Abstract
The SARS‐CoV‐2 infection, responsible for COVID‐19, has raised the interest for infection‐associated muco‐cutaneous symptoms. While dermatologic symptoms in general gained an increasing awareness, affection of the nail organ has been mentioned only recently. We provide a narrative review on COVID‐19 manifestation on the nail organ and add symptoms induced by personal protective measures and SARS‐CoV‐2 vaccination. Available treatment options are discussed.
Keywords: COVID‐19, COVID‐vaccination, nail diseases, nail organ, protective measures, SARS‐CoV‐2, treatment
1. INTRODUCTION
In 2019, a new highly contagious viral disease emerged in China. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was identified as the responsible pathogen and the disease was termed coronavirus disease‐19 (COVID‐19). On March 11, 2020, the WHO declared COVID‐19 as a pandemic disease. 1 Cardiovascular and pulmonary symptoms were major clinical symptoms, but soon it was observed that the systemic nature of this infection could affect all organs and tissues of the human body. Although cutaneous manifestations have only rarely been mentioned in the early reports from Wuhan, China, they gained increasing attention later when the pandemic affected Europe and America. 2 The dermatological signs of this systemic infection have been now categorized and have obtained a much better awareness. 3 , 4 , 5 , 6
In contrast to this, nail changes have only recently been identified as possible consequences of SARS‐CoV‐2‐infection. 7 We here review the current knowledge on this topic compared with that of 2020 and added treatment options for the various nail pathologies.
2. NAIL CHANGES INDUCED BY SARS‐COV‐2 INFECTION
2.1. Microvascular disturbances
Microvascular disturbances caused by SARS‐CoV‐2 can be observed by nailfold capillaroscopy. In an open trial with 82 COVID‐19 patients from Italy, pericapillary edema (80.5%), enlarged capillaries (61.0%), and sludge flow (53.7%) was reported. In addition, meandering capillaries and reduced capillary density has been observed in every second patient. Acute COVID‐19 infection was associated with a higher prevalence of hemosiderin deposits resulting from micro‐hemorrhages and micro‐thrombosis. Patients who recovered from COVID‐19 had a higher prevalence of capillary pathologies such as enlarged capillaries, meandering capillaries, loss of capillaries, and empty dermal papillae. 8
2.2. COVID‐toe or ‐finger
One of the most frequently observed cutaneous sign is the COVID‐toe or ‐finger, a pernio‐like periungual erythematous edema (Figure 1). Pernio‐like lesions on the fingers or toes typically occur as part of a resolution phase, milder or asymptomatic course of COVID‐19, whereas livedoid lesions and retiform purpura are associated with coagulopathy and more severe disease. 3 , 4 , 5 , 6 In one study on 54 patients cryofibrinogenemia was detected in 66.7%. 9
FIGURE 1.
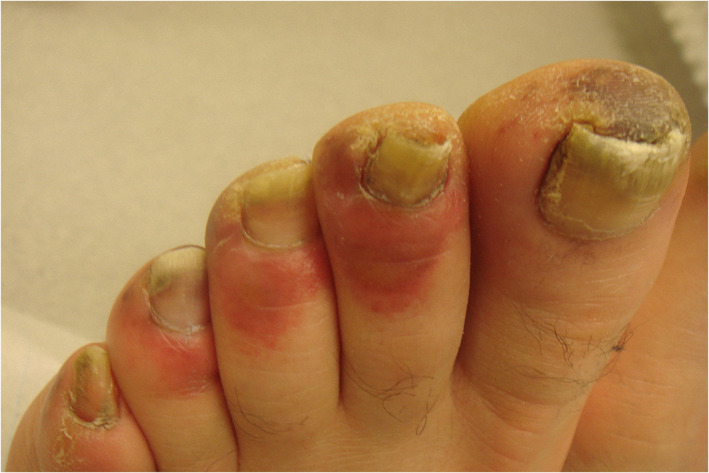
Pernio‐like lesion (“COVID‐toe”). Reproduced from Wollina U, et al. Cutaneous signs in COVID‐19 patients: A review. Dermatol Ther, 2020, 33 (5): e13549
Kanitakis et al. (2020) studied 17 patients with suspected but unconfirmed SARS‐CoV‐2 infection and pernio‐like acral lesions. Histopathological findings of skin biopsies included deep horizontal parakeratosis (71%), necrosis of epidermal keratinocytes (41.5%), dispersed or confluents basal cell vacuolation (18%), spongiosis (12%), lymphocytic exocytosis (12%), dermal edema (76.5%), perivascular dermal lymphocytic infiltrate (100%), perivascular eosinophils (23.5%), endothelial cell swelling (65%), dermal mucin deposits (41.5%), microthrombi in superficial capillaries (12%) or venules (6%), dermal fibrin deposits (12%), and fibrin deposits in venule walls (18%). The dermal infiltrate consisted mainly of CD3+ lymphocytes, but rare B‐lymphocytes or plasma cells and rare activated CD30+ cells were intermingled. Direct immunofluorescence was positive in 82% of samples with vascular deposits of IgM (53%), IgA, and/ or C3 (29% each) (Figures 2, 3, 4). 10
FIGURE 2.
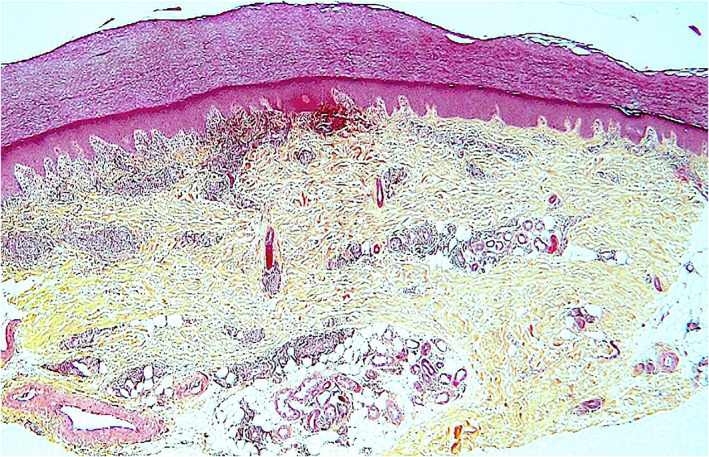
Pernio‐like lesion (“COVID‐toe”). Scanning magnification shows upper dermal edema and a dense, mononuclear cell perivascular, and periadnexal dermal infiltration (hematoxylin–eosin, original magnification ×40)
FIGURE 3.
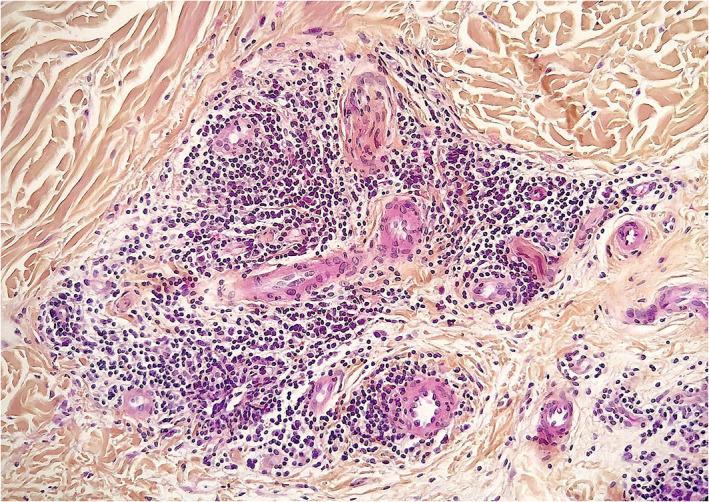
Pernio‐like lesion (“COVID‐toe”). Dense dermal perivascular and periadnexal lymphocytic infiltration (hematoxylin–eosin, original magnification ×100)
FIGURE 4.
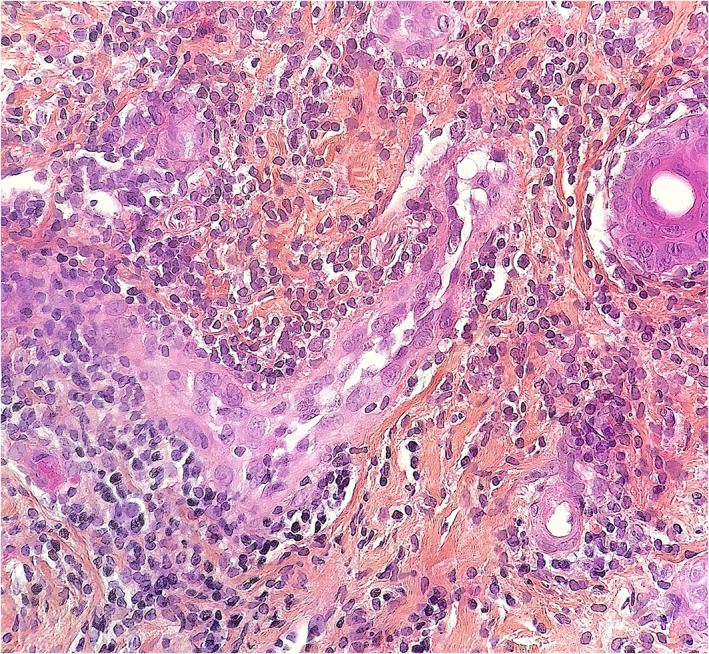
Pernio‐like lesion (“COVID‐toe”). Dense lymphocytic perivascular lymphocytic infiltration. Mild swelling of endothelial cells is seen (hematoxylin–eosin, original magnification ×250)
In another study of 17 adolescents (median age 13.2 years), 16 (94.1%) had bilaterally localized distal erythematous or cyanotic lesions. Dermatoscopy discovered red dots (100%), white rosettes (68.8%), and white streaks (62.5%). Histology demonstrated a remodeling of dermal blood vessels with a lobular arrangement, vascular wall thickening, and a mild perivascular lymphocytic infiltrate. In these patients, a SARS‐CoV‐2 infection was excluded by in situ hybridization and serologic testing. 11
Thirty‐three patients (mean age 23.4 years) with chilblains during the COVD‐19 pandemic presented with erythematous and purpuric papules on the toes or fingers with edema and pruritus or burning sensation. Histology (n = 5) revealed superficial dermal lymphocytic infiltrates around vessels and eccrine sweat glands. None of these patients tested positive with two different antibody assays for SARS‐CoV‐2. 12
These findings question a causative relationship to SARS‐CoV‐2 infection, while other studies claimed the presence of SARS‐CoV‐2 spike proteins within blood endothelial cells and eccrine sweat glands. About 50% of patients with COVID‐toes or ‐fingers were positive for SARS‐CoV‐2. Most patients were young. 13 , 14
The serologic negativity in patients with COVID‐toes or ‐fingers might be due to a later seroconversion in milder cases, limited antibody production, or induction of an early robust innate immune response to SARS‐CoV‐2. 15 The presence of microthrombi in COVID‐toes and ‐fingers suggests an association with the observed coagulopathy in COVID‐19. 10 , 16
The most important differential diagnoses include frostbites (perniones) and chilblain lupus erythematosus. In familial chilblain lupus, loss‐of‐function mutations in the nucleases TREX1 or SAMHD1 have been identified characteristic of a type I interferonopathy. 17
2.3. Acral gangrene
A rare but severe symptom is acral gangrene (Figure 5). It is a red flag sign of acute severe infection with multisystemic inflammation and cardiovascular malfunction. 18
FIGURE 5.
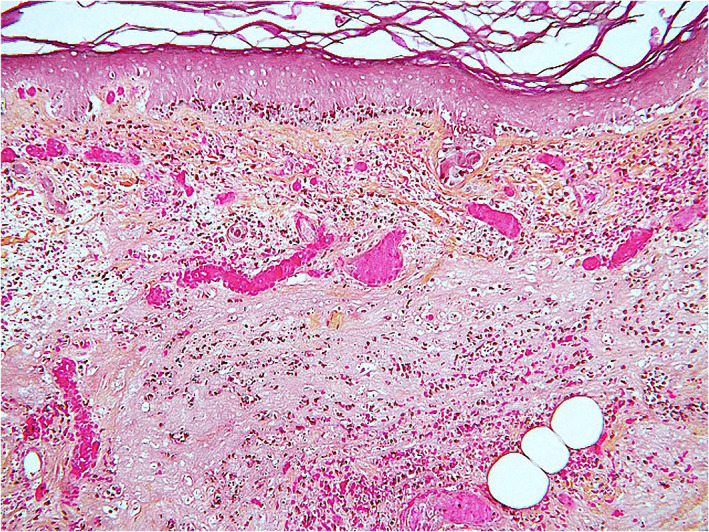
Acral purpuric/necrotic lesion from a patient with severe COVID‐19 infection. Thrombotic vasculopathy, dermal hemorrhage, and epidermal ischemic necrosis are seen (hematoxylin–eosin, original magnification ×250)
2.3.1. Treatment of microvascular symptoms
In case of young asymptomatic patients or patients without confirmation of SARS‐CoV‐2 infection COVID‐toe or finger is self‐limited and spontaneous resolution has been observed within 2–3 weeks. The basic treatment in early COVID‐19 stages consists of a combination of antiviral therapies (e.g., favipiravir, remdesivir, hydroxychloroquine, lopinavir plus ritonavir) with antithrombotic treatment, while in later advanced stages of the disease antithrombotic therapy should be combined with a treatment of the cytokine storm (e.g., tocilizumab, dexamethasone, interleukin‐1 or tumor necrosis factor‐beta antagonists). Antithrombotic treatment is using low‐molecular weight heparinoids or heparin. Heparin is capable of limiting endothelial damage such as glycocalyx shedding. These compounds have anticoagulant, antiviral, and anti‐inflammatory effects. 19 Oral corticosteroids in combination with low‐dose acetylsalicylic acid are the treatment of choice for cryofibrinogenemia. 20
2.4. Periungual desquamation
Periungual desquamation has been reported in children with severe Kawasaki‐like multisystemic inflammatory syndrome (MIS‐C) and adults recovering from severe COVID‐19. 21 , 22 Since coronary artery lesions have been observed in MIS‐C, it has been recommended to assess patients with severe COVID‐19 for such vascular symptoms. 23
2.4.1. Treatment
Moisturizers can be used to limit the symptoms.
2.5. Beau's lines and onychomadesis
Beau's lines are transverse grooves of the nails resulting from suppressed growth of the nail matrix by drugs or systemic infectious diseases. Beau's lines have been observed among children and adults with COVID‐19. 24 , 25 , 26 Single or multiple nails may be affected–mostly fingernails. Beau's lines can be accompanied by leukonychia. 24
Beau's lines may precede onychomadesis, characterized by separation of the nail plate from the nail matrix, with persistent attachment to the nail bed. Onychomadesis is a possible consequence of COVID‐19. 27 , 28
Another late manifestation of COVID‐19 disease is a heterogeneous red–white discoloration of the nail bed with distal onycholysis. 29
2.5.1. Treatment
Onychomadesis and Beau's lines are usually mild and self‐limited. In chemotherapy‐induced onychomadesis, high‐energy 633 nm red light (126 J/cm2 for 20 min every day) has been successful for fingernails but not toenails. 30
2.6. Discolorations of the lunula and the nail plate
A red‐violet band surrounding the distal margin of the nail lunula, is known as red half‐moon sign and has been observed in acute SARS‐CoV‐2 infection in adult patients. 31 , 32
Distal orange discolorations of the nail plates have been reported as a delayed response several weeks after COVID‐19 disease in elderly patients who developed later sarcopenia and anemia. 33
Nonblanchable transverse leukonychia (transverse white lines) was observed in a 47‐year‐old man with COVID‐19. These lines are due to abnormal keratinization of the nail plate. 34
2.6.1. Treatment of discolorations
There is no specific treatment of nail plate discolorations. It is most important to exclude infections (onychomycosis or bacterial infections). Colored nail lacquers can cover the symptoms. Transverse white lines may be persistent after severe damage of nail matrix.
2.7. Nail changes induced by COVID‐19 treatments
Favipiravir is a purine nucleoside precursor and competitive inhibitor of RNA‐dependent RNA polymerase. It is used in COVID‐19 off‐label with variable results. A yellow‐white fluorescence on the nails has been reported as an adverse event of favipiravir used for COVID‐19 disease. 35
Some patients on favipiravir developed a greenish fluorescence in the lunula and nail plate portion near the proximal nail fold. Green fluorescence has also been observed on the scalp hair made visible by Wood's lamp illumination. It is not yet clear, whether the fluorescence is mainly caused by drug metabolites or by additives of the tablets, such as yellow ferric oxide. 36
Hydroxychloroquine is an antimalarial drug with anti‐viral activity in vitro that may cause hyperpigmentation of tissues. Hydroxychloroquine has been widely used in prevention and treatment of SARS‐CoV‐2 infection but sound evidence for a clinical benefit is lacking. Hydroxychloroquine‐induced longitudinal melanonychia is a possible adverse event. 37
The most important differential diagnosis of drug‐induced melanonychia is subungual melanoma, which warrants a nail biopsy. 38
2.7.1. Treatment
Drug‐induced nail changes will most often spontaneously disappear after withdrawal of the responsible drug. No specific treatment exists.
2.8. Nail changes induced by vaccination
Recently, some cases of pernio‐like lesions were reported that developed on the hands and/ or feet a few days after vaccination against COVID‐19 with RNA‐based vaccines (BionTec/ Pfizer, Moderna). 39 , 40 , 41 , 42 Histologic examination revealed findings indistinguishable from idiopathic and COVID‐19‐related perniones. 40 , 42
2.8.1. Treatment
Low‐dose acetylsalicylic acid with oral corticosteroids can be used in symptomatic and painful lesions. Due to temporary character of the vaccine‐induced perniones, often treatment is unnecessary.
2.9. Nail changes by protective measures
The green nail syndrome or Goldman‐Fox syndrome is caused by nail infection or colonization with P. aeruginosa. It is known as an occupational disease in health care workers, especially in intensive care units. Green nail syndrome has recently been reported in health care workers during the COVID‐19 pandemic (Figure 6). 43 , 44
FIGURE 6.
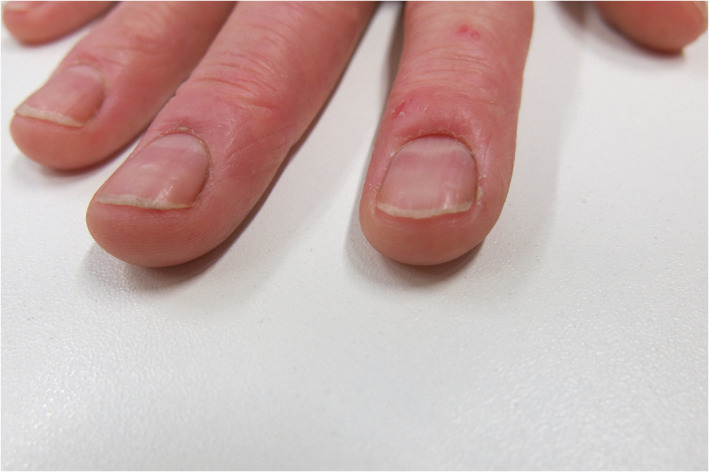
Irritant contact dermatitis with loss of cuticle and affection of the proximal nail fold by swelling, redness, and fine scaling
2.9.1. Treatment
Treatment consists of oral ciprofloxacin. Topical treatment includes removal of the onycholytic part of the nail and brushing of the nail bed with 2% sodium hypochlorite solution twice daily for at least 6 weeks, and topical nadifloxacin, tobramycin, or gentamycin. 43 , 45
Irritant hand dermatitis has been observed more frequently during the COVID‐19 pandemic especially on health care workers on COVID‐19 departments. One study reported adverse cutaneous reactions to frequent hand washing and frequent use of disinfectants in up to 80.2%. 46 Nails can be affected due to wet work leading to brittle nails. Loss of cuticle is often seen together with a chronic irritant proximal nail fold dermatitis (Figure 5). 47
Recommendations of skin care have been developed by various medical bodies including the European academy of dermatology and venereology task force on contact dermatitis to avoid adverse effects of sanitary procedures on hands and nails. 46 Limitation of wet work, use of protective gloves, and regular use of moisturizers during the day and emollients overnight are helpful.
3. CONCLUSIONS
Affection of the nail by COVID‐19 has only recently been reported. The nail organ can be affected by SARS‐CoV‐2 infection in a number of ways. Some of these symptoms are indicators of a mild course like COVID‐toe or‐finger, while others are a red flag for a serious course like acro‐ulcerative lesions. The medical treatment of COVID‐19 may also interfere with the nail organ often with color changes of the nail plate. Vaccination with RNA‐based vaccines can induce pernio‐like acral lesions of digits. Prolonged use of protective gloves and wet work bear a risk for bacterial infection of the nail plate with Pseudomonas leading to chloronychia. Health care staff with chloronychia is a potential source of transmissible infections, especially in intensive care units, transplant centers, and hemato‐oncology departments.
Informed consent was obtained from patients for clinical pictures.
CONFLICT OF INTEREST
Uwe Wollina, Jean Kanitakis, and Robert Baran declare no interest of conflicts.
AUTHORS CONTRIBUTION
Uwe Wollina, Jean Kanitakis, and Robert Baran developed the concept, collected data and analyzed published articles. Uwe Wollina wrote the first draft. Uwe Wollina, Jean Kanitakis, and Robert Baran contributed to the final draft, which was confirmed by all authors.
Wollina U, Kanitakis J, Baran R. Nails and COVID‐19 – A comprehensive review of clinical findings and treatment. Dermatologic Therapy. 2021;34(5):e15100. doi: 10.1111/dth.15100
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [PUBMED] at [https://pubmed.ncbi.nlm.nih.gov/].
REFERENCES
- 1. Do Borges Nascimento IJ, O'Mathúna DP, Von Groote TC, et al. Coronavirus disease (COVID‐19) pandemic: an overview of systematic reviews. BMC Infect Dis. 2021;21(1):525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larenas‐Linnemann D, Luna‐Pech J, Navarrete‐Rodríguez EM, et al. Cutaneous manifestations related to COVID‐19 immune dysregulation in the pediatric age group. Curr Allergy Asthma Rep. 2021;21(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wollina U, Karadağ AS, Rowland‐Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID‐19 patients: a review. Dermatol Ther. 2020;33(5):e13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wollina U, Chiriac A, Karadag AS. The dermatological spectrum of COVID‐19 disease: cutaneous signs for diagnostics and prognosis and an expanded classification. Open Access Maced J Med Sci. 2020;8:294‐303. [Google Scholar]
- 7. Ocampo‐Garza SS, Ocampo‐Candiani J, Camela E, et al. Nail changes as manifestation of systemic disease in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2021;35:e474‐e475. 10.1111/jdv.17273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID‐19 microvascular involvement. Microvasc Res. 2021;133:104071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gómez‐Fernández C, López‐Sundh AE, González‐Vela C, et al. High prevalence of cryofibrinogenemia in patients with chilblains during the COVID‐19 outbreak. Int J Dermatol. 2020;59(12):1475‐1484. [DOI] [PubMed] [Google Scholar]
- 10. Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain‐like acral lesions during the COVID‐19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain‐like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID‐19 pandemic. JAMA Netw Open. 2021;4(6):e2111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hébert V, Duval‐Modeste AB, Joly P, et al. Lack of association between chilblains outbreak and severe acute respiratory syndrome coronavirus 2: histologic and serologic findings from a new immunoassay. J Am Acad Dermatol. 2020;83(5):1434‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koschitzky M, Oyola RR, Lee‐Wong M, Abittan B, Silverberg N. Pediatric COVID toes and fingers. Clin Dermatol. 2021;39(1):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kashetsky N, Mukovozov IM, Bergman J. Chilblain‐like lesions (cll) associated with COVID‐19 (“COVID toes”): a systematic review. J Cutan Med Surg. 2021;12034754211004575. 10.1177/12034754211004575 [DOI] [PubMed] [Google Scholar]
- 15. Daneshjou R, Rana J, Dickman M, Yost JM, Chiou A, Ko J. Pernio‐like eruption associated with COVID‐19 in skin of color. JAAD Case Rep. 2020;6(9):892‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. König N, Fiehn C, Wolf C, et al. Familial chilblain lupus due to a gain‐of‐function mutation in STING. Ann Rheum Dis. 2017;76(2):468‐472. [DOI] [PubMed] [Google Scholar]
- 18. Chaudhary H, Mohan M, Jain A, et al. Acral gangrene: ugly cousin of “COVID toes” in multisystem inflammatory syndrome in children associated with SARS‐COV‐2? Pediatr Infect Dis J. 2021;40:e312‐e313. 10.1097/INF.0000000000003181 [DOI] [PubMed] [Google Scholar]
- 19. Asakura H, Ogawa H. COVID‐19‐associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113(1):45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michaud M, Pourrat J. Cryofibrinogenemia. J Clin Rheumatol. 2013;19(3):142‐148. [DOI] [PubMed] [Google Scholar]
- 21. Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS‐C) compared with severe acute COVID‐19. Jama. 2021;325(11):1074‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamoto T, Ishikane M, Sasaki R, Ohmagari N. Periungual desquamation in a Japanese adult recovering from severe COVID‐19. Int J Infect Dis. 2021;102:37‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abrams JY, Oster ME, Godfred‐Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS‐C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf GK, French LE. Beau‐lines of the fingernails in association with pediatric SARS‐CoV‐2 infections. J Dtsch Dermatol Ges. 2021;19(5):744‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ide S, Morioka S, Inada M, Ohmagari N. Beau's lines and leukonychia in a COVID‐19 patient. Intern Med. 2020;59(24):3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alobaida S, Lam JM. Beau lines associated with COVID‐19. CMAJ. 2020;192(36):E1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senturk N, Ozdemir H. Onychomadesis following COVID‐19 infection: is there a relationship? Dermatol Ther. 2020;33(6):e14309. [DOI] [PubMed] [Google Scholar]
- 28. Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID‐19 infection. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Demir B, Yuksel EI, Cicek D, Turkoglu S. Heterogeneous red‐white discoloration of the nail bed and distal onycholysis in a patient with COVID‐19. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li A, Li Y, Ge L, Li P, Li W. Onychomadesis associated with chemotherapy: case report and mini literature review. Drug des Devel Ther. 2017;11:2373‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Méndez‐Flores S, Zaladonis A, Valdes‐Rodriguez R. COVID‐19 and nail manifestation: be on the lookout for the red half‐moon nail sign. Int J Dermatol. 2020;59(11):1414. [DOI] [PubMed] [Google Scholar]
- 32. Neri I, Guglielmo A, Virdi A, Gaspari V, Starace M, Piraccini BM. The red half‐moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34(11):e663‐e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tammaro A, Adebanjo GAR, Erasmus HP, et al. Transverse orange nail lesions following SARS‐CoV‐2 infection. Dermatol Ther. 2021;34(1):e14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez‐Nieto D, Jimenez‐Cauhe J, Ortega‐Quijano D, Diaz‐Guimaraens B, Dominguez‐Santas M, Martinez‐Rubio J. Transverse leukonychia (Mees' lines) nail alterations in a COVID‐19 patient. Dermatol Ther. 2020;33(6):e13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gülseren D, Yalıcı‐Armagan B. Yellow‐white fluorescence on the nails: a novel finding of Favipiravir used for the treatment of COVID‐19. J Cosmet Dermatol. 2021;20:2392‐2393. 10.1111/jocd.14214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aslan Kayıran M, Cebeci F, Erdemir VA, Aksoy H, Akdeniz N, Gürel MS. Fluorescence of nails and hair on Wood's lamp examination in Covid pandemic; undefined effect of Favipiravir in humans. Dermatol Ther. 2021;34(1):e14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ricardo JW, Chikeka I, Silvers DN, Lipner SR. Longitudinal melanonychia and skin hyperpigmentation associated with hydroxychloroquine therapy. JAAD Case Rep. 2020;7:23‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an international Dermoscopy society study. J Eur Acad Dermatol Venereol. 2017;31(4):732‐736. [DOI] [PubMed] [Google Scholar]
- 39. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lesort C, Kanitakis J, Donzier L, Jullien D. Chilblain‐like lesions after BNT162b2 mRNA COVID‐19 vaccine: a case report suggesting that 'COVID toes' are due to the immune reaction to SARS‐CoV‐2. J Eur Acad Dermatol Venereol. 2021. 10.1111/jdv.17451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davido B, Mascitti H, Fortier‐Beaulieu M, et al. 'Blue toes' following vaccination with the BNT162b2 mRNA COVID‐19 vaccine. J Travel Med. 2021;28(4):taab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meara AS, Silkoski M, Quin K, Jarjour W. A case of chilblains‐like lesions post SARS‐CoV‐2 vaccine? J Rheumatol. 2021;210226. 10.3899/jrheum.210226 [DOI] [PubMed] [Google Scholar]
- 43. Schwartz RA, Kapila R. The Goldman‐fox syndrome: treating and preventing green pseudomonas nails in the era of COVID‐19. Dermatol Ther. 2021;34(1):e14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiriac AE, Chiriac A, Wollina U. Chloronychia in healthcare workers in COVID‐19 times. Skin Appendage Disord. 2021;7(1):80‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geizhals S, Lipner SR. Retrospective case series on risk factors, diagnosis and treatment of Pseudomonas aeruginosa nail infections. Am J Clin Dermatol. 2020;21(2):297‐302. [DOI] [PubMed] [Google Scholar]
- 46. Chernyshov PV, Kolodzinska L. Prospective study on hand dermatitis in nurses and doctors during COVID‐19 pandemic and its improvement by use of adopted recommendations of the European academy of dermatology and venereology task force on contact dermatitis. Dermatol Ther. 2020. Nov;33(6):e14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhir H. Hand dermatitis and nail disorders of the workplace. Clin Occup Environ Med. 2006;5(2):381‐396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [PUBMED] at [https://pubmed.ncbi.nlm.nih.gov/].


