Abstract
Nanomedicine usually refers to nanoparticles that deliver the functional drugs and siRNAs to treat cancer. Recent research has suggested that cancer cells can also make nanoparticles that also deliver functional molecules in promoting cancer metastasis, which is the leading cause of various cancer mortalities. This nanoparticle is called tumor-derived vesicles, or better-known as tumor-derived exosomes (TEXs). TEXs are nanoscale membrane vesicles (30–140 nm) that are released continuously by various types of cancer cells and contain tumor-derived functional biomolecules, including lipids, proteins, and genetic molecules. These endogenous TEXs can interact with host immune cells and epithelial cells locally and systemically. More importantly, they can reprogram the recipient cells in favor of promoting metastasis through facilitating tumor cell local invasion, intravasation, immune evasion, extravasation, and survival and growth in distant organs. Growing evidence suggests that TEXs play a key role in cancer metastasis. Here, we will review the most recent findings of how cancer cells harness TEXs to promote cancer metastasis through modulating vascular permeability, suppressing systemic immune surveillance, and creating metastatic niches. We will also summarize recent research in targeting TEXs to treat cancer metastasis.
KEY WORDS: Tumor-derived exosomes, Metastasis, Vasculature leaky, Immunosuppression, Pre-metastatic niche, Therapeutic implications, Exosome targeting, Nanocarrier
Graphical abstract
We reviewed recent progress on understanding how tumor-derived exosomes (TEXs) promote metastasis through facilitating tumor cell local invasion, intravasation, immune evasion, extravasation, and growth in distant organs.
1. Introduction
When cancer patients developed metastatic lesions in remote organs, the survival rate would be dramatically decreased compared to patients with local disease. Although it is known that cancer cells need to go through a series of steps to develop metastases, also known as the metastatic cascade1, the tumor-derived factors that modulate cancer cells to disseminate, survive in the circulation, and grow in the metastatic sites remain mostly unknown. Although several hypotheses about cancer metastasis have been proposed2, 3, 4, 5, no theory can fully explain the whole metastatic process; more importantly, there is currently no effective way to cure metastatic disease, which causes more than 90% of all cancer-related deaths.
Recently, tumor-derived exosomes (TEXs) have surfaced as critical tumor-derived factors that play a critical role in the metastatic process. A growing body of evidence has suggested that TEXs can interact with host immune cells6,7, epithelial cells8,9, and tumor cells10,11, to alter and reprogram the recipient cells to facilitate tumor progression and cancer metastasis. For example, it was found that TEXs manipulate the leakage of vascular barriers to not only facilitate tumor cell escape from primary tumor tissues but also promote tumor cells to live and grow in metastatic organs8. Recent studies also found that TEXs carry immunosuppressive proteins such as program death ligand 1 (PD-L1) on their surfaces that can suppress cytotoxic T-cells locally and systemically12,13. Studies also found that TEXs activate epithelial cells through payload RNAs to recruit myeloid cells and develop permissive lung pre-metastatic niches9. Although previously viewed as “trash bags,” TEXs are now known to be important mediators in cancer metastasis. Here, we will review the recent progress in current understanding of how TEXs promote cancer metastasis through delivering exosomal genetic molecules, proteins, and lipids with a focus on the role of tumor-derived exosomes in each step of the metastatic cascade. Understanding how TEXs modulate immune suppression and facilitate tumor dissemination and outgrowth in distant organs through their unique component will provide us novel insight to better design therapeutic strategies to fight against metastatic cancer.
2. TEXs and their interaction with host cells
TEXs are generally believed to be secreted from cancer cells by fusion of multivesicular bodies with the plasma membrane; a similar endocytic pathway is used for secreting exosomes by healthy cells14. However, due to TEXs' unique surface proteins, lipid composition, and contained genetic molecules, e.g., tumor-derived microRNAs (miRNAs), messenger RNAs, and DNAs (Fig. 1), TEXs show entirely different characteristics from normal cell-derived exosomes toward their biological functions15. Therefore, due to their nano-ranged size (30–140 nm) and their active biological functions, TEXs are much like nanomedicine made by cancer cells, except that they are pro-metastatic. TEXs enter host cells via various mechanisms (e.g., non-traditional endocytic pathways), depending on the target cells and the secreting cancer cells16. For example, glioblastoma cell-derived exosome uptake uses lipid Raft-mediated endocytosis and depends on undisturbed ERK1/2–HSP27 signaling17. Exosomes from brain-metastatic breast cancer cells use transcytosis to cross the brain endothelial cells18 and utilize the “CDC42-dependent clathrin-independent carrier/GPI-AP-enriched compartment (CLIC/GEEC) endocytic pathway” to enter astrocytes19.
Figure 1.
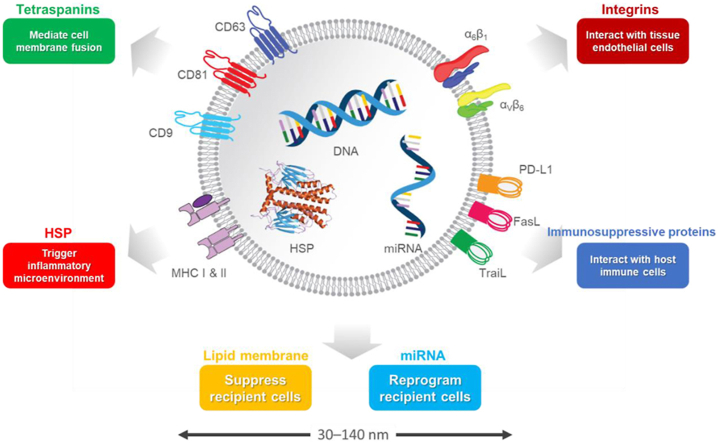
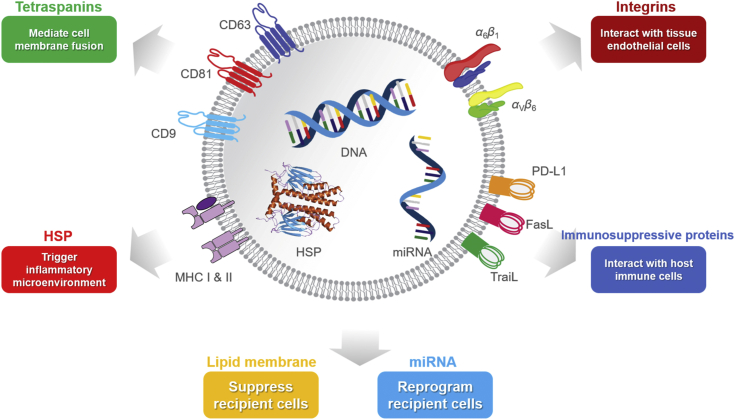
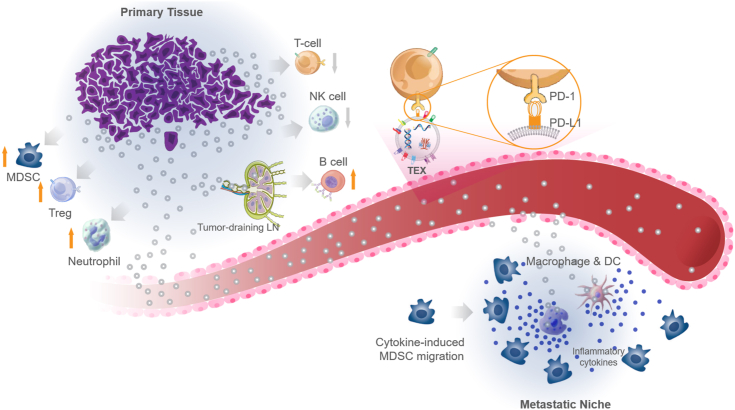
Schematic illustration of main characteristics of tumor-derived exosomes (TEXs). TEXs are membrane nanovesicles (30–140 nm) secreted by cancer cells. They carry specific tumor-derived integrins on their surfaces, which preferentially interact with certain endothelial cells. Once bound to the receptor cells, TEXs can deliver tumor-derived genetic molecules such as miRNAs and reprogram the recipient cells in the favor of cancer metastasis. TEXs also carry various immunosuppressive surface proteins such as PD-L1 that can directly interact with cytotoxic T-cells through PD-1/PD-L1 interaction locally and systemically, and inhibit T-cell anticancer function.
TEXs contain tumor-promoting content, both at the surface and in the lumen. For example, tetraspanins (which help mediate cell membrane fusion), MHC, integrins (which interact with tissue endothelial cells), and immunosuppressive proteins (e.g., PD-L1, FasL, TraiL) are in the phospholipid-bilayer membrane. HSPs (which trigger inflammatory microenvironments) and pro-tumor cytosolic proteins (e.g., enzymes, cytokines, and oncoproteins) and receptors (e.g., MET, gp130) are in the lumen20. TEXs also contain genetic materials (e.g., DNAs, miRNAs, mRNAs, IncRNAs) that reprograms recipient cells. For example, exosomal miR-105, miR-25-3p, and miR-103 can compromise vascular integrity, and exosomal miR-1246 and miR-92 participate in immunosuppression (Table 1).
Table 1.
Summary of functional molecules in TEXs.
| Functional molecule | Summary of mechanism |
|---|---|
| Makes vasculature leaky | |
| miR-105 | Lowers zonula occludens-1 (ZO-1) levels in endothelial cells |
| miR-25-3p | Silence Krüppel-like factor 2 (KLF2) and Krüppel-like factor 4 (KLF 4) in colorectal cancer |
| miR-103 | In liver cancer, inhibits the expression of the VE-cadherin, P120, and ZO-1 |
| Immunosuppression | |
| PD-L1 | Binds to PD-1 on cytotoxic T-cells, blocks signaling downstream of the T-cell receptor (TCR), and hence inactivates them. |
| Noncoding Y RNA hY4 | Helps reprogram monocytes in an immunosuppressive manner by activating endosomal TLR7/TLR8 signaling; increases TNF-alpha and PD-L1 expression |
| gp130 | An IL-6 receptor; in breast cancer, stimulates STAT3 signaling in bone-marrow derived macrophages and triggers IL-6 secretion |
| MET | A hepatocyte growth factor receptor; educates bone-marrow derived cells to make them pro-vasculogenic and pro-migratory |
| miR-1246 | Increases macrophages' TGF-β activity and makes them more motile and invasive, undergo epithelial-to-mesenchymal transition, degrade the extracellular matrix, and recruit immunosuppressive Tregs |
| miR-92a | By suppressing SMAD7 protein, increases TGF-β activity in hepatic stellate cells and deposition of extracellular matrix proteins in the pre-metastatic niche, promoting the recruitment of immunosuppressive BMDCs |
| Promotes metastatic niches | |
| Binding of disseminated tumor cells to metastatic niches | |
| ITGβ4 | On exosome's surface; binds to laminin in the laminin-rich lung microenvironment |
| ITGα6β4 | On exosome's surface; binds to epithelial cells and fibroblasts that belong to the lung |
| ITGαv | May selectively adhere to fibronectin in the fibronectin-rich microenvironment in the liver |
| Angiogenesis in metastatic niche | |
| Soluble E-cadherin | Binds to VE-cadherin on endothelial cells and hence activates NF-κB and β-catenin signaling |
| Carbonic anhydrase 9 (CA9) | Induces tube formation and migration and increases MMP2 production |
Once TEXs are released out into the extracellular microenvironment, their biological function is determined upon how they can be recognized by the host cells. Previous research has suggested that due to their tumor-derived unique surface proteins and lipid composition as well as their virus-like hydrodynamic sizes, TEXs may be quickly captured by host immune cells and epithelial cells21, 22, 23, 24. For example, using a luciferase-expressing mouse tumor cell line, Takahashi et al.21,22 demonstrated that B16-BL6-derived exosomes administered to syngeneic mice could be quickly distributed from blood circulation to tissues like liver, spleen, and lung with a half-life of around 2 min. Consistently, fluorescence-labeled TEXs could be detected in the blood vessels of lungs 5 min after tail vein injection23. Rapid clearance of intravenously-injected exosomes, which are derived from human prostate adenocarcinoma and labeled with a radiotracer, was also observed in nude mice24. Interestingly, by comparing exosome clearance rate in 4T1 tumor-bearing mice with different stains, namely BALB/c, nude, and NOD/SCID mice, Smyth et al.24 found that 4T1 exosomes were captured in liver and spleen as fast as 20 min in BALB/c mice after systemic administration and that the clearance rate is similar in both BALB/c mice and nude mice. However, the exosomes' clearance in immunocompromised mice was delayed, indicating that the innate immune system, not the adaptive immune system, plays a role in recognizing tumor exosomes in tumor-bearing hosts.
Given the similarity between TEXs and viruses, the human immune system might treat the constantly released TEXs as viruses and develop B-cell-mediated humoral immune responses against them25, which may trigger inflammatory cytokine release. The induced cytokines, which are not able to eliminate TEXs, may have pro-metastatic effects. By using similar luciferase-expressing B16F10 tumor models and monitoring the interaction between endogenously produced tumor vesicles and host cells, Pucci et al.25 found that tumor vesicles may be preferentially recognized by subcapsular sinus (SCS) CD169+ macrophages in tumor-draining lymph nodes. As these vesicles disrupt the SCS macrophage layer, they can access the B-cell follicles and trigger pro-tumor humoral immune responses against the TEXs25. This study suggests that host body may initiate humoral immune responses and treat TEXs as invaded viruses. This study also suggests that the recognition of TEXs by host cells may occur through a receptor-mediated capture of TEXs rather than through non-specific interactions26. More importantly, how TEXs interact with the recipient cells may also explain why the contained genetic molecules, especially miRNAs, can enter the cytosol and remain functional instead of being digested in endosomes inside cells.
3. Biofunctional role of tumor-derived exosomes in metastatic cascade
3.1. TEXs cause tumor vasculature leaky
One of the key questions in cancer biology is that why tumor cells can often leave primary tumor tissues regardless of their cellular origins, which results in the subsequent development of fatal metastasis. Recently, a pioneer study led by Dr. Wang has revealed that TEXs play a critical role in promoting tumor cell dissemination8. This study found that exosomes derived from metastatic MDA-MB-231 breast cancer cells cause adjacent endothelial cells with a down-regulation of the expression of zonula occludens-1 (ZO-1) protein8, which has a tight junction function. The TEX-induced ZO-1 inhibition makes the vasculature in primary tumor tissue leaky and therefore facilitates breast cancer cells intravasation. They further revealed that the expression of a miRNA called miR-105 is significantly higher in exosomes derived from MDA-MB-231 breast cancer cells compared to exosomes derived from normal breast cells (i.e., MCF-10A). Upon incubation with human microvascular endothelial cells (HMVECs), their data shows that exosomes with higher expressions of miR-105 cause the recipient endothelial cells to lose the function for ZO-1 protein secretion, but exosomes containing low miR-105 do not have that effect (Fig. 2a). More importantly, these miR-105-containing exosomes can travel to distant organs and increase vascular permeability at future metastatic sites, thereby facilitating metastasis by promoting extravasation8. This work highlighted the importance of TEXs in vascular remodeling locally and remotely; they help tumor cells to not only escape from the primary site but also grow in future metastatic sites.
Figure 2.
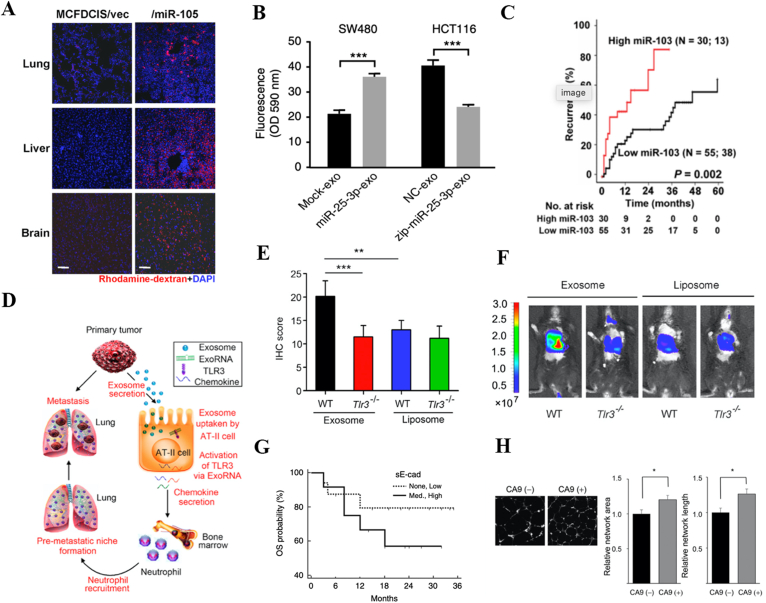
TEXs support pre-metastatic niches by compromising vascular integrity, suppressing the immune system, and promoting tumor angiogenesis. (A) Exosomal miR-105 promotes vascular permeability, as indicated by the increased “appearance of intravenously injected rhodamine-dextran (red) in various organs.” Reprinted from Ref. 8. Copyright © 2014 Elsevier Inc. (B) In colorectal cancer, exosomal miR-25-3p increases vascular permeability, as indicated by the increased permeability of the human umbilical vein endothelial cell (HUVEC) monolayers to rhodamine-dextran after exposure to exosomal miR-25-3p. Reprinted from Ref 27. Copyright © 2018, The Author(s). (C) In hepatocellular carcinoma, exosomal miR-103 is linked to higher recurrence rates. Reprinted from Ref 29. Copyright © 2018 by the American Association for the Study of Liver Diseases. (D) In breast cancer, exosomal RNAs enters alveolar type II cells (AT-II cells) and causes them to secrete chemokines, hence causing neutrophil recruitment and pre-metastatic niche formation in the lungs. Reprinted from Ref. 9. Copyright © 2016 Elsevier Inc. (E) In breast cancer, TEXs increase fibronectin expression in the lungs in wild-type littermates but not in Tlr3−/− mice. Liposomes do not seem to significantly increase fibronectin expression. Reprinted from Ref. 9. Copyright © 2016 Elsevier Inc. (F) Quantification of lung metastasis of Tlr3−/− mice or WT littermates after exosome or liposome administration. Reprinted from Ref. 9. Copyright © 2016 Elsevier Inc. (G) In patients with ovarian cancer, high levels of exosomal soluble E-cadherin (sE-cad) are linked to worse prognoses. Reprinted from Ref. 84. Copyright © 2018, The Author(s). (H) Tube formation and angiogenesis increase when human umbilical vein endothelial cells (HUVECs) are treated with exosomes containing carbonic anhydrase-9. Reprinted from Ref. 85. Copyright © 2017 Elsevier Inc.
Similar effects were also reported in both colorectal cancer (CRC) and hepatocellular carcinoma (HCC). In CRC, exosomal miR-25-3p can be transferred to vascular endothelial cells and silence Krüppel-like factor 2 (KLF2) and Krüppel-like factor 4 (KLF 4) in the receptor cells27 (Fig. 2b). Decreased KLF2 cannot inhibit the promoter activity of vascular endothelial growth factor receptor 2 (VEGFR2) and induce angiogenesis; meanwhile, decreased KLF4 inhibits the expression of tight-junction related proteins like ZO-1, occludin, and Claudin5, which leads to vascular leakiness and metastasis27,28. In HCC, exosomal miR-103 promotes vascular permeability and metastasis29 (Fig. 2c). Exosomal miR-103 is transferred to endothelial cells and inhibits the expression of VE-Cadherin, P120, and ZO-1, which are important in cell–cell adhesion and help maintain endothelial cell–cell contacts. When p120 expression is reduced, p120 cannot fulfill its normal function of stabilizing the E-cadherin (E-Cad)/β-catenin complex at the cell membrane to maintain intercellular adhesion29. Thus, vascular permeability, HCC cell migration, and the formation of hepatic and pulmonary metastases are promoted29.
Together, these studies have demonstrated that miRNA encapsulated in the TEXs plays a critical role in vascular permeability and contributes to tumor cell invasion at the primary site and extravasation at secondary sites (Fig. 3). In addition to the tumor vasculature disruption, studies also found that exosomal miRNAs play various roles in promoting cancer metastasis, including suppressing glucose uptake by niche cells, causing PTEN loss, and activating Toll-like receptor (TLR) (Table 2). Although various specific miRNA molecules in different cancer types have been reported, it remains unclear whether there is a more general molecular mechanism that helps modulate recipient cell function and tumor vasculature, such as TEX-mediated RNA sensing regardless of specific miRNA molecules. In addition, leaky tumor vasculature has long been confirmed and has been widely harnessed for drug delivery using human-made nanomedicine via taking advantage of the well-known enhanced permeability and retention (EPR) effect37. It was generally believed that the leaky tumor vasculature is due to poorly aligned defective endothelial cells during fast cancer angiogenesis. Recent findings from TEXs may provide a completely novel molecular mechanism for the EPR effect.
Figure 3.
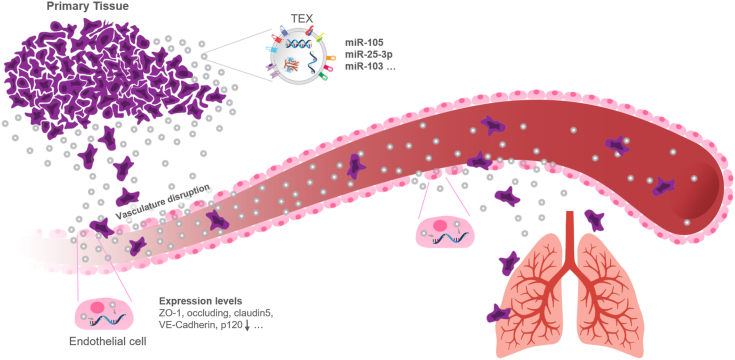
Tumor-derived exosomes (TEXs) promote metastasis by promoting vasculature disruption. Exosomes from metastatic cancer cells contain specific miRNAs, which cause endothelial cells to reduce their expression of important tight-junction proteins like ZO-1, cadherin, and P120. TEXs from the primary tumors leak into the bloodstream and travel to the metastatic sites. Upon arrival to the metastatic sites, they get taken in by endothelial cells there, down-regulate the expression of tight-junction proteins, and hence destroy vascular integrity. The destruction of vascular integrity facilitates metastasis.
Table 2.
Various exosomal miRNAs in cancer metastasis.
| Exosomal miRNA | Cancer type | Role in cancer metastasis | Ref. |
|---|---|---|---|
| miR-105 | Breast cancer | Down-regulate tight junction protein ZO-1 | 8 |
| miR-122 | Breast cancer | Down-regulate glycolytic enzyme pyruvate kinase | 30 |
| miR-19 | Breast cancer | PTEN loss | 31 |
| miR-939 | Breast cancer | Inhibit VE-cadherin | 32 |
| miR-21 | Lung cancer | Up-regulate STAT3 and VEGF | 33 |
| miR-23a | Lung cancer | Down-regulate ZO-1 | 34 |
| miR-21/29a | Lung cancer | Activating TLR7 and TLR8 | 35 |
| miR-25-3p | Color rector cancer | Down-regulate ZO-1, occludin, and claudin5 | 27 |
| miR-103 | Hepatocellular carcinoma | Down-regulate VE-Cadherin, p120, and ZO-1 | 29 |
| miR-222-3p | Ovarian cancer | Down-regulate SOCS3 | 36 |
3.2. TEXs suppress anti-tumor immunity
3.2.1. TEXs carry immunosuppressive PD-L1 on their surfaces and suppress activated T-cells
PD-L1 is a critical immunosuppressive protein that can bind to its receptor PD-1 on CD8+ effector T-cells to inhibit antitumor immune responses38. When PD-L1 binds to PD-1, signaling downstream of the heterodimeric T-cell receptor (TCR) is inhibited39.
It was initially thought that only tumor cells or tumor-associated lymphocytes such as antigen-presenting cells and bone marrow-derived myeloid cells are the major immunosuppressive factors that can present PD-L1 to inhibit cytotoxic CD8+ T-cell anticancer function38, 39, 40. Recent studies further found that TEXs in patient's blood also carry PD-L1 on their surfaces, revealing an entirely new understanding of TEX-mediated immune suppression12,13. For example, one group reported that exosomal PD-L1 has a significant immunosuppressive effect in melanoma patients12. By comparing PD-L1 expression on exosomes derived from a melanoma cell line, Chen et al.12 discovered that exosomes from metastatic melanoma cells express much higher levels of PD-L1 than their primary tumor cells. Their study further showed that the level of PD-L1 in peripheral circulating exosomes from metastatic melanoma patients is significantly higher than those from healthy donors, and those PD-L1 highly expressed exosomes can effectively deactivate cytotoxic T-cells and hence prevent the immune system from fighting the cancer cells. This result is consistent with previous findings, indicating that TEXs inhibited the proliferation of activated CD8+ effector T-cells41, 42, 43, 44, 45, 46. More importantly, they discovered that higher levels of exosomal PD-L1 before a pembrolizumab therapy (an anti-PD-1 therapy) is predictive of greater tumor burden and higher levels of IFN-γ, which are predictive of poor therapy outcomes12. Their results from clinical data further showed that high pretreatment levels of exosomal PD-L1 are indicative of immune dysfunction and severe exhaustion of T-cells, which makes them unable to be reinvigorated by the treatment12. This study further found that, in patients who responded to the therapy, the exosomal PD-L1 levels significantly increased (at least 2.43-fold) six weeks after the treatment started; this is likely because the treatment successfully invigorated the cytotoxic T-cells, and the tumor cells released more PD-L1 in a futile attempt to inactivate cytotoxic T-cells adaptively12.
Almost at the same time, another group also reported the suppressive function of exosomal PD-L1 on antitumor immunity in prostate cancer13. Their study demonstrated that if the syngeneic TRAMP-C2 prostate cancer cells lose the capability of secreting exosomal PD-L1 via CRISPR/Cas9-mediated depletion of Rab27a and PD-L1, the tumor growth will be significantly inhibited compared to its wild-type cell line in immunocompetent B6 mice. The molecular and cellular mechanism studies further reveal that exosomal PD-L1 can suppress antitumor immunity in the draining lymph nodes. Their study also found that administering exosomes derived from the wild-type cell line can rescue tumor growth of Rab27 and PD-L1 deficient cancer cells, confirming the exosomal PD-L1 immune suppression toward antitumor immunity13.
3.2.2. TEXs reprogram tumor-infiltrated immune cells into immunosuppressive phenotypes
Tumor-associated immune cells play an important role in promoting cancer metastasis due to their immunosuppressive characteristics, which are strongly associated with the tumor microenvironment38, 39, 40. Recent studies have found that TEXs contribute to reprograming the tumor-associated immune cells into pro-tumorigenic phenotypes6,7,36,47, 48, 49, 50, 51, 52. For example, it was reported that when monocytes were treated with chronic lymphocytic leukemia (CLL)-derived exosomes, the uptake of the exosomes trigger the release of inflammatory cytokines and PD-L1 expression, and skew monocytes into pro-tumorigenic phenotypes6. It was further revealed that the exosomal noncoding Y RNA hY4 result in the monocyte reprogramming by binding to endosomal TLR7/TLR86. This binding activates NF-κB expression, which promotes pro-tumor cytokine release (e.g., TNF-α secretion) and increases PD-L1 expression in monocytes6. Exosomal hY4 also stimulates TNF-α secretion6, an important cytokine that promotes cancer metastasis53. Another recent study also found that the IL-6-receptor-beta gp130 in breast cancer TEXs stimulates STAT3 signaling in bone-marrow derived macrophages (BMDMs) and triggers IL-6 secretion52, which promotes cancer cell proliferation, cancer cell invasion, and metastasis54. It was concluded that TEXs dominate tumor-associated macrophage polarization toward immunosuppressive M2-type macrophages in breast cancer52,55.
Given the fact that neutrophils are the most abundant innate immune cells in our body and the high immunogenicity of TEXs, the interaction between neutrophils and TEXs actively take place, and the exosome-mediated neutrophil activation may play a driving force for cancer metastasis progression56, 57, 58, 59. This idea was further confirmed by two recent studies revealing that TEXs can polarize myeloid-derived neutrophils into pro-tumor phenotypes7,60. For instance, Zhang et al.7 found that exosomes derived from gastric cancer can transport high mobility group box-1 (HMGB1) to neutrophils, which interacts with TLR4 and triggers neutrophil activation through NF-κB pathway. These findings demonstrated that TEXs play a role in polarizing tumor-associated neutrophils, which have been linked to the progression of cancer metastasis56,57.
TEXs also reprogram other types of tumor-infiltrated immune cells like tumor-associated macrophages. For example, colon cancer cells with specific mutp53 proteins secrete exosomes containing miR-1246 that reprogram neighboring macrophages, increasing their TGF-beta activity and hence their anti-inflammatory immunosuppressive activities. These macrophages become more motile and invasive, undergo epithelial–mesenchymal transition (EMT), degrade the extracellular matrix, have diminished phagocytotic abilities, and recruit immunosuppressive regulatory T-cells (Tregs). Their matrix metallopeptidase 9 (MMP-9) and vascular non-inflammatory molecule 1 (VNN-1) secretion also increases61. Mechanistically, exosomal miR-1246 originates from RNU2-1 degradation and its generation is dependent on neither Drosha nor Dicer62.
TEXs also suppress activity of tumor-infiltrated cytotoxic T lymphocytes. For example, the HSP70 on exosomes from renal cancer cells interact with TLR2 on the membrane of myeloid-derived suppressor cells (MDSCs). This interaction causes activation of downstream factors including MyD88, TRAF6, P38, and AP1l; hence, factors like arginase, reactive oxygen species (ROS), and nitric oxide (NO) that suppress T-cell activity and promote antigen-specific tumor immune escape are upregulated. This interaction also allows the exosomes to be uptaken by these MDSCs63.
3.2.3. TEXs suppress NK cell function
It was recently found that TEXs play a role in suppressing NK cells in the tumor microenvironment64. NK cells are believed to be important innate immune cells in controlling cancer metastasis65,66. NK cells can exert robust anti-metastatic functions via different pathways including the secretion of IFN-γ, granules, and the exposure of death inducing ligands such as Fas ligand (FasL) and TNF-related apoptosis inducing ligand (TRAIL)65. Upon co-culturing, NK cells have been shown to be able to kill cancer cell lines of different histological origins67. Improved NK cell cytotoxicity has been linked with good prognosis in patients bearing primary prostate carcinomas68. However, research found that NK cells have only a minimal inhibitory effect on established micro metastasis and these NK cells in the tumor microenvironment have been functionally suppressed64,66,69. Study further showed that TEXs derived from a highly-metastatic pancreatic cancer cell line inhibited the anti-tumor cytotoxicity of NK cells by downregulating their expression of NK group 2D (NKG2D) (an NK-cell activating receptor) and secretion of cytokines including TNF-α and IFN-γ64. Studies from other groups further confirmed that TEXs can deliver TGF-β to the surface of NK cells, and the engagement between TGF-β and its receptor on NK cells decreases NKG2D expression and suppresses NK cell cytotoxicity70,71, which has been found to contribute to the progression of cancer metastasis in various types of tumors65,69,72.
Taken together, these studies found that TEXs play a significant role in contributing to immunosuppression including enhancing exosomal immunosuppressive protein expression, suppressing antitumor immunity, polarizing tumor-associated immune cells into pro-tumor phenotype, and activating immunosuppressive population at primary tumor sites and remote metastatic organs (Fig. 4). So, disseminated tumor cells will be less likely to be caught by the immune system and hence will be able to settle, thrive, and proliferate in the metastatic sites. Recent studies even found that TEXs released from dying tumor cells following chemotherapy could promote Ly6C+CCR2+ monocyte expansion in pre-metastatic niche, thereby to facilitate cancer metastasis73.
Figure 4.
TEXs have various immunosuppressive functions. Cancer cells release tumor-derived exosomes (TEXs). TEXs suppress the activities of T-cells and natural killer (NK) cells. Exosomal PD-L1 (programmed death-ligand 1) suppresses T-cell activity by binding to PD-1 (programmed cell death protein 1) on T-cells. TEXs activate myeloid-derived suppressor cells (MDSCs), regulatory T-cells, and neutrophils in a pro-tumor manner. TEXs invade tumor-draining lymph nodes and disrupt SCS cells, which form the protective outer layer of the lymph nodes; then access B-cell zone to activate B-cells in a tumor-supportive manner. Next, TEXs leak into the blood stream and eventually develop the metastatic niche. In the metastatic sites, TEXs are uptaken by tissue resident epithelial cells and macrophages, which in turn release inflammatory cytokines. These cytokines cause MDSCs to migrate to the metastatic niche and hence make it more tumor-friendly.
3.3. TEXs promote pre-metastatic niche formation
3.3.1. Exosomes promote metastatic niches by recruiting and educating immune cells
Pre-metastatic niche formation was first proposed and investigated by a study led by Dr. Kaplan74. In their pioneer study, they found that bone marrow-derived dendritic cells (BMDCs) that express vascular endothelial growth factor receptor 1 (VEGFR1) home to pre-metastatic sites before the arrival of tumor cells. These VEGFR1+ cells express VLA-4 (also known as integrin α4β1); meanwhile, tumor-specific growth factors increase the expression of fibronectin (a VLA-4 ligand) in resident fibroblasts74. The interaction between fibronectin and VLA-4 enables BMDCs to home toward the pre-metastatic sites where these BMDCs produce proteinases like matrix metalloproteinase 9 (MMP9) that release Kit-ligand and VEGF-A74. Thereby, a permissive environment (pre-metastatic niche) for disseminated cancer cells to live and grow is prepared.
Later, another study further found that metastatic carcinomas like Lewis lung carcinoma (LLC) produce factors that induce bone marrow-derived dendritic cell (BMDC) production of pro-metastasis TNF-α through the activation of TLR2:TLR6 complexes53. TNF-α increases recruitment of leukocytes (in part because TNF-α increases vascular permeability); these leukocytes are later educated by the cancer cells and become pro-tumor. Hence, pre-metastatic niches become more tumor friendly39. But, the tumor-derived factors that caused the BMDCs to become activated and home to pre-metastatic niches remained unidentified in those studies53,74.
The tumor-derived factors were later identified as TEXs by different groups9,75. For instance, Liu et al.9 found that TEXs activate alveolar epithelial cells in a TLR3-dependent pathway and trigger myeloid cell attractive chemokine secretion, which leads to myeloid cell recruitment and pre-metastatic niche formation (Fig. 2d‒f). In addition to epithelial cell activation, researchers also found that TEXs can trigger NF-κB pathway activation in a TLR2-or MyD88-dependent pathway in tissue-resident macrophages, which results in the secretion of pro-inflammatory cytokines such as TNF-α, IL-6, G-CSF, and CCL276. Also, pancreatic cancer-derived exosomes, which have migration inhibitory factor (MIF), are phagocyted by Kupffer cells in the liver and release MIF into these cells76. So, these cells secrete more transforming growth factor-β (TGF-β) and produce more fibronectin, a contributor to the recruitment of bone marrow-derived macrophages, which contribute to the metastasis-supportive nature of hepatic pre-metastatic niches76. Furthermore, in melanoma, TEXs have MET (a hepatocyte growth factor receptor), which makes bone-marrow derived cells (BMDCs) pro-vasculogenic and pro-migratory. Then, these BMDCs exit the bone marrow and migrate to the pre-metastatic niches, where they promote vascular leakiness and secrete pro-inflammatory cytokines and chemokines23.
3.3.2. Exosomes determine organotropism (i.e., organ-specific metastasis)
It is a consistent clinical observation that patients with certain cancers tend to develop organ-specific metastasis. But what factors determine the metastasis organotropism has been a scientific mystery for a long time. Back in 1889, an English surgeon named Stephen Paget was asking this question77, “What is it that decides what organs shall suffer in a case of disseminated cancer?” Recently, Dr. Lyden's group found that TEXs prepare metastatic niches in specific organs and that exosomal surface integrins determine the organ-specific metastasis78. Remarkably, they found that exosomes derived from different cancer models interact preferentially with resident cells at the same future metastatic organs as their cell of origin, thus prepare the pre-metastatic niche78. Also, before being uptaken by the target cells, these exosomes first increase vascular leakiness at the pre-metastatic niche. For instance, in mice treated with 4175-LuT-derived exosomes (LuT, Lung-tropic), these exosomes increased the capability of 4175-LuT tumors to metastasize to the lungs and redirected the bone-tropic tumor cells to develop metastasis in the lung78. Treatment with liver-tropic exosomes derived from the pancreatic cancer cell line BxPC-3 increased their uptake in the liver and hence promoted liver metastases78.
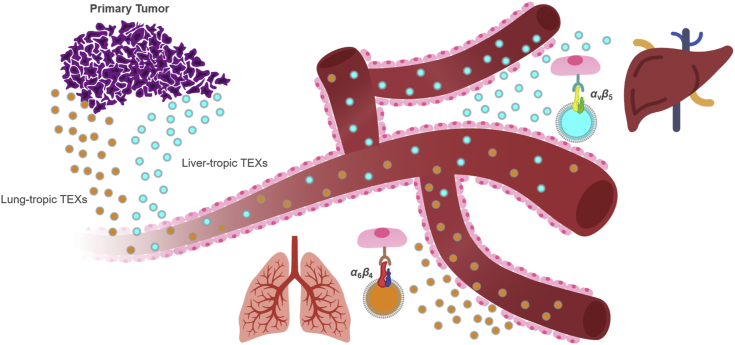
There is more evidence to support that exosomal surface integrins (ITGs) dictate metastatic organotropism by binding to specific ECM-rich areas in the target regions. For instance, ITGβ4 is expressed in especially high amounts in pre-metastatic breast cancer patients who later developed lung metastases. ITGβ4 (on the exosome's surface) binds to laminin in the laminin-rich lung microenvironment. Hence, the exosomes are preferentially uptaken by the lung, and lung metastases are promoted. Exosomal ITGαv (at time of diagnosis) is expressed in especially high amounts in pre-metastatic pancreatic cancer patients who later developed liver metastasis within 3 years of diagnosis. These exosomes may selectively adhere to fibronectin in the fibronectin-rich microenvironment in the liver. Exosomal ITGα6β4 and ITGα6β1 bind to epithelial cells and fibroblasts that belong to the lung78. Hence, they direct or redirect metastases to the lung. S100A4 helps cancer spread to the lungs79 and is regulated by ITGα6β480; so, in lung fibroblasts and when pre-metastatic niches are made, ITGα6β4 likely activates the Src-S100A4 axis78. These data suggest that TEXs express unique surface integrins that determine which organ recognizes the tumor-exosomes; therefore, pre-metastatic niches are preferentially prepared in that organ (Fig. 5).
Figure 5.
TEX surface integrins determine organotropic metastasis. Exosomes derived from different type of cancer cells can display different integrin proteins on their surfaces and those exosomal integrins determine which tissue endothelial cells can preferentially recognize them and develop pre-metastatic niche in that specific organ. For instance, ITGα6β4-expressing TEXs preferentially interact with epithelial cells in the lungs, and ITGαvβ5-expressing TEXs preferentially being recognized by Kupffer cells in the liver. This finding revealed how certain cancer tends to develop organ-specific metastasis.
3.3.3. Exosomes promote metastatic niches by increasing vascular permeability
For metastasis to occur, one of the key steps is to cross the endothelial barrier in the potential metastatic organs. TEXs help cancer cells cross the barrier by increasing vascular permeability in the metastatic organs. For example, metastatic breast cancer cells secrete miR-105-containing TEXs to pre-metastatic niche cells and hence increase vascular permeability there. Due to the TEXs, miR-105 levels in the pre-metastatic niche cells (which are endothelial cells) increased dramatically, and the expression of TJP1 (tight junction protein 1; also known as ZO-1) was down-regulated8. Consequently, these endothelial cells became better at migrating; the vascular integrity of endothelial layers became severely compromised8. Destruction of vascular integrity (mediated by miRNAs like miR-105) increases the penetration of pro-tumor exosome cargos (the particles that the exosomes contain), which leak into the pre-metastatic niches; hence, the cellular physiology of these organs becomes more metastasis-promoting and tumor-supportive8.
In breast cancer, TEXs can breach the blood–brain barrier (BBB) via transcytosis in endothelial cells. Although transcytosis is normally uncommon, they decrease Rab7 expression, hence facilitating their transport. The clathrin-dependent pathway and micropinocytosis, but not the caveolin-dependent pathway, were involved in the transcytosis18. Later on, the same group found out that, in breast cancer, after undergoing transcytosis in endothelial cells, these exosomes can enter astrocytes via the “Cdc42-dependent clathrin-independent carrier/GPI-AP-enriched compartment (CLIC/GEEC) endocytic pathway”19. The miR-301a in these exosomes downregulates tissue inhibitor of metalloproteinase-2 (TIMP-2) and upregulates ECM-remodelling proteins in astrocytes, contributing to astrocyte migration and the metastatic niche in the BBB19. Note that TEX uptake mechanisms are different in endothelial cells versus in astrocytes, suggesting that different cells have different exosome uptake mechanisms19.
Other studies support the role of TEXs in brain metastases. For example, Tominaga et al.81 discovered that, in breast cancer, TEXs trigger BBB destruction. The exosomal miR-181c downregulates PDPK1 and hence decreases phosphorylated cofilin, causing increased actin filament disassembly and disorganization and leading to BBB destruction. Exosomes derived from brain-metastatic breast cancer cells are uptaken by brain microvascular endothelial cells (BMECs) and have lncRNA GS1-600G8.5, which downregulates tight-junction proteins like ZO-1, N-cadherin, and claudin-5, and hence increases BBB permeability82. In breast and lung cancer, exosomal CEMIP (after uptake by microglia and brain endothelial cells) increases expression of the PTGS2, TNF, and CCL/CXCL cytokines, leading to increased branching of endothelial cells and inflammation in the brain's vascular niche83.
3.3.4. Exosomes promote metastatic niches by increasing angiogenesis
In ovarian cancer patients, exosomal soluble E-cadherin promotes tumor angiogenesis by binding to VE-cadherin on endothelial cells (Fig. 2g‒h); this event activates NF-κB and β-catenin signaling, which contributes to angiogenesis84.
In cancers such as renal cell carcinoma, TEXs overexpress carbonic anhydrase 9 (CA9)85. Its production is triggered by hypoxia-inducible factor 1 (HIF1), which is produced in response to hypoxic conditions85. Due to interacting with bicarbonate receptors in lamellipodia of tumor cells86, CA9 induces tube formation and migration and increases MMP2 production; hence, angiogenesis is promoted85. The formation of new blood vessels in the pre-metastatic niche helps tumor-derived secreted factors and pro-tumor extracellular vesicles to reach it. Hence, the microenvironment there becomes more tumor-friendly78.
Together, these studies have demonstrated that cancer cells do not randomly form metastases. They are more likely to make metastases when pre-metastatic niches are formed, where the microenvironments are tumor-friendly. TEXs promote pre-metastasis niche formation by increasing vascular permeability (in the pre-metastatic niche) and recruiting immune cells (e.g., bone marrow-derived cells, neutrophils).
4. Therapeutic implications via targeting TEXs
Based on these uncovered functional roles of TEXs in cancer metastasis, various strategies have been developed to target TEXs and to block exosome-mediated metastasis. In this section, we will mainly review the pilot studies in targeting TEXs to improve therapeutic efficacy (as summarized in Table 3). Previous reviews have summarized how to harness TEXs as therapeutic delivery cargos or as a liquid biopsy106, 107, 108.
Table 3.
Therapeutic implications via targeting TEXs.
| Mechanism | Case | Ref. |
|---|---|---|
| Inhibition of TEXs release | Targeting Rab27a | 23,87,88 |
| Targeting Munc13-4 | 89 | |
| Targeting nSMase2 | 90,91 | |
| Removal of circulating TEXs | Aethlon Hemopurifier® | 92 |
| Anti-CD9/anti-CD63 antibodies | 77,93 | |
| Blocking interaction of TEXs with recipient cells | Targeting fibronectin/heparan interaction | 11,94 |
| Targeting HSP70/TLR2 interaction | 95 | |
| Targeting exosome endocytosis | 17,96 | |
| Harnessing TEXs as therapeutic carriers to treat cancer and its metastasis | TEXs delivery with nucleic acids (e.g., siRNA against RAD51 and RAD52) | 97 |
| TEXs delivery with chemotherapeutics (e.g., doxorubicin, MTX, cisplatin and paclitaxel) | 98, 99, 100, 101 | |
| TEXs delivery with nanoparticles (e.g., iron oxide nanoparticles) | 102 | |
| TEXs to boost immune response | 103, 104, 105 |
4.1. Inhibition of TEXs release
Given the fact that tumor cells send out exosomes to help them survive and spread, the first idea is to inhibit the release of TEXs. Since Rab family proteins are well-described modulators of exosomes biogenesis109, Roma-Rodrigues and colleagues developed gold nanoparticles functionalized with thiolated oligonucleotides anti-Rab27a for selective silencing of Rab27a to decrease exosomes release in two breast cancer cell lines MCF-7 and MDA-MD-453, respectively87. Their results from in vitro studies showed an 80% decrease in exosome release after successful Rab27a gene silencing. However, this study did not provide the in vivo antitumor efficacy assessment. Using mouse models, Bobrie et al.88 reported that the Rab27a blockade in mammary carcinoma cells resulted in a decreased secretion of TEXs. Their results further indicate that the blockade of Rab27a in metastatic 4T1 cells significantly delayed tumor growth and lung metastases. In another study, Rab27a knockdown significantly decreased exosome secretion in melanoma cells and led to a decrease in tumor growth and lung metastasis23. In addition to increasing Rab protein-mediated exosome release, the protein Munc13-4 plays an essential role in promoting Ca2+-stimulated exosome release in highly aggressive breast carcinoma MDA-MB-231 cells, suggesting that Munc13-4 could be another molecular target for intervention in exosome-mediated metastasis89. It has also been reported that targeting neutral sphingomyelinase 2 (nSMase2) can inhibit cancer cell exosome secretion effectively90,91. Using xenograft breast cancer models, Kosaka et al.90 demonstrated that nSMase2 knockdown in cancer cells resulted in a significant decrease in lung metastasis, though not primary tumor growth, when compared with the parental cell line. nSMase2 knockdown inhibits exosomal miR-210 transfer to epithelial cells and diminishes the exosome-mediated angiogenic activity of endothelial cells in inoculated tumors.
4.2. Removal of circulating TEXs
Given the challenge of inhibiting TEX biogenesis, removal or disruption of circulating exosomes may serve as an alternative therapeutic strategy to inhibit cancer metastasis. It has been proposed that a device called Aethlon Hemopurifier® can capture and remove TEXs from the plasma of breast cancer patients92. The device comprises a hollow-fiber plasma separator cartridge containing a lectin affinity matrix or other affinity reagents, such as aptamers and proteins ligands, and can be integrated into standard dialysis machines. The idea is when the patient's blood goes through the device, the immobilized affinity reagents capture the targets such as TEXs. One of the biggest challenges associated with this technology lies in how to capture only TEXs while leaving normal cell-derived exosomes intact as there is currently no specific way to differentiate them. In addition, the entire blood dialysis strategy is complicated and leaves many uncertainties.
Alternatively, Nishida-Aoki et al.93 showed that the circulating exosomes in human breast cancer xenograft models could be depleted through administering human-specific anti-CD9 or anti-CD63 antibodies. They found this antibody treatment led to a significant decrease in lung and lymph node metastases though it did not affect inoculated tumor growth. Their study further showed that the antibody-tagged exosomes could be preferentially recognized and cleared by macrophages. Despite the promising effect, some questions remained. For instance, would antibody binding change the interaction of exosomes with host immune cells and epithelial cells? Why does antibody binding cause recognition of macrophages but not of other myeloid suppressive cells? The detailed molecular and cellular mechanisms that are responsible for the observed metastasis inhibition may be worthy of further investigation.
4.3. Blocking TEXs interaction with recipient cells
Studies revealed that TEXs may interact with recipient cells through specific binding followed by receptor-mediated endocytosis11,110, which provides a rationale to block exosome-mediated communication. It has been found that fibronectin on the surface of myeloma cell-derived exosomes acts as a ligand that can effectively bind to its receptor heparan sulfates on recipient cells11,94. Indeed, exosome uptake was specifically inhibited in the presence of free heparin sulfate, and exosome-mediated stimulation of glioblastoma cell migration was significantly reduced when the cells were treated with heparin94. TEXs can bind to Toll-like receptors on myeloid cells to induce myeloid cell activation and polarization. Using A8 peptide aptamer that can bind to heat shock protein 70 (HSP70), Gobbo et al.95 demonstrated that targeting TEXs can result in a decreased number of MDSCs in the spleen in B16F10 tumor-bearing C57BL/6 mice and a suppressed tumor progression. Other molecules, such as methyl-β-cyclodextrin (MβCD)17 and dynasore96, have also been reported to inhibit TEXs uptake by receptor cells.
4.4. Harnessing TEXs as therapeutic carriers to treat cancer and its metastasis
TEXs, as the nature reservoir of proteins or RNAs (siRNA, miRNA), are excellent carriers to solve suboptimal pharmaceutical properties of anti-cancer biomolecules such as instability, off-target toxicity, inefficient cell-uptake and so on111, 112, 113. In order to protect the degradation of siRNAs against RAD51 and RAD52, exosomes from Hela and ascites have been used as the carriers and cause better post-transcriptional gene silencing in recipient cells97. siRNA/shRNA which are specifically targeted to oncogenic KrasG12D have also demonstrated to have a longer circulation time and stronger inhibition against pancreatic cancer when delivered in exosomes114.
Another advantage to utilize TEXs as therapeutic carriers is their tumor targeting efficiency either by its intrinsic cell/organ tropism or extrinsic surface modification115. TEXs as summarized in this review have the ability to promote metastatic niches formation. Therapeutic cargos can be delivered easily to those niches by TEXs. Tang et al.98 isolated TEXs from HT1080 or Hela and found these exosomes not only fuse preferential with parent cancer cells in vitro but also show increased anti-cancer drugs (doxorubicin) in tumor lesion101. Another study also finds that exosomes from H22 cells can efficiently deliver chemotherapeutic drug molecules into tumor cells. It is known that pre-metastatic niche (PMN) in secondary organs create conductive environment for tumor metastasis. Zhao et al.116 developed biomimetic nanoparticles with exosome membranes and found higher lung PMN delivery. By incorporating siS100A4, these nanoparticles exhibited outstanding gene-silencing effects and suppressed postoperative breast cancer metastasis. Other strategies like active targeting of TEXs are also feasible due to easy protein/peptide expression, chemical conjugation or hydrophobic insertion on the surface. Researchers obtained large amounts of exosomes carrying iRGD by overexpressing iRGD-Lamp2b fused protein in cells. These exosomes are shown to specifically deliver doxorubicin to breast cancer cells through recognizing the surface αV integrin117. The ligand-based delivery system is capable of specific carry of siRNA to cells and block tumor growth in prostate, breast and colorectal cancers118.
It is also appealing to use TEXs as vaccines for immunotherapy, since large amount of tumor exosomes can be found in malignant effusions. These TEXs are able to carry and transfer tumor specific antigens to DCs, which is shown to induce potent cytotoxic antitumor effects. TS/A, MC38 and P815 cancer derived exosomes were studied to be loaded with BMDCs and injected back to established tumor models, promoting significantly tumor growth delay and up to 40% cure rate104. Morishita et al.103 modified the surface of TEXs with streptavidin and mixed with biotinylated CpG DNA to form antigens-adjuvant codelivery. They find stronger antitumor effects in B16BL6 mice after immunization with combined TEXs vaccines. Another study demonstrates that exosomes derived from HepG2 cells which are treated with resistant anticancer drug can induce superior HSP-specific NK cell responses105. A completed phase I clinical trial for recurrent malignant gliomas took patients’ own tumor cells and treated with antisense drug to induce exosomes generation when re-implanted into patients. These exosomes are supposed to activate the immune system against tumor as they slowly diffused out from small chambers. However, optimism of applying TEXs in immunotherapy should be conservative or at least the positive results should be treated case by case. There are other studies discover the immunosuppressive function of TEXs including impairment of monocyte differentiation and induction of a pro-inflammatory microenvironment. Under which condition that TEXs can be used to amplify the immune response should be treated cautiously.
Artificial nanoparticles used in medicine are largely known for its ability to deliver small molecule anti-cancer drugs, like doxorubicin (Doxil®) and paclitaxel (Abraxene®). Similarly, as a natural nanoparticle, TEXs have also been used as carriers for these small molecule drugs. Tang et al.98 incubate tumor cells (H22 or A2780 cells) with chemotherapeutics like doxorubicin, MTX, cisplatin and then irradiated with UBV to release TEXs. These exosomes are found to be more effective through efficiently delivering to tumors, inducing the formation of additional drug-packaging TEXs and enhancing tumor cell susceptibility to chemotherapy. Another study by the same group demonstrates that TEXs containing anti-tumor drugs are able to reverse drug resistance of tumor-repopulating cells through interfering with drug efflux and increasing nuclear uptake99. In a study to compare the difference of microvesicle- and exosome-mediated paclitaxel delivery, researchers found both TEXs with chemo-drugs are also uptook by the recipient cells through endocytosis100. Several clinical trials are ongoing to test the safety and effectiveness of TEXs loaded with chemotherapeutic drugs against malignant pleural effusion [NCT01854866 and NCT02657460].
5. Conclusions
Growing evidence has revealed that tumor-derived exosomes (TEXs), as a powerful nanoparticle secreted by cancer cells, play various roles in promoting cancer metastasis. First, TEXs can induce endothelial cells to down-regulate tight junction protein expression and hence cause leaky tumor vasculature, which contributes to cancer cells' local invasion and intravasation into the lymphatic system and the blood circulation. Various exosomal miRNAs contribute to cancer cell invasion and metastasis. Second, TEXs can suppress antitumor immunity to help tumor cells survive in circulation and metastatic sites. Third, TEXs can prepare tumor-friendly environments in distant organs to facilitate tumor cell extravasation into the parenchymal tissues (metastatic niche). Knowing these pro-metastatic roles of TEXs, scientists developed various strategies to target them, which include inhibiting the secretion of TEXs, removing circulating TEXs, and blocking TEXs' interactions with recipient cells. It is worth noting that we are still at the infant stages of targeting TEXs to interfere with their pro-tumor purposes. However, we believe that this is the right direction to pursue in order to find effective ways to cure cancer metastasis.
Future perspectives
A variety of molecular components of TEXs have been reported to be involved in cancer metastasis. However, many issues regarding the role of TEXs in cancer metastasis, immune regulation, and treatment resistance remain unanswered. Some important questions that need to be addressed include: (1) What is the key factor that regulates the interaction between TEXs and host immune cells and epithelial cells? (2) How exosomal miRNAs modulate the recipient cells? (3) How does the host immune system differentiate TEXs from normal cell-derived exosomes? (4) How to block TEXs communications with host immune cells to restore patient antitumor immunity?
Author contributions
Hongwei Chen conceived the idea and wrote the manuscript. Venkata Chengalvala wrote the manuscript. Hongxiang Hu re-graphed the Figures, wrote part of the manuscript and partially revised the manuscript. Duxin Sun reviewed the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Hongwei Chen, Email: hongweic@umich.edu.
Duxin Sun, Email: duxins@umich.edu.
References
- 1.Pantel K., Brakenhoff R.H. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 2.Goetz J.G. Metastases go with the flow. Science. 2018;362:999–1000. doi: 10.1126/science.aat9100. [DOI] [PubMed] [Google Scholar]
- 3.Cheung K.J., Ewald A.J. A collective route to metastasis: seeding by tumor cell clusters. Science. 2016;352:167–169. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turajlic S., Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- 5.Rankin E.B., Giaccia A.J. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haderk F., Schulz R., Iskar M., Cid L.L., Worst T., Willmund K.V. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aah5509. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Shi H., Yuan X., Jiang P.C., Qian H., Xu W.R. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. 2018;17:146. doi: 10.1186/s12943-018-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W.Y., Fong M.Y., Min Y.F., Somlo G., Liu L., Palomares M.R. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y.F., Gu Y., Han Y.M., Zhang Q., Jiang Z.P., Zhang X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto A., Takahashi Y., Nishikawa M., Sano K., Morishita M., Charoenviriyakul C. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci. 2017;108:1803–1810. doi: 10.1111/cas.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purushothaman A., Bandari S.K., Liu J., Mobley J.A., Brown E.E., Sanderson R.D. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem. 2016;291:1652–1663. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poggio M., Hu T.Y., Pai C.C., Chu B., Belair C.D., Chang A. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414–427. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49:347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Horibe S., Tanahashi T., Kawauchi S., Murakami Y., Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18:47. doi: 10.1186/s12885-017-3958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svensson K.J., Christianson H.C., Wittrup A., Bourseau-Guilmain E., Lindqvist E., Svensson L.M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 2013;288:17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morad G., Carman C.V., Hagedorn E.J., Perlin J.R., Zon L.I., Mustafaoglu N. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13:13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morad G., Daisy C.C., Otu H.H., Libermann T.A., Dillon S.T., Moses M.A. Cdc42-dependent transfer of mir301 from breast cancer-derived extracellular vesicles regulates the matrix modulating ability of astrocytes at the blood-brain barrier. Int J Mol Sci. 2020;21:3851. doi: 10.3390/ijms21113851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiteside T.L. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi Y., Nishikawa M., Shinotsuka H., Matsui Y., Ohara S., Imai T. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Charoenviriyakul C., Takahashi Y., Morishita M., Matsumoto A., Nishikawa M., Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur J Pharmaceut Sci. 2017;96:316–322. doi: 10.1016/j.ejps.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth T., Kullberg M., Malik N., Smith-Jones P., Graner M.W., Anchordoquy T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pucci F., Garris C., Lai C.P., Newton A., Pfirschke C., Engblom C. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352:242–246. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunderson S.C., Dunn A.C., Crocker P.R., McLellan A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng Z.C., Li Y.L., Pan Y.J., Lan X.L., Song F.Y., Sun J.B. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J., Wang P., Liu Y., Zhao L., Li Z., Xue Y. Kruppel-like factor 4 regulates blood-tumor barrier permeability via ZO-1, occludin and claudin-5. J Cell Physiol. 2014;229:916–926. doi: 10.1002/jcp.24523. [DOI] [PubMed] [Google Scholar]
- 29.Fang J.H., Zhang Z.J., Shang L.R., Luo Y.W., Lin Y.F., Yuan Y.F. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68:1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 30.Fong M.Y., Zhou W.Y., Liu L., Alontaga A.Y., Chandra M., Ashby J. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Zhan S.Y., Yao J., Lowery F.J., Zhang Q.L., Huang W.C. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Modica M., Regondi V., Sandri M., Iorio M.V., Zanetti A., Tagliabue E. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017;384:94–100. doi: 10.1016/j.canlet.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Luo F., Wang B.R., Li H.Q., Xu Y., Liu X.L. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370:125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Hsu Y.L., Hung J.Y., Chang W.A., Lin Y.S., Pan Y.C., Tsai P.H. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 35.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying X., Wu Q.F., Wu X.L., Zhu Q.Y., Wang X.J., Jiang L. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–43087. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Tang H.D., Liang Y., Anders R.A., Taube J.M., Qiu X.Y., Mulgaonkar A. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 2018;128:580–588. doi: 10.1172/JCI96061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin H., Wei S., Hurt E.M., Green M.D., Zhao L., Vatan L. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128:805–815. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinovink J.W., Marijt K.A., Schoonderwoerd M.J.A., van Hall T., Ossendorp F., Fransen M.F. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1294299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann T.K., Dworacki G., Tsukihiro T., Meidenbauer N., Gooding W., Johnson J.T. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 42.Kim J.W., Wieckowski E., Taylor D.D., Reichert T.E., Watkins S., Whiteside T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 43.Wieckowski E.U., Visus C., Szajnik M., Szczepanski M.J., Storkus W.J., Whiteside T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C.J., Kim S.H., Bianco N.R., Robbins P.D. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiteside T.L. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41:245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maybruck B.T., Pfannenstiel L.W., Diaz-Montero M., Gastman B.R. Tumor-derived exosomes induce CD8+ T cell suppressors. J Immunother Cancer. 2017;5:65. doi: 10.1186/s40425-017-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valenti R., Huber V., Iero M., Filipazzi P., Parmiani G., Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 48.Yu S.H., Liu C.R., Wang J.H., Liu Y.L., Zhang L.M., Cong Y.Z. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 49.Xiang X.Y., Poliakov A., Liu C., Liu Y.L., Deng Z.B., Wang J.H. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y.L., Xiang X.Y., Zhuang X.Y., Zhang S.Y., Liu C.R., Cheng Z.Q. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176:2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mrizak D., Martin N., Barjon C., Mustapha R., Niki T., Pancre V. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. Oral Oncol. 2015;51 doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 52.Ham S., Lima L.G., Chai E.P.Z., Muller A., Lobb R.J., Krumeich S. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front Immunol. 2018;9:871. doi: 10.3389/fimmu.2018.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S., Takahashi H., Lin W.W., Descargues P., Grivennikov S., Kim Y. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 55.Piao Y.J., Kim H.S., Hwang E.H., Woo J., Zhang M., Moon W.K. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. 2018;9:7398–7410. doi: 10.18632/oncotarget.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wculek S.K., Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S. IL-17-producing gamma delta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei B.J., Yao M.Y., Xing C.Y., Wang W., Yao J., Hong Y. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. OncoTargets Ther. 2016;9:5567–5575. doi: 10.2147/OTT.S108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takuwa H., Tsuji W., Yamamoto Y., Shintaku M., Yotsumoto F. Low neutrophil-lymphocyte ratio correlates with extended survival in patients with metastatic breast cancer who achieved clinically complete response following multidisciplinary therapy: a retrospective study. Oncol Lett. 2018;15:6681–6687. doi: 10.3892/ol.2018.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang W.L., Lan H.Y., Cheng W.C., Huang S.C., Yang M.H. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. 2019;12:10. doi: 10.1186/s13045-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooks T., Pateras I.S., Jenkins L.M., Patel K.M., Robles A.I., Morris J. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y.F., Hannafon B.N., Khatri U., Gin A., Ding W.Q. The origin of exosomal miR-1246 in human cancer cells. RNA Biol. 2019;16:770–784. doi: 10.1080/15476286.2019.1585738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Y., Xu H., Li N., Wang H., Ma L., Chen S. Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression. Cell Commun Signal. 2020;18:106. doi: 10.1186/s12964-020-00611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao J.G., Schlosser H.A., Wang Z.F., Qin J., Li J.H., Popp F. Tumor-derived extracellular vesicles inhibit natural killer cell function in pancreatic cancer. Cancers. 2019;11:874. doi: 10.3390/cancers11060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez-Soto A., Gonzalez S., Smyth M.J., Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Salup R.R., Herberman R.B. Role of natural-killer cells in control of metastases and for therapy of murine cancer. Eos (Washington, DC) 1988;8:151–158. [Google Scholar]
- 67.Ames E., Canter R.J., Grossenbacher S.K., Mac S., Chen M.Y., Smith R.C. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J Immunol. 2015;195:4010–4019. doi: 10.4049/jimmunol.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pasero C., Gravis G., Guerin M., Granjeaud S., Thomassin-Piana J., Rocchi P. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016;76:2153–2165. doi: 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 69.Spiegel A., Brooks M.W., Houshyar S., Reinhardt F., Ardolino M., Fessler E. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong C.-S., Sharma P., Yerneni S.S., Simms P., Jackson E.K., Whiteside T.L. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep. 2017;7:14684. doi: 10.1038/s41598-017-14661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szczepanski M.J., Szajnik M., Welsh A., Whiteside T.L., Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta 1. Haematologica. 2011;96:1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chockley P., Keshamouni V. Metastasis-specific, NK cell-mediated, immune surveillance of lung cancer. J Immunol. 2018;200:1384–1396. doi: 10.1172/JCI97611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun H.X., Hong M., Yang Q.Q., Li C., Zhang G.Z., Yue Q.L. Visualizing the down-regulation of hTERT mRNA expression using gold-nanoflare probes and verifying the correlation with cancer cell apoptosis. Analyst. 2019;144:2994–3004. doi: 10.1039/c9an00204a. [DOI] [PubMed] [Google Scholar]
- 74.Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow A., Zhou W., Liu L., Fong M.Y., Champer J., Van Haute D. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:3. [PubMed] [Google Scholar]
- 78.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Mark M.T. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grum-Schwensen B., Klingelhofer J., Grigorian M., Almholt K., Nielsen B.S., Lukanidin E. Lung metastasis fails in MMTV-PyMT oncomice lacking S100A4 due to a T-cell deficiency in primary tumors. Cancer Res. 2010;70:936–947. doi: 10.1158/0008-5472.CAN-09-3220. [DOI] [PubMed] [Google Scholar]
- 80.Chen M., Sinha M., Luxon B.A., Bresnick A.R., O'Connor K.L. Integrin alpha6beta4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem. 2009;284:1484–1494. doi: 10.1074/jbc.M803997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu Y., Chen L., Li L., Cao Y. Exosomes derived from brain metastatic breast cancer cells destroy the blood-brain barrier by carrying lncRNA GS1-600G8.5. BioMed Res Int. 2020;2020:7461727. doi: 10.1155/2020/7461727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodrigues G., Hoshino A., Kenific C.M., Matei I.R., Steiner L., Freitas D. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21:1403–1412. doi: 10.1038/s41556-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang M.K.S., Yue P.Y.K., Ip P.P., Huang R.L., Lai H.C., Cheung A.N.Y. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9:2270. doi: 10.1038/s41467-018-04695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horie K., Kawakami K., Fujita Y., Sugaya M., Kameyama K., Mizutani K. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun. 2017;492:356–361. doi: 10.1016/j.bbrc.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 86.Svastova E., Witarski W., Csaderova L., Kosik I., Skvarkova L., Hulikova A. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem. 2012;287:3392–3402. doi: 10.1074/jbc.M111.286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roma-Rodrigues C., Pereira F., de Matos A.P.A., Fernandes M., Baptista P.V., Fernandes A.R. Smuggling gold nanoparticles across cell types - a new role for exosomes in gene silencing. Nanomedicine (NY, NY, US) 2017;13:1389–1398. doi: 10.1016/j.nano.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 88.Bobrie A., Krumeich S., Reyal F., Recchi C., Moita L.F., Seabra M.C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 89.Messenger S.W., Woo S.S., Sun Z.Z., Martin T.F.J. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J Cell Biol. 2018;217:2877–2890. doi: 10.1083/jcb.201710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang Z., Yang M.L., Li Y.Z., Yang F., Feng Y. Exosomes derived from hypoxic colorectal cancer cells transfer Wnt4 to normoxic cells to elicit a prometastatic phenotype. Int J Biol Sci. 2018;14:2094–2102. doi: 10.7150/ijbs.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marleau A.M., Chen C.S., Joyce J.A., Tullis R.H. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishida-Aoki N., Tominaga N., Takeshita F., Sonoda H., Yoshioka Y., Ochiya T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol Ther. 2017;25:181–191. doi: 10.1016/j.ymthe.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J.P., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gobbo J., Marcion G., Cordonnier M., Dias A.M.M., Pernet N., Hammann A. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. JNCI, J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv330. [DOI] [PubMed] [Google Scholar]
- 96.Kawamoto T., Ohga N., Akiyama K., Hirata N., Kitahara S., Maishi N. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang K., Zhang Y., Zhang H., Xu P., Liu J., Ma J. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 99.Ma J., Zhang Y., Tang K., Zhang H., Yin X., Li Y. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713–727. doi: 10.1038/cr.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saari H., Lázaro-Ibáñez E., Viitala T., Vuorimaa-Laukkanen E., Siljander P., Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 101.Qiao L., Hu S., Huang K., Su T., Li Z., Vandergriff A. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10:3474–3487. doi: 10.7150/thno.39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y., Gao Y., Gong C., Wang Z., Xia Q., Gu F. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine (NY, NY, US) 2018;14:1973–1985. doi: 10.1016/j.nano.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 103.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 104.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 105.Lv L.H., Wan Y.L., Lin Y., Zhang W., Yang M., Li G.L. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnsen K.B., Gudbergsson J.M., Skov M.N., Pilgaard L., Moos T., Duroux M. A comprehensive overview of exosomes as drug delivery vehicles — endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta Rev Cancer. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. Acs Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luan X., Sansanaphongpricha K., Myers I., Chen H.W., Yuan H.B., Sun D.X. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 110.Gonda A., Kabagwira J., Senthil G.N., Wall N.R. Internalization of exosomes through receptor-mediated endocytosis. Mol Cancer Res. 2019;17:337–347. doi: 10.1158/1541-7786.MCR-18-0891. [DOI] [PubMed] [Google Scholar]
- 111.Wiklander O.P.B., Brennan M., Lötvall J., Breakefield X.O., El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sancho-Albero M., Medel-Martínez A., Martín-Duque P. Use of exosomes as vectors to carry advanced therapies. RSC Adv. 2020;10:23975–23987. doi: 10.1039/d0ra02414g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maia J., Caja S., Strano Moraes M.C., Couto N., Costa-Silva B. Exosome-based cell–cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao L., Gu C., Gan Y., Shao L., Chen H., Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15. doi: 10.1016/j.jconrel.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 117.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 118.Pi F., Binzel D.W., Lee T.J., Li Z., Sun M., Rychahou P. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol. 2018;13:82–89. doi: 10.1038/s41565-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]