Abstract
Objective: Our study aimed to evaluate the correlation of circular RNA SMARCA5 (circ-SMARCA5) and microRNA 432 (miR-432) with clinical characteristics and survival in bladder cancer patients. Methods: Preoperative clinicopathologic features and survival data of 156 bladder cancer patients were retrospectively reviewed. A total of 156 cases of tumor tissues, whereas 71 cases out of 156 available adjacent tissues were obtained from the Pathology Department for circ-SMARCA5 and miR-432 detections using real-time quantitative polymerase chain reaction. Results: Circ-SMARCA5 was upregulated but miR-432 was downregulated in tumor tissues compared with adjacent tissues; meanwhile, circ-SMARCA5 expression was negatively correlated with miR-432 in bladder cancer tissues. Circ-SMARCA5 high expression was correlated with larger tumor size, higher tumor stage, and lymph node (LYN) metastasis. However, miR-432 high expression was correlated with single multiplicity, smaller tumor size, lower tumor stage, less LYN metastasis in bladder cancer patients. Regarding survival, circ-SMARCA5 high expression was correlated with shorter disease-free survival (DFS) and overall survival (OS); whereas, miR-432 high expression was correlated with longer DFS and OS in bladder cancer patients. Further multivariate Cox's regression analysis displayed that circ-SMARCA5 high expression was an independent predictive factor for both worse DFS and OS in bladder cancer patients. Conclusion: Circ-SMARCA5 high expression but miR-432 low expression is correlated with advanced tumor features and poor survival of bladder cancer patients, which present as potential prognostic markers in bladder cancer.
Keywords: circ-SMARCA5, miR-432, bladder cancer, tumor feature, survival
Introduction
Bladder cancer is a heterogeneous disease as well as one of the most prevalent malignancies worldwide with annual new cases of 400 000.1 Vast majority of bladder cancer arises from urothelial cells lining the bladder and urinary tract exposed to potentially mutagenic chemicals filtered from kidneys.2 For the 70% to 80% bladder cancers that are nonmuscle invasive, transurethral resection of bladder tumor (TURBT) is the preliminary treatment with the addition of intravascular cytotoxic therapy in high-risk cases.3 Meanwhile, in terms of the muscle invasive bladder cancer, radical cystectomy is still the main stay of treatment, while neoadjuvant or adjuvant chemoradiotherapy is often added to reduce the risk of recurrence.4 Although surgical approaches and the use of chemoradiotherapy have standardized the treatment of bladder cancer, not all patients benefit from each treatment due to heterogeneity of the disease, which puts forth the importance of individual treatment strategy of bladder cancer.
As the typical noncoding RNA family member, circular RNAs (circRNAs) are noted by covalently closed loops with no polarity or polyadenylated tail.5 CircRNAs are highly conserved and stable in eukaryotes, and recent advances in researches reveal their high disease specificities as potential molecular biomarkers in cancers due to their involvement in tumorigenesis and metastasis via regulating microRNAs (miRNAs).5,6 CircRNA SWI/SNF-related matrix-associated actin-dependent regulator of chromatin A5 (circ-SMARCA5) is encoded by gene SMARCA5 on chromosome 4.7 Studies reveal that circ-SMARCA5 involves in the development and progression of various cancers through regulating multiple tumor-promoting or suppressive miRNAs, for instance, circ-SMARCA5 directly binds miR-432, which is a tumor-suppressive gene in prostate cancer.8-13 As for bladder cancer, circ-SMARCA5 is reported to promote cancer cell proliferation, migration, invasion, while suppress cell apoptosis, which enlightened the oncogenic role of circ-SMARCA5.7 However, the clinical implication of circ-SMARCA5 still lacks validation in bladder cancer. Based on the tumorigenic function of circ-SMARCA5 on bladder cancer cells, and its direct binding relationship with miR-432,7,12 we hypothesized that circ-SMARCA5 intercorrelated with miR-432 in bladder cancer as well, and they might have prognostic potential in bladder cancer patients.
Therefore, we evaluated the expressions of circ-SMARCA5 and miR-432, then explored their correlations with clinical characteristics and survival in bladder cancer patients.
Methods
Patients
A total of 156 bladder cancer patients were retrospectively reviewed in this study. All patients were admitted to our hospital for TURBT or radical cystectomy from January 2015 to December 2019. The screening criteria were (1) confirmed as bladder cancer by pathology, (2) age above 18 years old but less than 80 years old, (3) without neoadjuvant therapy, (4) fresh-frozen tumor tissue samples excised from surgery were available and eligible for reverse transcription quantitative polymerase chain reaction (RT-qPCR) detecting, (5) clinicopathologic features were complete, (6) follow-up data were available, and (7) without other tumors. This study was approved by the Ethics Committee of our hospital, and the written informed consents were acquired from patients or their families.
Data and Sample Collection
The preoperative clinicopathologic features including age, gender, multiplicity, tumor size, tumor stage, lymph node (LYN) metastasis status (determined by pathologic examination), and pathological grade were extracted from the electronic medical records. The tumor stage was assessed based on the degree of tumor invasion according to the criteria of Tumor, Node, Metastasis (TNM) stage in Union for International Cancer Control (UICC) version 7. The pathological grade was assessed according to the 2004 World Health Organization (WHO) classification of urothelial neoplasms tumor. The disease status and survival status of patient were obtained from follow-up data, and the last follow-up date was April 30, 2020. Disease-free survival (DFS) was calculated from the date of surgery to the date of disease relapsed or death. Overall survival (OS) was calculated from the date of surgery to the date of death. A total of 156 fresh-frozen tumor tissues were required from the Pathology Department. In addition, only 71 cases out of 156 patients had available adjacent tissues, and the 71 fresh-frozen adjacent tissue samples were obtained from the Pathology Department as well.
Circ-SMARCA5 and miR-432 Detection
The relative expressions of circ-SMARCA5 and miR-432 in tumor tissue and adjacent tissue were detected by the RT-qPCR. TRIzol™ Reagent (Thermo Fisher Scientific) was used to extract the total RNAs according to the manufacturer's instructions. Then the RNAs were delinearized using the RNase R (Epicentre) for the following detection of circ-SMARCA5, but not for miR-432 detection. After that, RNAs were reversely transcribed by the iScript™ cDNA Synthesis Kit (Bio-Rad) to cDNAs, and then underwent PCR using the THUNDERBIRD® SYBR® qPCR Mix (Toyobo). GAPDH was used as an internal reference for circ-SMARCA5 and U6 was used for miR-432. The primers were designed according to a previous study.7 Circ-SMARCA5 forward (5′-3′): AGATGGGCGAAAGTTCACTTAGA, reverse (5′-3′): GATTCTGATCCACAAGCCTCCT; miR-432 forward (5′-3′): ACACTCCAGCTGGGTCTTGGAGTAGGTCAT, reverse (5′-3′): TGTCGTGGAGTCGGCAATTC; GAPDH forward (5′-3′): GACCACAGTCCATGCCATCAC, reverse (5′-3′): ACGCCTGCTTCACCACCTT; U6 forward (5′-3′): CTCGCTTCGGCAGCACATATACTA, reverse (5′-3′): ACGAATTTGCGTGTCATCCTTGC. The binding site between circ-SMARCA5 and miR-432 was analyzed using the RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid), and presented in Supplemental Figure 1.
Statistical Analyses
Statistical analyses were performed using the SPSS 24.0 (IBM). Figures were made using the GraphPad Prism 8.01 (GraphPad Software Inc.). Comparison between 2 groups was determined by the Wilcoxon rank-sum test. Correlation was determined by Spearman's rank correlation test. DFS and OS were calculated and displayed using the Kaplan–Meier curve, and their differences between the 2 groups were analyzed by the Log-rank test. Factors related to DFS and OS were analyzed by the univariate and forward stepwise multivariate Cox's proportional hazard regression model. P value <.05 was considered as significant.
Results
Patients’ Characteristics
The mean age was 61.8 ± 10.5 years of bladder cancer patients, among whom 36 (23.1%) were female and 120 (76.9%) were male (Table 1). For tumor multiplicity, 106 (67.9%) patients were single and 50 (32.1%) patients were multiple. The mean tumor size was 2.5 ± 1.2 cm and 108 (69.2%) patients were at stage Ta and T1 and 48 (30.8%) patients were at T2 to T4. In addition, there were 13 (8.3%) patients with LYN metastasis; the patients with low and high pathological grades were 96 (61.5%) and 60 (38.5%), respectively.
Table 1.
Clinicopathologic Features.
| Items | Bladder cancer patients (N = 156) |
|---|---|
| Age (years), mean ± SD | 61.8 ± 10.5 |
| Gender, No. (%) | |
| Female | 36 (23.1) |
| Male | 120 (76.9) |
| Multiplicity, No. (%) | |
| Single | 106 (67.9) |
| Multiple | 50 (32.1) |
| Tumor size (cm), mean ± SD | 2.5 ± 1.2 |
| Tumor stage, No. (%) | |
| Ta&T1 | 108 (69.2) |
| T2-T4 | 48 (30.8) |
| LYN metastasis, No. (%) | |
| No | 143 (91.7) |
| Yes | 13 (8.3) |
| Pathological grade, No. (%) | |
| Low | 96 (61.5) |
| High | 60 (38.5) |
Abbreviations: SD, standard deviation; LYN, lymph node.
Circ-SMARCA5 and miR-432 in Bladder Cancer Patients
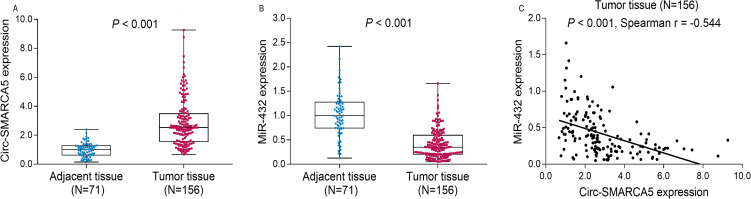
Circ-SMARCA5 expression was increased in tumor tissues (2.528 [1.534-3.487]) compared with adjacent tissue (1.000 [0.625-1.276]) (P < .001; Figure 1A), whereas miR-432 expression was reduced in tumor tissues (0.349 [0.205-0.595]) compared with adjacent tissues (1.000 [0.744-1.276]; P < .001) (Figure 1B). Besides, in bladder cancer tissues, circ-SMARCA5 was negatively correlated with miR-432 expression (P < .001, Spearman r = −0.544; Figure 1C).
Figure 1.
Expressions of circ-SMARCA5 and miR-432 in bladder cancer. Comparison of circ-SMARCA5 expression between tumor tissue and adjacent tissue (A). Comparison of miR-432 expression between tumor tissue and adjacent tissue (B). Correlation between circ-SMARCA5 and miR-432 in bladder cancer tissue (C).
Abbreviations: Circ-SMARCA5, circular RNA SWI/SNF-related matrix-associated actin-dependent regulator of chromatin A5; miR-432, microRNA 432.
Correlations of Circ-SMARCA5 and miR-432 with Patients’ Characteristics
Circ-SMARCA5 relative expression higher/lower than the mean expression in tumor tissue was determined as high/low, and the same for miR-432. Circ-SMARCA5 high expression was correlated with larger tumor size (P = .016), higher tumor stage (P = .015), LYN metastasis (P = .043), but not age (P = .188), gender (P = .254), multiplicity (P = .172), or pathological grade (P = .101) in bladder cancer patients (Table 2). As for miR-432, its high expression was correlated with single multiplicity (P = .040), smaller tumor size (P = .042), lower tumor stage (P = .005), reduced LYN metastasis (P = .043), but not age (P = .742), gender (P = .704), or pathological grade (P = .190) in bladder cancer patients (Table 2).
Table 2.
Correlations of Circ-SMARCA5 and miR-432 with Clinicopathologic Features.
| Items | Circ-SMARCA5 | P value | MiR-432 | P value | ||
|---|---|---|---|---|---|---|
| Low (n = 78) | High (n = 78) | Low (n = 78) | High (n = 78) | |||
| Age (years), No. (%) | .188 | .742 | ||||
| <60 years | 34 (43.6) | 26 (33.3) | 31 (39.7) | 29 (37.2) | ||
| ≥60 years | 44 (56.4) | 52 (66.7) | 47 (60.3) | 49 (62.8) | ||
| Gender, No. (%) | .254 | .704 | ||||
| Female | 15 (19.2) | 21 (26.9) | 19 (24.4) | 17 (21.8) | ||
| Male | 63 (80.8) | 57 (73.1) | 59 (75.6) | 61 (78.2) | ||
| Multiplicity, No. (%) | .172 | .040 | ||||
| Single | 57 (73.1) | 49 (62.8) | 47 (60.3) | 59 (75.6) | ||
| Multiple | 21 (26.9) | 29 (37.2) | 31 (39.7) | 19 (24.4) | ||
| Tumor size (cm), No. (%) | .016 | .042 | ||||
| <3 cm | 65 (83.3) | 52 (66.7) | 53 (67.9) | 64 (82.1) | ||
| ≥3 cm | 13 (16.7) | 26 (33.3) | 25 (32.1) | 14 (17.9) | ||
| Tumor stage, No. (%) | .015 | .005 | ||||
| Ta&T1 | 61 (78.2) | 47 (60.3) | 46 (59.0) | 62 (79.5) | ||
| T2-T4 | 17 (21.8) | 31 (39.7) | 32 (41.0) | 16 (20.5) | ||
| LYN metastasis, No. (%) | .043 | .043 | ||||
| No | 75 (96.2) | 68 (87.2) | 68 (87.2) | 75 (96.2) | ||
| Yes | 3 (3.8) | 10 (12.8) | 10 (12.8) | 3 (3.8) | ||
| Pathological grade, No. (%) | .101 | .190 | ||||
| Low | 53 (67.9) | 43 (55.1) | 44 (56.4) | 52 (66.7) | ||
| High | 25 (32.1) | 35 (44.9) | 34 (43.6) | 26 (33.3) | ||
Correlation was determined by Spearman's rank correlation test.
Abbreviations: circ-SMARCA5, circular RNA SMARCA5; miR-432, microRNA-432; LYN, lymph node.
Correlations of Circ-SMARCA5 and miR-432 With Survival of Bladder Cancer Patients
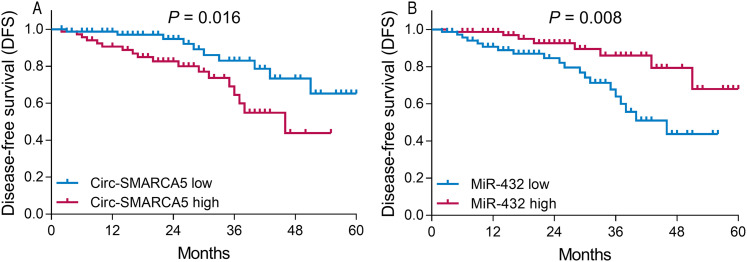
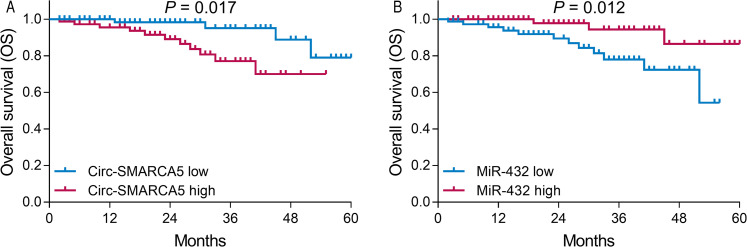
Patients with circ-SMARCA5 high expression presented shorter DFS than those with circ-SMARCA5 low expression (P = .016; Figure 2A). Whereas patients with miR-432 high expression presented longer DFS than those with miR-432 low expression (P = .008; Figure 2B). As for OS, patients with circ-SMARCA5 high expression had shorter OS compared with patients with circ-SMARCA5 low expression (P = .017; Figure 3A), while patients with miR-432 high expression had longer OS compared with those with miR-432 low expression (P = .012; Figure 3B).
Figure 2.
Correlation of circ-SMARCA5 and miR-432 with DFS. Comparison of DFS between circ-SMARCA5 high expression patients and circ-SMARCA5 low expression patients (A). Comparison of DFS between miR-432 high expression patients and miR-432 low expression patients (B).
Abbreviations: DFS, disease-free survival; Circ-SMARCA5, circular RNA SWI/SNF-related matrix-associated actin-dependent regulator of chromatin A5; miR-432, microRNA 432.
Figure 3.
Correlation of circ-SMARCA5 and miR-432 with OS. Comparison of OS between circ-SMARCA5 high expression patients and circ-SMARCA5 low expression patients (A). comparison of OS between miR-432 high expression patients and miR-432 low expression patients (B).
Abbreviations: OS, overall survival; Circ-SMARCA5, circular RNA SWI/SNF-related matrix-associated actin-dependent regulator of chromatin A5; miR-432, microRNA 432.
Factors Affecting Survival of Bladder Cancer Patients
The univariate Cox's regression analysis revealed that circ-SMARCA5 high expression (P = .020, hazard ratio [HR] = 2.554), multiplicity multiple (P = .011, HR = 2.633), larger tumor size (P = .028, HR = 2.348), higher tumor stage (P = .001, HR = 3.520), LYN metastasis (P = .017, HR = 3.315), and high pathological grade (P = .006, HR = 3.092) predicted more favorable DFS, while miR-432 high expression predicted less favorable DFS (P = .011, HR = 0.346) (Table 3). Further forward stepwise multivariate Cox's regression displayed that circ-SMARCA5 high expression (P = .032, HR = 2.379) and higher tumor stage (P = .002, HR = 3.368) were independent predictive factors for shorter DFS.
Table 3.
Analysis of Factors Predicting DFS.
| Items | Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| Circ-SMARCA5 expression (high vs low) | .020 | 2.554 | 1.160 | 5.626 |
| MiR-432 expression (high vs low) | .011 | 0.346 | 0.152 | 0.787 |
| Age (≥60 years vs <60 years) | .176 | 1.810 | 0.767 | 4.269 |
| Gender (male vs female) | .448 | 1.508 | 0.522 | 4.356 |
| Multiplicity (multiple vs single) | .011 | 2.633 | 1.248 | 5.557 |
| Tumor size (≥3.0 cm vs <3.0 cm) | .028 | 2.348 | 1.098 | 5.023 |
| Tumor stage (T2-T4 vs Ta&T1) | .001 | 3.520 | 1.655 | 7.486 |
| LYN metastasis (yes vs no) | .017 | 3.315 | 1.244 | 8.835 |
| Pathological grade (high vs low) | .006 | 3.092 | 1.391 | 6.877 |
| Forward stepwise multivariate Cox's regression | ||||
| Circ-SMARCA5 expression (high vs low) | .032 | 2.379 | 1.078 | 5.253 |
| Tumor stage (T2-T4 vs Ta&T1) | .002 | 3.368 | 1.572 | 7.214 |
Factors affecting DFS were analyzed by the univariate and forward stepwise multivariate Cox's proportional hazard regression model.
Abbreviations: DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; circ-SMARCA5, circular RNA SMARCA5; miR-432, microRNA-432; LYN, lymph node.
In regard to predictive factors for OS, circ-SMARCA5 high expression (P = .025, HR = 3.788), multiplicity multiple (P = .016, HR = 3.565), larger tumor size (P = .024, HR = 3.220), higher tumor stage (P = .006, HR = 4.547), LYN metastasis (P = .009, HR = 4.742), and high pathological grade (P = .029, HR = 3.593) predicted decreased OS, while miR-432 high expression (P = .021, HR = 0.225) predicted increased OS (Table 4). The further forward stepwise multivariate Cox's regression displayed that circ-SMARCA5 high expression (P = .044, HR = 3.370) and higher tumor stage (P = .010, HR = 4.126) independently predicted shorter OS.
Table 4.
Analysis of Factors Predicting OS.
| Items | Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| Circ-SMARCA5 expression (high vs low) | .025 | 3.788 | 1.178 | 12.179 |
| MiR-432 expression (high vs low) | .021 | 0.225 | 0.063 | 0.801 |
| Age (≥60 years vs <60 years) | .225 | 2.191 | 0.617 | 7.779 |
| Gender (male vs female) | .531 | 1.611 | 0.363 | 7.151 |
| Multiplicity (multiple vs single) | .016 | 3.565 | 1.263 | 10.067 |
| Tumor size (≥3.0 cm vs <3.0 cm) | .024 | 3.220 | 1.165 | 8.898 |
| Tumor stage (T2-T4 vs Ta&T1) | .006 | 4.547 | 1.551 | 13.336 |
| LYN metastasis (yes vs no) | .009 | 4.742 | 1.480 | 15.190 |
| Pathological grade (high vs low) | .029 | 3.593 | 1.141 | 11.315 |
| Forward stepwise multivariate Cox's regression | ||||
| Circ-SMARCA5 expression (high vs low) | .044 | 3.370 | 1.035 | 10.986 |
| Tumor stage (T2-T4 vs Ta&T1) | .010 | 4.126 | 1.396 | 12.192 |
Factors affecting OS were analyzed by the univariate and forward stepwise multivariate Cox's proportional hazard regression model.
Abbreviations: OS, overall survival; HR, hazard ratio; CI, confidence interval; circ-SMARCA5, circular RNA SMARCA5; miR-432, microRNA-432; LYN, lymph node.
Discussion
CircRNAs, a group of covalently closed RNA molecules, are produced by backsplicing from pre-mRNAs.14 Compared to their linear counterparts, circRNAs present a highly stable structure, therefore, the detection of circRNAs is more accessible in various samples, and the most studied property of circRNAs is their ability to inhibit miRNA via direct sponging.5 In cancer researches, circRNAs and their target miRNAs have been extensively investigated as potential biomarkers.14 From our observation in the present study, circ-SMARCA5 was overexpressed in bladder cancer tissues compared with adjacent tissues, and was negatively correlated with miR-432 expression in bladder cancer tissues. Moreover, circ-SMARCA5 high expression but miR-432 low expression was correlated with advanced tumor features, and circ-SMARCA5 was an independent predictive factor for poor survival in bladder cancer patients.
Circ-SMARCA5 has been revealed to involve in the progression of various cancers including prostate cancer, hepatocellular carcinoma, nonsmall cell lung cancer, cervical cancer, and gastric cancer.8-10,15 However, the role of circ-SMARCA5 in different cancers varies. For instance, it is upregulated and promotes proliferation in prostate cancer cells, while inhibits cervical cancer cell proliferation, migration, invasion, and induces cell cycle arrest.8,10 In addition, circ-SMARCA5 deteriorates prostate cancer via directly targeting miR-432/programmed cell death 10 (PDCD10) axis.12 As for bladder cancer, there has been only one experimental study reporting that circ-SMARCA5 is overexpressed in bladder cancer tissues, and it promotes cell proliferation, migration, invasion while inhibits cell apoptosis.7 Hence, we further investigated the correlation of circ-SMARAC5 with clinical features as well as survival in bladder cancer patients. We observed that circ-SMARCA5 was upregulated in tumor tissues compared with adjacent tissues, which was in accordance with the previous evidence.7 Moreover, miR-432 was downregulated in tumor tissues, and it was negatively correlated with circ-SMARCA5 expression. This might derive from that circ-SMARCA5 acted as a sponge to miR-432 in bladder cancer, therefore, their expressions were negatively correlated with each other. In addition, circ-SMARCA5 high expression but miR-432 low expression was correlated with advanced tumor features such as larger tumor size, higher tumor stage, and LYN metastasis, which could be explained by the following possible facts: (1) circ-SMARCA5 promoted cell proliferation, repressed cell apoptosis, facilitates cell migration, and enhanced cell invasion, thereby deteriorated the tumor features in bladder cancer patients.7 (2) Upregulation of circ-SMARCA5 might serve as a pool and increase the expression of its upstream gene SMARCA5 that damages DNA repair and promoted chromosomal instability in bladder cancer cells, thereby promoted tumor progression.13 (3) As the target of circ-SMARCA5, the downregulation of miR-432 was reported to activate signaling pathways such as Wnt/β-catenin and PI3K/AKT signaling pathways, thus, facilitated the tumor progression of bladder cancer.13,16,17
Although the molecular function of circ-SMARCA5 and miR-432 in bladder cancer has been partially shown, their possible clinical implications remain to be elusive. The previous report of other cancers observes that circ-SMARCA5, being tumor suppressive in intrahepatic cholangiocarcinoma, correlates with less severe clinical tumor features and prognosis.15 Therefore, we proposed that since circ-SMARCA5 promoted tumor progression, it might correlate with unfavorable survival in bladder cancer patients, and the opposite for miR-432. From our analysis, circ-SMARCA5 high expression but miR-432 low expression was correlated with shorter OS and DFS, and circ-SMARCA5 was an independent predictive factor for poor survival in bladder cancer patients. These findings were in accordance with our hypothesis, and here were some possible explanations: (1) circ-SMARCA5 high expression and miR-432 low expression were correlated with advanced tumor features, which might lead to poor treatment outcomes and thus an unfavorable survival. (2) Upregulated circ-SMARCA5 might harm chemotherapy sensitivity of bladder cancer cells like it was observed in intrahepatic cholangiocarcinoma, then worsened the response for adjuvant chemotherapy as well as prognosis of bladder cancer patients.15 However, the influence of circ-SMARCA5 on drug sensitivity in bladder cancer needed to be validated by further experiments. (3) The circ-SMARCA5/miR-432 axis might activate the aforementioned signaling pathways and promoted cell invasion, metastasis, thereby increased the tumor relapse after surgery.16-18
As the initial clinical investigation of circ-SMARCA5 and its target miR-432 in bladder cancer, the present study revealed their correlations with clinicopathological features and survival in bladder cancer patients, whereas limitations existed in this study. To note, the follow-up duration was relatively short in this study, therefore the associations of circ-SMARCA5 and miR-432 with survival of bladder cancer patients needed to be observed with prolonged follow-up. In addition, as a clinical study, the molecular mechanisms of circ-SMARCA5 and miR-432 were not investigated. Furthermore, this was a retrospective study, hence the influence of circ-SMARCA5 or miR-432 on prognosis could be better evaluated by prospective studies. Finally, a large-scaled and multicentered clinical study was needed to further validate our findings.
In conclusion, circ-SMARCA5 is upregulated but miR-432 is downregulated in bladder cancer, and circ-SMARCA5 high expression but miR-432 low expression is correlated with unfavorable tumor features as well as poor prognosis in bladder cancer patients.
Supplemental Material
Supplemental material, sj-tif-1-tct-10.1177_15330338211039110 for Negative Correlation Between Circular RNA SMARC5 and MicroRNA 432, and Their Clinical Implications in Bladder Cancer Patients by Zhijia Zhang, Yanxia Sang, Zhengan Liu and Jinkai Shao in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-2-tct-10.1177_15330338211039110 for Negative Correlation Between Circular RNA SMARC5 and MicroRNA 432, and Their Clinical Implications in Bladder Cancer Patients by Zhijia Zhang, Yanxia Sang, Zhengan Liu and Jinkai Shao in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338211039110 for Negative Correlation Between Circular RNA SMARC5 and MicroRNA 432, and Their Clinical Implications in Bladder Cancer Patients by Zhijia Zhang, Yanxia Sang, Zhengan Liu and Jinkai Shao in Technology in Cancer Research & Treatment
Acknowledgments
We would like to thank the language editing company Shanghai Qeejen Bio-tech Institution for their contribution to language polish, format editing, and submission of the article.
Abbreviations
- circRNAs
circular RNAs
- circ-SMARCA5
CircRNA SWI/SNF-related matrix-associated actin-dependent regulator of chromatin A5
- DFS
disease-free survival
- LYN
lymph node
- miRNAs
microRNAs
- OS
overall survival
- TURBT
transurethral resection of the bladder tumor
- UICC
Union for International Cancer Control
- WHO
World Health Organization
Footnotes
Authors’ Note: This study was approved by the Ethics Committee of our hospital (approval no. 20191202), and the written informed consents were acquired from patients or their families.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jinkai Shao https://orcid.org/0000-0002-4404-1125
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96-108. [DOI] [PubMed] [Google Scholar]
- 2.Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). 2020;8(1):15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(UK) NCCfC. Bladder cancer: diagnosis and management. Chir. Prax. 2015;75(1):139-150. [Google Scholar]
- 4.Nandagopal L, Sonpavde G. Circulating biomarkers in bladder cancer. Bladder Cancer. 2016;2(4):369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Yuan W, Yang X, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Wu Z, Wang Y, et al. Downregulated hsa_circ_0077837 and hsa_circ_0004826, facilitate bladder cancer progression and predict poor prognosis for bladder cancer patients. Cancer Med. 2020;9(11):3885-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Y, Zhang T, Liang C. Circular RNA SMARCA5 is overexpressed and promotes cell proliferation, migration as well as invasion while inhibits cell apoptosis in bladder cancer. Transl Cancer Res. 2019;8(5):1663-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong Z, Wan X, Zhang Y, et al. Androgen-responsive circular RNA circSMARCA5 is up-regulated and promotes cell proliferation in prostate cancer. Biochem Biophys Res Commun. 2017;493(3):1217-1223. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Xu QG, Wang ZG, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68(6):1214-1227. [DOI] [PubMed] [Google Scholar]
- 10.Tian JDC, Liang L. Involvement of circular RNA SMARCA5/microRNA-620 axis in the regulation of cervical cancer cell proliferation, invasion and migration. Eur Rev Med Pharmacol Sci. 2018;22(24):8589-8598. [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Chen Z, Zuo X. circSMARCA5 functions as a diagnostic and prognostic biomarker for gastric cancer. Dis Markers. 2019;2019:2473652. 10.1155/2019/2473652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C, Fan B, Ren Z, Liu B, Wang Y. CircSMARCA5 facilitates the progression of prostate cancer through miR-432/PDCD10 axis. Cancer Biother Radiopharm. 2020;36(1):70-83. [DOI] [PubMed] [Google Scholar]
- 13.Jin Q, Mao X, Li B, Guan S, Yao F, Jin F. Overexpression of SMARCA5 correlates with cell proliferation and migration in breast cancer . Tumour Biol. 2015;36(3):1895-1902. [DOI] [PubMed] [Google Scholar]
- 14.Vo JN, Cieslik M, Zhang Y, et al. The landscape of circular RNA in cancer. Cell. 2019;176(4):869-881.e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Q, Fang T. Circular RNA SMARCA5 correlates with favorable clinical tumor features and prognosis, and increases chemotherapy sensitivity in intrahepatic cholangiocarcinoma. J Clin Lab Anal. 2020;34(4):e23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang N, Chen WJ, Zhang JW, et al. Downregulation of miR-432 activates Wnt/beta-catenin signaling and promotes human hepatocellular carcinoma proliferation. Oncotarget. 2015;6(10):7866-7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Lu C, Zhou Y, Zhang Z, Sun L. Circular RNA hsa_circ_0008039 promotes breast cancer cell proliferation and migration by regulating miR-432-5p/E2F3 axis. Biochem Biophys Res Commun. 2018;502(3):358-363. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Cao X, Dong D, et al. Circular RNA TTN acts as a miR-432 sponge to facilitate proliferation and differentiation of myoblasts via the IGF2/PI3K/AKT signaling pathway. Mol Ther Nucleic Acids. 2019;18:966-980. 10.1016/j.omtn.2019.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tct-10.1177_15330338211039110 for Negative Correlation Between Circular RNA SMARC5 and MicroRNA 432, and Their Clinical Implications in Bladder Cancer Patients by Zhijia Zhang, Yanxia Sang, Zhengan Liu and Jinkai Shao in Technology in Cancer Research & Treatment
Supplemental material, sj-jpg-2-tct-10.1177_15330338211039110 for Negative Correlation Between Circular RNA SMARC5 and MicroRNA 432, and Their Clinical Implications in Bladder Cancer Patients by Zhijia Zhang, Yanxia Sang, Zhengan Liu and Jinkai Shao in Technology in Cancer Research & Treatment
Supplemental material, sj-docx-3-tct-10.1177_15330338211039110 for Negative Correlation Between Circular RNA SMARC5 and MicroRNA 432, and Their Clinical Implications in Bladder Cancer Patients by Zhijia Zhang, Yanxia Sang, Zhengan Liu and Jinkai Shao in Technology in Cancer Research & Treatment