Abstract
Background
People with chronic obstructive pulmonary disease (COPD) show considerable variation in symptoms, limitations, and well‐being; this often complicates medical care. A multi‐disciplinary and multi‐component programme that addresses different elements of care could improve quality of life (QoL) and exercise tolerance, while reducing the number of exacerbations.
Objectives
To compare the effectiveness of integrated disease management (IDM) programmes versus usual care for people with chronic obstructive pulmonary disease (COPD) in terms of health‐related quality of life (QoL), exercise tolerance, and exacerbation‐related outcomes.
Search methods
We searched the Cochrane Airways Group Register of Trials, CENTRAL, MEDLINE, Embase, and CINAHL for potentially eligible studies. Searches were current as of September 2020.
Selection criteria
Randomised controlled trials (RCTs) that compared IDM programmes for COPD versus usual care were included. Interventions consisted of multi‐disciplinary (two or more healthcare providers) and multi‐treatment (two or more components) IDM programmes of at least three months' duration.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. If required, we contacted study authors to request additional data. We performed meta‐analyses using random‐effects modelling. We carried out sensitivity analyses for the quality of included studies and performed subgroup analyses based on setting, study design, dominant intervention components, and region.
Main results
Along with 26 studies included in the 2013 Cochrane Review, we added 26 studies for this update, resulting in 52 studies involving 21,086 participants for inclusion in the meta‐analysis. Follow‐up periods ranged between 3 and 48 months and were classified as short‐term (up to 6 months), medium‐term (6 to 15 months), and long‐term (longer than 15 months) follow‐up. Studies were conducted in 19 different countries. The mean age of included participants was 67 years, and 66% were male. Participants were treated in all types of healthcare settings, including primary (n =15), secondary (n = 22), and tertiary care (n = 5), and combined primary and secondary care (n = 10). Overall, the level of certainty of evidence was moderate to high.
We found that IDM probably improves health‐related QoL as measured by St. George's Respiratory Questionnaire (SGRQ) total score at medium‐term follow‐up (mean difference (MD) ‐3.89, 95% confidence interval (CI) ‐6.16 to ‐1.63; 18 RCTs, 4321 participants; moderate‐certainty evidence). A comparable effect was observed at short‐term follow‐up (MD ‐3.78, 95% CI ‐6.29 to ‐1.28; 16 RCTs, 1788 participants). However, the common effect did not exceed the minimum clinically important difference (MCID) of 4 points. There was no significant difference between IDM and control for long‐term follow‐up and for generic QoL.
IDM probably also leads to a large improvement in maximum and functional exercise capacity, as measured by six‐minute walking distance (6MWD), at medium‐term follow‐up (MD 44.69, 95% CI 24.01 to 65.37; 13 studies, 2071 participants; moderate‐certainty evidence). The effect exceeded the MCID of 35 metres and was even greater at short‐term (MD 52.26, 95% CI 32.39 to 72.74; 17 RCTs, 1390 participants) and long‐term (MD 48.83, 95% CI 16.37 to 80.49; 6 RCTs, 7288 participants) follow‐up.
The number of participants with respiratory‐related admissions was reduced from 324 per 1000 participants in the control group to 235 per 1000 participants in the IDM group (odds ratio (OR) 0.64, 95% CI 0.50 to 0.81; 15 RCTs, median follow‐up 12 months, 4207 participants; high‐certainty evidence). Likewise, IDM probably results in a reduction in emergency department (ED) visits (OR 0.69, 95%CI 0.50 to 0.93; 9 RCTs, median follow‐up 12 months, 8791 participants; moderate‐certainty evidence), a slight reduction in all‐cause hospital admissions (OR 0.75, 95%CI 0.57 to 0.98; 10 RCTs, median follow‐up 12 months, 9030 participants; moderate‐certainty evidence), and fewer hospital days per person admitted (MD ‐2.27, 95% CI ‐3.98 to ‐0.56; 14 RCTs, median follow‐up 12 months, 3563 participants; moderate‐certainty evidence).
Statistically significant improvement was noted on the Medical Research Council (MRC) Dyspnoea Scale at short‐ and medium‐term follow‐up but not at long‐term follow‐up. No differences between groups were reported for mortality, courses of antibiotics/prednisolone, dyspnoea, and depression and anxiety scores. Subgroup analysis of dominant intervention components and regions of study suggested context‐ and intervention‐specific effects. However, some subgroup analyses were marked by considerable heterogeneity or included few studies. These results should therefore be interpreted with caution.
Authors' conclusions
This review shows that IDM probably results in improvement in disease‐specific QoL, exercise capacity, hospital admissions, and hospital days per person. Future research should evaluate which combination of IDM components and which intervention duration are most effective for IDM programmes, and should consider contextual determinants of implementation and treatment effect, including process‐related outcomes, long‐term follow‐up, and cost‐effectiveness analyses.
Plain language summary
Integrated disease management for people with chronic obstructive pulmonary disease
What are the effects of integrated disease management (IDM) programmes on quality of life, ability to exercise, and number of lung attacks compared to usual care in people with chronic obstructive pulmonary disease (COPD)?
Background
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease and is a major cause of ill health worldwide. People with COPD feel the impact of the disease in their daily life through symptoms such as breathlessness and coughing and acute worsening of symptoms in lung attacks.
Different healthcare providers, such as doctors, nurses, and physiotherapists, typically provide different types of care to people with COPD (e.g. prescribe medication, guide self‐management, provide education, present exercise training). Previously, people with COPD could visit one or more different healthcare providers, and these providers would work independently. The goal of an integrated disease management (IDM) programme is to include different components of care by which different healthcare providers are co‐operating and collaborating to provide more efficient care of better quality.
Study characteristics
We evaluated 52 studies involving 21,086 people with COPD. These studies were conducted in 19 countries spread all over the world. The average age of participants was 67 years, and 66% of participants were men. Some studies took place in general practices, some in hospitals, and some in both settings.
Key results
We found that people who participate in an IDM programme probably have better quality of life and their ability to exercise is probably improved compared to those receiving usual care. It is likely that people in an IDM programme have fewer hospital admissions for lung attacks and make fewer visits to an emergency department. When hospitalised, the total number of days people have to spend in hospital is reduced by two days. IDM programmes probably do not help to reduce the number of patients who die. The variety of available programmes makes it difficult to say if one IDM programme is the best.
Future studies should look at the most important components and the ideal length of the programme.
Certainty of the evidence
Overall, the certainty of our evidence was moderate to high but sometimes with large differences between studies.
This plain language summary is up‐to‐date as of February 2021.
Summary of findings
Summary of findings 1. Integrated disease management interventions compared to usual care for patients with chronic obstructive pulmonary disease.
| Integrated disease management interventions compared to usual care for patients with chronic obstructive pulmonary disease | ||||||
| Patient or population: patients with chronic obstructive pulmonary disease Setting: 15 studies in primary care, 22 studies in secondary care, 5 studies in tertiary care, 10 studies combination of primary and secondary care. 4 studies performed in North America, 9 studies in Northwestern Europe, 5 studies in Southern Europe, 3 studies in Oceania, 4 studies in East Asia, 3 studies in West Asia Intervention: integrated disease management interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with integrated disease management interventions | |||||
| Health‐related quality of life assessed with SGRQ (total) Scale from 0 to 100 (lower scores indicate better quality of life) Follow‐up: range 9 to 14 months; median 12 months | Mean change in SGRQ in control groups ranged from ‐6.77 to 6.24 points | MD 3.89 points lower (6.16 lower to 1.63 lower) | ‐ | 4321 (18 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | MCID for SGRQ is ‐4 points. Effect is not observed longer than 12 months |
| Functional exercise capacity assessed with 6MWD Follow‐up: range 9 to 14 months; median 12 months | Mean change in 6MWD in control groups ranged from ‐45.0 to 37.4 metres | MD 44.69 metres more (24.01 more to 65.37 more) | ‐ | 2071 (13 RCTs) | ⊕⊕⊕⊝ MODERATEa,c | MCID is 35 metres. The observed effect is consistent over time and is noticeable longer than 12 months |
| Respiratory‐related hospital admissions Follow‐up: range 3 to 36 months; median 12 months | Study population | OR 0.64 (0.50 to 0.81) | 4207 (15 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 324 per 1000 | 235 per 1000 (193 to 280) | |||||
| Hospital admissions, all causes Follow‐up: range 6 to 48 months; median 12 months | Study population | OR 0.75 (0.57 to 0.98) | 9030 (10 RCTs) | ⊕⊕⊕⊝ MODERATEd | ||
| 517 per 1000 | 445 per 1000 (379 to 512) | |||||
| Hospital days per patient, all causes Follow‐up: range 3 to 24 months; median 12 months | Mean hospital days per patient ranged from 1.6 to 25.5 days | MD 2.27 days fewer (3.98 fewer to 0.56 fewer) | ‐ | 3563 (14 RCTs) | ⊕⊕⊕⊝ MODERATEa | Mean change in hospital days ranged between an increase of 3.3 days and a reduction of 10.8 days |
| ED visits Follow‐up: range 3 to 48 months; median 12 months | Study population | OR 0.69 (0.50 to 0.93) | 8791 (9 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| 412 per 1000 | 326 per 1000 (259 to 394) | |||||
| *The basis for the assumed risk is provided in the footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the usual care group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walking distance; CI: confidence interval; ED: emergency department; IDM: integrated disease management; MCID: minimum clinically important difference; MD: mean difference; OR: odds ratio;RCT: randomised controlled trial;SGRQ: St. George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level because pooling showed substantial heterogeneity between studies, which could not or could only partially be explained by differences in the quality of studies.
bSubgroup analysis on the dominant component and region suggested intervention‐ and context‐specific effects.
cPooling of high‐quality studies showed a smaller non‐statistically significant difference of 6.51 metres (95% CI ‐7.53 to 20.55).
dDowngraded one level because pooling showed considerable heterogeneity and inconsistency in direction of effect between studies with statistical significantly fewer hospitalisations, with more hospitalisations, or with no differences between groups.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a heterogeneous, systemic condition characterised by restricted airflow that is not fully reversible. It is a major cause of morbidity because people with COPD experience chronic and progressive respiratory symptoms (i.e. dyspnoea and coughing) (GOLD 2020). The prevalence of COPD is currently estimated at 11.7% and is expected to increase substantially in the coming decades due to ageing of the world's population, continued use of tobacco, and exposure to indoor biomass pollution (GOLD 2020; Lopez 2006; Lozano 2012). According to the World Health Organization (WHO), COPD is the fourth leading cause of death in the world (Lopez 2006; WHO 2020). Additionally, COPD has important financial consequences, with high reported direct costs (e.g. healthcare resources, medication prescriptions) and indirect costs (e.g. absence from paid work, consequences of disability) (Britton 2003; FIRS 2017; Guarascio 2013).
Optimal management of COPD is complex as it is a multi‐component disease. Clinical, functional, and radiological presentations vary greatly from patient to patient, although patients may have a similar degree of airflow limitation (Agusti 2010; GOLD 2009; GOLD 2020; Wedzicha 2000). Previously, the sole focus in disease management lay on the degree of airflow limitation as a measure of disease severity (in the 2007 Global initiative for Chronic Obstructive Lung Disease (GOLD) classification of disease severity). This turned out to be a poor predictor of other important negative features of COPD, including health‐related quality of life (HRQoL) and exercise tolerance (Agusti 2010; Burgel 2010). These patient‐oriented outcomes are more important for people with COPD, given that COPD has a profound impact on HRQoL and exercise tolerance, even among those with modest airflow limitation (Engstrom 1996). Furthermore, impaired HRQoL (as shown in Domingo‐Salvany 2002, Fan 2002, and Martinez 2006) and exercise tolerance (as reported in Gerardi 1996 and Pinto‐Plata 2004) are associated with mortality (Cote 2009).
Some people are more prone than others to episodes of acute exacerbation, which is an important additional cause of morbidity, mortality, hospital admission, and impaired health status (Calverley 2003; Seemungal 1998; Wedzicha 2000). Although exacerbations become more severe and occur more frequently with increased severity of COPD, this is not always the case. There is evidence for a 'frequent‐exacerbation' phenotype (or group of people) with exacerbation more often than would be expected given disease 'severity' as predicted by lung function testing (Hurst 2010; Le Rouzic 2018).
Description of the intervention
Given that COPD is a disease with a clinically heterogeneous picture characterised by multiple disease components, treatment of patients with COPD requires that these different components of the disease be addressed in a comprehensive programme known as integrated disease management (IDM).
In the previous decade, the concept of IDM was introduced as a means of improving quality and efficiency of care for patients with chronic non‐communicable diseases such as COPD, heart failure, and diabetes mellitus. IDM interventions are aimed at reducing symptoms and avoiding fragmentation of care while containing costs. However, although IDM programmes are generally believed to be cost‐effective, evidence shows inconclusive results. Several systematic reviews have shown (partly) beneficial results for people with chronic heart failure (Gonseth 2004; Roccaforte 2005), diabetes (Bongaerts 2017; Knight 2005; Norris 2002; Pimouguet 2010), depression (Badamgarav 2003; Neumeyer‐Gromen 2004), and COPD (Cronin 2017).
It it important to note that there is no consensus in the literature about the definition of IDM. Several definitions have been proposed since the concept of 'disease management' was introduced. To facilitate communication between researchers, policy makers, and IDM program leaders, Schrijvers proposed a definition based on earlier reported definitions (Faxon 2004): "disease management consists of a group of coherent interventions designed to prevent or manage one or more chronic conditions using a systematic, multidisciplinary approach and potentially employing multiple treatment modalities. The goal of chronic disease management is to identify persons at risk for one or more chronic conditions, to promote self‐management by patients, and to address the illness or conditions with maximum clinical outcome, effectiveness, and efficiency regardless of treatment setting(s) or typical reimbursement patterns" (Schrijvers 2009). Peytremann‐Bridevaux and Burnand adapted the definition as follows: "chronic disease prevention and management consist of a group of coherent interventions, designed to prevent or manage one or more chronic conditions using a community‐wide, systematic, and structured multi‐disciplinary approach potentially employing multiple treatment modalities. The goal of chronic disease prevention and management is to identify persons with one or more chronic conditions, to promote self‐management by patients, and to address the illness or conditions according to disease severity and patient needs and based on the best available evidence, maximising clinical effectiveness and efficiency regardless of treatment setting(s) or typical reimbursement patterns. Routine process and outcome measurements should allow feedback to all those involved, as well as to adapt the programme" (Peytremann‐Bridevaux 2009).
Over the years, IDM programmes combining patient‐related, professional‐directed, and organisational interventions were developed with the goal of improving effectiveness and economic efficiency of long‐term care delivery (Lemmens 2009; Norris 2003; Wagner 2001). Since the previous version of this review of IDM for COPD patients (Kruis 2013), we have seen the advent of technology in IDM programmes, which potentially allows for continuously available and personalised types of patient guidance and monitoring (Kruse 2019).
Technology can be integrated into IDM programmes in different ways, such as use of SMS services, websites, apps, or home monitoring devices. Consequently, several different names are used to describe concepts within this area, such as telehealth, telemonitoring, telerehabilitation, eHealth, and mHealth, which have features that overlap. For the purposes of this systematic review, we adopted the term 'telemonitoring', defined as use of information and communication technologies to monitor and transmit items related to patient health status between geographically separated individuals (Maric 2009). Telemonitoring best describes the different interventions used in clinical studies, and is the term most studies have used themselves to describe their intervention. Hence, for this update, we have added telemonitoring as a possible additional component of IDM.
How the intervention might work
There is great variation in the symptoms, functional limitations, and degrees of psychological well‐being of patients with COPD, as well as in the speed of progression of COPD towards more severe stages (Agusti 2010). This calls for a multi‐faceted response, including different elements (e.g. smoking cessation, physiotherapeutic reactivation, self‐management, optimal medication adherence) targeted at the patient, the professional, and/or the organisation.
Ideally, COPD care is based on active self‐management to slow down progression of the disease, including daily self‐care, patient‐physician collaboration, and exacerbation management. Information should be tailored to patients' needs, knowledge level, and clinical profile and should be accessible to patients when they need it most (Bourbeau 2013; Tiep 1997)
Another potential benefit of IDM is that without proper self‐management, patients often refrain from reporting episodes of exacerbation to healthcare providers (Seemungal 2000). An important reason for this is fear of being sent to the hospital. Unfortunately, neglecting worsening of COPD leads to a negative spiral of increasing dyspnoea, deconditioning, and social deprivation. Eventually, this avoidant behaviour can lead to a respiratory crisis, which necessitates urgent referral to the hospital and might cause further damage to the lungs. To break through this self‐reinforcing negative spiral, healthcare professionals must collaborate with their patients. This requires focus on improving and maintaining self‐management skills, for example, by urging patients to respond rapidly and seek help to prevent further worsening (Chavannes 2008).
More recently, it has been argued that the addition of telemonitoring to IDM programmes allows for more continuous guidance and might lead to detection of deterioration earlier because of the potential for more frequent assessments. This could lead to more personalised management and prevention of exacerbations (Kruse 2019). However Kruse 2019 also concluded that it is unclear whether this approach enables people with COPD to self‐manage more easily. Telemonitoring for pulmonary rehabilitation showed effects similar to those seen with conventional face‐to‐face, centre‐based pulmonary rehabilitation for numerous outcomes (Cox 2021).
Why it is important to do this review
Review authors undertook the original version of this Cochrane Review in 2013 following a number of other (systematic) reviews that described beneficial effects of IDM for the health status of patients with COPD but were unable to draw firm conclusions due to large heterogeneity among interventions, study populations, outcome measurements, and methodological quality. This original review included 26 studies (Kruis 2013), and review authors concluded that IDM improved disease‐specific QoL and exercise capacity while reducing hospital admissions and hospital days per person.
An update of the review is required because since that time, many new studies have been conducted to evaluate the effects of IDM programmes on quality of life, exercise capacity, lung function, and exacerbation‐related outcomes such as respiratory‐related hospital admissions and emergency department (ED) visits. Also, COPD care globally has advanced tremendously. Advancements include greater financial reimbursement for pulmonary rehabilitation programmes and use of technological and digital opportunities. These have altered and potentially improved usual care and have resulted in new studies on the effectiveness of different types of IDM programmes, including telemonitoring interventions. Furthermore, the introduction of telemonitoring has allowed better assessment of actual adherence to IDM programmes due to logging of data entry in apps. This has reinforced the importance of long‐term follow‐up of outcomes, given that rates of adherence to the IDM programme vary widely and subsequently observed effects can be short‐lived (Cheikh‐Moussa 2020; Herbert 2018). Finally, the studies included in the previous review provided insufficient data to permit firm conclusions about the long‐term effectiveness of IDM.
In summary, in this update of the review, we aimed to summarise and assess evidence of short‐, medium‐, and long‐term effectiveness of IDM compared to usual care among patients with COPD.
Objectives
To compare the effectiveness of integrated disease management (IDM) programmes versus usual care for people with chronic obstructive pulmonary disease (COPD) in terms of health‐related quality of life (QoL), exercise tolerance, and exacerbation‐related outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐randomised trials in which IDM programmes or interventions were compared with control (i.e. usual care) in people with COPD. We excluded non‐randomised controlled trials and other intervention studies.
Types of participants
People with a clinical diagnosis of COPD according to the GOLD criteria were included: people with chronic respiratory symptoms (i.e. coughing, sputum, or dyspnoea) and a limited post‐bronchodilator forced expiratory volume in one second (FEV₁)‐to‐forced vital capacity (FVC) ratio < 0.7. Severity of airflow obstruction was classified by the GOLD stages of 2009 (GOLD 2009). All GOLD stages were accepted. Studies including participants with diagnoses other than COPD were only eligible if results for participants with COPD were available separately.
Types of interventions
We included studies in which the IDM intervention consisted of strategies to improve care for patients with COPD including organisational, professional, patient‐directed (e.g. self‐ management, education), and financial interventions. We classified these according to the Cochrane Effective Practice and Organisation of Care Group (EPOC) taxonomy of interventions (EPOC 2008), complemented with patient‐directed interventions. To be included in the review, a study had to include at least two of the following components of the IDM intervention.
Education/self‐management: education, self‐management, personal goals and/or action plan, exacerbation management.
Exercise: (home) exercise training and/or strength and/or endurance training.
Psychosocial component: cognitive‐behavioural therapy, stress management, other psychological assessment and/or treatment.
Smoking cessation.
Medication: optimisation medication regimen/prescription of medication adherence.
Nutrition: dietary intervention.
Follow‐up and/or communication: structural follow‐up and/or communication, case management by nurses, optimal diagnosis.
Multi‐disciplinary team: active participation and formation of teams of professional caregivers from different disciplines, revision of professional roles, integration of services, local team meetings.
Financial intervention: fees/payments/grants for providing IDM.
Furthermore, as IDM included different components, as mentioned above, different healthcare disciplines should be involved in delivery of the IDM programme. Hence, we included a study only if at least two different disciplines of healthcare providers were actively involved in the IDM programme.
Finally, a study should have a minimum duration of the IDM intervention of three months.
For all studies, we determined the dominant component of the programme by verifying with the study authors. If this was not possible, we decided based on the duration and intensity of each component. With the emergence of telemonitoring studies, we added telemonitoring as a separate dominant component post hoc.
Types of outcome measures
We specified the following outcomes a priori.
Primary outcomes
Health‐related quality of life (HRQoL), as reported by a validated disease‐specific questionnaire (e.g. St. George's Respiratory Questionnaire (SGRQ) ‐ Jones 1991; Jones 2005; Clinical COPD Questionnaire (CCQ) ‐ Kocks 2006, van der Molen 2003; Chronic Respiratory Questionnaire (CRQ) ‐ Guyatt 1987; Guyatt 2011; COPD Assessment Test (CAT) ‐ Jones 2009) or a generic quality of life questionnaire (e.g. Short Form‐36 (SF‐36) ‐ Ware 1992 EuroQol‐5D (EQ‐5D) ‐ EuroQol Group 1990)
Maximal or functional exercise capacity, as reported by peak capacity measured in the exercise laboratory by an incremental exercise test defined according to results of the 6‐minute walking distance test (6MWD) ‐ Redelmeier 1997 ‐ or the shuttle run test ‐ Singh 1992
Exacerbation‐related outcomes, as reported by one of the following: all‐cause hospital admissions, respiratory‐related hospital admissions, all‐cause hospital days, emergency department (ED) visits, patients with at least one exacerbation and patients with at least one prescription for prednisone and at least one for antibiotics. These outcomes follow the latest definitions of moderate and severe COPD exacerbations in the GOLD guideline and are also used in the two latest Cochrane Reviews assessing exacerbations as a primary outcome (GOLD 2020; Threapleton 2019; Walsh 2019)
Secondary outcomes
Clinical outcomes
Dyspnoea, as measured by the Medical Research Council (MRC) Dyspnea Scale ‐ Bestall 1999 ‐ or the Borg Scale ‐ Borg 1970
Survival (mortality)
Lung function (FEV₁, FVC)
Depression, as measured by the Hospital Anxiety and Depression Scale (HADS) ‐ Zigmond 1983 ‐ or the Beck Depression Inventory (BDI) ‐ Beck 1961
Process‐related outcomes
Coordination of care (e.g. accessibility of care, rate of patient participation in the disease management programme, patients' and healthcare professionals' satisfaction with the programme, extent to which disease management was implemented, from the perspective of the patient (PACIC) ‐ Glasgow 2005)
We evaluated outcomes at (1) short‐term (up to 6 months), (2) medium‐term (6 to 15 months), and (3) long‐term (longer than 15 months) endpoints, if possible.
Search methods for identification of studies
Electronic searches
The previously published version of this Review included studies up to April 2013. For the current update, we identified studies using the Cochrane Airways Group Register of trials; the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE (Ovid SP); Embase (Ovid SP); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO).
We used specific medical subject headings (from MeSH) and additional keywords to identify all trials on IDM in COPD patients. The search strategy was developed and conducted in collaboration with the Cochrane Airways Information Specialist. The initial strategy was developed for MEDLINE and was adapted for use in the other databases.
Complete search strategies for the database searches are provided in the appendices (MEDLINE ‐ Appendix 1; Embase ‐ Appendix 2; CINAHL ‐ Appendix 3; CENTRAL ‐ Appendix 4; Airways Register ‐ Appendix 5). The search period for this update covers April 2013 to September 2020. This includes an initial search on 4 January 2017 and updates in March 2018 and March 2019. We ran a final update search in September 2020.
Searching other resources
To identify all possible studies, we carried out an additional search for systematic reviews in the Cochrane Database of Systematic Reviews. We also screened reference lists of included studies and systematic reviews for potential studies for inclusion in the current review. To identify ongoing or new studies, we searched databases of ongoing studies, including ClinicalTrials.gov (up to September 2020) and the WHO International Clinical Trials Registry Platform (ICTRP) (up to March 2019). See Appendix 6 for those search terms.
Data collection and analysis
Selection of studies
The lead review author (CP) and one of two other review authors (EM, PH) independently assessed the title and abstract of each identified citation. If there was any doubt, we retrieved the full‐text article and examined it for inclusion eligibility. Disagreements were discussed during a consensus meeting. When consensus could not be reached, the third review author (AK ‐ the first author of the original 2013 review) adjudicated. Subsequently, the full text of the potential eligible abstract was read by two review authors (CP and EM or PH) before a decision was made regarding its inclusion in the review.
Data extraction and management
For the current update, we used Covidence to extract data and assess risk of bias for each included study (Covidence). The lead review author (CP) extracted data from all papers identified for inclusion using a digital data extraction form. Two other review authors (EM, PH) independently extracted data from an equal share of the same studies. We collected the following information: (1) study design (e.g. randomisation method, sample size, blinding); (2) participant characteristics (e.g. age, sex, COPD diagnosis); (3) interventions (i.e. setting, number of professionals involved, elements of IDM programme/intervention, frequency and duration of intervention); (4) outcome measures and timing of outcome assessment; and (5) results (e.g. loss to follow‐up, outcomes). Any discrepancies in data extraction between review authors were resolved through discussion. In case of missing data, we contacted the authors of these studies to request additional information or clarification.
Assessment of risk of bias in included studies
The lead review author (CP) assessed the risk of bias for all included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two other review authors (EM, PH) independently assessed risk of bias for an equal share of the same studies. Disagreements were resolved through discussion. The following risk of bias items were assessed.
Random sequence generation.
Concealment of allocation.
Blinding of participants and personnel, in relation to the intervention.
Blinding of outcome assessment (i.e. patient‐reported outcome, other outcomes).
Incomplete outcome data.
Selective outcome reporting.
Other bias.
As cluster‐randomised trials were also included, we added the following design‐related domains for these types of studies.
Recruitment bias (i.e. whether individuals were recruited after clusters had been randomised).
Baseline imbalance between groups (i.e. whether risk of baseline differences was reduced by using stratified or pair‐matched randomisation of clusters).
Loss of follow‐up of clusters (i.e. whether missing clusters and missing outcomes for individuals within clusters could lead to a risk of bias in cluster‐randomised trials).
Methods of analysis adequate for cluster‐randomised controlled trials (i.e. whether clustering was taken into account in the analysis) (Higgins 2011).
We judged all items as having high, low, or unclear risk of bias and provided a quote from the study and/or a justification for our decision.
Measures of treatment effect
We analysed results of the studies in RevMan 5, using random‐effects modelling. We used forest plots to compare results across trials. When possible, results were related to the minimum clinically important difference (MCID) for the respective variable. We undertook meta‐analysis only when this was meaningful, that is, when treatment, participants, and the underlying clinical question were similar enough for pooling to make sense, and when the results of at least two RCTs were available.
We used intention‐to‐treat data or the 'full analysis set' whenever reported. We used per‐protocol analysis when neither was reported. Normally, outcome measures that have been adjusted for baseline differences produce the most reliable outcomes. However, these can be analysed only by generic inverse variance (GIV). Also, we noted significant variation in the number of parameters adjusted for between studies. Hence, we used unadjusted values in our random‐effects modelling for studies with an RCT design, and values adjusted for potential clustering effects for studies with a cluster‐RCT design.
When multiple trial arms were reported in a single study (e.g. hospital‐based pulmonary rehabilitation and home‐based pulmonary rehabilitation), we included all relevant trial arms. We halved the control group in these cases to avoid double‐counting, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16.5.4) (Higgins 2019a).
Unit of analysis issues
When a study used a cluster‐RCT design, we calculated the estimate of effect by using the GIV whenever possible. We used the mean difference (MD) and the 95% confidence interval (CI) reported by study authors when the appropriate analyses were used and authors had adjusted for cluster effect. We calculated a dummy mean change and standard deviation (SD) based on the MD and its 95% CI for cluster‐RCT studies, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 23.1.3) (Higgins 2019b).
In case of a unit of analysis error in cluster‐RCTs, we adjusted for the design effect by reducing the size of the trial to its "effective sample size" (Rao 1992). The effective sample size of a single intervention group in a cluster‐randomised trial is its original sample size divided by a quantity called the 'design effect'. The design effect is 1 + (M ‐ 1) * ICC, where M is the average cluster size, and ICC is the intra‐cluster correlation coefficient. For dichotomous data, both the total number of participants and the number of participants experiencing the event were divided by the design effect. For continuous data, for which the GIV method could not be used, only sample sizes were reduced, and means and SDs were left unchanged (Higgins 2011).
Dealing with missing data
When a study paper missed important statistical information required for analysis, or required additional calculations that needed to be clarified, we attempted to contact study authors to gather the required information. When authors had not calculated relevant statistics but presented supporting data, we conducted calculations using methods described in the 2019 Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a). When studies did not report SDs for change from baseline but did provide information on means, standard errors (SEs), 95% CIs, P values, and population sizes across groups, we calculated SDs for change from baseline using the RevMan 5 internal calculator.
When we could not directly calculate the SD for change from baseline, we imputed the SD using a correlation coefficient as described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 6.5.2.8) (Higgins 2019a). We calculated the correlation coefficient by using the weighted mean (based on size of the study) of two or more studies that reported results for the respective variable in sufficient detail.
In the case that fewer than two studies provided sufficient information, a weighted mean correlation coefficient could not be calculated. In that case, we used data on post‐intervention measurements, as they are considered to be more precise.
For studies that reported a median instead of a mean, we estimated the mean and the SD using the method and open‐access calculator provided in Wan 2014.
Assessment of heterogeneity
We assessed heterogeneity in each meta‐analysis both visually through inspection of forest plots and statistically using tau², I², and the T statistic (Higgins 2019). We regarded heterogeneity as substantial when I² was greater than 50% or a low P value (< 0.10) was reported for the Chi² test for heterogeneity. We reported heterogeneity and explored the possible causes. In cases of substantial (I² > 50%) or considerable (I² > 75%) heterogeneity, we investigated sources for heterogeneity by conducting subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
The likelihood of publication bias was investigated by preparing a funnel plot only if ten or more studies were included in the meta‐analysis. Based on visual inspection, the likelihood of publication bias was evaluated. When asymmetry was observed, we attempted to identify possible reasons by considering the quality of the studies, the particular interventions included, and the contexts in which interventions were implemented.
Data synthesis
We performed statistical analyses using Review Manger software 5.3 (RevMan 5) and RevMan Web 2019 (RevMan Web 2019).
We pooled study results using the random‐effects model. For continuous data, we recorded mean change from baseline to endpoint and SD for each group and calculated the MD. For dichotomous data, we recorded the number of participants with each outcome event and calculated the odds ratio (OR). We used all results reported at short‐, medium‐, and/or long‐term follow‐up. Given that all interventions had a duration of 12 weeks at minimum, we analysed available data at 6 months for the short term. We analysed data measured most medial to the other time points (i.e. for medium term, we used results at 12 months when 9 and 12 months were given). When possible, we discussed the intervention effect estimate in the context of its MCID. If the meta‐analysis led to statistically significant overall estimates, we transformed these results back into measures that are clinically useful in daily practice, such as the number needed to treat for an additional beneficial outcome (NNTB).
Subgroup analysis and investigation of heterogeneity
To explain heterogeneity among study results, we planned the following subgroup analyses a priori (when data were available) to determine if outcomes differed among:
settings of the IDM intervention (e.g. primary, secondary, or tertiary care);
study designs (individually randomised patients versus cluster‐randomised patients); and
intervention groups, with regard to different components as listed by the EPOC classification (EPOC 2008).
We performed an additional post‐hoc subgroup analysis based on the region in which the study was conducted (i.e. North America, South America, Northwestern Europe, Southern Europe, East Asia, Central Asia) to account for regional differences in usual care and customs regarding hospitalisation, which proved to be large in Kessler 2018. The previous review authors planned to include an additional subgroup on disease severity (Kruis 2013), but they were unable to do so due to the poor quality of reporting. Also, Kruis 2013 performed an additional subgroup analysis based on control group (i.e. no treatment, treatment with one healthcare provider, treatment with one component, other disease management interventions). In the past decade, regular care has evolved in such a way that multiple individual 'intervention components' (e.g. exercise advice, educational flyers) are delivered to patients with COPD; therefore, classification would be too ambiguous, depending largely on what is reported. Hence, this review does not include different control groups as a subgroup analysis.
Sensitivity analysis
We performed sensitivity analyses on the basis of the methodological quality of studies. We did so by repeating our analysis among only studies judged to be of 'high quality'. For the purposes of this review, 'high‐quality studies' were defined as studies with low or unclear risk of bias due to allocation concealment, low or unclear risk of bias due to incomplete outcome data, and, in the case of cluster‐RCTs, studies with adequate analysis methods.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of this review in a 'Summary of findings' table, which includes an overall rating of the evidence using the GRADE approach, in accordance with recommendations laid out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This involves making separate ratings for quality of evidence for each patient‐important outcome by identifying five factors that can lower the quality of evidence, including study limitations, indirectness of evidence (also called clinical heterogeneity with regard to study population, intervention, control group, and outcomes), unexplained heterogeneity or inconsistency of results (i.e. statistical heterogeneity), imprecision of results (i.e. due to small sample sizes and few events), and high probability of publication bias. However, other factors can increase the quality of evidence; these include large magnitude of effect; plausible confounding, which could reduce the demonstrated effect; and the dose‐response gradient (GRADE Working Group 2004). We have presented footnotes to justify decisions made and have provided comments to support readers' understanding of this review.
We intended to present short‐, medium‐, and long‐term outcomes for all of our primary outcomes in the 'Summary of findings' table. However, because we were limited to a maximum of seven outcomes, we decided to present dichotomous outcomes for all time points and continuous outcomes for medium‐term follow‐up only, being most clinically relevant. For all outcomes, we presented the range and the median follow‐up.
Results
Description of studies
See Characteristics of included studies.
Results of the search
Our literature search yielded 6900 citations after duplicates were removed with potential for inclusion (see Figure 1). We excluded 6543 citations during the initial screening of titles and abstracts and assessed full texts of 357 citations. Eleven studies were ongoing at the time of this review (Ali 2020; Bourne 2017; Ding 2019; Drennan 2014; Foot 2017; Hajizadeh 2020a; Hansen 2017; NCT04136418; NCT04416295; NCT04533412; Steed 2017). One study had finished data collection, but as the results were not yet published, study authors wished to withhold results until after publication (Bourne 2017). A further seven provided insufficient detail to allow a decision on eligibility. We were unable to establish contact with the study authors, so some studies are still awaiting classification (Baumann 2012; Borji, 2018; Carcereny, 2016; Mao 2020; NCT04256070; Reguera 2017; Xu 2010). Thus, 26 new studies (57 citations) were added to this review, in addition to the 26 studies already included in the previous version of the review.
1.
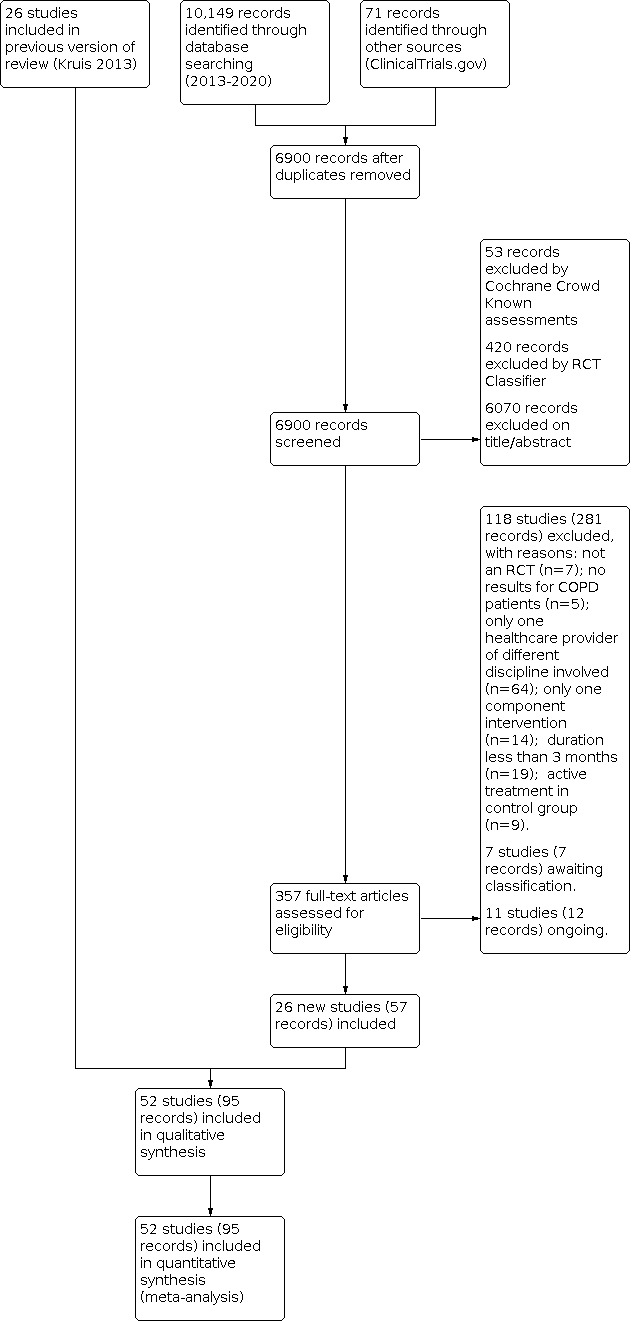
Study flow diagram
Included studies
We included the 26 RCTs from the 2013 version of the Cochrane Review (Kruis 2013). A total of 52 studies (represented by 95 citations) contributed to the current meta‐analysis, including 26 new studies (Aboumatar 2019; Bernocchi 2017; Fan 2012; Freund 2016; Haesum 2012; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Kennedy 2013; Kessler 2018; Khan 2019; Ko 2016; Kruis 2014; Lenferink 2019; Lilholt 2017; Lou 2015; Öztürk 2020; Rose 2017; Sanchez‐Nieto 2016; Silver 2017; Tabak 2014; Titova 2017; Vasilopoulou 2017; Vianello 2016; Wang 2017; Zhang 2020; Zwar 2016). The newly included studies were published between 2014 and 2020 and originated from across the globe. Four studies originated from China (Ko 2016; Lou 2015; Wang 2017; Zhang 2020), three from the USA (Aboumatar 2019; Fan 2012; Silver 2017), and one from Canada (Rose 2017). A total of nine studies were performed in Northwestern Europe ‐ three in the Netherlands (Kruis 2014; Lenferink 2019; Tabak 2014), two in Denmark (Haesum 2012; Lilholt 2017), and one each in Germany (Freund 2016), the UK (Kennedy 2013), and Norway (Titova 2017). Kessler 2018 was a multi‐national multi‐centre study performed in Germany, France, Italy, and Spain. Five studies were performed in Southern Europe ‐ Italy (Bernocchi 2017; Vianello 2016), Spain (Jimenez‐Reguera 2020; Sanchez‐Nieto 2016), and Greece (Vasilopoulou 2017). Three studies were performed in Western Asia ‐ one in Israel (Kalter‐Leibovici 2018), one in Pakistan (Khan 2019), and one in the Asian part of Turkey (Öztürk 2020). One study originated from Australia (Zwar 2016).
Of the 52 studies that met eligibility criteria, nine used a cluster‐RCT design, with general practices or healthcare regions as the unit of randomisation (Freund 2016; Kennedy 2013; Khan 2019; Kruis 2014; Lilholt 2017; Lou 2015; Rea 2004; Wood‐Baker 2006; Zwar 2016). All but two trials randomly assigned participants to either IDM or usual care. The other two trials had two different intervention groups and one usual care group (Vasilopoulou 2017; Wijkstra 1994). We included both intervention groups as separate comparisons and split the usual care group in half.
A description of the included studies is provided in Table 2Table 3, and Characteristics of included studies.
1. Characteristics of included studies.
| Study | Country | Region | N (randomised) | N (completed) | Number of intervention components | Number of healthcare providers | Dominant component intervention | Duration intervention | Setting | Control group |
| Aboumatar 2019 | USA | North America | 240 | 187 | 3 | 2 | SM | 3 months | SEC | U |
| Aiken 2006 | USA | North America | 41 | 18 | 5 | 2 | SF | 6 months | PRIM | U |
| Bendstrup 1997 | Denmark | Northwestern Europe | 42 | 32 | 4 | 7 | E | 3 months | SEC | U |
| Bernocchi 2017 | Italy | Southern Europe | 112 | 80 | 5 | 3 | TM | 3 months | PRIM/SEC | |
| Bourbeau 2003 | Canada | North America | 191 | 165 | 4 | 4 | SM | 8 weeks + 10 months maintenance | SEC | U |
| Boxall 2005 | Australia | Oceania | 60 | 46 | 2 | 3 | E | 3 months | PRIM | U |
| Cambach 1997 | Netherlands | Northwestern Europe | 43 | 23 | 2 | 2 | E | 3 months | PRIM | DRUG |
| Dheda 2004 | UK | Northwestern Europe | 33 | 25 | 4 | 2 | SF | 6 months | SEC | U |
| Engstrom 1999 | Sweden | Northwestern Europe | 55 | 50 | 4 | 5 | E | 4.5 months + 7.5 months maintenance | SEC | U |
| Fan 2012 | USA | North America | 426 | 426 | 4 | 2 | EDU | 4 weeks + 11 months follow‐up | SEC | U |
| Farrero 2001 | Spain | Southern Europe | 122 | 94 | 2 | 2 | SF | 12 months | SEC | U |
| Fernandez 2009 | Spain | Southern Europe | 50 | 41 | 2 | 2 | E | 11 months | PRIM | EDU |
| Freund 2016 | Germany | Northwestern Europe | 543 (COPD) | unknown | 5 | 2 | S | 12 months | PRIM | U |
| Gottlieb 2011 | Denmark | Northwestern Europe | 61 | 26 | 4 | Multi‐disciplinary team, not specified | E | 7 weeks + 6 months maintenance | PRIM | U |
| Güell 2000 | Spain | Southern Europe | 60 | 47 | 3 | 3 | E | 6 months + 6 months maintenance | SEC | U |
| Güell 2006 | Spain | Southern Europe | 40 | 25 | 2 | 4 | E | 4 months | TERT | DRUG |
| Haesum 2012 | Denmark | Northwestern Europe | 111 | 105 | 4 | Primary and secondary caregivers, not specified | TM | 4 months | PRIM/SEC | U |
| Jimenez‐Reguera 2020 | Spain | Southern Europe | 44 | 36 | 6 | 3 | SM | 8 weeks + 10 months maintenance | SEC | U |
| Kalter‐Leibovici 2018 | Israel | Western Asia | 1202 | 992 | 3 | SF | Minimum 2 years, maximum 5 years | SEC | U | |
| Kennedy 2013 | UK | Northwestern Europe | 1634 | 1146 | 2 | 2 | SM | PRIM | U | |
| Kessler 2018 | International (Germany, France, Italy, Spain) | Northwestern Europe, Southern Europe | 345 | 80 | 5 | 2 | SF | 12 months | SEC | U |
| Khan 2019 | Pakistan | Western Asia | 313 | 288 | 4 | 4 | SF | 6 months | PRIM | U |
| Ko 2016 | China | East Asia | 180 | 142 | 6 | 3 | SF | 8 weeks + 10 weeks maintenance | TERT | U |
| Koff 2009 | USA | North America | 40 | 38 | 4 | 2 | SM | 3 months | PRIM | U |
| Kruis 2014 | Netherlands | Northwestern Europe | 1086 | 810 | 6 | 5 | SM | 12 months | PRIM | U |
| Lenferink 2019 | Netherlands, Australia | Northwestern Europe, Oceania | 201 | 169 | 6 | 2 | SM | 9 months | SEC | U |
| Lilholt 2017 | Denmark | Northwestern Europe | 1125 | 574 | 4 | 2 | SF | 12 months | PRIM | U |
| Littlejohns 1991 | UK | Northwestern Europe | 152 | 133 | 4 | 3 | SF | 12 months | SEC | U |
| Lou 2015 | China | East Asia | 8171 | 6221 | 9 | 5 | EDU | 48 months | PRIM | U |
| Mendes 2010 | Brazil | South America | 117 | 85 | 2 | 2 | E | 3 months | PRIM/SEC | U |
| Öztürk 2020 | Turkey | Western Asia | 80 | 61 | 5 | 4 | SM | 3 months | SEC | U |
| Rea 2004 | New Zealand | Oceania | 135 | 117 | 5 | 4 | SM/SF | 12 months | PRIM/SEC | U |
| Rice 2010 | USA | North America | 743 | 743 | 3 | 2 | SM | 12 months | SEC | EDU |
| Rose 2017 | Canada | North America | 475 | 398 | 5 | 3 | SF | 9 months | SEC | U |
| Sanchez‐Nieto 2016 | Spain | Southern Europe | 96 | 85 | 7 | 3 | SM | 3 months | SEC | U |
| Silver 2017 | USA | North America | 428 | 423 | 5 | 2 | EDU | 6 months | SEC | U |
| Smith 1999 | Australia | Oceania | 96 | 36 | 8 | 3 | SF | 12 months | PRIM/SEC | U |
| Sridhar 2008 | UK | Northwestern Europe | 122 | 104 | 4 | 3 | E/SM | 8 weeks + 16 months maintenance | PRIM/SEC | U |
| Strijbos 1996 | Netherlands | Northwestern Europe | 50 | 41 | 3 | 3 | E | 3 months | PRIM/SEC | U |
| Tabak 2014 | Netherlands | Northwestern Europe | 29 | 12 | 8 | Primary and secondary caregivers, not specified | TM | 9 months | PRIM/SEC | U |
| Theander 2009 | Sweden | Northwestern Europe | 30 | 26 | 4 | 4 | E | 3 months | SEC | U |
| Titova 2017 | Norway | Northwestern Europe | 172 | 100 | 4 | 3 | SF | 24 months | PRIM/SEC | U |
| Trappenburg 2011 | Netherlands | Northwestern Europe | 233 | 193 | 3 | 3 | SM | 6 months | SEC | U |
| van Wetering 2010 | Netherlands | Northwestern Europe | 199 | 175 | 4 | 3 | E | 16 weeks + 20 months maintenance | SEC | U |
| Vasilopoulou 2017 | Greece | Southern Europe | 300 | 147 | 7 | 4 | TM (A), SF (B) | 8 weeks + 12 months maintenance | TERT | U |
| Vianello 2016 | Italy | Southern Europe | 334 | 262 | 5 | 3 | TM | 12 months | PRIM/SEC | U |
| Wakabayashi 2011 | Japan | East Asia | 102 | 85 | 4 | 2 | EDU | 6 months | SEC | EDU |
| Wang 2017 | China | East Asia | 130 | 120 | 4 | 3 | TM | 12 months | TERT | U |
| Wijkstra 1994 | Netherlands | Northwestern Europe | 45 | 43 | 2 | 3 | E | 3 months | PRIM | U |
| Wood‐Baker 2006 | Australia | Oceania | 135 | 112 | 3 | 2 | SM | 12 months | PRIM | EDU |
| Zhang 2020 | China | East Asia | 208 | 174 | 7 | 5 | SF | 24 months | TERT | U |
| Zwar 2016 | Australia | Oceania | 254 | 222 | 3 | 2 | EDU | 6 months (flexible) | PRIM | U |
Abbreviations. DRUG: optimisation of drug treatment; E: exercise; IT EDU: individual educational session; PRIM: primary care; SEC: secondary care; SF: structured follow‐up; SM: self‐management; TERT: tertiary care; TM: telemonitoring; U: usual care.
2. Components of IDM in each included study.
| Author | Education | Self‐ management | Exacerbation/Action plan | Telemonitoring | Exercise | Psychosocial/Occupational | Smoking | Optimal medication | Nutrition | Follow‐up | Case management | Multi‐disciplinary |
| Aboumatar 2019 | x | x | x | |||||||||
| Aiken 2006 | x | x | x | x | x | |||||||
| Bendstrup 1997 | x | x | x | x | ||||||||
| Bernocchi 2017 | x | x | x | x | x | |||||||
| Bourbeau 2003 | x | x | x | x | ||||||||
| Boxall 2005 | x | x | ||||||||||
| Cambach 1997 | x | x | ||||||||||
| Dheda 2004 | x | x | x | x | ||||||||
| Engstrom 1999 | x | x | x | x | ||||||||
| Fan 2012 | x | x | x | x | ||||||||
| Farrero 2001 | x | x | ||||||||||
| Fernandez 2009 | x | x | ||||||||||
| Freund 2016 | x | x | x | x | x | |||||||
| Gottlieb 2011 | x | x | x | x | ||||||||
| Güell 2000 | x | x | x | |||||||||
| Güell 2006 | x | x | ||||||||||
| Haesum 2012 | x | x | x | x | x | |||||||
| Jimenez‐Reguera 2020 | x | x | x | x | x | x | ||||||
| Kalter‐Leibovici 2018 | x | x | x | |||||||||
| Kennedy 2013 | x | x | ||||||||||
| Kessler 2018 | x | x | x | x | x | x | ||||||
| Khan 2019 | x | x | x | x | ||||||||
| Ko 2016 | x | x | x | x | x | x | ||||||
| Koff 2009 | x | x | x | x | ||||||||
| Kruis 2014 | x | x | x | x | x | x | ||||||
| Lenferink 2019 | x | x | x | x | x | x | ||||||
| Lilholt 2017 | x | x | x | x | ||||||||
| Littlejohns 1991 | x | x | x | x | ||||||||
| Lou 2015 | x | x | x | x | x | x | x | x | x | |||
| Mendes 2010 | x | x | ||||||||||
| Öztürk 2020 | x | x | x | x | x | |||||||
| Rea 2004 | x | x | x | x | x | |||||||
| Rice 2010 | x | x | x | |||||||||
| Rose 2017 | x | x | x | x | x | |||||||
| Sanchez‐Nieto 2016 | x | x | x | x | x | x | ||||||
| Silver 2017 | x | x | x | x | x | |||||||
| Smith 1999 | x | x | x | x | x | x | x | x | ||||
| Sridhar 2008 | x | x | x | x | ||||||||
| Strijbos 1996 | x | x | x | |||||||||
| Tabak 2014 | x | x | x | x | x | x | x | x | ||||
| Theander 2009 | x | x | x | x | ||||||||
| Titova 2017 | x | x | x | x | x | |||||||
| Trappenburg 2011 | x | x | x | |||||||||
| van Wetering 2010 | x | x | x | x | ||||||||
| Vasilopoulou 2017 | x | x | x | x | x | x | x | |||||
| Vianello 2016 | x | x | x | x | x | |||||||
| Wakabayashi 2011 | x | x | x | x | ||||||||
| Wang 2017 | x | x | x | x | ||||||||
| Wijkstra 1994 | x | x | ||||||||||
| Wood‐Baker 2006 | x | x | x | |||||||||
| Zhang 2020 | x | x | x | x | x | x | x | |||||
| Zwar 2016 | x | x | x |
Abbreviations. IDM: integrated disease management.
Participants
A total of 21,086 COPD patients were randomised in the 52 studies, with a range of 29 to 8171 patients per study. Of these, 16,390 (84%) patients completed the studies (range 23% to 100%). At the moment of inclusion, the mean age of the intervention population was 67.1 years (SD 9.27), with 65% male (range 25% to 99%). In the usual care group, mean age was 67.2 years (SD 9.26) and 67% (range 30 to 100%) were male.
Interventions
Patients were treated in all types of healthcare settings: primary care (15 studies), secondary care (22 studies), tertiary care (5 studies), and a combination of primary and secondary health care (10 studies). The numbers of healthcare professionals involved ranged from 2 to 7, with a mean number of 3. The number of components per programme ranged from 2 to 8, with a mean number of 4. Interventions also varied in terms of duration ‐ between 3 and 48 months ‐ with varying intensity of separate intervention components. Some interventions consisted of a clearly defined intensive intervention period and a subsequent maintenance or structural follow‐up period (Bourbeau 2003; Fan 2012; Gottlieb 2011; Güell 2000; Jimenez‐Reguera 2020; Ko 2016; Sridhar 2008; van Wetering 2010; Vasilopoulou 2017). One study had an intervention with a variable duration of 2 years minimum and 5 years maximum (Kalter‐Leibovici 2018).
Following the subgroup analysis performed in the previous version of this review, we determined the dominant component of the IDM programme from all newly included studies. The dominant component could be determined directly from the objective or title of the study for eight studies (Aboumatar 2019; Bernocchi 2017; Fan 2012; Haesum 2012; Kruis 2014; Öztürk 2020; Vasilopoulou 2017; Zwar 2016). For the remaining 18 studies, we contacted study authors to ask what they considered the dominant intervention component. Eleven study authors did not provide a response. Of the seven who responded, three indicated that the intervention did not have a dominant component. To perform a subgroup analysis on types of interventions, we chose the dominant component as the component with the greatest intensity in terms of duration. Given the increased use of telemonitoring and its distinguished features to monitor patients from a distance, we decided to include telemonitoring as a separate dominant component. In Vasilopoulou 2017, usual care was compared to two types of interventions: home‐based and hospital‐based pulmonary rehabilitation. As interventions were characterised by different dominant components (telemonitoring and structural follow‐up, respectively), we included both as separate interventions.
Including the dominant components identified by Kruis 2013, we arrived at the following categories of dominant components of IDM programmes.
Exercise (13 studies: Bendstrup 1997; Boxall 2005; Cambach 1997; Engstrom 1999; Fernandez 2009; Gottlieb 2011; Güell 2000; Güell 2006; Mendes 2010; Strijbos 1996; Theander 2009; van Wetering 2010; Wijkstra 1994).
Self‐management with an exacerbation action plan (12 studies: Aboumatar 2019; Bourbeau 2003; Jimenez‐Reguera 2020; Kennedy 2013; Koff 2009; Kruis 2014; Lenferink 2019; Öztürk 2020; Rice 2010; Sanchez‐Nieto 2016; Trappenburg 2011; Wood‐Baker 2006).
Structured follow‐up with healthcare professionals, including case management (15 studies: Aiken 2006; Dheda 2004; Farrero 2001; Freund 2016; Kalter‐Leibovici 2018; Kessler 2018; Khan 2019; Ko 2016; Lilholt 2017; Littlejohns 1991; Rose 2017; Smith 1999; Titova 2017; Vasilopoulou 2017; Zhang 2020).
Individualised educational sessions (5 studies: Fan 2012; Lou 2015; Silver 2017; Wakabayashi 2011; Zwar 2016).
Telemonitoring (6 studies: Bernocchi 2017; Haesum 2012; Tabak 2014; Vasilopoulou 2017; Vianello 2016; Wang 2017).
In addition, Kruis 2013 identified two studies that each had two dominant components. Sridhar 2008 included two components on which most of the intervention time was spent (i.e. exercise and self‐management with action plan). Rea 2004 included two dominant components: self‐management with action plan and structured follow‐up. Therefore we included these two studies in separate categories, namely, exercise and self‐management and self‐management and structural follow‐up.
Outcomes
We combined the outcomes of 26 recently included studies with the 26 already included studies. We recorded the number of studies reporting a specific outcome as follows.
Quality of life (46 studies).
Exercise capacity (28 studies).
Exacerbation‐related outcomes: measured by numbers of exacerbations, hospital admissions, hospitalisation days, emergency department (ED) visits, prednisolone or antibiotics courses (32 studies).
Lung function (21 studies).
Survival, mortality (15 studies).
Depression (10 studies).
Dyspnea (13 studies).
Process‐related outcomes (14 studies).
Details of the included studies and outcomes are provided in Characteristics of included studies, Table 4Table 5, Table 6, and Table 7.
3. Table of study characteristics/outcomes: quality of life.
| Author | Outcome domain | Outcome measure | Time points in months (time frame) | Data reported | Pooled |
| Aboumatar 2019 | Health‐related QoL | SGRQ | 4 (ST) | mean change, 95% CI, N/group | Yes |
| Aiken 2006 | Generic QoL | SF‐36 | 3 (ST); 6 (ST); 9 (MT); 12 (MT) | slopes of trajectories | No |
| Bendstrup 1997 | Health‐related QoL | CRQ, YGLQ | 1 (ST); 3 (ST) | mean change, SEM/group/P value | Yes |
| Bernocchi 2017 | Health‐related QoL | CAT score, Barthel score | 4 (ST); 6 (ST) | mean change, SD, N/group | No |
| Bourbeau 2003 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 6 (ST); 9 (MT) | mean, 95% CI, N/group | Yes |
| Boxall 2005 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 3 (ST); 12 (MT) | mean change, mean at follow‐up with SD, N/group/time point mean difference, 95% CI, P value | Yes |
| Cambach 1997 | Health‐related QoL | CRDQ (CRQ ‐ recalculated) | 3 (ST); 6 (MT) | mean at baseline, mean change, SD, N/group | No |
| Dheda 2004 | Health‐related QoL | SGRQ ‐ total | 12 (MT) | mean change, SE. N/group, P value | Yes |
| Engstrom 1999 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 12 (MT) | mean, SE, N/group/time point | Yes |
| Fan 2012 | Health‐related QoL; generic QoL | SGRQ ‐ total, SGRQ ‐ subtotals; SF‐12 MCS; SF‐12 PCS | 12 (MT) | mean change, SD, N/group | Yes |
| Farrero 2001 | Health‐related QoL | CRQ | 3 (ST); 12 (MT) | not reported | No |
| Fernandez 2009 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 12 (MT) | mean, SD, N/group/time point | Yes |
| Freund 2016 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Gottlieb 2011 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 6 (ST); 12 (MT); 18 (LT) | mean, SD, N/group/time point | Yes |
| Güell 2000 | Health‐related QoL; generic QoL | CRQ, BODE Index, VAS | 3 (ST); 6 (ST); 9 (MT); 12 (MT); 18 (LT); 24 (LT) | mean, SE/group/time point | Yes |
| Güell 2006 | Health‐related QoL | CRQ | 4 (ST) | mean, SD, N/group/time point | Yes |
| Haesum 2012 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Jimenez‐Reguera 2020 | Health‐related QoL; generic QoL | SGRQ, CAT, EQ‐5D, VAS | 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes |
| Kalter‐Leibovici 2018 | Health‐related QoL; generic QoL | SGRQ‐total, SF‐12 MCS, SF‐12 PCS | 12 (MT); 24 (LT) | mean change, SD, N/group | Yes |
| Kennedy 2013 | Generic QoL | EQ‐5D | 6 (ST); 12 (MT) | mean change, SD, N/group | No |
| Kessler 2018 | Health‐related QoL | SGRQ ‐ COPD specific, BODE Index | 12 (MT) | mean, SD, N/group (at 12 months) adjusted MD, 95% CI, N, P value | No |
| Khan 2019 | Health‐related QoL | BODE Index | 6 (ST) | MD, 95% CI, P value, N | No |
| Ko 2016 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 12 hs (MT) | mean change, SD, N/group | Yes |
| Koff 2009 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 3 (ST) | mean change, 95% CI, N/group | Yes |
| Kruis 2014 | Health‐related QoL; generic QoL | SGRQ ‐ total, SGRQ ‐ subscores, CCQ, SF‐36 PCS, SF‐36 MCS | 12 (MT) | mean change, 95% CI, N/group | Yes |
| Lenferink 2019 | Health‐related QoL | CRQ, CAT | 6 (ST); 12 (MT) | mean change, SD, N/group (additional data) | Yes |
| Lilholt 2017 | Generic QoL | SF‐36 MCS, SF‐36 PCS | 12 (MT) | MD, 95% CI, P value, N | Yes |
| Littlejohns 1991 | n.a. | n.a. | n.a. | n.a. | n.a |
| Lou 2015 | Health‐related QoL | BODE Index | 48 (LT) | mean change, SD, N/group adjusted median D, 95% CI, P value | No |
| Mendes 2010 | Health‐related QoL | BODE Index | 3 (ST) | mean, SD, N/group/time point ‐ box and whisker plots | No |
| Öztürk 2020 | Health‐related QoL; generic QoL | SGRQ, CAT, SF‐36 subdomains | 3 (ST) | mean, SD, N/group/time point | Yes |
| Rea 2004 | Health‐related QoL; generic QoL | CRQ, SF‐36 subdomains | 12 (MT) | mean, N/group/time point, P value difference | Yes |
| Rice 2010 | Health‐related QoL | SGRQ ‐ total | 12 (MT) | mean change/group mean difference (95% CI) | Yes |
| Rose 2017 | Health‐related QoL | SGRQ ‐ total, BODE Index | 6 (ST); 12 (MT) | mean, SD/group | Yes |
| Sanchez‐Nieto 2016 | n.a. | n.a. | n.a. | n.a. | n.a |
| Silver 2017 | n.a. | n.a. | n.a. | n.a. | n.a |
| Smith 1999 | Health‐related QoL | COOP | 12 (MT) | mean, SE, N/group/follow‐up | No |
| Sridhar 2008 | Health‐related QoL | CRQ | 24 (LT) | mean, SD, N/group/time point | Yes |
| Strijbos 1996 | n.a. | n.a. | n.a. | n.a. | n.a |
| Tabak 2014 | Health‐related QoL; generic QoL | CCQ, EQ‐5D Index, EQ‐5D VAS score | 1 (ST); 3 (MT) | mean, SD, N/group/time point | No |
| Theander 2009 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 3 (ST) | mean baseline, SD mean, SD/group | Yes |
| Titova 2017 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 6 (ST); 12 (MT); 4 (LT) | mean, 95% CI/group/time point MD, 95% CI, N, P value | Yes |
| Trappenburg 2011 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals, CCQ | 6 (ST) | mean change, SE, N/group | Yes |
| van Wetering 2010 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 4 (ST); 12 (MT); 24 (LT) | mean, SE, N/group MD, MD adjusted, SE, P value | Yes |
| Vasilopoulou 2017 | Health‐related QoL | SGRQ ‐ total, CAT score | 2 (ST); 14 (MT) | mean, SD, N/group/time point | Yes |
| Vianello 2016 | Generic QoL | SF‐36 PCS, SF‐36 MCS | 12 (MT) | mean, SD, N/group | Yes |
| Wakabayashi 2011 | Health‐related QoL | SGRQ ‐ total | 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes |
| Wang 2017 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 1 (ST); 3 (ST); 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes |
| Wijkstra 1994 | Health‐related QoL | CRQ | 3 (ST); 6 (ST); 12 (MT); 18 (LT) | mean change, SD, N/group | Yes |
| Wood‐Baker 2006 | Health‐related QoL | SGRQ ‐ total, SGRQ ‐ subtotals | 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes |
| Zhang 2020 | Health‐related QoL | CAT score | 3 (ST); 6 (ST); 12 (MT); 24 (LT) | mean, SD, N/group/time point | No |
| Zwar 2016 | Health‐related QoL | SGRQ ‐ total, CAT score | 12 (MT) | mean, SD, N, P value, t test statistic/group/time point | Yes |
Abbreviations. CAT: COPD Assessment Test; CCQ: Chronic COPD Questionnaire; CI: confidence interval; COOP: Dartmouth Primary Care Co‐operative Quality of Life Questionnaire; CRQ: Chronic Respiratory Questionnaire; EQ‐5D: EuroQol Quality of Life ‐ 5 domains; LT: long‐term follow‐up; MCS: Mental Component Score; MD: mean difference; MT: medium‐term follow‐up; QoL: quality of life; PCS: Physical Component Score; SD: standard deviation; SE: standard error; SGRQ: St. George's Respiratory Questionnaire; SF‐12: Short Form‐12; SF‐36: Short Form‐36; SIP: Sickness Impact Profile; ST: short‐term follow‐up; VAS: visual analogue scale; YGLQ: York Quality of Life Questionnaire.
4. Table of study characteristics outcomes: functional and maximum exercise capacity.
| Author | Outcome domain | Outcome measure | Time points in months (time frame) | Data presented | Pooled |
| Bendstrup 1997 | functional exercise capacity | 6MWD | 1 (ST); 3 (ST); 6 (ST) | mean change, SEM, group/P value | Yes |
| Bernocchi 2017 | functional exercise capacity | 6MWD | 4 (ST); | mean change, 95% CI, N/group | Yes |
| Bourbeau 2003 | functional exercise capacity | 6MWD | 4 (ST); 12 (MT) | not reported | No |
| Boxall 2005 | functional exercise capacity | 6MWD | 3 (ST) | mean, mean change, SD mean/group, P value | Yes |
| Cambach 1997 | functional and maximum exercise capacity | 6MWD, W‐max | 3 (ST); 6 (ST) | mean change, SD, N/group | Yes |
| Engstrom 1999 | functional exercise capacity | 6MWD | 12 (MT) | mean, SE, N/group/time point | Yes |
| Fernandez 2009 | functional exercise capacity | 6MWD, leg fatigue score | 12 (MT) | mean, SD, N/group/time point | Yes |
| Gottlieb 2011 | functional and maximum exercise capacity | 6MWD | 6 (ST); 12 (MT); 18 (LT) | mean, SD, N/group/time point | Yes |
| Güell 2000 | functional and maximum exercise capacity | 6MWD, W‐max | 3 (ST); 6 (ST); 9 (MT); 12 (MT); 18 (LT); 24 (LT) | mean, SE, group/time point | Yes |
| Güell 2006 | functional exercise capacity | 6MWD | 4 (ST) | mean, SD, N/group/time point | Yes |
| Jimenez‐Reguera 2020 | functional exercise capacity | 6MWD | 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes |
| Kalter‐Leibovici 2018 | functional exercise capacity | 6MWD | 12 (MT); 24 (LT) | mean change, SD, N/group | Yes |
| Kessler 2018 | functional exercise capacity | 6MWD | 12 (MT) | mean, SD, N/group (at 12 months) adjusted MD, 95% CI, N, P value | Yes |
| Khan 2019 | functional exercise capacity | 6MWD | 6 (ST) | mean change, SD, N/group, MD, 95% CI | Yes |
| Ko 2016 | functional exercise capacity | 6MWD | 12 (MT) | mean change, SD, N/group, P value | Yes |
| Littlejohns 1991 | functional exercise capacity | 6MWD | 12 (MT) | mean change, 95% CI, N/group | Yes |
| Lou 2015 | functional exercise capacity | 6MWD | 48 (LT) | mean change, SD, N/group adjusted median D, 95% CI, P value | Yes |
| Mendes 2010 | functional exercise capacity | 6MWD | 3 (ST) | mean, SD, N/group/time point ‐ box and whisker plots | Yes |
| Rea 2004 | functional and maximum exercise capacity | Shuttle walk test | 12 (MT) | mean, N/group/time point, P value difference | No |
| Strijbos 1996 | functional and maximum exercise capacity | W‐max, 4MWT, Borg scores during cycle test | 6 (ST); 12 (MT); 18 (LT) | mean, SD, N/group/time point | Yes |
| Tabak 2014 | functional exercise capacity | 6MWD | 1 (ST); 3 (MT) | mean, SD, N/group/time point | Yes |
| Theander 2009 | functional and maximum exercise capacity | 6MWD, grip strength | 3 (ST) | mean change, SD, N/group | Yes |
| van Wetering 2010 | functional and maximum exercise capacity | 6MWD, W‐max | 4 (ST); 12 (MT); 24 (LT) | mean change, SE, N/group | Yes |
| Vasilopoulou 2017 | functional and maximum exercise capacity | 6MWD, W‐max | 2 (ST); 14 (MT) | mean, SD, N/group/time point | Yes |
| Wakabayashi 2011 | functional exercise capacity | 6MWD | 6 (ST); 12 (MT) | mean change, SD, N/group/time point, P value | Yes |
| Wang 2017 | functional exercise capacity | 6MWD | 1 (ST); 3 (ST); 6 (ST); 12 (MT) | mean change, SD, N/group | Yes |
| Zhang 2020 | functional exercise capacity | 6MWD | 3 (ST); 6 (ST); 12 (MT); 24 (LT) | mean, SD, N/group/time point | Yes |
| Wijkstra 1994 | functional exercise capacity | 6MWD | 3 (ST); 6 (ST); 12 (MT); 18 (LT) | mean change, SD, N/group | Yes (3 months only) |
Abbreviations. 4MWT: 4‐minute walk test; 6MWD: 6‐minute walking distance; CI: confidence interval; LT: long‐term follow‐up; MD: mean difference; MT: medium‐term follow‐up; SD: standard deviation; SE: standard error; ST: short‐term follow‐up; W‐max: maximum exercise capacity (in watts).
5. Table of study characteristics outcomes: exacerbation outcomes.
| Author | Outcome domain | Outcome measure | Time points, months (time frame) | Data reported | Pooled |
| Aboumatar 2019 | hospitalisations; ED visit | respiratory‐related hospital admissions; hospital admissions (all causes); ED visits | 6 (ST) | Incidence rate, 95% CI, N/group | Yes |
| Bernocchi 2017 | hospitalisations | respiratory‐related hospital admissions; hospital admissions (all causes) | 6 (ST) | n, N/group | Yes |
| Bourbeau 2003 | hospitalisations; ED visit; exacerbation | respiratory‐related hospital admissions; hospital days per patient; ED visits; number of patients experiencing ≥ 1 exacerbation | 6 (ST); 9 (MT) | n, N/group, mean, SD/group | Yes |
| Boxall 2005 | hospitalisations | respiratory‐related hospital admissions; hospital days per patient | 3 (ST); 12 (MT) | n, N/group, mean, SD/group | Yes |
| Engstrom 1999 | hospitalisations | hospital days per patient | 12 (MT) | mean, SD/group | Yes |
| Fan 2012 | hospitalisations; ED visit; exacerbation | respiratory‐related hospital admissions; hospital days per patient; ED visits; patients using ≥ 1 course of oral steroids; patients using ≥ 1 course of antibiotics | 12 (MT) | n, N/group, rate per person‐year, mean, SD, N/group | Yes |
| Farrero 2001 | hospitalisations | hospital days per patient | 12 (MT) | mean, SD/group | Yes |
| Freund 2016 | hospitalisations | hospital days per patient | 12 (MT) | mean difference, 95% CI, N, P value | No |
| Güell 2000 | exacerbation | mean exacerbation rate | 24 (LT) | n as count data, mean, SD/group | Yes |
| Kalter‐Leibovici 2018 | hospitalisations | respiratory‐related hospital admissions; hospital admissions (all causes) | 36 (LT) | n, N/group | Yes |
| Kessler 2018 | hospitalisations, exacerbation | total hospital admissions (all causes); percentage of hospital days | 12 (MT) | n, N/group | Yes |
| total respiratory‐related hospital admissions | 12 (MT) | n, N/group | No | ||
| hospital days per patient; number of patients experiencing ≥ 1 exacerbation | 12 (MT) | n, N/group; mean, SD/group | Yes | ||
| Ko 2016 | hospitalisations | hospital days per patient | 12 (MT) | mean, SD/group | Yes |
| Koff 2009 | hospitalisations | respiratory‐related hospital admissions | 3 (ST) | n, N/group | Yes |
| Kruis 2014 | hospitalisations | hospital days per patient; mean exacerbation rate | 12 (MT) | mean, 95% CI/group, incidence rate ratio, 95% CI, N (for mild and severe exacerbations) | Yes |
| Lenferink 2019 | hospitalisations; exacerbation | respiratory‐related hospital admissions; hospital admissions (all causes); hospital days per patient; number of patients experiencing ≥ 1 exacerbation | 12 (MT) | n, N/group, mean, 95% CI, N/group | Yes |
| Littlejohns 1991 | hospitalisations; exacerbation | hospital admissions (all causes); patients using ≥ 1 course of oral steroids; patients using ≥ 1 course of antibiotics | 12 (MT) | n, N/group | Yes |
| Lou 2015 | hospitalisations; ED visit | hospital admissions (all causes); ED visits | 48 (LT) | %, N/group | Yes |
| Rea 2004 | hospitalisations; ED visit; exacerbation | respiratory‐related hospital admissions; hospital admissions (all causes); hospital days per patient; ED visits; patients using ≥ 1 course of oral steroids; patients using ≥ 1 course of antibiotics | 12 (MT) | n, N/group, mean/group | Yes |
| Rice 2010 | hospitalisations; ED visit | respiratory‐related hospital admissions; ED visits | 12 (MT) | n, N/group | Yes |
| Rose 2017 | hospitalisations; ED visit | hospital days per patient; ED visits | 12 (MT) | n, N/group, median, IQR/group | Yes |
| Sanchez‐Nieto 2016 | hospitalisations; ED visit; exacerbation | respiratory‐related hospital admissions; ED visits; patients using ≥ 1 course of oral steroids; patients using ≥ 1 course of antibiotics | 12 (MT) | n, N/group | Yes |
| Silver 2017 | hospitalisations; ED visit | hospital days per patient; ED visits | 12 (MT) | n, N/group, median, IQR/group | Yes |
| Smith 1999 | hospitalisations; ED visit | respiratory‐related hospital admissions; ED visits | 12 (MT) | n, N/group | Yes |
| Sridhar 2008 | hospitalisations; exacerbation | hospital admissions (all causes); number of patients experiencing ≥ 1 exacerbation | 24 (LT) | n, N/group | Yes |
| Tabak 2014 | ED visit | ED visits | 3 (MT) | median, IQR/group | No |
| Titova 2017 | hospitalisations | Total number of hospitalisations; categories of patients "HA category 1 (≤ 1 HA per year) and HA category 2 (≥ 2 HA per year)" | 12 (MT); 24 (LT) | n, N (count data) | No |
| Trappenburg 2011 | hospitalisations; exacerbation | respiratory‐related hospital admissions; hospital days per patient; number of patients experiencing ≥ 1 exacerbation | 6 (ST) | n, N; mean, SD, N/group/time point mean difference, 95% CI, P value | Yes |
| van Wetering 2010 | hospitalisations; exacerbation | hospital days per patient; number of patients experiencing ≥ 1 exacerbation | 24 (LT) | mean, SD/group, n, N/group | Yes |
| Vasilopoulou 2017 | hospitalisations; exacerbation | respiratory‐related hospital admissions; hospital admissions (all causes); hospital days per patient; number of patients experiencing ≥ 1 exacerbation; mean exacerbation rate | 14 (MT); 24 (LT) | n, N/ group/time point, mean, SD/group | Yes |
| Vianello 2016 | hospitalisations; ED visit | hospital days per patient (all causes); hospital days per patient (respiratory related); ED visits | 12 (MT) | mean, SD, N/group, rate per person‐year | Yes |
| Wakabayashi 2011 | ED visit | ED visits | 12 (MT) | mean, SD, N/group/time point | Yes |
| Zhang 2020 | hospitalisations; ED visit | admission rates; length of stay; ED visits (all outcomes for entire study population) | 3 (ST);6 (ST); 12 (MT); 24 (LT) | n, N (count data), median, IQR/group | No |
Abbreviations. CI: confidence interval; ED: emergency department; IQR: interquartile range; T: long‐term follow‐up; MD: mean difference; MT: medium‐term follow‐up; SD: standard deviation; SE: standard error; ST: short‐term follow‐up.
6. Table of study characteristics outcomes: secondary outcomes.
| Author | Outcome domain | Outcome measure | Time points, months (time frame) | Data reported | Pooled |
| Aboumatar 2019 | Mortality | Mortality | 4 (ST) | n, N | Yes |
| Bernocchi 2017 | Dyspnoea | mMRC | 4 (ST) | mean change, SD, N/group | Yes |
| Mortality | Mortality | 4 (MT) | n, N | Yes | |
| Bourbeau 2003 | Lung function | FEV₁, FEV₁% predicted | 12 (MT) | mean, SD, N/group/time point | Yes |
| Boxall 2005 | Dyspnoea | Borg score | 3 (ST); 12 (MT) | mean change, mean at follow‐up with SD, N/group/time point mean difference, 95% CI, P value | Yes |
| Engstrom 1999 | Depression | MACL | 12 (MT) | mean, SE, N/group/time point | No |
| Fan 2012 | Mortality | Mortality | 12 (MT) ‐ mean 250 days' follow‐up | n, N | Yes |
| Farrero 2001 | Lung function | FEV₁% predicted | 12 (MT) | mean, SD, N/group/time point, P value | Yes |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Fernandez 2009 | Lung function | FEV₁% predicted | 12 (MT) | mean, SD, N/group/time point | Yes |
| Gottlieb 2011 | Dyspnoea | Borg score | 6 (ST); 12 (MT); 18 (LT) | mean, SD, N/group/time point | Yes |
| Güell 2000 | Lung function | FEV₁% predicted | 3 (ST); 6 (ST); 9 (MT); 12 (MT); 18 (LT); 24 (LT) | mean, SE, group/time point | Yes |
| Borg score | 3 (ST); 6 (ST); 9 (MT); 12 (MT); 18 (LT); 24 (LT) | mean, SE, group/time point | Yes | ||
| Güell 2006 | Depression | Revised Symptom Checklist (SCL‐90‐R) | 4 (ST) | mean, SD, N/group/time point | No |
| Jimenez‐Reguera 2020 | Lung function | FEV₁% predicted (reported as FEV₁ in litres) | 6 (ST) | mean, SD, N/group/time point | Yes |
| Kalter‐Leibovici 2018 | Lung function | FEV₁% predicted | 12 (MT); 24 (LT) | mean change, SD, N/group | Yes |
| Dyspnoea | mMRC | 12 (MT); 24 (LT) | mean change, SD, N/group | Yes | |
| Kessler 2018 | Depression | HADS depression, HADS anxiety | 12 (MT) | mean, SD, N/group (at 12 months) adjusted MD, 95% CI, N, P value | Yes |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Khan 2019 | Lung function | FEV₁% predicted | 6 (ST) | mean change, SD, N/group, MD, 95% CI | Yes |
| Dyspnoea | mMRC | 6 (ST) | mean change, SD, N/group, MD, 95% CI | Yes | |
| Ko 2016 | Lung function | FEV₁% predicted | 12 (MT) | mean change, SD, N/group | Yes |
| Dyspnoea | mMRC | 12 (MT) | mean change, SD, N/group | Yes | |
| Koff 2009 | 3 (ST) | mean change, 95% CI, N/group | Yes | ||
| Kruis 2014 | Dyspnoea | mMRC | 12 (MT) | mean change, 95% CI, N/group | Yes |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Lenferink 2019 | Lung function | FEV₁% predicted | 12 (MT) | mean change, SD, N/group (additional data) | Yes |
| Dyspnoea | mMRC | 6 (ST); 12 (MT) | mean change, SD, N/group (additional data) | Yes | |
| Depression | HADS total score | 6 (ST); 12 (MT) | mean change, SD, N/group | Yes | |
| Littlejohns 1991 | Lung function | FEV₁% predicted | 12 (MT) | mean change, 95% CI, N/group | Yes |
| Depression | HADS depression, HADS anxiety | 12 (MT) | mean change, 95% CI, N/group | Yes | |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Lou 2015 | Lung function | FEV₁% predicted | 48 (LT) | mean change, SD, N/group adjusted median D, 95% CI, P value | No |
| Dyspnoea | mMRC | 48 (LT) | mean change, SD, N/group adjusted median D, 95% CI, P value | Yes | |
| Mortality | Mortality | 48 (MT) | n, N | Yes | |
| Mendes 2010 | Dyspnoea | mMRC | 3 (ST) | mean, SD, N/group/time point ‐ box and whisker plots | No |
| Öztürk 2020 | Lung function | FEV₁ | 3 (ST) | mean, SD, N/group/time | Yes |
| Dyspnoea | mMRC | 3 (ST) | mean, SD, N/group/time | Yes | |
| Depression | HADS depression, HADS anxiety | 3 (ST) | mean, SD, N/group/time | Yes | |
| Rice 2010 | Mortality | Mortality | 12 (MT) | n, N | Yes |
| Rose 2017 | Depression | HADS depression, HADS anxiety | 6 (ST); 12 (MT) | mean, SD/group | Yes |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Sanchez‐Nieto 2016 | Mortality | Mortality | 12 (MT) | n, N | Yes |
| Smith 1999 | Lung function | FEV₁, FEV₁% predicted | 12 (MT) | mean, SE/group | No |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Sridhar 2008 | Lung function | FEV₁, FEV₁% predicted | 48 (LT) | mean, SD, N/group/time point | Yes |
| Mortality | Mortality | 48 (MT) | n, N | Yes | |
| Titova 2017 | Depression | HADS depression, HADS anxiety | 6 (ST); 12 (MT); 24 (LT) | mean, 95% CI/group/time point MD, 95% CI, N, P value | Yes |
| Mortality | Mortality | 24 (MT) | n, N | Yes | |
| Trappenburg 2011 | Depression | HADS depression, HADS anxiety | 6 (ST) | mean change, SE, N/group | Yes |
| van Wetering 2010 | Lung function | FEV₁% predicted | 4 (ST) | mean, SE, N/group | Yes |
| Dyspnoea | MRC | 4 (ST); 12 (MT); 24 (LT) | mean, SE, N/group | Yes | |
| Vasilopoulou 2017 | Dyspnoea | mMRC | 2 (ST); 14 (MT) | mean, SD, N/group/time point | Yes |
| Vianello 2016 | Depression | HADS depression, HADS anxiety | 12 (MT) | mean, SD, N/group | Yes |
| Mortality | Mortality | 12 (MT) | n, N | Yes | |
| Wakabayashi 2011 | Lung function | FEV₁% predicted | 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes |
| Dyspnoea | mMRC | 6 (ST); 12 (MT) | mean, SD, N/group/time point | Yes | |
| Wang 2017 | Lung function | FEV₁ | 3 (ST); 6 (ST); 12 (MT) | mean, SD, N/group/time point | No (reporting error) |
| Dyspnoea | mMRC | 3 (ST); 6 (ST); 12 (MT) | mean, SD, N / group / time point | No (reporting error) | |
| Wijkstra 1994 | Lung function | FEV₁ | 3 (ST); 6 (ST); 12 (MT); 18 (LT) | mean change, SD, N/group (only for 3 months' follow‐up) | Yes |
| Wood‐Baker 2006 | Lung function | FEV₁, FEV₁% predicted | 6 (ST); 12 (MT) | mean, SD, N/group | Yes |
| Zhang 2020 | Lung function | FEV₁, FEV₁% predicted | 3 (ST); 6 (ST); 12 (MT); 24 (LT) | mean, SD, N/group/time point | Yes |
| Dyspnoea | mMRC | 3 (ST); 6 (ST); 12 (MT); 24 (LT) | mean, SD, N/group/time point | ||
| Zwar 2016 | Lung function | FEV₁% predicted | 12 (MT) | mean, SD, N/group/time point MD, 95% CI, P value, N | Yes |
Abbreviations. CI: confidence interval; FEV₁: forced expiratory volume in one second; HADS: Hospital Anxiety and Depression Scale; LT: long‐term follow‐up; MD: mean difference; mMRC: modified Medical Research Council Dyspnoea Scale; MT: medium‐term follow‐up; SD: standard deviation; SE: standard error; ST: short‐term follow‐up.
We requested additional data from 21 study authors; 14 (67%) responded. Nine studies provided additional data that we used in the analysis (Bernocchi 2017; Kalter‐Leibovici 2018; Kennedy 2013; Kessler 2018; Khan 2019; Lenferink 2019; Titova 2017; Vasilopoulou 2017; Wang 2017. Seven studies provided sufficient data for calculation of correlation coefficients used to impute missing data (Aboumatar 2019; Engstrom 1999; Fan 2012; Kalter‐Leibovici 2018; Lilholt 2017; Sridhar 2008; Vasilopoulou 2017) (see Dealing with missing data).
Excluded studies
We excluded 118 full‐text articles from the current update during the full‐text screening process. The Characteristics of excluded studies table provides full details on reasons for exclusion.
Risk of bias in included studies
Results of the risk of bias assessment are presented in Figure 2. All but one of the included studies were judged to be at high risk of bias for blinding of participants, which is a result of the nature of the intervention. With regard to the other domains, the likelihood that bias was present (high risk of bias) varied across studies, from 4% for random sequence generation (selection bias) to 27% for blinding of outcome assessment (detection bias).
2.

'Risk of bias' summary: review authors' judgments about each risk of bias item for each included study.
Allocation
We judged 43 included studies as having low risk of bias in sequence generation (Aboumatar 2019; Aiken 2006; Bernocchi 2017; Bourbeau 2003; Boxall 2005; Cambach 1997; Fan 2012; Fernandez 2009; Freund 2016; Gottlieb 2011; Haesum 2012; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Kennedy 2013; Kessler 2018; Khan 2019; Ko 2016; Koff 2009; Kruis 2014; Lenferink 2019; Lilholt 2017; Littlejohns 1991; Mendes 2010; Öztürk 2020; Rea 2004; Rice 2010; Rose 2017; Sanchez‐Nieto 2016; Silver 2017; Smith 1999; Sridhar 2008; Tabak 2014; Theander 2009; Trappenburg 2011; van Wetering 2010; Vasilopoulou 2017; Vianello 2016; Wakabayashi 2011; Wang 2017; Wijkstra 1994; Wood‐Baker 2006; Zhang 2020; Zwar 2016). Information from eight studies was insufficient to permit a decision (Bendstrup 1997; Dheda 2004; Engstrom 1999; Farrero 2001; Güell 2000; Güell 2006; Lou 2015; Strijbos 1996). One study was judged to have high risk of bias, as participants were randomised based on district (Titova 2017). With regard to allocation bias, we judged 27 studies as having low risk of bias and five studies as having high risk of bias. For the remaining 20 studies, provided information was insufficient to permit a firm conclusion (unclear risk of bias).
Blinding
The nature of the intervention makes blinding of participants and healthcare providers delivering the intervention impossible. Hence, we judged all studies, except Trappenburg 2011, which kept patients unaware of the primary study aim (postponed information), as having high risk of performance bias. Although blinding of patients and/or healthcare providers is impossible, outcome assessors in some cases could be blinded to participants' allocation. Twenty‐five studies were judged as having low risk. These studies had outcome assessors that were adequately blinded for allocation, reported only on outcomes that were objective (i.e. mortality, hospitalisations), or had an outcome committee judging the outcomes. This made risk of detection bias highly unlikely. Outcome assessors were unblinded in 15 studies (Boxall 2005; Cambach 1997; Farrero 2001; Kalter‐Leibovici 2018; Khan 2019; Koff 2009; Lenferink 2019; Lilholt 2017; Öztürk 2020; Rea 2004; Smith 1999; Tabak 2014; Theander 2009; Titova 2017; Vianello 2016), posing a high risk of bias. Twelve studies provided insufficient information and were judged as having unclear risk (Bendstrup 1997; Dheda 2004; Fernandez 2009; Gottlieb 2011; Kennedy 2013; Littlejohns 1991; Lou 2015; Mendes 2010; Sridhar 2008; Strijbos 1996; Wijkstra 1994; Wood‐Baker 2006). For the remaining 25 studies, outcome assessors were blinded to group allocation.
Incomplete outcome data
We judged 35 studies as having low risk of bias, as they had low dropout rates, or dropout rates were balanced across groups for similar reasons. We considered 13 studies to have high risk of bias (Bendstrup 1997; Bernocchi 2017; Cambach 1997; Farrero 2001; Gottlieb 2011; Kessler 2018; Lilholt 2017; Lou 2015; Mendes 2010; Smith 1999; Tabak 2014; Vianello 2016; Wang 2017). Four of these 13 studies had larger dropout in the control group than in the intervention group. In Lou 2015, 1217 participants dropped out from the control group compared to 779 from the intervention group. Reasons were death and inability to perform the walking test. In Bernocchi 2017, larger dropout rates in the control group were due to increased hospitalisations as a result of heart failure.
Selective reporting
We judged 39 studies to have low risk of reporting bias, meaning that all outcomes mentioned in the protocol or the clinical trial register were reported. Nine studies selectively reported outcomes specified in the protocol and/or in the methods section (Bourbeau 2003; Dheda 2004; Freund 2016; Gottlieb 2011; Jimenez‐Reguera 2020; Lilholt 2017; Littlejohns 1991; Smith 1999; Tabak 2014), or they changed operationalisation of the outcome (i.e. Physical Component Summary (PCS) subscore instead of SF‐36 score to measure QoL) (Lilholt 2017). In three studies (Bourbeau 2003; Dheda 2004; Öztürk 2020), the authors observed no statistically significant differences in outcomes and therefore did not present data. In Tabak 2014, outcomes were reported for only 3 months ‐ not for 6 and 9 months ‐ in contrast to the study protocol. This all points to the risk of selective outcome reporting.
With the exception of one outcome (hospital admission (in days)), funnel plots did not indicate that publication bias is likely. Observed asymmetry of the funnel plot for hospital admission is probably caused by the poor methodological quality of Farrero 2001.
Other potential sources of bias
We included nine cluster‐randomised trials, three of which introduced bias (Lou 2015; Rea 2004; Wood‐Baker 2006). In Wood‐Baker 2006, there was noticeable imbalance in differences between groups at baseline. Wood‐Baker 2006 and Lou 2015 did not account for clustering in statistical analyses of dichotomous outcomes. This may lead to over‐precise results and can result in much more weight in a meta‐analysis (Higgins 2011). Therefore, in our meta‐analyses, we adjusted for the design effect by reducing the size of the trial to its "effective sample size" for all dichotomous outcomes (Rao 1992), and we used the adjusted MD via the GIV approach for all continuous outcomes. In Rea 2004, there was loss to follow‐up of five clusters (four control and one intervention cluster). Other potential sources of bias were found in Titova 2017Kessler 2018Lenferink 2019Vasilopoulou 2017Vianello 2016, and Lou 2015. Lou 2015 was performed across four geographically distinct regions and based randomisation on geographical location, thereby potentially introducing cluster effects.
Effects of interventions
See: Table 1
Primary outcomes
1. Quality of life
Of the 52 studies included, 46 studies measured quality of life, that is, health‐related quality of life (34 studies), generic quality of life (four studies), or both (eight studies). In total, 11 different instruments were used (see Table 4).
Health‐related quality of life
St. George's Respiratory Questionnaire (SGRQ) (25 studies)
Chronic Respiratory Questionnaire (CRQ) (nine studies)
Clinical COPD Questionnaire (CCQ) (three studies)
COPD Assessment test (CAT) (six studies)
Body mass index (BMI), airflow obstruction, dyspnoea, and exercise capacity index (BODE) (six studies)
Barthel score (one study)
Dartmouth Primary Care Co‐operative Quality of Life Questionnaire (COOP) (one study)
Generic quality of life
Short Form‐36 (SF‐36) or Short Form‐12 (SF‐12) (eight studies)
EQ‐5D (four studies)
Sickness Impact Profile (SIP) (two studies)
York Quality of Life Questionnaire (YGLQ) (one study)
We performed a meta‐analysis combining the results of some or all of these questionnaires. The SGRQ and the CRQ are respiratory‐specific quality of life questionnaires and have become the recognised standards of HRQoL assessment amongst patients with COPD. However, pooling of these instruments into a meta‐analysis was impossible, as the CRQ is more responsive than the SGRQ (Puhan 2006). Furthermore, the included generic quality of life questionnaires (SF‐36, SIP, and COOP) measure other dimensions of generic quality of life; therefore combining these data in a meta‐analysis across tools is not recommended.
1.1. SGRQ total score (short‐term)
The SGRQ is a disease‐specific, validated questionnaire with a scale from 0 (good health) to 100 (worst health status). A negative sign on this questionnaire indicates improvement, and the minimum clinically important difference (MCID) is ‐4 points (Jones 1991). Sixteen studies with a total population of 1788 participants provided data on the SGRQ total score with follow‐up to 6 months (Aboumatar 2019; Bourbeau 2003; Boxall 2005; Dheda 2004; Gottlieb 2011; Jimenez‐Reguera 2020; Koff 2009; Öztürk 2020; Rose 2017; Theander 2009; Titova 2017; Trappenburg 2011; van Wetering 2010; Wakabayashi 2011; Wang 2017; Wood‐Baker 2006). The pooled mean difference (MD) in SGRQ total score was ‐3.78 (95% confidence interval (CI) ‐6.29 to ‐1.28) in favour of IDM. Pooling indicated substantial heterogeneity (I² = 72%) (Analysis 1.1; Figure 3). Heterogeneity could be explained in part by differences in the quality of the studies (I² = 46%). Sensitivity analysis of 'high‐quality studies' showed a comparable effect (MD ‐3.65, 95% CI ‐5.66 to ‐1.64), indicating a robustness of the overall effect estimate in favour of IDM.
1.1. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 1: SGRQ: short‐term (≤ 6 months)
3.

Forest plot of comparison: 1 Integrated disease management versus control, update, outcome: 1.34 SGRQ total score.
1.2. SGRQ total score (medium‐term)
Eighteen studies with a total population of 4321 participants provided data on the SGRQ total score with follow‐up between 6 and 15 months (Bourbeau 2003; Boxall 2005; Engstrom 1999; Fan 2012; Fernandez 2009; Gottlieb 2011; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Ko 2016; Kruis 2014; Rice 2010; Rose 2017; Titova 2017; Vasilopoulou 2017; Wakabayashi 2011; Wang 2017; Wood‐Baker 2006; Zwar 2016). Kessler 2018 used a COPD‐specific SGRQ, which could not be pooled. The pooled MD in SGRQ total score (MD ‐3.89, 95% CI ‐6.16 to ‐1.63) favoured IDM (Analysis 1.2; Figure 3). In other words, those treated with IDM reported 3.89 out of 100 points for improved quality of life. Pooling did indicate considerable heterogeneity (I² = 83%). Sensitivity analysis performed on high‐quality studies still showed a statistically significant effect in favour of IDM (MD ‐3.95, 95% CI ‐6.06 to ‐1.84). This effect was even more pronounced, indicating the robustness of our results. Sensitivity analysis of high‐quality studies only did not change the level of heterogeneity (I² = 79%). Pre‐defined and post‐hoc subgroup analyses were performed to investigate heterogeneity (see below).
1.2. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 2: SGRQ: medium‐term (> 6 to 15 months)
1.2.1. Subgroup analysis based on setting
Six studies reporting on SGRQ total score were performed in primary care (Boxall 2005; Fernandez 2009; Gottlieb 2011; Kruis 2014; Wood‐Baker 2006; Zwar 2016), nine studies in secondary care (Bourbeau 2003; Engstrom 1999; Fan 2012; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Rice 2010; Rose 2017; Titova 2017; Wakabayashi 2011), and three studies in tertiary care (Ko 2016; Vasilopoulou 2017; Wang 2017). A test for subgroup differences showed a statistically significant difference between subgroups (P = 0.001). Studies performed in primary and secondary care showed no statistically significant differences between IDM and control, and pooling of tertiary care studies showed a clinically and statistically significant improvement in favour of IDM (MD ‐14.58, 95% CI ‐21.56 to ‐7.61; Analysis 1.4). However, pooling indicated considerable heterogeneity for all three subgroups. Hence, results of the subgroup analysis should be interpreted with caution.
1.4. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 4: Subgroup analysis SGRQ (total score, medium‐term) based on type of setting
1.2.2. Subgroup analysis based on study design
We performed subgroup analysis based on study design and compared RCTs (total 2865 participants) with cluster‐RCTs (total 1420 participants) (Analysis 1.5). Tests for differences showed a statistically significant difference between both groups. Heterogeneity within the RCT remained considerable (I² = 83%).
1.5. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 5: Subgroup analysis SGRQ (total score, medium‐term) based on study design
1.2.3. Subgroup analysis based on dominant component of the programme
Two studies (total 294 participants) included individualised education as the dominant component (Fan 2012; Wakabayashi 2011), five studies (total 1825 participants) included self‐management as the dominant component (Bourbeau 2003; Jimenez‐Reguera 2020; Kruis 2014; Rice 2010; Wood‐Baker 2006), four studies (total 175 participants) included exercise as the dominant component (Boxall 2005; Engstrom 1999; Fernandez 2009; Gottlieb 2011), and five studies (total 1610 participants) included structural follow‐up as dominant component (Kalter‐Leibovici 2018; Ko 2016; Rose 2017; Titova 2017; Vasilopoulou 2017). Post hoc, we identified telemonitoring as an important dominant component in two studies (Vasilopoulou 2017; Wang 2017). Tests for subgroup differences showed a statistically significant result (Chi² = 17.89, df = 4, P = 0.001) indicating differences in effect between subgroups based on the dominant component. A statistically significant difference was found only in the group with telemonitoring as the dominant component (MD ‐18.33, 95% CI ‐26.72 to ‐9.94) (Analysis 1.6). However, the subgroup included only two studies. Also, heterogeneity remained moderate within subgroups. Hence, results should be interpreted with caution.
1.6. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 6: Subgroup analysis SGRQ (total score, medium‐term) based on dominant component of intervention
1.2.4. Subgroup analysis based on region of study
Four studies (total 1147 participants) were performed in North America (Bourbeau 2003; Fan 2012; Rice 2010; Rose 2017), four in Northwestern Europe (total 1286 participants) (Engstrom 1999; Gottlieb 2011; Kruis 2014; Titova 2017), three in Southern Europe (total 227 participants) (Fernandez 2009; Jimenez‐Reguera 2020; Vasilopoulou 2017), three in Oceania (total 380 participants) (Boxall 2005; Wood‐Baker 2006; Zwar 2016), three in East Asia (total 385 participants) (Ko 2016; Wakabayashi 2011; Wang 2017), and one in Western Asia (total 896 participants) (Wakabayashi 2011). Tests for subgroup differences showed a statistically significant difference in effect between groups (Chi² = 16.88, df = 5, P = 0.005) (Analysis 1.7). Closer inspection of the subgroups showed no differences between IDM and control for the Northwest Europe and Oceania subgroups. Heterogeneity remained substantial in the North America subgroup (I² = 56%) and the Southern Europe subgroup (I² = 61%) and were considerable in the East Asia subgroup (I² = 91%). Results for these subgroups should therefore be interpreted with caution.
1.7. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 7: Subgroup analysis SGRQ (total score, medium‐term) based on region
1.3. SGRQ total score ‐ long‐term
Four studies including 1090 participants measured the long‐term effect on SGRQ total score at 18 months (Gottlieb 2011), or at 24 months (Kalter‐Leibovici 2018; Titova 2017; van Wetering 2010). No statistically significant difference was noted between IDM and usual care (MD ‐0.69, 95% CI ‐3.31 to 1.93; I² = 31%) (Analysis 1.3; Figure 3).
1.3. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 3: SGRQ: long‐term (> 15 months)
1.4. SGRQ domain scores ‐ short‐term
Eleven studies with a total population of 1320 to 1327 participants reported scores on the SGRQ domains of symptoms, activity, and impact. For all domains, heterogeneity was substantial (I² between 46% and 71%) (Analysis 1.1). We found the following results: symptoms domain (MD ‐1.56, 95% CI ‐6.66 to 2.53), activity domain (MD ‐3.04, 95% CI ‐5.80 to ‐0.28), and impact domain (MD ‐3.76, 95% CI ‐5.94 to ‐1.57). Sensitivity analysis with only high‐quality studies showed a statistically significant effect in favour of IDM for the activity domain (MD ‐3.63, 95% CI ‐5.66 to ‐1.61; I² = 0%) and for the impact domain (MD ‐4.1, 95% CI ‐6.30 to ‐1.90; I² = 31%) of the SGRQ. There was no significant effect on the SGRQ symptoms domain (MD ‐1.94, 95% CI ‐5.26 to 1.38; I² = 41%). A portion of the heterogeneity could be explained by the difference in quality of studies, as heterogeneity decreased significantly across all domains when only high‐quality studies were pooled (Table 8).
7. Sensitivity analysis primary outcomes.
| Outcome | No. of studies | No. of participants | Studies omitted | Effect | Effect size | 95% CI | I² | P value |
| 1.1 SGRQ: short‐term (≤ 6 months) | ||||||||
| 1.1.1 SGRQ: total | 12 | 1386 | Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐3.65 | ‐5.66, ‐1.64 | 0.04 | 46 |
| 16 | 1788 | MD | ‐3.78 | ‐6.29, ‐1.28 | <0.00001 | 72 | ||
| 1.1.2 SGRQ: symptoms | 9 | 919 | Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐1.94 | ‐5.26, 1.38 | 0.09 | 41 |
| 13 | 1327 | MD | ‐1.56 | ‐5.66, 2.53 | <0.00001 | 71 | ||
| 1.1.3 SGRQ: activity | 9 | 916 | Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐3.63 | ‐5.66, ‐1.61 | 0.49 | 0 |
| 0 | 1320 | MD | ‐3.04 | ‐5.80, ‐0.28 | 0.02 | 50 | ||
| 1.1.4 SGRQ: impact | 9 | 917 | Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐4.1 | ‐6.30, ‐1.90 | 0.17 | 31 |
| 1322 | MD | ‐3.76 | ‐5.94,‐1.57 | 0.04 | 46 | |||
| 1.2 SGRQ: medium‐term (> 6 to 15 months) | ||||||||
| 1.2.1 SGRQ: total | 13 | 3889 | Engstrom 1999, Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐3.95 | ‐6.06, ‐1.84 | <0.00001 | 78 |
| 18 | 4321 | MD | ‐3.89 | ‐6.16, ‐1.63 | <0.00001 | 83 | ||
| 1.2.2 SGRQ: symptoms | 7 | 2195 | Engstrom 1999, Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐3.11 | ‐6.00, ‐0.21 | 0.04 | 55 |
| 12 | 2628 | MD | ‐3.88 | ‐7.75, ‐0.02 | <0.00001 | 79 | ||
| 1.2.3 SGRQ: activity | 7 | 2175 | Engstrom 1999, Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐3.09 | ‐5.98, ‐0.20 | 0.008 | 65 |
| 12 | 2608 | MD | ‐2.57 | ‐5.53,0.38 | < 0.0001 | 71 | ||
| 1.2.4 SGRQ: impact | 7 | 2178 | Engstrom 1999, Gottlieb 2011, Titova 2017, Wang 2017, Wood‐Baker 2006 | MD | ‐3.2 | ‐6.19, ‐0.21 | 0.95 | 0 |
| 12 | 2610 | MD | ‐3.34 | ‐6.26, ‐0.41 | <0.00001 | 77 | ||
| 1.3 SGRQ: long‐term (> 15 months) | ||||||||
| 1.3.1 SGRQ: total | 2 | 970 | Gottlieb 2011, Titova 2017 | MD | ‐1.02 | ‐4.30, 2.27 | 0.09 | 65 |
| 4 | 1090 | MD | ‐0.69 | ‐3.31, 1.93 | 0.22 | 31 | ||
| 1.3.2 SGRQ: symptoms | 1 | 157 | Gottlieb 2011, Titova 2017 | MD | n.a. | n.a. | n.a | n.a |
| 3 | 279 | MD | 2.35 | ‐5.49, 10.19 | 0.08 | 60 | ||
| 1.3.3 SGRQ: activity | 1 | 157 | Gottlieb 2011, Titova 2017 | MD | n.a. | n.a. | n.a. | n.a. |
| 3 | 278 | MD | ‐2.87 | ‐6.17, 0.43 | 0.55 | 0 | ||
| 1.3.4 SGRQ: impact | 1 | 150 | Gottlieb 2011, Titova 2017 | MD | n.a. | n.a. | n.a. | n.a. |
| 3 | 270 | MD | ‐2.21 | ‐4.71, 0.29 | 0.49 | 0 | ||
| 1.8 CRQ: short‐term (≤ 6 months) | ||||||||
| 1.8.1 CRQ: dyspnoea | 1 | 160 | Cambach 1997, Güell 2000, Güell 2006 | MD | n.a. | n.a. | n.a. | n.a. |
| 4 | 277 | MD | 0.8 | ‐0.01, 1.62 | 0.0001 | 86 | ||
| 1.8.2 CRQ: fatigue | 3 | 196 | Cambach 1997, Güell 2000, Güell 2006 | MD | 0.01 | ‐1.34, 1.35 | 0.28 | 21 |
| 6 | 314 | MD | 0.71 | ‐0.19, 1.62 | <0.00001 | 84 | ||
| 1.8.3 CRQ: emotion | 3 | 196 | Cambach 1997, Güell 2000, Güell 2006 | MD | ‐0.33 | ‐0.65, ‐0.01 | 0.37 | 0 |
| 6 | 314 | MD | 0.45 | ‐0.26, 1.17 | 0.003 | 72 | ||
| 1.8.4 CRQ: mastery | 3 | 196 | Cambach 1997, Güell 2000, Güell 2006 | MD | 0.38 | ‐1.20, 1.96 | 0.22 | 35 |
| 6 | 314 | MD | 0.72 | ‐0.08, 1.52 | 0.0002 | 79 | ||
| 1.9 CRQ: medium‐term (> 6 to 15 months) | ||||||||
| 1.9.1 CRQ: dyspnoea | 1 | 159 | Güell 2000 | MD | n.a. | n.a. | n.a. | n.a. |
| 2 | 219 | MD | 0.29 | ‐0.88, 1.46 | 0.004 | 88 | ||
| 1.9.2 CRQ: fatigue | 3 | 195 | Güell 2000 | MD | 0.35 | ‐1.22, 1.93 | 0.21 | 35 |
| 4 | 255 | MD | 0.37 | ‐0.53, 1.26 | 0.05 | 63 | ||
| 1.9.3 CRQ: emotion | 3 | 195 | Güell 2000 | MD | 0.26 | ‐1.91, 2.43 | 0.9 | 20 |
| 4 | 255 | MD | 0.36 | ‐0.84, 1.57 | 0.01 | 73 | ||
| 1.9.4 CRQ: mastery | 3 | 195 | Güell 2000 | MD | 1.06 | ‐1.19, 3.30 | 0.08 | 60 |
| 4 | 255 | MD | 0.76 | ‐0.41, 1.94 | 0.004 | 78 | ||
| 1.10 CRQ: long‐term (> 15 months) | ||||||||
| 1.10.1 CRQ: dyspnoea | 1 | 104 | Güell 2000 | MD | n.a. | n.a. | ||
| 2 | 151 | MD | 0.47 | ‐0.31, 1.25 | 0.07 | 70 | ||
| 1.10.2 CRQ: fatigue | 3 | 137 | Güell 2000 | MD | 0.42 | ‐0.05, 0.89 | 0.75 | 0 |
| 4 | 184 | MD | 0.46 | 0.06, 0.85 | 0.88 | 0 | ||
| 1.10.3 CRQ: emotion | 3 | 137 | Güell 2000 | MD | 0.52 | 0.00, 1.04 | 1 | 0 |
| 4 | 184 | MD | 0.52 | 0.10, 0.95 | 1 | 0 | ||
| 1.10.4 CRQ: mastery | 3 | 137 | Güell 2000 | MD | 0.75 | 0.22, 1.28 | 0.67 | 0 |
| 4 | 184 | MD | 0.83 | 0.41, 1.26 | 0.78 | 0 | ||
| 1.11 SF‐36 | ||||||||
| 1.11.1 SF‐36 MCS score | 3 | 2212 | Lilholt 2017, Vianello 2016 | MD | 0.44 | ‐0.43, 1.31 | 0.44 | 0 |
| 5 | 3699 | MD | 0.36 | ‐0.38,1.11 | 0.75 | 0 | ||
| 1.11.2 SF‐36 PCS score | 3 | 2217 | Lilholt 2017, Vianello 2016 | MD | ‐0.17 | ‐1.05, 0.71 | 0.33 | 0 |
| 5 | 3704 | 1.06 | ‐0.67, 2.79 | < 0.0001 | 84 | |||
| 1.13 Functional exercise capacity: 6MWD | ||||||||
| 1.13.1 6MWD: short‐term (≤ 6 months) | 8 | 886 | Bendstrup 1997, Bernocchi 2017Cambach 1997, Gottlieb 2011, Güell 2000, Güell 2006, Mendes 2010, Tabak 2014, Wang 2017 | MD | 41 | 4.40, 77.60 | < .00001 | 92 |
| 17 | 1390 | MD | 52.56 | 32.39,72.74 | < 0.0001 | 90 | ||
| 1.13.2 6MWD: medium‐term (> 6 months to 15 months) | 9 | 1576 | Engstrom 1999, Güell 2000, Kessler 2018, Wang 2017 | MD | 40.49 | 9.71, 71.27 | <0.00001 | 92 |
| 13 | 2071 | MD | 44.69 | 24.01, 65.37 | <0.00001 | 90 | ||
| 1.13.3 6MWD: long‐term (> 15 months) | 3 | 973 | Gottlieb 2011, Güell 2000, Lou 2015 | MD | 36.4 | ‐6.43, 79,24 | <0.00001 | 94 |
| 6 | 7288 | MD | 48.43 | 16.37, 80.49 | <0.00001 | 90 | ||
| 1.18 Respiratory‐related hospital admissions | ||||||||
| 1.18.1 Respiratory‐related hospital admissions: short‐term (≤ 6 months) | 2 | 265 | Bernocchi 2017 | OR | 0.71 | 0.28, 1.81 | 0.43 | 0 |
| 377 | OR | 0.6 | 0.30, 1.22 | 0.65 | 0 | |||
| 1.18.2 Respiratory‐related hospital admissions: medium‐term (> 6 to 15 months) | 8 | 2224 | Rea 2004, Smith 1999 | OR | 0.56 | 0.42, 0.76 | 0.06 | 48 |
| 2449 | OR | 0.6 | 0.44, 0.81 | 0.01 | 57 | |||
| 1.18.3 Respiratory‐related hospital admissions: long‐term (> 15 months) | 2 | 0 | n.a. | OR | n.a. | n.a. | n.a. | |
| 2 | 1381 | OR | 0.85 | 0.59, 1.23 | 0.23 | 29 | ||
| 1.22 All hospital admissions | ||||||||
| 1.22.2 All hospital admissions: medium‐ term (> 6 months to 15 months) | 3 | 760 | Rea 2004, Kessler 2018 | OR | 0.91 | 0.66, 1.26 | 0.74 | 0 |
| 5 | 1212 | OR | 0.93 | 0.71, 1.21 | 0.33 | 14 | ||
| 1.22.3 All hospital admissions: long‐term (> 15 months) | 3 | 1485 | Lou 2015 | OR | 0.88 | 0.61, 1.27 | 0.2 | 38 |
| 4 | 1920 | OR | 0.72 | 0.45, 1.04 | 0.007 | 55 | ||
| 1.23 Hospital days per patient (all causes) | ||||||||
| 1.23.1 Hospital days per patient (all causes): short‐term (≤ 6 months) | 2 | 0 | n.a. | MD | n.a. | n.a. | n.a. | |
| 2 | 273 | MD | ‐4.36 | ‐6.41, ‐2.31 | 0.16 | 49 | ||
| 1.23.2 Hospital days per patient (all causes): medium‐term (> 6 to 15 months) | 5 | 2086 | Engstrom 1999, Farrero 2001, Kessler 2018, Rea 2004, Vianello 2016 | MD | ‐1.01 | ‐3.41, 1.38 | 0.001 | 78 |
| 10 | 2944 | MD | ‐1.73 | ‐3.71, 0.25 | 0.0003 | 71 | ||
| 1.23.3 Hospital days per patient (all causes): long‐term (> 15 months) | 1 | 175 | Titova 2017 | MD | n.a. | n.a. | n.a. | n.a. |
| 2 | 346 | MD | ‐1.6 | ‐6.12, 2.92 | 0.1 | 63 | ||
| 1.24 ED visits | 6 | 2343 | Lou 2015, Rea 2004, Smith 1999, | OR | 0.69 | 0.50, 0.94 | 0.02 | 64 |
| 9 | 3005 | OR | 0.69 | 0.50, 0.93 | 0.02 | 68 | ||
| 1.26 Number of patients using ≥ 1 course of oral steroids | 2 | 218 | Farrero 2001, Rea 2004 | OR | 1.17 | 0.57, 2.40 | 0.19 | 42 |
| 4 | 433 | OR | 1.05 | 0.66, 1.64 | 0.25 | 27 | ||
| 1.27 Number of patients using ≥ 1 course of antibiotics | 2 | 218 | Rea 2004 | OR | 2.35 | 1.02, 5.42 | 0.15 | 53 |
| 3 | 321 | OR | 1.46 | 0.51, 4.18 | 0.007 | 80 | ||
Abbreviations. 6MWD: 6‐minute walking distance; CI: confidence interval; CRQ: Chronic Respiratory Questionnaire; ED: emergency department; MD: mean difference; OR: odds ratio; SF‐36: Short Form‐36; SGRQ: St. George's Respiratory Questionnaire.
1.5. SGRQ domain scores ‐ medium‐term
Twelve studies with a total population of 2608 to 2628 participants reported scores on the SGRQ domains after 6 to 15 months' follow‐up. We found the following results: symptoms domain: MD ‐3.88, 95% CI ‐7.75 to ‐0.02; I² = 79%; activity domain: MD ‐2.57, 95% CI ‐5.53 to 0.38; I² = 71%; and impact domain: MD ‐3.34, 95% CI ‐6.26 to ‐0.41; I² = 0%. Sensitivity analysis did not explain the heterogeneity observed (I² between 71% and 79%) but did show a statistically significant effect in favour of IDM. Effects were statistically significant for all domains (Analysis 1.2; Table 8).
1.6. SGRQ domain scores ‐ long‐term
Three studies measured the long‐term effect on SGRQ domains at 18 months (Gottlieb 2011), or at 24 months (Titova 2017; van Wetering 2010). As with the SGRQ total score, pooled effects did not show a statistically significant long‐term difference between both groups (Analysis 1.3).
1.7. CRQ domain scores ‐ short‐term
The Chronic Respiratory Disease Questionnaire (CRQ), with a scale from 0 to 7 and MCID of 0.5, was reported in nine studies (Bendstrup 1997; Cambach 1997; Farrero 2001; Güell 2000; Güell 2006; Lenferink 2019; Rea 2004; Sridhar 2008; Wijkstra 1994). Farrero 2001 administered the CRQ only to the first 40 consecutive patients, and therefore outcomes were not published. Bendstrup 1997 and Rea 2004 reported insufficient data to compute an estimation of effect and therefore were not included in the meta‐analysis. Wijkstra 1994 did not report on the dyspnoea dimension of the CRQ and compared two IDM interventions with usual care. We included both study arms in the meta‐analysis. Pooled results for the CRQ up to 6 months included 277 participants for the CRQ Dyspnea dimension and 314 for the other domains. There was no statistically significant difference between IDM and control for any dimension (Analysis 1.8). Heterogeneity was substantial for all dimensions (I² between 72% and 86%). Sensitivity analysis for CRQ Dyspnoea was not performed, as this would include only one high‐quality study. Sensitivity analysis for the other CRQ dimensions did not change the results but smaller heterogeneity was observed (I² between 0% and 35%). Thus, heterogeneity could be explained in part by the quality of the studies (see Table 8).
1.8. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 8: CRQ: short‐term (≤ 6 months)
1.8. CRQ domain scores ‐ medium‐term
Three of the four studies that reported CRQ up to 6 months also reported CRQ outcomes after 6 months (Güell 2000; Lenferink 2019; Wijkstra 1994). Pooled results, including 2 studies and 219 participants for the CRQ dyspnoea dimension, showed no statistically significant differences between IDM and control groups (MD 0.29, 95% CI ‐0.88 to 1.46). There also were no statistically significant differences between groups for the CRQ fatigue domain (MD 0.37, 95% CI ‐0.53 to 1.26), the CRQ emotion domain (MD 0.36, 95% CI ‐0.84 to 1.57), and the CRQ mastery domain (MD 0.76, 95% CI ‐0.41 to 1.94) (Analysis 1.9).
1.9. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 9: CRQ: medium‐term (> 6 to 15 months)
1.9. CRQ domain scores ‐ long‐term
Three studies reported on long‐term effects on the CRQ at 24 months' follow‐up, with a total of 184 participants (Güell 2000; Sridhar 2008; Wijkstra 1994) (Analysis 1.10). Pooled data showed no differences between groups on the CRQ dyspnoea domain (MD 0.47, 95% CI ‐0.31 to 1.25). In contrast, pooled data on the CRQ fatigue domain showed a statistically significant difference in favour of IDM (MD 0.46, 95% CI 0.06 to 0.85). Also, a significant difference in favour of IDM was observed for CRQ emotion (MD 0.53, 95% CI 0.10 to 0.95) and CRQ mastery (MD 0.83, 95% CI 0.41 to 1.26). With an MCID of 0.5, the differences were also clinically significant. Sensitivity analysis revealed that when Güell 2000 was excluded due to inadequate concealment of allocation, pooled differences on CRQ fatigue, emotion, and mastery remained in favour of IDM; however CRQ fatigue was not statistically significant (MD 0.42, 95% CI ‐0.05 to 0.89) (Table 8).
1.10. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 10: CRQ: long‐term (> 15 months)
1.10. General health‐related QoL
General HRQoL was measured with the SF‐36 in six studies (Aiken 2006; Kruis 2014; Lilholt 2017; Öztürk 2020; Rea 2004; Vianello 2016), or with the shorter SF‐12 in two studies (Fan 2012; Kalter‐Leibovici 2018). Aiken 2006 did not provide us with sufficient information and did not respond to our emails. Rea 2004 and Öztürk 2020 reported only on the separate dimensions of the SF‐36 and therefore could not be used for pooling. For the remaining studies, we pooled composite scores from the SF‐36 and the SF‐12. Hence, we pooled the data from studies for the Mental Component Summary (MCS) score with a total population of 3699 participants and of the Physical Component Summary (PCS) score with a total population of 3704 participants. Pooled MD on the MSC score showed no significant differences between both groups (MD 0.36, 95% CI ‐0.38 to 1.11; I² = 0%). Also no significant differences were observed on the PCS score (MD 1.06, 95% CI ‐0.67 to 2.79; I² = 84%). Substantial heterogeneity observed for the PCS score was due in part to differences in the quality of the studies. Sensitivity analysis excluding Vianello 2016 and Lilholt 2017 showed similar non‐significant effects (see Table 8). Two studies measured QoL with the Sickness Impact Profile (SIP) (Engstrom 1999; Littlejohns 1991) (Analysis 1.12). No between‐group differences were found in any domain of the SIP.
1.12. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 12: General health QoL: SIP mean difference
2. Exercise capacity
Twenty‐eight studies measured functional or maximum exercise capacity. Functional exercise capacity was measured through the 6MWD (26 studies) or the shuttle test (1 study). Maximal exercise capacity was measured using the cycle ergometer test expressed as W‐max (5 studies), leg fatigue score (1 study), and grip strength (1 study). The MCID on the 6MWD is estimated at 35 meters (Puhan 2008). No MCID for the cycle ergometer test is reported in the current literature. Results are shown in Figure 4.
4.

Forest plot of comparison: 1 Integrated disease management versus control, update, outcome: 1.13 Functional exercise capacity: 6MWD.
2.1. Functional exercise capacity ‐ short‐term
We pooled data from 17 studies using the 6MWD including 1390 participants (Bendstrup 1997; Bernocchi 2017; Boxall 2005; Cambach 1997; Gottlieb 2011; Güell 2000; Güell 2006; Jimenez‐Reguera 2020; Khan 2019; Mendes 2010; Tabak 2014; Theander 2009; van Wetering 2010; Wakabayashi 2011; Wang 2017; Wijkstra 1994; Zhang 2020). One study could not be pooled, as study authors reported no data because there was no significant difference between groups at 12 months' follow‐up (Bourbeau 2003). The pooled MD on the 6MWD outcome was 52.56 in favour of IDM (95% CI 32.39 to 72.74) and exceeded the MCID of 35. In other words, patients treated in an IDM programme were able to walk 52 meters more, on average, than those who received usual care. Pooling did indicate considerable heterogeneity (I² = 90%). Sensitivity analysis performed on high‐quality studies showed a smaller but still statistically and clinically significant effect in favour of IDM (MD 41.00, 95% CI 4.40 to 77.60, I² = 92%).
2.2. Functional exercise capacity ‐ medium‐term
Thirteen studies with a total population of 2071 participants provided data on the 6MWD after a medium‐term follow‐up period (between 6 and 15 months) (Engstrom 1999; Fernandez 2009; Gottlieb 2011; Güell 2000; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Kessler 2018; Ko 2016; Littlejohns 1991; Vasilopoulou 2017; Wakabayashi 2011; Wang 2017; Zhang 2020). Pooled MD showed a statistically and clinically significant effect of 44.69 in favour of IDM. The observed effect was statistically significant (95% CI 24.01. to 65.37) and exceeded the MCID of 35 meters. Sensitivity analysis showed that our results were robust (MD 40.49, 95% CI 9.71 to 71.27). However, heterogeneity remained substantial (I² = 92%). The heterogeneity among high‐quality studies and the large confidence interval for the pooled results of all studies indicate there may be substantial methodological or clinical differences between studies. Pre‐defined and post‐hoc subgroup analyses were performed to further investigate the existing heterogeneity (see below).
2.2.1. Subgroup analysis based on type of setting
Of the studies reporting 6MWD at 12 months, two were conducted in primary care (Fernandez 2009; Gottlieb 2011), seven in secondary care (Engstrom 1999; Güell 2000; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Kessler 2018; Littlejohns 1991; Wakabayashi 2011), and four in tertiary care (Ko 2016; Vasilopoulou 2017; Wang 2017; Zhang 2020). Tests for subgroup differences showed no difference in effect based on setting (Chi² = 4.49, df = 2, P = 0.11). However, heterogeneity remained considerable for the secondary care subgroup (I² = 80%) and for the tertiary care subgroup (I² = 87%). Therefore, results for these groups should be interpreted carefully (Analysis 1.14).
1.14. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 14: Subgroup analysis 6MWD (medium‐term) based on type of setting
2.2.2. Subgroup analysis based on dominant component of intervention
Four studies (102 participants) reporting on the 6MWD had some kind of exercise training as their dominant component (Engstrom 1999; Fernandez 2009; Gottlieb 2011; Güell 2000). In six studies, structural follow‐up was considered the dominant component (Kalter‐Leibovici 2018; Kessler 2018; Ko 2016; Littlejohns 1991; Vasilopoulou 2017; Zhang 2020). One study provided individualised education as the dominant component (Wakabayashi 2011), and another study included self‐management as the dominant component (Jimenez‐Reguera 2020). Therefore, these could not be pooled. A test for subgroup differences showed a statistically significant difference (Chi² = 10.56, df = 4, P = 0.03; Analysis 1.14).
Subgroup analysis for exercise training as the dominant component showed that the 6MWD improved by 68.21 metres (95% CI 44.75 to 91.68; I² = 3%). This effect was almost twice the MCID of 35 metres. Also, studies with telemonitoring as the dominant component showed a large improvement of 59.94 metres (95% CI 42.59 to 77.29; I² = 32%). Studies with structural follow‐up as the dominant component showed statistically significant differences in favour of IDM (MD 35.14, 95% CI 2.83 to 67.45). However, heterogeneity remained substantial.
2.2.3. Subgroup analysis based on region of study
Three studies reporting on 6MWD with medium‐term follow‐up were performed in Northwestern Europe (Engstrom 1999; Gottlieb 2011; Littlejohns 1991), five in Southern Europe (Fernandez 2009; Güell 2000; Jimenez‐Reguera 2020; Kessler 2018; Vasilopoulou 2017), four in East Asia (Ko 2016; Wakabayashi 2011; Wang 2017; Zwar 2016), and one in Western Asia (Kalter‐Leibovici 2018). A test for subgroup differences indicated statistically significant differences in effect between subgroups (Chi² = 19.09, df = 3, P = 0.00003).
Pooling of studies performed in Northwestern Europe showed no statistically significant difference between IDM and control (MD 18.18, 95% CI ‐7.87 to 44.24; I² = 4%). A statistically significant difference was found for the Southern Europe subgroup (MD of 61.73) and the East Asia subgroup (MD of 42.67). Pooling indicated considerable heterogeneity in the subgroup of studies from Southern Europe (I² = 68%) and East Asia (I² = 90%); results for these subgroups should therefore be interpreted carefully (Analysis 1.16).
1.16. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 16: Subgroup analysis 6MWD (medium‐term) based on region
2.3. Functional exercise capacity ‐ long‐term
Six studies on 7288 participants published long‐term results on the 6MWD (Gottlieb 2011; Güell 2000; Kalter‐Leibovici 2018; Lou 2015; van Wetering 2010; Zhang 2020). The MD was 48.83 metres in favour of IDM and was of statistically and clinically significant relevance (95% CI 16.37 to 80.49; I² = 90%) (Analysis 1.13). Sensitivity analysis could not explain heterogeneity and showed a smaller non‐statistically significant mean difference (MD 36.4; I² = 94%; Analysis 1.13) noted by a wide CI (95% CI ‐6.43.97 to 79.24).
1.13. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 13: Functional exercise capacity: 6MWD
2.4. Maximal exercise capacity
Four studies on 298 participants assessed maximum exercise capacity (in Watts) using the cycle ergometer test (Engstrom 1999; Strijbos 1996; van Wetering 2010; Wijkstra 1994). Pooling showed that IDM statistically significantly improved maximal exercise capacity by 7 Watts (MD 6.99, 95% CI 2.96 to 11.02; Analysis 1.17).
1.17. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 17: Maximal exercise capacity: cycle test (W‐max)
3. Exacerbation‐related outcomes
3.1. Respiratory‐related admissions
Fifteen studies including a total of 4207 participants reported on the number of patients with at least one respiratory‐related admission, which could be COPD‐related, exacerbation‐related, or of a respiratory nature in general. Pooling showed an effect in favour of the IDM intervention (OR 0.64, 95% CI 0.50 to 0.81). In other words, per 1000 patients, 89 fewer (range 131 fewer to 44 fewer) patients had a respiratory‐related (re‐)hospitalisation compared to patients given usual care (Analysis 1.18Figure 5).
1.18. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 18: Respiratory‐related hospital admissions
5.
In the usual care group, 32 out of 100 people had a respiratory‐related hospital admission over a period of 3 to 36 months, compared to 23 (95% CI 19 to 28) out of 100 people in the integrated disease management group.
3.2. Respiratory‐related admissions ‐ short‐term
We pooled data from three studies with 377 patients measuring respiratory‐related admissions until 6 months' follow‐up (Bernocchi 2017; Koff 2009; Trappenburg 2011). There were no statistically significant differences in the risk of respiratory‐related hospital admissions in the short term (OR 0.60; 95% CI 0.30 to 1.22). Studies were homogeneous, but the number of events was too small (ranging from 1 to 11) to allow firm conclusions based on the data.
3.3. Respiratory‐related admissions ‐ medium‐term
Nine studies with a total of 2449 participants reported on the number of patients with at least one respiratory‐related admission at 6 to 15 months' follow‐up (Bourbeau 2003; Fan 2012; Lenferink 2019; Rea 2004; Rice 2010; Sanchez‐Nieto 2016; Silver 2017; Smith 1999; Vasilopoulou 2017). Pooled estimates showed a statistically significant reduction in admissions in favour of IDM (OR 0.60, 95%CI 0.44 to 0.81). Data showed considerable heterogeneity (I² = 57%) (Analysis 1.18). Sensitivity analysis of only high‐quality studies showed similar results, with only a small reduction in heterogeneity (I² = 48%) (see Table 8). To further explore the reasons for heterogeneity, we performed three subgroup analyses.
3.3.1. Subgroup analysis based on setting
Heterogeneity remained substantial or considerable when we pooled all studies in which the intervention was delivered in a primary care setting (I² = 84%) and secondary or tertiary care settings combined (I² = 48%). A test for subgroup differences showed no differences between groups (Chi² = 0.38, df = 1, P = 0.54). In other words, there seems to be no convincing difference between primary care and secondary or tertiary care that can explain the observed heterogeneity (Analysis 1.19).
1.19. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 19: Subgroup analysis respiratory‐related hospital admissions (medium‐term) based on type of setting
3.3.2. Subgroup analysis based on dominant component of the programme
In five studies with a total of 1353 participants, the dominant component was self‐management (Bourbeau 2003; Lenferink 2019; Rea 2004; Rice 2010; Sanchez‐Nieto 2016). Two studies included education (Fan 2012; Silver 2017), two studies structural follow‐up (Smith 1999; Vasilopoulou 2017), and one study telemonitoring as the dominant intervention component (Vasilopoulou 2017). A test for subgroup difference showed no differences between groups (Chi² = 3.65, df = 3, P = 0.30). However, these results should be interpreted carefully, as only the self‐management subgroup pooled more than two studies, while the other subgroups pooled two or fewer studies. Among studies with self‐management as the dominant component, the effect on respiratory‐related admissions favoured IDM ((OR 0.55, 95% CI 0.43 to 0.71; I² = 0%) (Analysis 1.20).
1.20. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 20: Subgroup analysis respiratory‐related hospital admissions (medium‐term) based on dominant component of intervention
3.3.3. Subgroup analysis based on region
Four of the nine studies, with a total of 1788 participants, originated in North America (Bourbeau 2003; Fan 2012; Rice 2010; Silver 2017), two studies in Southern Europe (Sanchez‐Nieto 2016; Vasilopoulou 2017), one study in Northwestern Europe (Lenferink 2019), and one study in Oceania (Smith 1999). The effect estimate differed significantly between subgroups (Chi² = 10.93, df = 3, P = 0.01). Pooling of studies conducted in North America showed a significant reduction in respiratory‐related hospital admissions (OR 0.69, 95% CI 0.50 to 0.94; I² = 44), as did pooling of studies conducted in Southern Europe (OR 0.35, 95% CI 0.18 to 0.68; I² = 25%). Pooling of studies from Northwestern Europe and Oceania was not possible due to the small numbers (Analysis 1.21) (Lenferink 2019; Smith 1999). In addition to regional differences in effects of IDM on respiratory‐related hospital admissions, there was a marked difference in the mean rate of respiratory‐related hospital admissions per patient. Among IDM groups, the mean rate per patient was 0.19 admissions per patient in studies from North America, 0.21 per patient from Northwestern Europe, 0.59 per patient for Souhern Europe, and 0.70 per patient from Oceania. Similarly, for controls, the rate from North America was 0.26 per patient, from Northwestern Europe 0.26 per patient, from Southern Europe 0.64 per patient, and from Oceania 0.56 per patient.
1.21. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 21: Subgroup analysis respiratory‐related hospital admissions (medium‐term) based on region
3.4. Hospital admissions, all causes
We were able to pool ten studies that reported on patients experiencing at least one hospital admission for all causes and included a total of 9030 participants. Pooling showed an overall statistically significant effect in favour of IDM (OR 0.75, 95% CI 0.57 to 0.98). This means that compared with usual care, there were 72 fewer (range 138 fewer to 5 fewer) hospitalisations per 1000 with IDM. Pooling based on follow‐up period indicated slight differences in short‐, medium‐, and long‐term effects (Analysis 1.22).
1.22. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 22: All hospital admissions
3.5. Hospital admissions, all causes ‐ short‐term
Only one study including 112 participants reported on the number of hospital admissions for all causes after 6 months' follow‐up and therefore could not be pooled (Bernocchi 2017). Study authors reported a significant reduction in the number of patients having at least one hospital admission, in favour of the intervention group (OR 0.31, 95% CI 0.14 to 0.67).
3.6. Hospital admissions, all causes ‐ medium‐term
Five studies with a total of 1212 participants provided data on the number of participants admitted at least one time for all causes at 6 to 15 months' follow‐up (medium‐term) (Fan 2012; Kessler 2018; Lenferink 2019; Littlejohns 1991; Rea 2004). Kessler 2018 did not directly report the number of participants, so the number was approximated based on the percentage of people with 0 hospitalisation days. Pooling showed that results were homogeneous and there was no significant difference between groups (OR 0.93, 95% CI 0.71 to 1.21; I² = 14%). A sensitivity analysis of only high‐quality studies showed a similar result (OR 0.91, 95% CI 0.66 to 1.26; I² = 0%).
3.7. Hospital admissions, all causes ‐ long‐term
Four studies including a total of 7706 participants assessed the number of participants admitted after 15 months' follow‐up (Kalter‐Leibovici 2018; Lou 2015; Sridhar 2008; van Wetering 2010). Numbers of events and total numbers are lower for Lou 2015, as we reduced the size of the study to its 'effective sample size' to adjust for clustering effects. Pooled meta‐analysis showed no significant differences between groups (OR 0.72, 95% CI 0.45 to 1.16). Pooled results showed considerable heterogeneity (I² = 75%) and differences in direction of effect. Although Lou 2015 and van Wetering 2010 showed positive effects in favour of IDM, Kalter‐Leibovici 2018 and Sridhar 2008 showed no statistically significant differences. The different findings could have resulted from variation in follow‐up duration which ranged from 24 months in Sridhar 2008and van Wetering 2010 to 36 months in Kalter‐Leibovici 2018 to 48 months in Lou 2015. Finally, heterogeneity could be explained by the large differences in study size ranging from 104 participants in Sridhar 2008 to 6221 participants (435 effective sample size) in Lou 2015. Sensitivity analysis including only high‐quality studies did not show a statistically significant effect (OR 0.88, 95% CI 0.61 to 1.27; I² = 38%).
3.8. Hospital days per patient
We were able to pool 14 studies that reported on the number of hospital days among those (3563 participants) hospitalised during the study. Pooling showed an overall reduction of 2.27 days spent in the hospital in favour of IDM; this finding was statistically significant (MD ‐2.27, 95% CI ‐3.98 to ‐0.56; I² =7 8%) (see Figure 6).
6.

Forest plot of comparison: 1 Integrated disease management versus control, update, outcome: 1.24 Hospital days per patient (all causes).
3.9. Hospital days per patient ‐ short‐term
Two studies with a total of 273 participants reported on the difference in mean hospitalisation days per patient per group within the first six months (Boxall 2005; Trappenburg 2011). Pooling showed a significant reduction in days spent in the hospital per patient in favour of IDM (MD ‐4.36, 95% CI ‐6.41 to ‐2.31) (Analysis 1.23).
1.23. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 23: Hospital days per patient (all causes)
3.10. Hospital days per patient ‐ medium‐term
Ten studies including 2994 participants assessed the difference in mean hospitalisation days per patient per group from 6 to 15 months' follow‐up (Bourbeau 2003; Engstrom 1999; Farrero 2001; Kessler 2018; Ko 2016; Kruis 2014; Lenferink 2019; Rea 2004; Silver 2017; Vianello 2016). Pooling showed a non‐significant reduction in hospitalisation days in favour of IDM (MD ‐1.73, 95% CI ‐3.71 to 0.25), with moderate heterogeneity (I² = 71%). Heterogeneity could not be explained by differences in the quality of studies. Three studies showed a significant effect in favour of IDM (Farrero 2001; Ko 2016; Rea 2004), and one study showed a significant effect in favour of control (Smith 1999). Smith 1999 reported increased attention to disease and symptoms by the COPD nurse as a possible explanation. Mean hospitalisation days also varied substantially between studies and within the IDM study groups, with an average hospital stay ranging from 2 days in Silver 2017 to 25.5 days in Vianello 2016 (Analysis 1.23).
3.11. Hospital days per patient ‐ long‐term
Two studies with 346 participants reported the difference in mean hospitalisation days after 15 months' follow‐up (Titova 2017; van Wetering 2010). There was no significant difference between groups (MD ‐1.60, 95% CI ‐6.12 to 2.92) (Analysis 1.23).
3.12. Emergency department
Twelve studies assessed the number of participants with at least one ED visit (Bourbeau 2003; Fan 2012; Farrero 2001; Lou 2015; Rea 2004; Rice 2010; Rose 2017; Sanchez‐Nieto 2016; Silver 2017; Smith 1999; Trappenburg 2011; Wakabayashi 2011). To account for clustering, we reduced the study size in Lou 2015 to its 'effective sample size'. We were able to pool the data from nine studies with 8791 participants (Bourbeau 2003; Fan 2012; Lou 2015; Rea 2004; Rice 2010; Rose 2017; Sanchez‐Nieto 2016; Silver 2017; Smith 1999), which revealed a significant reduction in the number of participants with at least one ED visit in favour of IDM, with considerable heterogeneity (OR 0.69, 95% CI 0.50 to 0.93; I² = 68%) (Analysis 1.24). A sensitivity analysis including only high‐quality studies showed that the risk of an ED visit was still significantly reduced with IDM (OR 0.69, 95% CI 0.50 to 0.94; I² = 64%) but could not explain the heterogeneity. Further exploration to assess reasons for heterogeneity revealed that seven trials had decreased risk of ED visits in favour of IDM (Bourbeau 2003; Fan 2012; Lou 2015; Rea 2004; Rice 2010; Sanchez‐Nieto 2016; Silver 2017), of which three were statistically significant (Bourbeau 2003; Lou 2015; Rice 2010). Two studies showed a non‐significant increase in risk of ED visits for the IDM group (Rose 2017; Smith 1999). Silver 2017 reported in the discussion that lack of effect on ED visits "may be due to the emergency department functioning as an out‐patient or rescue clinic for patients with exacerbations of their disease". The fact that most of the participants enrolled in the study lacked access to a primary care provider could explain the observation that the effect was non‐significant.
1.24. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 24: ED visits
3.13. Number of patients experiencing at least one exacerbation
Seven studies reported on the number of patients experiencing at least one exacerbation during follow‐up. The definition of exacerbation differed slightly between studies. Trappenburg 2011 and Bourbeau 2003 defined an exacerbation as an increase in symptoms with deterioration of dyspnoea or purulent sputum. Lenferink 2019 used a similar definition (clear negative change in two symptoms classified as major symptoms (dyspnoea, sputum purulence, sputum volume) or in one major and one minor symptom (coughing, wheezing, fever) from baseline, for 2 or more consecutive days). Vasilopoulou 2017, Kruis 2014, and Sridhar 2008 defined exacerbation as an "unscheduled need for healthcare, or need for steroid tablets, or antibiotics for worsening of their COPD”. Vasilopoulou 2017 and Kruis 2014 defined exacerbation based on a visit to the general practitioner or the respiratory physician in combination with a prescription of antibiotics and/or prednisolone; Kruis 2014, Vasilopoulou 2017, and Kessler 2018 made a distinction between moderate and severe exacerbations. If provided, we included the results for severe exacerbations.
Pooling of all studies reporting on the number of participants experiencing at least one exacerbation during follow‐up showed no statistically significant difference between groups (OR 0.96, 95% CI 0.65 to 1.42). Pooling based on follow‐up periods showed consistent non‐significant results for medium‐term effects (OR 0.72, 95% CI 0.90 to 1.27; I² = 47%) and long‐term effects (OR 1.53, 95% CI 0.90 to 2.60; I² = 0%; Analysis 1.25). Trappenburg 2011, which reported results at 6 months' follow‐up, indicated that although exacerbation rates did not differ between groups, exacerbations within the IDM group were perceived as substantially milder by patients. Sridhar 2008, reporting on the number of participants experiencing at least one exacerbation at 24 months' follow‐up (long‐term), stated that patients in the intervention group were more likely to have exacerbations treated with oral steroids alone or oral steroids and antibiotics than patients in the control group. The initiator of treatment in the control group was statistically more likely to be the patient rather than the GP, and this could explain the absence of an effect.
1.25. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 25: Number of patients experiencing ≥ 1 exacerbation
3.14. Patients using at least one course of oral steroids
We pooled data from four studies including 433 participants reporting on the number of patients using at least one course of oral steroids during follow‐up (12 months) (Farrero 2001; Littlejohns 1991; Rea 2004; Sanchez‐Nieto 2016). Pooling showed homogeneity between studies and no differences between groups (OR 1.05, 95% CI 0.66 to 1.64; I² = 27%; Analysis 1.26).
1.26. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 26: Number of patients using ≥ 1 course of oral steroids
3.15. Patients using at least one course of antibiotics
Three studies with 321 participants reported on the number of patients using at least one course of antibiotics (Littlejohns 1991; Rea 2004; Sanchez‐Nieto 2016). The number of patients using at least one course of antibiotics was not statistically different between groups (OR 1.46, 95% CI 0.51 to 4.18; I² = 53%; Analysis 1.27). A sensitivity analysis of high‐quality studies showed decreased heterogeneity (I² = 53%) and significantly increased risk when a course of antibiotics was received by people in the IDM group (OR 2.35, 95% CI 1.02 to 5.42). Further exploration of these studies revealed that they provided the same follow‐up (12 months) but represented very different settings, as Rea 2004 was a cluster‐randomised trial in a primary care setting, and Littlejohns 1991 and Sanchez‐Nieto 2016 were RCTs conducted in a secondary care setting.
1.27. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 27: Number of patients using ≥ 1 course of antibiotics
Secondary outcomes
4. Dyspnoea
Fifteen studies reported on modified MRC Dyspnoea Scale scores as an outcome for dyspnoea (Bernocchi 2017; Gottlieb 2011; Kalter‐Leibovici 2018; Khan 2019; Ko 2016; Kruis 2014; Lenferink 2019; Lou 2015; Mendes 2010; Öztürk 2020; van Wetering 2010; Vasilopoulou 2017; Wakabayashi 2011; Wang 2017; Zhang 2020). Gottlieb 2011 did not publish any results, and results from Wang 2017 could not be included due to a reporting error. Outcomes were reported after 3 months, 4 months, 6 months, 12 months, and/or 24 months. The data allowed us to calculate the MRC Dyspnoea Scale score at short‐, medium‐, and long‐term follow‐up. Pooling showed significant improvement in favour of IDM for short‐term follow‐up (MD ‐0.33, 95% CI ‐0.52 to ‐0.15). Pooling of mMRC Dyspnoea Scale scores at medium‐ and long‐term follow‐up showed heterogeneity (I² = 96%) too large to be permit conclusions based on the results (Analysis 1.28). Dyspnoea as measured by Borg Scale score in three studies showed no differences between groups (MD 0.14, 95% CI ‐0.70 to 0.98; I² = 39%) (Boxall 2005; Gottlieb 2011; Güell 2000).
1.28. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 28: MRC dyspnoea score
5. Mortality
Fifteen studies assessed mortality as an outcome or as part of patient safety assessment. Of these studies, two assessed mortality at 6 months' follow‐up (Aboumatar 2019; Bernocchi 2017), nine at 12 months' follow‐up (Fan 2012; Farrero 2001; Kessler 2018; Littlejohns 1991; Rice 2010; Rose 2017; Sanchez‐Nieto 2016; Smith 1999; Vianello 2016), and four after more than 15 months' follow‐up (Kruis 2014; Lou 2015; Sridhar 2008; Titova 2017). The numbers for Lou 2015 are lower, taking clustering into account. Fan 2012 was temporarily stopped because all‐cause mortality was higher in the intervention group than in the usual care group. A thorough investigation of the circumstances of death by an independent and blinded panel showed that death was unrelated to the intervention, and a minority of deaths were due to COPD. Pooling of death events in IDM and control groups across all studies showed a non‐statistically significant effect in favour of the intervention (OR 0.86, 95%CI 0.59, to 1.25). Heterogeneity was substantial and could not be explained by duration of follow‐up, as outcomes were comparable after medium‐term (OR 0.80, 95% CI 0.45 to 1.43) and long‐term follow‐up (OR 0.87, 95% CI 0.48 to 1.57) (Analysis 1.30).
1.30. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 30: Mortality
6. Lung function
Lung function was expressed as FEV₁ in litres and as FEV₁% predicted. Following Kruis 2013, we pooled data from a total of six studies for FEV₁ (litre) (Bourbeau 2003; Kalter‐Leibovici 2018; Öztürk 2020; Sridhar 2008; Wood‐Baker 2006; Zhang 2020), and from 14 studies for FEV₁% predicted (Farrero 2001; Fernandez 2009; Güell 2000; Jimenez‐Reguera 2020; Kalter‐Leibovici 2018; Khan 2019; Ko 2016; Lenferink 2019; Littlejohns 1991; Lou 2015; van Wetering 2010; Wakabayashi 2011; Wood‐Baker 2006; Zhang 2020). Wang 2017 and Wood‐Baker 2006 reported on short‐term effects on FEV₁ in litres, but data from Wang 2017 could not be pooled due to reporting error. Pooling of FEV₁ in litres showed no differences between groups for medium‐ and long‐term follow‐up (Analysis 1.31). Pooled MDs in FEV₁% predicted showed a short‐term effect in favour of the IDM group (MD 2.88, 95% CI 1.35 to 4.40). This effect was statistically significant but was not clinically significant. Medium‐term effects were less pronounced and were not statistically significant (MD 0.95, 95% CI ‐0.20 to 2.11). After 24 months, there was no difference between groups (MD 1.18, 95% CI ‐0.82 to 3.18). Results were homogeneous across studies (Analysis 1.32). However, except for Lou 2015, 95% confidence intervals for the different studies were consistently large, suggesting large between‐patient variation.
1.31. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 31: FEV₁ (litre)
1.32. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 32: FEV₁ (% predicted)
7. Anxiety and depression
Ten studies assessed depression, anxiety, or both as an outcome (Engstrom 1999; Güell 2000; Kessler 2018; Lenferink 2019; Littlejohns 1991; Öztürk 2020; Rose 2017; Titova 2017; Trappenburg 2011; Vianello 2016). Engstrom 1999 used the Mood Adjective Check List (MACL), and Güell 2006 used a Revised Symptom Checklist. Kessler 2018 used the Hospital Anxiety and Depression Scale (HADS) but reported only the combined score. The other studies reported depression and anxiety scores from the HADS, and results were pooled. Pooled data from the anxiety domain of the HADS showed no differences between groups (MD 0.09, 95% CI ‐0.30 to 0.47; I² = 38%). Pooled data for the depression domain of the HADS showed a non‐significant effect in favour of the intervention group (MD ‐0.20, 95% CI ‐0.45 to 0.05; I² = 38%: Analysis 1.33).
1.33. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 33: Anxiety and depression (HADS)
8. Process‐related outcomes
8.1. Compliance/Adherence
Patient adherence to the programme or to intervention uptake was evaluated in five studies by review of programme attendance rate and programme completers (Bernocchi 2017; Rose 2017; Tabak 2014; Vasilopoulou 2017; Zwar 2016). Bernocchi 2017 reported a high adherence rate, with 93% of participants performing activities at home as part of the programme. Rose 2017 reported that 29% of participants were fully compliant and 22% were non‐compliant (< 50% compliant with separate components). In addition, only 7% of study participants attended respiratory rehabilitation despite this being a component of usual care. Study authors also noted that 38% of intervention group participants who met the eligibility criteria for pulmonary rehabilitation were unable to attend due to unavailability of classes. Tabak 2014 monitored use of the web portal and separate intervention modules and observed that use of the web portal differed greatly among participants; some used the diary almost every day, others used it on only half of the days. Varying levels of implementation were also reported by Kennedy 2013 and Zwar 2016. Zwar 2016 particularly reported low implementation rates by practitioners and low response to questionnaires caused by limited time.
8.2. Satisfaction
Eight studies assessed patient satisfaction with the IDM programme in some way (Bernocchi 2017; Fan 2012; Koff 2009; Kruis 2014; Littlejohns 1991; Rose 2017; Tabak 2014; Zwar 2016). Various questionnaires, either validated or self‐developed, were used to measure patient satisfaction; this made pooling impossible. Rose 2017 and Tabak 2014 used the eight‐item Client Satisfaction Questionnaire (CSQ‐8) (Attkisson 2004). Tabak 2014 measured lower satisfaction with the telehealth programme compared to usual care, and Rose 2017 found no differences between groups. Likewise, Fan 2012 found no differences between groups on the 21‐item Seattle Outpatient Satisfaction Questionnaire, and Littlejohns 1991 found no differences on its self‐developed questionnaire. Both Bernocchi 2017 and Koff 2009 reported high satisfaction scores for IDM, except for use of the pedometer, but did not compare satisfaction scores with those of the control group. Bernocchi 2017 saw that patients reported high satisfaction on all items of the self‐developed questionnaire, including service as a whole, use of the devices, and healthcare professionals’ willingness to respond to patient needs. Zwar 2016 included patient satisfaction as a secondary outcome in its protocol paper but for unknown reasons did not report on this.
8.3. Co‐ordination of care
Two studies assessed co‐ordination of care (Kruis 2014; Zwar 2016). Kruis 2014 measured the level of care integration from the view of patients using the Patient Assessment Chronic Illness Care (PACIC) and found a statistically significant increase and difference in favour of the IDM group (Glasgow 2005). Zwar 2016 included in its protocol the Collaborative Practice Scale to assess ‘interactions between nurses and GPs that enable synergistic influence of patient care' (WEISS 1985). For unknown reasons, these results were not reported.
Discussion
Summary of main results
This review summarised and meta‐analysed the results of 52 studies involving 21,086 participants with chronic obstructive pulmonary disease (COPD) who were randomly allocated to usual care or to an integrated disease management (IDM) programme with a minimum duration of 12 weeks. This review is an update of the review performed in 2013 (Kruis 2013). Studies were conducted in 19 different countries across multiple healthcare settings. All studies investigated an IDM programme. Studies differed in terms of intervention components, duration of intervention, healthcare professional involvement, follow‐up window, number of participants, and outcome reporting. Nonetheless, we were able to pool data on all primary outcomes for short‐term (up to 6 months), medium‐term (6 to 15 months), and long‐term (longer than 15 months) follow‐up. Results of the previous review support IDM for management of COPD. Results of this update reinforce these findings, providing evidence of higher certainty and including evidence on long‐term effects (up to 48 months).
First, this review showed that IDM probably improves health‐related quality of life (HRQoL) as indicated by a change in St. George's Respiratory Questionnaire (SGRQ) overall score by 3.89 points after 12 months without reaching the minimum clinically important difference (MCID) of ‐4 points. This improvement was more pronounced among high‐quality studies only, indicating the robustness of our conclusions. This effect was not observed after 15 months (mean difference (MD) ‐0.69). IDM probably leads to improvement after 12 months in the symptoms domain (MD ‐3.88) and in the impact domain (MD‐ 3.34) but not in the activity domain of the SGRQ. Across all outcomes, we observed considerable heterogeneity, which could be explained in part by differences in the quality of studies. Subgroup analysis suggested context‐specific effects with no differences among studies performed in Northwestern Europe and Oceania. Pooling of data from the Chronic Respiratory Questionnaire (CRQ), another measurement for HRQoL, showed statistically significant long‐term effects in favour of IDM in fatigue (MD 0.46), emotion (MD 0.53), and mastery (MD 0.83) domains. No significant effects were found for short‐ and medium‐term follow‐up, nor for generic quality of life.
Second, IDM probably results in a large improvement in maximum and functional exercise capacity as measured by the six‐minute walking distance test (6MWD), which exceeds the MCID of 35 metres. At short‐term follow‐up, pooling showed improvement of 48 metres. This effect was sustained over time, as shown by pooled data after 12 months (MD 44.69) and after 15 months' follow‐up (MD 60.41). Subgroup analysis indicated a considerable intervention‐specific effect, with a larger effect in studies with exercise, structural follow‐up, or telemonitoring as the dominant intervention component.
Third, the total number of patients with at least one respiratory‐related hospital admission receiving an IDM programme, after median follow‐up of 12 months, was on average 235 per 1000 patients compared to 324 per 1000 receiving usual care. Likewise the number of all‐cause hospital admissions decreased from 517 per 1000 for usual care to 445 per 1000 for IDM. Within the group of patients admitted to the hospital, IDM likely reduces the length of stay by 2.3 days after median follow‐up of 12 months. However, length of stay differed considerably between studies, ranging from a reduction of 10.8 days to an increase of 3.5 days in the IDM group compared to the usual care group. In terms of the number of emergency department (ED) visits, IDM probably reduces the number of visits by 86 per 1000 ED visits.
Effects on the aforementioned primary outcomes and details on level of certainty are summarised in Table 1. In addition to effects on our primary outcomes, we found a statistically significant improvement in lung function parameters without clinical relevance and in dyspnoea. We found no statistically significant differences between IDM and usual care in terms of generic quality of life (i.e. Short Form (SF)‐12/36 score), courses of antibiotics/prednisolone, mortality, or depression and anxiety scores.
Overall completeness and applicability of evidence
With the addition of 26 new studies resulting from the search update for the 2020 review, the number of people with COPD in this review increased from 2997 to 21,086. The large increase in terms of studies and participants has resulted in better precision and better generalisability of findings. In addition, we were able to distinguish short‐, medium‐, and long‐term effects. Unfortunately, we observed large heterogeneity within the primary analysis for almost all primary outcomes. Although part of the observed heterogeneity could probably be explained by variation in the quality of studies in some cases, our results are also marked by large clinical and methodological variations. Accordingly, the applicability of our evidence warrants some comments.
The COPD population in the included studies ranged from those with mild to very severe COPD, and trials were conducted across all types of healthcare settings in a range of different countries, each with a unique healthcare system. This improves generalisability and makes (parts of) the results of this review applicable to a large proportion of COPD patients worldwide. However, one should bear in mind that the precise applicability will depend on the context of the specific healthcare setting and the type of COPD patient. The IDM programmes included in this review also differed in types of healthcare providers involved, types of intervention components, and intervention duration and intensity, reflecting the diversity of daily practice. Overall, with subgroup analysis, we noticed intervention‐specific effects, that is, IDM programmes focused mainly on exercise probably result in greater improvement in exercise capacity, and programmes with self‐management as the dominant component probably lead to fewer respiratory‐related hospital admissions.
Besides clinical heterogeneity, our review also deals with significant methodological heterogeneity. We included studies with differences in duration and intensity of follow‐up. By dividing the follow‐up duration into short‐, medium‐, and long‐term follow‐up, we aimed to assess groups of studies with sufficient homogeneity. However, the intensity of the intervention could still differ between included studies. Also, it should be noted that an observed effect at long term does not necessarily indicate a sustained effect of the intervention because for some studies, the interventions continued throughout the study. Hence, further research is required to define the optimal combination, intensity, and duration of components of IDM programmes, taking into account the importance of methodological factors.
Our subgroup analysis results point towards beneficial effects among telemonitoring‐based IDM interventions in terms of health‐related quality of life, exercise capacity, and respiratory‐related hospital admissions. However, given the small number of studies (5 studies) including telemonitoring, no decisive conclusions or recommendations can be made regarding the overall beneficial effects of telemonitoring as an IDM programme. Future research should shed light on the beneficial effects of telemonitoring and its use in practice.
Also, the applicability of evidence depends on the healthcare context in which the IDM programme is implemented, which differed greatly among studies included in this review. Studies were conducted in many different countries across five different continents. Subgroup analysis pointed towards a context‐specific effect. This is in line with recent findings from the COMET study performed in Germany, France, Italy, and Spain (Kessler 2018), which reported significant country‐specific differences between study settings. Kessler attributed these to differences in routine care, such as country‐specific differences in baseline hospitalisation practices, admission criteria, and bed availability. Hence, effectiveness varying between study regions is likely related to variations in usual care that occur over time and are driven by national changes in policy and healthcare financing.
Also, country‐specific differences in terms of cultural and societal norms may play a role in terms of implementation fidelity and therefore outcomes (Marsiglia 2015). For example, the four‐year study in which the IDM group received a monthly one‐hour health lecture, performed by Lou 2015 in China, reported dropout (other than death) of only 7%, and noted that 87% of the study population attended all 48 COPD lectures.
Furthermore, the period in which included studies were published spanned 30 years, with the earliest published in 1991 and the latest in 2020. The clinical applicability of more recent studies is larger, given the embedding of IDM programmes into the healthcare system and the evolution of healthcare systems nationally and internationally. Hence, it would be worthwhile to investigate the relationship between advancements in usual healthcare over time and additional beneficial effects of IDM. Furthermore, it would be interesting to explore ways in which more weight could be given to more recent studies or older studies with limited applicability for current health care could be left out in a legitimate way.
Quality of the evidence
There was clinical and methodological heterogeneity among studies, which likely results (at least in part) from the complexity of IDM interventions. We have incorporated heterogeneity into estimated effects by using random‐effects analyses. Using the GRADE approach, we specified levels of quality of the evidence (high, moderate, low, and very low) in our 'Summary of findings' table. According to this approach, we checked whether included trials had limitations in terms of design, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results, or high probability of publication bias. Such limitations may impact the certainty of evidence for all outcomes that are relevant to guideline formation, health policy development, and clinical guidance.
We deemed the quality of evidence for HRQoL (as measured by the SGRQ) as moderate, and we observed a consistent effect in favour of the intervention group for all SGRQ domains at medium‐term follow‐up. We downgraded the quality of evidence due to large heterogeneity between studies. For outcomes of functional and maximum exercise capacity, we downgraded the certainty of evidence owing to large heterogeneity that may be caused by an intervention‐specific effect (i.e. IDM programmes with exercise as the dominant component showed more positive results for exercise capacity). We deemed the quality of evidence for respiratory‐related hospital admissions as high. We downgraded one level for all‐cause hospital admissions because of considerable heterogeneity and inconsistency in direction of effect. We also downgraded the certainty of evidence for outcomes of hospital days per patient and ED visits due to inconsistency in effects.
Potential biases in the review process
Several methodological strengths minimised the risk of bias in this review. As definitions of IDM are still under debate, we strictly determined the inclusion criteria for an IDM programme a priori and published this in our review protocol (Kruis 2011). Our definition was derived from definitions published in the literature (Peytremann‐Bridevaux 2009; Schrijvers 2009). Overall, researchers reported on "multiple interventions, designed to manage chronic conditions, with a focus on a multidisciplinary approach". Furthermore, these definitions suggest that IDM interventions should "focus on maximum clinical outcome, regardless of treatment setting(s) or typical reimbursement patterns". As a result, we chose to include all interventions, independent of treatment setting, and to keep our definition as simple as possible, to be easily understandable for readers and easy to use when readers check on all relevant literature. Therefore, we restricted included trials to multi‐component, multi‐disciplinary programmes of at least 12 weeks' duration. Furthermore, we performed comprehensive searches to identify possible studies, leading to identification of more than 10,000 potentially relevant abstracts. Subsequently, three different assessors assessed the abstracts. We reached consensus on all included studies. Final decisions of course are open to interpretation or criticism. However, we have applied a systematic approach to including and excluding studies in this review, have followed the criteria pre‐specified in the protocol, and have used robust methods for data collection and 'Risk of bias' assessment.
We were able to retrieve additional data from 17 study authors but did not receive a response from eight authors despite multiple reminders. This may have introduced bias. Another limitation of this review is inconsistent reporting in the included studies, in terms of adjusting for baseline differences. We decided on a conservative approach, using unadjusted mean differences for all randomised controlled trials (RCTs) and adjusted only values corrected for clustering effects, to overcome inconsistency between study authors' corrections. Inconsistency in reporting also resulted in the need for computing standard deviations of the mean change using appropriate analysis methods. Last, there may have been large heterogeneity in control groups, resulting from country‐specific healthcare systems and COPD regulations for COPD treatment (i.e. reimbursements). Because the level of detail in reporting usual care varied greatly between studies (possibly also due to journal guidelines), we decided it was more informative to further investigate differences between regions instead of differences between types of usual care, as was performed in the previous version of this review (Kruis 2013).
Agreements and disagreements with other studies or reviews
This review adds to the results of six earlier systematic reviews analysing IDM for COPD patients (Adams 2007; Lemmens 2009; Lemmens 2013; Niesink 2007; Peytremann‐Bridevaux 2008; Peytremann‐Bridevaux 2014). The current review brings together new trials that were not included in any of these reviews, and it provides an overview of multiple outcomes. Adams 2007 examined the effectiveness of programmes for COPD patients, including chronic care model components, and pooled six trials including at least two components. Pooled results did not demonstrate statistically significant differences on the SGRQ. Adams 2007 showed lower rates of hospitalisation and shorter length of stay in the intervention group, comparable to our results. Lemmens 2009 pooled data based on the number of components used in IDM and compared these to usual care. The effect on the SGRQ was optimal if three components of IDM were used (MD ‐4.69), which is comparable to our effect in the medium term (MD ‐3.89). Review authors also showed a decrease in the number of respiratory‐related hospitalisations for studies with multiple intervention components, with a pooled odds ratio (OR) of 0.58, which is comparable to the OR of 0.64 found in the current review. Niesink 2007 described the results of several studies that evaluated quality of life in IDM programmes among COPD patients. Five out of 10 studies showed clinically relevant improvement in quality of life. Peytremann‐Bridevaux 2008 examined the effectiveness of IDM in COPD patients for exercise tolerance, quality of life, hospital admissions, and mortality. Only data on hospital admissions and exercise tolerance were pooled. In line with the current review, positive effects on exercise capacity were found, but no significant effects were found for hospital admissions. Review authors demonstrated mean improvement of 32 metres on the 6MWD in five studies. Although we found overall improvement of 45 metres, this is largely attributable to the IDM programmes with a dominant exercise component. Furthermore, the pooled odds ratio of 0.85 (95% confidence interval (CI) 0.54 to 1.36) for mortality reported by review authors is comparable to that in our review (OR 0.86, 95% CI 0.59 to 1.25). Lemmens 2013 performed a meta‐analysis on existing reviews that focused on IDM programmes with two or more components for adult patients with COPD. They showed statistically significant improvements on the SGRQ in favour of IDM (P < 0.01) with moderate heterogeneity. In contrast to our review, these review authors did not find any significant changes in all‐cause hospitalisations (OR 0.95, 95% CI 0.76 to 1.14) or in numbers of ED visits (OR ‐0.11, 95% CI ‐0.26 to 0.04). Peytremann‐Bridevaux 2014 performed an additional analysis of studies in the previous version of this Cochrane systematic review, in which they specifically assessed potential differences in mortality between IDM and usual care. They found no effects of IDM on mortality (OR 1.00, 95% CI 0.79 to 1.28), which is in line with our current findings. Some of the observed differences can be explained by the fact that nearly all reviews used different definitions of IDM. Also, all aforementioned systematic reviews included study designs other than RCTs, except Peytremann‐Bridevaux 2014.
In addition to other reviews that assessed the effectiveness of IDM in COPD as described above, multiple systematic reviews have assessed the effectiveness of different components of IDM programmes.
Exercise
Two Cochrane Reviews examined pulmonary rehabilitation programmes for COPD patients in which the dominant component is generally exercise training. McCarthy 2015 assessed the effectiveness of pulmonary rehabilitation for COPD in general, although Puhan 2016 specifically assessed the effectiveness of pulmonary rehabilitation following an exacerbation of COPD. Similar to our review, McCarthy 2015 demonstrated statistically significant improvement in quality of life and exercise capacity (6MWD) in favour of pulmonary rehabilitation (SGRQ overall score MD ‐6.89; 6MWD MD 43.93 metres). Only one study in our review, Ko 2016, is also included in Puhan 2016, probably because of its selection of COPD patients with a recent exacerbation. The review authors also showed significant improvement in quality of life and exercise capacity in favour of pulmonary rehabilitation (SGRQ MD 7.80; 6MWD MD 62 metres) and a reduction in hospital admissions (OR 0.44).
Telemonitoring
The effectiveness of telemonitoring among COPD patients was assessed in a systematic review and meta‐analysis of 27 studies (Hong 2019). In contrast to results from our subgroup analysis with telemonitoring as the dominant component, Hong 2019 found no difference in SGRQ (MD ‐0.21; our review MD ‐18.33) or in hospitalisations (all‐cause and respiratory‐related). However, our analyses are based on a small number of studies, which makes it impossible to draw firm conclusions. Another recent systematic literature review showed inconclusive results for the effectiveness of telemonitoring in COPD (Kruse 2019). These review authors did not perform a meta‐analysis but described 29 articles, of which 13 (45%) showed favourable results, five (17%) negative outcomes, and 11 (38%) no differences in outcomes.
Self‐management
Two Cochrane systematic reviews reported on self‐management‐based interventions in COPD. Zwerink 2014 assessed self‐management training, which should allow patients to successfully manage their own disease. Follow‐up ranged between 2 and 24 months. Lenferink 2017 focused on self‐management interventions that are personalised and included action plans for the management of exacerbations. In line with our results, both reviews found significant improvement in HRQoL in favour of the intervention (Zwerink 2014 SGRQ overall score MD ‐3.51; Lenferink 2017 MD ‐2.69). In these reviews, respiratory‐related hospital admissions were assessed as the number of people with at least one respiratory‐related hospital admission. Still, both studies showed similar significantly reduced risk in favour of the intervention (Zwerink 2014 OR 0.57; Lenferink 2017 OR 0.69). It is interesting to note that in our review, we did not find a difference in the number of people prescribed at least one course of oral corticosteroids (OR 1.05), whereas in both of the other reviews, odds ratios appeared to be much higher in the intervention group, albeit with non‐statistically significant findings (Zwerink 2014 number of courses of steroids OR 4.42; Lenferink 2017 OR 4.38). This might have to do with the nature of the action plans incorporated into self‐management programmes, which stimulate patients to start a course of prednisolone in case of increased symptoms.
Education
A Cochrane systematic review from 2016 assessed the effectiveness of action plans with brief patient education for exacerbations in COPD (Howcroft 2016). Review authors showed that the intervention reduced the combined rate of hospitalisations and ED visits (rate ratio 0.59, 95% CI 0.44 to 0.79) and led to small but significant improvement in quality of life (SGRQ MD ‐2.8. 95% CI ‐4.8 to ‐0.8). One recent systematic review explored the effects of health coaching for people with COPD (Long 2019). According to the definition used in this review, health coaching programmes aim to improve self‐management and healthy behaviour by teaching and motivating patients to achieve personalised goals. Long 2019 showed that health coaching had a significantly positive effect on the SGRQ (MD ‐0.69). These review authors also found a significant reduction in COPD‐related hospital admissions (OR 0.45). In contrast to both of these reviews, our subgroup analysis on studies with education as the main component did not find significant differences in SGRQ (MD 0.15) nor in respiratory‐related hospital admissions (OR 0.83). This might be related to the content of the education, suggesting that action plans need to be an integral part of any educational component in IDM to be of benefit for patient outcomes. Additionally, as shown by Long 2019, education has a larger beneficial effect when it is personalised and includes motivational techniques and goal‐setting.
It is hard to draw conclusions on our subgroup analysis of the dominant component and the findings of earlier reviews because of the limited number of studies per dominant component and considerable variation among studies in terms of intervention duration. However, our findings suggest that to improve exercise capacity, IDM programmes with an exercise focus or with use of telemonitoring components are best suited. IDM programmes using telemonitoring can provide large benefit with regard to respiratory‐related admissions by monitoring the patient’s symptoms, providing tailored and individualised self‐management support (i.e. delivery of coping skills), and managing unexpected patient hospitalisations. For quality of life, most reviews on different components show improvement. Overall, this suggests that a multi‐component approach, such as that used in IDM programmes, should result in optimal benefit for multiple important outcomes.
Finally, when compared to pharmaceutical treatments such as long‐acting beta‐agonist (LABA)/long‐acting muscarinic antagonist (LAMA) treatment or use of phosphodiesterase‐4 inhibitors, our findings from the SGRQ showed improvement of comparable magnitude. Our review showed that IDM resulted in improvement of 3.89 points on the SGRQ compared to 4.08 points for LABA/LAMA treatment (Maqsood 2019), as well as 1.06 points for phosphodiesterase‐4 inhibitors (Janjua 2020). Although the confidence interval for IDM was wider (95% CI ‐6.16 to ‐1.63) compared to the confidence interval for LABA/LAMA treatment (95% CI ‐4.80 to ‐3.36), our results indicate clinical significance of the effects of IDM for a large group of patients.
Authors' conclusions
Implications for practice.
This review and meta‐analysis provides evidence that integrated disease management (IDM) programmes of at least 12 weeks' duration are generally effective for people with chronic obstructive pulmonary disease (COPD) and result in clinically beneficial outcomes. Effects are most pronounced on the short term and in the medium term. For the long term only, effects on six‐minute walking distance (6MWD) persist, although this may be explained in part by the smaller number of studies. Also, the effect size differs between studies and interventions. In practice, this means there is no one size fits all solution, and interventions should always be carefully designed and evaluated.
We calculated that 89 hospital admissions related to respiratory problems can be prevented for every 1000 patients treated with IDM, leading to a number needed to treat for additional beneficial outcome (NNTB) of 12 patients to prevent one from being admitted over follow‐up of 12 months. Although the numbers of patients admitted to hospital for all causes differed slightly between groups, time spent in the hospital decreased by two days in patients treated with IDM compared to those receiving usual care. This is of utmost importance, as hospitalisations contribute to the highest burden and costs among patients with COPD.
In our review, we do not provide the ideal combination of components that represent the optimal IDM programme. Rather, our results indicate that different dominant components of IDM have beneficial effects for specific outcomes. Our dominant component analysis showed that telemonitoring improves quality of life, whereas exercise tolerance is improved by IDM programmes with a dominant component of exercise, structural follow‐up, or telemonitoring, and respiratory‐related admissions are improved by self‐management. This means that IDM programmes should consist of several different components to reach the highest potential. Ideally, components of the IDM programme should be linked to personal goals of the patient.
Previously, Kessler 2018 and Marsiglia 2015 showed important differences in usual care between countries, and our review also found differences between regions. These differences might stem from a disparity in local availability of different components, from differences in the healthcare system, or from different customs. Furthermore, they are dependent on available resources and costs of interventions. Therefore, we suggest that policy makers and healthcare leaders should assess local needs and available interventions and use this overview to develop and implement an IDM programme in a context‐sensitive manner. This review suggests that an IDM programme with a combination of exercise training, self‐management, telemonitoring, and personalised education implemented in the right context should result in the best outcomes.
Implications for research.
Well‐designed and appropriately conducted studies are still needed to minimise bias, to allow measurement of the true intervention effect. Specifically, consistent reporting on exacerbation outcomes and on severity of exacerbations may overcome the difficulties we encountered in this review, for which we found a myriad of exacerbation definitions. Researchers are encouraged to use recent Global initiative for Chronic Obstructive Lung Disease (GOLD) guidelines to provide unambiguous definitions of disease severity and to evaluate effects of IDM programmes on mild, moderate, and severe exacerbations (GOLD 2020).
Subgroup analyses undertaken as part of this update stimulate new questions in relation to IDM and its contextual embedding. Differences in subgroups based on the dominant intervention component call for further research to identify which intervention component, or which combination of components, is most effective in IDM programmes, and for which patient groups. Similarly, the context‐specific effects we observed in the subgroup analysis suggest that the country in which the IDM programme is embedded and the level of usual care it is compared to greatly impact the magnitude of effect. This still means that the individual components of IDM programmes are important and will improve patient outcomes, as shown in this review. However, the contrast of a new IDM programme versus usual care becomes smaller when usual care itself already routinely contains several of the components. Other factors that remain uncertain are the optimal duration and intensity of the intervention and the combination of healthcare providers involved. These questions can be examined in a meta‐regression analysis, which could shed light on the contribution of each individual factor or combination of factors to observed treatment effects.
Although the observed effect of ‐ 3.89 on the SGRQ did not reach the proposed MCID of ‐4 points for medium‐term follow‐up, there could be a proportion of patients in the intervention group that does exceed the 4 points of improvement. These so called 'responders' would clinically benefit more from IDM than from usual care. In our review, only Bourbeau 2003 reported the proportion of people who improved by 4 points or more on the SGRQ. Hence, we echo Cates 2015 and urge trialists to also report, besides the mean difference, the spread of individual responses to the intervention or treatment. This information can be used for more complete assessment of clinical importance and helps to reveal the population benefit.
Last, process‐related outcomes raised issues that require consideration beyond this current review. For example, special attention should be given to evaluating the actual implementation of IDM programmes in existing healthcare structures, which should include outcomes related to patient satisfaction, feasibility, programme compliance, and assessment of personal and contextual determinants of implementation and treatment effects. Pragmatic, real‐life RCTs including both clinical and process‐related outcomes and qualitative assessment with long‐term follow‐up are needed to evaluate IDM programmes as comprehensive packages in routine primary and secondary care practice. As part of this, cost‐effectiveness remains an important outcome, to allow for reimbursement and to inform health policy development and clinical guidance.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2020 | New citation required and conclusions have changed | Abstract, plain language summary, results, discussion, and conclusions redrafted. Background and methods brought up‐to‐date, including use of current Cochrane risk of bias tool and dealing with missing data. Subgroup analyses revised. Summary of findings table updated Conclusions strengthened through the addition of 26 new studies. Conclusions based on short‐term (up to 6 months), medium‐term (6 to 15 months), and long‐term (longer than 15 months) effects |
| 21 September 2020 | New search has been performed | New literature search run |
History
Protocol first published: Issue 11, 2011 Review first published: Issue 10, 2013
Acknowledgements
We acknowledge the authors of the primary studies included in the meta‐analysis, especially those who have kindly provided additional data and clarification regarding their work. We acknowledge the contributions of Willem JJ Assendelft, Jacobijn Gussekloo, Melinde Boland, and Maureen Rutten‐van Möllken, who co‐authored the initial version of this review, and Nan van Geloven, who provided us with statistical guidance.
We would also like to thank Elizabeth Stovold (the Information Specialist for Cochrane Airways) for conducting all electronic searches, and Emma Denne for providing assistance during review and editorial processes.
The review authors and the Cochrane Airways’ Editorial Team are grateful to the following peer and patient reviewers for their time and comments.
Julia Robertson (Australia).
Hanan Aboumatar (USA).
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. exp Pulmonary Disease, Chronic Obstructive/ 2. Chronic Obstructive Pulmonary Disease.tw. 3. Chronic Obstructive Airway Disease.tw. 4. Chronic Obstructive Lung Disease.tw. 5. pulmonary emphysema.tw. 6. chronic bronchitis.tw. 7. (COPD or COAD or COBD or AECOPD).tw. 8. Chronic Airflow Obstruction.tw. 9. or/1‐8 10. disease management/ 11. Disease management.tw. 12. exp Managed Care Programs/ 13. managed care.tw. 14. insurance.tw. 15. case management.tw. 16. exp Patient Care Planning/ 17. "patient care plan$".tw. 18. "nursing care plan$".tw. 19. "goals of care".tw. 20. "care goal".tw. 21. exp "Delivery of Health Care, Integrated"/ 22. (integrated adj3 (health$ or care$ or delivery or system$)).tw. 23. disease state management.tw. 24. Comprehensive Health Care/ 25. "comprehensive health care".tw. 26. ((interdisciplin$ or multidisciplin$) adj3 (care or health$ or delivery or system$)).tw. 27. Primary Nursing/ 28. "primary nursing".tw. 29. "community based".tw. 30. exp Patient‐Centered Care/ 31. Patient Care Management/ 32. (patient adj3 (care or management)).tw. 33. practice guideline/ 34. education, medical, continuing/ or education, nursing, continuing/ 35. exp community health services/ 36. Primary Health Care/ 37. "patient care team".tw. 38. "critical pathways".tw. 39. Self Care/ 40. (continuity adj3 care).tw. 41. guideline$.tw. 42. "clinical protocol".tw. 43. "patient education".tw. 44. (self‐care or "self care").tw. 45. reminder system$.tw. or Reminder Systems/ 46. Health Education/ 47. Health Promotion/ 48. (health adj3 (education or promotion)).tw. 49. Community Health Planning/ 50. ambulatory care.tw. 51. feedback.tw. 52. or/10‐51 53. (clinical trial or controlled clinical trial or randomized controlled trial).pt. 54. (randomized or randomised).ab,ti. 55. placebo.ab,ti. 56. dt.fs. 57. randomly.ab,ti. 58. trial.ab,ti. 59. groups.ab,ti. 60. or/53‐59 61. Animals/ 62. Humans/ 63. 61 not (61 and 62) 64. 60 not 63 65. 9 and 52 and 64
Appendix 2. Embase (Ovid) search strategy
1. chronic obstructive lung disease/ 2. Chronic Obstructive Pulmonary Disease.tw. 3. Chronic Obstructive Airway Disease.tw. 4. Chronic Obstructive Lung Disease.tw. 5. pulmonary emphysema.tw. 6. chronic bronchitis.tw. 7. (COPD or COAD or COBD or AECOPD).tw. 8. Chronic Airflow Obstruction.tw. 9. or/1‐8 10. disease management/ 11. Disease management.tw. 12. managed care/ 13. managed care.tw. 14. (insurance and "case management").tw. 15. patient care planning/ 16. "patient care plan$".tw. 17. "nursing care plan$".tw. 18. "goals of care".mp. 19. "care goal".tw. 20. integrated health care system/ 21. (integrated adj3 (health$ or care$ or delivery or system$)).tw. 22. disease state management.tw. 23. health care/ 24. "comprehensive health care".tw. 25. ((interdisciplin$ or multidisciplin$) adj3 (care or health$ or delivery or system$)).tw. 26. primary nursing/ 27. "primary nursing".tw. 28. "community based".tw. 29. patient care/ 30. (patient adj3 (care or management)).tw. 31. practice guideline/ 32. medical education/ 33. exp community care/ 34. primary health care/ 35. "patient care team".tw. 36. "critical pathways".tw. 37. "case management".tw. 38. self care/ 39. (continuity adj3 "patient care").tw. 40. guideline$.tw. 41. "clinical protocol".tw. 42. "patient education".tw. 43. (self‐care or "self care").tw. 44. reminder system/ 45. reminder systems.tw. 46. health education/ 47. health promotion/ 48. (health adj3 (education or promotion)).tw. 49. health care planning/ 50. ambulatory care.tw. 51. feedback.tw. 52. or/10‐51 53. Randomized Controlled Trial/ 54. randomization/ 55. controlled clinical trial/ 56. Double Blind Procedure/ 57. Single Blind Procedure/ 58. Crossover Procedure/ 59. (clinica$ adj3 trial$).tw. 60. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 61. exp Placebo/ 62. placebo$.ti,ab. 63. random$.ti,ab. 64. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 65. (crossover$ or cross‐over$).ti,ab. 66. or/53‐65 67. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 68. human/ or normal human/ or human cell/ 69. 67 and 68 70. 67 not 69 71. 66 not 70 72. 9 and 52 and 71
Appendix 3. CINAHL (EBSCO) search strategy
S1 (MH "Pulmonary Disease, Chronic Obstructive+")
S2 COPD
S3 "chronic Obstructive Pulmonary Disease"
S4 "Chronic Obstructive Airway Disease"
S5 "Chronic Obstructive Lung Disease"
S6 "pulmonary emphysema"
S7 "chronic bronchitis"
S8 COAD
S9 "Chronic Airflow Obstruction"
S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9
S11 (MH "Disease Management")
S12 "Disease management"
S13 (MH "Managed Care Programs+")
S14 "managed care"
S15 insurance and "case management"
S16 (MH "Patient Care Plans+")
S17 "patient care plan*"
S18 "nursing care plan*"
S19 "goals of care"
S20 "care goal"
S21 (MH "Health Care Delivery, Integrated")
S22 (integrated and (health* or care* or delivery or system*))
S23 "disease state management"
S24 "Comprehensive Health Care"
S25 ((interdisciplin* or multidisciplin*) and (care or health* or delivery or system*))
S26 (MH "Primary Nursing")
S27 "primary nursing"
S28 "community based"
S29 (MH "Patient Centered Care")
S30 "patient care"
S31 "patient management"
S32 (MH "Education, Medical, Continuing")
S33 Education, Nursing, Continuing
S34 (MH "Community Health Services+")
S35 (MH "Primary Health Care")
S36 "patient care team"
S37 (MH "Critical Path")
S38 "case management"
S39 (MH "Self Care")
S40 (MH "Continuity of Patient Care")
S41 guideline*
S42 "clinical protocol"
S43 "patient education"
S44 self‐care or "self care"
S45 (MH "Reminder Systems")
S46 "reminder system*"
S47 (MH "Health Education")
S48 (MH "Health Promotion+")
S49 (health N3 educat*) or (health N3 promot*)
S50 "Community Health Planning"
S51 "ambulatory care"
S52 feedback
S53 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52
S54 S10 and S53
S55 (DE "RANDOMIZED CONTROLLED TRIALS")
S56 (MH "Double‐Blind Studies")
S57 (MH "Random Assignment")
S58 (MH "Placebos")
S59 placebo*
S60 random*
S61 crossover* or cross‐over*
S62 clinical* and (trial* or study or studies)
S63 (single* or double* or triple*) and blind*
S64 S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63
S65 S54 and S64 [Limiters ‐ Exclude MEDLINE records; Published Date from: 19900101‐20111231 ]
Appendix 4. CENTRAL search strategy
S1 (MH "Pulmonary Disease, Chronic Obstructive+") S2 COPD S3 "chronic Obstructive Pulmonary Disease" S4 "Chronic Obstructive Airway Disease" S5 "Chronic Obstructive Lung Disease" S6 "pulmonary emphysema" S7 "chronic bronchitis" S8 COAD S9 "Chronic Airflow Obstruction" S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 S11 (MH "Disease Management") S12 "Disease management" S13 (MH "Managed Care Programs+") S14 "managed care" S15 insurance OR "case management" S16 (MH "Patient Care Plans+") S17 "patient care plan*" S18 "nursing care plan*" S19 "goals of care" S20 "care goal" S21 (MH "Health Care Delivery, Integrated") S22 (integrated and (health* or care* or delivery or system*)) S23 "disease state management" S24 "Comprehensive Health Care" S25 ((interdisciplin* or multidisciplin*) and (care or health* or delivery or system*)) S26 (MH "Primary Nursing") S27 "primary nursing" S28 "community based" S29 (MH "Patient Centered Care") S30 "patient care" S31 "patient management" S32 (MH "Education, Medical, Continuing") S33 Education, Nursing, Continuing S34 (MH "Community Health Services+") S35 (MH "Primary Health Care") S36 "patient care team" S37 (MH "Critical Path") S38 "case management" S39 (MH "Self Care") S40 (MH "Continuity of Patient Care") S41 guideline* S42 "clinical protocol" S43 "patient education" S44 self‐care or "self care" S45 (MH "Reminder Systems") S46 "reminder system*" S47 (MH "Health Education") S48 (MH "Health Promotion+") S49 (health N3 educat*) or (health N3 promot*) S50 "Community Health Planning" S51 "ambulatory care" S52 feedback S53 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 S54 S10 and S53 S55 (DE "RANDOMIZED CONTROLLED TRIALS") S56 (MH "Double‐Blind Studies") S57 (MH "Random Assignment") S58 (MH "Placebos") S59 placebo* S60 random* S61 crossover* or cross‐over* S62 clinical* and (trial* or study or studies) S63 (single* or double* or triple*) and blind* S64 S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 S65 S54 and S64
Appendix 5. Cochrane Airways Group Register search strategy
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All AND INSEGMENT #2 MeSH DESCRIPTOR Bronchitis, Chronic AND INSEGMENT #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) AND INSEGMENT #4 COPD:MISC1 AND INSEGMENT #5 (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW AND INSEGMENT #6 #1 OR #2 OR #3 OR #4 OR #5 AND INSEGMENT #7 MeSH DESCRIPTOR Disease Management AND INSEGMENT #8 disease management AND INSEGMENT #9 MeSH DESCRIPTOR Managed Care Programs Explode All AND INSEGMENT #10 managed care AND INSEGMENT #11 insurance AND INSEGMENT #12 case management AND INSEGMENT #13 MeSH DESCRIPTOR Patient Care Planning Explode All AND INSEGMENT #14 patient care plan* AND INSEGMENT #15 nursing care plan* AND INSEGMENT #16 goals of care AND INSEGMENT #17 care goal AND INSEGMENT #18 MeSH DESCRIPTOR Delivery of Health Care, Integrated Explode All AND INSEGMENT #19 (integrated) NEAR3 (health* or care* or delivery or system*) AND INSEGMENT #20 disease state management AND INSEGMENT #21 MeSH DESCRIPTOR Comprehensive Health Care AND INSEGMENT #22 comprehensive health care AND INSEGMENT #23 ((interdisciplin* or multidisciplin*) NEAR3 (care or health* or delivery or system*)) AND INSEGMENT #24 MeSH DESCRIPTOR Primary Nursing AND INSEGMENT #25 primary nursing AND INSEGMENT #26 community based AND INSEGMENT #27 MeSH DESCRIPTOR Patient‐Centered Care AND INSEGMENT #28 MeSH DESCRIPTOR Patient Care Management AND INSEGMENT #29 patient care AND INSEGMENT #30 patient management AND INSEGMENT #31 MeSH DESCRIPTOR Education, Medical, Continuing AND INSEGMENT #32 MeSH DESCRIPTOR Education, Nursing, Continuing AND INSEGMENT #33 MeSH DESCRIPTOR Community Health Services Explode All AND INSEGMENT #34 MeSH DESCRIPTOR Primary Health Care AND INSEGMENT #35 patient care team AND INSEGMENT #36 critical pathway* AND INSEGMENT #37 MeSH DESCRIPTOR Self Care AND INSEGMENT #38 continuity NEAR3 care AND INSEGMENT #39 guideline* AND INSEGMENT #40 clinical protocol AND INSEGMENT #41 patient education AND INSEGMENT #42 self‐care or "self care" AND INSEGMENT #43 MeSH DESCRIPTOR Reminder Systems AND INSEGMENT #44 reminder system* AND INSEGMENT #45 MeSH DESCRIPTOR Health Education AND INSEGMENT #46 MeSH DESCRIPTOR Health Promotion AND INSEGMENT #47 (health) NEAR3 (educat* or promot*) AND INSEGMENT #48 MeSH DESCRIPTOR Community Health Planning AND INSEGMENT #49 ambulatory care AND INSEGMENT #50f eedback AND INSEGMENT #51 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 AND INSEGMENT #52 (#6 AND #51) AND (INREGISTER)
Appendix 6. ClinicalTrials.gov search strategy
| Field | Search term |
| Study type | interventional |
| Condition | COPD |
| Intervention | disease management OR integrated OR comprehensive |
Data and analyses
Comparison 1. Integrated disease management versus control, update.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 SGRQ: short‐term (≤ 6 months) | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 SGRQ: total | 16 | 1788 | Mean Difference (IV, Random, 95% CI) | ‐3.78 [‐6.29, ‐1.28] |
| 1.1.2 SGRQ: symptoms | 13 | 1327 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐5.66, 2.53] |
| 1.1.3 SGRQ: activity | 13 | 1320 | Mean Difference (IV, Random, 95% CI) | ‐3.04 [‐5.80, ‐0.28] |
| 1.1.4 SGRQ: impact | 13 | 1322 | Mean Difference (IV, Random, 95% CI) | ‐3.76 [‐5.94, ‐1.57] |
| 1.2 SGRQ: medium‐term (> 6 to 15 months) | 18 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 SGRQ: total | 18 | 4321 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐6.16, ‐1.63] |
| 1.2.2 SGRQ: symptoms | 12 | 2628 | Mean Difference (IV, Random, 95% CI) | ‐3.88 [‐7.75, ‐0.02] |
| 1.2.3 SGRQ: activity | 12 | 2608 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐5.53, 0.38] |
| 1.2.4 SGRQ: impact | 12 | 2610 | Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐6.26, ‐0.41] |
| 1.3 SGRQ: long‐term (> 15 months) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 SGRQ: total | 4 | 1090 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐3.31, 1.93] |
| 1.3.2 SGRQ: symptoms | 3 | 279 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐5.49, 10.19] |
| 1.3.3 SGRQ: activity | 3 | 278 | Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐6.17, 0.43] |
| 1.3.4 SGRQ: impact | 3 | 270 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐4.71, 0.29] |
| 1.4 Subgroup analysis SGRQ (total score, medium‐term) based on type of setting | 18 | 4321 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐6.16, ‐1.63] |
| 1.4.1 Primary care | 6 | 1545 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐3.55, 1.20] |
| 1.4.2 Secondary care | 9 | 2326 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐3.23, 0.47] |
| 1.4.3 Tertiary care | 3 | 450 | Mean Difference (IV, Random, 95% CI) | ‐14.58 [‐21.56, ‐7.61] |
| 1.5 Subgroup analysis SGRQ (total score, medium‐term) based on study design | 18 | 4321 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐6.16, ‐1.63] |
| 1.5.1 RCT | 15 | 2901 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐7.93, ‐2.02] |
| 1.5.2 Cluster‐RCT | 3 | 1420 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.60, 0.83] |
| 1.6 Subgroup analysis SGRQ (total score, medium‐term) based on dominant component of intervention | 17 | 4099 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐6.66, ‐1.73] |
| 1.6.1 Education | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐2.70, 3.00] |
| 1.6.2 Self‐management | 5 | 1825 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐4.01, 0.77] |
| 1.6.3 Telemonitoring | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐18.33 [‐26.72, ‐9.94] |
| 1.6.4 Exercise | 4 | 175 | Mean Difference (IV, Random, 95% CI) | ‐3.92 [‐9.95, 2.11] |
| 1.6.5 Structural follow‐up | 5 | 1610 | Mean Difference (IV, Random, 95% CI) | ‐4.19 [‐9.48, 1.09] |
| 1.7 Subgroup analysis SGRQ (total score, medium‐term) based on region | 18 | 4321 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐6.16, ‐1.63] |
| 1.7.1 North America | 4 | 1147 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐4.98, ‐0.02] |
| 1.7.2 Northwestern Europe | 4 | 1286 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.98, 1.74] |
| 1.7.3 Southern Europe | 3 | 227 | Mean Difference (IV, Random, 95% CI) | ‐11.42 [‐17.38, ‐5.45] |
| 1.7.4 Oceania | 3 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐2.61, 2.16] |
| 1.7.5 East Asia | 3 | 385 | Mean Difference (IV, Random, 95% CI) | ‐10.08 [‐21.59, 1.43] |
| 1.7.6 Western Asia | 1 | 896 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.95, 2.01] |
| 1.8 CRQ: short‐term (≤ 6 months) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 CRQ: dyspnoea | 4 | 277 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.01, 1.62] |
| 1.8.2 CRQ: fatigue | 5 | 314 | Mean Difference (IV, Random, 95% CI) | 0.71 [‐0.19, 1.62] |
| 1.8.3 CRQ: emotion | 5 | 314 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.26, 1.17] |
| 1.8.4 CRQ: mastery | 5 | 314 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.08, 1.52] |
| 1.9 CRQ: medium‐term (> 6 to 15 months) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 CRQ: dyspnoea | 2 | 219 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.88, 1.46] |
| 1.9.2 CRQ: fatigue | 3 | 255 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.53, 1.26] |
| 1.9.3 CRQ: emotion | 3 | 255 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.84, 1.57] |
| 1.9.4 CRQ: mastery | 3 | 255 | Mean Difference (IV, Random, 95% CI) | 0.76 [‐0.41, 1.94] |
| 1.10 CRQ: long‐term (> 15 months) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 CRQ: dyspnoea | 2 | 151 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.31, 1.25] |
| 1.10.2 CRQ: fatigue | 3 | 184 | Mean Difference (IV, Random, 95% CI) | 0.46 [0.06, 0.85] |
| 1.10.3 CRQ: emotion | 3 | 184 | Mean Difference (IV, Random, 95% CI) | 0.52 [0.10, 0.95] |
| 1.10.4 CRQ: mastery | 3 | 184 | Mean Difference (IV, Random, 95% CI) | 0.83 [0.41, 1.26] |
| 1.11 SF‐36 | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.11.1 SF‐36 MCS score | 5 | 3699 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.38, 1.11] |
| 1.11.2 SF‐36 PCS score | 5 | 3704 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.67, 2.79] |
| 1.12 General health QoL: SIP mean difference | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.12.1 SIP total | 2 | 183 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐3.00, 0.89] |
| 1.12.2 SIP: physical | 2 | 183 | Mean Difference (IV, Random, 95% CI) | ‐2.63 [‐5.55, 0.30] |
| 1.12.3 SIP: psychosocial | 2 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐3.17, 1.44] |
| 1.13 Functional exercise capacity: 6MWD | 25 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 6MWD: short‐term (≤ 6 months) | 17 | 1390 | Mean Difference (IV, Random, 95% CI) | 52.56 [32.39, 72.74] |
| 1.13.2 6MWD: medium‐term (> 6 months to 15 months) | 13 | 2071 | Mean Difference (IV, Random, 95% CI) | 44.69 [24.01, 65.37] |
| 1.13.3 6MWD: long‐term (> 15 months) | 6 | 7288 | Mean Difference (IV, Random, 95% CI) | 48.43 [16.37, 80.49] |
| 1.14 Subgroup analysis 6MWD (medium‐term) based on type of setting | 13 | 2071 | Mean Difference (IV, Random, 95% CI) | 43.21 [24.97, 61.44] |
| 1.14.1 Primary care | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 59.65 [21.96, 97.33] |
| 1.14.2 Secondary or tertiary care | 7 | 1368 | Mean Difference (IV, Random, 95% CI) | 25.01 [‐0.20, 50.21] |
| 1.14.3 Tertiary care | 4 | 624 | Mean Difference (IV, Random, 95% CI) | 60.41 [35.87, 84.96] |
| 1.15 Subgroup analysis 6MWD (medium‐term) based on dominant component of intervention | 13 | 2071 | Mean Difference (IV, Random, 95% CI) | 43.21 [24.97, 61.44] |
| 1.15.1 Education | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 16.30 [‐20.63, 53.23] |
| 1.15.2 Self‐management | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐52.65, 56.05] |
| 1.15.3 Telemonitoring | 2 | 195 | Mean Difference (IV, Random, 95% CI) | 59.94 [42.59, 77.29] |
| 1.15.4 Exercise | 4 | 189 | Mean Difference (IV, Random, 95% CI) | 68.21 [44.75, 91.68] |
| 1.15.5 Structural follow‐up | 6 | 1566 | Mean Difference (IV, Random, 95% CI) | 35.14 [2.83, 67.45] |
| 1.16 Subgroup analysis 6MWD (medium‐term) based on region | 13 | 2071 | Mean Difference (IV, Random, 95% CI) | 43.21 [24.97, 61.44] |
| 1.16.1 Northwestern Europe | 3 | 221 | Mean Difference (IV, Random, 95% CI) | 18.18 [‐7.87, 44.24] |
| 1.16.2 Southern Europe | 5 | 552 | Mean Difference (IV, Random, 95% CI) | 61.73 [36.74, 86.71] |
| 1.16.3 East Asia | 4 | 559 | Mean Difference (IV, Random, 95% CI) | 42.67 [13.94, 71.41] |
| 1.16.4 Western Asia | 1 | 739 | Mean Difference (IV, Random, 95% CI) | ‐4.50 [‐23.63, 14.63] |
| 1.17 Maximal exercise capacity: cycle test (W‐max) | 4 | 298 | Mean Difference (IV, Random, 95% CI) | 6.99 [2.96, 11.02] |
| 1.18 Respiratory‐related hospital admissions | 14 | 4207 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.81] |
| 1.18.1 Respiratory‐related hospital admissions: short‐term (≤ 6 months) | 3 | 377 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.30, 1.22] |
| 1.18.2 Respiratory‐related hospital admissions: medium‐term (> 6 to 15 months) | 9 | 2449 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.81] |
| 1.18.3 Respiratory‐related hospital admissions: long‐term (> 15 months) | 2 | 1381 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.23] |
| 1.19 Subgroup analysis respiratory‐related hospital admissions (medium‐term) based on type of setting | 9 | 2449 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.81] |
| 1.19.1 Primary care | 2 | 225 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.21, 3.76] |
| 1.19.2 Secondary or tertiary care | 7 | 2224 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.76] |
| 1.20 Subgroup analysis respiratory‐related hospital admissions (medium‐term) based on dominant component of intervention | 9 | 2449 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.81] |
| 1.20.1 Education | 2 | 854 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.45] |
| 1.20.2 Self‐management | 5 | 1353 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 1.20.3 Telemonitoring | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.09, 0.79] |
| 1.20.4 Structural follow‐up | 2 | 167 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.08, 5.55] |
| 1.21 Subgroup analysis respiratory‐related hospital admissions (medium‐term) based on region | 8 | 2316 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.44, 0.86] |
| 1.21.1 North America | 4 | 1788 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.50, 0.94] |
| 1.21.2 Northwestern Europe | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.82] |
| 1.21.3 Southern Europe | 2 | 235 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.18, 0.68] |
| 1.21.4 Oceania | 1 | 92 | Odds Ratio (M‐H, Random, 95% CI) | 1.89 [0.80, 4.45] |
| 1.22 All hospital admissions | 10 | 3244 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.57, 0.98] |
| 1.22.1 All hospital admissions: short‐term (≤ 6 months) | 1 | 112 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.67] |
| 1.22.2 All hospital admissions: medium‐term (> 6 months to 15 months) | 5 | 1212 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 1.22.3 All hospital admissions: long‐term (> 15 months) | 4 | 1920 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.16] |
| 1.23 Hospital days per patient (all causes) | 14 | 3563 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐3.98, ‐0.56] |
| 1.23.1 Hospital days per patient (all causes): short‐term (≤ 6 months) | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐4.36 [‐6.41, ‐2.31] |
| 1.23.2 Hospital days per patient (all causes): medium‐term (> 6 to 15 months) | 10 | 2944 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐3.71, 0.25] |
| 1.23.3 Hospital days per patient (all causes): long‐term (> 15 months) | 2 | 346 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐6.12, 2.92] |
| 1.24 ED visits | 9 | 3005 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.50, 0.93] |
| 1.25 Number of patients experiencing ≥ 1 exacerbation | 7 | 1378 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.42] |
| 1.25.1 Number of patients experiencing ≥ 1 exacerbation: short‐term (≤ 6 months) | 1 | 216 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.68, 1.99] |
| 1.25.2 Number of patients experiencing ≥ 1 exacerbation: medium‐term (> 6 months to 15 months) | 4 | 861 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.27] |
| 1.25.3 Number of patients experiencing ≥ 1 exacerbation: long‐term (> 15 months) | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.90, 2.61] |
| 1.26 Number of patients using ≥ 1 course of oral steroids | 4 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.66, 1.64] |
| 1.27 Number of patients using ≥ 1 course of antibiotics | 3 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.51, 4.18] |
| 1.28 MRC dyspnoea score | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.28.1 MRC dyspnoea score: short‐term (≤ 6 months) | 8 | 1132 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.52, ‐0.15] |
| 1.28.2 MRC dyspnoea score: medium‐term (> 6 months to 15 months) | 7 | 2753 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.98, ‐0.23] |
| 1.28.3 MRC dyspnoea score: long‐term (> 15 months) | 3 | 7252 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.88, 0.14] |
| 1.29 Borg score | 3 | 145 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.70, 0.98] |
| 1.30 Mortality | 15 | 4745 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.25] |
| 1.30.1 Mortality: short‐term (≤ 6 months) | 2 | 320 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.41, 2.93] |
| 1.30.2 Mortality: medium‐term (> 6 months to 15 months) | 9 | 2603 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.45, 1.43] |
| 1.30.3 Mortality: long‐term (> 15 months) | 4 | 1822 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.48, 1.57] |
| 1.31 FEV₁ (litre) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.31.1 FEV₁ (litre): short‐term (< 6 months) | 2 | 184 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.17, 0.55] |
| 1.31.2 FEV₁ (litre): medium‐term (> 6 months to 15 months) | 4 | 1344 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.05, 0.12] |
| 1.31.3 FEV₁ (litre): long‐term (> 15 months) | 3 | 1047 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.08, 0.18] |
| 1.32 FEV₁ (% predicted) | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.32.1 FEV₁ (% predicted): short‐term (≤ 6 months) | 7 | 954 | Mean Difference (IV, Random, 95% CI) | 2.88 [1.35, 4.40] |
| 1.32.2 FEV₁ (% predicted) medium‐term (> 6 to 15 months) | 10 | 1902 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐0.20, 2.11] |
| 1.32.3 FEV₁ (% predicted): long‐term (> 15 months) | 5 | 7328 | Mean Difference (IV, Random, 95% CI) | 1.18 [‐0.82, 3.18] |
| 1.33 Anxiety and depression (HADS) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.33.1 HADS: anxiety | 8 | 1580 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.30, 0.47] |
| 1.33.2 HADS: depression | 8 | 1584 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.45, 0.05] |
| 1.34 SGRQ total score | 25 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.34.1 Short‐term | 16 | 1788 | Mean Difference (IV, Random, 95% CI) | ‐3.78 [‐6.29, ‐1.28] |
| 1.34.2 Medium‐term | 18 | 4321 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐6.16, ‐1.63] |
| 1.34.3 Long‐term | 4 | 1090 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐3.31, 1.93] |
1.11. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 11: SF‐36
1.15. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 15: Subgroup analysis 6MWD (medium‐term) based on dominant component of intervention
1.29. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 29: Borg score
1.34. Analysis.

Comparison 1: Integrated disease management versus control, update, Outcome 34: SGRQ total score
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aboumatar 2019.
| Study characteristics | ||
| Methods | RCT; follow‐up: 6 months; control group: usual care | |
| Participants | Eligible: 417 Randomised: 240, I: 120, C: 120 Completed: 187, I: 93, C: 94 Mean age (SD): I: 63.9 years; C: 66.0 years Sex (% male): I: 40, C: 37 Inclusion criteria: hospitalised patients and their family‐caregivers who were admitted with a COPD‐related condition. Additional eligibility criteria included age 40 years or older; history of smoking more than 10 pack‐years; understands English language; has no terminal illness (< 6 months' life expectancy) unless end‐stage COPD; no severe cognitive dysfunction (able to follow simple instructions) Major exclusions: severe cognitive dysfunction; terminal illness (< 6 months' life expectancy) that is non‐COPD‐related; homelessness |
|
| Interventions | Hospital‐initiated programme that combines transition and long‐term self‐management support to patients and their family caregivers (the BREATHE programme). The BREATHE transitional care programme, which was co‐developed with COPD patients, family‐caregivers, and stakeholders Intervention components ‐ Tailored hospital‐to home transition support ‐ Individualised COPD self‐management education and support ‐ Facilitated access to community programmes and healthcare services The intervention is delivered by a new team member called "COPD Nurse Transition Guide". The new team member works with both hospital and outpatient care teams, is a registered nurse with home care service experience, and has received additional training in COPD self‐management and motivational interviewing. The nurse meets participants in the hospital and then follows up with them via home visits and phone calls. The intervention involves both patients and family caregivers (if available), is literacy adapted, and follows a tailored approach based on patient needs, priorities, and preferences Invervention duration: 3 months Disciplines involved: COPD nurse, treating physician Dominant component: none |
|
| Outcomes | Combined number of COPD‐related hospitalisations and ED visits per participant at 6 months (primary outcome); quality of life (SGRQ); combined number of ‘all‐cause’ hospitalisations and ED visits and individual components (hospital and ED visits separately); time to first event (re‐hospitalisation, first ED visit death); dyspnoea (mMRC); anxiety and depression; patient activation score; self‐efficacy and self‐care behaviours; patient perceptions of family‐caregivers' support; family‐caregivers' preparedness for caregiving and coping | |
| Notes | This article was retracted and re‐published due to programming error and other errors that affected the results of our article. This article was re‐published with complete corrected findings. These findings have been used in this review Dominant component: self‐management (investigator's judgement) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomised in a 1:1 ratio to either study group based on a pre‐generated sequence of assignments. Randomization was stratified by hospital unit, and a computer algorithm was used to perform blocked randomisation assignment..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "participants were randomised in a 1:1 ratio to either study group based on a pre‐generated sequence of assignments" Comment: unclear whether people screening for eligibility were aware of pre‐generated sequence of study group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...due to the nature of the intervention, participants and clinicians were not blinded" Comment: plausible that people being aware of group allocation could have biased results on SGRQ outcome, being more subjective. As noted by investigators, "increased communications with clinicians about exacerbation signs might have led to increased referrals to the emergency department (and subsequent hospitalizations)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: "data collectors and outcomes assessors were blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: loss to follow‐up balanced across arms (27 in intervention arm and 25 in usual care arm). Reasons for loss to follow‐up comparable between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all reports specified in protocol reported on. Additional post‐hoc analysis well supported by arguments. |
Aiken 2006.
| Study characteristics | ||
| Methods | RCT; follow‐up: unknown; control group: usual care, which means patients receiving care from managed care organisations (MCOs) | |
| Participants | Eligible: 192 (COPD and congestive heart failure) Randomised COPD: 61, I: 33, C: 28 Completed COPD: I; 14, C: 7 Mean age/sex: not reported separately for COPD patients Inclusion criteria: COPD or congestive heart failure, palliative treatment residing at home, receiving care by MCO, mean life expectancy 2 years, saturation < 88%, oxygen usage, marked limitation of physical functioning, recent exacerbation |
|
| Interventions | Phoenix Care palliative intervention services were added to treatment services of local MCOs. Registered nurse case managers (serving 30 to 35 patients) provided the intervention service. These nurses worked with protocols and held contact with attending physicians. Furthermore, they developed care plans, provided education to patients, and tailored self‐management of the disease. They supported services including assessing psychological and spiritual needs. During exacerbation episodes, nurses assessed medical status, implemented a symptom control intervention, and contacted the physician Intervention duration: 6 months Disciplines involved: GP, nurse case manager |
|
| Outcomes | SF‐36, medical utilisation | |
| Notes | Main component of programme: structured follow‐up with nurses/GP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was carried out within diagnosis, in blocks of 30 patients (15 intervention, 15 control) by a member of the project administration staff" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed‐envelopes, colour‐coded by diagnosis and containing the assignment to condition, were shuffled and assigned to participants in order of shuffling... the enroller, blinded to condition, opened the sealed envelope that identified the patients’ study condition" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all participants received an interview administered by a professional interviewing firm; interviewers were blind to condition and diagnosis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: s tudy authors performed an attrition analysis according to the Jurs and Glass procedure |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
Bendstrup 1997.
| Study characteristics | ||
| Methods | RCT; follow‐up: 24 weeks; control group: no treatment | |
| Participants | Randomised: 47, I: 22, C: 20 Completed: 32, I: 16, C: 16 Mean age: I: 64 years, C: 65 years Sex (% male) both groups: 56 Inclusion criteria: diagnosis of COPD according to GOLD, FEV₁ 25% to 55% of predicted value, Tiffeneau index < 70%, stable condition for 4 weeks (no change in exercise status, sputum colour/quantity, no change in medication) Major exclusions: heart disease, musculoskeletal disease limiting exercise, intermittent claudication limiting exercise |
|
| Interventions | Comprehensive outpatient rehabilitation programme ‐ Exercise training (strength training, backwards/sideways walking, endurance training): 3 times per week for 1 hour during 12 weeks. Patients were encouraged to train at home ‐ Occupational therapy: 2 group sessions ‐ Education: 12 sessions, including proper administration, inhalation techniques, psychological education, socioeconomic problems, and nutrition ‐ Smoking cessation: free nicotine patches, education Intervention duration: 12 weeks Involved disciplines: practice nurse, physiotherapist, dietician, psychologist, occupational therapist, social worker, physician |
|
| Outcomes | CRDQ, YQLQ, 6MWD, lung function, patient attendance, staff working hours | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomly allocated to either an intervention or a control group" Comment: no information on allocation procedure provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods used to conceal the sequence of treatment group allocation were not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: we could not ascertain how and whether outcome assessors were blinded to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high dropout rate (31%) |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Bernocchi 2017.
| Study characteristics | ||
| Methods | RCT; follow‐up: 6 months; control: usual care, multi‐centre (n = 3) | |
| Participants | Eligible: 319 Randomised: 112, I: 56, C: 56 Completed: 80, I: 45, C:35 Mean age: I: 71 years, C: 70 years Sex (% male): I: 88, C: 75 Inclusion criteria: older patients with COPD and cardiovascular heart disease: COPD new GOLD classification (B, C, and D classes) and spirometry in the previous year and systolic and/or diastolic CHF defined at least by an echocardiogram performed in clinical stability; II, III, and IV NYHA class and optimised drug therapy Major exclusions: physical activity limitations caused by non‐cardiac and/or pulmonary problems; obstructive cardiomyopathies and/or myocarditis; non‐cardiac and/or pulmonary pathologies that would cause the death of the patient during the study; poor adherence and compliance of the patient |
|
| Interventions | Home‐based telehealth and rehabilitation programme Intervention components ‐ Scheduled calls initiated by nurse (weekly) ‐ Unscheduled calls initiated by patients or caregivers through the service centre (24 hours/24 hours) to report any clinical problems in case of signs or symptoms ‐ Telemonitoring: during calls, patients can transmit via landline or mobile phone recordings from the 1‐lead ECG to a service centre, and talk to the nurse or doctor ‐ Home visit performed by therapist 7 days after hospital discharge by setting the daily physical activity and other home visits in case of need Home‐based rehabilitation programme ‐ Individual rehabilitative programme including ≥ 3 sessions/week of mini‐ergometer and exercises and 2 sessions/week of walking with pedometer ‐ Scheduled calls initiated by therapist performed weekly aimed at increasing workload and evaluating proper execution of exercises Duration intervention: 12 weeks Disciplines involved: nurse, therapist, treating physician |
|
| Outcomes | 6MWD, mMRC, PASE score, Barthel score, CAT score, number of hospitalisations total, hospitalisation ‐ respiratory‐related, mortality | |
| Notes | Dominant component: telerehabilitation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a computer generated tables to allocate patients in fixed blocks of 4” (Bernocchi 2016) |
| Allocation concealment (selection bias) | Low risk | Quote: “in order to prevent selection bias, the allocation sequence was concealed from the investigators enrolling and assessing patients, in sequentially numbered, opaque, sealed envelopes" (Bernocchi 2016) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “due to the nature of the intervention, neither the patients nor the physicians were blinded to patients’ group allocation” Comment: primary outcome functional exercise capacity, likely to be biased by difference in performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors and data analysts were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: missing outcome data were greater in control group (21/56) compared to intervention group (11/56). More loss to follow‐up in control group due to hospitalisation caused by heart failure. Loss to follow‐up likely to be related to intervention |
| Selective reporting (reporting bias) | Low risk | Comment: a secondary outcome in protocol defined as (1) reduction of hospitalisations for cardiovascular and/or respiratory disease; and (2) reduction of hospitalisations for all causes, in outcome paper reported as reduction to time‐to‐event, combining hospitalisation and mortality. Reasons for change in outcome paper not provided. Explanation sought and provided by study authors: "incidence of death was very low and we considered that the inclusion of the two events (hospitalisations and deaths) best described the effect of the treatment" Also feasibility measure adherence to ≥ 70% proposal rehabilitative sessions not reported; instead crude outcome reported. Unlikely to have biased outcomes |
Bourbeau 2003.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control group: usual care | |
| Participants | Eligible: 469 Randomised: 191, I: 95, C: 95 Completed: 165, I: 79, C: 86 Mean age: I: 69 years, C: 70 years Sex (% male): I: 52, C: 59 Inclusion criteria: stable COPD with ≥ 1 hospitalisation for an exacerbation in preceding year, age ≥ 50 years, pack‐years ≥ 10 years, FEV₁% predicted (post‐bronchodilator): 25% to 70%, FEV₁/FVC < 70% Major exclusions: no previous diagnosis of asthma or left congestive heart failure, terminal disease, dementia, uncontrolled psychiatric disease, no pulmonary rehab < 1 years ago, no long‐term facility stays |
|
| Interventions | Disease‐specific self‐management programme (Living Well With COPD) of 7 to 8 weeks' follow‐up including ‐ Individual sessions of education by an experienced health professional at the patient's home ‐ Content of education: COPD knowledge, breathing and coughing techniques, energy conservation during day‐by‐day activities, relaxation exercises; preventing and controlling symptoms through inhalation techniques, understanding and using a plan of action for acute exacerbation, adopting a healthy lifestyle, leisure activities and travelling, a simple home exercise programme. and long‐term home oxygen therapy ‐ An action plan for acute exacerbations was customised for each patient Intensity: education 1 hour per week during 7 to 8 weeks, follow‐up first 2 months' weekly telephone calls, then once‐a‐month telephone call. Exercise evaluation (not mandatory): 3 times per week, 30‐ to 45‐minutes/session + exercise teaching Intervention duration: 8 weeks followed by 10 months maintenance Involved disciplines: nurse, physiotherapist, physician, pulmonologist |
|
| Outcomes | SGRQ, exacerbations, spirometry, FEV₁ (L), forced vital capacity, hospital admissions, symptoms, emergency room visits, outpatients visits, 6MWD, walking distance | |
| Notes | Dominant component: self‐management (including action plan) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients underwent randomisation with the use of a central computer‐generated list of random numbers. Randomization was stratified per centre and in blocks of 6, and patients were assigned to the self management program (intervention group) or to usual care" |
| Allocation concealment (selection bias) | Low risk | Quote: "the blocking factor was not known by the investigators or their staff in each participating centre" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "since a double‐blind design was impossible ..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ".. an independent evaluator unaware of the patient assignment was responsible for the evaluation process in each centre. The evaluator was cautioned not to ask about the workbook modules and types of contact" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "an intention to treat analysis included all available study patients" |
| Selective reporting (reporting bias) | High risk | Comment: data on the 6MWD not presented but stated only as "not statistically significant"; study authors cannot provide us with additional data |
Boxall 2005.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 weeks; control group: usual care | |
| Participants | Eligible: not clear Randomised: 60, I: 30, C:30 (started intervention: I: 28, C: 26) Completed: 46, I: 23, C: 23 Mean age: I: 78 years, C: 76 years Sex (% male): I: 48, C: 65 Inclusion criteria: diagnosis of COPD by a respiratory specialist, age > 60 years, dyspnoea on exertion, live locally, motivated to exercise daily unsupervised, stable for 2 weeks, functionally housebound Major exclusions: attending outpatient‐based PR, restricted shoulder movement, living in nursing home, previous lung volume surgery, pain limiting mobility |
|
| Interventions | 12‐week home‐based pulmonary rehabilitation programme ‐ Exercise consisting of walking (level 1 to 10) and arm exercises (1 to 18) + educational sessions. Patients were required to carry out exercise daily. Weekly physiotherapy visits were scheduled for the first 6 weeks, and then visits were made until Week 12 of the programme. Visits were used to monitor exercise performance and progress in exercises, to retest 6MWD at regular intervals (Weeks 1, 4, 6, 8, and 12 of the programme) and to provide encouragement to patients ‐ Educational sessions for patients and carers were conducted by physiotherapists, nurses, and occupational therapy staff in their homes. Sessions covered anatomy and physiology of the lungs, use of respiratory devices, medications, breathing techniques, secretion removal techniques, energy conservation, use of adaptive aids, and stress management. Patients received on average 11 home visits during the programme Intervention duration: 12 weeks Disciplines involved: physiotherapist, nurse, occupational therapist |
|
| Outcomes | SGRQ, 6MWD, hospital admission, average length of stay, dyspnoea Borg Scale | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to equal groups using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "random numbers were coded into opaque envelopes by a person independent from the study, they retained the envelopes until initial assessment was completed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "neither assessors nor participants were blinded to group assignment in this study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "neither assessors nor participants were blinded to group assignment in this study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: m issing outcome data balanced in numbers (23/23 analysed in both groups) across intervention and control groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Cambach 1997.
| Study characteristics | ||
| Methods | RCT with cross‐over design; follow‐up: 6 months; control group: drug treatment only | |
| Participants | Eligible (asthma and COPD) : 89 Analysed (COPD) : 23 (COPD) , I: 15, C: 8 Mean age: I: 62 years, C: 62 years Sex (% male): I: 47, C: 75 Inclusion criteria: diagnosis of asthma or COPD according to guidelines, evidence of dyspnoea and decreased exercise tolerance as a result of obstructive lung disease, 18 to 75 years of age, ability to travel independently to the physiotherapy practice, medication prescribed by a pulmonary physician, motivation to improve self‐care, informed consent Major exclusions: manifested cardiac complaints, hypercapnia and/or hypoxia |
|
| Interventions | 3‐month rehabilitation programme including drug treatment Exercise group sessions of 3 to 4 participants including techniques of breathing retraining and evacuation of mucus, exercise training, patient education, relaxation techniques, and recreational activities. Training was provided 3 days a week for 90 minutes. Exercise training was performed twice a week on a cycle ergometer and by stair‐walking. Recreational activities were provided once a week for 45 minutes. Educational sessions were provided every week for 45 minutes Intervention duration: 12 weeks Involved disciplines: nurse, physiotherapist |
|
| Outcomes | 6MWD, incremental cycle ergometer test, CRQ | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomisation procedure; four closed envelopes for condition RC and four closed envelopes for condition CR" |
| Allocation concealment (selection bias) | Low risk | Quote: "four closed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: outcome assessors not likely to have been blinded to intervention, as patients were tested for exercise capacity in their practices, by their treated physiotherapist, who was probably not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “data obtained from patients who did not return for one or more of the assessments (i.e. baseline (t0), after 3 months (t3) and/or after 6 months (t6), or patients who were not measured within 3 weeks (from t0, t3 and t6) were excluded from data analysis" Comment: exclusion of non‐responders may have affected outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Dheda 2004.
| Study characteristics | ||
| Methods | RCT; follow‐up: 6 months; control group: primary care follow‐up | |
| Participants | Eligible: 33 Completed: 25, I: 10; C: 15 Mean age: I: 68 years, C: 71 years Sex (% male) both groups: unknown Inclusion criteria: diagnosis of COPD according to B TS guidelines, first admission to hospital with progressive symptoms, smoking history > 20 pack‐years Major exclusions: another dominant medical condition, mandatory reason for hospital follow‐up |
|
| Interventions | Intensive outpatient follow‐up program me following BTS guidelines Respiratory nurse and/or chest physician reviewed the intervention group ≥ 4 times in the 6‐month period (at 6, 8, 12, or 16 weeks). The following interventions were provided at some or all of these visits: spirometry with reversibility, review of inhaler technique and peak flow diary, ambulatory oxygen assessment, smoking cessation advice, steroid trial, nebuliser assessments, review of medication, advice about nutrition and exercise, and introduction to a patient support group Intervention duration: 6 months Involved disciplines: nurse, chest physician |
|
| Outcomes | SGRQ, SF‐36 | |
| Notes | Dominant component: structured follow‐up with nurse/GP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: methods used to conceal the sequence of treatment group allocation were not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blind to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not reported; therefore unclear who scored outcome assessments (patients, caregivers, or outcome assessors) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not clear whether results in SGRQ were described in the total population, as well as in patients who withdrew (n = 8) from the study |
| Selective reporting (reporting bias) | High risk | Comment: not all outcome measurements are given in measures; some are reported only as "there was no significant difference at 6 months in FEV1" |
Engstrom 1999.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control group: usual outpatient care | |
| Participants | Eligible: 58 Randomised: 55 Completed: 50, I: 26, C: 24 Mean age: I: 66 years, C: 67 years Sex (% male): I: 54, C: 50 Inclusion criteria: clinical diagnosis of COPD, developing after ≥ 10 years of smoking, FEV₁ < 50%, debut of symptoms after 40 years of age, dyspnoea mainly elicited by exercise or infection, no allergy Major exclusions: disabling or severe disease, coexistence of other causes of impaired pulmonary function |
|
| Interventions | 12‐month rehabilitation programme including ‐ Exercise training sessions (bicycle, arm, and breathing techniques), 2/week for 6 weeks, once weekly for 6 weeks, once every second week for 6 weeks, and then once a month for remaining period. Every session: 45 minutes. Furthermore, instructions for daily walks and an individualised daily 30‐minute home training programme ‐ Individualised educational programme with outpatient team (nurse and physician) on visit every 3 months ‐ Occupational therapist gave 2 group sessions about energy‐saving techniques and 2 global educational sessions ‐ Dietician gave information about nutrition for COPD patients and intervened in malnutrition Intervention duration: 12 months Involved disciplines: physiotherapist, nurse, physician, dietician, occupational therapist |
|
| Outcomes | SGRQ, 6MWD, W‐max, days in hospital, SIP, MACL | |
| Notes | Dominant c omponent: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information reported |
| Allocation concealment (selection bias) | High risk | Quote: "patients with COPD were recruited consecutively and, when a sufficient number had been collected, randomised to produce a rehab group and a control group of equal size" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all the physiological and QOL assessments were blinded, except the walking test, which was performed by the nurse in the rehabilitation team" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced in numbers across intervention and control groups (2 vs 3 persons) |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Fan 2012.
| Study characteristics | ||
| Methods | RCT; intended follow‐up: 12 months (terminated early, mean follow‐up 250 days); control: usual care (general information booklet COPD) | |
| Participants | Eligible: 426 Randomised: 426, I: 209, C: 217 Completed: 126, I: 197, C: 193 Mean age: I: 66.2 years, C: 65.8 years Sex (% male): I: 97.6, C: 96.3 Inclusion criteria: hospitalised for COPD in the 12 months before enrolment, post‐bronchodilator ratio of FEV₁ to FVC < 0.70 with FEV₁ < 80% predicted, older than 40 years, current or past history of cigarette smoking (> 10 pack‐years), ≥ 1 visit in the past year to a primary care or pulmonary clinic at a Veterans Affairs (VA) medical centre, no COPD exacerbation in the past 4 weeks, ability to speak English, access to a telephone Major exclusions: primary diagnosis of asthma or any medical condition that would impair ability to participate in the study or to provide informed consent |
|
| Interventions | Comprehensive care management programme ‐ COPD education during 4 individual 90‐minute weekly sessions and 1 group session ‐ Action plan for identification and treatment of exacerbations and scheduled proactive telephone calls for case management Patients in both intervention and usual care groups received a COPD informational booklet specially designed for the study; primary care providers received a copy of COPD guidelines and were advised to manage patients according to these guidelines Intervention duration: 4 week s followed by 11 months ' structural follow‐up Disciplines involved: primary care physician, case manager (nurse) |
|
| Outcomes | Time to first COPD hospitalisation (primary outcome); all‐cause hospitalisations; self‐reported COPD exacerbations; number of COPD exacerbations treated with prednisone/antibiotic; delay to prednisone/antibiotic treatment; all‐cause mortality; COPD‐related mortality; SGRQ; Veterans Medical Outcomes Study Short Form‐12; Patient Health Questionnaire (depressive symptoms); COPD‐related knowledge; self‐efficacy questionnaire | |
| Notes | Dominant component: self‐management Trial was stopped early when a safety monitoring board noted more deaths in the intervention group. Deaths due to COPD accounted for the largest difference: 10 in the intervention group vs 3 in the usual care group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the Cooperative Studies Program Coordinating Center in Boston, Massachusetts, randomly assigned eligible patients in equal numbers to 2 groups, stratifying patients by site to allow for possible regional differences in patient characteristics and clinical practice patterns" |
| Allocation concealment (selection bias) | Unclear risk | Comment: unclear whether investigators had access to randomisation lists |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the 2 groups differed on the basis of a complex behavioral intervention that made blinding impossible" Comment: majority of outcomes are self‐reported and may be affected by performance bias. However, not all outcomes (hospitalisation, mortality) are likely to be affected by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "research staff blinded to study group contacted patients every 2 months to determine whether they developed symptoms of a COPD exacerbation, along with details of treatment and health care use”; "3 blinded pulmonologists reviewed discharge summaries and other available information to determine the primary cause of all hospitalisations and classified them as COPD‐related (exacerbation or pneumonia), cardiovascular, or other” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "citing serious safety concerns, the data monitoring committee terminated the intervention before the trial’s planned completion after 426 (44%) of the planned total of 960 patients were enrolled"; "available data could not fully explain the excess mortality in the intervention group. Ability to assess the quality of the educational sessions provided by the case managers was limited" Comment: study was terminated before planned 12‐month follow‐up period. However, based on computed data, results are likely to be the same if the study would have been continued |
| Selective reporting (reporting bias) | Low risk | Comment: health care‐related costs, health service use, and medication adherence were not reported in the paper. Selective reporting probably due to early termination of the study. Given negative findings that were reported, it appears unlikely that selective reporting influences the conclusions reached. Full protocol requested from investigators but not received |
| Adequate analysis methods for CRT | Unclear risk | , |
Farrero 2001.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control group: usual care | |
| Participants | Randomised: 122, I: 60, C: 62 Completed: 94, I: 46, C: 48 Mean age: I: 69 years, C: 69 years Clinical diagnosis of COPD, requiring oxygen for ≥ 6 months, with willingness to participate in a hospital‐based home care programme, and with residence within easy reach of the hospital |
|
| Interventions | Hospital‐based home care programme of 12 months with the aim of combining home care management and easy access to hospital resources. Programme included ‐ Monthly telephone calls and 3‐monthly home visits from a nurse, working closely with a physician. Patients could also request an immediate response, which varied according to a home visit, a hospital visit, telephone advice, or a control visit Intervention duration: 12 months Involved disciplines: nurse and physician |
|
| Outcomes | CRQ, spirometry, mortality, hospital admissions, hospital days, ED visits | |
| Notes | Dominant component: structured follow‐up with nurses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "after this initial evaluation, informed consent was obtained and patients were allocated randomly to the HCP treatment group or to the control group" Comment: unclear if patients were randomised by sequence generated or based on, for example, date of admission |
| Allocation concealment (selection bias) | Low risk | Quote: "codes of randomisation were kept in sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "patients in the control group were evaluated by the HCP team at the outpatient department in the initial visit, and after 1 year" Comment: as the HCP team was the intervention team and was not blinded to which group a patient was randomised, it is likely that assessment can be influenced by no blinding of the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "quality of life was investigated in the first 40 consecutive patients included in the study (..) applied before the study and after 3 months and 12 months" Comment: reason for missing outcome data likely to be related to true outcome, with imbalance in numbers |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Fernandez 2009.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control group: education (mono‐disciplinary intervention) | |
| Participants | Eligible: 50 Randomised: 50, I: 30, C: 20 Mean age: 66 years, C: 70 years Sex: 100% male (both groups) Inclusion criteria: GOLD IV patients; younger than 80 years of age; stable COPD defined as a period of 2 months without any exacerbations, defined as signs of acute dyspnoea requiring medical attention, changes in the quantity and characteristics of sputum, an increase in pulmonary noise or an increase in the necessity for medication; correct administration of pharmacological treatment according to GOLD; home treatment with oxygen for ≥ 6 months before commencement of the study Major exclusions: severe cardiovascular pathology, unstable angina, acute myocardial infarction, cerebral vascular accident, physical or psychological disorder that impedes the practice of physical exercise |
|
| Interventions | Rehabilita tion programme of 11 months At the start: two 1‐hour sessions of respiratory re‐education in the hospital, where exercises at home were taught Home‐rehab programme ‐ One hour of exercise per day (respiratory re‐education, muscular inspiratory training, muscular training of upper and lower limbs) ‐ First 2 months: attendance of physiotherapist at home (who visited twice monthly for 1 hour) ‐ Months 2 to 9: single‐monthly visits to physiotherapist, including resistance training, respiratory re‐education, isotonic training, training of respiratory muscles ‐ Three respiratory education sessions by nursing staff (handling of inhalers, knowledge of illness, what to do in the event of an attack) Intervention duration: 11 months Disciplines involved: nurse, physiotherapist |
|
| Outcomes | Pulmonary function, SGRQ, 6MWD | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "50 patients were prospectively randomised to block of 5 patients and randomly divided into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout rates between groups comparable |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes in methods section provided |
Freund 2016.
| Study characteristics | ||
| Methods | Cluster‐RCT (115 clusters); follow‐up: 24 months; control: usual care | |
| Participants | Eligible: 3065 Randomised: total 2076, I: 1093, C: 983 Randomised: COPD: 543, I: 321, C: 222 Completed: total 1718, I: 874, C: 844 (24‐month follow‐up) Completed: COPD: not reported Mean age: I: 71.6 years, C: 72.4 years Sex (% male): I: 48, C: 48 Inclusion criteria: 18 years or older and received medical treatment for ≥ 1 of the following index conditions at time of inclusion: type 2 diabetes mellitus, COPD, or chronic heart failure ; risk for future hospitalisation (i.e. predicted likelihood of hospitalisation within the upper quartile of the total population of health plan patients, as determined by analysis of data from the preceding 18 months Major exclusion criteria: active cancer (cancer diagnosis and current receipt of radiotherapy or chemotherapy), moderate to severe dementia, permanent residency in a nursing home, participation in a concurrent clinical trial (including telemonitoring studies), severe physical and mental disorders (such as dementia, psychotic disorder, or palliative care needs), other problems that hindered active participation in the intervention (such as language barriers), as assessed by the primary care physician |
|
| Interventions | Protocol‐based care management, including structured assessment, action planning, and monitoring delivered by medical assistants Intervention components were self‐management (education, action planning, exacerbation management), assessment of medical and non‐medical needs and resources, goal‐setting, follow‐up/communication tailored to patients' heath status (minimum every 6 weeks), case management, multi‐disciplinary teams. PCPs and HCAs were trained jointly in communication techniques and goal‐setting to enhance communication within the care management team, weekly review of patient progress between primary care physician and medical assistant practice teams received $135 per enrolled patient per year to cover staff costs as financial incentive Duration intervention: 12 months Involved disciplines: primary care physician, GP or general internist, medical assistant |
|
| Outcomes | Number of all‐cause hospitalisations at 12 months at the patient level (primary outcome); number of days in the hospital; hospitalisations related to index conditions; patient‐reported quality of life (SF‐12); general health (EQ‐5D); all‐cause mortality Intervention costs (estimation based on g standard wages for medical assistants' and physicians' working time). Only number of all‐cause hospitalisations (12 months, 24 months) reported for COPD separately | |
| Notes | Unpublished data on COPD patients sought but not received Dominant component: self‐management |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used computer generated randomisation lists (SAS Version 9.2). Separate randomisation lists were prepared for urban and rural practices. A research assistant who was not otherwise involved in the project performed the central randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "we concealed the allocation to intervention or control groups until each practice completed patient enrolment and baseline assessment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "because of the nature of the intervention, blinding primary care physicians, medical assistants, and patients was not possible" Comment: unlikely to affect primary outcome (number of hospitalisations derived from insurance data) but may affect some of the secondary outcomes (e.g. self‐reported quality of life) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we blinded the assessment of the primary and secondary end points as well as the responsible statistician to study group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "for the quality‐of‐life measures, we performed analyses for the available cases (reported here) and used multiple imputation for incomplete data"; "results of the per protocol analysis and the multivariable models were similar to the results of the intention‐to‐treat analysis" Comment: furthermore, no missing data for 2 important outcome measures (all‐cause hospitalisation, number of days in hospital). Not all outcomes reported for participants with COPD specifically. Hence, impossible to conclude if missing outcome data are balanced in numbers across intervention and control groups |
| Selective reporting (reporting bias) | High risk | Comment: the PACIC, medication adherence, depression, self‐management capabilities, physical activity, activities of daily living, healthcare utilisation, total healthcare costs, blood pressure, MRC dyspnoea, forced expiratory volume, and number of exacerbations were mentioned in the protocol but were not reported in the results |
| Recruitment bias | Low risk | Quote: “we concealed the allocation to intervention or control groups until each practice completed patient enrolment and baseline assessment”; "we informed physicians about their allocation via an official letter and asked them to inform participating patients" |
| Baseline imbalance between groups | Unclear risk | Comment: practice and patient characteristics were similar between groups at baseline, with the exception of a slightly higher proportion of patients with COPD in the intervention group and a higher proportion from ethnic minorities in the usual care group. Investigators stratified randomisation according to population density of participating practice sites (urban vs rural) to minimise effects of population density on hospitalisation |
| Loss to follow‐up of clusters | Unclear risk | Comment: study authors describe a 10% attrition rate (see point 10 attrition), but loss to follow‐up of clusters is not mentioned, nor do study authors confirm that all clusters were present at follow‐up |
| Adequate analysis methods for CRT | Low risk | Quote: "we accounted for clustering within practices but were unable to account for clustering within physician/medical assistant teams within a practice (each of which had up to 2 teams)" Comment: intercluster correlation taken into account for sample size estimation |
Gottlieb 2011.
| Study characteristics | ||
| Methods | RCT; follow‐up: 18 months; control group: usual care | |
| Participants | Eligible: 133 Randomised: 61 , I: 35, C: 26 (started study I:22, C:20) Completed: 26, I: 16, C: 18 Mean age: I: 74 years, C: 73 years Sex (% male): I: 32, C: 35 Inclusion criteria: diagnosis of moderate COPD, FEV₁/FVC < 0.7 and 50% ≤ FEV₁ < 80% with motivation for pulmonary rehabilitation Exclusion criteria: comorbidity contraindicating rehabilitation, participation in PR within the last year, cognitive disorder limiting ability to participate in physical training and educational sessions |
|
| Interventions | Programme of intensive training for 7 weeks, with maintenance programme for 6 months, including ‐ Intensive 7‐week physical training and educational phase led by a multi‐disciplinary team. Furthermore, smoking cessation counselling given on an individual basis and a dietary intervention consisting of group cookery classes and individual sessions ‐ Final interview following completion of the programme, in which participants' achievements were compared to original goals ‐ Maintenance programme for 6 months, including a 90‐minute monthly session focusing on ways of incorporating exercise in daily life, 2 sessions on exercise activities in the local community, and another 2 sessions on exercise as well as on repetition of relevant topics Intervention duration: 7 weeks followed by 6 months ' maintenance Involved disciplines: multi‐disciplinary team, not further specified. Study authors were unreachable for further information |
|
| Outcomes | SGRQ, 6MWD, MRC, Borg Dyspnoea Scale, Sit‐to‐Stand test | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomised 1:1 to pulmonary rehabilitation and control" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was performed using sealed opaque envelopes randomly assigned to participants" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: w e could not ascertain how and whether outcome assessors were blinded to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: d ropout rate equally divided: 39% intervention group, 23% control group |
| Selective reporting (reporting bias) | High risk | Comment: r esults on MRC Dyspnea Scale not reported in results section |
Güell 2000.
| Study characteristics | ||
| Methods | RCT; follow‐up: 24 months; control group: usual care | |
| Participants | Eligible: 65 Randomised: 60, I: 30, C: 30 Completed (24 months): 47, I: 23, C: 24 Mean age: I: 66 years, C: 64 years Sex (% male) both groups: 100 Inclusion criteria: age ≤ 75 years, FEV₁ < 70%, FEV₁/FVC < 65%, PaO₂ > 55 mmHg at rest with no indication for prescribing home oxygen therapy Major exclusion criteria: clinically apparent heart disease, bone or joint disease; exacerbation or hospitalisation in previous month |
|
| Interventions | Outpatient pulmonary rehabilitation programme, followed by a 6‐month maintenance programme ‐ First 3 months: two 30‐minute sessions each week: breathing retraining, combined with low‐level home exercise programme. If indicated, patients also received chest physiotherapy, which involved teaching effective cough and postural drainage. Patients attended educational sessions on anatomy and basic physiology of the respiratory system as well as on the nature of their disease and of PR ‐ Months 3 to 6: exercise training programme of five 30‐minute sessions weekly on a stationary cycle ergometer. During this period, patients also began a programme of home exercise with either 30 minutes of pedaling on a stationary cycle or 1 hour of walking ‐ Months 6 to 12: single weekly session in groups during which patients performed exercises for breathing and leg‐arm co‐ordination ‐ Months 12 to 24: instructed to do home exercises without supervision Intervention duration: 6 months followed by 6 months ' maintenance Disciplines involved: nurse, physiotherapist, pulmonologist |
|
| Outcomes | Lung function, 6MWD, cycle ergometer, VAS, MRC, CRQ, exacerbations, hospital admissions | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was done at inclusion of consecutive patients" |
| Allocation concealment (selection bias) | High risk | Quote: "randomization was not concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "same physician saw patients at each visit" It is unlikely that the healthcare professional was blinded to treatment group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the technicians, who collected data for outcome measures at every visit, as explained below, were blinded to a patient’s allocation to PR or control groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: m issing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Güell 2006.
| Study characteristics | ||
| Methods | RCT; follow‐up: 4 months; control group: usual care | |
| Participants | Randomised: 40, I: 20, C: 29 Completed: 35, I: 18, C: 17 Mean age: I: 68 years, C: 66 years Male: I: 88%, C: 100% Inclusion criteria: age ≤ 75 years, FEV₁ < 70%, FEV₁/FVC < 65%, PaO₂ > 55 mmHg at rest with no indication for prescribing home oxygen therapy Exclusion criteria: psychiatric disturbance; no heart, bone, or joint disease; exacerbation or hospitalisation in previous 2 months |
|
| Interventions | Pulminary rehabilitation programme of 4 months, including ‐ First 2 months: two 30‐minute sessions each week, including relaxation techniques, breathing retraining, and chest wall and abdominal muscle wall work. Patients attended four 45‐ to 60‐minute educational sessions ‐ Month 2 to 4: five 30‐minute sessions weekly exercise training on cycle ergometer Intervention duration: 4 months Disciplines involved: nurse, physiotherapist, pulmonologist |
|
| Outcomes | MBHI, Revised Symptom Checklist (SCL‐90‐R), 6MWD, CRQ | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was done at inclusion of consecutive patients" Comment: it is not clear how the sequence was generated |
| Allocation concealment (selection bias) | High risk | Quote: "randomization was not concealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "neither patients nor clinicians were blinded to allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the technicians who collected the data were blinded to patient allocation, as were the data analysts, until the analysis was deemed complete" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: l oss to follow‐up comparable between groups (2 vs 3) |
| Selective reporting (reporting bias) | Low risk | Comment: a ll outcomes reported |
Haesum 2012.
| Study characteristics | ||
| Methods | RCT of a telerehabilitation programme; control: usual care (home exercises); follow‐up: 10 months | |
| Participants | Eligible: 114 Randomised: 111, I: 60, C: 51 Completed: 105, I: 57, C: 48 Mean age: I: 68 years, C: 68 years Sex (% male): 42.85 Inclusion criteria: over 18 years; can understand oral and written trial information; diagnosed COPD in stage III or IV (severe or very severe COPD); COPD as primary cause of reduction in function Major exclusion criteria: heart disease that could limit physical function; mental illness; terminal malignant disease; severe rheumatoid arthritis; pregnancy; living outside Aalborg Municipality |
|
| Interventions | Telerehabilitation with a telehealth monitoring device Intervention components: telemonitoring, home exercise, advice from healthcare professionals on disease and training, team video meetings with healthcare professionals from primary and secondary care (to co‐ordinate and discuss COPD patients’ individual rehabilitation programme) Duration intervention: 4 months Involved disciplines: GP, district nurse, nurse and doctor at healthcare centre or hospital |
|
| Outcomes | Admission rate per patient over a 10‐month period (primary outcome); cost of admission per patient (based on ambulatory contacts, GP contacts, emergency physician contacts, utilisation of other primary services, medicine consumption), SF‐36 | |
| Notes | Domi n ant component: telemonitoring ; SF‐36 not yet published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "after confirming eligibility and obtaining written informed consent, the patients drew envelopes to see which group they would attend" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the envelopes were sealed and therefore the allocation was blinded for health‐care professionals, patients and researchers" Comment: it remains unclear whether envelopes were sequentially numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants and personnel is not mentioned by study authors but is unlikely in light of the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors were blinded to group allocation (unpublished data) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data due to loss to follow‐up are balanced between groups (3 in intervention group, 3 in control group). Unlikely to have caused attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: according to study authors, QoL (SF‐36) will be reported in future publication |
Jimenez‐Reguera 2020.
| Study characteristics | ||
| Methods | RCT; follow‐up: 10 months post rehabilitation ; control group: usual care following 8 weeks PR | |
| Participants | Eligible: 44
Randomised: 44, I: 20, C: 24
Completed: 36; I: 17, C: 19
Mean age: I: 68 years, C: 68 years
Sex (% male): I: 41, C: 59
Inclusion criteria: COPD patient, between 55 and 85 years of age, with degree of severity II, III, or IV of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) scale, in a stable clinical situation (no exacerbations in the last 6 weeks) Major exclusion criteria: unstable cardiovascular disease or muscular or nervous system impairments that prevented performance of rehabilitation programme or evaluation tests; cognitive impairment that makes it difficult to understand the educational program and to manage the HappyAir system |
|
| Interventions | 10‐month PR maintenance programme following an integrated care plan using a mobile device with pulmonary care web‐based app (HappyAir app) HappyAir app comprised 2 components ‐ Educational programme providing patients advice about their disease ‐ Component for data collection for physical activity, medication intake, and disease HappyAir integrated plan was designed as a model of a therapeutic programme based on communication that introduced the figure of the therapeutic educator (physiotherapist or respiratory coach). Therapeutic educators had access to the platform for clinical evaluation assessment, recording weekly and monthly goals. Pulmonologist, physiotherapist had access to the platform to enter clinical data, communicate with therapeutic educator. Patients were made responsible for their self‐care and for management of their illness. Patient and educator shared responsibility Intervention duration: 8 weeks PR (both group); 10 months' maintenance programme Disciplines involved: physiotherapist or respiratory coach, pulmonologist |
|
| Outcomes | Adherence to maintenance program me (primary outcome); adherence to physical activity (Morisky‐Green Test) ; CAT; SGRQ; EQ‐5D; 6MWD | |
| Notes | Power calculation based on primary outcome (adherence to maintenance program me ); likely to be underpowered for other outcomes Dominant component: self‐management |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "p atients were recruited by convenience sampling through face‐to‐face interviews at participating hospitals. The recruitment of subjects was performed from patients attending pneumology consultations at the rehabilitation service of the hospitals participating in the study " Quote: "w e used a computer‐generated simple randomisation procedure, using the online randomisation tool Research Randomizer " Comment: selecti on preceded an initial face‐to‐face interview. Initial sel e ction of study pop ulation may be biased by willingness to participate in the interview. Adequate randomisation procedures used, so unlikely that selection bias was introduced between groups |
| Allocation concealment (selection bias) | Low risk | Quote: "b efore the beginning of the study, distribution was made in two groups through the Research Randomizer program, and a list of patients designated to each group was drawn up, considering a homogeneous distribution of groups for each hospital. This listing was sequentially numbered and coded " |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "d ue to the characteristics of the intervention, healthcare professionals and patients could not be blinded to the group assignment " |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: " t he follow‐up assessment of outcome measures of both groups was carried out by a blinded assessor " |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: s tudy dropout is balanced between groups (5/17 intervention; 5/19 control). Reasons for dropout are more or less comparable. Unclear if reasons are related to study allocation |
| Selective reporting (reporting bias) | High risk | Comment: study protocol is not available. Lung function outcomes (FEV ₁, FVC, FEV ₁ /FVC ratio) and VAS results reported that were not specified in the trial registration |
Kalter‐Leibovici 2018.
| Study characteristics | ||
| Methods | RCT; follow‐up: 24 months; control group: usual care; multi‐centre | |
| Participants | Eligible: 1333 Randomised: 1202, I: 600, C: 602 Completed: 992, I: 500, C: 492 Mean age: I: 66.7 years, C: 68.3 years Sex (% male): I: 69, C: 73 Inclusion criteria: aged 40 years or older; COPD patients with GOLD Stage III or IV (see table‐1), or patients with GOLD Stage II COPD, with past or current history of cigarette smoking, not history of childhood asthma, and with unstable disease (≥ 1 hospital admission or 2 visits to internal wing of emergency department for COPD exacerbation during past 12 months) Major exclusion criteria: permanent tracheostomy; heart failure with left ventricular ejection fraction < 40%; severe comorbidity; significant functional or cognitive impairment; communication problems; substance abuse; participating in another trial |
|
| Interventions | Disease management intervention delivered by trained COPD nurses in addition to recommended care Intervention components ‐ Face‐to‐face session with COPD nurse during visits; remote contact in between visits ‐ Symptom and adherence to treatment monitoring by COPD nurse, exacerbation management, lifestyle advice, treatment plan, and education ‐ Co‐ordination of care ‐ On‐call disease management nurse outside office hours Duration of intervention: duration of follow‐up; minimum 2 years, maximum 5 years Disicplines involved: trained COPD nurse, disease management nurse, programme director |
|
| Outcomes | Total number hospitalisation days (all‐cause and COPD‐related), number of patients with ≥ 1 hospitalisation (all‐cause and COPD‐related), hospitalisation rate, 6MWD, mMRC, SF‐12 MCS, SF‐12 PCS, SGRQ ‐ total, FEV₁% predicted | |
| Notes | Dominant component: structured follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "after completing eligibility and baseline assessment and providing signed informed consent, patients were randomly assigned either to the study intervention or to the control intervention, using a computerized randomisation program with permuted‐block design linked to the patients’ electronic medical record" |
| Allocation concealment (selection bias) | Low risk | Quote: "after completing eligibility and baseline assessment and providing signed informed consent, patients were randomly assigned either to the study intervention or to the control intervention" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study personnel at the COPD centres were not blinded to the patients’ assigned intervention during follow‐up assessments" Comment: primary outcomes less subjective; outcomes on health‐related quality of life, SGRQ, and depression symptoms may be biased by performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "hospital admissions were classified by two independent investigators, blinded to the patients’ assigned intervention" Comment: outcomes on hospitalisation assessed blinded to allocation. However, all other outcomes assessed by unblinded personnel at COPD centre with knowledge of allocation, likely to have biased results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all analyses were performed according to the intention‐to‐treat principle" Comment: 1 in 6 patients in both groups lost to‐follow up. Numbers and reasons for loss to‐follow‐up balanced between groups. In the control group, number of deaths (n = 91) slightly greater compared to control group (n = 72). Unlikely to have biased results |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in protocol reported. Protocol published as appendix to article |
Kennedy 2013.
| Study characteristics | ||
| Methods | Cluster‐RCT (44 clusters); follow‐up: 6 and 12 months; control group: usual care | |
| Participants | Eligible (diabetes, COPD, irritable bowel syndrome): 13,053, I: 5578, C: 7475 Randomised COPD: 1634, I: 1009, C: 625 Randomised total (diabetes, COPD, irritable bowel syndrome): 5599, I: 2295, C: 3304 Complete COPD: 1146, I: 424, C: 722 Completed total (diabetes, COPD, irritable bowel syndrome): 4076, I: 1649, C: 2427 Mean age: I: 68.89 (SD 10.08), C: 69.37 (SD 9.85) Sex COPD (% male): I: 51.0, C: 47.8 Inclusion criteria: patients with diabetes, COPD, or irritable bowel syndrome Major exclusions: under 18, insufficient English language, receiving palliative care, insufficient capacity to give written consent |
|
| Interventions | Practice level training in a whole systems approach to self‐management support. Practices were trained to use a range of resources: a tool to assess the support needs of patients, guidebooks on self‐management, and a web‐based directory of local self‐management resources Duration of intervention: cannot be defined Disciplines involved: GP, practice nurse |
|
| Outcomes | EQ‐5D, COPD‐specific quality of life, general health subscale of the Medical Outcomes Survey, healthcare utilisation, self‐efficacy, Medical Outcomes Survey (social or role limitations; energy and vitality; psychological well‐being; self‐care activity), COPD scale, Patient Enablement Questionnaire, enablement, HCCQ | |
| Notes | Dominant component: s elf‐manag e ment (investigator's judg e ment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used a wait list comparator group. Using a minimisation procedure based on practice size, area deprivation (the area index of multiple deprivation), and contractual status (contracted either to the National Health Service or to the local primary care trust), we allocated practices 1:1 to intervention or control groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "research staff recruiting practices are unaware of the next allocation in the sequence at the time of recruitment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of patients or personnel. Unclear whether patients were aware of allocation of practice |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “… and with the analyst (DR) blind to practice allocation” Comment: blinding of outcome assessor ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "we did not impute missing follow‐up data but used multivariate logistic regression to identify baseline covariates predictive of missing data and included these (disease, age, general health, deprivation index, and home ownership) as covariates" |
| Selective reporting (reporting bias) | Low risk | Comment: authors reported on a range of outcomes that did not show an effect. All primary outcomes and most secondary outcomes are reported. Primary and secondary outcomes for COPD study population were provided upon request |
| Recruitment bias | Low risk | Quote: "we intended to recruit patients before allocation, but this proved logistically impractical. Recruitment was through electronic health records rather than by professional invitation, but practitioners could exclude patients after identification" Comment: initial patient selection proceeded via existing disease registers. Recruitment could be influenced only by a request for exclusion of a patient. Proportion of excluded patients comparable between intervention and control |
| Baseline imbalance between groups | Low risk | Quote: "the two trial arms were well balanced on all variables at the patient level, although practices in the intervention group were o n average slightly smaller" |
| Loss to follow‐up of clusters | Low risk | Quote: "three practices randomised to the intervention group withdrew before data collection, leaving 19 intervention and 22 control practices" Comment: no practice were lost to follow‐up after the start of the trial |
| Adequate analysis methods for CRT | Low risk | Quote: "each outcome was subjected to analysis of covariance within a multi ‐ level regression framework. A 2‐level mixed model was used to account for clustering of patients within practices" |
Kessler 2018.
| Study characteristics | ||
| Methods | RCT, multi‐centre (n = 33), France (12), Germany (8), Italy (6), and Spain (7); follow‐up: 12 or 24 months; control group: usual care | |
| Participants | Randomised: 345, I: 172, C: 173 Completed: 265, I: 137, C: 128 Mean age: I: 67.3 years, C: 66.9 years Sex (% male): I: 69.4, C: 69.8 Inclusion criteria: COPD patients aged 35 years or older with post‐bronchodilator forced expiratory volume in 1 second (FEV₁)/forced vital capacity (FVC) ratio ≤ 70%; FEV₁ < 50% of predicted value; 10 pack‐year smoking history or more; ≥ 1 severe exacerbation in the previous year Major exclusions: not expected to survive longer than 6 months; cognitive/psychiatric disease; continuous treatment > 10 mg per day prednisone or equivalent longer than 6 weeks; living in a nursing home; unable to read or speak the country language |
|
| Interventions | Multi‐component home‐based COPD disease management intervention, specifically developed for patients with Gold III/IV COPD Intervention components ‐ Patient education (based on “Living Well With COPD”) and motivation by case managers, with the goal of attaining sustainable self‐management skills and behavioural changes ‐ Action plan to prevent exacerbations, with decision‐making and actions to be taken in case symptoms worsen ‐ Self‐monitoring of FEV₁, arterial oxygen saturation measured by pulse oximetry, and heart rate (HR). For patients on long‐term oxygen therapy, daily oxygen use and respiration rate (RR) were recorded by the NOWOX in‐line monitoring device ‐ Care co‐ordination through an e‐health platform for early detection of exacerbations by registration of status of well‐being, worsening, or alarm ‐ Reference to the investigator for same‐day medical assessment and follow‐up when confirmed alarm status ‐ During follow‐up consultation with physician, every 3 months Duration intervention: 12 or 24 months Disciplines involved: case manager, physician |
|
| Outcomes | Primary outcome: total number of all‐cause hospital days over 1 year Secondary outcome: COPD‐related hospital days, number of moderate to severe exacerbations, healthcare utilisation, death, HADS score, SGRQ, HRQoL, spirometry, ECG, 6MWD, BODE Index, fatal SAE cost‐effectiveness |
|
| Notes | Dominant component: structured follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were allocated to groups in a 1:1 fashion according to a pre‐specified randomisation list generated before the study by a partial‐minimisation computer algorithm under supervision of the study sponsor” |
| Allocation concealment (selection bias) | Low risk | Quote: “patients were assigned a randomisation number by study staff at each centre in sequential numerical order through a telephone‐based interactive voice response system" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “for practical reasons, the study was open; neither the patients nor the investigators were blinded to the COPD management strategy” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “hospitalisations were rigorously and blindly reviewed by the end‐point validation committee (EVC) and followed‐up with additional enquiries if necessary, ensuring the reliability of the outcomes”; "EVC members were 3 respiratory physicians independent from the sponsor and investigational sites” Comment: primary outcome assessed blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: missing outcome data were greater in control group (34/162) compared to intervention group (20/157). In control group patient death 23 compared to 3 in intervention group. Differences in missing outcome data potentially related to (absence) intervention. In addition, 23 patients in intervention group lost to follow‐up due to major protocol violations. Likely that outcomes are biased by loss to follow‐up, related to intervention |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes pre‐defined in protocol paper reported. However in addition, reported outcomes on smoking habits, daily use of LTOT, days to first exacerbation, number of patients who improved on 6MWD |
| Other bias | Unclear risk | Comment: main conclusions based on PP analysis instead of ITT. Analysis performed with ITT and PP populations. Potentially high risk of attrition bias with outcomes and reasons for missing data related to intervention. Multiple supportive outcomes that were not pre‐defined in protocol or trial register. ITT based on population at start of follow‐up period, after run‐in (5 weeks with intervention), instead of population at randomisation |
Khan 2019.
| Study characteristics | ||
| Methods | Cluster‐RCT (30 clusters); follow‐up: 6 months | |
| Participants | Eligible: not specified Randomised: 313, I: 159, C: 154 Completed: 288 , I: 147, C: 141 Mean age: I: 48 years, C: 48 years Sex (% male) : I: 77, C: 72 Inclusion criteria: newly diagnosed COPD given consent to participate in the trial, aged 18 years, currently residing (and expected to continue residing for the next 12 months) in the catchment area of the participating health facility Major exclusions: contraindication for trial procedures (e.g. people not fit for 6‐minute walk, advanced or complicated cases as per stage IV of National Institute for Health and Care Excellence (NICE)/Global Initiative for Chronic Obstructive Lung Disease (GOLD) ) |
|
| Interventions | Intervention components Overall quality of care, including enhanced screening and diagnosis, standardised prescription, follow‐up and adherence, referral linkage with district hospital ‐ 2‐day training of staff on screening, diagnosis, maintaining patient record, COPD education, follow‐up care, use of desk guides for staff ‐ Patient education using pictorial flipcharts on preventive measures ‐ Smoking cessation support ‐ Provision of free‐of‐charge inhalers and optimisation of medication Duration intervention: 6 months Disciplines involved: doctor and allied staff |
|
| Outcomes | BODE Index (primary outcome), COPD control, smoking status, follow‐up adherence. FEV, mMRC, 6MWD (elements of BODE Index), unpublished data | |
| Notes | Dominant component: structured follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the selection of the 30 trial facilities was carried out by listing all 41 eligible facilities in sealed opaque envelopes before shuffling and randomly selecting 30 of them. Then randomisation of the selected facilities (after obtaining district and communal consent) was done by again placing their names into sealed opaque envelopes and shuffling them, before a staff member of the provincial directorate randomly picked 15 envelopes for each treatment arm and opened them |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomisation of the selected facilities (after obtaining district and communal consent) was done by again placing their names into sealed opaque envelopes and shuffling them, before a staff member of the provincial directorate randomly picked 15 envelopes for each treatment arm and opened them" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "owing to the nature of the trial, it was not possible to blind individual patients or healthcare providers" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the facility doctor recorded the clinical data (that is, the diagnosis and prescription); ’paramedic’ staff recorded basic data (for example, name, age, sex, weight, height, peak expiratory flow rate result, and residential address)" Comment: outcomes assessed by facility staff, who were aware of allocation. Data analyst blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data were balanced in numbers across intervention (12/159) and control groups (13/154). Reasons for loss to follow‐up are unknown |
| Selective reporting (reporting bias) | Low risk | Quote: "the three secondary outcomes, which were all added post‐protocol..." Comment: outcomes were added post‐hoc. All outcomes specified in the protocol paper were reported |
| Recruitment bias | Low risk | Comment: patients were recruited after clusters were randomised. Patients and personnel were aware of the allocation. Unlikely that this has biased the results, as patients could not choose between facilities (region‐bound) and all new COPD cases aged 18 were eligible for participation |
| Baseline imbalance between groups | Low risk | Comments: mean cluster size was comparable, no large imbalances between groups |
| Loss to follow‐up of clusters | Low risk | Comment: no clusters were lost to follow‐up |
| Adequate analysis methods for CRT | Unclear risk | Quote: "to analyse the data, robust methods (suitable for cluster trials with relatively few clusters per arm) were used. For the continuous primary outcome, a crude analysis was initially carried out by calculating cluster‐level outcome values based on the mean of all outcome scores in each cluster. An independent t‐test was then used to estimate the treatment effect as the mean difference in the cluster level outcome values between treatment arms (intervention minus control), with the associated 95% CI and P value. To adjust for potentially confounding covariates, a two‐stage approach was used. First, a linear regression model was fitted to the individual‐level outcome data to adjust for covariates of interest, but excluding the treatment effect. A covariate adjusted difference‐residual for each cluster was then calculated from the model by calculating the mean difference between the observed and model predicted outcomes for each cluster. An independent t‐test was then used to estimate the covariate‐adjusted treatment effect as the mean difference in the cluster‐level difference‐residuals between treatment arms, with the associated 95% CI and P value" |
Ko 2016.
| Study characteristics | ||
| Methods | RCT, single‐centre; follow‐up: 12 months; control group: usual care | |
| Participants | Eligible: 230 Randomised: 180, I: 90, C: 90 Completed: 142, I: 73, C: 69 Mean age: I: 75 years, C: 75 years Sex (% male): I: 94, C: 97 Inclusion criteria: COPD patients who had been admitted with AECOPD. AECOPD defined as presentation with ≥ 2 major symptoms (increased dyspnoea, increased sputum purulence, increased sputum volume) or 1 major and 1 minor symptom (nasal discharge/congestion, wheeze, sore throat, cough) for ≥ 2 consecutive days Major exclusions: age < 40 years; asthma; chronic lung disease other than COPD; very severe medical illness that would affect patient’s ability to participate in this study |
|
| Interventions | Comprehensive COPD programme Intervention components ‐ Individualised care plan ‐ 1‐hour educational session from a respiratory nurse. Education included anatomy and physiology of the respiratory system, pathophysiology of COPD, smoking cessation, technique of using medications, dyspnoea management, nutrition, self‐management and exacerbation reduction skills, coping with psychological distress and relaxation techniques ‐ Social and community support ‐ Physiotherapist support for short‐course outpatient pulmonary rehabilitation or physical training programme to perform at home ‐ 3‐monthly telephone calls by a respiratory nurse over 1 year, and follow‐up at a respiratory clinic with a respiratory specialist once every 3 months for 1 year Durtion intervention: 12 months Disciplines involved: respiratory nurse, physiotherapist, respiratory specialist |
|
| Outcomes | Hospital re‐admission rate at 12 months (primary outcome); hospital days; health‐related quality of l ife (SGRQ); lung function (FEV₁% predicted, FVC% predicted, FEV₁/FVC ratio); exercise capacity (6MWD); dyspnoea ( m mMRC ), mortality | |
| Notes | Dominant component: structural follow‐up (author judgement) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a random number generator was used to assign the patient to the intervention or control group. A computer programme (allocation by minimisation) was used to assist the randomisation of subjects..." Comment: computer random number generator used with minimisation of age, sex, length of hospital admission, 6MWD, and predicted FEV₁ |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on concealment of allocation provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “owing to the nature of the intervention, this was an open study for the patients and therapists” Comment: participants and personnel were not blinded (QoL outcome might be influenced by this) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the research assistant performing the lung function, walking tests and questionnaire tests was neither involved in the delivery of patient care nor aware of the randomisation process” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “analyses were conducted according to the intention‐to‐treat principle” Comment: missing outcome data were more or less balanced in numbers across intervention (17/90) and control groups (21/90), with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: published report includes all primary and secondary outcomes that were pre‐specified. However additional outcomes such as exacerbations (treated with oral steroids or antibiotics), ED visits (obtained from participants and verified with medical record), m MRC, length of stay in hospital for COPD, length of stay for other causes reported on but not mentioned in trial register. No protocol published |
Koff 2009.
| Study characteristics | ||
| Methods | RCT; follow‐up 3 months; control group: usual care | |
| Participants | Eligible: 40 Randomised: 40, I: 20, C: 20 Completed: 38, I: 19, C: 19 Mean age: I: 67 years, C: 65 years Sex (% male): I: 45, C: 50 Inclusion criteria: clinical diagnosis of COPD, GOLD 3+4, with telephone land line Exclusion criteria: active treatment for lung cancer, illiteracy, non‐English‐speaking, inability to complete 6MWD |
|
| Interventions | Integrated self‐man a gement education al program me wit h proactive remote disease monitoring ‐ Disease‐specific education, by respiratory therapist at enrolment and daily by Health Buddy System (telehealthcare). Education included disease description, medications and their use, nutrition, breathing techniques ‐ Teaching of self‐management skills (use of an oximeter and increased awareness of clinical changes/problems). Patients could contact the co‐ordinator in case of deterioration ‐ Patients were remotely monitored 5 days per week with the Health Buddy system for changes in symptoms, saturation, 6MWD, and lung function. Study co‐ordinator reviewed these results and patients were contacted if they were at high risk for exacerbation, when they started exacerbation management or had contact with respiratory physician/GP Intervention duration: 3 months Disciplines involved: physician, pulmonologist |
|
| Outcomes | SGRQ, 6MWD, exacerbations, hospitalisations, ED visits, equipment satisfaction, number of calls | |
| Notes | Dominant component: self‐management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients randomly selected their group assignment (by choosing a blinded envelope that contained a group indicator" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients randomly selected their group assignment (by choosing a blinded envelope that contained a group indicator" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "because of the type of intervention, it was not possible to blind the subjects or investigators as to whether they were randomised to the treatment or control arms of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "primary end‐point was collected by the coordinator, and analysed by R.H. Jones" The co‐ordinator was also responsible for the intervention and therefore was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout rates balanced in numbers across groups |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
Kruis 2014.
| Study characteristics | ||
| Methods | Cluster‐RCT (40 clusters); follow‐up: 24 months; control: usual care; 40 clusters of primary care teams | |
| Participants | Eligible: 22698 Randomised: 1086, I: 554, C: 532 Completed: 810, I: 419, C: 391 Mean age: I: 68 years, C: 68 years Sex (% male): 54, I: 51, C: 57 Inclusion criteria: clinical diagnosis of COPD according to GOLD criteria, if possible and necessary (no spirometry data available) verified by available spirometry data or spirometry assessment Major exclusion criteria: terminally ill patients, dementia or cognitive impairment, inability to fill in Dutch questionnaires, hard drug or alcohol abuser |
|
| Interventions | Two‐day training of multi‐disciplinary team on all IDM components of intervention before implementation intervention. During training, the team redesigns the care process and defines responsibilities of different caregivers, and is trained in how to use feedback on process and outcome data to implement guideline‐driven integrated health care. The team sets up a time‐contingent individual practice plan, agreeing on steps to be taken to integrate a COPD IDM programme into daily practice. Practice‐tailored feedback reports are provided at baseline and at 6 and 12 months to each team. After 6 and 12 months, a refresher course is provided for all teams simultaneously to enable them to learn from each other’s experiences. Intensity of the IDM programme for individual patients depended on health status, personal needs, and preferences Intervention components ‐ Access to patient healthcare provider portal for process and outcome measures ‐ Optimal medication adherence ‐ Proper diagnosis ‐ Motivational interviewing ‐ Smoking cessation ‐ Self‐management ‐ Dietary intervention Duration intervention: 12 months Disciplines involved: GP, practice nurse, physiotherapist and dietician, consulting pulmonary physician |
|
| Outcomes | CCQ, SGRQ‐C, EQ‐5D, SF‐36, smoking behaviour (guided smoking attempts), IPAQ, SMAS‐30, MRC Dyspnoea, number of moderate exacerbations, number of severe exacerbations, level of care integration (PACIC and ACIC), satisfaction with healthcare providers, costs, healthcare utilisation, costs of productivity loss | |
| Notes | Dominant component: self‐management Additional comment: practices affiliated to Primary Care Research Network ‐ signed agreement to collaborate in scientific research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the same blinded researcher randomised matched clusters in pairs by using a computer generated list in four blocks of 10" |
| Allocation concealment (selection bias) | Low risk | Quote: “the clusters were matched and randomised by a researcher who was blinded to the identity of the practices” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "because of the nature of the intervention, participating healthcare providers and patients could not be blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "blinded research nurses assessed outcomes to minimise detection bias. Patients were instructed not to report on their type of management to these research nurses" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data was balanced in numbers across intervention (n = 135) and control groups (n = 141), with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes included in the protocol were reported, with the exception of ACIC (assessment of chronic illness care) and level of healthcare providers' satisfaction (intervention group only). Missing of outcomes most probably does not impact the quality of the evidence |
| Recruitment bias | High risk | Quote: “the GPs checked the selected patients against the formal inclusion and exclusion criteria before the recruitment procedure started” Comment: study flowchart suggests that patients were recruited after the cluster had been randomised |
| Baseline imbalance between groups | Low risk | Comment: most baseline characteristics did not differ significantly between intervention group and usual care group, although participants in the intervention group were significantly less likely to be male and had significantly higher functional CCQ scores |
| Loss to follow‐up of clusters | Unclear risk | Comment: insufficient information provided on practice level |
| Adequate analysis methods for CRT | Low risk | Quote: “we used linear mixed model analyses to assess differences within and between groups for all continuous outcomes, correcting for baseline scores, age, sex, proportion of patients with MRC score above 2, and clustering of patients per general practice. We used baseline scores as a dependent variable, the cluster was represented by a random effect, and the within patient covariance structure was unstructured. For dichotomous outcomes, we used logistic link generalised linear mixed models for repeated measurements to analyse differences within and between groups at all time points, correcting for the same covariates" |
Lenferink 2019.
| Study characteristics | ||
| Methods | RCT, multi‐centre (Netherlands (n = 2), Australia (n = 3)); follow‐up: 12 months; control group: usual care | |
| Participants | Eligible: 226 Randomised: 201, I: 102, C: 99 Completed: 169, I: 85, C: 84 Mean age: I: 69 years, C: 68 years Sex (% male): I: 65, C: 63 Inclusion criteria: diagnosis of COPD (GOLD criteria) with 1 to 5 highly prevalent comorbidities (i.e. ischaemic heart disease (history of myocardial infarction, angina pectoris)); heart failure; diabetes mellitus; active symptoms of anxiety and/or depression (≥ 11 Hospital Anxiety and Depression Scale (HADS) and/or anxiety or depression symptoms treated at the time of inclusion); ≥ 3 COPD exacerbations,defined as respiratory problems that required a course of oral corticosteroids/antibiotics; ≥ 1 hospitalisation for respiratory problems in the 2 years preceding study entry; ≥ 40 years of age Major exclusions: terminal cancer, end stage of COPD or another serious disease with expected survival < 12 months; other serious lung disease (e.g. α1‐antitrypsin deficiency; interstitial lung disease); cognitive impairment (MMSE < 24) |
|
| Interventions | Patient‐tailored multi‐disease exacerbation action plan Intervention components ‐ Depending on comorbidities 2 to 3 1‐ to 2‐hour group sessions (1 to 2 hours); 2 times individual hospital‐based self‐management session (1 hour) by trained case manager (respiratory nurse) and supported by cardiac, mental health, and/or diabetes nurses (first month) ‐ Group sessions including knowledge regarding COPD and comorbidities; symptom recognition and monitoring; self‐treatment (action plan linked to diary); breathing and relaxation exercises; extra session on how to check (and regulate) (Dutch patients only); blood glucose levels when necessary (diabetes patients); dietary and lifestyle behaviours ‐ Individual session: individualised action plan set for COPD and each comorbid symptom with colour coding, what are my “usual” symptoms card; diary training; exacerbation action plan training; mastery of skills (e.g. correct inhaler techniques; early recognition of exacerbations, self‐initiating correct and proper actions) ‐ Follow‐up phone calls by case manager to reinforce self‐management skills (Weeks 8, 20, 36) Duration intervention: 9 months Disciplines involved: respiratory nurse (case manager); cardiac, mental health, and/or diabetes nurse |
|
| Outcomes | Total number of COPD exacerbation days/patient/year (primary outcome); number of COPD exacerbations/patient/year; duration per COPD exacerbation/patient/year; severity of COPD exacerbation day (symptom diary); FEV₁, FEV₆, FVC; CAT; mMRC; health‐related QoL(EQ‐5D; VAS); Chronic Respiratory Diesease Questionnaire (CRQ); Fatigue (ICFS); Anxiety and Depression (HADS); Confidence and Competence (CSES, CRQ mastery domain); self‐management behaviour and knowledge (PIH); cost and healthcare utilisation; healthcare utilisation for COPD, all‐cause respiratory, cardiac, and diabetes; GP visits; specialist consultations and other services; number of hospitalisations; number of in‐hospital days; travel; costs of usual care; adherence qualitative outcomes | |
| Notes | Dominant component: self‐management with exacerbation plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “after baseline measurements, patients were allocated to self‐management or UC by an independent research assistant who was masked to treatment assignment and randomisation schedule, using a computerised minimisation program. Allocation was stratified per hospital for smoking status, modified Medical Research Council dyspnoea (mMRC) score, number of comorbidities, and being on a waiting list for pulmonary rehabilitation” |
| Allocation concealment (selection bias) | Low risk | Comment: concealment of allocation after baseline measurement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “blinding of patients and personnel to treatment group was not possible. Wherever possible, though, assessors of outcomes were blinded to treatment group” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “wherever possible, though, assessors of outcomes were blinded to treatment group" Comment: COPD exacerbation data (primary outcome) collected from symptom diary. Outcome self‐reported, hence outcomes likely to be biased by unb l inding of assessment of primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “if patients have less than three months of complete diary data over the course of the year they will be excluded from the analysis of the daily diaries”; “analyses were conducted on an intention‐to‐treat basis” Comment: missing outcome data were more or less balanced in numbers across intervention (17/102) and control groups (15/99), with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: all primary and secondary outcomes specified in protocol paper have been reported on. In addition, investigators report on analysis of COPD exacerbations and hospitalisations. Data on hospitalisation in protocol collected only for cost‐effectiveness |
| Other bias | Unclear risk | Comment: timely recognition of exacerbations through symptom diaries and tailored action plan ‐ part of intervention. So can be expected that number of reported exacerbations would be higher in intervention group than in usual care group (missed actual exacerbations). Outcomes may not present actual benefit of intervention |
Lilholt 2017.
| Study characteristics | ||
| Methods | Cluster‐RCT (13 clusters per study arm); follow‐up: 12 months; control group: usual care | |
| Participants | Eligible: not reported Randomised: 1125, I: 578, C: 647 Completed: 574, I: 258, C: 316 Mean age: I: 70 years, C: 70 years Sex (% male): I: 48; C: 43 Inclusion criteria: primary diagnosis of COPD based on spirometry, Medical Research Dyspnoea Council Scale (MRC) score ≥ 3 or modified Medical Research Dyspnoea Council Scale ( mMRC ) score ≥2 or COPD Assessment Test score ≥ 10, or ≥ 2 exacerbations during past 12 months; telephone connection; permanent residence; enrolled with participating GP; speaking Danish or living with Danish‐speaking relatives for support in use of telehealthcare system Major exclusions: cognitive impairment; no phone line or GSM coverage; inability to understand Danish to the extent allowing completion of study questionnaires |
|
| Interventions | Telehealthcare in addition to standard treatment and care Intervention components ‐ Self‐measurement of blood pressure, pulse, blood oxygen saturation, and weight ‐ Wireless transmission of vital health data to web portal, accessible to patients, relatives, and trained municipality healthcare personnel ‐ Monitoring of vital health data by trained municipality healthcare personnel (i.e. community nurses) based on individually determined threshold values. Monitoring frequency daily (first 2 weeks), once or twice weekly ‐ Contact by healthcare personnel with adverse changes in patient’s vital health values and responses (1‐way communication) ‐ Contact by healthcare personnel if measurements were not carried out as agreed or were not received as expected ‐ Follow‐up visit 3 to 4 weeks to review threshold values and tablet use Duration intervention: 12 months Disciplines involved: GP, healthcare personnel (i.e. community nurse) |
|
| Outcomes | Health‐related QoL (SF‐36) (primary outcome); mortality; diastolic blood pressure, systolic blood pressure, pulse, oxygen saturation, and weight; cost‐effectiveness ratio (ICER); cost per QALY | |
| Notes | Dominant component: self‐management (investigator judgement) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the municipality districts were matched 1:1 by the following variables: the total population size of the districts, the proportion of people with a higher education, the sum of the district’s total income, unemployment and the estimated number of patients with COPD”; “the districts were distributed randomly by a blinded volunteer with no relation to the trial, who performed the randomisation by throwing a dice" |
| Allocation concealment (selection bias) | Low risk | Quote: “the identification and recruitment of patients took place prior to random allocation of clusters in order to minimise biased recruitment" Comment: use of sealed envelope method by person not affiliated with the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: nature of intervention does not allow blinding. Primary outcome as subjective self‐reported measure likely to have biased outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: primary outcome subjective measure based on patient self‐report. Highly likely that knowledge of study allocation could have biased outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “reasons for withdrawing from the TeleCare North trial included complicated technology, concomitant health problems, not interested, leaving local geographical area, does not trust the equipment or disappointed over not being a part of the telehealth intervention” Comment: large proportion loss to follow‐up. 210/579 for intervention and 177/647 for control. Reason for loss to follow‐up for an intervention related to intervention (n = 101). Attrition rate at 12 months 53%, 110 interventions, 154 controls; patients had incomplete data. Analysis based on imputed data |
| Selective reporting (reporting bias) | High risk | Quote: “the primary outcome for theme 1 (effectiveness) is the change in health‐related quality of life (SF‐36) at the individual level from baseline to follow‐up at 12 months” (protocol paper)"; “the primary outcome measure was the adjusted mean differences in PCS summary scores between treatment groups at 12 month follow‐up" Comment: change in primary outcome with PCS as a subscore within SF‐36. No reason for change provided. No data on mortality |
| Recruitment bias | Low risk | Quote: “the identification and recruitment of patients took place prior to random allocation of clusters” Comment: in protocol paper: “the randomisation will not be undertaken until after all general practitioners have sent their lists of patients eligible for inclusion from their practice, and after all patients have given written consent to participation and completed baseline physical measurements and questionnaires” |
| Baseline imbalance between groups | Low risk | Comment: investigators minimised baseline imbalances through stratification on total population size of districts, proportion of people with a higher education, sum of district’s total income, unemployment and estimated number of patients with COPD. Baseline comparison provided. No large imbalances between groups |
| Loss to follow‐up of clusters | Low risk | Comment: no clusters lost to follow‐up |
| Adequate analysis methods for CRT | Low risk | Quote: “the clusters were assumed to be represented as random effects, and the models had robust covariance structures. ICC estimates of patient‐reported outcome variables were calculated for measurement of the variability within and across the clusters. The subgroup analyses applied the same statistical models and covariates as above, but with added treatment‐by‐covariate interaction for each subgroup” Comment: appropriate analysis applied to take clustering into account |
Littlejohns 1991.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control group: usual care | |
| Participants | Eligible: 166 Randomised: 152; I: 73, C: 79 Completed (12 months): 133, I: 68, C: 65 Mean age: I: 63 years, C: 63 years Sex (% male): I: 67, C: 63 Inclusion criteria: COPD diagnosed by spirometry, according to guidelines; age 30 to 75 years; pre‐bronchial FEV₁% < 60%; stable state; no change in medication for ≥ 6 weeks before recruitment; no other major disease |
|
| Interventions | Intervention group received care from the respiratory health worker while continuing with routine outpatient appointments during 12 months. Health worker provided ‐ Health education directed at the patient and the primary care team ‐ Monitoring of treatment compliance and optimising treatment by ensuring correct inhalation techniques and supervision of domiciliary oxygen ‐ Monitoring of the results of spirometry and of patients' symptoms to enable acute exacerbations and worsening heart failure to be detected and treated early ‐ Liaison between GP and hospital‐based services (including domiciliary physiotherapy services and social services) Intervention duration: 12 months Disciplines involved: GP, respiratory health worker |
|
| Outcomes | Mortality, spirometry, 6MWD, step test, MRC chronic bronchitis questionnaire, HADS, SIP, hospital admissions, drug prescriptions, visits to GP or clinic, satisfaction | |
| Notes | Dominant component: structured follow‐up with respiratory health worker | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers were generated by tables in permuted blocks of four, stratified by age and sex" |
| Allocation concealment (selection bias) | Low risk | Quote: "the groups to which successive patients were to be allocated were noted in sealed, numbered envelopes, which were kept centrally" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the physician was aware which group the patient was in" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout rates comparable between groups |
| Selective reporting (reporting bias) | High risk | Comment: outcomes on MRC chronic bronchitis questionnaire not reported |
Lou 2015.
| Study characteristics | ||
| Methods | Cluster‐ RCT; follow‐up: 4 years; control: usual care; 14 healthcare centres in rural areas of Xuzhou City, China | |
| Participants | Eligible: 8217 Randomised: 8171, I: 4172, C: 3999 Completed: 6221, I: 3418, C: 2803 Mean age: I: 62 years, C: 61 years Sex (% male): I: 48, C: 48 Inclusion criteria: clinical diagnosis of COPD according to GOLD criteria, verified by spirometry assessment Major exclusion criteria: presence of fever, active tuberculosis, changes in radiographic images or medication in the 4 weeks immediately preceding recruitment, primary diagnosis of asthma or obvious bronchiectasis, cystic fibrosis, interstitial lung disease, previous lung volume reduction surgery, lung transplantation, pneumonectomy, uncontrolled or serious conditions that could potentially affect spirometry tests, refusal to fill out psychological questionnaires |
|
| Interventions | Prior to implementation, health management intervention 2‐day training of GP. Training components included general information on COPD, pathogenesis, risk factors, clinical manifestations, clinical assessment, exacerbations, stable stages of treatment and rehabilitation of COPD, providing smoking cessation support, self‐management skills Intervention components ‐ Individual health management plan (based on baseline measurements) ‐ Attendance at educational lecture along with caregiver (every 2 weeks, 40 to 60 minutes per session): total 48 lectures (information on COPD, observation of inhaler techniques, medication, hospitalisation, smoking cessation, vaccination, exercise encouragement, rehabilitation, hand hygiene) ‐ Psychological counselling ‐ Face‐to‐face follow‐up visit (every 2 weeks) on treatment compliance: delivered by GP ‐ Monthly report by GP on patient condition for professional team (pulmonologist, psychiatrist, rehabilitation specialist, nutritionist, respiratory nurse), which provides feedback to GP on focus of action and supervises quality of care ‐ Meeting between professionals (every 2 months) Duration intervention: 48 months Disciplines involved: GP, pulmonologist, psychiatrist, rehabilitation specialist, nutritionist, respiratory nurse |
|
| Outcomes | BODE index, FEV₁% predicted, mMRC D yspnoea Scale, 6MWD, BMI, COPD knowledge, COPD‐related deaths, HADS, number of hospital admissions, number of ED visits, change in medication regimen | |
| Notes | Dominant component: e ducation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “centers with experience and those without were then randomly allocated separately into the health management and control groups…” Comment: insufficient detail on randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient detail on allocation procedure; additional information sought but not received |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: insufficient detail provided on blinding procedure; given nature of the intervention, participants and treating therapist not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not enough information provided to determine whether assessor was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “reasons for dropping out after randomisation were refusal to continue participation (25 subjects in the management group and 21 in the control group), lost to follow‐up (19 subjects in the management group and 32 in the control group), and death (610 subjects in the management group and 946 in the control group): Comment: statistically significant larger dropout rate in control group (1217) compared to intervention group (779). Reasons for dropout in control group were death, inability to perform walking test, and incomplete lung function test |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol in not available; published reports include all expected outcomes that were pre‐specified |
| Recruitment bias | Unclear risk | Comment: insufficient detail on whether people involved in recruitment knew about allocation. Additional information sought but not received |
| Baseline imbalance between groups | Low risk | Quote: "health‐care centers were classified into 2 groups: those with previous experience with health management counseling and those without" Comment: healthcare cent r es were stratified on experience to prevent baseline imbalance. No significant difference s between groups on healthcare cent r e level |
| Loss to follow‐up of clusters | Low risk | Comment: no clusters were lost to follow‐up |
| Adequate analysis methods for CRT | High risk | Comment: inadequate analysis for dichotomous outcomes, not accounting for possible clustering effects |
Mendes 2010.
| Study characteristics | ||
| Methods | RCT; follow‐up 12 weeks; 2 intervention groups (at‐home PR vs outpatient PR); 1 control group: usual care | |
| Participants | Eligible: 117 Randomised: 117 (intervention I: 42, intervention II: 46, control: 29) Analysed: 85 (intervention group I: 33, intervention II: 23, control: 29) Mean age: intervention I: 66 years, intervention II: 71, control: 71 Sex (% male): intervention I: 82, intervention II: 83, control: 66 Inclusion criteria: diagnosis of COPD according to GOLD, stable at inclusion Major exclusions: hospitalisation or COPD instability; presence of neuromuscular disease, associated respiratory disease, orthopaedic or neurological disease that affected gait; recent impairment due to comorbidities such as myocardial infarction, heart failure, stroke, or neoplasm; prior pneumonectomy or other thoracic surgery |
|
| Interventions | Home ‐ based or outpatient self‐monitored pulmonary rehabilitation program me ‐ Both intervention groups received 1 session of education about COPD, treatment and relevance of PR ‐ Both intervention groups trained 3 mornings a week for 3 months, with aerobic and strengthening exercises. Patients in the outpatient clinic trained under supervision; patients who trained at home were instructed in the clinic and received support through telephone calls Intervention duration: 3 months Disciplines involved: physiotherapist, pulmonologist |
|
| Outcomes | 6MWD, MRC, FEV₁, BMI, all included in BODE index (body mass, obstruction, dyspnoea, exercise tolerance) | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised electronically by a computer" |
| Allocation concealment (selection bias) | High risk | Comment: distribution of patients was unequal: 42 in at‐home group, 46 in outpatient group vs 29 in control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "two duly trained health care professionals were responsible for the evaluations, which were performed by the same evaluators for all patients" Comment: not clear whether these professionals were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "19 out of 46 of outpatient intervention group were lost to follow up, compared to 7 out of 42" Comment: reasons for missing outcome data likely to be related to true outcome, with imbalance in quantities of missing data |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Öztürk 2020.
| Study characteristics | ||
| Methods | RCT; follow‐up: 3 months; control group: usual care | |
| Participants | Eligible: 80 (consecutive inclusion) Randomised: 80, I: 40, C: 40 Completed: 63, I: 31, C: 30 Mean age: I: 65 years, C: 61 years Sex (% male): I: 94, C: 83 Inclusion criteria: aged 45 to 75 years with moderate and/or severe COPD Major exclusion criteria for patients: psychiatric, neurological, muscular, or decompensated chronic disease (congestive heart failure, chronic renal insufficiency, diabetes mellitus), mild COPD, respiratory disease other than COPD, acute exacerbation of COPD, exacerbation of COPD in the last 1 month |
|
| Interventions | Structure d self‐management educational programme provided by specified education team Intervention components ‐ 1 group educational session on activity and nutrition training (5 or fewer patients) ‐ Structural follow‐up by a chest disease specialist every 2 weeks, using motivational sentences and action plans ‐ Psychological assessment by a psychologist, on coping with chronic illness, leisure time, redirect to mental health support unit Duration intervention: 12 weeks Disciplines involved: chest disease specialist, physiotherapist, psychologist, dietician |
|
| Outcomes | CAT, SGRQ, SF‐36, HADS, mMRC | |
| Notes | Dominant component: self‐management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by using the random number table, 40 patients each were assigned to the self‐management training (case) and standard care (control) groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough detail provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no details provided; considering the nature of the study, unlikely that participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "a chest physician interviewed all included patients, and pulmonary function test, short form‐36 (SF‐36), St George’s respiratory questionnaire (SGRQ), and modified British Medical Research Council (mMRC) dyspnea scale were performed" Comment: no details provided; outcome assessors were the same personnel as those delivering the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "9 from the case and 10 from the control groups did not participate in the post‐training evaluation; therefore, 31 case and 30 control patients were included in the study" Comment: loss to follow‐up was balanced between groups; reason for loss to follow‐up was unclear but occurred prior to intervention period; therefore unlikely to be related to the intervention |
| Selective reporting (reporting bias) | Unclear risk | Quote: "in our study, we also found no significant differences between the two groups in terms of mortality and hospital readmission rates after one year" Comment: n o trial registration or protocol paper available; mortality and hospital admission rates not defined as outcomes in methods section of the paper. Reported only in the discussion |
Rea 2004.
| Study characteristics | ||
| Methods | Cluster RCT; follow‐up: 12 months; control: conventional care | |
| Participants | Eligible: 158 Randomised: 135; I: 83, C: 52 Completed: 117 Mean age of both groups: 68 years Sex (% male) of both groups: 41.5 Inclusion criteria: COPD diagnosed by ICD‐9‐CM codes and GP records for a clinical diagnosis of moderate to severe COPD Major exclusion criteria for patients: chronic asthma, bronchiectasis, comorbidity more significant than COPD, unable to give informed consent, prognosis < 12 months, long‐term oxygen therapy or too unwell, deceased Major exclusion criteria GP: no longer enrolled with participating GP practice or moved out of area, unable to contact patient, insufficient practice nurse resource |
|
| Interventions | Chronic disease management programme was implemented including ‐ An action plan, which was implemented by patient's own GP and practice nurse, with advice from respiratory nurse and specialist physician. The plan comprised a timetable for regular maintenance checks and achievable goals set for lifestyle changes ‐ Patients visited the nurse monthly, the GP 3‐monthly and at other times if worsening symptoms demanded more visits ‐ Patients received education about smoking cessation, medication. Annual influenza vaccination and pulmonary rehabilitation were recommended Intervention duration: 12 months Disciplines involved: GP, nurse, pulmonologist |
|
| Outcomes | Health status, SF‐36, CRQ, shuttle walk test, spirometry, hospital admissions, medication, courses of oral steroids, courses of antibiotics, smoking cessation Randomisation at cluster level, analysis at patient level |
|
| Notes | Dominant component: self‐management/action plan and structured follow‐up by GP/nurse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "practices were randomised, using a set of computer‐generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and healthcare providers not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: healthcare providers involved in the programme administered outcome measurements at visit |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced between groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported |
| Recruitment bias | Low risk | Quote: "written information about the trial was provided to patients and consent was obtained before patients knew whether they belonged to an intervention or control practice" |
| Baseline imbalance between groups | High risk | Comment: no stratified or pair‐matched randomisation was used, resulting in baseline imbalance of 99 eligible patients in the intervention group and 59 patients in the control group |
| Loss to follow‐up of clusters | High risk | Quote: "after randomisation, two practices declined to participate, and in three, changes of either GP's or practice nurses prevented participation before enrolment had begun" |
| Adequate analysis methods for CRT | High risk | Comment: inadequate methods of analysis: randomisation done at level of GP practice, analysis performed at level of patients |
Rice 2010.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control: single intervention (1 page of information and telephone number) | |
| Participants | Eligible: 743 Randomised: 743, I: 372, C: 371 Completed: 743, I: 323, C: 336 Mean age: I: 69 years, C: 71 years Sex (% male): I: 98, C: 98 Inclusion criteria: COPD diagnosed by spirometry; high risk for hospitalisation as predicted by 1 or more of the following during previous year; hospital admission or ED visit for COPD; long‐term home oxygen use or course of systemic corticosteroids for COPD Major exclusion criteria: any condition that might preclude effective participation in the study or that would reduce life expectancy to less than a year; no access to a telephone |
|
| Interventions | Chronic disease management programme of 12 months, including ‐ Group session (1‐1, 5‐hour): general information about COPD, medication, smoking cessation, vaccinations, and exercise ‐ All patients received an individualised written action plan including prescriptions for prednisone and antibiotics with contact information for a case manager. Participants were in possession of action plan medications at all times and were to refill prescriptions immediately upon initiating the action plan ‐ Case manager made monthly telephone calls Intervention duration: 12 months Disciplines involved: case manager, pharmacist |
|
| Outcomes | ED and hospital admissions related to COPD, SGRQ, mortality, number of telephone contacts | |
| Notes | Dominant component: self‐management/action plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we assigned subjects in equal proportions to each of the two treatment arms by permuted Block randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Comment: information not available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "blinded pulmonologists independently reviewed all discharge summaries and ED reports and assigned a primary cause for each" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all outcome data reported; concordance between outcome observers was tested in subsets and was 96.5% |
| Selective reporting (reporting bias) | Low risk | Comment: no missing outcome data |
Rose 2017.
| Study characteristics | ||
| Methods | RCT, multi‐centre (n = 2); follow‐up: 12 months; control group: usual care | |
| Participants | Eligible: 780 Randomised: 475, I: 237, C: 238 Completed: 398, I: 207, C: 191 Mean age: I: 71 years, C: 71 years Sex (% male): I: 44; C: 50 Inclusion criteria: COPD diagnosis (GOLD criteria) and published Canadian reference values confirmed by a respirologist or internist; 50 years of age or older; 1 of more ED visits or hospital admissions for COPD exacerbation in previous 12 months; 2 or more prognostically important COPD‐associated comorbidities (as defined by GOLD and Canadian Thoracic Society Guidelines) identified via medical record screening Major exclusions: primary diagnosis of asthma; terminal diagnosis; dementia; uncontrolled psychiatric illness; inability to understand English; no telephone access; inability to attend follow‐up; resident in a long‐term care facility; enrolled in provincial tele‐home monitoring programme; no family physician |
|
| Interventions | Multi‐component, case manager‐led intervention Intervention components ‐ Trained case manager delivered 40‐minute standardised educational session based on Living Well With COPD during study enrolment ‐ Individualised care and action plans for COPD exacerbation recognition, self‐management, and management of comorbidities ‐ Case manager‐initiated telephone consultations (12 weekly, monthly for subsequent 9 months; 21 sessions) comprising standardised reinforcement/motivational interviewing focused on health behaviours; action plan teach‐back sessions; assessment of symptoms/symptom monitoring, problems and problem solving strategies ‐ Ongoing case manager communication with family physicians and with hospital specialists including respirologists ‐ Priority access to ambulatory outpatient clinics Duration intervention: 9 months Disciplines involved: case manager, family physician, hospital specialist such as respirologist |
|
| Outcomes | Number of ED visits at 1 year after randomisation (primary outcome); time to first ED presentation; number of hospital admissions and number of hospitalised days at 1 year; mortality; BODE (body mass index, airflow obstruction, dyspnoea, and exercise capacity) index; health‐related QoL (EQ‐5D‐3L); disease‐specific QoL (SGRQ); Anxiety and Depression Scale (HADS), COPD Self‐Efficacy Scale (SES), Client Satisfaction Questionnaire‐8; Caregiver Impact Scale; adherence to chronic disease management measures; smoking cessation and vaccination status | |
| Notes | Dominant component: case management ( investigator's judgement) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomisation was performed according to a centralised, computer generated 1:1 randomisation schedule stratified by study site” |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on concealment of allocation provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “because of the nature of the intervention and co‐location of research staff within the respiratory clinics, healthcare providers, patients and outcome assessors were not blinded, though treating respirologists were not informed of study allocation” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “outcome assessors were not blinded” Comment: unlikely to affect primary outcome (number of ED visits) but may affect some secondary outcomes (e.g. self‐reported quality of life, HADS). Analysis performed by an independent statistician. Not explicitly stated but probably blinded to study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “intention‐to‐treat analysis according to a pre‐specified analysis plan” Comment: larger dropout in control group due to death; may by related to intervention but unlikely to have influenced outcomes |
| Selective reporting (reporting bias) | Low risk | Quote: “analysis according to a pre‐specified analysis plan” Comment: all pre‐specified outcomes are reported, except satisfaction with programme and caregiver impact. Reason provided by study authors is missing responses |
Sanchez‐Nieto 2016.
| Study characteristics | ||
| Methods | RCT; multi‐centre (n = 2); follow‐up: 12 months; control: usual care | |
| Participants | Eligible: 124 Randomised: 96, I: 54, C: 45 Completed: 85, I: 47, C: 38 Mean age: I: 69 years, C: 68 years Sex (% male): I: 6, C: 11 Inclusion criteria: clinical stability (at least in the 3 months before randomisation, with no change in medication or usual symptoms); active smoker or prior history of smoking of ≥ 10 pack‐years; post‐bronchodilator FEV₁/FVC ratio 70%; normal cognitive status to read and understand written texts and receive training in inhalation techniques or self‐care education sessions; physical status that allows for regular walking or exercise; no diagnosis of asthma, advanced heart failure, unstable ischaemic heart disease, terminal disease, dementia, or uncontrolled psychiatric disorders; ability to read texts; no participation in any pulmonary rehabilitation programme in previous year Major exclusion criteria: none reported (included in inclusion criteria) |
|
| Interventions | Self‐management programme consisting of several components ‐ Education: group education session on main characteristics COPD, specially designed for the SMP‐COPD programme ‐ Individual training session on inhalation techniques ‐ Written action plan with colour‐coded treatment instructions including recommendations for physical exercise, exacerbations ‐ Visit by respiratory nurse to check correct use of treatment instructions and inhalation techniques. Duration intervention: 12 weeks Disciplines involved: nurse, physiotherapist, medical specialist in respiratory medicine |
|
| Outcomes | Hospitalisation for COPD exacerbation (primary outcome), days at risk (primary outcome), A&E visits for COPD exacerbation, length of stay, antibiotic or glucocorticoid treatment, all‐cause mortality | |
| Notes | Dominant component: self‐management | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “simple randomisation was carried out separately at each site by means of a list of computer‐generated random numbers, assigning the patients to two groups” |
| Allocation concealment (selection bias) | Low risk | Quote: “simple randomisation was carried out separately at each site by means of a list of computer‐generated random numbers, assigning the patients to two groups” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, blinding of personnel and participants was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “because double‐blinding was not possible, an independent evaluator, who did not know the patients’ group assignments, was responsible for evaluating the outcome variables” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing data on outcomes and reasons for loss to follow‐up are balanced between groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol in not available; published reports include all expected outcomes that were pre‐specified |
Silver 2017.
| Study characteristics | ||
| Methods | RCT; follow‐up:6 months, control group: usual care | |
| Participants | Eligible: 574 Randomised: 428, I: 214, C: 214 Completed: 423, I: 211, C: 212 Mean age: I: 50 years, C: 57 years Sex (% male): I: 44, C: 50 Inclusion criteria: between 18 and 65 years of age; diagnosis of COPD based on FEV₁/FVC < 0.7 or FEV₁ < 80% predicted (performed before bronchodilator administration); at high risk for repeat hospitalisations or emergency department visits as predicted by hospital admission or emergency department visit in previous 12 months for a COPD exacerbation; long‐term home use of oxygen or treatment with a course of systemic corticosteroids in preceding 12 months Major exclusions: not expected to survive the hospitalisation; metastatic cancer, bed‐bound; non‐English‐speaking; inability to provide informed consent |
|
| Interventions | Respiratory therapist disease management transition team Intervention components ‐ 1‐hour educational in‐service by trained respiratory therapist case manager. Education included general information about COPD, direct observation of inhaler techniques, review and adjustment of outpatient COPD medications, smoking cessation counselling, recommendations concerning influenza and pneumococcal vaccinations, encouragement of regular exercise, instruction in hand hygiene ‐ Discussion with case manager and treating physician on need for pharmacotherapy ‐ Verification of COPD diagnosis with bedside spirometry if necessary ‐ Individualised written action plan ‐ Scheduled follow‐up telephone calls with case manager to address specific patient needs, concerns, and questions Duration intervention: 6 months Disciplines involved: respiratory therapist (case manager), treating physician |
|
| Outcomes | Combined number of non‐hospitalised ED visits and hospital admissions for a COPD exacerbation (primary outcome); ED visits for COPD exacerbations; hospital admissions for COPD exacerbations; ED visits for other causes; hospital admissions for other causes; ICU days; hospital days; all‐cause mortality | |
| Notes | Dominant component: s tructured follow‐up with c ase manager (investigator 's judgement) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “study subjects were randomly assigned to treatment groups in a 1:1 ratio using blocked randomisation (n = 4/block) |
| Allocation concealment (selection bias) | Unclear risk | Comment: n o details on concealment of allocation provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “we cannot exclude some form of bias in terms of the outcome assessment because this was not a blinded study” Comment: outcomes less subjective; however knowledge of allocation may have influenced participants' behaviour with regard to ED visit and other outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: study not blinded; however primary outcomes extracted from automated medical records and bi‐monthly telephone calls by study co‐ordinator to determine if participants had recent hospital or ED visits and medical indications for admission/ED visit. Outcome less subjective and based mostly on EHR, so unlikely to have biased results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: quantity of missing data minimum (3/214 intervention and 2/214 control) and balanced between groups |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐defined outcomes reported on. Reported on more than specified in trial register. For all outcomes, also reported on (1) number of participants with ≥ 1 COPD ED visit/non‐COPD ED visit/hospital admission; (2) median (IQR) per subject COPD ED visit/non‐COPD ED visit/hospital admission; (3) number of participants with ≥ 1 COPD ED visit/non‐COPD ED visit/hospital admission |
Smith 1999.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control: usual care | |
| Participants | Eligible: 105 Randomised: 96, I: 48, I: 48 Completed: 36 (data completed only for intervention group) Mean age: I: 70 years, C: 70 years Major inclusion criteria: COPD diagnosis according to guidelines, age > 40 years, FEV₁/FVC < 60%, stable state, carer involved in management, able to speak and read English and give written consent Major exclusion criterion: no other active illness |
|
| Interventions | Intervention of 12 months including ‐ Follow‐up planning for inpatients and outpatients with a nurse in shared care approach with GP and medical staff. Nurses discussed with GP goals for discharge and needs and facilitated involvement of domiciliary service. Goals were inserted into patient notes ‐ During 12 months every 2 to 4 weeks, there was a home visit including education, spirometry, optimal medication, exacerbation management, smoking cessation, and fitness advice Included HCPs: nurse, GP, social worker, hospital medical officer |
|
| Outcomes | COOP (HRQoL), mortality, hospital admissions, lung function | |
| Notes | Dominant component: structured follow‐up with nurse/GP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised as they were enrolled, following discharge from hospital (..), into the HBNI or control groups from two lists of randomly computer generated numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised as they were enrolled, following discharge from hospital" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "this study was unblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "this study was unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "attempts to perform questionnaires in the control subjects were unsuccessful due to a combination of (I) these subjects perceived no immediate benefit of the trial; and (ii) the burden of participating in a study" Comment: no outcomes reported in control group |
| Selective reporting (reporting bias) | High risk | Comment: 1 or more primary outcomes in the review (COOP, spirometry) are reported incompletely, so they cannot be entered into a meta‐analysis |
Sridhar 2008.
| Study characteristics | ||
| Methods | RCT; 104 weeks; control group: usual care | |
| Participants | Eligible: 297 Randomised: 122, I: 61, C: 61 Completed: 104, I: 55, C: 49 Mean age both groups: 70 years Sex (% male): both groups: 49 Inclusion criteria: diagnosis of COPD and admitted between 2000 and 2004 with acute exacerbation of COPD Exclusion criteria: significant comorbidity (severe heart disease or cancer, or any condition that would preclude participation in physical therapy component of PR programme) |
|
| Interventions | Nurse‐led intermediate care package ‐ Patients started with PR programme for 4 weeks, including general education about disease and treatment, and physical training programme ‐ After 4 weeks, patients received a home visit, including a written COPD action plan for exacerbations. GPs provided medication ‐ Patients received monthly telephone calls and a home visit every 3 months until 24 months' follow‐up. Calls reinforced advice regarding treatments, smoking cessation, need to continue exercise therapy; reinforced self‐management education Intervention duration: 24 months Disciplines involved: GP, nurse, physiotherapist |
|
| Outcomes | CRQ, mortality, exacerbations, hospital admissions, lung function | |
| Notes | Dominant component: exercise and action plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "122 patients were suitable and were recruited and randomised by the use of random numbers to the intervention and control group" |
| Allocation concealment (selection bias) | Unclear risk | Comment: n o information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: n o information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: d ropout rates comparable between groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Strijbos 1996.
| Study characteristics | ||
| Methods | RCT; 18 months; intervention group 1: hospital‐based PR, intervention group 2: home‐based PR, control group: usual care | |
| Participants | Eligible: 50 Randomised: 50, I group 1: 18, I group 2: 17, C: 15 Completed: 41, I group 1: 15, I group 2: 15, C: 15 Mean age: I 1: 61 years, I 2: 60 years, C: 63 Sex (% male): I 1: 93, I 2: 80, C: 80 Inclusion criteria: diagnosis of COPD as evidenced by history, physical examination, chest radiograph, and pulmonary function test results; PaCO₂ at rest < 6.5 kPa, and PaO₂ at rest > 7.5 kPa; FEV₁ < 65% predicted Major exclusion: ischaemic heart disease, musculoskeletal disorder or other disabling disease that could restrict rehab therapy |
|
| Interventions | 12‐week rehabilitation programme ‐ Both groups: exercise twice a week during 12 weeks, 1 hour each session ‐ In hospital group, exercise was administered by a physiotherapist (1 hour twice a week) and patients were instructed to practise daily exercise for ≥ 15 minutes. Patient education 3 times/1 hour by a respiratory nurse ‐ In home care group, exercise was carried out at home by local physiotherapist and home care nurse, under supervision of GP. Patients received individualised exercise programme from physiotherapist of 30 minutes (24 sessions) and were instructed to exercise ≥ 15 to 30 minutes. They received 3 times education by a nurse and 3 times visit by physician or GP ‐ Both groups were intended to continue exercise daily at home, after completion of the programme Intervention duration: 12 weeks Involved disciplines: nurse, physiotherapist, GP or pulmonologist |
|
| Outcomes | 4‐minute walking test (4MWT), cycle test (measured as maximum watts, W‐max), interviews | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to intervention or control group". Information is insufficient to be confident that the allocation sequence was genuinely randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: n o information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: w e were unable to ascertain whether outcome assessors were blinded to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: c omparable low dropout rates in both groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Tabak 2014.
| Study characteristics | ||
| Methods | RCT; follow‐up: 9 months; control: usual care (control group also received an activity sensor to register activity levels) | |
| Participants | Eligible: 101 Randomised: 29, I: 15, C: 14 Completed: 12, I: 10, C: 2 Mean age: I: 64 years, C: 63 years Sex (% male): 50, I: 50, C: 50 Inclusion criteria: COPE II criteria (e.g. no exacerbation in the month prior to enrolment and 3 or more exacerbations or 1 hospitalisation for respiratory problems in the 2 years preceding study entry), access to computer with Internet connection Major exclusion criteria: serious other disease with low survival rate; other disease influencing bronchial symptoms and/or lung function (e.g. cardiac insufficiency, sarcoidosis); uncontrolled diabetes mellitus during COPD exacerbation in the past or hospitalisation for diabetes mellitus in the 2 years preceding the study; need for regular oxygen therapy (> 16 hours per day or pO2 < 7.2 kPa); maintenance therapy with antibiotics; known alpha₁‐antitrypsin deficiency; impaired hand function causing inability to handle the application |
|
| Interventions | Components of telehealth programme ‐ Web‐based exercise programme (breathing exercise, relaxation, mobilisation, resistance and endurance training, muscle clearance) with individual exercise schemes created by physiotherapist, with feedback option for patients ‐ Individualised activity coach to monitor daily activity via an accelerometer‐based activity sensor and smartphone with encouraging motivational individualised daily messages ‐ Self‐management module on the web portal that enables patients to treat exacerbations themselves following a decision tree. Before use of self‐management module, attendance at 2 self‐management teaching sessions (90 minutes each) provided by nurse practitioner. Patients received recipes for their medication. Access to patient diary by chest physician and nurse practitioner ‐ Teleconsultation module allowing questions and comments between physiotherapist and patient Duration intervention: 9 months Disciplines involved: primary and secondary care professionals (physiotherapist, practice nurse, chest physician) |
|
| Outcomes | Adherence to intervention (primary outcome), satisfaction with received care (primary outcome), number of hospitalisations, duration of hospitalisations, number of ED visits, number of exacerbations, activity level (activity sensor), self‐perceived activity levels (Baecke Physical Activity Questionnaire), 6MWD, MFI (fatigue), CCQ, MRC Dyspnoea, EuroQol‐5D | |
| Notes | Dominant component: telemonitoring | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomised using a computer‐generated randomisation list where randomisation was applied in random blocks of two and four. Participants were allocated by a data manager in order of inclusion following the randomisation list” |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were allocated by a data manager in order of inclusion following the randomisation list, placed in a sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the intervention, it is not likely that participants and treating healthcare providers were blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not enough information provided to determine whether assessor was blinded to outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: large imbalance in dropout rates between intervention (n = 5) and control (n = 12) and reasons for missing data. Reasons for missing outcome data likely to be related to true outcome, being satisfied with received care |
| Selective reporting (reporting bias) | High risk | Quote: “data in Table 5 (clinical outcomes) are descriptive only, and present T0–T2” Comment: clinical outcomes reported only up to 3 months, not for 6 and 9 months Quote: “exacerbation data were not available for the control group”; “the temporary unavailability of one physiotherapy practice, were also a reason not all measurements were assessed. This made us unable to report the number of exacerbations in the control group” Comment: exacerbations/relapses reported for telehealth and usual care Comment: due to scope of study (pilot RCT) and size of groups, no statistical tests were performed. Furthermore MRC Dyspnoea was reported only for T1 (1 month). Hence, study fails to report all study outcomes as specified in the protocol |
Theander 2009.
| Study characteristics | ||
| Methods | RCT; 3 months; control group: usual care | |
| Participants | Eligible: 30 Randomised: 30, I: 15, C: 15 Completed: 26, I: 12, C: 14 Mean age: I: 66 years, C: 64 years Sex (% male): I: 25, C: 71 Inclusion criteria: diagnosis of COPD according to British guidelines, with FEV₁ between 60% and 25% post bronchodilation, age ≤ 75 years Major exclusions: disabling or severe disease other than COPD, impaired pulmonary function due to other disease, long‐term oxygen therapy, alpha₁‐antitrypsin deficiency, cancer disease, untreated obstructive sleep apnoea syndrome, no COPD‐related symptoms affecting activities of daily life |
|
| Interventions | Multi‐disciplinary programme ‐ Physiotherapy 2 days per week (1 hour) for 12 weeks, with additional home training after 1 month ‐ Dietician support (3 sessions of 1 hour): education and, if needed, additional nutritional supplementation ‐ Occupational therapist: education and teaching ‐ Nurse (2 sessions of 1 hour): education and self‐care advice Intervention duration: 3 months Disciplines involved: physiotherapist, dietician, occupational therapist, nurse |
|
| Outcomes | BMI, FEV₁, fatigue impact scale, 6MWD, grip strength, SGRQ, SF‐36 | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "for the randomisation we prepared 80 sealed opaque envelopes with assignment information: 40 for the rehabilitation group and 40 for the control group" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization procedures were performed by an independent person from the research group, who took a random envelope from the prepared box with sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the data collection was performed by members of the rehabilitation group. The data collection was not blinded to the data collector" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: c omparable dropout rates |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Titova 2017.
| Study characteristics | ||
| Methods | RCT, single‐centred; follow‐up: 3 years (initially 2 years planned); control: usual care | |
| Participants | Eligible: 199 Randomised:172, I: 91, C: 81 Completed: 100, I: 51, C: 49 Mean age: I: 74 years, C: 72 years Sex (% male): I: 43, C: 43 Inclusion criteria: admission due to COPD exacerbations; clinical diagnosis of COPD with GOLD stage III or IV; living in Trondheim municipality; ability to communicate in Norwegian; ability to sign informed consent Major exclusions: serious disease that might cause a very short life span (expected survival time < 6 months) |
|
| Interventions | Intervention components of COPD ‐ home intervention ‐ Call centre for support and communication with patients, home care nurses, co‐ordination between various levels of care ‐ Educational session for home care nurses and interactive e‐learning programme for patients ‐ Individualised self‐management plan for patients ‐ Joint visits at patients‘ homes by a specialist nurse who repeated the core element of the educational programme and reinforced specific health behaviours, as well as making necessary changes to patient's treatment programme Duration intervention: 24 months Disciplines involved: home care nurse, specialised nurse, GP |
|
| Outcomes | Number of hospital admissions caused by AECOPD (primary outcome), number of in‐hospital days due to AECOPD, all‐cause mortality, COPD‐related mortality, SGRQ, HADS, Patient Activation Measurement (PAM), use of medication, lung function, cost‐effectiveness | |
| Notes | Dominant compone n t: stru c tured follow‐up Study temporarily stopped after 2 years' follow‐up for 8 months due to increased mortality rates in intervention group. REC concluded that mortality was not related to intervention. Follow‐up continued, intervention not continued |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “it was decided by lottery that participants from District Pair 1 were assigned to the UC group, and participants from District Pair 2 were assigned to the IC group”; "the demography is quite similar according to age and disease panorama, i.e. the number of inhabitants 55–79 years old are the same in the two district pairs (Lerkendal/Heimdal; 15 800 and Østbyen/Midtbyen 15 200) Comment: randomisation was performed on district level, matched based on district size |
| Allocation concealment (selection bias) | High risk | Quote: "they were randomly allocated to either integrated care (IC) or usual care (UC) based on address of permanent residence Comment: insufficient detail on allocation concealment provided. Considering randomisation procedure on district level and following hospitalisation, it seems unlikely that participants and investigators could not foresee assignment to intervention or control conditions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was a prospective, open, single‐centre intervention study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the study was a prospective, open, single‐centre intervention study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “an increased number of deaths were registered among the patients in the IC group compared to the UC group.”; “data on the causes of death were analysed, and the REC concluded that the increased number of deaths in the IC group was not related to the COPD‐home intervention, but could be explained by pre‐study poorer health status and higher age” Comment: imbalance in number of deaths could have resulted in overall healthier health status among intervention group members at follow‐up. Insufficient details provided to be conclusive regarding effect on true outcome |
| Selective reporting (reporting bias) | Low risk | Comment: published reports include all expected outcomes that were pre‐specified, with the exception of lung function and cost‐effectiveness as included in the clinical trial register |
| Other bias | High risk | Comment: not defined as cluster‐RCT; however potential clustering effect, considering that level of randomisation is region (4 clusters) |
Trappenburg 2011.
| Study characteristics | ||
| Methods | RCT; follow‐up 6 months; control group: usual care | |
| Participants | Eligible: 391 Randomised: 233, I: 111, C: 122 Completed: 193, I: 91, C: 102 Mean age: I: 66 years, C: 65 years Sex (% male): I: 65, C: 69 Inclusion criteria: COPD diagnosed by spirometry, age > 40 years, smoking history > 20 years or 15 pack‐years, diagnosis of COPD as a major functionally limiting disease, current use of bronchodilator therapy Major exclusions: primary diagnosis of asthma, primary diagnosis of cardiac disease, presence of disease that could affect mortality or participation in the study |
|
| Interventions | 6‐month self‐management/action plan programme ‐ Individualised action plan with treatment prescriptions related to color‐coded symptom status to enhance adequate response to periods of symptom deterioration ‐ Action plan included ongoing support of case manager, in concordance with GP/respiratory physician. 2 reinforcement sessions provided by telephone at 1 and 4 months Intervention duration: 6 months Disciplines involved: GP, nurse, pulmonologist |
|
| Outcomes | Exacerbation rate and recovery time; SGRQ; HADS; courses of antibiotics, corticosteroids; ED visits for exacerbation; CCQ score during exacerbation | |
| Notes | Dominant component: self‐management/action plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was carried out using the minimization technique to balance the control and intervention groups for centre and gender" |
| Allocation concealment (selection bias) | Low risk | Quote: "to conceal the assignment sequence, a central web‐based service was used" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "an informed consent to postponed information procedure is used, keeping the patient unaware of the AP being the major study aim. This implies that all patients are informed about the fact that, besides the outcome assessment aiming at gaining more insight in daily symptom variations, the study has another purpose. Patients are told that they will be informed about this additional research question only after follow up because informing during recruitment would affect study results" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "investigators were blinded to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "monthly discontinuation rates and reasons for withdrawal are comparable in both study arms" |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
van Wetering 2010.
| Study characteristics | ||
| Methods | RCT; follow‐up: 24 months, control group: usual care | |
| Participants | Eligible: 199 Randomised: 199, I: 102, C: 97 Completed: I: 77, C: 81 Mean age: I: 66 years, C: 67 years Sex:: I: 71%, C: 71% Inclusion criteria: diagnosis of COPD according to guidelines, other inclusion criteria: impaired exercise capacity, W‐max < 70%, GOLD 2 + 3, clinically stable at inclusion Major exclusion criteria: prior rehabilitation, patients with serious comorbidity that precluded exercise therapy |
|
| Interventions | Community‐based COPD management programme ‐ Intensive 4‐month standardised, supervised physiotherapy 2/week (30 minutes), with home‐based exercise ‐ Participation in an individualised education programme ‐ All smokers were offered smoking cessation counselling ‐ Nutritionally depleted patients received counselling from a dietician ‐ During 20‐month active maintenance phase, patients were instructed to train at home and visited the physiotherapist once a month. Dietician support was continued Intervention duration: 16 weeks followed by 20 months ' maintenance Involved disciplines: nurse, physiotherapist, dietician |
|
| Outcomes | SGRQ, total score, number of exacerbations, mMRC, exercise performance (measured as maximum Watts: W‐max), 6MWD, muscle strength, isometric quadriceps peak torque, maximum inspiratory mouth pressure, fat‐free mass, lung function | |
| Notes | Dominant component of programme: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to INTERCOM or usual care using a computerised procedure with concealed patient allocation" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomised to INTERCOM or usual care using a computerised procedure with concealed patient allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all outcome measurements were assessed single blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: results were analysed by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Vasilopoulou 2017.
| Study characteristics | ||
| Methods | RCT; follow‐up: 14 months, control group: usual care (all study arms have 2 months' pulmonary rehab) | |
| Participants | Eligible: unknown Randomised: 150, I (A): 50, I (B): 50, C: 50 Completed: 147, I (A): 47, I (B): 50, C: 50 Mean age: I (A): 67 years, I (B): 67 years, C: 64 years Sex (% male): I (A): 94, I (B): 76, C: 74 Inclusion criteria: older than 40 years of age, diagnosis of COPD, FEV₁ to FVC < 0.7 with FEV₁ < 80% predicted, with optimal medical treatment according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) without regular use of systemic corticosteroids, history of acute exacerbations of COPD 1 year before entry into the study Major exclusions: diagnosis of orthopaedic, neurological, and other conditions that significantly impair exercise tolerance; respiratory disorder other than COPD; cognitive impairment and/or difficulties in managing electronic devices |
|
| Interventions | Intervention consisted of 2 months' outpatient rehabilitation followed by a 12‐month maintenance rehabilitation programme that is home‐based (group A) or hospital‐based (group B) Home‐based maintenance telerehabilitation (group A) Intervention components ‐ Individualised action plan ‐ Individually tailored physical exercise sessions to remote monitoring, adaption of exercise load based on exercise vital sign data (144 sessions) ‐ Self‐measurement of exercise vital sign data (heart rate and oxygen saturation) along with ratings related to symptoms of dyspnoea and leg discomfort immediately after completion of home exercise programme ‐ Manual entry of data into tablet and transmission of self‐collected data to web‐based platform 3 times per week (exercise vital sign data) or 2 times per week (pedometer, spirometry, oximetry, and responses to questionnaires (HRQoL, CAT, HADS, mMRC)) ‐ Review of transmitted data on secure web‐based server platform regularly by different healthcare professionals (3 or 4 times per week) ‐ Self‐management; psychological support and dietary and self‐management advice via scheduled weekly contacts with a physiotherapist, an exercise scientist, a dietician, and a physician through telephone or video conference ‐ Access to a pulmonologist at a call centre 5 days per week, 10 hours per day Hospital‐based maintenance rehabilitation (group B) Intervention components ‐ Continuation of rehabilitation programme twice weekly for 12 months, including exercise training, physiotherapy, dietary and psychological advice Intervention duration: 14 months (2 months ' outpatient rehabilitation + 12 months ' home‐based or hospital‐based maintenance rehabilitation) Involved disciplines: physiotherapist, dietician, physician (as case manager for home‐based telerehabilitation) |
|
| Outcomes | Rate of moderate to severe acute exacerbation (GOLD) (primary outcome), hospitalisations due to acute exacerbation of COPD (primary outcome), ED visits (primary outcome), rate of severe exacerbations (hospitalisations), rate of ED visits due to acute exacerbation of COPD that did not require hospital admission, functional capacity (peak work rate, 6MWD), daily physical activity (activity monitoring via accelerometer), health‐related quality of life (SGRQ), respiratory symptoms (CAT, mMRC); compliance with intervention | |
| Notes | Dominant component: (A) telemonitoring, (B) structural follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomised into three groups using a set of computer‐generated random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “our study design was not blinded” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: investigator aware of allocation. However primary endpoint objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: analysis performed per protocol. Dropouts (n = 3) in home‐based telerehabilitation group unlikely to affect outcome |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes have been reported |
| Other bias | Unclear risk | Comment: o utcome from baseline (Month 2, after outpatient PR) to 12 months. Usual care group also had no access to outpatient PR. Hence maintenance of benefits might be related to conduct of initial PR |
Vianello 2016.
| Study characteristics | ||
| Methods | RCT; follow‐up: 12 months; control: usual care | |
| Participants | Eligible: 458 Randomised: 334, I: 230, C: 104 (allocation using 2:1 ratio) Completed: 262, I: 181, C: 81 Mean age: I: 75.96 years, C: 76.48 years Sex (% male): I: 71.3, C: 73.1 Inclusion criteria: clinical diagnosis of class III to IV COPD according to GOLD guidelines, age ≥ 18 years, life expectancy > 12 months according to Multiparametric Prognostic Index (MPI), capability of using telemonitoring equipment Major exclusion criteria: concomitant significant lung disease, unwillingness to use telemonitoring technology, negative advice of GP, other serious social problems |
|
| Interventions | Tele‐self‐monitoring system: telemonitoring kit consisting of portable wrist clinic device for clinical parameters measuring heart rate and SpO₂ and gateway device for data transmission to a central data management unit every other day and/or with clinical worsening ‐ Patient‐customised threshold level of alert based on baseline values of pulmonary function test during routine visit, before hospitalisation ‐ Self‐management education material ‐ Access to data by pulmonary specialist. When alerted, pulmonary specialist contacts patients to verify and undertake appropriate action (e.g. modifying medication, sending district nurse for home visit, setting up office appointment with pulmonary specialist, taking patient to emergency department) Duration intervention: 12 months Disciplines involved: central data management unit operator, pulmonary specialist, nurse (for home visits) |
|
| Outcomes | SF‐36 PCS and MCS score (primary outcome), HADS, number of hospitalisations due to exacerbation, duration of hospitalisation due to exacerbation, number of hospitalisations for any cause, duration of hospitalisation for any cause, number of re‐admissions due to exacerbation, number of re‐admissions for any cause, number of appointments with pulmonary specialist, number of ED visits, mortality | |
| Notes | Dominant component: telemonitoring | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomisation was performed following standard procedures and checked for incorrect imbalances or meaningful baseline differences in variables using a dedicated algorithm provided by PASS 2008 software that took into account patient’s age and gender” Comment: randomisation to intervention or control group using 2:1 allocation. insufficient information about sequence generation process but imbalance checked using appropriate methods |
| Allocation concealment (selection bias) | Unclear risk | Quote: “randomisation was performed following standard procedures” Comment: insufficient information provided about concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “a pragmatic unblinded, parallel‐group, two arm, 12 month randomised controlled trial (RCT) was carried out” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: unblinded study design. Some outcomes were extracted from regional records and were less prone to detection bias (hospital admissions, healthcare service use including consultations with a pulmonary specialist and visits to ED service) and mortality |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “out of the 230 patients allocated to the study group, 19 did not actually participate in the study (and did not receive the TM equipment) for the following reasons: death (n = 1), withdrawal of consent (n = 9), administrative problems (n = 7), moving to a nursing home (n = 1), and another reason (n = 1)” |
| Selective reporting (reporting bias) | Low risk | Comment: published reports include all expected outcomes that were pre‐specified |
Wakabayashi 2011.
| Study characteristics | ||
| Methods | RCT; follow‐up 12 months; control group: single intervention (education) | |
| Participants | Eligible: 102 Randomised: 102; I: 52, C: 50 Completed: 85; I: 42, C: 43 Inclusion criteria: clinical diagnosis of COPD, > 65 years, exclusively visited clinic with monthly scheduled appointments, history of cigarette smoking Exclusion criteria: history of atopy or any apparent asthmatic features, illiterate or with cognitive impairment score < 26 on MMSE, lived in a residential care facility or nursing home, exacerbations during preceding 3 months, other respiratory disease such as bronchiectasis, any type of pulmonary fibrosis or congestive heart failure |
|
| Interventions | Patients underwent a programme of educational sessions for 6 months, individually tailored according to their domain scores on the LINQ questionnaire, which was designed to assess the need for information from the patient's perspective. Programme was given by respiratory nurses and pulmonary physicians. There were six domains: (1) understanding of COPD, (2) pharmacological treatments, (3) exercise, (4) avoidance of exacerbations, including action plan with instructions in the event of exacerbation, (5) smoking cessation, (6) nutrition. All patients were provided with a booklet that was used during each session. After intensive education period, each patient was followed up for 6 months in the same way as patients in the usual care group Intervention duration: 6 months Disciplines involved: nurse, pulmonologist |
|
| Outcomes | FEV₁, MRC, SGRQ, 6MWD, Lung Information Needs Questionnaire (LINQ), BMI, BODE Index (body mass index, dyspnoea, airflow obstruction, exercise capacity), activities of daily living (ADL), comorbidities, hospitalisations | |
| Notes | Dominant component: self‐management/action plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a case manager independent of the study randomly assigned patients to either group I or group U using a computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients' allocations were sealed in numbered envelopes by an independent evaluator, not involved in the interventions" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "an independent evaluator, who assessed outcomes at the beginning of the study, after initial integrated education (6 months), and after follow‐up period (6 months)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: c omparable dropout rates between groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
Wang 2017.
| Study characteristics | ||
| Methods | RCT, multi‐centre (n = 2); follow‐up: 12 months, control group: usual care | |
| Participants | Eligible: 162 Randomised: 130, I: 62, C: 68 Completed: 120, I: 55, C: 65 Mean age: I: 69 years, C: 72 years Sex (% male) I: 38, C: 55 Inclusion criteria: medically confirmed diagnosis of COPD based on Chinese Medical Association diagnostic criteria, including percentage forced expiratory volume for 1 second (FEV₁%) ≤ 80% and forced expiratory volume for 1 second divided by forced vital capacity (FEV₁/FVC) ≤ 70%; ability to speak Mandarin to communicate; discharged to a home where Internet and computer have been installed; ability to be reached by telephone post discharge Major exclusions: comorbidities (i.e. allergic rhinitis, myocardial infarction, severe heart failure, and malignant tumour); no access to a computer or Internet at home |
|
| Interventions | Web‐based coaching programme using EHRs, accessible for patients and medical staff Intervention components ‐ Web‐based HER system to allow for input of demographic information, record of admission, discharge, and community information ‐ Ability for patient to manage and control own record and enter health information ‐ Visual presentation of trajectory of disease to medical staff and patient ‐ Access to patient on information about the disease and health education content entered by administrator (medical staff). Health education included information about COPD and pulmonary rehabilitation instructions. Information related to COPD consisted of cause of disease, development, acute exacerbation, prognosis, medication information (name, route, dosage, and adverse reactions), oxygen therapy, diet, importance of smoking cessation ‐ Direct email‐like communication with community administrator ‐ Messaging function between medical team and patient (2‐way) ‐ Telephone call from research team every 2 weeks Duration intervention: 12 months Disciplines involved: community nurse, medical practitioner, clinical nurse |
|
| Outcomes | FEV₁ %, FVC%, FEV₁/FVC, peak expiratory flow and maximum mid‐expiratory flow, SGRQ, mMRC, 6MWD | |
| Notes | Dominant component: telemonitoring | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the patients who consented to participate were assigned to the intervention or control group using a computer‐generated randomised table” |
| Allocation concealment (selection bias) | Unclear risk | Comment: n o details on concealment of allocation provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “participants were not blinded to group assignment” Not explicitly mentioned whether medical staff was blinded. However, given nature of intervention, unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: in supplementary material – CONSORT eHEALTH checklist mentioned that all data were collected face‐to‐face by research assistant, who was blinded to allocation outcome. Researchers were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: loss to follow‐up unbalance d between groups: 7/62 in intervention lost to‐follow‐up with reason ‘could not contact’; 3/68 lost to follow‐up in control group. Analysis performed with only complete measurements |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not published nor trial registered in trial registry. All outcomes mentioned in protocol are reported, except FEV₁/FVC. No distinction between primary and secondary outcomes |
Wijkstra 1994.
| Study characteristics | ||
| Methods | RCT; follow‐up 12 weeks; control group: no treatment | |
| Participants | Randomised: 45 Completed: 43; I: 28, C: 15 Mean age I: 64 years, C: 62 years Sex (% male): I: 82, C: 93 Inclusion criteria: diagnosis of COPD with FEV₁ % < 60%, FEV₁/IVC < 50% Exclusion criteria: evidence of ischaemic heart disease, intermittent claudication, musculoskeletal disorder, other disabling disease that could restrict the rehab programme |
|
| Interventions | Comprehensive rehabilitation programme at home ‐ Patients were supervised by a multi‐disciplinary team: pulmonologist, physiotherapist, nurse, GP ‐ Patients visited physiotherapist twice a week for 12 weeks and programme consisted of conventional physiotherapy, upper limb training, inspiratory muscle training, exercise training. Patients had to practice twice a day for a half hour at home ‐ Furthermore, they received education at home from a nurse (once a month) ‐ They visited the GP once a month, who supervised clinical status and maintenance treatment Intervention duration: 3 months Disciplines involved: GP, physiotherapist, nurse |
|
| Outcomes | Lung function, CRQ, cycle ergometer test | |
| Notes | Dominant component: exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were stratified for their FEV₁ % predicted. After this stratification, the patients were randomly allocated" |
| Allocation concealment (selection bias) | Low risk | Quote: "(after randomisation), they were randomly allocated to one of three groups, each of 15 patients" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: p articipants and treating therapists not likely to have been blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: w e could not ascertain how and whether outcome assessors were blinded to treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: o nly 2 (out of 30) dropouts in rehabilitation group vs no dropouts in control group |
| Selective reporting (reporting bias) | Low risk | Comment: a ll outcomes reported |
Wood‐Baker 2006.
| Study characteristics | ||
| Methods | Cluster‐RCT; follow‐up 12 months, control group: education + usual care | |
| Participants | Eligible: 218
Randomised: 138; I: 67, C: 72
Completed (12 months): 112; I: 54, C: 58
Mean age: I: 69 years, C: 71 years
Sex (% male): I: 49, C: 71 Inclusion criteria: COPD diagnosed by spirometry, age > 50 years, tobacco smoking history > 10 pack‐years, FEV₁ < 65% predicted Major e xclusion criterion: nursing home residents |
|
| Interventions | Control + intervention group: COPD information booklet, individual educational session with nurse Intervention group: written self‐management plan, which was developed in consultation with the treating GP. Patients were encouraged to make early contact with GP during an exacerbation Intervention duration: 12 months Disciplines involved: GP, nurse |
|
| Outcomes | SGRQ, exacerbations (courses of antibiotics/prednisone), ED visits, hospital admissions, GP consultations, spirometry, mortality, physical exercise (pedometer) | |
| Notes | Before commencement of the randomisation process, only 50% of included GPs attended 1 of a series of educational workshops on management of COPD Dominant component: self‐management/action plan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "practices were randomised to the intervention or control group using a computer generated randomisation software package“ |
| Allocation concealment (selection bias) | Unclear risk | Comment: n o information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it is not likely that participants and personnel have been blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "baseline, 6‐ and 12‐month assessments involved face to face contact with a research nurse at the GP’s surgery or at patient's home“ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 13 intervention patients vs 14 control patients missing at 6 months; reasons similar |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were pre‐specified |
| Recruitment bias | Low risk | Comment: no information provided |
| Baseline imbalance between groups | High risk | Comment: baseline imbalance between groups |
| Loss to follow‐up of clusters | Low risk | Comment: no missing clusters |
| Adequate analysis methods for CRT | High risk | Comment: no adjustments for cluster‐randomised trials |
Zhang 2020.
| Study characteristics | ||
| Methods | RCT; follow‐up 24 months ; control group: u sual care | |
| Participants | Eligible: 702
Randomised: 208; I: 104, C: 104
Completed: 174; I: 85, C: 89
Mean age I: 65 years, C: 66 years
Sex (% male): I: 77, C: 75 Inclusion criteria: older than 45 years of age; diagnosis of GOLD stage II, III, or IV COPD as documented by pulmonary function testing; current or previous smoker with ≥ 10 pack‐years of cigarette smoking; hospitalised for an exacerbation of COPD Exclusion criteria: unable to provide accurate information or to follow instructions, unable to walk even during periods of COPD |
|
| Interventions | Hospital outreach PR program me after hospital discharge, delivered i n 2 p hases P h ase 1: 3‐month intensive intervention with i ntervention components ‐ S upervised physical exercise, 2 × per week, 50 minutes per session ‐ S moking cessation (2 sessions) ‐ S elf‐management education, including COPD knowledge, symptom management, instruction on medication intake and adherence, nutritional support. Session every 2 weeks ‐ P sychosocial support (2 sessions) P h ase 2: s tructural follow‐up by telephone (once every 1 to 2 weeks) and home visits (once every 1 to 3 months) up to 24 months by a respiratory nurse. Exercise diary to record daily exercise and symptoms Duration intervention: 3 months intensive with up to 24 months structural follow‐up Dis c iplines involved: respiratory nurse, physiotherapist, tai chi mentor, psychologist, nutritionist |
|
| Outcomes | Healthcare utili s ation costs (admission rates, admission days, ED visits) (primary outcome); lung function (FEV ₁, FVC, FEV ₁ % predicted; mMRC; 6MWD; CAT (health‐related QoL); COPD self‐management scale ) | |
| Notes | Dominant component: structured follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomization was used. Subjects were randomized after consent and collection of baseline data. Every two patients with the same level of COPD severity were allocated into one block according to their admission dates. In each block, the two patients were further allocated into treatment and control groups randomly based on allocation sequence" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were informed of the results of randomization in person or by phone after discharge" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blind trial. Given the nature of the intervention, blinding the subjects was not feasible, and the interventionist would also know that those contacted were in the intervention arm" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the statistician was blinded to individual results during the trial, and the allocation‐to‐trial‐arm coding was not revealed until the data set had been sealed. For outcome assessment, the assessor was also blinded to subject allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: c omparable dropout rates between groups (19/104 in intervention, 15/104 in control). Reasons for dropout are comparable between groups |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol is not available; all outcomes from trial registration are reported |
Zwar 2016.
| Study characteristics | ||
| Methods | Cluster‐RCT (36 clusters); follow‐up: 12 months, control group: usual care (copy COPD treatment guidelines) | |
| Participants | Newly diagnosed COPD patients Eligible: 287, I: 169, C: 118 Randomised: 254, I: 144, C: 110 Completed: 222, I: 126, C: 96 Mean age: I: 67 years, C: 65 years Sex (% male): I: 62 C: 58 Inclusion criteria: current and former smokers, aged 40 to 85 years, newly identified as having COPD on post‐bronchodilator spirometry (post‐bronchodilator FEV₁/FVC < 0.7), had attended the practice at least twice with ≥ 1 visit in the preceding 12 months Major exclusions: recorded diagnosis of COPD, unable to understand English sufficiently to complete study questionnaires or procedures, cognitive impairment |
|
| Interventions | Nurses and GPs in intervention practices were educated to work in partnership to identify patients with COPD and to initiate an evidence‐based early intervention programme Intervention components: care plan, education, optimal diagnosis, management of anxiety and depression, medication, influenza and pneumococcal vaccination, referral to PR and/or dietician if necessary/appropriate, smoking cessation advice and resources if necessary. Multidisciplinary teams; professional roles Duration intervention: intervention duration not fixed; expected to be completed within 6 months Disciplines involved: GP, nurse, physiotherapist, dietician |
|
| Outcomes | SGRQ, CAT, general health status, post‐bronchodilator FEV₁, COPD knowledge score, awareness of diagnosis of COPD, smoking status, immunisation status for influenza and pneumococcus, effective inhaler use (when prescribed), attendance at pulmonary rehabilitation, healthcare utilisation, intervention uptake | |
| Notes | Dominant component: e ducation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization and group allocation of GP practices was performed by an independent statistician using a computer‐generated randomisation program” |
| Allocation concealment (selection bias) | Low risk | Quote: “allocation concealment will be ensured as group allocation will be conducted at the same time as randomisation. Practices will be informed about their group allocation by fax (Bunker et al, 2012) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: " i n this pragmatic trial , participating GPs, PNs, and patients were not blind to the aims of the study n or to their randomisation group" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “project officers, who collected study outcome measures, and the statistician undertaking analyses were blind to group allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “the primary analysis was by intention to treat and relied on the diagnosis of COPD assigned by the PN/GP on the basis of case‐finding spirometry” Comment: 34 and 10 patients withdrew from intervention and control groups, respectively. Reasons for withdraw not likely to be related to true outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures described in the protocol, except patient satisfaction, are reported |
| Recruitment bias | High risk | Comment: patient inclusion following case‐finding procedures were performed after randomisation and group allocation of GP practices |
| Baseline imbalance between groups | Low risk | Comment: groups did not differ substantially in mean SGRQ nor in other characteristics |
| Loss to follow‐up of clusters | Unclear risk | Comment: 4 practices withdrew after randomisation, and 2 practices merged into 1 during the study period |
| Adequate analysis methods for CRT | Low risk | Quote: “intra‐cluster(practice) correlation coefficients (ICCs) were determined for all primary outcome variables” “The effect of the intervention on outcomes measured on a continuous scale (such as SGRQ score) were estimated and tested using mixed‐model analysis of variance in which time and treatment group were fixed effects and GP practice and subject nested within practice were random effects. The effect of the intervention on dichotomous variables was analysed using generalized estimating equations with a logistic link and a model structure that is analogous to that described above" |
4MWT: four‐minute walking test; 6MWD: six‐minute walking distance; ADL: activities of daily living; BMI: body mass index; BTS: British Thoracic Society; COOP: Dartmouth Primary Care Co‐operative Quality of Life Questionnaire; COPD: chronic obstructive pulmonary disease; CRDQ: Chronic Respiratory Disease Questionnaire; CRQ: Chronic Respiratory Questionnaire; ED: emergency department; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; HCCQ: Health Care Communication Questionnaire; HRQoL: health‐related quality of life; I: intervention; MACL: Mood Adjective Check List; MBHI: Millon Behavioral Health Inventory; MCO: managed care organisation; MMSE: Mini–Mental State Examination; MRC: Medical Research Council; NYHA: New York Heart Association; PR: pulmonary rehabilitation; RCT: randomised controlled trial; SGRQ: St. George's Respiratory Questionnaire; SIP: Sickness Impact Profile; VAS: visual analogue scale; VC: vital capacity; YQLQ: York Quality of Life Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ancochea 2018 | Fewer than 2 different healthcare providers included |
| Arbillaga‐Etxarri 2018 | Fewer than 2 different healthcare providers included |
| Bachmann 2018 | No results for COPD patients presented |
| Bachmann 2019 | Fewer than 2 component s of intervention |
| Bal 2016 | Fewer than 2 different healthcare providers included |
| Balaban 2017 | Fewer than 2 different healthcare providers included and duration < 3 months |
| Benzo 2016 | Fewer than 2 different healthcare providers included |
| Benzo 2019 | Fewer than 2 different healthcare providers included |
| Bischoff 2012 | No multi‐disciplinary intervention |
| Blumenthal 2014 | Fewer than 2 different healthcare providers included |
| Bringsvor 2018 | Duration < 3 months |
| Budnevskiy 2016 | Fewer than 2 different healthcare providers included |
| Cameron‐Tucker 2016 | Duration < 3 months |
| Carrieri 2005 | Active treatment in control group |
| Carron 2017 | Not an RCT |
| Casas 2006 | Intervention duration < 3 months |
| Collins 2019 | Fewer than 2 different healthcare providers and fewer than 2 intervention components included |
| Collinsworth 2018 | Fewer than 2 different healthcare providers included |
| Coultas 2016 | Fewer than 2 different healthcare providers included |
| Cox 2018 | Duration < 3 months |
| Csikesz 2016 | Duration < 3 months |
| De Godoy 2003 | Active treatment in control group |
| Drks 2019 | Fewer than 2 different healthcare providers included |
| Eaton 2009 | Intervention duration < 3 months |
| Effing 2009 | Active treatment in control group |
| Efraimsson 2008 | Fewer than 2 different healthcare providers included |
| Elliott 2004 | Fewer than 2 different healthcare providers included |
| Farmer 2017 | Fewer than 2 different healthcare providers included and duration < 3 months |
| Ferrone 2019 | Fewer than 2 different healthcare providers included |
| Flink 2017 | Fewer than 2 different healthcare providers included and duration < 3 months |
| Folch‐Ayora 2018 | Fewer than 2 different healthcare providers included |
| Fu 2018 | Fewer than 2 different healthcare providers included |
| Garcia 2007 | Duration of intervention < 3 months |
| George 2019 | Fewer than 2 different healthcare providers included |
| Gohl 2006 | Fewer than 2 different healthcare providers and fewer than 2 components included |
| Goldstein 1994 | Fewer than 2 components of intervention |
| Guell 2008 | Active treatment as control group |
| Hajizadeh 2020 | Fewer than 2 different healthcare providers included |
| Heidari 2018 | Fewer than 2 different healthcare providers included |
| Hernandez 2004 | Duration < 3 months |
| Hughes 2000 | No results solely for COPD |
| IRCT20160914029817N | Duration < 3 months |
| Jakobsen 2015 | Duration < 3 months |
| Jiang 2020 | Fewer than 2 intervention components |
| Jolly 2018 | Fewer than 2 different healthcare providers included |
| Jones 2009 | Fewer than 2 different healthcare providers included |
| JPRN‐UMIN000034582 | Fewer than 2 intervention components (pharmaceutical intervention) |
| Khdour 2011 | Fewer than 2 different healthcare providers included |
| Lahham 2018 | Duration < 3 months |
| Lainscak 2013 | Duration < 3 months |
| Lavoie 2017 | Fewer than 2 components of intervention (same study as Troosters 2017) |
| Li 2019 | Fewer than 2 different healthcare providers included |
| Liang 2019 | Duration < 3 months |
| Linden 2014 | Fewer than 2 different healthcare providers included |
| Liu 2006 | Not an RCT |
| Liu 2019 | Fewer than 2 components of intervention |
| Lorig 2006 | Fewer than 2 different healthcare providers included and duration < 3 months |
| Ly 2018 | Not an RCT |
| Maltais 2008 | No usual care as control group |
| Markun 2018 | Fewer than 2 components of intervention and duration < 3 months |
| Martin 2004 | Fewer than 2 components of intervention |
| Martinez 2014 | Fewer than 2 different healthcare providers included |
| McGeoch 2006 | Fewer than 2 components of intervention |
| Monninkhof 2003 | No usual care as control group |
| Moy 2014 | Fewer than 2 different healthcare providers included |
| Moy 2016 | Fewer than 2 different healthcare providers included |
| Muelepas 2007 | Not an RCT |
| NCT03794921 | Fewer than 2 intervention components |
| NCT03889054 | Fewer than 2 intervention components |
| NCT04260178 | Fewer than 2 different healthcare providers included |
| NCT04348344 | Fewer than 2 intervention components |
| NCT04437238 | Fewer than 2 different healthcare providers included |
| NCT04459546 | Fewer than 2 different healthcare providers included |
| Nguyen 2019 | Fewer than 2 different healthcare providers included |
| North 2018 | Fewer than 2 different healthcare providers included |
| Nyberg 2017 | Fewer than 2 different healthcare providers included |
| Rabinovich 2017 | Fewer than 2 different healthcare providers included |
| Radini 2017 | Not reported for COPD |
| Rausch‐Osthoff 2017 | Fewer than 2 different healthcare providers included |
| RBR‐533hht | Fewer than 2 different healthcare providers included and duration < 3 months |
| Renn 2018 | Fewer than 2 different healthcare providers included |
| Ries 2003 | Active treatment as control group |
| Ringbaek 2015 | Fewer than 2 different healthcare providers included |
| Rixon 2017 | Fewer than 2 different healthcare providers included |
| Robinson 2020 | Fewer than 2 different healthcare providers included |
| Rotter 2017 | Not an RCT |
| Schmidt 2018 | Fewer than 2 components of intervention (same study as Troosters 2017) |
| Scuffham 2018 | Fewer than 2 different healthcare providers included |
| Selzler 2019 | Duration < 3 months |
| Sidhu 2015 | Fewer than 2 different healthcare providers included |
| Soler 2006 | Active treatment as control group |
| Sorensen 2016 | Fewer than 2 different healthcare providers included |
| Soriano 2018 | Fewer than 2 different healthcare providers included |
| Stamenova 2020 | Fewer than 2 different healthcare providers included |
| Steele 2008 | Active treatment as control group |
| Stenlund 2019 | Fewer than 2 different healthcare providers included |
| Steurer‐Stey 2018 | Not an RCT |
| Thom 2019 | Fewer than 2 different healthcare providers included |
| Thurber 2018 | Not an RCT |
| Torre 2018 | Fewer than 2 different healthcare providers and fewer than 2 intervention components included |
| Troosters 2016 | Fewer than 2 intervention components |
| van der Weegen 2015 | Fewer than 2 different healthcare providers included |
| Van Genugten 2016 | Duration < 3 months and fewer than 2 different healthcare providers included |
| Varas 2018 | Duration < 3 months |
| Voncken‐Brewster 2015 | Fewer than 2 different healthcare providers included |
| Walker 2018 | Fewer than 2 different healthcare providers included |
| Walters 2013 | Fewer than 2 different healthcare providers included |
| Wang 2020 | Fewer than 2 different healthcare providers included |
| Waterhouse 2010 | Duration < 3 months and fewer than 2 different healthcare providers included |
| Welch 2020 | Fewer than 2 different healthcare providers included |
| Weldam 2017 | Fewer than 2 different healthcare providers included and duration < 3 months |
| Wootton 2019 | Fewer than 2 different healthcare providers included |
| Wu 2018 | Fewer than 2 intervention components |
| Xi 2014 | Fewer than 2 different healthcare providers included |
| Yoon 2018 | Not reported for COPD |
| Zakrisson 2019 | Duration < 3 months |
| Zhou 2010 | COPD diagnosis not an inclusion criterion |
| Zhou 2017 | Fewer than 2 different healthcare providers and fewer than 2 intervention components included |
COPD: chronic obstructive pulmonary disease. RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Baumann 2012.
| Methods | RCT with 26 weeks ' follow‐up to investigate whether relevant improvements in physical capabilities and quality of life for patients with COPD could be achieved by a long‐term, low‐intensity, once‐weekly rehabilitation programme using limited resources 100 patients with moderate to severe COPD were randomised to a continuous outpatient interdisciplinary rehabilitation programme or standard care |
| Participants | 100 patients with moderate to severe COPD |
| Interventions | Physiotherapy‐led supervised outpatient training sessions were performed once weekly in addition to educational elements |
| Outcomes | 6MWD, cycle ergometry, SGRQ |
| Notes |
Borji, 2018.
| Methods | RCT with 6 months ' follow‐up to determine the effect of the Adaptive Sustainability Care Model on re‐admission of patients with COPD |
| Participants | 80 COPD patients, randomised to intervention or routine care |
| Interventions | Adaptive sustainability model was performed for patients in the intervention group in 4 steps: investigation of the demographic characteristics of family, desensitisation, collaboration, and continuous monitoring during 6 months |
| Outcomes | R e‐admission rate |
| Notes | Additional details regarding intervention required to determine eligibility |
Carcereny, 2016.
| Methods | RCT with 6 months' follow‐up to evaluate the effectiveness of a therapeutic education programme on COPD in preventing exacerbations after discharge (APRENDEEPOC study) |
| Participants | COPD patients discharged from the hospital |
| Interventions | A Therapeutic Education Program (TEP) as a key component of the Integrated Care Model |
| Outcomes | H ospital (re) admissions, ED visits |
| Notes | Additional details regarding intervention required to determine eligibility |
Mao 2020.
| Methods | RCT with 3 months ' follow‐up to evaluate clinical outcomes and quality of life resulting from evidence‐based nursing in elderly patients with COPD and heart failure |
| Participants | 120 patients over 60 years of age with COPD or ECG and chest X‐ray diagnosed heart failure |
| Interventions | Evidence‐based nursing, which was p erformed by the selection of best evidence‐based practice by a multi‐disciplinary team, depending on the patient ' s condition. Evidence‐based practice included cognitive‐behaviour al intervention (i.e. health education, mental health status assessment), oxygen therapy, exercise tolerance, breathing function exercises, and dietary advice |
| Outcomes | Disease‐related adverse events, FVC, FEV ₁ /FVC, 6MWD, Minnesota Living With HA Questionnaire (MLWHFQ) , European Heart Failure Self‐care Behavior Scale (EHFScBS), nursing satisfaction, intervention compliance |
| Notes | Additional details regarding intervention required to determine eligibility. We were unable to contact study authors |
NCT04256070.
| Methods | RCT with 3 months ' follow‐up to eval u ate the effect of e ducation and teleconsultancy i ntervention based on Watson human care theory on self‐efficacy and quality of life of i ndividuals with COPD |
| Participants | 74 participants with COPD randomised to intervention or control |
| Interventions | Education, counselling , nursing care , and education booklet based on Watson h uman care theory; fixed teleconsultation appointments at 2, 4, 6, 8, and 10 weeks; 24‐hour teleconsultat ion if request ed by the individual |
| Outcomes | Chronic Obstructive Pulmonary Disease Self‐Efficacy Scale , SGRQ, FVC, FEV ₁, FEV ₁ /FVC, number of hospitalisations |
| Notes | Additional details regarding intervention required to determine eligibility |
Reguera 2017.
| Methods | RCT with 6 and 12 months' follow‐up to evaluate the efficacy of an integrated Internet programme (IIP) followed after conventional PR to maintain its benefits |
| Participants | COPD patients attending an ambulatory PR programme |
| Interventions | Integrated Internet programme consisting of plan of education, self‐care, physical activity, and behavioural modifications |
| Outcomes | SQRQ, CAT, 6MWD, dyspnoea |
| Notes | Full text not retrieved. Additional details regarding intervention required to determine eligibility |
Xu 2010.
| Methods | Four‐arm RCT with 3, 6, and 12 months' follow‐up to observe the efficacy of integrative respiratory rehabilitation training for exercise ability and quality of life of COPD patients in stable phase |
| Participants | O utpatients and inpatients with COPD from Department of Respiratory Medicine, Taihe Hospital, Yunyang Medical College |
| Interventions | Eighty outpatients and inpatients with COPD from Department of Respiratory Medicine, Taihe Hospital, Yunyang Medical College, were randomly divided into 4 groups, with 20 patients in each group. Patients in group A received only drug therapy, patients in group B received traditional qigong training, patients in group C received modern rehabilitation training, and patients in group D received integrative respiratory rehabilitation training |
| Outcomes | CRQ, Borg score, 6MWD |
| Notes | Full text not retrieved. Additional details regarding intervention required to determine eligibility |
6MWD: six‐minute walking distance. CAT: COPD Assessment Test. COPD: chronic obstructive pulmonary disease. CRQ: Chronic Respiratory Questionnaire. ECG: electrocardiogram. ED: emergency department. EQ‐5D: EuroQol Quality of Life ‐ 5 domains. FEV₁: forced expiratory volume in one second. FVC: forced vital capacity. PR: pulmonary rehabilitation. RCT: randomised controlled trial. SGRQ: St. George's Respiratory Questionnaire.
Characteristics of ongoing studies [ordered by study ID]
Ali 2020.
| Study name | Person‐centred Care at Distance (PROTECT) |
| Methods | Open‐label RCT with 3, 6, 12, and 24 months ' follow up Aim: to evaluate the effects of person‐centred care (PCC) by combined digital platform and structured telephone support for people with COPD and/or chronic heart failure |
| Participants | People with diagnosis of COP D or chronic heart failure |
| Interventions | Person‐centred care at a distance through an eHealth platform, used by professionals, patients, and relatives |
| Outcomes | General Self‐Efficacy Scale (GSE) , hospitalisation, mortality, healthcare utili s ation, EQ‐5D, HADS, shortness of breath in heart failure (SOB‐HF), CAT, mMRC |
| Starting date | Starting date: August 2017; estimated completion date: June 2021 |
| Contact information | Dr. Lilas Ali; lilas.ali@gu.se |
| Notes |
Bourne 2017.
| Study name | A Self‐Management Programme of Activity Coping and Education ‐ SPACE for COPD(C) ‐ in primary care |
| Methods | Prospective, multi‐site, single‐blinded RCT with follow‐up at 6 and 9 months |
| Participants | Patients with COPD; identified from General Practice COPD registers, responding to a poster advertisement displayed at GP practices and hospitals, or participating in previous research at the Respiratory Biomedical Research Unit at University Hospitals of Leicester |
| Interventions | Community‐based, HCP‐led, group‐based self‐management programme based on the Self‐Management Programme of Activity Coping and Education (SPACE for COPD(C)) |
| Outcomes | CAT, CRQ‐SR, incremental shuttle walk test, physical activity monitor, EQ‐5D, PAM, HADS, feasibility, acceptability, efficacy |
| Starting date | January 2015 |
| Contact information | Bourne@uhl‐tr.nhs.uk; clinical trial registration ISRCTN17942821 |
| Notes | Pre‐results published in trial register |
Ding 2019.
| Study name | Evaluation of an innovative mobile health program for the self‐management of chronic obstructive pulmonary disease (MH‐COPD) |
| Methods | Prospective open RCT with 3 and 6 months ' follow‐up Aim: to examine whether an innovative mobile health (mHealth)‐enabled care programme (MH‐COPD) will improve patient self‐management and relevant health outcomes |
| Participants | Patients with diagnosed COPD with chronic airflow limitation that is not fully reversible (post ‐ bronchodilator FEV ₁/ FVC < 70%, FEV ₁ < 80% predicted) and current o r former smokers (> 10 pack‐years) |
| Interventions | Innovative mHealth programme for COPD specifically designed to integrate an mHealth system within an existing COPD care service to deliver all core components advocated by evidence‐based clinical guidelines in Australia. The MH‐COPD programme includes health education, symptom monitoring, an electronic CIPD action plan, physical activity, and smoking cessation support i n cluding automatically generated motivational messages and inhaler technique. All data entries of participants recorded via the app, such as symptoms, action plan, and cigarettes, will be automatically uploaded to the online portal, accessible to research staff to mon i t or patient adherence |
| Outcomes | CAT, SGRQ, mMRC, test of adherence to inhalers, smoking cessation, Global Physical Activity Questionnaire, exacerbation rate, healthcare utilisation (hospital re ‐ admission s as ED visits) |
| Starting date | Starting date: June 2019; an t icipated end date: December 2021 |
| Contact information | Dr. Hang Ding; hang.ding@csiro.au |
| Notes |
Drennan 2014.
| Study name | Expanding Paramedicine in the Community (EPIC) study |
| Methods | Pragmatic, stratified RCT to compare a community paramedic intervention to standard of care for patients with COPD, heart failure (HF) , or diabetes mellitus (DM) |
| Participants | Patients with diagnosed DM, HF, or COPD, identified by the Family Health Care Team as being at high risk for hospital admission based on hospital admission rates over 3 years before study enrolment |
| Interventions | Intervention will consist of an initial visit and 3 follow‐up visits at 3‐month intervals over 1 year by a paramedic who has received additional training in chronic disease management. Visits include a medical history, a physical examination (recorded on an electronic assessment tool that is e‐linked to the patient care record for the entire family healthcare team), and disease‐specific education and counselling. If necessary, the community paramedic may initiate treatment in the home based on disease‐specific evidence‐based medical directives and/or may initiate telephone contact with the primary healthcare physician in accordance with the medical directive Control group: usual care from family healthcare team |
| Outcomes | Number of hospitalisations per patient after 1 year, number of 911 calls, number of clinical visits, length of hospital admission, mortality, EQ‐5D‐3L, intervention compliance and safety, cost‐effectiveness |
| Starting date | Starting date: June 2013; estimated completion date: December 2016 |
| Contact information | DrennanI@smh.ca; ClinicalTrials.gov identifier: NCT02034045 |
| Notes |
Foot 2017.
| Study name | REMAIN HOME ‐ REducing Medical Admissions INto Hospital through Optimising MEdication |
| Methods | Stepped‐wedged, cluster‐ RCT with 12‐month follow‐up. There will be 14 clusters, each representing a different general practice medical centre. A total of 2240 participants will be recruited from hospital who attend an enrolled medical centre, take 5 or more long‐term medicines, or whose reason for admission was related to heart failure or chronic obstructive pulmonary disease |
| Participants | Patients in hospital who are considered at risk of re‐admission, prescribed ≥ 5 long‐term medicines on discharge, or with primary discharge diagnosis of congestive heart failure or exacerbation of COPD |
| Interventions | A multi‐faceted and collaborative service involving a practice pharmacist integrated into a medical centre to assist patients in transitioning back into primary care after hospitalisation. Participants meet with the practice pharmacist and the GP after discharge to review and reconcile their medicines and discuss changes made in hospital. The pharmacist follows up with the participant and liaises with other health professionals involved in the patient's care |
| Outcomes | Rate of unplanned, all‐cause hospital re‐admissions; healthcare utilisation; cost‐effectiveness |
| Starting date | May 2017 ; actual end date: 14 April 2019 |
| Contact information | c.freeman4@uq.edu.au |
| Notes |
Hajizadeh 2020a.
| Study name | A RCT into a Telehealth Delivered Pulmonary Rehabilitation (TelePR) programme for Hispanic and African Patients hospitalized for COPD exacerbations |
| Methods | Single‐cent r e RCT with 8 weeks ' and 6 months ' follow‐up Aim: to test whether a referral to TelePRversus SPR resulted in decreased 6‐month re ‐ admission among Hispanic or African American patients hospitalised for COPD exacerbation |
| Participants | People with moderate COPD, African‐American/Hispanic, Spanish/English fluency, who are able to follow basic exercise instructions and use a stationary bike Important exclusion criteria are completion of pulmonary rehabilitation within the last year and weight < 300 lb |
| Interventions | Telehealth pulmonary rehabilitation, twice/week for 8 weeks Exercise bikes are equipped with software enabling respiratory therapist to remotely conduct pulmonary rehabilitation session with a patient while the patient is at home. Vital signs are continually monitored, and the RT is able to alert 911 (emergency services) if patient is in distress. Educational videos and stretches are also incorporated |
| Outcomes | Re ‐ hospitalisation following exacerbation of COPD, 2‐minute s tep test, CAT, mMRC, Bristol COPD Knowledge Questionnaire, depression, patient adherence, acceptability |
| Starting date | Starting date: April 2017; estimated completion date: November 2020 |
| Contact information | Prof Negin Hajizadeh; Nhajizadeh@northwell.edu |
| Notes |
Hansen 2017.
| Study name | COPD Online Rehabilitation (CORe) ‐ a Randomized, Multicenter Telemedicine Intervention Study |
| Methods | Multi‐centre (8 hospitals) RCT to compare the effects of supervised COPD online rehabilitation in groups, as delivered by health professionals in the patients’ own home via a computer, for patients with severe and very severe COPD with conventional supervised COPD rehabilitation programme Follow‐up duration: 12 months |
| Participants | Patients with severe and very severe (stage III or IV) COPD identified and recruited by respiratory nurses during outpatient COPD control visits at a respiratory and physiotherapy department |
| Interventions | Supervised online COPD rehabilitation, delivered in groups through a computer screen in the patient's own home. Rehabilitation contains exercise training and educational sessions 3 times per week for a duration of 10 weeks. Each session lasts 60 minutes (60% exercise, 40% education) |
| Outcomes | 6MWD, 30 s sit‐to‐stand test, EQ‐5D, CCQ, CAT, lung function, HADS, hospitalisation, exacerbation, mortality |
| Starting date | March 2016 ; actual completion date: 31 December 2019 |
| Contact information | Henrik.hansen.09@regionh.dk |
| Notes |
NCT04136418.
| Study name | A Randomised Designed Clinical Investigation of the Use of a Personalised Early Warning Decision Support System With Novel Saliva Bio‐profiling to Predict and Prevent Acute Exacerbations of Chronic Obstructive Pulmonary Disease |
| Methods | Multi‐centre, open label RCT with 12 months ' follow‐up Aim: t o investigate if a smart digital health intervention (COPDPredict™) can be used by both COPD patients and clinicians to improve self‐management, predict lung attacks early, intervene promptly, and avoid hospitalisation |
| Participants | People with clinically diagnosed and confirmed COPD with ≥ 2 acute exacerbations of COPD (AECOPD) in the previous 12 months according to the patient and/or ≥ 1 hospital admission for AECOPD |
| Interventions | COPDPredict™, which consists of a patient‐facing app and a clinician‐facing smart early warning decision support system. The app on a mobile device is used by patient s to track the status of their COPD and to inform the patient's care team |
| Outcomes | AECOPD‐related hospital admissions, inpatient days, COPD exacerbations, ED visits, symptom control markers, CAT, EQ‐5D, lifestyle choices, FEV ₁, C‐reactive protein during exacerbations |
| Starting date | Starting date: September 2020; estimation completion date: September 2022 |
| Contact information | Rachael O'Beney; Rachael.O'Beney@uhcw.nhs.uk |
| Notes |
NCT04416295.
| Study name | Selfcare MAnagement InteRvenTion in COPD (SMART COPD) |
| Methods | Single‐cent r e RCT with 6 and 12 months ' follow‐up Aim: to eval u ate a digital support and communication platform for COPD patients |
| Participants | People with diagnosed COPD |
| Interventions | LifePod: LifePod consists of a web‐based E‐health platform with 2 interfaces ‐ 1 medical for healthcare professionals and 1 patient interface. Patients can enter symptom parameters and vital parameters such as breathing and mucus status, weight, activity, medication, a nd other disease‐specific values. Several different validated questionnaires are sent regularly to the patient to obtain information about the patient's mood and activity. The platform contains a chat function between healthcare professionals and patients. A unique health profile is created in which patient s self‐report their health. P atient s receive direct feedback through the web application if they are within the interval given for the individual. Medical interfaces are designed so that patients are automatically placed in a priority order where by the person outside the given range is given top priority |
| Outcomes | mMRC, CAT, VAS, EQ‐5D, ho s pitalisations, lengt h of stay, healthcare visits |
| Starting date | Starting date: Augu s t 2019; estimated completion date: April 2021 |
| Contact information | Sofia Gerwards ; sofia.gerward@med.lu.se |
| Notes |
NCT04533412.
| Study name | Comprehensive Self‐management Support for COPD Patients (SAMBA COPD) |
| Methods | Single‐cent r e, double ‐ blind RCT with 6 months ' follow‐up |
| Participants | People aged 40 years or older with chart‐document severe or very severe COPD (FEV ₁ < 50% predicted) or COPD‐related ED/hospitalisation ≥ 1 visit within the past 12 month s and smoking history ≥ 10 pack‐years |
| Interventions | For the intervention, community health workers will assess barriers to good self‐management behavio u rs that lie within 4 domains: ( 1) social context, ( 2) physical health and functioning, ( 3) cognitive factors, and ( 4) psychological factors. They will work with participants for 6 months to help them work through their barriers to self‐management of COPD. Participants can also participate in home‐based pulmonary rehabilitation and can receive emergency pack/action pack medication for COPD exacerbation. The attention control is designed to isolate the impact of screening for self‐management barriers. The attention control will consist of 4 visits by a COPD educator who will review a COPD education booklet |
| Outcomes | CAT, Medication Adherence Report Scale, 6MWD |
| Starting date | Starting date: August 2020; estimated completion date: April 2022 |
| Contact information | Shynah James; shynah.james@mountsinai.org |
| Notes |
Steed 2017.
| Study name | TANDEM study ‐ Tailored intervention for ANxiety and DEpression Management in COPD |
| Methods | RCT with 6 and 12 months' follow‐up to investigate whether a cognitive‐behavioural approach (CBA) intervention, delivered prior to PR, can help to improve mild to moderate anxiety and/or depression in those with moderate to very severe COPD and consequently encourage PR uptake/completion (phase III of the TANDEM study) |
| Participants | Adults living with COPD recruited from primary care, community clinics, or secondary care clinics, or following referral to PR services, who have symptoms of mild to moderate comorbid anxiety or depression on screening; caregivers of patients |
| Interventions | A tailored, psychological intervention for mild to moderate anxiety and/or depression in people with chronic obstructive pulmonary disease (COPD) |
| Outcomes | Anxiety, depression (primary outcomes), dyspnoea, health‐related quality of life, functional activity, smoking status, process outcomes, cost‐effectiveness outcomes |
| Starting date | April 2016 |
| Contact information | s.j.c.taylor@qmul.ac.uk |
| Notes |
6MWD: six‐minute walking distance. AECOPD : acute exacerbation of COPD . ADL: activities of daily living. BMI: body mass index. BODE: BMI, airflow Obstruction, Dyspnoea, and Exercise Capacity. CAT: COPD Assessment Test . CCQ: COPD Control Questionnaire . COPD: chronic obstructive pulmonary disease. DM: diabetes mellitus . ED: emergency department . EQ‐5D: EuroQol Quality of Life ‐ 5 domain s. FEV₁: forced expiratory volume in one second . FVC: forced vital capacity . GOLD: Global Initiative for Chronic Obstructive Lung Disease. HADS: Hospital Anxiety and Depression S cale . HF: heart failure . IDM: integrated disease management. m MRC: modified Medical Research Council D yspnoea Scale . PAM: patient activation measure. RCT: randomised controlled trial. VAS: visual analogue scale .
Differences between protocol and review
We added Borg score next to MRC Dyspnea Score as an instrument to measure dyspnoea under secondary outcomes.
We did not search the DARE database for non‐Cochrane Reviews.
Update of 2020 allowed a more detailed evaluation of endpoints. We evaluated outcomes at the endpoints (1) short term (up to 6 months); (2) medium term (6 to 15 months), and (3) long term (more than 15 months), instead of at short‐ (12 months or less) and long‐term (longer than 12 months) follow‐up.
We presented results for continuous outcomes in the 'Summary of findings' table for medium‐term follow‐up only, instead of for short‐, medium‐, and long‐term follow‐up.
We included telemonitoring as a separate intervention component.
We included a definition of 'high‐quality' studies following our RoB judgement and performed sensitivity analysis on high‐quality studies only.
Contributions of authors
AK, NC, and NS wrote the protocol.
AK and NS wrote the previous version of this review.
CP, EM, and PH selected trials.
CP, EM, and PH extracted data and assessed risk of bias.
CP was responsible for data management and data analysis in RevMan.
CP and PH completed the clinical interpretation of results
All review authors contributed to and approved the final version of the review.
Contributions of editorial team
Chris Cates (Coordinating Editor and Contact Editor) checked data entry for the review update; edited the review update ; advised on methods, interpretation, and content; and approved the review update prior to publication.
Emma Dennett (Managing Editor) co‐ordinated the editorial process; advised on interpretation and content; and edited the review.
Emma Jackson (Assistant Managing Editor) conducted peer review and edited sections of the review.
Elizabeth Stovold (Information Specialist) designed the search strategy; ran the searches; and edited the search methods section.
Sources of support
Internal sources
-
LUMC, Leiden, Netherlands
Leiden University Medical Centre
External sources
-
All, Other
The authors declare that no funding such was received for this systematic review
Declarations of interest
NC is a senior researcher in the field of integrated disease management programmes who is involved in several initiatives promoting education, developing software applications, and providing e‐health solutions, which may be considered as a potential conflict of interest.
CP: none known.
EM: none known.
AK: was a PhD student on the RECODE trial, which investigates the effectiveness of integrated care for primary care COPD patients in a cluster‐randomised controlled trial in primary care. The Leiden University Medical Centre received a grant from ZonMW (Dutch governmental agency) and additional financial support from Achmea (Dutch Healthcare Insurer) for the RECODE trial. In the future, our RCT will be included in the Cochrane Review.
NS: none known.
PH: has received payment from E‐wise for development of a continuous medical education programme for general practitioners and pharmacists on severe asthma. E‐wise does not provide any type of disease management programme.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aboumatar 2019 {published data only}
- Aboumatar H, Naqibuddin M, Chung S, Adebowale H, Bone L, Brown T, et al. Better Respiratory Education and Treatment Help Empower (BREATHE) study: methodology and baseline characteristics of a randomized controlled trial testing a transitional care program to improve patient-centered care delivery among chronic obstructive pulmonary disease patients. Contemporary Clinical Trials 2017;62:159-67. [DOI] [PubMed] [Google Scholar]
- Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA 2019;322(14):1371-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboumatar H, Wise RA. Notice of retraction. Aboumatar H et al. Effect of a program combining transitional care and long-term self-management support on outcomes of hospitalized patients with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA 2018;320(22):2335-43. [DOI: 10.1001/jama.2019.11954] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Aiken 2006 {published and unpublished data}
- Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. Journal of Palliative Medicine 2006;9(1):111-26. [DOI] [PubMed] [Google Scholar]
Bendstrup 1997 {published data only}
- Bendstrup KE, Ingemann Jensen J, Holm S, Bengtsson B. Out-patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. European Journal of Respiratory Diseases 1997;10(12):2801-6. [DOI] [PubMed] [Google Scholar]
Bernocchi 2017 {published and unpublished data}
- Bernocchi P, Scalvini S, Galli T, Paneroni M, Baratti D, Turla O, et al. A multidisciplinary telehealth program in patients with combined chronic obstructive pulmonary disease and chronic heart failure: study protocol for a randomized controlled trial. Trials 2016;17(264):no pagination. [DOI: 10.1186/s13063-016-1584-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernocchi P, Vitacca M, La Rovere MT, Volterrani M, Galli T, Baratti D, et al. Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age and Ageing 2017;47(1):82-8. [DOI: 10.1093/ageing/afx146] [DOI] [PubMed] [Google Scholar]
Bourbeau 2003 {published data only}
- Bourbeau J, Collet JP, Schwartzman K, Ducruet T, Nault D, Bradley C. Economic benefits of self-management education in COPD. Chest 2006;130(6):1704-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Archives of Internal Medicine 2003;163(5):585-91. [DOI] [PubMed] [Google Scholar]
- Sedeno MF, Nault D, Hamd DH, Bourbeau J. A self-management education program including an action plan for acute COPD exacerbations. COPD 2009;6(5):352-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boxall 2005 {published data only}
- Boxall AM, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. Journal of Cardiopulmonary Rehabilitation 2005;25(6):378-85. [DOI] [PubMed] [Google Scholar]
Cambach 1997 {published data only}
- Cambach W, Chadwick-Straver RV, Wagenaar RC, Keimpema AR, Kemper HC. The effects of a community-based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. European Respiratory Journal 1997;10(1):104-13. [DOI] [PubMed] [Google Scholar]
Dheda 2004 {published data only}
- Dheda K, Crawford A, Hagan G, Roberts CM. Implementation of British Thoracic Society guidelines for acute exacerbation of chronic obstructive pulmonary disease: impact on quality of life. Postgraduate Medical Journal 2004;80(941):169-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engstrom 1999 {published data only}
- Engstrom CP, Persson LO, Larsson S, Sullivan M. Long-term effects of a pulmonary rehabilitation programme in outpatients with chronic obstructive pulmonary disease: a randomized controlled study. Scandinavian Journal of Rehabilitation Medicine 1999;31(4):207-13. [DOI] [PubMed] [Google Scholar]
Fan 2012 {published data only (unpublished sought but not used)}
- Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Annals of Internal Medicine 2012;156(10):673-83. [DOI: 10.7326/0003-4819-156-10-201205150-00003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Farrero 2001 {published data only}
- Farrero E, Escarrabill J, Prats E, Maderal M, Manresa F. Impact of a hospital-based home-care program on the management of COPD patients receiving long-term oxygen therapy. Chest 2001;119(2):364-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fernandez 2009 {published data only}
- Fernandez AM, Pascual J, Ferrando C, Arnal A, Vergara I, Sevila V. Home-based pulmonary rehabilitation in very severe COPD: is it safe and useful? Journal of Cardiopulmonary Rehabilitation and Prevention 2009;29(5):325-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freund 2016 {published data only (unpublished sought but not used)}
- Freund T, Peters-Klimm F, Boyd CM, Mahler C, Gensichen J, Erler A, et al. Medical assistant-based care management for high-risk patients in small primary care practices: a cluster randomized clinical trial. Annals of Internal Medicine 2016;164(5):323-30. [DOI: 10.7326/M14-2403] [26833209] [DOI] [PubMed] [Google Scholar]
Gottlieb 2011 {published data only}
- Gottlieb V, Lyngso AM, Nybo B, Frolich A, Backer V. Pulmonary rehabilitation for moderate COPD (GOLD 2) - does it have an effect? COPD 2011;8(5):380-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Güell 2000 {published and unpublished data}
- Güell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117(4):976-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Güell 2006 {published and unpublished data}
- Güell R, Resqueti V, Sangenis M, Morante F, Martorell B, Casan P, et al. Impact of pulmonary rehabilitation on psychosocial morbidity in patients with severe COPD. Chest 2006;129(4):899-904. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haesum 2012 {published and unpublished data}
- Dinesen B, Haesum LK, Soerensen N, Nielsen C, Grann O, Hejlesen O, et al. Using preventive home monitoring to reduce hospital admission rates and reduce costs: a case study of telehealth among chronic obstructive pulmonary disease patients. Journal of Telemedicine and Telecare 2012;18(4):221-5. [DOI: 10.1258/jtt.2012.110704] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jimenez‐Reguera 2020 {published data only}
- Jimenez-Reguera B, Maroto Lopez E, Fitch S, Juarros-Monteagudo L, Sanchez-Cortes M, Rodriguez-Hermosa JL, et al. Development and preliminary evaluation of the effects of an mhealth web-based platform (happyairtm) on adherence to a maintenance program after pulmonary rehabilitation in COPD patients: randomized controlled trial. JMIR mhealth and uhealth 2020;8(7):e18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalter‐Leibovici 2018 {published and unpublished data}
- Kalter-Leibovici O, Benderly M, Freedman LS, Kaufman G, Molcho Falkenberg Luft T, Murad H, et al. Disease management plus recommended care versus recommended care alone for ambulatory COPD patients. American Journal of Respiratory and Critical Care Medicine 2018;197(12):1565-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2013 {published and unpublished data}ISRCTN90940049
- Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a 'whole systems' model of self-management support for the management of long- term conditions in primary care: trial protocol. Implementation Science 2012;7:7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346:f2882. [DOI: 10.1136/bmj.f2882] [23670660] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2018 {published data only (unpublished sought but not used)}
- Bourbeau J, Kessler R, Casan P, Koehler D, Tognella S, Viejo JL, et al. An international randomised study of a home-based self-management program for severe COPD: the COPD patient management European trial. Canadian Journal of Respiratory Critical Care and Sleep Medicine 2017;2:98. [Google Scholar]
- Granados D, Bourbeau J, Durand-Zaleski I, Roze S, Kessler R. Cost-utility analysis of a home-based disease management intervention for patients with severe COPD in the COMET study. European Respiratory Journal 2018;Suppl 62:PA3162. [Google Scholar]
- Kessler R, Casan-Clara P, Koehler D, Tognella S, Viejo JL, Dal Negro RW, et al. COMET: a multicomponent home-based disease-management programme versus routine care in severe COPD. European Respiratory Journal 2018;51(1):1701612. [DOI: 10.1183/13993003.01612-2017] [DOI] [PubMed] [Google Scholar]
Khan 2019 {unpublished data only}
- Khan MA, Khan N, Walley JD, Khan MA, Hicks J, Ahmed M, et al. Effectiveness of delivering integrated COPD care at public healthcare facilities: a cluster randomised trial in Pakistan. BJGP Open 2019;3(1):bjgpopen18X101634. [DOI: 10.3399/bjgpopen18X101634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2016 {published data only}
- Ko FW, Cheung NK, Rainer TH, Lum C, Wong I, Hui DS. Comprehensive care programme for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2016;72(2):122-8. [DOI] [PubMed] [Google Scholar]
Koff 2009 {published data only}
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. European Respiratory Journal 2009;33(5):1031-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kruis 2014 {published and unpublished data}
- Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ 2014;349(7976):g5392. [DOI: 10.1136/bmj.g5392] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenferink 2019 {published and unpublished data}
- Lenferink A, Frith P, Valk P, Buckman J, Sladek R, Cafarella P, et al. A self-management approach using self-initiated action plans for symptoms with ongoing nurse support in patients with chronic obstructive pulmonary disease (COPD) and comorbidities: the COPE-III study protocol. Contemporary Clinical Trials 2013;36(1):81-9. [DOI] [PubMed] [Google Scholar]
- Lenferink A, Palen J, Valk Paul DLPM, Cafarella P, Veen A, Quinn S, et al. Exacerbation action plans for patients with COPD and comorbidities: a randomised controlled trial. European Respiratory Journal 2019;54(5):1802134. [DOI] [PubMed] [Google Scholar]
Lilholt 2017 {published data only (unpublished sought but not used)}
- Lilholt PH, Witt Udsen F, Ehlers L, Hejlesen OK. Telehealthcare for patients suffering from chronic obstructive pulmonary disease: effects on health-related quality of life: results from the Danish 'TeleCare North' cluster-randomised trial. BMJ Open 2017;7(5):e014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Littlejohns 1991 {published data only}
- Littlejohns P, Baveystock CM, Parnell H, Jones PW. Randomised controlled trial of the effectiveness of a respiratory health worker in reducing impairment, disability, and handicap due to chronic airflow limitation. Thorax 1991;46(8):559-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lou 2015 {published data only (unpublished sought but not used)}
- Lou P, Chen P, Zhang P, Yu J, Wang Y, Chen N, et al. A COPD health management program in a community-based primary care setting: a randomized controlled trial. Respiratory Care 2015;60(1):102-12. [DOI: 10.4187/respcare.03420] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mendes 2010 {published and unpublished data}
- Mendes de Oliveira JC, Studart Leitao Filho FS, Malosa Sampaio LM, Negrinho de Oliveira AC, Hirata RP, Costa D, et al. Outpatient vs. home-based pulmonary rehabilitation in COPD: a randomized controlled trial. Multidisciplinary Respiratory Medicine 2010;5(6):401-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Öztürk 2020 {published data only}
- Ozturk BO, Alpaydın AO, Ozalevli S, Guler N, Cimilli C. Self-management training in chronic obstructive lung disease improves the quality of life. Turkish Thoracic Journal 2020;21(4):266-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rea 2004 {published data only}
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Internal Medicine Journal 2004;34(11):608-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rice 2010 {published and unpublished data}
- Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2010;182(7):890-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rose 2017 {published data only}
- Rose L, Istanboulian L, Carriere L, Price A, Lee L, Rezaie S, et al. Program of integrated care for patients with chronic obstructive pulmonary disease and multiple comorbidities (pic COPD+): a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2017;195(Meeting Abstracts):A6739. [DOI] [PubMed] [Google Scholar]
Sanchez‐Nieto 2016 {published and unpublished data}
- Sanchez-Nieto JM, Andujar-Espinosa R, Bernabeu-Mora R, Hu C, Galvez-Martinez B, Carrillo-Alcaraz A, et al. Efficacy of a self-management plan in exacerbations for patients with advanced COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):1939-47. [DOI: 10.2147/COPD.S104728] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silver 2017 {published data only (unpublished sought but not used)}
- Silver PC, Kollef MH, Clinkscale D, Watts P, Kidder R, Eads B, et al. A respiratory therapist disease management program for subjects hospitalized with COPD. Respiratory Care 2017;62(1):1-9. [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith BJ, Appleton SL, Bennett PW, Roberts GC, Del Fante P, Adams R, et al. The effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD). Australian and New Zealand Journal of Medicine 1999;29(5):718-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sridhar 2008 {published data only}
- Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63(3):194-200. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strijbos 1996 {published data only}
- Strijbos JH, Postma DS, Altena R, Gimeno F, Koeter GH. A comparison between an outpatient hospital-based pulmonary rehabilitation program and a home-care pulmonary rehabilitation program in patients with COPD. A follow-up of 18 months. Chest 1996;109(2):366-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tabak 2014 {published data only (unpublished sought but not used)}
- Tabak M, Brusse-Keizer M, Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:935-44. [DOI: 10.2147/COPD.S60179] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Theander 2009 {published data only}
- Theander K, Jakobsson P, Jorgensen N, Unosson M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clinical Rehabilitation 2009;23(2):125-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Titova 2017 {published and unpublished data}
- Titova E, Salvesen O, Bentsen SB, Sunde S, Steinshamn S, Henriksen AH. Does an integrated care intervention for COPD patients have long-term effects on quality of life and patient activation? A prospective, open, controlled single-center intervention study. PLOS ONE 2017;12(1):e0167887. [DOI: 10.1371/journal.pone.0167887] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trappenburg 2011 {published data only}
- Trappenburg JC, Koevoets L, Weert-van Oene GH, Monninkhof EM, Bourbeau J, Troosters T, et al. Action plan to enhance self-management and early detection of exacerbations in COPD patients; a multicenter RCT. BMC Pulmonary Medicine 2009;9:52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappenburg JC, Monninkhof EM, Bourbeau J, Troosters T, Schrijvers AJ, Verheij TJ, et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax 2011;66(11):977-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Wetering 2010 {published and unpublished data}
- Hoogendoorn M, Wetering CR, Schols AM, Rutten-van Molken MP. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? European Respiratory Journal 2010;35(1):79-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van Molken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax 2010;65(1):7-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vasilopoulou 2017 {published and unpublished data}
- Kaltsakas G, Papaioannou AI, Vasilopoulou M, Spetsioti S, Gennimata SA, Palamidas AF, et al. Tele-monitoring intervention on COPD exacerbations. European Respiratory Journal 2016;48(Suppl 60):OA3045. [Google Scholar]
- Vasilopoulou M, Papaioannou AI, Kaltsakas G, Louvaris Z, Chynkiamis N, Spetsiotial S, et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. European Respiratory Journal 2017;49(5):1602129. [DOI: 10.1183/13993003.02129-2016] [DOI] [PubMed] [Google Scholar]
Vianello 2016 {published data only (unpublished sought but not used)}
- Vianello A, Fusello M, Gubian L, Rinaldo C, Dario C, Concas A, et al. Home telemonitoring for patients with acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulmonary Medicine 2016;16(1):157. [DOI: 10.1186/s12890-016-0321-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wakabayashi 2011 {published and unpublished data}
- Wakabayashi R, Motegi T, Yamada K, Ishii T, Jones RC, Hyland ME, et al. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatrics and Gerontology International 2011;11(4):422-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only (unpublished sought but not used)}
- Wang L, He L, Tao Y, Sun L, Zheng H, Zheng Y, et al. Evaluating a web-based coaching program using electronic health records for patients with chronic obstructive pulmonary disease in china: randomized controlled trial. Journal of Medical Internet Research 2017;19(7):e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijkstra 1994 {published data only}
- Wijkstra PJ, Ten Vergert EM, Altena R, Otten V, Kraan J, Postma DS, et al. Long term benefits of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease. Thorax 1995;50(8):824-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijkstra PJ, Van Altena R, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. European Respiratory Journal 1994;7(2):269-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wijkstra PJ, Mark TW, Kraan J, Altena R, Koeter GH, Postma DS. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1996;9(1):104-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wijkstra PJ, Mark TW, Kraan J, Altena R, Koeter GH, Postma DS. Long-term effects of home rehabilitation on physical performance in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1996;153(4 Pt 1):1234-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wood‐Baker 2006 {published data only}
- Wood-Baker R, McGlone S, Venn A, Walters EH. Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology 2006;11(5):619-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2020 {published data only (unpublished sought but not used)}
- Zhang A, Wang L, Long L, Yan J, Liu C, Zhu S, et al. Effectiveness and economic evaluation of hospital-outreach pulmonary rehabilitation for patients with chronic obstructive pulmonary disease. International Journal of COPD 2020;15:1071-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwar 2016 {published and unpublished data}
- Bunker JM, Reddel HK, Dennis SM, Middleton S, Van Schayck C, Crockett AJ, et al. A pragmatic cluster randomized controlled trial of early intervention for chronic obstructive pulmonary disease by practice nurse-general practitioner teams: Study Protocol. Implementation Science 2012;7:83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar NA, Bunker JM, Reddel HK, Dennis SM, Middleton S, Schayck OC, et al. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: a cluster randomized trial. Family Practice 2016;33(6):663-70. [DOI: 10.1093/fampra/cmw077] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ancochea 2018 {published data only}
- Ancochea J, Garcia-Rio F, Vazquez-Espinosa E, Hernando-Sanz A, Lopez-Yepes L, Galera-Martinez R, et al. Efficacy and costs of telehealth for the management of COPD: the PROMETE II trial. European Respiratory Journal 2018;51:5. [DOI: 10.1183/13993003.00354-2018] [DOI] [PubMed] [Google Scholar]
Arbillaga‐Etxarri 2018 {published data only}
- Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, Balcells E, Benet M, Borrellet E, et al. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. European Respiratory Journal 2018;52(4):1800063. [DOI: 10.1183/13993003.00063-2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachmann 2018 {published data only}
- Bachmann MO, Bateman ED, Stelmach R, Cruz AA, Pacheco de Andrade M, Zonta R, et al. Integrating primary care of chronic respiratory disease, cardiovascular disease and diabetes in Brazil: practical Approach to Care Kit (PACK Brazil): study protocol for randomised controlled trials. Journal of Thoracic Diseases 2018;10(7):4667-77. [DOI: 10.21037/jtd.2018.07.34] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachmann 2019 {published data only}
- Bachmann MO, Bateman ED, Stelmach R, Cruz AA, Pacheco de Andrade M, Zonta R, et al. Effects of PACK guide training on the management of asthma and chronic obstructive pulmonary disease by primary care clinicians: a pragmatic cluster randomised controlled trial in Florianopolis, Brazil. BMJ Global Health 2019;4(6):e001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bal 2016 {published data only}
- Bal Ozkaptan B, Kapucu S. Home nursing care with the self-care model improves self-efficacy of patients with chronic obstructive pulmonary disease. Japan Journal of Nursing Science 2016;13(3):365-77. [DOI] [PubMed] [Google Scholar]
Balaban 2017 {published data only}
- Balaban RB, Zhang F, Vialle-Valentin CE, Galbraith AA, Burns ME, Larochelle MR, et al. Impact of a patient navigator program on hospital-based and outpatient utilization over 180 days in a safety-net health system. Journal of General Internal Medicine 2017;32(9):981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzo 2016 {published data only}
- Benzo R, Vickers K, Novotny PJ, Tucker S, Hoult J, Neuenfeldt P, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization: a randomized study. American Journal of Respiratory and Critical Care Medicine 2016;194(6):672-80. [10.1164/rccm.201512-2503OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzo 2019 {published data only}
- Benzo R, McEvoy C. Effect of health coaching delivered by a respiratory therapist or nurse on self-management abilities in severe COPD: analysis of a large randomized study. Respiratory Care 2019;64(9):1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff 2012 {published data only}
- Bischoff EW, Akkermans R, Bourbeau J, Weel C, Vercoulen JH, Schermer TR. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ 2012;345:e7642. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenthal 2014 {published data only}
- Blumenthal JA, Emery CF, Smith PJ, Keefe FJ, Welty-Wolf K, Mabe S, et al. The effects of a telehealth coping skills intervention on outcomes in chronic obstructive pulmonary disease: primary results from the INSPIRE-II study. Psychosomatic Medicine 2014;76(8):581-92. [DOI: 10.1097/PSY.0000000000000101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bringsvor 2018 {published data only}
- Bringsvor HB, Langeland E, Oftedal BF, Skaug K, Assmus J, Berit Bentsen S, et al. Effects of a COPD self-management support intervention: a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:3677-88. [DOI: 10.2147/COPD.S181005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Budnevskiy 2016 {published data only}
- Budnevskiy AV, Chernov AV, Isaeva YV, Kozhevnikova SA. Clinical efficacy of pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease and metabolic syndrome [Russian]. Terapevticheskii Arkhiv 2016;88(8):25-9. [DOI: 10.17116/terarkh201688825-29] [DOI] [PubMed] [Google Scholar]
Cameron‐Tucker 2016 {published data only}
- Cameron-Tucker HL, Wood-Baker R, Joseph L, Walters JA, Schuz N, Walters EH. A randomized controlled trial of telephone-mentoring with home-based walking preceding rehabilitation in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:1991-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrieri 2005 {published data only}
- Carrieri-Kohlman V, Nguyen HQ, Donesky-Cuenco D, Demir-Deviren S, Neuhaus J, Stulbarg MS. Impact of brief or extended exercise training on the benefit of a dyspnea self-management program in COPD. Journal of Cardiopulmonary Rehabilitation 2005;25(5):275-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carron 2017 {published data only}
- Carron T, Bridevaux PO, Lorvall K, Parmentier R, Moix JB, Beytrison V, et al. Feasibility, acceptability and effectiveness of integrated care for COPD patients: a mixed methods evaluation of a pilot community-based programme. Swiss Medical Weekly 2017;147:w14567. [DOI: 10.4414/smw.2017.14567] [DOI] [PubMed] [Google Scholar]
Casas 2006 {published data only}
- Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. European Respiratory Journal 2006;28(1):123-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Collins 2019 {published data only}
- Collins EG, Jelinek C, O'Connell S, Butler J, Reda D, Laghi F. The effect of breathing retraining using metronome-based acoustic feedback on exercise endurance in COPD: a randomized trial. Lung 2019;197:181-8. [DOI: 10.1007/s00408-019-00198-4] [DOI] [PubMed] [Google Scholar]
Collinsworth 2018 {published data only}
- Collinsworth AW, Brown RM, James CS, Stanford RH, Alemayehu D, Priest EL. The impact of patient education and shared decision making on hospital readmissions for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2018;13:1325-32. [10.2147/COPD.S154414] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coultas 2016 {published data only}
- Coultas DB, Jackson BE, Russo R, Peoples J, Sloan J, Singh KP, et al. A lifestyle physical activity intervention for patients with COPD: a randomized controlled trial. Annals of the American Thoracic Society 2016;13(5):617-26. [DOI: 10.1513/AnnalsATS.201508-508OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2018 {published data only}
- Cox NS, McDonald CF, Alison JA, Mahal A, Wootton R, Hill CJ, et al. Telerehabilitation versus traditional centre-based pulmonary rehabilitation for people with chronic respiratory disease: protocol for a randomised controlled trial. BMC Pulmonary Medicine 2018;18(1):71. [DOI: 10.1186/s12890-018-0646-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Csikesz 2016 {published data only}
- Csikesz N, Nici L. A physician-led intervention to prevent COPD re-admissions. American Journal of Respiratory and Critical Care Medicine 2016;193(Meeting Abstracts):A3994. [Google Scholar]
De Godoy 2003 {published data only}
- De Godoy DV, De Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2003;84(8):1154-7. [DOI] [PubMed] [Google Scholar]
Drks 2019 {published data only}
- Huber S et al. Impact of a smartphone application (KAIA COPD-App) in combination with Activity Monitoring as maintenance program following pulmonary rehabilitation in COPD: an international multi-centered randomised controlled trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=DRKS00017275 (first received 27 June 2019). [DOI] [PMC free article] [PubMed]
Eaton 2009 {published data only}
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O'Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14(2):230-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Effing 2009 {published data only}
- Effing T, Kerstjens H, Valk P, Zielhuis G, Palen J. (Cost)-effectiveness of self-treatment of exacerbations on the severity of exacerbations in patients with COPD: the COPE II study. Thorax 2009;64(11):956-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Efraimsson 2008 {published data only}
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self-care management education at a nurse-led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Elliott 2004 {published data only}
- Elliott M, Watson C, Wilkinson E, Musk AW, Lake FR. Short- and long-term hospital and community exercise programmes for patients with chronic obstructive pulmonary disease. Respirology 2004;9(3):345-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farmer 2017 {published data only}
- Farmer A, Williams V, Velardo C, Shah SA, Yu LM, Rutter H, et al. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: randomized controlled trial. Journal of Medical Internet Research 2017;19(5):e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrone 2019 {published data only}
- Ferrone M, Masciantonio MG, Malus N, Stitt L, O'Callahan T, Roberts Z, et al. The impact of integrated disease management in high-risk COPD patients in primary care. NPJ Primary Care Respiratory Medicine 2019;29(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flink 2017 {published data only}
- Flink M, Lindblad M, Frykholm O, Kneck A, Nilsen P, Kristofer Årestedtet, et al. The Supporting Patient Activation in Transition to Home (sPATH) intervention: a study protocol of a randomised controlled trial using motivational interviewing to decrease re-hospitalisation for patients with COPD or heart failure. BMJ Open 2017;7:e014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folch‐Ayora 2018 {published data only}
- Folch-Ayora A, Orts-Cortes MI, Macia-Soler L, Andreu-Guillamon MV, Moncho J. Patient education during hospital admission due to exacerbation of chronic obstructive pulmonary disease: effects on quality of life - controlled and randomized experimental study. Patient Educating Counselling 2018;102(3):511-9. [DOI: 10.1016/j.pec.2018.09.013] [DOI] [PubMed] [Google Scholar]
Fu 2018 {published data only}
- Fu MMM, Lau KM. A self-management programme of rescue pack for chronic obstructive pulmonary disease (COPD) patients. Respirology 2017;22(104):AP048. [DOI: 10.1111/resp.13207_49] [DOI] [Google Scholar]
Garcia 2007 {published data only}
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101(7):1462-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
George 2019 {published data only}
- George J, Liang J, Abramson M, Russell G, Holland A, Zwar N, et al. Interdisciplinary model of care for COPD in Australian primary care. Respirology 2019;24(Suppl 1):79 [TO 107]. [Google Scholar]
Gohl 2006 {published data only}
- Gohl O, Linz H, Schonleben T, Otte B, Weineck J, Worth H. Benefits of a multimodular outpatient training program for patients with COPD [Effekte eines multimodularen ambulanten Trainingsprogramms fur Patienten mit COPD]. Pneumologie 2006;60(9):529-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldstein 1994 {published data only}
- Goldstein RS, Gort EH, Stubbing D, Avendano MA, Guyatt GH. Randomised controlled trial of respiratory rehabilitation. Lancet 1994;344(8934):1394-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guell 2008 {published data only}
- Guell MR, Lucas P, Galdiz JB, Montemayor T, Rodriguez Gonzalez-Moro JM, Gorostiza A, et al. Home vs hospital-based pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: a Spanish multicenter trial [Comparacion de un programa de rehabilitacion domiciliario con uno hospitalario en pacientes con EPOC: estudio multicentrico espanol]. Archivos de Bronconeumologia 2008;44(10):512-8. [PMID: ] [PubMed] [Google Scholar]
Hajizadeh 2020 {published data only}
- Hajizadeh N, Polo J, Ordonez K, Williams M, Tsang D, Zhang M, et al. Referral to telehealth delivered pulmonary rehabilitation (TelePR) versus standard pulmonary rehabilitation (SPR) in Hispanic and African patients hospitalized for COPD exacerbations: results of a randomized controlled trial. In: American Journal of Respiratory and Critical Care Medicine. Vol. Conference: American Thoracic Society International Conference, ATS 2020. United States. 2020.
Heidari 2018 {published data only}
- Heidari M, Fayazi S, Borsi SH, Mahmoud L, Moradbeigi K, Torabpour Torghi M, et al. Effect of the 5A model on clinical status indexes of COPD patients. Rehabilitation Nursing Journal 2018;43(3):158-66. [DOI: 10.1097/rnj.0000000000000012] [DOI] [PubMed] [Google Scholar]
Hernandez 2004 {published data only}
- Hernandez C, Pascual-Tape T, Soler N, Marti D, Esquerdo M, Olmos C, et al. Effects of an integrated care program on quality of life in patients with stable chronic obstructive pulmonary disease (COPD). Archivos de Bronconeumologia (Journal of the Spanish Society of Pulmonology and Thoracic Surgery) 2004;40:24. [Google Scholar]
Hughes 2000 {published data only}
- Hughes SL, Weaver FM, Giobbie-Hurder A, Manheim L, Henderson W, Kubal JD, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA 2000;284(22):2877-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
IRCT20160914029817N {published data only}
- IRCT20160914029817N. Effect of non-drug palliative care on quality of life in patients with chronic obstructive pulmonary disease. en.irct.ir/trial/31684 (first received 15 June 2018).
Jakobsen 2015 {published data only}
- Jakobsen AS, Laursen LC, Rydahl-Hansen S, Østergaard B, Gerds TA, Emme C, et al. Home-based telehealth hospitalization for exacerbation of chronic obstructive pulmonary disease: findings from "the virtual hospital" trial. Telemedicine and E Health 2015;21(1):364-73. [DOI: 10.1089/tmj.2014.0098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2020 {published data only}
- Jiang Y, Liu F, Guo J, Sun P, Chen Z, Li J, et al. Evaluating an intervention program using webchat for patients with chronic obstructive pulmonary disease: randomized controlled trial. Journal of Medical Internet Research 2020;22(4):e17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolly 2018 {published data only}
- Jolly K, Sidhu MS, Hewitt CA, Coventry PA, Daley A, Jordan R, et al. Self management of patients with mild COPD in primary care: randomised controlled trial. BMJ 2018;361:k2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2009 {published data only}
- Jones SM, Albert P, Warburton CJ, Calverley PMA, Davies L. Effect of a case management study on primary care use and prescribing for AECOPD. Thorax 2009;64(Suppl 4):A119 [P105]. [Google Scholar]
JPRN‐UMIN000034582 {published data only}
- JPRN-UMIN000034582. Randomized controlled trial of the ninjinyoeito for the COPD patient of frailty or prefrailty. rctportal.niph.go.jp/en/detail?trial_id=UMIN000034582 (first received 26 October 2017).
Khdour 2011 {published data only}
- Khdour MR, Agus AM, Kidney JC, Smyth BM, Elnay JC, Crealey GE. Cost-utility analysis of a pharmacy-led self-management programme for patients with COPD. International Journal of Clinical Pharmacy 2011;33(4):665-73. [DOI] [PubMed] [Google Scholar]
Lahham 2018 {published data only}
- Lahham A, McDonald CF, Mahal A, Lee AL, Hill CJ, Burge AT, et al. Acceptability and validity of a home exercise diary used in home-based pulmonary rehabilitation: a secondary analysis of a randomised controlled trial. Clinical Respiratory Journal 2018;12(6):20. [DOI] [PubMed] [Google Scholar]
Lainscak 2013 {published data only}
- Lainscak M, Kadivec S, Kosnik M, Benedik B, Bratkovic M, Jakhel T, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: a randomized controlled trial. Journal of the American Medical Directors Association 2013;14(6):450.e1-450.e6. [DOI: 10.1016/j.jamda.2013.03.003] [DOI] [PubMed] [Google Scholar]
Lavoie 2017 {published data only}
- Lavoie K, Sedeno M, Li P, Troosters T, Hamilton H, De Sousaet D, et al. Effects of bronchodilator therapy and exercise with self-management behaviour-modification on psychological and cognitive outcomes in COPD. European Respiratory Journal 2017;50(Suppl 61):OA4669. [DOI: 10.1183/1393003.congress-2017.OA4669] [DOI] [Google Scholar]
Li 2019 {published data only}
- Li XJ, Kang Y, Wang RR, Liao XL, Ou XF, Liu J, et al. The effects of safflower yellow on acute exacerbation of chronic obstructive pulmonary disease: a randomized, controlled clinical trial. Evidence-Based Complementary and Alternative Medicine 2019;2019:5952742. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2019 {published data only}
- Liang J, Abramson MJ, Russell G, Holland AE, Zwar NA, Bonevski B, et al. Interdisciplinary COPD intervention in primary care: a cluster randomised controlled trial. European Respiratory Journal 2019;53(4):1801530. [DOI] [PubMed] [Google Scholar]
Linden 2014 {published data only}
- Linden A, Butterworth SW. A comprehensive hospital-based intervention to reduce readmissions for chronically ill patients: a randomized controlled trial. American Journal of Managed Care 2014;20(10):783-92. [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu YF. Effects of the comprehensive pulmonary rehabilitation programme on the quality of life of the patients with COPD in recovery period (Chinese). Chinese Journal of Clinical Rehabilitation 2002;21:3170-1. [Google Scholar]
Liu 2019 {published data only}
- Liu H, Song M, Zhai ZH, Shi RJ, Zhou XL. Group singing improves depression and life quality in patients with stable COPD: a randomized community-based trial in China. Quality of Life Research 2019;28(3):725-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorig 2006 {published data only}
Ly 2018 {published data only}
- Ly O, Sibbald SL, Verma JY, Rocker G. Exploring role clarity in interorganizational spread and scale-up initiatives: the 'INSPIRED' COPD collaborative. BMC Health Services Research 2018;18(1):680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltais 2008 {published data only}
- Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2008;149(12):869-78. [PMID: ] [DOI] [PubMed] [Google Scholar]
Markun 2018 {published data only}
- Markun S, Rosemann T, Dalla-Lana K, Steurer-Stey C. Care in chronic obstructive lung disease (CAROL): a randomised trial in general practice. European Respiratory Journal 2018;51(5):1701873. [DOI: 10.1183/13993003.01873-2017] [DOI] [PubMed] [Google Scholar]
Martin 2004 {published data only}
- Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chronic Respiratory Disease 2004;1(4):191-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martinez 2014 {published data only}
- Martinez CH, Moy ML, Nguyen HQ, Cohen M, Kadri R, Roman P, et al. Taking Healthy Steps: rationale, design and baseline characteristics of a randomized trial of a pedometer-based Internet-mediated walking program in veterans with chronic obstructive pulmonary disease. BMC Pulmonary Medicine 2014;14:12. [DOI: 10.1186/1471-2466-14-12] [DOI] [PMC free article] [PubMed]
McGeoch 2006 {published data only}
- McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11(5):611-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Monninkhof 2003 {published data only}
- Monninkhof E, Valk P, Palen J, Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(5):815-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moy 2014 {published data only}
- Moy ML, Collins R, Martinez CH, Kadri R, Roman P, Holleman RG, et al. An internet-mediated, pedometer-based walking program improves HRQL in veterans with COPD (abstract). American Journal of Respiratory and Critical Care Medicine 2014;189:A3642. [Google Scholar]
Moy 2016 {published data only}
- Moy ML, Martinez CH, Kadri R, Roman P, Holleman RG, Hyungjin MK, et al. Long-term effects of an Internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. Journal of Medical Internet Research 2016;18(8):e215. [DOI: 10.2196/jmir.5622] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muelepas 2007 {published data only}
- Muelepas MS, Jacobs JE, Smeenk F, Smeele I, Lucas AEM, Bottema B, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scandinavian Journal of Primary Health Care 2007;25:186-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03794921 {published data only}
- NCT03794921. COPD access to pulmonary rehabilitation intervention (CAPRI) [Leveraging technology to address access and adherence to conventional hospital-based pulmonary rehabilitation in veterans with COPD]. clinicaltrials.gov/show/nct03794921 (first received 7 January 2019).
NCT03889054 {published data only}
- NCT03889054. Effect of education and respiratory rehabilitation on quality of life in COPD patients (EDUEPOC). clinicaltrials.gov/show/nct03889054 (first received 25 March 2019).
NCT04260178 {published data only}
- NCT04260178. Education-based intervention program for persons with chronic obstructive pulmonary disease (EBIPCOPD) [The impact of an education-based intervention program (EBIP) on dyspnea and chronic self-care management among COPD patients: a randomized controlled study]. clinicaltrials.gov/ct2/show/NCT03889054 (first received 4 March 2019).
NCT04348344 {published data only}
- NCT04348344. Study on the prevention and control system of chronic airway diseases, 2020. clinicaltrials.gov/show/NCT04348344 (first received 1 June 2020).
NCT04437238 {published data only}
- NCT04437238. Pilot evaluation of "KeepWell" using a hybrid effectiveness-implementation pragmatic randomized controlled trial [Effectiveness of an eHealth tool for older adults with multimorbidity ("KeepWell"): protocol for a hybrid effectiveness-implementation, pragmatic randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT04437238 (first received 18 June 2020).
NCT04459546 {published data only}
- NCT04459546. Nurse-led COPD self-management Intervention [The effect of rational drug use and symptom control training on self-efficacy, emotional status and clinical parameters in COPD (chronic obstructive pulmonary disease)]. clinicaltrials.gov/show/NCT04459546 (first received 7 July 2020).
Nguyen 2019 {published data only}
- Nguyen HQ, Liu I, Moy M, Fan VS, Gould MK, Desai S, et al. Effect of physical activity coaching on acute care utilization and survival in COPD: a pragmatic controlled trial (walk on!). American Journal of Respiratory and Critical Care Medicine 2019;199(9):A2645. [Google Scholar]
North 2018 {published data only}
- North M, Bourne S, Green B, Chauhan A, Brown T, Winter J, et al. A randomised controlled feasibility trial of an e-health platform supported care vs usual care after exacerbation of COPD. (Rescue COPD). Thorax 2018;73(Suppl 4):A231. [Google Scholar]
Nyberg 2017 {published data only}
- Nyberg A, Wadell K, Lindgren H, Tistad M. Internet-based support for self-management strategies for people with COPD-protocol for a controlled pragmatic pilot trial of effectiveness and a process evaluation in primary healthcare. BMJ Open 2017;7(7):e016851. [DOI: 10.1136/bmjopen-2017-016851] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabinovich 2017 {published data only}
- Demeyer H, Louvaris Z, Frei A, Rabinovich RA, Jong C, Gimeno-Santos E, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017;72(5):415-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radini 2017 {published data only}
- Radini D, Apuzzo GM, Stellato K, Sola G, Delli Quadri N, Fragiacomo E, et al. Home telemonitoring in patients with heart failure: the experience of a region of northern Italy in the EU funded project smartcare. European Journal of Heart Failure. 2017;19(suppl.S1):690. [Google Scholar]
Rausch‐Osthoff 2017 {published data only}
- Rausch-Osthoff AK, Greco N, Schwank A, Beyer S, Gisi D, Scheermesser M, et al. Effect of counselling during pulmonary rehabilitation on self-determined motivation towards physical activity in people with chronic obstructive pulmonary disease - protocol of a mixed methods study. BMC Pulmonary Medicine 2017;17:115. [DOI: 10.1186/s12890-017-0457-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
RBR‐533hht {published data only}
- RBR-533hht. Results of functional tests on supervised rehabilitation program and education program in patients with chronic obstructive pulmonary disease. www.ensaiosclinicos.gov.br/rg/RBR-533hht/ (first received 15 December 2018).
Renn 2018 {published data only}
- Renn BN, Hundt NE, Sansgiry S, Petersen NJ, Kauth MR, Kunik ME, et al. Integrated brief cognitive behavioral therapy improves illness intrusiveness in veterans with chronic obstructive pulmonary disease. Annals of Behavioral Medicine 2018;52(8):686-96. [DOI: 10.1093/abm/kax045] [DOI] [PubMed] [Google Scholar]
Ries 2003 {published data only}
- Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2003;167(6):880-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ringbaek 2015 {published data only}
- Ringbaek T, Green A, Laursen LC, Frausing E, Brondum E, Ulrik CS. Effect of tele health care on exacerbations and hospital admissions in patients with chronic obstructive pulmonary disease: a randomized clinical trial. International Journal of Chronic Obstructive Pulmonary Disease 2015;10(Suppl 59):1801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rixon 2017 {published data only}
Robinson 2020 {published data only}
- Robinson SA, Goldstein RL, Cruz Rivera PN, Kadri R, Cooper JD, Richardson CR, et al. Results from a multi-site web-based physical activity intervention in COPD: between group and site differences. American Journal of Respiratory and Critical Care Medicine 2020;201:A2507. [Google Scholar]
Rotter 2017 {published data only}
- Rotter T, Plishka C, Hansia MR, Goodridge D, Penz E, Kinsman L, et al. The development, implementation and evaluation of clinical pathways for chronic obstructive pulmonary disease (COPD) in Saskatchewan: protocol for an interrupted times series evaluation. BMC Health Services Research 2017;17(1):782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2018 {published data only}
- Schmidt B, Bourbeau J, Sedeno M, Li P, Troosters T, Hamilton A, et al. Impact of meeting behavioral targets in a self-management behaviour modification program designed to improve physical activity in COPD patients. In: Kongress der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin e.V; 2018 March 14-17; Dresden. 2018.
Scuffham 2018 {published data only}
- Scuffham PA, Byrnes JM, Pollicino C, Cross D, Goldstein S, Ng SK. The impact of population-based disease management services on health care utilisation and costs: results of the CAPICHe trial. Journal of General Internal Medicine 2018;34(1):41-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selzler 2019 {published data only}
- Selzler AM, Jourdain T, Wald J, Sedeno M, Janaudis-Ferreira T, et al. Evaluation of the Canadian standardized rehabilitation efficacy (CONSPIRE) trial. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine 2019;3(Suppl 1):47-8. [Google Scholar]
Sidhu 2015 {published data only}
- Sidhu MS, Daley A, Jordan R, Coventry PA, Heneghan C, Jowett S, et al. Patient self-management in primary care patients with mild COPD - protocol of a randomised controlled trial of telephone health coaching. BMC Pulmonary Medicine 2015;16:15. [DOI: 10.1186/s12890-015-0011-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soler 2006 {published data only}
- Soler JJ, Martinez-Garcia MA, Roman P, Orero R, Terrazas S, Martinez-Pechuan A. Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations [Eficacia de un programa especifico para pacientes con EPOC que presentan frecuentes agudizaciones]. Archivos de Bronconeumologia 2006;42(10):501-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sorensen 2016 {published data only}
- Sorensen SS, Pedersen KM, Weinreich UM, Ehlers L. Economic evaluation of community-based case management of patients suffering from chronic obstructive pulmonary disease. Applied Health Economics and Health Policy 2017;15(3):413-24. [DOI] [PubMed] [Google Scholar]
Soriano 2018 {published data only}
- Soriano JB, Garcia-Rio F, Vazquez Espinosa E, Díaz de Atauri J, López Yepes L, Galera Martínez R, et al. Efficacy and costs of telehealth for the management of COPD: a multicenter, randomized controlled trial. European Respiratory Journal 2018;50:OA4665. [DOI: 10.1183/1393003.congress-2017.OA4665] [DOI] [PubMed] [Google Scholar]
Stamenova 2020 {published data only}
- Stamenova V, Liang K, Yang R, Engel K, Lieshout F, Lalingo E, et al. Technology-enabled self-management of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: randomized controlled trial. Journal of Medical Internet Research 2020;22(7):e18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steele 2008 {published data only}
- Steele BG, Belza B, Cain KC, Coppersmith J, Lakshminarayan S, Howard J, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Archives of Physical Medicine and Rehabilitation 2008;89(3):404-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stenlund 2019 {published data only}
- Stenlund T, Nyberg A, Lundell S, Wadell K. Web-based support for self-management strategies versus usual care for people with COPD in primary healthcare: a protocol for a randomised, 12-month, parallel-group pragmatic trial. BMJ Open 2019;9(10):e030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steurer‐Stey 2018 {published data only}
- Steurer-Stey C, Lana KD, Braun J, Riet GT, Puhan MA. Effects of the "Living well with COPD" intervention in primary care: a comparative study. European Respiratory Journal 2018;51(1):1701375. [DOI: 10.1183/13993003.01375-2017] [DOI] [PubMed] [Google Scholar]
Thom 2019 {published data only}
- Thom DH, Willard-Grace R, Tsao S, Hessler D, Huang B, DeVore D, et al. Randomized controlled trial of health coaching for vulnerable patients with chronic obstructive pulmonary disease (COPD). Annals of the American Thoracic Society 2018;15(10):1159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thurber 2018 {published data only}
- Thurber EG, Aboumatar H. Do patient comorbidities impact the effectiveness of a COPD self-management program? Journal of Clinical and Translational Science 2018;2(Suppl 1):41. [DOI: 10.1017/cts.2018.163] [DOI] [Google Scholar]
Torre 2018 {published data only}
- Torre GLA, Cocchiara RA, Sordo ELO, Chiarini M, Siliquini R, Firenze A, et al. Counseling intervention to improve quality of life in patients with pre-existing acute myocardial infarction (AMI) or chronic obstructive pulmonary disease (COPD): a pilot study. Journal of Preventive Medicine and Hygiene 2018;59(2):E153-8. [PMC free article] [PubMed]
Troosters 2016 {published data only}
- Troosters T, Bourbeau J, Maltais F, Leidy N, Erzen D, De Sousa D, et al. Effect of 8 and 12 weeks' once-daily tiotropium and olodaterol, alone and combined with exercise training, on exercise endurance during walking in patients with COPD. European Respiratory Journal 2016;48(Suppl 60):PA976. [DOI: 10.1183/13993003.congress-2016.PA976] [DOI] [Google Scholar]
van der Weegen 2015 {published data only}
- Weegen S, Verwey R, Spreeuwenberg M, Tange H, Weijden T, Witte L. It's life! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. Journal of Medical Internet Research 2015;17(7):e184. [DOI: 10.2196/jmir.4579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Genugten 2016 {published data only}
- Van Genugten L, Priori R, Barretto C, Schonenberg H, Dekker M, Klee M, Saini P. An online intervention to maintain physical activity levels in COPD patients after pulmonary rehabilitation. Bulletin of the European Health Psychology Society 2016;18(Suppl):635. [Google Scholar]
Varas 2018 {published data only}
- Varas AB, Cordoba S, Rodriguez-Andonaegui I, Rocío Rueda M, García-Juez S, Vilaro J. Effectiveness of a community-based exercise training programme to increase physical activity level in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Physiotherapy Research International 2018;23(4):e1740. [10.1002/pri.1740] [DOI] [PubMed] [Google Scholar]
Voncken‐Brewster 2015 {published data only}
- Voncken-Brewster V, Tange H, Vries H, Nagykaldi Z, Winkens B, Weijden T. A randomized controlled trial evaluating the effectiveness of a web-based, computer-tailored self-management intervention for people with or at risk for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1061-73. [DOI: 10.2147/COPD.S81295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2018 {published data only}
- Walker PP, Pompilio PP, Zanaboni P, Bergmo TS, Prikk K, Malinovschi A. Telemonitoring in chronic obstructive pulmonary disease (CHROMED). A randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2018;198(5):620-8. [DOI: 10.1164/rccm.201712-2404OC] [DOI] [PubMed] [Google Scholar]
Walters 2013 {published data only}
- Walters J, Cameron-Tucker H, Wills K, Schuz N, Scott J, Robinson A, et al. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ Open 2013;3(9):e003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang LH, Zhao Y, Chen LY, Zhang L, Zhang YM. The effect of a nurse-led self-management program on outcomes of patients with chronic obstructive pulmonary disease. Clinical Respiratory Journal 2020;14(2):148-57. [DOI] [PubMed] [Google Scholar]
Waterhouse 2010 {published data only}
- Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA. A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up. Health Technology Assessment 2010;14(6):1-164. [DOI] [PubMed] [Google Scholar]
Welch 2020 {published data only}
- Welch L, Orlando R, Lin SX, Vassilev I, Rogers A. Findings from a pilot randomised trial of a social network self-management intervention in COPD. BMC Pulmonary Medicine 2020;20(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weldam 2017 {published data only}
- Weldam SWM, Schuurmans MJ, Zanen P, Heijmans M, Sachs APE, Lammers JJ. The effectiveness of a nurse-led illness perception intervention in COPD patients: a cluster randomised trial in primary care. ERJ Open Research 2017;3(4):00115-2016. [DOI: 10.1183/23120541.00115-2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wootton 2019 {published data only}
- Wootton SL, Hill K, Alison JA, Ng LWC, Jenkins S, Eastwood PR, et al. Effects of ongoing feedback during a 12-month maintenance walking program on daily physical activity in people with COPD. Lung 2019;197(3):315-19. [DOI] [PubMed] [Google Scholar]
Wu 2018 {published data only}
- Wu W, Liu X, Li P, Li N. Effect of liuzijue exercise combined with elastic band resistance exercise on patients with COPD: a randomized controlled trial. Evidence-Based Complementary and Alternative Medicine 2018;6:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xi 2014 {published data only}
- Li JM, Cheng SZ, Cai W, Zhang ZH, Liu QH, Xie BZ, et al. Transitional care for patients with chronic obstructive pulmonary disease. International Journal of Nursing Sciences 2014;1(2):157-64. [DOI: 10.1016/j.ijnss.2014.05.004] [DOI] [Google Scholar]
Yoon 2018 {published data only}
- Yoon J, Chang E, Rubenstein LV, Park A, Zulman D, Stockdale S, et al. Impact of primary care intensive management on high-risk veterans' costs and utilization: a randomized quality improvement trial. Annals of Internal Medicine 2018;168(12):846-54. [DOI: 10.7326/M17-3039] [DOI] [PubMed] [Google Scholar]
Zakrisson 2019 {published data only}
- Zakrisson A, Theander K, Arne M, Hasselgren M, Lisspers K, Stallberg B. A complex intervention of self‐management for patients with COPD or CHF in primary care improved performance and satisfaction with regard to own selected activities: a longitudinal follow‐up. Journal of Advanced Nursing 2019;75(1):175-86. [DOI] [PubMed] [Google Scholar]
Zhou 2010 {published data only}
- Zhou Y, Hu G, Wang D, Wang S, Wang Y, Liu Z, et al. Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. BMJ 2010;341:c6387. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2017 {published data only}
- Zhou L, Li X, Guan L, Chen J, Guo B, Wu W, et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:1279-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baumann 2012 {published data only}
- Baumann HJ, Kluge S, Rummel K, Klose H, Hennigs JK, Schmoller T, et al. Low intensity, long-term outpatient rehabilitation in COPD: a randomised controlled trial. Respiratory Research 2012;13:86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borji, 2018 {published data only}
- Borji M, Salimi E. The effect of adaptive sustainability care model on readmission in patients with COPD: a randomized clinical trial. Iranian Journal of Allergy, Asthma and Immunology 2018;17:p204-5.
Carcereny, 2016 {published data only}
- Carcereny CH, Seijas N, Folch A, Roman M, Orts I, Aibar J, et al. Impact of therapeutic education program for COPD patients on preventing readmissions (APRENDEEPOC). European Respiratory Journal 2016;48(suppl 60):PA1614. [DOI: 10.1183/13993003.congress-2016.PA1614] [DOI] [Google Scholar]
Mao 2020 {published data only}
- Mao Q, Yan L. The clinical efficacy of evidence-based nursing in elderly patients with chronic obstructive pulmonary disease combined with heart failure. International Journal of Clinical and Experimental Medicine 2020;13(2):1140-7. [Google Scholar]
NCT04256070 {published data only}
- NCT04256070. Effect of education and tele-consultancy intervention based on Watson human care theory individuals with COPD. clinicaltrials.gov/show/NCT04256070 (first received 5 February 2020).
Reguera 2017 {published data only}
- Reguera BJ, Lopez EM, Martin ML, Monteagudo LJ, Gutierrez NG, Sanchez M, et al. Efficacy of an integrated internet community program after pulmonary rehabilitation for COPD patients: a pilot randomized control trial RTY. In: European Respiratory Journal. Vol. 50. 2017:OA514.
Xu 2010 {published data only}
- Xu YH, Wang JH, Li HF, Zhu XH, Wang G. Efficacy of integrative respiratory rehabilitation training in exercise ability and quality of life of patients with chronic obstructive pulmonary disease in stable phase: a randomized controlled trial]. [Chinese]. Zhong Xi Yi Jie He Xue Bao 2010;8(5):432-7. [DOI: 10.3736/jcim20100506] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Ali 2020 {published data only}
- Ali L, Wallstrom S, Barenfeld E, Fors A, Fredholm E, Gyllensten H, et al. Person-centred care by a combined digital platform and structured telephone support for people with chronic obstructive pulmonary disease and/or chronic heart failure: study protocol for the PROTECT randomised controlled trial. BMJ Open 2020;10(7):e036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourne 2017 {published data only}
- Bourne CLA, Kanabar P, Mitchell K, Schreder S, Houchen-Wolloff L, Bankart M, et al. A self-management programme of activity coping and education - SPACE for COPD(C) - in primary care: the protocol for a pragmatic trial. BMJ Open 2017;7(7):e014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ding 2019 {published data only}ACTRN12618001091291
- Ding H, Karunanithi M, Ireland D, McCarthy L, Hakim R, Phillips K, et al. Evaluation of an innovative mobile health programme for the self-management of chronic obstructive pulmonary disease (MH-COPD): protocol of a randomised controlled trial. BMJ Open 2019;9(4):e025381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drennan 2014 {published data only}
- Expanding Paramedicine in the Community (EPIC) study. Ongoing study. Starting date: June 2013; estimated completion date: December 2016. Contact author for more information.
Foot 2017 {published data only}
- Foot H, Freeman C, Hemming K, Scott I, Coombes ID, Williams ID, et al. Reducing Medical Admissions into Hospital through Optimising Medicines (REMAIN HOME) Study: protocol for a stepped-wedge, cluster-randomised trial. BMJ Open 2017;7(4):e015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajizadeh 2020a {published data only}
- Hajizadeh N, Polo J, Ordonez K, Williams M, Tsang D, Zhang M, et al. Referral to telehealth delivered pulmonary rehabilitation (TelePR) versus standard pulmonary rehabilitation (SPR) in Hispanic and African patients hospitalized for COPD exacerbations: results of a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2020;201(1):A6127. [Google Scholar]
Hansen 2017 {published data only}
- Hansen H, Bieler T, Beyer N, Godtfredsen N, Kallemose T, Frølich A. COPD online-rehabilitation versus conventional COPD rehabilitation - rationale and design for a multicenter randomized controlled trial study protocol (CORe trial). BMC Pulmonary Medicine 2017;17(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04136418 {published data only}
- NCT04136418. Predict & Prevent: use of a personalised early warning decision support system to predict and prevent acute exacerbations of COPD [A randomised designed clinical investigation of the use of a personalised early warning decision support system with novel saliva bio-profiling to predict and prevent acute exacerbations of chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT04136418 (first received 23 October 2019).
NCT04416295 {published data only}
- NCT04416295. Selfcare MAnagement InteRvenTion in COPD (SMART COPD) [Evaluation of a digital support and communication platform for COPD]. clinicaltrials.gov/ct2/show/NCT04416295 (first received 4 June 2020).
NCT04533412 {published data only}
- NCT04533412. Comprehensive self-management support for COPD patients (SAMBA COPD). clinicaltrials.gov/ct2/show/NCT04533412 (first received 31 August 2020).
Steed 2017 {published data only}
- Steed L, Sohanpal R, Heslop-Marshall K, Saqi-Waseem S, Barradell A, Pinnock H, et al. Development of the TANDEM intervention for chronic obstructive pulmonary disease: reporting on the pre-pilot phase. European Respiratory Journal 2017;50(Suppl 61):PA944. [DOI: 10.1183/1393003.congress-2017.PA944] [DOI] [Google Scholar]
Additional references
Adams 2007
- Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Archives of Internal Medicine 2007;167(6):551-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agusti 2010
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research 2010;11:122. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Attkisson 2004
- Attkisson C, Greenfield T. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Mahwah, NJ: Lawrence Erlbaum Associates, 2004. [Google Scholar]
Badamgarav 2003
- Badamgarav E, Weingarten SR, Henning JM, Knight K, Hasselblad V, Gano A Jr, et al. Effectiveness of disease management programs in depression: a systematic review. American Journal of Psychiatry 2003;160(12):2080-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bestall 1999
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54(7):581-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bongaerts 2017
- Bongaerts BWC, Mussig K, Wens J, Lang C, Schwarz P, Roden M, et al. Effectiveness of chronic care models for the management of type 2 diabetes mellitus in Europe: a systematic review and meta-analysis. BMJ Open 2017;7(3):e013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borg 1970
- Borg G. Perceived exertion as an indicator of somatic stress. Scandinavian Journal of Rehabilitation Medicine 1970;2(2):92-8. [PMID: ] [PubMed] [Google Scholar]
Bourbeau 2013
- Bourbeau J, Saad N. Integrated care model with self-management in chronic obstructive pulmonary disease: from family physicians to specialists. Chronic Respiratory Disease 2013;10:93-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Britton 2003
- Britton M. The burden of COPD in the UK: results from the Confronting COPD survey. Respiratory Medicine 2003;97(Suppl C):S71-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burgel 2010
- Burgel PR, Paillasseur JL, Caillaud D, Tillie-Leblond I, Chanez P, Escamilla R, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. European Respiratory Journal 2010;36(3):531-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Calverley 2003
- Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet 2003;362(9389):1053-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cates 2015
- Cates C, Karner C. Clinical importance cannot be ruled out using mean difference alone. BMJ 2015/11/20;351:h5496. [DOI] [PubMed] [Google Scholar]
Chavannes 2008
- Chavannes NH. Integrated chronic obstructive pulmonary disease management in primary care. Disease Management and Health Outcomes 2008;16(5):1. [Google Scholar]
Cheikh‐Moussa 2020
- Cheikh-Moussa K, Mira JJ, Orozco-Beltran D. Improving engagement among patients with chronic cardiometabolic conditions using mHealth: critical review of reviews. JMIR mHealth uHealth 2020;8(4):e15446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cote 2009
- Cote CG, Celli BR. BODE index: a new tool to stage and monitor progression of chronic obstructive pulmonary disease. Pneumonologia i Alergologia Polska 2009;77(3):305-13. [PMID: ] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence systematic review software. Melbourne, Australia, Veritas Health Innovation (accessed 2 December 2019). Available at www.covidence.org.
Cox 2021
- Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013040. [DOI: 10.1002/14651858.CD013040.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cronin 2017
- Cronin J, Murphy A, Savage E. Can chronic disease be managed through integrated care cost-effectively? Evidence from a systematic review. Iranian Journal of Medial Science 2017;186:827-34. [DOI] [PubMed] [Google Scholar]
Domingo‐Salvany 2002
- Domingo-Salvany A, Lamarca R, Ferrer M, Garcia-Aymerich J, Alonso J, Felez M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(5):680-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Engstrom 1996
- Engstrom CP, Persson LO, Larsson S, Ryden A, Sullivan M. Functional status and well being in chronic obstructive pulmonary disease with regard to clinical parameters and smoking: a descriptive and comparative study. Thorax 1996;51(8):825-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2008
- Cochrane Effective Practice and Organization of Care Review Group. Data collection checklist. methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC%20Data%20Collection%20Checklist.pdf (accessed 4 October 2013).
EuroQol Group 1990
- The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fan 2002
- Fan VS, Curtis JR, Tu SP, McDonell MB, Fihn SD. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest 2002;122(2):429-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
FIRS 2017
- European Respiratory Society, on behalf of the Forum of International Respiratory Societies (FIRS). The global impact of respiratory disease, Second Edition. 2017. www.firsnet.org/images/publications/The_Global_Impact_of_Respiratory_Disease.pdf (accessed prior to 17 November 2020).
Gerardi 1996
- Gerardi DA, Lovett L, Benoit-Connors ML, Reardon JZ, ZuWallack RL. Variables related to increased mortality following out-patient pulmonary rehabilitation. European Respiratory Journal 1996;9(3):431-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glasgow 2005
- Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Medical Care 2005;43(5):436-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
GOLD 2009
- GOLD 2009. Global initiative for chronic obstructive pulmonary disease. Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2009. goldcopd.org/gold-reports/ (accessed 4 October 2013 ).
GOLD 2020
- GOLD 2020. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2020 report). goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (accessed 5 May 2020).
Gonseth 2004
- Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. European Heart Journal 2004;25(18):1570-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group 2004. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guarascio 2013
- Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoeconomic and Outcomes Research 2013;5:235-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 1987
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [DOI] [PubMed] [Google Scholar]
Herbert 2018
- Herbert RD, Kasza J, Bo K. Analysis of randomised trials with long-term follow-up. BMC Medical Research Methodology 2018;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2019
- Higgins JT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Chapter 9.5.2. Identifying and measuring heterogeneity. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. S eco nd edition. Chichester (UK): John Wiley & Sons, 2019:205-28. [Google Scholar]
Higgins 2019a
- Higgins JT, Li T, Deeks JJ. Chapter 23.1.3 . Methods of analysis for cluster-randomized trials. In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. S eco nd edition. Chichester (UK): John Wiley & Sons, 2019:143-76. [Google Scholar]
Higgins 2019b
- Higgins J T, Eldridge S, Li T. Chapter 16.5.4 . How to include multiple groups from one study c hapter ? In: Higgins JT, Thomas J, Chandler J, Cumpston M, Li J, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. S eco nd edition. Chichester (UK ): John Wi ley & Sons, 2019:569-93. [Google Scholar]
Hong 2019
- Hong Y, Lee SH. Effectiveness of tele-monitoring by patient severity and intervention type in chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. International Journal of Nursing Studies 2019;92:1-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Howcroft 2016
- Howcroft M, Walters EH, Wood-Baker R, Walters JAE. Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005074. [DOI: 10.1002/14651858.CD005074.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurst 2010
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Janjua 2020
- Janjua S, Fortescue R, Poole P. Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD002309. [DOI: 10.1002/14651858.CD002309.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85 Suppl B:25-31; discussion 33-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. European Respiratory Journal 2009;34(3):648-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Knight 2005
- Knight K, Badamgarav E, Henning JM, Hasselblad V, Gano AD Jr, Ofman JJ, et al. A systematic review of diabetes disease management programs. American Journal of Managed Care 2005;11(4):242-50. [PMID: ] [PubMed] [Google Scholar]
Kocks 2006
- Kocks JW, Tuinenga MG, Uil SM, den Berg JW, Stahl E, Molen T. Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respiratory Research 2006;7:62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruse 2019
- Kruse C, Pesek B, Anderson M, Brennan K, Comfort H. Telemonitoring to manage chronic obstructive pulmonary disease: systematic literature review. JMIR Medical Informatics 2019;7(1):e11496. [DOI: 10.2196/11496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemmens 2009
- Lemmens KM, Nieboer AP, Huijsman R. A systematic review of integrated use of disease-management interventions in asthma and COPD. Respiratory Medicine 2009;103(5):670-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lemmens 2013
- Lemmens KMM, Lemmens LC, Boom JHC, Drewes HW, Meeuwissen JAC, Steuten LMG, et al. Chronic care management for patients with COPD: a critical review of available evidence. Journal of Evaluation in Clinical Practice 2013;19(5):734-52. [DOI] [PubMed] [Google Scholar]
Lenferink 2017
- Lenferink A, Brusse-Keizer M, Valk PDLPM, Frith PA, Zwerink M, Monninkhof EM, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD011682. [DOI: 10.1002/14651858.CD011682.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Rouzic 2018
- Le Rouzic O, Roche N, Cortot AB, Tillie-Leblon, Masure F, Perez T, et al. Defining the "Frequent Exacerbator" phenotype in COPD: a hypothesis-free approach. Chest 2018;153(5):1106-15. [DOI] [PubMed] [Google Scholar]
Long 2019
- Long H, Howells K, Peters S, Blakemore A. Does health coaching improve health-related quality of life and reduce hospital admissions in people with chronic obstructive pulmonary disease? A systematic review and meta-analysis. British Journal of Health Psychology 2019;24:515-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. European Respiratory Journal 2006;27(2):397-412. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2095-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maqsood 2019
- Maqsood U, Ho TN, Palmer K, Eccles FJR, Munavvar M, Wang R, et al. Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD012930. [DOI: 10.1002/14651858.CD012930.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maric 2009
- Maric B, Kaan A, Ignaszewski A, Lear SA. A systematic review of telemonitoring technologies in heart failure. European Journal of Heart Failure 2009;11:506-17. [DOI] [PubMed] [Google Scholar]
Marsiglia 2015
- Marsiglia FF, Booth JM. Cultural adaptation of interventions in real practice settings. Research on Social Work Practice 2015;25(4):423-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2006
- Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. American Journal of Respiratory and Critical Care Medicine 2006;173(12):1326-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumeyer‐Gromen 2004
- Neumeyer-Gromen A, Lampert T, Stark K, Kallischnigg G. Disease management programs for depression: a systematic review and meta-analysis of randomized controlled trials. Medical Care 2004;42(12):1211-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Niesink 2007
- Niesink A, Trappenburg JC, Weert-van Oene GH, Lammers JW, Verheij TJ, Schrijvers AJ. Systematic review of the effects of chronic disease management on quality-of-life in people with chronic obstructive pulmonary disease. Respiratory Medicine 2007;101(11):2233-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Norris 2002
- Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. American Journal of Preventive Medicine 2002;22(Suppl 4):15-38. [PMID: ] [DOI] [PubMed] [Google Scholar]
Norris 2003
- Norris SL, Glasgow RE, Engelgau MM, O'Connor PJ, McCulloch D. Chronic disease management: a definition and systematic approach to component interventions. Disease Management and Health Outcomes 2003;11(8):477-88. [Google Scholar]
Peytremann‐Bridevaux 2008
- Peytremann-Bridevaux I, Staeger P, Bridevaux PO, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease-management programs: systematic review and meta-analysis. American Journal of Medicine 2008;121(5):433-43.e4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peytremann‐Bridevaux 2009
- Peytremann-Bridevaux I, Burnand B. Letter to the editor. Disease management: a proposal for a new definition. International Journal of Integrated Care 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peytremann‐Bridevaux 2014
- Peytremann-Bridevaux I, Taffe P, Burnand B, Bridevaux PO, Puhan MA. Mortality of patients with COPD participating in chronic disease management programmes: a happy end? Thorax 2014;69(9):865-6. [DOI] [PubMed] [Google Scholar]
Pimouguet 2010
- Pimouguet C, Le Goff M, Thiebaut R, Dartigues JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. Canadian Medical Association Journal 2010;183(2):E115-27. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto‐Plata 2004
- Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. European Respiratory Journal 2004;23(1):28-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puhan 2006
- Puhan MA, Soesilo I, Guyatt GH, Schunemann HJ. Combining scores from different patient reported outcome measures in meta-analyses: when is it justified? Health and Quality of Life Outcomes 2006;4:94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2008
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. European Respiratory Journal 2008;32(3):637-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puhan 2016
- Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005305. [DOI: 10.1002/14651858.CD005305.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 1992
- Rao JNK, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48:577-85. [PubMed] [Google Scholar]
Redelmeier 1997
- Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1278-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevManWeb). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Roccaforte 2005
- Roccaforte R, Demers C, Baldassarre F, Teo KK, Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. European Journal of Heart Failure 2005;7(7):1133-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schrijvers 2009
- Schrijvers G. Disease management: a proposal for a new definition. International Journal of Integrated Care 2009;9:e06. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seemungal 1998
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(5 Pt 1):1418-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Seemungal 2000
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1608-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 1992
- Singh S. The use of field walking tests for assessment of functional capacity in patients with chronic airways obstruction. Physiotherapy 1992;78:102-4. [Google Scholar]
Threapleton 2019
- Threapleton CJD, Janjua S, Fortescue R, Baker EH. Head-to-head oral prophylactic antibiotic therapy for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013024. [DOI: 10.1002/14651858.CD013024.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiep 1997
- Tiep BL. Disease management of COPD with pulmonary rehabilitation. Chest 1997;112(6):1630-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
van der Molen 2003
- Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health and Quality of Life Outcomes 2003;1:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2001
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affairs (Project Hope) 2001;20(6):64-78. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2019
- Walsh A, Perrem L, Khashan AS, Henry MT, Ni Chroinin M. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD011959. [DOI: 10.1002/14651858.CD011959.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Wedzicha 2000
- Wedzicha JA. The heterogeneity of chronic obstructive pulmonary disease. Thorax 2000;55(8):631-2. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WEISS 1985
- Weiss SJ, Davis HP. Validity and reliability of the collaborative practice scales. Nursing Research 1985;34(5):299-305. [PubMed] [Google Scholar]
WHO 2020
- World Health Organization. Global burden of disease. www.who.int/gho/mortality_burden_disease/en/ (accessed 22 May 2020).
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zwerink 2014
- Zwerink M, Brusse‐Keizer M, Valk PDLPM, Zielhuis GA, Monninkhof EM, Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD002990. [DOI: 10.1002/14651858.CD002990.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kruis 2011
- Kruis AL, Smidt N, Assendelft WJJ, Gussekloo J, Boland MRS, Rutten-van Mölken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD009437. [DOI: 10.1002/14651858.CD009437] [DOI] [PubMed] [Google Scholar]
Kruis 2013
- Kruis AL, Smidt N, Assendelft WJJ, Gussekloo J, Boland MRS, Rutten-van Mölken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD009437. [DOI: 10.1002/14651858.CD009437.pub2] [DOI] [PubMed] [Google Scholar]


