Abstract
The World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (WHO 2017) included updated criteria for diagnosis and classification of post-transplant lymphoproliferative disorders (PTLDs). This study evaluated the clinicopathologic spectrum using WHO 2017 criteria and adult PTLD patients’ outcomes over 30 years between 1987 and 2017 at Mayo Clinic (Rochester, MN). Patients were retrospectively reviewed for clinical features, outcomes, and diagnostic pathology material and classified based on WHO 2017 criteria. A total of 227 patients were diagnosed with PTLD, with a median time from transplant to PTLD of 45 months. PTLD occurred >1 year after transplant in 149 (66%) patients. Monomorphic PTLD was the most common subtype (173, 76%), with diffuse large B cell lymphoma as the commonest morphology (n = 137). Epstein-Barr virus was positive in 61% of total cases and 90% of PTLD that developed within 1 year from transplant. The median event-free survival (EFS) and overall survival for the entire cohort were 21 months (95% confidence interval [CI]: 9–35) and 82 months (95% CI: 39–115), respectively. The EFS or overall survival was not impacted by Epstein-Barr virus status but differed based on WHO subtypes and year of diagnosis. Management changed over time with increased use of rituximab or chemotherapy + immunosuppression reduction as initial therapy. When compared to the matched general population and de novo diffuse large B cell lymphoma, patients not achieving EFS 24 status (no progression/treatment or death within 24 mo of diagnosis) had a worse standardized mortality ratio 16.75 (95% CI: 13.91–20) versus SMR 1.72 (95% CI: 1.26–2.28) in those who achieved EFS24. Cause of death was mostly attributed to non-lymphoma–related causes in those achieving EFS 24.
Introduction
Post-transplant lymphoproliferative disorders (PTLDs) encompass a heterogeneous group of lymphoid or plasmacytic proliferations that occur in patients following solid organ or allogeneic stem cell transplantation (ASCT).1 The incidence of PTLD has risen over the past 2 decades, secondary to numerous factors, including an increased number of transplants, use of new immunosuppressive agents, improved diagnostic methodologies, and recognition of new types of PTLD.2,3 In 2017, the revised 4th edition of the World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (WHO 2017)1 was published, which included updated criteria and terminology for diagnosis and classification of PTLD. Classified under the general heading of “Immunodeficiency-associated lymphoproliferative disorders,” PTLDs encompass a diagnostically challenging group of heterogeneous lesions in their morphology, phenotype, clonality, and Epstein-Barr virus (EBV) status. EBV is implicated in 60%–80% of PTLDs, although this number varies widely depending on the histopathologic subtype, patient demographics, and time from transplant.1,2,4
In the WHO 2017 classification, proliferations previously designated as “early lesions” in the WHO 2008 have been retitled “non-destructive PTLD” to emphasize their pathologic characteristics and aid in distinguishing these from cases of PTLD which occur shortly after transplant (and can be of any subtype). Nondestructive PTLDs (ND-PTLD) may be of 3 histopathologic subtypes: (1) plasmacytic hyperplasia (PH), (2) infectious mononucleosis (IM), and (3) florid follicular hyperplasia (FFH), with this latter group being a 2017 addition to the classification.1,5 The diagnostic criteria for polymorphic PTLD (P-PTLD) remained largely unchanged in the 2017 update, but with the acknowledgment that EBV-positive diffuse large B cell lymphomas (DLBCLs), and also peripheral T cell lymphomas (PTCL), may have a polymorphous background, which should not exclude them from being classified as monomorphic PTLD.1 Monomorphic PTLDs include lymphoid or plasmacytic proliferations that would fulfill criteria for a WHO-entity (DLBCL, plasmacytoma, etc.) outside of the post-transplant setting.1 While low-grade B-cell lymphomas such as follicular lymphoma, chronic lymphocytic leukemia, and marginal zone lymphoma were historically excluded from this group; the 2017 update now includes EBV-positive cases of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), typically occurring in the skin or subcutaneous tissue, as PTLD.6 Finally, the relatively recently described indolent entity, EBV-positive mucocutaneous ulcer, has been shown to occur in the post-transplant setting but is presently classified outside of the PTLD category. However, as this entity may mimic other types of PTLD, consideration of this in the differential diagnosis is essential in the proper clinicopathologic evaluation of PTLD patients.7,8
Significant progress has been made in the treatment of PTLD over the last 3 decades. With phase II studies showing promising responses to single-agent rituximab and response-adapted sequential treatment of rituximab monotherapy followed by CHOP-based chemotherapy, treatment patterns have evolved.9–12 Given the changes in both diagnostic criteria as well as therapeutic approaches over the past several decades, the goal of our study was to evaluate the clinicopathologic spectrum (using WHO 2017 criteria) and outcomes of adult patients with PTLD at a large solid organ transplant center over 30 years.
Methods
Patients with a history of solid organ transplant who were diagnosed with PTLD between 1987 and 2017 at Mayo Clinic (Rochester, MN, USA) were identified through the Mayo Clinic Lymphoma Database and the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER). Clinical data were obtained and recorded for all patients as available using a standard protocol. Diagnostic pathology material was retrospectively reviewed when available (RLK) and classified according to the WHO 2017 criteria using H&E, immunohistochemical stains, EBER in situ hybridization, and fluorescent in situ hybridization (FISH) from the time of diagnosis. Cases were considered “PTLD, NOS” if the histology could not be confidently classified based on pathology material available for review. As per WHO 2017, indolent small B-cell lymphomas were not included among the PTLDs except for EBV-positive marginal zone lymphoma (no cases identified).
Statistical analysis
Event-free survival (EFS) was defined as the time from diagnosis of PTLD to relapse, progression, retreatment (second-line therapy), or death due to any cause. Overall survival (OS) was defined as the time from diagnosis until death due to any cause. The EFS and OS analyses were performed using the Kaplan–Meier method. Cox proportional hazards models were used to assess the association of clinical factors with OS. Baseline clinical and pathological characteristics and management categories by EBV status were compared using the Chi-square test, and P values of <0.05 were considered significant. OS stratified by event within 24 months was compared to the general US population as previously described for newly diagnosed DLBCL.13 The study was approved by Mayo Clinic IRB and conducted according to the Declaration of Helsinki. All analyses were performed using R version 3.6.2 and SAS version 9.4M5 software.
Results
Patient characteristics
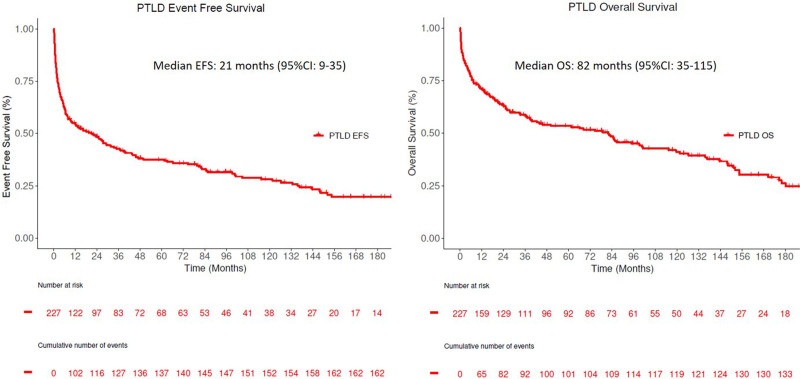
Between 1987 and 2017, a total of 227 patients were diagnosed or managed with PTLD at Mayo Clinic, Rochester. The median age at PTLD diagnosis was 55 years (IQR 45–64 y). The baseline characteristics of the entire cohort are shown in Table 1. Sixty-seven percent (n = 153) of the cohort were male. The median time from transplant to PTLD was 45 months (IQR: 7–112). PTLD occurred >1 year after transplant in 149 (66%) patients. The most common transplanted organs were the kidney (41%) and liver (29%). Extranodal involvement was present in 84% at presentation, and 19% involved the engrafted organ. With a median follow-up of 85 months (IQR 33.7–151.6), 73% (166/227) had disease relapse/progression, and 60% (136/227) patients were deceased. One hundred sixteen (55%) patients had progression within 2 years from diagnosis. The median EFS and OS for the entire cohort were 21 months (95% CI: 9–35) and 82 months (95% CI: 39–115), respectively (Figure 1; see Supplemental Digital Figure S1, http://links.lww.com/HS/A194 for monomorphic PTLD and DLBCL).
Table 1.
Baseline Characteristics of the Entire Cohort (n = 227)
| N = 227 | |
|---|---|
| Median age at diagnosis (y) | 55 (IQR 45–64) |
| ≥60 | 84 (37%) |
| Males (%) | 153 (67.4%) |
| Median age at transplant (years) | 49 (IQR 36–60) |
| Median time from transplant to PTLD (mo) | 45.4 (1.0–499.8) |
| Transplant type, n (%) | |
| Renal | 93 (41%) |
| Liver | 66 (29.1%) |
| Heart | 21 (9.3%) |
| Lung | 16 (7.0%) |
| Pancreas | 12 (5.3%) |
| Renal + Pancreas | 11 (4.8%) |
| Heart + Lung | 3 (1.3%) |
| Other | 5 (2.1%) |
| WHO subtype, n (%) | |
| Monomorphic B-cell | 168 (74%) |
| Polymorphic | 13 (5.7%) |
| Classical HL | 6 (2.6%) |
| Monomorphic T-cell | 5 (2.2%) |
| Nondestructive | 3 (1.3%) |
| Mucocutaneous ulcer | 1 (0.4%) |
| PTLD NOS | 31 (13.7%) |
| EBV positive | 139 (61.2%) |
| Grafted organ involvement | 44 (19.4%) |
| Stage III/IV | 134 (61.2%) |
| Extranodal sites ≥ 1 | 191 (84.1%) |
| PTLD ≥ 1 y after transplant | 149 (65.6%) |
| Elevated LDH | 50 (29.1%) |
EBV = Epstein-Barr virus; LDH, lactate dehydrogenase; NOS, not otherwise specified; PTLD = post-transplant lymphoproliferative disorder.
Figure 1.
Kaplan Meier estimates of entire PTLD cohort. (A) Event-free survival and (B) overall survival. PTLD = post-transplant lymphoproliferative disorder.
Pathologic features
Original pathology materials were available for review in 177 cases (78%). The remaining 50 cases (22%) were reviewed by RLK and TMH and, if possible, classified based on clinical notes and pathology reports. Cases, where pathology material was unavailable and available documentation was insufficient to classify PTLD subtype were designated PTLD, NOS. Similarly, cases where the available pathology material was sufficient to diagnose PTLD but insufficient to subclassify the lesion were also classified as PTLD, NOS. The breakdown of the histopathologic subtypes is shown in Table 1.
Monomorphic PTLD was the most common subtype (173, 76.2%) followed by polymorphic (13, 5.7%), non-destructive (3, 1.3%), and classic Hodgkin lymphoma (6, 2.6%) (CHL-PTLD). One case of EBV-positive mucocutaneous ulcer was identified in the cohort. This case was initially diagnosed as monomorphic PTLD, DLBCL, and was reclassified upon this study’s review. Within the monomorphic PTLD cohort, 168 were B-cell lineage, and 5 were T-cell lineage neoplasms. One hundred thirty-seven (79.2%) cases were morphologically DLBCL. An additional 9 cases were histopathologically high-grade B-cell lymphoma (HGBL), and 8 were plasma cell myeloma or plasmacytoma (Table 2). The 5 T-cell lymphomas included 3 PTCL NOS, 1 T-cell large granular lymphocytic leukemia (T-LGL), and 1 ALK-negative anaplastic large cell lymphoma (ALCL). Three cases were histologically monomorphic on the H&E slide but did not have sufficient phenotyping performed to classify them further. No EBV-positive low-grade B-cell lymphomas were identified.
Table 2.
Subtypes of Monomorphic B-cell PTLD
| Subtype | N (%) |
|---|---|
| DLBCL | 137 (81.6%) |
| High-grade B-cell lymphoma | 9 (5.4%) |
| Plasma cell myeloma/plasmacytoma | 8 (4.8%) |
| Plasmablastic lymphoma | 5 (3.0%) |
| Burkitt lymphoma | 2 (1.2%) |
| Primary CNS Lymphoma | 2 (1.2%) |
| Unclassifiable | 5 (3.0%) |
CNS = central nervous system; DLBCL = diffuse large B cell lymphoma; PTLD = post-transplant lymphoproliferative disorder.
Among the monomorphic PTLD cases with DLBCL morphology (n = 137), the median age was slightly older at 57 years (IQR 46–65) (see Supplemental Digital Table S1, http://links.lww.com/HS/A194). Renal (46%) and liver (22%) were the 2 most common transplant organ types associated with monomorphic PTLD, DLBCL. Graft organ involvement was seen in 17% (23/137) of patients. FISH studies to exclude MYC rearrangements were performed on only 14 (10%) of DLBCL cases (all negative for a MYC rearrangement). Therefore, it is possible that a small subset of untested DLBCL cases harbors MYC and either BCL2 or BCL6 rearrangements that would reclassify them as “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements” (double/triple hit lymphomas) based on the WHO 2017. The cell of origin testing by the Hans algorithm was available on 35 cases (28 non-GCB and 7 GCB).
EBV status
Using EBER in situ hybridization at the time of diagnosis, EBV was positive in 139/227 (61%) total cases and 78/137 (57%) monomorphic PTLD-DLBCL cases. There was no association between EBV status and clinical factors (age, gender, stage, LDH, and extranodal site involvement) in the entire cohort or within monomorphic PTLD, DLBCL type (Table 3 and see Supplemental Digital Table 2, http://links.lww.com/HS/A194). In the PTLD cohort developed within 1 year from transplant, 70/78 (90%) were EBV positive, compared with 69/149 (46%) of the PTLDs developed >1-year post-transplant (P < 0.001). The involvement of the grafted organ by PTLD was seen in 44/227 cases (19%) of which, 33/44 (75%) were EBV positive, as compared to those without grafted organ involvement, where EBV positivity was seen in 106/182 (58%) cases. In multivariable analysis, features independently associated with EBV positivity included time from transplant (<1 y) and involvement of the grafted organ by PTLD (both P < 0.001). Among the monomorphic PTLD, DLBCL cohort that was diagnosed <1 year from transplant (51/137), 90% (46/51) were EBV positive. Of the 28 non-GCB monomorphic PTLD, DLBCL 50% (14/28) were EBV positive compared to only 1/7 (14%) (P = 0.091) for GCB cases. Only 13 polymorphic PTLDs were diagnosed over the study period, of which 11 (84%) were EBV positive. Of the 3 ND-PTLDs, 2 were IM, 1 was PH, and none was FFH. All ND-PTLDs were EBV positive, by definition.
Table 3.
Comparisons of Baseline Characteristics of PTLD Patients Based on EBV Statusa
| EBV Positive (N =139) | EBV Negative (N =78) | P | |
|---|---|---|---|
| Age > 60 y | 48 (34.5%) | 34 (43.6%) | 0.21 |
| Male gender | 91 (65.5%) | 55 (70.5%) | 0.73 |
| LDH > ULN | 29 (27.9%) | 20 (30.8%) | 0.91 |
| Stage III/IV | 80 (59.7%) | 49 (64.5%) | 0.74 |
| IPI > 1 | 80 (60.6%) | 46 (63.0%) | 0.91 |
| Extranodal disease | 120 (86.3%) | 62 (79.5%) | 0.36 |
| Grafted organ | 33 (23.7%) | 9 (11.5%) | <0.001 |
| PTLD < 1 y after transplant | 70 (50.4%) | 5 (6.4%) | <0.001 |
aUnknown = 10.
EBV = Epstein-Barr virus; IPI = International Prognostic Index; PTLD = post-transplant lymphoproliferative disorder; ULN = upper limit of normal.
Therapeutic strategies and clinical outcomes
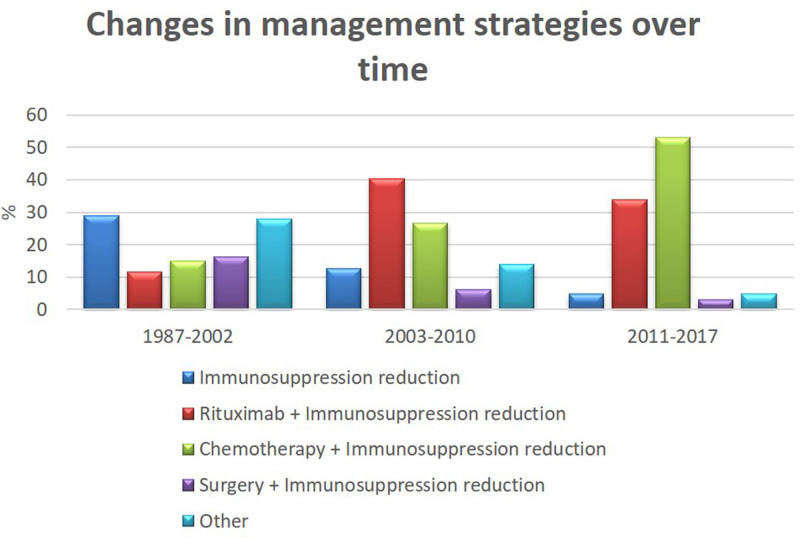
Details of the initial management approach among various WHO subtypes are shown in Table 4. Interventions included immunosuppression reduction alone (N = 38, 17%), chemotherapy/immunochemotherapy with or without immunosuppression reduction (N = 67, 30%), rituximab alone or with immunosuppression reduction (N = 63, 28%), and surgery alone or with immunosuppression reduction (n = 21, 9%). Only 8 patients received either radiation alone or in combination with decreased immunosuppression; this therapy was classified as “other.” The treatment preference changed over time with the increased use of rituximab + immunosuppression reduction and chemotherapy + immunosuppression reduction as initial therapy seen in the last decade (Figure 2). Use of rituximab + immunosuppression reduction as initial treatment increased from 11.6% in patients diagnosed between 1987 and 2002 (n = 86), to 37.6% in those diagnosed between 2003 and 2017 (n = 141).
Table 4.
Various Initial Treatment Strategies Based on PTLD Subtype in the Total Cohort
| Treatment Category | Nondestructive (n = 3) | Polymorphic (n = 13) | Monomorphic DLBCL Type (n = 137) | Monomorphic non-DLBCL Type (n = 37) | PTLD NOS (n = 31) | Classic Hodgkin (n = 6) | Total (n = 227) |
|---|---|---|---|---|---|---|---|
| DIS alone | 2 (66.7%) | 5 (38.5%) | 17 (12.4%) | 8 (21.6%) | 5 (16.1%) | 1 (16.7%) | 38 (16.7%) |
| Rituximab ± DIS (includes rituximab alone) | 0 | 5 (38.5%) | 47 (34.3%) | 3 (8.1%) | 8 (25.8%) | 0 | 63 (27.8%) |
| Chemotherapy ± DIS (includes chemo alone) | 0 | 0 | 45 (32.8%) | 15 (40.5%) | 4 (12.9%) | 3 (50%) | 67 (29.5%) |
| Surgery ± DIS (includes surgery alone) | 1 (33.3%) | 1 (7.7%) | 10 (7.3%) | 6 (16.2%) | 3 (9.7%) | 0 | 21 (9.3%) |
| Othera | 0 | 2 (15.4%) | 18 (13.1%) | 5 (13.5%) | 11 (35.5%) | 2 (33.3%) | 38 (16.7%) |
aInterferon +DIS, no treatment—dead before treatment initiation, observation, radiation, radiation+DIS.
DIS = decreased immunosuppression; DLBCL = diffuse large B-cell lymphoma.
Figure 2.
Bar graph showing changes in treatment strategies over time divided in 3 eras (1987–2002, 2003–2010, and 2011–2017).
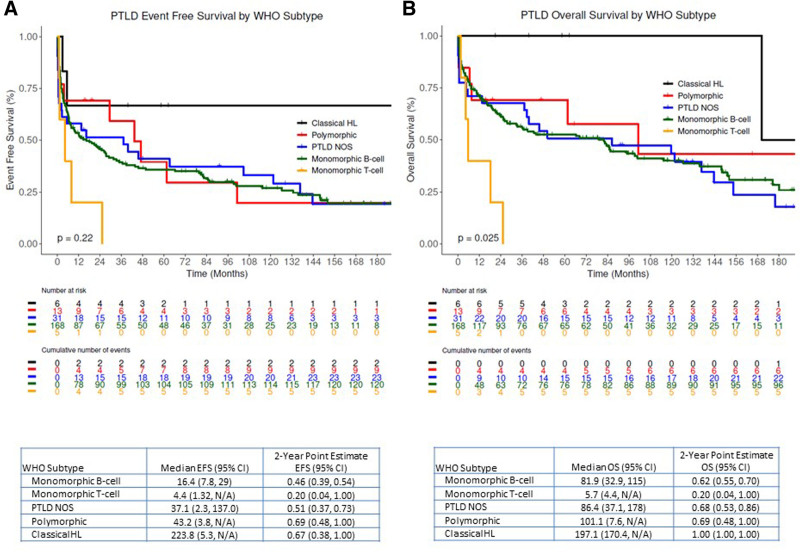
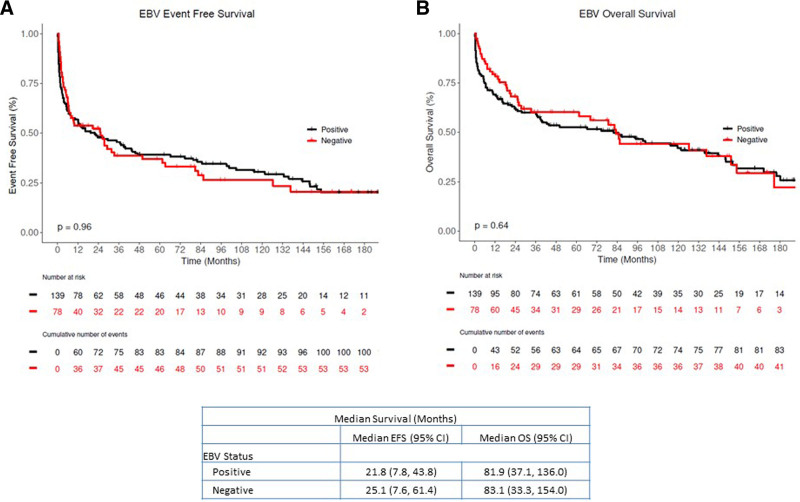
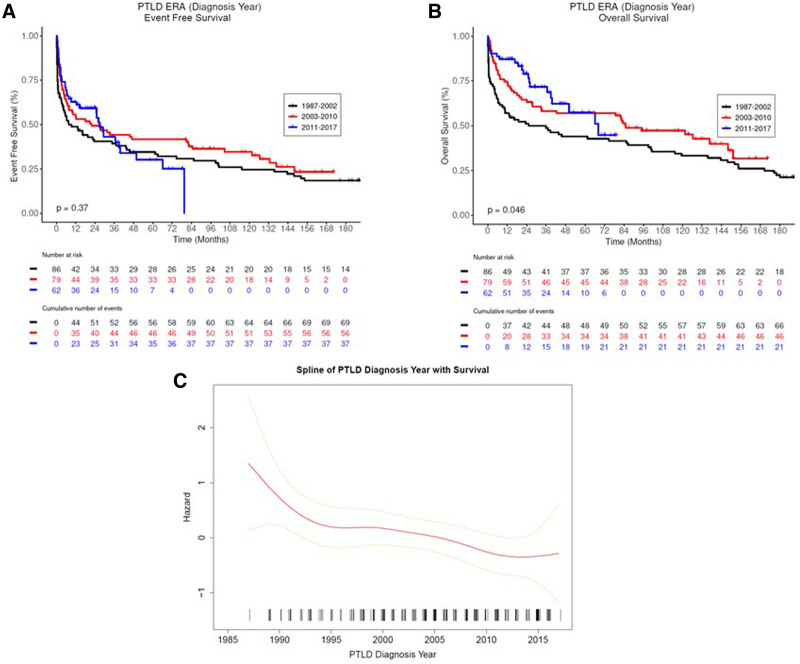
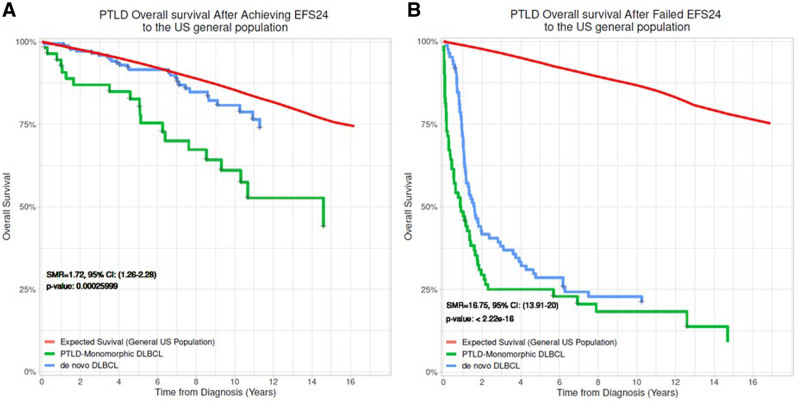
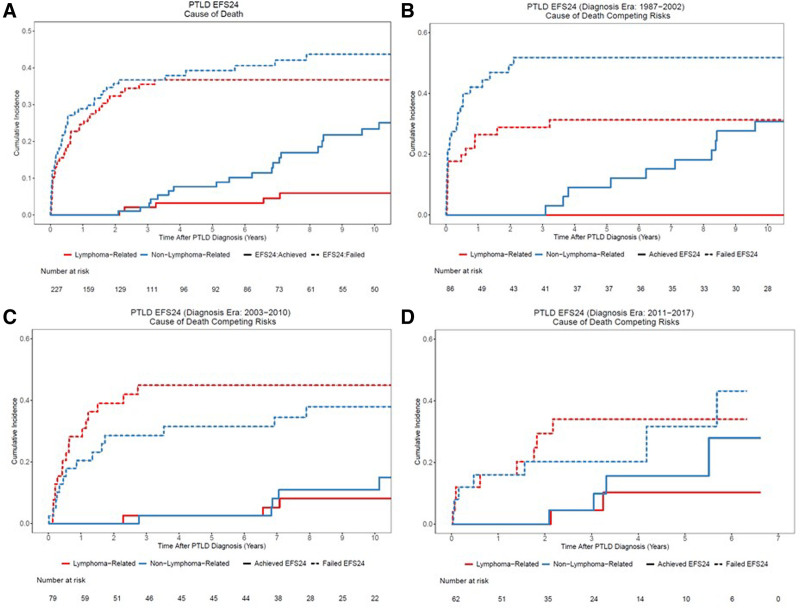
PTLD-CHL patients had the best OS, and the monomorphic T-cell PTLDs had the most inferior outcomes (2-y OS rate 100% versus 20%), albeit based on small numbers (Figure 3). The EBV status was not associated with either EFS or OS in both the entire PTLD cohort and monomorphic PTLD, DLBCL type cohort (Figure 4, and see Supplemental Digital Figure S2, http://links.lww.com/HS/A194). Additionally, the EFS and OS were analyzed based on the eras used to categorize the management changes over the years (Figure 5A and B). The OS improved significantly over the years as also depicted in the hazard ratio spline curve based on the year of PTLD diagnosis (Figure 5C). We then compared the observed survival in patients achieving event-free survival at 24 months (EFS24) versus those failing to achieve EFS24 in both monomorphic PTLD DLBCL, and de novo DLBCL versus expected survival in age- and sex-matched US population (Figure 6). Patients who failed to achieve EFS24 did much worse with a standardized mortality ratio (SMR) of 16.75 (95% CI: 13.91–20) than patients who achieved EFS24 status with an SMR of 1.72 (95% CI: 1.26–2.28). Moreover, for DLBCL PTLD, the overall survival in patients who achieved EFS24 still remained significantly below the background population, as opposed to in de novo DLBCL where survival approaches the age- and sex-matched population. When evaluating the entire cohort, cumulative incidence analysis of cause of death for those achieving EFS24 showed significantly lower 5-year lymphoma-related deaths at 0.03 (95% CI: 0.01–0.10) as compared to 0.37 (95% CI: 0.29–0.47) in those failing EFS24 (Figure 7). The non-lymphoma–related deaths showed a decline over the years when the cumulative incidence analyses for cause of death were done based on 3 different eras (1987–2002 [Figure 7B], 2003–2010 [Figure 7C], and 2011–2017 [Figure 7D]).
Figure 3.
Kaplan Meier estimates of entire PTLD Cohort based on WHO subtypes. (A) Event-free survival and (B) overall survival. PTLD = post-transplant lymphoproliferative disorder; WHO = World Health Organization.
Figure 4.
Kaplan Meier estimates of the entire PTLD Cohort based on EBV status. (A) Event-free survival and (B) overall survival (black: EBV positive, red: EBV negative). EBV = Epstein-Barr virus; PTLD = post-transplant lymphoproliferative disorder.
Figure 5.
PTLD survival based on era of PTLD diagnosis (1987–2002, 2003–2010, and 2011–2017). (Kaplan Meier estimates of (A) event-free survival and (B) overall survival. Functional form of association between diagnosis year and overall survival (C). PTLD = post-transplant lymphoproliferative disorder.
Figure 6.
Overall survival in PTLD based on EFS24 status. (A) Kaplan Meier estimates for PTLD patients achieving EFS24 compared with US general population with PTLD and de novo (non-PTLD) DLBCL. Red: Expected survival (general US population) for PTLD; Green: Monomorphic DLBCL, PTLD achieving EFS24; Blue: De novo (non-PTLD) DLBCL achieving EFS24. (B) Overall survival from early event within 24 months of diagnosis in cohort who failed EFS24. Red: Expected survival (general US population) for PTLD, Green: Monomorphic DLBCL PTLD not achieving EFS24, Blue: De novo (non-PTLD) DLBCL not achieving EFS24. DLBCL = diffuse large B cell lymphoma; EFS, event-free survival; PTLD = post-transplant lymphoproliferative disorder.
Figure 7.
Cumulative incidence of cause of death in the PTLD cohort. (A) Entire PTLD cohort; and (B)–(D) based on EFS24 status in 3 different eras (1987–2002, 2003–2010, and 2011–2017). PTLD = post-transplant lymphoproliferative disorder.
Discussion
This report describes a 30-year clinicopathological experience with PTLD at a single center, based on the recent 2017 WHO classification. It also provides survival estimates based on new WHO subtypes and describes various management strategies and their outcomes. PTLD comprises a morphologically heterogeneous group of lymphoproliferative conditions, of which monomorphic PTLD constitutes up to 60%–80%.1 Monomorphic PTLD fulfills specific WHO criteria for either a B or T/NK-cell NHL described in immunocompetent patients, except for small B-cell lymphoid neoplasms that are not designated as PTLDs. Of the monomorphic PTLDs, DLBCL is the most common, with the less common occurrences of Burkitt lymphoma or a plasma cell neoplasm. Our study also predominantly comprised of monomorphic PTLD B-cell type (168/227, 74%), of which 137/168 (85%) cases were DLBCL. The monomorphic PTLD, T-cell type is rare and consists of 2.2% (5/227) of our patient cohort. Of the 3 ND-PTLDs, 2 were IM, 1 was PH, and none was FFH. ND-PTLDs are usually seen in children or adults with solid organ transplant without prior EBV infection.5,14,15 These typically involve lymph nodes or tonsils and adenoids rather than extranodal sites.14,15 Since our series only included adult patients, the proportion of ND-PTLD was lower. Polymorphic PTLD forms a minority of PTLDs and is also seen more commonly in children.16–18 It is seen after the primary EBV infection post-transplant and tends to occur earlier than monomorphic PTLD. Only 13 (5.7%) patients in our cohort were recognized as P-PTLD with age at PTLD diagnosis ranging between 22 and 62 years. This is consistent with another large single-center study of 140 biopsy-proven PTLD cases after solid organ transplant or ASCT, where P-PTLD was seen in 6% of patients despite pediatric patients’ inclusion.19
EBV-positive mucocutaneous ulcer (EBV-MCU) is an indolent entity that has been recently recognized and occurs in patients with age-related or iatrogenic immunosuppression.7 It can be seen in solid organ transplant recipients and needs to be considered while diagnosing PTLD. While one series showed higher percentages, up to 10%, of EBV-MCU when retrospectively evaluating their PTLD cohort, we reclassified only one case originally diagnosed as monomorphic PTLD, DLBCL to EBV-MCU. As this is a relatively recently described entity, further prospective series are needed to establish EBV-MCU frequency in the post-transplant setting.7 EBV+ MALT lymphomas typically occur in the skin and subcutaneous tissue, which is different than the typical EBV-negative MALT lymphomas seen more commonly in the stomach or parotid gland in the immunocompetent hosts. EBV+ MALT tends to occur late after transplant and is usually solitary with an overall good prognosis.6,20,21 EBV+MALTs are rare in occurrence, and no cases were identified in our study. In our report, the cases where the available pathology material was sufficient to diagnose PTLD but insufficient to subclassify the lesion were classified as PTLD, NOS. These constituted about 13.7% of the total 227 cases highlighting the need for substantial tissue for accurate categorization of PTLD.
Recent studies have shown a longer median time from solid organ transplant to PTLD diagnosis to be approximately four years.19,22,23 Dierickx et al19 in 2013 reported that 71% of 140 biopsy-proven PTLD cases were diagnosed >1 year after solid organ transplant, with a median time of 4 years. Similarly, in a more recent multicenter collaboration of 877 adult PTLD patients after solid organ transplant, the median time from solid organ transplant to PTLD diagnosis was 57 months.23 Our findings are similar with a median time of 45.4 months (range: 1.0–499.8), with 66% of cases of PTLD diagnosed >1 years from solid organ transplant. Historically, this has been much shorter, with most (80%) cases occurring within the first year.24
EBV positivity is more often seen in the early-onset PTLD (<1 y) than late-onset.15,22,25,26 EBV positivity rate in our study was 61% in the entire cohort and 90% in those with early-onset PTLD.
These findings are consistent with studies reported in the last decade.19,22,23 EBV negative PTLD has been on the rise, possibly due to newer immunosuppressive regimens, improved knowledge of risk factors of EBV+ PTLD, better awareness, delayed onset in the diagnosis, and enhanced diagnostic techniques.15,22
PTLD frequently involves extranodal sites and presents with advanced-stage disease. A report by Dierickx et al19 had 78% cases with one or more sites of extranodal involvement and 72% with advanced-stage disease (Ann Arbor stage III/IV). In several studies, the most frequent extranodal sites are the gastrointestinal tract, including the liver (up to 30%) and grafted organ (10%–20%).2,19,23,27,28 Our report had 84% of cases with extranodal involvement and 61% with advanced-stage disease. CNS involvement is low in PTLD and ranges between 5% and 20% in the reported series.2,19,28 Our numbers are lower for CNS involvement at 1.2% and similar for the grafted organ involvement at 19% compared to other reports.
Treatment of PTLD is individualized based on the PTLD type, solid organ transplant type, risk of graft rejection, clinical presentation, tumor burden of PTLD, and patient age/comorbidities. The majority of available data for PTLD treatment rely on retrospective studies and a handful of prospective trials.9–11 Additionally, most of the data pertains to the treatment of CD 20+ B-cell PTLD. Due to these reasons, no standard treatment guidelines exist. Reduction of immunosuppression alone was the first step in management in PTLD, with most success in EBV+, early-onset, and nonmonomorphic PTLD (ND and polymorphic).22,29–31 Complete remission (CR) rates with immunosuppression reduction alone have been reported between 5% and 50%, with durable responses in only 5%–30% in these predominantly retrospective reports.22,29,30 Rituximab monotherapy was subsequently utilized as salvage therapy post RIS failure, with overall response rates (ORRs) up to 70%.32–35 The results of the largest prospective phase II PTLD-1 trial utilizing sequential treatment (ST) of rituximab followed by CHOP-21 chemotherapy were published in 2012, with an ORR of 90% and median OS of 6.6 years.11 This was a significant improvement over the rituximab monotherapy trials with reported OS between 1.2 and 3.5 years.9,33,35 These results have changed the treatment paradigm of B-cell PTLD. Our study depicts this evolution in management, with an increasing number of patients receiving rituximab ± chemotherapy in combination with immunosuppression reduction in the cohorts diagnosed between 2003–2010 and 2011–2017. A subsequent risk-stratified ST (RSST) approach in B-cell PTLD, where patients in CR after initial rituximab × 4 weekly cycles induction were stratified to rituximab consolidation, also showed a similar ORR of 88% and median OS of 6.6 years.10 This approach is now favored due to its lower treatment-related mortality (TRM) in PTLD patients (8%), compared to that of the ST (TRM: 13%) and other retrospective series with first-line chemotherapy (TRM: 31%).9–11,32,36
Median OS in our study is 82 months (95% CI: 35–115), which is much longer than studies reported in the early 2000s and like the estimates of the recently reported phase II trials. Monomorphic PTLD, DLBCL subtype also showed a similar median OS of 85 months, likely owing to the increased use of rituximab and chemotherapy in addition to immunosuppression reduction and improved TRM over the years. This is further confirmed by evaluating the OS based on the year of PTLD diagnosis and cumulative incidence analyses in different eras for cause of death in these patients. Both our entire PTLD cohort and monomorphic PTLD, DLBCL cohort did not show a significant difference in EFS and OS based on the EBV status. This mimics more recently reported phase II trial data where EBV status did not show a difference in survival.10,11,19 Some reports conflict with this notion where EBV positive status is associated with more prolonged survival.15,22,23,32,37 This conflict can be explained because most series are retrospective, single-center, and do not have centrally reviewed pathology specimens. These reports also span over decades during which management preferences, diagnostic techniques, immunosuppression regimens, and supportive care measures have evolved. The patient cohorts are heterogeneous and vary significantly between different reports, including adult or pediatric patients alone or combined. Some reports evaluating the use of rituximab after RIS’s failure may include more patients with M-PTLD than ND or polymorphic subtype.
Our study provides EFS and OS based on the new 2017 WHO subtypes, with cHL PTLD having the best OS (2-y OS rate 100%) and monomorphic PTLD, T-cell subtype with remarkably inferior outcomes (2-y OS rate 20%), despite small numbers in both these cohorts. This has not been described before in the literature and could help prognosticate patients. To further aid in the prognostication of patients with monomorphic PTLD, DLBCL we compared the OS based on EFS24 status with that of de novo DLBCL and age- and sex-matched US general population, and there was a significant impact of EFS24 status on monomorphic PTLD, DLBCL survival. The 2- and 5-year OS rates in those achieving EFS 24 were 88% and 80.4%, respectively, compared to 38.3% and 34.6% in patients who failed EFS24. This highlights significant early mortality and emphasizes the need for optimal treatment as a frontline strategy. Furthermore, in contrast to the de novo DLBCL, where survival approached that of general population after achieving EFS24, monomorphic PTLD-DLBCL’s survival lagged behind.38,39 Based on the cause of death data, this is mostly contributed by the nonlymphoma deaths which are likely from complications related to ongoing immunosuppression, graft failure, surgical complications, other non-PTLD malignancies, and other comorbidities. This highlights the need for continued multidisciplinary care of these patients.
This study has the inherent bias and limitations associated with a retrospective, single-center cohort study, including disease heterogeneity, lack of control over confounding factors, treatment choices, and timing of follow-ups and radiologic studies. We also acknowledge that not having MYC FISH on many of our DLBCL cases is a limitation to 100% accurate classification by the WHO 2017. However, within a PTLD cohort, this is a relatively minor limitation since the majority of PTLD DLBCLs are of the non-GCB type. Indeed, among our cases in which Hans algorithm was performed, as expected, the vast majority of our DLBCL cases were non-GCB (80%) which have a much lower rate (<2% in prior studies) of being a double-hit lymphoma.40 Overall, central review of pathology remains a strength of our study in addition to a dataset that spans over 30 years and homogenously depicts practice changes over different decades based on PTLD research’s evolution. We can also provide survival estimates based on the WHO subtypes and have identified EFS24 as an additional prognostication tool.
Conclusions
In summary, our study confirms the improvement in survival of PTLD patients and changes in the treatment preferences over time. The use of rituximab with or without chemotherapy increased over the years as frontline therapy in addition to RIS. New WHO subtypes and EFS24 status aid in predicting outcomes in patients with PTLD. EFS 24 status is prognostic in monomorphic PTLD, DLBCL but patients show consistent increase in late non-lymphoma–related mortality.
Disclosures
TMH held a consulting or advisory role in Celgene, Kite/Gilead, Seattle Genetics. MJM received research funding from BMS, Morphosys, Nanostring, Genentech, and held a consulting or advisory role in Pfizer, Kite, and Morphosys. SMA received honoraria WebMD and Research to Practice, and received research funding from Bristol-Myers Squibb, Seattle Genetics, Affimed Therapeutics, Regeneron, Trillium Therapeutics, AI Therapeutics, and ADC Therapeutics. JRC held a consulting or advisory role in Janssen, received research funding from NanoString Technologies, Celgene, and Genentech. NNB held a consulting or advisory role in Purdue Pharma (Institutional), Adicet Bio (Institutional), Verastem (Institutional), and Seattle Genetics (Institutional), and received research funding from Kite Pharma (Institutional).
Sources of funding
Supported in part by the University of Iowa/Mayo Clinic Lymphoma SPORE CA97274-19 and the Lymphoma Epidemiology Outcomes U01 CA195568.
Supplementary Material
Footnotes
RLK and AK are co-first authors.
Previous Presentation: Presented as an oral presentation at the American Society of Hematology 60th Annual Meeting and Exposition, San Diego, CA, USA, December 1–4, 2018.
Supplemental digital content is available for this article.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed.Lyon, France: International Agency for Research on Cancer (IARC);2017. [Google Scholar]
- 2.Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–562. [DOI] [PubMed] [Google Scholar]
- 3.Asleh R, Clavell AL, Pereira NL, et al. Incidence of malignancies in patients treated with sirolimus following heart transplantation. J Am Coll Cardiol. 2019;73:2676–2688. [DOI] [PubMed] [Google Scholar]
- 4.Schober T, Framke T, Kreipe H, et al. Characteristics of early and late PTLD development in pediatric solid organ transplant recipients. Transplantation. 2013;95:240–246. [DOI] [PubMed] [Google Scholar]
- 5.Vakiani E, Nandula SV, Subramaniyam S, et al. Cytogenetic analysis of B-cell posttransplant lymphoproliferations validates the World Health Organization classification and suggests inclusion of florid follicular hyperplasia as a precursor lesion. Hum Pathol. 2007;38:315–325. [DOI] [PubMed] [Google Scholar]
- 6.Gibson SE, Swerdlow SH, Craig FE, et al. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: a distinct type of posttransplant lymphoproliferative disorder? Am J Surg Pathol. 2011;35:807–815. [DOI] [PubMed] [Google Scholar]
- 7.Hart M, Thakral B, Yohe S, et al. EBV-positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lymphoproliferative disorder. Am J Surg Pathol. 2014;38:1522–1529. [DOI] [PubMed] [Google Scholar]
- 8.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107:3053–3057. [DOI] [PubMed] [Google Scholar]
- 10.Trappe RU, Dierickx D, Zimmermann H, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase II trial. J Clin Oncol. 2017;35:536–543. [DOI] [PubMed] [Google Scholar]
- 11.Trappe R, Oertel S, Leblond V, et al. ; German PTLD Study Group; European PTLD Network. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196–206. [DOI] [PubMed] [Google Scholar]
- 12.Trappe RU, Koenecke C, Dreyling MH, et al. Treatment stratification in B-cell PTLD after solid organ transplantation (SOT) by international prognostic index (IPI) and response to rituximab: Interim results from the PTLD-2 trial. J Clin Oncol. 2020;38(15_suppl):8045. [Google Scholar]
- 13.Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lones MA, Mishalani S, Shintaku IP, et al. Changes in tonsils and adenoids in children with post-transplant lymphoproliferative disorder: report of three cases with early involvement of Waldeyer’s ring. Hum Pathol. 1995;26:525–530. [DOI] [PubMed] [Google Scholar]
- 15.Nelson BP, Nalesnik MA, Bahler DW, et al. Epstein-Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000;24:375–385. [DOI] [PubMed] [Google Scholar]
- 16.Webber SA, Naftel DC, Fricker FJ, et al. ; Pediatric Heart Transplant Study. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367:233–239. [DOI] [PubMed] [Google Scholar]
- 17.Mynarek M, Schober T, Behrends U, et al. Posttransplant lymphoproliferative disease after pediatric solid organ transplantation. Clin Dev Immunol. 2013;2013:814973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke AJ, Bishnoi R, Bajwa R, et al. Outcomes in pediatric patients with post-transplant lymphoproliferative disorder (PTLD): analysis of a 20-year single-institutional experience. Hematol Oncol. 2017;35:352–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dierickx D, Tousseyn T, Sagaert X, et al. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54:2433–2440. [DOI] [PubMed] [Google Scholar]
- 20.Nassif S, Ozdemirli M. EBV-positive low-grade marginal zone lymphoma in the breast with massive amyloid deposition arising in a heart transplant patient: a report of an unusual case. Pediatr Transplant. 2013;17:E141–E145. [DOI] [PubMed] [Google Scholar]
- 21.Gong S, Crane GM, McCall CM, et al. Expanding the spectrum of EBV-positive marginal zone lymphomas: a lesion associated with diverse immunodeficiency settings. Am J Surg Pathol. 2018;42:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evens AM, David KA, Helenowski I, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagadeesh D, Tsai DE, Wei W, et al. Post-transplant lymphoproliferative disorder (PTLD) after solid organ transplant (SOT): a multicenter real world analysis (RWA) of 877 patients (pts) treated in the modern era. J Clin Oncol. 2020;38(15_suppl):e20026. [Google Scholar]
- 24.Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342:1514–1516. [DOI] [PubMed] [Google Scholar]
- 25.Dotti G, Fiocchi R, Motta T, et al. Lymphomas occurring late after solid-organ transplantation: influence of treatment on the clinical outcome. Transplantation. 2002;74:1095–1102. [DOI] [PubMed] [Google Scholar]
- 26.Leblond V, Davi F, Charlotte F, et al. Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? J Clin Oncol. 1998;16:2052–2059. [DOI] [PubMed] [Google Scholar]
- 27.Aull MJ, Buell JF, Trofe J, et al. Experience with 274 cardiac transplant recipients with posttransplant lymphoproliferative disorder: a report from the Israel Penn International Transplant Tumor Registry. Transplantation. 2004;78:1676–1682. [DOI] [PubMed] [Google Scholar]
- 28.Caillard S, Lamy FX, Quelen C, et al. ; French Transplant Centers. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682–693. [DOI] [PubMed] [Google Scholar]
- 29.Ghobrial IM, Habermann TM, Maurer MJ, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders. J Clin Oncol. 2005;23:7574–7582. [DOI] [PubMed] [Google Scholar]
- 30.Tsai DE, Hardy CL, Tomaszewski JE, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076–1088. [DOI] [PubMed] [Google Scholar]
- 31.Rees L, Thomas A, Amlot PL. Disappearance of an Epstein-Barr virus-positive post-transplant plasmacytoma with reduction of immunosuppression. Lancet. 1998;352:789. [DOI] [PubMed] [Google Scholar]
- 32.Elstrom RL, Andreadis C, Aqui NA, et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006;6:569–576. [DOI] [PubMed] [Google Scholar]
- 33.González-Barca E, Domingo-Domenech E, Capote FJ, et al. ; GEL/TAMO (Grupo Español de Linfomas); GELCAB (Grupo para el Estudio de los Linfomas Catalano-Balear); GOTEL (Grupo Oncológico para el Tratamiento y Estudio de los Linfomas). Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. 2007;92:1489–1494. [DOI] [PubMed] [Google Scholar]
- 34.Blaes AH, Peterson BA, Bartlett N, et al. Rituximab therapy is effective for posttransplant lymphoproliferative disorders after solid organ transplantation: results of a phase II trial. Cancer. 2005;104:1661–1667. [DOI] [PubMed] [Google Scholar]
- 35.Oertel SH, Verschuuren E, Reinke P, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant. 2005;5:2901–2906. [DOI] [PubMed] [Google Scholar]
- 36.Taylor AL, Bowles KM, Callaghan CJ, et al. Anthracycline-based chemotherapy as first-line treatment in adults with malignant posttransplant lymphoproliferative disorder after solid organ transplantation. Transplantation. 2006;82:375–381. [DOI] [PubMed] [Google Scholar]
- 37.Leblond V, Dhedin N, Mamzer Bruneel MF, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772–778. [DOI] [PubMed] [Google Scholar]
- 38.Maurer MJ, Jais JP, Ghesquières H, et al. Personalized risk prediction for event-free survival at 24 months in patients with diffuse large B-cell lymphoma. Am J Hematol. 2016;91:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakobsen LH, Bøgsted M, Brown PN, et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: a Danish population-based study. J Clin Oncol. 2017;35:778–784. [DOI] [PubMed] [Google Scholar]
- 40.Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131:2060–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.