Abstract
Pain due to osteoarthritis (OA) often occurs during locomotion in the vertical direction when joints are subjected to high mechanical load, e.g. during standing up from a chair or using stairs. To investigate joint pain in OA rat models, dynamic weight-bearing or gait analysis is traditionally conducted during horizontal walking on a flat surface. However, in chronic models of OA, which are of particular translational relevance for the disease, differences in the readouts between OA and control rats are often weak and of high variability leading to an insufficient assay window for drug profiling. To measure pain-related symptoms more sensitively, we conducted a dynamic weight-bearing test in the moment of a strong voluntary mechanical load. For that, we permanently housed rats in a four-story rat colony cage (RCC) and determined hind paw forces during voluntary jumping from one level to the next. This outcome measure was named jump incapacitance. After inducing OA by destabilizing the medial meniscus (DMM), we found that during jumps the average ipsilateral over contralateral hind paw forces were significantly reduced compared with healthy controls (jump incapacitance) from early- (day 7) to late-stage disease (day 90). An intra-articular injection of Zilretta (triamcinolone acetonide extended-release injectable suspension) attenuated OA-induced jump incapacitance after 8 days compared with DMM rats receiving vehicle (p = 0.069). In contrast, a CatWalk test for gait disturbance failed to detect any significant alterations in the chronic course of the DMM model. In conclusion, the dynamic weight-bearing test during jumping represents a novel method to characterize joint pain symptoms even in a slowly progressive OA rat model. It is sensitive, observer independent, relates to clinically relevant endpoints and demonstrates backtranslation of a drug that is approved for the treatment of OA knee pain.
Keywords: Osteoarthritis, Weight bearing, Pain, Behavior test, Destabilization of the medial meniscus (DMM)
Osteoarthritis, Weight bearing, Pain, Behavior test, Destabilization of the medial meniscus (DMM).
1. Introduction
Osteoarthritis (OA) is a leading cause of disability worldwide and was ranked second in recent estimate of global years lived with a disability [1]. The life-time risk of symptomatic knee OA is 40% for men and 47% for women [2]. The majority of OA patients suffer from frequent joint pain symptoms and pain relief is a major goal for novel drugs. In early disease, joint pain most often arises during motion activities (such as walking up or down stairs or standing up from a chair) as joints receive a certain degree of mechanical burden. In clinical trials, pain severity is scored in the context of these activities by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire, the Visual Analog Scale for Pain (VAS), or the Intermittent and Constant Osteoarthritis Pain Index (ICOAP) [3, 4].
Experimentally in rats, OA can be induced by surgical destabilization of the medial meniscus (DMM), causing joint instability with abnormal load on the medial tibial condyle, leading to subchondral bone defects and site-specific cartilage degeneration [5]. In this model, pain-related behavior is not always present despite significant damage to the medial tibial plateau cartilage and synovitis [6]. An objective quantification of pain-related OA symptoms in these models can be achieved by measuring the incapacitance of relative ground reaction forces between the ipsilateral and contralateral hind limbs [7]. During a static incapacitance test, rats are assessed when standing upright after being offered a food reward, presupposing a direct interaction with the experimenter and the necessity of confining the rat to a small chamber [8]. In dynamic incapacitance tests, rats are assessed when moving voluntarily on four legs across a platform [9]. In contrast to the static test, the rat can be investigated independently of the observer. However, apart from spontaneous rearing, the body weight is mostly borne via all four legs and, therefore, the hind limb joints are subject to less mechanical load. Another established and common test, the CatWalk test, investigates gait disturbance since joint pain can result in limping during walking. In this test, temporal and spatial gait parameters are conveyed from paw prints recorded during movement along a walkway [10]. However, there are limitations due to intraindividual variances from spontaneous exploratory behavior and weight distribution across four legs. Finally, other strategies, such as a pressure application measure after directly pinching the knee joint or determining secondary hyperalgesia by using the classical von Frey test, were used to investigate evoked pain [11]. These tests are limited by the lack of observer independence and their poor relation to the clinical situation [12].
To rely on clinical endpoints, such as those assessed during WOMAC, symptomatic investigations should be sensitive, observer independent and mimic relevant motions, including walking along a flat surface, standing up from a chair or using stairs, as closely as possible [3].
In slowly progressing OA models such as DMM, which exhibits many relevant features of structural changes seen in OA patients, the above-mentioned weight-bearing test rarely delivers robust results even with a reasonable number of animals per group. To circumvent this problem, OA symptoms are often characterized in separate, additional models with more transient but stronger pain symptoms by, for example, intra-articular (IA) injection of Mono Iodo Acetate (MIA) or Complete Freund's Adjuvant (CFA) [13]. However, these models exhibit no relevant OA etiology and thereby have a rather limited utility for the characterization of drugs targeting the disease (disease-modifying osteoarthritis drugs [DMOADs]) instead of symptoms only.
To overcome the problem of a pain phenotyping within slowly progressing models, such as after DMM, one strategy could be to exaggerate pain transiently by spontaneous locomotion activities that challenge the moderately diseased joint by transient mechanical burden. Instead of measuring pain during standing, sitting static or vertical walking on a flat surface, as per the CatWalk or dynamic weight-bearing tests, we aimed to determine incapacitance during spontaneous jumping. With regular housing conditions in type IV cages, rats do not have the opportunity to jump. We developed the rat colony cage (RCC) in which up to 48 rats can be housed over 4 levels with different functionalities in one socially connected community [14]. The levels are connected by jump holes or a staircase. In this large and complex habitat with a social environment, rats show a much higher level of spontaneous activity compared with regular type IV cages [15, 16]. While the rats live in the cage for 4 weeks, they learn to frequently jump a vertical distance of 40 cm to the next level, allowing us to determine incapacitance at a moment of very high hind paw ground reaction forces. Here we describe the validation of this novel method, which has been named jump incapacitance, in the DMM model of chronic OA and reference treatment with Zilretta, a slow release formulation of triamcinolone, approved for the treatment of OA knee pain.
2. Methods
2.1. Animals and housing conditions
Ninety-five female Sprague Dawley rats aged 9–10 weeks were purchased from Envigo RMS GmbH (Rossdorf, Germany), and 47/95 were used in the present study (see section 2.2). The mean ± standard deviation body weight was 176 ± 5.5 g. On arrival, rats were directly transferred to the RCC, which is a modified ferret cage (Tecniplast, Cat. No. 4P02B700RAT) with four levels interconnected by jump holes and a staircase (Figure 1A). The cage can home a social group of up to 48 rats and automatically track level changes of each individual rat by radio frequency identification (RF-ID) technology. The temperature of the housing room was 22 °C ± 2 °C, with 45–65% humidity, and a 12/12 day–night rhythm was inverted with red light from 6 am to 6 pm.
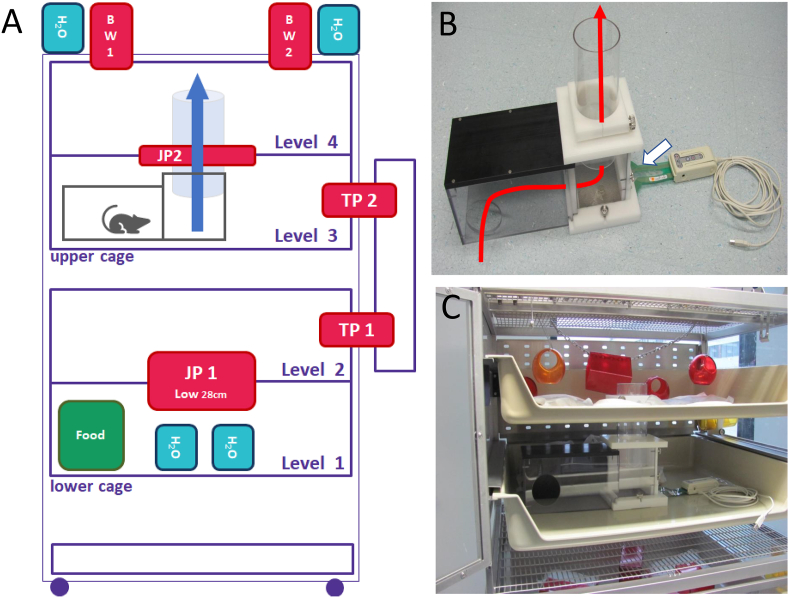
Figure 1.
Rat colony cage (RCC) with installation of the jump incapacitance recording system. (A) Scheme of RCC. Forty-eight female Sprague Dawley rats were housed in RCCs for 4 weeks of habituation and 13 weeks' experiment. (B) Assembled jump incapacitance unit with shelter (left), cover around jump hole (middle) and USB sensor mat (white arrow). The red line indicates the direction the rat is moving to reach the next level. C) Integration of assembled jump incapacitance recording unit into level 3. TP = Tracking point; JP = Jump Hole; BW = Body weighing station.
Rats lived in the RCC for at least 4 weeks before surgery. Technical details and the general effects on rats, such as stress reduction, have been described previously [14]. All procedures were approved by the animal protection authorities of the local district government (Approval ANZ DA4_1009, Regional Authorities of Hessen, Darmstadt, Germany).
2.2. Groups and cage allocation
The 95 rats were allocated to two RCCs (47 and 48 rats, respectively). Fifteen of the 95 rats, seven and eight per RCC, did not receive surgery but had a single IA injection of vehicle (healthy and vehicle group). The remaining 80 rats (40 per cage) underwent DMM surgery. At day 6 after surgery, a CatWalk test was performed and, based on the results, the rats were allocated to five groups of 16 rats (eight per cage) with an almost equal degree of gait disturbance in each group. Here we report the findings from three groups: healthy and vehicle (n = 15); DMM and vehicle (n = 16); and DMM and Zilretta (n = 16). The remaining 48 rats (24/RCC), which were part of the two social communities in the RCCs, received a novel test item, the results of which will be reported elsewhere.
2.3. Surgery
Approximately 30 minutes before surgery, each rat received buprenorphine (0.06 mg/kg subcutaneous [SC]; Temgesic, RB Pharmaceuticals Limited, Berkshire, United Kingdom). During surgery, rats were anesthetized with 1.5–2.5% isoflurane (Baxter, Unterschleissheim, Germany) in 0.5 L/min carbogen (95% oxygen and 5% carbon dioxide). To induce DMM, a skin incision was made from the distal patella proximal to the tibial plateau (of the right joint). The muscle layer was opened at the knee flexion with a scalpel and the medial meniscus tendon, which was ligated with scissors, was exposed. The joint capsule, associated muscles and connective tissue were sutured in layers. For post-surgical analgesia, rats received meloxicam (0.5 mg/kg SC; Metacam injection solution, Boehringer Ingelheim, Biberach, Germany). See Brenneis et al., 2017 for more details on surgery [14].
2.4. Drug application
Seven days following DMM surgery, rats received one IA injection (30 μL) of either 192 μg Zilretta (National Drug Code: 70801-003-01, Flexion Therapeutics, Burlington, MA, USA) or standard suspension vehicle (0.5% Methocel and 0.25% Tween 20 in water).
2.5. Jump incapacitance
2.5.1. Habituation with voluntary training
When the rats arrived from the breeder, where they had been housed in regular small cages (e.g. type IV), they did not easily jump through the jump holes in the RCC, so they needed habituation and training. Following arrival, they were socialized in a group of 48 rats in the RCC. To train the rats to jump, for the first five days a platform was placed under the lower jump hole (jump hole 1) to support first jumps.
2.5.2. Recording system
The jump incapacitance recording system consists of a custom made shelter that directs the rat to the jumping area, a cover that fits the top of the jump hole (both form Hugo Wohnig, Muster-und Modellbau, Niedernhausen, Germany) and the K-Scan™ sensor mat (Map#5101 from Savecomp Megascan GmbH, Hannover, Germany) with 1936 single sensor cells (15.5 cells/cm2) (Figure 1B). The sensor mat was connected to a laptop via a USB device (K-Scan™ Evolution Handle). Prior to data acquisition, the sensor mat was calibrated by three weights (5606.99 g, 25606 g, 45606 g).
2.5.3. Data acquisition
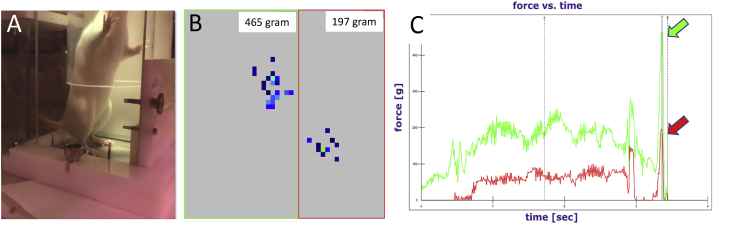
To habituate the rats to the system, the shelter and cover were both installed in the RCC between levels three and four from the beginning of the experiment (Figure 1A, C). For data acquisition, all animals were transferred from the RCC to type IV cages and the sensor mat was installed. Next, single rats were placed into the shelter from which they spontaneously walked below the jump hole, stepped onto the sensor mat and jumped to the upper level (Figure 2A and supplementary video 1). Each animal was introduced to the shelter multiple times until three jumps per animal and time points were recorded. Using the I-Scan™ software (Version 7.60–18I), a single jump was recorded with a scanning speed of 100Hz. Each image gives information on the position, area and pressure of footprints (Figure 2B). The recording was started manually by the observer just before the animal was ready to jump and it was stopped manually when the rat reached the upper level. Data acquisition at all timepoints was carried out with the same sensor mat calibrated before the experiment.
Figure 2.
Recording and analysis of peak forces. (A) Image of a rat on the sensor mat immediately before jumping to the next level. (B) Activated units on sensor mat at a certain time point. Forces are conveyed at both hind paws and shown with the resolution of single detectors (blue squares). The region of interest of the ipsilateral and contralateral paw is allocated manually (see large red and green rectangle). (C) Dynamic forces detected at both hind paws over the past 4 seconds before jumping. Arrows indicate the maximum force directly before the rat is leaving the sensor mat when jumping to the next level. The value of the max. force at the very end of the trace is used for further analysis (% of contralateral).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.heliyon.2021.e07906
The following is the supplementary data related to this article:
Demonstration of jump incapacitance recording and analysis. The video shows an example of a rat performing the jump incapacitance test and the resultant data recording.
2.5.4. Data evaluation
In order to recognize the exact moment of the jump, each recording was analyzed in a force versus time graph. When an animal jumped upwards, the recording ended with a high peak. To connect the peaks to the respective right and left hind paws, peaks were immediately aligned with the rat's actual position on the mat during the jump. Next, the value of the maximal force applied on each hind paw was transferred from I-Scan™ (Version 7.60–18I) software to Excel. Here, the maximum force of the right leg was calculated as a percentage of the left leg.
2.6. Gait analysis
On days 6, 34, 62 and 90 after surgery, the CatWalk test (CatWalk XT 10.0 system, Noldus, Wageningen, Netherlands) was performed to investigate gait characteristics according to time and surface parameters of paw prints (Figure 3A). To adapt rats to the test system, they were trained twice with raspberry syrup placed in the target cage behind the CatWalk walkway. During testing, the paw prints of rats were visualized by boundary surface optics while walking completely voluntarily. Three runs per time point and rat were acquired between 7 am and 3 pm. Only runs with a minimum of three sequential steps without stopping were taken for the analysis. For an estimation of gait disturbance, the relative print length of the ipsilateral hind paw over the contralateral hind paw was calculated and expressed as percent of contralateral. Within the multiple gait parameters calculated by the CatWalk XT system, the print length parameter has been identified to best discriminate (lowest p value) between healthy and surgery-induced OA rats [15].
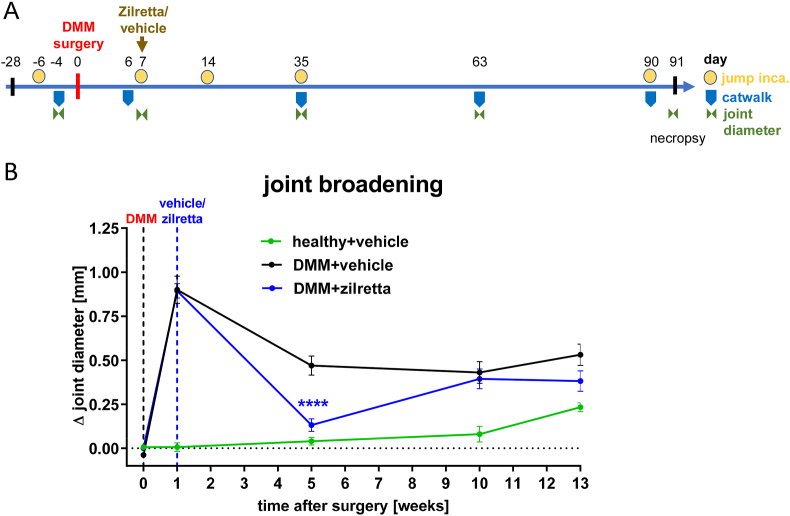
Figure 3.
Chronic rat model of osteoarthritis. (A) Overview of the experimental design: Joint instability has been induced surgically by destabilization of the medial meniscus (DMM). Zilretta or vehicle has been injected intra-articularly (IA) at day 7. Various parameters were measured longitudinally as indicated. (B) Joint broadening over time. Knee joint diameter was determined with caliper. Shown is the SEM of the ipsilateral minus the contralateral joint diameter of n = 15–16 rats/group. ∗∗∗∗p < 0.0001 with two-way ANOVA (mixed model) and Tukey post hoc test.
2.7. Quantification of the joint diameter
Anesthetized (isoflurane 3%–4.5%) rats were placed in a sideway position and the investigated leg was slightly stretched. While the leg was fixed by hand, an electronic caliper (Quick Mini, Mitutoyo, Neuss, Germany) was placed from the ventral to the middle of the joint space (at the widest location between the lateral and medial femoral condyles) and closed. The leg was then released and moved up and down. The joint diameter was read when it was stable (±0.1 mm), and the measurement was repeated once. The joint diameter was determined at both knee joints in weeks 0, 1, 5, 10 and 13 (Figure 3A).
2.8. Statistical analysis
The joint diameter, CatWalk and jump incapacitance data were analyzed by a two-way ANOVA (mixed model for repeated time points) with a Tukey post hoc test using GraphPad Prism (Version 8.2.0).
3. Results
3.1. Joint broadening after OA induction by DMM surgery
At 1-week post-surgery the ipsilateral minus contralateral knee joint diameter had increased by approximately 0.9 mm (Figure 3B). With some decrease over time, this DMM-induced joint broadening remained until week 13 after surgery. Compared with DMM rats who received vehicle, Zilretta-treated rats showed a significantly (p < 0.0001) reduced joint broadening at week 5 after surgery. However, this treatment effect had disappeared at the next time point investigated (week 10).
3.2. Jump performance
After 5 days in the RCC, the platforms that supported first voluntary jumps through the jump holes during the training phase were removed as more rats started to jump without it. Before baseline jump incapacitance recordings were taken at day 21 after arrival in the RCC, all rats had frequently performed voluntary jumps through the jump holes between all levels of the cage. This was confirmed by the RF-ID-based tracking system (data not shown).
3.3. Jump incapacitance before and during DMM-induced OA
Baseline jump incapacitance values were generated after 3 weeks of habituation and 6 days before DMM surgery by calculating the ratio of the maximum forces measured during jumps for the ipsilateral versus the contralateral hind paw (Figure 4A). There were no significant differences of the mean baseline values among the three groups, although the healthy rats showed some trend for putting slightly more weight on the right hind paw throughout the study.
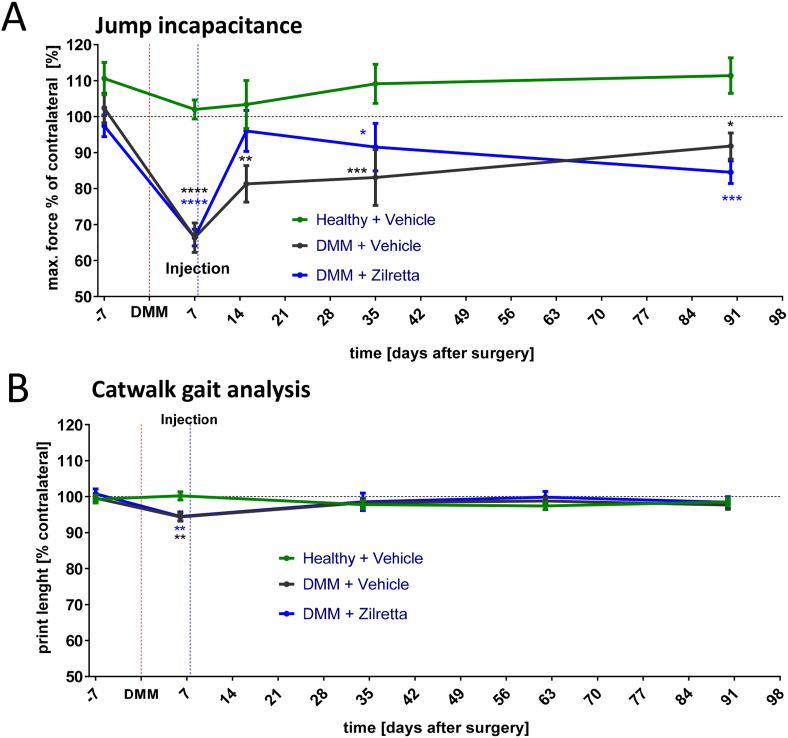
Figure 4.
Comparison of jump incapacitance and CatWalk gait analysis after DMM. (A) Jump incapacitance over time. The % of the ipsilateral over the contralateral hind paw max is shown. Force detected during jumps. Mean ± SEM of n = 15–16 rats. ∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001 with two-way ANOVA (mixed model) and Tukey post hoc test compared with the healthy and vehicle group. (B) CatWalk gait analysis over time. Shown is the % of the ipsilateral over the contralateral hind paw max. Force detected during walking along the walkway. ∗∗p < 0.01 with two-way ANOVA (mixed model) and Tukey post hoc test compared with the healthy and vehicle group.
At day 7 after DMM (measured before IA injection), the mean ± SEM weight-bearing ratio dropped from 102 ± 4.2% to 66 ± 4.1% in the DMM and vehicle group and from 97 ± 3.3% to 66 ± 2.4% in the DMM and Zilretta group. The weight-bearing ratio for both groups on day 7 was significantly different to the healthy control group (p < 0.0001). Importantly, DMM-induced jump incapacitance in the vehicle-treated group remained significantly different from the healthy control group during the chronic OA phase (day 15 = 81 ± 4.9% [p < 0.01], day 35 = 83 ± 7.2% [p < 0.001] and day 90 = 92 ± 3.4% [p < 0.05]).
In rats that received IA Zilretta on day 7, jump incapacitance on day 15 (8 days after injection) was almost normal (96 ± 5.7%) and, in contrast to the DMM and vehicle group, was not statistically different (p = 0.45) to healthy controls (103 ± 6.7%) and almost significantly different the DMM and vehicle group (p = 0.069). At later time points, the weight-bearing ratio of the DMM and Zilretta group declined and became statistically different to healthy controls (day 35 = 91.5 ± 6.6%, p < 0.05; day 90 = 84.5 ± 3.1%, p < 0.001).
3.4. Gait disturbance before and during DMM-induced OA
Baseline values for the CatWalk gait analysis were measured 4 days before DMM surgery by calculating the ratio of ipsilateral over the contralateral paw print length (Figure 4B). Mean values of all three groups were close to 100 without any significant differences. At day 6 after DMM surgery (1 day before IA injection), the mean ± SEM paw print length ratio was 94.4 ± 1.1% in the DMM and vehicle group, and 94.5 ± 1.3% in the DMM and Zilretta group. Both were significantly lower compared with the healthy control group (p < 0.01). However, during weeks 5, 9 and 13, there were no significant differences in ratios between both DMM groups and the healthy control group, i.e. no gait disturbance was detectable.
4. Discussion
We hypothesized that during chronic OA in rats, pain symptoms may be more sensitively monitored when high load onto knee joints is transiently induced. With the novel jump incapacitance outcome measure, we demonstrated a significant difference in weight bearing in the chronic and slowly progressive DMM model compared with healthy controls from an early (day 7) to a very late point (day 63) after surgical induction of joint instability.
During the development of potential DMOADs, preclinical proof of concept needs to be demonstrated, for which rodent models are typically used [17]. Ideally, both structural damage and relevant pain-like symptoms should be measurable in a rodent OA model, in order to support dose selection and dosing regimens for a drug candidate with efficacy in both dimensions, i.e. cartilage structure and pain symptoms. However, it has been shown by us and other groups that the detection of relevant pain-related behavior in chronic models of OA is challenging [13,15]. To achieve a therapeutic window and demonstrate structural improvement, for example with a drug candidate that exhibits an anti-catabolic mode of action, OA disease should be slowly progressing. This is the case for the DMM model. Due to its relatively mild joint instability, less cartilage damage is observed until a much later phase (week 20) in OA progression than the anterior cruciate ligament transection plus medial meniscus transection (ACLT+tMx) or medial meniscal tear (MMT) models [15, 18]. Although it has been shown that DMM induces significant changes in medial tibial plateau cartilage and synovitis [6, 19], it is uncertain whether there are constant periods of measurable pain. Using a dynamic weight-bearing test, no pain-related behavior could be detected, until a very late phase (week 13) [6].
In the present study, a typical joint broadening symptom was observed after DMM surgery that peaked during week 1, persisted until the end of the study, and was sensitive to anti-inflammatory Zilretta treatment. Joint broadening can potentially be a consequence of surgery, synovitis, bone remodeling or another pathology [16, 20]. The effect has been shown to be exaggerated when rats are housed in RCCs compared with type IV cages [15]. Over the complete time course of this model, we determined weight bearing-related gait parameters using the CatWalk test and compared the outcome with the novel jump incapacitance measure obtained in the dynamic weight-bearing test during jumping. During the CatWalk test a significant assay window was present in the early post-surgical phase on day 7; however, no difference to healthy animals could be detected at later time points. This is in accordance with Ferland et al., who reported that after anterior cruciate ligament transection with partial medial meniscectomy (ACLT+pMMx) only a minor and probably insufficient assay window was measurable using the CatWalk test [13]. These results suggest that in certain joint instability models, gait analysis during horizontal walking is probably not sensitive enough and, therefore, does not detect pain behavior at most of the investigated time points. In contrast, gait analysis revealed a robust assay window during the sub-acute OA-pain phase after IA MIA injection (data on file and [21]), indicating that, in general, the CatWalk method is reliable in detecting joint pain, although it appears that a pronounced level of symptomatic severity in the model is needed.
Using the novel jump incapacitance outcome measure in the slowly progressing DMM model, we demonstrated a significant and large assay window to compare healthy and diseased animals from the first time point (day 7) until the last time point (day 63) measured following surgery. Weight bearing is quantified during walking in the CatWalk test, in contrast to the dynamic weight-bearing test in which weight bearing is quantified during jumping. We suggest that this difference is essential for the ability to detect an assay window using jump incapacitance. One further confounding variable in the comparison between the CatWalk test and our jump incapacitance measure is that different units are used for measurement; paw print intensity (pixel number and intensity) is used in the CatWalk test and weight (force) is used in jump incapacitance. Therefore, a direct comparison of the jump incapacitance measure with the dynamic weight-bearing test, which uses weight forces during horizontal movements with weight forces during vertical movements, in the DMM model would further strengthen this hypothesis.
From the perspective of the principles of the 3Rs (Replacement, Reduction and Refinement), the improved assay window can be expected to reduce the group size necessary to detect significant changes with certain power and, therefore, will reduce animal numbers [22]. In addition, the use of RCCs refines animal housing by reducing stress due to social interactions and a more complex, enriched environment, which is closer to the natural habitat of rats.
Traditional methods, measuring transiently evoked pain by, for example, von Frey filaments, also produce a robust assay window during DMM-induced OA [23]. This suggests that, by artificially amplifying the force towards the thresholds of nociceptors, these tests exhibit enough sensitivity to detect chronic OA pain. However, since the fine tuning of pain stimulation depends on the experimenter and also the test subjects (e.g. rats), they can be influenced by the experimenter in a variable manner and a standardized comparison between experiments and labs may be limited [12, 24]. In contrast, automated recording during a spontaneous behavior in jump incapacitate tests enables observer-independent results [12].
Another strength of measuring jump incapacitance is that the investigated behavior relates to clinically relevant movement evoked pain, such as standing up from a chair or using stairs. Both are important real-life situations that were reflected in the WOMAC questionnaire, which is often used in clinical OA trials [25]. The most important analogy is that rat jumping is predominantly a movement in a vertical direction that is exclusively borne by the hind paws. Consequently, more force has to be captured by the joint. In patients, during using stairs and standing up from a chair, to a large extent the movement goes in the vertical direction and this causes pain due to more mechanical pressure on the condyles, which have sensitized nerve endings and low protection due to cartilage erosion. Finally, Zilretta is approved to treat pain in knee OA and here we have demonstrated that there is a clear trend for a therapeutic benefit of Zilretta in the rat DMM model of OA. We have, therefore, demonstrated backtranslation in a preclinical model, which validates the novel jump incapacitance method [26]. The strongest benefit was seen 8 days following injection (day 15 post-surgery). At the next investigated time point, day 28 post-injection or day 35 post-surgery, the benefit was reduced and no significant incapacitance compared with the healthy and vehicle control group was observed. This could be due to a washout of Zilretta from the knee joint leading to very low local exposure at the relevant tissue. Interestingly, at the very late time point of 83 days after injection (day 90 after surgery), there was a trend for a worse outcome in the Zilretta group compared with the DMM and vehicle group. Here, the beneficial anti-inflammatory effect had most likely been washed out completely while disease progression may have been accelerated. To finally confirm that Zilretta worsens disease progression, further histopathological investigations would be necessary. Finally, it could not be excluded that Zilretta had a negative impact on healthy joints. However, since the focus of this study was on transient symptomatic improvement after DMM, an additional group of healthy rats receiving Zilretta could not be justified considering animal welfare.
The described advantages of the jump incapacitance readout may not be restricted to the DMM OA model. All rat models in which pain is induced unilaterally at the hind limb that do not lead to an avoidance of voluntary jumping by any functional impairment or severe pain are of relevance, including joint disease models (e.g. ACLT+tMx, MMT, IA MIA or collagen-induced arthritis), inflammatory pain models (e.g. intraplantar CFA, Zymosan or Carrageenan), and even mild neuropathic pain models with low motoric deficits (such as the chronic constrictive injury model), all of which exhibit pain at one hind paw.
5. Conclusion
In summary, the jump incapacitance test represents a novel behavioral assessment tool to assess pain symptoms in various rat models with unilateral pain in the hind extremities. It is sensitive, observer independent, relates to clinically relevant endpoints and was validated using a drug that is approved for the treatment of OA knee pain.
Declarations
Author contribution statement
Andreas Westhof: Performed the experiments; Analyzed and interpreted the data.
Kerstin Kleinschmidt-Doerr: Conceived and designed the experiments.
Martin Michaelis: Analyzed and interpreted the data; Wrote the paper.
Christian Brenneis: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This study was sponsored by Merck Healthcare KGaA, Darmstadt, Germany (CrossRef Funder ID:10.13039/100009945).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors Christian Brenneis, Andreas Westhof and Martin Michaelis are employees of Merck Healthcare KGaA, Darmstadt, Germany. Kerstin Kleinschmidt-Doerr is an employee of Merck KGaA, Darmstadt, Germany.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Murphy L., Schwartz T.A., Helmick C.G., Renner J.B., Tudor G., Koch G., Dragomir A., Kalsbeek W.D., Luta G., Jordan J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy N. The WOMAC knee and hip osteoarthritis indices: development, validation, globalization and influence on the development of the AUSCAN hand osteoarthritis indices. Clin. Exp. Rheumatol. 2005;23:S148–153. [PubMed] [Google Scholar]
- 4.Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain: visual analog Scale for pain (VAS pain), numeric rating Scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade Scale (CPGS), short form-36 bodily pain Scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res. 2011;63(Suppl 11):S240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 5.Iijima H., Aoyama T., Ito A., Tajino J., Nagai M., Zhang X., Yamaguchi S., Akiyama H., Kuroki H. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22:1036–1043. doi: 10.1016/j.joca.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Gowler P.R.W., Mapp P.I., Burston J.J., Shahtaheri M., Walsh D.A., Chapman V. Refining surgical models of osteoarthritis in mice and rats alters pain phenotype but not joint pathology. PloS One. 2020;15 doi: 10.1371/journal.pone.0239663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piel M.J., Kroin J.S., van Wijnen A.J., Kc R., Im H.J. Pain assessment in animal models of osteoarthritis. Gene. 2014;537:184–188. doi: 10.1016/j.gene.2013.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H.T., Uchimoto K., Duellman T., Yang J. Automated assessment of pain in rats using a voluntarily accessed static weight-bearing test. Physiol. Behav. 2015;151:139–146. doi: 10.1016/j.physbeh.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quadros A.U., Pinto L.G., Fonseca M.M., Kusuda R., Cunha F.Q., Cunha T.M. Dynamic weight bearing is an efficient and predictable method for evaluation of arthritic nociception and its pathophysiological mechanisms in mice. Sci. Rep. 2015;5:14648. doi: 10.1038/srep14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira-Gomes J., Adaes S., Castro-Lopes J.M. Assessment of movement-evoked pain in osteoarthritis by the knee-bend and CatWalk tests: a clinically relevant study. J. Pain. 2008;9:945–954. doi: 10.1016/j.jpain.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Leuchtweis J., Imhof A.K., Montechiaro F., Schaible H.G., Boettger M.K. Validation of the digital pressure application measurement (PAM) device for detection of primary mechanical hyperalgesia in rat and mouse antigen-induced knee joint arthritis. Methods Find. Exp. Clin. Pharmacol. 2010;32:575–583. doi: 10.1358/mf.2010.32.8.1532102. [DOI] [PubMed] [Google Scholar]
- 12.Cobos E.J., Portillo-Salido E. Bedside-to-Bench" behavioral outcomes in animal models of pain: beyond the evaluation of reflexes. Curr. Neuropharmacol. 2013;11:560–591. doi: 10.2174/1570159X113119990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferland C.E., Laverty S., Beaudry F., Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol. Biochem. Behav. 2011;97:603–610. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Brenneis C., Westhof A., Holschbach J., Michaelis M., Guehring H., Kleinschmidt-Doerr K. Automated tracking of motion and body weight for objective monitoring of rats in colony housing. J. Am. Assoc. Lab Anim. Sci. 2017;56:18–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Brenneis C., Menges S., Westhof A., Lindemann S., Thudium C.S., Kleinschmidt-Doerr K. Colony housing promotes structural and functional changes during surgically induced osteoarthritis in rats. Osteoarthritis Cartilage Open. 2020;2:100100. doi: 10.1016/j.ocarto.2020.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Y.M., Menges S., Westhof A., Kleinschmidt-Doerr K., Brenneis C., Pitsillides A.A. Systematic analysis reveals that colony housing aligns gait profiles and strengthens link between histological and micro-CT bone markers in rat models of osteoarthritis. Faseb. J. 2021;35 doi: 10.1096/fj.202002009R. [DOI] [PubMed] [Google Scholar]
- 17.Malfait A.M., Little C.B. On the predictive utility of animal models of osteoarthritis. Arthritis Res. Ther. 2015;17:225. doi: 10.1186/s13075-015-0747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller R.E., Malfait A.M. Osteoarthritis pain: what are we learning from animal models? Best Pract. Res. Clin. Rheumatol. 2017;31:676–687. doi: 10.1016/j.berh.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iijima H., Aoyama T., Ito A., Yamaguchi S., Nagai M., Tajino J., Zhang X., Kuroki H. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthritis Cartilage. 2015;23:1563–1574. doi: 10.1016/j.joca.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Gigout A., Harazin D., Topping L.M., Merciris D., Lindemann S., Brenneis C., Nissim A. Early detection of osteoarthritis in the rat with an antibody specific to type II collagen modified by reactive oxygen species. Arthritis Res. Ther. 2021;23:113. doi: 10.1186/s13075-021-02502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs B.Y., Dunnigan K., Pires-Fernandes M., Allen K.D. Unique spatiotemporal and dynamic gait compensations in the rat monoiodoacetate injection and medial meniscus transection models of knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:750–758. doi: 10.1016/j.joca.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusche B. The 3Rs and animal welfare - conflict or the way forward? ALTEX. 2003;20:63–76. [PubMed] [Google Scholar]
- 23.Miller R.E., Belmadani A., Ishihara S., Tran P.B., Ren D., Miller R.J., Malfait A.M. Damage-associated molecular patterns generated in osteoarthritis directly excite murine nociceptive neurons through Toll-like receptor 4. Arthritis Rheum. 2015;67:2933–2943. doi: 10.1002/art.39291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesler E.J., Wilson S.G., Lariviere W.R., Rodriguez-Zas S.L., Mogil J.S. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci. Biobehav. Rev. 2002;26:907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 25.Goggins J., Baker K., Felson D. What WOMAC pain score should make a patient eligible for a trial in knee osteoarthritis? J. Rheumatol. 2005;32:540–542. [PubMed] [Google Scholar]
- 26.Paik J., Duggan S.T., Keam S.J. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79:455–462. doi: 10.1007/s40265-019-01083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of jump incapacitance recording and analysis. The video shows an example of a rat performing the jump incapacitance test and the resultant data recording.
Data Availability Statement
Data will be made available on request.