Abstract
Alzheimer's disease is associated with poor sleep, but the impact of tau and β-amyloid (Aβ) pathology on sleep remains largely unknown. Here, we test the hypothesis that tau and Aβ predict unique impairments in objective and self-perceived human sleep under real-life, free-living conditions. Eighty-nine male and female cognitively healthy older adults received 18F-FTP-tau and 11C-PIB-Aβ PET imaging, 7 nights of sleep actigraphy and questionnaire measures, and neurocognitive assessment. Tau burden, but not Aβ, was associated with markedly worse objective sleep. In contrast, Aβ and tau were associated with worse self-reported sleep quality. Of clinical relevance, Aβ burden predicted a unique perceptual mismatch between objective and subject sleep evaluation, with individuals underestimating their sleep. The magnitude of this mismatch was further predicted by worse executive function. Thus, early-stage tau and Aβ deposition are linked with distinct phenotypes of real-world sleep impairment, one that includes a cognitive misperception of their own sleep health.
SIGNIFICANCE STATEMENT Alzheimer's disease is associated with sleep disruption, often before significant memory decline. Thus, real-life patterns of sleep behavior have the potential to serve as a window into early disease progression. In 89 cognitive healthy older adults, we found that tau burden was associated with worse wristwatch actigraphy-measured sleep quality, and that both tau and β-amyloid were independently predictive of self-reported sleep quality. Furthermore, individuals with greater β-amyloid deposition were more likely to underestimate their sleep quality, and sleep quality underestimation was associated with worse executive function. These data support the role of sleep impairment as a key marker of early Alzheimer's disease, and offer the possibility that actigraphy may be an affordable and scalable tool in quantifying Alzheimer's disease-related behavioral changes.
Keywords: aging, Alzheimer's disease, amyloid, sleep, tau
Introduction
The association between sleep disturbance and Alzheimer's disease (AD) is multidimensional (Mander et al., 2016; Wang and Holtzman, 2020). Brain regions critical to the regulation of sleep-wake behavior are highly vulnerable to and among the earliest regions affected by AD pathology (Serrano-Pozo et al., 2011; Stern and Naidoo, 2015; Theofilas et al., 2015; Oh et al., 2019). Furthermore, the aggregation of tau and β-amyloid (Aβ) pathology in cortical regions is associated with specific deficits in neural oscillatory activity during sleep, both in humans and rodents (Roh et al., 2012; Menkes-Caspi et al., 2015; Lucey et al., 2019; Winer et al., 2019). However, it remains unclear how the early deposition of both tau and Aβ affects real-life human sleep behavior (i.e., an individual's everyday sleep activity), their related subjective perception of sleep, and any mismatch between objective and subjective sleep health. The widespread availability of accelerometers enables the quantification of ecological sleep-wake behaviors outside the laboratory using continuous recording of actigraphy (Ancoli-Israel et al., 2003). When coupled with validated biomarkers, actigraphy may thus represent a meaningful tool in determining early sleep changes associated with disease.
AD pathology is detectable before the onset of observable symptoms through the use of PET imaging (Jagust, 2018). Pairing actigraphy with PET imaging in asymptomatic, cognitively healthy older adults therefore provides a unique opportunity to identify associations between tau and Aβ pathologic burden and sleep measures that occur early on in the progression of AD (Winer and Mander, 2018), and can reflect real-life sleep behavior. Moreover, such quantification is needed if sleep disruption is to become a scalable biomarker tool for early AD disease risk and detection, in order to quantify the efficacy of intervention strategies that modify sleep with the goal of reducing AD risk.
In humans, both objective (actigraphy-estimated) and subjective (questionnaire-based) approaches to sleep assessment have demonstrated that poor sleep predicts worse outcomes in the context of aging and AD (Lim et al., 2013; Diem et al., 2016; McSorley et al., 2019). In addition, seminal findings have revealed a link between greater AD pathology (particularly Aβ) and worse sleep quality (Ju et al., 2013; Spira et al., 2013; Sprecher et al., 2015, 2017; Branger et al., 2016; Brown et al., 2016; Ettore et al., 2019). However, there are limited data establishing a link between objective measures of sleep behavior and in vivo markers of AD progression. Considering the recent advent of tau PET imaging, there is a hastened need to investigate the contributions of AD pathology to disrupted sleep behavior, and whether AD pathology further disrupts the precision of older adults' evaluation of their sleep quality relative to objective measures.
Here, we addressed these questions by combining tau and Aβ PET imaging with multiple nights of at-home naturalistic sleep actigraphy-estimated measurement in combination with validated subjective sleep assessment tools in a cohort of cognitively healthy older adults. More specifically, we sought to test three inter-related hypotheses: that the early accumulation of tau and Aβ in cognitive normal older adults are associated (1) with worse patterns of objective sleep in a real-life setting, (2) with impairments in self-reported perception of sleep quality, and (3) because of AD pathologic burden within key cognitive brain networks, with a greater mismatch between self-assessed perception of sleep quality relative to objective sleep amount.
Materials and Methods
Participants
Eighty-nine cognitively normal older adults participated in the study. Subjects underwent actigraphy recording, 18F-FTP (tau) and 11C-PiB PET (Aβ) imaging (Figs. 1, 2), structural MRI, neuropsychological testing, and standard laboratory blood tests, including APOE genotyping. Eighty-nine cognitively normal older adults participated in the study.
Figure 1.
Actigraphy, tau PET, and Aβ PET in 3 sample participants. A, Seven 24 h periods of raw actigraphy data display accelerometer counts in 15 s epochs. B, 18F-FTP coronal images demonstrate tau deposition. C, 11C-PiB sagittal images demonstrate Aβ deposition.
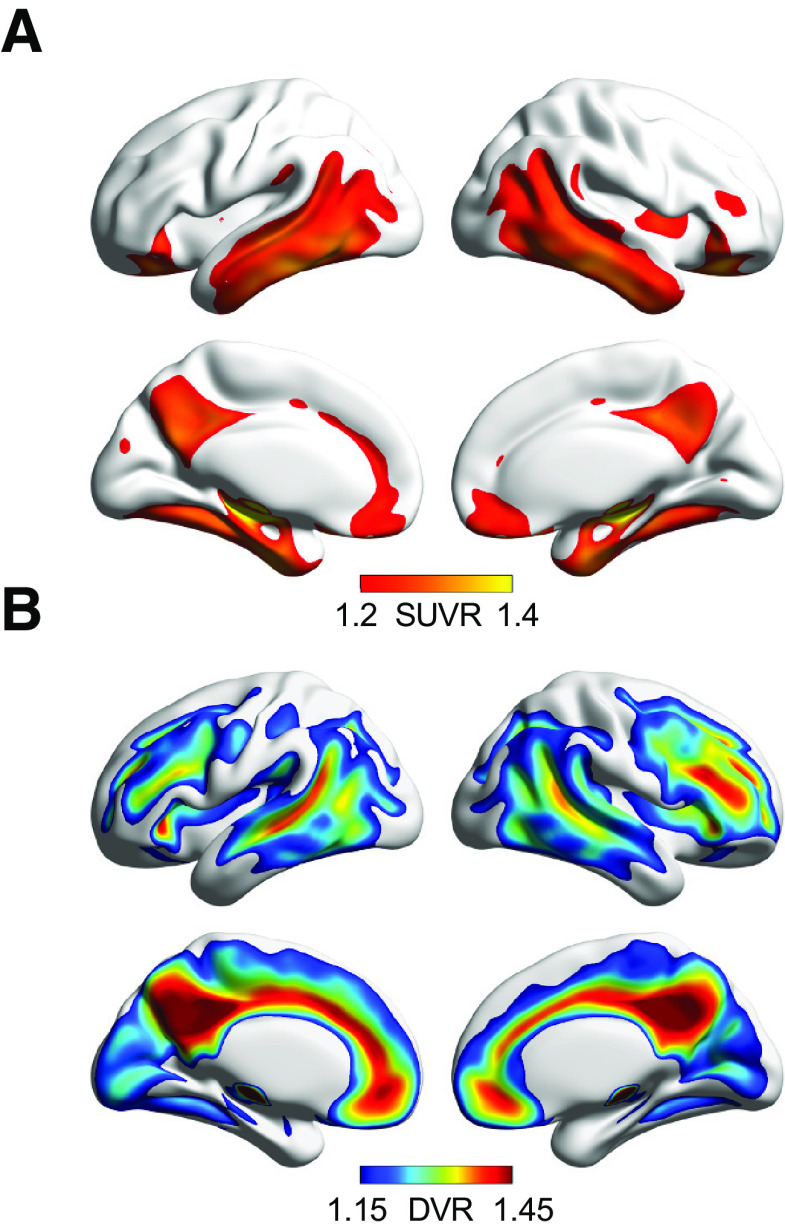
Figure 2.
Voxelwise tau and Aβ PET maps at the group level. A, Mean voxelwise 18F-FTP SUVR in n = 89 individuals, representing tau distribution in healthy older adults. B, Mean voxelwise 11C-PiB DVR in the same n = 89 individuals, representing Aβ distribution.
Participants were free of neurologic or psychiatric disorders, without use of antipsychotic or depression medication, scored ≥25 on the Mini-Mental State Examination (Folstein et al., 1975), and displayed normal performance on neuropsychological testing (1.5 SDs within age, education, and sex adjusted means). Participants were not selected for sleep concerns. The study was approved by the Institutional Review Boards at University of California, Berkeley and Lawrence Berkeley National Laboratories (LBNL). All participants provided written informed consent.
Actigraphy data
Participants were asked to wear a wristwatch actigraph (Micro Motionlogger, Ambulatory Monitoring) on the nondominant wrist for seven consecutive 24 h periods. Data were excluded if the watch was taken off during the night, and participants were removed from the analysis if they had <4 (of 7) complete nights of data, resulting in a study mean of 6.8 ± 1.0 nights per subject. Accelerometer data were recorded in 15 s epochs. Sleep parameters were derived in zero crossing mode in Action W-2 software (version 2.7.3045, Ambulatory Monitoring).
Sleep journals that included information on sleep and wake timing (distinct from the subjective sleep assessment, described below) were completed by every participant for each night they wore the watch. Journals and event markers noting when participants started trying to fall asleep and awoke in the morning were used as a guide to determine down (in bed) periods. The actigraphy-estimated measures are referred to as objective measures of sleep but rely on (subjective) participant reports of sleep timing to estimate sleep period time.
Sleep analysis was quantified using the Sadeh algorithm (Sadeh et al., 1994). The sleep period was defined as the first minute of at least 3 consecutive minutes scored as sleep within the down period (sleep onset) until the last minute of 5 continuous minutes scored as sleep within the down period (sleep offset). Nocturnal sleep duration was calculated as total minutes scored as sleep within the sleep period. Sleep efficiency was calculated as total minutes scored as sleep divided by duration of the sleep period. Sleep fragmentation was calculated as number of awakenings divided by the duration of the sleep period, multiplied by 100.
For every participant, each of these three measures was averaged across the total number of nights of data collected. The three parameters were then standardized (Z scored) and averaged, resulting in a composite global sleep quality score for each participant (Landry et al., 2015). The fragmentation score was inverted before averaging, so that higher values meant “better quality” sleep for all measures.
Sleep questionnaire data
Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). The PSQI is a well-validated questionnaire assessing 7 domains of sleep quality over the past month to provide a global score (0-21) of overall sleep quality, with scores >5 indicating poor sleep quality. In addition to the global score, reported sleep duration and sleep efficiency (sleep duration divided by the duration of the interval between reported bedtime and wake time) were included in analyses with PET data. PSQI data were collected within 2 weeks of the first night of actigraphy data for every participant.
Cognitive data
All participants received neuropsychological assessment to quantify standardized cognitive domain performance related to verbal memory, working memory, and executive function. Specifically, (1) assessment and analyses targeted long-delay (after 20 min) memory free recall using the California Verbal Learning Test (Delis et al., 2000) assessing episodic memory performance, (2) WMS-III Digit Span backwards score (Wechsler, 1997) quantifying working memory, and (3) Trail B minus A score from the Trail Making Test (Reitan and Wolfson, 1985) measuring executive function. Neuropsychological data were obtained within 1.3 ± 3.4 months of the first actigraphy night.
PET data
11C-PiB PET imaging, quantifying Aβ burden, was conducted in 3D acquisition mode using a BIOGRAPH PET/CT Truepoint 6 scanner (Siemens Medical Systems), with 11C-PiB synthesized at the LBNL Biomedical Isotope Facility. Immediately after intravenous injection of ∼15 mCi of PiB, 90 min of dynamic acquisition frames was obtained (4 × 15, 8 × 30, 9 × 60, 2 × 180, 10 × 300, and 2 × 600 s). For each 11C-PiB scan, CT scan was obtained for attenuation correction. 11C-PiB PET images were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation, scatter correction, and smoothed with a 4 mm Gaussian kernel. 11C-PiB scans were collected within 8.6 ± 7.5 months of actigraphy data.
11C-PiB data were realigned, and frames from the first 20 min of acquisition were averaged and coregistered to participants' corresponding structural MRI (see below for MRI details). Distribution volume ratios (DVRs) for 11C-PiB images were generated with Logan graphical analysis on 11C-PiB frames corresponding to 35-90 min after injection using a cerebellar gray matter reference region (Logan et al., 1996; Price et al., 2005). Cortical 11C-PiB DVR was calculated as a weighted mean across FreeSurfer-derived native space frontal, temporal, parietal, and posterior cingulate cortical regions. Participants were classified as Aβ-positive if their cortical 11C-PiB DVR was ≥1.065, a cutoff adapted from previous thresholds developed in our laboratory (Villeneuve et al., 2015) (Table 1).
Table 1.
Participant demographics, PET, and sleep characteristicsa
| Demographics (n = 89) | |
|---|---|
| Age, yr | 78.1 ± 7.6 |
| Female, n (%) | 56 (63) |
| APOE e4 carriers,b n (%) | 23 (27) |
| Education, yr | 17.0 ± 2.0 |
| Mini-Mental State Examination | 28.6 ± 1.4 |
| Body mass index | 25.6 ± 4.2 |
| PET imaging | |
| PiB status, n (%) | PiB+ 45 (51) PiB- 44 (49) |
| FTP Braak staging, n (%) | Braak 0: 16 (18) Braak I/II: 57 (64) Braak III/IV: 16 (18) |
| PiB+ within FTP Braak stage, n (%) | Braak 0: 4 PiB+ (25) Braak I/II: 29 PiB+ (51) Braak III/IV: 12 PiB+ (75) |
| Actigraphy-measured sleep | |
| Nights recorded | 6.8 ± 1.0 |
| Sleep period time, h | 7.9 ± 1.0 |
| Sleep duration, h | 7.2 ± 1.1 |
| Sleep efficiency, % | 90.9 ± 7.8 |
| Fragmentation index | 0.87 ± 0.62 |
| Self-reported sleep | |
| PSQI global score | 4.6 ± 2.9 |
| Sleep duration, h | 7.2 ± 1.1 |
| Sleep efficiency, % | 87.0 ± 9.7 |
aData are mean ± SD. PiB status was determined based on a DVR threshold of 1.065 (Villeneuve et al., 2015). FTP Braak staging, indexing the progression of tau pathology, was assigned to one of four stages based on FTP uptake in Braak-based composite regions, described previously (Maass et al., 2017).
bAPOE genotyping data are not available for 5 subjects.
18F-FTP PET imaging, assessing tau burden, was conducted on the same PET scanner in 3D acquisition mode, with 18F-FTP also synthesized at the LBNL Biomedical Isotope Facility using a GE TracerLab FXN-Pro synthesis module with a modified protocol supplied by Avid Radiopharmaceuticals. Participants were injected with 10 mCi of tracer and were scanned from 75 to 115 min in list mode; data from 80 to 100 min were reconstructed as 4 × 5 min frames. A CT scan was performed before the start of each emission acquisition. Images were reconstructed in the same manner as the 11C-PiB data. 18F-FTP scans were collected within 8.6 ± 6.8 months of actigraphy data.
After realigning, calculating the mean, and coregistering and reslicing the mean to the structural MRI, the 18F-FTP standard uptake value ratio (SUVR) was calculated by intensity normalizing the mean image by mean inferior cerebellar gray matter uptake. SUVR images were coregistered and resliced to structural MRI. To account for partial volume effects because of atrophy and spillover signal, the Geometric Transfer Matrix approach (Rousset et al., 1998) was used for partial volume correction (PVC) based on FreeSurfer-derived ROIs, including corrections for extracerebral signal as previously described in detail (Baker et al., 2017). For descriptive purposes, participants were assigned to one of four FTP Braak stages based on previously determined thresholds in ROIs approximating tau neuropathological staging (Braak and Braak, 1991; Schöll et al., 2016; Maass et al., 2017) (Table 1).
Analyses between sleep variables and tau PET focused on 18F-FTP SUVR within two a priori ROIs: (1) bilateral entorhinal cortex, because of its known sensitivity to age-related tau deposition (Buckley et al., 2017; Maass et al., 2018), and (2) a composite ROI (“meta-ROI”) because of its representation of pathologic severity of tau accumulation in the context of Alzheimer's disease (Jack et al., 2017; Maass et al., 2017). The meta-ROI consisted of weighted mean PVC 18F-FTP SUVR within bilateral entorhinal, amygdala, parahippocampal, fusiform, inferior temporal, and middle temporal FreeSurfer regions (Baker et al., 2017; Maass et al., 2017).
18F-FTP and 11C-PiB maps from all participants were warped to MNI space (Schöll et al., 2016; Maass et al., 2018). These warped images were used to create group mean images of 18F-FTP and 11C-PiB.
MRI data
High-resolution T1-weighted MPRAGE images were acquired for every subject on a 1.5T Siemens Magnetom Avanto scanner at LBNL (TR/TE = 2110/3.58 ms, FA = 15°, 1 × 1 × 1 mm resolution). T1 MPRAGE scans were processed with FreeSurfer version 5.3.0 to derive ROIs in each subject's native space using the Desikan-Killiany atlas.
FreeSurfer ROIs were used to calculate global 11C-PiB PET and 18F-FTP PET ROI measures in native space for each subject. FreeSurfer gray matter cerebellum mask was used as a reference region in calculating 11C-PiB DVRs. The intercept of cerebellar gray matter and a reverse-normalized mask of inferior cerebellum (derived from SUIT template) was used to calculate 18F-FTP SUVRs. MR images were also segmented into tissue types using SPM12 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology). SPM-derived segments for noncerebral tissues were subsequently used for PVC (Baker et al., 2017). Imaging results were displayed on 3D brain surfaces using BrainNet Viewer (Xia et al., 2013).
APOE genotyping
Determination of APOE alleles was performed using a TaqMan Allelic Discrimination Assay using a Real-Time PCR system (Applied Biosystems). APOE genotyping was completed for 84 participants.
Statistical analysis
Two-sided bivariate correlation was used to test associations between PET, sleep, and cognition metrics. Comparisons of correlation strength for sleep and PET variable associations were performed using the Robust Correlation Toolbox (Pernet et al., 2013). Multiple linear regression models were used to examine PET, sleep, and cognition relationships with age, sex, and APOE e4 allele carrier status included as covariates. For linear regressions with actigraphy-derived measures as an outcome, the sleep medication score from the PSQI was used as an additional covariate to adjust for medication use. This medication score was not used in analyses with PSQI measures as an outcome since the score is used in calculating the PSQI global score (Buysse et al., 1989). For linear regressions with PET imaging measures, the time interval between sleep assessment and PET scan was additionally included as a covariate. Similarly, for regressions with neuropsychological test scores, the time interval between sleep assessment and neuropsychological assessment was included as a covariate. Outliers >3 or <3 SDs were removed from analyses of sleep duration, sleep efficiency, and sleep fragmentation. All participants were included in analyses of actigraphy composite score.
Results
Participant demographics, PET, and sleep characteristics are summarized in Table 1. A total of 609 nights of actigraphy data were collected, with a mean (±SD) of 6.8 (±1.0) nights per participant. Twenty-four subjects reported having taken prescription or over-the-counter medicine to help them fall asleep in the month preceding actigraphy data collection. Five participants reported a history of sleep apnea. Two participants had actigraphy-estimated sleep efficiency values >3 SDs below the mean and were removed from correlations with actigraphy-estimated sleep efficiency as the outcome. One participant had a sleep duration value >3 SDs above the mean and was removed from correlations with actigraphy-estimated sleep duration as the outcome.
Actigraphy composite and PSQI global score did not differ on the basis of APOE e4 carrier status (t < 0.41, p > 0.68).
Associations between objectively measured sleep, tau, and Aβ
Focusing first on objective sleep, analyses sought to test the hypothesis that tau and Aβ burden are predictive of worse sleep quality reflected by the actigraphy composite score (Landry et al., 2015). All bivariate correlation (r) values between sleep measures and tau (18F-FTP SUVR) and Aβ (11C-PiB DVR) are presented in Table 2.
Table 2.
Correlations between regional PET uptake and sleep variables of interesta
| Correlation with entorhinal FTP SUVR (r) | Correlation with meta-ROI FTP SUVR (r) | Correlation with cortical PiB DVR (r) | |
|---|---|---|---|
| Actigraphy-measured sleep | |||
| Global actigraphy composite | −0.23* | −0.31** | −0.01 |
| Sleep duration | −0.08 | −0.11 | −0.03 |
| Sleep efficiency | −0.18 | −0.29** | 0.02 |
| Sleep fragmentation | 0.11 | 0.19 | −0.07 |
| Self-reported sleep | |||
| PSQI global | 0.34*** | 0.28** | 0.36*** |
| Sleep duration | −0.18 | −0.07 | −0.13 |
| Sleep efficiency | −0.18 | −0.16 | −0.28** |
aColumns represent each brain ROI. Rows represent two-sided bivariate correlations between sleep metric and PET uptake (see Materials and Methods). PiB DVR ROIs are comprised of weighted PiB DVR means within FreeSurfer regions as described by Villeneuve et al. (2015). FTP SUVR ROIs are comprised of weighted FTP SUVR means within FreeSurfer regions after PVC, as described by Maass et al. (2017).
*p < 0.05;
**p < 0.01;
***p < 0.001; significant r value.
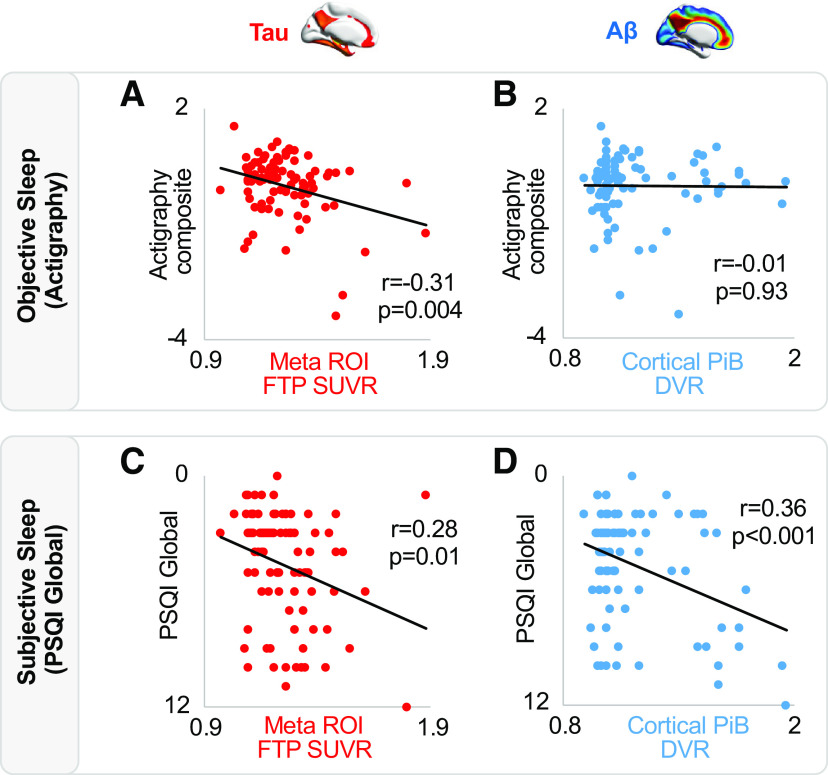
Consistent with our hypothesis, greater tau deposition in the Alzheimer's-sensitive meta-ROI was significantly associated with worse quality of objective sleep, defined by the actigraphy composite score (Fig. 3A; r = −0.31, p = 0.004). This was similarly true for the entorhinal region, predicting worse sleep indexed by the actigraphy composite score (r = −0.23, p = 0.03). Thus, greater tau burden in brain regions reflecting both age-related accumulation in the entorhinal cortex, but also early AD progression defined in the meta-ROI, predicted worse objective sleep quality. Moreover, associations for both these anatomic regions remained significant in linear regressions that included age, sex, sleep medication usage, APOE e4 carrier status, and time interval between actigraphy and PET scan as covariates (both p < 0.02).
Figure 3.
Associations between tau, Aβ, and sleep. A, Negative association between tau PET and objective sleep (actigraphy composite score). B, No association between Aβ PET and actigraphy composite score. C, Positive association between tau PET and subjective sleep (PSQI global score). D, Positive association between Aβ PET and PSQI global score. PSQI score is inverted on y axis reflecting that greater scores indicate worse sleep quality. Two-sided bivariate correlations were used to test for significant associations with r and p values displayed on each panel.
Next, analyses tested whether Aβ deposition was similarly related to worse real-life objective sleep. Unlike tau, there was no association between cortical Aβ burden and the actigraphy composite score (Fig. 3B; r = −0.01, p = 0.93). That is, objective sleep quality was not significantly worse in individuals with high Aβ deposition. Confirming a dissociation between tau and Aβ in predicting worse objective sleep, comparison of these relationships (based on 1000 bootstrap samples of correlation coefficient pairs) revealed that the actigraphy composite score correlation with meta-ROI tau was significantly stronger than the correlation with Aβ (Δr = 0.30, p = 0.01), although the correlation with entorhinal tau was not significantly dissociable from the correlation with Aβ (Δr = 0.22, p = 0.18).
Next, analyses explored relationships with each actigraphy factor that comprised the composite score, thereby testing whether individual metrics of objective sleep disturbance were associated with tau and Aβ. Greater meta-ROI tau burden was significantly related to worse sleep efficiency (r = −0.29, p = 0.01), although this measure was only marginally associated with entorhinal cortex tau (r = −0.18, p = 0.09), suggesting a link to AD-related tau deposition, rather than simply normative age-related accumulation. Demonstrating tau specificity, cortical Aβ showed no relationship with sleep efficiency (r = 0.02, p = 0.86). The meta-ROI tau showed a marginal association with worse continuity of sleep (indexed by greater sleep fragmentation; r = 0.19, 0.07), but this measure was not related to entorhinal tau or Aβ (p > 0.33). Finally, neither tau (entorhinal nor meta-ROI) nor cortical Aβ was associated with total sleep duration (all p > 0.29). The significance (and lack thereof) of these results remained unchanged in linear regressions that included age, sex, sleep medication usage, APOE e4 carrier status, and time interval between actigraphy and PET scan as covariates, with the exception of meta-ROI significantly now predicting sleep fragmentation when including these covariates (p = 0.02).
Therefore, greater tau burden, and not Aβ, is associated with worse objective human sleep quality as expressed in a real-world, at-home environment—an AD-pathologic link that was independent of age, sex, or APOE e4 status. The relationships held for tau in the entorhinal cortex, thought to represent early age-related tau deposition, and was stronger for the larger AD meta-ROI, representing a preclinical/prodromal pattern of AD progression. While these relationships were robust for the composite of actigraphy-estimated sleep quality, sleep efficiency was the only stand-alone component of this composite that was significantly related to tau.
Associations between subjectively measured sleep, tau, and Aβ
Having demonstrated that tau burden is associated with objective sleep quality, analyses next tested the second hypothesis: that both measures of AD pathology (tau, Aβ) similarly predict participants' own subjective assessment of their sleep quality, quantified using the PSQI global score. The PSQI global score represents a multidimensional subjective sleep disturbance metric (Buysse et al., 1989) analogous to the objective actigraphy composite score. A complete list of bivariate correlation (r) values is presented in Table 2.
Greater tau burden in the entorhinal cortex was significantly associated with higher PSQI global score, reflecting worse sleep quality (r = 0.34, p = 0.001). This association with worse sleep quality was similarly true for the meta-ROI tau measure (r = 0.28, p = 0.01; Fig. 3C). Unlike objective sleep measures that were specific to tau, these relationships were also observed for Aβ. Specifically, greater cortical Aβ burden, like tau burden, was also associated with worse sleep quality (i.e., higher PSQI global score; r = 0.36, p < 0.001; Fig. 3D). All three associations remained significant in linear regressions that adjusted for age, sex, APOE e4 carrier status, and time interval between PSQI assessment and PET scan (all p < 0.02).
However, and demonstrating unique contributions, when cortical Aβ, entorhinal tau, and meta-ROI tau were entered into the same linear model predicting PSQI global score (along with age, sex, APOE e4 carrier status, and time interval between PSQI assessment and PET scan), Aβ and entorhinal tau separately and significantly predicted worse sleep quality (p < 0.03), although not so for the AD-sensitive meta-ROI tau (p = 0.43). When an interaction term for Aβ and entorhinal tau was added to the model, it was not significant (p = 0.10). Therefore, both cortical Aβ and entorhinal cortex tau each independently (and without interaction) predicted an individual's subjective perception of their sleep quality.
Beyond the global summary score, further analyses tested whether tau or Aβ was associated with individual sleep factors assessed by the PSQI questionnaire. Neither of the two tau ROIs was associated with self-reported sleep efficiency nor self-reported sleep duration (all p > 0.10). However, greater cortical Aβ predicted worse self-reported sleep quality, indexed by perceived sleep efficiency (r = −0.28, p = 0.01). This Aβ relationship was not observed for self-reported sleep duration (p > 0.10). All results remained unchanged when adjusting for age, sex, APOE e4 carrier status, and time interval between PSQI assessment and PET scan.
As an additional test of the study hypothesis, we entered both the actigraphy composite and PSQI global score as predictors in three regression models predicting entorhinal tau, meta-ROI tau, and cortical Aβ, respectively, with age, sex, and APOE e4 status as covariates in each model. For entorhinal tau and meta-ROI tau, both lower actigraphy composite (indexing worse objective sleep) and higher PSQI global score (indexing worse subjective sleep) were significantly associated with greater tau burden (p < 0.03). However, in the cortical amyloid model, PSQI global score was a significant predictor (p < 0.001) and actigraphy composite was not (p = 0.85), confirming the main study findings.
Therefore, both tau and Aβ burden were associated with significantly worse self-reported sleep, with the relationships most pronounced for perceived sleep quality, more than perceived sleep quantity.
Associations between subjective-objective sleep mismatch, tau, and Aβ
Having tested AD pathology associations between objective and subjective measures of sleep quality separately, a next set of analyses examined the third hypothesis: related to age- and AD-pathology-related impact on networks linked to cognitive function, the severity of AD pathology would further be associated with the magnitude of inaccuracy between objective and subjective sleep, indexed in the mismatch value.
First, confirming a mismatch in these older adults, the actigraphy composite score of objective sleep and the PSQI global score of subjective sleep were not significantly correlated (r = −0.07, p = 0.49). That is, self-reported perception of sleep quality did not accurately reflect objectively measured sleep quality. Such findings indicate that cognitively normal older adults are inaccurate in their self-evaluation of overall objective sleep quality.
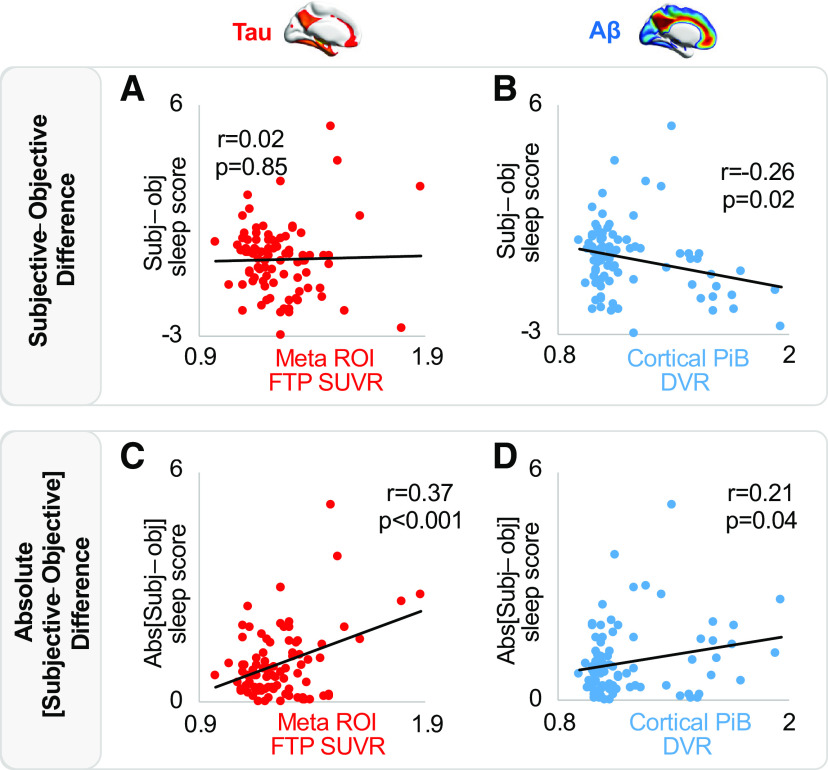
With the lack of a significant association between these two main sleep quantification methods confirming a subjective-objective mismatch, analyses next focused on whether the severity of tau and/or Aβ burden predicted this measure of perceptual sleep inaccuracy mismatch. To do so, difference scores were quantified by standardizing PSQI global and actigraphy composite scores, and then subtracting the actigraphy z score from the PSQI global z score (subjective – objective). This mismatch difference score represents an instrument-dependent measure allowing for exploring factors which may contribute to individual differences in self-evaluating sleep quality. Here, a mismatch difference score of 0 indicates perfect agreement between objective and subjective sleep evaluation. Negative values indicate an underestimation of sleep quality, and positive values indicate an overestimation of sleep quality. By this calculation scheme, 41 (46%) participants underestimated their sleep quality, and 48 (54%) overestimated.
Tau burden was not associated with this subjective-objective mismatch score (both tau ROIs, all p > 0.45; Fig. 4A). In contrast, cortical Aβ negatively predicted the degree of sleep perception mismatch (difference score, r = −0.26, p = 0.02; Fig. 4B). That is, individuals with greater cortical Aβ were significantly worse in accurately self-assessing their own sleep quality, indeed perceiving their sleep quality to be worse than objective sleep quality assessment indicated it to be (i.e., perceptual sleep-quality under-estimators). A comparison of these relationships (based on 1000 bootstrap samples of correlation coefficient pairs) showed that the strength of the Aβ correlation was significantly stronger than the meta-ROI tau correlation (Δr = −0.26, p = 0.03).
Figure 4.
Associations between subjective-objective sleep mismatch, tau, and Aβ. A, No association between tau PET and subjective-objective sleep quality mismatch. B, Negative association between Aβ PET and subjective-objective sleep quality mismatch. C, Positive association between tau PET and absolute misestimation of sleep quality. D, Positive association between Aβ PET and absolute misestimation of sleep quality.
To further investigate the hypothesis, we sought to determine whether the accuracy of self-assessment calculated as the absolute value of (PSQI global z score – actigraphy composite z score) was also related to AD pathologic burden. This absolute difference score, composed of only positive values, reflects the inaccuracy of an individual's self-evaluation regardless of the direction of the mismatch. For this quantification of the sleep mismatch score, greater tau burden in both the entorhinal cortex (r = 0.23, p = 0.03) and in the AD-related meta-ROI predicted greater mismatch inaccuracy (r = 0.37, p < 0.001; Fig. 4C). Cortical Aβ similarly predicted a significantly greater inaccuracy mismatch (r = 0.21, p = 0.04; Fig. 4D). In a model that included entorhinal tau, meta-ROI tau, and cortical Aβ, only tau in the meta-ROI remained significant in predicting absolute subjective-objective difference (p = 0.01). The same was true when including age, sex, APOE e4 carrier status, and time interval between sleep assessment and PET imaging as covariates (p = 0.01). These results suggest that individuals with higher levels of AD pathologic burden show worse absolute inaccuracy in self-evaluating their sleep quality.
Associations between subjective-objective sleep mismatch and cognition
AD pathologic burden was associated with the magnitude of subjective-objective mismatch in sleep quality. To better understand potential underlying mechanistic explanations, the final analysis sought to test whether neuropsychological test performance was associated with the subjective-objective mismatch in sleep quality, specifically the cognitive domains of executive function, episodic memory and working memory.
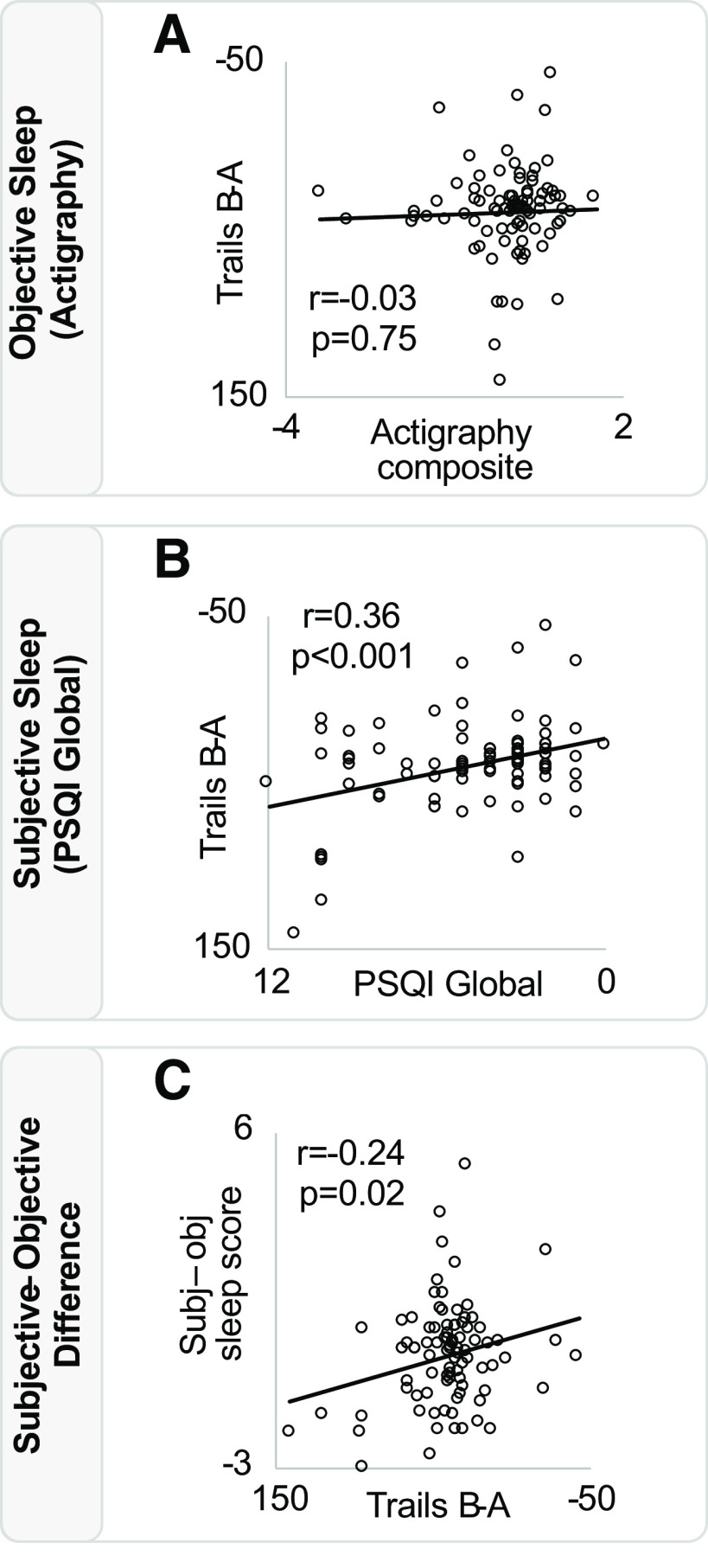
For executive function (Trail Making Test B minus A), worse performance was significantly associated with a greater mismatch in sleep evaluation (subjective-objective) (r = −0.24, p = 0.02; Fig. 5). This association remained significant in a model which included age, sex, APOE e4 carrier status, and time interval between neuropsychological assessment and sleep assessment (p = 0.01). Unlike executive function, episodic memory (verbal memory) and working memory (backward digit span) measures did not predict the subjective-objective mismatch score (p > 0.36), suggesting a relationship specific to executive function and underlying associated networks.
Figure 5.
Associations between sleep and cognition. A, Objective sleep (actigraphy composite score) is not associated with performance on the executive function task Trail Making Test (B minus A). B, Subjective sleep (PSQI global score) is positively associated with performance on the trails task. C, Trails task performance is negatively associated with the difference in subjective and objective sleep quality across individuals. PSQI score is inverted on its axis, reflecting that greater scores indicate worse sleep quality. Trail Making Test (B minus A) is also inverted on its axis, reflecting that higher scores indicate worse executive function.
In addition, a linear regression, which included both cortical Aβ and executive function (Trail Making Test score) (in addition to age, sex, APOE e4 carrier status, and time interval between neuropsychological assessment and sleep assessment), showed that Aβ and executive performance significantly and independently predicted subjective-objective mismatch (Aβ: p = 0.04; Trail Making Test: p = 0.02). When an interaction term for Aβ × Trail Making Test score was added to the model, it was not a significant predictor (p = 0.45), suggesting that the two contribute independently without interaction. The selectivity of the association with an executive performance measure may thus offer one potential candidate mechanism accounting for this sleep perceptual mismatch phenomenon linked to fronto-parietal networks that underlie executive function (Rosen et al., 2010; D'Argembeau et al., 2012), one that occurs in conjunction with, though potentially independent of, the impact of Aβ.
Associations between each of the three cognitive tests with the actigraphy composite, PSQI global score, and tau and Aβ burden are presented for completeness in Table 3.
Table 3.
Correlations between cognitive test performance, sleep quality measures, and PET uptakea
| CVLT Long Delay Free Recall (r) | Digit Span Backwards (r) | Trail Making Test B – A (r) | |
|---|---|---|---|
| Actigraphy composite | −0.02 | 0.09 | −0.03 |
| PSQI global | −0.11 | 0.00 | 0.36*** |
| Entorhinal FTP SUVR | −0.24* | −0.01 | 0.09 |
| Meta-ROI FTP SUVR | −0.25* | 0.13 | −0.08 |
| Cortical PiB DVR | −0.09 | 0.16 | 0.07 |
aColumns represent each cognitive test. Rows represent two-sided bivariate correlations between cognitive test performance and sleep metric or PET uptake (see Materials and Methods). PiB DVR ROIs are comprised of weighted PiB DVR means within FreeSurfer regions as described by Villeneuve et al. (2015). FTP SUVR ROIs are comprised of weighted FTP SUVR means within FreeSurfer regions after PVC, as described by Maass et al. (2017).
*p < 0.05;
**p < 0.01;
***p < 0.001; significant r value.
Discussion
These data collectively demonstrate that the severity of tau burden in both age-related and AD-related anatomic regions of the human brain are associated with an impaired profile of human sleep, measured in a real-world, ecological context. Specifically, these pathologic AD proteins were significantly linked to worse objective sleep quality, and beyond objective measures, that tau as well as the magnitude of cortical Aβ burden each independently predicted worse self-reported sleep quality on average. Moreover, individuals with greater cortical Aβ burden, though not tau burden, expressed a greater inaccuracy perceptual disparity, or mismatch, between their subjective estimate of sleep and their objectively measured sleep, leading to an underestimation of actual sleep quality. Finally, and potentially informative of an underlying neural mechanism, the magnitude of this AD pathology-related inaccuracy was selectively associated with worse executive function, and not episodic memory or working memory.
Greater tau burden, both within the entorhinal cortex and the temporal meta-ROI, was associated with worse objective sleep quality, as estimated by actigraphy. The entorhinal cortex is among the earliest brain regions to accumulate tau pathology (Braak and Braak, 1991), and tau tangles within entorhinal cortex may reflect either age-related aggregation or early AD (Maass et al., 2018). Beyond the entorhinal cortex, however, tau within the meta-ROI encompassing multiple temporal lobe regions is considered more indicative of AD progression, wherein pathologic tau begins to spread outside of the medial temporal lobe (Jack et al., 2017). The findings of an association between impaired sleep and the earliest stage of tau deposition suggest that measures of real-life sleep impairment may reflect a link with preclinical/prodromal AD disease risk. Indeed, tau burden even in cognitively healthy older adults is predictive of future cognitive decline (Sperling et al., 2019). The current association identified between tau and sleep quality therefore suggests that objective, at-home sleep assessment could ultimately serve as one passive, noninvasive tool for detecting early tau accumulation and identifying AD risk (Mander et al., 2016, 2017). Further, given the mechanistic links, some causal, between impaired sleep, cognitive decline, and the accumulation of Aβ and tau (Lim et al., 2013; Diem et al., 2016; McSorley et al., 2019), the treatment of such disrupted sleep may help reduce AD risk or delay disease onset.
Interestingly, the relationships between objective sleep quality were most related to tau, with cortical Aβ showing no association with worse objective sleep quality that reached significance (though Aβ was predictive of the mismatch between objective and subjective sleep, discussed later). In AD, Aβ plaque accumulation throughout neocortex is thought to signify the beginning of a pathologic cascade that, in turn, leads to widespread tau deposition and neurodegeneration (Hardy and Selkoe, 2002; Jagust, 2018). The lack of a relationship between Aβ and the actigraphy composite measure of sleep quality in this cognitively normal cohort suggests that the presence of Aβ in the cortex may not, by itself, disrupt patterns of actigraphy-measured nocturnal sleep behavior in cognitively normal older adults yet to transition into MCI or AD.
Disrupted patterns of sleep-wake behavior in healthy individuals predict greater risk of subsequent dementia (Lim et al., 2013; Diem et al., 2016; Lysen et al., 2020). This is true of other markers of abnormal brain aging, including white matter integrity (Oosterman et al., 2008; Zuurbier et al., 2015; Baillet et al., 2017) and arteriolosclerosis (Lim et al., 2016). Especially notable is that Aβ-positive older adults designated on the basis of CSF measures (i.e., low levels of Aβ peptide 42; CSF Aβ42), have worse sleep efficiency than Aβ-negative individuals (Ju et al., 2013; Ettore et al., 2019). Our data advance such links in two directions. First, we reveal unique associations between several sleep measures (objective, subjective, the mismatch between the two), Aβ in the cortex, and tau in several temporal lobe regions. Second, by treating Aβ as a continuous (rather than categorical) variable, our data explain what may otherwise be seen as a discrepancy between studies. Specifically, low-CSF Aβ42 individuals are likely to have elevated tau deposition (La Joie et al., 2018), meaning that Aβ-positive individuals likely also have substantial temporal lobe tau. Therefore, common across studies (Ju et al., 2013) is that individuals farther along in the progression of AD, determined within the brain or in circulating CSF, experience worse sleep efficiency with no observed difference in sleep duration.
Independent of objective sleep, a greater burden of both tau and cortical Aβ was associated with worse subjective evaluation of sleep quality. These associations remained significant when tau and Aβ were included in the same model, meaning that the relationships between tau and poor sleep, and Aβ and poor sleep, are independent of one another—they each contribute distinctly.
Such a finding therefore supports previous reports of worse subjectively perceived sleep quality in the presence of greater AD pathology, particularly Aβ (Spira et al., 2013; Sprecher et al., 2015, 2017; Branger et al., 2016; Brown et al., 2016; Fjell et al., 2018), but adds to them by establishing that tau, and independent of Aβ, also contributes to worse subjective sleep quality. Despite incongruence between PET associations across modalities (i.e., the lack of an association between Aβ and objective sleep), these AD pathology and subjective sleep associations are themselves additionally relevant. Healthy sleep is multidimensional, and subjective sleep may carry unique information about an individual's well-being relative to objective sleep measures (Buysse, 2014; Watson et al., 2015). Our findings support this concept by demonstrating distinct AD pathologic correlates of tau and Aβ.
A third finding of the current study was that greater Aβ burden was linked to a significantly greater mismatch between participants' subjective evaluation of sleep, relative to their actual objective sleep, resulting in an underestimation of sleep quality. As with our findings, objective and subjective measures of sleep quality are not typically correlated with one another in older adult cohorts (Grandner et al., 2006; Buysse et al., 2008; Landry et al., 2015; Baillet et al., 2016; DiNapoli et al., 2017; Kaplan et al., 2017; Matthews et al., 2018). The current results provide one potentially novel mechanistic explanation account for this sleep disconnect: AD pathologic burden reflects part of a brain mechanism that impairs the ability for accurate self-quantification of nightly sleep quality. As a result, individuals express a subjective underestimation of their actual objectively quality of sleep. While this mismatch measure is dependent on the instruments used, identifying contributors to sleep mismatch may help inform future studies of preclinical and clinical AD so as to measure both objective and subjective sleep assessments, rather than relying on one or the other. If replicated, this finding may serve as added evidence that objective sleep measures are needed when evaluating sleep in older populations, especially those at higher AD risk.
The magnitude of this sleep perceptual mismatch effect was additionally associated with worse executive function, yet was not related to episodic memory function. Executive function is linked to both frontotemporal and frontoparietal networks (Rosen et al., 2010; D'Argembeau et al., 2012), and both are affected early in AD pathology development (Maass et al., 2019). Therefore, degradation of executive cognition, potentially linked to AD pathology or independent of it, may impair functional brain networks necessary for the subjective awareness of one's sleep health, thereby leading to the reported mismatch inaccuracy.
Our current findings must be appreciated within the context of several important limitations. First, the study did not formally evaluate sleep disordered breathing using in-laboratory sleep apnea assessment. Obstructive sleep apnea is an established risk factor for AD (Yaffe et al., 2011; Osorio et al., 2015) and has been linked to higher levels of tau and Aβ in cognitively healthy older adults (Ju et al., 2016; Sharma et al., 2018; Bubu et al., 2019; André et al., 2020; Carvalho et al., 2020). Therefore, the added contribution of apnea (self-reported in 5 participants) in the context of the current results remains unknown. Second, study analyses focused on nocturnal sleep and thus did not collect information on daytime napping. Actigraphy is known to lack specificity in differentiating daytime napping from sedentary behavior (Kanady et al., 2011); thus, only nocturnal actigraphy data were used in this study. Notably, increases in daytime napping have been linked to Aβ burden (Ju et al., 2013; Musiek et al., 2018) and cognitive impairment in older men (Leng et al., 2019). Our findings demonstrate a link between AD pathology and nocturnal sleep quality, but are not able to quantify the potential influence of napping on these relationships. Third, this study did not include electrophysiological measures of sleep microarchitecture that could potentially explain individual differences in self-evaluation of sleep quality. For example, it is not known whether the reduction of slow wave activity in individuals with high amyloid burden (Winer et al., 2020) might contribute to the observed mismatch in perception of sleep quality. Finally, this study did not include measures of depression or anxiety, which are known to contribute to both sleep disturbance (Buysse, 2004) as well as risk of cognitive decline (Byers and Yaffe, 2011) in the context of aging.
The relationship between disrupted sleep and AD is considered to be a bidirectional process (Mander et al., 2016; Wang and Holtzman, 2020). The cross-sectional design of this study means that the data cannot determine the directionality or causality of the reported associations. Longitudinal studies that assess sleep and markers of AD, each at multiple time points, will be needed to distinguish the relative timeline of increasing brain pathology and worsening sleep quality. Sleep and/or pathology intervention studies will further be required for establishing directional causality.
In conclusion, these data support an association between AD pathology and the disruption of real-life sleep patterns in cognitively healthy older adults. Moreover, the presence of tau and Aβ pathology predicted greater inaccuracy in self-assessment of sleep quality. These findings add to a growing understanding of the sleep-wake behavioral correlates of early AD disease processes within the human brain, and offer the possibility that sleep, measured both objectively and subjectively, may serve as both a scalable indicator of AD pathology progression and a possible modifiable target for therapeutic intervention.
Footnotes
M.P.W. serves as an advisor to and has equity interest in Bryte, Shuni, and StimScience. W.J.J. serves as a consultant to Genentech, Biogen, Bioclinica, CuraSen, and Grifols. The remaining authors declare no competing financial interests.
This work was supported by National Institutes of Health F31AG063428 to J.R.W., F32AG057107 to T.M.H., R01AG034570 to W.J.J., and R01AG031164, RF1AG054019, and RF1AG054106 to M.P.W. Avid Radiopharmaceuticals enabled the use of the 18F-flortaucipir radiotracer, but did not provide direct funding and were not involved in data analysis or interpretation. We thank the participants of the Berkeley Aging Cohort Study; and Jared Saletin, Kailin Zhuang, Kaitlin Swinnerton, Victoria Tennant, and Taylor Mellinger for assistance.
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP (2003) The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26:342–392. 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- André C, Rehel S, Kuhn E, Landeau B, Moulinet I, Touron E, Ourry V, Le Du G, Mézenge F, Tomadesso C, de Flores R, Bejanin A, Sherif S, Delcroix N, Manrique A, Abbas A, Marchant NL, Lutz A, Klimecki OM, Collette F, et al. (2020) Association of sleep-disordered breathing with Alzheimer disease biomarkers in community-dwelling older adults: a secondary analysis of a randomized clinical trial. JAMA Neurol 77:716–724. 10.1001/jamaneurol.2020.0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet M, Cosin C, Schweitzer P, Pérès K, Catheline G, Swendsen J, Mayo W (2016) Mood influences the concordance of subjective and objective measures of sleep duration in older adults. Front Aging Neurosci 8:181. 10.3389/fnagi.2016.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet M, Dilharreguy B, Pérès K, Dartigues JF, Mayo W, Catheline G (2017) Activity/rest cycle and disturbances of structural backbone of cerebral networks in aging. Neuroimage 146:814–820. 10.1016/j.neuroimage.2016.09.051 [DOI] [PubMed] [Google Scholar]
- Baker SL, Maass A, Jagust WJ (2017) Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief 15:648–657. 10.1016/j.dib.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, de Flores R, Mutlu J, de La Sayette V, Eustache F, Chételat G, Rauchs G (2016) Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging 41:107–114. 10.1016/j.neurobiolaging.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, Laws SM, Taddei K, Macaulay SL, Ames D, Fowler C, Maruff P, Masters CL, Rowe CC, Martins RN, AIBL Research Group (2016) The relationship between sleep quality and brain amyloid burden. Sleep 39:1063–1068. 10.5665/sleep.5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, Hogan MM, Shim AM, Mukhtar F, Sharma N, Mbah AK, Seixas AA, Kam K, Zizi F, Borenstein AR, Mortimer JA, Kip KE, Morgan D, Rosenzweig I, Ayappa I, et al. (2019) Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep 42:zsz048. 10.1093/sleep/zsz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, Jackson JD, Mormino EC, Rentz DM, Sperling RA, Johnson KA, Amariglio RE (2017) Region-specific association of subjective cognitive decline with tauopathy independent of global β-amyloid burden. JAMA Neurol 74:1455–1463. 10.1001/jamaneurol.2017.2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ (2004) Insomnia, depression and aging: assessing sleep and mood interactions in older adults. Geriatrics 59:47–51; quiz 52. [PubMed] [Google Scholar]
- Buysse DJ (2014) Sleep health: can we define it? Does it matter? Sleep 37:9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, Matthews KA (2008) Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med 4:563–571. 10.5664/jcsm.27351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K (2011) Depression and risk of developing dementia. Nat Rev Neurol 7:323–331. 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DZ, St Louis EK, Schwarz CG, Lowe VJ, Boeve BF, Przybelski SA, Reddy A, Mielke MM, Knopman DS, Petersen RC, Jack CR, Vemuri P (2020) Witnessed apneas are associated with elevated tau-PET levels in cognitively unimpaired elderly. Neurology 94:e1793–e1802. 10.1212/WNL.0000000000009315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E (2012) Valuing one's self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cereb Cortex 22:659–667. 10.1093/cercor/bhr144 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California Verbal Learning Test, Ed 2 (CVLT-II). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Tranah G, Cauley JA, Ancoli-Israel S, Redline S, Spira AP, Hillier TA, Ensrud KE (2016) Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry 24:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNapoli EA, Gebara MA, Kho T, Butters MA, Gildengers AG, Albert SM, Dew MA, Erickson KI, Reynolds CF, Karp JF (2017) Subjective-objective sleep discrepancy in older adults with MCI and subsyndromal depression. J Geriatr Psychiatry Neurol 30:316–323. 10.1177/0891988717731827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettore E, Bakardjian H, Solé M, Levy Nogueira M, Habert MO, Gabelle A, Dubois B, Robert P, David R (2019) Relationships between objectives sleep parameters and brain amyloid load in subjects at risk for Alzheimer's disease: the INSIGHT-preAD Study. Sleep 42:zsz137. 10.1093/sleep/zsz137 [DOI] [PubMed] [Google Scholar]
- Fjell AM, Idland AV, Sala-Llonch R, Watne LO, Borza T, Brækhus A, Lona T, Zetterberg H, Blennow K, Wyller TB, Walhovd KB (2018) Neuroinflammation and tau interact with amyloid in predicting sleep problems in aging independently of atrophy. Cereb Cortex 28:2775–2785. 10.1093/cercor/bhx157 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Yoon IY, Youngstedt SD (2006) Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms 4:129–136. 10.1111/j.1479-8425.2006.00207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297:353–356. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci K, Machulda MM, Mielke MM, Roberts RO, Vemuri P, Reyes DA, Petersen RC (2017) Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement 13:205–216. 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W (2018) Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci 19:687–700. 10.1038/s41583-018-0067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM (2013) Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70:587–593. 10.1001/jamaneurol.2013.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, Crimmins DL, Fagan AM, Holtzman DM (2016) Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol 80:154–159. 10.1002/ana.24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanady JC, Drummond SPA, Mednick SC (2011) Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res 20:214–222. 10.1111/j.1365-2869.2010.00858.x [DOI] [PubMed] [Google Scholar]
- Kaplan KA, Hardas PP, Redline S, Zeitzer JM, Sleep Heart Health Study Research Group (2017) Correlates of sleep quality in midlife and beyond: a machine learning analysis. Sleep Med 34:162–167. 10.1016/j.sleep.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Bejanin A, Fagan AM, Ayakta N, Baker SL, Bourakova V, Boxer AL, Cha J, Karydas A, Jerome G, Maass A, Mensing A, Miller ZA, O'Neil JP, Pham J, Rosen HJ, Tsai R, Visani AV, Miller BL, Jagust WJ, et al. (2018) Associations between [18F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology 90:e282–e290. 10.1212/WNL.0000000000004860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GJ, Best JR, Liu-Ambrose T (2015) Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci 7:166. 10.3389/fnagi.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Redline S, Stone KL, Ancoli-Israel S, Yaffe K (2019) Objective napping, cognitive decline, and risk of cognitive impairment in older men. Alzheimers Dement 15:1039–1047. 10.1016/j.jalz.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA (2013) Sleep fragmentation and the risk of incident Alzheimer's disease and cognitive decline in older persons. Sleep 36:1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Schneider JA, Bennett DA, Buchman AS (2016) Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community-dwelling older people. Stroke 47:516–518. 10.1161/STROKEAHA.115.011608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840. 10.1097/00004647-199609000-00008 [DOI] [PubMed] [Google Scholar]
- Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TL, Holtzman DM (2019) Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med 11:eaau650. 10.1126/scitranslmed.aau6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA (2020) Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement 16:1259–1267. 10.1002/alz.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, Rabinovici GD, Jagust WJ, Alzheimer's Disease Neuroimaging Initiative (2017) Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage 157:448–463. 10.1016/j.neuroimage.2017.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, Baker SL, Rabinovici GD, Jagust WJ (2018) Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci 38:530–543. 10.1523/JNEUROSCI.2028-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Berron D, Harrison TM, Adams JN, La Joie R, Baker S, Mellinger T, Bell RK, Swinnerton K, Inglis B, Rabinovici GD, Düzel E, Jagust WJ (2019) Alzheimer's pathology targets distinct memory networks in the ageing brain. Brain 142:2492–2509. 10.1093/brain/awz154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, Walker MP (2016) Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends Neurosci 39:552–566. 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Walker MP (2017) Sleep and human aging. Neuron 94:19–36. 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Patel SR, Pantesco EJ, Buysse DJ, Kamarck TW, Lee L, Hall MH (2018) Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health 4:96–103. 10.1016/j.sleh.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley VE, Bin YS, Lauderdale DS (2019) Associations of sleep characteristics with cognitive function and decline among older adults. Am J Epidemiol 188:1066–1075. 10.1093/aje/kwz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkes-Caspi N, Yamin HG, Kellner V, Spires-Jones TL, Cohen D, Stern EA (2015) Pathological tau disrupts ongoing network activity. Neuron 85:959–966. 10.1016/j.neuron.2015.01.025 [DOI] [PubMed] [Google Scholar]
- Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YE (2018) Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol 75:582–590. 10.1001/jamaneurol.2017.4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Eser RA, Ehrenberg AJ, Morales D, Petersen C, Kudlacek J, Dunlop SR, Theofilas P, Resende ED, Cosme C, Alho EJL, Spina S, Walsh CM, Miller BL, Seeley WW, Bittencourt JC, Neylan TC, Heinsen H, Grinberg LT (2019) Profound degeneration of wake-promoting neurons in Alzheimer's disease. Alzheimers Dement 15:1253–1263. 10.1016/j.jalz.2019.06.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman J, van Harten B, Vogels R, Gouw A, Weinstein H, Scheltens P, Scherder E (2008) Distortions in rest-activity rhythm in aging relate to white matter hyperintensities. Neurobiol Aging 29:1265–1271. 10.1016/j.neurobiolaging.2007.02.014 [DOI] [PubMed] [Google Scholar]
- Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, Wohlleber ME, Ducca EL, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport DM, de Leon MJ, Weiner MW, Aisen P, Petersen R, Jack C, Jagust W, Morris JC, et al. (2015) Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84:1964–1971. 10.1212/WNL.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Wilcox RR, Rousselet GA (2013) Robust correlation analyses: false positive and power validation using a new Open Source Matlab toolbox. Front Psychol 3:606. 10.3389/fpsyg.2012.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA (2005) Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 25:1528–1547. 10.1038/sj.jcbfm.9600146 [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Tucson, AZ: Neuropsychological Press. [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM (2012) Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med 4:150ra122. 10.1126/scitranslmed.3004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O, Rothlind J, Sturm V, Kramer JH, Weiner M, Miller BL (2010) Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage 49:3358–3364. 10.1016/j.neuroimage.2009.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC (1998) Correction for partial volume effects in PET: principle and validation. J Nucl Med 39:904–911. [PubMed] [Google Scholar]
- Sadeh A, Sharkey M, Carskadon MA (1994) Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 17:201–207. 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ (2016) PET imaging of tau deposition in the aging human brain. Neuron 89:971–982. 10.1016/j.neuron.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, Wohlleber M, Miller MD, Andrade A, Lewis C, Tweardy S, Buj M, Yau PL, Sadda R, Mosconi L, Li Y, Butler T, Glodzik L, Fieremans E, Babb JS, et al. (2018) Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med 197:933–943. 10.1164/rccm.201704-0704OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, Hanseeuw BJ, Buckley R, Chhatwal J, Hedden T, Marshall GA, Quiroz YT, Donovan NJ, Jackson J, Gatchel JR, Rabin JS, Jacobs H, Yang HS, Properzi M, Kirn DR, et al. (2019) The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol 85:181–193. 10.1002/ana.25395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM (2013) Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol 70:1537–1543. 10.1001/jamaneurol.2013.4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM (2015) Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 36:2568–2576. 10.1016/j.neurobiolaging.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Sager MA, Asthana S, Johnson SC, Benca RM, Bendlin BB (2017) Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 89:445–453. 10.1212/WNL.0000000000004171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AL, Naidoo N (2015) Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus 4:25. 10.1186/s40064-014-0777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P, Dunlop S, Heinsen H, Grinberg LT (2015) Turning on the light within: subcortical nuclei of the isodentritic core and their role in Alzheimer's disease pathogenesis. J Alzheimers Dis 46:17–34. 10.3233/JAD-142682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, La Joie R, Arthur-Bentil SK, Vogel JW, Marks SM, Lehmann M, Rosen HJ, Reed B, Olichney J, Boxer AL, Miller BL, Borys E, Jin LW, Huang EJ, Grinberg LT, et al. (2015) Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain 138:2020–2033. 10.1093/brain/awv112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Holtzman DM (2020) Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45:104–120. 10.1038/s41386-019-0478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, Malhotra RK, Martin JL, Patel SR, Quan SF, Tasali E, Consensus Conference Panel (2015) Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: methodology and discussion. Sleep 38:1161–1183. 10.5665/sleep.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997) Wechsler Memory Scale, Ed 3. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Winer JR, Mander BA (2018) Waking up to the importance of sleep in the pathogenesis of Alzheimer disease. JAMA Neurol 75:654–656. 10.1001/jamaneurol.2018.0005 [DOI] [PubMed] [Google Scholar]
- Winer JR, Mander BA, Helfrich RF, Maass A, Harrison TM, Baker SL, Knight RT, Jagust WJ, Walker MP (2019) Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J Neurosci 39:6315–6324. 10.1523/JNEUROSCI.0503-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JR, Mander BA, Kumar S, Reed M, Baker SL, Jagust WJ, Walker MP (2020) Sleep Disturbance Forecasts β-Amyloid Accumulation across Subsequent Years. Curr Biol 30:4291–4298.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8:e68910. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL (2011) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306:613–619. 10.1001/jama.2011.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier LA, Ikram MA, Luik AI, Hofman A, Van Someren EJ, Vernooij MW, Tiemeier H (2015) Cerebral small vessel disease is related to disturbed 24-h activity rhythms: a population-based study. Eur J Neurol 22:1482–1487. 10.1111/ene.12775 [DOI] [PubMed] [Google Scholar]