Abstract
Purpose of review
The current review highlights the complexity of the pediatric and adolescent gynecology subspecialty as well as the recent and exciting opportunities for innovation within the field.
Recent findings
The opportunities for concept, treatment, instrument, and knowledge-transfer innovation to better serve the specific needs of pediatric gynecology patients include novel approaches to neovagina creation using magnets, improving postoperative vaginal wound healing through newly designed and degradable vaginal stents, and complex Mullerian reconstructive surgical planning using virtual reality immersive experiential training.
Summary
There is a significant window of opportunity to address the needs of pediatric, adolescent and adult gynecological patients with new innovative concepts and tools.
Keywords: innovation, pediatric adolescent gynecology, science and technology
INTRODUCTION
Pediatric and adolescent gynecology (PAG) focuses on a unique subset of gynecologic disorders from infancy to young adulthood, and the combination of medical and surgical approaches applied to diagnose, treat, and manage them [1]. Though relatively new as a distinct discipline, the pathophysiology specific to pediatric and adolescent females have challenged physicians for over 100 years [2]. The obstacles inherent to a discipline that must master aspects of surgery, pediatrics, endocrinology, and gynecology are necessarily multifaceted. Past the intrinsic complexity and sensitive nature of these diagnoses, these difficulties can include anatomical and surgical challenges requiring significant approach modifications over a large patient age range, taking into account pubertal status and developmental changes. All of these issues can be aggravated by congenital anomalies, growing organs, reproductive potential, abuse, trauma, sexuality, and level of maturity, which highlight the subsequent need for specialized PAG training centers. A successful pediatric gynecologist must be able to adapt and modify their approach not only to the individual patient encounter, but their current and future surgical and postoperative care plans as well.
Pediatric medicine as a whole is often disadvantaged by the application of adult-designed technology solutions to rare pediatric abnormalities. Innovation in PAG-guided personalized precision therapies, however, is often opposed by low incidence rates of pediatric gynecologic diseases, the resultant smaller market size, and an overarching lack of physician–engineering–commercialization pipelines. This combination inevitably leads PAG surgeons to use adult devices for off-label pediatric indications with the potential for legal, ethical, or medical ramifications [3■]. One hopes that, with increasing recognition of the uniqueness of this population, more accurate diagnoses and more effective treatments and devices specifically tailored to the physiology and anatomy of pediatric patients could emerge [3■].
As in any pediatric surgical subspeciality, opportunities for concept, treatment, instrument and knowledge-transfer innovation to better serve the specific needs of pediatric gynecology patients abound. Despite the areas of advancement in this evolving field, innovation is often stymied by the reductionist concept of limited incidence. The fact that there are as many as 50 000 pediatric and 213 000 adult female patients [4–7] per year undergoing significant pelvic reconstructive surgeries should highlight the commonalities of the themes seen in PAG patients across all diagnoses and gynecologic subspecialties, including neovaginal creation, surgical vs. nonsurgical considerations and limitations, postoperative recovery, and long-term vaginal outcomes.
HISTORICAL APPROACHES TO NEOVAGINA CREATION
In a recent historical review of topics pertinent to modern PAG by Yordan and Yordan [2], between the years 1869 and 1895, the most common subject was the surgical management of congenital absence or atresia of the vagina and associated or adjacent organs. Although rare, these anomalies were considered important given their association with retention of menses and inability to achieve intercourse [2]. The creation of a neovagina with positioning of the vaginal opening between the bladder and rectum by a single-stage procedure of blunt dissection was first described by Thomas Addis Emmet in 1887. He also demonstrated that the immediate placement of a glass vaginal dilator provided the best chance of a favorable outcomes [2]. The use of graduated dilators for neovagina creation was next proposed by Frank in 1938 and modified by Ingram in 1981 to utilize a bicycle seat mounted on a stool to facilitate vaginal dilation [8]. Though effective, these techniques are time-intensive for both the patient and physician/educator, requiring patient commitment and readiness and often with considerable coaching from the training provider.
Surgical procedures have changed [9] from utilization of labia majora skin flaps (Williams procedure) to the use of bowel loop to create a vaginal lumen (McIndoe procedure) [10] to the Sheares procedure utilizing a postoperative vaginal mould [11]; from laparoscopic approaches such as the Davydov procedure, which uses peritoneum to constitute the neovaginal walls and vault [12] to the Vecchietti procedure, involving laparoscopic placement of abdominal device tractioned through the pelvis to a vaginal olive [13]. Despite these advances, neovaginal surgical approaches have not significantly evolved in concept or approach. Moreover, even minimally invasive and laparoscopic surgical approaches, though associated with a significant reduction in postoperative recovery times, involve substantial risk and sometimes a prolonged and cumbersome postoperative period [13].
CURRENT APPROACHES TO NEOVAGINA CREATION
In patients with a diagnosis of utero-vaginal agenesis, the creation of a neovaginal canal currently follows one of two approaches, both of which involve applying force to the vaginal dimple so as to elongate it to an appropriate size to enable sexual function. The nonsurgical approach uses progressive dilatation with increasingly larger dilators with steady pressure applied over repeated periods of time. This approach requires patient maturity, patience, and willingness to dilate, an ability to withstand vaginal discomfort, and a consistent effort to apply dilatory pressure. This approach can create an adequate neovaginal canal without surgical intervention within 3–6 months, depending on patient motivation. Patients who are not sexually active will need to continue using the vaginal dilators to maintain the caliber of the vagina until sexual activity is initiated [14■].
The surgical approach to neovagina creation also involves applying force to the vaginal dimple, wherein a vaginal ‘pouch’ is created out of peritoneum, bowel, or buccal mucosa [8,15]. The Vecchietti procedure [8] is one of the more commonly used surgical approaches, which involves an acrylic vaginal ‘olive’ placed in the vaginal dimple. This olive is then dually sutured to an external ratcheting system with intracorporeal tractioning, a system that ultimately applies consistent and progressive pressure over time to the levered vaginal dimple (Fig. 1: Laparoscopic Vecchietti Device; Manufacturer Karl Storz Endoscopy, Tuttlingen, Germany). The significant drawbacks of this approach include the technical challenges involved in its creation, the limited number of centers providing training and utilization of the technique, and patient discomfort. Though a neovaginal canal of adequate size can be accomplished within only 5 days, these patients are hospitalized while under traction, requiring epidural pain relief and anticoagulants while recumbent. This technique then requires a second procedure to remove the tractioning device and ‘olive’, and subsequent management requires the patient to continue to wear a vaginal stent and to dilate progressively thereafter if not sexually active.
FIGURE 1.
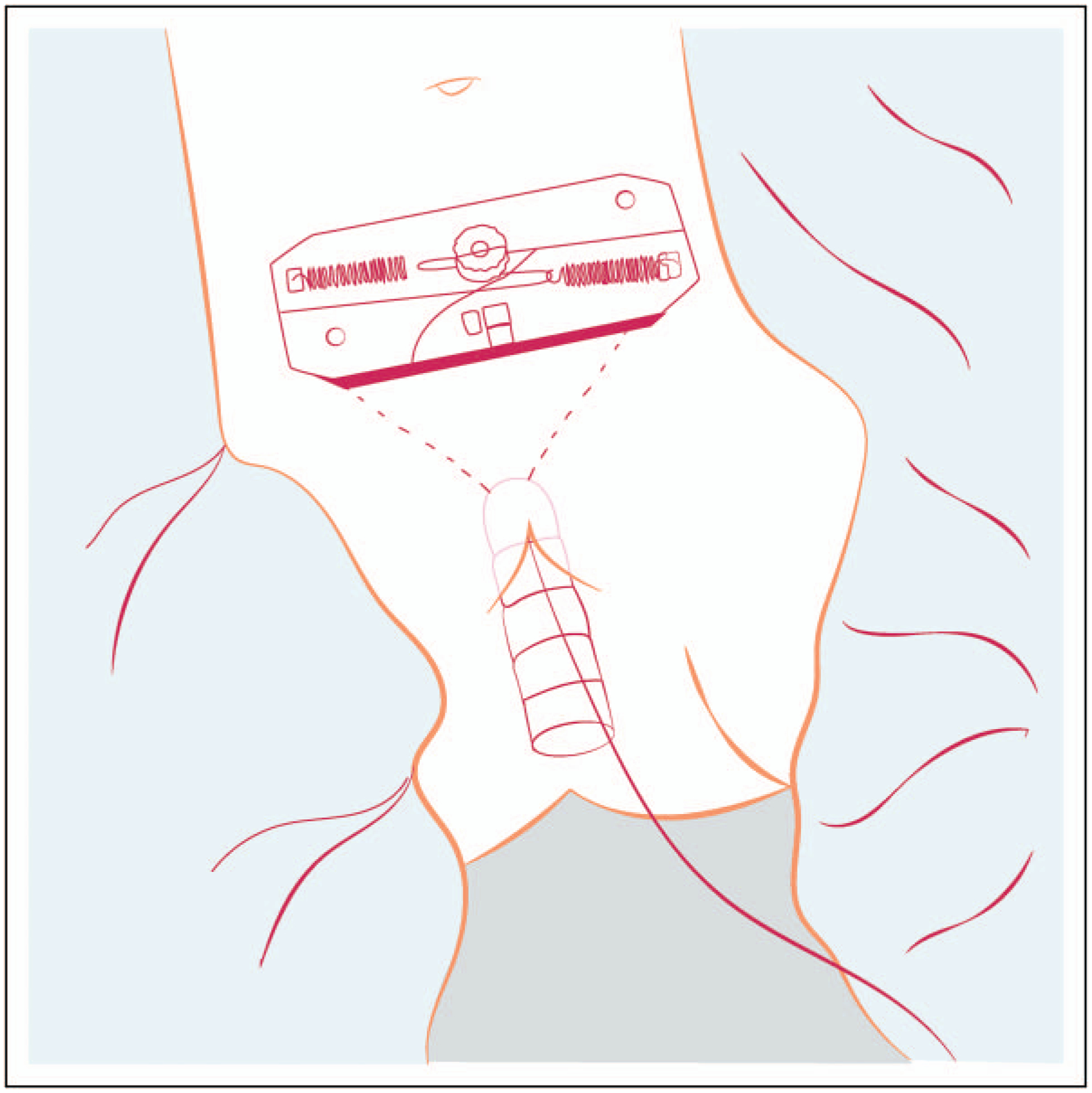
Laparoscopic Vecchietti device.
Though both surgical and nonsurgical approaches will accomplish neovagina creation, both also entail significant limitations and complications [13]. Each approach for the surgical creation of a neovagina has its own inherent risk profile associated to the specific technique. With a nonsurgical approach, the application of force to the vaginal dimple with vaginal dilators can lead to discomfort, pain, bleeding, vaginal or bladder perforation, and early discontinuation. Moreover, postoperatively there is a risk of restenosis or vaginal fibrosis in up to 73% of patients undergoing neovagina creation surgery [4] if vaginal stents and dilators are not used consistently thereafter. Stenosis of the vagina can have life-long implications for pediatric and adult patients including pain, inability to tolerate vaginal exams, retention of menstrual fluids and vaginal secretions, dyspareunia, and reduction in quality of life [16]. When neovaginal grafts are used, the risk of restenosis increases and autograft tissues (e.g., bowel mucosa) may cause chronic vaginal discharge [10]. It is therefore unfortunately unsurprising to find that many pediatric surgical centers quote a 50% risk of surgical revision after vaginal reconstruction [6].
VAGINAL WOUND HEALING
Within the field of gynecology, an understanding of vaginal wound healing and postoperative or post-radiation fibrotic tissue formation is limited. To facilitate wound healing and prevent fibrotic tissue repair, gynecologists use vaginal stents in the immediate postoperative setting, transitioning to vaginal dilators for intermittent but continued use until sexual activity is resumed. A vaginal stent is a medical device placed into the vaginal canal postoperatively such that the vaginal walls do not adhere to each other during the healing process, in an effort to reduce vaginal tissue fibrosis and to maintain the vaginal caliber. These are comprised of a silicone membrane surrounding a foam inner core operated with balloon inflation by a syringe connected to a plastic catheter hanging from the distal end of the stent, with a second plastic tube also outside the patient vaginal cavity to facilitate the egress of vaginal secretions (Figure 2: Previous Mentor (Silimed) Vaginal Stent; PMT Corporation, MN, USA). However, there are no currently available vaginal stents that are designed for the pediatric population, and those available historically come in sizes incongruent with the adolescent vagina, much less that of an infant. PAG physicians are thus currently relegated to using suboptimal stents such as Foley balloons [17] or creating their own makeshift stents using either finger slots from sterile gloves and gauze, sterile cement coated in bone wax and placed in a condom, or plastic moulding [18].
FIGURE 2.
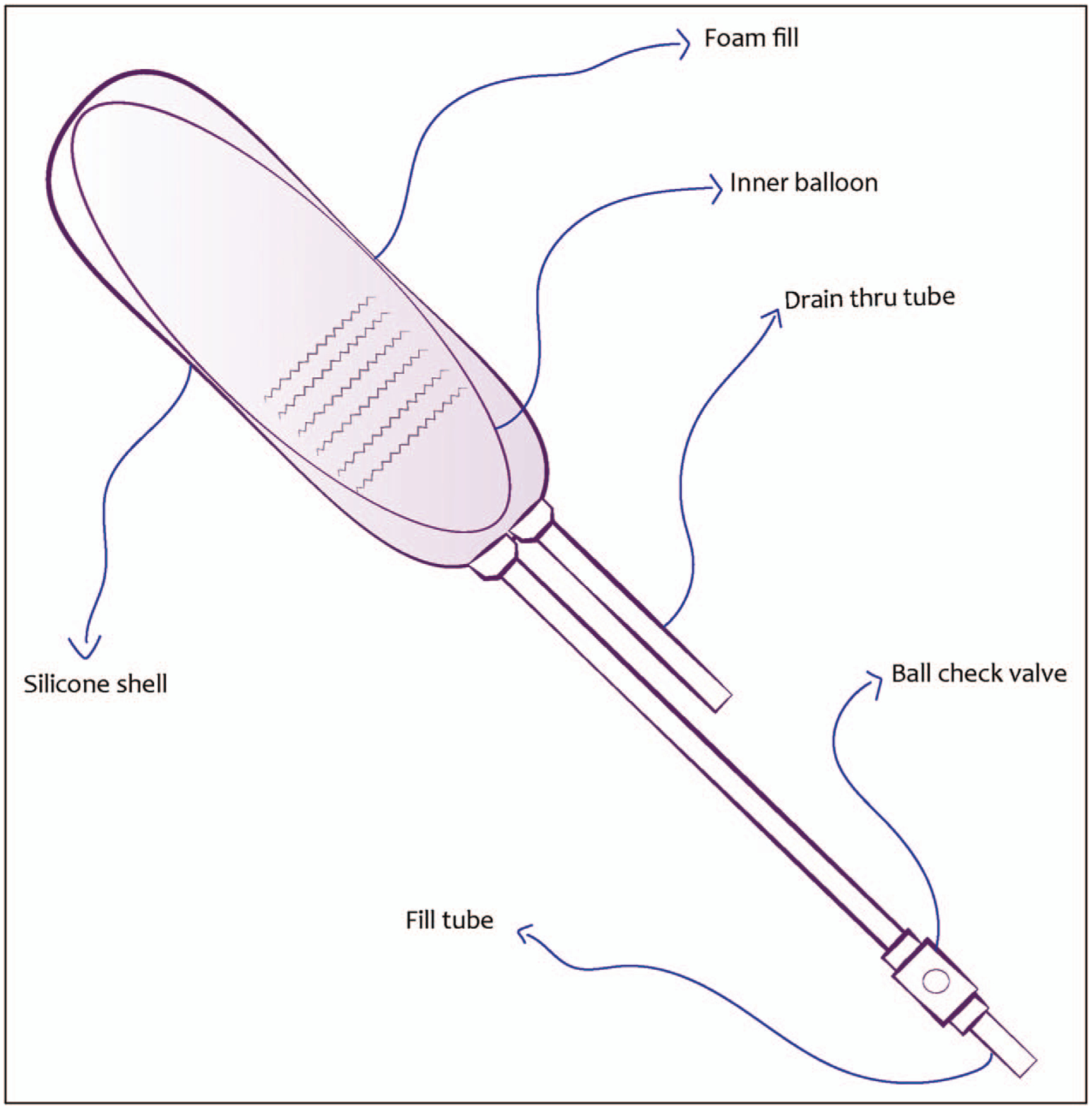
Schematic of Previous Mentor (Silimed) Vaginal Stent.
In addition to stents, gynecologists also routinely use estrogen creams, primarily conjugated equine estrogen (Premarin; Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer, PA, USA), to enhance vaginal tissue repair. This cream is either directly applied to the vagina postoperatively or as a coating to vaginal packing stents [18,19]. Patients experiencing vaginal bleeding or erosion after dilator use [14■] are also instructed to use short-term local estrogen application to relieve these symptoms. Although the exact mechanism of action of estrogen cream is still largely unknown, postoperative vaginal estrogen is known to have a trophic or proliferative effect on the vaginal mucosa, inhibits proteolytic degradation of the mucosa and increasing vaginal tissue compliance via promoting the production of extra-cellular matrix components such as type I and III collagen [20,21].
Despite its abundant clinical use, there are critical issues limiting the application of estrogen cream. One of these issues includes the creams’ inability to effectively ‘adhere’ to the wound, resulting in unmeasurable loss, dose variability, and ultimately incomplete therapy. Dosing is further complicated by endogenous changes in estrogen production, namely at prepubertal, adult and postmenopausal stages [5], which translates into variations in estrogen receptor signal transduction [22] and tissue responsiveness to wound healing [19,23]. For this reason, estrogen cream application has been shown to have paradoxically divergent tissue-level outcomes when administered pre or postoperatively in different patient demographics [19,24], albeit without current clinical guidelines for precision therapeutic use. As such, stents, dilators, and topical estrogens are suboptimal options, leading to low patient compliance, uneven dosing, high rates of emergency room visits and hospital admissions for replacements and complications, and poor patient outcomes.
OPPORTUNITIES FOR INNOVATION IN NEOVAGINA CREATION
The surgical field has always been receptive to disruptive new technologies, especially when they enable physician and patient flexibility, personalization of care, reduction of cost, and improved patient outcomes [25]. In addition, a push towards outpatient, minimally invasive, and wearable technologies exists in multiple other disciplines past medicine and health care, offering venues for collaboration and retrofitting of ideas. Just within the limited subspecialty field of PAG there are multiple opportunities for innovation in and improvement of neovagina creation, postoperative care, outcome optimization, and knowledge transfer. Though we have traditionally approached neovagina creation as a binary – surgical or nonsurgical – there may be a hybrid middle ground allowing for a streamlined outpatient approach modeled after other disciplines that could then be applied to pediatric gynecology.
‘WEARABLE SURGERY’
The art and science of marrying two existing concepts in novel ways is the heart of and challenge inherent to clinico-medical innovation [3■,25]. The creation of neo-lumens in different organs using magnetic anastomoses has a history of success in other surgical applications, and its application in pediatric gynecology with regard to neovagina lumen creation is apparent, though as-yet unrealized. As an example, Flourish (Cook Medical, Bloomington, IN, USA) is an Food & Drug Administration-approved device designed for use in esophageal atresia, using precise placement of rare earth magnets to appose the upper and lower ends of an atretic infant esophagus [26,27]. Other minimally invasive magnet-based approaches include the ever-linQendoAVF system (Manufacturer Becton, Dickinson and Company, NJ, USA), used to create an arteriovenous fistula for hemodialysis access without open surgery [28]. In light of these advances and the success of these examples, it becomes apparent how this concept can be modified for the PAG patient population. Magnetizing both specially designed undergarments and an expandable vaginal dilator specifically designed for vaginal dimples could significantly increase the efficiency of dilator use, or alternatively implanting nontoxic degradable ferromagnetic materials directly to the vaginal dimple mucosa, would allow elongation of the vaginal dimple into an adult sized vagina [29]. Creating a vaginal segment using only magnets could convert a complex surgical technique performed at a few specialized centers into a low-impact outpatient procedure that could be applied on a broader scale. By reducing patient effort from long-term (e.g., dilatation) to short-term (either with implanted magnet repulsion/attraction alone or in combination with magnetized dilators), the increased efficiency of neovagina creation and maintenance, enabled by this kind of technology, could significantly reduce patient frustration, discomfort, and early discontinuation.
LOOKING AHEAD: ‘NEXT GEN’ VAGINAL STENTS AND IMMERSIVE SURGICAL LEARNING
Vaginal stents previously existed on the market in large, nonergonomic designs poorly tolerated in the pediatric population. Perplexingly, those designs recently became available again after being removed from production for more than 5 years [30]. This void in the gynecological medical device market led PAG physicians to investigate and obtain a more robust understanding of vaginal shape geometry, the beneficial effects of tissue stretch on vaginal healing, and patient-specific design criteria such that a novel and appropriate adolescent vaginal stent for postoperative use could be created [31]. The new pediatric/adolescent specific vaginal stent is designed to maintain important parameters that include appropriate sizing, mechanical coupling to the vaginal wall, and application of constant pressure to the boundaries of the neovagina. These measures will limit stent egress with Valsalva, maintain flexibility to allow for patient comfort, reduce mucosal erosion, and facilitate easy insertion and retrieval with improved ergonomics (Table 1: Comparison of Previous Mentor (Silimed) Vaginal Stent to Novel Pediatric Vaginal Stent).
Table 1.
Comparison of Previous Mentor (Silimed) Vaginal Stent to Novel Pediatric Vaginal Stent
| Currently available vaginal stent | Novel vaginal stent being developed | |
|---|---|---|
| Appropriate size for adult patients | ✓ | ✓ |
| Appropriate size for pediatric/adolescence patients | ✓ | |
| Silicone tubing outside of body | ✓ | |
| Integrated inflation/deflation (no tubing outside of body) | ✓ | |
| Center lumen for vaginal secretions | ✓ | ✓ |
| Magnetic insertion handle for deployment, inflation/deflation, retrieval | ✓ | |
| Improved materials for patient comfort and to prevent dislodgement | ✓ |
In addition to pediatric/adolescent-specific stent creation, the development of ‘next generation’ vaginal stents further improve on current designs by eliminating the need for postoperative stent removal after wear-time is complete. Resorbable stents are already in therapeutic use for cardiovascular applications [32] and are being developed for other organ lumens such as esophageal [33], gastrointestinal [34], and ureteral [35] applications. Transfer of this technology to pediatric gynecology in the form of vaginal stents that degrade slowly over time would allow for the same basic functionality – application of traction to the vaginal walls after surgery, maintenance of vaginal caliber during wound healing, and egress of exudative fluids, blood, and vaginal discharge to limit infection and wound breakdown – but without requiring a second procedure for removal. Controlled drug delivery can also be incorporated into the stent design through physical entrapment within the material, with release mediated by diffusion or polymer erosion [36]. The addition of controlled-release estrogen to the stent material, for example, would eliminate the need for cumbersome external estrogen cream application.
In the design of a resorbable vaginal stent, incorporating mechanical properties that ensure that the stents maintain vaginal patency (e.g., sufficient radial strength) while avoiding adverse biological responses such as tissue overgrowth [37] are critical. Careful tuning of the stent degradation rate is also needed to ensure the retention of appropriate mechanical properties and load transfer to the neo-tissue throughout healing. As these stents dissolve, the release of degradation products must not compromise healing or increase inflammation in the surrounding tissue [38]. Polymeric materials are most commonly selected for resorbable stent applications (e.g., polylactides, polycaprolactone, polyanhydrides) due to the tight control of degradation rate afforded by tuning polymer chemistry and molecular weight. As an alternative to polymeric stents, magnesium-based alloys are under investigation as bioresorbable metallic stents [37]. In addition to base material choice, the geometry of the stent itself, with struts of varying thicknesses and architecture, can be used to further control degradation, mechanical properties, and deployment variables. Although pediatric/adolescent vaginal stents can build off the design framework established in other applications, more research is needed to address gynecologic-specific design considerations, such as differences in shape and sizing options, shorter time-scales for dissolution, deployment outside of the operating room, and biomechanics balancing patency retention, patient comfort, and optimal vaginal healing.
SURGICAL PLANNING AND TEACHING OF COMPLEX PEDIATRIC GYNECOLOGIC INTERVENTIONS
An often-neglected facet of subspecialty medicine is that of knowledge transfer and learner engagement [39]. Teaching difficult surgical concepts in a limited number of centers with a relatively small number of surgical opportunities [25] can lead to inexperienced subspecialists and poor outcomes. Surgical simulation has long been used to preplan and teach surgical concepts, techniques and technicity to learners. Combining this with advances in computer simulation technology will undoubtedly facilitate learner engagement and competence. Adoption of virtual reality simulation by other high-stakes/high-cost industries such as architecture design have allowed customers a virtual experience in a yet-to-be-built facility [40]. Borrowing from other industries and surgical disciplines, where immersive experiential preplanning is now considered standard, the art of teaching surgical technicity may no longer involve a real patient but a simulated one within an immersive virtual reality experience. The advent of Google Glasses and Oculus Rift Head Mount Display over the last decade have brought experiential surgery into the forefront of medicine, and the addition of haptic (tactile) feedback allows for an even more realistic surgical training experience, where learners can see, feel, and study tissues and anatomy, and develop surgical pathways to practice and plan the most effective roadmap of care. Many companies specifically target resident surgical teaching with resultant decreases in surgical errors [25], rare disease surgical planning and practice [41], novel medical device testing [42] and improvements in patient satisfaction. Surgical teaching in pediatric gynecology using patient-specific MRIs and virtual surgical tools, will allow surgeons and learners to visualize, plan, coordinate, and practice in advance for improved outcomes to treat complex Mullerian, cloacal, and pelvic anomalies.
CONCLUSION
There is a significant window of opportunity to address the needs of pediatric, adolescent and adult gynecological patients with new innovative concepts and tools. Combining existing ideas and concepts will allow for streamlined approaches and drastic improvements in pediatric and adolescent patient care. Advancing the field of women’s health through applications of science, technology, and engineering to medicine will inevitably create new and exciting arenas for innovation in PAG.
KEY POINTS.
There are opportunities for concept, treatment, instrument and knowledge-transfer innovation to better serve the specific needs of pediatric gynecology patients.
Novel approaches to neovagina creation can involve use of magnets as an outpatient procedure.
Postoperative vaginal wound healing can be improved with novel and degradable vaginal stents.
Complex Mullerian reconstructive surgical planning can be enhanced through virtual reality immersive experiential training.
Acknowledgements
We would like to thank Drs Hector Martinez-Valdez and Monica Farenholtz for their assistance with this article.
Financial support and sponsorship
The novel pediatric and adolescent vaginal stents are supported in part through awards by the National Institutes of Health (1R43HD092156-01A1) and SouthWest Pediatric Device Consortium.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Hertweck P, Yoost J. Common problems in pediatric and adolescent gynecology. Expert Rev Obstet Gynecol 2010; 5:311–328. [Google Scholar]
- 2.Yordan EE, Yordan RA. Mini-review. The early historical roots of pediatric and adolescent gynecology. J Pediatr Adolesc Gynecol 1997; 10:183–191. [DOI] [PubMed] [Google Scholar]
- 3.■.Sack BS, Elizondo RA, Huang GO, et al. Pediatric medical device development by surgeons via capstone engineering design programs. J Pediatr Surg 2018; 53:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article reviews of the critical need for pediatric-specific medical devices. Describes a successful partnership between a children’s hospital and engineering schools to facilitate the pipeline for pediatric medical device development.
- 4.Raya-Rivera A, Esquiliano D, Fierro-Pastrana R, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 2014; 384:329–336. [DOI] [PubMed] [Google Scholar]
- 5.Emans SJ, Laufer MR, Goldstein DP. Pediatric and adolescent gynecology. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 6.Godbole P, Koyle M, Wilcox D, editors. Pediatric urology: surgical complications and management, 2nd ed. Wiley Blackwell; 2015. [Google Scholar]
- 7.Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006; 33:621–630. [DOI] [PubMed] [Google Scholar]
- 8.Fedele L, Bianchi S, Borruto F, et al. A new laparoscopic procedure for creation of a neovagina in Mayer–Rokitansky–Kuster–Hauser syndrome. Fertil Steril 1996; 66:854–857. [DOI] [PubMed] [Google Scholar]
- 9.Laufer MR. Congenital absence of the vagina: in search of the perfect solution. When, and by what technique, should avagina be created? Curr Opin Obstet Gynecol 2002; 14:441–444. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Li B, Li W, et al. Laparoscopic vaginal reconstruction using an ileal segment. Int J Gynecol Obstet 2009; 107:258–261. [DOI] [PubMed] [Google Scholar]
- 11.Schätz T, Huber J, Wenzl R. Creation of a neovagina according to Wharton–Sheares–George in patients with Mayer–Rokitansky–Küster–Hauser syndrome. Fertil Steril 2005; 83:437–441. [DOI] [PubMed] [Google Scholar]
- 12.Allen LM, Lucco KL, Brown CM, Spitzer RF. Psychosexual and functional outcomes after creation of a neovagina with laparoscopic Davydov in patients with vaginal agenesis. Fertil Steril 2010; 94:2272–2276. [DOI] [PubMed] [Google Scholar]
- 13.Rall K, Schickner MC, Barresi G, et al. Laparoscopically assisted neovaginoplasty in vaginal agenesis: a long-term outcome study in 240 patients. J Pediatr Adolesc Gynecol 2014; 27:379–385. [DOI] [PubMed] [Google Scholar]
- 14.■.Patel V, Hakim J, Gomez-Lobo V, Amies A. Provider’s experiences with vaginal dilator use for progressive perineal dilatation (PPD). J Pediatr Adolesc Gynecol 2018; 31:45–47. [DOI] [PubMed] [Google Scholar]; This is the first multicenter study on providers’ experiences using vaginal dilators for patients requiring neovagina creation. This article highlights the limitations of vaginal dilator use in patients and the need for education, training, and alternative modalities for outpatient neovagina creation.
- 15.Grimsby GM, Bradshaw K, Baker L. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol 2014; 123:947–950. [DOI] [PubMed] [Google Scholar]
- 16.Bradford A, Fellman B, Urbauer D, et al. Assessment of sexual activity and dysfunction in medically underserved women with gynecologic cancers. Gynecol Oncol 2015; 139:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler KG, Mohindra P, Kim AG-LV. Foley catheter as a vaginal stent in a toddler with vaginal rhabdomyosarcoma. J Pediatr Adolesc Gynecol 2018; 31:315–317. [DOI] [PubMed] [Google Scholar]
- 18.Adamson CD, Naik BJ, Lynch DJ. Ideas and innovations the vacuum expandable condom mold: a simple vaginal stent for mcindoe-style vaginoplasty. Plast Reconstr Surg 2004; 113:664–666. [DOI] [PubMed] [Google Scholar]
- 19.Ripperda CM, Maldonado PA, Acevedo JF, et al. Vaginal estrogen: a dual-edged sword in postoperative healing of the vaginal wall. Menopause 2017; 24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balgobin S, Montoya TI, Shi H, et al. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol Reprod 2013; 89:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montoya TI, Maldonado PA, Acevedo JF, Word RA. Effect of vaginal or systemic estrogen on dynamics of collagen assembly in the rat vaginal wall. Biol Reprod 2015; 92:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Press M, Nousek-Goebl N, Bur M, Greene G. Estrogen receptor localization in the female genital tract. Am J Pathol 1986; 123:280–292. [PMC free article] [PubMed] [Google Scholar]
- 23.Cotreau MM, Chennathukuzhi VM, Harris HA, et al. A study of 17B-estradiol-regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas 2007; 58:366–376. [DOI] [PubMed] [Google Scholar]
- 24.Karp D, Jean-Michel M, Johnston Y, et al. A randomized clinical trial of the impact of local estrogen on postoperative tissue quality after vaginal reconstructive surgery. Female Pelvic Med Reconstr Surg 2012; 18:211–215. [DOI] [PubMed] [Google Scholar]
- 25.Fink C: How Vr saves lives in the OR. Forbes 2017. Available from: www.forbes.com/sites/charliefink/2017/09/28/how-vr-saves-lives-in-the-or/#311e196f3099. [Accessed 3 May 2019] [Google Scholar]
- 26.Zaritzky M, Ben R, Johnston K. Magnetic gastrointestinal anastomosis in pediatric patients. J Pediatr Surg 2014; 49:1131–1137. [DOI] [PubMed] [Google Scholar]
- 27.Zaritzky M Cook Medical’s Flourish TM receives authorization for pediatric esophageal atresia. Cook Medical 2017; Available from: https://www.cookme-dical.com/newsroom/cook-medicals-flourish-receives-authorization-for-pediatric-esophageal-atresia/. [Accessed 3 May 2019] [Google Scholar]
- 28.TVAMedical: Transforming vascular access. Changing lives. Available from: https://tvamedical.com.
- 29.Hakim J NeoVagina creation with magnets. 2019.
- 30.Recalls and safety alerts: vaginal stent- inflatable (2015-10-17). Health Canada 2015. Available from: http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2015/55656r-eng.php. [Accessed 3 May 2019] [Google Scholar]
- 31.Hakim J, Dietrich JE, Smith PA, Buskmiller C. Vaginal stents, vaginal dilators, and methods of fabricating the same. United States US20180071502A1, filed April 4, 2016, and issued March 15, 2018.Available from: https://patents.google.com/patent/US20180071502A1/en?inventor=hakim+ju-lie&oq=hakim+julie. [Accessed 3 May 2019] [Google Scholar]
- 32.Haude M, Ince M, Kische S, et al. Safety and clinical performance of a drug eluting absorbable metal scaffold in the treatment of subjects with de novo lesions in native coronary arteries: Pooled 12-month outcomes of. Catheter Cardiovasc Interv 2018; 92:E502–E511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutau H, Musani AI, Laroumagne S, et al. Biodegradable airway stents – bench to bedside: a comprehensive review. Respiration 2015; 20:512–521. [DOI] [PubMed] [Google Scholar]
- 34.Al Lehibi A, Al Balkhi A, Al Mtawa A, Al Otaibi N. Endoscopic biodegradable stents as a rescue treatment in the management of post bariatric surgery leaks: a case series Authors Endoscopic stents used in this series Case presentation. Endosc Int Open 2018; 06:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barros A, Olivera C, Lima E, et al. Ureteral stents technology: biodegradable and drug-eluting perspective. In Comprehensive Biomaterials II, 7: 793–812. Amsterdam: Elsevier, 2017. [Google Scholar]
- 36.Htay T, Liu MW. Drug-eluting stent: a review and update. Vasc Health Risk Manag 2005; 1:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mcmahon S, Bertollo N, Cearbhaill EDO, et al. Progress in polymer science bio-resorbable polymer stents: a review of material progress and prospects. Prog Polym Sci 2018; 83:79–96. [Google Scholar]
- 38.Lyu S, Untereker D. Degradability of polymers for implantable biomedical devices. Int J Mol Sci 2009; 10:4033–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palaszewski DM, Miladinovic B, Caselnova PM, Holmstrom S. In-training impact of a pediatric and adolescent gynecology curriculum on an obstetrics and gynecology residency. J Pediatr Adolesc Gynecol 2016; 29:668–672. [DOI] [PubMed] [Google Scholar]
- 40.Virtual reality for architects. ArchDaily. Available from: https://www.archdaily.com/tag/virtual-reality-for-architects. [Accessed 3 May 2019]
- 41.Holley P How doctors used virtual reality to save the lives of conjoined twin sisters. Washington Post 2017. Available from: https://www.washington-post.com/news/innovations/wp/2017/07/21/how-doctors-used-virtualreality-to-save-the-lives-of-conjoined-twin-sisters/?utm_term=.eb35c20cd424. [Accessed 3 May 2019] [Google Scholar]
- 42.Medical virtual reality: work and collaborate on medical data in the virtual & augmented reality. Medical Holodeck 2019. Available from: https://www.medicalholodeck.com. [Accessed 3 May 2019] [Google Scholar]


