Abstract
Do people who have low-quality sleep tend to have more negative affect? This question is of great public interest, and many would assume the answer is “yes.” However, previous findings have been mixed, possibly due to differing measures of sleep and affect, or to a failure to separately examine negative affect reactivity and regulation. Across two studies, we assessed adults’ perceived sleep quality for at least two weeks and tested their negative affect reactivity and regulation in response to unpleasant pictures (Study 1) or painful thermal stimulation (Study 2) using both self-report and physiological measures. The relationships between perceived sleep quality, on the one hand, and negative affect reactivity and regulation, on the other, were non-significant. Furthermore, a Bayesian approach unanimously favored the null hypothesis. These results suggest that individual differences in perceived sleep quality may not predict negative affect reactivity or regulation across adult individuals.
Keywords: sleep, negative affect, affect reactivity, affect regulation, Bayesian analysis
Introduction
People have long been interested in the relationship between sleep and negative affect (Goldstein & Walker, 2014; Tempesta, Socci, De Gennaro, & Ferrara, 2018). One common intuition is that poor sleep leads to feeling bad (Baglioni, Spiegelhalder, Lombardo, & Riemann, 2010), and some recent studies seem to bear out this intuition (e.g., Ben Simon, Vsallat, Barnes, & Walker, 2020; Yoo, Gujar, Hu, Jolesz, & Walker, 2007). However, findings have been mixed (e.g., Shermohammed, Kordyban, & Somerville, 2020). One possible explanation for the mixed findings is that there are many ways to operationalize sleep (e.g., sleep quality, sleep duration, total sleep loss) and negative affect (e.g., affect reactivity, mood, stress). In addition, poor sleep has its impact by compromising the ability to successfully regulate negative affect when needed (e.g., Mauss, Troy, & LeBourgeois, 2013), which may explain why poor sleep sometimes (but not always) lead to greater negative affect. However, here too findings are mixed (e.g., Shermohammed et al., 2020). The state of the literature is thus currently unsettled, and it is not clear whether, when confronted with an unpleasant stimulus, individuals with poor sleep (1) respond with greater levels of negative affect and (2) have a diminished capacity to regulate negative affective states.
Sleep and Negative Affect Reactivity
Prior studies have examined the relationship between various subfacets of sleep and different forms of negative affect. Several studies have reported that experimental sleep loss leads to amplified negative affect. Using functional magnetic resonance imaging (fMRI), Yoo and colleagues (2007) found that after sleep deprivation, individuals had greater behavioral and amygdalar reactivity to unpleasant pictures than individuals whose sleep was not experimentally restricted. A night of total sleep deprivation also increases the threat value of faces with ambiguous expressions (Goldstein-Piekarski, Greer, Saletin, & Walker, 2015). An event-related potential (ERP) study reported that sleep-deprived participants had larger ERP-based reactivity to negative pictures than sleep-control participants (Cote, Jancsar, & Hunt, 2015). Studies using autonomic measures found heightened pupillary and cardiovascular reactivity to negative affect stimulation after sleep deprivation (Franzen, Buysse, Dahl, Thompson, & Siegle, 2009; Franzen et al., 2011). Sleep restriction (4h) was also found to increase reactivity to negative pictures compared with sleep extension (9.5h) in adolescents (Reddy, Palmer, Jackson, Farris, & Alfano, 2017). In addition, with correlational designs, some sleep diary studies found that poorer/shorter sleep was associated with more self-reported daily stressors (Vigoureux, Lee, Buxton, & Almeida, 2019; Yap, Slavish, Taylor, Bei, & Wiley, 2020). It was also found that compared to good sleepers, individuals with insomnia perceived greater impact from daily stressors (Morin, Rodrigue, & Ivers, 2003). Among shift nurses, higher levels of emotional disturbance was reported to be associated with poor sleep quality (Lee, Chen, Meg Tseng, Lee, & Huang, 2015).
Other studies have failed to replicate this effect. For example, two studies failed to find effects of sleep deprivation on experienced reactivity to negative pictures (Pilcher, Callan, & Posey, 2015; Tempesta et al., 2010). Studies using objective measures of affect reactivity also have failed to find this effect. For example, Shermohammed et al. (2020) using fMRI and Alfarra et al. (2015) using ERP both reported no effect of sleep deprivation on self-reported or neural reactivity to negative pictures. Even after five consecutive nights of sleep restriction, sleep did not influence participants’ affect reactivity to negative pictures (Tempesta, Salfi, De Gennaro, & Ferrara, 2020). Furthermore, several studies have reported the opposite effect. Sleep deprivation was found to decrease the perceived emotional level of angry faces (Van Der Helm, Gujar, & Walker, 2010) and ERP-based reactivity to unpleasant pictures (Zhang, Lau, & Hsiao, 2019b). In correlational diary studies, there are also reports that sleep quality or quantity did not predict daily negative affect reactivity (e.g., Sin, Wen, Klaiber, Buxton, & Almeida, 2020). In another study that tracked sleep and daily affect across 2 weeks in young women, neither sleep quality or sleep duration predicted negative affect (Kalmbach, Pillai, Roth, & Drake, 2014). Additionally, in a study tracking sleep and daily life across a week, it was found that negative affect and stressors were unrelated to subsequent sleep quality or duration (Sin et al., 2017).
Why might findings be mixed? One possibility is that both sleep and affect are heterogeneous constructs whose subfacets are imperfectly correlated. Given that different studies have focused on different facets of each construct, mixed findings may be inevitable. Most prior studies measured either sleep duration or sleep quality, and studies examining both these facets of sleep might help clarify some of the mixed findings. In addition, due to resource limitations, many sleep studies – such as sleep deprivation studies – are usually constrained in terms of sample size (e.g., n = 26 in Yoo et al., 2007; n = 18 in Goldstein-Piekarski et al., 2015; n = 42 in Tempesta et al., 2020), which may have contributed to the finding of a negative sleep-negative affect relationship in some studies and a null or positive relationship in others. A second possible reason for mixed findings is that poor sleep may have its primary impact on negative affect via compromised affect regulation (Palmer & Alfano, 2017). If individuals only occasionally engage in affect regulation when facing negative-affect eliciting situations, this might help to explain why sleep variation only occasionally predicts differences in negative affect between sleep-deprived and non-sleep-deprived participants. In the next section, we consider the empirical status of this idea.
Sleep and Negative Affect Regulation
Despite repeated suggestions made by various authors regarding a possible relationship between sleep and negative affect regulation in both empirical (e.g., Yoo et al., 2007; Zhang, Lau, & Hsiao, 2019a) and theoretical articles (e.g., Gruber & Cassoff, 2014; Palmer & Alfano, 2017), only a handful of studies have explicitly assessed sleep and affect regulation.
In a correlational study, Mauss and colleagues (2013) found an association between poorer sleep quality and less success in using one affect regulation strategy (reappraisal) to regulate experimentally induced sadness. A sleep-restriction study reported the first causal evidence for the detrimental impact of sleep loss on affect regulation, but the assessment of affect regulation was based on self-reports (Baum et al., 2014). More recently, one study found that sleep deprivation impaired two affect regulation strategies (reappraisal and distraction) based on ERP data (Zhang et al., 2019b). However, two other studies that examined the influence of sleep deprivation (Shermohammed et al., 2020) and sleep restriction (Reddy et al., 2017) on reappraisal did not find an effect of sleep loss based on self-report or fMRI data. In one correlational fMRI study, it was found that only one subscale of the Pittsburgh Sleep Quality Index, use of sleep medications, but no other sleep aspects including self-reported sleep quality and sleep duration, was related to activation of neural regions supporting emotion regulation (Minkel et al., 2012). Furthermore, mixed findings were reported within one single study: better self-reported sleep quality was associated with greater regulation-related frontal activation while higher sleep efficiency was associated with less activation in the same region (Klumpp et al., 2017). Thus, here too, as in the case of sleep and negative affect reactivity, findings are mixed.
Affect regulation can occur via an early disengagement from processing emotional content or a late engagement with elaborated emotional processing (Sheppes, Scheibe, Suri, & Gross, 2011; Sheppes et al., 2014). Disengagement-based regulation strategies (e.g., distraction, mindfulness) involve attention selection while engagement-based strategies (e.g., reappraisal) involve elaborating emotional information and cognitively modulating the semantic meaning of the situation. If sleep influences different forms of affect regulation differently (McCoy & Strecker, 2011), this might help to explain inconsistencies in the literature sleep and affect regulation.
The Present Research
Sleep and negative affect are both multi-faceted constructs. To gain clarity about the complex relationship between sleep and negative affect, one needs to be specific about which aspects of sleep and negative affect are related to one another. The goal of the present research is to examine the relationships between perceived sleep quality and negative affect reactivity and regulation across adult individuals. Unlike most prior studies, we focus on perceived sleep quality because, first, observation of natural variation in sleep quality has greater ecological validity than observation of the effects of artificial sleep deprivation; and second, the overall perception of how well one has slept is often of great psychological importance to individuals. We focus on the reactivity and regulation aspects of negative affect because responding to unpleasant stimuli and regulating that are the core components of individuals’ negative affective experience as they interact with the world. The current research sought to address two questions: (1) Do individual differences in perceived sleep quality predict differences in negative affect reactivity? and (2) Do individual differences in perceived sleep quality predict differences in the ability to regulate negative affect? We take a between-subject approach to tackle these questions with a focus on experimentally manipulated affect reactivity and regulation. Across two studies, we followed individuals for at least two weeks and measured their perceived sleep quality as well as their negative affect reactivity and regulation. Using unpleasant pictures with adults who varied in sleep quality (Study 1), or using painful heat stimulation in adults with lower back pain (Study 2), we engaged participants in tasks to either naturally react to the stimuli or to regulate the induced negative affect using engagement- and disengagement-based strategies, while we obtained self-report as well as physiological measures of affective response. Previous studies have all relied on frequentist analysis which cannot support a null hypothesis. In this research, we built Bayesian regression models in addition to frequentist analyses to better understand the relationships among sleep quality, negative affect, and negative affect regulation. We have shared the data and analysis scripts on the Open Science Framework (OSF) for Study 1 (https://osf.io/5w2n7/) and Study 2 (https://osf.io/x9e5t/).
Study 1:
Using Unpleasant Pictures to Induce Negative Affect
In Study 1, participants were shown unpleasant pictures and asked either to react naturally or to regulate negative affect using reappraisal or distraction. We measured participants’ affective responses using self-report, facial electromyography (EMG), and skin conductance level (SCL). Following prior studies, we hypothesized that poor sleep quality and shorter sleep duration would be associated with greater negative affect reactivity and less successful negative affect regulation. For affect regulation, we assessed both engagement-based (reappraisal) and disengagement-based (distraction) strategies.
Method
Participants
This study is part of the Sleep and Affect Study at Stanford University, which investigates links among affect, affect regulation, and sleep bruxism (defined as clenching or grinding teeth during sleep). This research does not focus on the clinical aspect of the sample (i.e. sleep bruxism). Research comparing individuals with and without sleep bruxism will be reported elsewhere. We recruited participants with and without sleep bruxism symptoms using Facebook/Instagram advertisements, flyers on campus, local hospitals, and dental clinics. Participants were not recruited if they reported any medical conditions, medication treatment, dental conditions other than sleep bruxism, psychiatric conditions, sleep disorders, caffeine use disorder, intake of sleep medicines, or significant exposure to tobacco or nicotine products. For present purposes, we considered only those participants who completed the sleep diary and the laboratory affective task. This sample consisted of 68 participants (20 males, 46 females, and 2 other) whose ages ranged from 21 to 48 years (Mean = 30.2, SD = 7.8). There were 29 Caucasian (42.6%), 31 Asians (45.6%), 1 African American (1.5%), 1 Pacific Islander (1.5%), 1 Native American (1.5%), and 5 in other races (7.4%). In our sample, there were 20 participants (29.4%) with sleep bruxism. All participants were informed about the study procedures and gave written consent before participation.
Sleep Measures
During a two-week sleep tracking period, participants filled out a morning sleep diary within 60 minutes after waking on each day. Participants reported their perceived sleep quality on a 5-point scale (1-very poor, 2-poor, 3-fair, 4-good, 5-very good). They also estimated the time they tried to fall asleep, sleep onset latency, time awake after sleep onset, and morning wake-up time (items drawn from the Consensus Sleep Diary; Carney et al., 2012). Based on this information, we calculated participants’ sleep duration (sleep duration = duration between the time to try to fall sleep and morning wake-up − sleep onset latency − time awake after sleep onset). Because we were interested in the effect of individual differences in sleep, we needed to get a trait-level estimate of how well a participant sleeps generally. Therefore, we calculated the average perceived sleep quality and duration across the sleep tracking period. In the same sleep diary, participants also reported their levels of positive and negative affect, bodily and emotional arousal levels, sleepiness, and plans for the day. Because we were interested in induced negative affect in a controlled setting, we do not report on the measures of state affect from the diary here. While keeping a sleep diary, participants also wore an actigraph watch during the study period. Before the sleep tracking period, participants slept at their residence with ambulatory polysomnography (PSG) recording for one night. The actigraph and PSG data are not reported because valid data were available for only a fraction of the sample.
Laboratory Affect Reactivity and Regulation Task
At the end of the two weeks of sleep diary, participants visited the laboratory to complete an affect reactivity and regulation task. The task had a 2 (intensity: neutral, negative) × 3 (instruction: WATCH, RETHINK, DISTRACT) design. In the task, participants viewed picture stimuli of different emotional intensities from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008), Emotional Picture System (EmoPicS; Wessa, M. et al., 2010), Nencki Affective Picture System (NAPS; Marchewka, Żurawski, Jednoróg, & Grabowska, 2014), and EmoMadrid emotional pictures database (EmoMadrid; https://www.psicologiauam.es/CEACO/EmoMadrid.htm) on a computer screen. Before the actual task, participants were instructed about how to carry out instructions and were tested on 6 practice trials. Prior to the picture stimulus in each trial, participants were cued either to WATCH (“simply pay attention to the depicted situation and allow any thoughts and feelings to arise as they naturally would”), RETHINK (i.e., reappraisal, “think about what is going on in the depicted situation in a way that helps you feel less negative”), or DISTRACT (“think about something that is completely unrelated to the depicted situation and helps you feel less negative”).
Each trial sequence was as follows (Figure 1): first, the cue word to WATCH, RETHINK, or DISTRACT appeared at the center of the screen for 2s. A picture was then presented on the screen for 6s, during which participants implemented the cued instruction. At the end of each trial, participants rated the strength of their current emotional valence (i.e. “How negative did you feel by the time the picture left the screen?”) and arousal (i.e. “How emotionally charged or activated did you feel by the time the picture left the screen?”) on a 1–5 scale (1: no negative feeling or emotional arousal; 5: very strong negative feeling or emotional arousal). The duration of valence and arousal ratings was fixed to 4s. Participants completed 180 trials across 5 blocks each lasting approximately 12 min. Each block consisted of 36 trials, with 9 neutral stimuli and 27 negative stimuli. In the trials for each stimulus type, there were equal numbers of WATCH-, RETHINK-, and DISTRACT-trials. The trial order in each block was pseudo-randomized.
Figure 1. Trial structure of the laboratory task in Study 1.

A WATCH trial is presented here as an example. In WATCH trials, participants were instructed to react to the picture stimulus naturally.
Affect ratings were averaged across trials for each condition in each participant. Following prior studies (e.g., Mauss et al., 2013; Reddy et al., 2017; Shermohammed et al., 2020), negative affect reactivity was operationalized as the difference in the affect ratings between negative and neutral WATCH-trials, and negative affect regulation was operationalized as the difference in the affect ratings from negative WATCH-trials to negative RETHINK- or DISTRACT-trials. As the valence and arousal ratings were highly correlated across participants (r > .90, p < .001), we averaged them to derive a composite affect rating score. The results based on individual ratings are the same as those based on the composite score (Table S7).
Physiological Data Recording and Data Reduction
During the laboratory task, physiological data were recorded and amplified with a multichannel BioNex 8-slot chassis (Mindware Technologies, Grahanna, OH) with modules for facial EMG, skin conductance, electrocardiogram (ECG), impedance cardiography (ICG), respiration, finger pulse, and finger temperature. Data were sampled at 1000 Hz, 16-bit digitized, and transmitted to a computer for viewing and storage using the Mindware computer software BioLab 3.3. Before conducting analyses, we determined that we would examine only the corrugator supercilii EMG and skin conductance level for this study because we expected them to be most directly linked to negative affect. Specifically, corrugator supercilii EMG is well characterized to be related to the valence dimension and skin conductance to be related to the arousal dimension of negative affect (Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000). Similar to the self-report data, the physiological data were averaged across trials for each condition within each participant. The difference in physiological responses between negative and neutral WATCH-trials was used to index affect reactivity, and the attenuation from WATCH-trials to RETHINK- or DISTRACT-trials was used to index negative affect regulation.
Corrugator supercilii EMG was recorded with 4-mm miniature Beckman Ag/AgCl electrode pairs filled with SignaGel electrode gel (Parker Laboratories, Inc., NJ) from the corrugator supercilii muscle on the left side of the face. The experimenter cleaned the skin with alcohol pads (Curity, Kendall Company, Mansfield, MA), abraded with Nuprep (Weaver and Company, Aurora, CO), and washed with water and cotton pads before electrode application. The signal was subjected to a 500-Hz antialiasing hardware filter. Afterwards, we applied a 60-Hz notch filter, a 20–500-Hz digital band-pass filter to the signal. Finally, we rectified and smoothed the data using a running average with 10-ms time constant. For each trial, a change ratio from the baseline (cue word) to the stimulus presentation was calculated as the trial-level EMG response (Kreibig, Samson, & Gross, 2013), which was used to index affect reactivity and regulation.
Skin conductance was recorded by applying constant 0.5 volts DC through two disposable 1 cm-diameter Ag/AgCl electrodes pre-gelled with isotonic paste (EL507, Biopac, Goleta, CA). The electrodes were attached to the palmar surface of the middle phalanges of the index and ring fingers of the nondominant hand. We down-sampled the data to 10 Hz with a 5-Hz low-pass filter to calculate SCL (in μSiemens). For each trial, a change score from the baseline (cue word) to the stimulus presentation was calculated as the trial-level SCL response (Kreibig et al., 2013), which was used to index affect reactivity and regulation.
Procedures
For two weeks, participants were instructed to sleep at their residence while tracking their sleep using a sleep diary and other tools (see Sleep Measures for details). After this two-week period, they were permitted to continue filling out the sleep diary until their laboratory session if they wished. On average, participants had valid sleep data for 14.3 days (SD = 1.8, range = [10, 19]). Afterwards, they came to the laboratory to complete a computerized task to measure their affect reactivity and regulation to unpleasant pictures. All participants received a financial compensation for participation. The study protocol was approved by the Institutional Review Board at Stanford University.
Data Analysis
Data analysis was performed in R 3.5.1 (R Core Team, 2018). We first calculated the frequentist Pearson’s correlations between perceived average sleep quality/duration and affect measures (negative affect reactivity, negative affect regulation via reappraisal, and negative affect regulation via distraction) with p-values. Then, we built Bayesian linear regression models to predict an affect measure using perceived average sleep quality/duration with the brms package (Bürkner, 2017) which is based on Stan (Carpenter et al., 2017). All variables were standardized before being entered into the models. We used unbiased weakly informative priors in the Bayesian models: a Gaussian distribution (μ = 0, σ = 1) as the prior for the intercept and slope coefficients and a positive half Cauchy distribution (x0 = 0, γ = 1) for the standard deviation of residuals. Applying these priors is equivalent to an L2 regularization of the regression model. The Bayesian models were fit in 4 Markov chains with 2000 iterations (500 warmups) using the Hamiltonian Monte Carlo (HMC) sampling algorithm. All models converged well with sufficient effective samples. The Bayes Factors (BF) were estimated based on the bridge sampling method (Bürkner, 2017). A Bayes Factor is the ratio of the marginal likelihoods under the null hypothesis (H0) and the alternative hypothesis (H1), indicating the relative plausibility of the data under the two competing hypotheses (Jeffreys, 1961). Therefore, a BF = 1 indicates equal evidence for H0 and H1, a BF < 1/3 indicates substantial evidece in favor of H0, and a BF > 3 indicates substantial evidence in favor of H1 (Wetzels & Wagenmakers, 2012). In secondary analyses, we performed the same analyses for the perceived sleep quality/duration of the night before the laboratory task.
Results
Sample Characteristics
As expected, there was considerable variation in participants’ sleep. Participants’ average perceived sleep quality ranged from 2.4 to 4.5 on a 1–5 scale (Mean = 3.6, SD = .50). On average, the intraindividual standard deviation of perceived sleep quality during the sleep tracking period was 0.7 (SD = .21). Their average perceived sleep duration across two weeks was well distributed between 5.9 and 8.8 hours (Mean = 7.3, SD = .64). In addition, the task was successful at inducing negative affect and participants also varied in their induced negative affect. Participants’ average negative affect ratings across negative-WATCH trials were well distributed above 1 from 1.2 to 4.3 on a 1–5 scale (Mean = 2.5, SD = .75). As expected, affect reactivity (the change from neutral-WATCH to negative-WATCH trials) measured by self-report (mean = 1.17, SD = 0.56) and corrugator EMG activity (mean = 0.05, SD = 0.16) was significantly above 0 (self-report: t(67) = 17.30, p < .001; EMG: t(67) = 2.36, p = .021), suggesting a successful induction of negative affect reactivity. In contrast, skin conductance level was not sensitive enough to capture the manipulation of affect reactivity (mean = −0.004, SD = 0.05), t(67) = −0.61, p = .547. As expected, affect regulation (the decrease from negative-WATCH trials to negative-RETHINK/DISTRACT trials) measured by self-report (reappraisal: mean = 0.47, SD = 0.40; distraction: mean = 0.56, SD = 0.49) and corrugator EMG activity (reappraisal: mean = 0.04, SD = 0.13; distraction: mean = 0.09, SD = 0.16) was significantly above 0 (self-report for reappraisal: t(67) = 9.68, p < .001; EMG for reappraisal: t(67) = 2.48, p = .016; self-report for distraction: t(67) = 9.40, p < .001; EMG for distraction: t(67) = 4.54, p < .001), suggesting successful regulation by reappraisal and distraction. Skin conductance level was not sensitive enough to capture the manipulation of affect regulation of reappraisal (mean = −0.004, SD = 0.04), t(67) = −0.76, p = .447, or distraction (mean = −0.007, SD = 0.03), t(67) = −1.83, p = .071.
Sleep and Negative Affect Reactivity
Figure 2 shows the relationship between perceived sleep quality/duration and negative affect reactivity measured by self-report, corrugator EMG activity, and skin conductance level. Using the frequentist approach, the correlations between those measures were below .10 in magnitude with p > .40. That is, none of the sleep-affect relationships were statistically significant in the frequentist approach. The exact p-values of each correlation were presented in the Supplemental Materials (Table S1).
Figure 2. Scatterplots of perceived sleep quality/duration and negative affect reactivity in Study 1.
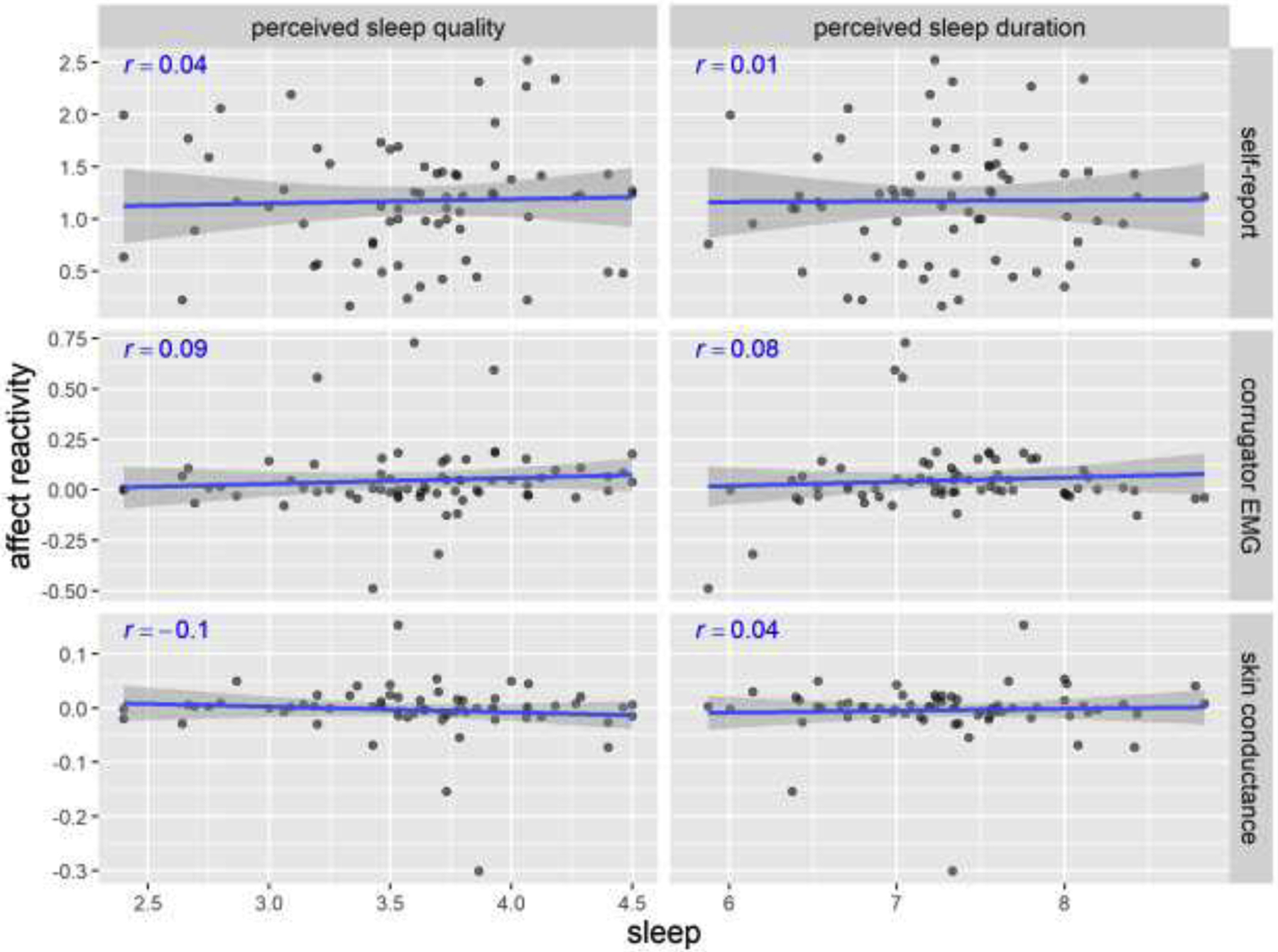
Pearson’s r’s and fitted lines with standard error shades are added to the scatter plots. Sleep quality is on a 1–5 scale. Sleep duration is in hours. Self-report reactivity is the change from in affect rating (on a 1–5 scale) from neutral-WATCH to negative-WATCH trials. Corrugator EMG represents the percentage change from baseline. Skin conductance represents the absolute change from baseline (in μSiemens).
The Bayesian analysis corroborated the frequentist analysis results. As an example, in Figure 3 we present the prior vs. posterior sample distribution of the standardized coefficient of perceived sleep quality in prediction of self-reported negative affect reactivity. The prior distribution of the standardized coefficient of perceived sleep quality was widely distributed around 0 (95% CI = [−1.96, 1.96]). After fitting to the empirical data, its posterior distribution became much denser in the close range around 0 (Mean = .03, 95% CI = [−.21, .28]). This distribution pattern indicates that, when run on empirical data, the model finds a high likelihood that there was near-zero association between perceived sleep quality and negative affect reactivity, and much lower likelihood that there was a significantly positive or negative association. The prior vs. posterior distributions for other models were similar and can be found in the Supplemental Materials (Figure S2). The results of Bayesian linear regression models predicting negative affect reactivity from perceived sleep quality and duration are presented in Table 1. As expected, with the weakly informative priors, the posterior standardized coefficients β were close to our initially calculated Pearson’s r’s and small in magnitude (< .10). All Bayes Factors were smaller than 1/5, suggesting substantial support for the null hypothesis about the relationship between perceived sleep quality and negative affect reactivity.
Figure 3. The prior and posterior sample distribution of the standardized coefficient of perceived sleep quality in prediction of self-report negative affect reactivity in Study 1.
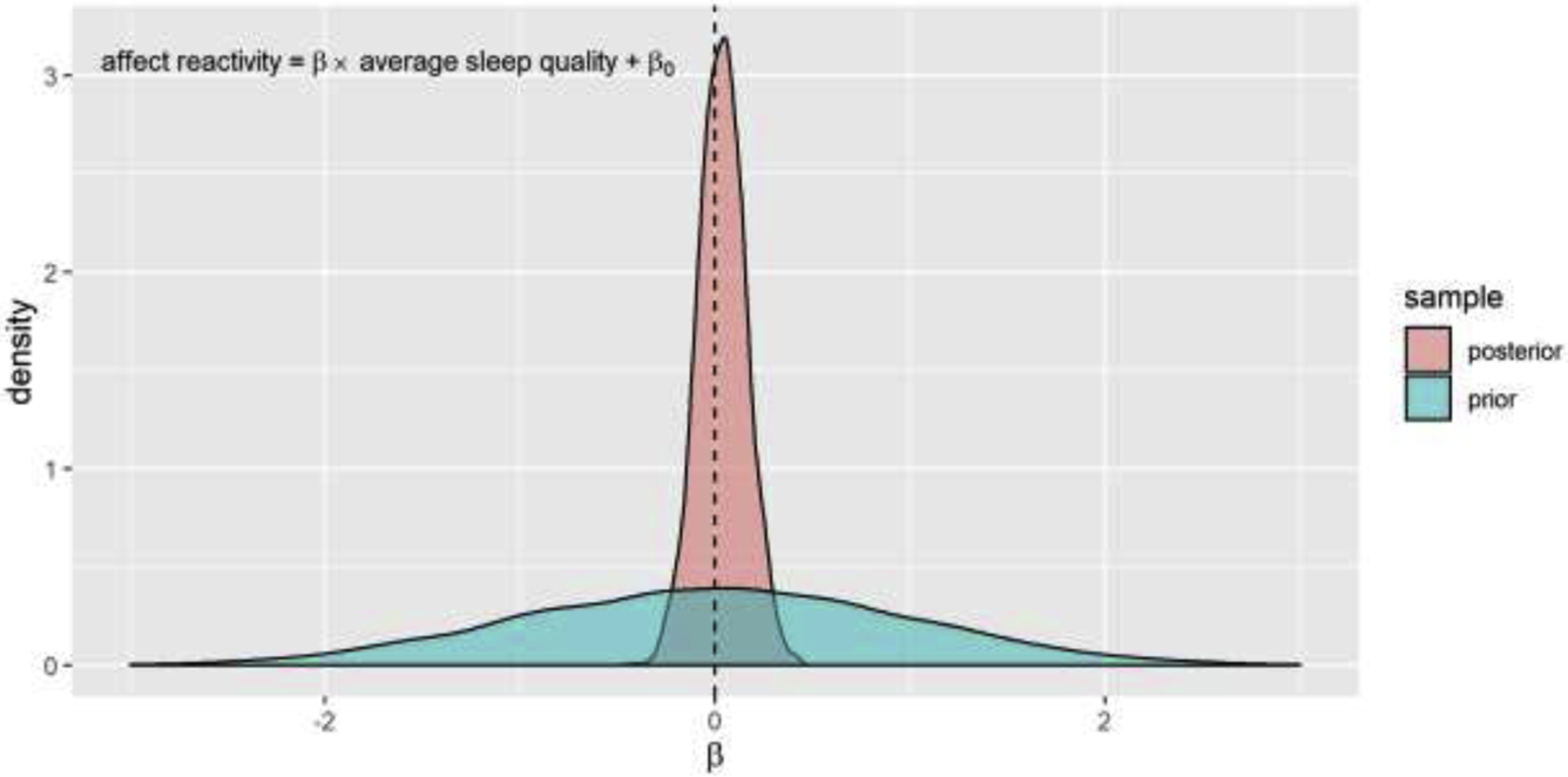
The posterior distribution indicates a strong likelihood based on the empirical data that there is near-zero association between perceived sleep quality and negative affect reactivity. Bayesian sampling n = 6000. The dash line indicates β = 0.
Table 1.
Results of Bayesian linear regression models of perceived sleep quality/duration predicting negative affect reactivity in Study 1.
| Independent variable | ||
|---|---|---|
| Perceived sleep quality | Perceived sleep duration | |
| Self-report rating | β = .03, 95% CI = [−.21, .28], BF = .128* | β = .01, 95% CI = [−.24, .25], BF = .123* |
| Corrugator EMG | β = .09, 95% CI = [−.16, .34], BF = .159* | β = .08, 95% CI = [−.17, .32], BF = .151* |
| Skin conductance | β = −.10, 95% CI = [−.34, .14], BF = .174* | β = .04, 95% CI = [−.20, .28], BF = .130* |
β: standardized coefficient posterior estimate; CI: credible interval; BF: Bayes Factor
BF < 1/3;
BF < 1/10;
Sleep and Negative Affect Regulation (Reappraisal)
Figure 4 shows the relationship between perceived sleep quality/duration and affect regulation via reappraisal. Five out of the 6 correlations were small in magnitude and non-significant, r < .10, p > .50. The largest correlation was the one between perceived sleep duration and regulation via reappraisal measured by skin conductance, r = .21, p = .09. However, it remained statistically non-significant in the frequentist approach. The exact p-values of all Pearson’s correlations can be found in the Supplemental Materials (Table S3).
Figure 4. Scatterplots of perceived sleep quality/duration and negative affect regulation via reappraisal in Study 1.
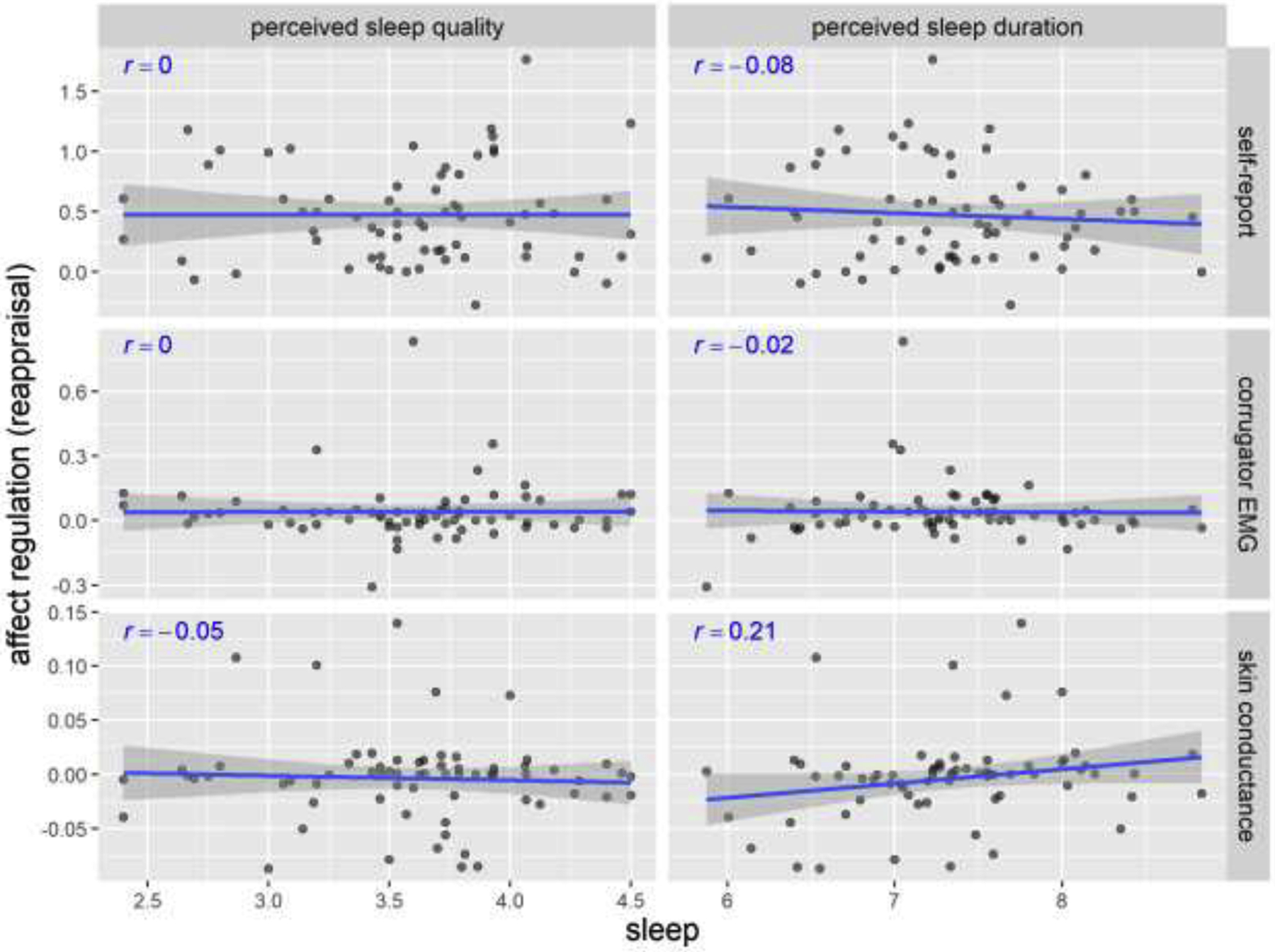
Pearson’s r’s and fitted lines with standard error shades are added to the scatter plots. Sleep quality is on a 1–5 scale. Sleep duration is in hours. Self-report regulation (reappraisal) is the change from in affect rating (on a 1–5 scale) from negative-WATCH to negative-RETHINK trials. Corrugator EMG represents the percentage change from baseline. Skin conductance represents the absolute change from baseline (in μSiemens).
The results of Bayesian linear regression models of predicting affect regulation via reappraisal from perceived sleep quality and duration are presented in Table 2. Similar to the frequentist analysis, five out of the 6 posterior stardardized coefficient β were smaller than .10 and their Bayes Factors were smaller than 1/6, providing substantial support for the null hypothesis about the relationship between sleep quality/duration and affect regulation via reappraisal. Of note, for the only Bayes Factor that was larger than 1/3 (i.e. perceived sleep duration in prediction of SCL-measured affective regulation via reappraisal), it was still smaller than 1, suggesting relatively stronger evidence for the null hypothesis than the alternative hypothesis. The prior vs. posterior distributions of the coefficients for all models were similar to the pattern in Figure 3 and can be found in the Supplemental Materials (Figure S4).
Table 2.
Results of Bayesian linear regression models of perceived sleep quality/duration predicting affect regulation (reappraisal) in Study 1.
| Independent variable | ||
|---|---|---|
| Perceived sleep quality | Perceived sleep duration | |
| Self-report | β = 9.1×10−4, 95% CI = [−.25, .25], BF = .12.2* | β = −.08, 95% CI = [−.33, .17], BF = .150* |
| Corrugator EMG | β = 3.3×10−3, 95% CI = [−.25, .25], BF = .123* | β = −.02, 95% CI = [−.26, .22], BF = .124* |
| Skin conductance | β = −.05, 95% CI = [−.29, .19], BF = .134* | β =.21, 95% CI = [−.03, .45], BF = .537 |
β: standardized coefficient posterior estimate; CI: credible interval; BF: Bayes Factor
BF < 1/3;
BF < 1/10;
Sleep and Negative Affect Regulation (Distraction)
Results were similar for affect regulation via distraction (Figure 5). The correlations between perceived sleep quality/duration and affect regulation via distraction were small and non-significant, r < .12, p > .30. The exact p-values of each correlation are presented in the Supplemental Materials (Table S5). None of the correlations were statistically significant in the frequentist approach.
Figure 5. Scatterplots of perceived sleep quality/duration and affect regulation via distraction in Study 1.
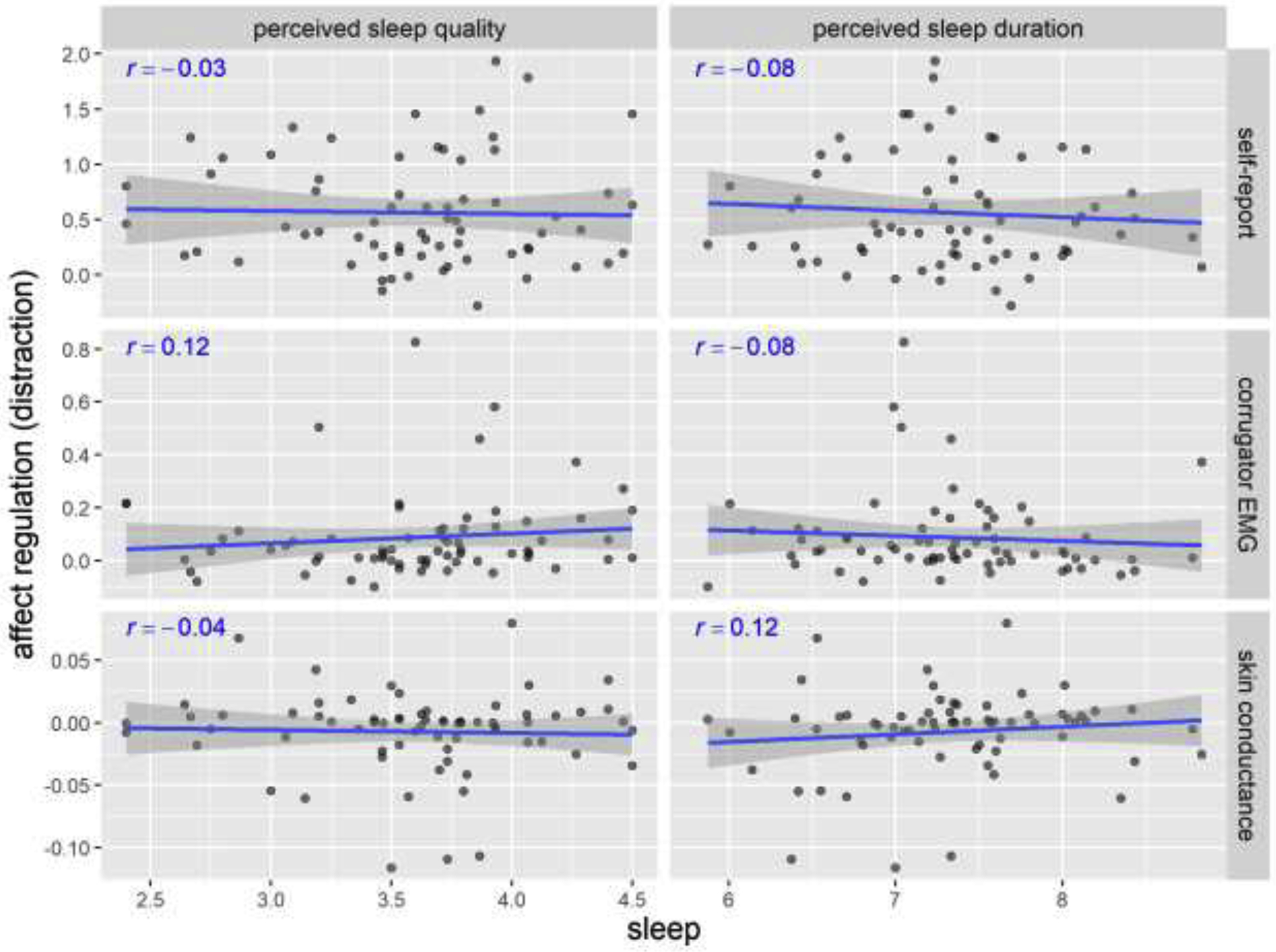
Pearson’s r’s and fitted lines with standard error shades are added to the scatter plots. Sleep quality is on a 1–5 scale. Sleep duration is in hours. Self-report regulation (distraction) is the change from in affect rating (on a 1–5 scale) from negative-WATCH to negative-DISTRACT trials. Corrugator EMG represents the percentage change from baseline. Skin conductance represents the absolute change from baseline (in μSiemens).
The results of Bayesian linear regression models of predicting affect regulation via distraction from perceived sleep quality/duration corroborated the frequentist results (Table 3). All posterior stardardized coefficient β were smaller than .12 and all Bayes Factors were smaller than 1/5, suggesting substantial support for the null hypothesis about the relationship between perceived sleep quality/duration and affect regulation via distraction. The prior vs. posterior distributions of the coefficients for all models were similar to the pattern in Figure 3 and can be found in the Supplemental Materials (Figure S6).
Table 3.
Results of Bayesian linear regression models of perceived sleep quality/duration predicting affect regulation (distraction) in Study 1.
| Independent variable | ||
|---|---|---|
| Perceived sleep quality | Perceived sleep duration | |
| Self-report | β = −.03, 95% CI = [−.26, .22], BF = .126* | β = −.08, 95% CI = [−.32, .17], BF = .149* |
| Corrugator EMG | β = .11, 95% CI = [−.13, .35], BF = .190* | β = −.08, 95% CI = [−.32, .16], BF = .150* |
| Skin conductance | β = −.04, 5% CI = [−.28, .20], BF = .129* | β = .11, 95% CI = [−.12, .35], BF = .192* |
β: standardized coefficient posterior estimate; CI: credible interval; BF: Bayes Factor
BF < 1/3;
BF < 1/10;
In summary, contrary to our initial expectations, Study 1 was consistent with the null hypothesis of the relationship between perceived sleep quality/duration and negative affect reactivity and regulation (reappraisal and distraction) based both on self-report and physiological data. To rule out the potential confounding role of sleep bruxism, we further tested whether sleep bruxism moderated the relationships between perceived sleep quality and negative affect reactivity and regulation. None of the moderation effects were significant with Bonferroni correction (corrected p’s > .05). In our secondary analysis, we also assessed the relationship between other sleep measures and negative affect reactivity and regulation. Specifically, the results for sleep quality of the previous night of the assessment of affect reactivity and regulation (i.e. last-night sleep quality), and sleep duration of the previous night of the assessment of affect reactivity and regulation (i.e. last-night sleep duration) were similar to those of perceived sleep quality (see Supplemental Materials for details).
Study 2:
Using Heat Stimulation to Induce Negative Affect
In Study 1, we observed null relationships between perceived sleep quality and negative affect reactivity and regulation. To assess the generalizability of this finding, we used data from a second and larger sample of individuals with low back pain and induced negative affect via heat stimulation to the lower back, which we hoped would maximize self-relevance of the negative affect stimulation. For affect regulation, we retained our focus on reappraisal, but replaced distraction with another disengagement-based mindfulness-like strategy (i.e. acceptance, see Method for details). Based on prior literature and Study 1, we expected weak to modest relationships between perceived sleep quality and negative affect reactivity and regulation.
Method
Participants
This study is part of the NIH funded Stanford Center for Back Pain project (ref. P01AT006651). The participants were adults diagnosed with chronic low back pain. This research does not focus on the clinical aspect of the sample. Research focusing on the role of chronic low back pain will be reported elsewhere. For present purposes, we considered only those participants who completed the laboratory task (n = 204). We excluded from analysis participants who did not have at least 14 days of daily questionnaire data (18 participants), leaving a final sample of 186 participants. The average age of the final sample was 39.8 years (SD = 11.7, range = [21, 64]). Among them, 99 were females (53.2%), 81 were males (43.5%), and the other 6 participants’ gender information was unknown (3.2%). There were 81 Caucasian (43.5%), 49 Asians (26.3%), 10 African Americans (5.4%), 1 Pacific Islander (0.5%), and 45 in other races (24.2%). All participants were informed about the study procedures and gave written consent before participation.
Sleep Quality Measures
We obtained two measures of perceived sleep quality: one from the daily questionnaires and the other from the Patient-Reported Outcomes Measurement Information System (PROMIS; https://www.healthmeasures.net/) Sleep Disturbance Scale. In the daily questionnaire, participants rated the sleep quality of the previous night on a continuous scale of 0 (very poor) to 100 (very good). We calculated the average perceived sleep quality across days. In addition to the daily questionnaire, the PROMIS Sleep Disturbance was used to retrospectively assess self-reported perceptions of sleep quality, sleep depth, and restoration associated with sleep over a 7-day period of time (Buysse et al., 2010). This includes perceived difficulties and concerns of time with getting to sleep or staying asleep, as well as perceptions of the adequacy of and satisfaction with sleep. PROMIS instruments are based on an item response theory-based assessment that utilizes item level responses rather than composite scale responses. PROMIS measures are normed against the U.S. population and have a mean t-score of 50 points and a standard deviation of 10 points. For each Sleep Disturbance item, participants rated on a 1–5 scale and an adaptive t-score was generated automatically. The scale has been validated with excellent measurement properties (Buysse et al., 2010). As expected, sleep disturbance had a strong negative correlation with daily-questionnaire sleep quality in our sample, r = −.57, p < .001. Thus, we reversed the coding sleep disturbance to be a second measure of sleep quality.
Laboratory Affect Reactivity and Regulation Task
In the task, there were 4 types of trials: RESPOND (number of trials = 11; equivalent to WATCH in Study 1), REFRAME (number of trials = 10; equivalent to RETHINK in Study 1, reappraisal), OBSERVE (number of trials = 10; acceptance), and REST (number of trials = 10). Participants practiced each type of trial with the same stimulus before the real test. In every non-REST trial, participants received heat stimulation to the low back to induce thermal pain. The temperature of the heat stimulation was calibrated for each participant (Mean = 47.2 °C, SD = 0.9) to evoke pain. In RESPOND trials, participants responded to the stimulation in a natural way without regulating their affect. In REFRAME trials, participants used reappraisal (i.e. reinterpreted the way they thought about the current pain experience) to regulate their affect reaction to the heat stimulation. In OBSERVE trials, participants regulated their affect reaction to the heat stimulation in using the acceptance strategy by noticing their moment-to-moment experience, such as thoughts, physical sensations, and feelings without modulating their experience (i.e. mindfulness; Dixon et al., 2020; Goldin, Moodie, & Gross, 2019). In REST trials, there was no heat stimulus and patients were instructed not to give self-report ratings.
The task began with a 10-s fixation cross (+) at the center of screen and one RESPOND trial to give participants baseline sensation of the heat stimulation. The data of the first trial were discarded, leaving 10 RESPOND trials for analysis. After that, each trial began with a fixation cross for 1s, 3s, or 7s (randomly determined). Then a cue word (RESPOND, REFRAME, OBSERVE, or REST) appeared for 12s to identify the trial type. The thermal stimulation was applied together with the presentation of the cue word. At the end of each trial, participants provided a pain intensity rating and an unpleasantness rating on a scale from 0 (no pain or unpleasantness) to 10 (most imaginable pain or unpleasantness) with a time limit of 5s. After the first trial, the order of the trials was pseudorandomized. Similar to Study 1, affect ratings were averaged across trials for each condition within each participant. Negative affect reactivity was operationalized as the pain intensity and unpleasantness ratings during the RESPOND trials. Affect regulation was operationalized as the reduction in the pain intensity and unpleasantness ratings from the RESPOND condition to the REFRAME or OBSERVE conditions. As the pain intensity ranting and unpleasantness rating were highly correlated (r > .80, p < .001), we averaged them to derive a composite affect rating score. The results based on individual ratings are the same as those based on the composite score (Table S9).
Procedures
After recruitment, participants were instructed to complete daily questionnaires about their pain, perceived sleep quality, negative emotion, and positive emotion until the laboratory experiment. Participants with data shorter than 2 weeks were not included in analysis (Mean = 33.4 days, SD = 12.8). Because our focus was on the negative affect induced by stimuli in a controlled setting, the measures of state affect in the daily questionnaires are not reported here. Prior to the laboratory visit, participants also completed a battery of baseline questionnaires including the Sleep Disturbance Scale in the PROMIS bank. Then they came to the laboratory to complete a task measuring negative affect reactivity and regulation in a magnetic resonance imaging (MRI) scanner. Only a subset of the sample had valid fMRI data and the fMRI data for the laboratory affect reactivity and regulation task will be reported in a separate paper.
Statistical Analysis
For Study 2, the data analysis was performed using the same statistical methods as Study 1. Our primary focus was the relationship of perceived sleep quality (one measure from the daily questionnaires and the other from the PROMIS Sleep Disturbance Scale) with negative affect reactivity and regulation, and we performed pre-registered frequentist multiple regressions (OSF registration: https://osf.io/nk329). In the pre-registered frequentist regression models, we used daily-questionnaire sleep quality and sleep disturbance (reversed) as two simultaneous predictors to predict each of the affect measures (affect reactivity, affect regulation via reappraisal, and affect regulation via acceptance).
Results
Sample Characteristics
Similar to Study 1, there was considerable variation in perceived sleep quality among participants in the sample. Their average daily-questionnaire sleep quality varied from 16.5 to 86.2 on a 0–100 scale (Mean = 53.2, SD = 13.8). On average, the intraindividual standard deviation of perceived sleep quality during the sleep tracking period was 15.5 (SD = 5.3). In addition, the task was successful at inducing negative affect. Participants’ negative affect induced by heat stimulation (ratings of pain intensity and unpleasantness) was widely distributed above zero from 1.1 to 9.0 on a 0–10 scale (Mean = 5.2, SD = 1.8). As expected, participants’ negative affect was effectively attenuated by the two affect regulation strategies, reappraisal (attenuation mean = 1.1, SD = 0.9, range = [−0.4, 4.0]) and acceptance (attenuation mean = 0.8, SD = 0.9, range = [−1.4, 4.6]).
Sleep and Negative Affect Reactivity
The relationship between perceived sleep quality measures (one measure from the daily questionnaires and the other from the PROMIS Sleep Disturbance Scale) and negative affect reactivity is shown in Figure 6. Following the pre-registration, we built linear regression models to predict negative affect reactivity using daily-questionnaire sleep quality and sleep disturbance as the two predictors in the model (Table 4). The results showed that neither of the predictors nor the whole model significantly predicted negative affect reactivity (p > .30). More importantly, the model explained very little variance in negative affect reactivity, R2 = .01, p = .461.
Figure 6. Scatterplots of perceived sleep quality and negative affect reactivity in Study 2.
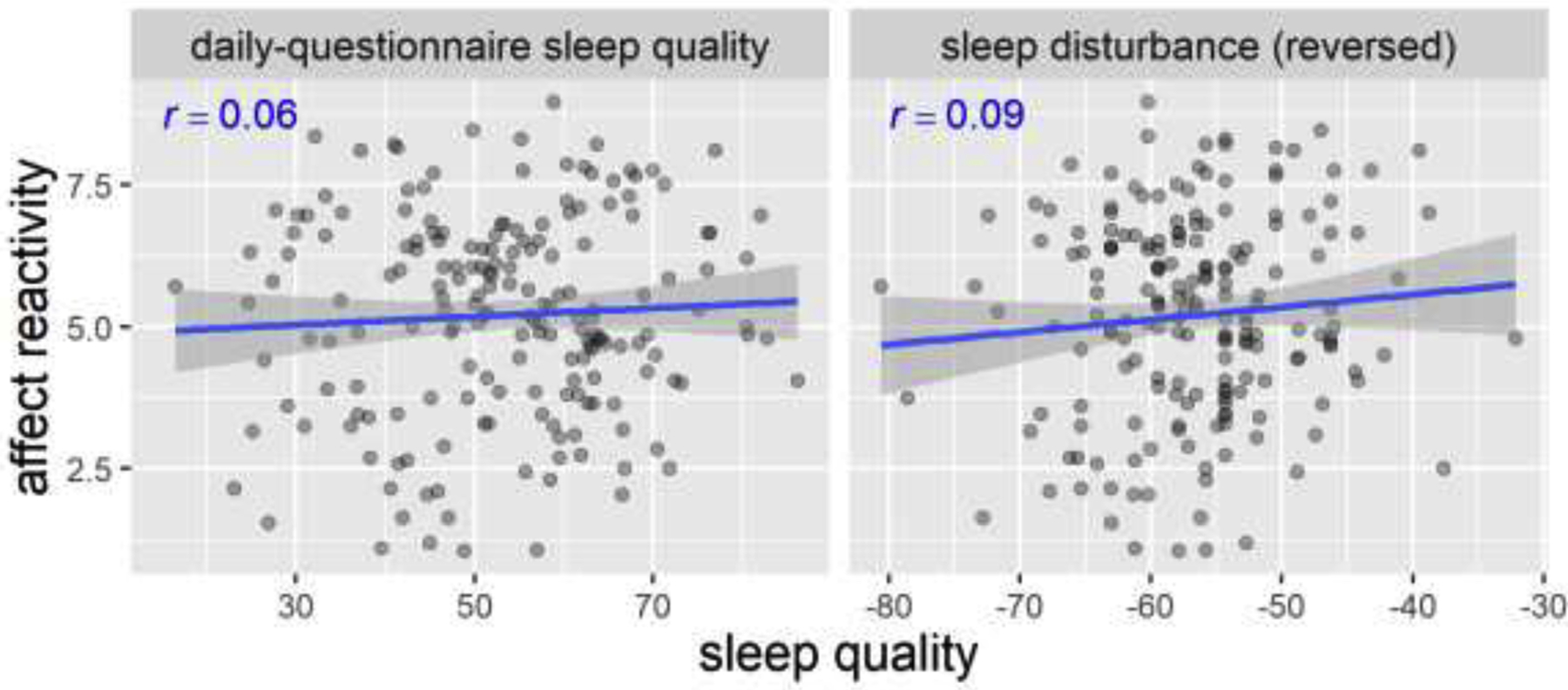
Pearson’s r’s and fitted lines with standard error shades are added to the scatter plots. Daily-questionnaire sleep quality is on a 0–100 scale. Sleep disturbance uses an adaptive t-score. Affect reactivity is the affect rating during the RESPOND trials (on a 0–10 scale).
Table 4.
Results of pre-registered frequentist linear regression model of perceived sleep quality predicting negative affect reactivity in Study 2.
| DV: Negative affect reactivity | ||||
|---|---|---|---|---|
| β | t/F | p | R 2 | |
| Daily-questionnaire sleep quality | .01 | t(183) = .09 | .931 | |
| Sleep disturbance (reversed) | .08 | t(183) = .98 | .330 | |
| Model | F(2, 183) = .78 | .461 | .01 | |
β: standardized coefficient;
Similar to Study 1, we built Bayesian linear regression models to predict negative affect reactivity using each of the perceived sleep quality measures as the single predictor (first row in Table 5). Similar to Study 1, the posterior standardized coefficients β were small in magnitude (< .10) and all Bayes Factors were below 1/5, providing evidence for the null relationship between perceived sleep quality and negative affect reactivity. The prior vs. posterior distributions of the coefficients for all models were similar to the pattern in Figure 3 and can be found in the Supplemental Materials (Figure S8).
Table 5.
Results of Bayesian linear regression models of perceived sleep quality predicting negative affect reactivity and regulation in Study 2.
| Perceived sleep quality measure | ||
|---|---|---|
| Daily-questionnaire sleep quality | Sleep disturbance (reversed) | |
| Affect reactivity | β = .06, 95% CI = [−.09, .20], BF = .099** | β = .09, 95% CI = [−.05, .24], BF = .158* |
| Affect regulation (reappraisal) | β = .06, 95% CI = [−.09, .20], BF = .099** | β = .10, 95% CI = [−.04, .25], BF = .197* |
| Affect regulation (acceptance) | β = .09, 95% CI = [−.05, .23], BF = .162* | β = .14, 95% CI = [.00, .28], BF = .459 |
β: standardized coefficient posterior estimate; CI: credible interval; BF: Bayes Factor
BF < 1/3;
BF < 1/10;
Sleep and Negative Affect Regulation
The relationship between the perceived sleep quality measures and affect regulation (via reappraisal and acceptance) is shown in Figure 7. As pre-registered, we built linear regression models to predict affect regulation using daily-questionnaire sleep quality and sleep disturbance (reversed) as the two predictors in the model (Table 6 and 7). The results showed that neither of the predictors nor the whole model significantly predicted affect regulation (p > .14). More importantly, the models explained very little variance in affect regulation (reappraisal: R2 = .01, p = .372; acceptance: R2 = .02, p = .156).
Figure 7. Scatterplots of perceived sleep quality and affect regulation in Study 2.
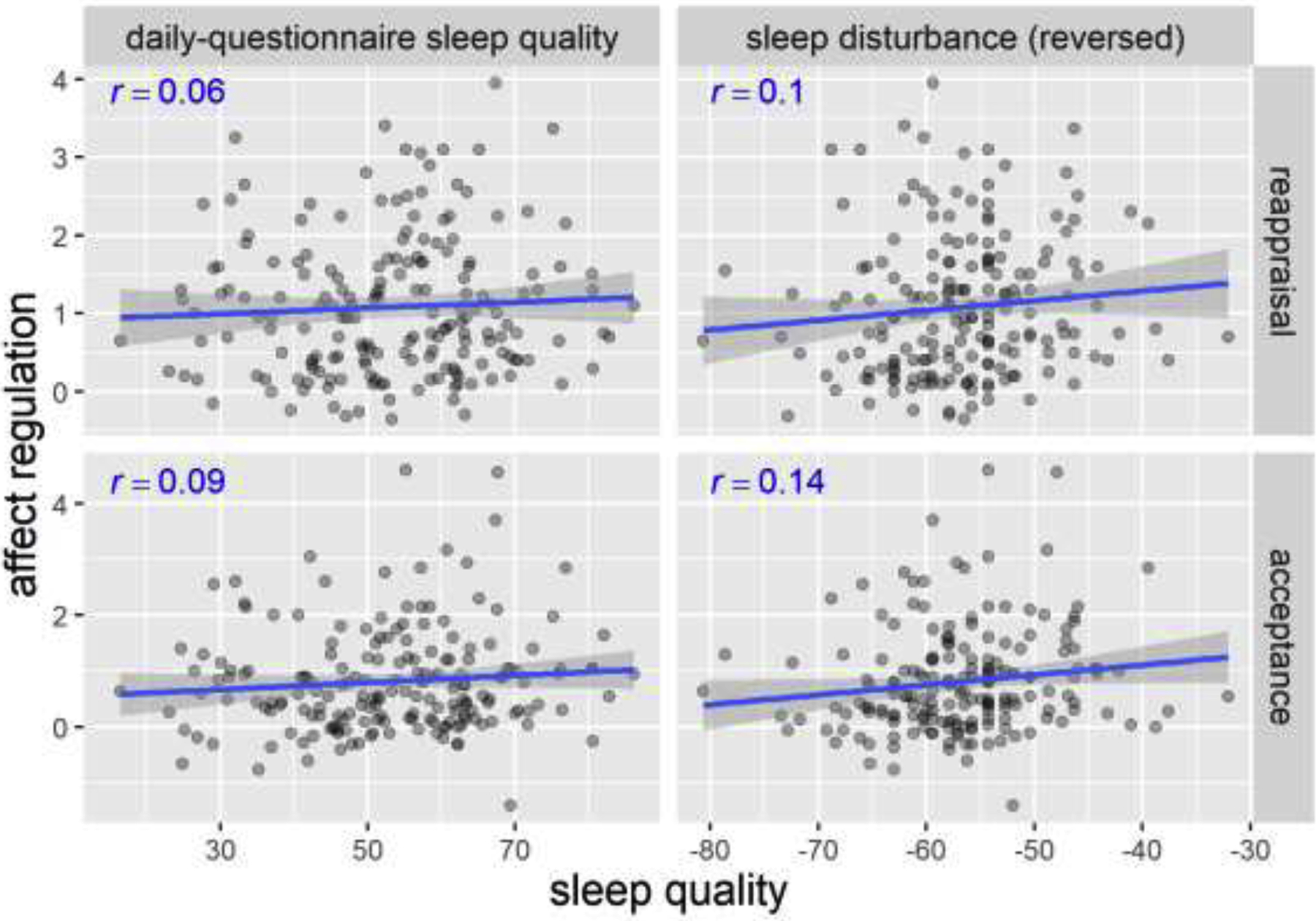
Pearson’s r’s and fitted lines with standard error shades are added to the scatter plots. Daily-questionnaire sleep quality is on a 0–100 scale. Sleep disturbance uses an adaptive t-score. Affect regulation is the change in affect rating (on a 0–10 scale) from the RESPOND condition to the REFRAME/OBSERVE condition.
Table 6.
Results of pre-registered frequentist linear regression models of perceived sleep quality predicting affect regulation (reappraisal) in Study 2.
| DV: Affect regulation (reappraisal) | ||||
|---|---|---|---|---|
| β | t/F | p | R 2 | |
| Daily-questionnaire sleep quality | −.002 | t(183) = −.02 | .984 | |
| Sleep disturbance (reversed) | −.10 | t(183) = −1.17 | .242 | |
| Model | F(2, 183) = 1.00 | .372 | .01 | |
β: standardized coefficient;
Table 7.
Results of pre-registered frequentist linear regression models of perceived sleep quality predicting affect regulation (acceptance) in Study 2.
| DV: Affect regulation (acceptance) | ||||
|---|---|---|---|---|
| β | t/F | p | R 2 | |
| Daily-questionnaire sleep quality | .02 | t(183) = .22 | .828 | |
| Sleep disturbance (reversed) | .13 | t(183) = 1.46 | .145 | |
| Model | F(2, 183) = 1.88 | .156 | .02 | |
β: standardized coefficient;
The Bayesian linear regression models corroborated the frequentist results (last two rows in Table 5). As expected, the posterior standardized coefficients β were small in magnitude. Three out of the 4 Bayes Factors for perceived sleep quality were below 1/5. Of note, for the only Bayes Factor that was larger than 1/3 (i.e. reverse-coded sleep disturbance in prediction of affect regulation via acceptance, β =.14, BF = .459), it was still smaller than 1/5, suggesting relatively stronger evidence for the null hypothesis than the alternative hypothesis. The prior vs. posterior distributions of the coefficients for all models were similar to the pattern in Figure 3 and can be found in the Supplemental Materials (Figure S8).
To summarize, in Study 2 we assessed the relationships between two measures of perceived sleep quality (one direct measure from the daily questionnaires and the other from the PROMIS Sleep Disturbance Scale) and self-report affect reactivity and regulation (via reappraisal and acceptance). To rule out the potential confounding role of chronic back pain symptoms, we further tested whether one’s average pain intensity moderated the relationships between perceived sleep quality and negative affect reactivity and regulation. None of the moderation effects were significant (p’s > .05). The results were consistent with Study 1 in favoring the null relationships between perceived sleep quality and affect reactivity and regulation. In secondary analyses, we also assessed the relationships between last-night sleep quality (i.e., sleep quality of the night before the laboratory assessment of affect reactivity and regulation) and negative affect reactivity and regulation. The results were similar to those of average perceived sleep quality (see Figure S7 and Table S8 for details).
Discussion
In this research, we examined the relations among perceived sleep quality, negative affect reactivity, and negative affect regulation. We not only distinguished affect reactivity from regulation, but also assessed various forms of affect regulation (engagement- and disengagement-based). Across two studies, we used unpleasant pictures and heat stimulation to induce negative affect and utilized self-report and physiological (EMG and SCL) measures to assess negative affect. Our frequentist analyses indicated that the strength of the relationships between perceived sleep quality, on the one hand, and negative affect reactivity and regulation, on the other, were small and none were statistically significant. Furthermore, our Bayesian analysis unanimously favored the null hypotheses over the alternative hypotheses. Overall, these findings suggest that perceived sleep quality does not predict negative affect reactivity or regulation across individuals in these contexts. In other words, if a person perceives his or her sleep quality to be generally bad, it does not imply this person would show greater or smaller reactivity when encountering an unpleasant stimulus compared to other people. It does not imply this person would be better or worse at regulating such negative affect than other people either.
Sleep and Negative Affect Reactivity
Variation in perceived sleep quality across individuals was not associated with self-report or physiological affect reactivity to negative stimuli. In addition, it was found in Study 1 that individual differences in perceived sleep duration did not predict affect reactivity either. While these results may appear surprising in the face of lay assumptions, several between-subject correlational diary studies have reported the same null relationship between perceived sleep quality and daily negative affect (e.g., Sin et al., 2020; Yap et al., 2020). Of note, this was not because the participants had limited variability in sleep or negative affect. Instead, there was wide variation in both sleep and negative affect in our data. It is generally thought that the relationship between sleep and negative affect is bi-directional and subject to various moderators (Kahn, Sheppes, & Sadeh, 2013). One explanation for the null relationship observed here may be that their direct link is diluted by other factors (i.e., low signal-to-noise ratio). One individual who habitually sleeps poorly may be emotionally supported by success in career or a good family relationship, whereas another individual who habitually sleeps poorly may have none of these resources. Another explanation is that the range of natural sleep variation may not be extreme enough to influence negative affect (e.g., Yoo et al., 2007). In other words, being deprived of sleep for a full night might increase negative affect, whereas lesser disruptions may not.
Sleep may influence negative affect, negative affect in turn may influence sleep (Kahn et al., 2013). Given the bi-directional relationship between sleep and negative affect, future research may benefit from manipulating sleep while observing negative affect and vice versa. In our studies, we observed a null relationship between sleep and negative affect among individuals without psychiatric disorders. However, the sleep-negative affect link could be more pronounced in individuals with clinical affective disruptions. It would be interesting for future research to examine the relationship between sleep and negative affect in individuals with anxiety or mood disorders.
Sleep and Negative Affect Regulation
Although many have suggested that poor-quality or limited-duration sleep is associated with poor affect regulation (Palmer & Alfano, 2017), our findings suggest that perceived sleep quality variation across individuals is not associated with the capacity to regulate negative affect (at least in the context of the three types of regulation we assessed across these two studies). This is consistent with anecdotal observations that some individuals who have generally short or poor sleep are also able to regulate negative affect well. These individuals might be good at negative affect regulation to begin with or might have learned to cope with negative affect over chronic poor sleep. Nevertheless, a null relationship between sleep quality and affect regulation capacity does not mean a null relationship between sleep quality and how frequently individuals regulate negative affect or what regulation strategies they choose to use, and this possibility warrants future research.
Limitations and Future Directions
A few limitations need to be acknowledged. First, our research focuses on the between-individual relationship between sleep quality and negative affect and cannot speak to their within-individual relationship. Future studies are encouraged to study the variations in sleep and negative affect within individuals. Second, although our two samples show sleep quality variation and negative affect reactivity, they both have clinical symptoms (i.e. sleep bruxism and low back pain) that are often not present in the general population. Therefore, our findings should be generalized with caution to other populations. Third, although the relationships between sleep quality and negative affect reactivity and regulation across individuals are of interest and significance themselves, our two studies did not manipulate sleep quality and thus are unable to support a causal conclusion. One direction for future experimental studies is to manipulate perceived sleep quality. For example, researchers might provide false feedback regarding objective sleep parameters, thereby leading participants to reassess their perceived sleep quality. Fourth, we tracked participants’ sleep for two weeks, but the sleep measures in our studies were self-reported. Future studies are needed that track sleep using objective measures such as PSG for multiple nights in order to examine the associations between objective sleep parameters and negative affect. Fifth, while our research focuses on the reactivity to affectively negative stimuli as one form of negative affect, there are other types of negative affect (e.g., negative mood, stress) that may be related to sleep quality. Similarly, our research has studied only three forms of affect regulation and the findings may not generalize to other regulation strategies. To more fully understand the relationship between sleep and negative affect, future studies are needed to assess a wider range of negative affect and regulation strategies and to examine their relationships with sleep. Sixth, although we intended to separate affect reactivity and regulation in our paradigm, there was still a possibility that participants automatically engaged affect regulation during reactivity trials. As a result, affect reactivity and regulation might both be underestimated. Last but not least, it should be noted that out study used only two physiological measures of negative affect. Our physiological finding based on corrugator EMG and SCL may not generalize to other relevant physiological systems such as cardiovascular activation and respiration.
Conclusion
In two studies, we found null relationships between individual differences in perceived sleep quality, on the one hand, and negative affect reactivity and regulation, on the other, across individuals. Future research is encouraged to further study the within-individual relationship between sleep and negative affect with the use of both subjective and objective measures of these two constructs.
Supplementary Material
Highlights:
We induced negative affect using generic and self-relevant stimuli in laboratories.
We used both self-report and physiological measures to assess negative affect.
We separated the processes of negative affect reactivity and regulation.
We used Bayesian analysis to confirm the null hypotheses.
Acknowledgments
This research was supported by National Institute of Dental and Craniofacial Research R01 DE026771 awarded to James J. Gross (Study 1) and National Center for Complementary and Alternative Medicine P01 AT00665101 awarded to Sean Mackey (Study 2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Links to data and code: Study 1 (https://osf.io/5w2n7/) and Study 2 (https://osf.io/x9e5t/)
References
- Alfarra R, Fins AI, Chayo I, & Tartar JL (2015). Changes in attention to an emotional task after sleep deprivation: Neurophysiological and behavioral findings. Biological Psychology, 104, 1–7. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Spiegelhalder K, Lombardo C, & Riemann D (2010). Sleep and emotions: a focus on insomnia. Sleep Medicine Reviews, 14(4), 227–238. [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, & Beebe DW (2014). Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry and Allied Disciplines, 55(2). 10.1111/jcpp.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Simon E, Vallat R, Barnes CM, & Walker MP (2020). Sleep Loss and the Socio-Emotional Brain. Trends in Cognitive Sciences, 24(6), 435–450. 10.1016/j.tics.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Bürkner PC (2017). brms: An R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80(1), 1–28. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, … Pilkonis PA (2010). Development and Validation of Patient-Reported Outcome Measures for Sleep Disturbance and Sleep-Related Impairments. Sleep, 33(6), 781–792. 10.1093/sleep/33.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. In Handbook of emotions (2nd ed., pp. 173–191). [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The Consensus Sleep Diary: Standardizing Prospective Sleep Self-Monitoring. Sleep, 35(2), 287–302. 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, … Riddell A (2017). Stan : A Probabilistic Programming Language. Journal of Statistical Software, 76(1). 10.18637/jss.v076.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote K, Jancsar C, & Hunt B (2015). Event-related neural response to emotional picture stimuli following sleep deprivation. Psychology & Neuroscience, 8(1), 102. [Google Scholar]
- Dixon ML, Moodie CA, Goldin PR, Farb N, Heimberg RG, & Gross JJ (2020). Emotion Regulation in Social Anxiety Disorder: Reappraisal and Acceptance of Negative Self-beliefs. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(1), 119–129. 10.1016/j.bpsc.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, & Siegle GJ (2009). Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology, 80(3), 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, & Buysse DJ (2011). Cardiovascular Reactivity to Acute Psychological Stress Following Sleep Deprivation. Psychosomatic Medicine, 73(8), 679–682. 10.1097/PSY.0b013e31822ff440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Moodie CA, & Gross JJ (2019). Acceptance versus reappraisal: Behavioral, autonomic, and neural effects. Cognitive, Affective, & Behavioral Neuroscience, 19(4), 927–944. 10.3758/s13415-019-00690-7 [DOI] [PubMed] [Google Scholar]
- Goldstein-Piekarski AN, Greer SM, Saletin JM, & Walker MP (2015). Sleep deprivation impairs the human central and peripheral nervous system discrimination of social threat. Journal of Neuroscience, 35(28), 10135–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, & Walker M (2014). The Role of Sleep in Emotional Brain Function. Annual Review of Clinical Psychology, 10, 679–708. 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, & Cassoff J (2014). The Interplay Between Sleep and Emotion Regulation: Conceptual Framework Empirical Evidence and Future Directions. Current Psychiatry Reports, 16(11). 10.1007/s11920-014-0500-x [DOI] [PubMed] [Google Scholar]
- Jeffreys H (1961). Theory of Probability. Oxford: UK Oxford University Press. [Google Scholar]
- Kahn M, Sheppes G, & Sadeh A (2013). Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology, 89(2), 218–228. 10.1016/j.ijpsycho.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Pillai V, Roth T, & Drake CL (2014). The interplay between daily affect and sleep: A 2-week study of young women. Journal of Sleep Research, 23(6), 636–645. 10.1111/jsr.12190 [DOI] [PubMed] [Google Scholar]
- Klumpp H, Roberts J, Kapella MC, Kennedy AE, Kumar A, & Phan KL (2017). Subjective and objective sleep quality modulate emotion regulatory brain function in anxiety and depression. Depression and Anxiety, 34(7), 651–660. 10.1002/da.22622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD, Samson AC, & Gross JJ (2013). The psychophysiology of mixed emotional states. Psychophysiology, 50(8), 799–811. 10.1111/psyp.12064 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. In Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- Lee CY, Chen HC, Meg Tseng MC, Lee HC, & Huang LH (2015). The relationships among sleep quality and chronotype, emotional disturbance, and insomnia vulnerability in shift nurses. Journal of Nursing Research, 23(3), 225–235. 10.1097/jnr.0000000000000095 [DOI] [PubMed] [Google Scholar]
- Marchewka A, Żurawski Ł, Jednoróg K, & Grabowska A (2014). The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior Research Methods, 46(2), 596–610. 10.3758/s13428-013-0379-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Troy AS, & LeBourgeois MK (2013). Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition & Emotion, 27(3), 567–576. 10.1080/02699931.2012.727783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, & Strecker RE (2011). The cognitive cost of sleep lost. Neurobiology of Learning and Memory, 96(4), 564–582. 10.1016/j.nlm.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel JD, McNealy K, Gianaros PJ, Drabant EM, Gross JJ, Manuck SB, & Hariri AR (2012). Sleep quality and neural circuit function supporting emotion regulation. Biology of Mood & Anxiety Disorders, 2(1), 1–9. 10.1186/2045-5380-2-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S, & Ivers H (2003). Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic Medicine, 65(2), 259–267. 10.1097/01.PSY.0000030391.09558.A3 [DOI] [PubMed] [Google Scholar]
- Palmer CA, & Alfano CA (2017). Sleep and emotion regulation: An organizing, integrative review. Sleep Medicine Reviews, 31, 6–16. 10.1016/j.smrv.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Callan C, & Posey JL (2015). Sleep deprivation affects reactivity to positive but not negative stimuli. Journal of Psychosomatic Research, 79(6), 657–662. 10.1016/j.jpsychores.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Reddy R, Palmer CA, Jackson C, Farris SG, & Alfano CA (2017). Impact of sleep restriction versus idealized sleep on emotional experience, reactivity and regulation in healthy adolescents. Journal of Sleep Research, 26(4), 516–525. 10.1111/jsr.12484 [DOI] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, & Gross JJ (2011). Emotion-Regulation Choice. Psychological Science, 22(11), 1391–1396. 10.1177/0956797611418350 [DOI] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, Radu P, Blechert J, & Gross JJ (2014). Emotion regulation choice: A conceptual framework and supporting evidence. Journal of Experimental Psychology: General, 143(1), 163–181. 10.1037/a0030831 [DOI] [PubMed] [Google Scholar]
- Shermohammed M, Kordyban LE, & Somerville LH (2020). Examining the Causal Effects of Sleep Deprivation on Emotion Regulation and Its Neural Mechanisms. Journal of Cognitive Neuroscience, 1–12. [DOI] [PubMed] [Google Scholar]
- Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, & Buxton OM (2017). Bidirectional, Temporal Associations of Sleep with Positive Events, Affect, and Stressors in Daily Life Across a Week. Annals of Behavioral Medicine, 51(3), 402–415. 10.1007/s12160-016-9864-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Wen JH, Klaiber P, Buxton OM, & Almeida DM (2020). Sleep Duration and Affective Reactivity to Stressors and Positive Events in Daily Life. Health Psychology. 10.1037/hea0001033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempesta D, Couyoumdjian A, Curcio G, Moroni F, Marzano C, De Gennaro L, & Ferrara M (2010). Lack of sleep affects the evaluation of emotional stimuli. Brain Research Bulletin, 82(1–2), 104–108. 10.1016/j.brainresbull.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Tempesta D, Salfi F, De Gennaro L, & Ferrara M (2020). The impact of five nights of sleep restriction on emotional reactivity. Journal of Sleep Research, (February), e13022. 10.1111/jsr.13022 [DOI] [PubMed] [Google Scholar]
- Tempesta D, Socci V, De Gennaro L, & Ferrara M (2018). Sleep and emotional processing. Sleep Medicine Reviews, 40, 183–195. 10.1016/j.smrv.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Van Der Helm E, Gujar N, & Walker MP (2010). Sleep deprivation impairs the accurate recognition of human emotions. Sleep, 33(3), 335–342. 10.1093/sleep/33.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigoureux TFD, Lee S, Buxton OM, & Almeida DM (2019). Stressor reactivity to insufficient sleep and its association with body mass index in middle‐ aged workers. Journal of Sleep Research. 10.1111/jsr.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Neumeister P, Bode K, Heissler J, & Schönfelder S (2010). EmoPicS: subjective and psychophysiological evaluation of new imagery for clinical biopsychological research. Research. Journal of Clinical Psychology and Psychotherapy, Supplement, 1, 11–77. [Google Scholar]
- Wetzels R, & Wagenmakers EJ (2012). A default Bayesian hypothesis test for correlations and partial correlations. Psychonomic Bulletin and Review, 19(6), 1057–1064. 10.3758/s13423-012-0295-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap Y, Slavish DC, Taylor DJ, Bei B, & Wiley JF (2020). Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: a daily intensive longitudinal study. Sleep, 43(3). 10.1093/sleep/zsz250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, Gujar N, Hu P, Jolesz FA, & Walker MP (2007). The human emotional brain without sleep—a prefrontal amygdala disconnect. Current Biology, 17(20), R877–R878. 10.1016/j.cub.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lau EYY, & Hsiao JH (2019a). Sleep deprivation compromises resting-state emotional regulatory processes: An EEG study. Journal of Sleep Research, 28(3), 1–8. 10.1111/jsr.12671 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lau EYY, & Hsiao JH (2019b). Using emotion regulation strategies after sleep deprivation: ERP and behavioral findings. Cognitive, Affective, & Behavioral Neuroscience, 19(2), 283–295. 10.3758/s13415-018-00667-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


