ABSTRACT
Temperature variation—through time and across climatic gradients—affects individuals, populations, and communities. Yet how the thermal response of biological systems is altered by environmental stressors is poorly understood. Here, we quantify two key features—optimal temperature and temperature breadth—to investigate how temperature responses vary in the presence of antibiotics. We use high-throughput screening to measure growth of Escherichia coli under single and pairwise combinations of 12 antibiotics across seven temperatures that range from 22°C to 46°C. We find that antibiotic stress often results in considerable changes in the optimal temperature for growth and a narrower temperature breadth. The direction of the optimal temperature shifts can be explained by the similarities between antibiotic-induced and temperature-induced damage to the physiology of the bacterium. We also find that the effects of pairs of stressors in the temperature response can often be explained by just one antibiotic out of the pair. Our study has implications for a general understanding of how ecological systems adapt and evolve to environmental changes.
IMPORTANCE The growth of living organisms varies with temperature. This dependence is described by a temperature response curve that is described by an optimal temperature where growth is maximized and a temperature range (termed breadth) across which the organism can grow. Because an organism’s temperature response evolves or acclimates to its environment, it is often assumed to change over only evolutionary or developmental timescales. Counter to this, we show here that antibiotics can quickly (over hours) change the optimal growth temperature and temperature breadth for the bacterium Escherichia coli. Moreover, our results suggest a shared-damage hypothesis: when an antibiotic damages similar cellular components as hot (or cold) temperatures do, this shared damage will combine and compound to more greatly reduce growth when that antibiotic is administered at hot (or cold) temperatures. This hypothesis could potentially also explain how temperature responses are modified by stressors other than antibiotics.
KEYWORDS: Escherichia coli, antibiotic resistance, antibiotics, climate change, environmental microbiology, microbial ecology, multiple stressors, systems biology, temperature, thermal response
INTRODUCTION
Many environments experience daily and seasonal temperature fluctuations that affect rates of physiological processes. These changes in turn affect biological and ecological traits and ultimately impact the behavior of communities (1–10). In this manner temperature fluctuations can drive the evolution of organisms through variation in thermal sensitivity—the ability to function and survive at different temperatures (2, 11–16).
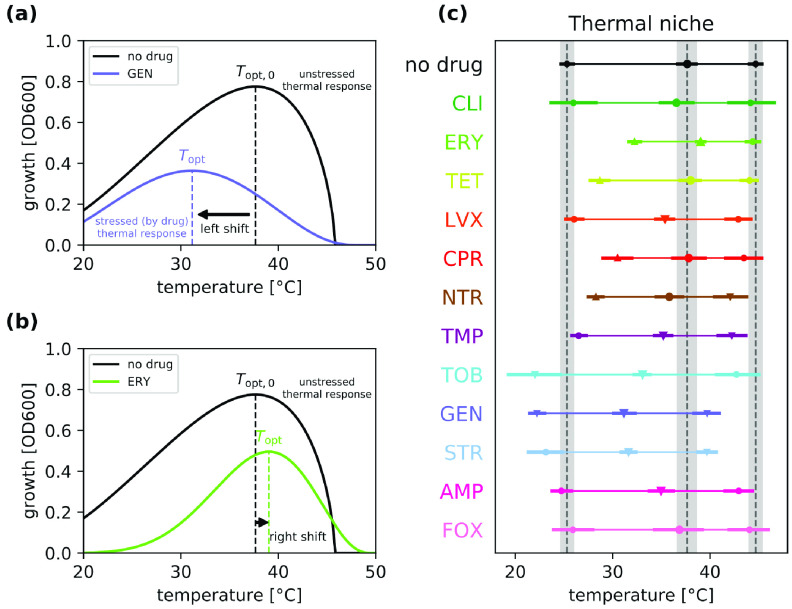
Measuring the growth of a living organism at different temperatures yields a temperature response curve (Fig. 1a). Typically, temperature response curves have a single peak, corresponding to an optimal temperature where growth is maximized (2). As the temperature changes away from the optimum in either direction, the growth rate decreases, with an especially steep decline at higher temperatures. The range of temperatures in which an organism can grow to a certain extent (e.g., at least half of the maximum growth) is termed the temperature breadth. Living organisms are said to experience either cold or heat stress at extreme temperatures where growth is substantially less than optimum.
FIG 1.
Temperature response curves change under antibiotic stress. (a) An example of a left shift of optimal temperature with antibiotic GEN. (b) An example of a right shift of optimal temperature with antibiotic ERY. (c) Optimal growth temperature (middle marker) and temperature niche (thin line joining the half-maximal growth temperatures, left and right markers) observed under each antibiotic used in this study. Point estimates for the optimal and half-maximal growth temperatures are shown as markers (circles or triangles). To show the uncertainty in the estimates, 95% credible intervals (CIs; see Materials and Methods) are drawn as thick lines. The CIs for the no-drug condition are shaded in the plot to facilitate comparison. The markers indicate whether the CI under the corresponding antibiotic overlaps the CI of the control condition (circles) or not (triangles). When the marker is a triangle, it points upward if the estimated parameter is higher than the control condition and downward if it is lower.
Thermal response curves are fundamental to grasping the variability of physiological and ecological traits in response to temperature changes in different taxonomic groups and habitats. Because shifts in the thermal response curves are representative of average fitness performance and temporal niches (17), optimal temperature and thermal breadth are indicative of evolution and acclimation patterns based on how species’ performance contributes to survivorship or fecundity (15). For instance, seasonal variation in temperature could lead to an evolution of different attack and escape speeds that would allow individuals to perform best when they are predator or prey (18). Another example is given by the Micromonas genus of marine phytoplankton, where niche partitioning has been observed, with various thermal responses according to their geographic location (19).
At a cellular level the performance of an organism across different temperatures can lead to various genetic and physiological adaptation mechanisms. For example, in bacteria thermal sensitivity is related to many physiological and genetic modulations in metabolism, including outer membrane rigidity (20, 21), chemotaxis (22, 23), enzymatic thermostability (24, 25), and other general adaptive responses (26, 27). Moreover, the heat shock response—a cellular mechanism to deal with the deleterious effects of high temperatures, such as protein misfolding and aggregation—is highly conserved in both prokaryotes and eukaryotes (27, 28). Understanding responses to temperature changes is important to infer general patterns of how organisms, species, communities, and ecosystems are adapting to fluctuations in climate patterns and different environmental conditions.
Any environmental feature that kills a living organism or reduces its growth can be considered a stressor. Temperature can interact with other environmental stressors such as light, precipitation, pH, and salinity. Exposure to different stressor types and intensities can lead to phenotypic variation in an organism’s ability to respond to temperature changes (29, 30). Nevertheless, how the effects of environmental stressors interact with temperature responses is not well understood. Therefore, insights on whether temperature responses—as described by optimal temperatures and temperature breadths—can change rapidly and plastically in the presence of other environmental stressors have been lacking. In fact, it has been commonly assumed that thermal responses are not altered in the presence of other stressors (31–33).
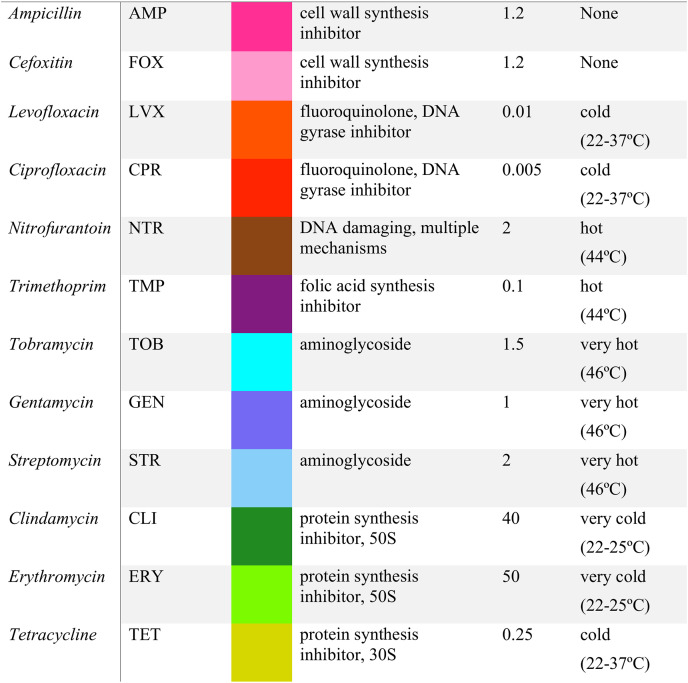
A systematic approach that informs how optimal temperatures and temperature breadths are shifted by stressors (Fig. 1) is needed to uncover these ambiguities and provide additional insights on fitness tradeoffs and thermal adaptation strategies. Here, we use a combined empirical-theoretical approach to study if the characteristics of thermal response curves change in response to additional environmental stressors. In particular, we use an experimental system of Escherichia coli and antibiotics as stressors in order to investigate how a physiological trait—growth of the bacterium—responds to variation in temperature in the presence of different stressor conditions. We obtain temperature response data for E. coli in 12 single-drug and 66 two-drug combination environments, where antibiotics are chosen to cover a wide range of mechanisms of action (Table 1). We then quantify both the optimal temperature and the temperature breadth of E. coli in the presence of these different environments.
TABLE 1.
List of antibioticsa
The antibiotics used are listed with their abbreviation, mechanism of action, dose, and our color scheme throughout the paper. Similar colors are chosen for drugs belonging to the same class/mechanism of action. For example, blue tones are chosen for aminoglycosides. The similarity of each antibiotic to temperature stress according to their interactions with other stressors (34) is also shown, along with the corresponding range of growth temperatures that show similarity. For the purposes of cold/heat similarity, we consider any antibiotic with a similar temperature range lower than the optimum as cold similar. For example, there are two groups of cold-similar antibiotics, which we call cold (22 to 37°C) and very cold (22 to 25°C). These terms are used to distinguish the groups by the relative strength of the cold stress to which they are similar but not necessarily the severity of the cold stress in an absolute sense.
We first show that individual stressors can have a substantial impact on the optimal temperature and temperature breadth. Next, we evaluate if the directions of the shifted thermal responses are related to the mechanism of action of the antibiotics. Previously, we determined that some specific classes of antibiotics have similar physiological effects as either heat or cold stress in E. coli (34). This classification was based on comparing the experimentally determined interaction profile (synergies and antagonisms with other stressors) of antibiotics and various growth temperatures (Table 1). We find that in most cases the direction of the shifts in the thermal responses under antibiotic stress can be explained through these groups. Lastly, we investigate how pairs of stressors move optimal temperatures in different directions compared to the optimal temperatures under single-stressor conditions. In particular, we evaluate the extent to which the optimal temperatures result from integrated effects of both stressors, or whether a single stressor is the key driver of the temperature response. We infer from our results that a single drug often plays a dominant role in determining the optimal temperature response of a combined treatment.
Our experimental and theoretical framework on temperature response curves of E. coli presented here allows us to better understand how thermal sensitivities change in response to stressors. Therefore, our analysis will shed light on fundamental features shaping the ecological and evolutionary responses of organisms facing complex environmental conditions. By using antibiotics as stressors and a bacterium as a model organism, our study system is particularly valuable for its experimental tractability and reproducibility.
(This article was submitted to an online preprint archive [35].)
RESULTS
In this paper we investigate how different stressors (antibiotics) alter an organism’s response to temperature, both in isolation and in combination. To do this, we determine the temperature optimum and temperature niche/breadth of E. coli by fitting the modified Briere model (developed here, see Materials and Methods) to experimental data of bacterial growth collected under different (unstressed and stressed) growth environments across seven temperatures (22°C, 25°C, 30°C, 37°C, 41°C, 44°C, and 46°C). The entire data set and model fits are shown in Fig. S1 in the supplemental material. More details about the model and fitting procedure can be found in Materials and Methods and Text S1.
Includes additional methodological details regarding the modified Briere model for temperature responses and the statistical approaches used in this paper. Download Text S1, PDF file, 0.2 MB (207.1KB, pdf) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Full data set and model fits. (Lower half) The growth data for all antibiotic combinations (blue dots) and the fitted modified Briere model (black lines) are shown, as well as 95% credible intervals for the modified Briere model. The upper left corner corresponds to the no-drug case. (Upper half) The fitted curves corresponding to each drug combination are shown in black, and the fits corresponding to each single drug are shown in their corresponding color. In the uppermost row, the growth curve in the absence of antibiotics is shown in black and the single-drug curves are shown in the corresponding color. The entries corresponding to FOX and STR+FOX in the upper quadrant are labeled to aid interpretation. Download FIG S1, EPS file, 2.9 MB (2.9MB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
First, we explore how the optimal growth temperature of E. coli changes under single-stressor conditions (Fig. 1a and b). We find that the majority of the single-drug environments exhibit left shifts—meaning the optimal temperature (Topt) is lower (Fig. 1c)—compared to the no-drug condition, Topt = 37.7°C, 95% credible interval (CI) (36.7°C, 38.6°C). Right shifts are both less common and of lower magnitude than the observed left shifts. In addition, we find that the thermal niche breadth typically becomes narrower under antibiotic stress, meaning that E. coli can survive and properly function at a reduced temperature range.
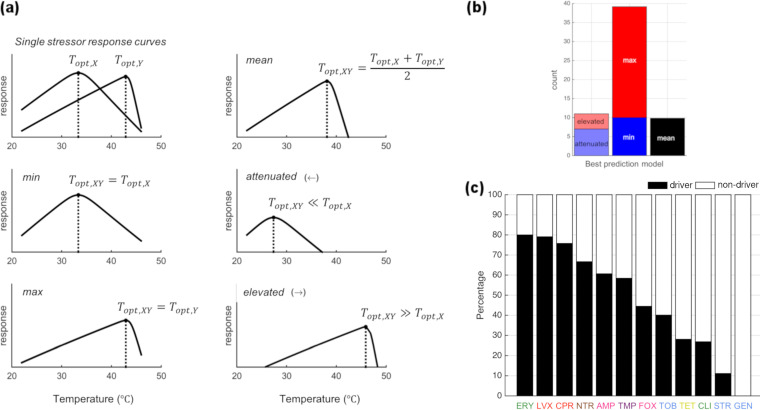
Next, we investigate whether the physiological effects of antibiotics bear any relation to the direction of the observed shifts in the temperature responses (Fig. 2). To do this, we group the antibiotics according to the similarity of their physiological effects to those of low or high temperatures, as determined previously (34). We observe the direction of the shifts for both single drugs (Fig. 2a, left panels) and drug combinations that contain one or more of the antibiotics in the group (Fig. 2a, right panels). We find that—for both single drugs and combinations—cold-similar antibiotics (i.e., with effects on bacteria similar to those caused by low temperatures) tend to either leave the optimal temperature unchanged or shift it slightly to the right (i.e., to higher optimal temperatures). In contrast, heat-similar antibiotics (i.e., with effects on bacteria similar to those caused by high temperatures) tend to result in unchanged optimal temperatures or shifts to the left (i.e., to lower optimal temperatures). In fact, bacteria exposed to aminoglycosides (tobramycin [TOB], gentamicin [GEN], and streptomycin [STR]), which induce misfolding of membrane proteins and have similar physiological effects as very high temperatures (Fig. 2), show the greatest shifts toward the left. This is not the case for other protein synthesis inhibitors such as erythromycin (ERY) or clindamycin (CLI) that are similar to cold. Interestingly, beta-lactams shift the temperature curves in a similar way as heat-similar drugs when used in combinations, despite them having a different mechanism of action (inhibition of cell wall synthesis) that was not found to be heat similar.
FIG 2.
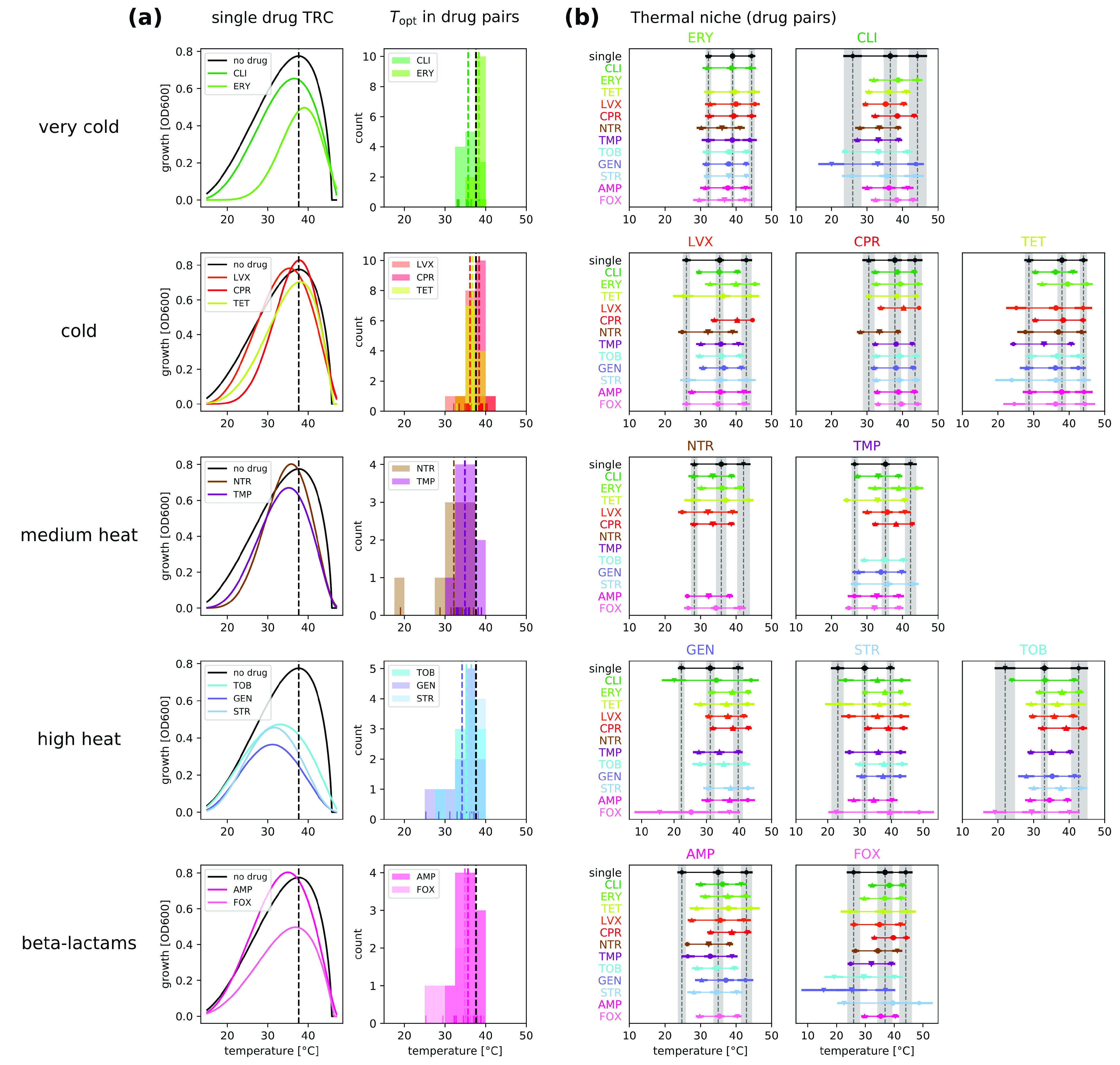
Physiological effects of antibiotics predict the direction of shifts in the optimal temperature. (a) (Left) The fitted temperature response curve (TRC) in the presence of single antibiotics is compared to the unstressed growth condition. Drugs are grouped according to the similarity of their effects to temperature (34), as shown in the left of the plots, except beta-lactams, which did not show similarity to temperature. (Right) Histogram of shifts in the optimal temperature under all pairwise drug combinations involving the drugs in the group. The individual estimates are shown as short lines in the bottom. The mean of the optimal temperature estimates involving each drug (including combinations) is shown as a dashed colored line. The unstressed optimal temperature is shown as a black dashed line in both sets of plots. For both single drugs and combinations, the direction of the optimal temperature shifts depends on whether the drug is similar to cold or to heat. (b) Optimal growth temperature and temperature niche observed under each antibiotic combination used in this study. The first drug in the combination is shown at the top of the plot. The second drug is shown on the y axis using its assigned line color. The single-drug conditions are shown with shaded 95% credible intervals to facilitate comparisons and the point estimates are marked as in Fig. 1c. Conditions under which the maximum growth was too small to estimate parameters reliably were removed.
Furthermore, to evaluate the extent to which these changes are concentration dependent, we measure the growth of E. coli at 12 growth temperatures and increasing antibiotic concentrations (ranging from no antibiotic to near inhibitory) for two antibiotics: ERY and trimethoprim (TMP) (Fig. S4 and S5). We find that growth decreases with increasing antibiotic concentration, but this decrease is not uniform at all temperatures. For both antibiotics, the growth decrease at temperature extremes is sharper than at the central part of the curve. However, for TMP (a heat-similar drug), growth at high temperatures decreases more sharply than at low temperatures. In contrast, for ERY (cold similar), growth decreases more sharply at low temperatures than at high temperatures. The observed shifts in optimal temperature seem to be due to this asymmetry in the growth decrease which causes the position of the central part of the curve to change. From these experiments, we conclude that both the magnitude of the optimal temperature shifts and the extent of the decrease in temperature breadth depend on the antibiotic concentration. However, the direction of the optimal temperature shifts seems to remain consistent when varying antibiotic concentrations.
The optimal temperature model yielding the best prediction for each drug pair is shown. The blue oval frame represents the min model, the red rectangular frame represents the max model, the light red circle frame with the arrow pointing right represents the elevated model, and the light blue frame with the arrow pointing left represents the attenuated model. Four pairs—specifically, AMP+TOB, CLI+AMP, TMP+LVX, and CPR+TET—exhibited multiple models showing the best model prediction, with all results showing mean and max to be the best models. These pairs are displayed as “multiple” in the figure. Download FIG S3, EPS file, 0.7 MB (721.8KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of increasing antibiotic concentration for erythromycin in the temperature response curve. The temperature curves for E. coli under increasing ERY concentration c are shown. The concentrations c are shown as a proportion of the maximum concentration used (where c = 1 corresponds to 1,000 μg/μl). The individual data points are shown as blue dots. The mean at each measured temperature is shown as a gray line in each panel. The curves for the mean growth for all concentrations are shown simultaneously in the bottom right panel. The curves are color coded from low (purple) to high (yellow) concentration. Download FIG S4, EPS file, 0.5 MB (517.3KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of increasing antibiotic concentration for trimethoprim on the temperature response curve. The temperature curves for E. coli under increasing TMP concentration are shown. The concentrations c are shown as a proportion of the maximum concentration used (0.14 μg/μl). The individual data points are shown as blue dots. The mean at each measured temperature is shown as a gray line in each panel. The curves for the mean growth for all concentrations are shown simultaneously in the bottom right panel. The curves are color coded from low (purple) to high (yellow) concentration. Download FIG S5, EPS file, 0.6 MB (588.5KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We then compare the optimal temperature and temperature niche—the range between the temperatures that result in half-maximum growth—for bacteria under all antibiotic combinations to those under the single-drug conditions (Fig. 2b). For some antibiotics (e.g., ERY and ciprofloxacin [CPR]), the optimal temperature and the thermal niche range are similar to those of the single drug when combined with most other antibiotics. In contrast, there are other antibiotics for which these features show much more variation when combined with others (e.g., GEN, STR, TOB, and cefoxitin [FOX]). This suggests that some antibiotics may act as the main drivers of the temperature response curve of antibiotic combinations.
Following this idea, we further explored how the optimal growth temperature is determined under combinations of stressors relative to the optimal temperature under single-stressor conditions. We contrast the observed optimal temperatures with the predictions of five candidate models of how the combination optimal temperature could be determined from that of the single stressors (see Materials and Methods) (Fig. 3a). The min and max models assume that the optimal temperature of the combination is determined by the optimal temperature of a single drug (the minimum or the maximum of the pair, respectively). These models best describe most (65%) multidrug combinations (Fig. 3b). The attenuated and elevated models assume that the optimal temperature of the combination is either lower or higher, respectively, than for both single drugs. These models best describe 18% of the combinations. Lastly, the mean model assumes that the temperature of the combination is determined by the average of the single-drug optimal temperatures. This model best described only 17% of the drug combinations. These results suggest that the optimal temperature of antibiotic combinations is often determined by a single drug.
FIG 3.
The optimal growth temperature under stressor combinations is often determined by a single stressor. (a) Schematic illustration of models to determine the optimal growth temperature under two stressors (Topt, XY) given the single-stressor optimal temperatures (Topt, X, Topt, Y). (b) The frequency at which each model is the best fit, across all drug combinations. (c) Proportion of time that each antibiotic is the main driver of the optimal temperature when combined with other antibiotics. This is based on cases where an individual model (min, max, attenuated, or elevated) best describes the optimal temperature under an antibiotic combination. In these cases, the driver of the combination is the antibiotic for which the optimal temperature (when present on its own) is closer to the optimal temperature of the combination.
Finally, we explore cases where single-driver models (min, max, attenuated, and elevated) represent the best optimal temperature model over the mean model, where both stressors compromise to result in the optimal temperature of an organism in the presence of combined stressors (Fig. 3c). Interestingly, we rarely observe aminoglycosides (GEN, STR, and TOB), antibiotics similar to high heat, being drivers. In contrast, some cold-similar drugs (ERY, LVX, CPR), but not all (CLI, TET), frequently drive the optimal temperature of the combination. To account for the possibility that some antibiotics appear to be a driver more often than others purely by chance, we used a permutation test to evaluate our data against the null model that all drugs are equally likely to be a driver (see D-statistic in Text S1 for details). This test provides strong evidence (P = 0.002) that some antibiotics have a greater tendency to be drivers than others by testing the entire data set simultaneously. We also tested if specific antibiotics are drivers more often than expected by chance (see M-statistic in Text S1). However, we did not obtain statistically significant results for individual drugs, after correcting for multiple comparisons. We believe this may be due to a lack of statistical power to detect differences due to the smaller number of observations for individual antibiotics than for the full data set.
DISCUSSION
Through a systematic analysis of growth response curves of bacteria across different temperatures and under different stressor environments, we investigate the effects of stressors on the phenotypic variation in temperature response traits—optimal temperature and temperature breadth. We see that stressors often decrease the temperature breadth and shift the optimal temperature in a direction that depends on their physiological mechanism of harm. In addition, our results suggest that left shifts—where the optimal temperature in a stressed environment is lower than the optimal temperature under unstressed environmental conditions—are more common and dramatic as opposed to right shifts toward higher optimal temperatures. This may be partially due to the asymmetry of temperature response curves, where the interval between the minimum and optimal growth temperatures is larger than that between the optimal and maximum growth temperatures.
High temperature harms living organisms through multiple mechanisms, including misfolding and aggregating proteins, damaging nucleic acids, and increasing membrane permeability (27). The heat shock response attempts to prevent and/or repair this damage by producing chaperones that aid the correct folding of proteins (36). It has previously been shown that certain kinds of antibiotics can activate components of the heat shock response (34, 37, 38). Moreover, heat shock chaperones have been shown to help combat aminoglycoside-induced protein misfolding (39). However, adding antibiotics to heat stress is unlikely to help the cell survive high temperatures, since the heat shock response is already induced by the high temperature alone. Thus, right shifts in the optimal temperature may be rare because it is unlikely that adding a second stressor can reduce or repair the high-temperature-induced damage. In most cases where we do observe a right shift, it seems to be due to asymmetrical effects on the temperature response curve, where the left portion (i.e., below the unstressed optimal temperature Topt) is more depressed by the antibiotic than the right portion (Fig. 2a; see also Fig. S1 and S4 in the supplemental material).
In contrast, cold temperatures predominantly slow down cell growth by suppressing DNA replication or protein translation (40, 41). Since the effects of low temperature seem to be primarily mediated by slowing down metabolism and growth rather than the accumulation of physical damage, it seems more likely that stressors can shift the optimal temperature to the left, especially when the stressor is more harmful at higher temperatures. In some cases, cold temperatures might allow cells to sustain antibiotic killing because certain antibiotics are effective against only actively growing cells (42). Low temperatures have also been shown to alter the structural stability (43) or the global uptake of some antibiotics such as gentamicin, thus impairing killing efficiency (44).
Antibiotics damage bacteria through various mechanisms of action, such as interfering with cell wall synthesis (45), binding the ribosome to either inhibit protein translation or increase the translational error rate (46), and modifying DNA supercoiling (47). Based on network clustering methods (48, 49), we previously found that certain antibiotic classes have similar physiological effects as either heat or cold in E. coli (34). These temperature-drug groups were shown to correlate with changes in drug sensitivity of high-temperature-adapted strains obtained by Rodríguez-Verdugo et al. (50) and are consistent with previous work comparing the protein expression profile of E. coli under various antibiotics with that induced under heat shock and cold shock (37). Interestingly, here we find that in most cases the direction of the shifts in the optimal temperature can be predicted from these groups. Cold-similar drugs tend either to leave the optimal temperature unchanged or to shift it slightly to the right. In contrast, heat-similar drugs tend to result in larger shifts to the left or leave the optimal temperature unchanged (Fig. 2). Similar trends are exhibited by antibiotic combinations containing drugs in these groups.
We propose a shared-damage hypothesis to explain this phenomenon: antibiotics that damage the same cellular functions as those damaged by temperature stress (heat or cold) will cause an increased burden to the cell machinery that repairs this damage. For example, simultaneous exposure to aminoglycosides and high temperatures will result in more misfolded proteins than either stressor on its own. Upon addition of an antibiotic, the stress-response machinery of the cell could be overwhelmed at less extreme temperatures, causing a greater reduction in growth at temperatures that cause similar physiological damage to the drug. The effect of these kinds of antibiotics in the temperature response curve will thus be asymmetrical. Growth will be more strongly reduced in the direction (heat or cold relative to Topt) where the drug and temperature damage overlap, and the optimal temperature will often shift toward the opposite direction because it suffers less from growth reduction. In terms of stressor interactions based on the Bliss independence framework, the shared-damage hypothesis implies that stressors (e.g., antibiotics and temperatures) with overlapping damage mechanisms will tend to interact synergistically, decreasing growth more than what would be expected given the effects of the individual stressors (Fig. S7).
Relationship between antibiotic-temperature interactions based on Bliss independence and temperature response curves. The black curve corresponds to the fitted temperature response in the absence of antibiotic g0(T) in E. coli. The colored line corresponds to the response under an antibiotic ga(T) (ERY, a cold-similar antibiotic, left, and GEN, a heat-similar antibiotic, right). The temperature response curve predicted by the Bliss independence null model g˜a(T) is shown in gray (see Text S1 for details). A temperature interacts synergistically with the antibiotic if growth is reduced more than predicted by the null model (red-shaded area) and antagonistically if growth is higher than predicted (green-shaded area). Note the opposite patterns in interactions below and above the optimal temperature in the absence of drugs. Download FIG S7, EPS file, 0.3 MB (346.6KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The most pronounced shifts in optimal temperature tend to occur in cases with lower peak growth (Fig. 2a). This suggests that perhaps these shifts become more pronounced when increasing antibiotic concentration, as we see for ERY and TMP (Fig. S4 and S5). However, when evaluated across all antibiotic backgrounds in our study, maximum growth is uncorrelated with the magnitude of the optimal temperature shifts (Fig. S6). Our results suggest that not all antibiotics elicit optimal temperature shifts, but for those that do, the magnitude of shifts is concentration dependent. We hypothesize that increasing the concentration of heat-similar drugs will result in greater shifts to the left and that doing so for cold-similar drugs will result in greater shifts to the right.
The magnitude of optimal temperature shifts is uncorrelated with maximum growth. (Left) The maximum growth rate gmax for all conditions is plotted against the absolute magnitude of the shift in the optimal temperature. The maximum growth in the absence of antibiotics is shown as a dotted line. The line of best fit is shown in orange. There is no apparent correlation between the maximum growth rate and the magnitude of the temperature shifts. All point estimates shown in the plot correspond to mean posterior values. (Right) The estimated maximum growth rate gmax is shown as a function of the estimated optimal temperature for each antibiotic background (including both single drugs and pairs). For comparison purposes, the minimum (Tmin), optimum (Topt), and maximum (Tmax) temperatures in the absence of drugs are shown as vertical lines. The antibiotic backgrounds are color coded (purple, a heat-similar and a cold-similar drug are present simultaneously; red, heat-similar but not cold-similar drugs are present; blue, cold-similar but not heat-similar drugs are present; gray, neither heat-similar nor cold-similar drugs are present). Note that the color scheme is different than in Table 1: we are grouping cold- and heat-similar antibiotics rather than showing individual drugs. As noted in the text, drug combinations with heat-similar drugs tend to exhibit left shifts, and combinations with cold-similar drugs tend to either leave the optimal temperature unchanged or shift it to the right. Our results suggest that shifts do not exist for all drugs. However, when a shift exists, its magnitude may be concentration dependent. Download FIG S6, EPS file, 0.2 MB (244.8KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Notably, although the aminoglycosides (TOB, GEN, and STR) share the same cellular target—the ribosome—as the other protein synthesis inhibitors (CLI, ERY, and TET) used in our study, they result in distinct effects on the thermal response. Previously, differences in the effects of aminoglycosides and other protein synthesis inhibitors at different growth rates have been attributed to the reversibility of ribosomal binding (51). In that study, the authors found that STR is more effective when the growth rate is lower, which does not agree with our results at low temperatures. This discrepancy may be because the reduction in growth was previously manipulated by nutrient limitation as opposed to the temperature variation in our study.
Instead of binding reversibility, we could explain the different effects of these drugs by their mechanisms of action being qualitatively different, with the aminoglycosides being heat similar and the other protein synthesis inhibitors being cold similar. This is because aminoglycosides, unlike other protein synthesis inhibitors, induce mistranslation by the ribosome that decreases translational accuracy and causes protein misfolding (52). Cold temperatures may counteract this effect by slowing down ribosomal activity and increasing accuracy (37), thus causing aminoglycosides to be less effective when bacterial growth is suppressed at lower temperatures, as we observe. Reduced drug uptake at low temperatures could also play a role (44).
Interestingly, beta-lactams have a similar effect in the temperature response as heat-similar drugs. We speculate that this may be due to increased effectiveness at high temperatures due to a synergy between the cell wall damage caused by the antibiotic and the increased membrane permeability caused by high temperatures. Further disentangling these processes in future studies will help increase our understanding of the connection between antibiotic susceptibility and bacterial physiology.
The breadth of the temperature niche is typically reduced in the presence of antibiotics, both in isolation and in combination. The molecular mechanisms involved in this reduction of the temperature breadth by antibiotics have not yet been studied experimentally. However, in the absence of antibiotics the lower and upper limits of growth are believed to be set by chemical and physical limits on the biological processes necessary for bacterial physiology, growth, and cell division (2). Since drugs introduce physiological damage in addition to that caused by temperature extremes, it seems likely that in most cases antibiotics would further constrain the temperature response by killing off barely surviving populations at the extreme temperatures. Previous studies have also observed the temperature niche being reduced upon exposure to stressors (53, 54). From our results it is apparent that stressors can reduce the temperature niche of living organisms at either temperature extreme. Thus, species that experience a wide range of stressful conditions at different times could perhaps experience selection for a broader temperature breadth that can be adapted to various environmental stressors (17).
The temperature niche is measured as the range between the low and high temperatures for half-maximum growth: its definition is therefore relative to the maximum growth. Consequently, conditions that decrease the right and central (i.e., near Topt) portions of the curve more than the left portion can result in a temperature niche that is shifted to the left (and vice versa) in the absence of increased growth at temperature extremes. These effects can lead to apparently surprising cases where adding a drug extends the limits for the thermal niche of the unstressed condition without increased growth at temperature extremes. This suggests that microbial communities may experience shifted thermal niches—giving rise to a different competitive landscape (e.g., due to reduced invasibility of high-temperature habitats under aminoglycosides)—in the presence of certain antibiotics. These effects could be particularly important in the presence of variation in adaptations to antibiotics within microbial communities, which might cause the severity of their effects on the temperature curves to be species dependent. We expect antibiotics to change the competitive landscape the most in mixed populations with both antibiotic-resistant and nonresistant microbes, since the latter would be expected to show larger antibiotic-induced changes in the thermal response than the former under the shared-damage hypothesis.
We find that in most cases the shift in optimal temperature of E. coli due to antibiotic pairs is primarily determined by a single antibiotic. However, we also found cases where interactions between antibiotics seem to be important for determining the optimal temperature. For example, aminoglycosides (TOB, GEN, and STR) show the largest degree of downshifting of the optimal temperature. However, when a second drug is added, this downshifting tends to be alleviated. Thus, in combinations of stressors, aminoglycosides are not the dominant driver for changing optimal temperature despite their large effects when used alone. When a shift in the thermal optimum is alleviated by addition of a second antibiotic, this does not imply that the reduced growth is also alleviated. Typically, these shifts are due to the second antibiotic decreasing some regions of the temperature response curve more sharply than others (Fig. S1). A notable exception involves interactions between certain aminoglycosides (GEN and STR) and other protein synthesis inhibitors (ERY, TET, and other aminoglycosides), possibly because inhibition of protein synthesis reduces the aminoglycoside-induced production of misfolded protein aggregates.
The growth response to multiple environmental factors such as temperature, CO2, and pH has been measured in green algae (55). Under conditions with a large number of factors present simultaneously, the response is dominated by a single, severely detrimental driver. In contrast, in environments with a smaller number of factors, specific interactions between drivers were found to determine overall growth rather than the response to an overriding factor. These results were explained by the authors by the presence of a severely detrimental driver limiting the growth reduction that can be obtained by additional stressors, making the severe driver the primary determinant of the response. In contrast, we find that the effects of an antibiotic can sometimes be partially undone by another (e.g., aminoglycosides and other protein synthesis inhibitors). This suggests that, while identifying a dominant environmental driver can be a simplified approach to understanding organismal response to a complex system, this needs to be done with care since interactions between drivers can be a contributing factor as well.
The thermal optimum is often below the mean environmental temperature. This is because thermal response curves typically decrease sharply at high temperatures, so the penalty of going above the optimal temperature is much steeper than going below. The exact distance between the thermal optimum and the mean is determined by the temperature variability in the environment (31–33). It is thus often tacitly assumed that the optimum temperature of individuals closely aligns with the environment in which the individual has been reared and/or the species has evolved. For this reason, the optimal temperature is not expected to quickly shift in response to other stressful conditions. This is a common assumption in mathematical models that describe the combined effects of temperature and other stressors such as pH (56), nutrient limitation (57), and humidity (58). In contrast, we observe that stressors can substantially and quickly change the optimal temperature for growth of a bacterium. A study that evaluated the combined effects of temperature and salt in slime molds (59) also found shifts in the thermal optimum, suggesting that this phenomenon is not limited solely to antibiotics.
Any physical or chemical environmental feature that kills or slows the population growth of a living organism can be considered a stressor. Antibiotics are stressors to bacteria in clinical settings, but they may not always take this role in nature. It has been proposed that some antibiotics may participate in communication or be by-products of metabolism in their natural environments (60–62). Since we explain thermal optimum shifts through differential growth reduction, our shared-damage hypothesis predicts antibiotic-induced thermal optimum shifts will occur when antibiotics are acting as stressors. However, this will not necessarily happen when antibiotics have a different role at much lower concentrations than those relevant in the clinic. In these cases, we would expect thermal optima to change only if there is a nonnegligible fitness decrease caused by the antibiotic. Further work could test this by measuring the effects of an antibiotic on the thermal responses of microbes that naturally occur in the same environments as the antibiotic.
An exciting potential application of the shared-damage hypothesis is in predicting the effect of other stressors on the thermal optima of living organisms. To do this, further studies are necessary to evaluate the extent to which the physiological damage caused by other environmental stressors—such as pressure and pH—is similar to either temperature stress or antibiotics. This can be done by comparing either the gene-expression profile or the interaction profile (i.e., synergies and antagonisms with other stressors) of the environmental stressor of interest with those of extreme temperatures and/or antibiotics, as has been done to explore the overlap between antibiotics and temperature (34, 37). Our hypothesis would then predict that stressors that induce similar damage as high temperature will result in left shifts in Topt (and vice versa). Moreover, the direction of the shift induced by a stressor should be the same as that of other stressors (e.g., antibiotics) that cause similar physiological damage. For example, beta-lactam antibiotics compromise the integrity of the bacterial cell wall, so we speculate that the induced damage to the cell could have certain similarities to osmotic shock. If this were true, it seems possible that osmotic shock might change the temperature responses in a similar way as beta-lactams.
Although there has been substantial interest in understanding thermal response curves because of their potential to predict responses to climate change (18, 19, 63, 64), the implications might be even broader. For example, an intriguing recent study showed that increased local temperatures were associated with increasing antibiotic resistance (65). This may be because temperature or seasonality affects environmental growth of resistant strains (66, 67) and horizontal gene transfer—one method of facilitating resistance transmission (68, 69). Another study showed that adaptations to long-term temperature changes unexpectedly coincided with mutations conferring resistance to rifampin, an antibiotic that impairs RNA polymerase (50). Climate change has also been linked to changes in host-parasite dynamics that alter the frequency and severity of many infectious diseases (70, 71). Our work here and elsewhere shows that certain classes of antibiotics are more effective at different temperatures and that there is substantial overlap in the response mechanisms to temperature and some kinds of antibiotics (34, 37, 39, 72). This suggests the hypothesis that climate change might favor the evolution of resistance to specific (i.e., heat-similar) antibiotics indirectly due to its overlap with resistance to high-temperature stress.
From our results it also appears that drugs can be used to modify temperature response curves in predictable ways. A temperature-drug system could perhaps be used to examine scenarios for biological responses to climate change via a variety of thermal responses in a laboratory setting. Going forward, such a system could serve as a simplified model for examining changes in response to temperature across seasons, geographic gradients, and climate change.
Finally, it is worth nothing that exact estimates of the optimal temperature and temperature niche depend somewhat on the choice of mathematical model (73). This is why the abilities of a model to provide a good fit to the data and have biologically interpretable parameters are important considerations in choosing an appropriate model (10). In order to fit the variety of shapes and skews we observe here for temperature responses, we developed a novel flexible temperature response model that is reparametrized in terms of biologically meaningful parameters (Materials and Methods and Text S1).
Temperature is one of the fundamental drivers of biological processes. By using antibiotics as stressors, our study system is particularly valuable for its tractability, reproducibility, and potential to study temperature-stressor interactions beyond the pairwise level. Our results provide insights into the interactions between temperature and other stressors. Particularly, we show that stressors can modify the temperature response curves of a living organism and that these changes can be predicted from the way the stressor harms its physiology. More broadly, our results imply that the chemical environment of a living organism—or potentially the presence of other stressors—can influence how it interacts with both abiotic (temperature) and biotic (modified competition due to changes in its thermal niche) factors. Investigating stressor effects on the physiological and ecological trait responses to temperature changes under this framework could lead to future research directions in exploring other environmental stressors that may aid in predicting the stability and diversity of ecological systems.
MATERIALS AND METHODS
Experimental framework.
(i) Bacterial strain and growth medium. The study used BW25113, a derivative of the F− λ− E. coli K-12 strain BD792 (CGSC6159) (74). Bacterial cultures were grown in LB broth (10 g/liter tryptone, 5 g/liter yeast extract, and 10 g/liter NaCl) and maintained in 25% glycerol at −80°C. Fresh cultures were started by adding 20 μl of thawed bacterial glycerol stock into 2 ml of LB followed by incubation at 37°C. Cultures were grown to exponential growth phase and diluted to maintain 104 cells per experimental condition.
(ii) Antibiotics. A total of 12 antibiotics were included in the study as representatives of all major drug classes. Ciprofloxacin (CPR) from MP Biomedicals (Santa Ana, CA) and gentamicin (GEN), levofloxacin (LVX), tetracycline (TET), tobramycin (TOB), erythromycin (ERY), ampicillin (AMP), clindamycin (CLI), streptomycin (STR), nitrofurantoin (NTR), cefoxitin (FOX), and trimethoprim (TMP)—all from Sigma (St. Louis, MO)—were used. Stock solution at 20 mg/ml of each antibiotic was stored in 50-μl aliquots at −20°C. Each aliquot was only frozen and thawed once to preserve potency.
(iii) Growth experiments. Antibiotics used in all experiments inhibited bacterial growth at sublethal concentrations (50% to 90% growth). The desired concentrations were first determined by a 12-step concentration series of 2-fold at each step in 96-well plates (Costar). Antibiotic stock solutions were prepared in a total volume of 5 ml at 10-fold their respective concentrations (Table 1). Experiments with pairwise drug combinations were prepared by adding 10 μl of each component drug followed by the addition of 80 μl cell inoculum. Ten microliters of LB medium was added in replacement of a second drug for single-drug experiments. Each experimental condition was conducted in 4 replicates from the same antibiotic stock solution. The 96-well plates were incubated at various temperatures (22°C, 25°C, 30°C, 37°C, 41°C, 44°C, and 46°C) with aeration at 300 rpm. Cell density was measured at 4 h, 8 h, 12 h, and 24 h by reading optical density at 600 nm (OD600). The optical density measurements (used as a proxy for bacterial growth) at 24 h were used to infer the temperature curves.
The E. coli growth data set used to infer the temperature response curves (see Fig. S1 in the supplemental material) was partially used in a previous publication (34). The overlapping data (less than 30% of the data points) correspond to growth under no antibiotic and single antibiotics at all measured temperatures as well as growth under antibiotic pairs at a single temperature (41°C).
(iv) Growth experiments at various antibiotic concentrations. For a representative cold-similar (ERY) and heat-similar (TMP) antibiotic, we conducted additional growth experiments at various antibiotic concentrations and temperatures (Fig. S4 and S5). Ten antibiotic concentrations were chosen as a linear gradient ranging from the absence of drug to a near-inhibitory drug concentration (ERY, 1,000 μg/μl; TMP, 0.14 μg/μl) in order to clearly see changes in the shape of the temperature response. Four replicates of each antibiotic concentration were incubated at each of 14 temperatures, ranging from 18°C to 50°C.
Mathematical framework.
(i) Modified Briere model for characterizing temperature response curves. Briere et al. (75) defines a simple model for the temperature dependence of a trait, such as growth, denoted by g(T) as follows:
where Tmin and Tmax are the minimum and maximum temperatures of growth, respectively. This model is not flexible enough to describe the antibiotic growth curves we found empirically. As a more general alternative, we propose the modified Briere model:
where a, b ≥ 0 are parameters that determine the shape of the curve. In this modified Briere model, we have Topt = αTmax + (1 − α)Tmin, where corresponds to the location of the optimal temperature relative to the minimum and maximum growth temperatures.
Consequently, our model can be reparametrized as
where gmax is the maximum value of the trait, i.e., growth, and s = a + b is a parameter that determines how steeply the temperature response curve increases (see the supplemental material for details). We use this parametrization for parameter fitting of temperature response curves of the bacterium across different drug combination treatments (Table 1 lists chosen drugs in our study).
(ii) Bayesian parameter fitting. The modified Briere model was fitted to the temperature growth curve for the bacterium under all conditions through a Bayesian methodology with the PyMC3 library of the Python programming language (76). The following methodology is used for obtaining Bayesian estimates for the model parameters. Let yi be the ith observed data point for growth after 24 h, and let Ti be the temperature at which it was observed. The observed values were assumed to be Gamma distributed with
where the Gamma distribution is parametrized in terms of the mean μ and standard deviation σ. The data are clearly heteroskedastic, and multiple measurements were taken at the same temperature, enabling estimates of the standard deviation at each measured temperature. Because of this, a different standard deviation was modeled for each measured temperature. The following hierarchical model was used for the standard deviation:
The prior distributions for the modified Briere model parameters and a justification for all priors used are given in the supplemental material. A variational method (full-rank ADVI) (77) was used to obtain approximate posterior distributions for the model parameters. These posterior distributions were used to construct point estimates—the expected value of the posterior distribution—and 95% credible intervals for all parameters to evaluate the uncertainty in the estimates. Credible intervals are the Bayesian analog to confidence intervals. A credible interval contains the true value of the parameter of interest with the specified (e.g., 95%) probability, given the observed data.
(iii) Models for predicting optimal temperatures for multidrug responses. We denote single drugs as X and Y and the combination of drugs as XY. We use these drug notations as a subscript for the corresponding optimal temperatures, i.e., Topt, X, Topt, Y, and Topt, XY. For predicting the optimal temperature of multidrug combination treatments, we define five different models by the choice of simple yet biologically meaningful scenarios (Fig. 3).
(a) A single drug is playing a major role in determining the optimal temperature of the bacterial response. The optimal temperature of the combination is given by either of the two individual stressors (min or max model). Topt, XY = min(Topt, X, Topt, Y) or Topt, XY = max(Topt, X, Topt, Y).
(b) The optimal temperature of the bacterium in the presence of drug combinations is shifted to lower or higher temperatures than both single drugs’ optimal temperature values. Along the lines of these extreme behaviors, we define attenuated and elevated optimal temperature models Topt, XY << min(Topt, X, Topt, Y) or Topt, XY >> max(Topt, X, Topt, Y).
(c) Temperature tolerance is determined by both of the drugs in the combination. To uncover such cases, we define our fifth model, namely, the mean optimal temperature model. This model expresses the optimal temperature of the combined treatment as the average of the two single drug optimal temperatures (Fig. 1). In other words, the mean model is equivalent to .
To determine the best optimal temperature model, we measured the difference between the actual value and the predicted value of each of the min, mean, and max models. We considered the best-fit model as the one with the smallest absolute difference between actual and predicted values. When this absolute difference is greater than the cutoff value of 2.20°C (see Fig. S2), then the best model is determined to be either the attenuated or elevated model depending on the direction of the optimal temperature shift.
Threshold for distinguishing single-driver models (min versus attenuated or max versus elevated). The distribution of model prediction errors across the min, mean, and max models is plotted to decide a cutoff to determine if the optimal temperature shift of a pairwise combination is large enough to decide that the attenuated or elevated model is a better fit. When the prediction error (as defined by an absolute value of difference of optimal temperature prediction and actual optimal temperature) is higher than 2.20°C, the best model is either the attenuated model or elevated model based on the direction. Download FIG S2, EPS file, 0.4 MB (364.5KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data availability.
The data and code used in this paper are available in the Dryad Digital Repository (78).
ACKNOWLEDGMENTS
We are grateful for funding from the Hellman Foundation (P.J.Y.), a KL2 Fellowship (P.J.Y.) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI grant number UL1TR001881, UC Mexus and CONACYT (M.C.-L.), and a James F. McDonnell Foundation Complex Systems Scholar Award (V.M.S.).
We thank Nina Singh for comments on the manuscript. We thank Rina Watanabe for laboratory assistance.
Contributor Information
Pamela J. Yeh, Email: pamelayeh@ucla.edu.
James C. Stegen, Pacific Northwest National Laboratory
REFERENCES
- 1.Chadwick LE, Rahn H. 1954. Temperature dependence of rattling frequency in the rattlesnake, Crotalus v. viridis. Science 119:442–443. doi: 10.1126/science.119.3092.442. [DOI] [PubMed] [Google Scholar]
- 2.Huey RB, Kingsolver JG. 1989. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol 4:131–135. doi: 10.1016/0169-5347(89)90211-5. [DOI] [PubMed] [Google Scholar]
- 3.Lynch M, Gabriel W. 1987. Environmental tolerance. Am Nat 129:283–303. doi: 10.1086/284635. [DOI] [Google Scholar]
- 4.Fenberg PB, Self A, Stewart JR, Wilson RJ, Brooks SJ. 2016. Exploring the universal ecological responses to climate change in a univoltine butterfly. J Anim Ecol 85:739–748. doi: 10.1111/1365-2656.12492. [DOI] [PubMed] [Google Scholar]
- 5.Somero G. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners' and 'losers’. J Exp Biol 213:912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- 6.Ohlberger J, Mehner T, Staaks G, Holker F. 2008. Temperature-related physiological adaptations promote ecological divergence in a sympatric species pair of temperate freshwater fish, Coregonus spp. Funct Ecol 22:501–508. doi: 10.1111/j.1365-2435.2008.01391.x. [DOI] [Google Scholar]
- 7.Bennett AF. 1980. The thermal dependence of lizard behaviour. Anim Behav 28:752–762. doi: 10.1016/S0003-3472(80)80135-7. [DOI] [Google Scholar]
- 8.Savage VM, Gilloly JF, Brown JH, Charnov EL. 2004. Effects of body size and temperature on population growth. Am Nat 163:429–441. doi: 10.1086/381872. [DOI] [PubMed] [Google Scholar]
- 9.Ali RM. 1970. The influence of suspension density and temperature on the filtration rate of Hiatella arctica. Mar Biol 6:291–302. doi: 10.1007/BF00353662. [DOI] [Google Scholar]
- 10.Adams MP, Collier CJ, Uthicke S, Ow YX, Langlois L, O’Brien KR. 2017. Model fit versus biological relevance: evaluating photosynthesis-temperature models for three tropical seagrass species. Sci Rep 7:39930. doi: 10.1038/srep39930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padfield D, Yvon-Durocher G, Buckling A, Jennings S, Yvon-Durocher G. 2016. Rapid evolution of metabolic traits explains thermal adaptation in phytoplankton. Ecol Lett 19:133–142. doi: 10.1111/ele.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 13.Beaugrand G, Reid PC, Ibanez F, Lindley JA, Edwards M. 2002. Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296:1692–1694. doi: 10.1126/science.1071329. [DOI] [PubMed] [Google Scholar]
- 14.Buckley LB, Huey RB. 2016. How extreme temperatures impact organisms and the evolution of their thermal tolerance. Integr Comp Biol 56:98–109. doi: 10.1093/icb/icw004. [DOI] [PubMed] [Google Scholar]
- 15.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford Scholarship Online, Oxford, United Kingdom. [Google Scholar]
- 16.Angilletta MJ, Bennett AF, Guderley H, Navas CA, Seebacher F, Wilson RS. 2006. Coadaptation: a unifying principle in evolutionary thermal biology. Physiol Biochem Zool 79:282–294. doi: 10.1086/499990. [DOI] [PubMed] [Google Scholar]
- 17.Gaitan-Espitia JD, Belen Arias M, Lardies MA, Nespolo RF. 2013. Variation in thermal sensitivity and thermal tolerances in an invasive species across a climatic gradient: lessons from the land snail Cornu aspersum. PLoS One 8:e70662. doi: 10.1371/journal.pone.0070662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell AI, Pawar S, Savage VM. 2011. Systematic variation in the temperature dependence of physiological and ecological traits. Proc Natl Acad Sci U S A 108:10591–10596. doi: 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demory D, Baudoux AC, Monier A, Simon N, Six C, Ge P, Rigaut-Jalabert F, Marie D, Sciandra A, Bernard O, Rabouille S. 2019. Picoeukaryotes of the Micromonas genus: sentinels of a warming ocean. ISME J 13:132–146. doi: 10.1038/s41396-018-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell NJ, Sandercock SP. 1980. The regulation of bacterial membrane fluidity by modification of phospholipid fatty acyl chain length, p 181–190. In Kates M, Kuksis A (ed), Membrane fluidity, vol 1. Humana Press, Totowa, NJ. [Google Scholar]
- 21.Mansilla MC, Cybulski LE, Albanesi D, Mendoza D. 2004. Control of membrane lipid fluidity by molecular thermosensors. J Bacteriol 186:6681–6688. doi: 10.1128/JB.186.20.6681-6688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda K, Imae Y, Shioi JI, Oosawa F. 1976. Effect of temperature on motility and chemotaxis of Escherichia coli. J Bacteriol 127:1039–1046. doi: 10.1128/jb.127.3.1039-1046.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulick A, Jakovljevic V, Zhang S, Erickstad M, Groisman A, Meir Y, Ryu WS, Wingreen NS, Sourjik V. 2017. Mechanism of bidirectional thermotaxis in Escherichia coli. Elife 6:e26607. doi: 10.7554/eLife.26607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takami H, Takaki Y, Chee GJ, Nishi S, Shimamura S, Suzuki H, Matsui S, Uchiyama I. 2004. Thermoadaptation trait revealed by the genome sequence of thermophilic Geobacillus kaustophilus. Nucleic Acids Res 32:6292–6303. doi: 10.1093/nar/gkh970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao H, McKenzie T, Hageman R. 1986. Isolation of a thermo-stable enzyme variant by cloning and selection in a thermophile. Proc Natl Acad Sci U S A 83:576–580. doi: 10.1073/pnas.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Farrell AP, Matala A, Narum SR. 2018. Mechanisms of thermal adaptation and evolutionary potential of conspecific populations to changing environments. Mol Ecol 27:659–674. doi: 10.1111/mec.14475. [DOI] [PubMed] [Google Scholar]
- 27.Richter K, Haslbeck M, Buchner J. 2010. The heat shock response: life on the verge of death. Mol Cell 40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Mayer MP, Bukau B. 2005. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed TE, Schindler DE, Waples RS. 2011. Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conserv Biol 25:56–63. doi: 10.1111/j.1523-1739.2010.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas MK, Aranguren-Gassis M, Kremer CT, Gould MR, Anderson K, Klausmeier CA, Litchman E. 2017. Temperature-nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob Chang Biol 23:3269–3280. doi: 10.1111/gcb.13641. [DOI] [PubMed] [Google Scholar]
- 31.Martin TL, Huey RB. 2008. Why “suboptimal” is optimal: Jensen’s inequality and ectotherm thermal preferences. Am Nat 171:E102–E118. doi: 10.1086/527502. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci U S A 105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarasekare P, Savage V. 2012. A framework for elucidating the temperature dependence of fitness. Am Nat 179:178–191. doi: 10.1086/663677. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Loya M, Kang TM, Lozano NA, Watanabe R, Tekin E, Damoiseaux R, Savage VM, Yeh PJ. 2019. Stressor interaction networks suggest antibiotic resistance co-opted from stress responses to temperature. ISME J 13:12–23. doi: 10.1038/s41396-018-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruz-Loya M, Tekin E, Kang TM, Rodriguez-Verdugo A, Savage VM, Yeh PJ. 2020. Antibiotics shift the temperature response curve of Escherichia coli growth. bioRxiv doi: 10.1101/2020.04.04.025874. [DOI] [PMC free article] [PubMed]
- 36.Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. 2010. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol 2:a004390. doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanBogelen RA, Neidhardt FC. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A 87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardoso K, Gandra RF, Wisniewski ES, Osaku CA, Kadowaki MK, Felipach-Neto V, Haus LFA, Simão RCG. 2010. DnaK and GroEL are induced in response to antibiotic and heat shock in Acinetobacter baumannii. J Med Microbiol 59:1061–1068. doi: 10.1099/jmm.0.020339-0. [DOI] [PubMed] [Google Scholar]
- 39.Goltermann L, Good L, Bentin T. 2013. Chaperonins fight aminoglycoside-induced protein misfolding and promote short-term tolerance in Escherichia coli. J Biol Chem 288:10483–10489. doi: 10.1074/jbc.M112.420380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann S, Cherkasova V, Bankhead P, Bukau B, Stoecklin G. 2012. Translation suppression promotes stress granule formation and cell survival in response to cold shock. Mol Biol Cell 23:3786–3800. doi: 10.1091/mbc.E12-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha AK, Pavankumar TL, Kamisetty S, Mittal P, Ray MK. 2013. Replication arrest is a major threat to growth at low temperature in Antarctic Pseudomonas syringae Lz4W. Mol Microbiol 89:792–810. doi: 10.1111/mmi.12315. [DOI] [PubMed] [Google Scholar]
- 42.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell SM, Ullman JL, Teel AL, Watts RJ. 2014. pH and temperature effects on the hydrolysis of three beta-lactam antibiotics: ampicillin, cefalotin and cefoxitin. Sci Total Environ 466–467:547–555. doi: 10.1016/j.scitotenv.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Loughman K, Hall J, Knowlton S, Sindeldecker D, Gilson T, Schmitt DM, Birch JW, Gajtka T, Kobe BN, Florjanczyk A, Ingram J, Bakshi CS, Horzempa J. 2016. Temperature-Dependent gentamicin resistance of Francisella tularensis is mediated by uptake modulation. Front Microbiol 7:37. doi: 10.3389/fmicb.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J, Zhou D, Steitz TA, Polikanov YS, Gagnon MG. 2018. Ribosome-targeting antibiotics: modes of action, mechanisms of resistance, and implications for drug design. Annu Rev Biochem 87:451–478. doi: 10.1146/annurev-biochem-062917-011942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeBel M. 1988. Ciprofloxacin: chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacotherapy 8:3–33. doi: 10.1002/j.1875-9114.1988.tb04058.x. [DOI] [PubMed] [Google Scholar]
- 48.Yeh P, Tschumi AI, Kishony R. 2006. Functional classification of drugs by properties of their pairwise interactions. Nat Genet 38:489–494. doi: 10.1038/ng1755. [DOI] [PubMed] [Google Scholar]
- 49.Segrè D, Deluna A, Church GM, Kishony R. 2005. Modular epistasis in yeast metabolism. Nat Genet 37:77–83. doi: 10.1038/ng1489. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Verdugo A, Gaut BS, Tenaillon O. 2013. Evolution of Escherichia coli rifampicin resistance in an antibiotic-free environment during thermal stress. BMC Evol Biol 13:50. doi: 10.1186/1471-2148-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greulich P, Scott M, Evans MR, Allen RJ. 2015. Growth-dependent bacterial susceptibility to ribosome-targeting antibiotics. Mol Syst Biol 11:796. doi: 10.15252/msb.20145949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. 1999. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother 43:727–737. doi: 10.1128/AAC.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okie JG, Van Horn DJ, Storch D, Barrett JE, Gooseff MN, Kopsova L, Takacs-Vesbach CD. 2015. Niche and metabolic principles explain patterns of diversity and distribution: theory and a case study with soil bacterial communities. Proc Biol Sci 282:20142630. doi: 10.1098/rspb.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer K, Dierks A, Franke K, Geister TL, Liszka M, Winter S, Pflicke C. 2010. Environmental effects on temperature stress resistance in the tropical butterfly Bicyclus anynana. PLoS One 5:e15284. doi: 10.1371/journal.pone.0015284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brennan G, Collins S. 2015. Growth responses of a green alga to multiple environmental drivers. Nat Clim Chang 5:892–897. doi: 10.1038/nclimate2682. [DOI] [Google Scholar]
- 56.Rosso L, Lobry JR, Bajard S, Flandrois JP. 1995. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl Environ Microbiol 61:610–616. doi: 10.1128/aem.61.2.610-616.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kovárová K, Zehnder AJ, Egli T. 1996. Temperature-dependent growth kinetics of Escherichia coli ML 30 in glucose-limited continuous culture. J Bacteriol 178:4530–4539. doi: 10.1128/jb.178.15.4530-4539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parra R, Magan N. 2004. Modelling the effect of temperature and water activity on growth of Aspergillus niger strains and applications for food spoilage moulds. J Appl Microbiol 97:429–438. doi: 10.1111/j.1365-2672.2004.02320.x. [DOI] [PubMed] [Google Scholar]
- 59.Cuppers HG, Oomes S, Brul S. 1997. A model for the combined effects of temperature and salt concentration on growth rate of food spoilage molds. Appl Environ Microbiol 63:3764–3769. doi: 10.1128/aem.63.10.3764-3769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mlot C. 2009. Microbiology. Antibiotics in nature: beyond biological warfare. Science 324:1637–1639. doi: 10.1126/science.324_1637. [DOI] [PubMed] [Google Scholar]
- 61.Martínez JL. 2008. Antibiotics and antibiotic resistance genes in natural environments. Science 321:365–367. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 62.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. 2008. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321:1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huey RB, Kingsolver JG. 2011. Variation in universal temperature dependence of biological rates. Proc Natl Acad Sci U S A 108:10377–10378. doi: 10.1073/pnas.1107430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI. 2018. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol Lett 21:1425–1439. doi: 10.1111/ele.13107. [DOI] [PubMed] [Google Scholar]
- 65.MacFadden DR, McGough SF, Fisman D, Santillana M, Brownstein JS. 2018. Antibiotic resistance increases with local temperature. Nat Clim Chang 8:510–514. doi: 10.1038/s41558-018-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gautam R, Bani-Yaghoub M, Neill WH, Dopfer D, Kaspar C, Ivanek R. 2011. Modeling the effect of seasonal variation in ambient temperature on the transmission dynamics of a pathogen with a free-living stage: example of Escherichia coli O157:H7 in a dairy herd. Prev Vet Med 102:10–21. doi: 10.1016/j.prevetmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Mermel LA, Machan JT, Parenteau S. 2011. Seasonality of MRSA infections. PLoS One 6:e17925. doi: 10.1371/journal.pone.0017925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lorenz MG, Wackernagel W. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev 58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 11:355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 70.Molnar PK, Kutz SJ, Hoar BM, Dobson AP. 2013. Metabolic approaches to understanding climate change impacts on seasonal host-macroparasite dynamics. Ecol Lett 16:9–21. doi: 10.1111/ele.12022. [DOI] [PubMed] [Google Scholar]
- 71.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 72.Rodríguez-Verdugo A, Lozano-Huntelman N, Cruz-Loya M, Savage V, Yeh P. 2020. Compounding effects of climate warming and antibiotic resistance. iScience 23:101024. doi: 10.1016/j.isci.2020.101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Low-Décarie E, Boatman TG, Bennett N, Passfield W, Gavalás-Olea A, Siegel P, Geider RJ. 2017. Predictions of response to temperature are contingent on model choice and data quality. Ecol Evol 7:10467–10481. doi: 10.1002/ece3.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Briere J-F, Pracros P, Le Roux A-Y, Pierre J-S. 1999. A novel rate model of temperature-dependent development for arthropods. Environ Entomol 28:22–29. doi: 10.1093/ee/28.1.22. [DOI] [Google Scholar]
- 76.Salvatier J, Wiecki TV, Fonnesbeck C. 2016. Probabilistic programming in Python using PyMC3. PeerJ Prepr 4:e1686v1. https://peerj.com/preprints/1686/. [Google Scholar]
- 77.Kucukelbir A, Tran D, Ranganath R, Gelman A, Blei DM. 2017. Automatic differentiation variational inference. J Mach Learn Res 18:430–474. [Google Scholar]
- 78.Cruz-Loya M, Tekin E, Kang TM, Cardona N, Lozano-Huntelman N, Rodriguez-Verdugo A, Savage VM, Yeh PJ. 2021. Data from “Antibiotics shift the temperature response curve of Escherichia coli growth.” Dryad Digital Repository doi: 10.5068/D14T2B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes additional methodological details regarding the modified Briere model for temperature responses and the statistical approaches used in this paper. Download Text S1, PDF file, 0.2 MB (207.1KB, pdf) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Full data set and model fits. (Lower half) The growth data for all antibiotic combinations (blue dots) and the fitted modified Briere model (black lines) are shown, as well as 95% credible intervals for the modified Briere model. The upper left corner corresponds to the no-drug case. (Upper half) The fitted curves corresponding to each drug combination are shown in black, and the fits corresponding to each single drug are shown in their corresponding color. In the uppermost row, the growth curve in the absence of antibiotics is shown in black and the single-drug curves are shown in the corresponding color. The entries corresponding to FOX and STR+FOX in the upper quadrant are labeled to aid interpretation. Download FIG S1, EPS file, 2.9 MB (2.9MB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The optimal temperature model yielding the best prediction for each drug pair is shown. The blue oval frame represents the min model, the red rectangular frame represents the max model, the light red circle frame with the arrow pointing right represents the elevated model, and the light blue frame with the arrow pointing left represents the attenuated model. Four pairs—specifically, AMP+TOB, CLI+AMP, TMP+LVX, and CPR+TET—exhibited multiple models showing the best model prediction, with all results showing mean and max to be the best models. These pairs are displayed as “multiple” in the figure. Download FIG S3, EPS file, 0.7 MB (721.8KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of increasing antibiotic concentration for erythromycin in the temperature response curve. The temperature curves for E. coli under increasing ERY concentration c are shown. The concentrations c are shown as a proportion of the maximum concentration used (where c = 1 corresponds to 1,000 μg/μl). The individual data points are shown as blue dots. The mean at each measured temperature is shown as a gray line in each panel. The curves for the mean growth for all concentrations are shown simultaneously in the bottom right panel. The curves are color coded from low (purple) to high (yellow) concentration. Download FIG S4, EPS file, 0.5 MB (517.3KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effects of increasing antibiotic concentration for trimethoprim on the temperature response curve. The temperature curves for E. coli under increasing TMP concentration are shown. The concentrations c are shown as a proportion of the maximum concentration used (0.14 μg/μl). The individual data points are shown as blue dots. The mean at each measured temperature is shown as a gray line in each panel. The curves for the mean growth for all concentrations are shown simultaneously in the bottom right panel. The curves are color coded from low (purple) to high (yellow) concentration. Download FIG S5, EPS file, 0.6 MB (588.5KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relationship between antibiotic-temperature interactions based on Bliss independence and temperature response curves. The black curve corresponds to the fitted temperature response in the absence of antibiotic g0(T) in E. coli. The colored line corresponds to the response under an antibiotic ga(T) (ERY, a cold-similar antibiotic, left, and GEN, a heat-similar antibiotic, right). The temperature response curve predicted by the Bliss independence null model g˜a(T) is shown in gray (see Text S1 for details). A temperature interacts synergistically with the antibiotic if growth is reduced more than predicted by the null model (red-shaded area) and antagonistically if growth is higher than predicted (green-shaded area). Note the opposite patterns in interactions below and above the optimal temperature in the absence of drugs. Download FIG S7, EPS file, 0.3 MB (346.6KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The magnitude of optimal temperature shifts is uncorrelated with maximum growth. (Left) The maximum growth rate gmax for all conditions is plotted against the absolute magnitude of the shift in the optimal temperature. The maximum growth in the absence of antibiotics is shown as a dotted line. The line of best fit is shown in orange. There is no apparent correlation between the maximum growth rate and the magnitude of the temperature shifts. All point estimates shown in the plot correspond to mean posterior values. (Right) The estimated maximum growth rate gmax is shown as a function of the estimated optimal temperature for each antibiotic background (including both single drugs and pairs). For comparison purposes, the minimum (Tmin), optimum (Topt), and maximum (Tmax) temperatures in the absence of drugs are shown as vertical lines. The antibiotic backgrounds are color coded (purple, a heat-similar and a cold-similar drug are present simultaneously; red, heat-similar but not cold-similar drugs are present; blue, cold-similar but not heat-similar drugs are present; gray, neither heat-similar nor cold-similar drugs are present). Note that the color scheme is different than in Table 1: we are grouping cold- and heat-similar antibiotics rather than showing individual drugs. As noted in the text, drug combinations with heat-similar drugs tend to exhibit left shifts, and combinations with cold-similar drugs tend to either leave the optimal temperature unchanged or shift it to the right. Our results suggest that shifts do not exist for all drugs. However, when a shift exists, its magnitude may be concentration dependent. Download FIG S6, EPS file, 0.2 MB (244.8KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Threshold for distinguishing single-driver models (min versus attenuated or max versus elevated). The distribution of model prediction errors across the min, mean, and max models is plotted to decide a cutoff to determine if the optimal temperature shift of a pairwise combination is large enough to decide that the attenuated or elevated model is a better fit. When the prediction error (as defined by an absolute value of difference of optimal temperature prediction and actual optimal temperature) is higher than 2.20°C, the best model is either the attenuated model or elevated model based on the direction. Download FIG S2, EPS file, 0.4 MB (364.5KB, eps) .
Copyright © 2021 Cruz-Loya et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The data and code used in this paper are available in the Dryad Digital Repository (78).