Abstract
Objectives
To investigate the regulatory handling of cancer drugs that were granted accelerated approval by the US Food and Drug Administration (FDA) but failed to improve the primary endpoint in post-approval trials and to evaluate the extent to which negative post-approval trials changed the recommendations in treatment guidelines.
Design
Retrospective observational study.
Setting
FDA and National Comprehensive Cancer Network (NCCN) reports.
Included drugs
Cancer drugs that received accelerated approval from the FDA and had negative post-approval trials.
Main outcome measures
Regulatory outcomes, including withdrawal, conversion to regular approval, and no action.
Results
18 indications for 10 cancer drugs that received accelerated approval but failed to improve the primary endpoint in post-approval trials were identified. Of these, 11 (61%) were voluntarily withdrawn by the manufacturer and one (bevacizumab for breast cancer) was revoked by the FDA. Of the 11 withdrawals, six occurred in 2021 alone. The remaining six (33%) indications remain on the label. The NCCN guidelines provide a high level of endorsement (category 1 endorsement for one and category 2A endorsement for seven) for accelerated approval drugs that have failed post-approval trials, sometimes even after the approval has been withdrawn or revoked.
Conclusion
Cancer drug indications that received accelerated approval often remained on formal FDA approved drug labelling and continued to be recommended in clinical guidelines several years after statutorily required post-approval trials showed no improvement in the primary efficacy endpoint. Clinical guidelines should better align with the results of post-approval trials of cancer drugs that received accelerated approval.
Introduction
Patients with cancer need timely access to drugs that meaningfully improve how they feel, function, or survive.1 However, measuring clinical endpoints such as improvement in overall survival can take time, and patients may be willing to tolerate uncertainty about such benefits in exchange for early access to promising cancer drugs, particularly for cancers that lack other treatment options. The accelerated approval pathway of the US Food and Drug Administration (FDA) covers this situation. The FDA may grant approval to any drug—although it is most often used in cancer—that has shown improvements in surrogate measures in clinical testing that are only reasonably likely to predict actual clinical benefit (that is, improved survival or quality of life). Cancer related surrogate measures include changes in tumour size or time to progression of cancer. These surrogate measures may ultimately be shown to predict meaningful clinical benefit, but this is often not the case.2 3
The law mandates that drugs granted accelerated approval be tested in post-approval trials showing the putative clinical benefit expected from improvement in surrogate measures. The rationale for such a requirement is to ensure that all drugs granted accelerated approval eventually have their clinical benefit established for the patients relying on them.4 Central to the accelerated approval concept is the need for these post-approval trials to be conducted in a reasonable timeframe, that the trials use meaningful clinical endpoints, and that the approved indication be withdrawn if a drug fails to show clinical benefit that outweighs its risks. However, post-approval trials are often delayed, with half still underway three years after approval.5 Among the trials that have been completed, approximately 40% used surrogate measures, including the same surrogate measure that led to accelerated approval.6
No previous study has examined what regulatory steps are taken with respect to drugs that received accelerated approval when the post-approval trials are conducted and fail to confirm clinical benefit. The FDA considers withdrawal of drug approvals to be administratively and socially challenging,7 as was evident in the case of the revoking of accelerated approval for bevacizumab for metastatic breast cancer.8 We investigated the regulatory consequences for cancer drugs that were previously granted accelerated approval by the FDA but failed to show improvement in the primary efficacy endpoint in post-approval trials. We also investigated the extent to which negative post-approval trials affected recommendations in clinical practice guidelines.
Methods
We systematically searched the Drugs@FDA database for all cancer drugs granted accelerated approval from the inception of the programme until December 2020.9 We verified the status of statutorily required post-approval trials for these drugs for the given indications by searching the FDA Database of Postmarketing Requirements and Commitments.10 This FDA website allows the user to search the accelerated approval status for a given product, and it provides information on the post-approval requirement for each indication and categorises the status of requirement as “ongoing,” “pending,” “delayed,” “terminated,” “submitted,” “fulfilled,” or “released.” In this website, the confirmatory trial may also be described with its name (for example, IMVigor211), but ClinicalTrials.gov identifiers are usually not provided.
Building on our previous work,6 we also searched PubMed and Google Scholar for published post-approval trials by using the trial name and other identifiers described in the FDA database. As some trials with null results may not have been published but may have been announced by press release, we also searched Google News with various combinations of search terms including the drug name, trial name, FDA, accelerated approval, and post-approval trial to identify updates on the status of post-approval trials. We cross referenced the trial description (and trial name, when applicable) to information available from the FDA database to ensure that these were the confirmatory trials conducted to fulfil the FDA’s post-marketing requirement. We did these searches in February 2021 and updated them on 10 March 2021. We verified our findings by searching ClinicalTrials.gov on 10 May 2021. Confirmatory trials may be conducted not in the exact clinical setting in which the accelerated approval was granted but in a slightly different patient population if the FDA allows these trials to serve dual goals of confirmation of accelerated approval and expanded indication. BG did all the searches and data extractions, and BNR verified them.
Our study cohort included cancer drugs that had initially received accelerated approval but ultimately failed to establish benefit, either because the post-approval trials failed to show improvement in the primary endpoint or because the post-approval trials were not completed/conducted, leading to the withdrawal of the indication. Our objective was to assess the regulatory consequences for cancer drugs that failed to improve the stated primary endpoint in the confirmatory trials. Thus, to be maximally conservative, if the confirmatory trial showed improvement in a surrogate measure as the primary endpoint but no improvement in overall survival, we did not include it in our cohort.
For the cohort of cancer drugs that failed to improve the primary endpoint in the confirmatory trials, we extracted characteristics of pre-approval and confirmatory trials, including the primary endpoint that formed the basis of accelerated approval, the primary endpoint of the confirmatory trial, and the consequence after the negative confirmatory trial results emerged. Categories of consequences included remaining on the label, voluntary withdrawal by the manufacturer, regular FDA approval granted, and approval revoked by the FDA. Drugs that have received accelerated approval can be converted to regular approval with confirmation of clinical benefit. Without such confirmation, the industry can withdraw the approval voluntarily or the FDA can enforce withdrawal by taking the necessary administrative steps to revoke the accelerated approval. We determined these consequences by using the FDA database as well as the most recent FDA drug labelling. We also measured the time, in years, from the granting of accelerated approval to the earliest known date when negative trial results were announced (for example, by industry press release, conference presentation, or publication) and the time from this announcement until the regulatory consequence or until 31 May 2021 if no regulatory action had been taken by this time.
To assess the consequences of regulatory actions or negative confirmatory trial results on clinical practice, we searched the National Comprehensive Cancer Network (NCCN) guidelines to determine the recommendation for each accelerated approval indication on 12 May 2021. The NCCN guidelines are the most comprehensive and highly used clinical practice guidelines in oncology and serve as one of five compendia used by the Centers for Medicare and Medicaid Services to make decisions on coverage.11 NCCN recommendation categories include category 1 (high level of evidence, uniform NCCN consensus), category 2A (lower level of evidence, uniform NCCN consensus), category 2B (lower level of evidence, NCCN consensus), and category 3 (any level of evidence, NCCN disagreement). We also noted the date when the latest revision of the NCCN guidelines was published relative to the date of the availability of negative confirmatory evidence. The NCCN guidelines also indicate whether a certain indication’s recommendation category has been changed or removed compared with the previous version of the guidelines.
On 27-29 April 2021, the FDA held a meeting of the Oncology Drug Advisory Committee (ODAC) to discuss six of the accelerated approvals that had failed to improve the primary endpoint in the confirmatory trials and vote on what regulatory actions might be appropriate.12 The FDA is not bound by recommendations from its advisory committees but usually follows them. We also included the outcome of that ODAC meeting vote for each indication. Finally, to provide a comparison of the FDA’s approach to these drugs with those of international regulators, we searched for each drug in the European Medicines Agency (EMA) database and assessed whether each drug had received marketing authorisation by the EMA for the given indication.
Patient and public involvement
Patients or the public were not involved in the design, conduct, or reporting of this study, as this was a study conducted solely from publicly available records. However, this study was inspired by listening to patient advocates’ and public voices and confusions in several media about the accelerated approval process for cancer drugs. During our talks/seminars, we have spoken to several members of the patient community who suggested that we pursue this work for clarification of the evidence base for cancer drugs that receive accelerated approval from the US FDA.
Results
We identified 18 indications for 10 different cancer drugs that had initially received accelerated approval but ultimately either failed to establish benefit because the post-approval trials failed to improve the primary endpoint (n=16) or were withdrawn because these studies were not conducted/completed (n=2) (table 1). More than half (11; 61%) had received accelerated approval in the previous five years (2016-20), of which all but one were immunotherapies. The most common type of cancer was urothelial cancer (four) followed by small cell lung cancer, hepatocellular cancer, and breast cancer (two each). Most (15; 83%) of these indications had received accelerated approval on the basis of studies showing a change in tumour response rate, and the remaining accelerated approval indications were granted on the basis of progression-free survival (n=2) and overall survival from a phase II trial (n=1).
Table 1.
Characteristics of accelerated approval trials that have failed post-approval trials and had indication withdrawn/revoked
| Characteristics | Failed confirmatory trials (n=18) | Indication withdrawn or revoked* (n=10) |
|---|---|---|
| Primary endpoint for accelerated approval | Response rate (15); progression-free survival (2); overall survival in phase II trial (1) | Response rate (8); progression-free survival (1); overall survival in phase II trial (1) |
| Primary endpoint for post-approval trial | Overall survival, as primary or co-primary endpoint (16); trials not completed (2) | Overall survival, as primary or co-primary endpoint (8); trials not completed (2) |
| Tumour type | Urothelial (4); haematological (3); lung (3); breast (2); hepatocellular (2); glioblastoma (1); melanoma (1); sarcoma (1); gastrointestinal (1) | Haematological (3); lung (3); urothelial (2); breast (1); sarcoma (1) |
| Drug type | Checkpoint inhibitor (11); monoclonal antibody (4); targeted therapy (1); chemotherapy (1); antibody drug conjugate (1) | Checkpoint inhibitor (4); monoclonal antibody (3); targeted therapy (1); chemotherapy (1); antibody drug conjugate (1) |
10 trials that led to withdrawal or revoking of approvals are subset of overall 18 failed confirmatory trials.
For the 16 indications with completed post-approval trials, the primary efficacy endpoint was either overall survival alone or overall survival as a co-primary endpoint with progression-free survival. In each case, the post-approval trials showed that the drugs failed to improve overall survival. In four indications, the trials showed that the drugs improved progression-free survival but failed to improve the (co-)primary endpoint of overall survival. In seven indications, the trials showed that the drugs failed to improve both progression-free survival and overall survival. In two other cases (olaratumab in sarcoma and durvalumab in urothelial cancer), the effect of the drug on progression-free survival was detrimental. One trial (bevacizumab in glioblastoma) showed that the drug failed to improve both overall survival and quality of life, and one trial (gemtuzumab in acute myeloid leukaemia) showed that the drug failed to improve overall survival, disease-free survival, and response rates. In the case of nivolumab in small cell lung cancer, results from two different confirmatory trials were available. Nivolumab failed to improve overall survival in both the trials, but the effects of the drug on progression-free survival were beneficial in one trial and detrimental in the other. The other two indications were voluntarily withdrawn, as the post-approval trials were not completed: tositumomab to treat follicular lymphoma (for which a different trial showed no benefit for progression-free survival and overall survival)13 and fludarabine to treat chronic leucocytic leukaemia.
Regulatory outcomes after negative post-approval trial
The most common outcome for cancer drugs that received accelerated approval but failed to improve the primary endpoint in post-approval trials was voluntary withdrawal of the indication (n=9; 50%). In two cases, after voluntary withdrawals, the FDA subsequently granted a new regular approval for the same drug for the same disease at a different dose or for a biomarker defined population. Only in one case (bevacizumab for metastatic breast cancer) did the FDA revoke the approved indication (table 2). All of the drugs with withdrawn or revoked indications remain on the market for other indications, except for olaratumab, which had no other indication, and tositumomab, which was withdrawn from the US market for commercial reasons, citing lack of demand.13
Table 2.
Cancer drug indications that initially received accelerated approval but failed to improve primary endpoint in confirmatory trials
| Drug | Indication | Endpoint leading to accelerated approval | Post-approval trial results | Outcome as of May 2021 | Status in current NCCN guidelines* |
|---|---|---|---|---|---|
| Gemtuzumab | AML | RR | OS, DFS, RR all not improved; concerning safety signals | Voluntarily withdrawn; subsequently approved with lower dose and new schedule in different population | NA |
| Gefitinib | NSCLC | RR | 3 confirmatory trials failed to confirm benefit: one closed early, one non-inferiority study, one failed to improve primary endpoint of OS | Voluntarily withdrawn; subsequently approved in different population (EGFR mutations positive only) | NA |
| Tositumomab | Follicular lymphoma | RR | Confirmatory trial not completed; other trial suggested no survival benefit | Voluntarily withdrawn | NA |
| Bevacizumab | HER2 negative breast | PFS | Three trials with no improvement in OS; PFS improved | Revoked by FDA over objection of manufacturer | Yes: category 2A |
| Fludarabine | B cell CLL | RR | Confirmatory trial not completed | Voluntarily withdrawn | NA |
| Bevacizumab | Glioblastoma | RR | OS (primary endpoint) and quality of life not improved; PFS improved | Converted to regular approval | Category 2A |
| Nivolumab | Melanoma after ipilimumab or BRAF inhibitor | RR | OS and PFS both not improved | Converted to regular approval on basis of results from other trials | Category 2A |
| Pembrolizumab | PDL1 + gastric or gastro-oesophageal cancer | RR | OS and PFS not improved in 2 trials | Voluntarily withdrawn; ODAC voted 6-2 in favour of withdrawal | Category 2A |
| Atezolizumab | Urothelial, second line | RR | OS and PFS both not improved | Voluntarily withdrawn | Removed |
| Atezolizumab | Urothelial, first line | RR | OS not improved; PFS improved | Pending; ODAC voted 10-1 against withdrawal | Category 2A |
| Pembrolizumab | Urothelial, first line | RR | OS not improved; PFS not improved | Pending; ODAC voted 5-3 against withdrawal | Category 2A |
| Olaratumab | Soft tissue sarcoma | OS from phase II trial | OS not improved; PFS detrimental | Voluntarily withdrawn | Removed |
| Atezolizumab | PDL1+ TNBC | PFS | OS and PFS both not improved | Pending; ODAC voted 7-2 against withdrawal | Category 1 |
| Durvalumab | Urothelial | RR | OS not improved; PFS detrimental | Voluntarily withdrawn | Removed |
| Nivolumab | Hepatocellular cancer | RR | OS not improved; PFS not improved | Voluntarily withdrawn; ODAC voted 5-4 in favour of withdrawal | Category 2A |
| Nivolumab | SCLC | RR | OS not improved in 2 confirmatory trials; PFS improved in one and detrimental in another | Voluntarily withdrawn | Category 3 |
| Pembrolizumab | Hepatocellular cancer | RR | OS and PFS both not improved | Pending; ODAC voted 8-0 against withdrawal | Category 2B |
| Pembrolizumab | SCLC | RR | OS not improved; PFS improved | Voluntarily withdrawn | Category 3 |
AML=acute myeloid leukaemia; CLL=chronic lymphocytic leukaemia; EGFR=epidermal growth factor receptor; FDA=US Food and Drug Administration; NA=not applicable; ODAC=Oncology Drug Advisory Committee; OS=overall survival; PFS=progression-free survival; RR=response rate; SCLC=small cell lung cancer; TNBC=triple negative breast cancer.
As of 12 May 2021.
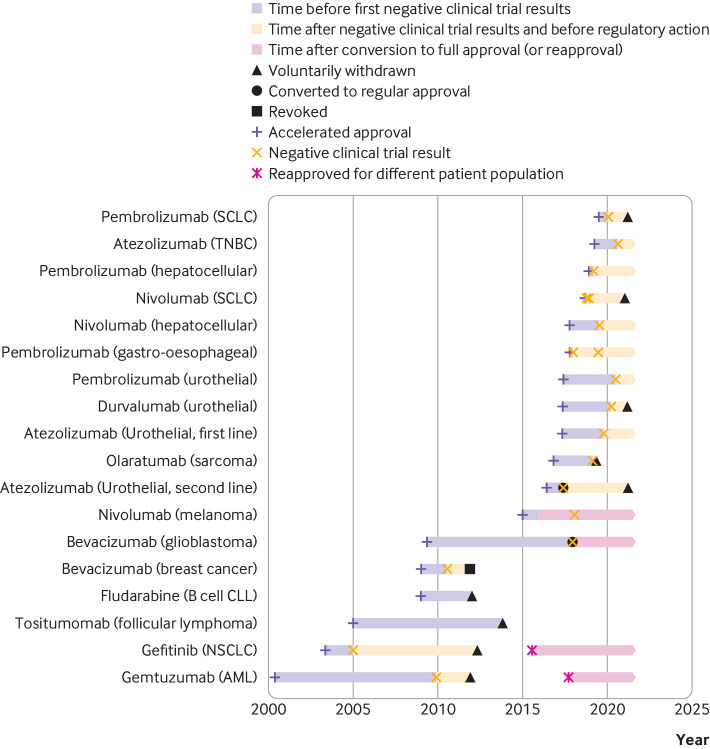
In the case of bevacizumab in glioblastoma, the FDA converted accelerated approval to regular approval on the basis of improvement in the secondary endpoint of progression-free survival despite the drug failing to improve both overall survival (the primary endpoint of the trial) and quality of life. In the case of nivolumab as a second line treatment for melanoma after progression on ipilimumab or BRAF inhibitors, although the statutorily required confirmatory trial failed to show improved overall survival,14 the FDA accepted results from other positive trials of the same drug as supporting evidence for converting the accelerated approval to regular approval. In six (33%) cases, the indication with accelerated approval remained on the drug’s labelling as of May 2021. After the completion of this study, two more indications have been voluntarily withdrawn by the industry in July 2021—pembrolizumab for gastro-oesophageal cancers and nivolumab monotherapy for hepatocellular cancer. Thus, as of July 2021, six (33%) of 18 drug indications either remain on the drug’s labelling or were converted from accelerated approval to regular approval despite the negative results from the required trial. The time from accelerated approval until the regulatory outcome—which included withdrawal, revocation, conversion to regular approval, or the end of the study time period on 31 May 2021—ranged from 1.7 to 11.5 years, with a median of 3.9 years (fig 1).
Fig 1.
Timeline showing dates of accelerated approval, announcement of negative results from confirmatory trials, and regulatory action, if any. Note that pembrolizumab for PDL1+ gastro-oesophageal cancer and nivolumab for hepatocellular cancer were subsequently voluntarily withdrawn by industry in July 2021. AML=acute myeloid leukaemia; CLL=chronic lymphocytic leukaemia; NSCLC=non-small cell lung cancer; SCLC=small cell lung cancer; TNBC=triple negative breast cancer
Among the withdrawn indications, the time from the announcement of negative confirmatory trial results to withdrawal ranged from 0.25 years (olaratumab in sarcoma) to 3.75 years (atezolizumab in urothelial cancer), with a median of 1.6 years, excluding the case of gefitinib in lung cancer, for which the FDA restricted access to the drug within six months but the manufacturer did not formally withdraw the indication for another eight years. Among the six indications with no regulatory action, the time from the announcement of negative results to 31 May 2021 ranged from 0.7 years (atezolizumab in breast cancer) to 3.3 years (pembrolizumab in gastro-oesophageal cancers), with a median of 1.7 years.
Ten of the negative post-approval trials had results announced since 2018, of which nine involve immunotherapy agents. Of these 10, four (40%) indications have been voluntarily withdrawn by the manufacturer—olaratumab for soft tissue sarcoma, durvalumab for urothelial carcinoma, nivolumab for small cell lung cancer, and pembrolizumab for small cell lung cancer. The other six accelerated approval indications remain as labelled indications—nivolumab and pembrolizumab for hepatocellular cancer, pembrolizumab and atezolizumab as first line treatment for urothelial cancer, pembrolizumab for gastro-oesophageal cancer, and atezolizumab for triple negative breast cancer. However, as noted above, nivolumab for hepatocellular cancer and pembrolizumab for gastro-oesophageal cancer have subsequently been withdrawn after our study period was complete.
In the ODAC meeting that reviewed the six immunotherapy drug indications that had received accelerated approval but shown no improvement in the primary endpoint in confirmatory trials, the ODAC committee voted to withdraw approval of the indication in two cases and maintain accelerated approval status until another confirmatory trial is completed in the other four cases (table 2). In July 2021, the industry decided to voluntarily withdraw the approval for the indications in two cases in which the ODAC had voted in support of withdrawal (nivolumab for hepatocellular cancer and pembrolizumab for gastro-oesophageal cancer).
Therefore, as of July 2021, of the 18 accelerated approval indications with negative post-approval trials, 11 (61%) were voluntarily withdrawn, one (5%) was revoked by the FDA, and six (33%) remain on the label (two via conversion to regular approval status and four as continued “dangling” accelerated approval status).
Impact on clinical practice guidelines
After excluding two indications for which the confirmatory trials were not completed (tositumomab and fludarabine) and two indications for which the drug was reapproved at a different dose or schedule (gemtuzumab and gefitinib), we examined the status of 14 accelerated approval indications in NCCN guidelines as of May 2021. The NCCN guidelines deleted the recommendation for the drug after a negative post-approval trial and withdrawal of the indication in three (21%) cases (olaratumab in sarcoma, and durvalumab and atezolizumab in urothelial cancer) and decreased the category of recommendation from 2A to category 3 in two (14%) cases (nivolumab and pembrolizumab in small cell lung cancer). Pembrolizumab in hepatocellular cancer remains in the NCCN guidelines as a category 2B recommendation.
In seven (50%) other cases, the category of recommendation remained 2A (the second highest level of recommendation) despite the drugs not improving the primary endpoint in post-approval trials. In one case (atezolizumab in triple negative breast cancer), the drug continues to hold a category 1 recommendation despite the failure to improve outcomes in the post-approval trial. All the NCCN guidelines related to the drug indications in this study had their latest revision between 19 February 2021 and 28 April 2021, meaning that in all cases the guidelines were subject to a formal review by the NCCN after the availability of negative confirmatory evidence (table 2). This is also confirmed by the presence of citations to the negative confirmatory trials in the Discussion section of these guidelines.
Comparison with EMA
Of the 18 drug indications in the cohort, seven (39%) had not been approved by the EMA as of May 2021—bevacizumab for glioblastoma, pembrolizumab for gastro-oesophageal cancers, pembrolizumab for hepatocellular cancer, pembrolizumab for small cell lung cancer, nivolumab for hepatocellular cancer, nivolumab for small cell lung cancer, and druvalumab for urothelial cancer. In two cases (bevacizumab for breast cancer and atezolizumab as second line treatment for urothelial cancer), the indications remain approved by the EMA although they were withdrawn in the US. The remaining indications had no discrepancies between the regulatory outcomes of the drugs in the two jurisdictions. In the case of olaratumab for soft tissue sarcoma, the EMA conditional marketing authorisation was later withdrawn on the basis of the negative results from a confirmatory study, consistent with the voluntary withdrawal in the US. Tositumomab has also been withdrawn from the EMA on request from the sponsor, consistent with the FDA.
Discussion
In this study of cancer drugs that received accelerated approval but subsequently failed to show improvement in the primary clinical efficacy endpoint in confirmatory trials, we found that the indication often remains on the drug’s label. Even in cases in which the indication was withdrawn, the withdrawal was sometimes delayed for several years after initial approval and after the results of post-approval trial were announced. These indications also frequently continue to be recommended in clinical practice guidelines, sometimes after the manufacturer has withdrawn the indication from the FDA. Although some cancer drugs get reapproved at a different doses and dosing schedules or in slightly different populations, cases also exist in which the accelerated approval has simply been converted into regular approval or the post-approval trial requirement has been waived when the post-approval trial has failed to meet the primary endpoint.
The FDA’s accelerated approval programme is intended to balance speed of access and quality of evidence for promising new drugs. A fundamental premise of this balance is withdrawal of the approved indication if the drug does not show clinical benefit in the post-approval trial, but we found that this often does not occur. Half of all the withdrawals of approved indications for cancer drugs occurred in the past two years, which may suggest that the FDA is taking a stronger stance in recent years in enforcing the policy compromise at the heart of the accelerated approval programme. However, the recent withdrawals could also simply be a result of increased numbers of accelerated approvals or relaxed standards for accelerated approval in recent years. At an ODAC meeting in April 2021, four of the six “dangling” accelerated approval indications were recommended to remain on the drug’s label. In July 2021, the industry voluntarily withdrew the approval of two of the six “dangling” accelerated approval indications that received a recommendation against approval from the ODAC meeting, but the regulatory action for the remaining four indications that received support from ODAC voting remains to be seen. The FDA is not required to follow the recommendations of the ODAC meeting.
Eighty per cent of the drugs that failed in post-approval trials initially received accelerated approval on the basis of response rates, highlighting the poor surrogacy of response rates for overall survival. This has two important implications. Firstly, the FDA Table of Surrogate Endpoints lists response rate as a surrogate for several tumour types.15 The FDA states that the surrogate measures will be removed from the table if the surrogacy is disproven. Despite these cases, response rate remains on the table for these indications. Secondly, many drugs have recently received regular (not accelerated) approval on the basis of response rates alone, meaning that no post-approval confirmatory trials will be required to confirm these drugs’ benefit to patients.16 The FDA should carefully evaluate the Table of Surrogate Endpoints and remove those that have been shown not to correlate with clinical endpoints.
Most confirmatory trials continue to use surrogate measures—and often the same surrogate measure as the pre-approval trials—to assess clinical benefit after accelerated approval; we previously found that only 20% of confirmatory trials used overall survival as the endpoint.6 In this study, all of the drugs that failed to show efficacy on the primary endpoint used overall survival as the endpoint. However, we have previously found that 40% of post-approval trials use surrogate measures as the primary endpoint, so our results may underestimate the actual negative performance of accelerated approval drugs in post-approval trials. This may have the unintended consequence of serving as a further disincentive for manufacturers to choose overall survival as the primary endpoint in confirmatory trials, as the likelihood of failing to meet the primary endpoint is higher when the primary endpoint is overall survival. As most surrogates have weak correlation with overall survival, and all withdrawals of accelerated approvals have happened in cases in which the confirmatory trials have used overall survival as the primary endpoint, this underscores the need for mandating overall survival as the primary endpoint in post-approval trials for cancer drugs.
Policy implications
Our results also show that the FDA often does not take immediate action on accelerated approval cancer drugs even when post-approval trials are negative. Without proactive steps from the FDA, drugs that have no proven clinical benefit and known toxicities will continue to be used by patients and clinicians who rely on the FDA to assess the risks and benefits of drugs.
Some of the FDA’s decisions may have also been related to the public pressure as seen during the revoking of bevacizumab’s indication for breast cancer.8 Some patients and physicians may contend that they received clinical benefit from using these drugs even though such benefit was not proven in trials. Complexity is also added when the industry does not accept the FDA’s request to withdraw the approval (of the 10 recent negative post-approval trials, four indications were voluntarily withdrawn by the industry at the request of the FDA and the remaining six were presented to the ODAC meeting). Clearly establishing the consequences of delayed or negative confirmatory trials at the time of granting accelerated approval would avoid confusion.17 There may also be some concerns that higher rates of withdrawal may be detrimental to the FDA’s reputation; however, we believe that failure to take appropriate actions when results from confirmatory trials are negative is far more detrimental to the public’s confidence in the agency and in the accelerated approval pathway. The ethics of regulatory inaction when confirmatory trials are negative may also reflect betrayal of patients’ sacrifice and consent when participating in these trials.
Clinical practice guidelines continue to endorse use of these drugs even after the drugs have had negative results in post-approval trials. In some cases, as with bevacizumab in metastatic breast cancer, the guidelines continue to provide a high level of endorsement even after the FDA revoked the approved indication. This means that patients and clinicians will likely continue using the drugs despite no evidence of benefit. Additionally, the NCCN compendia recommendations are used to support Medicare coverage in the US, meaning that Medicare will be required to reimburse use of these drugs despite regulatory action. This has important financial consequences for patients and the healthcare system, as these drugs are invariably expensive.18 NCCN guidelines are known to make off-label recommendations based on lower evidentiary standards than the FDA,11 and our findings that the drugs with indications that have been withdrawn or revoked owing to lack of efficacy continue to receive a high level of endorsement raise further concerns about how NCCN guidelines are produced and their role in establishing coverage for cancer drugs in the US.
Although the accelerated approval pathway is designed to hasten patients’ access to promising drugs, delays in regulatory actions after negative confirmatory trials mean that patients will continue to use these therapies that lack evidence of clinical benefit. Some accelerated approval drugs with negative confirmatory trials were given a “second chance” in another confirmatory trial while the accelerated approval indication remained on the drug’s labelling. Suggestions for second chances for the dangling accelerated approvals were also discussed at the April 2021 ODAC meeting. The FDA should take actions to assure that risks and benefits are clearly communicated to patients and their physicians, including the fact that some of the approvals remain on the label despite the negative results from the confirmatory trials.
Strengths and limitations of this study
This is the most comprehensive study so far of all the post-approval trials in oncology that have failed to show improvement in the primary endpoint after the drug initially received accelerated approval. One of the limitations of our study is that we relied on publicly available information, and private communication between drug manufacturers and the FDA may have affected these decisions. In those cases, the key information underlying such decisions should be made publicly available. We reviewed only the most recent NCCN guidelines, but all guidelines were updated after the confirmatory trial results were available and cited these trial results, meaning that the guideline committees were aware of them. However, the guidelines could change in the future, especially once regulatory actions are taken on indications that are still dangling accelerated approvals.
Conclusion
Six of 18 cancer drugs that initially received accelerated approval have indications that remain on the labelling and are recommended in clinical guidelines despite no improvement in the primary endpoint in post-approval trials. These findings reflect the lack of fulfilment of the compromise between speed and evidence that underpins the accelerated approval pathway. A recent flurry of regulatory action suggests that the FDA has paid greater attention to these situations in the past two years, although additional guidance and reforms of the accelerated approval pathway are needed to assure that all FDA approved drugs are shown to be safe and effective for patients.
What is already known on this topic
Cancer drugs that receive accelerated approval from the US Food and Drug Administration on the basis of improvement in surrogate measures are subject to confirmatory trials
Most confirmatory trials that verify the benefit of accelerated approval cancer drugs use surrogate endpoints for verification
Confirmatory trials are sometimes not completed until several years after accelerated approval
What this study adds
All confirmatory trials that failed to show improvement in the primary endpoint had used overall survival as the primary endpoint
Accelerated approval is not always withdrawn, even when the confirmatory trial results are negative
Clinical guidelines continue to recommend cancer drugs for indications for which the confirmatory trials have failed to show clinical benefit, sometimes despite the withdrawal of approval
Contributors: BG, BNR, and ASK conceptualized the study. BG collected and analyzed data. All authors ensured the accuracy of analysis. BG wrote the first draft of the manuscript, which was revised, edited, and agreed for submission by all authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. BG is the guarantor.
Funding: Arnold Ventures funded this study. The funder had no role in the conceptualisation, design, analysis, and reporting of the study or in the decision to publish the manuscript. BG receives salary support from the Ontario Institute for Cancer Research funded by the government of Ontario.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support for the study from Arnold Ventures; ASK is principal investigator on a grant from the FDA to Brigham and Women’s Hospital on an unrelated topic; BG has received consulting fees from Vivio Health, outside the submitted work; BNR has received a grant from Anthem Public Policy Institute and personal fees from Blue Cross Blue Shield of Massachusetts, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
All authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We have plans to disseminate the results of this study via media and online webinars to the patient community after its publication. Those online webinars will also be recorded and posted on YouTube. We will also post a blog and share the results through our personal and institutional social media accounts for wider dissemination of the results.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
No additional data available.
References
- 1.FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource. US Food and Drug Administration, National Institutes of Health, 2016. [PubMed] [Google Scholar]
- 2.Gyawali B, Hey SP, Kesselheim AS. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine 2020;21:100332. 10.1016/j.eclinm.2020.100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang TJ, Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer 2019;144:1746-51. 10.1002/ijc.31957 [DOI] [PubMed] [Google Scholar]
- 4.Gyawali B, Kesselheim AS. Reinforcing the social compromise of accelerated approval. Nat Rev Clin Oncol 2018;15:596-7. 10.1038/s41571-018-0066-3 [DOI] [PubMed] [Google Scholar]
- 5.Beaver JA, Howie LJ, Pelosof L, et al. A 25-Year Experience of US Food and Drug Administration Accelerated Approval of Malignant Hematology and Oncology Drugs and Biologics: A Review. JAMA Oncol 2018;4:849-56. 10.1001/jamaoncol.2017.5618 [DOI] [PubMed] [Google Scholar]
- 6.Gyawali B, Hey SP, Kesselheim AS. Assessment of the Clinical Benefit of Cancer Drugs Receiving Accelerated Approval. JAMA Intern Med 2019;179:906-13. 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herder M. Pharmaceutical Drugs of Uncertain Value, Lifecycle Regulation at the US Food and Drug Administration, and Institutional Incumbency. Milbank Q 2019;97:820-57. 10.1111/1468-0009.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med 2011;365:e3. 10.1056/NEJMp1107201 [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. FDA In Brief: FDA Oncologic Drugs Advisory Committee to Review Status of Six Indications Granted Accelerated Approval. 2021. https://www.fda.gov/news-events/fda-brief/fda-brief-fda-oncologic-drugs-advisory-committee-review-status-six-indications-granted-accelerated.
- 10.US Food and Drug Administration. Postmarket Requirements and Commitments. https://www.accessdata.fda.gov/Scripts/cder/pmc/index.cfm
- 11.Wagner J, Marquart J, Ruby J, et al. Frequency and level of evidence used in recommendations by the National Comprehensive Cancer Network guidelines beyond approvals of the US Food and Drug Administration: retrospective observational study. BMJ 2018;360:k668. 10.1136/bmj.k668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaver JA, Pazdur R. “Dangling” Accelerated Approvals in Oncology. N Engl J Med 2021;384:e68. 10.1056/NEJMp2104846 [DOI] [PubMed] [Google Scholar]
- 13.Prasad V. The withdrawal of drugs for commercial reasons: the incomplete story of tositumomab. JAMA Intern Med 2014;174:1887-8. 10.1001/jamainternmed.2014.5756 [DOI] [PubMed] [Google Scholar]
- 14.Larkin J, Minor D, D’Angelo S, et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol 2018;36:383-90. 10.1200/JCO.2016.71.8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration. Table of Surrogate Endpoints That Were the Basis of Drug Approval or Licensure. https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure.
- 16.Gyawali B, D’Andrea E, Franklin JM, Kesselheim AS. Response Rates and Durations of Response for Biomarker-Based Cancer Drugs in Nonrandomized Versus Randomized Trials. J Natl Compr Canc Netw 2020;18:36-43. 10.6004/jnccn.2019.7345 [DOI] [PubMed] [Google Scholar]
- 17.Gyawali B, Ross JS, Kesselheim AS. Fulfilling the Mandate of the US Food and Drug Administration’s Accelerated Approval Pathway: The Need for Reforms. JAMA Intern Med 2021. 10.1001/jamainternmed.2021.4604 [DOI] [PubMed] [Google Scholar]
- 18.Gellad WF, Kesselheim AS. Accelerated Approval and Expensive Drugs - A Challenging Combination. N Engl J Med 2017;376:2001-4. 10.1056/NEJMp1700446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data available.