Abstract
Background:
Huachansu injection (HCS) is a widely used traditional Chinese medicine for advanced non-small cell lung cancer (NSCLC) to alleviate the adverse drug reactions (ADRs) and enhance the clinical efficacy of chemotherapy.
Objective:
To evaluate the efficacy and safety of HCS as an adjunctive treatment to platinum-based chemotherapy (PBC) for advanced NSCLC.
Methods:
A systematic review and meta-analysis were conducted according to PRISMA guidelines. A total of nine databases were searched to select randomized controlled trials (RCTs) of HCS plus PBC to treat NSCLC from inception to October 10, 2020. RCTs on HCS plus PBC vs PBC alone for advanced NSCLC were included. Dichotomous data were pooled as risk ratio (RR) with 95% confidence intervals. RCTs compared to HCS plus PBC vs PBC alone were included. Primary outcomes were objective response rate (ORR) and disease control rate (DCR), and secondary outcomes were survival rate, quality of life (QOL), and adverse drug reactions (ADRs). GRADE software was used to access the quality of evidence.
Results:
A total of 32 RCTs, including 2753 patients, were included. Compared to PBC alone, HCS plus PBC improved the ORR, DCR, 1- and 2-year survival rates, and QOL and alleviated neutropenia, thrombocytopenia, nausea, vomiting, anemia, liver injury, renal injury, and alopecia.
Conclusions:
Compared to PBC alone, HCS plus PBC improved the clinical efficacy and alleviated the ADRs in advanced NSCLC patients. Considering the limitations of the included RCTs, high-quality trials with longer follow-ups are needed to further confirm the results.
Keywords: huachansu injection, meta-analysis, non-small cell lung cancer, platinum-based chemotherapy, systematic review
1. Introduction
Lung cancer accounts for 11.6% of all diagnosed cancers and is the leading cause of 18.4% of overall cancer-related mortalities, with 5% as the 5-year survival rate.[1] Lung cancer is of two major types: non-small cell lung cancer (NSCLC) that accounts for approximately 85%, and small cell lung cancer (SCLC) that accounts for 15% of all lung cancers.[2] Therefore, advanced progression and metastasis NSCLC patients are not suitable for surgery and have to receive chemotherapy, radiotherapy, or chemoradiotherapy that significantly improves the clinical efficacy.[3–6] However, patients receiving chemoradiotherapy often suffer from multiple toxic effects and adverse drug reactions (ADRs), such as neutropenia, neurotoxicity, hepatorenal dysfunction, and other toxicities.[7–9] All these ADRs lead to poor survival and affect the quality of life (QOL) in patients.[10,11] Hence, the development of novel treatment strategies to improve tumor responses and reduce the risk of ADRs is a salient issue.
Platinum-based chemotherapy (PBC) is the standard first-line therapy for advanced NSCLC,[12] which consists of several types of platinum regimens, such as cisplatin, carboplatin, or oxaliplatin and several types of chemotherapy regimens, such as paclitaxel, docetaxel, vinorelbine, or gemcitabine.[13–15] PBC doublet chemotherapy prolongs the median survival by approximately 1.5–2 months compared to single-agent PBC or other monotherapy in patients with NSCLC.[16–18]
Traditional Chinese medicine (TCM) combined with PBC has been widely used in China to treat advanced NSCLC. Previous meta-analyses[19–21] published in Chinese, have assessed the efficacy and safety, but no definitive conclusions were reached. Also, the dosage and optimal concentration for combination with PBC to achieve the best efficacy and safety of the treatment are yet to be determined. Huachansu injection (HCS) is a traditional Chinese medicine extract, which has a long-term adjuvant effect on chemotherapy in patients with NSCLC.[19–21] HCS is an injectable form of the sterilized hot-water extract of the skin glands of B. gargarizans,[19] and the vital bioactive compounds of B. gargarizan include cinobufagin, resibufogenin, bufotenine, cinobufotenine, and serotonin.[21] HCS was injected intravenously with 10–20 ml once a day. The treatment time per cycle was 7–28 days and treatment cycles were one to four cycles. Some clinical trials that evaluated the efficacy and safety of HCS combined with PBC for advanced NSCLC have been published. Therefore, the efficacy and safety of HCS plus PBC for patients with advanced NSCLC are yet to be clarified. Herein, we performed this systematic review and meta-analysis to provide evidence for the efficacy and safety of HCS plus PBC for the treatment of advanced NSCLC.
2. Materials and methods
We implemented this systematic review and meta-analysis following the Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) guidelines.[22] The protocol was pre-registered on the International Prospective Register of Systematic Reviews (PROSPERO): CRD42020212821. The study was approved by the ethics institutional review board of the People's Hospital of Guangxi Zhuang Autonomous Region.
2.1. Search strategy
PubMed, Medline (via OVID SP), Embase (via OVID SP), Cochrane Central Register of Controlled Trials (CENTRAL), Nursing and Allied Health Literature (CINAHL), China National Knowledge Infrastructure (CNKI), China Biological Medicine Database (CBM), Chinese Scientific Journals Full-Text Database (VIP), and Wanfang databases were searched systematically from their inception until October 10, 2020. The specific search strategies of Pubmed database are shown in Item S1 Supplemental Digital Content (see Item S1, which illustrates the specific search strategies of Pubmed database). No language restrictions were imposed.
2.2. Inclusion criteria
Participants, interventions, control/comparisons, outcomes, and study (PICOS) strategy was used to guide the researchers on the selection of randomized controlled trials (RCTs) for this meta-analysis. All studies were required to fulfill the following inclusion criteria:
-
1.
participants: Patients with advanced NSCLC and age ≥ 18 years. Patients should meet all of the following criteria: cytological or pathological examination diagnosis of NSCLC; at least one bidimensional measurable lesion; stage III/IV; Karnofsky performance status (KPS) scale ≥ 60;[23] life expectancy ≥ 3 months; not received chemotherapy, radiotherapy, or surgery recently.
-
2.
interventions and comparisons: HCS combined with PBC; intervention in the control group: PBC alone and different types of PBC regimens are eligible.
-
3.
outcome: the primary outcomes were objective response rate (ORR) and disease control rate (DCR), while the secondary outcomes were survival rate, QOL, and ADRs;
-
4.
study design: RCTs.
2.3. Exclusion criteria
The studies that the following criteria were excluded from this meta-analysis:
-
1.
non-RCTs, reviews, meta-analysis, non-clinical studies, case reports, meeting abstracts, and observations studies;
-
2.
duplicated studies;
-
3.
incomplete, incorrect, or unavailable data, or the study did not provide any primary or secondary outcomes;
-
4.
treatment combined with any other herbs;
-
5.
inappropriate outcome reports.
2.4. Selection of studies
Two researchers (Liang and Xi) independently searched the databases comprehensively. Then, the duplicate records were deleted separately, and the eligible articles were screened and selected by reading the titles, abstracts, and necessary assessment of the full-text of the studies. The references of previous reviews and retrieved articles were comprehensively checked to identify additional eligible studies. Discrepancies were resolved by consensus.
2.5. Data collection and quality assessment
Two reviewers (Liang and Xi) independently extracted the data and the study information, such as age, gender, KPS, and sample size; intervention protocol of HCS, such as dosage, frequency, and course; concurrent PBC regimens; primary and secondary outcomes. The third reviewer examined the consistency of the extracted data. The methodological quality of the included studies was assessed by Xi and Liang using the Cochrane risk-of-bias tool.[24]
2.6. Statistical analysis
The statistical analysis of data was performed in Review Manager 5.4.0. (Cochrane Collaboration Software).[24] Dichotomous outcomes were shown as risk ratios (RR) with 95% confidence intervals (CI). Considering the potential heterogeneity between the trials, I2 was used to quantitate the heterogeneity. I2≥ 50% or P ≤ .05 indicated a high statistical heterogeneity among trials. If no heterogeneity was observed (I2 < 50% or P > .05), the fixed-effects model was used, otherwise the data were evaluated using random-effects model. Subgroup analyses were performed based on the usage of HCS, different types of PBC regimens, and evaluation criteria of primary outcomes. Sensitivity analysis was performed by a leave-one-out analysis[25] to observe the magnitude of influence of each study on the pooled RR. The significance level for this meta-analysis model was P value less than 0.05.
To objectively measure the presence of publication bias, a Harbord test was performed.[26] Duval and Tweedie trim-and-fill methods[27] were used to evaluate the publication/reporting biases visually. In order to assess the confidence of the evidence and determine whether additional studies are required for sufficient conclusion, we conducted the trial sequential analysis (TSA) to guarantee against false positive (type I) or false negative (type II) errors. Two reviewers (Liang and Xi) independently evaluated the reliability of the evidence related to each outcome using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE).[28] The quality of the evidence was graded at the four following levels: high, moderate, low, and very low.
3. Results
3.1. Study identification and selection
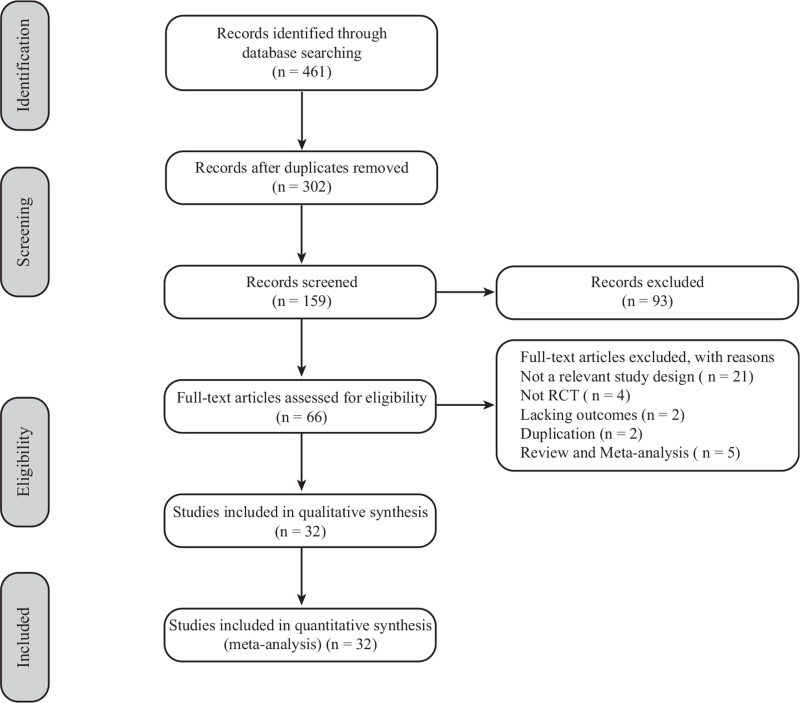
A total of 461 studies were retrieved by literature search, and 302 duplicate articles were excluded. Then, 159 studies remained for further analysis. After evaluation of the titles and abstracts, 93 irrelevant articles were excluded. After reading the remaining 66 full-text papers, 34 studies were excluded for the following reasons: studies with an irrelevant study design (n = 21), non-RCTs (n = 4), lacking outcomes (n = 2), duplication of published articles (n = 2), and review and meta-analysis (n = 4). Finally, 32 studies were included in the current meta-analysis. Figure 1 showed the PRISMA schematic.
Figure 1.
Flow diagram of study selection.
3.2. Characteristics of included studies
The main characteristics of the included studies are described in Table 1. Overall, 32 trials encompassing 2753 patients were included in the present meta-analysis. Patients received PBC as the pemetrexed plus cisplatin (AP, 1 trial), docetaxel plus carboplatin (DC, 1 trial), docetaxel plus cisplatin (DP, 4 trials), etoposide plus cisplatin (EP, 1 trial), gemcitabine plus cisplatin (GP, 6 trials), vinorelbine plus cisplatin (NP, 10 trials), paclitaxel plus carboplatin (TC, 1 trial), and paclitaxel plus cisplatin (TP, 8 trials). A total of 17 trials assessed the tumor responses based on the World Health Organization (WHO) guidelines,[29] and 11 trials evaluated the same according to the response evaluation criteria in solid tumors (RECIST).[30] Next, 8 trials assessed the ADRs based on WHO criteria, and 2 trials evaluated them based on Common Toxicity Criteria for Adverse Events.
Table 1.
Characteristics of the included trials.
| NSCLC(III-IV) | Intervention and control protocol | ||||||
| First author, year | Sample size (M/F) | E/C | Age | Experimental | Control | Dose/Days/Cycles | Outcomes (evaluation criteria) |
| Bian et al 2015 | 63 (33/30) | 32/31 | 25–56 | HCS + GP | GP | 20 ml × 21d × 2 | ORR (RECIST), DCR (RECIST), QOL, ADRs (NR) |
| Bao et al 2011 | 93 | 45/48 | 54 (34–76) | HCS + GP | GP | 20 ml × 15d × 2 | ORR (WHO), DCR (WHO), QOL, ADRs (WHO) |
| Cao 2009 | 50 (28/22) | 25/25 | 58 (40–75) | HCS + NP | NP | 20 ml × 21d × 3 | ORR (WHO), DCR (WHO), Survival rate |
| Cao et al 2016 | 80 (49/31) | 40/40 | 57.41 ± 9.04 | HCS + DP | DP | 10–20 ml × 28d × 1 | ORR (RECIST), DCR (RECIST), Survival rate, ADRs (NR) |
| Chen 2016 | 90 (50/40) | 45/45 | 59.75 (39–76) | HCS + GP | GP | 20 ml × 28d × 2 | ORR (RECIST), DCR (RECIST), Survival rate, ADRs (WHO) |
| Chi et al 2019 | 98 (54/44) | 49/49 | 54.5 ± 10 | HCS + DP | DP | 10–20 ml × 28d × 3 | ORR (RECIST), DCR (RECIST), QOL, ADRs (CTCAE v4.0) |
| Deng 2018 | 68 (43/25) | 34/34 | 53.23 ± 7.05 | HCS + TC | TC | 20 ml × 21d × 2 | ORR (NR), DCR (NR) |
| Dong 2013 | 86 (47/39) | 46 /40 | 46-69 | HCS + AP | AP | 30 ml × 21d × 4 | ORR (RECIST), DCR (RECIST), QOL |
| Hao et al 2016 | 92 (64/28) | 42/50 | 58.13 ± 9.05 | HCS + TP | TP | 20 ml × 5d × 3 | ORR (WHO), ADRs (NR) |
| Hu 2012 | 74 (43/31) | 36/38 | NR | HCS + TP | TP | 20 ml × 14d × 4–6 | ORR (RECIST), DCR (RECIST), Survival rate, QOL, ADRs (WHO) |
| Huang 2009 | 62 (35/27) | 32/30 | 61.5 (49–74) | HCS + GP | GP | 20 ml × 21d × 2 | ORR (WHO), DCR (WHO) |
| Ji et al 2017 | 98 (45/53) | 49/49 | 54.2 (25–75) | HCS + DC | DC | 20 ml × 14d × 4 | ORR (RECIST), DCR (RECIST), QOL, ADRs (CTCAE v4.0) |
| Jin 2007 | 60 (42/18) | 32/28 | 65 (52–77) | HCS + NP | NP | 20 ml × 28d × 2 | ORR (WHO), DCR (WHO), QOL, ADRs (NR) |
| Lan 2017 | 96 (51/45) | 48/48 | 43-73 | HCS + TP | TP | 20 ml × 28d × 2 | ORR (NR), DCR (NR), ADRs (NR) |
| Li 2015 | 60 (29/31) | 30/30 | 36-55 | HCS + TP | TP | 20 ml × 21d × 2 | ORR (WHO), DCR (WHO) |
| Li et al 2009 | 62 | 30/32 | 34-76 | HCS + NP | NP | 20 ml × 10–15d × 2 | ORR (WHO), DCR (WHO), QOL, ADRs (WHO) |
| Liu et al 2007 | 62 (42/20) | 32/30 | 49.7 (33–68) | HCS + NP | NP | 20 ml × 21–28d × 2 | ORR (WHO), DCR (WHO), ADRs (NR) |
| Lu and Lu 2015 | 62 (37/25) | 31/31 | 57 (41–71) | HCS + NP | NP | 20 ml × 10d × 2 | ORR (RECIST), DCR (RECIST) |
| Ma and Lu 2011 | 217 (114/103) | 109/108 | 45.8 (40–73) | HCS + GP | GP | 20 ml × 28d × 3 | ORR (RECIST), DCR (RECIST), Survival rate, ADRs (NR) |
| Miao et al 2007 | 87 (50/37) | 43/44 | 53.5 (34–74) | HCS + NP | NP | 20 ml × 5d × 3–6 | ORR (WHO), DCR (WHO), QOL, ADRs (WHO) |
| Qiao et al 2006 | 120 (87/33) | 60/60 | 69.5 (60–76) | HCS + NP | NP | 20 ml × 28d × 1 | ORR (RECIST), DCR (RECIST), Survival rate, QOL, ADRs (NR) |
| Wang 2006 | 60 (42/18) | 30/30 | 59.5 (38–72) | HCS + TP | TP | 20 ml × 28d × 2 | ORR (WHO), ADRs (WHO) |
| Wang and Shu 2009 | 120 (67/53) | 60/60 | 58.5 (37–77) | HCS + TP | TP | 20 ml × 21d × 2 | ORR (WHO), DCR (WHO), QOL |
| Wang et al 2018 | 77 (43/34) | 38/39 | 68 | HCS + TP | TP | 20 ml × 28d × 2 | Survival rate, QOL, ADRs (WHO), |
| Xiong and Li 2005 | 62 (42/20) | 32/30 | 49.7 (33–68) | HCS + NP | NP | 20 ml × 21–28d × 2 | ORR (WHO), DCR (WHO), ADRs (WHO) |
| Yang and Sun 2016 | 44 | 32/12 | 67.98 (61–81) | HCS + GP | GP | 20 ml × 8d × 4 | ORR (WHO), DCR (WHO) |
| Yang and Xi 2006 | 60 (38/22) | 30/30 | 52 (35–69) | HCS + NP | NP | 15 ml × 21d × 2 | ORR (WHO), DCR (WHO), QOL, ADRs (NR) |
| Yao et al 2018 | 200 (121/79) | 100/100 | 34-75 | HCS + DP | DP | 20 ml × 5d × 3 | ORR (NR), DCR (NR), Survival rate |
| Ying and Hong 2018 | 120 (83/37) | 60/60 | 63 (45–75) | HCS + EP | EP | 20 ml × 28d × 3 | ORR (RECIST), DCR (RECIST) |
| Yu et al 2012 | 64 (39/25) | 32/32 | 62 (49–71) | HCS + DP | DP | 20 ml × 28d × 2 | ORR (WHO), DCR (WHO), QOL, ADRs (NR) |
| Zhang et al 2001 | 72 (49/23) | 37/35 | 50 (20–74) | HCS + NP | NP | 20–30 ml × 28d × 3 | ORR (WHO), DCR (WHO), QOL, Survival rate |
| Zhou 2014 | 94 (49/45) | 47/47 | 59-82 | HCS + TP | TP | 20 ml × 14d × 3 | ORR (WHO), DCR (WHO) |
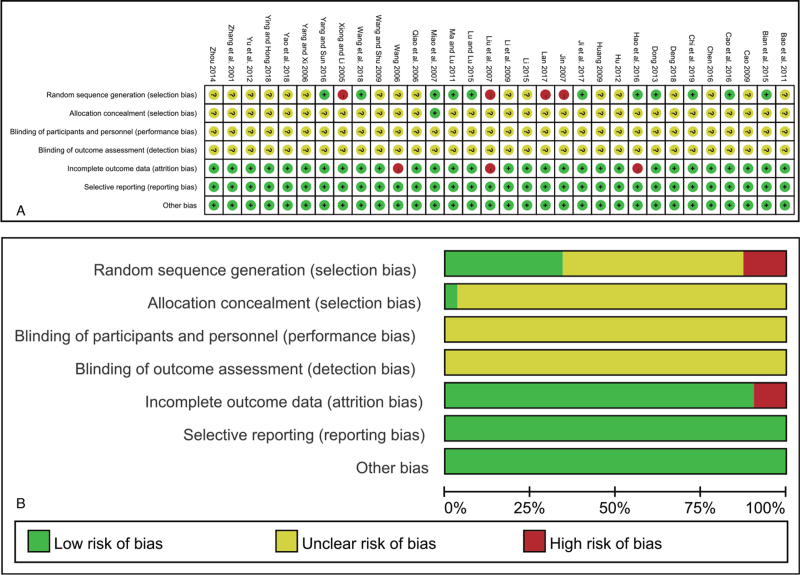
3.3. Risk of methodological bias
The risk of bias of all included trials was evaluated and is summarized in Figure 2. The random sequence generation of 11 studies used the random number table, and 4 trials used hospital or clinic record numbers. The allocation concealment and blinding method were not clear in most of the included trials, except in one that used a sealed envelope. All trials had complete follow-up. Selective reporting existed in 2 trials[31,32] without completely report the DCR and another 1 trial[33] did not have a complete report on ADRs.
Figure 2.
Risk of bias of included studies. (A) Risk of bias summary: judgments about each bias item for each study; (B) Risk of bias summary graph.
3.4. Outcome measures
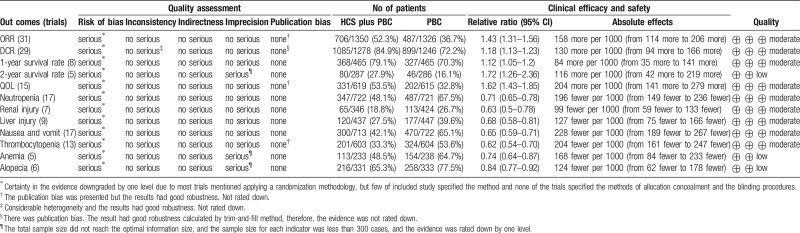
The findings of this meta-analysis are summarized in Table 2. The subgroup analysis results are summarized in Table 3. GRADE assessments are described in Table 4.
Table 2.
Summary of the meta-analysis.
| Outcomes | Trials | HCS plus PBC (Evens/Total) | PBC (Evens/Total) | RR (95% CI) | I2 | P |
| ORR | 31 | 706/1350 | 487/1326 | 1.43 (1.31, 1.56) | 15% | <.0001∗ |
| DCR | 29 | 1085/1278 | 899/1246 | 1.18 (1.13, 1.23) | 41% | <.0001∗ |
| Survival rate | ||||||
| 1-year survival rate | 8 | 368/465 | 327/465 | 1.12 (1.05, 1.20) | 31% | .0007∗ |
| 2-year survival rate | 5 | 80/287 | 46/286 | 1.72 (1.26, 2.36) | 0% | .0007∗ |
| QOL | 15 | 331/619 | 202/615 | 1.62 (1.43, 1.85) | 45% | <.0001∗ |
| ADRs | ||||||
| Neutropenia | 17 | 347/722 | 487/721 | 0.71 (0.65, 0.78) | 36% | <.0001∗ |
| Thrombocytopenia | 13 | 201/603 | 324/604 | 0.62 (0.54, 0.70) | 24% | <.0001∗ |
| Nausea and vomit | 17 | 300/713 | 470/722 | 0.65 (0.59, 0.71) | 28% | <.0001∗ |
| Anemia | 5 | 113/233 | 154/238 | 0.74 (0.64, 0.87) | 0% | .0002∗ |
| Liver injury | 9 | 120/437 | 177/447 | 0.68 (0.58, 0.81) | 0% | <.0001∗ |
| Renal injury | 7 | 65/346 | 113/424 | 0.63 (0.50, 0.78) | 0% | <.0001∗ |
| Alopecia | 6 | 216/331 | 258/333 | 0.84 (0.77, 0.92) | 46% | .0002∗ |
Table 3.
Subgroups analysis of primary outcomes.
| Objective response rate (ORR) | Disease control rate (DCR) | |||||||||||
| Study event rates | Trials | Study event rates | ||||||||||
| Subgroups | Trials | HCS plus PBC (Evens/Total) | PBC (Evens/Total) | RR (95% CI) | I2 | P | HCS plus PBC (Evens/Total) | PBC (Evens/Total) | RR (95% CI) | I 2 | P | |
| Subgroups analysis via doses | ||||||||||||
| HCS (< 20 ml/times) | 3 | 42/119 | 28/119 | 1.50 (1.00–2.25) | 0% | .05 | 3 | 90/119 | 73/119 | 1.23 (1.04, 1.46) | 0% | 0.02∗ |
| HCS (20 ml/times) | 26 | 617/1148 | 424/1132 | 1.44 (1.32–1.58) | 26% | < .0001∗ | 24 | 920/1076 | 766/1052 | 1.18 (1.13, 1.23) | 31% | < 0.0001∗ |
| HCS (> 20 ml/times) | 2 | 47/83 | 35/75 | 1.20 (0.89, 1.61) | 0% | .23 | 2 | 75/83 | 60/75 | 1.12 (0.99, 1.28) | 88% | 0.08 |
| Subgroups analysis via treatment time | ||||||||||||
| <14 days | 6 | 164/278 | 108/269 | 1.52 (1.28, 1.82) | 82% | <.0001∗ | 5 | 207/236 | 170/219 | 1.14 (1.05, 1.25) | 52% | 0.003∗ |
| 14 days | 3 | 72/132 | 46/134 | 1.59 (1.22, 2.08) | 0% | .0007∗ | 3 | 107/132 | 78/134 | 1.39 (1.19, 1.63) | 75% | < 0.0001∗ |
| 15 days | 1 | 24/45 | 21/48 | 1.22 (0.80, 1.86) | NA | .36 | 1 | 39/45 | 40/48 | 1.04 (0.88, 1.23) | NA | 0.65 |
| 21 days | 10 | 192/353 | 131/340 | 1.40 (1.19, 1.64) | 0% | < .0001∗ | 10 | 307/353 | 269/340 | 1.10 (1.03, 1.17) | 0% | 0.006∗ |
| 28 days | 11 | 254/542 | 181/535 | 1.38 (1.19, 1.59) | 0% | < .0001∗ | 10 | 425/512 | 342/505 | 1.22 (1.14, 1.31) | 0% | < 0.0001∗ |
| Subgroups analysis via treatment cycles | ||||||||||||
| 1 | 2 | 41/100 | 36/100 | 1.14 (0.80, 1.62) | 0% | .47 | 2 | 79/100 | 67/100 | 1.18 (0.99, 1.40) | 0% | 0.058 |
| 2 | 16 | 310/575 | 227/569 | 1.35 (1.19, 1.53) | 0% | < .0001∗ | 15 | 475/545 | 420/539 | 1.12 (1.06, 1.18) | 0% | < 0.001∗ |
| 3 | 9 | 266/512 | 178/518 | 1.54 (1.34, 1.76) | 62% | < .0001∗ | 8 | 396/470 | 327/468 | 1.21 (1.12, 1.29) | 37% | < 0.001∗ |
| 4 | 4 | 89/163 | 46/139 | 1.65 (1.25, 2.17) | 48% | .0003∗ | 4 | 135/163 | 85/139 | 1.34 (1.16, 1.55) | 92% | < 0.001∗ |
| Subgroups analysis via chemotherapy | ||||||||||||
| HCS plus AP vs AP | 1 | 33/46 | 23/40 | 1.25 (0.90, 1.72) | NA | .18 | 1 | 43/46 | 38/40 | 0.98 (0.89, 1.09) | NA | 0.76 |
| HCS plus DC vs DC | 1 | 17/49 | 8/49 | 2.13 (1.01, 4.46) | NA | .05 | 1 | 34/49 | 17/49 | 2.00 (1.31, 3.06) | NA | 0.001∗ |
| HCS plus DP vs DP | 4 | 103/221 | 55/221 | 1.87 (1.43, 2.45) | 53% | < .0001∗ | 4 | 180/221 | 142/221 | 1.27 (1.13, 1.42) | 0% | < 0.0001∗ |
| HCS plus EP vs EP | 1 | 15/60 | 9/60 | 1.67 (0.79, 3.51) | NA | .18 | 1 | 47/60 | 31/60 | 1.52 (1.15, 2.00) | NA | 0.003∗ |
| HCS plus GP vs GP | 6 | 164/295 | 101/274 | 1.53 (1.26, 1.85) | 0% | < .0001∗ | 6 | 253/295 | 205/274 | 1.16 (1.06, 1.26) | 2% | 0.0007∗ |
| HCS plus NP vs NP | 10 | 177/352 | 141/345 | 1.22 (1.04, 1.44) | 0% | .01 | 10 | 301/352 | 273/345 | 1.08 (1.01, 1.16) | 0% | 0.03∗ |
| HCS plus TC vs TC | 1 | 11/34 | 10/34 | 1.10 (0.54, 2.24) | NA | .79 | 1 | 32/34 | 25/34 | 1.28 (1.03, 1.59) | NA | 0.03∗ |
| HCS plus TP vs TP | 7 | 186/293 | 140/303 | 1.39 (1.21, 1.60) | 38% | < .0001∗ | 5 | 195/221 | 168/223 | 1.17 (1.07, 1.28) | 0% | 0.0005∗ |
| Subgroups analysis via evaluation criteria | ||||||||||||
| WHO criteria | 17 | 357/611 | 260/593 | 1.35 (1.21, 1.51) | 0% | < .0001∗ | 15 | 470/539 | 405/513 | 1.11 (1.05, 1.17) | 0% | 0.0003∗ |
| RECIST | 11 | 262/557 | 185/551 | 1.39 (1.21, 1.61) | 0% | < .0001∗ | 11 | 453/557 | 362/551 | 1.23 (1.15, 1.32) | 72% | < 0.0001∗ |
Table 4.
GRADE evidence profile of clinical efficacy and safety.
3.5. Objective response rate (ORR)
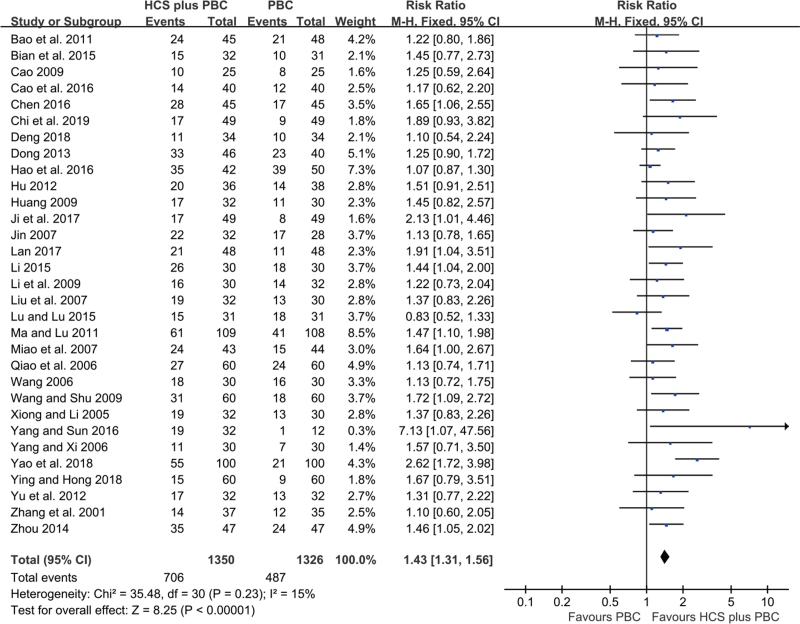
A total of 31 trials, including 2676 participants, reported the ORR (Fig. 3). No statistical heterogeneity was detected among the trials (I2 = 15%). Therefore, we applied the fixed-effects model for the analysis. Compared to PBC alone, HCS plus PBC significantly increased the ORR (RR = 1.43, 95% CI: 1.31–1.56, P < .00001; Fig. 3).
Figure 3.
Forest plot of improved ORR with HCS plus PBC versus PBC alone.
3.6. Disease control rate (DCR)
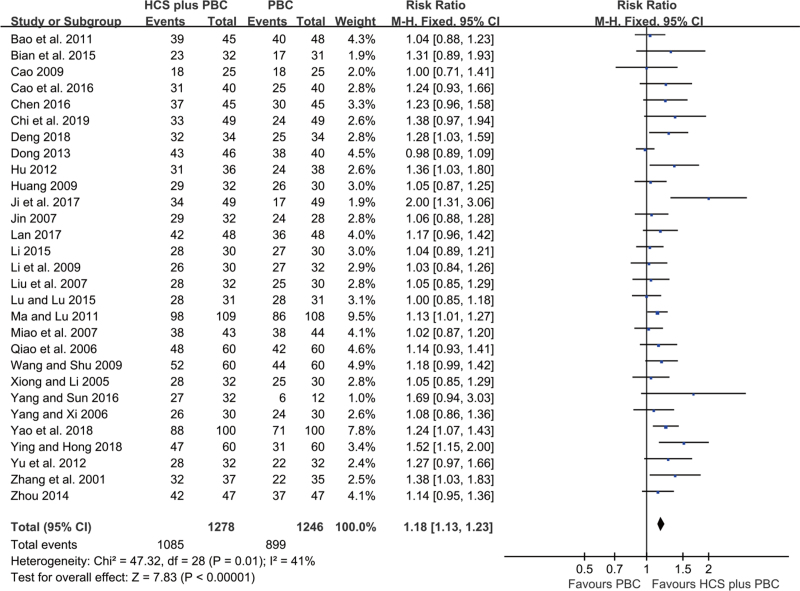
A total of 29 trials with 2524 participants reported the DCR. Acceptable statistical heterogeneity was observed among the trials (I2 = 41%); hence, a fixed-effects model was used to evaluate the data. The results showed that HCS plus PBC significantly increased the DCR (RR = 1.18, 95% CI: 1.13–1.23, P < .00001; Fig. 4) compared with PBC alone.
Figure 4.
Forest plot of improved DCR with HCS plus PBC versus PBC alone.
3.7. Survival rate
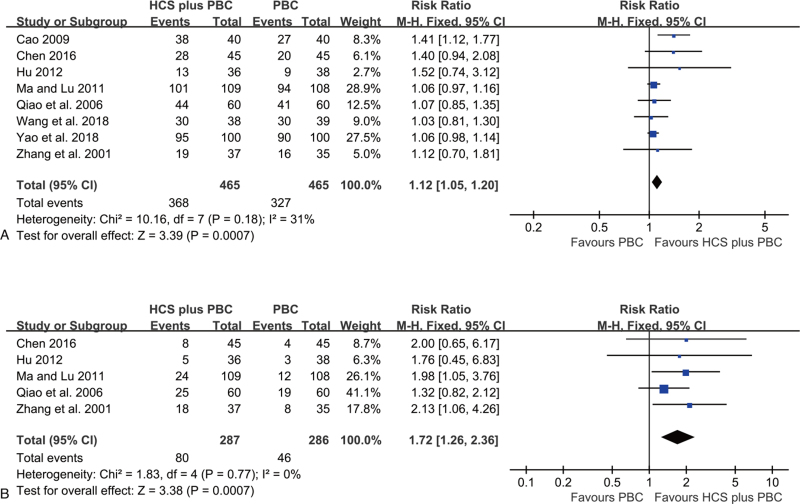
The summary estimates of 8 trials reported data on 1- and 2- year survival rates. The results revealed that compared to PBC alone, HCS plus PBC improved the 1-year survival rate (RR = 1.12, 95% CI: 1.05–1.20, P = .0007; Fig. 5A) and 2-year survival rate (RR = 1.72, 95% CI: 1.26–2.36, P = .0007; Fig. 5B), without significant heterogeneity (I2 = 31% and 0%, respectively). The fixed effects model was applied.
Figure 5.
Forest plot of survival rate with HCS plus PBC versus PBC alone. (A) 1-year survival rate; (B) 2-year survival rate.
3.8. Improvement of QOL
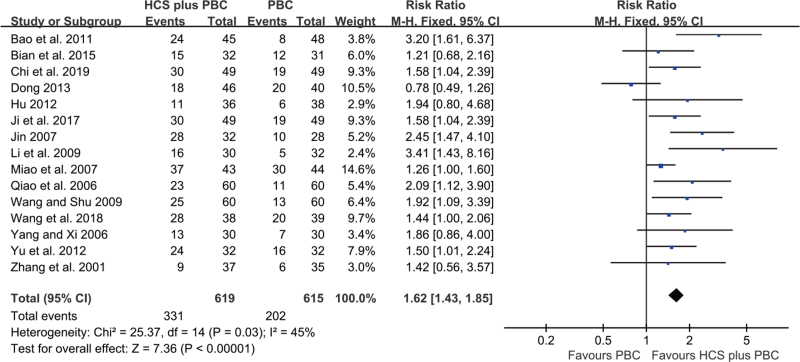
A total of 15 trials with 1234 participants reported data on QOL. These trials were pooled, and compared to PBC, HCS plus PBC was significantly improved the QOL (RR = 1.62, 95% CI: 1.43–1.85, P < .00001; Fig. 6), without significant heterogeneity (I2 = 45%). The fixed-effects model was applied.
Figure 6.
Forest plot of improved QOL with HCS plus PBC versus PBC alone.
3.9. ADRs
A total of 20 trials reported the ADRs with respect to neutropenia, thrombocytopenia, nausea and vomiting, anemia, liver injury, renal injury, and alopecia (see Figure S1–7 Supplemental Digital Contents, which illustrate the forest plots of different types of ADRs). This pooled analysis showed that HCS plus PBC was related to lower risk of neutropenia (RR = 0.71, 95% CI: 0.65–0.78, P < .00001), thrombocytopenia (RR = 0.62, 95% CI: 0.54–0.70, P < .00001), nausea and vomiting (RR = 0.65, 95% CI: 0.59–0.71, P < .00001), anemia (RR = 0.74, 95% CI: 0.64–0.87, P = .0002), liver injury (RR = 0.68, 95% CI: 0.58–0.81, P < .0001), renal injury (RR = 0.63, 95% CI: 0.50–0.78, P < .0001), and alopecia (RR = 0.84, 95% CI: 0.77–0.92, P = .0002) compared with PBC alone. No significant heterogeneity was detected (I2 = 36%, 24%, 28%, 0%, 0%, 0%, and 46%, respectively), and hence, the fixed effects model was applied.
3.10. Subgroup analysis of ORR and DCR
Subgroup analyses were conducted to explore and explain the sources of heterogeneity (Table 3). The doses were divided into < 20 mL/times, 20 mL/times, and >20 mL/times, respectively. The results of subgroup analysis revealed that patients who received < 20 mL/times and 20 mL/times of HCS showed improved ORR and DCR, respectively (Table 3 and Figure S8-9 Supplemental Digital Contents, which illustrate the forest plots of subgroups analysis of ORR and DCR via different doses of HCS). The treatment time in one cycle was divided into <14 days, 14 days, 15 days, 21 days, and 28 days, respectively. These findings suggested that except for treatment with 15 days, all different treatment times of HCS in one cycle could improve the ORR and DCR (Table 3 and Figure S10-11 Supplemental Digital Contents, which illustrate the forest plots of subgroups analysis of ORR and DCR via different treatment time of HCS). The treatment cycles were divided into 1–4 cycles, respectively. The results showed that except for treatment with one cycle, all the different treatment cycles of HCS increased the ORR and DCR (Table 3 and Figure S12-13 Supplemental Digital Contents, which illustrate the forest plots of subgroups analysis of ORR and DCR via different treatment cycles of HCS). The included type of PBC regimens was divided into AP, DC, DP, EP, GP, NP, TC, and TP, respectively. The results showed that except for AP, the other types of PBC regimens plus HCS improved the ORR and DCR (Table 3 and Figure S14-15 Supplemental Digital Contents, which illustrate the forest plots of subgroups analysis of ORR and DCR via different types of chemotherapy regimens of HCS). Finally, tumor responses were assessed according to WHO or RECIST guidelines. The subgroup analysis based on the above criteria revealed that HCS plus PBC improved the ORR and DCR (Table 3 and Figure S16-17 Supplemental Digital Contents, which illustrate the forest plots of subgroups analysis of ORR and DCR via different evaluation criteria of HCS).
3.11. Publication bias
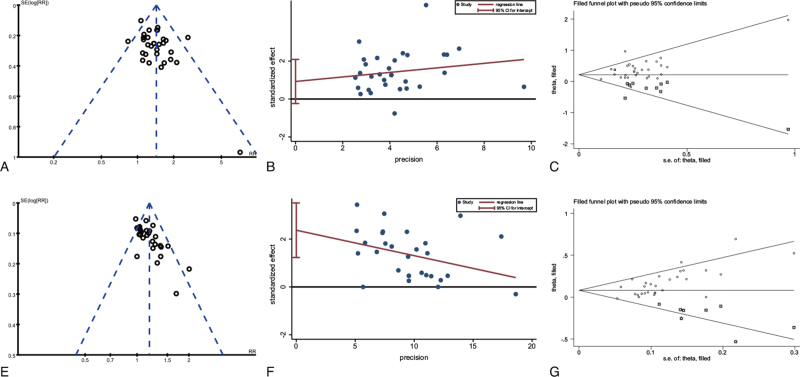
Three types of funnel plots of primary and secondary outcomes are shown in Figure 7 and Figure S18 Supplemental Digital Content (see Figure S18, which illustrates the funnel plots of secondary outcomes), respectively. Most of the funnel plots displayed asymmetry on visual inspection. ORR results from the Harbord test did not reveal any significant publication bias (P = .115, Figure 7 and Table S1 Supplemental digital Content, which illustrates the evaluation results of publication bias). However, a significant publication bias was detected in DCR (P < .001, Figure 7 and Table S1 Supplemental Digital Content Table S1, which illustrates the evaluation results of publication bias). A sensitivity analysis was implemented using the trim-and-fill method, which yielded a symmetrical funnel plot (Fig. 7, Figure S18 Supplemental Digital Content, which illustrates the funnel plots of secondary outcomes and Table S1 Supplemental Digital Content, which illustrates the evaluation results of publication bias). The results of the trim-and-fill method exhibited robust RRs (Table S1 Supplemental Digital Content, which illustrates the evaluation results of publication bias), and the potential publication bias did not influence the significance of our results.
Figure 7.
Funnel plots, Harbord test funnel plots to assess publication bias and trim-and-fill funnel plots of primary outcomes. (A) funnel plot of ORR; (B) Harbord test funnel plot of ORR; (C) trim-and-fill funnel plot of ORR; (D) funnel plot of DCR; (E) Harbord test funnel plot of DCR; (F) trim-and-fill funnel plot of DCR. Trim-and-filled funnel plot of RR from studies that investigated the association between insomnia and the risk of depression. The circles alone are real studies and the circles enclosed in boxes are ‘filled’ studies. The horizontal line represents the summary effect estimates, and the diagonal lines represent pseudo-95% CI limits.
3.12. Sensitivity and meta-regression analysis
Sensitivity analyses were used to explore the potential sources of heterogeneity in primary outcomes and secondary outcomes, assess the influence of various exclusions on the pooled RRs, and evaluate the stability of the quantitative synthesis results. In the leave-one-out analysis by excluding each study sequentially, the overall pooled RRs did not change substantially (see Figure S19 Supplemental Digital Content, which illustrates the results of sensitivity analysis), indicating that the results were related to robustness.
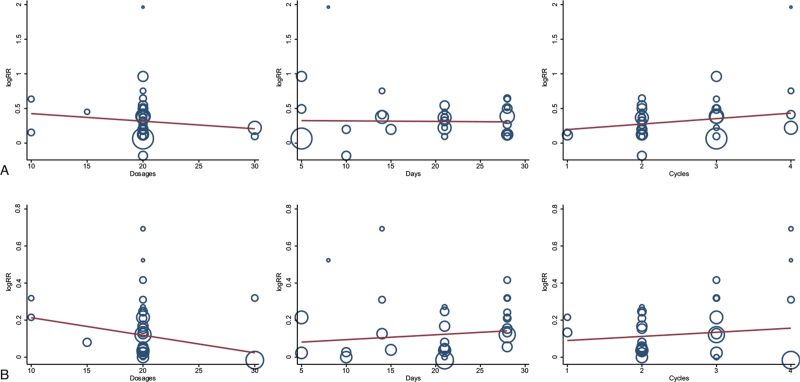
The meta-regression analysis suggested that the ORR and DCR were not improved as the HCS dosage (from 10 mL to 30 mL) or treatment time (from 5 days to 28 days) or cycle number increased (from 1 to 4) (Fig. 8).
Figure 8.
Meta-regression analysis showing that the ORR and DCR was not improved with increased dosages, treatment time, and cycle number of HCS.
3.13. TSA
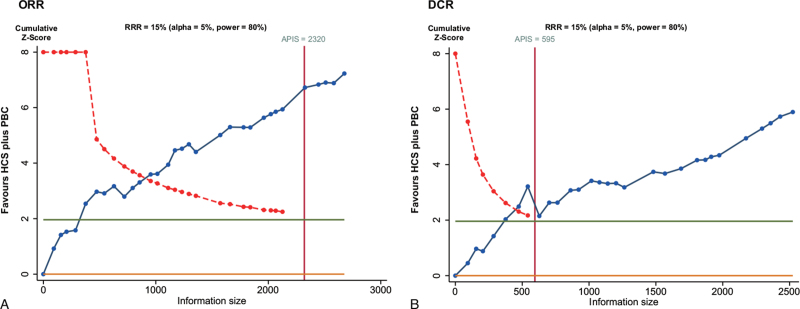
We used TSA boundaries to evaluate the robustness of the results and calculated the a priori information size (APIS) in the meta-analysis. As shown in Figure 9, the Z-score curve (blue line) crossed the statistical significance boundary (red polylines), the required information size (vertical red line), and the conventional statistical significance boundary corresponding to a two-sided P-value of .05 (dark green lines). The results indicated that HCS plus PBC increased the ORR and DCR in NSCLC patients.
Figure 9.
Trial sequential analysis. (A) ORR; (B) DCR.
Also, as shown in Figure S20 Supplemental Digital Content (see Figure S20 which illustrates the results of trial sequential analysis), the improvement in the 1-year survival rate and QOL and the reduction in neutropenia, thrombocytopenia, nausea and vomiting, anemia, and liver injury are definite and well-documented. The improvement in the 2-year survival rate and the decrease in the risk of renal injury need to be substantiated by additional studies (see Figure S20 Supplemental Digital Content, which illustrates the results of trial sequential analysis). However, although the graph results showed a significant difference between HCS plus PBC and PBC alone, the reduction in the risk of alopecia might be a false-positive result (see Figure S20 Supplemental Digital Content, which illustrates the results of trial sequential analysis).
3.14. Quality of evidence
We used GRADE to assess the quality of evidence with respect to the clinical efficacy and safety of HCS. Due to some uncertainty about the methodological risk of bias, and since the evidence was rated down by only one level, we were moderately confident in the outcomes of ORR, DCR, 1-year survival rate, QOL, neutropenia, renal injury, liver injury, nausea and vomiting, and thrombocytopenia. On the other hand, we had low confidence in the outcomes of 2-year survival rate, anemia, and alopecia, because except for the possible risk of bias, other factors need to be considered to assess the level of evidence, including insufficient sample size and few relevant studies (Table 4).
4. Discussion
This systematic review and meta-analysis encompassing 32 trials with 2753 patients demonstrated that PBC combination with HCS in the treatment of advanced NSCLC significantly improves the ORR, DCR, and QOL and decreases the risk of ADRs compared to PBC alone treatment. These findings are objective and completely unaffected by any focus groups, such as health professionals, users, policymakers, and providers.
PBC is one of the standards first-line chemotherapy regimens for NSCLC. Despite continual improvements in chemotherapy agents, the clinical efficacy is unsatisfactory, with limited benefits, and patients suffer from chemotherapy-induced toxicity. Furthermore, the prognosis of advanced NSCLC remains poor, and hence, novel therapeutic strategies are urgently required. Prior to our study, one meta-analysis[19] evaluated the effects of HCS plus first-line PBC, and two meta-analyses[20,21] evaluated the effects of HCS plus chemotherapy on lung cancer, but no definitive conclusions were drawn because of the lower methodological quality of the RCTs included. Moreover, the latest published meta-analysis by Xu et al.[19] did not determine the objective response, and insufficient data on ADRs, incomplete trials, and small sample size might weaken the statistical characteristics and reduce the credibility of the evidence. Therefore, we performed a comprehensive search and included several recently published RCTs to achieve clinical advancement and provide convincing evidence for the clinical application of a combination of HCS and PBC in the treatment of advanced NSCLC.
Although most of the included trials had an unclear methodological risk of bias, the quality of the methods in the trials was consistent. TSA performed based on most of the outcomes revealed that the required information for a robust meta-analysis was collected, and the efficacy and safety of HCS plus PBC were significantly superior to those of PBC alone. The confidence of our study and the quality of the evidence were assessed by GRADE. The results of GRADE assessment suggested that the quality of evidence was moderate in most of the outcomes, including ORR, DCR, 1-year survival rate, QOL, neutropenia, renal injury, liver injury, nausea, vomiting, and thrombocytopenia.
According to WHO and RECIST guidelines, the evaluation of the objective response and disease progression are crucial for the clinical assessment of cancer therapeutics.[34] Both DCR and ORR are valuable endpoints for the evaluation of the efficacy of cancer treatment. Especially, the definition of DCR, including complete response (CR), partial response (PR), and no change (NC, stable disease), has been suggested to be the best response outcome to predict the overall survival (OS) and progression-free survival (PFS). Therefore, DCR was considered the primary outcome in our study.[35,36] The current study revealed that HCS plus PBC significantly improves the 1- and 2-year survival rates in patients with advanced NSCLC compared to PBC alone. A previously published meta-analysis[19] suggested that HCS combined with PBC significantly improves the QOL of patients with advanced NSCLC. Our results also demonstrated a significant improvement in the QOL in HCS plus PBC compared to PBC alone. PBC is related to many toxic ADRs in patients with NSCLC, which can severely reduce the QOL and decrease clinical efficacy. Thus, methods to reduce the risk of ADRs of chemotherapy while maintaining clinical efficacy are currently a research hotspot. This study confirmed that compared to PBC alone, HCS plus PBC significantly reduced the ADRs in patients with advanced NSCLC.
B. gargarizans (Bufonidae family) is a small amphibian traditional medicinal animal for the pharmaceutical value of Chansu and Chanpi, and HCS (cinobufacini) is an injectable form of the sterilized hot-water extract of the skin glands of B. gargarizans.[19,37] According to the principles of TCM, HCS is commonly used to counteract toxicity, alleviate pain, and induce resuscitation.[38–40] More than 30 components have been discovered in the skin extract of B. gargarizans, and the vital bioactive compounds include cinobufagin, resibufogenin, bufotenine, cinobufotenine, and serotonin. Modern pharmacological published studies demonstrated that resibufogenin, cinobufagin, and bufalin inhibit tumor cells, and bufotenine, cinobufotenine, and serotonin regulate the nervous system.[41,42] However, to clarify the function of HCS as an adjunct to chemotherapy, the specific mechanisms need to be elucidated.
Although the present meta-analysis revealed favorable outcomes, several limitations cannot be ignored. First, the quality of the original studies was generally not high because most of the included studies reported inadequate detailed information of generating random sequences methods, allocation concealment methods, and blinding study design. Second, although we searched all the mainstream Chinese and English electronic databases, all the included trials were conducted in China, and thus, it is unclear whether the conclusions of this meta-analysis could be applied to advanced NSCLC patients worldwide, which reduces the universality of the conclusions. Third, GRADE revealed that the quality of 2-year survival rate, anemia, and alopecia was “low” due to the risk of bias and insufficient sample sizes. Despite the above limitations, the results of the current meta-analysis revealed a systematic evaluation of the efficacy and safety in multiple outcomes and provided clinical evidence of HCS plus PBC for the treatment of advanced NSCLC patients.
5. Conclusion
In summary, the moderate-quality evidence reveals that the combination of HCS with PBC is beneficial for the clinical efficacy and QOL and reduces the risk of chemotherapy-induced ADRs for patients with advanced NSCLC. HCS may be a valuable adjunctive treatment to PBC for the treatment of advanced NSCLC. However, eligible RCTs lack high methodological quality and potential risk of bias, and HCS needs to be further explored with respect to these outcomes. To further confirm the conclusion of the current study, high-quality, larger sample size, and well-designed RCTs are an urgent requisite.
Author contributions
Conceptualization: Xueyan Liang, Jiaxi Xi, Xinmei Tan.
Data curation: Xueyan Liang, Jiaxi Xi, Xinmei Tan.
Formal analysis: Xueyan Liang, Xinmei Tan.
Funding acquisition: Xiaoyu Chen, Yan Li.
Investigation: Xiaoyu Chen, Yan Li, Xinmei Tan.
Methodology: Xueyan Liang, Jiaxi Xi, Xinmei Tan, Sitong Guo, Mingyu Meng, Xiaoyu Chen, Yan Li.
Software: Xueyan Liang, Jiaxi Xi, Xinmei Tan, Sitong Guo, Mingyu Meng, Xiaoyu Chen, Yan Li.
Validation: Jiaxi Xi, Sitong Guo, Yan Li.
Visualization: Xueyan Liang, Mingyu Meng, Xiaoyu Chen.
Writing – original draft: Xueyan Liang, Jiaxi Xi.
Writing – review & editing: Xiaoyu Chen, Yan Li, Xinmei Tan.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ADRs = adverse drug reactions, AP = pemetrexed plus cisplatin, CBM = China Biological Medicine Database, CI = confidence interval, CNKI = China National Knowledge Infrastructure, DC = docetaxel plus carboplatin, DCR = disease control rate, DP = docetaxel plus cisplatin, EP = etoposide plus cisplatin, GP = gemcitabine plus cisplatin, GRADE = Grading of Recommendations Assessment, Development, and Evaluation, HCS = Huachansu injection, NP = vinorelbine plus cisplatin, NSCLC = non-small cell lung cancer, ORR = objective response rate, PFS = progression-free survival, PRISMA guidelines = Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, QOL = quality of life, RCT = randomized controlled trial, RECIST = Response Evaluation Criteria in Solid Tumors, RevMan5.4 = Review Manager 5.4, Taxol = paclitaxel, TBC = paclitaxel-based chemotherapy, TC = paclitaxel and carboplatin, TO = paclitaxel and oxaliplatin, TP = paclitaxel and cisplatin, VIP = Chinese Scientific Journals Full-Text Database, WHO = World Health Organization.
How to cite this article: Tan X, Liang X, Xi J, Guo S, Meng M, Chen X, Li Y. Clinical efficacy and safety of Huachansu injection combination with platinum-based chemotherapy for advanced non-small cell lung cancer: A systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100:36(e27161).
XMT, XYL and JXX contributed equally to this work.
This project was supported by the National Natural Science Foundation of Guangxi Province (No.2018GXNSFAA281159).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are publicly available.
Supplemental digital content is available for this article.
ADRs = adverse drug reactions, AP = pemetrexed plus cisplatin, CTCAE = Common terminology criteria for adverse events version, DC = docetaxel plus carboplatin, DCR = disease control rate, DP = docetaxel plus cisplatin, E/C = experimental group (Huachansu injection plus paclitaxel-based chemotherapy)/control group (paclitaxel-based chemotherapy), EP = etoposide plus cisplatin, GP = gemcitabine plus cisplatin, HCS = Huachansu injection, M/F = male/female, NP = vinorelbine plus cisplatin, NSCLC = non-small cell lung cancer, ORR = objective response rate, QOL = quality of life, RECIST = Response Evaluation Criteria in Solid Tumors, TC = paclitaxel plus carboplatin, TP = paclitaxel plus cisplatin, WHO = World Health Organization guidelines for solid tumor responses.
DCR = disease control rate, HCS = Huachansu injection, ORR = objective response rate, PBC = paclitaxel-based chemotherapy, QOL = quality of life, RR = relative ratio.
Favours HCS plus PBC with statistical significance.
AP = pemetrexed plus cisplatin, DC = docetaxel plus carboplatin, DCR = disease control rate, DP = docetaxel plus cisplatin, EP = etoposide plus cisplatin, GP = gemcitabine plus cisplatin, HCS = Huachansu injection, NP = vinorelbine plus cisplatin, ORR = objective response rate, RECIST = Response Evaluation Criteria in Solid Tumors, TC = paclitaxel plus carboplatin, TP = paclitaxel plus cisplatin, WHO = World Health Organization guidelines for solid tumor responses.
Favours HCS plus PBC with statistical significance.
References
- [1].Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction—evidence, pitfalls and future perspectives. Nat Rev Clin Oncol 2021;18:135–51. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:07–30. [DOI] [PubMed] [Google Scholar]
- [3].Jumeau R, Vilotte F, Durham AD, Ozsahin EM. Current landscape of palliative radiotherapy for non-small-cell lung cancer. Transl Lung Cancer Res 2019;8: Suppl 2: S192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liang H, Deng H, Liang W, et al. Perioperative chemoimmunotherapy in a patient with stage IIIB non-small cell lung cancer. Ann Transl Med 2020;8:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trodella L, D’Angelillo RM, Ramella S, et al. Chemo-radiotherapy in non-small cell lung cancer: the role of gemcitabine. Ann Oncol 2006;17: Suppl 5: v52–4. [DOI] [PubMed] [Google Scholar]
- [6].Wujanto C, Vellayappan B, Siva S, et al. Stereotactic body radiotherapy for oligometastatic disease in non-small cell lung cancer. Front Oncol 2019;9:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol 2012;13:671–8. [DOI] [PubMed] [Google Scholar]
- [8].Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509–20. [DOI] [PubMed] [Google Scholar]
- [9].Takimoto T, Nakabori T, Osa A, et al. Tubular nephrotoxicity induced by docetaxel in non-small-cell lung cancer patients. Int J Clin Oncol 2012;17:395–8. [DOI] [PubMed] [Google Scholar]
- [10].Michael M, Wirth A, Ball DL, et al. A phase I trial of high-dose palliative radiotherapy plus concurrent weekly Vinorelbine and Cisplatin in patients with locally advanced and metastatic NSCLC. Br J Cancer 2005;93:652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salama JK, Vokes EE. New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. J Clin Oncol 2013;31:1029–38. [DOI] [PubMed] [Google Scholar]
- [12].de Castro J, Tagliaferri P, de Lima VCC, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care 2017;26:e12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: a meta-analysis. Lung Cancer 2019;135:196–204. [DOI] [PubMed] [Google Scholar]
- [14].Gridelli C, Sacco PC. Novel cytotoxic drugs in advanced nonsmall cell lung cancer. Curr Opin Oncol 2016;28:110–4. [DOI] [PubMed] [Google Scholar]
- [15].Belani CP, Langer C. First-line chemotherapy for NSCLC: an overview of relevant trials. Lung Cancer 2002;38:13–9. [DOI] [PubMed] [Google Scholar]
- [16].Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2000;18:122–30. [DOI] [PubMed] [Google Scholar]
- [17].Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 1998;16:2459–65. [DOI] [PubMed] [Google Scholar]
- [18].Lilenbaum RC, Herndon JE, 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190–6. [DOI] [PubMed] [Google Scholar]
- [19].Xu Y, Han D, Feng F, et al. Meta-analysis of Cinobufacini injection combined with platinum-contained first-line chemotherapy in treatment of non-small cell lung cancer. China J Chin Mater Med 2019;44:4728–37. [DOI] [PubMed] [Google Scholar]
- [20].Huang R, Su Y. Clinical efficacy of cinobufacin capsules combined with chemotherapeutic drugs in the treatment of non-small cell lung cancer: a meta-analysis. Clin Med J 2018;16:59–63. [Google Scholar]
- [21].Tu C, Yin J, He J. Meta-Analysis of cinobufacini injection plus chemotherapy in the treatment of non-small-cell lung cancer. Antitumor Pharm 2012;2:67–72. [Google Scholar]
- [22].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220–4. [DOI] [PubMed] [Google Scholar]
- [24].Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Available from www.training.cochrane.org/handbook. Accessed November 30, 2020. [Google Scholar]
- [25].Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res 2009;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–57. [DOI] [PubMed] [Google Scholar]
- [27].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [28].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–14. [DOI] [PubMed] [Google Scholar]
- [30].Watanabe H, Yamamoto S, Kunitoh H, et al. Tumor response to chemotherapy: the validity and reproducibility of RECIST guidelines in NSCLC patients. Cancer science 2003;94:1015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hao Y, Liu Y, Dai Z. Clinical efficacy of cinobufacini injection for advanced non-small cell lung cancer. Inner Mongolia Med J 2016;48:233–5. [Google Scholar]
- [32].Wang S. Clinical research on non-small cell lung cancer treated by cinobutacini injection plus chemotherapy. Central Plains Med J 2006;33:70. [Google Scholar]
- [33].Liu W, Sun P, Zhang P. Clinical study of combined huachansu with chemotherapy on advanced non-small cell lungs cancer. Chin J Clin Oncol Rehabil 2007;14:463–4. [Google Scholar]
- [34].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [35].Claret L, Gupta M, Han K, et al. Prediction of overall survival or progression free survival by disease control rate at week 8 is independent of ethnicity: Western versus Chinese patients with first-line non-small cell lung cancer treated with chemotherapy with or without bevacizumab. J Clin Pharmacol 2014;54:253–7. [DOI] [PubMed] [Google Scholar]
- [36].Paesmans M, Sculier JP, Libert P, et al. Response to chemotherapy has predictive value for further survival of patients with advanced non-small cell lung cancer: 10 years experience of the European Lung Cancer Working Party. Eur J Cancer 1997;33:2326–32. [DOI] [PubMed] [Google Scholar]
- [37].Zhao H, Wu X, Wang H, et al. Qualitative and quantitative analysis of cinobufacini injection using rapid separation liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry and HPLC-photodiode array detection, a feasible strategy for the quality control of Chinese medicine injections. J Sep Sci 2013;36:492–502. [DOI] [PubMed] [Google Scholar]
- [38].Sakurai K, Yoshii E, Hashimoto H, Kubo K. Gas liquid chromatography of steroids of Ch’an Su. II. Reinvestigation on the determination of bufadienolides. Chem Pharm Bull 1968;16:1140–3. [DOI] [PubMed] [Google Scholar]
- [39].Xu R, Xie HQ, Deng LL, et al. A new bufadienolide with cytotoxic activity from the Chinese traditional drug Ch’an Su. Chin J Nat Med 2014;12:623–7. [DOI] [PubMed] [Google Scholar]
- [40].Cheng CS, Wang J, Chen J, et al. New therapeutic aspects of steroidal cardiac glycosides: the anticancer properties of Huachansu and its main active constituent Bufalin. Cancer Cell Int 2019;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McBride MC. Bufotenine: toward an understanding of possible psychoactive mechanisms. J Psychoactive Drugs 2000;32:321–31. [DOI] [PubMed] [Google Scholar]
- [42].Qi F, Li A, Inagaki Y, et al. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int Immunopharmacol 2011;11:342–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.