Supplemental Digital Content is available in the text.
Background.
We aimed to characterize patterns of differences in heart graft failure rates by recipient sex, accounting for modifying effects of donor sex and recipient age.
Methods.
We evaluated 69 246 first heart transplant recipients (1988–2019; Scientific Registry of Transplant Recipients). We used multivariable time-varying Cox models, considering recipient sex by donor sex by recipient age interaction and adjusting for potential confounders. Using the hazard ratio (HR) from the models and a fixed profile of recipient and donor characteristics, we also compared fitted absolute failure rates by recipient sex.
Results.
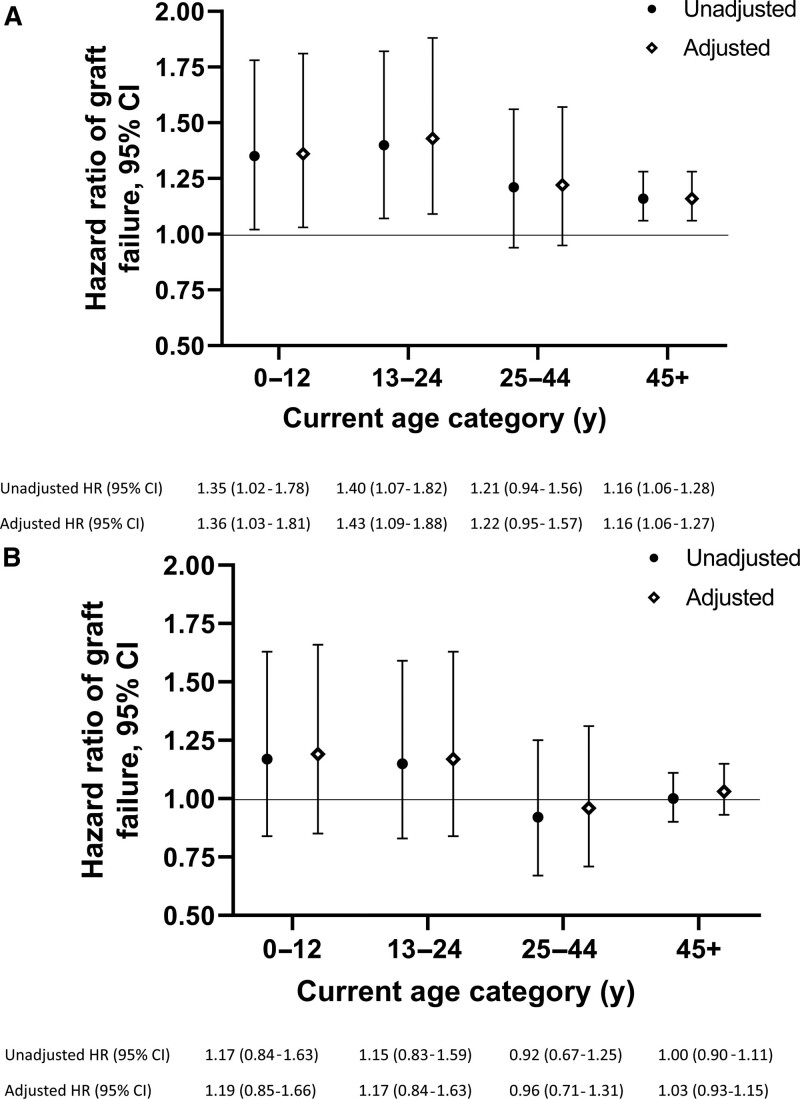
Among recipients of male donors, female recipients of all ages had higher failure rates than males (0–12 y: HR 1.36 (95% confidence interval [CI], 1.03-1.81); 13–24 y: 1.43 [1.09-1.88]; 25–44 y: 1.22 [0.95-1.57]; ≥45 y: 1.16 [1.06-1.27]); differences were statistically significant in all age intervals except 25–44 y. When the donor was male, 13 to 24-y-olds showed the largest absolute difference in fitted absolute failure rates, with rates higher by 11.3 failures per 1000 person-y in female than male recipients. Among recipients of female donors, there were no statistically significant differences in graft failure rates between female and male heart recipients of any age. Although point estimates suggested higher failure rates in female than male recipients <25 y (0–12 y: HR 1.19 [95% CI, 0.85-1.66]; 13–24 y: 1.17 [0.84-1.63]), these were not statistically significant.
Conclusions.
Female recipients tended to have poorer outcomes than males, particularly at younger ages and when the donor was male, consistent with observations in kidney transplants.
Identification of sex differences in the prevalence and severity of a wide range of conditions has led to fundamental work uncovering a new understanding of disease mechanisms.1-6 Several factors that differ between the sexes, some of which change with age, may be important determinants of heart graft survival. These include immunosuppressive medication adherence,7-9 pharmacokinetics and pharmacodynamics of immunosuppressive medications, immune reaction against the HY antigen,10-13 and sex differences in immune-related gene expression.14-16 Sex differences in immune reactivity, driven in part by sex hormones15,16 and further influenced by differential expression of hormone receptors, may also play a role. Currently, immunosuppression strategies do not differ by recipient sex. Characterization of similarities or differences in graft outcomes by recipient sex, in different organ types, may provide clues as to mechanisms and is an important first step to more personalized immunosuppression strategies.
The manifestations of sex differences change with age. For example, sex hormone levels are similar for pre-pubertal boys and girls but differ dramatically by sex after puberty until after menopause, when sex hormone profiles among men and women are more similar. Given that sex hormones influence immune reactivity,15,16 it is likely that the magnitude of any sex differences in graft failure risk differs by age.
Several prior studies showed that the relationship between recipient sex and graft outcomes is modified by donor sex.10,17-21 The most important reason for this interaction may be the presence of the HY-antigen on male, but not female, tissues. This antigen may provoke an immunologic reaction in female recipients of a male donor.13,15,22,23
We showed donor sex-dependent and recipient age-dependent differences in the risk of graft failure by recipient sex among kidney transplant recipients.10 Among recipients of male donors, females of all ages had poorer graft survival than males, whereas, among recipients of female donors, only adolescent and young adult females had poorer outcomes than males of the same age. In the setting of a female donor, female recipients ≥45 y old had significantly better graft survival than males of the same age. These findings point to a potential role for sex differences in immune reactivity mediated by sex hormones,22 in the observed sex differences in graft outcomes.
It is not clear whether similar sex differences in graft outcomes exist in heart transplant recipients. Prior studies comparing outcomes in male and female heart transplant recipients considered overall mortality rates rather than graft failure rates.17,18,20,21 Comparisons of absolute mortality rates between males and females are uninterpretable24 and may not exclusively reflect differences in graft survival. Overall mortality includes death with graft function and excludes retransplants following graft failure. The mechanisms underlying sex differences in graft survival may differ from those underlying sex differences in patient survival. Furthermore, the expected age-specific mortality risk is lower for females than males.25 No prior studies of heart transplant recipients considered the potentially modifying effect of recipient age. We hypothesized that the pattern of differences in heart graft survival by recipient sex would be similar in nature and magnitude to that observed for kidney transplant recipients. We aimed to characterize patterns of difference in graft survival between male and female heart transplant recipients in the pre-pubertal, adolescent and young adult, mid-adulthood, and post-menopausal age ranges, accounting for the potentially modifying effect of donor sex.
MATERIALS AND METHODS
Data Source and Population
This was a retrospective cohort study of individuals recorded in the Scientific Registry of Transplant Recipients (SRTR) who received a first, single-organ heart transplant in the United States between January 1, 1988, and June 1, 2019. Patients were followed until June 1, 2019. The SRTR includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight to the activities of the Organ Procurement and Transplantation Network and SRTR contractors.
Exposure and Outcome Definitions
The primary exposure was recipient sex. Because interactions between donor and recipient sex have been shown in numerous prior studies,10,26 we considered donor–recipient sex combinations: male donor–male recipient (MM), male donor–female recipient (MF), female donor–male recipient (FM), and female donor–female recipient (FF). Sex differences in graft outcomes may also differ by recipient current age because of age-related differences in expression of sexual dimorphism15,22; therefore, we included a donor–recipient sex combination by recipient current age interaction term. Current age was a time-varying variable and categorized as 0–12 (pre-pubertal), 13–29 (adolescence and young adulthood), 30–44 (middle adulthood), and ≥45 y (post-menopausal). Heart, liver, and kidney graft failure rates were previously shown to vary by recipient current age with an inverted U–shaped relationship, peaking in adolescence and young adulthood.27-29
Primary Outcome
The primary outcome was graft failure, defined as retransplantation or death following graft failure.28 Graft status (failed vs functioning) is reported annually to the SRTR. When a death is reported, it is required to indicate whether the death was a result of graft failure or due to some other factor unrelated to graft failure (ie, death with graft function). It was important to exclude death with graft function from the definition of graft failure because of the potentially different mechanisms underlying sex differences in graft survival and those underlying sex differences in patient survival, as well as the expected sex differences age-specific mortality risks.25 Therefore, observation was censored at death with graft function.
Statistical Analyses
Association Between Recipient Sex and Graft Survival
We used Cox models with time-varying covariates to assess the associations between recipient sex and graft failure. Time zero was the date of transplant. Unadjusted analyses were followed by multivariable analyses adjusted for potential confounders. The models included the following covariates: donor and recipient race (White, Black, other), donor age, donor:recipient weight ratio (a measure of donor–recipient body size match or mismatch), primary heart disease (categorized as congenital heart disease, coronary/ischemic disease, myocarditis, cardiomyopathy, other), medical condition at transplant (ICU/hospital/no hospital stay), use of a ventricular assist device (VAD), and era of transplant (1988–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2014, 2015–2019). Use of VADs was not common until the early 2000s; although some VAD use is captured in the database before 2003, there were large amounts of missing data before 2003, whereas after 2003 there was almost no missing data. The amount of missing data on VAD use decreased from 98% in 1988 to 65% in 2002 to virtually zero thereafter. We assumed that all patients with missing data on VAD use had not used a VAD. Transplant era categories were based on changes in immunosuppression practices over time.30 Panel reactive antibodies (a marker of sensitization) are not available so could not be included. We considered including insurer (public, private, none) and HLA mismatch in the models, but insurer was missing in up to 26%, and HLA mismatch was missing in up to 22%; therefore these were excluded. Missing variables were imputed using multiple imputation methods based on the joint distributions of all other variables in the model.31
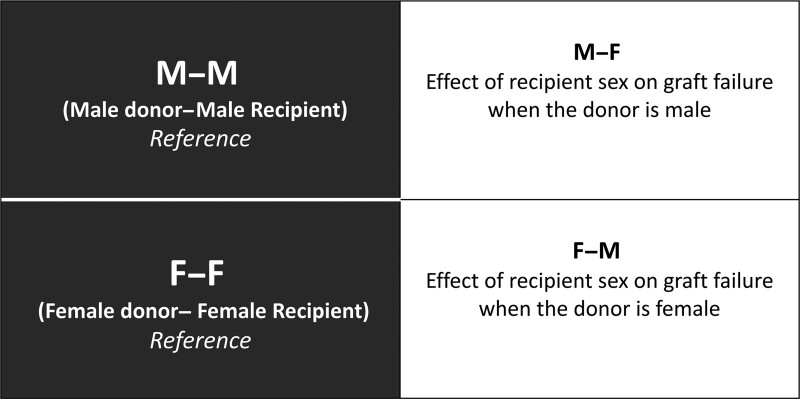
We first fitted the models setting MM as the reference category. This allowed us to compare graft failure rates between male and female recipients of a male donor (MF vs MM). We then re-fitted the same model setting FF as the reference category. This allowed us to compare graft failure rates between male and female recipients of a female donor (FM vs FF) (Figure 1). Hazard ratios (HRs) were always expressed as the hazard for female relative to male. HR and adjusted HR (aHR) are presented with 95% confidence intervals (CIs).
FIGURE 1.
Interpretation of contrasts between donor–recipient sex combinations. The two black boxes on the left show the two different reference groups considered: male donor–male recipient (MM) and female donor–female recipient (FF). The gray boxes to the right of each reference group represent the contrasts estimating the effects of recipient sex. For example, the contrast between the MM reference and male donor–female recipient (MF) gives the effect of recipient sex when the donor is male. FM, female donor–male recipient.
To determine the proportionality of hazards, we used Kaplan-Meier plots comparing graft survival by donor–recipient sex combination. In addition, proportionality was assessed by refitting the models, censoring all observations at 5 and 10 y. Results were unchanged, indicating that hazards were proportional.
Sensitivity Analyses
We fitted the model including insurer and HLA mismatch using multiple imputation for missing values. We also fitted a model limited to patients transplanted in 2003 or later when only 10 of the 36 813 transplants had missing data on VAD use. In addition, we fitted a model that included donor:recipient predicted heart mass ratio32 (classified as <0.8, 0.8–0.9, 0.9–1.1, 1.1–1.3, >1.3) as a measure of size mismatch rather than donor:recipient weight ratio. The predictive equation to estimate heart mass is only valid in adults, so this analysis was restricted to recipients >18 y old who had a donor >18 y old.
Fitted Absolute Graft Failure Rates by Current Age Stratified on Donor–Recipient Sex Combination
Crude failure rates do not account for either the changing failure risk over time since transplant or for the impact of confounders and therefore may be misleading. Crude failure rate estimates are highly influenced by the proportion of person-years contributed by incident transplant recipients, who have much higher failure rates in the first 1–3 mo post-transplant than they do thereafter. Incident transplant may differ by sex, leading to bias. To avoid this problem, we calculated fitted failure rates for each current age interval based on absolute failure rates in male recipients of male donors with a fixed profile of other patient and donor characteristics (failures per 1000 person-y of observation within that interval) and the HRs from the models described above. We also calculated crude graft failure rates for each current-age interval (failures per 1000 person-y of observation within that interval) for each donor–recipient sex combination. Individuals could contribute person-time to multiple current-age intervals.
We performed data analyses using Statistical Analysis Software 9.4 (SAS Institute, Cary, NC) and S-plus (version 6.1). The study was approved by the McGill University Health Center Research Ethics Board.
RESULTS
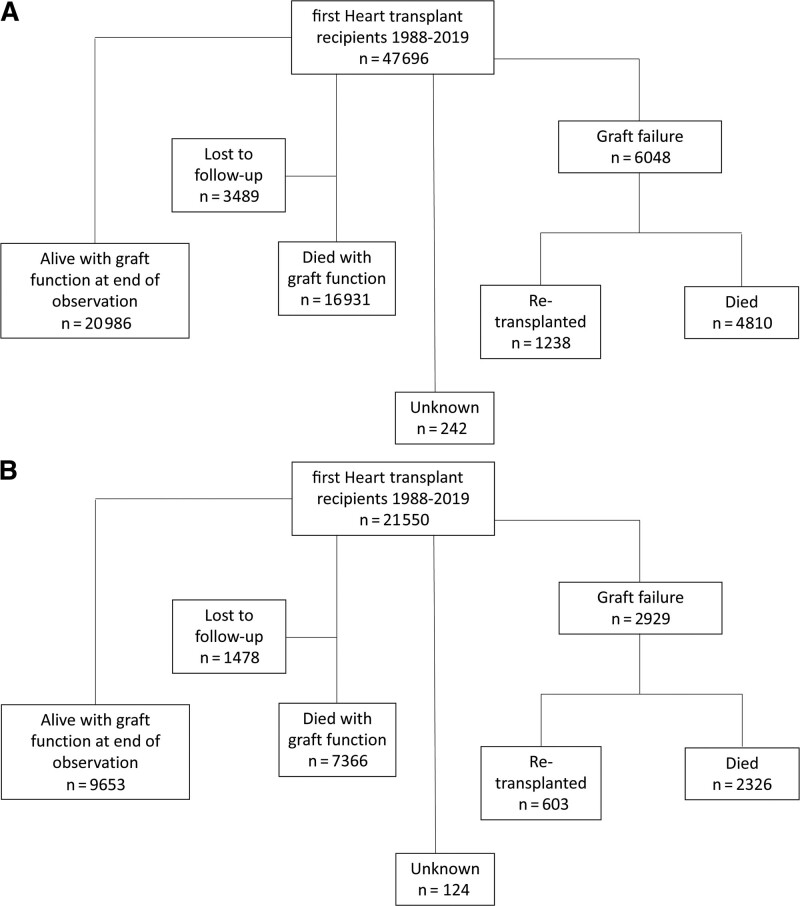
We identified 69 319 individuals who had a first, single-organ heart transplant between January 1, 1988, and June 1, 2019. We excluded 72 for whom the status of the graft could not be determined (graft recorded as failed, but no record of death or retransplant), and 1 with unknown donor sex, leaving 69 246 (47 696 with a male donor; 21 550 with a female donor). Patients were followed for a median of 6.0 (interquartile range 2.0–11.5) y, with a total of 520 212 person-y of observation. The outcomes of heart recipients by donor sex are shown in Figure 2A and B.
FIGURE 2.
Flow diagrams of heart recipient outcomes—outcomes of recipients of a (A) male donor and (B) female donor are shown.
Recipient and Transplant Characteristics
Tables 1 and 2 summarize the composition of the observed experience within each age interval for male and female recipients of male donors and female donors, respectively. The distribution of most characteristics was similar for males and females, but there were a few differences. Among recipients of a male donor, males were slightly older than females at transplant and received slightly older donors than females. Across most ages, a greater proportion of the observation time of males than females was contributed by Whites and a greater proportion of observation time of females than males was contributed by Blacks. Across most ages, a greater proportion of the observation time of males than females was contributed by people who had used a VAD. The distribution of primary heart disease differed across age categories and sex differences in primary disease differed by age. In the youngest age interval, a greater proportion of the observation time of males than females was contributed by those with congenital heart disease. In the oldest two age intervals, a greater proportion of the observation time of males than females was contributed by those with coronary/ischemic disease. In both the youngest and oldest age intervals, a greater proportion of observation time of females than males was contributed by those with cardiomyopathy.
TABLE 1.
Composition of the contrasted experience by heart recipient sex and age among recipients of a male donor (N = 47 696)
| 0–12 y | 13–24 y | 25–44 y | ≥45 y | |||||
|---|---|---|---|---|---|---|---|---|
| Females (MF) | Males (MM) | Females (MF) | Males (MM) | Females (MF) | Males (MM) | Females (MF) | Males (MM) | |
| Person-y of observation | 9414 | 10 888 | 7334 | 13 267 | 10 985 | 32 511 | 39 070 | 238 313 |
| Retransplants | 98 | 89 | 103 | 106 | 97 | 177 | 83 | 466 |
| Deaths after failure | 209 | 174 | 192 | 290 | 211 | 591 | 466 | 2445 |
| Age at transplant (y) | ||||||||
| Median (IQR) | 1 (0–3) | 0 (0–3) | 12 (5–16) | 14 (9–17) | 31 (25–36) | 33 (26–38) | 54 (48–59) | 56 (50–61) |
| Race (%) | ||||||||
| White | 76.4 | 80.9 | 76.7 | 77.5 | 72.6 | 76.1 | 78.6 | 87.8 |
| Black | 16.8 | 14.9 | 17.7 | 18.6 | 24.1 | 20.2 | 19.1 | 10.0 |
| Other | 6.9 | 4.2 | 5.6 | 3.9 | 3.4 | 3.7 | 2.4 | 2.2 |
| Primary disease (%) | ||||||||
| Congenital heart disease | 45.7 | 58.5 | 33.5 | 35.0 | 13.9 | 11.3 | 5.3 | 3.3 |
| Cardiomyopathy | 45.6 | 33.8 | 55.4 | 53.3 | 67.7 | 65.9 | 63.6 | 36.6 |
| Coronary/ischemic | 0.5 | 0.7 | 1.0 | 1.2 | 8.0 | 13.5 | 25.2 | 57.5 |
| Myocarditis | 5.1 | 3.4 | 5.4 | 5.7 | 4.0 | 5.8 | 2.4 | 1.3 |
| Others | 3.2 | 3.1 | 4.6 | 4.2 | 6.0 | 3.1 | 3.2 | 1.1 |
| Missing (%) | 0.0 | 0.1 | 0.2 | 0.5 | 0.5 | 0.3 | 0.3 | 0.2 |
| Insurer (%) | ||||||||
| Private | 40.4 | 42.0 | 43.5 | 47.6 | 42.5 | 42.8 | 46.4 | 44.7 |
| Public | 41.4 | 41.8 | 34.2 | 32.3 | 29.6 | 29.7 | 29.0 | 29.3 |
| No coverage | 2.2 | 1.2 | 2.1 | 1.3 | 1.9 | 1.2 | 1.1 | 0.7 |
| Missing | 16.3 | 15.0 | 20.3 | 18.9 | 26.0 | 26.4 | 23.5 | 25.3 |
| Era (%) | ||||||||
| 1988–1994 | 17.8 | 17.4 | 22.1 | 21.7 | 28.0 | 28.4 | 27.1 | 27.5 |
| 1995–1999 | 15.4 | 18.8 | 21.5 | 20.5 | 19.7 | 17.2 | 22.6 | 21.8 |
| 2000–2004 | 17.2 | 17.1 | 20.7 | 18.1 | 20.0 | 17.6 | 19.6 | 19.0 |
| 2005–2009 | 22.5 | 20.1 | 18.2 | 17.8 | 15.6 | 17.7 | 16.3 | 16.1 |
| 2010–2014 | 19.4 | 18.4 | 13.2 | 15.8 | 12.5 | 13.2 | 10.9 | 11.2 |
| 2015–2019 | 7.7 | 8.2 | 4.2 | 6.2 | 4.3 | 5.9 | 3.6 | 4.4 |
| Ventricular assist device | 8.8 | 9.0 | 10.8 | 20.4 | 18.4 | 29.3 | 14.3 | 21.5 |
| Medical condition | ||||||||
| ICU | 58.0 | 57.9 | 50.0 | 47.1 | 43.7 | 41.4 | 38.2 | 38.3 |
| Hospitalized/not ICU | 13.7 | 11.7 | 14.0 | 14.2 | 15.3 | 16.5 | 13.7 | 14.0 |
| Not hospitalized | 28.3 | 30.4 | 36.0 | 38.6 | 40.8 | 42.0 | 48.0 | 47.5 |
| Missing (%) | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| Donor:recipient weight ratio | ||||||||
| Median (IQR) | 1.4 (1.1–1.8) | 1.4 (1.1–1.8) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 1.1 (0.9–1.3) | 1.0 (0.8–1.1) | 1.0 (0.9–1.2) | 1.0 (0.8–1.1) |
| Missing (%) | 7.2 | 6.4 | 7.9 | 7.5 | 10.1 | 11.8 | 8.4 | 9.5 |
| Donor age (y) | ||||||||
| Median (IQR) | 1 (0–4) | 1 (0–4) | 12 (5–17) | 16 (8–22) | 21 (17–30) | 24 (19–34) | 23 (18–34) | 26 (20–37) |
| Missing (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| HLA MM | ||||||||
| Median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) |
| Missing (%) | 22.1 | 22.0 | 20.1 | 17.8 | 15.1 | 15.8 | 14.8 | 14.8 |
| Donor race | ||||||||
| White (%) | 76.7 | 73.4 | 80.1 | 79.2 | 81.3 | 83.9 | 83.7 | 85.3 |
| Black (%) | 20.3 | 23.8 | 16.7 | 18.9 | 16.6 | 14.2 | 13.7 | 12.9 |
| Other (%) | 3.0 | 2.8 | 3.2 | 1.9 | 2.2 | 1.9 | 2.6 | 1.8 |
Because the unit of analysis was person-time, rather than person, the characteristics presented are weighted by a factor derived from the number of person-y of observation and number of events and presented as weighted mean ± SD, weighted median (IQR), or percent (%). For example, 76.4% of the person-y contributed by females between 0–12 y were by white recipients.
FF, female donor–female recipient; FM, female donor–male recipient; ICU, intensive care unit; IQR, interquartile range; MF, male donor–female recipient; MM, male donor–male recipient.
TABLE 2.
Composition of the contrasted experience by heart recipient sex and age among recipients of a female donor (N = 21550)
| 0–12 y | 13–24 y | 25–44 y | ≥45 y | |||||
|---|---|---|---|---|---|---|---|---|
| Females (MF) | Males (MM) | Females (MF) | Males (MM) | Females (MF) | Males (MM) | Females (MF) | Males (MM) | |
| Person-y of observation | 7079 | 8538 | 6087 | 7826 | 10 702 | 9535 | 41 928 | 66 681 |
| Retransplants | 60 | 44 | 62 | 72 | 67 | 68 | 92 | 128 |
| Deaths after failure | 133 | 154 | 134 | 144 | 200 | 186 | 471 | 764 |
| Age at transplant (y) | ||||||||
| Median (IQR) | 0 (0–2) | 0 (0–3) | 12 (6–16) | 12 (6–16) | 32 (25–37) | 31 (23–37) | 55 (49–60) | 56 (50–62) |
| Race (%) | ||||||||
| White | 76.4 | 80.9 | 76.7 | 77.5 | 72.6 | 76.1 | 78.6 | 87.8 |
| Black | 16.8 | 14.9 | 17.7 | 18.6 | 24.0 | 20.2 | 19.1 | 10.0 |
| Other | 6.9 | 4.2 | 5.6 | 3.9 | 3.4 | 3.7 | 2.4 | 2.2 |
| Primary disease (%) | ||||||||
| Congenital heart disease | 45.7 | 58.5 | 33.5 | 35.0 | 13.9 | 11.3 | 5.3 | 3.3 |
| Cardiomyopathy | 45.6 | 33.8 | 55.4 | 53.3 | 67.6 | 65.9 | 63.6 | 36.6 |
| Coronary/ischemic | 0.5 | 0.7 | 1.0 | 1.2 | 8.0 | 13.5 | 25.2 | 57.5 |
| Myocarditis | 5.1 | 3.4 | 5.4 | 5.7 | 4.0 | 5.8 | 2.4 | 1.3 |
| Others | 3.2 | 3.5 | 4.6 | 4.2 | 6.0 | 3.1 | 3.3 | 1.1 |
| Missing (%) | 0.0 | 0.1 | 0.2 | 0.5 | 0.5 | 0.3 | 0.3 | 0.2 |
| Insurer (%) | ||||||||
| Private | 40.4 | 42.0 | 43.5 | 47.6 | 42.5 | 42.8 | 46.4 | 44.7 |
| Public | 41.4 | 41.8 | 34.2 | 32.3 | 29.6 | 29.7 | 29.0 | 29.3 |
| No coverage | 2.2 | 1.2 | 2.1 | 1.3 | 1.9 | 1.2 | 1.1 | 0.7 |
| Missing | 16.0 | 15.0 | 20.3 | 18.9 | 26.0 | 26.4 | 23.5 | 25.3 |
| Era (%) | ||||||||
| 1988–1994 | 17.8 | 17.4 | 22.1 | 21.7 | 28.0 | 28.4 | 27.1 | 27.5 |
| 1995–1999 | 15.4 | 18.8 | 21.5 | 20.5 | 19.7 | 17.2 | 22.6 | 21.8 |
| 2000–2004 | 17.2 | 17.1 | 20.7 | 18.1 | 20.0 | 17.6 | 19.6 | 19.0 |
| 2005–2009 | 22.5 | 20.1 | 18.2 | 17.8 | 15.6 | 17.1 | 16.3 | 16.1 |
| 2010–2014 | 19.4 | 18.4 | 13.2 | 15.8 | 12.5 | 13.2 | 10.9 | 11.2 |
| 2015–2019 | 7.7 | 8.2 | 4.2 | 6.2 | 4.3 | 5.9 | 3.6 | 4.4 |
| Ventricular assist device | 9.2 | 7.2 | 11.8 | 12.7 | 19.6 | 18.7 | 14.3 | 15.5 |
| Medical condition | ||||||||
| ICU | 58.0 | 57.9 | 50.0 | 47.1 | 43.7 | 41.4 | 38.2 | 38.3 |
| Hospitalized/not ICU | 13.7 | 11.7 | 14.0 | 14.2 | 15.3 | 16.5 | 13.7 | 14.0 |
| Not hospitalized | 28.3 | 30.4 | 36.0 | 38.6 | 40.8 | 42.0 | 48.0 | 47.5 |
| Missing (%) | 0.0 | 0.1 | 0.0 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| Donor:recipient weight ratio | ||||||||
| Median (IQR) | 1.4 (1.1–1.7) | 1.4 (1.1–1.8) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.8–1.1) |
| Missing (%) | 6.0 | 6.0 | 7.4 | 7.7 | 8.5 | 12.3 | 7.0 | 10.1 |
| Donor age (y) | ||||||||
| Median (IQR) | 1 (0–4) | 1 (0–4) | 15 (7–22) | 15 (5–22) | 29 (18–39) | 29 (19–40) | 34 (21–44) | 35 (23–45) |
| Missing (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| HLA MM | ||||||||
| Median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) |
| Missing (%) | 21.1 | 23.1 | 19.3 | 22.1 | 15.5 | 19.1 | 14.0 | 15.7 |
| Donor race | ||||||||
| White (%) | 76.7 | 73.4 | 80.1 | 79.3 | 81.3 | 83.9 | 83.7 | 85.3 |
| Black (%) | 20.3 | 23.8 | 16.7 | 18.9 | 16.6 | 14.2 | 13.7 | 12.9 |
| Other (%) | 3.0 | 2.8 | 3.2 | 1.9 | 2.2 | 1.9 | 2.6 | 1.8 |
FF, female donor–female recipient; FM, female donor–male recipient; ICU, intensive care unit; IQR, interquartile range; MF, male donor–female recipient; MM, male donor–male recipient.
Comparison of Graft Survival by Recipient Sex
Figure 3 shows the relative hazards of graft failure for female compared—with male recipients of a (A) male donor or (B) female donor at different recipient ages. Among recipients of a male donor, graft failure rates were higher in female than male recipients in all age intervals. The differences were statistically significant and largest for those 0–12 y (aHR 1.36 [95% CI, 1.03-1.81]) and 13–24 y (aHR 1.43 [95% CI, 1.09-1.88]). Although the point estimate for 25- to 44-y-old recipients of a male donor suggested higher failure rates in females than males, the difference was not statistically significant (aHR 1.22 [95% CI, 0.95-1.57]). Among ≥45-y-old recipients of a male donor, females showed significantly higher hazards of graft failure than males (aHR 1.16 [95% CI, 1.06-1.27]).
FIGURE 3.
Comparisons of heart failure rates by recipient sex–relative hazards of graft failure in female vs male recipients stratified by donor sex. (A) When the donor was male, adjusted hazards of graft failure were higher in female than male recipients of all ages. (B) When the donor was female, female recipients <25-y-old had higher graft failure rates than male recipients of the same age, though these estimates are uncertain. There were no clear differences by recipient sex among those ≥25 y. Hazards ratios (HRs) and adjusted HRs are shown with 95% confidence intervals (CIs). Final models were adjusted for recipient race, primary heart disease, donor age, donor race, donor:recipient weight ratio, medical condition at transplant, use of a ventricular assist device, and era of transplant.
When the donor was female (Figure 3b), there were no statistically significant differences by recipient sex. Point estimates for the two youngest age intervals suggested 17–19% higher failure rates in females than males, but confidence intervals included 1.00. There were no evident differences in failure rates between males and females ≥25 y; the aHR was 0.96 for those 25–44 y, and 1.03 for those ≥45 y, with CIs that included 1.00.
Sensitivity Analyses
The model including insurer and HLA mismatch with multiple imputations for missing values returned results almost identical to those shown in Figure 3 (not shown). In a cohort limited to transplants from 2003 or later (when VAD data were not missing), comparisons of VAD use by sex were similar to what was observed in the entire cohort, with slightly higher VAD use in males than females in most age intervals among recipients of both male and female grafts (Tables S1a and S1b, SDC, http://links.lww.com/TXD/A356). The model limited to transplants 2003 or later returned results very similar to those in the primary model (where missing VAD use data were assumed to represent no VAD use) (Table S2, SDC, http://links.lww.com/TXD/A356). There were some differences in point estimates (young recipients of a female donor) and substantially wider confidence intervals owing to the much smaller sample, but the pattern of sex differences across ages was similar. The model that adjusted for donor:recipient predicted heart mass ratio instead of donor:recipient weight ratio included the 43 069 recipients >18 y old for whom heart mass could be predicted for both donor and recipient. Results were similar (Table S3, SDC, http://links.lww.com/TXD/A356). However, these are difficult to interpret because of a large amount of missing data.
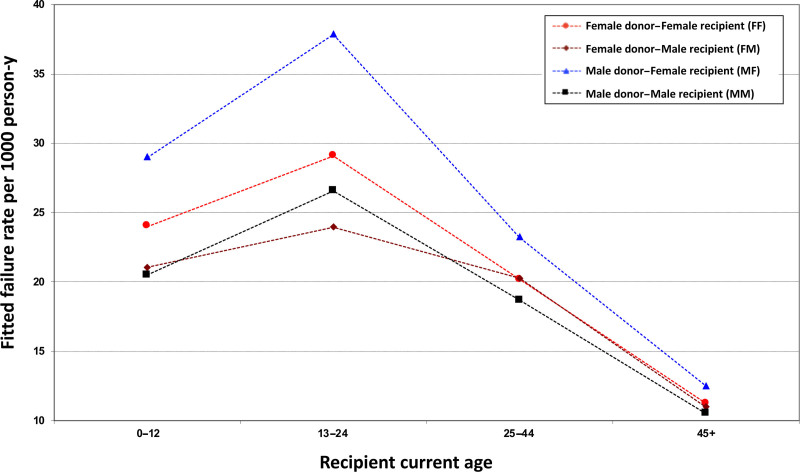
Fitted Absolute Graft Failure Rates by Donor–Recipient Sex Combination and Recipient Age
Figure 4 shows fitted graft failure rates by donor–recipient sex combination in the four current-age intervals among those with fixed characteristics (see Figure 4 caption). When the donor was male, failure rates were higher in female than male recipients across all ages except those ≥45 y; 13–24 y-olds showed the largest absolute difference, with rates higher by 11.3 failures per 1000 person-y. In the setting of a female donor, the estimated difference in failure rates between female and male recipients was smaller; the largest difference (3.9 failures per 1000 person-y) was seen among 13- to 24-y-olds. Comparisons of the fitted absolute failure rates between male and female recipients (adjusted for potential confounders) are provided by the models used to calculate these rates.
FIGURE 4.
Fitted absolute heart graft failure rates by donor–recipient sex combination. Fitted graft failure rates (failures per 1000 person-y) within each current age interval (0–12, 13–29, 30–44, ≥45 y) are shown for each donor–recipient sex combination. These estimates are based on the following profile of recipient and donor characteristics: White recipient race, donor age ≤35 y, White donor race, donor:recipient weight ratio ≥0.9, no use of a ventricular assist device, primary disease coronary/ischemic, medical condition not hospitalized, and transplant era 2005–2009.
Crude graft failure rates by donor–recipient sex combination in the four current-age intervals are shown in Figure S1, SDC, http://links.lww.com/TXD/A356.
DISCUSSION
This was the first study to compare heart graft failures by recipient sex, accounting for the potentially modifying effects of both donor sex and recipient age. When the donor was male, the pattern of recipient sex differences in heart graft survival was similar to that observed in kidney transplant.10 The magnitudes of the differences in each age interval were in the same range as those observed in kidney transplant; however, confidence intervals were wide, indicating uncertainty. Power to precisely estimate effect size was limited because of the relatively small number of person-years of observation and low failure rates, particularly in the youngest age intervals.
Among recipients of female donors, the pattern in heart transplant recipients differed somewhat from that seen in other organs. The point estimates suggested higher failure rates for female than male heart recipients <25 y. Although higher failure rates were also seen in female than male kidney transplant recipients in the adolescent and young adult age range, kidney recipients showed no clear sex differences in failure rate among children.10 However, there was substantial uncertainty around the estimates for both heart and kidney transplants. Whereas female heart recipients of post-menopausal age who received a female donor showed no evident difference in graft failure rates compared with their male counterparts who also received a female organ, older female kidney and liver recipients showed lower failure rates than males.10
Our findings in heart transplant recipients are similar to those of prior studies that compared mortality rates by heart recipient sex. These studies showed higher mortality among female than male adult18,20,33 and pediatric21 recipients of a male donor. No prior studies compared graft failures or mortality between male and female recipients of female donors. Several factors may contribute to the observed differences in outcomes by recipient sex.
There is some evidence that the observed sex differences are immune-mediated. The X-chromosome contains the largest number of immune-related genes in the genome (eg, FOXP3, IL-2 gamma chain, TLR7, CD40L).34 Because up to 23% of genes on the “inactivated” Xs in females are expressed,34 females may have greater immune reactivity than males because of greater expression of these immune-related genes. The immune-stimulating influence of estrogen and the inhibiting effects of androgens15,16,35 may also contribute to the pattern of poorer outcomes among female than male recipients in the adolescent and young adult age interval. Three studies of adult heart transplant recipients showed higher risks of acute rejection among females than males. A study of 160 recipients showed 3.2 times higher odds of antibody-mediated rejection in females than males.36 Female recipients (vs male) had an odds ratio of 1.55 (1.33, 1.82) for acute rejection in the first post-transplant year in a study of 31 634 recipients,20 and a greater proportion of female (52.4%) than male (45.7%) recipients required treatment for acute rejection within the first 3 y after transplant in a study of 165 recipients.26
Given the consistently poorer outcomes among female than male recipients of a male donor, it seems likely that immune recognition of the HY antigen (present only on male tissues) by female recipients of a male donor plays a major role in the observed differences. Higher levels of sensitization among women than men due to prior pregnancies may certainly contribute to poorer outcomes in females. However, this would not play a role in children and adolescents. In addition, it is informative to note that adult female recipients only appear to have a higher risk of graft failure than male recipients in the setting of a male donor; there was no apparent difference when the donor was female. If sensitization from prior pregnancies played a major role in the observed sex differences in graft failure rates between male and female recipients, then female recipients should have a higher risk of graft failure than male recipients regardless of donor sex; this is not the case.
Sex hormones, the levels of which are age-related, may influence the pharmacokinetics and pharmacodynamics of immunosuppressive medications, further contributing to sex differences in outcomes. Finally, women have been repeatedly shown to have better immunosuppressive medication adherence than men.7-9 Better medication adherence in women may mitigate the higher risks related to greater immune reactivity in adolescents and younger adults and lead to lower risks in women than men in the post-menopausal age group. Importantly, sex differences in adherence vary by age: no sex differences in adherence were observed among kidney transplant recipients under 17 y old.7 Therefore, sex differences in medication adherence are unlikely to contribute to sex difference in graft outcomes in children. It should be emphasized that this study cannot identify mechanisms for sex differences. The SRTR provides no information on sex hormone levels, measures of immune activation, or medication adherence.
It is useful to consider the magnitude of the HR for graft failure risk associated with recipient sex that represents a clinically meaningful difference. Trivedi et al identified donor and recipient factors that predict heart graft survival and used these to generate risk scores that they proposed could be used to make clinical decisions.37 Ischemia time >4 h (vs <4 h: HR 1.13), which contributed 2 points, and sex mismatch (HR 1.07), which contributed 1 point (out of a maximum of 6 points), were included among the variables contributing to the donor risk score. The recipient risk score included 9 variables (maximum score 16); included among these were previous transplant (HR 1.16) and previous cancer (HR 1.08), which contributed 2 points each. A 2-point difference in risk score corresponded to the difference between a “very low risk” recipient and an “intermediate risk” recipient or between a “low risk” donor and an “intermediate risk” donor; these risk categories were considered clinically meaningful. Based on the HR associated with variables considered clinically meaningful in the risk scores, an HR ≥1.07–1.16 would likely be thought clinically relevant.
This study has limitations. The study included a long time period over which both transplant management and outcomes have changed. We included era as a covariate in an effort to account for this but cannot exclude residual confounding. However, it is unlikely that the long timeframe would introduce bias in comparisons between males and females. Biologic differences between males and females have not changed over time. Furthermore, transplant management strategies have never differed for males and females; as organ allocation and transplant management changed, new approaches were equally applied to both sexes.
Power to generate precise HR estimates was limited in a model that considered the potentially modifying effects of recipient age and donor sex. However, these interactions have been previously observed in kidney,10 liver, heart, and pancreas transplant26-28,33,38 and have a strong biologic rationale; they cannot be ignored. Estimation of sex differences in graft outcomes ignoring the modifying effects of recipient age and donor sex may be more precise but likely meaningless. As the largest heart transplant cohort worldwide, the SRTR provides the best estimates currently available. Future studies combining data from multiple large databases are needed to get more precise estimates.
The age intervals were selected to represent pre-pubertal, adolescence and young adulthood, middle adulthood, and post-menopausal ages. However, these ages do not always align with these developmental periods; misclassification was possible. Information on puberty and menopause was not available. We believe that the age intervals chosen minimize misclassification.
It is possible that some outcomes may have been misclassified, whereby deaths classified as occurring with graft function (and therefore censored) may actually have been related to immune-mediated transplant vasculopathy. If such misclassifications were more (or less) likely in females than males, our estimates may be biased. It is somewhat reassuring that prior studies that considered recipient sex differences in mortality risk had similar findings.17,18,21,33
Finally, our study was restricted to heart transplant recipients in the United States; conclusions cannot be generalized to recipients in other countries. Biologic differences between the sexes will be consistent across countries. However, sex and gender biases in medical care and gender-related adherence behaviors may vary by country.
This study provides further evidence of the importance of recipient sex to graft outcomes. The pattern seen in heart transplant recipients was consistent with that observed in kidney and other transplants when the donor was male, with higher failure rates in females than males, and the largest sex differences seen in children, adolescents, and young adults. The pattern of sex differences is uncertain in the setting of a female donor and less consistent across organs. These observations suggest that reaction of female recipients against the HY antigen present on male tissues may be among the most important contributors to the higher failure rates seen in female than male recipients of a male donor. Larger studies, combining data from multiple data sources from around the world, are needed to get more precise estimates of sex differences in graft outcomes. It will also be important to compare rates of acute rejection and of cardiac allograft vasculopathy by recipient sex, taking the modifying effects of age and donor sex into account. Fundamental studies comparing the immune systems of males and females at different ages and exploring sex differences in pharmacokinetics and pharmacodynamics of immunosuppressive agents are also needed.
ACKNOWLEDGMENTS
The data that support the findings of this study are openly available from the SRTR; the SRTR requires a formal request for data and a data use agreement. The data reported here have been supplied by the Chronic Disease Research Group of the Hennepin Healthcare Research Institute as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Supplementary Material
Footnotes
Published online 7 September, 2021.
This work was funded by the Canadian Institutes of Health Project Grant PJT165832.
B.J.F. and R.S.-P. are both members of the Centre for Outcomes Research and Evaluation of the Research Institute of the McGill University Health Centre. R.S.-P. and H.C. are the recipients of FRQS Chercheurs-boursier clinicien awards.
A.D.S. participated in data analysis and in the writing of the article. X.Z. and M.D. participated in research design, in the performance of the research, and in data analysis. R.S.-P. and H.C. participated in research design and in the writing of the article. L.W. participated in the writing of the article. B.J.F. participated in research design, in the writing of the article, in the performance of the research, and in data analysis
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Pinn VW. Sex and gender factors in medical studies: implications for health and clinical practice. JAMA. 2003;289:397–400. [DOI] [PubMed] [Google Scholar]
- 2.Shah ASV, Griffiths M, Lee KK, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler S, Altfeld M. Sex differences in HIV-1-mediated immunopathology. Curr Opin HIV AIDS. 2016;11:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep. 2018;15:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferretti MT, Iulita MF, Cavedo E, et al. ; Women’s Brain Project and the Alzheimer Precision Medicine Initiative. Sex differences in Alzheimer disease – the gateway to precision medicine. Nat Rev Neurol. 2018;14:457–469. [DOI] [PubMed] [Google Scholar]
- 7.Boucquemont J, Pai ALH, Dharnidharka VR, et al. Gender differences in medication adherence among adolescent and young adult kidney transplant recipients. Transplantation. 2019;103:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spivey CA, Chisholm-Burns MA, Damadzadeh B, et al. Determining the effect of immunosuppressant adherence on graft failure risk among renal transplant recipients. Clin Transplant. 2014;28:96–104. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm-Burns MA, Spivey CA, Tolley EA, et al. Medication therapy management and adherence among US renal transplant recipients. Patient Prefer Adherence. 2016;10:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepeytre F, Dahhou M, Zhang X, et al. Association of sex with risk of kidney graft failure differs by age. J Am Soc Nephrol. 2017;28:3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melk A, Babitsch B, Claas F, et al. Equally interchangeable? How sex and gender affect transplantation. Transplantation. 2019;103:1094–1110. [DOI] [PubMed] [Google Scholar]
- 12.Gratwohl A, Döhler B, Stern M, et al. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet. 2008;372(9632):49–53. [DOI] [PubMed] [Google Scholar]
- 13.Tan JC, Kim JP, Chertow GM, et al. Donor-recipient sex mismatch in kidney transplantation. Gend Med. 2012;9:335–347. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 16.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11:A479–A485. [DOI] [PubMed] [Google Scholar]
- 17.Kemna M, Albers E, Bradford MC, et al. Impact of donor-recipient sex match on long-term survival after heart transplantation in children: an analysis of 5797 pediatric heart transplants. Pediatr Transplant. 2016;20:249–255. [DOI] [PubMed] [Google Scholar]
- 18.Kittleson MM, Shemin R, Patel JK, et al. Donor-recipient sex mismatch portends poor 10-year outcomes in a single-center experience. J Heart Lung Transplant. 2011;30:1018–1022. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Li B, Wei Y-G, et al. Female-to-male match predicted poor survival after living-donor liver transplantation—some issues needed to be clarified. Transplantation. 2012;94:e35–e36. [DOI] [PubMed] [Google Scholar]
- 20.Reed RM, Netzer G, Hunsicker L, et al. Cardiac size and sex-matching in heart transplantation: size matters in matters of sex and the heart. JACC Hear Fail. 2014;2:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tosi L, Federman M, Markovic D, et al. The effect of gender and gender match on mortality in pediatric heart transplantation. Am J Transplant. 2013;13:2996–3002. [DOI] [PubMed] [Google Scholar]
- 22.Lau A, West L, Tullius SG. The impact of sex on alloimmunity. Trends Immunol. 2018;39:407–418. [DOI] [PubMed] [Google Scholar]
- 23.Tan JC, Wadia PP, Coram M, et al. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008;86:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapir-Pichhadze R, Pintilie M, Tinckam KJ, et al. Survival analysis in the presence of competing risks: the example of waitlisted kidney transplant candidates. Am J Transplant. 2016;16:1958–1966. [DOI] [PubMed] [Google Scholar]
- 25.Owens IPF. Ecology and evolution. Sex differences in mortality rate. Science. 2002;297: 2008–2009. [DOI] [PubMed] [Google Scholar]
- 26.Zeier M, Döhler B, Opelz G, et al. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–2576. [DOI] [PubMed] [Google Scholar]
- 27.Foster BJ, Dahhou M, Zhang X, et al. High risk of graft failure in emerging adult heart transplant recipients. Am J Transplant. 2015;15:3185–3193. [DOI] [PubMed] [Google Scholar]
- 28.Foster BJ, Dahhou M, Zhang X, et al. High risk of liver allograft failure during late adolescence and young adulthood. Transplantation. 2016;100:577–584. [DOI] [PubMed] [Google Scholar]
- 29.Foster BJ, Dahhou M, Zhang X, et al. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011;92:1237–1243. [DOI] [PubMed] [Google Scholar]
- 30.Gulati A, Sarwal MM. Pediatric renal transplantation: an overview and update. Curr Opin Pediatr. 2010;22:189–196. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kransdorf EP, Kittleson MM, Benck LR, et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant. 2019;38:156–165. [DOI] [PubMed] [Google Scholar]
- 33.Weiss ES, Allen JG, Patel ND, et al. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail. 2009;2:401–408. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Syrett CM, Kramer MC, et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci USA. 2016;113:E2029–E2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294:87–94. [DOI] [PubMed] [Google Scholar]
- 36.Grupper A, Nestorovic EM, Daly RC, et al. Sex related differences in the risk of antibody-mediated rejection and subsequent allograft vasculopathy post-heart transplantation: a single-center experience. Transplant Direct. 2016;2:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trivedi JR, Cheng A, Ising M, et al. Heart transplant survival based on recipient and donor risk scoring: a UNOS database analysis. Asaio J. 2016;62:297–301. [DOI] [PubMed] [Google Scholar]
- 38.Marino IR, Doyle HR, Aldrighetti L, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22:1754–1762. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.