Background:
Central nervous system complications are reported in an increasing number of patients with Coronavirus Disease 2019 (COVID-19). COVID-19–related Guillain-Barré syndrome (GBS) is of particular importance given its association with higher mortality rates and prolonged respiratory failure.
Review Summary:
We conducted a systematic review of published cases for COVID-19–related GBS, and provide a summary of clinical management strategies for these cases. Sixty-three studies, including 86 patients, were included. Seventy-six cases with reported outcome data were eligible for the outcome analysis. Ninety-nine percent of patients were diagnosed with COVID-19 before diagnosis of GBS (median: 14 d prior, interquartile range: 7 to 20). Intravenous immunotherapy (intravenous immunoglobulin: 0.4 g/kg/d for 5 d) was the most frequently used treatment approach. The review indicated that the outcome was not favorable in 26% of cases (persistent neurological deficits). A mortality rate of 3.5% was observed in patients with COVID-19–related GBS.
Conclusions:
Although evidence to support specific treatments is lacking, clinicians should consider the benefits of immunotherapy and plasma exchange in addition to the standard antimicrobial and supportive therapies for patients who meet the diagnostic criteria for acute sensory and motor polyradiculoneuritis. Intravenous immunoglobulin treatment alone is not shown to result in improved outcomes or mortality. More extensive studies aimed at exploring the neurological manifestations and complications of COVID-19 and distinctive treatment options for COVID-19–related GBS are warranted.
Key Words: COVID-19, coronavirus, Guillain-Barré syndrome, plasma exchange, intensive care units, immunotherapy, IVIG
An increasing body of evidence has emerged to establish the link between Coronavirus Disease 2019 (COVID-19) infection and major neurological complications such as cerebrovascular accidents, acute transverse myelitis, encephalitis, and Guillain-Barré syndrome (GBS).
Angiotensin-converting enzyme 2 (ACE2) has been identified as an important severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor, mediating its entry into the cell.1 ACE2 receptors are widely expressed in the lungs, heart, and brain.2 The expression of the ACE2 receptors on the endothelial cells of the blood-brain barrier facilitates the viral binding and entry into the central nervous system (CNS).3–6 ACE2 receptors are highly expressed in the ventrolateral medulla and the nucleus of the solitary tract.5 In addition to the direct viral binding and cell entry, activation of inflammatory mediators is thought to result in a proinflammatory state within the CNS.7 In addition, COVID-19 is suggested to trigger a molecular mimicry phenomenon on the affected endothelial cells, where cross-reactions occur between antibodies and a large number of proteins present on the plasma membrane surface due to COVID-19 induced stress.8,9 As a result of the above mechanisms various pathways within the CNS can lead to direct injury to nerve tissue, in addition to a cytokine storm across the blood-brain barrier, hypoxia from COVID-19–related lung injury, and an uncontrolled immune response.6,10–13 Figure 1 demonstrates different mechanisms through which SARS-CoV-2 may cause neuronal injuries.
FIGURE 1.
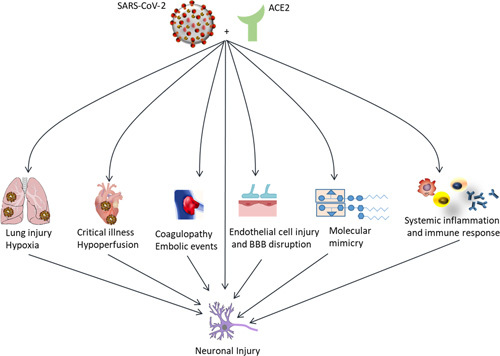
ACE2-binding SARS-CoV-2 causes various complications in different organs that can lead to neurological complications. ACE2 indicates angiotensin-converting enzyme 2; BBB, blood-brain barrier; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Neurological manifestations are reported in up to 36% of patients with COVID-19. Among COVID-19-associated CNS conditions, GBS has emerged in an increasing number of case reports as an additional hazard with a significant risk of mortality or prolonged respiratory failure.4,11,12,14–20
We herein present an in-depth systematic review of COVID-19–related GBS cases with analysis. The purpose of this systematic review is to recapitulate the available treatments for COVID-19–related GBS and to provide a summary of clinical management strategies for this complication. We explore management obstacles in the intensive care unit (ICU) for COVID-19–related GBS patients during the pandemic.
METHODS
Search Strategy and Selection Criteria
All articles in English and Spanish languages, including adult patients, and published in PubMed-indexed scientific journals were considered eligible. Randomized controlled trials, prospective and retrospective cohorts, case series, and case reports, as well as cross-sectional studies involving patients with COVID-19–related GBS were eligible for inclusion.
We performed a systematic search on databases PubMed, EMBASE, and Web of Science to identify studies with the following subject heading terms: “COVID” OR “Coronavirus” AND “Guillain-Barre.” We extracted the data from reports, with adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.21 Details of the patient population, COVID-19 symptoms and management, GBS symptoms, management, and outcomes were recorded. The search occurred from May 19, 2020, through January 31, 2021 which captured a total of 99 studies. Thirteen additional reports were captured from reference lists of retrieved reports and Google Scholar searches. At the time of conducting this study there were no published randomized trials or cross-sectional studies. We identified 3 systematic reviews, 1 cohort, and 1 observational study. All case reports and case series were included in the analysis. There were also 3 correspondence letters eligible for inclusion in the qualitative synthesis (Table 1).
TABLE 1.
COVID-19–Related GBS Correspondence Letters
| References | Title | Question | Conclusion |
|---|---|---|---|
| Gupta et al22 | Is COVID-19–related Guillain-Barré syndrome different? | How does COVID-19–related Guillain-Barré syndrome compare against other presentations of GBS? | Anti-ganglioside antibody was not found in patients with COVID-19– and Zika virus–related GBS. The neuropathy in viral infections–related GBS could be due to other autoantibodies that are not detected as yet or the viruses produced nerve damage due to other neurotoxic effects |
| Cappello8 | COVID-19 and molecular mimicry: the Columbus’ Egg? | Does molecular mimicry explain both the acute pulmonary embolism and the multi-organ microvascular thrombosis that some patients experience? | It would be appropriate if this Journal would stimulate the scientific community on the fact that molecular mimicry phenomena can occur in SARS-CoV-2 It is also urgent to start the search for human epitopes that turn into autoantigens, and to remind this risk to all those who are currently working on vaccines |
| Gigli et al23 | Guillain‑Barré syndrome in the COVID‑19 era: just an occasional cluster? | Compare the frequency of GBS cases during the March-April months of the last 3 y and to admissions for GBS during the same months of the current year in Friuli Venezia-Giulia, Italy | Compared with years 2017-2019, the increase of GBS cases in 2020 is 5.41-fold The suspicion that this striking difference could be due to the pandemic curve in our region is, therefore, legitimate |
COVID-19 indicates Coronavirus Disease 2019; GBS, Guillain-Barré syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data Analysis
Descriptive statistics were tabulated for the analytic cohort. Continuous data were reported as median with interquartile ranges and compared using the Kruskal-Wallis test or Wilcoxon rank test. Categorical data were expressed as proportions and compared using the χ2 test. The published outcome data for each case were classified into 2 categories. Clinical improvement, defined as neurological or autonomic, or respiratory symptoms improvement, weaning off the ventilator, or improvement of oxygen requirement and inflammatory markers. No improvement is defined as no sign of clinical improvement, worsening of the neurological examination, hemodynamic instability, and death. All analyses were conducted using R (The R Foundation for Statistical Computing, Vienna, Austria).24 P-values <0.05 were considered to be significant.
RESULTS
To graphically summarize the studies’ inclusion processes, we constructed a PRISMA diagram (Fig. 2) which demonstrates the selection mechanism of among the total of 99 discovered publications.21 From a total of the final 63 publications (55 case reports and 8 case series), 86 cases were included in this study. Most of the cases were reported from Italy (30%), the United States (19%), and Spain (9%) (Fig. 3). The reported in-hospital mortality rate among a total of 86 patients were 3.5%. Seventy-six cases reported the outcome of their management and were included in the final analysis; among them, 74% reported clinical improvement, while 26% reported no improvement. Demographic and clinical data stratified by patients’ outcome are shown in Table 2. Patients with no improvement were older (P=0.003) and had a higher incidence of quadriplegia (P=0.02), areflexia (P=0.02) and respiratory failure (P=0.004) (Table 2).
FIGURE 2.
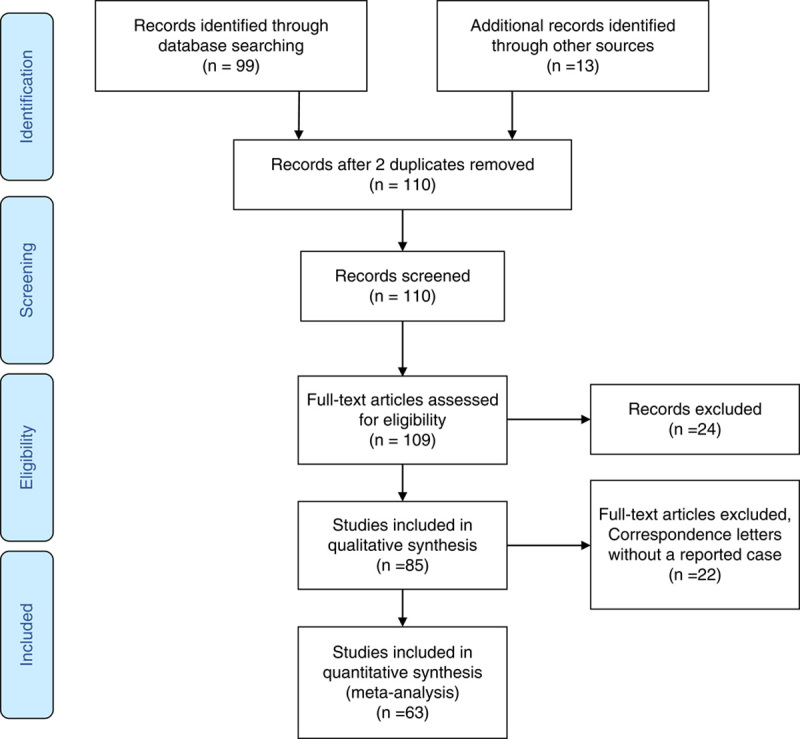
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
FIGURE 3.
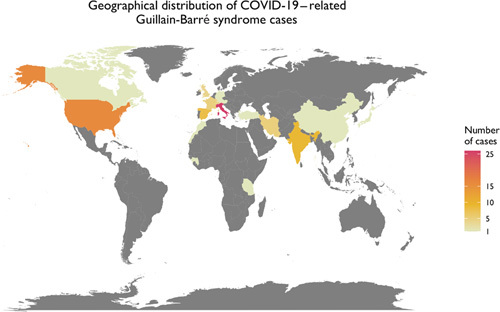
Geographical distribution of the Coronavirus Disease 2019 (COVID-19)-related Guillain-Barré syndrome cases.
TABLE 2.
Demographic and Clinical Features of the Cases With Reported Outcome (N=76)
| Clinical Improvement (N=57) | No Improvement (N=21) | P | |
|---|---|---|---|
| Age (y) | 55 (49-64) | 66.5 (55-72) | 0.003 |
| Sex* | |||
| Female | 17 (30) | 8 (40) | 0.6 |
| Male | 39 (70) | 12 (60) | |
| Comorbidities† | |||
| Yes | 20 (61) | 9 (82) | 0.3 |
| No | 13 (39) | 2 (18) | |
| COVID-19 symptoms | |||
| Fever | 35 (61) | 15 (71) | 0.4 |
| Cough | 35 (61) | 17 (81) | 0.2 |
| Dyspnea | 13 (22) | 4 (20) | 0.5 |
| Anosmia/ageusia | 14 (24) | 1 (4) | 0.05 |
| GBS subtype‡ | |||
| AIDP | 20 (54) | 5 (31) | 0.5 |
| AMSAN | 9 (24) | 6 (35) | |
| FDP | 1 (3) | 1 (6) | |
| MFS | 4 (11) | 1 (6) | |
| GBS symptoms | |||
| Tetraparesis | 15 (27) | 8 (40) | 0.4 |
| Paresthesia | 23 (41) | 11 (55) | 0.4 |
| Hypoesthesia | 7 (12) | 2 (10) | 1 |
| Ataxia | 8 (14) | 1 (5) | 0.4 |
| Areflexia | 11 (20) | 10 (50) | 0.02 |
| Quadriplegia | 0 | 3 (15) | 0.02 |
| Paraplegia | 1 (2) | 2 (10) | 0.3 |
| Paraparesis | 17 (30) | 4 (20) | 0.8 |
| Facial paresis | 8 (14) | 3 (15) | 1 |
| Facial diplegia | 4 (7) | 1 (5) | 1 |
| Respiratory failure | 1 (2) | 5 (23) | 0.004 |
| Time from onset of COVID-19 diagnosis to GBS (d)§ | 14 (10-20) | 10 (6-14) | 0.1 |
| Time from neurological symptoms to hospital admission (d)∥ | 3 (2-4) | 1 (1-3) | 0.2 |
| Ventilator support | 22 (39) | 16 (80) | 0.004 |
| Time from hospital admission to ICU admission (d)¶ | 3 (2-3) | 2 (1.5-3.5) | 0.6 |
| COVID-19 treatment# | |||
| Hydroxychloroquine | 20 (43) | 8 (47) | 1 |
| Lopinavir/ritonavir | 9 (20) | 7 (41) | 0.1 |
| Remdesivir | 0 | 0 | 1 |
| Antiviral agents | 15 (32) | 7 (41) | 1 |
| Antibiotics | 14 (30) | 7 (41) | 0.7 |
| Corticosteroids | 8 (17) | 3 (18) | 1 |
| Convalescent plasma | 0 | 0 | 1 |
| Tocilizumab (the only reported monoclonal antibody) | 4 (8) | 0 | 0.4 |
| GBS treatment** | |||
| IVIG | 49 (87) | 13 (65) | 0.6 |
| Plasmapheresis | 3 (5) | 1 (5) | 1 |
| Plasmapheresis and IVIG | 1 (2) | 2 (10) | 0.3 |
| Prednisone | 0 | 2 (10) | 0.1 |
| No treatment | 2 (4) | 1 (5) | 1 |
| In-hospital mortality | 0 | 3 (15) | 0.02 |
| Time from neurological symptoms to start of IVIG treatment (d)†† | 4 (2-7) | 3 (3-4) | 0.6 |
| IVIG days‡‡ | 5 (5-5) | 5 (5-5) | 0.4 |
| IVIG dose§§ | |||
| 0.40 g/kg/d for 5 d | 34 (81) | 10 (83) | 1 |
| 2 g/kg for 5 d | 6 (14) | 2 (17) | 1 |
| 30 g for 5 cycles | 2 (5) | 0 | 1 |
| Hospital length of stay (d)∥∥ | 12.5 (9-23.7) | 7 (4-31) | 0.6 |
| ICU length of stay (d)¶¶ | 0 (0-5) | 4 (1-14) | 0.02 |
Bold values are indicates statistically significant.
Data are presented as median (interquartile range), or n (%), and compared using Kruskal-Wallis test, or Wilcoxon signed-rank test and χ2 test, respectively.
76 cases reported patients’ sex.
44 cases reported comorbidities.
54 cases reported the GBS subtype.
70 cases reported time from onset of COVID-19 diagnosis to GBS.
47 cases reported time from neurological symptoms to hospital admission.
23 cases reported time from hospital admission to ICU admission.
64 cases reported their COVID-19 managements.
75 cases reported their GBS treatments.
32 cases reported time from neurological symptoms to start of IVIG treatment.
56 cases reported IVIG days.
54 cases reported exact used IVIG dose.
37 cases reported hospital length of stay.
41 cases reported ICU length of stay.
AIDP indicates acute inflammatory demyelinating polyneuropathy; AMSAN, acute motor and sensory axonal neuropathy; COVID-19, Coronavirus Disease 19; FDP, facial diplegia; GBS, Guillain-Barré syndrome; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MFS, Miller-Fisher syndrome.
Demographic data of published cases, as well as the reported clinical data for each COVID-19 case, is demonstrated in Table 3. Among a total of 86 cases, the most-reported comorbidity was hypertension (20%) and type 2 diabetes or prediabetes (9%). Cough (70%), fever (63%), dyspnea (24%), anosmia or ageusia (17%), diarrhea (16%), pharyngitis or upper respiratory infection (URI) symptoms (15%), and fatigue, myalgia, or arthralgia (12%) were the first COVID-19 infection symptoms reported among the patients, respectively. A majority of the cases (83%) were diagnosed using the reverse transcription-polymerase chain reaction technique; and 86% of the specimens were collected through nasopharyngeal (NP) swab. Forty-five percent of the cases reported cerebrospinal fluid polymerase chain reaction for COVID-19 results with no positive report. Seventy-eight percent of the cases reported their choice of treatment for COVID-19. These treatments included hydroxychloroquine (45%), antibiotics (34%), lopinavir/ritonavir (25%), darunavir and antiretroviral therapy (7%), umifenovir (3%), oseltamivir (3%), tocilizumab (1%), and corticosteroids (16%). There was no reported use of remdesivir among the cases we reviewed. Similarly, the use of Regeneron monoclonal antibodies against SARS-CoV-2 (casirivimab with imdevimab) has not been reported in any cases with COVID-19–related GBS; the only reported monoclonal antibody in this population was tocilizumab, a monoclonal antibody against the interleukin-6 receptor. One case reported at the Chest annual meeting reported the use of tocilizumab together with convalescent plasma, but the patient did not improve and remained dependent on ventilatory support. Among 92% of the cases that reported ventilator support status, 48% reported failure of weaning trials during the treatment period.
TABLE 3.
Characteristic and COVID-19–Related Data Among COVID-19–Related GBS Cases
| References | Age (y) | Sex | Medical History/Comorbidities | COVID-19 Symptoms | COVID-19 Dx Method | COVID-19 Management | ICU Required | Ventilation Required | Time |
|---|---|---|---|---|---|---|---|---|---|
| Alberti et al17 | 71 | M | HTNAAA (T)Lung cancer (T) | FeverDyspnea | RT-PCRNP swab | Lopinavir/ritonavirHydroxychloroquine | Yes | Yes | <24 h |
| Camdessanche et al18 | 64 | M | Rotator cuff tear at admission | FeverCough | RT-PCRNP swab | ParacetamolLopinavir/ritonavir | Yes | Yes | 12 d |
| El Otmani et al19 | 70 | F | RA | Cough | RT-PCROP swab | HydroxychloroquineAzithromycin | No | No | NA |
| Juliao et al25 | 61 | M | NR | FeverCough | RT-PCRNP swab | Lopinavir/ritonavirHydroxychloroquine | No | No | NA |
| Marta-Enguita et al26 | 76 | F | None | FeverCough | RT-PCRSite NR | Amoxicillin/clavulanateAzithromycin | Yes | Yes | 4 h |
| Ottaviani et al15 | 66 | F | HTN | FeverCoughDorsal rash | RT-PCRNP swab | Lopinavir/ritonavirHydroxychloroquine | Yes | Yes | NR |
| Padroni et al16 | 70 | F | NR | FeverCough | RT-PCRNP swab | Supportive | Yes | Yes | 4 d |
| Scheidl et al4 | 54 | F | None | HypogeusiaHyposmia | RT-PCROP swab | None | No | No | NA |
| Sedaghat and Karimi14 | 65 | M | DM2 | FeverCoughDyspnea | RT-PCROP swab | Lopinavir/ritonavirHydroxychloroquineAzithromycin | No | No | NA |
| Zhao et al27 | 61 | F | NR | FeverCough | RT-PCROP swab | UmifenovirLopinavir/ritonavir | No | No | NA |
| Toscano et al28 | 77 | F | NR | FeverCoughAgeusia | RT-PCRNP swab | Acetaminophen | Yes | Yes | NR |
| Toscano et al28 | 23 | M | NR | FeverPharyngitis | RT-PCRNP swab | Amoxicillin | No | No | NA |
| Toscano et al28 | 55 | M | NR | FeverCough | RT-PCRNP swab | Azithromycin | Yes | Yes | 2d |
| Toscano et al28 | 76 | M | NR | CoughHyposmia | RT-PCRNP swab | NR | No | No | NA |
| Toscano et al28 | 61 | M | NR | CoughAgeusiaAnosmia | Serum IgG | NA | Yes | Yes | 5 d |
| Gigli et al23 | 53 | M | NR | FeverDiarrhea | IgM/IgGSerum and CSF | NR | NR | NR | NR |
| Galan et al29 | 43 | M | NR | URTIDiarrhea | RT-PCRSite NR | Lopinavir/ritonavirHydroxychloroquineAmoxicillinCorticosteroids | No | No | NA |
| Virani et al20 | 54 | M | Clostridium difficile colitis | FeverCoughDyspnea | RT-PCRNP swab | AmoxicillinCorticosteroidsHydroxychloroquine | Yes | Yes | NR |
| Coen et al30 | 70 | M | None | CoughFatigueMyalgia | RT-PCRNP swab | NR | No | No | NA |
| Rana et al31 | 54 | M | Clostridium difficile colitisHLDRLS | FeverRhinorrheaOdynophagia | RT-PCRSite NR | AmoxicillinCorticosteroidsHydroxychloroquineAzithromycin | Yes | Yes | <24 h |
| Arnaud et al32 | 64 | M | NR | CoughDyspneaDiarrheaFever | RT-PCRNP swab | CefotaximeAzithromycinHydroxychloroquine | No | No | NA |
| Chan et al33 | 58 | M | None | None | RT-PCROP swab | CeftriaxoneAzithromycin | No | No | NA |
| Molina et al34 | 55 | F | Dyslipidemia, active smoking | FeverNonproductive coughDyspnea | RT-PCRNP swab | HydroxychloroquineCeftriaxoneAzithromycin | Yes | No | 2 d |
| Farzi et al35 | 41 | M | DM2 | CoughDyspneaFever | RT-PCRNP swab | Lopinavir/RitonavirHydroxychloroquine | No | No | NA |
| Helbok et al36 | 68 | M | None | Dry coughHeadacheFatigueMyalgiaFeverAnosmiaAgeusia | Antibody testing | Oral methylprednisoloneC-reactive proteinElevated erythrocyte sedimentationPlasma exchange | Yes | Yes | 36 h |
| Hutchins et al37 | 21 | M | HTN, prediabetes, class I obesity | FeverCoughDyspneaDiarrheaNauseaHeadacheSinonasal congestion | RT-PCRNP/OP swab | Plasma exchange | No | No | NA |
| Lantos et al38 | 36 | M | left eye strabismus (asymptomatic for 30 y) | FeverChillsMyalgia | RT-PCRNP swab | Hydroxychloroquine | No | No | NA |
| Lascano et al39 | 52 | F | None | Dry coughFeverOdynophagiaArthralgiaDiarrhea | IgM/IgG, followed by RT-PCRNP swab | None | No | Yes | NA |
| Lascano et al39 | 63 | F | DM2 | Dry coughShiveringOdynophagiaBreathing difficultiesChest pain | RT-PCRNP swab | None | No | No | NA |
| Lascano et al39 | 61 | F | None | Productive coughFeverMyalgiaVasovagal syncopeDiarrheaNauseaVomiting | RT-PCRNP swab | None | No | No | NA |
| Reyes-Bueno et al40 | 51 | F | None | DiarrheaOdynophagiaCough | IgG | Gabapentin | No | No | NA |
| Su et al41 | 72 | M | Coronary artery disease, HTN, alcohol abuse | Mild diarrheaAnorexiaChills | RT-PCRNP swab | Sulfamethoxazole-trimethoprim | Yes | Yes | 3 d |
| Webb et al42 | 57 | M | HTN and psoriasis | CoughHeadacheMyalgiaMalaiseFeverDiarrhea | RT-PCRNP swab | Co-amoxiclav | Yes | Yes | 3 d |
| Bigaut et al43 | 48 | M | NR | CoughAstheniaMyalgia in legsAnosmiaAgeusiaDiarrhea | RT-PCRNP swab | None | No | No | NA |
| Bigaut et al43 | 70 | F | NR | AnosmiaAgeusiaDiarrheaAstheniaMyalgia | RT-PCRNP swab | None | Yes | Yes | 3 d |
| Assini et al44 | 55 | M | NR | AnosmiaAgeusiaFeverCough | RT-PCROP swab | IdrossichlorochineArbidolRitonavirLopinavir | Yes | Yes | 3 d |
| Assini et al44 | 60 | M | NR | FeverCough | RT-PCRNP swab | HydroxychloroquineAntiretroviral therapyTocilizumab | No | Yes | NA |
| Bracaglia et al45 | 66 | F | None | None | RT-PCRNP swab | RitonavirDarunavirHydroxychloroquine | No | No | NA |
| Ebrahimzadeh et al46 | 46 | M | NR | FeverSore throatDry coughDyspnea | RT-PCRNP swab | Hydroxychloroquine | No | No | NA |
| Ebrahimzadeh et al46 | 65 | M | NR | NR | RT-PCRNP swab | NR | No | No | NA |
| Chan et al47 | 68 | M | NR | FeverUpperrespiratory symptoms | RT-PCRNP swab | Plasmapheresis | No | No | NA |
| Chan et al47 | 84 | M | NR | Fever | RT-PCRNP swab | Plasmapheresis | No | Yes | NA |
| Sancho-Saldaña et al48 | 56 | F | NR | FeverDry coughShortness of breath | RT-PCRNP swab | NR | Yes | No | 5 d |
| Kilinc et al49 | 50 | M | None | Dry cough | Fecal PCR, serumIgM, IgG | None | No | No | NA |
| Oguz-Akarsu50 | 53 | F | None | Fever | RT-PCRNP swab | HydroxychloroquineAzithromycin | No | No | NA |
| Pfefferkorn et al51 | 51 | M | NR | FeverFlu-like symptomsFatigueDry cough | RT-PCRNP swab | Plasma exchange | No | Yes | NA |
| Hirayama et al52 | 54 | F | Asthma | CoughFever | RT-PCROP swab | Betamethasone | No | No | NA |
| Korem et al53 | 58 | F | Cervical spondylosis and disk herniation | FeverCoughBack pain | NR | Azithromycin | No | No | NA |
| Tiet and AlShaikh54 | 49 | M | Sinusitis | DyspneaHeadacheCough | RT-PCROP swab | None | Yes | No | NR |
| Defabio et al55 | 70 | F | Reflex sympathetic dystrophyFibromyalgiaGERDHiatal herniaAsthma | FeverDyspneaCough | NR | NR | No | No | NA |
| Curtis et al56 | 8 | M | None | DyspneaCough | NR | None | Yes | Yes | NR |
| Gale et al57 | 58 | M | HTNHypercholesterolemiaMyocardial infarction | Coryzal symptoms | RT-PCRTracheal aspirate | Dexamethasone | Yes | Yes | 2 |
| Ameer et al58 | 30s | M | None | FeverCough | RT-PCRNP, OP swabs | None | No | No | NA |
| Manganotti et al59 | 72 | M | NR | FeverDyspneaHyposmiaAgeusia | RT-PCRNP swab | HydroxychloroquineOseltamivirDarunavirMethylprednisoloneTocilizumab | Yes | Yes | NR |
| Manganotti et al59 | 72 | M | NR | FeverCough DyspneaHyposmiaAgeusia | RT-PCRNP swab | HydroxychloroquineLopinavir-ritonavirMethylprednisolone | Yes | Yes | NR |
| Manganotti et al59 | 49 | F | NR | FeverCough DyspneaHyposmiaAgeusia | RT-PCRNP swab | HydroxychloroquineLopinavir-ritonavirMethylprednisolone | NR | No | NR |
| Manganotti et al59 | 94 | M | NR | FeverCoughGI symptoms | RT-PCRNP swab | Methylprednisolone | NR | No | NR |
| Manganotti et al59 | 76 | M | NR | FeverCoughDysuriaHyposmiaAgeusia | RT-PCRNP swab | HydroxychloroquineOseltamivirDarunavirMethylprednisoloneTocilizumabMeropenamLinezolidClarithromycin, doxycyclineFluconazole | Yes | Yes | NR |
| McDonnell et al60 | 54 | M | DM2Herniated nucleus pulposus at C6-C7, L2-L3, L3-L4, L4-L5 with disk bulges | FeverAgeusia | NP swab RT-PCR | Hydroxychloroquine 400 mg for 4 d | Yes | No | 0 |
| Diez-Porras et al61 | 54 | M | HTNObesity | Febrile syndromeCoughMyalgia | RT-PCRNP swab | Azithromycin, hydroxychloroquine, lopinavir/ritonavir | Yes | Yes | NR |
| Manji et al62 | 12 | M | NR | FeverCoughRespiratory distressHypoxiaTachycardia | RT-PCRNP swab | Empiric antibiotic coverage and other treatment modalities as required | Yes | Yes | NR |
| Bueso et al63 | 60 | F | Migraines | FeverCoughMyalgiaDysgeusiaDyspnea | RT-PCRNP swab | AzithromycinHydroxychloroquine | No | No | N/A |
| Zito et al64 | 57 | M | NR | DysgeusiaCoughFever | Positive serum SARS-CoV-2 IgG | NR | No | No | NA |
| Garnero et al65 | 65 | M | NR | Pneumonia | NR | NR | NR | NR | NR |
| Garnero et al65 | 73 | M | NR | Pneumonia | NR | NR | NR | NR | NR |
| Garnero et al65 | 55 | M | NR | Pneumonia | NR | NR | NR | NR | NR |
| Garnero et al65 | 46 | F | NR | Diarrhea | NR | NR | NR | NR | NR |
| Garnero et al65 | 60 | M | NR | Pneumonia | NR | NR | NR | NR | NR |
| Garnero et al65 | 63 | F | NR | Pneumonia | NR | NR | NR | NR | NR |
| Lowery et al66 | 45 | M | DyslipidemiaHTNCrohn disease on adalimumab | Sinus congestionCoughDyspnea | RT-PCRNP swab | 200 mg hydroxychloroquine bid for 5 d | Yes | Yes | 2 |
| Hutchins et al37 | 21 | M | HTNPrediabetesObesity | FeverCough DyspneaDiarrheaNausea, HeadacheSinonasal congestionDizzinessTachycardia | RT-PCRNP and OP swab | Supplemental O2 | No | No | NA |
| Atakla et al67 | 41 | M | NR | Influenza syndromeDigestive disorderAnosmia Ageusia | RT-PCRNP swab | Azithromycin | Yes | Yes | NR |
| Abrams et al68 | 67 | F | Breast cancer (T) | CoughNausea | RT-PCRNP swab | NR | Yes | Yes | NR |
| Agha Abbaslou et al69 | 55 | F | Unknown chronic lung disease | CoughFeverChillsDyspnea | RT-PCRNP swab | Hydroxychloroquine (lopinavir/ritonavir) | Yes | Yes | 2 |
| Assini et al70 | 60 | M | NR | CoughFever | RT-PCRNP swab | HydroxychloroquineAntiretroviral therapyTocilizumab | Yes | Yes | 3 |
| Assini et al70 | 55 | M | NR | CoughFeverAnosmiaAgeusia | RT-PCRNP swab | HydroxychloroquineUmifenovirRitonavirLopinavir | Yes | Yes | 3 |
| Chakraborty and Kumar71 | 75 | M | NR | Dyspnea | RT-PCRNP swab | Culture-based antibiotics | Yes | Yes | <1 |
| Garcia-manzanedo et al72 | 77 | M | HTNHLDCOPD | NR | RT-PCRNP swab | Hydroxychloroquine lopinavir/ritonavirPiperacillin/tazobactam | Yes | Yes | NR |
| Liberatoret al73 | 49 | M | HTNTesticular seminoma (T) | CoughFever | RT-PCRNP swab | HydroxychloroquineLopinavir/ritonavirCeftriaxone | Yes | Yes | 4 |
| Tard et al74 | 76 | M | Isquemic cardiomyopathyAAAHTNHLD | CoughAsthenia | RT-PCRNP swab | NR | Yes | Yes | 1 |
| Dufour et al75 | 36 | F | Obesity | DyspneaAnosmia | RT-PCRNP swab | Supportive | No | No | NA |
| Nanda et al76 | 55 | F | DM2HTNCLT | FeverAbdominal pain | RT-PCRNP swab | NR | No | No | NA |
| Nanda et al76 | 72 | M | HTN | CoughFever | RT-PCRNP swab | Supportive | Yes | Yes | NR |
| Nanda et al76 | 55 | M | DM2HTNCKD | CoughSore throat | RT-PCRNP swab | NR | No | No | NA |
| Nanda et al76 | 49 | M | HTN | Fever | RT-PCRNP swab | NR | No | No | NA |
| Raahimi et al77 | 46 | M | HTN | NR | RT-PCRNP swab | Supportive | No | Yes | NR |
AAA indicates abdominal aortic aneurysm; CKD, chronic kidney disease; CLT, chronic lymphocytic thyroiditis; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus Disease 19; CSF, cerebrospinal fluid; DM2, type 2 diabetes mellitus; Dx, diagnostic; F, female; GBS, Guillain-Barré syndrome; GERD, gastroesophageal reflux disease; GI, gastrointestinal; HLD, hyperlipidemia; HTN, hypertension; ICU, intensive care unit; M, male; MRI, magnetic resonance imaging; NA, not applicable; NE, not evocable; NP, nasopharyngeal; NR, not reported; OP, oropharyngeal; RA, rheumatoid arthritis; RLS, restless leg syndrome; RT-PCR, reverse transcription-polymerase chain reaction; T, treated; Time, time between hospital admission and ICU admission; URTI, upper respiratory tract infection.
Ninety-nine percent of the patients were diagnosed with COVID-19 before GBS symptoms were recorded, with 1 patient who had GBS symptoms 7 days before the COVID-19 diagnosis. The median interval between COVID-19 diagnosis and the first recorded neurological symptoms was 14 (interquartile range=7 to 20) days. Paresthesia (41%), quadriparesis (28%), areflexia (27%), paraparesis (26%), dysphagia (15%), facial paresis (14%), ataxia (12%), asthenia (12%), hypoesthesia (10%), respiratory failure (7%), facial diplegia (6%), paraplegia (3%), and quadriplegia (3%) were the GBS symptoms reported among the patients respectively. Forty-four percent of the cases reported performing biological tests for other viral infections. Among these patients, human immunodeficiency virus (68%), followed by influenza viruses (21%) were the most common tested viruses. Nineteen percent of the cases reported performing magnetic resonance imaging. Twelve percent of these cases did not detect any GBS-related findings. However, 31% reported enhancement of caudal nerve roots, and 12% reported abnormal enhancement of facial nerve. A motor nerve conduction study was performed in 76% of the cases. Among these cases, the most frequently examined nerves for velocity assessment were tibial nerve (54%), common peroneal nerve (37%), and the median nerve (37%). For those cases in which the tibial nerve was tested, 49% showed bilateral absent or decreased velocity, 26% showed unilateral decreased velocity, and 17% showed normal velocity at the tibial nerve. Among cases who reported common peroneal nerve testing, 71% had bilateral absent or decreased velocity, 21% had normal velocity, and 8% had unilateral decreased velocity at the common peroneal nerve. For cases with reported median nerve testing, 50% had bilateral absent or decreased velocity, 25% had normal velocity, and 25% had unilateral absent or decreased velocity at the median nerve. Sixty-five percent of the cases reported the type of GBS; among them, 54% were acute inflammatory demyelinating polyneuropathy (AIDP), 32% were acute motor-sensory axonal neuropathy (AMSAN), 11% were MFS and 4% had isolated facial diplegia. Almost all of the cases (98%) reported their choice of GBS management. Intravenous immunoglobulin (IVIG) (87%) was the most used treatment approach followed by plasma exchange (8%). Four percent of patients who received IVIG also underwent plasmapheresis; 2% received low molecular weight heparin (LMWH) or enoxaparin, and 1% Gabapentin. Two percent of patients were treated only with prednisone, and 5% received no specific GBS treatments. Detailed GBS clinical and management data are demonstrated in Table 4 and diagnostic data in Table 5.
TABLE 4.
GBS-related Data Among COVID-19–Related GBS Cases
| References | GBS Symptoms | ND | CN Involvement | AD Symptoms | Time | MRC and DTR | CSF | GBS Subtype | GBS Management | IVIG-D | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | Paresthesia Tetraparesis Hypesthesia Areflexia | 3 | No | No | 4 | 3/5 UE 2/5 LE DTR absent global (S) | P: 54 mg/dL L: 9 cells/μL | NR | IVIG (0.40 g/kg/d for 5 d) | 3 | Deceased (severe respiratory failure) |
| 18 | Paresthesia Tetraparesis Areflexia | 9 (I) | Dysphagia | No | 11 | 2/5 PUE 4/5 DUE 2/5 LE DTR absent global (S) | P: 166 mgl/dL L: NR | AIDP | IVIG (0.40 g/kg/d for 5 d); LMWH | 3 | ICU admission and mechanically ventilated (respiratory insufficiency) |
| 19 | Paresthesia Quadriplegia Areflexia | 10 | No | No | 3 | NR | P: 100 mg/dL L: NR | AMSAN | IVIG (2 g/kg for 5 d) | 10 | No significant neurological improvement after 1 wk of treatment |
| 25 | FDP | 1 | FDP | No | 10 | NR Absent blink reflex bilaterally (S) | P: 44 mg/dL L: absent | FDP | Prednisone | NA | Small improvement of symptoms bilaterally after 2 wk |
| 26 | Lumbago Paresthesia Tetraparesis Areflexia | 10 | Dysphagia | No | 8 | 0/5 PUE 4/5 DUE 0/5 PLE 2-3/5 DLE DTR absent global (S) | P: NR L: NR | NR | None | NA | Deceased (severe respiratory failure) |
| 15 | Paraparesis Paraplegia Areflexia | 3 | Unilateral FNP | No | 10 | Initial 4/5 DUE(S) | P: 108 mg/dL L: absent | NR | IVIG (0.4 g/kg for 5 d) | 3 | Did not improve with treatment, progressively developed proximal weakness in all extremities, dysesthesia, and unilateral facial palsy |
| 16 | Paresthesia Tetraparesis Areflexia | 1 | No | No | 24 | 4/5 DUE 4/5 DLE DTR absent global (S) | P: 48 mg/dL L: 1 cell/L | NR | IVIG (400 mg/die for 5 d) | 3 | Worsening of muscle weakness causing respiratory failure |
| 4 | Paresthesia Paraparesis Areflexia | 10 | Dysphagia | No | 21 | 3/5 PLE 4/5 DLE DTR absent LE(S) | P: 140 g/L L: normal | AIDP | IVIG (0.40 g/kg/d for 5 d) | 12 | Almost complete recovery of neurological symptoms after the treatment |
| 14 | Tetraparesis FDP Areflexia | 5 | FDP | No | 9 | 2/5 PUE 3/5 DUE 1/5 PLE 2/5 DLE Grade 3 HB DTR absent global (S) | Not performed | AMSAN | IVIG (0.40 g/kg/d for 5 d) | 14 | NR |
| 27 | Tetraparesis Areflexia | 1 | No | No | 7(I) | 4/5 PUE 4/5 DUE 3/5 PLE 3/5 DLE DTR absent LE(S) | P: 124 mg/dL L: 5 cells/dL | AIDP | IVIG (dose NR) | 4 | Normal muscle strength in both UE and LE and return of DTR in LE |
| 27 | Paresthesia Tetraplegia Facial paresis Areflexia | 1 | Dysphagia Tongue weakness | No | 7 | NR | P: 101 mg/dL L: 4 cells/mm3 | AMSAN | IVIG (2 cycles; dose NR) | 2 | Persistence of severe UL weakness, LL paraplegia and dysphagia |
| 27 | Paresthesia FDP Ataxia Areflexia | <1 | FDP | No | 10 | NR | P: 123 mg/dL L: absent | AMSAN | IVIG (1 cycle; dose NR) | 1 | Decreased ataxia, disappearance of limb paresthesia, and mild decrease of facial weakness |
| 27 | Tetraparesis Facial paresis Respiratory failure Areflexia | 1 | FDP | No | 10 | NR | P: 193 mg/dL L: absent | AMAN | IVIG (2 cycles; dose NR) | 4 | Neuromuscular respiratory failure, progression to flaccid tetraplegia. His condition remained critical after 1 mo of neurological onset |
| 27 | Tetraparesis Ataxia Areflexia | 1 | No | No | 5 | NR | P: normal L: absent | AIDP | IVIG (1 cycle; dose NR) | 7 | Mild motor improvement after treatment, more evident in UE. However, patient unable to stand 1 mo after symptoms onset |
| 27 | Facial paresis Paraplegia Respiratory failure | 1 | Facial paresis Dysphagia | No | 7 | NR | P: 40 mg/dL L: 3 cells/mm3 | AIDP | IVIG (1 cycle; dose NR); plasmapheresis | 2 | Neuromuscular respiratory failure with concomitant Acinetobacter pneumonia during IVIG treatment. Patient still tetraplegic and ventilation dependent 4 wk after neurological onset |
| 28 | Paresthesia Ataxia | NR | No | No | NR | NR | P: 1928 mg/dL L: 2.6 cells/μL | AIDP | NR | NA | NR |
| 23 | Tetraparesis Hypoesthesia Facial paresis Dysphagia Areflexia | NR | Facial paresis Dysphagia | No | 10 | 3/5 PUE 3/5 PLE 4/5 DUE 4/5 DLE DTR absent global (S) | NR | NR | IVIG (dose NR) | NR | Worsening of motor function during the first 2 d of hospitalization, adding facial paresis and dysphagia to the previous symptoms. Slight improvement of neurologic and respiratory symptoms afterwards |
| 29 | Tetraparesis Areflexia | 2 | No | UR | 8 | 3/5 UE 2/5 LE DTR absent global (S) | Not performed | NR | IVIG (0.40 g/kg/d for 5 d) | 2 | Improvement of respiratory symptoms and UE weakness. LE weakness persisted after treatment |
| 20 | Paraparesis Allodynia Areflexia | 4 | No | UR Constipation | 10 | MRC NR DTR absent global (S) | Albumin-cytologic dissociation. Levels not reported | AIDP | IVIG (0.40 g/kg/d for 5 d) | 5 | Rapid improvement of neurological symptoms after treatment |
| 30 | Tetraparesis Areflexia FDP | NR | FDP | UR Resting tachycardia | 14 | 3/5 PUE 4/5 DLE 0-1/5 PLE 0-1/5 DLE(S) | Not performed | AIDP | IVIG (0.40 g/kg/d for 5 d) | NR | Improvement of respiratory symptoms. Worsening of neurological symptoms at follow up progressing to tetraparesis and FDP |
| 31 | Areflexia Paraparesis Decreased proprioception | 4 | No | No | 21 | NR | P: 1.65 g/L L: absent | NR | IVIG for 5 d (dose NR) | NR | NR |
| 32 | Areflexia FDP Dysarthria | NR | Yes | No | No COVID-19 symptoms at onset of neurological symptoms | NR | P: 1.00 g/L L: 4×106 cells/L | AIDP | IVIG (0.40 g/kg/d for 5 d) | 2 | The patient was discharged from hospital 2 d after completing IVIG. At that time, he had slight movements of his facial muscles, and the distal paresthesias of his lower extremities were unchanged |
| 33 | Paresthesias Quadriparesis FDP Dysphagia | 1 | Yes | No | 14 | 2/5 left UE 3/5 right UE 4/5 LE DTR absent global (S) | P: 0.86 g/L L: 3 cells/mm3 | AMSAN | IVIG (0.40 g/kg/d for 5 d) | NR | After 5 d of ICU admission, she was discharged to the neurology ward for clinical improvement with a motor balance of 5/5 (right arm), 3/5 (left arm), and 4/5 (both legs), with paresthesias persisting |
| 34 | Hyporeflexia Hypoesthesia Decreased proprioception | 7 | No | No | 10 | 4/5 UE 3/5 LE DTR absent LE(S) | NR | AIDP | IVIG (0.40 g/kg/d for 5 d) | 7 | On discharge patient could ambulate but with some residual weakness in lower extremities, so was referred for rehabilitation clinic |
| 35 | Hypoesthesia Dysesthesia Ataxia Paraparesis | 2 | No | No | 14 | 2/5 PUE 4/5 DUE 2/5 PLE 4/5 DLE DTR absent global (S) | P: 64 mg/dL L: 2 cells/mm3 | AIDP | IVIG 30g total dose for 1 d, followed by 4 cycles of plasma exchange | 3 | The patient improved gradually and was transferred to a neurorehabilitation facility 4 wk after symptom onset, where he regained mobility without significant help another 4 wk later |
| 36 | Dysarthria Hypogeusia Facial paresis Hypoesthesia Paraparesis | 1 | Yes | No | 16 | 4/5 PUE 4/5 PLE DTR absent global (S) | P: 46 mg/dL L: absent | Bifacial weakness with paresthesias (BFP) | 5 cycles plasma exchange | NR | Tolerated plasma exchange well with slight improvement in facial weakness and paresthesia. Discharged to inpatient rehabilitation |
| 37 | Ophthalmoparesis Ataxia Hyporeflexia Hypoesthesia | NR | Yes | No | 2 | NR | NR | MFS | IVIG (dose and duration NR) | NR | Subsequent improvement of neurological symptoms after IVIG treatment. Patient was discharged after 4 d of hospitalization |
| 38 | Quadriparesis Ataxia Paresthesia Dysgeusia Cacosmia | NR | No | Yes | 15 | NR | P: 60 mg/dL L: 3 cells/mm3 | AIDP | IVIG (0.40 g/kg/d for 5 d) | 2 | At 5 d, improvement of tetraparesis. Able to stand up with assistance |
| 38 | Tetraparesis Paresthesia Areflexia | NR | No | No | 7 | NR | P: 40 mg/dL L: 2 cells/mm3 | AIDP | IVIG (0.40 g/kg/d for 5 d) | 10 | At 5 d, dismissal with full motor recovery. Persistence of lower limb areflexia and distal paresthesia |
| 38 | Facial diplegia Paresthesia Paraparesis Dysphagia Areflexia | NR | Yes | Yes | 22 | NR | P: 140 mg/dL L: 4 cells/mm3 | AIDP | IVIG (0.40 g/kg/d for 5 d) | 2 | At 5 d, improvement of tetraparesis and ability to walk with assistance. Persistence of neuropathic pain and distal paresthesia |
| 39 | Diplopia Paraparesis Facial paresis Areflexia | 12 | Yes | Yes | 15 | 3/5 PLE 2/5 DLE DTR absent global (S) | P: 70 mg/dL L: 5 cells/mm3 | MFS | IVIG (0.40 g/kg/d for 5 d); gabapentin 900 mg/d | 13 | Progressive improvement in facial and limb paresis, diplopia and pain. Patient still on neurological rehabilitation |
| 40 | Paresthesia Quadriplegia Areflexia | 1 | No | Yes | 6 | 3/5 PUE 3/5 PLE DTR absent global (S) | P: 313 mg/dL L: 1 cell/mm3 | AIDP | IVIG (2 g/kg divided over 3 d) | 3 | Transferred to ICU and intubated. Developed ventilator-associated pneumonia (Stenotrophomonas maltophilia). Remains in the ICU with severe weakness |
| 41 | Quadriparesis Hypoesthesia | 1 | No | No | 6 | 4/5 UE 3/5 PLE 2/5 DLE DTR absent global (S) | P: 51 mg/dL L: normal cell counts | AIDP | IVIG (0.40 g/kg/d for 5 d) | 2 | Intubated and ventilated in the ICU. Treated for aspiration pneumonia. Oxygen requirements and inflammatory markers have improved; patient currently being weaned-off ventilation |
| 42 | Paresthesia Ataxia FNP | 4 | Yes | NR | 21 | 4/5 UE 3/5 DLE DTR absent global (S) | P: 0.94 g/L L: normal cell count | NR | IVIG started on day 5 (2 g/kg) | 5 | Discharged home with progressive improvement |
| 42 | Tetraparesis Dyspnea FNP | 3 | Yes | NR | 10 | 2/5 PLE 4/5 DLE DTR absent LE(S) | P: 1.06 g/L 6×106/L | NR | IVIG (2 g/kg) started day 4 of neurological symptoms | 4 | Condition improved slowly with physiotherapy, needing transfer to rehabilitation center |
| 43 | Dysphagia Facial paresis | NR | Yes | NR | 20 | NR | P: normal L: NR | GBS/MFS overlap syndrome | IVIG (0.4 g/kg/d for 5 d) | NR | Very rapid clinical response in swallowing, speech, tongue mobility and strength, and eyelid ptosis |
| 44 | Paraparesis | NR | NR | Paralytic ileus Loss of blood pressure control, | 23 | NR | P: normal L: NR | ASMAN | IVIG (0.4 g/kg/d for 5 d) | 3 | Autonomic symptomatology significantly improved—remission of gastroplegia and recovery of intestinal functions. Persistent osteotendinous hyporeflexia but slight improvement in foot drop |
| 45 | Hyposthenia Paresthesia Dysphagia Dysarthria FDP | NR | Yes | NR | 0 | 4/5 PUE 3/5 DUE 2/5 PLE 1/5 DLE DTR absent global (S) | P: 245 mg/dL L: 13/mm3 | NR | IVIG for 5 d | NR | Immediately after IVIG, improved to MRC scale of 4/5 in distal upper limbs and 3/5 in both proximal and distal lower limbs, FDP developed, ultimately transferred to rehabilitaiton care |
| 46 | Paraparesis Paresthesia FNP | 2 | Yes | NR | 18 | 4/5 UE 4/5 PLE 3/5 DLE DTR absent global (S) | P: 78 mg/dL L: 4/mm3 | NR | Did not receive treatment | NA | After 16 d of close monitoring, his muscle forces improved to near normal |
| 46 | Paraparesis Paresthesia | 4 | No | NR | 10 | 4/5 UE 2/5 PLE 3/5 DLE DTR absent at LE, decreased at UE(S) | NR | NR | IVIG (dose NR) | NR | Discharged after 14 d, muscle forces were 4/5 in all extremities |
| 47 | Paraparesis Paresthesia Facial paresis Dysphagia Dysarthria | 5 | Yes | NR | 18 | 4/5 PLE DTR absent at LE(S) | P: 226 mg/dL L: 3 cells/mm3 | NR | Plasmapheresis | NA | dysphagia has resolved and 28 d after GBS symptom onset, he can now ambulate with minimal assistance |
| 47 | Paraparesis Paresthesia Facial paresis Respiratory failure | 7 | Yes | NR | 23 | 3/5 PUE 4/5 PLE DTR absent at LE(S) | P: 67 mg/dL L: 1 cell/mm3 | NR | IVIG (dose NR); plasmapheresis | NR | Underwent tracheostomy and 25 d after GBS symptom onset, he remains quadriparetic with intermittent autonomic dysfunction, but is slowly being weaned from the ventilator |
| 48 | Tetraparesis Paresthesia FNP Dysphagia | 2 (I) | Yes | NR | 15 | 2/5 all extremities DTR absent global (S) | P: 0.86 g/L L: 3 cells/mm3 | NR | IVIG (2 g/kg/5 d) | NR | Started recovering by day 7 after the onset of weakness |
| 49 | FDP Paraparesis | 4 | Yes | NR | 28 | MRC NR DTR absent global (S) | P: normal L: normal | AMSAN | IVIG (2 g/kg/5 d) | 7 | Recovery started within days of treatment. On day 14 the patient was discharged with a mild proximal weakness in the lower extremities and FDP |
| 78 | Dysarthria Paraparesis | 3 | Yes | NR | (I)# NR | 4/5 LE DTR absent at LE(S) | P: 32.6 mg/dL L: normal | NR | Plasmapheresis | NA | Two weeks after the onset of symptoms, the neurological findings had improved markedly and she was able to walk without assistance |
| 51 | Tetraparesis Paresthesia FDP | 2 | Yes | NR | 14 | 2-4/5 all extremities DTR absent global (S) | P: normal L: 9 cell/μL | AIPD | IVIG (30 g daily for 5 d) | <1 | Thirty-one days after admission signs of motor improvement with regressive facial and hypoglossal paresis but still needed mechanical ventilation |
| 52 | Paresthesia Asthenia | NR | No | NR | 20 | 4/4 PLE 5/5 DLE 4/4 UE | NR | NR | Did not receive treatment | NA | Symptoms improved with discharge home on day 18 |
| 53 | Paresthesia Asthenia Lumbago Ascending quadriparesis | NR | No | No | 14 | 3/5 LE 4/5 UE | P: 117 mg/dL L: 2 cumm | NR | 2 mg/kg IVIG for 4 d | NR | Symptoms improved significantly, discharged to acute rehabilitation facility |
| 54 | Paresthesia Facial diplegia Asthenia | NR | Facial diplegia | None | 21 | 1/5 LE 3/5 PUE 2/5 DUE DTR absent global (S) | P: >1.25 g/L L: 1×106 cells/L | AIDP | IVIG 0.4 g/kg for 5 d | NR | Gradually improved, able to mobilize unassisted with neurorehabilitation and 15 wk after IVIG treatment |
| 55 | Paresthesia Dysautonomia | NR | No | UR | 3 mo | 4/5 LE DTR absent LE (S) | P: 127 mg/dL L: 8/cmm | NR | IVIG | NR | Motor and sensation largely returned at discharge |
| 56 | Paraplegia Urinary retention | NR | Esotropia, dysconjugate gaze | UR | NR | 3/5 UE 2/5 LE DTR absent global (S) | P: 620 mg/dL L: 1 cell/cumm | AIDP | IVIG 2 g/kg over 48 h | 2 | Extubated on hospital day 5, transferred to inpatient rehabilitation 3 wk after IVIG completion |
| 57 | Asthenia Paresthesia | 2 | Weak cough Dysphagia Dysarthria | Labile blood pressure Fecal retention | 13 | 5/5 UE 4/5 LE | P: 1.5 g/L L: absent | AIDP | IVIG 0.4 g/kg | NR | Extubated on hospital day 18, discharged to community rehabilitation unit, then to home |
| 58 | Asthenia Areflexia Paresthesia | 1 | NR | NR | 4 | 3/5 PUE 2/5 DUE 3/5 LE DTR absent global (S) | P: 1.14 g/L L: <1/mm3 | AMSAN | IVIG 0.4 g/kg/d for 5 d | NR | Discharged on hospital day 12, significant improvement with residual weakness in hands and feet |
| 59 | Tetraparesis | NR | Facial paresis | NR | 18 | MRC: NR DTR absent global (S) | P: 52 mg/dL L: 1 cell/mm3 | NR | IVIG cycle (0.4 g/kg for 5 d) | NR | Progressive improvement of tetraparesis after initiating IVIG therapy |
| 59 | Tetraparesis | NR | None | NR | 30 | MRC: NR DTR absent global (S) | P: 40 mg/dL L: 1 cell/mm3 | NR | IVIG cycle (0.4 g/kg for 5 d) | NR | Progressive improvement of asthenia after initiating IVIG therapy |
| 59 | Ophthalmoplegia Ataxia | NR | Ophthalmoplegia Facial hypoesthesia | NR | 14 | MRC: NR DTR absent global (S) | P: 72 mg/dL L: 5 cell/mm3 | NR | IVIG cycle (0.4 g/kg for 5 d) | NR | Progressive improvement of neurological symptoms after initiating IVIG therapy |
| 59 | Lower extremity Asthenia | NR | None | NR | 33 | MRC: NR DTR diminished global (S) | Not performed | NR | Methylprednisolone 60 mg for 5 d | NR | Stationary; no significant improvement of neurological symptoms after initiating IVIG therapy |
| 59 | Asthenia Facial paresis Diplopia | NR | Facial paresis Diplopia | NR | 22 | MRC: NR DTR absent global (S) | P: 53 mg/dL L: 2 cell/mm3 | NR | IVIG cycle (0.4 g/kg for 5 d) | NR | Progressive improvement of neurological symptoms after initiating IVIG therapy |
| 60 | Dysphagia Asthenia Paresthesias Facial diplegia Dysphagia Dysarthria | 2 | Facial diplegia and paresthesias Dysphagia Dysarthria | NR | 1 | 4/5 UE 3/5 PLE DTR: +1 throughout | P: 74 mg/dL L: absent | Recurrent GBS secondary to COVID-19 infection or CIDP | IVIG cycle (0.4 g/kg for 5 d) | 3 | Residual asthenia and hypoxia resolved weeks after discharge; regained full muscle strength but severe persistent paresthesias of the medial left knee up to the medial thigh |
| 61 | Hypoesthesia Paraparesis | 1 | None | NR | 5 | 2/5 left UE 3/5 right DUE DTR absent global (S) | P: 52 mg/dL L: absent | ADP | IVIG cycle (0.4 g/kg for 5 d) | NR | Discharged from ICU 14 d after intubation with residual severe flaccid tetraparesis, bilateral facial palsy, and dysphagia; underwent 7 wk of rehabilitation and now able to walk independently with support |
| 62 | Quadriparesis Facial paresis Asthenia | 5 | NR | NR | 7 | 1/5 LE 2/5 UE DTR absent global (S) | NR | NR | IVIG cycle (0.4 g/kg for 5 d) | NR | Respiratory and neurological status improved 5 d after admission after course of IVIG; planned for weaning and extubation on day 6 but patient unintentionally self-extubated and expired from cardiac arrest |
| 63 | Paresthesias Asthenia Respiratory failure | NR | NR | Loss of blood pressure and heart rate control Fecal incontinence Urinary retention | 22 | 2/5 LE 3/5 UE 3/5 neck flexion and extension DTR: absent in LE, diminished in UE | P: 197 mg/dL L: absent | NR | IVIG cycle (0.4 g/kg for 5 d), enoxaparin 30 mg bid | NR | Improvement in respiratory and neurological function; ambulating with assistance 2 mo after admission; persistent neuropathic pain in lower extremities |
| 64 | Paresthesias Asthenia Gait disturbance | 13 | No | No | 18 | 3/5 right DLE 4/5 left DLE 4/5 DUE DTR: diminished global (S) | P: normal L: normal | AMSAN | IVIG cycle (0.4 g/kg for 5 d) | 16 | After IVIG, significant improvement in asthenia but persistent gait disturbance; patient transferred to rehabilitation and slowly regained ability to walk unassisted after 1 mo at discharge |
| 65 | NR | NR | NR | NR | NR | NR | NR | AIDP | IVIG | NR | NR |
| 65 | NR | NR | NR | NR | 0 | NR | P: 0.6 g/L L: NR | Classical GBS | IVIG | NR | NR |
| 65 | NR | NR | Yes | NR | 20 | NR | P: 0.3 g/L L: NR | NFS-GBS Overlap | IVIG | NR | NR |
| 65 | NR | NR | NR | NR | 3 | NR | P: 1 g/L L: NR | Classical GBS | IVIG | NR | NR |
| 65 | NR | NR | NR | NR | 20 | NR | P: 0.2 g/L L: NR | AMSAN | IVIG | NR | NR |
| 65 | NR | NR | NR | NR | 15 | NR | P: 0.9 g/L L: NR | AMSAN | IVIG | NR | NR |
| 66 | Ataxia Asthenia Paresthesias Dysphagia Quadriparesis Respiratory failure | NR | Bilateral ptosis, CN 3,4,6 deficits Dysphagia | NR | 14 | 2/5 right UE, LE 0/5 left UE DTR absent global (S) | P: normal L: normal | MFS-GBS overlap | IVIG cycle (0.4 g/kg for 5 d) | 4 | 5 wk after admission, transferred to LTAC for vent weaning and PT, now 5.5 postdiagnosis and tolerating few hours per day of pressure support; patient able to control head, some distal extremity, extraocular, and tongue movements |
| 79 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 67 | FDP Pharyngeal paralysis Dysphagia Quadriparesis | 4 | FDP Pharyngeal paralysis Dysphagia | Urinary incontinence | 5 to 10 | 2/5 PLE 1/5 DLE 3/5 UE DTR diminished global (S) | P: 64 mg/dL L: normal | AIDP | IVIG cycle (0.4 g/kg for 5 d) | NR | Marked neurological improvement on hospital day 16 with residual urinary incontinence; patient transferred to physiotherapy unit for rehabilitation |
| 68 | Paresthesia Tetraparesis | 1 | No | UR | 5 | 3/5 UE 3/5 LE DTR absent global (S) | P: 222 mg/dL L: 0 cells/μL | NR | 5 cycles plasma exchange | NA | Hospitalization subsequently complicated by streptococcal bacteremia requiring antibiotics. Discharged at day 30 with improved neurological condition |
| 69 | Paraparesis | 26 (I) | NR | No | 31 | 3/5 LE DTR absent LE (S) | P: 48 mg/dL L: 0 cells/μL | AMSAN | IVIG (dose NR) | 5 | Deceased due to ARDS |
| 70 | Paraparesis | NR | NR | UR Loss of blood pressure control | 20 | MRC NR DTR absent global (S) | P: normal range L: NR | AMSAN | IVIG (0.4 g/kg/d) | NR | After 5 d, the vegetative symptomatology significantly improved, with the remission of gastroplegia and recovery of intestinal functions |
| 70 | Ptosis Dysphagia Dysphonia | 20 (I) | Yes | No | 20 | 5/5 both UE and LE DTR decreased (S) | P: normal L: NR | MFS | IVIG (0.4 g/kg/d) 5 d | NR | The first clinical improvements occurred during the fifth day of treatment, with progressively improving trend and complete remission on swallowing and feeding |
| 71 | Tetraparesis | 1 | NR | No | <1 | 2/5 both UE and LE DTR decreased global (S) | P: 39 mg/dL L: 1 cell/μL | AIPD | IVIG (0.4 g/kg/d) 5 d | NR | Patient was extubated on the 17th day of illness. Subsequently, he was discharged from the hospital 24th day of illness with no residual muscle weakness |
| 72 | FDP Dysarthria Dysphagia | <1 | Yes | No | 7 | 5/5 both UE and LE | P: 77 mg/dL L: NR | AIPD | IVIG (0.4 g/kg/d)5 d | NR | Progressive clinical improvement was observed after 2nd dose of IVIG, leading to discharge |
| 73 | Paraparesis | 27 (I) | Yes | Hypertensive crisis Tachyarrhythmia-bradyarrythmia | 36 | 3/5 UE 4/5 LE DTR decreased global (S) | P: 48 mg/dL L: 0 cells/μL | AIPD | Supportive | NA | Forty days from fever the patient showed a spontaneous improvement of the clinical picture, at day 56 after admission only mild weakness of the deltoid bilaterally and left biceps was evident |
| 74 | Tetraplegia Paresthesis FDP | <1 | Yes | No | 10 | Initial MRC NR DTR absent global (S) | P: 1 g/dL L: 0 cells/μL | NR | IVIG (0.4 g/kg/d) 5 d Plasma exchange (4 cycles) | NR | Following IVIG and steroids, a partial clinical improvement was seen. Two months after onset, FDP was still severe but improvements in muscle strength continued in axial, proximal and distal segments |
| 75 | Paraparesis | 3 | No | No | 21 | 5/5 UE 3/5 LE DTR absent at LE (S) | P: 20 mg/dL L: 0 cells/μL | NR | IVIG (0.4 g/kg/d) 5 d | NR | After 1 wk of hospitalization, her strength began to improve. She was eventually discharged home after 10 d in the hospital. A follow-up phone call after 3 wk, found that that patient was already ambulating short distances with minor help |
| 76 | Tetraparesis | 3 | No | No | 10 | 4/5 UE 2/5 LE DTR absent global (S) | P: 54 mg/dL L: 5 cells/μL | AMAN | IVIG (0.4 g/kg/d) 5 d | NR | Patient was discharged after 10 d of hospital stay with grade 4/5 power in both lower limbs and grade 4+/5 power in both upper limbs |
| 76 | Tetraparesis | 3 | No | No | 6 | 3/5 UE 2/5 LE DTR absent global (S) | P: 74 mg/dL L: 0 cells/μL | AMSAN | IVIG (0.4 g/kg/d) 5 d | NR | Worsening respiratory distress, patient expired after 7 d of hospitalization |
| 76 | Tetraparesis | 3 | No | No | 7 | 4/5 UE 3/5 LE DTR absent at LE (S) | P: 84 mg/dL L: 5 cells/dL | AMSAN | IVIG (0.4 g/kg/d) 5 d | NR | Good improvement (able to walk independently at discharge) |
| 76 | FNP Paraparesis | 4 | Yes | No | 10 | 5/5 UE 3/5 LE DTR absent at LE | P: 52 mg/dL L: 5 cells/dL | AMAN | IVIG (0.4 g/kg/d) 5 d | NR | Good improvement (able to walk independently at discharge) |
| 77 | Paraparesis Parestesis | 7 | No | No | 53 | 5/5 UE 3/5 LE DTR absent at LE | P: 127 mg/dL L: <2 cells/dL | AIDP | IVIG (0.4 g/kg/d) 5 d | NR | Three months after his hospital discharge, he has been able to walk independently, occasionally using a stick for longer distances |
AAA indicates abdominal aortic aneurysm; AD, autonomic dysregulation; AIDP, acute inflammatory demyelinating polyradiculoneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor-sensory axonal neuropathy; ARDS, acute respiratory distress syndrome; CN, cranial nerve; COVID-19, Coronavirus Disease 19; CSF, cerebrospinal fluid; DLE, distal lower extremities; DTR, deep tendon reflexes; DUE, distal upper extremities; FDP, facial diplegia; FNP, facial nerve palsy; GBS, Guillain-Barré syndrome; HB, House-Brackmann Facial Paralysis Scale; (I), symptoms started inversely; ICU, intensive care unit; IVIG, intravenous immunoglobulin; IVIG-D, days between neurological symptom’s onset and the start of IVIG treatment; L, leukocytes; LE, lower extremities; LMWH, low–molecular-weight heparin; LTAC, Long-term acute care; MFS, Miller-Fisher syndrome; MRC, Medical Research Council Scale for Muscle Strength; NA, not applicable; ND, days between neurological symptoms and hospital admission; NR, not reported; P, protein; PLE, proximal lower extremities; PT, physical therapy; PUE, proximal upper extremities; (S), symmetric; Time, days between the onset of COVID-19 symptoms and onset of neurological symptoms; UE, upper extremities; UR, urinary retention.
TABLE 5.
GBS Diagnostic Data Among COVID-19–Related GBS Cases
| References | Antigangliosides Antibodies in Serum | CSF PCR Analysis for COVID-19 | Motor nerve Conduction Study (V, F Waves) | Biological Test for Infections Other Than COVID-19 | MRI Findings Related to GBS |
|---|---|---|---|---|---|
| 17 | NR | Negative | V=decreased at CPN, RN Absent at TN F waves=not performed at UE and LE | NR | Not performed |
| 18 | Negative | NR | V=decreased at right MN, as well as bilateral UN, CPN, and TN F waves=absent at CPN, TN bilaterally | Negative for Campylobacter jejuni, Mycoplasma pneumoniae, Salmonella enterica, CMV, EBV, HSV1, and 2, VZV, influenza virus A and B, HIV, and hepatitis E | Not performed |
| 19 | NR | Negative | V=normal in all extremities F waves=NR Marked reduction or absence of EP in both motor and sensory nerves | NR | Not performed |
| 25 | NR | Negative | Not performed | NR | None |
| 26 | NR | NR | Not performed | NR | Not performed |
| 15 | Negative | Negative | V=decreased at left TN and left CPN F waves=absent at left TN, CPN and right MN | NR | Not performed |
| 16 | NR | NR | V=decreased at MN, UN and TN bilaterally, NE at CPN bilaterally F waves=absent at MN, UN, TN, and CPN bilaterally | Negative for Mycoplasma pneumoniae, Legionella pneumophila, Streptococcus pneumoniae, HSV, VZV, EBV, CMV, HIV-1, Borrelia burgdorferi | Not performed |
| 4 | NR | NR | V=normal at TN, MN, UN bilaterally F waves=normal at TN with pathologic intermediate latency responses (complex A-waves) bilaterally | Negative for Lyme disease, Campylobacter jejuni, HIV | None |
| 14 | NR | NR | V=No response at MN, UN and CPN F waves=no response at TN | NR | None |
| 27 | NR | NR | V=normal in UE and LE F waves=absent at left UN, and TN bilaterally | NR | Not performed |
| 28 | Negative | NR | V=normal at UN and TN F waves=absent at UN and TN | NR | Enhancement of caudal nerve roots |
| 28 | NR | NR | V=decreased at TN F waves=absent at TN | NR | Enhancement of FN bilaterally |
| 28 | Negative | NR | V=normal at UN and TN F waves=absent at UN and TN | NR | Enhancement of caudal nerve roots |
| 28 | NR | NR | V=decreased at TN F waves==normal at UN and TN | NR | None |
| 28 | Negative | NR | V=decreased at TN F waves=absent at TN | Negative for Campylobacter jejuni, EBV, CMV, HSV, VZV, influenza, HIV Patient developed Acinetobacter pneumonia during ICU stay | None |
| 23 | Negative | Negative | NR | Negative for influenza virus A and B, Borrelia and TBE | NR |
| 29 | NR | NR | V=decreased, nerves NR F waves=increased minimal latency at right L5 and S1 roots | NR | Not performed |
| 20 | NR | NR | Not performed | Positive for Rhinovirus (NP swab) | None |
| 30 | Negative | Negative | V=NR F waves=decreased persistence or absence in tested nerves Nerves NR | NR | None |
| 31 | NR | NR | V=decreased Nerves NR F waves=absent Nerves NR | Negative for Influenza virus A and B | None |
| 32 | Negative | Negative | V=decreased in bilateral MN, UN, TN, CPN F waves=Absent in all 4 limbs | Negative for Campylobacter jejuni, HIV, syphilis, CMV, and EBV | Not performed |
| 33 | NR | Negative | V=NR F waves=Absent in the left TN | NR | Bilateral facial nerve enhancement involving the labyrinthine segment, tympanic segment, mastoid segment, and extracranial facial nerve |
| 34 | NR | Negative | V=NR F waves=Absent in the bilateral TN and UN | NR | Slight leptomeningeal enhancement at the brainstem and cervical cord |
| 35 | NR | NR | V=Decreased at left TN, bilateral MN and UN F waves=Absent in the bilateral TN and CPN | NR | NR |
| 36 | Negative | Negative (positive for anti-SARS-CoV-2-antibodies) | V=Normal at right MN, UN, CPN, TN F waves=Absent in right CPN and right TN | Negative for CMV, EBV, influenza virus A/B, respiratory syncytial virus, Chlamydia pneumonia, Mycoplasma pneumonia | None |
| 37 | Negative | NR | V=Decreased at left MN, CPN F waves=NR | Positive for HSV Negative for adenovirus, influenza a/b, parainfluenza 1/2/3/4, Chlamydia pneumonia, Mycoplasma pneumonia, bocavirus, coronavirus, respiratory syncytial virus A/B, metapneumovirus, rhinovirus/enterovirus | Abnormal enhancement of the facial (CNVII) and abducens (CNVI) nerves bilaterally, as well as the right oculomotor nerve (CNIII) |
| 38 | Negative | NR | V=NR F waves=NR | NR | Striking enlargement, prominent enhancement with gadolinium, and T2 hyperintense signal of the left cranial nerve (CN) III |
| 39 | Negative | Negative | V=Decreased at MN, UN, TN, CPN F waves=Absent | NR | Spinal cord: no nerve root gadolinium enhancement |
| 39 | NR | NR | V=Decreased at TN F waves=Absent | NR | Not performed |
| 39 | NR | Negative | V=Decreased at MN, UN, TN, CPN F waves=Absent | NR | None |
| 40 | Negative | NR | V=NR F waves=Anomalies in lower limbs | NR | NR |
| 41 | Negative | Negative | V=Decreased at TN, CPN, right UN F waves=Absent | Negative for JSV, VZV, and CMV | NR |
| 42 | Negative | Negative | V=Decreased at TN, CPN, right UN and MN F waves=Absent TN, CPN right MN | Negative for syphilis, HIV, HBV, and HCV | NR |
| 43 | Negative | Negative | V=decreased at PN and TN bilaterally F=increased latency | Negative for HIV, Lyme disease, syphilis | Report shows multiple cranial neuritis, radiculitis, plexitis of both brachial and lumbar plexus |
| 43 | Negative | Negative | V=decreased at left MN F waves=NR | NR | Not performed |
| 44 | Negative | Negative | V=NR F waves=NR | NR | None |
| 44 | Negative | Negative | V=normal NR not reported F waves=NR | NR | NR |
| 45 | Negative | NR | V=decreased at left UN and bilateral PN F waves=absent at TN and PN bilaterally | Negative for HSV 1-2, EBV, VZV, CMV, HIV, Mycoplasma pneumoniae, Borrelia | NR |
| 46 | Negative | NR | V=decreased at UN bilaterally and right TN F waves=absent at MN, UN and TN bilaterally | Negative for Campylobacter jejuni, HIV, EBV, CMV, influenza virus (type A and B), and HCV | None |
| 46 | Negative | NR | V=decreased at TN bilaterally F waves=absent at MN, UN and TN bilaterally | Negative for Campylobacter jejuni, HIV, EBV, CMV, influenza virus (type A and B), and HCV | Not performed |
| 47 | Negative | NR | Not performed | Not performed | None |
| 47 | Positive | NR | Not performed | NR | NR |
| 48 | Negative | Negative | V=NR F waves=absent bilaterally | NR | Brainstem and cervical meningeal enhancement |
| 49 | Negative | Negative | V=NR F waves=NR | Negative for Borrelia burgdorferi, syphilis, Campylobacter jejuni, CMV, hepatitis E, Mycoplasma pneumonia, and EBV | None |
| 50 | NR | Negative | V=decreased at right UN and right PN F waves=normal minimal latencies with decreased persistence | Negative for HIV | Asymmetrical thickening and hyperintensity of postganglionic roots supplying the brachial and lumbar plexuses |
| 51 | Negative | Negative | V=decreased at left MN, left TN F waves=absent at UE and LE | NR | Symmetrical contrast enhancement of the spinal nerve roots at all levels of the spine including the cauda equina |
| 52 | Negative | NR | V=normal at left MN, UN, RN, CPN F waves=normal at left MN, UN | NR | Not performed |
| 53 | NR | NR | Not performed | NR | Moderate bilateral and moderate left-sided neural foraminal narrowing at L2-3 and L3-4 |
| 54 | NR | Negative | V=Decreased at right MN, UN, TN F waves=NR | NR | NR |
| 55 | NR | NR | NR | Negative for meningitis, HSV, VZV, Lyme, VDRL, West Nile, Enterovirus, CMV, HIV | NR |
| 56 | NR | Negative | V=Decreased at left MN, CPN F waves=NR | Negative CSF Gram stain/culture, rapid meningitis-encephalitis multiplex panel; negative respiratory viral PCR and culture Negative blood, urine, stool cultures | Abnormal enhancement of posterior nerve roots from T11 through cauda equina |
| 57 | NR | NR | V=Decreased at MN, UN F waves=NR | Positive IgG and IgM to Campylobacter jejuni Negative CSF PCR analysis of fungal, viral, bacterial pathogens, negative HBV | None |
| 58 | Negative | Negative | V=Decreased right TN F waves=NR | Negative HSV1, HSV2, VZV, enterovirus in CSF, negative serum HBV, HCV, HIV, syphilis, CMV, EBV, Mycoplasma, Lyme, Legionella, pneumococcus | Not performed |
| 59 | Negative | Negative | V=decreased at both MN and CPN bilaterally F waves=normal | Negative for HIV, HCV, HBV | Not performed |
| 59 | Negative | Negative | V=decreased at both MN and CPN bilaterally F waves=absent at CPN bilaterally | Negative for HIV, HCV, HBV | Negative |
| 59 | Negative | Negative | V=normal at both UE and LE bilaterally F waves=normal | Negative for HIV, HCV, HBV | Negative |
| 59 | Not performed | Not performed | V=NR F waves=Absent at CPN bilaterally | Negative for HIV, HCV, HBV | Not performed |
| 59 | Negative | Negative | V=normal at CPN bilaterally F waves=decreased at CPN bilaterally | Negative for HIV, HCV, HBV | Not performed |
| 60 | Negative | NR | NR | Negative for Lyme, HIV, viral hepatitis, ANA, RF | NR |
| 61 | Positive IgM for GM2 and GD3, weak IgG band for GT1b | NR | conduction blocks, absence of F waves in right ulnar and axon potentials in the F response of the right tibial nerve | NR | NR |
| 62 | NR | NR | NR | NR | NR |
| 63 | NR | NR | NR | NR | NR |
| 64 | Negative anti-GM1, anti-GD1b, anti-GQ1b IgG and IgM | NR | V=not evocable at TN bilaterally and CPN F waves=absent at LE bilaterally | Positive anti-EBV, anti-CMV, and anti-Mycoplasma pneumoniae IgG Negative HIV, syphilis, CMV, EBV, Mycoplasma pneumoniae | NR |
| 65 | Negative | NR | NR | NR | NR |
| 65 | Negative | Negative | NR | NR | NR |
| 65 | Negative | Negative | NR | NR | NR |
| 65 | Negative | Negative | NR | NR | NR |
| 65 | Negative | Negative | NR | NR | NR |
| 65 | Negative | NR | NR | NR | NR |
| 66 | Positive anti-GQ1B antibodies | Not performed | NR | Tracheal aspirate grew beta-lactamase resistant Haemophilus influenzae | Intrathecal caudal-equina enhancement consistent with GBS |
| 79 | NR | NR | NR | NR | NR |
| 67 | NR | Negative | V=normal at MN, UN and TN F waves=TN F-wave latencies with pathologic intermediate latency responses of complex A-wave bilaterally | Patient treated amoxicillin and ciprofloxacin for a GI disorder related to salmonellosis around 2 wk before admission | MRI C-spine showed no pathologic findings |
| 68 | Negative | Negative | Not performed | Negative antibodies for Lyme, and HIV | None |
| 69 | NR | Not performed | V=absent at TN and CPN bilaterally F waves=NR | NR | NR |
| 70 | Negative | Negative | V=decreased in both UE and LE bilaterally F waves=NR | NR | NR |
| 70 | Negative | Negative | V=NR F waves=NR | NR | None |
| 71 | NR | NR | V=decreased at UN and MN, TN, and CPN unexcitable bilaterally F waves=absent at UN, MN, TN, and CPN bilaterally | NR | NR |
| 72 | NR | NR | V=NR F waves=NR | NR | None |
| 73 | Negative | Negative | V=normal at both UE and LE F waves=normal at both UN and TN | Negative for herpesviruses | None |
| 74 | Negative | Negative | V=decreased at both UE and LE F waves=NR | NR | None |
| 75 | Positive | NR | NR | NR | None |
| 76 | NR | NR | V=NR F waves=NR | Negative for HIV, HBV, HCV | None |
| 76 | NR | NR | V=NR F waves=NR | Negative for HIV, HBV, HCV | None |
| 76 | NR | NR | V=NR F waves=NR | Negative for HIV, HBV, HCV | None |
| 76 | NR | NR | V=NR F waves=NR | Negative for HIV, HBV, HCV | None |
| 77 | NR | Negative | V=decrease at TN and CPN bilaterally F waves=NR | Negative for HIV, syphilis and Lyme disease | None |
ANA indicates antinuclear antibody; CMV, cytomegalovirus; COVID-19, Coronavirus Disease 19; CPN, common peroneal nerve; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; EMG, electromyography; EP, electrical potential; FN, facial nerve; GBS, Guillain-Barré syndrome; GI, gastrointestinal; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTN, hypertension; ICU, intensive care unit; Ig, immunoglobulin; LE, lower extremities; MN, median nerve; MRI, magnetic resonance imaging; NE, not evocable; NR, not reportable; PCR, polymerase chain reaction; RF, rheumatoid factor; RN, radial nerve; SN, sural nerve; TBE, tick-borne encephalitis; TN, tibial nerve; UE, upper extremities; UN, ulnar nerve; V, conduction velocity (m/s); VZV, varicella zoster virus.
DISCUSSION
In this systematic review of reported COVID-19 cases, we did not identify a consensus on the diagnostic approach and treatment of patients with superimposed GBS. The most commonly reported treatment was IVIG, in addition to therapies aimed at the COVID-19 infection such as antibiotics and antiviral agents. Our findings confirm that quadriplegia, areflexia, and respiratory failure are associated with poor outcome among COVID-19–related GBS patients (P=0.02, 0.02, and 0.004, respectively), but GBS subtypes and treatment strategies including IVIG and systemic steroids are not. Moreover, this review indicated a mortality rate of 3.5% in patients with COVID-19–related GBS, which is more than twice the WHO reported mortality rate of 2.2% among general COVID-19 cases.80 We also found a significantly higher rate of acute respiratory failure requiring mechanical ventilation in this population (47% vs. 16% in the general COVID-19 cases81) and persistent neurological deficits (26% vs. 18.3%82). Although available case reports do not provide evidence of causation, the poor outcome and high mortality rate in COVID-19–related GBS patients underscores the importance of early diagnosis and effective treatment of neurological complications in this population.
GBS Diagnosis in COVID-19 Patients
Clinical diagnosis of GBS can be particularly challenging in patients with severe COVID-19 symptoms.2,20 A considerable variety of early neurological symptoms have been reported after the onset of COVID-19 symptoms. The interval between the first reported signs of viral infection and the onset of neurological symptoms ranged from 0 to 60 days, with 1 case reporting GBS symptoms 7 days before COVID-19 symptoms occurred.27
Types of GBS in COVID-19 Patients
The geographical distribution of reported COVID-19–related GBS resembles the worldwide distribution80 of COVID-19 infections at the time of this report (Fig. 3). The most commonly diagnosed type of GBS in this population is reported to be AIDP, as is typical in dengue or Zika virus–related GBS. However, one study reported that COVID-19–related GBS patients were mainly diagnosed with acute motor axonal neuropathy (AMAN) and AMSAN. The authors further stated that patients with AIDP had better outcomes than those diagnosed with AMAN or AMSAN.22 We could not corroborate these findings, nevertheless, and failed to validate an association between GBS types and patient outcomes in this review. Gupta and colleagues also speculated that COVID-19–related GBS may have a different pathogenetic mechanism compared with other types of GBS. However, the findings reported in this review indicate a common clinical and pathogenetic characteristics between COVID-19–related GBS and other types of GBS.22
General Treatments for COVID-19
Management of the COVID-19 infection is overshadowed by many epidemiological, clinical and social factors and a lack of effective therapies and accepted treatment protocols. While several experimental strategies have been used to treat patients with significant symptoms, current management of COVID-19 primarily focuses on providing supportive therapies including mechanical ventilation.83 Recent experimental therapies have shown some promise, including antiviral agents such as the adenosine analogue remdesivir and the protease inhibitors lopinavir and ritonavir.84–88 Chloroquine and hydroxychloroquine have been shown to inhibit COVID-19 in vitro and were widely used in patients with COVID-19 until newer studies proved their lack of clinical efficiency.84,89 While hydroxychloroquine has largely fallen out of favor as a primary therapeutic option for COVID-19,90 a significant percentage of existing case studies in our review have documented its use in treatment. In the COVID-19-GBS cases included in this review, 25% were treated with lopinavir or ritonavir, and 43% were treated with hydroxychloroquine.14,15,17–20,25,27,29 Further, antibiotics such as azithromycin and amoxicillin were also used in 33% of the cases we analyzed.14,19,20,26,28,29 The body of evidence is increasing in support of the use of monoclonal antibodies (tocilizumab, casirivimab, and imdevimab) in general COVID-19 patients,91,92 but given the limited data available in patients with COVID-19–related GBS, it is impossible to determine their clinical importance and outcome effects in this population.
Steroids for COVID-19–Related GBS
Steroids were administered to 12% of the cases that were included in our analysis.20,29,83 Recent guidelines on the management of critically ill adults with COVID-19 recommends against routine use of steroids in mechanically ventilated adults without acute respiratory distress syndrome; however, they can be used in the presence of acute respiratory distress syndrome and in patients experiencing a refractory shock.93 Studies suggest that corticosteroids may lead to prolonged viral shedding, hence the need to limit their routine use.12,94 Available literature argues, nevertheless, that steroids could mitigate the fatal immune system activation seen in COVID-19,83 based on their positive effects during the Ebola epidemic and as the first-line therapy for the postviral autoimmune response to herpes virus encephalitis.83,95–97 A recent study showed that intravenous dexamethasone therapy for 10 days was associated with decreased 28-day mortality in COVID-19 patients receiving respiratory support but no benefit in those who did not require respiratory support. These findings suggest that the benefits of diminished immunopathologic activation may outweigh possible prolonged viral shedding in the subset of COVID-19 patients requiring ventilatory support.98
The effects of steroid use during the management of typical GBS patients has also been widely studied.99 Consistent with earlier reports from GBS in general population,99 our review indicates that systemic steroids does not affect the outcome, defined as mortality or ICU admission, in patients with COVID-19–related GBS.
IVIG Treatment for COVID-19–Related GBS
IVIG was used in 87% of the GBS cases included in our review. When treating the parainfectious form of GBS that co-occurs with COVID-19, IVIG, or plasma exchange may not only mitigate the neuroinflammatory response but may also prove beneficial in controlling the associated systemic inflammation and sepsis.12,100,101 In this setting, however, a clinical concern is related to the association between IVIG and the risk of thromboembolism.102 COVID-19 is commonly associated with a prothrombotic state, as evident from an increase in the D-dimer levels103 and reported cases of venous thromboembolism and embolic strokes.104 Current guidelines support the use of thromboprophylaxis with low molecular weight heparin, in COVID-19 patients without contraindications. Those with clinical evidence of a venous thromboembolism should be treated with therapeutic doses of anticoagulation.105
The most common dose for IVIG was 0.4 g/kg/d for 5 days.4,11,14–18,20 Overall, outcomes with these medications were greatly variable and unpredictable. In patients treated with IVIG and some combination of hydroxychloroquine, antivirals, and antibiotics, outcomes ranged from complete recovery4 to persistent lower extremity weakness20 to death from progressive respiratory failure.17 Despite uncertainty regarding COVID-19 and GBS management, one report recommended antiviral agents and IVIG as a reasonable therapeutic strategy at this point.14
Convalescent Plasma and Plasma Exchange Therapy
Only 7 reported cases of COVID-19–associated GBS have been treated with plasmapheresis alone or in addition to IVIG.28,47 A randomized controlled trial in 2014 suggested that IVIG and plasmapheresis are equally effective in treating GBS.106 Some experts, however, believe that plasmapheresis may be a better therapeutic approach and should be considered before IVIG in GBS. Historically, IVIG has been more widely used because of its availability and simplicity; it requires no specialized equipment and has a relatively low risk of adverse events.106 There is also no evidence at this time that a combination therapy with IVIG and plasmapheresis is associated with better long-term or short-term outcomes compared with standard therapy in GBS patients.106–109 Although the Surviving Sepsis Campaign panel of experts recommend against routine use of IVIG in COVID-19 patients, it may be reasonable to consider these treatment options in the subgroup of COVID-19 patients with a suspected or confirmed GBS.
Out of the cases we reviewed, none reported the use of convalescent plasma therapy in the treatment of COVID-19–related GBS. However, the use of convalescent serum therapy for COVID-19 is a rapidly emerging but controversial area of research. Plasma is collected from previously infected individuals to passively transfer antibodies to an infected patient, with the goal to improve clinical symptoms and mortality.110 Plasma exchange with convalescent serum could be an innovative approach to the management of COVID-19–associated GBS. While current randomized controlled trials have not shown a significantly beneficial or detrimental effect of convalescent plasma on mortality in COVID-19 patients, lower mortality rates have been associated with those who receive plasma containing higher concentrations of neutralizing antibodies. Some studies suggest convalescent exchange may have the greatest benefit when initiated early in the disease course before complications of the infection occur.111 A study of patients with severe COVID-19 pneumonia showed no difference in 30-day mortality or clinical status between those assigned to convalescent plasma versus placebo.112 In regard to possible adverse effects, the largest safety study to date has noted transfusion reactions occurring in <1% of patients and causing death in 0.3%. Possible reactions include transfusion-associated circulatory overload, transfusion-related acute lung injury, and severe allergic reaction.113
While the use of convalescent plasma has not demonstrated significant effects on mortality in general COVID-19 patients, further research is needed to assess its impact on COVID-19–related GBS.114 One case report has documented the use of convalescent plasma in a patient COVID-19–related GBS; however, its efficacy is unclear as the patient developed worsening respiratory failure and was unable to be weaned from ventilatory support at time of publication.115 In addition, addressing the use of convalescent plasma in COVID-19–related GBS presents unique challenges apart from its use in general COVID-19 patients. One study reported deferring the use of convalescent plasma out of concern for potential parainfectious antibody-mediated peripheral nerve damage from donor plasma.68 Due to the ambiguity of current evidence in this subset of COVID-19 patients, further research is needed to assess its efficacy in this population. It should be noted, however, that the most recent guidelines suggest using convalescent plasma in the management of COVID-19 patients only in the setting of a clinical trial.113
Critical Care in COVID-19 Patients With GBS
In our study, patients with poor outcomes or who showed no clinical improvement were associated with longer ICU stays (P=0.02, Table 2). The ICU management of COVID-19-related GBS presents a unique set of challenges and obstacles. Poorer outcomes and longer ICU admissions highlight the increased mortality risk in this population and the potential burden on hospital resources. As mentioned above and presented in Table 1, there is a highly variable temporal relationship between the development of neurological and respiratory symptoms of GBS and COVID-19. In particular, evidence from several case reports suggests that possible symptom overlap portends more considerable obstacles in its management.17,28 As both diagnoses may be complicated by severe respiratory failure and the need for early respiratory support, ICU admission is often indicated but may not be feasible in some centers given the scarcity of resources during a pandemic.17
It is unclear if the development of ventilator-dependent respiratory failure in reported patients is caused by the sequelae of GBS-related neuromuscular dysfunction or COVID-19 respiratory symptoms. Out of the cases we analyzed, 47% required mechanical ventilation and ICU admission, highlighting the need for critical care resources in these patients.15–18,20,26,28,31 A retrospective study in Wuhan, China reported that 71% of 52 COVID-19 patients with unspecified GBS status admitted to the ICU required mechanical ventilation.116 Similarly, a retrospective study of 76 GBS patients admitted to the ICU showed that 78% required mechanical ventilation.117 Although the precise pathophysiology of respiratory failure in COVID-19 patients with GBS remains unclear, the increased prevalence of ventilator dependency among cases we reviewed suggests a possible synergistic response associated with a worst outcome (P=0.004), which warrants further investigation. Indeed, recent literature does support this hypothesis and suggest that the presence of significant respiratory symptoms in the acute phase of COVID-19 may be associated with more severe forms of GBS.4 As such, respiratory complications in COVID-19 patients with GBS, including prolonged ventilator dependence and bacterial superinfection, pose a significant obstacle to patient recovery, particularly in areas with limited ICU resources. Current guidelines advise managing mechanically ventilated adults with COVID-19 similar to patients with other causes of acute respiratory failure. These ventilation strategies include low tidal volume ventilation at 4 to 8 mL/kg of predicted body weight, titrated positive end-expiratory pressure and reduction of barotrauma by restricting the peak and plateau inspiratory pressures.94 However, the efficacy of other mechanical ventilation strategies in COVID-19 patients, as well as COVID-19–related GBS patients, has not yet been extensively investigated. The documented association between mechanical ventilation and no clinical improvement in this review (P=0.007) underscores the need for carefully designed studies of ventilation strategies among this group of patients.
Study Limitations
The main limitation of the current systematic review of the literature is the low number of available cases worldwide and an even lower number of cases with the reported outcome. To facilitate further studies, we suggest that while reporting cases, authors report the outcome of the case as well. Despite the limitations, this systematic review and analysis is the first systematic study that rigorously assesses the effect of treatment outcomes and discusses the ICU challenges and management of COVID-19–related GBS patients during the COVID-19 pandemic.
CONCLUSIONS
To conclude, GBS should be considered as a potential high-risk complication in critically ill COVID-19 patients with early-onset weakness and pulmonary findings that are inconsistent with the severity of their respiratory status. Although evidence to support specific treatments are lacking, clinicians should consider the benefits of immunotherapy and plasma exchange, in addition to standard antimicrobial and supportive therapies, if the diagnostic criteria for an acute sensory and motor polyradiculoneuritis are met. This review indicated that IVIG treatment alone did not result in improved outcomes or mortality. Hence, the effects of more aggressive treatment options including plasmapheresis and convalescent plasma exchange should be examined further for this group of high-risk patients. More extensive studies aimed at exploring neurological manifestations and complications of COVID-19, together with distinctive treatment options for COVID-19–related GBS are warranted.
Footnotes
S.G. and S.E. contributed equally.
The authors declare no conflict of interest.
Contributor Information
Sogand Goudarzi, Email: sgoudarz@bidmc.harvard.edu.
Shooka Esmaeeli, Email: sh.esmaeeli@gmail.com.
Juan D. Valencia, Email: jvalenc1@bidmc.harvard.edu.
Maegan E. Lu, Email: mlu@bu.edu.
Riley R. Hales, Email: rhales@bu.edu.
Corey R. Fehnel, Email: cfehnel@bidmc.harvard.edu.
Christopher M. Conley, Email: Christopher.Conley@bmc.org.
Sadeq A. Quraishi, Email: saq@mac.com.
Ala Nozari, Email: Ala.Nozari@bmc.org.
REFERENCES
- 1.Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a review. Acta Neurol Scand. 2020;142:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonck K, Garrez I, De Herdt V, et al. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020;27:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheidl E, Canseco DD, Hadji-Naumov A, et al. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38:1549.e3–1549.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappello F. COVID-19 and molecular mimicry: the Columbus’ egg? J Clin Neurosci. 2020;77:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappello F. Is COVID-19 a proteiform disease inducing also molecular mimicry phenomena? Cell Stress Chaperones. 2020;25:381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehghani Firouzabadi M, Dehghani Firouzabadi F, Goudarzi S, et al. Has the chief complaint of patients with COVID-19 disease changed over time? Med Hypotheses. 2020;144:109974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh AK, Bhushan B, Maurya A, et al. Novel coronavirus disease 2019 (COVID-19) and neurodegenerative disorders. Dermatol Ther. 2020;33:e13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Needham EJ, Chou SHY, Coles AJ, et al. Neurological implications of COVID-19 Infections. Neurocrit Care. 2020;32:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firouzabadi FD, Firouzabadi MD, Ghalehbaghi B, et al. Have the symptoms of patients with COVID-19 changed over time during hospitalization? Med Hypotheses. 2020;143:110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottaviani D, Boso F, Tranquillini E, et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41:1351–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padroni M, Mastrangelo V, Asioli GM, et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020;267:1877–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camdessanche JP, Morel J, Pozzetto B, et al. COVID-19 may induce Guillain-Barré syndrome. Rev Neurol (Paris). 2020;176:516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Otmani H, El Moutawakil B, Rafai MA, et al. Covid-19 and Guillain-Barré syndrome: more than a coincidence!. Rev Neurol (Paris). 2020;176:518–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virani A, Rabold E, Hanson T, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20:e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). J Chinese Integr Med. 2009;7:889–896. [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Paliwal VK, Garg RK. Is COVID-19-related Guillain-Barré syndrome different? Brain Behav Immun. 2020;87:177–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gigli GL, Bax F, Marini A, et al. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J Neurol. 2020;268:1195–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 25.Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77:230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marta-Enguita J, Rubio-Baines I, Gastón-Zubimendi I. Síndrome de Guillain-Barré fatal tras infección por virus SARS-CoV2 [Fatal Guillain-Barre syndrome after infection with SARS-CoV-2]. Neurologia. 2020;35:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galán AV, Saucedo PDS, Postigo FP, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Neurologia. 2020;35:268–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coen M, Jeanson G, Culebras Almeida LA, et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. 2020;87:111–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rana S, Lima AA, Chandra R, et al. Novel coronavirus (COVID-19)-associated Guillain-Barré syndrome: case report. J Clin Neuromuscul Dis. 2020;21:240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaud S, Budowski C, Ng Wing Tin S, et al. Post SARS-CoV-2 Guillain-Barré syndrome. Clin Neurophysiol. 2020;131:1652–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan JL, Ebadi H, Sarna JR. Guillain-Barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can J Neurol Sci. 2020;47:852–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteban Molina A, Mata Martínez M, Sánchez Chueca P, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Med Intensiva. 2020;44:513–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farzi MA, Ayromlou H, Jahanbakhsh N, et al. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. 2020;346:577294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helbok R, Beer R, Löscher W, et al. Guillain-Barré syndrome in a patient with antibodies against SARS-COV-2. Eur J Neurol. 2020;27:1754–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutchins KL, Jansen JH, Comer AD, et al. COVID-19-associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. AJNR Am J Neuroradiol. 2020;41:1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lantos JE, Strauss SB, Lin E. COVID-19-associated Miller Fisher syndrome: MRI findings. AJNR Am J Neuroradiol. 2020;41:1184–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lascano AM, Epiney JB, Coen M, et al. SARS-CoV-2 and Guillain-Barré syndrome: AIDP variant with favorable outcome. Eur J Neurol. 2020;27:1751–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes-Bueno JA, García-Trujillo L, Urbaneja P, et al. Miller-Fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27:1759–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su XW, Palka SV, Rao RR, et al. SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020;62:E48–E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb S, Wallace VC, Martin-Lopez D, et al. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 2020;13:e236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigaut K, Mallaret M, Baloglu S, et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assini A, Benedetti L, Di Maio S, et al. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. 2020;41:1657–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bracaglia M, Naldi I, Govoni A, et al. Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J Neurol. 2020;267:3166–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebrahimzadeh SA, Ghoreishi A, Rahimian N. Guillain-Barré syndrome associated with the coronavirus disease 2019 (COVID-19). Neurol Clin Pract. 2020;11:e196–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan M, Han SC, Kelly S, et al. A case series of Guillain-Barré syndrome following COVID-19 infection in New York. Neurol Clin Pract. 2020. DOI: 10.1212/CPJ.0000000000000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sancho-Saldaña A, Lambea-Gil Á, Liesa JLC, et al. Guillain–Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med (Lond). 2020;20:e93–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilinc D, van de Pasch S, Doets AY, et al. Guillain-Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27:1757–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oguz-Akarsu E, Ozpar R, Mirzayev H, et al. Guillain-Barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. 2020;62:E54–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, et al. Acute polyradiculoneuritis with locked-in syndrome in a patient with COVID-19. J Neurol. 2020;267:1883–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirayama T, Hongo Y, Kaida K, et al. Guillain-Barré syndrome after COVID-19 in Japan. BMJ Case Rep. 2020;13:e239218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korem S, Gandhi H, Dayag DB. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. 2020;13:e237215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tiet MY, AlShaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13:e236536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Defabio AC, Scott TR, Stenberg RT, et al. Guillain-Barré syndrome in a patient previously diagnosed with COVID-19. Am J Emerg Med. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtis M, Bhumbra S, Felker MV, et al. Guillain-Barré syndrome in a Child With COVID-19 infection. Pediatrics. 2020:e2020015115. [DOI] [PubMed] [Google Scholar]
- 57.Gale A, Sabaretnam S, Lewinsohn A. Guillain-Barré syndrome and COVID-19: association or coincidence. BMJ Case Rep. 2020;13:e239241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ameer NA-O, Shekhda KA-O, Cheesman A. Guillain-Barré syndrome presenting with COVID-19 infection. BMJ Case Rep. 2020;13:e236978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manganotti P, Bellavita G, D’Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 2021;93:766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDonnell EP, Altomare NJ, Parekh YH, et al. COVID-19 as a trigger of recurrent Guillain-Barré syndrome. Pathogens. 2020;9:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diez-Porras L, Vergés E, Gil F, et al. Guillain-Barré-Strohl syndrome and COVID-19: case report and literature review. Neuromuscul Disord. 2020;30:859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manji HK, George U, Mkopi NP, et al. Guillain-Barré syndrome associated with COVID-19 infection. Pan Afr Med J. 2020;35(suppl 2):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bueso T, Montalvan V, Lee J, et al. Guillain-Barre syndrome and COVID-19: a case report. Clin Neurol Neurosurg. 2021;200:106413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zito A, Alfonsi E, Franciotta D, et al. COVID-19 and Guillain-Barré syndrome: a case report and review of literature. Front Neurol. 2020;11:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garnero M, Del Sette M, Assini A, et al. COVID-19-related and not related Guillain-Barré syndromes share the same management pitfalls during lock down: the experience of Liguria region in Italy. J Neurol Sci. 2020;418:117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowery MM, Taimur Malik M, Seemiller J, et al. Atypical variant of Guillain Barre syndrome in a patient with COVID-19. J Crit Care Med. 2020;6:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atakla HG, Noudohounsi M, Sacca H, et al. Acute Guillain-Barré polyradiculoneuritis indicative of COVID-19 infection: a case report. Pan Afr Med J. 2020;35(suppl 2):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abrams RMC, Kim BD, Markantone DM, et al. Severe rapidly progressive Guillain-Barré syndrome in the setting of acute COVID-19 disease. J Neurovirol. 2020;26:797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agha Abbaslou M, Karbasi M, Mozhdehipanah H. A rare axonal variant of Guillain-Barré syndrome as a neurological complication of COVID-19 infection. Arch Iran Med. 2020;23:718–721. [DOI] [PubMed] [Google Scholar]
- 70.Assini A, Benedetti L, Di Maio S, et al. Correction to: New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. 2020;41:2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakraborty N, Kumar H. Intravenous Immunoglobulin may reverse multisystem inflammation in COVID-19 pneumonitis and Guillain-Barré syndrome. Indian J Crit Care Med. 2020;24:1264–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.García-Manzanedo S, López de la Oliva Calvo L, Ruiz Álvarez L. Guillain-Barré syndrome after COVID-19 infection. Med Clin (Barc). 2020;155:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liberatore G, De Santis T, Doneddu PE, et al. Clinical reasoning: a case of COVID-19-associated pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Neurology. 2020;95:978–983. [DOI] [PubMed] [Google Scholar]
- 74.Tard C, Maurage CA, de Paula AM, et al. Anti-pan-neurofascin IgM in COVID-19-related Guillain-Barré syndrome: evidence for a nodo-paranodopathy. Neurophysiol Clin. 2020;50:397–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dufour C, Co TK, Liu A. GM1 ganglioside antibody and COVID-19 related Guillain Barre syndrome—a case report, systemic review and implication for vaccine development. Brain Behav Immun Health. 2021;12:100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nanda S, Handa R, Prasad A, et al. COVID-19 associated Guillain-Barre syndrome: contrasting tale of four patients from a tertiary care centre in India. Am J Emerg Med. 2021;39:125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raahimi MM, Kane A, Moore CE, et al. Late onset of Guillain-Barré syndrome following SARS-CoV-2 infection: part of ‘long COVID-19 syndrome’? BMJ Case Rep. 2021;14:e240178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oguz-Akarsu E, Ozpar R, Mirzayev H, et al. Guillain–Barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. 2020;62:E54–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foresti C, Servalli MC, Frigeni B, et al. COVID-19 provoking Guillain-Barré syndrome: the Bergamo case series. Eur J Neurol. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tzotzos SJ, Fischer B, Fischer H, et al. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pilotto A, Cristillo V, Piccinelli SC, et al. COVID-19 severity impacts on long-term neurological manifestation after hospitalisation. medRxiv. 2021;2020:12. [Google Scholar]
- 83.Neri P, Pichi F. COVID-19 and the eye immunity: lesson learned from the past and possible new therapeutic insights. Int Ophthalmol. 2020;40:1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carod-Artal FJ. Complicaciones neurológicas por coronavirus y COVID-19 [Neurological complications of coronavirus and COVID-19]. Rev Neurol. 2020;70:311–322. [DOI] [PubMed] [Google Scholar]
- 85.Cao B, Zhang D, Wang C. A trial of lopinavir–ritonavir in COVID-19. N Engl J Med. 2020;382:e68. [DOI] [PubMed] [Google Scholar]
- 86.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2020;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan S, Siddique R, Shereen MA, et al. Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J Clin Microbiol. 2020;58:e00187-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh B, Ryan H, Kredo T, et al. Chloroquine or hydroxychloroquine for prevention and treatment of COVID‐19. Cochrane Database Syst Rev. 2021;2:CD013587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J, Tan DY, Fau YY, et al. Do corticosteroids have a role in treating Ebola virus disease? Sci China Life Sci. 2015;58:111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stahl JP, Mailles A. Herpes simplex virus encephalitis update. Curr Opin Infect Dis. 2020;32:239–243. [DOI] [PubMed] [Google Scholar]
- 97.Shang L, Zhao J, Hu Y, et al. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2020;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hughes R. Corticosteroids for treating Guillain-Barré syndrome. Cochrane Database Syst Rev. 2000;2:CD001446. [DOI] [PubMed] [Google Scholar]
- 100.Rimmer E, Houston BL, Kumar A, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busani S, Damiani E, Cavazzuti I, et al. Intravenous immunoglobulin in septic shock: review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. 2016;82:559–572. [PubMed] [Google Scholar]
- 102.Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27:171–178. [DOI] [PubMed] [Google Scholar]
- 103.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2014;2014:CD002063. [DOI] [PubMed] [Google Scholar]
- 107.van den Berg B, Walgaard C, Drenthen J, et al. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2000;10:469–489. [DOI] [PubMed] [Google Scholar]
- 108.Farcas P, Avnun L, Frisher S, et al. Efficacy of repeated intravenous immunoglobulin in severe unresponsive Guillain-Barré syndrome. Lancet. 2000;350:1747. [DOI] [PubMed] [Google Scholar]
- 109.Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. [DOI] [PubMed] [Google Scholar]
- 110.Rajendran KA-O, Krishnasamy N, Rangarajan J, et al. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol. 2000;92:1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2020;384:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2020:ciaa478. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma; 2020.
- 115.Yaqoob H, Jain A, Epelbaum O. A unique case of Guillain-Barré syndrome related to COVID-19 infection. Chest. 2020;158:A771. [Google Scholar]
- 116.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siow WT, Liew MF, Shrestha BR, et al. Managing COVID-19 in resource-limited settings: critical care considerations. Crit Care. 2020;24:167. [DOI] [PMC free article] [PubMed] [Google Scholar]


