Supplemental digital content is available in the text.
Key words/Abbreviations: autism spectrum disorder; major depressive disorder; anorexia nervosa; bipolar disorder; schizophrenia; attention-deficit/hyperactivity disorder; gut microbiota; ADHD = attention-deficit/hyperactivity disorder; AN = anorexia nervosa; ANX = anxiety disorders; ASD = autism spectrum disorder; BMI = body mass index; BPD = bipolar disorder; DSM = Diagnostic and Statistical Manual for Psychiatric Disorders; ED = eating disorders; ICD-10 = International Classification of Disease, Tenth Revision; MDD = major depressive disorder; MNOS = modified Newcastle-Ottawa Scale; NGS = next-generation sequencing; SCFA = short-chain fatty acids; SCZ = schizophrenia; Microbiota Terminology: High-throughput sequencing = a group of sequencing techniques that allow for large-scale genome sequencing by processing multiple DNA sequences in parallel; Marker-gene analysis = amplification of target genes to determine the presence and abundance of microbes. 16S rRNA is a highly conserved gene among bacteria and archaea commonly used as a marker gene in studies of microbiota. This technique uses universal primers targeting the hypervariable regions (V1–V9) of ribosomal RNA; Metagenome analysis (shotgun) = uses the entire genome of all microorganisms for whole-genome sequencing, which offers higher resolution compared with marker-gene analysis and allows for functional characterization of microbial communities; Alpha diversity = refers to the diversity within a sample. It can measure richness of species (observed species, Chao1, ACE index) or how evenly distributed the microbes are (Shannon and Simpson index); Beta diversity = refers to diversity between samples. Different metrics can be used to measure beta diversity. Bray-Curtis dissimilarity measures differences in microbial abundance between samples. Unifrac takes phylogenetics or relatedness of species into account, and it can be weighted to the relative abundance of species (weighted Unifrac)
ABSTRACT
Objective
This systematic review sought to comprehensively summarize gut microbiota research in psychiatric disorders following PRISMA guidelines.
Methods
Literature searches were performed on databases using keywords involving gut microbiota and psychiatric disorders. Articles in English with human participants up until February 13, 2020, were reviewed. Risk of bias was assessed using a modified Newcastle-Ottawa Scale for microbiota studies.
Results
Sixty-nine of 4231 identified studies met the inclusion criteria for extraction. In most studies, gut microbiota composition differed between individuals with psychiatric disorders and healthy controls; however, limited consistency was observed in the taxonomic profiles. At the genus level, the most replicated findings were higher abundance of Bifidobacterium and lower abundance of Roseburia and Faecalibacterium among patients with psychiatric disorders.
Conclusions
Gut bacteria that produce short-chain fatty acids, such as Roseburia and Faecalibacterium, could be less abundant in patients with psychiatric disorders, whereas commensal genera, for example, Bifidobacterium, might be more abundant compared with healthy controls. However, most included studies were hampered by methodological shortcomings including small sample size, unclear diagnostics, failure to address confounding factors, and inadequate bioinformatic processing, which might contribute to inconsistent results. Based on our findings, we provide recommendations to improve quality and comparability of future microbiota studies in psychiatry.
INTRODUCTION
The gut microbiota has extensive reciprocal connections with the human brain through microbial metabolites, the vagus nerve and hormonal and immunological signaling, collectively forming the microbiome-gut-brain axis (1,2). Animal studies suggest a possible influence of gut microbiota on behavior. Using microbiota-depleted or axenic rodents, researchers were able to replicate pathognomonic behaviors in animals after transplantation with fecal microbiota from humans with depression (3,4), schizophrenia (SCZ) (5), or attention-deficit/hyperactivity disorder (ADHD) (6). Similarly, colonization of axenic mice with fecal microbiota from children with autism spectrum disorder (ASD) induced autism-like behavior (7). However, these studies are commonly limited by the small number of human donors, and findings from animal experiments are not easily transferable to humans (8).
Targeting the gut microbiota might provide new therapeutic avenues for psychiatric disorders, but whether and how the microbiota differs in individuals with psychiatric disorders is insufficiently understood (7). The number of studies investigating the differences in gut microbiota between healthy controls and patients with psychiatric disorders has accelerated with increased interest and affordability of high-throughput sequencing techniques (9). A number of systematic reviews have sought to compile these findings; however, most focused on a limited number of related psychiatric disorders rather than a comprehensive overview of the field (10–17).
Although the field is relatively young and many studies use different experimental designs and methodology, we contend that there is a need for a comprehensive systematic review of microbiota studies, including all major categories of psychiatric disorders, at this stage of the science. We included all case-control studies that used high-throughput sequencing techniques for analysis, which, to our knowledge, has not been done before.
Our primary objective is to compare gut microbiota in patients with a psychiatric disorder (i.e., ADHD, anxiety disorders [ANX], ASD, bipolar disorder [BPD], eating disorders (EDs), major depressive disorder [MDD], tics, obsessive-compulsive disorders, posttraumatic stress disorder, SCZ) with healthy controls. Alpha diversity, average species diversity in a sample, and beta diversity, similarities in diversity between samples and taxonomic differences across the psychiatric disorders, are the primary outcome measures. Secondary outcome measures are differences in microbial metabolites and associations between taxonomic differences and clinical features.
METHODS
Protocol and Registration
This systematic review has been preregistered at PROSPERO under the identification number CRD42019132642. PRISMA guidelines for a systematic review or meta-analysis have been followed (18).
Eligibility Criteria
The primary objective was to compile evidence of differences in fecal microbiota as a proxy for microbiota of the lower gastrointestinal tract between patients with psychiatric disorders and healthy individuals. Therefore, only original observational studies using a case-control, cohort, or cross-sectional design with a healthy control group were included. We had no restriction regarding the age of participants. Animal studies, studies without a diagnosed psychiatric disorder, interventional studies, and studies that did not use high-throughput sequencing techniques were excluded.
Information Sources
Human studies in English up until February 13, 2020, were searched in Medline, Embase, Cochrane, and Web of Science and Psychinfo, with support from Karolinska Institutet University Library, using the following search string (for Web of Science): ((anxiety or “obsessive-compulsive” or paranoi* or panic or phobi* or “anorexia nervosa” or “appetite disorder*” or “binge-eating” or bulimia or “compulsive eat*” or “eating disorder*” or “feeding disorder*” or attention-deficit/hyperactivity disorder or asperger* or “attention deficit” or autistic or autism or “bipolar disorder” or depression or “depressive disorder*” or mania* or manic or ptsd or “post-traumatic*” or schizophrenia or schizophrenic* or “stress disorder*” or tic or tics or tourette*)) AND ((“enteric bacteria” or “gastr* flora” or “gut flora” or “intestinal flora” or microbiom* or microbiot* or microbial* or microflora* or mycobiome*)) NOT ((“animal*” or “guinea pig*” or “horse*” or “mice” or “mouse” or “rat” or “rats”)) (Supplementary Material 1, http://links.lww.com/PSYMED/A743). The gathered articles were deduplicated. Gray literature, such as dissertations, reports, and manuscripts, was included if inclusion criteria were fulfilled. Reference lists from other reviews were used for reference checking.
Study Selection and Data Collection Process
A single Endnote file was exported to Rayyan.com (19). Two reviewers, blinded to each other’s assessments, sorted articles based on title and abstract. Reasons for exclusion were standardized according to the terms: review, animal studies, gray literature that did not meet eligibility criteria, not relevant, case series/case reports, and not retrievable. Subsequently, full-text articles were assessed for eligibility. If the article did not meet the inclusion criteria, it was excluded according to the following terms: not a microbiota study, not a study of a psychiatric disorder, or irrelevant study design. If the reviewers had disparate opinions about inclusion, group consensus was achieved through discussion. A priori determined outcome variables were subdivided into four categories: general information about each study, study design and demographic variables, methodological information, and outcome variables (alpha and beta diversity, and taxonomic abundance of bacterial groups; microbial metabolites; and association with clinical features). No assumptions or simplifications were made during extraction. If the reported data from the study were absent or ambiguous, the cell was filled in as not available (NA). No deviations from the prespecified protocol occurred. All extracted information was double checked by another reviewer to ensure that it was correctly extracted.
Assessment of Risk of Bias
Two reviewers independently conducted quality assessments of all included studies using the Newcastle-Ottawa Scale (NOS) (20). The original NOS is a tool to assess the quality of nonrandomized studies in systematic reviews and meta-analyses. However, the exposure items are not applicable for most observational cross-sectional studies. We therefore modified the scale to more adequately represent the study quality by replacing exposure items with methodological items (Supplementary Material 2, http://links.lww.com/PSYMED/A744). Because this is a field that relies heavily on emerging technology, consistency of methodological reporting is critical to ensure reproducibility. Thus, the modified Newcastle-Ottawa Scale (MNOS) contains eight items, categorized into three topics: selection, comparability, and methodology. We chose age as the most important confounding factor to examine comparability across studies (21). Discrepancies in quality assessment were resolved by discussion among the reviewers.
Statistical Analysis
Spearman correlation was used for correlation analysis between study quality (MNOS score) and time of publication. R packages were used for statistical analysis and figures (22). The reported taxonomic differences between cases and healthy controls across psychiatric disorders were summarized as a heat-map to identify replicated patterns despite differences in hypervariable regions and bioinformatic processes. We only included studies that used 16S rRNA sequencing as the sequencing method. We chose to map genus-level differences based on the resolution of the sequencing method (23). If a genus was not reported in more than one study, it was excluded. The following values were assigned to “increase,” “decrease,” and “no report/no change” in abundance, respectively: +1, −1, and 0. Next, the values were weighted by the sample size in a disorder-specific manner. Finally, the sum of weighted values of each genus in different studies per disorder was visualized in the heat-map, with disorders being listed in decreasing order according to number of studies included.
RESULTS
Study Selection
Of 4231 identified studies, 2682 articles were screened, and 341 articles went to full-text assessment for eligibility. Sixty-nine articles met the inclusion criteria and were included in this review (4,5,24–89) (Figure 1).
FIGURE 1.
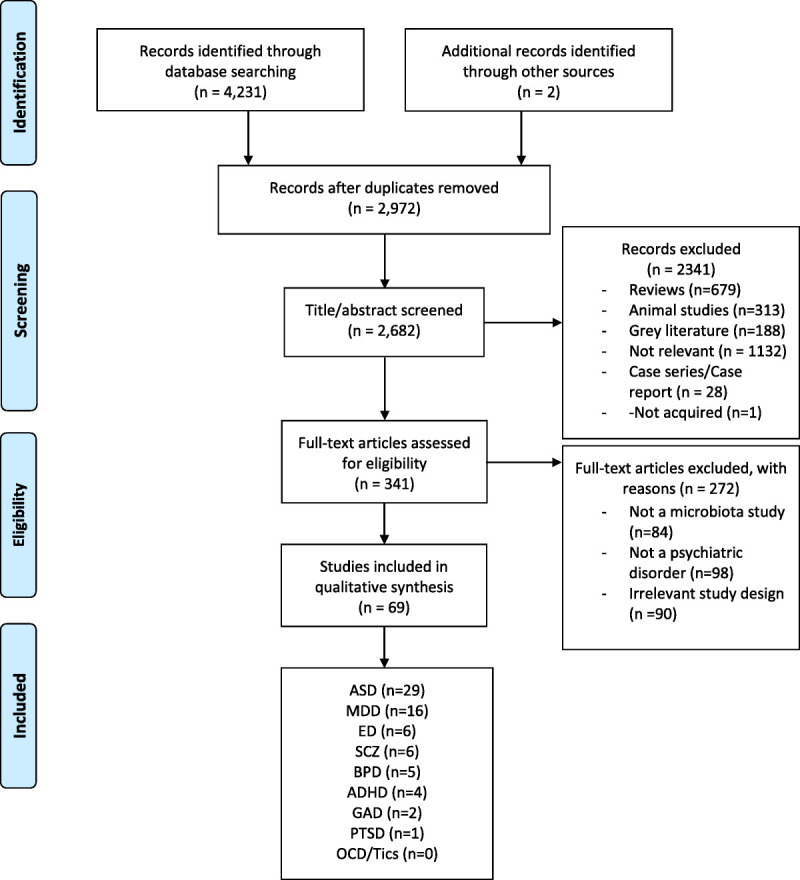
PRISMA flow diagram. Attention-deficit/hyperactivity disorder (ADHD), anxiety disorders (ANX), autism spectrum disorder (ASD), bipolar disorder (BPD), eating disorders (ED), major depressive disorder (MDD), tics, obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and schizophrenia (SCZ). Color image is available online only with this article at www.psychosomaticmedicine.org.
Study Characteristics
From 2015 forward, the number of studies in this field increased rapidly. A Spearman correlation test showed a weak positive correlation between overall study quality as measured with MNOS and date of publication, which was not statistically significant (rs = 0.025, p = .84; Figure 2). Although the difference in study quality was large between individual studies, the general study quality has not improved over time (Supplementary Table 1, http://links.lww.com/PSYMED/A745). Most studies were conducted in Europe, the United States, and, more recently, China, contributing approximately 30% each to the total number of publications (Supplementary Table 2, http://links.lww.com/PSYMED/A746).
FIGURE 2.
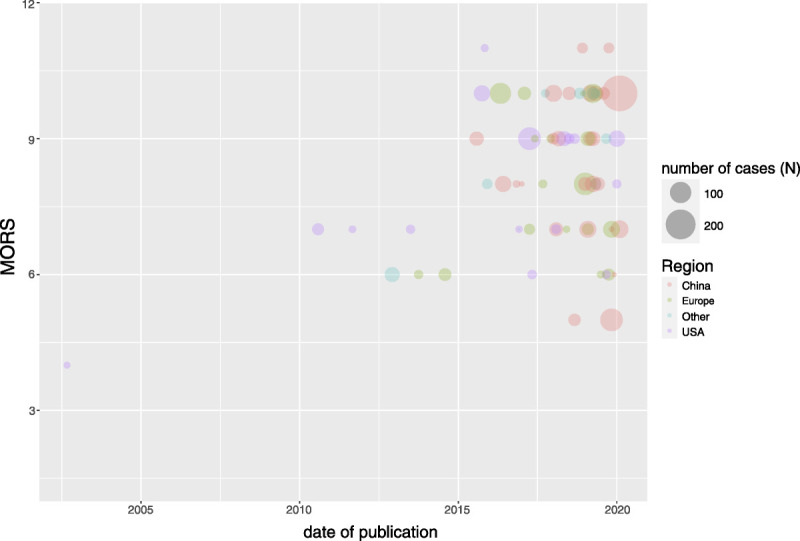
Risk of bias visualized with a bubble chart. Modified Newcastle-Ottawa Score Score (MORS) is shown in the y axis and date of publication in the x axis. Each study is represented with a bubble, and the size of the bubble corresponds to the number of cases in the studies. “Region” represents the place where the study was conducted. Color image is available online only with this article at www.psychosomaticmedicine.org.
Demographic characteristics of each study are presented in Supplementary Table 3, http://links.lww.com/PSYMED/A747. A total of 2880 cases, with a mean number of 42 cases per study, have been included in these 69 studies. The average number of controls for every case was less than 1:1. In fact, 37 of the articles had more cases than controls. Excluding ASD studies, the mean age was 34.5 years, ranging from 8.4 to 52.9 years. Because ASD studies mainly recruited children, the mean age was 6.5 years, ranging from 2 to 14.4 years. Notably, 47 of 69 studies did not use age-matched controls. The sex in ASD studies was heavily weighted toward boys (male-to-female ratio, >5:1), which could be due to earlier age of onset of ASD in boys. In contrast, ED studies only recruited female cases. For the remaining diagnoses, the sex (male-to-female) ratio was approximately 2:3. Body mass index (BMI) was reported in 44 of 69 studies. Mean BMI for adult cases (ED not included) was 23 kg/m2. Eleven of 69 studies specifically reported distribution of race in study participants. Most participants were identified as White, followed by Asian and Black (Supplementary Table 3, http://links.lww.com/PSYMED/A747).
Methodological Characteristics
Exclusion Criteria
The 42 different exclusion criteria listed across the studies differ considerably (Supplementary Table 2, http://links.lww.com/PSYMED/A746). For example, the required antibiotic-free period before sampling ranged from 2 weeks to 6 months (35,63). A quarter of the studies did not exclude participants who had taken antibiotics recently. Information on smoking and physical activity was collected in 16% and 9% of the studies, respectively (3,27,29,38,47,54,56,62,63,72,73,80,87).
Dietary Assessment
Forty-two percent of the studies assessed the diet of participants in any way, 7% used a food frequency questionnaire, and 10% used food diaries (3,26,28,30,36,51,52,54,58,79,85,89) (Supplementary Table 3, http://links.lww.com/PSYMED/A747).
Diagnosis
Eighty-four percent of the studies reported use of diagnostic systems, 43% according to the Diagnostic and Statistical Manual for Psychiatric Disorders, Fourth Edition (DSM-IV), 28% of the studies according to the DSM-5, 7% according to the International Classification of Disease, Tenth Revision (ICD-10), and 6% according to both the DSM and ICD (90–92). However, only 26% of the studies reported use of a structured diagnostic interview for assessment of diagnoses in study participants (Supplementary Table 3, http://links.lww.com/PSYMED/A747).
Sampling and Sequencing
In the majority of studies, samples were frozen at −80°C before extraction (Supplementary Table 4, http://links.lww.com/PSYMED/A748). Eighty-six percent of the studies used marker-gene analysis methods, and 9% used metagenome analysis (62,66,67,71,76,81). For the studies that used marker-gene analysis, 16S ribosomal RNA was the most amplified gene, with the hypervariable regions V3–V4 being the most frequently amplified regions (Figure 3A). All amplicons were sequenced with high-throughput sequencing methods using six different sequencing platforms in which MiSeq, Roche 454, and HiSeq were the most used in falling order (Figure 3B). Six studies specifically reported using blank samples during extraction and amplification steps to assess potential contamination of samples or equipment (25,31,33,44,75,82).
FIGURE 3.
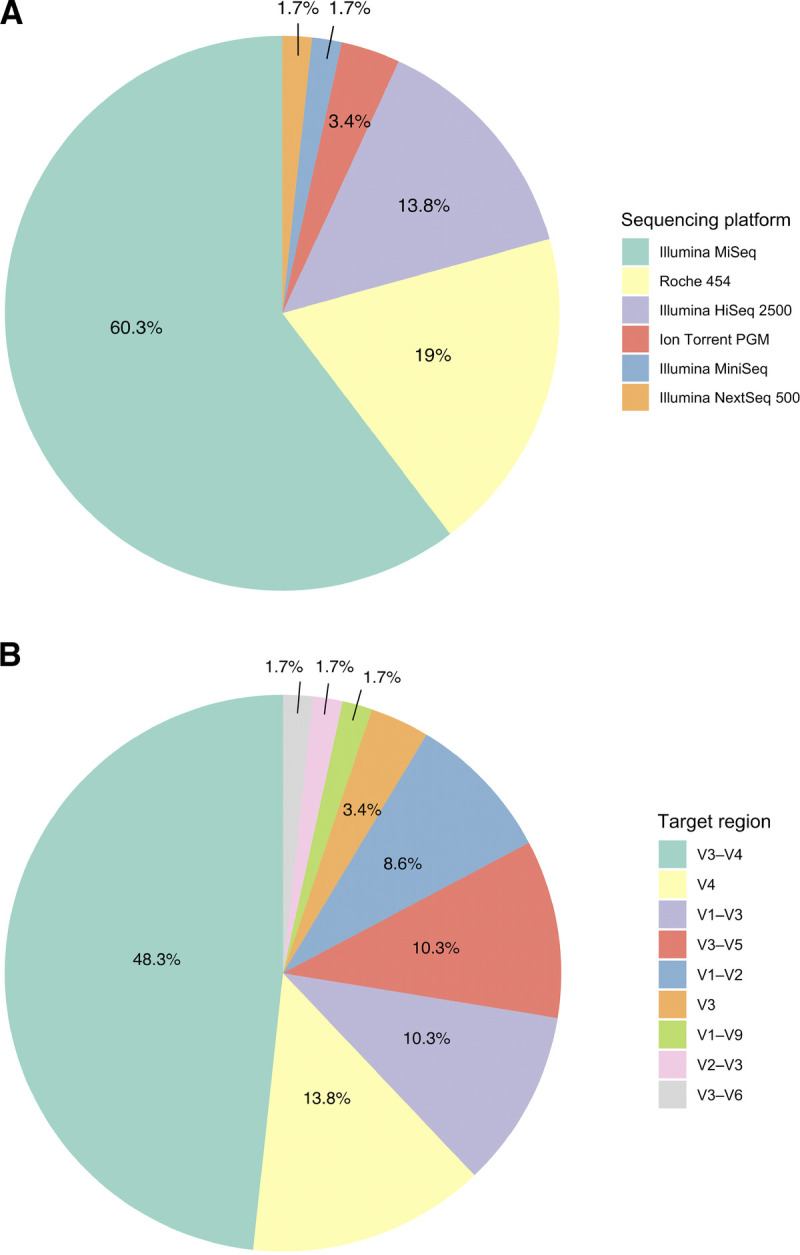
Amplification regions and sequencing platforms used in the 16S marker-gene studies. A, Sequencing primers for variable regions (V) shown in percentage. B, Sequencing platform used for high-throughput sequencing shown in percentage. The sequencing platforms and and target regions are listed from highest to lowest percentages. Color image is available online only with this article at www.psychosomaticmedicine.org.
Bioinformatic Processing
Fifty-one percent of the studies used QIIME, 19% used Mothur, 7% used R vegan package, and 3% used MEGAN5 for bioinformatic processing (Supplementary Table 4, http://links.lww.com/PSYMED/A748). For taxonomic assignment, 49% of the studies used closed-reference picking, 23% used open-reference picking, and 12% used de novo reference picking, whereas the rest of the studies did not report how taxonomic assignments were done or had ambiguous information. The most frequently used reference database for taxonomic assignment was GreenGenes (32%) followed by The Ribosomal Database Project (22%) and Silva (16%). Notably, 30% of the studies rarefied and 12% normalized the data set before calculating alpha or beta diversity indices.
Microbial Diversity in Psychiatric Disorders
Alpha Diversity
Among the 57 studies that reported one or several alpha diversity indices, 12% showed increased diversity in cases and 18% found decreased diversity in cases, whereas the majority of studies, 44%, did not detect any significant differences between cases and controls. Twenty-six percent showed mixed results (Supplementary Table 5, http://links.lww.com/PSYMED/A749). All alpha diversity indices, separated by disorder, are summarized in Figure 4. Detailed results per disorder are presented in Supplementary Material 3, http://links.lww.com/PSYMED/A750.
FIGURE 4.
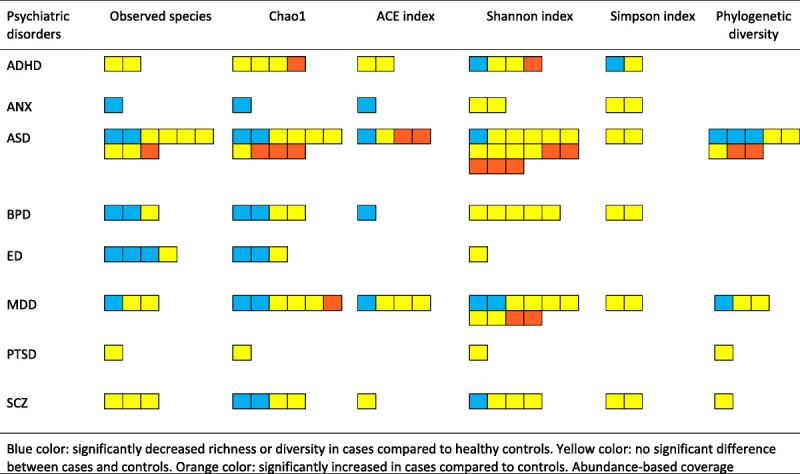
Alpha diversity indices reported for each psychiatric disorder. Blue indicates significantly decreased richness of diversity in cases compared with between cases and controls; yellow, no significant difference between cases and controls; and orange, significantly increased in cases compared with controls. ACE = abundance-based coverage estimator; ADHD = attention-deficit/hyperactivity disorder; ANX = anxiety disorder; ASD = autism spectrum disorder; BPD = bipolar disorder; ED = eating disorder; MDD = major depressive disorder; PTSD = posttraumatic stress disorder; SCZ = schizophrenia. Color image is available online only with this article at www.psychosomaticmedicine.org.
Beta Diversity
Among the 45 studies that reported beta diversity indices, 67% of the studies detected significant dissimilarity in microbiota composition between cases and controls, 27% showed no significant difference, and 6% had mixed findings (Supplementary Table 5, http://links.lww.com/PSYMED/A749). The results are most coherent in ANX, ED, SCZ, and MDD, where all but one study showed significant dissimilarity between cases and controls (40). In ASD, 57% (8 of 14 studies) found significantly dissimilar beta diversity indices between cases and healthy controls (25,30,43,65,69,70,77,89). The results are mixed for BPD and ADHD (Figure 5).
FIGURE 5.
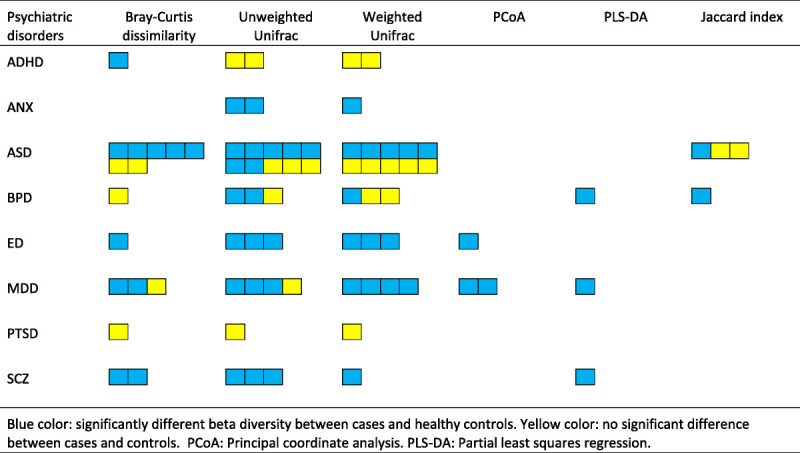
Beta diversity indices reported for each psychiatric disorder. Blue indicates significantly different beta diversity between cases and healthy controls; yellow color indicates no significant difference between cases and controls. PCoA = principal coordinate analysis; PLS-DA = partial least squares regression; ADHD = attention-deficit/hyperactivity disorder; ANX = anxiety disorder; ASD = autism spectrum disorder; BPD = bipolar disorder; ED = eating disorder; MDD = major depressive disorder; PTSD = posttraumatic stress disorder; SCZ = schizophrenia. Color image is available online only with this article at www.psychosomaticmedicine.org.
Comparison of Altered Microbiota Among Psychiatric Disorders at Genus Level
To identify patterns in taxonomic differences at genus level among the disorders, we visualized the genus-level alterations in a heat-map (Figure 6). Alterations in the relative abundance of Bifidobacterium, Faecalibacterium, and Roseburia were shared among several psychiatric disorders. Higher abundance of Bifidobacterium was found in ADHD, ASD, AN, and MDD (24,27,28,52,58,60,76,88), and lower abundance of Roseburia was reported in studies of ASD, AN, BPD, and SCZ (4,26,36,52,63,74,78,80). Furthermore, lower abundance of Faecalibacterium was reported in all psychiatric disorders (25,32,40–42,44,45,57,66,74,82,87). There were few replicated findings within each psychiatric disorder, and the abundance of specific genera was contradictory in many cases (Supplementary Material 3, http://links.lww.com/PSYMED/A750).
FIGURE 6.
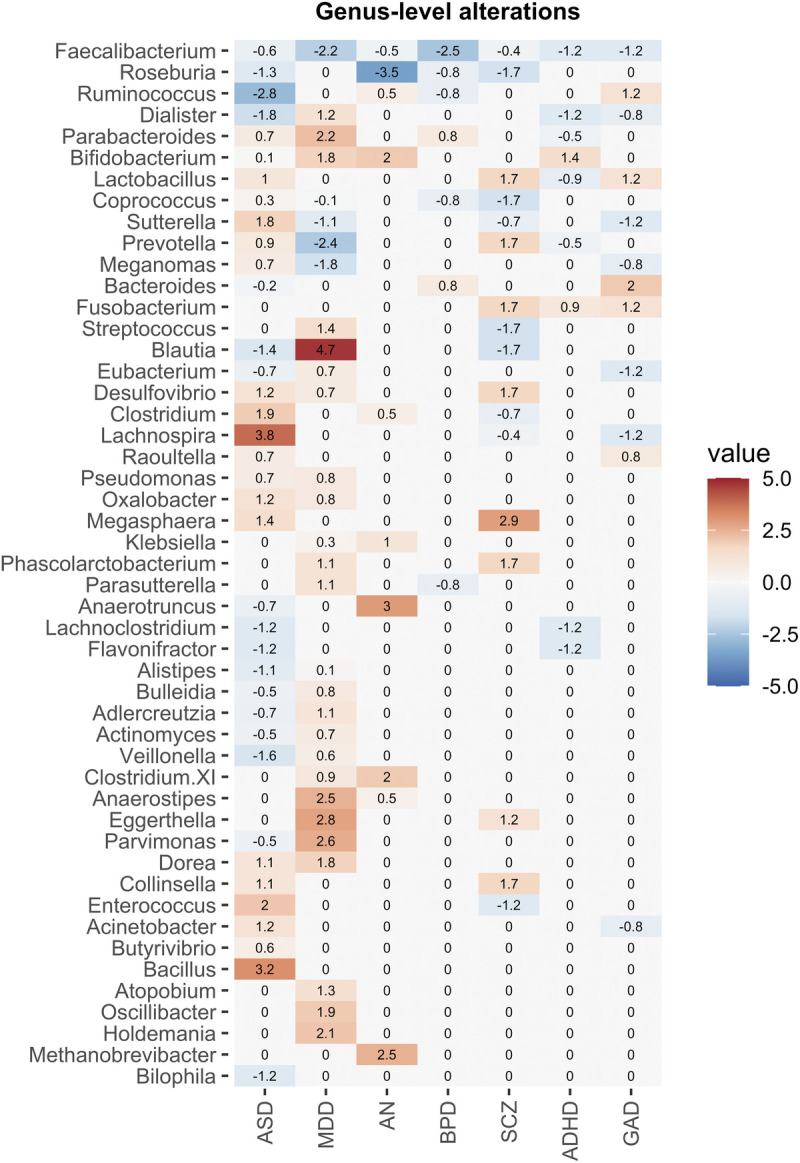
Heat-map of the gut microbiota alterations at genus level across psychiatric disorders. Only studies that used 16S rRNA sequencing and genera that were reported in more than one study were included. The values were weighted by sample size in a disorder-specific manner and represent the consistency of the results across the studies. The disorders are listed in order of number of studies included in the heat-map: attention-deficit/hyperactivity disorder (ADHD), Generalized anxiety disorder (GAD), autism spectrum disorder (ASD), bipolar disorder (BPD), anorexia nervosa (AN), major depressive disorder (MDD), tics, obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), schizophrenia (SCZ). Color image is available online only with this article at www.psychosomaticmedicine.org.
Association Between Gut Microbiota and Clinical Features
Twenty-eight studies reported positive associations between 38 clinical features and microbial alterations in cases (Supplementary Table 5, http://links.lww.com/PSYMED/A749). Replicated correlations between studies were generally scarce. Prevotella was negatively correlated with depressive symptoms according to Hamilton Depression Rating Scale (47,73). Higher abundance of Faecalibacterium was associated with improved quality of life, positively correlated with better sleep, and negatively correlated with depressive symptoms, social deficit, and hyperactivity (25,32,40,42,66).
Association Between Short-Chain Fatty Acids and Psychiatric Disorders
In eight studies, levels of short-chain fatty acids (SCFAs) were measured in stool samples (Supplementary Table 5, http://links.lww.com/PSYMED/A749). Borgo et al. (26) found significantly decreased levels of total SCFAs, specifically butyrate and propionate, in patients with AN compared with normal-weight controls. Morita et al. (53) supported the finding of decreased levels of propionate in addition to acetate but did not detect significantly lower levels of total SCFA or butyrate. In contrast, Mack et al. (52) found no significant differences in SCFA levels, although the proportion of butyrate was lower in the AN group considering higher levels of branched-chain fatty acids, such as valerate. The only study on SCFAs in stool samples from patients with MDD found no significant difference in SCFA levels in cases compared with healthy controls (3). The results of SCFAs in studies of ASD were ambiguous. Whereas Liu et al. (48) found lower levels of butyrate and acetate, but higher levels of valerate, Berding and Donovan (25) identified higher levels of butyrate and acetate and propionate. Coretti et al. (30) report higher levels of butyrate, although the levels were within normal range. However, Kang et al. (43) were unable to detect any significant differences in butyrate or propionate concentration between cases and controls.
DISCUSSION
In this systematic review, we explored 69 articles across psychiatric disorders to determine if there are consistent differences in gut microbiota between patients with psychiatric illness and healthy controls. Our findings were limited by the methodological differences at various levels across the studies. Here, we summarize the findings, discuss the methodological differences, and provide recommendations for improving microbiota studies in psychiatric disorders.
Summary of Evidence
Alpha and Beta Diversity Between Psychiatric Disorders and Controls
In summary, either there was no significant difference in alpha diversity between patients with psychiatric disorders and controls or the results were ambiguous. Across all reviewed disorders, the most consistent alpha diversity results emerged in AN, with three of four studies reporting a significant decrease in species richness in patients with AN (36,45,54). AN is associated with prolonged caloric restriction. Decreased alpha diversity in patients with AN could partly reflect prolonged restricted energy consumption. Dietary components such as carbohydrates and proteins nourish microbes in the gastrointestinal tract. When there is low dietary intake, like in AN, it may create a nonconducive environment for some microbes, subsequently affecting diversity (93).
In the majority of studies reviewed, beta diversity analysis showed a separation between patients with a psychiatric disorder and healthy controls; however, there is only limited consensus on which bacteria that differ in abundance between cases and controls. The most replicated findings are lower abundance of butyrate-producing genera, Roseburia and Faecalibacterium, and higher abundance of commensal bacteria, Bifidobacterium.
Decreased Abundance of Butyrate-Producing Genera in Psychiatric Disorders
The abundance of Roseburia was decreased in four psychiatric disorders (ASD, AN, BPD, SCZ). Roseburia includes five species that produce SCFAs, especially butyrate, and is associated with metabolic disorders such as type 2 diabetes and obesity (94,95). We also observed decreased abundance of Faecalibacterium across all psychiatric disorders. Interestingly, the abundance of Faecalibacterium is associated with various health benefits, such as less depressive symptoms, and better sleep, and improved quality of life (25,32,40,42,66). Faecalibacterium has a sole known species, Faecalibacterium prausnitzii, which constitutes between 5% and 15% of the human gut microbiota. It has anti-inflammatory properties and promotes intestinal barrier function through modulation of tight-junction protein expression and is one of the main butyrate producers (96,97). Depletion of F. prausnitzii is evident in inflammatory bowel diseases and even in patients with COVID-19 (98,99). The abundance of F. prausnitzii was inversely correlated with disease severity of COVID-19 (100).
Butyrate, one of the most abundant SCFAs, is a metabolite from the bacterial fermentation of dietary fibers. It is a crucial energy source for colonocytes and has anti-inflammatory properties of its own (101). In mice, oral administration of sodium butyrate was shown to have antidepressive effects in forced swim and tail suspension tests (102). In addition, supplementation of sodium butyrate can attenuate social deficits and decrease repetitive behavior (103). It has been suggested that butyrate could influence the brain monoaminergic pathway through modulation of the histone deacetylase inhibitor, thus affecting gene expression (104). In patients with AN, lower levels of butyrate compared with healthy controls have been documented (26,52,53,105). The mechanistic effect of butyrate in psychiatric disorders requires further inquiry.
Increased Abundance of Commensal Genera Bifidobacterium in Psychiatric Disorders
Bifidobacterium, a genus of commensal bacteria colonizing the gut, was elevated in several psychiatric disorders. There are more than 50 different known strains of Bifidobacterium. Because of various ascribed health benefits (106), different strains of Bifidobacterium are used as probiotics (107), despite insufficient scientific evidence (108). In fact, gut commensal bacteria may have complex effects on the host depending on the host genetics, environmental factors, and the overall gut microbiota composition. Thereby, beneficial bacteria can turn pathobiotic in some circumstances (109). Deeper sequencing at the species level, functional profiling, and mapping diet and host genetics might take us closer to elucidating the role of altered levels of Bifidobacterium in psychiatric disorders.
Recommendations
Most reviewed studies suffered from methodological limitations that affect the generalizability of the findings. Prominent methodological issues included small sample size, differences in patient selection and diagnosis, failure to exclude or account for confounding factors, and shortcomings in bioinformatics analysis. Therefore, we propose recommendations to improve the quality and comparability of future studies (Figure 7).
FIGURE 7.
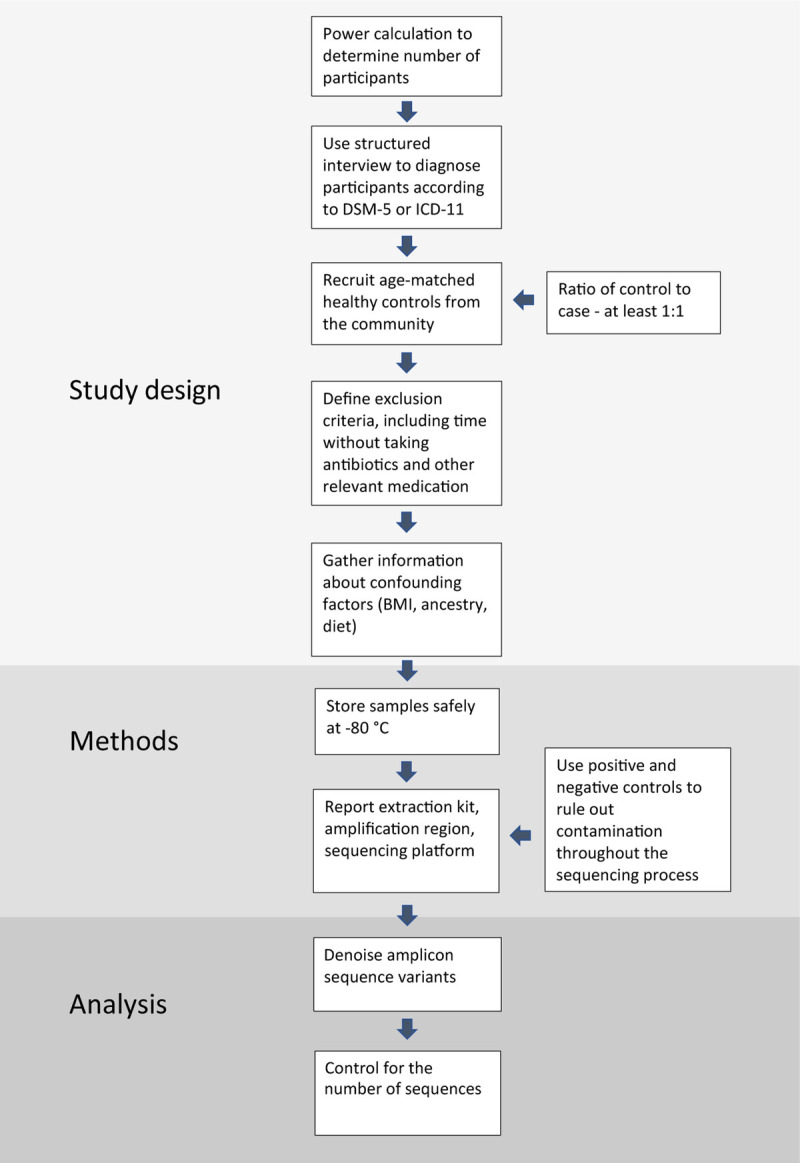
Recommendations on how to conduct a microbiota study of psychiatric disorders. DSM-5 = Diagnostic and Statistical Manual for Psychiatric Disorders, Fifth Edition; ICD-11 = International Classification of Disease, 11th Revision; BMI = body mass index. Color image is available online only with this article at www.psychosomaticmedicine.org.
Statistical Power and Case/Control Ratio
None of the studies included in this systematic review reported any kind of power calculation. Assessing the number of study participants needed to avoid type II error is fundamental in all scientific disciplines. However, in microbiome studies, power analyses remain challenging because of unknown true effect size and unknown composition of the microbiome in case and control groups. Nevertheless, several tools exist for calculating power in microbiota studies, for example, the HMP package or the micropower package (110). Importantly, power increases significantly by stratified sampling of controls on critical confounding factors, such as age, sex, and BMI. In addition, it is generally recommended that the ratio of controls to cases is at least 1:1 or higher (111).
Diagnostics
A minority of studies adopted validated structured diagnostic interviews for cases and controls (112). The recommendation is to use structured diagnostic interviews to assess diagnosis and comorbidities in cases and to rule out psychiatric illness in healthy controls.
Confounding Factors
There are two main groups of confounders in microbiota research: demographic variables including age, sex, ethnicity, and BMI, and environmental factors that include diet, medication, and life-style factors.
Age
The gut microbiota is not static and changes throughout life (21). In addition, aging has been linked to gain of disease-associated gut microbes in many diseases, such as diabetes and colorectal cancer (113). Therefore, we recommend frequency-based matching of controls based on important confounding factors, such as age.
Sex
Women have been reported to have higher alpha diversity than men, and microbial composition seems to vary significantly between sexes after puberty (114). In this review, AN and ASD groups have the most skewed sex ratios, underscoring the importance of matching the sex of cases to controls or adjusting for this confounding factor in disorders with considerable sex imbalances (115).
Ancestry and Geography
Gut microbiota may also be influenced by ancestry and geographical location (116–118). For example, healthy Black women have higher abundance of Bacteroides compared with White women (119). Because we cannot assume that all participants belong to the predominant ancestry group of that country, ancestry and geographic location of the study participants are important confounders to report.
Body Mass Index
Gut microbial diversity and composition have been shown to be associated with BMI, even after controlling for confounding factors such as sex and age (120,121). BMI is typically lower in AN, for example, which could be associated with the lower alpha diversity in the studies we reviewed. Given that BMI is known to deviate from the general population in individuals with many mental illnesses, BMI should be taken into consideration in microbiota studies of psychiatric disorders (122).
Diet
Microbial community structure and activity can be influenced by both short-term changes in diet (123) and long-term dietary patterns (93). In the studies reviewed here, only ~40% performed dietary assessments using a variety of approaches such as habitual food frequency questionnaires, 24-hour food recall, conventional macronutrient and micronutrient profiles, for example. Lack of standardized and comparable methods to assess the effects of food intake on the gut microbiota remains a limitation in microbiome research (124).
Medication
Antibiotics have a major impact on gut microbiota, and it can take up to 6 months for the gut microbiota to recover (125). Although requiring 6 months without antibiotics before inclusion could curtail the number of eligible participants in a study, fewer than 3 months without antibiotics before participation is not advisable. Of particular interest to this review, microbiota alterations associated with antipsychotic medication have been described in both animal and human studies (126,127). However, excluding patients on antipsychotic medication in studies of psychotic or BPDs might exclude most of eligible patients, whereas controlling for medication would be a more tenable option. Finally, when conducting a study on microbiota in psychiatric disorders, it is also important to document and, if possible, adjust for unusual medications used by individuals with specific disorders such as laxatives in AN and commonly abused substances from nicotine to opiates (128,129).
Bioinformatic Processing
It has been shown that differences in methodology regarding extraction, amplification, sequencing, and library preparation are sources of confounding. For example, the variation in microbiota profiles was greater in the same individual using different amplification regions than variation in stool samples from different individuals using the same amplification regions (130). As a result, it is recommended to harmonize amplification regions across studies. Further guidelines on methodology for microbiota studies have already been covered extensively (131–133). Here we highlight two aspects of data analysis that we came across in several studies in this systematic review.
Next-generation sequencing (NGS) entails unequal library sizes. Rarefying is commonly adopted to estimate uncertainty in NGS count data by selecting a minimum library size, discard libraries that are smaller than the set size, and subsample the remaining libraries without replacement. However, there are crucial flaws with this approach. It lowers power by discarding samples that cannot be classified, and it does not account for overdispersion, thus providing inferior sensitivity compared with an infinite mixture model, such as negative binomial or Gamma-Poisson (134).
It is customary to use operational taxonomic unit (OTU) picking where reads are clustered according to a set dissimilarity threshold that is arbitrarily chosen. An alternative is amplicon sequence variants, which infers sequences exactly instead of constructing OTUs. It not only offers resolution down to a single nucleotide difference; more importantly, the results are comparable between studies without sacrificing reads when using closed-reference OTU picking with reference databases. Comparable in performance to amplicon sequence variants using DADA2 is denoising with Deblur (135). In summary, ecological validity can be seriously hampered by both rarefaction and OTU picking, which has pivotal implications for downstream analysis, for example, microbiota diversity.
Lastly, we encourage researchers to be selective in their hypotheses testing and using carefully considered microbiota analysis. Preregistration of study design and analysis methods should be mandatory.
Future Perspectives
Large sample sizes are critical for adequate power, but it requires large-scale collaboration and proper funding. For now, one way to ramp up sample size is to use an open-source database depository such as Qiita (136). It allows sharing and reusing data sets from previous studies, in addition to performing new analyses according to current best practices. Although the gut microbiota is fairly stable during adulthood, its diversity and composition can change with diet, exercise, use of antibiotics, and so on. Therefore, it is important to collect longitudinal data to determine whether the differences in microbiota between patients with a psychiatric illness and controls are a state or a trait (2). Finally, as the price drops for shotgun metagenomic sequencing, the popularity of marker-gene sequencing might be diminished. Shotgun metagenomics has the benefit of higher resolution, down to the species level, enabling a more specific taxonomic and functional characterization of the gut microbes.
Limitations
Several limitations of our systematic review should be considered. First, by excluding interventional studies, we may have overlooked some contributions to the knowledge base. Second, our review was limited to English-language reports. Third, although a strength of our review is the comprehensive review across psychiatric disorders, it also introduced complexities as the psychiatric illnesses studied naturally vary by sex and age (e.g., samples from patients with AN were skewed toward women, and samples from patients with ADHD were skewed toward young men), meaning that it was not always possible to disambiguate diagnosis from age and sex. In addition, comorbidities between psychiatric disorders are common. It is possible that unmeasured comorbidities increased similarity of findings across different psychiatric disorders. Future studies should consider and control for co-occurring presentations whenever possible.
Conclusions
Our review suggests that the study of the intestinal microbiota in psychiatric disorders is characterized by minimal cohesion and few reliable replications. In part, this reflects the stage of the field as methods are still being optimized and standardized. Given this context, the limited evidence suggests no consistent differences in alpha diversity between patients with psychiatric disorders (AN being an exception) and healthy controls. However, we found more consistent differences in beta diversities, indicating dissimilar microbiota composition between patients with a psychiatric disorder and healthy controls. The most replicated findings at genus level include decreased abundance of SCFA-producing microbes, such as F. prausnitzii and Roseburia species, and increased abundance of Bifidobacterium in patients with psychiatric disorders, which provide grounds for further functional and mechanistic investigations. Finally, to improve study quality, collaboration between psychiatrists and microbiologists is imperative in planning and executing microbiome studies in psychiatric disorders.
Supplementary Material
Acknowledgments
We would like to thank Gun Brit Knutssön and Magdalena Svanberg, two librarians at the Karolinska Institutet University Library who performed the database searches.
Source of Funding and Conflicts of Interest: C.M. Bulik reports the following: Shire (grant recipient, Scientific Advisory Board member), Idorsia (consultant), and Pearson (author, royalty recipient). Dr. Bulik is supported by the National Institute of Mental Health (R01MH120170, R01MH119084, R01MH118278, U01 MH109528), Brain and Behavior Research Foundation Distinguished Investigator Grant, Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864), and Lundbeck Foundation (grant no. R276-2018-4581). The other named authors have no conflict of interest, financial or otherwise.
Author Contribution: C.M.B., L.L.C., and A.A. conceptualized the project. L.L.C., A.A., G.F.M., and D.D. performed the literature review, including sorting and extracting the articles, in addition to writing the first draft of the manuscript. All authors contributed to the writing and revising of the manuscript.
Footnotes
L.L.C. and A.A. contributed equally to this work.
Supplemental Digital Content
Contributor Information
Afrouz Abbaspour, Email: afrouz.abbaspour@ki.se.
George F. Mkoma, Email: george.mkoma@sund.ku.dk.
Cynthia M. Bulik, Email: cynthia_bulik@med.unc.edu.
Christian Rück, Email: christian.ruck@ki.se.
Diana Djurfeldt, Email: diana.djurfeldt@ki.se.
REFERENCES
- 1.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The microbiota-gut-brain axis. Physiol Rev 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 2.Aroniadis OC, Drossman DA, Simren M. A perspective on brain-gut communication: The American Gastroenterology Association and American Psychosomatic Society joint symposium on brain-gut interactions and the intestinal microenvironment. Psychosom Med 2017;79:847–56. [DOI] [PubMed] [Google Scholar]
- 3.Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 4.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96. [DOI] [PubMed] [Google Scholar]
- 5.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, Zhou X, Gui S, Perry SW, Wong ML, Licinio J, Wei H, Xie P. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-gaba cycle and schizophrenia-relevant behaviors in mice. Sci Adv 2019;5:eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tengeler AC, Dam SA, Wiesmann M, Naaijen J, van Bodegom M, Belzer C, Dederen PJ, Verweij V, Franke B, Kozicz T, Arias Vasquez A, Kiliaan AJ. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 2020;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci 2016;39:763–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, Ford CB, Bryant JA, Henn MR, Hohmann EL. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med 2020;17:e1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuter JA, Spacek DV, Snyder MP. High-throughput sequencing technologies. Mol Cell 2015;58:586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezawada N, Phang TH, Hold GL, Hansen R. Autism spectrum disorder and the gut microbiota in children: a systematic review. Ann Nutr Metab 2020;76:16–29. [DOI] [PubMed] [Google Scholar]
- 11.Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry 2019;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho LKH, Tong VJW, Syn N, Nagarajan N, Tham EH, Tay SK, Shorey S, Tambyah PA, Law ECN. Gut microbiota changes in children with autism spectrum disorder: a systematic review. Gut Pathog 2020;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TT, Hathaway H, Kosciolek T, Knight R, Jeste DV. Gut microbiome in serious mental illnesses: a systematic review and critical evaluation. Schizophr Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schalla MA, Stengel A. Gastrointestinal alterations in anorexia nervosa—a systematic review. Eur Eat Disord Rev 2019;27:447–61. [DOI] [PubMed] [Google Scholar]
- 15.Vindegaard N, Speyer H, Nordentoft M, Rasmussen S, Benros ME. Gut microbial changes of patients with psychotic and affective disorders: a systematic review. Schizophr Res 2020. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Xu X, Li J, Li F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry 2019;10:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanada K, Nakajima S, Kurokawa S, Barcelo-Soler A, Ikuse D, Hirata A, Yoshizawa A, Tomizawa Y, Salas-Valero M, Noda Y, Mimura M, Iwanami A, Kishimoto T. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord 2020;266:1–13. [DOI] [PubMed] [Google Scholar]
- 18.Moher D Liberati A Tetzlaff J Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute Web site: Oxford; 2000. [Google Scholar]
- 21.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel D, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H. Welcome to the tidyverse. J Open Source Softw 2019;4. [Google Scholar]
- 23.Johnson JS, Spakowicz DJ, Hong BY, Petersen LM, Demkowicz P, Chen L, Leopold SR, Hanson BM, Agresta HO, Gerstein M, Sodergren E, Weinstock GM. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun 2019;10:5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, Smeekens SP, Netea MG, Buitelaar JK, Franke B, van Hijum SAFT, Arias Vasquez A. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 2017;12:e0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berding K, Donovan SM. Diet can impact microbiota composition in children with autism spectrum disorder. Front Neurosci 2018;12:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borgo F, Riva A, Benetti A, Casiraghi MC, Bertelli S, Garbossa S, Anselmetti S, Scarone S, Pontiroli AE, Morace G, Borghi E. Microbiota in anorexia nervosa: the triangle between bacterial species, metabolites and psychological tests. PLoS One 2017;12:e0179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, Xie P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat 2018;14:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung YE, Chen HC, Chou HL, Chen IM, Lee MS, Chuang LC, Liu YW, Lu ML, Chen CH, Wu CS, Huang MC, Liao SC, Ni YH, Lai MS, Shih WL, Kuo PH. Exploration of microbiota targets for major depressive disorder and mood related traits. J Psychiatr Res 2019;111:74–82. [DOI] [PubMed] [Google Scholar]
- 29.Coello K, Hansen TH, Sorensen N, Munkholm K, Kessing LV, Pedersen O, Vinberg M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav Immun 2019;75:112–8. [DOI] [PubMed] [Google Scholar]
- 30.Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, Borrelli L, Corrado G, Comegna M, Buommino E, Castaldo G, Bravaccio C, Chiariotti L, Berni Canani R, Lembo F. Gut microbiota features in young children with autism spectrum disorders. Front Microbiol 2018;9:3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 2013;8:e76993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB, Ellingrod VE, McInnis MG. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res 2017;87:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA, 3rd. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010;16:444–53. [DOI] [PubMed] [Google Scholar]
- 34.Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, Collins MD, Lawson PA, Summanen P, Baysallar M, Tomzynski TJ, Read E, Johnson E, Rolfe R, Nasir P, Shah H, Haake DA, Manning P, Kaul A. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 2002;35(Suppl 1):S6–s16. [DOI] [PubMed] [Google Scholar]
- 35.Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res 2012;5:419–27. [DOI] [PubMed] [Google Scholar]
- 36.Hanachi M, Manichanh C, Schoenenberger A, Pascal V, Levenez F, Cournede N, Dore J, Melchior JC. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: an explicative factor of functional intestinal disorders? Clin Nutr 2019;38:2304–10. [DOI] [PubMed] [Google Scholar]
- 37.Hemmings SMJ, Malan-Müller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, Marotz CA, Siebler PH, Braspenning M, Van Criekinge W, Hoisington AJ, Brenner LA, Postolache TT, McQueen MB, Krauter KS, Knight R, Seedat S, Lowry CA. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 2017;79:936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, Shi X, Li Z, Shen Y, Shi X, Wang L, Li G, Yuan Y, Wang J, Zhang Y, Zhao L, Zhang M, Kang Y, Liang Y. Possible association of firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr Dis Treat 2018;14:3329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue R, Sakaue Y, Sawai C, Sawai T, Ozeki M, Romero-Perez GA, Tsukahara T. A preliminary investigation on the relationship between gut microbiota and gene expressions in peripheral mononuclear cells of infants with autism spectrum disorders. Biosci Biotechnol Biochem 2016;80:2450–8. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015;48:186–94. [DOI] [PubMed] [Google Scholar]
- 41.Jiang HY, Zhang X, Yu ZH, Zhang Z, Deng M, Zhao JH, Ruan B. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res 2018;104:130–6. [DOI] [PubMed] [Google Scholar]
- 42.Jiang HY, Zhou YY, Zhou GL, Li YC, Yuan J, Li XH, Ruan B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav Brain Res 2018;347:408–13. [DOI] [PubMed] [Google Scholar]
- 43.Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, Lozupone CA, Hahn J, Adams JB, Krajmalnik-Brown R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 2018;49:121–31. [DOI] [PubMed] [Google Scholar]
- 44.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 2013;8:e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, Carroll IM. The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med 2015;77:969–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushak RI, Winter HS, Buie TM, Cox SB, Phillips CD, Ward NL. Analysis of the duodenal microbiome in autistic individuals: association with carbohydrate digestion. J Pediatr Gastroenterol Nutr 2017;64:e110–6. [DOI] [PubMed] [Google Scholar]
- 47.Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, Lv H, Guo X, Dong K, Zhu Y, Li Q. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord 2017;207:300–4. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, Yu Y, Mei L, Yang P, Tang Y, Zheng P. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep 2019;9:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Zhang L, Wang X, Wang Z, Zhang J, Jiang R, Wang X, Wang K, Liu Z, Xia Z, Xu Z, Nie Y, Lv X, Wu X, Zhu H, Duan L. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin Gastroenterol Hepatol 2016;14:1602–11.e5. [DOI] [PubMed] [Google Scholar]
- 50.Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell Mol Gastroenterol Hepatol 2017;3:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma B, Liang J, Dai M, Wang J, Luo J, Zhang Z, Jing J. Altered gut microbiota in Chinese children with autism spectrum disorders. Front Cell Infect Microbiol 2019;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mack I, Cuntz U, Gramer C, Niedermaier S, Pohl C, Schwiertz A, Zimmermann K, Zipfel S, Enck P, Penders J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 2016;6:26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, Miyazaki K, Sudo N. Gut dysbiosis in patients with anorexia nervosa. PLoS One 2015;10:e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morkl S, Lackner S, Muller W, Gorkiewicz G, Kashofer K, Oberascher A, Painold A, Holl A, Holzer P, Meinitzer A, Mangge H, Holasek S. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Disord 2017;50:1421–31. [DOI] [PubMed] [Google Scholar]
- 55.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 2014;26:1155–62. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, Knight R, Jeste DV. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res 2019;204:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Painold A, Morkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, Birner A, Fellendorf F, Platzer M, Queissner R, Schutze G, Schwarz MJ, Moll N, Holzer P, Holl AK, Kapfhammer HP, Gorkiewicz G, Reininghaus EZ. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord 2019;21:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plaza-Díaz J, Gómez-Fernández A, Chueca N, Torre-Aguilar MJ, Gil Á, Perez-Navero JL, Flores-Rojas K, Martín-Borreguero P, Solis-Urra P, Ruiz-Ojeda FJ, Garcia F, Gil-Campos M. Autism spectrum disorder (ASD) with and without mental regression is associated with changes in the fecal microbiota. Nutrients 2019;11:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, Fischer A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS One 2018;13:e0200728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pulikkan J, Maji A, Dhakan DB, Saxena R, Mohan B, Anto MM, Agarwal N, Grace T, Sharma VK. Gut microbial dysbiosis in indian children with autism spectrum disorders. Microb Ecol 2018;76:1102–14. [DOI] [PubMed] [Google Scholar]
- 61.Rose DR, Yang H, Serena G, Sturgeon C, Ma B, Careaga M, Hughes HK, Angkustsiri K, Rose M, Hertz-Picciotto I, Van de Water J, Hansen RL, Ravel J, Fasano A, Ashwood P. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun 2018;70:354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res 2018;192:398–403. [DOI] [PubMed] [Google Scholar]
- 63.Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, Zhang M, Hu S, Liang Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res 2018;197:470–7. [DOI] [PubMed] [Google Scholar]
- 64.Son JS, Zheng LJ, Rowehl LM, Tian X, Zhang Y, Zhu W, Litcher-Kelly L, Gadow KD, Gathungu G, Robertson CE, Ir D, Frank DN, Li E. Comparison of fecal microbiota in children with autism spectrum disorders and neurotypical siblings in the simons simplex collection. PLoS One 2015;10:e0137725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabro A, De Filippo C. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 2019;4:623–32. [DOI] [PubMed] [Google Scholar]
- 67.Wang M, Wan J, Rong H, He F, Wang H, Zhou J, Cai C, Wang Y, Xu R, Yin Z, Zhou W. Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with autism spectrum disorder. mSystems 2019;4:e00321–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 2011;6:e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhai Q, Cen S, Jiang J, Zhao J, Zhang H, Chen W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: a pilot study of Chinese children. Environ Res 2019;171:501–9. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M, Ma W, Zhang J, He Y, Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep 2018;8:13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carissimi C, Laudadio I, Palone F, Fulci V, Cesi V, Cardona F, Alfonsi C, Cucchiara S, Isoldi S, Stronati L. Functional analysis of gut microbiota and immunoinflammation in children with autism spectrum disorders. Dig Liver Dis 2019;51:1366–74. [DOI] [PubMed] [Google Scholar]
- 72.Chen JJ, He S, Fang L, Wang B, Bai SJ, Xie J, Zhou CJ, Wang W, Xie P. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging (Albany NY) 2020;12:2764–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen YH, Bai J, Wu D, Yu SF, Qiang XL, Bai H, Wang HN, Peng ZW. Association between fecal microbiota and generalized anxiety disorder: severity and early treatment response. J Affect Disord 2019;259:56–66. [DOI] [PubMed] [Google Scholar]
- 74.Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, Lu H, Lu Q, Du Y, Hu Z, Ng CH, Zhang H, Lu J, Mou T, Lu S, Wang D, Duan J, Hu J, Huang M, Wei N, Zhou W, Ruan L, Li MD, Xu Y. Gut microbiota changes in patients with bipolar depression. Adv Sci (Weinh) 2019;6:1900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong X, Liu J, Cetinbas M, Sadreyev R, Koh M, Huang H, Adeseye A, He P, Zhu J, Russell H, Hobbie C, Liu K, Onderdonk AB. New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 2019;11:2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai WT, Deng WF, Xu SX, Zhao J, Xu D, Liu YH, Guo YY, Wang MB, He FS, Ye SW, Yang QF, Liu TB, Zhang YL, Wang S, Li MZ, Yang YJ, Xie XH, Rong H. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in major depressive disorder patients. Psychol Med 2019;1–12. [DOI] [PubMed] [Google Scholar]
- 77.Li N, Yang J, Zhang J, Liang C, Wang Y, Chen B, Zhao C, Wang J, Zhang G, Zhao D, Liu Y, Zhang L, Yang J, Li G, Gai Z, Zhang L, Zhao G. Correlation of gut microbiome between ASD children and mothers and potential biomarkers for risk assessment. Genomics Proteomics Bioinformatics 2019;17:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mason BL, Li Q, Minhajuddin A, Czysz AH, Coughlin LA, Hussain SK, Koh AY, Trivedi MH. Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J Affect Disord 2020;266:394–401. [DOI] [PubMed] [Google Scholar]
- 79.McIntyre RS, Subramaniapillai M, Shekotikhina M, Carmona NE, Lee Y, Mansur RB, Brietzke E, Fus D, Coles AS, Iacobucci M, Park C, Potts R, Amer M, Gillard J, James C, Anglin R, Surette MG. Characterizing the gut microbiota in adults with bipolar disorder: a pilot study. Nutr Neurosci 2019;1–8. [DOI] [PubMed] [Google Scholar]
- 80.Niu M, Li Q, Zhang J, Wen F, Dang W, Duan G, Li H, Ruan W, Yang P, Guan C, Tian H, Gao X, Zhang S, Yuan F, Han Y. Characterization of intestinal microbiota and probiotics treatment in children with autism spectrum disorders in China. Front Neurol 2019;10:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rong H, Xie XH, Zhao J, Lai WT, Wang MB, Xu D, Liu YH, Guo YY, Xu SX, Deng WF, Yang QF, Xiao L, Zhang YL, He FS, Wang S, Liu TB. Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res 2019;113:90–9. [DOI] [PubMed] [Google Scholar]
- 82.Stevens BR, Roesch L, Thiago P, Russell JT, Pepine CJ, Holbert RC, Raizada MK, Triplett EW. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun H, You Z, Jia L, Wang F. Autism spectrum disorder is associated with gut microbiota disorder in children. BMC Pediatr 2019;19:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tomova A, Soltys K, Repiska G, Palkova L, Filcikova D, Minarik G, Turna J, Prochotska K, Babinska K, Ostatnikova D. Specificity of gut microbiota in children with autism spectrum disorder in slovakia and its correlation with astrocytes activity marker and specific behavioural patterns. Physiol Behav 2020;214:112745. [DOI] [PubMed] [Google Scholar]
- 85.Wang LJ, Yang CY, Chou WJ, Lee MJ, Chou MC, Kuo HC, Yeh YM, Lee SY, Huang LH, Li SC. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry 2020;29:287–97. [DOI] [PubMed] [Google Scholar]
- 86.Xu R, Wu B, Liang J, He F, Gu W, Li K, Luo Y, Chen J, Gao Y, Wu Z, Wang Y, Zhou W, Wang M. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun 2020;85:120–7. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Pan LY, Zhang Z, Zhou YY, Jiang HY, Ruan B. Analysis of gut mycobiota in first-episode, drug-naïve Chinese patients with schizophrenia: a pilot study. Behav Brain Res 2020;379:112374. [DOI] [PubMed] [Google Scholar]
- 88.Zheng P, Yang J, Li Y, Wu J, Liang W, Yin B, Tan X, Huang Y, Chai T, Zhang H, Duan J, Zhou J, Sun Z, Chen X, Marwari S, Lai J, Huang T, Du Y, Zhang P, Perry SW, Wong ML, Licinio J, Hu S, Xie P, Wang G. Gut microbial signatures can discriminate unipolar from bipolar depression. Adv Sci (Weinh) 2020;7:1902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zurita MF, Cardenas PA, Sandoval ME, Pena MC, Fornasini M, Flores N, Monaco MH, Berding K, Donovan SM, Kuntz T, Gilbert JA, Baldeon ME. Analysis of gut microbiome, nutrition and immune status in autism spectrum disorder: a case-control study in ecuador. Gut Microbes 2020;11:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Pub; 2013. [Google Scholar]
- 91.Bell CC. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. JAMA 1994;272:828–9. [Google Scholar]
- 92.World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: clinical descriptions and diagnostic guidelines. Wkly Epidemiol Rec Relev Epidémiol Hebd 1992;67:227. [Google Scholar]
- 93.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, Galván-Rodríguez FM, Miranda-Brito C, Romano MC, Piña-Escobedo A, Pizano-Zárate ML, Hoyo-Vadillo C, García-Mena J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis 2015;34:1337–46. [DOI] [PubMed] [Google Scholar]
- 95.Jamshidi P, Hasanzadeh S, Tahvildari A, Farsi Y, Arbabi M, Mota JF, Sechi LA, Nasiri MJ. Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut Pathog 2019;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiu X, Zhang M, Yang X, Hong N, Yu C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohns Colitis 2013;7:e558–68. [DOI] [PubMed] [Google Scholar]
- 97.Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 2013;16:255–61. [DOI] [PubMed] [Google Scholar]
- 98.Pittayanon R, Lau JT, Leontiadis GI, Tse F, Yuan Y, Surette M, Moayyedi P. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology 2020;158:930–46.e1. [DOI] [PubMed] [Google Scholar]
- 99.Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70:698–706:gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020;159:944–55.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- 102.Sun J, Wang F, Hong G, Pang M, Xu H, Li H, Tian F, Fang R, Yao Y, Liu J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett 2016;618:159–66. [DOI] [PubMed] [Google Scholar]
- 103.Kratsman N, Getselter D, Elliott E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology 2016;102:136–45. [DOI] [PubMed] [Google Scholar]
- 104.Yamawaki Y, Fuchikami M, Morinobu S, Segawa M, Matsumoto T, Yamawaki S. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J Biol Psychiatry 2012;13:458–67. [DOI] [PubMed] [Google Scholar]
- 105.Speranza E, Cioffi I, Santarpia L, Del Piano C, De Caprio C, Naccarato M, Marra M, De Filippo E, Contaldo F, Pasanisi F. Fecal short chain fatty acids and dietary intake in italian women with restrictive anorexia nervosa: a pilot study. Front Nutr 2018;5:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bottacini F, van Sinderen D, Ventura M. Omics of bifidobacteria: research and insights into their health-promoting activities. Biochem J 2017;474:4137–52. [DOI] [PubMed] [Google Scholar]
- 107.Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: a randomized, placebo-controlled trial. Biomark Insights 2015;10:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ng QX, Peters C, Ho CYX, Lim DY, Yeo WS. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord 2018;228:13–9. [DOI] [PubMed] [Google Scholar]
- 109.Fine RL, Mubiru DL, Kriegel MA. Chapter two—friend or foe? Lactobacillus in the context of autoimmune disease. In: Alt FW, editor. Advances in Immunology, Volume 146. Cambridge, MA: Academic Press; 2020:29–56. [DOI] [PubMed] [Google Scholar]
- 110.Kelly BJ, Gross R, Bittinger K, Sherrill-Mix S, Lewis JD, Collman RG, Bushman FD, Li H. Power and sample-size estimation for microbiome studies using pairwise distances and permanova. Bioinformatics 2015;31:2461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewallen S, Courtright P. Epidemiology in practice: case-control studies. Community Eye Health 1998;11:57–8. [PMC free article] [PubMed] [Google Scholar]
- 112.First MB. Structured Clinical Interview for DSM-IV Axis I Disorders SCID-I: Clinician Version, Scoresheet. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 113.Ghosh TS, Das M, Jeffery IB, O’Toole PW. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013;39:400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet 2008;9:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, Mujagond P, Chen XJ, Rong ZH, Chen P, Lyu LY, Wang X, Wu CB, Yu N, Xu YJ, Yin J, Raes J, Knight R, Ma WJ, Zhou HW. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med 2018;24:1532–5. [DOI] [PubMed] [Google Scholar]
- 117.Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, Tremaroli V, Bakker GJ, Attaye I, Pinto-Sietsma SJ, van Raalte DH, Snijder MB, Nicolaou M, Peters R, Zwinderman AH, Bäckhed F, Nieuwdorp M. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med 2018;24:1526–31. [DOI] [PubMed] [Google Scholar]
- 118.Fortenberry JD. The uses of race and ethnicity in human microbiome research. Trends Microbiol 2013;21:165–6. [DOI] [PubMed] [Google Scholar]
- 119.Carson TL, Wang F, Cui X, Jackson BE, Van Der Pol WJ, Lefkowitz EJ, Morrow C, Baskin ML. Associations between race, perceived psychological stress, and the gut microbiota in a sample of generally healthy Black and White women: a pilot study on the role of race and perceived psychological stress. Psychosom Med 2018;80:640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow STE, Schröder H. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol 2018;2018:4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr Obes 2019;14:e12480. [DOI] [PubMed] [Google Scholar]
- 122.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 2013;45:92–9. [DOI] [PubMed] [Google Scholar]
- 123.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnson AJ Vangay P Al-Ghalith GA Hillmann BM Ward TL Shields-Cutler RR Kim AD Shmagel AK Syed AN; Personalized Microbiome Class Students, Walter J Menon R Koecher K Knights D. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe 2019;25:789–802.e5. [DOI] [PubMed] [Google Scholar]
- 125.Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, Pyl PT, Coelho LP, Yang H, Wang J, Typas A, Nielsen MF, Nielsen HB, Bork P, Wang J, Vilsbøll T, Hansen T, Knop FK, Arumugam M, Pedersen O. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol 2018;3:1255–65. [DOI] [PubMed] [Google Scholar]
- 126.Flowers SA, Baxter NT, Ward KM, Kraal AZ, McInnis MG, Schmidt TM, Ellingrod VL. Effects of atypical antipsychotic treatment and resistant starch supplementation on gut microbiome composition in a cohort of patients with bipolar disorder or schizophrenia. Pharmacotherapy 2019;39:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skonieczna-Żydecka K, Łoniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, Galling B. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology (Berl) 2019;236:1491–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tropini C, Moss EL, Merrill BD, Ng KM, Higginbottom SK, Casavant EP, Gonzalez CG, Fremin B, Bouley DM, Elias JE, Bhatt AS, Huang KC, Sonnenburg JL. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 2018;173:1742–54.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cussotto S, Clarke G, Dinan TG, Cryan JF. Psychotropics and the microbiome: a chamber of secrets…. Psychopharmacology (Berl) 2019;236:1411–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huttenhower C Gevers D Knight R Abubucker S Badger JH Chinwalla AT Creasy HH Earl AM FitzGerald MG Fulton RS Giglio MG Hallsworth-Pepin K Lobos EA Madupu R Magrini V Martin JC Mitreva M Muzny DM Sodergren EJ Versalovic J Wollam AM Worley KC Wortman JR Young SK Zeng Q Aagaard KM Abolude OO Allen-Vercoe E Alm EJ Alvarado L Andersen GL Anderson S Appelbaum E Arachchi HM Armitage G Arze CA Ayvaz T Baker CC Begg L Belachew T Bhonagiri V Bihan M Blaser MJ Bloom T Bonazzi V Paul Brooks J Buck GA Buhay CJ Busam DA Campbell JL Canon SR Cantarel BL Chain PSG Chen IMA Chen L Chhibba S Chu K Ciulla DM Clemente JC Clifton SW Conlan S Crabtree J Cutting MA Davidovics NJ Davis CC DeSantis TZ Deal C Delehaunty KD Dewhirst FE Deych E Ding Y Dooling DJ Dugan SP Michael Dunne W Scott Durkin A Edgar RC Erlich RL Farmer CN Farrell RM Faust K Feldgarden M Felix VM Fisher S Fodor AA Forney LJ Foster L Di Francesco V Friedman J Friedrich DC Fronick CC Fulton LL Gao H Garcia N Giannoukos G Giblin C Giovanni MY Goldberg JM Goll J Gonzalez A Griggs A Gujja S Kinder Haake S Haas BJ Hamilton HA Harris EL Hepburn TA Herter B Hoffmann DE Holder ME Howarth C Huang KH Huse SM Izard J Jansson JK Jiang H Jordan C Joshi V Katancik JA Keitel WA Kelley ST Kells C King NB Knights D Kong HH Koren O Koren S Kota KC Kovar CL Kyrpides NC La Rosa PS Lee SL Lemon KP Lennon N Lewis CM Lewis L Ley RE Li K Liolios K Liu B Liu Y Lo CC Lozupone CA Dwayne Lunsford R Madden T Mahurkar AA Mannon PJ Mardis ER Markowitz VM Mavromatis K McCorrison JM McDonald D McEwen J McGuire AL McInnes P Mehta T Mihindukulasuriya KA Miller JR Minx PJ Newsham I Nusbaum C O’Laughlin M Orvis J Pagani I Palaniappan K Patel SM Pearson M Peterson J Podar M Pohl C Pollard KS Pop M Priest ME Proctor LM Qin X Raes J Ravel J Reid JG Rho M Rhodes R Riehle KP Rivera MC Rodriguez-Mueller B Rogers YH Ross MC Russ C Sanka RK Sankar P Fah Sathirapongsasuti J Schloss JA Schloss PD Schmidt TM Scholz M Schriml L Schubert AM Segata N Segre JA Shannon WD Sharp RR Sharpton TJ Shenoy N Sheth NU Simone GA Singh I Smillie CS Sobel JD Sommer DD Spicer P Sutton GG Sykes SM Tabbaa DG Thiagarajan M Tomlinson CM Torralba M Treangen TJ Truty RM Vishnivetskaya TA Walker J Wang L Wang Z Ward DV Warren W Watson MA Wellington C Wetterstrand KA White JR Wilczek-Boney K Wu Y Wylie KM Wylie T Yandava C Ye L Ye Y Yooseph S Youmans BP Zhang L Zhou Y Zhu Y Zoloth L Zucker JD Birren BW Gibbs RA Highlander SK Methé BA Nelson KE Petrosino JF Weinstock GM Wilson RK White O, The Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Allaband C, McDonald D, Vázquez-Baeza Y, Minich JJ, Tripathi A, Brenner DA, Loomba R, Smarr L, Sandborn WJ, Schnabl B, Dorrestein P, Zarrinpar A, Knight R. Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol 2019;17:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim D, Hofstaedter CE, Zhao C, Mattei L, Tanes C, Clarke E, Lauder A, Sherrill-Mix S, Chehoud C, Kelsen J, Conrad M, Collman RG, Baldassano R, Bushman FD, Bittinger K. Optimizing methods and dodging pitfalls in microbiome research. Microbiome 2017;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall LI, McDonald D, Melnik AV, Morton JT, Navas J, Quinn RA, Sanders JG, Swafford AD, Thompson LR, Tripathi A, Xu ZZ, Zaneveld JR, Zhu Q, Caporaso JG, Dorrestein PC. Best practices for analysing microbiomes. Nat Rev Microbiol 2018;16:410–22. [DOI] [PubMed] [Google Scholar]
- 134.McMurdie PJ, Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol 2014;10:e1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nearing JT, Douglas GM, Comeau AM, Langille MGI. Denoising the denoisers: an independent evaluation of microbiome sequence error-correction approaches. PeerJ 2018;6:e5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gonzalez A, Navas-Molina JA, Kosciolek T, McDonald D, Vázquez-Baeza Y, Ackermann G, DeReus J, Janssen S, Swafford AD, Orchanian SB, Sanders JG, Shorenstein J, Holste H, Petrus S, Robbins-Pianka A, Brislawn CJ, Wang M, Rideout JR, Bolyen E, Dillon M, Caporaso JG, Dorrestein PC, Knight R. Qiita: rapid, web-enabled microbiome meta-analysis. Nat Methods 2018;15:796–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


