Abstract
Objective: To investigate the potential miRNA-mRNA network co-expressed in polycystic ovary syndrome (PCOS) and diabetes, and explore the molecular mechanism of traditional acupuncture treatment of PCOS. Methods: Patients with PCOS and diabetes who had undergone acupuncture treatment from January 2019 to June 2020 were recruited in this study. The potential miRNA-mRNA network co-expressed in PCOS and diabetes was obtained through bioinformatics analysis. The expression levels of candidate gen es were determined using quantitative qRT-PCR to study the effectiveness of acupuncture approach. Further, the mechanism of action of acupuncture method was determined using luciferase assay. Results: A total of 44 patients were included in this study. The miRNA-mRNA network for PCOS was then constructed based on the results of the bioinformatics analysis. Acupuncture treatment could significantly down-regulate miR-32-3p levels and up-regulate expression of PLA2G4A. Luciferase experiments showed that miR-32-3p could affect glucose metabolism in PCOS patients through down-regulating PLA2G4A expression. Functional and pathway enrichment analysis further suported this finding. Conclusions: MiR-32-3p regulates PLA2G4A protein expression, which is vital in the pathogenesis of PCOS and diabetes. Further, this research proved that the potential mechanism of traditional acupuncture treatment may be the downregulation of miR-32-3p, thus inhibiting PCOS and diabetes progression.
Keywords: Acupuncture therapy, polycystic ovary syndrome, diabetes mellitus, group IV phospholipases A2 (PLA2G4A), microRNAs
Introduction
Polycystic ovary syndrome (PCOS) is a worldwide hormonal and metabolic disorder of women [1,2]. Hyperandrogenemia, irregular or non-ovulation, and polycystic ovaries are the clinical manifestations of PCOS. A previous study reports that genetic and environmental factors play pivotal roles in onset and progression of PCOS [3]. The etiology of PCOS and mechanism of progression are not fully understood yet [4]. Therefore, there is a need to explore effective diagnostic and therapeutic approaches for PCOS.
PCOS is associated with metabolic-related complications, such as gestational diabetes, oxidative stress, IR (insulin resistance), and T2DM (type 2 diabetes) [5,6]. Although the relationship between insulin resistance and PCOS has been reported in previous studies, details on how the two conditions co-exist have not been fully explored. Excess POCS-mediated androgen inhibits insulin activity. Notably, insulin resistance and hyperinsulinemia can in turn aggravate the deterioration of PCOS endocrine as well as reproductive functions. The interplay between high androgen levels and insulin resistance makes PCOS and diabetes presenting a vicious circle process. Therefore, treatment of PCOS with diabetes is challenging.
In recent years, several studies have explored the link between regulatory miRNAs and various diseases [7]. MicroRNA is a single-stranded, non-coding and endogenous RNA molecule that inhibits mRNA translation by binding to target mRNA, thereby stopping translation of post-transcriptional proteins [8,9]. MicroRNAs mainly exist in tissue microenvironment where they regulate various key biological functions [10-13]. Studies have reported that miRNAs are potential biomarkers of diabetes [14,15]. Further, studies report that miRNAs in the circulatory system are implicated in the pathogenesis of PCOS [16]. Therefore, miRNA is a potential therapeutic target and a diagnostic biomarker for PCOS [17]. Regulation of miRNA expression is thought to serve a key part in disrupting the vicious circle of PCOS and diabetes, however, this postulation should be explored further. Acupuncture is a common therapeutic approach in East Asian medicine with a filament needle penetrating specific points on the skin called acupuncture points. Previous studies have proved that acupuncture regulates endocrine system, and it has been used in the treatment of female infertility and diabetes-related diseases worldwide [18,19]. Interestingly, animal experiments verified that miRNAs play an essential part in acupuncture treatment approach [20]. Therefore, acupuncture may block the vicious circle of diabetes and PCOS by regulating miRNA.
The aim for this work was to retrieve potential miRNA-mRNA network co-expressed in PCOS and diabetes utilizing microarray data in the Gene Expression Omnibus (GEO) database. Further, we purposed to elucidate the biological mechanism of acupuncture treatment in inhibiting the vicious cycle of PCOS/diabetes. In addition, we used pathogenesis and potential molecular targets of PCOS to understand the molecular mechanism of traditional acupuncture treatment of PCOS.
Materials and methods
Subjects
A total of 44 women with PCOS and diabetes who had been treated in our hospital from January 2019 to June 2020 were recruited in this study. These women were allocated randomly to the acupuncture group (n=23) and the normal control group (n=21). According to the ovulation conditions, these women also were divided into the ovulation group (n=34) and the no ovulation control group (n=10).
Inclusion criteria: (1) Aged over 18. (2) Patients with PCOS and diabetes were diagnosed for the first time by clinical manifestations and imaging examination. (3) Patients with consciousness and could actively cooperate. Exclusion criteria: (1) Patients with other gynecological diseases. (2) Patients with mental disorders and severe cerebrovascular disease. (3) Patients with severe cardiopulmonary, hepatorenal dysfunction and renal insufficiency. The acupoints were informed by traditional Chinese medicine theory and classic acupoint consensus and references [21]. All clinical samples were endometrial tissues collected from patients. Informed consent was obtained from participants before sample collection. The Ethics Committee of the Women and Children’s Hospital of Guangdong Medical University (No. 2020004) approved the study.
We carried out acupuncture treatment twice a week for 3 months on all the participants in the acupuncture group. Acupuncture procedure was carried out as follows: the participant lay in a supine position and a 25 to 40 mm long needle was inserted at different acupoints. The needle was maintained for 30 s upon arrival of qi, followed by twirling at 90°. Further, it was lifted and thrust 60-100 times/min with a range of 2 mm. The needle was maintained at each acupoint for 30 minutes. The stimulation intensity was adjusted depending on the patient’s condition. For Zusanli (ST36) and Guanyuan (CV4), 2 cm of the needle was inserted and retained for 10 min. Acupuncture was suspended when the patient reported discomfort.
Microarray analysis of differentially expressed genes and miRNA target genes prediction
Gene expression profiles were retrieved from GEO database, followed by normalization by the “normalize between array” function from “LIMMA” package [22]. Further, DEMs and DE-miRNAs of normal and diseased samples from GSE34526, GSE70318 and GSE25462 datasets were identified. miRNA target genes were predicted using the miRWalk3.0 web resource, RNAhybrid, TargetScan and miRTarBase (Version 7.0) [23].
Western blot experiments
Cell lysing was performed with the RIPA buffer enriched with a protease inhibitor cocktail. Afterwards, quantification of the total protein was conducted with the Bicinchoninic Acid kit (Pierce, USA). Then, we aliquoted 8 µg of the total protein and employed SDS-PAGE gel electrophoresis to resolve the proteins. The fractionated proteins were then electro-blotted onto a 0.45-µm PV membrane (Immobilon™; Merck Millipore, Darmstadt, Germany). Subsequently, blocking of the membranes and probing with the antibodies β-actin (1:500, #05-665; Merck Millipore) or anti-PLA2G4A (1:1000, #ab58375; Abcam, USA) for overnight were conducted. Thereafter, we conjugated the membranes with HRP-conjugated secondary antibodies (1:5000, #ab205719, Abcam) via incubation. An enhanced chemiluminescence was used to detect the bound antibodies. Finally, the Image J (NIH, Bethesda, MD, USA) software was employed to quantify the intensities of the bands.
Quantitative real-time PCR
Isolation of the total RNA was accomplished with the Mini-BEST Universal RNA Extraction kit (TaKaRa). Afterwards, the Prime-Script RT Master Mix (TaKaRa) was employed to convert the RNA into cDNA via reverse transcription. Then, the SYBR Green Master Mix (TaKaRa) was utilized in the qPCR, which was performed on the PCR LightCycler480 (Roche Diagnostics). At the same time, we used the TRIzol® reagent (Gibco/Life 270 Technologies, Thermo Fisher Scientific) to isolate the total miRNA from the cell cultures. The quantity and quality of miRNA were determined using stem-loop quantitative qRT-PCR (TaqMan probe approach). First-strand cDNA synthesis of purified miRNA was performed using M-MLV reverse transcriptase and primers as per the manufacturer provided protocol (Promega, Fitchberg, MA, USA). Relative gene expression was determined using U6 gene as internal reference. All qRT-PCR analyses were replicated thrice. The primers used were as follows: PLA2G4A, forward: 5’-AGGAAAAGGTCAATGCCGCC-3’, reverse: 5’-GCCCCAGACACCCATCAAGA-3’. GAPDH, forward: 5’-GCACCGTC AAGGCTGAGAAC-3’, reverse: 5’-TGGTGAAGACGCCAGTGG A-3’. MiR-32-3p forward: 5’-TTTCTCTATCGATAGGTACCGGCAGTTACCATTTCACAC-3’; reverse: 5’-CACGCCGAATCAACATCAGTCTGATAA-3’.
Transfection of miRNAs
As show in previous studies, miR-32-3p was upregulated or inhibited by a chemically synthesized miRNA mimics or inhibitors (Gene Pharma) [24,25]. Cells were seeded and transfected using a riboFECT™ CP transfection kit for 24 h as per the manufacturer provided protocol (Ribobio). RT-qPCR was used to determine transfection efficiency 48 h after transfection.
Dual-luciferase reporter assay
Gene-Chem (Shanghai, China) constructed the PLA2G4A reporter plasmids. Co-transfection of the reporter vector with PLA2G4A-WT or PLA2G4A-Mut into HEK293 cells was conducted with the Lipofectamine 2000 system (Invitrogen, Carlsbad, CA, USA) for 48 h. We already know that HEK293 is a cell line with high transfection efficiency and ease of culture. Luciferase enzyme activities were then assessed by the Dual Luciferase Reporter Assay System (Promega) as per the manufacturer provided protocol.
Enrichment analysis and GTEX databases analysis
KEGG enrichment and gene ontology (GO) analysis were employed to explore potential mechanism of PCOS pathogenesis using DAVID [26]. P<0.05 signified statistical significance. Association analysis of PLA2G4A with SMG1 and PTEM retrieved from GTEX database was also conducted with the R software. The “gganatogram” and “ggpubr” packages were utilized to plot the human protein expression map.
Statistical analysis
RNA sequence data were assessed using R software as indicated above. Other statistical analyses were performed using the GraphPad Prism software. The measurement data conforming to the normal distribution were expressed by mean ± standard deviation. Differences between two groups were compared via the two-tailed Students’ t-test. The enumeration data was expressed as percentage or rate. And chi square test was used for comparisons between two groups. P<0.05 was considered as difference with statistical significance.
Result
Patient’s characteristics
A total of 44 patients were recruited in this study. 23 participants were grouped in the acupuncture treatment group whereas 21 participants were grouped in the control group. The female characteristics of the two groups are shown in Table 1. Differences between the indicators in each group were negligible at the baseline level. However, as shown in Table 2, after acupuncture adjuvant treatment, there were significant differences in LH, FSH, LH/FSH, E2, P, FPG and TNF-α (pg/ml) between the two groups (P<0.05). Further, long-term acupuncture treatment increased LH, FSH, LH/FSH, E2 and P levels in patients with PCOS and diabetes which implied improvement in endocrine function. Moreover, acupuncture treatment also regulated blood sugar level in diabetes and reduced blood inflammation indicators such as TNF-α (P<0.05). In addition, the indexes of average age, duration of infertility, and BMI between the two groups were statistically comparable (P>0.05).
Table 1.
Basic information of patients in two groups
| Parameters | Control group (n=21) | Acupuncture group (n=23) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (year) | 34.0±2.81 | 33.48±2.74 | 0.621 | 0.538 |
| Infertility duration (year) | 3.48±1.83 | 3.48±1.38 | 0.001 | 0.999 |
| Ovulation (Cases) | 7 (33.3%) | 5 (26.1%) | 0.744 | 0.388 |
| BMI (kg/m2) | 25.85±1.09 | 25.64±1.14 | 0.623 | 0.536 |
| LH (mIU/ml) | 7.94±0.72 | 7.66±0.61 | 1.396 | 0.170 |
| FSH (mIU/ml) | 6.85±0.81 | 6.72±0.71 | 0.567 | 0.574 |
| LH/FSH | 1.16±0.08 | 1.15±0.09 | 0.388 | 0.700 |
| E2 (pg/ml) | 81.42±5.75 | 78.86±5.04 | 1.574 | 0.123 |
| P (pg/ml) | 0.66±0.09 | 0.64±0.07 | 0.827 | 0413 |
| FPG (mg/dL) | 93.62±9.26 | 97.39±14.17 | 1.034 | 0.307 |
| 2 h Glucose OGTT (mg/dL) | 144.92±34.5 | 142.91±31.71 | 0.201 | 0.841 |
| HbA1c (%) | 7.4±0.36 | 7.34±0.37 | 0.544 | 0.589 |
| IL-6 (pg/ml) | 59.16±4.17 | 57.67±5.54 | 1.000 | 0.323 |
| TNF-α (pg/ml) | 40.48±3.14 | 41.19±3.2 | 0.742 | 0.462 |
| CRP (mg/l) | 4.05±0.35 | 4.0±0.32 | 0.495 | 0.623 |
Table 2.
Characteristics of the study population at 3 months after acupuncture
| Parameters | Control group (n=21) | Acupuncture group (n=23) | t/χ2 value | P value |
|---|---|---|---|---|
| Ovulation (Cases) | 13 (61.9%) | 21 (91.3%) | 5.403 | 0.020 |
| BMI (kg/m2) | 25.6±0.59 | 25.57±0.57 | 0.172 | 0.865 |
| LH (mIU/ml) | 7.13±0.51 | 7.62±0.41 | 3.527 | 0.001 |
| FSH (mIU/ml) | 7.67±0.53 | 7.16±0.43 | 3.519 | 0.001 |
| LH/FSH | 0.93±0.08 | 1.07±0.07 | 6.191 | <0.001 |
| E2 (pg/ml) | 81.68±2.96 | 77.95±3.36 | 3.891 | <0.001 |
| P (pg/ml) | 0.61±0.07 | 0.65±0.05 | 2.196 | 0.034 |
| FPG (mg/dL) | 89.03±6.87 | 94.7±8.76 | 2.373 | 0.022 |
| 2 h Glucose OGTT (mg/dL) | 142.9±20.53 | 142.48±21.15 | 0.067 | 0.947 |
| HbA1c (%) | 7.26±0.13 | 7.33±0.2 | 1.362 | 0.181 |
| IL-6 (pg/ml) | 57.59±5.3 | 58.41±3.28 | 0.623 | 0.537 |
| TNF-α (pg/ml) | 38.7±3.12 | 41.33±2.89 | 2.903 | 0.006 |
| CRP (mg/l) | 3.9±0.23 | 4.0±0.21 | 1.508 | 0.139 |
Identification of DEMs and DEmiRNAs
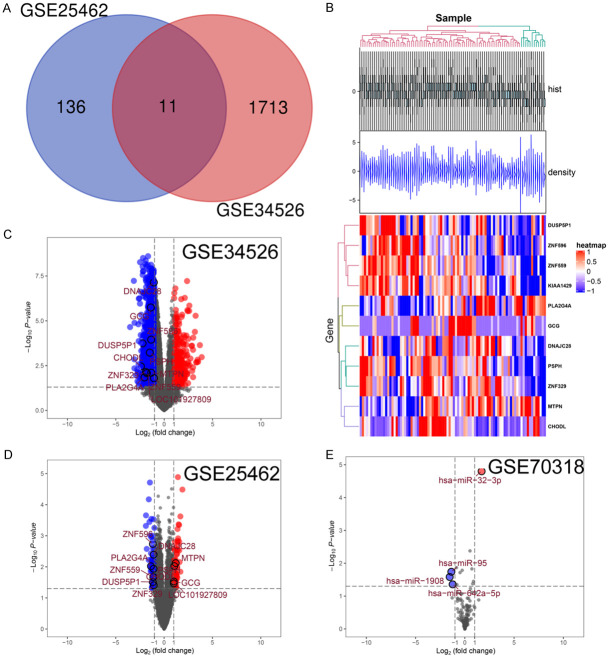
R software package was used to analyze PCOS data set GSE34526 (3 PCOS, 7 normal) and diabetes mRNA data set GSE25462 (10 diabetes, 25 normal), miRNA data set GSE70318 (19 diabetes, 17 normal) to explore differentially expressed genes involved in PCOS and diabetes. There is currently no dataset on the effectiveness of acupuncture in the treatment of PCOS. Our aim was to discover potential miRNA-mRNA networks with these datasets (GSE34526, GSE25462 and GSE70318) in PCOS patients with diabetes and provide guidance for subsequent experimental studies. Veen diagrams were then generated using GSE34526 and GSE25462 datasets to identify mRNAs involved PCOS and diabetes (Figure 1A). Eleven genes were differentially expressed including: ZNF596, PLA2G4A, DUSP5P1, CHODL, LOC101927809, ZNF329, DNAJC28, MTPN, ZNF559, GCG, and PSPH. A heatmap was constructed using GSE25462 data set for visualization of clustering and expression of identified genes (Figure 1B). Differentially expressed genes from each data set were used to generate volcano maps (Figure 1C-E). ZNF596, PLA2G4A, DUSP5P1, CHODL, ZNF329, DNAJC28 and ZNF559 genes were positively correlated with PCOS and diabetes. Higher expression level of miR-32-3p was observed in diabetic samples as compared with the normal samples.
Figure 1.
Identification of DEMs and DEmiRNAs. Green dots indicate the down-regulated genes and red dots denote the genes that are upregulated. A: Venn diagram showing intersection of DEMs in GSE25462 and GSE34526 datasets; B: Heat map of DEMs from GSE25462 dataset; C: Volcano plot of GSE34526 (21655 RNAs); D: Volcano plot of GSE25462 (20486 RNAs); E: Volcano plot of GSE70318 (153 miRNAs).
Construction of miRNA-mRNA network
The results of Cytoscape software (version 3.8.0) showed that miRNA-mRNA network consists of up-regulated and down-regulated miRNAs and their target genes (Figure 2A). For example, high expression levels of hsa-miR-32-3p may down-regulate transcription of PLA2G4A, ZNF559, ZNF329, CHODL, ZNF596, MTPN and GCG. And down-regulation of hsa-miR-642a-5p may up-regulate PSPH transcription. These findings imply that miR-32-3p affects the pathological process of PCOS and diabetes by down-regulating PLA2G4A, ZNF559, ZNF329, CHODL and ZNF596.
Figure 2.
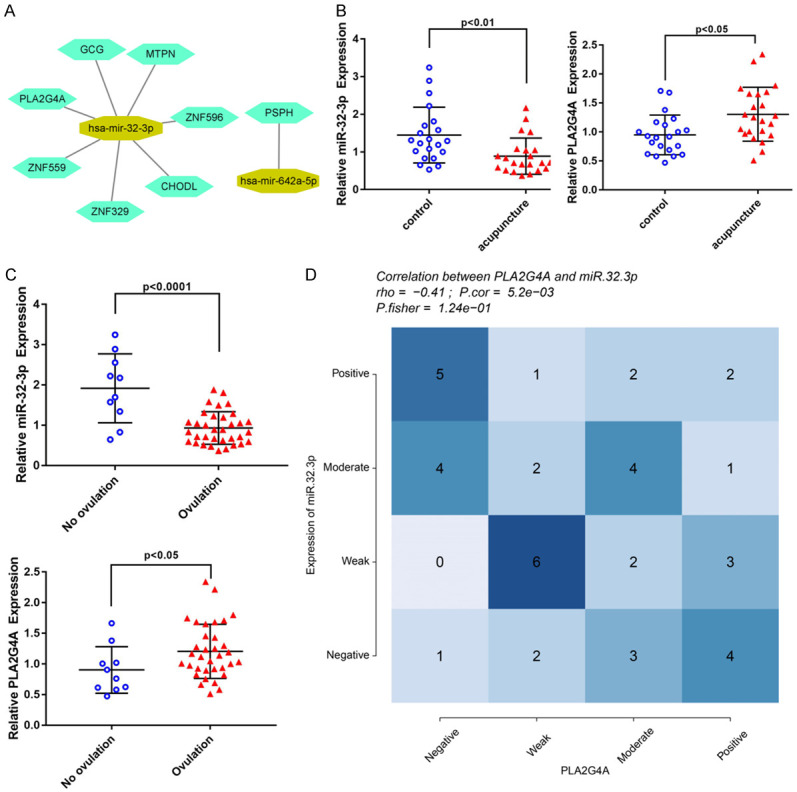
Construction of miRNA-mRNA network and analysis of clinical correlation. A: The miRNA-mRNA network for DE-miRNAs and DEMs; B: Relative expression of PLA2G4A and miR-32-3p detected by qPCR. Significant differences were observed between control and acupuncture groups; C: Relative expression of PLA2G4A and miR-32-3p detected by qPCR. Significant differences were observed between groups with or without ovulation after acupuncture treatment. D: Correlation of miR-32-3p and PLA2G4A expression levels in clinical samples. The relative expression of miR-32-3p and PLA2G4A in clinical samples is negatively correlated.
Functional and pathways enrichment analysis
Enrichment analysis of DEMs showed enrichment of phospholipase A1 activity, phospholipase A2 activity consuming 1,2-dioleoylphosphatidylethanolamine, calcium-dependent phospholipase A2 activity, and Lysophospholipase activity. Pathway analysis indicated that DEMs were involved in: ether lipid metabolism; glycine, serine and threonine metabolism; linoleic acid metabolism; arachidonic acid metabolism; VEGF signaling pathway; alpha-linolenic acid metabolism; biosynthesis of amino acids; Fc epsilon RI signaling pathway; long-term depression; insulin secretion; ovarian steroidogenesis; glucagon signaling pathway; and herpes simplex virus 1 infection (P<0.05; Table 3). The most enriched gene in identified functions and pathways was PLA2G4A, and it may be implicated in pathogenesis of PCOS as well as diabetes. Therefore, miR-32-3p may affect the pathogenesis of PCOS and diabetes by regulating PLA2G4A.
Table 3.
GO function and KEGG pathway enrichment result
| ID | Term | Gene | P value |
|---|---|---|---|
| GO:0004622 | Lysophospholipase activity | PLA2G4A | 0.011 |
| GO:0008970 | Phospholipase A1 activity | PLA2G4A | 0.006 |
| GO:0047498 | Calcium-dependent phospholipase A2 activity | PLA2G4A | 0.008 |
| GO:0102568 | Phospholipase A2 activity consuming 1,2-dioleoylphosphatidylethanolamine | PLA2G4A | 0.011 |
| hsa00260 | Glycine, serine and threonine metabolism | PSPH | 0.025 |
| hsa00565 | Ether lipid metabolism | PLA2G4A | 0.03 |
| hsa00590 | Arachidonic acid metabolism | PLA2G4A | 0.039 |
| hsa00591 | Linoleic acid metabolism | PLA2G4A | 0.018 |
| hsa00592 | Alpha-Linolenic acid metabolism | PLA2G4A | 0.015 |
| hsa01230 | Biosynthesis of amino acids | PSPH | 0.045 |
| hsa04370 | VEGF signaling pathway | PLA2G4A | 0.036 |
| hsa04664 | Fc epsilon RI signaling pathway | PLA2G4A | 0.042 |
| hsa04730 | Long-term depression | PLA2G4A | 0.037 |
| hsa04911 | Insulin secretion | GCG | 0.042 |
| hsa04913 | Ovarian steroidogenesis | PLA2G4A | 0.031 |
| hsa04922 | Glucagon signaling pathway | GCG | 0.044 |
| hsa05168 | Herpes simplex virus 1 infection | ZNF559/ZNF596 | 0.033 |
miR-32-3p and PLA2G4A levels were related to acupuncture treatment
The expression levels of miR-32-3p and PLA2G4A in tissue samples were detected by qRT-PCR. The levels of hsa-miR-32-3p expressions in the acupuncture group were remarkably lower than those of the control group (P<0.01). On the contrary, expression level of PLA2G4A in the acupuncture treatment group was remarkably higher than that of the control group (P<0.05) (Figure 2B). Moreover, the expression levels of hsa-miR-32-3p in the ovulation group were remarkably lower than those of the no ovulation group (P<0.0001). On the contrary, expression levels of PLA2G4A in the ovulation group remarkably higher than those of the no ovulation group (P<0.05) (Figure 2C). The expression level of miR-32-3p and PLA2G4A were negatively correlated with human endometrial tissue (Figure 2D). Univariate logistic regression of miR-32-3p and PLA2G4A suggested that high expression level of PLA2G4A or low expression level of miR-32-3p may influence the probability of ovulation (Table 4). Therefore, these findings imply that PCOS patients may benefit from acupuncture which can up-regulate PLA2G4A and down-regulate the expression of miR-32-3p.
Table 4.
Univariate logistic regression of factors that affect ovulation
| Variable | Univariate analysis | |
|---|---|---|
|
| ||
| OR (95% CI) | P value | |
| miR-32-3p | 0.069 (0.012-0.392) | 0.003 |
| PLA2G4A | 8.060 (1.054-76.054) | 0.048 |
miR-32-3p negatively regulated PLA2G4A expression
The results predicted by RNA hybrid 2.12 showed that there is a docking site between miR-32-3p and PLA2G4A, as shown in Figure 3A. In Figure 3B, miR-32-3p was remarkably elevated by miR-32-3p mimics. The results of miR-32-3p mimic transfection showed decreased expression levels of PLA2G4A as well as decreased mRNA levels of PTEN and SMG1 in HEK-293 cells (Figure 3B-E). Besides, SMG1 and PTEM both showed a significant positive correlation with PLA2G4A in the GTEX cohort (Figure 3F, 3G). Luciferase reporter assay results showed low luciferase activity in HEK-293 cells co-transfected with PLA2G4A-WT and miR-NC control (P<0.05) (Figure 3H). These results show that miR-32-3p is involved in down-regulation of PLA2G4A expression (Figure 3I). And miR-32-3p may act as a sponge of PLA2G4A and down-regulates PLA2G4A’s expression.
Figure 3.
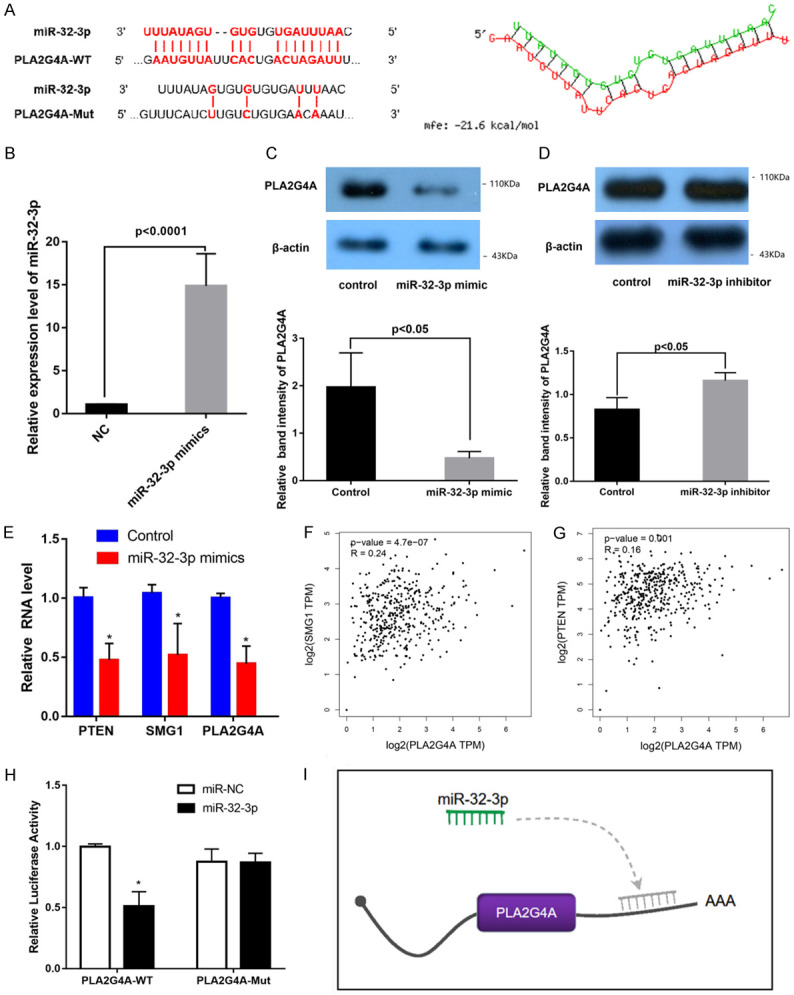
miR-32-3p downregulates PLA2G4A expression. (A) miR-32-3p as the target miRNA of PLA2G4A was determined by RNAhybrid tool. Minimum free energy (MFE); (B) The relative expression of miR-32-3p in HEK-293 cells was detected by qPCR after transfected with miR-32-3p mimics; (C, D) The result of WB showed expression of PLA2G4A in HEK-293 cells transfected with miR-32-3p inhibitor or miR-32-3p mimic; (E) Results of PCR showed mRNAs level of PTEN, SMG1 and PLA2G4A in HEK-293 cells transfected with miR-32-3p inhibitor or miR-32-3p mimic; (F, G) The correlations shown are for ovarian tissues from GTEX cohorts. Correlation analysis of PLA2G4A with PTEN (F) and SMG1 (G) in ovarian tissue. (H) Analysis of ALP2G4A regulation by miR-32-3p by Luciferase reporter assays; (I) A model showing downregulation of PLA2G4A expression by miR-32-3p.
Expression of PLA2G4A in human tissues and cells
Besides, the immunofluorescent analysis of PLA2G4A was carried out. And PLA2G4A was mainly detected in cytosol according to the information from THE HUMAN PROTEIN ATLAS (Figure 4A, 4B). In the endometrial tissue, PLA2G4A was mainly detected in glandular cells according to the information from THE HUMAN PROTEIN ATLAS (Figure 4C, 4D). To further explore the expression profiles of PLA2G4A, we constructed human tissue-enriched protein map, which showed that PLA2G4A was significantly enriched in ovary, uterus and vagina (Figure 4E).
Figure 4.
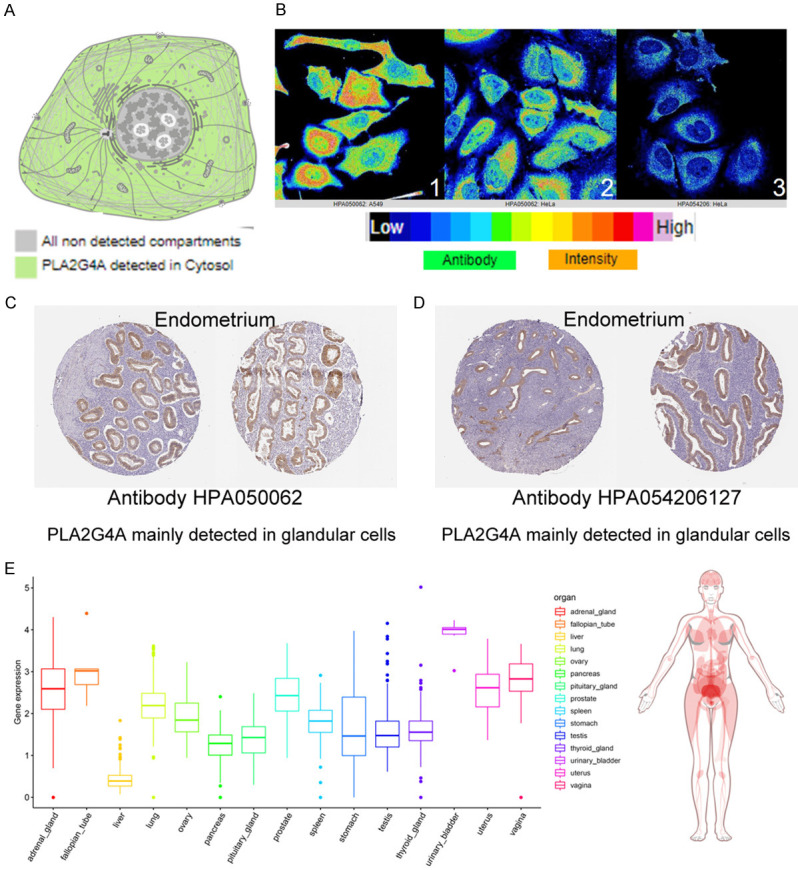
Expression of PLA2G4A in human tissues and cells. A, B: The immunofluorescent analysis of PLA2G4A was carried out. And PLA2G4A was mainly detected in cytosol; C, D: In the endometrial tissue, PLA2G4A was mainly detected in glandular cells; E: PLA2G4A was shown by human protein expression map (GTEX cohort, n=1884).
GSEA analysis
GO function analysis of GSEA demonstrated that PLA2G4A-linked signaling functions in ovulation cycle, modulation of glucose metabolic process, modulation of pri-miRNA transcription by RNA polymerase II, proton-transporting two-sector ATPase complex (catalytic domain), glucose catabolic process, sodium: potassium-exchanging ATPase complex, complex of collagen trimers, mitochondrial proton-transporting ATP synthase complex, sodium: potassium-exchanging ATPase activity, adrenergic receptor activity and high voltage-gated calcium channel activity were enriched (Figure 5A). Furthermore, PLA2G4A-related pathways revealed by KEGG pathways analysis of GSEA (including: thiamine metabolism pathway, apoptosis - multiple species pathway, type I diabetes mellitus pathway, insulin secretion pathway, glycolysis/gluconeogenesis pathway, steroid hormone biosynthesis cascade, endocrine, as well as other factor-regulated calcium reabsorption cascade, type II diabetes mellitus and insulin resistance pathway) were enriched (Figure 5B). Enrichment of these biological functions and pathways implied that PLA2G4A may be able to interrupt the vicious cycle in POCS and diabetes.
Figure 5.
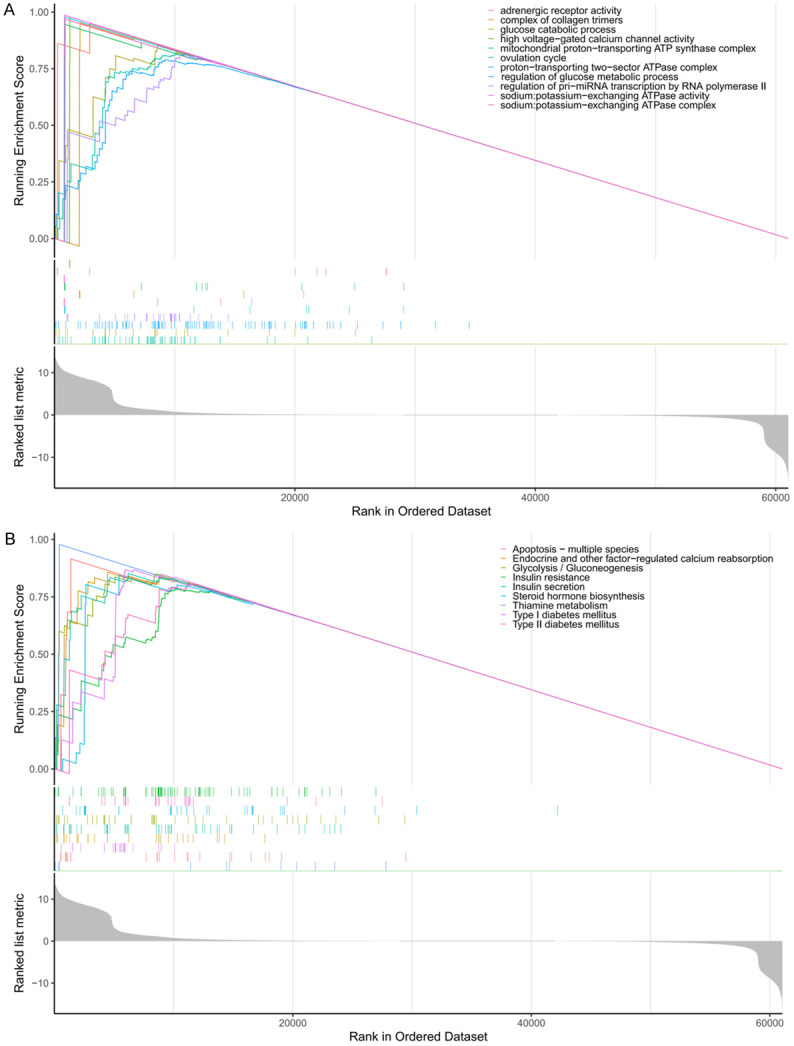
The KEGG pathway analysis and GO function analysis of pathways and functions associated with PLA2G4A were visualized by GSEA. And significant correlations were observed. A: GO enrichment analysis; B: KEGG enrichment analysis. Note: GSEA: Gene Set Enrichment Analysis.
Discussion
In the current study, we used published microarray data and bioinformatics tools to explore the DEMs that are commonly associated with PCOS and diabetes. Potential miRNA-mRNA regulatory network was identified. Further, we observed that miR-32-3p might inhibit transcription of PLA2G4A, which may be implicated in the vicious circle of PCOS and diabetes. Our systematic analyses provide information on the complex pathogenesis of PCOS at molecular level.
GO and KEGG enrichment analyses indicated that PLA2G4A played an important biological function in pathogenesis of PCOS and diabetes. The results showed that PLA2G4A was involved in various metabolic processes including ether lipid metabolism, arachidonic acid metabolism, linoleic acid metabolism, as well as alpha-Linolenic acid metabolism. Moreover, PLA2G4A participated in signal transduction pathways related to sex hormones functions and metabolism (for example: ovarian steroidogenesis, Fc epsilon RI signaling pathway as well as VEGF signaling pathway). Therefore, we speculated that PLA2G4A plays a vital role in energy metabolism and hormone regulation. GSEA analysis based on GO and KEGG databases further showed the role of PLA2G4A in metabolism and hormone regulation. KEGG pathways analysis of GSEA showed that PLA2GA was implicated in diabetes mellitus pathway, metabolism related pathway, insulin resistance and endocrine related pathway. GO function analysis of GSEA showed that PLA2G4A played a key role in ATP-related energy metabolism. Dysregulation of endocrine and energy metabolism are important mechanisms in pathogenesis of PCOS and diabetes [27].
Previous studies reported that PLA2G4A gene served a pivotal role in sugar metabolism and lipid metabolism [28,29]. Functional analysis of BanI polymorphism suggested that presence of the G allele in the PLA2G4A genotype positively correlated with high cPLA2 activity [30]. Interestingly, higher cPLA2 activity conferred protective effect in female diabetic patients [31]. Estrogen activated cPLA2 by inducing increase in extracellular Ca2+ and free Ca2+ in the cytoplasm [32]. Therefore, down-regulation of cPLA2 activity caused by down-regulation of estrogen may explain insulin resistance in PCOS patients. Up-regulation of PLA2G4A affects activity of cPLA2 thus inhibiting the vicious circle of PCOS and diabetes.
We have demonstrated that miR-32-3p downregulates PLA2G4A transcription in in vitro experiments. Previous studies reported that PCOS-related miRNAs were highly expressed in ovaries. These miRNAs regulate adipogenesis, steroid hormone synthesis, follicle development and maturation, and insulin signaling pathways. Shen et al. reported that overexpression of miR-32 induced by hyperglycaemic microenvironment enhanced cardiac fibroblast apoptosis, implying that miR-32 may be involved in diabetes-induced cardiomyopathy [33]. Further, studies reported that miR-32 might be related to the pathophysiological process of cardiovascular diseases [34]. High expression levels of miR-32 have been reported in PCOS patients [35]. The ability of miR-32-3p to act as a response element for directly regulating the transcription of PLA2G4A was confirmed by a luciferase reporter gene assay. These findings show that acupuncture procedure down-regulates expression of miR-32-3p in PCOS and diabetes patients. Therefore, downregulation of miR-32-3p may play a pivotal role in the traditional acupuncture treatment of PCOS and diabetes-related diseases.
Diagnosis and treatment of PCOS is a common challenge encountered by clinicians and medical professionals. Previous studies have characterized and identified that various miRNAs could be potential therapeutic targets and a diagnostic biomarkers for PCOS [17]. Further, studies reported that regulation of the metabolic and androgenic system by miRNAs might be implicated in the mechanism of acupuncture treatment [20]. Notably, acupuncture is not recommended in Western medicine, mainly because the precise mechanism of action is unknown. We explored potential molecular mechanisms of acupuncture in the treatment of diabetic PCOS by constructing miRNA-mRNA networks using bioinformatics methods and clinical data in this study. This research provided information on acupuncture in the treatment of PCOS patients. However, this study has certain limitations. Future studies should incorporate additional factors to increase the sample size and further clarify the mechanism of acupuncture treatment for PCOS. In addition, targeted regulation of PLA2G4A by miR-32-3p requires further animal and cytological experiments. Moreover, genetic polymorphism of PLA2G4A may also affect protein function; therefore, further research should be carried out [36]. Nonetheless, our study has identified miR-32-3p and PLA2G4A as potential independent risk factors affecting PCOS prognosis. Interestingly, our research proved that miR-32-3p and PLA2G4A were regulated by acupuncture treatment. Transcriptional regulation of PLA2G4A by miRNA-32-3p was also confirmed in this study.
There are still some limitations in the present study, such as a single center study, small sample size, unclear about miR-32-3p and PLA2G4A expression in PCOS patients with diabetes, and no supply of the pathways for PLA2G4A by the molecular and biological examination. More samples are needed in future studies, and more prospective, controlled, multi-center studies with long follow-up are also required for further validation.
In summary, miR-32-3p targets PLA2G4A protein which may be a target for the treatment of the vicious cycle of PCOS and diabetes. The underlying mechanism of traditional acupuncture treatment may be through inhibition of the vicious circle of PCOS and diabetes by down-regulation of miR-32-3p.
Disclosure of conflict of interest
None.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Actkins KV, Singh K, Hucks D, Velez Edwards DR, Aldrich M, Cha J, Wellons M, Davis LK. Characterizing the clinical and genetic spectrum of polycystic ovary syndrome in electronic health records. J Clin Endocrinol Metab. 2021;106:153–167. doi: 10.1210/clinem/dgaa675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Melo AS, Dias SV, Cavalli Rde C, Cardoso VC, Bettiol H, Barbieri MA, Ferriani RA, Vieira CS. Pathogenesis of polycystic ovary syndrome: multifactorial assessment from the foetal stage to menopause. Reproduction. 2015;150:R11–24. doi: 10.1530/REP-14-0499. [DOI] [PubMed] [Google Scholar]
- 4.Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3:1545–1573. doi: 10.1210/js.2019-00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu S, Xu B, Long R, Jin L. The effect of polycystic ovary syndrome without hyperandrogenism on pregnancy-related outcomes: a retrospective cohort study. BJOG. 2020;128:1003–1010. doi: 10.1111/1471-0528.16557. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell R, Hart RJ. Pregnancy-related outcomes for women with polycystic ovary syndrome. Womens Health (Lond) 2017;13:89–97. doi: 10.1177/1745505717731971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141:1202–1207. doi: 10.1016/j.jaci.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma HK, Ratre YK, Bhaskar L, Colombatti R. Erythrocyte microRNAs: a tiny magic bullet with great potential for sickle cell disease therapy. Ann Hematol. 2021;100:607–614. doi: 10.1007/s00277-020-04390-y. [DOI] [PubMed] [Google Scholar]
- 9.Singh GB, Cowan DB, Wang DZ. Tiny regulators of massive tissue: microRNAs in skeletal muscle development, myopathies, and cancer cachexia. Front Oncol. 2020;10:598964. doi: 10.3389/fonc.2020.598964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrie CH, Saunders NJ, Soneji S, Palazzo S, Dunlop HM, Cooper CD, Brown PJ, Troussard X, Mossafa H, Enver T, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS. MicroRNA expression in lymphocyte development and malignancy. Leukemia. 2008;22:1440–1446. doi: 10.1038/sj.leu.2405083. [DOI] [PubMed] [Google Scholar]
- 11.Bar M, Wyman SK, Fritz BR, Qi J, Garg KS, Parkin RK, Kroh EM, Bendoraite A, Mitchell PS, Nelson AM, Ruzzo WL, Ware C, Radich JP, Gentleman R, Ruohola-Baker H, Tewari M. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells. 2008;26:2496–2505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He HX, Ji AQ, Han N, Zhao YX, Hu S, Kong QL, Liu Y, Sun QF. Identification of peripheral blood and menstrual blood based on the expression level of microRNAs and discriminant analysis. Fa Yi Xue Za Zhi. 2020;36:514–518. doi: 10.12116/j.issn.1004-5619.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, Lv J, Xu P, Gu L, Cao J, Xu L, Xue K, Li Q. Identification of microRNAs and genes associated with hyperandrogenism in the follicular fluid of women with polycystic ovary syndrome. J Cell Biochem. 2018;119:3913–3921. doi: 10.1002/jcb.26531. [DOI] [PubMed] [Google Scholar]
- 15.Ali Beg MM, Verma AK, Saleem M, Saud Alreshidi F, Alenazi F, Ahmad H, Joshi PC. Role and significance of circulating biomarkers: miRNA and E2F1 mRNA expression and their association with type-2 diabetic complications. Int J Endocrinol. 2020;2020:6279168. doi: 10.1155/2020/6279168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devarbhavi P, Telang L, Vastrad B, Tengli A, Vastrad C, Kotturshetti I. Identification of key pathways and genes in polycystic ovary syndrome via integrated bioinformatics analysis and prediction of small therapeutic molecules. Reprod Biol Endocrinol. 2021;19:31. doi: 10.1186/s12958-021-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdalla M, Deshmukh H, Atkin SL, Sathyapalan T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): a review. Life Sci. 2020;259:118174. doi: 10.1016/j.lfs.2020.118174. [DOI] [PubMed] [Google Scholar]
- 18.Jang JH, Lee DJ, Bae CH, Ha KT, Kwon S, Park HJ, Hahm DH, Lee H, Kim S. Changes in small intestinal motility and related hormones by acupuncture stimulation at Zusanli (ST 36) in mice. Chin J Integr Med. 2017;23:215–220. doi: 10.1007/s11655-016-2609-8. [DOI] [PubMed] [Google Scholar]
- 19.Sui M, Xue L, Ying X. Association of acupuncture treatment with mortality of type 2 diabetes in china: evidence of a real-world study. Int J Environ Res Public Health. 2020;17:7801. doi: 10.3390/ijerph17217801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko JH, Kim SN. MicroRNA in acupuncture studies: does small RNA shed light on the biological mechanism of acupuncture? Evid Based Complement Alternat Med. 2019;2019:3051472. doi: 10.1155/2019/3051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mu Y, Li Q, Cheng J, Shen J, Jin X, Xie Z, Gao Z, Zhang W, Hua Q, Xia L, Gao Y, Xia Y. Integrated miRNA-seq analysis reveals the molecular mechanism underlying the effect of acupuncture on endometrial receptivity in patients undergoing fertilization: embryo transplantation. 3 Biotech. 2020;10:6. doi: 10.1007/s13205-019-1990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Q, Wang J, Sun R, Gu W, He Z, Chen Q, Liu W, Chen Y, Wang J, Zhang Y. Identification of circulating hub long noncoding RNAs associated with hypertrophic cardiomyopathy using weighted correlation network analysis. Mol Med Rep. 2020;22:4637–4644. doi: 10.3892/mmr.2020.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolazzi G, Benos PV, Tumminello M, Coronnello C. An improvement of ComiR algorithm for microRNA target prediction by exploiting coding region sequences of mRNAs. BMC Bioinformatics. 2020;21:201. doi: 10.1186/s12859-020-3519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Zhou J, Zhao J, Hou D, Zhang H, Li L, Zou D, Hu J, Zhang Y, Jing Z. MiR-18a-downregulated RORA inhibits the proliferation and tumorigenesis of glioma using the TNF-alpha-mediated NF-kappaB signaling pathway. EBioMedicine. 2020;52:102651. doi: 10.1016/j.ebiom.2020.102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YS, Kang XR, Zhou ZH, Yang J, Xin Q, Ying CT, Zhang YP, Tao J. MiR-1908/EXO1 and MiR-203a/FOS, regulated by scd1, are associated with fracture risk and bone health in postmenopausal diabetic women. Aging (Albany NY) 2020;12:9549–9584. doi: 10.18632/aging.103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021;44:233–244. doi: 10.1007/s40618-020-01351-0. [DOI] [PubMed] [Google Scholar]
- 28.Wolford JK, Konheim YL, Colligan PB, Bogardus C. Association of a F479L variant in the cytosolic phospholipase A2 gene (PLA2G4A) with decreased glucose turnover and oxidation rates in Pima Indians. Mol Genet Metab. 2003;79:61–66. doi: 10.1016/s1096-7192(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay BL, Cormier H, Rudkowska I, Lemieux S, Couture P, Vohl MC. Association between polymorphisms in phospholipase A2 genes and the plasma triglyceride response to an n-3 PUFA supplementation: a clinical trial. Lipids Health Dis. 2015;14:12. doi: 10.1186/s12944-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbosa NR, Junqueira RM, Vallada HP, Gattaz WF. Association between BanI genotype and increased phospholipase A2 activity in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:340–343. doi: 10.1007/s00406-007-0736-0. [DOI] [PubMed] [Google Scholar]
- 31.Wu HT, Chuang YW, Huang CP, Chang MH. Loss of angiotensin converting enzyme II (ACE2) accelerates the development of liver injury induced by thioacetamide. Exp Anim. 2018;67:41–49. doi: 10.1538/expanim.17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45:209–224. doi: 10.1007/s11745-010-3391-6. [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Xing W, Liu R, Zhang Y, Xie C, Gong F. MiR-32-5p influences high glucose-induced cardiac fibroblast proliferation and phenotypic alteration by inhibiting DUSP1. BMC Mol Biol. 2019;20:21. doi: 10.1186/s12867-019-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai Y, Yan T, Gao Y. Silence of miR-32-5p promotes endothelial cell viability by targeting KLF2 and serves as a diagnostic biomarker of acute myocardial infarction. Diagn Pathol. 2020;15:19. doi: 10.1186/s13000-020-00942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:355–362. doi: 10.1007/s10815-013-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadalin S, Buretic-Tomljanovic A. An association between the BanI polymorphism of the PLA2G4A gene for calcium-dependent phospholipase A2 and plasma glucose levels among females with schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2018;135:39–41. doi: 10.1016/j.plefa.2018.06.007. [DOI] [PubMed] [Google Scholar]