Abstract
Autism spectrum disorder (ASD) is a common set of heterogeneous neurodevelopmental disorders resulting from a variety of genetic and environmental risk factors. A core feature of ASD is impairment in prosocial interactions. Current treatment options for individuals diagnosed with ASD are limited, with no current FDA-approved medications that effectively treat its core symptoms. We recently demonstrated that enhanced serotonin (5-HT) activity in the nucleus accumbens (NAc), via optogenetic activation of 5-HTergic inputs or direct infusion of a specific 5-HT1b receptor agonist, reverses social deficits in a genetic mouse model for ASD based on 16p11.2 copy number variation. Furthermore, the recreational drug MDMA, which is currently being evaluated in clinical trials, promotes sociability in mice due to its 5-HT releasing properties in the NAc. Here, we systematically evaluated the ability of MDMA and a selective 5-HT1b receptor agonist to rescue sociability deficits in multiple different mouse models for ASD. We find that MDMA administration enhances sociability in control mice and reverses sociability deficits in all four ASD mouse models examined, whereas administration of a 5-HT1b receptor agonist selectively rescued the sociability deficits in all six mouse models for ASD. These preclinical findings suggest that pharmacological enhancement of 5-HT release or direct 5-HT1b receptor activation may be therapeutically efficacious in ameliorating some of the core sociability deficits present across etiologically distinct presentations of ASD.
Subject terms: Experimental models of disease, Social neuroscience
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition defined by impairments in social interactions and communication, in addition to stereotyped and restricted behaviors [1]. Diagnosis of ASD is increasingly common, with a prevalence rate in the United States of 1 in 54 [2]. Clinical presentation of ASD individuals can vary markedly, likely because the etiology of ASD is complex and heterogeneous [3, 4]. Genetic contributions are important and include copy number variations, mutations in highly penetrant alleles, combinations of common risk variants, and mutations in components of the neuron-specific nBAF chromatin-remodeling complex [3, 5–7]. Similar to virtually all other neuropsychiatric disorders, environmental factors during prenatal and postnatal development interact with genetic risk to define ASD pathophysiology [8, 9].
Despite the broad range of clinical presentations that meet diagnostic criteria for ASD and its complex etiology, there are several core features of ASD that are universal, notably deficits in social interaction [10, 11] first characterized in 1943 by Kanner as “extreme autistic aloneness” [12]. Common behavioral features among disparate forms of ASD suggest that there may be common pathological neural mechanisms upon which different genetic and environmental factors converge. For example, abnormalities in serotonin (5-HT)-mediated modulation of brain functions in ASD was first suggested by studies reporting abnormal blood levels of 5-HT in ASD individuals [13–18]. Although it is unclear how abnormalities in blood 5-HT are related to 5-HT function in the brain, neuroimaging and postmortem studies in children and adults with ASD have reported decreased brain 5-HT production, 5-HT transporter (SERT) binding, and 5-HT receptor binding [19–24].
We first became interested in the possible role of 5-HT in prosocial behaviors because of the finding that oxytocin-induced release of 5-HT in the nucleus accumbens (NAc), a key node of classic mesolimbic reward circuitry, was critical for social reward as assessed by a conditioned place preference (CPP) assay [25]. We confirmed and extended the support for this hypothesis by finding that bidirectional optogenetic manipulation of 5-HT release in the NAc bidirectionally influenced sociability, which was assessed using a three-chamber social preference assay and juvenile intruder assay [26]. To explore the potential role of this mechanism in ASDs, we used a conditional knockout mouse model of the 16p11.2 deletion syndrome, one of the most prominent genetic variations found in ASD [27–29]. Restricted genetic deletion of the 16p11.2 syntenic region in 5-HT neurons caused sociability deficits, which were rescued by optogenetic release of 5-HT in the NAc or infusion of a 5-HT1b receptor agonist into the NAc [26]. Consistent with these findings, the recreational drug (±)3,4-methylenedioxymethamphetamine (MDMA), which has powerful prosocial effects in human subjects [30], promotes sociability in mice by enhancing 5-HT release in the NAc, an effect that, like the optogenetic release of 5-HT in the NAc, requires activation of 5-HT1b receptors [26, 31].
These findings suggest that enhancement of 5-HT signaling, perhaps by targeting specific 5-HT receptor subtypes, might be an effective treatment in ameliorating social deficits present in ASD. To address this possibility, we initially examined the effects of intraperitoneal administration of MDMA in four different ASD mouse models with very different etiologies: homozygous genetic deletion of 16p11.2 [32], homozygous single gene deletions of Cntnap2 [33] and Fmr1 [34], and an environmental model that involves exposure to valproic acid (VPA) in utero [35–37]. We then examined the effects of systemic administration of the 5-HT1b receptor agonist, CP-94,253, in these four ASD mouse models as well as two additional genetic models generated by genetic mutation of subunits of the neuron-specific nBAF chromatin-remodeling complex, Actl6b and Arid1b [38–40]. All of the ASD mouse models exhibited sociability deficits, which were rescued by both MDMA and CP-94,253. These unexpected results suggest that targeting 5-HT1b receptors may be a valuable therapeutic strategy for treating sociability deficits in ASDs independent of their underlying etiologies.
Materials and methods
Animals
Male and female C57BL/6 mice (obtained from Jackson Laboratory, stock number 000664) and the following transgenic lines were used as experimental subjects at 8–16 weeks of age. To minimize the possibility that unknown genetic background differences between the experimental lines and their controls account for observed behavioral differences, all of the following lines were backcrossed extensively (>8 generations) to C57BL/6 mice prior to their use in generating mice for this study, except for line no. 7, which was on a mixed C57BL/6 and CD1 background.
Tg(Slc6a4-cre)ET33Gsat (Sert-Cre, GENSAT Project at Rockefeller University; MGI: 3836639) [41]
B6N.129P2(Cg)-Igs13tm1Dolm Igs14tm1Dolm/J (16p11.2flx/flx, gifted by R. Dolmetsch. This line is available from Jackson Laboratory, stock number 025330) [32]: CD1 background.
B6.129(Cg)-Cntnap2tm1Pele/J (Jackson Laboratory, stock number 028635) [33]
B6.129P2-Fmr1tm1Cgr/J (males: Fmr1–/y, females: Fmr1−/−, Jackson Laboratory, stock number 003025) [34]
B6.129S6-Actl6btm1Grc/J (gift from G. Crabtree, available from Jackson Laboratory, stock number 018783) [38]
C57BL/6-Arid1bem2Hzhu/J (Arid1bflx, Jackson Laboratory, stock number 032061) [39]
Sert-Cre+/–:16p11.2flx/flx male and female mice were generated at Stanford using a breeding strategy previously described [26]. The mice used in this study were homozygous for 16p11.2flx and heterozygous for Sert-Cre.
Sert-Cre+/–:Arid1bflx/+ male and female mice were generated at Stanford using a breeding strategy previously described [26]. The mice used in this study were heterozygous for Arid1bflx and Sert-Cre.
VPA mice were generated as previously described [35–37]. In brief, C57BL/6 mice were mated overnight and pregnancy was determined by the presence of a vaginal plug. Pregnant female mice were injected subcutaneously on gestational day E12.5 with either 600 mg/kg of VPA sodium salt (P4543 Sigma) or vehicle for controls. Sert-Cre+/–:16p11.2flx/flx mice were on a mixed background of C57BL/6 and CD1. All other transgenic mice were maintained on a C57BL/6 background. For all transgenic models, controls were littermates that did not have the transgene of interest deleted or for Fmr1−/− females, age-matched, in-house bred C57BL/6 (Supplementary Table S1). Novel juvenile mice used for the juvenile interaction and three-chamber sociability tests were conspecifics of the same sex as the test mouse, 3–5 weeks of age and were not treated with drug or vehicle. Each novel juvenile was a wild-type of the same background as the model under investigation and bred in house. Mice were housed on a 12-h light/dark cycle with food and water ad libitum. All procedures complied with the animal care standards set forth by the National Institute of Health and were approved by Stanford University’s Administrative Panel on Laboratory Animal Care and Administrative Panel of Biosafety. No statistical methods were used to predetermine sample size, which was based on extensive prior experience with the assays used. All experiments were conducted in a blinded manner such that assays were conducted and analyzed without knowledge of the specific manipulation being performed and with animals being randomized by cage before behavioral experiments.
Drugs
Drugs were administered intraperitoneally at a volume of 0.01 ml/g. 5-Propoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-pyrrolo[3,2-b]pyridine hydrochloride (CP-94,253 hydrochloride) (10 mg/kg, Tocris) and MDMA (7.5 mg/kg, Organix) were dissolved in 0.9% normal saline.
Design of behavioral tests with drug administration
For all behavioral tests conducted to assess the effects of drug administration (three-chamber, juvenile interaction, novel object and open field), mice were counterbalanced for drug versus vehicle such that half the mice received a drug injection on the first day and the other half received vehicle, 20 min prior to testing. One week later, the behavior was repeated, with mice that received drug on day 1, receiving vehicle and vice versa. For clarity, individual subjects are graphically represented as lines in the left panels in each figure, indicating an individual mouse that received both vehicle and drug on separate days. Different cohorts of animals were used for each behavioral test that examined the effects of MDMA. Specifically, a single cohort of mice underwent one behavioral test that was conducted across 2 weeks to ensure mice were counterbalanced. The same cohort of mice was used for all four behavioral experiments that involved CP-94,253, with one cohort of mice being run over the course of 8 weeks in total. In addition, behavioral tests with CP-94,253 were conducted in a counterbalanced manner to ensure that the order of behavioral tests was not a confound.
Three-chamber sociability test
This assay was performed in an arena with three separate chambers, as previously described [26]. On the first day, mice were habituated to the apparatus for 5 min. On the second day, a conspecific juvenile of the same sex as the test mouse (3–5 weeks old) was placed under a wire mesh cup with square holes that were 0.8 cm × 0.8 cm in one of the outer chambers. An empty wire mesh cup was placed in the opposite outer chamber. The tops of the cups were covered to prevent test mice from crawling on top. The test mouse was placed in the center chamber for 2 min. The barriers were then raised, and the test mouse was allowed to freely explore for a duration of 20 min. Placement of the juvenile mice in one of the outer chambers was also counterbalanced across sessions and a novel juvenile introduced during both sessions. Location of the test mouse was assayed automatically using a video tracking system (BIOBSERVE). Social preference was calculated as: [(time in juvenile side − time in empty side)/(time in juvenile side + time in empty side)].
Juvenile interaction test
The juvenile interaction test was performed in the home cage of the test animal as previously described [26]. In brief, cage mates were temporarily moved to a holding container and the test mouse was habituated to isolation in its home cage for 1 min, at which time a novel conspecific juvenile mouse of the same sex as the test mouse (3–5 weeks old) was placed into the home cage for 2 min of free interaction. All sessions were video recorded using a ceiling-mounted digital camera and analyzed manually without knowledge of the experimental manipulation that had been performed. Social investigation was defined by active pursuit, grooming, and sniffing body regions such as snout, body, and anogenital area. Individual social interactions were not assayed independently. For drug administration experiments, each test mouse underwent two rounds of the juvenile interaction test, counterbalanced for the order of drug versus vehicle administration and with a novel juvenile introduced during each session.
Novel object interaction test
This test was performed in the exact same manner as the juvenile interaction test, except that a novel toy mouse or plastic toy was placed into the home cage of the test mice instead of a novel juvenile.
Open field test
To assay locomotor activity, mice were place in an open field arena (40 cm × 40 cm) and allowed to move freely for a duration of 18 min . Total distance traveled was assayed automatically using a video tracking system (BIOBSERVE).
CP-94,253 dose-response
Dose-response experiments were conducted using either the three-chamber sociability or juvenile interaction assays in two different ways: (1) in a single cohort of mice, a progressive dose series (1, 3, 10, and 30 mg/kg) of CP-94,253 was administered to an individual mouse, with each dose separated by 2 days. For the entire cohort, drug and vehicle were administered in a counterbalanced manner for each dose as described in design of behavioral tests with drug administration. The three-chamber sociability and juvenile interaction assays were conducted in separate cohorts. (2) In separate cohorts of mice, only a single dose (1, 3, 10, or 30 mg/kg) of CP-94,253 was administered to an individual mouse, with each cohort being exposed to either the three-chamber sociability or juvenile interaction assay twice per dose (drug or vehicle), again in a counterbalance manner.
Conditioned place preference (CPP) test
The CPP chamber (Med Associates, VT, USA) was an acrylic box, with internal dimensions of 28 cm × 28 cm × 20 cm. Two equal-sized compartments were created within each testing chamber. Each compartment had a distinct floor, either clear, textured acrylic or smooth, black acrylic and distinct walls, vertical lines or circles. Left/right positioning of the floors and walls was alternated between conditioning chambers. Conditioning experiments involving CP-94,253 took place over 4 consecutive days. On day 1, a baseline preference was conducted. Subjects were placed in the left compartment and the subject mouse was allowed to freely explore the entire chamber for 30 min. On days 2–3, one conditioning session was conducted per day, drug or vehicle were administered in a counterbalanced manner. On day 2, the mouse was confined to one chamber for 1 h, 20 min after receiving an injection of drug or vehicle. On day 3, the mouse was confined to opposite chamber for 1 h, 20 min after receiving an injection of drug if previously administered vehicle or vice versa. On day 4, the post-conditioning test was conducted in the same manner as the baseline preference test. Mouse movement was tracked by an array of infrared beam break counters, recorded using Med Associates software, and analyzed offline.
Principal component analysis
Principal component analysis (PCA) was independently performed in R on scaled and centered data from the following groups:
ASD model mice under vehicle condition (Fig. 5A),
ASD model mice and control mice under vehicle condition (Fig. 5B),
ASD model mice and control mice under 5-HT1b agonist condition (Fig. 5C),
All of the groups listed above (used to calculate the Euclidean distances in Fig. 5D, E).
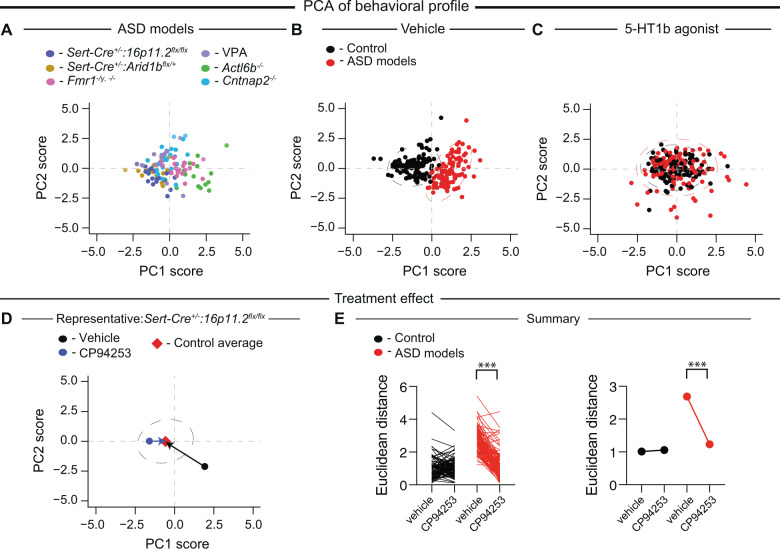
Fig. 5. Systemic administration of CP-94,253 normalizes the global behavioral profile of all ASD models.
A PCA of sociability and control assay data from ASD model mice following vehicle treatment (individual models are color coded). B PCA of control (black) and ASD model (red) mice following vehicle treatment. C PCA of control (black) and ASD model (red) mice following CP-94,253 treatment. Individual data points shown, dashed lines represent 95th % quantile intervals. D Representative example of Euclidean distance calculation to control average following systemic administration of either vehicle or CP-94,253 in a Sert-Cre+/–:16p11.2flx/flx mouse. E Quantification of the Euclidean distance to the center of the control group for each mouse following systemic administration of either vehicle or CP-94,253 (F1,209 = 285.5, P < 0.0001, n = 105–106). If error bars are not clearly visible, they are smaller than the symbol used to represent the SEM. Data are mean ± SEM. ***P < 0.001; two-way ANOVA with Sidak’s multiple comparison post hoc test.
The first two principal components together accounted for >60% of the variance in each of the plots shown. To quantify the effect of CP-94,253 on the global behavior profile of each subject, we first estimated the center of the distribution of the control cohort administered vehicle (control average) by finding the center of a multivariate t-distribution fit onto the PC1 and PC2 scores for these mice. Then, for each mouse in the ASD model and control cohorts, we calculated the Euclidean distances from that mouse’s score under CP-94,253 treatment to the control average and from that mouse’s score under vehicle treatment to the control average.
Statistical analyses
For all data acquisition and analysis, investigators were blinded to the manipulation that the experimental subject had received and the genotype of the subject. Student’s t-tests were used to compare two groups. Two-way ANOVA was used for the analysis of multiple groups with Sidak’s multiple comparison post hoc test, when appropriate. For analysis of each experiment by sex, a multivariable three-way ANOVA was used to compare: drug administration, genotype, and sex, with Sidak’s multiple comparison post hoc test used to determine if there were any significant sex differences. For all experiments, drug administration equally affected males and females (Supplementary Table S2) and thus the data were pooled as represented in all figures. Statistical analyses were performed using Prism 8.4 (GraphPad Software) except for the PCA analysis that was conducted using custom code in R. All data were tested for normality and equal variances. All data are express as mean ± SEM except for PCA.
Results
Sociability deficits are conserved across multiple mouse models for ASD
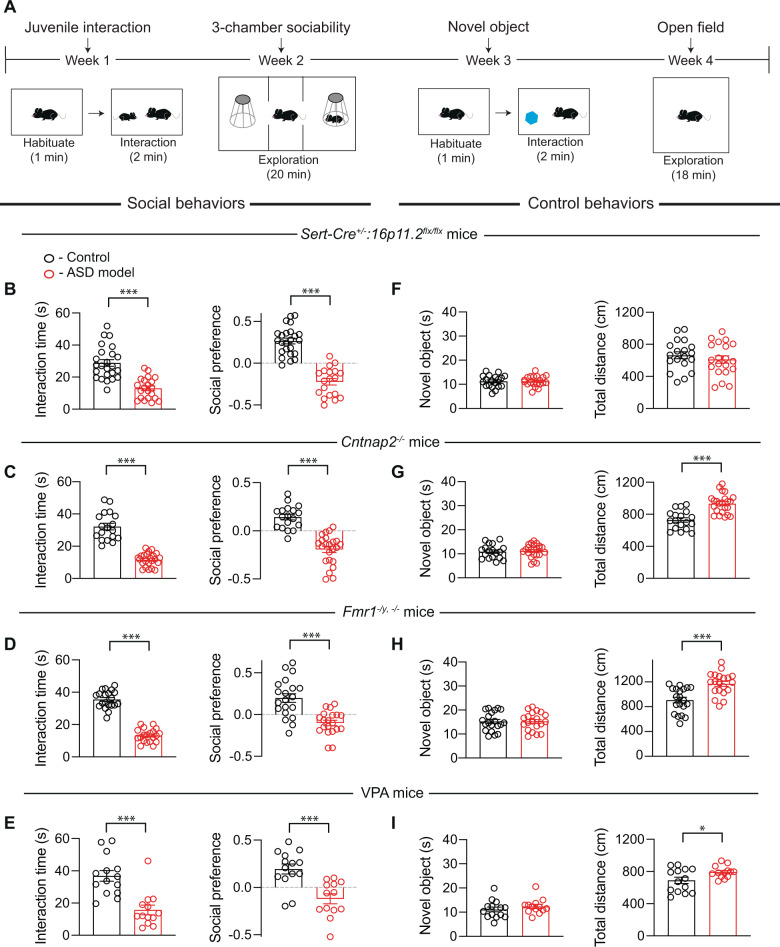
To assess basal sociability behavior, we performed juvenile interaction and three-chamber sociability tests (Fig. 1A) in four different ASD mouse models: (1) 16p11.2flx/flx mice crossed with Sert-Cre+/– mice (Sert-Cre+/–:16p11.2flx/flx) yielding mice in which the syntenic region on mouse chromosome 7F3 is deleted from 5-HT neurons [26], (2) a model of Fragile X syndrome in which Fmr1 is constitutively deleted (Fmr1–/y, −/−) [34], (3) a constitutive knockout of Cntnap2 [33], which has been associated with ASDs [42, 43] (Cntnap2−/−), and (4) mice whose mothers were treated with VPA throughout pregnancy (VPA mice) [35], a manipulation that mimics a potential environmental cause of ASD [36, 37]. We chose these mouse models because they have been reported to have social behavior deficits yet represent very different genetic and environmental etiologies for ASDs. In all four lines, mice spent less time interacting with a novel conspecific juvenile mouse when introduced into their home cage compared to controls and during the three-chamber sociability test, spent less time in the chamber that contained a novel conspecific juvenile under an enclosure that allowed for physical interaction (Fig. 1B–E). We also assessed general novelty seeking using the novel object interaction assay and locomotion in an open field (Fig. 1A). None of the mouse lines showed changes in the time spent exploring a novel object while all the lines, except the Sert-Cre+/–:16p11.2flx/flx line, exhibited hyperactivity (Fig. 1F–I). There were no differences between male and female mice for any of these results or for subsequent results (Supplementary Table S2) and therefore results from male and female mice were combined.
Fig. 1. Behavior deficits present in four mouse models for ASD.
A Sample behavioral assay timeline. Behavioral assays were conducted in counterbalanced manner to ensure that the order of behavioral tests was not a confound. B Quantification of sociability during juvenile interaction (t43 = 6.072, P < 0.001, n = 21–24) and three-chamber sociability (t41 = 9.353, P < 0.001, n = 19–24) in control or Sert-Cre+/–:16p11.2flx/flx mice. For all panels, juvenile interaction is on the left and three-chamber sociability is on the right with controls in black and ASD models in red. C Quantification of sociability during juvenile interaction (t39 = 10.01, P < 0.001, n = 18–23) and three-chamber sociability (t39 = 7.581, P < 0.001, n = 18–23) in control or Cntnap2−/− mice. D Quantification of sociability during juvenile interaction (t38 = 14.70, P < 0.001, n = 20) and three-chamber sociability (t38 = 4.935, P < 0.001, n = 20) in control or Fmr1–/y, −/− deletion mice. E Quantification of sociability during juvenile interaction (t25 = 4.721, P < 0.001, n = 13–14) and three-chamber sociability (t25 = 4.239, P < 0.001, n = 13–14) in control mice or mice exposed to valproic acid in utero. F Sert-Cre+/–:16p11.2flx/flx does not alter novel objection interaction (t37 = 0.09247, P = 0.9268, n = 19–20) or locomotion (t37 = 0.8478, P = 0.4020, n = 19–20). G Cntnap2−/− mice do not show altered novel object interaction (t39 = 0.3320, P = 0.7416, n = 18–23), but have increases in locomotor activity (t39 = 5.475, P < 0.001, n = 18–23). H Fmr1–/y, −/− mice do not have altered novel object interaction (t38 = 0.02767, 0.9781, n = 20), but have increases in locomotor activity (t38 = 4.338, P < 0.001, n = 20). I VPA mice have no alterations in novel object interaction (t25 = 0.9353, 0.3586, n = 13–14), but do have increases in locomotion (t25 = 2.282, P < 0.05, n = 13–14). Data are mean ± SEM. *P < 0.05, ***P < 0.001; unpaired t-test. For all figures, comparisons with no asterisk had P > 0.05 and were considered not significant.
MDMA reverses sociability deficits in multiple mouse models for ASD
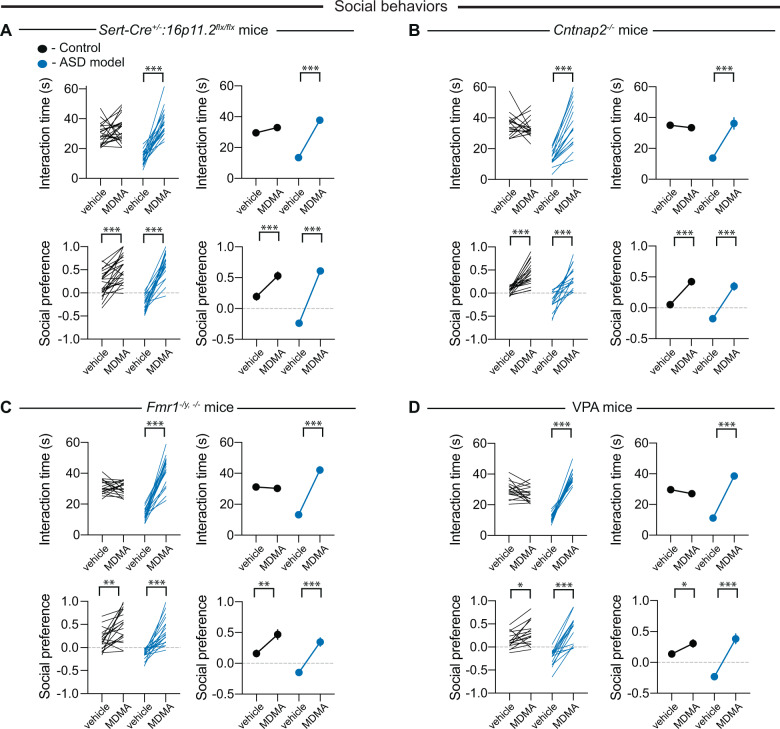
To test whether systemic administration of MDMA could rescue sociability deficits, all four ASD mouse lines as well as control mice were administered MDMA at a dose (7.5 mg/kg i.p.) that promotes sociability but does not elicit reinforcement [31] or vehicle 20 min prior to performance of the juvenile interaction and three-chamber sociability assays. All subjects received MDMA or vehicle in a counterbalanced, blinded fashion such that the behavioral assays and their analyses were performed without knowledge of the substance that had been administered to the subject. Administration of MDMA robustly reversed the sociability deficits in both assays in all four mouse lines models while vehicle administration had no effect (Fig. 2A–D). In control mice, MDMA enhanced preference for the social chamber in the three-chamber test, as previously reported [31], but did not increase the time spent interacting with a juvenile (Fig. 2A–D). Novel object interaction time was not affected by MDMA in any of the mouse lines (Supplementary Fig. S1A–D).
Fig. 2. Systemic administration of MDMA reverses social deficits in four mouse models for ASD.
A Quantification of juvenile interaction (F1,42 = 43.17, P < 0.001, n = 20–24) and three-chamber sociability (F1,42 = 28.06, P < 0.001, n = 20–24) in control or Sert-Cre+/–:16p11.2flx/flx mice with systemic administration of vehicle or MDMA. For all panels, juvenile interaction is on the top and three-chamber sociability is on the bottom with controls in black and ASD models in blue. Individual mice are represented as lines in the left panels and indicate an individual mouse that received vehicle and drug on separate days. If error bars are not clearly visible, they are smaller than the symbol used to represent the SEM. B Quantification of juvenile interaction (F1,33 = 45.46, P < 0.001, n = 16–19) and three-chamber sociability (F1,33 = 10.53, P < 0.001, n = 16–19) in control or Cntnap2−/− mice with systemic administration of vehicle or MDMA. C Quantification of juvenile interaction (F1,36 = 123.1, P < 0.001, n = 19) and three-chamber sociability (F1,36 = 12.34, P < 0.001, n = 19) in control or Fmr1–/y, −/− mice with systemic administration of vehicle or MDMA. D Quantification of juvenile interaction (F1,30 = 172.4, P < 0.001, n = 15–17) and three-chamber sociability (F1,30 = 24.84, P < 0.001, n = 15–17) in control or VPA mice with systemic administration of vehicle or MDMA. In this and all subsequent figures, the left panels illustrate individual subjects; right panels display mean ± SEM. *P < 0.05, ***P < 0.001; two-way ANOVA with Sidak’s multiple comparison post hoc test.
A 5-HT1b receptor agonist reverses behavioral deficits in multiple mouse models for ASD
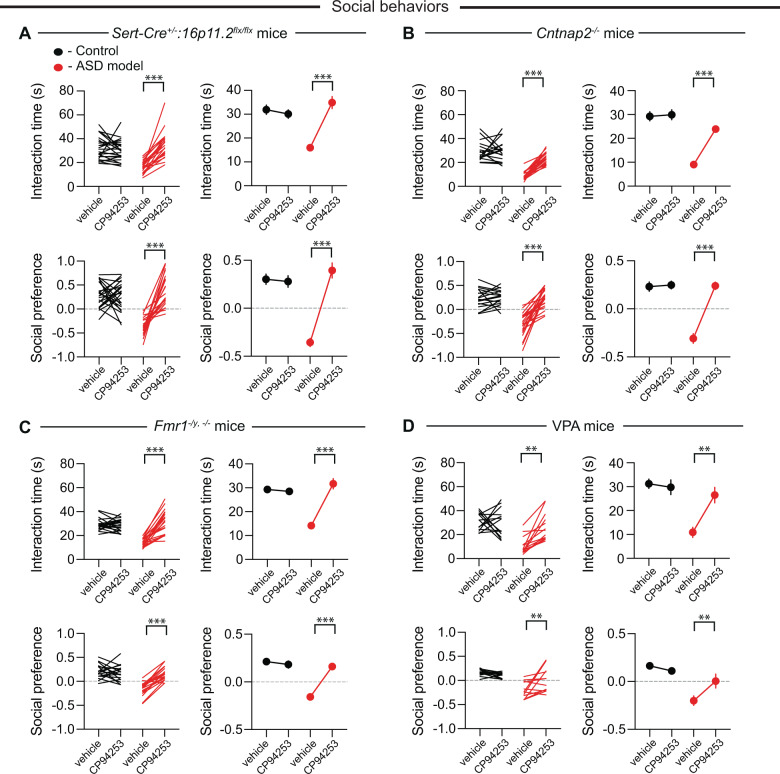
Although MDMA is in advanced clinical trials as an adjunct to therapy for treatment of post-traumatic stress disorder (PTSD) [44] and social anxiety in ASD [45], it has a long history of abuse potential and there are concerns regarding its toxicity [46]. These effects are thought to be due to MDMA’s molecular interactions with the 5-HT and dopamine (DA) transporters to elicit robust release of these neuromodulators. Given that we have previously shown that MDMA’s prosocial effect is dependent on 5-HT release in the NAc, while its reinforcing effects are primarily mediated by DA release [31], drugs that directly target the receptor(s) upon which 5-HT acts in the NAc to enhance sociability may recapitulate MDMA’s prosocial effects while mitigating MDMA’s associated health risks. Three different studies from our lab have consistently found that a 5-HT1b receptor antagonist prevents the social reward and sociability effects of 5-HT release in the NAc [25, 26, 31]. Furthermore, infusion of a 5-HT1b receptor agonist directly into the NAc enhanced sociability in Sert-Cre+/–:16p11.2flx/flx mice [26]. Therefore, we assessed whether systemic administration of the highly specific 5-HT1b receptor agonist CP-94,253 [47] could reverse sociability deficits in ASD mouse models in a manner similar to MDMA. Again, all assays and analyses were performed blindly. In all genetic ASD mouse lines, administration of CP-94,253 (10 mg/kg i.p.) reversed the sociability deficits seen in both the juvenile interaction and three-chamber tests while having no effects in control mice (Fig. 3A–C). In VPA mice, administration of CP-94,253 reversed sociability deficits in the juvenile interaction test but in the three-chamber assay, it reversed the social aversion displayed by these mice without enhancing sociability.
Fig. 3. Systemic administration of CP-94,253 reverses social deficits in four mouse models for ASD.
A Quantification of juvenile interaction (F1,43 = 45.58, P < 0.001, n = 21–24) and three-chamber sociability (F1,41 = 34.56, P < 0.001, n = 19–24) in control or Sert-Cre+/−:16p11.2flx/flx mice with systemic administration of vehicle or CP-94,253. For all panels, juvenile interaction is on the top and three-chamber sociability is on the bottom with controls in black and ASD model in red. If error bars are not clearly visible, they are smaller than the symbol used to represent the SEM. B Quantification of juvenile interaction (F1,39 = 28.92, P < 0.001, n = 18–23) and three-chamber sociability (F1,39 = 41.17, P < 0.001, n = 18–23) in control or Cntnap2−/− mice with systemic administration of vehicle or CP-94,253. C Quantification of juvenile interaction (F1,38 = 54.62, P < 0.001, n = 20) and three-chamber sociability (F1,36 = 38.14, P < 0.001, n = 19) in control or Fmr1–/y, −/− mice with systemic administration of vehicle or CP-94,253. D Quantification of juvenile interaction (F1,25 = 10.75, P < 0.01, n = 13–14) and three-chamber sociability (F1,24 = 10.42, P < 0.01, n = 12–14) in control or VPA mice with systemic administration of vehicle or CP-94,253. Data are mean ± SEM. **P < 0.01, ***P < 0.001; two-way ANOVA with Sidak’s multiple comparison post hoc test. .
To assess if 10 mg/kg of CP-94,253 was an appropriate dose to reverse the observed sociability deficits, we performed dose-response experiments in wild-type and Cntnap2−/− mice. Administration of CP-94,253 at four different doses (1, 3, 10, and 30 mg/kg) dose-dependently reversed the sociability deficits seen in both juvenile interaction and three-chamber tests when given progressively every 2 days in the same cohort of Cntnap2−/− mice (Supplementary Fig. S2A, B). Consistent with our previous results, CP-94,253 at doses up to 10 mg/kg had no effects in wild-type mice but at 30 mg/kg induced a minor increase in sociability in both assays (Supplementary Fig. S2A, B). To test for possible longer-term, “carry-over” effects of prior administration of CP-94,253, we repeated dose-response experiments in separate cohorts of wild-type and VPA mice such that each individual subject received only a single dose of CP-94,253. The results of these experiments were essentially identical to those of the previous dose-response assays (Supplementary Fig. S2C, D).
To determine if CP-94,252 at the dose tested in all four ASD models (10 mg/kg) might be acutely reinforcing and therefore exhibits abuse liability we performed CPP assays in wild-type and Cntnap2−/− mice. However, CP-94,253 did not elicit CPP or conditioned place aversion in either wild-type or Cntnap2−/− mice (Supplementary Fig. S3). Surprisingly, CP-94,253 reduced the hyperactivity exhibited by the Cntnap2−/−, Fmr1–/y, −/−, and VPA mice but had no effect on the normal locomotion in the Sert-Cre+/–:16p11.2flx/flx (Supplementary Fig. S4). Similarly, the drug had no effect on performance in the novel object interaction assay in any of the mouse lines. (Supplementary Fig. S5).
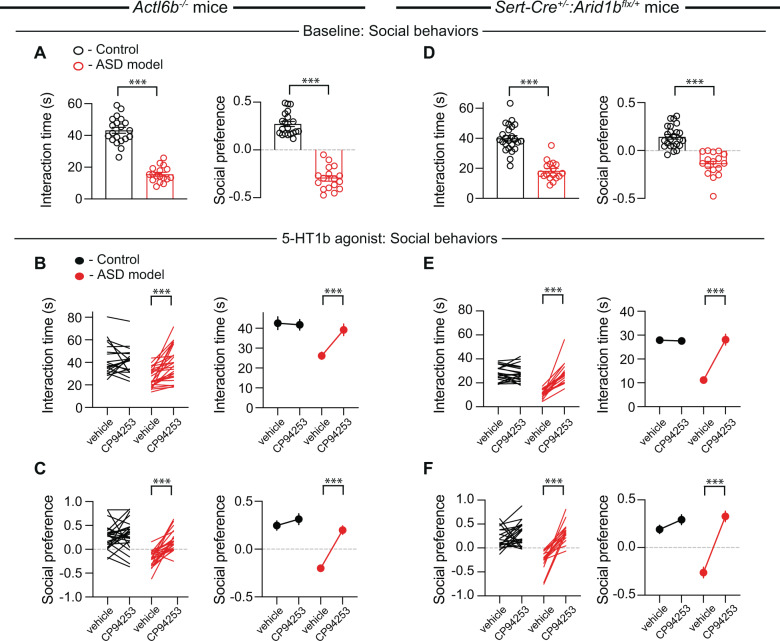
To further examine the surprisingly consistent effects of CP-94,253, we extended our investigation of 5-HT1b receptor agonism to genetic deletion ASD models of two subunits of the neuron-specific nBAF chromatin remodeling complex, Actl6b and Arid1b, which are among the most frequently mutated genes in ASD and non-syndromic intellectual disability [48–54]. Consistent with a previous report [40], Actl6b−/− mice exhibited impaired sociability in both assays (Fig. 4A). Administration of CP-94,253 reversed these sociability deficits (Fig. 4B, C) as well as the hyperactivity observed in this mouse line (Supplementary Fig. S6A) but had no effects in the novel object interaction assay (Supplementary Fig. S6B). Because of the availability of mice in which Arid1b is floxed (Arid1bflx) and our interest in the role of 5-HT signaling in ASD, rather than examining a global Arid1b heterozygous deletion, we crossed heterozygous Arid1bflx/+ mice with Sert-Cre+/+ mice to generate mice with heterozygous Arid1b deletion only in 5-HT neurons (Sert-Cre+/–:Arid1bflx/+). Similar to Sert-Cre+/–:16p11.2flx/flx mice, these mice exhibited deficits in sociability in both assays (Fig. 4D) with no abnormalities in the novel object interaction assay or locomotion (Supplementary Fig. S7A). Administration of CP-94,253 in Sert-Cre+/–:Arid1bflx/+ mice reversed the social deficits observed in both the juvenile interaction and three-chamber sociability tests, again with no effects on control mice (Fig. 4E, F), or alterations in novel object interaction or locomotion (Supplementary Fig. S7B).
Fig. 4. Systemic administration of CP-94,253 reverses social deficits in Actl6b−/− and Sert-Cre+/–:Arid1bflx/+ mice.
A Quantification of sociability during juvenile interaction (t35 = 12.05, P < 0.001, n = 17–20) and three-chamber sociability (t35 = 14.35, P < 0.001, n = 17–20) in control (black) or Actl6b−/− (red) mice. If error bars are not clearly visible, they are smaller than the symbol used to represent the SEM. B, C Quantification of juvenile interaction (B: F1,35 = 31.12, P < 0.001, n = 17–20) and three-chamber sociability (C: F1,50 = 16.38, P < 0.001, n = 24–28) in control or Actl6b−/− mice with systemic administration of vehicle or CP-94,253. D Quantification of sociability during juvenile interaction (t39 = 8.468, P < 0.001, n = 17–24) and three-chamber sociability (t39 = 7.573, P < 0.001, n = 17–24) in control (black) or Sert-Cre+/–:Arid1bflx/+ (red) mice. E, F Quantification of juvenile interaction (E: F1,37 = 52.43, P < 0.001, n = 17–22) and three-chamber sociability (F: F1,35 = 22.22, P < 0.001, n = 16–22) in control or Sert-Cre+/–:Arid1bflx/+ mice with systemic administration of vehicle or CP-94,253. Data are mean ± SEM. ***P < 0.001; two-way ANOVA with Sidak’s multiple comparison post hoc test. .
Principal component analysis of the behavioral profiles of mouse models for ASD
While our standard methods of analysis allow us to assess the effect of drug administration in a single assay, we cannot readily examine the global effects of this manipulation across multiple behaviors. To address this limitation, we performed dimensionality reduction using PCA to better understand the overall effect of CP-94,253 on the global behavioral profiles of individual mice. This allowed us to visualize and quantify the impact of CP-94,253 administration for hundreds of behavioral measurements in all six ASD models and their controls. PCA of ASD model mice treated with vehicle indicated that the behavioral profiles of the six ASD models were similar and did not form distinguishable clusters (Fig. 5A, PC1 and PC2 accounted for ~66% of the variance). However, PCA of both ASD model and control cohorts treated with vehicle revealed that the behavioral profiles of ASD model mice are clearly distinguishable from those of control animals, as evidenced by their nearly nonoverlapping distributions (Fig. 5B).
PC1 was most strongly anti-correlated with performance on the sociability assays, while PC2 was most strongly correlated with the control assays. Together PC1 and PC2 explained ~72% of the variance (Supplementary Table S3). Notably, when these same subjects received CP-94,253, their behavioral profiles were no longer distinguishable from those of controls (Fig. 5C). Finally, we quantified the extent to which each subject’s behavior was rescued by CP-94,253 by calculating the Euclidean distances from the subject’s PCA score under CP-94,253 or vehicle treatment to the control vehicle average (Fig. 5D). CP-94,253 treatment significantly reduced this distance for individuals in the ASD model cohorts without affecting controls (Fig. 5E). This quantification emphasizes the dramatic behavioral effects of this straightforward pharmacological manipulation and suggests that systemic administration of CP-94,253 results in an overall normalization of behavior in all of the ASD mouse models examined.
Discussion
In the present study, we demonstrate that enhancing 5-HT activity, via MDMA or with a selective 5-HT1b receptor agonist, reverses impairments in sociability across multiple mouse models for ASD. Although both agents ameliorated sociability deficits, there were two differences in the drugs’ behavioral effects. First, MDMA, but not CP-94,253, promoted sociability in control mice. Second, CP-94,253 reduced the hyperactivity observed in the four ASD mouse models in which it occurred but, consistent with previous reports [55–57], had no effect on locomotor activity in control mice. In contrast, MDMA increases locomotor activity at higher doses [31]. The differences in the drugs’ behavioral effects are likely due to their very different mechanisms of actions. MDMA binds to SERT with high affinity and causes supraphysiological release of 5-HT through a reverse transport mechanism that is independent of action potential activity and very different than the slower, modest increases in 5-HT caused by 5-HT specific reuptake inhibitors (SSRIs) [58–62]. MDMA also binds to the DA transporter with lower affinity and the consequent increase in DA levels likely accounts for its locomotor stimulatory and reinforcing properties at high doses [31, 63–65]. In contrast, CP-94,253 is a highly specific 5-HT1b receptor agonist that, unlike MDMA, should have minimal effects on the many other 5-HT receptor subtypes that will be influenced by the 5-HT released by MDMA.
We intentionally examined multiple ASD mouse models based on different genetic or environmental syndromes, which are well described in human subjects. Although we extensively backcrossed all of our mouse lines to C57BL/6 mice, it is formally possible that unknown background genetic differences between the experimental and control mice may have contributed to the observed behavioral differences. Even if this occurred, however, it does not change the fact that two different drugs, which influence 5-HT signaling, were effective in all models suggesting that 5-HT-mediated modulation of key targets such as the NAc may be one critical convergent target upon which the different causal genetic or environmental factors act. Consistent with this proposal, deletion of 16p11.2 or Arid1b only from 5-HT neurons was sufficient to cause robust sociability deficits but not the hyperactivity observed in all of the other ASD models, which involved brain-wide genetic deletions or prenatal exposure to VPA. This suggests that the hyperactivity was generated by alterations in brain circuity other than that involving 5-HT neurons.
Previous work provides additional evidence that several of the ASD mouse models we used exhibit abnormalities in 5-HT signaling. Fmr1–/y mice have reduced SERT mRNA during postnatal development [66] and recently FMR1 was identified as a novel SERT-interacting protein [67]. Prenatal exposure of VPA in rodents results in decreased 5-HT levels and abnormal 5-HT neuron differentiation and innervation patterns [68, 69]. While studies have not directly identified 5-HT dysfunction in Cntnap2−/− mice, both CNTNAP2 and its ligand CNTN1, which is highly expressed in 5-HT neurons, are neurexin-family adhesion molecules important in axon development [70, 71]. Similarly, while the role of the BAF complex, which contains subunits encoded by Actl6b and Arid1b, in 5-HT signaling is unclear, cortical neurons cultured from Actl6b−/− mice have altered levels of 5-HT receptors [40] and mutations in another BAF subunit impairs 5-HTergic neuron differentiation [72]. Endogenous 5-HT signaling has also been suggested to be altered in the 16p11.2 deletion mouse model [73] and selectively deleting 16p11.2 from 5-HT neurons influences their electrophysiological properties [26]. Two additional mouse lines that influence 5-HT and exhibit abnormal social behaviors are mice lacking or harboring mutations in Slc6a4, the gene that encodes SERT [74, 75] and mice lacking monoamine oxidase A (MAOA), the gene responsible for degradation of 5-HT in the brain [76].
Although SSRIs are commonly prescribed to treat symptoms associated with ASD, such as anxiety and depression, they do not appear to ameliorate core ASD symptoms, including social deficits [77]. Nevertheless, individuals with ASD have been reported to express abnormalities in 5-HT system physiology and genetics, including alterations in 5-HT synthesis, as well as receptor and transporter binding capacity [78–81]. Indeed, the earliest biomarker found in ASD was alterations in peripheral 5-HT levels [13–18]. While peripheral mechanisms such as tactile abnormalities can contribute to behavioral abnormalities in ASD mice [82] and 5-HT1b receptors can regulate vasoconstriction and possibly nociceptive neurotransmission [83], the fact that direct infusion of a CP-94,253 into the NAc reverses social deficits present in mice with 16p11.2 selectively deleted from 5-HT neurons [26] suggests that CP-94,253 is acting centrally, not peripherally. In light of the minimal efficacy of SSRIs and our current findings, we suggest that the magnitude, kinetics, and specificity of enhancement of 5-HT-mediated signaling in pivotal brain target regions, such as the NAc, are critical components of effective 5-HT based therapeutic interventions.
What might be mechanisms that contribute to the prosocial effects of 5-HT1b receptor activation in ASD mouse models? 5-HT inhibits excitatory synaptic transmission in the NAc via activation of presynaptic 5-HT1b receptors [25, 31, 84]. Given that inhibition of basolateral amygdala excitatory inputs to NAc reverses social deficits in a Shank3 deletion ASD model [85], the modulation of excitatory inputs to NAc, a key node of reward circuity that influences social behaviors [86, 87], may be one important mechanism contributing to the prosocial effects of CP-94,253 and MDMA. However, given the complexity of the circuitry that mediates even the simplest prosocial behavior in mice [86, 87], much work remains to be done to fully understand how these drugs mediate their prosocial behavioral effects.
An important consideration when examining compounds for potential therapeutic benefit is whether they elicit effects that outlast their acute actions. For example, there is potential for sensitization to the prosocial effects of MDMA [88]. While long-term effects of CP-94,253 cannot be definitively ruled out, this seems unlikely given that evidence of sustained effects in the sociability assays was never observed. Indeed, there were no differences in the dose-response assays in mice given all four doses (each dose separated by 2 days) versus mice that each received only a single dose.
We hope our findings will draw attention to the possibility that a relatively simple, targeted pharmacological intervention might be useful in ameliorating some of the core deficits seen in individuals with ASD. MDMA is already being investigated as an adjunctive treatment for PTSD and social anxiety in ASD. However, because of its potential toxicity and abuse potential, it seems unlikely that MDMA will be useful as a prolonged, daily treatment to reduce sociability deficits in ASD. We are more enthusiastic about the potential utility of a 5-HT1b receptor agonist in the treatment of ASD. Our PCA revealed that following treatment with CP-94,253 the global behavioral profile of all ASD models was indistinguishable from that of controls. This normalization occurred despite the dramatically different genetic modifications and environmental manipulations generating the pathological behavioral changes. Furthermore, unlike manipulations that enhance DA neuron activity or DA release (e.g., MDMA or other psychostimulants), manipulations that increase 5-HT neuron activity or 5-HT release (e.g., SSRIs) are not acutely reinforcing and do not induce hyperactivity [33], both of which are strongly associated with abuse potential in rodent models. An important limitation of our study is that we examined only acute actions of MDMA and CP-94,253. Furthermore, 5-HT plays an important role in brain development [79, 89], and therefore appropriate caution must be taken in testing any 5-HTergic agent in children. Nevertheless, we hope that our findings will stimulate further work defining the effectiveness of these simple, but potentially powerful therapeutic interventions.
Funding and disclosure
This work was supported by the following grants: F32 MH103949 (to JJW); HHMI Gilliam Fellowship (to DFCP and RCM); NSF Graduate Research Fellowship (to DFCP); K99 DK115895 (to DJC); F31 MH116588 and F99 NS118735 (to WW); SFARI 514863 and R01 NS046789 (to GRC); K08 MH110610 (to BDH); and the Stanford University Wu Tsai Neurosciences Institute (to RCM). RCM is on the scientific advisory boards of MapLight Therapeutics, Cerevance, Cyclerion, AZTherapies, MindMed, and Aelis Farma. GRC is a founder of Foghorn Therapeutics. The remaining authors have no existing conflicts.
Supplementary information
Supplementary Material Figures and Tables
Acknowledgements
We thank the members of the Malenka and Heifets labs for helpful comments on the project. We thank Claire Ellis for her help with breeding, maintaining, and delivering many of the mouse lines. We also thank Jason M. Tucciarone for his expertise in timed pregnancies for the generation of the valproic acid mice. MDMA was a gift from R. Doblin, Multidisciplinary Association for Psychedelic Studies (MAPS).
Author contributions
JJW performed and analyzed the majority of the experiments with assistance of PL. DFCP designed the PCA code and with DJC performed the PCA analysis. DJC assisted in behavioral analysis and design. WW and GRC helped select the mouse lines to study and bred, maintained, and delivered a significant proportion of the cohorts used in the studies. BDH and JS assisted in the design of the MDMA studies. JJW and RCM developed the project, designed the experiments, interpreted results, and wrote the paper, which was edited by all authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01091-6.
References
- 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- 2.Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, et al. Prevalence of autism spectrum disorder among children aged 8 years – Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69:1–12. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geschwind DH, State MW. Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 2015;14:1109–20. doi: 10.1016/S1474-4422(15)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10:74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolen G, Sahin M. Editorial: essential pathways and circuits of autism pathogenesis. Front Neurosci. 2016;10:182. doi: 10.3389/fnins.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180:568–84. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–33. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollander JA, Cory-Slechta DA, Jacka FN, Szabo ST, Guilarte TR, Bilbo SD, et al. Beyond the looking glass: recent advances in understanding the impact of environmental exposures on neuropsychiatric disease. Neuropsychopharmacology. 2020;45:1086–96. doi: 10.1038/s41386-020-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuccillo MV. Striatal circuits as a common node for autism pathophysiology. Front Neurosci. 2016;10:27. doi: 10.3389/fnins.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JA, Penagarikano O, Belgard TG, Swarup V, Geschwind DH. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 2015;10:111–44. doi: 10.1146/annurev-pathol-012414-040405. [DOI] [PubMed] [Google Scholar]
- 11.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–50. [PubMed] [Google Scholar]
- 13.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–20. doi: 10.1016/S0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 14.Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24:919–29. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–9. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: evidence from a family study of multiple-incidence autism families. Am J Psychiatry. 1999;156:557–63. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann N Y Acad Sci. 1990;600:331–40. doi: 10.1111/j.1749-6632.1990.tb16893.x. [DOI] [PubMed] [Google Scholar]
- 18.Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–54. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Chandana SR, Behen ME, Juhasz C, Muzik O, Rothermel RD, Mangner TJ, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–82. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Chugani DC. Role of altered brain serotonin mechanisms in autism. Mol Psychiatry. 2002;7:S16–7. doi: 10.1038/sj.mp.4001167. [DOI] [PubMed] [Google Scholar]
- 21.Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–7. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- 23.Oblak A, Gibbs TT, Blatt GJ. Reduced serotonin receptor subtypes in a limbic and a neocortical region in autism. Autism Res. 2013;6:571–83. doi: 10.1002/aur.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy DG, Daly E, Schmitz N, Toal F, Murphy K, Curran S, et al. Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger’s syndrome: an in vivo SPECT study. Am J Psychiatry. 2006;163:934–6. doi: 10.1176/ajp.2006.163.5.934. [DOI] [PubMed] [Google Scholar]
- 25.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–84. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature. 2018;560:589–94. doi: 10.1038/s41586-018-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 28.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–38. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 29.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamilar-Britt P, Bedi G. The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neurosci Biobehav Rev. 2015;57:433–46. doi: 10.1016/j.neubiorev.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heifets BD, Salgado JS, Taylor MD, Hoerbelt P, Cardozo Pinto DF, Steinberg EE, et al. Distinct neural mechanisms for the prosocial and rewarding properties of MDMA. Sci Transl Med. 2019;11:eaaw6435. [DOI] [PMC free article] [PubMed]
- 32.Portmann T, Yang M, Mao R, Panagiotakos G, Ellegood J, Dolen G, et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep. 2014;7:1077–92. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–60. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed]
- 35.Chaliha D, Albrecht M, Vaccarezza M, Takechi R, Lam V, Al-Salami H, et al. A systematic review of the valproic-acid-induced rodent model of autism. Dev Neurosci. 2020;42:12–48. doi: 10.1159/000509109. [DOI] [PubMed] [Google Scholar]
- 36.Castro K, Baronio D, Perry IS, Riesgo RDS, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr Neurosci. 2017;20:343–50. doi: 10.1080/1028415X.2015.1133029. [DOI] [PubMed] [Google Scholar]
- 37.Al-Amin MM, Rahman MM, Khan FR, Zaman F, Mahmud Reza H. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav Brain Res. 2015;286:112–21. doi: 10.1016/j.bbr.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 38.Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Celen C, Chuang JC, Luo X, Nijem N, Walker AK, Chen F, et al. Arid1b haploinsufficient mice reveal neuropsychiatric phenotypes and reversible causes of growth impairment. Elife. 2017;6:e25730. [DOI] [PMC free article] [PubMed]
- 40.Wenderski W, Wang L, Krokhotin A, Walsh JJ, Li H, Shoji H, et al. Loss of the neural-specific BAF subunit ACTL6B relieves repression of early response genes and causes recessive autism. Proc Natl Acad Sci USA. 2020;117:10055–66. doi: 10.1073/pnas.1908238117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacai H, Sakoori K, Konno K, Nagahama K, Suzuki H, Watanabe T, et al. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat Commun. 2020;11:5140. doi: 10.1038/s41467-020-18861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, et al. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol. 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danforth AL, Struble CM, Yazar-Klosinski B, Grob CS. MDMA-assisted therapy: a new treatment model for social anxiety in autistic adults. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:237–49. doi: 10.1016/j.pnpbp.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 46.McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–7. doi: 10.1016/S0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- 47.Koe BK, Nielsen JA, Macor JE, Heym J. Biochemical and behavioral studies of 5-HT1b receptor agonist, CP-94,253. Drug Dev Res. 1992;26:241–50. doi: 10.1002/ddr.430260305. [DOI] [Google Scholar]
- 48.Bell S, Rousseau J, Peng H, Aouabed Z, Priam P, Theroux JF, et al. Mutations in ACTL6B cause neurodevelopmental deficits and epilepsy and lead to loss of dendrites in human neurons. Am J Hum Genet. 2019;104:815–34. doi: 10.1016/j.ajhg.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoyer J, Ekici AB, Endele S, Popp B, Zweier C, Wiesener A, et al. Haploinsufficiency of ARID1B, a member of the SWI/SNF-a chromatin-remodeling complex, is a frequent cause of intellectual disability. Am J Hum Genet. 2012;90:565–72. doi: 10.1016/j.ajhg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santen GW, Aten E, Sun Y, Almomani R, Gilissen C, Nielsen M, et al. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat Genet. 2012;44:379–80. doi: 10.1038/ng.2217. [DOI] [PubMed] [Google Scholar]
- 51.Tsurusaki Y, Okamoto N, Ohashi H, Kosho T, Imai Y, Hibi-Ko Y, et al. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet. 2012;44:376–8. doi: 10.1038/ng.2219. [DOI] [PubMed] [Google Scholar]
- 52.Santen GW, Clayton-Smith J, consortium ABC. The ARID1B phenotype: what we have learned so far. Am J Med Genet C Semin Med Genet. 2014;166C:276–89. doi: 10.1002/ajmg.c.31414. [DOI] [PubMed] [Google Scholar]
- 53.Moffat JJ, Jung EM, Ka M, Smith AL, Jeon BT, Santen GWE, et al. The role of ARID1B, a BAF chromatin remodeling complex subunit, in neural development and behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:30–38. doi: 10.1016/j.pnpbp.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krupp DR, Barnard RA, Duffourd Y, Evans SA, Mulqueen RM, Bernier R, et al. Exonic mosaic mutations contribute risk for autism spectrum disorder. Am J Hum Genet. 2017;101:369–90. doi: 10.1016/j.ajhg.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- 56.Nasehi M, Ghadimi F, Khakpai F, Zarrindast MR. Interaction between harmane, a class of beta-carboline alkaloids, and the CA1 serotonergic system in modulation of memory acquisition. Neurosci Res. 2017;122:17–24. doi: 10.1016/j.neures.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Der-Ghazarian TS, Call T, Scott SN, Dai K, Brunwasser SJ, Noudali SN, et al. Effects of a 5-HT1B receptor agonist on locomotion and reinstatement of cocaine-conditioned place preference after abstinence from repeated injections in mice. Front Syst Neurosci. 2017;11:73. doi: 10.3389/fnsys.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharm Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 59.Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharm Ther. 2002;95:73–88. doi: 10.1016/S0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 60.Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA. 1992;89:1817–21. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–9. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- 62.Hagino Y, Takamatsu Y, Yamamoto H, Iwamura T, Murphy DL, Uhl GR, et al. Effects of MDMA on extracellular dopamine and serotonin levels in mice lacking dopamine and/or serotonin transporters. Curr Neuropharmacol. 2011;9:91–5. doi: 10.2174/157015911795017254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan KA, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administration in rats. Behav Pharm. 2009;20:688–94. doi: 10.1097/FBP.0b013e328333a28d. [DOI] [PubMed] [Google Scholar]
- 64.Vidal-Infer A, Roger-Sanchez C, Daza-Losada M, Aguilar MA, Minarro J, Rodriguez-Arias M. Role of the dopaminergic system in the acquisition, expression and reinstatement of MDMA-induced conditioned place preference in adolescent mice. PLoS ONE. 2012;7:e43107. doi: 10.1371/journal.pone.0043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol. 2001;16:589–98. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- 66.Uutela M, Lindholm J, Rantamaki T, Umemori J, Hunter K, Voikar V, et al. Distinctive behavioral and cellular responses to fluoxetine in the mouse model for Fragile X syndrome. Front Cell Neurosci. 2014;8:150. doi: 10.3389/fncel.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinlan MA, Robson MJ, Ye R, Rose KL, Schey KL, Blakely RD. Ex vivo quantitative proteomic analysis of serotonin transporter interactome: network impact of the SERT Ala56 coding variant. Front Mol Neurosci. 2020;13:89. doi: 10.3389/fnmol.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyazaki K, Narita N, Narita M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int J Dev Neurosci. 2005;23:287–97. doi: 10.1016/j.ijdevneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Dufour-Rainfray D, Vourc’h P, Le Guisquet AM, Garreau L, Ternant D, Bodard S, et al. Behavior and serotonergic disorders in rats exposed prenatally to valproate: a model for autism. Neurosci Lett. 2010;470:55–9. doi: 10.1016/j.neulet.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 70.Spencer WC, Deneris ES. Regulatory mechanisms controlling maturation of serotonin neuron identity and function. Front Cell Neurosci. 2017;11:215. doi: 10.3389/fncel.2017.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubio-Marrero EN, Vincelli G, Jeffries CM, Shaikh TR, Pakos IS, Ranaivoson FM, et al. Structural characterization of the extracellular domain of CASPR2 and insights into its association with the novel ligand contactin1. J Biol Chem. 2016;291:5788–802. doi: 10.1074/jbc.M115.705681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinberg P, Flames N, Sawa H, Garriga G, Hobert O. The SWI/SNF chromatin remodeling complex selectively affects multiple aspects of serotonergic neuron differentiation. Genetics. 2013;194:189–98. doi: 10.1534/genetics.112.148742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panzini CM, Ehlinger DG, Alchahin AM, Guo Y, Commons KG. 16p11.2 deletion syndrome mice perseverate with active coping response to acute stress - rescue by blocking 5-HT2A receptors. J Neurochem. 2017;143:708–21. doi: 10.1111/jnc.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–71. doi: 10.1016/S0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 75.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci USA. 2012;109:5469–74. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, et al. Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol. 2013;16:869–88. doi: 10.1017/S1461145712000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Politte LC, Henry CA, McDougle CJ. Psychopharmacological interventions in autism spectrum disorder. Harv Rev Psychiatry. 2014;22:76–92. doi: 10.1097/HRP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 78.Zafeiriou DI, Ververi A, Vargiami E. The serotonergic system: its role in pathogenesis and early developmental treatment of autism. Curr Neuropharmacol. 2009;7:150–7. doi: 10.2174/157015909788848848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience. 2016;321:24–41. doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takumi T, Tamada K, Hatanaka F, Nakai N, Bolton PF. Behavioral neuroscience of autism. Neurosci Biobehav Rev. 2020;110:60–76. doi: 10.1016/j.neubiorev.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Dolen G. Autism: oxytocin, serotonin, and social reward. Soc Neurosci. 2015;10:450–65. doi: 10.1080/17470919.2015.1087875. [DOI] [PubMed] [Google Scholar]
- 82.Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell. 2019;178:867–86. doi: 10.1016/j.cell.2019.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loyd DR, Henry MA, Hargreaves KM. Serotonergic neuromodulation of peripheral nociceptors. Semin Cell Dev Biol. 2013;24:51–7. doi: 10.1016/j.semcdb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathur BN, Capik NA, Alvarez VA, Lovinger DM. Serotonin induces long-term depression at corticostriatal synapses. J Neurosci. 2011;31:7402–11. doi: 10.1523/JNEUROSCI.6250-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Folkes OM, Baldi R, Kondev V, Marcus DJ, Hartley ND, Turner BD, et al. An endocannabinoid-regulated basolateral amygdala-nucleus accumbens circuit modulates sociability. J Clin Invest. 2020;130:1728–42. doi: 10.1172/JCI131752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walsh JJ, Christoffel DJ, Wu X, Pomrenze MB, Malenka RC. Dissecting neural mechanisms of prosocial behaviors. Curr Opin Neurobiol. 2020;68:9–14. doi: 10.1016/j.conb.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klawonn AM, Malenka RC. Nucleus accumbens modulation in reward and aversion. Cold Spring Harb Symp Quant Biol. 2018;83:119–29. doi: 10.1101/sqb.2018.83.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Curry DW, Berro LF, Belkoff AR, Sulima A, Rice KC, Howell LL. Sensitization to the prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA) Neuropharmacology. 2019;151:13–20. doi: 10.1016/j.neuropharm.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Niederkofler V, Asher TE, Dymecki SM. Functional interplay between dopaminergic and serotonergic neuronal systems during development and adulthood. ACS Chem Neurosci. 2015;6:1055–70. doi: 10.1021/acschemneuro.5b00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material Figures and Tables