Abstract
Objective: To investigate the effect of remifentanil combined with sevoflurane inhalation anesthesia on coagulation function and postoperative recovery of patients undergoing endoscopic selective varices devascularization (ESVD). Methods: Altogether 116 patients undergoing ESVD in our hospital were randomly divided into two groups. The control group received propofol combined with remifentanil anesthesia, while the observation group received remifentanil combined with sevoflurane inhalation anesthesia. Results: There was no statistical difference in coagulation function indexes between the control group and the observation group (P>0.05), and the clinical anesthesia effect of the observation group was better (P<0.001). Five minutes after intubation, compared with the control group, the clinical stress response index was better (all P<0.001), the quality of anesthesia recovery was higher (all P<0.001), the heart rate was better after anesthesia (P<0.001), and the incidence of adverse reactions was higher (P<0.01) in the observation group. Conclusion: Compared with remifentanil combined with propofol anesthesia, remifentanil combined with sevoflurane inhalation anesthesia for ESVD can effectively improve the clinical anesthesia effect, improve the quality of anesthesia recovery, and reduce stress reactions, but the incidence of adverse reactions is increased. Therefore the clinical application still needs to be strengthened.
Keywords: Remifentanil, propofol, sevoflurane, endoscopic selective varices devascularization, anesthetic effect, coagulation function, postoperative recovery
Introduction
Endoscopic selective varices devascularization (ESVD) is a common minimally invasive surgical treatment. Compared with other general anesthesia operations, it has the advantages of having a small wound, less bleeding and a quick recovery [1]. ESVD can continuously alleviate damage to other organs caused by an open surgery and it promotes the postoperative rehabilitation of patients [2]. The key to relieve pain and improve prognosis is to select reasonable and appropriate anesthesia methods [3]. At present, remifentanil combined with sevoflurane or propofol is widely used for general anesthesia in the clinic. Although both of the modes have the advantages of a quick onset, easy measurement of anesthesia degree, similar anesthesia effects and safety, remifentanil combined with sevoflurane can promote patients’ rapid awakening and obviously relieve patients’ pain [4,5]. However, the effects of the two drugs on the anesthesia for ESVD and their influence on coagulation function and safety have not been reported. In this research, the effects of remifentanil combined with sevoflurane inhalation anesthesia on coagulation function and postoperative recovery of patients undergoing ESVD were studied.
Materials and methods
General information
From January 2017 to May 2018, altogether 116 patients undergoing ESVD in our hospital were selected. Inclusion criteria: (1) Patients who had not received ESVD within 1 month; (2) Patients with complete clinical data; (3) Informed consent forms was obtained from the patients [6]. Exclusion criteria: (1) Those with other serious important organ diseases; (2) Those with severe consciousness disorders; (3) Those with diseases of the blood and immune systems; (4) Those were allergic to narcotic drugs in this research [7].
Research participants were randomly divided into two groups by a random number table method. The control group (58 cases, 28 males and 30 females) was given propofol combined with remifentanil anesthesia, aged 45 to 69 years, with an average of (57.0±1.5) years. According to Child-Pugh classification, there were 20 cases of Grade A, 27 cases of Grade B and 11 cases of Grade C. According to vein’s classification, there were 48 cases of GOV1, 7 cases of GOV2 and 3 cases of GOV3. The observation group (58 cases, 29 males and 29 females) was given remifentanil combined with sevoflurane inhalation anesthesia, aged 46 to 69 years, with an average of (57.5±1.4) years. According to Child-Pugh classification, there were 18 cases of Grade A, 29 cases of Grade B and 11 cases of Grade C. According to vein’s classification, there were 46 cases of GOV1, 9 cases of GOV2 and 3 cases of GOV3. There was no significant difference in general data between the two groups, and they were comparable (P>0.05). This research has been approved by Ethics Committee of our hospital.
Methods
Patients in both groups were intubated under general anesthesia.
Control group
All patients received target-controlled infusion anesthesia of propofol combined with remifentanil, in which the induced concentration of propofol (AstraZeneca Pharmaceutical Co., Ltd.) was 1 mg/kg, the plasma concentration of propofol was 1.5-2.5 μg/mL during operation, and remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd., 1 mg; 0.4 μg/kg) was induced to 6 ng/mL, and the plasma concentration was maintained to 2-8 ng/mL during operation. Target-controlled infusion was used for anesthesia. After the target-controlled infusion device was connected, age, body weight and target concentration were input, and the drug was administered under computer control. Propofol was stopped 5 min before the operation, and remifentanil was stopped at the end of the operation.
Observation group
All patients received remifentanil combined with sevoflurane (Hebei Yipin Pharmaceutical Co., Ltd., China) inhalation anesthesia. The application method of remifentanil anesthetic was the same as that of the control group. Sevoflurane was inhaled at a concentration of 1.5%-4.5%, and the dosage was adjusted according to the anesthesia depth and hemodynamic changes.
After operation, 2.0 mg of neostigmine (Zhejiang Xianju Pharmaceutical Co., Ltd., China) and 1.0 mg of atropine (Zhejiang Ruixin Pharmaceutical Co., Ltd., China) were given intravenously. To reduce the awareness rate during operation, 0.01 mg/kg of midazolam was added intravenously every 1 h when the operation time was longer than 2 h, and stopped 30 min before the end of operation. The tidal volume of two groups of patients during operation was 6-8 mL/kg, and the respiratory frequency was 12 times/min-14 times/min.
Outcome measures
Main outcome measures
Clinical anesthesia effect, coagulation function and anesthesia recovery quality. Secondary outcome measures: clinical stress reaction, hemodynamic indexes and adverse reactions.
Operation conditions
The operation time, blood loss, anesthesia time and remifentanil dosage were compared between the two groups.
Blood coagulation function
Venous blood from the elbow of patients was collected before operation and 1 h after operation, and thrombin time, activated thrombin time, fibrinogen, prothrombin time and other indexes were detected by CoaguChek XS whole coagulation tester, made in Switzerland [8,9].
Clinical stress response
The clinical stress response refers to stress response indicators at 5 minutes before anesthesia induction and 5 minutes after intubation, including diastolic blood pressure, systolic blood pressure, adrenaline, cortisol and other indicators. Among them, diastolic blood pressure and systolic blood pressure in the body were detected by ambulatory blood pressure monitoring, usually once every 15-30 minutes, and the final result was the average of the values at the two time points. Three milliliters of elbow venous blood were collected at the two time points, and adrenaline, cortisol and other hormones were detected by high performance liquid chromatography, with Agilent.
Anesthesia recovery quality
The recovery of clinical indicators of patients was recorded in detail, which mainly included spontaneous breathing recovery time, complete instruction action time, speech response time and extubation time.
Hemodynamic index
The change of blood oxygen saturation of patients was detected by using blood oxygen saturation detector, and the change of HR (heart rate) was observed.
Adverse reactions
The adverse reactions included nausea, vomiting, respiratory depression and other adverse reactions.
Statistical methods
SPSS 20.0 was used to analyze the data, and the measurement data were expressed by mean ± standard deviation (x̅ ± sd). Two independent samples t test was used to compare the mean between the two groups, and paired t test was used to compare the mean before and after intervention in the same group. The counting data were expressed by rate and tested by χ2 test, and the difference was statistically significant with P<0.05.
Results
Operation conditions
There were no significant differences in operation time, intraoperative blood loss, postoperative plasma drainage and anesthesia time between the two groups (all P>0.05). The dosage of remifentanil in the observation group was significantly less than that in the control group (P<0.001; Table 1).
Table 1.
Comparison of baseline data (x̅ ± sd)
| Index | Observation group (n=58) | Control group (n=58) | t/χ2 | P |
|---|---|---|---|---|
| Operation time (min) | 175.31±15.54 | 172.57±14.66 | 0.977 | 0.331 |
| Intraoperative blood loss (mL) | 317.09±32.85 | 321.24±30.97 | 0.700 | 0.485 |
| Postoperative drainage fluid volume (mL) | 479.17±42.96 | 466.81±45.92 | 1.497 | 0.132 |
| Anesthesia time (min) | 256.14±22.85 | 247.36±31.08 | 1.733 | 0.086 |
| Remifentanil dosage (μg) | 2127.55±297.84 | 2382.97±288.14 | 4.694 | 0.000 |
Coagulation function
There were no significant differences in thrombin time, activated thromboplastin time, prothrombin time and fibrinogen between the observation group and the control group (all P>0.05), as shown in Table 2.
Table 2.
Indexes of coagulation function (x̅ ± sd)
| Group | Thrombin time (s) | Activated partial thromboplastin time (s) | Fibrinogen (g/L) | Prothrombin time (s) |
|---|---|---|---|---|
| Control group (n=58) | 20.21±1.02 | 36.45±2.39 | 3.54±0.25 | 12.87±2.01 |
| Observation group (n=58) | 19.94±1.15 | 35.81±2.02 | 3.37±0.98 | 12.52±1.97 |
| t | 1.536 | 1.558 | 1.280 | 0.941 |
| P | 0.127 | 0.122 | 0.203 | 0.346 |
Clinical stress response
Five minutes before anesthesia induction, there were no significant differences in the clinical stress response indexes between the two groups (all P>0.05), and five minutes after intubation, the clinical stress response indexes of the observation group were significantly better than those of the control group (all P<0.001), as shown in Table 3 and Figure 1.
Table 3.
Clinical stress response indicators (score, x̅ ± sd)
| Group | Diastolic pressure (mmHg) | Systolic blood pressure (mmHg) | Adrenaline (ng/L) | Cortisol (µg/L) |
|---|---|---|---|---|
| Control group (n=58) | ||||
| 5 minutes before induction of anesthesia | 125.21±9.82 | 78.67±4.91 | 70.56±12.04 | 94.94±2.35 |
| 5 minutes after intubation | 130.24±11.02*** | 86.45±3.54*** | 78.65±6.87*** | 108.64±10.65*** |
| Observation group (n=58) | ||||
| 5 minutes before induction of anesthesia | 124.91±7.81 | 78.58±4.01 | 69.88±17.47 | 94.85±2.07 |
| 5 minutes after intubation | 126.82±5.23***,### | 79.62±6.47***,### | 67.48±8.54***,### | 91.24±6.90***,### |
Note: Compared with before treatment;
P<0.001.
Compared with control group;
P<0.001.
Figure 1.
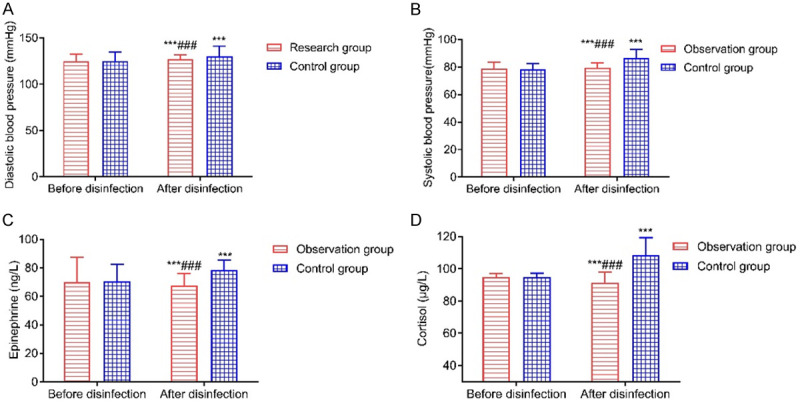
Comparison of clinical stress response indicators. A: Diastolic pressure; B: Systolic blood pressure; C: Adrenaline; D: Cortisol. Compared with before disinfection, ***P<0.001; compared with the control group, ###P<0.001.
Anesthesia recovery quality
Compared with the control group, the quality of anesthesia recovery in the observation group was significantly higher (all P<0.001), as shown in Table 4.
Table 4.
Anesthesia recovery quality (min, x̅ ± sd)
| Group | Spontaneous breathing recovery time | Complete command action time | Speech response time | Extubation time |
|---|---|---|---|---|
| Control group (n=58) | 5.57±1.05 | 7.88±1.17 | 11.54±1.85 | 15.38±1.22 |
| Observation group (n=58) | 4.36±1.17 | 6.05±1.26 | 7.86±1.07 | 11.98±1.04 |
| t | 5.862 | 8.105 | 13.113 | 16.152 |
| P | 0.000 | 0.000 | 0.000 | 0.000 |
Hemodynamic conditions
Before anesthesia, there was no significant difference in blood oxygen saturation and HR between the two groups (P>0.05). After anesthesia, there was no significant difference in blood oxygen saturation between the two groups (P>0.05), but the heart rate increased significantly (P<0.05). Compared with the control group, there were no significant changes in oxygen saturation after anesthesia in the observation group (P>0.05), and the heart rate was lower (P<0.001), as shown in Table 5.
Table 5.
Hemodynamic indicators (x̅ ± sd)
| Group | Blood oxygen saturation (%) | HR (times/min) | ||
|---|---|---|---|---|
|
|
|
|||
| Before anesthesia | After anesthesia | Before anesthesia | After anesthesia | |
| Control group (n=58) | 96.27±1.42 | 97.17±2.53 | 76.90±9.82 | 83.48±3.50* |
| Observation group (n=58) | 96.55±1.32 | 97.88±2.77 | 76.56±8.57 | 80.74±2.10* |
| t | 1.100 | 1.441 | 0.199 | 5.112 |
| P | 0.274 | 0.152 | 0.843 | 0.000 |
Note: Compared with before treatment;
P<0.05.
HR: heart rate.
Adverse reactions
Compared with the control group, the incidence of adverse reactions in the observation group was significantly higher (P<0.01), as shown in Table 6.
Table 6.
Adverse reactions (cases, %)
| Group | nausea | Vomiting | Respiratory depression | Incidence |
|---|---|---|---|---|
| Control group (n=58) | 5 | 4 | 3 | 20.69% |
| Observation group (n=58) | 12 | 10 | 6 | 48.28% |
| χ2 | 3.377 | 2.924 | 0.482 | 10.747 |
| P | 0.066 | 0.087 | 0.488 | 0.001 |
Discussion
ESVD is mainly applied to alleviate the bleeding of esophageal varices in the body, it can continuously reduce the risk of postoperative rebleeding, and the effect is remarkable [10,11]. It is still necessary to select appropriate and effective narcotic drugs for analgesia during and after operations [12,13]. At present, remifentanil combined with sevoflurane inhalation anesthesia, as an effective and safe anesthesia scheme, and is widely used in ESVD [14,15].
The results of this study showed that compared with the control group, the clinical anesthesia effect of the observation group was obviously improved. At the same time, 5 minutes after intubation, compared with the control group, the clinical stress response index of the observation group was significantly better, and the dosage of remifentanil in the observation group was significantly less than that in the control group. This suggested that sevoflurane combined with remifentanil for general anesthesia can effectively improve the stress response index of the body and it has ideal anesthetic effect. This may be because sevoflurane anesthesia can assist analgesia and reduce blood pressure, and remifentanil combined with sevoflurane anesthesia is more effective, which significantly reduces the dosage of remifentanil, thus reducing its damage to other important organs and tissues and therefore reducing the stress response of the body [16-19].
The quality of anesthesia recovery in the observation group was significantly higher than that in the control group. After anesthesia, compared with the control group, the heart rate of the observation group was significantly better. Compared with the control group, there was no obvious difference in coagulation function, suggesting that sevoflurane combined with remifentanil anesthesia had little influence on coagulation function and hemodynamic indexes, thus improving the quality of anesthesia recovery. The main reason is that the application of sevoflurane combined with remifentanil can effectively expand the bronchus and inhibit the autonomic reflex, at the same time reduce the blood-gas partition coefficient and the stimulation of the organism to ensure the stability of the anesthesia process and promote the rapid awakening of patients after operation [20,21]. At the same time, combined inhalation of both groups can continuously improve the quality of clinical anesthesia, reduce the minimum effective concentration of sevoflurane in the alveoli, reduce the incidence of hypodose-dependent blood pressure reduction of remifentanil, and ensure the safety of patients [22,23]. However, the results of this study showed that the incidence of adverse reactions in the observation group was significantly higher than that in the control group. This suggested that sevoflurane combined with remifentanil anesthesia significantly increased the incidence of nausea and vomiting, which should be closely observed in the clinical application.
There are still some shortcomings in this research, such as having only a few study samples and a lack of scientific basis. In the future, it is still necessary to collect a larger number of clinical cases as research subjects, so as to improve the rationality and scientific method [24,25].
To sum up, compared with remifentanil combined with propofol anesthesia, remifentanil combined with sevoflurane inhalation anesthesia can improve the clinical anesthesia effect, improve the quality of anesthesia recovery, and reduce the impact of the stress response and overall hemodynamics, but the incidence of the adverse reactions is increased. Clinical application still needs to be strengthened.
Disclosure of conflict of interest
None.
References
- 1.Ren LQ, Sun XX, Guan Y. Effects of sevoflurane or propofol combined with remifentanil anesthesia on clinical efficacy and stress response in pregnant women with pregnancy-induced hypertension. Eur Rev Med Pharmacol Sci. 2018;22:1825–1829. doi: 10.26355/eurrev_201803_14602. [DOI] [PubMed] [Google Scholar]
- 2.Hong YP, Wei W, Ding J, Chen YP, Teng WJ, Guo MD, Hua HJ, Ye XH, Wu ZP, Li ZM. Clinical study on the choice of retreatment time for precise devascularization under endoscopic esophagogastric connected varicose veins. Chin J Gastroenterol. 2020;25:40–42. [Google Scholar]
- 3.Aguado D, Bustamante R, Gómez de Segura IA. Toll-like receptor 4 deficient mice do not develop remifentanil-induced mechanical hyperalgesia: an experimental randomised animal study. Eur J Anaesthesiol. 2018;35:505–510. doi: 10.1097/EJA.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 4.Şimşek HO, Kocatürk O, Demetoğlu U, Gürsoytrak B. Propofol based total intravenous anesthesia versus sevoflurane based inhalation anesthesia: the postoperative characteristics in oral and maxillofacial surgery. J Craniomaxillofac Surg. 2020;48:880–884. doi: 10.1016/j.jcms.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q, Chen K. Sevoflurane inhalation anesthesia for uncooperative pediatric outpatients in the treatment of ankyloglossia: a retrospective study of 137 Cases. J Invest Surg. 2019;34:1–5. doi: 10.1080/08941939.2019.1609141. [DOI] [PubMed] [Google Scholar]
- 6.Wanderer JP, Rathmell JP. Multi-dimensional anesthesia: the effects of sevoflurane or propofol with remifentanil. Anesthesiology. 2019;131:A17. doi: 10.1097/ALN.0000000000003052. [DOI] [PubMed] [Google Scholar]
- 7.Godet T, Guérin R, Verlhac C, Cayot S, Jabaudon M, Bazin JE, Futier E, Constantin JM. Enteral versus total parenteral nutrition in patients undergoing pancreaticoduodenectomy: a randomized multicenter controlled trial (Nutri-DPC): let’s take a closer look at the pancreas. Ann Surg. 2018;197:e70. doi: 10.1097/SLA.0000000000002153. [DOI] [PubMed] [Google Scholar]
- 8.Kwon JH, Shin YH, Gil NS, Park J, Chung YJ, Hahm TS, Jeong JS. Effect-site concentration of remifentanil for smooth emergence from sevoflurane anesthesia in patients undergoing endovascular neurointervention. PLoS One. 2019;14:e0218074. doi: 10.1371/journal.pone.0218074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Yook K, Yoo KY, Choi JI, Bae HB, You Y, Jin BY, Jeong S. Comparison of the effect of sevoflurane or propofol anesthesia on the regional cerebral oxygen saturation in patients undergoing carotid endarterectomy: a prospective, randomized controlled study. BMC Anesthesiol. 2019;19:157. doi: 10.1186/s12871-019-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Y, Han L, Dong MM, Sun QC, Ding K, Zhang ZF, Cao JL, Zhang YY. Comparison of a loading dose of dexmedetomidine combined with propofol or sevoflurane for hemodynamic changes during anesthesia maintenance: a prospective, randomized, double-blind, controlled clinical trial. BMC Anesthesiol. 2018;18:12. doi: 10.1186/s12871-018-0468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu MQ, Wu HY, Yang DL, Li FX, Li ZC, Wang S, He RL. Effects of small-dose remifentanil combined with index of consciousness monitoring on gastroscopic polypectomy: a prospective, randomized, single-blinded trial. Trials. 2018;19:392. doi: 10.1186/s13063-018-2783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Q, Li J, Dai CL, Li HC, Iqbal K, Liu F, Gong CX. Anesthesia with sevoflurane or isoflurane induces severe hypoglycemia in neonatal mice. PLoS One. 2020;15:e0231090. doi: 10.1371/journal.pone.0231090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong Y, Lu Z, Ye M, Yu H. Early postoperative recovery in operating room after desflurane anesthesia combined with bispectral index (BIS) monitoring and warming in lengthy abdominal surgery: a randomized controlled study. BMC Anesthesiol. 2018;18:110. doi: 10.1186/s12871-018-0577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia L, Xie M, Zhang J, Guo JY, Tong T, Xing YY. Efficacy of different dose of dexmedetomidine combined with remifentanil in colonoscopy: a randomized controlled trial. BMC Anesthesiol. 2020;20:225. doi: 10.1186/s12871-020-01141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Timea B, Csaba L, Zoltan V, Gabor W, Tihamer M, Lajos B, Laszlo L. Cost-effectiveness of anesthesia maintained with sevoflurane or propofol with and without additional monitoring: a prospective, randomized controlled trial. BMC Anesthesiol. 2018;18:100. doi: 10.1186/s12871-018-0563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortea JI, Puerto M, Fernández-Mena C, Asensio I, Arriba M, Almagro J, Bañares J, Ripoll C, Bañares R, Vaquero J. Sevoflurane versus ketamine + diazepam anesthesia for assessing systemic and hepatic hemodynamics in rats with non-cirrhotic portal hypertension. PLoS One. 2020;15:e0233778. doi: 10.1371/journal.pone.0233778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu YY, Li HY, Shi CM, Qian M, Yang N, Wang LW, Gao XY, Ni C. lncRNAs are involved in sevoflurane anesthesia-related brain function modulation through affecting mitochondrial function and aging process. Biomed Res Int. 2020;2020:1–14. doi: 10.1155/2020/8841511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariana O, Fernandes AL, Susana V. Using sevoflurane in a pediatric patient with nemaline rod myopathy. Pediatr Anesth. 2018;28:749–750. doi: 10.1111/pan.13458. [DOI] [PubMed] [Google Scholar]
- 19.Ferenc P, Walid H. Macgyver or machiavellian approaches to delivery of sevoflurane in neonates. Pediatr Anesth. 2018;28:756–757. doi: 10.1111/pan.13453. [DOI] [PubMed] [Google Scholar]
- 20.Samani S, Kaur D, Li KK. PTU-115 timing of endoscopy and 30 day mortality in patients admitted with variceal haemorrhage. Gut. 2019;68:A52. [Google Scholar]
- 21.Moon AM, Kim HP, Barritt AS, Darling J, Arora S. A quality improvement initiative results in improved rates of timely postvariceal bleeding surveillance endoscopy. Am J Gastroenterol. 2020;115:625–628. doi: 10.14309/ajg.0000000000000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshihiro F, Yoshitaka K, Hirohito T, Yuu Y, Katsutoshi S, Takao I. Sa1911 dual red imaging enables to decrease recurrence rate of esophageal varices by accurate intra-variceal sclerotherapy injection. Gastrointest Endosc. 2018;87:AB246. [Google Scholar]
- 23.Chen N, Li YW, Fan HZ, Tian AR, Yuan H, Jiang ZY, Yu YX, Ruan LL, Hu PP, Yue M, Li J, Zhu CL. Analysis of dynamic disturbance in blood coagulation function of patients with coronavirus disease 2019: a retrospective observational study. Medicine. 2020;99:e22635. doi: 10.1097/MD.0000000000022635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao HF, Dong ZG, Xu WQ, Chen XY, Xin PL, Zhang JY. Evaluation and comparison of thromboelastography and conventional coagulation tests for blood coagulation function in children with Henoch-Schönlein Purpura. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27:850–854. doi: 10.19746/j.cnki.issn.1009-2137.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Poniedziaek B, Siwulski M, Wiater A, Komaniecka I, Komosa A, Gąsecka M, Magdziak Z, Mleczek M, Niedzielski P, Proch J, Ropacka-Lesiak M, Lesiak M, Henao E, Rzymski P. The Effect of mushroom extracts on human platelet and blood coagulation: in vitro screening of eight edible species. Nutrients. 2019;11:3040. doi: 10.3390/nu11123040. [DOI] [PMC free article] [PubMed] [Google Scholar]


