Abstract
Transformation of pre-B cells by Abelson murine leukemia virus (Ab-MLV) involves a balance between positive, growth-stimulatory signals from the v-Abl oncoprotein and negative regulatory cues from cellular genes. This phenomenon is reflected by the clonal selection that occurs during Ab-MLV-mediated transformation in vivo and in vitro. About 50% of all Ab-MLV-transformed pre-B cells express mutant forms of p53 as they emerge from this process, suggesting that this protein may play an important role in the transformation process. Consistent with this idea, expression of p19Arf, a protein whose function depends on the presence of a functional p53, is required for the apoptotic crisis that characterizes primary Ab-MLV transformants. To test the role of p53 in pre-B-cell transformation directly, we examined the response of Trp53−/− mice to Ab-MLV. The absence of p53 shortens the latency of Abelson disease induction but does not affect the frequency of cells susceptible to Ab-MLV-induced transformation. However, primary transformants derived from the null animals bypass the apoptotic crisis that characterizes the transition from primary transformant to fully malignant cell line. These effects do not require p21Cip-1, a major downstream target of p53; however, consistent with a role of p19Arf, transformants expressing mutant p53 and abundant p19 retain wild-type p19 sequences.
Abelson murine leukemia virus (Ab-MLV) is a highly oncogenic transforming retrovirus. The activity of the single protein product of the virus, the v-Abl protein tyrosine kinase, is required to transform cells in vitro and to induce pre-B-cell lymphomas in vivo (34, 50). Despite the presence of a dominantly acting oncogene, Ab-MLV-induced transformation is a multistep process. Even though tumors arise rapidly in vivo, an early polyclonal phase is followed by a later mono- or oligoclonal phase (14, 15). In vitro, primary lymphoid transformants undergo a period of crisis in which many cells die before permanently established, highly malignant cell lines arise (30, 35, 47, 51, 52). Because changes in expression or activity of the v-Abl protein do not occur during this phase (52), the process appears to be mediated by changes in cellular genes that impact the transformation process.
One cellular gene involved in Ab-MLV transformation is Trp53, which encodes the p53 tumor suppressor protein; about 50% of all Ab-MLV-transformed pre-B cells express mutant forms of this molecule (30, 47). p53 responds to cellular insults, including oncogenic stimuli (20, 27, 41). Activation of p53 can induce G1 arrest by inducing the p21Cip-1 cyclin-dependent kinase inhibitor and other target molecules (5, 8, 10, 11, 16, 55). The protein can also mediate apoptosis, probably by stimulating certain p53-responsive genes and suppressing others (1, 13, 22, 36, 37, 40). All of these responses appear to be important for the tumor suppressor functions of p53 (20, 27, 41).
Activation of p53 following oncogenic stimuli, including those from Myc and E1A, requires a functional p19Arf protein (9, 29, 56), as does the ability of p53 to mediate senescence and block immortalization of mouse embryo fibroblasts (MEF) (18, 56). Abundant expression of p19Arf often precedes the emergence of Trp53 mutations in Ab-MLV-transformed pre-B cells, and primary transformed pre-B cells derived from Ink4a/Arf−/− mice do not undergo crisis in vitro (30), suggesting that oncogenic signals from v-Abl activate the same pathway during transformation of pre-B cells. Consistent with this idea, upregulation of p19Arf occurs in response to signals from c-Myc (56), an obligate downstream target of v-Abl (38, 54, 57). Thus, the outcome of Ab-MLV infection may reflect the balance achieved by opposing positive growth-stimulatory signals and negative apoptotic signals.
The p19Arf-p53 regulatory loop provides an attractive mechanism by which the crisis characteristic of Ab-MLV-mediated lymphoid transformation may be induced. This model predicts that p53 is a critical element. To test this hypothesis, we examined the response of pre-B cells from Trp53−/− mice to Ab-MLV. Our data reveal that the absence of p53 shortens the latent period for induction of Ab-MLV-mediated lymphoma but does not affect the frequency of primary Ab-MLV-induced transformation. However, p53 is required for the crisis that characterizes the pre-B-cell transformation process. Consistent with the predicted role of p19Arf in this process, cells expressing mutant forms of p53 and abundant levels of p19Arf retain the wild-type p19Arf sequence. These data demonstrate that p53 is an important downstream element mediating the cellular response to oncogenic signals from the v-Abl protein and reinforce the role of p19Arf in the process.
MATERIALS AND METHODS
Cells and mice.
Pre-B-cell lines were routinely maintained in supplemented RPMI 1640 medium (50 μM 2-mercaptoethanol, 2 mM l-glutamine, 50 μg of streptomycin per ml, and 50 U of penicillin per ml) containing 10% heat-inactivated fetal calf serum (Sigma) at 37°C in a 6% humidified CO2 atmosphere. Bone marrow transformation assays were performed as described previously by using the Ab-MLV-P160 strain (12, 35). To derive cell lines, primary transformants were removed from agar at 10 days postinfection and plated in 24-well plates in supplemented RPMI 1640 medium containing 20% fetal calf serum. The cultures were monitored daily for cell density and viability. When the cells filled the well, half of the cells were transferred to a new well. This process was continued until the cells grew to confluence and were >85% viable; then, the cells were transferred to a 35-mm dish and subcultured as before. When the cultures were consistently greater than 90% viable and could be routinely subcultured, the cells were considered established. Trp53−/− mice were derived from a BALB/cJ Trp53+/− breeding pair that had been backcrossed to BALB/cJ five times and inbred for three generations (Jackson Laboratories). The mice were bred by brother-sister mating at the Tufts University School of Medicine animal facility. Genotypes were assessed by PCR amplification of DNA prepared from tail fragments and a combination of primers specific for Trp53 and the neomycin resistance gene present in the targeted allele, as recommended by the supplier. Animals were injected intravenously via the tail vein with Ab-MLV-P160 and monitored for disease induction; animals were sacrificed when evidence of tumors, such as hind limb paralysis, lymphadenopathy, splenomegaly, cachexia, or general ill health, was noted. Animals were examined for the characteristic features of Abelson disease, including tumors affecting the lower spinal column and lymph nodes and sparing the thymus. The (p21Cip-1)−/− mice were of a mixed background (8).
Apoptosis analyses.
For merocyanin 540 (MC540) staining of apoptotic cells (31), cells were collected and washed twice in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) (Sigma). A 1-mg/ml stock of MC540 in 50% ethanol (Molecular Probes) was added to a final concentration of 0.05 μg/ml in PBS–0.1% BSA, and the cells were analyzed immediately by flow cytometry with a FACScan instrument. Apoptotic cells are stained specifically with MC540 (31). To analyze the integrity of DNA in dying cells, the cells were washed in PBS and resuspended in DNA lysis buffer (100 mM Tris [pH 8], 20 mM EDTA, 0.8% sodium lauryl sarcosinate), and 3.3 mg of RNase per ml was added (21). The mixture was incubated for 30 min at 37°C, proteinase K was added to a final concentration of 5 mg/ml, and the samples were incubated for an additional 2 h at 50°C. The samples were extracted with phenol-chloroform, precipitated with ethanol, and analyzed by electrophoresis through 2% agarose gels containing ethidium bromide.
Nucleic acid preparation and sequence analysis.
RNA was prepared from Ab-MLV-transformed cells by using an RNeasy kit (Qiagen) according to the manufacturer’s instructions. To prepare cDNA, 10 μg of total RNA was mixed with 1 μM primer, heated to 70°C for 10 min, and chilled on ice for 5 min. Then, 40 U of RNasin, 5 mM dithiothreitol, and 25 mM deoxynucleoside triphosphate (dNTP) mix were added and the mixture was heated at 42°C for 5 min. Synthesis was conducted for 1 h at 42°C with Moloney murine leukemia virus reverse transcriptase (GIBCO-BRL). The reaction was stopped by incubating the samples at 75°C for 10 min. The cDNA was then amplified by PCR with 250 μM dNTP mix (Pharmacia), 1× PCR buffer (Perkin-Elmer Cetus), 1 μM (each) primer, and 2.5 U of Taq polymerase (Perkin-Elmer Cetus). The samples were incubated in a programmable Thermal Controller (MJ Research) for 34 cycles of 94°C for 1 min, 57°C for 2 min, and 72°C for 2 min, followed by a 5-min incubation at 72°C. Control reaction mixtures lacking DNA or from reverse transcription reactions carried out in the absence of RNA did not give rise to specific products. Reverse transcription was primed with the Ink/Arf common antisense primer 5′-GCAAAGCTTGAGGCCGGATTTAGCTCTGCTC-3′ (29). To amplify p16Ink4a cDNA, this primer was used in combination with the exon 1α primer 5′-CGGGATCCGCTGCAGACAGACTGGCCAG-3′ (29); p19Arf cDNA was amplified with the common primer and the exon 1β primer 5′-CGCCGCTGAGGGAGTAC-3′. Ink/Arf locus sequences were amplified from BALB/cByJ kidney DNA. Exon 1β sequences were amplified with 5′-GTCCAGGATTCCGGTGC-3′ and the exon 1β primer used for cDNA synthesis; exon 2 sequences were amplified with 5′-ACATAGGGCTTCTTTCTTGGGTCC-3′ and 5′-GGACCAACTATGCTCACCTGGGC-3′. Each PCR mixture contained 100 to 200 ng of DNA, 125 μM dNTP mix (Pharmacia), 1× PCR buffer (Perkin-Elmer Cetus), 0.5 μM (each) primer, and 1 U of Taq polymerase (Perkin-Elmer Cetus). The samples were incubated in a programmable Thermal Controller for 30 cycles of 94°C for 1 min, 55°C for 1.5 min, and 72°C for 1.5 min, followed by a 10-min incubation at 72°C. All amplified products were cloned into the TA cloning vector (Invitrogen) and sequenced on an ABI373-stretch machine (Perkin-Elmer) at the DNA Facility, Department of Physiology, Tufts University School of Medicine.
Protein analysis.
For Western analysis, cells were washed in PBS and cell pellets were lysed in a solution of 10 mM Tris (pH 7.4), 1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate (25). The lysates were heated at 95°C for 5 min and sheared through a 25-gauge needle. The amount of protein in each lysate was quantified by using a bicinchoninic acid protein assay kit (Pierce), and 50 μg of each sample was fractionated through an SDS-polyacrylamide gel. Proteins were electrotransferred to polyvinylidene difluoride membranes (Millipore), which were probed with anti-p19Arf (26) or anti-Gag/v-Abl (H548) (7) antibodies. The blots were developed with a chemiluminescence kit (Tropix), according to the manufacturer’s instructions.
RESULTS
Absence of Trp53 accelerates Abelson disease in vivo.
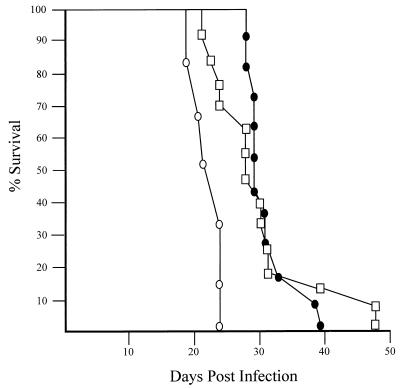
Mutation of Trp53 is a frequent occurrence in Ab-MLV-transformed pre-B-cell lines (47). To determine if the presence of a functional p53 affected the induction of Abelson disease, 5- to 8-week-old Trp53+/+, Trp53+/−, or Trp53−/− mice were injected with Ab-MLV and monitored for the development of tumors (Fig. 1). As expected for adult mice on the highly Ab-MLV-susceptible BALB/c background (33), all of the injected animals developed tumors. Irrespective of p53 status, the diseased animals displayed pathological features characteristic of typical Abelson disease, including hind limb paralysis, enlarged peripheral lymph nodes, and moderate splenomegaly with sparing of the thymus (24, 33). However, all of the null animals developed tumors more rapidly, with a mean latency of 23 days, than did heterozygous or wild-type animals, which displayed mean latencies of 30 and 31 days, respectively. An even more dramatic shift in latency has been observed by Zou et al., who have analyzed p53 null, heterozygous, and wild-type mice on a mixed 129 background (5a). Thus, the lack of p53 accelerates the development of Ab-MLV-induced tumors in vivo.
FIG. 1.
Accelerated tumor induction in Trp53−/− mice. Age-matched Trp53−/− (○), Trp53+/− (□), and Trp53+/+ (●) mice were injected with Ab-MLV-P160 and monitored for tumor development. Animals were sacrificed when tumors were evident; each point represents a single mouse.
Absence of p53 does not affect pre-B-cell transformation frequency.
To determine whether the initiation of Ab-MLV-mediated transformation is affected by p53 status, bone marrow cells from Trp53+/+, Trp53+/−, or Trp53−/− mice were infected with Ab-MLV, plated in soft agar, and monitored for transformation (35). All samples gave rise to morphologically typical transformed pre-B-cell colonies. The colonies obtained from Trp53−/− bone marrow were slightly larger than those found in other samples. Cell counts performed on 10 of these colonies revealed that they contained between 5 × 105 and 1.8 × 106 cells, with an average of 1.1 × 106 cells/colony; 10 colonies from Trp53+/+ mice contained between 2 × 105 and 1.1 × 106 cells and averaged 6 × 105 cells/colony. The frequency of primary transformants was slightly higher in the samples from Trp53−/− and Trp53+/− animals in most experiments (Table 1). However, differences in transformation frequencies of two- to threefold are common when individual mice from inbred strains are examined (our unpublished data), suggesting that these differences may not reflect the Trp53 status of the mice. Thus, the initial transformation frequency by Ab-MLV was not markedly affected by the Trp53 gene. This is consistent with the observation that Trp53 mutations arise late in the transformation process in the Ab-MLV system (30, 47) and in other types of tumors, including the BCR/ABL-induced chronic myelogenous leukemia (2, 20, 28, 46).
TABLE 1.
Absence of p53 does not affect transformation frequency
| Genotype | No. of colonies/106 nucleated bone marrow cells ± SEMa
|
|||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | |
| Trp53+/+ | 37 ± 5 | 21 ± 4 | 38 ± 4 | 57 ± 5 |
| Trp53+/− | 46 ± 5 | 42 ± 4 | 59 ± 6 | NT |
| Trp53−/− | 49 ± 8 | 40 ± 5 | 96 ± 8 | 70 ± 5 |
Bone marrows from age-matched Trp53+/+, Trp53+/−, and Trp53−/− mice were infected with Ab-MLV-P160 and plated in soft agar; macroscopically visible primary transformed pre-B-cell colonies were scored 10 days later. NT, not tested.
p53 influences the establishment of primary pre-B-cell transformants.
Only a fraction of primary pre-B-cell transformants from normal mice become established cell lines (30, 47, 52). To determine if p53 affects this parameter, primary transformants from Trp53+/+, Trp53+/−, and Trp53−/− mice were removed from agar and cultured in liquid medium. About 10% of the transformants from Trp53+/+ mice became established after an average of 29 days in culture; none were established earlier than 16 days postexplant (Table 2). However, all 87 of the primary transformants isolated from Trp53−/− mice gave rise to established cell lines 4 to 6 days after explant from agar. Interestingly, primary transformants from Trp53+/− mice displayed an intermediate phenotype, with 50% or more of these becoming fully established in about 2 weeks (Table 2).
TABLE 2.
p53 influences the establishment of primary transformantsa
| Genotype | No. of transformants established/no. explanted (%) | Time to establishment (days)
|
|
|---|---|---|---|
| Range | Avg | ||
| Trp53+/+ | |||
| Expt 1 | 0/24 (<1) | ||
| Expt 2 | 4/46 (9) | 16–42 | 29 |
| Expt 3 | 4/40 (10) | 24–47 | 36 |
| Trp53+/− | |||
| Expt 1 | 12/24 (50) | 5–14 | 10 |
| Expt 2 | 16/24 (67) | 12–18 | 15 |
| Expt 3 | 15/20 (75) | 6–16 | 11 |
| Trp53−/− | |||
| Expt 1 | 24/24 (100) | 4–5 | 5 |
| Expt 2 | 39/39 (100) | 4–5 | 5 |
| Expt 3 | 24/24 (100) | 4–6 | 5 |
Primary transformants from three different genotypes were explanted from agar and cultured in liquid medium, as described in Materials and Methods. Primary transformants were considered to be established when they maintained viability of >90% and could be subcultured on a routine basis.
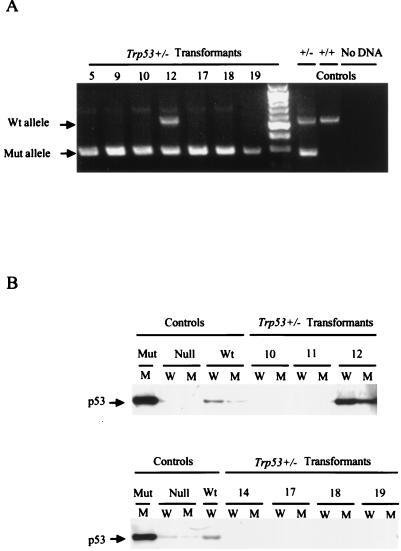
Accelerated loss of the single wild-type p53 allele is a common feature of tumors that arise in Trp53+/− mice (3, 4, 17). To determine if similar events might account for the high frequency with which the Trp53+/− cell lines became established, the presence of wild-type and targeted alleles in the cell lines was assessed. PCR analysis revealed that six of seven cell lines examined had lost the wild-type copy of Trp53 (Fig. 2A). Consistent with these data, immunoprecipitation and Western analysis revealed that p53 could not be detected in cell lines that lacked a functional Trp53 allele (Fig. 2B). Interestingly, sample 12, from the only cell line that retained a copy of the wild-type gene, expressed a p53 protein that reacted with antibodies specific for both wild-type and mutant forms of p53 (Fig. 2B). Based on analyses of other cell lines (47), this pattern likely reflects the emergence of cells expressing a mutant form of p53 in this population. These analyses and the high frequency with which primary transformants from Trp53−/− mice become established demonstrate that p53 expression has a major effect on the ability of primary transformants to evolve into established cell lines.
FIG. 2.
Trp53+/− transformants lose their remaining Trp53 allele rapidly. (A) DNAs from representative Trp53+/− transformants were amplified with primers specific for the wild-type and targeted alleles, and products were fractionated through an agarose gel containing ethidium bromide. The numbers above each lane identify the cell clone from which the sample was derived. DNAs from Trp53+/− and Trp53+/+ mice and a reaction mix containing no DNA were used as controls. The unmarked lane contains a 100-bp DNA ladder, used as a marker. Arrows denote positions of the wild-type (Wt) and mutant (Mut) specific PCR products. (B) Lysates from the cell lines analyzed in panel A and control cell lines were immunoprecipitated with anti-p53 antibody Ab-4, specific for wild-type p53 (lanes W), or anti-p53 antibody Ab-3, specific for mutant forms of p53, (lanes M) and the immunoprecipitates were analyzed by Western blotting with anti-p53 antibody Ab-7, which recognizes both mutant and wild-type p53 forms on Western blots. The p53 statuses of the control wild-type (204-3-1), mutant (143-2M), and null (L1-2) cell lines were characterized previously (47).
p53 is required for induction of the apoptotic crisis.
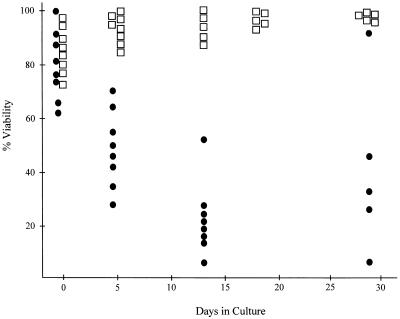
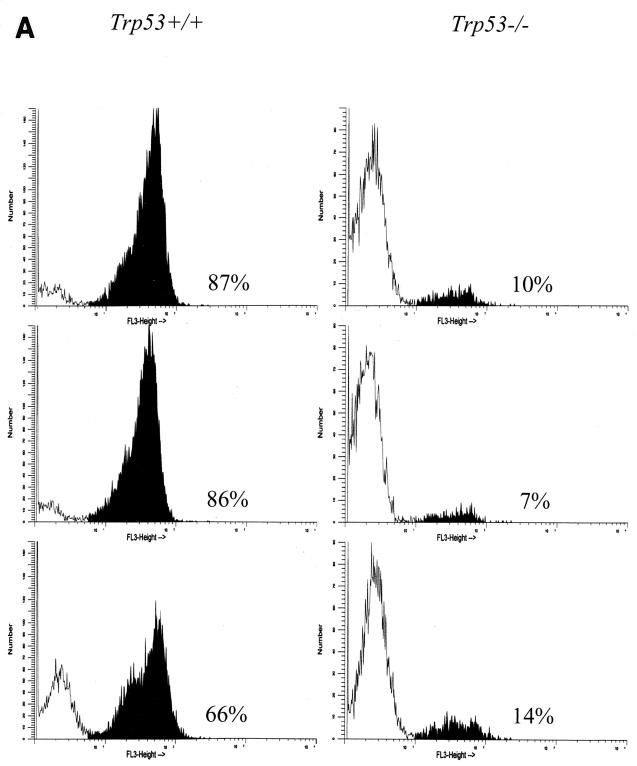
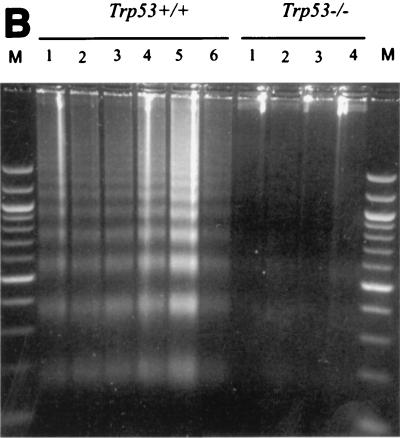
Primary Ab-MLV-transformed pre-B cells undergo a crisis marked by apoptotic cell death several days after they are explanted from agar (30). To determine if Trp53 plays a role in crisis induction, the health and viability of primary transformants derived from Trp53−/− and Trp53+/+ mice were analyzed. As expected, based on analysis of primary transformants from many strains of mice, the Trp53+/+ transformants were highly viable during the first few days following explant from agar (Fig. 3). However, the viability of most clones decreased by day 5 and often continued to decrease over the next 3 to 4 weeks; a high frequency of these cells underwent apoptosis, as judged by staining with MC540 (31) and the presence of the DNA ladder pattern characteristic of programmed cell death (Fig. 4). In contrast to this pattern, the Trp53−/− transformants displayed viabilities in excess of 85% throughout the experiment (Fig. 3), and levels of apoptosis were very low (Fig. 4). These data demonstrate that expression of a functional p53 is required for induction of the crisis that characterizes the transformation process.
FIG. 3.
Trp53 is required for crisis induction. Primary transformants from Trp53+/+ (●) and Trp53−/− (□) mice were assessed for viability by using trypan blue staining when they were removed from agar cultures and at regular intervals thereafter.
FIG. 4.
Trp53+/+ transformants undergo apoptotic crisis during outgrowth. (A) Three independent Trp53+/+ and Trp53−/− transformants were stained with MC540 (31) and analyzed by flow cytometry. The percentages of apoptotic cells, represented by black peaks, are noted. The data shown are representative of analyses of more than 10 additional independent transformants from each background. (B) DNA was prepared as described in Materials and Methods from representative Trp53+/+ and Trp53−/− transformants and fractionated through an agarose gel containing ethidium bromide. The data shown are representative of analyses of 10 independent transformants from each background. Lane M, 100-bp ladder marker.
p53-dependent crisis occurs in the absence of p21Cip-1.
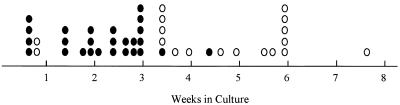
One important mediator on the p53 pathway is the cyclin-dependent kinase inhibitor p21Cip-1 (10, 11, 16, 55). To determine if p21Cip-1 plays a role in p53-mediated crisis, primary transformants were prepared from the bone marrow of p21Cip-1−/− mice. These cells, similar to those from virtually all strains of mice, were highly viable when explanted from agar. However, like cells from wild-type mice, all of the primary transformants underwent an apoptotic crisis, as judged by MC540 staining and DNA laddering analysis (data not shown), beginning about 5 days after explant and lasting for 30 to 35 days. Many primary transformants succumbed to crisis during this period, and 39% (18 of 46) became established (Fig. 5). Although the frequency with which primary transformants from these mice became established was somewhat higher than that observed for Trp53+/+ mice, these animals are on different genetic backgrounds, making direct comparisons problematic. The frequencies with which primary transformants become established varies among strains; analyses of cells from mixed backgrounds similar to that of p21Cip-1 null mice reveal establishment frequencies ranging from 20 to 80% (16a). In all of these instances, the transformation process is accompanied by a profound apoptotic crisis. Thus, while p21Cip-1 may play some role in the frequency with which primary transformants become established, the uniform presence of apoptotic crisis in cells from p21Cip-1−/− mice demonstrates that the protein is not required for this phenomenon. Consistent with this idea, four of eight p21Cip-1−/− transformants tested acquired Trp53 mutations during expansion (data not shown); this frequency is similar to that observed with primary transformants from other strains of mice (47).
FIG. 5.
p21Cip-1−/− transformants undergo crisis. Growth and viability of primary transformants from p21Cip-1−/− mice were monitored as described in Materials and Methods. The times at which primary transformants succumbed to crisis (●) and at which cell lines that survived crisis and became established (○) are indicated.
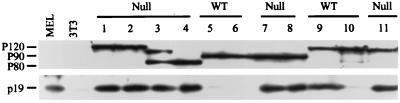
p19Arf expression is elevated in Trp53 null transformants.
Our earlier work established a correlation between expression of Ink4a/Arf locus products and expression of mutant forms of p53 (30) and suggested that a functional p19Arf-p53 pathway (27, 41) is required for crisis. This model predicts that expression of p19Arf is not deleterious to the growth of transformants from Trp53 null mice. To test this idea, a panel of transformants was examined for p19Arf expression by Western blotting. All 12 transformants tested, including the representatives shown (Fig. 6), expressed readily detectable p19Arf. As shown previously (30), none of the transformants from control animals expressing wild-type p53 expressed p19. The sample in lane 9 is representative of a transformant from wild-type mice that expresses a mutant p53. This pattern of expression is similar to that observed in other types of cells which lack a functional p53 protein (18, 29, 45, 56) and is consistent with the absence of apoptosis following p19Arf overexpression in transformants lacking a functional p53 (30).
FIG. 6.
p19Arf is expressed in transformants from Trp53 null mice. Lysates were prepared from transformants from Trp53 null animals or wild-type (WT) mice and examined by Western blotting for the presence of the p19Arf protein. The cells used were derived with either the P120, P90, or P80 strains of Ab-MLV (24). The transformation properties of these viruses are similar in wild-type and Trp53 null mice (our unpublished data). Lysates from NIH 3T3 (p19Arf-negative) cells and from the p19Arf-positive cell line MEL (29) were used as controls. The blots were also probed with the H548 anti-Gag/v-Abl monoclonal antibody (7) to control for protein loading.
The hypothesis that p19Arf functions through p53 in the Ab-MLV system also predicts that p19Arf retains functional potential in cells expressing mutant p53. To assess the status of p19 in these cells, p19Arf cDNAs from two independent cell lines expressing mutant p53 were amplified, cloned, and sequenced. In addition, because published sequence information indicates that p19Arf sequences are not identical in all inbred strains (23), the genomic sequences of Ink4a/Arf exon 1β and exon 2, which encode the majority of the p19Arf protein, were determined by amplifying these regions from BALB/cByJ liver DNA. Consistent with published information (23), comparison of the BALB/cByJ sequence with that expressed by the MEL erythroleukemia cell line from which p19Arf was originally cloned (29) revealed the presence of a sense variant (T75C) and a missense variant (G257A), resulting in the substitution of a histidine for an arginine, in BALB/cByJ. Comparison of the p19Arf sequences from both transformants revealed that they were identical to the BALB/cByJ sequence obtained from liver DNA. Similar analyses revealed that the p16Ink4a sequences expressed by these transformants were also identical to the published BALB/c sequence. These data suggest that both Ink/Arf locus products retain their full functional potential and support the view that p19Arf functions through p53 in Ab-MLV transformants.
DISCUSSION
Our results demonstrate that expression of wild-type p53 plays a key role in the selection process that characterizes the early phases of Ab-MLV-induced pre-B-cell transformation. The protracted period of apoptotic crisis that characterizes the transition from primary pre-B transformant to a fully malignant cell does not occur when primary transformants are derived from Trp53 null mice. Furthermore, transformants from heterozygous animals display an intermediate level of crisis and rapid loss of heterozygosity at Trp53. This pattern is similar to that obtained in tumors arising in Trp53+/− mice (3, 4, 17) but contrasts with the pattern observed in transformants from normal mice (47, 53). Loss of Trp53 sequence is rare in the latter circumstance, probably because both copies of Trp53 would have to be altered for the cells to have a selective advantage. In contrast, a single dominant-negative mutation, such as those found in Ab-MLV-transformed pre-B cells, is sufficient to ablate protein function.
Despite its dramatic effect on the establishment of primary Ab-MLV transformants and the slightly higher numbers of B220-positive, immunoglobulin M-negative pre-B cells reported for Trp53−/− mice (42), the absence of p53 did not affect the frequency of Ab-MLV-susceptible target cells in a marked way. BALB/c mice, the strain used here, are highly sensitive to Ab-MLV transformation in vitro (35), raising the possibility that effects of p53 on transformation frequency may be less dramatic in this strain. Enhanced transformation of myeloid progenitors from Trp53−/− mice on a C57BL/6 background has been reported (43), and recent work with Trp53−/− animals on a mixed 129 background demonstrated higher frequencies of Ab-MLV target cells in null animals (5a). The absence of p53 also does not affect the phenotype of the transformants; similar to Ab-MLV transformants from normal mice, Trp53 null cells express the B220 pre-B-cell differentiation antigen and have rearranged their immunoglobulin heavy chain genes but not their light chain genes (our unpublished data).
In contrast to the results obtained in vitro, Trp53 null animals develop tumors somewhat more rapidly than those which retain a functional p53 protein. The relatively small differences in the latency period most likely reflect the high susceptibility of BALB/c mice to Abelson disease (33). Consistent with this view, a more dramatic impact on tumor latency was observed when animals on a mixed 129 background were tested (5a). These differences probably reflect the impact of other genes on Ab-MLV tumorigenesis; two genes affecting the susceptibility of laboratory mice to Ab-MLV have been identified, but the mechanisms underlying their effects remain unknown (32, 33). Consistent with the phenotype of in vitro transformants, the pattern of tumor development and the morphology of the tumor cells suggest that tumor cells are similar in the wild-type and null animals. The differences in latency and the observation that Abelson disease normally involves clonal selection (14, 15) suggest that the latter process may be reduced or absent in tumors arising in Trp53 null animals.
p21Cip-1, an important downstream effector of p53 (10, 11, 16, 55), does not appear to play a major role in Ab-MLV-induced pre-B-cell transformation. Although we could not compare the frequencies of target cells in null animals to genetically matched control animals, comparisons with other mice of similar mixed background suggest that the null animals have normal numbers of Ab-MLV-susceptible cells. p21Cip-1 also does not play a major role in crisis induction, demonstrating that this protein is not required for the p53-mediated effects on transformation. In addition, expression of p21Cip-1 induces neither apoptosis nor obvious changes in cell cycle parameters in Trp53 null transformants (our unpublished data). These data are consistent with the fact that p53-mediated apoptosis is p21Cip-1 independent in other types of cells (1, 6, 19, 49).
Primary transformants derived from Trp53−/− mice are strikingly similar to those derived from Ink4a−/− mice; in both cases, virtually all primary transformants become established very rapidly and the apoptotic crisis that characterizes the transformation process does not occur (30). These data and the correlation between p19Arf expression and p53 status (30) suggest that these two proteins function in an interdependent fashion during transformation. A similar relationship has been observed when MEF undergo senescence in vitro (18, 56). In this instance, loss of either p19Arf or p53 permits the emergence of immortalized cells. However, senescence is a normal cell process characterized by specific changes in gene expression and cell cycle arrest (44, 48). Most normal lymphoid cells do not grow extensively in vitro, even in the presence of lymphokines, and a process similar to senescence has not been documented in this cell type. Nonetheless, because expression of some oncogenes induces changes in MEF that resemble senescence (39), determining if primary transformants undergoing crisis share features with senescent cells could help to illuminate the mechanisms involved.
The relationship between p19Arf expression and the emergence of cells expressing wild-type p53 suggests that the p19Arf-p53 regulatory loop (27, 41) is an important cellular response to Ab-MLV pre-B-cell infection. This response may be triggered by v-Abl-mediated activation of c-Myc, an important downstream target of v-Abl (38, 54, 57), which in turn can activate p19Arf (56). Indeed, because the vast majority of Ab-MLV transformants either express mutant p53 or fail to express p19Arf once they are fully established (30), this pathway may serve as the major cellular gatekeeper modulating Ab-MLV transformation.
ACKNOWLEDGMENTS
We thank Philip Leder, Ronald DePinho, and Andrew Beavis for providing reagents critical to this work; Henry Wortis and Allen Parmelee for assistance with flow cytometry; Zohar Sachs for assistance with mice; and Anne Halgren for technical support.
This work was supported by grant CA33771 from the National Institutes of Health.
REFERENCES
- 1.Attardi L D, Lowe S W, Brugarolas J, Jacks T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 1996;15:3693–3701. [PMC free article] [PubMed] [Google Scholar]
- 2.Baker S, Markowitz S, Fearson E, Wilson J, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–914. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 3.Baxter E W, Blyth K, Donehower L A, Cameron E R, Onions D E, Neil J C. Moloney murine leukemia virus-induced lymphomas in p53-deficient mice: Overlapping pathways in tumor development? J Virol. 1996;70:2095–2100. doi: 10.1128/jvi.70.4.2095-2100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blyth K, Terry A, O’Hara M, Baxter E W, Campbell M, Stewart M, Donehower L A, Onions D E, Neil J C, Cameron E R. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene. 1995;10:1717–1723. [PubMed] [Google Scholar]
- 5.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature (London) 1995;377:552–556. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 5a.Calame, K. Personal communication.
- 6.Chen X, Ko L J, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro B, Wehrly K, Cloyd M, Britt W, Portis J, Collins J, Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology. 1981;112:131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- 8.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21Cip1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 9.de Stanchina E, McCurrach M E, Zindy F, Shieh S-Y, Ferbeyre G, Samuelson A V, Prives C, Roussel M F, Sherr C J, Lowe S W. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 1998;12:2434–2442. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulic V, Kaufmann W K, Wilson S J, Tisty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 11.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 12.Engelman A, Rosenberg N. Temperature-sensitive mutants of Abelson murine leukemia virus deficient in protein tyrosine kinase activity. J Virol. 1990;64:4242–4251. doi: 10.1128/jvi.64.9.4242-4251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedlander P, Haupt Y, Prives C, Oren M. A mutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol Cell Biol. 1996;16:4961–4971. doi: 10.1128/mcb.16.9.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green P L, Kaehler D A, Bennett L M, Risser R. Multiple steps are required for the induction of tumors by Abelson murine leukemia virus. J Virol. 1989;63:1989–1994. doi: 10.1128/jvi.63.5.1989-1994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green P L, Kaehler D A, Risser R. Clonal dominance and progression in Abelson murine leukemia virus lymphomagenesis. J Virol. 1987;61:2192–2197. doi: 10.1128/jvi.61.7.2192-2197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1-cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 16a.Jenab, J., and N. Rosenberg. Unpublished data.
- 17.Jones J M, Attardi L, Godley L A, Laucirica R, Medina D, Jacks T, Varmus H E, Donehower L A. Absence of p53 in a mouse mammary tumor model promotes tumor cell proliferation without affecting apoptosis. Cell Growth Differ. 1997;8:829–838. [PubMed] [Google Scholar]
- 18.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Consoli U, Audreeff M, Shiku H, Deisseroth A B, Zhang W. Activation of p21WAF1/CIP1 expression in a temperature-sensitive mutant of human p53 does not lead to apoptosis. Oncogene. 1995;11:2311–2316. [PubMed] [Google Scholar]
- 20.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 21.McGahon A J, Nishioka W K, Martin S J, Mahboubi A, Cotter T G, Green D R. Regulation of Fas apoptotic cell death by Abl. J Biol Chem. 1995;270:22625–22631. doi: 10.1074/jbc.270.38.22625. [DOI] [PubMed] [Google Scholar]
- 22.Miyashita T, Reed J C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 23.Mock B A, Ramsey E S, Mock B A. Cdkn2, the cyclin-dependent kinase inhibitor encoding p16INK4a and p19ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr. Proc Natl Acad Sci USA. 1998;95:2429–2434. doi: 10.1073/pnas.95.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmar K, Huebner R C, Rosenberg N. Carboxyl-terminal determinants of Abelson protein important for lymphoma induction. J Virol. 1991;65:6478–6485. doi: 10.1128/jvi.65.12.6478-6485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar K, Rosenberg N. Ras complements the carboxyl terminus of v-Abl protein in lymphoid transformation. J Virol. 1996;70:1009–1015. doi: 10.1128/jvi.70.2.1009-1015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomerantz J, Schreiber-Agus N, Liegeois N J, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H W, Cordon-Cardo C, DePinho R A. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 27.Prives C. Signaling to p53: breaking the MDM2-p53 circuit. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 28.Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994;84:2391–2411. [PubMed] [Google Scholar]
- 29.Quelle D E, Zindy F, Ashmun R A, Sherr C J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 30.Radfar A, Unnikrishnan I, Lee H-W, DePinho R A, Rosenberg N. P19Arf induces p53-dependent apoptosis during Abelson virus-mediated pre-B cell transformation. Proc Natl Acad Sci USA. 1998;95:13194–13199. doi: 10.1073/pnas.95.22.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid S, Cross R, Snow E. Combined Hoechst 33342 and merocyanin 540 staining to examine murine B cell cycle stage, viability and apoptosis. J Immunol Methods. 1996;192:43–54. doi: 10.1016/0022-1759(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 32.Risser R, Kaehler D, Lamph W W. Different genes control the susceptibility of mice to Moloney or Abelson murine leukemia viruses. J Virol. 1985;55:547–553. doi: 10.1128/jvi.55.3.547-553.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risser R, Potter M, Rowe W P. Abelson virus-induced lymphomagenesis in mice. J Exp Med. 1978;148:714–726. doi: 10.1084/jem.148.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg N. abl oncogenes. Semin Virol. 1991;2:365–374. [Google Scholar]
- 35.Rosenberg N, Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976;143:1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan K, Vousden K. Characterization of structural p53 mutants which show selective defects in apoptosis but no cell cycle arrest. Mol Cell Biol. 1998;18:3692–3698. doi: 10.1128/mcb.18.7.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabbatini P, Chiou S-K, Rao L, White E. Modulation of p53-mediated transcriptional repression and apoptosis by adenovirus E1B 19K protein. Mol Cell Biol. 1995;15:1060–1070. doi: 10.1128/mcb.15.2.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawyers C L, Callahan W, Witte O N. Dominant negative myc blocks transformation by abl oncogenes. Cell. 1992;70:901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- 39.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y Q, Shenk T. Relief of p53-mediated transcriptional repression by the adenovirus E1B 19K protein or the cellular bcl-2 protein. Proc Natl Acad Sci USA. 1994;91:8940–8944. doi: 10.1073/pnas.91.19.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherr C J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 42.Shick L, Carman J H, Somasundaram K, Burrell M, Hill D E, Zeng Y-X, Wang Y, Wiman K G, Salhany K, Kadesch T R, Monroe J G, Donehower L A, El-Deiry W S. Decreased immunoglobulin deposition in tumors and increased immature B cells in p53-null mice. Cell Growth Differ. 1997;8:121–131. [PubMed] [Google Scholar]
- 43.Skorski T, Nieborowska-Skorska M, Wlodarski P, Perrotti P, Martinez R, Wasik M A, Calabretta B. Blastic transformation of p53-deficient bone marrow cells by P210 bcr/abl tyrosine kinase. Proc Natl Acad Sci USA. 1996;93:13137–13142. doi: 10.1073/pnas.93.23.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein D H, Dulic V. Origins of G1 arrest in senescent human diploid fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 45.Stott F J, Bates S, James M C, McConnell B B, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden K H, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuppia L, Calabrese G, Peila R, Guanciali-Franchi P, Morizio E, Spadano A, Palka G. p53 loss and point mutations are associated with suppression of apoptosis and progression of CML into myeloid blastic crisis. Cancer Genet Cytogenet. 1997;98:28–35. doi: 10.1016/s0165-4608(96)00413-x. [DOI] [PubMed] [Google Scholar]
- 47.Thome K C, Radfar A, Rosenberg N. Mutation of Tp53 contributes to the malignant phenotype of Abelson virus-transformed lymphoid cells. J Virol. 1997;71:8149–8156. doi: 10.1128/jvi.71.11.8149-8156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Votja P J, Barrett J C. Genetic analysis of cellular senescence. Biochim Biophys Acta. 1995;1242:29–41. doi: 10.1016/0304-419x(95)00002-w. [DOI] [PubMed] [Google Scholar]
- 49.Wagner A, Kokontis J M, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994;8:2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- 50.Wang J Y J. Abl tyrosine kinase in signal transduction and cell-cycle regulation. Curr Opin Genet Dev. 1993;3:35–43. doi: 10.1016/s0959-437x(05)80338-7. [DOI] [PubMed] [Google Scholar]
- 51.Whitlock C A, Witte O N. Abelson virus-infected cells exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981;40:577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitlock C A, Ziegler S F, Witte O N. Progression of the transformed phenotype in clonal lines of Abelson virus-infected lymphocytes. Mol Cell Biol. 1983;3:596–604. doi: 10.1128/mcb.3.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf D, Rotter V. Inactivation of p53 gene expression by an insertion of Moloney murine leukemia virus-like DNA sequences. Mol Cell Biol. 1984;4:1402–1410. doi: 10.1128/mcb.4.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong K K, Zou X, Merrell K T, Patel A J, Marcu K B, Chellappan S, Calame K. v-Abl activates c-myc transcription through the E2F site. Mol Cell Biol. 1995;15:6535–6544. doi: 10.1128/mcb.15.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature (London) 1993;366:707–710. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 56.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou X, Rudchenko S, Wong K-K, Calame K. Induction of c-myc transcription by the v-Abl tyrosine kinase requires Ras, Raf1, and cyclin-dependent kinases. Genes Dev. 1997;11:654–662. doi: 10.1101/gad.11.5.654. [DOI] [PubMed] [Google Scholar]