Abstract
Background:
Recovery from alcohol use disorders (AUDs) consists of salutary changes in behavior and affect. While evidence suggests that recovery-related behavioral changes, such as abstinence, emerge in tandem with both neural and affective changes, the precise relationships among these changes remain unknown. To understand these relationships, we examined associations between duration of abstinence (DOA), affective states, and neuroimaging-based structural measures of the brain reward system (BRS) in AUD men (AUDM) and AUD women (AUDW).
Methods:
Participants were community respondents from the Boston area comprising right-handed abstinent AUD individuals (n=60; 30 men) and non-AUD controls (NC; n=60; 29 men). Multivariate linear regressions compared short-/mid-term abstainers (≤five years), long-term abstainers (>five years), and the NC group on measures of BRS volume (3T magnetic resonance imaging (MRI) scans) and on measures of affect (Profile of Mood States (POMS); Multiple Affect Adjective Checklist (MAACL); Hamilton Rating Scale for Depression (HRSD)). Analyses contrasted sex differences and accounted for age, education, drinking severity, and verbal IQ.
Results:
Compared to the NC group, short-/mid-term abstainers exhibited larger posterior insular volume (total (ß=0.019, 95%CI: 0.004,0.034)), higher negative affect (POMS Mood Disturbance (ß=27.8, 95%CI: 11.56,44.04), and lower positive affect (POMS Vigor (ß=−4.89, 95%CI: −9.06,−0.72)). Long-term abstainers exhibited significantly smaller volumes of aggregate anterior cingulate cortex (ß=−0.06, 95%CI: −0.113,−0.008), and higher HRSD scores (ß=1.56, 95%CI: 0.14,2.98). Relative to AUDM, AUDW exhibited significantly larger right anterior insular volumes (ß=0.03, 95%CI: 0.01,0.06) and significantly greater MAACL Positive Affect scores (ß=7.56, 95%CI: 0.59,11.55) in association with DOA.
Conclusions:
The findings suggest that differences in abstinence from alcohol are correlated with differences in neural recovery and differences in affective dimensions of recovery from AUDs. The observed sex differences extend evidence of dimorphic effects of AUDs and recovery on brain structure and function. Future longitudinal research will substantiate inferences concerning the directionality of these relationships.
Keywords: alcohol use disorders, recovery, sex differences, insula, cingulate cortex
Introduction
Recovery from alcohol use disorders (AUDs) is a multidimensional process consisting of a variety of salutary changes in both behavioral and affective patterns (NIAAA2020). Recovery-related behavioral changes range from cessation of heavy drinking to complete abstinence (White & Kurtz, 2006). The affective changes that manifest throughout recovery include improvements in dimensions of well-being, such as subjective happiness (Kelly et al., 2018) and emotional regulation (Kober, 2014). Although there is mounting clinical evidence that the affective improvements characterizing recovery generally emerge over the course of continued abstinence or moderation (Kelly et al., 2018; Witkiewitz et al., 2020), and there is a substantial body of neuroimaging research demonstrating that alcohol-related damage to the brain is at least partially reversible with abstinence (Bartsch et al., 2007; Demirakca et al., 2011; Durazzo et al., 2017; Durazzo et al., 2011; Fein & Cardenas, 2015; Makris et al., 2008; Rosenbloom & Pfefferbaum, 2008; Zou et al., 2018), it remains unknown how these recovery-related improvements in affect are related to the observed brain changes and how they relate to recovery-related behaviors. By contrast, the relationship between abstinence, corresponding brain changes, and affective impairments has been relatively well-established (Oscar-Berman et al., 2014). In general, it is hypothesized that acute and protracted abstinence from alcohol is accompanied by intense negative affective states (e.g., dysphoria, anxiety), along with the inability to experience positive affective states (i.e., anhedonia), either because of premorbid brain abnormalities (Blum et al., 1996) or because of neuroadaptations that emerged to neutralize the noxious effect of alcohol and to enable the body to maintain homeostasis (George & Koob, 2017; Koob, 2013; Koob et al., 2014; Wise, 2008). There is considerable evidence (Fritz et al., 2019; Heinz et al., 2005; Pando-Naude et al., 2021; Tupala & Tiihonen, 2004; Volkow et al., 2007) suggesting that chronic alcohol exposure is associated with a downregulation of the mesocorticolimbic dopamine system (Bailey et al., 2001; Koob, 2013; Melis et al., 2005; Wise, 2008) and an upregulation of both the hypothalamus-pituitary-adrenal (HPA) axis and the brain stress system (Adinoff et al., 1998; Chavkin & Koob, 2016; Koob, 2013; Koob et al., 2014; Koob & Le Moal, 2008). In the absence of alcohol, that is, during early abstinence, these alcohol-related dysregulations of the brain reward and stress circuitries relay signals that frequently motivate a return to addictive involvement with alcohol (i.e., relapse) among individuals with histories of AUDs (Brown et al., 1995; Koob, 2013; Marlatt & Gordon, 1980). This process describes the withdrawal stage of the addiction cycle and has been referred to as the “emotional dark side of addiction” (Koob, 2015; Koob & Moal, 2005). It is thought to characterize the neural and motivational factors underlying the perpetuation of addictive behavior. Importantly, recent neuroimaging research has revealed sex differences with respect to the types of negative affect that tend to be experienced during withdrawal from addictive substances, such as alcohol, suggesting sex-specific motivations for relapse behavior (Becker & Koob, 2016; Potenza et al., 2012; Sawyer et al., 2019; Seo et al., 2011) – a tendency that is at least partly related to inherent sex differences in the neural substrates underlying affective processing (Moriguchi et al., 2014). Hence, while there is a relatively detailed neural portrait of how abstinence, affect, and sex are related to addiction, in general, a model for how these dynamics are related to recovery is, for the most part, lacking.
While there is some evidence indicating that the negative affect motivating a return to hazardous drinking resolves within the first few months to one year of abstinence (Brown & Schuckit, 1988; Brown et al., 1995), improvements in these abnormalities may actually take much longer to manifest. For example, individuals with less than five years of abstinence present a significantly greater risk of becoming addictively involved with alcohol, compared to people without a history of AUDs (Dawson et al., 2015; Dennis et al., 2007; Grant et al., 2016; Kelly et al., 2018). Conversely, for AUD individuals, the risk of becoming addictively involved with alcohol returns to that of the general population after approximately five years of abstinence (Dawson et al., 2015; Dennis et al., 2007; Grant et al., 2016; Kelly et al., 2018). Thus, there is reason to infer that it may take as long as five years before affective and corresponding neural profiles among AUD individuals become more normative. Additionally, much of the neuroscientific research on recovery from AUDs has centered primarily on relatively short-term alcohol abstinence (<five years) mostly among AUD men (AUDM) (Sawyer et al., 2017). Therefore, it remains unknown whether the affective changes that characterize long-term alcohol abstinence (>five years) correspond with discrete neural changes, and whether there are sex differences among such trends.
The present study was designed to test whether differences in DOA among AUDM and AUDW are linked to differences in neural recovery and predicted differences in affective dimensions of recovery. We used magnetic resonance imaging (MRI) to test whether DOA was related to differences in volumetric measures of the brain reward system (BRS). First, we tested the hypothesis that short-/mid-term abstainers (i.e., ≤ five years) would exhibit significantly greater negative affect/lower positive affect and volumetric abnormalities in BRS regions involved in affective processing, relative to long-term abstainers (i.e., > five years) and a non-AUD control (NC) group. Next, we tested the hypothesis that long-term abstainers would exhibit no difference on measures of affective states and BRS volumes, relative to the NC group. Because it is unknown whether or how recovery-related behaviors, such as DOA, might exert an influence on affective improvements through discrete brain changes, we conducted separate exploratory analyses to detect possible mediational relationships between these variables. Lastly, we tested the hypothesis that there would be sex differences between AUDM and AUDW with respect to affective states and BRS volumes in relation to different DOA. In particular, based upon results of our prior research (Rivas-Grajales et al., 2017; Ruiz et al., 2013; Sawyer et al., 2017), the AUDW were expected to exhibit evidence of normative volumetric and affective patterns earlier than AUDM. In addition to potentially suggesting neural indices of successful recovery (Humphreys & Bickel, 2018), such information may be relevant for augmenting existing strategies for remediation.
Materials and Methods
Participants
Participants included in this study were involved in a previous study conducted at the Boston University Laboratory of Neuropsychology (Sawyer et al., 2017). All of the participants were right-handed (Briggs & Nebes, 1975) and included 60 abstinent AUD individuals (30 men) and 60 NC individuals (29 men) from the Boston area, who were solicited from newspaper and web-based advertisements, and posts on bulletin boards at Boston University Medical Center, Boston Veterans Affairs (VA) Healthcare System, VA after-care programs, and community locations. The Institutional Review Boards of the participating institutions approved the research. Informed consent was obtained prior to neuropsychological testing. Participants were reimbursed for time and travel expenses. Data collection took place between 2010 and 2012.
Clinical Evaluation
Participants underwent a medical history interview and vision testing, plus a series of questionnaires (e.g., handedness, medical history, alcohol and drug use) to ensure they met inclusion criteria. Participants performed a computer assisted, shortened version of the Diagnostic Interview Schedule (DIS) (Robins et al., 2000) that provides lifetime psychiatric diagnoses according to DSM-IV (APA1994) criteria. Tests of intelligence and memory were conducted, including the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) (Wechsler, 1997a) for Full-Scale IQ (FSIQ), Verbal IQ (VIQ), Performance IQ (PIQ), and Working Memory Index (WMI), as well as the Wechsler Memory Scale, Third Edition (WMS-III) (Wechsler, 1997b) for Delayed Memory, a neuropsychological measure of response initiation in impulsive behavior.
Inclusion and Exclusion Criteria
AUD participants were included if they had a lifetime drinking history of five or more years of consuming at least 21 drinks per week (Cahalan, 1968), a level that has been established as constituting heavy drinking, in addition to meeting the diagnostic criteria for AUDs, that is, alcohol abuse and/or dependence, according to the Diagnostic and Statistical Manual-IV (APA1994). Additionally, AUD participants were required to be abstinent for at least four weeks before testing and scanning.
Participants were excluded from further participation if any source (DIS scores, hospital records, referrals, or personal interviews) indicated that English was not one of their first languages, or if they had any of the following: corrected visual acuity worse than 20/50 in both eyes; Korsakoff’s syndrome; HIV; cirrhosis; major head injury with loss of consciousness greater than 30 minutes unrelated to AUDs; stroke; epilepsy or seizures unrelated to AUDs; schizophrenia; electroconvulsive therapy; history of illicit drug use once per week or more within the past four years. The study excluded from its analysis individuals with Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) scores greater than 16 (i.e., meeting the criteria for moderate-to-severe depression (Hamilton, 1967; Zimmerman et al., 2013)), which may represent a smaller proportion of AUD individuals in the general population. Importantly, although a substantial proportion of individuals with AUD have a comorbid affective disorder (i.e., Bipolar Disorders and Major Depressive Disorder (MDD)) (Pettinati et al., 2013), such disorders are associated with distinctive morphological abnormalities of the BRS (Alloy et al., 2016; Lopez-Larson et al., 2002; O’Connor & Agius, 2015; Strakowski et al., 2002; Takahashi et al., 2010). Because the present study is aimed at identifying affective correlates of recovery from alcohol-related brain changes, exclusion of AUD individuals with more severe affective disorders will reduce confounding effects.
Drinking Variables
In addition to meeting the DSM-IV criteria for alcohol abuse or dependence, AUD status and severity among the AUD participants was determined as described below.
Duration of Heavy Drinking.
Participants received a structured interview regarding their drinking patterns, including duration of heavy drinking (DHD – i.e., 21 or more drinks per week (one drink: 355 ml beer, 148 ml wine, or 44 ml hard liquor)).
Daily Drinks.
A Quantity Frequency Index (Cahalan et al., 1969), was used to calculate the amount, type, and frequency of alcohol usage (roughly corresponding to number of daily drinks (DD)). Calculations of DD for the NC participants were based on alcohol consumed over the last six months; for the AUD participants, DD represented alcohol consumption over the six months preceding cessation of drinking.
Recovery-Related Variables
Duration of Abstinence.
In addition to providing information about the stability of AUD-related sequalae in the absence of the acute effects of ethanol and its detoxification, consideration of duration of abstinence (DOA) from alcohol provides a measure of recovery-related behavior in that, for AUD-individuals, it commonly represents a purposeful, active, and intentional decision not to drink (Amodeo et al., 1992). DOA was estimated as the length of time between the date of last drink and the date of the MRI scan and/or relevant assessment (see below). DOA was ascertained with several comprehensive interviews over multiple testing sessions, in which the date of last drink was obtained and examined for consistency across sessions.
MRI Acquisition
MRI scans were obtained on a Siemens 3-Tesla TIM Trio scanner with an 8-channel head coil. Image acquisitions included sagittal scout and two T1-weighted MP-RAGE series for volumetric analysis: TR=2530 ms TE=3.39 ms, TI=1100 ms, flip-angle=7°, Field-of-View=256, slice-thickness=1.33 mm, number-of-slices=128 contiguous, sagittal images of the entire brain, matrix=256×256, number-of-excitations=2. The two MP-RAGE series were averaged, then the averaged series was re-sliced in a standard coronal three-dimensional brain coordinate system (Kennedy et al., 1989). Images were reformatted to standard spatial orientation, but not rescaled in size.
MRI Morphometric Analyses
Image analyses followed semi-automated procedures developed by the Center for Morphometric Analysis at Massachusetts General Hospital (MGH) (Caviness, Kennedy, et al., 1996; Filipek et al., 1994; Makris et al., 1999). Images were inspected for gross abnormalities, and cortical gray matter, white matter, subcortical structures, and ventricles were manually segmented on T1-weighted images using a computer-assisted approach (Filipek et al., 1994). Neocortex was subdivided further into parcellation units, involving a number of manual and computer-assisted operations (Caviness, Meyer, et al., 1996). Cortical subregions of the reward network were divided: dorsolateral prefrontal cortex (DLPFC; defined as the sum of the dorsolateral superior frontal and middle-frontal gyri, approximating Brodmann areas 8, 9, and 46), insular, subcallosal, orbitofrontal, and cingulate cortices, parahippocampal gyrus, and temporal pole. Gray matter subcortical structures in the reward network included the nucleus accumbens (NAc), amygdala, hippocampus, and ventral diencephalon (VDC). For volumetric comparisons to reward network regions, we included analyses of sensory cortex (cuneus) and subcortical (dorsal striatum) regions.
Segmentation and cortical parcellation were supervised by a neuroanatomist. Blindness to group assignment was maintained during analyses. High inter-rater and intra-rater reliability of these methods have been established (Caviness, Meyer, et al., 1996; De Fosse et al., 2004; Frazier et al., 2005; Goldstein et al., 1999; Goldstein et al., 2001; Herbert et al., 2003; Makris et al., 2004; Seidman et al., 1999). Estimated total intracranial volume was obtained using FreeSurfer 5.3, an automated procedure (Fischl et al., 2004).
Measures of Affective States
Negative and positive affective states were assessed through several multi-item measures of distinct affect, distinct mood states, and specific emotions over varying durations of time.
Negative Affective States.
The Multiple Affect Adjective Check List (MAACL) (Zuckerman & Lubin, 1965) was used to evaluate negative affective states. For this measure, participants are asked to select from a list of 132 adjectives that may relate to how he/she is feeling on the day of the assessment, including in the present moment. Negative affective states are categorized into anxiety (e.g., afraid, fearful, frightened, panicky, shaky, and tense), depression (e.g., alone, destroyed, forlorn, lonely, lost, and miserable), and hostility (e.g., annoyed, critical, cross, cruel, and disagreeable). “Dysphoria,” as measured by the MAACL, represents a composite of the other individual measures of negative affect.
Negative mood states were evaluated using the Profile of Mood States (POMS) (McNair et al., 1971) The POMS measures five different dimensions of mood swings over a period of time, including Tension-Anxiety, Anger-Hostility, Fatigue-Inertia, Depression-Dejection, and Confusion-Bewilderment. Participants are asked to rate their mood over the past week using a five-point Likert Scale, ranging from zero (not at all) to four (extremely). Total Mood Disturbance (TMD), which refers to a global indicator of emotional disturbance or psychological distress, is calculated by summing the scores from the other five independent measures of negative mood assessed by the POMS.
The severity of depressed mood also was evaluated using the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960). HRSD scores of 8, 16, and 25 or above indicate mild, moderate, or severe depression, respectively (Zimmerman et al., 2013). Participants were presented with a series of questions related to affective and somatic indices of depression and anxiety relevant to the past two weeks, which require the assessment and interpretation of a trained scale administrator to score.
Positive Affective States.
The MAACL (Zuckerman & Lubin, 1965) also was used to evaluate positively valenced affective states of low arousal (e.g., happy, joyful, and pleasant), as well as a positive level of activation (e.g., adventurous, daring, and energetic). Together, these scales compose the “Positive Affect Sensation Seeking” measure on the MAACL.
The POMS measure of Vigor-Activity (e.g., lively, active, energetic, alert, full of pep, carefree, and vigorous) also was used as a proxy for positive affective states.
Statistical Analyses
Descriptive statistics were generated to characterize the mean and standard deviation of demographic, drinking/recovery, neuropsychological, BRS volumetric, and affective variables stratified by AUD status and sex. Independent sample t-tests were employed to identify any significant differences between AUD and NC groups, as well as between subgroups of AUD participants according to DOA and sex. There were four primary sets of analyses using multivariable linear regressions. The first set of analyses examined the association between DHD and DD and BRS total and regional volumetric measures (normalized to the total intracranial volume), and adjusted for the covariates age, education, and VIQ. In the context of neurological and psychiatric disorders, and AUDs in particular, VIQ, rather than the PIQ or FIQ, is generally regarded as being a more stable measure of intelligence over time (Parsons, 1977). The second set of analyses examined the association between DHD, DD, and measures of negative and positive affective states, and adjusted for the same covariates as in the first analyses. The third set of analyses assessed the association between DOA and BRS total and regional volumetric measures (normalized to the total intracranial volume) in AUD individuals only, and the fourth set of analyses assessed the association between DOA and measures of affective states in AUD individuals only. Both the third and fourth set of analyses adjusted for age, education, VIQ, DHD, DD, and included an interaction term for sex and DOA. DOA was operationalized in the regression models in two different ways: (a) as a continuous variable and (b) a dichotomous variable, dichotomized into ≤ five years (short-/mid-term abstainers) and > five years (long-term abstainers). We report the beta estimates and their corresponding 95% confidence interval as a measure of the strength of association. In addition, significant interactions between any BRS volumetric measure, affective measure, and DOA were plotted to visualize sex difference. Locally weighted scatterplot smoothing (LOWESS) curves also were created to visualize a smooth line through the relationship of DOA and measures of affective states for the AUD participants broken down by sex. All measures of affective states were standardized to their mean and standard deviation, and measures can be compared on the same standardized scale. For all analyses, the significance level was set at 0.05 and the analyses were conducted in RStudio version 1.2.1335 (R Development Core Team, 2019).
Results
Sample
Of the 120 participants completing the study, (n = 2) AUD participants were found to meet the exclusion criteria for illicit drug use (one AUDW who had smoked marijuana within the past six months and one AUDM who had used cocaine within the past eight months) more than once a week within the past four years, and therefore were excluded from the analysis. Of the remaining 118 participants, n = 33 were either taking medications for a variety of conditions or had used illicit drugs earlier than four years before enrollment or had a potentially confounding medical history. We did not exclude these individuals and included them in our analyses so that our sample would be more representative of the conditions present in the United States, thereby allowing for greater generalizability of the findings. However, we separately analyzed data from an unconfounded subgroup of 85 participants (31 AUD (16 men); 54 NC (26 men)) consisting of participants who were not currently taking psychotropic medications. Additionally, the unconfounded subgroup was restricted to individuals for whom no source indicated: hepatitis; or any of the following disorders: Major Depressive Disorder; Bipolar Disorder I or II; schizoaffective disorder; schizophreniform disorder; or Generalized Anxiety Disorder. Additionally, because we aimed to eliminate the confounding protentional of even moderate depressive symptoms within our secondary analyses, we excluded individuals with HRSD scores of greater than 13, thereby including only those with mild or no depressive symptoms. No Results of the analyses of the data from the unconfounded subgroup of participants were statistically consistent with those as reported in the text for the total sample.
Participant Characteristics
Table 1 provides demographic information (i.e., age and education) and drinking and recovery variables (i.e., DD, DHD, DOA). Of those, only age, education, and DD were compared between the AUD and NC groups. Education and DD were significantly different between the AUD and NC groups (education p = 0.009; DD p < 0.001). The NCW had significantly more education (p = 0.015; M = 16.10, SD = 2.31) than the NCM (M =14.69, SD = 2.02). NCW also had significantly higher Delayed Memory scores (p = 0.001; M = 116.81, SD = 15.31) than the NCM (M = 104.29, SD = 12.96). This pattern was similar to that observed in the AUD group (Delayed Memory p < 0.001; AUDW: M = 117.55, SD = 17.00; AUDM: M = 100.97, SD = 11.49), suggesting that the difference is attributable to the influence of sex rather than the influence of AUD. With respect to the within sex comparisons, the NCW had significantly more education (p = 0.02; M = 16.10, SD = 2.31) than the AUDW (M = 14.68, SD = 2.45). AUDW had significantly higher DD (p < 0.001; M = 7.69, SD = 6.83) than the NCW (M = 0.18, SD = 0.34). AUDM had higher DD (p < 0.001; M = 11.04, SD = 8.31) than NCM (M = 0.33, SD = 0.48), whereas NCM had higher PIQ (p = 0.04; M = 106.93, SD = 11.55) than AUDM (M = 100.43, SD = 12.16), with no other statistically significant differences between the groups.
Table 1.
Demographic, Behavioral, and Neuropsychological Characteristics of the Sample.
| Alcohol Use Disorder (AUD) | p-value AUDW vs AUDM | Normal Control (NC) | p-value NCW vs NCM | p-value AUDW vs NCW | p-value AUDM vs NCM | p-value AUD vs NC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women (n=30) | Men (n=30) | Women (n=31) | Men (n=29) | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Demographic Variables | |||||||||||||
| Age | 51.57 | 9.77 | 51.78 | 11.09 | 0.939 | 55.82 | 13.81 | 51.89 | 12.23 | 0.247 | 0.17 | 0.970 | 0.299 |
| Education | 14.68 | 2.45 | 13.88 | 2.27 | 0.194 | 16.10 | 2.31 | 14.69 | 2.02 | 0.015 | 0.02 | 0.154 | 0.009 |
| Drinking/Recovery Variables | |||||||||||||
| Daily Drinks (ounces ethanol per day) | 7.69 | 6.83 | 11.04 | 8.31 | 0.093 | 0.18 | 0.34 | 0.33 | 0.48 | 0.169 | 0.00 | 0.000 | 0.000 |
| Duration of Heavy Drinking (years) | 13.03 | 6.31 | 16.57 | 8.25 | 0.068 | - | - | - | - | - | - | - | - |
| Duration of Abstinence (years) | 7.19 | 9.37 | 4.81 | 9.32 | 0.329 | - | - | - | - | - | - | - | - |
| Neuropsychological Variables | |||||||||||||
| FSIQb | 108.00 | 18.28 | 104.20 | 11.43 | 0.345 | 111.29 | 14.36 | 109.25 | 10.34 | 0.531 | 0.44 | 0.083 | 0.100 |
| PIQc | 106.59 | 18.49 | 100.43 | 12.16 | 0.139 | 110.39 | 15.30 | 106.93 | 11.55 | 0.329 | 0.39 | 0.041 | 0.054 |
| VIQd | 107.93 | 17.86 | 106.80 | 11.77 | 0.776 | 110.48 | 14.03 | 109.86 | 11.37 | 0.851 | 0.54 | 0.319 | 0.271 |
| WMIe | 105.90 | 17.04 | 106.07 | 13.24 | 0.966 | 108.03 | 12.94 | 104.57 | 9.94 | 0.252 | 0.59 | 0.627 | 0.870 |
| Delayed Memoryf | 117.55 | 17.00 | 100.97 | 11.49 | 0.000 | 116.81 | 15.13 | 104.29 | 12.96 | 0.001 | 0.86 | 0.308 | 0.554 |
Mean and standard deviation (SD) are presented for AUD and NC men (NCM) and women separately. The p-values of the mean differences are presented. Effects significant at p < 0.05 are in bold.
Neuropsychological scores were unavailable for 1 AUD woman (AUDW), 1 AUD man (AUDM), and 1 NC woman (NCW). The number of DD was not obtained for 1 AUDM.
Wechsler Adult Intelligence Scale, Full Scale IQ.
Wechsler Adult Intelligence Scale, Performance IQ.
Wechsler Adult Intelligence Scale, Verbal IQ.
Wechsler Adult Intelligence Scale, Working Memory Index.
Wechsler Memory Scale, Delayed Memory.
Drinking/Recovery Patterns
The AUDW and the AUDM did not differ significantly by DHD (Table 1). The AUD participants drank heavily (i.e., ≥ 21 drinks per week) for a period of at least five years during their lifetimes (range of DHD: five to 37 years). None of the NC participants drank heavily. The AUDW and the AUDM did not differ significantly by DD. The AUD individuals were abstinent for extended lengths, an average of six years (range of DOA: four weeks to 38 years). Because we were investigating the stable and persistent sequelae of AUDs, which were independent of current drinking and withdrawal, all of the AUD participants had abstained from alcohol for at least four weeks prior to testing, except for one AUDW and two AUDM with shorter abstinence periods, who were excluded from the unconfounded subgroup. The DOA variable did not apply to the NC group, who did not exhibit AUD drinking behaviors. AUDM and AUDW did not differ significantly by DOA (Table 1).
Duration of Abstinence and Brain Reward Volumes
Multivariable linear regression analyses were used to assess the relationship between DOA and volumetric measures of the BRS. The results are presented in Table 2, Supplemental Material – Tables S1. The regression model included age, education, VIQ, DHD, and DD as covariates. Relative to the NC group, the AUD group had significantly smaller volumes in the total (95% Confidence Interval (CI): −0.04, 0) and right ratios (95% CI: −0.04, 0) of the anterior insula. Additionally, in the AUD group, there was a negative relationship between DOA and the volumes of the posterior insula. Sex differences among the AUD group also were detected: Relative to the AUDM, the AUDW exhibited significantly greater volumes of the total BRS (95% CI: 0.35, 1.75) and the temporal pole (total ratio (95% CI: 0.1, 0.26), left ratio (95% CI: 0.09, 0.29), and right ratio (95% CI: 0.09, 0.24)), as well as the right ratio (95% CI: 0.01, 0.07) of the anterior insula. Post-hoc analyses were conducted to determine differences between the short-term abstainers (i.e., < one year) and the mid-term abstainers (i.e., > one year, < five years). No significant differences were found between these groups. When the DOA variable was dichotomized (i.e., ≤ five years and > five years), the short-/mid-term abstainers (i.e., ≤ five years) showed a significantly larger volume of the entire posterior insula (total (95% CI: 0.004, 0.034), left (95% CI: 0.003, 0.033), right (95% CI: 0.002, 0.039)), relative to the NC group, whereas the long-term abstainers (> five years) exhibited significantly less volume in the total (95% CI: −0.113, −0.008) and left ratio (95% CI: −0.151, −0.022) of the anterior cingulate cortex (ACC), relative to the NC group. Short-/mid-term abstainers had significantly greater volume in the left ratio (95% CI: −0.003, 0) of the posterior cingulate cortex (PCC) than the long-term abstainers. Additionally, long-term AUDW abstainers had significantly greater volumes than long-term AUDM abstainers in the total BRS (95% CI: 0.31, 1.69), ventral diencephalon (total ratio (95% CI: 0, 0.06) and left ratio (95% CI: 0, 0.06)), temporal pole (total ratio (95% CI: 0.1, 0.26), left ratio (95% CI: 0.09, 0.29), and right ratio (95% CI: 0.09, 0.24)), and the right ratios of the anterior insula (95% CI: 0.01, 0.07) and posterior insula (95% CI: 0, 0.06). Further, significant interactions were observed between DOA and sex, with AUDM exhibiting a negative association between DOA and volume in the left ratio of the parahippocampal gyrus, relative to AUDW, who exhibited a positive association (95% CI: 0, 0). Both AUDM and AUDW exhibited negative associations between volume in the entire dorsal striatum and DOA, but the AUDW exhibited this trend more strongly in all three ratios (total (95% CI: −0.2, −0.03), left (95% CI: −0.2, −0.03), right (95% CI: −0.21, −0.03), relative to AUDM (Supplemental Material – Figure S1).
Table 2. Morphometric Analyses.
Adjusted Linear Regression of the Effect of History of AUDs and Duration of Abstinence on Volumetric Measures of Insular and Cingulate Cortices.
| Anterior Cingulate Cortex | Posterior Cingulate Cortex | Anterior Insular Cortex | Posterior Insular Cortex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Ratio | Left Ratio | Right Ratio | Total Ratio | Left Ratio | Right Ratio | Total Ratio | Left Ratio | Right Ratio | Total Ratio | Left Ratio | Right Ratio | ||
| NC (n=60) | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| AUD (n=60) | −0.013 [−0.052, 0.025] | −0.023 [−0.07, 0.025] | −0.004 [−0.049, 0.041] | −0.011 [−0.04, 0.015] | −0.005 [−0.032, 0.021] | −0.017 [−0.05, 0.015] | −0.02 [−0.04, 0] | −0.02 [−0.04, 0] | −0.02 [−0.04, 0] | 0.01 [0, 0.02] | 0.01 [−0.01, 0.02] | 0.01 [−0.01, 0.03] | |
| Duration of Abstinence (categorical)a | |||||||||||||
| NC | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| ≤ 5 years | 0.017 [−0.027, 0.06] | 0.013 [−0.041–0.067] | 0.021 [−0.031, 0.072] | −0.005 [−0.04, 0.028] | 0 [−0.033, 0.032] | −0.009 [−0.047, 0.03] | −0.019 [−0.04, 0.002] | −0.022 [−0.046, 0.002] | −0.016 [−0.037, 0.006] | 0.019 [0.004, 0.034] | 0.018 [0.003, 0.033] | 0.021 [0.002, 0.039] | |
| NC | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| > 5 years | −0.06 [−0.113, 0.008] | −0.086 [−0.15, −0.022] | −0.034 [−0.101, 0.033] | −0.022 [−0.06, 0.012] | −0.015 [−0.052, 0.02] | −0.03 [−0.071, 0.01] | −0.018 [−0.043, 0.008] | −0.01 [−0.04, 0.019] | −0.025 [−0.051, 0.002] | −0.009 [−0.027, 0.009] | −0.008 [−0.027, 0.01] | −0.009 [−0.032, 0.014] | |
| Duration of Abstinence (categorical)b | |||||||||||||
| ≤ 5 years | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| > 5 years | −0.08 [−0.003, −0.001] | −0.147 [−0.003, 0] | −0.014 [−0.004, −0.001] | 0.003 [−0.004, 0] | −0.028 [−0.003, 0] | 0.034 [−0.005, 0] | 0.01 [−0.03, 0.05] | 0.01 [−0.03, 0.06] | 0.01 [−0.03, 0.05] | −0.03 [−0.07, 0] | −0.03 [−0.06, 0.01] | −0.04 [−0.08, 0] | |
| Sex | |||||||||||||
| ≤ 5 years men | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| ≤ 5 years women | −0.001 [−0.02, −0.085] | −0.001 [−0.02, −0.07] | −0.001 [−0.027, −0.14] | −0.021 [−0.01, −0.11] | −0.014 [−0.01, −0.063] | −0.029 [−0.01, −0.17] | 0.02 [−0.01, 0.05] | 0.01 [−0.03, 0.04] | 0.04 [0.01, 0.07] | 0.02 [0, 0.05] | 0.01 [−0.01, 0.04] | 0.03 [0, 0.06] | |
| Duration of Abstinence (years) b | |||||||||||||
| −0.001 [−0.001, −0.004] | −0.005 [0, −0.004] | 0.002 [−0.001, −0.005] | 0 [0, −0.004] | −0.002 [0, −0.003] | 0.001 [0, −0.005] | 0.0003 [−0.002, 0.002] | 0.0009 [−0.003, 0] | −0.0003 [−0.002, 0.002] | −0.0018 [−0.0036, 0] | −0.0017 [−0.0035, 0] | −0.002 [−0.004, 0] | ||
| Sex | |||||||||||||
| men | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| women | 0.004 [−0.019, −0.006] | 0.003 [−0.02, −0.005] | 0.005 [−0.025, −0.01] | −0.031 [−0.003,-0.004] | −0.018 [−0.003,-0.002] | −0.043 [−0.01, −0.006] | 0.02 [0, 0.05] | 0.01 [−0.02, 0.04] | 0.03 [0.01, 0.06] | 0.01 [−0.01, 0.04] | 0 [−0.02, 0.03] | 0.02 [−0.01, 0.05] | |
The 95% confidence intervals of the mean differences are presented. Effects significant at p < 0.05 are in bold. Ref = reference group
Adjusted for age, education, and VIQ
Adjusted for age, education, VIQ, DHD, and DD
Affective States
Table S2 provides results of comparisons among affective states between group (AUD vs. NC) and sex (men vs. women). Whereas the NCW reported higher positive affective states than the NCM on MAACL Positive Affect Sensation Seeking, MAACL Positive Affect, MAACL Sensation Seeking, POMS Vigor, only MAACL Positive Affect was higher among AUDW (p = 0.015), relative to AUDM. There were no within group sex differences on measures of negative affective states in either the NC or the AUD groups. However, relative to the NC group, the AUD group had higher POMS Mood Disturbance (p = 0.001), POMS Anger (p = 0.006), POMS Confusion (p = 0.003), POMS Depression (p = 0.002), POMS Fatigue (p = 0.032), POMS Tension (p = 0.003), HRSD (p < 0.001), and POMS Vigor (p = 0.018). Examination of within sex differences revealed that, compared to the NCW, the AUDW had higher scores on POMS Mood Disturbance (p = 0.01), POMS Confusion (p = 0.01), POMS Tension (p = 0.02), HRSD (p = 0.02), MAACL Sensation Seeking (p = 0.01), and POMS Vigor (p = 0.01). However, compared to AUDW, the AUDM scored higher on POMS Mood Disturbance (p = 0.039), POMS Anger (p = 0.040), POMS Depression (p = 0.008), and HRSD (p = 0.001).
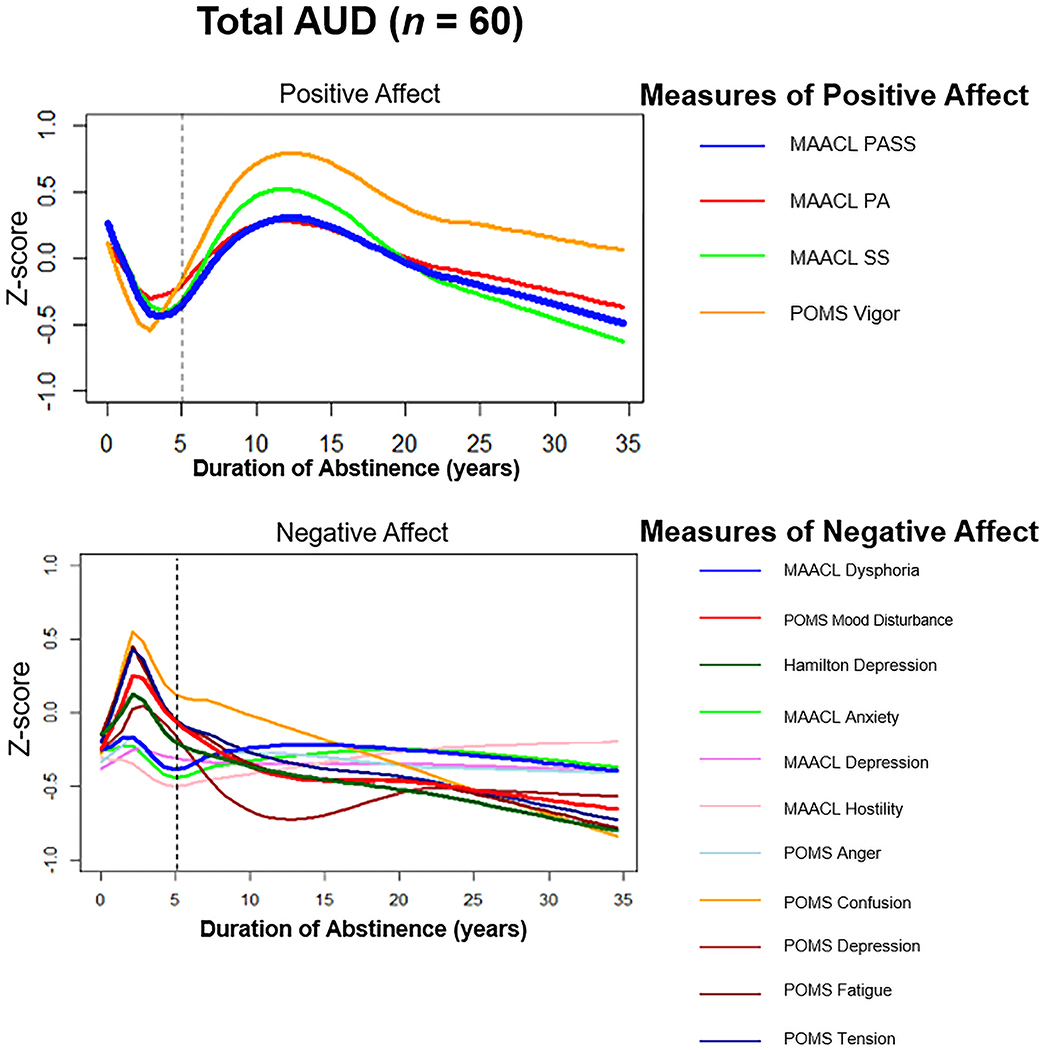
We plotted LOWESS curves (Figure 2) for the standardized composite measures of affective states relative to DOA among the AUD cohort. During the early years of abstinence (i.e., ≤ five years), there was a steady decrease in the positive affective variables (MAACL Positive Affect Sensation Seeking and POMS Vigor), and a steady increase of negative affective variables (MAACL Dysphoria, POMS Mood Disturbance, and HRSD). Beyond five years of abstinence, scores on the measures of positive affective states increased before stabilizing, while the negative affective scores continually decreased. All individual standardized measures of positive affective states and negative affective states were plotted separately, and all measures had similar curves over the DOA. In addition, the LOWESS curves for all measures corresponded similarly to the composite measures.
Figure 2:
Locally Weighted Scatterplot Smoothing (LOWESS) analysis representing measures of affective states (standardized) during the first 35 years of abstinence.
We also plotted LOWESS curves in relation to sex among the AUD group (Figure 3). Whereas the AUDW reported an increase in negative affective states during the early years of abstinence (i.e., ≤ five years), the AUDM reported a decrease in negative affect during the first part of the same period, until approximately three years of abstinence. Subsequently, negative affective states tended to decrease in the AUDW and increase in the AUDM. There was greater variability among both AUDW and AUDM with respect to affective states with increasing DOA (i.e., beyond 10 years). In measures of positive affective states, a somewhat predictable trend emerged for the AUDW, with a decline that lasted for approximately the same amount of time than the increase in negative affective states did. In the AUDM, there was less correspondence between the self-report of positive affective states and negative affective states during the early phases of abstinence, but a general upward trend emerged among positive affective states after approximately 10 years of abstinence.
Figure 3:
Locally Weighted Scatterplot Smoothing (LOWESS) analysis representing sex differences in measures of affective states (standardized) during the first 35 years of abstinence.
Duration of Abstinence and Affective States
A multivariable linear regression analysis (Tables 3 and 4) was used to assess the relationship between DOA and measures of affective states. The regression model contained age, education, VIQ, DHD, and DD as covariates. The results revealed that, relative to the NC group, the AUD group reported significantly higher POMS Mood Disturbance (95% CI: 5.39, 32.57), POMS Anger (95% CI: 0.81, 5.29), POMS Confusion (95% CI: 0.75, 6.05), POMS Depression (95% CI: 0.91, 5.88), POMS Tension (95% CI: 0.84, 6.25), and HRSD (95% CI: 1.19, 3.67). This pattern of results appeared to be driven primarily by the short-/mid-term abstainers, who exhibited significantly higher POMS Mood Disturbance (95% CI: 11.56, 44.04), POMS Anger (95% CI: 1.58, 6.98), POMS Confusion (95% CI: 1.34, 7.57), POMS Depression (95% CI: 2.28, 8.04), POMS Fatigue (95% CI: 0.62, 7.31), POMS Tension (95% CI: 1.85, 8.26), HRSD (95% CI: 2.04, 4.65), in addition to significantly lower POMS Vigor (95% CI: −9.06, −0.72), compared to the NC group. Accordingly, there were no significant differences on any affective measure between the long-term abstainers and the NC group, except with respect to relatively higher scores on the HRSD (95% CI: 0.14, 2.98). The AUDW reported significantly higher MAACL Positive Affect (95% CI: 0.59, 11.55) and MAACL Sensation Seeking (95% CI: −0.93, 8.68) in association with DOA, relative to the AUDM. No differences were detected in the effect of DOA between the AUDW and the AUDM except for the MAACL Positive Affect, which remained higher among the AUDW, relative to the AUDM, after five years of abstinence. However, when comparing the effect of long-term abstinence on affective states with the effect of short-/mid-term abstinence on affective states, significant sex interactions were observed. The AUDW reported lower scores on the MAACL Dysphoria (95% CI: −49.77, −4.68), MAACL Depression (95% CI: −33.74, −0.85), and MAACL Anxiety (95% CI: −70.92, −5.55) associated with the longer DOA, but the AUDM showed the opposite effect (Supplemental Material – Figure S2).
Table 3.
Adjusted Linear Regression of the Effect of History of AUDs and Duration of Abstinence on Measures of Negative Affective States.
| MAACL Dysphoria (Composite) | MAACL Anxiety | MAACL Depression | MAACL Hostility | POMS Mood Disturbance (Composite) | POMS Anger | POMS Confusion | POMS Depression | POMS Fatigue | POMS Tension | Hamilton | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC (n=60) | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| AUD (n=60) | 4.23 [−1.81, 10.28] | 2.84 [−1.85, 7.53] | 6.29 [−1.3, 13.89] | 1.39 [−1.88, 4.65] | 18.98 [5.39, 32.57] | 3.05 [0.81, 5.29] | 3.4 [0.75, 6.05] | 3.4 [0.91, 5.88] | 2.46 [−0.36, 5.29] | 3.54 [0.84, 6.25] | 2.43 [1.19, 3.67] | |
| Duration of Abstinence (categorical) a | ||||||||||||
| NC | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| ≤ 5 years | 5.23 [−1.65, 12.1] | 3.63 [−1.86, 9.12] | 7.02 [−0.69, 14.73] | 1.73 [−2, 5.46] | 27.8 [11.56, 44.04] | 4.28 [1.58, 6.98] | 4.45 [1.34, 7.57] | 5.16 [2.28, 8.04] | 3.97 [0.62, 7.31] | 5.06 [1.85, 8.26] | 3.34 [2.04, 4.65] | |
| NC | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| > 5 years | 4.36 [−3.45, 12.18] | 2.73 [−3.41, 8.87] | 8.22 [−1.48, 17.91] | 1.4 [−3.52, 6.32] | 6.37 [−8.48, 21.21] | 1.62 [−0.65, 3.9] | 1.99 [−1.26, 5.24] | 1.25 [−1.55, 4.06] | 0.48 [−2.88, 3.83] | 1.45 [−1.71, 4.6] | 1.56 [0.14, 2.98] | |
| Duration of Abstinence (categorical) b | ||||||||||||
| ≤ 5 years | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| > 5 years | 13.72 [−4, 31.44] | 6.86 [−6.07, 19.78] | 23.72 [−1.97, 49.41] | 4.23 [−3.99, 12.44] | −16.92 [−55.55, 21.71] | −2.73 [−9.5, 4.03] | −2.32 [−9.77, 5.13] | −1.87 [−9, 5.25] | −4.3 [−12.14, 3.5] | −3.79 [−11.49, 3.9] | −1.55 [−5.55, 2.44] | |
| Sex | ||||||||||||
| ≤ 5 years men | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| ≤ 5 years women | 10.45 [−1.47, 22.38] | 7.41 [−1.29, 16.12] | 9.45 [−7.84, 26.75] | 2.75 [−2.78, 8.28] | 6.46 [−21.9, 34.82] | 0.52 [−4.44, 5.5] | 0.8 [−4.67, 6.27] | −0.85 [−6.08, 4.38] | −1.03 [−6.78, 4.72] | 1.68 [−3.97, 7.33] | −0.04 [−2.97, 2.9] | |
| Sex/DOA interaction | ||||||||||||
| ≤ 5 years men | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| ≤ 5 years women | −27.22 [−49.77, −4.68] | −17.29 [−33.74, −0.9] | −38.23 [−70.92, −5.55] | −6.46 [−16.92, 3.99] | −15.32 [−66.29, 35.66] | −0.77 [−9.68, 8.2] | −0.59 [−10.43, 9.24] | −3.74 [−13.15, 5.66] | 0.79 [−9.55, 11.1] | −1.72 [−11.9, 8.44] | 0.26 [−5.02, 5.53] | |
| Duration of Abstinence (years) b | 0.18 [−0.68, 1.05] | 0.04 [−0.58, 0.67] | 0.23 [−1.03, 1.5] | 0.14 [−0.24, 0.53] | −1.26 [−3.28, 0.76] | −0.23 [−0.6, 0.11] | −0.21 [−0.59, 0.18] | −0.25 [−0.62, 0.12] | −0.37 [−0.77, 0.03] | −0.21 [−0.61, 0.19] | −0.19 [−0.39, 0] | |
| Sex | ||||||||||||
| men | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | |
| women | 7.76 [−4.42, 19.94] | 5.72 [−3.07, 14.51] | 5.15 [−12.61, 22.9] | 2.93 [−2.53, 8.4] | 0.01 [−28.17, 28.19] | −0.24 [−5.08, 4.6] | 0.18 [−5.15, 5.52] | −2.29 [−7.43, 2.86] | −2.28 [−7.83, 3.28] | 1.12 [−4.47, 6.71] | −0.16 [−2.93, 2.61] |
The 95% confidence intervals of the mean differences are presented. Effects significant at p < 0.05 are in bold. Ref = reference group
Adjusted for age, education, and VIQ
Adjusted for age, education, VIQ, DHD, and DD
Table 4.
Adjusted Linear Regression of the Effect of History of AUDs and Duration of Abstinence on Measures of Positive Affective States.
| MAACL PASS (Composite) | MAACL Positive Affect | MAACL Sensation Seeking | POMS Vigor | ||
|---|---|---|---|---|---|
| NC (n=60) | ref | ref | ref | ref | |
| AUD (n=60) | −2.14 [−5.07, 0.78] | −1.41 [−4.41, 1.59] | −2.22 [−5.05, 0.61] | −3.13 [−6.68, 0.42] | |
| Duration of Abstinence (categorical) a | |||||
| NC | ref | ref | ref | ref | |
| ≤ 5 years | −2.44 [−5.86, 0.98] | −1.97 [−5.46, 1.51] | −1.96 [−5.27, 1.35] | −4.89 [−9.06, −0.72] | |
| NC | ref | ref | ref | ref | |
| > 5 years | −1.91 [−6.01, 2.19] | −0.99 [−5.18, 3.2] | −2.18 [−6.29, 1.93] | 0.41 [−4.01, 4.84] | |
| Duration of Abstinence (categorical) b | |||||
| ≤ 5 years | ref | ref | ref | ref | |
| > 5 years | −1.51 [−9.48, 6.45] | −2.48 [−10.63, 5.66] | 1.11 [−6.45, 8.68] | 1.91 [−7.55, 11.37] | |
| Sex | |||||
| ≤ 5 years men | ref | ref | ref | ref | |
| ≤ 5 years women | 5.33 [−0.03, 10.7] | 6.07 [0.59, 11.55] | 2.27 [−2.83, 7.36] | −5.35 [−12.29, 1.6] | |
| Sex/DOA interaction | |||||
| ≤ 5 years men | ref | ref | ref | ref | |
| ≤ 5 years women | 0.05 [−10.09, 10.18] | 2.05 [−8.31, 12.41] | −3.43 [−13.05, 6.2] | 9.28 [−3.21, 21.76] | |
| Duration of Abstinence (years) b | −0.07 [−0.44, 0.29] | −0.09 [−0.47, 0.3] | −0.01 [−0.35, 0.33] | 0 [−0.52, 0.51] | |
| Sex | |||||
| men | ref | ref | ref | ref | |
| women | 7.16 [2.02, 12.3] | 7.56 [2.19, 12.92] | 3.88 [−0.93, 8.68] | −3.51 [−10.67, 3.64] | |
The 95% confidence intervals of the mean differences are presented. Effects significant at p < 0.05 are in bold. Ref = reference group
Adjusted for age, education, and VIQ
Adjusted for age, education, VIQ, DHD, and DD
Discussion
Early Abstinence
As predicted, we found that the short-/mid-term abstainers exhibited significantly greater negative affect and lower positive affect, compared to the NC group. This group of abstainers also had significantly greater posterior insular volume than the NC group. Previous studies have demonstrated that structural abnormalities of the insula are associated with affective disorders (Barrett & Simmons, 2015), in addition to chronic alcohol consumption and increased chance of relapse during early abstinence from alcohol (Cardenas et al., 2011; Durazzo et al., 2017; Durazzo et al., 2011; Zou et al., 2018). Interestingly, that research has observed volumetric deficits of the entire insula (anterior and posterior) among short-term abstainers (Durazzo et al., 2010; Durazzo et al., 2011; Makris et al., 2008; Zou et al., 2018), which tend to exhibit morphological “recovery” over the course of early abstinence (Durazzo et al., 2015; Zou et al., 2018). Part of the reason for the difference in our findings and those of the previous studies may be that most studies only considered relatively short DOA of less than one year, exclusively among AUDM, and studies that considered longer DOA of one year or more did not control for factors that may have had a bearing on the effect of DOA, such as measures of drinking severity (e.g., DHD, DD). Hence, the insular “recovery” observed in studies with narrower parameters may have been a precursor to the trend that we detected using a wider lens, namely, the abnormally higher posterior insular volume among short-/mid-term abstainers, but not among long-term abstainers. Given the role that the posterior insula plays in the processing of interoceptive pain/stress-related inputs from the body, and the increased tendency to feel negatively valenced somataffective experiences during early abstinence (Heilig et al., 2010), we suggest that the relatively larger volume we observed among the short-/mid-term abstainers potentially represents a compensatory mechanism that provides increased cortical supply to cope with increased interoceptive demand. The fact that there was no significant difference in posterior insula between long-term abstainers and the NC group may indicate that its volume may become more normative with continued abstinence, which might be related to the relatively normal affective profile we observed among this group. Further support for this hypothesis comes from a separate mediation analysis we conducted, which demonstrated that the effect of abstinence on the lower scores in depression, as measured by the HRSD, was mediated by the smaller volumes of the posterior insula (see Appendix).
The negative affective profile that characterized short-/mid-term abstainers may also have been related to the influence of the PCC, in which we found that the short-/mid-term abstainers exhibited significantly greater volume than the long-term abstainers. Again, previous studies have shown that chronic alcohol consumption has been associated with decreased volume of the PCC (Zakiniaeiz et al., 2017), which make the finding from this study surprising. However, the difference in the patterns that we observed and those of previous studies may also be attributable to methodological differences, as observed above. The relationship between increased negative affect and increased PCC volume may be explainable in similar terms to our previous interpretation. That is, increased cortical volume of this region may be an index of the functional role that it is playing in the facilitation of affective experiences. As a central node of the default mode network, the PCC is involved in processing awareness of the “self” in relation to the present moment (Brewer et al., 2013). Increased functional connectivity of the PCC has been observed to be related to rumination in depression (Berman et al., 2011; Cheng et al., 2018). Similarly, we suggest that the increased volume observed in the PCC among the short-/mid-term abstainers may represent the disproportionate influence of a brain region responsbile for self-awareness (excessive preoccupation with how one is feeling/doing) to a degree that can be experienced as negative (e.g., social anxiety). Accordingly, this regional difference between short-/mid-term abstainers and long-term abstainers may partially explain the relative difference between these groups and the NC group in terms of their affective profiles, as well as their relative risk of becoming relapse (Grant et al., 2016; Oliveira et al., 2018). This notion is supported by a separate mediation analysis we conducted, in which the lower volume of the PCC mediated the effect of abstinence on higher scores on a measure of positive affect (i.e., POMS Vigor) (see Appendix).
We also detected sex differences in the association between DOA and affect within early abstinence. Our results, which complemented the findings of Kelly and colleagues (Kelly et al., 2018), revealed that AUDM with short-term abstinence tended to exhibit higher positive affective states and lower negative affective states than AUDM within mid-term abstinence (i.e., > one year, < five year), who exhibited higher negative affective states and lower positive affective states. In some cases, negative affect was highest among AUDM with much lengthier DOA (i.e., MAACL Hostility scores are highest much later, at year 12). In contrast, the AUDW exhibited peak negative affect during early abstinence, which trended downward thereafter. The observed differences may be representative of sex-specific differences in brain regions involved in affective processing and regulation. For example, in our analyses, we observed that the volume of the anterior insula and temporal poles was significantly greater among the AUDW, relative to AUDM – a trend that corresponded with significantly higher scores on measures of positive affect (i.e., MAACL Positive Affect and MAACL Sensation Seeking) – with each year of abstinence among the AUDW. Additional research confirming and explaining these sex differences will be crucial to providing sex-specific treatment for affective dysregulation in recovery.
Long-term Abstinence
Consistent with our hypotheses, affective states among the long-term abstainers were relatively normal, with the exception of higher levels of depression as measured by the HRSD. However, this group exhibited significantly lower volumes of the ACC, relative to the NC group. This finding corresponds with those from previous studies demonstrating that reduced gray matter volume in the ACC is associated with chronic alcohol consumption (Sawyer et al., 2017; Xiao et al., 2015), in addition to depressed mood (Boes et al., 2008; Singh et al., 2012), which reinforces what is currently known about the role of the ACC in facilitating affective and motivational processes (Holroyd & Yeung, 2012; Touroutoglou et al., 2019). It is interesting, however, that in our sample, the AUD group with the most negative affect (i.e., short-/mid-term abstainers) showed no volumetric difference from the NC group within the ACC, while decreased ACC volume characterized the AUD group with the least negative affect (i.e., long-term abstainers). To interpret this nuanced finding, we highlight that previous studies have observed an association between gray matter volume of the ACC and a unique variety of dispositional intentionality (i.e., tenacity in the face of a challenge) (Mulert et al., 2005; Touroutoglou et al., 2019; Van Schuerbeek et al., 2011). Given that abstinence, especially early abstinence, commonly represents intentional behavior in the face of pathologically conditioned responses to external and internal cues (Amodeo et al., 1992), we suggest that the relatively normal ACC volume among the short-/mid-term abstainers may represent a type of compensatory mechanism that could serve as a self-regulatory behavioral function (i.e., tenacity) during early abstinence, when negative affect is at its height. After peak negative affect abates, through the transition into long-term abstinence, perhaps the demand for intensive effort to abstain attenuates and this regulatory function is eventually offloaded onto cortical resources more appropriate to the task, which may partially explain why the long-term abstainers exhibited lower volume of the ACC.
Sex differences were also observed among the long-term abstainers. For example, compared to long-term abstinent AUDM, long-term abstinent AUDW had significantly greater volume of the ventral diencephalon. However, because this region comprises multiple subregions (i.e., basal forebrain, ventral tegmental area, and hypothalamus), limitations of the segmentation method we employed prevented us from analyzing in detail. Nevertheless, we found that long-term abstinent AUDW exhibited significantly greater positive affect, as measured by the MAACL, in addition to significantly greater posterior insula volume, relative to the long-term abstinent AUDM. As observed previously, differences in the morphological profile of the posterior insula has been linked to differences in affective processing (Barrett & Simmons, 2015). The morphological sex differences we observed in the posterior insula may be at least partially attributable to the influence of gonadal hormones (Becker & Koob, 2016), which may have had some bearing on the affective sex differences detected. In the context of long-term abstinence, a relatively larger posterior insula among the AUDW may represent a transition to a more normative sex-specific affective style. Perhaps through continued abstinence, affective states are processed in ways that are considered more typical for each sex (Moriguchi et al., 2014). Alternatively, and/or concomitantly, these sex differences may be endemic to the dynamic interplay between gender and sociocultural influences related to different life stages that overlap with different DOA.
Limitations
All of these findings should be considered in light of several limitations of this study. First, due to the cross-sectional and correlational nature of the data, we can detect interindividual differences only, rather than intraindividual changes in volumetric and affective differences associated with DOA. Future longitudinal studies are therefore needed to augment our current understanding of the observed patterns. Additionally, socioeconomic status (SES), a factor that has been shown to have a significant bearing on affective states (Navarro-Carrillo et al., 2020), was not considered directly, but rather, by proxy, via level of education. More comprehensive indices of SES might provide greater insight into the link between affect, drinking/recovery-related behaviors, and the brain. This link may also have been influenced by other factors not assessed in this study, including, but not limited to, personality traits, diet/nutrition, exercise, subclinical liver dysfunction, genetic predispositions, nonpharmacological therapeutic influences (e.g., psychotherapy, mutual-help organization activity), as well as the co-occurrence of any other non-drug “behavioral” addictions (e.g., food addiction, gambling addiction, sex addiction), which are common among individuals with AUDs (Grant et al., 2006). Beyond adhering to our exclusion criteria as specified in the Results section, we recognize the potentially confounding effects of other potential variables, and therefore, in our analyses, we specifically considered two subgroups of AUD individuals. We called these subgroups “confounded” (i.e., those with minor medical conditions) and “unconfounded” (i.e., those with no such conditions). These analyses revealed no significant differences between the subgroups on the measures assessed. This study excluded from its analyses individuals with HRSD scores greater than 16 (i.e., those who met the criteria for moderate-to-severe depression), which likely yielded less difference between the AUD groups and the NC group in terms of depression than may have been observed otherwise. Future studies could include AUD individuals with comorbid mood disorders to provide a more comprehensive representation of the affective profile characterizing individuals in recovery from AUDs. Finally, no consideration was made of whether abstinence was intentional or incidental, as is commonly the case for those who “mature out” of their addictions (Lopez-Quintero et al., 2011). Parsing out the dynamic interrelationship between brain, behavior, affect, and sex-related influences in the context of recovery will require future research in this area to account for each of these potential influences.
Conclusions
Recent advances in the study of recovery from addictions have revealed that this process consists of more than behavior change (Kelly et al., 2018; Witkiewitz et al., 2020). Changes in the affective dimensions of life appear to be among the most salient features of recovery from AUDs (NIAAA2020). To our knowledge, this is the first study to investigate the neural correlates of the affective profiles related to varying DOA, especially in relation to longer term abstinence. As such, our findings provide valuable insight into the neural correlates of the affective dimensions of an important variety of recovery from AUDs, namely, abstinence. This is a necessary step in the direction of characterizing the way in which changes in the brain are related to the multidimensional changes that occur in and through the broader process of recovery. In addition to suggesting potentially fruitful strategies to augmenting traditional treatment approaches (e.g., cogntivie-behavioral therapy) with respect to relevant factors, such as sex and phase of recovery, the association we observed between volumetric measures of BRS regions, affect, and different DOA may hold promise for informing more novel neurotherapeutic treatment approaches (e.g., real-time neurofeedback, transcranial magnetic stimulation). Future research is needed to substatiate this inference and to determine the clinical relevance of these findings.
Supplementary Material
Figure 1:
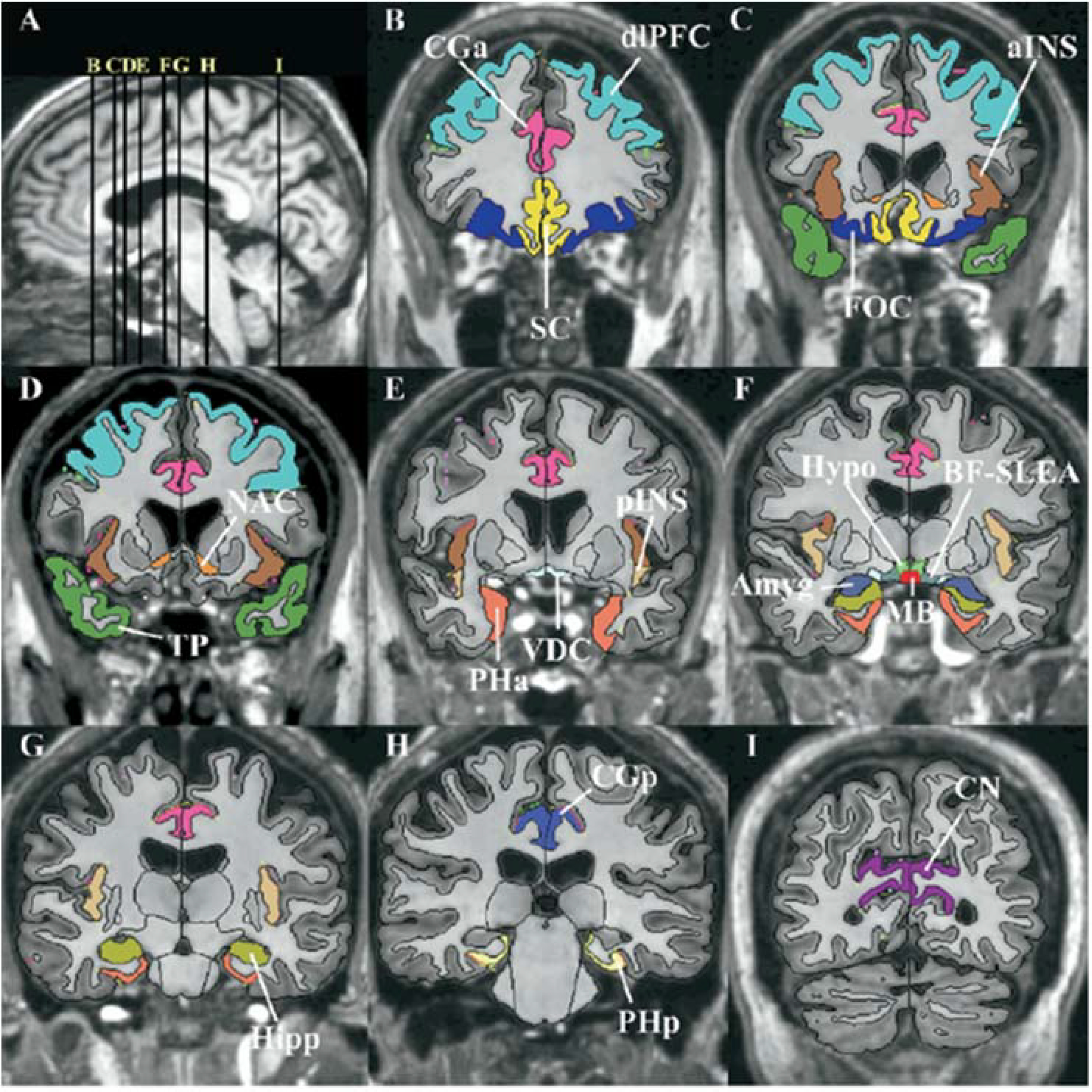
Segmentation method of the cortical and subcortical structures composing the reward system, shown in T1-weighted magnetic resonance images.
Acknowledgements
This work was supported by grants awarded to Marlene Oscar-Berman from the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, US Department of Health and Human Services (R01AA07112 and K05AA00219) and from the US Department of Veterans Affairs Clinical Science Research and Development Service (I01CX000326), as well as grant P41RR14075 from the National Center for Research Resources at the Center for Functional Neuroimaging Technologies, Massachusetts General Hospital. We gratefully thank Kayle Sawyer for his consultation, and for his help with data collection and analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the United States Government.
Footnotes
Competing Interests
The authors declare no financial or non-financial competing interests.
References
- Adinoff B, Iranmanesh A, Veldhuis J, & Fisher L (1998). Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health and Research World, 22(1), 67–72. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15706736 [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Olino T, Freed RD, & Nusslock R (2016). Role of Reward Sensitivity and Processing in Major Depressive and Bipolar Spectrum Disorders. Behavior Therapy, 47(5), 600–621. doi: 10.1016/j.beth.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Washington, DC. [Google Scholar]
- Amodeo M, Kurtz N, & Cutter HS (1992). Abstinence, reasons for not drinking, and life satisfaction. International Journal of the Addictions, 27(6), 707–716. doi: 10.3109/10826089209068762 [DOI] [PubMed] [Google Scholar]
- Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, & Little HJ (2001). Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology, 41(8), 989–999. doi: 10.1016/s0028-3908(01)00146-0 [DOI] [PubMed] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews: Neuroscience, 16(7), 419–429. doi: 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, & Bendszus M (2007). Manifestations of early brain recovery associated with abstinence from alcoholism. Brain, 130(Pt 1), 36–47. doi: 10.1093/brain/awl303 [DOI] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex Differences in Animal Models: Focus on Addiction. Pharmacological Reviews, 68(2), 242–263. doi: 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, & Jonides J (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. doi: 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, & Comings DE (1996). The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine, 89(7), 396–400. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8774539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, McCormick LM, Coryell WH, & Nopoulos P (2008). Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biological Psychiatry, 63(4), 391–397. doi: 10.1016/j.biopsych.2007.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, & Whitfield-Gabrieli S (2013). What about the “Self” is Processed in the Posterior Cingulate Cortex? Frontiers in Human Neuroscience, 7, 647. doi: 10.3389/fnhum.2013.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs GG, & Nebes RD (1975). Patterns of hand preference in a student population. Cortex, 11(3), 230–238. doi: 10.1016/s0010-9452(75)80005-0 [DOI] [PubMed] [Google Scholar]
- Brown SA, & Schuckit MA (1988). Changes in depression among abstinent alcoholics. Journal of Studies on Alcohol, 49(5), 412–417. doi: 10.15288/jsa.1988.49.412 [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, & Schuckit MA (1995). Stress, vulnerability and adult alcohol relapse. Journal of Studies on Alcohol, 56(5), 538–545. doi: 10.15288/jsa.1995.56.538 [DOI] [PubMed] [Google Scholar]
- Cahalan D (1968). American Drinking Practices: Summary of Findings from a National Probability Sample. I. Extent of Drinking by Population Subgroups. Quarterly Journal of Studies on Alcohol, 29(1), 130–151. [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, & Crossley HM (1969). American Drinking Practices: A National Study of Drinking Behavior and Attitudes. Monographs of the Rutgers Center of Alcohol Studies, 6. [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, & Meyerhoff DJ (2011). Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biological Psychiatry, 70(6), 561–567. doi: 10.1016/j.biopsych.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS Jr., Kennedy DN, Richelme C, Rademacher J, & Filipek PA (1996). The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cerebral Cortex, 6(5), 726–736. doi: 10.1093/cercor/6.5.726 [DOI] [PubMed] [Google Scholar]
- Caviness VS Jr., Meyer J, Makris N, & Kennedy DN (1996). MRI-Based Topographic Parcellation of Human Neocortex: An Anatomically Specified Method with Estimate of Reliability. Journal of Cognitive Neuroscience, 8(6), 566–587. doi: 10.1162/jocn.1996.8.6.566 [DOI] [PubMed] [Google Scholar]
- Chavkin C, & Koob GF (2016). Dynorphin, Dysphoria, and Dependence: the Stress of Addiction. Neuropsychopharmacology, 41(1), 373–374. doi: 10.1038/npp.2015.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Xie X, Wei D, Huang CC, Yang AC, Tsai SJ, Li Q, Meng J, Lin CP, Xie P, & Feng J (2018). Increased functional connectivity of the posterior cingulate cortex with the lateral orbitofrontal cortex in depression. Transl Psychiatry, 8(1), 90. doi: 10.1038/s41398-018-0139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Saha TD, & Grant BF (2015). Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug and Alcohol Dependence, 148, 56–61. doi: 10.1016/j.drugalcdep.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fosse L, Hodge SM, Makris N, Kennedy DN, Caviness VS Jr., McGrath L, Steele S, Ziegler DA, Herbert MR, Frazier JA, Tager-Flusberg H, & Harris GJ (2004). Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology, 56(6), 757–766. doi: 10.1002/ana.20275 [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, & Mann K (2011). Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcoholism, Clinical and Experimental Research, 35(9), 1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x [DOI] [PubMed] [Google Scholar]
- Dennis ML, Foss MA, & Scott CK (2007). An eight-year perspective on the relationship between the duration of abstinence and other aspects of recovery. EvaluationReview, 31(6), 585–612. doi: [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, & Meyerhoff DJ (2017). Regional brain volume changes in alcohol-dependent individuals during early abstinence: associations with relapse following treatment. Addiction Biology, 22(5), 1416–1425. doi: 10.1111/adb.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Yeh PH, & Meyerhoff DJ (2015). Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addiction Biology, 20(5), 956–967. doi: 10.1111/adb.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pathak V, Gazdzinski S, Mon A, & Meyerhoff DJ (2010). Metabolite levels in the brain reward pathway discriminate those who remain abstinent from those who resume hazardous alcohol consumption after treatment for alcohol dependence. Journal of Studies on Alcohol and Drugs, 71(2), 278–289. doi: 10.15288/jsad.2010.71.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, & Meyerhoff DJ (2011). Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcoholism, Clinical and Experimental Research, 35(6), 1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, & Cardenas VA (2015). Neuroplasticity in Human Alcoholism: Studies of Extended Abstinence with Potential Treatment Implications. Alcohol Res, 37(1), 125–141. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26259093 [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, & Caviness VS Jr. (1994). The young adult human brain: an MRI-based morphometric analysis. Cerebral Cortex, 4(4), 344–360. doi: 10.1093/cercor/4.4.344 [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, & Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, & Biederman J (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry, 162(7), 1256–1265. doi: 10.1176/appi.ajp.162.7.1256 [DOI] [PubMed] [Google Scholar]
- Fritz M, Klawonn AM, & Zahr NM (2019). Neuroimaging in alcohol use disorder: From mouse to man. Journal of Neuroscience Research. doi: 10.1002/jnr.24423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, & Koob GF (2017). Individual differences in the neuropsychopathology of addiction. Dialogues in Clinical Neuroscience, 19(3), 217–229. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29302219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS Jr., Faraone SV, & Tsuang MT (1999). Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Archives of General Psychiatry, 56(6), 537–547. doi: 10.1001/archpsyc.56.6.537 [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr., Faraone SV, & Tsuang MT (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex, 11(6), 490–497. doi: 10.1093/cercor/11.6.490 [DOI] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, & Hasin DS (2016). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry, 73(1), 39–47. doi: 10.1001/jamapsychiatry.2015.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Brewer JA, & Potenza MN (2006). The neurobiology of substance and behavioral addictions. CNS Spectr, 11(12), 924–930. doi: 10.1017/s109285290001511x [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1967). Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology, 6(4), 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, & Becker HC (2010). Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology, 15(2), 169–184. doi: 10.1111/j.1369-1600.2009.00194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, & Bartenstein P (2005). Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. American Journal of Psychiatry, 162(8), 1515–1520. doi: 10.1176/appi.ajp.162.8.1515 [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, & Caviness VS Jr. (2003). Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain, 126(Pt 5), 1182–1192. doi: 10.1093/brain/awg110 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Yeung N (2012). Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences, 16(2), 122–128. doi: 10.1016/j.tics.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Humphreys K, & Bickel WK (2018). Toward a Neuroscience of Long-term Recovery From Addiction. JAMA Psychiatry, 75(9), 875–876. doi: 10.1001/jamapsychiatry.2018.0956 [DOI] [PubMed] [Google Scholar]
- Kelly JF, Greene MC, & Bergman BG (2018). Beyond Abstinence: Changes in Indices of Quality of Life with Time in Recovery in a Nationally Representative Sample of U.S. Adults. Alcoholism, Clinical and Experimental Research, 42(4), 770–780. doi: 10.1111/acer.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DN, Filipek PA, & Caviness VR (1989). Anatomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Transactions on Medical Imaging, 8(1), 1–7. doi: 10.1109/42.20356 [DOI] [PubMed] [Google Scholar]
- Kober H (2014). Emotion Regulation in Substance Use Disorders. In Gross J (Ed.), Handbook of Emotion Regulation (2nd ed.). New York, NY: Guilford. [Google Scholar]
- Koob GF (2013). Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Current Topics in Behavioral Neurosciences, 13, 3–30. doi: 10.1007/7854_2011_129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2015). The dark side of emotion: the addiction perspective. European Journal of Pharmacology, 753, 73–87. doi: 10.1016/j.ejphar.2014.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Arends MA, & Moal ML (2014). Drugs, Addiction, and the Brain. Oxford, UK: Elsevier. [Google Scholar]
- Koob GF, & Le Moal M (2008). Addiction and the brain antireward system. Annual Review of Psychology, 59, 29–53. doi: 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Moal ML (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nature Neuroscience, 8(11), 1442–1444. doi: 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, & Strakowski SM (2002). Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry, 52(2), 93–100. doi: 10.1016/s0006-3223(02)01350-1 [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, & Blanco C (2011). Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction, 106(3), 657–669. doi: 10.1111/j.1360-0443.2010.03194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS Jr., Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, & Breiter HC (2004). Decreased absolute amygdala volume in cocaine addicts. Neuron, 44(4), 729–740. doi: 10.1016/j.neuron.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, & Caviness VS (1999). MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage, 9(1), 18–45. doi: 10.1006/nimg.1998.0384 [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, & Harris GJ (2008). Decreased Volume of the Brain Reward System in Alcoholism. Biological Psychiatry, 64(3), 192–202. doi: 10.1016/j.biopsych.2008.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, & Gordon JR (1980). Determinants of relapse: Implications for the maintenance of behavior change. In Davidson PO & Davidson SM (Eds.), Behavioral Medicine: Changing Health Lifestyles (pp. 410–452). New York: Brunner/Mazel [Google Scholar]
- McNair DM, Lorr M, & Droppleman LF (1971). Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Melis M, Spiga S, & Diana M (2005). The dopamine hypothesis of drug addiction: hypodopaminergic state. International Review of Neurobiology, 63, 101–154. doi: 10.1016/s0074-7742(05)63005-x [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Touroutoglou A, Dickerson BC, & Barrett LF (2014). Sex differences in the neural correlates of affective experience. Social Cognitive and Affective Neuroscience, 9(5), 591–600. doi: 10.1093/scan/nst030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, & Hegerl U (2005). Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. International Journal of Psychophysiology, 56(1), 65–80. doi: 10.1016/j.ijpsycho.2004.10.002 [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2020). Division of Treatment and Recovery Research. Retrieved from https://www.niaaa.nih.gov/division-treatment-recovery-research
- Navarro-Carrillo G, Alonso-Ferres M, Moya M, & Valor-Segura I (2020). Socioeconomic Status and Psychological Well-Being: Revisiting the Role of Subjective Socioeconomic Status. Frontiers in Psychology, 11, 1303. doi: 10.3389/fpsyg.2020.01303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, & Agius M (2015). A systematic review of structural and functional MRI differences between psychotic and nonpsychotic depression. Psychiatria Danubina, 27 Suppl 1, S235–239. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26417770 [PubMed] [Google Scholar]
- Oliveira LM, Bermudez MB, Macedo MJA, & Passos IC (2018). Comorbid social anxiety disorder in patients with alcohol use disorder: A systematic review. Journal of Psychiatric Research, 106, 8–14. doi: 10.1016/j.jpsychires.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of Impaired, Spared, and Recovered Neuropsychological Processes in Alcoholism. In P. A& S. EV (Eds.), Handbook of Clinical Neurology: Alcohol and the Nervous System (pp. 183–210). Edinburgh: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pando-Naude V, Toxto S, Fernandez-Lozano S, Parsons CE, Alcauter S, & Garza-Villarreal EA (2021). Gray and white matter morphology in substance use disorders: a neuroimaging systematic review and meta-analysis. Transl Psychiatry, 11(1), 29. doi: 10.1038/s41398-020-01128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA (1977). Neuropsychological deficits in alcoholics: facts and fancies. Alcoholism, Clinical and Experimental Research, 1(1), 51–56. doi: 10.1111/j.1530-0277.1977.tb05767.x [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, & Dundon WD (2013). Current status of co-occurring mood and substance use disorders: a new therapeutic target. American Journal of Psychiatry, 170(1), 23–30. doi: 10.1176/appi.ajp.2012.12010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, & Sinha R (2012). Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. American Journal of Psychiatry, 169(4), 406–414. doi: 10.1176/appi.ajp.2011.11020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rivas-Grajales AM, Sawyer KS, Karmacharya S, Papadimitriou G, Camprodon J, Harris GJ, Kuicki M, Oscar-Berman M, & Makris N (2017). Sexually Dimorphic Structural Abnormalities in Major Connections of the Medial Forebrain Bundle in Alcoholism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, & Rourke KM (2000). Diagnostic Interview Schedule for the DSM-IV (DIS-IV). St. Louis, MO: Washington University School of Medicine. [Google Scholar]
- Rosenbloom MJ, & Pfefferbaum A (2008). Magnetic resonance imaging of the living brain: evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res Health, 31(4), 362–376. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23584010 [PMC free article] [PubMed] [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, & Harris GJ (2013). Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism, Clinical and Experimental Research, 37(1), 110–122. doi: 10.1111/j.1530-0277.2012.01862.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer KS, Maleki N, Urban T, Marinkovic K, Karson S, Ruiz SM, Harris GJ, & Oscar-Berman M (2019). Alcoholism gender differences in brain responsivity to emotional stimuli. Elife, 8. doi: 10.7554/eLife.41723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer KS, Oscar-Berman M, Barthelemy OJ, Papadimitriou GM, Harris GJ, & Makris N (2017). Gender dimorphism of brain reward system volumes in alcoholism. Pyschiatry Research: Neuroimaging, 263, 15–25. doi: 10.1016/j.pscychresns.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, & Tsuang MT (1999). Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biological Psychiatry, 46(7), 941–954. doi: 10.1016/s0006-3223(99)00075-x [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, & Sinha R (2011). Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping, 32(11), 1998–2013. doi: 10.1002/hbm.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Chen MC, Kelley RG, Garrett A, Mitsunaga MM, Bararpour L, Howe M, Reiss AL, & Gotlib IH (2012). Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar Disord, 14(6), 585–596. doi: 10.1111/j.1399-5618.2012.01043.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, & DelBello MP (2002). Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord, 4(2), 80–88. doi: 10.1034/j.1399-5618.2002.01160.x [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yucel M, Lorenzetti V, Tanino R, Whittle S, Suzuki M, Walterfang M, Pantelis C, & Allen NB (2010). Volumetric MRI study of the insular cortex in individuals with current and past major depression. Journal of Affective Disorders, 121(3), 231–238. doi: 10.1016/j.jad.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Andreano JM, Adebayo M, Lyons S, & Barrett LF (2019). Motivation in the Service of Allostasis: The Role of anterior Mid Cingulate Cortex. Advanced Motivational Science, 6, 1–25. doi: 10.1016/bs.adms.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupala E, & Tiihonen J (2004). Dopamine and alcoholism: neurobiological basis of ethanol abuse. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28(8), 1221–1247. doi: 10.1016/j.pnpbp.2004.06.022 [DOI] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, & Luypaert R (2011). Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Research, 1371, 32–42. doi: 10.1016/j.brainres.2010.11.073 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, & Wong C (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. Journal of Neuroscience, 27(46), 12700–12706. doi: 10.1523/jneurosci.3371-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997a). WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1997b). WMS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- White WL, & Kurtz E (2006). The varieties of recovery experience. International Journal of Self-Help & Self-Care, 3(1–2), 21–61. [Google Scholar]
- Wise RA (2008). Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity Research, 14(2–3), 169–183. doi: 10.1007/BF03033808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Montes KS, Schwebel FJ, & Tucker JA (2020). What Is Recovery? Alcohol Res, 40(3), 01. doi: 10.35946/arcr.v40.3.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, & Pan P (2015). Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug and Alcohol Dependence, 153, 22–28. doi: 10.1016/j.drugalcdep.2015.05.030 [DOI] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, & Constable RT (2017). Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin, 13, 181–187. doi: 10.1016/j.nicl.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, & Dalrymple K (2013). Severity classification on the Hamilton Depression Rating Scale. Journal of Affective Disorders, 150(2), 384–388. doi: 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]
- Zou X, Durazzo TC, & Meyerhoff DJ (2018). Regional Brain Volume Changes in Alcohol-Dependent Individuals During Short-Term and Long-Term Abstinence. Alcoholism, Clinical and Experimental Research, 42(6), 1062–1072. doi: 10.1111/acer.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, & Lubin B (1965). Multiple Affect Adjective Check List. San Diego, CA: Educational and Industrial Testing Services. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.