Abstract
Objectives:
Define clinical spectrum and long-term outcomes of gut malrotation. With new insights, an innovative procedure was introduced and predictive models were established.
Methods:
Over 30-years, 500 patients were managed at 2 institutions. Of these, 274 (55%) were children at time of diagnosis. At referral, 204 (41%) patients suffered midgut-loss and the remaining 296 (59%) had intact gut with a wide range of digestive symptoms. With midgut-loss, 189 (93%) patients underwent surgery with gut transplantation in 174 (92%) including 16 of 31 (16%) who had autologous gut reconstruction. Ladd's procedure was documented in 192 (38%) patients with recurrent or de novo volvulus in 41 (21%). For 80 patients with disabling gastrointestinal symptoms, gut malrotation correction (GMC) surgery “Kareem's procedure” was offered with completion of the 270° embryonic counterclockwise-rotation, reversal of vascular-inversion, and fixation of mesenteric-attachments. Concomitant colonic dysmotility was observed in 25 (31%) patients.
Results:
The cumulative risk of midgut-loss increased with volvulus, prematurity, gastroschisis, and intestinal atresia whereas reduced with Ladd's and increasing age. Transplant cumulative survival was 63% at 10-years and 54% at 20-years with best outcome among infants and liver-containing allografts. Autologous gut reconstruction achieved 78% and GMC had 100% 10-year survival. Ladd's was associated with 21% recurrent/de novo volvulus and worsening (P > 0.05) of the preoperative National Institute of Health patient-reported outcomes measurement information system gastrointestinal symptom scales. GMC significantly (P ≤ 0.001) improved all of the symptomatology domains with no technical complications or development of volvulus. GMC improved quality of life with restored nutritional autonomy (P < 0.0001) and daily activities (P < 0.0001).
Conclusions:
Gut malrotation is a clinicopathologic syndrome affecting all ages. The introduced herein definitive correction procedure is safe, effective, and easy to perform. Accordingly, the current standard of care practice should be redefined in this orphan population.
Keywords: autologous gut reconstruction, colonic dysmotility, congenital anomalies, embryonic rotation, gastrointestinal symptoms, gastroschisis, gut malrotation, gut malrotation correction surgery, Kareem's procedure, gut transplantation, intestinal atresia, intestinal malrotation, Ladd's procedure, mesenteric fixation, mid-gut volvulus, oro-cecal transit time, radiology
The twentieth century witnessed great interest in gut development particularly the complex evolution of midgut.1–7 The different anomalies of embryonic rotation and mesenteric fixation were described. The midgut from the fixed point of the duodenum to the mid-transverse colon is attached to the posterior abdominal wall by a narrow mesenteric hilum.8 This incites the easy development of midgut volvulus with the superior mesenteric artery being the central axis of such a life-threatening event. More recently, advances have also been made concerning the molecular and genetic defects associated with these enigmatic abnormalities.9–11 Despite modern surgical advances, little progress has been made in the management of such a life-long potentially lethal inherited disorder.12–24
The clinical significance of embryonic midgut anomalies was revealed in the early 1920s allowing the 1930s seminal introduction of the life-saving Ladd's procedure.8,25,26 The operation aimed to detorse volvulus and treat duodenal obstruction by releasing the Ladd bands. At the same time, the malrotation was reverted to an earlier stage, rather than corrected, with widening of the mesentery to reduce risk of recurrent volvulus.
Over the years, a few short-lived modifications were introduced to the gold standard Ladd's procedure. The intent was to amend the anatomic deviation with limited stabilization of the mesentery.27–30 More recently, a plethora of scientific publications has emerged, mainly as case series with a few review articles.31–51 These reports attempted to better understand the disease spectrum and management strategies particularly among the adult population.
In 1990s, a new dimension was added to the management of patients with catastrophic midgut-loss.52 With the observed life-threatening complications associated with total parenteral nutrition (TPN), gut transplantation was introduced. Subsequently, autologous gut reconstruction has evolved as part of an integrated management strategy.53,54 In the light of these repercussions, a novel procedure was conceptualized by the primary author and judiciously implemented to treat patients with disabling digestive symptoms and prevent midgut volvulus. The operation stemmed from 30 years of experience in transplant and digestive surgery combined with recent revelations in mesenteric and neuroenteric embryonic development.53–56
This is the largest worldwide series that comprehensively addresses the clinicopathologic spectrum of gut malrotation (GM) in both children and adults. The long-term efficacy of gut transplantation and autologous reconstruction is also assessed. The technical details of the new procedure are illustrated and fully described. Lastly, outcome risk factors are identified and predictive models are established to guide the future management of this perplexing population.
METHODS
Study Design
This ambispective cohort study comprised of 500 gut malrotation (GM) patients. The retrospective group consisted of 204 (41%) patients referred with catastrophic midgut-loss. The prospective group included 125 (25%) with intact gut and disabling gastrointestinal symptoms that were referred for digestive surgery. The remaining 171 (34%) were retrieved from Cleveland Clinic electronic database with confirmed radiologic diagnosis utilizing an integrated natural language processing algorithm. The majority of gut-loss patients were referred for gut transplantation (GT) and/or autologous gut reconstruction (AGR) at the University of Pittsburgh Medical Center and most of the intact gut symptomatic patients were referred to Cleveland Clinic Center for Gut Rehabilitation and Transplantation.
The retrospective group (n = 204) was examined to assess long-term efficacy of GT and AGR. The prospective group (n = 125) was studied to define the clinical spectrum of the GM disorder and durability of the newly introduced gut malrotation correction (GMC) surgery. The collected data of the total 500 patients were stratified and analyzed to measure risk of midgut-loss and identify the distinctive clinical features of GM among those with intact gut.
The technical concept of the GMC procedure was born as the result of the primary author's cumulative experience with organ transplant and digestive surgery. The instigated techniques of retrieving the intestinal allograft, preserving the recipient pancreatico-duodenal-complex, and reconstructing native gut were seminal to the inception of GMC.57,58 The primary surgical principle of the procedure has been completion of the embryonic 270° counterclockwise-midgut rotation with establishment of the mesenteric-attachments. Colon resection was performed for patients with concomitant colonic dysmotility.
Core data including patient characteristics and pertinent clinical features were pooled from a computerized database. Chart review was conducted to obtain further relevant information. The nationally shared medical records were also accessed. Telehealth visits and telephone interviews were conducted for complete follow-up of the GT, AGR, and GMC patients especially in the midst of COVID-19 pandemic. Institutional Review Board approvals were obtained with an honest broker for data management.
Definitions
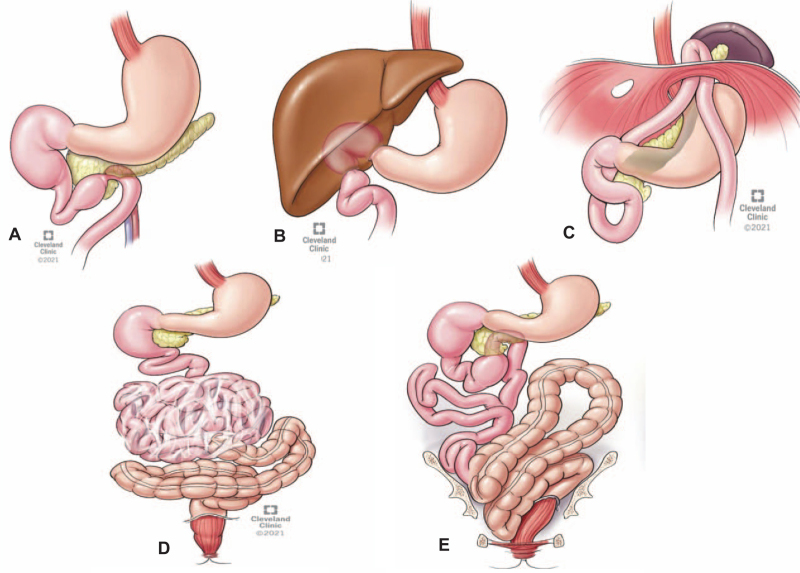
The diagnosis was established utilizing different imaging techniques and was further confirmed during surgery.59 Supplementary Figure-1 identifies different abnormal locations and configurations of the midgut visceral (Fig. 1A) and vascular (Fig. 1B) structures. The type of malrotation was difficult to be categorized because of prereferral midgut-loss or prior Ladd's procedure.
FIGURE 1.
The observed intraoperative anatomic abnormalities and configurations of duodenum, small intestine, and large bowel. Note tethering of the duodenum anteriorly with the uncinate process and posteriorly between inferior pancreatic surface and retroperitoneal cava (A). In a patient with repaired duodenal atresia at birth, the liver grew over the anterior duodenal wall (B) and another patient with proximal jejunum herniating into a missed diaphragmatic defect (C). Internal hernia with cocoon encasement was discovered in 3 patients with situs-inversus (n=1) and redo Ladd's procedure (n=2) (D). A very convoluted transverse colon with contracted mesentery and left colon with a floppy mesenteric attachment were common findings (E ). Note sagging of descending and sigmoid colon into the pelvic cavity.
Gut malrotation syndrome (GMS) was defined by a cluster of disabling digestive symptoms. Dysmotility was diagnosed in symptomatic patients with delayed orocecal and colonic transit time. GT was indicated for irreversible gut failure with TPN-associated complications including liver failure.54 AGR is a digestive surgery that restores gut continuity and remodels intestinal transit time.54
Study Population
The study was conceived in the mid-2000s with the observed steady GM-associated midgut-loss referral to transplantation and was launched with the 2010 development of GMC surgery. Of the 500 collated patients, 274 (55%) were children at the time of GM diagnosis. Midgut-loss was present in 204 (41%) whereas 296 (59%) had intact gut with a wide range of digestive symptoms and other extra-gastrointestinal associated pathology. The clinical features of the total population according to age of diagnosis and status of midgut are summarized in Tables 1 and 2, respectively.
TABLE 1.
Clinical Features of the 500 Patients According to Age at Time of Gut Malrotation Diagnosis
| Variable | Total | Children (<18 yr) | Adults (≥ 18 yr) | P Value |
| Number of patients (%) | 500 | 274 (55) | 226 (45) | NA |
| Geographic distribution (%) | 0.5 | |||
| National | 469 (94) | 255 (93) | 214 (95) | |
| International | 31 (6) | 19 (7) | 12 (5) | |
| Sex (Female : Male) | 1.2 : 1 | 0.8 : 1 | 2.1 : 1 | <0.0001 |
| Race/Ethnicity (White : African-American) | 8.6 : 1 | 8.1 : 1 | 9.3 : 1 | <0.0001 |
| Prematurity (%) | 126 (25) | 115 (42) | 11 (5) | <0.0001 |
| Associated abdominal congenital anomalies (%) | 216 (43) | 174 (64) | 42 (19) | <0.0001 |
| Gastroschisis | 77 (15) | 65 (24) | 12 (5) | <0.0001 |
| Intestinal atresia | 65 (13) | 57 (21) | 8 (4) | <0.0001 |
| Gastroschisis + intestinal atresia | 15 (3) | 14 (5) | 1 (0.4) | 0.3080 |
| Accessory gut organs | 59 (12) | 38 (14) | 21 (9) | 0.1114 |
| Genetic syndromes (%) | 51 (10) | 36 (13) | 15 (7) | 0.015 |
| Thrombophilia (%) | 25 (5) | 9 (3) | 16 (7) | 0.003 |
| Age at time of diagnosis (median [IQR], yr) | 13 [0–37] | 0.1 [0–6] | 40 [28–57] | <0.0001 |
| Duration between symptoms and diagnosis (yr) | <0.0001 | |||
| 0–5 | 341 (68) | 219 (80) | 122 (54) | |
| >5 | 159 (32) | 55 (20) | 104 (46) | |
| Clinical presentation at referral (%) | <0.0001 | |||
| Midgut-loss | 204 (41) | 174 (64) | 30 (13) | |
| Disabling digestive symptoms | 125 (25) | 40 (15) | 85 (38) | |
| Nonspecific digestive symptoms | 171 (34) | 60 (22) | 111 (49) | |
| History of Volvulus (%) | 254 (51) | 196 (72) | 58 (26) | <0.0001 |
| Connective tissue/autoimmune (%) | 25 (5) | 5 (2) | 20 (9) | 0.0003 |
| Gut dysmotility (%) | 56 (11) | 20 (7) | 36 (16) | 0.002 |
| Total parenteral nutrition (%) | 228 (46) | 190 (69) | 38 (17) | <0.0001 |
| Prior abdominal surgery | ||||
| Number of patients (%) | 441 (88) | 260 (95) | 181 (80) | <0.0001 |
| Number of procedures (mean ± SD) | 3 ± 3 | 3 ± 2 | 2 ± 2 | 0.07 |
| Ladd's procedure (%) | 192 (38) | 116 (42) | 76 (34) | 0.05 |
| Open | 143 (74) | 100 (86) | 43 (57) | <0.0001 |
| Laparoscopic | 49 (26) | 16 (14) | 33 (43) | |
| Prior organ/cell transplant (%) | 26 (5) | 14 (5) | 12 (5) | 0.5 |
| Surgical management (%) | 269 (54) | 187 (68) | 82 (36) | <0.0001 |
| Gut transplantation (GT) | 174 (65)∗ | 157 (84) | 17 (21) | |
| Autologous gut reconstruction (AGR) | 15 (5) | 8 (4) | 7 (8) | |
| Gut malrotation correction (GMC) surgery | 80 (30) | 22 (12) | 58 (71) | |
| Overall survival (%)† | 383 (77) | 197 (72) | 186 (82) | 0.01 |
Sixteen patients had prior autologous gut reconstruction (AGR).
As of February 15, 2021.
TABLE 2.
Descriptive Features of the 500 Gut Malrotated Patients According to Status of Gut Anatomy at Time of Referral
| Variable | Midgut-loss | Intact Midgut | P Value |
| Number of patients (%) | 204 (41) | 296 (59) | NA |
| Geographic distribution (%) | 0.322 | ||
| National | 190 (93) | 279 (94) | |
| International | 14 (7) | 17 (6) | |
| Sex (Female / Male) | 90 / 114 | 186 / 110 | <0.0001 |
| Race (White / African-American) | 180 / 24 | 268 / 28 | 0.002 |
| Age at time of diagnosis (median [IQR], yr) | 1 [0–8] | 29 [10–52] | <0.0001 |
| ≤1 | 131 (64) | 42 (14) | |
| >1 to < 18 | 43 (21) | 58 (20) | |
| 18 to ≤ 40 | 24 (12) | 96 (32) | |
| >40 to ≤60 | 6 (3) | 55 (19) | |
| ≥60 | 0 (0) | 45 (15) | |
| Prematurity (%) | 96 (47) | 30 (10) | <0.0001 |
| Associated abdominal congenital anomalies (%) | 143 (70) | 73 (25) | <0.0001 |
| Gastroschisis | 67 (47) | 10 (14) | <0.0001 |
| Intestinal atresia | 52 (36) | 13 (18) | <0.0001 |
| Gastroschisis + intestinal atresia | 15 (11) | 0 (0) | <0.0001 |
| Accessory gut organs | 9 (6) | 50 (68) | <0.0001 |
| Time between symptoms and diagnosis (yr) | |||
| 0–5 | 172 (84) | 169 (57) | <0.0001 |
| >5 | 32 (16) | 127 (43) | |
| History of volvulus (%) | 198 (97) | 56 (19) | <0.0001 |
| History of Ladd's procedure (%) | 50 (25) | 142 (48) | <0.0001 |
| Open | 45 (90) | 98 (69) | |
| Laparoscopic | 5 (10) | 44 (31) | |
| Prior abdominal surgery | |||
| Number of patients (%) | 200 (98) | 241 (81) | <0.0001 |
| Number of procedures (median [IQR]) | 3 [2–5] | 2 [1–4] | <0.0001 |
| Prior organ/cell transplant (%) | 6 (3) | 20 (7) | 0.025 |
| Gut dysmotility (%) | 16 (8) | 40 (14) | <0.0001 |
| Connective tissue / autoimmune (%) | 5 (2) | 20 (7) | <0.0001 |
| Genetic syndromes (%) | 15 (7) | 36 (12) | 0.104 |
| Thrombophilia (%) | 19 (10) | 6 (2) | 0.228 |
| Total parenteral nutrition (%) | 199 (98) | 29 (10) | <0.0001 |
| Surgical management (%) | 189 (93) | 80 (27) | <0.0001 |
| Gut transplantation (GT) | 174 (92)∗ | 0 (0) | |
| Autologous gut reconstruction (AGR) | 15 (8) | 0 (0) | |
| Gut malrotation correction (GMC) surgery | 0 (0) | 80 (100) | |
| Overall survival (%)† | 123 (60) | 260 (88) | <0.0001 |
NA indicates non-applicable.
Sixteen patients failed prior autologous gut reconstruction.
as of February 15, 2021.
Of the 204 midgut-loss patients, 189 (93%) underwent surgical intervention; AGR in 31 (16%) (Table 3) and GT in 174 (92%) including 16 of the AGR patients (Table 4). The remaining 15 (7%) continued to receive TPN because of poor surgical candidacy or unwillingness to proceed with transplant. Of the 125 prospectively-studied patients with disabling digestive symptoms, 80 (64%) underwent GMC surgery (Table 5) with the complexity of a few patients as shown in Supplementary Figure-2. Surgery was deferred in the remaining 45 (36%) because of national/international health insurance denial (n = 16), covid-19 pandemic (n = 14), socioeconomic barriers (n = 8) or patient/parent desire (n = 7).
TABLE 3.
Clinical Features and Surgical Anatomy of the Autologous Gut Reconstruction (AGR) Patients
| Variable | Total No. | AGR-only | AGR-Gut Transplant | P Value |
| No. patients (%) | 31 | 15 (48) | 16 (52) | |
| Age at time of gut-loss (median [IQR], yr) | 1 [0–25] | 25 [15–40] | 0 [0–1] | <0.0001 |
| Age at time of surgery (median [IQR], yr) | 16 [5–31] | 30 [19–44] | 5 [2–12] | <0.0001 |
| Children (%) | 22 (71) | 6 (40) | 16 (100) | 0.0002 |
| Gender (Female : Male) | 1.1 : 1 | 1.5 : 1 | 0.8 : 1 | 0.3656 |
| Perinatal diagnosis (≤7 d) | 15 (48) | 3 (20) | 12 (75) | 0.0022 |
| Prematurity (%) | 17 (55) | 6 (40) | 11 (69) | 0.1080 |
| Associated congenital anomalies (%) | 24 (77) | 8 (53) | 16 (100) | 0.001 |
| Gastroschisis | 14 (45) | 3 (20) | 11 (69) | 0.006 |
| Intestinal atresia | 8 (26) | 4 (27) | 4 (25) | 0.915 |
| Gastroschisis + intestinal atresia | 2 (6) | 1 (7) | 1 (6) | 0.924 |
| Genetic disorders (%) | 6 (19) | 6 (40) | 0 (0) | 0.0048 |
| Length of residual intestine | ||||
| Small bowel (mean ± SD, cm) | 47 ± 43 | 65 ± 52 | 26 ± 10 | 0.014 |
| Large bowel | 0.474 | |||
| ≥50% | 8 (26) | 3 (20) | 5 (31) | |
| <50% | 23 (74) | 12 (80) | 11 (69) | |
| Prior abdominal surgery (median [IQR]) | 4 [3–6] | 4 [3–67] | 4 [2–6] | 0.6910 |
| Prior stem cell transplant (%) | 1 (3) | 1 (7) | 0 (0) | NA |
| Liver pathology (n) | 27 (87) | 12 (80) | 16 (100) | 0.0272 |
| Steatosis/cholestasis (%) | 12 (44) | 6 (50) | 6 (38) | |
| Mild/moderate Fibrosis (%) | 15 (56) | 6 (50) | 10 (63) | |
| Surgical management (%) | ||||
| Gut reconstruction | 11 (35) | 8 (53) | 3 (19) | 0.0258 |
| Bowel lengthening | 23 (74) | 9 (60) | 14 (88) | 0.037 |
| Longitudinal (Bianchi) | 6 (26) | 0 (0) | 6 (43) | |
| Transverse (STEP) | 17 (74) | 9 (100) | 8 (57) | |
| Enterotrophic (GLP-2) treatment | 1 (3) | 1 (7) | 0 (0) | 0.29 |
| Overall survival (%) | 22 (71) | 12 (80) | 10 (63) | 0.28 |
| TPN-free survival (%) | 12 (55) | 5 (42) | 7 (70)∗ | 0.032 |
| Follow-up (median [IQR], yr) | 3 [1–11] | 3 [0.3–7] | 5 [1–13] | 0.36 |
TPN indicates total parenteral nutrition.
All patients were TPN dependent before transplantation.
TABLE 4.
Transplantation for Irreversible Gut Failure after Loss of the Malrotated Intestine
| Variable | Total | Liver-Free | Liver-Containing | P Value |
| Number of recipients/allografts | 174 / 200 | 77 / 86 | 97 / 114 | NA |
| Recipient age (mean ± SD, yr) | 9 ± 7 | 13 ± 12 | 4 ± 4 | <0.0001 |
| Children / adults | 150 / 24 | 59 / 18 | 91 / 6 | 0.001 |
| Recipient sex (Female : Male) | 1: 1.5 | 1: 1.5 | 1: 1.4 | 0.896 |
| Recipient age at time of gut failure (yr) | 4 ± 4 | 9 ± 8 | 2 ± 2 | <0.0001 |
| ≤1 yr | 114 (66) | 33 (43) | 81 (84) | <0.001 |
| >1 to ≤ 18 | 40 (23) | 29 (38) | 11 (11) | |
| ≥18 | 20 (11) | 15 (19) | 5 (5) | |
| Prematurity (%) | 88 (51) | 31 (40) | 57 (59) | 0.018 |
| Other congenital anomalies (%) | 138 (79) | 46 (60) | 92 (95) | <0.0001 |
| Abdominal wall/gut | 103 (59) | 34 (44) | 69 (71) | 0.003 |
| Cardiopulmonary/neurocognitive | 35 (20) | 12 (16) | 23 (24) | 0.184 |
| Thrombophilia (%) | 17 (10) | 10 (13) | 4 (4) | 0.005 |
| Prior liver/stem cell transplant | 5 (3) | 2 (3) | 3 (3) | 0.853 |
| Total abdominal surgery (mean ± SD) | 3 ± 2 | 4 ± 2 | 3 ± 2 | 0.36 |
| Length of residual midgut | ||||
| Small bowel (mean ± SD, cm) | 20 ± 17 | 20 ± 17 | 20 ± 16 | 0.95 |
| Large bowel (≤ 50%) | 145 (83) | 60 (78) | 85 (88) | 0.08 |
| TPN duration (median [IQR], month) | 20 [12–42] | 25 [13–67] | 18 [12–33] | 0.006 |
| Gut dysmotility (%) | 8 (5) | 8 (10) | 0 (0) | <0.0001 |
| Prior autologous gut reconstruction (%) | 16 (9) | 6 (8) | 10 (10) | 0.56 |
| Total serum bilirubin (mean ± SD, mg/dl) | 11 ± 13 | 2 ± 2 | 17 ± 13 | <0.0001 |
| Liver pathology (steatosis/fibrosis/cirrhosis) | 54 / 75 / 43 | 40 / 35 / 0 | 14 / 40 / 43 | 0.001 |
| Primary allograft (%) | ||||
| Intestine/modified multivisceral | 76 / 1 | 76 / 1 | NA | NA |
| Liver-intestine/full multivisceral | 87 / 10 | NA | 87 / 10 | NA |
| Retransplantation (%) | 22 (13) | 13 (17) | 9 (9) | 0.057 |
| Positive T/B cell cross-match (n = 169, %) | 23 (14) | 9 (12) | 14 (15) | 0.626 |
| Splenectomy (%) | 42 (24) | 2 (3) | 40 (41) | <0.0001 |
| Thymoglobulin/campath-1H induction (%) | 103 (59) | 52 (68) | 51 (53) | 0.046 |
| Portal drainage (%) | NA | 28 (36) | NA | NA |
| Cold ischemia time (mean ± SD, hour) | 8 ± 2 | 7 ± 2 | 8 ± 2 | 0.001 |
| Operative time (mean ± SD, hour) | 12 ± 3 | 10 ± 3 | 13 ± 3 | 0.002 |
| Length of hospital stay (mean ± SD, week) | 8 ± 6 | 7 ± 5 | 9 ± 6 | 0.011 |
| Graft loss (death/graft failure) (%) | 110 (55) | 60 (70) | 50 (44) | 0.0004 |
| Chronic allograft rejection | 31 (18) | 22 (29) | 9 (9) | 0.001 |
| Lymphoproliferative disorder (PTLD) | 28 (16) | 11 (14) | 17 (18) | 0.56 |
| Graft versus host disease (GVHD) | 14 (8) | 6 (8) | 8 (8) | 0.9 |
| Overall patient survival (%) | 101 (58) | 40 (52) | 61 (63) | 0.146 |
| TPN-free survival (%) | 90 (89) | 32 (80) | 58 (95) | 0.017 |
| Follow-up (mean ± SD, yr) | 11 ± 8 | 9 ± 7 | 11 ± 8 | 0.06 |
NA indicates non-applicable; modified multivisceral includes stomach, duodenum, and pancreas en bloc with the intestine; full multivisceral is en bloc inclusion of the stomach, duodenum, pancreas, intestine, and liver. TPN, total parenteral nutrition.
TABLE 5.
Clinical Features and Operative Details of the Gut Malrotation Correction (GMC) Surgery Patients
| Colonic Dysmotility | ||||
| Variable | Total | No | Yes | P Value |
| Study patients (%) | 80 | 55 (69) | 25 (31) | NA |
| Children / Adults | 6 / 74 | 4 / 51 | 2 / 23 | 0.437 |
| Sex (Female : Male) | 3 : 1 | 2.2 : 1 | 7.3 : 1 | 0.05 |
| Race (White / African-American) | 79 / 1 | 54 / 1 | 25 / 0 | 0.5 |
| International patients (%) | 9 (11) | 7 (13) | 2 (8) | 0.257 |
| Hospital to hospital transfer (%) | 11 (14) | 9 (16) | 2 (8) | 0.3 |
| Age at onset of symptoms (mean ± SD, yr) | 22 ± 17 | 21 ± 17 | 25 ± 17 | 0.31 |
| Age at time of diagnosis (mean ± SD, yr) | 29 ± 17 | 27 ± 18 | 32 ± 14 | 0.015 |
| Age at time of surgery (mean ± SD, yr) | 36 ± 14 | 35 ± 14 | 37 ± 13 | 0.58 |
| Time from diagnosis to surgery (median [IQR], yr) | 3 [1–9] | 2 [1–9] | 4 [1–6] | 0.06 |
| Duration of symptoms (mean ± SD, yr) | 14 ± 15 | 14 ± 14 | 12 ± 11 | 0.05 |
| Total parenteral nutrition requirement (%) | 13 (16) | 8 (15) | 5 (20) | 0.5 |
| Recurrent bowel obstruction/volvulus (%) | 23 (29) | 14 (25) | 9 (36) | 0.3 |
| Scores of NIH-PROMIS-GI symptom scales (mean ± SD) | 29 ± 9 | 28 ± 9 | 32 ± 9 | 0.19 |
| Small bowel bacterial overgrowth (%) | 20 (25) | 10 (18) | 10 (40) | 0.04 |
| Prior abdominal surgery | ||||
| Number of patients (%) | 68 (85) | 48 (87) | 20 (80) | 0.2 |
| Number of procedures (mean ± SD) | 2 ± 2 | 2 ± 2 | 3 ± 2 | 0.2 |
| Number of prior Ladd's procedure (%) | 45 (56) | 28 (52) | 17 (68) | 0.2 |
| Elective | 34 (76) | 20 (71) | 14 (82) | 0.147 |
| Open | 25 (56) | 14 (50) | 11 (65) | 0.2 |
| Prior liver transplant | 2 (3) | 2 (4) | 0 (0) | NA |
| Prior bariatric surgery (%) | 3 (4) | 0 (0) | 3 (12) | NA |
| Connective tissue/autoimmune disorders (%) | 16 (20) | 5 (9) | 11 (44) | 0.0005 |
| Total surgical procedures (%) | ||||
| Completion of upper midgut rotation | 79 (99)∗ | 54 (98) | 25 (100) | 0.373 |
| Foregut reconstruction | 6 (8) | 2 (4) | 4 (16) | 0.0314 |
| Duodenoplasty | 4 (5) | 2 (4) | 2 (8) | 0.228 |
| Reduction of jejunal intussusception | 2 (3) | 2 (4) | 0 (0) | NA |
| Colon resection | 52 (65) | 31 (56) | 21 (84) | 0.007 |
| Pyloroplasty | 3 (4) | 0 (0) | 3 (12) | 0.002 |
| Diverting stoma | 4 (5) | 0 (0) | 4 (16) | 0.002 |
| Operative time (mean ± SD, hour) | 6.5 ± 2.1 | 6.5 ± 2.1 | 6.3 ± 2.3 | 0.5 |
| Operative blood loss (mean ± SD, ml) | 75 ± 41 | 75 ± 41 | 75 ± 43 | 0.9 |
| Length of hospital stay (mean ± SD, d) | 13 ± 5 | 13 ± 6 | 12 ± 3 | 0.3 |
| Clavien-Dindo complication grade (%) | 7 (9) | 5 (9) | 2 (8) | 0.44 |
| Grade I-II | 4 (5) | 3 (5) | 1 (4) | |
| Grade IIIa-IVa | 3 (4) | 2 (4) | 1 (4) | |
| Readmission (%)† | 6 (8) | 3 (5) | 3 (12) | 0.0019 |
| Total loaded cost (mean ± SD, $)‡ | 64 ± 22 | 63 ± 21 | 70 ± 23 | 0.6 |
| Overall survival (%) | 100 | 100 | 100 | 1.0 |
| Follow-up (mean ± SD, mo) | 37 ± 23 | 37 ± 21 | 35 ± 28 | 0.7 |
NA indicates non-applicable.
The liberated duodenum of the remaining patient was maintained and fixed on the left side (See Supplementary Figure 8).
Within first postoperative year.
In thousands.
Evaluation
The referred patients with midgut-loss underwent a thorough initial evaluation to assess nutritional status, gut anatomy, and associated pathology.53,54 The prereferral diagnostic studies and operative reports were independently reviewed by 2 of the coauthors to confirm the history of malrotation. Targeted clinical, laboratory, radiologic, endoscopic and histopathologic examinations were established to assess candidacy for gut rehabilitation and/or transplantation.54
Patients with intact gut and disabling symptoms were typically referred after needless work-up for eating, somatoform, and mental health disorders. Accordingly, a few specific studies were conducted to confirm the diagnosis and exclude other gastrointestinal/systemic disorders. Motility studies were conducted for constipated patients including sitz markers and orocecal transit time with gastric emptying, anorectal manometry, and defecography in selected cases (Supplementary Figure-3).
The well-established National Institute of Health (NIH) patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales were modified and self-reported by the GMC surgery patients.60 The 8 symptomatology domains were abdominal pain, gastroesophageal reflux, nausea/vomiting, bloating, constipation, diarrhea, pelvic floor dysfunction, and restricted oral intake (Supplementary Figure-4). With a total of 65 points, frequency and severity were measured before surgery and during the last follow-up. Lower scores indicate improvement of the GI symptoms. Patients with prior Ladd's (n = 45) were asked to answer the questionnaire to the best of their recollection assessing the symptoms before and after the procedure. Patients with early childhood Ladd's (n = 11) were not able to assess the impact of Ladd's on the symptomatology scales.
Assessment of the 171 Cleveland Clinic electronic database-identified patients was based upon the retrieved medical information including demographics and pertinent clinical features. The diagnosis was confirmed by an expert radiologist with supportive clinical and operative data. Medical history was carefully reviewed to determine onset of digestive symptoms, time of diagnosis, history of volvulus, and prior abdominal surgeries including Ladd's procedure. Special focus was directed towards details of the digestive symptoms and development of volvulus with and without midgut-loss.
SURGICAL PROCEDURES
Autologous Gut Reconstruction
The remedial procedures including bowel lengthening were recently described.54 A total of 31 patients underwent AGR as a definitive treatment or bridge to transplant. Intestinal lengthening was performed in 23 (74%) patients; Bianchi in 6 (26%) and serial transverse enteroplasty in 17 (74%). The freely mobile part of the duodenum was also lengthened. Correction of the residual gut anatomic location with mesenteric fixation was performed after completion of the reconstructive procedure(s). With descriptive features summarized in Table 3, 16 (52%) patients ultimately required GT.
Gut Transplantation
The donor and recipient operative techniques were previously published.52 Of the 174 patients, 77 (44%) required primary liver-free allografts with most being isolated intestine (Table 4). The remaining 97 (56%) received liver-containing allografts with 10 multivisceral including stomach, duodenum, pancreas, intestine, and liver. With an overall retransplantation rate of 13%, 4 recipients successfully received a third allograft. Transplant types are illustrated in Supplementary-Figure-5 with clinical data featured in Table 4.
GMC Surgery
Contrary to Ladd's (Supplementary-Figure-6), the 2 essential steps of the GMC “Kareem's” procedure were liberation of the duodenum with completion of the 270° counterclockwise-rotation and establishment of all mesenteric-attachments. The aim was to alleviate gastrointestinal symptoms and prevent volvulus. Resection of the convoluted colon was performed in patients with distorted or shortened mesocolon and colonic dysmotility.
With a few exceptions, most of the procedures were elective. Through a midline incision, the abdomen was explored with identification of the midgut anatomy including duodenum, cecocolon, and mesenteric hilum. Tissue dissection was sharp with judicious use of thermal hemostasis. Omentectomy and adhesiolysis were often required because of prior abdominal surgeries. Ladd bands were never seen in any of the patients.
Attention was first directed towards dissection of the duodenum through a combined anterior and posterior approach. In most cases, the duodenum was tethered anteriorly by the uncinate process/gastrocolic ligament and trapped posteriorly between the posterior pancreatic surface and retroperitoneal cava (Fig. 1A). There was a single example of the proximal duodenum being imprisoned within the liver parenchyma and proximal jejunum being detained in a diaphragmatic defect because of prior reconstructive surgery during infancy (Fig. 1B,C). Internal hernia with cocoon of the midgut was observed in 3 cases with situs ambiguous in one and history of multiple Ladd's in 2 (Fig. 1D).
The next step was dissection of the colon that was mostly located in the center or left side of the abdomen (Fig. 1E). The transverse and/or left colon were commonly tortuous with contracted mesentery (Fig. 1E). In some patients, the descending and sigmoid colon were sagging into the pelvic cavity due to a floppy mesorectum (Fig. 1E).
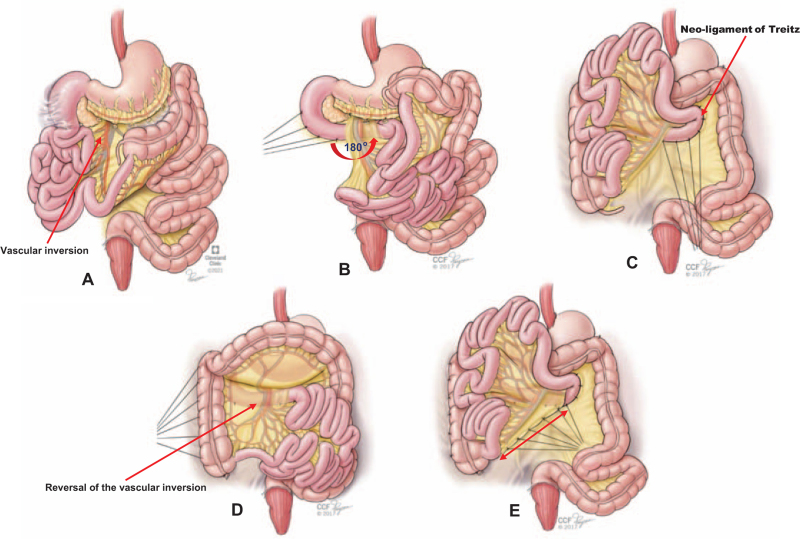
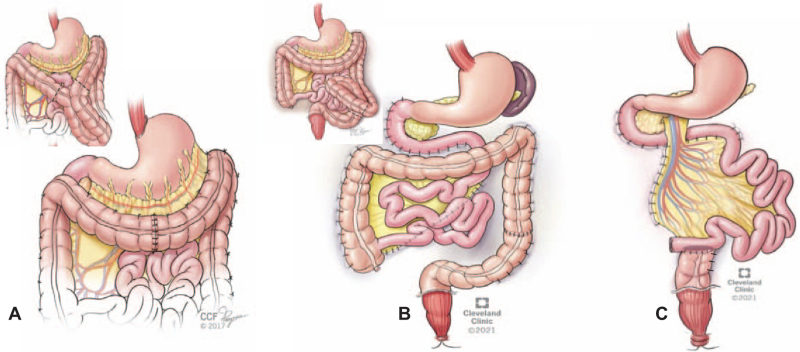
When indicated, colectomy was performed before the counterclockwise-rotation of the duodenum posterior to the mesenteric vessels with duodenopexy and creation of a neo-ligament of Treitz (Fig. 2A–C). With completion of the 180° counterclockwise enteromesenteric-rotation, vascular-inversion was consequentially reversed (Fig. 2D). The right-sided newly-oriented cecum and ascending colon were then fixed to the posterior and parietal peritoneum (Fig. 1D). Subsequently, the root of the mesentery was snugly anchored to the posterior peritoneum along the long diagonal axis between the fixed lower right cecum and upper left neo-ligament of Treitz (Fig. 1E). Segmental transverse or left colon resection was required for patients with contracted mesentery and redundant colon (Figure 3A–B). Subtotal colectomy was required for patients with severe colonic dysmotility (Figure 3C). After restoration of gut continuity, colopexy and sigmoidopexy were completed (Fig. 3). Full technical details are available at Cleveland Clinic video platform. https://www.youtube.com/user/ClevelandClinic
FIGURE 2.
The technical steps of the gut malrotation correction (GMC) surgery “Kareem's procedure”. After dissection of the duodenum, the third and fourth part were rotated (curved arrow) to the left side 180° behind the mesenteric hilum (superior mesenteric artery and vein) to complete the embryonic 270° counterclockwise midgut rotation (A-B). With proper vascular orientation, duodenopexy was completed with interrupted silk sutures creating a neo-ligament of Treitz (red arrow) in the left upper abdominal compartment (B-C). After complete dissection and freeing of the colon, the cecum and right colon were placed in the right side of the abdominal cavity and fixed into the posterior and lateral peritoneum, respectively (D). Note the resultant subsequent reversal of the vascular inversion (red arrow) . After colonic resection, when indicated, the mesenteric root is fixed to the posterior peritoneum along the diagonal long axis (double arrow line) between the cecum and neo-ligament of Treitz with interrupted silk sutures (E).
FIGURE 3.
Concomitant colon resection with the gut malrotation correction (GMC) surgery. A) Segmental resection of the transverse colon in patients with convoluted and contracted transverse mesocolon (insert). B) Segmental left colon resection in patients with convoluted and redundant descending / sigmoid colon (insert). C) Subtotal colectomy in patients with severe colonic dysmotility with a colo-ileal anastomosis in a side to end fashion. Note completion of the colopexy after the colonic resection. In patients with pelvic floor dysfunction, sigmoidopexy as well as rectopexy are indicated (B-C).
Postoperative Care
Infection prophylaxis was utilized for all patients. Thromboprophylaxis was universal and life-long anticoagulation was needed for thrombophilic individuals. Prokinetic and antidiarrheal agents were required for AGR patients with periodic treatment of bacterial overgrowth in selected cases. GLP-2 was given to a single AGR patient who failed TPN weaning.54
The complex management of transplant recipients stemmed from the high intestinal allograft immunogenicity and intricacy of the surgical procedures52–54 Immunosuppression was tacrolimus-steroid based with induction/preconditioning in 103 (59%) (Table 4). Postoperative monitoring included early diagnosis and treatment of rejection, graft versus host disease (GVHD), posttransplant lymphoproliferative disorders (PTLD), and cytomegaloviral infection.
Long-term follow-up included regular visits which were more frequent and comprehensive for the transplant recipients. The GMC surgery patients required yearly follow-up with upper gastrointestinal contrast series for certain patients (Supplementary Figure-7).
Quality of Life Assessment
The assessment was limited to the surgical patients. A chart review was completed for the transplant survivors with special focus on neurocognitive functions, mental health issues, and socioeconomic status documented by mental health professionals.61 The GMC surgery patients were prospectively evaluated for changes in the modified eight NIH-PROMIS gastrointestinal symptom domains and TPN requirement. Postoperative complications, hospital readmissions, reoperations, and current body mass index status were used as surrogate markers of global health. The physical performance status of transplant survivors and GMC surgery patients was assessed utilizing the Karnofsky/Lansky scale system.
Data Management and Statistical Analysis
Data were collated into a master file and stratified according to age at time of GM diagnosis and status of midgut. The prospectively collected data of the midgut-loss patients were classified according to the type of surgical intervention. Both AGR and GT patients were sub-grouped according to subsequent need for transplant and type of required allograft, respectively. The GMC surgery group was classified according to status of colonic motility.
Data were summarized as mean ± standard deviation or median (interquartile range, IQR) for continuous and percentages for categorical variables. Group differences were assessed with paired/unpaired t test, ANOVA, and nonparametric Kruskal-Wallis rank-sum. Noncontinuous variables were examined using the Pearson chi-square test.
Time to development of midgut volvulus with and without midgut-loss was illustrated in a scatter plot. Survival and cumulative risk of midgut-loss were calculated using Kaplan-Meier product limit and group comparison was with log-rank test. All events were computed as of February 15, 2021.
Predictive Modeling
The total population was computed to develop midgut-loss predictive and GMS defined models. For midgut-loss, Cox proportional hazard model was used with time to event being calculated from date of birth to date of midgut-loss or last follow-up. Age at time of GM diagnosis, sex, race, prematurity, gastroschisis, intestinal atresia, autoimmune/systemic disorders, Ladd's, and volvulus were computed as exposure variables. For the GMS model, the 125 patients with disabling gastrointestinal symptoms were computed in reference to the rest of the 296 intact gut patients. The exposure variables were abdominal pain, gastro-esophageal reflux, nausea/vomiting, bloating, and altered bowel habits. The stepwise variable selection and univariate/multivariate analyses methods were used to develop the generalized linear regression model. All analyses were done using R. software package (R studio, version 3.5.2, Boston, MA).
RESULTS
Total Population Descriptive Analysis
The complexity of the total study population was indicated by history of volvulus in 51% and associated abdominal congenital anomalies in 43% with genetic, connective tissue, and gut motility disorders in 26%. History of abdominal surgery was documented in 88%. Prior transplants included liver (n = 17), kidney (n = 3), stem cell (n = 3), heart (n = 2), and double-lung (n=1). History of Ladd's was documented in 192 (38%) patients.
Sex, race, prematurity, associated abdominal congenital anomalies, duration of symptoms, prior abdominal surgeries, and volvulus were significant features of both early GM diagnosis (Table 1) and midgut-loss (Table 2). History of Ladd's, connective tissue disease, autoimmune disorders, and dysmotility were observed at a significantly (P < 0.0001) higher rate among patients with intact gut. The digestive symptoms among the 296 patients with intact gut were abdominal pain (67%), gastro-esophageal reflux (36%), nausea/vomiting (58%), bloating (38%), constipation (45%), and/or diarrhea (29%). GMS was identified in 42% of the study population.
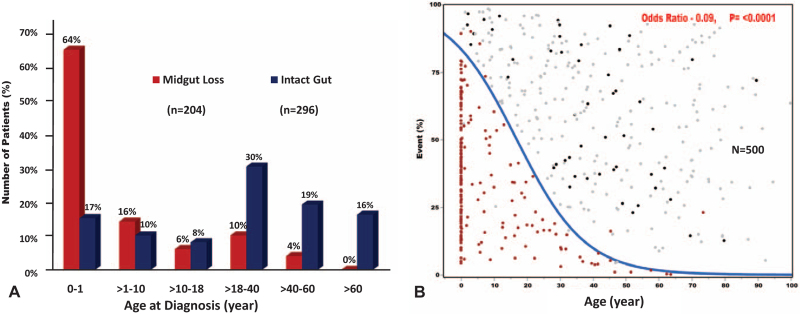
The distribution of midgut-loss according to age is shown in Figure 4A. The inverse correlation between age and volvulus development is illustrated in Figure 4B. Note the highest incidence of volvulus and midgut-loss during the first year of life, including 30 neonates.
FIGURE 4.
The clinical presentation of the 500 gut malrotation patients according to age. A) Incidence of midgut-loss according to age categories. Note the highest incidence among infants including 30 neonates. Most of the patients with intact gut had at least one gastrointestinal symptom with 125 (42%) had gut malrotation syndrome (GMS) according to the newly introduced modified NIH-PROMIS-GI symptom scales-based model. B) The significant correlation between age and development of volvulus with ( dark red dots) and without ( black dots) catastrophic midgut-loss. The patients who did not develop volvulus were presented with gray dots. Note that midgut-loss was clustered at early age but still occurred among older patients.
Volvulus after Ladd's Procedure
With the intent to treat, volvulus was documented in 97 of the 192 patients who underwent Ladd's procedure with an overall incidence of 51%. The midgut was rescued in 50 patients with an overall success rate of 52%. With a mean follow-up of 16 ± 3 years (range: 1–74), recurrent or de novo volvulus was documented in 41 of the 192 patients with an overall risk of 21%. Of these, 18 (44%) ultimately lost the midgut.
Autologous Gut Reconstruction
Of the 31 AGR patients, 22 (71%) were children with none of the patients having hepatic cirrhosis (Table 3). The ultimate need for GT was denoted by higher percentages of perinatal diagnosis, younger age, associated gut anomalies particularly gastroschisis, and shorter bowel length. In the AGR-only patients, the number of primary reconstructive procedures was significantly higher with less need for bowel lengthening (Table 3).
With a median follow-up of 3 years (range: 1–11), 22 (71%) were alive including those who were rescued with transplant achieving an overall TPN-free survival of 55%. TPN-dependent survivors were under evaluation or not candidates for transplant. The 3 AGR-only mortalities were due to line-infection, pneumonia-associated severe combined immune deficiency (SCID), and GVHD after stem cell transplant for SCID. The overall Kaplan-Meier cumulative survival was 78% at 1, 5, and 10 years.
Gut Transplantation
At the time of transplant, 150 (86%) patients were children with a higher need for liver-containing allograft (61%) particularly among infants (84%) (Table 4). Interestingly, liver-containing transplant was required at a significantly higher rate among premature children and patients with abdominal wall and/or gut anomalies. With a maximum of 13 prior abdominal surgeries, 5 patients had prior liver (n = 4) or stem cell (n = 1) transplant. A few years after transplant, the 4 liver recipients developed midgut volvulus complicated with mesenteric infarction requiring retransplantation with liver-free (n = 1) and liver-containing (n = 3) visceral allografts. Operative and postoperative details are given in Table 4.
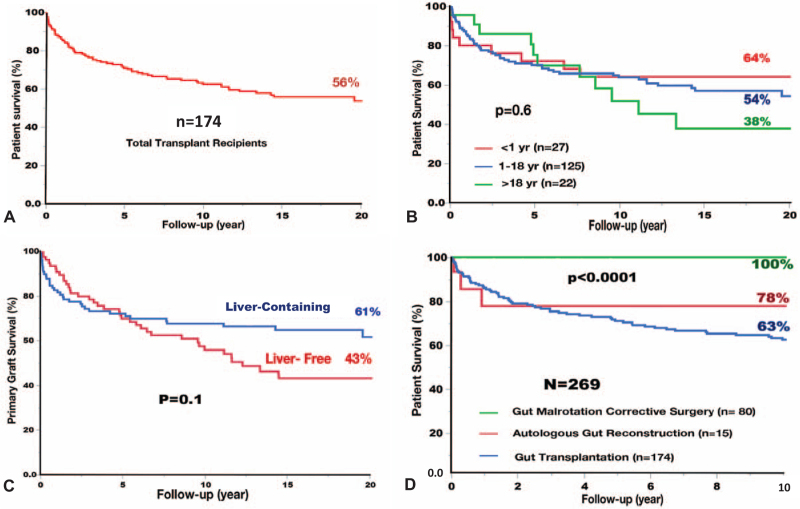
With a mean follow-up of 11 ± 8 years, 101 (58%) patients were alive with TPN-free survival of 89%. Leading causes of death were sepsis (32%), rejection (23%), PTLD (11%), technical complications (10%), and GVHD (8%). With a maximum follow-up of 30 years, cumulative patient survival was 86% at 1-year, 71% at 5-years, 63% at 10-years, and 54% at 20-years (Fig. 5A). Infants (Fig. 5B) and liver-containing allografts (Fig. 5C) had the best survival with 20-year rates of 64% and 61%, respectively.
FIGURE 5.
Kaplan-Meier cumulative survival among the 269 surgically treated patients: A) Overall transplant patient survival , B) Recipient survival according to age, C) Survival of the liver-free and liver-containing allografts, and D) Survival of the gut malrotation correction (GMC) surgery patients compared to autologous reconstructive and transplant surgery. Note best transplant survival among infants and liver-containing allografts with no mortalities among GMC surgery patients.
Neurocognitive and mental health disorders were documented in 45% and 60% of 89 current survivors, respectively. Common impairments were intellectual disability, developmental delay, anxiety, depression, and autism. Risk factors were age at time of midgut-loss (odds ratio = 1.1, P = 0.017) and associated congenital disorders (odds ratio = 2.4, P = 0.04).
Most adult survivors completed high school or higher education with the younger age group continuing to attend schools with an overall education index of 97%. With 56% still being students, 30% were fully employed with 3% homemakers. The remaining 11% were either unemployed (6%) or in preschool/day care (5%). Equally impressive was the achievement of 80% to 100% Lansky/Karnofsky performance score in 85% of total survivors. Remarkably, 2 female recipients gave birth to a total of 3 healthy children and 4 male patients fathered 6 children.
GMC Surgery “Kareem's Procedure”
Of the 80 GMC surgery patients, 74 (92%) were adults and 6 (8%) were children including a 13-month old baby. Associated colonic dysmotility was observed in 25 (31%) patients (Table 5). The dysmotility patients were all White with female predominance. Other distinguishing features were concomitant connective tissue disease and autoimmune disorders including Ehlers Danlos syndrome, older age at time of diagnosis, bacterial overgrowth, and shorter duration of symptoms.
Surgical correction of the midgut anatomic abnormalities was complete in all but 1 adult patient with long-segment duodenal atresia requiring duodenojejunal reconstruction at birth. As such, all the steps of GMC surgery were performed with the exception of fixing the entire duodenum to the right of the mesenteric hilum (Supplementary Figure-8). Simultaneous foregut reconstruction was performed in 6 patients; gastrogastric (n = 2), gastroplasty (n = 1), jejunal interposition (n = 1), reversal of fundoplication (n = 1), and simultaneous gastric-bypass (n = 1). Prior bariatric surgery was documented in 3 patients with sleeve (n = 2) and Roux-en Y (n = 1). Duodenoplasty was required for 4 incidental duodenal diverticulae with ectopic gastric mucosa in one. With reduction of 2 jejunal intussusception, midgut reconstruction was required in another case with enterocutaneous fistula due to a technically-flawed operation before referral. Colon resection was required at a significantly (P = 0.007) higher rate among dysmotility patients with concomitant need for pyloroplasty and diverting stoma in few cases (Table 5). It is imperative to emphasize that these adjunct digestive surgeries were performed in the patients who needed colon resection.
With the perioperative data given in Table 5, there were no technical complications. With minimal operative blood loss, the mean operative time was 6.5 hours. In patients with no history of multiple abdominal operations or need for simultaneous foregut and colonic surgeries, the mean operative time was 4.1 ± 1 hour with a minimum of 2.9 and maximum of 6 hours. Nonetheless, all procedures were open with hand-sewn anastomoses in the milieu of a teaching environment. The postoperative hospital recovery was relatively slow among patients with motility disorders.
Postoperative complications developed in a total of 7 (9%) patients with 4 experiencing Clavien-Dindo grade I-II due to wound and line infections. The remaining 3 had grade IIIa-IVa with short-lived respiratory insufficiency due to fluid overload, line placement-induced pneumothorax, and intra-abdominal infection with percutaneous drainage. Hospital readmission within the first 90 postoperative days was required for 3 (5%) of the non-colonic dysmotility patients due to line-induced bacteremia (n = 2) and vague abdominal pain (n = 1). Another 3 (16%) gut dysmotility patients were admitted within the first year for uncomplicated sigmoid diverticulitis, line infection, and radiologically-proven functional bowel obstruction. None of the patients required surgical intervention. However, a total of 11 (10%) patients with recurrent refractory constipation required completion colectomy during the study period with concomitant ventral hernia repair in 5 cases.
With a mean follow-up of 36 ± 23 months, all patients were alive with no single example of de novo or recurrent volvulus. The long-term survival was better compared to those who developed midgut-loss and underwent AGR and/or GT (Fig. 5D).
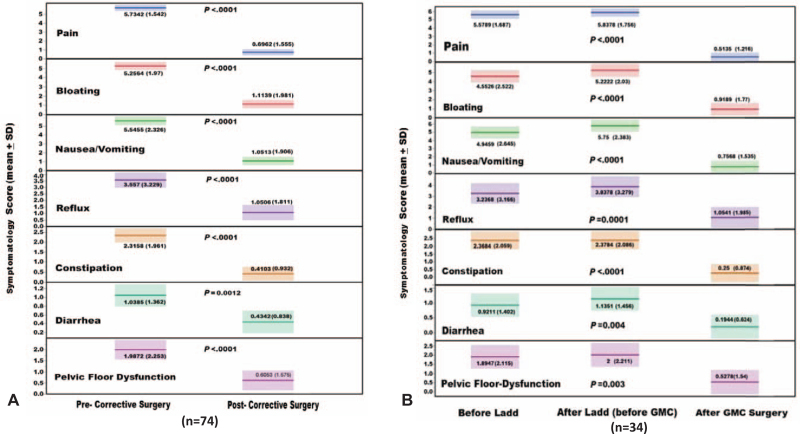
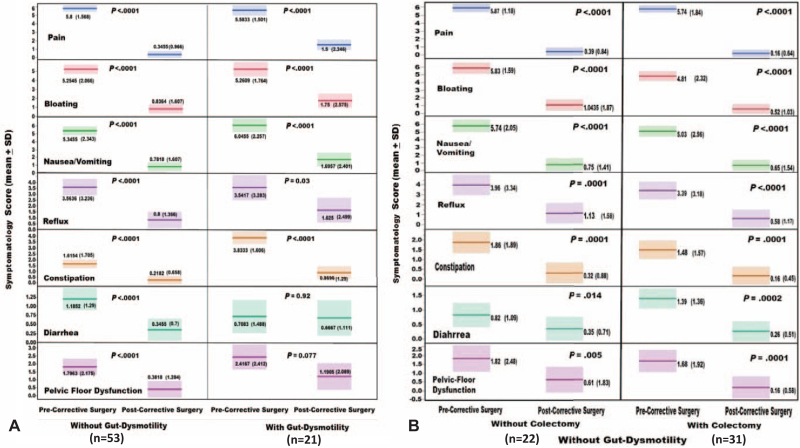
With a maximum of 10 year follow-up and exclusion of patients with adjunct major foregut reconstruction, the preoperative scores of the eight NIH-PROMIS gastrointestinal symptom scales significantly (P < 0.001) improved in all of the 74 study patients (Fig. 6A). The same level of significance was also observed among patients with worsening symptoms after Ladd's (Fig. 6B). Similar results were observed among patients with and without gut dysmotility (Fig. 7A). Furthermore, such a significant improvement was maintained among the 22 patients without colon resection (Fig. 7B). These testimonial results leave no doubt concerning the therapeutic efficacy of GMC surgery in treating the GM-associated digestive symptoms (Fig. 7B). All of the 13 patients with preoperative-TPN achieved full nutritional autonomy maintaining an average body mass index of 25 kg/m2.
FIGURE 6.
The impact of gut malrotation correction (GMC) surgery on the modified preoperative National Institute of Health (NIH) patient-reported outcomes measurement information system (PROMIS) gastrointestinal Symptom Scales. A) The total study patients (n=74) with highly significant improvement in each of the symptom domains. B) The sub-cohort with prior Ladd's procedure and complete study points (n=34). Note worsening of the symptom scales after Ladd's with significant improvement after GMC surgery. The significant improvement in the eighth oral restricted intake scale is not shown in the figure because of data limitations in the utilized illustration computer program.
FIGURE 7.
The impact of gut malrotation correction (GMC) surgery on the preoperative modified National Institute of Health (NIH) patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. A) The study patients without and with gut dysmotility. B) The non-dysmotility patients with and without adjunct colectomy. Note the universal significant improvement among all study patients including those who did not undergo colon resection confirming the sole therapeutic efficacy of the GMC surgery.
The preoperative Karnofsky/Lansky performance scores significantly (P < 0.0001) improved after GMC surgery. The preoperative scores were below 50% in 52 (65%) patients and between 50% and 70% in the remaining 28 (35%). At the last follow-up, most patients experienced performance scores of 80% to 100% resuming full daily activities.
Outcome Analysis and Predictive Models
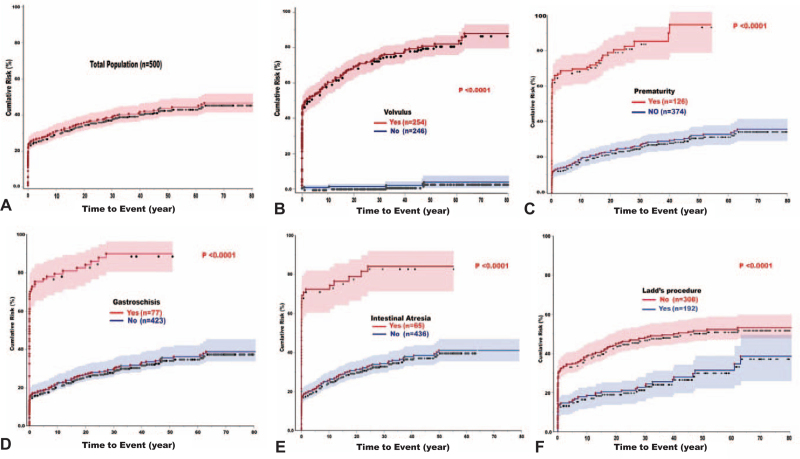
With univariate analysis, volvulus, prematurity, gastroschisis, intestinal atresia, and male sex were risk factors for midgut-loss. Ladd's, autoimmune/systemic disorders, White race, and increasing age were associated with reduced probability of midgut-loss. The overall cumulative risk of midgut-loss among the total population was 30% at 10 years, 43% at 50 years, and 47% at 80 years (Fig. 8A). The cumulative risk with each variable is depicted in Figure 8B to F. With the intent to treat, midgut-loss still occurred with Ladd's at a cumulative incidence of 18% at 10-years, 31% at 50-years, and 38% at 80-years (Fig. 8F). With multivariate analysis, volvulus, prematurity, gastroschisis, and intestinal atresia continued to be significant midgut-loss risk factors while Ladd's and increasing age continued to be associated with reduced risk of gut-loss (Table 6). Full details including the receiving operating characteristics (ROC) curve and the linear predictive equation are exhibited in Supplementary Figure-9A.
FIGURE 8.
The Kaplan-Meier cumulative risk of midgut-loss in patients with gut malrotation. A) Total population, B) According to development of volvulus, C) Prematurity, D) Gastroschisis, E) Intestinal atresia, and F) Ladd's procedure. Volvulus was the most significant risk factor and Ladd's procedure was protective. Solid lines are the curves for cumulative risk, dotted lines are patients at risk, and shaded areas are the confidence interval (CI).
TABLE 6.
Predictors of Midgut-loss and Development of Gut Malrotation Syndrome (GMS)
| Midgut Loss (N = 500) | |||
| Hazard Ratio (HR) | 95% Confidence Interval | P Value | |
| Volvulus | 27.144 | 11.861–62.120 | <0.001 |
| Prematurity | 2.137 | 1.556–2.937 | <0.001 |
| Gastroschisis | 1.667 | 1.209–2.299 | 0.002 |
| Intestinal atresia | 1.445 | 1.028–2.032 | 0.034 |
| Ladd's procedure | 0.323 | 0.228–0.457 | <0.001 |
| Age at time of diagnosis | 0.945 | 0.931–0.958 | <0.001 |
| Gut Malrotation Syndrome (n = 296) | |||
| Odds Ratio (OR) | 95% Confidence Interval | P Value | |
| Gut dysmotility | 14.99 | 4.9–45 | 0.003 |
| Race/Ethnicity (White) | 4.11 | 1.23–13.7 | 0.021 |
| Ladd's procedure | 1.8 | 1.06–3.06 | 0.028 |
| Sex (female) | 1.76 | 1.01–3.08 | 0.046 |
The generalized linear predictive model defined GMS with abdominal pain, nausea/vomiting, and bloating being the distinctive clinical symptoms. The syndrome was identified in 42% of the study patients. With the overall probability of 0.96, bloating had the highest statistical weight. With multivariate analysis, gut dysmotility, White race, Ladd's procedure, and female sex were significant predictors of GMS (Table 6). The model was formulated with 91% sensitivity and 94% specificity. Full details including the linear predictive equation are provided in Supplementary Figure-9B.
DISCUSSION
With great wisdom, Professor Ladd warned us that GM is rare enough so that it is likely to escape the mind and it is common enough to be important.8 He also stated that timely and suitable surgical intervention offers the only chance for cure and restoration of the health of these patients.26 Accordingly, scientists and clinicians across the world continued to tackle such a puzzling and potentially lethal disorder.2–7,9–24,62–64
During the last century, the field has experienced certain misconceptions and controversies. Most physicians continued to believe that GM is a childhood disorder.44–46 The congenital syndrome was also considered as a simple morphologic anomaly resulting in misnomenclature of the type of malrotation.1 Of utmost importance, have been the surgical controversies concerning restitution of the mesenteric-attachments and management of the asymptomatic patients.15–20,36 This study and other recently published data emphasized the clinicopathologic diversity of the syndrome with subtle and often overlooked symptoms in a considerable number of patients.13–24,65,66 With increased awareness and frequent use of dedicated imaging studies, it is anticipated that there will be less misdiagnosis with increased recognition of GM as a clinical syndrome particularly in adults.
Recent years witnessed new paradigms in the pathogenesis of GM. The role of the mesentery in the embryonic development and support of the human digestive organs has been elucidated.4–7,9,55,56 In addition, human biologists and geneticists advocated an interplay between the aberrant enteric nervous system and the abnormally rotated midgut67,68 Such a notion was supported by the recent documentation of intrinsic neuropathologic abnormalities in a malrotated human intestine.69 A similar study is currently in progress at our institution with intriguing initial results. Accordingly, it is time for clinicians and surgeons to recognize GM as an enteromesenteric syndrome with intrinsic motility disorders.
This large series addressed the full clinical spectrum of GM in both children and adults. There was an observed wide gap between onset of symptoms, diagnosis, and surgical treatment. Associated genetic defects and other congenital abdominal anomalies seemed to drive the earlier childhood diagnosis.70–72 Connective tissue, autoimmune, and gut motility disorders were common among patients with adulthood diagnosis. These commonalities could be partially explained by the differential disruption of the intercellular molecular and genetic signals that regulate migration, differentiation and maturation of the endodermic, mesodermic, and neural crest cells. It has also been speculated that intrauterine vascular insults could be another contributing factor.4–7,9
The many faces of malrotated gut were portrayed with special emphasis on clinical presentations in milieu of age at diagnosis and necessity for surgical intervention. The development of midgut volvulus with the imminent risk of mesenteric infarction was the most devastating feature particularly among the pediatric population.73,74 This study is the first to document the cumulative incidence of midgut-loss with identification of several risk factors including volvulus, prematurity, gastroschisis, and intestinal atresia. Along with other scattered publications, Ladd's procedure and increasing age reduced but did not prevent the risk of volvulus.15–24,31–48
Patients with intact gut experienced digestive symptoms which were commonly incapacitating. With the first adult case being reported in 1960s, recent literature highlighted the common development of GM symptoms in both adults and children.37–41 This study is the first to define the GMS comprising of pain, nausea/vomiting, and bloating. The frequency and severity of these symptoms varied according to the distorted anatomy and altered motility of midgut with the development of intermittent volvulus.17–19,49–51 The designed herein patient-generated report captured the breadth and depth of the patient illness experience.
With the largest series and the longest follow-up ever reported in the literature, this study featured recent advances in the surgical management of midgut malrotated patients. With massive midgut-loss, integrative surgical management with AGR and GT achieved long-term 10-year survival of 78% and 63%, respectively. Infants experienced the best outcome with 20-year survival rate of 64%. Overall, 2 decades of functional survival were attainable with better quality of life.
One of the primary objectives of this study was to introduce the new operation and assess its therapeutic efficacy. The surgical principles of the GMC surgery stemmed from the normal embryonic development and rotation of the human midgut. In contrast to Ladd's, the procedure normalizes the enteromesenteric structural and vascular anatomy with restoration of the defective interface between the mesentery and retroperitoneum.
The anatomically-based procedure is safe, effective, and easy to perform in all ages. It should be recognized as an integral part of the surgical armamentarium. Collective efforts should be directed towards training both pediatric and adult digestive surgeons as a part of the surgical training curriculum with establishment of a current procedural terminology (CPT) code. It remains to be seen if the procedure can be laparoscopically performed.
This article calls for 2 evidence-based recommendations. First, the current favorable long-term outcomes with gut rehabilitation and transplantation substantiate a definitive rather than a comfort care for neonates and infants with midgut infarction.61,75,76 Second, the proven herein therapeutic efficacy of GMC warrants utilization of the procedure for the GM symptomatic patients, those with volvulus after Ladd's, and expectantly the asymptomatic patients.77 Despite the need for additional long-term follow-up, the entailed enteromesenteric corrections are proven to preclude the risk of midgut volvulus. Furthermore, new advances in the perinatal diagnosis of midgut malrotation/volvulus with prompt surgical intervention are expected to enhance the outcome of such a potentially life-threatening complication.78–80
Supplementary Material
Acknowledgments
The authors would like to thank their patients for their great efforts in increasing public and medical community awareness about congenital malrotation underscoring its potential life-threatening and disabling effects on digestive health. They extend their gratitude to the Cleveland Clinic, the University of Pittsburgh medical team, Center of Medical Art, and Ms. Sabrina Phillips.
DISCUSSANT
Dr. Gail Besner
Thank you for allowing a pediatric surgeon to comment on Dr. Abu-Elmagd's beautiful presentation of 500 patients with a history of intestinal malrotation. In this report, patients with significant intestinal loss mainly underwent transplantation, and those without intestinal loss but with disabling GI symptoms, underwent a newly described procedure called “gut malrotation correction.”
Ladd described his procedure to correct malrotation almost a century ago. Very interestingly however, the procedure that we as pediatric surgeons do is very different than the GMC that the authors describe. We detorse the bowel if there is a volvulus, divide Ladd's bands and completely straighten out the duodenum, and broaden the base of the small bowel mesentery by putting all of the small bowel on the right and all of the colon on the left, like opening the pages of a book. We do not try to recreate normal rotational anatomy. Although the Ladd's procedure does not decrease the chance of a future midgut volvulus to zero, it makes it extremely unlikely, probably in part because of the development of postoperative adhesions.
My questions are regarding the GMC performed in 80 patients. The authors describe that some of their patients had tethered duodenum or internal small bowel hernias, and two thirds had redundant colon requiring partial colectomy. These abnormalities were corrected at the same time as the duodenopexy, colopexy, and mesentericopexy. Is it possible that correction of the small bowel or colonic problems alone, without recreation of rotation, may have been enough? Also, do the authors recommend any changes in the operative technique performed by pediatric surgeons when we operate for malrotation, which is often in the first months of life? Thank you again for a lovely presentation.
Response Dr. Kareem M. Abu-Elmagd
Thank you Dr. Besner for reviewing the manuscript and I greatly appreciate your kind remarks. First, I would like to address 2 controversial issues in your comments. The Ladd's is not a correction procedure as clearly stated in the presentation and the manuscript. The procedure primarily aimed to rescue patients, particularly infants, with midgut volvulus and duodenal obstruction. Along with widening of the mesentery, the malrotated distal midgut was reverted to an earlier stage with 90° clockwise rotation to separate the cecum and right colon from the duodenum and proximal bowel. In essence, the midgut rotational anomaly was converted from asynchronous to synchronous mode.
Your statement that future midgut volvulus is extremely unlikely to occur after Ladd's is an interesting one. In fact, this evidence-based study showed the contrary. Despite its potential nonelective therapeutic benefits and with the intent to treat, Ladd's procedure was still associated with a cumulative risk of recurrent or de-novo midgut volvulus with mesenteric infarction of 10% at 10 years and up to 38% at 80 years. The manuscript fully addressed such a pivotal point with the longest ever published follow-up time. Nonetheless, we all must agree that every gut and every life matters.
To answer your 2 questions, I would like to clarify certain relevant points stated in your discussion. The tethered duodenum was found in almost every patient and the internal hernia was observed in only a few cases. The colon was redundant but coiled because of shortened and contracted mesentery. To fully address the first question, the gastrointestinal symptomatology domains data were analyzed in patients with and without colon resection and highlighted in the final manuscript. The improvement in each of the symptom scales continued to be highly significant in the cohort without colon resection. Accordingly, surgical recreation of the embryonic normal rotational anatomy is the essence of symptoms improvement. In a nutshell, GM is an enteromesenteric disorder that it is hard to explain but easy to fix.
I truly believe, based on the data presented today, that all patients with malrotation including infants and adults with incidental diagnosis should be offered the new operation and for the lack of a better term you can call it “Kareem's procedure.” The operation is easy to perform. The total operative time ranges from 3 to 4 hours particularly in patients without prior multiple abdominal surgeries and those who do not require adjunct procedures or extensive colon resection. I am committed to provide both the pediatric and adult surgeons with the exposure needed to perform such a simple procedure with the hope for imminent introduction of a safe laparoscopic approach.
If he were with us today, I believe that Professor Ladd would certainly welcome such an important contribution to the field since his landmark presentation at the Boston Surgical Society nearly a century ago.
Dr. Andreas Tzakis (Cleveland, OH)
I want to thank Dr. Abu-Elmagd for sharing his manuscript with me. This is a landmark paper. It not only reviews the author's unique lifelong experience with the care of patients with GM but establishes guidelines for diagnosis and innovative treatment. A first principle is to stop applying just comfort measures for babies who lost their gut! Agree 100%. The first year of life is when most of these patients lose their gut. With transplantation more than half of them will live long term. There is a paradox: despite of hospitalizations, surgeries, immunosuppression, most survivors complete education, maintain full employment, and some gave childbirth.
I have 2 questions regarding the surgical treatment: Patients who underwent gut reconstruction suffered 22% mortality in the first postoperative year. Do you have a plan or proposals on how to mitigate these early losses? Losses after transplantation are continuous and present even more than 10 years after transplantation. The liver has a protective effect. The losses are for the most part due to undiagnosed rejections. We think that these undiagnosed rejections are due to lack of monitoring and for this reason we introduced serum Citrulline as a practical marker of intestinal damage. Like other markers we use in transplantation, it indicates damage and is not specific for rejection. If not Citrulline, do you have other suggestions for effective monitoring?
Dr. Kareem M. Abu-Elmagd
Thank you Andy for your kind remarks and your significant contribution to the field of intestinal and multivisceral transplantation. First, I would like to emphasize that there was no mortalities after the GMC surgery. The 3 deaths among the AGR patients were inevitable due to SCID. Two patients died of infection and the third was a victim of fatal GVHD after stem cell transplant. It is imperative to emphasize that none of these 3 patients were candidate for GT because of the prohibitive risk of post-transplant GVHD due to the combined immune deficiency.
With GT, I agree that the field is waiting for a reliable, sensitive, and specific biomarker for early detection with prompt treatment of acute intestinal allograft rejection. However, sepsis, PTLD, GVHD, and the sinister problem of chronic rejection continued to play an important role in early and late graft loss. The favorable long-term outcome observed among the liver-containing allografts is a testimony of the immunoprotective effect of the liver that we have revealed more than two decades ago. Innovative tactics including achievement of clinical allograft tolerance are still needed to overcome the long-term hazards of chronic rejection among liver-free allografts and infection in multivisceral recipients. The annual meeting of this prestigious association witnessed, on at least 6 occasions, our contribution to the evolution of the field with the introduction of novel surgical techniques, effective immunosuppressive strategies, and better postoperative care.
REFERENCES
- 1.Dott NM. Anomalies of intestinal rotation: their embryology and surgical aspects: with report of five cases. Br J Surg 1923; 11:251–286. [Google Scholar]
- 2.Snyder WH, Jr, Chaffin L. Embryology and pathology of the intestinal tract: presentation of 40 cases of malrotation. Ann Surg 1954; 140:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firor HV, Harris VJ. Rotational abnormalities of the gut: re-emphasis of a neglected facet, isolated incomplete rotation of the duodenum. Am J Roentgenol 1974; 120:315–321. [DOI] [PubMed] [Google Scholar]
- 4.Kluth D, Jaeschke-Melli S, Fiegel H. The embryology of gut rotation. In seminars in pediatric surgery. Elsevier 2003. 275–279. [DOI] [PubMed] [Google Scholar]
- 5.Soffers JH, Hikspoors JP, Mekonen HK, et al. The growth pattern of the human intestine and its mesentery. BMC Dev Biol 2015; 15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WK, Kim H, Ahn DH, et al. Timetable for intestinal rotation in staged human embryos and fetuses. Birth Defects Res A Clin Mol Teratol 2003; 67:941–945. [DOI] [PubMed] [Google Scholar]
- 7.Elumalai G, ALL. Malrotation of midgut” embryological basis and its clinical significance. Elixir Embryol 2016; 100:43420–43424. 103: 45657-45660. [Google Scholar]
- 8.Ladd WE. Congenital obstruction of the duodenum in children. N Engl J Med 1932; 206:277–283. [Google Scholar]
- 9.Martin V, Shaw-Smith C. Review of genetic factors in intestinal malrotation. Pediatr Surg Int 2010; 26:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Chen S, Yan W, et al. Congenital short-bowel syndrome: clinical and genetic presentation in China. J Parenter Enter Nutr 2020; doi: 10.1002/jpen.1974. [DOI] [PubMed] [Google Scholar]
- 11.Lv X, Chen H, Sun X, et al. Assessment of plasma microRNAs in congenital intestinal malrotation. Mol Med Rep 2020; 22:3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welch G, Azmy A, Ziervogel M. The surgery of malrotation and midgut volvulus: a nine year experience in neonates. Ann Royal Coll Surg Engl 1983; 65:244–247. [PMC free article] [PubMed] [Google Scholar]
- 13.Mehall JR, Chandler JC, Mehall RL, et al. Management of typical and atypical intestinal malrotation. J Pediatr Surg 2002; 37:1169–1172. [DOI] [PubMed] [Google Scholar]
- 14.Kapfer SA, Rappold JF. Intestinal malrotation—not just the pediatric surgeon's problem. J Am Coll Surg 2004; 199:628–635. [DOI] [PubMed] [Google Scholar]
- 15.Malek MM, Burd RS. Surgical treatment of malrotation after infancy: a population-based study. J Pediatr surg 2005; 40:285–289. [DOI] [PubMed] [Google Scholar]
- 16.Malek MM, Burd RS. The optimal management of malrotation diagnosed after infancy: a decision analysis. Am J Surg 2006; 191:45–51. [DOI] [PubMed] [Google Scholar]
- 17.McVay MR, Kokoska ER, Jackson RJ, et al. The changing spectrum of intestinal malrotation: diagnosis and management. Am J Surg 2007; 194:712–719. [DOI] [PubMed] [Google Scholar]
- 18.Lampl B, Levin TL, Berdon WE, et al. Malrotation and midgut volvulus: a historical review and current controversies in diagnosis and management. Pediatr Radiol 2009; 39:359–366. [DOI] [PubMed] [Google Scholar]
- 19.Nehra D, Goldstein AM. Intestinal malrotation: varied clinical presentation from infancy through adulthood. Surgery 2011; 149:386–393. [DOI] [PubMed] [Google Scholar]
- 20.Lodwick DL, Minneci PC, Deans KJ. Current surgical management of intestinal rotational abnormalities. Curr Opin Pediatr 2015; 27:383–388. [DOI] [PubMed] [Google Scholar]
- 21.Covey SE, Putnam LR, Anderson KT, et al. Prophylactic versus symptomatic Ladd procedures for pediatric malrotation. J Surg Res 2016; 205:327–330. [DOI] [PubMed] [Google Scholar]
- 22.Langer JC. Intestinal rotation abnormalities and midgut volvulus. Surg Clin 2017; 97:147–159. [DOI] [PubMed] [Google Scholar]
- 23.Kinlin C, Shawyer AC. The surgical management of malrotation: a Canadian Association of Pediatric Surgeons survey. J Pediatr Surg 2017; 52:853–858. [DOI] [PubMed] [Google Scholar]
- 24.Neville JJ, Gallagher J, Mitra A, et al. Adult presentations of congenital Midgut Malrotation: a systematic review. World J Surg 2020; 44:1771–1778. [DOI] [PubMed] [Google Scholar]
- 25.Ladd WE. Congenital obstruction of the small intestine. J Am Med Assoc 1933; 101:1453–1458. [Google Scholar]
- 26.Ladd WE. Surgical diseases of the alimentary tract in infants. N Engl J Med 1936; 215:705–708. [Google Scholar]
- 27.Wangensteen O. New operative techniques in the management of bowel obstruction. Surg Gynec Obst 1942; 75:675–692. [Google Scholar]
- 28.Estrada RL. Anomalies of Intestinal Rotation and Fixation: Including Mesentericoparietal Hernias. Illinois, USA: Thomas, Springfield; 1958. [Google Scholar]
- 29.Rees JR, Redo SF. Anomalies of intestinal rotation and fixation. Am J Surg 1968; 116:834–841. [DOI] [PubMed] [Google Scholar]
- 30.Gohl ML, DeMeester TR. Midgut nonrotation in adults: an aggressive approach. Am J Surg 1975; 129:319–323. [DOI] [PubMed] [Google Scholar]
- 31.Haak BW, Bodewitz ST, Kuijper CF, et al. Intestinal malrotation and volvulus in adult life. Int J Surg Case Rep 2014; 5:259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higashi Y, Onishi I, Kayahara M, et al. A case of midgut volvulus related to adult intestinal malrotation found with weight loss after streptococcus infection: a case report and literature review. Int J Surg Case Rep 2021; 79:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotobi H, Tan V, Lefèvre J, et al. Total midgut volvulus in adults with intestinal malrotation. Report of eleven patients. J Visc Surg 2017; 154:175–183. [DOI] [PubMed] [Google Scholar]
- 34.Strouse PJ. Disorders of intestinal rotation and fixation (“malrotation”). Pediatric Radiol 2004; 34:837–851. [DOI] [PubMed] [Google Scholar]
- 35.Negri E, Coletta R, Morabito A. Congenital short bowel syndrome: systematic review of a rare condition. J Pediatric Surg 2020; 55:1809–1814. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira MS, Simões J, Folgado A, et al. Recurrent midgut volvulus in an adult patient—the case for pexy? A case report and review of the literature. Int J Surg Case Rep 2020; 66:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moldrem AW, Papaconstantinou H, Broker H, et al. Late presentation of intestinal malrotation: an argument for elective repair. World J Surg 2008; 32:1426–1431. [DOI] [PubMed] [Google Scholar]
- 38.Chong E, Liu DS, Rajagopal V, et al. Midgut volvulus secondary to congenital malrotation in pregnancy. BMJ Case Rep CP 2020; 13:234664–234667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamichhane A, Sharma R, Rajkarnikar R, et al. A five years old child with failure to thrive and vomiting presenting as a diagnostic dilemma: a case report. JNMA: J Nepal Med Assoc 2020; 58:600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devlin H. Midgut malrotation causing intestinal obstruction in adult patients. Ann Royal Coll Surg Engl 1971; 48:227–237. [PMC free article] [PubMed] [Google Scholar]
- 41.Janik JS, Ein SH. Normal intestinal rotation with non-fixation: a cause of chronic abdominal pain. J Pediatr Surg 1979; 14:670–674. [DOI] [PubMed] [Google Scholar]
- 42.Al-Hadidi A, Gonzalez DO, Besner GE, et al. Multiple recurrences of mesenteric narrowing following Ladd procedure. J Pediatr Surg Case Rep 2020; 61:101588–101591. [Google Scholar]
- 43.Zengin A, Uçar Bİ, Düzgün ŞA, et al. Adult midgut malrotation presented with acute bowel obstruction and ischemia. Int J Surg Case Rep 2016; 22:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penco JM, Murillo JC, Hernández A, et al. Anomalies of intestinal rotation and fixation: consequences of late diagnosis beyond two years of age. Pediatr Surg Int 2007; 23:723–730. [DOI] [PubMed] [Google Scholar]
- 45.Spigland N, Brandt ML, Yazbeck S. Malrotation presenting beyond the neonatal period. J Pediatr Surg 1990; 25:1139–1142. [DOI] [PubMed] [Google Scholar]
- 46.Ford EG, Senac MO, Jr, Srikanth M, et al. Malrotation of the intestine in children. Ann Surg 1992; 215:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Husberg B, Salehi K, Peters T, et al. Congenital intestinal malrotation in adolescent and adult patients: a 12-year clinical and radiological survey. SpringerPlus 2016; 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekonenko C, Sujka JA, Weaver K, et al. The identification and treatment of intestinal malrotation in older children. Pediatr Surg Int 2019; 35:665–671. [DOI] [PubMed] [Google Scholar]
- 49.Morris G, Kennedy A, Cochran W. Small bowel congenital anomalies: a review and update. Curr Gastroenterol Rep 2016; 18:16–27. [DOI] [PubMed] [Google Scholar]
- 50.Stringer MD. Intestinal malrotation. Pediatric Surgery 2009; Berlin, Heidelberg: Springer, 393–403. [Google Scholar]
- 51.von Flüe M, Herzog U, Ackermann C, et al. Acute and chronic presentation of intestinal nonrotation in adults. Dis Colon Rectum 1994; 37:192–198. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Elmagd K, Bond G, Reyes J, et al. Intestinal transplantation: a coming of age. Adv Surg 2002; 36:65–101. [PubMed] [Google Scholar]
- 53.Abu-Elmagd KM, Costa G, Bond GJ, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009; 250:567–581. [DOI] [PubMed] [Google Scholar]
- 54.Abu-Elmagd KM, Armanyous SR, Fujiki M, et al. Management of five hundred patients with gut failure at a single center: surgical innovation versus transplantation with a novel predictive model. Ann Surg 2019; 270:656–674. [DOI] [PubMed] [Google Scholar]
- 55.Coffey JC, Walsh D, Byrnes KG, et al. Mesentery—a ‘New’organ. Emerg Top Life Sci 2020; 4:191–206. [DOI] [PubMed] [Google Scholar]
- 56.Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol-Gastrointest Liver Physiol 2013; 305:G1–G24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abu-Elmagd K, Fung J, Bueno J, et al. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg 2000; 232:680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abu-Elmagd KM. Preservation of the native spleen, duodenum, and pancreas in patients with multivisceral transplantation: nomenclature, dispute of origin, and proof of premise. Transplantation 2007; 84:1208–1209. [DOI] [PubMed] [Google Scholar]
- 59.Pickhardt PJ, Bhalla S. Intestinal malrotation in adolescents and adults: spectrum of clinical and imaging features. Am J Roentgenol 2002; 179:1429–1435. [DOI] [PubMed] [Google Scholar]
- 60.Spiegel BM, Hays RD, Bolus R, et al. Development of the NIH patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol 2014; 109:1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abu-Elmagd KM, Kosmach-Park B, Costa G, et al. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg 2012; 256:494–508. [DOI] [PubMed] [Google Scholar]
- 62.Stauffer U, Herrmann P. Comparison of late results in patients with corrected intestinal malrotation with and without fixation of the mesentery. J Pediatr Surg 1980; 15:9–12. [DOI] [PubMed] [Google Scholar]
- 63.Murphy FL, Sparnon AL. Long-term complications following intestinal malrotation and the Ladd's procedure: a 15 year review. Pediatr Surg Int 2006; 22:326–329. [DOI] [PubMed] [Google Scholar]
- 64.El-Gohary Y, Alagtal M, Gillick J. Long-term complications following operative intervention for intestinal malrotation: a 10-year review. Pediatr Surg Int 2010; 26:203–206. [DOI] [PubMed] [Google Scholar]
- 65.Powell DM, Othersen HB, Smith C. Malrotation of the intestines in children: the effect of age on presentation and therapy. J Pediatr Surg 1989; 24:777–780. [DOI] [PubMed] [Google Scholar]
- 66.Durkin ET, Lund DP, Shaaban AF, et al. Age-related differences in diagnosis and morbidity of intestinal malrotation. J Am Coll Surg 2008; 206:658–663. [DOI] [PubMed] [Google Scholar]
- 67.Coombs R, Buick R, Gornall P, et al. Intestinal malrotation: the role of small intestinal dysmotility in the cause of persistent symptoms. J Pediatr Surg 1991; 26:553–556. [DOI] [PubMed] [Google Scholar]
- 68.Khen N, Jaubert F, Sauvat F, et al. Fetal intestinal obstruction induces alteration of enteric nervous system development in human intestinal atresia. Pediatr Res 2004; 56:975–980. [DOI] [PubMed] [Google Scholar]
- 69.Devane S, Coombes R, Smith V, et al. Persistent gastrointestinal symptoms after correction of malrotation. Arch Dis Child 1992; 67:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunter AG, Stevenson RE. Gastroschisis: clinical presentation and associations. Am J Med Genet Part C Semin Med Genet 2008; 148C:219–230. [DOI] [PubMed] [Google Scholar]
- 71.Abdelhafeez A, Alagtal M, Tareen F, et al. The incidence of symptomatic malrotation post gastroschisis repair. Eur J Pediatr Surg 2011; 21:375–376. [DOI] [PubMed] [Google Scholar]
- 72.Fawley JA, Abdelhafeez AH, Schultz JA, et al. The risk of midgut volvulus in patients with abdominal wall defects: a multi-institutional study. J Pediatr Surg 2017; 52:26–29. [DOI] [PubMed] [Google Scholar]
- 73.Do WS, Marenco CW, Horton JD, et al. Predictors of bowel resection during nonelective ladd procedure for pediatric malrotation. J Surg Res 2019; 243:419–426. [DOI] [PubMed] [Google Scholar]
- 74.Hong CR, Han SM, Staffa SJ, et al. Long-term outcomes of ultrashort bowel syndrome due to malrotation with midgut volvulus managed at an interdisciplinary pediatric intestinal rehabilitation center. J Pediatr Surg 2019; 54:964–967. [DOI] [PubMed] [Google Scholar]
- 75.Duggan CP, Jaksic T. Pediatric intestinal failure. N Engl J Med 2017; 377:666–675. [DOI] [PubMed] [Google Scholar]
- 76.Pet GC, McAdams RM, Melzer L, et al. Attitudes surrounding the management of neonates with severe necrotizing enterocolitis. J Pediatr 2018; 199:186–193. e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graziano K, Islam S, Dasgupta R, et al. Asymptomatic malrotation: diagnosis and surgical management: an American Pediatric Surgical Association outcomes and evidence based practice committee systematic review. J Pediatr Surg 2015; 50:1783–1790. [DOI] [PubMed] [Google Scholar]
- 78.Li X, Zhao Z, Li X, et al. Appearance of fetal intestinal obstruction on fetal MRI. Prenat Diagn 2020; 40:1398–1407. [DOI] [PubMed] [Google Scholar]
- 79.Sciarrone A, Teruzzi E, Pertusio A, et al. Fetal midgut volvulus: report of eight cases. J Matern Fetal Neonatal Med 2016; 29:1322–1327. [DOI] [PubMed] [Google Scholar]
- 80.Molvarec A, Bábinszki Á, Kovács K, et al. Intrauterine intestinal obstruction due to fetal midgut volvulus: a report of two cases. Fetal Diagn Ther 2007; 22:38–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.