Abstract
It is now well understood that the eukaryotic host has evolved multiple mechanisms to monitor and respond to the diverse and biochemically active microbiota that thrives in a symbiotic fashion in the gut and other tissues. Generally, these mechanisms are based on traditional notions of innate and adaptive immune processes, which are mediated by recognition of, and response to, microbially derived macromolecules. Microbes themselves are metabolically active and contribute a vast array of small molecules, not present in germ-free model systems, with diverse putative and unknown biological function, and intensive work is ongoing to unravel their roles in physiological systems. Metazoans have evolved and maintain distinct gene regulatory networks to detect and respond to environmental, non-self-molecules (xenobiotics), and interestingly, recent investigation has shown that these pathways are operational in the detection and response to microbiota-derived small metabolites. These processes likely represent a general mechanism of host-microbe crosstalk, and they have clinical implications in drug and xenobiotic metabolism.
Keywords: detoxification, lactobacilli, Nrf2, signaling
Eukaryotic-Prokaryotic Interactions
As a moment’s thought demonstrates, multicellular organisms have interfaced with their external environment continuously throughout evolutionary history. Part of the external environment must necessarily have included the myriad prokaryotic microbes and viral particles that are capable of occupying virtually all available niches, including sites external and internal to an organism. Necessarily, metazoans have had to evolve diverse and specific mechanisms to protect themselves from the threats posed by exogenous microbes, mechanisms now generally referred to as immunity. Indeed, our fundamental conception of immunology is based on the notion of defense against external invasion and subversion, and it is often illustrated and taught with corresponding martial metaphors. Traditionally, immunology is usually defined in terms of subfields of intrinsic, innate, and adaptive immunity, all of which are conceptualized as multifaceted and interactive cell-based defensive processes variably shared by most eukaryotic taxa and deployed in specific tissues.
Intrinsic defenses are represented by physical and chemical obstacles that can include secreted products, such as polymeric cuticle and mucus, epithelial barrier enhancing cellular junctions and cell walls, chemical defenses, such as antimicrobial cationic peptides, and reactive oxygen species (ROS), among others. Typically, intrinsic defenses are seen in epithelial tissues that interface directly with the external environment or line the lumen of gut or respiratory surfaces (37). Intrinsic defenses are often static, but can be induced in certain situations, such as the transcriptional activation of antimicrobial peptide (AMP) synthesis or posttranslational release of mucins (47). Intrinsic defenses, which are seen in all metazoans, remain a key component of human physiology, e.g., AMP secretion from Paneth cells in small intestinal epithelial crypt cells, mucins from goblet cells, ROS production via Nox/Duox enzymes, and extensive barrier structures in the epithelial monolayer (43, 47).
Innate immunity similarly has an ancient history and is present in lower invertebrates and plants. Innate immunity is based on receptor-mediated detection of macromolecules derived from, and characteristic of, microbes, a class of molecules now operationally referred to as “MAMPs”, for microbe-associated molecular patterns, which include bacterial lipopolysaccharides, peptidoglycans, flagellin, and viral nucleic acids (1, 3). MAMPs are bound by a functional class of transmembrane or intracytoplasmic receptors termed pattern recognition receptors (PRR) and includes the well-studied Toll-like receptors, Nod-like receptors, and others. PRR recognition of MAMPs results in activation of cytoplasmic signaling cascades and involve relays of phosphorylation and/or proteolytic cleavage and can result in transcriptional activation of inflammatory mediators (or intrinsic processes, such as AMP production) and/or variations on programmed cell death.
Finally, adaptive immunity, which is specific to higher chordates, occurs in animals where genetic recombinatorial machinery allows for the ability to detect and respond to a diverse array of foreign macromolecules (antigens), far beyond the dozen or so structures recognizable by the innate immune system. Adaptive immunity perceives and mediates responses via the activities of a specific class of effector cell (lymphocytes). B-cell dependent humoral and cell-mediated (T cell) components have been well. Prokaryotes profoundly affect adaptive immunity, as germ-free mice characteristically show hypoplastic lymphoid tissues and exhibit a spectrum of abnormalities in adaptive immune function (38).
A commonality of these various components of immunity is their ability to defend against both specific bacterial or viral whole organisms (e.g., intrinsic defenses to thwart active invasion and cell damage) and bacterial or viral products, including MAMPs/antigens (e.g., in simulation of inflammation, or B- and T-cell responses). Beyond these processes, multicellular organisms have evolved systems to perceive and respond to other forms of environmental stresses, including physiochemical threats from changes in abundance of O2 (hypoxia), H+ (pH), and e− (redox and electrophilic stress). Obviously, the ancestral and proverbial “primordial soup” was awash in extremes of chemistry, and understandably, cellular processes have evolved to respond and manage this physiochemical stress, including the hypoxia-inducible factor (HIF) pathway and redox signaling mechanisms. Similarly, small organic molecules were a feature of the prebiotic world, and simple metazoans would have been (and are) constantly exposed to a complex chemical milieu. Specific signaling pathways, such as nuclear factor E2-related factor (Nrf2), aryl hydrocarbon (AHR), and others, act as cellular or organismic transducers to coordinate defenses against such environmental challenges. In humans and higher vertebrates, these ancient and conserved pathways are fully operational, have been well studied in the context of exposure to environmental chemicals, and are, thus, of significant interest to pulmonary physiologists and toxicologists. It can be postulated that the vast array of extreme chemistry, small molecules, and metabolites produced by microbial fermentation of ingested foodstuffs can act as an internal “primordial soup”, from which we derive not just nutritional value, but a panoply of functional signaling molecules, which provides the host with a “preimmune” mechanism of mediating host-microbial interactions.
Symbiotic Microbiota
Recent years have seen increased interest in the microbiota—the microbial occupants invariably associated with metazoans (18, 22, 36, 59). Beginning with the endosymbiotic origin of mitochondria, wherein the enzymatic machinery of oxidative phosphorylation and, thus, energy production was outsourced to ancestral prokaryotes, numerous biochemical functions provided by microbes have been described, resulting in a diversity of symbiotic partnerships (4). The pervasiveness of these arrangements has suggested the concept of the “holobiont”. Holobiont was originally a term found in ecology literature, meaning “an assemblage of a host and the many other species living in or around it, which together form a discrete ecological unit” (48). The concept has gained traction in the host-microbial context, as the degree to which symbiosis occurs among living systems has become more apparent. Clearly, for example, the ecological role of termites in their woody environment would be moot if these insects were deprived of their symbiotic cellulolytic, gut-dwelling bacteria. Similarly, the existence of many herbivorous mammals and their ecological roles (and the environmental effects of modern cattle ranching) could not exist without a similar arrangement with the host and symbiotic gut microbes.
Interest in the role of the microbiota on mammalian physiology and, thus, human health has also burgeoned. The vast number of microbes (10 to 100 trillion) that reside in the mammalian gut and other anatomical locations serves numerous beneficial functions that includes stimulation of adaptive immune system development and competitive exclusion of pathogenic microorganisms (“colonization resistance”) (18, 22). Experiments in germ-free mice have shown a compelling role of the microbiota in influencing a wide range of innate and adaptive immune and metabolic processes (53). Quantitative and/or qualitative abnormalities of the microbiota—dysbiosis—have been associated with inflammatory bowel disease (IBD) (15), other allergic systemic immune conditions, metabolic/hepatic disorders, and even neuropsychiatric conditions (5, 19, 21). Bacteria administered as a therapeutic—probiotics—have been reported to dampen inflammation, improve barrier function, and promote reparative responses, and they have shown promise in both gut and systemic functional and inflammatory disorders (45, 61).
Mechanistically, the realization of the role of the microbiota in host physiology and pathology has been driven by advances in technical and informatics platforms that have allowed the accurate tabulation and classification of the microbial diversity in many biological systems. Current metagenomic studies have characterized the vast genetic diversity encoded in the microbiome, and mass spectroscopy-based methods have defined a prodigious “metabolome” of small molecules and fermentative products (54, 63). Thus, it is now apparent that microbial communities are capable of influencing the physiochemical environment they live in, whether the environment is a marine, aquatic, soil niche, or in the case of higher vertebrates, the gut lumen and other anatomic sites with stable microbial commensal populations. In mammals, specific microbially derived small-molecule metabolites are increasingly recognized as having regulatory functions in multiple host physiological processes (23, 34, 42, 50, 51). Metabolites influenced by the microbiota may be perceived by traditionally conceptualized immune receptors, for example, microbially modified metabolites taurine, histamine, and spermine can stimulate intracytoplasmic Nod-like NLRP6 inflammasome activation, and contribute to abnormal inflammatory signaling and colitis (34). In summary, there is increasing evidence of microbiota-encoded biochemical processes playing significant roles in human disease (17).
Thus, our interaction with the microbial world, traditionally defined by host perception of macromolecules/antigens, must now be expanded to include perception of xenobiotic-derived small molecules and metabolites. This review is intended to establish a context wherein the traditional view of immunity in the perception and response to microbes, must be extended to encompass biochemical/metabolic influences of the resident prokaryotes, and consideration of how the eukaryotic host has evolved to perceive them.
Xenobiotic Signaling
A xenobiotic, (as opposed to endobiotic) broadly defined, refers to substance that is exogenous to the body or to an ecological system (54). Xenobiotics can be considered to extend to dietary phytochemicals, environmental toxins, and pollutants, and the entire small-molecule pharmacopeia.
In the context of host-microbial interactions, chemical products of bacterial metabolism may be considered xenobiotics, and be confirmed as such if they are not detectable in germ-free whole animal systems.
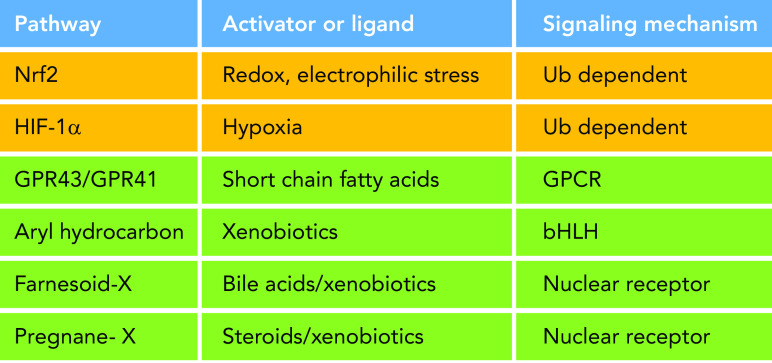
Microbial metabolites can be detected by the host via a number of specific and dedicated membrane-bound and nuclear receptors. A primary example are the short-chain fatty acids, microbial fermentation products of less than six carbons, which include butyrate, propionate, and acetate. These molecules are well-known effectors of microbial functions, as butyrate is considered a major energy source for intestinal epithelial cells (12). Significantly, butyrate can also function as a potent regulatory molecule, affecting epithelial homeostasis via the G protein-coupled receptors (GPCRs) GPR43 and GPR41 (55). Furthermore, butyrate, a four-carbon carboxylic acid, is thought to represent a mimetic of histone modification and, thus, function as a histone deacetylase inhibitor at the chromatin level, bestowing the ability to stimulate gene expression via alteration of chromatin dynamics in immunocompetent cells (7, 27) (FIGURE 1).
FIGURE 1.
Physiochemical and xenobiotic response pathways Multiple eukaryotic signaling mechanisms enable the host to perceive microbial environmental changes and/or small molecules; see text for description. These include pathways that can detect physiochemical signals (Nrf2 and HIF-1α) and small molecules of microbial origin. bHLH, basic helix-loop-helix transcription factor; GPCR, G protein-coupled receptor; HIF-1α, hypoxia-inducible factor-1α; Nrf2, nuclear factor E2-related factor.
Additionally, microbial metabolites can act as ligands for intracytoplasmic nuclear receptors. For example, secondary bile acids—bile acids derivatized by members of the microbiota (deoxycholate and lithocholate)—are perceived by the farnesoid-X receptor (23). The AHR (6) and pregnane X nuclear receptors (60) detect indole tryptophan derivatives, another general class of microbial metabolites with potent host signaling effects, and can stimulate various gene regulatory programs that affect aspects of mucosal homeostasis. The AHR can detect and activate detoxification pathways in response to a range of xenobiotic molecules (46). Taken together, these examples define the existence of specific cellular receptors dedicated to the perception of microbia-derived small metabolites.
Hypoxic and Redox Signaling
Beyond microbia-derived metabolites, the host can detect microbiota-derived physiochemical stimuli. For example, bacterial respiration can stimulate epithelial responses via the oxidant sensor HIF (2, 58). Hif-1α is a cytoplasmic transcription factor that under basal normoxic conditions is constitutively modified by prolyl hydroxylases, allowing binding to the Von Hippel Lindau (VHL) ubiquitin ligase and consequent tonic proteasome degradation. During hypoxia, loss of prolyl hydroxylation releases Hif-1α from VHL, thus allowing Hif-1α accumulation, translocation to the nucleus, binding to specific hypoxia response element promoter motifs, and activation of a battery of genes to allow the cell to respond and adapt to hypoxic conditions, such as EPO, VEGF, and enzymes necessary for glucose metabolism. Early motile metazoans presumably would have used this pathway to spatially adapt to changing environmental conditions. As metabolically active prokaryotes are inseparable components of the environment, such organisms could have used the pathway to detect and respond to microbes. These events may exist in mammals; studies in germ-free mice have shown that the measurable Po2 in the intestine is significantly higher than in conventional mice, and microbial growth can deplete luminal mucosal O2 content (58). Interestingly, butyrate stimulates of O2 utilization, greater anaerobiosis and compensatory Hif-1α activation that culminates in improved epithelial barrier function (28). Additionally, the commensal Bacteroides thetaiotaomicron can induce Hif-1α and result in colonization resistance to fungal infection (14).
Additionally, bacteria can stimulate enzymatic reactive oxygen species (ROS) production via NADPH oxidases and produce ROS as a byproduct of their own respiration, thus activating redox-signaling events. ROS are unstable intermediates of O2 catabolism and are used as signaling messengers in virtually all multicellular life, including both plants and animals (32). In mammals, close contact of commensal Lactobacilli with the intestinal epithelial surface activates redox-signaling pathways, resulting in a wide range of downstream-signaling events (24–26). This redox signaling occurs by the transient reversible oxidation of low pKa sensor cysteines present in the active site of a number of regulatory enzymes, including enzymes involved in MAPK activation (DUSPs) (62), NF-κB signaling (nedd8) (9), and cytoskeletal dynamics/motility (LMW-PTPases) (56). Overall, these conserved systems allow cellular reaction to microbial physiochemical stimuli and likely evolved contemporaneously with the earliest interaction between prokaryotes and metazoans, antedating the evolution of immunity (32).
Nrf2/ARE Pathway
The Nrf2/ARE signaling module is another evolutionarily conserved signal transduction pathway, one that responds to electrophilic and oxidative stresses (including ROS), whether derived from intrinsic (energy production) or extrinsic (xenobiotic) stimuli and, thus, represent a mechanism, whereby the host can react to a variety of environmental signals, including redox stress, electrophiles, and xenobiotics (33, 52) (FIGURE 2). Nrf2 is a member of the CNC (cap’n’collar) family of b-Zip (basic leucine zipper) transcription factors (41) and is posttranslationally regulated in a manner broadly analogous to Hif-1α activation. Under basal conditions present in the cytosol, Nrf2 is bound by the Keap1 subunit of a cullin-dependent E3 ubiquitin ligase that promotes Nrf2 cytoplasmic degradation. Prooxidant or electrophilic stress within the cytoplasm causes rapid oxidation of redox-sensitive cysteines on Keap1, leading to conformational change, suppression of proteasomal degradation, and consequent accumulation of Nrf2. Nrf2 then translocates to the nucleus, dimerizes with the small protein Maf, and binds to a well-defined antioxidant response element (ARE) promoter sequence, subsequently activating expression of an extensive gene network. These genes include antioxidants, efflux pumps, and detoxification enzymes, which when activated, serve to reestablish an optimal redox milieu and, thus, provide a cellular cytoprotective function (57).
FIGURE 2.
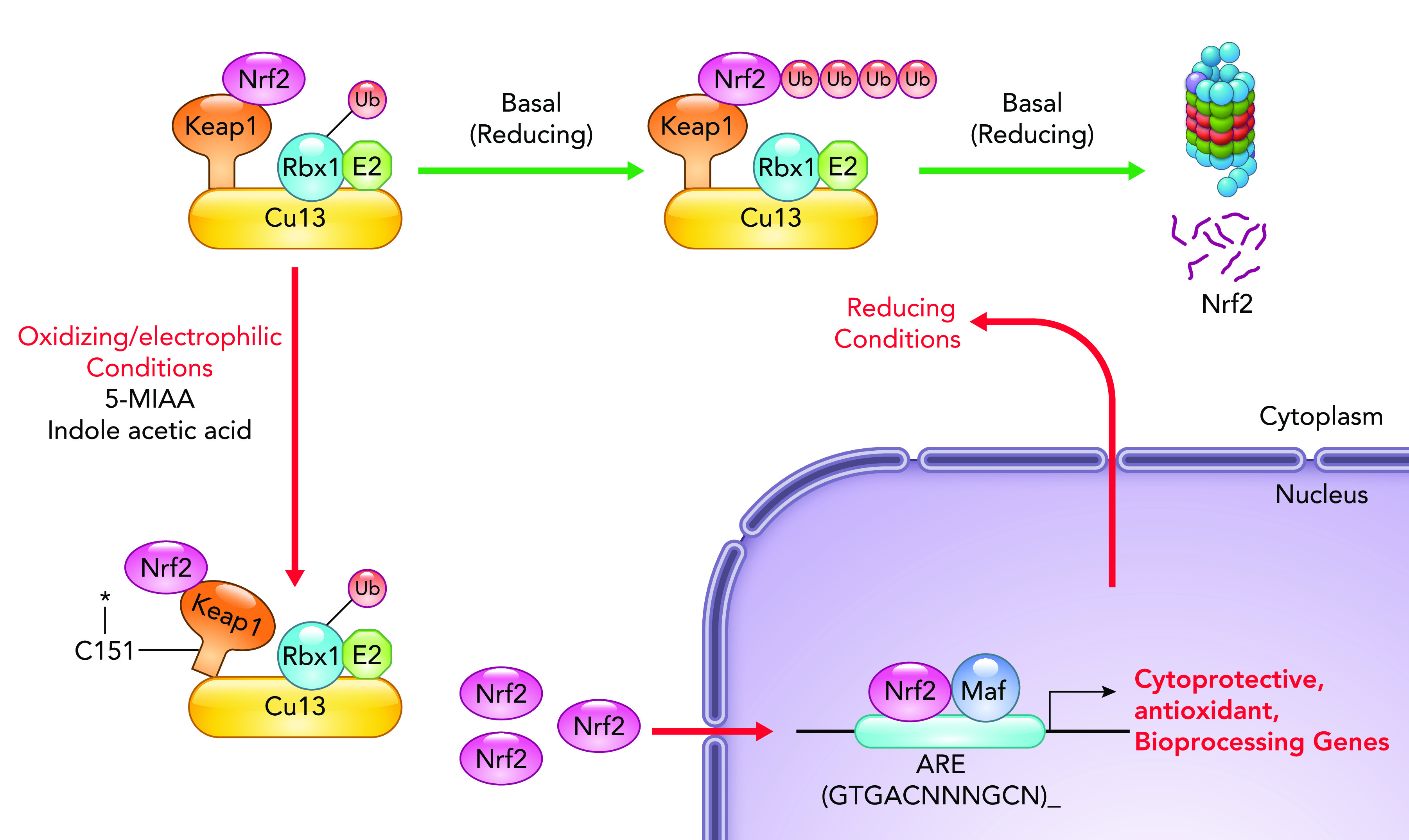
The Nrf2/ARE pathway: activation of the Nrf2/ARE signaling module At homeostasis, Nrf2 interacts with Keap1 (physically associated with a ubiquitin ligase), which results in constitutive ubiquitination and proteasomal degradation. Exposure to oxidant or electrophilic stress in the cytoplasm or microbially derived small molecules (e.g., 5-MIAA, indole acetic acid) results in transient and reversible modification of regulatory cysteine residues in Keap1, leading to Keap1 conformational change and Nrf2 release, stabilization, and cytoplasmic accumulation. Free Nrf2 translocates to the nucleus, where, as a dimer with the small DNA-binding protein Maf, it binds to antioxidant response element and transcriptionally activates the expression of a class of genes that includes cytoprotective factors and detoxification enzymes. Nrf2-induced gene products then act to reestablish cellular redox homeostasis and protect against subsequent insults. Nrf2, nuclear factor E2-related factor.
Model organisms with Nrf2 loss of function fail to upregulate these antioxidant or detoxification effector genes and are hypersensitive to a variety of exogenous insults, including UV radiation, sepsis, and inhaled xenobiotic oxidants and toxins (16, 29). Nrf2-null mice are hypersensitive to dextran sodium sulfate colitis, indicating protective function in barrier epithelia (30). Evidence is emerging that the Nrf2 pathway may play a role in interaction of the gut epithelia of multiple organisms with their resident microbes. In C. elegans, enzymatically generated ROS in response to bacterial infection stimulates the activation of the Nrf2 isolog SKN-1, resulting in cytoprotective effects (20). Drosophila Nrf2 (cncC) is stimulated by bacteria in the fly gut, and gut-directed overexpression of cncC, or suppression of Keap1 protects flies from oxidant-induced mortality (24). In mice, beneficial effects of probiotic Lactobacilli on epithelial injury are abrogated in Nrf2-null animals (24), and Peptostreptococcus produces the tryptophan metabolite indole acetic acid, which acts as an Nrf2 inducer and, consequently, stimulates beneficial barrier and restitution effects (64).
These observations have prompted increasing interest in the therapeutic exploitation Nrf2 as a mediator of cytoprotection (10). The rationale for exploiting Nrf2 is based on stimulation of hormesis. Hormesis is a concept originally derived from studies of unicellular eukaryotes (i.e., yeasts) wherein low, near-threshold levels of a stressor, such as UV exposure, is protective against more intense or prolonged stimuli (35). In this case, perception of the stressor, via the production and recognition of structural DNA alteration, results in upregulation of a battery of genes with DNA repair function. In a sense, a hormetic response is akin to a “training effect” well known to exercise physiologists (and athletes of all stripes!), wherein environmental stress results in adaptive responses, generally the result of compensatory gene expression. The microbiota, even when possessing a beneficial relationship with the host, nevertheless is an extrinsic influence, and xenobiotics produced by microbes are a potent signaling stressor. Thus, hormesis, as a response to xenobiotic and environmental stimuli, likely extends to the acquisition of, and adaptation to exogenous bacteria.
Systemic Nonimmune Microbial Signaling
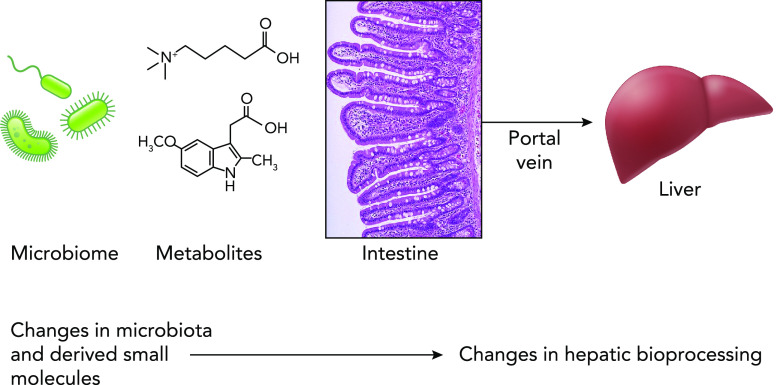
Clearly, much microbial perception and signaling occur at epithelial surfaces. Epithelia, by definition, are derived from endodermal or ectodermal tissues, and thus function as interface with the outside world, whether the environmental matrix or luminal contents. Microbial preimmune signaling can affect systemic tissues. Recent data suggest the mammalian liver can function as a central coordinator of microbial xenobiotic perception (49). The liver is supplied by the portal vein, a vascular channel draining the vast majority of the intestinal watershed. This system is physiologically unique in that it is the only venous system that does not drain directly into the heart. Most digested macromolecules absorbed across the mucosa of the large and small intestine are delivered directly to the liver (a prominent exception is lipids, which are absorbed in mucosal lymphatic vessels and distribute systemically). In the liver, portal blood perfuses the hepatic sinusoids, and myriad biotransformations occur within the highly metabolically active hepatocytes. (In parallel, the bone marrow-derived Kupffer cells lining the sinusoids allow immune perception of MAMPs in the draining portal circulation) (FIGURE 3). Along with processing of dietary peptides and carbohydrates, the hepatocytes also metabolize ingested environmental small molecules, be they inadvertent toxicants, prescribed pharmacological agents, or, relevant to our discussion, small metabolites of microbial origin. This initial hepatic processing of ingested xenobiotics is referred to as “first pass metabolism”, a concept familiar to any introductory pharmacology student, but has not typically been considered in the context of host-microbial interactions.
FIGURE 3.
The gut-liver axis The microbiota produces a vast and diverse range of small-molecule metabolites that are absorbed across the intestinal barrier epithelia. The portal vein is a unique vascular channel that drains most of the intestinal tract and shunts the vast majority of digested dietary macronutrients and micronutrients, as well as ingested environmental toxins and administered pharmacology agents. The expansive metabolic capacity of the liver then mediates appropriate bioprocessing. What has been overlooked are macro and small molecules derived from the gut microbiota. It has long been understood that microbiota macromolecules (or MAMPs) are perceived by innate and adaptive signaling in the Kupffer and stellate cells. Recent data suggest microbial small molecules can be perceived by conserved xenobiotic response pathway operating in hepatocytes. Responses to the small molecules can influence host response to toxins or drugs, and perhaps mediate other physiological processes.
The Nrf2/ARE system operates in the liver and can transcriptionally activate drug-metabolizing enzymes (44, 65). Mice null in Nrf2, or biochemically unable to activate the pathway, fail to upregulate the typical effector genes and are hypersensitive to exposure to hepatotoxic drugs (13). Interestingly, Saeedi et al. (49) showed mice supplemented with oral Lactobacilli induced hepatic Nrf2 and typical Nrf2-responsive genes, resulting in animals resistant to subsequent exposure to several chemical oxidant challenges. Furthermore, specific bacteria were shown to activate hepatic Nrf2 via the metabolite 5-methoxy-indole acetic acid (or 5-MIAA). In parallel work with an invertebrate system, treatment of germ-free Drosophila with a fly-specific symbiont, L. plantarum, was effective in induction of fly Nrf2 (CnC) in the fat body of the fly, a systemic tissue that performs biochemical transformation homologous of the liver. Significantly, flies thus treated were resistant to the oxidant toxin paraquat (49). Thus, preimmune recognition of microbial metabolites can have effects on systemic detoxification pathways.
Implications
These data suggest changes in the microbiota community structure and abundance (dysbiosis), from many causes, including diet and antibiotic use, may result in consequent and idiosyncratic responses to potentially hepatotoxic agents. In the field of human medicine and pharmacology, all clinicians are aware of markedly different responses of individual patients to a standard dosage a given therapeutic. This includes unpredictable toxic effects, often manifesting as hepatocellular injury. Although it has been recognized that microbes can detoxify or otherwise biotransform ingested pharmacological agents by virtue of microbially encoded enzymes (8, 11, 31, 34, 39), it has been suggested that microbes could indirectly affect biotransformation of drugs via activation of host-encoded enzymes in the liver (and likely other tissues) via Nrf2 and other preimmune signaling. This implication is significant in that alteration of the microbiota could, thus, affect metabolism of parenterally administered agents, which is often the case in critically ill patients. Additionally, modulation of xenobiotic biotransformation in the liver due to variable exposure to microbial signals is a double-edged sword, as different enzymatic complements may act to detoxify one ingested agent, while also biotransform another into a toxic intermediate. For example, while acetaminophen is converted to an inert metabolite by one complement of inducible enzymes, other enzymes yield NAPQI, the highly hepatotoxic intermediate (40). Thus, it is difficult to conceptualize a specific probiotic approach to correct what could be described as a pharmacologically dysbiotic microbiota. However, preservation of a metabolic status quo could perhaps be preserved by autologous fecal microbiota transplant (FMT) in patients subjected to major drug-induced challenges (e.g., sepsis, transplant patients).
Overall, all metazoans, indeed, all living things, have evolved the capability of perceiving and responding/adapting to small molecules and physiochemical stimuli from the environment, which as we are rapidly realizing, includes the resident microbial communities. The realization that microbes have increasingly diverse mechanisms to affect our physiology will hopefully allow potential use of them as therapeutic agents. Additionally, such physiochemical, preimmune signaling by the microbiota may be considered a normal part of physiology in the gut and beyond.
Acknowledgments
This article was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases Grant AI64462.
No conflicts of interest, financial or otherwise, are declared by the authors.
A.S.N. drafted manuscript; A.S.N. edited and revised manuscript; A.S.N. approved final version of manuscript.
References
- 1.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10: 131–144, 2010. doi: 10.1038/nri2707. An erratum for this article is available at https://doi.org/10.1038/nri2728. [DOI] [PubMed] [Google Scholar]
- 2.Alam A, Leoni G, Quiros M, Wu H, Desai C, Nishio H, Jones RM, Nusrat A, Neish AS. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol 1: 15021, 2016. doi: 10.1038/nmicrobiol.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8: 411–420, 2008. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 4.Bosch TCG, Guillemin K, McFall-Ngai M. Evolutionary “experiments” in symbiosis: the study of model animals provides insights into the mechanisms underlying the diversity of host-microbe interactions. BioEssays 41: e1800256, 2019. doi: 10.1002/bies.201800256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DA, Paik YH, Schnabl B. Role of gut microbiota in liver disease. J Clin Gastroenterol 49, Suppl 1: S25–S27, 2015. doi: 10.1097/MCG.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 357: 806–810, 2017. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111: 2247–2252, 2014. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen F, Stappenbeck TS. Microbiome control of innate reactivity. Curr Opin Immunol 56: 107–113, 2019. doi: 10.1016/j.coi.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol 175: 4194–4198, 2005. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 10.Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, Dinkova-Kostova AT. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 18: 295–317, 2019. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 11.Currò D. The role of gut microbiota in the modulation of drug action: a focus on some clinically significant issues. Expert Rev Clin Pharmacol 11: 171–183, 2018. doi: 10.1080/17512433.2018.1414598. [DOI] [PubMed] [Google Scholar]
- 12.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13: 517–526, 2011. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci 59: 169–177, 2001. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 14.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 21: 808–814, 2015. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785, 2007. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuse Y, Kobayashi M. Conservation of the Keap1-Nrf2 system: an evolutionary journey through stressful space and time. Molecules 22: 436, 2017. doi: 10.3390/molecules22030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geva-Zatorsky N, Elinav E, Pettersson S. When cultures meet: the landscape of “social” interactions between the host and its indigenous microbes. BioEssays 41: e1900002, 2019. doi: 10.1002/bies.201900002. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med 24: 392–400, 2018. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 535: 94–103, 2016. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 20.van der Hoeven R, McCallum KC, Cruz MR, Garsin DA. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog 7: e1002453, 2011. doi: 10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146: 1449–1458, 2014. doi: 10.1053/j.gastro.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214, 2012. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain U, Lai CW, Xiong S, Goodwin VM, Lu Q, Muegge BD, Christophi GP, VanDussen KL, Cummings BP, Young E, Hambor J, Stappenbeck TS. Temporal regulation of the bacterial metabolite deoxycholate during colonic repair is critical for crypt regeneration. Cell Host Microbe 24: 353–363.e355, 2018. doi: 10.1016/j.chom.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RM, Desai C, Darby TM, Luo L, Wolfarth AA, Scharer CD, Ardita CS, Reedy AR, Keebaugh ES, Neish AS. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep 12: 1217–1225, 2015. doi: 10.1016/j.celrep.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, Lambeth JD, Denning PW, Neish AS. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J 32: 3017–3028, 2013. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RM, Neish AS. Redox signaling mediated by the gut microbiota. Free Radic Biol Med 105: 41–47, 2017. doi: 10.1016/j.freeradbiomed.2016.10.495. [DOI] [PubMed] [Google Scholar]
- 27.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165: 1708–1720, 2016. doi: 10.1016/j.cell.2016.05.018. A correction for this article is available at https://doi.org/10.1016/j.cell.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 30.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66: 11580–11584, 2006. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 31.Klaassen CD, Cui JY. Review: Mechanisms of how the intestinal microbiota alters the effects of drugs and bile acids. Drug Metab Dispos 43: 1505–1521, 2015. doi: 10.1124/dmd.115.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9: 119–145, 2014. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 33.Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol 30: 871–884, 2010. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, Pevsner-Fischer M, Shapiro H, Christ A, Harmelin A, Halpern Z, Latz E, Flavell RA, Amit I, Segal E, Elinav E. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163: 1428–1443, 2015. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Yang T, Sun Z. Hormesis in health and chronic diseases. Trends Endocrinol Metab 30: 944–958, 2019. doi: 10.1016/j.tem.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230, 2012. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151: 616–632, 2016. doi: 10.1053/j.gastro.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macpherson AJ, McCoy KD. Standardised animal models of host microbial mutualism. Mucosal Immunol 8: 476–486, 2015. doi: 10.1038/mi.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 152: 39–50, 2013. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazaleuskaya LL, Sangkuhl K, Thorn CF, FitzGerald GA, Altman RB, Klein TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics 25: 416–426, 2015. doi: 10.1097/FPC.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 91: 9926–9930, 1994. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, Miller CN, Pollack JL, Nagana Gowda GA, Fontana MF, Erle DJ, Anderson MS, Locksley RM, Raftery D, von Moltke J. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49: 33–41.e37, 2018. doi: 10.1016/j.immuni.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14: 9–21, 2017. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339: 79–88, 2006. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 45.Pace F, Pace M, Quartarone G. Probiotics in digestive diseases: focus on Lactobacillus GG. Minerva Gastroenterol Dietol 61: 273–292, 2015. [PubMed] [Google Scholar]
- 46.Pernomian L, Duarte-Silva M, de Barros Cardoso CR. The Aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol. In press. doi: 10.1007/s12016-020-08789-3. [DOI] [PubMed] [Google Scholar]
- 47.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153, 2014. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 48.Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26: 110–130, 2017. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saeedi BJ, Liu KH, Owens JA, Hunter-Chang S, Camacho MC, Eboka RU, Chandrasekharan B, Baker NF, Darby TM, Robinson BS, Jones RM, Jones DP, Neish AS. Gut resident Lactobacilli activate hepatic Nrf2 and protect against oxidative injury. Cell Metab 31: 956–968.e5, 2020. doi: 10.1016/j.cmet.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 22: 1079–1089, 2016. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 51.Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Specialized metabolites from the microbiome in health and disease. Cell Metab 20: 719–730, 2014. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res 44: 1267–1288, 2010. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- 53.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19: 59–69, 2007. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 14: 273–287, 2016. doi: 10.1038/nrmicro.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52: 1–8, 2017. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swanson PA II, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci USA 108: 8803–8808, 2011. doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taguchi K, Kensler TW. Nrf2 in liver toxicology. Arch Pharm Res 43: 337–349, 2020. doi: 10.1007/s12272-019-01192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol 17: 774–785, 2017. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 449: 804–810, 2007. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: 296–310, 2014. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract 2012: 872716, 2012. doi: 10.1155/2012/872716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wentworth CC, Alam A, Jones RM, Nusrat A, Neish AS. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J Biol Chem 286: 38448–38455, 2011. doi: 10.1074/jbc.M111.268938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wikoff WRA, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106: 3698–3703, 2009. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O'Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 22: 25–37.e26, 2017. doi: 10.1016/j.chom.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu KC, Cui JY, Klaassen CD. Effect of graded Nrf2 activation on phase-I and -II drug metabolizing enzymes and transporters in mouse liver. PLoS One 7: e39006, 2012. doi: 10.1371/journal.pone.0039006. [DOI] [PMC free article] [PubMed] [Google Scholar]