Abstract
Introduction
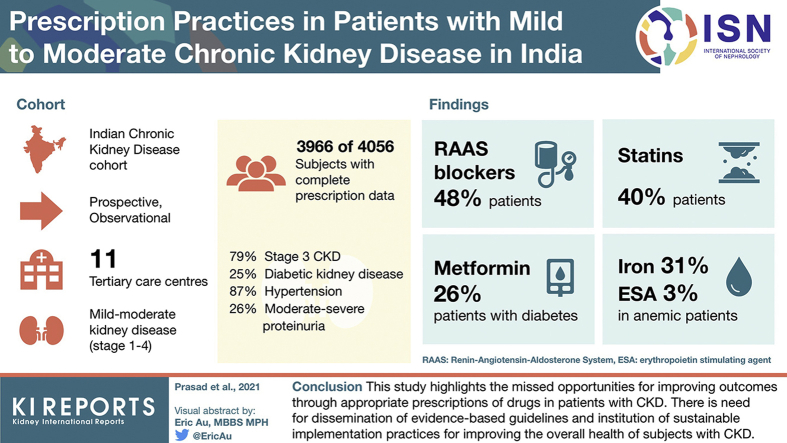
Patients with chronic kidney disease (CKD) require multiple medications. There is no information on prescription patterns or the use of evidence-based therapies for management of CKD from low-middle-income countries. Using baseline data from the Indian CKD (ICKD) cohort, we describe the drug prescription practices in patients with mild to moderate CKD.
Methods
The ICKD study is a prospective, observational cohort study of mild to moderate kidney disease across 11 centers in India. We analyzed all the prescriptions captured at enrollment in the ICKD study. Drugs were categorized into 11 different groups. We provide descriptive data on prescription details and evaluate the appropriateness of medication use.
Results
Complete prescription data were available in 3966 out of 4056 (97.8%) subjects enrolled in the ICKD database. Most patients had stage 3 CKD, 24.9% had diabetic kidney disease, 87% had hypertension, and 25.5% had moderate to severe proteinuria. Renin-angiotensin-aldosterone system blockers were prescribed in less than half (47.9%) and in 58.8% of patients with proteinuric CKD. Metformin was prescribed in 25.7% of diabetic subjects with CKD. Only 40.4% of patients were taking statins; 31.1% and 2.8% subjects with anemia were receiving iron and erythropoiesis-stimulating agents, respectively.
Conclusion
This study highlights the missed opportunities for improving outcomes through appropriate prescriptions of drugs in patients with CKD. There is need for dissemination of evidence-based guidelines and institution of sustainable implementation practices for improving the overall health of patients with CKD.
Keywords: antihypertensives, chronic kidney diseases, indigenous medicines, prescription pattern
Graphical abstract
Chronic kidney disease (CKD) is an important global public health problem.1, 2, 3 The Global Burden of Disease (GBD) study has highlighted the disproportionate burden of CKD in emerging countries. India is a lower-middle-income country with almost 16% of the world’s population. About 115 million people had CKD in India in 2017, with about 230,000 deaths attributable to CKD.1, 2, 3 The Million Death Study4 documented a 50% increase in deaths caused by kidney failure in India between 2001 to 2003 and 2011 to 2013.
There are several evidence-based approaches—both nonpharmacologic and pharmacologic—to slow down or halt the progression of kidney disease and prevent or treat CKD-associated complications, such as cardiovascular disease, anemia, and mineral and bone disorders.5, 6, 7 They include maintaining a healthy lifestyle, blood pressure and blood sugar control, the use of renin-angiotensin blockers, lipid-lowering therapies, other cardioprotective therapies like antiplatelet agents, and correction of anemia and mineral and bone disorders. Patients may also require symptomatic treatment, for example, for the management of fluid retention.8,9
As a result, patients with CKD are taking multiple medications, making rational drug prescription an arduous task.10, 11, 12, 13 Patients with CKD are also at higher risk of medication-related problems.10,13,14 Therefore, medication management in CKD offers unique challenges but presents providers with opportunities to enhance the quality of care for this high-risk population. Implementing strategies based on the risks and benefits of all prescribed agents, and deprescribing and prescribing as indicated, may improve patient outcomes. The use of multiple medications increases the cost of therapy as well as the risk of nonadherence. Therefore, it is important to ensure that evidence-based treatments that improve the outcomes are prioritized over those not supported by high-quality evidence.
Studies on prescriptions in CKD and other chronic conditions like heart failure show that multiple factors, including socioeconomic conditions, visit to the general physicians versus specialists, and prevalent practice in that country affect the prescription pattern. 15, 16, 17, 18
There are no large data sets on the prescription pattern of the medications in patients with CKD from the developing world. We describe prescription patterns at enrollment in patients with mild to moderate CKD included in the large ICKD cohort study.
Methods
The ICKD study is a longitudinal study of subjects with mild to moderate CKD recruited at 11 centers across India. All centers except 2 are public sector hospitals. The study design has been previously published.19 Briefly, the study recruits adult subjects between 18 and 70 years of age who have mild to moderate CKD and who are attending outpatient nephrology clinics at participating centers. Both incident and prevalent patients are eligible for enrollment. Patients undergoing dialysis, organ transplant recipients, those with malignancy, on immunosuppressive drug therapy, or with poor functional status are excluded. Subjects are being followed-up annually, and prescription as well as outcome data are being collected.
Demographic details, diagnosis, comorbidities, clinical features, laboratory findings, and treatment details are recorded and stored anonymously in a secure database. The study has approval from the institutional review boards at all centers, and all participants provide written informed consent.
We included subjects with complete prescription data at recruitment in this analysis. Patients were categorized into different stages of CKD stages 1 to 4 as per estimated glomerular filtration rate (eGFR) used in Kidney Disease: Improving Global Outcomes (KDIGO) staging. eGFR was calculated using creatinine-based CKD Epidemiology Collaboration (CKD-EPICr) equation.20 Educational status, employment status, living in rural or urban residence, hazardous occupational exposure, and alternative medication use (defined as use of indigenous, ayurvedic, or other unregulated medications) were captured. Directed questions to ascertain occupational exposure, the use of alternative drugs, and the use of nonsteroidal anti-inflammatory drugs after diagnosis of CKD were incorporated in the study questionnaire. Data regarding the use of alternative drugs in relation to onset of kidney disease were also recorded.
The diagnosis of CKD and comorbidities were made after a review of history and medical records. Diabetes, hypertension, and cardiovascular disease were defined as per prevailing standards for diagnosis. Anemia was diagnosed and classified as per World Health Organization criteria.21
Data were collected from the most recent prescription slip and verbal inquiry and then classified into 11 different categories used for the management of CKD or its complications: antihypertensives, antidiabetics, phosphate binders, iron supplementation, erythropoiesis-stimulating agent (ESA) therapy, lipid-lowering therapy, antiplatelet drugs, uric acid–lowering drugs, vitamin D analogues, sodium bicarbonate, and multivitamins. The antihypertensives were further categorized as drugs that block angiotensin system, that is, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), calcium channel blockers, beta blockers, central sympatholytics, and diuretics. The antidiabetics insulin or oral hypoglycemic agents, such as sulfonylureas, metformin, or dipeptidyl-peptidase 4 inhibitors, were also noted. Phosphate binders were divided into calcium or non–calcium-based phosphate binders. The use of phosphate binder in patients with or without hyperphosphatemia was also analyzed. The distribution of proportions of these categories of medicines as per CKD stages 1 to 4 was analyzed.
Statistical Analysis
The statistical analysis was descriptive. Data were analyzed using Stata statistical software (version 14; Stata Corp LP, College Station, TX). Both descriptive and analytical statistics were performed showing sociodemographic, clinical, and biochemical characteristics of participants. The continuous variables were expressed as mean ± SD and median (25th, 75th percentile), and the categorical values were expressed in number and percentage in parenthesis. Distribution of various drugs across the CKD stages or in other subcategories are descriptive and presented as number (percentage).
Results
Of 4056 patients recruited in the ICKD database, complete prescription data at baseline were available in 3966 (97.8%) patients, who constitute the study population for this paper. Table 1 shows the clinical characteristics and causes of CKD in the cohort. The mean age of the cohort participants was 50.3 ± 11.8 years, and 67.2% were male. A majority were either uneducated (26.9%) or had only completed primary school but did not pass high school education (34%). A total of 64.7% patients had a rural background, and 50.4% had a history of hazardous occupational exposure. The median annual income was $1680 USD (interquartile range $1008–$4200 USD), and 32.1% had access to some form of medical insurance. Patients spent 11.7% (4.7%–26.0%) of their total annual household income on medical care. In 57%, the cost of care was >10% of their nonfood expenditure.
Table 1.
Baseline characteristics of participants in the Indian Chronic Kidney Disease study
| Characteristics | Overall (n = 4056) | Missing prescription data (n = 90) |
|---|---|---|
| Socioeconomic factors | ||
| Male gender | 2725 (67.2) | 54.0 (60.0) |
| Age, yrs | 50.3 ± 11.8 | 44.6 ± 13.7 |
| Education | ||
| Uneducated | 1088 (27.0) | 27 (30.3) |
| Below high school | 1374 (34.0) | 29 (32.6) |
| Completed school | 538 (13.3) | 14 (15.7) |
| College and above | 1038 (25.7) | 19 (21.4) |
| Rural residence | 2626 (64.7) | 65 (72.2) |
| Hazardous occupational exposurea | 2035 (50.4) | 24 (27.0) |
| Hypertension | 3487 (87.0) | 52 (59.1) |
| Diabetes | 1485 (37.5) | 18 (20.5) |
| Cardiovascular disease | 876 (21.8) | 16 (19.5) |
| Tobacco use | 747 (18.6) | 10 (11.5) |
| Medical insurance available | 1276 (32.1) | 21 (23.9) |
| Annual income, USD | $1680 ($1008–$4200) | $1680 ($756–$3780) |
| Annual medication cost, USD | $285.6 ($84.0–$571.2) | $319.2 ($161.3–$537.6) |
| BMI, kg/m2 | 24.4 (21.6–27.4) | 22.6 (20.4–26.7) |
| Systolic blood pressure, mm Hg | 130 (120–144) | 133 (122–146) |
| Diastolic blood pressure, mm Hg | 80 (78–90) | 84 (70–90) |
| eGFR, ml/min/1.73m2 | 40.5 (33.7–50.8) | 37.0 (30.8–49.0) |
| uACR, mg/g | 29.2 (10.7–304.3) | 11.3 (8.2–194.6) |
| <300 | 2817 (74.5) | 60 (81.1) |
| 300–1000 | 583 (15.4) | 10 (13.5) |
| >1000 | 384 (10.1) | 4 (5.4) |
| Causes of CKD | ||
| CAKUT | 37 (0.9) | 1 (1.1) |
| Chronic glomerulonephritis | 598 (14.8) | 18 (20.0) |
| Chronic interstitial nephritis | 940 (23.2) | 18 (20.0) |
| Diabetic kidney disease | 1011 (24.9) | 17 (18.9) |
| Hypertensive nephrosclerosis | 320 (7.9) | 3 (3.3) |
| Other | 208 (5.1) | 11 (12.2) |
| Polycystic kidney disease | 139 (3.4) | 2 (2.2) |
| Renovascular disease | 15 (0.4) | 0.00 |
| Unknown | 788 (19.4) | 20 (22.2) |
BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; uACR, urine albumin to creatinine ratio; USD, U.S. dollar.
Data shown as mean ± SD, median (25th–75th percentile), and n (%).
Work performed around sand, dust, chemicals, or animals, or working barefoot in fields.
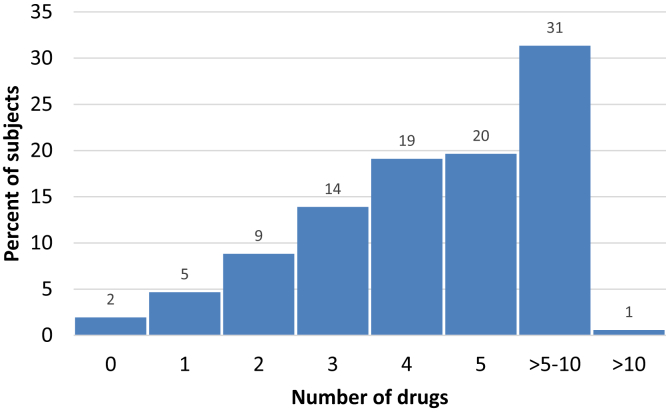
The study cohort included 100 (2.5%) patients in stage 1; 309 (7.8%) in stage 2; 3131 (79%) in stage 3, and 426 (10.7%) in stage 4 CKD. The most commonly identified causes of CKD were diabetic kidney disease (24.9%) and chronic interstitial nephritis (23.2%), whereas the cause of CKD could not be determined in approximately 788 (19.4%) participants. Hypertension was the most common associated comorbidity, reported by 87% of participants. This was followed by anemia (64.7%), diabetes (37.5%), and other cardiovascular morbidities (21.8%). The medication burden with the number of different drug classes is shown in Figure 1. The median (25th–75th percentile) number of prescribed drugs was 5 (3–6) in the study cohort. More than half of patients were taking >5 different drug classes. A total of 924 (22.7%) patients admitted using alternative therapies. Five hundred twenty-two (56.5%) patients had used these drugs within 4 weeks before their diagnosis of kidney disease, and 252 (27.3%) patients had used these drugs for the treatment of kidney disease. A total of 624 (15.7%) participants admitted to using nonsteroidal anti-inflammatory drugs even after their diagnosis of CKD. A detailed description of prescription pattern is shown in Table 2.
Figure 1.
Frequency of use of different classes of drugs in the study cohort.
Table 2.
Distribution of classes of drugs prescribed in different stages of CKD
| Drugs, n (%) | CKD stage (n) |
Total (N = 3966) | |||
|---|---|---|---|---|---|
| 1 (100) | 2 (309) | 3 (3131) | 4 (426) | ||
| Antihypertensive drugs | 89 (89.0) | 256 (82.9) | 2525 (80.7) | 339 (79.6) | 3209 (80.9) |
| Beta-blockers | 13 (13.0) | 64 (20.7) | 881 (28.1) | 118 (27.7) | 1076 (27.1) |
| Calcium channel blockers | 18 (18.0) | 119 (38.5) | 1348 (43.1) | 203 (47.7) | 1688 (42.6) |
| Alpha blockers | 7 (7.0) | 26 (8.4) | 349 (11.2) | 40 (9.4) | 422 (10.7) |
| ACE inhibitors | 31 (31.0) | 78 (25.2) | 711 (22.7) | 73 (17.1) | 893 (22.5) |
| ARBs | 47 (47.0) | 111 (35.9) | 793 (25.3) | 57 (13.4) | 1008 (25.4) |
| Diuretics | 14 (14.0) | 70 (22.7) | 905 (28.9) | 148 (34.7) | 1137 (28.7) |
| Central sympatholytic | 2 (2.0) | 6 (1.9) | 93 (3.0) | 17 (4.0) | 118 (3.0) |
| Phosphate binders | 36 (36.0) | 119 (38.5) | 1090 (34.8) | 210 (49.3) | 1455 (36.7) |
| Calcium-based | 36 (36.0) | 114 (36.9) | 979 (31.3) | 165 (38.7) | 1294 (32.6) |
| Non–calcium based | 0 (0) | 5 (1.6) | 122 (3.9) | 48 (11.3) | 175 (4.4) |
| Iron supplements | 21 (21.0) | 64 (20.7) | 820 (26.2) | 140 (32.9) | 1045 (26.4) |
| Oral | 21 (21.0) | 63 (20.4) | 817 (26.1) | 138 (32.4) | 1039 (26.2) |
| Intravenous | 0 (0) | 1 (0.3) | 6 (0.2) | 2 (0.5) | 9 (0.2) |
| Erythropoiesis-stimulating agents | 1 (1.0) | 9 (2.9) | 79 (2.5) | 23 (5.4) | 112 (2.8) |
| Erythropoietin | 1 (1.0) | 8 (2.6) | 65 (2.1) | 17 (4.0) | 91 (2.3) |
| Darbepoietin | 0 (0) | 1 (0.3) | 10 (0.3) | 4 (0.9) | 15 (0.4) |
| Others | 0 (0) | 0 (0) | 4 (0.1) | 2 (0.5) | 6 (0.2) |
| Sodium bicarbonate | 11 (11.0) | 92 (29.8) | 1367 (43.7) | 230 (54.0) | 1700 (42.9) |
| Antiplatelet therapy | 8 (8.0) | 53 (17.2) | 707 (22.6) | 92 (21.6) | 860 (21.7) |
| Clopidogrel | 1 (1.0) | 7 (2.3) | 129 (4.1) | 16 (3.8) | 153 (3.9) |
| Aspirin | 8 (8.0) | 53 (17.2) | 659 (21.1) | 84 (19.7) | 804 (20.3) |
| Uric acid–lowering agent | 3 (3.0) | 37 (12.0) | 498 (15.9) | 60 (14.1) | 598 (15.1) |
| Allopurinol | 0 (0) | 5 (1.6) | 31 (1.0) | 3 (0.7) | 39 (1.0) |
| Febuxostat | 3 (3.0) | 32 (10.4) | 469 (15.0) | 57 (13.4) | 561 (14.2) |
| Statins | 27 (27.0) | 110 (35.6) | 1312 (41.9) | 153 (35.9) | 1602 (40.4) |
| Vitamin D3 compounds | 10 (10.0) | 17 (5.5) | 387 (12.4) | 55 (12.9) | 469 (11.8) |
| Cholecalciferol or ergocalciferol | 9 (9.0) | 15 (4.9) | 248 (7.9) | 25 (5.9) | 297 (7.5) |
| Calcitriol | 1 (1.0) | 2 (0.7) | 149 (4.8) | 29 (6.8) | 181 (4.6) |
| Others | 0 (0) | 0 (0) | 4 (0.1) | 1 (0.24) | 5 (0.1) |
| Multivitamin | 5 (5.0) | 50 (16.2) | 845 (27.0) | 147 (34.5) | 1047 (26.4) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CKD, chronic kidney disease.
Antihypertensive Therapies
Overall, 80.9% patients were taking antihypertensive medications. Of those with a diagnosis of hypertension, 92.5% were taking antihypertensive drugs, with a similar proportion between males and females. Subjects <60 years of age were more likely to be taking antihypertensive drugs compared with patients ≥60 years of age. Among those undergoing treatment, the recorded blood pressure was <140/90 mm Hg in 43%. The frequency of antihypertensive medications in subjects with hypertension is shown in Supplementary Table S1. The most commonly prescribed antihypertensive agents were renin-angiotensin-aldosterone system (RAAS) blockers in 47.9% (ACEIs 22.5%, ARBs 25.4%), followed by calcium channel blockers (42.6%), diuretics (28.7%), beta-blockers (27.1%), alpha-blockers (10.7%), and central sympatholytic drugs (3%; Table 2). A total of 72% of patients with stage 1 CKD, 59% of patients with stage 2 CKD, 47% of patients with stage 3 CKD, and 30% of patients with stage 4 CKD were prescribed RAAS blockers. The use of the calcium channel blockers increased and the use of ACEIs/ARBs decreased with advancing CKD stages. The number of antihypertensive drugs prescribed were: 1 in 1221 (30.8%), 2 in 1134 (28.6%), 3 in 598 (15.1%), 4 in 211 (5.3), and ≥5 to 7 in 45 (1.1%) patients with CKD. Of the 1467 subjects with diabetes, 55.8% were taking ACEIs/ARBs. Of the 967 patients with proteinuric illnesses (albumin to creatinine ratio >300 mg/g), 58.8% were prescribed ACEIs/ARBs.
Antidiabetic Prescriptions
Of 1467 participants with a diagnosis of diabetes (all type 2 disease), 959 (65.4%) were receiving ≥1 antidiabetic medicine: 27.7% were taking sulfonylureas, followed by insulin (26.4%), metformin (25.7%), dipeptidyl-peptidase 4 inhibitors (9.5%), and others (3.6%; Table 3). More than half (52.9%) of patients with stage 1 CKD, 30.1% of patients with stage 2 CKD, 26.2% of patients with stage 3 CKD, and 16.6% of patients with stage 4 CKD with diabetes were taking metformin (Table 3). Of 1310 and 157 patients with diabetes with GFR ≥30 ml/min/1.73m2 and <30 ml/min/1.73m2, 351 (26.80%) and 26 (16.60%) were taking metformin, respectively. More than one third (34.5%) of patients with diabetes were not receiving any antidiabetic drugs. Among those with a diagnosis of diabetes, the use of antidiabetic drugs was similar across sex and age groups, defined by ≥60 or <60 years of age.
Table 3.
CKD stagewise distribution of antidiabetic drugs in subjects with diabetes
| Drugs, n (%) | CKD stage (n) |
Total (N = 1467) | |||
|---|---|---|---|---|---|
| 1 (17) | 2 (93) | 3 (1200) | 4 (157) | ||
| Overall | 13 (76.5) | 57 (61.3) | 802 (66.8) | 87 (55.4) | 959 (65.4) |
| Insulin | 5 (29.4) | 13 (14.0) | 331 (27.6) | 39 (24.8) | 388 (26.4) |
| Sulfonylureas | 5 (29.4) | 33 (35.5) | 337 (28.1) | 32 (20.4) | 407 (27.7) |
| Metformin | 9 (52.9) | 28 (30.1) | 314 (26.2) | 26 (16.6) | 377 (25.7) |
| DPP4 inhibitors | 2 (11.8) | 11 (11.8) | 118 (9.8) | 9 (5.7) | 140 (9.5) |
| Others | 0 (0) | 6 (6.8) | 38 (3.2) | 10 (6.4) | 54 (3.6) |
| Not taking any antidiabetic drug | 4 (23.5) | 36 (38.7) | 398 (33.2) | 70 (44.5) | 508 (34.6) |
CKD, chronic kidney disease; DPP4, dipeptidyl-peptidase 4.
Statins
Lipid-lowering therapy with a statin was prescribed to a total of 40.4% of patients. A majority (58.1%) of patients with diabetes were receiving a statin. Of those >50 years of age, only 47.4% (59.5% of patients with diabetes and 35.2% of patients without diabetes) were prescribed a statin.
Phosphate Binders
Of 1455 (36.7%) patients who had been prescribed phosphate binders, a majority (1294; 89%) were given a calcium-based binder. The use of calcium-based phosphate binders was higher across all CKD stages (Table 2). The phosphate levels in subjects taking phosphate binder were 4.5 ± 1.4 mg/dl compared with 3.9 ± 1.2 mg/dl for those who were not taking a phosphate binder. Moreover, 52.1% of subjects with serum phosphate levels ≥4.5 mg/dl were not prescribed any phosphate binder.
Anemia Management
A total of 26.4% of patients were prescribed any iron compounds. Only 772 (31.1%) anemic subjects were taking iron supplements. Over 99% of the prescriptions were for oral iron agents. Only 112 (2.8%) had been prescribed an ESA with a majority (91; 2.3%) taking epoetin alfa. Of 181 patients with CKD with hemoglobin levels <9 g/dl, 14 (7.7%) were taking ESAs.
Other Therapies
Only 11.8% of the patients had a prescription of a vitamin D compound or its analogs. Calcitriol was prescribed to 4.6% patients and cholecalciferol to 7.5%. Overall, 42.9% of the patients had been prescribed sodium bicarbonate. A total of 21.7% were receiving any antiplatelet therapy, the majority (20.3%) taking aspirin. More than half (57.3%) of subjects with a history of cardiovascular disease (CVD) were receiving antiplatelet therapy. Overall, 15.1% subjects were receiving uric acid–lowering therapy—a majority (14.2%) were taking febuxostat (Table 2).
Discussion
This is the first and the largest study documenting pharmacologic prescription practices among patients with CKD in the developing world. Compared with Western cohorts with CKD, the ICKD population is relatively young and predominantly from rural regions.22, 23, 24, 25, 26 We provide insight into treatment preferences and identify gaps with regard to the use of evidence-based therapies for the management of kidney disease in the context of slowing progression and the development of cardiovascular complications in resource-limited settings. A notable finding is the suboptimal use of drugs that block the renin-angiotensin pathway and lipid-lowering agents. On the other hand, we identify the use of non–evidence-based therapies, such as uric acid lowering agents. These identify important knowledge gaps that need to be addressed through targeted education of nephrologists and general physicians as well as the implementation of incentives. Adoption of evidence-based approaches will not only improve patient outcomes but will also result in cost savings by preventing the development of kidney failure and/or complications that require expensive therapies. Compelling evidence to show that drugs that block renin-angiotensin-aldosterone pathway have a salutary effect on hard clinical outcomes in patients with CKD is available from large-scale, randomized clinical trials.3 Less than half of the prescriptions in the ICKD cohort included ACEIs and/or ARBs. Even among patients with proteinuric illnesses, where the indication for their use is the strongest (class I recommendation by KDIGO Blood Pressure Guidelines), only 59% had been prescribed an ACEI/ARB.27 Similarly, only 56% of those with diabetic kidney disease were taking these agents. We also noted a decline in the use of RAAS blockers with increasing CKD severity. Only 30% of patients with stage 4 CKD were taking these agents. In the Chronic Renal Insufficiency Study (CRIC) cohort, 70% were receiving RAAS blockers, with as much as 75% and 37% of those in stage 3 and stage 5 CKD, respectively.28 In the German CKD study, about 83% received RAAS blockers.29 In addition to retarding CKD progression, RAAS blockers reduce the risk of heart failure and death regardless of disease severity. In a meta-analysis, the International Network of CKD cohorts documented wide variations in antihypertensive prescription patterns and blood pressure control internationally.30 RAAS blocker use ranged from 54% to 91%. Calcium channel blockers were more frequently used in Asian cohorts. Whether this is related to drug preference, availability, or ease of monitoring is not known.
Among patients with diabetes, sulfonylureas were the most frequently prescribed oral hypoglycemic agents. Only a minority of patients were taking metformin, even among those with an eGFR >30 ml/min/1.73m2. This differs from the dominant use of metformin (55%–91%) in the general diabetes population in India.31, 32, 33 Despite the recommendation by guidelines to use metformin in view of its multiple advantages, <25% of people with diabetes were taking this agent. Concern for side effects could have influenced its restricted use. However, the current UK National Institute of Health and Clinical Practice Guidelines on the treatment of type 2 diabetes mellitus and KDIGO Clinical Practice Guideline for Diabetes Management in CKD allow metformin use up to a GFR of 30 ml/min/1.73 m2, with dose reduction advised at 45 ml/min/1.73 m2.34,35 In the United States, metformin is contraindicated for men with serum creatinine ≥1.5 mg/dl and women with serum creatinine ≥1.4 mg/dl.36 However, in the ICKD cohort, only 27% diabetic subjects in stages 1 to 3 were taking metformin. The use of other newer oral hypoglycemic agents, like dipeptidyl-peptidase 4 inhibitors, was low. The nonuse of sodium glucose cotransporter 2 inhibitors was likely because recruitment in the study had occurred before the overwhelming evidence favoring their use in patients with CKD had accrued.
The Study of Heart and Renal Protection trial established the role of lipid-lowering therapy in the management of non–dialysis-dependent CKD.37 KDIGO lipid guidelines38 recommend that all adults ≥50 years of age with CKD be treated with a statin or a combination of statin plus ezetimibe. In the ICKD cohort, 40% overall and 47.4% of ≥50-year-olds received statins. Underuse of statins in CKD has been noted elsewhere—only 52% of those >50 years of age were receiving statins in the GCKD study.39
Among other cardioprotective therapies, about 20% of patients were receiving aspirin, including 51% patients with established CVD. Although the role of routine aspirin use for primary prevention of cardiovascular complications in CKD is debated,40,41 it is indicated for secondary prevention in those with high CVD risk or established CVD.42,43 The neglect of cardioprotective therapies in patients with CKD has been noted.44
We noticed a low use of iron and ESA therapy even in patients with anemia. The KDIGO anemia management guideline45 recommends iron therapy before the initiation of ESA therapy. The majority among those taking ESAs were receiving conventional ESAs despite being on outpatient treatment, perhaps because of the higher cost of the longer-acting ESAs. This low use might reflect ignorance about the evidence-based approaches for anemia treatment among the treating physicians.
Interestingly, we found that about 15% of patients with CKD were treated with uric acid–lowering agents, mainly febuxostat. None of them had a diagnosis of gout. No guideline recommends the routine use of uric acid–lowering drugs, reinforced through 2 recent randomized controlled trials46,47 that did not show this strategy to be effective in slowing the progression of CKD.
Sodium bicarbonate was prescribed in >42% of patients. According to current recommendations, bicarbonate use is recommended only for the correction of acidosis in those with serum bicarbonate values <22 mmol/l,48 which is unlikely in a vast number of subjects in this cohort—with >50% having an eGFR >30 ml/min/1.73m2.
Another interesting finding was the use of herbal medicines in 22.7% of the patients in the cohort. Patients in India often consult practitioners of alternative systems of medicine alongside allopathic doctors. The deep belief in the population about the utility of these therapies is driven by information circulated through word of mouth and on social media.49, 50, 51 A significant proportion of patients turn to these therapies out of desperation upon learning about the irreversible nature of CKD.
Given that we captured prescription details at the time of recruitment, the data presented here represent practice patterns in the wider community practice in India rather than those in recruiting nephrology centers. This might explain the gap between the medication use and best evidence-informed practice. Given the large burden of CKD and relative shortage of nephrologists, it is inevitable that most of the patients with mild to moderate CKD will continue to be managed by non-nephrologists, who should also receive education on the use of evidence-based therapies. Because we only included patients that had been referred to the recruiting centers, these data might not be generalizable to the broader population of Indian patients with CKD managed in the community. We did not capture data regarding compliance with prescription and reasons for noncompliance. The longitudinal component of the study will allow exploration of the impact of these differences on outcomes.
In conclusion, this study provides insights into the prescription patterns for the treatment of CKD in India. We highlight missed opportunities for improving outcomes through appropriate prescriptions of RAAS blockers, lipid-lowering therapy, cholecalciferol, and metformin. We also identify possible instances of inappropriate medication use. There is a need for wider dissemination of evidence-based guidelines and institution of sustainable implementation practices for improving the overall health of subjects with CKD.
Disclosure
VJ has received research grants from Baxter and GSK and consultancy and advisory board honoraria from Baxter Healthcare and AstraZeneca, outside the published work. All the other authors declared no competing interests.
Acknowledgments
This work was supported by grants from the Department of Biotechnology, Ministry of Science and Technology, Govt of India (BT/PR3150/MED/30/1345/2014).
Footnotes
Supplementary Table S1. Use of antihypertensives in subject with hypertension.
STROBE statement.
Supplementary Material
Supplementary Table S1. Use of antihypertensives in subject with hypertension.
STROBE statement.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey A.S., Atkins R., Coresh J. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 3.Xie X., Liu Y., Perkovic V. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Dare A.J., Fu S.H., Patra J. Renal failure deaths and their risk factors in India 2001-13: nationally representative estimates from the Million Death Study. Lancet Glob Health. 2017;5:e89–e95. doi: 10.1016/S2214-109X(16)30308-4. [DOI] [PubMed] [Google Scholar]
- 5.Levey A.S., Eckardt K.U., Tsukamoto Y. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 6.Levin A., Hemmelgarn B., Culleton B. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–1162. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moe S., Drueke T., Cunningham J. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 8.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.KDOQI, National Kidney F KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 suppl 3):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Cardone K.E., Bacchus S., Assimon M.M. Medication-related problems in CKD. Adv Chronic Kidney Dis. 2010;17:404–412. doi: 10.1053/j.ackd.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty S., Ghosh S., Banerjea A. Prescribing patterns of medicines in chronic kidney disease patients on maintenance hemodialysis. Indian J Pharmacol. 2016;48:586–590. doi: 10.4103/0253-7613.190760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tozawa M., Iseki K., Iseki C. Analysis of drug prescription in chronic haemodialysis patients. Nephrol Dial Transplant. 2002;17:1819–1824. doi: 10.1093/ndt/17.10.1819. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker C.F., Miklich M.A., Patel R.S. Medication safety principles and practice in CKD. Clin J Am Soc Nephrol. 2018;13:1738–1746. doi: 10.2215/CJN.00580118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz M.B., Refiker M., Guray Y. Prescription patterns in patients with systolic heart failure at hospital discharge: why beta blockers are underprescribed or prescribed at low dose in real life? Int J Clin Pract. 2007;61:225–230. doi: 10.1111/j.1742-1241.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Ramahi R. Medication prescribing patterns among chronic kidney disease patients in a hospital in Malaysia. Saudi J Kidney Dis Transpl. 2012;23:403–408. [PubMed] [Google Scholar]
- 16.Faggiano P., Opasich C., Tavazzi L. Prescription patterns of diuretics in chronic heart failure: a contemporary background as a clue to their role in treatment. J Card Fail. 2003;9:210–218. doi: 10.1054/jcaf.2003.25. [DOI] [PubMed] [Google Scholar]
- 17.Mamadi R.K., Sathish R., Selvaraj D.R. Prescription pattern, short-term outcomes, and its determinants in patients with chronic kidney disease attending a tertiary care hospital. Indian J Pharmacol. 2019;51:55–60. doi: 10.4103/ijp.IJP_350_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhana Israt Jahan M.J.R., Khanam Keya, Parvin Limu. A survey on prescription pattern for patient of kidney diseases. DIU J Allied Health Sci. 2016;3:27–32. [Google Scholar]
- 19.Kumar V., Yadav A.K., Gang S. Indian Chronic Kidney Disease Study: design and methods. Nephrology (Carlton) 2017;22:273–278. doi: 10.1111/nep.12789. [DOI] [PubMed] [Google Scholar]
- 20.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization website Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. https://www.who.int/vmnis/indicators/haemoglobin.pdf Available at:
- 22.Imai E., Matsuo S., Makino H. Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol. 2010;14:558–570. doi: 10.1007/s10157-010-0328-6. [DOI] [PubMed] [Google Scholar]
- 23.Kang E., Han M., Kim H. Baseline general characteristics of the Korean chronic kidney disease: report from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) J Korean Med Sci. 2017;32:221–230. doi: 10.3346/jkms.2017.32.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin A., Rigatto C., Brendan B. Cohort profile: Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT) BMC Nephrol. 2013;14:121. doi: 10.1186/1471-2369-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan J., Zou X.R., Han S.P. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: results from the Chinese cohort study of chronic kidney disease (C-STRIDE) BMC Nephrol. 2017;18:23. doi: 10.1186/s12882-017-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung A.K., Chang T.I., Cushman W.C. Blood pressure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:1027–1036. doi: 10.1016/j.kint.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Ku E., McCulloch C.E., Vittinghoff E. Use of antihypertensive agents and association with risk of adverse outcomes in chronic kidney disease: focus on angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider M.P., Hilgers K.F., Schmid M. Blood pressure control in chronic kidney disease: a cross-sectional analysis from the German Chronic Kidney Disease (GCKD) study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alencar de Pinho N., Levin A., Fukagawa M. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96:983–994. doi: 10.1016/j.kint.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Muhas C., Salim C.M., Mufeeda T.P. Prescription pattern of anti-diabetic drugs in a rural area of South Malabar region of Kerala, South India. Int J Res Med Sci. 2018;6:4082–4086. [Google Scholar]
- 32.Seshadri K.G., Venkataraman S., Manikandan C.D. Antidiabetes drug prescription in Indian scenario—a cross-sectional analysis from a large, pan India database of the Apollo Sugar Clinics. Diabetes. 2018;67(suppl 1) 1204–P. [Google Scholar]
- 33.Singla R., Bindra J., Singla A. Drug prescription patterns and cost analysis of diabetes therapy in India: audit of an endocrine practice. Indian J Endocrinol Metab. 2019;23:40–45. doi: 10.4103/ijem.IJEM_646_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4 suppl):S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Health and Clinical Excellence website The management of type 2 diabetes: 2010 NICE guidelines. London, UK: National Institute for Health and Clinical Excellence, 2010. http://www.nice.org.uk/nicemedia/live/12165/44320/44320.pdf Available at.
- 36.American Diabetes Association Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetes—2017. Diabetes Care. 2017;40:S64–S74. doi: 10.2337/dc17-S011. [DOI] [PubMed] [Google Scholar]
- 37.Baigent C., Landry M. Study of Heart and Renal Protection (SHARP) Kidney Int Suppl. 2003;84:S207–S210. doi: 10.1046/j.1523-1755.63.s84.4.x. [DOI] [PubMed] [Google Scholar]
- 38.Wanner C., Tonelli M., Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 39.Schneider M.P., Hubner S., Titze S.I. Implementation of the KDIGO guideline on lipid management requires a substantial increase in statin prescription rates. Kidney Int. 2015;88:1411–1418. doi: 10.1038/ki.2015.246. [DOI] [PubMed] [Google Scholar]
- 40.Goicoechea M., de Vinuesa S.G., Quiroga B. Aspirin for primary prevention of cardiovascular disease and renal disease progression in chronic kidney disease patients: a multicenter randomized clinical trial (AASER study) Cardiovasc Drugs Ther. 2018;32:255–263. doi: 10.1007/s10557-018-6802-1. [DOI] [PubMed] [Google Scholar]
- 41.Ittaman S.V., VanWormer J.J., Rezkalla S.H. The role of aspirin in the prevention of cardiovascular disease. Clin Med Res. 2014;12:147–154. doi: 10.3121/cmr.2013.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne R.A., Colleran R. Aspirin for secondary prevention of cardiovascular disease. Lancet. 2020;395:1462–1463. doi: 10.1016/S0140-6736(20)30799-6. [DOI] [PubMed] [Google Scholar]
- 43.Qu B., He Y., Wu L. Is there a cardiovascular protective effect of aspirin in chronic kidney disease patients? A systematic review and meta-analysis. Int Urol Nephrol. 2020;52:315–324. doi: 10.1007/s11255-019-02350-8. [DOI] [PubMed] [Google Scholar]
- 44.Tonelli M., Karumanchi S.A., Thadhani R. Epidemiology and mechanisms of uremia-related cardiovascular disease. Circulation. 2016;133:518–536. doi: 10.1161/CIRCULATIONAHA.115.018713. [DOI] [PubMed] [Google Scholar]
- 45.Locatelli F., Bárány P., Covic A. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28:1346–1359. doi: 10.1093/ndt/gft033. [DOI] [PubMed] [Google Scholar]
- 46.Badve S.V., Pascoe E.M., Tiku A. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 47.Doria A., Galecki A.T., Spino C. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382:2493–2503. doi: 10.1056/NEJMoa1916624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens P.E., Levin A., Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 49.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jha V. Herbal medicines and chronic kidney disease. Nephrology (Carlton) 2010;15(suppl 2):10–17. doi: 10.1111/j.1440-1797.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 51.SocialMediaToday website. Why is word of mouth marketing so important? https://www.socialmediatoday.com/marketing/why-word-mouth-marketing-so-important Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.