJ, Wong WJ, and Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40: 294–309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Makino A, Scott BT, and Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53: 1783–1794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Maletic SD, Dragicevic-Djokovic LM, Zikic RV, Stajn AS, Milenkovic P, and Kostic MM. Effects of nitric oxide donors on energy metabolism of rat erythrocytes. J Environ Pathol Toxicol Oncol 19: 383–390, 2000 [PubMed] [Google Scholar]
- 225.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, and Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Martinez-Reyes I and Chandel NS. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov 4: 1371–1373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, and Medford RM. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest 92: 1866–1874, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Matsui R, Xu S, Maitland KA, Hayes A, Leopold JA, Handy DE, Loscalzo J, and Cohen RA. Glucose-6 phosphate dehydrogenase deficiency decreases the vascular response to angiotensin II. Circulation 112: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 229.Matsui R, Xu S, Maitland KA, Mastroianni R, Leopold JA, Handy DE, Loscalzo J, and Cohen RA. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E(−/−) mice. Arterioscler Thromb Vasc Biol 26: 910–916, 2006 [DOI] [PubMed] [Google Scholar]
- 230.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, Moretti M, De Smaele E, Beg AA, Tergaonkar V, Chandel NS, and Franzoso G. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol 13: 1272–1279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.McCabe TJ, Fulton D, Roman LJ, and Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000 [DOI] [PubMed] [Google Scholar]
- 232.McCarty MF, Barroso-Aranda J, and Contreras F. AMP-activated kinase may suppress NADPH oxidase activation in vascular tissues. Med Hypotheses 72: 468–470, 2009 [DOI] [PubMed] [Google Scholar]
- 233.McNeill E, Crabtree MJ, Sahgal N, Patel J, Chuaiphichai S, Iqbal AJ, Hale AB, Greaves DR, and Channon KM. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic Biol Med 79: 206–216, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.McNeill E, Stylianou E, Crabtree MJ, Harrington-Kandt R, Kolb AL, Diotallevi M, Hale AB, Bettencourt P, Tanner R, O'Shea MK, Matsumiya M, Lockstone H, Muller J, Fletcher HA, Greaves DR, McShane H, and Channon KM. Regulation of mycobacterial infection by macrophage Gch1 and tetrahydrobiopterin. Nat Commun 9: 5409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mescka CP, Guerreiro G, Donida B, Marchetti D, Wayhs CA, Ribas GS, Coitinho AS, Wajner M, Dutra-Filho CS, and Vargas CR. Investigation of inflammatory profile in MSUD patients: benefit of L-carnitine supplementation. Metab Brain Dis 30: 1167–1174, 2015 [DOI] [PubMed] [Google Scholar]
- 236.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, and Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481: 380–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, and Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186: 3299–3303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Dabritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, and O'Neill LA. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167: 457.e13–470.e13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mishra P and Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol 212: 379–387, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, and Imaizumi T. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis [in Japanese]. J Cardiol 33: 105–106, 1999 [PubMed] [Google Scholar]
- 241.Mourier A, Motori E, Brandt T, Lagouge M, Atanassov I, Galinier A, Rappl G, Brodesser S, Hultenby K, Dieterich C, and Larsson NG. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J Cell Biol 208: 429–442, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Muller FL, Liu Y, and Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004 [DOI] [PubMed] [Google Scholar]
- 243.Murphy MP and Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity 38: 201–202, 2013 [DOI] [PubMed] [Google Scholar]
- 244.Nagata D, Mogi M, and Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003 [DOI] [PubMed] [Google Scholar]
- 245.Nash DT and Nash SD. Ranolazine for chronic stable angina. Lancet 372: 1335–1341, 2008 [DOI] [PubMed] [Google Scholar]
- 246.Nishimura A, Shimauchi T, Tanaka T, Shimoda K, Toyama T, Kitajima N, Ishikawa T, Shindo N, Numaga-Tomita T, Yasuda S, Sato Y, Kuwahara K, Kumagai Y, Akaike T, Ide T, Ojida A, Mori Y, and Nishida M. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci Signal 11: eaat5185, 2018 [DOI] [PubMed] [Google Scholar]
- 247.Noguchi T, Inoue H, and Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem 261: 13807–13812, 1986 [PubMed] [Google Scholar]
- 248.O'Neill LAJ and Artyomov MN. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat Rev Immunol 19: 273–281, 2019 [DOI] [PubMed] [Google Scholar]
- 249.Okar DA, Wu C, and Lange AJ. Regulation of the regulatory enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Adv Enzyme Regul 44: 123–154, 2004 [DOI] [PubMed] [Google Scholar]
- 250.Osman I, He X, Liu J, Dong K, Wen T, Zhang F, Yu L, Hu G, Xin H, Zhang W, and Zhou J. TEAD1 (TEA domain transcription factor 1) promotes smooth muscle cell proliferation through upregulating SLC1A5 (solute carrier family 1 member 5)-mediated glutamine uptake. Circ Res 124: 1309–1322, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Packer MA and Murphy MP. Peroxynitrite formed by simultaneous nitric oxide and superoxide generation causes cyclosporin-A-sensitive mitochondrial calcium efflux and depolarisation. Eur J Biochem 234: 231–239, 1995 [DOI] [PubMed] [Google Scholar]
- 252.Palacios-Callender M, Quintero M, Hollis VS, Springett RJ, and Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A 101: 7630–7635, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquiere B, Maio N, Rice CM, Rouault TA, Cassel T, Higashi RM, Lane AN, Fan TW, Wink DA, and McVicar DW. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun 11: 698, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Panieri E and Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7: e2253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Paulsen CE and Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol 5: 47–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pearce EL and Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 38: 633–643, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, and Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460: 103–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pell VR, Chouchani ET, Frezza C, Murphy MP, and Krieg T. Succinate metabolism: a new therapeutic target for myocardial reperfusion injury. Cardiovasc Res 111: 134–141, 2016 [DOI] [PubMed] [Google Scholar]
- 259.Peralta D, Bronowska AK, Morgan B, Doka E, Van Laer K, Nagy P, Grater F, and Dick TP. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol 11: 156–163, 2015 [DOI] [PubMed] [Google Scholar]
- 260.Peyton KJ, Liu XM, Yu Y, Yates B, Behnammanesh G, and Durante W. Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells. Biochem Pharmacol 156: 204–214, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pike LS, Smift AL, Croteau NJ, Ferrick DA, and Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta 1807: 726–734, 2011 [DOI] [PubMed] [Google Scholar]
- 262.Pircher A, Treps L, Bodrug N, and Carmeliet P. Endothelial cell metabolism: a novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis 253: 247–257, 2016 [DOI] [PubMed] [Google Scholar]
- 263.Poole LB and Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol 12: 18–24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Prysyazhna O, Rudyk O, and Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med 18: 286–290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Qi X, Qvit N, Su YC, and Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126: 789–802, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Quinlan CL, Goncalves RL, Hey-Mogensen M, Yadava N, Bunik VI, and Brand MD. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J Biol Chem 289: 8312–8325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, and Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem 287: 27255–27264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S, Raissi TC, Pause A, St-Pierre J, and Jones RG. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep 21: 1–9, 2017 [DOI] [PubMed] [Google Scholar]
- 269.Radi R, Rodriguez M, Castro L, and Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys 308: 89–95, 1994 [DOI] [PubMed] [Google Scholar]
- 270.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, and Simon MC. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev Cell 11: 81–92, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Randle PJ, Garland PB, Hales CN, and Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963 [DOI] [PubMed] [Google Scholar]
- 272.Rees ML, Subramaniam J, Li Y, Hamilton DJ, Frazier OH, and Taegtmeyer H. A PKM2 signature in the failing heart. Biochem Biophys Res Commun 459: 430–436, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ren R, Guo J, Shi J, Tian Y, Li M, and Kang H. PKM2 regulates angiogenesis of VR-EPCs through modulating glycolysis, mitochondrial fission, and fusion. J Cell Physiol 235: 6204–6217, 2020 [DOI] [PubMed] [Google Scholar]
- 274.Richard E, Gallego-Villar L, Rivera-Barahona A, Oyarzabal A, Perez B, Rodriguez-Pombo P, and Desviat LR. Altered redox homeostasis in branched-chain amino acid disorders, organic acidurias, and homocystinuria. Oxid Med Cell Longev 2018: 1246069, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, and Hue L. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J 381: 561–579, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Riganti C, Gazzano E, Polimeni M, Aldieri E, and Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med 53: 421–436, 2012 [DOI] [PubMed] [Google Scholar]
- 277.Robinson AJ, Hopkins GL, Rastogi N, Hodges M, Doyle M, Davies S, Hole PS, Omidvar N, Darley RL, and Tonks A. Reactive oxygen species drive proliferation in acute myeloid leukemia via the glycolytic regulator PFKFB3. Cancer Res 80: 937–949, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Roth D, Krammer PH, and Gulow K. Dynamin related protein 1-dependent mitochondrial fission regulates oxidative signalling in T cells. FEBS Lett 588: 1749–1754, 2014 [DOI] [PubMed] [Google Scholar]
- 279.Rovira-Llopis S, Banuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, and Victor VM. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol 11: 637–645, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Saleme B, Gurtu V, Zhang Y, Kinnaird A, Boukouris AE, Gopal K, Ussher JR, and Sutendra G. Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity. Sci Transl Med 11: eaau8866, 2019 [DOI] [PubMed] [Google Scholar]
- 281.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, and Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446: 444–448, 2007 [DOI] [PubMed] [Google Scholar]
- 282.Santel A and Frank S. Shaping mitochondria: the complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 60: 448–455, 2008 [DOI] [PubMed] [Google Scholar]
- 283.Santoro MM.Fashioning blood vessels by ROS signalling and metabolism. Semin Cell Dev Biol 80: 35–42, 2018 [DOI] [PubMed] [Google Scholar]
- 284.Sanzey M, Abdul Rahim SA, Oudin A, Dirkse A, Kaoma T, Vallar L, Herold-Mende C, Bjerkvig R, Golebiewska A, and Niclou SP. Comprehensive analysis of glycolytic enzymes as therapeutic targets in the treatment of glioblastoma. PLoS One 10: e0123544, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Scarbrough PM, Mapuskar KA, Mattson DM, Gius D, Watson WH, and Spitz DR. Simultaneous inhibition of glutathione- and thioredoxin-dependent metabolism is necessary to potentiate 17AAG-induced cancer cell killing via oxidative stress. Free Radic Biol Med 52: 436–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Godde L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquiere B, Lunt SY, Fendt SM, and Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520: 192–197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouche A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, and Carmeliet P. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 19: 37–48, 2014 [DOI] [PubMed] [Google Scholar]
- 288.Schrepfer E and Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell 61: 683–694, 2016 [DOI] [PubMed] [Google Scholar]
- 289.Schroder K, Zhou R, and Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327: 296–300, 2010 [DOI] [PubMed] [Google Scholar]
- 290.Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner B, Steinberg P, Pfeiffer AF, and Ristow M. Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem 281: 977–981, 2006 [DOI] [PubMed] [Google Scholar]
- 291.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BA, Sobel RA, Wipke BT, Steinman L, Scannevin RH, and Zamvil SS. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A 113: 4777–4782, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Scialo F, Fernandez-Ayala DJ, and Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol 8: 428, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Oresic M, Pich S, Burcelin R, Palacin M, and Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A 109: 5523–5528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Segal AW.NADPH oxidases as electrochemical generators to produce ion fluxes and turgor in fungi, plants and humans. Open Biol 6: 160028, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Semenza GL.Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta 1813: 1263–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sena LA and Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Seo M and Lee YH. PFKFB3 regulates oxidative stress homeostasis via its S-glutathionylation in cancer. J Mol Biol 426: 830–842, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Serasinghe MN, Wieder SY, Renault TT, Elkholi R, Asciolla JJ, Yao JL, Jabado O, Hoehn K, Kageyama Y, Sesaki H, and Chipuk JE. Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol Cell 57: 521–536, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shah A, Xia L, Goldberg H, Lee KW, Quaggin SE, and Fantus IG. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 288: 6835–6848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shen T, Zheng M, Cao C, Chen C, Tang J, Zhang W, Cheng H, Chen KH, and Xiao RP. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem 282: 23354–23361, 2007 [DOI] [PubMed] [Google Scholar]
- 301.Shen Y, Kapfhamer D, Minnella AM, Kim JE, Won SJ, Chen Y, Huang Y, Low LH, Massa SM, and Swanson RA. Bioenergetic state regulates innate inflammatory responses through the transcriptional co-repressor CtBP. Nat Commun 8: 624, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, and Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124: 444–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, and Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shin CS, Mishra P, Watrous JD, Carelli V, D'Aurelio M, Jain M, and Chan DC. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun 8: 15074, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, and Weyand CM. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med 213: 337–354, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shutt T, Geoffrion M, Milne R, and McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep 13: 909–915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sinha RA, Singh BK, Zhou J, Wu Y, Farah BL, Ohba K, Lesmana R, Gooding J, Bay BH, and Yen PM. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 11: 1341–1357, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, and Kimmelman AC. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496: 101–105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sreedhar A, Wiese EK, and Hitosugi T. Enzymatic and metabolic regulation of lysine succinylation. Genes Dis 7: 166–171, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.St-Pierre J, Buckingham JA, Roebuck SJ, and Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 311.Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, and Heller R. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem 285: 10638–10652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Stanhewicz AE and Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev 75: 61–70, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Staniek K and Nohl H. Are mitochondria a permanent source of reactive oxygen species? Biochim Biophys Acta 1460: 268–275, 2000 [DOI] [PubMed] [Google Scholar]
- 314.Stockwell BR, Jiang X, and Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol 30: 478–490, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Stone OA, El-Brolosy M, Wilhelm K, Liu X, Romao AM, Grillo E, Lai JKH, Gunther S, Jeratsch S, Kuenne C, Lee IC, Braun T, Santoro MM, Locasale JW, Potente M, and Stainier DYR. Loss of pyruvate kinase M2 limits growth and triggers innate immune signaling in endothelial cells. Nat Commun 9: 4077, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, and Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104: 2569–2575, 2001 [DOI] [PubMed] [Google Scholar]
- 317.Su YC and Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum Mol Genet 22: 4545–4561, 2013 [DOI] [PubMed] [Google Scholar]
- 318.Sydow K and Munzel T. ADMA and oxidative stress. Atheroscler Suppl 4: 41–51, 2003 [DOI] [PubMed] [Google Scholar]
- 319.Tanada Y, Shioi T, Kato T, Kawamoto A, Okuda J, and Kimura T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci 137: 20–27, 2015 [DOI] [PubMed] [Google Scholar]
- 320.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, and O'Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496: 238–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Tejero J, Shiva S, and Gladwin MT. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev 99: 311–379, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordonez A, Corral-Escariz M, Soro I, Lopez-Bernardo E, Perales-Clemente E, Martinez-Ruiz A, Enriquez JA, Aragones J, Cadenas S, and Landazuri MO. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab 14: 768–779, 2011 [DOI] [PubMed] [Google Scholar]
- 323.Tengan CH and Moraes CT. NO control of mitochondrial function in normal and transformed cells. Biochim Biophys Acta Bioenerg 1858: 573–581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tennant DA, Duran RV, and Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10: 267–277, 2010 [DOI] [PubMed] [Google Scholar]
- 325.Thaher O, Wolf C, Dey PN, Pouya A, Wullner V, Tenzer S, and Methner A. The thiol switch C684 in Mitofusin-2 mediates redox-induced alterations of mitochondrial shape and respiration. Neurochem Int 117: 167–173, 2018 [DOI] [PubMed] [Google Scholar]
- 326.Theodorou K and Boon RA. Endothelial cell metabolism in atherosclerosis. Front Cell Dev Biol 6: 82, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Therade-Matharan S, Laemmel E, Carpentier S, Obata Y, Levade T, Duranteau J, and Vicaut E. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol 289: R1756–R1762, 2005 [DOI] [PubMed] [Google Scholar]
- 328.Thwe PM and Amiel E. The role of nitric oxide in metabolic regulation of Dendritic cell immune function. Cancer Lett 412: 236–242, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tomas L, Edsfeldt A, Mollet IG, Perisic Matic L, Prehn C, Adamski J, Paulsson-Berne G, Hedin U, Nilsson J, Bengtsson E, Goncalves I, and Bjorkbacka H. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur Heart J 39: 2301–2310, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tong M, Zablocki D, and Sadoshima J. The role of Drp1 in mitophagy and cell death in the heart. J Mol Cell Cardiol 142: 138–145, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Tornatore L, Thotakura AK, Bennett J, Moretti M, and Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol 22: 557–566, 2012 [DOI] [PubMed] [Google Scholar]
- 332.Tretter L and Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci 360: 2335–2345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Tseng HH, Vong CT, Kwan YW, Lee SM, and Hoi MP. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci Rep 6: 35016, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Tuder RM, Davis LA, and Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 185: 260–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Upchurch GR Jr., Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF Jr., and Loscalzo J.. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 272: 17012–17017, 1997 [DOI] [PubMed] [Google Scholar]
- 336.Vallance P, Leone A, Calver A, Collier J, and Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992 [DOI] [PubMed] [Google Scholar]
- 337.Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, and Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733–739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Venema RC, Ju H, Zou R, Ryan JW, and Venema VJ. Subunit interactions of endothelial nitric-oxide synthase. Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem 272: 1276–1282, 1997 [DOI] [PubMed] [Google Scholar]
- 339.Verhaar MC, Stroes E, and Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol 22: 6–13, 2002 [DOI] [PubMed] [Google Scholar]
- 340.Verhaar MC, Wever RM, Kastelein JJ, van Dam T, Koomans HA, and Rabelink TJ. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation 97: 237–241, 1998 [DOI] [PubMed] [Google Scholar]
- 341.Wada J and Nakatsuka A. Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med Okayama 70: 151–158, 2016 [DOI] [PubMed] [Google Scholar]
- 342.Wai T and Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab 27: 105–117, 2016 [DOI] [PubMed] [Google Scholar]
- 343.Walker HA, McGing E, Fisher I, Boger RH, Bode-Boger SM, Jackson G, Ritter JM, and Chowienczyk PJ. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol 38: 499–505, 2001 [DOI] [PubMed] [Google Scholar]
- 344.Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, Xu Y, Zhou L, Chu Y, Zhang C, Qin X, Yang P, and Yu H. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1beta production and to prevent DSS-induced colitis in mice. Cell Rep 19: 2331–2344, 2017 [DOI] [PubMed] [Google Scholar]
- 345.Wang H, Nicolay BN, Chick JM, Gao X, Geng Y, Ren H, Gao H, Yang G, Williams JA, Suski JM, Keibler MA, Sicinska E, Gerdemann U, Haining WN, Roberts TM, Polyak K, Gygi SP, Dyson NJ, and Sicinski P. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 546: 426–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wang J, Tong C, Yan X, Yeung E, Gandavadi S, Hare AA, Du X, Chen Y, Xiong H, Ma C, Leng L, Young LH, Jorgensen WL, Li J, and Bucala R. Limiting cardiac ischemic injury by pharmacological augmentation of macrophage migration inhibitory factor-AMP-activated protein kinase signal transduction. Circulation 128: 225–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, and Cerione RA. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18: 207–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wang L, Ishihara T, Ibayashi Y, Tatsushima K, Setoyama D, Hanada Y, Takeichi Y, Sakamoto S, Yokota S, Mihara K, Kang D, Ishihara N, Takayanagi R, and Nomura M. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia 58: 2371–2380, 2015 [DOI] [PubMed] [Google Scholar]
- 349.Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, and Zou MH. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One 6: e25436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, and Zou MH. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes 66: 193–205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, and Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35: 871–882, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wang S, Peng Z, Wang S, Yang L, Chen Y, Kong X, Song S, Pei P, Tian C, Yan H, Ding P, Hu W, Liu CH, Zhang X, He F, and Zhang L. KRAB-type zinc-finger proteins PITA and PISA specifically regulate p53-dependent glycolysis and mitochondrial respiration. Cell Res 28: 572–592, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wappler EA, Institoris A, Dutta S, Katakam PV, and Busija DW. Mitochondrial dynamics associated with oxygen-glucose deprivation in rat primary neuronal cultures. PLoS One 8: e63206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Warburg O, Wind F, and Negelein E. The metabolism of tumors in the body. J Gen Physiol 8: 519–530, 1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, and Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol 168: 1737–1748, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Watanabe T, Saotome M, Nobuhara M, Sakamoto A, Urushida T, Katoh H, Satoh H, Funaki M, and Hayashi H. Roles of mitochondrial fragmentation and reactive oxygen species in mitochondrial dysfunction and myocardial insulin resistance. Exp Cell Res 323: 314–325, 2014 [DOI] [PubMed] [Google Scholar]
- 357.Weber C and von Hundelshausen P. CANTOS trial validates the inflammatory pathogenesis of atherosclerosis: setting the stage for a new chapter in therapeutic targeting. Circ Res 121: 1119–1121, 2017 [DOI] [PubMed] [Google Scholar]
- 358.Wende AR, Brahma MK, McGinnis GR, and Young ME. Metabolic origins of heart failure. JACC Basic Transl Sci 2: 297–310, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, and Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472: 476–480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Westermann B.Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884, 2010 [DOI] [PubMed] [Google Scholar]
- 361.Westermann B.Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta 1817: 1833–1838, 2012 [DOI] [PubMed] [Google Scholar]
- 362.Whitsett J, Rangel Filho A, Sethumadhavan S, Celinska J, Widlansky M, and Vasquez-Vivar J. Human endothelial dihydrofolate reductase low activity limits vascular tetrahydrobiopterin recycling. Free Radic Biol Med 63: 143–150, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Willems PH, Rossignol R, Dieteren CE, Murphy MP, and Koopman WJ. Redox homeostasis and mitochondrial dynamics. Cell Metab 22: 207–218, 2015 [DOI] [PubMed] [Google Scholar]
- 364.Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, and Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A 108: 19611–19616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wolf A, Zalpour C, Theilmeier G, Wang BY, Ma A, Anderson B, Tsao PS, and Cooke JP. Dietary L-arginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J Am Coll Cardiol 29: 479–485, 1997 [DOI] [PubMed] [Google Scholar]
- 366.Wolin MS, Ahmad M, and Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–L173, 2005 [DOI] [PubMed] [Google Scholar]
- 367.Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, Garcia-Caballero M, Missiaen R, Huang H, Bruning U, Blacher S, Vinckier S, Goveia J, Knobloch M, Zhao H, Dierkes C, Shi C, Hagerling R, Moral-Darde V, Wyns S, Lippens M, Jessberger S, Fendt SM, Luttun A, Noel A, Kiefer F, Ghesquiere B, Moons L, Schoonjans L, Dewerchin M, Eelen G, Lambrechts D, and Carmeliet P. The role of fatty acid beta-oxidation in lymphangiogenesis. Nature 542: 49–54, 2017 [DOI] [PubMed] [Google Scholar]
- 368.Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH, Kong HK, Park EY, Kim HK, Han J, Chang M, and Park JH. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1alpha axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PLoS One 10: e0132285, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wu D, Huang RT, Hamanaka RB, Krause M, Oh MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, and Mutlu GM. HIF-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 6: e25217, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Wu H and Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res 126: 1549–1564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Xiao W and Loscalzo J. Metabolic responses to reductive stress. Antioxid Redox Signal 32: 1330–1347, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Xiao W, Wang RS, Handy DE, and Loscalzo J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal 28: 251–272, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Xu J, Wu Y, Song P, Zhang M, Wang S, and Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 116: 944–953, 2007 [DOI] [PubMed] [Google Scholar]
- 374.Xu MJ, Song P, Shirwany N, Liang B, Xing J, Viollet B, Wang X, Zhu Y, and Zou MH. Impaired expression of uncoupling protein 2 causes defective postischemic angiogenesis in mice deficient in AMP-activated protein kinase alpha subunits. Arterioscler Thromb Vasc Biol 31: 1757–1765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, Caldwell RB, Fulton DJ, Su Y, Hoda MN, Zhou G, Wu C, and Huo Y. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol 34: 1231–1239, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Yang Q, Xu J, Ma Q, Liu Z, Sudhahar V, Cao Y, Wang L, Zeng X, Zhou Y, Zhang M, Xu Y, Wang Y, Weintraub NL, Zhang C, Fukai T, Wu C, Huang L, Han Z, Wang T, Fulton DJ, Hong M, and Huo Y. PRKAA1/AMPKalpha1-driven glycolysis in endothelial cells exposed to disturbed flow protects against atherosclerosis. Nat Commun 9: 4667, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Yang W and Lu Z. Pyruvate kinase M2 at a glance. J Cell Sci 128: 1655–1660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Yang WS and Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26: 165–176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Yang X, Li Y, Li Y, Ren X, Zhang X, Hu D, Gao Y, Xing Y, and Shang H. Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Front Physiol 8: 600, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J, Finley LW, Lu C, Lindsten T, Cross JR, Qing G, Liu Z, Simon MC, Rabinowitz JD, and Thompson CB. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov 4: 1406–1417, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ye J, Mancuso A, Tong X, Ward PS, Fan J, Rabinowitz JD, and Thompson CB. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A 109: 6904–6909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, and Yodoi J. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol 4: 514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yu T, Jhun BS, and Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal 14: 425–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Yu T, Robotham JL, and Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 103: 2653–2658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Yue W, Chen Z, Liu H, Yan C, Chen M, Feng D, Yan C, Wu H, Du L, Wang Y, Liu J, Huang X, Xia L, Liu L, Wang X, Jin H, Wang J, Song Z, Hao X, and Chen Q. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res 24: 482–496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, and Chilian WM. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal 11: 1961–1974, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Zaric BL, Radovanovic JN, Gluvic Z, Stewart AJ, Essack M, Motwalli O, Gojobori T, and Isenovic ER. Atherosclerosis linked to aberrant amino acid metabolism and immunosuppressive amino acid catabolizing enzymes. Front Immunol 11: 551758, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Zhang BB, Zhou G, and Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9: 407–416, 2009 [DOI] [PubMed] [Google Scholar]
- 389.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, and Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 390.Zhang J, Muri J, Fitzgerald G, Gorski T, Gianni-Barrera R, Masschelein E, D'Hulst G, Gilardoni P, Turiel G, Fan Z, Wang T, Planque M, Carmeliet P, Pellerin L, Wolfrum C, Fendt SM, Banfi A, Stockmann C, Soro-Arnaiz I, Kopf M, and De Bock K. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab 31: 1136.e7–1153.e7, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zhang J, Pavlova NN, and Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J 36: 1302–1315, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, and Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol 28: 1627–1633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Zhang R, Li R, Liu Y, Li L, and Tang Y. The glycolytic enzyme PFKFB3 controls TNF-alpha-induced endothelial proinflammatory responses. Inflammation 42: 146–155, 2019 [DOI] [PubMed] [Google Scholar]
- 394.Zhao RZ, Jiang S, Zhang L, and Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int J Mol Med 44: 3–15, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Zhao Y, Dai XY, Zhou Z, Zhao GX, Wang X, and Xu MJ. Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice. Acta Pharmacol Sin 37: 196–203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zhou HL, Zhang R, Anand P, Stomberski CT, Qian Z, Hausladen A, Wang L, Rhee EP, Parikh SM, Karumanchi SA, and Stamler JS. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 565: 96–100, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zhou R, Tardivel A, Thorens B, Choi I, and Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140, 2010 [DOI] [PubMed] [Google Scholar]
- 398.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]
- 399.Zhou S, Kachhap S, Sun W, Wu G, Chuang A, Poeta L, Grumbine L, Mithani SK, Chatterjee A, Koch W, Westra WH, Maitra A, Glazer C, Carducci M, Sidransky D, McFate T, Verma A, and Califano JA. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proc Natl Acad Sci U S A 104: 7540–7545, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Zhu Y, Han XQ, Sun XJ, Yang R, Ma WQ, and Liu NF. Lactate accelerates vascular calcification through NR4A1-regulated mitochondrial fission and BNIP3-related mitophagy. Apoptosis 25: 321–340, 2020 [DOI] [PubMed] [Google Scholar]
- 401.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, and Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]