Key Points
Question
What is the effect of screen time in the first 48 hours after concussion on the duration of concussive symptoms?
Findings
In this randomized clinical trial including 125 patients with concussion aged 12 to 25 years, those who abstained from screen time during the first 48 hours of recovery had a statistically significant shorter duration of symptoms (3.5 days) than those who were permitted screen time (8 days).
Meaning
This study provides preliminary evidence supporting clinical recommendations to limit screen time in the acute period after concussion.
Abstract
Importance
There are limited data to guide screen time recommendations after concussion.
Objective
To determine whether screen time in the first 48 hours after concussion has an effect on the duration of concussive symptoms.
Design, Setting, and Participants
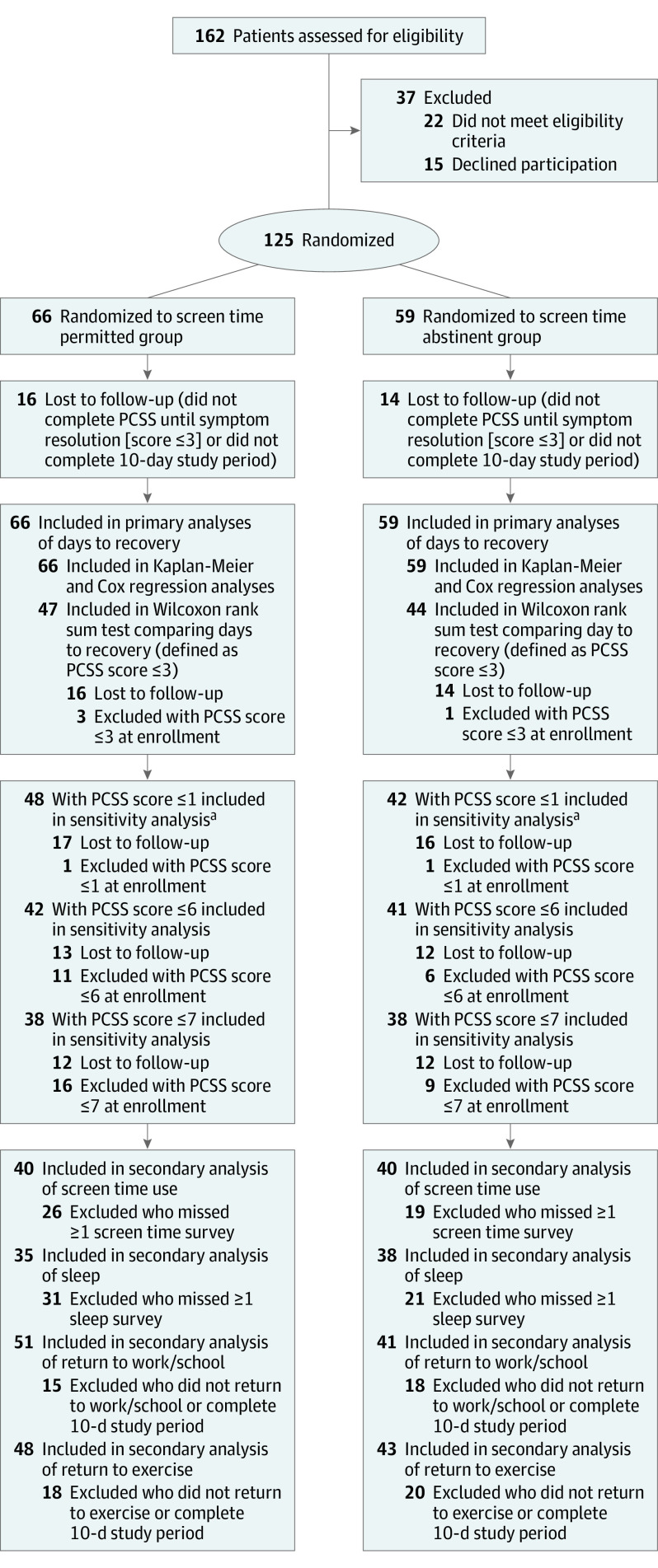
This randomized clinical trial was conducted in the pediatric and adult emergency departments of a tertiary medical center between June 2018 and February 2020. Participants included a convenience sample of patients aged 12 to 25 years presenting to the emergency department within 24 hours of sustaining a concussion. A total of 162 patients were approached, 22 patients met exclusion criteria, and 15 patients declined participation; 125 participants were enrolled and randomized.
Interventions
Patients were either permitted to engage in screen time (screen time permitted group) or asked to abstain from screen time (screen time abstinent group) for 48 hours after injury.
Main Outcomes and Measures
The primary outcome was days to resolution of symptoms, defined as a total Post-Concussive Symptom Scale (PCSS) score of 3 points or lower. Patients completed the PCSS, a 22-symptom scale that grades each symptom from 0 (not present) to 6 (severe), each day for 10 days. Kaplan-Meier curves and Cox regression modeling were used to compare the 2 groups. A Wilcoxon rank sum test was also performed among participants who completed the PCSS each day through recovery or conclusion of the study period.
Results
Among 125 patients with concussion, the mean (SD) age was 17.0 (3.4) years; 64 participants (51.2%) were male. A total of 66 patients were randomized to the screen time permitted group, and 59 patients were randomized to the screen time abstinent group. The Cox regression model including the intervention group and the patient’s self-identified sex demonstrated a significant effect of screen time (hazard ratio [HR], 0.51; 95% CI, 0.29-0.90), indicating that participants who engaged in screen time were less likely to recover during the study period. In total, 91 patients were included in the Wilcoxon rank sum test (47 patients from the screen time permitted group, and 44 patients from the screen time abstinent group). The screen time permitted group had a significantly longer median recovery time of 8.0 days (interquartile range [IQR], 3.0 to >10.0 days) compared with 3.5 days (IQR, 2.0 to >10.0 days; P = .03) in the screen time abstinent group. The screen time permitted group reported a median screen time of 630 minutes (IQR, 415-995 minutes) during the intervention period compared with 130 minutes (IQR, 61-275 minutes) in the screen time abstinent group.
Conclusions and Relevance
The findings of this study indicated that avoiding screen time during acute concussion recovery may shorten the duration of symptoms. A multicenter study would help to further assess the effect of screen time exposure.
Trial Registration
ClinicalTrials.gov Identifier: NCT03564210
This randomized clinical trial examines whether engaging in vs abstaining from screen time within the first 48 hours after concussion has an effect on the duration of concussive symptoms among patients aged 12 to 25 years.
Introduction
An estimated 2.5 million people present to the emergency department (ED) in the US for traumatic brain injuries annually.1 Children and adolescents aged 10 to 19 years have the highest incidence of concussion.2,3 In 2017, 15% of students in high school reported having at least 1 diagnosed concussion.4
The International Concussion in Sports Group and the Centers for Disease Control and Prevention recommend a period of complete cognitive and physical rest for the first 24 to 48 hours after sustaining a concussion followed by a structured return to activity.5,6 There is practice and guideline variability regarding which activities are considered cognitive rest during the acute recovery period.7,8,9,10 Parents and patients frequently ask whether screen time is permitted. Many clinicians and guidelines caution against screen time.7 Other clinicians permit limited or certain types of screen time if that engagement does not induce symptoms, believing it is one of the few forms of safe distraction permitted during this acute recovery period.9,11 US teenagers engage in more than 7 hours of screen time daily, not including time spent on schoolwork.12 Inhibiting the use of electronic screens has been associated with dysregulation, social isolation, and a heightened sense of illness anxiety.7,13,14
To our knowledge, no randomized clinical trial has specifically assessed the effect of screen time on concussive symptoms. We conducted a randomized clinical trial examining the effect of screen time on the time until symptom recovery in the first 10 days after injury. We hypothesized that abstaining from screen time would shorten the duration of concussive symptoms.
Methods
Study Design
We conducted a single-center parallel-design randomized clinical trial between June 2018 and February 2020 among patients who presented to the pediatric and adult EDs of the University of Massachusetts Medical Center in Worcester, within 24 hours of sustaining a concussion. Participants were randomized to 2 intervention groups; 1 group was permitted to engage in screen time during the first 48 hours of recovery, and 1 group was asked to abstain from screen time during the same period. The primary outcome was the number of days until functional resolution of concussive symptoms, which was defined as the first day with a total score of 3 points or lower on the Post-Concussive Symptom Scale (PCSS), regardless of the level of activity in which they were engaged. This study was reviewed and approved by the University of Massachusetts Medical School Institutional Review Board. Patients and/or their guardians provided written informed consent and assent, when applicable. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Study Population
Patients were screened for study eligibility if they were between ages 12 and 25, presented to the ED within 24 hours of a head injury, and met the criteria for concussion on the Acute Concussion Evaluation–Emergency Department tool.15,16 This instrument is used by ED clinicians to diagnose a concussion and identify risk factors for prolonged recovery.14,15,16,17 A total of 162 patients with concussion were approached for participation; of those, 22 patients were excluded, and 15 patients declined participation. The remaining 125 patients were enrolled sequentially during periods when study staff were available.
Patients were excluded if their attending physician declined participation, their guardian was not present (if the patient was younger than 18 years), or they (or their parent or guardian) were not fluent in English. In addition, patients were excluded if they were intoxicated; had a Glasgow Coma Scale18 score of less than 15 points; had intracranial abnormalities identified on imaging; had preexisting intellectual disability, severe psychiatric illness, severe neurological conditions, or substantial previous neurological surgery; or required neurosurgical intervention, intubation, or hospital admission.
Study Protocol
The trial protocol appears in Supplement 1. Study staff, including medical students, residents, and attending physicians, enrolled patients and assisted them in completing standardized intake forms using the Research Electronic Data Capture (REDCap) system,19 an online secure data management system hosted by the University of Massachusetts Medical School. Enrollment forms included a survey of demographic characteristics, the Acute Concussion Evaluation–Emergency Department tool, and the PCSS, which patients completed in the ED.
After forms were completed, participants were randomized into 2 groups (screen time permitted or screen time abstinent) through REDCap using a random number list, which was generated and uploaded by the principal investigator (T.M.); the patient’s intervention group was blinded to both the participant and study staff during the completion of enrollment forms. Using a standard script, the study staff instructed the intervention (screen time abstinent) group to abstain from engaging with any form of screen time for the first 48 hours after discharge. The control (screen time permitted) group was instructed by study staff that they were allowed to engage with any form of screen time, as tolerated, for the first 48 hours. All participants were advised to avoid attending work or school or completing remote work for the first 48 hours. Study staff provided participants with written group-specific instructions and standard discharge information about concussion.20 Participants were encouraged to follow up with the concussion clinic or their primary physician within the next 3 days, and they were given a paper log to assist in tracking screen time.
Participants completed the PCSS at the time of enrollment, then daily for the 10-day study duration (based on the median time to recovery reported in a previous ED-based observational study21). For 3 calendar days after discharge, participants completed a daily screen time survey and the PCSS. On days 4 through 10, participants completed an activity survey and the PCSS. Participants received daily surveys via REDCap at 8:00 am and had until midnight to complete them. If a survey had not been completed by late afternoon, the study staff promoted retention with a text message (most common), an email, or a phone call per the participant’s preferred communication method. Standardized language was used in communications to minimize bias from study staff being unblind to group assignment.
Primary Outcome Measure
Our primary outcome was days to concussion recovery, with recovery defined as the first day a patient reported a PCSS score of 3 points or lower. The PCSS is a 22-symptom scale that grades each symptom from 0 (not present) to 6 (severe) and reliably detects change over time in patients with concussion.14 Scores on the PCSS range from 0 to 132 points, with higher scores indicating higher severity of postconcussive symptoms. The PCSS cutoff score of 3 points or lower was chosen based on the results of a study comprising more than 30 000 male and female high school athletes, who demonstrated median PCSS scores of 1 point and 3 points, respectively, in the absence of recent head injury.22
Secondary Outcome Measures
As a secondary outcome, we conducted a sensitivity analysis using 3 additional thresholds for recovery (PCSS scores of ≤7 points, ≤6 points, and ≤1 point). These values were chosen based on previously examined PCSS cutoff scores for recovery in normative and concussion literature.22,23,24,25,26 Other secondary outcomes included the amount of screen and sleep time during the intervention period, the day of return to school or work after the intervention period, the day of return to exercise after the intervention period, and daily PCSS scores. To evaluate the underlying mechanism of protection in screen time absence, we performed an exploratory analysis of the PCSS symptoms of photophobia, visual disturbance, and sleep-related symptoms for each intervention group during the time of intervention.
Statistical Analysis
Our primary outcome was time to symptom recovery over the study period, which was measured by the PCSS. Using comparative literature to estimate a clinically meaningful effect, we hypothesized that screen time avoidance would decrease a participant’s daily PCSS score by 12 points, resulting in approximately 2 fewer days until recovery (equivalent to a moderate effect size based on an estimated SD of 22 points on the PCSS).24 Using the means procedure in the G Power program, with 2-sided α = .05 and power (1-β) = 0.80, the calculated sample needed was 106 participants (53 participants for each intervention group). Our initial analysis plan was based on a study conducted by Thomas et al24 that compared median days to recovery among those who completed follow-up, with recovery defined by a PCSS score threshold. We later decided to perform survival curve analyses to include censored data and reflect an intention-to-treat approach that allowed for analysis of all randomized participants.
Our primary outcome of days until concussion recovery (defined as a PCSS score of ≤3 points) was examined using 2 different analyses: Kaplan-Meier survival curves including all enrolled participants and a Wilcoxon rank sum test comparing median days to recovery. Kaplan-Meier survival curves were constructed for each intervention group up to day 10, using censoring of participants unavailable for follow-up as appropriate. Data from patients who did not meet recovery criteria on or before day 10 were censored at the last follow-up date or day 10. The difference between intervention groups was estimated using hazard ratios (HRs) with 95% CIs obtained from a Cox proportional hazards model that was adjusted for self-identified sex, which has previously been found to be associated with recovery.27 Age was not significantly associated with outcomes in univariate modeling and was therefore not included in the multivariable Cox model. Violations to model assumptions were tested, and influential observations were assessed.
A 1-tailed Wilcoxon rank sum test was used to compare median days to recovery between the intervention groups. This test included only participants who had a PCSS score higher than 3 points at enrollment and who either recovered from their concussion during the study period or completed daily surveys through study completion. Participants who had not completed surveys for 3 consecutive calendar days and who had not yet met the criteria for concussion recovery were considered unavailable for follow-up and were not included in the Wilcoxon rank sum test. The day of recovery was defined as the first day a participant had a total PCSS score of 3 points or lower even if symptoms worsened in later days. Those with concussions lasting longer than 10 days were categorized as experiencing recovery on day 11.
For secondary analyses, the amount of screen time and sleep during the intervention period was calculated for the 2 groups, including only participants who reported these data on all of the first 3 daily surveys. For the first 3 days, the proportion of participants reporting sleep-related symptoms, photophobia, or visual disturbances was compared between the 2 groups using χ2 tests. Median days until return to work/school or exercise were calculated for each group, including only those participants who either returned to the activity during the study period or completed surveys through study completion. All data were analyzed using SAS software, version 9.4 (SAS Institute Inc), and Stata software, version 16 (StataCorp LLC).
Results
Baseline Participant Characteristics
We enrolled 125 patients (mean [SD] age, 17.0 [3.4] years; 64 male participants [51.2%] and 61 [48.8%] female participants) and stopped enrollment in March 2020 because of institutional restrictions resulting from the COVID-19 pandemic (Figure 1). The screen time permitted group comprised 66 participants; of those, 37 participants (56.1%) were male, and 29 participants (43.9%) were female, with a mean (SD) age of 17.1 (4.0) years. The screen time abstinent group included 59 participants; of those, 27 participants (45.8%) were male, and 32 participants (54.2%) were female, with a mean (SD) age of 16.9 (3.3) years. The median PCSS score at enrollment was 21.0 points (IQR, 8.0-39.0 points) in the screen time permitted group and 24.5 points (IQR, 11.0-38.0 points) in the screen time abstinent group. Most concussions occurred during sports activities (62 total participants [49.6%]). Additional demographic characteristics, risk factors for severe concussion, and injury characteristics are shown in Table 1.
Figure 1. CONSORT Flow Diagram.
PCSS indicates Post-Concussive Symptom Scale.
aThe secondary analysis was performed using a Wilcoxon rank sum test at different PCSS cutoff scores for concussion recovery.
Table 1. Demographic Characteristics, Risk Factors for Prolonged Concussions, and Injury Characteristics of Patient Groups.
| Characteristic | No. (%) | |
|---|---|---|
| Screen time permitted group | Screen time abstinent group | |
| Total patients, No. | 66 | 59 |
| Sex | ||
| Male | 37 (56.1) | 27 (45.8) |
| Female | 29 (43.9) | 32 (54.2) |
| Age, mean (SD), y | 17.1 (4.0) | 16.9 (3.3) |
| History | ||
| Concussion | 23 (34.8) | 16 (27.1) |
| Headaches | 22 (33.3) | 24 (40.7) |
| Loss of consciousness | 17 (25.8) | 10 (16.9) |
| Amnesia | ||
| Retrograde | 9 (13.6) | 12 (20.3) |
| Anterograde | 19 (28.8) | 19 (32.2) |
| Mechanism of injury | ||
| Motor vehicle crash | 13 (19.7) | 14 (23.7) |
| Sports | 34 (51.5) | 28 (47.5) |
| Other | 19 (28.8) | 17 (28.8) |
| PCSS score at enrollment, median (IQR) | 21.0 (8.0-39.0) | 24.5 (11.0-38.0) |
Abbreviations: IQR, interquartile range; PCSS, Post-Concussive Symptom Scale.
Effect of Screen Time on Time to Recovery
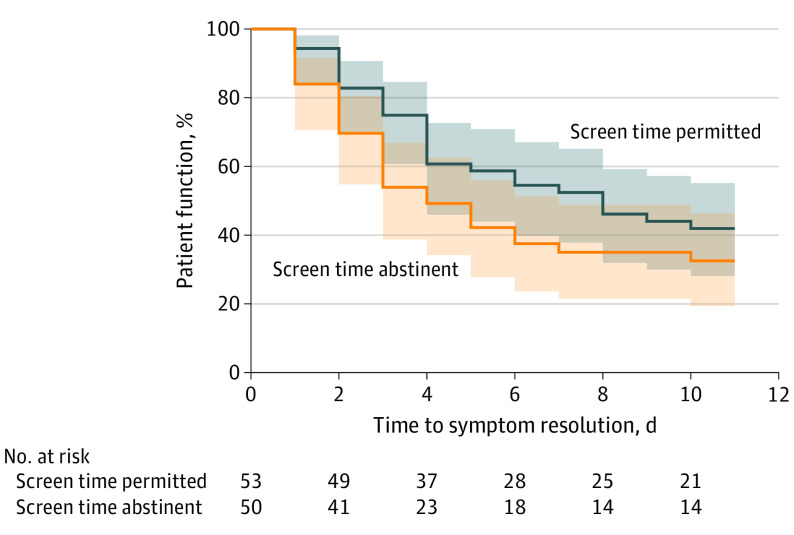
The primary analysis using a Cox regression model that included intervention group and sex indicated that female participants (HR, 0.34; 95% CI, 0.19-0.60) and participants in the screen time permitted group (HR, 0.51; 95% CI, 0.29-0.90) were less likely to recover over the study period compared with male participants and participants in the screen time abstinent group (Figure 2).
Figure 2. Kaplan-Meier Survival Curve Comparing Days Until Concussion Recovery of Screen Time Abstinent vs Screen Time Permitted Groups.
Days until concussion recovery defined as a daily Post-Concussive Symptom Scale score of 3 points or lower. Shading represents 95% CIs.
A total of 91 patients were included in the primary analysis using a Wilcoxon rank sum test (47 patients from the screen time permitted group, and 44 patients from the screen time abstinent group), in which a PCSS score of 3 points or lower was used to define recovery. Among 125 participants, 95 patients (50 in the screen time permitted group and 45 in the screen time abstinent group) completed surveys until either concussion resolution (a PCSS score of ≤3 points) or the conclusion of the study period. The percentage of participants who were unavailable for follow-up for inclusion in the Wilcoxon rank sum test was similar between the 2 groups (16 of 66 patients [24.2%] in the screen time permitted group and 14 of 59 patients [23.7%] in the screen time abstinent group). Four patients (3 in the screen time permitted group and 1 in the screen time abstinent group) had a PCSS score of 3 points or lower at enrollment and were not included in the analysis because they already met the definition of recovery. The screen time permitted group had a significantly longer median time until recovery compared with the screen time abstinent group (8.0 days [IQR, 3.0 to >10.0 days] vs 3.5 days [IQR, 2.0 to >10.0 days], respectively; P = .03). Twelve patients in each group reported a daily PCSS score greater than 3 points after they had been defined as recovered on a previous day, with a maximum recorded score of 11 points.
Adherence to Intervention
In total, 80 participants (40 per group) completed all 3 days of screen time surveys. The screen time permitted group reported more screen time overall (median, 630 minutes [IQR, 415-995 minutes]) compared with the screen time abstinent group (median, 130 minutes [IQR, 61-275 minutes]). The screen time permitted group spent more time on all screen modalities, especially television and phones (eTable in Supplement 2). No adverse events or effects of the intervention were reported in either group.
Secondary Analyses
Sensitivity analyses using different definitions of recovery (PCSS scores of ≤1 point, ≤6 points, and ≤7 points) demonstrated a persistent effect of screen time on days to recovery using both the Cox regression model (eFigure in Supplement 2) and the Wilcoxon rank sum test (Table 2). In the Wilcoxon rank sum test, the median number of days to recovery was longer in the screen time permitted group for all PCSS cutoff scores. For example, when a PCSS cutoff score of 7 points or lower was used, the median number of days to recovery was 8.0 (IQR, 3.0 to >10.0) in the screen time permitted group and 3.5 (IQR, 2.0 to >10.0) in the screen time abstinent group (Table 2). At this cutoff score, 25 of 38 patients (65.8%) in the screen time permitted group and 27 of 38 patients (71.1%) in the screen time abstinent group experienced resolution of symptoms by day 10. The median daily total PCSS scores for both groups are shown in Figure 3.
Table 2. Comparison of Median Days to Recovery Between Patient Groups Based on Different Cutoff Scores on the PCSS.
| PCSS cutoff score | Screen time permitted group | Screen time abstinent group | ||
|---|---|---|---|---|
| Patients, No. | Median (IQR) d to recovery | Patients, No. | Median (IQR) d to recovery | |
| ≤1 | 48 | 9.5 (4.0 to >10.0) | 42 | 6.5 (3.0 to >10.0) |
| ≤3 | 47 | 8.0 (3.0 to >10.0) | 44 | 3.5 (2.0 to >10.0) |
| ≤6 | 42 | 8.0 (3.0 to >10.0) | 41 | 4.0 (2.0 to >10.0) |
| ≤7 | 38 | 8.0 (3.0 to >10.0) | 38 | 3.5 (2.0 to >10.0) |
Abbreviations: IQR, interquartile range; PCSS, Post-Concussive Symptom Scale.
Figure 3. Unadjusted Comparison of Total Daily Post-Concussive Symptom Scale (PCSS) Scores of Screen Time Permitted vs Screen Time Abstinent Groups.
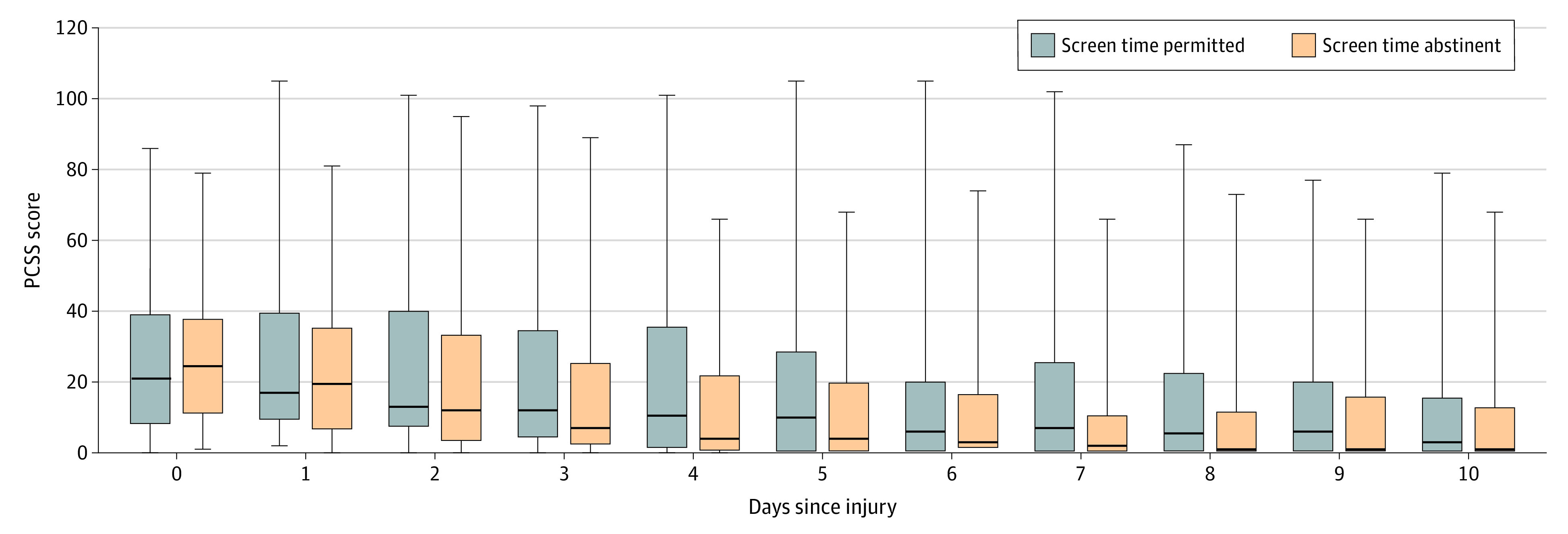
A total of 66 patients were in the screen time permitted group, and 59 patients were in the screen time abstinent group. Boxes reflect medians and IQRs, and whiskers represent the range of total PCSS scores in the groups at each day after injury.
The most common symptoms during the intervention period were headache, fatigue, light sensitivity, and feeling slow. No significant differences in the proportion of patients reporting sleep-related symptoms, light sensitivity, or visual disturbance during the first 3 days of recovery were observed between the screen time permitted and screen time abstinent groups.
A total of 73 participants (35 in screen time permitted group and 38 in the screen time abstinent group) reported sleep duration on the first 3 daily surveys. No difference in sleep duration was found between the screen time permitted group (median, 29 hours [IQR, 22-32 hours]) vs the screen time abstinent group (median, 29 hours [IQR, 24-34 hours]).
Among 125 patients enrolled, 92 patients (51 in the screen time permitted group and 41 in the screen time abstinent group) completed surveys until they either returned to work or school or completed the 10-day study period. The median time to return to work or school was 7.0 days (IQR, 5.0 to >10.0 days) in the screen time permitted group and 6.0 days (IQR, 4.0-9.5 days) in the screen time abstinent group. A total of 91 patients (48 in the screen time permitted group and 43 in the screen time abstinent group) completed surveys until they returned to exercise or completed the study period. The median time to return to exercise was 8.0 days (IQR, 4.0 to >10.0 days) in the screen time permitted group and 7.0 days (IQR, 5.0 to >10.0 days) in the screen time abstinent group.
Discussion
This randomized clinical trial found that a brief screen time abstinence after concussion was associated with a significantly faster recovery. We did not assess whether further reductions in screen time beyond the 2-day intervention period would confer additional benefits. However, we are not aware of any study that has directly tested the effect of screen time on outcome after concussion. This issue is important because screen time use is ubiquitous among adolescents and young adults and is considered a form of rest by many clinicians.
An acute rest period is widely recommended for patients with concussion; however, recommendations are inconsistent regarding which screen time activities are permitted as part of cognitive rest.5,9,28 It is unknown how the cognitive burden of television or other screen time compares with other activities that patients with concussion may choose when strict rest is prescribed. We suspect increased eye strain and photic stimulation with screen time use may worsen concussive symptoms. Television is a migraine trigger,29 and an association has been found between migraines and the development of concussive symptoms.30 Several studies have suggested that early return to cognitive and physical activity may be beneficial to concussion recovery.24,31,32,33 Replacing activity with screen time could adversely affect recovery. Sleep is thought to be beneficial to concussion recovery and may be adversely affected by screen time, although our study did not find a difference in sleep outcomes.34
Our primary outcome was the day of recovery defined as a PCSS score of 3 points or lower. Because previous studies have used different PCSS cutoff scores to define recovery, we performed a secondary sensitivity analysis to examine a range of definitions of recovery and evaluate whether there was a persistent effect of the screen time intervention using different PCSS cutoff scores.22,23,24,25,26 By investigating different PCSS cutoff scores, we were able to compare our population with those in other pediatric randomized clinical trials. For example, our study found that at a PCSS cutoff score of 7 points or lower for recovery, 65.8% of patients in the screen time permitted group and 71.1% of patients in the screen time abstinent group experienced resolution of symptoms by day 10. This result is consistent with those reported by Thomas et al,24 who compared 1 to 2 days vs 5 days of rest (with no school, work, or physical activity), finding that 67% and 63% of the patients in the respective cohorts experienced concussion resolution by 10 days after injury using a PCSS cutoff score for resolution of 7 points or lower.
Limitations
This study has limitations. The study was conducted in a single center, which could limit the generalizability of results. We enrolled a convenience sample during hours when study staff was available. Because of pandemic restrictions, enrollment was halted with 15 patients fewer than the 106 participants we calculated a priori to power the Wilcoxon rank sum test, which increases the risk of a type II error. Outcome data are missing from loss to follow-up, although missing data were similar between intervention groups. In addition, we used survival methods that accommodate censored observations, which is an acceptable approach for handling missing data on a time to event outcome variable.35 By ranging our analyses across various PCSS cutoff scores, we were able to report multiple outcomes. We acknowledge that this approach increases the likelihood of a type I error; however, we consider this an acceptable tradeoff because it enables us to compare our results with those of previous studies, replicate these or similar findings in future studies, and further validate the best PCSS cutoff score to define clinically meaningful recovery from concussion.
Our study relied on self-reported screen time, which may be subject to recall or social desirability bias to conform to a group assignment. Only 40 participants in each group completed all 3 screen time surveys, which raises a question of reporting bias for those who adhered to the study protocol, which, if present, could challenge the internal validity of the study. Studies comparing user self-reporting with device-tracking data have reported that participants both overestimate and underestimate actual screen time use.36,37,38 It is worth noting that our screen time abstinent group did engage in 5 to 10 minutes of screen time daily to complete the study surveys.
Future clinical trials might consider exploring the role of screen time in a larger and more diverse population to examine whether the effect of screen time on recovery after concussion is consistent in a multicenter study. It would also be of interest to investigate the effect of specific screen time activities (ie, video games) on concussive symptoms. Screen time tracking applications might be employed to more accurately measure screen time and explore whether there is a dose-dependent effect.39
Conclusions
This randomized clinical trial found that abstaining from screen time in the acute period after concussion may be associated with a shorter duration of symptoms, which supports clinical recommendations to limit screen time in the acute period after concussion. A multicenter study would help to further assess the effect of screen time exposure.
Trial Protocol
eTable. Comparison of Screen Time Activity Between Screen Time Abstinent and Screen Time Permitted Groups in First 3 Calendar Days After Concussion
eFigure. Kaplan-Meier Survival Curves Comparing Days Until Concussion Recovery Between Screen Time Abstinent and Screen Time Permitted Groups at 4 Different Post-Concussive Symptom Scale (PCSS) Cutoff Scores
Data Sharing Statement
References
- 1.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16. doi: 10.15585/mmwr.ss6609a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang AL, Sing DC, Rugg CM, Feeley BT, Senter C. The rise of concussions in the adolescent population. Orthop J Sports Med. 2016;4(8):2325967116662458. doi: 10.1177/2325967116662458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowacki R, van Eldik N, Eikens M, et al. Evaluation of a follow-up program for mild traumatic brain injury in schoolchildren. Eur J Paediatr Neurol. 2017;21(2):382-387. doi: 10.1016/j.ejpn.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 4.DePadilla L, Miller GF, Jones SE, Peterson AB, Breiding MJ. Self-reported concussions from playing a sport or being physically active among high school students—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(24):682-685. doi: 10.15585/mmwr.mm6724a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Recovery from concussion. Centers for Disease Control and Prevention; 2019. Accessed October 12, 2020. https://www.cdc.gov/headsup/basics/concussion_recovery.html.
- 7.DiFazio M, Silverberg ND, Kirkwood MW, Bernier R, Iverson GL. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila). 2016;55(5):443-451. doi: 10.1177/0009922815589914 [DOI] [PubMed] [Google Scholar]
- 8.Arbogast KB, McGinley AD, Master CL, Grady MF, Robinson RL, Zonfrillo MR. Cognitive rest and school-based recommendations following pediatric concussion: the need for primary care support tools. Clin Pediatr (Phila). 2013;52(5):397-402. doi: 10.1177/0009922813478160 [DOI] [PubMed] [Google Scholar]
- 9.Halstead ME, McAvoy K, Devore CD, Carl R, Lee M, Logan K; Council on Sports Medicine and Fitness; Council on School Health . Returning to learning following a concussion. Pediatrics. 2013;132(5):948-957. doi: 10.1542/peds.2013-2867 [DOI] [PubMed] [Google Scholar]
- 10.Schneider KJ, Leddy JJ, Guskiewicz KM, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. 2017;51(12):930-934. doi: 10.1136/bjsports-2016-097475 [DOI] [PubMed] [Google Scholar]
- 11.Halstead ME, Walter KD, Moffatt K; Council on Sports Medicine and Fitness . Sport-related concussion in children and adolescents. Pediatrics. 2018;142(6):e20183074. doi: 10.1542/peds.2018-3074 [DOI] [PubMed] [Google Scholar]
- 12.Rideout V, Robb M. The common sense census: media use by tweens and teens, 2019. Common Sense Media; 2020. Accessed December 20, 2020. https://www.commonsensemedia.org/research/the-common-sense-census-media-use-by-tweens-and-teens-2019
- 13.Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(2):217-223. doi: 10.1136/jnnp-2011-300767 [DOI] [PubMed] [Google Scholar]
- 14.Rivara FP, Graham R. Sports-related concussions in youth: report from the Institute of Medicine and National Research Council. JAMA. 2014;311(3):239-240. doi: 10.1001/jama.2013.282985 [DOI] [PubMed] [Google Scholar]
- 15.Gioia GA, Collins M, Isquith PK. Improving identification and diagnosis of mild traumatic brain injury with evidence: psychometric support for the acute concussion evaluation. J Head Trauma Rehabil. 2008;23(4):230-242. doi: 10.1097/01.HTR.0000327255.38881.ca [DOI] [PubMed] [Google Scholar]
- 16.Gioia GA, Schneider JC, Vaughan CG, Isquith PK. Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br J Sports Med. 2009;43(suppl 1):i13-i22. doi: 10.1136/bjsm.2009.058255 [DOI] [PubMed] [Google Scholar]
- 17.Zuckerbraun NS, Atabaki S, Collins MW, Thomas D, Gioia GA. Use of modified acute concussion evaluation tools in the emergency department. Pediatrics. 2014;133(4):635-642. doi: 10.1542/peds.2013-2600 [DOI] [PubMed] [Google Scholar]
- 18.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84. doi: 10.1016/s0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schutzman S. Patient education: head injury in children and adolescents (beyond the basics). UpToDate; 2018. Accessed February 1, 2018. https://www.uptodate.com/contents/head-injury-in-children-and-adolescents-beyond-the-basics
- 21.Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132(1):8-17. doi: 10.1542/peds.2013-0432 [DOI] [PubMed] [Google Scholar]
- 22.Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132-1140. doi: 10.1001/jamapediatrics.2015.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haider MN, Leddy JJ, Pavlesen S, et al. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med. 2018;52(18):1179-1190. doi: 10.1136/bjsports-2016-096551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223. doi: 10.1542/peds.2014-0966 [DOI] [PubMed] [Google Scholar]
- 25.Iverson GL, Lovell MR, Collins MW. Interpreting change on ImPACT following sport concussion. Clin Neuropsychol. 2003;17(4):460-467. doi: 10.1076/clin.17.4.460.27934 [DOI] [PubMed] [Google Scholar]
- 26.Lau BC, Collins MW, Lovell MR. Sensitivity and specificity of subacute computerized neurocognitive testing and symptom evaluation in predicting outcomes after sports-related concussion. Am J Sports Med. 2011;39(6):1209-1216. doi: 10.1177/0363546510392016 [DOI] [PubMed] [Google Scholar]
- 27.Zemek R, Barrowman N, Freedman SB, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014-1025. doi: 10.1001/jama.2016.1203 [DOI] [PubMed] [Google Scholar]
- 28.Brown NJ, Mannix RC, O’Brien MJ, Gostine D, Collins MW, Meehan WP III. Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299-e304. doi: 10.1542/peds.2013-2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montagni I, Guichard E, Carpenet C, Tzourio C, Kurth T. Screen time exposure and reporting of headaches in young adults: a cross-sectional study. Cephalalgia. 2016;36(11):1020-1027. doi: 10.1177/0333102415620286 [DOI] [PubMed] [Google Scholar]
- 30.Eckner JT, Seifert T, Pescovitz A, Zeiger M, Kutcher JS. Is migraine headache associated with concussion in athletes? a case-control study. Clin J Sport Med. 2017;27(3):266-270. doi: 10.1097/JSM.0000000000000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moor HM, Eisenhauer RC, Killian KD, et al. The relationship between adherence behaviors and recovery time in adolescents after a sports-related concussion: an observational study. Int J Sports Phys Ther. 2015;10(2):225-233. [PMC free article] [PubMed] [Google Scholar]
- 32.Grool AM, Aglipay M, Momoli F, et al. ; Pediatric Emergency Research Canada (PERC) Concussion Team . Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316(23):2504-2514. doi: 10.1001/jama.2016.17396 [DOI] [PubMed] [Google Scholar]
- 33.Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2016;31(4):233-241. doi: 10.1097/HTR.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50-58. doi: 10.1016/j.smrv.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharkow M. The accuracy of self-reported internet use—a validation study using client log data. Commun Methods Meas. 2016;10(1):13-27. doi: 10.1080/19312458.2015.1118446 [DOI] [Google Scholar]
- 37.Araujo T, Wonneberger A, Neijens P, de Vreese C. How much time do you spend online? understanding and improving the accuracy of self-reported measures of internet use. Commun Methods Meas. 2017;11(3):173-190. doi: 10.1080/19312458.2017.1317337 [DOI] [Google Scholar]
- 38.Orben A, Przybylski AK. Screens, teens, and psychological well-being: evidence from three time-use-diary studies. Psychol Sci. 2019;30(5):682-696. doi: 10.1177/0956797619830329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11(11):e0165331. doi: 10.1371/journal.pone.0165331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Comparison of Screen Time Activity Between Screen Time Abstinent and Screen Time Permitted Groups in First 3 Calendar Days After Concussion
eFigure. Kaplan-Meier Survival Curves Comparing Days Until Concussion Recovery Between Screen Time Abstinent and Screen Time Permitted Groups at 4 Different Post-Concussive Symptom Scale (PCSS) Cutoff Scores
Data Sharing Statement