Abstract
Gastric Helicobacter infection in healthy pet cats is not well characterized. We performed endoscopy with gastric biopsy on 15 healthy pet cats that were rigorously screened to exclude underlying or concurrent diseases that might affect Helicobacter colonization. Gastric mucosa biopsy specimens were examined by histology, culture, and PCR for the presence of Helicobacter infection and by histology for the presence of gastritis. Of 15 cats, all but 1 had gastric Helicobacter-like organisms (GHLOs) on examination by light microscopy, and in the one histologically negative cat, GHLOs were detected by PCR. Gastric inflammation was mild or was absent for all cats. No Helicobacter species were identified by culture. Analysis of the 16S rRNA sequence from Helicobacter strains from 10 cats showed that all bacteria were closely related to Helicobacter felis, although there was heterogeneity among the sequences. These results suggest that the gastric mucosa of healthy pet cats is commonly colonized with an uncultivated Helicobacter that is closely related to H. felis, is associated with little or no gastritis, and shows heterogeneity in its 16S rRNA sequence. The epithet “Helicobacter heilmannii” continues to be an appropriate working designation for these bacteria.
Helicobacter spp. have commonly been isolated from the gastric mucosa of humans, nonhuman primates, dogs, cats, ferrets, cheetahs, and pigs (6, 16, 31, 46). In humans, Helicobacter pylori infection has been associated with chronic gastritis, gastroduodenal ulceration, and gastric lymphoma and adenocarcinoma (3, 30, 36, 40). The pathogenicity of Helicobacter infection in cats is less clearly understood; there is considerable debate as to whether feline helicobacters are commensal or pathogenic organisms. Since gastritis is a common gastrointestinal disease in the cat and because Helicobacter infection has been implicated in human chronic gastritis, the presence of Helicobacter in feline gastric biopsy specimens has raised the question of its potential causal role in gastritis. Several studies to date have examined this relationship, because the cat may serve as a useful animal model of human disease. The prevalence of Helicobacter infection in cats with symptomatic gastrointestinal disease has been reported to be 53 to 76% (18, 25, 37). In comparison, studies performed with clinically healthy cats have shown infection rates ranging from 42 to 100% (18, 20, 23, 29, 35, 38, 46). However, in the latter studies the cats were from research colonies or animal shelters, where the high prevalence of Helicobacter may have been due to close contact. This hypothesis is consistent with the finding of an increased prevalence of H. pylori in humans living under crowded or poor hygienic conditions (6). In a recent study of Helicobacter colonization in cats, animals presented for surgical procedures in situations in which the possibility of an underlying disease or immunosuppression leading to Helicobacter colonization was not rigorously excluded (35).
Reports of domestic animal-to-human transmission and isolation of H. pylori from domestic cats (20) have led to speculation that cats and dogs may serve as a reservoir for human infection (1, 29, 33, 44, 45). Studies that characterize gastric Helicobacter infection in clinically normal, privately owned pet cats are lacking. To further elucidate the role of Helicobacter infection in feline gastritis, we performed a well-controlled, prospective study with privately owned, healthy pet cats. The objectives of this study were (i) to assess the prevalence of Helicobacter spp. in pet cats that were rigorously screened for concurrent or underlying diseases and that had no history of anorexia, vomiting, diarrhea, or weight loss; (ii) to determine the association between Helicobacter infection and gastritis; and (iii) to determine if the Helicobacter spp. isolated from these cats represented one or more distinct species on the basis of morphology, culture, and 16S rRNA sequence analysis.
(Results of this study were presented in part as an abstract at the 15th American College of Veterinary Internal Medicine Forum, Orlando, Fla., 1997.)
MATERIALS AND METHODS
Animals.
Fifteen healthy, privately owned pet cats (six females and nine males) between 1 and 11 years old (median age, 3 years) were studied. All cats belonged to staff members, students, and veterinarians working at the Veterinary Medical Teaching Hospital, University of California, Davis, and featured 13 mixed breeds and 2 purebreds. The consent of the owners of all cats was obtained. All cats had been asymptomatic in terms of vomiting, diarrhea, inappetence, or weight loss for at least 6 months prior to evaluation. In addition, all cats had a normal physical examination, a normal minimum database (complete blood count, serum biochemical profile, and urinalysis), and negative serology for feline leukemia virus and feline immunodeficiency virus. Fecal specimens collected from all cats were negative for intestinal parasites or ova on a direct fecal smear and fecal flotation.
Gastroduodenoscopy.
All cats were anesthetized for flexible endoscopy and biopsy of the cardiac, fundic, and antral regions of the stomach. Representative biopsy specimens from each location were placed into 0.5 ml of urea containing 147 mM l-tryptophan, 74 mM KH2PO4, 57 mM K2HPO4, 0.8 M NaCl, 3.3 M urea, 10% (vol/vol) ethanol, and 0.025% (wt/vol) phenol red for rapid urease testing. Representative biopsy specimens were also placed in 0.3 ml of sterile saline for culture, sterile microcentrifuge tubes frozen at −70°C for PCR, and modified Karnovsky’s fixative for scanning and transmission electron microscopy. One biopsy specimen from each location was also used to make impression smears on glass slides. In addition, two biopsy specimens from each site and one biopsy specimen from the duodenum were immersed in 10% neutral buffered formalin for histology.
Rapid urease test.
Gastric mucosal biopsy specimens were incubated in urea broth at room temperature. They were scored positive if the indicator turned red within 24 h.
Culture.
Gastric mucosal biopsy specimens were minced in saline with a sterile glass rod. A drop of the material was placed onto brucella agar containing 5% fetal calf serum (Gibco, Gaithersberg, Md.), 5 mg of trimethoprim per liter, 10 mg of vancomycin per liter, 4 mg of amphotericin B per liter, and 2,500 IU of polymxin B (Sigma, St. Louis, Mo.) per liter; brain heart infusion blood agar (Difco, Detroit, Mich.) containing trimethoprim, vancomycin, polymxin B, and amphotericin B; and brain heart infusion blood agar containing no antibiotics. All plates were incubated in an AnaeroPack jar (Remel, Lenexa, Kans.) with an AnaeroPack-Campylo microaerophilic gas generating system (Remel) at 37°C. The biopsy specimens were incubated and observed for up to 1 week.
Cytology and histology.
Gram-stained impression smears were viewed in 10 different fields under light microscopy, and the presence of gastric Helicobacter-like organisms (GHLOs) was recorded. Formalin-fixed gastric mucosal biopsy specimens were embedded in paraffin and sectioned to a thickness of 5 μm. These specimens were stained with hematoxylin and eosin and with Warthin-Starry stain. Hematoxylin- and eosin-stained biopsy specimens were examined for the presence of tortuous gastric glands, mononuclear inflammatory cell infiltrates (lymphocytes, plasma cells, and monocytes), neutrophils, lymphoid follicles, and fibrosis of the lamina propria. Severity was graded according to the following scale: 0, none; 1, mild multifocal; 2, mild widespread; 3, mild widespread and moderate multifocal; 4, moderate widespread; 5, moderate widespread and severe multifocal; 6, severe widespread. Warthin-Starry-stained sections were examined for the presence of GHLOs on the surface (in surface mucus and gastric pits) and deep in the gastric glands. Sections were scored for intensity of colonization by using the same six-point scale described above. If colonization was confined to the surface mucus or the deep glands or if colonization was different in the two locations, the locations were scored separately. Otherwise, each section received a single score encompassing both surface and deep colonization. All sections were scored without knowledge of their source.
Amplification and sequencing of 16S rRNA.
PCR and 16S rRNA sequencing were performed for the first 10 cats enrolled in the study by previously described methods (42). Briefly, about 25 mg of cat stomach tissue was minced under sterile conditions and placed in 200 μl of digestion buffer (50 mM Tris [pH 9], 1 mM EDTA) containing 1% Laureth 12 (PPG/Mazer Chemicals, Gurnee, Ill.) and 0.2 mg of proteinase K (Sigma) per ml. The samples were incubated at 37°C for 16 h and then 94°C for 10 min to denature the proteinase K. The remaining cellular debris was sedimented, and the supernatant was withdrawn and frozen at −20°C.
DNA extracts were thawed on ice, and 2 μl was added to a 100-μl reaction volume containing standard amounts of GeneAmp reagents (Perkin-Elmer Cetus, Norwalk, Conn.), 25 pmol of each primer (Table 1), and 1.5 mM MgCl2. Amplification (94°C for 1 min; 35 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 to 2 min; and 72°C for 10 min) was performed in a Perkin-Elmer thermocycler (model 2400), with a negative control (water substituted for DNA extract) included with each reaction. The PCR products were visualized by agarose gel electrophoresis and purified with a Centricon-30 concentrator according to the manufacturer’s instructions (Amicon, Beverly, Mass.), and both strands were sequenced completely with an ABI 377 automated DNA sequencer. Amplification and sequencing were performed for each of the 10 cats with a universal primer (8F) designed to amplify all known bacterial 16S rRNA genes (48) and a primer (274R) specific for the Helicobacter genus (4) (Table 1). For one cat, we also performed PCR and sequencing with a primer (133F) determined from the first sequencing reaction together with a universal primer (1429R). Additional primers (not shown in Table 1) were synthesized as needed in order to obtain nearly the entire sequence of the 16S rRNA gene from the organism infecting this animal.
TABLE 1.
Oligonucleotide primers used for amplification and sequencing of 16S rRNA
| Primer designation | Sequence (5′ to 3′) | E. coli 16S rRNA positions |
|---|---|---|
| 8F | TGCAGAGTTTGATCCTGGCTCAG | 8–27 |
| 133F | CGGGTGAGTAACGCATAGATGACA | 110–133 |
| 1492F | CCGGGTTACCTTGTTACGACTT | 1510–1492 |
| 274R | TCTCAGGCCGGATACCCGTCATAGCCT | 300–274 |
16S rRNA sequence analysis.
DNA sequences were compared to sequences in the GenBank database by using FASTA, aligned with PILEUP, and compared with DISTANCES (Wisconsin Sequence Analysis Package; Genetics Computer Group). Similarity matrices were constructed from aligned sequences by using only the sequence positions for which data were available for at least 90% of the strains. Similarity matrices were corrected for multiple base changes by the method of Jukes and Cantor (28), and a phylogenetic tree was constructed with TREEVIEW (39).
Electron microscopy.
Biopsy samples were fixed and stored in modified Karnovsky’s fixative at 4°C for up to 12 weeks until processing. The fixative consisted of 2.0% paraformaldehyde and 2.5% glutaraldehyde in 0.06 M Sorensen’s phosphate buffer. The specimens were then rinsed briefly in 0.1 M Sorensen’s phosphate buffer. Specimens for scanning electron microscopy were dehydrated in a graded acetone series (50 to 100%) for 10 min each, dried to the critical point with bone-dry-grade liquid carbon dioxide, and then mounted on specimen support stubs with silver suspension paste. A Polaron E5000 sputter coater was used to coat the specimens with 5-nm gold particles. A Philips PSEM501 scanning electron microscope was used to view and photograph the samples at 10 to 15 kV.
Specimens for transmission electron microscopy were postfixed in 1% osmium tetroxide in 0.1 M Sorensen’s phosphate buffer for 1 h at 4°C, dehydrated as described above for scanning electron microscopy, and infiltrated and embedded in epoxy resin. Sections for light microscopy were cut to 1 μm in thickness and were stained with methylene blue-Azure II. Appropriate areas were then selected and sectioned at 60 to 90 nm, stained with uranyl acetate and lead citrate, and viewed and photographed in a Philips EM-400 transmission electron microscope.
Statistical analysis.
Friedman’s two-way analysis of variance was used to compare gastritis scores and GHLO scores for the three gastric regions. Kruskal-Wallis one-way analysis of variance was used to compare GHLO scores between groups defined by the severity of gastritis. A P value of <0.05 was considered significant.
Nucleotide sequence accession numbers.
The rRNA sequences reported here have been deposited with GenBank under accession nos. AF058768 (1,406 bp) and AF058769 to AF05877 (inclusive; 164 bp each).
RESULTS
Rapid urease test, cytology, and culture.
The gross endoscopic appearance of the gastric mucosa was unremarkable in all cats. The rapid urease test and touch cytology were positive for samples from one or more gastric sites for 13 of 15 and 11 of 15 cats, respectively. Helicobacter organisms were not cultured from any cat.
Histology.
GHLOs were detectable in Warthin-Starry-stained sections from all except one of the cats. Bacteria were present in surface mucus, gastric pits, and glands, and severity ranged from 1 (rare bacteria) to 6 (many bacteria packed in glands or in gastric mucus). Bacteria were most consistently present in fundic biopsy specimens (13 of 14 cats) but for some cats were found in all gastric sites examined (cardia, 7 of 14 cats; antrum, 10 of 14 cats). Despite this finding, differences in the intensity of colonization between the three gastric regions were not significant (P = 0.64). No GHLOs were visualized in the duodenum.
Gastric inflammation was absent from eight cats, four cats had grade 1 inflammation in one or more sections, and three cats had grade 2 inflammation in at least one section. There were no significant differences in the severity of gastric inflammation among the three gastric regions (P = 0.63) or any effects of age on the severity of the inflammation. Seven cats had at least a few mononuclear inflammatory cells in the stomach (scored 1 or 2), and eight cats had mild fibrosis of the gastric lamina propria. Three cats had lymphoid follicles in the gastric lamina propria. Neutrophilic infiltrates and tortuous glands were not found in any stomach, although one cat had moderate eosinophilic infiltration in the gastric mucosa. There was no correlation between the presence of histologic lesions and the presence or intensity of colonization with GHLOs for each of the three gastric regions (P = 0.9). Colonization scores in sections without inflammation ranged from 0 to 6 (mean ± standard deviation, 3.1 ± 3.7), and colonization scores in sections with moderate inflammation ranged from 0 to 4 (mean ± standard deviation, 2.3 ± 2.1).
Electron microscopy.
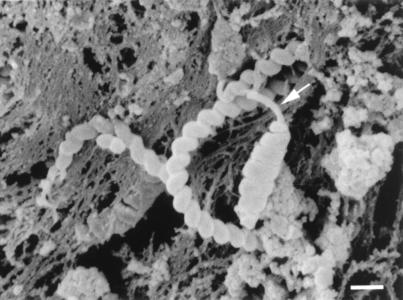
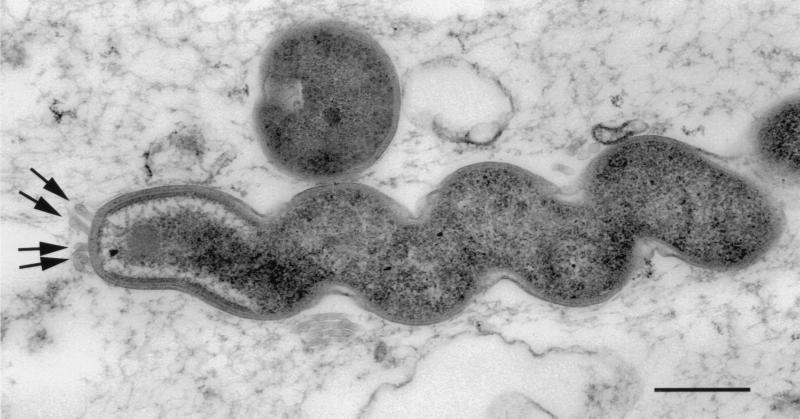
In the large majority of sections evaluated, we observed a long bacterium that measured 0.5 to 0.6 μm in single-filament diameter by 4 to 10 μm in length (Fig. 1). The coiled profile (10 to 15 turns) measured 0.6 to 0.8 μm in overall diameter and did not taper at the ends. The coils were relatively loose, with a pitch of 40 to 70° from the longitudinal axis. At least one terminal tuft of 6 to 10 flagella was observed on all organisms (Fig. 2).
FIG. 1.
Scanning electron micrograph of two different morphologic forms of bacteria. The two forms are a long, thin, loosely coiled type and a shorter, tightly coiled form. The arrow indicates prominent polar flagella. Bar, 1.0 μm.
FIG. 2.
Transmission electron micrograph of a typical helical bacterium. The cell wall is that of a gram-negative bacterium. Arrows indicate bases of individual flagella. Bar, 0.5 μm.
Occasional sections showed a relatively short form of helical bacteria, measuring 0.5 to 0.6 μm in single-filament diameter by 3 to 5 μm in length (Fig. 1). The coiled profile (six to eight turns) measured 0.8 to 1.0 μm in diameter at the center and tapered to a diameter of 0.5 to 0.7 μm at the ends. The coils were quite tight, with a pitch of 75 to 85° from the longitudinal axis. At least one terminal tuft of 6 to 10 flagella was observed on all organisms. All bacteria were located within the mucus and other contents of the gastric lumen. No association or attachment was noted with gastric epithelial cells. No periplasmic or axial fibrils were observed within any of the bacteria.
PCR.
Partial 16S rRNA sequences obtained from bacteria from the stomachs of the first 10 cats enrolled in the study yielded 164 bp of readable sequence that excluded primer regions. On the basis of this partial sequence, all 10 cats were determined to be infected with bacteria that fell in the Helicobacter genus, and all were most closely related to Helicobacter felis. However, sequences amplified from bacteria from the 10 cats were not identical to one another. Of 164 bp, there were four positions at which sequences showed heterogeneity. For bacteria from 7 of the 10 cats, sequence data for all four positions were unambiguous. Pairwise comparisons with sequences from bacteria from these seven cats and with the H. felis sequence are shown in Table 2. Although the sequences are closely related, in general they are not identical, and in some cases they differ by more than 2%. Comparison with 16S rRNA sequences from “Helicobacter heilmannii” yielded similar results (data not shown). In contrast, the 16S rRNA sequences from different H. pylori strains differed by 0.6% or less, including strains isolated from humans, cats, and rhesus monkeys (5, 13, 20, 26).
TABLE 2.
Similarity matrix of partial 16S rRNA sequences
| Cat no. or strain (accession no.) | % Similaritya
|
||||||
|---|---|---|---|---|---|---|---|
| Cat 2 | Cat 3 | Cat 5 | Cat 6 | Cat 9 | Cat 10 | H. felis | |
| 1 (AF058768) | 98.2 | 98.8 | 98.8 | 99.4 | 97.6 | 97.6 | 98.2 |
| 2 (AF058769) | 99.4 | 99.4 | 98.8 | 99.4 | 99.4 | 100 | |
| 3 (AF058770) | 98.8 | 99.4 | 98.8 | 98.8 | 99.4 | ||
| 5 (AF058772) | 99.4 | 98.8 | 98.8 | 99.4 | |||
| 6 (AF058773) | 98.2 | 98.2 | 98.8 | ||||
| 9 (AF057776) | 100 | 99.4 | |||||
| 10 (AF058777) | 99.4 | ||||||
| H. felis CS1 (M57398) | |||||||
Percentages are based on comparisons of the 164 bp of unambiguous sequence amplified from the gastric tissue of seven cats and from the corresponding region of the 16S rRNA gene from H. felis CS1.
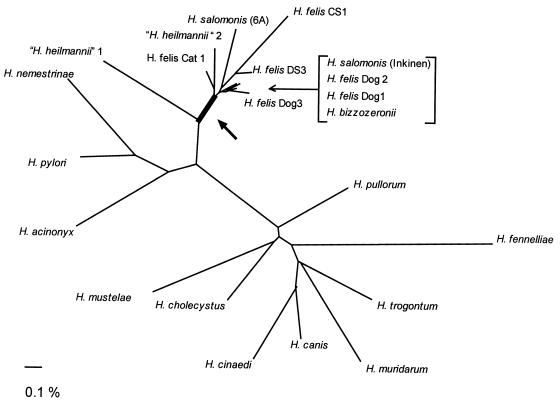
The partial sequence analysis performed with the 16S rRNA genes amplified from bacteria from the 10 cats was sufficient to determine that the bacteria observed histologically were closely related to H. felis. However, we could not make an unequivocal species designation because of sequence variability. Furthermore, we could not be sure that the variability that we observed over the 164 bp was characteristic of the entire gene. We therefore sequenced 1,406 bp (91%) of the 16S rRNA gene amplified from the bacteria in the stomach of one cat. Comparison of this sequence to the sequences in the GenBank database with FASTA showed that it was 99.1% identical to Helicobacter salomonis Inkinen, which was recently isolated from dogs and is also in the H. felis group (27). Phylogenetic analysis (Fig. 3) showed that the organism identified in this cat falls within the group of closely related organisms identified in dogs, cats, and occasionally, humans (42).
FIG. 3.
Phylogenetic tree showing the genetic relationship among Helicobacter species on the basis of 16S rRNA sequences. The branch of the “H. felis species group” is shown by the black bar and is marked with a thick arrow. The bracketed strains branch closely in the position designated by the thin arrow. Accession numbers for each of the Helicobacter species are as follows: H. acinonyx, M88148; H. bizzozeronii, V09404; H. canis, L13464; H. cholecystus, U46129; H. cinaedi, M88150; H. felis Cat 1 AF058768; H. felis CS1, M57398; H. felis DS3, M37643; H. felis Dog1, U51870; H. felis Dog2, U51871; H. felis Dog3, U51872; H. fennelliae M88154; “H. heilmannii” 1, L10079; “H. heilmannii” 2, L10080; H. muridarum, M80205; H. mustelae, M35048; H. nemestrinae, X67854; H. pullorum, L36141; H. pylori, M88157; H. salomonis Inkinen, U89351; H. salomonis 6A, V09405; H. trogontum, U65103. The scale (0.1%) indicates percent difference in 16S rRNA sequences.
DISCUSSION
Since the discovery that H. pylori is a pathogen in humans, many studies have evaluated the link between Helicobacter infection and gastric pathology in other animals. Interest in the cat is prompted in part by its potential as an animal model for human disease (21) and because there has been speculation about the zoonotic potential of this domestic species living in such close proximity to humans (9, 14, 29, 33, 38, 44). Earlier studies were performed with cats from research colonies or animal shelters or from a veterinary hospital where they presented for surgical procedures or euthanasia (14, 20, 23, 49). Our study was unique because the cats were privately owned pets that were recruited for participation in the study and that were rigorously screened to exclude underlying or concurrent diseases that may have altered Helicobacter colonization in the gastrointestinal tract.
Our data taken together with data from other recent reports permit several conclusions regarding the bacterial flora of cats. Gastric infection with “H. heilmanii” is common in healthy pet cats, and it is generally associated with minimal inflammation. The host-parasite relationship in such cases may be simply commensal (the bacterium benefits and the host is unharmed) or it may be symbiotic (the host and the bacterium benefit), such as occurs when a bacterium provides a probiotic effect or contributes to competitive exclusion of more pathogenic bacteria (8). The spectrum of the host-pathogen relationship in Helicobacter infection is probably quite broad across a variety of hosts and Helicobacter species. It may range from commensal, as appears to be the case with “H. heilmanii” in cats and perhaps also in nonhuman primates (7, 41), to chronic histologic gastritis and eventual disease in some, such as occurs with “H. heilmanii” and H. pylori infection in humans (24, 43). Occasionally, acute, symptomatic gastritis may occur, as has been observed with Helicobacter acinonyx (10–12) and rarely in humans infected with H. felis (29).
The gastric Helicobacter seen most commonly in cats (and probably many other animals) is not generally cultivable by standard methods that have been successful with other Helicobacter species. Although one group has reported successful cultivation of “H. heilmannii”-like organisms from dogs, which have been called H. salomonis (27) and Helicobacter bizzozeronii (22), our findings are consistent with those of several other groups that have examined large series of cats and dogs and found that cultures are rarely positive (9, 35). When cultures are positive, the organisms frequently do not resemble morphologically the “H. heilmannii”-like bacteria that are more commonly seen on histology (35), which was in fact the case in the initial description of H. felis (32). Despite its name, a cultivated organism that morphologically resembles the originally described H. felis (32) is not the Helicobacter most commonly seen in healthy cats. Rather, one sees organisms such as those seen in Fig. 1, although occasionally, other morphologies may be found (Fig. 1). Whether these less common morphologies represent a different form of the same species or different organisms cannot be determined from the present study, although their infrequent occurrence suggests the former.
There appears to be a large group of gastric helicobacters that is closely related but not identical to H. felis by 16S rRNA sequence analysis (Fig. 3). Although we determined a large portion of the 16S rRNA gene from the bacterium from only one cat, our partial sequence data taken in the context of the literature suggest that one could probably amplify from morphologically identical bacteria very large numbers of unique 16S rRNA genes whose sequences would vary from the H. felis sequence by 1 to 2%. DNA hybridization studies with DNAs from two cultivated organisms in this cluster (H. bizozzeroni and H. salomonis) suggest that they may be distinct species, even though their 16S rRNA genes are very closely related to that of H. felis (22, 27). Similar instances in which 16S rRNA genes are nearly identical but in which DNA hybridization results suggest that two organisms are different species have been described previously with other bacterial genera (15). However, DNA hybridization is technically demanding, and it has sometimes been difficult to identify a percent relatedness that reliably groups species (47).
In our original report which confirmed by 16S rRNA sequencing that “Gastrospirillum” was in fact a Helicobacter, we tentatively proposed the name “H. heilmannii.” However, since the two clones that we sequenced were only 96.5% similar, we suggested that “H. heilmannii” may represent multiple species (42). We do not propose to identify as a novel species the organism whose 16S rRNA gene that we have sequenced in the study described in this report. Since most of the organisms identified in cats (and probably dogs) are uncultivated and resemble “H. heilmannii,” whose 16S rRNA gene is 98.7% similar to that of H. felis CS1 (42), this and similar organisms identified in cats and dogs with very closely related 16S rRNA genes may be appropriately assigned to an “H. felis species group” (Fig. 3). Similar organisms seen in nonhuman primates and other hosts may also belong to this group. The presence of periplasmic fibers, which usually distinguishes “H. heilmannii” from H. felis, is not always reliable since they are sometimes lost on subculture (9). The “H. felis species group” may represent a somewhat heterogeneous group of organisms that sometimes is cultivable and that other times is not, that sometimes has periplasmic fibers and that other times does not, and that sometimes has a 16S rRNA gene that is virtually identical to that of H. felis CS1 and that other times shows divergence of 1 to 2%. On the other hand, cultivation of some of these organisms may confirm that they are indeed novel species. For the present, however, the epithet “H. heilmannii” is commonly used in the literature to refer to an uncultivated H. felis-like organism that lacks periplasmic fibers, and it is probably appropriate to continue to use this as a working designation (47).
It is now clear that cats are not a reservoir for infection of humans with H. pylori. Although an initial report found H. pylori in a colony of cats from one commercial vendor (17, 20), our results for 15 pet cats and a recent report of a study involving 58 pet cats (35) did not find H. pylori in a single animal, which is consistent with most epidemiologic evidence (2, 19). Given the ubiquitous nature of Helicobacter in cats, the close contact with their human owners, and the overall low prevalence of “H. heilmannii” and H. felis infections from human endoscopy specimens (44), it is unlikely that these bacteria present a significant health risk to humans.
In summary, the stomach of healthy cats is commonly colonized with organisms that resemble H. felis in terms of 16S rRNA and microscopy but that are uncultivable by methods routinely used for other Helicobacter species. The epithet “H. heilmannii” is convenient as a working designation for these organisms. They are associated with minimal inflammation and probably have a commensal or perhaps even symbiotic relationship with their host. These organisms likely are genetically heterogeneous, although the use of novel species designations should be approached cautiously. The recent proposal (34) that uncultivated bacteria be included in a new category, Candidatus (L. candidatus, a candidate), pending further identification is interesting but has not yet become widely applied.
ACKNOWLEDGMENTS
This project was supported in part by a grant from the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis. J.V.S. is supported in part by NIH grant AI-01399-02.
We thank Michael Syvanen for helpful discussions on phylogenetic analysis.
REFERENCES
- 1.Al-Himyary A J, Zabaneh R I, Zabaneh S S, Barnett S. Gastrospirillum hominis in acute gastric erosion. South Med J. 1994;87:1147–1150. doi: 10.1097/00007611-199411000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Ansorg R, Vonheinegg E H, Vonrecklinghausen G. Cat owner’s risk of acquiring a Helicobacter pylori infection. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1995;283:122–126. doi: 10.1016/s0934-8840(11)80898-4. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree J E, Spencer J. Immunologic aspects of Helicobacter pylori infection and malignant transformation of B cells. Semin Gastrointest Dis. 1996;7:30–40. [PubMed] [Google Scholar]
- 4.Dewhirst F, Seymour C, Fraser G J, Paster B J, Fox J G. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int J Syst Bacteriol. 1994;44:553–560. doi: 10.1099/00207713-44-3-553. [DOI] [PubMed] [Google Scholar]
- 5.Drazek E S, Dubois A, Holmes R K. Characterization and presumptive identification of Helicobacter pylori isolates from rhesus monkeys. J Clin Microbiol. 1994;32:1799–1804. doi: 10.1128/jcm.32.7.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois A. Spiral bacteria in the human stomach: the gastric Helicobacters. Emerg Infect Dis. 1995;1:79–85. doi: 10.3201/eid0103.950302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois A, Fiala N, Heman-Ackah L M, Drazek E S, Tarnawski A, Fishbein W N, Perez-Perez G I, Blaser M J. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 8.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B, Paola J, Sherding A. Prevalence and varieties of Helicobacter spp. in dogs from random sources and pet dogs: animal and public healthy implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton K A, Dewhirst F E, Radin M J. Helicobacter acinonyx sp. nov., isolated from cheetahs with gastritis. Int J Syst Bacteriol. 1993;43:99–106. doi: 10.1099/00207713-43-1-99. [DOI] [PubMed] [Google Scholar]
- 11.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Fox J G, Morgan D R. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus) Vet Pathol. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- 12.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Morgan D R. Gastric spiral bacilli in captive cheetahs. Scand J Gastroenterol Suppl. 1991;181:38–42. doi: 10.3109/00365529109093206. [DOI] [PubMed] [Google Scholar]
- 13.Eckloff B W, Poszorski R P, Kline B C, Cockerill R F. A comparison of 16S ribosomal DNA sequences from five isolates of Helicobacter pylori. Int J Syst Bacteriol. 1994;44:320–323. doi: 10.1099/00207713-44-2-320. [DOI] [PubMed] [Google Scholar]
- 14.El-Zaatari F A K, Woo J S, Badr A, Osato M S, Serna H, Lichtenberger L M, Genta R M, Graham D Y. Failure to isolate Helicobacter pylori from stray cats indicated that H. pylori is an anthroponosis—an animal infection with a human pathogen. J Med Microbiol. 1997;46:372–376. doi: 10.1099/00222615-46-5-372. [DOI] [PubMed] [Google Scholar]
- 15.Fox G E, Wisotzkey J D, Jurtshuk P J. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 16.Fox J G, Lee A. The role of Helicobacter spp. in newly recognized gastrointestinal diseases of animals. Lab Anim Sci. 1997;47:222–255. [PubMed] [Google Scholar]
- 17.Fox J G, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geyer C, Colbatzky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993;133:18–19. doi: 10.1136/vr.133.1.18. [DOI] [PubMed] [Google Scholar]
- 19.Graham D Y, Malaty H M, Evans D G, Evans D J, Jr, Klein P D, Adam E. Epidemiology of Helicobacter pylori in an aysmptomatic population in the United States: effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 20.Handt L K, Fox J G, Dewhirst F E, Fraser G J, Paster B J, Yan L L, Rozmiarek H, Rufo R, Stalis I H. Helicobacter pylori isolated from the domestic cat: public health implications. Infect Immun. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handt L K, Fox J G, Stalis I H, Rosemarie R, Lee G, Linn J, Li X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995;33:2280–2289. doi: 10.1128/jcm.33.9.2280-2289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanninen M L, Happonen I, Saari S, Jalava K. Culture and characteristics of Helicobacter bizzozeronii, a new canine gastric Helicobacter sp. Int J Syst Bacteriol. 1996;46:160–166. doi: 10.1099/00207713-46-1-160. [DOI] [PubMed] [Google Scholar]
- 23.Happonen I, Saari S, Castren L, Tyni O, Hanninen M L, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. J Vet Med A. 1996;43:305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 24.Heilmann K L, Borchard F. Gastritis due to spiral shaped bacteria other than H. pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 26.Hook-Nikanne J, Solin M L, Kosunen T U, Kaartinen M. Comparison of partial 16S rRNA sequences of different Helicobacter pylori strains, Helicobacter mustelae and a gastric Campylobacter-like organism (GCLO) Syst Appl Microbiol. 1991;14:270–274. [Google Scholar]
- 27.Jalava K, Kaartinen M, Utriainen M, Happonen I, Hanninen M L. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int J Syst Bacteriol. 1997;47:975–982. doi: 10.1099/00207713-47-4-975. [DOI] [PubMed] [Google Scholar]
- 28.Jukes T H, Cantor C R. Evolution of protein molecules. In: Monro H N, editor. Mammalian protein metabolism. Vol. 3. New York, N.Y: Academic Press, Inc.; 1969. pp. 21–132. [Google Scholar]
- 29.Lavelle J P, Landas S, Mitros F A, Conklin J L. Acute gastritis associated with spiral organisms from cats. Dig Dis Sci. 1994;39:744–750. doi: 10.1007/BF02087417. [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Fox J, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee A, O’Rourke J. Gastric bacteria other than Helicobacter pylori. Gastroenterol Clin N Am. 1993;22:21–42. [PubMed] [Google Scholar]
- 32.Lee A, Hazell S L, O’Rourke J, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzucchelli I, Wilder-Smith C H, Ruchti C, Meyer-Wyss B, Merki H S. Gastrospirillum hominis in asymptomatic, healthy individuals. Dig Dis Sci. 1993;38:2087–2089. doi: 10.1007/BF01297089. [DOI] [PubMed] [Google Scholar]
- 34.Murray R G E, Schleifer K H. Taxonomic notes: a proposal for recording the properties of putative taxa of prokaryotes. Int J Syst Bacteriol. 1994;44:174–176. doi: 10.1099/00207713-44-1-174. [DOI] [PubMed] [Google Scholar]
- 35.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthesy-Theulaz I, Halter F, Lauerburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomura A, Stemmermann G N, Chyou P, Kato I, Perez G I, Blaser M J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 37.Novo R C, Magne M L. Proceedings of the 13th Annual ACVIM Veterinary Forum. 1995. Current concepts in the management of Helicobacter-associated gastritis; pp. 57–61. [Google Scholar]
- 38.Otto G, Hazell S H, Fox J G, Howlett C R, Murphy J C, O’Rourke J L, Lee A. Animal and public health implications of gastric colonization of cats by Helicobacter-like organisms. J Clin Microbiol. 1994;32:1043–1049. doi: 10.1128/jcm.32.4.1043-1049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 40.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 41.Solnick, J. V., D. R. Canfield, and J. Parsonnet. 1996. Seroprevalence of Helicobacter pylori infection in rhesus monkeys. Gastroenterology 110(Suppl.):A261.
- 42.Solnick J V, O’Rourke J, Lee A, Paster B, Dewhirst F E, Tompkins L S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- 43.Solnick J V, Tompkins L S. Helicobacter pylori and gastroduodenal disease: pathogenesis and host-parasite interaction. Infect Agents Dis. 1992;1:294–309. [PubMed] [Google Scholar]
- 44.Stolte M, Wellens E, Bethke B, Ritter M, Eidt H. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand J Gastroenterol. 1994;29:1061–1064. doi: 10.3109/00365529409094888. [DOI] [PubMed] [Google Scholar]
- 45.Thompson M A, Storey P, Greer R, Cleghorn G J. Canine-human transmission of Gastrospirillum hominis. Lancet. 1994;343:1605–1607. doi: 10.1016/s0140-6736(94)93060-0. [DOI] [PubMed] [Google Scholar]
- 46.Vandamme P, Pot B, Gillis M, DeVos P, Kersters K, Swings J. Polyphasic taxonomy: a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber A F, Hasa O, Sautter J H. Some observations concerning the presence of spirilla in the fundic glands of dogs and cats. Am J Vet Res. 1958;19:422–426. [PubMed] [Google Scholar]
- 48.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Assoc. 1998;212:529–533. [PubMed] [Google Scholar]