Abstract
Background:
COVID-19 outbreak in 2019 took the entire world by a storm with the medical fraternity struggling to understand and comprehend its complex nature. A number of patients who are COVID positive have reported oral lesions. However, there is still a lingering question, whether these lesions are because of coronavirus infection or they are secondary to the patient’s systemic condition. This article aims to report the oral findings of an observational study of 713 patients diagnosed with COVID-19.
Materials and Methods:
A singlssswe-institution, short-term observational study was conducted on patients admitted to Symbiosis University Hospital and Research Centre, Lavale, Pune who were positive to coronavirus, who presented varied oral findings such as herpes simplex, candidiasis, geographic tongue, and aphthous ulcer.
Results:
A total of 713 patients, 416 males and 297 females, who were positive to coronavirus, were screened from April 2020 to June 30, 2020, for oral ulcers. In this group, nine patients reported oral discomfort due to varied forms of oral lesions ranging from herpes simplex ulcers to angular cheilitis (1.26%).
Conclusion:
This study supports the hypothesis that oral manifestations in patients diagnosed with COVID-19 could be secondary lesions resulting from local irritants or from the deterioration of systemic health or could be just coexisting conditions. No specific pattern or characteristic oral lesions were noted in a study of 713 COVID-positive patients in our study to qualify these lesions as oral manifestations of SARS-CoV-2 infection.
Key Words: Candidiasis, COVID-19, glossitis, herpes simplex, oral ulcer
INTRODUCTION
The World Health Organization named “2019 novel coronavirus” on January 12, 2020 after its discovery in Wuhan, Hubei Province with the cluster of new types of viral pneumonia cases.[1] COVID-19 outbreak in 2019 took the entire world by a storm with the medical fraternity struggling to understand and comprehend its complex nature. Patients infected with the SARS-CoV-02 virus presented the symptoms such as fever, fatigue, dry cough, and dyspnea, with or without nasal congestion, runny nose, or other upper respiratory symptoms.[1,2,3] Other atypical symptoms including gastrointestinal symptoms such as nausea, diarrhea, vomiting, loss of smell (anosmia), or taste (ageusia) also add to the symptoms list recently reported among COVID-19.[4]
Dentists are at a higher risk of contracting this infection and also there is growing interest due to the route of transmission through aerosols produced by saliva during procedures.[5] The oral cavity is a mirror of health and disease. Many systemic diseases are accompanied by oral manifestations.[6] Oral mucosa is generally the first site affected by viral infections, acting as a natural barrier to infection.[7]
In April 2020, Chaux-Bodard et al.[8] reported a COVID-19-positive case with painful inflammation of lingual papilla and skin lesion. They proposed that study should be done with a larger sample size in the population. Soares et al.[9] reported a case of oral reddish lesions and ulceration that occurred in a 42-year-old male positive for SARS-CoV-2. The biopsy was performed on the buccal mucosa. The biopsied lesion had the epithelium with severe vacuolization and occasional exocytosis. They suggested that SARS-CoV-2 can cause oral lesions and all COVID-19 positive patients should undergo full mouth examination. Martín Carreras-Presas et al.[10] published a report of three cases, of which two were suspect and one confirmed case infected by the SARS-CoV-2 virus. They also suggested carrying out the intraoral examination in suspected as well as patients diagnosed with COVID-19 infection with all the necessary precautions for diagnosis and appropriate treatment for the oral lesions. Oral lesions can result from the systemic deterioration or local conditions or a combination of both.
This study was conducted to answer the research Query, whether Oral lesions are a manifestation of COVID or they occur because of systemic disease.
MATERIALS AND METHODS
An observational study was conducted in the period between May 2020 and June 2020. A total of 713 patients were included for this study, which comprised 416 males and 297 females among the patients admitted to the Symbiosis University Hospital and Research Centre IPD after being detected as corona positive infected with SARS-CoV-2 virus. Patients with comorbidities such as diabetes mellitus and hypertension as well as patients without comorbidities were screened for oral ulcers.
Inclusion criteria
All patients of both genders infected with SARS-CoV-2 virus, diagnosed on a real-time polymerase chain reaction test in the age group of 12–80 years, were admitted to the hospital.
Exclusion criteria
Patients of age <12 and more than 80. These patients were excluded as patient compliance in these age categories would be less favorable
Also patients not willing to give written informed consent. These patients were not pursued, as many of them felt it was a social stigma being tagged as COVID positive and not willing to participate in any form of the study
Seriously ill patients requiring intensive care were also excluded.
These patients presented with complaints ranging from mild fever, sore throat, to difficulty in breathing. Those patients who verbally complained about oral discomfort and pain were screened for oral lesions.
Methodology
The study consisted of two stages. Initially, all the patients were verbally questioned about any oral discomfort or pain. Stage two consisted of detailed questioning of the patients who had complaints of oral lesions or ulcers, limited examination, and photographing of the lesions whenever permissible/possible.
During Stage 1, the investigators wore face shield, N95 mask, surgical gowns as well as a double pair of gloves, patients were screened for initial eligibility based on inclusion and exclusion criteria as mentioned above. After identification of the patients with oral manifestations, Stage 2 was executed. Stage 2 was conducted, by physically recording the case history of patients on pen and paper after obtaining their informed consent and then photographing the oral lesion from a safe distance utilizing a mobile camera placed in a strip seal bag or zip-lock plastic bag. No other dental instruments were used such as mouth mirror, probe, or cheek retractors. Patients were made to retract their lips or cheek and the photographs were taken. The entire operation was done in the COVID isolation ward of the hospital, and these patients were not taken to the dental department or seated on a dental chair. No advanced lighting equipment or special cameras were used, due to the prevailing conditions. The photographs along with the case history were reviewed by an Oral Medicine, Diagnosis, and Radiology specialist.
In this study, there were many variables such as age, sex, educational status, dental hygiene status, habits, presence or absence of comorbidities, the severity of the comorbidity, and investigator observations. The dependent variable analyzed in this study was the presence or absence of oral manifestations in these patients.
This research was approved by the Independent Ethics Committee of the Symbiosis International Deemed University as under number SIU/IEC/154. The principles and considerations for the conduct of research involving humans were duly followed.
RESULTS
The study was based on the Research Query, “COVID patients and oral ulcers are dependent?” OR, “Covid patients and oral ulcers are independent?” Among 713 patients positive to coronavirus, who were screened for oral lesions, only nine patients had complaints (1.26%). This means that only 1.26% of the COVID patient presented with an oral lesion. Table 1 shows that patients were followed up till they were discharged from the COVID centre.
Table 1.
Distribution of oral lesions in COVID-positive patients
| Comorbidity | Number of males | Number of females |
|---|---|---|
| Comorbidity present | 3 | 4 |
| Comorbidity absent | 0 | 2 |
| Total | 3 | 6 |
Total nine patients with oral lesions
Description of lesion
Patient 1
A 58-year-old male with uncontrolled diabetes mellitus presented with ulcers on the left buccal mucosa corresponding to 35, 36, and 37 regions. Roughly 4 cm × 3 cm which were irregular in shape. The ulcer appears to have punched-out borders with tissue tags and is surrounded by inflammation. A similar ulcer was also present in the right buccal mucosa with 45, 46, and 47 [Figure 1].
Figure 1.
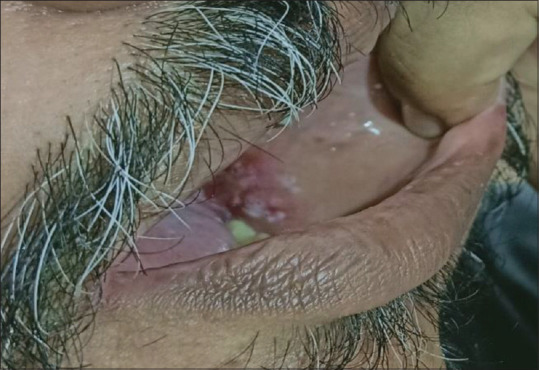
Patient number 1: Traumatic ulcer on the left buccal mucosa corresponding to 35, 36, and 37 regions, irregular in shape with punched-out borders with tissue tags.
Differential diagnosis: Traumatic ulcer secondary to cheek bite.
Patient 2
A 70-year-old female with uncontrolled diabetes mellitus presented with complaints of burning mouth. Generalized mucositis was seen on the lower labial mucosa extending from commissure to commissure (probably related to the anemic condition). Indentations were noted along anterior and lateral borders of the tongue corresponding to the gaps in her teeth, along with erythema of tongue margins. This could be correlated to her nutritional state, Hb, and her uncontrolled diabetes. Detailed questioning revealed that the patient had prior episodes of oral ulcers [Figure 2].
Figure 2.
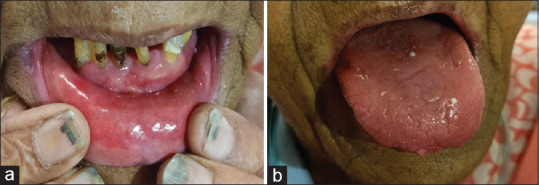
(a and b) Patient number 2: Generalized mucositis with indentations along anterior and lateral borders and erythema of tongue margins.
Differential diagnosis: Diabetic mucositis.
Patient 3
A 43-year-old male with a history of diabetes mellitus presented with solitary ulcer on both buccal mucosa. Dorsum of the tongue shows an area of papillary atrophy close to the junction of anterior two-third and posterior one-third of the tongue surrounded by an area of regenerating papillae. Bilateral angular cheilitis was noted. A solitary ulcer with ragged borders and tissue tags was present on both sides of mucosa corresponding to the sharp teeth margins. Intraorally poor oral hygiene was noted along with complete attrition of teeth [Figure 3].
Figure 3.
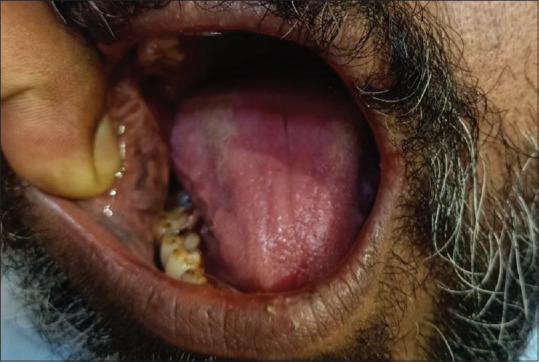
Patient number 3: Geographic tongue with traumatic ulcers on buccal mucosa and bilateral angular cheilitis.
Differential diagnosis: Geographic tongue (related to psychosomatic disorders) and traumatic ulcers.
Patient 4
A 50-year-old female with a history of hypertension and hyperthyroidism presented reddish-white spots on the palate. Red and white areas were seen irregularly scattered on the palate in the rugae region and the posterior hard palate. White areas appeared like flecks of candida pseudomembrane and the red areas appeared irregular where the pseudomembrane appeared to be scraped off.
Differential diagnosis: Acute/chronic pseudomembranous candidiasis (thrush).
Patient 5
A 65-year-old female with no relevant medical history presented with complaint of burning sensation on the tip of the tongue and inability to eat food. Mucositis on upper and lower labial mucosa in the anterior region with generalized papillary atrophy was observed. Ulcer could be related to nutritional deficiency. Patient refused retraction for taking clinical photographs.
Differential diagnosis: Geographic tongue and nutritional deficiency mucositis.
Patient 6
A 69-year-old male with a history of diabetes mellitus presented with lesions on the right side of the lower lip. Lower lip shows the presence of a solitary intact vesicle, roughly 1 cm by 1 cm along with an erosion corresponding to a burst vesicle with irregular margins. Furthermore, angular cheilitis was noted [Figure 4].
Figure 4.
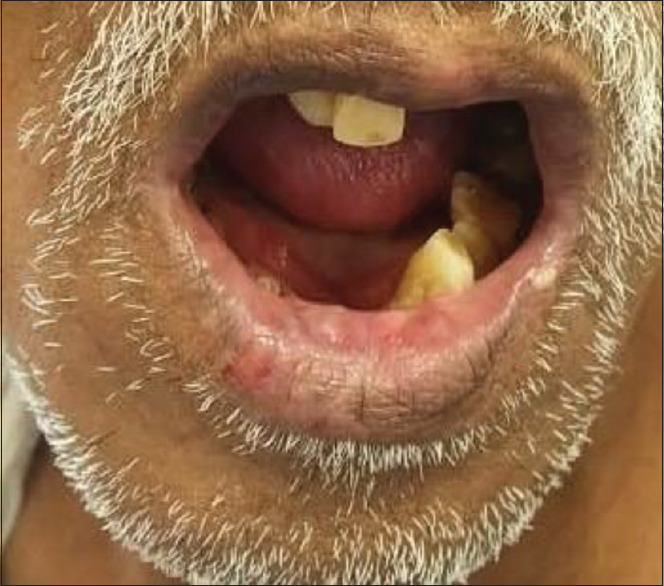
Patient number 6: Recurrent herpetic labialis and angular cheilitis.
Differential diagnosis: Recurrent herpetic labialis. Nutritional deficiency angular cheilitis.
Patient 7
A 50-year-old female with history of diabetes mellitus and hypertension presented with ulcers on her lower lip. Lower lip appears to be swollen. A necrotic area on the lower lip extending from the right lateral incisor to the left commissure. Whitish pseudomembrane along with neighboring erosive areas close to the midline along with bloody encrustations on the left side closer to the commissure [Figure 5].
Figure 5.
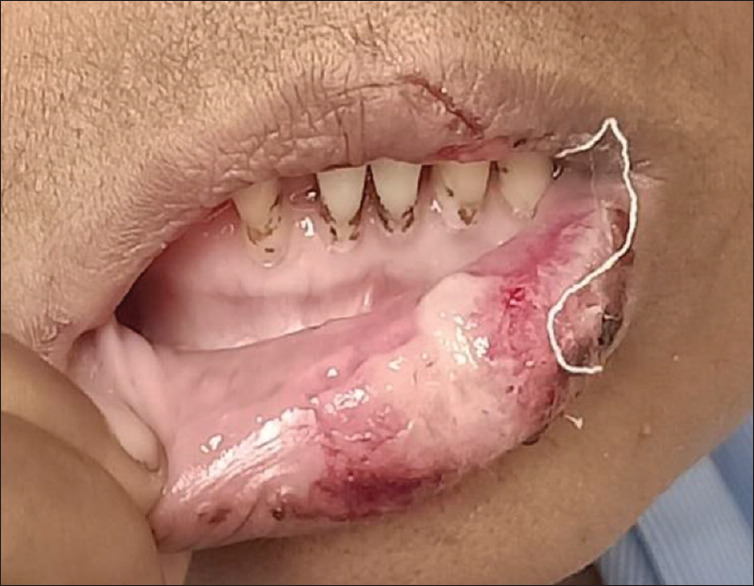
Patient number 7: Traumatic ulcer with bloody encrustations.
Differential diagnosis: Traumatic ulcer with bloody encrustations (history of intubation).
Patient 8
A 60-year-old female with no relevant medical history presented with complaint of ulcers on her mouth and inability to eat. The dorsal surface of the tongue shows generalized erythema with large areas showing complete papillary atrophy with small surrounding zones of papillary regeneration. Bilateral angular cheilitis was noted. Geographic tongue along with nutritional deficiency and xerostomia was observed. A lack of salivary pool in the mouth was noted [Figure 6].
Figure 6.
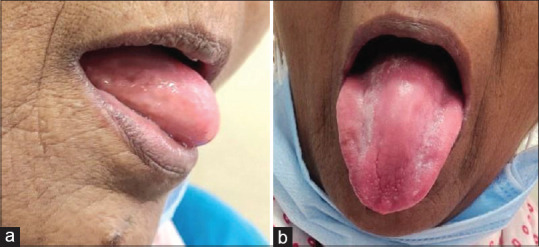
(a and b) Patient number 8: Bilateral angular cheilitis, ulceration secondary to nutritional deficiency, benign migratory glossitis.
Differential diagnosis: Ulceration secondary to nutritional deficiency/benign migratory glossitis.
Patient 9
A 50-year-old female patient with a history of diabetes mellitus presented with complaint of pin pricking sensation on the back of her throat. Examination revealed glazed inelastic tissue. Lip mucosa shows a generalized pallor with a marked lack of salivary pool and the patient complains of dysphagia.
Differential diagnosis: Mucositis secondary to anemia and xerostomia.
DISCUSSION
This research highlights the oral findings of patients infected with the SARS-CoV-02 virus. There have been reports in the literature regarding oral manifestations in COVID patients. Chaux-Bodard et al.[8] reported a case in April 2020 where the patient initially had painful inflammation of the papilla of the tongue, which ultimately healed as an asymptomatic ulcer in 10 days without a scar. This patient however had an erythematous skin lesion on the big toe on day 3. This patient tested positive on Day 8.
This led to the thought that this could probably be an inaugural symptom for COVID. Amorim Dos Santos et al.[11] reported a 67-year-old Caucasian male who tested positive to coronavirus and presented oral manifestations such as recurrent herpes simplex, candidiasis, and geographic tongue. Soares et al.[9] reported a 42-year-old patient, who tested positive for SARS-CoV-2 with a painful ulcer on the buccal mucosa. Oral examination showed that, besides the ulcerated lesion, multiple reddish macules of different sizes were scattered along the hard palate, tongue, and lips. They believed that SARS-CoV-2 can cause oral lesions. Martín Carreras-Presas et al.[10] reported three cases with ulcers, of which 1 was infected by the SARS-CoV-2 virus and two were suspected patients infected by the SARS-CoV-2 virus. The lesions resemble herpes simplex infection but were not confirmed by biopsies. They think that these may be COVID patients presenting with oral manifestations.
A total of 713 patients, 416 males and 297 females, who were positive to coronavirus were screened between April 2020 and June 30, 2020, for oral ulcers. In this population, nine patients reported oral discomfort due to varied forms of oral lesions ranging from herpes simplex ulcers to angular cheilitis.
All the patients were under institutional care. They were administered multivitamins and Vitamin C along with their regular medicines for their existing comorbidities, following prescribed treatment norms for the care of COVID-positive patients. The additional vitamin supplements[12] could be one of the reasons for having observed only nine patients with oral ulcers from a total of 713 patients. Being admitted in the wards, they were ensured a good diet, rest, and stress-free environment as they were away from work and other stress related to family or traveling. They were simultaneously being treated for their existing comorbidities too.
There were a total of only nine patients who complained of oral ulcers. There may have been other patients too who had ulcers, but since they did not complain of pain and did not report any discomfort on being verbally questioned, we can safely presume that their conditions were not that painful or significant.
Six patients who had ulcers were females and three were males. All the six females were in the age group >50. In this age group, women have generally attained menopause and have decreased secretions, frequently complaining of xerostomia. Such an environment is more conducive to the occurrence of oral ulcers. Furthermore, four of the females who had oral conditions had comorbidity of diabetes mellitus/hypertension, which again contributes to exaggeration of response to local irritants. Two of the female patients did not have any relevant medical history. Santosh et al.,[13] Shigli and Giri,[14] and Brahmankar[15] too in their studies have reported the various oral manifestations in menopausal women. Bajaj et al.[16] have elaborated on the oral complication in diabetic patients. Thus, similar results were reported by Santosh, Shigli, and Brahmankar as well as Bajaj in their study where oral manifestations were more common in menopausal women and patients having existing comorbidities such as diabetes.
In our study, the three male patients who had oral ulcers were diabetic. Furthermore, two of the ulcers were related to sharp teeth and could be classically called traumatic ulcers due to cheek biting. One male patient had classical herpes simplex ulcer.
All the patients were from a lower socioeconomic background. Their oral hygiene status was average to poor. The presentation of the lesions also did not have any characteristic distinctive features. They could be easily identified and diagnosed as known lesions of geographic tongue or mucositis rather than being identified as new or novel oral presentation of SARS CoV-2 virus infection. Other additional observations made in the study were male:female ratio of COVID-positive patients. It was observed that there were more male than female patients infected by the SARS-CoV-2 virus. This is in concurrence with the review article published by Gebhard et al.[17] In the review by Jiang et al., they reported a greater incidence of COVID-19 in older male patients with existing comorbidities as a result of weaker immune function.[18] However, the oral manifestations seen in the nine patients showed a higher incidence in female patients. This higher incidence in female patients was noted in the older age group (>50).[13,14,15] Some of the patients complained of alteration of taste, smell, and dry mouth. Among these nine patients, five reported alterations in smell, seven reported alterations in taste, and all nine patients reported decreased salivation. Similar findings were also reported by Yan et al.[4]
Oral ulcers may be manifestations of many systemic conditions or responses to local irritants. Even oral ulcers of viral origin have varied presentations and features as Van Heerden,[19] Schubert,[20] and many others have discussed. Thus, calling oral lesions in patients infected by the SARS CoV-2 virus as COVID ulcers is not justified. Zhang reported similar findings in a study of 140 hospitalized COVID-19 patients. Older age, the high number of comorbidities, and more prominent laboratory abnormalities were associated with older patients.[21] A much more detailed study of longer duration in larger sample size may give varied results and recommended.
CONCLUSION
COVID-19 patients present with varied forms of oral lesions ranging from small ulcers to large vesicles, bald tongue to geographic tongue. These lesions cannot be referred to as oral manifestations of COVID-19 infections. We support the argument that some oral conditions reported could be coincidental presentations due to local causes or other reasons, such as systemic conditions or due to stress. These lesions cannot be called as “COVID” ulcers. Among 713 patients positive to coronavirus, who were screened for oral lesions, only nine patients had complaints. This study supports the argument that oral manifestations could be secondary lesions resulting from the deterioration of systemic health, or due to treatments for COVID-19, or could be just coexisting conditions. No specific pattern or characteristic oral lesions were noted in a study of 713 COVID-positive patients.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10:806–13. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira LJ, Pereira CV, Murata RM, Pardi V, Pereira-Dourado SM. Biological and social aspects of Coronavirus Disease 2019 (COVID-19) related to oral health. Braz Oral Res. 2020;34:e041. doi: 10.1590/1807-3107bor-2020.vol34.0041. [DOI] [PubMed] [Google Scholar]
- 6.Mehrotra V, Devi P, Bhovi TV, Jyoti B. Mouth as a mirror of systemic diseases. Gomal J Med Sci. 2010;8:235–41. [Google Scholar]
- 7.Earar K, Arbune M, Schipor O, Dorobat CM, Stefanescu V, Gurau G, et al. Oral mucosa- Gate for covid-19 infection and correlation with chemical structures of the biocides. Revista de Chimie. 2020;71:410–5. [Google Scholar]
- 8.Chaux-Bodard AG, Deneuve S, Desoutter A. Oral manifestation of Covid-19 as an inaugural symptom? J Oral Med Oral Surg. 2020;26:18. [Google Scholar]
- 9.Soares CD, Carvalho RA, Carvalho KA, Carvalho MG, Almeida OP. Letter to Editor: Oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. 2020;25:e563–4. doi: 10.4317/medoral.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Diseases. 2020;00:1–3. doi: 10.1111/odi.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amorim Dos Santos J, Normando AG, Carvalho da Silva RL, De Paula RM, Cembranel AC, Santos-Silva AR, et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis. 2020;97:326–8. doi: 10.1016/j.ijid.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan A, McIntosh WB, Allam BF, Lamey PJ. Recurrent aphthous ulceration: Vitamin B1, B2 and B6 status and response to replacement therapy. J Oral Pathol Med. 1991;20:389–91. doi: 10.1111/j.1600-0714.1991.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 13.Santosh P, Nidhi S, Sumita K, Farzan R, Bharati D, Ashok K. Oral findings in postmenopausal women attending dental hospital in Western part of India. J Clin Exp Dent. 2013;5:e8–12. doi: 10.4317/jced.50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigli KA, Giri PA. Oral manifestations of menopause. J Basic Clin Reprod Sci. 2015;4:4–8. [Google Scholar]
- 15.Brahmankar U. Taos of late 50: A review on postmenopausal oral discomfort in women. SRM J Res Dent Sci. 2015;6:116–20. [Google Scholar]
- 16.Bajaj S, Prasad S, Gupta A, Singh VB. Oral manifestations in type-2 diabetes and related complications. Indian J Endocrinol Metab. 2012;16:777–9. doi: 10.4103/2230-8210.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–9. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Heerden WF. Oral manifestations of viral infections. SA Fam Pract. 2006;48:20–4. [Google Scholar]
- 20.Schubert MM. Oral manifestations of viral infections in immunocompromised patients. Curr Opin Dent. 1991;1:384–97. [PubMed] [Google Scholar]
- 21.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]


