Abstract
Lipids make up a diverse subset of biomolecules that are responsible for mediating a variety of structural and functional properties as well as modulating cellular functions such as trafficking, regulation of membrane proteins and subcellular compartmentalization. In particular, phospholipids are the main constituents of biological membranes and play major roles in cellular processes like transmembrane signaling and structural dynamics. The chemical and structural variety of lipids makes analysis using a single experimental approach quite challenging. Research in the field relies on the use of multiple techniques to detect and quantify components of cellular lipidomes as well as determine structural features and cellular organization. Understanding these features can allow researchers to elucidate the biochemical mechanisms by which lipid–lipid and/or lipid–protein interactions take place within the conditions of study. Herein, we provide an overview of essential methods for the examination of lipids, including extraction methods, chromatographic techniques and approaches for mass spectrometric analysis.
Keywords: phospholipids, chromatography, extraction, mass spectrometry, lipidomics
Introduction
Lipids make up a diverse subset of biomolecules that can be grouped into the following categories: fatty acids, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids and polyketides (Fahy et al., 2009). They are responsible for mediating a variety of structural and functional properties, and reside predominantly in cell membranes as the primary constituents of the bilayer. Lipid bilayers also house various embedded proteins that contribute to the cell membrane functionality. Lipids are not only are essential for cellular architecture, but are involved in processes that modulate cellular function such as trafficking, regulation of membrane proteins and subcellular compartmentalization. Of the categories mentioned above, phospholipids (PLs) are one of the most abundant constituents of biological membranes and play important roles in cellular processes like transmembrane signaling and structural dynamics. The major PL classes (Fig. 1) are: (1) phosphatidylcholines (PC); (2) phosphatidylethanolamines (PE); (3) phosphatidylinositols (PI); (4) phosphatidylserines (PS); (5) phosphatidylglycerols (PG); and (6) phosphatidic acids (PA). Of these, (1)–(5) are predominantly found in the membranes of mammalian cells. These can often exist as phospholipid variants, with a diverse array of alkyl chains, such as sphingolipids, plasmalogens, glycerol diesters and poly-phosphorylated species. PLs are amphipathic molecules that can be distinguished by two hydrophobic fatty acyl molecules esterified at the sn-1 and sn-2 positions of glycerol and a polar head group linked by a phosphate residue at the sn-3 position. PL classes differ in terms of size, shape, charge and chemical composition, which gives rise to an asymmetric distribution within the membrane. The chemical and physical properties of membranes are greatly determined by the PL composition. PLs can also stabilize membrane-bound proteins and serve as cofactors for enzymatic reactions. The generation of first and second messengers, for example, inositol trisphosphate, phosphatidylinositol 4,5-bisphosphate and platelet-activating factorm, are responsible for regulating PL metabolism, secretion, cellular architecture and membrane fusion. Although the structural building blocks for lipid molecules are not very complex, they have the potential to generate almost 100,000 different molecular species (van Meer, 2005; Yetukuri et al., 2008). This lipid diversity is regulated and synthesized by a considerable part of the genome, and the role of this compositional complexity remains broadly defined.
Figure 1.
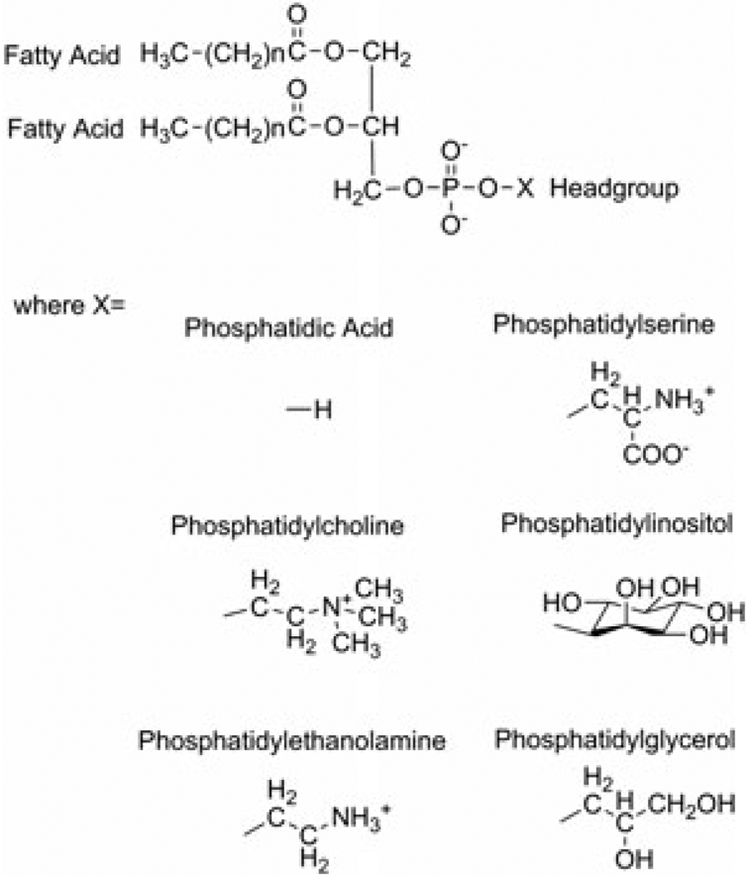
Structures of phospholipid classes.
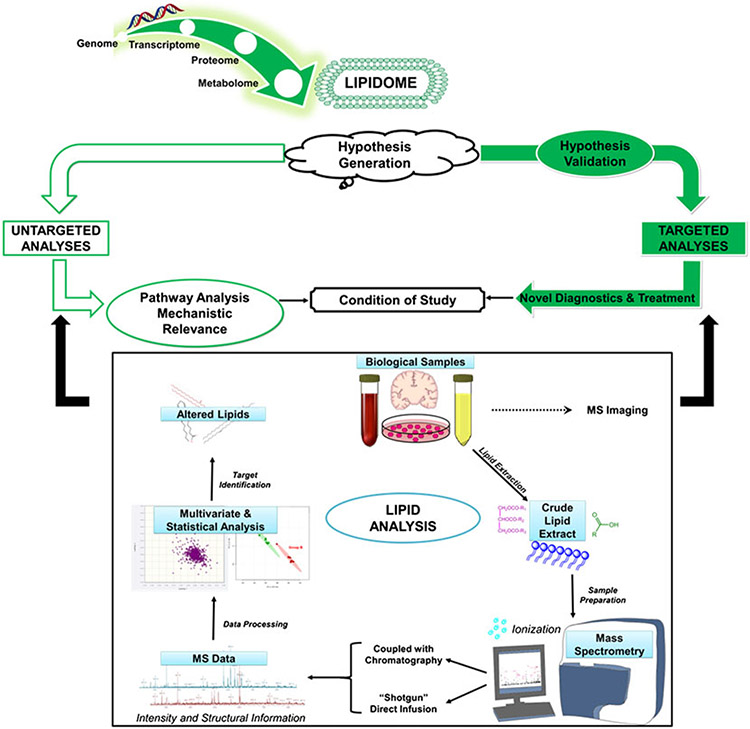
The complete profile of lipid species present in a cell, organelle or tissue refers to the lipidome, whereas the study of lipid profiles within biological systems can be referred to as lipidomics (Gross and Han, 2011). Lipidomics seeks to identify lipid alterations within a target system, characterize molecular species and determine the roles of genes and proteins involved in lipid metabolism and lipid-mediated signaling (Gross and Han, 2011; Wenk, 2010). Identifying the biological significance of lipid alterations can provide insight into processes that regulate cellular homeostasis in health and disease states. Although lipids stand out among cellular metabolites in the sheer number of unique species, this class of biomolecules remained in the shadow of the ongoing ‘omics’ revolution for a long time. The chemical and structural variety of lipids makes analysis using a single experimental approach quite challenging (Shevchenko and Simons, 2010; Wenk, 2010). Research in the field relies on the use of multiple techniques to detect and quantify components of cellular lipidomes as well as determine structural features and cellular organization. Understanding these features can allow researchers to elucidate the biochemical mechanisms by which lipid–lipid, lipid–protein interactions take place within the conditions of the study. Lipidomics studies, both untargeted and targeted, use similar experimental protocols that have been heavily bolstered by recent developments in modern mass spectrometry (MS)-based analytical platforms (Fig. 2). Lipidomics is a rapidly expanding field of research, which has emerged as a valuable approach to understanding lipid biology in the biosciences.
Figure 2.
Lipidomic workflow.
Herein, we will provide an overview of essential methods for the analysis of lipids, including extraction methods, chromatographic techniques and approaches for mass spectrometric analysis.
Extraction techniques
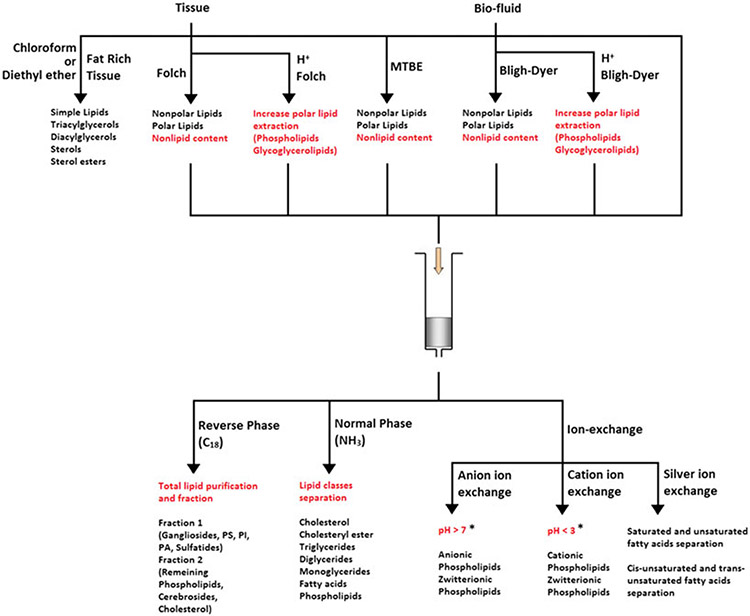
The complexity of biological samples, such as tissues, cells and body fluids, often requires optimization of techniques for sample preparation. For the extraction and analysis of lipids, there are two major challenges to overcome, which are extraction efficiency and complete removal of nonlipid contents. By optimizing sample preparation, it is possible to increase throughput and analyses reproducibility. This section will discuss organic solvent extraction and solid-phase extraction (SPE), which have been used extensively for lipid sample preparation. Figure 3 provides a summary of these extraction techniques.
Figure 3.
Overall applications of extraction techniques on lipids research. *pH values are approximated, pH adjustment depends on analytes
Organic solvent extraction
Proper organic solvent extraction relies heavily on the selection of lipid-soluble solvents. For polar lipids containing both hydrophilic and hydrophobic groups, appropriate organic solvents include chloroform and methanol. Alkane solvents are well suited for extraction of nonpolar lipids lacking hydrophilic groups, such as triacyglycerols and sterols. Lipid solubility can be quantified directly using pure lipid standards; however, predicting or measuring extraction efficiencies is often a challenge when extracting lipids from biological samples. This is due mainly to the strong interactions between lipids and cell biopolymers such as proteins and polysaccharides. Extraction solvents should be selected carefully in order to disrupt these molecular associations, with a balance of both polar and nonpolar characteristics. Polar solvents such as methanol have high dielectric constants that are able to access regions of ion–dipole interactions and hydrogen bonds, and can interrupt such associations.
The Folch method, published in 1957, is the most commonly used lipid extraction technique (Folch et al., 1957). It uses a mixture of chloroform and methanol in a 2:1 ratio, which can simultaneously overcome both hydrophobic and polar interactions between lipids and biopolymers. A wash step is incorporated following extraction to remove nonlipid components using small volumes of water. It is critical that the ratio of chloroform–methanol–water is maintained at 8:4:3 to prevent the loss of polar lipids as a result of excess water. This method yields higher lipid extraction efficiency than any other single solvent method.
The Bligh and Dyer method was introduced as a variation of the Folch method (Bligh and Dyer, 1959), which incorporated water present within samples into the solvent system and made the extraction procedure convenient and economical. Generally, the Folch method is used for the extraction of lipids from solid tissue whereas the Bligh–Dyer method is advantageous for biological fluids (Schiller et al., 2004).
Charged, polar lipids such as glycerophospholipids are able to bind to various biopolymers through ionic interactions that cannot be easily disrupted by polar organic solvents. In such cases, pH adjustments to the aqueous medium prior to extraction can be beneficial for achieving quantitative extraction. Acidification is an effective way to increase extraction efficiency. The addition of acid can convert negatively charged ionized molecules (lipids or biopolymers) to non-ionized forms, interrupting ionic interactions along with increasing lipid hydrophobicity. This was demonstrated in a study where the extraction of polar lipids from M. thermoautotrophicum cells was six times greater by replacing the water in the Bligh–Dyer solvent system with 5% trichloroacetic acid (Nishihara and Yosuke, 1987). We also determined that acidified extraction is not only beneficial for maximal recovery, but can also increase the ionization efficiency of lipids in mass spectrometric analysis (Fig. 4). However, acidification of extraction procedures must be used cautiously, where the pH range is maintained at 2–4. Ester bonds are vulnerable to acid, and hydrolysis can take place after long exposures to concentrated acid, resulting in the loss of lipids. To overcome this phenomenon, Nishihara and Yosuke (1987) used 5% trichloroacetic acid in the place of hydrochloric acid (2 m).
Figure 4.
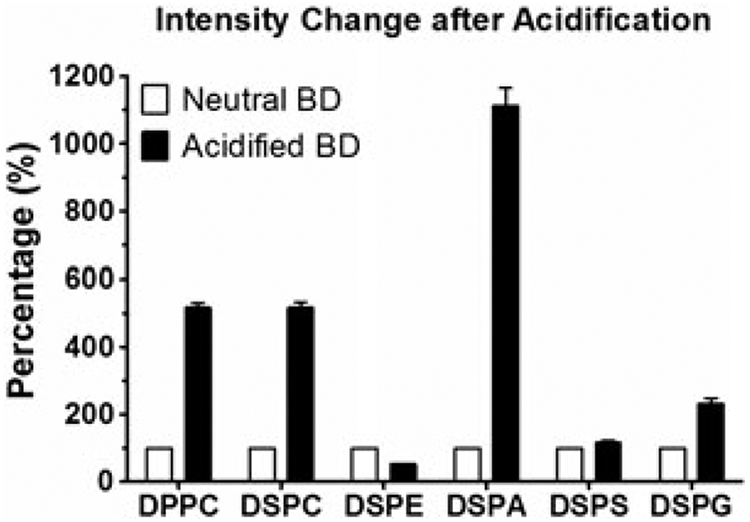
Effect of acidification on the signal intensity of DPPC, DSPC, DSPE, DSPA, DSPS, and DSPG after acidified Bligh–Dyer (BD) extraction. Six different phospholipids were prepared, extracted using neutral or acidified BD extraction, then analyzed using ESI-MS. The intensity changes after acidification were shown via comparing to control. The conventional BD extraction was set as control and made 100%. Data are presented as mean ± SEM (n = 3).
Temperature is another feature that should be controlled. Low temperatures are essential for large sample sizes undergoing acidified extraction and quantitation. Our laboratory, along with many others, showed that reduced temperatures reduce degradation and improve lipid stability (Fig. 5). The control of both pH and temperature can prevent the occurrence or reduce the rate of hydrolysis. It is important to note that acidified extraction methods are not suitable for the long-term storage of samples, thus requiring analysis immediately following extraction.
Figure 5.
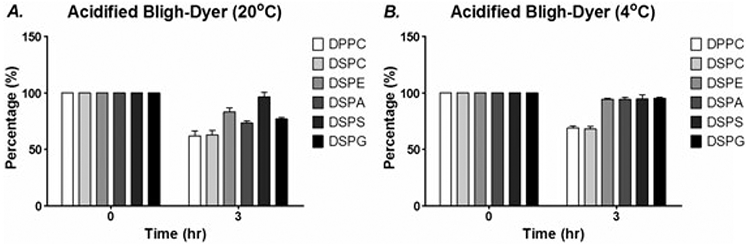
Effect of acidification on stability of phospholipids after acidified BD extraction. Six different phospholipids were prepared, extracted using neutral or acidified BD extraction. The extracts were allowed to incubate at either 20 or 4°C for 0 or 20 h prior to analysis using ESI-MS. With acidified BD extraction, a varying extent of degradation was observed with all phospholipids except DSPS at 20°C (A). At 4°C (B), only phosphatidylcholines (PCs) show significant degradation. No significant degradation was observed in samples extracted using conventional BD extraction. The time point of 0 h was set as a control with the value being set at 100%. Data are presented as mean ± SEM (n = 3).
A liquid–liquid extraction method utilizing methyl tert-butyl ether (MTBE)/methanol was developed recently and simplified the handling of samples in comparison to the Folch and Bligh–Dyer methods (Matyash et al., 2008). In this method, the low density of MTBE causes the lipid-containing organic phase to form the upper layer during liquid-phase extraction. In the Folch and Bligh–Dyer methods, the lipid-containing phase forms the lower layer due to the high density of chloroform, which can make lipid layer collection difficult. Also, non-extractable matrices such as denatured proteins can be removed by centrifugation easily since they form a dense pellet at the bottom of the extraction tube instead of in between the upper and lower phases as observed with the Folch and Bligh–Dyer methods. This suggests that the MTBE method yields similar or better recoveries of most major lipid classes compared with Folch or Bligh–Dyer. It can also be used for the simultaneous extraction of lipids and metabolites for metabolic profiling and lipidomic analysis. MTBE extractions can be performed on limited amounts of tissue samples (i.e. 2.5 mg) and are suitable for the handling of clinical specimens (Chen et al., 2013).
Organic solvent extraction, such as chloroform and methanol, has been used widely for lipid research and while there are various advantages, limitations persist. Some of these include labor-intensive and time-consuming procedures such as multiple extractions per sample for optimal recovery. Extraction efficiencies can be inconsistent, especially in the presence of emulsifiers, a common occurrence in complex biological samples. Emulsions can result in the partial or complete loss of complex glycolipids and polar prostaglandins in the aqueous layers (Chen et al., 2013). Further, the need for high volumes of toxic, organic solvents requires researchers to utilize extra preparative measures for safety.
It is important to point out that methanol, mentioned in the methods above, does not typically serve as an extraction reagent for lipids since it is miscible with water. Rather, it is used as a reagent that disrupts the interaction between lipids and biopolymers. Similar solvents would be ethanol, 1-propanol, 2-propanol and n-butanol. While ethanol may offer a similar disruption effect as methanol, 1- and 2-propanol may be weaker owing to the larger hydrophobic moiety. n-Butanol is the weakest in disruption among these four solvents, but it is only partially miscible with water, and can sometimes be used to extract lipids without the aid of other organic solvents. Altering solvents with the goal of providing a cleaner matrix is usually more applicable when the analyte is fully understood. In this case, the solvents can be chosen accordingly to inhibit the extraction of undesirable lipids and nonlipid content. However, this is not always the case in most lipid research, especially in lipidomics where hundreds, if not thousands, of lipids are extracted at once. Generally, a recommended approach would be to extract total lipids as much as possible, then further clean the sample using SPE if necessary. In such a scenario, methanol is usually the best choice.
Solid-phase extraction
Solid-phase extraction was first introduced in the mid-1970s as a method for quick and efficient sample preparation for lipid analysis (Thurman and Mills, 1998). This technique uses principles similar to those in liquid chromatography where lipids in hydrophilic media are retained on the SPE column, while the nonlipid impurities are allowed to pass through. Lipids are then eluted and collected using organic solvents with lower polarities. SPE is a useful method for the isolation and purification of targeted lipids along with the enrichment of minor lipid species. The cartridges come in a variety of formats for the handling of different lipids, matrices, volumes and sample concentrations. Some of these include the syringe barrel, large volume capacity barrel, 96-well format and pipette tip format. SPE cartridges, like columns used in high-performance liquid chromatography (HPLC), can be classified as reversed phase, normal phase and ion exchange. SPE is powerful for a broad range of applications and when combined with a variety of elution procedures, is useful for total lipid purification as well as class separation.
With reversed-phase SPE (C18) cartridges, crude lipid extracts obtained from the Folch or Bligh–Dyer method can be diluted with water/methanol, and then loaded on the activated SPE cartridge. As the polarity of the solvent is increased, lipids remain bound to the column through hydrophobic interactions while water-soluble impurities can pass through. The remaining lipids can be collected by elution using chloroform–methanol (1:2, v/v). Alternatively, a two-step elution can permit two fractions to be collected. This would require the first elution to be performed with methanol–water (12:1, v/v), and the second elution using chloroform–methanol (1:2, v/v). In doing so, the first fraction contains gangliosides, phosphatidylserine, phosphatidylinositol, phosphatidic acid and sulfatides, while the second fraction contains all phospholipids, cerebrosides and cholesterol (Kyrklund, 1987).
Normal-phase SPE bind lipids via polar interactions, such as hydrogen-bonding and ion-dipole forces, and can used to separate polar lipids from nonpolar lipids that lack polar functional groups. The cartridges available for normal-phase SPE include amino- (NH2), cyano- (CN) and silica (Si) phases. Kaluzny et al. (1985) demonstrated that aminopropyl (NH2) normal-phase SPE can serve as an effective technique to separate different lipid classes including cholesterol, cholesteryl esters, triglycerides, diglycerides, monoglycerides, fatty acids and phospholipids. For example, Kaluzny et al. reported the performance of three NH2-SPE cartridges loaded with crude lipid extracts. The first elution was performed with chloroform–isopropanol (2:1, v/v), ethanol–acetic acid (98:2, v/v) and methanol, which sequentially produced three fractions. The first contained cholesterol, cholesteryl esters, tryglycerides, diglycerides and monoglycerides, which were then loaded onto a second cartridge. The second and third fractions contained fatty acids and phospholipids, respectively. Additional elution steps were carried out sequentially with hexane (100%), hexane–dichloromethane–ethanol (89:10:1, v/v), hexane–ethyl acetate (95:5, v/v), hexane–ethyl acetate (85:15, v/v) and chloroform–methanol (2:1, v/v). This yielded five fractions: (1) cholesterol esters, and later fractions with (3) 90% of cholesterol, (4) diglycerides and (5) monoglycerides. A third cartridge was then able to separate the second fraction (2) into triglycerides and 10% cholesterol. A modified procedure was also developed for separating chlorinated fatty acid methyl esters from the unchlorinated counterparts (Åkesson-Nilsson, 2003).
Ion-exchange SPE, another useful technique for the isolation and separation of lipid species, can be classified into weak or strong cation and anion exchangers. Strong cation exchangers contain negatively charged benzenesulfonic acids or propylsulfonic acids, while weak cation exchangers have carboxylic acids that are charged at high pH ranges and neutral at low pH ranges. Strong anion exchangers possess positively charged quaternary amines, while weak anion exchangers have primary, secondary or tertiary amines that are positively charged at low pH but neutral at high pH. An earlier method used silver ion-exchangers that have silica-based benzenesulfonic acid SPE cartridges, which can be loaded with a solution of silver nitrate in acetonitrile–water (10:1, v/v) for conversion to the ionic form of silver. The elution scheme for isolating fractions from these ion-exchange cartridges uses varying ratios of dichloromethane, acetone and acetonitrile to separate saturated monoenes, dienes, trienes, tetraenes, pentaenes, hexaenes-fatty acid methyl ester derivatives (Christie, 1989). A similar study was performed to achieve separation of saturated, unsaturated and trans-unsaturated fatty acids (Goto et al., 2012). Ion-exchange SPE cartridges are used widely by researchers for the removal of lipids from analytes of interest (Shen et al., 2005; Yoon et al., 2015). Method development can be optimized easily for separating positively or negatively charged lipids, and is especially appropriate for phospholipids. Anionic phospholipids such as phosphatidic acid and phosphatidylserine are negatively charged at high pH ranges, permitting them to be retained on strong anion-exchange SPE cartridges, allowing cationic phospholipids (neutral at high pH ranges) to pass through. Eluting solvent pH is easily adjusted to <3, where anionic phospholipids are neutral and can be easily collected from the SPE cartridge. Similar procedures can be performed for cationic phospholipids using strong cation exchange SPE. Zwitterionic phospholipids are negatively charged at high pH and positively charged at low pH, which makes them good candidates for either strong anion or cation exchange SPE with pH adjustments. Mixed mode ion-exchange SPE is a combination of reversed-phase and ion-exchange modes and provides an additional dimension of separation for charged and neutral lipids using proper strengths of organic solvents.
SPE is not only an effective tool for analysis of crude lipid extracts, but can be also applied directly to some biofluid samples such as serum, urine and cerebrospinal fluid. It is important to note that acidification is an essential step prior to sample loading onto SPE cartridges in order to increase lipid retention. Further, low loading and reduced flow rates should be employed for maximal retention. Online SPE liquid chromatography (SPC-LC) is the preferred method when compared with manual SPE (offline). It is highly reproducible, less time consuming and labor intensive, and reduces the potential of exposure to potentially hazardous biological samples. Although online SPE has several advantages including high-throughput features for large sample sizes, clinical research, and diagnostics, it has higher instrumentation costs (de Jong et al., 2007).
Chromatographic methods
One method employed for many lipidomic studies involves the direct infusion of whole lipid extracts into ESI sources. (Han and Gross, 2005b; Han et al., 2005, 2006; Hsu et al., 1998; Koulman et al., 2007; Schwudke et al., 2006). This is referred to as a ‘shotgun’ approach (Han and Gross, 2005a) and involves no chromatographic separation of lipid classes prior to infusion. This platform permits analysis of the lipidome directly from crude lipid extracts where lipids undergo intrasource separation and can be analyzed using precursor ion and neutral loss scans to identify key lipid fragments. While shotgun lipidomics is simple, high-throughput and quick, requiring short data acquisition times, a significant disadvantage of this approach is that many compounds, such as major phospholipid molecular species and their counter-ions, can compete for ionization upon simultaneous infusion into the ESI source and result in ion suppression (Roberts et al., 2008; Wolf and Quinn, 2008). As a result of this competition, ion-suppression can interfere with the detection of minor species within a sample matrix. Detection and sensitivity can also be limited by the presence of isobaric species (Ivanova et al., 2009). Introducing a preliminary separation step prior to infusion, such as HPLC, high-performance thin-layer chromatography (HP-TLC) or gas chromatography (GC), can minimize suppression, allowing compounds to be resolved sequentially with greater ionization yields and increased sensitivity (Petković et al., 2001; Roberts et al., 2008; Wenk et al., 2003; Wolf and Quinn 2008).
Gas chromatography can be used for phospholipid analysis; however, this is not an optimal choice given that separation is dependent on analyte volatility (Axelsen and Murphy, 2010; Buyer and Sasser, 2012). For this technique, phospholipids can be hydrolyzed to diacyglycerols with phospholipase C and derivatized for GC analysis (Tserng and Griffin, 2003). A further drawback is that these analyses require high column temperatures that can result in sample degradation, compromised sensitivity and data quality. Capillary columns are commonly used and provide chromatographic resolution as well as reproducible retention times (Dunn et al., 2011). Although there is a wide range of stationary phases available for GC, methyl-phenyl columns are typically used, and flame ionization detectors serve as the most common detection method (Dunn et al., 2011).
Thin-layer chromatography (TLC) was the earliest and widely established chromatographic method used for lipid assessment that continues to be employed today (Fuchs et al., 2007; Touchstone, 1995; Weerheim et al., 2002). Recent advancements in the application of TLC and HP-TLC have resulted in greater separation efficiencies during lipid analysis (Carrasco-Pancorbo et al., 2009). TLC has proven to be a highly effective and versatile technique for phospholipid separation (Touchstone, 1995). The majority of TLC-based phospholipid separations are performed using a silica gel stationary phase, in conjunction with organic solvents such as chloroform, methanol, water and modifiers as the mobile phase (Handloser et al., 2008). Separation takes place on layers of silica gel adsorbent on glass plates where lipid samples are applied as discrete spots. Lipid samples travel in the mobile phase at different rates based on their affinity for the silica adsorbent. One-dimensional TLC is useful for simple mixtures, while two-dimensional TLC (2D-TLC) yields better resolution for complex lipid mixtures. HP-TLC is commonly coupled with densitometric quantification and various detection reagents can be used to identify resolved lipid classes (Grizard et al., 2000; Helmy, 2004; Moe et al., 2004; Sommerer et al., 2004; Zhong et al., 2000). TLC and HP-TLC are advantageous because of simplicity, high resolving power, and affordability, but are limited by low resolution, sensitivity and lipid recovery (Carrasco-Pancorbo et al., 2009; Cartwright, 1993). Although TLC is a quick method with no sample carryover as each analyte has a new stationary phase, liquid chromatography typically gives better separation (Touchstone, 1995) and may be coupled to a variety of detectors.
High-performance liquid chromatography, in comparison to other chromatographic methods, is the most popular method and has very broad applications in lipid analysis. This is possibly due to the ability to utilize multiple HPLC methodologies for isolation and analysis of phospholipids. Partition chromatography is defined by two polarity modes: normal-phase liquid chromatography (NP-LC) and reversed-phase liquid chromatography (RP-LC) (Carrasco-Pancorbo et al., 2009). Generally, NP-LC separates lipid classes, while RP-LC separates based on fatty-acyl composition (Lin, 2007). For this reason, NP-LC is useful for separating phospholipids with differing head groups, while RP-LC can separate phospholipids of the same class. Both normal-phase and reversed-phase based separation methods have been used for the separation of lipid classes; however, complete class separation has not been shown (Castro-Perez et al., 2010; Peterson and Cummings, 2006). While the mobile phases selected for HPLC vary depending on the column of choice, typical solvents used for chromatographic elution include alcohols, such as methanol and 2-propanol, acetonitrile, hexane, chloroform and/or water (Uran et al., 2001). For subsequent mass spectrometric analyses, acetic or formic acid are added along with an amino-base (ammonia, methylamine, diisopropylamine and piperidine) in order to facilitate ion-pairing in both negative and positive ionization modes (Wolf and Quinn, 2008).
Normal-phase methods are used in HPLC to accomplish initial separation of phospholipid classes through either gradient or isocratic elution (Hermansson et al., 2005; Houjou et al., 2005; Ivanova et al., 2007; Taguchi et al., 2000). In NP chromatography, phospholipids elute in order from the most hydrophobic to the most hydrophilic using a polar stationary phase and a less polar mobile phase (Houjou et al., 2005). Elution patterns are based largely on the polar properties of the phospholipid head group. During instances where head groups possess similar polarities, separation is accomplished based on differences in their backbone. Commonly used NP stationary phases include silica, alumina and cyano columns (Meyer, 1998; Snyder et al., 1997). Silica is the most common of the nonbonded phases and yields greater selectivity; however, this method can be limited by retention time reproducibility across runs owing to water adsorption (Meyer, 1998; Snyder et al., 1997). Alumina is not as common because of its low theoretical plate number, as well as retention time variability and poor sample recovery. Cyano stationary phases are quite stable and can also be used for phospholipid analysis. Selection of solvents for NP chromatography requires consideration of several factors, including solvent strength, localization, basicity and cutoffs for UV detection (Meyer, 1998; Snyder et al., 1997). Disadvantages to using normal phases include lengthy elution and equilibration times, resulting in low throughput. Chloroform and hexane are commonly used mobile phases, both of which introduce ionization challenges when coupled with MS (Henderson and McIndoe, 2006). A more recently identified approach that can be used to retain amphiphilic compounds such as phospholipids is hydrophilic interaction liquid chromatography (HILIC) (Schwalbe-Herrmann et al., 2010; Zhu et al., 2012). This technique is a combination of a polar stationary phase with a highly organic mobile phase that increases the retention of solutes as the percentage of organic solvent is increased (Zhu et al., 2012). Although HILIC is a variant of NP chromatography, RP solvent systems are frequently used to avoid the issues commonly encountered with conventional normal phase methods. Some of the advantages HILIC presents are lower back pressures and higher sensitivity when coupled with ESI-MS.
Reversed-phase chromatography uses a nonpolar stationary phase and a polar mobile phase and is based on lipophilicity, where lipids within the same class separate according to carbon chain length and quantity of double bonds (Perona and Ruiz-Gutierrez, 2003; Pietiläinen et al., 2007). C18, C8 and octadecylsilyl RP columns have been employed using eluting solvents such as methanol, isopropanol, acetonitrile, hexane and chloroform (Medina-Gomez et al., 2007; Rainville et al., 2007; Wolf and Quinn, 2008). When using these columns, hydrophobic C18 groups interact with the hydrophobic fatty acid chains of phospholipids (Zhai and Reilly, 2002). As a result, phospholipids with longer fatty acid chains possess greater solute hydrophobicity, and retention times are decreased as the number of double bonds on fatty acid chains is increased (Zhai and Reilly, 2002). Resolution following separation and speed optimization can be implemented with higher column temperatures with or without gradients, along with column particle sizes <2 μm (Rainville et al., 2007). A disadvantage to using RP conditions is that hydrophilic compounds are often not as well retained as those observed in NP-LC (Meyer, 1998; Snyder et al., 1997). As an alternative, two-dimensional liquid chromatography (2D-LC), made up of a combination of separation systems, i.e. NP-RP-LC, can offer increased chromatographic selectivity as well as optimized lipid separation.
Detectors
The most common chromatographic detection methods include refractive index detection (RID), evaporative light-scattering detection (ELSD), electrochemical detection, suppressed conductivity detection and charged aerosol detection (Carrasco-Pancorbo et al., 2009). ELSD utilizes a universal detector that responds to any analyte different from the mobile phase through continuous monitoring, resulting in minimal background signal. ELSD is compatible with a broad range of solvents as well as gradient elution, although isocratic elution, a feature not permitted by RID, is optimal. Furthermore, unlike ultra-violet (UV) detection, the output signal from ELSD is independent of acyl chain length and degree of saturation (Carrasco-Pancorbo et al., 2009). UV detectors are used very frequently for HPLC, as they are relatively inexpensive and easily accessible. These detectors are sensitive, selective, and are optimal for natural lipids containing conjugated double bonds. While UV detection is widely used for phospholipid analysis (Christie, 1987), fluorescence-based detection is not, since only a few rare lipids possess natural fluorescence. Fluorescence can be useful, however, for analysis of fatty acids following conversion to suitable derivatives. Similarly, infrared (IR) detectors are limited to nonpolar lipids with a narrow window for absorbance detection (Hamilton et al., 1987). Recently, detectors with laser light sources have emerged and exceeded the capabilities of previous technologies. One example is the evaporative laser light scattering detector, which has outperformed ELSD in terms of sensitivity, stability and reproducibility (Carrasco-Pancorbo et al., 2009). Charged aerosol detection, developed only in 2004, has also demonstrated greater sensitivity than ELSD with a broader dynamic range (Eom et al., 2010; Moreau, 2009). Normal-phase chromatography is often coupled to ELSD (Hvattum et al., 2006) or UV detection (Saldanha et al., 2006). These detectors are restricted by the lack of selectivity and choice of mobile phase, making MS the preferred detector for lipidomic studies (Malavolta et al., 2004; Wang et al., 2004).
Mass spectrometry
During MS analysis lipid samples undergo ionization and vaporization upon entering the mass spectrometer, and are sorted by mass to charge ratio (m/z) within the mass analyzer (Griffiths and Wang, 2009). Introduction of crude lipid extracts with no prior separation via direct infusion is called shotgun lipidomics (Han et al., 2005; Schwudke et al., 2007b; Shevchenko and Simons, 2010). The shotgun approach, discussed in detail above, is a rapid and effective way to assess lipid profiles. Although an informative and high-throughput approach, shotgun analyses can often be complicated by ion suppression (Blanksby and Mitchell, 2010), as well as inherent bias towards more abundant and easily ionized lipids (Griffiths and Wang, 2009; Han et al., 2012). The alternative to the shotgun method is incorporation of a chromatographic separation prior to MS analysis, resulting in reduced ion suppression and greater sensitivity (Murphy and Gaskell, 2011). However, ionization conditions and column memory effect, the carryover of sample constituents across analysis, can pose significant challenges. Major lipidomic approaches are either the targeted study of individual lipid classes or their subclasses (Han et al., 2012; Lee et al., 2003), or global, broad spectrum profiling of crude lipid extracts. Unfortunately, global lipidomics often results in complex spectra containing numerous isobaric species, and targeted approaches often encounter low abundances of desired lipid classes. Chromatography can address both of these issues by separating target analytes from complex matrices (Blanksby and Mitchell, 2010).
Crude lipid extracts can also be used for direct identification of lipids based on mass via high-resolution mass spectrometers such as the Fourier transform ion cyclotron resonance and FT-Orbitrap (Schwudke et al., 2007a; Shevchenko and Simons, 2010). This is known as top-down lipidomics and these instruments facilitate rapid scanning and high mass accuracy (Griffiths and Wang, 2009; Han et al., 2005). The top-down approach is primarily used for determining alterations in lipid patterns. For the majority of lipid studies, however, tandem mass spectrometry (MS/MS) is the dominant method (Shevchenko and Simons, 2010). The application of tandem MS experiments is referred to as bottom-up lipidomics, where various scanning options (precursor ion scan, product ion scan, neutral loss scan) are used to generate specific fragment ions (Schuhmann et al., 2011; Shevchenko and Simons, 2010). Selected reaction monitoring or multiple reaction monitoring can be used to maximize sensitivity for specific precursors or fragment mass pairs (Griffiths and Wang, 2009). There have been newer developments using nanospray technologies, which have increased the sensitivity of lipid analysis as well as sample throughput using chip-based nanospray array devices (Ejsing et al., 2009). For example, nano-electropray sources (Wilm and Mann, 1994, 1996; Wilm et al., 1996) are able to allow analysis of picomolar or sub-picomolar levels of lipids (Brügger et al., 1997).
Over the past 40 years, GC-MS coupled with electron ionization (EI) has served as a basis for lipid identification and quantification. Although widely used, limitations with this method persist (Berry, 2004). Throughout the mid-1970s, field ionization (FI) was employed; however, lower and less stable ion currents made this method unpopular (Wood and Lau, 1974). Recently, FI has been used in conjunction with orthogonal acceleration time-of-flight MS. This combination has overcome some of the previous challenges posed by FI (Hejazi et al., 2009). Another earlier method used for the analysis of complex, high molecular weight lipids was fast atom bombardment (FAB) MS. It is important to note that findings from studies conducted using FAB, coupled with tandem MS, have laid the foundation for our understanding of the fragmentation of lipid ionization ( Jensen et al., 1987). However, the drawbacks of FAB, including low sensitivity, matrix ions and source fragmentation, greatly limited analysis and quantitation (Blanksby and Mitchell, 2010). The introduction of electrospray ionization (ESI) MS applied to the analysis of glycerophospholipids addressed many of these limitations, including increasing sensitivity by two orders of magnitude, thereby permitting the detection of more than 50 phospholipid species (Han and Gross, 1994; Kerwin et al., 1994; Kim et al., 1994). ESI allows two approaches for lipidomic studies: shotgun lipidomics and chromatography-coupled MS for phospholipid identification and quantitation. While both approaches possess their strengths, the combination of the two can be the most effective (Blanksby and Mitchell, 2010; Ivanova et al., 2009).
Techniques based on ambient ionization, such as desorption electrospray ionization (DESI), have recently emerged and been applied to lipid analysis (Cooks et al., 2006a; Takats et al., 2004). DESI offers direct sampling from biological tissues and does not require high-vacuum conditions (Wiseman et al., 2005). Rapid evaporative ionization mass spectrometry represents another method for lipid analysis, in which ions are formed from the rapid thermal evaporation of biological tissue from a surgical electrode and then extracted into the MS (Schäfer et al., 2009).
The use of tandem MS techniques, such as parent ion and neutral loss scanning (Hayes and Gross, 1989; Yost and Enke, 1979), in combination with ESI and matrix-assisted laser desorption and ionization (MALDI), has been essential in advancing the field of lipidomics (Blanksby and Mitchell, 2010). Tandem MS with CID is an excellent tool for elucidating lipid structures, as well as enhancing analytical sensitivity in both global and targeted lipidomics (Blanksby and Mitchell, 2010).
Ionization methods
The distinction between ‘hard’ and ‘soft’ ionization MS methods is heavily dependent on the properties of the analyte of interest (Fuchs et al., 2010). Most modern ionization methods are considered to be soft ionization when compared with EI (Chapman, 1995). EI makes use of fast electrons in order to ionize the analyte molecules in the gas phase by the removal of one electron. This method has been used exclusively for decades, mainly because of its suitability for the investigation of small and/or volatile compounds (Gross, 2004). However, EI is not very effective for analyzing larger molecules with low volatilities. While detection of phospholipid molecular ions is possible using EI, the abundance of fragment ions makes it difficult to analyze complex mixtures (Klein, 1971). For this reason, EI-MS is now used for assessing free fatty acids in lipid samples. Chemical ionization (CI) is another ionization method used frequently for lipid analyses, and can be used alone or in combination with atmospheric pressure (APCI) (Byrdwell, 2001; Fuchs et al., 2010).
The advent of soft-ionization methods should be considered a milestone in the history of modern mass spectrometry as it enabled analyses of molecules refractive to EI. These methods include ESI and MALDI. ESI and MALDI are the most popular ion sources for lipidomics studies and are commercially available in the majority of MS devices (Fuchs et al., 2010). It is important to note that they are often regarded as competitive methods; however, they should be considered complementary (Fuchs et al., 2010).
Electrospray ionization
The most widely used atmospheric pressure ionization modes are ESI and APCI (Byrdwell, 2001; Peterson and Cummings, 2006). ESI is an appropriate technique for polar compounds including phospholipids (Hunt and Postle, 2006; Kim et al., 2009; Min et al., 2010; Postle 2009; Postle et al., 2007), while APCI is better suited for less polar molecules (Byrdwell and Emken, 1995; Farwanah et al., 2009; Murphy et al., 2011; Xu and Brenna 2007). The soft ionization of ESI is quite effective for polar lipids since they are readily ionized in both negative and positive ion modes, i.e. protonation [M + H] + or deprotonation [M − H]−, resulting in a single intact ion (Blanksby and Mitchell, 2010). ESI-MS performed on samples containing phospholipids generate unique fragment ions, indicating a loss of the polar head group, which can be diagnostic for the presence of individual classes. For example, m/z 184 is indicative of choline-containing phospholipids and can be detected during product ion analysis of protonated choline-containing phospholipid molecular species. Table 1 provides a summary of unique fragments including adducts, in both positive and negative ion mode, which can be used for phospholipid identification. While saturated and monounsaturated fatty acids ionize well in the negative ion mode, it can be difficult to detect fragment ions. Our approach for detection and MS/MS quantification is to utilize precursor ion scanning as the product ion.
Table 1.
Headgroup fragments for phospholipid fragments
| Class | Characteristic headgroup fragments (m/z) | |
|---|---|---|
| Positive ion mode (ESI+) |
Negative ion mode (ESI−) |
|
| PA | — | — |
| PC | 184, 60 | 224 |
| PE | 141 | 196 |
| PG | — | 227 |
| PI | — | 223, 241, 259, 297, 315 |
| PS | 87 | 185 |
ESI, electrospray ionization; PA, phosphatidic acids; PC, phosphatidylcholine; PE, phosphatidylethanolamines; PG, phosphatidylglycerols; PI, phosphatidylinositols; PS, phosphatidylserines.
Another method for lipid analysis is atmospheric pressure photoionization (APPI), which can extend the range of ionizable compounds (Delobel et al., 2005, 2006; Marchi et al., 2009). ESI, APCI and APPI were recently compared (Cai and Syage, 2006) with regard to their quantitative accuracy and sensitivity for neutral lipids such as fatty acids, diacylgycerols and triacylglycerols. The results proved LC-APPIMS to be useful for quantitative analysis, and ESI and APCI more effective for the analysis of polar lipids, with ESI excelling in reproducibility (Cai and Syage, 2006). Analysis using APPI and ESI coupled with HPLC can be particularly useful for determining information about the polar range of lipids present within complex samples (Cai and Syage, 2006).
MALDI
Following lipid studies using ESI-MS, there were initial reports using MALDI of glycerophospholipid and sphingolipid species (Harvey, 1995a, 1995b; Marto et al., 1995). Given that MALDI is a soft-ionization technique, it yields quasi-molecular ions, instead of radical cations, from abstraction of an electron from the analyte of interest (Fuchs and Schiller, 2009). While APCI (Byrdwell, 2001) and ESI (Pulfer and Murphy, 2003) are commonly used and often preferred for lipidomics studies (Hou et al., 2008), MALDI-MS has proven to be an effective method and is gaining popularity (Fuchs and Schiller, 2008; Schiller et al., 2006). This is partly due to the growing interest in MALDI-MS imaging for lipid studies (McDonnell and Heeren, 2007). The MALDI-MS approach relies on the utilization of a matrix that initially absorbs the energy of the laser and mediates the generation of ions (Fuchs et al., 2010), where the suitability of a certain compound as a matrix is determined by the type of laser and its emission wavelength (Fuchs et al., 2010). Factors that constitute a good MALDI matrix include excellent signal-to-noise ratio for the peaks of the analyte of interest, high absorbance at the laser emission wavelength, low background to avoid interferences between the matrix and the analyte ions, specificity for a single analyte adduct, and low tendency for cluster formation (Fuchs et al., 2010; Lou et al., 2009). Some advantages of MALDI are that measurements are high throughput, time efficiency, relative inexpensiveness and require simpler sample purification owing to high tolerance for salts and impurities. Separation by ion mobility has also been used along with MALDI-MS to fractionate lipids (Jackson et al., 2005; McLean et al., 2007; Woods, 2006).
The phosphatidylcholine (PC) class of phospholipids has been studied most frequently using MALDI-MS. This is primarily because PCs are the most abundant constituents of eukaryotic cellular membranes and are commercially available with a wide range of fatty acid residues and ether derivatives. Additionally, PCs can be easily detected in positive ion mode by their quaternary ammonia group with a permanent positive charge (Fuchs et al., 2010). Unlike ESI, MALDI analysis of phospholipids can result in positive ions for all subclasses, permitting a broader range of detection per acquisition (Caprioli et al., 1997; Petkovicć et al., 2001). Phosphatidylinositol (PI), phosphatidylserine (PS) and phosphatidic acid (PA) are inherently less abundant and have high negative charge densities, thus are not easily detected (Gellermann et al., 2006). It was recently demonstrated that performing a preliminary PC removal step through binding with a small column filled with a silica gel cation exchanger, allows easy monitoring and quantification of other phospholipids such as PI (Johanson and Berry, 2009; Johanson et al., 2007). See Gellerman et al. (2006 for a review on the detection limitations of various phospholipid classes. Detection limitations are particularly important for direct infusion lipid studies using crude lipid extracts from biological samples. In such samples, phospholipids containing quaternary ammonia groups like PCs tend to suppress other classes (Petković et al., 2001). Various lipid classes have also been shown to influence the relative intensities of others when recorded at varying analyte concentrations (Fuchs et al., 2010). The use of an acidic add-on, such as DHB, or an alkaline add-on, such as 9-aminoacridine (9AA) is recommended for acquiring spectra in the positive or negative mode, respectively (Fuchs et al., 2010).
Mass spectrometric imaging
Two-dimensional (2D) MS-based imaging (Boxer et al., 2009; McDonnell and Heeren, 2007; Pacholski and Winograd, 1999; Seeley and Caprioli, 2008) has proven to be a powerful technique for investigating spatial distribution of lipid within substructures of tissues, in which an analyte’s properties are presented as a function of its spatial distribution within the x–y plane. This is an advantageous approach because it does not rely on tedious histochemical labeling protocols (Lane et al., 2009; Nemes et al., 2009). Structure-specific lipid analysis is an especially striking feature of mass spectrometric imaging (MSI), given that lipid analysis is typically performed following extraction protocols that remove information about tissue location (Murphy et al., 2009).
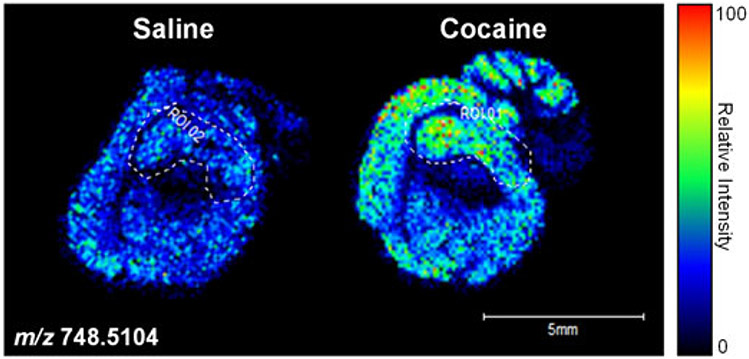
Based on the 2D data obtained, three-dimensional (3D) images can also be recapitulated (Sinha et al., 2008). MALDI-MS is well-established and has become a popular technique to employ for imaging. The advent of MALDI imaging observed a paradigm shift from earlier methods with the ability to analyze secondary ions from biological tissue slices (Jones et al., 2014; Murphy et al., 2009). This method is employed by fixing tissue slices onto MALDI targets, which are then covered by a matrix so that spectral intensities can be used to create an image. The site of the laser spot results in the formation of both positive and negative ions as the biomolecules and neutral matrix analytes are lifted from the underlying tissue. Phospholipids tend to generate an abundant number of negative ions during MALDI-MSI owing to the phosphodiester moiety that can exist as a very stable gas phase anion (Murphy et al., 2009), and is utilized more so for the observation of acidic phospholipids such as PI, PA, PG and PS (Murphy et al., 2009). The resulting mass spectra translate to image pixels based on specific x–y coordinates. Contributors to pixel size are altered by the size of MALDI matrix crystals as well as the laser spot itself (Murphy et al., 2009). Information regarding x–y coordinates, m/z ratios and ion intensity can be obtained from the acquired mass spectra. Thin tissue sections (15 μm) are usually used, resulting in a lateral resolution of 25–100 μm (Murphy et al., 2009). For concentration quantitation, certain lipid classes can be detected, although quaternary ammonia group compounds are often overestimated in the positive ion mode. This can make absolute lipid quantitation cumbersome (Murphy et al., 2009). The presence of matrix compounds and prior chromatographic separation can be incorporated to overcome such issues. Figure 6 shows an example of MS-based imaging using MALDI-FTICR-MS investigating the phospholipid distribution within substructures such as the hippocampus and cerebellum of sagittal brain slices from cocaine- and saline-treated rats. This technique can provide essential information regarding the localization of lipid changes within specific regions of whole tissue, such as the brain
Figure 6.
MALDI-FTICR-MS relative expression and distribution of phospholipid species (m/z 748.5104). MALDI-FTICR-MS was performed on adult mouse sagittal brain sections acquired in the negative ion mode. Left panel: saline-treated; Right panel: cocaine-treated. The color scale to the right of the panel represents the relative intensity normalized to total ion count. Scale bar: 5 mm.
In addition to MALDI, secondary ion mass spectrometry (SIMS) has been used widely for molecular imaging. SIMS involves the use of ion beams that are focused upon inorganic surfaces, which result in the emission of secondary ions that can be analyzed for mass (Murphy et al., 2009). Both MALDI and SIMS are carried out under a vacuum. DESI is a newer, user-friendly method, based on ambient ionization that requires minimal sample preparation and simple analysis (Cooks et al., 2006b; Ifa et al., 2007; Wiseman et al., 2006). Using DESI-MS imaging, a recent study was able to assemble a 3D molecular reconstruction of a mouse brain (Eberlin et al., 2010).
Challenges in lipid research
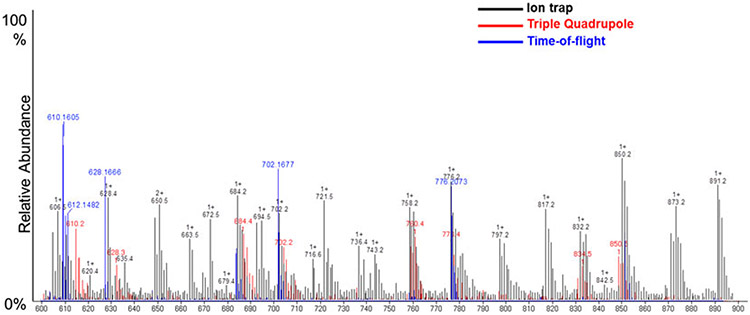
As discussed earlier, complete lipid extraction and resolved chromatographic separation pose difficult challenges for researchers. Another aspect of lipid research that can be difficult in lipid analysis is the validation of structurally characterized molecular species. There are many mass spectrometric platforms used with various ionization techniques; however, the extent of crossover in terms of detection, sensitivity and feature identification is quite limited. Figure 7 demonstrates such variability, in positive ion mode analysis, across three different platforms of ESI-MS: iontrap, time-of-flight and triple-quadrupole MS. Databases for identification of lipid feature assignment are also limited since they are platform specific and are primarily suited for LC-MS lipid analysis rather than direct infusion data. In recent years, there has been an increasing number of research consortia along with individual laboratories that are focusing their efforts toward standardizing experimental protocols and establishing lipid networks (Murphy and Nicolaou, 2013). The advent of newer bioinformatics tools, protocols and instrumentation provides advanced lipidomics approaches for users to perform comprehensive analysis (Quehenberger et al., 2010).
Figure 7.
Comparison of different MS platforms with ESI-MS. The following platforms were used: Trap XCT ion-trap (Agilent Technologies, Santa Clara, CA, USA), LCT Premier time-of-flight (Waters, MA, USA) and triple-quadrupole (Agilent 6460 LC-MS QQQ System). ESI-MS was performed as described previously (Kinsey et al., 2008; Peterson et al., 2008; Zhang et al., 2005) with a nitrogen drying gas flow-rate of 8 L/min at 350°C and a nebulizer pressure of 30 psi. The scanning range was from 600 to 900 m/z on 5 μL of the sample scanned in positive ion mode for 2.5 min with a mobile phase of acetonitrile–methanol–water (2:3:1) in 0.1% ammonium formate.
Conclusion
The development of chromatographic techniques, novel ionization methods and fragmentation strategies has greatly advanced the field of lipidomics, making it now possible to acquire the entire lipidome of cells or individual organelles. These advances have led to the development of sophisticated bioinformatics approaches for automated analyses of complex datasets. There are a growing number of programs and/or software packages that can be used for robust data processing of -omic data, which can be used for spectral filtering, peak detection, alignment, normalization and multivariate analysis, along with mechanistic relevance through metabolic pathway analyses. The available software packages and programs were developed to be dependent on queries of ion masses and elution times, and can be used for both shotgun and chromatography-coupled lipidomics. Examples of such software include LIMSA, LipidXplorer, MetaboAnalyst, XCMS and MZMine (Haimi et al., 2006; Herzog et al., 2012; Katajamaa et al., 2006; Tautenhahn et al., 2012; Xia et al., 2009). The use of such analyses can provide essential information for identifying targets for biomarker development along with the mechanistic roles of lipid mediation/association in disease states and progression. Furthermore, findings from lipidomic studies can be integrated with genomic and proteomic data to provide a better understanding of the role lipids play in various biological and disease processes.
Acknowledgments
This research was funded in part by NIH NIBIB (1R01EB016100) to B.S.C./R.D.A, and Auburn Internal Grants Program.
Abbreviations used:
- 2D-TLC
two-dimensional TLC
- APCI
atmospheric pressure chemical ionization
- API
atmospheric pressure ionization
- APPI
atmospheric pressure photoionization
- CAD
charged aerosol detection
- CI
chemical ionization
- DESI
desorption electrospray ionization
- ECD
electrochemical detection
- EI
electron ionization
- ELLSD
evaporative laser light scattering detector
- ELSD
evaporative light-scattering detection
- ESI
electrospray ionization
- FAB
fast atom bombardment
- FI
field ionization
- FID
flame ionization detectors
- GC
gas chromatography
- HILIC
hydrophilic interaction liquid chromatography
- HPLC
high-performance liquid chromatography
- IR
infrared
- HP-TLC
high-performance thin-layer chromatography
- MALDI
matrix-assisted laser desorption/ionization
- MSI
mass spectrometric imaging
- MRM
multiple reaction monitoring
- MS/MS
tandem mass spectrometry
- MTBE
methyl tert-butyl ether
- NP-LC
normal phase liquid chromatography
- OSE
organic solvent extraction
- PA
phosphatidic acids
- PC
phosphatidylcholines
- PE
phosphatidylethanolamines
- PG
phosphatidylglycerols
- PI
phosphatidylinositols
- PLs
phospholipids
- PS
phosphatidylserines
- REIMS
rapid evaporative ionization mass spectrometry
- RID
refractive index detection
- RP-LC
reversed-phase liquid chromatography
- SCD
suppressed conductivity detection
- SPC-LC
SPE liquid chromatography
- SPE
solid-phase extraction
- SIMS
secondary ion mass spectrometry
- TLC
thin-layer chromatography
- TOF
time-of-flight
References
- Åkesson-Nilsson G Isolation of chlorinated fatty acid methyl esters derived from cell-culture medium and from fish lipids by using an aminopropyl solid-phase extraction column. Journal of Chromatography A 2003; 996(1): 173–80. [DOI] [PubMed] [Google Scholar]
- Axelsen PH and Murphy RC. Quantitative analysis of phospholipids containing arachidonate and docosahexaenoate chains in microdissected regions of mouse brain. Journal of Lipid Research 2010; 51(3): 660–71. DOI: 10.1194/jlr.D001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S Lipid Analysis: isolation, separation, identification and structural analysis of lipids. Nutrition Bulletin 2004; 29(1): 72–3. DOI: 10.1111/j.1467-3010.2003.00361.x. [DOI] [Google Scholar]
- Blanksby SJ and Mitchell TW. Advances in mass spectrometry for lipidomics. Annual Review of Analytical Chemistry (Palo Alto, CA) 2010; 3: 433–65. DOI: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- Bligh EG and Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 1959; 37(8): 911–7. [DOI] [PubMed] [Google Scholar]
- Boxer SG, Kraft ML and Weber PK. Advances in imaging secondary ion mass spectrometry for biological samples. Annual Review of Biophysics 2009; 38: 53–74. DOI: 10.1146/annurev.biophys.050708.133634. [DOI] [PubMed] [Google Scholar]
- Brügger B, Erben G, Sandhoff R, Wieland FT and Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proceedings of the National Academy of Sciences 1997; 94(6): 2339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyer JS and Sasser M. High throughput phospholipid fatty acid analysis of soils. Applied Soil Ecology 2012; 61: 127–30. DOI: 10.1016/j.apsoil.2012.06.005. [DOI] [Google Scholar]
- Byrdwell WC. Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids 2001; 36(4): 327–46. [DOI] [PubMed] [Google Scholar]
- Byrdwell WC and Emken E. Analysis of triglycerides using atmospheric pressure chemical ionization mass spectrometry. Lipids 1995; 30(2): 173–5. DOI: 10.1007/BF02538272. [DOI] [PubMed] [Google Scholar]
- Cai S-S and Syage JA. Comparison of atmospheric pressure photoionization, atmospheric pressure chemical ionization, and electrospray ionization mass spectrometry for analysis of lipids. Analytical Chemistry 2006; 78 (4): 1191–9. DOI: 10.1021/ac0515834. [DOI] [PubMed] [Google Scholar]
- Caprioli RM, Farmer TB and Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Analytical Chemistry 1997; 69(23): 4751–60. [DOI] [PubMed] [Google Scholar]
- Carrasco-Pancorbo A, Navas-Iglesias N and Cuadros-Rodríguez L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: modern lipid analysis. TrAC Trends in Analytical Chemistry 2009; 28(3): 263–78. DOI: 10.1016/j.trac.2008.12.005. [DOI] [Google Scholar]
- Cartwright I. Separation and Analysis of Phospholipids by Thin Layer Chromatography, Biomembrane Protocols, Graham J and Higgins J (eds). Humana Press: Totawa, NJ, 1993. [DOI] [PubMed] [Google Scholar]
- Castro-Perez JM, Kamphorst J, DeGroot J, Lafeber F, Goshawk J, Yu K, Shockcor JP, Vreeken RJ and Hankemeier T. Comprehensive LC-MSE lipidomic analysis using a shotgun approach and its application to biomarker detection and identification in osteoarthritis patients. Journal of Proteome Research 2010; 9(5): 2377–89. DOI: 10.1021/pr901094j. [DOI] [PubMed] [Google Scholar]
- Chapman J. Encyclopedia of Analytical Science. Academic Press: London, 1995. [Google Scholar]
- Chen S, Hoene M, Li J, Li Y, Zhao X, Häring H-U, Schleicher ED, Weigert C, Xu G and Lehmann R. Simultaneous extraction of metabolome and lipidome with methyl tert-butyl ether from a single small tissue sample for ultra-high performance liquid chromatography/mass spectrometry. Journal of Chromatography A 2013; 1298: 9–16. [DOI] [PubMed] [Google Scholar]
- Christie WW. High-performance Liquid Chromatography and Lipids: a Practical Guide, 1st edn. Pergamon Press: Oxford, 1987. [Google Scholar]
- Christie WW. Silver ion chromatography using solid-phase extraction columns packed with a bonded-sulfonic acid phase. Journal of Lipid Research 1989; 30(9): 1471–3. [PubMed] [Google Scholar]
- Cooks RG, Ouyang Z, Takats Z and Wiseman JM. Ambient mass spectrometry. Science 2006a; 311(5767): 1566–70. [DOI] [PubMed] [Google Scholar]
- Cooks RG, Ouyang Z, Takats Z and Wiseman JM. Detection technologies. Ambient mass spectrometry. Science 2006b; 311(5767): 1566–70. DOI: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA, de Vries EG and Kema IP. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC-tandem mass spectrometry. Clinical Chemistry 2007; 53(9): 1684–93. [DOI] [PubMed] [Google Scholar]
- Delobel A, Touboul D and Laprévote O. Structural characterization of phosphatidylcholines by atmospheric pressure photoionization mass spectrometry. European Journal of Mass Spectrometry 2005; 11(4): 409–17. [DOI] [PubMed] [Google Scholar]
- Delobel A, Roy S, Touboul D, Gaudin K, Germain DP, Baillet A, Brion F, Prognon P, Chaminade P and Laprévote O. Atmospheric pressure photoionization coupled to porous graphitic carbon liquid chromatography for the analysis of globotriaosylceramides. Application to Fabry disease. Journal of Mass Spectrometry 2006; 41(1): 50–8. DOI: 10.1002/jms.945. [DOI] [PubMed] [Google Scholar]
- Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, Brown M, Knowles JD, Halsall A, Haselden JN, Nicholls AW, Wilson ID, Kell DB and Goodacre R. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nature Protocols 2011; 6(7): 1060–83. DOI: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- Eberlin LS, Ifa DR, Wu C and Cooks RG. Three-dimensional vizualization of mouse brain by lipid analysis using ambient ionization mass spectrometry. Angewandte Chemie International Edition 2010; 49(5): 873–6. DOI: 10.1002/anie.200906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroos K, Klemm RW, Simons K and Shevchenko A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proceedings of the National Academy of Sciences 2009; 106(7): 2136–41. DOI: 10.1073/pnas.0811700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom HY, Park SY, Kim MK, Suh JH, Yeom H, Min JW, Kim U, Lee J, Youm JR and Han SB. Comparison between evaporative light scattering detection and charged aerosol detection for the analysis of saikosaponins. Journal of Chromatography A 2010; 1217(26): 4347–54. DOI: 10.1016/j.chroma.2010.04.047. [DOI] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ and Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. Journal of Lipid Research 2009; 50(Supplement): S9–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwanah H, Wirtz J, Kolter T, Raith K, Neubert RHH and Sandhoff K. Normal phase liquid chromatography coupled to quadrupole time of flight atmospheric pressure chemical ionization mass spectrometry for separation, detection and mass spectrometric profiling of neutral sphingolipids and cholesterol. Journal of Chromatography B 2009; 877 (27): 2976–82. DOI: 10.1016/j.jchromb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M and Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 1957; 226(1): 497–509. [PubMed] [Google Scholar]
- Fuchs B and Schiller J. MALDI-TOF MS Analysis of Lipids from Cells, Tissues and Body Fluids. Lipids in Health and Disease. Springer: Berlin, 2008. [DOI] [PubMed] [Google Scholar]
- Fuchs B and Schiller J. Application of MALDI-TOF mass spectrometry in lipidomics. European Journal of Lipid Science and Technology 2009; 111 (1): 83–98. DOI: 10.1002/ejlt.200800223. [DOI] [Google Scholar]
- Fuchs B, Schiller J, Suss R, Schurenberg M and Suckau D. A direct and simple method of coupling matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) to thin-layer chromatography (TLC) for the analysis of phospholipids from egg yolk. Analytical and Bioanalytical Chemistry 2007; 389(3): 827–34. DoI: 10.1007/s00216-007-1488-4. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Süß R and Schiller J. An update of MALDI-TOF mass spectrometry in lipid research. Progress in Lipid Research 2010; 49(4): 450–75. DOI: 10.1016/j.plipres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Gellermann GP, Appel TR, Davies P and Diekmann S. Paired helical filaments contain small amounts of cholesterol, phosphatidylcholine and sphingolipids. Biological Chemistry 2006; 387(9): 1267–74. [DOI] [PubMed] [Google Scholar]
- Goto H, Shionoya N, Sugie M, Tominaga M, Shimelis O, Taniguchi M, Igarashi T and Hirata Y. Novel pre-fractionation method of trans fatty acids by gas chromatography with silver-Ion cartridge column. Journal of Oleo Science 2012; 61(2): 49–56. [DOI] [PubMed] [Google Scholar]
- Griffiths WJ and Wang Y. Mass spectrometry: from proteomics to metabolomics and lipidomics. Chemical Society Reviews 2009; 38(7): 1882–96. [DOI] [PubMed] [Google Scholar]
- Grizard G, Sion B, Bauchart D and Boucher D. Separation and quantification of cholesterol and major phospholipid classes in human semen by high-performance liquid chromatography and light-scattering detection. Journal of Chromatography B Biomedical Science Applications 2000; 740(1): 101–7. [DOI] [PubMed] [Google Scholar]
- Gross JH. Mass Spectrometry: a Textbook. Springer: Berlin, 2004. [Google Scholar]
- Gross RW and Han X Lipidomics at the interface of structure and function in systems biology. Chemistry and Biology 2011; 18(3): 284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimi P, Uphoff A, Hermansson M and Somerharju P. Software tools for analysis of mass spectrometric lipidome data. Analytical Chemistry 2006; 78(24): 8324–31. DOI: 10.1021/ac061390w. [DOI] [PubMed] [Google Scholar]
- Hamilton RJ, Mitchell SF and Sewell PA. Techniques for the detection of lipids in high-performance liquid chromatography. Journal of Chromatography 1987; 395: 33–46. [DOI] [PubMed] [Google Scholar]
- Han X and Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proceedings of the National Academy of Sciences 1994; 91 (22): 10635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X and Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrometry Reviews 2005a; 24(3): 367–412. DOI: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- Han X and Gross RW. Shotgun lipidomics: multidimensional MS analysis of cellular lipidomes. Expert Review of Proteomics 2005b; 2(2): 253–64. DOI: 10.1586/14789450.2.2.253. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Cheng H, Yang K, Abendschein DR and Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 2005; 44(50): 16684–94. DOI: 10.1021/bi051908a. [DOI] [PubMed] [Google Scholar]
- Han X, Yang K, Yang J, Cheng H and Gross RW. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. Journal of Lipid Research 2006; 47(4): 864–79. DOI: 10.1194/jlr.D500044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang K and Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrometry Reviews 2012; 31(1): 134–78. DOI: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handloser D, Widmer V and Reich E. Separation of phospholipids by HPTLC – an investigation of important parameters. Journal of Liquid Chromatography and Related Technologies 2008; 31(13): 1857–70. DOI: 10.1080/10826070802188940. [DOI] [Google Scholar]
- Harvey D Matrix-assisted laser desorption/ionization mass spectrometry of phospholipids. Journal of Mass Spectrometry 1995a; 30(9): 1333–46. [Google Scholar]
- Harvey DJ. Matrix-assisted laser desorption/ionization mass spectrometry of sphingo-and glycosphingo-lipids. Journal of Mass Spectrometry 1995b; 30(9): 1311–24. [Google Scholar]
- Hayes RN and Gross ML. Collision-induced dissociation. Methods in Enzymology 1989; 193: 237–63. [DOI] [PubMed] [Google Scholar]
- Hejazi L, Ebrahimi D, Hibbert DB and Guilhaus M. Compatibility of electron ionization and soft ionization methods in gas chromatography/orthogonal time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry 2009; 23(14): 2181–9. [DOI] [PubMed] [Google Scholar]
- Helmy FM. Comparative studies of the endogenous phospholipids and their in vitro hydrolysis by endogenous phospholipases of various tissues from 7-day-old chicks: a thin layer chromatographic and densitometric analysis. Cell Biochemistry and Functions 2004; 22(6): 389–98. DOI: 10.1002/cbf.1170. [DOI] [PubMed] [Google Scholar]
- Henderson MA and McIndoe JS. Ionic liquids enable electrospray ionisation mass spectrometry in hexane. Chemical Communications 2006; 27: 2872–4. DOI: 10.1039/B606938J. [DOI] [PubMed] [Google Scholar]
- Hermansson M, Uphoff A, Käkelä R, and Somerharju P. Automated quantitative analysis of complex lipidomes by liquid chromatography/mass spectrometry. Analytical Chemistry 2005; 77(7): 2166–75. DOI: 10.1021/ac048489s. [DOI] [PubMed] [Google Scholar]
- Herzog R, Schuhmann K, Schwudke D, Sampaio JL, Bornstein SR, Schroeder M and Shevchenko A. LipidXplorer: a software for consensual cross-platform lipidomics. PLoS One 2012; 7(1): e29851. DOI: 10.1371/journal.pone.0029851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Zhou H, Elisma F, Bennett SA and Figeys D. Technological developments in lipidomics. Briefings in Functional Genomics and Proteomics 2008; 7(5): 395–409. [DOI] [PubMed] [Google Scholar]
- Houjou T, Yamatani K, Imagawa M, Shimizu T and Taguchi R. A shotgun tandem mass spectrometric analysis of phospholipids with normal-phase and/or reverse-phase liquid chromatography/electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry 2005; 19(5): 654–66. DOI: 10.1002/rcm.1836. [DOI] [PubMed] [Google Scholar]
- Hsu F-F, Bohrer A and Turk J. Formation of lithiated adducts of glycerophosphocholine lipids facilitates their identification by electrospray ionization tandem mass spectrometry. Journal of the American Society for Mass Spectrometry 1998; 9(5): 516–26. DOI: 10.1016/S1044-0305(98)00012-9. [DOI] [PubMed] [Google Scholar]
- Hunt AN and Postle AD. Mass spectrometry determination of endonuclear phospholipid composition and dynamics. Methods 2006; 39(2):104–11. DOI: 10.1016/j.ymeth.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Hvattum E, Uran S, Sandbæk AG, Karlsson AÅ and Skotland T. Quantification of phosphatidylserine, phosphatidic acid and free fatty acids in an ultrasound contrast agent by normal-phase high-performance liquid chromatography with evaporative light scattering detection. Journal of Pharmaceutical and Biomedical Analysis 2006; 42(4): 506–12. DOI: 10.1016/j.jpba.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Ifa DR, Wiseman JM, Song Q and Cooks RG. Development of capabilities for imaging mass spectrometry under ambient conditions with desorption electrospray ionization (DESI). International Journal of Mass Spectrometry 2007; 259(1): 8–15. [Google Scholar]
- Ivanova PT, Milne SB, Byrne MO, Xiang Y and Brown HA. Glycerophospholipid Identification and Quantitation by Electrospray Ionization Mass Spectrometry, Brown HA (ed.). Methods in Enzymology. Academic Press: London, 2007. [DOI] [PubMed] [Google Scholar]
- Ivanova PT, Milne SB, Myers DS and Brown HA. Lipidomics: a mass spectrometry based systems level analysis of cellular lipids. Current Opinion in Chemical Biology 2009; 13(5–6): 526–31. DOI: 10.1016/j.cbpa.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SN, Wang HYJ and Woods AS. Direct tissue analysis of phospholipids in rat brain using MALDI-TOFMS and MALDI-ion mobility-TOFMS. Journal of the American Society of Mass Spectrometry 2005; 16: 133–8. [DOI] [PubMed] [Google Scholar]
- Jensen NJ, Tomer KB and Gross ML. FAB MS/MS for phosphatidylinostitol,-glycerol,-ethanolamine and other complex phospholipids. Lipids 1987; 22(7): 480–9. [DOI] [PubMed] [Google Scholar]
- Johanson RA and Berry GT. Brain Phosphoinositide Extraction, Fractionation, and Analysis by MALDI-TOF MS. Lipidomics. Springer: Berlin, 2009. [DOI] [PubMed] [Google Scholar]
- Johanson RA, Buccafusca R, Quong JN, Shaw MA and Berry GT. Phosphatidylcholine removal from brain lipid extracts expands lipid detection and enhances phosphoinositide quantification by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Analytical Biochemistry 2007; 362(2): 155–67. [DOI] [PubMed] [Google Scholar]
- Jones EE, Powers TW, Neely BA, Cazares LH, Troyer DA, Parker AS and Drake RR. MALDI imaging mass spectrometry profiling of proteins and lipids in clear cell renal cell carcinoma. Proteomics 2014; 14(7–8): 924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzny M, Duncan L, Merritt M and Epps D. Rapid separation of lipid classes in high yield and purity using bonded phase columns. Journal of Lipid Research 1985; 26(1): 135–40. [PubMed] [Google Scholar]
- Katajamaa M, Miettinen J and Orešič M. MZmine: toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics 2006; 22(5): 634–6. [DOI] [PubMed] [Google Scholar]
- Kerwin JL, Tuininga AR and Ericsson L. Identification of molecular species of glycerophospholipids and sphingomyelin using electrospray mass spectrometry. Journal of Lipid Research 1994; 35(6): 1102–14. [PubMed] [Google Scholar]
- Kim H, Min HK, Kong G and Moon MH. Quantitative analysis of phosphatidylcholines and phosphatidylethanolamines in urine of patients with breast cancer by nanoflow liquid chromatography/tandem mass spectrometry. Analytical and Bioanalytical Chemistry 2009; 393(6–7): 1649–56. [DOI] [PubMed] [Google Scholar]
- Kim H-Y, Wang T-CL and Ma Y-C. Liquid chromatography/mass spectrometry of phospholipids using electrospray ionization. Analytical Chemistry 1994; 66(22): 3977–82. [DOI] [PubMed] [Google Scholar]
- Kinsey GR, Blum JL, Covington MD, Cummings BS, McHowat J and Schnellmann RG. Decreased iPLA2gamma expression induces lipid peroxidation, cell death, and sensitizes cells to oxidant-induced apoptosis. Journal of Lipid Research 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R Mass spectrometry of the phosphatidylcholines: dipalmitoyl, dioleoyl, and stearoyl-oleoyl glycerylphosphorylcholines. Journal of Lipid Research 1971; 12(2): 123–31. [PubMed] [Google Scholar]
- Koulman A, Tapper BA, Fraser K, Cao M, Lane GA and Rasmussen S. High-throughput direct-infusion ion trap mass spectrometry: a new method for metabolomics. Rapid Communications in Mass Spectrometry 2007; 21(3): 421–8. DOI: 10.1002/rcm.2854. [DOI] [PubMed] [Google Scholar]
- Kyrklund T Two procedures to remove polar contaminants from a crude brain lipid extract by using prepacked reversed-phase columns. Lipids 1987; 22(4): 274–7. [DOI] [PubMed] [Google Scholar]
- Lane AL, Nyadong L, Galhena AS, Shearer TL, Stout EP, Parry RM, Kwasnik M, Wang MD, Hay ME, Fernandez FM and Kubanek J. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proceedings of the National Academy of Science, USA 2009; 106(18): 7314–9. DOI: 10.1073/pnas.0812020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Williams MV, DuBois RN and Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Communications in Mass Spectrometry 2003; 17(19): 2168–76. [DOI] [PubMed] [Google Scholar]
- Lin JT. HPLC Separation of Acyl Lipid Classes. Journal of Liquid Chromatography and Related Technologies 2007; 30(14): 2005–20. DOI: 10.1080/10826070701435020. [DOI] [Google Scholar]
- Lou X, van Dongen JL, Vekemans JA and Meijer E. Matrix suppression and analyte suppression effects of quaternary ammonium salts in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: an investigation of suppression mechanism. Rapid Communications in Mass Spectrometry 2009; 23(19): 3077–82. [DOI] [PubMed] [Google Scholar]
- Malavolta M, Bocci F, Boselli E and Frega NG. Normal phase liquid chromatography–electrospray ionization tandem mass spectrometry analysis of phospholipid molecular species in blood mononuclear cells: application to cystic fibrosis. Journal of Chromatography B 2004; 810(2): 173–86. DOI: 10.1016/j.jchromb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marchi I, Rudaz S and Veuthey J-L. Atmospheric pressure photoionization for coupling liquid-chromatography to mass spectrometry: a review. Talanta 2009; 78(1): 1–18. DOI: 10.1016/j.talanta.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Marto JA, White FM, Seldomridge S and Marshall AG. Structural characterization of phospholipids by matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry. Analytical Chemistry 1995; 67(21): 3979–84. [DOI] [PubMed] [Google Scholar]
- Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A and Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. Journal of Lipid Research 2008; 49(5): 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell LA and Heeren R. Imaging mass spectrometry. Mass Spectrometry Reviews 2007; 26(4): 606–43. [DOI] [PubMed] [Google Scholar]
- McLean JA, Ridenour WB and Caprioli RM. Profiling and imaging of tissues by imaging ion mobility–mass spectrometry. Journal of Mass Spectrometry 2007; 42: 1099–105. [DOI] [PubMed] [Google Scholar]
- Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GSH, Lopez M, Seppänen-Laakso T, Ashcroft FM, Orešič M and Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genetics 2007; 3(4): e64. DOI: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V Practical high-performance liquid chromatography, 3rd edn. Wiley: Chichester, 1998. [Google Scholar]
- Min HK, Kong G and Moon MH. Quantitative analysis of urinary phospholipids found in patients with breast cancer by nanoflow liquid chromatography–tandem mass spectrometry: II. Negative ion mode analysis of four phospholipid classes. Analytical and Bioanalytical Chemistry 2010; 396(3): 1273–80. [DOI] [PubMed] [Google Scholar]
- Moe MK, Anderssen T, Strom MB and Jensen E. Vicinal hydroxylation of unsaturated fatty acids for structural characterization of intact neutral phospholipids by negative electrospray ionization tandem quadrupole mass spectrometry. Rapid Communications in Mass Spectrometry 2004; 18(18): 2121–30. DOI: 10.1002/rcm.1601. [DOI] [PubMed] [Google Scholar]
- Moreau RA. Lipid analysis via HPLC with a charged aerosol detector. Lipid Technology 2009; 21(8–9): 191–4. DOI: 10.1002/lite.200900048. [DOI] [Google Scholar]
- Murphy RC and Gaskell SJ. New applications of mass spectrometry in lipid analysis. Journal of Biological Chemistry 2011; 286(29): 25427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RC, Hankin JA and Barkley RM. Imaging of lipid species by MALDI mass spectrometry. Journal of Lipid Research 2009; 50(suppl.): S317–22. DOI: 10.1194/jlr.R800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RC, Leiker TJ and Barkley RM. Glycerolipid and cholesterol ester analyses in biological samples by mass spectrometry. Biochimica et Biophysica Acta – Molecular and Cell Biology of Lipids 2011; 1811(11): 776–83. DOI: 10.1016/j.bbalip.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA and Nicolaou A. Lipidomics applications in health, disease and nutrition research. Molecular Nutrition and Food Research 2013; 57(8): 1336–46. [DOI] [PubMed] [Google Scholar]
- Nemes P, Barton AA and Vertes A. Three-dimensional imaging of metabolites in tissues under ambient conditions by laser ablation electrospray ionization mass spectrometry. Analytical Chemistry 2009; 81 (16): 6668–75. DOI: 10.1021/ac900745e. [DOI] [PubMed] [Google Scholar]
- Nishihara M and Yosuke K. Extraction and composition of polar lipids from the archaebacterium, Methanobacterium thermoautotrophicum: effective extraction of tetraether lipids by an acidified solvent. Journal of Biochemistry 1987; 101(4): 997–1005. [DOI] [PubMed] [Google Scholar]
- Pacholski ML and Winograd N. Imaging with mass spectrometry. Chemistry Review 1999; 99(10): 2977–3006. [DOI] [PubMed] [Google Scholar]
- Perona JS and Ruiz-Gutierrez V. Simultaneous determination of molecular species of monoacylglycerols, diacylglycerols and triacylglycerols in human very-low-density lipoproteins by reversed-phase liquid chromatography. Journal of Chromatography B: Analytical Technology in Biomedicine and Life Sciences 2003; 785(1): 89–99. [DOI] [PubMed] [Google Scholar]
- Peterson B, Stovall K, Monian P, Franklin JL and Cummings BS. Alterations in phospholipid and fatty acid lipid profiles in primary neocortical cells during oxidant-induced cell injury. Chemical and Biological Interactions 2008; 174(3): 163–76. [DOI] [PubMed] [Google Scholar]
- Peterson BL and Cummings BS. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomedical Chromatography 2006; 20(3): 227–43. DOI: 10.1002/bmc.563. [DOI] [PubMed] [Google Scholar]
- Petković M, Schiller J, Müller M, Benard S, Reichl S, Arnold K and Arnhold J. Detection of individual phospholipids in lipid mixtures by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: phosphatidylcholine prevents the detection of further species. Analytical Biochemistry 2001; 289(2): 202–16. DOI: 10.1006/abio.2000.4926. [DOI] [PubMed] [Google Scholar]
- Pietiläinen KH, Sysi-Aho M, Rissanen A, Seppänen-Laakso T, Yki-Järvinen H, Kaprio J and Orešič M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects – a monozygotic twin study. PLoS One 2007; 2(2): e218. DOI: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle AD. Phospholipid lipidomics in health and disease. European Journal of Lipid Science and Technology 2009; 111 (1): 2–13. [Google Scholar]
- Postle AD, Wilton DC, Hunt AN and Attard GS. Probing phospholipid dynamics by electrospray ionisation mass spectrometry. Progress in Lipid Research 2007; 46(3–4): 200–24. DOI: 10.1016/j.plipres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Pulfer M and Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrometry Reviews 2003; 22(5): 332–64. [DOI] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S and Shaner RL. Lipidomics reveals a remarkable diversity of lipids in human plasma. Journal of Lipid Research 2010; 51(11): 3299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville PD, Stumpf CL, Shockcor JP, Plumb RS and Nicholson JK. Novel application of reversed-phase UPLC-oaTOF-MS for lipid analysis in complex biological mixtures: a new tool for lipidomics. Journal of Proteome Research 2007; 6(2): 552–8. DOI: 10.1021/pr060611b. [DOI] [PubMed] [Google Scholar]
- Roberts LD, McCombie G, Titman CM and Griffin JL. A matter of fat: an introduction to lipidomic profiling methods. Journal of Chromatography B 2008; 871(2): 174–81. DOI: 10.1016/j.jchromb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Saldanha T, Sawaya ACHF, Eberlin MN and Bragagnolo N. HPLC Separation and determination of 12 cholesterol oxidation products in fish: comparative study of RI, UV, and APCI-MS detectors. Journal of Agricultural and Food Chemistry 2006; 54(12): 4107–13. DOI: 10.1021/jf0532009. [DOI] [PubMed] [Google Scholar]
- Schäfer KC, Dénes J, Albrecht K, Szaniszló T, Balog J, Skoumal R, Katona M, Tóth M, Balogh L and Takáts Z. In vivo, in situ tissue analysis using rapid evaporative ionization mass spectrometry. Angewandte Chemie International Edition 2009; 48(44): 8240–2. [DOI] [PubMed] [Google Scholar]
- Schiller J, Süß R, Arnhold J, Fuchs B, Lessig J, Müller M, Petković M, Spalteholz H, Zschörnig O and Arnold K. Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Progress in Lipid Research 2004; 43(5): 449–88. [DOI] [PubMed] [Google Scholar]
- Schiller J, Suss R, Fuchs B, Muller M, Zschornig O and Arnold K. MALDI-TOF MS in lipidomics. Frontiers in Bioscience: a Journal and Virtual Library 2006; 12: 2568–79. [DOI] [PubMed] [Google Scholar]
- Schuhmann K, Herzog R, Schwudke D, Metelmann-Strupat W, Bornstein SR and Shevchenko A. Bottom-up shotgun lipidomics by higher energy collisional dissociation on LTQ Orbitrap mass spectrometers. Analytical Chemistry 2011; 83(14): 5480–7. [DOI] [PubMed] [Google Scholar]
- Schwalbe-Herrmann M, Willmann J and Leibfritz D. Separation of phospholipid classes by hydrophilic interaction chromatography detected by electrospray ionization mass spectrometry. Journal of Chromatography A 2010; 1217(32): 5179–83. DOI: 10.1016/j.chroma.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Schwudke D, Oegema J, Burton L, Entchev E, Hannich JT, Ejsing CS, Kurzchalia T and Shevchenko A. Lipid profiling by multiple precursor and neutral loss scanning driven by the data-dependent acquisition. Analytical Chemistry 2006; 78(2): 585–95. DOI: 10.1021/ac051605m. [DOI] [PubMed] [Google Scholar]
- Schwudke D, Hannich JT, Surendranath V, Grimard V, Moehring T, Burton L, Kurzchalia T and Shevchenko A. Top-down lipidomic screens by multivariate analysis of high-resolution survey mass spectra. Analytical Chemistry 2007a; 79(11): 4083–93. [DOI] [PubMed] [Google Scholar]
- Schwudke D, Liebisch G, Herzog R, Schmitz G and Shevchenko A. Shotgun lipidomics by tandem mass spectrometry under data-dependent acquisition control. Methods in Enzymology 2007b; 433:175–91. [DOI] [PubMed] [Google Scholar]
- Seeley EH and Caprioli RM. Imaging mass spectrometry: towards clinical diagnostics. Proteomics and Clinical Applications 2008; 2(10–11): 1435–43. DOI: 10.1002/prca.200800013. [DOI] [PubMed] [Google Scholar]
- Shen JX, Motyka RJ, Roach JP and Hayes RN. Minimization of ion suppression in LC-MS/MS analysis through the application of strong cation exchange solid-phase extraction (SCX-SPE). Journal of Pharmaceutical and Biomedical Analysis 2005; 37(2): 359–67. [DOI] [PubMed] [Google Scholar]
- Shevchenko A and Simons K. Lipidomics: coming to grips with lipid diversity. Nature Reviews Molecular Cell Biology 2010; 11(8): 593–8. [DOI] [PubMed] [Google Scholar]
- Sinha TK, Khatib-Shahidi S, Yankeelov TE, Mapara K, Ehtesham M, Cornett DS, Dawant BM, Caprioli RM and Gore JC. Integrating spatially resolved three-dimensional MALDI IMS with in vivo magnetic resonance imaging. Nature Methods 2008; 5(1): 57–9. DOI: 10.1038/nmeth1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LR, Kirkland JJ and Glajch JL. Practical HPLC Method Development, 2nd edn. Wiley: New York, 1997. [Google Scholar]
- Sommerer D, Süß R, Hammerschmidt S, Wirtz H, Arnold K and Schiller J. Analysis of the phospholipid composition of bronchoalveolar lavage (BAL) fluid from man and minipig by MALDI-TOF mass spectrometry in combination with TLC. Journal of Pharmaceutical and Biomedical Analysis 2004; 35(1): 199–206. DOI: 10.1016/j.jpba.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Taguchi R, Hayakawa J, Takeuchi Y and Ishida M. Two-dimensional analysis of phospholipids by capillary liquid chromatography/electrospray ionization mass spectrometry. Journal of Mass Spectrometry 2000; 35(8): 953–66. DOI:. [DOI] [PubMed] [Google Scholar]
- Takats Z, Wiseman JM, Gologan B and Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004; 306(5695): 471–3. [DOI] [PubMed] [Google Scholar]
- Tautenhahn R, Patti GJ, Rinehart D and Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Analytical Chemistry 2012; 84(11): 5035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman EM and Mills MS. Solid-phase Extraction: Principles and Practice. Wiley: New York, 1998. [Google Scholar]
- Touchstone JC. Thin-layer chromatographic procedures for lipid separation. Journal of Chromatography B: Biomedical Sciences and Applications 1995; 671(1–2): 169–95. DOI: 10.1016/0378-4347(95)00232-8. [DOI] [PubMed] [Google Scholar]
- Tserng K-Y and Griffin R. Quantitation and molecular species determination of diacylglycerols, phosphatidylcholines, ceramides, and sphingomyelins with gas chromatography. Analytical Biochemistry 2003; 323(1): 84–93. DOI: 10.1016/j.ab.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Uran S, Larsen Å, Jacobsen PB and Skotland T. Analysis of phospholipid species in human blood using normal-phase liquid chromatography coupled with electrospray ionization ion-trap tandem mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications 2001; 758(2): 265–75. DOI: 10.1016/S0378-4347(01)00188-8. [DOI] [PubMed] [Google Scholar]
- van Meer G Cellular lipidomics. The EMBO Journal 2005; 24(18): 3159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Xie S, Yang J, Yang Q and Xu G. Structural identification of human blood phospholipids using liquid chromatography/quadrupole-linear ion trap mass spectrometry. Analytica Chimica Acta 2004; 525(1): 1–10. DOI: 10.1016/j.aca.2004.07.065. [DOI] [Google Scholar]
- Weerheim AM, Kolb AM, Sturk A and Nieuwland R. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Analytical Biochemistry 2002; 302(2): 191–8. DOI: 10.1006/abio.2001.5552. [DOI] [PubMed] [Google Scholar]
- Wenk MR. Lipidomics: new tools and applications. Cell 2010; 143(6): 888–95. [DOI] [PubMed] [Google Scholar]
- Wenk MR, Lucast L, Di Paolo G, Romanelli AJ, Suchy SF, Nussbaum RL, Cline GW, Shulman GI, McMurray W and De Camilli P. Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry. Nature Biotechnology 2003; 21(7): 813–7. [DOI] [PubMed] [Google Scholar]
- Wilm M and Mann M. Analytical properties of the nanoelectrospray ion source. Analytical Chemistry 1996; 68(1): 1–8. [DOI] [PubMed] [Google Scholar]
- Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T and Mann M. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. 1996. [DOI] [PubMed] [Google Scholar]
- Wilm MS and Mann M. Electrospray and Taylo–Cone theory, Dole’s beam of macromolecules at last? International Journal of Mass Spectrometry and Ion Processes 1994; 136(2–3): 167–80. [Google Scholar]
- Wiseman JM, Puolitaival SM, Takáts Z, Cooks RG and Caprioli RM. Mass spectrometric profiling of intact biological tissue by using desorption electrospray ionization. Angewandte Chemie 2005; 117(43): 7256–9. [DOI] [PubMed] [Google Scholar]
- Wiseman JM, Ifa DR, Song Q and Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angewandte Chemie International Edition 2006; 45(43): 7188–92. DOI: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- Wolf C and Quinn PJ. Lipidomics: Practical aspects and applications. Progress in Lipid Research 2008; 47(1): 15–36. DOI: 10.1016/j.plipres.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Wood GW and Lau PY. Analysis of intact phospholipids by field desorption mass spectrometry. Biological Mass Spectrometry 1974; 1(2): 154–5. [DOI] [PubMed] [Google Scholar]
- Woods AS. IR-MALDI-LDI combined with ion mobility orthogonal time-of-flight mass spectrometry. Journal of Proteome Research 2006; 5: 1484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Psychogios N, Young N and Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Research 2009; 37(suppl. 2): W652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y and Brenna JT. Atmospheric pressure covalent adduct chemical ionization tandem mass spectrometry for double bond localization in monoene- and diene-containing triacylglycerols. Analytical Chemistry 2007; 79(6): 2525–36. DOI: 10.1021/ac062055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetukuri L, Ekroos K, Vidal-Puig A and Orešič M. Informatics and computational strategies for the study of lipids. Molecular BioSystems 2008; 4(2): 121–7. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Kim M-S, Kim SH, Park HM, Pyo H, Lee YM, Lee K-T and Hong J. Effective application of freezing lipid precipitation and SCX-SPE for determination of pyrrolizidine alkaloids in high lipid foodstuffs by LC-ESI-MS/MS. Journal of Chromatography B 2015; 992: 56–66. [DOI] [PubMed] [Google Scholar]
- Yost R and Enke C. Triple quadrupole mass spectrometry for direct mixture analysis and structure elucidation. Analytical Chemistry 1979; 51 (12): 1251–64. [DOI] [PubMed] [Google Scholar]
- Zhai D and Reilly P. Effect of FA. chain length on normal- and reversed-phase HPLC of phospholipids. Journal of the American Oil Chemists Society 2002; 79(12): 1187–90. DOI: 10.1007/s11746-002-0625-0. [DOI] [Google Scholar]
- Zhang L, Peterson BL and Cummings BS. The effect of inhibition of Ca2+-independent phospholipase A2 on chemotherapeutic-induced death and phospholipid profiles in renal cells. Biochemical Pharmacology 2005; 70(11): 1697–706. [DOI] [PubMed] [Google Scholar]
- Zhong JH, Guo QY, Ye RG, Lindholm B and Wang T. Phospholipids in dialysate and the peritoneal surface layer. Advances in Peritoneal Dialysis 2000; 16: 36–41. [PubMed] [Google Scholar]
- Zhu C, Dane A, Spijksma G, Wang M, van der Greef J, Luo G, Hankemeier T and Vreeken RJ. An efficient hydrophilic interaction liquid chromatography separation of 7 phospholipid classes based on a diol column. Journal of Chromatography A 2012; 1220: 26–34. DOI: 10.1016/j.chroma.2011.11.034. [DOI] [PubMed] [Google Scholar]