Abstract
Alleviating the multiple problems of children with autism spectrum disorder (ASD) and its comorbid conditions presents major challenges for the affected children, parents, and therapists. Because of a complex psychopathology, structured therapy and parent training are not always sufficient, especially for those patients with intellectual disability (ID) and multiple comorbidities. Moreover, structured therapy is not available for a large number of patients, and pharmacological support is often needed, especially in those children with additional attention deficit/hyperactivity and oppositional defiant, conduct, and sleep disorders.
Keywords: Autism spectrum disorder, ADHD, Children and adolescents, Pharmacotherapy
Abstract
Die Linderung der vielfältigen Probleme von Kindern mit Autismus-Spektrum-Störung (ASS) und ihrer Begleiterkrankungen stellt für die betroffenen Kinder, Eltern und Therapeuten eine große Herausforderung dar. Aufgrund einer komplexen Psychopathologie reichen strukturierte Therapie und Elterntraining nicht immer aus, insbesondere bei Menschen mit geistiger Behinderung (GB) und multiplen Komorbiditäten. Darüber hinaus steht für viele Patienten keine strukturierte Therapie zur Verfügung, und häufig ist pharmakologische Unterstützung erforderlich, insbesondere bei Kindern, bei denen eine Aufmerksamkeitsdefizit‑/Hyperaktivitätsstörung und oppositionelle Trotz‑, Verhaltens- oder Schlafstörungen hinzukommen.
Schlüsselwörter: Autismus-Spektrum-Störung, ADHS, Kinder und Jugendliche, Pharmakotherapie
Introduction
Autism spectrum disorder (ASD) is a common [73], complex, genetically based, disabling disorder [15] that needs specific knowledge and parenting skills [165] and burdensome, costly treatment. The complex clinical picture is characterized in ICD-11 6A02 [320] by
Persistent deficits in the ability to initiate and sustain reciprocal social interaction and social communication,
A range of restricted, repetitive, and inflexible patterns of behavior and interests, and
A high prevalence of intellectual disability, language impairments, and other comorbid disorders
and a number of comorbid conditions such as attention deficit/hyperactivity disorder (ADHD), sleep disorders, convulsions, oppositional defiant disorder (ODD), anxieties, obsessions and compulsions (OCD), depression, and numerous other symptoms and conditions that are discussed as to whether they represent “core” or comorbid problems [281]. These conditions differ in symptomatology, prevalence, and treatability from those of normally developing children. These differences, partly related to the reduced flexibility (for change), partly to genetic and social conditions, may render therapy and its prognosis difficult, and will increase the impairments of self-worth/self-efficacy and the tendency for depression in the children on the spectrum. Comorbid conditions also seem to contribute to the increased mortality of children with ASD [304].
Table 1.
Abbreviations
| Abbrev. | Definition | Abbrev. | Definition |
|---|---|---|---|
| ABA | Applied behavioral analysis | IQ | Intelligence (Quotient) |
| ACTH | Adrenocorticotropic hormone, corticotropin | LGS | Lennox–Gastaut syndrome |
| AD | Antidepressant | LKS | Landau–Kleffner syndrome |
| AD | MAOI | Monoamino oxidase inhibitor | |
| ADHD | Attention deficit/hyperactivity syndrome | MPEP | 2‑methyl-6- (phenylethynyl)pyridine |
| BD | Bipolar disorder | MT1 | Melatonin 1 (receptor) |
| ASD | Autism spectrum disorder | NDRI | Norepinephrine-dopamine reuptake inhibitor |
| BPD | Borderline personality disorder | NMDA | N‑methyl-D-aspartate |
| CBT | Cognitive behavioral therapy | OCD | Obsessive compulsive disorder |
| CSWS | Continuous spike waves during slow-wave sleep | ODD/CD | Oppositional defiant disorder/conduct disorder |
| DSM‑5 | Diagnostic and Statistic Manual for Mental Disorders, 5th edition | PE | Partial epilepsy |
| DRESS | Drug rash with eosinophilia and systemic symptoms | PECS | Picture exchange communication system |
| EF | Executive functions (functioning) | REM sleep | Rapid eye movement sleep |
| ESES | Electrical status epilepticus during slow-wave sleep | RLS | Restless legs syndrome |
| FDA | Food and Drug Administration | SGA | Second generation antipsychotic |
| FGA | First generation antipsychotic | SSRI | Selective serotonin reuptake inhibitor |
| FXS | Fragile X syndrome | SNRI | Selective serotonin and norepinephrine reuptake inhibitor |
| GABA | Gamma-amino-butyric acid | SE | Side effects |
| GAD | Generalized anxiety disorder | Half life | |
| CBT | Cognitive behavioral therapy | TCA | Tricyclic antidepressant |
| ICD | International Classification of Diseases | TCM | Traditional Chinese medicine |
| ID | Intellectual disability | TEACCH | Treatment and education of autistic and related communication handicapped children |
| IGF‑1 | Insulin-like growth factor – 1 | VPS | Valproic acid |
ASD comprises persons with a very low functional level up to a normal or even supranormal level with relatively low impairment. The disorder may not be cured but largely ameliorated by therapy and guided intrafamilial support [36, 165]. Especially in children with a low functional level, structured behavioral therapies [178] such as ABA1 and its variants, TEACCH2 or PECS3 have been proven to be beneficial. Therapeutic success will depend on the level of impairment, the intrafamilial and peer relation support, the availability, quality and quantity of therapeutic support [183, 192], the age at diagnosis [86, 119, 229, 263, 299], the types and number of comorbid conditions, and the financial support provided by the state or the social insurance, because an individual family will usually not dispose of the necessary means. Less affected children will present with flexibility problems and may easily be overburdened with social problems [166]. Additional challenges may be caused by comorbid conditions like ADHD, dysexecutive problems, depression, anxiety disorders, or seizures [10, 18, 24, 38, 105, 106, 187, 201, 281] (Table 2 [187, 223, 281]). Therapy should aim at attaining autonomy, flexibility, social competence, an educational level that is appropriate to the individual intellectual capacity of the child, and provide the basis for a self-determined and socially integrated life.
Table 2.
ASD: relevant comorbid disorders
| Disorders | Normotypic Children % | ASD Children % | References |
|---|---|---|---|
| Anxiety disorders | 20–40 | 11–84 | [281] |
| Sensory integration/EF | 7.5–15 | [126, 198] | |
| Sleep disorder | 22–32 | 40–80 | [175] |
| ADHD | 5–7 | 30–75 | [10, 58, 266] |
| ODD/CD | 30–90 | [264] | |
| Intellectual disability | 2–3 | 25–70 | [163] |
| OCD | 2.5 | 8–37 | [187] |
| Epilepsy | 1–3 | 20–34 | [24, 105, 261] |
| Depression/BPD | 2–3 | 11–20 | [161, 201] |
| Tic disorder | 1–2 | 9–20 | [260] |
| Central auditory processing disorder | 2–5 | ? | [16] |
“Conventional” pharmacotherapy is targeted to reduce inappropriate behavior and the associated burden for family, school, and the social environment, to limit inattention, impulsivity, and hyperactivity associated with ADHD, and to reduce the risk of seizures. Up to two-thirds of children with ASD are treated with psychotropics, and a third with multiple drugs [92, 156, 288]. Newer trends aim at improving social communication [21] or at transferring experimental therapies into real life [81, 171]. Examples include improving the imbalance between excitatory (glutamatergic) and inhibitory (GABA-ergic) neurotransmission [180, 216] or synaptic plasticity [34]. Among the most promising candidate substances are [171], NMDA4 antagonists [33], memantine [139], and d‑Cycloserine [68, 214], the GABA agonists, baclofen or arbaclofen [77, 130], oxytocin [17, 21, 47, 113, 313], vasopressin [235] or balovaptan [27], and insulin-like growth factors (IGF-I) [44, 301]. Among these, only the binding hormone oxytocin has gained widespread attention, stimulating a considerable number of clinical studies, although with inconsistent results [228].
In order to improve the multiple medical, social, behavioral, learning, or sleep-related problems, a number of drugs have been recommended and studied in clinical trials [241]. In addition, a number of experimental therapies, such as diets and brain extracts, were tried, most of them without any clinical evidence. Because the individual reaction to pharmacotherapy varies considerably [28], individualized treatment is mandatory [218]. We, therefore, performed a systematic review of the current literature, aiming at providing an overview on recommended pharmacotherapy for ASD and its most important comorbid disorders. The review is divided into three sections:
Pharmacologic agents
Therapy for common problems of ASD and comorbid disorders
Other substances, supplementary and alternative therapies.
Methods
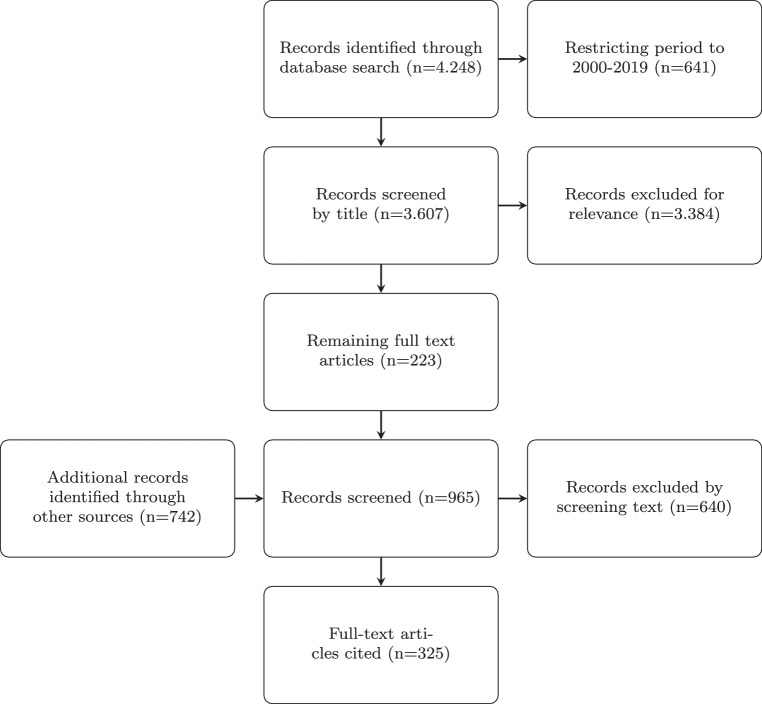
We searched the database PubMed/Medline for the following terms: autism AND pharmacotherapy OR medication, and retrieved 4.248 citations. Restricting the period covered to the years 2000–2019 and the language to English OR French OR German; 3.607 citations remained, including 1120 reviews. Selecting relevant titles, primarily taking into account the contents and quality of the papers, and secondarily the authors, publication media (impact factor), and date (selecting newer references), 223 remained. These were carefully studied in detail and supplemented by 742 additional relevant articles retrieved by specific topic searches that were considered important for understanding during the writing process. This added to 965 references of which 325 were cited in this article, depending on their subjectively estimated significance5, and aiming at not overloading the chapter with citations (see Fig. 1). The relationship between reviews and meta-analyses and original papers in the cited references was .
Fig. 1.
Processing of records
Pharmacotherapy of ASD
In the following, we will discuss the various groups of pharmaceuticals used in children and adolescents with ASD, namely antipsychotics, antidepressants, and anticonvulsants.
Antipsychotics
Antipsychotics influence dopamine neurotransmission, act sedating in lower, antipsychotic in medium, and narcotic in high doses. First generation antipsychotics (FGA), especially haloperidol, have been shown to influence stereotypic and hyperactive behavior, to reduce temper tantrums and social isolation [9]. FGAs should no longer be used because of an inappropriate risk–benefit ratio related to cognitive as well as early and late (e.g., dyskinetic) side effects. As an alternative, second generation antipsychotics (SGAs), especially risperidone, aripiprazole, and quetiapine, are substances of choice for treating aggression, self-injuring behavior, temper tantrums, withdrawal, tics, and rituals.
This is also true for the SGA clozapine because of its dangerous hematologic side effects [152]. As an alternative, SGAs, especially risperidone, aripiprazole, and quetiapine, are substances of choice for treating aggression, self injuring behavior, temper tantrums, withdrawal, tics and rituals [35, 43, 62, 68, 103, 122, 153, 170, 221, 231, 241, 249, 262, 272, 290, 295, 319]. Other SGAs (such as asenapine and iloperidone) may also be used off-label but do not offer advantages [326]. Positive effects should be balanced against (metabolic, endocrine, neurologic, and cardiac) side effects [61, 273]. Therefore, mainly low-dose application should be tried. Recommended dosages and specific features are listed in Table 4. Adding topiramate to risperidone therapy was more effective on overall behavior when compared to risperidone monotherapy [257]. A potential adverse effect of topiramate on language development [227] has, nevertheless, to be considered.
Table 4.
Selected antipsychotics used in children and adolescents with ASD
| Drug | Recommended Dose (mg/kg/d) | Spec. remarks | References | |
|---|---|---|---|---|
| Risperidone | 22 h | 0.005–0.02 also available as syrup | Standard therapy | [42, 64, 153, 207, 278] |
| Aripiprazole | 60–80 h | 0.05–0.1 | Standard therapy | [46, 62, 66, 82, 196, 231] |
| Olanzapine | 30–60 h | 0.1 | SE: sedation, metabolic | [93, 136, 291] |
| Paliperidone | 0.5–2 | No advantage over risperidone | [98] | |
| Quetiapine | 7 h | 0.5–4 | Also acts against GAD | [109, 122, 200] |
| Ziprasidone | 6 h | 0.02–0.4 | Cardiac SE (QTc ) | [69, 195] |
| Pimozide | 55 h | 0.02–0.08 | FGA, therapy resistant tics | [79] |
[110], as related to ASD, 9-hydroxyrisperidone, also available as syrup FDA approved from age 5 years on, also available as solution, FDA approved from age 6 years on, GAD – generalized anxiety disorder
Antidepressants
In normally developing children, selective serotonin antagonists (SSRIs) are effective against depressive symptoms with substance-related differences in effectivity and side effects. SSRIs also act against anxiety disorders in lower dosages and against OCD in higher dosages, compared to the treatment of depression. In children with ASD, SSRIs are widely prescribed, but their therapeutic effect is less evident [319]. Other AD agents, such as MAOIs, mirtazapine, hypericum, etc., also seem to produce only little effect, possibly because of elevated peripheral serotonin blood levels in a number of children and adolescents with ASD [100, 232, 309, 319].
A few studies suggest improvements of repetitive and stereotypic behavior with AD therapy in children with ASD [221], although this was not reported by King et al. [168] or Williams et al. [319]. Side effects of SSRIs usually are mild but may be exaggerated in children with ASD, especially when children are restless and agitated [173]. Bupropion, a NDRI6 acts like a stimulant, may create dependence, and should not be used in adolescents. Mirtazapine [243], a tricyclic AD, has modest antidepressant effects and further acts as a sedative and hypnotic agent by stimulating H1 receptors but is slowly eliminated ( 37 h), strongly increases appetite, and leads to significant weight gain [143]. Studies in autistic children are scarce (e.g., [243]), and long-term studies are not available. Mirtazapine, therefore, should not be used or only used for a limited period and in low doses. Clomipramine and tricyclic antidepressants should only be used with care because of their severe side effects, and duloxetine and pregabaline have not been systematically studied in children and adolescents with ASD.
In summary, although AD medication, especially SSRIs, is widely prescribed in children and adolescents, its effectiveness is limited to not evident in children with ASD, and side effects may be more exaggerated in these patients. Therefore, the use of ADs in ASD can generally not be recommended. Because of their widespread use, pharmacologic data on AD medication are nevertheless summarized in Table 5.
Table 5.
Selected antidepressants used in children and adolescents with ASD to treat depression, anxiety, and OCD
| Drug | Recommended Dose (mg/kg/d) | Specific remarks | Literature | |
|---|---|---|---|---|
| Fluoxetine | 1–6 d | 0.4–0.8 | SE: sleep & eating problems | [135, 169, 253] |
| Paroxetine | 12–22 h | 0.4 | Also effective against anxiety disord. and drug treatment | [242] |
| Sertraline | 23–26 h | 1 | Well tolerated | [292] |
| Agomelatin | 2.3 h | 0.5–1 | MT1 & agonist, no systematic studies in adolescents | [224] |
| Duloxetin | 8–17 h | 0.4–1.2 | SNRI | [224] |
| Pregabalin | 6 h | 3–6–10 | GABA analogon, pain killer, anticonvulsant, anxiolytic | No studies in ASD patients |
[110], as related to ASD
Anticonvulsants
Anticonvulsants may be used to treat epilepsies, bipolar disorders, and externalizing behavioral problems7. Anticonvulsant treatment of children with ASD [83, 133, 261], like in other patients with convulsions, depends on the type of convulsions and should always be combined with psychosocial support [261].
The most commonly used pharmacotherapeutics are valproic acid, lamotrigine, levetiracetam, and ethosuximide [96], cf. Table 6. In select syndromes such as Landau–Kleffner syndrome or ESES8, corticosteroids, ACTH, or immunoglobulin therapy may be considered [303]. Additional nonpharmacological therapeutic options for therapy-resistant epilepsies include vagus nerve stimulation [184], ketogenic diet, and neurosurgical interventions [114]. It is not clear whether an interictal epileptiform EEG may be a cofactor contributing to neurologic deterioration or progressing developmental retardation [310]. Pharmacologic treatment should always be considered if symptoms get worse.
Table 6.
Anticonvulsants selected
| Drug | (h) | Recommended Dose (mg/kg/d) | Comments | References |
|---|---|---|---|---|
| Ethosuximide | 53 | 10–20–40 | Absences, well tolerated | [95]e |
| No effect on behavior, additive to VPS | ||||
| Valproic acid | 12–16 | 10–15–30 | Enhances GABA-ergic inhibition | [96, 136] |
| Cortical hyperconnectivity, increases risk | ||||
| Of ASD and malformation when | ||||
| Administered during pregnancy | ||||
| Lamotrigine | 25–50 | 0.5–4 | Against gen. and PE, well tolerated | [23] |
| Against BSD, no effect on behavior | ||||
| Levetiracetam | 7 | 20–40–60 | Against generalized and PE, SE tiredness | [96] |
| No effect on behavior | ||||
| Clobazam | 18 | 0.2–0.8 | Add-on against prim. generalized and PE | [83] |
| Clonazepam | 18–50 | 0.01–0.4 | Against myoclonus epilepsy, SE: dizziness, ataxia | [83] |
| Gabapentin | 10–40 | Add-on against PE and sec. generalized | ||
| Epilepsy, SE tiredness, DRESS | [115] | |||
| Sultiame | 24 | 5–6 | SE: ataxia, paresthesia, anorexia | |
| Topiramate | 19–25 | 1– | Against PE and generalized epilepsy, | |
| LGS, SE tiredness | ||||
| Weight loss, cognitive | [68, 133] | |||
| impairment | ||||
| Vigabatrin | 5–8 | 20– |
[110], as related to ASD, DRESS drug rash with eosinophilia and systemic symptoms, LGS Lennox–Gastaut syndrome
Therapy for Common Problems of ASD and Comorbid Disorders
Pharmacotherapy for patients with ASD aims at reducing inappropriate behavior and the related intrafamiliar and psychological stress, at improving engagement in therapy, health-related quality of life, performance at school and work, social integration and participation, and at treating comorbid problems such as ADHD or seizures [14, 53, 67, 72, 154, 156, 164, 180, 210, 220, 245, 274]. Limitations include inconsistent evidence of efficiency and side effects, especially with long-term use [107]. A recent study [53] compared the benefits and adverse effects of the pharmacological treatment of a number of targeted symptoms in 505 children with ASD. The authors found small to medium benefits to adverse effects ratios and concluded that individualized treatment is mandatory. Table 3 summarizes the medical indications and available drugs.
Table 3.
ASD Symptoms, comorbid disorders and (off-label) pharmacotherapy
| Symptoms | Available drugs |
|---|---|
| Behavioral problems, restlessness, temper tantrums, self-injuring behavior | Antipsychotics, (anticonvulsants) |
| Social problems | Oxytocin, D‑cycloserin, memantine (experimental) |
| Sleeping problems | Melatonin, antipsychotics, antihistaminics |
| ADHD | Atomoxetin, methylphenidate, amphetamines, (guanfacine ER) |
| Tics | Antipsychotics, ( sympathomimetics, SSRIs) |
| Depression | SSRIs, SNRIs, (+ antipsychotics) |
| Bipolar disorder | Antipsychotics, (lithium) |
| Anxiety & OCD | SSRIs (higher dosage needed), pregabaline |
| Seizures | Valproic acid, levetiracetam, lamotrigine (and others) |
| Psychosis | Antipsychotics |
| GI problems | Diet? probiotics? |
ADHD
ASD and ADHD share genetic, neurophysiological, and clinical similarities [10, 181]. Both disorders affect attention, flexibility, planning, and response inhibition, have a high heritability, early onset, overlapping comorbidities, and prevail in males [50, 58]. Hans Asperger already described attention problems as “almost regularly occurring in children of this type” [13]. Ronald et al. [265] found significant correlations between ASD and ADHD pheno and genotypes in their twins’ early development study, and a probability of 41% for co-occurrence ADHD in ASD patients. Nijmijer et al. [225] found genetic linkages between ASD and ADHD on chromosomes 7, 12, 15, 16, and 18. The “dual disorder” is characterized by increased psychopathology and psychosocial stress, more compromised cognitive and daily functions, including maladaptive behaviors, and poorer effects of therapy [48, 125, 147, 160, 246, 251]. ASD and ADHD share multiple comorbidities, such as dysexecutive problems, increased anxiety, sensory integration, sleep, affective and central hearing processing disorders, developmental delay, OCD, and epilepsy [187, 223, 281]. These comorbid conditions will largely determine the clinical picture. Unfortunately, ADHD in autistic patients is generally not appropriately treated [160]. This could be due to the fact that ADHD was excluded in autism diagnosis in ICD-10, a path that has now been changed in DSM‑5 and ICD-11.
Treatment of ADHD in patients with ASD should follow the same multimodal algorithms as for ADHD alone and should include psychoeducation [87, 219, 238], parental training [41, 85, 87], school-based measures (such as daily record cards [70, 80, 97], structured task organization, physical activity [39, 158, 302]), and medication [31, 285, 296]. ADHD medication is usually less effective, and SE are more pronounced in ASD patients, especially in those with ID [48, 85, 241, 255]. Cognitive training [56] and neurofeedback [88, 212, 252] are less effective and more complex. Occupational therapy [49] is useful as an adjunct for improving comorbid sensory integration and dysexecutive problems.
Medication for ASD/ADHD targets modulating dopamine and epinephrinergic transmitter systems, thereby increasing dopamine availability in frontal areas and striatum, and downregulating dopamine moderators. Usually, two types of medication are distinguished: stimulants (methylphenidate, amphetamine, lis-dexamphetamine) and nonstimulants (atomoxetine and alpha‑2 agonists).
Stimulants. Effectiveness and compatibility of methylphenidate, the most frequently used ADHD medication, have multiply been proven in patients with ASD and ADHD, with and without ID [11, 255, 282, 298]. In addition to the main ADHD symptoms, executive and nonexecutive memory, reaction time, reaction time variability, response inhibition, social communication, and self-regulation are significantly improved with methylphenidate [51, 149, 298] with somewhat lower effect sizes (around 0.5) in children with ASD and ADHD, compared to normally developing children with ADHD. Because of the short of about 2 hours, stimulants are usually administered in a slow-release formulation, acting for 10–14 hours, depending on the preparation. About 70% of the normally developing children and half of the children with ASD and ID respond by improved behavior, especially with decreased impulsivity, improved cooperation and attention, and less hyperactivity. Behavioral improvement is more pronounced in children presenting with hyperactivity and normal IQ [4]. Careful dosage titration is recommended because of the large variability of efficacy that may be explained genetically [206]. The effect of methylphenidate on growth has been divergently debated with height deficits ranging from 0 to 4.7 cm with consistent use [258]. In children with severe side effects or decreased responsiveness to methylphenidate, amphetamine [284], or lisdexamphetamine [52, 54, 127, 145], an inactive amphetamine precursor that is activated in the erythrocytes may be recommended because of their larger effect sizes. Amphetamines, and especially lisdexamphetamine, also improve mood while acting.
Emotional dysregulation (irritability) is a common problem in children with ADHD and with ASD, with rates around 78% for both disorders [179]. Stimulants and atomoxetine act effectively but may also increase emotional dysregulation, although at a much lower prevalence of about 17% [104]. In addition, effects on sleep (longer sleep latency, decreased sleep efficiency, and shorter sleep duration) were observed with stimulant medication [167].
Atomoxetine. The norepinephrine reuptake inhibitor and NMDA receptor antagonist possesses good effectiveness [123, 124] and (compared to methylphenidate) a considerably longer of 35 hours and 99% plasma albumin binding. Because of its nearly continuous action, atomoxetine is a recommendable alternative to methylphenidate, although with a smaller effect size [5, 236, 244], especially in children who respond with pronounced SE to stimulants or are very difficult to handle in the morning and evening hours, when methylphenidate does not act. It may also be recommended in children with comorbid depression, tics, or anxiety disorders [3, 5]. Atomoxetine needs a longer dosing period (up to 12 weeks) and may cause initial fatigue, headache, and gastrointestinal SE, wherefore the medication should initially be started in the evening hours. About 15% of the patients may react with increased aggression, requiring discontinuation of atomoxetine and either addition of risperidone [207] or aripiprazole [231] or switching to extended-release guanfacine [269, 270] or lisdexamphetamine [52].
Comparing atomoxetine and amphetamine derivates, higher effect sizes of methyplhenidate slow release preparations have been reported [121]. Small but significant cardiovascular effects have been reported for stimulant and atomoxetine medication [132], mainly small increases of the heart rate and of systolic or diastolic blood pressure [132]. Because significant cardiovascular effects may not be excluded in a small subgroup of patients (e.g., with slow drug metabolism), occasional blood pressure checks are recommended.
Alpha-2-agonists. Clonidine and extended-release guanfacine are less effective medications against ADHD core symptoms with some antitic potential, pronounced tiredness, and gastrointestinal SE, which may lead to discontinuing the medication. Hyperactivity and impulsivity are improved in about 45% of cases [144, 199, 241, 270, 294].
Other treatments for ADHD. Mindfulness-based [1, 259, 268] and neurofeedback therapies [138] have been tried with some success in children with ASD and ADHD.
Affective Disorders
Due to the fact that antidepressant medication is of questionable effect in children and adolescents with ASD, their use may generally not be recommended. There is no clear-cut evidence that this recommendation is also valid for patients with severe depression, and the widespread use of antidepressant medication reflects this challenge, especially in the light that the prevalence of comorbid depression in autistic patients is fourfold compared to the nonautistic population [318]. Combining antidepressants with (low-dose) antipsychotic medication may generally be recommended for augmenting antidepressant effects in therapy resistant depressive patients and–although with low evidence [78]–in suicidal patients. This relates to the long period needed for antidepressant drug effects to become evident and to the effect of antipsychotics to reduce initially present internal drive and suicidality. Psychotherapy adds to antidepressant therapy for light to medium severe depression in the short term but better in the long term. For severe depression, combining psycho and pharmacotherapy is recommended in normotypic children [40, 65].
Suicidality has been reported in 21.3% (7–47%) of patients with ASD [142, 324]. Suicidal ideation is very common in adolescents with ASD, especially in Asperger’s autists, and is largely related to their increased vulnerability to stress, anxiety, and depression, their inflexibility, and their proneness to become bullied or sexually abused [142].
Bipolar disorders are detected in 6–21% of adult ASD patients [307], and 30% of bipolar I patients meet the criteria for ASD [161]. Data for children and adolescents are still lacking. Therapeutic options include SGA, valproic acid, AD medication if severe depressive symptoms are present, and lithium. Lithium medication also improves social functioning in animals and adults [190]. Its use may be especially limited in children because of the narrow therapeutic range, its effect on thyroid function, the resulting need of a highly compliant and supportive environment, and the considerable and poorly tolerated emotional indifference created by the drug [208, 277].
Anxiety Disorders
About 40% of children with ASD present with various anxiety disorders, phobias including social phobia, general, and separation anxiety disorder, and OCD [323]. They also often react with symptoms of anxiety or even panic in reaction to changes in their environment. An early study [292] reported beneficial effects with low-dose AD medication against anxieties. Stachnik et al [290] reviewed the beneficial effect of neuroleptics for anxiety disorders in children with ASD. High doses of antidepressants may reduce OCD symptoms in normotypic children. Unfortunately, their effectiveness is not confirmed in children with ASD [169, 222, 253], possibly because of the background similarities of ASD and OCD [271].
In general, the treatment methods of choice for fears and OCD are parent training, play therapy, and cognitive behavioral therapy (CBT) [6, 60]. Antidepressants in higher dosages may be tried in individual patients as an adjunct to cognitive therapies. Because of the poor flexibility of patients with ASD, CBT may be very laborious in autistic children and adolescents.
Medication Against Sleep Disorders
Medication may be helpful in inducing and improving disturbed sleep but should be provided with caution: melatonin will improve sleep rhythm in 85% of the children with ASD even in those without disturbed melatonin circadian rhythm at a daily dosage of 1–6 mg given 30 minutes before bedtime [108, 267]. Advancing sleep onset will require a smaller dose of 0.2–0.5 mg given 3–5 h prior to the desired sleep time [32, 175]9.
Other sleep stimulating agents, like valerian, passion flower, and hops provide placebo support; benzodiazepines, zolpidem, and zaleplon act on GABA receptors, helping in inducing sleep but usually have a long , decrease REM sleep phases, but lead to habituation, to losing sleep induction effects during prolonged use, and to promoting anxiety [234]. Sleep-inducing antidepressants like trazodone are commonly used. For contraindications (tricyclics, mirtazapine), see Sect. 3.2.
Restless legs syndrome [59, 280]10, another syndrome disturbing sleep and quality of life based on a genetic predisposition, dysregulation of iron metabolism, and the dopaminergic system, suggest considering iron deficiency as a cause of sleep disturbance [308].
Other sleep-stimulating agents, like valerian, passion flower, and hops, provide placebo support; benzodiazepines, zolpidem, and zaleplon act on GABA receptors, helping in inducing sleep but usually have a long , decrease REM sleep phases, lead to habituation, may lose sleep induction effects and promote anxiety during prolonged use [234]. Sleep-inducing antidepressants like trazodone11 are commonly used. For contraindications (tricyclics, mirtazapine), see Sect. 3.2.
Benzodiazepines, especially those targeting receptor subtypes, may attenuate ASD symptoms [216]. The clinical significance of this effect is not known at present12.
Convulsions and Epilepsy
Epilepsy (more than one convulsion) occurs in about 5–46% of children with ASD, (compared to 1–2% in children not on the spectrum), depending on the clinical sample and the severity of ID [287]. Comorbid epilepsy adds to the impact of ASD on quality of life [303] because of a number of additional problems, such as cognitive, speech developmental, sleep, affective, medical, social, and behavioral issues [90, 118]. Phenotypes and causes are still insufficiently researched.
Mitochondrial respiratory chain defects have been detected as an important link between epilepsy and ASD [315]. In addition, three ASD associated syndromes with known genetic cause, tuberous sclerosis, Rett’s syndrome, and fragile X syndrome, are associated with epilepsy. Another group of disorders, epileptic encephalopathies, have been described in the context of brain dysfunction and increasing autistic symptomatology [74], affecting about 40% of children with convulsions in early childhood. These include early myoclonic encephalopathies, West, Dravet, Lennox Gastaud, and Landau–Kleffner syndromes, myoclonus epilepsy in nonprogressive encephalopathies, and continuous spike waves in slow-wave sleep (CSWS) [303]. Risk factors include epilepsies with known structural defects, bilateral frontal EEG changes, and persistent hypsarrhythmia [303].
Gastrointestinal Issues
Gastrointestinal distress related to constitutional, behavioral, and inflammatory causes is frequently observed in children with ASD and may be related to altered ASD severity [140]. Alterations of the intestinal microbiota, permeability, and functioning may, for example, alter intestinal serotonin metabolism and cause hyperserotoninemia, alter immune responses, and even brain functioning and behavior via the gut–brain axis [12, 193]. Attempts to influence these disturbances by diets (such as a gluten-free diet), probiotics, antibiotic or other “treatments” such as detoxification, would need careful prospective randomized clinical trials, precise diagnostics, and well-established clinical algorithms. At present, this clinical evidence is not available [240]
Irritability, Aggression, Disruptive, and Self-Injuring Behavior
Impulsive aggression and related disruptive behavior, as well as self-injuring behavior are frequently observed in ASD/ADHD and are the leading cause for school suspension, clinical referrals, and ward admissions [182]. Positive parenting [71], early intensive psychosocial and behavioral interventions [60, 76], specific multisystemic programs, such as multisystemic therapy [131] or the Fast Track program [25, 55], and psychosocial interventions such as T‑MAY [279] or TRAAY [276], and group sessions for social competence [101] lead to significant improvements of adaptive behavior. Recommendations for medical treatment include stimulants (in the case of comorbid ADHD) and nonstimulant medication, SGAs (cf. Sect. 3.1), antidepressant and mood stabilizing agents [48, 68, 75, 91, 116, 159]. In addition to pharmacotherapy, behavioral and social competence training, and parental counselling are strongly recommended.
Sleep Disorders
Independently of their intellectual capacity, up to of children with ASD suffer from sleep problems: delayed sleep onset, frequent night awakenings, reduced total sleep time, dys and parasomnias [26, 57, 63, 157, 175, 189, 197, 205, 256, 308, 317]. These problems often persist into adulthood. The causes range from poor sleep hygiene and inconsistent parental behavior [317], (self) regulatory problems and central excitatory/inhibitory imbalance, delayed sleep pattern maturation, a disturbed hypothalamic-pituitary-adrenal axis, and decreased and dysrhythmic melatonin secretion to decreased binding of melatonin to its transporter protein and melatonin receptor dysfunction [57, 141, 202]. Recently, slow-release melatonin13 was approved by the European Medicines Agency for the treatment of sleep disorders in children with ASD from the age of 2. In addition, anxiety [305], ADHD/ASD associated sleep and sensory integration problems [126] leading to increased external stimulation (or decreased stimulus filtering), and cerebral convulsions may disturb sleep and quality of life of affected children and, consequently, of the whole family. Therefore, sleep diagnostics and treatment are important for both children with ASD and their families [174, 308].
Restless legs syndrome [59, 280], another syndrome disturbing sleep and quality of life based on a genetic predisposition, dysregulation of iron metabolism, and the dopaminergic system, suggest considering iron deficiency as a cause of sleep disturbance [308].
Behavioral measures [30, 283, 314] like fixed bedtime routine, providing sleeping cues and a low stimulation evening routine, supporting self-soothing behavior, light therapy14 [84], avoiding daytime sleeping, etc., and sensory integration therapy [325] have proven to be helpful, although with little evidence [30].
Chronic Tic Disorders, Tourette Syndrome, and Stereotypies
Chronic tic disorders and motor stereotypies are common comorbid movement disorders in children and adolescents with ASD [249]. The prevalence of chronic tic disorder is about 6.5% [281], about 10 times higher than in normally developing children. It is characterized by involuntary movements or utterings that vary in onset and frequency, depending on daytime and seasonal variations and stress exposure. Treatment is necessary if severity and frequency exceed subjective or environmental tolerance. Effective treatment options [249] (besides relaxation, stress reduction, and bio or neurofeedback) include antipsychotics such as risperidone, aripiprazole, or pimozide, eventually with added pentoxyfylline, and the anticonvulsant topiramate are effective, whereas haloperidole, levetiracetam, guanfacine, and atomoxetine, as well as metoclopramide and odansetron, have not proven effective [249, 262].
Other Substances, Supplementary and Alternative Therapies
Among the “newer” pharmacologic concepts (such as IGF‑1, memantine, D‑cycloserine, arbaclofen, and oxytocin [240, 300]), only three show promise for the future: oxytocin with the objective to improve sociogenic behavior, beta blockers to reduce stress, and the glutamate antagonist, 2‑methyl-6-(phenylethynyl)pyridine (MPEP), to reduce stereotypic behavior [94]. For the latter substance, it is feared that sociogenic behavior may deteriorate during treatment [297].
In the short term, intranasal oxytocin enhances motivation and attention to social stimuli, improves social initiative, understanding, learning [8, 22, 176], and better recognition of emotions [111]. Unfortunately, these improvements were not substantiated in long-term trials [7, 112, 313, 321, 322]. A meta-analysis [248] reported medium-effect sizes for prolonged oxytocin therapy in small samples. Reasons for the variation in oxytocin response include time dependency of the oxytocin response [230], single nucleotide polymorphisms of the oxytocin receptor [148], and lasting effects of postnatal stimulation of the oxytocin system [300]. When studying oxytocin effects patients and targets must be carefully selected. Therefore, the clinical usefulness of oxytocin is still a matter of debate [228, 306]. Melanocortin, stimulating oxytocin release, could be a useful alternative [215], but large clinical trials are lacking. Still, a special edition of “Brain Research”15 provides a comprehensive overview about the state of research.
There is only limited evidence for using beta blockers for reducing stress-related autoaggressive behavior [312] or memantine for improving language and memory functions [233]. Defects of GABA-A receptors, leading to deficient synaptogenesis, have been demonstrated in fragile X syndrome, a pervasive developmental disorder with known genetic defect16. Ganaxolone, a strong GABA-A agonist, was used in a controlled clinical study [29, 188] and was found to be safe but only effective in a subgroup of patients with fragile X syndrome, high levels of anxiety, and low intellectual capacity.
Medical cannabis, especially for ADHD, tics, sleep problems, behavioral problems, and anxiety [2, 134, 247], may improve symptoms but does not lead to remission. Treatment evidence at present is limited to anecdotical reports and a few small studies; three further studies are to be expected. Treatment options should, therefore, be restricted to single patients in whom standard treatment did not improve severe symptoms.
Various behavioral and functional therapies, such as structured behavioral therapies [178, 254, 299], communication and social skills training [177, 213], occupational therapy [49, 194], mindfulness [259], play teaching [162], music [217, 289], and speech therapy, have been shown to have beneficial effects in improving development, behavior, speech, social functioning, and quality of life [146, 191, 192, 220, 221, 275]. Physical exercise is an effective treatment option, especially in children with dual disorder, ASD and ADHD [128, 286, 302].
Alternative, “natural” treatments seem less invasive, safer (there are no reports on dangerous action), more intuitive to understand, and easier to procure. Parents are concerned with the safety or side effects (listed in the package leaflet) of medication or are disappointed because conventional medication did not change the core symptoms of ASD [120]. Therefore, alternative therapies are very popular [186, 191, 316]; a third of the parents of children with ASD have tried “alternative”, “integrative”, or “complementary”17 therapies [185, 186, 191]. A higher educational level of the mothers predicted the use of alternative therapies [120]. Half of the families use alternative therapies, although they do not rate them as useful.
Most of these therapies are used as an adjunct to conventional therapy. Biologically based therapies (such as diet[239, 293], vitamins and minerals, food supplements such as omega‑3 fatty acids [150], herbal remedies, secretin), and mind–body interventions (such as prayer, shamanism, biofeedback, meditation, and relaxation) are more often perceived efficacious than body-based methods (such as sensory integration therapy [325], massage, craniosacral therapy, neurofeedback, and special exercises) or energy therapies (healing touch, energy transfer) [120]. Technology based interventions seem promising because of the attention sustaining potential, but, at present, evidence of the success of such approaches is poor [172, 250]. Examples are interventions for acquiring language skills [226], for differentiating facial expressions [19], treating food selectivity [20], or anxiety or stress management [37].
A number of physicians encourage multivitamins (49%), essential fatty acids (25%), melatonin (25%), and probiotics (19%), and discourage withholding (76%) or delaying immunizations (55%), chelation (61%), anti-infectives (57%), or secretin (43%) [120]. It has to be stated that there is no clinical evidence for applying specific (e.g., gluten-free or pro-biotic) diets [203], vitamins18 [155, 237], oligominerals, herbal medicine [311], transfer of energy, chelates19 [151], or biologicals such as secretin [180, 186]. It has been found that 10% of parents even use potentially dangerous “medication” such as “whole-brain extracts” [185]. Medication from the Far East, such as traditional Chinese medicine or acupuncture, or osteopathy may be useful in the short-term run in improving single symptoms (restlessness, sleep disturbance); the long-term outcome is rather dubious [45].
Discussion
Pharmacotherapy in children and adolescents with ASD may be helpful in overcoming otherwise not resolvable behavioral and attentional problems (see Table 2 for an overview of indications and classes of useful substances). Individualized treatment is always mandatory, Reviewing the extensive literature on pharmacotherapy of ASD, a few trends may be recognized:
Conventional therapy, although mostly funded on extensive controlled studies, has its limits, especially when treating irritability and temper tantrums. These problems should be restricted by early behavioral treatment. Unfortunately, these treatments are tedious and not available everywhere. In addition, the question of the impact of comorbid conditions has not been solved as yet.
Pharmacologic treatments are not sufficient; the primary ASD treatment, especially for children with intellectual disabilities, will remain structured and functional therapy, as well as parental empowerment and support.
Therapies aiming at improving the core symptoms of ASD, such as social communication: novel therapies, e.g., oxytocin, are encumbered with the complex functioning of our social brain, which is outlined in the first days of life or even before.
At present, genetically based therapies are not visible on the horizon, mostly because the genetic background of ASD is so complex that it will probably need further years of intensive research to link clinical pictures to genetic variants and establish repair options.
Behavioral problems, including irritability, reactive and proactive aggression, disruptive and self-stimulating behavior, restlessness, and temper tantrums, are among the most important therapeutic targets in children with ASD. Because of their very limited flexibility [102] and working memory problems [117], children with ASD easily become despaired and helpless and express this in externalizing behavior that can become difficult to control. Pharmacologic treatment, mostly using antipsychotics, must find a compromise between behavioral control, oversedation, and (mostly metabolic) side effects.
Depressed mood and anxiety disorders call for psychotherapy and, in selected patients, for treatment with antidepressants. The problems with antidepressant medication are its reduced efficacy in autistic vs. normally developing children (see Sect. 3.2), and, again, walking the tightrope between brightening mood or reducing anxiety or obsessions and compulsions and an increased behavioral activation.
Sleep problems are observed in a majority of patients with ASD. Sleep hygiene and bedtime routines should be tried before trying medication, and sleep-related side effects of stimulant therapy should also be considered as a promoting factor of sleep dysfunction. Melatonin is the first-line drug, especially for difficulties in falling asleep. It is effective in about two-thirds and counterbalances inherited melatonin dysfunction. It should be noted that falling asleep with lights on (especially from computer or mobile phone screens) counteracts the action of melatonin medication.
Treatment of ADHD, one of the most prominent comorbid conditions of ASD with overlapping symptoms, is often a key factor in enabling social and intellectual learning, school attendance, and fighting restlessness and impulsivity. Problems are related to the reduced efficacy of pharmacotherapy compared to normotypic patients and a multitude of interacting problems, e.g., bipolar disorder and ADHD.
Convulsions, most frequently observed in children with ASD and ID, should be treated like in normally developing children (see Sect. 3.2.1). Attention should be paid to sedation, metabolic, learning inhibition side effects, and, and in adolescents, to teratogenic side effects for the offspring.
The rediscovery of the gut–brain axis is a relatively new field of research and might, therefore, be overestimated by parents. More prospective studies will shed light on the effects of dietary and probiotic measures. Alternative treatments are comprehensively largely overestimated for their effects, ranging from dietary to physical and possibly endangering measures. Because alternative “medications” are not controlled for their action in prospective randomized trials, it is difficult to argue against the use of such substances in the general public, mostly because “natural” substances are considered harmless and innocuous (see Sect. 3.4).
In summary, we compiled an overview on substances that may be advantageously used in children with ASD with the aim of improving social behavior, learning ability, and quality of life of the children and their environment. The approach is rather defensive, mostly targeting undesired symptoms. Future work and experience should focus on desired changes of core symptoms, on long-term efficacy, on reducing polypragmasia and undesired drug effects, and on avoiding overtreatment, especially if behavioral therapies are available as an alternative. On the other hand, the benefits of carefully prescribed medication should always be recognized.
Funding
Open access funding provided by Medical University of Vienna.
Conflict of Interest
The authors state that no author has a conflict of interest to declare.
Footnotes
applied behavioral analysis [209].
Treatment and Education of Autistic and related Communication Handicapped Children [211].
Picture Exchange Communication System [89].
N‑methyl-D-aspartate.
Again, selecting more carefully performed studies, more recent, often cited papers, and preferring reviews, if available, over original studies.
norepinephrine and dopamine reuptake inhibitor.
Electrical status epilepticus during slow-wave sleep.
These two references do not primarily refer to children with ASD.
General description.
Trittico®.
Alterations of the excitatory/inhibitory CNS imbalance in children with ASD? [99].
Slenyto®.
10.000 lux for h in the early evening and/or morning in order to synchronize the circadian rhythm better.
Vol. 1580:1–232(2015).
Fragile X mental retardation 1 (FMR1) gene on chromosome X (Xa27.3).
Alternative and conventional medication.
For heavy metal detoxication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Popow, Email: christian.popow@meduniwien.ac.at.
Susanne Ohmann, Email: susanne.ohmann@meduniwien.ac.at.
Paul Plener, Email: paul.plener@meduniwien.ac.at.
References
- 1.Aadil M, Cosme RM, Chernaik J. Mindfulness-based cognitive behavioral therapy as an adjunct treatment of Attention Deficit Hyperactivity disorder in young adults: A literature review. Cureus. 2017;9(5):e1269. doi: 10.7759/cureus.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Burke SL, Maddux M. Current state of evidence of cannabis utilization for treatment of autism spectrum disorders. BMC Psychiatry. 2019;19(328):1–10. doi: 10.1186/s12888-019-2259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen A, Kurlan R, Gilbert D, Dunn D, Dallee FR, Spencer T. 16th World Congress of IACAPAP. Darmstadt: Steinkopff; 2004. Atomoxetine treatment in children with ADHD and comorbid tic disorders; pp. 311–331. [Google Scholar]
- 4.Aman MG, Buican B, Arnold LE. Methylphenidate treatmentin children with borderline IQ and mental retardation: analysis of three aggregated studies. J Child Adolesc Psychopharmacol. 2003;13(1):29–40. doi: 10.1089/104454603321666171. [DOI] [PubMed] [Google Scholar]
- 5.Aman MG, Smith T, Arnold LE, Corbett-Dick P, Tumuluru R, Hollway JA, Hyman SL, Mendoza-Burcham M, Pan X, Mruzek DW, Lecavalier L, Levato L, Silverman LB, Handen B. A review of atomoxetine effects in young people with developmental disabilities. Res Dev Disabil. 2014;35(6):1412–1424. doi: 10.1016/j.ridd.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Child and Adolescent Psychiatry Committee on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with obsessive–compulsive disorder. J Am Acad Child Adoles Psych. 2012;51(1):98–113. [DOI] [PubMed]
- 7.Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S, et al. Intranasal oxytocin in the treatment of autism spectrum disorder: a review of literature and early safety and efficacy data in youth. Brain Res Brain Res Protoc. 1580;2014:188–198. doi: 10.1016/j.brainres.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci Usa. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson LT, Campbell M, Grega DM, Perry R, Small AM, Green WH. Haloperidol in the treatment of infantile autism: effects on learning and behavioral symptoms. Am J Psychiatry. 1984;141:1195–1202. doi: 10.1176/ajp.141.10.1195. [DOI] [PubMed] [Google Scholar]
- 10.Antshel KM, Zhang-James Y, Faraone SV. The comorbidity of ADHD and autism spectrum disorder. Expert Rev Neurother. 2013;13(10):1117–1128. doi: 10.1586/14737175.2013.840417. [DOI] [PubMed] [Google Scholar]
- 11.Arnold LE. Commentary: filling out the evidence base for treatment of attention-deficit hyperactivity disorder symptoms in children with intellectual and developmental disability: conclusions for clinicians - response to Simonoff et al. J Child Psychol Psychiatry Allied Discip. 2013;54(6):701–704. doi: 10.1111/jcpp.12097. [DOI] [PubMed] [Google Scholar]
- 12.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asperger H. Die „Autistischen Psychopathen” im Kindesalter [The “Autistic Psychopaths” in Childhood] Arch Psychiatr. 1944;117:76–136. doi: 10.1007/BF01837709. [DOI] [Google Scholar]
- 14.Bachmann CJ, Manthey T, Kamp-Becker I, Glaeske G, Hoffmann F. Psychopharmacological treatment in children and adolescents with autism spectrum disorders in Germany. Res Dev Disabil. 2013;34(9):2551–2563. doi: 10.1016/j.ridd.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Bai D, Hon Kei Yip B, Windham GC, Sourander A, Francis R, Yoffe R, Glasson E, Mahjani B, Suominen A, Leonard H, Gissler M, Buxbaum JD, Wong K, Schendel D, Kodesh A, Breshnahan M, Levine SZ, Parner ET, Hansen SN, Hultman C, Reichenberg A, Sandin S. Association of genetic and environmental factors with autism in a 5-country cohort. Jama Psychiatry. 2019;76(10):1035–1043. doi: 10.1001/jamapsychiatry.2019.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey T. Beyond DSM: The role of auditory processing in attention and its disorders. Appl Neuropsychol Child. 2012;1(2):112–120. doi: 10.1080/21622965.2012.703890. [DOI] [PubMed] [Google Scholar]
- 17.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banaschewski T, Poustka L, Holtmann M. Autismus und ADHS über die Lebensspanne. Differenzialdiagnosen oder Komorbidität? [Autism and ADHD across the life span. Differential diagnoses or comorbidity?] Nervenarzt. 2011;82(5):573–580. doi: 10.1007/s00115-010-3239-6. [DOI] [PubMed] [Google Scholar]
- 19.Banire B, Al Thani D, Makki M, Qaraqe M, Anand K, Olcay C, Khowaja K, Mansoor B. Universal access in human-computer interaction. Multimodality and assistive environments. Berlin Heidelberg: Springer; 2019. Attention assessment: Evaluation of facial expressions of children with autism spectrum disorder. In; pp. 32–48. [Google Scholar]
- 20.Banire B, Khowaja K, Mansoor B, Qaraqe M, Al Thani D. Advances in neurobiology. Berlin Heidelberg: Springer; 2020. Reality-based technologies for children with autism spectrum disorder: a recommendation for food intake intervention; pp. 679–693. [DOI] [PubMed] [Google Scholar]
- 21.Baribeau DA, Anagnostou E. Social communication is an emerging target for pharmacotherapy in autism spectrum disorder – a review of the literature on potential agents. J Can Acad Child Adolesc Psychiatry. 2014;23(1):20–30. [PMC free article] [PubMed] [Google Scholar]
- 22.Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- 23.Belsito KM, Law PA, Kirk KS, Landa RJ, Zimmerman AW. Lamotrigine therapy for autistic disorder: a randomized, double-blind, placebo-controled trial. J Autism Dev Disord. 2001;31:175–181. doi: 10.1023/A:1010799115457. [DOI] [PubMed] [Google Scholar]
- 24.Besag FM. Epilepsy in patients with autism: links, risks and treatment challenges. NDT. 2017;14:1–10. doi: 10.2147/NDT.S120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bierman KL, Coie J, Dodge K, Greenberg M, Lochman J, McMohan R, Pinderhughes E, Conduct Problems Prevention Research Group School outcomes of aggressive-disruptive children: prediction from kindergarten risk factors and impact of the fast track prevention program. Aggr Behav. 2013;39(2):114. doi: 10.1002/ab.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackmer AB, Feinstein JA. Management of sleep disorders in children with neurodevelopmental disorders: A review. Pharmacotherapy. 2016;36(1):84–98. doi: 10.1002/phar.1686. [DOI] [PubMed] [Google Scholar]
- 27.Bolognani F, Del Valle Rubido M, Squassante L, Wandel C, Derks M, Murtagh L, Sevigny J, Khwaja O, Umbricht D, Fontoura P. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci Transl Med. 2019;11(491):eaat7838. doi: 10.1126/scitranslmed.aat7838. [DOI] [PubMed] [Google Scholar]
- 28.Bowers K, Lin PI, Erickson C. Pharmacogenomic medicine in autism: challenges and opportunities. Paediatr Drugs. 2015;17(2):115–124. doi: 10.1007/s40272-014-0106-0. [DOI] [PubMed] [Google Scholar]
- 29.Braat S, Kooy RF. Insights into GABA‑A ergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology. 2015;88:48–54. doi: 10.1016/j.neuropharm.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Brown CA, Kuo M, Phillips L, Berry R, Tan M. Non-pharmacological sleep interventions for youth with chronic health conditions: A critical review of the methodological quality of the evidence. Disab Rehab. 2013;35(15):1221–1255. doi: 10.3109/09638288.2012.723788. [DOI] [PubMed] [Google Scholar]
- 31.Brown KA, Samuel S, Patel DR. Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: a review for practitioners. Translat Pediatr. 2018;7(1):36–47. doi: 10.21037/tp.2017.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruni O, Alonso-Alconada D, Besag F, Biran V, Braam W, Cortese S, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paed Neurol. 2015;19(2):122–133. doi: 10.1016/j.ejpn.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Burnashev N, Szepetowski P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol. 2015;20:73–82. doi: 10.1016/j.coph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Canitano R. New experimental treatments for core social domain in autism spectrum disorders. Front Pediatr. 2014;2(Article 61):1–6. doi: 10.3389/fped.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canitano R, Scandurra V. Psychopharmacology in autism: an update. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):18–28. doi: 10.1016/j.pnpbp.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Carbone PS. Moving from research to practice in the primary care of children with autism spectrum disorders. Acad Pediatr. 2013;13(5):390–399. doi: 10.1016/j.acap.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Carlier S, Van der Paelt S, Ongenae F, De Backere F, De Turck Empowering children with ASD and their parents: design of a serious game for anxiety and stress reduction. Sensors. 2020;20(4):966. doi: 10.3390/s20040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoglund Carlsson L, Norrelgen F, Kjellmer L, Westerlund J, Gillberg C, Fernell E. Coexisting disorders and problems in preschool children with autism spectrum disorders. Sci World J. 2013;213979. 10.1155/2013/213979. [DOI] [PMC free article] [PubMed]
- 39.Cerrillo-Urbina AJ, García-Hermoso A, Sánchez-López M, Pardo-Guijarro MJ, Santos Gómez JL, Martínez-Vizcaíno V. The effects of physical exercise in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis of randomized control trials. Child Care Health Dev. 2015;41(6):779–788. doi: 10.1111/cch.12255. [DOI] [PubMed] [Google Scholar]
- 40.Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685–716. doi: 10.1146/annurev.psych.52.1.685. [DOI] [PubMed] [Google Scholar]
- 41.Charach A, Carson P, Fox S, Ali MU, Beckett J, Lim CG. Interventions for preschool children at high risk for ADHD: a comparative effectiveness review. Pediatrics. 2013;131(5):e1584–e1604. doi: 10.1542/peds.2012-0974. [DOI] [PubMed] [Google Scholar]
- 42.Chavez B, Chavez-Brown M, Rey JA. Role of risperidone in children with autism spectrum disorder. Ann Pharmacother. 2006;40(5):909–916. doi: 10.1345/aph.1G389. [DOI] [PubMed] [Google Scholar]
- 43.Chavez B, Chavez-Brown M, Sopko MA, Jr., Rey JA. Atypical antipsychotics in children with pervasive developmental disorders. Paediatr Drugs. 2007;9(4):249–266. doi: 10.2165/00148581-200709040-00006. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Alberts I, Li X. Dysregulation of the IGF-I/PIeK/AKT/TOR signaling pathway in autism spectrum disorders. Int J Dev Neurosci. 2014;35:35–41. doi: 10.1016/j.ijdevneu.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Cheuk DK, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD007849.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ching H, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD009043.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Chini B, Leoncino M, Gigliucci V. Oxytocin in the developing brain: Relevance as disease-modifying treatment in autism spectrum disorders. In Carlo Sala and Chiara Verpelli, editors, Neu Syn Dysfunct Autism Spect Dis Intellect Disab. 2016;253–266. London, San Diego, Oxford: Academic Press.
- 48.Clark B, Bélanger SA. ADHD in children and youth: part 3‑assessment and treatment with comorbid ASD, ID, or prematurity. Paediatr Child Health. 2018;23(7):485–490. doi: 10.1093/pch/pxy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark GF, Watling R, Parham LD, Schaaf R. Occupational. 2019;73(3):1–7303390010p. doi: 10.5014/ajot.2019.733001. [DOI] [PubMed] [Google Scholar]
- 50.Clarke TK, Lupton MK, Fernandez-Pujals AM, Starr J, Davies G, Cox S, et al. Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol Psychiatry. 2015;21(3):419–425. doi: 10.1038/mp.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry. 2014;76:603–615. doi: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Coghill DR, Banaschewski T, Nagy P, Hernández Otero I, Soutullo C, Yan B, Caballero B, Zuddas A. Long-term safety and efficacy of lisdexamfetamine dimesylate in children and adolescents with ADHD: A phase IV, 2‑year, open-label study in Europe. CNS Drugs. 2017;31(7):625–638. doi: 10.1007/s40263-017-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman DM, Adams JB, Anderson AL, Frye RE. Rating of the effectiveness of 26 psychiatric and seizure medications for autism spectrum disorder: results of a national survey. J Child Adolesc Psychopharmacol. 2019;29(2):107–123. doi: 10.1089/cap.2018.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Comiran E, Kessler FH, Froehlich PE, Lisdexamfetamine LRP. A pharmacokinetic review. Eur. J Pharmaceut Sci. 2016;89:172–179. doi: 10.1016/j.ejps.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 55.Conduct Problems Prevention Research Group. The effects of the Fast Track preventive intervention on the development of conduct disorder across childhood. Child Dev. 2011;82(1):331–345. doi: 10.1111/j.1467-8624.2010.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, et al. Cognitive training for attention-deficit/hyperactivity disorder: Meta- analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adoles Psych. 2015;54(3):164–174. doi: 10.1016/j.jaac.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortesi F, Giannotti F, Ivanenko A, Johnson K. Sleep in children with autistic spectrum disorder. Sleep M. 2011;11(7):659–664. doi: 10.1016/j.sleep.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Craig F, Savino R, Trabacca A. A systematic review of comorbidity between cerebral palsy, autism spectrum disorders and attention deficit hyperactivity disorder. Eur J Paediatr Neurol. 2019;23(1):31–42. doi: 10.1016/j.ejpn.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Dauvilliers Y, Winkelmann J. Restless legs syndrome: update on pathogenesis. Curr Opinoion Pulm Med. 2013;19(6):594–600. doi: 10.1097/MCP.0b013e328365ab07. [DOI] [PubMed] [Google Scholar]
- 60.Dawson G, Burner K. Behavioral interventions in children and adolescents with autism spectrum disorder: a review of recent findings. Curr Opin Pediatr. 2011;23(6):616–620. doi: 10.1097/MOP.0b013e32834cf082. [DOI] [PubMed] [Google Scholar]
- 61.De Hert M, Dobbelaere M, Sheridan EM, Cohen D, Correll CU. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur psychiatr. 2011;26(3):144–158. doi: 10.1016/j.eurpsy.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Deb S, Farmah BK, Arshad E, Deb T, Roy M, Unwin GL. The effectiveness of aripiprazole in the management of problem behaviour in people with intellectual disabilities, developmental disabilities and/or autistic spectrum disorder–a systematic review. Res Dev Disabil. 2014;35(3):711–725. doi: 10.1016/j.ridd.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Devnani PA, Hegde AU. Autism and sleep disorders. J Pediatr Neurosci. 2015;10(4):304–307. doi: 10.4103/1817-1745.174438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dinissen M, Dietrich A, van den Hoofdakker BJ, Hoekstra Clinical and pharmacokinetic evaluation of risperidone for the management of autism spectrum disorder. Expert Opin Drug Metab Toxicol. 2015;11(1):111–124. doi: 10.1517/17425255.2015.981151. [DOI] [PubMed] [Google Scholar]
- 65.Dolle K, Schulte-Körne G. Evidenztabelle Psycho- und Pharmakotherapie im Vergleich und in Kombination zur Leitlinie „Behandlung von depressiven Störungen bei Kindern und Jugendlichen“ [Table of evidence Psycho- and pharmacotherapy, comparing and combining the guideline Treatment of depressive disorders in children and adolescents. 2012. http://www.awmf.org/leitlinien/aktuelle-leitlinien.html. Accessed: 17 Jun 2021.
- 66.Douglas-Hall P, Curran S, Bird V, Taylor D. Aripiprazole: a review of its use in the treatment of irritability associated with autistic disorder patients aged 6–17. J Cent Nerv Syst Disord. 2011;12(3):143–153. doi: 10.4137/JCNSD.S4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Pediatrics. 2012;130(4):717–726. doi: 10.1542/peds.2012-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dia Clin. Neurosci. 2012;14(3):263–279. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duggal HS. Ziprasidone for maladaptive behavior and attention-deficit/hyperactivity disorder symptoms in autistic disorder. J Child Adolesc Psychopharmacol. 2007;2:261–263. doi: 10.1089/cap.2006.00136. [DOI] [PubMed] [Google Scholar]
- 70.DuPaul GJ, Gormley MJ, Laracy SD. School-based interventions for elementary school students with ADHD. Child Adolescent Psych Clin N. Am. 2014;23(4):687–697. doi: 10.1016/j.chc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Dyches TT, Smith TB, Korth BB, Roper SO, Mandleco B. Positive parenting of children with developmental disabilities: a meta-analysis. Res Develop Disabil. 2012;33(6):2213–2220. doi: 10.1016/j.ridd.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Earle JF. An introduction to the psychopharmacology of children and adolescents with autism spectrum disorder. J Child Adoles Psych Nurs. 2016;29(2):62–71. doi: 10.1111/jcap.12144. [DOI] [PubMed] [Google Scholar]
- 73.Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Engel J., Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ilae task force on classification and terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 75.Epstein R, Fonnesbeck C, Williamson E, Kuhn T, Lindegren ML, Rizzone K, Krishnaswami S, Sathe N, Ficzere CH, Ness GL, Wright GW, Raj M, Potter S, McPheeters M. Psychosocial and pharmacologic interventions for disruptive behavior in children and adolescents. comparative effectiveness review number 154. 2015. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/disruptive-beha vior-disorder_research.pdf. Accessed: 17 Jun 2021 [PubMed]
- 76.Epstein RA, Fonnesbeck C, Potter S, Rizzone KH, McPheeters M. Psychosocial interventions for child disruptive behaviors: a meta-analysis. Pediatrics. 2015;136(5):947–960. doi: 10.1542/peds.2015-2577. [DOI] [PubMed] [Google Scholar]
- 77.Erickson CA, Veenstra-Vanderweele JM, Melmed RD, McCracken JT, Ginsberg LD, Sikich L, Scahill L, Cherubini M, Zarevics P, Walton-Bowen K, Carpenter RL, Bear MF, Wang PP, King BH. Stx209 (Arbaclofen) for autism spectrum disorders: an 8‑week open-label study. J Autism Dev Disord. 2014;44(4):958–964. doi: 10.1007/s10803-013-1963-z. [DOI] [PubMed] [Google Scholar]
- 78.Ernst CL, Goldberg JF. Antisuicide properties of psychotropic drugs: a critical review. Harv Rev Psychiatry. 2004;12(1):14–41. doi: 10.1080/10673220490425924. [DOI] [PubMed] [Google Scholar]
- 79.Ernst M, Magee HJ, Gonzalez NM, Locascio JJ, Rosenberg CR, Campbell M. Pimozide in autistic children. Psychopharmacol Bull. 1992;28(2):187–191. [PubMed] [Google Scholar]
- 80.Evans SW, Owens JS, Wymbs BT, Ray AR. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157–198. doi: 10.1080/15374416.2017.1390757. [DOI] [PubMed] [Google Scholar]
- 81.Farmer C, Thurm A, Grant P. Pharmacotherapy for the core symptoms in autistic disorder: current status of the research. Drugs. 2013;73(4):303–314. doi: 10.1007/s40265-013-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farmer CA, Aman MG. Aripiprazole for the treatment of irritability associated with autism. Expert Opin Pharmacother. 2011;12(4):635–640. doi: 10.1517/14656566.2011.557661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faulkner MA, Singh SP. Neurogenetic disorders and treatment of associated seizures. Pharmacotherapy. 2013;33(3):330–343. doi: 10.1002/phar.1201. [DOI] [PubMed] [Google Scholar]
- 84.Faulkner SM, Bee PE, Meyer N, Dijk DJ, Drake RJ. Light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuro-psychiatric illness: a systematic review and meta-analysis. Sleep Med Rev. 2019;46:108–123. doi: 10.1016/j.smrv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Feldman ME, Charach A, Bélanger SA. ADHD in children and youth: part 2—treatment. Paediatr Child Health. 2018;23:462–472. doi: 10.1093/pch/pxy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernell E, Eriksson MA, Gillberg C. Early diagnosis of autism and impact on prognosis: a narrative review. Clin Epidemiol. 2013;5:33–43. doi: 10.2147/clep.s41714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrin M, Moreno-Granados JM, Salcedo-Marin MD, Ruiz-Veguilla M, Perez-Ayala V, Taylor E. Evaluation of a psychoeducation programme for parents of children and adolescents with ADHD: Immediate and long-term effects using a blind randomized controlled trial. Eur Child Adolesc Psychiatry. 2014;23(8):637–647. doi: 10.1007/s00787-013-0494-7. [DOI] [PubMed] [Google Scholar]
- 88.Flatz T, Gleußner M. Neurofeedbacktherapie bei ADHS und Autismus [neurofeedback therapy for ADHD and Autism] Paediatr Paedol. 2014;49:22–27. doi: 10.1007/s00608-013-0132-0. [DOI] [Google Scholar]
- 89.Flippin M, Reszka S, Watson LR. Effectiveness of the picture exchange communication system (PECS) on communication and speech for children with autism spectrum disorders: a meta-analysis. Am J Speech Lang Pathol. 2010;19(2):178–195. doi: 10.1044/1058-0360(2010/09-0022. [DOI] [PubMed] [Google Scholar]
- 90.Francis A, Msall M, Obringer E, Kelley K. Children with autism spectrum disorder and epilepsy. Pediat Ann. 2013;42(12):255–260. doi: 10.3928/00904481-20131122-10. [DOI] [PubMed] [Google Scholar]
- 91.Frazier TW, Youngstrom EA, Haycook T, Sinoff A, Dimitrou F, Knapp J, Sinclair L. Effectiveness of medication combined with intensive behavioral intervention for reducing aggression in youth with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2010;20(3):167–177. doi: 10.1089/cap.2009.0048. [DOI] [PubMed] [Google Scholar]
- 92.Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J Child Adolesc Pharmacol. 2011;21(6):571–579. doi: 10.1089/cap.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frémaux T, Reymann JM, Chevreuil C, Bentué-Ferrer D. Prescription de l’olanzapine chez l’enfant et l’adolescent [Verschreibung von Olanzapin bei Kindern und Jugendlichen] Encephale. 2007;33(2):188–196. doi: 10.1016/S0013-7006(07)91549-3. [DOI] [PubMed] [Google Scholar]
- 94.Frye RE. Social skills deficits in autism spectrum disorder: potential biological origins and progress in developing therapeutic agents. CNS Drugs. 2018;32(8):713–734. doi: 10.1007/s40263-018-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frye RE, Sreenivasula S, Adams JB. Traditional and non-traditional treatments for autism spectrum disorder with seizures: an on-line survey. BMC Pediatr. 2011;11:37. doi: 10.1186/1471-2431-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frye RE, Rossignol D, Casanova MF, Brown GL, Martin V, Edelson S, Coben R, Lewine J, Slattery JC, Lau C, Hardy P, Fatemi SH, Folsom TD, MacFabe D, Adams JB. A review of traditional and novel treatments for seizures in autism spectrum disorder: findings from a systematic review and expert panel. Front Public Health. 2013;1(31):1–26. doi: 10.3389/fpubh.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaastra GF, Groen Y, Tucha L, Tucha O. The effects of classroom interventions on off-task and disruptive classroom behavior in children with symptoms of attention-deficit/hyperactivity disorder: A meta-analytic review. PLOS ONE. 2016;11(2):e0148841:1–19. 10.1371/journal.pone.0148841. [DOI] [PMC free article] [PubMed]
- 98.Gahr M, Kölle MA, Schönfeldt-Lecuona C, Lepping P, Freudenmann RW. Paliperidone extended-release: does it have a place in antipsychotic therapy? DDDT. 2011;11(5):125–146. doi: 10.2147/DDDT.S17266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Molec Med. 2015;15(2):146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garbarino VR, Gilman LT, Daws LC, Gould GG. Extreme enhancement or depletion of serotonin transporter function and serotonin availability in autism spectrum disorder. Pharmacol Res. 2019;140:85–99. doi: 10.1016/j.phrs.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gates JA, Kang E, Lerner MD. Efficacy of group social skills interventions for youth with autism spectrum disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2017;52:164–181. doi: 10.1016/j.cpr.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends Cogn Sci. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of Aripiprazole and Risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum Dev. 2014;45(2):185–192. doi: 10.1007/s10578-013-0390-x. [DOI] [PubMed] [Google Scholar]
- 104.Ghanizadeh A, Molla MAS, Olango GJ. The effect of stimulants on irritability in autism comorbid with ADHD: a systematic review. Neuropsych Dis Treat. 2019;15:1547–1555. doi: 10.2147/ndt.s194022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gilby KL, O’Brien TJ. Epilepsy, autism, and neurodevelopment: kindling a shared vulnerability? Epilepsy Behav. 2013;26(3):370–374. doi: 10.1016/j.yebeh.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Gjevik E, Sandstad B, Andreassen OA, Myhre AM, Sponheim E. Exploring the agreement between questionnaire information and DSM-IV diagnoses of comorbid psychopathology in children with autism spectrum disorders. Autism. 2014;19(4):433–442. doi: 10.1177/1362361314526003. [DOI] [PubMed] [Google Scholar]
- 107.Goel R, Hong JS, Findling RL, Ji NY. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psych. 2018;30(1):78–95. doi: 10.1080/09540261.2018.1458706. [DOI] [PubMed] [Google Scholar]
- 108.Goldman SE, Adkins KW, Calcutt MW, Carter MD, Goodpaster RL, Wang L, Shi Y, Burgess HJ, Hachey DL, Malow BA. Melatonin in children with autism spectrum disorders: endogenous and pharmacokinetic profiles in relation to sleep. J Autism Dev Disord. 2014;44:2525–2535. doi: 10.1007/s10803-014-2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golubchik P, Sever J, Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin Neuropharmacol. 2011;34(6):216–219. doi: 10.1097/WNF.0b013e31823349ac. [DOI] [PubMed] [Google Scholar]
- 110.Gründer G, Benkert O. Handbuch der Psychopharmakatherapie [Handbook of Psychopharmacotherapy] Heidelberg: Springer; 2008. [Google Scholar]
- 111.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 112.Guastella AJ, Hickie IB, McGuiness MM, Otis M, Woods EA, Disinger HM, Chan HK, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 113.Guastella AJ, Hickie IB. Oxytocin treatment, circuitry and autism: a critical review of the literature placing oxytocin into the austism context. Biol Psychiatry. 2015;79(3):234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 114.Guenot M. Indications et risques des techniques neuro-chirurgicales chez l’enfant présentant une épilepsie partielle pharmaco-résistante. [Surgical treatment for epilepsy in children wihth treatment resistant partial epilepsy: indications and complications] Rev Neurol. 2004;160(Supplement 1):203–209. doi: 10.1016/S0035-3787(04)71201-1. [DOI] [PubMed] [Google Scholar]
- 115.Guglielmo R, Ioime L, Grandinetti P, Janiri L. Managing disruptive and compulsive behaviors in adult with autistic disorder with Gabapentin. J Clin Psychopharmacol. 2013;33(2):273–274. doi: 10.1097/JCP.0b013e318285680c. [DOI] [PubMed] [Google Scholar]
- 116.Gurnani T, Ivanov I, Newcorn JH. Pharmacotherapy of aggression in child and adolescent psychiatric disorders. J Child Adol. Psychopharmacol. 2016;26(1):65–73. doi: 10.1089/cap.2015.0167. [DOI] [PubMed] [Google Scholar]
- 117.Habib A, Harris L, Pollick F, Melville C. A meta-analysis of working memory in individuals with autism spectrum disorders. PLoS ONE. 2019;14(4e0216198):1–25. doi: 10.1371/journal.pone.0216198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hamiwka LD, Wirrell EC. Comorbidities in pediatric epilepsy: beyond “just” treating the seizures. J Child Neurol. 2009;24(6):734–742. doi: 10.1177/0883073808329527. [DOI] [PubMed] [Google Scholar]
- 119.Handleman JS, Harris SL. Preschool education programs for children with autism. Austin: ProEd; 2001. [Google Scholar]
- 120.Hanson E, Kalish LA, Bunce E, Curtis C, McDaniel S, Ware J, Petry J. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. J Autism Dev Disord. 2007;37(4):628–636. doi: 10.1007/s10803-006-0192-0. [DOI] [PubMed] [Google Scholar]
- 121.Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11(176):1–8. doi: 10.1186/1471-244X-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hardan AY, Jou RJ, Handen BL. Retrospective study of quetiapine in children and adolescents with pervasive developmental disorders. J Autism Dev Disord. 2005;35(3):387–391. doi: 10.1007/s10803-005-3306-1. [DOI] [PubMed] [Google Scholar]
- 123.Harfterkamp M, van de Loo-Neus G, Minderaa RB, van der Gaag RJ, Escobar R, Schacht A, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adol Psych. 2012;51(7):733–741. doi: 10.1016/j.jaac.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Harfterkamp M, Buitelaar JK, Minderaa RB, van de Loo-Neus G, van der Gaag RJ, Hoekstra PJ. Long-term treatment with atomoxetine for attention-deficit/ hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: An open-label extension study. J Child Adolesc Psychopharmacol. 2013;23(3):194–199. doi: 10.1089/cap.2012.0012. [DOI] [PubMed] [Google Scholar]
- 125.Hartley SL, Sikora DM. Which DSM-IV-TR criteria best differentiate high-functioning autism spectrum disorder from ADHD and anxiety disorders in older children? Autism. 2009;13(5):485–509. doi: 10.1177/1362361309335717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hazen EP, Stornelli JL, O’Rourke JA, Koesterer K, McDougle CJ. Sensory symptoms in autism spectrum disorders. Harv Rev Psychiatry. 2014;22(2):112–124. doi: 10.1097/01.HRP.0000445143.08773.58. [DOI] [PubMed] [Google Scholar]
- 127.Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Den Heijer AE, Groen Y, Tucha L, Fuermaier ABM, Janneke Koerts, Lange KW, Thome J, Tucha O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: a systematic literature review. J Neural Transm. 2017;124(Suppl 1):S3–S26. doi: 10.1007/s00702-016-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hellings JA, Weckbaugh M, Nickel EJ, Cain SE, Zarcone JR, Reese RM, Hall S, Ermer DJ, Tsai LY, Schroeder SR, Cook EH. A double-blind, placebo-controlled study of valproate for aggression in youth with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2005;15(4):682–692. doi: 10.1089/cap.2005.15.682. [DOI] [PubMed] [Google Scholar]
- 130.Henderson C, Wietjunge I, Kinoshita MN, Shumway M, Hammond RS, Postma FR, Brynczka C, Rush R, Thomas A, Paylor R, Warren ST, Vanderklish PW, Kind PC, Carpenter RL, Bear MF, Healy AM. Reversal of disease-related pathologies in the Fragile X mouse model by selective activation of GABA‑B receptors with arbaclofen. Sci Transl Med. 2012;4(152):1–11. doi: 10.1126/scitranslmed.3004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Henggeler SW, Schaeffer CM. Multisystemic therapy: clinical overview, outcomes, and implementation research. Fam Proc. 2016;55(3):514–528. doi: 10.1111/famp./12232. [DOI] [PubMed] [Google Scholar]
- 132.Hennissen L, Bakker MJ, Banaschewski T, Carucci S, Coghill D, Danckaerts M, et al. Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: A systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs. 2017;31(3):199–215. doi: 10.1007/s40263-017-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirota T, Veenstra-Vanderweele J, Hollander E, Kishi T. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948–957. doi: 10.1007/s10803-013-1952-2. [DOI] [PubMed] [Google Scholar]
- 134.Hoch E, Niemann D, von Keller R, Schneider M, Friemel CM, Preuss UW, Hasan A, Pogarell O. How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269(1):87–105. doi: 10.1007/s00406-019-00984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582–589. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- 136.Hollander E, Soorya L, Wasserman S, Esposito K, Chaplin W, Anagnostou E. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol. 2006;9:209–213. doi: 10.1017/S1461145705005791. [DOI] [PubMed] [Google Scholar]
- 137.Hollander E, Wasserman S, Swanson EN, Chaplin W, Schapiro ML, Zagursky K, Novotny S. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J Child Adolesc Psychopharmacol. 2006;16(5):541–548. doi: 10.1089/cap.2006.16.541. [DOI] [PubMed] [Google Scholar]
- 138.Holtmann M, Steiner S, Hohmann S, Poustka L, Banaschewski T, Bötelte S. Neurofeedback in autism spectrum disorders. Dev Med Child Neurol. 2011;53:986–993. doi: 10.1111/j.1469-8749.2011.04043.x. [DOI] [PubMed] [Google Scholar]
- 139.Hosenbocus S, Chahal R. Memantine: a review of possible uses in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22(2):166–171. [PMC free article] [PubMed] [Google Scholar]
- 140.Hsiao EY. Gastrointestinal issues in autism spectrum disorder. Harv Rev Psychiatry. 2014;22(2):104–111. doi: 10.1097/HRP.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 141.Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, Luu T, Lai Y, Lee NH. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009;2(2):78–97. doi: 10.1002/aur.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huguet G, Contrejean Y, Doyen C. Troubles du spectre autistique et suicidalité. Encephale. 2014;41(4):362–369. doi: 10.1016/j.encep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 143.Hurwitz R, Blackmore R, Hazell P, Williams K, Woolfenden S. Tricyclic antidepressants for autism spectrum disorders (ASD) in children and adolescents. Coch Data. Syst Rev. 2012;14(3):1–31. doi: 10.1002/14651858.CD008372.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huss M, Chen W, Ludolph AG. Guanfacine extended release: A new pharmacological treatment option in. Europe. Clin Drug Investigat. 2016;36(1):1–25. doi: 10.1007/s40261-015-0336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hutson PH, Pennick M, Secker R. Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: a novel d‑amphetamine pro-drug. Neuropharmacology. 2014;87:41–50. 10.1016/j.neuropharm.2014.02.014. [DOI] [PubMed]
- 146.Hyman SL, Levy SE, Myers SM, Councl on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. Identification, evaluation, and management of children with autism spectrum disorders. Pediatrics. 2019;145(1):e20193447:1–64. 10.1542/peds.2019-3447. [DOI] [PubMed]
- 147.Iizuka C, Yamashita Y, Nagamitsu S, Yamashita T, Araki Y, Ohya T, Hara M, Shibuya I, Kakuma T, Matsuishi T. Comparison of the strengths and difficulties questionnaire (SDQ) scores between children with high-functioning autism spectrum disorder (HFASD) and attention-deficit/hyperactivity disorder (AD/HD) Brain Dev. 2010;32(8):609–612. doi: 10.1016/j.braindev.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 148.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr. Association of the oxytocin receptor gene (oxtr) Let. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, McDougle CJ, et al. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Development Dis. 2009;39(3):395–404. doi: 10.1007/s10803-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.James S, Montgomery P, Williams K. Omega‑3 fatty acids supplementation for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD007992.pub2. [DOI] [PubMed] [Google Scholar]
- 151.James S, Stevenson SW, Silove N, Williams K. Chelation for autism spectrum disorder (ASD). Coch Data Sys Rev. 2015;11(5):1–27, 2015. [DOI] [PMC free article] [PubMed]
- 152.Jensen PS, Buitelaar J, Pandina GJ, Binder C, Haas M. Management of psychiatric disorders in children and adolescents with atypical antipsychotics: a systematic review of published clinical trials. Eur J Child Adolesc Psychiatry. 2007;16(2):104–120. doi: 10.1007/s00787-006-0580-1. [DOI] [PubMed] [Google Scholar]
- 153.Jesner OS, Aref-Adib M, Coren E. Risperidone for autism spectrum disorder. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ji NY, Findling RL. An update on pharmacotherapy for autism spectrum disorder in children and adolescents. Curr Opin Psychiatry. 2015;28(2):91–101. doi: 10.1097/YCO.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 155.Jia F, Wang B, Schan L, Xu Z, Staal WG, Du L. Core symptoms of autism improved after vitamin D supplementation. Pediatrics. 2015;135(1):e196–e198. doi: 10.1542/peds.2014-2121. [DOI] [PubMed] [Google Scholar]
- 156.Jobski K, Hofer J, Hoffmann F, Bachmann C. Use of psychotropic drugs in patients with autism spectrum disorders: a systematic review. Acta Psych Scand. 2017;135(1):8–28. doi: 10.1111/acps.12644. [DOI] [PubMed] [Google Scholar]
- 157.Johnson CR, Turner KS, Foldes EL, Malow BA, Wiggs L. Comparison of sleep questionnaires in the assessment of sleep disturbances in children with autism spectrum disorders. Sleep Med. 2012;13:795–801. doi: 10.1016/j.sleep.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones RA, Downing K, Rinehart NJJ, Barnett LM, May T, McGillivray JA, et al. Physical activity, sedentary behavior and their correlates in children with autism spectrum disorder: A systematic review. PLOS One. 2017;12(2):1–23. doi: 10.1371/journal.pone.0172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Joshi G. Are there lessons to be learned from the prevailing patterns of psychotropic drug use in patients with autism spectrum disorder? Acta Psychiatr Scand. 2017;135(1):5–7. doi: 10.1111/acps.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Joshi G, Faraone SV, Wozniak J, Tarko L, Fried R, Galdo M, Furtak SL, Biederman J. Symptom profile of ADHD in youth with high-functioning autism spectrum disorder: a comparative study in psychiatrically referred populations. J Atten Disord. 2017;21(10):846–855. doi: 10.1177/1087054714543368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Joshi G, Biederman J, Petty C, Goldin RL, Furtak SL, Wozniak J. Examining the comorbidity of bipolar disorder and autism spectrum disorders: a large controlled analysis of phenotypic and familial correlates in a referred population of youth with bipolar I disorder with and without autism spectrum disorders. J Clin Psych. 2013;74(6):578–586. doi: 10.4088/JCP.12m07392. [DOI] [PubMed] [Google Scholar]
- 162.Jung S, Sainato DM. Teaching play skills to young children with autism. J Intellec Develop Disabil. 2013;38(1):74–90. 10.3109/13668250.2012.732220. [DOI] [PubMed]
- 163.Kantzer A‑K, Fernell E, Gillberg C, Miniscalco C. Autism in community pre-schoolers: developmental profiles. Res Develop Disabil. 2013;34(9):2900–2908. doi: 10.1016/j.ridd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 164.Kaplan G, McCracken JT. Psychopharmacology of autism spectrum disorders. Pediat Clin N. Am. 2012;59(1):175–187. doi: 10.1016/j.pcl.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 165.Karst JS, Van Hecke AV. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin Child Fam. Psych Rev. 2012;15(3):247–277. doi: 10.1007/s10567-012-0119-6. [DOI] [PubMed] [Google Scholar]
- 166.Kasari C, Patterson S. Interventions addressing social impairment in autism. Cur Psych Rep. 2012;14(6):713–725. doi: 10.1007/s11920-012-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD. Stimulant medications and sleep for youth with ADHD: A meta-analysis. Pediatrics. 2015;136(6):1144–1153. doi: 10.1542/peds.2015-1708. [DOI] [PubMed] [Google Scholar]
- 168.King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, Donelly CL, Anagnostou E, Dukes K, Sullivan L, Hirtz D, Wagner A, Ritz L, STAART Psychopharmacology Network Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior. Arch Gen Psychiatry. 2009;66(6):583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.King BH. Fluoxetine and repetitive behaviors in children and adolescents with autism spectrum disorder. JAMA. 2019;322(16):1557–1558. doi: 10.1001/jama.2019.11738. [DOI] [PubMed] [Google Scholar]
- 170.Kirino E. Efficacy and tolerability of pharmacotherapy options for the treatment of irritability in autistic children. Clin Med Insights Pediatr. 2014;25(8):17–30. doi: 10.4137/CMPed.S8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kleijer KTE, Schmeisser MJ, Krueger DD, Boeckers TM, Scheiffele P, Bourgeron T, et al. Neurobiology of autism gene products: towards pathogenesis and drug targets. Psychopharmacology (Berl.) 2014;231(6):1037–1062. doi: 10.1007/s00213-013-3403-3. [DOI] [PubMed] [Google Scholar]
- 172.Knight V, McKissick BR, Saunders A. A review of technology-based interventions to teach academic skills to students with autism spectrum disorder. J Autism Develop Disord. 2013. 10.1007/s10803-013-1814-y. [DOI] [PubMed]
- 173.Kolevzon A, Mathewson KA, Hollander E. Selective serotonin reuptake inhibitors in autism: a review of efficacy and tolerability. J Clin Psychiatry. 2006;67(3):407–414. doi: 10.4088/JCP.v67n0311. [DOI] [PubMed] [Google Scholar]
- 174.Kotagal S. Treatment of dyssomnias and parasomnias in childhood. Curr Treat Options Neurol. 2012;14(6):630–649. doi: 10.1007/s11940-012-0199-0. [DOI] [PubMed] [Google Scholar]
- 175.Kotagal S, Broomall A. Sleep in children with autism spectrum disorder. Pediat. Neurol. 2012;47(4):242–251. doi: 10.1016/j.pediatrneurol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 176.Kruppa JA, Gossen A, Oberwelland Weiß E, Kohls G, Großheinrich N, Cholemkery H, Freitag CM, Karges W, Wölfle E, Sinzig J, Fink GR, Herpertz-Dahlmann B, Konrad K, Schulte-Rüther M. Neural modulation of social reinforcement learning by intranasal oxytocin in male adults with high-functioning autism spectrum disorder: a randomized trial. Neuropsychopharmacology. 2019;44(4):749–756. doi: 10.1038/s41386-018-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Review LEA. social skills groups may improve social competence in children and adolescents with autism spectrum disorder. Evidence-Based Mental Health. 2013;16(1):11. doi: 10.1136/eb-2012-100985. [DOI] [PubMed] [Google Scholar]
- 178.LeBlanc LA, Gillis JM. Behavioral interventions for children with autism spectrum disorders. Ped Clin N. Am. 2012;59(1):147–164. doi: 10.1016/j.pcl.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 179.Lee DO, Ousley OY. Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. J Child Adoles. Psychopharmacol. 2006;16(6):737–746. doi: 10.1089/cap.2006.16.737. [DOI] [PubMed] [Google Scholar]
- 180.Lee YJ, Oh SH, Park C, Hong M, Lee AR, Yoo HJ, Shin CY, Cheon K-A, Bahn GH. Advanced pharmacotherapy evidenced by pathogenesis of autism spectrum disorder. Clin Psychopharmacol Neurosci. 2014;12(1):19–30. doi: 10.9758/cpn.2014.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children – What do we know? Front Hum Neurosci. 2014;8:268. doi: 10.3389/fnhum.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lerner MD, Haque OS, Northrup EC, Lawer L, Bursztajn HJ. Emerging perspectives on adolescents and young adults with high-functioning autism spectrum disorders, violence, and criminal law. J Am Acad Psych Law. 2012;40(2):177–190. [PubMed] [Google Scholar]
- 183.Lerner MD, White SW, McPartland JC. Mechanisms of change in psychosocial interventions for autism spectrum disorders. Dialog Clin. Neurosci. 2012;14(3):307–318. doi: 10.31887/DCNS.2012.14.3/mlerner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Levy ML, Levy KM, Hoff D, Amar AP, Park MS, Conklin JM, Baird L, Apuzzo MLJ. Vagus nerve stimulation therapy in patients with autism spectrum disorder and intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. J Neurosurg Pediatr. 2010;5(5):595–602. doi: 10.3171/2010.3.PEDS09153. [DOI] [PubMed] [Google Scholar]
- 185.Levy S. Complementary and alternative medicine among children recently diagnosed with Autistic Spectrum Disorder. J Dev Behav Pediatr. 2003;24:418–423. doi: 10.1097/00004703-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 186.Levy SE, Hyman SL. Complementary and alternative medicine treatments for children with autism spectrum disorders. Child Adolesc Psychiatry. 2015;24(1):117–143. doi: 10.1016/j.chc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 187.Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 188.Ligsay A, Van Dijck A, Nguyen DV, Lozano R, Chen Y, Bickel ES, et al. Neurodevelop. 2017;9(1):1–13. doi: 10.1186/s11689-017-9207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu X, Hubbard JA, Fabes RA, Adam JB. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry Hum Dev. 2006;37:179–191. doi: 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- 190.Liu Z, Smith CB. Lithium: a promising treatment for fragile X syndrome. ACS Chem Neurosci. 2014;18(5):477–483. doi: 10.1021/cn500077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lofthouse N, Hendren R, Hurt E, Arnold LE, Butter E. A review of complementary and alternative treatments for autism spectrum disorders. Autism Res Treat. 2012;2012:870391. doi: 10.1155/2012/870391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lord C, McGee JP. Educating children with autism. Washington, DC: National Academic Press; 2001. [Google Scholar]
- 193.Louis P. Does the human gut microbiota contribute to the etiology of autism spectrum disorders? Dig Dis Sci. 2012;57(8):1987–1989. doi: 10.1007/s10620-012-2286-1. [DOI] [PubMed] [Google Scholar]
- 194.Mahdi F, Setiawati Y. Occupational therapy for children with attention deficit hyperactivity disorder: a literature review. J Child Adolesc Psychiatry. 2019;3(1):1–3. [Google Scholar]
- 195.Malone RP, Delaney MA, Hyman SB, Cater JR. Ziprasidone in adolescents with autism: an open-label pilot study. J Child Adolesc Psychopharmacol. 2007;17(6):779–790. doi: 10.1089/cap.2006.0126. [DOI] [PubMed] [Google Scholar]
- 196.Maloney A, Mick EO, Frazier J. Aripiprazole decreases irritability in 12 out of 14 youth with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2014;24(6):357–359. doi: 10.1089/cap.2013.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Malow BA, Byars K, Johnson K, Weiss S, Bernal P, Goldman SE, et al. A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(Suppl 2):106–124. doi: 10.1542/peds.2012-0900I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Martínez K, Martínez-García M, Marcos-Vidal L, Janssen J, Castellanos FX, Pretus C, et al. Sensory-to-cognitive systems integration is associated with clinical severity in autism spectrum disorder. J Am Acad Child Adoles Psych. 2019; electronic preprint:1–11, 2019. 10.1016/j.jaac.2019.05.033. [DOI] [PubMed]
- 199.Martinez-Raga J, Knecht C, de Alvaro R. Profile of guanfacine extended release and its potential in the treatment of attention-deficit hyperactivity disorder. Neuropsych Dis Treat. 2015;11:1359–1370. doi: 10.2147/ndt.s65735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Masi G, Milone A, Veltri S, Iuliano R, Pfanner C, Pisano S. Use of quetiapine in children and adolescents. Paediatr Drugs. 2015;17(2):125–140. doi: 10.1007/s40272-015-0119-3. [DOI] [PubMed] [Google Scholar]
- 201.Matson JL, Cervantes PE. Commonly studied comorbid psychopathologies among persons with autism spectrum disorder. Res Dev Disabil. 2014;35(5):952–962. doi: 10.1016/j.ridd.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 202.Matsuura H, Tateno K, Aou S. Dynamical properties of the two-process model for sleep-wake cycles in infantile autism. Cogn Neurodyn. 2008;2:221–228. doi: 10.1007/s11571-008-9051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? Bioassays. 2014;36(10):933–939. doi: 10.1002/bies.201400075. [DOI] [PubMed] [Google Scholar]
- 204.Mazahery H, Camargo CA, Jr, Conlon C, Beck KL, Kruger MC, von Hurst PR. Nutrients. 2016;8(4):236. doi: 10.3390/nu8040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mazurek MO, Dovgan K, Neumeyer AM, Malow BA. Course and predictors of sleep and co-occurring problems in children with autism spectrum disorder. J Autism Dev Disord. 2019;49(5):2101–2115. doi: 10.1007/s10803-019-03894-5. [DOI] [PubMed] [Google Scholar]
- 206.McCracken JT, Badashova KK, Posey DJ, Aman MG, Scahill L, Tierney E, Arnold LE, Vitiello B, Whelan F, Chuang SZ, Davies M, Shah B, McDougle CJ, Nurmi EL. Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics. 2014;14(3):295–302. doi: 10.1038/tpj.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, et al., and for Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. New Eng J Med. 2002;347(5):314–21. 10.1056/NEJMoa013171. [DOI] [PubMed]
- 208.McDougle CJ, Scahill L, McCracken JT, Aman MG, Tierney E, Arnold LE, Freeman BJ, Martin A, McGough JJ, Cronin P, Posey DJ, Riddle MA, Ritz L, Swiezy NB, Vitiello B, Volkmar FR, Votolato NA, Walson P. Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. Background and rationale for an initial controlled study of risperidone. Child Adolesc Psychiatr Clin N Am. 2000;9(1):201–224. doi: 10.1016/S1056-4993(18)30142-1. [DOI] [PubMed] [Google Scholar]
- 209.McEachin JJ, Smith T, Lovaas OI. Long-term outcome for children with autism who received early intensive behavioral treatment. Am J Ment Retard. 1993;97(4):359–372. [PubMed] [Google Scholar]
- 210.McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127(5):e1312–e1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- 211.Mesibov GB, Shea V, Schopler E. The TEACCH approach to autism spectrum disorders. Issues in clinical child psychology. New York: Springer; 2004. [Google Scholar]
- 212.Micoulaud-Franchi JA, Geoffroy PA, Fond G, Lopez R, Bioulac S, Philip P. EEG neurofeedback treatments in children with ADHD: An updated meta-analysis of randomized controlled trials. Front Hum Neurosci. 2014 doi: 10.3389/fnhum.2014.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mikami AY, Jia M, Na JJ. Social skills training. Child Adoles Psych Clin N. Am. 2014;23(4):775–788. doi: 10.1016/j.chc.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 214.Minshawi NF, Wink LK, Shaffer R, Plawecki MH, Posey DJ, Liu H, Hurwitz S, McDougle CJ, Swiezy NB, Erickson CA. A randomized, placebo-controlled trial of d‑cycloserine for the enhancement of social skills training in autism spectrum disorders. Mol Autism. 2016;7:2. doi: 10.1186/s13229-015-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, Young LJ. Melanocortin receptor agonists facilitate oxytocin-dependent partner preference formation in the prairie vole. Neuropsychopharmacology. 2015;40:1856–1865. doi: 10.1038/npp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Möhler H. The legacy of the benzodiazepine receptor: from flumazenil to enhancing cognition in Down syndrome and social interaction in autism. Adv Pharmacol. 2015;72:1–36. doi: 10.1016/bs.apha.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 217.Molnar-Szakacs I, Music HP. a unique window into the world of autism. Ann New York Acad Sci. 2012;1252:318–324. doi: 10.1111/j.1749-6632.2012.06465.x. [DOI] [PubMed] [Google Scholar]
- 218.Molteni M, Nobile M, Cattaneo D, Radice S, Clementi E. Potential benefits and limits of psychopharmacological therapies in pervasive developmental disorders. CCP. 2014;9(4):365–376. doi: 10.2174/15748847113086660070. [DOI] [PubMed] [Google Scholar]
- 219.Montoya A, Colom F, Ferrin M. Is psychoeducation for parents and teachers of children and adolescents with ADHD efficacious? A systematic literature review. Eur psychiatr. 2011;26(3):166–175. doi: 10.1016/j.eurpsy.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 220.Moyal WN, Lord C, Walkup JT. Quality of life in children and adolescents with autism spectrum disorders: what is known about the effects of pharmacotherapy? Paediatr Drugs. 2014;16(2):123–128. doi: 10.1007/s40272-013-0050-4. [DOI] [PubMed] [Google Scholar]
- 221.Myers SM, Plauché Johnson C, and the Council on Children With Disabilities. Management of children with autism spectrum disorders. Pediatrics. 2007;120(5):1162–82. 10.1542/peds.2007-2362. [DOI] [PubMed]
- 222.Nadeau J, Sulkowski ML, Ung D, Wood JJ, Lewin AB, Murphy TK, Ehrenreich JM, Storch EA. Treatment of comorbid anxiety and autism spectrum disorders. Neuropsychiatry (london) 2011;1(6):567–578. doi: 10.2217/npy.11.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Newcorn JH, Halperin JM, Jensen PS, Abikoff HB, Arnold LE, Cantwell DP, et al. Symptom profiles in children with ADHD: Effects of comorbidity and gender. J Am Acad Child Adoles Psych. 2001;40(2):137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 224.Niederhofer H. Efficacy of Duloxetine and Agomelatine does not exceed that of other antidepressants in patients with autistic disorder: preliminary results in 3 patients. Prim Care Companion Cns Disord. 2011;13(1):PCC.10I0138. doi: 10.4088/PCC.10l01038blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nijmeijer JS, Arias-Vásquez A, Rommelse NNJ, Altink ME, Anney RJL, Asherson P, et al. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. J Am Acad Child Adoles Psych. 2010;49(7):675–685. doi: 10.1016/j.jaac.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Novack MN, Hong E, Dixon DR, Granpeesheh D. An evaluation of a mobile application designed to teach receptive language skills to children with autism spectrum disorder. Behav Analysis Practice. 2018;12(1):66–77. doi: 10.1007/s40617-018-00312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ojemann LM, Ojemann GA, Dodrill CB, Crawford CA, Holmes MD, Dudley DL. Language disturbances as side effects of topiramate and zonisamide therapy. Epilepsy Behav. 2001;2(6):579–584. doi: 10.1006/ebeh.2001.0285. [DOI] [PubMed] [Google Scholar]
- 228.Ooi YP, Weng S-J, Kossowsky J, Gerger H, Sung M. Oxytocin and autism spectrum disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2017;50(1):5–13. doi: 10.1055/s-0042-109400. [DOI] [PubMed] [Google Scholar]
- 229.Orinstein AJ, Helt M, Troyb E, Tyson KE, Barton ML, Eigsti I-M, Naigles L, Fein DA. Intervention history of children and adolescents with high- functioning autism and optimal outcomes. J Dev Behav Pediatr. 2014;35(4):247–256. doi: 10.1097/DBP.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Owada K, Okada T, Munesue T, Kuroda M, Fujioka T, Uno Y, Matsumoto K, Kuwabara H, Mori D, Okamoto Y, Yoshimura Y, Kawakubo Y, Arioka Y, Kojima M, Yuhi T, Yassin W, Kushima I, Benner S, Ogawa N, Kawano N, Eriguchi Y, Uemura Y, Yamamoto M, Kano Y, Kasai K, Higashida H, Ozaki N, Kosaka H, Yamasue H. Quantitative facial expression analysis revealed the efficacy and time course of oxytocin in autism. Brain. 2019;142(7):2127–2136. doi: 10.1093/brain/awz126. [DOI] [PubMed] [Google Scholar]
- 231.Owen R, Linmarie Sikich RNM, Corey-Lisle P, Manos G, McQuade RD, Carson WH, Findling RL. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124(6):1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- 232.Owley T, Walton L, Salt J, Guter S, Winnega M, Leventhal BL, Cook EH., Jr. An open-label trial of excitalopram in pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(4):343–348. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- 233.Owley T, Salt J, Guter S, Grieve A, Walton L, Ayuyao N, Leventhal BL, Cook EH., Jr. A prospective, open-label trial of memantine in the treatment of cognitive, behavioral, and memory dysfunction in pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):517–524. doi: 10.1089/cap.2006.16.517. [DOI] [PubMed] [Google Scholar]
- 234.Pagel JF, Parnes BL. Medications for the treatment of sleep disorders: an overview. Prim Care Companion J Clin Psychiatry. 2001;3(3):118–125. doi: 10.4088/PCC.v03n0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Parker KJ, Oztan O, Libove RA, Mohsin DS, Karhson N, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Fung LK, Garner JP, Hardan AY. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med. 2019;11(491):eaau7356. doi: 10.1126/scitranslmed.aau7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Patra S, Nebhinani N, Viswanathan A, Kirubakaran R. Atomoxetine for attention deficit hyperactivity disorder in children and adolescents with autism: A systematic review and meta-analysis. Autism Res. 2019;12(4):542–552. doi: 10.1002/aur.2059. [DOI] [PubMed] [Google Scholar]
- 237.Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28(6):2398–2413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 238.Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, et al. Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (adhd) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J Child Adoles Psychopharmacol. 2013;23(5):337–351. doi: 10.1089/cap.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pelsser LM, Frankena K, Toorman J, Rodrigues Pereira R. Diet and ADHD, reviewing the evidence: A systematic review of meta-analyses of double-blind placebo-controlled trials evaluating the efficacy of diet interventions on the behavior of children with ADHD. PLOS ONE. 2017;12(1):e0169277. doi: 10.1371/journal.pone.0169277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Penagarikano O. New therapeutic options for autism spectrum disorder: Experimental evidences. Exp Neurobiol. 2015;24(4):301–311. doi: 10.5607/en.2015.24.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Politte LC, Henry CA, McDougle CJ. Psychopharmacological interventions in autism spectrum disorder. Harv Rev Psychiatry. 2014;22(2):76–92. doi: 10.1097/HRP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 242.Posey DI, Litwiller M, Koburn A, McDougle CJ. Paroxetine in autism. J Am Acad Child Adolesc Psychiatry. 1999;38(2):111–112. doi: 10.1097/00004583-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 243.Posey DJ, Guenin KD, Kohn AE, Swiezy NB, McDougle CJ. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11:267–277. doi: 10.1089/10445460152595586. [DOI] [PubMed] [Google Scholar]
- 244.Posey DJ, Wiegand RE, Wilkerson J, Maynard M, Stigler KA, McDougle CJJ. Open-label atomoxetine for attention-deficit/hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):599–610. doi: 10.1089/cap.2006.16.599. [DOI] [PubMed] [Google Scholar]
- 245.Poustka L, Bender F, Bock M, Bölte S, Möhler E, Banaschewski T, Goth K. Temperament und soziale Reaktiviät bei Autismus-Spektrum-Störungen und ADHS [Personality and social responsiveness in autism spectrum disorders and attention deficit/hyperactivity disorder] Z Kinder Jugendpsychiatr Psychother. 2011;39:133–141. doi: 10.1024/1422-4917/a000099. [DOI] [PubMed] [Google Scholar]
- 246.Poustka L, Brandeis D, Hohmann M, Bölte S, Banaschewski T. Neurobiologically based interventions for autism spectrum disorders - rationale neurobiology based interventions for autism spectrum disorders - rationale and new directions. RNN. 2014;32(1):197–212. doi: 10.3233/RNN-139010. [DOI] [PubMed] [Google Scholar]
- 247.Premoli M, Aria F, Bonini SA, Maccarinelli G, Gianoncelli A, Pina SD, Tambaro S, Memo M, Mastinu A. Cannabidiol: recent advances and new insights for neuropsychiatric disorders treatment. Life. 2019;224:120–127. doi: 10.1016/j.lfs.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 248.Preti A, Melis M, Siddi S, Vellante M, Doneddu G, Fadda R. Oxytocin and autism: a systematic review of randomized controlled trials. J Child Adolesc Psychopharmacol. 2014;24(2):54–68. doi: 10.1089/cap.2013.0040. [DOI] [PubMed] [Google Scholar]
- 249.Rajapakse T, Pringsheim T. Pharmacotherapeutics of Tourette syndrome and stereotypies in autism. Semin Pediatr Neurol. 2010;17(4):254–260. doi: 10.1016/j.spen.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 250.Ramdoss S, Machalicek W, Rispoli M, Mulloy A, Lang R, O’Reilly M. Computer-based interventions to improve social and emotional skills in individuals with autism spectrum disorders: a systematic review. Dev Neurorehabil. 2012;15(2):119–135. doi: 10.3109/17518423.2011.651655. [DOI] [PubMed] [Google Scholar]
- 251.Rao PA, Landa RJ. Association between severity of behavioral phenotype and comorbid attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Autism. 2013;18(3):272–280. doi: 10.1177/1362361312470494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Razoki B. Neurofeedback versus psychostimulants in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a systematic review. Neuropsych Dis Treat. 2018;14:2905–2913. doi: 10.2147/ndt.s178839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reddihough DS, Marraffa C, Mouti A, O’Sullivan M, Lee KJ, Orsini F, Hazell P, Granich J, Whitehouse AJO, Wray J, Dossetor D, Santosh P, Silove N, Kohn M. Effect of fluoxetine on obsessive-compulsive behaviors in children and adolescents with autism spectrum disorders. A randomized clinical trial. JAMA. 2019;322(16):1561–1569. doi: 10.1001/jama.2019.14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD) Cochrane Database Sys Rev. 2012;10(CD009260):1–63. doi: 10.1002/14651858.CD009260.pub2. [DOI] [PubMed] [Google Scholar]
- 255.Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. A randomized controlled crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psych. 2005;62:1266–1274. [DOI] [PubMed]
- 256.Reynolds AM, Soke GN, Sabourin KR, Hepburn S, Katz T, Wiggins LD, et al. Sleep problems in 2‑ to 5‑year-olds with autism spectrum disorder and other developmental delays. Pediatrics. 2019;14(3):pii:e20180492. 10.1542/peds.2018-0492. [DOI] [PMC free article] [PubMed]
- 257.Rezaei V, Mohammadi MR, Ghanizadeh A, Sahraian A, Tabrizi M, Rezzadeh SA, Akondzadeh S. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1269–1272. doi: 10.1016/j.pnpbp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 258.Richardson E, Seibert T, Uli NK. Growth perturbations from stimulant medications and inhaled corticosteroids. Transl Pediatr. 2017;6(4):237–247. doi: 10.21037/tp.2017.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ridderinkhof A, de Bruin EI, Blom R, Bögels SM. Mindfulness-based program for children with autism spectrum disorder and their parents: Direct and long-term improvements. Mindfulness. 2018;9(3):773–791. doi: 10.1007/s12671-017-0815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ringman JM, Jankovic J. Occurrence of tics in Asperger’s syndrome and autistic disorder. J Child Neurol. 2000;21(8):1081–1109. doi: 10.1177/088307380001500608. [DOI] [PubMed] [Google Scholar]
- 261.Robinson SJ. Childhood epilepsy and autism spectrum disorders: psychiatric problems, phenotypic expression, and anticonvulsants. Neuropsychol Rev. 2012;22(3):271–279. doi: 10.1007/s11065-012-9212-3. [DOI] [PubMed] [Google Scholar]
- 262.Roessner V, Schoenefeld K, Buse J, Wanderer S, Rothenberger A. Therapie der Tic-Störungen [Therapy of tic disorders] Z Kinder Jugendpsychiatr. 2012;40(4):217–237. doi: 10.1024/1422-4917/a000176. [DOI] [PubMed] [Google Scholar]
- 263.Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37(1):8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rommelse NNJ, Altink ME, Fliers EA, Martin NC, Buschgens CJM, Hartman CA, et al. Comorbid problems in ADHD: degree of association, shared endophenotypes, and formation of distinct subtypes. implications for a. future DSM. J Abnorm Child Psych. 2009;37(6):793–804. doi: 10.1007/s10802-009-9312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 266.Ronald A, Larsson H, Anckarsäter H, Lichtenstein P. Symptoms of autism and ADHD: a Swedish twin study examining their overlap. J Abnorm Psychol. 2014 doi: 10.1037/a0036088. [DOI] [PubMed] [Google Scholar]
- 267.Rossignol DA, Frye RE. Melatonin in autism spectrum disorders. CCP. 2014;9(4):326–334. doi: 10.2174/15748847113086660072. [DOI] [PubMed] [Google Scholar]
- 268.Saunders DC. Mindfulness-based ADHD treatment for children: a pilot feasibility study. J Acad Child Adoles Psych. 2019;58(Suppl 10):312. American Academy of Child & Adolescent Psychiatry (AACAP) 66th Annual Meeting.
- 269.Scahill L, Aman MG, McDougle CJ, McCracken JT, Tierney E, Dziura J, Arnold LE, Posey D, Young C, Shah B, Ghuman J, Ritz L, Vitiello B. A prospective open trial of guanfacine in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):589–598. doi: 10.1089/cap.2006.16.589. [DOI] [PubMed] [Google Scholar]
- 270.Scahill L, McCracken JT, King BH, Rockhill C, Shah B, Politte L, Sanders R, Minjarez M, Cowen J, Mullett J, Page C, Ward D, Deng Y, Loo S, Dziura J, McDougle CJ, Research Units on Pediatric Psychopharmacology Autism Network Extended release guanfacine for hyperactivity in children with autism spectrum disorder. Am J Psychiatry. 2015;172(12):1197–1206. doi: 10.1176/appi.ajp.2015.15010055. [DOI] [PubMed] [Google Scholar]
- 271.Scahill L, McDougle CJ, Williams SK, Dimitropoulos A, Aman MG, McCracken JT, et al. Children’s yale-brown obsessive compulsive scale modified for pervasive developmental disorders. J Am Acad Child Adoles Psych. 2006;45(9):1114–1123. doi: 10.1097/01.chi.0000220854.79144.e7. [DOI] [PubMed] [Google Scholar]
- 272.Scheltema Beduin A, de Haan L. Off-label second generation antipsychotics for impulse regulation disorders: a review. Psychopharmacol Bull. 2010;43(3):45–81. [PubMed] [Google Scholar]
- 273.Schmeck K. Antipsychotika im Kindes- und Jugendalter: pro und contra [Pros and cons of antipsychotics in children and adolescents] Prax Schweiz Rundsch Med. 2015;104(16):859–864. doi: 10.1024/1661-8157/a002089. [DOI] [PubMed] [Google Scholar]
- 274.Schneider BN, Enenbach M. Managing the risks of ADHD treatments. Current Psych Rep. 2014;16(10):479. doi: 10.1007/s11920-014-0479-3. [DOI] [PubMed] [Google Scholar]
- 275.Schreibman L. Intensive behavioral/psychoeducational treatments for autism: research needs and future direction. J Autism Dev Disord. 2000;30(5):373–378. doi: 10.1023/A:1005535120023. [DOI] [PubMed] [Google Scholar]
- 276.Schur SB, Sikich L, Findling RL, Malone RP, Crismon ML, Derivan A, Macintyre JC, II, Pappadopulos E, Greenhill L, Schooler N, van Orden K, Jensen PS. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part ii. A review. J Am Acad Child Adolesc Psychiatry. 2003;43(2):132–144. doi: 10.1097/01.CHI.0000037017.34553.2E. [DOI] [PubMed] [Google Scholar]
- 277.Scott J, Etain B, Bellivier F. Can an integrated science approach to precision medicine research improve lithium treatment in bipolar disorders? Front Psychiatry. 2018;9(360):1–10. doi: 10.3389/fpsyt.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Scott LJ, Dhillon S. Risperidone: a review of its use in the treatment of irritability associated with autistic disorder in children and adolescents. Paediatr Drugs. 2007;9(5):343–354. doi: 10.2165/00148581-200709050-00006. [DOI] [PubMed] [Google Scholar]
- 279.Scotto Rosato N, Correll CU, Pappadopulos E, Chait A, Crystal S, Jensen PS on behalf of the Treatment of Maladaptive Aggressive in Youth Steering Committee. Treatment of maladaptive aggression in youth: CERT guidelines II. treatments and ongoing management. Pediatrics. 2012;129(6):e1577–586. 10.1542/peds.2010-1361. [DOI] [PubMed]
- 280.Silva GE, Goodwin JL, Vana KD, Vasquez MM, Wilcox PG, Quan SF. Restless legs syndrome, sleep, and quality of life among adolescents and young adults. J Clin Sleep Med. 2014;10(7):779–786. doi: 10.5664/jcsm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 282.Simonoff E, Taylor E, Baird G, Bernard S, Chadwick O, Liang H, Whitwell S, Riemer K, Sharma K, Pandey Sharma S, Wood N, Kelly J, Golaszewski A, Kennedy J, Rodney L, West N, Walwyn R, Jichi F. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry. 2013;54(5):527–535. doi: 10.1111/j.1469-7610.2012.02569.x. [DOI] [PubMed] [Google Scholar]
- 283.sleepjunkie. The ultimate guide to helping children with autism sleep soundly at night. https://www.sleepjunkie.org/autism-and-sleep/, 2019. Accessed: 17 Jun 2021.
- 284.Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;98(1):127–152. doi: 10.1016/S0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 285.Southammakosane C, Schmitz K. Pediatric psychopharmacology for treatment of ADHD, depression, and anxiety. Pediatrics. 2015;136(2):351–359. doi: 10.1542/peds.2014-1581. [DOI] [PubMed] [Google Scholar]
- 286.Sowa M, Meulenbroek K. Effects of physical exercise on autism spectrum disorders: a meta-analysis. Res Autrism Spectr Disord. 2012;6(1):46–57. doi: 10.1016/j.rasd.2011.09.001. [DOI] [Google Scholar]
- 287.Spence SJ, Schneider MT. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr Res. 2009;65:599–606. doi: 10.1203/PDR.0b013e31819e7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Spencer D, Marchall J, Post B, Kulakodlu M, Newschaffer C, Dennen T, Azocar F, Jain A. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833–840. doi: 10.1542/peds.2012-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Srinivasan SM, Bhat AN. A review of “music and movement” therapies for children with autism: embodied interventions for multisystem development. Front Integrat Neurosci. 2013;7(22):1–15. 10.3389/fnint.2013.00022. [DOI] [PMC free article] [PubMed]
- 290.Stachnik JM, Nunn-Thompson C. Use of atypical antipsychotics in the treatment of autistic disorder. Ann Pharmacother. 2007;41(4):626–634. doi: 10.1345/aph.1H527. [DOI] [PubMed] [Google Scholar]
- 291.Stavrakaki C, Antochi R, Emery PC. Olanzapine in the treatment of pervasive developmental disorders: a case series analysis. JPN. 2004;29(1):57–60. [PMC free article] [PubMed] [Google Scholar]
- 292.Steingard RJ, Zimnitzky B, DeMaso DR, Bauman ML, Bucci JP. Sertraline treatment of transition-associated anxiety and agitation in children with autistic disorder. J Child Adolesc Psychopharmacol. 1997;7(1):9–15. doi: 10.1089/cap.1997.7.9. [DOI] [PubMed] [Google Scholar]
- 293.Stevenson J, Buitelaar J, Cortese S, Ferrin M, Konofal E, Lecendreux M, et al., and on behalf of the European ADHD Guidelines Group. Research review: The role of diet in the treatment of attention-deficit/hyperactivity disorder – an appraisal of the evidence on efficacy and recommendations on the design of future studies. J Child Psychol Psychiat. 2014;55(5):416–27. https://doi.org/0.1111/jcpp.12215. [DOI] [PubMed]
- 294.Stigler KA. Psychopharmacologic management of serious behavioral disturbance in ASD. Child Adolesc Psychiatr Clin N Am. 2014;23(1):73–82. doi: 10.1016/j.chc.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 295.Stigler KA, McDougle CJ. Pharmacotherapy of irritability in pervasive developmental disorders. Child Adolesc Psychiatr Clin N Am. 2008;17(4):739–752. doi: 10.1016/j.chc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 296.Sturman N, Deckx L, van Driel ML. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Sys Rev. 2017;11(Cd011144):1–98. doi: 10.1002/14651858.CD011144.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sung M, Chin CH, Lim CG, Liew HSA, Lim CS, Kashala E, Weng S-J. What’s in the pipeline? Drugs in development for autism spectrum disorder. NDT. 2014;10:371–381. doi: 10.2147/NDT.S39516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tarrant N, Roy M, Deb S, Odedra S, Retzer A, Roy A. The effectiveness of methylphenidate in the management of attention deficit hyperactivity disorder (ADHD) in people with intellectual disabilities: A systematic review. Res Development Disabil. 2018;83:217–232. 10.1016/j.ridd.2018.08.017. [DOI] [PubMed]
- 299.Tonge BJ, Bull K, Brereton A, Wilson R. A review of evidence-based early intervention for behavioural problems in children with autism spectrum disorder: the core components of effective programs, child-focused interventions and comprehensive treatment models. Curr Opin Psychiatry. 2014;27(2):158–165. doi: 10.1097/YCO.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 300.Torres N, Martins D, Ant’onio JS, Prata D, Veríssimo M. How do hypothalamic nonapeptides shape youth’s sociality? a systematic review on oxytocin, vasopressin and human socio-emotional development. Neurosci Biobehav Rev. 2018;90:309–331. doi: 10.1016/j.neubiorev.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 301.Torres-Aleman I. Toward a comprehensive neurobiology of IGF‑I. Devel Neurobio. 2010;70(5):384–396. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- 302.Toscano CVA, Carvalho HM, Ferreira JP. Exercise effects for children with autism spectrum disorder: metabolic health, autistic traits, and quality of life. Percept Mot Skills. 2018;125(1):126–146. doi: 10.1177/0031512517743823. [DOI] [PubMed] [Google Scholar]
- 303.Tuchman R, Alessandri M, Cuccaro M. Autism spectrum disorder and epilepsy: Moving towards a comprehensive approach to treatment. Brain Dev. 2010;32:719–730. doi: 10.1016/j.braindev.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 304.Tye C, Runicles AK, Whitehouse AJO, Alvares GA. Characterizing the interplay between autism spectrum disorder and comorbid medical conditions: an integrative review. Front Psychiatry. 2019 doi: 10.3389/fpsyt.2018.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Uren J, Richdale AL, Cotton SM, Whitehouse AJO. Sleep problems and anxiety from 2 to 8 years and the influence of autistic traits: a longitudinal study. Eur Child. 2019;28(8):1117–1127. doi: 10.1007/s00787-019-01275-y. [DOI] [PubMed] [Google Scholar]
- 306.van Ijzendoorn MH, Bakermans-Kranenburg MJ. The role of oxytocin in parenting and as augmentative pharmacotherapy; critical issues and bold conjectures. J Neuroendocrinology. 2015;27(1):1–8. doi: 10.1111/jne.12355. [DOI] [PubMed] [Google Scholar]
- 307.Vanucchi G, Masi G, Toni C, Dell’Osso L, Erfurth A, Perugi G. Bipolar disorder in adults with Asperger’s Syndrome: a systematic review. J Affect Disord. 2014;168:151–160. doi: 10.1016/j.jad.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 308.Veatch OJ, Maxwell-Horn AC, Malow BA. Sleep in autism spectrum disorders. Curr Sleep Medicine Rep. 2015;1(2):131–140. doi: 10.1007/s40675-015-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceed Nat Acad. Sci. 2012;109(14):5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Venkateswaran S, Shevell M. The case against routine encephalography in specific language impairment. Pediatrics. 2008;122:e911–e916. doi: 10.1542/peds.2008-0257. [DOI] [PubMed] [Google Scholar]
- 311.Wang L, Conion MA, Christophersen CT, Sorich MJ, Angley MT. Gastrointestinal microbiota and metabolite biomarkers in children with autism spectrum disorders. Biomarkers Med. 2014;8(3):331–344. doi: 10.2217/bmm.14.12. [DOI] [PubMed] [Google Scholar]
- 312.Ward F, Tharian P, Roy S, Deb M, Unwin GL. Efficacy of beta blockers in the management of problem behaviours in people with intellectual disabilities: a systematic review. Res Dev Disabil. 2013;34(12):4293–4303. doi: 10.1016/j.ridd.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 313.Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, Takao H, Nippashi Y, Kawakubo Y, Kunimatsu A, Kasai K, Yamasue H. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 314.Weiskop S, Richdale A, Matthews J. Behavioural treatment to reduce sleep problems in children with autism or fragile x syndrome. Dev Med Child Neurol. 2005;47(2):94–104. doi: 10.1017/S0012162205000186. [DOI] [PubMed] [Google Scholar]
- 315.Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, Kern RL, Natowicz MR. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3(11):e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Whitehouse AJ. Complementary and alternative medicine for autism spectrum disorders: Rationale, safety and efficacy. J Paediatrics Child Health. 2013. 10.1111/jpc.12242. [DOI] [PubMed]
- 317.Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev Med Child Neurol. 2004;46:372–380. doi: 10.1017/S0012162204000611. [DOI] [PubMed] [Google Scholar]
- 318.Wijnhoven LAMW, Niels-Kessels H, Creemers DHM, Vermulst AA, Otten R, Engels RCME. Prevalence of comorbid depressive symptoms and suicidal ideation in children with autism spectrum disorder and elevated anxiety symptoms. J Child Adolesc Ment Health. 2019;31(1):77–84. doi: 10.2989/17280583.2019.1608830. [DOI] [PubMed] [Google Scholar]
- 319.Williams K, Brignell A, Randall M, Silove P, Hazell N. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD004677.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.World Health Organization. ICD-11 for mortality and morbidity statistics (Version : 04/2019). https://icd.who.int/browse11/l-m/en, 2019. Accessed: 17 Jun 2021
- 321.Yamasue H, Domes G. Behav Pharmacol Neuropep: Oxytocin, volume 35 of Current Topics in Behavioral Neurosciences, chapter Oxytocin and Autism Spectrum Disorders, pages 449–465. Springer, 2019. 10.1007/7854_2017_24. [DOI] [PubMed]
- 322.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2016;21(9):1225–1231. doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zaboski BA, Storch EA. Comorbid autism spectrum disorder and anxiety disorders: a brief review. Future Neurol. 2018;13(1):31–7. 10.2217/fnl-2017-0030. [DOI] [PMC free article] [PubMed]
- 324.Zahid S, Upthegrove R. Suicidality in autistic spectrum disorders. Crisis. 2017;38(4):237–246. doi: 10.1027/0227-5910/a000458. [DOI] [PubMed] [Google Scholar]
- 325.Zimmer M, Desch L. Sensory integration therapies for children with developmental and behavioral disorders. Pediatrics. 2012;129(6):1186–1189. doi: 10.1542/peds.2012-0876. [DOI] [PubMed] [Google Scholar]
- 326.Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600–620. doi: 10.1016/j.euroneuro.2011.04.001. [DOI] [PubMed] [Google Scholar]