Abstract
Results of immunotherapy in childhood solid cancer have been so far, with the exception of neuroblastoma, quite disappointing. Lack of knowledge of the immune contexture of these tumors may have contributed to the failure of immunotherapies so far. Here, we systematically reviewed the literature regarding the immunology of Wilms tumor (WT), one of the most frequent pediatric solid tumors of the abdomen. In Wilms tumor patients the high cure rate of >90%, achieved by the combination of surgery and radio-chemotherapy, is at the expense of a high early and late toxicity. Moreover, treatment-resistant entities, such as diffuse anaplastic tumors or recurrent disease, still pose unsolved clinical problems. Successful immunotherapy could represent a novel and possibly less-toxic treatment option. Employing the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) method of literature search, we analyzed the current knowledge of the immunological landscape of Wilms tumors in terms of tumor microenvironment, prognostic implications of single biomarkers, and immunotherapy response.
Graphical abstract
The corresponding author and colleagues address the role of immunotherapy in Wilms tumor, in the attempt to evaluate the issues at the basis of the so-far disappointing results of immunomodulation in childhood solid cancer. On the basis of the current knowledge, novel reasonable areas of research are highlighted.
Introduction
In adult oncology, research on the tumor immune microenvironment has already shown great promise in unravelling novel prognostic markers1 as well as novel therapeutic possibilities,2 with the advantage of reducing the toxicity of the conventional chemotherapy-based treatments.
Attempts to translate the successes of immunotherapy in the field of pediatric oncology has been enthusiastically endeavored, although with a certain delay. Immunotherapy might serve as an option to cure the 10% of tumors resistant to standard therapy. Moreover, early and late toxicity of cytotoxic chemotherapy and radiotherapy regimens pose serious problems in pediatric oncology, as it affects patients up to adolescence and adulthood. Immunotherapy could offer a unique chance to reduce the high morbidity in children treated according to standard-of-care therapies. Studies on survivors of childhood cancer have shown that at least 60% of young adults develop chronic health conditions (CHCs), and by age 45 years, 80% experience at least one serious disabling or life-threatening CHC, as conventional cancer treatment is not cell specific.3,4 This burden of disease is double if compared to the general population.4
In contrast to adult oncology, the achievements of immunotherapy in the field of pediatric solid tumors have revealed themselves to be not quite as remarkable. In fact, most immunotherapies in solid childhood cancer so far failed to show an objective anti-tumor effect. While, for example, treatments with checkpoint inhibitor anti-PD-L1 have shown even superior results to traditional chemotherapy for adult cancers,5 the interim results of the largest trial on pediatric patients, KEYNOTE-051, showed a low to absent antitumor effect of pembrolizumab, an anti PD-1 monoclonal antibody.6 The only exception is the cytotoxic anti-GD2 monoclonal antibody (mAb) therapy given for neuroblastoma. The latter is now a US Food and Drug Administration (FDA)-approved therapy and has reached the ranking of standard treatment.7
The failure of immunomodulating immunotherapy with any checkpoint inhibitor correlates with the fact that pediatric solid tumors are far less immunogenic than adult cancers.8 The reason might be that pediatric tumors usually descend from embryonal cells rather than mature epithelial cells and are presumably the result of transcriptional abnormalities (i.e, single-gene inactivation or amplification) and not of the gradual accumulation of genetic mutations over time.8
The last pediatric strategy forum, organized by the European Society for Pediatric Oncology and Innovative Therapies for Children with Cancer in Europe Consortium, revealed that the lack of knowledge regarding the immunological landscape of solid childhood tumors is probably responsible for the failure of most immunotherapies tested so far.9 Future translational studies should help improve the understanding of immune evasion mechanisms in childhood cancers, providing a rational base for novel treatment concepts. It appears essential to remember that children are not small adults. Correspondingly, childhood cancer most likely follows unique immunological pathways, which remain to be explored.
Wilms tumor (WT), or nephroblastoma, is the most common renal malignancy in childhood and the second most common solid abdominal tumor presenting in infants and children after neuroblastoma, with an incidence of 1:10,000 infants. WT typically arises between the ages of 2 and 5 years, with 95% of diagnoses before the age of 10 years.10
The prognosis of WT has significantly improved in the last four decades, resulting in a 5-year survival rate that exceeds 90%.11 However, 1 out of 4 WT patients present either unfavorable histologic and molecular features or metastatic, bilateral, or recurrent disease, whose survival rates remain well below this standard.12,13
Similar to other childhood cancers, chemo- and radiotherapy regimens applied for WT expose patients to an increased risk of adverse outcomes. Those include long-term heart disease, pulmonary disease, renal failure, infertility, and secondary cancers, as well as short-term mortality due to therapy-induced toxicity (Table 1).14 Consequently, clinical research focuses on reducing these high secondary morbidity and mortality rates. For example, as anthracyclines are known to be potentially cardiotoxic, there have been efforts to reduce their employment in therapy.15 In this line, the SIOP (Societè Internationale d’Oncologie Pédiatrique)-2001 trial showed that doxorubicin can be safely omitted in the treatment regimens of stage II to III intermediate-risk tumors, without changes in overall survival (OS) and with non-significant decrease in the event-free survival.16
Table 1.
Schematic evaluation of late effects of treatment in Wilms tumor survivors
| Morbidity | Mortality | |
|---|---|---|
| Heart disease | ||
| Congestive heart failure | cumulative incidence after 20 years: 4.4% of patients treated with DXO at initial diagnosis;102 17.4% DXO at relapse102 | |
| Subclinical cardiac abnormalities | frequency at 7 years follow-up: 24/97 (25%) of patients treated with DXO3 | |
| Any cardiovascular CHC (grade 1–5) | cumulative burden at age 50 years: 4.13 (3.57–4.69)4 | |
| Pulmonary disease | ||
| 1 or more abnormal pulmonary function test results (FEV1, VC, DLCO) and fibrosis on X-ray | cumulative incidence at 15 years: <0.5% with no RT;103 4.0% RT for initial treatment;103 5.5% RT for relapse103 | |
| Pulmonary fibrosis | >5 year follow up: 0.2%14 | |
| Any pulmonary CHC (grade 1-5) | cumulative burden at age 50 years: 1.96 (1.53–2.39)4 | |
| Renal dysfunction | ||
| End-stage renal disease (ESRD) | cumulative incidence at 20 years: 0.6% after unilateral disease;104 12% bilateral disease104 | |
| Any renal CHC (grade 1–5) | cumulative burden at age 50 years: 0.48 (0.25–0.70)4 | |
| Secondary malignant neoplasms | ||
| cumulative incidence at 15 years: 1.6% (73% within the radiation field)105 | mortality 5 years follow-up: 1.1% of deaths14 | |
| mortality >5 years follow-up: 2.7% of deaths14 | ||
| cumulative burden at age 50 years: 0.61 (0.32–0.90)4 | ||
| Reproductive health problems | ||
| Primary ovarian failure | 20/25 female survivors (80%)106 | |
| Premature menopause (<36 years) | 4/25 female survivors (16%)106 | |
| Spontaneous abortion or stillbirths | 2% of the recorded pregnancies107 | |
| Premature births (20–36 weeks of gestation) | 33.3% of children of female with WART107 | |
| Low birth weight (<2.5 kg) | 33.3% of children of females with WART | |
| Any reproductive CHCs | cumulative burden at age 50 years: 1.16 (0.82–1.49)4 | |
CHC, chronic health condition; DXO, doxorubicin; RT, radiotherapy; WART, whole abdomen radiotherapy. Cumulative incidence (%) expresses disease frequency in a given period of time. Cumulative burden expresses the mean number of recurrent/multiple health events a cohort member experiences by a given time point in the presence of competing risk events. The grades are according to the Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE): mild (grade 1), moderate (grade 2), severe/disabling (grade 3), life-threatening (grade 4), or death (grade 5).4
In the last decade, immunotherapy has been thought to hold great promise in improving survival with limited side effects.17 However, unlike neuroblastoma or sarcomas, WT has only been investigated in a few ongoing clinical trials.18
To set the stage for future development of immunotherapy in WTs, we reviewed the literature on the immunology of this cancer (Figure 1). We evaluated studies on the tumor microenvironment, its prognostic implications and main immunotherapeutic clinical trials. A comprehensive understanding should enable a rational design for effective immunotherapeutic concepts. To the best of our knowledge, this is the first systematic review focusing on the immunology of WTs so far.
Figure 1.
PRISMA flow diagram
Schematic view of the search strategy according to PRISMA flow diagram. WT, Wilms tumor.
Immune cell infiltration
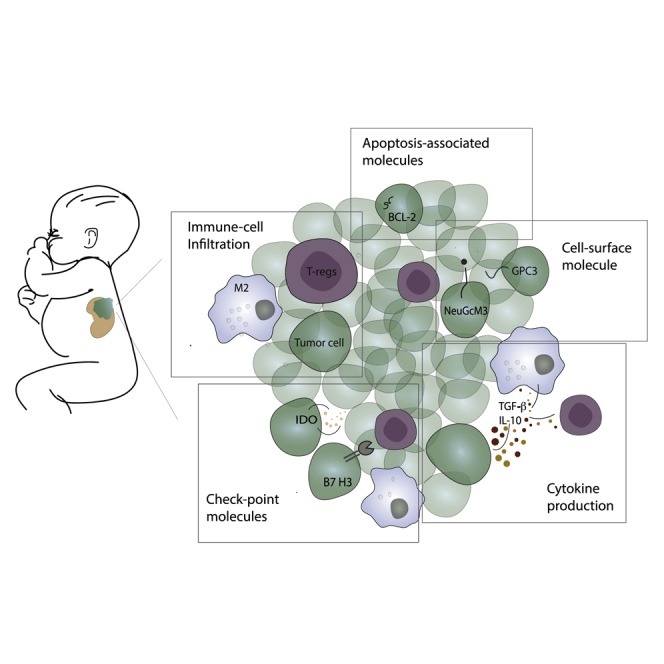
All studies underline the presence of an immunosuppressive microenvironment in Wilms tumor.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 The cell infiltrate is usually detected in the stromal component of the tumor, and the most abundant cells are tumor-associated macrophages (TAMs).19,24,25,29 There has been an attempt to correlate the presence of such cells to clinicopathological features of WT. In this line, Liou et al.25 evaluated that mean macrophage count densities (CD68+) were higher in stage IV than in stage I (p = 0.021). Concerning prognosis, a statistically significant correlation between TAM count in stage II WT and decreased disease-free survival (DFS) (p = 0.035) was found.24 Correspondingly, vascular invasion, another unfavorable prognostic factor, also correlated with the number of macrophages.24 With respect to macrophage polarization subtypes, either pro- (M1) or anti-inflammatory (M2), Tian et al.29 demonstrated in 61 samples of WT that TAMs were usually M2. An increased ratio of M2/M1 correlated with disease progression from stage I to stage III, prognosis and unfavorable histology.29 Thus, the macrophage infiltrate might be employed to refine the staging algorithm.
Regarding tumor-infiltrating lymphocytes (TIL), both the quantitative as well as the qualitative analysis indicated an immunosuppressive environment in all the evaluated studies.19, 20, 21, 22, 23,26,27 WTs usually present with a low number of CD3+ lymphocytes, and CD3+ cells were usually stromal.26,27 Moreover, CD3-positive cells were predominantly T-regulatory (T-reg) and T helper 2 (Th2) polarized cells. The immunosuppressive nature of the tumor is further supported by expression of interleukin (IL)-10 and indoleamine 2,3-dioxygenase (IDO).27 Thus, WT must be regarded as an immunologically cold tumor, usually lacking a higher number of activated CD8+ cytotoxic T cells.
Despite the general lack of a CD8+ immune T cell infiltrate, Mardanpour et al.30 recently found that higher CD8+ tumor-infiltrating lymphocyte counts at the invasive margins were correlated with lower tumor recurrency rates. In particular, early-stage tumors were found to present significantly higher TILs both in the center and in the invasive border of the tumor.
Furthermore, Holl et al.28 could detect the presence of effector memory cells characterized by high CD57, PD-1, and HLA DR expression. This activated T cell phenotype was not found in circulating T cells, suggesting that T cells can be activated in WTs if appropriately stimulated.
However, TILs were found to be not only sparse but also difficult to expand in vitro and lacking a specific autologous anti-tumor cytotoxic activity.21,22 It has been hypothesized that a low expression of immunomodulatory molecules on tumor cells or the production of immunosuppressive factors may be responsible for the reduced activation and expansion of TILs in pediatric tumors.21 Thus, the low expression of major histocompatibility complex (MHC) class I and of ICAM/LFA3 adhesion molecules as well as a concurrent high expression of TGF-β may explain the difficulties in pediatric TIL cultures. However, pediatric tumors characterized by high expression of MHC I and low concentrations of TGF-β readily allow TIL cultures. This supports the hypothesis that when immunosuppressive elements of childhood cancers are antagonized, stimulation of an ablative T cell response is feasible.
It should be noted that defects in T cell responsiveness are not limited to TILs. Most recently, Das et al.31 reported both qualitative and quantitative deficits in the circulating naive T cells in 195 pediatric patients with solid or hematological malignancies including WTs. Such findings might become relevant when potential CAR-T cell therapy is considered.
Due to the lack of T cells in the microenvironment of WT, immunotherapeutic strategies targeting T cells in WTs are rare. Obviously, tumor-specific cytotoxic T cells have to be generated by other means, such as T cell vaccines or the generation of CAR-T cells. In this line, a phase I study on tumor-associated antigen cytotoxic T cells (TAA-Ts) has been conducted on relapsed or refractory solid pediatric tumors, with remarkable success (NCT02789228).32 The trial was based on the generation and ex vivo expansion of TAA-Ts directed toward three different tumor-associated antigens (TAAs): WT1, preferentially expressed antigen of melanoma (PRAME), and survivin. All three of them are target antigens overexpressed on tumor cells.33, 34, 35 Of the 15 patients who were included in the study, 73% were defined as responders. Furthermore, 5 of the 7 WT patients who received the infusions were defined as responders. No infusion-correlated adverse events have been reported.
With respect to vaccines, we identified only one approach done for advanced solid pediatric cancer in which WT patients were included. The vaccine was based on an IL-12-secreting dendritic cell (DC)-based vaccine using tumor lysates.36 In one of the WT patients, the vaccine was associated with the transition of a progressive disease to stable disease 6 months after vaccine application.
Expression of immune checkpoint molecules
PD-L1 is the immune checkpoint molecule that has been most frequently analyzed in solid pediatric cancer and correspondingly in WTs. With the exception of pediatric Hodgkin’s lymphoma and pediatric sarcomas,37 PD-L1 expression was found to be generally low.38, 39, 40, 41, 42 Routh et al.40 analyzed 81 WT samples by immunohistochemistry: PD-L1 was expressed by only 14% of nephroblastomas and could be correlated with increased cancer recurrence. The study was done in tumors with favorable histology, which account for 90% of WTs (relative risk [RR], 3.65%; p value, 0.02). The number of anaplastic tumors was too small to draw solid conclusions. Pinto et al.38 broadened the analysis evaluating the expression of PD-1, PD-L1, and PD-L2 in combination with gene expression analyzed via NanoString. The study was conducted on a series of 124 pediatric solid tumors, including 25 nephroblastomas. Expression of immune checkpoint molecules was mostly absent or present at only low levels, even though gene transcripts were always detectable. Silva et al.42 confirmed that WT showed the lowest levels of PD-L1 expression within the group of pediatric solid tumors (ganglioneuroblastoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma). Low PD-L1 expression correlated with low tumor-infiltrating lymphocytes.
Correspondingly, the interim results of the KEYNOTE-051 study, the ongoing clinical trial on 155 pediatric patients, revealed that pembrolizumab (anti-PD-1) only led to an anti-tumor activity in patients with relapsed or refractory Hodgkin’s lymphoma but not any other solid tumor.6 Objective responses were only observed in 5.9% of other solid tumors, including adrenocortical carcinoma, mesothelioma, malignant ganglioglioma, epithelioid sarcoma, lymphoepithelial carcinoma, and malignant rhabdoid, in contrast to 60% of Hodgkin’s lymphoma patients. Similar to studies done in adults, the trial included only patients with a positive PD-L1 expression, defined as the expression of the ligand in at least 1% of the tumor cells. Reasons for the poor activity of PD-1inhibition in these tumor types are not fully understood. The final results of KEYNOTE-051 are expected by September 2022 and may shed further light on the matter.
Apart from the PD-1/PD-L1 axis, several other immune checkpoint pathways have been reported to regulate the immune responses in the tumor microenvironment. Mochizuki et al.41 evaluated the expression of (1) alternative checkpoint molecules expressed on TILs as B- and T-lymphocyte attenuator (BTLA), TIM3, and LAG3; and (2) their respective ligands expressed on tumor cells such as Herpes Virus Entry Mediator (HVEM), GAL9, and MHC II on common solid pediatric tumors (n = 65). Expression of the above-mentioned molecules was rare (<30%) in the 10 WT samples included in the study.41 Certainly, a higher number of samples would better allow any conclusions.
Lately, rising interest has been addressed to B7-H3 (CD276), another checkpoint molecule of the B7 superfamily. This is a widely overexpressed surface molecule in adult solid tumors, implicated in immune escape mechanisms such as inhibition of naive T cell activation and production of cytotoxic cytokines, as well as in pro-metastatic activities.43 With respect to childhood cancer, its expression has been found to be high in 388 tumor samples. Most importantly, WT samples stained high in 100% of the analyzed cases.44 Due to this overwhelming high expression, B7-H3 successfully served as a target molecule for CAR-T cell therapy in a preclinical murine tumor model. Intravenous injection of B7-H3 directed CAR-T cell-eradicated xenografts of pediatric solid tumors.44
High expression of B7-H3 on childhood cancer also promoted the development of the respective antibody as radioimmunotherapy, when conjugated to iodine124. A phase I study performed on desmoplastic small round cell tumors including WT revealed that the therapy was well tolerated (NCT01099644) and is about to be followed by a phase II trial.45
Moreover, a further phase I study (NCT02982941) has been initiated, which evaluates the potential of enoblituzumab, a pure anti-B7-H3 mAb, in children with relapsed or refractory malignant B7-H3-positive solid tumors. Results of the above-mentioned trials have not been published yet.
Recent studies found that response to immune checkpoint inhibitors (ICIs) is higher in tumors presenting microsatellite instability (MSI) or deficiency in the mismatch repair system (dMMR).46 Accelerated approval of immunotherapy trials (i.e., pembrolizumab) based on the presence of these biomarkers has been already granted from the FDA both for adult and pediatric cancers. Whether the latter group expresses such markers is, however, still unclear.
Considering the relative “quiet” nature of the genome of childhood solid tumors, it is not surprising that MSI was not found to be common feature in WT.47, 48, 49 However, Diniz et al.47 encountered the presence of deficits in MMR genes in up to 42% of WT samples (19/45), correlating significantly to both stage and survival rates. These results are in contrast with the previous findings of Segers et al.,48 who found a normal expression of MMR proteins in all the evaluated samples (100), leading them to conclude that dMMR does not play a role in WT if not in the context of constitutional mismatch repair syndrome (CMMR-D). Further studies are required, however, to better establish the role of dMMR and its possible role in the immunotherapy response of childhood solid tumors.
Cell surface molecules
Proteoglycans, glycoproteins and glycolipids present in the cell membrane and extracellular matrix play vital roles in cell adhesion, migration, proliferation, and signaling pathways. The differential glycosylation of glycoproteins or their aberrant expression in cancer cells as compared to normal cells promotes tumor growth. Thus, tumor-associated glycosylation provides a rational concept for targeted therapy.50 The only immunotherapy that became part of standard regimen in pediatric solid tumors is based on a cytotoxic anti-GD2 antibody targeting the glycan structure of specific gangliosides in tumor cells.
GD2 is physiologically involved in neural differentiation and proliferation during embryogenesis and has been found to be highly expressed on a variety of pediatric cancers such as neuroblastoma, retinoblastoma, Ewing’s sarcoma, and osteosarcoma.51
The incorporation of anti-GD2 treatment into maintenance therapy of high-risk neuroblastoma led to significantly higher event-free survival (66% versus 46%; p = 0.01) and OS (86% versus 75%; p = 0.02) compared with isotretinoin-based chemotherapy alone, leading to approval by the FDA.7,8
In the search for gangliosides other than GD2, particular attention has been given to GM3(Neu5Gc)-containing gangliosides. These molecules are normal components of cell membranes in normal tissues of all mammals except human beings. In humans, their expression has only been observed in malignant cells. This would therefore make such molecules perfect targets for immunotherapy. The expression of GM3(Neu5Gc) on pediatric solid tumors has been evaluated by Scursoni et al.52 The group analyzed patients who have undergone neoadjuvant chemotherapy according to the SIOP 2001 protocol. Expression of GM3(Neu5Gc) was found in 88% of WTs but was absent in fetal or normal kidneys. Interestingly, antibodies that recognize these gangliosides were also found in patients’ serum. Thus, this ganglioside could be considered as neoantigen associated with renal malignant transformation in future clinical trials. There was a significant inverse correlation between mutated p53 and detection of GM3(Neu5Gc), which could suggest an abundance of the ganglioside associated with intact tumor p53-associated suppressor signal cascade in WT. Correspondingly, anaplastic tumors, frequently characterized by an abnormal p53 protein, have a lower expression of GM3(Neu5Gc). This could be the premise to employ this molecule as prognostic biomarker.
Moreover, racotumomab, a cytotoxic antibody directed toward GM3(Neu5Gc)-containing gangliosides, has been developed. Racotumomab revealed promising results in breast cancer patients who also express GM3(Neu5GC).53 With respect to childhood cancer, a phase I study has been conducted on high-risk refractory pediatric solid tumors, including one WT patient.54 Racotumomab was found safe and immunogenic in pediatric patients, but only 2/14 patients had stable disease after receiving the antibody, and no regression was reported. Potentially, immune-modulating agents enhancing antibody-dependent cellular phagocytosis (ADCP) or antibody-dependent cellular cytotoxicity (ADCC) might be added to improve efficacy in childhood cancer.55
Glycolipids are not the only cell surface molecules identified as potential target structures for cytotoxic antibodies. An alternative target structure is the carcinoembryonic antigen glypican-3 (GPC3), a heparan sulfate proteoglycan that is associated with cell growth, development, and the responses to various growth factors. Inactivation of GPC3 is responsible for X-linked Simpson-Golabi-Behmel overgrowth syndrome. 10% of these patients develop an embryonal malignancy, including WT. Furthermore, GPC3 overexpression has been found in pediatric tumors that derive from tissues that express such antigens during development, like liver, kidney, and gonads.56
A phase I study investigating a GPC3-derived peptide vaccine for patients with refractory pediatric solid tumors revealed encouraging data.57 In particular, hepatoblastomas were found responsive to this vaccine (progression-free survival, p ≤ 0.01), but also the single patient with nephroblastoma included in the trail was listed in the “responsive” group of patients.
GPC3 might be a very attractive target for WT-directed therapy, as this proteoglycan is expressed in more than 30% of the analyzed nephroblastoma samples.58,59 Tretiakova et al.60 even reported a positive expression in 77% of primary and in 93% of metastatic WTs. As GPC3 is not expressed by other renal tumors or other small blue round cell tumors, with the exception of malignant rhabdoid tumors, GPC3 expression could further serve as a marker for differential diagnosis and follow-up of WT.
However, GPC3-based therapy will most likely be only applicable for children aged over 1 year. The reason is that GPC3 is an essential protein in nephrogenesis, expressed in the fetal ureteric bud and collecting system in a time-specific manner, and is constitutively expressed in children up to 1 year of age.56
Cytokine production
Proinflammatory cytokines have been shown to promote tumor development.61 However, in contrast to adult renal carcinoma cell lines, which express high levels of classical pro-inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor (TNF)-α, WT cell lines were found devoid of those factors but tested markedly positive for TGF-β by ELISA.62 Correspondingly, the tumor microenvironment of WT is also characterized by a high production of immunosuppressive cytokines, of which TGF-β appears to have the most dominant role in tumor progression.62, 63, 64, 65 Its role in kidney development and renal architecture control might explain its expression in a malignant kidney tumor. Moreover, high TGF-β levels are generally associated with epithelial-to-mesenchymal transition (EMT) mediated by E-cadherin downregulation. Correspondingly, TGF-β expression was highly correlated with invasiveness and metastasis in WT, and patients with higher TGF-β levels presented a shorter disease-free survival (p = 0.022).64
A further tumor-promoting role of TGF-β in Wilms is the fact that this cytokine induces Forkhead box P3 (FOXP3) expression on T-reg cells.65 This is a transcription factor known to be crucial in conferring immunosuppressive functions to the T-regs.66 Correspondingly, FOXP3+ T-reg cells have been described to be diffusely present in WTs, and their infiltration is significantly higher than in control kidneys.27 The high expression of TGF-β also correlated with the inability of expanding TILs in vitro. Given its central role, TGF-β expression might serve as a potential therapeutic target. To our knowledge, no trial has focused its attention on TGF-β inhibition in pediatric solid tumors.
The expression of other immunosuppressive cytokines has been less investigated in WT so far. Maturu et al.27 identified an upregulation of IL-10 and IDO. Since both of those cytokines induce the suppression of effector T cell activation, the WT-associated cytokine pattern correlates well with the absence of tumor infiltrating cytotoxic T cells.67,68 No study has further evaluated the role of the latter immunosuppressive cytokines on prognosis or as possible targets of therapy.
Neoantigen production
In contrast to adult cancers, which frequently derive from epithelial cells, pediatric tumors usually descend from embryonal cells and are presumably the result of epigenetic and transcriptional reprogramming by a few mutations rather than a consequence of the gradual accumulation of an increasing number of genetic mutations over time. This explains the far lower mutational burden and the expression of fewer potential neoantigens, which limits the susceptibility to antigen-specific immune targeting.69
It should be noted that there is a group of pediatric hypermutant hematological, brain, and intestinal cancers arising in the context of a constitutional mismatch repair deficiency (CMMRD). A recent comprehensive analysis of hypermutation in cancer revealed that up to 5% of pediatric tumors, including nephroblastoma, present a high mutational burden, all with defective MMR systems.70 This syndrome is currently under investigation for neoantigen-based vaccine approaches also involving checkpoint inhibitors,71
TAAs do not exclusively derive from mutated proteins but might become targets for the immune system due to over- or atypical expression. One example of the success of such antigen targeting is the above-mentioned clinical trial on TAA-Ts directed toward three TAAs: WT1, PRAME, and survivin (NCT02789228).32 Its striking response rate of 73% demonstrates that new T cell therapies, which areable to target multiple antigens, may have the valuable potential to overcome antigen depletion in pediatric solid tumors.
Apart from being targets of a cytotoxic T cell response, such aberrantly expressed TAAs can also serve as targets for autoantibodies. Tumor-associated autoantibodies can be used as diagnostic tools. Despite that nephroblastomas have a much lower expression of autoantibodies than neuroblastomas,72,73 differential autoantibody signatures allowed separation between WT patients, neuroblastoma patients, and healthy controls with sensitivity and specificity of more than 86%.72 In WT patients not subjected to neoadjuvant chemotherapy, the autoantibodies contributing the most to the identification of WT patients were directed to the proteins ZFP 346 and fascin.73 Surprisingly, fascin- and ZFP 346-specific autoantibodies were not found in previously treated patients, suggesting that specific changes in the autoantibody repertoire occurred under chemotherapy. Since, according to the SIOP protocol, WT patients are treated without a previous diagnostic biopsy, autoantibody signatures may aid radiological and clinical identification of nephroblastoma. However, currently available data are insufficient to allow diagnosis of nephroblastoma solely based on antibody titers. Similarly, current cohorts of serum analysis are yet too small to allow translation to clinical decision making.
Apoptosis-associated proteins
Cell death signaling is relevant in any inflammation-relevant process. Thus, dysregulation of cell death pathways may contribute to tumorigenesis by altering the resistance of tumor cells to cell death induced by the immune system. For example, BCL-2 protects cells from programmed cell death, and its overexpression has been proposed to be tumorigenic and to mediate resistance to therapy. In this line, protein expression of BCL-2 in WT had a strong negative prognostic value.39,74 Pro-apoptotic BCL-2 family members such as Bcl-x and Bax transcripts were not associated with prognosis of nephroblastoma. During normal development, BCL-2 is known to regulate complex apoptotic processes during nephrogenesis and is transiently expressed in the blastema during induction by the ureteric bud, when malignant transformation is supposed to occur. Thus, its expression fits well with a less-differentiated state of kidney cells. Interestingly, although WTs express significant amounts of BCL-2 mRNA and protein, anaplastic tumors do not, regardless of p53 mutations.75
Most recently, the Wilms tumor-suppressing peptide (WTSP) was identified.76 WTSP is usually highly expressed in the serum of healthy children and suppressed in nephroblastoma patients. Serum levels of this peptide increased after radical nephrectomy but remained low in patients with partially resected WT or recurrent/metastatic disease.77 WTSP inhibited the proliferation of WT cells and induced cell cycle arrest in a dose- and time-dependent manner.77 In addition, WTSP induced apoptosis and necrosis of WT cells, partly through the downregulation of beta-catenin, a key factor in cell proliferation. Moreover, WTSP reduced BCL-2 and increased BAX expression in tumor cells, suggesting that the peptide also induced cell apoptosis partly via BCL-2 family members. Thus, the induction of WTSP or WTSP analog could serve as further novel alternative therapeutic approach, reducing the burden of classical chemotherapy for affected children.
With respect to apoptosis-related therapeutic approaches, TNF-α has gained some attention as a combination therapy in nephroblastoma, even though its expression in the tumor has not been separately evaluated. TNF-α is a cytostatic molecule produced by macrophages and lymphocytes, which has demonstrated in vitro antitumor activity in many tumor cells, such as melanoma and colorectal and bladder carcinoma. In pediatric solid tumors, TNF-α demonstrated single-agent antitumor activity in a phase I clinical trial and a synergistic effect when administered with actinomycin D in vitro.78 Using this combination, a phase II trial on 21 patients with recurrent or refractory WT has been performed.79 The combination was well tolerated; 3 patients (15.8%) had complete response and 5 (26.3%) had stable disease.
Similarities between placenta and childhood cancer
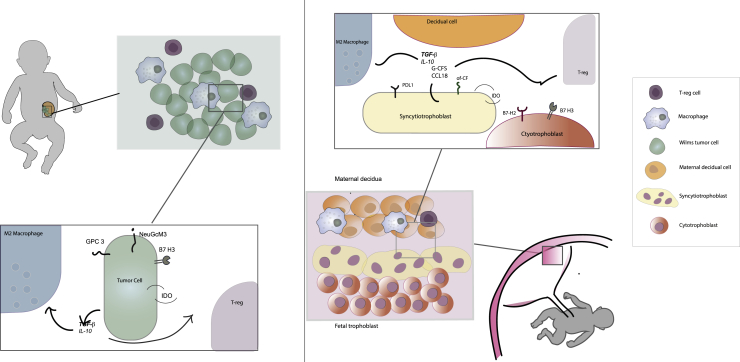
Childhood solid cancers, including WT, appear to be immunologically cold, little to non-inflamed entities, with a paucity of T cell and dendritic cell infiltration. This predominantly immunosuppressive microenvironment of pediatric malignancies resembles in a number of aspects the immune-privileged site of the placenta. Similarities between carcinogenesis and placentation have long been noticed80 (Figure 2).
Figure 2.
Similarities between Wilms tumor and placenta microenvironment
On the left, schematic view of the immune microenvironment of Wilms tumor and its main actors. On the right, schematic view of the immune microenvironment of the placenta with some of its main actors. On the far right, figure legend.
Trophoblastic cells of the placenta indeed have the inherent ability to invade healthy tissues, to form new vessels, and to promote an environment that is protected from the immune system. Strikingly, these are all hallmarks of malignant tissues. It has been demonstrated that several proto-oncogenes involved in tumorigenesis and cancer progression, such as EGFR, MYC, FOS, and RAS, are expressed during the peak of the development of trophoblastic cells and therefore promote its invasive potential.81 In this line, combined expression of MYC and RAS in malignant cells has been shown to promote a tumor microenvironment devoid of CD8+ T cells and rich in M2-like macrophages, resembling the immune landscape of childhood cancers.
Strong feto-maternal tolerance is achieved by a multitude of immunomodulatory properties of the feto-maternal interface, in absence of which the pregnancy would be terminated. These characteristics might now be evaluated to identify novel therapeutic targets for pediatric malignancies. Since these entities mostly arise form embryonal cells, it has been hypothesized that they could also reactivate trophoblastic placental programs responsible for immune escape.82 These involve cytokine secretion, checkpoint molecule expression, and glycan patterns.
With respect to cytokines, trophoblastic cells produce high levels of TGF-β and IL-10, which polarize the decidual macrophages versus the M2 type and promote the differentiation of T-reg cells.83 It has been demonstrated that both these cells are essential for the maintenance of pregnancy and that their induction is directly determined by fetal-derived molecules, which also include other cytokines such as macrophage colony-stimulating factor (M-CSF) and CCL18.84 Since the role of macrophages and T-reg cells seems to be prominent in the immune escape of childhood cancer, the investigation of cytokine patterns expressed by trophoblastic cells could further elucidate the immunosuppressive mechanisms of pediatric solid tumors.
The role of co-signaling molecules at the feto-maternal interface, such as checkpoint molecules of the B7 family, has been recently highlighted.85 The outer layer of the placenta, formed by completely differentiated syncytiotrophoblastic cells, expresses PD-L1 abundantly. As mentioned above, this molecule is unfortunately only rarely expressed in childhood cancer. It is to be noted, however, that this molecule is almost absent in cytotrophoblasts, which represent the inner pluripotent layer of the placenta. Cytotrophoblastic cells present, on the other hand, a constant expression of B7-H3, a checkpoint molecule highly expressed in pediatric tumors, including WT. Thus, blastemic cells of childhood cancer potentially better resemble the undifferentiated population of the cytotrophoblasts rather than the syncytiotrophoblasts. In this respect, it will be interesting to evaluate other checkpoint molecules expressed in this population. For example, B7-H2 (also known as ICOS-B7h) has been recently revealed to be an important costimulatory molecule of cytotrophoblastic cells.86 It was demonstrated that B7-H2 blockade causes decline of TGF-β and IDO levels in the placenta and an increase of IFN-γ levels. This correlated with a reversed ratio of T-reg/CD 8+ T cells and interruption of pregnancy in mouse models. It has been therefore postulated that this molecule might have a prime role in maintaining tolerance at the feto-maternal interface. Thus, analysis of B7-H2 in childhood cancer might offer novel possibilities for treatment.
IDO itself is one of the most relevant mediators of the immunosuppression at the feto-maternal interface.87 It is well established that the fetus actively defends itself from maternal T cell attack through the expression of IDO, and its direct inhibition allows maternal lymphocytes to mediate fetal rejection. This protein has been only sparsely investigated in pediatric solid tumors so far but was found to be expressed in WTs.27 Its prime role in the placenta might underline the urgency of its further investigation as therapeutic target in childhood cancer.
With respect to the glycan structures, differential glycosylation patterns have been found to be shared between placenta, tumors, and fetal tissue. These offer particularly interesting therapeutic possibilities, as the embryonic/placental glycosylation is absent or minimally expressed in normal tissues.81 In this regard, the group of Mads Dougaard identified the oncofetal chondroitin sulfate antigen (CSA), which is physiologically expressed in the syncytiotrophoblast, as well as in various malignant tissues.88 Furthermore, the group was able to successfully target CSA in murine models, without adverse effects. Since also other glycoproteins and glycolipids involved in embryogenesis, such as GPC3 and GD2, have already shown therapeutic value in childhood cancer, we believe that further evaluation of CSA as therapeutic target for pediatric cancers should be taken under consideration.
Conclusions and future directions
Our search clearly indicates that most studies investigating the tumor microenvironment in WT have only been done in a very limited number of patients, obviously due to its orphan disease character. Despite the lack of larger cohorts, the data still present a fairly clear picture. WTs appear as immunologically cold malignancies, sparsely infiltrated by CD8+ T cells, and, if immune cells are present, they are usually T-reg cells and M2-like macrophages. This tumor immune cell infiltrate correlates well with the cytokine pattern, which is predominated by IL-10, TGF-β, and IDO, all factors that suppress a cytotoxic T cell response. Such a pattern correlates with a lack of T cell-dependent neoantigens, due to a low mutational burden.
It has been speculated that the cold microenvironment of childhood cancer is ascribable to the developing immune system, which may play an integral role in immune response to cancer.24 Changes in the immunological response with age are actually not surprising, if one considers the reactions to infectious stimuli. The Epstein-Barr virus (EBV), for example, typically produces an asymptomatic disease in children under the age of 6 years but is the cause of infectious mononucleosis and higher risk of Hodgkin’s lymphoma in adolescents and adults.89 Furthermore, this could be related to the simultaneous timing of carcinogenesis and immune system maturation, which could lead to the ignoring of the tumor by the immune cells.24
In adult cancer, it has been proposed that an effective immunotherapy should be stratified depending on the baseline steady-state immune microenvironment (hot, altered-immunosuppressed, altered-excluded, and cold immune tumors).90 Thus, immunotherapy employed for WT faces the challenge that cold tumors are the most arduous to eradicate, and the recommended approach is to turn them into hot tumors. This can be achieved by providing pro-inflammatory stimuli attracting immune cells to the tumor bed in combination with immune checkpoint inhibitors.91 Such pro-inflammatory stimuli might be provided by novel experimental approaches such as oncolytic viruses or bispecific antibodies linking T cells to tumor cells. Further options to induce an immunogenic tumor cell death include selected chemotherapies or low doses of conventional radiotherapy. Lately, a number of reports specifically documented immune-enhancing aspects of radiotherapy in combination with checkpoint inhibition.92 Radiotherapy was shown to induce a re-programming of M2-like macrophages into M1-like macrophages.93,94 The latter aspect could be specifically relevant in WT, as M2 macrophages are predominant. As neo-adjuvant radio- and chemotherapy are already included in the standard treatment for many pediatric solid cancers, it would be crucial to understand their putative immunomodulatory effects in this tumor entity and eventually consider them for a coherent combination therapy with checkpoint inhibitors.
A second reasonable immunotherapeutic strategy appears to be the development of cytotoxic monoclonal antibodies against glycolipids or glycoproteins, as anti-GD2 mAb therapy has shown remarkable success in neuroblastoma. In this line, identification of novel glycan antigens on WT such as CSA will be of interest. Moreover, antibody-dependent cellular cytotoxicity or antibody-dependent phagocytosis might be evaluated as a mechanism of action of the antibody-mediated cytotoxic effect.95 Cytotoxic antibody therapy might be further enhanced by the blockade of macrophage checkpoints such as the CD47/SIRPα axis96,97 or HDAC-inhibitors.55
It has been shown that the inhibition of pro-apoptotic signaling mediated by the BCL-2 family could potentially further enhance immunotherapy.98 As BCL-2 appears to be of prognostic relevance in WTs, inhibition of BCL-2 might be considered to be combined with cytotoxic antibody therapy or other means.
Furthermore, the identification of specific targets, expressed on tumor cells and absent or minimally expressed on normal cells, is crucial to minimize the potential off-tumor toxicities of immunotherapy.99 Immunotherapy is indeed not to be considered risk free. Adverse events such as aplastic anemia, hypothyroidism, or hypophysitis have been described in association with mAb therapy.100 As these could have lifelong consequences, especially on the developing child, they need to be accurately taken into consideration. However, in contrast to standard chemotherapy, immunotherapy has the potential to be extremely cell-specific, therefore lowering the risk of long-term adverse events. This could have, in the authors’ opinion, important implications, especially for high-risk pediatric tumors, which usually undergo heavier treatment courses.
It is clear that its orphan disease character constitutes great constraint for the possibility of conducting statistically significant preclinical and clinical studies of WT. For this reason, we advocate for larger multicenter diagnostic and therapeutic studies, which could allow the inclusion of a sufficient number of patients, in order to broaden the much-needed knowledge of the immune contexture of childhood solid cancer. In agreement with the pediatric strategy forum,9 we believe that this is crucial to conceive clinical trials with a higher possibility of success and finally fulfill the promise of immunotherapy in reducing mortality and morbidity in childhood cancer.
Materials and methods
Research strategy
The search was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Checklist.101 We used PubMed and the Cochrane Library database with the MeSH headings “Wilms Tumor” or “Nephroblastoma” and the subheading “immunology” along with terms “immune microenvironment” and “inflammatory.” The limiters were humans, “infants,” and “childhood” and 1990–2020. The resulting abstracts were then manually curated by the authors. The immunotherapy results were separately investigated with the MeSH headings “Immunotherapy” and “Pediatric Solid Tumors” in order to have the broadest dataset available. The titles and abstracts were manually reviewed for pertinence, and when in doubt the full text was consulted to ascertain the involvement of nephroblastoma patients in the trials. Studies not in English, on adult patients (>18 years), where WT was only exploited as a control group without results displayed, and where the focus was directed on genetic biomarkers (WT1) or differential diagnostic markers were excluded. Pertinent articles cited in the studies identified through database search were also included in our review and are listed as emerging from “other sources.”
Of the 141 studies that matched our research string, 50 were found eligible for full-text evaluation, and, after application of our exclusion criteria, a total of 31 studies matched the purposes of our study (Figure 1). The majority (28/31) of the articles found were translational clinical studies. Due to the orphan disease character, studies were frequently done on a very limited number of patients (median n = 16). Our systematic review still revealed a surprisingly uniform picture independent of the study modality (IHC on FFPE samples, cell line culture, xenograft, flow cytometry), patient number, or patient characteristics. Tables 2 and 3 list the evaluated studies.
Table 2.
Studies evaluating immunological parameters in Wilms tumors
| Study | Immunological parameter evaluated | Methodology used | No. patients |
|---|---|---|---|
| Liou et al. Vasc. Cell 201325 | immune infiltration: (TAM ↓∗) | IHC | 124 |
| Maturu et al. Translational Oncology 201426 | immune infiltration (CTL, B cells, TAM, TIN, MC) and inflammatory markers (COX2, HIF1, pStat3, pErk, iNOS, NT, VEGF) | IHC on FFPE patient samples + mouse model (WT1 gene ablation) | 16 |
| Maturu et al. Neoplasia 201727 | immune infiltration (CTL, T-reg, NK, pDC, B cells), inflammatory cytokines (IL-10, TGF-β, TNF-α, IL-1β, IL-4, IL-6, IL-8, IL-12, IL-23, CCL3, CCL4, CCL5, CCL17, CCL22, CCL20, CXCL12), inflammatory markers (COX2, pStat3, HIFa) and IDO. | IHC on FFPE patient samples + mouse model (WT1 gene ablation) | 16 |
| Holl et al. J. Pediatr. Urol. 201928 | immune infiltration (CTL, TAM, T-reg, CD45+, DC, NK) and checkpoint molecules (PD-L1) | IHC | 5 |
| Tian et al. J. Pediatr. Urol. 202029 | immune infiltration (M1 and M2-type TAM↓∗) | IHC, IF, western blot | 61 |
| Vakkila et al. Clinical Cancer Res. 200624 | immune infiltration (CTL, TAM, DC, NK cells) | IHC | 5 |
| Nagai et al. Pediatr. Res. 199723 | immune infiltration (CTL) | tumor culture: CD3+CD4−CD8+ CTL line established from patient TILs | 1 |
| Nanno et al. Eur. J. Immunol. 199222 | immune infiltration (CTL-γ/δ T cell antigen receptors) | Tumor cell culture, IF, northern blotting, DNA-amplification, cloning, and sequencing | 1 |
| Droz et al. Hum. Pathol. 199019 | immune infiltration (CTL, B cells, TAM) | IHC on frozen samples | 12 |
| Haas et al. Cancer Immunol. Immunother. 199020 | immune infiltration (CTL, B cells, TAM) | ICC, cell culture | 9 |
| Rivoltini et al. Cancer Immunol. Immunother. 199221 | immune infiltration (CTL-γ/δ T cell antigen receptors, B cells), cytokine expression (TGF- β), and adhesion molecules (ICAM1, LFA3) | cell culture | 5 |
| Mardanpour et al. Tumour Biol. 202030 | immune infiltration (CD8+∗) | IHC in FFPE samples | 42 |
| Routh et al. J. Urol. 200840 | checkpoint molecules (PD-L1↓∗) | IHC on FFPE samples | 81 |
| Silva et al. J. Pathol. Clin. Res. 202042 | immune infiltration (CTLs, T-reg, TAM) and checkpoint molecules (PD-1/PD-L1) | 30 | |
| Mochizuki et al. Pediatr. Hematol. Oncol. 201941 | checkpoint molecules (TIM3, LAG 3, BTLA, GAL9, MCH II, HVEM) | IHC on FFPE samples | 10 |
| Pinto et al. Pediatr Blood Cancer 201738 | checkpoint molecules (PD-1, PD-L1, PD-L2) | IHC on FFPE samples, mRNA expression | 25 |
| Majzner et al. Clin. Cancer Res. 201944 | checkpoint molecules (B7-H3) | IHC on FFPE samples, flow cytometry | 12 |
| Diniz et al. Pediatr. Hematol. Oncol. 201347 | dMMR/MSI | IHC on FFPE, RT-PCR, FCE | 45 |
| Segers et al. Pediatr. Dev. Pathol. 201248 | dMMR/MSI | IHC on FFPE, PCR | 100 |
| Mason et al. J. Ped. Surg. 200049 | MSI | PCR | 14 |
| Scursoni et al. Pediatr. Dev. Pathol. 201052 | cell surface molecules (NeuGc-GM3) | IHC on FFPE samples | 25 |
| Kinoshita et al. Eur. J. Pediatr. Surg. 201459 | cell surface molecules (GPC3) | IHC on FFPE samples, ELISA | 30 |
| Tretiakova et al. Virchows Arch. 201560 | cell surface molecules (GPC3) | IHC on FFPE samples, microarray analysis | 21 |
| Maeurer et al. Cancer Immunol. Immunother. 199562 | cytokines (IL-1, IL-2, IL-4, IL-6, IL-10, TGF-β, TNF-α, INF-γ, GM-CSF, TCR-α, TCR-β) and immune infiltration (CTL, NK, B cells) | cultured cell lines: IHC, flow cytometry, cDNA extraction and amplification | 0 |
| Amarante et al. Int. Rev. Immunol. 201765 | cytokines (TGF- β) | review on the role of TGF-β in WT | 0 |
| Zhang et al. J. Pediatr. Urol. 201464 | cytokines (TGF- β↓∗) | IHC | 51 |
| Schmitt et al. PLoS ONE 201172 | autoantibody signature | seral protein microarray screening | 88 |
| Schmitt et al. Int. J. Cancer 201273 | autoantibody signature | seral protein microarray screening | 110 |
| Zhao et al. J. Cancer Res. Clin. Oncol. 201976 | apoptosis-associated molecules (WTSP) | IHC, cell culture, western blot | 0 |
| Ghanem et al. Br. J. Cancer 200174 | apoptosis-associated molecules (BCL-2↓∗, BAX, BCL-XS/L) | IHC of FFPE samples, protein extraction, western blot | 61 |
| Re et al. Int. J. Cancer 199975 | apoptosis-associated molecules (BCL-2∗, BCL-XL) | IHC on FFPE and cryostat samples, IF, RNA and northern blot hybridization, RT-PCR, western blot | 13 |
Arrow down (↓) indicates negative prognostic value. Absence of arrow indicates that no significant correlation to overall survival (OS) or recurrence free survival (RFS) could be found. IHC, immunohistochemistry; FFPE, formalin-fixed paraffin-embedded; IF, immunofluorescence; FCE, fluorescence capillary electrophoresis; RT-PCR, real-time polymerase chain reaction; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; CTL, cytotoxic T lymphocytes; TAM, tumor-associated macrophages; TIN, tumor-infiltrating neutrophils; MC, mast cells; NK , natural killer cells; pDC, plasmacytoid dendritic cells; DC, dendritic cells; TILs, tumor-infiltrating lymphocytes.
∗Prognostic relevant factor.
Table 3.
Immunotherapy trials including Wilms tumor patients
| Study/trial | Immunotherapeutic agent | Targeted molecule | Phase | Patients enrolled | No. of Wilms tumor patients | Results |
|---|---|---|---|---|---|---|
| Hont et al. J. Clin. Oncol. 201932 | TAA-Ts | WT1 PRAME survivin | phase I | relapsed and refractory solid tumors | 9 | safe and immunogenic; response rate: 73% |
| Dohnal et al. Cytotherapy 200736 | DC-based vaccine | – | phase I | advanced solid pediatric malignancies | 2 | safe and immunogenic |
| Geoerger et al. Lancet Oncol. 20206/KEYNOTE-051 trial | pembrolizumab (mAb) | PD-L1 | phase I–II | PD-L1+, advanced relapsed or refractory solid pediatric tumor or lymphoma | 3 | ongoing trial; response only in Hodgkin’s lymphoma |
| Merchant et al. Clin. Cancer Res. 2016108 | ipilimumab (mAb) | CTLA4 | phase I | advanced solid pediatric malignancies | 3 | no objective tumor regressions |
| Modak et al. Cancer Res. 2018 NCT0109964445 | IP 131I-8H9 (radio-conjugated mAb) | B7-H3 | phase I | desmoplastic round cell tumors | not disclosed | satisfactory safety profile and promising antitumor activity; a phase II is ongoing. |
| NCT02982941 | enoblituzumab (mAb) | B7-H3 | phase I | B7-H3+ relapsed or refractory malignant solid tumors | not disclosed | ongoing trial; no results published |
| Cacciavillano et al. Pediatr. Blood Cancer 201554 | racotumomab (mAb) | NeuGc-GM | phase I | pediatric refractory malignancies | 1 | safe and immunogenic; no regression reported. |
| Tsuchiya et al. OncoImmunology 201757 | GPC3-peptide vaccination | GPC3 | phase I | pediatric refractory solid malignancies | 1 | safe and immunogenic; induced response in 1/2 WT patients |
| Seibel et al. J. Immunother. Emphasis Tumor Immunol. 199478 | rTNF-α + actinomycin | TNF-α | phase I | pediatric refractory malignancies | 6 | tumor response in 1/6 WT patients |
| Meany et al. J. Immunother. 200879 | rTNF-α + actinomycin | TNF-α | phase II | recurrent Wilms tumor | 19 | safe, complete tumor response in 3/19 patients |
Main immunotherapy clinical trials including WT patients are listed. TAA-Ts, tumor-associated antigen cytotoxic T cells; mAb, monoclonal antibody.
Acknowledgments
The authors acknowledge the valuable contribution of all the members of the Bergmann group of the surgical research laboratory of Anna Spiegel through useful discussion.
Author contributions
Conceptualization: M.B., M.M., and F.P.; Methodology: M.B., M.M., H.K., L.K., G.A., and F.P.; Investigation: F.P. and M.B.; Writing – original draft: F.P. and M.B.; Writing – review & editing: M.M., H.K., L.K., and G.A.
Declaration of interests
The authors declare no competing interests. M.B. receives research funds and consultant fees from Boehringer Ingelheim and Bristol Myers Squibb.
References
- 1.Pagès F., Mlecnik B., Marliot F., Bindea G., Ou F.-S., Bifulco C., Lugli A., Zlobec I., Rau T.T., Berger M.D. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 2.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019;38:255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright K.D., Green D.M., Daw N.C. Late effects of treatment for wilms tumor. Pediatr. Hematol. Oncol. 2009;26:407–413. doi: 10.1080/08880010903019344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakta N., Liu Q., Ness K.K., Baassiri M., Eissa H., Yeo F., Chemaitilly W., Ehrhardt M.J., Bass J., Bishop M.W. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390:2569–2582. doi: 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., OAK Study Group Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geoerger B., Kang H.J., Yalon-Oren M., Marshall L.V., Vezina C., Pappo A., Laetsch T.W., Petrilli A.S., Ebinger M., Toporski J. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020;21:121–133. doi: 10.1016/S1470-2045(19)30671-0. [DOI] [PubMed] [Google Scholar]
- 7.Keyel M.E., Reynolds C.P. Spotlight on dinutuximab in the treatment of high-risk neuroblastoma: development and place in therapy. Biologics. 2018;13:1–12. doi: 10.2147/BTT.S114530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones D.T.W., Banito A., Grünewald T.G.P., Haber M., Jäger N., Kool M., Milde T., Molenaar J.J., Nabbi A., Pugh T.J. Molecular characteristics and therapeutic vulnerabilities across paediatric solid tumours. Nat. Rev. Cancer. 2019;19:420–438. doi: 10.1038/s41568-019-0169-x. [DOI] [PubMed] [Google Scholar]
- 9.Pearson A.D.J., Rossig C., Lesa G., Diede S.J., Weiner S., Anderson J., Gray J., Geoerger B., Minard-Colin V., Marshall L.V. ACCELERATE and European Medicines Agency Paediatric Strategy Forum for medicinal product development of checkpoint inhibitors for use in combination therapy in paediatric patients. Eur. J. Cancer. 2020;127:52–66. doi: 10.1016/j.ejca.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Carachi R., Grosfeld J.L., editors. The Surgery of Childhood Tumors. Springer; 2016. [Google Scholar]
- 11.Irtan S., Ehrlich P.F., Pritchard-Jones K. Wilms tumor: “State-of-the-art” update, 2016. Semin. Pediatr. Surg. 2016;25:250–256. doi: 10.1053/j.sempedsurg.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Dome J.S., Graf N., Geller J.I., Fernandez C.V., Mullen E.A., Spreafico F., Van den Heuvel-Eibrink M., Pritchard-Jones K. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol. 2015;33:2999–3007. doi: 10.1200/JCO.2015.62.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Termuhlen A.M., Tersak J.M., Liu Q., Yasui Y., Stovall M., Weathers R., Deutsch M., Sklar C.A., Oeffinger K.C., Armstrong G. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer. 2011;57:1210–1216. doi: 10.1002/pbc.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotton C.A., Peterson S., Norkool P.A., Takashima J., Grigoriev Y., Green D.M., Breslow N.E. Early and late mortality after diagnosis of wilms tumor. J. Clin. Oncol. 2009;27:1304–1309. doi: 10.1200/JCO.2008.18.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Pal H.J., van Dalen E.C., van Delden E., van Dijk I.W., Kok W.E., Geskus R.B., Sieswerda E., Oldenburger F., Koning C.C., van Leeuwen F.E. High risk of symptomatic cardiac events in childhood cancer survivors. J. Clin. Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard-Jones K., Bergeron C., de Camargo B., Oldenburger F., Boccon-Gibod L., Leuschner I., Vujanic G., Sandstedt B., de Kraker J., van Tinteren H. Omission of doxorubicin from the treatment of stage II–III, intermediate-risk Wilms’ tumour (SIOP WT 2001): an open-label, non-inferiority, randomised controlled trial. Lancet. 2015;386:1156–1164. doi: 10.1016/S0140-6736(14)62395-3. [DOI] [PubMed] [Google Scholar]
- 17.Wayne A.S., Capitini C.M., Mackall C.L. Immunotherapy of childhood cancer: from biologic understanding to clinical application. Curr. Opin. Pediatr. 2010;22:2–11. doi: 10.1097/MOP.0b013e3283350d3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry R.L., Meyran D., Ziegler D.S., Haber M., Ekert P.G., Trapani J.A., Neeson P.J. Immune profiling of pediatric solid tumors. J. Clin. Invest. 2020;130:3391–3402. doi: 10.1172/JCI137181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Droz D., Rousseau-Merck M.F., Jaubert F., Diebold N., Nezelof C., Adafer E., Mouly H. Cell differentiation in Wilms’ tumor (nephroblastoma): an immunohistochemical study. Hum. Pathol. 1990;21:536–544. doi: 10.1016/0046-8177(90)90011-s. [DOI] [PubMed] [Google Scholar]
- 20.Haas G.P., Solomon D., Rosenberg S.A. Tumor-infiltrating lymphocytes from nonrenal urological malignancies. Cancer Immunol. Immunother. 1990;30:342–350. doi: 10.1007/BF01786883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivoltini L., Arienti F., Orazi A., Cefalo G., Gasparini M., Gambacorti-Passerini C., Fossati-Bellani F., Parmiani G. Phenotypic and functional analysis of lymphocytes infiltrating paediatric tumours, with a characterization of the tumour phenotype. Cancer Immunol. Immunother. 1992;34:241–251. doi: 10.1007/BF01741792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanno M., Seki H., Mathioudakis G., Suzuki R., Itoh K., Ioannides C.G., Suzuki S., Chen P.-F., Platsoucas C.D. γ/δ T cell antigen receptors expressed on tumor-infiltrating lymphocytes from patients with solid tumors. Eur. J. Immunol. 1992;22:679–687. doi: 10.1002/eji.1830220310. [DOI] [PubMed] [Google Scholar]
- 23.Nagai K., Yamada A., Eguchi H., Kato H., Itoh K. HLA-A2402-restricted and tumor-specific cytotoxic T lymphocytes from tumor-infiltrating lymphocytes of a child with Wilms’ tumor. Pediatr. Res. 1997;42:122–127. doi: 10.1203/00006450-199707000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Vakkila J., Jaffe R., Michelow M., Lotze M.T. Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: a major nosologic difference with adult tumors. Clin. Cancer Res. 2006;12:2049–2054. doi: 10.1158/1078-0432.CCR-05-1824. [DOI] [PubMed] [Google Scholar]
- 25.Liou P., Bader L., Wang A., Yamashiro D., Kandel J.J. Correlation of tumor-associated macrophages and clinicopathological factors in Wilms tumor. Vasc. Cell. 2013;5:5. doi: 10.1186/2045-824X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maturu P., Overwijk W.W., Hicks J., Ekmekcioglu S., Grimm E.A., Huff V. Characterization of the inflammatory microenvironment and identification of potential therapeutic targets in wilms tumors. Transl. Oncol. 2014;7:484–492. doi: 10.1016/j.tranon.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maturu P., Jones D., Ruteshouser E.C., Hu Q., Reynolds J.M., Hicks J., Putluri N., Ekmekcioglu S., Grimm E.A., Dong C., Overwijk W.W. Role of Cyclooxygenase-2 Pathway in Creating an Immunosuppressive Microenvironment and in Initiation and Progression of Wilms’ Tumor. Neoplasia. 2017;19:237–249. doi: 10.1016/j.neo.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holl E.K., Routh J.C., Johnston A.W., Frazier V., Rice H.E., Tracy E.T., Nair S.K. Immune expression in children with Wilms tumor: a pilot study. J. Pediatr. Urol. 2019;15:441.e1–441.e8. doi: 10.1016/j.jpurol.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Tian K., Wang X., Wu Y., Wu X., Du G., Liu W., Wu R. Relationship of tumour-associated macrophages with poor prognosis in Wilms’ tumour. J. Pediatr. Urol. 2020;16:376.e1–376.e8. doi: 10.1016/j.jpurol.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Mardanpour K., Rahbar M., Mardanpour S., Mardanpour N., Rezaei M. CD8+ T-cell lymphocytes infiltration predict clinical outcomes in Wilms’ tumor. Tumour Biol. 2020;42 doi: 10.1177/1010428320975976. 1010428320975976. [DOI] [PubMed] [Google Scholar]
- 31.Das R.K., Vernau L., Grupp S.A., Barrett D.M. Naïve T-cell Deficits at Diagnosis and after Chemotherapy Impair Cell Therapy Potential in Pediatric Cancers. Cancer Discov. 2019;9:492–499. doi: 10.1158/2159-8290.CD-18-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hont A.B., Cruz C.R., Ulrey R., O’Brien B., Stanojevic M., Datar A., Albihani S., Saunders D., Hanajiri R., Panchapakesan K. Immunotherapy of Relapsed and Refractory Solid Tumors With Ex Vivo Expanded Multi-Tumor Associated Antigen Specific Cytotoxic T Lymphocytes: A Phase I Study. J. Clin. Oncol. 2019;37:2349–2359. doi: 10.1200/JCO.19.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.B., Haber D.A. Wilms tumor and the WT1 gene. Exp. Cell Res. 2001;264:74–99. doi: 10.1006/excr.2000.5131. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda S., Pelus L.M. Survivin, a cancer target with an emerging role in normal adult tissues. Mol. Cancer Ther. 2006;5:1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 35.Toledo S.R.C., Zago M.A., Oliveira I.D., Proto-Siqueira R., Okamoto O.K., Severino P., Vêncio R.Z.N., Gamba F.T., Silva W.A., Moreira-Filho C.A. Insights on PRAME and osteosarcoma by means of gene expression profiling. J. Orthop. Sci. 2011;16:458–466. doi: 10.1007/s00776-011-0106-7. [DOI] [PubMed] [Google Scholar]
- 36.Dohnal A.M., Witt V., Hügel H., Holter W., Gadner H., Felzmann T. Phase I study of tumor Ag-loaded IL-12 secreting semi-mature DC for the treatment of pediatric cancer. Cytotherapy. 2007;9:755–770. doi: 10.1080/14653240701589221. [DOI] [PubMed] [Google Scholar]
- 37.Lucchesi M., Sardi I., Puppo G., Chella A., Favre C. The dawn of “immune-revolution” in children: early experiences with checkpoint inhibitors in childhood malignancies. Cancer Chemother. Pharmacol. 2017;80:1047–1053. doi: 10.1007/s00280-017-3450-2. [DOI] [PubMed] [Google Scholar]
- 38.Pinto N., Park J.R., Murphy E., Yearley J., McClanahan T., Annamalai L., Hawkins D.S., Rudzinski E.R. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr. Blood Cancer. 2017;64:e26613. doi: 10.1002/pbc.26613. [DOI] [PubMed] [Google Scholar]
- 39.Ring E.K., Markert J.M., Gillespie G.Y., Friedman G.K. Checkpoint Proteins in Pediatric Brain and Extracranial Solid Tumors: Opportunities for Immunotherapy. Clin. Cancer Res. 2017;23:342–350. doi: 10.1158/1078-0432.CCR-16-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Routh J.C., Ashley R.A., Sebo T.J., Lohse C.M., Husmann D.A., Kramer S.A., Kwon E.D. B7-H1 expression in Wilms tumor: correlation with tumor biology and disease recurrence. J. Urol. 2008;179:1954–1959, discussion 1959–1960. doi: 10.1016/j.juro.2008.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mochizuki K., Kawana S., Yamada S., Muramatsu M., Sano H., Kobayashi S., Ohara Y., Takahashi N., Hakozaki M., Yamada H. Various checkpoint molecules, and tumor-infiltrating lymphocytes in common pediatric solid tumors: Possibilities for novel immunotherapy. Pediatr. Hematol. Oncol. 2019;36:17–27. doi: 10.1080/08880018.2019.1578843. [DOI] [PubMed] [Google Scholar]
- 42.Silva M.A., Triltsch N., Leis S., Kanchev I., Tan T.H., Van Peel B., Van Kerckhoven M., Deschoolmeester V., Zimmermann J. Biomarker recommendation for PD-1/PD-L1 immunotherapy development in pediatric cancer based on digital image analysis of PD-L1 and immune cells. J. Pathol. Clin. Res. 2020;6:124–137. doi: 10.1002/cjp2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melaiu O., Lucarini V., Giovannoni R., Fruci D., Gemignani F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.07.001. Published online July 10, 2020. [DOI] [PubMed] [Google Scholar]
- 44.Majzner R.G., Theruvath J.L., Nellan A., Heitzeneder S., Cui Y., Mount C.W., Rietberg S.P., Linde M.H., Xu P., Rota C. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019;25:2560–2574. doi: 10.1158/1078-0432.CCR-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Modak S., Carrasquillo J., LaQuaglia M., Pat Z., Heaton T., Cheung N.-K., Pandit-Taskar N. Abstract CT006: Intraperitoneal radioimmunotherapy for desmoplastic small round cell tumor: Results of a phase I study ( NCT01099644) Cancer Res. 2018;78(Suppl 13):CT006. [Google Scholar]
- 46.Chang L., Chang M., Chang H.M., Chang F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl. Immunohistochem. Mol. Morphol. 2018;26:e15–e21. doi: 10.1097/PAI.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 47.Diniz G., Aktas S., Cubuk C., Ortac R., Vergin C., Olgun N. Tissue expression of MLH1, PMS2, MSH2, and MSH6 proteins and prognostic value of microsatellite instability in Wilms tumor: experience of 45 cases. Pediatr. Hematol. Oncol. 2013;30:273–284. doi: 10.3109/08880018.2013.780274. [DOI] [PubMed] [Google Scholar]
- 48.Segers H., van den Heuvel-Eibrink M.M., de Krijger R.R., Pieters R., Wagner A., Dinjens W.N.M. Defects in the DNA mismatch repair system do not contribute to the development of childhood wilms tumors. Pediatr. Dev. Pathol. 2013;16:14–19. doi: 10.2350/12-09-1249-OA. [DOI] [PubMed] [Google Scholar]
- 49.Mason J.E., Goodfellow P.J., Grundy P.E., Skinner M.A. 16q loss of heterozygosity and microsatellite instability in Wilms’ tumor. J. Pediatr. Surg. 2000;35:891–896, discussion 896–897. doi: 10.1053/jpsu.2000.6911. [DOI] [PubMed] [Google Scholar]
- 50.Li N., Gao W., Zhang Y.-F., Ho M. Glypicans as Cancer Therapeutic Targets. Trends Cancer. 2018;4:741–754. doi: 10.1016/j.trecan.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki M., Cheung N.-K.V. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin. Ther. Targets. 2015;19:349–362. doi: 10.1517/14728222.2014.986459. [DOI] [PubMed] [Google Scholar]
- 52.Scursoni A.M., Galluzzo L., Camarero S., Pozzo N., Gabri M.R., de Acosta C.M., Vázquez A.M., Alonso D.F., de Dávila M.T.G. Detection and characterization of N-glycolyated gangliosides in Wilms tumor by immunohistochemistry. Pediatr. Dev. Pathol. 2010;13:18–23. doi: 10.2350/08-10-0544.1. [DOI] [PubMed] [Google Scholar]
- 53.Mulens V., de la Torre A., Marinello P., Rodríguez R., Cardoso J., Díaz R., O’Farrill M., Macias A., Viada C., Saurez G. Immunogenicity and safety of a NeuGcGM3 based cancer vaccine: Results from a controlled study in metastatic breast cancer patients. Hum. Vaccin. 2010;6 doi: 10.4161/hv.6.9.12571. Published online September 14, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Cacciavillano W., Sampor C., Venier C., Gabri M.R., de Dávila M.T.G., Galluzzo M.L., Guthmann M.D., Fainboim L., Alonso D.F., Chantada G.L. A Phase I Study of the Anti-Idiotype Vaccine Racotumomab in Neuroblastoma and Other Pediatric Refractory Malignancies. Pediatr. Blood Cancer. 2015;62:2120–2124. doi: 10.1002/pbc.25631. [DOI] [PubMed] [Google Scholar]
- 55.Laengle J., Kabiljo J., Hunter L., Homola J., Prodinger S., Egger G., Bergmann M. Histone deacetylase inhibitors valproic acid and vorinostat enhance trastuzumab-mediated antibody-dependent cell-mediated phagocytosis. J. Immunother. Cancer. 2020;8:e000195. doi: 10.1136/jitc-2019-000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortiz M.V., Roberts S.S., Glade Bender J., Shukla N., Wexler L.H. Immunotherapeutic Targeting of GPC3 in Pediatric Solid Embryonal Tumors. Front. Oncol. 2019;9:108. doi: 10.3389/fonc.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuchiya N., Hosono A., Yoshikawa T., Shoda K., Nosaka K., Shimomura M., Hara J., Nitani C., Manabe A., Yoshihara H. Phase I study of glypican-3-derived peptide vaccine therapy for patients with refractory pediatric solid tumors. OncoImmunology. 2017;7:e1377872. doi: 10.1080/2162402X.2017.1377872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan E.S., Pawel B.R., Corao D.A., Venneti S., Russo P., Santi M., Sullivan L.M. Immunohistochemical expression of glypican-3 in pediatric tumors: an analysis of 414 cases. Pediatr. Dev. Pathol. 2013;16:272–277. doi: 10.2350/12-06-1216-OA.1. [DOI] [PubMed] [Google Scholar]
- 59.Kinoshita Y., Tanaka S., Souzaki R., Miyoshi K., Kohashi K., Oda Y., Nakatsura T., Taguchi T. Glypican 3 expression in pediatric malignant solid tumors. Eur. J. Pediatr. Surg. 2015;25:138–144. doi: 10.1055/s-0034-1393961. [DOI] [PubMed] [Google Scholar]
- 60.Tretiakova M., Zynger D.L., Luan C., Andeen N.K., Finn L.S., Kocherginsky M., Teh B.T., Yang X.J. Glypican 3 overexpression in primary and metastatic Wilms tumors. Virchows Arch. 2015;466:67–76. doi: 10.1007/s00428-014-1669-4. [DOI] [PubMed] [Google Scholar]
- 61.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maeurer M.J., Martin D.M., Castelli C., Elder E., Leder G., Storkus W.J., Lotze M.T. Host immune response in renal cell cancer: interleukin-4 (IL-4) and IL-10 mRNA are frequently detected in freshly collected tumor-infiltrating lymphocytes. Cancer Immunol. Immunother. 1995;41:111–121. doi: 10.1007/BF01527407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gülhan B., Orhan D., Kale G., Besbas N., Özen S. Studying cytokines of T helper cells in the kidney disease of IgA vasculitis (Henoch-Schönlein purpura) Pediatr. Nephrol. 2015;30:1269–1277. doi: 10.1007/s00467-015-3051-4. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Liu W., Qin Y., Wu R. Expression of TGF-β1 in Wilms’ tumor was associated with invasiveness and disease progression. J. Pediatr. Urol. 2014;10:962–968. doi: 10.1016/j.jpurol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Amarante M.K., de Oliveira C.E.C., Ariza C.B., Sakaguchi A.Y., Ishibashi C.M., Watanabe M.A.E. The predictive value of transforming growth factor-β in Wilms tumor immunopathogenesis. Int. Rev. Immunol. 2017;36:233–239. doi: 10.1080/08830185.2017.1291639. [DOI] [PubMed] [Google Scholar]
- 66.Wing J.B., Tanaka A., Sakaguchi S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity. 2019;50:302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 67.Munn D.H., Mellor A.L. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 69.Hutzen B., Ghonime M., Lee J., Mardis E.R., Wang R., Lee D.A., Cairo M.S., Roberts R.D., Cripe T.P., Cassady K.A. Immunotherapeutic Challenges for Pediatric Cancers. Mol. Ther. Oncolytics. 2019;15:38–48. doi: 10.1016/j.omto.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell B.B., Light N., Fabrizio D., Zatzman M., Fuligni F., de Borja R., Davidson S., Edwards M., Elvin J.A., Hodel K.P. Comprehensive Analysis of Hypermutation in Human Cancer. Cell. 2017;171:1042–1056.e10. doi: 10.1016/j.cell.2017.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westdorp H., Kolders S., Hoogerbrugge N., de Vries I.J.M., Jongmans M.C.J., Schreibelt G. Immunotherapy holds the key to cancer treatment and prevention in constitutional mismatch repair deficiency (CMMRD) syndrome. Cancer Lett. 2017;403:159–164. doi: 10.1016/j.canlet.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 72.Schmitt J., Keller A., Nourkami-Tutdibi N., Heisel S., Habel N., Leidinger P., Ludwig N., Gessler M., Graf N., Berthold F. Autoantibody signature differentiates Wilms tumor patients from neuroblastoma patients. PLoS ONE. 2011;6:e28951. doi: 10.1371/journal.pone.0028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmitt J., Heisel S., Keller A., Leidinger P., Ludwig N., Habel N., Furtwängler R., Nourkami-Tutdibi N., Wegert J., Grundy P. Multicenter study identified molecular blood-born protein signatures for Wilms Tumor. Int. J. Cancer. 2012;131:673–682. doi: 10.1002/ijc.26419. [DOI] [PubMed] [Google Scholar]
- 74.Ghanem M.A., Van der Kwast T.H., Den Hollander J.C., Sudaryo M.K., Van den Heuvel M.M., Noordzij M.A., Nijman R.J.M., Soliman E.H., van Steenbrugge G.J. The prognostic significance of apoptosis-associated proteins BCL-2, BAX and BCL-X in clinical nephroblastoma. Br. J. Cancer. 2001;85:1557–1563. doi: 10.1054/bjoc.2001.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Re G.G., Hazen-Martin D.J., Bahtimi R.E., Brownlee N.A., Willingham M.C., Garvin A.J. Prognostic significance of Bcl-2 in Wilms’ tumor and oncogenic potential of Bcl-XL in rare tumor cases. Int. J. Cancer. 1999;84:192–200. doi: 10.1002/(sici)1097-0215(19990420)84:2<192::aid-ijc17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Zhao W., Li J., Li P., Guo F., Gao P., Zhang J., Yan Z., Wang L., Zhang D., Qin P. Wilms tumor-suppressing peptide inhibits proliferation and induces apoptosis of Wilms tumor cells in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2019;145:2457–2468. doi: 10.1007/s00432-019-03003-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Zhang B., Wang J., Yu J., Zheng S. A serum protein fingerprint in the diagnosis and prognosis of Wilms’ tumors in children. Life Sci. J. 2009;6:27–32. [Google Scholar]
- 78.Seibel N.L., Dinndorf P.A., Bauer M., Sondel P.M., Hammond G.D., Reaman G.H. Phase I study of tumor necrosis factor-alpha and actinomycin D in pediatric patients with cancer: a Children’s Cancer Group study. J. Immunother. Emphasis Tumor Immunol. 1994;16:125–131. doi: 10.1097/00002371-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Meany H.J., Seibel N.L., Sun J., Finklestein J.Z., Sato J., Kelleher J., Sondel P., Reaman G. Phase 2 trial of recombinant tumor necrosis factor-alpha in combination with dactinomycin in children with recurrent Wilms tumor. J. Immunother. 2008;31:679–683. doi: 10.1097/CJI.0b013e3181826d72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953;7:320–338. [Google Scholar]
- 81.Khazamipour N., Al-Nakouzi N., Oo H.Z., Ørum-Madsen M., Steino A., Sorensen P.H., Daugaard M. Oncofetal Chondroitin Sulfate: A Putative Therapeutic Target in Adult and Pediatric Solid Tumors. Cells. 2020;9:818. doi: 10.3390/cells9040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costanzo V., Bardelli A., Siena S., Abrignani S. Exploring the links between cancer and placenta development. Open Biol. 2018;8:180081. doi: 10.1098/rsob.180081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ander S.E., Diamond M.S., Coyne C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019;4:eaat6114. doi: 10.1126/sciimmunol.aat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Svensson-Arvelund J., Mehta R.B., Lindau R., Mirrasekhian E., Rodriguez-Martinez H., Berg G., Lash G.E., Jenmalm M.C., Ernerudh J. The Human Fetal Placenta Promotes Tolerance against the Semiallogeneic Fetus by Inducing Regulatory T Cells and Homeostatic M2 Macrophages. J. Immunol. 2015;194:1534–1544. doi: 10.4049/jimmunol.1401536. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Y., Zheng Q., Jin L. The Role of B7 Family Molecules in Maternal-Fetal Immunity. Front. Immunol. 2020;11:458. doi: 10.3389/fimmu.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riella L.V., Dada S., Chabtini L., Smith B., Huang L., Dakle P., Mfarrej B., D’Addio F., Adams L.-T., Kochupurakkal N. B7h (ICOS-L) maintains tolerance at the fetomaternal interface. Am. J. Pathol. 2013;182:2204–2213. doi: 10.1016/j.ajpath.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 88.Salanti A., Clausen T.M., Agerbæk M.Ø., Al Nakouzi N., Dahlbäck M., Oo H.Z., Lee S., Gustavsson T., Rich J.R., Hedberg B.J. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell. 2015;28:500–514. doi: 10.1016/j.ccell.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rostgaard K., Balfour H.H., Jr., Jarrett R., Erikstrup C., Pedersen O., Ullum H., Nielsen L.P., Voldstedlund M., Hjalgrim H. Primary Epstein-Barr virus infection with and without infectious mononucleosis. PLoS ONE. 2019;14:e0226436. doi: 10.1371/journal.pone.0226436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 91.Ott P.A., Hodi F.S., Kaufman H.L., Wigginton J.M., Wolchok J.D. Combination immunotherapy: a road map. J. Immunother. Cancer. 2017;5:16. doi: 10.1186/s40425-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Twyman-Saint Victor C., Rech A.J., Maity A., Rengan R., Pauken K.E., Stelekati E., Benci J.L., Xu B., Dada H., Odorizzi P.M. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dovedi S.J., Adlard A.L., Lipowska-Bhalla G., McKenna C., Jones S., Cheadle E.J., Stratford I.J., Poon E., Morrow M., Stewart R. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 94.Stary V., Wolf B., Unterleuthner D., List J., Talic M., Laengle J., Beer A., Strobl J., Stary G., Dolznig H., Bergmann M. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J. Immunother. Cancer. 2020;8:e000667. doi: 10.1136/jitc-2020-000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petricevic B., Laengle J., Singer J., Sachet M., Fazekas J., Steger G., Bartsch R., Jensen-Jarolim E., Bergmann M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013;11:307. doi: 10.1186/1479-5876-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Veillette A., Chen J. SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018;39:173–184. doi: 10.1016/j.it.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Ring N.G., Herndler-Brandstetter D., Weiskopf K., Shan L., Volkmer J.-P., George B.M., Lietzenmayer M., McKenna K.M., Naik T.J., McCarty A. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA. 2017;114:E10578–E10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kasper S., Breitenbuecher F., Reis H., Brandau S., Worm K., Köhler J., Paul A., Trarbach T., Schmid K.W., Schuler M. Oncogenic RAS simultaneously protects against anti-EGFR antibody-dependent cellular cytotoxicity and EGFR signaling blockade. Oncogene. 2013;32:2873–2881. doi: 10.1038/onc.2012.302. [DOI] [PubMed] [Google Scholar]
- 99.Casey D.L., Cheung N.V. Immunotherapy of Pediatric Solid Tumors: Treatments at a Crossroads, with an Emphasis on Antibodies. Cancer Immunol. Res. 2020;8:161–166. doi: 10.1158/2326-6066.CIR-19-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 101.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Green D.M., Grigoriev Y.A., Nan B., Takashima J.R., Norkool P.A., D’Angio G.J., Breslow N.E. Congestive heart failure after treatment for Wilms’ tumor: a report from the National Wilms’ Tumor Study group. J. Clin. Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 103.Green D.M., Lange J.M., Qu A., Peterson S.M., Kalapurakal J.A., Stokes D.C., Grigoriev Y.A., Takashima J.R., Norkool P., Friedman D.L., Breslow N.E. Pulmonary disease after treatment for Wilms tumor: a report from the national wilms tumor long-term follow-up study. Pediatr. Blood Cancer. 2013;60:1721–1726. doi: 10.1002/pbc.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breslow N.E., Collins A.J., Ritchey M.L., Grigoriev Y.A., Peterson S.M., Green D.M. Vol. 174. J. Urol; 2005. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the U.S. Renal Data System; pp. 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Breslow N.E., Takashima J.R., Whitton J.A., Moksness J., D’Angio G.J., Green D.M. Second malignant neoplasms following treatment for Wilm’s tumor: a report from the National Wilms’ Tumor Study Group. J. Clin. Oncol. 1995;13:1851–1859. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 106.Wallace W.H., Shalet S.M., Crowne E.C., Morris-Jones P.H., Gattamaneni H.R. Ovarian failure following abdominal irradiation in childhood: natural history and prognosis. Clin. Oncol. (R. Coll. Radiol.) 1989;1:75–79. doi: 10.1016/s0936-6555(89)80039-1. [DOI] [PubMed] [Google Scholar]
- 107.Green D.M., Lange J.M., Peabody E.M., Grigorieva N.N., Peterson S.M., Kalapurakal J.A., Breslow N.E. Pregnancy outcome after treatment for Wilms tumor: a report from the national Wilms tumor long-term follow-up study. J. Clin. Oncol. 2010;28:2824–2830. doi: 10.1200/JCO.2009.27.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merchant M.S., Wright M., Baird K., Wxlaer L.H., Rodriguez-Galindo C., Bernstein D. Phase I clinical trial of Ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res. 2016;22:1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]