Abstract
Thiazolidinediones (TZD) are peroxisome proliferator-activated receptor γ (PPARγ) agonists that may reduce hepatic steatosis through their effects in adipose tissue and therefore have been assessed as potential therapies to treat nonalcoholic fatty liver disease (NAFLD) in humans. However, some studies suggest that expression and activation of hepatocyte PPARγ promotes steatosis and that would limit the benefits of TZD as a NAFLD therapy. To further explore this possibility, we examined the impact of short-term rosiglitazone maleate treatment after the development of moderate or severe diet-induced obesity, in both control and adult-onset hepatocyte-specific PPARγ knockout (PpargΔHep) mice. Independent of the level of obesity and hepatic PPARγ expression, the TZD treatment enhanced insulin sensitivity, associated with an increase in white adipose tissue (WAT) fat accumulation, consistent with clinical observations. However, TZD treatment increased hepatic triglyceride content only in control mice with severe obesity. Under these conditions, PpargΔHep reduced diet-induced steatosis and prevented the steatogenic effects of short-term TZD treatment. In these mice, subcutaneous WAT was enlarged and associated with increased levels of adiponectin, while hepatic levels of phosphorylated adenosine 5′-monophosphate–activated protein kinase were also increased. In addition, in mice with severe obesity, the expression of hepatic Cd36, Cidea, Cidec, Fabp4, Fasn, and Scd-1 was increased by TZD in a PPARγ-dependent manner. Taken together, these results demonstrate that hepatocyte PPARγ expression offsets the antisteatogenic actions of TZD in mice with severe obesity. Therefore, in obese and insulin resistant humans, TZD-mediated activation of hepatocyte PPARγ may limit the therapeutic potential of TZD to treat NAFLD.
Keywords: thiazolidinediones, AAV8-TBGp-Cre, PPARγ knockout mouse model, obesity, insulin resistance
Nonalcoholic fatty liver disease (NAFLD) is the main cause of chronic liver disease, with an estimated prevalence of 25% in the general population (1). The main feature of NAFLD is hepatic steatosis, which is the accumulation of triglycerides (TG) in more than 5% of the parenchyma. Although steatosis is considered a benign condition, it can progress into nonalcoholic steatohepatitis (NASH), which is an advanced stage of NAFLD associated with liver injury, inflammation, and hepatocyte ballooning with or without fibrosis (2). Of note, the onset of steatosis and the subsequent progression to NASH is closely related with obesity and type 2 diabetes in humans. Since the rate of obesity continues increasing in the Western population, NAFLD cases are expected to rise and become a serious burden on our healthcare system (1,3).
While the etiology of NAFLD is multifactorial (2), chronic consumption of fat and carbohydrate-rich foods, and the lack of physical activity in individuals with obesity and type 2 diabetes can significantly contribute to the development of NAFLD. Therefore, lifestyle modifications to reduce caloric intake and increase physical activity, combined with drugs that reduce insulin resistance and improve peripheral metabolism are considered potential NAFLD therapies (4-6). Specifically, thiazolidinediones (TZD), a class of heterocyclic compounds that selectively activate peroxisome proliferator-activated receptor γ (PPARγ) and are approved to treat type 2 diabetes, may also be effective in treating NAFLD (7). PPARγ belongs to a family of nuclear receptors that are expressed in multiple tissues where they regulate different metabolic processes (8). There are 2 isoforms of PPARγ, PPARγ1 and PPARγ2. PPARγ1 has 30 amino acids less in the N-terminal domain than PPARγ2, but both isoforms have the same DNA binding domain, and both can be activated by rosiglitazone and pioglitazone (Food and Drug Administration–approved TZD to treat type 2 diabetes). The activation of PPARγ with TZD in muscle and adipose tissue improves insulin sensitivity, glucose disposal, and reduces the levels of lipids in the circulation (7-9). Based on these positive actions of TZD, long-term trials were conducted to examine the efficacy of TZD in NAFLD patients (5,6). Briefly, the meta-analysis of trials <24 months indicate that TZD reduce steatosis in NAFLD patients (10-12). However, the extension of the TZD treatment >24 months does not correlate with a progressive reduction of steatosis (5,13) and is speculated to be due to the “exhaustion” of the effects of TZD on insulin sensitivity (5). In general, although TZD have been used in different trials with promising effects, the limited efficacy, coupled with potential side effects, dampens the use of TZD to treat NAFLD.
The therapeutic effects of TZD to reduce steatosis may be offset by their actions on hepatocyte function. To date, the direct and indirect effects of TZD on the liver remain controversial (8,14,15). In fact, it remains to be determined if TZD affect liver steatosis directly by activating hepatocyte PPARγ in vivo in a model of established diet-induced obesity, which is an experimental condition that mimics NAFLD, insulin resistance, and obesity. This is a relevant question because the expression of hepatic PPARγ is increased in NAFLD patients (16,17), and in mice with diet-induced obesity (18,19). To better understand the specific role that hepatocyte PPARγ plays in the response to TZDs, we have used an adult-onset, hepatocyte-specific PPARγ knockout mouse model (PpargΔHep) to determine whether the direct effects of TZD on hepatocytes reduce their therapeutic effects on the liver, in a PPARγ-dependent fashion, after diet-induced obesity and insulin resistance are established.
Methods
Mouse Studies
These studies were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago. PPARγ floxed breeders in a C57Bl/6J background were originally purchased from Jackson Laboratories [Ppargfl/fl, Stock # 004584, Jackson Laboratories, Bar Harbor, ME, USA (20)] and maintained as a homozygous breeding colony, with standard chow diet and a 14-h light/10-h dark cycle (lights on at 6:00 am). At 4 to 6 weeks of age, a group of chow-fed Ppargfl/fl mice were switched to a low-fat diet (LFD) containing 10% kcal from fat (D12450H, D12450J, Research Diets, New Brunswick, NJ, USA) or a high-fat diet (HFD) containing 45% or 60% kcal from fat (D12451 (HFD 45%) or D12492 (HFD 60%) (Research Diets) and fed for 7 weeks. Another subgroup of Ppargfl/fl male mice were switched to the LFD or HFD 60% at 10 weeks of age and fed for 16 weeks. After this period of feeding, adult-onset hepatocyte-specific PPARγ knockout (PpargΔHep) mice were generated by injecting adeno-associated virus serotype 8 (AAV8) vectors that contain a thyroxine-binding globulin-driven (TBGp) Cre recombinase (AAV8-TBGp-Cre, Penn Vector Core, University of Pennsylvania, Philadelphia, PA, USA, and Addgene, Watertown, MA, USA) as previously described (19,21,22). Ppargfl/fl littermates were injected with AAV8-TBGp-null to generate control mice. One week later, half of the control and PpargΔHep mice fed a HFD were then switched to a HFD with the same percentage of kcal from fat and supplemented with 70 mg of rosiglitazone maleate/kg of diet (HFD/TZD, Cat# D1711502, D18061406, Research Diets) for 5 or 6 additional weeks. Rosiglitazone maleate was purchased from AdipoGen Life Sciences (San Diego, CA, USA). Diet composition is provided in Table 1.
Table 1.
Composition of the diets used in this study
| LFD | HFD (45% Kcal from fat), % | HFD/TZD (45% Kcal from fat), % | LFD | HFD (60% Kcal from fat), % | HFD/TZD (60% Kcal from fat), % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D12450H | D12451 | D1711502 | D12450J | D12492 | D18061406 | |||||||
| g | kcal | g | kcal | g | kcal | g | kcal | g | kcal | g | kcal | |
| Protein | 19 | 20 | 24 | 20 | 24 | 20 | 19 | 20 | 26 | 20 | 26 | 20 |
| Carbohydrate | 67 | 70 | 41 | 35 | 41 | 35 | 67 | 70 | 26 | 20 | 26 | 20 |
| Fat | 4 | 10 | 24 | 45 | 24 | 45 | 4 | 10 | 35 | 60 | 35 | 60 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | ||||||
| kcal/gm | 3.8 | 4.7 | 4.7 | 3.8 | 5.2 | 5.2 | ||||||
| Ingredients | ||||||||||||
| Casein | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 |
| L-cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 452.2 | 1809 | 72.8 | 291 | 72.8 | 291 | 506.2 | 2025 | 0 | 0 | 0 | 0 |
| Maltodextrin 10 | 75 | 300 | 100 | 400 | 100 | 400 | 125 | 500 | 125 | 500 | 125 | 500 |
| Sucrose | 172.8 | 691 | 172.8 | 691 | 172.8 | 691 | 68.8 | 275 | 68.8 | 275 | 68.8 | 275 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 |
| Soybean oil | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 |
| Lard | 20 | 180 | 177.5 | 1598 | 177.5 | 1598 | 20 | 180 | 245 | 2205 | 245 | 2205 |
| Mineral mix S10026 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 |
| DiCalcium phosphate | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate 1H2O | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 |
| Vit Mix V100001 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| Cholesterol | 0.054 | 0.167 | 0.167 | 0.054 | 0.216 | 0.216 | ||||||
| Rosiglitazone maleate | 0 | 0 | 0.0601 | 0 | 0 | 0.0542 | ||||||
| Total | 1055.05 | 4057 | 858.15 | 4057 | 858.21 | 4057 | 1055.05 | 4057 | 773.85 | 4057 | 773.85 | 4057 |
Abbreviations: HFD, high-fat diet; LFD, low-fat diet; TZD, thiazolidinedione.
One week after injection of AAV vectors in the groups of mice that were fed a LFD or HFD 60% for 16 weeks, whole body nuclear magnetic resonance–based fat and lean mass was measured with a minispec LF50 Body Composition Analyzer (Bruker, Billerica, MA, USA) every 2 weeks. In all groups of mice, an insulin tolerance test (ITT; 1-1.5 mU insulin ip/g) was performed 4 h after food withdrawal at 7:00 am, 5-6 weeks after injection of AAV vectors. Blood glucose was measured through lateral tail vein bleeds with a glucometer (Accu-check, Roche) over a 2-h timespan, as described previously (23). Mice were euthanized by decapitation, after a 4-h food withdrawal at 7:00 am, and blood was collected into EDTA-coated microtainers to obtain plasma after centrifugation. Tissues were weighed, fixed in formalin, or snap-frozen in liquid nitrogen for further analysis.
Plasma Endpoints
Plasma TG, nonesterified fatty acids, cholesterol, ketone bodies (Fujifilm Wako Diagnostics, Richmond, VA, USA), alanine transaminase (ALT) and aspartate aminotransferase (AST) (Pointe Scientific, Canton, MI, USA) were measured with colorimetric assays. Plasma insulin (Mercodia, Uppsala, Sweden) (24) and adiponectin (Abcam, Cambridge, MA) (25) were measured with commercial enzyme-linked immunosorbent assay kits. All kits were used according to the manufacturer’s instructions and measured using a Synergy H1 Hybrid plate Reader (BioTek, Winooski, VT, USA).
Liver TG Content
Small pieces of frozen livers were homogenized in isopropanol, incubated at room temperature while shaken for 45 min, followed by a 10-min centrifugation to collect cellular debris. The supernatant was then used to measure TG levels as described previously (26).
Hepatic Gene Expression
RNA was isolated with Trizol Reagent (Life Technologies, Carlsbad, CA, USA). Total RNA was treated with RNAse-free DNAse (Promega, Madison, WI, USA), and retrotranscribed to complimentary (cDNA) with First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). cDNA samples were used to measure gene expression using gene-specific primers provided in Table 2, and Brilliant III Ultra-Fast QPCR Master mix (Agilent Technologies, Santa Clara, CA, USA), as described previously (26).
Table 2.
Sequence of primers used for qPCR in this study
| Name | NM_ID | Sense | Antisense |
|---|---|---|---|
| Pparg | NM_001127330 | AGACCACTCGCATTCCTTTG | CCTGTTGTAGAGCTGGGTCTTT |
| Cd36 | NM_001159558 | GGAGCCATCTTTGAGCCTTC | TGGATCTTTGTAACCCCACAAG |
| Cidea | NM_007702 | GCAGCCTGCAGGAACTTATC | TCATGAAATGCGTGTTGTCC |
| Cidec | NM_178373 | AAGATGGCACAATCGTGGAG | TTAGTTGGCTTCTGGGAAAGG |
| Fabp4 | NM_024406 | AAGAAGTGGGAGTGGGCTTT | GCTCTTCACCTTCCTGTCGT |
| Mogat1 | NM_026713 | TCTGGTTCTGTTTCCCGTTG | ACATTGCCACCTCCATCCTT |
| Acc1 | NM_133360 | ATCCTGCGAACCTGGATTCT | CCCACCAGAGAAACCTCTCC |
| Fasn | NM_007988 | TGAGCACACTGCTGGTGAAC | CAGGTTCGGAATGCTATCCA |
| Scd1 | NM_009127 | ATCGCCCCTACGACAAGAAC | GTTGATGTGCCAGCGGTACT |
| Cpt1a | NM_013495 | ACAACAACGGCAGAGCAGAG | GGACACCACATAGAGGCAGAAG |
| Hmgsc2 | NM_008256 | TCAGGGGTCTAAAGCTGGAA | TAAGCCTGAGCCGTAGGAGA |
| Beta actin | NM_007393 | CTGGGACGACATGGAGAAGA | ACCAGAGGCATACAGGGACA |
| Peptidylprolyl isomerase A | NM_008907 | TGGTCTTTGGGAAGGTGAAAG | TGTCCACAGTCGGAAATGGT |
Abbreviation: qPCR, quantitative polymerase chain reaction.
Western Blots
Proteins were obtained from frozen livers, separated in 26-well Criterion TGX gels and transferred onto nitrocellulose membranes as previously described (21,23). The membranes were incubated overnight at 4°C with either adenosine monophosphate–activated protein kinase C (AMPK) #D5A2 (27) or phosphorylated AMPK T172 #D4D6D (28), and PPARγ #C26H12 (29) (Cell Signalling Technologies, Danvers, MA, USA) at a dilution 1/1000. Goat antirabbit immunoglobulin G (H+L)-horseradish peroxidase conjugate [#172-1019, 1/2000 for 1 h (30); Bio-Rad Laboratories, Hercules, CA, USA] was used as secondary antibody, and the specific signal was detected and quantified as previously reported (21,23).
Histology and Immunohistochemistry
The Research Histology Core at the University of Illinois at Chicago paraffin-embedded and stained (hematoxylin and eosin) 5-µm liver sections. The Human Tissue Resource Center of the University of Chicago paraffin-embedded and generated 5-µm subcutaneous adipose tissue sections. Deparaffined sections of the adipose tissue were blocked with 10% normal donkey serum in a humidity chamber for 20 min at room temperature and incubated with caveolin-1 antibody [#ab2910, 1:200, Abcam (31)] at 4°C. Then, the sections were incubated with 488 goat antirabbit [#4412S, 1:1000 (32); Cell Signaling Technologies] for 1 h. After multiple wash steps with phosphate buffered saline, slides were counterstained with 4′,6-diamidino-2-phenylindole and mounted with DAKO fluorescent mounting medium. Images of the stained sections were taken with a Leica DMi8 microscope with a DFC3000 (fluorescent images) or DMC5400 (hematoxylin and eosin) camera (Leica Microsystems, Wetzlar, Germany). To measure cell frequency and cell size of the subcutaneous adipose tissue (33), images were processed with Cellprofiler image analysis software [Broad Institute (34)].
Statistical Analysis
Data are represented as means ± standard error of the mean. Data were analyzed by 2-way analyses of variance followed by Tukey or Bonferroni post-hoc test. In each group, 2 different analysis were performed to test the effect of HFD and PpargΔHep between LFD- and HFD-fed mice and the effect of TZD and PpargΔHep in HFD-fed mice. The statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). In the tables and figures, daggers (†) and double daggers (‡) indicate significant differences between LFD- and HFD-fed control and PpargΔHep mice, respectively. Letters a to d indicate significant differences induced by TZD in HFD-fed mice. Asterisks (*) indicated significant differences induced by PpargΔHep. P-value < 0.05 is considered significant.
Results
The Obesogenic Effects of Rosiglitazone Are Dependent on the Level of Obesity and Independent of Hepatocyte PPARγ Expression in Male Mice
We tested whether TZD (rosiglitazone maleate) increased insulin sensitivity and obesity in a hepatocyte-specific PPARγ-dependent manner in different groups of diet-induced obese mice. In 2 groups of mice in which HFD feeding was started at 4 to 6 weeks of age, 7 weeks of HFD modestly increased body weight above LFD: 4 g (HFD 45%) and 3.1 g (HFD 60%). Whereas 10 weeks of HFD 60%, starting at 10 weeks of age, increased body weight above LFD by 11.25 g (Fig. 1A-1C). After these periods of HFD feeding, control and PpargΔHep mice were generated (Fig. 1A-1C, AAV arrows), and 1 week later half of the mice in each group were maintained on the HFD or switched to the HFD/TZD diet for an additional 5 to 6 weeks (Fig. 1A-1C, TZD arrows). PpargΔHep did not affect weight gain in all diet groups (Fig. 1D-1F). Consistent with the actions of TZD in humans (7), TZD had an overall obesogenic effect [Effect of TZD, P-value = 0.0024; HFD 45% Fig. 1D)], 0.033 [HFD 60% (Fig. 1E)], and <0.0001 [HFD 60% (Fig. 1F)]. Similarly, TZD increased subcutaneous fat weight [Effect of TZD, P-value = 0.001; HFD 45% (Fig. 1D)], 0.075 (HFD 60%) (Fig. 1E), and <0.0001 (HFD 60%) (Fig. 1F)] and brown adipose tissue (BAT) in all groups (Table 3). However, the most dramatic impact of TZD was observed in mice that were fed HFD 60% for 16 weeks before the TZD treatment (Fig. 1F). In this group, TZD had an overall significant impact on the sum of WAT (<0.0001) (Table 3) and increased nuclear magnetic resonance–based fat mass but not lean mass (Fig. 1G). The obesogenic effect of TZD may be due in part to an increase of food intake (not measured in this study) as previously reported in mice with established diet-induced obesity (35). Of note, PpargΔHep enhanced the impact of HFD/TZD on subcutaneous fat weight only in the group of mice in which HFD was started at 10 weeks of age (Fig. 1F), indicative of a stronger adipogenic effect of TZD on this group of mice.
Figure 1.
Loss of hepatocyte peroxisome proliferator-activated receptor γ (PPARγ) expression did not have a major effect in diet-induced obesity or thiazolidinedione (TZD)-mediated adiposity. Body weight curves of 4- to 6-week-old Ppargfl/fl mice were fed a HFD containing 45% kcal from fat (A) or 60% kcal from fat (B) for 7 weeks or a high-fat diet (HFD) containing 60% kcal from fat (C) for 16 weeks before being treated with adeno-associated virus (AAV; AAV arrow) to generate control (C) or PpargΔHep knockout (KO) mice. A week later, half of the mice fed a HFD in (A) and (B) were switched to a HFD/TZD for 5 weeks (TZD arrow), and mice in (C) were switched to a HFD/TZD for 6 weeks (TZD arrow). Body weight and subcutaneous fat (SC-fat) weight at the end of the study in mice fed a HFD containing 45% kcal from fat for 13 weeks (D), 60% kcal from fat for 13 weeks (E), or 60% kcal from fat for 23 weeks (F). Dotted lines in (D)-(F) show average weight of mice fed a HFD or HFD/TZD at the end of the study. Nuclear magnetic resonance–based fat mass and lean mass (G) in mice fed a HFD containing 60% kcal from fat. † and ‡ indicate significant differences induced by HFD as compared as low-fat diet (LFD)-fed mice within group: †differences in control (C) mice; ‡differences in KO mice. Letters a to d indicate significant differences induced by HFD/TZD as compared to HFD-fed mice within group. Asterisks indicate significant differences induced by PpargΔHep as compared to control mice within diet. a,†,‡ P < 0.05; **, P < 0.01; c, †††,‡‡‡ P < 0.001; d, ††††,‡‡‡‡, P < 0.0001. n = 6-10 mice/group.
Table 3.
Weight of white adipose tissue and brown adipose tissue in the different groups of mice used in this study
| LFD | HFD | HFD/TZD | ||||
|---|---|---|---|---|---|---|
| WAT (g) | Control | PpargΔHep | Control | PpargΔHep | Control | PpargΔHep |
| Cohort 1 (HFD 45%) | 1.50 ± 0.15 | 1.50 ± 0.16 | 2.59† ± 0.39 | 2.75‡‡ ± 0.19 | 2.71 ± 0.25 | 3.83 ± 0.51 |
| Cohort 2 (HFD 60%) | 2.07 ± 0.4 | 2.55 ± 0.35 | 4.95† ± 0.78 | 4.93‡ ± 0.7 | 6.16 ± 0.28 | 5.71 ± 0.82 |
| Cohort 3 (HFD 60%) | 1.88 ± 0.23 | 1.57 ± 0.23 | 4.73††† ± 0.22 | 5.01‡‡‡‡ ± 0.26 | 6.59c ± 0.22 | 7.33d ± 0.35 |
| BAT (g) | Control | Pparg ΔHep | Control | Pparg ΔHep | Control | Pparg ΔHep |
| Cohort 1 (HFD 45%) | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.18b ± 0.01 | 0.21d ± 0.01 |
| Cohort 2 (HFD 60%) | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.22 ± 0.03 | 0.21 ± 0.03 | 0.34 ± 0.04 | 0.39a ± 0.04 |
| Cohort 3 (HFD 60%) | 0.16 ± 0.02 | 0.15 ± 0.01 | 0.38†††† ± 0.03 | 0.345‡‡‡‡ ± 0.03 | 0.70b ± 0.05 | 0.92d ± 0.11 |
WAT is the sum of urogenital, mesenteric, perirenal and inguinal (subcutaneous) adipose tissue. Cohort 1: mice fed a LFD or HFD containing 45% kcal from fat for 13 weeks and half switched to HFD/TZD in the last 5 weeks. Cohort 2: mice fed a LFD or HFD 60% kcal from fat for 13 weeks and half switched to HFD/TZD in the last 5 weeks. Cohort 3: mice fed a LFD or HFD 60% kcal from fat for 23 weeks and half switched to HFD/TZD in the last 6 weeks. † and ‡ indicate significant differences induced by HFD as compared as LFD-fed mice within group: †Differences in control mice; ‡Differences in knockout mice. Letters a to d indicate significant differences induced by HFD/TZD as compared to HFD-fed mice within group. a,†,‡ P < 0.05; **, P < 0.01; c,†††,‡‡‡ P < 0.001; d,††††,‡‡‡‡, P < 0.0001 (n = 6-10 mice/group).
Abbreviations: BAT, brown adipose tissue; HFD, high-fat diet; LFD, low-fat diet; TZD, thiazolidinedione; WAT, white adipose tissue.
HFD impaired insulin sensitivity in all diet groups (Fig. 2). Consistent with the well-known actions of TZD as insulin sensitizers (7), there was an overall effect of HFD/TZD on the response to ITT and the levels of insulin (Fig. 2A, 2C, 2F, and 2H). However, there was minimal effects of TZD on basal blood glucose and plasma lipids in control mice (Table 4). Interestingly, the TZD-mediated reduction in plasma insulin in PpargΔHep mice, with prolonged 60% HFD feeding, was not associated with an improved response to the ITT (Fig. 2H, and 2I). This suggests that hepatocyte PPARγ may contribute to the insulin-sensitizing effects of TZD in obese mice. In fact, this negative effect of PpargΔHep on insulin sensitivity was previously reported in muscle and adipose tissue of ob/ob mice with hepatocyte PPARγ knockout (36).
Figure 2.
Loss of hepatocyte peroxisome proliferator-activated receptor γ (PPARγ) expression did not have a major effect in the insulin sensitizing effects of thiazolidinedione (TZD). Insulin tolerance tests (ITT) performed in control (C) or PpargΔHep knockout (KO) mice fed a high-fat diet (HFD) containing 45% kcal from fat (A), 60% kcal from fat (B) for 11 weeks, or a HFD containing 60% kcal from fat (C) for 22 weeks. Area under the curve (AUC) of the ITT and plasma insulin of mice fed a low-fat diet (LFD), a HFD containing 45% kcal from fat (D and E), 60% kcal from fat for 13 weeks (F and G), or a HFD containing 60% kcal from fat for 23 weeks (H and I). † and ‡ indicate significant differences induced by HFD as compared as LFD-fed mice within group: †differences in control (C) mice; ‡differences in KO mice. Letters a to d indicate significant differences induced by HFD/TZD as compared to HFD-fed mice within group. Asterisks indicate significant differences induced by PpargΔHep as compared to control mice within diet. *,a,†,‡ P < 0.05; b, P < 0.01; c,†††,‡‡‡ P < 0.001; ‡‡‡‡, P < 0.0001. (n = 6-10 mice/group).
Table 4.
Plasma endpoints analyzed in this study
| LFD | HFD | HFD/TZD | ||||
|---|---|---|---|---|---|---|
| Control | PpargΔHep | Control | PpargΔHep | Control | PpargΔHep | |
| Glucose, mg/dL | ||||||
| Cohort 1 (HFD 45%) | 192.0 ± 9.8 | 172.0 ± 17.0 | 185.5 ± 11.1 | 188.5 ± 8.0 | 158.3 ± 8.9 | 180.3 ± 10.1 |
| Cohort 2 (HFD 60%) | 181.3 ± 2.7 | 194.7 ± 10.4 | 213.5 ± 18.0 | 194.7 ± 8.4 | 202.1 ± 3.2 | 204.4 ± 4.6 |
| Cohort 3 (HFD 60%) | 149.1 ± 9.6 | 141.2 ± 11.6 | 189.8† ± 6.5 | 177.2‡ ± 7.8 | 159.8a ± 5.3 | 159.1 ± 7.6 |
| NEFA, mEq/L | ||||||
| Cohort 1 (HFD 45%) | 1.26 ± 0.08 | 1.17 ± 0.09 | 0.98 ± 0.15 | 0.98 ± 0.05 | 0.95 ± 0.14 | 0.79 ± 0.06 |
| Cohort 2 (HFD 60%) | 0.83 ± 0.04 | 0.86 ± 0.07 | 0.51†† ± 0.05 | 0.49‡‡‡ ± 0.04 | 0.41 ± 0.01 | 0.46 ± 0.03 |
| Cohort 3 (HFD 60%) | 0.82 ± 0.09 | 0.71 ± 0.07 | 0.60 ± 0.04 | 0.51 ± 0.03 | 0.63 ± 0.08 | 0.71 ± 0.07 |
| TG, mg/dL | ||||||
| Cohort 1 (HFD 45%) | 25.8 ± 2.4 | 40.1 ± 4.9 | 21.6† ± 3.2 | 29.4 ± 4.7 | 29.6 ± 4.3 | 26.9 ± 0.9 |
| Cohort 2 (HFD 60%) | 37.8 ± 6.0 | 35.9 ± 3.3 | 21.4 ± 2.9 | 33.6 ± 2.7 | 22.0 ± 2.0 | 28.0 ± 2.1 |
| Cohort 3 (HFD 60%) | 44.6 ± 7.0 | 47.2 ± 4.8 | 38.8 ± 4.4 | 38.9 ± 3.9 | 28.3 ± 2.8 | 39.2 ± 4.1 |
| Cholesterol, mg/dL | ||||||
| Cohort 1 (HFD 45%) | 87.1 ± 4.0 | 71.1 ± 11.4 | 111.8 ± 12.8 | 106.6 ± 8.4 | 83.5 ± 10.6 | 88.5 ± 5.7 |
| Cohort 2 (HFD 60%) | 102.8 ± 16.4 | 117.4 ± 10.5 | 152.0 ± 14.9 | 139.3 ± 7.0 | 137.2a ± 5.3 | 90.9* ± 11.9 |
| Cohort 3 (HFD 60%) | 177.1 ± 29.1 | 254.2 ± 33.4 | 202.3 ± 17.8 | 257.5 ± 20.5 | 193.8 ± 20.6 | 198.2 ± 17.0 |
Cohort 1: Mice fed a LFD or HFD containing 45% kcal from fat for 13 weeks and half switched to HFD/TZD in the last 5 weeks. Cohort 2: mice fed a LFD or HFD 60% kcal from Fat for 13 weeks and half switched to HFD/TZD in the last 5 weeks. Cohort 3: mice fed a LFD or HFD 60% kcal from fat for 23 weeks and half switched to HFD/TZD in the last 6 weeks. † and ‡ indicate significant differences induced by HFD as compared as LFD-fed mice within group: †differences in control mice; ‡differences in knockout mice. Letters a to d indicate significant differences induced by HFD/TZD as compared to HFD-fed mice within group. asterisks indicate significant differences induced by PpargΔHep as compared to control mice within diet. a,†,‡ P < 0.05; **, P < 0.01; c,†††,‡‡‡ P < 0.001; d,††††,‡‡‡‡, P < 0.0001 (n = 6-10 mice/group).
Abbreviations: HFD, high-fat diet; LFD, low-fat diet; NEFA, nonesterified fatty acids; TG, triglycerides; TZD, thiazolidinedione.
Expression of Hepatocyte PPARγ Serves to Enhance Liver Steatosis in Severely Obese Mice Treated With Rosiglitazone for 6 Weeks
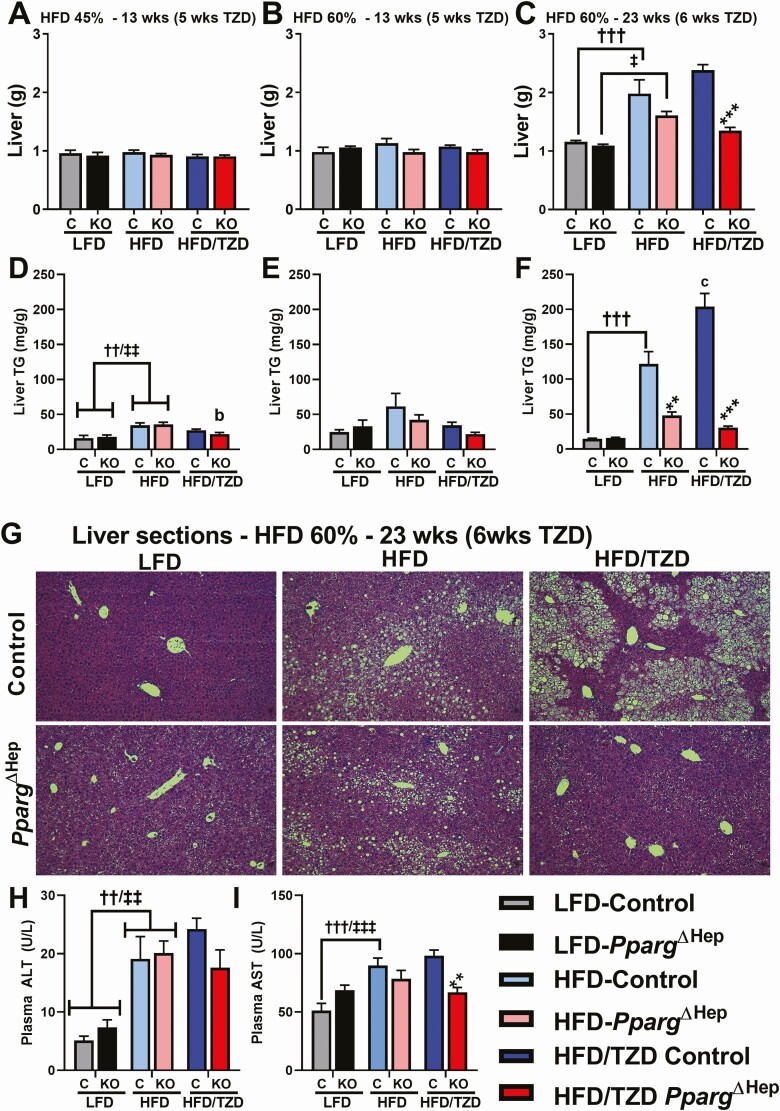
A HFD did not increase liver weight in young mice after 13 weeks of feeding, but it increased liver weight in adult mice after 23 weeks of feeding (Fig. 3A-3C). Of note, HFD 45% modestly increased hepatic TG content in young mice, which was reduced by TZD only in PpargΔHep mice (Fig. 3D and 3E). Interestingly, the increase in liver weight induced by prolonged HFD 60% was associated with a dramatic increase in liver TG content, which was even further enhanced by the TZD treatment (Fig. 3F). The loss of hepatocyte PPARγ expression in this group (mice fed a HFD 60% for 23 weeks) reduced liver weight and TG content (Fig. 3C and 3F), as reflected by representative micrographs of hematoxylin and eosin–stained liver sections (Fig. 3G). Also in this diet group, we measured circulating markers of liver injury: ALT and AST. Although these markers were increased in control mice by HFD, this TZD treatment did not reduce ALT or AST in control mice (Fig. 3H and 3I). However, TZD did reduce AST levels in PpargΔHep mice (Fig. 3I).
Figure 3.
Loss of hepatocyte peroxisome proliferator-activated receptor γ (PPARγ) expression reduces liver weight and steatosis in obese mice treated with rosiglitazone. Liver weight of control (C) or PpargΔHep knockout (KO) mice fed a high-fat diet (HFD) containing 45% kcal from fat (A), 60% kcal from fat (B) for 13 weeks, or a HFD containing 60% kcal from fat (C) for 23 weeks. Liver triglycerides (TG) of control (C) or PpargΔHep (KO) mice fed a HFD containing 45% kcal from fat (D), 60% kcal from fat (E) for 13 weeks, or a HFD containing 60% kcal from fat (F) for 23 weeks. Representative pictures of hematoxylin and eosin-stained liver sections (G), and plasma alanine transaminase (ALT) (H) and aspartate aminotransferase (AST) (I) levels of mice fed a HFD for 23 weeks. † and ‡indicate significant differences induced by HFD as compared as low-fat diet (LFD)-fed mice within group: †differences in control (C) mice; ‡differences in KO mice. Letters a to d indicate significant differences induced by HFD/thiazolidinediones (TZD) as compared to HFD-fed mice within group. Asterisks indicate significant differences induced by PpargΔHep as compared to control mice within diet. *,‡, P < 0.05; **,b,††,‡‡ P < 0.01; ***,c,†††,‡‡‡ P < 0.001 (n = 8-9 mice/group).
PPARγ is a transcription factor known to regulate the expression of genes associated with lipid metabolism and the development of lipid droplets in hepatocytes (37). Consistent with previous reports (18), prolonged HFD 60% feeding in PPARγ-intact control mice dramatically increased hepatic PPARγ messenger RNA levels, compared to control LFD-fed mice (Fig. 4A) while 6 weeks of HFD/TZD did not increase PPARγ expression further. Since the polymerase chain reaction primers used could not differentiate between the PPARγ isoforms, western blot analysis was performed and revealed the PPARγ2 isoform was undetectable in LFD-fed control mice but dramatically increase in response to HFD, while TZD had no further stimulatory actions (Fig. 4B). In contrast, the PPARγ1 isoform was detectable in LFD-fed control mice and was reduced by HFD feeding. Since PPARγ2 was completely eliminated in PpargΔHep mice, but PPARγ1 expression remained low but detectable, we can conclude PPARγ2 is the primary isoform expressed in the hepatocyte and responsive to HFD feeding, while PPARγ1 isoform is largely expressed in nonparenchymal cells (Fig. 4B). Interestingly, PpargΔHep reduced HFD-induced expression of the PPARγ target genes related to fatty acid uptake and processing: fatty acid translocase (Cd36), cell death activator A and C (Cidea and Cidec), fatty acid–binding protein 4 (Fabp4), and monoacylglycerol O-acyltransferase 1 (Mogat1) (Fig. 4C). Also, PpargΔHep blocked the positive effect of TZD on the expression of Cd36, Cidea, Cidec, and Fabp4, indicating that TZD directly activates these steatogenic factors and contributes to the increase of hepatic TG levels through the direct activation of hepatocyte PPARγ. Since steatosis can also be promoted by enhanced de novo lipogenesis (DNL) and/or reduced fatty acid oxidation, we examined the expression levels of genes key in these processes. The expression of genes critical for DNL—acetyl-CoA carboxylase (Acc1), fatty acid synthase (Fasn), and stearoyl-CoA desaturase (Scd1)—was not upregulated by HFD (Fig. 4D), consistent with a previous report showing long-term HFD feeding reduces DNL (38). However, TZD did increase the expression of Fasn and Scd-1 in a hepatocyte PPARγ-dependent manner. This suggests that TZD-mediated activation of PPARγ in hepatocytes could increase DNL in obese mice and contribute to increased steatosis. The expression of genes critical for fatty acid oxidation—carnitine palmitoyltransferase 1A (Cpt1a) and 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2)—was not significantly altered by HFD or TZD. However, there was an overall positive effect (P < 0.0001) of PpargΔHep on the expression of hepatic Cpt1a, which was significantly increased in HFD fed mice (Fig. 4E). This result suggests that enhanced fatty acid oxidation in PpargΔHep mice could contribute to reduce steatosis.
Figure 4.
Expression of hepatic genes in mice fed a high-fat diet (HFD) for 23 weeks. (A and B) Hepatic expression of peroxisome proliferator-activated receptor γ (PPARγ) and the isoforms PPARγ1 and 2. (C) Hepatic expression of PPARγ-target genes: Cd36, Cidea, Cidec, Fabp4, and Mogat1. (D) Hepatic expression of de novo lipogenesis (DNL) genes: Acc1, Fasn, and Scd1. (E) Hepatic expression of genes involved in fatty acid oxidation: Cpt1a and Hmgsc2. † indicates significant differences induced by HFD as compared as low-fat diet (LFD)-fed control mice. Letters a to d indicate significant differences induced by HFD/thiazolidinediones (TZD as compared to HFD-fed mice within group. Asterisks indicate significant differences induced by PpargΔHep as compared to control mice within diet. a,*,† P < 0.05; b,**,†† P < 0.01; c,***,††† P < 0.001; ****,††††, P < 0.0001 [n = 3-4 mice/group in (B); n = 8-9 mice/group in (A, C-E).
Loss of Hepatocyte PPARγ Increased Phosphorylation of Hepatic AMPK and Enhanced the Effects of Rosiglitazone in Adipose Tissue
We previously reported that phosphorylation of AMPK was reduced in mice with NASH but restored by PpargΔHep (21). In the current study, PpargΔHep increased the hepatic levels of phosphorylated (activated) AMPK in HFD-fed mice in the absence or presence of TZD (Fig. 5A and 5B). Phosphorylated AMPK (activated) increases fatty acid oxidation and reduces DNL (39); the increased activity of AMPK in PpargΔHep mice may explain the reduction of steatosis observed in these groups (Fig. 3F). Plasma ketones are markers of hepatic fatty acid oxidation and they were not increased in PpargΔHep mice under basal (not fasted) conditions. Interestingly, TZD reduced plasma ketones (Fig. 5C) and increased the expression of lipogenic genes Fasn and Scd1 (Fig. 4C) in obese control mice, which may be indicative of reduced AMPK activity due to activation of PPARγ in hepatocytes of control mice.
Figure 5.
Loss of hepatocyte peroxisome proliferator-activated receptor γ (PPARγ) expression is associated with increased hepatic phosphorylated adenosine 5′-monophosphate–activated protein kinase (pAMPK) and adiponectin levels in mice fed a high-fat diet (HFD) for 23 weeks. (A) Western blots of AMPK (adenosine 5′-monophosphate–activated protein kinase; top) and pAMPK (bottom). Ponceau S stained membranes were used to normalize AMPK and pAMPK levels. (B) Hepatic pAMPK/AMPK ratio. (C) Plasma ketone levels. (D) Plasma adiponectin levels. (E) Relative frequency of the size of adipocytes of subcutaneous adipose tissue. Letters a to d indicate significant differences induced by HFD/TZD as compared to HFD-fed mice within group. Asterisks indicate significant differences induced by PpargΔHep as compared to control mice within diet. a,* P < 0.05; b, P < 0.01; d, P < 0.0001 (n = 3-9 mice/group).
Adiponectin is a key adipokine that activates hepatic AMPK to reduce TG content in the liver (40). Plasma adiponectin levels are increased with TZD treatments (41). The short-term and low dose of TZD used in this study increased plasma adiponectin in obese mice, but that rise was more pronounced in PpargΔHep mice (Fig. 5D). Interestingly, this effect was associated with an increase in adipocyte size (Fig. 5E) and adipose tissue mass (Fig. 1F), suggesting that the adipogenic effects of TZD were increased in PpargΔHep mice and that could contribute to reduce hepatic TG via adiponectin/AMPK pathway. Taken together, the expression of PPARγ in hepatocytes of control mice may suppress AMPK-mediated regulation of hepatic metabolism and contribute to sustain liver steatosis, as well as alter the effects of TZD on adipose tissue. However, this latter effect requires further investigation.
Discussion
To date, the hepatocyte-specific actions of TZD in NAFLD are not completely understood and remain controversial because clinical reports show variable effects (8,14,15). Some studies suggest that PPARγ, the primary effector of TZD, is not significantly expressed in the human liver and TZD should reduce NAFLD by increasing insulin sensitivity in peripheral tissues without activating PPARγ in hepatocytes (14). However, the expression of PPARγ is increased in liver biopsies of NAFLD patients and in the steatotic livers of diet-induced obese mice (16-19). Similarly, hepatic PPARγ expression is increased in mice with leptin deficiency (ob/ob mice), lipodystrophy (A-ZIP mice), and diet-induced obesity that have steatosis (36,42,43). In these preclinical models, TZD treatment further increases hepatic TG content, leading the authors to conclude that the steatogenic actions of TZD were due to its direct actions on PPARγ in liver hepatocytes (36,42). These direct actions are supported by the fact that hepatic PPARγ2 is increased by HFD (44) in a hepatocyte-specific manner, TZD promotes lipid accumulation and enhances PPARγ-target gene expression, indicative of lipid uptake and storage, in isolated mouse hepatocytes (21). Therefore, although TZD increases insulin sensitivity in peripheral tissues in patients with NAFLD, TZD-mediated activation of hepatocyte PPARγ reduces the positive effect of increased insulin sensitivity in NAFLD. This may represent a critical limitation for the use of TZD as potential drugs to treat NAFLD patients.
To further study the interrelationship between TZD and PPARγ on hepatocyte function, our group used a mouse model with inducible hepatocyte-specific knockout of PPARγ: PpargΔHep. This mouse model allows us to avoid the confounding effects of congenital loss of hepatocyte PPARγ (albumin-driven Cre recombinase-mediated inactivation of PPARγ) and to assess the specific contribution of TZD-mediated activation of hepatocyte PPARγ after the liver has developed NAFLD. Also, we have used diet-induced obese mice, which is a more translational model than A-ZIP or ob/ob mice, to study the development and/or regression of NAFLD associated with diet-induced insulin resistance. It should be noted that in the current study, the effect of HFD paradigm on liver steatosis and obesity was dependent of the age at which diets were introduced: 5 to 6 weeks of age (peripubertal) vs 10 to 12 weeks of age (postpubertal). In fact, we previously reported that HFD-induced metabolic dysfunction was less severe in mice in which HFD was started at peripubertal age, compared to HFD started in adult mice (10-12 weeks), and was attributed to higher levels of Insulin-like growth factor 1 and lean mass (45). Also, 14 weeks of HFD 60% initiated in adult mice (19) induced similar steatosis and obesity as the prolonged (23 weeks) HFD 60% of this study. Therefore, in the current study, comparing the impact of hepatocyte PPARγ loss and TZD treatment after different diet regimens show for the first time that the deleterious effects of activating hepatocyte PPARγ are dependent on the severity of diet-induced obesity/steatosis.
Our data complement previous work showing short-term, low-dose TZD treatment enhances insulin sensitivity in mice with moderate and severe obesity. However, under severe obesogenic conditions induced only by prolonged HFD 60%, TZD promotes steatosis in a hepatocyte-specific PPARγ-dependent fashion. These findings further support the hypothesis that the direct actions of TZD in hepatocytes reduce their potential as effective therapies to treat NAFLD, and the potential negative actions of TZD/PPARγ on hepatocyte function should be weighted in the design of therapies for NAFLD. Based on our overall findings, our main question was how hepatocyte PPARγ sustains and TZD enhances established diet-induced steatosis to contribute to the development of NAFLD.
Hepatocyte PPARγ is strongly associated with DNL and steatosis in multiple models (37,46). However, in this and previous reports (19,47), adult-onset loss of hepatocyte PPARγ had minimal impact on expression of DNL genes. Nonetheless, TZD enhanced the expression of a subset of DNL genes (Fasn and Scd1) in PPARγ-intact mice despite the reduction in insulin levels (direct insulin-sensitizing effect of TZD). Furthermore, our study neither challenged the mice to increase hepatic DNL nor measured the rate of DNL in vivo, and so hepatocyte PPARγ may contribute to sustain DNL and promote steatosis in the obese mice of this study. Hepatocyte PPARγ also promotes fatty acid uptake and re-esterification. We and others reported that hepatocyte PPARγ directly increases hepatic Cd36 and Mogat1 expression, among other genes, to increase steatosis in diet-induced obese mice (19,48). Overexpression of PPARγ in the liver increases hepatic Cd36, Cidea, Cidec, Fabp4, and Mogat1, among other genes, to increase fatty acid uptake and storage in hepatocytes (37,48,49) and is correlated with steatosis in male and female mice (50). Loss of hepatocyte PPARγ expression reduced the expression of these genes and steatosis, while TZD upregulated them in a hepatocyte PPARγ-dependent manner to promote steatosis despite the improvement of insulin sensitivity. Therefore, our results indicate that fatty acid uptake and storage are key mechanisms regulated by PPARγ in hepatocytes, which sustains steatosis in obese mice and is enhanced with TZD treatments.
Hepatic DNL and fatty acid uptake are key mechanisms that promote NAFLD in humans. The direct effects of TZD on DNL and fatty acid uptake in hepatocytes of obese individuals may reduce the therapeutic benefits of TZD on NAFLD. Our results are in agreement with other studies that show the steatogenic effect of TZD treatments on diet-induced obese mice (49), and diabetic KKAy obese mice (51), as well as those in AZIP and ob/ob mice (36,42). However, these results are in stark contrast with the antisteatogenic effect of TZD in humans (5,6), and lean mice (49). Here, it should be mentioned lifestyle modifications adopted by humans treated with TZD could contribute to the different hepatic responses (steatogenic vs antisteatogenic) of TZD in humans and preclinical models. In humans, TZD are provided along with reduced caloric intake and increased physical activity, whereas in mice TZD are provided with HFD ad libitum. Also, the different hepatic responses to TZD treatments may be due to the degree of obesity and the cell-specific level of PPARγ expression: PPARγ2 in hepatocytes (16-19,43,44). In this sense, mouse studies showed that loss of hepatocyte PPARγ expression (21,52) or the constitutive deacetylation of PPARγ (53) enhanced the antisteatogenic properties of TZD in HFD-fed obese mice. Therefore, if TZD cannot promote DNL and fatty acid uptake in hepatocytes (as occurs in PpargΔHep mice), the insulin-sensitizing effects of TZD in peripheral tissues will reduce liver steatosis efficiently. The lack of steatogenic effects of TZD in the liver of PpargΔHep mice is consistent with RNAseq results from our group as well as others, showing rosiglitazone and pioglitazone upregulates gene ontology terms related to fatty acid metabolism in control mice but not in hepatocyte-specific PPARγ knockout mice (GEO: GSE162276, GSE140607) (21,52). Therefore, despite the fact that TZD improves insulin sensitivity, the collective work of ourselves and others suggest that the therapeutic potential of TZD in treating NAFLD patients should be considered with caution due to its ability to activate hepatocyte PPARγ and exacerbate steatosis.
In conclusion, our results support that hepatocyte PPARγ is an important regulator of steatosis in a translational mouse model of NAFLD with diet-induced obesity and insulin resistance, and HFD- and TZD-mediated activation of hepatocyte PPARγ regulates similar mechanisms that contribute to increase the storage of fat in hepatocytes in vivo. Importantly, our study provides additional evidence related to the design of the HFD feeding paradigm selected to induce obesity and to assess the effect of TZD on liver physiology.
Acknowledgments
We thank Dr. Gregory Norris and Danielle Pins for their technical assistance. AAV vectors were obtained from Penn Vector Core in the Gene Therapy Program of the University of Pennsylvania. We thank the Research Histology Core at the University of Illinois at Chicago established with the support of the Vice Chancellor of Research and the University of Chicago Human Tissue Resource Center for their assistance.
Financial Support : National Institutes of Health K01DK115525, Early Career Development Award of the Central Society for Clinical and Translational Research, and UIC start-up funds (J.C.C.), Comunidad de Madrid-Talento Grant 2018-T1/BMD-11966 (A.D.R.), Latin@s Gaining Access to Networks for Advancement in Science Research Fellowship HSI-STEM grant #P031C160237 from USA ED, 2020 Endocrine Society Summer Research Fellowship (J.M.), and VA Merit BX004448 (R.D.K.).
Additional Information
Disclosures: Authors do not have any conflict of interest.
Data Availability
Data and analysis generated in this study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Younossi ZM. Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol. 2019;70(3):531-544. [DOI] [PubMed] [Google Scholar]
- 2. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Younossi ZM, Tampi RP, Racila A, et al. Economic and clinical burden of nonalcoholic steatohepatitis in patients with type 2 diabetes in the U.S. Diabetes Care. 2020;43(2):283-289. [DOI] [PubMed] [Google Scholar]
- 4. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149(2):367-378.e5; quiz e314-365. [DOI] [PubMed] [Google Scholar]
- 5. Ratziu V, Charlotte F, Bernhardt C, et al. ; LIDO Study Group . Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51(2):445-453. [DOI] [PubMed] [Google Scholar]
- 6. Sanyal AJ, Chalasani N, Kowdley KV, et al. ; NASH CRN . Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmadian M, Suh JM, Hah N, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azzu V, Vacca M, Virtue S, Allison M, Vidal-Puig A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology 2020;158(7):1899-1912. [DOI] [PubMed] [Google Scholar]
- 10. Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He L, Liu X, Wang L, Yang Z. Thiazolidinediones for nonalcoholic steatohepatitis: a meta-analysis of randomized clinical trials. Medicine (Baltimore). 2016;95(42):e4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177(5):633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165(5):305-315. [DOI] [PubMed] [Google Scholar]
- 14. Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127(4):1202-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finck BN. Targeting Metabolism, Insulin Resistance, and Diabetes to Treat Nonalcoholic Steatohepatitis. Diabetes. 2018;67(12):2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lima-Cabello E, García-Mediavilla MV, Miquilena-Colina ME, et al. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond). 2011;120(6):239-250. [DOI] [PubMed] [Google Scholar]
- 17. Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96(5):1424-1430. [DOI] [PubMed] [Google Scholar]
- 18. Inoue M, Ohtake T, Motomura W, et al. Increased expression of PPARγ in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336(1):215-222. [DOI] [PubMed] [Google Scholar]
- 19. Wolf Greenstein A, Majumdar N, Yang P, Subbaiah PV, Kineman RD, Cordoba-Chacon J. Hepatocyte-specific, PPARγ-regulated mechanisms to promote steatosis in adult mice. J Endocrinol. 2017;232(1):107-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712-15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee SM, Pusec CM, Norris GH, et al. Hepatocyte-specific loss of PPARγ protects mice from NASH and increases the therapeutic effects of rosiglitazone in the liver. Cell Mol Gastroenterol Hepatol. 2021;11(5):1291-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cordoba-Chacon J. Loss of hepatocyte-specific PPARγ expression ameliorates early events of steatohepatitis in mice fed the methionine and choline-deficient diet. PPAR Res. 2020;2020:9735083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cordoba-Chacon J, Majumdar N, List EO, et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015;64(9):3093-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. RRID:AB_2783837. https://antibodyregistry.org/search.php?q=AB_2783837. [Google Scholar]
- 25. RRID:AB_2891131. https://antibodyregistry.org/search.php?q=AB_2891131. [Google Scholar]
- 26. Cordoba-Chacon J, Gahete MD, McGuinness OP, Kineman RD. Differential impact of selective GH deficiency and endogenous GH excess on insulin-mediated actions in muscle and liver of male mice. Am J Physiol Endocrinol Metab. 2014;307(10):E928-E934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. RRID:AB_10622186. https://antibodyregistry.org/search.php?q=AB_10622186. [Google Scholar]
- 28. RRID:AB_2799368. https://antibodyregistry.org/search?q=AB_2799368. [Google Scholar]
- 29. RRID:AB_2166051. https://antibodyregistry.org/search?q=AB_2166051. [Google Scholar]
- 30. RRID:AB_11125143. https://antibodyregistry.org/search?q=AB_11125143. [Google Scholar]
- 31. RRID:AB_303405. https://antibodyregistry.org/search?q=AB_303405. [Google Scholar]
- 32. RRID:AB_1904025. https://antibodyregistry.org/search?q=AB_1904025. [Google Scholar]
- 33. Liew CW, Boucher J, Cheong JK, et al. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat Med. 2013;19(2):217-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones TR, Kang IH, Wheeler DB, et al. CellProfiler Analyst: data exploration and analysis software for complex image-based screens. BMC Bioinformatics. 2008;9:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu M, Sarruf DA, Talukdar S, et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17(5):618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsusue K, Haluzik M, Lambert G, et al. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111(5):737-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARγ1) overexpression. J Biol Chem. 2003;278(1): 498-505. [DOI] [PubMed] [Google Scholar]
- 38. Duarte JA, Carvalho F, Pearson M, et al. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J Lipid Res. 2014;55(12):2541-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia D, Hellberg K, Chaix A, et al. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep 2019;26:192-208.e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288-1295. [DOI] [PubMed] [Google Scholar]
- 41. Combs TP, Wagner JA, Berger J, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARγ agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143(3):998-1007. [DOI] [PubMed] [Google Scholar]
- 42. Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278(36):34268-34276. [DOI] [PubMed] [Google Scholar]
- 43. Morán-Salvador E, López-Parra M, García-Alonso V, et al. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. Faseb J. 2011;25(8):2538-2550. [DOI] [PubMed] [Google Scholar]
- 44. Vidal-Puig A, Jimenez-Liñan M, Lowell BB, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97(11):2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cordoba-Chacon J, Gahete MD, Pozo-Salas AI, et al. Peripubertal-onset but not adult-onset obesity increases IGF-I and drives development of lean mass, which may lessen the metabolic impairment in adult obesity. Am J Physiol Endocrinol Metab. 2012;303(9):E1151-E1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang YL, Hernandez-Ono A, Siri P, et al. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem. 2006;281(49):37603-37615. [DOI] [PubMed] [Google Scholar]
- 47. Kineman RD, Majumdar N, Subbaiah PV, Cordoba-Chacon J. Hepatic PPARγ is not essential for the rapid development of steatosis after loss of hepatic GH signaling, in adult male mice. Endocrinology. 2016;157(5):1728-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee YJ, Ko EH, Kim JE, et al. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc Natl Acad Sci U S A. 2012;109(34):13656-13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gao M, Ma Y, Alsaggar M, Liu D. Dual outcomes of rosiglitazone treatment on fatty liver. AAPS J. 2016;18(4):1023-1031. [DOI] [PubMed] [Google Scholar]
- 50. de Conti A, Tryndyak V, Willett RA, et al. Characterization of the variability in the extent of nonalcoholic fatty liver induced by a high-fat diet in the genetically diverse Collaborative Cross mouse model. FASEB J. 2020;34(6):7773-7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng J, Huan Y, Jiang Q, Sun SJ, Jia CM, Shen ZF. Effects and potential mechanisms of pioglitazone on lipid metabolism in obese diabetic KKAy mice. PPAR Res. 2014;2014:538183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kulkarni S, Huang J, Tycksen E, Cliften PF, Rudnick DA. Diet modifies pioglitazone’s influence on hepatic PPARγ-regulated mitochondrial gene expression. PPAR Res. 2020;2020:3817573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kraakman MJ, Liu Q, Postigo-Fernandez J, et al. PPARγ deacetylation dissociates thiazolidinedione’s metabolic benefits from its adverse effects. J Clin Invest. 2018;128(6):2600-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and analysis generated in this study are not publicly available but are available from the corresponding author on reasonable request.