Abstract
Hypoxia, the most common feature in the tumor microenvironment, is closely related to tumor malignant progression and poor patient’s prognosis. Exosomes, initially recognized as cellular “garbage dumpsters”, are now known to be important mediums for mediating cellular communication in tumor microenvironment. However, the mechanisms of hypoxic tumor cell-derived exosomes facilitate colorectal cancer progression still need further exploration. In the present study, we found that exosomes from hypoxic colorectal cancer cells (H-Exos) promoted G1-S cycle transition and proliferation while preventing the apoptosis of colorectal cancer cells by transmitting miR-210-3p to normoxic tumor cells. Mechanistic investigation indicated that miR-210-3p from H-Exos elicited its protumoral effect via suppressing CELF2 expression. A preclinical study further confirmed that H-Exos could promote tumorigenesis in vivo. Clinically, the expression of miR-210-3p in circulating plasma exosomes was markedly upregulated in colorectal cancer patients, which were closely associated with multiple unfavorable clinicopathological features. Taken together, these results suggest that hypoxia may stimulate colorectal cancer cells to secrete miR-210-3p-enriched exosomes in tumor microenvironment, which elicit protumoral effects by inhibiting CELF2 expression. These findings provide new insights on the mechanism of colorectal cancer progression and potential therapeutic targets for colorectal cancer.
Keywords: Colorectal cancer, hypoxia, exosomes, miR-210-3p, CELF2, progression
Impact statement
This work expanded the molecular mechanisms underlying colorectal cancer (CRC) progression by exploring the role of hypoxic CRC cells-secreted exosomal miR-210-3p in the viability of normoxic CRC cells. The results showed that exosomes from hypoxic CRC cells could promote cell cycle progression and proliferation while inhibiting the apoptosis of CRC cells by transmitting miR-210-3p to normoxic tumor cells. Further mechanistic investigation indicated that miR-210-3p from hypoxic CRC cell exosomes targeted Dicer 3′ UTR of CELF2 mRNA for silencing, thereby eliciting a protumoral effect. These findings indicate that exosomal miR-210-3p acts as a CRC oncogene via directly suppressing tumor suppressor gene, providing new insights into the mechanism of CRC progression.
Introduction
According to GLOBOCAN 2018 data, colorectal cancer (CRC) is the third most commonly diagnosed cancer but the second leading cause of cancer death worldwide. 1 Over the past few decades, although great improvement has been made in screening programs, diagnosis, and treatment strategies, the therapeutic effect and overall prognosis of CRC still fail to be satisfactory.2,3 Therefore, exploring and uncovering the potential mechanisms underlying the development and progression of CRC continue to be important.
Increasing and accumulating evidence indicates that the occurrence and development of CRC are remarkedly influenced by the local tumor microenvironment (TME).4,5 Hypoxia, as a well-recognized feature of TME, is defined that the oxygen pressure in tissues is less than 5 to 10 mm Hg.6,7 Numerous studies have shown that hypoxia is essential for tumor progression.8,9 The hypoxic TME plays master regulatory functions in each step of tumor development, from tumor initiation to the ultimate metastatic colonization, and the processes it regulates include tumor proliferation, apoptosis, migration, invasion, and metastasis.6,10,11 On one hand, hypoxic tumor cells can educate TME by secreting a variety of biologically active substances, thereby creating a favorable environment for self-growth;12,13 on the other hand, hypoxic tumor cells can also regulate the biological behaviors of tumor cells in normoxic region to promote tumor progression.14,15 Thus, in-depth exploration of the mechanisms by which hypoxia regulates tumor progression will help us to further understand the process of CRC development.
Exosomes, as one of small extracellular vesicles, is composed of 30 to 200 nm lipid bilayer, which is released by nearly all types of cells and is initially recognized as cellular “garbage dumpsters”.16,17 Currently, exosomes are identified as important mediators for cellular communication, 18 which can transport multiple biological components from parental cells to recipient cells, thereby influencing recipient cell functions.16,17 Interestingly, a growing body of studies has indicated that hypoxia can enhance the malignant state of tumor cells to alter the release and contents of exosomes from tumors.19,20 In hypoxic conditions, tumor cells can remodel TME to support tumor growth by establishing communication with surrounding stromal cells in TME by secreting large amounts of exosomes.19,20 Hsu et al. found that hypoxia exosomes-delivered miRNAs promoted angiogenesis and vascular permeability of endothelial cells in lung cancer. 21 More recently, numerous studies have shown that exosomes-derived from hypoxic cancer cells are effective inducers of immunosuppression by promoting M2-subtype polarization of macrophages, and this includes exosomes derived from glioma, 22 lung cancer, 23 pancreatic cancer, 24 and epithelial ovarian cancer. 25 These results provide ample evidence presenting the important role of tumor-derived exosomes in mediating hypoxia-induced TME evolution. In fact, although there are a number of stromal cells in TME, tumor cells are still the predominant cellular component. Therefore, it is reasonably hypothesized that hypoxic tumor cells can also modulate the biological behaviors of normal tumor cells by delivering exosomes, thereby promoting CRC progression.
In this study, we explored the role of exosomes derived from hypoxic CRC cells (H-Exos) on the proliferation and apoptosis of normoxic CRC cells in TME. Our results demonstrated that H-Exos could promote G1-S cell cycle transition and proliferation while inhibiting the apoptosis of CRC cells. Further mechanistic dissection indicated that H-Exos altered normoxic CRC cells toward protumoral activity by delivering oncogenic microRNA-210-3p (miR-210-3p) to suppress the expression of CUGBP, Elav-like family member 2 (CELF2). Clinically, the levels of circulating plasma exosomal miR-210-3p were markedly upregulated in CRC patients, which were closely associated with multiple unfavorable clinicopathological features of CRC patients. Together, these results provide a novel insight on the mechanism of CRC progression and potential therapeutic targets for CRC.
Materials and methods
Patients and clinical samples
Fifty CRC patients who were hospitalized at Zhongnan Hospital of Wuhan University were recruited for this study. Inclusion criteria: (1) pathologically diagnosed with CRC; (2) undergone radical surgery; and (3) intact clinicopathological data. Exclusion criteria: (1) other malignant tumors and (2) antitumor treatment before surgery. Meanwhile, 25 healthy donors were recruited for control. A total of 5 mL peripheral blood were collected from CRC patients and placed in anticoagulant tubes before and after surgery, and equal volumes of blood samples were collected from healthy donors. All samples were processed within 2 h after collection. This study was conducted with the approval from the Medical Ethics Committees of Zhongnan Hospital of Wuhan University, and all included objects provided written informed consent before sample collection.
Cell culture and hypoxia treatment
The human CRC cell line HCT116 was grown in RPMI 1640 (Gibco, USA) medium containing 10% fetal bovine serum (FBS; HyClone, USA) and incubated in a 5% CO2, 37°C incubator. Cells were cultured in a hypoxic incubator (Thermo Scientific, USA) containing a low oxygen concentration (1% O2, 5% CO2 and 94% N2) for hypoxia treatment.
Exosome isolation and identification
For exosome isolation, HCT116 cells were cultured in RPMI 1640 medium containing 10% exosome-depleted FBS (SBI, USA) under normoxic (20% O2) or hypoxic (1% O2) conditions. Then, the cell culture medium from normoxic and hypoxic HCT116 cells was harvested after two days, and exosomes were isolated using ExoQuick reagent (SBI) according to the manufacturer’s instructions. Briefly, ExoQuick reagent were incubated with culture medium (1:5) for over 12 h, and then centrifuged at 1500g for 30 min; pelleted exosomes were resuspended in 100 μL PBS and stored at −80°C for further use. In addition, plasma exosomes were isolated by using the total exosome isolation reagent (Magen, China). The reagent was added to the serum samples and incubated at 4°C for 30 min. After that, the plasma samples were centrifuged at 2000g for 30 min to remove cells and debris. Exosomes were obtained by centrifugation at 10,000g for 10 min and resuspended in PBS. Hypoxic or normoxic CRC cell-derived exosomes were named H-Exos or N-Exos, respectively. A bicinchoninic acid protein assay kit (Millipore, USA) was used to measure the protein content of exosomes, and a microfiltration ExoMir PLUS Kit (Bio Scientific, USA) was applied to deplete the exosomes from the culture medium.
Exosomes were first identified by transmission electron microscopy (TEM). Briefly, exosomes were suspended in glutaraldehyde, dropped in carbon-coated copper grids, stained with 2% uranyl acetate, dried, and imaged. In addition, the size and concentration of exosomes were analyzed using a Nanosight LM10 System (Nanosight Ltd), which was equipped with fast video capture and particle-tracking software by measuring the rate of Brownian motion to calculate nanoparticle concentrations and size distribution. Moreover, exosomes were further analyzed by Western blot to detect the expression of CD9, CD63, and TSG101 protein, while tubulin was used as a control.
Exosome labeling and tracking
Purified exosomes labeled with PKH67 were resuspended and cultured with unstained HCT116 cells for exosome uptake experiments. After incubation for 30 min, 2 h, or 12 h at 37°C, HCT116 cells were stained with DiIC16 (Sigma-Aldrich) and DAPI (BioGems, USA) and then imaged under a fluorescence microscope.
Cell transfection
MiR-210-3p mimic, inhibitor, and corresponding control were purchased from Ribo (Guangzhou, China). The pcDNA-3.1-CELF2 and blank plasmids were obtained from GenePharma (Shanghai, China). Cell transfection was conducted with Lipofectamine 2000 (Invitrogen, USA) when cells reached to 80% confluence in 6-well plates.
Quantitative RT-PCR
Total RNA was extracted by TRIzol reagent (Invitrogen). The reverse transcription of mRNA or miRNA was conducted with a reverse transcription kit (Takara, Japan) or Bulge-Loop miRNA RT-PCR Starter Kit (Ribio), respectively. Quantitative RT-PCR (qRT-PCR) was carried out using SYBR Green PCR Mix (Takara). The relative mRNA and miRNA expression levels were calculated with the 2−ΔΔCt method. GAPDH or U6 was used as a normalization control for miRNA or mRNA. The relative primers were as follows: miR-210-3p, RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGCC‑3′; miR-210-3p, F: 5′-CTGTGCGTGTGACAG‑3', R: 5′-GTGCAGGGTCCGAGGT‑3′; CELF2, F: 5′-TTTGAGCCTTACGGAGCCG‑3′, R: 5′-AACAACCTTTACTCTGCGGAG‑3′; U6, F: 5′-ATTGGAACGATACAGAGAAGATT-3′, R: 5′-GGAACGCTTCACGAATTTG-3′; and GAPDH, F: 5′-GGAGCGAGATCCCTCCAAAAT-3′, R: 5′-GGCTGTTGTCATACTTCTCATGG-3′.
Western blot
Samples were lysed with RIPA lysis buffer (Beyotime, China) supplemented with protease inhibitor cocktail (Roche, Switzerland), and the concentrations were determined with a BCA Protein Assay Kit (Beyotime). An 8%–10% SDS acrylamide gel was used for detecting CD9 (Abcam, UK), CD63 (Abcam), TSG101 (Abcam), Tubulin (Abcam), HIF-1α (Proteintech, USA), CELF2 (Proteintech, USA), and GAPDH (Abcam).
Cell proliferation and apoptosis assays
To assess proliferation, a cell counting kit (CCK-8) assay was conducted. Cells were plated in 96-well plates, and 10 μL of CCK-8 (Sangon Biotech, China) was added into each well and cultured for 2 h. The absorbance value was analyzed with a Spectra microplate reader (BioTek, USA). For the apoptosis assay, cells were digested with 0.25% trypsin without EDTA and stained with a FITC Annexin V/PI apoptosis detection kit (DOJINDO, Japan). Briefly, cells were collected after transfection and stained with propidium iodide/FITC Annexin V for 10 min at 4°C in the dark, followed by the addition of the binding buffer and then analysis by flow cytometry (FACS Canto II, BD Company, USA) within 10 min.
Cell cycle analysis
After pre-treatment or transfection, CRC cells were cultured in serum-free medium and then fixed with 70% ethanol. The cells were treated with 2 mg/mL of bovine pancreatic RNase (Sigma) and then incubated in propidium iodide (20 mg/mL; Sigma) for in the dark. Cell analysis was conducted with FACScan (Becton-Dickinson, Franklin Lakes, NJ, USA), while quantification of G0/G1, S, and G2/M phase with ModFit software (Becton-Dickinson).
Luciferase reporter assay
Wild-type or mutant CELF2 3′ UTR psiCHECK-2 plasmid (Promega, USA) and miR-210-3p mimic or control and miR-210-3p inhibitor or control (RiboBio) were cotransfected with HCT116 cells by using Lipofectamine 3000 (Invitrogen). Additionally, HCT116 cells were treated with RNA isolates from H-Exos (exosRNA) or treated by concomitating use of antagomiRNA (RiboBio) with exoRNA isolates. After transfection or treatment for 48 h, according to the manufacturer’s instructions, the luciferase activity of cells was measured by using a dual luciferase reporter assay kit (Promega), and Renilla luciferase activity was used for normalization.
Immunohistochemistry
Tumor samples from xenograft models were fixed with formalin and embedded in paraffin. Then, tissue sections with a thickness of 4 μm were obtained from paraffin-embedded samples. After dewaxing, rehydration, and antigen retrieval, sections were incubated with CELF2 antibody (1:200 dilution; BD Biosciences, USA) and Ki-67 antibody (1:200 dilution, Cell Signalling Technology, USA) at 4°C overnight. Then, sections were incubated with secondary biotinylated antibody for 30 min at 37°C and finally visualized with DAB solution and counterstained with hematoxylin. Pictures were taken under a light microscope, and the proportion of positively stained tumor cells was independently assessed by two observers.
Animal experiments
BALB/c female nude mice were housed in the Animal Laboratory Unit of Zhongnan Hospital of Wuhan University (Wuhan, China). Twelve mice were randomly divided into three groups (four mice per group), and 3 × 106 HCT116 cells were subcutaneously injected into the hind limb region of each mouse. After 10 days, N-Exos or H-Exos mixed with 200 μL PBS were injected into the implanted tumor. Thirty days later, the mice were euthanized. The xenograft tumors were collected, and the weight and volume of tumors were calculated. The animal experiments were conducted under the approval from the Institutional Animal Care and Use Committee of Zhongnan Hospital of Wuhan University.
Statistical analysis
SPSS 22.0 was used for data analyses, and data visualization was conducted by using GraphPad Prism 6.0. Continuous data are presented as the means ± SD and were compared by using Student’s t-test or analysis of variance, as appropriate. The association between plasma exosomal miR-210-3p expression and clinicopathological parameters was analyzed using the Pearson χ2 test. P < 0.05 was defined as a statistically significant difference.
Results
H-Exos promotes the proliferation and cycle transition while inhibiting the apoptosis of normoxic tumor cells in vitro
To examine the effect of hypoxia on exosomes release, identical numbers of HCT116 cells were cultured under normoxia and hypoxia (1% O2). HIF-1α, a classic hypoxic responsive gene, was used to verify the hypoxic response in CRC cells. Western blot found that HIF-1α expression in hypoxia CRC cells was significantly upregulated compared with normoxia cells, indicating that 1% O2 could induce CRC cells to exhibit the hypoxic status (Figure S1). Then, H-Exos and N-Exos were isolated and further quantitated by TEM, NTA, and Western blot. TEM presented that both H-Exos and N-Exos presented typical rounded particles ranging from 50 to 150 nm in diameter, and NTA exhibited a similar size distribution and concentrations of exosomes (Figure 1(a) and (b)). Western blot showed the presence of the well-known exosome marker proteins CD9, CD63, and TSG101 (Figure 1(c)). Moreover, TEM, NTA, and Western blot demonstrated that the concentrations of exosomes harvested from hypoxic HCT116 cells were significantly higher than those from the normoxic control (Figure 1(a) to (c)). Altogether, these data indicate that hypoxia promotes the exosomes secretion from CRC cells.
Figure 1.
Hypoxia induces exosome secretion from CRC cells to promote proliferation while inhibiting apoptosis in normoxic tumor cells in vitro. (a) Transmission electron microscopy images of exosomes isolated from conditioned medium of HCT116 cells under normoxia (N-Exos) and hypoxia (H-Exos); Scale bar, 200 nm. (b) NTA of N/H-Exos isolated by ultracentrifugation. (c) Western blot analysis for exosomal proteins CD9, CD63, and TSG101. Tubulin in cell extracts was used as a control for cell density. (d) Flow cytometry analyzed the effect of H/N-Exos on the cell cycle of normoxic CRC cells. (e) CCK-8 assay detected the effect of H/N-Exos on the proliferation of normoxic CRC cells. (f) Annexin V-FITC/PI assay detected the effect of H/N-Exos on the apoptosis of normoxic CRC cells. (g–i) The effect of H-Exos depletion on the cell cycle, proliferation, and apoptosis of normoxic CRC cells. Each experiment was carried out at least three times. *P < 0.05; **P < 0.01; and ***P < 0.001. (A color version of this figure is available in the online journal.)
Then, the effects of H-Exos on cell cycle, proliferation, and apoptosis of normoxic CRC cells were explored. Flow cytometry analysis showed that H-Exos markedly decreased G0/G1 peak cells percentage and increased S peak cells, but did not affect the G2/M phase cells ratio (Figure 1(d)). Additionally, CCK8 assays showed that H-Exos significantly enhanced the proliferation of CRC cells (Figure 1(e)). In contrast, cell apoptosis was significantly decreased by H-Exos compared with N-Exos (Figure 1(f)). Moreover, the exosomes of conditioned media from hypoxic HCT116 cells were depleted by a microfilter and then added exosome-free conditioned media to HCT116 cells cultured under 20% O2. Further functional experiments demonstrated that depletion of exosomes reduced the enhancement effect of H-Exos on the cell cycle (Figure 1(g)) and proliferation of normoxic HCT116 cells (Figure 1(h)) while reducing the inhibitory effect of H-Exos on the apoptosis of normoxic HCT116 cells (Figure 1(i)). Collectively, these results suggest that H-Exos can promote G1 to S phase transition and proliferation while inhibiting the apoptosis of normoxic CRC cells in vitro.
H-Exos-encapsulated miR-210-3p can be transferred to normoxic CRC cells
It has been proven that miRNAs delivered from exosomes play important roles in exosome-mediated intercellular communication.26,27 Previous study has demonstrated that hypoxia could up-regulate the expression of miR-210-3p in CRC cells..28,29 Therefore, we speculated whether H-Exos regulate the behaviors of normoxic cells by transferring miR-210-3p. We first detected the levels of miR-210-3p in equal weights of H-Exos and N-Exos, and found that the levels of miR-210-3p in H-Exos were significantly higher than those in N-Exos (Figure S2(a)). Further results showed that the levels of mature miR-210-3p were obviously elevated (Figure 2(a)), but the levels of pri-/pre-miR-210-3p had no significant change in recipient CRC cells after treatment with H-Exos (Figure 2(b)). Consistently, the levels of pri/pre-miR-210-3p were also not significantly different in H-Exos or N-Exos (Figure S2(b)). In addition, after treatment with RNase A+Triton-X, H-Exos failed to increase the levels of miR-210-3p in recipient CRC cells compared with the control and RNase A alone groups (Figure 2(c)). Treatment of recipient cells with RNA polymerase II inhibitor did not affect the increase of miR-210-3p in recipient CRC cells mediated by H-Exos (Figure 2(c)). The above results demonstrate that miR-210-3p is protected by H-Exos and that increased cellular levels of miR-210-3p in normoxic CRC cells arise from H-Exos-mediated miRNA transport but not endogenous miR-210-3p induction. Furthermore, when normoxic CRC cells were incubated with exosomes derived from normoxic or hypoxic HCT116 cells transfected with miR-210-3p inhibitor, the levels of miR-210-3p in recipient CRC cells were no longer increased (Figure 2(d)). Additionally, in the treatment of normoxic CRC cells with PKH67-labeled N-Exos or H-Exos, PKH67 green fluorescence was observed in the recipient cells (Figure 2(e)). Taken together, these data indicate that H-Exos-encapsulated miR-210-3p can be transferred to normoxic CRC cells.
Figure 2.
miR-210-3p is highly enriched in H-Exos and can be transferred to normoxic CRC cells via exosomes. (a) qRT-PCR analysis of mature miR-210 levels in normoxic HCT116 cells treated with N/H-Exos. (b) qRT-PCR analysis of pri/pre miR-210 levels in normoxic HCT116 cells treated with N/H-Exos. (c) qRT-PCR analysis of the effect of RNase, RNase A+Triton-X, and RNA POLY II inh on miR-210 expression in normoxic HCT116 cells mediated by N/H-Exos. (d) qRT-PCR analysis of mature miR-210 levels in normoxic HCT116 cells treated with exosomes derived from hypoxic or normoxic CRC cells transfected with miR-210 inhibitor. (e) Immunofluorescence staining showing the uptake of H-Exos in normoxic HCT116 cells; Scale bar, 50 μm. Each experiment was carried out at least three times. ***P < 0.001. n.s.: not significant. (A color version of this figure is available in the online journal.)
H-Exos-transferred miR-210-3p promotes the cell cycle transition and proliferation while inhibiting the apoptosis in normoxic CRC cells in vitro
The effect of miR-210-3p on the proliferation and apoptosis of normoxic CRC cells was further evaluated. First, hypoxic HCT116 cells were transfected with miR-210-3p inhibitor to reduce the levels of miR-210-3p in H-Exos, and then H-Exos was used to treat normoxic CRC cells. Further functional experiments showed that the cell cycle and proliferation-promoting effect and apoptosis-inhibiting effect of H-Exos on normoxic CRC cells were prevented by transfecting hypoxic HCT116 cells with a miR-210-3p inhibitor (Figure 3(a) to (c)). Moreover, the effect of miR-210-3p on the biological behaviors of normoxic CRC cells was further validated by transfecting miR-210-3p mimic into recipient CRC cells. The results demonstrated that transfection of the miR-210-3p mimic accelerated G1-S cell cycle transition and enhanced the proliferative ability while inhibiting the apoptosis of normoxic CRC cells compared to those cells transfected with control mimics (Figure 3(d) to (f)). Taken together, H-Exos-transferred miR-210-3p promotes the cell cycle transition and proliferation while inhibiting the apoptosis in normoxic CRC cells in vitro.
Figure 3.
H-Exos-transferred miR-210-3p promotes proliferation while inhibiting apoptosis in normoxic CRC cells in vitro. (a) Transfection of miR-210 inhibitor prevented the effect of H-Exos on the cell cycle-promoting effect of HCT116 cells. (b) Transfection of miR-210 inhibitor prevented the effect of H-Exos on the promotion of HCT116 proliferation. (c) Transfection of miR-210 inhibitor prevented the effect of H-Exos on the inhibition of HCT116 apoptosis. (d) Transfection with a miR-210 mimic accelerated G1-S cell cycle transition of normoxic HCT116 cells. (e) Transfection with a miR-210 mimic enhanced the proliferation of normoxic HCT116 cells. (f) Transfection with a miR-210 mimic reduced the apoptosis of normoxic HCT116 cells. Each experiment was carried out at least three times. **P < 0.01, ***P < 0.001. n.s.: not significant. (A color version of this figure is available in the online journal.)
miR-210-3p promotes cell cycle transition and proliferation while inhibiting apoptosis in normoxic CRC cells by directly targeting CELF2
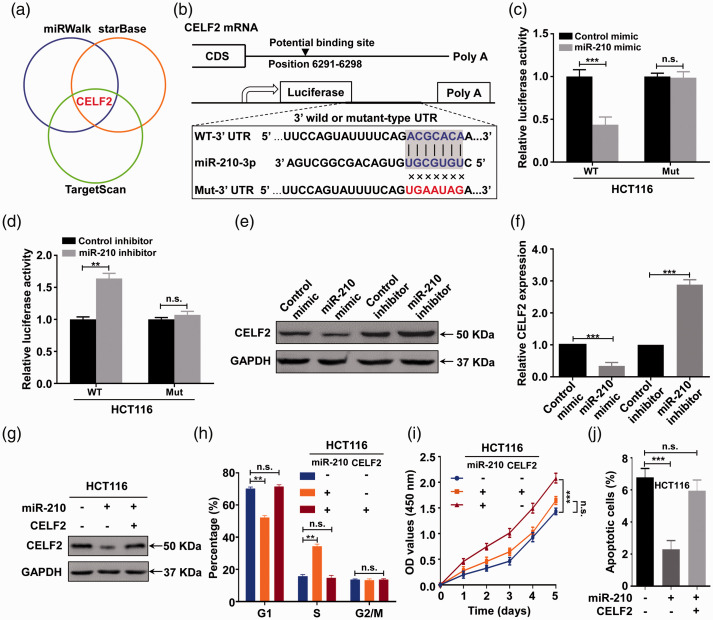
A growing body of evidence indicates that miRNAs perform their functions mainly by suppressing downstream genes. 30 Thus, we performed in silico analyses to determine possible targets of miR-210-3p by using three predicted miRNA online databases, including TargetScan, miRWalk, and starBase (Figure 4(a)). Among these predicted common targets, CELF2 has been shown to be a tumor suppressor gene and was therefore selected as the candidate object. 31 To confirm that CELF2 was the direct target of miR-210-3p, luciferase reporter assays were first performed in normoxic CRC cells after confirming the potential binding site of miR-210-3p in the 3′ UTR of CELF2 mRNA (Figure 4(b)). The results showed that the luciferase activity was significantly inhibited when miR-210-3p mimics were cotransfected with the wild-type luciferase reporters, while the relative luciferase activity was noticeably increased when miR-210-3p inhibitors were used. However, the above effects were abolished when a plasmid carrying a mutated sequence was used instead (Figure 4(c) and (d)). In order to further verify the above results, RNA extracted from H-Exos was used to pretreat HCT116 cells, the results found that the luciferase activity of HCT116 cells in wild-type group was significantly reduced after treatment, but the mutant-type group had no significant change. Furthermore, when HCT116 cells were treated by concomitating use of antagomiRNA with exoRNA isolates, the inhibition of luciferin activity mediated by exoRNA isolates was reversed (Figure S3). These data demonstrate that miR-210-3p can directly bind to the 3′ UTR of CELF2 mRNA. Moreover, Western blot results showed that the miR-210-3p mimic down-regulated CELF2 protein expression, while the miR-210-3p inhibitor presented the opposite effect (Figure 4(e)). Consistent with the Western blot results, CELF2 mRNA was downregulated by the miR-210-3p mimic but upregulated by the miR-210-3p inhibitor (Figure 4(f)). Together, these results suggest that miR-210-3p suppresses CELF2 expression in CRC cells in post-transcription level by directly targeting its 3′ UTR to degrade it.
Figure 4.
miR-210-3p promotes proliferation while inhibiting apoptosis in normoxic CRC cells by directly targeting CELF2. (a) CELF2 was predicted as a potential target of miR-210-3p via three miRNA databases, including miRWalk, starBase, and TargetScan. (b) A schematic representation of the predicted duplex sequences between the WT or Mut 3ʹ UTR of CELF2 mRNA and miR-210-3p. (c) Relative luciferase activity of the CELF2 3′ UTR in HCT116 cells cotransfected with the indicated reporters and miR-210-3p mimic oligonucleotides. (d) Relative luciferase activity of the CELF2 3′ UTR in HCT116 cells cotransfected with the indicated reporters and miR-210-3p inhibitor oligonucleotides. (e) Western blot detection of CELF2 protein expression in HCT116 cells transfected with miR-210-3p mimic and miR-210-3p inhibitor. (f) qRT-PCR analysis of CELF2 mRNA expression in HCT116 cells transfected with miR-210-3p mimic and miR-210-3p inhibitor. (g) Western blot analysis of CELF2 protein expression in HCT116 cells transfected with the CELF2 plasmid or negative control, along with a miR-210-3p mimic or corresponding control. (h) The cell cycle change of HCT116 cells transfected with CELF2 plasmid or a negative control, along with miR-210-3p mimic or corresponding control, as determined by flow cytometry analysis. (i) The cell proliferation capacity of HCT116 cells transfected with CELF2 plasmid or a negative control, along with miR-210-3p mimic or corresponding control, as determined by CCK-8 assay. (j) Apoptosis rate of pretreated HCT116 cells transfected with CELF2 plasmid or a negative control, along with miR-210-3p mimic or corresponding control, as determined by Annexin V-FITC/PI assay. Each experiment was carried out at least three times. **P < 0.01, and ***P < 0.001. n.s.: not significant. (A color version of this figure is available in the online journal.)
To further evaluate whether CELF2 was required for the effect of miR-210-3p on the malignant behaviors of normoxic CRC cells, CELF2 plasmid and miR-210-3p mimic were cotransfected into normoxic HCT116 cells. Western blot results showed that the repression of CELF2 mediated by the miR-210-3p mimic could be partly attenuated by CELF2 upregulation (Figure 4(g)). Further, overexpression of CELF2 could partly attenuate the promotive effects on cell cycle and proliferation, and the inhibitive function on cell apoptosis induced by miR-210-3p overexpression in normoxic HCT116 cells (Figure 4(h) to (j)). Collectively, these data suggest that miR-210-3p promotes cell cycle progression and proliferation while inhibiting the apoptosis of normoxic CRC cells by directly suppressing CELF2 expression.
H-Exos promotes the tumorigenesis of normoxic CRC cells in vivo
Furthermore, the effect of H-Exos on the tumorigenesis of normoxic CRC cells in vivo was investigated via a nude mouse xenograft model. As shown in Figure 5(a), morphological analysis of subcutaneous xenografts found that treatment with H-Exos significantly promoted tumor growth in nude mice compared with N-Exos or the control group. Further results showed that the tumor volume and tumor weight were significantly increased in the H-Exos group (Figure 5(b) and (c)). Then, qRT-PCR and IHC showed that the expression levels of CELF2 mRNA and protein were significantly downregulated in tumor tissues from the H-Exos group (Figure 5(d) and (e)). Moreover, IHC staining revealed that the Ki-67 index in tumor tissues was obviously increased in the H-Exos treatment group (Figure 5(f)). Moreover, the expression level of Cleaved Caspase-3 protein, an indicator of cell apoptosis, was significantly downregulated in tumors from H-Exos group (Figure 5(g)). Taken together, these results suggest H-Exos promotes normoxic CRC cells tumorigenesis via down-regulating CELF2 expression in vivo.
Figure 5.
H-Exos promote the tumorigenesis of normoxic CRC cells in vivo. (a) Morphological images of tumor xenografts in the HCT116 alone, HCT116+N-Exos, and HCT116+H-Exos groups. (b) Tumor volume and (c) tumor weight of xenografts in the HCT116 alone, HCT116+N-Exos, and HCT116+H-Exos groups. (d) qRT-PCR analysis of CELF2 mRNA expression in tumors from the HCT116 alone, HCT116+N-Exos, and HCT116+H-Exos groups. (e, f, and g) IHC detection of CELF2, Ki-67 and Cleaved Caspase-3 protein expression in tumors from the HCT116 alone, HCT116+N-Exos, and HCT116+H-Exos groups, respectively. Scale bars, 100 µm. Each in vitro experiment was carried out at least three times. ***P < 0.001. n.s.: not significant. (A color version of this figure is available in the online journal.)
Upregulation of miR-210-3p levels in plasma exosomes is related to CRC progression
Next, we determined the levels of miR-210-3p expression in plasma exosomes from CRC patients. qRT-PCR results showed that the expression levels of miR-210-3p in plasma exosomes of CRC patients were significantly upregulated compared to those of healthy donors (t = 9.222, P < 0.001; Figure 6(a)). Further analyses found that exosomal miR-210-3p was markedly increased in tumors with low differentiation (t = 4.169, P < 0.001; Figure 6(b)) and advanced TNM stage (t = 5.345, P < 0.001; Figure 6(c)). After radical surgery to remove the tumor, plasma exosomal miR-210-3p level was significantly decreased (t = 9.981, P < 0.001; Figure 6(d)).
Figure 6.
Upregulation of miR-210-3p levels in plasma exosomes is associated with CRC progression. (a) qRT-PCR detection of miR-210-3p levels in plasma exosomes from healthy donors and CRC patients. (b, c) qRT-PCR analysis of miR-210-3p levels in plasma exosomes from CRC with different tumor grades and TNM stages. (d) The effect of radical surgery on the miR-210-3p levels in plasma exosomes from CRC. Each experiment was carried out at least three times. (A color version of this figure is available in the online journal.)
Furthermore, based on the median level of miR-210-3p expression, included CRC patients were divided into high (n = 25) and low groups (n = 25). The Chi square test showed that upregulation of miR-210-3p expression in plasma exosomes was significantly associated with poor tumor differentiation (P = 0.023), lymphovascular invasion (P = 0.004), perineural invasion (P = 0.018), late N stage (P = 0.011), and advanced TNM stage (P = 0.002) but not with gender, age, tumor site, or T stage (P > 0.05, respectively; Table 1). Together, these results indicate that upregulation of miR-210-3p expression in plasma exosomes is related to CRC progression.
Table 1.
Correlation between miR-210-3p levels in plasma exosomes and clinicopathological characteristics in patients with CRC (n = 50).
| Parameter | Case | miR-210-3p expression |
χ2 value | P value | |
|---|---|---|---|---|---|
| Low (n = 25) | High (n = 25) | ||||
| Gender | 0.368 | 0.544 | |||
| Male | 34 | 18 | 16 | ||
| Female | 16 | 7 | 9 | ||
| Age, years | 0.725 | 0.395 | |||
| ≤60 | 23 | 10 | 13 | ||
| >60 | 27 | 15 | 12 | ||
| Tumor site | 0.739 | 0.390 | |||
| Colon | 29 | 16 | 13 | ||
| Rectum | 21 | 9 | 12 | ||
| Tumor differentiation | 5.195 | 0.023* | |||
| Middle and high | 22 | 15 | 7 | ||
| Low | 28 | 10 | 18 | ||
| Lymphovascular invasion | 8.333 | 0.004* | |||
| Absence | 30 | 20 | 10 | ||
| Presence | 20 | 5 | 15 | ||
| Perineural invasion | 5.556 | 0.018* | |||
| Absence | 32 | 20 | 12 | ||
| Presence | 18 | 5 | 13 | ||
| T stage | 1.587 | 0.208 | |||
| T1 and T2 | 14 | 9 | 5 | ||
| T3 and T4 | 36 | 16 | 20 | ||
| N stage | 6.522 | 0.011* | |||
| N0 and N1 | 23 | 16 | 7 | ||
| N2 and N3 | 27 | 9 | 18 | ||
| TNM stage | 9.680 | 0.002* | |||
| I and II | 25 | 18 | 7 | ||
| III | 25 | 7 | 18 | ||
CRC: colorectal cancer; TNM: tumor-node-metastasis.
*P<0.05.
Discussion
Exosomes, originally described in the 1980s, were considered at the time to be a kind of nonbiological garbage waste excreted by cells. 17 Increasing studies have gradually suggested that exosomes are biologically active small molecules that are widely involved in regulating various pathophysiological processes of the human body.16,17 In particular, exosome-mediated tumor progression have become a hot spot in the cancer research field16,18,19 A growing body of evidence indicates that exosomes encompass multiple types of biologically active substances (protein, lipids, etc.), of which small noncoding RNAs (ncRNAs) are the most abundant.26,27 In this study, our results found that hypoxia increased CRC-derived exosomal miR-210-3p levels, which promoted proliferation while inhibiting apoptosis in normoxic tumor cells by directly targeting CELF2 expression. These results suggest that hypoxic TME may promote CRC cells to produce miR-210-3p-enriched exosomes, which may drive nonhypoxic CRC cells toward a protumoral phenotype. This is the first study, to our knowledge, indicating that exosomes act as a mediator between hypoxic and normoxic CRC cells in TME by delivering miR-210-3p.
Hypoxia, as a common feature of the TME, plays as a main driving power in tumor progression by favoring heterogeneity, invasiveness, angiogenesis, and metastatic potency.6,7 Recently, increasing data have demonstrated exosomes function as a key role in hypoxia-mediated tumor malignant evolution.10,19 Under hypoxic conditions, tumor cells can secrete more exosomes to enhance interaction with other cells in TME, thereby gaining the advantage of survival and metastasis. 19 There are numerous studies which show that exosomes derived from hypoxic glioma, 22 lung cancer, 21 pancreatic cancer, 24 and epithelial ovarian cancer 25 can promote macrophage M2 polarization to establish an immunosuppressive TME. In addition, exosomes derived from hypoxic glioblastoma multiforme 32 and lung cancer 23 cells may induce endothelial cells angiogenesis. Herein, we demonstrated that H-Exos promoted tumor proliferation while inhibiting tumor apoptosis of recipient normoxic cells. Our results, combined with previous data, indicate that exosomes act as the messenger transport signals between cells. Through exosome-mediated transfer, tumor cells in the hypoxic zone can enhance the malignant proliferation ability of receptor tumor cells in the normoxic region.
miR-210-3p, as an intronic miRNA located within the genomic locus of transcript AK123483, is involved in numerous biological programs, including proliferation, apoptosis, angiogenesis, invasion, metastasis, and metabolism. 33 It has been reported to function as either an oncogene or a tumor suppressor, depending on tumor type.33,34 In recent years, miR-210-3p has been regarded as an important regulator of the cellular response to hypoxia, which can induce miR-210 expression in a HIF-1α-dependent manner.34,35 As a hypoxia-regulated miRNA (HRM), miR-210-3p is consistently upregulated in both normal and tumoral hypoxic cells. 36 In CRC, growing evidence has demonstrated that miR-210 overexpression in CRC, and its high expression are closely related to multiple unfavorable clinicopathological features and poor prognosis.29,37 Moreover, hypoxia can induce miR-210 expression in CRC cells to mediate hypoxia-induced CRC progression. Qu et al. 28 found that under hypoxic conditions, miR-210 promoted the migration and invasion of CRC cells by directly targeting VMP1. In addition, Sun et al. 38 demonstrated that hypoxia could induce autophagy via the HIF-1α/miR-210/Bcl-2 pathway to affect radiosensitivity in colon cancer cells. In this study, we found that hypoxia induced the expression of miR-210-3p in CRC cell-derived exosomes, which promoted proliferation and cell cycle transition while inhibiting the apoptosis of normoxic tumor cells. Moreover, miR-210-3p level was upregulated in circulating plasma exosomes, which was significantly correlated with poor tumor differentiation, lymphovascular invasion, perineural invasion, late N stage, and advanced TNM stage in patients with CRC. These data indicate that miR-210-3p acts as an oncogene during the initiation and development of CRC, which can be transferred from hypoxic tumor cells to normal tumor cells via exosomes, thereby promoting its malignant biological behaviors and mediating CRC progression.
CELF2 belongs to the CELF family of proteins, as an RNA binding protein (RBP), which can bind to double or single-stranded RNA to regulate various posttranscriptional events, including RNA splicing, shuttling, editing, stabilization, and translation. 31 Genomic analysis found that CELF2 is predominantly downregulated in cancers compared with normal tissues. 39 Consistent with other RBPs, CELF2 has been demonstrated to regulate cancer-related transcripts.40,41 More recently, Yeung et al. found that CELF2 suppressed non-small cell lung carcinoma progression by suppressing PREX2-PTEN interaction. 42 In CRC, CELF2 has also been shown to play a tumor-suppressor role in tumor development. 31 However, the underlying mechanism of CELF2 downregulation in CRC remains unclear. Previously, miRNAs have been demonstrated to regulate CELF2 expression in numerous human cancers. For example, Wang et al. showed that miR-615-3p promoted proliferation and migration while inhibiting apoptosis by suppressing CELF2 expression in gastric cancer; 43 Liao et al. 44 found that miR-20a promoted proliferation and invasion while inhibiting apoptosis of glioma cells by downregulating CELF2. In the present study, we found that CELF2 played a critical role in hypoxic CRC-derived exosome-mediated CRC progression. Through exosome transfer, miR-210-3p was transported from hypoxic tumor cells to normoxic tumor cells, which promoted proliferation while inhibiting the apoptosis of normoxic tumor cells by directly targeting CELF2 expression. Combined with previous results, our data lead to the reasonable conclusion that the miRNA-CELF2 axis plays important roles in tumor progression.
In conclusion, our study suggests that the hypoxic TME may stimulate CRC cells to secrete miR-210-3p-enriched exosomes that can elicit protumoral effects of normoxic cells. We therefore conclude that tumor-derived exosomes may act as messengers, which transport miRNAs between hypoxic and normoxic cancer cells to remodel the TME of CRC. These findings provide new insights into the mechanism of CRC progression and potential therapeutic targets for CRC patients.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211011576 for Hypoxic colorectal cancer-secreted exosomes deliver miR-210-3p to normoxic tumor cells to elicit a protumoral effect by Liuqing Ge, Feng Zhou, Jiayan Nie, Xiaobing Wang and Qiu Zhao in Experimental Biology and Medicine
Footnotes
AUTHORS’ CONTRIBUTIONS: GLQ conceived and designed this study; GLQ, ZF, NJY, and WXB carried out experiments, analyzed experimental results; GLQ wrote this manuscript; GLQ, ZF, and ZQ revised this manuscript. All authors and participants reviewed the paper and approved the final version of the article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Fund Youth Fund of China (No. 81300274).
ORCID iD: Liuqing Ge https://orcid.org/0000-0003-0552-2732
SUPPLEMENTAL MATERIAL: Supplemental material for this article is available online.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 2.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet 2019; 394:1467–80 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70:145–64 [DOI] [PubMed] [Google Scholar]
- 4.Tauriello DVF, Batlle E. Targeting the microenvironment in advanced colorectal cancer. Trends Cancer 2016; 2:495–504 [DOI] [PubMed] [Google Scholar]
- 5.Fessler E, Medema JP. Colorectal cancer subtypes: developmental origin and microenvironmental regulation. Trends Cancer 2016; 2:505–18 [DOI] [PubMed] [Google Scholar]
- 6.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science 2016; 352:175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 2019; 18:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015; 5:378–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther 2016; 164:152–69 [DOI] [PubMed] [Google Scholar]
- 10.Schito L, Semenza GL. Hypoxia-Inducible factors: master regulators of cancer progression. Trends Cancer 2016; 2:758–70 [DOI] [PubMed] [Google Scholar]
- 11.Noman MZ, Hasmim M, Lequeux A, Xiao M, Duhem C, Chouaib S, Berchem G, Janji B. Improving cancer immunotherapy by targeting the hypoxic tumor microenvironment: new opportunities and challenges. Cells 2019; 8:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest 2016; 126:3672–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov 2016; 6:235–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Yang M, Li Y, Yang F, Feng Y. Exosomes derived from hypoxic colorectal cancer cells transfer Wnt4 to normoxic cells to elicit a prometastatic phenotype. Int J Biol Sci 2018; 14:2094–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, Li X, Chen J, Liu K, Li C, Zhu G. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res 2016; 76:1770–80 [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367:eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 2019; 88:487–514 [DOI] [PubMed] [Google Scholar]
- 18.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Rel 2015; 219:278–94 [DOI] [PubMed] [Google Scholar]
- 19.Meng W, Hao Y, He C, Li L, Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer 2019; 18:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, Shen H. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer 2018; 17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017; 36:4929–42 [DOI] [PubMed] [Google Scholar]
- 22.Qian M, Wang S, Guo X, Wang J, Zhang Z, Qiu W, Gao X, Chen Z, Xu J, Zhao R, Xue H, Li G. Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-kappaB pathways. Oncogene 2020; 39:428–42 [DOI] [PubMed] [Google Scholar]
- 23.Hsu YL, Hung JY, Chang WA, Jian SF, Lin YS, Pan YC, Wu CY, Kuo PL. Hypoxic lung-cancer-derived extracellular vesicle MicroRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol Ther 2018; 26:568–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T, Liu B, Su L, Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kgamma to promote pancreatic cancer metastasis. Cancer Res 2018; 78:4586–98 [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett 2018; 435:80–91 [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinform 2015; 13:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 2018; 17:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu A, Du L, Yang Y, Liu H, Li J, Wang L, Liu Y, Dong Z, Zhang X, Jiang X, Wang H, Li Z, Zheng G, Wang C. Hypoxia-inducible MiR-210 is an independent prognostic factor and contributes to metastasis in colorectal cancer. PLoS One 2014; 9:e90952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Yang Y, Qu A, Wu Q, Zhang X, Wang L, Li C, Dong Z, Du L, Wang C. Prognostic value of a hypoxia-related microRNA signature in patients with colorectal cancer. Aging 2020; 12:35–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol 2018; 141:1202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramalingam S, Ramamoorthy P, Subramaniam D, Anant S. Reduced expression of RNA binding protein CELF2, a putative tumor suppressor gene in colon cancer. Immunogastroenterology 2012; 1:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 2013; 110:7312–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bavelloni A, Ramazzotti G, Poli A, Piazzi M, Focaccia E, Blalock W, Faenza I. MiRNA-210: a current overview. Anticancer Res 2017; 37:6511–21 [DOI] [PubMed] [Google Scholar]
- 34.Qin Q, Furong W, Baosheng L. Multiple functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer Res 2014; 33:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noman MZ, Janji B, Berchem G, Chouaib S. miR-210 and hypoxic microvesicles: two critical components of hypoxia involved in the regulation of killer cells function. Cancer Lett 2016; 380:257–62 [DOI] [PubMed] [Google Scholar]
- 36.Devlin C, Greco S, Martelli F, Ivan M. miR-210: more than a silent player in hypoxia. IUBMB Life 2011; 63:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Z, Di M, Fu W, Tang Q, Liu Y, Lei P, Gu X, Liu T, Sun M. Integrated analysis identifies a nine-microRNA signature biomarker for diagnosis and prognosis in colorectal cancer. Front Genet 2020; 11:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Xing X, Liu Q, Wang Z, Xin Y, Zhang P, Hu C, Liu Y. Hypoxia-induced autophagy reduces radiosensitivity by the HIF-1alpha/miR-210/bcl-2 pathway in Colon cancer cells. Int J Oncol 2015; 46:750–6 [DOI] [PubMed] [Google Scholar]
- 39.Wang ZL, Li B, Luo YX, Lin Q, Liu SR, Zhang XQ, Zhou H, Yang JH, Qu LH. Comprehensive genomic characterization of RNA-Binding proteins across human cancers. Cell Rep 2018; 22:286–98 [DOI] [PubMed] [Google Scholar]
- 40.Subramaniam D, Natarajan G, Ramalingam S, Ramachandran I, May R, Queimado L, Houchen CW, Anant S. Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol 2008; 294:G1025–32 [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell 2003; 11:113–26 [DOI] [PubMed] [Google Scholar]
- 42.Yeung YT, Fan S, Lu B, Yin S, Yang S, Nie W, Wang M, Zhou L, Li T, Li X, Bode AM, Dong Z. CELF2 suppresses non-small cell lung carcinoma growth by inhibiting the PREX2-PTEN interaction. Carcinogenesis 2020; 41:377–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Liu L, Sun Y, Xue Y, Qu J, Pan S, Li H, Qu H, Wang J, Zhang J. miR-615-3p promotes proliferation and migration and inhibits apoptosis through its potential target CELF2 in gastric cancer. Biomed Pharmacother 2018; 101:406–13 [DOI] [PubMed] [Google Scholar]
- 44.Liao C, Chen W, Wang J. MicroRNA-20a regulates glioma cell proliferation, invasion, and apoptosis by targeting CUGBP Elav-Like family member 2. World Neurosurg 2019; 121:e519.e27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211011576 for Hypoxic colorectal cancer-secreted exosomes deliver miR-210-3p to normoxic tumor cells to elicit a protumoral effect by Liuqing Ge, Feng Zhou, Jiayan Nie, Xiaobing Wang and Qiu Zhao in Experimental Biology and Medicine