Abstract
Weight loss is an effective strategy for management of hypertension and bariatric surgery is the most effective weight loss and maintenance strategy for obesity. The importance of bariatric surgery in the long-term management of hypertension and which operation is most effective is less clear. We compared the effectiveness of vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) for remission and relapse of hypertension after surgery in the Effectiveness of Gastric Bypass vs. Gastric Sleeve for Cardiovascular Disease (ENGAGE CVD) cohort study. Operations were done by 23 surgeons across 9 surgical practices. Hypertension remission and relapse were assessed in each year of follow-up beginning 30 days and up to 5 years post-surgery. We used a local instrumental variable approach to account for selection bias in the choice of VSG or RYGB. The study population included 4,964 patients with hypertension at the time of surgery (n = 3,186 VSG; n= 1,778 RYGB). At 1 year, 27% of patients with RYGB and 28% of patients with VSG achieved remission. After 5 years, without accounting for relapse, 42% of RYGB and 43% of VSG patients had experienced hypertension remission. After accounting for relapse, only 17% of RYGB and 18% of VSG patients remained in remission 5 years after surgery. There were no statistically significant differences between VSG and RYGB for hypertension remission, relapse, or mean systolic and diastolic blood pressure (BP) at any time during follow-up.
Keywords: Bariatric surgery, Gastric bypass, Sleeve gastrectomy, Hypertension, Blood pressure, Comparative study
INTRODUCTION
Severe obesity (body mass index [BMI] ≥ 35 kg/m2) has increased over the past several decades with rates as high as 30% for middle-aged Black women compared to 17% for their white counterparts in the U.S.1 Studies throughout the world have shown that there is a direct relationship between BMI and blood pressure (BP),2–5 and obesity may be responsible for 65%−78% of hypertension in the U.S.6, 7 Weight loss is recommended to reduce BP in adults with obesity4, 8, 9 but weight loss through lifestyle modification alone is challenging.10, 11 Only 50% of studies testing intensive lifestyle interventions for weight loss and maintenance show at least 5% weight loss (considered clinically meaningful), and most of the participants gain back at least half of this lost weight over 18–30 months.12 In contrast, surgical weight loss also known as bariatric surgery results in 18%−25% weight loss after 5 years, with a regain of 3%−15% in this same period of time.13, 14
Bariatric surgery also has promise as an effective treatment for cardiovascular risk factors.15 Systematic reviews and meta-analyses have reported 1-year hypertension remission rates ranging from 43% to 83%,15–19 and that hypertension remission may depend on the type of bariatric operation.16 Roux-en-Y Gastric Bypass (RYGB) in randomized trials had higher rates of hypertension remission within 5 years compared to vertical sleeve gastrectomy (VSG), whereas observational study results found no differences between operations.16 Few studies have been published on hypertension relapse rates following bariatric surgery. Those that have been done suggest that most patients who experience remission will relapse within 5–10 years and resume antihypertensive medication.20
The published literature for the impact of bariatric surgery on hypertension has several limitations: (1) a paucity of long-term outcomes, (2) examination of the rates of relapse as well as remission to understand the durable effect of surgery on hypertension in real-world clinical practices, (3) inclusion of populations that could benefit most from this treatment such as non-Hispanic Black patients, and (4) comparative studies reflecting current bariatric practice which includes over 60% VSG operations in the U.S.21 Additionally, many studies do not incorporate information about how surgeons and patients make decisions regarding which operation to have, which can bias the comparative findings.22 To address these limitations, we compared the effectiveness of VSG and RYGB for hypertension remission and relapse up to 5 years following surgery in the Effectiveness of Gastric Bypass vs. Gastric Sleeve for Cardiovascular Disease (ENGAGE CVD) study cohort. The ENGAGE CVD study was designed to use rigorous comparative effectiveness methods and an extensive process for stakeholder engagement to understand the factors that led to decisions between RYGB and VSG so that the analyses could account for these differences.23
METHODS
Anonymized data that support the findings of this study may be made available in the following conditions: (1) agreement to collaborate with the study team on all publications, (2) provision of external funding for the collaboration, (3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and (4) agreement to abide by the terms outlined in all data use agreements. This study was approved by the healthcare system institutional review board with a waiver of informed consent.
Identification of the Study Population
The ENGAGE CVD study design and methods have been previously described in detail.23 Briefly, we used health plan data to identify adult members of a large integrated healthcare system with ~4.7 million members in the Southern California region of the U.S., who underwent VSG or RYGB between 2009 and 2016 (N=22,095). Eligibility for weight loss surgery within the healthcare system is based upon national recommendations and includes having a BMI ≥40 kg/m2 or a BMI of 35–39 kg/m2 with at least one major obesity-related comorbid condition such as type 2 diabetes (T2DM), sleep apnea or heart disease.24 Only adults ≥18 years have coverage for bariatric surgery within the healthcare system. During the study period, bariatric operations were performed by 23 surgeons across 9 surgical practices, some internal to the organization (n = 4) and others not (n = 5). Long-term care was solely the responsibility of the healthcare system while patients were members of the health plan.
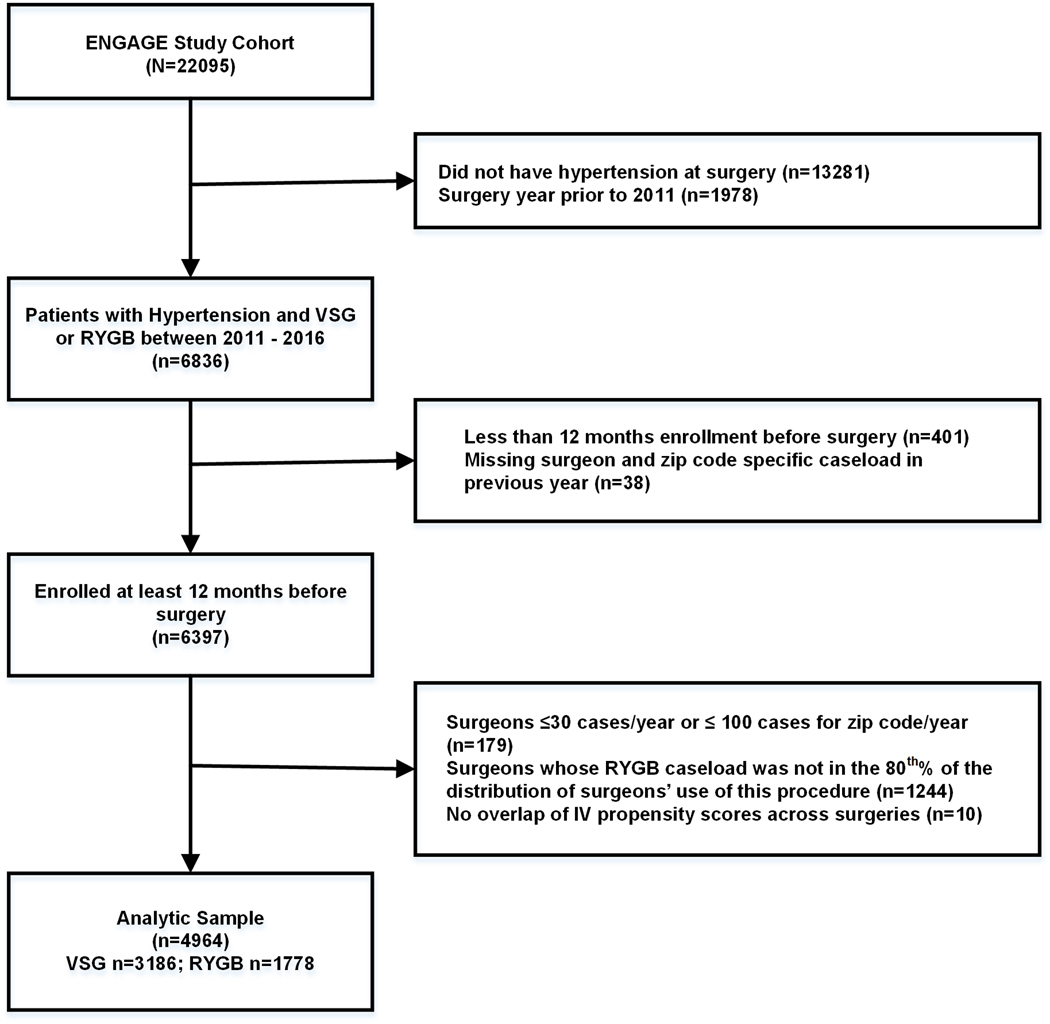
Figure 1 shows the derivation of the analytic sample. Beginning with the larger cohort (N=22,095), we restricted our analysis to patients with hypertension at surgery and to operations performed after 2010 when all surgeons were approved to perform VSG (a necessary condition of the Instrumental Variable [IV] analysis). From these 6,836 patients, we excluded patients who were missing data on surgeon and zip code-specific caseload in the previous year (n=38; a necessary condition of the IV analysis) and had <1 year of follow-up (n=401).
Figure 1.
Flow diagram for selection of patients with hypertension in the ENGAGE CVD cohort study.
For the IV analyses we also excluded: (1) patients for whom their surgeon did not have at least 30 cases and/or the surgeon’s own practice 3-digit zip code did not have at least 100 cases in the year before the surgery (n=179), (2) patients whose surgeons’ caseload for RYGB in the last year was in the bottom or top 10th percentile of the distribution of surgeons’ use of this operation (n=1,244), and (3) patients with the same IV-based propensity score to receive RYGB but who did not have a match receiving VSG (n=10). The rationale for these IV-based exclusions is provided in the Online Supplement. The final analytic sample included 4,964 patients (n=3,186 VSG and n=1,778 RYGB).
Patient Demographic and Clinical Characteristics
Data for the study were obtained from patient electronic medical records and billing claims for outside services. Baseline information was extracted in the 12 to 24 months before the date of surgery and included: (1) patient demographics (self-reported date of birth, gender, race/ethnicity, zip code of primary residence), (2) smoking status (never, current, former), (3) vital signs from outpatient visits (height, weight, BP), (4) prescription medications (antihypertensive medications, insulin, oral hypoglycemic agents, statins), (5) aspirin and nonsteroidal anti-inflammatory (NSAID) use, (6) comorbid conditions (T2DM, gastroesophageal reflux disease [GERD], gastritis duodenitis, dyspepsia, hiatal hernia, sleep apnea, chronic kidney disease [CKD], mental illness), (7) healthcare utilization (outpatient and emergency department visits, hospitalizations), and (8) bariatric operation type, location, and surgeon. The Elixhauser Comorbidity Index25 was calculated using diagnoses codes.
Outcome Measures
Hypertension was defined as:26, 27 (1) the two most recent and consecutive BP measurements on separate days, at least 1 week apart, in an outpatient/non-urgent care or non-surgical setting prior to surgery were elevated (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg) or (2) antihypertensive medication use at the time of surgery plus 1 outpatient encounter with hypertension diagnosed (International Classification of Diseases, Ninth Edition or Tenth Edition diagnostic codes: ICD-9: 401.x – 405.x or ICD-10: I10-I15) within 1 year of the medication dispense. A patient was considered on antihypertensive medication at the time of surgery if the medication sold date + (days supply × 1.25) included the surgery date. This formula allowed for patients who did not take their medications as instructed.28 We used the BP criteria of 140/90 mm Hg to reflect the clinical practice guidelines in the target healthcare system. Furthermore, the study period was primarily before the American College of Cardiology/American Heart Association (ACC/AHA) hypertension guideline in 20174 that lowered the threshold to 130/80 mm Hg.
Hypertension remission and relapse were assessed in each year of follow-up beginning 30 days after surgery and up to 5 years post-surgery until December 31, 2018. Remission and relapse criteria were created from previous research in this area27 as well as guidelines for outcomes research in bariatric surgery created by the American Society for Metabolic and Bariatric Surgery.26 Remission of hypertension was defined as meeting all three of the following criteria: (1) two systolic BPs <140 mm Hg at least 1 week apart in an outpatient/non-urgent care setting, (2) two diastolic BPs <90 mm Hg at least 1 week apart in an outpatient /non-urgent care setting, and (3) no evidence of antihypertensive medications that overlapped the dates of these BP measures. The date of remission was defined as the date when the patient met the second BP criteria. Relapse was defined as follows for those patients who experienced remission: (1) any dispense for antihypertensive medication regardless of BP measures, or (2) 2 elevated BP measurements (systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg) at least 1 week apart in an outpatient/non-urgent care setting. We also examined mean systolic and diastolic BP at the time of surgery and in each year of follow-up as well as weight loss (lbs) and percent total weight loss (%TWL) calculated as ((weight at surgery – weight at follow-up)/weight at surgery)*100 in each year of follow-up.
Statistical Analysis
We used a local Instrumental Variable (LIV) approach to balance measured and unmeasured confounders and covariates across VSG and RYGB operations.29–34 To choose the confounders and covariates we used previous literature in this area13, 35–40 as well as extensive collaboration with an advisory board of bariatric surgeons.23 Details about the LIV methods are provided in the Online Supplement. A brief overview of the methods is described below. The confounders and covariates chosen as a result of this process are shown in Table 1.
Table 1.
Descriptive statistics for patients with hypertension in the ENGAGE CVD cohort study. Unadjusted differences between sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) are presented as well as findings after adjustment using low (< Median) and high (≥ Median) levels of the Instrumental Variable (IV).
| Characteristics | VSG n=3,186 | RYGB n=1,778 | p-value | < IV Median n=2,491 | ≥ IV Median n=2,473 | p-value* | ||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) age, years | 50.7 | 10.1 | 50.9 | 9.7 | .65 | 50.5 | 51.1 | .10 |
| Women | 2,427 | 76.1% | 1,281 | 72.0% | <.001 | 74.9% | 74.5% | .79 |
| Non-Hispanic White | 1,212 | 38.0% | 701 | 39.4% | .336 | 37.8% | 39.2% | .34 |
| Non-Hispanic Black | 835 | 26.2% | 337 | 19.0% | <.001 | 24.3% | 22.9% | .07 |
| Hispanic | 1,039 | 32.6% | 664 | 37.3% | <.001 | 34.0% | 34.6% | .67 |
| Ever Smoker | 1,174 | 36.8% | 650 | 36.6% | .839 | 37.7% | 35.7% | .21 |
| Mean (SD) BMI, kg/m2 | 43.8 | 6.8 | 43.5 | 6.9 | .20 | 43.6 | 43.8 | .39 |
| Mean (SD) Systolic BP, mm Hg | 131.1 | 14.1 | 130.0 | 14.1 | .009 | 131.0 | 130.4 | .15 |
| Mean (SD) Diastolic BP, mm Hg | 76.3 | 10.2 | 74.8 | 10.1 | <.001 | 76.2 | 75.2 | .003 |
| Mean (SD) Elixhauser score | 2.85 | 9.8 | 4.10 | 10.3 | <.001 | 3.76 | 3.77 | .42 |
| Gastro-esophageal reflux disease † | 1,031 | 32.4% | 684 | 38.5% | <.001 | 33.9% | 35.1% | .46 |
| Gastritis or duodenitis † | 550 | 17.3% | 87 | 4.9% | <.001 | 14.6% | 11.0% | .001 |
| Dyspepsia † | 111 | 3.5% | 69 | 3.9% | .474 | 3.2% | 3.9% | .48 |
| Hiatal hernia | 118 | 3.7% | 78 | 4.4% | .236 | 4.0% | 3.8% | .83 |
| Sleep Apnea | 564 | 17.7% | 352 | 19.8% | .068 | 18.8% | 18.1% | .54 |
| Type 2 diabetes mellitus | 1153 | 36.2% | 1,196 | 67.3% | <.001 | 47.4% | 47.2% | .92 |
| Chronic kidney disease | 571 | 17.9% | 376 | 21.1% | .006 | 19.1% | 19.0% | .94 |
| Severe mental illness | 146 | 4.6% | 100 | 5.6% | .105 | 4.7% | 5.1% | .64 |
| Severe depression and/or anxiety | 195 | 6.1% | 125 | 7.0% | .211 | 6.5% | 6.4% | .98 |
| Mild-to-moderate depression and/or anxiety | 1,467 | 46.0% | 801 | 45.1% | .50 | 45.7% | 45.6% | .92 |
| BMI ≥ 50 kg/m2 | 531 | 16.7% | 281 | 15.8% | .431 | 16.0% | 16.7% | .60 |
| Weight change 12 months before surgery | −18.9 | 15.1 | −18.0 | 14.7 | .048 | −19.0 | −18.1 | .05 |
| Mean (SD) scheduled visit attendance rate ‡ | 0.76 | 0.12 | 0.76 | 0.13 | .34 | 0.76 | 0.76 | .71 |
| Mean (SD) number of inpatient days ‡ | 0.10 | 0.40 | 0.08 | 0.35 | .132 | 0.086 | 0.082 | .74 |
| Mean (SD) number of ED visits ‡ | 0.35 | 0.83 | 0.37 | 0.97 | .469 | 0.38 | 0.36 | .54 |
| Aspirin use ‡ | 882 | 27.7% | 719 | 40.4% | <.001 | 33.0% | 31.5% | .31 |
| Aspirin use § | 595 | 18.7% | 487 | 27.4% | <.001 | 24.2% | 19.4% | <.001 |
| NSAID use ‡ | 1,558 | 48.9% | 782 | 44.0% | .001 | 47.3% | 46.8% | .75 |
| NSAID use § | 564 | 17.7% | 273 | 15.4% | .034 | 16.4% | 17.3% | .46 |
SD = standard deviation; BMI = body mass index; BP = blood pressure; NSAID = nonsteroidal anti-inflammatory drug; ED = emergency department; Values are No., % unless otherwise indicated; Variables are at the time of surgery unless otherwise indicated;
After controlling for year of surgery and 3-digit zip code indicators, zip code-level surgery volume, and surgeon-specific caseload in previous year;
In the 2 years before surgery;
In the 12 months before surgery;
In the 3 months before surgery
Instrumental variable selection and testing
IV methods are designed to mimic the conditions of random assignment of treatments occurring in real-world clinical care settings. An IV must be correlated with the exposure (bariatric surgery) but not associated with the outcome (hypertension remission/relapse) except through its correlation with the exposure. The IV chosen for the current study was the rate of RYGB per surgeon during the 12 months prior to a patient’s bariatric operation calculated as (# RYGB per surgeon/# RYGB and VSG operations per surgeon = RYGBrate). Validation of the IV was done by comparing imbalances in patient-level confounders and covariates between operations across the median of the IV. A valid IV would reduce any imbalances. Based upon the validation findings, we restricted our analytical sample to patients treated by surgeons in the central 80th percentile of the distribution (Figure 1 and Table 1) due to observed covariate imbalance across most levels of the IV.41–43 The Online Supplement provides detailed analyses that support this choice.
Local instrumental variable approach
The LIV approach was carried out in two steps. In the first step, the choice of RYGB versus VSG was modelled as a function of the RYGBrate IV, after controlling for the following potential confounders: all baseline covariates (Table 1), 3-digit zip-code, year of surgery, and the denominator of the RYGBrate using a probit regression model. A propensity for RYGB was estimated from this first-step regression.
In the second step, we modeled the outcome as a three-level ordinal variable (original hypertension status [remaining hypertensive throughout the follow-up period], remission, or relapse) with two separate logit models using generalized estimating equations with a logit link function and exchangeable correlation structure.44, 45 The first model represented the log-odds of remaining hypertensive or experiencing remission versus experiencing relapse. The second logit model represented the log-odds of remaining hypertensive versus experiencing relapse or remission. In addition to the confounders from the first step, clinical factors (see Table 1) could interact with the propensity of RYGB selection from the first step and were tested for an appropriate polynomial of the propensity score itself. The partial derivative of the predicted probability of remaining hypertensive, remitting, or relapsing with respect to the propensity score was used as an estimator of the marginal treatment effects.32
Heterogeneity of treatment effects (HTE)
These effects were then aggregated to form the population average and subgroup-specific average treatment effects that were used for the heterogeneity of treatment (HTE) analyses.30, 46–48 Sub-groups tested for these effects included age (< 65 years and ≥ 65 years), BMI (< 50 kg/m2 and ≥ 50 kg/m2), and race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic). All standard errors were calculated using non-parametric bootstrapping and allowed for clustering of individual outcomes over time.
Sensitivity analyses
To compare our findings to other literature in this area, we performed three sensitivity analyses: (1) a standard multivariate regression analysis, (2) a standard inverse-probability weighted propensity score analysis, and (3) a standard IV analysis using a two-stage residual inclusion approach.49, 50 All analyses were performed in Stata (StataCorp), version 15.1.
RESULTS
Study Population
Table 1 presents the results for the comparison of patients who had RYGB (n=3,186) and VSG (n=1,778) without IV adjustment. Compared to patients who had a VSG operation, patients who had RYGB were more likely to be Hispanic, have a higher comorbidity burden, have GERD, T2DM, and CKD, and to use aspirin before surgery. Compared to patients who had RYGB, patients who had a VSG operation were more likely to be women, non-Hispanic Black, have higher mean systolic and mean diastolic BP, have gastritis or duodenitis, and to use NSAIDs before surgery.
The IV (RYGBrate) was significantly predictive of having a RYGB operation (F-statistic: 272, p<0.001) indicating this was a valid proxy for the assignment of patients to bariatric operation. When confounders and covariates were stratified by the IV median, the balance improved for most covariates (Table 1) with the exception of mean diastolic BP (p=.003), rates of gastritis or duodenitis (p=.001), and aspirin use (p<.001). These variables were included in all outcome analyses to account for these differences.
Hypertension Remission and Relapse
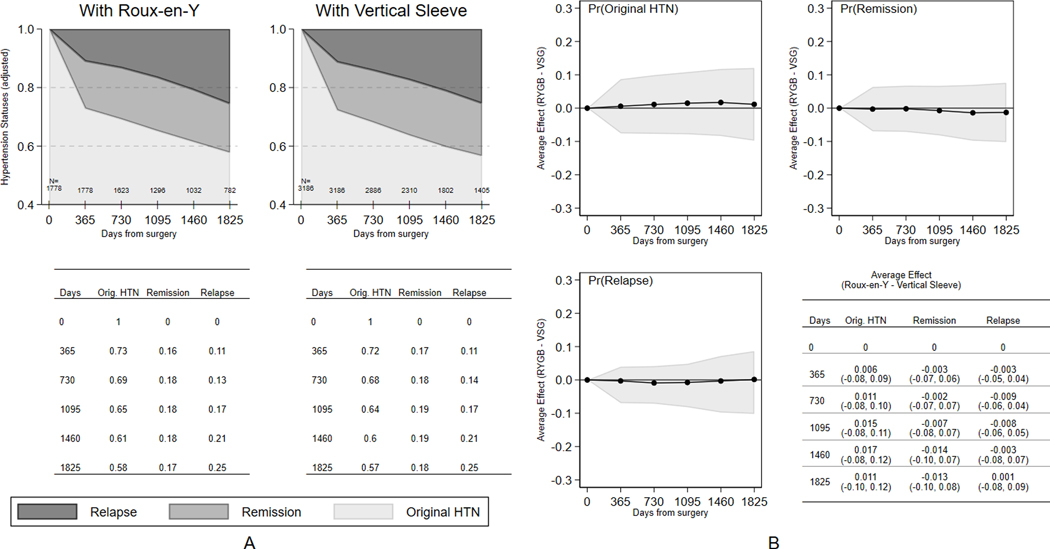
Five-year retention rates overall were 77.8% (76.4% RYGB and 78.7% VSG). Most patients were lost to follow-up because of loss of membership in the health plan (86.4%) with 12.0% loss due to death. Only 1.6% were missing data in the follow-up period. There were no differences between bariatric operations in loss to followup (Figure S7, Online Supplement). Figure 2 shows the hypertension status in each year of follow-up by operation. There were no statistically significant differences between patients having VSG or RYGB in the likelihood of hypertension remission or relapse throughout the 5-year period. At 12 months, 27% of RYGB and 28% of VSG patients achieved remission with an additional 1%−2% of patients achieving hypertension remission each year. Without accounting for relapse, 42% of patients having RYGB and 43% having VSG had experienced hypertension remission at some point within 5 years after surgery. After accounting for relapse, only 17% of patients having RYGB and 18% having VSG remained in remission 5 years after surgery. Antihypertensive medication was restarted in 64.5% of patients who experienced hypertension remission.
Figure 2.
Hypertension status (original hypertension state [remained hypertensive], remission, relapse) for patients after having Sleeve Gastrectomy (VSG) or Roux-en-Y Gastric Bypass (RYGB). Data are presented at baseline and in each year of follow-up for: (a) absolute rates and (b) difference in absolute rates between VSG and RYGB after adjustment using a local instrumental variable (LIV) approach.
Findings for the sensitivity analyses using different statistical methods to address comparative effectiveness are shown in Figure S8 in the Online Supplement. Findings were similar to the main results using LIV methods; there were no differences between RYGB and VSG on hypertension remission and relapse.
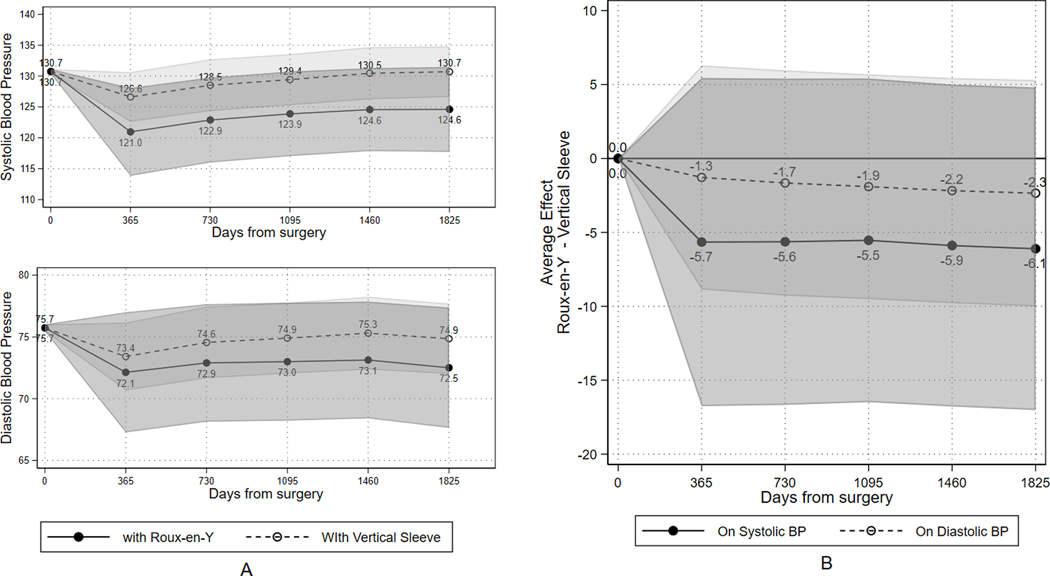
Blood Pressure and Weight Loss Over Time
Figure 3 shows mean systolic and diastolic BP in each year of follow-up by operation. Although mean BP appeared higher in each year of follow-up for patients having VSG when compared to those having RYGB, these differences were not statistically significant. The descriptive patterns for weight loss are shown in Figures S13–S15 and Tables S5–S7 in the Online Supplement. In general, %TWL was highest at 12 months following surgery (28.0% TWL for RYGB and 23.3% TWL for VSG) and patients gradually regained some weight throughout the follow-up period resulting in 21.7% TWL (95% CI: 22.4%, 21.0%) for patients having RYGB and 16.6% (17.1%,16.0%) having VSG at the end of 5 years.
Figure 3.
Mean systolic and diastolic blood pressure (BP) after adjustment using a local instrumental variable (LIV) approach. Data are presented at baseline and in each year of follow-up for: (a) absolute mean BP for Sleeve Gastrectomy (VSG) and Roux-en-Y Gastric Bypass (RYGB) and (b) difference in mean BP between RYGB and VSG.
Heterogeneity of Treatment Effects
There were no statistical differences between VSG and RYGB for hypertension remission and relapse across subgroups of age, BMI at time of surgery, and race/ethnicity (Figure S9, Online Supplement). The findings for BP were also consistent across subgroups of age, BMI, and race/ethnicity (Figure S10, Online Supplement).
DISCUSSION
In one of the largest contemporary samples in the literature of bariatric patients undergoing VSG, we found a similar rate of hypertension remission for VSG (43%) compared to RYGB (42%) and similar BP measurements after 5 years of follow-up. We also found that the majority (58%−60%) of patients who achieved hypertension remission, regardless of bariatric operation, experienced relapse of their hypertension by 5 years, such that only 17% of patients having RYGB and 18% of those having VSG remained in remission long-term.
While there is strong evidence that bariatric surgery is the most effective treatment for severe obesity and metabolic disorders such as T2DM,51 there is limited evidence on bariatric surgery for the management hypertension. As such, current hypertension guidelines recommend considering bariatric surgical procedures only in those who cannot meet weight loss goals with nonpharmacological interventions.4 Recent clinical trials, which were primarily designed to evaluate the effects of bariatric surgery on T2DM,52–54 have assessed BP following bariatric surgery. For example the GATEWAY (Gastric Bypass to Treat Obese Patients With Steady Hypertension) trial found that 55 after three years, 73% of RYGB patients (n=50) achieved the primary endpoint of a greater than 30% reduction in the number of antihypertensive medications with BP <140/90 mm Hg compared with 11% of patients who received medical therapy (n=50). Further, 35% of RYGB patients achieved hypertension remission without the need for continued antihypertensive medication compared with only 2% of patients who received medical therapy. However, there was no difference between the groups in maintaining BP below 140/90 mm Hg.
Few studies have compared VSG and RYGB on hypertension remission and relapse. A recent meta-analysis of 32 studies comparing 5-year VSG and RYGB hypertension remission rates found that 52% of VSG and 62% of RYGB patients experienced hypertension remission; a 26% higher rate of remission for RYGB compared to VSG.16 Similar to our study findings, when restricted to observational studies (n=2) comparing 5-year hypertension remission and BP changes between VSG and RYGB, there were no differences between operations. In a recent retrospective observational analysis of U.S. claims data for RYGB and VSG operations, where only medication discontinuation and restart were used to define hypertension remission and relapse, there also were no differences between operations at 4 years following surgery.56 This study found higher rates of durable remission than our study, however, this difference could be due to the fact that they did not use BP values in the determination of remission and relapse. In our study, over 35% of patients experienced relapse due to BP values alone (no medication restart). Finally, our findings are supported by the Swedish Obese Subject (SOS) study.20 Although there were no VSG operations, most of the patients in the SOS study restarted antihypertension medications 10 years following surgery.20
Our study had several limitations, most notably that it was a retrospective observational design with non-random assignment to operation; however, we used the LIV approach to address this. To mitigate confounding and account for (1) decisions surgeons made when using certain operations for particular patients, as well as (2) patients’ decisions, we worked closely with our advisory board of bariatric surgeons and our patient and provider co-investigators to identify factors critical to why patients would choose/undergo VSG or RYGB.23 Although the rigorous LIV approach was designed to mimic the effect of random assignment in randomized trials, it also resulted in a loss of 1,244 patients who were different than those upon which the findings were based (Table S2, Online Supplement), potentially limiting the generalizability of the LIV results. To address this concern, we conducted a traditional propensity score analysis including these 1,244 patients and findings were similar (Figure S11, Online Supplement).
Another limitation of our study was that we used older guidelines to define hypertension and its remission and relapse since this reflected clinical practice at the time of the study (2009 – 2016). If we had used the newer guidelines (BP threshold of 130/80 mm Hg) for all patients, it is likely we would have found lower rates of remission after bariatric surgery. In addition, although patterns of weight loss (Figures S13–S15, Online Supplement) generally followed the changes in hypertension remission and relapse over time (Figure 2), we did not formally test if weight loss mediated these changes in hypertension. We also did not test whether changes in comorbidities such as sleep apnea were responsible for hypertension remission and relapse. These mediational analyses require different statistical models57, 58 than those used in the present study for comparative effectiveness and future research should explore the mechanisms responsible for changes in hypertension remission and relapse following bariatric surgery.
Despite these limitations, our study has notable strengths. To our knowledge, the ENGAGE CVD study is one of the largest and most diverse (over 50% of the cohort is non-Hispanic Black and Hispanic) observational cohort studies in a real-world clinical setting on the comparative effectiveness of VSG and RYGB for hypertension remission and relapse with 5 years of follow-up. We used rigorous IV methods to overcome selection bias in choice of surgical operation to mitigate the effect of both observed and unobserved confounding on our results. Our findings were consistent in sensitivity analyses using different analytic methods and were supported by the limited observational studies in this area.16, 56, 59
PERSPECTIVES
Our study demonstrated no difference between VSG and RYGB for hypertension remission and relapse. Both operations resulted in substantial proportions of patients achieving hypertension remission; however, most patients experienced a relapse of their hypertension in the 5-year follow-up period. The return of patients to normotensive status would have substantial impact on mortality from CVD,60 however, based upon our findings and those of others,20, 56 bariatric surgery alone does not guarantee a patient will achieve and maintain remission of hypertension.
Bariatric surgery is a highly effective treatment for severe obesity and cardiovascular conditions. Much like other surgical treatments for cardiovascular conditions, such as coronary artery disease, the surgery itself is only a tool to assist with the large, sustained weight loss and reset of the metabolic system that is necessary to resolve hypertension. Lifestyle changes such as diet and exercise, similar to that needed after coronary bypass, are necessary to maintain the changes from bariatric surgery. Based on our findings and the recent findings from the GATEWAY trial55, demonstrating that bariatric surgery is an effective strategy for reducing the number of antihypertensive while maintaining BP control, we would not recommend restarting or maintenance of hypertensive medications following surgery, but a systematic monitoring of patient vitals and lifetime counseling for how to maintain the changes seen with surgery.
Future clinical trials and observational comparative effectiveness studies are warranted to understand why patients experience hypertension relapse after bariatric surgery. Such studies could explore the relationship between BP, weight change, comorbidity resolution, and other patient-level characteristics and hypertension remission and relapse. The use of different BP cut points should be examined as well to provide evidence for surgical effectiveness with the newest hypertension treatment and control guidelines.4 Cost-effectiveness and long-term studies that compare RYGB and SG to conventional medical treatment for hypertension remission and relapse, as well as patient-centered outcomes such as quality of life are also warranted. Finally, studies that identify interventions to help patients achieve and maintain hypertension remission after bariatric surgery are needed.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
Long-term hypertension remission and relapse rates for the two most common bariatric operations in the world.
What is Relevant?
Long-term hypertension remission rates after bariatric surgery do not differ by bariatric operation and may be much lower than previously reported because 58%−60% relapse after remission.
Summary
Future studies are warranted to understand why patients experience hypertension relapse after bariatric surgery, such as the relationship between BP and weight regain, and to identify interventions to help patients achieve and maintain hypertension remission after bariatric surgery.
ACKNOWLEDGEMENTS
We would like to acknowledge the bariatric patients who contributed data for this study without whom the work would not be possible. In addition, we would like to thank Mr. Mark Hinson, from Graphics of Distinction, who designed our graphic abstract.
SOURCE OF FUNDING
Support for this study was provided by the National Heart, Lung, and Blood Institute (5R01HL130462). The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
CONFLICTS OF INTEREST/DISCLOSURES
KR: Received funding from the National Institutes of Health (NIH) for this work and funding for other research and research support through her institution from Merck & Co., Vital Strategies, Novartis, and CSL Behring, LLC unrelated to the current manuscript.
AB: Received consulting fees through Salutis Consulting LLC unrelated to the current manuscript.
KJC: Received funding from the NIH for this work and other research.
DEA: Received funding from the NIH for this work; and NIH and PCORI funding for other research. Received support for personal travel to conferences from the World Congress for Interventional Therapy for Diabetes and the IFSO Latin America Chapter.
Footnotes
All other authors report no conflicts of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA. 2014;311:806–814: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-Induced Hypertension: Interaction of Neurohumoral and Renal Mechanisms. Circ Res. 2015;116:991–1006: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harsha DW, Bray GA. Weight Loss and Blood Pressure Control (Pro). Hypertension. 2008;51:1420–1425; discussion 1425: 10.1161/HYPERTENSIONAHA.107.094011. [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 Acc/Aha/Aapa/Abc/Acpm/Ags/Apha/Ash/Aspc/Nma/Pcna Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 5.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-Related Hypertension: Pathogenesis, Cardiovascular Risk, and Treatment: A Position Paper of the Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15:14–33: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman JP, Stampfer MJ, Curhan GC. Diet and Lifestyle Risk Factors Associated with Incident Hypertension in Women. JAMA. 2009;302:401–411: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrison RJ, Kannel WB, Stokes J 3rd, Castelli WP. Incidence and Precursors of Hypertension in Young Adults: The Framingham Offspring Study. Prev Med. 1987;16:235–251: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 8.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of Weight Reduction on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Hypertension. 2003;42:878–884: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 9.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, et al. Long-Term Weight Loss and Changes in Blood Pressure: Results of the Trials of Hypertension Prevention, Phase Ii. Ann Intern Med. 2001;134:1–11: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 10.Aucott L, Rothnie H, McIntyre L, Thapa M, Waweru C, Gray D. Long-Term Weight Loss from Lifestyle Intervention Benefits Blood Pressure?: A Systematic Review. Hypertension. 2009;54:756–762: 10.1161/HYPERTENSIONAHA.109.135178. [DOI] [PubMed] [Google Scholar]
- 11.Straznicky N, Grassi G, Esler M, Lambert G, Dixon J, Lambert E, Jordan J, Schlaich M, European Society of Hypertension Working Group on O, Australian NZOS. European Society of Hypertension Working Group on Obesity Antihypertensive Effects of Weight Loss: Myth or Reality? J Hypertens. 2010;28:637–643: 10.1097/HJH.0b013e32833778e1. [DOI] [PubMed] [Google Scholar]
- 12.Loveman E, Frampton GK, Shepherd J, Picot J, Cooper K, Bryant J, Welch K, Clegg A. The Clinical Effectiveness and Cost-Effectiveness of Long-Term Weight Management Schemes for Adults: A Systematic Review. Health Technol Assess. 2011;15:1–182: 10.3310/hta15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arterburn D, Wellman R, Emiliano A, Smith SR, Odegaard AO, Murali S, Williams N, Coleman KJ, Courcoulas A, Coley RY, et al. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A Pcornet Cohort Study. Ann Intern Med. 2018;169:741–750: 10.7326/M17-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arterburn DE, Johnson E, Coleman KJ, Herrinton LJ, Courcoulas AP, Fisher D, Li RA, Theis MK, Liu L, Fraser JR, Haneuse S. Weight Outcomes of Sleeve Gastrectomy and Gastric Bypass Compared to Nonsurgical Treatment. Ann Surg. 2020: 10.1097/SLA.0000000000003826. [DOI] [PubMed] [Google Scholar]
- 15.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric Surgery and Cardiovascular Outcomes: A Systematic Review. Heart. 2012;98:1763–1777: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 16.Climent E, Goday A, Pedro-Botet J, Sola I, Oliveras A, Ramon JM, Flores-Le Roux JA, Checa MA, Benaiges D. Laparoscopic Roux-En-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy for 5-Year Hypertension Remission in Obese Patients: A Systematic Review and Meta-Analysis. J Hypertens. 2020;38:185–195: 10.1097/HJH.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 17.Owen JG, Yazdi F, Reisin E. Bariatric Surgery and Hypertension. Am J Hypertens. 2017;31:11–17: 10.1093/ajh/hpx112. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm SM, Young J, Kale-Pradhan PB. Effect of Bariatric Surgery on Hypertension: A Meta-Analysis. Ann Pharmacother. 2014;48:674–682: 10.1177/1060028014529260. [DOI] [PubMed] [Google Scholar]
- 19.Heneghan HM, Meron-Eldar S, Brethauer SA, Schauer PR, Young JB. Effect of Bariatric Surgery on Cardiovascular Risk Profile. Am J Cardiol. 2011;108:1499–1507: 10.1016/j.amjcard.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, et al. Bariatric Surgery and Long-Term Cardiovascular Events. JAMA. 2012;307:56–65: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 21.Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in Utilization of Bariatric Surgery in the United States from 1993 to 2016. Ann Surg. 2020;271:201–209: 10.1097/SLA.0000000000003554. [DOI] [PubMed] [Google Scholar]
- 22.Bosco JL, Silliman RA, Thwin SS, Geiger AM, Buist DS, Prout MN, Yood MU, Haque R, Wei F, Lash TL. A Most Stubborn Bias: No Adjustment Method Fully Resolves Confounding by Indication in Observational Studies. J Clin Epidemiol. 2010;63:64–74: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman KJ, Fischer H, Arterburn DE, Barthold D, Barton LJ, Basu A, Courcoulas A, Crawford CL, Fedorka P, Kim B, et al. Effectiveness of Gastric Bypass Versus Gastric Sleeve for Cardiovascular Disease: Protocol and Baseline Results for a Comparative Effectiveness Study. JMIR Res Protoc. 2020;9:e14936: 10.2196/14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Potential Candidates for Bariatric Surgery. https://www.niddk.nih.gov/health-information/weight-management/bariatric-surgery/potential-candidatesAccess. April 30, 2020
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Med Care. 1998;36:8–27: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, Ballem N, Kligman M, Kothari S. Standardized Outcomes Reporting in Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2015;11:489–506: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Selby JV, Peng T, Karter AJ, Alexander M, Sidney S, Lian J, Arnold A, Pettitt D. High Rates of Co-Occurrence of Hypertension, Elevated Low-Density Lipoprotein Cholesterol, and Diabetes Mellitus in a Large Managed Care Population. Am J Manag Care. 2004;10:163–170 [PubMed] [Google Scholar]
- 28.Coleman KJ, Huang YC, Koebnick C, Reynolds K, Xiang AH, Black MH, Alskaf S. Metabolic Syndrome Is Less Likely to Resolve in Hispanics and Non-Hispanic Blacks after Bariatric Surgery. Ann Surg. 2014;259:279–285: 10.1097/SLA.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 29.Angrist J, Imbens G, Rubin D. Identification of Causal Effects Using Instrumental Variables. J Amer Stat Assoc. 1996;91:444–455 [Google Scholar]
- 30.Basu A. Estimating Person-Centered Treatment (Pet) Effects Using Instrumental Variables: An Application to Evaluating Prostate Cancer Treatments. Journal of Applied Econometrics. 2014;29:671–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu A, Gore JL. Are Elderly Patients with Clinically Localized Prostate Cancer Overtreated? Exploring Heterogeneity in Survival Effects. Med Care. 2015;53:79–86: 10.1097/MLR.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu A, Heckman JJ, Navarro-Lozano S, Urzua S. Use of Instrumental Variables in the Presence of Heterogeneity and Self-Selection: An Application to Treatments of Breast Cancer Patients. Health Econ. 2007;16:1133–1157: 10.1002/hec.1291. [DOI] [PubMed] [Google Scholar]
- 33.Grieve R, O’Neill S, Basu A, Keele L, Rowan KM, Harris S. Analysis of Benefit of Intensive Care Unit Transfer for Deteriorating Ward Patients: A Patient-Centered Approach to Clinical Evaluation. JAMA Netw Open. 2019;2:e187704: 10.1001/jamanetworkopen.2018.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kent DM, Steyerberg E, van Klaveren D. Personalized Evidence Based Medicine: Predictive Approaches to Heterogeneous Treatment Effects. BMJ. 2018;363:k4245: 10.1136/bmj.k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arterburn D, Bogart A, Coleman KJ, Haneuse S, Selby JV, Sherwood NE, Sidney S, Theis MK, Campos GM, McCulloch D, PJ OC. Comparative Effectiveness of Bariatric Surgery Vs. Nonsurgical Treatment of Type 2 Diabetes among Severely Obese Adults. Obes Res Clin Pract. 2013;7:e258–268: 10.1016/j.orcp.2012.08.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arterburn D, Schauer DP, Wise RE, Gersin KS, Fischer DR, Selwyn CA Jr., Erisman A, Tsevat J. Change in Predicted 10-Year Cardiovascular Risk Following Laparoscopic Roux-En-Y Gastric Bypass Surgery. Obes Surg. 2009;19:184–189: 10.1007/s11695-008-9534-7. [DOI] [PubMed] [Google Scholar]
- 37.Coleman KJ, Haneuse S, Johnson E, Bogart A, Fisher D, O’Connor PJ, Sherwood NE, Sidney S, Theis MK, Anau J, Schroeder EB, O’Brien R, Arterburn D. Long-Term Microvascular Disease Outcomes in Patients with Type 2 Diabetes after Bariatric Surgery: Evidence for the Legacy Effect of Surgery. Diabetes Care. 2016;39:1400–1407: 10.2337/dc16-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA, DeLany JP, Lang W, Jakicic JM. Three-Year Outcomes of Bariatric Surgery Vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015;150:931–940: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O’Connor PJ, O’Brien R, Bogart A, Theis MK, Anau J, Schroeder EB, Sidney S. Association between Bariatric Surgery and Macrovascular Disease Outcomes in Patients with Type 2 Diabetes and Severe Obesity. JAMA. 2018;320:1570–1582: 10.1001/jama.2018.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien R, Johnson E, Haneuse S, Coleman KJ, O’Connor PJ, Fisher DP, Sidney S, Bogart A, Theis MK, Anau J, Schroeder EB, Arterburn D. Microvascular Outcomes in Patients with Diabetes after Bariatric Surgery Versus Usual Care: A Matched Cohort Study. Ann Intern Med. 2018;169:300–310: 10.7326/M17-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu A, Jones AM, Dias PR. Heterogeneity in the Impact of Type of Schooling on Adult Health and Lifestyle. J Health Econ. 2018;57:1–14: 10.1016/j.jhealeco.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baiocchi M, Small DS, Lorsh S, Rosenbaum PR. Building a Stronger Instument in an Observational Study of Perinatal Care for Premature Infants. J Am Stat Assoc. 2010;105:1285–1296 [Google Scholar]
- 43.Keele L, Harris S, Pimentel SD, Grieve R. Stronger Instruments and Refined Covariate Balance in an Observational Study of the Effectiveness of Prompt Admission to Intensive Care Units. J Royal Stat Soc. 2018 [Google Scholar]
- 44.Basu A, Manca A. Regression Estimators for Generic Health-Related Quality of Life and Quality-Adjusted Life Years. Med Decis Making. 2012;32:56–69: 10.1177/0272989X11416988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papke LE, Wooldridge JM. Panel Data Methods for Fractional Response Variables with an Application to Test Pass Rates. J Econometrics. 2008;145:121–133 [Google Scholar]
- 46.Heckman JJ, Vytlacil EJ. Local Instrumental Variables and Latent Variable Models for Identifying and Bounding Treatment Effects. Proc Natl Acad Sci U S A. 1999;96:4730–4734: 10.1073/pnas.96.8.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heckman JJ, Vytlacil EJ. Structural Equations, Treatment Effects and Econometric Policy Evaluation. Econometrica. 2005;73:669–738 [Google Scholar]
- 48.Newhouse JP, McClellan M. Econometrics in Outcomes Research: The Use of Instrumental Variables. Annu Rev Public Health. 1998;19:17–34: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 49.Basu A, Coe NB, Chapman CG. 2sls Vs 2sri: Appropriate Methods for Rare Outcomes and/or Rare Exposures. Health Econ. 2018;27:937–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terza JV, Basu A, Rathouz PJ. Two-Stage Residual Inclusion Estimation: Addressing Endogeneity in Health Econometric Modeling. J Health Econ. 2008;27:531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA. 2020;324:879–887: 10.1001/jama.2020.12567. [DOI] [PubMed] [Google Scholar]
- 52.Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of Laparoscopic Roux-En-Y Gastric Bypass Surgery on Type 2 Diabetes Mellitus with Hypertension: A Randomized Controlled Trial. Diabetes Res Clin Pract. 2013;101:50–56: 10.1016/j.diabres.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-Metabolic Surgery Versus Conventional Medical Treatment in Obese Patients with Type 2 Diabetes: 5 Year Follow-up of an Open-Label, Single-Centre, Randomised Controlled Trial. Lancet. 2015;386:964–973: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 54.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR, Investigators S. Bariatric Surgery Versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641–651: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiavon CA, Bhatt DL, Ikeoka D, Santucci EV, Santos RN, Damiani LP, Oliveira JD, Machado RHV, Halpern H, Monteiro FLJ, et al. Three-Year Outcomes of Bariatric Surgery in Patients with Obesity and Hypertension : A Randomized Clinical Trial. Ann Intern Med. 2020;173:685–693: 10.7326/M19-3781. [DOI] [PubMed] [Google Scholar]
- 56.Nudotor RD, Canner JK, Haut ER, Prokopowicz GP, Steele KE. Comparing Remission and Recurrence of Hypertension after Bariatric Surgery: Vertical Sleeve Gastrectomy Versus Roux-En-Y Gastric Bypass. Surg Obes Relat Dis. 2020: 10.1016/j.soard.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 57.Naimi AI, Kaufman JS, MacLehose RF. Mediation Misgivings: Ambiguous Clinical and Public Health Interpretations of Natural Direct and Indirect Effects. Int J Epidemiol. 2014;43:1656–1661: 10.1093/ije/dyu107. [DOI] [PubMed] [Google Scholar]
- 58.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 59.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric Surgery Versus Conventional Medical Therapy for Type 2 Diabetes. New England Journal of Medicine. 2012;366:1577–1585:doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 60.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. Dash Collaborative Research Group. N Engl J Med. 1997;336:1117–1124: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.