Supplemental digital content is available in the text.
Key words/Abbreviations: depressive disorders, gut microbiota, NMR, SCFA, gut symptoms, BMI = body mass index, IBS = irritable bowel syndrome, GSRS-IBS = Gastrointestinal Symptom Rating Scale for IBS, MADRS-S = Montgomery-Åsberg Depression Rating Scale—Self-Assessment, MDD = major depressive disorder, NMR = nuclear magnetic resonance, SCFAs = short-chain fatty acids
ABSTRACT
Objective
Short-chain fatty acids (SCFAs) are produced by the gut microbiota and may reflect health. Gut symptoms are common in individuals with depressive disorders, and recent data indicate relationships between gut microbiota and psychiatric health. We aimed to investigate potential associations between SCFAs and self-reported depressive and gut symptoms in young adults.
Methods
Fecal samples from 164 individuals (125 were patients with psychiatric disorders: mean [standard deviation] age = 21.9 [2.6] years, 14% men; 39 nonpsychiatric controls: age = 28.5 [9.5] years, 38% men) were analyzed for the SCFA acetate, butyrate, and propionate by nuclear magnetic resonance spectroscopy. We then compared SCFA ratios with dimensional measures of self-reported depressive and gut symptoms.
Results
Depressive symptoms showed a positive association with acetate levels (ρ = 0.235, p = .003) and negative associations with both butyrate (ρ = −0.195, p = .014) and propionate levels (ρ = −0.201, p = .009) in relation to total SCFA levels. Furthermore, symptoms of diarrhea showed positive associations with acetate (ρ = 0.217, p = .010) and negative associations with propionate in relation to total SCFA levels (ρ = 0.229, p = 0–007). Cluster analysis revealed a heterogeneous pattern where shifts in SCFA ratios were observed in individuals with elevated levels of depressive symptoms, elevated levels of gut symptoms, or both.
Conclusions
Shifts in SCFAs are associated with both depressive symptoms and gut symptoms in young adults and may have of relevance for treatment.
INTRODUCTION
The World Health Organization lists major depressive disorder (MDD) as a leading cause of disability in the world, and it is currently associated with an annual suicide rate of approximately 800,000 individuals (1). Furthermore, MDD and related disorders affect about 300 million people worldwide (2). However, knowledge of the underlying pathophysiology is lacking, resulting in poor treatment and outcome for many patients (3).
It is clear that several neurotransmitter systems, including serotonergic, dopaminergic, and glutamatergic systems, may have altered function in MDD. Postprandial serotonin levels in plasma are altered in irritable bowel syndrome (IBS) compared with controls; elevated serotonin was found when diarrhea predominant and low when constipation is predominant (4). Young adult patients seeking psychiatric care reported more gastrointestinal (GI) symptoms than controls and the level of GI symptoms were associated with depressive symptom severity and trait anxiety (5). We have also shown links between IBS symptoms and circulating levels of melatonin as well as symptoms of anxiety and depression (5–7). The intestinal microbiota influences both neural, endocrine signaling and local and systemic immune responses. For example, many of the common neurotransmitters in our nervous system and GI tract can also be synthesized by commensal bacteria (8). Furthermore, hypothalamic-pituitary-adrenal axis abnormalities have been identified in individuals with depression (9). Previous studies have shown the potential influence of the gut microbiota and its effects on central regulatory systems that may influence body and brain function (10) including the hypothalamic-pituitary-adrenal axis (11). Corticotropin-releasing hormone administration results in higher ACTH in patients with IBS compared with controls and genetic variations in a CRH receptor gene have been linked to high anxiety trait and an increased risk of developing stress-induced psychopathology (12,13). Another proposed mechanism via CRH is mast cell activation and the release of proteases (14). Altered gut microbiotia composition, increased gut permeability, changes in enteroendocrine activity, and dysregulation of the immune system all interact and influence brain function (15,16).
Short-chain fatty acids (SCFAs) are bacterial fermentation products that have the potential to influence the intestinal immune responses (17). In the gut, the most abundant SCFAs are acetate, propionate, and butyrate in a molar ratio of 3:1:1 accounting for >95% of the SCFA content (18). Acetate, propionate, and butyrate have multiple functions including energy supply, stimulation of epithelial cell proliferation, and activation of G protein–coupled cell surface receptors, as well as direct effects on substrate and energy metabolism in peripheral tissues such as the liver and skeletal muscles (18). SCFAs can also stimulate gut endocrine cells to increase serotonin production (19).
A new field of research indicates that bacterial products including SCFAs may also play a major role in stress-induced disorders including anxiety- and depressive-like behavior. A recent rodent study showed that SCFA supplementation could counter the effects of chronic psychosocial stress on anxiety- and depressive-like symptoms and behavior (20). However, most of the studies investigating the role of SCFAs and their potential beneficial functions on brain and behavior are based on rodent models increasing the need of human studies on this topic. There are numerous reports linking IBS with depressive/anxiety disorders (21,22). Because microbial dysbiosis has been implicated as causative to gut-related symptoms in IBS, it is possible that shifts in SCFAs will be associated with both depressive symptoms and IBS.
The aim of the present study was to test if the levels of acetate, propionate, and butyrate in feces are related to self-reported symptoms of depression and GI symptoms in young adults with and without psychiatric disorders.
METHODS
Participants
Participants in this study were patients recruited to participate in “Uppsala Psychiatric Patient Samples,” a project for collection of biological material from patients seeking psychiatric care at the Department of General Psychiatry at Uppsala University Hospital, Sweden. The patients in this study were recruited to Uppsala Psychiatric Patient Samples between September 2012 and February 2015. During this period, 388 patients were recruited to the biobank (23), and fecal samples from these 125 patients were available for SCFA analysis. Moreover, samples from 39 control individuals were available for SCFA analysis. The controls included staff members of the Uppsala University Hospital and students from Uppsala University. Assessment of psychiatric diagnoses was performed by trained personnel using the Swedish version of the Mini-International Neuropsychiatric Interview.
Ethical Approval
The study was approved by the Regional Ethics Committee in Uppsala (2012/081 and 2013/219). All participants signed an informed consent form.
Depressive Symptoms (Montgomery-Åsberg Depression Rating Scale—Self-Assessment)
The self-rating version of the Montgomery-Åsberg Depression Rating Scale—Self-Assessment (MADRS-S) was used for rating depressive symptoms (24–27). The MADRS-S has been demonstrated to be a reliable patient-administered tool for depressive symptoms and consists of nine questions rated on a 0 to 6 Likert scale, with a possible score range from 0 to 54 (28).
Gastrointestinal Symptoms
We used the Swedish translation of the Gastrointestinal Symptom Rating Scale for IBS (GSRS-IBS) to evaluate GI symptoms. This is a validated self-assessment instrument for evaluating IBS symptoms that is able to discriminate symptom severity and frequency (29). The participants (both patients and controls) reported GI symptoms during the last week and scored the symptoms from 1 to 7 using the Likert scale, with possible total scores ranging from 13 to 91 points. The questionnaire includes 13 questions that are grouped into symptom clusters: pain syndrome, bloating syndrome, constipation syndrome, diarrhea syndrome, and satiety.
Analysis of SCFAs
SCFA has been analyzed using nuclear magnetic resonance (NMR) spectroscopy. Between 100 and 500 mg of stool sample was diluted with 1.5 mL sodium phosphate buffer (0.4 M, pH 7.0), homogenized by vortex mixing and centrifuged at 6300 rpm at 4°C for 15 minutes. From the supernatant, 1.5 mL of fecal water has been transferred to a fresh tube and subjected to centrifugation at 20,000g at 4°C for 15 minutes. This step was repeated once with 1 mL of supernatant recovered from the previous centrifugation. Finally, 525 μL of the supernatant was mixed with 45 μL D2O and 30 μL internal standard. Each sample solution (560 μL) was transferred to a 5-mm NMR tube, and the 1H NMR spectra were acquired using a Bruker Advance III spectrometer operating at 600-MHz proton frequency and equipped with a cryogenically cooled probe and an autosampler. Each spectrum was recorded (25°C, 128 transients, acquisition time of 1.8 seconds, relaxation delay of 4 seconds) using a zgesgp pulse sequence (Bruker Biospin) using excitation sculpting with gradients for suppression of the water resonance. For each spectrum, 65,536 data points were collected over a spectral width of 17,942 Hz (30). All NMR spectra were processed using Bruker TopSpin 4.1 software. The data were Fourier transformed after multiplication by a line broadening of 0.3 Hz and referenced to internal standard peak TSP at 0.0 ppm. Baseline and phase were corrected manually. Each spectrum was integrated using Amix 3.7.3 (Bruker BioSpin GmbH, Rheinstetten, Germany) into 0.01-ppm integral regions between 0.41 and 10 ppm, in which areas between 4.52 and 5.00 ppm were excluded. Each integral region was referenced to the internal standard. The integral regions corresponding to SCFAs were adjusted for milligrams of stool samples extracted and used for further statistical analysis. Few samples were analyzed with lesser sample amounts. Absolute numbers and calculation can be found in the Supplemental Digital Content file, http://links.lww.com/PSYMED/A756. Relative ratios of SCFAs were used for statistical analysis, as these were less dependent on time or temperature differences during sample handling (31).
Statistical Analysis
Before all analyses, the variables were screened for the distribution of normality with Shapiro-Wilk test of normality (p > .05) and visually estimated for normal distribution (histogram, Q-Q-plot box-plot). We calculated acetate, butyrate, and propionate reference values as a ratio each to the total SCFAs (sum of acetate, butyrate, and propionate) in the sample. We performed Mann-Whitney tests to investigate the statistical difference between patient and controls regarding ratios of acetate, butyrate, and propionate; sex; and psychiatric medication. Bivariate analysis was performed to test if body mass index (BMI) and age were associated with SCFAs with Spearman rank correlation.
Correlation analysis (partial Spearman rank-order correlations) was performed to analyze if acetate, butyrate, and propionate ratios were related to depressive symptoms severity (MADRS-S total score) and self-reported GI symptoms (GSRS-IBS). In all partial correlations analyses, we controlled for BMI and use of psychiatric medication (yes/no). Individuals with incomplete or missing symptomatic data were also excluded from the respective statistical analyses (missing data for MADRS-S [n = 2], GSRS-IBS [n = 20], and BMI [n = 5]). The significance level was set to a p value less than .05, and because we considered our study as an extensive pilot study, with low statistical power, we did not perform any corrections for multiple testing. We did dimensional analyses and combined cases and controls because controls were also displaying a range of both depressive and gut-related symptoms.
A hierarchical linear regression model was performed with MADRS-S total score as the dependent variable. Predictors included in the first model were SCFA ratios, BMI, age, and psychiatric medication (yes/no), and then the GI symptoms were added in the second model and the change in R2 was noted. These statistical analyses were conducted using the SPSS Statistics Version 25.
Cluster analysis was performed using the statistical software R. A heat map was created to visualize the relationship between the individual gut and depressive symptoms and the SCFA ratios (using the package ggplots2). For the cluster analysis, only 137 individuals had complete data from GSRS-IBS, MADRS-S, and SCFA ratios, which were respectively transformed to z scores. To visualize the distribution of data, high levels or scores are coded orange, whereas low levels or scores are coded purple. The BMI on the left side of the heat map is colored blue, where low BMI is colored light blue. To illustrate patients and controls, on the right-side values that represent patients are in black and controls are marked gray. Furthermore, to visualize the clusters better, we colored the columns showing MADRS-S items in pink, the SCFA ratios in green, and the GSRS-IBS items in turquoise.
RESULTS
The sample’s characteristics are described in Table 1. In the bivariate analyses, acetate ratios were negatively associated with BMI (−0.156, p = .049), but no associations were found to butyrate ratios (0.103, p = .196) or propionate ratios (0.132, p = .097). No relationship to SCFA ratios was found between age and sex. Patients did not differ as a group from controls regarding acetate, butyrate, and propionate ratios (Table S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A756). However, we could see a tendency toward an association between acetate and butyrate ratios and psychiatric medication and use of antidepressants (Table S1). Based on these findings, the partial correlations below were adjusted for BMI and psychiatric medication (Y/N).
TABLE 1.
Relevant Characteristics of the Cohort
| Patients (n = 125) | Controls (n = 39) | |
|---|---|---|
| Age, mean (SD), y | 21.9 (2.6) | 28.5 (9.5) |
| Sex, men/women (% men) | 17/108 (14) | 15/24 (38) |
| BMI, median (range), kg/m2 | 22.4 (15.1–46.6) | 23.0 (18.1–37.8) |
| MADRS-S total score, median (range) | 22 (0–49) | 5 (0–20) |
| GSRS-IBS total score, median (range) | 31.5 (13–78) | 23.0 (13–47) |
| Medication, n (%) | ||
| Any medication | 106 (84.8) | 20 (51.2) |
| Any psychiatric medication | 82 (66) | 3 (8) |
| Other anxiolytic medicationa | 29 (23) | 0 (0) |
| Antidepressive treatmentb | 58 (46) | 3 (7.7) |
| Antipsychotics | 7 (5.6) | 0 (0) |
| Antiepileptics | 11 (8.8) | 0 (0) |
| Benzodiazepines | 4 (3.2) | 0 (0) |
| Z analogs | 7 (5.6) | 0 (0) |
| Immune modulating | 8 (6.4) | 0 (0) |
| Diagnosis, n (%) | ||
| Current depressive episode (UP/BP) | 68 (54.4) | 0 (0) |
| Any bipolar disorderc | 23 (18.4) | 0 (0) |
| Celiac disease | 1 (0.8) | 0 (0) |
| Systemic inflammatory diseased | 4 (3.2) | 0 (0) |
| Lifetime depressive episode (UP) | 73 (58.4) | 5 (13.8) |
| Any anxiety disorder, DSM-IV | 85 (68) | 1 (2.5) |
| Anorexia | 0 (0) | 0 (0) |
| Bulimia nervosa | 10 (8) | 0 (0) |
| SCFAs | ||
| Acetate ratio, median (range) | 0.77 (0.45–0.98) | 0.76 (0.44–0.87) |
| Butyrate ratio, median (range) | 0.05 (0.01–0.12) | 0.05 (0.03–0.09 |
| Propionate ratio, median (range) | 0.18 (0.01–0.43) | 0.18 (0.08–0.51) |
SD = standard deviation; BMI = body mass index (missing data for 4 patients); MADRS-S = Montgomery-Åsberg Depression Rating Scale—Self-Assessment (missing data for 1 patient); GSRS-IBS = Gastrointestinal Symptom Rating Scale for Irritable Bowel Syndrome (missing data for 14 patients and 4 controls); UP/BP = unipolar/bipolar disorder; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; SCFAs = short-chain fatty acids.
a Sedating antihistamines, phenothiazines.
b Selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitor, mood stabilizers, and atypical antidepressants.
c Bipolar types I and II and nonspecified.
d Systemic inflammatory disease, that is, four cases of inflammatory bowel disorder.
One patient had comorbid high functioning autism and dyslexia, one patient had comorbid attention-deficit/hyperactivity disorder, one case presented psychotic symptoms, and one patient has since this study started committed suicide.
Depressive symptoms severity (total MADRS-S score) was correlated with acetate ratio (ρ = 0.235, p = .003), butyrate ratio (ρ = −0.195, p = .014), and propionate ratio (ρ = −0.207, p = .009) when adjusting for BMI and psychiatric medication. Reanalysis of the patient data alone gave similar results (Table 2). BMI, butyrate and propionate ratios, age, and medication were the included predictors in a linear regression model with MADRS-S as the dependent factor (R2 = 0.307, p = 1.3726E − 9).
TABLE 2.
Relation of Depressive Symptoms and Acetate, Butyrate, and Propionate Ratios, Shown by Correlation Coefficients and Two-Tailed p Values for Partial Bivariate Associations Between the SCFA Ratios and the Montgomery-Åsberg Depression Rating Scale—Self-Assessment
| Population | Acetate | Butyrate | Propionate | |
|---|---|---|---|---|
| MADRS-S | P and C | 0.235 (0.003) | −0.195 (0.014) | −0.207 (0.009) |
| MADRS-S | P | 0.231 (0.012) | −0.246 (0.007) | −0.181 (0.050) |
Adjusted for body mass index, age, and use of psychiatric medications (Y/N). Bold values indicate an association with a p value of less than .05. Reanalysis of the patient (P) data excluding controls (C) gave comparable results.
MADRS-S = Montgomery-Åsberg Depression Rating Scale—Self-Assessment; SCFA = short-chain fatty acid.
Exploratory analyses to investigate the associations with the separate MADRS-S items (nine questions) and the three SCFA ratios were performed in a post hoc analysis (shown in Table 2). Positive associations were found between acetate ratio and five of the MADRS-S items (mood, feelings of unease, initiative, emotional involvement, and pessimism), but no significant associations were found for sleep, ability to concentrate, appetite, and zest for life. Butyrate and propionate showed an inverse pattern of associations with the same items as acetate (Table S2, Supplemental Digital Content, http://links.lww.com/PSYMED/A756).
The subsequent exploratory analysis of correlations between SCFA ratios and diverse GI symptom items (GSRS-IBS) is shown in Table 3. A positive association was found between acetate ratios and the GSRS-IBS items diarrhea and bloating, whereas negative associations were found between constipation and butyrate ratios and diarrhea and propionate ratios (Table 3.) These findings were not affected by adjustment factors of BMI and psychiatric medications (data not shown). The total scores for of MADRS-S and GSRS-IBS were correlated (ρ = 0.320. p = .0001). To test what percentage of variance of the MADRS-S–SCFA association is “accounted for” by GI symptoms, a hierarchical linear regression model showed that adding GI symptoms to the aforementioned original model increased the explained variance with nearly 6% (change in R2 = 0.065, p = .052). Excluding patients with bulimia nervosa from the analysis did not change the results.
TABLE 3.
Post Hoc Analysis of Acetate, Butyrate, and Propionate Ratios in Relation to Gastrointestinal Symptoms Measured Using the Gastrointestinal Symptom Rating Scale for IBS (GSRS-IBS)
| GSRS-IBS Symptoms | Acetate | Butyrate | Propionate |
|---|---|---|---|
| Satiety | 0.076 (n.s.) | −0.113 (n.s.) | −0.053 (n.s.) |
| Diarrhea | 0.217 (.010) | −0.048 (n.s.) | −0.229 (.007) |
| Constipation | −0.031 (n.s.) | −0.169 (.046) | 0.082 (n.s.) |
| Bloating | 0.172 (.043) | −0.156 (n.s.) | −0.148 (n.s.) |
| Pain | 0.117 (n.s.) | −0.120 (n.s.) | −0.098 (n.s.) |
Correlation coefficients and two-tailed p values in parentheses for associations between short-chain fatty acid ratios and the separate GSRS-IBS items. n = 152. Adjusted for body mass index, age, and use of psychiatric medications (Y/N). Bold values indicate an association with a p value of less than .05.
IBS =irritable bowel syndrome; GSRS-IBS = GSRS-IBS = Gastrointestinal Symptom Rating Scale for IBS.
To visualize the relationships between the symptoms and SCFA ratios, we performed cluster analyses. As shown in Figure 1, a heat map illustrates the clusters of the separate items of MADRS-S and GSRS-IBS, as well as the acetate, butyrate, and propionate ratios (Figure 1). Six clusters are highlighted in the heat map, illustrated as black rectangles around the respective grouping. Cluster I contains patients with mixed depressive symptoms and GI symptoms with predominance of diarrhea and lower propionate ratios. Cluster II includes patients with high depressive scores and lower scores on other gut symptoms and shows lower butyrate and propionate ratios. Cluster III describes a third group of individuals with both low gut and depressive symptoms. Interestingly, this small group has very low acetate ratios and higher butyrate and propionate ratios. Cluster IV includes patients with both depressive symptoms and gut-related issues with predominance of pain and obstipation and lower butyrate ratios. Cluster V includes controls and patients with some GI symptoms and elevated acetate ratios. Cluster VI, in the lowest rectangle, controls with low depressive scores but with some GI symptoms also seem to have lower acetate and propionate ratios and somewhat higher butyrate ratios.
FIGURE 1.
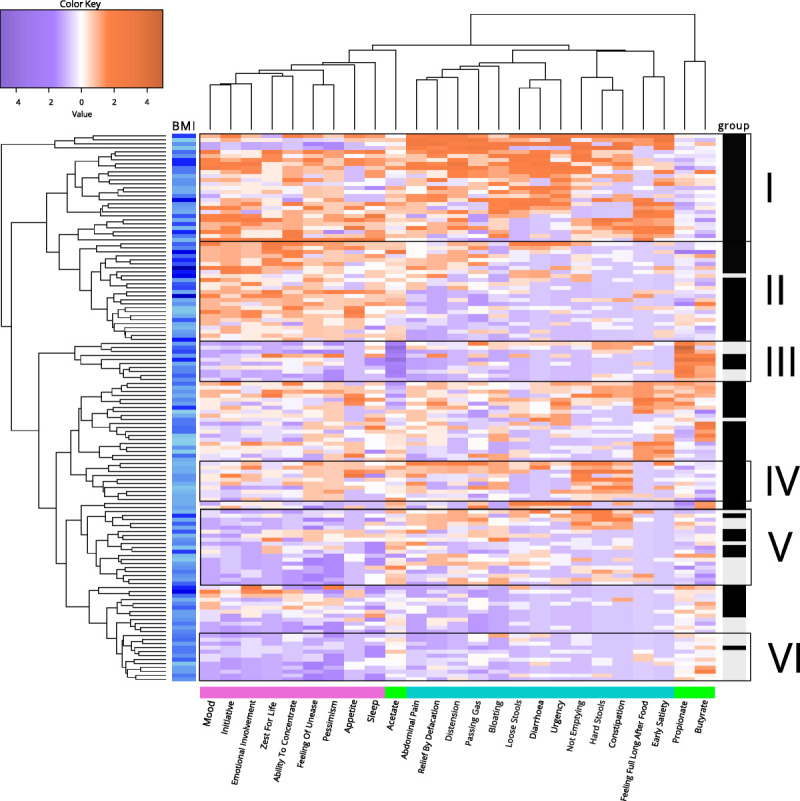
Heat map of the separate MADRS-S and GSRS-IBS items and acetate, butyrate, and propionate ratios. A heat map visualizing the relationship between individual gut and depressive symptoms and SCFA ratios. For the cluster analysis, only 137 individuals had complete data from GSRS-IBS, MADRS-S, and SCFA analysis. Orange boxes represent high levels/scores of the respective items, and purple boxes represent low levels/scores of these items. BMI levels are indicated in blue, where light blue indicates low BMI (left panel). To illustrate individual groups, patients are colored with black boxes and controls in gray boxes (right panel). The MADRS-S items are in pink, SCFAs in green, and the GSRS-IBS items in turquoise (lower panel). MADRS-S = Montgomery-Åsberg Depression Rating Scale—Self-Assessment; GSRS-IBS = Gastrointestinal Symptom Rating Scale for Irritable Bowel Syndrome; SCFA = short-chain fatty acid; BMI = body mass index. Color image is available online only at www.psychosomaticmedicine.org.
DISCUSSION
This pilot study found relationships between depressive and GI symptoms and the ratios of three major SCFAs measured in fecal material from a mixed cohort of young adults with and without psychiatric diagnoses. These associations were independent from BMI and psychiatric medication. Furthermore, exploration of the data revealed groups of individuals with depressive symptoms, GI symptoms alone, or in combination may show shifts in SCFA ratios with relevance for both patients and controls.
Our results indicate that shifts in SCFA ratios are associated with both IBS and depression. We have previously explored the correlation between GI and depressive symptoms in young adults and shown high correlations (5). Our results indicate that the associations between SCFAs and depressive symptoms are both stronger than and independent from GI symptoms. A bidirectional interaction between gut symptoms and the brain mediated by the gut microbiota and their respective metabolites has been proposed (32–34). SCFAs in addition to local influences on epithelial cells (35) activate G protein–coupled receptors (36) and are involved in neurotransmitter production (37). Moreover, SCFAs can penetrate the blood-brain barrier (38). Differences in the SCFA composition, because of changes in microbiota, correlate with neural activity and brain structure in humans, as assessed by functional and structural magnetic resonance imaging (39).
Although studies examining SCFAs differ in their design by measuring different populations, feces, or blood and calculating absolute or relative levels, which makes comparison between studies more difficult, some findings are consistent. Our study showed that acetate and butyrate are associated with depressive and GI symptoms, but the relationships seem to go in opposite directions. In agreement with earlier work, acetate was positively correlated with BMI in our material. Elevated levels of acetate in feces have been shown to be associated with obesity in several studies and were recently summarized in a meta-analysis but do not reach significance after corrections for cofactors in other studies on older populations (40). The relationship between depressive and GI symptoms was independent from BMI in this young adult population, raising an interesting hypothesis that these symptoms in combination with SCFA disruption may predispose for future metabolic syndrome and obesity in later life. The negative association between butyrate ratio and depressive GI symptoms in this study was weak and is in line with earlier findings and proposed positive impact of butyrate on health (41). These findings raise questions as to the potential future therapies. Oral administration of butyrate has been suggested to be beneficial for a wide spectrum of diseases, ranging from metabolic diseases to colorectal cancers (42). Fiber-rich diets such as the “Mediterranean style” diet are also reported to increase butyrate and, in theory, may reduce the risk of several diseases linked to aging and the immune system (43). As we can conclude from the clustering data, several groups of individuals with different combinations of SCFA distribution, depressive, and or GI symptoms exist. Whether symptoms in these groups respond to dietary or supplement-based interventions in a uniform or differential manner remains to be seen. Some indications of highly individual reactions to dietary interventions are emerging (44).
The present study has some limitations. The sample is small, and individuals with psychiatric morbidity are overrepresented. The management of samples during collection may have varied between individuals; however, our previous data show that the use of SCFA ratios instead of absolute values reduced the influence of time and temperature on the results (31). Furthermore, dietary intake is also an important contributor to SCFA levels, and we do not have information on dietary pattern. An assessment of systemic SCFA concentrations was not conducted and would be interesting for future studies. A strength of the study is the use of dimensional measures of depressive and GI symptoms, and we have a full range of depressive symptoms represented in the cohort of young adults. The recruitment base was an ambulatory care unit within general psychiatry in a university town; most patients were therefore students, as were the controls. Patients with severe autism spectra disorders, severe eating disorders, or psychosis are not represented because they are generally referred to other units.
This study demonstrates clear interactions between psychiatric and GI symptoms with SCFA composition in young adults with primarily affective and/or anxiety disorders and controls without a psychiatric diagnosis. Biological heterogeneity exists within psychiatric diagnostic groups; this study provides insight into the potential beneficial effects after dietary interventions among potential subgroups with high acetate and low butyrate and propionate ratios, which should be further explored.
Supplementary Material
Acknowledgments
The authors thank Ulla Nordén for her excellent research assistance, Hans Arinell for excellent statistical advice, and Uppsala Biobank for collaboration with the sample management.
Source of Funding and Conflicts of Interest: This work was supported by a grant from the Ekhaga Foundation. Material collection was supported by grants from Märta och Nicke Näsvells fund, Stiftelsen Söderström–Königska sjukhemmet, Swedish Medical Association, and Medical Training and Research Agreement (ALF) Funds from Uppsala University Hospital. The funders had no role in study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Footnotes
Supplemental Digital Content
Contributor Information
Bettina Müller, Email: Bettina.Muller@slu.se.
Annica J. Rasmusson, Email: Annica.Rasmusson@neuro.uu.se.
David Just, Email: david.just@abbvie.com.
Shishanthi Jayarathna, Email: shishanthi.jayarathna@slu.se.
Ali Moazzami, Email: Ali.Moazzami@slu.se.
Zorana Kurbalija Novicic, Email: zknovicic@gmail.com.
REFERENCES
- 1.World Health Organization . Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: WHO; 2017. Licence: CC BY-NC-SA 30 IGO. [Google Scholar]
- 2.Gotlib IH, Hammen CL. Handbook of Depression. 2nd ed. New York NY: The Guilford Press; 2009. [Google Scholar]
- 3.Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psych 2019;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:349–57. [DOI] [PubMed] [Google Scholar]
- 5.Soderquist F, Syk M, Just D, Kurbalija Novicic Z, Rasmusson AJ, Hellstrom PM, Ramklint M, Cunningham JL. A cross-sectional study of gastrointestinal symptoms, depressive symptoms and trait anxiety in young adults. BMC Psychiatry 2020;20:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soderquist F, Sundberg I, Ramklint M, Widerstrom R, Hellstrom PM, Cunningham JL. The relationship between daytime salivary melatonin and gastrointestinal symptoms in young adults seeking psychiatric care. Psychosom Med 2019;81:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundberg I, Ramklint M, Stridsberg M, Papadopoulos FC, Ekselius L, Cunningham JL. Salivary melatonin in relation to depressive symptom severity in young adults. PLoS One 2016;11:e0152814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galland L. The gut microbiome and the brain. J Med Food 2014;17:1261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J Psychiatr 2017;27:101–11. [DOI] [PubMed] [Google Scholar]
- 10.Ming X, Chen N, Ray C, Brewer G, Kornitzer J, Steer RA. A gut feeling: a hypothesis of the role of the microbiome in attention-deficit/hyperactivity disorders. Child Neurol Open 2018;5:2329048X18786799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012;37:1885–95. [DOI] [PubMed] [Google Scholar]
- 12.Weger M, Sandi C. High anxiety trait: a vulnerable phenotype for stress-induced depression. Neurosci Biobehav Rev 2018;87:27–37. [DOI] [PubMed] [Google Scholar]
- 13.Kano M, Muratsubaki T, Van Oudenhove L, Morishita J, Yoshizawa M, Kohno K, Yagihashi M, Tanaka Y, Mugikura S, Dupont P, Ly HG, Takase K, Kanazawa M, Fukudo S. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep 2017;7:12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One 2012;7:e39935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohman L, Tornblom H, Simren M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol 2015;12:36–49. [DOI] [PubMed] [Google Scholar]
- 16.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009;58:196–201. [DOI] [PubMed] [Google Scholar]
- 17.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 19.Reigstad CS, Salmonson CE, Rainey JF, 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015;29:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol 2018;596:4923–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, Creed F. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology 2006;130:1447–58. [DOI] [PubMed] [Google Scholar]
- 22.Karling P, Maripuu M, Wikgren M, Adolfsson R, Norrback KF. Association between gastrointestinal symptoms and affectivity in patients with bipolar disorder. World J Gastroenterol 2016;22:8540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham JL, Zanzi M, Willebrand M, Ekselius L, Ramklint M. No regrets: young adult patients in psychiatry report positive reactions to biobank participation. BMC Psychiatry 2017;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svanborg P, Asberg M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatr Scand 1994;89:21–8. [DOI] [PubMed] [Google Scholar]
- 25.Mattila-Evenden M, Svanborg P, Gustavsson P, Asberg M. Determinants of self-rating and expert rating concordance in psychiatric out-patients, using the affective subscales of the CPRS. Acta Psychiatr Scand 1996;94:386–96. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham JL, Wernroth L, von Knorring L, Berglund L, Ekselius L. Agreement between physicians’ and patients’ ratings on the Montgomery-Asberg Depression Rating Scale. J Affect Disord 2011;135:148–53. [DOI] [PubMed] [Google Scholar]
- 27.Nejati S, Ariai N, Bjorkelund C, Skoglund I, Petersson EL, Augustsson P, Hange D, Svenningsson I. Correspondence between the neuropsychiatric interview M.I.N.I. and the BDI-II and MADRS-S self-rating instruments as diagnostic tools in primary care patients with depression. Int J Gen Med 2020;13:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fantino B, Moore N. The self-reported Montgomery-Asberg Depression Rating Scale is a useful evaluative tool in major depressive disorder. BMC Psychiatry 2009;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiklund IK, Fullerton S, Hawkey CJ, Jones RH, Longstreth GF, Mayer EA, Peacock RA, Wilson IK, Naesdal J. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol 2003;38:947–54. [DOI] [PubMed] [Google Scholar]
- 30.Rohnisch HE, Eriksson J, Mullner E, Agback P, Sandstrom C, Moazzami AA. AQuA: an automated quantification algorithm for high-throughput NMR-based metabolomics and its application in human plasma. Anal Chem 2018;90:2095–102. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham JL, Bramstang L, Singh A, Jayarathna S, Rasmusson AJ, Moazzami A, Muller B. Impact of time and temperature on gut microbiota and SCFA composition in stool samples. PLoS One 2020;15:e0236944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013;155:1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 2011;23:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 37.DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, La Gamma EF. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res 2005;142:28–38. [DOI] [PubMed] [Google Scholar]
- 38.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 2014;817:221–39. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Real JM, Serino M, Blasco G, Puig J, Daunis-i-Estadella J, Ricart W, Burcelin R, Fernandez-Aranda F, Portero-Otin M. Gut microbiota interacts with brain microstructure and function. J Clin Endocrinol Metab 2015;100:4505–13. [DOI] [PubMed] [Google Scholar]
- 40.Muller M, Hernandez MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, van Eijk H, Canfora EE, Blaak EE. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep 2019;9:12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: a double-edged sword for health? Adv Nutr 2018;9:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011;17:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph J, Depp C, Shih PB, Cadenhead KS, Schmid-Schonbein G. Modified Mediterranean diet for enrichment of short chain fatty acids: potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front Neurosci 2017;11:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


