Abstract
This nonrandomized, multicenter cohort, open-label clinical trial evaluated the efficacy and safety of combined chemotherapy with arsenic trioxide (ATO) in children with stage 4/M neuroblastoma (NB). We enrolled patients who were newly diagnosed with NB and assessed as stage 4/M and received either traditional chemotherapy or ATO combined with chemotherapy according to their own wishes. Twenty-two patients were enrolled in the trial group (ATO combined with chemotherapy), and 13 patients were enrolled in the control group (traditional chemotherapy). Objective response rate (ORR) at 4 weeks after completing induction chemotherapy was defined as the main outcome, and adverse events were monitored and graded in the meantime. Data cutoff date was December 31, 2019. Finally, we found that patients who received ATO combined with chemotherapy had a significantly higher response rate than those who were treated with traditional chemotherapy (ORR: 86.36% vs. 46.16%, p = 0.020). Reversible cardiotoxicity was just observed in three patients who were treated with ATO, and no other differential adverse events were observed between the two groups. ATO combined with chemotherapy can significantly improve end-induction response in high-risk NB, and our novel regimen is well tolerated in pediatric patients. These results highlight the superiority of chemotherapy with ATO, which creates new opportunity for prolonging survival. In addition, this treatment protocol minimizes therapeutic costs compared with anti-GD2 therapy, MIBG, and proton therapy and can decrease the burden to families and society. However, we also need to evaluate more cases to consolidate our conclusion.
Key words: Chemotherapy, Arsenic trioxide (ATO), Neuroblastoma (NB), Children, Clinical Trial
INTRODUCTION
Neuroblastoma (NB), originating from the nerve crest of the sympathetic nervous system, is one of the most common malignant extracranial solid tumors in children, accounting for approximately 10% of pediatric malignancies and contributing to 15% of all pediatric cancer mortality1,2. About 40% of patients are defined as high-risk NB (HRNB) with distant metastasis diseases at diagnosis. Although new strategies have been developed for treatment in recent years, the outcome of patients with HRNB remains poor, with long-term survival less than 50%3. Currently, chemotherapy still plays a key role in the treatment of NB, and it is reported that improved end-induction response in HRNB is associated with longer survival4. Therefore, searching for innovative regimens of induction chemotherapy is strategic to improve prognosis of patients with HRNB.
Arsenic trioxide (ATO) is an ancient drug used in traditional Chinese medicine for more than 2000 years, and currently, it is a Food and Drug Administration (FDA)-approved drug to treat acute promyelocytic leukemia (APL)5,6. In recent years, ATO has been reported to exert potent cytotoxic activity against a large variety of cancer cells of solid tumor including NB7. Particularly, our previous studies8,9 have confirmed that ATO could inhibit proliferation of NB cells by retarding cell cycle in the G0 or G2/M phase and has synergetic cytotoxic effects to NB cells when used in combination with other chemotherapy drug like etoposide, cisplatin, vinorelbine, and docetaxel. Recently, ATO is demonstrated as a Hedgehog (HH) pathway inhibitor acting at the level of GLI. The HH signaling pathway plays an important role in the development of neural crest stem cells10. It is reported that the signaling molecules of this pathway such as SHH, PTCH1, SMO, and GLI are highly expressed in NB patients, and activated HH pathway accounts for the poor prognosis of NB patients11. Evidence has shown that ATO could suppress tumor growth of NB by blocking HH/GLI both in vitro and in vivo12,13. Therefore, we conducted an innovative clinical study to evaluate the efficacy and safety of ATO combined with chemotherapy for patients with newly diagnosed stage 4/M NB. Here we report the preliminary results.
MATERIALS AND METHODS
Ethics Statement
All patients and/or guardians gave written informed consent approved by the Ethics Committee of Sun Yet-Sen Memorial Hospital, and the study was approved by both the protocol review committee and the institutional review board of each institution. This trial was also registered with ClinicalTrial.gov (NCT03503864) and Chinese Clinical Trial Registry (ChiCTR1800014748).
Patients
Children ≤14 years of age were eligible for this study if they were newly diagnosed with NB and assessed as stage 4 according to the International Neuroblastoma Staging System (INSS) or stage M according to the International Neuroblastoma Risk Group (INRG), respectively14,15. Patients were divided into two groups: (a) trial group: patients received traditional chemotherapy combined with ATO; (b) control group: patients received traditional chemotherapy alone.
Treatment
Patients in the control group received traditional comprehensive treatment following SMHPO-N-2012 NB protocol, a protocol based on N7 and NB2004 protocols (Table 1). Traditional chemotherapy was a nine-cycle treatment and consisted of three regimens: CAV (cycles 1, 2, 4, and 6), PVP (cycles 3, 5, and 7), and CT (cycles 8 and 9). The CAV regiment was composed of vincristine 0.022 mg/kg or 0.67 mg/m2 daily for 2 h on days 1 and 2, doxorubicin 25 mg/m2 over 3 h, and cyclophosphamide 1.2 g/m2 for 3 h daily on days 1 to 3. The PVP regimen consisted of cisplatin 50 mg/m2 daily for 6 h over days 1 to 4 and etoposide (light resistant) 200 mg/m2 daily for more than 4 h over days 1 to 3. The CT regimen consisted of cyclophosphamide 1.2 g/m2 for 3 h daily on days 1 and 2 and topotecan (light resistant) 2 mg/m2 daily as a continuous infusion over days 1 to 3 (72 h in total).
Table 1.
Dose and Usage of Chemotherapeutics
| Regimen/Drug | Dose and Usage | Time |
|---|---|---|
| ATO | ||
| Arsenic trioxide | 0.16 mg/kg/day, IV drip, PI > 4 h | d (−2)–d7 |
| CAV | ||
| CTX | 1.2 g/m2/day, IV drip, PI = 3 h | d1–d2 |
| THP | 25 mg/m2/day, IV drip, PI > 3 h | d1–d3 |
| VCR* | 0.022 mg/kg/day or 0.67 mg/m2/day, IV drip, PI = 2 h | d1–d3 |
| PVP | ||
| DDP | 50 mg/m2/day, IV drip, PI = 6 h (light resistant) | d1–d4 |
| VP-16 | 200 mg/m2/day, IV drip, PI > 4 h | d1–d3 |
| CT | ||
| CTX | 1.2 g/m2/day, IV drip, PI = 3 h | d1–d2 |
| Topotecan | 2 mg/m2/day, IV drip, PI = 24 h (light resistant) | d1–d3 |
CTX, cyclophosphamide; THP, pyranodomycin; VCR, vincristine; DDP, carboplatin; VP-16, etoposide.
Take the lower value of the two calculation methods; maximum dose = 0.67 mg/day and total dose of 3 days ≤2 mg.
In the trial group, patients received ATO combined with chemotherapy for nine courses in total (Fig. 1). ATO was administered 2 days in advance at a dose of 0.16 mg/kg per day for 10 days (Table 1). The ATO injection was administered at a constant rate over 8 h in 250–500 ml of normal saline or a 5% glucose solution through a central venous catheter. Simultaneously, patients received 0.5–1.0 g ascorbic acid along with 5% 100- to 250-ml glucose injections in another vein channel. Surgery could be performed at one of the two different time points if necessary: before treatment or after four courses of our protocols. As for surgery, we consulted the HR-NBL1/SIOPEN study16 and strongly encouraged complete macroscopic excision (CME) of the primary tumor, including all visible and palpable tumor and related involved lymph nodes. If surgery was performed, evaluation of patient’s status should be conducted before surgery and at least 2 weeks after it.
Figure 1.
Arsenic trioxide (ATO) combined with induction chemotherapy of SMHPO-N-2012 neuroblastoma (NB) protocol.
General imaging examinations such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) CT were conducted every two or four courses of chemotherapy. In our assessment, if patients presented for surveillance PET CT, high standard uptake value (SUV) of tumors (>2.5) was interpreted as “a suspicious malignant process,” and further examinations should be required to fully understand this distinction from other malignant tumors.
Serum neuron-specific enolase (NSE) and urinary vanillylmandelic acid/creatinine (VMA/Cr) ratio was measured at each course. Bone marrow examination was performed at each course until bone marrow metastasis converted negative.
Assessments
The primary analytic end point was objective response rate (ORR). Time to event was defined as time from diagnosis until time of 4 weeks after completing nine cycles of chemotherapies or the time of first occurrence of relapse, progression. Effective evaluation was performed using the International NB Staging System criteria14 at the end point. (1) A complete response (CR) was defined as the complete resolution of all clinical evidence of disease for at least 4 weeks. (2) A partial response (PR) was defined as a 50%–90% reduction in the sum of the products of the perpendicular diameters of all measurable lesions for at least 4 weeks and no appearance of new lesions. (3) Stable disease (SD) was defined as a decrease <50% in tumor size less than a PR, but no disease progression or any lesion enlarged by <25%. (4) Progressive disease (PD) was defined as the appearance of new lesions or a 25% increase in the product of the two longest perpendicular diameters in any previously measurable lesion (excluding bone).
The toxicities associated with ATO include cardiotoxicity, hepatotoxicity, nephrotoxicity, neurotoxicity, metabolic disturbance, fluid retention, skin discoloration, xeroderma, conjunctivitis, etc17. Adverse events (AEs) were monitored and graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0 (https://ctep.cancer.gov).
Statistical Analyses
Categorical variables (efficiency of induction chemotherapy and determination of cytotoxic activity) were compared by using Fisher’s exact test, and count data were analyzed by using nonparametric test as appropriate. Statistical significance was defined as a value of p < 0.05. All analyses were performed with SPSS 25.0.
RESULTS
Patient Characteristics
During January 1, 2018, and December 31, 2019, a total of 22 patients were enrolled in the trial group, while 13 patients were included in the control group during January 1, 2011 and December 31, 2019. All of these 35 patients were evaluated as high risk, and patient demographics and baseline characteristics were comparable between groups (Tables 2 and 3).
Table 2.
Patient Demographic and Baseline Clinical Characteristics
| Trial Group (N = 22) | Control Group (N = 13) | Total (N = 35) | p Value* | |
|---|---|---|---|---|
| Age | 0.384 | |||
| Median (years) | 3.00 | 4.00 | 4.90 | |
| Range (IQR) (years) | 0.7–7.0 (3.49) | 0.1–8.0 (3.41) | 0.1–8.0 (2.85) | |
| <18 months | 3 (13.64%) | 1 (7.69%) | 4 (11.43%) | |
| ≥18 months | 19 (86.36%) | 12 (92.31%) | 31 (88.57%) | |
| Gender | 0.488 | |||
| Male | 12 (54.55%) | 9 (69.23%) | 21 (60.00%) | |
| Female | 10 (45.45%) | 4 (30.77%) | 14 (40.00%) | |
| Stage of disease | 1.000 | |||
| INSS stage 4 | 20 (90.91%) | 12 (92.31%) | 32 (91.43%) | |
| INRG stage M | 2 (9.09%) | 1 (7.69%) | 3 (8.57%) | |
| Primary site | 1.000 | |||
| Adrenal gland | 17 (77.27%) | 10 (76.92%) | 27 (77.14%) | |
| Mediastinum | 2 (9.09%) | 0 (0%) | 2 (5.71%) | |
| Bone | 2 (9.09%) | 3 (23.08%) | 5 (14.29%) | |
| Cervical region | 1 (4.55%) | 0 (0%) | 1 (2.86%) | |
| BM metastasis | 1.000 | |||
| Yes | 15 (68.18%) | 9 (69.23%) | 24 (68.57%) | |
| No | 7 (31.82%) | 4 (30.77%) | 11 (31.43%) | |
| VMA/Cr | 0.432 | |||
| Median | 42.60 | 44.70 | 44.70 | |
| Range (IQR) | 6.18–108.6 (43.92) | 17.8–388 (66.03) | 6.18–388 (51.00) | |
| MYCN amplification | 1.000 | |||
| Positive | 13 (59.09%) | 8 (61.54%) | 21 (60.00%) | |
| Negative | 9 (40.91%) | 5 (38.46%) | 14 (40.00%) |
IQR, Interquartile range; INSS, International Neuroblastoma Staging System; INRG, International Neuroblastoma Risk Group; BM, bone marrow; VMA/Cr, urinary vanillylmandelic acid/creatinine ratio.
Categorical variables were compared using Fisher’s exact test, and count data were analyzed using nonparametric test.
Table 3.
Treatment Responses of End-Induction Chemotherapy
| Group | Enrolled Cases (N) | Response | ORR (%) | ||
|---|---|---|---|---|---|
| CR (N) | PR (N) | NR/PD (N) | |||
| ATO combined with chemotherapy | 22 | 12 | 7 | 3 | 86.36 |
| Conventional chemotherapy | 13 | 3 | 3 | 7 | 46.16 |
CR, complete response; PR, partial response; NR, no response; PD, progressive disease; ATO, arsenic trioxide. Using Fisher’s exact probabilities: p = 0.020.
Primary NB sites were adrenal gland (n = 17, 77.27%), mediastinum (n = 2, 9.09%), bone (n = 2, 9.09%), and cervical region (n = 1, 4.55%) in the trial group, and adrenal gland (n = 10, 76.92%) and bone (n = 3, 23.08%) in the control group. The most common metastasis was bone marrow in both groups (trial group: 15/22; control group: 9/13). MYCN amplification detection was performed in all patients, and positive rates were 59.09% (13/22) and 61.54% (8/13) in the trial group and control group, respectively.
In the trial group, 22 patients received ATO combined with chemotherapy, and 13 patients in the control group were treated with traditional chemotherapy.
Efficacy
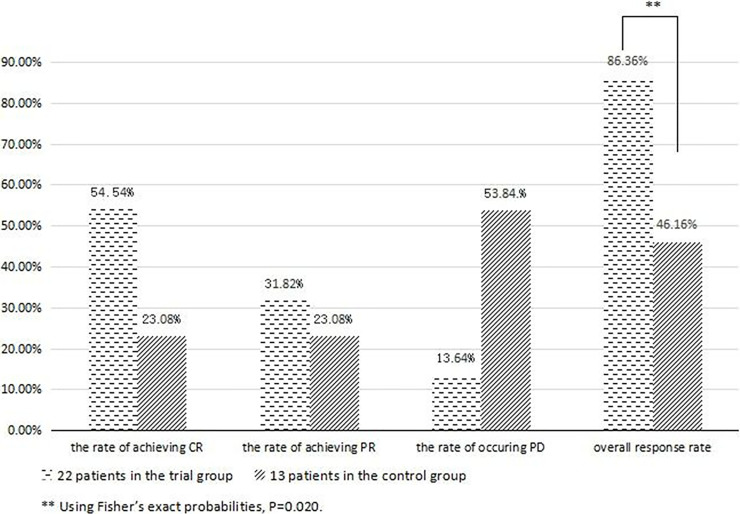
Among patients treated with combined chemotherapy, 12 patients achieved CR, 7 were PR, and 3 were PD after induction therapy. In the control group, 3 patients achieved CR and 3 were PR, and 7 patients were assessed as PD. Patients who received ATO combined with chemotherapy had a higher response rate than those who were treated with traditional chemotherapy (ORR: 86.36% vs. 46.16%, p = 0.020) (Table 3, Figs. 2 and 3).
Figure 2.
Magnetic resonance imaging (MRI) of a 3-year-old girl in the trial group (A: before treatment; B: after ATO combined with chemotherapy and surgery). MRI of a 2-year-old girl in the trial group (C: before treatment; D: after ATO combined with chemotherapy and surgery).
Figure 3.
Different results between the trial group and control group in evaluation. **Patients who received ATO combined with chemotherapy had a higher overall response rate (ORR) than those who were treated with traditional chemotherapy.
Toxicity
There were no treatment-related deaths. Table 4 lists all grade 3 or 4 AEs that occurred in 35 patients (22 in the trial group and 13 in the control group) who were enrolled in this trial.
Table 4.
Incidence and Severity of Adverse Events
| Adverse Event | Trial Group (N = 22) | Control Group (N = 13) | p Value* |
|---|---|---|---|
| Hematologic | |||
| Leukopenia | 1.000 | ||
| Grade 3 | 0 (0.00%) | 0 (0.00%) | |
| Grade 4 | 22 (100.00%) | 13 (100.00%) | |
| Neutropenia | 1.000 | ||
| Grade 3 | 0 (0.00%) | 0 (0.00%) | |
| Grade 4 | 22 (100.00%) | 13 (100.00%) | |
| Anemia | 0.259 | ||
| Grade 3 | 14 (63.64%) | 11 (84.62%) | |
| Grade 4 | 8 (36.36%) | 2 (15.38%) | |
| Thrombocytopenia | 1.000 | ||
| Grade 3 | 18 (81.82%) | 10 (76.92%) | |
| Grade 4 | 4 (18.18%) | 3 (23.08%) | |
| Nonhematologic | |||
| AST elevation | 1.000 | ||
| Grade 3 | 3 (13.64%) | 3 (23.08%) | |
| Grade 4 | 0 (0.00%) | 0 (0.00%) | |
| Others | 19 (86.36%) | 10 (76.92%) | |
| ALT elevation | 1.000 | ||
| Grade 3 | 3 (13.64%) | 3 (23.08%) | |
| Grade 4 | 0 (0.00%) | 0 (0.00%) | |
| Others | 19 (86.36%) | 10 (76.92%) | |
| Infection | 0.734 | ||
| Grade 3 | 13 (59.09%) | 7 (53.85%) | |
| Grade 4 | 0 (0.00%) | 0 (0.00%) | |
| Others | 9 (40.91%) | 6 (46.15%) | |
Categorical variables were compared using Fisher’s exact test, and count data were analyzed using nonparametric test.
All patients had grade 4 leukopenia and neutropenia during chemotherapy, with no significant difference observed between groups. The nonhematologic grade 4 toxicities were not found in our study. The nonhematologic grade 3 toxicities included infection, alanine aminotransferase (ALT) elevation, and aspartate aminotransferase (AST) elevation. The most common grade 3 nonhematologic AE was infection, and there were 59.09% (13/22) and 53.85% (7/13) in the trial group and control group, respectively. In addition, AST and ALT elevation simultaneously occurred in six patients (each three in the trial group and control group) but could be reversed by using hepatinica. Reversible cardiotoxicity was observed in three patients who received ATO combined with chemotherapy, presenting as increased heart rates in one case and asymptomatic serum increased heart type creative arouses enzyme (CK-MB) in another two cases (the CK-MB levels of two patients were less than 2.5 times the upper limit of normal and evaluated as grade 1 according to NCI-CTCAE). Initial cardiac workup of these two patients with elevated CK-MB, including electrocardiogram (ECG), echocardiography, and Holter monitoring, yielded normal results. We found no significant difference in systolic ejection fraction (range: 59%–64%) with baseline prior to treatment (range: 60%–66%) either. These cardiac side reactions disappeared after suspension of ATO, and no abnormality was observed in careful reexamination.
DISCUSSION
NB is the most common extracranial solid tumors in children that can occur anywhere in the sympathetic nervous system, accounting for about 8% to 10% of childhood malignancies and 15% of childhood tumor-related mortality2. HRNB is a tumor with a high degree of malignancy with 5 years overall survival (OS) rate of less than 40% before the multimodality therapy, such as chemotherapy, surgery, radiotherapy, and hematopoietic stem cell transplantation2. Worse still, the recurrence rate of HRNB within 2 years (median time: 4.5–18.7 months) after the initial diagnosis has exceeded 80%, and only 7% to 12.7% of relapsed cases could survive more than 5 years18,19. In an effort to enhance the survival of HRNB, scholars have focused on immunotherapy and targeted therapy. Dinutuximab, an anti-GD2 monoclonal antibody, was reported to raise 2-year event-fee survival (EFS) and OS rates to 66 ± 5% and 86 ± 4%, respectively, compared to the standard treatment in a phase III study20. It is important to note that anti-GD2 therapy may enhance the incidence of low GD2-expressing tumor, decreasing the effectiveness of maintenance therapy21. A study in 2015 (NCT01355679) in which therapy is assigned based on results of molecular profiling for patients with relapsed disease, and only 1 of 14 subjects achieved partial remission (the median time of EFS was 59 days)22. Association studies indicated that some patients had responses to other molecularly guided approaches for MYCN, ALK, and PI3K/AKT/mTOR pathways in the short term; however, the efficacy of these treatments showed a gradual decline due to drug resistance and reduction in target cells23. With this situation, the molecularly guided approaches are not as satisfied until now in clinical practices.
In our study, 13 patients in the control group received traditional comprehensive treatment, a protocol based on N7 and NB2004 protocols. Of these patients, the value of the response rate (6/13, 46.16%) was significantly smaller compared to literature-reported results. N7 protocol was tested by Cheung et al.24, and 24 patients more than 1 year of age with newly diagnosed HRNB were enrolled in their study. Among these patients, the overall response after induction chemotherapy consisted of 21 CR/very good partial response (VGPR) (87.50%), 2 PR (8.33%), and 1 PD (4.17%). According to Kholer et al.25, of the 30 patients with positive mIBG scans, there were 16 in CR giving a response rate of 53.3% [95% confidence interval (CI): 36.1%–69.8%]. This difference might be explained as follows. Firstly, this might be related to the small sample size, resulting in greater fluctuation of the predicted value. Secondly, in the control group of our study, the positive rate of MYCN amplification (61.54%) was higher than the other two studies (45.83% reported by Cheung et al.24 and 28.13% reported by Kholer et al.25), and it indicated that patients in our group might have a higher degree of malignancy with poor therapeutic effects. The overall response rate of the trial group was higher than might be expected that 86.36% of patients achieved PR or better, which significantly surpassed the control group without any obvious difference in the toxicities. Compared with published data of the Children’s Oncology Group (COG)4, the ORR of our induction effect was higher than COG (86.36% vs. 78.4%). Our investigation defined the antitumor efficiency and safety of this regimen, suggesting a potential role for ATO in the management of patients with HRNB. In our study, all patients had grade 4 leukopenia and neutropenia. Infection occurred about 59.09% (13/22) and 53.85% (7/13) in the trial group and control group, respectively. AST and ALT elevation simultaneously went up in six patients (each three in the trial group and control group). Three patients who received ATO combined with chemotherapy were found with cardiotoxicity. After corresponding symptomatic treatment and suspension chemotherapy, all these AEs could be reversed.
ATO has been used as a drug for the treatment of various diseases in ancient China. As early as the 1970s, Chinese scholars discovered that ATO can induce APL tumor cell differentiation and apoptosis to achieve remarkable results in initial/relapse tumor APL6. Dozens of vitro and vivo studies have shown the broad-spectrum antitumor activities of ATO in multiple tumors26–30, suggesting the clinical application value of ATO. Our previous studies have found some mechanisms of ATO cytotoxicity in NB cells. First is the G2/M cell cycle arrest at 48 h in the SKNSH cell line after ATO administration, which can significantly enhance the cytotoxic efficiency of M phase-specific chemotherapy drugs (vinorelbine, docetaxel, etc)9. Second is a dose-dependent upregulation of TrkA and TrkC receptors related to the good prognosis of NB31. Furthermore, ATO could induce the downregulation of glycoprotein P (P-gp) in SK-N-SH cells9. Relapsing NB has frequently gained multidrug resistance (MDR), so that benefits little from conventional regimens. The P-gp and multidrug resistance-associated protein 1 (MRP1) are the pumps commonly found to confer MDR in cancers. P-gp and MRP1 have been demonstrated to regulate the export of cytotoxic drugs, including vincristine, etoposide, daunorubicin, and others32. It has been reported that MRP1 is a direct transcriptional target of MYCN in NB, which enhanced MRP1-mediated drug resistance33. In addition to MRP1, an increased expression of other MRP family members MRP4 was confirmed as predictive of poor clinical outcome in aggressive NB34. Research studies demonstrate upregulation of P-gp, and MDR1 gene expression was strongly upregulated during standard chemotherapy in multifocal HRNB35. Karlsson et al. demonstrated that ATO could efficiently kill NB cells, while the chemotherapeutics of HRNB, i.e., etoposide, doxorubicin, carboplatin, and vincristine, failed to kill multidrug-resistant NB cells36. This result is in agreement with our study demonstrating that ATO has advantages for the treatment of relapsing NB.
Mechanisms underlying the ATO cytotoxic actions are various, such as inducing PML-RARα fusion protein degradation combined with retinoic acid. Retinoic acid targets the RAR portion of the fusion protein, whereas ATO targets the PML part of the protein, which resulted in apoptosis and partial differentiation of the leukemic cells37. Furthermore, ATO can induce ROS production to mediate activation of the downstream caspase-dependent apoptosis pathways38; upregulate tumor apoptosis genes bax, bak, and Fas; inhibit tumor angiogenesis; and so on39. Recently, some considered that the cytotoxic actions of ATO are probably via inhibition of the HH signaling pathway by targeting Gli protein. HH signaling pathway is critical for embryonic patterning and pathologically associated with oncogenesis, maintenance of the tumorigenicity, and prognosis in rhabdomyosarcoma40,41, medulloblastoma42, osteosarcoma43, etc. A high proportion of abnormal activation of HH signaling pathway in surgical specimens of embryonic tumors has been confirmed. The positive expression rates of SHH, PTCH, and Gli1 are 96%, 100%, and 68% in NB; 78%, 100%, and 78% in rhabdomyosarcoma; and 71%, 100%, and 43% in Wilms tumor, respectively11, suggesting that the components of the HH pathway may be a target for treatment. Several studies indicated that HH pathway downstream module Gli inhibitors can availably inhibit the proliferation of NB cells44–47 by modulating the expression of cell cycle proteins such as cyclin D1 or p2148, and decrease the transcription of Gli1 downstream gene MYCN, which is closely related to the invasiveness and poor prognosis of HRNB49. Antagonism of ATO to Gli1 was confirmed via replacing zinc finger of Gli1 protein or preventing the accumulation of cilium, resulting in the inactivation of Gli150,51. All in all, ATO may antagonize NB tumors by means of multiple pathways mentioned above.
CONCLUSION
From our study, we concluded that the ATO combined with chemotherapy has an obvious superiority in end-induction response (ORR: 86.36%) for newly diagnosed stage 4/M NB in children, with mild ATO-related toxicities observed. These results highlight the superiority of chemotherapy with ATO, which creates new opportunity for prolonging survival. Besides, this treatment protocol minimizes therapeutic costs compared with anti-GD2 therapy, MIBG, and proton therapy and can decrease the burden to families and society. Further exploration is needed to bring in more cases to consolidate our conclusion and enrich the fundamental research of ATO’s cytotoxic effect in the NB cell line. We will continue to track the long-term prognosis of patients in this study.
ACKNOWLEDGMENTS
This work was supported by grants 2017A030313806 and 2020A1515010127 from the Guang Dong Natural Science Foundation and grant SYS-C-202007 from Sun Yat-Sen Clinical Research Cultivating Program. Yang Li designed the trial and revised the manuscript; Chunmou Li, Xiaomin Peng, and Chuchu Feng carried out this trial and analyzed the data; Chunmou Li, Xiaomin Peng, Chuchu Feng, and Xilin Xiong organized the figures and wrote the manuscript; other authors participated in this clinical trial. All authors read and approved the final manuscript. Trial Registration: ClinicalTrial.gov (NCT03503864) and Chinese Clinical Trial Registry (ChiCTR1800014748).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007;369(9579):2106–20. [DOI] [PubMed] [Google Scholar]
- 2.Smith V, Foster J. High-risk neuroblastoma treatment review. Children 2018;5(9):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, Cohn SL. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33(27):3008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto N, Naranjo A, Hibbitts E, Kreissman SG, Granger MM, Irwin MS, Bagatell R, London WB, Greengard EG, Park JR, DuBois SG. Predictors of differential response to induction therapy in high-risk neuroblastoma: A report from the Children’s Oncology Group (COG). Eur J Cancer 2019;112(5):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au W, Li C, Lee V, Yuen Hui L, Yau J, Chan G, Ha S, Kwong Y. Oral arsenic trioxide for relapsed acute promyelocytic leukemia in pediatric patients. Pediatr Blood Cancer 2012;58(4):630–2. [DOI] [PubMed] [Google Scholar]
- 6.Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A, Reynolds J, Di IJ, Tiley C, Taylor K, Filshie R, Seldon M, Taper J, Szer J, Moore J, Bashford J, Seymour JF, Australasian Leukaemia and Lymphoma Group. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 2012;120(8):1570–80. [DOI] [PubMed] [Google Scholar]
- 7.Gazitt Y, Akay C. Arsenic trioxide: An anti cancer missile with multiple warheads. Hematology 2005;10(3):205–13. [DOI] [PubMed] [Google Scholar]
- 8.Qi K, Li Y, Huang K, Xiong X, Chuchu F, Zhang C, Weng W. Pre-application of arsenic trioxide may potentiate cytotoxic effects of vinorelbine/docetaxel on neuroblastoma SK-N-SH cells. Biomed Pharmacother. 2019;113:108665. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Li Y, Xiong X, Qi K, Zhang C, Fang J, Guo H. Low dose of arsenic trioxide inhibits multidrug resistant-related p-glycoprotein expression in human neuroblastoma cell line. Int J Oncol. 2016;49(6):2319–30. [DOI] [PubMed] [Google Scholar]
- 10.Schiapparelli P, Shahi MH, Enguita-Germán M, Johnsen JI, Kogner P, Lázcoz P. Castresana JS. Inhibition of the sonic hedgehog pathway by cyclopamine reduces the CD133+/CD15+ cell compartment and the in vitro tumorigenic capability of neuroblastoma cells. Cancer Lett. 2011;310(2):222–31. [DOI] [PubMed] [Google Scholar]
- 11.Oue T, Yoneda A, Uehara S, Yamanaka H, Fukuzawa M. Increased expression of the hedgehog signaling pathway in pediatric solid malignancies. J Pediatr Surg. 2010;45(2):387–92. [DOI] [PubMed] [Google Scholar]
- 12.Beauchamp EM, Ringer Lymor BG, Sajwan KP, Hall MD, Lee YC, Peaceman D, Ozdemirli M, Rodriguez O, Macdonald TJ, Albanese C, Toretsky JA, Uren A. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121(1):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickström M, Dyberg C, Shimokawa T, Milosevic J, Baryawno N, Fuskevåg OM, Larsson R, Kogner P, Zaphiropoulos PG, Johnsen JI. Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int J Cancer 2013;132(7):1516–24. [DOI] [PubMed] [Google Scholar]
- 14.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77. [DOI] [PubMed] [Google Scholar]
- 15.Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK, Nuchtern JG, von Schweinitz D, Simon T, Cohn SL, Pearson AD, INRG Task Force. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol. 2009;27(2):289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes K, Pötschger U, Pearson ADJ, Sarnacki S, Cecchetto G, Gomez-Chacon J, Squire R, Freud E, Bysiek A, Matthyssens LE, Metzelder M, Monclair T, Stenman J, Rygl M, Rasmussen L, Joseph JM, Irtan S, Avanzini S, Godzinski J, Björnland K, Elliott M, Luksch R, Castel V, Ash S, Balwierz W, Laureys G, Ruud E, Papadakis V, Malis J, Owens C, Schroeder H, Beck-Popovic M, Trahair T, Forjaz de Lacerda A, Ambros PF, Gaze MN, McHugh K, Valteau-Couanet D, Ladenstein RL. International Society of Paediatric Oncology Europe Neuroblastoma Group (SIOPEN). Influence of surgical excision on the survival of patients with stage 4 high-risk neuroblastoma: A report from the HR-NBL1/SIOPEN study. J Clin Oncol. 2020;38(25):2902–15. [DOI] [PubMed] [Google Scholar]
- 17.Quezada G, Kopp L, Estey E, Wells RJ. All-trans-retinoic acid and arsenic trioxide as initial therapy for acute promyelocytic leukemia. Pediatr Blood Cancer 2008;51(1):133–5. [DOI] [PubMed] [Google Scholar]
- 18.Basta NO, Halliday GC, Makin G, Birch J, Feltbower R, Bown N, Elliott M, Moreno L, Barone G, Pearson AD, James PW, Tweddle DA, McNally RJ. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br J Cancer 2016;115(9):1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.London WB, Bagatell R, Weigel BJ, Fox E, Guo D, Van RC, Naranjo A, Park JR. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer 2017;123(24):4914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ploessl C, Pan A, Maples KT, Lowe DK. Dinutuximab: An anti-GD2 monoclonal antibody for high-risk neuroblastoma. Ann Pharmacother. 2016;50(5):416–22. [DOI] [PubMed] [Google Scholar]
- 21.Keyel ME, Reynolds CP. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: Development and place in therapy. Biologics 2019;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saulnier Sholler GL, Bond JP, Bergendahl G, Dutta A, Dragon J, Neville K, Ferguson W, Roberts W, Eslin D, Kraveka J, Kaplan J, Mitchell D, Parikh N, Merchant M, Ashikaga T, Hanna G, Lescault PJ, Siniard A, Corneveaux J, Huentelman M, Trent J. Feasibility of implementing molecular-guided therapy for the treatment of patients with relapsed or refractory neuroblastoma. Cancer Med. 2015;4(6):871–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsen JI, Dyberg C, Fransson S, Wickström M. Molecular mechanisms and therapeutic targets in neuroblastoma. Pharmacol Res. 2018;13:164–76. [DOI] [PubMed] [Google Scholar]
- 24.Cheung NK, Kushner BH, LaQuaglia M, Kramer K, Gollamudi S, Heller G, Gerald W, Yeh S, Finn R, Larson SM, Wuest D, Byrnes M, Dantis E, Mora J, Cheung IY, Rosenfield N, Abramson S, O’Reilly RJ. N7: A novel multi-modality therapy of high risk neuroblastoma in children diagnosed over 1 year of age. Med Ped Oncol. 2001;36 (1):227–30. [DOI] [PubMed] [Google Scholar]
- 25.Kholer JA, Ellershaw C, Machin D. Response to N7 induction chemotherapy in children more than 1 year of age diagnosed with metastatic neuroblastoma treated at UKCCSSG centers. Pediatr Blood Cancer 2007;49:234–9. [DOI] [PubMed] [Google Scholar]
- 26.Emadi A, Gore SD. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010;24:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm SA, Marymont M, Chandler JP, Muro K, Newman SB, Levy RM, Jovanovic B, McCarthy K, Raizer JJ. Phase I study of arsenic trioxide and temozolomide in combination with radiation therapy in patients with malignant gliomas. J Neurooncol. 2012;110(2):237–43. [DOI] [PubMed] [Google Scholar]
- 28.Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, Yang CH. Arsenic trioxide in patients with hepatocellular carcinoma: A phase II trial. Invest New Drugs 2007;25(1):77–84. [DOI] [PubMed] [Google Scholar]
- 29.Wei W, Zhou F, Zhang Y, Guo L, Shi H, Hou J. A combination of thalidomide and arsenic trioxide is effective and well tolerated in patients with myelodysplastic syndromes. Leukemia Res. 2012;36(6):715–19. [DOI] [PubMed] [Google Scholar]
- 30.Roboz GJ, Ritchie EK, Curcio T, Provenzano J, Carlin R, Samuel M, Wittenberg B, Mazumdar M, Christos PJ, Mathew S, Allen-Bard S, Feldman EJ. Arsenic trioxide and low-dose cytarabine in older patients with untreated acute myeloid leukemia, excluding acute promyelocytic leukemia. Cancer 2008;113(9):2504–11. [DOI] [PubMed] [Google Scholar]
- 31.Xiong X, Li Y, Liu L, Qi K, Zhang C, Chen Y, Fang J. Arsenic trioxide induces cell cycle arrest and affects Trk receptor expression in human neuroblastoma SK-N-SH cells. Biol Res. 2018;51(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher JI, Williams RT, Henderson MJ, Norris MD, Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat. 2016;26:1–9. [DOI] [PubMed] [Google Scholar]
- 33.Manohar CF, Bray JA, Salwen HR, Madafiglio J, Cheng A, Flemming C, Marshall GM, Norris MD, Haber M, Cohn SL. MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma. Oncogene 2004;23(3):753–62. [DOI] [PubMed] [Google Scholar]
- 34.Norris MD, Smith J, Tanabe K, Tobin P, Flemming C, Scheffer GL, Wielinga P, Cohn SL, London WB, Marshall GM, Allen JD, Haber M. Expression of multidrug transporter MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and confers resistance to irinotecan in vitro. Mol Cancer Ther. 2005;4(4):547–53. [DOI] [PubMed] [Google Scholar]
- 35.Oue T, Yoneda A, Uehara S, Yamanaka H, Fukuzawa M. Increased expression of multidrug resistance-associated genes after chemotherapy in pediatric solid malignancies. J Pediatr Surg. 2009;44(2):377–80. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson J, Ora I, Porn-Ares I, Pahlman S. Arsenic trioxide-induced death of neuroblastoma cells involves activation of Bax and does not require p53. Clin Cancer Res. 2004;10(9):3179–88. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Lallemand-Breitenbach V, de The H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission. Oncogene 2001;20(49):7257–65. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Yel L, Kim D, Kim C, Chiplunkar S, Gollapudi S. Arsenic trioxide induces apoptosis in peripheral blood T lymphocyte subsets by inducing oxidative stress: A role of Bcl-2. Mol Cancer Ther. 2003;2(8):711–9. [PubMed] [Google Scholar]
- 39.Hoonjan M, Jadhav V, Bhatt P. Arsenic trioxide: Insights into its evolution to an anticancer agent. J Biol Inorg Chem. 2018;23(3):313–29. [DOI] [PubMed] [Google Scholar]
- 40.Ridzewski R, Rettberg D, Dittmann K, Cuvelier N, Fulda S, Hahn H. Hedgehog inhibitors in rhabdomyosarcoma: A comparison of four compounds and responsiveness of four cell lines. Front Oncol. 2015;5:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang WQ, Hei Y, Kang L, Xiao LH. Heparanase-1 and components of the hedgehog signalling pathway are increased in untreated alveolar orbital rhabdomyosarcoma. Clin Experiment Ophthalmol. 2014;42(2):182–9. [DOI] [PubMed] [Google Scholar]
- 42.Marino S. Medulloblastoma: Developmental mechanisms out of control. Trends Mol Med. 2005;11(1):17–22. [DOI] [PubMed] [Google Scholar]
- 43.Hirotsu M, Setoguchi T, Sasaki H, Matsunoshita Y, Gao H, Nagao H, Kunigou O, Komiya S. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer 2010;9(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao L, Xia YP, Zhou YN, Dai RL, Yang X, Duan SJ, Qiao X, Mei YW, Hu B, Cui H. A critical role of Sonic Hedgehog signaling in maintaining the tumorigenicity of neuroblastoma cells. Cancer Sci. 2009;100(10):1848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahi M, Schiapparelli P, Afzal M, Sinha S, Rey JA, Castresana JS. Expression and epigenetic modulation of sonic hedgehog–GLI1 pathway genes in neuroblastoma cell lines and tumors. Tumour Biol. 2011;32(1):113–27. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Gu S, Huang J, Chen S, Zhang Z, Xu M. Inhibition of autophagy potentiates the efficacy of Gli inhibitor GANT-61 in MYCN-amplified neuroblastoma cells. BMC Cancer 2014;14(1):768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan H, Luo H, Wang J, Ji X, Zhang Z, Wu J, Zhang X, Wu X. Smoothened-independent activation of hedgehog signaling by rearranged during transfection promotes neuroblastoma cell proliferation and tumor growth. Biochim Biophys Acta 2016;1860(9):1961–72. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Wang X, Wan J, Li T, Gong X, Zhang K, Yi L, Xiang Z, Xu M, Cui H. Sonic Hedgehog pathway is essential for neuroblastoma cell proliferation and tumor growth. Mol Cell Biochem. 2012;364(1–2):235–41. [DOI] [PubMed] [Google Scholar]
- 49.Boehme KA, Zaborski JJ, Riester R, Schweiss SK, Hopp U, Traub F, Kluba T, Handgretinger R, Schleicher SB. Targeting hedgehog signalling by arsenic trioxide reduces cell growth and induces apoptosis in rhabdomyosarcoma. Int J Oncol. 2016;48(2):801–12. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Lee JJ, Kim J, Gardner D, Beachy PA. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci USA 2010;107(30):13432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han JB, Sang F, Chang JJ, Hua YQ, Shi WD, Tang LH, Liu LM. Arsenic trioxide inhibits viability of pancreatic cancer stem cells in culture and in a xenograft model via binding to SHH-Gli. Onco Targets Ther. 2013;6:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]