Summary
ATP- and GTP-dependent molecular switches are extensively used to control functions of proteins in a wide range of biological processes. However, CTP switches are rarely reported. Here, we report that a nucleoid occlusion protein Noc is a CTPase enzyme whose membrane-binding activity is directly regulated by a CTP switch. In Bacillus subtilis, Noc nucleates on 16 bp NBS sites before associating with neighboring non-specific DNA to form large membrane-associated nucleoprotein complexes to physically occlude assembly of the cell division machinery. By in vitro reconstitution, we show that (1) CTP is required for Noc to form the NBS-dependent nucleoprotein complex, and (2) CTP binding, but not hydrolysis, switches Noc to a membrane-active state. Overall, we suggest that CTP couples membrane-binding activity of Noc to nucleoprotein complex formation to ensure productive recruitment of DNA to the bacterial cell membrane for nucleoid occlusion activity.
Keywords: nucleoid occlusion protein, Noc, ParB, CTP, membrane-binding protein, bacterial cell division, X-ray crystallography, in vitro reconstitution
Graphical abstract
Highlights
-
•
CTP is required for Noc to form a higher-order nucleoprotein complex on DNA
-
•
CTP binding switches DNA-entrapped Noc to a membrane-active state
-
•
CTP hydrolysis likely reverses the association between Noc-DNA and the membrane
-
•
The membrane-targeting helix adopts an autoinhibitory conformation in apo-Noc
Jalal et al. report that the nucleoid occlusion protein Noc is a CTPase enzyme whose membrane-binding activity is directly regulated by CTP. CTP binding switches the Noc-DNA complex from a membrane-inactive state to an active state, thus ensuring productive recruitment of DNA to the bacterial cell membrane.
Introduction
While ATP and GTP switches are ubiquitous in biology, CTP switches have rarely been identified but may be more widespread than previously appreciated. A recent discovery showed that ParB, a crucial protein for bacterial chromosome segregation, is the founding member of a new class of CTP-dependent molecular switches (Osorio-Valeriano et al., 2019; Soh et al., 2019). ParB nucleates on a parS DNA sequence and associates with neighboring DNA, a process known as spreading, to enable faithful chromosome segregation (Breier and Grossman, 2007; Funnell, 2016; Graham et al., 2014; Jalal and Le, 2020; Murray et al., 2006; Sanchez et al., 2015). CTP induces ParB self-dimerization to create a clamp-like molecule (Soh et al., 2019). The ParB clamp self-loads at parS, then spreads by sliding to neighboring DNA while still entrapping DNA (Jalal et al., 2020a; Soh et al., 2019). Essentially, CTP serves to switch ParB from a parS-nucleating open clamp to a DNA-sliding closed-clamp state (Jalal et al., 2020a; Soh et al., 2019). The result is the formation of a higher-order nucleoprotein complex with multiple ParB-CTP clamps entrapped in the vicinity of the parS locus. The higher-order nucleoprotein complex stimulates the ATPase activity of ParA, a partner of ParB, driving the segregation of replicated chromosomes to daughter cells (Hwang et al., 2013; Jalal and Le, 2020; Lim et al., 2014; Vecchiarelli et al., 2012, 2013, 2014).
In Firmicutes, the nucleoid occlusion protein Noc is a paralog of ParB (Jalal et al., 2020b; Sievers et al., 2002; Wu and Errington, 2011); however, the role of Noc is different from that of a canonical ParB (Pang et al., 2017; Veiga et al., 2011; Wu and Errington, 2004). Noc helps direct the assembly of the cell division machinery toward the middle of a dividing cell where the concentration of chromosomal DNA (the nucleoid) is the least, thus ensuring a binary cell division (Adams et al., 2015; Rodrigues and Harry, 2012; Wu and Errington, 2004; Yu et al., 2021). Noc does so by nucleating on 16 bp NBS (Noc-binding site) sites scattered around the chromosome before spreading to neighboring DNA to form large Noc-DNA nucleoprotein complexes (Wu et al., 2009). Unusually, Noc is also a peripheral membrane protein that directly associates with the cell membrane via a predicted N-terminal amphipathic helix (Adams et al., 2015; Figure 1A). The recruitment of the chromosomal DNA to the membrane is crucial for preventing the assembly of the division machinery over the chromosome; indeed, a Noc variant lacking the amphipathic helix is impaired in nucleoid occlusion activity (Adams et al., 2015). In Bacillus subtilis, Noc was observed to associate with the cell membrane in a transient manner in vivo (Wu et al., 2009). It was thought that a strong membrane-binding activity of Noc might have been selected against, as a stable association with the membrane might hamper chromosome replication and segregation (Adams et al., 2015), yet it is unclear how the membrane-binding activity of Noc is modulated. Furthermore, Noc must bring the chromosomal DNA to the membrane to physically inhibit the assembly of the division machinery (Adams et al., 2015); an unregulated membrane-binding activity would likely confine apo-Noc permanently to the cell membrane, thus unfavorably limiting the recruitment of DNA to the membrane (Adams et al., 2015). Again, it remains unclear whether the membrane-binding activity of Noc is regulated and, if so, how.
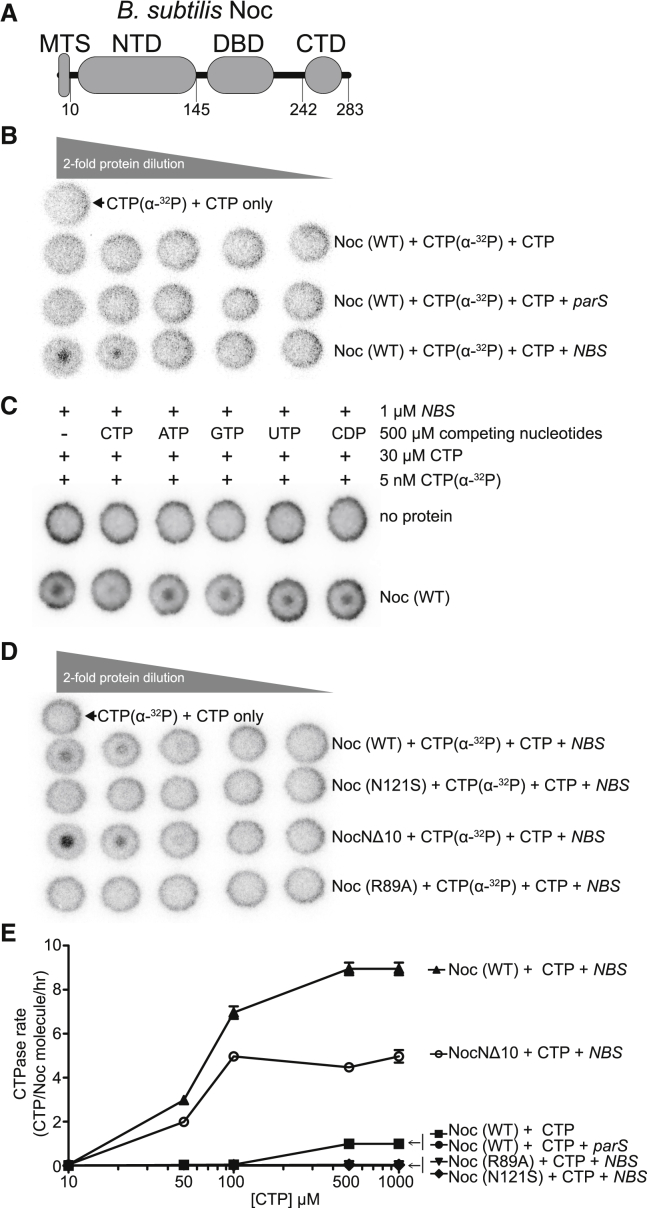
Figure 1.
Noc binds and hydrolyzes CTP in the presence of NBS DNA
(A) The domain architecture of B. subtilis Noc: a membrane-targeting sequence (MTS), an N-terminal domain (NTD), a central DNA-binding domain (DBD), and a C-terminal domain (CTD).
(B–D) CTP binding as monitored by DRaCALA assay using radiolabeled CTP α-P32. The bull’s-eye staining indicates CTP binding due to a more rapid immobilization of protein-ligand complexes compared with free ligands. The starting concentration of Noc used in all panels was 30 μM. The concentrations of CTP α-P32, unlabeled CTP, and a 22 bp parS/NBS DNA used in all panels were 5 nM, 30 μM, and 1 μM, respectively.
(E) CTP hydrolysis rates of Noc (WT) and variants were measured by continuous detection of released inorganic phosphates (see STAR Methods). CTPase rates were measured at increasing concentrations of CTP. All reactions contained 1 μM Noc (WT/variants) ± 1 μM 22 bp NBS or parS DNA and an increasing concentration of CTP (0, 10, 50, 100, 500, and 1,000 μM). Experiments were triplicated, and the SDs of the CTPase rates were presented.
To investigate further, we have biochemically reconstituted NBS-dependent Noc spreading and membrane association events using purified B. subtilis Noc protein and phospholipid vesicles. We show that, similar to a canonical ParB, Noc is a CTPase enzyme that binds CTP to form a protein clamp that can slide and entrap DNA. Importantly, CTP binding, but not hydrolysis, is required to switch Noc-DNA from a membrane-inactive to an active state, thus locking Noc into a pathway in which it must spread before associating with the cell membrane. We solve an X-ray crystal structure of a C-terminal domain truncated apo-Noc from Geobacillus thermoleovorans, in which its membrane-targeting amphipathic helix adopts an autoinhibitory conformation, restricted from interacting with the membrane. We suggest that CTP binding might liberate the amphipathic helix, thereby switching Noc to a membrane-active state. Altogether, we demonstrate that CTP directly regulates the membrane-binding activity of the nucleoid occlusion protein Noc, further expanding the role of CTP switches in biology.
Results
NBS DNA increases the CTP binding and hydrolysis rate of Noc
Given the shared ancestry between ParB and Noc, we wondered if B. subtilis Noc also binds and hydrolyses CTP. To investigate, we used a membrane-spotting assay (DRaCALA), and the result showed that B. subtilis Noc binds radiolabeled CTP, but only in the presence of a cognate 22 bp NBS DNA (Figure 1B). An excess of unlabeled CTP, but no other NTP or CDP, outcompeted radiolabeled CTP for binding to Noc, suggesting that B. subtilis Noc binds CTP specifically (Figure 1C). Similarly, an N-terminally truncated Noc variant lacking the 10 amino acid (AA) membrane-targeting sequence (NocNΔ10) also bound radiolabeled CTP in the presence of NBS DNA (Figure 1D). However, the Noc (R89A) and Noc (N121S) variants, whose equivalent substitutions in ParB have been shown to impair spreading and CTP binding (Jalal et al., 2020a; Osorio-Valeriano et al., 2019; Soh et al., 2019; Figure S1A), did not bind radiolabeled CTP at the tested concentration (Figure 1D). Next, we performed a quantitative nucleotide-binding assay using isothermal titration calorimetry (ITC) with a non-hydrolyzable CTP analog (CTPɣS) to ensure the heat exchange was due solely to nucleotide binding but not hydrolysis or NBS DNA binding. We found that B. subtilis Noc binds CTPɣS with a moderate affinity (Kd = 68 ± 23 μM), while Noc (N121S) bound CTPɣS more weakly at Kd = 232 ± 66 μM, and Noc (R89A) did not detectably bind nucleotide (Figure S1B). Consistent with a previous report (Soh et al., 2019), B. subtilis Noc also showed CTP hydrolysis activity, albeit at a low rate of about one CTP per Noc per hour when only the purified protein and CTP were included (Figure 1E; Soh et al., 2019). The addition of a 22 bp NBS DNA, but not a non-cognate 22 bp parS DNA, increased the CTP hydrolysis rate 9-fold to about nine CTP per Noc per hour (Figure 1E; Soh et al., 2019). The Noc (R89A) and Noc (N121S) variants did not show noticeable CTP-hydrolyzing activity (Figure 1E). Last, despite binding CTP equally or more strongly than the wild-type (WT) (Figure 1D), NocNΔ10 hydrolyzed CTP at a reduced rate of about five CTP per Noc per hour (Figure 1E; see Discussion). Altogether, our data suggest that B. subtilis Noc is a CTPase enzyme that binds and hydrolyses CTP in the presence of cognate DNA.
CTP and NBS DNA stimulate the engagement of the N-terminal domain of Noc in vitro
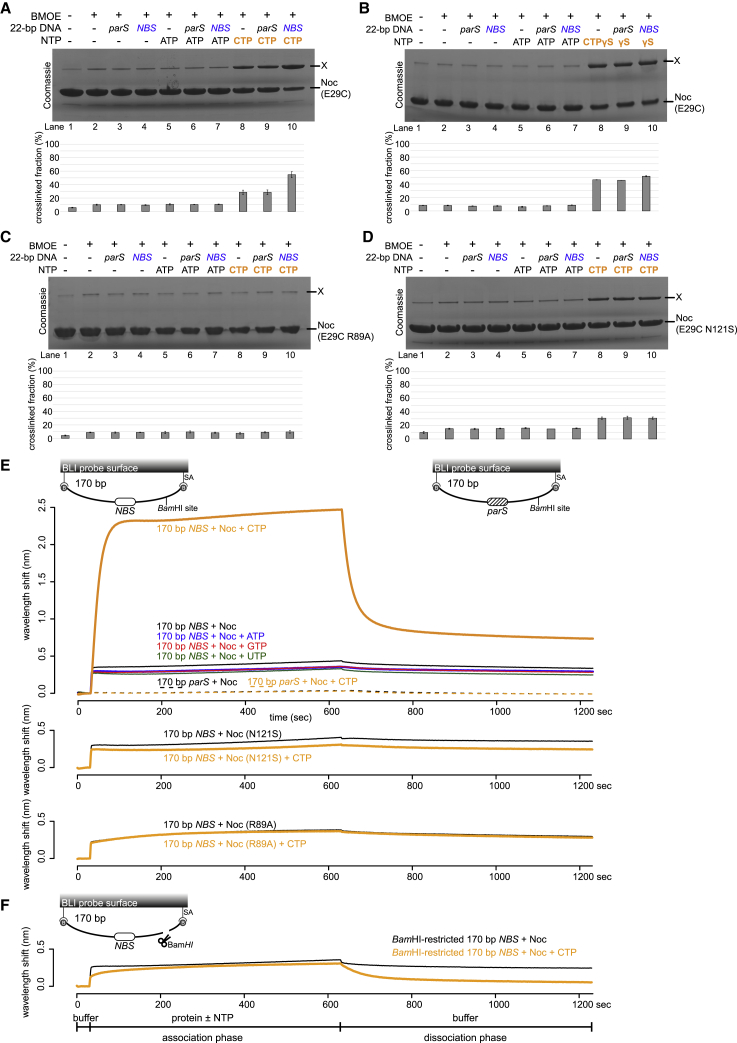
In the presence of CTP, ParB self-engages at the N-terminal domain (NTD) to create a clamp-like molecule (Jalal et al., 2020a; Soh et al., 2019). To investigate whether CTP elicits a similar response in Noc, we used site-specific crosslinking of a purified B. subtilis Noc (E29C) variant by a sulfhydryl-to-sulfhydryl crosslinker bismaleimidoethane (BMOE). On the basis of a sequence alignment between B. subtilis ParB and Noc (Figure S1A), residue E29 at the NTD was selected and substituted by cysteine on an otherwise cysteine-less Noc (WT) background (Figure S2A) to create a variant in which symmetry-related cysteines become covalently linked together if they are within 8 Å of each other. The crosslinked form was detectable as a protein form with reduced mobility on SDS-PAGE (labeled X in Figure 2). In the absence of CTP, Noc (E29C) crosslinked minimally (∼10% crosslinked fraction; Figure 2A, lanes 2–4). The crosslinking efficiency increased threefold (∼30%) in the presence of CTP (Figure 2A, lane 8), but not CDP or ATP (Figure 2A; Figure S2B). The crosslinking efficiency further increased fivefold (∼55%) when both CTP and a 22 bp NBS were included (Figure 2A, lane 10; Figure S2D). However, the addition of a non-cognate 22 bp parS DNA did not result in the same high level of crosslinking, even when CTP was present (Figure 2A, lane 9). Noticeably, non-hydrolyzable CTPɣS readily promoted crosslinking (∼45% crosslinked fraction) regardless of the presence or absence of NBS DNA (Figure 2B, lanes 8–10; Figure S2E). Therefore, our data suggest that CTP binding, but not hydrolysis, is required for the NTD engagement of B. subtilis Noc. Consistent with the requirement of CTP binding for NTD engagement, the Noc (E29C R89A) variant, in which the R89A substitution incapacitates CTP binding, did not crosslink beyond the background level in any tested condition (Figure 2C). The Noc (E29C N121S) variant, which binds CTP (albeit at a reduced affinity) but cannot hydrolyze CTP, crosslinked similarly to Noc (E29C) (∼30% crosslinked fraction) at the saturating concentration of 1 mM CTP, although the NBS-stimulated crosslinking was abolished (Figure 2D). Last, the NocNΔ10 (E29C) variant, which lacks the N-terminal membrane-targeting sequence, crosslinked similarly to Noc (E29C) in the presence of CTP and NBS DNA (Figure S2C).
Figure 2.
CTP and NBS DNA promote the self-engagement of the N-terminal domain of Noc
(A and B) SDS-PAGE analysis of BMOE crosslinking products of 10 μM B. subtilis Noc (E29C) ± 1 μM 22 bp parS/NBS DNA ± 1.0 mM NTP. All crosslinking reactions were performed at 22°C unless indicated otherwise. X indicates a crosslinked form of Noc (E29C). Quantification of the crosslinked fraction is shown below each representative image. Error bars represent SEM from three replicates.
(C) Same as (A), but Noc (E29C R89A) was used instead.
(D) Same as (A), but Noc (E29C N121S) was used instead.
(E) CTP facilitates the association of Noc with a closed NBS DNA substrate beyond nucleation. Bio-layer interferometry (BLI) analysis of the interaction between a premix of 1.0 μM B. subtilis Noc ± 1.0 mM NTP and a 170 bp dual biotin-labeled DNA that contains either an NBS or a non-cognate parS site. Interactions between a dual biotinylated DNA and a streptavidin (SA)-coated probe created a closed DNA molecule where both ends were blocked (Jalal et al., 2020a) (see also the schematic diagram of the BLI probes). Other Noc variants, Noc (R89A) and Noc (N121S), were also analyzed in the same assay.
(F) BLI analysis of the interaction between a premix of 1.0 μM B. subtilis Noc ± 1.0 mM CTP and a BamHI-restricted dual biotinylated NBS DNA. The 170 bp NBS DNA substrate was also designed with a BamHI recognition site (see the schematic diagram of the probes). The intact dual biotinylated DNA was first immobilized onto the probe surface, then an open end was generated by digestion with BamHI (see STAR Methods). Afterward, the probe was used in a BLI analysis with a premix of Noc ± CTP. Each experiment was triplicated, and a representative sensorgram was shown.
Noc associates with a closed NBS DNA substrate in a CTP-dependent manner
To further investigate the roles of the NTD engagement, we followed the spreading of Noc in real time. We used a 170 bp dual biotin-labeled NBS DNA that had been tethered at both ends onto a streptavidin-coated probe to form a closed DNA and measured the bio-layer interferometry (BLI) signal (Figures 2E and 2F; Jalal et al., 2020a). BLI monitors wavelength shifts resulting from changes in the optical thickness of the probe during the association and dissociation of Noc from the closed NBS DNA substrate. In the absence of CTP, we observed only the nucleation event on NBS DNA with 1 μM purified Noc (Figure 2E). Premixing Noc with ATP, GTP, or UTP did not change the sensorgram markedly; however, the addition of 1 mM CTP increased the BLI response by ∼6-fold (Figure 2E), consistent with Noc-CTP spreading from the NBS to accumulate more on the 170 bp closed DNA substrate than by nucleation alone. We did not observe a noticeable BLI response when a 170 bp closed parS DNA substrate was used instead (Figure 2E), confirming that nucleation and spreading by B. subtilis Noc is strictly dependent on the NBS. We also observed that DNA-bound Noc-CTP dissociated readily into the solution when the BLI probe was returned to a protein-free buffer without CTP (Figure 2E; Figure S2F, dissociation phase). However, the dissociation of pre-bound Noc-CTP from DNA was slowed by ∼5-fold if the probe was returned to a buffer supplemented with CTP (Figure S2F). Furthermore, we observed that pre-bound Noc-CTPɣS was more stable and dissociated slowly into a buffer only solution (Figure S2G).
We then tested the mutant proteins by BLI assay and observed that both the Noc (R89A) and Noc (N121S) variants could nucleate but could not spread to accumulate on the closed NBS substrate even in the presence of 1 mM CTP (Figure 2E), suggesting that NTD engagement is required for spreading (see also Figures 2C and 2D).
Next, we investigated whether a DNA substrate with a free end (an open DNA) could also support Noc accumulation in our BLI setup. The 170 bp dual biotin-labeled DNA was designed with a unique BamHI recognition site flanking the NBS site (Figure 2F). To generate a free end on the DNA, the DNA-coated probe was immersed in buffer containing BamHI restriction enzyme. Before BamHI digestion, Noc showed an enhanced association on a closed DNA substrate in the presence of CTP (Figure 2E). However, after BamHI digestion, the addition of CTP did not affect the BLI response beyond the nucleation of Noc at the NBS (Figure 2F). We reasoned that, similar to the canonical ParB clamp (Jalal et al., 2020a), Noc spreads but quickly escapes by sliding off a free DNA end. Overall, our BLI analyses support the idea of a clamp-like Noc-CTP that can spread and accumulate on a closed DNA substrate, most likely by entrapping DNA.
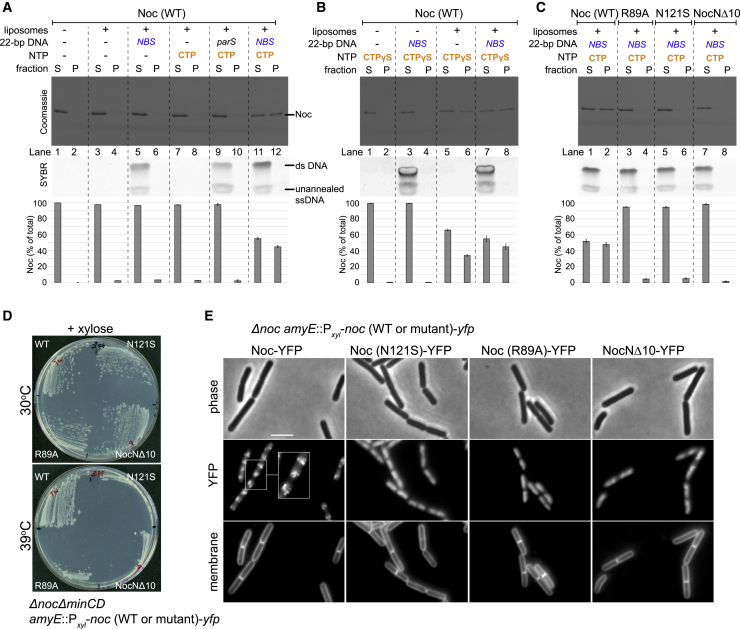
Noc binds liposomes in the presence of CTP
It has been shown previously in vivo that Noc possesses membrane-binding activity and that it brings chromosomal DNA to the cell membrane to prevent cell division (Adams et al., 2015). Puzzlingly, however, we could not observe any noticeable association between purified B. subtilis Noc and liposomes by a co-sedimentation assay (Figure 3A, lanes 1–4). We wondered if CTP might be the missing co-factor that activates the membrane-binding activity of Noc. To test this possibility, purified Noc was incubated with liposomes with or without CTP and 22 bp NBS DNA and ultracentrifuged (Figure 3A; Figure S3A). The pellet contained sedimented liposomes and associating protein, while the supernatant contained unbound protein; protein and DNA species from both fractions were analyzed by polyacrylamide gel electrophoresis. We did not observe a significant increase in the amount of Noc in the pellet when CTP alone, CTP and a non-cognate 22 bp parS DNA (Figure 3A, lanes 5–10), or other nucleotides were used (Figure S3B). However, in the presence of both CTP and a 22 bp NBS DNA, ∼45% of the Noc protein was detected robustly in the pellet (Figure 3A, lane 11 and 12), suggesting that the in vitro membrane-binding activity of Noc is CTP and NBS dependent. NBS DNA is most likely required to promote CTP binding and the membrane-binding activity of Noc, rather than to concentrate a large amount of Noc molecules in the vicinity of NBS. Supporting this proposition, the short length of a 22 bp NBS DNA duplex should allow only a dimer of Noc or Noc-CTP complex to occupy the DNA at a time. Furthermore, nearly all of the 22 bp NBS DNA was present in the supernatant (instead of in the pellet) after centrifugation (Figure 3A), most likely because Noc-CTP clamps rapidly escaped the open linear NBS DNA (see also Figure 2F). This result suggests that individual Noc-CTP possesses a substantial membrane-binding capability.
Figure 3.
Noc binds liposomes in the presence of CTP and NBS DNA, and the phenotypic effects of the Noc variants
(A) Analysis of B. subtilis Noc binding to membranes by a liposome co-sedimentation assay. A premix of 0.75 μM B. subtilis Noc protein ± 1.0 μM 22 bp linear parS/NBS DNA ± 1.0 mM CTP ± 1.0 mg/mL liposomes was incubated at 22°C for 5 min before ultracentrifugation. The resulting supernatant (S) and pellet (P) fractions were analyzed using SDS-PAGE. Samples were also loaded onto a 20% TBE PAGE, and the gel was subsequently stained with Sybr Green for DNA. Quantification of Noc in each fraction is shown below each representative image. Error bars represent SEM from three replicates.
(B) Same as (A), but 1.0 mM CTPɣS was used instead.
(C) Other Noc variants, Noc (R89A), Noc (N121S), and NocNΔ10, were also analyzed in a liposome co-sedimentation assay.
(D) Complementation of noc in a ΔnocΔminCD background. Strains ΔnocΔminCD amyE::Pxyl-noc (WT or mutant)-yfp were streaked on nutrient agar plates supplemented with 0.5% xylose and incubated at 30°C or 39°C.
(E) Cellular localization of YFP-labeled Noc (WT or mutants). Representative images of Δnoc amyE::Pxyl-noc (WT or mutant)-yfp cells grown in the presence of 0.5% xylose. Cell membranes were stained with FM5-95. Scale bar, 3 μm. Inset shows a magnification of a section of cells.
Next, we observed that the addition of CTPɣS alone caused ∼35% of Noc to associate with the pelleted vesicles (Figure 3B, lanes 5 and 6). The vesicle-bound fraction further increased to ∼45% when both CTPɣS and a 22 bp NBS DNA were present (Figure 3B, lanes 7 and 8). We infer that CTP binding, but not hydrolysis, is required for the in vitro Noc-liposome interaction. Consistent with the requirement of CTP binding for membrane-binding activity, the Noc (R89A) and Noc (N121S) variants that do not bind CTP or bind CTP weakly failed to co-sediment with liposomes even when CTP and NBS were included (Figure 3C, lanes 3 and 4 and lanes 5 and 6). The 10 AA N-terminal peptide was previously shown in vivo to be the membrane-targeting determinant of B. subtilis Noc (Adams et al., 2015). Here, we also confirmed that a purified NocNΔ10 lacking this segment was unable to co-sediment with liposomes in vitro regardless of the presence or absence of CTP or NBS DNA (Figure 3C, lanes 7 and 8). Last, in another control experiment, C. crescentus ParB, which binds CTP but not the cell membrane (Jalal et al., 2020a; Lim et al., 2014; Toro et al., 2008), did not co-sediment with liposomes in the presence of CTP ± parS or NBS DNA (Figure S3C).
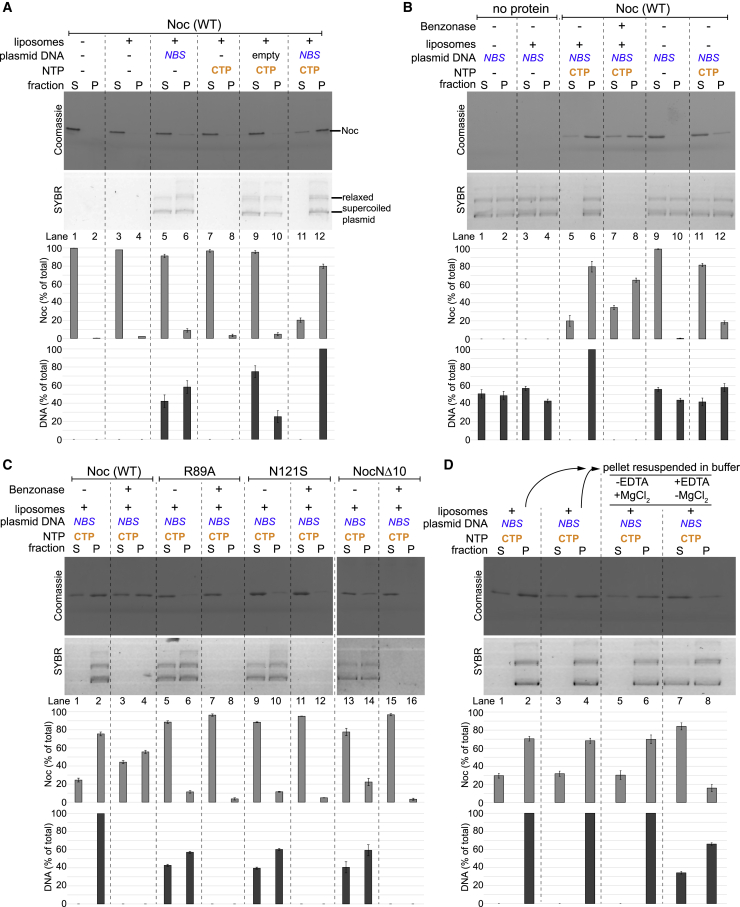
Noc recruits NBS plasmid to liposomes in the presence of CTP
The recruitment of chromosomal DNA to the membrane is essential for Noc to exert nucleoid occlusion activity in vivo (Adams et al., 2015). Indeed, ectopic expression of noc (R89A), noc (N121S), or nocNΔ10 could not rescue the synthetic cell division defect of a B. subtilis ΔnocΔminCD double mutant at elevated temperature (Figure 3D; Adams et al., 2015). Epi-fluorescence microscopy of B. subtilis cells harboring yfp-tagged noc mutant alleles also confirmed that Noc (R89A), Noc (N121S), and NocNΔ10 failed to form punctate foci near the cell periphery (i.e., were defective in the formation of large membrane-associated nucleoprotein complexes) (Figure 3E; Adams et al., 2015). We wondered if the Noc-dependent recruitment of DNA to the membrane could be biochemically reconstituted. To this end, we assembled a reaction containing purified Noc, liposomes, CTP, and a ∼5 kb circular NBS-harboring plasmid before ultracentrifugation (Figure 4). Unlike the 22 bp NBS DNA, the NBS plasmid is topologically closed and therefore should robustly retain closed Noc-CTP clamps. Unfortunately, because of its high molecular weight, ∼45%–55% of the circular plasmid sedimented independently of the liposomes (Figure 4A, lanes 5 and 6; Figure 4B, lanes 1–4). Nevertheless, in the presence of liposomes and CTP, the NBS plasmid completely co-sedimented with Noc (Figure 4A, lanes 11 and 12), demonstrating that Noc can recruit plasmid DNA to liposomes in the presence of CTP. The pellet/supernatant distribution of a control plasmid with no NBS (“empty”) was unaffected by the presence of Noc and CTP, and only ∼2% of Noc was found in the pellet (Figure 4A, lanes 9 and 10). Next, in an attempt to minimize the sedimentation of a plasmid by itself, we performed a vesicle flotation assay in which liposomes and associating protein/DNA migrate up a sucrose gradient to the topmost faction rather than down into the pellet (Figure S4A). Despite the basal level of ∼20% total NBS plasmid in the top fraction even when liposomes were omitted (Figure S4B, lane 3), we again observed ∼80% of total NBS plasmid being recruited to the liposomes when purified Noc and CTP were also present (Figure S4C, lane 12).
Figure 4.
Noc recruits NBS plasmid to liposomes in the presence of CTP
(A) Analysis of B. subtilis Noc binding to membranes and the recruitment of plasmid DNA to the membranes by a liposome co-sedimentation assay. A premix of 0.75 μM B. subtilis Noc protein ± 100 nM 5-kb plasmid DNA ± 1.0 mM CTP ± 1.0 mg/mL liposomes was incubated at 22°C for 5 min before ultracentrifugation. Either an empty plasmid or an NBS-harboring plasmid was used in this assay. The resulting supernatant (S) and pellet (P) fractions were analyzed using SDS-PAGE. Samples were also loaded onto a 1% agarose gel and were subsequently stained with Sybr Green for DNA. Quantification of Noc or DNA in each fraction is shown below each representative image. Error bars represent SEM from three replicates.
(B) Similar to (A), a premix of 100 nM NBS plasmid ± 0.75 μM Noc ± 1.0 mM CTP ± 1.0 mg/mL liposomes was first assembled and incubated at 22°C for 5 min. However, before ultracentrifugation, a non-specific DNA nuclease (Benzonase) was either added or omitted from the samples, as indicated.
(C) Other Noc variants, Noc (R89A), Noc (N121S), and NocNΔ10, were also analyzed in a liposome co-sedimentation assay. Benzonase was either added or omitted, as indicated, before ultracentrifugation.
(D) The association of Noc-NBS DNA with liposomes is reversible. A premix of 0.75 μM B. subtilis Noc protein + 100 nM NBS plasmid + 1.0 mM CTP + 1.0 mg/mL liposomes was ultracentrifuged, and the resulting fractions were analyzed for protein and DNA contents (lanes 1 and 2 and lanes 3 and 4). The resulting pellets (lanes 2 and 4) were subsequently resuspended in either a binding buffer (− EDTA + 1 mM MgCl2) or a stripping buffer (+ 10 mM EDTA − MgCl2). The resuspensions were ultracentrifuged for the second time, and the resulting fractions were analyzed for protein and DNA contents (lanes 5 and 6 and lanes 7 and 8).
In light of the above results, we wondered if the role of NBS was to stimulate the membrane-binding activity of Noc-CTP. To test this possibility, we first assembled a co-sedimentation reaction as described above. Subsequently, a non-specific DNase (Benzonase) was added to eliminate the NBS plasmid before ultracentrifugation (Figure 4B, lanes 7 and 8). The nuclease treatment eliminated intact NBS plasmid from both the supernatant and the pellet fractions; however, ∼65% of the total amount of Noc still co-sedimented to the pellet in comparison with ∼80% when nuclease was omitted (Figure 4A, lanes 7 and 8 versus lanes 5 and 6). In parallel, we tested spreading-defective Noc (R89A) and Noc (N121S), and a membrane-binding-defective NocNΔ10 for their ability to recruit the NBS plasmid to liposomes in a co-sedimentation assay (Figure 4C) as well as in a flotation assay (Figure S4D). Consistent with previous and the above in vivo data (Adams et al., 2015; Figure 3E), these mutants could not recruit DNA to the pellet fraction beyond the basal level (Figure 4C; Figure S4D). Altogether, these results suggest that the NBS specifically activates the membrane-binding activity of Noc in the presence of CTP, thereby recruiting Noc-DNA complexes to the membrane.
The association of Noc-NBS DNA with liposomes is reversible
Once the membrane-associated Noc-DNA nucleoprotein complexes form, can this process be reversed? To investigate, we used a buffer supplemented with EDTA to sequester Mg2+, thereby disrupting CTP binding in preformed liposome-bound Noc-DNA complexes (Figure 4D). In this experiment, the pellet containing preformed liposome-bound Noc-DNA complexes (Figure 4D, lane 2 or 4) was either resuspended in EDTA-minus or EDTA-plus buffer before being ultracentrifuged again. After the second round of centrifugation, both the pellet and the supernatant fractions were analyzed for the presence of protein and DNA (Figure 4D, lanes 5–8). We observed that although nearly all NBS plasmid remained in the pellet when an EDTA-minus buffer was used (Figure 4D, lane 6), ∼38% of the total plasmid returned to the supernatant in the presence of EDTA (Figure 4D, lane 7). These results demonstrate that the membrane-binding activity of Noc can be reversed and suggest a possible inhibitory mechanism that keeps apo-Noc in the membrane-inactive mode in the absence of CTP.
Crystal structure of Geobacillus thermoleovorans NocΔCTD shows the membrane-targeting amphipathic helix in an autoinhibitory conformation
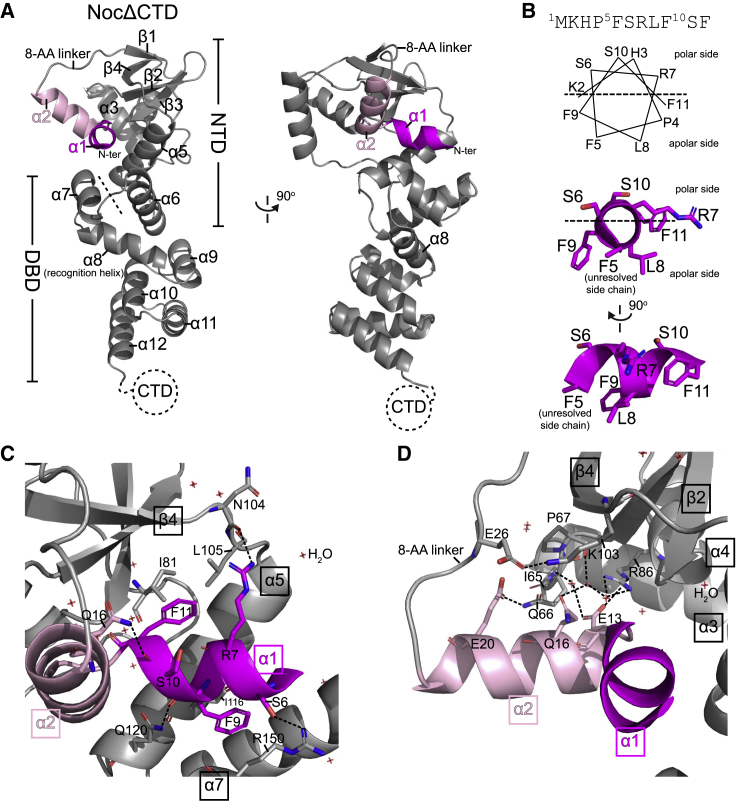
To gain further insights into the membrane-inactive state, we sought to solve a crystal structure of Noc. We could not obtain high-quality crystals of B. subtilis Noc either in full-length or truncated forms despite extensive efforts. However, we could grow and collect diffraction data for a C-terminal domain truncated apo-Noc (NocΔCTD) from a thermophilic bacterium, Geobacillus thermoleovorans, to 2.5 Å resolution. B. subtilis Noc and G. thermoleovorans Noc share 72% sequence identity (see the sequence alignment in Figure S5A). The structure was solved by iodide SAD phasing, as no other Noc protein family structure was available as a template for molecular replacement. The asymmetric unit contains two similar copies of monomeric apo-Noc (root-mean-square deviation [RMSD] = 0.9 Å) (Figure S5B), hence we used the more complete subunit for all further analysis.
Each NocΔCTD subunit contains an NTD (helices α1–α6) and an NBS-specific DNA-binding domain (DBD) (helices α7–α12) (Figure 5A; Jalal et al., 2020b; Wu et al., 2009). The primary dimerization domain at the C-terminal side of Noc was truncated in the NocΔCTD and hence was not present in this structure. Most notably, electron density for five of the 10 AA comprising the membrane-targeting helix α1 were visible in a 310 helical conformation (Figures 5A and 5B). From the structure, it is apparent that the visible membrane-targeting sequence (AA 5–10) of Noc is indeed amphipathic, with distinct polar and hydrophobic faces (Figure 5B). The amphipathic helix α1 is immediately followed by helix α2 and subsequently by an 8 AA α2-β1 loop that precedes the main NTD (Figure 5A). By sequence comparison with a canonical ParB (Soh et al., 2019), the main NTD (β1-α6) of Noc contains the CTP-binding motifs, while the amphipathic α1, α2, and the α2-β1 loop are specific to the Noc protein family (Figure S5A). We noted that the hydrophobic face of the amphipathic α1 helix is buried toward α5 and α7 at the core of Noc (Figures 5A and 5C) and thus is unexposed and unlikely to be available for membrane interaction. Specifically, the side chain of S6 hydrogen bonds with the side chain of R150, and the side chain of R7 hydrogen bonds with the main chain oxygen of N104 (Figure 5C). Additionally, the main chain oxygen of S10 hydrogen bonds with the side chain of Q16, and last, the main chain oxygen of F11 hydrogen bonds with the side chain of Q120 (Figure 5C). Sidechains of F9 and F11 also interact hydrophobically with the side chains of I116 and I81, respectively (Figure 5C). These interactions thus bury α1 in a potential autoinhibitory conformation. We further noted that α2, which does not target the membrane per se but is conserved among Noc homologs (Figure S5A; Adams et al., 2015), also contributes to holding α1 in the repressed state (Figure 5D). Specifically, the side chains of both E13 and Q16 form water-mediated contacts with the side chains of I65, R86, and K103, while the side chain of E20 forms a hydrogen bond with Q66 (Figure 5D). Overall, our apo-NocΔCTD structure suggests a repressed state that might keep Noc in the membrane-inactive state in the absence of CTP.
Figure 5.
Crystal structure of Geobacillus thermoleovorans NocΔCTD shows the membrane-targeting amphipathic helix in an autoinhibitory conformation
(A) Crystal structure of a G. thermoleovorans NocΔCTD monomer (gray) with an N-terminal amphipathic helix α1 (magenta) and helix α2 (pink). Helix α2 is connected to the main N-terminal domain (NTD) via an 8 amino acid loop. The dashed line demarcates the NTD from the DNA-binding domain (DBD). Helix α8 at the DBD is the recognition helix that contributes to the specific recognition of the NBS site (Jalal et al., 2020b; Wu et al., 2009).
(B) The membrane-targeting amphipathic helix α1. A helical wheel representation of the 10 amino acid at the N terminus of G. thermoleovorans Noc. Although the first 5 amino acids were unresolved in the NocΔCTD crystal structure, the next 5 amino acids adopt a 310 helical conformation with distinct polar and hydrophobic sides.
(C and D) Helices α1 (magenta) and α2 (pink) pack themselves into the core N-terminal domain (gray). Hydrogen bonds are shown as dashed lines, water molecules are also shown.
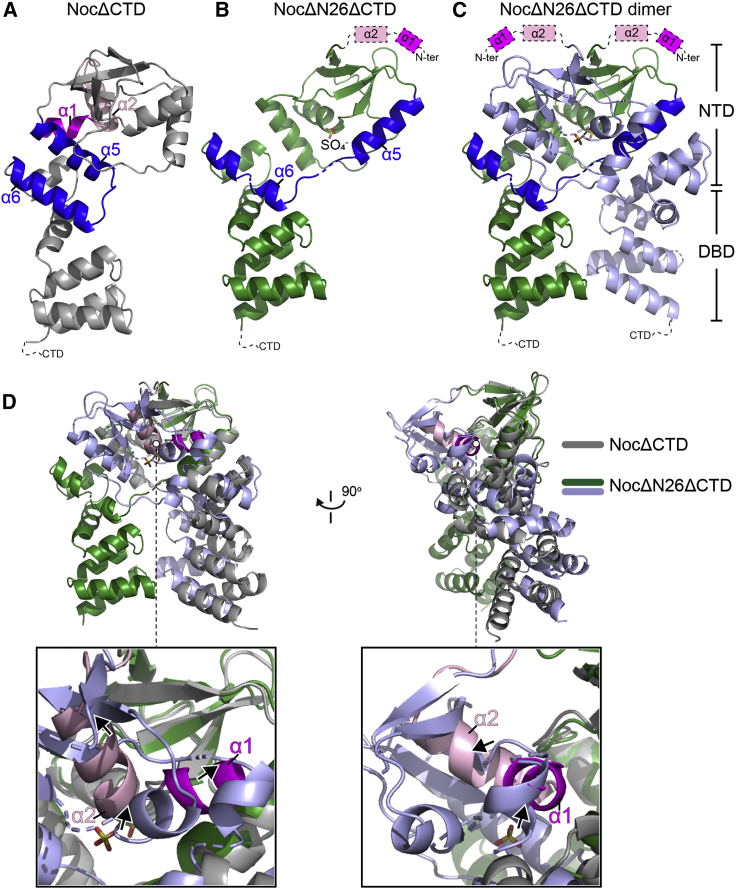
Crystal structure of the G. thermoleovorans NocNΔ26ΔCTD variant is incompatible with an autoinhibitory conformation of the amphipathic helix
Next, we attempted to obtain a co-crystal structure of Noc in complex with nucleotides but were not successful when NocΔCTD or NocNΔ10ΔCTD protein variants were used. However, in the presence of CTPɣS, we were able to grow and collect 2.95 Å diffraction data for a crystal of a further truncated G. thermoleovorans NocNΔ26ΔCTD variant, which lacks both the N-terminal membrane-targeting helix and the C-terminal domain. After solving its structure, it was apparent that NocNΔ26ΔCTD had adopted an alternative conformation to that of NocΔCTD (Figure 6A versus Figures 6B and 6C). This alternative conformation of NocΔN26ΔCTD is compatible with homodimer formation, giving an interfacial area of ∼2,700 Å2 (as evaluated with jsPISA), which resembles that observed for a co-crystal structure of B. subtilis ParBΔCTD with bound cytidine diphosphate (CDP) (RMSD = 2.17 Å) (Figure S6A; Soh et al., 2019). However, there was no clear electron density for a bound nucleotide in our structure; instead a sulfate anion from the crystallization solution occupies a position equivalent to that of the β-phosphate of the nucleotide in the B. subtilis ParBΔCTD-CDP structure (Figure S6B; Soh et al., 2019). We further observed that helix α5 in the NocΔN26ΔCTD structure swings outward by 104° and no longer forms a bundle with helix α6 from the same subunit (Figure 6A versus Figure 6B) and that this movement might drive the self-dimerization at the NTD of Noc (i.e., the NTD engagement) (Figure 6C). By superimposing the NTDs of NocΔCTD and NocNΔ26ΔCTD (RMSD = 1.49 Å), we detected severe clashes between α1, α2, and the opposite subunit of NocNΔ26ΔCTD (Figure 6D; Figures S6C and S6D). Therefore, it is clear that the autoinhibitory state of α1 and α2 (as observed in NocΔCTD) is not compatible with the alternative conformation in the NocNΔ26ΔCTD structure. We speculate that the amphipathic helix α1 and helix α2 might be liberated from the autoinhibitory conformation to be compatible with the NTD-engagement conformation in the NocNΔ26ΔCTD structure.
Figure 6.
The conformation of Noc in the crystal structure of G. thermoleovorans NocNΔ26ΔCTD is incompatible with an autoinhibitory state of the amphipathic helix
(A) Crystal structure of G. thermoleovorans NocΔCTD (same as Figure 5A), with helices α5 and α6 highlighted in blue. The amphipathic helix α1 and α2 are shown in magenta and pink, respectively.
(B) Crystal structure of a G. thermoleovorans NocNΔ26ΔCTD variant that lacks both the C-terminal domain (CTD) and helices α1 and α2 (dashed boxes). Helices α5 and α6 are shown in blue.
(C) A dimer of G. thermoleovorans NocNΔ26ΔCTD that self-dimerizes at the N-terminal domain (NTD). Helices α5 and α6 in one of the subunits are shown in blue.
(D) A superimposition at the NTDs of NocΔCTD monomer (gray) and NocNΔ26ΔCTD dimer (green and light blue) shows a severe clash (arrows) between α1 (magenta), α2 (pink), and the opposite subunit of NocNΔ26ΔCTD (light blue).
Last, we overexpressed and purified six B. subtilis Noc variants to investigate the effects of N-terminal deletions and substitutions on the membrane-binding ability (Figure S7). Removing a lysine residue at position 2 (NocΔK2) or the first 4 AA (NocΔ4) had a mild effect on the membrane-binding activity of Noc, as judged by liposome co-sedimentation assays (Figure S7, lanes 3 and 4 and lanes 7 and 8). However, hydrophobicity-reducing substitutions such as K2E, F5A, and F5E had a negative effect on Noc-liposome binding (Figure S7, lanes 5 and 6, lanes 9 and 10, and lanes 11 and 12). In contrast, when the hydrophobicity of the first 4 AA segment was increased, as in the S4L variant, Noc (S4L) binding to the liposomes increased compared with Noc (WT) (Figure S7, lanes 13 and 14). Overall, although the conformation of the N-terminal 10 AA sequence of Noc in a membrane-bound state is not yet known, our results suggest that the properties of these amino acids are fine-tuned for membrane-binding activity.
Discussion
Assembly of the membrane-associated Noc-DNA nucleoprotein complex: Roles of CTP
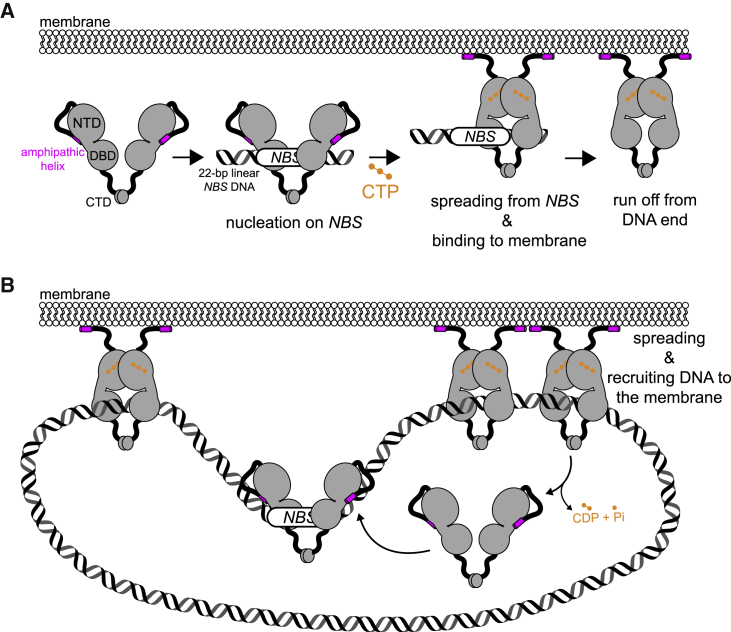
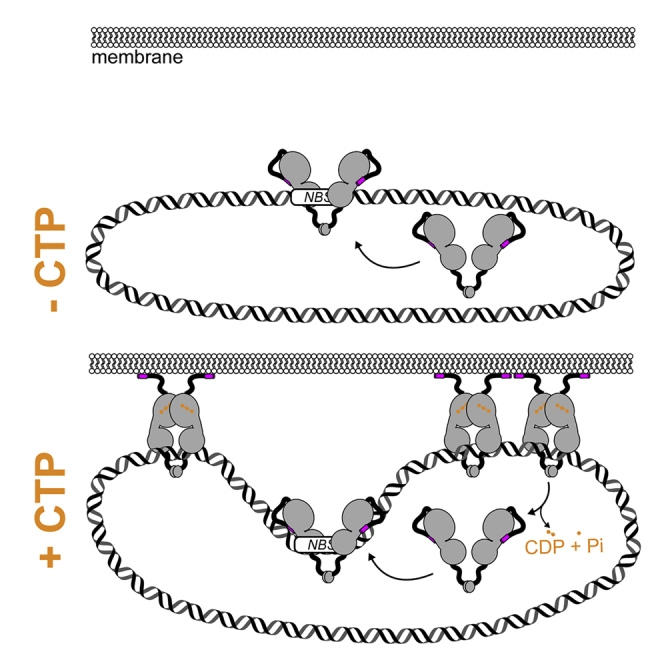
The nucleoid occlusion protein Noc increases cell division efficiency (Rodrigues and Harry, 2012) by directing the division machinery toward mid-cell either by inhibiting FtsZ formation over the nucleoid (Adams et al., 2015; Wu and Errington, 2004) and/or by concentrating FtsZ in the vicinity of a pre-existing mid-cell Z-ring (Yu et al., 2021). The extensive Noc-mediated DNA-membrane interaction is at the heart of both models for nucleoid occlusion (Adams et al., 2015; Yu et al., 2021). In this study, we show that CTP regulates the nucleoid occlusion activity of Noc. We provide evidence that (1) CTP is required for Noc to form the NBS-dependent nucleoprotein complex, and (2) CTP binding switches Noc from a membrane-inactive auto-repressed state to a membrane-active state. We propose that the dual dependency of Noc’s membrane-binding activity on NBS and CTP might ensure productive recruitment of DNA to the bacterial cell membrane to exert the nucleoid occlusion activity (Figure 7). It has been estimated that the intracellular concentrations of Noc and CTP are ∼5 μM and ∼1 mM, respectively (Buckstein et al., 2008; Wu et al., 2009). At these concentrations, if the membrane-binding activity of Noc were solely dependent on CTP, most intracellular DNA-unbound Noc would be in the CTP-bound state and confined to the cell membrane, thus potentially limiting the recruitment of chromosomal DNA to the cell membrane. We therefore reason that the NBS-stimulated Noc-CTP interaction provides a mechanism to commit Noc into a pathway in which only DNA-entrapped Noc molecules are able to associate with the cell membrane (Figure 7B). Another consequence of coupling membrane activity to NBS binding is that membrane-associated nucleoprotein complexes are spatially confined near the vicinity of NBS sites. This spatial confinement is important for directing division machinery formation toward mid-cell, where the concentration of NBS sites, hence the nucleoid occlusion activity, is lowest (Wu et al., 2009).
Figure 7.
A model for a CTP-dependent regulation of membrane-binding activity of Noc
(A) Noc binds specifically to NBS site to nucleate on DNA. In the apo- or NBS-associated form, the amphipathic helix (magenta) adopts an autoinhibitory conformation and thus cannot bind to the membrane. NBS-binding stimulates Noc to bind CTP (orange). Concomitantly, CTP induces a sliding clamp conformation in Noc that can run off open ends of a linear 22 bp NBS DNA. In this state, the amphipathic helix is likely liberated from the autoinhibitory conformation, thus enables Noc-CTP to bind to the membrane.
(B) When a circular NBS plasmid with no open end was used, a sliding clamp of Noc entraps plasmid DNA and recruit DNA to the membrane. In the presence of CTP, a tripartite membrane-protein-DNA linkage is formed. CTP hydrolysis is not required for membrane binding or DNA recruitment but might have a role in releasing Noc from DNA and the membrane.
The lack of a bound nucleotide in the NocNΔ26ΔCTD structure prevents us from drawing a conclusion on the role of CTP and the possible conformational liberation of the amphipathic helix α1. However, several lines of evidence suggest that CTP might favor the NTD-engagement conformation as observed in our NocNΔ26ΔCTD structure: (1) CTP/CTPɣS promoted the crosslinking between symmetrical E29C residues, a readout for NTD engagement (Figure 2A); (2) the conformation of NocNΔ26ΔCTD is highly similar to that of a nucleotide-bound B. subtilis ParB (Figure S6A; Soh et al., 2019); and (3) CTP/CTPɣS enables Noc (WT) to co-sediment with liposomes (Figure 3). Altogether, it is not unreasonable to speculate that the CTP-induced NTD engagement might liberate the amphipathic helix from its autoinhibitory state to interact with the cell membrane. The 8 AA loop that connects the amphipathic helix to the rest of Noc might offer the flexibility to orient the amphipathic helix parallel to the membrane plane for binding (Figure 7).
This study also provides evidence that B. subtilis Noc possesses CTPase activity, but CTP hydrolysis is required for neither clamp formation nor membrane association. What might the role of CTP hydrolysis be? Similar to the counterpart ParB (Jalal et al., 2020a; Osorio-Valeriano et al., 2019; Soh et al., 2019), CTP hydrolysis and/or the subsequent release of hydrolytic products might disengage the NTD to open the clamp to release entrapped DNA (Figure 7B). Concomitantly, Noc might revert to the membrane-inactive state, enabling its extraction from the cell membrane. Our experiment that used EDTA to sequester coordinated Mg2+ to artificially promote the dissociation of CTP from Noc supports the proposal that membrane association can be reversible (Figure 4D). Furthermore, B. subtilis Noc foci associate with the cell membrane in a transient manner, perhaps suggesting a weak and fast on/off membrane interaction in vivo (Adams et al., 2015). The transient association with the membrane, possibly endowed by a CTP hydrolysis event that facilitates the release of Noc, might be advantageous for the cells because a strong and permanent mode of binding might hamper chromosome replication or damage DNA. Supporting this view, fusing NocNΔ10 to a synthetic transmembrane helix led to broken, bisected chromosomes and eventually chromosome segregation defects in many B. subtilis cells (Adams et al., 2015; Nyholm et al., 2007). Last, we noted that NocNΔ10 binds CTP equally well as, or slightly stronger than Noc (WT) (Figure 1D), yet its CTPase rate is reduced by half (Figure 1E). It is still unclear why this is the case; however, we speculate that the autoinhibitory conformation of this N-terminal-most region might play a role in CTP binding/hydrolysis. Lending support to our speculation, this N-terminal-most region interacts with residues Q66 and R86 (apo-NocΔCTD structure; Figure 5D) whose equivalent residues in B. subtilis ParB are known to be critical for CTP binding (Soh et al., 2019).
Overall, we envision a dynamic cycle inside the cells in which CTP hydrolysis converts a closed clamp Noc-CTP to Noc-CDP. The CDP-bound Noc might exist very briefly, as CDP likely dissociates rapidly from Noc (because of its low affinity to Noc; Figure S1B), thus opening the clamp to release entrapped Noc from the chromosome and the membrane (Figure 7B). The resulting apo-Noc might also be short-lived because it quickly re-nucleates at NBS sites (because of its nanomolar binding affinity to NBS; Jalal et al., 2020b); nucleating Noc at NBS then rebinds CTP to close the clamp to spread and to rebind to the cell membrane, thus essentially restarting the cycle.
The expanding roles of CTP switches in biology
ATP and GTP switches that control membrane activity are widespread in all domains of life. For example, ATP binding promotes the dimerization of MinD (role in bacterial cell division site selection) and concomitantly increases its affinity for the cell membrane (Hu and Lutkenhaus, 2003; Hu et al., 2002; Ramm et al., 2019; Wu et al., 2011). MinE, a partner of MinD, stimulates the ATPase activity of MinD, promoting MinD dissociation from the membrane (Hu et al., 2002; Lutkenhaus, 2012; Park et al., 2012; Ramm et al., 2019). In eukaryotes, both the Ras-related protein (Sar) and ADP-ribosylation factor (Arf) (role in vesicle trafficking) function as GTP-dependent switches, cycling between the active GTP-bound form and the inactive GDP-bound form (Beck et al., 2008; Bielli et al., 2005; Bos et al., 2007; Dodonova et al., 2017; Hanna et al., 2016; Krauss et al., 2008; Lee et al., 2005; Zhukovsky et al., 2019). In the GDP-bound form, the amphipathic helix of Sar/Arf1 adopts a repressed conformation by burying itself into a hydrophobic pocket (Goldberg, 1998; Zhukovsky et al., 2019). The exchange of GDP for GTP induces conformational changes that push the myristoylated amphipathic helix out of the hydrophobic pocket, enabling membrane association (Goldberg, 1998; Zhukovsky et al., 2019). Our study provides the first evidence for a CTP switch that controls membrane-binding activity (Figure 7), suggesting that CTP switches are likely to control more diverse functions in biology than previously appreciated.
Limitations of the study
Several unanswered questions warrant future investigations. First, the conformation (or conformations) of the 10 AA membrane-targeting sequence in a membrane-bound state of Noc-CTP has not been resolved. Second, it is unknown how the Noc-NBS DNA binding event mechanistically promotes CTP binding and the subsequent NTD engagement. Relatedly, how a non-hydrolyzable CTP analog (CTPɣS) can bypass the requirement for NBS DNA to bind to Noc is also not yet clear. Solving a co-crystal structure of a Noc-NBS DNA complex; studying the in-solution dynamics of clamp opening and closing in the presence or absence of NBS, CTP, or CTPɣS; and the availability of new types of non-hydrolyzable CTP analogs will be required to answer these questions.
Final perspectives
Our work unveils a nucleotide-dependent regulatory layer, in addition to the previously described DNA-dependent regulation (Adams et al., 2015), in the activity of the nucleoid occlusion protein Noc. The dual dependency on nucleotide and DNA guarantees a productive formation of the tripartite DNA-protein-membrane super-complex for nucleoid occlusion while allowing efficient cycling of Noc between the membrane-bound and unbound states. In this work, we also provide evidence for a CTP switch that controls membrane-binding activity, adding the control of membrane association in Noc to the role of ParB-CTP in bacterial chromosome segregation. It is likely that CTP switches are pervasive in biology but have so far been underappreciated (Basu and Koonin, 2005; Osorio-Valeriano et al., 2019; Soh et al., 2019). Finally, evolution is replete with examples of functional domains being adapted to diversify the functions of proteins. The gene encoding Noc apparently resulted from duplication and neo-functionalization of parB (Jalal et al., 2020b; Sievers et al., 2002; Wu and Errington, 2011), our work furthers the understanding of how a CTP switch has been adapted to a new function, and hence might have important implications in understanding biological innovations by evolution.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Please refer to Table S1 | N/A | |
| Chemicals, peptides, and recombinant proteins | ||
| cOmplete Mini EDTA-free Protease Inhibitor Cocktail | Roche Applied Science | Cat# 11836170001 |
| BamHI | NEB | Cat# R0136S |
| Bismaleimidoethane (BMOE) | ThermoFisher | Cat# 22323 |
| CTPγS | Jena Bioscience and in-house synthesis (Rejzek and Le, 2021) | N/A |
| E. coli total lipid extract | Avanti/Merck | Cat# 100500C-100MG |
| FM 5-95 | ThermoFisher | Cat# T23360 |
| NTP | ThermoFisher | Cat# R0481 |
| P32-α-CTP | Perkin Elmer | Cat# BLU008H250UC |
| Critical commercial assays | ||
| Amersham Protran supported Western blotting membranes, nitrocellulose | GE Healthcare | Cat# GE10600016 |
| Dip-and-Read Streptavidin (SA) biosensors | Molecular Devices | Cat# 18-5019 |
| EnzChek Phosphate Assay Kit | ThermoFisher | Cat# E6646 |
| Gateway BP Clonase II enzyme mix | ThermoFisher | Cat# 11789020 |
| Gibson Assembly Master Mix | NEB | Cat# E2611S |
| HiLoad 16/600 Superdex 75pg column | GE Healthcare | Cat# GE28989333 |
| HIS-Select Cobalt Affinity Gel | Sigma-Aldrich | Cat# H8162 |
| HisTrap High Performance column | GE Healthcare | Cat# GE17524801 |
| HiTrap Heparin High Performance column | GE Healthcare | Cat# GE17040601 |
| PD 10 Desalting Columns | Sigma-Aldrich | Cat# GE17085101 |
| Deposited data | ||
| Crystal structures | This study | PDB: 7NFU and PDB: 7NG0 |
| Mendeley data | This study | https://doi.org/10.17632/px2k9hr94c.1 |
| Oligonucleotides | ||
| Please refer to Table S2 | N/A | |
| Recombinant DNA | ||
| Please refer to Table S2 | N/A | |
| Software and algorithms | ||
| AIMLESS | (Evans and Murshudov, 2013) | https://www.ccp4.ac.uk/ |
| BLItz Pro | Molecular Devices | Cat# 50-0156 |
| BUCCANEER | (Cowtan, 2006) | https://www.ccp4.ac.uk/ |
| CCP4i2 | (Potterton et al., 2018) | https://www.ccp4.ac.uk/ |
| COOT | (Emsley and Cowtan, 2004) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| CRANK2 | (Skubák and Pannu, 2013) | https://www.ccp4.ac.uk/ |
| DIALS | (Winter et al., 2018) | https://dials.github.io/ |
| Excel 2016 | Microsoft | RRID: SCR_016137 |
| ImageJ | NIH | https://imagej.net/RRID:SCR_003070 |
| Image Studio Lite | LI-COR Biosciences | RRID: SCR_013715 |
| jsPISA | (Krissinel, 2015) | http://www.ccp4.ac.uk/pisa/ |
| MetaMorph | Molecular Devices | RRID:SCR_002368 |
| MolProbity | (Williams et al., 2018) | http://molprobity.biochem.duke.edu/ |
| PHASER | (McCoy et al., 2007) | https://phenix-online.org/ |
| PyMOL | The PyMOL Molecular Graphics System | https://pymol.org/2/ |
| R | R Foundation for Statistical Computing | https://www.r-project.org/ |
| REFMAC5 | (Murshudov et al., 1997) | https://www.ccp4.ac.uk/ |
| XIA2 | (Winter, 2010) | https://xia2.github.io/index.html |
Resource availability
Lead contact
Questions about or requests for methods, strains, and resources generated in this study can be directed to the Lead Contact, Tung Le (tung.le@jic.ac.uk).
Materials availability
Plasmids and strains generated in this study are available upon request to the Lead Contact.
Data and code availability
-
•
The crystallographic structure of G. thermoleovorans NocΔCTD and G. thermoleovorans NocNΔ26ΔCTD have been deposited in the PDB with accession codes PDB: 7NFU and PDB: 7NG0, respectively. All images and source data presented in figures are available in Mendeley Data at https://data.mendeley.com/datasets/px2k9hr94c/1.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.
Experimental model and subject details
Escherichia coli and B. subtilis were grown in LB and CH medium (Partridge and Errington, 1993), respectively. Carbenicillin (100 μg/mL), chloramphenicol (50 μg/mL), and tetracycline (12 μg/mL) were used for selection in E. coli, as required. Kanamycin (5 μg/mL), spectinomycin (50 μg/mL), and tetracycline (10 μg/mL) were used for selection in B. subtilis, as required. Xylose was added as needed at the concentration indicated.
Method details
Plasmid and strain construction
Construction of pET21b::noc (WT)-his6
A double-stranded DNA (dsDNA) fragment containing a codon-optimized B. subtilis noc gene was chemically synthesized (gBlocks, IDT). The pET21b plasmid backbone was generated via a double digestion of pET21b::Caulobacter crescentus parB-his6 with NdeI and HindIII (Lim et al., 2014). The resulting backbone was subsequently gel-purified and assembled with the noc gBlocks fragment using a 2x Gibson master mix. Briefly, 2.5 μL of the gBlocks fragment and 2.5 μL of NdeI-HindIII-cut pET21b at equimolar concentration were added to 5 μL of a 2x Gibson master mix (NEB). The mixture was incubated at 50°C for 60 min. Subsequently, 5 μL was used to transform chemically competent E. coli DH5α cells. Gibson assembly was possible owing to a 23-bp sequence shared between the NdeI-HindIII-cut pET21b backbone and the gBlocks fragment. The resulting plasmid was verified by Sanger sequencing (Eurofins, Germany).
Construction of pET21b::noc (Δ2K, Δ4, K2E, S4L, F5A, or F5E)-his6
Same procedure as above, except that dsDNA fragments containing codon-optimized B. subtilis noc (Δ2K, Δ4, K2E, S4L, F5A, or F5E) variants were chemically synthesized instead (gBlocks, IDT).
Construction of pET21b::nocNΔ10-his6
The coding sequence of a 10-amino-acid N-terminally truncated Noc (NocNΔ10) was amplified by PCR using primers AJ65 and AJ66, and pET21b::noc-his6 as a template. The resulting PCR product was gel-purified and assembled into an NdeI-HindIII-cut pET21b using a 2x Gibson master mix. Gibson assembly was possible owing to a 23-bp sequence shared between the NdeI-and-HindIII cut pET21b backbone and the PCR amplified fragment. The 23-bp homologous region was introduced during the synthesis of primers AJ65 and AJ66. The resulting plasmid was verified by Sanger sequencing (Eurofins, Germany).
Construction of pET21b::noc (R89A)-his6
To introduce the R89A mutation into the coding sequence of Noc, primers P3296 and AJ73, and primers P3297 and AJ74 were used in PCR reactions to amplify the left half and the right half of noc (R89A), respectively, from the pET21b::noc-his6 template. A 15-bp overlapping region between the two PCR fragments contained the point mutation and also enabled their assembly by a Gibson master mix. Briefly, 1.7 μL of each PCR-amplified DNA fragment and 1.6 μL of a gel-purified NdeI-HindIII-cut pET21b at equimolar concentration were added to 5 μL of a 2x Gibson master mix. The mixture was incubated at 50°C for 60 min. Subsequently, 5 μL was used to transform chemically competent E. coli DH5α cells. The resulting plasmid was verified by Sanger sequencing (Eurofins, Germany).
Construction of pET21b::noc (N121S)-his6
The same procedure as above was used to introduce the N121S mutation into the coding sequence of Noc, except that primers P3296 and AJ87, and primers P3297 and AJ86 were used to amplify the left half and the right half of noc (N121S), respectively, from the pET21b::noc-his6 template.
Construction of pET21b::noc (E29C)-his6
The same procedure as above was used to introduce the E29C mutation into the coding sequence of Noc, except that primers P3296 and AJ85, and primers P3297 and AJ84 were used to amplify the left half and the right half of noc (E29C), respectively, from the pET21b::noc-his6 template.
Construction of pET21b::noc (E29C R89A)-his6
The same procedure as above was used to introduce the R89A mutation into the coding sequence of Noc (E29C), except that primers P3296 and AJ73, and primers P3297 and AJ74 were used to amplify the left half and the right half of noc (E29C R89A), respectively, from the pET21b::noc (E29C)-his6 template.
Construction of pET21b::noc (E29C N121S)-his6
The same procedure as above was used to introduce the N121S mutation into the coding sequence of Noc (E29C), except that primers P3296 and AJ86, and primers P3297 and AJ87 were used to amplify the left half and the right half of noc (E29C N121S), respectively, from the pET21b::noc (E29C)-his6 template.
Construction of pET21b::Geobacillus thermoleovorans nocΔCTD-his6
A dsDNA fragment containing the coding sequence of a 42-amino-acid C-terminally truncated G. thermoleovorans Noc was chemically synthesized (gBlocks, IDT). The gBlocks fragment was assembled into an NdeI-HindIII-cut pET21b using a 2x Gibson master mix. Gibson assembly was possible owing to a 23-bp sequence shared between the NdeI-HindIII-cut pET21b backbone and the gBlocks fragment. The resulting plasmid was verified by Sanger sequencing (Eurofins, Germany).
Construction of pET21b::Geobacillus thermoleovorans nocNΔ26ΔCTD-his6
The coding sequence of a 26-amino-acid N-terminally truncated and 42-amino-acid C-terminally truncated G. thermoleovorans Noc was amplified by PCR using primers AJ76 and AJ81, and pET21b::Geobacillus thermoleovorans NocΔCTD-his6 as a template. The resulting PCR product was gel-purified and assembled into an NdeI-HindIII-cut pET21b using a 2x Gibson master mix. Gibson assembly was possible owing to a 23-bp sequence shared between the NdeI-and-HindIII cut pET21b backbone and the PCR amplified fragment. The 23-bp homologous region was introduced during the synthesis of primers AJ76 and AJ81. The resulting plasmid was verified by Sanger sequencing (Eurofins, Germany).
Construction of pMCS5-4xNBS
A dsDNA fragment containing four NBS sites were chemically synthesized (gBlocks, IDT). The gBlocks fragment was assembled into an EcoRI-cut pMCS5 (Thanbichler et al., 2007) using a 2x Gibson master mix. Gibson assembly was possible owing to a 23-bp sequence shared between the EcoRI-cut pMCS5 backbone and the gBlocks fragment. The resulting plasmid was verified by Sanger sequencing (Eurofins, Germany).
Construction of pSG4926N121S and B. subtilis strains harboring the noc (N121S)-yfp fusion
The N121S substitution was introduced into the noc(WT)-yfp(mut1) plasmid pSG4926 by site-directed mutagenesis using PFU Turbo DNA polymerase (Agilent Technologies), and primers Noc(N121S)-F and Noc(N121S)-R. After verification of the mutation by DNA sequencing, the mutagenized plasmid (pSG4926N121S) was introduced into the B. subtilis Δnoc mutant (DWA117) by transformation (Jenkinson, 1983) to generate strain 4746. To construct strain 4747, the ΔminCminD deletion was introduced into strain 4746 by transforming strain 4746 with the chromosomal DNA of strain DWA564, with selection for kanamycin resistance. The transformation plates were incubated at 30°C. Note that fusions to YFP(mut1) or mYFP gave a similar localization pattern and were both functional for the wild-type Noc.
DNA preparation for in vitro assays
A 22-bp palindromic single-stranded DNA (ssDNA) oligomer (NBS: GGATATTTCCCGGGAAATATCC or parS: GGATGTTTCACGTGAAACATCC) [dissolved to 100 μM in buffer containing 1 mM Tris-HCl pH 8.0 and 5 mM NaCl] was heated at 98°C for 5 min before being left to cool down to room temperature overnight to form 50 μM parS or NBS DNA duplex. The core sequences of NBS and parS are underlined.
Construction of biotinylated DNA substrates for bio-layer interferometry (BLI) analysis
DNA constructs were chemically synthesized (gBlocks dsDNA fragments, IDT) with M13F and M13R homologous regions at each end. To generate a dual biotin-labeled DNA substrate, PCR reactions were performed using a 2x GoTaq PCR master mix (Promega), biotin-labeled M13F and biotin-labeled M13R primers, and gBlocks fragments as templates. PCR products were gel purified, and subsequently used in BLI assays.
Protein overexpression and purification
Plasmids pET21b::noc-his6 (WT or mutants) were introduced into E. coli Rosetta (DE3) competent cells by heat-shock transformation. A 10-mL overnight culture was used to inoculate 1 L of LB medium + carbenicillin + chloramphenicol. Cells were grown at 37°C with shaking at 250 rpm to an OD600 of ∼0.4. The culture was then left to cool down to 4°C before isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM. The culture was shaken for an additional 3 hr at 28°C before the cells were pelleted by centrifugation. Pelleted cells were resuspended in a buffer containing 100 mM Tris-HCl pH 8.0, 250 mM NaCl, 10 mM imidazole, 5% (v/v) glycerol, 10 mg of lysozyme, and an EDTA-free protease inhibitor tablet (Merck). Cells were lysed by sonication (10 cycles of 15 s with 10 s resting on ice in between each cycle). The cell debris was removed through centrifugation at 28,000 g for 30 min and the supernatant was filtered through a 0.45 μm filter (Sartorius). The lysate was then loaded into a 1-mL HisTrap column (GE Healthcare) that had been equilibrated with buffer A [100 mM Tris-HCl pH 8.0, 250 mM NaCl, 10 mM imidazole, and 5% (v/v) glycerol]. Protein was eluted from the column using an increasing gradient of imidazole (10 mM to 500 mM) in the same buffer. Noc-containing fractions were pooled together and diluted to a conductivity of 16 mS/cm before being loaded onto a 1-mL Heparin HP column (GE Healthcare) that had been equilibrated with 100 mM Tris-HCl pH 8.0, 25 mM NaCl, and 5% (v/v) glycerol. Protein was eluted from the Heparin column using an increasing gradient of NaCl (25 mM to 1 M) in the same buffer. Lastly, proteins were polished using a gel filtration column. To do so, Noc-containing fractions were concentrated using an Amicon Ultra-15 10 kDa cut-off spin filter (Merck) before being loaded onto a Superdex-75 gel filtration column (GE Healthcare). The gel filtration column was pre-equilibrated with buffer containing 10 mM Tris-HCl pH 8.0 and 250 mM NaCl. Eluted protein fractions were analyzed for purity by SDS-PAGE.
Noc variants that were used in crosslinking experiments were purified using a one-step Ni-affinity column, and all buffers were adjusted to pH 7.4 which was optimal for crosslinking reactions. Purified proteins were desalted using a PD-10 column (Merck), concentrated using an Amicon Ultra-4 10 kDa cut-off spin column (Merck), and stored at −80°C in a storage buffer [100 mM Tris-HCl pH 7.4, 250 mM NaCl, 10% (v/v) glycerol, and 1 mM TCEP].
Measurement of NTPase activity by EnzChek phosphate assay
NTP hydrolysis was monitored using an EnzChek Phosphate Assay Kit (Thermo Fisher). Samples (100 μL) containing a reaction buffer ± 0 to 1 mM of CTP ± 1 μM NBS/parS dsDNA + 1 μM of purified Noc (WT or mutants) were assayed in a Biotek EON plate reader for 15 hr with readings every minute. The reaction buffer (1 mL) typically contained 640 μL ultrapure water, 100 μL 10x customized reaction buffer [100 mM Tris pH 8.0, 2 M NaCl, and 20 mM MgCl2], 200 μL MESG substrate solution, and 10 μL purine nucleoside phosphorylase (1 unit). Reactions with buffer only, buffer + protein only or buffer + NTP only were also included as controls. The plates were shaken at 280 rpm continuously for 15 hr at 25°C. The inorganic phosphate standard curve was also constructed according to the manual. Each assay was triplicated. The NTPase rates were calculated using a linear regression fitting in Excel.
In vitro crosslinking using a sulfhydryl-to-sulfhydryl crosslinker bismaleimidoethane (BMOE)
A 50 μL mixture of 10 μM Noc (WT/mutants) ± 1 mM NTP ± 1 μM 22-bp NBS/parS dsDNA was assembled in a reaction buffer [10 mM Tris-HCl pH 7.4, 200 mM NaCl, and 1 mM MgCl2] and was incubated for 10 min at 22°C or for 1, 5, 10, 15, and 30 min at 4°C. Subsequently, BMOE was added to the final concentration of 1 mM, and the reaction was quickly mixed by three pulses of vortexing. SDS-PAGE sample buffer containing 23 mM β-mercaptoethanol was then added immediately to quench the crosslinking reaction. Samples were heated to 50°C for 10 min before being loaded on 12% WedgeWell Tris-Glycine polyacrylamide gels (Thermo Fisher). Each experiment was triplicated. Polyacrylamide gels were stained in an InstantBlue Coomassie solution (Abcam) and band intensity was quantified using Image Studio Lite (LI-COR Biosciences). The crosslinked fractions were averaged, and their standard errors were calculated in Excel.
CTPɣS was custom synthesized either in-house (Rejzek and Le, 2021) or by Jena Biosciences.
Measurement of protein-DNA interactions by bio-layer interferometry (BLI)
Bio-layer interferometry (BLI) experiments were conducted using a BLItz system equipped with Dip-and-Read Streptavidin (SA) Biosensors (Molecular Devices). BLItz monitors wavelength shifts (nm) resulting from changes in the optical thickness of the sensor surface during association or dissociation of the analyte. All BLI experiments were performed at 22°C. Briefly, the streptavidin biosensor was hydrated in a low-salt binding buffer [100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM MgCl2, and 0.005% Tween 20] for at least 10 min before each experiment. Biotinylated double-stranded DNA (dsDNA) was immobilized onto the surface of the SA sensor through a cycle of Baseline (30 s), Association (120 s), and Dissociation (120 s). Briefly, the tip of the biosensor was dipped into a binding buffer for 30 s to establish the baseline, then to 1 μM biotinylated dsDNA for 120 s, and finally to a low salt binding buffer for 120 s to allow for dissociation. After the immobilization of DNA on the sensor, association reactions were monitored at 1 μM dimer concentration of Noc (WT/mutants) (with or without 1mM NTP) for 600 s. At the end of each binding step, the sensor was transferred into a protein-free binding buffer to follow the dissociation kinetics for 600 s. The sensor can be recycled by dipping in a high-salt buffer [100 mM Tris-HCl pH 8.0, 2 M NaCl, 1mM EDTA, and 0.005% Tween 20] for 20 min to remove bound Noc.
For experiments where a closed DNA substrate was cleaved to generate a free DNA end, DNA-coated sensors were dipped into 300 μL of restriction solution [266 μL of water, 30 μL of 10x CutSmart buffer (NEB), and 4 μL of BamHI-HF restriction enzyme (20,000 units/mL)] for 30 min at 37°C.
Differential radial capillary action of ligand assay (DRaCALA)
Purified Noc (WT and variants) (final concentration: 30 μM) were incubated with 5 nM radiolabeled P32-α-CTP (Perkin Elmer), 30 μM of unlabeled CTP (Thermo Fisher), 1 μM of 22 bp NBS or parS DNA in the reaction buffer [100 mM Tris pH 8.0, 100 mM NaCl, and 5 mM CaCl2] for 5 min at room temperature. For the NTP competition assay, the mixture was further supplemented with 500 μM of either unlabeled CTP, CDP, ATP, GTP, or UTP. Four μL of samples were spotted slowly onto a dry nitrocellulose membrane (Amersham Protran 0.45 μm) and air-dried. Subsequently, membranes were exposed to a phosphor screen (GE Healthcare) for two minutes. Each DRaCALA assay was triplicated, and a representative autoradiograph was shown.
Measurement of Noc-CTPɣS/CDP interaction by isothermal titration calorimetry (ITC)
All ITC experiments were recorded using a MicroCal PEAQ ITC instrument (Malvern Panalytical, UK). Experiments were performed at 4°C and both protein and ligand were in the buffer 100 mM Tris-HCl pH 8.0, 100 mM NaCl, and 5 mM CaCl2. The calorimetric cell was filled with 100 μM monomer concentration of either B. subtilis Noc (WT), Noc (R89A), or Noc (N121S), and was titrated with 3 mM CTPɣS. For each ITC run, a single injection of 0.5 μL of 3 mM CTPɣS or CDP was performed first, followed by 19 injections of 2 μL each. Injections were carried out at 120 s intervals with a stirring speed of 750 rpm. Each experiment was run in duplicate. The raw titration data were integrated and fitted to a one-site binding model using the built-in software of the MicroCal PEAQ ITC. Controls (CTPɣS/CDP into buffer and buffer into protein) were performed and no signal was observed.
Liposomes preparation
E. coli total lipid extract (25 mg/mL in chloroform, Avanti) was used to generate model liposomes. Briefly, an argon stream was used to evaporate chloroform from the lipids, and the resulting lipid cake was further dried under vacuum for 2 hr. The lipids were subsequently re-suspended in a buffer containing 100 mM Tris-HCl pH 7.4 and 200 mM NaCl. The mixture was incubated at 30°C for 30 min with vigorous vortexing every 5 min. The final concentration of the resuspended lipids was 50 mg/mL. The resuspended lipids were then extruded to ∼100 nm single unilamellar vesicles (SUV) using a mini-extruder (Avanti) equipped with polycarbonate membranes (0.1 μm pore size). The size of the resulting SUVs was confirmed by dynamic light scattering.
Liposome sedimentation assays
A 500 μL mixture of 0.75 μM Noc (WT/mutants) ± 1.0 mg/mL liposomes ± 1 mM NTP ± DNA (either 1 μM of 22-bp NBS/parS dsDNA or 100 nM of NBS-harboring/empty plasmid) was assembled in a binding buffer [100 mM Tris-HCl pH 7.4, 200 mM NaCl, and 1 mM MgCl2]. The mixture was incubated at 22°C for 20 min before being centrifuged at 50,000 rpm for 20 min at 22°C (TLA120.2 rotor, Optima Max-E Benchtop Ultracentrifuge). After centrifugation, the supernatant was transferred to a new 1.5-mL Eppendorf tube. The pellet was resuspended in 500 μL of binding buffer before being transferred to another 1.5-mL Eppendorf tube. SDS-PAGE sample buffer was then added, and the samples were heated at 70°C for 5 min before being loaded onto either 12% WedgeWell Tris-Glycine polyacrylamide gels, Novex 20% TBE polyacrylamide gels, or 1% agarose gels. Gels were subsequently stained in an InstantBlue Coomassie solution (to detect protein bands) or in a Sybr Green solution (to detect DNA bands). Each assay was triplicated. Protein/DNA band intensity was quantified using Image Studio Lite (LI-COR Biosciences). The protein/DNA fractions were averaged, and their standard errors were calculated in Excel.
For nuclease treatment, a 500 μL mixture of 0.75 μM Noc (WT/mutants) ± 1.0 mg/mL liposomes ± 1 mM NTP ± 100 nM of NBS-harboring/empty plasmid was incubated at room temperature for 10 min. Afterward, 1 μL of Benzonase (250 units/ μL) was added, and the mixture was incubated for a further 10 min at 22°C before ultracentrifugation.
For re-sedimentation experiments (Figure 4D), the pellet (from the first round of ultracentrifugation) was resuspended either in 500 μL of binding buffer [100 mM Tris-HCl pH 7.4, 200 mM NaCl, and 1 mM MgCl2] or in a stripping buffer [100 mM Tris-HCl pH 7.4, 200 mM NaCl, and 10 mM EDTA]. The resuspended pellet was centrifuged for the second time at 50,000 rpm for 20 min at 22°C. After the second centrifugation, the supernatant was transferred to a new 1.5-mL Eppendorf tube. The pellet was resuspended in 500 μL of binding buffer before being transferred to another 1.5-mL Eppendorf tube. SDS-PAGE sample buffer was then added to the supernatant and the pellet fractions, and the samples were analyzed on denaturing polyacrylamide gels.
Liposome flotation assays
A 200 μL mixture of 0.75 μM Noc (WT/mutants) ± 1.0 mg/mL liposomes ± 1 mM NTP ± 20 nM NBS-harboring/empty plasmid was assembled in a 30% sucrose buffer [100 mM Tris-HCl pH 7.4, 200 mM NaCl, 1 mM MgCl2, and 30% sucrose]. The mixture was incubated at 22°C for 5 min before being overlaid with 250 μL of a 25% sucrose buffer [100 mM Tris-HCl pH 7.4, 200 mM NaCl, 1 mM MgCl2, and 25% sucrose]. Finally, 150 μL of a 0% sucrose buffer [100 mM Tris-HCl pH 7.4, 200 mM NaCl, and 1 mM MgCl2] was added as the top layer. The solution was incubated for 15 min at 22°C before being centrifuged at 70,000 rpm at 22°C for 20 min (TLA120.2 rotor, Optima Max-E Benchtop Ultracentrifuge). After centrifugation, three equal fractions (200 μL each) were gently drawn sequentially from the bottom of the ultracentrifugation tube using a Hamilton syringe. SDS-PAGE sample buffer was added to each fraction, and samples were heated at 70°C for 5 min before being loaded onto either 12% WedgeWell Tris-Glycine polyacrylamide gels or 1% agarose gels. Gels were subsequently stained in an InstantBlue Coomassie solution (to detect protein bands) or in a Sybr Green solution (to detect DNA bands). Each assay was triplicated. Protein/DNA band intensity was quantified using Image Studio Lite (LI-COR Biosciences). The protein/DNA fractions were averaged, and their standard errors were calculated in Excel.
Protein crystallization, structure determination, and refinement
Crystallization screens were set up in sitting-drop vapor diffusion format in MRC2 96-well crystallization plates with drops comprised of 0.3 μL precipitant solution and 0.3 μL of protein and incubated at 293 K. After optimization of initial hits, suitable crystals were cryoprotected and mounted in Litholoops (Molecular Dimensions) before flash-cooling by plunging into liquid nitrogen. X-ray data were recorded either on beamline I04 or I04-1 at the Diamond Light Source (Oxfordshire, UK) using either an Eiger2 XE 16M or a Pilatus 6M-F hybrid photon counting detector (Dectris), with crystals maintained at 100 K by a Cryojet cryocooler (Oxford Instruments). Diffraction data were integrated and scaled using DIALS (Winter et al., 2018) via the XIA2 expert system (Winter, 2010) then merged using AIMLESS (Evans and Murshudov, 2013). Data collection statistics are summarized in Table S3. The majority of the downstream analysis was performed through the CCP4i2 graphical user interface (Potterton et al., 2018).
Geobacillus thermoleovorans NocΔCTD
His-tagged NocΔCTD Noc (∼10 mg/mL) was premixed with 1 mM CTP and 1 mM MgCl2 in buffer [10 mM Tris-HCl pH 8.0 and 250 mM NaCl] before crystallization. Crystals grew in a solution containing 2.0 M ammonium sulfate and 50 mM tri-sodium citrate, and were cryoprotected in the crystallization solution supplemented with 20% (v/v) glycerol, 1 mM CTP and 1 mM MgCl2. For iodide derivatization, the cryoprotectant comprised the crystallization solution supplemented with 25% (v/v) ethylene glycol, 1 mM CTP, 1 mM MgCl2, and 500 mM potassium iodide; crystals were soaked in this solution for less than 30 s before cryo-cooling. Three 360° passes of X-ray data were taken at a wavelength of 1.8 Å from different parts of a single crystal and merged to give a highly redundant dataset to 3.4 Å resolution in space group P213 with cell parameters a = b = c = 136.6 Å. Solvent content estimation gave a value of 66% for two copies of the 29 kDa subunit per asymmetric unit (ASU). The structure was subsequently solved by single-wavelength anomalous dispersion using the CRANK2 pipeline (Skubák and Pannu, 2013), which located 12 iodide sites, and BUCCANEER (Cowtan, 2006) was able to build and sequence 339 residues in two chains corresponding to 67% of those expected for two monomers, giving Rwork and Rfree values of 0.318 and 0.362, respectively, to 3.4 Å resolution after refinement with REFMAC5 (Murshudov et al., 1997). At this point, this preliminary model was used as a starting point for refinement against a native dataset processed to 2.5 Å resolution in the same space group, but with a significantly longer cell edge of 146.8 Å corresponding to a solvent content of 72.6%. Thus, it was necessary to resolve the structure by molecular replacement using PHASER (McCoy et al., 2007) before further refinement in REFMAC5. After a complete rebuild in BUCCANEER, several iterations of model building in COOT (Emsley and Cowtan, 2004) and REFMAC5 refinement jobs yielded the final model with Rwork and Rfree values of 0.210 and 0.240, respectively, to 2.5 Å resolution.
Despite the presence of CTP in the crystallization buffer, no density for CTP was found in the NocΔCTD structure, presumably because NBS DNA was not included to facilitate CTP-binding, or the high concentrations of sulfate (2M) from the precipitant excluded the ligand.
Geobacillus thermoleovorans NocNΔ26ΔCTD
His-tagged NocNΔ26ΔCTD Noc (∼10 mg/mL) was premixed with 1 mM CTPɣS and 1 mM MgCl2 in buffer [10 mM Tris-HCl pH 8.0 and 250 mM NaCl] before crystallization. Crystals grew in a solution containing 0.2 M di-ammonium phosphate and 2.3 M ammonium sulfate and were cryoprotected in this solution supplemented with 20% (v/v) glycerol. X-ray data were processed to a resolution of 2.95 Å in space group C2221 with cell parameters of a = 105.1, b = 106.6, c = 42.2 Å. Analysis of the likely composition of the ASU suggested that it contained a single copy of the 26 kDa NocNΔ26ΔCTD Noc monomer, giving an estimated solvent content of 46%. The structure was solved by molecular replacement with PHASER (McCoy et al., 2007) using chain A from the G. thermoleovorans NocΔCTD structure above as the template. The search model was split into three separate ensembles comprising residues 26-100, 101-140, and 141-230, respectively. PHASER successfully placed all three ensembles, although one of these had to be interchanged with a symmetry mate to restore the connectivity of the starting template. Several iterations of model building in COOT and refinement REFMAC5 yielded the final model with Rwork and Rfree values of 0.267 and 0.288, respectively, to 2.95 Å resolution.
Fluorescence microscopy
Cells containing fluorescent protein fusions were grown at 30°C. Xylose (0.5% w/v) was included in the media to induce the expression of YFP fusions in B. subtilis. Cell membranes were stained by mixing 15 μL of culture with 0.5 μL of membrane dye FM5-95 (200 μg/ml, Invitrogen). Cells were mounted on microscope slides covered with a thin agarose pad (1.2% w/v in dH2O) and images were acquired with a Rolera EM-C2 (Q-imaging) camera attached to a Nikon Ti microscope using METAMORPH version 6 (Molecular Devices), with an exposure time of 400 ms for YFP and 1000 ms for membranes. Images were prepared for publication using ImageJ.
Quantification and statistical analysis
Information about statistical analysis and sample size for each experiment are detailed in the relevant STAR Methods sections.
Acknowledgments
This study was funded by the Royal Society University Research Fellowship Renewal (URF\R\201020 to T.B.K.L.), and a Wellcome Trust Investigator grant (209500) to J.E. that supported L.J.W. A.S.B.J.’s PhD studentship was funded by the Royal Society (RG150448), and N.T.T. was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) grant-in-add (BBS/E/J/000C0683 to the John Innes Centre). We thank Diamond Light Source for access to beamlines I04 and I04-1 under proposals MX18565 and MX25108 with support from the European Community’s Seventh Framework Program (FP7/2007–2013) under grant agreement 283570 (BioStruct-X).
Author contributions
Conceptualization, A.S.B.J. and T.B.K.L.; formal analysis, A.S.B.J., N.T.T., L.J.W., C.E.M.S., and D.M.L.; investigation, A.S.B.J., N.T.T., L.J.W., K.R., G.B., C.E.M.S., D.M.L., J.E., and T.B.K.L.; resources, M.R.; writing, A.S.B.J., L.J.W., D.M.L., J.E., and T.B.K.L.; supervision, T.B.K.L.
Declaration of interests
The authors declare no competing interests.
Published: July 15, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.molcel.2021.06.025.
Supplemental information
References
- Adams D.W., Wu L.J., Errington J. Nucleoid occlusion protein Noc recruits DNA to the bacterial cell membrane. EMBO J. 2015;34:491–501. doi: 10.15252/embj.201490177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M.K., Koonin E.V. Evolution of eukaryotic cysteine sulfinic acid reductase, sulfiredoxin (Srx), from bacterial chromosome partitioning protein ParB. Cell Cycle. 2005;4:947–952. doi: 10.4161/cc.4.7.1786. [DOI] [PubMed] [Google Scholar]
- Beck R., Sun Z., Adolf F., Rutz C., Bassler J., Wild K., Sinning I., Hurt E., Brügger B., Béthune J., Wieland F. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc. Natl. Acad. Sci. U S A. 2008;105:11731–11736. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielli A., Haney C.J., Gabreski G., Watkins S.C., Bannykh S.I., Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J. Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J.L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Breier A.M., Grossman A.D. Whole-genome analysis of the chromosome partitioning and sporulation protein Spo0J (ParB) reveals spreading and origin-distal sites on the Bacillus subtilis chromosome. Mol. Microbiol. 2007;64:703–718. doi: 10.1111/j.1365-2958.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- Buckstein M.H., He J., Rubin H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 2008;190:718–726. doi: 10.1128/JB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- Dodonova S.O., Aderhold P., Kopp J., Ganeva I., Röhling S., Hagen W.J.H., Sinning I., Wieland F., Briggs J.A.G. 9Å structure of the COPI coat reveals that the Arf1 GTPase occupies two contrasting molecular environments. eLife. 2017;6:e26691. doi: 10.7554/eLife.26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evans P.R., Murshudov G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell B.E. ParB partition proteins: complex formation and spreading at bacterial and plasmid centromeres. Front. Mol. Biosci. 2016;3:44. doi: 10.3389/fmolb.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- Graham T.G.W., Wang X., Song D., Etson C.M., van Oijen A.M., Rudner D.Z., Loparo J.J. ParB spreading requires DNA bridging. Genes Dev. 2014;28:1228–1238. doi: 10.1101/gad.242206.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M.G., 4th, Mela I., Wang L., Henderson R.M., Chapman E.R., Edwardson J.M., Audhya A. Sar1 GTPase activity is regulated by membrane curvature. J. Biol. Chem. 2016;291:1014–1027. doi: 10.1074/jbc.M115.672287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Lutkenhaus J. A conserved sequence at the C-terminus of MinD is required for binding to the membrane and targeting MinC to the septum. Mol. Microbiol. 2003;47:345–355. doi: 10.1046/j.1365-2958.2003.03321.x. [DOI] [PubMed] [Google Scholar]
- Hu Z., Gogol E.P., Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc. Natl. Acad. Sci. U S A. 2002;99:6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L.C., Vecchiarelli A.G., Han Y.-W., Mizuuchi M., Harada Y., Funnell B.E., Mizuuchi K. ParA-mediated plasmid partition driven by protein pattern self-organization. EMBO J. 2013;32:1238–1249. doi: 10.1038/emboj.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal A.S.B., Le T.B.K. Bacterial chromosome segregation by the ParABS system. Open Biol. 2020;10:200097. doi: 10.1098/rsob.200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal A.S., Tran N.T., Le T.B. ParB spreading on DNA requires cytidine triphosphate in vitro. eLife. 2020;9:e53515. doi: 10.7554/eLife.53515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal A.S.B., Tran N.T., Stevenson C.E., Chan E.W., Lo R., Tan X., Noy A., Lawson D.M., Le T.B.K. Diversification of DNA-Binding specificity by permissive and specificity-switching mutations in the ParB/Noc protein family. Cell Rep. 2020;32:107928. doi: 10.1016/j.celrep.2020.107928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson H.F. Altered arrangement of proteins in the spore coat of a germination mutant of Bacillus subtilis. J. Gen. Microbiol. 1983;129:1945–1958. doi: 10.1099/00221287-129-6-1945. [DOI] [PubMed] [Google Scholar]
- Krauss M., Jia J.-Y., Roux A., Beck R., Wieland F.T., De Camilli P., Haucke V. Arf1-GTP-induced tubule formation suggests a function of Arf family proteins in curvature acquisition at sites of vesicle budding. J. Biol. Chem. 2008;283:27717–27723. doi: 10.1074/jbc.M804528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E. Stock-based detection of protein oligomeric states in jsPISA. Nucleic Acids Res. 2015;43(W1):W314–W319. doi: 10.1093/nar/gkv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.C.S., Orci L., Hamamoto S., Futai E., Ravazzola M., Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Lim H.C., Surovtsev I.V., Beltran B.G., Huang F., Bewersdorf J., Jacobs-Wagner C. Evidence for a DNA-relay mechanism in ParABS-mediated chromosome segregation. eLife. 2014;3:e02758. doi: 10.7554/eLife.02758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. The ParA/MinD family puts things in their place. Trends Microbiol. 2012;20:411–418. doi: 10.1016/j.tim.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H., Ferreira H., Errington J. The bacterial chromosome segregation protein Spo0J spreads along DNA from parS nucleation sites. Mol. Microbiol. 2006;61:1352–1361. doi: 10.1111/j.1365-2958.2006.05316.x. [DOI] [PubMed] [Google Scholar]
- Murshudov G.N., Vagin A.A., Dodson E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Nyholm T.K.M., Özdirekcan S., Killian J.A. How protein transmembrane segments sense the lipid environment. Biochemistry. 2007;46:1457–1465. doi: 10.1021/bi061941c. [DOI] [PubMed] [Google Scholar]
- Osorio-Valeriano M., Altegoer F., Steinchen W., Urban S., Liu Y., Bange G., Thanbichler M. ParB-type DNA segregation proteins are CTP-dependent molecular switches. Cell. 2019;179:1512–1524.e15. doi: 10.1016/j.cell.2019.11.015. [DOI] [PubMed] [Google Scholar]
- Pang T., Wang X., Lim H.C., Bernhardt T.G., Rudner D.Z. The nucleoid occlusion factor Noc controls DNA replication initiation in Staphylococcus aureus. PLoS Genet. 2017;13:e1006908. doi: 10.1371/journal.pgen.1006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.-T., Wu W., Lovell S., Lutkenhaus J. Mechanism of the asymmetric activation of the MinD ATPase by MinE. Mol. Microbiol. 2012;85:271–281. doi: 10.1111/j.1365-2958.2012.08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S.R., Errington J. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol. 1993;8:945–955. doi: 10.1111/j.1365-2958.1993.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Potterton L., Agirre J., Ballard C., Cowtan K., Dodson E., Evans P.R., Jenkins H.T., Keegan R., Krissinel E., Stevenson K. CCP4i2: the new graphical user interface to the CCP4 program suite. Acta Crystallogr. D Struct. Biol. 2018;74:68–84. doi: 10.1107/S2059798317016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm B., Heermann T., Schwille P. The E. coli MinCDE system in the regulation of protein patterns and gradients. Cell. Mol. Life Sci. 2019;76:4245–4273. doi: 10.1007/s00018-019-03218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejzek M., Le T.B.K. Chemical synthesis and purification of a non-hydrolyzable CTP analog CTPγS. BioRxiv. 2021 doi: 10.1101/2021.01.13.426546. [DOI] [Google Scholar]
- Rodrigues C.D.A., Harry E.J. The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization. PLoS Genet. 2012;8:e1002561. doi: 10.1371/journal.pgen.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A., Cattoni D.I., Walter J.-C., Rech J., Parmeggiani A., Nollmann M., Bouet J.-Y. Stochastic self-assembly of ParB proteins builds the bacterial DNA segregation apparatus. Cell Syst. 2015;1:163–173. doi: 10.1016/j.cels.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Sievers J., Raether B., Perego M., Errington J. Characterization of the parB-like yyaA gene of Bacillus subtilis. J. Bacteriol. 2002;184:1102–1111. doi: 10.1128/jb.184.4.1102-1111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubák P., Pannu N.S. Automatic protein structure solution from weak X-ray data. Nat. Commun. 2013;4:2777. doi: 10.1038/ncomms3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh Y.-M., Davidson I.F., Zamuner S., Basquin J., Bock F.P., Taschner M., Veening J.-W., De Los Rios P., Peters J.-M., Gruber S. Self-organization of parS centromeres by the ParB CTP hydrolase. Science. 2019;366:1129–1133. doi: 10.1126/science.aay3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M., Iniesta A.A., Shapiro L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 2007;35:e137. doi: 10.1093/nar/gkm818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro E., Hong S.-H., McAdams H.H., Shapiro L. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc. Natl. Acad. Sci. U S A. 2008;105:15435–15440. doi: 10.1073/pnas.0807448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A.G., Mizuuchi K., Funnell B.E. Surfing biological surfaces: exploiting the nucleoid for partition and transport in bacteria. Mol. Microbiol. 2012;86:513–523. doi: 10.1111/mmi.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A.G., Hwang L.C., Mizuuchi K. Cell-free study of F plasmid partition provides evidence for cargo transport by a diffusion-ratchet mechanism. Proc. Natl. Acad. Sci. U S A. 2013;110:E1390–E1397. doi: 10.1073/pnas.1302745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A.G., Neuman K.C., Mizuuchi K. A propagating ATPase gradient drives transport of surface-confined cellular cargo. Proc. Natl. Acad. Sci. U S A. 2014;111:4880–4885. doi: 10.1073/pnas.1401025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga H., Jorge A.M., Pinho M.G. Absence of nucleoid occlusion effector Noc impairs formation of orthogonal FtsZ rings during Staphylococcus aureus cell division. Mol. Microbiol. 2011;80:1366–1380. doi: 10.1111/j.1365-2958.2011.07651.x. [DOI] [PubMed] [Google Scholar]
- Williams C.J., Headd J.J., Moriarty N.W., Prisant M.G., Videau L.L., Deis L.N., Verma V., Keedy D.A., Hintze B.J., Chen V.B. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018;27:293–315. doi: 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G. xia2: an expert system for macromolecular crystallography data reduction. J. Appl. Cryst. 2010;43:186–190. [Google Scholar]
- Winter G., Waterman D.G., Parkhurst J.M., Brewster A.S., Gildea R.J., Gerstel M., Fuentes-Montero L., Vollmar M., Michels-Clark T., Young I.D. DIALS: implementation and evaluation of a new integration package. Acta Crystallogr. D Struct. Biol. 2018;74:85–97. doi: 10.1107/S2059798317017235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.J., Errington J. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell. 2004;117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Wu L.J., Errington J. Nucleoid occlusion and bacterial cell division. Nat. Rev. Microbiol. 2011;10:8–12. doi: 10.1038/nrmicro2671. [DOI] [PubMed] [Google Scholar]
- Wu L.J., Ishikawa S., Kawai Y., Oshima T., Ogasawara N., Errington J. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 2009;28:1940–1952. doi: 10.1038/emboj.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Park K.-T., Holyoak T., Lutkenhaus J. Determination of the structure of the MinD-ATP complex reveals the orientation of MinD on the membrane and the relative location of the binding sites for MinE and MinC. Mol. Microbiol. 2011;79:1515–1528. doi: 10.1111/j.1365-2958.2010.07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhou J., Gueiros-Filho F.J., Kearns D.B., Jacobson S.C. Noc corrals migration of FtsZ protofilaments during cytokinesis in Bacillus subtilis. MBio. 2021;12 doi: 10.1128/mBio.02964-20. e02964-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukovsky M.A., Filograna A., Luini A., Corda D., Valente C. Protein amphipathic helix insertion: a mechanism to induce membrane fission. Front. Cell Dev. Biol. 2019;7:291. doi: 10.3389/fcell.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The crystallographic structure of G. thermoleovorans NocΔCTD and G. thermoleovorans NocNΔ26ΔCTD have been deposited in the PDB with accession codes PDB: 7NFU and PDB: 7NG0, respectively. All images and source data presented in figures are available in Mendeley Data at https://data.mendeley.com/datasets/px2k9hr94c/1.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.