Abstract
Decades of research have revealed numerous risk factors for mental disorders beyond genetics, but their consistency and magnitude remain uncertain. We conducted a “meta‐umbrella” systematic synthesis of umbrella reviews, which are systematic reviews of meta‐analyses of individual studies, by searching international databases from inception to January 1, 2021. We included umbrella reviews on non‐purely genetic risk or protective factors for any ICD/DSM mental disorders, applying an established classification of the credibility of the evidence: class I (convincing), class II (highly suggestive), class III (suggestive), class IV (weak). Sensitivity analyses were conducted on prospective studies to test for temporality (reverse causation), TRANSD criteria were applied to test transdiagnosticity of factors, and A Measurement Tool to Assess Systematic Reviews (AMSTAR) was employed to address the quality of meta‐analyses. Fourteen eligible umbrella reviews were retrieved, summarizing 390 meta‐analyses and 1,180 associations between putative risk or protective factors and mental disorders. We included 176 class I to III evidence associations, relating to 142 risk/protective factors. The most robust risk factors (class I or II, from prospective designs) were 21. For dementia, they included type 2 diabetes mellitus (risk ratio, RR from 1.54 to 2.28), depression (RR from 1.65 to 1.99) and low frequency of social contacts (RR=1.57). For opioid use disorders, the most robust risk factor was tobacco smoking (odds ratio, OR=3.07). For non‐organic psychotic disorders, the most robust risk factors were clinical high risk state for psychosis (OR=9.32), cannabis use (OR=3.90), and childhood adversities (OR=2.80). For depressive disorders, they were widowhood (RR=5.59), sexual dysfunction (OR=2.71), three (OR=1.99) or four‐five (OR=2.06) metabolic factors, childhood physical (OR=1.98) and sexual (OR=2.42) abuse, job strain (OR=1.77), obesity (OR=1.35), and sleep disturbances (RR=1.92). For autism spectrum disorder, the most robust risk factor was maternal overweight pre/during pregnancy (RR=1.28). For attention‐deficit/hyperactivity disorder (ADHD), they were maternal pre‐pregnancy obesity (OR=1.63), maternal smoking during pregnancy (OR=1.60), and maternal overweight pre/during pregnancy (OR=1.28). Only one robust protective factor was detected: high physical activity (hazard ratio, HR=0.62) for Alzheimer’s disease. In all, 32.9% of the associations were of high quality, 48.9% of medium quality, and 18.2% of low quality. Transdiagnostic class I‐III risk/protective factors were mostly involved in the early neurodevelopmental period. The evidence‐based atlas of key risk and protective factors identified in this study represents a benchmark for advancing clinical characterization and research, and for expanding early intervention and preventive strategies for mental disorders.
Keywords: Risk factors, protective factors, mental disorders, dementia, psychotic disorders, mood disorders, autism spectrum disorder, attention‐deficit/hyperactivity disorder, early intervention, preventive strategies
Mental disorders are complex conditions of uncertain aetiopathology. Although a genetic predisposition is evident (e.g., for psychotic disorders1, 2, 3, bipolar disorders4, 5, depressive and anxiety disorders6, 7), even polyrisk genetic scores, on their own, explain only a small proportion of the phenotypic variance8, 9, 10. There is strong evidence that environmental factors underlie much of the variation in clinical and neurobiological phenotypes of mental disorders and their outcomes11, and there are suggestions for dynamic three‐dimensional gene‐by‐environment‐by‐time interactions.
Aetiopathological knowledge in psychiatry has often been plagued by scientific pessimism. However, there have been recent exponential developments in research, to the point that numerous non‐purely genetic risk factors for mental disorders have been identified. The timing of their effect encompasses prenatal or perinatal, childhood, later (adolescent/young adult) or antecedent (shortly preceding the onset of a disorder) phases.
The number of individual studies exploring risk or protective factors for mental disorders has grown over the past decades, and several meta‐analyses have been published. More recently12, umbrella review methods (i.e., systematic reviews of meta‐analyses13) have allowed comparisons between different meta‐analyses, by summarizing the findings with a uniform approach for all risk/protective factors, including expected variability in the quality, focus of interest, and several types of biases in the meta‐analyses14, 15, 16.
Umbrella reviews can also apply robust classification criteria17 to rank the credibility of the evidence, controlling at the same time for several biases18, 19, 20, 21, which helps overcome conflicting meta‐analytic findings on complex topics13. Accordingly, umbrella reviews with a classification of the credibility of evidence are employed to help synthesize the available literature in order to guide both clinical care and public health policies. Collectively, umbrella reviews are at the top of the hierarchy in the evaluation of evidence16, 22.
While several recent umbrella reviews have evaluated the consistency and magnitude of risk and protective factors for each specific mental disorder, no systematic synthesis has yet collectively appraised the evidence across all existing mental disorders. Therefore, the extent to which these factors may differently exert their influence within specific disorders or across different disorders is currently unknown.
We present here the first systematic synthesis of umbrella reviews of non‐purely genetic risk and protective factors for mental disorders. This approach has been termed “meta‐umbrella” and offers an overarching field‐wide overview to comprehensively assess a certain topic23. Our aims were to provide an evidence‐synthesis comparative atlas of the consistency and magnitude of risk and protective factors for mental disorders beyond genetics, and to formulate recommendations for the next generation of aetiopathological research and preventive psychiatry.
METHODS
Search strategy and selection criteria
We conducted a meta‐umbrella systematic review of umbrella reviews23. The search strategy followed the PRISMA guidelines24. A multi‐step systematic literature search was performed by independent researchers to explore Web of Science (Clarivate Analytics) databases (including the Web of Science Core Collection, BIOSIS Citation Index, MEDLINE, KCI‐Korean Journal Database, SciELO Citation Index, and Russian Science Citation Index), PubMed, the Cochrane Central Register of Reviews, and Ovid/PsycINFO databases, from inception to January 1, 2021.
The following broad search terms were applied: “umbrella review” and (“risk” OR “protect*”). Papers identified were initially screened based on title and abstract reading. After the exclusion of those which were not relevant based on the topic investigated, full texts of the remaining papers were further assessed for inclusion. The references of umbrella reviews included in the final dataset were also reviewed to identify additional eligible papers.
Studies included were: a) umbrella reviews, defined as systematic collections and assessments of multiple systematic reviews and/or meta‐analyses published on a specific research topic14, 15, b) reporting quantitative data from observational individual studies (i.e., case‐control, cohort, cross‐sectional or ecological studies) on non‐purely genetic risk and/or protective factors for mental disorders based on established criteria for classifying the credibility of the evidence18, 19, 20, 21 (see below), and c) primarily investigating the association between these risk and/or protective factors and ICD (any version) or DSM (any version) mental disorders.
Mental disorders were stratified by using the corresponding ICD‐10 diagnostic blocks: organic, including symptomatic, mental disorders; mental and behavioural disorders due to psychoactive substance use; schizophrenia, schizotypal and delusional disorders; mood (affective) disorders; neurotic, stress‐related and somatoform disorders; behavioural syndromes associated with psychological disturbances and physical factors; disorders of adult personality and behaviour; mental retardation; disorders of psychological development; and behavioural and emotional disorders with onset usually occurring in childhood and adolescence.
Studies excluded were: a) systematic reviews or meta‐analyses other than umbrella reviews, individual studies (including Mendelian randomization studies and randomized controlled trials), clinical cases, conference proceedings, and study protocols; b) umbrella reviews not reporting quantitative data; c) umbrella reviews addressing outcomes other than the onset of an established mental disorder (e.g., those related to clinical outcomes such as relapse, remission or treatment response15, 23, or biomarkers); d) umbrella reviews employing other classification approaches, such as GRADE25, because these mostly apply to interventional effects, not aetiology26.
We did not include pure genetic factors or biomarkers, because genetic/biomarker causality is tested with other analytical approaches (such as genome‐wide association studies and meta/mega‐analyses). When there were two or more umbrella reviews from the same centre, authors were contacted to clarify overlaps. When two papers presented overlapping datasets on the same risk/protective factor for the same disorder, only the paper with the largest dataset was retained for the analysis. Disagreements in search and selection were resolved through discussion and consensus.
Measures and data extraction
At least two independent researchers extracted a predetermined set of variables characterizing each umbrella review, including the first author and year of publication, the corresponding ICD‐10 diagnostic block(s), the number of meta‐analyses included, the median number of individual studies and of cases (with interquartile range) per association, the overall number of risk/protective factors investigated, and the range of years for which the evidence was reviewed.
Further variables were extracted to characterize the association between each specific risk/protective factor and each mental disorder. We recorded each risk/protective factor (if the timing of effect was specified, this was additionally reported, e.g., childhood, midlife, elderhood). Following a pragmatic approach, each risk/protective factor was defined as originally operationalized by each individual study, without redefining it unless strictly necessary to improve the clarity of reporting. Since each factor (e.g., smoking) can be associated with multiple outcomes (e.g., lung and pancreatic cancer), the total number of associations tested in umbrella reviews typically exceeds that of factors27.
We recorded the specific mental disorder which was the focus of each umbrella review and matched it with the corresponding ICD‐10 diagnostic block. Furthermore, we recorded the number of individual studies and cases analyzed per each association, the strength of the association and its measurement – odds ratio (OR), risk ratio (RR), incidence rate ratio (IRR), hazard ratio (HR), Hedges’ g, Cohen’s d, and r – with the corresponding 95% confidence intervals (CI). A value of OR, RR, IRR or HR and its 95% CI higher than 1, or a value of Hedges’ g, Cohen's d, or r higher than 0 indicates an association with an increased likelihood of a mental disorder (i.e., risk factor). A value of OR, RR, IRR or HR and its 95% CI lower than 1, or a value of Hedges’ g, Cohen's d, or r lower than 0 indicates an association with a reduced likelihood of a mental disorder (i.e., protective factor). We also provided the equivalent OR (eOR) for all metrics: an eOR higher than 1 indicates an association with an increased likelihood of a mental disorder (i.e., risk factor), while an eOR lower than 1 indicates an association with a reduced likelihood of a mental disorder (i.e., protective factor)15. Finally, we extracted the overall class of evidence as reported for each association and the class of evidence reported in prospective studies of each association (see below).
Strategy for data synthesis
The results were systematically stratified across the corresponding ICD‐10 diagnostic blocks and described across three sections: a) evidence for associations between risk/protective factors and individual mental disorders, b) evidence for transdiagnostic associations of risk/protective factors, c) evidence for factors that have both risk and protective associations with various mental disorders.
For the first analysis, we reported the classification of the credibility of the evidence in the included umbrella reviews according to established criteria13, 18, 19, 20: class I, convincing (number of cases >1,000, p<10–6, I2<50%, 95% prediction interval excluding the null, no small‐study effects, and no excess significance bias); class II, highly suggestive (number of cases >1,000, p<10–6, largest study with a statistically significant effect, and class I criteria not met); class III, suggestive (number of cases >1,000, p<10–3, and class I‐II criteria not met); class IV, weak (p<0.05 and class I‐III criteria not met); and non‐significant (p>0.05). We considered only factors with a class of evidence from I to III, and primarily focused on those with robust evidence (i.e., class I and II). We additionally reported the class of evidence for each association when the analyses were restricted to prospective studies (if provided by the umbrella reviews included). This sensitivity analysis deals with the problem of reverse causation that may affect, for example, case‐control studies20. Furthermore, we indicated whether the associations involving medical treatments were likely confounded by underlying conditions which might themselves increase the risk of mental disorders (confounding by indication)28. We also reported the quality of the included meta‐analyses measured by the AMSTAR (A Measurement Tool to Assess Systematic Reviews) tool29.
The second analysis (transdiagnostic associations) was conducted only for those risk factors that were shared by at least two disorders. We applied the TRANSD criteria, which empirically evaluate the consistency and extent of putative transdiagnostic constructs across six domains30, 31. In order to be validated, a transdiagnostic association had to adopt a transparent (criterion T) diagnostic definition according to the gold standard; clearly report (criterion R) the primary outcome of the study; be appraised (criterion A) as “across diagnoses and within spectrum” or “across diagnostic spectra”; numerate (criterion N) the corresponding ICD‐10 diagnostic categories and spectra; and show (criterion S) a transdiagnostic class of evidence of at least III, and not inferior to the lowest class of evidence for the corresponding disorder‐specific associations. The transdiagnostic class of evidence within prospective studies was additionally reported in order to demonstrate (criterion D) the generalizability of the transdiagnostic factor.
The third analysis was based on a systematic description of the findings.
RESULTS
Database
Overall, 1,361 records were retrieved, 800 suitable papers were screened, and 14 umbrella reviews were eligible6, 15, 27, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 (see Figure 1). The eligible umbrella reviews were published between 2017 and 2021, and reviewed individual studies published from 1995 to 2020. The 14 eligible umbrella reviews (Table 1) included 390 meta‐analyses. The median number of meta‐analyses per umbrella review was 26 (interquartile range: 9‐43).
Figure 1.
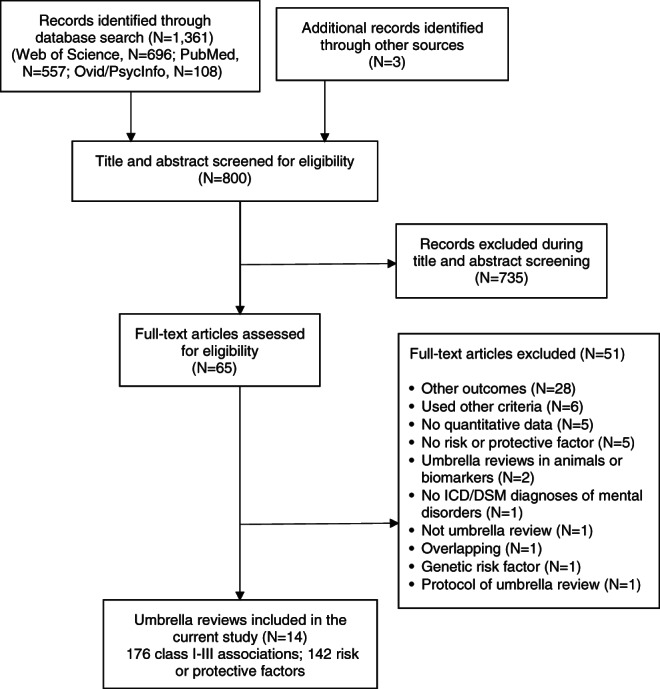
PRISMA flow chart outlining study selection process
Table 1.
Overall characteristics of the umbrella reviews included in the current study
| Risk or protective factor | Mental disorder | Number of individual studies (cases) | Strength of association, measure | 95% CI | Class of evidence (prospective evidence class) | Quality (AMSTAR) | eOR |
|---|---|---|---|---|---|---|---|
| Organic, including symptomatic, mental disorders | |||||||
| Type 2 diabetes mellitus | Vascular dementia | 14 (1,396) | 2.28, RR | 1.94‐2.66 | I (I) | High | 2.28 |
| Depression in elderhood | Any dementia | 25 (4,957) | 1.85, RR | 1.67‐2.05 | I (I) | Medium | 1.85 |
| Depression in elderhood | Alzheimer's disease | 16 (3,358) | 1.65, RR | 1.42‐1.92 | I (I) | Medium | 1.65 |
| Low frequency of social contacts | Any dementia | 8 (1,122) | 1.57, RR | 1.32‐1.85 | I (I) | Medium | 1.57 |
| Type 2 diabetes mellitus | Alzheimer's disease | 21 (3,537) | 1.54, RR | 1.39‐1.72 | I (I) | High | 1.54 |
| Benzodiazepines use* | Any dementia | 5 (11,741) | 1.49, RR | 1.30‐1.72 | I (I) | High | 1.49 |
| Depression | Alzheimer's disease | 25 (5,101) | 1.77, RR | 1.48‐2.13 | II (II) | High | 1.77 |
| Type 2 diabetes mellitus | Any dementia | 22 (15,707) | 1.60, RR | 1.43‐1.79 | II (II) | High | 1.60 |
| High physical activity | Alzheimer's disease | 9 (1,358) | 0.62, HR | 0.52‐0.72 | II (II) | Medium | 0.62 |
| History of cancer | Alzheimer's disease | 7 (4,635) | 0.62, HR | 0.53‐0.74 | II (II) | Medium | 0.62 |
| Obesity in midlife | Any dementia | 5 (1,914) | 1.91, RR | 1.40‐2.62 | III (NA) | Medium | 1.91 |
| Low education | Any dementia | 23 (8,739) | 1.88, RR | 1.51‐2.33 | III (NA) | High | 1.88 |
| Low education | Alzheimer's disease | 16 (2,769) | 1.82, RR | 1.36‐2.43 | III (NA) | High | 1.82 |
| Low frequency electromagnetic fields | Alzheimer's disease | 25 (3,238) | 1.74, RR | 1.37‐2.21 | III (NA) | High | 1.74 |
| Aluminium exposure | Alzheimer's disease | 8 (1,383) | 1.72, OR | 1.33‐2.21 | III (NA) | Medium | 1.72 |
| Depression in childhood | Any dementia | 9 (3,538) | 1.63, RR | 1.27‐2.11 | III (NA) | High | 1.63 |
| Herpes viruses infection | Alzheimer's disease | 33 (1,330) | 1.38, OR | 1.14‐1.65 | III (NA) | Medium | 1.38 |
| Statins use | Any dementia | 12 (37,798) | 0.83, RR | 0.76‐0.91 | III (NA) | High | 0.83 |
| High physical activity | Any dementia | 21 (3,845) | 0.76, RR | 0.66‐0.86 | III (NA) | Medium | 0.76 |
| NSAID use | Alzheimer's disease | 16 (53,372) | 0.74, RR | 0.64‐0.86 | III (NA) | High | 0.74 |
| Mental and behavioural disorders due to psychoactive substance use | |||||||
| Tobacco smoking | Opioid use disorder | 10 (2,447) | 3.07, OR | 2.27‐4.14 | II (II) | Low | 3.07 |
| Impulsivity‐related personality traits in college adolescents | Alcohol related disorder | 15 (NA) | 0.53, d | 0.43‐0.64 | III (NA) | Medium | 2.63 |
| ADHD | Tobacco related disorder | 4 (NA) | 2.36, OR | 1.71‐3.27 | III (NA) | Medium | 2.36 |
| Impulsivity‐related personality traits in community adolescents | Alcohol related disorder | 9 (NA) | 0.45, d | 0.33‐0.56 | III (NA) | Medium | 2.26 |
| Impulsivity‐related personality traits in school adolescents | Alcohol related disorder | 12 (NA) | 0.43, d | 0.34‐0.52 | III (NA) | Medium | 2.18 |
| Parental alcohol supply | Alcohol related disorder | 8 (NA) | 2.00, OR | 1.72‐2.32 | III (NA) | Medium | 2.00 |
| Peer smoking behaviour | Tobacco related disorder | 71 (NA) | 1.92, OR | 1.76‐2.09 | III (NA) | Medium | 1.92 |
| Externalizing symptoms in adolescents | Alcohol related disorder | 23 (NA) | 1.63, OR | 1.39‐1.90 | III (NA) | Medium | 1.63 |
| Smoking in movies | Tobacco related disorder | 9 (4,398) | 1.46, RR | 1.23‐1.73 | III (NA) | Medium | 1.46 |
| Surviving childhood cancer | Alcohol related disorder | 3 (1,348) | 0.78, OR | 0.68‐0.88 | III (NA) | Medium | 0.78 |
| Surviving childhood cancer | Tobacco related disorder | 6 (2,064) | 0.54, OR | 0.42‐0.70 | III (NA) | Medium | 0.54 |
| Parental stricter alcohol rules | Alcohol related disorder | 2 (NA) | 0.41, OR | 0.33‐0.51 | III (NA) | Medium | 0.41 |
| Schizophrenia, schizotypal and delusional disorders | |||||||
| Clinical high‐risk state for psychosis | Any non‐organic psychotic disorder | 9 (1,226) | 9.32, OR | 4.91‐17.72 | I (I) | High | 9.32 |
| Black‐Caribbean ethnicity in England | Any non‐organic psychotic disorder | 9 (3,446) | 4.87, IRR | 3.96‐6.00 | I (IV) | High | 4.87 |
| Obstetric complications | Schizophrenia spectrum disorders | 18 (1,000) | 1.97, OR | 1.55‐2.50 | I (NA) | Low | 1.97 |
| Minor physical anomalies | Any non‐organic psychotic disorder | 14 (1,212) | 0.92, g | 0.61‐1.23 | II (NA) | Medium | 5.30 |
| Trait anhedonia | Any non‐organic psychotic disorder | 44 (1,601) | 0.82, g | 0.72‐0.92 | II (NA) | Medium | 4.41 |
| Cannabis use | Schizophrenia spectrum disorders | 10 (4,036) | 3.90, OR | 2.84‐5.35 | II (II) | High | 3.90 |
| Ethnic minority in low ethnic density area | Any non‐organic psychotic disorder | 5 (1,328) | 3.71, IRR | 2.47‐5.58 | II (IV) | High | 3.71 |
| Stressful events | Schizophrenia spectrum disorders | 13 (2,218) | 3.11, OR | 2.31‐4.18 | II (NA) | Medium | 3.11 |
| Adversities in childhood | Schizophrenia spectrum disorders | 34 (7,738) | 2.80, OR | 2.34‐3.34 | II (II) | Medium | 2.80 |
| Second generation immigrant | Any non‐organic psychotic disorder | 26 (28,753) | 1.68, IRR | 1.42‐1.92 | II (IV) | High | 1.68 |
| Premorbid IQ | Any non‐organic psychotic disorder | 16 (4,459) | −0.42, g | −0.52 to −0.33 | II (IV) | Medium | 0.47 |
| Olfactory identification ability | Any non‐organic psychotic disorder | 55 (1,703) | −0.91, g | −1.05 to −0.78 | II (NA) | High | 0.19 |
| Social withdrawal in childhood | Any non‐organic psychotic disorder | 15 (1,810) | 0.59, g | 0.33‐0.85 | III (IV) | High | 2.91 |
| Tobacco smoking | Schizophrenia spectrum disorder | 17 (NA) | 2.34, OR | 1.65‐3.33 | III (NA) | High | 2.34 |
| North African immigrant in Europe | Any non‐organic psychotic disorder | 12 (2,577) | 2.22, IRR | 1.58‐3.12 | III (IV) | High | 2.22 |
| Urbanicity | Any non‐organic psychotic disorder | 8 (45,791) | 2.19, OR | 1.55‐3.09 | III (III) | Medium | 2.19 |
| Ethnic minority in high ethnic density area | Any non‐organic psychotic disorder | 5 (1,328) | 2.11, IRR | 1.39‐3.20 | III (IV) | High | 2.11 |
| First generation immigrant | Any non‐organic psychotic disorder | 42 (25,063) | 2.10, IRR | 1.72‐2.56 | III (IV) | High | 2.10 |
| Toxoplasma gondii IgG | Any non‐organic psychotic disorder | 42 (8,796) | 1.82, OR | 1.51‐2.18 | III (IV) | High | 1.82 |
| Non‐right handedness | Any non‐organic psychotic disorder | 41 (2,652) | 1.58, OR | 1.35‐1.86 | III (NS) | Medium | 1.58 |
| Paternal age >35 | Schizophrenia spectrum disorders | 10 (NA) | 1.28, OR | 1.11‐1.48 | III (NA) | Medium | 1.28 |
| Winter/spring season of birth in the Northern hemisphere | Any non‐organic psychotic disorder | 27 (115,010) | 1.04, OR | 1.02‐1.06 | III (NA) | High | 1.04 |
| Mood (affective) disorders | |||||||
| Widowhood | Depressive disorders | 5 (2,720) | 5.59, RR | 3.79‐8.23 | I (I) | Low | 5.59 |
| Sexual dysfunction | Depressive disorders | 6 (5,488) | 2.71, OR | 1.93‐3.79 | I (I) | High | 2.71 |
| Irritable bowel syndrome | Bipolar disorders | 6 (177,117) | 2.48, OR | 2.35‐2.61 | I (NA) | High | 2.48 |
| Four or five metabolic risk factors | Depressive disorders | 8 (1,191) | 2.06, OR | 1.59‐2.68 | I (I) | Low | 2.06 |
| Physical abuse in childhood | Depressive disorders | 10 (3,886) | 1.98, OR | 1.68‐2.33 | I (I) | Medium | 1.98 |
| Job strain | Depressive disorders | 7 (1,909) | 1.77, OR | 1.46‐2.13 | I (I) | Medium | 1.77 |
| Obesity | Depressive disorders | 8 (7,673) | 1.35, OR | 1.21‐1.50 | I (I) | Low | 1.35 |
| Dietary zinc | Depressive disorders | 8 (3,708) | 0.65, RR | 0.57‐0.75 | I (NA) | Medium | 0.65 |
| Tea intake | Depressive disorders | 13 (4,373) | 0.68, RR | 0.61‐0.77 | I (NA) | Medium | 0.68 |
| Dry eye disease with Sjögren's syndrome | Depressive disorders | 7 (3,062) | 4.25, OR | 2.67‐6.76 | II (NA) | Low | 4.25 |
| Poor physical health | Depressive disorders in elderhood | 11 (8,630) | 4.08, OR | 3.25‐5.12 | II (NA) | Low | 4.08 |
| Adversities in childhood | Bipolar disorders | 13 (1,146) | 2.86, OR | 2.03‐4.04 | II (NA) | High | 2.86 |
| Emotional abuse in childhood | Depressive disorders | 8 (4,112) | 2.78, OR | 1.89‐4.09 | II (III) | Medium | 2.78 |
| Chronic disease | Depressive disorders in elderhood | 10 (9,090) | 2.59, OR | 1.78‐3.76 | II (III) | Low | 2.59 |
| Intimate partner violence against women | Depressive disorders | 9 (3,003) | 2.57, RR | 2.25‐2.94 | II (NA) | Low | 2.57 |
| Sexual abuse in childhood | Depressive disorders | 14 (4,586) | 2.42, OR | 1.94‐3.02 | II (II) | Medium | 2.42 |
| Gulf war veterans | Depressive disorders | 11 (16,826) | 2.37, OR | 1.91‐2.93 | II (NA) | Low | 2.37 |
| Asthma | Depressive disorders in childhood | 7 (2,828) | 2.08, OR | 1.56‐2.77 | II (NA) | Low | 2.08 |
| Three metabolic risk factors | Depressive disorders | 8 (3,014) | 1.99, OR | 1.60‐2.48 | II (II) | Low | 1.99 |
| Poor vision | Depressive disorders in elderhood | 12 (11,066) | 1.94, OR | 1.67‐2.25 | II (NA) | Medium | 1.94 |
| Sleep disturbances | Depressive disorders in elderhood | 11 (2,610) | 1.92, RR | 1.59‐2.33 | II (II) | High | 1.92 |
| Psoriasis | Depressive disorders | 9 (86,945) | 1.64, OR | 1.41‐1.90 | II (NA) | Medium | 1.64 |
| Low education | Depressive disorders in elderhood | 24 (16,590) | 1.58, OR | 1.38‐1.82 | II (IV) | Low | 1.58 |
| Metabolic syndrome | Depressive disorders | 27 (20,924) | 1.42, OR | 1.28‐1.57 | II (IV) | Medium | 1.42 |
| Sedentary behaviour | Depressive disorders | 24 (60,526) | 1.25, RR | 1.16‐1.35 | II (NA) | Medium | 1.25 |
| Neglect in childhood | Depressive disorders | 6 (1,668) | 2.75, OR | 1.59‐4.74 | III (NA) | Medium | 2.75 |
| Insomnia | Depressive disorders | 21 (NA) | 2.60, OR | 1.98‐3.42 | III (NA) | Low | 2.60 |
| Chronic lung disease | Depressive disorders | 4 (297,031) | 2.38, RR | 1.47‐3.85 | III (NA) | Medium | 2.38 |
| Dry eye disease without Sjögren's syndrome | Depressive disorders | 6 (611,517) | 2.24, OR | 1.50‐3.34 | III (NA) | Low | 2.24 |
| Vitamin D deficiency | Depressive disorders | 3 (NA) | 2.22, HR | 1.42‐3.47 | III (III) | High | 2.22 |
| Asthma | Bipolar disorders | 4 (50,358) | 2.12, OR | 1.57‐2.87 | III (NA) | Medium | 2.12 |
| Maltreatment in childhood | Depressive disorders in childhood | 5 (1,400) | 2.03, OR | 1.37–3.01 | III (NA) | High | 2.03 |
| Terrorist act exposure | Depressive disorders | 6 (NA) | 2.02, OR | 1.38‐2.96 | III (NA) | High | 2.02 |
| Diabetes | Depressive disorders in elderhood | 9 (1,814) | 1.88, OR | 1.31‐2.70 | III (NA) | Medium | 1.88 |
| Heart disease | Depressive disorders in elderhood | 6 (1,911) | 1.81, OR | 1.41‐2.31 | III (NA) | Medium | 1.81 |
| Obesity | Bipolar disorders | 9 (12,259) | 1.77, OR | 1.40‐2.23 | III (NA) | Low | 1.77 |
| Hearing impairment | Depressive disorders in elderhood | 7 (4,448) | 1.71, OR | 1.28‐2.27 | III (NA) | Medium | 1.71 |
| Age >65 | Depressive disorders in elderhood | 6 (15,017) | 1.63, OR | 1.24‐2.16 | III (NA) | Low | 1.63 |
| Living alone | Depressive disorders in elderhood | 16 (10,478) | 1.55, OR | 1.23‐1.95 | III (NA) | Low | 1.55 |
| Age >85 | Depressive disorders in elderhood | 12 (4,559) | 1.52, OR | 1.20‐1.93 | III (NA) | Low | 1.52 |
| Two metabolic risk factors | Depressive disorders | 8 (6,691) | 1.45, OR | 1.17‐1.80 | III (NA) | Low | 1.45 |
| Low birth weight (≤2,500 g) | Depressive disorders | 21 (NA) | 1.38, OR | 1.16‐1.65 | III (NA) | Low | 1.38 |
| Age >75 | Depressive disorders in elderhood | 19 (11,219) | 1.35, OR | 1.17‐1.56 | III (NA) | Low | 1.35 |
| Type 2 diabetes mellitus | Depressive disorders | 11 (37,964) | 1.24, OR | 1.09‐1.40 | III (NA) | Medium | 1.24 |
| Unemployment | Depressive disorders | 13 (40,679) | 1.16, OR | 1.09‐1.23 | III (NA) | Medium | 1.16 |
| Fruit intake | Depressive disorders | 8 (NA) | 0.85, RR | 0.77‐0.93 | III (NA) | Low | 0.85 |
| Traditional/healthy dietary patterns | Depressive disorders | 17 (NA) | 0.76, RR | 0.68‐0.86 | III (NA) | Low | 0.76 |
| Iron intake | Depressive disorders | 3 (1,045) | 0.40, RR | 0.24‐0.65 | III (NA) | Medium | 0.40 |
| Neurotic, stress‐related and somatoform disorders | |||||||
| Physical abuse in childhood | Social anxiety disorder | 4 (1,191) | 2.59, OR | 2.17‐3.10 | I (IV) | High | 2.59 |
| Physical disease history | PTSD | 4 (2,161) | 2.29, OR | 2.07‐2.52 | I (NA) | High | 2.29 |
| Family history of psychiatric disorder | PTSD | 12 (1,765) | 1.80, OR | 1.48‐2.19 | I (NA) | Medium | 1.80 |
| Being an Indigenous American | PTSD | 5 (3,214) | 1.47, OR | 1.28‐1.69 | I (NA) | High | 1.47 |
| Cumulative exposure to potentially traumatic experiences | PTSD | 17 (3,094) | 5.24, OR | 3.54‐7.76 | II (NA) | High | 5.24 |
| Trauma severity | PTSD | 25 (2,017) | 0.66, g | 0.44‐0.88 | II (IV) | Medium | 3.32 |
| Being trapped in an earthquake | PTSD | 1 (2,028 | 2.86, OR | 2.52‐3.25 | II (NA) | High | 2.86 |
| Female sex | PTSD | 112 (9,137) | 1.65, OR | 1.45‐1.87 | II (NA) | Medium | 1.65 |
| Torture exposure | PTSD | 10 (1,357) | 4.46, OR | 2.39‐8.31 | III (NA) | Low | 4.46 |
| Sexual abuse in childhood | Social anxiety disorder | 5 (1,239) | 3.18, OR | 1.73‐5.86 | III (IV) | High | 3.18 |
| Personal psychiatric history | PTSD | 27 (1,753) | 2.45, OR | 1.67‐3.61 | III (IV) | Medium | 2.45 |
| Overprotection from father | Obsessive‐compulsive disorder | 6 (716) | 0.44, g | 0.21‐0.68 | III (NA) | High | 2.24 |
| Behavioural syndromes associated with physiological disturbances and physical factors | |||||||
| Appearance‐related teasing victimization | Any eating disorder | 10 (1,341) | 2.91, OR | 2.05‐4.12 | II (NA) | Medium | 2.91 |
| Sexual abuse in childhood | Bulimia nervosa | 26 (1,103) | 2.73, OR | 1.96‐3.79 | II (NA) | Medium | 2.73 |
| ADHD | Any eating disorder | 12 (3,618) | 4.24, OR | 2.62‐6.87 | III (NA) | Medium | 4.24 |
| Physical abuse in childhood | Binge eating disorder | 4 (NA) | 3.10, OR | 2.48‐3.88 | III (NA) | Medium | 3.10 |
| Sexual abuse in childhood | Binge eating disorder | 7 (NA) | 2.31, OR | 1.66‐3.20 | III (NA) | Medium | 2.31 |
| Self‐reported dieting | Bulimia nervosa | 7 (NA) | 0.22, r | 0.14‐0.30 | III (NA) | Medium | 2.26 |
| Body dissatisfaction | Any eating disorder | 11 (NA) | 0.14, r | 0.11‐0.17 | III (NA) | Medium | 1.67 |
| Perceived pressure to be thin | Any eating disorder | 4 (NA) | 0.11, r | 0.08‐0.14 | III (NA) | Medium | 1.51 |
| Negative affect | Any eating disorder | 11 (NA) | 0.09, r | 0.06‐0.12 | III (NA) | Medium | 1.38 |
| 5‐min Apgar score <7 | Anorexia nervosa | 33 (2,701) | 1.32, OR | 1.17‐1.49 | III (NA) | Medium | 1.32 |
| Disorders of adult personality and behaviour | |||||||
| Emotional abuse in childhood | Borderline personality disorder | 27 (3,525) | 28.15, OR | 17.46‐53.68 | II (NA) | Medium | 28.15 |
| Emotional neglect in childhood | Borderline personality disorder | 21 (3,225) | 22.86, OR | 11.55‐45.22 | II (NA) | Medium | 22.86 |
| Adversities in childhood | Borderline personality disorder | 97 (16,098) | 14.32, OR | 10.80‐18.98 | II (NA) | Medium | 14.32 |
| Physical abuse in childhood | Borderline personality disorder | 30 (2,869) | 9.30, OR | 6.57‐13.17 | II (NA) | Medium | 9.30 |
| Sexual abuse in childhood | Borderline personality disorder | 31 (3,748) | 7.95, OR | 6.21‐10.17 | II (NA) | Medium | 7.95 |
| Physical neglect in childhood | Borderline personality disorder | 20 (3,072) | 5.73, OR | 3.21‐10.21 | II (NA) | Medium | 5.73 |
| Mental retardation | |||||||
| None of the factors was supported by class I, II or III evidence | |||||||
| Disorders of psychological development | |||||||
| Maternal SSRI use during pregnancy* | Autism spectrum disorder | 7 (19,670) | 1.84, OR | 1.60‐2.11 | I (II) | Medium | 1.84 |
| Maternal pre‐pregnancy antidepressantuse* | Autism spectrum disorder | 7 (22,877) | 1.48, RR | 1.29‐1.71 | I (NA) | Medium | 1.48 |
| Maternal chronic hypertension | Autism spectrum disorder | 4 (22,864) | 1.48, OR | 1.29‐1.70 | I (NA) | Medium | 1.48 |
| Maternal gestational hypertension | Autism spectrum disorder | 9 (4,334) | 1.37, OR | 1.21‐1.54 | I (NA) | Medium | 1.37 |
| Maternal pre‐eclampsia | Autism spectrum disorder | 10 (10,699) | 1.32, RR | 1.20‐1.45 | I (NA) | Medium | 1.32 |
| Maternal age ≥35 years | Autism spectrum disorder | 11 (>1,000) | 1.31, RR | 1.18‐1.45 | I (NA) | Low | 1.31 |
| Maternal overweight pre/during pregnancy | Autism spectrum disorder | 5 (7,872) | 1.28, RR | 1.19‐1.36 | I (II) | Low | 1.28 |
| Highest paternal age group vs. reference group | Autism spectrum disorder | 20 (2,920) | 1.55, OR | 1.39‐1.73 | II (NA) | Medium | 1.55 |
| Paternal age >45 years | Autism spectrum disorder | 18 (>1,000) | 1.43, OR | 1.33‐1.53 | II (III) | High | 1.43 |
| Highest maternal age group vs. reference group | Autism spectrum disorder | 19 (2,254) | 1.42, OR | 1.29‐1.55 | II (IV) | Medium | 1.42 |
| Paternal age 40‐45 years | Autism spectrum disorder | 12 (>1,000) | 1.37, OR | 1.23‐1.53 | II (IV) | High | 1.37 |
| Maternal autoimmune disease | Autism spectrum disorder | 10 (9,775) | 1.37, OR | 1.21‐1.54 | II (NA) | Medium | 1.37 |
| Higher paternal age (per 10‐years increase) | Autism spectrum disorder | 17 (47,373) | 1.21, OR | 1.18‐1.24 | II (NA) | Medium | 1.21 |
| Maternal paracetamol use during pregnancy* | Autism spectrum disorder | 5 (>100) | 1.20, RR | 1.14‐1.26 | II (NA) | Medium | 1.20 |
| Maternal age 30‐34 | Autism spectrum disorder | 8 (>1,000) | 1.14, RR | 1.09‐1.18 | II (NA) | Low | 1.14 |
| Hearing impairment | Autism spectrum disorder | 7 (4,370) | 14.16, RR | 4.53‐44.22 | III (NA) | Medium | 14.16 |
| 5‐min Apgar score <7 | Autism spectrum disorder | 6 (3,676) | 1.67, OR | 1.34 ‐2.09 | III (NA) | Medium | 1.67 |
| Family history of psoriasis | Autism spectrum disorder | 8 (>1,000) | 1.59, OR | 1.28‐1.97 | III (NA) | Medium | 1.59 |
| Family history of rheumatoid arthritis | Autism spectrum disorder | 8 (>1,000) | 1.51, OR | 1.19‐1.91 | III (NA) | Medium | 1.51 |
| Maternal diabetes | Autism spectrum disorder | 16 (8,872) | 1.49, RR | 1.28‐1.74 | III (NA) | High | 1.49 |
| Family history of type 1 diabetes | Autism spectrum disorder | 13 (>1,000) | 1.49, OR | 1.23‐1.81 | III (NA) | Medium | 1.49 |
| Maternal infection requiring hospitalization | Autism spectrum disorder | 3 (34,547) | 1.30, OR | 1.14‐1.50 | III (NA) | Medium | 1.30 |
| Family history of any autoimmune disease | Autism spectrum disorder | 17 (1,894) | 1.28, OR | 1.12‐1.48 | III (NA) | Medium | 1.28 |
| Reference group vs. lowest paternal age group | Autism spectrum disorder | 15 (2,295) | 1.24, OR | 1.12‐1.37 | III (NA) | Medium | 1.24 |
| Higher maternal age (per 10‐years increase) | Autism spectrum disorder | 14 (46,025) | 1.18, OR | 1.10‐1.26 | III (NA) | Medium | 1.18 |
| Paternal age 35‐40 years | Autism spectrum disorder | 16 (>1,000) | 1.14, OR | 1.08‐1.21 | III (NA) | High | 1.14 |
| Behavioural and emotional disorders with onset usually occurring in childhood and adolescence | |||||||
| Maternal pre‐pregnancy obesity | ADHD | 11 (40,880) | 1.63, OR | 1.49‐1.77 | I (I) | Low | 1.63 |
| Eczema in childhood | ADHD | 6 (10,636) | 1.31, OR | 1.20‐1.44 | I (IV) | Low | 1.31 |
| Maternal hypertensive disorders during pregnancy | ADHD | 8 (37,128) | 1.29, OR | 1.22‐1.36 | I (NA) | High | 1.29 |
| Maternal pre‐eclampsia | ADHD | 6 (>1,000) | 1.28, OR | 1.21‐1.35 | I (NA) | High | 1.28 |
| Maternal paracetamol use during pregnancy* | ADHD | 8 (>1,000) | 1.25, RR | 1.17‐1.34 | I (I) | High | 1.25 |
| Maternal smoking during pregnancy | ADHD | 20 (50,044) | 1.60, OR | 1.45‐1.76 | II (II) | High | 1.60 |
| Asthma in childhood | ADHD | 11 (32,539) | 1.51, OR | 1.40‐1.63 | II (NA) | High | 1.51 |
| Maternal overweight pre/during pregnancy | ADHD | 9 (23,525) | 1.28, OR | 1.21‐1.35 | II (I) | Low | 1.28 |
| Preterm birth | ADHD | 11 (1,542) | 1.84, OR | 1.36‐2.49 | III (NA) | High | 1.84 |
| Maternal stress during pregnancy | ADHD | 8 (25,547) | 1.72, OR | 1.27‐2.34 | III (NA) | High | 1.72 |
| Maternal SSRI use during pre‐pregnancyperiod* | ADHD | 3 (39,097) | 1.59, RR | 1.23‐2.06 | III (NA) | High | 1.59 |
| Maternal non‐SSRI antidepressants use during pregnancy* | ADHD | 6 (23,064) | 1.50, RR | 1.24‐1.82 | III (NA) | High | 1.50 |
| Maternal SSRI use during pregnancy* | ADHD | 5 (56,502) | 1.37, RR | 1.16‐1.63 | III (NA) | High | 1.37 |
| Child 4 months younger than school classmates | ADHD | 30 (>1,000) | 1.36, RR | 1.25‐1.47 | III (NA) | High | 1.36 |
| Maternal diabetes | ADHD | 2 (>1,000) | 1.36, HR | 1.19‐1.55 | III (NA) | High | 1.36 |
| 5‐min Apgar score <7 | ADHD | 7 (37,414) | 1.30, OR | 1.11‐1.52 | III (NA) | High | 1.30 |
| High frequency of maternal cell phone use during pregnancy | ADHD | 5 (6,922) | 1.29, OR | 1.12‐1.48 | III (NA) | Low | 1.29 |
| Caesarean delivery | ADHD | 14 (92,426) | 1.17, OR | 1.08‐1.26 | III (NA) | High | 1.17 |
| Breech/transverse presentation | ADHD | 5 (29,051) | 1.14, OR | 1.06‐1.22 | III (NA) | High | 1.14 |
IQR – interquartile range
Evidence for association between risk/protective factors and mental disorders
Altogether, 1,180 associations between putative risk or protective factors and mental disorders were analyzed. Among them, 497 were non‐significant and 507 of class IV, leaving 176 risk/protective associations of class I‐III, which were included in the current study. Twenty‐one associations met class I or II from prospective designs (most robust associations). Table 2 summarizes the associations of risk/protective factors and mental disorders, stratified by ICD‐10 diagnostic blocks.
Table 2.
Evidence for associations between non‐purely genetic risk or protective factors and mental disorders
| Risk or protective factor | Mental disorder | Number of individual studies (cases) | Strength of association, measure | 95% CI | Class of evidence (prospective evidence class) | Quality (AMSTAR) | eOR |
|---|---|---|---|---|---|---|---|
| Organic, including symptomatic, mental disorders | |||||||
| Type 2 diabetes mellitus | Vascular dementia | 14 (1,396) | 2.28, RR | 1.94‐2.66 | I (I) | High | 2.28 |
| Depression | Any dementia | 33 (25,106) | 1.99, RR | 1.84‐2.16 | I (I) | High | 1.99 |
| Depression in elderhood | Any dementia | 25 (4,957) | 1.85, RR | 1.67‐2.05 | I (I) | Medium | 1.85 |
| Depression in elderhood | Alzheimer’s disease | 16 (3,358) | 1.65, RR | 1.42‐1.92 | I (I) | Medium | 1.65 |
| Low frequency of social contacts | Any dementia | 8 (1,122) | 1.57, RR | 1.32‐1.85 | I (I) | Medium | 1.57 |
| Type 2 diabetes mellitus | Alzheimer’s disease | 21 (3,537) | 1.54, RR | 1.39‐1.72 | I (I) | High | 1.54 |
| Benzodiazepines use* | Any dementia | 5 (11,741) | 1.49, RR | 1.30‐1.72 | I (I) | High | 1.49 |
| Depression | Alzheimer’s disease | 25 (5,101) | 1.77, RR | 1.48‐2.13 | II (II) | High | 1.77 |
| Type 2 diabetes mellitus | Any dementia | 22 (15,707) | 1.60, RR | 1.43‐1.79 | II (II) | High | 1.60 |
| High physical activity | Alzheimer’s disease | 9 (1,358) | 0.62, HR | 0.52‐0.72 | II (II) | Medium | 0.62 |
| History of cancer | Alzheimer’s disease | 7 (4,635) | 0.62, HR | 0.53‐0.74 | II (II) | Medium | 0.62 |
| Obesity in midlife | Any dementia | 5 (1,914) | 1.91, RR | 1.40‐2.62 | III (NA) | Medium | 1.91 |
| Low education | Any dementia | 23 (8,739) | 1.88, RR | 1.51‐2.33 | III (NA) | High | 1.88 |
| Low education | Alzheimer’s disease | 16 (2,769) | 1.82, RR | 1.36‐2.43 | III (NA) | High | 1.82 |
| Low frequency electromagnetic fields | Alzheimer’s disease | 25 (3,238) | 1.74, RR | 1.37‐2.21 | III (NA) | High | 1.74 |
| Aluminium exposure | Alzheimer’s disease | 8 (1,383) | 1.72, OR | 1.33‐2.21 | III (NA) | Medium | 1.72 |
| Depression in childhood | Any dementia | 9 (3,538) | 1.63, RR | 1.27‐2.11 | III (NA) | High | 1.63 |
| Herpes viruses infection | Alzheimer’s disease | 33 (1,330) | 1.38, OR | 1.14‐1.65 | III (NA) | Medium | 1.38 |
| Statins use | Any dementia | 12 (37,798) | 0.83, RR | 0.76‐0.91 | III (NA) | High | 0.83 |
| High physical activity | Any dementia | 21 (3,845) | 0.76, RR | 0.66‐0.86 | III (NA) | Medium | 0.76 |
| NSAID use | Alzheimer’s disease | 16 (53,372) | 0.74, RR | 0.64‐0.86 | III (NA) | High | 0.74 |
| Mental and behavioural disorders due to psychoactive substance use | |||||||
| Tobacco smoking | Opioid use disorder | 10 (2,447) | 3.07, OR | 2.27‐4.14 | II (II) | Low | 3.07 |
| Impulsivity‐related personality traits in college adolescents | Alcohol related disorder | 15 (NA) | 0.53, d | 0.43‐0.64 | III (NA) | Medium | 2.63 |
| ADHD | Tobacco related disorder | 4 (NA) | 2.36, OR | 1.71‐3.27 | III (NA) | Medium | 2.36 |
| Impulsivity‐related personality traits in community adolescents | Alcohol related disorder | 9 (NA) | 0.45, d | 0.33‐0.56 | III (NA) | Medium | 2.26 |
| Impulsivity‐related personality traits in school adolescents | Alcohol related disorder | 12 (NA) | 0.43, d | 0.34‐0.52 | III (NA) | Medium | 2.18 |
| Parental alcohol supply | Alcohol related disorder | 8 (NA) | 2.00, OR | 1.72‐2.32 | III (NA) | Medium | 2.00 |
| Peer smoking behaviour | Tobacco related disorder | 71 (NA) | 1.92, OR | 1.76‐2.09 | III (NA) | Medium | 1.92 |
| Externalizing symptoms in adolescents | Alcohol related disorder | 23 (NA) | 1.63, OR | 1.39‐1.90 | III (NA) | Medium | 1.63 |
| Smoking in movies | Tobacco related disorder | 9 (4,398) | 1.46, RR | 1.23‐1.73 | III (NA) | Medium | 1.46 |
| Surviving childhood cancer | Alcohol related disorder | 3 (1,348) | 0.78, OR | 0.68‐0.88 | III (NA) | Medium | 0.78 |
| Surviving childhood cancer | Tobacco related disorder | 6 (2,064) | 0.54, OR | 0.42‐0.70 | III (NA) | Medium | 0.54 |
| Parental stricter alcohol rules | Alcohol related disorder | 2 (NA) | 0.41, OR | 0.33‐0.51 | III (NA) | Medium | 0.41 |
| Schizophrenia, schizotypal and delusional disorders | |||||||
| Clinical high‐risk state for psychosis | Any non‐organic psychotic disorder | 9 (1,226) | 9.32, OR | 4.91‐17.72 | I (I) | High | 9.32 |
| Black‐Caribbean ethnicity in England | Any non‐organic psychotic disorder | 9 (3,446) | 4.87, IRR | 3.96‐6.00 | I (IV) | High | 4.87 |
| Obstetric complications | Schizophrenia spectrum disorders | 18 (1,000) | 1.97, OR | 1.55‐2.50 | I (NA) | Low | 1.97 |
| Minor physical anomalies | Any non‐organic psychotic disorder | 14 (1,212) | 0.92, g | 0.61‐1.23 | II (NA) | Medium | 5.30 |
| Trait anhedonia | Any non‐organic psychotic disorder | 44 (1,601) | 0.82, g | 0.72‐0.92 | II (NA) | Medium | 4.41 |
| Cannabis use | Schizophrenia spectrum disorders | 10 (4,036) | 3.90, OR | 2.84‐5.35 | II (II) | High | 3.90 |
| Ethnic minority in low ethnic density area | Any non‐organic psychotic disorder | 5 (1,328) | 3.71, IRR | 2.47‐5.58 | II (IV) | High | 3.71 |
| Stressful events | Schizophrenia spectrum disorders | 13 (2,218) | 3.11, OR | 2.31‐4.18 | II (NA) | Medium | 3.11 |
| Adversities in childhood | Schizophrenia spectrum disorders | 34 (7,738) | 2.80, OR | 2.34‐3.34 | II (II) | Medium | 2.80 |
| Second generation immigrant | Any non‐organic psychotic disorder | 26 (28,753) | 1.68, IRR | 1.42‐1.92 | II (IV) | High | 1.68 |
| Premorbid IQ | Any non‐organic psychotic disorder | 16 (4,459) | –0.42, g | –0.52 to –0.33 | II (IV) | Medium | 0.47 |
| Olfactory identification ability | Any non‐organic psychotic disorder | 55 (1,703) | –0.91, g | –1.05 to –0.78 | II (NA) | High | 0.19 |
| Social withdrawal in childhood | Any non‐organic psychotic disorder | 15 (1,810) | 0.59, g | 0.33‐0.85 | III (IV) | High | 2.91 |
| Tobacco smoking | Schizophrenia spectrum disorder | 17 (NA) | 2.34, OR | 1.65‐3.33 | III (NA) | High | 2.34 |
| North African immigrant in Europe | Any non‐organic psychotic disorder | 12 (2,577) | 2.22, IRR | 1.58‐3.12 | III (IV) | High | 2.22 |
| Urbanicity | Any non‐organic psychotic disorder | 8 (45,791) | 2.19, OR | 1.55‐3.09 | III (III) | Medium | 2.19 |
| Ethnic minority in high ethnic density area | Any non‐organic psychotic disorder | 5 (1,328) | 2.11, IRR | 1.39‐3.20 | III (IV) | High | 2.11 |
| First generation immigrant | Any non‐organic psychotic disorder | 42 (25,063) | 2.10, IRR | 1.72‐2.56 | III (IV) | High | 2.10 |
| Toxoplasma gondii IgG | Any non‐organic psychotic disorder | 42 (8,796) | 1.82, OR | 1.51‐2.18 | III (IV) | High | 1.82 |
| Non‐right handedness | Any non‐organic psychotic disorder | 41 (2,652) | 1.58, OR | 1.35‐1.86 | III (NS) | Medium | 1.58 |
| Paternal age >35 | Schizophrenia spectrum disorders | 10 (NA) | 1.28, OR | 1.11‐1.48 | III (NA) | Medium | 1.28 |
| Winter/spring season of birth in the Northern hemisphere | Any non‐organic psychotic disorder | 27 (115,010) | 1.04, OR | 1.02‐1.06 | III (NA) | High | 1.04 |
| Mood (affective) disorders | |||||||
| Widowhood | Depressive disorders | 5 (2,720) | 5.59, RR | 3.79‐8.23 | I (I) | Low | 5.59 |
| Sexual dysfunction | Depressive disorders | 6 (5,488) | 2.71, OR | 1.93‐3.79 | I (I) | High | 2.71 |
| Irritable bowel syndrome | Bipolar disorders | 6 (177,117) | 2.48, OR | 2.35‐2.61 | I (NA) | High | 2.48 |
| Four or five metabolic risk factors | Depressive disorders | 8 (1,191) | 2.06, OR | 1.59‐2.68 | I (I) | Low | 2.06 |
| Physical abuse in childhood | Depressive disorders | 10 (3,886) | 1.98, OR | 1.68‐2.33 | I (I) | Medium | 1.98 |
| Job strain | Depressive disorders | 7 (1,909) | 1.77, OR | 1.46‐2.13 | I (I) | Medium | 1.77 |
| Obesity | Depressive disorders | 8 (7,673) | 1.35, OR | 1.21‐1.50 | I (I) | Low | 1.35 |
| Dietary zinc | Depressive disorders | 8 (3,708) | 0.65, RR | 0.57‐0.75 | I (NA) | Medium | 0.65 |
| Tea intake | Depressive disorders | 13 (4,373) | 0.68, RR | 0.61‐0.77 | I (NA) | Medium | 0.68 |
| Dry eye disease with Sjögren’s syndrome | Depressive disorders | 7 (3,062) | 4.25, OR | 2.67‐6.76 | II (NA) | Low | 4.25 |
| Poor physical health | Depressive disorders in elderhood | 11 (8,630) | 4.08, OR | 3.25‐5.12 | II (NA) | Low | 4.08 |
| Adversities in childhood | Bipolar disorders | 13 (1,146) | 2.86, OR | 2.03‐4.04 | II (NA) | High | 2.86 |
| Emotional abuse in childhood | Depressive disorders | 8 (4,112) | 2.78, OR | 1.89‐4.09 | II (III) | Medium | 2.78 |
| Chronic disease | Depressive disorders in elderhood | 10 (9,090) | 2.59, OR | 1.78‐3.76 | II (III) | Low | 2.59 |
| Intimate partner violence against women | Depressive disorders | 9 (3,003) | 2.57, RR | 2.25‐2.94 | II (NA) | Low | 2.57 |
| Sexual abuse in childhood | Depressive disorders | 14 (4,586) | 2.42, OR | 1.94‐3.02 | II (II) | Medium | 2.42 |
| Gulf war veterans | Depressive disorders | 11 (16,826) | 2.37, OR | 1.91‐2.93 | II (NA) | Low | 2.37 |
| Asthma | Depressive disorders in childhood | 7 (2,828) | 2.08, OR | 1.56‐2.77 | II (NA) | Low | 2.08 |
| Three metabolic risk factors | Depressive disorders | 8 (3,014) | 1.99, OR | 1.60‐2.48 | II (II) | Low | 1.99 |
| Poor vision | Depressive disorders in elderhood | 12 (11,066) | 1.94, OR | 1.67‐2.25 | II (NA) | Medium | 1.94 |
| Sleep disturbances | Depressive disorders in elderhood | 11 (2,610) | 1.92, RR | 1.59‐2.33 | II (II) | High | 1.92 |
| Psoriasis | Depressive disorders | 9 (86,945) | 1.64, OR | 1.41‐1.90 | II (NA) | Medium | 1.64 |
| Low education | Depressive disorders in elderhood | 24 (16,590) | 1.58, OR | 1.38‐1.82 | II (IV) | Low | 1.58 |
| Metabolic syndrome | Depressive disorders | 27 (20,924) | 1.42, OR | 1.28‐1.57 | II (IV) | Medium | 1.42 |
| Sedentary behaviour | Depressive disorders | 24 (60,526) | 1.25, RR | 1.16‐1.35 | II (NA) | Medium | 1.25 |
| Neglect in childhood | Depressive disorders | 6 (1,668) | 2.75, OR | 1.59‐4.74 | III (NA) | Medium | 2.75 |
| Insomnia | Depressive disorders | 21 (NA) | 2.60, OR | 1.98‐3.42 | III (NA) | Low | 2.60 |
| Chronic lung disease | Depressive disorders | 4 (297,031) | 2.38, RR | 1.47‐3.85 | III (NA) | Medium | 2.38 |
| Dry eye disease without Sjögren’s syndrome | Depressive disorders | 6 (611,517) | 2.24, OR | 1.50‐3.34 | III (NA) | Low | 2.24 |
| Vitamin D deficiency | Depressive disorders | 3 (NA) | 2.22, HR | 1.42‐3.47 | III (III) | High | 2.22 |
| Asthma | Bipolar disorders | 4 (50,358) | 2.12, OR | 1.57‐2.87 | III (NA) | Medium | 2.12 |
| Maltreatment in childhood | Depressive disorders in childhood | 5 (1,400) | 2.03, OR | 1.37–3.01 | III (NA) | High | 2.03 |
| Terrorist act exposure | Depressive disorders | 6 (NA) | 2.02, OR | 1.38‐2.96 | III (NA) | High | 2.02 |
| Diabetes | Depressive disorders in elderhood | 9 (1,814) | 1.88, OR | 1.31‐2.70 | III (NA) | Medium | 1.88 |
| Heart disease | Depressive disorders in elderhood | 6 (1,911) | 1.81, OR | 1.41‐2.31 | III (NA) | Medium | 1.81 |
| Obesity | Bipolar disorders | 9 (12,259) | 1.77, OR | 1.40‐2.23 | III (NA) | Low | 1.77 |
| Hearing impairment | Depressive disorders in elderhood | 7 (4,448) | 1.71, OR | 1.28‐2.27 | III (NA) | Medium | 1.71 |
| Age >65 | Depressive disorders in elderhood | 6 (15,017) | 1.63, OR | 1.24‐2.16 | III (NA) | Low | 1.63 |
| Living alone | Depressive disorders in elderhood | 16 (10,478) | 1.55, OR | 1.23‐1.95 | III (NA) | Low | 1.55 |
| Age >85 | Depressive disorders in elderhood | 12 (4,559) | 1.52, OR | 1.20‐1.93 | III (NA) | Low | 1.52 |
| Two metabolic risk factors | Depressive disorders | 8 (6,691) | 1.45, OR | 1.17‐1.80 | III (NA) | Low | 1.45 |
| Low birth weight (≤2,500 g) | Depressive disorders | 21 (NA) | 1.38, OR | 1.16‐1.65 | III (NA) | Low | 1.38 |
| Age >75 | Depressive disorders in elderhood | 19 (11,219) | 1.35, OR | 1.17‐1.56 | III (NA) | Low | 1.35 |
| Type 2 diabetes mellitus | Depressive disorders | 11 (37,964) | 1.24, OR | 1.09‐1.40 | III (NA) | Medium | 1.24 |
| Unemployment | Depressive disorders | 13 (40,679) | 1.16, OR | 1.09‐1.23 | III (NA) | Medium | 1.16 |
| Fruit intake | Depressive disorders | 8 (NA) | 0.85, RR | 0.77‐0.93 | III (NA) | Low | 0.85 |
| Traditional/healthy dietary patterns | Depressive disorders | 17 (NA) | 0.76, RR | 0.68‐0.86 | III (NA) | Low | 0.76 |
| Iron intake | Depressive disorders | 3 (1,045) | 0.40, RR | 0.24‐0.65 | III (NA) | Medium | 0.40 |
| Neurotic, stress‐related and somatoform disorders | |||||||
| Physical abuse in childhood | Social anxiety disorder | 4 (1,191) | 2.59, OR | 2.17‐3.10 | I (IV) | High | 2.59 |
| Physical disease history | PTSD | 4 (2,161) | 2.29, OR | 2.07‐2.52 | I (NA) | High | 2.29 |
| Family history of psychiatric disorder | PTSD | 12 (1,765) | 1.80, OR | 1.48‐2.19 | I (NA) | Medium | 1.80 |
| Being an Indigenous American | PTSD | 5 (3,214) | 1.47, OR | 1.28‐1.69 | I (NA) | High | 1.47 |
| Cumulative exposure to potentially traumatic experiences | PTSD | 17 (3,094) | 5.24, OR | 3.54‐7.76 | II (NA) | High | 5.24 |
| Trauma severity | PTSD | 25 (2,017) | 0.66, g | 0.44‐0.88 | II (IV) | Medium | 3.32 |
| Being trapped in an earthquake | PTSD | 1 (2,028 | 2.86, OR | 2.52‐3.25 | II (NA) | High | 2.86 |
| Female sex | PTSD | 112 (9,137) | 1.65, OR | 1.45‐1.87 | II (NA) | Medium | 1.65 |
| Torture exposure | PTSD | 10 (1,357) | 4.46, OR | 2.39‐8.31 | III (NA) | Low | 4.46 |
| Sexual abuse in childhood | Social anxiety disorder | 5 (1,239) | 3.18, OR | 1.73‐5.86 | III (IV) | High | 3.18 |
| Personal psychiatric history | PTSD | 27 (1,753) | 2.45, OR | 1.67‐3.61 | III (IV) | Medium | 2.45 |
| Overprotection from father | Obsessive‐compulsive disorder | 6 (716) | 0.44, g | 0.21‐0.68 | III (NA) | High | 2.24 |
| Behavioural syndromes associated with physiological disturbances and physical factors | |||||||
| Appearance‐related teasing victimization | Any eating disorder | 10 (1,341) | 2.91, OR | 2.05‐4.12 | II (NA) | Medium | 2.91 |
| Sexual abuse in childhood | Bulimia nervosa | 26 (1,103) | 2.73, OR | 1.96‐3.79 | II (NA) | Medium | 2.73 |
| ADHD | Any eating disorder | 12 (3,618) | 4.24, OR | 2.62‐6.87 | III (NA) | Medium | 4.24 |
| Physical abuse in childhood | Binge eating disorder | 4 (NA) | 3.10, OR | 2.48‐3.88 | III (NA) | Medium | 3.10 |
| Sexual abuse in childhood | Binge eating disorder | 7 (NA) | 2.31, OR | 1.66‐3.20 | III (NA) | Medium | 2.31 |
| Self‐reported dieting | Bulimia nervosa | 7 (NA) | 0.22, r | 0.14‐0.30 | III (NA) | Medium | 2.26 |
| Body dissatisfaction | Any eating disorder | 11 (NA) | 0.14, r | 0.11‐0.17 | III (NA) | Medium | 1.67 |
| Perceived pressure to be thin | Any eating disorder | 4 (NA) | 0.11, r | 0.08‐0.14 | III (NA) | Medium | 1.51 |
| Negative affect | Any eating disorder | 11 (NA) | 0.09, r | 0.06‐0.12 | III (NA) | Medium | 1.38 |
| 5‐min Apgar score <7 | Anorexia nervosa | 33 (2,701) | 1.32, OR | 1.17‐1.49 | III (NA) | Medium | 1.32 |
| Disorders of adult personality and behaviour | |||||||
| Emotional abuse in childhood | Borderline personality disorder | 27 (3,525) | 28.15, OR | 17.46‐53.68 | II (NA) | Medium | 28.15 |
| Emotional neglect in childhood | Borderline personality disorder | 21 (3,225) | 22.86, OR | 11.55‐45.22 | II (NA) | Medium | 22.86 |
| Adversities in childhood | Borderline personality disorder | 97 (16,098) | 14.32, OR | 10.80‐18.98 | II (NA) | Medium | 14.32 |
| Physical abuse in childhood | Borderline personality disorder | 30 (2,869) | 9.30, OR | 6.57‐13.17 | II (NA) | Medium | 9.30 |
| Sexual abuse in childhood | Borderline personality disorder | 31 (3,748) | 7.95, OR | 6.21‐10.17 | II (NA) | Medium | 7.95 |
| Physical neglect in childhood | Borderline personality disorder | 20 (3,072) | 5.73, OR | 3.21‐10.21 | II (NA) | Medium | 5.73 |
| Mental retardation | |||||||
| None of the factors was supported by class I, II or III evidence | |||||||
| Disorders of psychological development | |||||||
| Maternal SSRI use during pregnancy* | Autism spectrum disorder | 7 (19,670) | 1.84, OR | 1.60‐2.11 | I (II) | Medium | 1.84 |
| Maternal pre‐pregnancy antidepressant use* | Autism spectrum disorder | 7 (22,877) | 1.48, RR | 1.29‐1.71 | I (NA) | Medium | 1.48 |
| Maternal chronic hypertension | Autism spectrum disorder | 4 (22,864) | 1.48, OR | 1.29‐1.70 | I (NA) | Medium | 1.48 |
| Maternal gestational hypertension | Autism spectrum disorder | 9 (4,334) | 1.37, OR | 1.21‐1.54 | I (NA) | Medium | 1.37 |
| Maternal pre‐eclampsia | Autism spectrum disorder | 10 (10,699) | 1.32, RR | 1.20‐1.45 | I (NA) | Medium | 1.32 |
| Maternal age ≥35 years | Autism spectrum disorder | 11 (>1,000) | 1.31, RR | 1.18‐1.45 | I (NA) | Low | 1.31 |
| Maternal overweight pre/during pregnancy | Autism spectrum disorder | 5 (7,872) | 1.28, RR | 1.19‐1.36 | I (II) | Low | 1.28 |
| Highest paternal age group vs. reference group | Autism spectrum disorder | 20 (2,920) | 1.55, OR | 1.39‐1.73 | II (NA) | Medium | 1.55 |
| Paternal age >45 years | Autism spectrum disorder | 18 (>1,000) | 1.43, OR | 1.33‐1.53 | II (III) | High | 1.43 |
| Highest maternal age group vs. reference group | Autism spectrum disorder | 19 (2,254) | 1.42, OR | 1.29‐1.55 | II (IV) | Medium | 1.42 |
| Paternal age 40‐45 years | Autism spectrum disorder | 12 (>1,000) | 1.37, OR | 1.23‐1.53 | II (IV) | High | 1.37 |
| Maternal autoimmune disease | Autism spectrum disorder | 10 (9,775) | 1.37, OR | 1.21‐1.54 | II (NA) | Medium | 1.37 |
| Higher paternal age (per 10‐years increase) | Autism spectrum disorder | 17 (47,373) | 1.21, OR | 1.18‐1.24 | II (NA) | Medium | 1.21 |
| Maternal paracetamol use during pregnancy* | Autism spectrum disorder | 5 (>100) | 1.20, RR | 1.14‐1.26 | II (NA) | Medium | 1.20 |
| Maternal age 30‐34 | Autism spectrum disorder | 8 (>1,000) | 1.14, RR | 1.09‐1.18 | II (NA) | Low | 1.14 |
| Hearing impairment | Autism spectrum disorder | 7 (4,370) | 14.16, RR | 4.53‐44.22 | III (NA) | Medium | 14.16 |
| 5‐min Apgar score <7 | Autism spectrum disorder | 6 (3,676) | 1.67, OR | 1.34 ‐2.09 | III (NA) | Medium | 1.67 |
| Family history of psoriasis | Autism spectrum disorder | 8 (>1,000) | 1.59, OR | 1.28‐1.97 | III (NA) | Medium | 1.59 |
| Family history of rheumatoid arthritis | Autism spectrum disorder | 8 (>1,000) | 1.51, OR | 1.19‐1.91 | III (NA) | Medium | 1.51 |
| Maternal diabetes | Autism spectrum disorder | 16 (8,872) | 1.49, RR | 1.28‐1.74 | III (NA) | High | 1.49 |
| Family history of type 1 diabetes | Autism spectrum disorder | 13 (>1,000) | 1.49, OR | 1.23‐1.81 | III (NA) | Medium | 1.49 |
| Maternal infection requiring hospitalization | Autism spectrum disorder | 3 (34,547) | 1.30, OR | 1.14‐1.50 | III (NA) | Medium | 1.30 |
| Family history of any autoimmune disease | Autism spectrum disorder | 17 (1,894) | 1.28, OR | 1.12‐1.48 | III (NA) | Medium | 1.28 |
| Reference group vs. lowest paternal age group | Autism spectrum disorder | 15 (2,295) | 1.24, OR | 1.12‐1.37 | III (NA) | Medium | 1.24 |
| Higher maternal age (per 10‐years increase) | Autism spectrum disorder | 14 (46,025) | 1.18, OR | 1.10‐1.26 | III (NA) | Medium | 1.18 |
| Paternal age 35‐40 years | Autism spectrum disorder | 16 (>1,000) | 1.14, OR | 1.08‐1.21 | III (NA) | High | 1.14 |
| Behavioural and emotional disorders with onset usually occurring in childhood and adolescence | |||||||
| Maternal pre‐pregnancy obesity | ADHD | 11 (40,880) | 1.63, OR | 1.49‐1.77 | I (I) | Low | 1.63 |
| Eczema in childhood | ADHD | 6 (10,636) | 1.31, OR | 1.20‐1.44 | I (IV) | Low | 1.31 |
| Maternal hypertensive disorders during pregnancy | ADHD | 8 (37,128) | 1.29, OR | 1.22‐1.36 | I (NA) | High | 1.29 |
| Maternal pre‐eclampsia | ADHD | 6 (>1,000) | 1.28, OR | 1.21‐1.35 | I (NA) | High | 1.28 |
| Maternal paracetamol use during pregnancy* | ADHD | 8 (>1,000) | 1.25, RR | 1.17‐1.34 | I (I) | High | 1.25 |
| Maternal smoking during pregnancy | ADHD | 20 (50,044) | 1.60, OR | 1.45‐1.76 | II (II) | High | 1.60 |
| Asthma in childhood | ADHD | 11 (32,539) | 1.51, OR | 1.40‐1.63 | II (NA) | High | 1.51 |
| Maternal overweight pre/during pregnancy | ADHD | 9 (23,525) | 1.28, OR | 1.21‐1.35 | II (I) | Low | 1.28 |
| Preterm birth | ADHD | 11 (1,542) | 1.84, OR | 1.36‐2.49 | III (NA) | High | 1.84 |
| Maternal stress during pregnancy | ADHD | 8 (25,547) | 1.72, OR | 1.27‐2.34 | III (NA) | High | 1.72 |
| Maternal SSRI use during pre‐pregnancy period* | ADHD | 3 (39,097) | 1.59, RR | 1.23‐2.06 | III (NA) | High | 1.59 |
| Maternal non‐SSRI antidepressants use during pregnancy* | ADHD | 6 (23,064) | 1.50, RR | 1.24‐1.82 | III (NA) | High | 1.50 |
| Maternal SSRI use during pregnancy* | ADHD | 5 (56,502) | 1.37, RR | 1.16‐1.63 | III (NA) | High | 1.37 |
| Child 4 months younger than school classmates | ADHD | 30 (>1,000) | 1.36, RR | 1.25‐1.47 | III (NA) | High | 1.36 |
| Maternal diabetes | ADHD | 2 (>1,000) | 1.36, HR | 1.19‐1.55 | III (NA) | High | 1.36 |
| 5‐min Apgar score <7 | ADHD | 7 (37,414) | 1.30, OR | 1.11‐1.52 | III (NA) | High | 1.30 |
| High frequency of maternal cell phone use during pregnancy | ADHD | 5 (6,922) | 1.29, OR | 1.12‐1.48 | III (NA) | Low | 1.29 |
| Caesarean delivery | ADHD | 14 (92,426) | 1.17, OR | 1.08‐1.26 | III (NA) | High | 1.17 |
| Breech/transverse presentation | ADHD | 5 (29,051) | 1.14, OR | 1.06‐1.22 | III (NA) | High | 1.14 |
AMSTAR – A Measurement Tool to Assess Systematic Reviews, OR – odds ratio, RR – risk ratio, IRR – incidence rate ratio, HR – hazard ratio, eOR – equivalent OR, NA – not available, ADHD – attention‐deficit/hyperactivity disorder, PTSD – post‐traumatic stress disorder, NSAID – nonsteroidal anti‐inflammatory drug, SSRI – selective serotonin‐reuptake inhibitor, * documented or likely confounding by indication
Organic, including symptomatic, mental disorders
Twenty‐one associations with any dementia, Alzheimer’s disease, or vascular dementia were evaluated within this ICD‐10 diagnostic block27. Seven associations were supported by class I evidence (Table 2). Four risk factors were involved in these associations: type 2 diabetes mellitus (with vascular dementia, RR=2.28, and with Alzheimer’s disease, RR=1.54); depression (with any dementia, RR=1.99); depression in elderhood (with any dementia, RR=1.85, and with Alzheimer's disease, RR=1.65); low frequency of social contacts (with any dementia, RR=1.57); and benzodiazepine use (with any dementia, RR=1.49; likely confounding by indication such as difficulties with sleep and chronic anxiety with or without depression).
Four associations were supported by class II evidence (Table 2). These involved two risk factors, namely depression at any age (with Alzheimer's disease, RR=1.77) and type 2 diabetes mellitus (with any dementia, RR=1.60); and two protective factors, i.e. history of cancer (with Alzheimer's disease, HR=0.62, possibly due to survival bias) and high physical activity (with Alzheimer's disease, HR=0.62).
Ten associations were supported by class III evidence (Table 2), involving six risk factors (obesity in midlife, low education, low frequency electromagnetic fields, aluminium exposure, depression in childhood, and herpes viruses infection); and three protective factors (statin use, high physical activity, and non‐steroidal anti‐inflammatory drug use).
All factors with class I and II evidence remained at the same level of evidence in prospective analyses. For factors with class III evidence, no prospective analysis data were available (Table 2).
Mental and behavioural disorders due to psychoactive substance use
Twelve associations across tobacco related disorder, alcohol related disorder and opioid use disorder were evaluated within this ICD‐10 diagnostic block38, 41. None of the associations was supported by class I evidence. Only one association was supported by class II evidence, involving tobacco smoking as a risk factor for opioid use disorder (OR=3.07).
Eleven associations were supported by class III evidence (Table 2), involving eight risk factors and two protective factors. The three risk factors for tobacco related disorder were attention‐deficit/hyperactivity disorder (ADHD), peer smoking behaviour, and smoking in movies; the five risk factors for alcohol related disorder were impulsivity‐related personality traits in college or school or community adolescents, parental alcohol supply, and externalizing symptoms in adolescents. The two protective factors were surviving childhood cancer (for alcohol and tobacco related disorder) and parental stricter alcohol rules (for alcohol related disorder).
For class II evidence, the prospective analysis showed that tobacco smoking remained at the same level of evidence as a risk factor for opioid use disorder. For the remaining class III evidence factors, no prospective analysis data were available (Table 2).
Schizophrenia, schizotypal and delusional disorders
Twenty‐two associations with any non‐organic psychotic disorder and schizophrenia spectrum disorders were evaluated within this ICD‐10 diagnostic block15, 33. Only three associations were supported by class I evidence (Table 2). These all included risk factors: clinical high risk state for psychosis (with any non‐organic psychotic disorder, OR=9.32), Black‐Caribbean ethnicity in England (with any non‐organic psychotic disorder, IRR=4.87), and obstetric complications (with schizophrenia spectrum disorders, OR=1.97).
Nine associations were supported by class II evidence (Table 2). Seven of these involved risk factors, namely minor physical anomalies (Hedges’ g = 0.92), trait anhedonia (Hedges’ g = 0.82), ethnic minority in low ethnic density area (IRR=3.71), and being a second generation immigrant (IRR=1.68), with any non‐organic psychotic disorder; and cannabis use (OR=3.90), stressful events (OR=3.11), and adversities in childhood (OR=2.80), with schizophrenia spectrum disorders. Two associations involved protective factors: premorbid IQ (Hedges’ g = –0.42) and olfactory identification ability (Hedges’ g = –0.91) with any non‐organic psychotic disorder.
Ten associations were supported by class III evidence (Table 2). These all involved risk factors: social withdrawal in childhood, tobacco smoking, being a North African immigrant in Europe, urbanicity, ethnic minority in high ethnic density area, being a first generation immigrant, Toxoplasma gondii IgG, non‐right handedness, paternal age >35, and winter/spring season of birth in the Northern hemisphere.
For class I evidence, the prospective analysis of risk factors showed that only clinical high risk state for psychosis remained at the same level of evidence, while Black‐Caribbean ethnicity in England was downgraded to class IV evidence, and for obstetric complications the level of evidence was not available. For class II evidence, the prospective analysis of risk factors showed that cannabis use and adversities in childhood remained at the same level of evidence, while ethnic minority in low ethnic density area and being a second generation immigrant were downgraded to class IV evidence. One class II evidence protective factor, premorbid IQ, was also downgraded to class IV evidence. For the remaining class II factors, the level of evidence in prospective studies was not available.
For class III evidence risk factors, the prospective analysis showed that only urbanicity remained at the same level of evidence, while social withdrawal in childhood, being a North African immigrant in Europe, ethnic minority in high ethnic density area, being a first generation immigrant and Toxoplasma gondii IgG were downgraded to class IV evidence. The remaining factors were either downgraded to the non‐significant level or the level of evidence was not available (Table 2).
Mood (affective) disorders
Forty‐eight associations with depressive or bipolar disorders were evaluated within this ICD‐10 diagnostic block32, 34. Nine associations were supported by class I evidence (Table 2). Of these, six were risk factors for depressive disorders: widowhood (RR=5.59), sexual dysfunction (OR=2.71), four or five metabolic risk factors (OR=2.06), physical abuse in childhood (OR=1.98), job strain (OR=1.77), and obesity (OR=1.35). One was a risk factor for bipolar disorders: irritable bowel syndrome (OR=2.48). Two were protective factors for depressive disorders: dietary zinc (RR=0.65) and tea intake (RR=0.68).
Sixteen associations were supported by class II evidence (Table 2). These included nine risk factors for depressive disorders: dry eye disease with Sjögren’s syndrome (OR=4.25), emotional abuse in childhood (OR=2.78), intimate partner violence against women (RR=2.57), sexual abuse in childhood (OR=2.42), being a Gulf War veteran (OR=2.37), three metabolic risk factors (OR=1.99), psoriasis (OR=1.64), metabolic syndrome (OR=1.42), and sedentary behaviour (RR=1.25). There were five risk factors for depressive disorders in elderhood: poor physical health (OR=4.08), chronic disease (OR=2.59), poor vision (OR=1.94), sleep disturbances (RR=1.92), and low education (OR=1.58). There was one risk factor for depressive disorders in childhood: asthma (OR=2.08). There was one risk factor for bipolar disorders: adversities in childhood (OR=2.86).
Twenty‐three associations were supported by class III evidence (Table 2). These included ten risk factors for depressive disorders: neglect in childhood, insomnia, chronic lung disease, dry eye disease without Sjögren's syndrome, vitamin D deficiency, terrorist act exposure, two metabolic risk factors, low birth weight (≤2,500 g), type 2 diabetes mellitus, and unemployment. There was one risk factor for depressive disorders in childhood (maltreatment), and seven risk factors for depressive disorders in elderhood (diabetes, heart disease, hearing impairment, age >65, living alone, age >85, and age >75). There were two risk factors for bipolar disorders: asthma and obesity. There were also three protective factors for depressive disorders: fruit intake, traditional/healthy dietary patterns, and iron intake.
For class I evidence, the prospective analysis showed that six risk factors for depressive disorders – widowhood, sexual dysfunction, four or five metabolic risk factors, physical abuse in childhood, job strain, and obesity – remained at the same level of evidence, while dietary zinc and tea intake, as well as irritable bowel syndrome, which was associated with bipolar disorders, were either downgraded to the non‐significant level, or the level of evidence was not available. For class II evidence, the prospective analysis showed that two risk factors for depressive disorders (sexual abuse in childhood, and three metabolic risk factors), and one risk factor for depressive disorders in elderhood (sleep disturbances) remained at the same level of evidence. Two class II risk factors for depressive disorders (emotional abuse in childhood, and metabolic syndrome), and two risk factors for depressive disorders in elderhood (chronic disease and low education) were downgraded to class III or IV evidence. For the remaining class II factors, the level of evidence in prospective studies was not available. For class III evidence, the prospective analysis showed that one risk factor for depressive disorders (vitamin D deficiency) remained at the same level of evidence, while all the other factors were either downgraded to the non‐significant level or the level of evidence was not available (Table 2).
Neurotic, stress‐related and somatoform disorders
Twelve associations across three mental disorders – social anxiety disorder, obsessive‐compulsive disorder, and post‐traumatic stress disorders (PTSD) – were evaluated within this ICD‐10 diagnostic block6, 36. Four associations were supported by class I evidence (Table 2). These involved one risk factor for social anxiety disorder, namely physical abuse in childhood (OR=2.59); and three risk factors for PTSD: physical disease history (OR=2.29), family history of psychiatric disorder (OR=1.80), and being an indigenous American (OR=1.47).
Four associations were supported by class II evidence (Table 2). These all involved risk factors for PTSD: cumulative exposure to potentially traumatic experiences (OR=5.24), trauma severity (Hedges’ g = 0.66), being trapped in an earthquake (OR=2.86), and female sex (OR=1.65).
Four associations were supported by class III evidence (Table 2), involving two risk factors for PTSD (torture exposure and personal psychiatric history); one risk factor for social anxiety disorder (sexual abuse in childhood); and one risk factor for obsessive‐compulsive disorder (overprotection from father).
For class I evidence, the prospective analysis showed that no factor retained its class of evidence. Physical abuse in childhood as a risk factor for social anxiety disorder was downgraded to class IV evidence, while the other factors were downgraded to the non‐significant level or were not computable or available. For class II evidence, the prospective analysis showed that trauma severity as a risk factor for PTSD was downgraded to class IV evidence. For class III evidence, the prospective analysis showed that personal psychiatric history as a risk factor for PTSD, and sexual abuse in childhood as a risk factor for social anxiety disorder, were downgraded to class IV evidence. For the remaining class II and III evidence factors, no prospective analysis data were available (Table 2).
Behavioural syndromes associated with physiological disturbances and physical factors
Ten associations with eating disorders (any eating disorder, bulimia nervosa, anorexia nervosa, binge eating disorder) were evaluated within this ICD‐10 diagnostic block40. None of the associations was supported by class I evidence. Two associations were supported by class II evidence (Table 2), involving two risk factors: appearance‐related teasing victimization (with any eating disorder, OR=2.91) and sexual abuse in childhood (with bulimia nervosa, OR=2.73).
Eight associations were supported by class III evidence (Table 2), involving ADHD, physical and sexual abuse in childhood, self‐reported dieting, body dissatisfaction, perceived pressure to be thin, negative affect, and 5‐min Apgar score <7.
No prospective analysis data were available for any of the factors (Table 2).
Disorders of adult personality and behaviour
Six associations with borderline personality disorder were evaluated within this ICD‐10 diagnostic block42. The associations were all supported by class II evidence, involving emotional (OR=28.15), physical (OR=9.30) and sexual (OR=7.95) abuse; emotional (OR=22.86) and physical (OR=5.73) neglect; and adversities in childhood (OR=14.32) (Table 2).
The level of evidence in prospective studies was not available.
Mental retardation
No class I‐III risk factor for mental retardation was identified.
Disorders of psychological development
Within this ICD‐10 diagnostic block, 26 associations with autism spectrum disorder were evaluated35. Seven associations were supported by class I evidence (Table 2). These involved seven risk factors: maternal selective serotonin reuptake inhibitor (SSRI) use during pregnancy (OR=1.84, confounding by indication such as underlying maternal mental disorders), maternal pre‐pregnancy antidepressant use (RR=1.48, confounding by indication as above), maternal chronic hypertension (OR=1.48), maternal gestational hypertension (OR=1.37), maternal pre‐eclampsia (RR=1.32), maternal age ≥35 years (RR=1.31), and maternal overweight pre/during pregnancy (RR=1.28).
Eight associations were supported by class II evidence (Table 2), all involving risk factors. These were: highest paternal age group vs. reference group (OR=1.55), paternal age >45 years (OR=1.43), highest maternal age group vs. reference group (OR=1.42), paternal age 40‐45 years (OR=1.37), maternal autoimmune disease (OR=1.37), higher paternal age per 10‐years increase (OR=1.21), maternal paracetamol use during pregnancy (RR=1.20, likely confounding by indication such as maternal comorbidities involving inflammation or infection), and maternal age 30‐34 (RR=1.14).
Eleven associations were supported by class III evidence (Table 2), all involving risk factors: hearing impairment, 5‐min Apgar score <7, family history of psoriasis, family history of rheumatoid arthritis, maternal diabetes, family history of type 1 diabetes, maternal infection requiring hospitalization, family history of any autoimmune disease, reference group vs. lowest paternal age group, higher maternal age per 10‐years increase, and paternal age 35‐40 years.
For class I evidence, the prospective analysis showed that none of the risk factors remained at the same level. Maternal SSRI use during pregnancy (confounding by indication) and maternal overweight pre/during pregnancy were downgraded to class II evidence, while all other class I factors were downgraded to non‐significant levels or prospective evidence was not available. For class II evidence, the prospective analysis showed that none of the factors retained the same level of evidence. Paternal age >45 years, highest maternal age group vs. reference group, and paternal age 40‐45 years were downgraded to class III or IV evidence. For the remaining class II evidence factors and all class III evidence factors, no prospective analysis data were available (Table 2).
Behavioural and emotional disorders with onset usually occurring in childhood and adolescence
Nineteen associations with ADHD were evaluated within this ICD‐10 diagnostic block37. Five associations were supported by class I evidence (Table 2), all including risk factors: maternal pre‐pregnancy obesity (OR=1.63), eczema in childhood (OR=1.31), maternal hypertensive disorders during pregnancy (OR=1.29), maternal pre‐eclampsia (OR=1.28), and maternal paracetamol use during pregnancy (OR=1.25, likely confounding by indication).
Three associations were supported by class II evidence (Table 2), involving three risk factors: maternal smoking during pregnancy (OR=1.60), asthma in childhood (OR=1.51), and maternal overweight pre/during pregnancy (OR=1.28).
Eleven associations, all involving risk factors, were supported by class III evidence (Table 2). They were: preterm birth, maternal stress during pregnancy, maternal SSRI use during pre‐pregnancy period, maternal non‐SSRI antidepressant use during pregnancy, maternal SSRI use during pregnancy (confounding by indication for all antidepressant exposures), child 4 months younger than school classmates, maternal diabetes, 5‐min Apgar score <7, high frequency of maternal cell phone use during pregnancy, caesarean delivery, and breech/transverse presentation.
For class I evidence, the prospective analysis showed that maternal obesity pre‐pregnancy and maternal paracetamol use during pregnancy (likely confounding by indication) remained at the same level of evidence, while eczema in childhood was downgraded to class IV evidence, and there were no prospective data for the remaining factors. For class II evidence, the prospective analysis showed that maternal smoking during pregnancy remained at the same level of evidence, while maternal overweight pre/during pregnancy was upgraded to class I level factor (there were no more small‐study effects). For the remaining class II and all class III evidence factors, no prospective analysis data were available (Table 2).
Quality assessment
Based on the AMSTAR evaluation, 58 associations (32.9%) met the high‐quality level, 86 (48.9%) were of medium quality, and 32 (18.2%) were of low quality (Table 2).
Evidence for transdiagnostic risk/protective factors
Eighteen risk factors had a consistent definition across umbrella reviews and were associated with different mental disorders, enabling us to pool them and test their transdiagnosticity against TRANSD criteria (Table 3).
Table 3.
Evidence for transdiagnostic risk factors
| Factor | Mental disorders | Transdiagnostic class of evidence (prospective evidence class) | Transdiagnostic odds ratio (95% CI) | Number of individual studies (cases) | TRANSD criteria met or not |
|---|---|---|---|---|---|
| Sexual abuse in childhood | Borderline personality disorder | II (NA) | 3.92 (3.33‐4.61) | 83 (>10,676) | Yes |
| Bulimia nervosa | |||||
| Binge eating disorder | |||||
| Depressive disorders | |||||
| Social anxiety disorder | |||||
| Physical abuse in childhood | Depressive disorders | II (NA) | 4.82 (3.92‐5.91) | 48 (>7,946) | Yes |
| Social anxiety disorder | |||||
| Borderline personality disorder | |||||
| Binge eating disorder | |||||
| Adversities in childhood | Borderline personality disorder | II (NA) | 13.83 (10.49‐18.23) | 144 (24,982) | Yes (for two disorders only) |
| Bipolar disorders | |||||
| Schizophrenia spectrum disorders | |||||
| 5‐min Apgar score <7 | Autism spectrum disorder | III (III) | 1.27 (1.11‐1.46) | 46 (43,791) | Yes |
| Anorexia nervosa | |||||
| ADHD | |||||
| Type 2 diabetes mellitus | Alzheimer’s disease | II (III) | 1.53 (1.39‐1.69) | 46 (42,897) | No |
| Vascular dementia | |||||
| Depressive disorders | |||||
| Obesity | Depressive disorders | II (NA) | 1.58 (1.40‐ 1.79) | 22 (21,846) | No |
| Bipolar disorders | |||||
| Any dementia | |||||
| Asthma | Depressive disorders in childhood | II (NA) | 1.79 (1.62‐ 1.97) | 22 (85,725) | Yes (for two disorders only) |
| Bipolar disorders | |||||
| ADHD | |||||
| Low education | Depressive disorders in elderhood | II (NA) | 1.68 (1.46‐1.93) | 40 (19,359) | No |
| Alzheimer’s disease | |||||
| ADHD | Any eating disorder | III (NA) | 3.58 (2.50‐5.14) | 16 (>3,618) | Yes |
| Tobacco related disorder | |||||
| Tobacco smoking | Opioid use disorder | II (II) | 2.61 (2.04‐3.33) | 27 (>2,447) | No |
| Schizophrenia spectrum disorders | |||||
| Emotional abuse in childhood | Borderline personality disorder | II (NA) | 15.22 (10.02‐23.10) | 35 (7,637) | Yes |
| Depressive disorders | |||||
| Hearing impairment | Autism spectrum disorder | III (NA) | 4.98 (2.17‐ 11.45) | 14 (8,818) | No |
| Depressive disorders in elderhood | |||||
| Maternal pre‐eclampsia | Autism spectrum disorder | I (II) | 1.29 (1.22‐1.36) | 16 (>11,699) | Yes |
| ADHD | |||||
| Maternal paracetamol use during pregnancy* | Autism spectrum disorder | II (II) | 1.23 (1.17‐1.28) | 13 (>2,000) | Yes |
| ADHD | |||||
| Maternal SSRI use during pregnancy* | Autism spectrum disorder | I (II) | 1.62 (1.44‐ 1.82) | 12 (76,112) | Yes |
| ADHD | |||||
| Maternal overweight pre/during pregnancy | Autism spectrum disorder | I (I) | 1.26 (1.22‐ 1.30) | 14 (31,397) | No |
| ADHD | |||||
| Maternal diabetes | Autism spectrum disorder | III (III) | 1.44 (1.27‐1.65) | 18 (>9,872) | No |
| ADHD | |||||
| Surviving childhood cancer | Tobacco related disorder | III (NA) | 0.61 (0.50‐0.75) | 9 (3,412) | No |
| Alcohol related disorder |
ADHD – attention‐deficit/hyperactivity disorder, SSRI – selective serotonin‐reuptake inhibitor, NA – not available, * documented or likely confounding by indication
Sexual abuse in childhood met TRANSD transdiagnostic criteria across at least five mental disorders: borderline personality disorder42, bulimia nervosa40, binge eating disorder40, depressive disorders34, and social anxiety disorder36 (class II evidence; OR=3.92).
Physical abuse in childhood met TRANSD transdiagnostic criteria across at least four mental disorders: depressive disorders34, social anxiety disorder36, borderline personality disorder42, and binge eating disorder40 (class II evidence; OR=4.82).
Adversities in childhood were associated with at least three mental disorders: borderline personality disorder42, bipolar disorders32, and schizophrenia spectrum disorders33 (class II evidence; OR=13.83). However, bipolar disorders did not meet the criterion T of the TRANSD framework, because the ICD/DSM gold standard was not acknowledged32.
Five‐min Apgar score <7 met TRANSD transdiagnostic criteria across three mental disorders: autism spectrum disorder35, anorexia nervosa40, and ADHD37 (class III evidence; OR=1.27).
Type 2 diabetes mellitus was associated with Alzheimer’s disease27, vascular dementia27, and depressive disorders34 (class II evidence; OR=1.53); and obesity was associated with depressive disorders34, bipolar disorders32, and any dementia27 (class II evidence; OR=1.58). However, they did not meet the TRANSD criterion T27, 32, 34.
Asthma was associated with depressive disorders in childhood34, bipolar disorders32, and ADHD37 (class II evidence; OR=1.79). However, bipolar disorders did not met the criterion T of the TRANSD framework32. Several other risk factors were associated with at least two mental disorders, as shown in Table 3.
When the transdiagnostic class of evidence was restricted to prospective analyses, 5‐min Apgar score <7 remained in class III, while type 2 diabetes mellitus was downgraded from class II to class III. Prospective data were not available for the remaining transdiagnostic factors associated with at least three mental disorders.
Evidence for factors having both risk and protective associations with various mental disorders
No factors were found to have both risk and protective associations with various mental disorders. There were only reciprocal operationalizations of the same factor showing risk‐increasing or protective effects (e.g., high physical activity vs. sedentary behaviour, or parental alcohol supply vs. parental stricter alcohol rules).
DISCUSSION
This is the largest available systematic evidence‐based risk atlas of mental disorders. Its main strength is the rigorous assessment of the credibility of the evidence, which is essential to overcome several types of biases in aetiopathological research. Furthermore, we have adopted a lifespan approach spanning from the pre/perinatal period to childhood, adulthood and elderhood.
A first overarching finding is that 176 associations between risk/protective factors and mental disorders met the criteria for class I‐III evidence. These associations reflected large‐scale observational studies conducted worldwide, thus representing consolidated risk signatures for mental disorders and countering replication crisis43 and scientific pessimism in psychiatry.
At the same time, it is essential to acknowledge that association is not necessarily causation. In particular, reverse causation can confound aetiopathological research44. Accordingly, assessing temporality between exposures and outcome is one of the core Bradford Hill criteria that may be considered when navigating the difficult question of causation vs. plain association45, 46. This potential bias was controlled in sensitivity analyses. Some factors were additionally excluded because of survival biases (i.e., history of cancer27). Others were excluded because of confounding by indication, as documented in previous umbrella reviews and meta‐analyses21, 47 (i.e., maternal SSRI use before and during pregnancy35, 37, maternal antidepressant use before pregnancy35, maternal non‐SSRI antidepressant use during pregnancy37) or acknowledged as likely (benzodiazepine use27, maternal paracetamol use during pregnancy35, 37). We found that 26 associations, relating to 20 risk factors and one protective factor, retained convincing or highly suggestive credibility of evidence (i.e., class I or II) in prospective analyses. The provision of such robust knowledge is essential to allow a more detailed characterization of mental disorders which overcomes the current diagnostic limitations48, 49, 50, and a prerequisite for evidence‐based preventive and early intervention approaches51, 52, because most of the identified risk factors are, at least theoretically, modifiable.
Specifically, we have found that type 2 diabetes mellitus, depression and low frequency of social contacts are consistently associated with dementia. These exposures should be systematically screened in the elderly and could be considered part of refined management strategies in the early phases of dementia. At the same time, our finding of the protective role of high‐intensity exercise is consistent with meta‐analytic evidence that this exercise improves some outcomes of dementia, such as motor performance and daily functioning53.
Beyond dementia, impaired physical health emerged as an overarching core cluster, with three or four‐five metabolic risk factors and obesity being associated with depressive disorders; maternal overweight before/during pregnancy with autism spectrum disorder; and maternal overweight or obesity before/during pregnancy with ADHD. These findings reflect the close interplay between environmental factors and early brain development, as well as the close interconnection of mental and physical domains54. The latter has the potential to offset the numerator of efforts and costs for preventive and early intervention by a denominator of multiple mental and physical disease endpoints. Physical activity is recommended55 for improving outcomes across several mental disorders, including substance related disorders56, and is also indicated to protect physical health of people with mental disorders57. The emerging field of lifestyle psychiatry recommends physical activity together with other “lifestyle factors”, even beyond clinical populations, as a universal tool for public health strategies58.
A related risk domain points to the potential impact of reducing tobacco smoking41 or maternal smoking during pregnancy37 in order to prevent opioid use disorder and ADHD, respectively; similarly, reducing cannabis use33 emerges as an accessible mainstream approach to prevent psychosis59. Effective public health (e.g., community pharmacy‐delivered interventions60), psychoeducation61 and pharmacological interventions (e.g., varenicline62, 63, 64) are available to reduce tobacco smoking, but no interventions have yet been consolidated to reduce maternal smoking65 or cannabis use66, 67.
A further cluster includes risk factors related to environmental stressors, with childhood adversities being associated with psychosis, and widowhood, childhood physical or sexual abuse, and job strain with depressive disorders. Early traumatic experiences have been suggested to be associated with a pro‐inflammatory state in adulthood, with specific inflammatory profiles depending on the type of trauma68. Unfortunately, the current evidence is insufficient to recommend specific interventions to prevent early traumatic experiences69. Future research should prioritize population‐level actions on social determinants of mental health (demographic, economic, neighbourhood, environmental events, social and cultural domains) to replace negative cycles of poverty, abuse, violence, environmental degradation and high personal stress with virtuous cycles of mental health, well‐being, and sustainable development52, 70.
Another important finding is that the strongest level I risk factor surviving prospective analyses was the clinical high risk state for psychosis15, 71, with an eOR of about 9. However, this state may be better conceptualized as a risk marker, because it represents the result of different interacting risk factors72, 73 that accumulate during the recruitment phase74 of these individuals. The clinical high risk state for psychosis is also the prototypical example of antecedent conditions75, for which the boundaries with the onset of the disorder itself may become blurred76, 77, 78, 79.
According to methodological guidelines, ORs greater than 4.72 are to be considered large (assuming a prevalence rate of mental disorders in the non‐exposed ranging from 1% to 5%)80. The vast majority of identified class I‐III factors (independently of prospective sensitivity analyses) had only a small to medium effect size, with a few exceptions mostly relating to childhood trauma. This finding indicates that future aetiopathological studies need to move away from univariable analyses to rather augment polygenic risk prediction by multivariable measurements of environmental exposures in the same individuals.
In fact, mental disorders exhibit both equifinality (multiple factors can lead to the same disorder) and multifinality (the same aetiological factor can result in different mental disorders). For example, recent genome‐wide association, copy number variant and exome sequencing studies have detected shared genetic risk loci among schizophrenia, bipolar disorder and autism, indicating a broad genetic vulnerability to mental disorders (i.e., genetic pleiotropy)81, 82. On the other hand, recent transdiagnostic approaches in psychiatry have explored multifinality of environmental exposures. However, to date, transdiagnostic approaches have been limited by several methodological caveats, mostly involving reporting inaccuracies83.
Our approach of combining robust classification of evidence with the TRANSD recommendations30 has addressed these biases to deliver robust transdiagnostic evidence inasmuch as data were available. As shown in Table 3, we failed to identify a universal transdiagnostic factor that could account for most mental disorders (such as the “p” factor marker for general psychopathology83). This finding is supported by the lack of convincing evidence supporting the existence of a truly transdiagnostic biomarker84. However, it is important to acknowledge that transdiagnostic aetiopathological research is still an emerging field and that only a few observational studies have conducted multivariable measurements that both lump (transdiagnostic) and split (specific) risk/protective factors across diagnostic dimensions85. The factors identified in Table 3 could represent the starting set of exposures to be tested across different mental disorders or intermediate phenotypes (e.g., those proposed by the Research Domain Criteria86).
Notably, about one‐third of any class I‐II factors listed in Table 2 and the vast majority of transdiagnostic factors listed in Table 3 impact the early neurodevelopment. This finding confirms that the maximal window of opportunity for discovering and therapeutically addressing transdiagnostic risk or protective factors is during the very early phases of neurodevelopment, where the chances of impacting the course of multiple disorders are the highest. Conceptually, these results corroborate the essential neurodevelopmental nature of many mental disorders and suggest that pre/perinatal psychiatry should become a mainstream focus of future applied clinical research and prevention psychiatry.
Genetic factors can be measured en masse with high precision, building on variation in specific single nucleotides in exact positions in the genome, and thus are unambiguously defined at all ages for all individuals and across all studies. In contrast, massive measurements of multiple environmental (or epigenetic) factors are challenging.
First, environmental factors pose logistic barriers, because their assessment may be particularly time consuming and lead to missing data. Recent developments in digital technologies (e.g., electronic medical records, mobile apps)87, 88 and sequential testing frameworks89, as well as the recent availability of poly‐environmental risk scores (e.g., psychosis poly‐risk score87, 90 or exposome91), may make it possible to record multiple exposures in the same individuals in a deep phenotyping approach and over time.
Second, the distinction between clear‐cut genetic and environmental factors in several circumstances may be spurious. For example, family history of mental disorders and socioeconomic status comprise both a genetic and an environmental component90, genetic disposition for ADHD increases the risk of exposure to adverse environments92, and polygenic risk scores for psychosis impact certain behavioural traits and risk exposures93. Epigenetic factors at the crossroads between genes and the environment94 add another level of complexity. A pragmatic approach could be to define environmental factors as non‐purely genetic factors, in line with the current study.
Third, while some risk factors are clearly operationalized (e.g., 5‐min Apgar score <7 and low birth weight ≤2,500 g), numerous others (e.g., stressful events, childhood adversities) are not. Specifically, some of them are imprecisely defined, assessed through different instruments, or include contextual specifiers. For example, stressful events can be ascertained through multiple psychometric instruments, generally falling into two categories: checklists (e.g., the Life Events Checklist) and semi‐structured interviews (e.g., the Life Events and Difficulties Schedule)95. While pooling these different instruments is legitimate within meta‐analytical approaches, their empirical interchangeability for future use in research or clinical settings remains questionable. Similarly, while we found that advanced paternal age has been associated with autism, some associations have defined this factor by comparing the highest paternal age group vs. a reference group96. Interestingly, the authors themselves acknowledged that, as the reference groups were heterogeneous, it was “impossible to define a specific age range as the reference group”96. Because an unclear reference group is used for this factor, it is not truly measurable.
The associated caveat is that using loose operationalizations of factors will inevitably inflate their non‐specificity of association across mental disorders, and therefore lead to an observed artificial transdiagnosticity across different dimensions. For example, psychotic experiences97 measured through self‐administered questionnaires98 are relatively frequent at the population level (prevalence about 8% in young adults99) but poorly predictive of psychosis onset (risk of psychosis: 0.5‐1% per year99). These manifestations cannot be conflated with the clinical high risk state for psychosis, which requires detection by an experienced and trained clinician100, is not common in the general population (only 0.3% of individuals101), and is highly predictive of psychosis onset (risk of psychosis: 20% at 2 years71, 102). The trivialization of the contextual significance of complex phenomena and their operationalization may result in non‐specificity, triggering illusions of continuity and transdiagnostic phenomena103.
In a similar vein, other factors may require temporal (e.g., childhood, midlife, elderhood) or contextual specifiers (e.g., Black‐Caribbean ethnicity in England or indigenous Americans), since their validity may depend on their timing of action or different cultural scenarios. We also found that some factors may be influenced by changes in the contextual environment (e.g., cumulative exposure to potentially traumatic experiences), which may impact their durability over time. A further important methodological limitation is that there are several spurious risk markers (beyond the clinical high risk state for psychosis). For example, some experiences included among “childhood adversities”, such as bullying, may be a marker of early vulnerability in social contexts104.
The lack of standardized assessment measures to reliably record environmental exposures may prevent their usability in research and clinical settings. Accordingly, a significant advancement of knowledge would likely be reached by a global collaborative harmonization effort to standardize the multimodal (e.g., psychopathological, neurobiological, neurocognitive) measurement of these exposures, as well as a specific support from funders to achieve these goals. The set of exposures provided in Table 2 may represent the starting point for emerging international efforts promoted by research funders, such as the Common Measures in Mental Health Science Governance Board105, which aims to drive the adoption of harmonized data collection instruments that are transferable to a variety of locations and areas of mental health research, considering aspects of diversity, inclusivity, cultural and geographical appropriateness.
The main limitation of the current study is that, because confounding (e.g., by indication, as highlighted above21, 47) cannot be ruled out in findings of observational studies, it is not possible to establish causation from the associations. More robust epidemiological methods are needed to control for confounders and better identify causal risk factors for major mental disorders that would enhance the precision and generalizability of the current evidence106. Nevertheless, our findings represent an important agenda for experimental research that can do this, including intervention trials for treatments and prevention. Second, the observed risk factors have been mostly measured in univariable analyses that cannot control for their intercorrelation. Third, gene‐by‐environment correlations and interactions have been inadequately reported. Fourth, we could only identify a small number of protective factors (only 9% of the 176 analyzed factors), likely because current research has been disease‐centred, with resilience factors and good mental health outcomes being investigated only more recently107, 108.
Finally, the umbrella review approach favours the selection of more commonly and readily studied factors, which are more likely to be meta‐analyzed. However, although some emerging risk or protective factors may not have a corresponding eligible meta‐analysis to be included in an umbrella review, this possibility is unlikely, since meta‐analyses are now being performed frequently. In any case, for most of these emerging factors, the current grade of evidence is unlikely to be remarkable, given the limited data. Furthermore, the primary aim of the current study was to provide an evidence‐based classification of the existing knowledge, as opposed to appraising emerging factors that may be consolidated by future research. The rapid progress in aetiopathological meta‐research in this field will nevertheless require periodic updates of knowledge via umbrella reviews, which could leverage the methodological framework validated in the current study.
In conclusion, the evidence‐based atlas of key risk and protective factors identified in the current study equips clinicians and researchers with a solid benchmark for advancing aetiopathological research and for expanding early intervention and preventive strategies for mental disorders.
REFERENCES
- 1.Rasic D, Hajek T, Alda M et al. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta‐analysis of family high‐risk studies. Schizophr Bull 2014;40:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo LE, Kaur R, Meiser B et al. Risk of schizophrenia in relatives of individuals affected by schizophrenia: a meta‐analysis. Psychiatry Res 2020;286:112852. [DOI] [PubMed] [Google Scholar]
- 3.Pepper EJ, Pathmanathan S, McIlrae S et al. Associations between risk factors for schizophrenia and concordance in four monozygotic twin samples. Am J Med Genet B Neuropsychiatr Genet 2018;177:503‐10. [DOI] [PubMed] [Google Scholar]
- 4.Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience 2009;164:331‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MH, Hsu JW, Huang KL et al. Risk and coaggregation of major psychiatric disorders among first‐degree relatives of patients with bipolar disorder: a nationwide population‐based study. Psychol Med 2019;49:2397‐404. [DOI] [PubMed] [Google Scholar]
- 6.Tortella‐Feliu M, Fullana MA, Perez‐Vigil A et al. Risk factors for posttraumatic stress disorder: an umbrella review of systematic reviews and meta‐analyses. Neurosci Biobehav Rev 2019;107:154‐65. [DOI] [PubMed] [Google Scholar]
- 7.McGuffin P, Katz R, Watkins S et al. A hospital‐based twin register of the heritability of DSM‐IV unipolar depression. Arch Gen Psychiatry 1996;53:129‐36. [DOI] [PubMed] [Google Scholar]
- 8.Wray NR, Lin T, Austin J et al. From basic science to clinical application of polygenic risk scores: a primer. JAMA Psychiatry 2021;78:101‐09. [DOI] [PubMed] [Google Scholar]
- 9.The Schizophrenia Working Group of the Psychiatric Genomics Consortium . Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. medRxiv 2020;20192922.
- 10.Marsman A, Pries LK, Ten Have M et al. Do current measures of polygenic risk for mental disorders contribute to population variance in mental health? Schizophr Bull 2020;46:1353‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uher R, Zwicker A. Etiology in psychiatry: embracing the reality of poly‐gene‐environmental causation of mental illness. World Psychiatry 2017;16:121‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moe RH, Haavardsholm EA, Christie A et al. Effectiveness of nonpharmacological and nonsurgical interventions for hip osteoarthritis: an umbrella review of high‐quality systematic reviews. Phys Ther 2007;87:1716‐27. [DOI] [PubMed] [Google Scholar]
- 13.Fusar‐Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health 2018;21:95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis JP. Integration of evidence from multiple meta‐analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta‐analyses. CMAJ 2009;181:488‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radua J, Ramella‐Cravaro V, Ioannidis JPA et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry 2018;17:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aromataris E, Fernandez R, Godfrey CM et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132‐40. [DOI] [PubMed] [Google Scholar]
- 17.Theodoratou E, Tzoulaki I, Zgaga L et al. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellou V, Belbasis L, Tzoulaki I et al. Environmental risk factors and Parkinson’s disease: an umbrella review of meta‐analyses. Parkinsonism Relat Disord 2016;23:1‐9. [DOI] [PubMed] [Google Scholar]
- 19.Belbasis L, Bellou V, Evangelou E et al. Environmental factors and risk of multiple sclerosis: findings from meta‐analyses and Mendelian randomization studies. Mult Scler 2020;26:397‐404. [DOI] [PubMed] [Google Scholar]
- 20.Belbasis L, Bellou V, Evangelou E et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta‐analyses. Lancet Neurol 2015;14:263‐73. [DOI] [PubMed] [Google Scholar]
- 21.Dragioti E, Solmi M, Favaro A et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry 2019;76:1241‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fusar‐Poli P, Hijazi Z, Stahl D et al. The science of prognosis in psychiatry: a review. JAMA Psychiatry 2018;75:1289‐97. [DOI] [PubMed] [Google Scholar]
- 23.Mentis AA, Dardiotis E, Efthymiou V et al. Non‐genetic risk and protective factors and biomarkers for neurological disorders: a meta‐umbrella systematic review of umbrella reviews. BMC Med 2021;19:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009;151:264‐9. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383‐94. [DOI] [PubMed] [Google Scholar]
- 27.Bellou V, Belbasis L, Tzoulaki I et al. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta‐analyses. Alzheimers Dement 2017;13:406‐18. [DOI] [PubMed] [Google Scholar]
- 28.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016;316:1818‐9. [DOI] [PubMed] [Google Scholar]
- 29.Shea BJ, Reeves BC, Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fusar‐Poli P.TRANSD recommendations: improving transdiagnostic research in psychiatry. World Psychiatry 2019;18:361‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fusar‐Poli P, Solmi M, Brondino N et al. Transdiagnostic psychiatry: a systematic review. World Psychiatry 2019;18:192‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bortolato B, Kohler CA, Evangelou E et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta‐analyses. Bipolar Disord 2017;19:84‐96. [DOI] [PubMed] [Google Scholar]
- 33.Belbasis L, Kohler CA, Stefanis N et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta‐analyses. Acta Psychiatr Scand 2018;137:88‐97. [DOI] [PubMed] [Google Scholar]
- 34.Kohler CA, Evangelou E, Stubbs B et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta‐analyses and Mendelian randomization studies. J Psychiatr Res 2018;103:189‐207. [DOI] [PubMed] [Google Scholar]
- 35.Kim JY, Son MJ, Son CY et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry 2019;6:590‐600. [DOI] [PubMed] [Google Scholar]
- 36.Fullana MA, Tortella‐Feliu M, Fernandez de la Cruz L et al. Risk and protective factors for anxiety and obsessive‐compulsive disorders: an umbrella review of systematic reviews and meta‐analyses. Psychol Med 2020;50:1300‐15. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Kim JY, Lee J et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry 2020;7:955‐70. [DOI] [PubMed] [Google Scholar]
- 38.Solmi M, Civardi S, Corti R et al. Risk and protective factors for alcohol and tobacco related disorders: an umbrella review of observational studies. Neurosci Biobehav Rev 2020;121:20‐8. [DOI] [PubMed] [Google Scholar]
- 39.Solmi M, Dragioti E, Arango C et al. Risk and protective factors for mental disorders with onset in childhood/adolescence: an umbrella review of published meta‐analyses of observational longitudinal studies. Neurosci Biobehav Rev 2021;120:565‐73. [DOI] [PubMed] [Google Scholar]
- 40.Solmi M, Radua J, Stubbs B et al. Risk factors for eating disorders: an umbrella review of published meta‐analyses. Braz J Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solmi M, Dragioti E, Croatto G et al. Risk and protective factors for cannabis, cocaine, and opioid use disorders: an umbrella review of meta‐analyses of observational studies. Neurosci Biobehav Rev 2021;126:243‐51. [DOI] [PubMed] [Google Scholar]
- 42.Solmi M, Dragioti E, Croatto G et al. Risk and protective factors for personality disorders: an umbrella review of published meta‐analyses of case‐control and cohort studies. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 43.Salazar de Pablo G, Studerus E, Vaquerizo‐Serrano J et al. Implementing precision psychiatry: a systematic review of individualized prediction models for clinical practice. Schizophr Bull 2021;47:284‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010;468:203‐12. [DOI] [PubMed] [Google Scholar]
- 45.Hofler M.The Bradford Hill considerations on causality: a counterfactual perspective. Emerg Themes Epidemiol 2005;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas RM, McMichael AJ. Association or causation: evaluating links between “environment and disease”. Bull World Health Organ 2005;83:792‐5. [PMC free article] [PubMed] [Google Scholar]
- 47.Halvorsen A, Hesel B, Ostergaard SD et al. In utero exposure to selective serotonin reuptake inhibitors and development of mental disorders: a systematic review and meta‐analysis. Acta Psychiatr Scand 2019;139:493‐507. [DOI] [PubMed] [Google Scholar]
- 48.Maj M, van Os J, De Hert M et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021;20:4‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maj M, Stein DJ, Parker G et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020;19:269‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds CF 3rd.Optimizing personalized management of depression: the importance of real‐world contexts and the need for a new convergence paradigm in mental health. World Psychiatry 2020;19:266‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leavell H, Clark E. Preventive medicine for the doctor in his community: an epidemiologic approach, 1st ed.New York: McGraw‐Hill, 1958. [Google Scholar]
- 52.Arango C, Diaz‐Caneja CM, McGorry PD et al. Preventive strategies for mental health. Lancet Psychiatry 2018;5:591‐604. [DOI] [PubMed] [Google Scholar]
- 53.Yeh SW, Lin LF, Chen HC et al. High‐intensity functional exercise in older adults with dementia: a systematic review and meta‐analysis. Clin Rehabil 2021;35:169‐81. [DOI] [PubMed] [Google Scholar]
- 54.Vancampfort D, Firth J, Schuch FB et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta‐analysis. World Psychiatry 2017;16:308‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stubbs B, Vancampfort D, Hallgren M et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta‐review of the evidence and position statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry 2018;54:124‐44. [DOI] [PubMed] [Google Scholar]
- 56.Ashdown‐Franks G, Firth J, Carney R et al. Exercise as medicine for mental and substance use disorders: a meta‐review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med 2020;50:151‐70. [DOI] [PubMed] [Google Scholar]
- 57.Firth J, Siddiqi N, Koyanagi A et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 2019;6:675‐712. [DOI] [PubMed] [Google Scholar]
- 58.Firth J, Solmi M, Wootton RE et al. A meta‐review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020;19:360‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Forti M, Marconi A, Carra E et al. Proportion of patients in south London with first‐episode psychosis attributable to use of high potency cannabis: a case‐control study. Lancet Psychiatry 2015;2:233‐8. [DOI] [PubMed] [Google Scholar]
- 60.Brown TJ, Todd A, O’Malley C et al. Community pharmacy‐delivered interventions for public health priorities: a systematic review of interventions for alcohol reduction, smoking cessation and weight management, including meta‐analysis for smoking cessation. BMJ Open 2016;6:e009828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wray JM, Funderburk JS, Acker JD et al. A meta‐analysis of brief tobacco interventions for use in integrated primary care. Nicotine Tob Res 2018;20:1418‐26. [DOI] [PubMed] [Google Scholar]
- 62.Pearsall R, Smith DJ, Geddes JR. Pharmacological and behavioural interventions to promote smoking cessation in adults with schizophrenia and bipolar disorders: a systematic review and meta‐analysis of randomised trials. BMJ Open 2019;9:e027389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siskind DJ, Wu BT, Wong TT et al. Pharmacological interventions for smoking cessation among people with schizophrenia spectrum disorders: a systematic review, meta‐analysis, and network meta‐analysis. Lancet Psychiatry 2020;7:762‐74. [DOI] [PubMed] [Google Scholar]
- 64.Peckham E, Brabyn S, Cook L et al. Smoking cessation in severe mental ill health: what works? An updated systematic review and meta‐analysis. BMC Psychiatry 2017;17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Claire R, Chamberlain C, Davey MA et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2020;3:CD010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coronado‐Montoya S, Morissette F, Abdel‐Baki A et al. Preventive interventions targeting cannabis use and related harms in people with psychosis: a systematic review. Early Interv Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 67.O’Connor E, Thomas R, Senger CA et al. Interventions to prevent illicit and nonmedical drug use in children, adolescents, and young adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2020;323:2067‐79. [DOI] [PubMed] [Google Scholar]
- 68.Baumeister D, Akhtar R, Ciufolini S et al. Childhood trauma and adulthood inflammation: a meta‐analysis of peripheral C‐reactive protein, interleukin‐6 and tumour necrosis factor‐alpha. Mol Psychiatry 2016;21:642‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.US Preventive Services Task Force , Curry SJ, Krist AH et al. Interventions to prevent child maltreatment: US Preventive Services Task Force recommendation statement. JAMA 2018;320:2122‐8. [DOI] [PubMed] [Google Scholar]
- 70.Lund C, Brooke‐Sumner C, Baingana F et al. Social determinants of mental disorders and the sustainable development goals: a systematic review of reviews. Lancet Psychiatry 2018;5:357‐69. [DOI] [PubMed] [Google Scholar]
- 71.Catalan A, Salazar de Pablo G, Vaquerizo Serrano J et al. Annual research review: Prevention of psychosis in adolescents – systematic review and meta‐analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry 2021;62:657‐73. [DOI] [PubMed] [Google Scholar]
- 72.Fusar‐Poli P, Tantardini M, De Simone S et al. Deconstructing vulnerability for psychosis: meta‐analysis of environmental risk factors for psychosis in subjects at ultra high‐risk. Eur Psychiatry 2017;40:65‐75. [DOI] [PubMed] [Google Scholar]
- 73.Oliver D, Reilly T, Baccaredda Boy O et al. What causes the onset of psychosis in individuals at clinical high risk? A meta‐analysis of risk and protective factors. Schizophr Bull 2020;46:110‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fusar‐Poli P, Schultze‐Lutter F, Cappucciati M et al. The dark side of the moon: meta‐analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull 2016;42:732‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salazar de Pablo G, Catalan A, Fusar‐Poli P. Clinical validity of DSM‐5 attenuated psychosis syndrome: advances in diagnosis, prognosis, and treatment. JAMA Psychiatry 2020;77:311‐20. [DOI] [PubMed] [Google Scholar]
- 76.Fusar‐Poli P, De Micheli A, Chalambrides M, et al. Unmet needs for treatment in 102 individuals with brief and limited intermittent psychotic symptoms (BLIPS): implications for current clinical recommendations. Epidemiol Psychiatr Sci 2019;29:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fusar‐Poli P, Cappucciati M, De Micheli A et al. Diagnostic and prognostic significance of brief limited intermittent psychotic symptoms (BLIPS) in individuals at ultra high risk. Schizophr Bull 2017;43:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fusar‐Poli P, Cappucciati M, Bonoldi I et al. Prognosis of brief psychotic episodes: a meta‐analysis. JAMA Psychiatry 2016;73:211‐20. [DOI] [PubMed] [Google Scholar]
- 79.Fusar‐Poli P, Salazar De Pablo G, Rajkumar R et al. Diagnosis, prognosis and treatment of brief psychotic episodes: a review and research agenda. Lancet Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 80.Chen H, Cohen P, Chen S. How big is a big odds ratio? interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat‐Simul C 2010;39:860‐4. [Google Scholar]
- 81.Henriksen MG, Nordgaard J, Jansson LB. Genetics of schizophrenia: overview of methods, findings and limitations. Front Hum Neurosci 2017;11:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cross‐Disorder Group of the Psychiatric Genomics Consortium . Identification of risk loci with shared effects on five major psychiatric disorders: a genome‐wide analysis. Lancet 2013;381:1371‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caspi A, Houts RM, Belsky DW et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci 2014;2:119‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carvalho AF, Solmi M, Sanches M et al. Evidence‐based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry 2020;10:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartman CA. The important gain is that we are lumpers and splitters now; it is the splitting that needs our hard work. World Psychiatry 2021;20:72‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanislow CA. RDoC at 10: changing the discourse for psychopathology. World Psychiatry 2020;19:311‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oliver D, Spada G, Englund A et al. Real‐world digital implementation of the Psychosis Polyrisk Score (PPS): a pilot feasibility study. Schizophr Res 2020;226:176‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hickie IB. The role of new technologies in monitoring the evolution of psychopathology and providing measurement‐based care in young people. World Psychiatry 2020;19:38‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt A, Cappucciati M, Radua J et al. Improving prognostic accuracy in subjects at clinical high risk for psychosis: systematic review of predictive models and meta‐analytical sequential testing simulation. Schizophr Bull 2017;43:375‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oliver D, Radua J, Reichenberg A et al. Psychosis Polyrisk Score (PPS) for the detection of individuals at‐risk and the prediction of their outcomes. Front Psychiatry 2019;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pries LK, Erzin G, van Os J et al. Predictive performance of exposome score for schizophrenia in the general population. Schizophr Bull 2021;47:277‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zwicker A, MacKenzie LE, Drobinin V et al. Neurodevelopmental and genetic determinants of exposure to adversity among youth at risk for mental illness. J Child Psychol Psychiatry 2020;61:536‐44. [DOI] [PubMed] [Google Scholar]
- 93.Socrates A, Maxwell J, Glanville KP et al. Investigating the effects of genetic risk of schizophrenia on behavioural traits. NPJ Schizophr 2021;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schiele MA, Gottschalk MG, Domschke K. The applied implications of epigenetics in anxiety, affective and stress‐related disorders – a review and synthesis on psychosocial stress, psychotherapy and prevention. Clin Psychol Rev 2020;77:101830. [DOI] [PubMed] [Google Scholar]
- 95.Beards S, Gayer‐Anderson C, Borges S et al. Life events and psychosis: a review and meta‐analysis. Schizophr Bull 2013;39:740‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu S, Wu F, Ding Y et al. Advanced parental age and autism risk in children: a systematic review and meta‐analysis. Acta Psychiatr Scand 2017;135:29‐41. [DOI] [PubMed] [Google Scholar]
- 97.van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry 2016;15:118‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karcher NR, Barch DM, Avenevoli S et al. Assessment of the Prodromal Questionnaire‐Brief Child Version for measurement of self‐reported psychoticlike experiences in childhood. JAMA Psychiatry 2018;75:853‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sullivan SA, Kounali D, Cannon M et al. A population‐based cohort study examining the incidence and impact of psychotic experiences from childhood to adulthood, and prediction of psychotic disorder. Am J Psychiatry 2020;177:308‐17. [DOI] [PubMed] [Google Scholar]
- 100.Fusar‐Poli P, Raballo A, Parnas J. What is an attenuated psychotic symptom? On the importance of the context. Schizophr Bull 2017;43:687‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schultze‐Lutter F, Michel C, Ruhrmann S et al. Prevalence and clinical significance of DSM‐5‐attenuated psychosis syndrome in adolescents and young adults in the general population: the Bern Epidemiological At‐Risk (BEAR) study. Schizophr Bull 2014;40:1499‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fusar‐Poli P, Salazar de Pablo G, Correll C et al. Prevention of psychosis: advances in detection, prognosis and intervention. JAMA Psychiatry 2020;77:755‐65. [DOI] [PubMed] [Google Scholar]
- 103.Parnas J, Henriksen MG. Epistemological error and the illusion of phenomenological continuity. World Psychiatry 2016;15:126‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varese F, Smeets F, Drukker M et al. Childhood adversities increase the risk of psychosis: a meta‐analysis of patient‐control, prospective‐ and cross‐sectional cohort studies. Schizophr Bull 2012;38:661‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.International Alliance of Mental Health Research Funders . Driving the adoption of shared measures. https://www.iamhrf.org/projects.
- 106.Firth J, Wootton RE, Carvalho AF. Toward preventive psychiatry: the role of advanced epidemiological methods. Am J Psychiatry 2020;177:888‐90. [DOI] [PubMed] [Google Scholar]
- 107.Salazar de Pablo G, De Micheli A, Nieman DH et al. Universal and selective interventions to promote good mental health in young people: systematic review and meta‐analysis. Eur Neuropsychopharmacol 2020;41:28‐39. [DOI] [PubMed] [Google Scholar]
- 108.Fusar‐Poli P, Salazar de Pablo G, De Micheli A et al. What is good mental health? A scoping review. Eur Neuropsychopharmacol 2020;31:33‐46. [DOI] [PubMed] [Google Scholar]


