Abstract
The fragment crystallizable (Fc) domain of antibodies is responsible for their protective function and long-lasting serum half-life via Fc-mediated effector function, transcytosis, and recycling through its interaction with Fc receptors (FcRs) expressed on various immune leukocytes, epithelial, and endothelial cells. Therefore, the Fc–FcRs interaction is a control point of both endogenous and therapeutic antibody function. There are a number of reported genetic variants of FcRs, which include polymorphisms in (i) extracellular domain of FcRs, which change their affinities to Fc domain of antibodies; (ii) both cytoplasmic and intracellular domain, which alters the extent of signal transduction; and (iii) the promoter region of the FcRs gene, which affects the expression level of FcRs, thus being associated with the pathogenesis of disease indications. In this review, we firstly describe the correlation between the genetic variants of FcRs and immunological disorders by individual differences in the extent of FcRs-mediated regulations. Secondly, we discuss the influence of the genetic variants of FcRs on the susceptibility to infectious diseases or cancer in the perspective of FcRs-induced effector functions. Overall, we concluded that the genetic variants of FcRs are one of the key elements in the design of antibody therapeutics due to their variety of clinical outcomes among individuals.
Keywords: FcRs, genetic variants, immunological disorders, infectious diseases, cancer, antibody engineering
1. Introduction
In the top 10 best-selling drugs in 2020, a total of 5 were monoclonal antibodies (mAbs), including the first and second rank drugs [1]. In addition, 57.3% of the expected top 10 drug sales in 2021 were therapeutic mAbs, and this proportion is predicted to rise even higher [2]. By May 2021, the Food and Drug Administration (FDA) authorized 102 therapeutic mAbs. As for disease indications, treatments of therapeutic mAbs were carried out mainly in cancers (43.1%), autoimmune diseases (25.5%), and infectious diseases (6.9%) [3]. The major reasons why the market of therapeutic antibodies is growing so rapidly are due to their high specificity, low toxicity, and long serum half-life, similar to their biological properties in our body [4,5,6,7].
Antibodies play protective roles by specific binding to pathogens, infected cells, and tumor cells via antibody-mediated functions, including neutralization [8,9], agglutination [10,11], antibody-dependent cellular cytotoxicity (ADCC) [12,13,14], antibody-dependent cellular phagocytosis (ADCP) [15,16], and complement-mediated cytotoxicity (CDC) [17,18]. These functions stem from the cooperation of the fragment antigen-binding (Fab) that recognizes antigens in a specific manner and the Fc that triggers the effector functions by binding to the FcRs [19]. In addition to the major function of antibodies as pivotal parts in humoral immunity, the interaction between the Fc-portion of antibodies and FcRs can effectively cooperate with FcRs-expressed cellular immunity.
FcRs-mediated antibody effector function, transcytosis, and recycling are initialized by the Fc–FcRs interactions. These events are sophisticated outcomes, being harmonized with the following three features. The first is the inherency of FcRs. The existence of various types of FcRs on the surface of immune leukocytes results in intrinsic affinities and specificity to the Fc domain of antibodies, leading to diverse antibody-mediated immune reactions. FcRs are classified into five types (FcαRs, FcδRs, FcγRs, FcεRs, FcμRs), interacting with the isotypes of immunoglobulins (Igs) [20,21]. Either FcγRs or FcεRs are further categorized into FcγRI, FcγRIIa, FcγRIIb, FcγRIIc, FcγRIIIa, and FcγRIIIb, or FcεRI and FcεRII, respectively [22]. Fcα/μR is a dual binding receptor protein to both IgA and IgM. Despite its unknown function, it is clear that Fcα/μR is related to both positive and negative immune responses, based on recent research [23]. While FcγRs, FcεRs, and FcαRs regulate immune regulation, the neonatal Fc receptor (FcRn) interacts with IgGs and enables them to be recycled in serum [24]. In addition, polymeric immunoglobulin receptor (pIgR) interacts with both dimeric IgA and pentameric IgM and exerts them to be transported from the basolateral surface of the epithelium to the apical side [24,25]. The second characteristic which contributes to immune effector functions is the distribution of FcRs on various cell types. FcRs are expressed on the membranes of not only immune cells, but also epithelial and endothelial cells (Table 1), and their expression profiles are variable in response to surrounding cytokines. The last hallmark is the diversity of FcRs ligands that are distinctive isotypes and subclasses of Igs. Igs are divided into five isotypes (IgA, IgD, IgE, IgG, and IgM) and six further subclasses (IgA1, IgA2, IgG1, IgG2, IgG3, and IgG4) [26,27,28]. Each of them has its own unique structural and functional characteristics, thus triggering various immune responses. Furthermore, B-cells can alter their production of Ig isotypes, regardless of antigen-specificity, through the unique process called immunoglobulin isotype switching, which is caused by recombination of the immunoglobulin heavy chain (IGH) gene. Because each Ig isotype has an intrinsic affinity to FcRs, isotype switching also remodels the recruitment of effector cells, regulated by cytokines with regards to their functional requirements [29,30].
Table 1.
Ig binding affinity, function, and expression of individual FcRs.
| Receptor | Ig Isotype and Subclass Binding Affinity | Function | Expression | |||
|---|---|---|---|---|---|---|
| Myeloid Cell | Lymphoid Cell | Non-Immune Cell | ||||
| FcγRI | IgG1,2,3,4 | High affinity (IgG1 = IgG3 > IgG4 >>> IgG2) |
Activation | Monocyte, Macrophage, Neutrophil, DC, Mast cell, Eosinophil |
- | - |
| FcγRIIa | Low affinity (H131: IgG3 > IgG1 > IgG2 > IgG4 R131: IgG3 > IgG1 > IgG4 > IgG2) |
Activation/Inhibition | Monocyte, Macrophage, Neutrophil, DC, Mast cell, Basophil, Eosinophil |
T cell | Platelet, Endothelium | |
| FcγRIIb | Low affinity (IgG3 > IgG1 > IgG4 >> IgG2) |
Inhibition | Monocyte, Macrophage, Neutrophil, DC, Mast cell, Basophil, Eosinophil |
B cell, Plasma cell |
- | |
| FcγRIIc | Low affinity (IgG3 > IgG1 > IgG4 >> IgG2) |
Activation | Monocyte, Macrophage, Neutrophil | NK cell | - | |
| FcγRIIIa | Low affinity (IgG3 >> IgG1 > IgG4 > IgG2) |
Activation/Inhibition | Monocyte, Macrophage | NK cell, T cell |
- | |
| FcγRIIIb | Low affinity (IgG1 = IgG3 >>> IgG2 = IgG4) |
Activation | Neutrophil, Basophil, Eosinophil | - | - | |
| FcRn | High affinity (pH ≤ 6.5)/Low affinity (pH 7.4) (IgG4 > IgG1 > IgG3 > IgG2) |
Transcytosis/Recycling | Monocyte, Macrophage, Neutrophil, DC | - | Endothelium, Epithelium | |
| FcεRI | IgE | High affinity | Activation | Langerhans cell, Eosinophil, Mast cell, Basophil, DC, Monocyte |
- | Platelet |
| FcεRII | Low affinity | Activation | Langerhans cell, Monocyte, DC, Macrophage, Eosinophil, | B cell, T cell, NK cell |
Platelet | |
| FcαRI | IgA1,2 | Low affinity (IgA1 = IgA2) |
Activation/Inhibition | Monocyte, Macrophage, Neutrophil, DC, Kupffer cell | - | - |
| FcμRI | IgM | Low affinity (monomeric IgM)/High avidity (pentameric IgM) | NA | - | B cell, T cell, NK cell |
- |
| Fcα/μR | IgA/IgM | High affinity (IgM > IgA) |
NA | Macrophage, DC | B cell | - |
| FcδR | IgD | NA | ||||
| plgR | Dimeric IgA/pentameric IgM | High affinity | Transcytosis | - | - | Endothelium, Epithelium |
DC: dendritic cells, NK cells: natural killer cells, NA: not analyzable.
When the immune effector functions are excessive by the cooperative and synergistic effects of the three features, immune self-attack arises and can lead to autoimmune, inflammatory, or allergic diseases by the unbalanced activation of FcRs [21,22,31]. Consequently, these abnormal immune reactions by either autoantibodies or autoreactive lymphocytes target self-antigens with hypersensitivity to harmless environmental antigens, respectively [32,33]. Conversely, if the immune effector functions are insufficient to meet the activation threshold, the susceptibility to pathogens is increased by the non-induction of effector functions, leading to less clearances of immune complexes (ICs) [26,34]. Likewise, immunosurveillance against precancerous or cancerous cells can be attenuated [35,36].
In this review, we discuss (i) the correlations between genetic diversity of FcRs among individuals and pathogenesis of immunological disorders and (ii) the effect of FcRs genetic variants on susceptibility to infectious diseases and cancer. With regards to diverse FcRs variants, antibody engineering strategies for improving therapeutic efficacy are also reviewed.
2. Immune Disorders and Fc Receptor Genetic Variants
The genetic variety of FcRs can affect individual FcRs-Ig affinities, the strength of FcRs’ intracellular signaling, and their expression levels, which can lead to the distinctive immunological outcomes. In other words, FcRs’ polymorphisms are closely related to a variety of overactive immune responses such as autoimmunity, inflammation, and allergic reactions. This section describes three different types of FcR variants, which are FcγRIIb, FcεRI, and FcαRI, and their consequent immune activation mechanisms.
2.1. FcγRIIb Genetic Variants: Insufficient Inhibitory Function of FcγRIIb Cause Autoimmune Diseases
FcγRIIb, the sole inhibitory receptor of FcγRs known so far, serves as a negative regulator of humoral immunity via the inhibition of B-cell activation or antigen phagocytosis of dendritic cells (DCs). FcγRIIb is expressed on various immune cells such as DCs and macrophages (Table 1), and notably, it is the only FcγR expressed on B-cells and plasma cells as well [21,22,37]. Activated signaling pathways of immune cells are suppressed via the dephosphorylation of activation motifs of B-cell receptors (BCRs) or T-cell receptors (TCRs) through the co-engagement of immunoreceptor tyrosine-based inhibitory motif (ITIM) in the cytoplasmic tail of FcγRIIb. The IgG binding ratio of activating FcγRs to FcγRIIb (A/I ratio) on the immune cells determines the activation threshold, and this can be altered by the extent of FcγRIIb expression [38,39]. In clinical perspectives, FcγRIIb is reported to be involved in the pathogenesis of autoimmune diseases [40,41,42,43]. For example, one of the main hallmarks of autoimmune diseases such as systemic lupus erythematosus (SLE) is the overproduction of autoantibodies due to the defect in the downregulation of the humoral immune response affected by FcγRIIb-dependent inhibitory mechanisms [37,44].
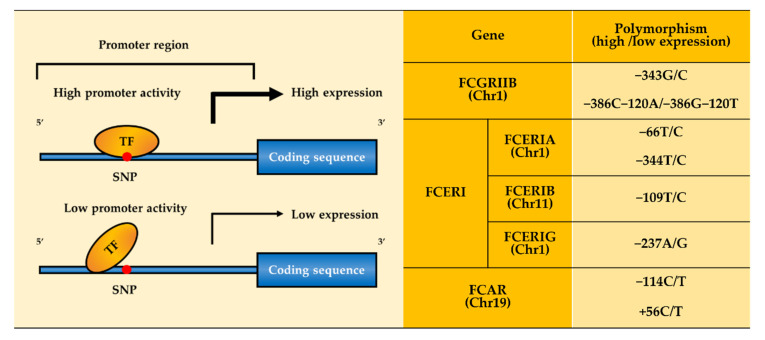
Single nucleotide polymorphisms (SNPs) in the promoter region of the FCGRIIB gene are reported to change the affinity to transcription factors and result in expression differences of FcγRIIb on the immune cell surface (Figure 1) [37,39,45]. Three SNPs in the promoter are identified: −386G/C, −343G/C, and −120T/A promoter polymorphisms in the relative positions from the transcription start site (TSS: Position +1) (Figure 1 and Table 2) [46,47]. Interestingly, because the −386C allele mostly accompanies the −120A allele, −386C allele and −120A alleles may form a haplotype [46]. Each promoter with −386C−120A haplotype and −343G allele showed a higher transcription strength of promoter activity with the higher FcγRIIb expression level than that of −386G−120T and −343C allele, respectively (Figure 1). This may be the reason why an insufficient FcγRIIb expression affected by the SNPs is associated with autoimmune diseases (Table 2). The homozygous −343C/C genotype was observed at a higher frequency in SLE patients (7.9%) than in healthy controls (0.8%) [47]. In addition, SLE patients reported decreased expression of FcγRIIb in both memory B cells and DCs [48,49]. Based on these results, immunological disorders can be caused by decreased FcγRIIb expression due to the genetic variations in the promoter region of the FCGRIIB gene.
Figure 1.
Polymorphisms influence gene expression. Polymorphism in the promoter region induces differences in promoter activity and therefore changes the expression level of Fc receptors. −386C−120A and −343G of FCGRIIB increase the expression level of FcγRIIb. −66T and −344T of FCERIA, −109T of FCERIB, and −237A of FCERIG increase the expression level of α, β, and γ subunit, respectively. −114C and +56C of FCAR increase the expression level of FcαRI.
Table 2.
Summary of genetic variants of individual FcRs and related diseases.
| Fc Receptor | Genetic Variant | Related Diseases | |||||
|---|---|---|---|---|---|---|---|
| Polymorphism | Copy Number Variant | ||||||
| Promoter Region | Extracellular Domain | Transmembrane Domain | Intracellular Domain | ||||
| FcγRIIa | H131R | R131 allele: Bacterial infection (Low affinity to IgG2) |
|||||
| FcγRIIb | −120T/A −343G/C −386G/C |
−343G, −386C−120A allele: SLE (High FcγRIIb expression) |
|||||
| FcγRIIIa | 48L/R/H V158F |
F131 allele: Cancer (Low affinity to IgG) |
|||||
| FcγRIIIb | NA1 NA2 SH |
NA2 allele: CGD (Low affinity to IgG1 and IgG3) |
|||||
| FcRn | VNTR1- VNTR5 | NA | |||||
| FcεRI | α-subunit | −66T/C −344C/T |
−66T, −344T allele: Allergic diseases (High FcεRI expression) |
||||
| β-subunit | −109C/T | I181L V183L |
E237G | −109T allele: Asthma (High FcεRI expression) L181, L183 allele: Atopy, Asthma (Altered signaling pathway leading to cell activation) G237 allele: Allergy diseases (Activating signal induction) |
|||
| γ-subunit | −237A/G | −237A allele: AIA (High FcεRI expression) |
|||||
| FcαRI | −114C/T +56C/T |
S248G | −114C, +56C allele: IgAN (High FcαRI expression) G248 allele: SLE (Altered signaling pathway leading to cell activation) |
||||
NA: not analyzable.
The aforementioned research indicates that reinforcing the affinity of FcγRIIb with engineered Fc might have an advantage in the treatment of autoimmune diseases by the suppression of the activating signal of abnormal immune responses via the FcγRIIb-dependent inhibitory mechanisms [37,48,49,50,51,52,53,54,55,56,57]. For example, Szymkowski and his colleagues constructed an anti-CD19 antibody, XmAb5871, with engineered Fc that exhibits a >400-fold increased FcγRIIb affinity, relative to native IgG1 Fc. XmAb5871 suppressed B cell proliferation and antigen-presenting cell (APC) function by overcoming the FcγRIIb dysfunction of both SLE and rheumatoid arthritis (RA) B cells. Moreover, in both cases, the abnormal production of antibodies was decreased and an improved survival rate was observed after administration of XmAb5871 in both SLE and RA peripheral blood mononuclear cell (PBMC)-engrafted mice [58,59].
2.2. FcεRI Genetic Variants: Hyperactive FcεRI Results in Allergic Diseases
FcεRI is one of the high-affinity receptors that have been studied as a critical component in allergic responses. FcεRI is an Fc receptor for IgE isotype antibodies, and the cross-linking of FcεRI by the binding of IgE activates mast cells to secrete allergic mediators such as histamine, leukotrienes, and a number of cytokines and chemokines to induce allergic responses. The FcεRI is observed on the surface of not only mast cells but also epidermal Langerhans cells, eosinophils, basophils, and even antigen-presenting cells such as DCs and monocytes (Table 1) [60,61,62,63].
The heterotetrameric FcεRI is composed of α, β, and two γ subunits linked by disulfide bonds. Each subunit contributes to the function of the FcεRI, and they are reported to have several SNPs in their promoter regions, which affect allergic responses (Figure 1). As for the α subunit, −66T/C and −344T/C alleles have been identified as the promoters of the FCERIA gene (Figure 1 and Table 2). The higher strength of promoter activity due to the high affinity of transcription factors to the FcεRI α subunit gene was detected in the −66T and −344T alleles, compared to that of the −66C and −344C alleles, respectively (Figure 1) [64,65]. On one hand, people with a heterozygous −66T/C genotype are generally healthy, while those with a homozygous −66T/T genotype tend to hold allergic diseases, which indicates that people who have −66C allele presents resistance to allergic diseases [65]. On the other hand, −344T allele carriers expressed FcεRI on mast cells, resulting in a high degree of histamine release to provoke IgE-mediated allergic responses [66] and elevation of total serum IgE level [67,68]. As for now, the detailed relationship between the elevated IgE level and SNPs in the promoter is still unknown. In another report, among aspirin-intolerant chronic urticaria (AICU) and aspirin-intolerant asthma (AIA) patients, those who have −344T allele not only indicated a higher atopy rate (among AIA patients, those who had −344T allele were 59.46%; among AICU patients, those who had −344T allele were 84.1%) but also total serum IgE concentrations compared to those with the homozygous −344C/C genotype [66,69]. Collectively, the prevalence of −344T allele with relatively high affinity to transcription factor to the other −344C allele in both AICU and AIA patients indicates that SNPs at the promoter of the FCERIA gene plays a significant role in the development of allergic diseases.
In the β subunit, three SNPs have been reported: the promoter region (−109T/C), intracellular domain (G237E), and transmembrane domain (L181I and L183V) of the FCERIB gene (Figure 1 and Figure 2, and Table 2). Firstly, individuals with the −109T allele showed a higher expression level of the FcεRI β subunit than those with −109C allele (Figure 1), and also asthma patients with homozygous −109T/T genotype showed significantly higher total IgE level, compared to those with −109T/C or −109C/C genotype [70,71,72]. Secondly, the G237E polymorphism also affects allergic responses which can be explained through the following two hypotheses: (1) G237E polymorphism is placed in the intracellular tail of β subunit close to an immunoreceptor tyrosine-based activation motif (ITAM) (Figure 2). The substitution of glutamic acid to glycine residue introduces a critical hydrophobicity adjacent to ITAM in the C-terminus of the β subunit, which can affect the signaling for FcεRI function [73]. (2) G237E polymorphism, which is close to an alternative splicing site, may also affect expression level of the receptor on the cell surface. Kinet and his colleagues identified a new transcript of the β subunit that contains an in-frame stop codon generated by an alternative splicing of the FcεRI β transcript, which would be translated into a truncated β subunit [74]. Compared to the full-length β subunit, the truncated one is significantly less expressed and therefore the G237E polymorphism might affect the expression level of the β chain, which can lead to the diverse susceptibility of allergic responses among individuals [74,75,76]. Lastly, L181I and L183V polymorphism is located in the transmembrane domain (Figure 2). Detailed mechanisms have not yet been fully studied; however, all the infants who have maternally inherited the FcεRI-L181 allele revealed a higher incidence of atopy than those with the I181 and homozygous FcεRI–L/L181 and L/L183 genotypes showed a higher prevalence in asthma, compared with the counterpart genotype [77,78,79]. In the γ subunit, −237A/G polymorphism on the promoter region in the FCERIG gene has been identified (Figure 1). The function of the γ subunit is associated with the signal-transduction pathway of FcεRI. For example, the ITAM of the γ subunit is pivotal for FcεRI-mediated mast cell activation. Individuals with −237A allele showed higher expression of the γ chain than those with −237G allele, which subsequently upregulates the mast cell activation more easily and release inflammatory mediator and cytokines (Figure 1) [66,79,80]. AIA patients with the −237A/A genotype are reported to exhibit higher total serum IgE level than those with the A/G, G/G genotype (IgE concentration of AIA patients with the −237A/A genotype were Log2.25 ± 0.57 IU/mL), which indicates that the −237A/G polymorphism plays a significant role in the development of AIA [69].
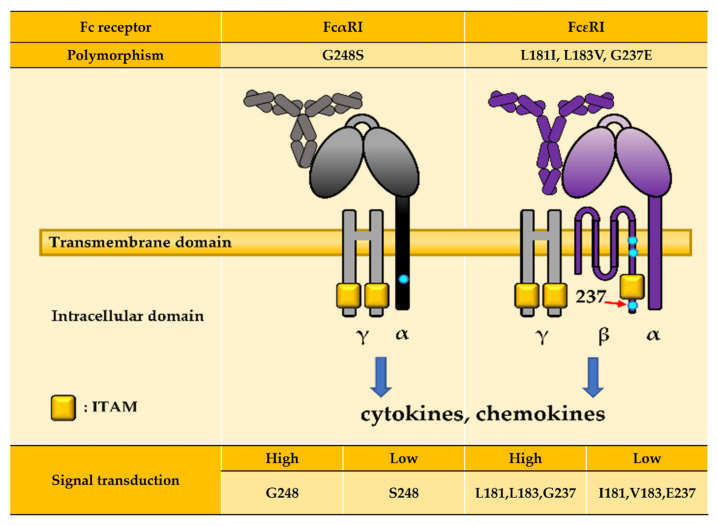
Figure 2.
Polymorphisms alter signal transduction. Turquoise dots indicate amino acid sequences that affect downstream signaling pathways. G248 located in the cytoplasmic domain of FcαRI, produces pro-inflammatory cytokines without γ-chain, compared to S248. L181 and L183, located in the transmembrane domain of FcεRI, are more easily seen in patients with atopy and asthma, compared to I181 and V183, respectively. G237, located in the intracellular domain of FcεRI, causes an excessive allergic response compared to E237.
Based on these results, preventing the cross-linking of IgE with the FcεRI can be one of the attractive treatments for IgE-mediated allergic diseases. Indeed, omalizumab (Xolair®) which is an FDA and European Medicines Agency (EMA)-approved recombinant humanized anti-IgE mAbs that neutralize IgE by blocking IgE–FcεRI interaction [81]. Of note, omalizumab was also approved for self-injection across all the approved allergic indications as of April 2021 [82].
2.3. FcαRI Genetic Variants: Upregulated FcαRI Leads to Autoimmune Diseases
FcαRI is expressed on neutrophils, eosinophils, monocytes/macrophages, DCs, and Kupffer cells (Table 1) [83]. This receptor is specific to IgA and can bind to both human IgA1 and IgA2 subclasses [84,85]. Among three of the FcαRI polymorphisms, two exist in the promoter region (−114C/T and +56C/T on the promoter in the FCAR gene, Figure 1) and the other in the cytoplasmic domain (G248S, Figure 2), each of which causes diverse immunological impact (Table 2).
Two polymorphisms exist in the FcαRI promoter and have been reported to be associated with the etiology of IgA nephropathy (IgAN) [86]. IgAN is the most common disease of primary glomerulonephritis (GN) by the accumulation of unglycosylated IgA1-FcαRI complexes in the glomerular mesangium [87,88,89]. −114C and +56C alleles were more frequent in IgAN patients than in other GN patients and healthy adults [86]. The FCAR gene promoter with these two alleles has a significantly higher promoter activity than those with −114T and +56T alleles (Figure 1) [90], which upregulate the expression of soluble FcαRI in the serum of IgAN patients, consequently leading to development into IgAN via the increased deposition of the IgA1–FcαRI complex in the mesangium [91,92]. Consistent with these results, removing soluble FcαRI from the serum of transgenic mice expressing human FcαRI in macrophages/monocytes can eliminate effects such as massive mesangial IgA deposition, glomerular, and interstitial macrophage infiltration, mesangial matrix expansion, hematuria, and mild proteinuria, which are the initial symptoms of IgAN [93,94,95].
G248S polymorphism in the cytoplasmic domain of FcαRI affects intracellular signals, thereby leading to different biological outcomes (Figure 2) [96]. FcαRI-S248 allele requires FcαRI γ-chain to mediate cytokine production, whereas FcαRI-G248 allele can mediate pro-inflammatory cytokine production without the γ-chain binding. Genotype analysis confirmed that the G248 allele is significantly enriched in SLE patients (36% for African American, 19% for Caucasian), compared to control populations (26% for African American, 14% for Caucasian). Therefore, the G248 allele may be considered as a candidate risk factor in autoimmune diseases such as SLE [97].
Combined with these studies, the therapeutic direction of IgAN and SLE can be offered with a method to target FcαRI or IgA, which can neutralize soluble FcαRI or unglycosylated IgA1 to interrupt the IgA–FcαRI interaction.
3. Therapeutic Efficacy of Antibodies and Fc Receptor Genetic Variants
The genetic variety of FcRs induces different effector functions by both endogenous and therapeutic antibodies, conferring tolerance to biological invasions and malignant cells. Thus, the genetic variants of FcRs are correlated with susceptibility to infectious diseases and cancers as well as the extent of clinical benefits from therapeutic antibodies against pathogens or malignancy. Indeed, examples of Fc engineering efforts that altered FcRs’ affinity showed improved clinical efficacy or prolonged half-life of antibodies [98,99,100,101,102,103,104,105]. This section introduces the variants of four different FcRs, which are FcγRIIa, FcγRIIIa, FcγRIIIb, and FcRn and affect the susceptibility to pathogens or cancer. This can be informative as to strategies for clinical application of antibody therapeutics.
3.1. FcγRIIa Genetic Variants: High-Affinity FcγRIIa for IgG2 Induces ADCP and Confers Low Susceptibility to Infection and Vaccine Effect
FcγRIIa is expressed on APCs such as DCs, macrophages, and B cells, and operates activating signaling pathway of effector functions by its own ITAM in the intracellular domain (Table 1) [22]. Recently, several studies suggested that FcγRIIa polymorphism affect the ‘vaccine effect’ through the FcγRIIa-mediated phagocytosis, which is part of the antigen presenting and hence can contribute to adaptive immune systems [106,107].
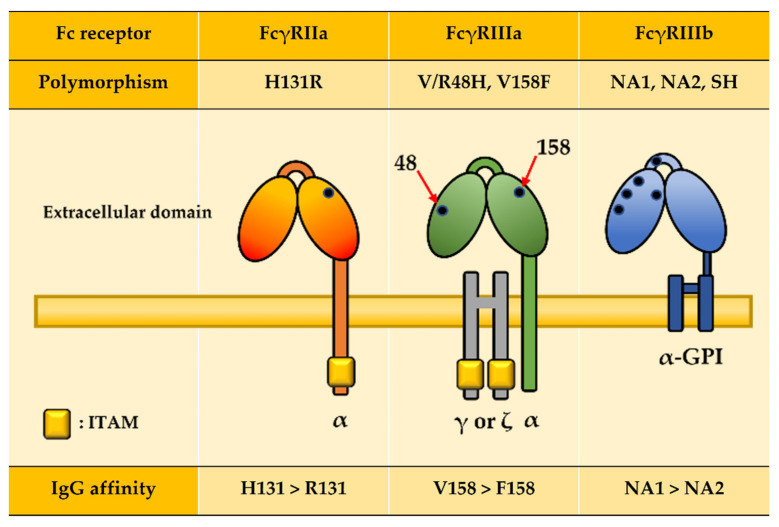
FcγRIIa polymorphism exists in the 131 residue of the C’E loop, which directly contacts with the Fc domain of IgG antibodies and results in the affinity difference (Figure 3 and Table 2) [108,109,110,111]. H131R polymorphism is amino acid sequence change from histidine (H; codon CAT) to arginine (R; codon CGT), and FcγRIIa-H131 has a higher affinity to the Fc domain than FcγRIIa-R131 (Figure 3). A notable feature of the FcγRIIa-H131 is its efficient binding with IgG2 as opposed to low or non-binding with the FcγRIIa-R131 [27]. Under bacterial infections, IgG2 subclasses are induced by bacterial capsular polysaccharide, which provokes ADCP by the interaction of IgG2-opsonized bacteria and the FcγRIIa-H131 [26]. For this reason, homozygous FcγRIIa-H/H131 patients showed lower susceptibility to bacterial infection than patients with other genotypes. Indeed, among pediatric patients who suffer from pneumococcal sepsis, the proportion of the R/R genotype (43%) was higher than the H/H genotype (22%) [112,113,114,115].
Figure 3.
Polymorphisms affect immunoglobulin affinity. Black dots indicate amino acid residues that affect IgG affinity. H131 of FcγRIIa, V158 of FcγRIIIa, and NA1 of FcγRIIIb havehigher IgG affinity than the other alleles.
Ravetch and his colleagues demonstrated the feasibility of utilizing the FcγRIIa-mediated vaccine effect for both infection and cancer therapy in which the strengthened FcγRIIa affinity resulted in DCs maturation and induction of CD8+ T-cell response. Anti-influenza mAbs engineered to increase FcγRIIa affinity showed enhanced pharmacokinetic activity both in the prevention and treatment of influenza infection. In addition, the vaccine effect induced by FcγRIIa-expressing DCs was shown to lead to long-term anti-cancer activity [106,107].
3.2. FcγRIIIa Genetic Variants: High-Affinity FcγRIIIa for IgG Is Associated with Low Susceptibility to Cancer and Results in a Higher Response of NK Cell-Mediated ADCC of Therapeutic Antibodies
FcγRIIIa is expressed on natural killer (NK) cells, γδT cells, and subsets of monocytes and macrophages (Table 1) [116]. Once FcγRIIIa interacts with its ligand, ITAM in its cytoplasmic domain initiates the signaling pathway via phosphorylation by Src family kinases (SFKs) and spleen tyrosine kinases, thereby activating the effector function of the immune cells themselves, producing cytokines or chemokines, and inducing cell migration [117]. The effector function induced by FcγRIIIa includes ADCC, which is triggered by the interaction between multiple Fcs in ICs and the FcγRIIIa expressed on NK cells [118].
Two polymorphisms of FcγRIIIa have been identified in each extracellular domain 1 (EC1) and EC2 (Figure 3 and Table 2). In EC1, there is a tri-allelic polymorphism in which the residue 48 of the EC1 can be leucine (L; codon CTC), arginine (R; codon CGC), or histidine (H; codon CAC) [119]. In EC2, the 158 residue can be either valine (V; codon GTT) or phenylalanine (F; codon TTT). As the 158 residue directly contacts with the lower hinge region of IgG, this polymorphism affects the binding affinity to IgG (Figure 3) [120]. The V158 allele exhibits approximately twofold higher affinity for IgG than the F158 allele, which makes the NK cells more ready to induce ADCC [27]. Statistical analysis showed that carriers of the FcγRIIIa F158 allele appear to be more susceptible to cancer incidence. Indeed, among the patients with ERBB2/HER2-positive breast cancer, patients with the F/F158 genotype (46%) and F/V158 genotype (42%) have greater prevalence relative to those with the V/V158 genotype (12%). This proportion is consistent in the metastatic colorectal cancer patients: the F/F158 genotype (48.5%) and F/V158 genotype (41.4%) were observed for a higher proportion than the V/V158 genotype (10.1%) [35,36]. Notably, in addition to the statistics that FcγRIIIa polymorphism affects cancer prognosis, patients with V/V158 genotype also display more clinical benefits than those with F/F158 and F/V158 genotype in the treatment of therapeutic antibodies, including rituximab (Rituxan®), trastuzumab (Herceptin®), cetuximab (Erbitux®), and avelumab (Bavencio®) [35,121,122,123]. These results are presumed to be resulting from higher responses of NK cell-mediated ADCC due to the higher affinity to mAbs in V158 allele carriers compared with people with the F/F158 homozygote [124].
To improve the efficacy of cancer immunotherapy with regards to NK cell-mediated ADCC, increasing the FcγRIIIa affinity to the antibody Fc can be a powerful strategy. Fc-engineered anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody, AGEN1181, enhanced its binding to both F158 and V158 alleles, thus showing improved therapeutic efficacy in patients who carry the F158 allele, compared with the first generation anti-CTLA-4 antibody treatment [125]. Therefore, patients with low affinity FcγRIIIa allele, the remaining 40% of patients who had insignificant treatment effects with the first generation anti-CTLA-4 antibody, would be able to receive clinical benefits from the Fc-engineered antibodies.
3.3. FcγRIIIb Genetic Variants: High-Affinity FcγRIIIb for IgG1 and IgG3 Induces Neutrophil-Mediated Phagocytosis and Contributes to Low Susceptibility to Infections
FcγRIIIb is an IgG receptor with a glycosylphosphatidylinositol anchor in the transmembrane domain of neutrophils, basophils, and eosinophils (Table 1) [126]. The activation of FcγRIIIb leads to neutrophil degranulation and oxidative burst and induces the phagocytosis of IgG-opsonized pathogens by neutrophils [127].
FcγRIIIb has three polymorphisms in the membrane-distal Ig-like extracellular domain, each of which is a haplotype, comprising five respective amino acid residues: NA1 (R36, N65, A78, D82, and V106), NA2 (S36, S65, A78, N82, and I106), and SH (S36, S65, D78, N82, and I106) (Figure 3 and Table 1) [128]. The two co-dominantly expressed allelic variants, NA1 and NA2, show the difference in four of the five amino acids, which changes the number of glycosylation sites: NA1 has four and NA2 has six, which affects their interactions with IgG (Figure 3) [129]. The NA1 allele has a higher affinity for IgG1 and IgG3, and this allows the NA1 allele to more efficiently induce the neutrophil-mediated phagocytosis of IgG-opsonized particles and bind IgG3 more effectively than the NA2 allele [130]. The polymorphism of FcγRIIIb also affects the susceptibility to immune disorder or malaria infection. The risk of developing immune-mediated complications for chronic granulomatous disease (CGD) was lower in NA1 homozygous patients, compared with those with NA1/NA2 or NA2/NA2 [131]. Severe malaria infection in children was shown more in people carrying the FcγRIIIb-NA2 allele than those with NA1: patients with NA2/NA2 were 51.6%; NA1/NA2, 38.5%; and NA1/NA1, 9.9% [132].
Increasing FcγRIIIb affinity is expected to improve anti-cancer efficacy through neutrophil-mediated phagocytosis. Obinutuzumab (Gazyva®) is a type 2 anti-CD20 antibody approved for chronic lymphocytic leukemia (CLL). Obinutuzumab, the glycoengineered anti-CD20 for enhanced binding to FcγRIIIb showed a seven-fold higher affinity relative to the wild-type rituximab, which results in 47% higher phagocytosis of opsonized CLL by activating the FcγRIIIb-expressing polymorphonuclear neutrophils (PMNs) [133].
3.4. FcRn Genetic Variants: High-Expression of FcRn Increases IgG Half-Life
Heterodimeric FcRn is a family of major histocompatibility complex (MHC) class I molecule, which comprises the α-chain and β2m. It is expressed in monocytes, macrophages, neutrophils, DCs, and on the surface of epithelial and endothelial cells (Table 1). The FcRn interacts with both IgG and albumin at the acidic environment (pH ≤ 6.5) to be sorted into vesicles so as to be released back into the bloodstream without being decomposed in the lysosome. Consequently, the sorted vesicles are released out to the serum at a neutral pH (pH 7.4), which is how FcRn contributes to an important role in maintaining the high amount of IgG and albumin in circulation [24].
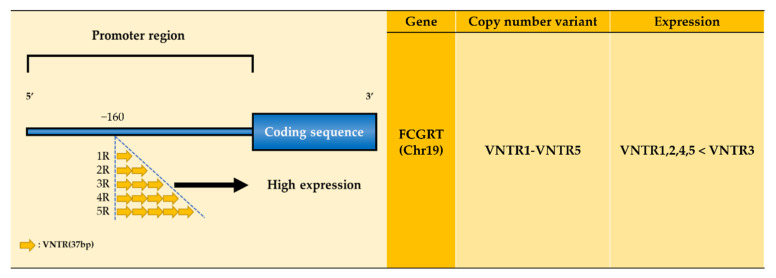
The expression level of FcRn depends upon a variable number of tandem repeats (VNTR) polymorphisms (Figure 4 and Table 2). VNTR identified within the promoter of the FCGRT gene may influence the transcriptional activity of the FCGRT gene promoter by changing the length of the transcription regulatory region, thereby altering the expression level of FcRn and affecting IgG catabolism (Figure 4) [134,135]. The VNTR region in the promoter consisted of one to five tandem repeats (VNTR1–VNTR5). VNTR3, the most common allele, is associated with an increase in promoter activity and the FcRn protein level, compared with the VNTR2 allele. In practice, monocytes from VNTR3 homozygotes displayed increased FcRn expression and led to recycling IgG more efficiently than the VNTR2/VNTR3 heterozygotes [134]. The IgG replacement of common variable immunodeficiency disorder (CVID) patients treated with intravenous immunoglobulin (IVIg) was more efficacious in VNTR3 homozygous patients than in VNTR2/VNTR3 heterozygous patients. Therefore, high IgG efficiency was found in CVID patients with VNTR3 homozygotes [136,137].
Figure 4.
Repeat 3 of VNTR in the promoter of the FCGRT gene shows the highest FcRn expression.
Since the expression level of FcRn depends on the VNTR variants and it is associated with IgG half-life in blood, the modulation of FcRn-mediated IgG recycling can be applied to various diseases with two opposite strategies. The first is to increase the half-life of therapeutic antibodies for infection and cancer treatment, thus expecting the advantage of less frequent and lower doses of therapeutic antibody administration. Fc variants with M428L/N434S mutations showed an 11-fold improvement in FcRn binding affinity at pH 6.0 and a 3-fold improvement in serum half-life than native IgG1 in cynomologus monkeys [138]. Furthermore, Georgiou and his colleagues developed an IgG1 Fc variant containing the L309D/Q311H/N434S substitutions that bind strongly at the acidic pH and simultaneously are released from FcRn at neutral pH showed improved pharmacokinetics relative to both endogenous IgG1 and widely used half-life extension variants [99]. The second is to reduce circulating IgG by blocking FcRn-IgG interaction for autoimmune disease treatment by decreasing the presence of endogenous pathogenic autoantibodies [139]. Rozanolixizumab, an anti-FcRn antibody, lasted dose-dependent reduction in serum IgG concentrations [140]. Additionally, in a phase 2 clinical trial, the clinical efficacy and safety of rozanolixizumab were confirmed in the moderate-to-severe generalized myasthenia gravis (gMG) patients, and phase 3 is ongoing [141]. Another anti-FcRn antibody, HBM9161, resulted in sustained and dose-dependent IgG reduction in a phase 1 clinical trial [142].
4. Conclusions
We classified the mutation positions of FcR variants into three groups in this article. Firstly, those in the ectodomain of FcγRs directly affect the interaction between FcRs and antibody Fc, which can differ in the extent of the recruitment of immune effector leukocytes such as NK cells for ADCC activity or macrophages for ADCP activity. This interaction can be strengthened by antibody Fc engineering with enhanced affinity to distinct FcγRs. Secondly, polymorphisms in the transmembrane or cytoplasmic domain of FcεRs or FcαRs on mast cells or PMNs, respectively, determine whether the downstream intracellular signaling would be turned on or off. For example, the G237 allele in the FcεR β subunit of cytoplasmic tail in proximity to ITAM motif on γ subunit is more easily activated and leads to hypersensitive immune response, relative to the E237 allele. Lastly, FcRs variants in the transcription regulatory region can affect the expression of the respective genes, which can change the activation threshold of immune effector functions [31,45,143].
In the aspect of disease susceptibility, the enhancement of not only FcRs’ affinities to Igs but also FcR-mediated signal transduction, as well as FcRs transcription via FcRs variants, would increase the risk of autoimmune diseases. Conversely, lowering the affinities, downstream signaling, or transcription level of FcRs weaken the protectiveness against the pathogenesis of infections or outbreaks of malignancy. Especially, the Fc–FcRs affinities can be therapeutically surmountable through Fc engineering for either boosting or attenuating the interaction. However, the pharmacologic activities of therapeutic antibodies that harnesses the effector functions is dependent upon patient genotype [144,145,146]. Attempts to increase effector functions by engineered Fc have shown a remarkably improved therapeutic potency in low-affinity receptor genotype patients. For example, Margetuximab (Margenza®) was designed to target the same epitope with trastuzumab (Herceptin®) with enhanced affinity to FcγRIIIa. In a SOPHIA phase 3 clinical trial, the FcγRIIIa-V/F158 and FcγRIIIa-F/F158 genotype patients showed an improved therapeutic potency of HER-2 positive breast cancer but did not show a significant effect in the v/v genotype patients [147,148]. This result showed that the treatment effect can differ depending on the FcRs’ genotype. Therefore, the clinical benefits of engineered antibodies can be maximized only when the genotypes of FcRs in patients are well-studied and considered in the treatment of mAbs with a proper therapeutic window for each individual.
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
| AIA | Aspirin-intolerant asthma |
| AICU | Aspirin-intolerant chronic urticarial |
| APC | Antigen presenting cell |
| BCR | B-cell receptor |
| CDC | Complement-mediated cytotoxicity |
| CGD | Chronic granulomatous disease |
| CLL | Chronic lymphocytic leukemia |
| CTLA-4 | Cytotoxic T lymphocyte associated protein-4 |
| CVID | Common variable immunodeficiency disorder |
| DC | Dendritic cell |
| EC | Extracellular |
| EMA | European Medicines Agency |
| Fab | Fragment antigen-binding |
| Fc | Fragment crystallizable |
| FcRs | Fc receptors |
| FcRn | Neonatal Fc receptor |
| FDA | Food and Drug Administration |
| gMG | Generalized myasthenia gravis |
| GN | Glomerulonephritis |
| IC | Immune complex |
| Ig | Immunoglobulin |
| IgAN | IgA nephropathy |
| IGH | Immunoglobulin heavy chain |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| IVIg | Intravenous immunoglobulin |
| mAbs | Monoclonal antibodies |
| MHC | Major histocompatibility complex |
| NK | Natural killer |
| PBMC | Peripheral blood mononuclear cell |
| pIgR | Polymeric immunoglobulin receptor |
| PMNs | Polymorphonuclear neutrophils |
| RA | Rheumatoid arthritis |
| SLE | Systemic lupus erythematosus |
| SNP | Single nucleotide polymorphism |
| TCR | T-cell receptor |
| TSS | Transcription start site |
| VNTR | Variable number of tandem repeats |
Author Contributions
Conceptualization, J.K. and T.H.K.; visualization, J.Y.L.; writing, J.K., J.Y.L., H.G.K., M.W.K. and T.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NRF-2020R1F1A1072124 grant funded by the Korean government and the Korea Environmental Industry and Technology Institute (KEITI) grant funded by the Ministry of Environment of Korea.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Urquhart L. Top companies and drugs by sales in 2020. Nat. Rev. Drug Discov. 2021;19:228–229. doi: 10.1038/d41573-020-00047-7. [DOI] [PubMed] [Google Scholar]
- 2.Urquhart L. Top product forecasts for 2020. Nat. Rev. Drug Discov. 2020;19:86. doi: 10.1038/d41573-020-00011-5. [DOI] [PubMed] [Google Scholar]
- 3.Antibody Society. [(accessed on 25 May 2021)]; Available online: https://www.antibodysociety.org/resources/approved-antibodies/
- 4.Chames P., Van Regenmortel M., Weiss E., Baty D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler M.J., Dimitrov D.S. Therapeutic Antibodies Against Cancer. Hematol. Clin. N. Am. 2012;26:447–481. doi: 10.1016/j.hoc.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang T.H., Jung S.T. Reprogramming the Constant Region of Immunoglobulin G Subclasses for Enhanced Therapeutic Potency against Cancer. Biomolecules. 2020;10:382. doi: 10.3390/biom10030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X., Ng S.M., Hassouna E., Warrington A., Oh S.-H., Rodriguez M. Human-derived natural antibodies: Biomarkers and potential therapeutics. Future Neurol. 2015;10:25–39. doi: 10.2217/fnl.14.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang S., Zhang X., Yang Y., Hotez P.J., Du L. Neutralizing antibodies for the treatment of COVID-19. Nat. Biomed. Eng. 2020;4:1134–1139. doi: 10.1038/s41551-020-00660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan J.R., Wilkie B.N., Winter A.J. Natural and Immune Antibodies for Vibrio fetus in Serum and Secretions of Cattle. Infect. Immun. 1972;5:728–733. doi: 10.1128/iai.5.5.728-733.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche A.M., Richard A.L., Rahkola J., Janoff E.N., Weiser J.N. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2014;8:176–185. doi: 10.1038/mi.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavilio D., Hosmalin A., Scott-Algara D. Natural Killer Cells. Elsevier; Amsterdam, The Netherlands: 2010. Natural killer cells and human immunodeficiency virus; pp. 481–497. [Google Scholar]
- 13.Román V.R.G., Murray J.C., Weiner L.M. Antibody Fc. Elsevier; Amsterdam, The Netherlands: 2014. Antibody-dependent cellular cytotoxicity (ADCC) pp. 1–27. [Google Scholar]
- 14.Jochems C., Hodge J.W., Fantini M., Fujii R., Ii Y.M.M., Greiner J.W., Padget M.R., Tritsch S.R., Tsang K.Y., Campbell K.S., et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–86373. doi: 10.18632/oncotarget.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay M.Z., Wiehe K., Pollara J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019;10:332. doi: 10.3389/fimmu.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamen L., Myneni S., Langsdorf C., Kho E., Ordonia B., Thakurta T., Zheng K., Song A., Chung S. A novel method for determining antibody-dependent cellular phagocytosis. J. Immunol. Methods. 2019;468:55–60. doi: 10.1016/j.jim.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Thornton B.P., Vĕtvicka V., Ross G.D. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J. Immunol. 1994;152:1727–1737. [PubMed] [Google Scholar]
- 18.Terajima M., Cruz J., Co M.D.T., Lee J.-H., Kaur K., Wilson P.C., Ennis F.A. Complement-Dependent Lysis of Influenza A Virus-Infected Cells by Broadly Cross-Reactive Human Monoclonal Antibodies. J. Virol. 2011;85:13463–13467. doi: 10.1128/JVI.05193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Erp E.A., Luytjes W., Ferwerda G., Van Kasteren P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruhns P., Jönsson F. Mouse and human FcR effector functions. Immunol. Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 21.Frank M.M., Lawley T.J., Hamburger M.I., Brown E.J. Immunoglobulin G Fc Receptor-Mediated Clearance in Autoimmune Diseases. Ann. Intern. Med. 1983;98:206–218. doi: 10.7326/0003-4819-98-2-218. [DOI] [PubMed] [Google Scholar]
- 22.Boross P., Arandhara V.L., Martin-Ramirez J., Santiago-Raber M.-L., Carlucci F., Flierman R., Van Der Kaa J., Breukel C., Claassens J.W.C., Camps M., et al. The Inhibiting Fc Receptor for IgG, FcγRIIB, Is a Modifier of Autoimmune Susceptibility. J. Immunol. 2011;187:1304–1313. doi: 10.4049/jimmunol.1101194. [DOI] [PubMed] [Google Scholar]
- 23.Ghumra A., Shi J., McIntosh R.S., Rasmussen I.B., Braathen R., Johansen F.-E., Sandlie I., Mongini P.K., Areschoug T., Lindahl G., et al. Structural requirements for the interaction of human IgM and IgA with the human Fcα/μ receptor. Eur. J. Immunol. 2009;39:1147–1156. doi: 10.1002/eji.200839184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borrok M.J., Wu Y., Beyaz N., Yu X.-Q., Oganesyan V., Dall’Acqua W.F., Tsui P. pH-dependent binding engineering reveals an FcRn affinity threshold that governs IgG recycling. J. Biol. Chem. 2015;290:4282–4290. doi: 10.1074/jbc.M114.603712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaetzel C.S., Robinson J.K., Chintalacharuvu K.R., Vaerman J.P., Lamm M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc. Natl. Acad. Sci. USA. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidarsson G., Dekkers G., Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruhns P., Iannascoli B., England P., Mancardi D.A., Fernandez N., Jorieux S., Daëron M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 28.Bengtén E., Wilson M., Miller N., Clem L.W., Pilström L., Warr G.W. Immunoglobulin Isotypes: Structure, Function, and Genetics. Orig. Evol. Vertebr. Immune Syst. 2000;248:189–219. doi: 10.1007/978-3-642-59674-2_9. [DOI] [PubMed] [Google Scholar]
- 29.Stavnezer J. Immunoglobulin class switching. Curr. Opin. Immunol. 1996;8:199–205. doi: 10.1016/S0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 30.Geha R.S., Rosen F.S. The Genetic Basis of Immunoglobulin-Class Switching. N. Engl. J. Med. 1994;330:1008–1009. doi: 10.1056/NEJM199404073301412. [DOI] [PubMed] [Google Scholar]
- 31.Binstadt B.A., Geha R.S., Bonilla F.A. IgG Fc receptor polymorphisms in human disease: Implications for intravenous immunoglobulin therapy. J. Allergy Clin. Immunol. 2003;111:697–703. doi: 10.1067/mai.2003.1380. [DOI] [PubMed] [Google Scholar]
- 32.Marrack P., Kappler J.W., Kotzin B.L. Autoimmune disease: Why and where it occurs. Nat. Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- 33.Ermann J., Fathman C.G. Autoimmune diseases: Genes, bugs and failed regulation. Nat. Immunol. 2001;2:759–761. doi: 10.1038/ni0901-759. [DOI] [PubMed] [Google Scholar]
- 34.Wallace P.K., Howell A.L., Fanger M.W. Role of Fcγ receptors in cancer and infectious disease. J. Leukoc. Biol. 1994;55:816–826. doi: 10.1002/jlb.55.6.816. [DOI] [PubMed] [Google Scholar]
- 35.Gavin P.G., Song N., Kim S.R., Lipchik C., Johnson N.L., Bandos H., Finnigan M., Rastogi P., Fehrenbacher L., Mamounas E.P. Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2–positive breast cancer: Analysis of the NSABP B-31 trial. JAMA Oncol. 2017;3:335–341. doi: 10.1001/jamaoncol.2016.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kjersem J.B., Skovlund E., Ikdahl T., Guren T., Kersten C., Dalsgaard A.M., Yilmaz M.K., Fokstuen T., Tveit K.M., Kure E.H. FCGR2A and FCGR3A polymorphisms and clinical outcome in metastatic colorectal cancer patients treated with first-line 5-fluorouracil/folinic acid and oxaliplatin+/− cetuximab. BMC Cancer. 2014;14:1–9. doi: 10.1186/1471-2407-14-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith K.G.C., Clatworthy M.R. FcγRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbrugge A., Meyaard L. Signaling by ITIM-Bearing Receptors. Curr. Immunol. Rev. 2005;1:201–212. doi: 10.2174/1573395054065160. [DOI] [Google Scholar]
- 39.Ben Mkaddem S., Benhamou M., Monteiro R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019;10:811. doi: 10.3389/fimmu.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbeek J.S., Hirose S., Nishimura H. The Complex Association of FcγRIIb with Autoimmune Susceptibility. Front. Immunol. 2019;10:2061. doi: 10.3389/fimmu.2019.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willcocks L.C., Carr E.J., Niederer H.A., Rayner T.F., Williams T.N., Yang W., Scott J.A.G., Urban B.C., Peshu N., Vyse T.J. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA. 2010;107:7881–7885. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuyama H., Nimmerjahn F., Ravetch J.V. The inhibitory Fcγ receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 2004;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 43.McGaha T.L., Karlsson M.C., Ravetch J.V. FcγRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J. Immunol. 2008;180:5670–5679. doi: 10.4049/jimmunol.180.8.5670. [DOI] [PubMed] [Google Scholar]
- 44.Pendergraft W.F., Badhwar A.K., Preston G.A. Autoantigen complementarity and its contributions to hallmarks of autoimmune disease. J. Theor. Biol. 2015;375:88–94. doi: 10.1016/j.jtbi.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Bournazos S., Woof J.M., Hart S.P., Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin. Exp. Immunol. 2009;157:244–254. doi: 10.1111/j.1365-2249.2009.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su K., Wu J., Edberg J.C., Li X., Ferguson P., Cooper G.S., Langefeld C.D., Kimberly R.P. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcγRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J. Immunol. 2004;172:7186–7191. doi: 10.4049/jimmunol.172.11.7186. [DOI] [PubMed] [Google Scholar]
- 47.Blank M.C., Stefanescu R.N., Masuda E., Marti F., King P.D., Redecha P.B., Wurzburger R.J., Peterson M.G., Tanaka S., Pricop L. Decreased transcription of the human FCGR2B gene mediated by the -343 G/C promoter polymorphism and association with systemic lupus erythematosus. Qual. Life Res. 2005;117:220–227. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 48.Mackay M., Stanevsky A., Wang T., Aranow C., Li M., Koenig S., Ravetch J.V., Diamond B. Selective dysregulation of the FcγIIB receptor on memory B cells in SLE. J. Exp. Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carreño L.J., Pacheco R., Gutiérrez M.A., Jacobelli S., Kalergis A.M. Disease activity in systemic lupus erythematosus is associated with an altered expression of low-affinity Fcγ receptors and costimulatory molecules on dendritic cells. Immunology. 2009;128:334–341. doi: 10.1111/j.1365-2567.2009.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fong D.C., Malbec O., Arock M., Cambier J.C., Fridman W.H., Daëron M. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated FcγRIIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol. Lett. 1996;54:83–91. doi: 10.1016/S0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 51.Malbec O., Fridman W.H., Daëron M. Negative Regulation of Hematopoietic Cell Activation and Proliferation by FcγRIIB. Immunoreceptor Tyrosine-Based Inhib. Motifs. 1999;244:13–27. doi: 10.1007/978-3-642-58537-1_2. [DOI] [PubMed] [Google Scholar]
- 52.Kepley C.L., Cambier J.C., Morel P.D., Lujan D., Ortega E., Wilson B.S., Oliver J.M. Negative regulation of FcϵRI signaling by FcγRII costimulation in human blood basophils. J. Allergy Clin. Immunol. 2000;106:337–348. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 53.Daëron M., Malbec O., Latour S., Arock M., Fridman W.H. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J. Clin. Investig. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tam S.W., Demissie S., Thomas D., Daëron M. A bispecific antibody against human IgE and human FcγRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59:772–780. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 55.Allen L.C., Kepley C.L., Saxon A., Zhang K. Modifications to an Fcγ-Fcɛ fusion protein alter its effectiveness in the inhibition of FcɛRI-mediated functions. J. Allergy Clin. Immunol. 2007;120:462–468. doi: 10.1016/j.jaci.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Cemerski S., Chu S.Y., Moore G.L., Muchhal U.S., Desjarlais J.R., Szymkowski D.E. Suppression of mast cell degranulation through a dual-targeting tandem IgE–IgG Fc domain biologic engineered to bind with high affinity to FcγRIIb. Immunol. Lett. 2012;143:34–43. doi: 10.1016/j.imlet.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Chu S.Y., Horton H.M., Pong E., Leung I.W., Chen H., Nguyen D.-H., Bautista C., Muchhal U.S., Bernett M.J., Moore G.L., et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcγRIIb with Fc-engineered antibody. J. Allergy Clin. Immunol. 2012;129:1102–1115. doi: 10.1016/j.jaci.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Horton H.M., Chu S.Y., Ortiz E.C., Pong E., Cemerski S., Leung I.W.L., Jacob N., Zalevsky J., DesJarlais J.R., Stohl W., et al. Antibody-Mediated Coengagement of FcγRIIb and B Cell Receptor Complex Suppresses Humoral Immunity in Systemic Lupus Erythematosus. J. Immunol. 2011;186:4223–4233. doi: 10.4049/jimmunol.1003412. [DOI] [PubMed] [Google Scholar]
- 59.Chu S.Y., Yeter K., Kotha R., Pong E., Miranda Y., Phung S., Chen H., Lee S.H., Leung I., Bonzon C. Suppression of Rheumatoid Arthritis B Cells by XmAb5871, an Anti-CD19 Antibody That Coengages B Cell Antigen Receptor Complex and Fcγ Receptor IIb Inhibitory Receptor. Arthritis Rheumatol. 2014;66:1153–1164. doi: 10.1002/art.38334. [DOI] [PubMed] [Google Scholar]
- 60.Ra C., Jouvin M.H., Kinet J.P. Complete structure of the mouse mast cell receptor for IgE (Fc ϵ RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J. Biol. Chem. 1989;264:15323–15327. doi: 10.1016/S0021-9258(19)84829-9. [DOI] [PubMed] [Google Scholar]
- 61.Blank U., Ra C., Miller L., White K., Metzger H., Kinet J.-P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 62.Osterhoff B., Rappersberger K., Wang B., Koszik F., Ochiai K., Kinet J.-P., Stingl G. Immunomorphologic Characterization of Fc∈RI-Bearing Cells Within the Human Dermis. J. Investig. Dermatol. 1994;102:315–320. doi: 10.1111/1523-1747.ep12371789. [DOI] [PubMed] [Google Scholar]
- 63.Maurer D., Fiebiger E., Reininger B., Wolff-Winiski B., Jouvin M.H., Kilgus O., Kinet J.P., Stingl G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J. Exp. Med. 1994;179:745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanada S., Nakano N., Potaczek D.P., Maeda K., Shimokawa N., Niwa Y., Fukai T., Sanak M., Szczeklik A., Yagita H. Two different transcription factors discriminate the −315C> T polymorphism of the FcεRIα gene: Binding of Sp1 to−315C and of a high mobility group-related molecule to −315T. J. Immunol. 2008;180:8204–8210. doi: 10.4049/jimmunol.180.12.8204. [DOI] [PubMed] [Google Scholar]
- 65.Hasegawa M., Nishiyama C., Nishiyama M., Akizawa Y., Mitsuishi K., Ito T., Kawada H., Furukawa S., Ra C., Okumura K. A novel −66T/C polymorphism in FcεRI α-chain promoter affecting the transcription activity: Possible relationship to allergic diseases. J. Immunol. 2003;171:1927–1933. doi: 10.4049/jimmunol.171.4.1927. [DOI] [PubMed] [Google Scholar]
- 66.Bae J.-S., Kim S.-H., Ye Y.-M., Yoon H.J., Suh C.-H., Nahm D.-H., Park H.-S. Significant association of FcɛRIα promoter polymorphisms with aspirin-intolerant chronic urticaria. J. Allergy Clin. Immunol. 2007;119:449–456. doi: 10.1016/j.jaci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Pawankar R. Mast cells as orchestrators of the allergic reaction: The IgE-IgE receptor mast cell network. Curr. Opin. Allergy Clin. Immunol. 2001;1:3–6. doi: 10.1097/01.all.0000010977.11360.f4. [DOI] [PubMed] [Google Scholar]
- 68.Rosenwasser L.J. Mechanisms of IgE Inflammation. Curr. Allergy Asthma Rep. 2011;11:178–183. doi: 10.1007/s11882-011-0179-6. [DOI] [PubMed] [Google Scholar]
- 69.Palikhe N.S., Kim S.-H., Cho B.-Y., Ye Y.-M., Hur G.-Y., Park H.-S. Association of three sets of high-affinity IgE receptor (FcepsilonR1) polymorphisms with aspirin-intolerant asthma. Respir. Med. 2008;102:1132–1139. doi: 10.1016/j.rmed.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 70.Kim S.H., Bae J.S., Holloway J., Lee J.T., Suh C.H., Nahm D.H., Park H.S. A polymorphism of MS4A2 (−109T> C) encoding the β-chain of the high-affinity immunoglobulin E receptor (FcεR1β) is associated with a susceptibility to aspirin-intolerant asthma. Clin. Exp. Allergy. 2006;36:877–883. doi: 10.1111/j.1365-2222.2006.02443.x. [DOI] [PubMed] [Google Scholar]
- 71.Hizawa N., Yamaguchi E., Jinushi E., Kawakami Y. A common FCER1B gene promoter polymorphism* influences total serum IgE levels in a Japanese population. Am. J. Respir. Crit. Care Med. 2000;161:906–909. doi: 10.1164/ajrccm.161.3.9903128. [DOI] [PubMed] [Google Scholar]
- 72.Hill M., Cookson W. A new variant of the β subunit of the high-affinity receptor for immunoglobulin E (FcεRI-β E237G): Associations with measures of atopy and bronchial hyper-responsiveness. Hum. Mol. Genet. 1996;5:959–962. doi: 10.1093/hmg/5.7.959. [DOI] [PubMed] [Google Scholar]
- 73.Xiaozhu Z., Weidong Z., Diwen Q., Andrew S., Cheng T.W. The E237G polymorphism of the high-affinity IgE receptor β chain and asthma. Ann. Allergy Asthma Immunol. 2004;93:499–503. doi: 10.1016/S1081-1206(10)61419-6. [DOI] [PubMed] [Google Scholar]
- 74.Donnadieu E., Jouvin M.-H., Rana S., Moffatt M.F., Mockford E.H., Cookson W.O., Kinet J.-P. Competing functions encoded in the allergy-associated FcϵRIβ gene. Immunity. 2003;18:665–674. doi: 10.1016/S1074-7613(03)00115-8. [DOI] [PubMed] [Google Scholar]
- 75.Shirakawa T., Mao X.-Q., Sasaki S., Enomoto T., Kawai M., Morimoto K., Hopkin J. Association between atopic asthma and a coding variant of FcεRIβ in a Japanese population. Hum. Mol. Genet. 1996;5:1129–1130. doi: 10.1093/hmg/5.8.1129. [DOI] [PubMed] [Google Scholar]
- 76.Hill M.R., James A.L., Faux J.A., Ryan G., Hopkin J.M., Le Souef P., Musk A.W., Cookson W. Fc(epsilon)RI-(beta) polymorphism and risk of atopy in a general population sample. BMJ. 1995;311:776–779. doi: 10.1136/bmj.311.7008.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shirakawa T., Li A., Dubowitz M., Dekker J.W., Shaw A.E., Faux J.A., Ra C., Cookson W.O., Hopkin J.M. Association between atopy and variants of the β subunit of the high–affinity immunoglobulin E receptor. Nat. Genet. 1994;7:125–130. doi: 10.1038/ng0694-125. [DOI] [PubMed] [Google Scholar]
- 78.Hijazi Z., Haider M.Z., Khan M., Ai-Dowaisan A.A. High frequency of IgE receptor FcεRIß variant (Leu181/Leu183) in Kuwaiti Arabs and its association with asthma. Clin. Genet. 2008;53:149–152. doi: 10.1111/j.1399-0004.1998.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 79.Kinet J.-P. THE HIGH-AFFINITY IgE RECEPTOR (FcεRI): From Physiology to Pathology. Annu. Rev. Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 80.Sakurai D., Yamasaki S., Arase K., Park S.Y., Arase H., Konno A., Saito T. FcεRIγ-ITAM is differentially required for mast cell function in vivo. J. Immunol. 2004;172:2374–2381. doi: 10.4049/jimmunol.172.4.2374. [DOI] [PubMed] [Google Scholar]
- 81.Holgate S., Smith N., Massanari M., Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–1736. doi: 10.1111/j.1398-9995.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 82.Cision PR Newswire. [(accessed on 25 May 2021)]; Available online: https://www.prnewswire.com/news-releases/novartis-receives-fda-approval-of-xolair-omalizumab-self-injection-with-prefilled-syringe-across-all-indications-for-appropriate-patients-301266937.html.
- 83.Monteiro R.C., Van De Winkel J.G. IgA Fc receptors. Annu. Rev. Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 84.Maliszewski C.R., March C.J., Schoenborn M.A., Gimpel S., Shen L. Expression cloning of a human Fc receptor for IgA. J. Exp. Med. 1990;172:1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monteiro R.C., Kubagawa H., Cooper M.D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J. Exp. Med. 1990;171:597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsuge T., Shimokawa T., Horikoshi S., Tomino Y., Ra C. Polymorphism in promoter region of Fcα receptor gene in patients with IgA nephropathy. Qual. Life Res. 2001;108:128–133. doi: 10.1007/s004390100458. [DOI] [PubMed] [Google Scholar]
- 87.Zhang C., Zeng X., Li Z., Wang Z., Li S. Immunoglobulin A nephropathy: Current progress and future directions. Transl. Res. 2015;166:134–144. doi: 10.1016/j.trsl.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Xie Y., Chen X., Nishi S., Narita I., Gejyo F. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 2004;65:1135–1144. doi: 10.1111/j.1523-1755.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 89.Xie Y., Nishi S., Ueno M., Imai N., Sakatsume M., Narita I., Suzuki Y., Akazawa K., Shimada H., Arakawa M., et al. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003;63:1861–1867. doi: 10.1046/j.1523-1755.2003.00935.x. [DOI] [PubMed] [Google Scholar]
- 90.Shimokawa T., Tsuge T., Okumura K., Ra C. Identification and characterization of the promoter for the gene encoding the human myeloid IgA Fc receptor (FcαR, CD89) Immunogenetics. 2000;51:945–954. doi: 10.1007/s002510000226. [DOI] [PubMed] [Google Scholar]
- 91.Monteiro R.C., Grossetête B., Nguyen A.T., Jungers P., Lehuen A. IgA Nephropathy. Volume 111. Karger Publishers; Basel, The Switzerland: 1995. Dysfunctions of Fcα Receptors by Blood Phagocytic Cells in IgA Nephropathy1; pp. 116–122. [PubMed] [Google Scholar]
- 92.Kashem A., Endoh M., Yano N., Yamauchi F., Nomoto Y., Sakai H., Kurokawa K. Glomerular FcαR expression and disease activity in IgA nephropathy. Am. J. Kidney Dis. 1997;30:389–396. doi: 10.1016/S0272-6386(97)90284-5. [DOI] [PubMed] [Google Scholar]
- 93.Launay P., Grossetête B., Arcos-Fajardo M., Gaudin E., Torres S.P., Beaudoin L., de Serre N.P.-M., Lehuen A., Monteiro R.C. Fcα Receptor (Cd89) Mediates the Development of Immunoglobulin a (Iga) Nephropathy (Berger’s Disease) Evidence for Pathogenic Soluble Receptor–Iga Complexes in Patients and Cd89 Transgenic Mice. J. Exp. Med. 2000;191:1999–2010. doi: 10.1084/jem.191.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berger J. IgA glomerular deposits in renal disease. Transplant. Proc. 1969;1:939–944. [PubMed] [Google Scholar]
- 95.Galla J.H. IgA nephropathy. Kidney Int. 1995;47:377–387. doi: 10.1038/ki.1995.50. [DOI] [PubMed] [Google Scholar]
- 96.Jasek M., Manczak M., Sawaryn A., Obojski A., Wiśniewski A., Łuszczek W., Kuśnierczyk P. A novel polymorphism in the cytoplasmic region of the human immunoglobulin A Fc receptor gene. Eur. J. Immunogenet. 2004;31:59–62. doi: 10.1111/j.1365-2370.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- 97.Wu J., Ji C., Xie F., Langefeld C.D., Qian K., Gibson A.W., Edberg J.C., Kimberly R.P. FcαRI (CD89) Alleles Determine the Proinflammatory Potential of Serum IgA. J. Immunol. 2007;178:3973–3982. doi: 10.4049/jimmunol.178.6.3973. [DOI] [PubMed] [Google Scholar]
- 98.Kang T.H., Jung S.T. Boosting therapeutic potency of antibodies by taming Fc domain functions. Exp. Mol. Med. 2019;51:1–9. doi: 10.1038/s12276-019-0345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee C.-H., Kang T.H., Godon O., Watanabe M., Delidakis G., Gillis C.M., Sterlin D., Hardy D., Cogné M., Macdonald L.E. An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-13108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu R., Oldham R.J., Teal E., Beers S.A., Cragg M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies. 2020;9:64. doi: 10.3390/antib9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grevys A., Bern M., Foss S., Bratlie D.B., Moen A., Gunnarsen K.S., Aase A., Michaelsen T.E., Sandlie I., Andersen J.T. Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions. J. Immunol. 2015;194:5497–5508. doi: 10.4049/jimmunol.1401218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kellner C., Otte A., Cappuzzello E., Klausz K., Peipp M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemother. Ther. 2017;44:327–336. doi: 10.1159/000479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Mathieu M., Brezski R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2017;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sondermann P., Szymkowski D.E. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr. Opin. Immunol. 2016;40:78–87. doi: 10.1016/j.coi.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Kubota T., Niwa R., Satoh M., Akinaga S., Shitara K., Hanai N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009;100:1566–1572. doi: 10.1111/j.1349-7006.2009.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DiLillo D.J., Ravetch J.V. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell. 2015;161:1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bournazos S., Corti D., Virgin H.W., Ravetch J.V. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature. 2020;588:485–490. doi: 10.1038/s41586-020-2838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woof J.M., Burton D.R. Human antibody–Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 2004;4:89–99. doi: 10.1038/nri1266. [DOI] [PubMed] [Google Scholar]
- 109.Maxwell K.F., Powell M.S., Hulett M.D., Barton P.A., McKenzie I.F., Garrett T.P., Hogarth P.M. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat. Genet. 1999;6:437–442. doi: 10.1038/8241. [DOI] [PubMed] [Google Scholar]
- 110.Powell M.S., Barnes N.C., Bradford T.M., Musgrave I.F., Wines B.D., Cambier J.C., Hogarth M. Alteration of the FcγRIIa Dimer Interface Affects Receptor Signaling but Not Ligand Binding. J. Immunol. 2006;176:7489–7494. doi: 10.4049/jimmunol.176.12.7489. [DOI] [PubMed] [Google Scholar]
- 111.Ramsland P.A., Farrugia W., Bradford T.M., Sardjono C.T., Esparon S., Trist H.M., Powell M.S., Tan P.S., Cendron A.C., Wines B.D., et al. Structural Basis for FcγRIIa Recognition of Human IgG and Formation of Inflammatory Signaling Complexes. J. Immunol. 2011;187:3208–3217. doi: 10.4049/jimmunol.1101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Domingo P., Muñiz-Diaz E., Baraldès M.A., Arilla M., Barquet N., Pericas R., Juarez C., Madoz P., Vázquez G. Associations between Fc gamma receptor IIA polymorphisms and the risk and prognosis of meningococcal disease. Am. J. Med. 2002;112:19–25. doi: 10.1016/S0002-9343(01)01047-6. [DOI] [PubMed] [Google Scholar]
- 113.Bazilio A.P., Viana V.S., Toledo R., Woronik V., Bonfa E., Monteiro R.C. FcγRIIa polymorphism: A susceptibility factor for immune complex-mediated lupus nephritis in Brazilian patients. Nephrol. Dial. Transplant. 2004;19:1427–1431. doi: 10.1093/ndt/gfh121. [DOI] [PubMed] [Google Scholar]
- 114.Clatworthy M.R. Antibody Fc. Elsevier; Amsterdam, The Netherlands: 2014. Fcγ Receptor Polymorphisms and Susceptibility to Infection; pp. 217–237. [Google Scholar]
- 115.Yuan F.F., Sullivan J.S. FcγRIIA polymorphism as a risk factor for invasive Streptococcus pneumoniae. Clin. Appl. Immunol. Rev. 2005;5:397–403. doi: 10.1016/j.cair.2005.11.001. [DOI] [Google Scholar]
- 116.Ravetch J.V., Bolland S. Igg fc receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 117.Gillis C., Gouel-Chéron A., Jönsson F., Bruhns P. Contribution of human FcγRs to disease with evidence from human polymorphisms and transgenic animal studies. Front. Immunol. 2014;5:254. doi: 10.3389/fimmu.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Taeye S.W., Bentlage A.E.H., Mebius M.M., Meesters J.I., Lissenberg-Thunnissen S., Falck D., Sénard T., Salehi N., Wuhrer M., Schuurman J., et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front. Immunol. 2020;11:740. doi: 10.3389/fimmu.2020.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barb A.W. Fc γ receptor compositional heterogeneity: Considerations for immunotherapy development. J. Biol. Chem. 2021;296:100057. doi: 10.1074/jbc.REV120.013168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shields R.L., Namenuk A.K., Hong K., Meng Y.G., Rae J., Briggs J., Xie D., Lai J., Stadlen A., Li B. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J. Biol. Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 121.Kim N.H., Jung H.D., Kim J.G., Lee J.-J., Yang D.-H., Park Y.H., Do Y.R., Shin H.J., Kim M.K., Hyun M.S., et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108:2720–2725. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 122.Boyerinas B., Jochems C., Fantini M., Heery C.R., Gulley J.L., Tsang K.Y., Schlom J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calemma R., Ottaiano A., Trotta A.M., Nasti G., Romano C., Napolitano M., Galati D., Borrelli P., Zanotta S., Cassata A., et al. Fc gamma receptor IIIa polymorphisms in advanced colorectal cancer patients correlated with response to anti-EGFR antibodies and clinical outcome. J. Transl. Med. 2012;10:232. doi: 10.1186/1479-5876-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahaweni N.M., Olieslagers T.I., Rivas I.O., Molenbroeck S.J.J., Groeneweg M., Bos G.M.J., Tilanus M.G.J., Voorter C.E.M., Wieten L. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-34258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Day S., Ramamurthy C., Bullock A.J., El-Khoueiry A.B., Ohanjanian L., Wijatyk A., Feliu W.I.O., Shapiro I., Ancukiewicz M., Chand D., et al. AGEN1181, a clinical stage Fc-engineered anti-CTLA-4 antibody with improved therapeutic potential for the treatment of patients with advanced malignancies. J. Clin. Oncol. 2020;38:TPS3157. doi: 10.1200/JCO.2020.38.15_suppl.TPS3157. [DOI] [Google Scholar]
- 126.Patel K.R., Roberts J.T., Barb A.W. Multiple Variables at the Leukocyte Cell Surface Impact Fc γ Receptor-Dependent Mechanisms. Front. Immunol. 2019;10:223. doi: 10.3389/fimmu.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Der Pol W.-L., Van De Winkel J.G.J. IgG receptor polymorphisms: Risk factors for disease. Immunogenetics. 1998;48:222–232. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 128.Bux J., Stein E.-L., Bierling P., Fromont P., Clay M., Stroncek D., Santoso S. Characterization of a New Alloantigen (SH) on the Human Neutrophil Fcγreceptor IIIb. Blood. 1997;89:1027–1034. doi: 10.1182/blood.V89.3.1027. [DOI] [PubMed] [Google Scholar]
- 129.Ory P.A., Clark M.R., Kwoh E.E., Clarkson S.B., Goldstein I.M. Sequences of complementary DNAs that encode the NA1 and NA2 forms of Fc receptor III on human neutrophils. J. Clin. Investig. 1989;84:1688–1691. doi: 10.1172/JCI114350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salmon J.E., Millard S.S., Brogle N.L., Kimberly R.P. Fc gamma receptor IIIb enhances Fc gamma receptor IIa function in an oxidant-dependent and allele-sensitive manner. J. Clin. Investig. 1995;95:2877–2885. doi: 10.1172/JCI117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Foster C.B., Lehrnbecher T., Mol F., Steinberg S.M., Venzon D.J., Walsh T.J., Noack D., Rae J., Winkelstein J.A., Curnutte J.T., et al. Host defense molecule polymorphisms influence the risk for immune-mediated complications in chronic granulomatous disease. J. Clin. Investig. 1998;102:2146–2155. doi: 10.1172/JCI5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nasr A., Aljada A., Hamid O., Elsheikh H.A., Masuadi E., Al-Bawab A., Alenazi T.H., Abushouk A., Salah A.M. Significant Differences in FcγRIIa, FcγRIIa and FcγRIIIb Genes Polymorphism and Anti-Malarial IgG Subclass Pattern Are Associated with Severe Malaria in Saudi Children. Research Square; Durham, NC, USA: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Golay J.T., Da Roit F., Bologna L., Ferrara C., Leusen J.H., Rambaldi A., Klein C., Introna M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122:3482–3491. doi: 10.1182/blood-2013-05-504043. [DOI] [PubMed] [Google Scholar]
- 134.Sachs U.J.H., Socher I., Braeunlich C.G., Kroll H., Bein G., Santoso S. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor α-chain promoter. Immunology. 2006;119:83–89. doi: 10.1111/j.1365-2567.2006.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sabol S.Z., Hu S., Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Qual. Life Res. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 136.Passot C., Azzopardi N., Renault S., Baroukh N., Arnoult C., Ohresser M., Boisdron-Celle M., Gamelin E., Watier H., Paintaud G. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. mAbs. 2013;5:614–619. doi: 10.4161/mabs.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gouilleux-Gruart V., Chapel H., Chevret S., Lucas M., Malphettes M., Fieschi C., Patel S., Boutboul D., Marson M.-N., Gérard L., et al. Efficiency of immunoglobulin G replacement therapy in common variable immunodeficiency: Correlations with clinical phenotype and polymorphism of the neonatal Fc receptor. Clin. Exp. Immunol. 2012;171:186–194. doi: 10.1111/cei.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zalevsky J., Chamberlain A.K., Horton H.M., Karki S., Leung I.W.L., Sproule T.J., Lazar G.A., Roopenian D.C., Desjarlais J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li X., Kimberly R.P. Targeting the Fc receptor in autoimmune disease. Expert Opin. Ther. Targets. 2014;18:335–350. doi: 10.1517/14728222.2014.877891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kiessling P., Lledo-Garcia R., Watanabe S., Langdon G., Tran D., Bari M., Christodoulou L., Jones E., Price G., Smith B., et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci. Transl. Med. 2017;9:eaan1208. doi: 10.1126/scitranslmed.aan1208. [DOI] [PubMed] [Google Scholar]
- 141.Bril V., Benatar M., Andersen H., Vissing J., Brock M., Greve B., Kiessling P., Woltering F., Griffin L., Van den Bergh P. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: A phase 2 randomized control trial. Neurology. 2021;96:e853–e865. doi: 10.1212/WNL.0000000000011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yap D.Y., Hai J., Lee P.C., Zhou X., Lee M., Zhang Y., Wang M., Chen X. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a Phase 1 Study for Healthy Chinese Volunteers. Clin. Transl. Sci. 2021;1:1–11. doi: 10.1111/cts.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mellor J.D., Brown M.P., Irving H.R., Zalcberg J.R., Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J. Hematol. Oncol. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaifu T., Nakamura A. Polymorphisms of immunoglobulin receptors and the effects on clinical outcome in cancer immunotherapy and other immune diseases: A general review. Int. Immunol. 2017;29:319–325. doi: 10.1093/intimm/dxx041. [DOI] [PubMed] [Google Scholar]
- 145.Van Sorge N., Van Der Pol W.L., Van de Winkel J. FcγR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens. 2003;61:189–202. doi: 10.1034/j.1399-0039.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 146.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2017;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gyawali B., Kesselheim A.S. FDA approval standards for anticancer agents—Lessons from two recent approvals in breast cancer. Nat. Rev. Clin. Oncol. 2021;18:397–398. doi: 10.1038/s41571-021-00504-1. [DOI] [PubMed] [Google Scholar]
- 148.Markham A. Margetuximab: First Approval. Drugs. 2021;81:599–604. doi: 10.1007/s40265-021-01485-2. [DOI] [PubMed] [Google Scholar]