Significance Statement
Variants in the APOL1 gene are thought to be important contributors to a disparity in the incidence of ESKD among Black people, which is approximately three-fold higher than among White people. No specific treatment or management protocol for APOL1-associated nephropathy currently exists. Using a Delphi consensus process supported by a systematic literature review, a multidisciplinary group agreed on practical measures for care of patients who may have APOL1-associated nephropathy. The recommendations address three areas: (1) counseling, genotyping, and diagnosis; (2) disease awareness and education; and (3) a future vision for the management of patients with APOL1 high-risk genotypes. These recommendations may help clinicians improve awareness and diagnosis of APOL1-associated nephropathy and by doing so, may provide opportunities to reduce health disparities related to kidney disease.
Keywords: chronic kidney disease, end stage kidney disease, focal segmental glomerulosclerosis, genetic renal disease, HIV nephropathy, hypertension, lupus nephritis, pediatrics, polymorphisms, APOL1
Visual Abstract
Abstract
Background
APOL1 variants contribute to the markedly higher incidence of ESKD in Blacks compared with Whites. Genetic testing for these variants in patients with African ancestry who have nephropathy is uncommon, and no specific treatment or management protocol for APOL1-associated nephropathy currently exists.
Methods
A multidisciplinary, racially diverse group of 14 experts and patient advocates participated in a Delphi consensus process to establish practical guidance for clinicians caring for patients who may have APOL1-associated nephropathy. Consensus group members took part in three anonymous voting rounds to develop consensus statements relating to the following: (1) counseling, genotyping, and diagnosis; (2) disease awareness and education; and (3) a vision for management of APOL1-associated nephropathy in a future when treatment is available. A systematic literature search of the MEDLINE and Embase databases was conducted to identify relevant evidence published from January 1, 2009 to July 14, 2020.
Results
The consensus group agreed on 55 consensus statements covering such topics as demographic and clinical factors that suggest a patient has APOL1-associated nephropathy, as well as key considerations for counseling, testing, and diagnosis in current clinical practice. They achieved consensus on the need to increase awareness among key stakeholders of racial health disparities in kidney disease and of APOL1-associated nephropathy and on features of a successful education program to raise awareness among the patient community. The group also highlighted the unmet need for a specific treatment and agreed on best practice for management of these patients should a treatment become available.
Conclusions
A multidisciplinary group of experts and patient advocates defined consensus-based guidance on the care of patients who may have APOL1-associated nephropathy.
ESKD affects approximately 750,000 people in the United States and represents a major health disparity, with incidence approximately three-fold higher among Black people than among White people.1,2 Social determinants of health, racism, and genetic factors are understood to contribute to this disparity.3,4 Genetic risk factors include two independent coding variants in the APOL1 gene, G1 and G2, which are found almost exclusively in people with recent West sub-Saharan African ancestry.5,6 Two copies of the APOL1 variants (G1/G1, G1/G2, G2/G2) are commonly referred to as a “high-risk” genotype, although the resulting risk of CKD is estimated to be 15%–20%, which is moderate when compared with Mendelian forms of inherited nephropathy, such as polycystic kidney disease.1 Approximately 10%–15% of the Black population has a high-risk APOL1 genotype,7 and so, we can estimate that up to 3% of the Black population will be affected.1 The high-risk genotypes are found in approximately 16%–23% of Black people with nondiabetic CKD,8 indicating that APOL1 genotype alone does not account for the entirety of the excess incidence of CKD in the Black community.
APOL1 high-risk genotypes have been associated with several kidney diseases, including FSGS, HIV-associated nephropathy, hypertension-attributed ESKD, lupus nephritis, and sickle cell disease nephropathy.9 Here, we use the term “APOL1-associated nephropathy” to describe any nephropathy in people with a high-risk APOL1 genotype when there is no other known cause of kidney disease, although “APOL1 nephropathy” is also used by nephrologists in both research and clinical settings, and both terms are widely used in the literature. APOL1-associated nephropathy reflects a spectrum of kidney diseases caused by APOL1 risk variants. It can be superimposed on other forms of kidney disease, but often, it presents as glomerulosclerosis with accompanying tubulointerstitial and vascular changes.
A decade after the discovery of the APOL1 G1 and G2 variants, the mechanism by which they cause kidney injury in some individuals has not been fully elucidated, and interactions with environmental exposures and comorbidities remain unclear.10 Although the majority of people with a high-risk APOL1 genotype will not develop CKD, we cannot currently predict who will be affected. The two-hit hypothesis suggests that a high-risk APOL1 genotype could act as a first hit, but a second hit, such as modifier genes, HIV infection, SLE, or other comorbidity, may be required to initiate nephropathy.11 There is an urgent need for mathematical models that provide age-dependent predictions of CKD development in individuals with a high-risk APOL1 genotype and that also incorporate other CKD risk factors.
Genetic testing for APOL1 in African ancestry patients with nephropathy is currently uncommon. As such, many patients with APOL1-associated nephropathy who present with subnephrotic proteinuria are misclassified as having unspecified CKD or hypertension-attributed CKD, although intensive BP control does not slow kidney disease progression.9 Although genetic testing for APOL1 is performed in many transplantation centers for living kidney donors and recipients, significant controversy exists over whether APOL1 testing should be considered standard of care. There is currently no specific treatment or management protocol for APOL1-associated nephropathy, and so, the clinical value of APOL1 testing in routine clinical practice has not been established. The majority of Black people with CKD do not have APOL1 high-risk genotypes and so, are likely at lower risk for rapid progression to ESKD. This may provide a rationale for genetic testing to distinguish these individuals from those with APOL1-associated forms of kidney disease. Despite the current absence of a specific treatment for APOL1-associated nephropathy, most participants in community deliberations (all of whom identified as Black) have expressed a desire to be informed of their APOL1 genotype.12,13 However, there are serious ethical, practical, and financial issues to be considered before APOL1 genetic testing becomes routine.10 The ethical issues include the potential for discrimination in health care insurance, employment, and life insurance; stigmatization; and psychologic burdens. Research is needed to ensure the views and experiences of researchers, clinicians, and members of the Black public are identified and incorporated into a cohesive and inclusive testing and management protocol that includes robust patient protections.
This manuscript addresses the absence of a testing and management protocol by presenting consensus recommendations from a multidisciplinary group of experts on APOL1-associated nephropathy. Our consensus recommendations cover (1) counseling, genotyping, and diagnosis; (2) disease awareness and education; and (3) a future vision for the management of APOL1-associated nephropathy. These recommendations were established using a Delphi consensus process and are supported by evidence from a systematic literature search.
Methods
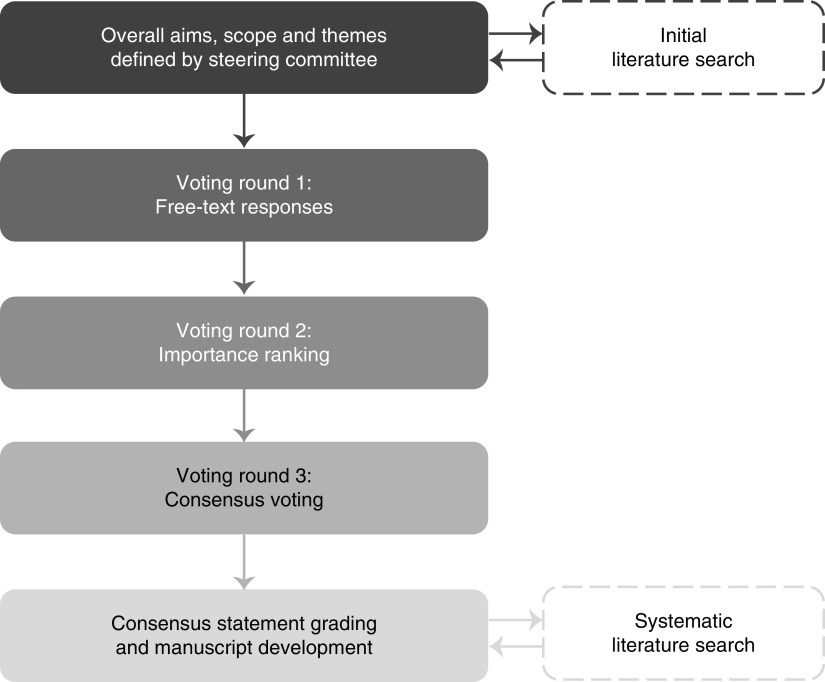
Consensus statements on APOL1-associated nephropathy were developed using a Delphi process, summarized in Figure 1. The overall aims, scope, and themes were defined by the Steering Committee: B.I.F. (nephrologist; nonvoting chair) and E.B. (bioethicist; voting member). Counseling, testing, and diagnosis of APOL1-associated nephropathy; disease awareness and education; and future vision were the central themes. Screening of the healthy population or kidney donors and recipients in transplantation was deemed outside the scope of this process.
Figure 1.
Overview of the Delphi consensus process. The Consensus Group (n=14) completed online surveys from July to December 2019 (rounds 1 and 2) and voted anonymously using electronic keypads at a face-to-face meeting in February 2020 (round 3; three participants were unable to attend and so, completed a blinded online survey instead).
Expert Panel participants, chosen by the Steering Committee to represent diverse specialties and experience in APOL1-associated nephropathy, were invited by email. The 13 members of the Expert Panel included two patient advocates and 11 specialists in adult and pediatric nephrology, cardiology, population health, medical genetics, biostatistics, and bioethics. This group was racially diverse and included eight persons of African descent. The Steering Committee guided the development of the surveys for each voting round, reviewed the collated responses and summaries, validated the systematic literature search, and critically evaluated the evidence. PharmaGenesis London supported the Steering Committee by carrying out literature searches, drafting and distributing surveys, and collating and analyzing responses. Surveys were completed by the voting members of the Steering Committee and the Expert Panel, collectively referred to as the Consensus Group. All responses were anonymous to the Steering Committee and members of the Expert Panel.
The Delphi process consisted of three rounds. Rounds 1 and 2 were conducted via online surveys (SurveyMonkey, San Mateo, CA) in July–December 2019, and the final round was conducted face to face at a meeting in February 2020. At this meeting, moderated by the nonvoting chair, the Steering Committee and the Expert Panel discussed the proposed consensus statements, and the nonvoting chair updated the wording as required on the basis of the group feedback before the group voted again and achieved consensus. The Consensus Group used electronic keypads to anonymously register their level of agreement with each statement on a five-point Likert scale: strongly agree, agree, neither agree nor disagree, disagree, or strongly disagree. These anonymous results were then shared with the group in real time. Three members of the Expert Panel were unable to attend the face-to-face meeting. Following the meeting, they reviewed the final statements and voted via a blinded online survey, which did not reveal the group results. The threshold for consensus was predefined as at least 75% of the Consensus Group voting “agree” or “strongly agree.”
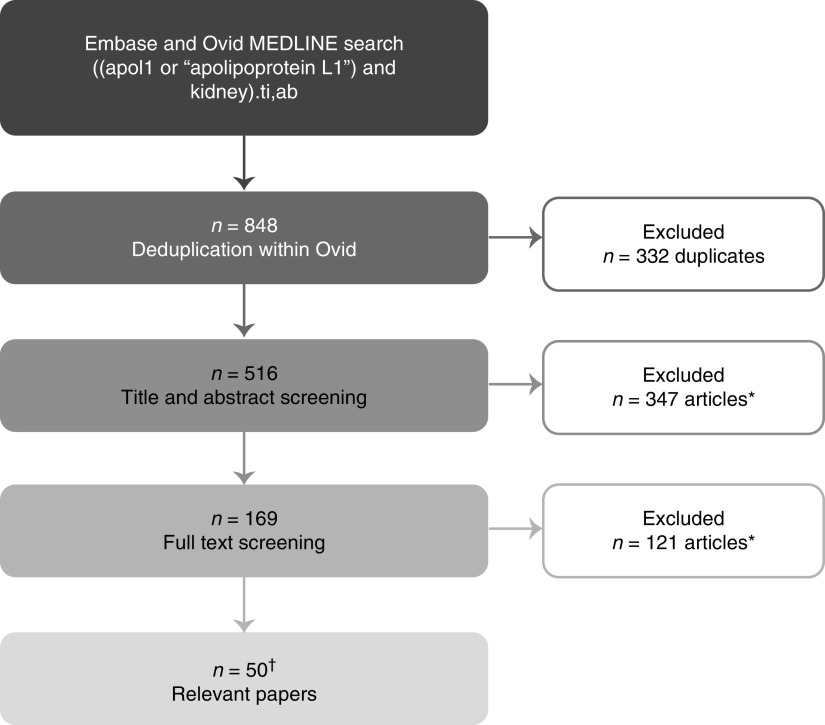
A systematic literature review was performed to identify evidence supporting the consensus statements (Figure 2, Table 1). The literature search was performed using Ovid (Walters Kluwer, South Holland, The Netherlands). The MEDLINE and Embase databases were screened using the search terms (title/abstract) apol1 or apolipoprotein L1 AND kidney, and search results were limited to articles published from January 1, 2009 to July 14, 2020. Initial results were exported and categorized using a traffic light system (red: not likely to be relevant; amber: potentially relevant; and green: likely to be relevant); this was reviewed by the Steering Committee. The evidence was graded using the Grading of Recommendations Assessment, Development and Evaluation system.14 This was reviewed and agreed on by the Steering Committee and Expert Panel (Table 1).
Figure 2.
Systematic literature search strategy. The literature search was performed on the date range from January 1, 2009 to July 14, 2020. *Exclusion criteria: non-English language, review article, editorial, protocol, no human participants (i.e., in vitro or animal studies), no participants of African descent, not about APOL1 G1 or G2, not about kidney disease or renal function, investigating molecular mechanisms of kidney injury only, or otherwise not considered relevant; †two additional relevant papers were identified from the reference lists of the full texts screened.
Table 1.
Consensus statements on APOL1-associated nephropathy: Counseling, genotyping, and diagnosis; disease awareness and education; and future vision
| Statement | Consensus Votea | GRADE Scoreb | Key Supporting Evidence |
|---|---|---|---|
| Section A: Counseling, genotyping, and diagnosis | |||
| A1. APOL1-associated nephropathy should be considered in all patients with kidney disease who have African ancestry, particularly when there is a family history of CKD | 100%: A+=57%; A=43% | 1B | Refs. 5,15,16 |
| A2. Regardless of ancestry, APOL1-associated nephropathy should be considered in all patients with kidney disease and a family member with a confirmed APOL1 high-risk genotype | 86%: A+=50%; A=36%; N=14% | 1C | Ref. 17 |
| A3. Clinical factors that are relevant to considering APOL1-associated nephropathy include hypertension, nondiabetic nephropathy, and rapid progression of CKD | 100%: A+=57%; A=43% | 1B | Hypertensionc; 18 nondiabetic nephropathyc; 5,19 rapid progression of CKDc8,15,18,20–31 |
| A4. APOL1-associated nephropathy includes a spectrum of kidney diseases in patients with a high-risk APOL1 genotype | 100%: A+=79%; A=21% | 1B | Refs. 5,15,16,18,27,29,31–42 |
| A5. FSGS, HIV-associated nephropathy, nondiabetic kidney disease, purported “hypertensive nephrosclerosis,” and ESKD are all associated with a high-risk APOL1 genotype | 93%: A+=64%; A=29%; D=7% | 1B | FSGSc; 5,15,32,34 HIV-associated nephropathyc; 15,16,33,35,37 nondiabetic kidney diseasec; 5,19,38,40,42 hypertension-attributed ESKDc; 5,18,32,34,39 ESKDc27,29,31,32,36,39,41 |
| A6. If a physician is unaware that a patient has APOL1-associated nephropathy, this may lead to: | 100%: A+=57%; A=43% | 1C | Expert opinion |
| Lack of preparation for possible rapid progression to ESKD | |||
| Inadequate monitoring of disease progression | |||
| A lost opportunity for the patient to participate in a clinical trial testing a treatment for APOL1-associated nephropathy | |||
| A7. Identifying an APOL1 high-risk genotype in a patient with kidney disease may give the physician a better understanding of the likelihood of rapid progression of kidney disease and guide monitoring | 100%: A+=36%; A=64% | 1C | Expert opinion |
| A8. For a patient with APOL1-associated nephropathy, important advantages of learning their APOL1 status may include: | 86%: A+=29%; A=57%; N=14% | 1C | Hypertensive patientsd43–45 |
| A better understanding of the likelihood of rapid disease progression | |||
| An awareness that they are not to blame for their disease | |||
| Greater knowledge about their ancestry | |||
| Increased motivation to live a healthy lifestyle | |||
| The opportunity to participate in a clinical trial testing a treatment for APOL1-associated nephropathy | Donorsd46,47 | ||
| The potential for family members to learn their own APOL1 status | Community membersd13,48 | ||
| A9. Clinicians’ lack of confidence in communicating the findings from APOL1 testing to patients in a clear and effective way that minimizes patient confusion and misunderstanding is a major barrier to APOL1 testing in patients with nephropathy | 93%: A+=57%; A=36%; D=7% | 1C | Expert opinion; PCPsc44 |
| A10. Causing patients fear, distress, anxiety, or a sense of futility or creating misunderstanding and confusion about the meaning of high-risk APOL1 status are seen as significant potential disadvantages of APOL1 testing | 93%: A+=86%; A=7%; D=7% | 1C | Ref. 46 |
| A11. Potential concerns related to APOL1 testing that individual patients should be aware of include: | 100%: A+=71%; A=29% | 1C | Refs. 12,13,46,47,49–51 |
| Cost associated with testing | |||
| Implications of a positive test | |||
| Fear of being diagnosed with APOL1-associated nephropathy | |||
| Concern about disease progression | |||
| Future health insurance access and cost | |||
| Discrimination (e.g., job, life insurance) | |||
| Concern that there is no treatment and therefore, no value in knowing | |||
| Concern for family members who might have a high-risk APOL1 genotype | |||
| A12. APOL1 testing is not common in routine clinical practice; it is carried out in only a few specialist centers, often as part of APOL1 research, and as such, there is currently no “typical pathway” for APOL1 testing | 100%: A+=79%; A=21% | 1C | Refs. 17,49,52–54 |
| A13. High-quality materials, developed for health care professionals and patients by genetics professionals, bioethicists, clinicians, and members of the Black community, could increase support among stakeholders for counseling and testing of APOL1 genotype in appropriate patients | 86%: A+=36%; A=50%; N=14% | 1C | Refs. 13,44,49,55 |
| A14. Nephrologists should be responsible for counseling patients in their practice about the APOL1 high-risk genotypes as appropriate, ordering the APOL1 test, and returning results to patients | 100%: A+=57%; A=43% | 1C | Ref. 56; PCPs feel poorly preparedd44 |
| A15. Regarding the current scientific understanding of APOL1-associated nephropathy, it is important that patients considering APOL1 testing are aware: | 93%: A+=57%; A=36%; N=7% | 1C | Expert opinion |
| Of what is known about the natural history of APOL1-associated nephropathy | |||
| Of the implications of a positive or negative test for the APOL1 high-risk genotype | |||
| That the majority of people with high-risk APOL1 genotypes will not develop CKD | |||
| That the biology underlying the disease is still under study | |||
| Of the greater risk of morbidity and mortality associated with CKD in African ancestry Americans compared with Americans of other ancestries | |||
| Of the current knowledge regarding APOL1 and kidney donation | |||
| A16. Patients considering APOL1 testing should be aware that there: | 100%: A+=79%; A=21% | 1C | |
| Is currently no specific treatment to prevent APOL1-associated nephropathy or alter the course of disease progression | Expert opinion | ||
| Are many things that patients, regardless of APOL1 genotype, can do to protect their kidneys and promote general health (e.g., eat a healthy diet, exercise, avoid smoking) | Living donorsd47,57 | ||
| Is the potential for people with a high-risk APOL1 genotype to take part in clinical trials | People without CKDd55 | ||
| A17. Patients considering APOL1 testing should also be informed about the potential implications associated with learning their APOL1 genotype (e.g., difficulty obtaining insurance) and that family members may also have the APOL1 high-risk genotype | 100%: A+=71%; A=29% | 1C | Ref. 12 |
| A18. When health care professionals discuss APOL1 testing with their patients, they should be honest and transparent about the current understanding of APOL1, take time to discuss APOL1 with patients, listen to their concerns, answer questions, and provide information sources that are balanced, easy to use and understand, and reliable | 100%: A+=100% | 1C | Expert opinion |
| A19. Patients should have a clear understanding of the purpose of the APOL1 test and the potential implications of the result before the test is taken | 100%: A+=86%; A=14% | 1C | Expert opinion; living donorsd57 |
| A20. After a patient with CKD is confirmed to have a high-risk APOL1 genotype, the clinician should: | 100%: A+=86%; A=14% | 1C | Refs. 12,13,55; strict BP controlc58 |
| Offer to tell the patient his or her genotype | |||
| Discuss the implications of the patient’s APOL1 genotype and long-term prognosis | |||
| Discuss the implications for family members, emphasizing that only a fraction of carriers will go on to develop kidney disease | |||
| Aim to lower patient anxiety | |||
| Explain that there are currently no specific treatments | |||
| Encourage the patient to participate in clinical trials for which they are eligible | |||
| Inform the patient about other modifiable risk factors and renoprotective measures (lifestyle changes, BP control) | |||
| A21. After a patient with CKD is confirmed to have a low-risk APOL1 genotype, the clinician should: | 100%: A+=100% | 1C | Ref. 12 |
| Offer to tell the patient his or her genotype | |||
| Discuss the implications of the patient’s APOL1 genotype and their long-term prognosis | |||
| Discuss the possibility that other family members could still have the APOL1 high-risk genotype | |||
| Aim to lower patient anxiety | |||
| Inform the patient about other modifiable risk factors and renoprotective measures (lifestyle changes, BP control) | |||
| A22. There is no established best practice for counseling patients about APOL1 testing; further research is required to define best practice | 100%: A+=86%; A=14% | 1C | Ref. 49 |
| A23. Nephrology trainees need to be educated to be able to confidently deliver effective counseling to patients about APOL1 testing | 100%: A+=93%; A=7% | 1C | Expert opinion |
| A24. No specific treatment is currently available for patients with APOL1-associated nephropathy | 100%: A+=100% | 1C | Expert opinion |
| Section B: Disease awareness and education | |||
| B1. Awareness of health disparities related to kidney disease in Black people is variable among key stakeholders | 100%: A+=79%; A=21% | 1C | Expert opinion |
| B2. Research is required to assess the awareness of health disparities related to kidney disease in Black people among key stakeholders | 93%: A+=86%; A=7%; N=7% | 1C | Expert opinion |
| B3. Lack of Black representation among key stakeholders exacerbates health disparities related to kidney disease | 100%: A+=93%; A=7% | 1C | Expert opinion |
| B4. Many nephrologists are aware of APOL1-associated nephropathy, but awareness among other key stakeholders (including regulators, payers, policy makers, and nonspecialist health care professionals) is inadequate | 100%: A+=36%; A=64% | 1C | Ref. 49 |
| B5. Research is required to assess the current state of awareness of APOL1-associated nephropathy among key stakeholders | 86%: A+=57%; A=29%; N=14% | 1C | Expert opinion |
| B6. There are low levels of awareness of APOL1-associated nephropathy in the Black community | 93%: A+=64%; A=29%; N=7% | 1C | Refs. 12,43,44,48 |
| B7. Low levels of awareness of APOL1-associated nephropathy among patients contribute to low participation in research and clinical trials | 86%: A+=57%; A=29%; N=7%; D+=7% | 1C | Expert opinion |
| B8. Low levels of awareness of APOL1-associated nephropathy in the Black community mean that kidney transplant recipients and donors may not be aware of their APOL1 status and the potential associated risk | 100%: A+=43%; A=57% | 1C | Refs. 46,47,57 |
| B9. Use of APOL1 genotypes in the Kidney Donor Risk Index, instead of race, is under study to determine if this better defines the quality of Black deceased donor kidneys | 93%: A+=64%; A=29%; N=7% | 1C | Ref. 59 |
| B10. The lack of specific treatment and concerns regarding insurance reimbursement for genetic testing and potential discrimination on the basis of APOL1 genotype (e.g., life insurance, health insurance, job security) may be barriers to physicians educating patients about APOL1-associated nephropathy | 100%: A+=79%; A=21% | 1C | Refs. 49,54 |
| B11. The lack of education about APOL1-associated nephropathy and testing in the Black community reinforces low levels of trust in the medical establishment | 79%: A+=50%; A=29%; N=7%; D=14% | 1C | Ref. 12 |
| B12. Low levels of trust in the medical establishment could be a barrier to APOL1 testing in the Black community | 100%: A+=79%; A=21% | 1C | Refs. 13,46,47,51 |
| B13. Nephrologists, primary care providers, nurses, community leaders, and patient networks all have an important role to play in educating Black patients with nephropathy about the APOL1 gene | 100%: A+=86%; A=14% | 1C | Expert opinion |
| B14. Persons responsible for educating Black patients with nephropathy about the APOL1 high-risk genotype should be knowledgeable about the current understanding of APOL1 risk, appropriately trained to effectively deliver information on genetic risk, and trained in cultural and structural competency | 100%: A+=86%; A=14% | 1C | Expert opinion |
| B15. Educators should be able to communicate medical information in lay terms, deliver culturally sensitive information regarding ancestry-based risk, facilitate questions, encourage open discussion, and inspire trust | 100%: A+=93%; A=7% | 1C | Refs. 43,55 |
| B16. Clear, accurate educational materials should be given to patients; ideally, these should feature Black people | 100%: A+=79%; A=21% | 1C | Refs. 13,47,51,55 |
| B17. Educators should be able to refer patients to trusted sources for information (e.g., medical societies, patient advocacy organizations, health care systems) | 100%: A+=79%; A=21% | 1C | Ref. 43 |
| B18. To improve awareness of APOL1 among the patient community, health care professionals should work with community leaders and community groups and should provide education in a setting that is familiar and comfortable for patients | 100%: A+=93%; A=7% | 1C | Expert opinion |
| Section C: Future vision | |||
| C1. A specific, effective treatment for APOL1-associated nephropathy would meet an unmet patient need | 100%: A+=93%; A=7% | 1C | Regulatory perspectived60 |
| C2. There is a pressing need for specific, effective treatments for patients with APOL1-associated nephropathy | 100%: A+=100% | 1C | Expert opinion |
| C3. If a treatment for APOL1-associated nephropathy becomes available, all patients suspected of having APOL1-associated nephropathy should be offered genetic testing with appropriate counseling | 100%: A+=79%; A=21% | 1C | Expert opinion; in the absence of treatmentd12 |
| C4. If a treatment for APOL1-associated nephropathy becomes available, all Black people (or those with sub-Saharan African ancestry) with CKD should be offered genetic testing with appropriate counseling | 79%: A+=43%; A=36%; N=7%; D=14% | 1C | Expert opinion; in the absence of treatmentd12 |
| C5. If a treatment for APOL1-associated nephropathy becomes available, intervention should begin as early as possible in patients with APOL1-associated nephropathy on the basis of data from clinical trials | 93%: A+=64%; A=29%; N=7% | 1C | Expert opinion |
| C6. If a treatment for APOL1-associated nephropathy was available, a kidney biopsy should be performed to confirm the diagnosis in individuals with high-risk APOL1 genotypes | 86%: A+=50%; A=36%; N=14% | 1C | Expert opinion |
| C7. Important potential benefits of a treatment for APOL1-associated nephropathy becoming available include: | 100%: A+=93%; A=7% | 1C | Expert opinion |
| Slowing kidney disease progression | |||
| Extending life expectancy | |||
| Reducing the incidence of ESKD in the Black population | |||
| Reducing the number of people requiring a kidney transplant | |||
| Reducing health disparities associated with kidney disease in the Black population | |||
| C8. Other potential benefits of a treatment for APOL1-associated nephropathy becoming available include: | 86%: A+=57%; A=29%; N=14% | 1C | |
| Reducing the costs associated with management of ESKD (dialysis/transplant) | |||
| Reducing cardiovascular morbidity and mortality in the Black community | |||
| C9. Failure of insurance companies to cover treatment-related costs for APOL1-associated nephropathy could exacerbate disparities | 100%: A+=79%; A=21% | 1C | Expert opinion |
| C10. Classification of the APOL1 high-risk genotype as a preexisting condition by insurance companies is a concern for patients and their families, particularly because more widespread genotyping is anticipated if treatments become available | 93%: A+=79%; A=14%; D=7% | 1C | Ref. 12; donorsd46,47,51 |
| C11. Inappropriate treatment and inconsistency in clinical practice are potential concerns related to a treatment for APOL1-associated nephropathy becoming available | 100%: A+=79%; A=21% | 1C | Expert opinion |
| C12. APOL1-associated nephropathy should be recognized in the classification guidelines as an adjunct to the clinical diagnosis (e.g., “FSGS, APOL1 variant”) | 93%: A+=71%; A=21%; N=7% | 1C | Expert opinion |
| C13. The cost to a patient for health insurance should not increase as a result of their APOL1 genotype; this should be ensured through legislation | 100%: A+=79%; A=21% | 1C | Expert opinion |
The final statements and consensus voting results were obtained via discussion and anonymous voting by the Consensus Group at the face-to-face meeting following presentations about current clinical science relevant to APOL1-associated nephropathy; three participants were unable to attend and responded via a blinded online survey after the meeting. GRADE, Grading of Recommendations Assessment, Development and Evaluation; PCP, primary care physician.
Consensus: A+, strongly agree; A, agree; N, neither agree nor disagree; D, disagree; D+, strongly disagree.
Grade of recommendation: 1A, strong recommendation, high-quality evidence; 1B, strong recommendation, moderate-quality evidence; 1C, strong recommendation, low-quality or very low–quality evidence; 2A, weak recommendation, high-quality evidence; 2B, weak recommendation, moderate-quality evidence; 2C, weak recommendation, low-quality or very low–quality evidence.
Supports part of the statement only.
Indirect evidence.
Results
Consensus Statements
The Consensus Group (n=14) agreed on 55 consensus statements, which are presented in Table 1. A guide to assist the identification of statements relevant to particular themes is provided in Table 2. A summary of the statements is provided herein, and a full discussion of each statement and the supporting evidence is included in Supplemental Material.
Table 2.
Guide to the consensus statements on APOL1-associated nephropathy in Table 1
| Theme | Statements |
|---|---|
| Genetic testing | A1, A2, A6–A14, B11, B12, C3, C4 |
| Diagnosis | A1–A7 |
| Patient counseling | A8–A11, A13–A23, C3, C4 |
| Disease awareness | A13, B1–B9, B18 |
| Disease education | A8, A13, B10–B18 |
| Unmet need for treatment | A24, C1, C2 |
| Vision of future treatment | C3–C8, C12, C13 |
| Concerns regarding future treatment | B10, C9–C11 |
Section A: Counseling, Testing, and Diagnosis
First, key considerations for counseling, testing, and diagnosis in current clinical practice were agreed upon (Table 1, statements A1–A24). On the basis of the known genetic associations, the Consensus Group agreed that APOL1-associated nephropathy and therefore, APOL1 testing should be considered in all patients of African ancestry with kidney disease and in any patient with kidney disease and a family member with a confirmed APOL1 high-risk genotype (Table 1, statements A1 and A2).5,15–17 Hypertension, nondiabetic nephropathy, and rapid progression of CKD were agreed to be relevant clinical factors, although APOL1-associated nephropathy can also present as any one of a spectrum of kidney diseases (Table 1, statements A3–A5).5,8,15,16,18–42
APOL1 testing in the current clinical setting has potential advantages and disadvantages both for clinicians and for patients (Table 1, statements A6–A11).12,13,43–50 However, it is not common at present in routine clinical practice (Table 1, statement A12).17,49,52–54 To help to address some of the barriers to testing, the Consensus Group defined key aspects of appropriate counseling for patients who are considering APOL1 testing (Table 1, statements A13–A19) and discussion of results after testing (Table 1, statements A20 and A21).12,13,44,47,49,55–58 We recognize that there is currently no established best practice and that additional professional education may be required to enable nephrologists to counsel patients about APOL1 (Table 1, statements A22 and A23).49 Given that no specific treatment is currently available for APOL1-associated nephropathy (Table 1, statement A24), it is important that patients be made aware of this fact during counseling, testing, and diagnosis.
Section B: Disease Awareness and Education
Awareness of a disease is a crucial step toward bringing about improvements in care. Therefore, the Consensus Group considered levels of awareness among key stakeholders and agreed on important features of an education program to raise awareness of APOL1-associated nephropathy among the patient community (Table 1, statements B1–B18).
Health disparities related to kidney disease in the Black community persist despite substantial research effort, and the group agreed a lack of sufficient Black representation among key stakeholders was a contributing factor (Table 1, statement B3). The Consensus Group believes that awareness of health disparities varies among key stakeholders, and apart from among nephrologists, awareness of APOL1-associated nephropathy is inadequate (Table 1, statements B1 and B4), although research is required to fully assess awareness (Table 1, statements B2 and B5).49 Knowledge of APOL1-associated nephropathy in the Black community is low, which affects participation in research and clinical trials and affects kidney transplant donors and recipients (Table 1, statements B6–B9).12,43,44,46–48,57,59 Clinicians face barriers in educating patients about APOL1-associated nephropathy; the Consensus Group agreed that this lack of education reinforces low levels of trust in the medical establishment within the Black community on the basis of historical and ongoing mistreatment of Black people by the medical establishment and other major institutions, which is itself a barrier to APOL1 testing (Table 1, statements B10–B12).12,13,46,47,49,51,54
There was consensus that nephrologists, primary care providers, nurses, community leaders, and patient networks all have important roles to play in educating patients about APOL1 (Table 1, statement B13). Persons responsible for education should have appropriate knowledge, skills, and training, and they should be prepared to share educational materials, refer patients to trusted sources for further information, and provide education in a setting that is familiar and comfortable for patients (Table 1, statement B14–B18).13,43,47,51,55
Section C: Future Vision
Finally, the Consensus Group considered the need for treatment, agreed on key features of an ideal future clinical scenario in which a treatment is available, and identified potential barriers to accessing treatment (Table 1, statements C1–C13).
There is a pressing, unmet patient need for a specific, effective treatment for APOL1-associated nephropathy (Table 1, statements C1 and C2).60 The Consensus Group agreed that if a safe and effective treatment becomes available, all patients suspected of having APOL1-associated nephropathy, including all Black patients with CKD, should be offered genetic testing and that treatment should begin as early as possible following a confirmatory kidney biopsy (Table 1, statements C3–C6).12 Although an effective treatment has the potential to transform care for patients with APOL1-associated nephropathy and reduce health disparities, uncertainties regarding insurance coverage, access to treatment, and inconsistency in clinical practice remain (Table 1, statements C7–C11 and C13).12,46,47,51 To enable clinicians to identify which patients could benefit from a specific and effective treatment, the Consensus Group recommended that APOL1-associated nephropathy should be recognized as an adjunct to the clinical diagnosis (e.g., “FSGS, APOL1 variant”) (Table 1, statement C12).
Discussion
We set out to gather expert opinions on the care of people who may have APOL1-associated nephropathy using a Delphi consensus process supported by a systematic literature review. For the first time, a multidisciplinary group of experts and patient advocates has agreed on (1) a framework for counseling, genotyping, and diagnosis; (2) key considerations for educating patients and other stakeholders about APOL1-associated nephropathy and raising awareness in the patient community; and (3) a vision for the care of patients in a future where an effective treatment is available. The 55 consensus statements provide an overview of current expert opinion and may be considered as recommendations for centers across the United States when implementing APOL1 genotyping or participating in APOL1 research studies or clinical trials.
The multidisciplinary group of experts agreed on a broad approach to counseling of patients who may have APOL1-associated nephropathy. Given that there is currently no specific treatment, the Consensus Group agreed that there is a need for health care professionals to be open and honest when discussing APOL1-associated nephropathy in order to ensure that patients are well informed and comprehend the limitations and benefits of genetic testing before deciding to proceed. Further work is needed to define best practice fully both in counseling patients about APOL1 and genotyping and in training health care professionals to provide this counseling.
With regard to which individuals should be considered for APOL1 genetic testing, consensus was reached that APOL1-associated nephropathy should be considered in all patients with kidney disease who have African ancestry, particularly where there is a family history of CKD, and that, regardless of ancestry, APOL1-associated nephropathy should be considered in all patients with kidney disease and a family member with a confirmed APOL1 high-risk genotype. Consensus on APOL1 testing in individuals without kidney disease but who are at risk for nephropathy on the basis of a family history of CKD or who have high BP remains to be reached. The experts agreed that there is a critical need for specific, effective treatments for patients with APOL1-associated nephropathy. In a future clinical scenario in which an effective treatment is available, the Consensus Group agreed that APOL1 testing should be offered to all patients with a clinical picture of APOL1-associated nephropathy as well as all Black patients with CKD and that intervention should begin as early as possible. At present, however, there remains a lack of evidence to guide decisions regarding which patients should be considered for testing. APOL1 high-risk genotypes markedly increase the likelihood of developing CKD, particularly in younger and middle-aged individuals, and the age when this risk may decline is unknown. The typical age at onset of ESKD in patients with APOL1-associated nephropathy is relatively young, often before 55 years. Given that modifying factors often trigger nephropathy in genetically susceptible individuals, older individuals with high-risk genotypes who have not developed CKD are presumed to have escaped exposure to such modifiers and now face lower risk for nephropathy. There is a clear need to gather data and to develop models that incorporate other CKD risk factors and support age-dependent prediction.
On a population level, there is a clear association between high-risk APOL1 genotypes and various kidney diseases, but the molecular mechanism behind the genotype–phenotype relationship is not yet well understood.61 Accurately communicating the risk to patients while acknowledging the limitations of our current knowledge requires careful consideration, and given that there is currently no specific treatment, there has understandably been a reluctance among medical professionals to discuss APOL1 with patients. However, recent publications have shown that there is support among the Black community for testing.12,43 In one series of community deliberations, 95% of participants supported offering APOL1 testing to African ancestry patients with kidney disease, even though they were aware that no treatment was available; specifically, 64% supported offering tests to all Black patients, 21% supported offering tests to those with risk factors for CKD, and 10% supported offering tests only to patients with signs of CKD.12 Here, we have sought to provide clinicians with tools to help them to address this call for more widespread APOL1 testing.
The experts agreed that awareness of APOL1-associated nephropathy is low among the Black community and key decision makers, as noted in several studies,12,43,44,48 and this is not surprising given that there have been no major educational initiatives in this area. The panel agreed on various factors that may contribute to this lack of awareness and that this may reinforce a lack of trust among Black people in the medical establishment. Key features of an education program were outlined, including the need for coordination between health care professionals and community groups; the availability of clear and accurate information; and well-informed, trusted experts to educate the community about APOL1.
The Black community experiences significant health disparities related to kidney disease.4 Conditions associated with socioeconomic deprivation and racism (e.g., discrimination, residential segregation, medical mistrust, and being uninsured and underinsured) contribute to the onset and progression of CKD and ESKD.3,62 However, APOL1 genetic risk is also a contributing factor, particularly in the progression from CKD to ESKD.8 Widespread identification of patients with APOL1-associated nephropathy, coupled with access to an effective treatment, could help to reduce poor health outcomes by targeting one of the biologic factors contributing to kidney disease in Black people. There are currently no Food and Drug Administration–approved treatments available that specifically target APOL1-associated nephropathy; however, preclinical studies and phase 1 and 2 trials of potential new therapies are underway. Therefore, the medical community should prepare for the clinical and ethical issues that may arise as a result of a treatment becoming available. The case for offering APOL1 genotyping in routine clinical practice will be strengthened as patients with APOL1-associated nephropathy have more opportunities to participate in clinical trials of novel treatments. The potential of any treatment to reduce health disparities must be viewed in light of the significant nonbiologic factors associated with social class and race that also contribute to CKD in Black people.4 Addressing these social factors on a population level, through government and community intervention and action, will be needed before racial disparities in CKD can be eradicated.
The Delphi method was originally developed to gather expert opinion on cutting edge topics in which there are little or no relevant data in the scientific literature. As expected, our systematic review highlighted that most of the consensus statements agreed here are supported by low-quality, very low–quality or no relevant evidence. However, by using the Delphi method to anonymously gather expert opinion and give equal weighting to all opinions, we have consolidated the multidisciplinary group’s different expertise, experiences, and viewpoints on APOL1-associated nephropathy. Participants were able to debate each statement at the face-to-face meeting to find common ground, but anonymity was maintained in the voting process to avoid any peer pressure. We recognize that the agreed consensus statements are not exhaustive and do not constitute clinical practice guidelines. Indeed, without data from clinical trials, it would be premature to develop treatment guidelines. However, in the absence of further research and formal guidance, these results provide an overview of current expert opinion in the field (albeit the panel included only a few specialists in each area so may not represent the opinion of the entire kidney disease community). The consensus statements may be of use to nephrologists and other health care professionals who are considering implementing APOL1 testing in their clinics.
Advances in our understanding of APOL1-associated nephropathy provide an opportunity to reduce health disparities related to kidney disease. Here, we have highlighted key steps needed to improve awareness and diagnosis of APOL1-associated nephropathy and outlined the opportunities for clinical care in the present era and a vision for the future.
Disclosures
W. Burke reports honoraria from the University of Minnesota (related to a National Institutes of Health [NIH]–funded grant) and Columbia University (related to an NIH-funded grant) and scientific advisor or membership as advisor to the editorial board of Annals of Internal Medicine. J. Divers reports honoraria from NIH and Oak Ridge Associated Universities. L. Eberhard is an employee of Oxford PharmaGenesis Ltd. B.I. Freedman is a consultant for AstraZeneca and RenalytixAI. Wake Forest University Health Sciences and B.I. Freedman have rights to an issued United States patent related to APOL1 genetic testing. B.I. Freedman also reports research funding from AstraZeneca and RenalytixAI. C.A. Gadegbeku is a site investigator for a clinical trial in anemia sponsored by Akebia and serves as Fresenius Medical Care Episcopal Hospital Dialysis Medical Director in Philadelphia, Pennsylvania. C.A. Gadegbeku also reports research funding from Vertex and scientific advisor or membership as an American Society of Nephrology (ASN) council member. R. Gbadegesin reports consultancy agreements with Keryx Biopharmaceuticals and research funding from AstraZeneca, Bristol Myers Squibbs, and Goldfinch Bio. M.E. Hall reports consultancy agreements from the Clinical Events Committee for the Baroreflex Activation Therapy for Heart Failure ( BeAT-HF) trial and with Relypsa for the Patiromer for the Management of Hyperkalemia in Subjects Receiving RAASi Medications for the Treatment of Heart Failure (DIAMOND) trial, and scientific advisor or membership as an editorial board member of Hypertension. T. Jones-Smith reports consultancy agreements from Dynamic Life Health and Dynamic Life Press; research funding from Nova Biomedical; and other interests/relationships in Dynamic Life Press and Salem Media. R. Knight reports honoraria from American Kidney Fund, Johns Hopkins Center for Health Equity, Northwestern University, and Personalized Medicine Coalition; scientific advisor or membership on the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Advisory Council, the Scientific Advisory Board for the “Rescuing Kidneys at Risk of Discard” project, and the Scientific Registry of Transplant Recipients (SRTR) Visiting Committee; speakers bureau for American Association of Kidney Patients (AAKP); and other interests/relationships with AAKP as president, the Bowie State University Board of Advisors, the Quality Insights Patient Advisory Committee as a member, NIDDK in the Health Information Technology Workgroup, the National Renal Administrators Association (NRAA)/ESKD Forum Health Information Technology Project as a member, and the SRTR Visiting Committee. J.B. Kopp reports patents and inventions for Vpr antibodies, NIH; scientific advisor or membership with editorial boards for American Journal of Nephrology and American Journal of Physiology–Renal Physiology, as a board member for the National Kidney Foundation serving the National Capitol Area, and with the NIH Foundation for the Advancement of Education in the Sciences; and other interests/relationships, including with NIDDK; having or is negotiating confidentiality disclosure agreement with InterMune, Ionis, and Third Rock; collaboration research and development agreement with Sanofi; and research collaboration agreements with AstraZeneca, Genentech, Merck, and Third Rock. C.P. Kovesdy is a consultant for AstraZeneca, Bayer, Cara Therapeutics, Reata, Takeda, and Tricida. C.P. Kovesdy also reports consultancy agreements with Amgen; research funding with Akebia, Bayer, Gilead, and GlaxoSmithKline; honoraria from Amgen, AstraZeneca, Bayer, Cara, Reata, Takeda, and Tricida; scientific advisor or membership as an associate editor of CJASN and Nephron and as an editorial board of American Journal of Kidney Disease, International Urology Nephrology, Kidney International Reports, Kidney Medicine, and Nephrology Dialysis Transplantation; and royalties from UpToDate for authorship of two chapters. K. Norris reports consultancy agreements with Atlantis Healthcare (compliance, research, and quality care for dialysis and CKD care in Puerto Rico); scientific advisor or membership with AAKP, Association of American Medical Colleges (AAMC), Atlantis Healthcare, CJASN, Ethnicity and Disease, the International Nephrology Society (ISN), JASN, and National Kidney Foundation (NKF) Kidney Early Evaluation Program (KEEP); and other interests/relationships with AAKP and NKF. O. Olabisi reports consultancy agreements with Icagen. G.V. Roberts reports consultancy agreements with Options Unlimited International LLC; ownership interest in Microsoft via stock and 50% of Options Unlimited International; research funding from the Veterans Administration, the Center for Disease Innovation, and the Kidney Precision Medicine Project funded by NIDDK; honoraria from the APOL1 Long-term Kidney Transplantation Outcomes Consortium (APOLLO) Community Advisory Committee, the APOL1 Community Advisory Board, and the Kidney Precision Medicine Project; patient advisor for the Community Advisory Committee; scientific advisor or membership with the APOLLO Community Advisory Board, the ASN Coronavirus Disease 2019 (COVID-19) Response Team, the ASN COVID-19 Transplant Subcommittee, the Canadians Seeking Solutions and Innovations to Overcome CKD (Can-SOLVE CKD) International Research Advisory Committee, the Center for Dialysis Innovation Patient Advisory Board, the Home Dialyzors United Advisory Committee, the Kidney Health Initiative (KHI) Patient and Family Partnership Council, and the Kidney Research Institutes Patient Advisory Committee; speakers bureau for the American Association of Kidney Patients Speakers Bureaus; and other interests/relationships with the American Association of Kidney Patients, the APOLLO Recruitment Committee, the ASN COVID-19 Response Team and Transplant Subcommittee, the Can-SOLVE CKD International Research Advisory Committee, the ISN Patient Group, the KHI Patient Family Partnership Council, NKF as an ambassador, and the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Diseases. J.R. Sedor is a consultant for Maze. J.R. Sedor also reports consultancy agreements with Boehringer Ingelheim, Goldfinch Bio and Sanofi Genzyme; research funding from Calliditas and Novartis for clinical trials; honoraria from Chugai Pharmaceutical Co. (Next Generation Kidney Research Meeting, Tokyo), Drexel University, Maze, NKF Arizona, the University of Maryland, the University of Michigan, and the University of Minnesota; patents and inventions with APOL1 transgenic mice licensed to Sanofi Genzyme; invention disclosure for machine learning analysis of kidney biopsies; scientific advisor or membership with the Kidney Foundation of Ohio (kidney patient organization for direct aid; on the board of directors) and NephCure Kidney International; and other interests/relationships on the editorial boards of American Journal of Nephrology, JASN, and Seminars in Nephrology and with ISN as a member. All remaining authors have nothing to disclose.
Funding
This initiative was funded by an educational grant from AstraZeneca. The consensus process was conducted and managed by an independent third-party agency, PharmaGenesis London (London, United Kingdom), funded by the educational grant from AstraZeneca, under the guidance of the steering committee. No fees were provided to the Steering Committee or the Expert Panel for participating in this initiative.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Richard Claes of PharmaGenesis London for providing editorial support for the manuscript, which was funded by AstraZeneca. The authors also acknowledge Ms. Sally Janani and Mr. Brian McMunn (PharmaGenesis London) for their support with the Delphi consensus process.
This work is endorsed by the Renal Association, NephCure Kidney International, and the Texas Kidney Foundation.
AstraZeneca was not involved in the study design, the data collection or analysis, the writing or editing of the manuscript, or the decision to submit the paper for publication.
Dr. E. Blacksher and Dr. B.I. Freedman contributed to the conception and design of the study; Dr. E. Blacksher, Dr. L. Eberhard, and Dr. B.I. Freedman contributed to drafting of the manuscript; Dr. L. Eberhard contributed to administrative, technical, and material support; all authors contributed to acquisition, analysis, and/or interpretation of data and critical revision of the manuscript for important intellectual content; and all authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020101399/-/DCSupplemental.
Supplemental Material. Additional information to support the consensus statements and references.
References
- 1.Dummer PD, Limou S, Rosenberg AZ, Heymann J, Nelson G, Winkler CA, et al.: APOL1 kidney disease risk variants: An evolving landscape. Semin Nephrol 35: 222–236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System : 2019. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Available at: https://www.usrds.org/annual-data-report. Accessed March 17, 2021
- 3.Nicholas SB, Kalantar-Zadeh K, Norris KC: Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis 22: 6–15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laster M, Shen JI, Norris KC: Kidney disease among African Americans: A population perspective. Am J Kidney Dis 72[Suppl 1]: S3–S7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al.: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umeukeje EM, Young BA: Genetics and ESKD disparities in African Americans. Am J Kidney Dis 74: 811–821, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limou S, Nelson GW, Kopp JB, Winkler CA: APOL1 kidney risk alleles: Population genetics and disease associations. Adv Chronic Kidney Dis 21: 426–433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al.; AASK Study Investigators; CRIC Study Investigators: APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BI, Limou S, Ma L, Kopp JB: APOL1-associated nephropathy: A key contributor to racial disparities in CKD. Am J Kidney Dis 72[Suppl 1]: S8–S16, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young BA, Fullerton SM, Wilson JG, Cavanaugh K, Blacksher E, Spigner C, et al.: Clinical genetic testing for APOL1: Are we there yet? Semin Nephrol 37: 552–557, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer ND, Freedman BI: APOL1 and progression of nondiabetic nephropathy. J Am Soc Nephrol 24: 1344–1346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umeukeje EM, Young BA, Fullerton SM, Cavanaugh K, Owens D, Wilson JG, et al.: You are just now telling us about this? African American perspectives of testing for genetic susceptibility to kidney disease. J Am Soc Nephrol 30: 526–530, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young BA, Blacksher E, Cavanaugh KL, Freedman BI, Fullerton SM, Kopp JB, et al.; APOL1 Stakeholders Project: Apolipoprotein L1 testing in African Americans: Involving the community in policy discussions. Am J Nephrol 50: 303–311, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al.; GRADE Working Group: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al.: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al.: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neugut YD, Mohan S, Gharavi AG, Kiryluk K: Cases in precision medicine: APOL1 and genetic testing in the evaluation of chronic kidney disease and potential transplant. Ann Intern Med 171: 659–664, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, et al.; SK Investigators: Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman BI, Langefeld CD, Turner J, Núñez M, High KP, Spainhour M, et al.: Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int 82: 805–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen TK, Choi MJ, Kao WH, Astor BC, Scialla JJ, Appel LJ, et al.: Examination of potential modifiers of the association of APOL1 alleles with CKD progression. Clin J Am Soc Nephrol 10: 2128–2135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jotwani V, Shlipak MG, Scherzer R, Parekh RS, Kao WH, Bennett M, et al.: APOL1 genotype and glomerular and tubular kidney injury in women with HIV. Am J Kidney Dis 65: 889–898, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanji Z, Powe CE, Wenger JB, Huang C, Ankers E, Sullivan DA, et al.: Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol 22: 2091–2097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng DK, Robertson CC, Woroniecki RP, Limou S, Gillies CE, Reidy KJ, et al.: APOL1-associated glomerular disease among African-American children: A collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant 32: 983–990, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamrat R, Peralta CA, Tajuddin SM, Evans MK, Zonderman AB, Crews DC: Apolipoprotein L1, income and early kidney damage. BMC Nephrol 16: 14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tin A, Grams ME, Estrella M, Lipkowitz M, Greene TH, Kao WH, et al.: Patterns of kidney function decline associated with APOL1 genotypes: Results from AASK. Clin J Am Soc Nephrol 11: 1353–1359, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzur S, Rosset S, Skorecki K, Wasser WG: APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 27: 1498–1505, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, et al.: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TK, Tin A, Peralta CA, Appel LJ, Choi MJ, Lipkowitz MS, et al.: APOL1 risk variants, incident proteinuria, and subsequent eGFR decline in Blacks with hypertension-attributed CKD. Clin J Am Soc Nephrol 12: 1771–1777, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, et al.: Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peralta CA, Bibbins-Domingo K, Vittinghoff E, Lin F, Fornage M, Kopp JB, et al.: APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol 27: 887–893, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riella C, Siemens TA, Wang M, Campos RP, Moraes TP, Riella LV, et al.: APOL1-associated kidney disease in Brazil. Kidney Int Rep 4: 923–929, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bajaj A, Ihegword A, Qiu C, Small AM, Wei WQ, Bastarache L, et al.: Phenome-wide association analysis suggests the APOL1 linked disease spectrum primarily drives kidney-specific pathways. Kidney Int 97: 1032–1041, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekrikpo UE, Mnika K, Effa EE, Ajayi SO, Okwuonu C, Waziri B, et al.: Association of genetic polymorphisms of TGF-β1, HMOX1, and APOL1 with CKD in Nigerian patients with and without HIV. Am J Kidney Dis 76: 100–108, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Anyaegbu EI, Shaw AS, Hruska KA, Jain S: Clinical phenotype of APOL1 nephropathy in young relatives of patients with end-stage renal disease. Pediatr Nephrol 30: 983–989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekulu PM, Nkoy AB, Betukumesu DK, Aloni MN, Makulo JRR, Sumaili EK, et al.: APOL1 risk genotypes are associated with early kidney damage in children in Sub-Saharan Africa. Kidney Int Rep 4: 930–938, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, et al.: APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol 23: 343–350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, et al.: APOL1 risk variants are strongly associated with HIV-associated nephropathy in Black South Africans. J Am Soc Nephrol 26: 2882–2890, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer H, Tayo BO, Salako B, Gottesman O, Ogunniyi A, Bottinger EP, et al.: Genetic variation in APOL1 gene is associated with chronic kidney disease (CKD) in Nigerians. Presented at the NKF 2012 Spring Clinical Meetings, National Harbor, MD, USA, May 9-13, 2012 [Google Scholar]
- 39.Sumaili EK, Shemer R, Kruzel-Davila E, Cohen EP, Mutantu PN, Bukabau JB, et al.: G1 is the major APOL1 risk allele for hypertension-attributed nephropathy in Central Africa. Clin Kidney J 12: 188–195, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tayo BO, Kramer H, Salako BL, Gottesman O, McKenzie CA, Ogunniyi A, et al.: Genetic variation in APOL1 and MYH9 genes is associated with chronic kidney disease among Nigerians. Int Urol Nephrol 45: 485–494, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, et al.: Effect of genetic African ancestry on eGFR and kidney disease. J Am Soc Nephrol 26: 1682–1692, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulasi II, Tzur S, Wasser WG, Shemer R, Kruzel E, Feigin E, et al.: High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract 123: 123–128, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Horowitz CR, Ferryman K, Negron R, Sabin T, Rodriguez M, Zinberg RF, et al.: Race, genomics and chronic disease: What patients with African ancestry have to say. J Health Care Poor Underserved 28: 248–260, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horowitz C, Ferryman K, Negron R, Rodriguez M, Bottinger E, Sanderson S, et al.: What happens when African ancestry patients learn they have a genetically increased risk for a chronic disease? What do their doctors think? J Gen Intern Med 29[1]: S255–S256, 2014 [Google Scholar]
- 45.Horowitz CR, Fei K, Ramos MA, Hauser D, Ellis SB, Calman N, et al.: Receipt of genetic risk information significantly improves blood pressure control among African ancestry adults with hypertension: Results of a randomized trial. J Gen Intern Med 33: S322, 2018 [Google Scholar]
- 46.Gordon EJ, Amόrtegui D, Blancas I, Wicklund C, Friedewald J, Sharp RR: African American living donors’ attitudes about APOL1 genetic testing: A mixed methods study. Am J Kidney Dis 72: 819–833, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon EJ, Amórtegui D, Blancas I, Wicklund C, Friedewald J, Sharp RR: A focus group study on African American living donors’ treatment preferences, sociocultural factors, and health beliefs about apolipoprotein L1 genetic testing. Prog Transplant 29: 239–247, 2019 [DOI] [PubMed] [Google Scholar]
- 48.Berrigan M, Austrie J, Fleishman A, Tercyak KP, Pollak MR, Pavlakis M, et al.: Opinions of African American adults about the use of apolipoprotein L1 (ApoL1) genetic testing in living kidney donation and transplantation. Am J Transplant: 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon EJ, Wicklund C, Lee J, Sharp RR, Friedewald J: A national survey of transplant surgeons and nephrologists on implementing apolipoprotein L1 (APOL1) genetic testing into clinical practice. Prog Transplant 29: 26–35, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horowitz CR, Abul-Husn NS, Bottinger E, Ramos MA, Fei K: What people with African ancestry have to say about genomics, disparities and chronic disease. Presented at the 39th Annual Meeting of the Society of General Internal Medicine, Hollywood, FL, USA, May 11-14, 2016 [Google Scholar]
- 51.Gordon EJ, Amortegui D, Wicklund C, Friedewald J, Sharp R: African American living kidney donors’ perceptions of APOL1 genetic testing and its impact on ethnic identity. Am J Transplant 19[Suppl 3]: 392–393, 2019 [Google Scholar]
- 52.Newell KA, Formica RN, Gill JS, Schold JD, Allan JS, Covington SH, et al.: Integrating APOL1 gene variants into renal transplantation: Considerations arising from the American Society of Transplantation Expert Conference. Am J Transplant 17: 901–911, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Garg N, Lentine KL, Inker LA, Garg AX, Rodrigue JR, Segev DL, et al.: The kidney evaluation of living kidney donor candidates: US practices in 2017. Am J Transplant 20: 3379–3389, 2020 [DOI] [PubMed] [Google Scholar]
- 54.McIntosh T, Mohan S, Sawinski D, Iltis A, DuBois JM: Variation of ApoL1 testing practices for living kidney donors. Prog Transplant 30: 22–28, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horowitz CR, Abul-Husn NS, Ellis S, Ramos MA, Negron R, Suprun M, et al.: Determining the effects and challenges of incorporating genetic testing into primary care management of hypertensive patients with African ancestry. Contemp Clin Trials 47: 101–108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas CP, Freese ME, Ounda A, Jetton JG, Holida M, Noureddine L, et al.: Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genet Med 22: 1025–1035, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mena-Gutierrez AM, Reeves-Daniel AM, Jay CL, Freedman BI: Practical considerations for APOL1 genotyping in the living kidney donor evaluation. Transplantation 104: 27–32, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ku E, Lipkowitz MS, Appel LJ, Parsa A, Gassman J, Glidden DV, et al.: Strict blood pressure control associates with decreased mortality risk by APOL1 genotype. Kidney Int 91: 443–450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freedman BI, Moxey-Mims MM, Alexander AA, Astor BC, Birdwell KA, Bowden DW, et al.: APOL1 long-term kidney transplantation outcomes network (APOLLO): Design and rationale. Kidney Int Rep 5: 278–288, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panico C, Thompson A: African Americans, kidney disease, and drug development: A regulatory perspective. Am J Kidney Dis 72[Suppl 1]: S33–S36, 2018 [DOI] [PubMed] [Google Scholar]
- 61.Reidy KJ, Hjorten R, Parekh RS: Genetic risk of APOL1 and kidney disease in children and young adults of African ancestry. Curr Opin Pediatr 30: 252–259, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins D, Agodoa L, Norris K: Kidney disease in disadvantaged populations. Int J Nephrol 2012: 469265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.