Abstract
Background:
Critically-ill patients and their families suffer a high burden of psychological symptoms due, in part, to many transitions among clinicians and settings during and after critical illness, resulting in fragmented care. Communication facilitators may help.
Design and Intervention:
We are conducting two cluster-randomized trials, one in the U.S. and one in France, with the goal of evaluating a nurse facilitator trained to support, model, and teach communication strategies enabling patients and families to secure care consistent with patients’ goals, beginning in ICU and continuing for 3 months.
Participants:
We will randomize 376 critically-ill patients in the US and 400 in France to intervention or usual care. Eligible patients have a risk of hospital mortality of greater than15% or a chronic illness with a median survival of approximately 2 years or less.
Outcomes:
We assess effectiveness with patient- and family-centered outcomes, including symptoms of depression, anxiety, and post-traumatic stress, as well as assessments of goal-concordant care, at 1-, 3-, and 6-months post-randomization. The primary outcome is family symptoms of depression over 6 months. We also evaluate whether the intervention improves value by reducing utilization while improving outcomes. Finally, we use mixed methods to explore implementation factors associated with implementation outcomes (acceptability, fidelity, acceptability, penetration) to inform dissemination. Conducting the trial in U.S. and France will provide insights into differences and similarities between countries.
Conclusions:
We describe the design of two randomized trials of a communication facilitator for improving outcomes for critically ill patients and their families in two countries.
Introduction
The impact of critical illness is increasing due to our aging population and advances in effectiveness and availability of critical care.1,2 Critically ill patients and their families suffer a high burden of symptoms of depression, anxiety, and post-traumatic stress due, in part, to fragmented medical care that is often poorly aligned with patients’ goals.3–6 Fragmented care may arise from the numerous transitions patients and families experience across clinicians and settings, starting in the ICU and extending to acute care, often including returning to the ICU as well as transitioning to inpatient rehabilitation, skilled nursing facilities, or home.7–9 Through these transitions, patients and families often struggle to navigate the spectrum of goals of care to match their goals with treatments, communicate goals to their clinicians, and make difficult medical decisions. Unfortunately, poor communication compounds an already stressful experience.1,2,7,8,10–14 and can lead to high intensity and unwanted care.15,16 Ineffective communication has been documented in many countries, including U.S. and France, and differences between these countries have been described.17 We are testing whether an intervention to improve communication for critically ill patients and their families can reduce family distress in the U.S. and France.
Building on social cognitive theory18–21 and our prior work,3,22 we designed an intervention to improve outcomes for patients’ family using nurse facilitators to support, model, and teach communication strategies that enable patients and families to secure care aligned with patients’ goals over an acute episode of illness, beginning in the ICU. In a prior version of this intervention, facilitators were trained to apply communication, attachment, and mediation strategies to facilitate social cognitive components to support families. The social cognitive components were designed to: 1) build self-efficacy for communicating with clinicians; 2) improve outcome expectations that this communication can improve care; and 3) enhance behavioral capability through skill building to resolve barriers to effective communication and mediate conflict.22 In our prior trial, the intervention reduced symptoms of depression among family members of critically ill patients with a predicted mortality of at least 30% at 6 months and reduced hospital length of stay and costs, even after accounting for intervention costs.22–24
Feedback from families identified limitations of our prior intervention. This feedback contributed to four specific improvements: (1) include critically ill patients with a lower severity of illness to increase proportion of eligible patients; (2) extend the intervention over 3-months, rather than restricting to the hospital stay, to enable support across transitions; (3) focus on eliciting and implementing patients’ goals of care; and (4) examine factors that can impede or facilitate future implementation and dissemination using a type 1 hybrid effectiveness-implementation trial.25
These two trials, conducted simultaneously in the U.S. and France, have three specific aims. First, we will evaluate the effectiveness of the intervention on patient- and family-centered outcomes after critical illness. The primary outcome is family members’ burden of symptoms of depression over the 6 months post-randomization. Second, we evaluate the intervention’s effectiveness for increasing healthcare value. Finally, we use mixed-methods to understand factors and outcomes related to the implementation of this intervention. We chose to conduct two separate trials because of the dramatic differences in the healthcare cultures, the organization of healthcare systems, and the research funding mechanisms.
Methods
Overview:
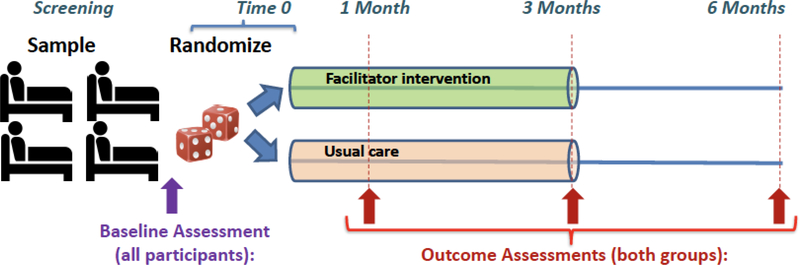
We are conducting two cluster-randomized trials of a single intervention designed to improve outcomes for critically ill patients and their family. The trials are conducted in four hospitals in Seattle, Washington and four hospitals in France, including 2 hospitals in Paris ad one hospital in Brest and one in Nantes. In the US, we aim to randomize 376 patients to intervention (n=188) or control (n=188) and we estimate 564 family members (approximately 1.5 family member per patient). In France, we aim to randomize 400 patients to intervention (n=200) or control (n=200) and estimate 400 family members. US and French investigators choose slightly different numbers of patients and family members based on loss to follow-up and numbers of family members per patient from prior studies from each country.3,22,26–30 The facilitators are experienced ICU nurses with specialized training to: 1) define and discuss goals of care with patients and families, with attention to their emotional needs; 2) support and model successful approaches for discussing goals with clinicians; 3) set expectations for success for these discussions; and 4) reduce barriers by identifying and mediating conflict. Training included at least 2 days of combined didactic and practice sessions including role play with simulated family members. A training manual will be available after study completion. Patient- and family-centered outcomes are assessed at randomization, and 1, 3, and 6 months after randomization (Figure 1).
Figure 1:
Overview of the study timeline
Study population and participant eligibility
A. Patients:
Using the EHR and daily ICU rounds (Monday to Friday), study staff identify consecutive patients in the ICU who meet all four of the following eligibility criteria: 1) age ≥18 years, 2) English-speaking (in the U.S.) or French-speaking (in France), 3) a family member available to participate, and 4) a chronic life-limiting illness suggesting a median survival of 2 years or a severe acute illness with a risk of hospital mortality of >15%. Chronic life-limiting illnesses, similar to our prior trial,22 target a median survival (without critical illness) of two years (see Appendix). Acute illness criteria include an APACHE-II (in the U.S.) or SOFA (in France) predicting a ≥15% risk of hospital mortality (the different severity of illness scores was based on tradition of prior research in each country). After initial screen and a “opt out” text page is sent to the patient’s attending physician to confirm appropriateness for the study, study staff contact the patient or, if the patient doesn’t have decisional capacity, a legal next of kin in-person or by phone. Decisional capacity is determined by the attending physician (or designee) to align with clinical practice.31 Patients without decisional capacity who had no eligible family are excluded. We anticipate that the majority of eligible patients will not have decisional capacity and the intervention will focus on family participants.
C. Family:
Family participants are identified by the patient if the patient has capacity. If the patient does not have decisional capacity, family are identified via the EHR or by a legal surrogate decision-maker. “Family” is not confined to immediate family. Any family or friend is eligible if they are 18 years or older and identified by the patient or legal surrogate as involved in care for the patient, provided they have primary language proficiency and no cognitive deficits limiting their ability to complete surveys. Family members are recruited by phone or in person. We do not limit the number of family members who can participate but anticipate 1–3 family members per patient.
Theoretical basis of the intervention:
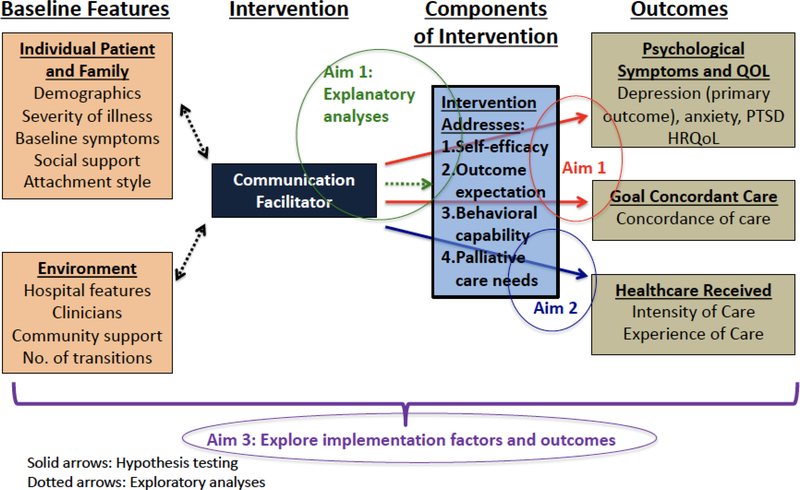
This intervention draws on social cognitive theory,18–21 which posits that the impetus for behavior change arises from interaction between people, their behaviors, and their environments. Self-efficacy, outcome expectations, and behavioral capabilities are key targets that support behavior change. Facilitators address each in our intervention:32 1) self-efficacy by discussing ways that patients and families have had difficult conversations in the past and what worked for them; 2) outcome expectations by discussing how having effective conversations with clinicians can help assure treatment is aligned with goals; and 3) behavioral capabilities by teaching communication skills, addressing barriers, and mediating conflict. Figure 2 shows the role of social cognitive theory within a commonly-used model for designing behavioral interventions to improve quality of life, as well as how our Aims fit in this model.33–37
Figure 2:
Conceptual Model for study
Operationalization of intervention:
Facilitators follow each intervention patient and family for 3 months following randomization and implement the key components of the intervention summarized in Table 1. Since most eligible patients won’t have decisional capacity, intervention components focus on family members.
Table 1:
Overview of the Key Components of the Intervention
| GOALS | FACILITATOR SKILLS OR TASKS | TOOLS |
|---|---|---|
| A. Increase self-efficacy for identifying and discussing goalsof-care | Support discussions of prognosis and of values, goals and preferences | Facilitated values history51 |
| B. Improve outcome expectations by identifying and resolving barriers | Discuss how effective communication can alter and improve treatment plans | Attachment theory to adapt communication177,178 |
| C. Increase behavioral capacity with skills in communication & mediation | Address barriers; identify and mediate conflict; teach mediation skill | Mediation training for facilitators73,74 |
A. Increase self-efficacy:
Based on our prior research,38–45 and the work of others,46–51 we train facilitators to support, model, and teach family members to identify and integrate patients’ values into goals of care, preferences, and decisions. Facilitators discuss times family members have navigated difficult conversations, praising successful behaviors. Activities to identify and integrate goals of care include: 1) facilitating and supporting discussions of prognosis among patients, families and clinicians;52–56 2) facilitating and supporting discussions of treatment preferences;15,46,57,58 and 3) obtaining a facilitated values history for incapacitated patients from family and translate this into treatment preferences.51 Obtaining a facilitated values history includes a tool to enhance patient-centered discussions of prognosis, goals of care, and treatment preferences: the Facilitated Values History51 for family-clinician communication that focuses family on the patient’s values and goals of care. Facilitators participate in these discussions and provide guidance with this content. To increase patients’ and families’ capacity to define, understand and convey their goals, facilitators also elicit strong emotion and respond with empathy to allow participants to feel heard and supported. The decision psychology literature suggests that strong emotions can impair information processing and reasoning.51 Therefore, having a facilitator elicit strong emotion with unhurried listening and empathy can help a person transition to cognitive processing and decision making.
B. Improve outcome expectations for achieving goals of care:
The facilitators model and teach strategies to family members to support their expectations for having successful and effective conversations and how communicating well can improve their care. The focus on goals of care is a new addition based on feedback from the prior trial. These strategies include exploring prior experiences and the outcomes of those experiences. (Did respondents feel encouraged or discouraged? What would have helped them manage the discussion successfully? How can the facilitator help?) Additionally, using attachment theory,59 the facilitators identify family members’ interpersonal communication style to ensure that outcome expectations are appropriately tailored to each patient and family. Attachment theory provides a framework to address individuals’ capacities to communicate with others and influences communication with healthcare teams.23,60–63 Three of the 4 attachment styles pertain to about half the general population64–66 who may derive particular benefit from the use of targeted communication skills that support difficult decision-making. For example, individuals who are predominantly “self-reliant” in stressful situations (25% of the population) may be reluctant to ask questions or participate in shared decision-making; they may benefit from being given options and feeling in charge. Those who are predominantly “support-seeking” in stressful situations (10% of the population) often have high emotional needs that may be inadequately addressed in harried healthcare settings; setting a structured schedule to have questions answered may be reassuring. Individuals with a “cautious” attachment style (10% of the population) may exhibit approach-avoidance behavior under stress so that attempts at collaborating may be abandoned out of fear of relying on others; communication that is non-judgmental and responsive can enhance trust.63 Facilitators use a brief, valid measure of attachment styles (the Relationship Scales Questionnaire)65 that we used successfully in our prior trial.22 Importantly, facilitators use these styles to adapt communication approaches to meet the needs of each patient and family member.
C. Increase behavioral capability through skill acquisition and conflict mediation:
Facilitators model and teach skills that support and advance successful discussions. For example, facilitators help patients and families prepare for discussions by reviewing their goals of care, writing down unanswered questions, and anticipating and planning responses to likely barriers (e.g., “clinicians are too busy”, “I don’t like asking questions because I don’t want to be one of those annoying people who asks too many questions”). Facilitators also model and teach ways to address conflict, a common occurrence in healthcare that frequently involves goals of care and is a key barrier to communication.67–70 Incorporating mediation into healthcare can identify and resolve conflicts, thereby improving communication, as well as patient and family outcomes.71–74 If patients and families feel supported and experience less conflict, they may be more able to process information and make decisions. We train facilitators in mediation principles including: summarizing the issues contributing to conflict, avoiding unwarranted assumptions that are underlying the conflict, framing issues neutrally, focusing on issues rather than personalities, and making proposals for resolution.75–77 Facilitators model these skills for family members during the discussions about goals of care and around transitions in care settings that occur during the study period. We target all of the following types of conflict: clinician-patient, clinician-family, and intra-family.
Description of the conduct of the intervention
A. Implementation of intervention during and after the ICU:
During the ICU stay, facilitators interact in person, and by phone and videoconference, with family members and many types of clinicians (physicians, nurses, social workers, etc.). Following the ICU stay, facilitators interact with patients, family and clinicians in person and by phone for 3 months from randomization (when most first readmissions occur78–80) or for 1 month after a patient’s death occurring in the first 3 months. In-person contacts include visits to patients’ homes and/or care facilities; phone contacts include calls to family members. Because prior studies suggest frequent contact is important, the schedule for contact is a minimum of every 48 hours in the ICU, every 72 hours in the acute care setting,79,81–85 within 72 hours of change in care setting, weekly for a month after hospital discharge, and then twice monthly. The facilitators use clinical judgment if they feel more frequent contact is warranted, and family members have access to facilitators through phone and email 5 days per week. In addition to checking directly with patients/families during regular contacts (calls, visits), facilitators also access the medical record to ensure they have accurate information about appointments and treatment plans. Facilitators encourage referral to inpatient or outpatient palliative care services when they identify palliative care needs (i.e., communication, decision-making, and symptom needs). We record all facilitator contacts, including the number and type of contacts for each patient and family to assess intervention fidelity, “dose”, and costs. Facilitators complete a checklist of study activities after each contact (see Appendix.)
B. Training facilitators:
Training is provided by the investigators and consultants with expertise in the following areas: clinical communication skills, use of attachment theory, and mediation. Training addresses the use of these skills across care settings (e.g., inpatient, outpatient, home, care facility). Communication training includes identification of goals of care, incorporating principles of advance care planning and the facilitated values history. Attachment theory training includes understanding the four attachment styles, the consequences of these styles for communication, and the approaches most appropriate for each style. Mediation training covers skills associated with assessment and preparation: rapport building; information gathering and exchange; development and evaluation of options; shuttle diplomacy; and resolution. Facilitators participate in role-playing exercises during training with standardized family members, and they are required to demonstrate mastery of intervention skills before engagement and during fidelity checks. We will enhance and expand our current training manual as the trial goes on, enhancing dissemination of the intervention to other institutions.
C. Intervention fidelity:
To ensure scientific rigor, intervention fidelity is assessed with methods outlined by the NIH Behavioral Treatment Fidelity Workgroup on consistency in dose, providers, delivery, receipt, and enactment of interventions.86,87 Facilitators meet weekly with investigators to discuss intervention patients and ensure intervention fidelity overall and between facilitators. Formal fidelity checks will be completed for 10% of patients/families, in which encounters are audio recorded and then compared with an intervention checklist for completion of intervention components (Appendix).
Control group:
Participants randomized to control complete the same study measures at all data collection points, but the facilitator is not involved. Contacts with study staff at all data collection points are also designed to enhance survey return rates.
Randomization:
The unit of randomization is the patient. The potential for contamination due to individual clinicians caring for patients in both groups is minimized because the focus of the intervention is specific to the individual patient and family and tailored to their needs. Randomization occurs in variable-sized blocks stratified by hospital, separately for each trial. Family members are clustered within patients. In the US and France, we allow enrollment of multiple family members per patient with no maximum. Based on prior studies, we expect 1.5 family members per patient in the US and 1.0 per patient in France.3,22,26–30
Outcomes and mediators:
Figure 2 shows a conceptual model for assessment of outcomes, baseline individual and environment factors, and potential mediators influencing the intervention. The outcomes and potential mediators are summarized in Table 2.
Table 2:
Main study measures and data collection protocol
| MAIN OUTCOME MEASURES | CONCEPT | DATA COLLECTION: SOURCE & TIME |
|---|---|---|
| Aim 1: Outcomes | ||
| Primary Outcome: HADS depression score | Depressive and anxiety symptoms | Patients and/or Families: Enrollment (if able),1, 3, 6, months post-randomization for all; 1 month after death for family members |
| SUPPORT question109 | Goal-concordant care | |
| Impact of event scale (IES)88,105,106 | Post-traumatic stress symptoms | |
| QUAL-E and QUAL-E (Fam)114–116 | Quality of life | |
| Aim 1: Potential Mediators of the Intervention | ||
| Perceived Competence Scale (PCS)117,118 | Efficacy/capability/expectations for health behavior | Patients and/or Families: Enrollment (if able),1, 3, 6 months post-randomization |
| Aim 2: Outcomes | ||
| Hospitalizations, ICU admits,, palliative care consultations | Healthcare utilization; use of palliative care | Medical record: After-death or 6 months |
| Hospital and ICU costs | Inpatient costs of care from index hospitalization (direct and indirect) | Medical record/financial databases(US only) |
| Aim 3: Implementation Factors and Outcomes | ||
| Domains: intervention, settings, individual, process | Key factors identified from CFIR125 | Patients/families/clinicians: Qualitative interviews |
| Outcomes: acceptability, fidelity, penetration | Key outcomes identified by Proctor126 |
Aim 1:
For Aim 1, we will evaluate the effectiveness of the intervention on one primary and several secondary outcomes. Although we will assess psychological symptoms (depression, anxiety, and PTSD) in patients and family, most critically ill patients are unable to participate. Therefore, the intervention and the outcomes focus on the family. The primary outcome is the depression subscale of the HADS over 6 months (including 1-, 3-, and 6-month assessments). These outcomes were selected based on evidence supporting their importance to patients and families.88
A. Depression and anxiety (with depression as the primary outcome):
We assess patient and family member symptoms of depression and anxiety with the Hospital Anxiety and Depression Scale (HADS), which has become standard for ICU and post-ICU studies.4,88–90 The HADS is a reliable and valid 14-item, 2-domain (anxiety and depression) tool used to assess symptoms of psychological distress.91,92 Each item is scored on a 4-point scale (ranging from 0–3) with scores for each 7 item subscale (anxiety and depression) ranging from 0–21. HADS has been used in over 700 studies with evidence of reliability, validity and responsiveness among critically ill patients and their family.3,93–104
B. Post-traumatic stress:
We assess patient’s and family’s symptoms of PTSD with the Impact of Events Scale-6 (IES-6). It is derived from IES and IES-R, PTSD measures that are recommended for use with ICU survivors to evaluate PTSD88,105,106 (Cronbach’s Alpha, .80–.96 for total scores, .46–.56 for subscales; IES-R (0.96).107,108 Each item is scored on a 4-point scale that addresses symptom severity ranging from “not at all” to “extremely”, and it may be scored either as a summary score or as a mean of the 6 items.
C. Goal-concordant care:
We measure concordance between the care patients want and the care they are receiving with two questions.109 The first defines patients’ goals: “If (you/the patient) had to make a choice at this time, would (you/the patient) prefer a course of treatment focused on extending life as much as possible, even if it means having more pain and discomfort, or would (you/the patient) want a plan of care focused on relieving pain and discomfort as much as possible, even if that means not living as long?” The second question assesses perceptions of current treatment using the same two options. The outcome is a dichotomous variable of whether the preference matches the report of care received. Although this creates a “false dichotomy” in that many patients want both; this “forced choice” helps identify patients’ top priority.110–112 This approach mirrors clinical practice in which goals of care are determined by the legal surrogate decision-maker when patients are unable to respond for themselves. Based on prior studies, we expect 50–60% of controls will report goal-concordant care.109,113
D. Quality of life:
We use the QUAL-E and the QUAL-E (Fam) to assess quality of life. These companion questionnaires, one completed by patients and the other by family members, were developed systematically using focus groups, interviews and surveys to identify items, and then factor analyzed to identify quality of life domains. The QUAL-E includes 20 items in 4 domains (life completion, relationship with the healthcare system, preparation, symptom impact) with acceptable levels of fit (CFI= 0.89, GFI= 0.88, RMSEA =.06) and high levels of internal consistency (Cronbach’s alpha >= 0.70 for 3 of the 4 factors). It demonstrated convergent and discriminant validity analyses against the FACIT-SP and other measures. The QUAL-E (Fam) includes 17 items in two domains (relationship with healthcare providers, completion) and additional items assessing aspects of preparedness. The QUAL-E (Fam)’s convergent and discriminant validity were supported with findings that were in line with predicted associations with the PCAS, FACIT-Sp and other measures.114 Like other quality of life measures in which subscales assess domains that are conceptually distinct and therefore are not easily summed,114 we selected domains and items with relevance for the current study. For the QUAL-E, these include all domains except symptom impact; for the QUAL-E (Fam), we included all 17 items.114–116
E. Patient and family report of self-efficacy, outcome expectations, and behavioral capacity:
Participants complete the Perceived Competence Scale (PCS) as a measure of these three components derived from social cognitive theory. It is a 4-item questionnaire that has been used to measure respondent’s perceptions of competency and agency at enacting the specific behaviors being assessed.117,118 Its validity has been supported by significant associations between improved PCS scores and changes in a variety of healthcare behaviors, including short and long term tobacco cessation,119–121 glycemic control,118,122 and oral health.123 Reliabilities are reported consistently at alpha > 0.80.118,123
Aim 2:
We will examine the effectiveness of the intervention for reducing ICU and hospital readmissions and reducing costs of care. The intervention is designed to affect these outcomes by improving overall communication and, more specifically, clarifying and communicating goals of care. In France, because of universal healthcare and limited availability of cost data, this aim will focus on utilization only.
We will measure hospital readmission after initial hospital discharge through the available electronic health records (EHR), institutional billing systems, and patient/family self-reports. By using all three sources for data, we expect to capture hospitalizations that may occur outside of the healthcare system from which the patient was enrolled. Our primary focus will be readmission within 30 days of hospital discharge as this is a national standard,124 but we will also collect hospital and ICU length of stay, emergency department visits and outpatient clinic visits from the EHR or surveys.
Aim 3:
Aim 3 enables us to evaluate the factors and outcomes that influence implementation of the intervention. We will use semi-structured interviews with a purposive sample of family members, clinicians, and administrators, as well as facilitators, to identify diverse interview participants with experience with the intervention and continue recruitment until thematic saturation is achieved.
A. Key factors for implementation:
Key factors that influence implementation are explored through semi-structured interviews with family members, clinicians, and administrators. Using the Consolidated Framework for Implementation Research (CFIR), questions are modified to provide a tailored assessment of the intervention and the context in which it is implemented; discussions focus on the effect of inner and outer settings, individual characteristics, and processes of care.125
B. Key implementation outcomes:
Outcomes will be guided by the Outcomes for Implementation Research126 and use both qualitative and quantitative measures. Qualitatively, semi-structured interviews will explore three key implementation outcomes (acceptability, fidelity, appropriateness).126 Quantitative measures will include the proportion of eligible patients enrolled, the proportion of patients randomized to intervention who receive the intervention (facilitators talk with family members), and assessments of intervention dose (examining time spent by facilitators and intervention components delivered).
Additional measures and data
A. Description of participants:
For all participants, we collect age, gender, race/ethnicity, education, employment, and social support. For patients, we collect comorbidities and severity of illness with APACHE-II (in the U.S.)127–129 or SOFA (in France.)130 For family, we collect relationship with the patient and whether they reside with the patient.
B. Attachment style:
To identify attachment styles, we administer the Relationship Scales Questionnaire (RSQ)65 which we successfully used as part of the prior facilitator intervention.22
Data collection:
Study staff involved in outcome data collection are blinded to treatment assignment.
A. Surveys:
To enhance response rates, surveys may be completed in person, by phone, by mail, or online, depending on participant preference. Strategies to enhance response rates include: 1) use of study staff known to participants to contact participants at each data collection point; 2) inclusion of small monetary gratuity ($10) with each follow-up questionnaire (in the U.S. only); and 3) reminder contacts prior to, and following each distribution time point.131,132 Families complete the same measures at the same intervals as patients with two exceptions: 1) families complete the baseline measures at randomization, while for patients there may be a delay for them to regain decisional capacity; and 2) families of patients who die receive modified surveys 4–6 weeks after a patient’s death, which include the HADS, IES-6, and QUAL-E (Fam).
B. Electronic health record (EHR):
We will use the EHR to supplement family report of healthcare utilization. We will also use the EHR to collect disease characteristics and specific processes of care during and after the ICU stay including treatment intensity (e.g., CPR, mechanical ventilation), transitions in care, and palliative care consults.
C. Hospital financial databases:
For hospitals in the U.S., we will obtain costs associated with inpatient utilization from hospital financial databases and will link this with our EHR data.
D. Qualitative data collection:
Aim 3 collects data with approximately 30 semi-structured interviews with family members (n=15), clinicians (n=10), and administrators (n=5). We use purposive sampling with the goal of a diverse group based on race/ethnicity, age, gender, and, for clinicians, specialty and year of training.
Overview of Analytic Methods
Aim 1 Analyses:
Our primary outcome is family members’ symptoms of depression over 6 months. We will follow the intention-to-treat principle. Our primary analysis will use a linear mixed effects model with family member symptoms at all time points (1,3, 6 months) as the response, main effects for intervention and time points, and random effects to account for multiple measurements (time points) per family member and multiple family members per patient. We will also adjust for hospital, since randomization is stratified by hospital, and for response at randomization to improve precision. This model allows the average response to be different at 1, 3, and 6 months, but assumes the intervention has the same effect at each of these times. We will also explore whether the effect of intervention is different across time by including an interaction between time and intervention. The advantage of using the data at all 3 time points and a mixed model approach is that we can gain precision; it also allows missing responses, assuming the responses are missing at random. We will use a similar approach for the other continuous outcomes, and a generalized linear mixed effects model for the binary outcome of goal-concordant care.
Aim 2 Analyses:
This aim will evaluate the effect of intervention on costs (in the U.S.) and utilization (in the U.S. and France) during the 6-month study period from the EHR, hospital financial databases and surveys. Utilization measures, including ICU-free days and hospital-free days to day 30, days in ICU, and days in hospital, will be evaluated with linear regression. As is standard for cost analyses, we plan to use generalized linear models with a gamma error distribution and identity link function, to explore the difference in mean healthcare costs for total inpatient hospital costs and disaggregated costs (direct and indirect costs), between intervention and control participants during the initial hospitalization and up to 30 days after randomization.133 To estimate the cost of providing the intervention, we will collate study staff records of the time involved in implementing and sustaining the intervention during the trial, not including research activities such as obtaining informed consent and the evaluation of the interventions. We will then determine the average full time equivalent (FTE) over intervention patients, and convert FTE to costs based on average yearly salaries at the study sites, including benefit load. All costs will be adjusted for inflation through the study period.
Aim 3 analyses:
For quantitative analyses of implementation, we will describe the proportion eligible who enroll and the fidelity with the intervention components. For qualitative analyses exploring intervention implementation, we will perform thematic analysis of transcribed interviews to explore feedback on the intervention and ways to improve the intervention delivery and implementation.134,135 Analyses will be guided by the Consolidated Framework for Implementation Research (CFIR) in exploration of factors affecting implementation125 and by Outcomes for Implementation Research in exploration of implementation outcomes.126 For example, we can examine facilitators by “high” and “low” fidelity and sites by “high” and “low” penetration to examine cross-case patterns in CFIR constructs that differentiate these groups. Qualitative data will be imported into analytic software (DeDoose and NVivo), where investigators will perform iterative, inductive coding to identify recurrent themes, categories, and relationships among themes and categories. To ensure trustworthiness (a qualitative concept similar to reliability in quantitative analysis135–138), we will perform a “member check” of the results with prior participants selected for diversity of participant type.
Missing data:
Our goal is to minimize missing data by minimizing respondent burden, offering multiple methods for survey completion, and having trained staff with repeated contact with participants. However, data could still be missing due to skipping individual items on a survey, omissions in the EHR, lack of follow-up, or death. We will quantify the amount of missing data and apply appropriate methods to account for missing data.139,140
Sample size estimates
The focus for sample size estimation is the primary outcome: family member depression as assessed by the HADS depression subscale. For all calculations, we assume a two-sided test with a significance level of 0.05. If we assume 300 total family members (1 family member per patient and 150 per arm), a standard deviation of HADS depression scores of 4.2 points141 in both arms, 3 measurements of depression (at 1, 3, and 6 months), and an intraclass correlation (ICC) of 0.2, we will be able to detect a difference in mean depression of at least 0.93 points with 80% power and 1.07 points with 90% power. If we had only 1 measurement instead of 3 per family member or, equivalently, if the ICC were 1, we would have 80% power to detect a difference of at least 1.36 points. If we have more than 1 family member per patient (1.5 is expected), we will be able to detect smaller differences at the same power. Across all of these varying conditions, detectable differences are slightly within or below the estimated minimally clinically important difference for the HADS depression scale of 1.6 to 2.0, suggesting we will have adequate power to identify a minimally clinically important difference. In the US we anticipate ≥80% complete data and in France we anticipate >75% complete data, so we aim to randomize 376 patients in the US and 400 in France to achieve at least 300 patients and at least 300 family members with complete data for each trial (U.S. and France).
Adaptations for the COVID-19 pandemic
These trials were underway when the COVID-19 pandemic started in 2020. Both trials stopped enrollment for the initial surge of COVID-19 in the US and France. Once the caseloads of COVID-19 stabilized, both trials resumed with recruitment and intervention activities occurring virtually – by phone or videoconferencing. Both trials are tracking the amount of contact occurring in person as compared to phone or videoconferencing.
Discussion
We have described two parallel randomized trials that are being conducted, one in the U.S. and one in France. These trials provide innovation and advance the science of palliative care in critical care settings in three important ways: enhancements to the intervention based on prior studies, innovation in the model of care coordination the intervention is promoting, and innovation in the use of a type 1 hybrid effectiveness-implementation trial to examine implementation processes and outcomes.25
This intervention is based on the intervention in a prior ICU communication facilitator study22 and builds on prior work evaluating navigators or facilitators in a variety of settings.142–147 However, our intervention is novel by its inclusion of nurse facilitators with innovative training that supports, models, and teaches communication skills to patients and family facing critical illness and also continues to follow patients and families beyond the ICU for three months. Since this intervention will be implemented both during and after critical illness, it addresses key gaps identified in recent systematic reviews4,148–151 by: 1) beginning early in the high stress time of critical illness and following patients after ICU; 2) focusing on communication about goals of care; and 3) targeting a diverse population (e.g. age, diagnoses, SES) to enhance generalizability, scalability, and cost-effectiveness.
One of the major challenges for interventions designed to improve communication about goals of care has been a limited number of validated outcomes.152–155 We address this with standardized measures with good psychometric characteristics. The effectiveness outcomes— reducing the burden of psychological symptoms and impaired quality of life—will be assessed with well-validated measures. These measures are standard for ICU and post-ICU studies.4,88–90 Our intervention extends over 3 months and we elected to include outcomes extending beyond the intervention to 6 months to assess whether benefit persists beyond the intervention as seen our prior study showing benefit at 6 months.22 In addition, we assess social cognitive outcomes of behavior change (i.e., patient/family self-efficacy, outcome expectations, behavioral capacity) that are targeted by this intervention with a validated measure that will provide insights into the mechanisms that mediate the study’s outcomes.156–159
Our primary outcome is family member symptoms of depression over the 6 months after randomization. The importance of improving outcomes for family caregivers and the value this brings to patient outcomes is well-established.160,161 When family members suffer from the burden of critical illness, patients also suffer. Much of the suffering for family members comes from the difficulties of surrogate decision-making and the fact that most critically ill patients do not have decisional capacity.162 Interventions that reduce family member distress also improve the care they can provide to patients.3,22 In addition, stress extends beyond the ICU as family bear a significant burden of caregiving after the ICU.3,93,163 As many as 20–60% of families and patients suffer a high burden of psychological symptoms during and after the ICU.3,93,164–168 Improving communication is key because poor communication worsens distress,169 and interventions that improve communication reduce distress.3,22 Further, caregiver stress increases healthcare use and reduces quality of life.170–174
We have designed a hybrid implementation-effectiveness trial to use the innovation of implementation science to promote implementation and dissemination of effective interventions. Implementation science, or T4 translation research, is the systematic study of methods to promote the uptake and integration of health interventions.25,126,175,176 Hybrid effectiveness-implementation trials, such as this, represent an innovative design that can facilitate more rapid translation of research into clinical practice while also evaluating the effectiveness of the intervention.25 Improving communication in complex and diverse settings is an ideal intervention for a hybrid effectiveness-implementation trial because evidence of the value of communication is clear, but the core issue is how to efficiently implement interventions that promote effective care.151 We are collecting data on implementation factors and outcomes in order to facilitate implementation and dissemination in the future and advance implementation science for palliative care and communication interventions.
In this paper, we have described the protocol that is being implemented in two randomized trials designed to evaluate a communication facilitator intervention to improve patient- and family-centered outcomes for critically ill patients and their families during and after a patient’s critical illness. Conducting these parallel studies in the U.S. and France provides a unique opportunity to evaluate effectiveness and implementation in two very different medical and social cultures.
Supplementary Material
Funding:
US: Funded by the National Institute of Nursing Research (R01 NR018161)
France: Funded by the French Ministry of Health, Programme Hospitalier de Recherche Clinique (AOM 18139)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: an analysis of bed numbers, use, and costs. Critical care medicine 2004;32:1254–9. [DOI] [PubMed] [Google Scholar]
- 2.Teno JM, Gozalo P, Khandelwal N, et al. Association of Increasing Use of Mechanical Ventilation Among Nursing Home Residents With Advanced Dementia and Intensive Care Unit Beds . JAMA Intern Med 2016;176:1809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 2007;356:469–78. [DOI] [PubMed] [Google Scholar]
- 4.Davidson JE, Aslakson RA, Long AC, et al. Guidelines for Family-Centered Care in the Neonatal, Pediatric, and Adult ICU. Crit Care Med 2017;45:103–28. [DOI] [PubMed] [Google Scholar]
- 5.Long AC, Kross EK, Davydow DS, Curtis JR. Posttraumatic stress disorder among survivors of critical illness: creation of a conceptual model addressing identification, prevention, and management. Intensive Care Med 2014;40:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 2009;35:796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA 2002;288:2151–62. [DOI] [PubMed] [Google Scholar]
- 8.Wachter RM, Goldman L. The hospitalist movement 5 years later. JAMA 2002;287:487–94. [DOI] [PubMed] [Google Scholar]
- 9.Pantilat SZ, Alpers A, Wachter RM. A new doctor in the house: ethical issues in hospitalist systems. JAMA 1999;282:171–4. [DOI] [PubMed] [Google Scholar]
- 10.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013;309:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff JL, Giovannetti ER, Boyd CM, et al. Effects of guided care on family caregivers. The Gerontologist 2010;50:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wootton R, Gramotnev H, Hailey D. Telephone-supported care coordination in an Australian veterans population: a randomized controlled trial. Journal of telemedicine and telecare 2010;16:57–62. [DOI] [PubMed] [Google Scholar]
- 13.Amaravadi RK, Dimick JB, Pronovost PJ, Lipsett PA. ICU nurse-to-patient ratio is associated with complications and resource use after esophagectomy. Intensive Care Med 2000;26:1857–62. [DOI] [PubMed] [Google Scholar]
- 14.Pronovost PJ, Jenckes MW, Dorman T, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA 1999;281:1310–7. [DOI] [PubMed] [Google Scholar]
- 15.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botti S, Orfali K, Iyengar SS. Tragic choices: Autonmy and emotional responses to medical decisions. J Consum Res 2009;36:337–52. [Google Scholar]
- 18.Bandura A Social Learning Theory. New Jersey: Prentice Hall; 1977. [Google Scholar]
- 19.Bandura A Self-Efficacy: Toward a unifying theory of behavior change. Psychological Reviews 1977;84:191–215. [DOI] [PubMed] [Google Scholar]
- 20.Bandura A Self-efficacy mechanism in human agency. American Psychology 1982;37:122–47. [Google Scholar]
- 21.Strecher VJ, DeVellis BE, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Education Quarterly 1986;13:73–91. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JR, Treece PD, Nielsen EL, et al. Randomized Trial of Communication Facilitators to Reduce Family Distress and Intensity of End-of-Life Care. Am J Respir Crit Care Med 2016;193:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Ciechanowski PS, Downey L, et al. Development and evaluation of an interprofessional communication intervention to improve family outcomes in the ICU. Contemp Clin Trials 2012;33:1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khandelwal N, Benkeser D, Coe NB, Engelberg RA, Curtis JR. Economic feasibility of staffing the intensive care unit with a communication facilitator. Ann Am Thorac Soc 2016;13:2190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox CE, Hough CL, Carson SS, et al. Effects of a Telephone- and Web-based Coping Skills Training Program Compared with an Education Program for Survivors of Critical Illness and Their Family Members. A Randomized Clinical Trial. Am J Respir Crit Care Med 2018;197:66–78. [DOI] [PubMed] [Google Scholar]
- 27.Cox CE, Wysham NG, Kamal AH, et al. Usability Testing of an Electronic Patient-Reported Outcome System for Survivors of Critical Illness. Am J Crit Care 2016;25:340–9. [DOI] [PubMed] [Google Scholar]
- 28.Cox CE, Wysham NG, Walton B, et al. Development and usability testing of a Web-based decision aid for families of patients receiving prolonged mechanical ventilation. Ann Intensive Care 2015;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azoulay E, Pochard F, Chevret S, et al. Impact of a family information leaflet on effectiveness of information provided to family members of intensive care unit patients: a multicenter, prospective, randomized, controlled trial. Am J Respir Crit Care Med 2002;165:438–42. [DOI] [PubMed] [Google Scholar]
- 30.Kentish-Barnes N, Chevret S, Champigneulle B, et al. Effect of a condolence letter on grief symptoms among relatives of patients who died in the ICU: a randomized clinical trial. Intensive Care Med 2017;43:473–84. [DOI] [PubMed] [Google Scholar]
- 31.Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007;357:1834–40. [DOI] [PubMed] [Google Scholar]
- 32.Glanz K, Rimer BK. Theory at a Glance: A Guide for Health Promotion Practice. Bethesda, MD: National Institutes of Health, National Cancer Institute; 1997. [Google Scholar]
- 33.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995;273:59–65. [PubMed] [Google Scholar]
- 34.Zubritsky C, Abbott KM, Hirschman KB, Bowles KH, Foust JB, Naylor MD. Health-related quality of life: expanding a conceptual framework to include older adults who receive long-term services and supports. Gerontologist 2013;53:205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrans CE. Differences in what quality-of-life instruments measure. J Natl Cancer Inst Monogr 2007:22–6. [DOI] [PubMed] [Google Scholar]
- 36.Sousa KH, Kwok OM. Putting Wilson and Cleary to the test: analysis of a HRQOL conceptual model using structural equation modeling. Qual Life Res 2006;15:725–37. [DOI] [PubMed] [Google Scholar]
- 37.Wyrwich K, Harnam N, Revicki DA, Locklear JC, Svedsater H, Endicott J. Assessing health-related quality of life in generalized anxiety disorder using the Quality Of Life Enjoyment and Satisfaction Questionnaire. Int Clin Psychopharmacol 2009;24:289–95. [DOI] [PubMed] [Google Scholar]
- 38.Curtis JR, Engelberg RA, Wenrich MD, et al. Studying communication about end-of-life care during the ICU family conference: Development of a framework. J Crit Care 2002;17:147–60. [DOI] [PubMed] [Google Scholar]
- 39.Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med 2005;171:844–9. [DOI] [PubMed] [Google Scholar]
- 40.Curtis JR, Patrick DL, Shannon SE, Treece PD, Engelberg RA, Rubenfeld GD. The family conference as a focus to improve communication about end-of-life care in the intensive care unit: Opportunities for improvement. Crit Care Med 2001;29:N26–N33. [DOI] [PubMed] [Google Scholar]
- 41.Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. Chest 2008;134:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White DB, Braddock CH 3rd, Bereknyei S, Curtis JR. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med 2007;167:461–7. [DOI] [PubMed] [Google Scholar]
- 43.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med 2007;35:442–8. [DOI] [PubMed] [Google Scholar]
- 44.White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. The language of prognostication in intensive care units. Med Decis Making 2010;30:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White DB, Malvar G, Karr J, Lo B, Curtis JR. Expanding the paradigm of the physician’s role in surrogate decision-making: an empirically derived framework. Crit Care Med 2010;38:743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for endof-life decision making. Ann Intern Med 2010;153:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med 2007;167:453–60. [DOI] [PubMed] [Google Scholar]
- 48.Back AL, Arnold RM, Tulsky JA, Baile WF, Fryer-Edwards KA. Teaching communication skills to medical oncology fellows. J Clin Oncol 2003;21:2433–6. [DOI] [PubMed] [Google Scholar]
- 49.Walling A, Lorenz KA, Dy SM, et al. Evidence-based recommendations for information and care planning in cancer care. J Clin Oncol 2008;26:3896–902. [DOI] [PubMed] [Google Scholar]
- 50.Weissman DE. Decision making at a time of crisis near the end of life. JAMA 2004;292:1738–43. [DOI] [PubMed] [Google Scholar]
- 51.Scheunemann LP, Arnold RM, White DB. The facilitated values history: helping surrogates make authentic decisions for incapacitated patients with advanced illness. American journal of respiratory and critical care medicine 2012;186:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Back AL, Arnold RM. Discussing prognosis: “how much do you want to know?” talking to patients who do not want information or who are ambivalent. J Clin Oncol 2006;24:4214–7. [DOI] [PubMed] [Google Scholar]
- 53.Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc 2003;51:1398–403. [DOI] [PubMed] [Google Scholar]
- 54.Hancock K, Clayton JM, Parker SM, et al. Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review. Palliat Med 2007;21:507–17. [DOI] [PubMed] [Google Scholar]
- 55.Hough CL, Curtis JR. Long-term sequelae of critical illness: memories and health-related quality of life. Crit Care 2005;9:145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med 2009;361:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammes BJ. What does it take to help adults successfully plan for future medical decisions? J Palliat Med 2001;4:453–6. [DOI] [PubMed] [Google Scholar]
- 58.Hammes BJ, Rooney BL, Gundrum JD. A comparative, retrospective, observational study of the prevalence, availability, and specificity of advance care plans in a county that implemented an advance care planning microsystem. J Am Geriatr Soc 2010;58:1249–55. [DOI] [PubMed] [Google Scholar]
- 59.Bowlby J The making and breaking of affectional bonds. II. Some principles of psychotherapy. The fiftieth Maudsley Lecture. Br J Psychiatry 1977;130:421–31. [DOI] [PubMed] [Google Scholar]
- 60.Ciechanowski P, Russo J, Katon W, et al. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med 2004;66:720–8. [DOI] [PubMed] [Google Scholar]
- 61.Ciechanowski P, Wagner E, Schmaling K, et al. Community-integrated home-based depression treatment in older adults: a randomized controlled trial. JAMA 2004;291:1569–77. [DOI] [PubMed] [Google Scholar]
- 62.Kaya N Effect of attachment styles of individuals discharged from an intensive care unit on intensive care experience. J Crit Care 2012;27:103 e7–14. [DOI] [PubMed] [Google Scholar]
- 63.Tan A, Zimmermann C, Rodin G. Interpersonal processes in palliative care: an attachment perspective on the patient-clinician relationship. Palliat Med 2005;19:143–50. [DOI] [PubMed] [Google Scholar]
- 64.Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. Am J Psychiatry 2001;158:29–35. [DOI] [PubMed] [Google Scholar]
- 65.Griffin D, Bartholomew K. The metaphysics of measurement: The case of adult attachment. Advances in Personal Relationships 1994;5:17–52. [Google Scholar]
- 66.Scharfe J, Bartholomew K. Reliability and stability of adult attachment patterns. Personal Relationships 1994;1:23–43. [Google Scholar]
- 67.Breen CM, Abernethy AP, Abbott KH, Tulsky JA. Conflict associated with decisions to limit life-sustaining treatment in intensive care units. J Gen Intern Med 2001;16:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azoulay E, Timsit JF, Sprung CL, et al. Prevalence and factors of intensive care unit conflicts: the conflicus study. Am J Respir Crit Care Med 2009;180:853–60. [DOI] [PubMed] [Google Scholar]
- 69.Piers RD, Azoulay E, Ricou B, et al. Perceptions of appropriateness of care among European and Israeli intensive care unit nurses and physicians. JAMA : the journal of the American Medical Association 2011;306:2694–703. [DOI] [PubMed] [Google Scholar]
- 70.Piers RD, Van den Eynde M, Steeman E, Vlerick P, Benoit DD, Van Den Noortgate NJ. End-of-life care of the geriatric patient and nurses’ moral distress. J Am Med Dir Assoc 2012;13:80 e7–13. [DOI] [PubMed] [Google Scholar]
- 71.Dubler NN. Mediating disputes in managed care: resolving conflicts over covered services. J Health Care Law Policy 2002;5:479–501. [PubMed] [Google Scholar]
- 72.Dubler NN. Conflict and consensus at the end of life. Hastings Cent Rep 2005;Spec No:S19–25. [DOI] [PubMed] [Google Scholar]
- 73.Morreim H Conflict Resolution in the Clinical Setting: A Story Beyond Bioethics Mediation. J Law Med Ethics 2015;43:843–56. [DOI] [PubMed] [Google Scholar]
- 74.Morreim H Story of a Mediation in the Clinical Setting. J Clin Ethics 2016;27:43–50. [PubMed] [Google Scholar]
- 75.Gold J Advanced Mediation Manual: Using a Faciltative Approach. Washington DC: Washington Legal Foundation; 2002. [Google Scholar]
- 76.Dubler NN, Liebman CB. Bioethics Mediation: A Guide to Shaping Shared Solutions. New York: United Hospital Fund; 2004. [Google Scholar]
- 77.Baruch Bush RA, Folger JP. The Promise of Mediation: Jossey Bass, Inc.; 1994. [Google Scholar]
- 78.Donze J, Aujesky D, Williams D, Schnipper JL. Potentially Avoidable 30-Day Hospital Readmissions in Medical Patients: Derivation and Validation of a Prediction Model. JAMA internal medicine 2013:1–7. [DOI] [PubMed] [Google Scholar]
- 79.Feltner C, Jones CD, Cene CW, et al. Transitional Care Interventions to Prevent Readmissions for Persons With Heart Failure: A Systematic Review and Meta-analysis. Ann Intern Med 2014. [DOI] [PubMed] [Google Scholar]
- 80.van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 2011;183:E391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown RS, Peikes D, Peterson G, Schore J, Razafindrakoto CM. Six features of Medicare coordinated care demonstration programs that cut hospital admissions of high-risk patients. Health affairs 2012;31:1156–66. [DOI] [PubMed] [Google Scholar]
- 82.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA 2009;301:603–18. [DOI] [PubMed] [Google Scholar]
- 83.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: The importance of transitional care in achieving health reform. Health affairs 2011;30:746–54. [DOI] [PubMed] [Google Scholar]
- 84.Naylor MD, Bowles KH, McCauley KM, et al. High-value transitional care: translation of research into practice. J Eval Clin Pract 2013;19:727–33. [DOI] [PubMed] [Google Scholar]
- 85.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. Journal of the American Geriatrics Society 2004;52:675–84. [DOI] [PubMed] [Google Scholar]
- 86.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443–51. [DOI] [PubMed] [Google Scholar]
- 87.Robb SL, Burns DS, Docherty SL, Haase JE . Ensuring treatment fidelity in a multi-site behavioral intervention study: implementing NIH Behavior Change Consortium recommendations in the SMART trial. Psychooncology 2011;20:1193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Needham DM, Sepulveda KA, Dinglas VD, et al. Core Outcome Measures for Clinical Research in Acute Respiratory Failure Survivors. An International Modified Delphi Consensus Study. Am J Respir Crit Care Med 2017;196:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turnbull AE, Sepulveda KA, Dinglas VD, Chessare CM, Bingham CO 3rd, Needham DM. Core Domains for Clinical Research in Acute Respiratory Failure Survivors: An International Modified Delphi Consensus Study. Crit Care Med 2017;45:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Connolly B, Hough CL. Coloring by Number? Core Outcome Measures and the Canvas of Intensive Care Unit Survivorship. Am J Respir Crit Care Med 2017;196:1087–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 92.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 93.Pochard F, Azoulay E, Chevret S, et al. Symptoms of anxiety and depression in family members of intensive care unit patients: Ethical hypothesis regarding decision-making capacity. Crit Care Med 2001;29:1893–7. [DOI] [PubMed] [Google Scholar]
- 94.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med 2005;171:987–94. [DOI] [PubMed] [Google Scholar]
- 95.Azoulay E, Pochard F, Chevret S, et al. Half the family members of intensive care unit patients do not want to share in the decision-making process: a study in 78 French intensive care units. Crit Care Med 2004;32:1832–8. [DOI] [PubMed] [Google Scholar]
- 96.Azoulay E, Pochard F, Chevret S, et al. Meeting the needs of intensive care unit patient families: a multicenter study. Am J Respir Crit Care Med 2001;163:135–9. [DOI] [PubMed] [Google Scholar]
- 97.Anderson WG, Arnold RM, Angus DC, Bryce CL. Posttraumatic stress and complicated grief in family members of patients in the intensive care unit. J Gen Intern Med 2008;23:1871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson WG, Arnold RM, Angus DC, Bryce CL. Passive decision-making preference is associated with anxiety and depression in relatives of patients in the intensive care unit. Journal of critical care 2009;24:249–54. [DOI] [PubMed] [Google Scholar]
- 99.Fumis RR, Deheinzelin D. Family members of critically ill cancer patients: assessing the symptoms of anxiety and depression. Intensive Care Med 2009;35:899–902. [DOI] [PubMed] [Google Scholar]
- 100.Fumis RR, Ranzani OT, Faria PP, Schettino G. Anxiety, depression, and satisfaction in close relatives of patients in an open visiting policy intensive care unit in Brazil. J Crit Care 2015;30:440 e1–6. [DOI] [PubMed] [Google Scholar]
- 101.Fumis RR, Ranzani OT, Martins PS, Schettino G. Emotional disorders in pairs of patients and their family members during and after ICU stay. PLoS One 2015;10:e0115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garrouste-Orgeas M, Coquet I, Perier A, et al. Impact of an intensive care unit diary on psychological distress in patients and relatives*. Crit Care Med 2012;40:2033–40. [DOI] [PubMed] [Google Scholar]
- 103.Garrouste-Orgeas M, Philippart F, Timsit JF, et al. Perceptions of a 24-hour visiting policy in the intensive care unit. Crit Care Med 2008;36:30–5. [DOI] [PubMed] [Google Scholar]
- 104.Garrouste-Orgeas M, Willems V, Timsit JF, et al. Opinions of families, staff, and patients about family participation in care in intensive care units. J Crit Care 2010;25:634–40. [DOI] [PubMed] [Google Scholar]
- 105.Turnbull AE, Rabiee A, Davis WE, et al. Outcome Measurement in ICU Survivorship Research From 1970 to 2013: A Scoping Review of 425 Publications. Crit Care Med 2016;44:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bienvenu OJ, Williams JB, Yang A, Hopkins RO, Needham DM. Posttraumatic stress disorder in survivors of acute lung injury: evaluating the Impact of Event Scale-Revised. Chest 2013;144:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hosey MM, Bienvenu OJ, Dinglas VD, et al. The IES-R remains a core outcome measure for PTSD in critical illness survivorship research. Crit Care 2019;23:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thoresen S, Tambs K, Hussain A, Heir T, Johansen VA, Bisson JI. Brief measure of posttraumatic stress reactions: impact of Event Scale-6. Soc Psychiatry Psychiatr Epidemiol 2010;45:405–12. [DOI] [PubMed] [Google Scholar]
- 109.Teno JM, Fisher ES, Hamel MB, Coppola K, Dawson NV. Medical care inconsistent with patients’ treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc 2002;50:496–500. [DOI] [PubMed] [Google Scholar]
- 110.Coast J, Huynh E, Kinghorn P, Flynn T. Complex Valuation: Applying Ideas from the Complex Intervention Framework to Valuation of a New Measure for End-of-Life Care. Pharmacoeconomics 2016;34:499–508. [DOI] [PubMed] [Google Scholar]
- 111.Finkelstein EA, Bilger M, Flynn TN, Malhotra C. Preferences for end-of-life care among community-dwelling older adults and patients with advanced cancer: A discrete choice experiment. Health Policy 2015;119:1482–9. [DOI] [PubMed] [Google Scholar]
- 112.Flynn TN, Bilger M, Malhotra C, Finkelstein EA. Are Efficient Designs Used in Discrete Choice Experiments Too Difficult for Some Respondents? A Case Study Eliciting Preferences for End-of-Life Care. Pharmacoeconomics 2016;34:273–84. [DOI] [PubMed] [Google Scholar]
- 113.Curtis JR, Downey L, Back AL, et al. A patient and clinician communication-priming intervention increases patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized trial. JAMA Intern Med 2018;178:930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steinhauser KE, Voils CI, Bosworth HB, Tulsky JA. Validation of a measure of family experience of patients with serious illness: the QUAL-E (Fam). J Pain Symptom Manage 2014;48:1168–81. [DOI] [PubMed] [Google Scholar]
- 115.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre LM, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000;284:2476–82. [DOI] [PubMed] [Google Scholar]
- 116.Steinhauser KE, Clipp EC, Bosworth HB, et al. Measuring quality of life at the end of life: validation of the QUAL-E. Palliat Support Care 2004;2:3–14. [DOI] [PubMed] [Google Scholar]
- 117.Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutr Phys Act 2012;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care 1998;21:1644–51. [DOI] [PubMed] [Google Scholar]
- 119.Williams GC, Niemiec CP, Patrick H, Ryan RM, Deci EL. The importance of supporting autonomy and perceived competence in facilitating long-term tobacco abstinence. Ann Behav Med 2009;37:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams GC, McGregor H, Sharp D, et al. A self-determination multiple risk intervention trial to improve smokers’ health. J Gen Intern Med 2006;21:1288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams GC, McGregor HA, Sharp D, et al. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol 2006;25:91–101. [DOI] [PubMed] [Google Scholar]
- 122.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol 2004;23:58–66. [DOI] [PubMed] [Google Scholar]
- 123.Munster Halvari AE, Halvari H, Bjornebekk G, Deci EL. Self-determined motivational predictors of increases in dental behaviors, decreases in dental plaque, and improvement in oral health: a randomized clinical trial. Health Psychol 2012;31:777–88. [DOI] [PubMed] [Google Scholar]
- 124.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Annals of internal medicine 2011;155:520–8. [DOI] [PubMed] [Google Scholar]
- 125.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE -- Acute physiology and chronic health evaluation: A physiologically based classification system. Critical Care Medicine 1981;9:591–7. [DOI] [PubMed] [Google Scholar]
- 128.Knaus WA, Wagner DP, Draper EA, et al. APACHE III prognostic systems: Risk prediction of hospital mortality for criticall ill hospitalized adults. Chest 1991;100:1619–36. [DOI] [PubMed] [Google Scholar]
- 129.Kajdacsy-Balla Amaral AC, Andrade FM, Moreno R, Artigas A, Cantraine F, Vincent JL. Use of the sequential organ failure assessment score as a severity score. Intensive care medicine 2005;31:243–9. [DOI] [PubMed] [Google Scholar]
- 130.Peres Bota D, Melot C, Lopes Ferreira F, Nguyen Ba V, Vincent JL. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive care medicine 2002;28:1619–24. [DOI] [PubMed] [Google Scholar]
- 131.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 132.Dotolo D, Nielsen EL, Curtis JR, Engelberg RA. Strategies for Enhancing Family Participation in Research in the ICU: Findings From a Qualitative Study. J Pain Symptom Manage 2017;54:226–30 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ 2005;24:465–88. [DOI] [PubMed] [Google Scholar]
- 134.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. [DOI] [PubMed] [Google Scholar]
- 135.Miles MB, Huberman AM. Qualitative Data Analysis: An expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994. [Google Scholar]
- 136.Giacomini M, Cook DJ, for the Evidence-Based Medicine Working Group. Qualitative research in health care: Are the results of the study valid? JAMA 2000;284:357–62. [DOI] [PubMed] [Google Scholar]
- 137.Stange KC, Miller WL, Crabtree BJ, O’Connor PJ, Zyzanski SJ. Multimethod research: Approaches for integrating qualitative and quantitative research. Journal of General Internal Medicine 1994;9:278–82. [DOI] [PubMed] [Google Scholar]
- 138.Strauss AL, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks: Sage Publications; 1998. [Google Scholar]
- 139.Little R, Rubin D. Statistical analysis with missing data. Third ed. New York: Wiley; 2019. [Google Scholar]
- 140.Khandelwal N, Brumback LC, Halpern SD, Coe NB, Brumback B, Curtis JR. Evaluating the Economic Impact of Palliative and End-of-Life Care Interventions on Intensive Care Unit Utilization and Costs from the Hospital and Healthcare System Perspective. J Palliat Med 2017;20:1314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carson SS, Cox CE, Wallenstein S, et al. Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. JAMA 2016;316:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balaban RB, Galbraith AA, Burns ME, Vialle-Valentin CE, Larochelle MR, Ross-Degnan D. A Patient Navigator Intervention to Reduce Hospital Readmissions among High-Risk Safety-Net Patients: A Randomized Controlled Trial. J Gen Intern Med 2015;30:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chillakunnel Hussain Rawther S, Pai MS, Fernandes DJ, et al. A Randomized controlled trial to evaluate the impact of a Nurse Navigator Programme on outcomes of people with breast cancer: study protocol. J Adv Nurs 2017;73:977–88. [DOI] [PubMed] [Google Scholar]
- 144.Green BB, Anderson ML, Wang CY, et al. Results of nurse navigator follow-up after positive colorectal cancer screening test: a randomized trial. J Am Board Fam Med 2014;27:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med 2009;24:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ranaghan C, Boyle K, Meehan M, Moustapha S, Fraser P, Concert C. Effectiveness of a patient navigator on patient satisfaction in adult patients in an ambulatory care setting: a systematic review. JBI Database System Rev Implement Rep 2016;14:172–218. [DOI] [PubMed] [Google Scholar]
- 147.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aslakson RA, Reinke LF, Cox C, Kross EK, Benzo RP, Curtis JR. Developing a Research Agenda for Integrating Palliative Care into Critical Care and Pulmonary Practice To Improve Patient and Family Outcomes. J Palliat Med 2017;20:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Aslakson R, Cheng J, Vollenweider D, Galusca D, Smith TJ, Pronovost PJ. Evidence-based palliative care in the intensive care unit: a systematic review of interventions. J Palliat Med 2014;17:219–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med 2015;43:1102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tulsky JA, Beach MC, Butow PN, et al. A Research Agenda for Communication Between Health Care Professionals and Patients Living With Serious Illness. JAMA Intern Med 2017;177:1361–6. [DOI] [PubMed] [Google Scholar]
- 152.Lo B Improving care near the end of life: Why is it so hard? JAMA 1995;274:1634–6. [PubMed] [Google Scholar]
- 153.Lorenz K, Lynn J. Morton SC. End-of-Life Care and Outcomes. Summary, Evidence Report/Technology Assessment: Number 110. AHRQ Publication Number 05-E004–1.Agency for Healthcare Research and Quality, 2004. (Accessed September 17, 2018, at https://archive.ahrq.gov/downloads/pub/evidence/pdf/eolcare/eolcare.pdf.) [Google Scholar]
- 154.Curtis JR, Engelberg RA. Measuring success of interventions to improve the quality of end-of-life care in the intensive care unit. Crit Care Med 2006;34:S341–7. [DOI] [PubMed] [Google Scholar]
- 155.Patterson ES, Wears RL. Patient handoffs: standardized and reliable measurement tools remain elusive. Joint Commission journal on quality and patient safety / Joint Commission Resources 2010;36:52–61. [DOI] [PubMed] [Google Scholar]
- 156.Smith MS, Wallston KA, Smith CA. The development and validation of the Perceived Health Competence Scale. Health Educ Res 1995;10:51–64. [DOI] [PubMed] [Google Scholar]
- 157.Marks GR, Lutgendorf SK. Perceived health competence and personality factors differentially predict health behaviors in older adults. Journal of Aging and Health 1999;11:221–39. [DOI] [PubMed] [Google Scholar]
- 158.Arnold R, Ranchor AV, DeJongste MJ, et al. The relationship between self-efficacy and self-reported physical functioning in chronic obstructive pulmonary disease and chronic heart failure. Behav Med 2005;31:107–15. [DOI] [PubMed] [Google Scholar]
- 159.Kryworuchko J, Stacey D, Bennett C, Graham ID. Appraisal of primary outcome measures used in trials of patient decision support. Patient Educ Couns 2008;73:497–503. [DOI] [PubMed] [Google Scholar]
- 160.Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA 2014;311:1052–60. [DOI] [PubMed] [Google Scholar]
- 161.Lynn J Strategies to ease the burden of family caregivers. JAMA 2014;311:1021–2. [DOI] [PubMed] [Google Scholar]
- 162.Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med 1997;155:15–20. [DOI] [PubMed] [Google Scholar]
- 163.Siegel MD, Hayes E, Vanderwerker LC, Loseth DB, Prigerson HG. Psychiatric illness in the next of kin of patients who die in the intensive care unit. Crit Care Med 2008;36:1722–8. [DOI] [PubMed] [Google Scholar]
- 164.Desbiens NA, Mueller-Rizner N, Connors AF Jr., Wenger NS, Lynn J The symptom burden of seriously ill hospitalized patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcome and Risks of Treatment. J Pain Symptom Manage 1999;17:248–55. [DOI] [PubMed] [Google Scholar]
- 165.Jones C, Skirrow P, Griffiths RD, et al. Post-traumatic stress disorder-related symptoms in relatives of patients following intensive care. Intensive Care Med 2004;30:456–60. [DOI] [PubMed] [Google Scholar]
- 166.Kross EK, Curtis JR. Burden of psychological symptoms and illness in family of critically ill patients: what is the relevance for critical care clinicians? Crit Care Med 2008;36:1955–6. [DOI] [PubMed] [Google Scholar]
- 167.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Critical care medicine 2012;40:502–9. [DOI] [PubMed] [Google Scholar]
- 168.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Critical care medicine 2012;40:618–24. [DOI] [PubMed] [Google Scholar]
- 169.Tilden VP, Tolle SW, Garland MJ, Nelson CA. Decisions about life-sustaining treatment: Impact of physicians’ behaviors on the family. Arch Intern Med 1995;155:633–8. [PubMed] [Google Scholar]
- 170.Sharpe M, Burton C, Sawhney A, McGorm K, Weller D. Is co-morbid depression adequately treated in patients repeatedly referred to specialist medical services with symptoms of a medical condition? Journal of psychosomatic research 2012;72:419–21. [DOI] [PubMed] [Google Scholar]
- 171.Luppa M, Sikorski C, Motzek T, Konnopka A, Konig HH, Riedel-Heller SG. Health Service Utilization and Costs of Depressive Symptoms in Late Life - A Systematic Review. Current pharmaceutical design 2012;18:5936–57. [DOI] [PubMed] [Google Scholar]
- 172.Schillerstrom JE, Royall DR, Palmer RF. Depression, disability and intermediate pathways: a review of longitudinal studies in elders. Journal of geriatric psychiatry and neurology 2008;21:183–97. [DOI] [PubMed] [Google Scholar]
- 173.McKnight PE, Kashdan TB. The importance of functional impairment to mental health outcomes: a case for reassessing our goals in depression treatment research. Clinical psychology review 2009;29:243–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer 1998;6:101–8. [DOI] [PubMed] [Google Scholar]
- 175.Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ 2013;347:f6753. [DOI] [PubMed] [Google Scholar]
- 176.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ciechanowski PS, Russo JE, Katon WJ, et al. The association of patient relationship style and outcomes in collaborative care treatment for depression in patients with diabetes. Med Care 2006;44:283–91. [DOI] [PubMed] [Google Scholar]
- 178.Ciechanowski PS, Walker EA, Katon WJ, Russo JE. Attachment theory: a model for health care utilization and somatization. Psychosom Med 2002;64:660–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.