Abstract
Background
Cutaneous reactions after COVID-19 vaccination have been commonly reported; however, histopathologic features and clinical correlations have not been well characterized.
Methods
We evaluated for a history of skin biopsy all reports of reactions associated with COVID-19 vaccination identified in an international registry. When histopathology reports were available, we categorized them by reaction patterns.
Results
Of 803 vaccine reactions reported, 58 (7%) cases had biopsy reports available for review. The most common histopathologic reaction pattern was spongiotic dermatitis, which clinically ranged from robust papules with overlying crust, to pityriasis rosea-like eruptions, to pink papules with fine scale. We propose the acronym “V-REPP” (vaccine-related eruption of papules and plaques) for this spectrum. Other clinical patterns included bullous pemphigoid-like (n = 12), dermal hypersensitivity (n = 4), herpes zoster (n = 4), lichen planus-like (n = 4), pernio (n = 3), urticarial (n = 2), neutrophilic dermatosis (n = 2), leukocytoclastic vasculitis (n = 2), morbilliform (n = 2), delayed large local reactions (n = 2), erythromelalgia (n = 1), and other (n = 5).
Limitations
Cases in which histopathology was available represented a minority of registry entries. Analysis of registry data cannot measure incidence.
Conclusion
Clinical and histopathologic correlation allowed for categorization of cutaneous reactions to the COVID-19 vaccine. We propose defining a subset of vaccine-related eruption of papules and plaques, as well as 12 other patterns, following COVID-19 vaccination.
Key words: Ad26.COV2.S, AZD1222, BNT162b2, bullous pemphigoid, chilblains, COVID-19, delayed large local, dermal hypersensitivity reaction, dermatology, dermatopathology, erythema multiforme, erythromelalgia, Johnson & Johnson Janssen, lichen planus, Moderna, morbilliform, mRNA-1273, Oxford-AstraZeneca, papular, papulosquamous, pathology, pernio, Pfizer-BioNTech, pityriasis rosea, psoriasis, registry, SARS-CoV-2, Stevens-Johnson syndrome, urticaria, vaccine, zoster
Capsule Summary.
-
•
In this registry-based study, we observed diverse COVID-19 vaccine-associated cutaneous reactions, including papulovesicular, pityriasis rosea-like, and papulosquamous eruptions classified as vaccine-related eruption of papules and plaques.
-
•
This detailed study using the clinical and histopathologic correlation of 13 reaction patterns may aid with the diagnosis of cutaneous side effects from the COVID-19 vaccine.
Introduction
As of June 2021, a total of 1.84 billion doses of COVID-19 vaccines have been administered globally.1 The Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2) vaccines, which use a novel mRNA technology, have been reported to cause various dermatologic side effects, such as delayed large local reactions, local injection site reactions, urticaria, morbilliform reactions, erythromelalgia, zoster, pernio, and cosmetic filler reactions.2, 3, 4 The Johnson and Johnson (Ad26.COV2.S) vaccine, which uses a nonreplicating viral vector, appears to have relatively fewer dermatologic side effects, with the clinical trial reporting only local injection site reactions.5 The Oxford-AstraZeneca (AZD1222) trial reported local injection site reactions and 1 case each of psoriasis, rosacea, vitiligo, and Raynaud's syndrome, although real-world studies are lacking.4 , 6
Although clinicopathologic correlation is key to understanding the pathophysiology, to our knowledge there have been no systematic studies examining the clinicopathologic correlations between the cutaneous reactions associated with COVID-19 vaccine across a broad spectrum of reaction patterns and their accompanying histopathology. Hence, the purpose of this study was to improve the characterization of dermatologic reactions to COVID-19 vaccination through an analysis of biopsy reports and corresponding clinical photographs from cases entered into an international COVID-19 dermatology registry. Given that a growing percentage of the world's population is being vaccinated, such data may aid with the diagnosis of cutaneous side effects of COVID-19 vaccination.
Methods
In December 2020, our international COVID-19 dermatology registry, established in collaboration with the American Academy of Dermatology and the International League of Dermatological Societies, began collecting reports of patients with cutaneous reactions to COVID-19 vaccination (www.aad.org/covidregistry).2 , 7 Entry of de-identified patient cases was restricted to only health care workers. The Massachusetts General Brigham Institutional Review Board exempted this study as not human subject research.
The registry collected data regarding COVID-19 vaccination and characteristics of the cutaneous reactions.2 As in prior work, we defined a wheal on the vaccinated arm as a local injection site reaction if it occurred within 3 days of the first dose of vaccination and a delayed large local reaction if it occurred more than 4 days after vaccination.2 Additionally, the registry queried whether a skin biopsy report was available and asked for full details of any biopsy reports. Physicians and other health care providers who entered pending or incomplete biopsy reports were contacted for updates and additional clarifying information. For records where full biopsy reports were available, health care providers were then contacted to request de-identified patient photos.
Biopsy reports, and clinical photographs when available, were reviewed by 4 board-certified dermatologists (Drs Kovarik, Damsky, Fox, and Freeman), 2 of whom are dermatopathologists (Drs Kovarik and Damsky); these were organized by group reaction patterns and histopathologic findings into harmonized clinical and histopathologic entities. We used Stata (version 16, StataCorp LLC) to analyze data.
Results
From December 24, 2020 to May 19, 2021, health care providers entered 803 cases of COVID-19 vaccine-related cutaneous reactions into the American Academy of Dermatology or International League of Dermatological Societies registry. A portion of these cases (n = 414) have been previously reported without pathology.2 Of the 803 cases, vaccine manufacturers overall were Moderna (69%), Pfizer (25%), Johnson and Johnson (1.0%), Oxford-AstraZeneca (0.6%), and unspecified (4.4%). The most commonly reported morphologies were local injection site reactions, delayed large local reactions, urticaria, morbilliform, zoster, and papulosquamous eruptions (Supplemental Table I; available via Mendeley at https://data.mendeley.com/datasets/cyxcbmc5zc/1.) Cases were reported by dermatologists (46%), other physicians (22%), midlevel providers (9.2%), nurses (9.1%), and other health care providers (13%).
Of the 803 cases, 78 providers (9.7%) indicated that a skin biopsy was performed. Records listed as pending (n = 15) or incomplete (n = 5) were not included, leaving 58 (7%) complete biopsy reports for review (Table I ). The median age of these 58 patients was 61 years (interquartile range [IQR], 44-77); 62% were women, 75% were White, and 95% were from the United States. The majority of cases for whom skin biopsy reports were available were reported by dermatologists (94%). Vaccine manufacturers were Moderna (46%), Pfizer (42%), Johnson and Johnson (1.7%), Oxford/AstraZeneca (1.7%), and unspecified (8.6%).
Table I.
Categorization of clinical and histopathologic features of COVID-19 vaccine cutaneous reactions∗
| Clinical reaction pattern | Age (range), y | Vaccine brand (%) | Distribution | Morphology (based on clinical photograph review) | Histopathology | |
|---|---|---|---|---|---|---|
| V-REPP (n = 15) | ||||||
| Robust | Papulovesicular (n = 3) | 29-81 | Moderna (33%), Pfizer (67%) | Trunk, extremities > neck, face, head | Discrete edematous papules, some with central vesiculation and crusting | Spongiotic dermatitis as robust intercellular edema with intraepidermal vesicles, papillary dermal edema, and dermal eosinophils; interface changes may or may not be present |
| Moderate | Pityriasis rosea-like (n = 8) | 41-82 | Moderna (38%), Pfizer (50%), Oxford-AstraZeneca (12%) | Trunk, extremities > face | Oval, pityriasis rosea-like pink edematous papules and plaques, some with central crust and some with trailing scale | Spongiotic dermatitis >> interface changes and dermal eosinophils are often present |
| Mild | Papulosquamous with subtle scale (n = 4) | 31-71 | Moderna (50%), Pfizer (25%), Unspecified (25%) | Trunk, extremities | Oval or annular pink thin papules coalescing into plaques, with mild surface changes and subtle scale | Spongiosis as mild intercellular edema and vacuolar interface changes are present and may be focal; eosinophils may or may not be present |
| Bullous pemphigoid-like (n = 12) | ||||||
| 42-97 | Moderna (36%), Pfizer (64%) | Trunk, extremities > face, head, neck, oral mucosa, genital mucosa | Tense unilocular clear fluid-filled bullae on an erythematous base | Subepidermal blister formation and mixed inflammation with eosinophils
|
||
| Dermal hypersensitivity reaction (n = 4) | ||||||
| 34-83 | Moderna (25%), Pfizer (25%), Unspecified (25%) | Trunk, extremities > face, neck | Pink edematous papules coalescing into plaques without surface change; individual lesions last >24 h | Perivascular infiltrate with mixed inflammation, which may include lymphocytes, neutrophils, and eosinophils | ||
| Herpes zoster (n = 4) | ||||||
| 39-78 | Moderna (50%), Pfizer (12%), Johnson and Johnson (25%) | Trunk, extremities, face | Grouped vesicles on an erythematous base not crossing the midline | All with viral cytopathic changes present. Involvement of the hair follicle in 2 of 4 cases | ||
| Lichen planus-like (n = 4) | ||||||
| 31-72 | Moderna (25%), Pfizer (75%) | Trunk, extremities | No clinical images | Lichenoid interface dermatitis; dermal eosinophils may be present | ||
| Pernio (n = 3) | ||||||
| 22-60 | Moderna (33%), Pfizer (67%) | Fingers, toes | Pink to violaceous papules of the toes? fingers too? Maybe just say toes > fingers or digits? | Perivascular lymphocytic infiltrate with papillary dermal edema and interface changes | ||
| Urticaria (n = 2) | ||||||
| 47-68 | Moderna (50%), Pfizer (50%) | Trunk, extremities, face | Erythematous, well-circumscribed papules and plaques without surface change lasting < 24 h | Dermal edema with sparse perivascular lymphocytes, neutrophils and eosinophils | ||
| Neutrophilic dermatosis (n = 2) | ||||||
| 68-93 | Moderna (50%), Pfizer (50%) | Trunk, extremities, face | Bright red to violaceous dermal papules and plaques | Dense dermal neutrophilic infiltrate with papillary dermal edema; leukocytoclasis and secondary vasculitic changes may be present | ||
| Leukocytoclastic vasculitis (n = 2) | ||||||
| 57-61 | Moderna (50%), Pfizer (50%) | Lower extremities | Deep red to maroon palpable purpura | Epidermal infiltrate of neutrophils and extravasated erythrocytes with perivascular neutrophils and leukocytoclasis | ||
| Morbilliform (n = 2) | ||||||
| 50-85 | Moderna (100%) | Trunk, extremities | No clinical images | Perivascular mixed infiltrate; interface changes may be present | ||
| Delayed large local reactions (n = 2) | ||||||
| 27-35 | Moderna (100%) | Vaccinated arm | Indurated, erythematous plaque | Superficial perivascular and perifollicular lymphocytic infiltrate with rare eosinophils and scattered mast cells | ||
| Erythromelalgia (n = 1) | ||||||
| 27 | Moderna (100%) | Hands, feet | Erythematous, edematous hands and feet (with burning sensation) | Superficial and deep perivascular inflammation and edema | ||
| Other (n = 5) | ||||||
| Stevens-Johnson Syndrome | 46 | Moderna (100%) | All skin surfaces, oral and genital mucosa | Atypical targetoid papules with duskiness, bullae, and epidermal necrosis in the center; hemorrhagic crusting on the vermillion lips; lesions involved palms and soles | Full-thickness epidermal necrosis | |
| Erythema multiforme | 42 | Moderna (100%) | Arms, hands | Erythematous, targetoid papules and plaques | Spongiotic and vacuolar interface dermatitis | |
| Granuloma annulare | 85 | Pfizer (100%) | Trunk | No clinical images | Interstitial granulomatous reaction | |
| Tattoo sarcoidal reaction | 38 | Moderna (100%) | Leg | No clinical images | Tattoo with suppurativa granulomatous inflammation | |
| New onset psoriasis | 67 | Moderna (100%) | Trunk, extremities, head, neck, face | Well demarcated erythematous papules and plaques with overlying silvery scale | Epidermal acanthosis, confluent parakeratosis with trapped clusters of neutrophils, and focal spongiform pustule formation. Diminished thickness of granular layer | |
BMZ, Basement membrane zone; BP, blood pressure; DIF, direct immunofluorescence; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; V-Repp, vaccine-related eruptions of papules and plaques.
These data from 58 biopsy reports represent a subset of the overall 803 cases in the registry, where biopsy was performed and the report was available for review. For clinical photos of these reactions see Supplemental Fig 1.
For patients receiving vaccines requiring 2 doses (ie, primarily Moderna/Pfizer), 55% of biopsy reports were taken following the first dose. Of note, 8 patients biopsied after the first dose were not planning to receive the second dose, given the severity of the cutaneous reaction. These included 2 cases of leukocytoclastic vasculitis, 2 cases of papulosquamous eruptions, 1 case of urticaria, 1 case of dermal hypersensitivity reaction, 1 case of bullous pemphigoid, and 1 case of Stevens-Johnson syndrome.
Clinicopathologic correlation revealed 13 different COVID-19 vaccine reaction patterns where biopsy reports were evaluable: vaccine-related eruption of papules and plaques (V-REPP) (n = 15), bullous pemphigoid-like (n = 12), dermal hypersensitivity reactions (n = 4), herpes zoster (n = 4), lichen planus-like (n = 4), pernio (n = 3), urticaria (n = 2), neutrophilic dermatosis (n = 2), leukocytoclastic vasculitis (n = 2), morbilliform (n = 2), delayed large local reactions (n = 2), erythromelalgia (n = 1), and other (n = 5), including Stevens-Johnson syndrome (n = 1) and erythema multiforme (n = 1). Clinical photographs correlated with histopathology are shown in Supplemental Fig 1 (available via Mendeley at https://data.mendeley.com/datasets/cyxcbmc5zc/1.)
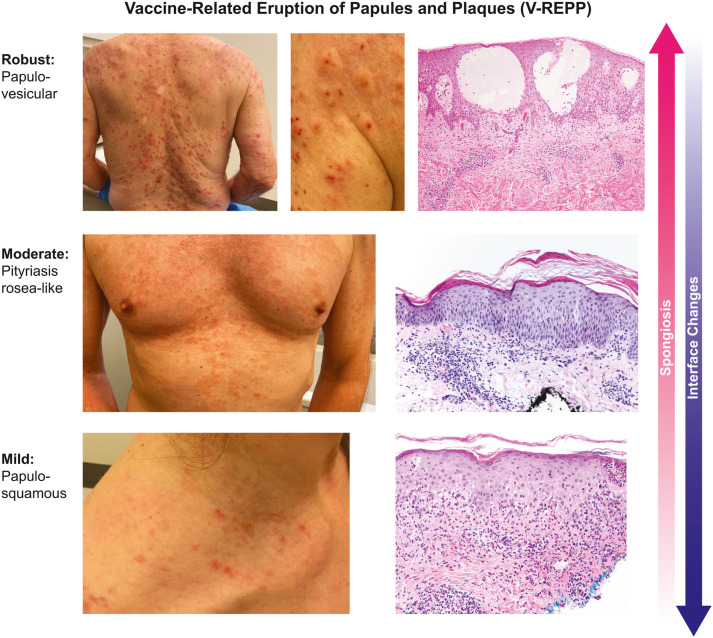
The histologic reaction pattern most commonly biopsied was a spectrum of spongiotic dermatitis after Moderna (40%), Pfizer (47%), Oxford-AstraZeneca (6.7%), and unspecified (6.7%) vaccines. These vaccine-related eruptions of papules and plaques, which we call V-REPP (Fig 1 ), clinically had papules and/or plaques with surface changes. They ranged on a clinical spectrum from edematous and crusted papules (robust), to edematous and erythematous scaly papules and plaques resembling pityriasis rosea-like changes (moderate), to subtle scaly papules and plaques (mild). The findings were clinically diverse, but had similar histopathology, which existed on a spectrum related to the degree of spongiosis present on the biopsy compared to the degree of interface changes. Robust V-REPP on biopsy showed marked spongiosis with intraepidermal vesicles and minimal to no interface changes (biopsy reports, n = 3). Moderate V-REPP showed moderate spongiosis more often than interface changes (n = 8). Mild V-REPP demonstrated mild spongiosis and more-prominent interface changes (n = 4). Eosinophils were commonly present in the cases with marked spongiosis and were less likely to be present in the cases with minimal spongiosis.
Fig 1.
Spectrum of V-REPP following COVID-19 vaccination by degree of spongiosis and interface changes present on histopathology. V-REPP, Vaccine-related eruption of papules and plaques.
The median time to V-REPP was 12 (IQR, 4-16) days after COVID-19 vaccination. Robust V-REPP occurred at a median of 5.5 (IQR, 4-7) days after vaccination and lasted up to 49 days at the time of reporting. However, because 100% of these eruptions were ongoing at the time of reporting, the natural history of the cutaneous reaction has yet to be determined. Moderate V-REPP occurred a median of 13 (IQR, 4-19) days after vaccination and lasted up to 90 days, with 88% ongoing at the time of reporting. For several of the cases that were pityriasis rosea-like, V-REPP started after the first dose of the mRNA vaccine and then flared with the second dose. Mild V-REPP occurred a median of 16 (IQR, 14-18) days after vaccination and lasted up to 18 days, with 50% ongoing at the time of reporting.
Discussion
In this registry-based study, we grouped 58 biopsy reports and clinical photographs of COVID-19 vaccine reactions into 13 patterns, with the most common categories including V-REPP, bullous pemphigoid-like, dermal hypersensitivity reactions, herpes zoster, lichen planus-like, and pernio. The relative frequency of these biopsy-proven categories differs from the overall 803 dermatologic vaccine reactions in the registry, possibly because providers were less likely to biopsy common and well-described vaccine side effects, such as local injection site reactions, delayed large local reactions, morbilliform eruptions, and urticaria. For example, for delayed large local reactions occurring 4 days or more after vaccination, there were 301 total reports in the registry but just 2 were biopsied.
The most commonly biopsied reactions in the registry were what we describe here as V-REPP (Fig 1). The histopathologic spectrum of V-REPP all showed some degree of spongiosis, ranging from significant spongiosis with intraepidermal vesicle formation (robust V-REPP), to pityriasis rosea-like changes (moderate V-REPP), to minimal spongiosis (mild V-REPP). One previously reported case of pityriasis rosea-like eruption following a second dose of the Pfizer vaccine similarly showed spongiosis with interface changes.8 Pityriasis rosea-like eruptions have previously been described after vaccination for smallpox, tuberculosis, polio, influenza, papillomaviruses, diphtheria, tetanus, hepatitis B, pneumococcus, and yellow fever.9 Unlike classic pityriasis rosea, pityriasis rosea-like reactions following vaccination or medications may lack herald patches and may feature a more diffuse papulosquamous exanthem, similar to what we observed in the registry.9 Although the more robust papulovesicular spectrum of V-REPP can clinically mimic an id reaction, there are several key distinctions. An id reaction or autoeczematization, is generally a dermatitis distant to an initial site of inflammation or infection; it is not usually seen after vaccination, and would not typically have the same spectrum of clinical and pathologic changes.10
The mechanism of V-REPP after COVID-19 vaccination is unknown, but the delayed occurrence of these reactions suggests 2 potential mechanisms: (1) delayed hypersensitivity response to vaccination; or (2) T-cell-mediated skin reaction due to molecular mimicry with a viral epitope. In fact, infection with SARS-CoV-2 itself has been associated with pityriasis rosea-like eruptions.11 Histopathology of pityriasis rosea-like eruptions following SARS-CoV-2 infection have similarly been reported to demonstrate spongiosis and a superficial perivascular lymphocytic infiltrate.12 However, robust V-REPP, in which papulovesicles may be observed due to exuberant spongiosis, appear to be different from other vesicular eruptions caused by true SARS-CoV-2 infection. Vesicular eruptions by SARS-CoV-2 infection show vacuolar degeneration on biopsy and have been proposed to relate to direct cytotoxic effects of the virus.13 , 14 Lesional biopsy demonstrated interface changes, with parakeratosis and scattered dyskeratotic keratinocytes.
Histopathologic features of the more-common reaction patterns to Moderna and Pfizer vaccines have been previously described in the literature. Similar to our findings, histopathology of delayed large local reactions showed perivascular lymphocytic infiltrates with eosinophils and mast cells, consistent with a delayed T-cell-mediated hypersensitivity reaction.15, 16, 17 Morbilliform eruptions after vaccination similarly demonstrated perivascular lymphocytic inflammation.18 Additionally, some cutaneous vaccine reactions, such as pernio/chilblains, had similar morphology and histopathology to pernio described in association with SARS-CoV-2 infection.11 , 19, 20, 21
Biopsy reports of additional dermatologic morphologies were also reported in the registry, such as lichen planus, neutrophilic dermatoses, and psoriasis (Supplemental Fig 1). COVID-19 vaccines can elicit strong T- and B-cell responses against SARS-CoV-2. Their role and the mechanism by which they might elicit off-target immune-stimulatory effects, including provoking T-cell dependent disorders, requires further study.22 , 23 We also observed other immune-mediated dermatologic disorders, such as bullous pemphigoid and leukocytoclastic vasculitis, potentially driven by off-target immune activation following COVID-19 vaccination.24 These shifts in the immune response after vaccination may also be associated with reactivation of other viruses; eg, 4 cases of confirmed herpes zoster with viral cytopathic changes observed in the registry.25 , 26
Although these cutaneous reactions may lead to hesitation in receiving future vaccine doses, it is important for patients and providers alike to recognize that in the cases of 2-dose vaccines, most eruptions, across a broad range of different reaction patterns, did not lead to anaphylaxis or severe adverse events with the second dose. It is important to distinguish cutaneous reactions that can be managed after a second dose (the majority of cases) versus the rare reactions that represent absolute contraindications.2 , 27 We did receive 1 report of biopsy confirmed Stevens-Johnson syndrome following vaccination, which represents an absolute contraindication to second-dose vaccination.28
Our observational registry-based study has multiple limitations. Our overall registry case numbers may not be representative of the true incidence or prevalence of vaccine-associated cutaneous reactions, as providers may be more likely to submit more-severe or uncommon cases to the registry. Additionally, biopsy reports may be less representative of cutaneous vaccine reactions overall, given provider predilection to reserve biopsies for unusual and/or previously undescribed conditions, rather than taking biopsies of more-common or easily recognized conditions. This study is also limited in generalizability, because patients in the registry were predominantly from the United States where vaccine roll out has been greatest for mRNA-based vaccines. Additionally, this study relied on the text entered from biopsy reports and clinical photographs, which may oversimplify the interpretation of histopathologic and in-person evaluations. Another limitation is that the V-REPP classification was not part of the original registry entry choices, because this classification was developed during data analysis. These cases were reclassified after review of photographs and pathology reports by the authors of the study.
In conclusion, this case series demonstrated the clinical and histopathologic characteristics of multiple dermatologic conditions after COVID-19 vaccination. We hope these data will aid physicians and other providers in the diagnosis of dermatologic conditions associated with the COVID-19 vaccine, which will likely be encountered more frequently as vaccine distribution expands globally.
Conflicts of interest
Drs Freeman, Hruza, Rosenbach, Lipoff, Fox, and Thiers are members of the American Academy of Dermatology COVID-19 Ad Hoc Task Force. Dr French is the President and Dr Lim a board member of the International League of Dermatological Societies. Dr Thiers is the Immediate Past President of the American Academy of Dermatology. Dr Freeman is an author of COVID-19 dermatology for UpToDate. Drs McMahon, Kovarik, Damsky, Nazarian, Desai, and Blumenthall and Authors Tyagi, Chamberlin, and Fathy have no conflicts to declare.
Acknowledgments
We acknowledge the following individuals for providing clinical and histology photographs and/or clinical discussion of cases: Jayne Bird, Shannon Foster, Elizabeth Goodwin, Tia Janicki, Anu Jayaraman, Richard Johnson, Eva Kerby, Cheryl Levin, Josette McMichael, Alyssa Miceli, Pamela Scheinman, Courtney Struthers, Mark Swick, and Artur Zembowicz. We thank all the health care providers worldwide who entered cases in this registry.
Footnotes
Funding sources: The COVID-19 dermatology registry is supported by a grant from the International League of Dermatological Societies and by in-kind support from the American Academy of Dermatology.
IRB approval status: The registry was reviewed by the Partners Healthcare (Massachusetts General Hospital) Institutional Review Board and it was determined that it did not meet the definition of human subjects research.
Reprints not available from the authors.
References
- 1.Coronavirus (COVID-19) vaccinations Our World in Data. https://ourworldindata.org/covid-vaccinations
- 2.McMahon D.E., Amerson E., Rosenbach M., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Q., Ramie F., McMahon D.E., Freeman E.E. COVID-19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. May 31, 2021;39(4):653–673. doi: 10.1016/j.det.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., Le Gars M., Shukarev G., et al. Interim results of a phase 1–2a trial of Ad26.COV2.s Covid-19 vaccine. N Engl J Med. 2021;384(19):1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman E.E., McMahon D.E., Fitzgerald M.E., et al. The American Academy of Dermatology COVID-19 registry: crowdsourcing dermatology in the age of COVID-19. J Am Acad Dermatol. 2020;83(2):509–510. doi: 10.1016/j.jaad.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyrenne B.M., Al-Mohammedi F., DeKoven J.G., Alhusayen R. Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(9):e546–e548. doi: 10.1111/jdv.17342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drago F., Ciccarese G., Javor S., Parodi A. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30(3):544–545. doi: 10.1111/jdv.12942. [DOI] [PubMed] [Google Scholar]
- 10.Ilkit M., Durdu M., Karakaş M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38(3):191–202. doi: 10.3109/1040841X.2011.645520. [DOI] [PubMed] [Google Scholar]
- 11.Freeman E.E., McMahon D.E., Lipoff J.B., et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh E., Cardenas-de la Garza J.A., Cuellar-Barboza A., Franco-Marquez R., Arvizu-Rivera R.I. SARS-CoV-2 spike protein positivity in pityriasis rosea-like and urticaria-like rashes of COVID-19. Br J Dermatol. 2021;184(6):1194–1195. doi: 10.1111/bjd.19833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Nieto D., Ortega-Quijano D., Jimenez-Cauhe J., et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45(7):872–875. doi: 10.1111/ced.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marzano A.V., Genovese G., Fabbrocini G., et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenthal K.G., Freeman E.E., Saff R.R., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Nieto D., Hammerle J., Fernandez-Escribano M., et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. 'COVID-arm': a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35(7):e425–e427. doi: 10.1111/jdv.17250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed localized hypersensitivity reactions to the moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157(6):716–720. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackerman M., Henry D., Finon A., Binois R., Esteve E. Persistent maculopapular rash after the first dose of Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(7):e423–e425. doi: 10.1111/jdv.17248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez S., Vakharia P., Vandergriff T., Freeman E.E., Vasquez R. Pernio after COVID-19 vaccination. Br J Dermatol. 2021;185(2):445–447. doi: 10.1111/bjd.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colmenero I., Santonja C., Alonso-Riano M., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of 7 paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kha C., Itkin A. New-onset chilblains in close temporal association to mRNA-1273 (Moderna) vaccination. JAAD Case Rep. 2021;12(1):12–14. doi: 10.1016/j.jdcr.2021.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 23.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6(1):74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomayko M.M., Damsky W., Fathy R., et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148(3):750–751. doi: 10.1016/j.jaci.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C., Cotter D., Basa J., Greenberg H.L. 20 post-COVID-19 vaccine-related shingles cases seen at the Las Vegas dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;30(7):1960–1964. doi: 10.1111/jocd.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) April 12, 2021:Keab345. doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson L.B., Landman A.B., Shenoy E.S., et al. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2021;9(8):3200–3202. doi: 10.1016/j.jaip.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dash S., Sirka C.S., Mishra S., Viswan P. COVID-19 vaccine induced Steven-Johnson syndrome: a case report. Clin Exp Dermatol. June 3, 2021 doi: 10.1111/ced.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]