Abstract
Human cell survival requires function of the Na+/K+ pump; the heteromeric protein that hydrolyzes ATP to extrude Na+ and import K+ across the plasmalemma, thereby building and maintaining these ions’ electrochemical gradients. Numerous dominant diseases caused by mutations in genes encoding for Na+/K+ pump catalytic (α) subunit isoforms highlight the importance of this protein. Here, we review literature describing disorders caused by missense mutations in ATP1A1, the gene encoding the ubiquitously expressed α1 isoform of the Na+/K+ pump. These various maladies include primary aldosteronism with secondary hypertension, an endocrine syndrome, Charcot-Marie-Tooth disease, a peripheral neuropathy, complex spastic paraplegia, another neuromuscular disorder, as well as hypomagnesemia accompanied by seizures and cognitive delay, a condition affecting the renal and central nervous systems. This article focuses on observed commonalities among these mutations’ functional effects, as well as on the special characteristics that enable each particular mutation to exclusively affect a certain system, without affecting others. In this respect, it is clear how somatic mutations localized to adrenal adenomas increase aldosterone production without compromising other systems. However, it remains largely unknown how and why some but not all de novo germline or familial mutations (where the mutant must be expressed in numerous tissues) produce a specific disease and not the other diseases. We propose hypotheses to explain this observation and the approaches that we think will drive future research on these debilitating disorders to develop novel patient-specific treatments by combining the use of heterologous protein-expression systems, patient-derived pluripotent cells, and gene-edited cell and mouse models.
Keywords: active transport; dominant-negative; gain-of-function mutation; haploinsufficiency; Na, K-ATPase
INTRODUCTION: THE Na+/K+ PUMP AND ITS ISOFORMS
The Na+/K+ pump, also known as the Na+,K+-ATPase, exports three Na+ ions and imports two K+ ions against their electrochemical gradients at the expense of one ATP molecule. Nearly all mammalian cells rely on the proper function of this pump to maintain ionic, osmotic, and electrical homeostasis. Excitable cells like neurons and myocytes use the Na+ and K+ gradients to properly fire action potentials, maintain resting potential, and reuptake neurotransmitters. Cells in other organs require the Na+/K+ pump not only for their own cellular homeostasis, but also to perform their whole body homeostatic function. For example, cells in the kidney express the enzyme at extremely high levels because Na+/K+ pump function drives the reabsorption of glucose and amino acids, regulates electrolyte balance, and maintains acid/base balance (1).
The physiologic importance of the Na+/K+ pump is demarcated by the pharmacological use of cardiotonic steroids (aka digitalis derivatives) to treat congestive heart failure for more than two centuries (2). These drugs are specific Na+/K+ pump inhibitors that act by reducing the electrochemical Na+ gradient, reducing Ca2+ extrusion through Na+/Ca2+ exchanger (NCX), thereby allowing Ca2+ replenishment in the sarcoplasmic reticulum (3, 4). Historically, digitoxin has been the most commonly used digitalis derivative, although new variations of these steroids are currently in clinical trials (5). Experimentally, ouabain is the digitalis derivative most commonly used to specifically identify the enzymatic and transport activity of the Na+/K+ pump in biochemical and electrophysiological experiments.
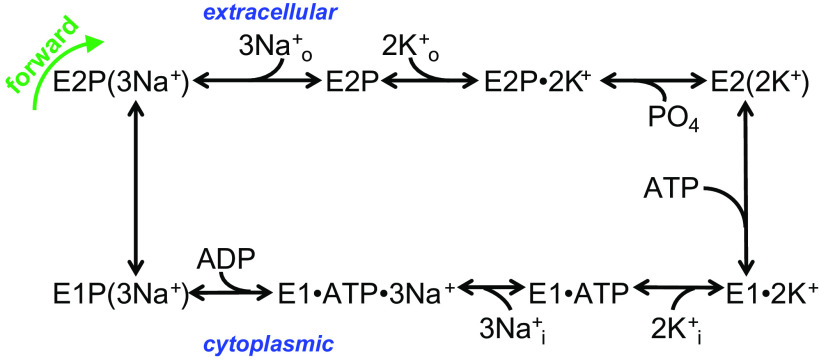
Enzymes in the P-type ATPase family, to which the Na+/K+ pump belongs, are characterized by a kinetic cycle composed of several fully reversible partial reactions in which the protein is transiently phosphorylated at a strictly conserved aspartic acid residue in the amino acid sequence DKTG. According to the Post-Albers kinetic scheme (6), the pump alternates between two major conformations, E1 and E2. E1 has intracellular facing ion binding sites, a higher affinity for Na+, and its phosphorylated intermediate, E1P, can be dephosphorylated in the reverse direction when excess ADP is added in the presence of Na+ (see Fig. 1, bottom left reaction). E2 has extracellular facing ion-binding sites with a high affinity for K+. Binding of three cytoplasmic Na+ ions to the protein in E1 catalyzes phosphorylation by MgATP producing the transient E1P(3Na+) occluded form, which spontaneously converts to the E2P conformation releasing Na+ ions to the extracellular side, one at a time (7). Following the release of Na+, two K+ ions bind, closing access to the external side and accelerating dephosphorylation of the phosphorylated E2 intermediate (E2P), becoming occluded in the E2(2K+) state. The transition from E2(2K+) to E1 that releases K+ to the intracellular side is accelerated by low-affinity binding of ATP, resetting the cycle (8). The Na+/K+ pump uses 20%–30% of the cellular ATP at rest (9) and this fraction increases in active cells, particularly in active excitable cells under increased Na+ load.
Figure 1.
Post-Albers scheme of Na+/K+ pump function. See explanation in the text.
The Na+/K+ pump is an obligatory heterodimer composed of an α subunit (∼100 kDa) and a glycosylated β subunit (∼55 kDa). The αβ dimer frequently associates with a third regulatory subunit of the FXYD family (10, 11). Humans have four α, three β, and seven FXYD isoforms (1, 12), which combine to form tissue and cell-specific pumps with variable kinetic parameters (13). The catalytic α subunit has ten transmembrane segments (M1–M10) that house the ion-translocation sites, and three well-defined cytoplasmic domains. The A (actuator) domain consists of the N-terminal portion of the polypeptide chain and the first intracellular loop between M2 and M3. The P (phosphorylation) and N (nucleotide-binding) domains, which contain the phosphorylatable aspartic acid and the ATP-binding site, respectively, are located in the long intracellular loop between M4 and M5 (12). During the transport cycle, the relationship between the N and P domains varies mildly but the association and dissociation of the A domain is a notable feature that is essential for the endogenous phosphatase activity of the protein (14, 15). The auxiliary β subunit has a single transmembrane segment with an intracellular N-terminus and is thought to be important for enzyme maturation, as it has stabilizing effects on the α subunit promoting proper folding and membrane insertion during endoplasmic reticulum protein processing (16). The FXYD proteins have a single transmembrane segment with an intracellular C-terminus and they fine-tune the ion affinities of the complex (17, 18).
Each isoform has tissue-specific expression patterns. The α1 subunit is ubiquitously expressed in all tissues, the α2 subunit is expressed in glia and muscle, the α3 subunit is almost exclusively neuronal, and the α4 subunit (encoded by ATP1A4) is found exclusively in the testes (19). To date, the only Na+/K+ pump-subunit mutations associated with diseases are ATP1A1 (α1), ATP1A2 (α2), ATP1A3 (α3), and FXYD2 (the γ subunit, the FXYD2 protein). Depending on the location of the malfunctioning mutant subunits, the resulting disease can impact one or more systems, including the adrenal gland, kidney, skeletal muscle, and both the peripheral and central nervous systems. This review focuses on the diseases associated with α1 mutations, which generally manifest with a set of symptoms distinct from those of diseases caused by mutations in α2 or α3 subunits. Given that all Na+/K+ pump isozymes perform very similar tasks (i.e., transport 3 Na+ out in exchange for 2 K+ in, at the expense of 1 ATP), this phenotypic distinction between diseases caused by mutations in different α-isoforms is an indication that the resulting pathophysiology is due to a disruption in the native function of the particular cells and tissues that express the mutated isoform. Sometimes expressing different α-isoforms within the same cell may result in distinct phenotypes depending on where the mutant α-isoform is located (e.g., in the soma versus axon within the same neuron). Mutations in the α2 isoform are associated with hemiplegic migraine (for review, see Ref. 20), epileptic syndrome (21), and hypokalemic periodic paralysis (22). Mutations in the neuronal α3 subunit [recently reviewed by Holm and Lykke-Hartmann (23)] are associated with alternating hemiplegia of childhood (24), rapid-onset-dystonia Parkinsonism (25), and a condition that presents with cerebral ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) (26).
Because of the importance of Na+/K+ pump function for cell survival, and the ubiquitous tissue distribution of α1 subunit-containing pumps, disease-association with α1 mutations was only recently discovered, as most α1 mutations render cells nonviable. Recently, however, α1 somatic mutations (i.e., mutations in cells other than germline cells) in adenomas of the adrenal gland (27) and aldosterone-producing cell clusters (28) were shown to induce primary aldosteronism. Even more recently, distinct α1 mutations were shown to produce familial (i.e., inherited) Charcot-Marie-Tooth (CMT) type 2 (29) and subsequently de novo germinal mutations (i.e., mutations in germline cells which produce mutant gametes) were associated with a form of hypomagnesemia presenting with seizures and cognitive delay (30), as well as complex spastic paraplegia (CSP) (31). This review focuses on the mechanisms by which these various mutations of ATP1A1 are thought to induce clearly distinct diseases. To facilitate the discussion, Table 1 summarizes the diseases described so far that have been associated with mutations in the ATP1A1 gene and its renal interacting partner FXYD2.
Table 1.
Summary of the diseases caused by mutation in two Na+/K+ pump genes
| Na+/K+ Pump gene | Somatic vs Germline | Mutation | Na+/K+ Pump Function Loss | Inward Current | Dominant Negative | |
|---|---|---|---|---|---|---|
| Primary aldosteronism (PA) | ATP1A1 | Somatic de novo recurrent | L104R, V332G delF100_L104 delM102_L103 delM102_I106 delL103_L104 del delI322_I325 delF956_E961 delF959_E961 delE960_L964 EETA963S |
Yes | Yes | Unlikely |
| G99R I327S |
Yes | No | ||||
| Charcot-Marie Tooth (CMT) 2 | ATP1A1 | Germline familial | L48R P600A P600T A597T D601F D811A |
Yes | No | Unknown |
| Intermediate CMT | ATP1A1 | Germline familial | F207S G877S |
Yes | Unknown | Likely |
| Hypomagnesemia accompanied by seizures and cognitive delay (HASCD) | ATP1A1 | Germline de novo | L302R G303R |
Yes | Likely | Unknown |
| M857R | Yes | Unclear | ||||
| Complex spastic paraplegia (CSP) | ATP1A1 | Germline de novo | L337P | Yes | Unknown | Unknown |
| Borderline learning impairment/ sleep disorder/poor emotional control | ATP1A1 | Germline de novo | G864R | Not tested | Unknown | Unknown |
| Severe developmental delay/ focal seizures | ATP1A1 | Germline de novo | D933N | Yes | No | Unknown |
| Isolated dominant hypomagnesemia (IDH) | FXYD2 | Germline familial | G41R | Yes | Unclear | Likely |
PRIMARY ALDOSTERONISM
The most common cause of secondary hypertension is primary aldosteronism (PA) (32). Aldosterone is a steroid hormone primarily associated with maintaining Na+ and K+ balance. Its production and secretion by the zona glomerulosa of the adrenal cortex is regulated by the renin-angiotensin system which is activated by reduction of the effective extracellular fluid volume and falls in blood pressure, or when extracellular K+ levels rise (33). Aldosterone acts directly on the principal cells of the renal collecting tubules to increase Na+ reabsorption and K+ secretion by several mechanisms that include increased synthesis and membrane insertion of the luminal epithelial sodium channel (ENaC) and basolateral Na+/K+ pump. The increased Na+/K+ pump density in the basolateral membrane may also involve increased trafficking toward the plasma membrane triggered by the rise in intracellular Na+, which may reflect a cAMP-independent and protein kinase A (PKA)-dependent pathway (34). However, whether this reflects direct kinase phosphorylation of the α1 subunit at the conserved S941 residue remains to be shown; kinase-dependent regulation of the Na+/K+ pump is particularly complex as reviewed some time ago by Therien and Blostein (35). Despite the uncertainties regarding the involvement of PKA-dependent phosphorylation, this tightly regulated negative feedback system contributes to both acute and long-term homeostatic blood pressure regulation.
In PA, aldosterone is secreted without regulatory control. Patients with PA present as hypertensive with hypokalemia and alkalosis, with high aldosterone and low renin levels. The reported prevalence of this disease varies between 5% and 20% of hypertensive patients (28, 36, 37). With the advent of exome and next-generation sequencing, mutations in ATP1A1 and other genes encoding membrane proteins have been associated with the most common presentations of PA due to increased aldosterone production by aldosterone-producing adenomas, bilateral adrenal hyperplasia, and aldosterone-producing cell clusters (28, 36).
Aldosterone-producing cells of the zona glomerulosa show high levels of aldosterone synthase (CYP11B2). These cells have very negative “resting” membrane potentials (−80 to −85 mV) and undergo periodic depolarizations (38). Membrane potential oscillation triggers an oscillating Ca2+ signal that is key for the modulation of aldosterone production (39). The primary physiological stimuli to increase aldosterone production above basal levels are angiotensin II and extracellular K+, which regulate the “resting” potential and the voltage oscillation frequency (38). Angiotensin II and K+ intricately control the opening of voltage-gated T-, L-, and N-type Ca2+ channels, thus regulating the strength of the Ca2+ signal [reviewed in (39)].
Mutations of membrane proteins associated with PA disrupt ion homeostasis. The first of these mutations was reported by Choi et al. (40) who identified substitutions in an inwardly rectifying K+ channel gene (KCNJ5) in cells from aldosterone-producing adenomas from patients with inherited hypertension. Since then, a wealth of other channel and ATPase mutations that contribute to PA have been identified and characterized.
PA-associated mutations identified so far in ion-channel genes, which include somatic and germline mutations, lead to increased inward currents, therefore promoting resting potential depolarization, increased voltage-oscillation frequency, and aldosterone production. Mutations of KCNJ5, which is highly expressed in the adrenal glands, are found in roughly 40% of aldosterone-producing adenomas (41, 42). These mutations alter the ion-selectivity filter of the channel, resulting in Na+ permeation and an inward current (40, 43–45). Mutations in CACNA1D, which encodes the α-1D subunit of CaV1.3, a voltage-gated Ca2+ channel highly expressed in endocrine, kidney/urinary, and male reproductive tissues, lead to enhanced voltage-dependent Ca2+ channel activity (41, 46, 47). Finally, mutations of voltage-dependent Cl− channel CLC2 gene (CLCN2), the gene encoding the voltage-dependent Cl− channel CLC2, with high levels of protein expression in endocrine, lung, gastrointestinal tract, liver, gallbladder, male reproductive tissues, and lymphoid tissues, lead to enhanced activity due to voltage-dependent gating (48, 49). These mutations are commonly called gain-of-function mutations1. Interestingly, loss-of-function mutations that reduce the activity of some of these channels are associated with other disease processes but not PA (50).
Somatic mutations of two genes that encode distinct P-type ATPases, ATP2B3 and ATP1A1, produce heterogeneous functional effects leading to PA. ATP2B3, which encodes the third isoform of the plasma membrane Ca2+ ATPase (PMCA3), is expressed throughout the central nervous system and in the adrenal cortex. The PA-inducing mutations identified in PMCA3 are within transmembrane segment M4 (27, 51, 52), a region conserved in all P-type 2 ATPases, as amino acids in this segment participate in the binding of the transported ions. Detailed functional studies of the effects of these mutations are missing due to the lack of specific inhibitors and a suitable expression system for PMCA3. However, the depolarization in cultured aldosterone-producing-adenoma cells from patients carrying these mutations (27) and in cell lines overexpressing mutant PMCA3 pumps (53) suggests an aberrant function carrying an inward current. The other P-type ATPase gene whose mutation causes PA is ATP1A1.
It appears that race and gender may contribute to determine which PA-driver gene is mutated (42, 54–56). Mutations in KCNJ5 are more frequent in females and usually are diagnosed at younger ages, whereas mutations in ATP1A1, ATP2B3, and CACNA1D are more frequent in smaller tumors found in males and diagnosed at older ages. Although the criteria for inclusion in the study may vary across reports, KCNJ5 is the most frequently reported mutation in East Asians (57–60) and Caucasians (41, 42, 51, 54, 55), whereas CACNA1D is the most frequently mutated gene in people of African descent (56). These studies indicate that 5%–8% of patients with aldosterone-producing adenomas (APAs) from Caucasian (∼1,000 adenomas) and African descent (73 adenomas) harbor ATP1A1 mutations, whereas less than 1% of East Asian patients (∼450 patients) carry a mutation in this gene. Next we discuss the different disease states caused by mutations of the Na+/K+ pump α1 subunit and suggest the perturbed cell mechanism that contributes to the pathophysiology.
ATP1A1 Mutations Associated with Primary Aldosteronism
Numerous mutations of ATP1A1, the ubiquitous α1 Na+/K+ pump subunit, have been identified in adrenal adenomas of patients with PA. It is important to note that these somatic mutations only affect the pumps in adenoma cells and not those in other parts of the body, like the collecting tubule, and therefore the mutations’ effects are mediated by and limited to changes in the cellular physiology of the cells carrying the mutations. Most of these mutations are scattered through M1, M4, and M9 of the α1 subunit (27, 28, 41, 51), as illustrated in the Na+/K+ pump two-dimensional (2-D) map (Fig. 2A, green residues) and Na+-bound crystal structure (61) (Fig. 2B, green residues). In the article that discovered the link between an ATP1A1 mutation and PA, Beuschlein et al. (27) described that primary cultured cells isolated from two patients with aldosterone-producing adenomas harboring these Na+/K+ pump mutations were depolarized by 20 mV when compared with cultured cells from normal adjacent tissue. Shortly thereafter, Azizan et al. (41) discovered that four of these single substitutions (L104R, delF100-L104, V332G, and EETA963S) induce inward currents in the Na+/K+ pumps that carry them, under conditions that produced outward currents (due to the 3Na+/2K+ stoichiometry) in wild-type pumps. Therefore, it was expected that all PA-inducing ATP1A1 mutations would lead to aberrant inward currents in the Na+/K+ pumps that carry them, representing a similar “gain of function” as in all ion-channel mutations.
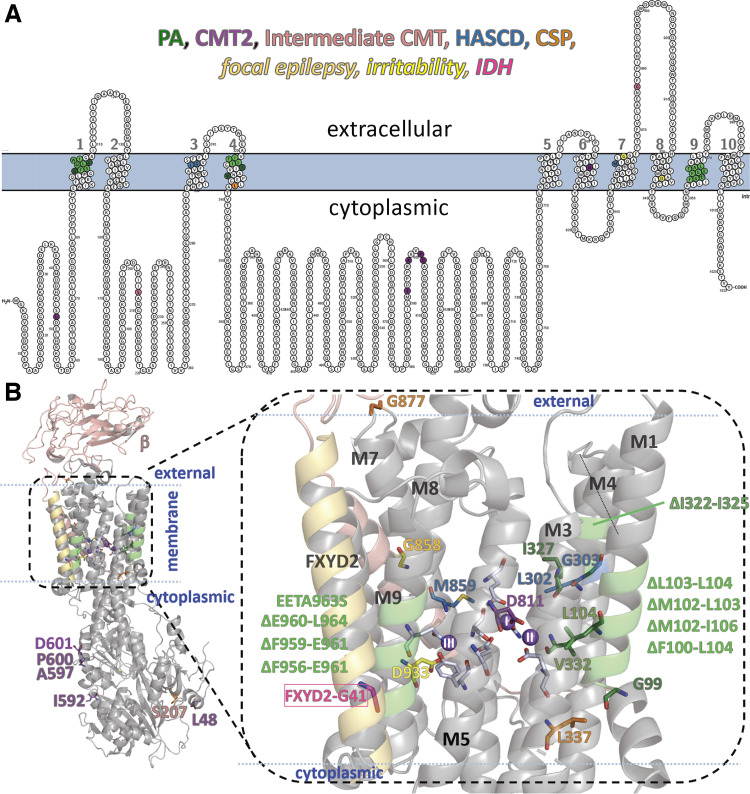
Figure 2.
Location of disease-linked mutations in the Na+/K+ pump α subunit. The same residue (or carbon) colors are used to group the mutations in both panels. Green for primary aldosteronism (PA, with single substitutions in dark green and deletions in lighter green), blue for hypomagnesemia with seizures and cognitive delay (HASCD), orange for complex spastic paraplegia (CSP), purple for Charcot-Marie-Tooth type 2 (CMT2), pink for intermediate CMT, dark yellow for developmental delay with focal seizures, and light yellow for sleep disorder with irritability. For completion, the FXYD2 residue that causes isolated dominant hypomagnesemia (IDH) is highlighted in hot pink. A: topographic two-dimensional (2-D) map of the Na+/K+ pump α subunit indicating the residues whose mutation is associated with each disease. The membrane is indicated by the light blue shade. Large numbers correspond to transmembrane segments. Every 10th residue is numbered. B: crystal structure of the Na+/K+ pump in the E1P state with three Na+ ions bound; pdb access code 3WGV (61). The three Na+ ions are purple spheres marked I, II, and III according to the site that they occupy. The residues mutated in ATP1A1 related diseases (labeled and colored as indicated above) and the residues contributing sidechain oxygens to the ion-binding sites with gray carbons are shown in a stick representation.
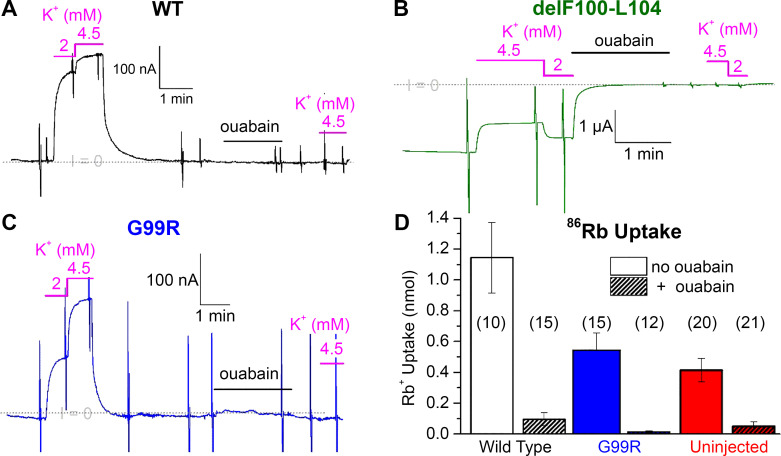
Our laboratory performed detailed analyses of the functional consequences of the PA-inducing mutations described earlier, as well as several mutations reported in subsequent articles. We found that deletions delM102-L103, delL103-L104, and delM102-I106 in M1, delI322-I325 in M4, and delF956-E961, delF959-E961, and delE960-L964 in M9 produce aberrant inward currents of variable amplitude that would be consistent with the hypothesized “gain-of-function” mechanism (62, 63). The main ionic species carrying the inward current in all of these mutants was Na+ (cf. figure 6 in Ref. 62), which contrasts with the transport of protons carried out by wild-type pumps when the extracellular Na+ and K+ concentrations are reduced (64, 65). However, we also found that such aberrant inward currents were absent in G99R in M1 (62) and I327S in M4 (63) and therefore these cannot explain how these two mutations cause PA. Actually, both mutations reduce the apparent affinity for transported ions (particularly intracellular Na+), which likely underlies the pathophysiology of PA caused by these mutations (62, 63). Along these lines, the inward currents (measured as current per Na+/K+ pump molecule) through EETA963S and certain other mutants appeared to be too small to induce significant depolarization. The total level of Na+/K+ pump expression is unchanged in aldosterone-producing adenomas compared with normal tissues (27), indicating that the EETA963S mutant Na+/K+ pump allele is expressed to the same level as the wild type. Normal expression, combined with the loss of function, suggests that haploinsufficiency suffices to produce the PA associated with this mutant, without the need for dominant-negative effects (discussed in the CMT section). The functional characteristics of some PA-mutant pumps heterologously expressed in Xenopus oocytes are illustrated in Fig. 3. As indicated in the figure legend, representative current recordings from oocytes expressing wild type (Fig. 3A), delF100-L104 (Fig. 3B), or G99R (Fig. 3C) were obtained following an increase in intracellular Na+ to maximize Na+/K+ pump activity. At −50 mV, in an extracellular solution containing Na+, when wild-type pumps are activated by addition of extracellular K+ ([Na+] + [K+] = 150 mM), a large outward current (upward current deflection Fig. 3A) is activated, in a K+ concentration-dependent manner. This current is irreversibly inhibited by treatment with ouabain demonstrating it is due to the Na+/K+ pump. In contrast, whether in the presence or absence of external K+, an oocyte expressing delF100-L104 displays a large inward current (Fig. 3B). The inward current is also completely inhibited by ouabain, again demonstrating that this aberrant current is carried by the mutant pumps. Under these conditions of high intracellular Na+, an oocyte expressing the loss-of-function mutation G99R also shows a K+-concentration-dependent outward current (Fig. 3C). The larger jump in outward current from 2 mM to 4.5 mM K+ is due to the lower apparent affinity for K+ of this mutant. The reduction of affinity for transported ions, particularly for intracellular Na+ (62) constitutes a loss of function for G99R. To demonstrate that this constitutes a significant loss of function at physiological intracellular Na+, we measured the Na+/K+ pump-mediated uptake of radioactive Rb+ (an ion that is transported like K+) in oocytes that were not Na+-loaded (Fig. 3D).
Figure 3.
Malfunction of Na+/K+ pump primary aldosteronism (PA)-inducing mutants. Current recorded at −50 mV in Xenopus oocytes coexpressing the human β subunit with either wild-type α1 (A), delF100-L104-α1 (B), or G99R-α1 (C). The gray dotted line indicates the zero-current level (I = 0). The current in 150 mM external Na+ is close to zero for wild type and for G99R, but it is negative (inward) in the oocyte expressing the deletion mutant delF100-L104. In all cases, application of extracellular K+ (at the indicated concentrations) induces a positive deflection of the current. In the presence of K+, the net current is positive (outward) for wild type and G99R, but it is inward for delF100-L104. Note that the Na+/K+ pump specific inhibitor ouabain blocks the current deflections induced by subsequent application of K+ and eliminated the inward current carried by delF100-L104 mutant. Also, note that the K+-induced currents in G99R require higher K+ concentrations than wild type. The intracellular Na+ concentration of these three oocytes was increased by a 30-min incubation in a sodium-loading solution containing (in mM) 90 NaOH, 20 tetraethylammonium-OH, 0.2 EGTA, and 40 HEPES (pH 7.2 with sulfamic acid). D: oocytes expressing wild type, G99R, or only endogenous pumps (uninjected) were incubated for 15 min in a solution composed of (in mM) 4.5 RbCl (with 86RbCl), 133 methane sulfonic acid (MS), 125 mM NaOH, 10 HEPES, 5 Ba(OH)2, 1 Mg(OH)2, 0.5 Ca(OH)2, 0.05 bumetanide (pH 7.4). The experiment was performed without and with 100 µM ouabain to obtain the Na+/K+ pump-specific uptake of Rb+, which is imported like K+. Note at normal concentrations of intracellular Na+ (i.e., without preincubation in Na+-loading solution), the oocytes injected with G99R cRNA were indistinguishable from uninjected oocytes, indicating that the G99R present a loss of function under these physiological conditions. Data in D are the average from independent determinations in the number of oocytes indicated in parenthesis, replotted form Fig. 11B in Ref. 62 with error bars representing SD instead of SE; experiments in A–C are distinct from those previously published, but illustrate the same properties described in Ref. 62. For further experimental details see Ref. 62.
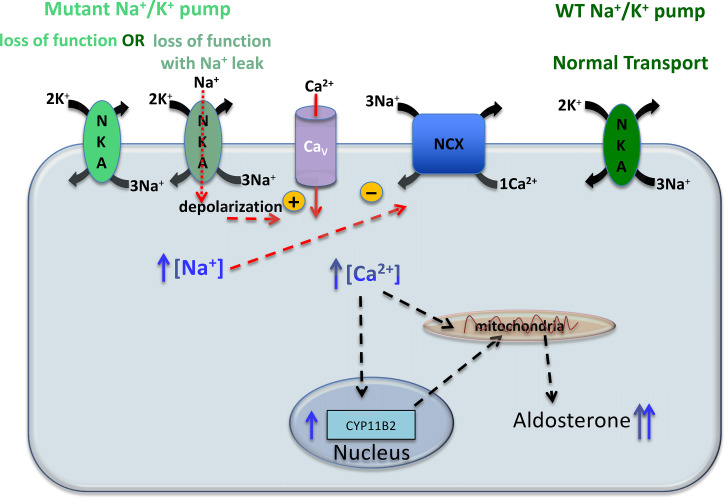
As mentioned earlier, oscillating Ca2+ entry is required for the normal regulation of aldosterone production. This means that removal of free Ca2+ from the cytoplasm following each voltage oscillation is also critical, as prolonged cytoplasmic Ca2+ elevations would lead to overproduction of aldosterone. Cytosolic Ca2+ is cleared via sequestration into the endoplasmic reticulum by the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) and extruded from the cell by the plasma membrane Ca2+ ATPase (PMCA) and NCX. PMCA uses ATP hydrolysis to export one Ca2+ ion against its steep plasma membrane gradient and has high affinity and low capacity. Complementary to PMCA is NCX. The low Ca2+ affinity and high-transport capacity of NCX make it suitable for managing large increases in cytosolic Ca2+ that occur during depolarization, and its activity is intimately linked with Na+/K+ pump activity. Although the usual function of NCX is to remove Ca2+ from the cell using the electrochemical Na+ gradient (1 Ca2+ out, 3 Na+ in), a sufficient elevation of intracellular Na+ or depolarization of the membrane potential may slow its transport, or even reverse it to Ca2+ entry and Na+ extrusion (66). Therefore, given the large oscillations in intracellular Ca2+ concentration, simple haploinsufficiency due to a malfunctioning Na+/K+ pump produced by a mutant allele might easily reduce the Na+ gradient and subsequently alter Ca2+ dynamics within aldosterone-producing zona glomerulosa cells, leading to increased aldosterone production, as depicted in Fig. 4.
Figure 4.
Simplified representation of an aldosterone producing adenoma (APA) cell with Na+/K+ pump mutations. The normal allele expresses Na+/K+ pumps (NKA) with normal transport. There are two phenotypes for the mutations that cause primary aldosteronism (PA), both with impaired Na+/K+ pump function that leads to increased intracellular Na+ concentration leading to increased intracellular [Ca2+] due to reduced Ca2+ extrusion through the Na+/Ca2+ exchanger (NCX). In most mutants, the loss of function is accompanied by an inward leak current that increases depolarization facilitating activation of voltage-dependent Ca2+ channels (CaV) further increasing intracellular [Ca2+]. Elevated intracellular [Ca2+] leads to an increase in mitochondrial and nuclear [Ca2+] where it activates transport of cholesterol into the mitochondria and transcription of CYP11B2 (the aldosterone synthase gene), respectively. Aldosterone synthase localizes to the inner membrane of the mitochondria, where aldosterone is produced, for more details on aldosterone synthesis see the excellent review by Spät and Hunyady (33).
Given the central role of the Na+/K+ pump in cellular homeostasis, it is also possible that secondary active transporters other than, or in addition to, NCX mediate the increased aldosterone production. In this regard, Stindl et al (67) failed to observe increased intracellular Ca2+ concentration in adrenocortical carcinoma cell line (NCI-H295R) cells heterologously expressing rat G99R, L104R, or V332G mutant pumps compared with cells expressing rat wild-type pumps. However, the approach used in such study does not reflect the haploid pathophysiological situation, with half the pumps being mutants. Instead, the experiment reports what happens to cells that overexpress, either mutant or wild-type ATPases. The authors suggest that the effects of L104R and V332G may be mediated by proton fluxes through the mutant pumps, instead of NCX. However, this interpretation is difficult to reconcile with the fact that the main permeant ion in those mutant leaks is Na+ (cf. figure 6 in Ref. 62), although protons appear to leak through L104R (27, 41). This means that if protons are responsible for the effects of ATP1A1 mutants these are probably mediated by Na+/H+ exchanger. In any case, the study by Stindl et al. demonstrates that the effects of G99R cannot be explained by the leak hypothesis, as they observe a much smaller cellular depolarization with this mutant than the other two. Such small depolarization could be explained by the reduced intracellular Na+ affinity of G99R (62).
Although more research will be required to conclusively prove that Ca2+ dysregulation due to haploinsufficiency is solely responsible for increased aldosterone production, as we propose, other strong evidence supporting that haploinsufficiency is able to induce hyperaldosteronism autonomously is scattered throughout the literature. Yingst et al. demonstrated that ouabain inhibition of ATP1A1 resulted in increased intracellular [Ca2+] and aldosterone production in rat adrenal glands (68). The peak effect occurred at ouabain concentrations around the IC50 for rat α1 Na+/K+ pump inhibition (∼100 μM); that is, at ouabain concentrations where only half of the pumps were active, analogous to haploinsufficiency due to a loss-of-function mutation. Furthermore, heterozygous knockout ATP1A1+/− mice, with constitutive whole body haploinsufficiency had increased aldosterone levels, although the animals were normotensive (69). Further studies will be needed to evaluate the compensation mechanisms that prevent a rise in blood pressure in the setting of elevated aldosterone in heterozygous knockout mice.
CHARCOT-MARIE-TOOTH DISEASE
Hereditary sensory motor neuropathies vary in their severity, age of onset, and genetic causes and sometimes form part of a syndrome (70). Charcot-Marie-Tooth (CMT) disease comprises a group of nonsyndromic genetically heterogeneous peripheral and sensory neuropathies that affect ∼1 in 2,500 individuals with the age of onset ranging from childhood to adulthood (71). Most patients with CMT present with weakness, atrophy, sensory loss, and absence of reflexes in their limbs, as well as high-arched feet (pes cavus). There are two main types of CMT; CMT1 and CMT2, characterized by different motor nerve conduction velocities. Patients with CMT1 have severely reduced conduction velocities (below 25 m/s) due to defects in the myelin sheath. This demyelinating form is usually more severe and has an age of onset within the first two decades of age. In contrast, patients with CMT2 present normal conduction velocities (above 45 m/s) but have reduced compound muscle action potential amplitude due to reduced numbers of functional neuromuscular junctions following axonal loss in the nerve fibers (72). In addition, the neuropathy in patients with milder reductions in conduction velocity (between 25 and 45 m/s) is classified as intermediate CMT (73).
Mutations in over 90 genes are associated with CMT (74). The most common genes whose associated with CMT1 encode proteins exclusively expressed in the Schwann cells that produce the myelin sheet; PMP22 (peripheral myelin protein) and MPZ (myelin protein zero). The genetic cause of CMT1 can be identified in more than 90% of patients, with ∼70% of these cases being attributed to mutation or duplication in the PMP22 gene (75), located in chromosome 17 (76). The gene most commonly mutated in CMT2 patients is MFN2 (mitofusin-2) (75), a protein that is abundant in the outer membrane of the mitochondria and in the endoplasmic reticulum in regions called mitochondrial associated membranes (77). Other genes whose mutations have been associated with CMT2 are HSPB1, MPZ, K1F1B, RAB7A, LMNA, MED25 (78), and ATP1A1 (29).
ATP1A1 Mutations Associated with Charcot-Marie-Tooth Disease
In their seminal article, Lassuthova et al. (29) reported the evaluation of seven families from the Czech Republic, Italy, USA, Australia, and South Korea affected with CMT2 and identified seven novel ATP1A1 missense mutations associated with the disease: L48R, I592T, A597T, P600A, P600T, D601F, and D811A (Fig. 2, purple residues). Five of the six substitutions located within intracellular loops (I592T, A597T, P600A, P600T, and D601F) reside in the helical linker region that couples the N and P domains, suggesting that this region is a hotspot for CMT2 missense mutations. The other intracellular ATP1A1 missense mutation, L48R, is in the A domain, which modulates interactions of the N and P domains. Given the location of these six cytoplasmic mutations, it is thought that the disruption of the collaborative movement and interactions of the three cytoplasmic domains causes loss of function by impeding the auto phosphorylation/dephosphorylation reactions required for active ion pumping. On the other hand, the mutant D811A (in transmembrane segment 6) alters the coordination of ions (both Na+ and K+) in two of the three ion-binding sites (site I and site II, Fig. 2B), leading to a malfunctioning Na+/K+ pump. Interestingly, mutations of the equivalent residue in the α3 subunit, D801, have been found in patients with sporadic (nonhereditary) forms of rapid-onset dystonia parkinsonism, D801Y (25), or alternating hemiplegia of childhood, D801Y (79), D801N (24, 80), D801E (81), and D801V (82).
To evaluate the functional effects of CMT-causing ATP1A1 mutations, Lassuthova et al. (29) used two approaches. First, they examined the electrophysiological characteristics of human wild-type ATP1A1 and the different CMT2-inducing variants in Xenopus oocytes, where most mutants showed reduced currents (particularly P600A and D811A), suggesting a loss of function. In addition, they evaluated cell survival in the presence of the specific Na+/K+ pump inhibitor ouabain in U20S cells heterologously expressing pumps with reduced ouabain affinity. Expressing ouabain-resistant Na+/K+ pumps [due to the double mutation Q118RN129D that occurs naturally to confer ouabain resistance to the rat enzyme (83)] in cells containing endogenous ouabain-sensitive Na+/K+ pumps is a longstanding technique for studying this enzyme. Because U20S cells have endogenous Na+/K+ pumps with high affinity for ouabain, they die in the presence of micromolar concentrations of the drug. However, heterologous expression of functional ouabain-resistant pumps can rescue cells grown in the presence of the same ouabain concentration. U20S cells expressing wild-type ouabain-resistant pumps survived in the presence of ouabain, while cell viability was significantly decreased in cells expressing the ouabain-resistant mutants L48R, I592T, P600T, and D811A, again suggesting loss of function. Also, by performing immunostaining in rat nerve preparations, the authors found that peripheral nerves express high levels of the Na+/K+ pump α1 subunit, which is consistent with these ATP1A1 mutations causing CMT2.
A threonine to alanine mutation (T991A) in the electroneutral K+-Cl− cotransporter KCC3 has been shown to cause a case of CMT2-like sporadic neuropathy (84). KCC3 uses the K+ gradient formed by the Na+/K+ pump to drive the extrusion of KCl maintaining cell volume upon cell swelling (85, 86). T991A leads to a gain-of-function mutation with a constitutively active transporter (84, 87). In contrast to a gain of function, it is possible that a loss of function of ATP1A1 is mediated by KCC3 inability to extrude KCl due to a reduced K+ gradient. In any case, the effect of ATP1A1 mutations appears unlikely to be mediated by alteration of kinase-dependent modulation of Na+/K+ pump function because CMT mutations occur at sites far from kinase-regulatory-site targets or their signature sequences, with no obvious structural way for allosteric effects to be transmitted to those sites. However, it has been shown recently in Xenopus oocytes expressing human α1β1FXYD1 that the regulatory protein induces an important retention in intracellular compartments and that this effect is relieved by injection with PKA catalytic subunit (18). Similar levels of mRNA transcripts for FXYD1 and ATP1A1 are found in the spinal cord (humanproteinatlas.org) and the structural interaction between the cytoplasmic helix of FXYD1 and the α subunit remains unknown. Therefore, it is possible that some of the mutations in α1 intracellular loops alter FXYD1/α1 interactions that determine the PKA-regulated trafficking effect. Although further studies will be required to evaluate this possibility, a critical role of FXYD1 remains elusive (for review, see Ref. 11).
More recently, mutations S207F and G877S in the Na+/K+ pump α1 subunit (colored pink in Fig. 2) were found in two Chinese families with intermediate CMT (88). Sural nerve biopsies from patients revealed a pronounced loss of large myelinated axons, groups of regenerating axons, and several thinly myelinated axons. The authors concluded that these mutations do not affect ATP1A1 mRNA levels but downregulated Na+/K+ pump expression by increasing proteasome degradation, suggesting that these ATP1A1 mutant alleles have a dominant-negative effect, with lower than haploid protein expression. Such severe reduction in Na+/K+ pump activity would significantly reduce the K+ gradient required to regulate volume and avoid nerve burst through KCC3, and the Na+ gradient required for Na+/Ca2+ exchangers to extrude Ca2+ (88, 89), avoiding apoptosis due to a prolonged rise in basal cytosolic Ca2+. A recent study evaluating pluripotent stem cells (iPSCs) isolated from a patient carrying the P600A mutation (89) also supports dominant-negative effects underlying CMT pathophysiology. Preneuronal cells carrying P600A were unable to differentiate into fully mature neuronal cells, but they were able to differentiate into other cell types. Furthermore, an antibody against the α1 subunit was unable to detect its expression in P600A preneuronal cells that produced neurites, but detected Na+/K+ pumps in the neurites from control subjects.
Dominant-negative effects where the reduced membrane insertion of the mutant allele impairs insertion (and therefore function) of the normal allele may suggest that Na+/K+ pump quaternary structure containing multiple α1 subunits, e.g., a diprotomer containing 2α1/2β1 subunits could contribute to the pathophysiology. In this model, any pumps containing a nonfunctioning mutant α1 subunit would be targeted for proteasome degradation. If ATP1A1 gene products are transcribed and translated equally from both alleles, then a random pairing would result in a 75% reduction in functional pumps being delivered to the plasma membrane. However, it must be pointed out that although the multimeric association of Na+/K+ pumps has been reported in preparations with high Na+/K+ pump density (90), the functional consequence and importance of such interaction remains unresolved (8). Further studies will be required to corroborate whether this dominant-negative effect is necessary for CMT2 development and how non-neuronal cells that certainly express the mutant ATP1A1 may avoid being affected.
HYPOMAGNESEMIA ACCOMPANIED BY SEIZURES AND COGNITIVE DELAY
Germline mutations in ATP1A1 have been linked to a condition found in three infants, characterized by hypomagnesemia accompanied by seizures and cognitive delay (HASCD) (30). We refer the reader to an excellent comprehensive review discussing previously known genetic causes of hypomagnesemia (91). Here, we focus on the presentations of the disease induced by mutations in ATP1A1 and compare them with mutations in other genes, particularly mutation of FXYD2, the Na+/K+-pump γ-subunit gene highly expressed in the kidney.
Magnesium, the 4th most abundant ion in the body and the second most abundant intracellular ion (92), is an essential cofactor for many enzymes, participates in regulating fluxes of other ions across membranes, and is required for the biological activity of ATP (92, 93). Mg2+ balance is maintained via absorption in the gastrointestinal tract and excretion by the kidneys (94, 95). Thirty percent of the plasma Mg2+ is bound to either albumin or globulin, and only the remaining 70% filters across the glomerulus (94, 95). Reabsorption of the filtered Mg2+ is predominantly paracellular, with ∼15% of the filtered Mg2+ reabsorbed in the proximal convoluted tubule and ∼70% in the thick ascending limb (94, 96). The latter is driven by the lumen-positive transepithelial voltage generated by transporters and channels whose activities depend on the proper functioning of the Na+/K+ pump (94, 95). Ten percent of the remaining Mg2+ is actively reabsorbed in the distal convoluted tubule (91, 92, 94). It seems that the apical-membrane TRPM6 channel allows Mg2+ entry into the cell (94, 95), but the mechanism of exit across the basolateral membrane is unclear, although a Na+/Mg2+ exchanger driven by the Na+ gradient has been proposed (91, 97–99).
Hypomagnesemia occurs when the mechanisms of reabsorption mentioned earlier are altered due to mutations in any of the genes encoding the proteins involved. Different mutations are associated with distinct presentations, which Viering et al. (91) classified into four groups: hypercalciuric hypomagnesemias caused by mutations in claudin 16 (CLDN16), claudin 19 (CLDN19), calcium sensing receptor gene (CASR), CLCNKB; Gitelman-like hypomagnesemias caused by mutations in CLCNKB, SLC12A3, renal protein “barttin” gene (BSND), ATP-sensitive inward rectifier potassium channel Kir4.1 (KCNJ10), FYXD2, hepatocyte nuclear factor-1 β gene (HNF1B), pterin-4 α-carbinolamine dehydratase 1 (PCBD1); mitochondrial hypomagnesemias caused by mutations in SARS2, MT-TI, and deletions in mitochondrial DNA that also cause Kearns–Sayre syndrome; and other hypomagnesemias linked to mutations in TRPM6, CNMM2, EGF, EGFR, KCNA1, FAM111A. The hypomagnesemia induced by mutations in ATP1A1 falls in the latter category (30).
Given that resident Mg2+ ions block the N-methyl-d-aspartate (NMDA) type of ionotropic glutamate receptors involved in neuronal excitability, seizures are one important corollary of reduced serum Mg2+. Although Mg2+ supplementation is required to stop the seizures, in some cases this treatment is insufficient. For instance, patients with a mutation in either FXYD2 or ATP1A1, two Na+/K+ pump genes, differ in their sensitivity to Mg2+ supplementation. Patients with isolated dominant hypomagnesemia (IDH) with seizures and tetany due to a FXYD2 mutation respond to Mg2+ supplementation (100), whereas patients with hypomagnesemia accompanied by seizures and cognitive delay (HASCD) due to an ATP1A1 mutation are refractory to this treatment (30). Before we discuss the mechanisms involved in these disease states, we should point out that the distinct effect of Mg2+ supplementation is consistent with the different distribution of the two subunits. Although the γ subunit (FXYD2) is mostly found in the kidney, the α1 subunit encoded by ATP1A1 is distributed throughout the body, including the brain. Two other diseases that are refractory to Mg2+ supplementation are due to mutations of proteins that are expressed in the kidney and brain; hypomagnesemia with seizures and mental retardation caused by recessive mutations in the putative divalent metal cation transporter CNNM2 (101), and EAST syndrome due to mutations in KCNJ10 (102, 103).
The treatment-refractory central nervous system (CNS) symptoms of these diseases are seemingly distinct from the hypomagnesemia caused by renal malfunction as the hypomagnesemia in these instances appears to be a lingering symptom of these mutations rather than the cause of the pathology. For instance, Bockenhauer et al. (103) speculate that a nonfunctional KCNJ10, which is expressed in glial cells, may impair the spatial buffering of K+ and lead to extended neuronal depolarization, a situation prone to induce seizures. Given that elevated extracellular K+ concentrations resulting from the KCNJ10 mutation likely cause seizures, it is not surprising that Mg2+ supplementation is without effect. The function of CNNM2 remains controversial (91, 97, 98, 104), but its hypomagnesemia-causing mutations are accompanied by brain developmental malformations which are thought to underlie the continuity of seizures even after Mg2+ serum concentration is returned to normal with supplementation (101, 105). Thus, it stands to reason that there is also a separate CNS mechanism underlying the seizures in patients with HASCD caused by ATP1A1 mutations and that hypomagnesemia is a symptom of the deleterious effects caused by Na+/K+ pumps containing a mutant α1 subunit.
Mechanisms of the Hypomagnesemia by Na+/K+ Pump-Isoform Mutants
The FXYD2 mutation G41R causes hypomagnesemia (100, 106). It is thought that the hypomagnesemia requires a dominant-negative effect because two individuals lacking one copy of the FXYD2 gene (11q23.3-ter deletion) had normal Mg2+ levels (100). This indicates that FXYD2-haploinsufficiency cannot explain IDH and that the aberrant protein must be present to produce the IDH phenotype. Yet, it remains unclear whether the dominant-negative effect occurs through disrupted trafficking (100) or if an aberrant inward current is carried by these trimeric pumps (107). In contrast, the functional effects of ATP1A1 mutations associated with hypomagnesemia (L302R, G303R, and M859R, indicated in blue in Fig. 2) have been evaluated in more detail with biochemical and electrophysiological techniques (30). A ouabain survival assay like the one described earlier for the analysis of CMT mutations indicated that all three mutations produce a dramatic loss of Na+/K+ pumping activity. Biochemical evaluation of heterologously expressed mutants showed impaired ATPase activity due to alteration of the partial reactions composing the enzymatic cycle. For example, all three mutants reduced the maximal phosphorylation from ATP by 20%–70%, reduced the K+ apparent affinity by 5- to 36-fold, and altered the interaction with intracellular Na+ by reducing either the cooperativity or the affinity for the ion. In addition, electrophysiological evaluation of cells heterologously expressing L302R or G303R presented a Na+-dependent inward current that induced depolarization when compared with cells expressing wild-type pumps. Conversely, the electrophysiological characteristics of M859R expressing cells were indistinguishable from wild-type expressing ones (30). Further studies are required to determine whether both an aberrant inward current mechanism (L302R and G303R) and a loss-of-function mechanism (M859R) can induce HASCD or, alternatively, an aberrant inward current may have been masked in the heterologous expression system used. As in the case of CMT, a dominant negative effect may also explain disease induction, but if this was the case, these effects should be tissue dependent to explain the different phenotypes, as discussed in the perspective section. It seems unlikely that simple haploinsufficiency can explain hypomagnesemia as heterozygous ATP1+/− knockout mice lack the condition and have normal serum electrolytes.
COMPLEX SPASTIC PARAPLEGIA
A recent report showed that the ATP1A1 mutation L337P in M4 produces a form of spastic paraplegia with congenital onset (93). The patient with this mutation presented with a spastic gait at the onset of walking (18 mo) accompanied by upper motor neuron signs including hyperreflexia, bilateral Babinski, and ankle clonus. The neurocognitive examination revealed a moderate developmental delay, sleep disorder, and irritability with hyperactivity. To evaluate the mutant protein, the authors used a ouabain-survival assay to show that the mutation produces a loss of function that leads to impeded survival, similar to that observed with the CMT2-causing mutation D811A (29). The introduction of a proline in the intracellular half of M4 (Fig. 2B, orange residue), about one turn of a helix from the ion-coordinating residue E334, probably disrupts ion binding allosterically. However, the pathophysiological mechanism, whether simple haploinsufficiency, an aberrant inward leak, or a dominant-negative effect may be involved, was not evaluated.
PERSPECTIVE: WHAT WE KNOW AND WHAT WE NEED TO LEARN REGARDING ATP1A1 DISEASES
An important remaining question, recently discussed in detail by Sweadner et al. (108), is why is there only a partial overlap between the three orthologues, ATP1A1, ATP1A2, and ATP1A3, in the position of mutations that cause disease. Given that the functional domains are conserved in all isoforms, one might expect that highly conserved regions and residues would be the most relevant for mutations to cause disease; however, this is not the case. A partial explanation for this observation [also suggested by Sweadner et al. (2019)] may lie in the distinct location of these isoforms and on the Na+/K+ transport demands of the regions where they are expressed (e.g., glia versus neuron, soma versus dendrites, etc.). However, it is also possible that the cellular mechanisms responsible for assembling Na+/K+ pump subunits and delivering the functional multimeric complex to its appropriate membrane location in different cell types are differentially altered by the mutations. In this context, it is interesting that higher-order protein/protein interactions, including Na+/K+ pump multimers, may play an important role in determining localization of the enzyme. Such yet to be identified multimeric complexes (whether composed of only pumps or associated with other membrane proteins (e.g., NCX), may provide an explanation for major dominant-negative effects within pluripotent cells from patients with a CMT mutation (89) and for several α1 and α2 mutations that have relatively minor functional effects when these mutations are expressed in Xenopus oocytes, an expression system known to overexpress misfolded proteins, such as some cystic fibrosis transmembrane conductance regulator mutants that cause cystic fibrosis (109).
Regarding ATP1A1 in particular, the two most important remaining questions are: 1) Why do different mutations of the same protein lead to diseases with clearly distinct characteristics? and 2) what mechanisms are involved in each pathophysiological phenotype? The answers to these questions overlap. Obviously, in primary aldosteronism (PA) the mutated ATP1A1 is circumscribed to a small group of cells within the adrenal gland (either in adenomas or in aldosterone-producing clusters) without affecting other tissues in the body. This explains why mutations that induce PA do not produce neurologic or renal disorders. In addition, although one might expect PA in patients with CMT, complex spastic paraplegia, or hypomagnesemia due to ATP1A1 mutations, this has not been reported. Possibly, the young age of the few patients described with complex spastic paraplegia and hypomagnesemia may reduce the likelihood of detecting hypertension. However, patients with CMT2 comprise a wider age range with larger numbers of affected individuals and they appear normotensive. It is possible that the loss of Na+/K+ pump function induced by ATP1A1 mutations causing neurological diseases increases aldosterone production, but that the effect of aldosterone on blood pressure is compensated by mechanisms triggered early in development. In keeping with this hypothesis, constitutive heterozygous knockout ATP1A1+/− mice have increased aldosterone production, but maintain normal blood pressure (69). In this context, it appears important to know whether patients with ATP1A1 inducing neuropathies have elevated aldosterone levels to determine whether a compensatory mechanism is responsible for their normal blood pressure.
The aberrant inward “leak” current observed with many PA mutations (often called a gain of function; 41, 64) can explain their dominant character. However, it remains to be determined decisively whether simple haploinsufficiency or a dominant-negative effect underlies the disease observed with mutations that only produce a loss of function without inward currents, like G99R and I327S (62, 63). The use of inducible CRE-LOX models may help solve this conundrum in the near future.
An even larger puzzle is what makes a mutation cause either CMT, complex spastic paraplegia, or hypomagnesemia accompanied by seizures and cognitive delay. In all three disease types, the mutations affect half of all α1 subunits in all cells and tissues in the body. Obviously, the functional effects of these mutations must be distinct to induce such different diseases. Functional evaluation of CMT2 mutations in Xenopus oocytes indicates loss of function. It is therefore interesting that sensorimotor deficits in heterozygous ATP1A1+/− knockout mice have yet to be reported, even though these mice have been studied for at least 20 years (110–114). This observation may reflect: 1) that mice have shorter peripheral nerves than humans, therefore mitigating the deleterious effect of reduced intracellular Na+ clearance capacity, 2) that differences in motor activity between laboratory mice and the human patients contribute to this difference, or 3) a dominant-negative effect where expression of the mutant allele is required. However, as these mutations do not produce hypertension or electrolyte imbalances, if a dominant-negative hypothesis suggests changes in axonal membrane targetting, then one would expect that the determinants of such targetting be different in neurons than in other cells whose homeostasis also depends critically on Na+/K+ pump α1 subunits. Certainly, it is conceivable that yet to be identified cell-specific targetting determinants are responsible for the differences between neurological disorders. Alternatively, the differences may stem from the different physiological functions of the cells themselves. For example, high expression of Na+/K+ pumps in neurons is required to limit the refractory period after an impulse conduction. If a mutant ATP1A1 allele produces nonfunctional pumps that result in a dominant-negative effect on Na+/K+ pump expression, the reduction in functioning pumps at the plasma membrane (∼75% reduction assuming random assembly) would be debilitating for proper neuronal function. In contrast, the mutationally induced dominant-negative loss of Na+/K+ pump efficiency may have little to no effect in tissues where Na+/K+ pumps play more of a “housekeeping” role.
Regarding the other two neurological diseases, there is evidence that two-out-of-three mutations inducing hypomagnesemia accompanied with seizures present an aberrant inward current that may explain their distinction from CMT-inducing mutations. It is conceivable that the one mutation that did not induce external Na+-dependent depolarization when expressed in heterologous expression systems induced such inward currents under conditions that may be encountered within the cerebrospinal fluid (e.g., transient changes in pH or ion concentrations) that were not evaluated in the original article describing these mutations. In addition to explaining the difference between mutations that induce CMT and mutations that induce hypomagnesemia with seizures, such an inward current would also provide a straightforward explanation to the refractoriness of the CNS symptoms to normalizing Mg2+ levels. This is because the presence of a depolarizing inward current in the neuron could be responsible for the hyperexcitability underlying the seizures. The limited scope of experiments with the complex spastic paraplegia mutation reported to date is insufficient to reach conclusions beyond this mutation inducing a major loss of pump function.
In closing, it is important to stress that it is highly likely that novel ATP1A1-mutation pathologies will be described in the near future. In keeping with this, as this review article was being revised, two more de novo germline mutations (yellow tones in Fig. 2) were reported to cause two phenotypes distinct from previously described mutations. A female patient with the mutation D933N (in TM8, light yellow in Fig. 2), equivalent to D927N in ATP1A3, a mutation known to cause rapid-onset dystonia parkinsonism (25) and Na+/K+ pump loss of function (115, 116), presented with borderline intellectual performance, sleep disorder, and irritability, therefore sharing some, but not all symptoms with the patient with CSP. A boy carrying the mutation G864R (in TM7, dark yellow in Fig. 2) presented with severe developmental delay with focal seizures with normal serum magnesium (117). Exciting new studies aimed to determine what is special about each ATP1A1 mutation linked to disease will probably require a combination of varied heterologous expression systems, patient-derived, or genetically modified pluripotent cells, and genetically modified mouse models, to distinguish between the pathophysiologic mechanisms. Creating these cellular and animal models will be a particularly important development in the quest to find new treatments to help patients with these complex diseases.
GRANTS
This work was supported by the National Institute of Neurological Diseases and Stroke 1-R03 NS116433-01 (to P. Artigas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S., E.D.B., and P.A. prepared figures; E.D.B., K.S., G.A., L.M., and P.A. drafted manuscript; E.D.B., K.S., G.A., L.M., and P.A. edited and revised manuscript; E.D.B., K.S., G.A., L.M., and P.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Craig Gatto at Illinois State University and Guillermo Altenberg at TTUHSC for insightful comments on the manuscript and Dylan J. Meyer (currently at Harvard Medical School) for comments on the manuscript and the experiments used in Fig. 3.
Footnotes
It is customary to use gain of function for two types of effect: 1) one that enhances the function of the protein or 2) one that gives the protein a novel function. The term can be particularly confusing in cases where the “gain of function” implies a loss of the normal function with the appearance of a novel function, as when ATP1A1 mutations induce a passive ion pathway through the mutant pumps, which that at the same time dissipates the gradient that the pump normally build (i.e., an effective loss of normal function). Similar confusions could arise from the effect of these gain-of-function mutations in ion channels. Throughout this review, we will reserve gain of function to refer to higher activity of the protein in question and we will use aberrant function to refer to novel functions of a protein that imply an abnormal function (related or not to the normal function of the protein).
REFERENCES
- 1.Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol 8: 371, 2017. doi: 10.3389/fphys.2017.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Withering W. An Account of the Foxglove and Some of its Medical Uses: With Practical Remarks on Dropsy and Other Diseases. Birmingham: M. Swinney for J. G. J. and J. Robinson, 1785. [Google Scholar]
- 3.Stanley CM, Gagnon DG, Bernal A, Meyer DJ, Rosenthal JJ, Artigas P. Importance of the voltage dependence of cardiac Na/K ATPase isozymes. Biophys J 109: 1852–1862, 2015. doi: 10.1016/j.bpj.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Repke KR, Sweadner KJ, Weiland J, Megges R, Schon R. In search of ideal inotropic steroids: recent progress. Prog Drug Res 47: 9–52, 1996. doi: 10.1007/978-3-0348-8998-8_1. [DOI] [PubMed] [Google Scholar]
- 5.Kanai R, Cornelius F, Ogawa H, Motoyama K, Vilsen B, Toyoshima C. Binding of cardiotonic steroids to Na(+),K(+)-ATPase in the E2P state. Proc Natl Acad Sci USA 118: e2020438118, 2021. doi: 10.1073/pnas.2020438118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post RL, Sen AK, Rosenthal AS. A phosphorylated intermediate in adenosine triphosphate-dependent sodium and potassium transport across kidney membranes. J Biol Chem 240: 1437–1445, 1965. [PubMed] [Google Scholar]
- 7.Moreno C, Yano S, Bezanilla F, Latorre R, Holmgren M. Transient electrical currents mediated by the Na(+)/K(+)-ATPase: a tour from basic biophysics to human diseases. Biophys J 119: 236–242, 2020. doi: 10.1016/j.bpj.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 9.Lingrel JB, Kuntzweiler T. Na+,K(+)-ATPase. J Biol Chem 269: 19659–19662, 1994. [PubMed] [Google Scholar]
- 10.Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens 17: 526–532, 2008. doi: 10.1097/MNH.0b013e3283036cbf. [DOI] [PubMed] [Google Scholar]
- 11.Yap JQ, Seflova J, Sweazey R, Artigas P, Robia SL. FXYD proteins and sodium pump regulatory mechanisms. J Gen Physiol 153, 2021. doi: 10.1085/jgp.202012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen PL, Hakansson KO, Karlish SJ. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol 65: 817–849, 2003. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- 13.Blanco G. The NA/K-ATPase and its isozymes: what we have learned using the baculovirus expression system. Front Biosci 10: 2397–2411, 2005. doi: 10.2741/1705. [DOI] [PubMed] [Google Scholar]
- 14.Dyla M, Kjærgaard M, Poulsen H, Nissen P. Structure and mechanism of P-type ATPase ion pumps. Annu Rev Biochem 89: 583–603, 2020. doi: 10.1146/annurev-biochem-010611-112801. [DOI] [PubMed] [Google Scholar]
- 15.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys 40: 243–266, 2011. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 16.Gatto C, McLoud SM, Kaplan JH. Heterologous expression of Na(+)-K(+)-ATPase in insect cells: intracellular distribution of pump subunits. Am J Physiol Cell Physiol 281: C982–C992, 2001. doi: 10.1152/ajpcell.2001.281.3.C982. [DOI] [PubMed] [Google Scholar]
- 17.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290: F241–F250, 2006. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- 18.Meyer DJ, Bijlani S, de Sautu M, Spontarelli K, Young VC, Gatto C, Artigas P. FXYD protein isoforms differentially modulate human Na/K pump function. J Gen Physiol 152: e202012660, 2020. doi: 10.1085/jgp.202012660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 25: 292–303, 2005. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich T, Tavraz NN, Junghans C. ATP1A2 mutations in migraine: seeing through the facets of an ion pump onto the neurobiology of disease. Front Physiol 7: 239, 2016. doi: 10.3389/fphys.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deprez L, Weckhuysen S, Peeters K, Deconinck T, Claeys KG, Claes LR, Suls A, Van Dyck T, Palmini A, Matthijs G, Van Paesschen W, De Jonghe P. Epilepsy as part of the phenotype associated with ATP1A2 mutations. Epilepsia 49: 500–508, 2008. doi: 10.1111/j.1528-1167.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 22.Sampedro Castaneda M, Zanoteli E, Scalco RS, Scaramuzzi V, Marques Caldas V, Conti Reed U, da Silva AMS, O'Callaghan B, Phadke R, Bugiardini E, Sud R, McCall S, Hanna MG, Poulsen H, Mannikko R, Matthews E. A novel ATP1A2 mutation in a patient with hypokalaemic periodic paralysis and CNS symptoms. Brain 141: 3308–3318, 2018. doi: 10.1093/brain/awy283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm TH, Lykke-Hartmann K. Insights into the pathology of the α3 Na(+)/K(+)-ATPase ion pump in neurological disorders; lessons from animal models. Front Physiol 7: 209, 2016. doi: 10.3389/fphys.2016.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet 44: 1030–1034, 2012. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MAJ, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia Parkinsonism. Neuron 43: 169–175, 2004. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Demos MK, van Karnebeek CD, Ross CJ, Adam S, Shen Y, Zhan SH, Shyr C, Horvath G, Suri M, Fryer A, Jones SJ, Friedman JM; FORGE Canada Consortium. A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet J Rare Dis 9: 15, 2014. doi: 10.1186/1750-1172-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 45: 440–444, 444e1-2, 2013. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 28.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci USA 112: E4591–E4599, 2015. doi: 10.1073/pnas.1505529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lassuthova P, Rebelo AP, Ravenscroft G, Lamont PJ, Davis MR, Manganelli F, et al. Mutations in ATP1A1 cause dominant Charcot-Marie-Tooth type 2. Am J Hum Genet 102: 505–514, 2018. doi: 10.1016/j.ajhg.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlingmann KP, Bandulik S, Mammen C, Tarailo-Graovac M, Holm R, Baumann M, Konig J, Lee JJY, Drogemoller B, Imminger K, Beck BB, Altmuller J, Thiele H, Waldegger S, Van't Hoff W, Kleta R, Warth R, van Karnebeek CDM, Vilsen B, Bockenhauer D, Konrad M. Germline de novo mutations in ATP1A1 cause renal hypomagnesemia, refractory seizures, and intellectual disability. Am J Hum Genet 103: 808–816, 2018. doi: 10.1016/j.ajhg.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stregapede F, Travaglini L, Rebelo AP, Cintra VP, Bellacchio E, Bosco L, Alfieri P, Pro S, Zuchner S, Bertini E, Nicita F. Hereditary spastic paraplegia is a novel phenotype for germline de novo ATP1A1 mutation. Clin Genet 97: 521–526, 2020. doi: 10.1111/cge.13668. [DOI] [PubMed] [Google Scholar]
- 32.Monticone S, Tetti M, Burrello J, Buffolo F, De Giovanni R, Veglio F, Williams TA, Mulatero P. Familial hyperaldosteronism type III. J Hum Hypertens 31: 776–781, 2017. doi: 10.1038/jhh.2017.34. [DOI] [PubMed] [Google Scholar]
- 33.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84: 489–539, 2004. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- 34.Vinciguerra M, Deschênes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin P-Y, Féraille E. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell 14: 2677–2688, 2003. doi: 10.1091/mbc.e02-11-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol 279: C541–C566, 2000. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Sanchez CE, Kuppusamy M, Gomez-Sanchez EP. Somatic mutations of the ATP1A1 gene and aldosterone-producing adenomas. Mol Cell Endocrinol 408: 213–219, 2015. doi: 10.1016/j.mce.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amar L, Lepoutre C, Bobrie G, Plouin PF. [Endocrine hypertension]. Rev Med Interne 31: 697–704, 2010. doi: 10.1016/j.revmed.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest 122: 2046–2053, 2012. doi: 10.1172/JCI61996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett PQ, Guagliardo NA, Klein PM, Hu C, Breault DT, Beenhakker MP. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J Physiol 594: 5851–5860, 2016. doi: 10.1113/JP271896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RK. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331: 768–772, 2011. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azizan EAB, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LS, Brighton CA, Teo AED, Davenport AP, Dekkers T, Tops B, Kusters B, Ceral J, Yeo GSH, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P, Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 45: 1055–1060, 2013. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 42.Åkerström T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, Maharjan R, Robinson B, Iwen KA, Dralle H, D Volpe C, Bäckdahl M, Botling J, Stålberg P, Westin G, Walz MK, Lehnert H, Sidhu S, Zedenius J, Björklund P, Hellman P. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer 22: 735–744, 2015. doi: 10.1530/ERC-15-0321. [DOI] [PubMed] [Google Scholar]
- 43.Zheng FF, Zhu LM, Zhou WL, Zhang Y, Li MY, Zhu YC, Wang JG, Zhu DL, Gao PJ. A novel somatic mutation 145–147delETEinsK in KCNJ5 increases aldosterone production. J Hum Hypertens 31: 756–759, 2017. doi: 10.1038/jhh.2017.50. [DOI] [PubMed] [Google Scholar]
- 44.Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, Wang W-H, Lifton RP. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA 109: 2533–2538, 2012. doi: 10.1073/pnas.1121407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE. A Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab 98: E1861–E1865, 2013. doi: 10.1210/jc.2013-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniil G, Fernandes-Rosa FL, Chemin J, Blesneac I, Beltrand J, Polak M, Jeunemaitre X, Boulkroun S, Amar L, Strom TM, Lory P, Zennaro M-C. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine 13: 225–236, 2016. doi: 10.1016/j.ebiom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholl UI, Stölting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C, Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife 4: e06315, 2015. doi: 10.7554/eLife.06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholl UI, Stolting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, Yoo T, Choi J, Xu S, Wu A, Kirchner M, Mertins P, Rump LC, Onder AM, Gamble C, McKenney D, Lash RW, Jones DP, Chune G, Gagliardi P, Choi M, Gordon R, Stowasser M, Fahlke C, Lifton RP. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet 50: 349–354, 2018. doi: 10.1038/s41588-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandes-Rosa FL, Daniil G, Orozco IJ, Goppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, Schwarzmayr T, Strom TM, Jentsch TJ, Zennaro MC. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet 50: 355–361, 2018. doi: 10.1038/s41588-018-0053-8. [DOI] [PubMed] [Google Scholar]
- 50.Tevosian SG, Fox SC, Ghayee HK. Molecular mechanisms of primary aldosteronism. Endocrinol Metab (Seoul) 34: 355–366, 2019. doi: 10.3803/EnM.2019.34.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, Lucatello B, Ronconi V, Fallo F, Bernini G, Maccario M, Giacchetti G, Veglio F, Warth R, Vilsen B, Mulatero P. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension 63: 188–195, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01733. [DOI] [PubMed] [Google Scholar]
- 52.Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab 101: 494–503, 2016. doi: 10.1210/jc.2015-3284. [DOI] [PubMed] [Google Scholar]
- 53.Tauber P, Aichinger B, Christ C, Stindl J, Rhayem Y, Beuschlein F, Warth R, Bandulik S. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca(2+)-ATPase ATP2B3. Endocrinology 157: 2489–2499, 2016. doi: 10.1210/en.2015-2029. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala M-V, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro M-C. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 64: 354–361, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- 55.Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis A-C, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf) 83: 779–789, 2015. doi: 10.1111/cen.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, Thompson LDR, Cohen DL, Luther JM, Gellert L, Vaidya A, Barletta JA, Else T, Giordano TJ, Tomlins SA, Rainey WE. Genetic characteristics of aldosterone-producing adenomas in Blacks. Hypertension 73: 885–892, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, Shin CS, Kim SW, Kim SY. Genetics of aldosterone-producing adenoma in Korean patients. PLoS One 11: e0147590, 2016. doi: 10.1371/journal.pone.0147590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, Chen J, Zhou WL, Shen ZJ, Zhu YC, Wang JG, Zhu DL, Gao PJ. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 65: 622–628, 2015. doi: 10.1161/HYPERTENSIONAHA.114.03346. [DOI] [PubMed] [Google Scholar]
- 59.Wu V-C, Huang K-H, Peng K-Y, Tsai Y-C, Wu C-H, Wang S-M, Yang S-Y, Lin L-Y, Chang C-C, Lin Y-H, Lin S-L, Chu T-S, Wu K-D. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep 5: 11396, 2015. doi: 10.1038/srep11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, Fan Y, Ouyang J. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore) 94: e708, 2015. doi: 10.1097/MD.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanai R, Ogawa H, Vilsen B, Cornelius F, Toyoshima C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature 502: 201–206, 2013. doi: 10.1038/nature12578. [DOI] [PubMed] [Google Scholar]
- 62.Meyer DJ, Gatto C, Artigas P. On the effect of hyperaldosteronism-inducing mutations in Na/K pumps. J Gen Physiol 149: 1009–1028, 2017. doi: 10.1085/jgp.201711827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer DJ, Gatto C, Artigas P. Na/K pump mutations associated with primary hyperaldosteronism cause loss of function. Biochemistry 58: 1774–1785, 2019. doi: 10.1021/acs.biochem.9b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanley KS, Meyer DJ, Gatto C, Artigas P. Intracellular requirements for passive proton transport through the Na+,K+-ATPase. Biophys J 111: 2430–2439, 2016. doi: 10.1016/j.bpj.2016.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell TJ, Zugarramurdi C, Olivera JF, Gatto C, Artigas P. Sodium and proton effects on inward proton transport through Na/K pumps. Biophys J 106: 2555–2565, 2014. doi: 10.1016/j.bpj.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brini M, Carafoli E. The plasma membrane Ca(2+) ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 3: a004168, 2011. doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stindl J, Tauber P, Sterner C, Tegtmeier I, Warth R, Bandulik S. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na(+)/K(+)-ATPase. Endocrinology 156: 4582–4591, 2015. doi: 10.1210/en.2015-1466. [DOI] [PubMed] [Google Scholar]
- 68.Yingst DR, Davis J, Krenz S, Schiebinger RJ. Insights into the mechanism by which inhibition of Na,K-ATPase stimulates aldosterone production. Metabolism 48: 1167–1171, 1999. doi: 10.1016/s0026-0495(99)90133-6. [DOI] [PubMed] [Google Scholar]
- 69.Moseley AE, Huddleson JP, Bohanan CS, James PF, Lorenz JN, Aronow BJ, Lingrel JB. Genetic profiling reveals global changes in multiple biological pathways in the hearts of Na, K-ATPase alpha 1 isoform haploinsufficient mice. Cell Physiol Biochem 15: 145–158, 2005. doi: 10.1159/000083647. [DOI] [PubMed] [Google Scholar]
- 70.Suter U, Scherer SS. Disease mechanisms in inherited neuropathies. Nat Rev Neurosci 4: 714–726, 2003. doi: 10.1038/nrn1196. [DOI] [PubMed] [Google Scholar]
- 71.Stavrou M, Sargiannidou I, Christofi T, Kleopa KA. Genetic mechanisms of peripheral nerve disease. Neurosci Lett 742: 135357, 2021. doi: 10.1016/j.neulet.2020.135357. [DOI] [PubMed] [Google Scholar]
- 72.Gemignani F, Marbini A. Charcot-Marie-Tooth disease (CMT): distinctive phenotypic and genotypic features in CMT type 2. J Neurol Sci 184: 1–9, 2001. doi: 10.1016/s0022-510x(00)00497-4. [DOI] [PubMed] [Google Scholar]
- 73.Berciano J, García A, Gallardo E, Peeters K, Pelayo-Negro AL, Álvarez-Paradelo S, Gazulla J, Martínez-Tames M, Infante J, Jordanova A. Intermediate Charcot-Marie-Tooth disease: an electrophysiological reappraisal and systematic review. J Neurol 264: 1655–1677, 2017. doi: 10.1007/s00415-017-8474-3. [DOI] [PubMed] [Google Scholar]
- 74.Timmerman V, Strickland AV, Zuchner S. Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the human genome project success. Genes (Basel) 5: 13–32, 2014. doi: 10.3390/genes5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szigeti K, Lupski JR. Charcot-Marie-Tooth disease. Eur J Hum Genet 17: 703–710, 2009. doi: 10.1038/ejhg.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol 47: 673–698, 2013. doi: 10.1007/s12035-012-8370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernard-Marissal N, van Hameren G, Juneja M, Pellegrino C, Louhivuori L, Bartesaghi L, Rochat C, El Mansour O, Médard J-J, Croisier M, Maclachlan C, Poirot O, Uhlén P, Timmerman V, Tricaud N, Schneider BL, Chrast R. Altered interplay between endoplasmic reticulum and mitochondria in Charcot–Marie–Tooth type 2A neuropathy. Proc Natl Acad Sci USA 116: 2328–2337, 2019. doi: 10.1073/pnas.1810932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bird TD. Charcot-Marie-Tooth (CMT) hereditary neuropathy overview. In: GeneReviews((R)), edited by Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A.. Seattle (WA): University of Washington, 1993. [Google Scholar]
- 79.Viollet L, Glusman G, Murphy KJ, Newcomb TM, Reyna SP, Sweney M, et al. Alternating hemiplegia of childhood: retrospective genetic study and genotype-phenotype correlations in 187 subjects from the US AHCF Registry. PLoS One 10: e0127045, 2015. [Erratum in PLoS One 10: e0137370, 2015]. doi: 10.1371/journal.pone.0127045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmüller J, Frommolt P, Zirn B, Ebinger F, Siemes H, Nürnberg P, Brockmann K, Gärtner J. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol 11: 764–773, 2012. doi: 10.1016/S1474-4422(12)70182-5. [DOI] [PubMed] [Google Scholar]
- 81.Hoei-Hansen CE, Dali CÍ, Lyngbye TJB, Duno M, Uldall P. Alternating hemiplegia of childhood in Denmark: clinical manifestations and ATP1A3 mutation status. Eur J Paediatr Neurol 18: 50–54, 2014. doi: 10.1016/j.ejpn.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Panagiotakaki E, De Grandis E, Stagnaro M, Heinzen EL, Fons C, Sisodiya S, et al. Clinical profile of patients with ATP1A3 mutations in Alternating Hemiplegia of Childhood—a study of 155 patients. Orphanet J Rare Dis 10: 123, 2015. doi: 10.1186/s13023-015-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Price EM, Lingrel JB. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry 27: 8400–8408, 1988. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- 84.Kahle KT, Flores B, Bharucha-Goebel D, Zhang J, Donkervoort S, Hegde M, Hussain G, Duran D, Liang B, Sun D, Bonnemann CG, Delpire E. Peripheral motor neuropathy is associated with defective kinase regulation of the KCC3 cotransporter. Sci Signal 9: ra77, 2016. doi: 10.1126/scisignal.aae0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flores B, Schornak CC, Delpire E. A role for KCC3 in maintaining cell volume of peripheral nerve fibers. Neurochem Int 123: 114–124, 2019. doi: 10.1016/j.neuint.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kahle KT, Khanna AR, Alper SL, Adragna NC, Lauf PK, Sun D, Delpire E. K-Cl cotransporters, cell volume homeostasis, and neurological disease. Trends Mol Med 21: 513–523, 2015. doi: 10.1016/j.molmed.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adragna NC, Ravilla NB, Lauf PK, Begum G, Khanna AR, Sun D, Kahle KT. Regulated phosphorylation of the K-Cl cotransporter KCC3 is a molecular switch of intracellular potassium content and cell volume homeostasis. Front Cell Neurosci 9: 255, 2015. doi: 10.3389/fncel.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He J, Guo L, Lin S, Chen W, Xu G, Cai B, Xu L, Hong J, Qiu L, Wang N, Chen W. ATP1A1 mutations cause intermediate Charcot-Marie-Tooth disease. Hum Mutat 40: 2334–2343, 2019. doi: 10.1002/humu.23886. [DOI] [PubMed] [Google Scholar]
- 89.Manganelli F, Parisi S, Nolano M, Miceli F, Tozza S, Pisciotta C, Iodice R, Provitera V, Cicatiello R, Zuchner S, Taglialatela M, Russo T, Santoro L. Insights into the pathogenesis of ATP1A1-related CMT disease using patient-specific iPSCs. J Peripher Nerv Syst 24: 330–339, 2019. doi: 10.1111/jns.12357. [DOI] [PubMed] [Google Scholar]
- 90.Taniguchi K, Kaya S, Abe K, Mårdh S. The oligomeric nature of Na/K-transport ATPase1. J Biochem 129: 335–342, 2001. doi: 10.1093/oxfordjournals.jbchem.a002862. [DOI] [PubMed] [Google Scholar]
- 91.Viering D, de Baaij JHF, Walsh SB, Kleta R, Bockenhauer D. Genetic causes of hypomagnesemia, a clinical overview. Pediatr Nephrol 32: 1123–1135, 2017. doi: 10.1007/s00467-016-3416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vetter T, Lohse MJ. Magnesium and the parathyroid. Curr Opin Nephrol Hypertens 11: 403–410, 2002. doi: 10.1097/00041552-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 5: i3–i14, 2012. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brenner BM. Brenner & Rector's the Kidney. Philadelphia: W.B. Saunders Company, 2000, vol. 1. [Google Scholar]
- 95.Ahmed F, Mohammed A. Magnesium: the forgotten electrolyte—a review on hypomagnesemia. Med Sci (Basel) 7: 56, 2019. doi: 10.3390/medsci7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boron WF, Boulpaep EL. Medical Physiology. Philadelphia, PA: Elsevier, 2017, p. 1312. [Google Scholar]
- 97.Corral-Rodríguez MÁ, Stuiver M, Abascal-Palacios G, Diercks T, Oyenarte I, Ereño-Orbea J, de Opakua AI, Blanco FJ, Encinar JA, Spiwok V, Terashima H, Accardi A, Müller D, Martínez-Cruz LA. Nucleotide binding triggers a conformational change of the CBS module of the magnesium transporter CNNM2 from a twisted towards a flat structure. Biochem J 464: 23–34, 2014. doi: 10.1042/BJ20140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stuiver M, Lainez S, Will C, Terryn S, Günzel D, Debaix H, Sommer K, Kopplin K, Thumfart J, Kampik NB, Querfeld U, Willnow TE, Němec V, Wagner CA, Hoenderop JG, Devuyst O, Knoers NVAM, Bindels RJ, Meij IC, Müller D. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet 88: 333–343, 2011. doi: 10.1016/j.ajhg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A, Schweigel M. SLC41A1 is a novel mammalian Mg2+ carrier. J Biol Chem 283: 16235–16247, 2008. doi: 10.1074/jbc.M707276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meij IC, Koenderink JB, De Jong JC, De Pont JJ, Monnens LA, Van Den Heuvel LP, Knoers NV. Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase gamma-subunit. Ann N Y Acad Sci 986: 437–443, 2003. doi: 10.1111/j.1749-6632.2003.tb07226.x. [DOI] [PubMed] [Google Scholar]
- 101.Arjona FJ, de Baaij JHF, Schlingmann KP, Lameris ALL, van Wijk E, Flik G, Regele S, Korenke GC, Neophytou B, Rust S, Reintjes N, Konrad M, Bindels RJM, Hoenderop JGJ. CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet 10: e1004267, 2014. doi: 10.1371/journal.pgen.1004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sponder G, Mastrototaro L, Kurth K, Merolle L, Zhang Z, Abdulhanan N, Smorodchenko A, Wolf K, Fleig A, Penner R, Iotti S, Aschenbach JR, Vormann J, Kolisek M. Human CNNM2 is not a Mg(2+) transporter per se. Pflugers Arch 468: 1223–1240, 2016. doi: 10.1007/s00424-016-1816-7. [DOI] [PubMed] [Google Scholar]
- 105.Accogli A, Scala M, Calcagno A, Napoli F, Di Iorgi N, Arrigo S, Mancardi MM, Prato G, Pisciotta L, Nagel M, Severino M, Capra V. CNNM2 homozygous mutations cause severe refractory hypomagnesemia, epileptic encephalopathy and brain malformations. Eur J Med Genet 62: 198–203, 2019. doi: 10.1016/j.ejmg.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 106.de Baaij JHF, Dorresteijn EM, Hennekam EAM, Kamsteeg E-J, Meijer R, Dahan K, Muller M, van den Dorpel MA, Bindels RJM, Hoenderop JGJ, Devuyst O, Knoers NVAM. Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol Dial Transplant 30: 952–957, 2015. doi: 10.1093/ndt/gfv014. [DOI] [PubMed] [Google Scholar]
- 107.Sha Q, Pearson W, Burcea LC, Wigfall DA, Schlesinger PH, Nichols CG, Mercer RW. Human FXYD2 G41R mutation responsible for renal hypomagnesemia behaves as an inward-rectifying cation channel. Am J Physiol Renal Physiol 295: F91–F99, 2008. doi: 10.1152/ajprenal.00519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sweadner KJ, Arystarkhova E, Penniston JT, Swoboda KJ, Brashear A, Ozelius LJ. Genotype-structure-phenotype relationships diverge in paralogs ATP1A1, ATP1A2, and ATP1A3. Neurol Genet 5: e303, 2019. doi: 10.1212/NXG.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Csanady L, Vergani P, Gadsby DC. Structure, gating, and regulation of the CFTR anion channel. Physiol Rev 99: 707–738, 2019. doi: 10.1152/physrev.00007.2018. [DOI] [PubMed] [Google Scholar]
- 110.He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB. The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol 281: R917–R925, 2001. doi: 10.1152/ajpregu.2001.281.3.R917. [DOI] [PubMed] [Google Scholar]
- 111.Barcroft LC, Moseley AE, Lingrel JB, Watson AJ. Deletion of the Na/K-ATPase alpha1-subunit gene (Atp1a1) does not prevent cavitation of the preimplantation mouse embryo. Mech Dev 121: 417–426, 2004. doi: 10.1016/S0925-4773(04)00086-3. [DOI] [PubMed] [Google Scholar]
- 112.Dostanic I, Schultz JEJ, Lorenz JN, Lingrel JB. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem 279: 54053–54061, 2004. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 113.Moseley AE, Cougnon MH, Grupp IL, El Schultz J, Lingrel JB. Attenuation of cardiac contractility in Na,K-ATPase alpha1 isoform-deficient hearts under reduced calcium conditions. J Mol Cell Cardiol 37: 913–919, 2004. doi: 10.1016/j.yjmcc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 114.Kutz LC, Mukherji ST, Wang X, Bryant A, Larre I, Heiny JA, Lingrel JB, Pierre SV, Xie Z. Isoform-specific role of Na/K-ATPase alpha1 in skeletal muscle. Am J Physiol Endocrinol Metab 314: E620–E629, 2018. doi: 10.1152/ajpendo.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Einholm AP, Toustrup-Jensen MS, Holm R, Andersen JP, Vilsen B. The rapid-onset dystonia parkinsonism mutation D923N of the Na+, K+-ATPase alpha3 isoform disrupts Na+ interaction at the third Na+ site. J Biol Chem 285: 26245–26254, 2010. doi: 10.1074/jbc.M110.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poulsen H, Khandelia H, Morth JP, Bublitz M, Mouritsen OG, Egebjerg J, Nissen P. Neurological disease mutations compromise a C-terminal ion pathway in the Na(+)/K(+)-ATPase. Nature 467: 99–102, 2010. doi: 10.1038/nature09309. [DOI] [PubMed] [Google Scholar]
- 117.Lin Z, Li J, Wu JT, Gao Y, Jiang KY. . ATP1A1 de novo mutation-related disorders: clinical and genetic features. Front Pediatr 9: 657256, 2021. doi: 10.3389/fped.2021.657256. [DOI] [PMC free article] [PubMed] [Google Scholar]