Abstract
Members of the recently discovered SOCS/CIS/SSI family have been proposed as regulators of cytokine signaling, and while targets and mechanisms have been suggested for some family members, the precise role of these proteins remains to be defined. To date no SOCS proteins have been specifically implicated in interleukin-2 (IL-2) signaling in T cells. Here we report SOCS-3 expression in response to IL-2 in both T-cell lines and human peripheral blood lymphocytes. SOCS-3 protein was detectable as early as 30 min following IL-2 stimulation, while CIS was seen only at low levels after 2 h. Unlike CIS, SOCS-3 was rapidly tyrosine phosphorylated in response to IL-2. Tyrosine phosphorylation of SOCS-3 was observed upon coexpression with Jak1 and Jak2 but only weakly with Jak3. In these experiments, SOCS-3 associated with Jak1 and inhibited Jak1 phosphorylation, and this inhibition was markedly enhanced by the presence of IL-2 receptor beta chain (IL-2Rβ). Moreover, following IL-2 stimulation of T cells, SOCS-3 was able to interact with the IL-2 receptor complex, and in particular tyrosine phosphorylated Jak1 and IL-2Rβ. Additionally, in lymphocytes expressing SOCS-3 but not CIS, IL-2-induced tyrosine phosphorylation of STAT5b was markedly reduced, while there was only a weak effect on IL-3-mediated STAT5b tyrosine phosphorylation. Finally, proliferation induced by both IL-2- and IL-3 was significantly inhibited in the presence of SOCS-3. The findings suggest that when SOCS-3 is rapidly induced by IL-2 in T cells, it acts to inhibit IL-2 responses in a classical negative feedback loop.
T-lymphocyte proliferation, a crucial component of immune responses, is controlled by a number of cytokines, in particular interleukin-2 (IL-2). IL-2 signals via a receptor composed of three chains IL-2Rα, IL-2Rβ, and γc (the common γ chain, shared by the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15) (22). These receptor components have no intrinsic kinase activity but when stimulated dimerize and induce tyrosine phosphorylation of intracellular substrate(s) via the receptor-associated Janus kinases (Jaks). Binding of IL-2 to its receptor results in activation of Jak1 and Jak3, which in turn induce phosphorylation of associated signaling molecules including the STATs (signal transducers and activators of transcription), leading to the expression of target genes (see reference 11a for a review).
While these signaling responses have been well characterized, how they are controlled is not clear. A number of cytokines (notably IL-2, IL-3, and erythropoietin [EPO]) have been shown to recruit the inhibitory tyrosine phosphatase SHP-1 (19). More recently, the cytokine-inducible gene CIS (for cytokine-induced SH2 [Src homology 2] protein) was shown to associate with the IL-3 and EPO receptors (IL-3R and EPOR) (29) and later shown to inhibit signaling through these receptors in an apparent classical negative feedback loop (13). Subsequently, SOCS-1/JAB/SSI-1 was shown to inhibit IL-6-induced macrophage differentiation of M1 cells and to bind to Jak2 and inhibit signaling through it. A number of cytokine-inducible genes that along with CIS and SOCS-1 encode a family of proteins that can modulate cytokine signaling variably called SOCS (suppressors of cytokine signalling) (26), or CIS (7), or SSI (STAT-induced STAT inhibitors) (17) have now been cloned. SOCS proteins are characterized by a central SH2 domain and a unique motif in their C-terminal region which has been designated a SOCS box (or CIS homology domain). The SH2 domain, while enabling interaction with phosphorylated proteins involved in signal transduction and cell activation, has been shown at least in the case of CIS and SOCS-1 to be insufficient to inhibit cytokine signaling (7, 18). The SOCS box is found in a number of other families of proteins in association with other motifs (WD40 repeats, ankyrin repeats, Spry domains, and GTPase domains) (10, 12, 20).
SOCS-3 mRNA has been detected in various tissues in response to a number of cytokines, including IL-2, IL-3, IL-6, leukemia-inhibitory factor (LIF) (10, 26), growth hormone (GH) (1), and leptin (3). Preliminary data has suggested that SOCS-3 may play a role in inhibitory responses to LIF (12, 14), GH (1), and leptin (3). In vivo northern analyses have shown induction of SOCS-3 in spleen and thymus, but the expression and modulation of the SOCS-3 protein in lymphocytes have not been examined. In this report, we show that SOCS-3 protein expression and tyrosine phosphorylation are highly regulated by IL-2 in T cells. Significantly, we have found that SOCS-3 can associate with components of the IL-2R complex, specifically Jak1 (but not Jak3), and IL-2Rβ. We further show that SOCS-3 can inhibit Jak1 phosphorylation, that this is accentuated by the presence of IL-2Rβ, and that expression of SOCS-3 can inhibit IL-2 responses.
MATERIALS AND METHODS
Antibodies.
Polyclonal rabbit anti-CIS antiserum was previously described (29). Polyclonal rabbit anti-SOCS-3 antiserum was generated by using amino acids 5 to 21 of human SOCS-3 protein. Anti-Myc monoclonal antibody (MAb) 9E10 was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.), anti-FLAG MAb M2 was purchased from Eastman Kodak (Rochester, N.Y.), antiphosphotyrosine MAb 4G10 was purchased from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Polyclonal rabbit anti-Jak3 and anti-STAT5b antisera were generous gifts of J. O’Shea (National Institutes of Health). Jak1 MAb was obtained from Transduction Laboratories (Lexington, Ky.). MAb 561 was used for immunoprecipitation of IL-2Rβ as described previously (11); antisera to IL-2Rβ (Santa Cruz Biotechnology) and IL-3Rβc (Upstate Biotechnology) were used for Western blotting. A MAb was used for immunoblotting Jak1 (Transduction Laboratories).
Constructs.
The full-length human SOCS-3 and SOCS-2 cDNAs were generated by PCR from a human T-cell library and subcloned into the mammalian expression vector pME18S (in which expression is driven by the SRα promoter [27]). Human recombinant SOCS-3 (hrSOCS-3), hrSOCS-2, murine recombinant Jak1, and hrJak3 cDNA were generated by PCR and subcloned into pME18S with a single copy of the FLAG sequence at their carboxy termini (4). IL-2Rβ and IL-2Rγ cDNAs were also cloned into pME18S. A previously described (7, 29) SOCS-1/JAB construct was also used.
Cells and transfections.
Peripheral blood lymphocytes (PBLs) were prepared from normal donors by using standard protocols and stimulated for 72 h in Yessel’s medium supplemented with 10% human serum, 2 mM glutamine, 100 U of penicillin and 100 U of streptomycin per ml, and 2 mg of phytohemagglutinin (PHA-L; Boehringer Mannheim) per ml. They were then washed and rested for 12 h in RPMI 1640 supplemented with 2% human serum without PHA or IL-2. YT cells were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 U of penicillin and 100 U of streptomycin per ml. The IL-2-dependent T-cell lines HT-2 and Kit-225 were grown as described above in medium containing 50 U of IL-2 per ml. Cytokine-dependent cells were rested in RPMI 1640 medium–2% FBS for 4 to 12 h prior to cytokine stimulation. BaF3 transfectants (maintained in IL-3 [10 ng/ml]) expressing IL-2Rβ and SOCS-3 were generated by electroporation (4 × 106 cells/ml) with plasmids containing IL-2Rβ, using a Bio-Rad Gene Pulser (260 V, 960 mF), and were selected in neomycin (2 mg/ml; Gibco BRL) or puromycin (200 μg/ml; Bio-Rad). Resistant clones were tested for IL-2Rβ and SOCS-3 expression by Western blotting using IL-2Rβ antiserum (Santa Cruz Biotechnology) or antiserum generated to the amino terminus of SOCS-3. Ba/F3 cells expressing the tetracycline-responsive transcriptional activator protein were generated by electroporation with pUHD15-1 modified to contain a puromycin resistance gene (15). This clone was transfected with pUHD10-3 containing SOCS-3 by electroporation as described above and selected by using 1.2 mg of hygromycin per ml. Tetracycline (1 μg/ml) was replaced every 48 h; to induce expression of SOCS-3, cells were grown in the absence of tetracycline for 36 h. 293T cells were cultured in Dulbecco modified Eagle medium medium with 10% FBS, 2 mM glutamine, and 100 U of penicillin and streptomycin per ml and transfected at 40 to 50% confluency, using calcium phosphate precipitation reagents by standard protocols (5′-3′, Inc., Boulder, Colo.); cells were harvested at 36 to 48 h. To test cytokine responses, cells were stimulated with IL-2 (50 U/ml), IL-3 (10 ng/ml), IL-4 (100 U/ml), IL-10 (100 U/ml), or gamma interferon (50 U/ml).
RNA preparation and Northern blot analyses.
Using an RNeasy midi kit (Qiagen, Valencia, Calif.), we extracted total RNA from PBLs or cell lines either treated with cytokine or untreated and quantitated RNA by spectrophotometry. Then 20 μg of total RNA from each sample was subjected to electrophoresis overnight in a 1% agarose formaldehyde gel. Gels were washed three times with water and then denatured for 30 min in 50 mM NaOH–10 mM NaCl, rinsed with 100 mM Tris HCl (pH 7.5) for 30 min, and finally placed in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 30 min. Gels were transferred overnight onto nylon membranes (Schleicher & Schleicher, Keene, N.H.) that had been pretreated with water and then 10× SSC. Following transfer, membranes were rinsed with 2× SSC and cross-linked in a UV Stratalinker 2400 (Stratagene, La Jolla, Calif.). Membranes were prehybridized in 5 ml of Rapid-hyb (Amersham, Arlington Heights, Ill.) and then hybridized with cDNAs labeled by random priming using the Rediprime DNA labeling system (Amersham). Following hybridization, membranes were subjected to a final wash in 0.1× SSC–0.1% sodium dodecyl sulfate (SDS) at 65°C.
Immunoprecipitations and immunoblotting.
Following treatment, cells were washed in phosphate-buffered saline lysed in Brij 97 lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% Nonidet P-40, 1 mM Na3VO4, 5 mM NaF, 10 mg each of leupeptin and aprotonin per ml), and centrifuged at 18,000 × g at 4°C for 15 min. Lysates were immunoblotted as described below or immunoprecipitated by incubating with one of the antibodies described above bound to protein A-agarose beads or with a SOCS-3–glutathione S-transferase (GST) fusion protein bound to glutathione-Sepharose, for 2 to 4 h at 4°C. Lysates and immunoprecipitates were washed, boiled in reducing SDS sample buffer, resolved on SDS-polyacrylamide gels (Novex, San Diego, Calif.), and transferred onto Immobilon membranes (Millipore). Immunoblotting was performed with antibodies as described above, and blots were visualized by enhanced chemiluminescence (Pierce, Rockford, Ill.).
In vitro kinase assays.
Following immunoprecipitation as described above, precipitates were washed twice with MSH buffer (100 mM NaCl, 10 mM HEPES) and then incubated for 15 min with kinase reaction buffer (20 mM Tris [pH 7.5], 5 mM MgCl2, 5 mM MnCl2, 1 μM ATP) containing 10 μCi of [γ-32P]ATP on ice. After two washes with MSH buffer, samples were boiled in reducing SDS buffer, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and the gels were dried and subjected to autoradiography.
Thymidine incorporation assay.
Cells from two BaF3 clones inducibly expressing SOCS-3 were rested in cytokine-free medium as described above, with half the cells first being removed from tetracycline for 24 h. Cells (4 × 104 per well) were plated in a 96-well plate in RPMI 1640 medium–5% FBS, with or without tetracycline (1 μg/ml) and with no cytokine or 50 U of IL-2 per ml. After 20 h, [3H]thymidine (1 mCi/ml) was added for 4 h, cells were harvested onto glass fiber filters, and incorporated [3H]thymidine was quantitated as described elsewhere (15).
RESULTS
SOCS-3 and CIS are rapidly induced by IL-2 in T cells.
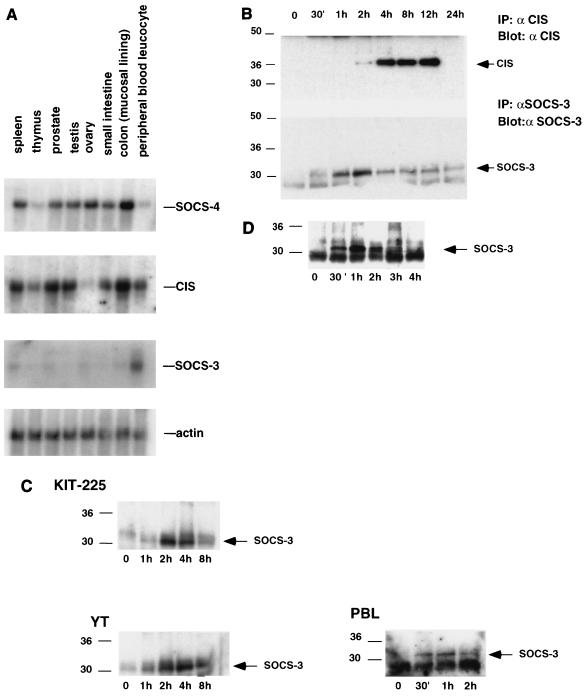
To assess the potential role of SOCS proteins in lymphocytes, we initially examined their expression by Northern blot analysis. Probing of multitissue mRNA blots revealed strong expression of CIS in a number of tissues, including thymus, spleen, prostate, and PBLs (Fig. 1A). While SOCS-4 mRNA was not detected in PBLs or the thymus, significant levels of expression were detected in the spleen and colon. Interestingly, although SOCS-3 expression was detectable in the spleen, highest levels were seen in PBLs, with lower levels detected in other tissues examined (Fig. 1A). The more selective expression of SOCS-3 in comparison to other SOCS proteins suggests an important role in leukocytes.
FIG. 1.
SOCS-3 is expressed in lymphocytes and regulated by IL-2 and IL-3. (A) Tissue mRNA blots were probed with cDNAs to CIS, SOCS-3, and SOCS-4, with β-actin as a control. (B) Kit-225 cells were treated as described above, and lysates were immunoprecipitated (IP) with antiserum to either SOCS-3 (αSOCS3) or CIS (αCIS) prior to SDS-PAGE. Filters were blotted with the corresponding antibody. (C) Kit-225 cells, YT cells, or PHA blasts were rested overnight in 2% FBS, stimulated with IL-2 for between 30 min and 8 h, lysed, immunoprecipitated, and immunoblotted with the antiserum to SOCS-3. (D) BaF3 cells were placed in cytokine-free medium overnight and treated with IL-3 for the indicated times, and the lysates were immunoprecipitated and immunoblotted with antiserum to SOCS-3. Sizes are indicated in kilodaltons.
The expression of SOCS proteins has been shown to be highly regulated by cytokines (7, 10, 17, 26). To further study the regulation of SOCS-3 and CIS proteins in lymphocytes, Kit-225 cells were placed in cytokine-free, reduced-serum conditions for 12 h before treatment with IL-2 for periods of up to 24 h. The cells were then lysed, and SOCS-3 and CIS were immunoprecipitated for Western blotting. In these experiments, SOCS-3 protein was detected within 30 min of IL-2 stimulation, peaked at 2 to 4 h, and returned to basal levels within 8 h; CIS was first seen at 2 h, was strongly expressed between 4 and 12 h, and was again undetectable at 24 h (Fig. 1B). Interestingly, we observed only the 37-kDa form of CIS in Kit-225 cells, although 32-, 37-, and 45-kDa forms of this protein have been reported (13, 29). In the YT NK-like cell line, SOCS-3 expression peaked between 2 and 4 h (Fig. 1C); in human PBL-derived blasts, SOCS-3 expression was noted within 30 min of IL-2 stimulation, peaked at 1 h, and declined thereafter (Fig. 1C). Alpha interferon was also able to induce low levels of SOCS-3 protein expression in Kit-225 cells (data not shown).
To determine if SOCS-3 protein could be induced by other cytokines in lymphocytes, IL-3-responsive BaF3 cells were examined. The cells were incubated in cytokine-free, reduced-serum medium for 12 h and then treated with IL-3. SOCS-3 protein was initially seen at 30 min, peaked at 1 h, and was absent by 4 h (Fig. 1D). These findings suggest that SOCS-3 is a short-lived immediate-early gene product, induced by IL-2, and IL-3 in lymphocytes.
SOCS-3 is tyrosine phosphorylated in response to IL-2.
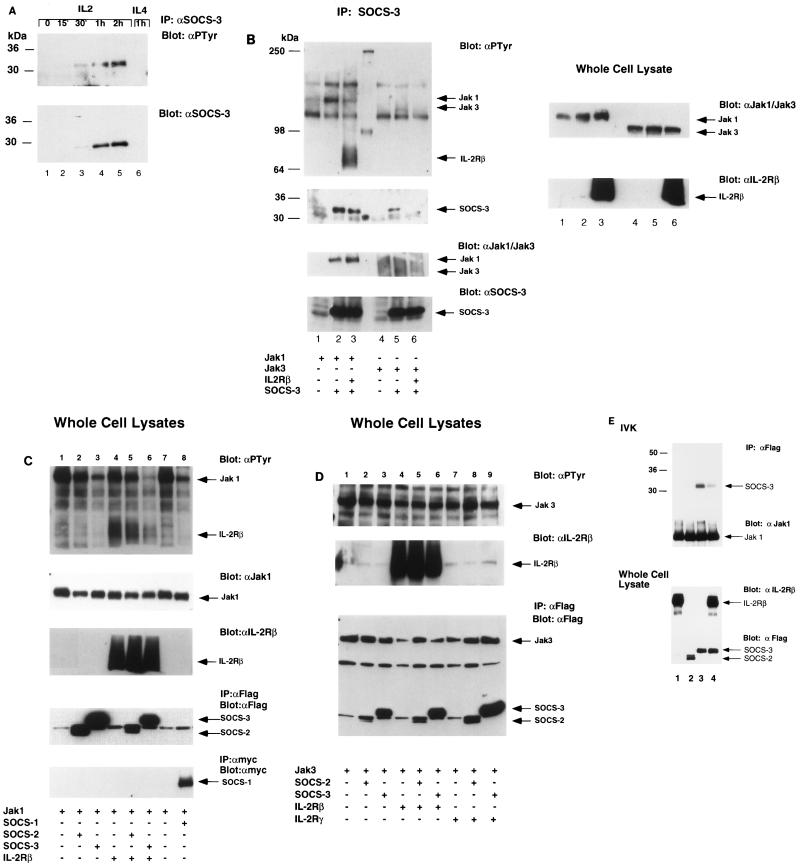
SOCS-1/JAB/SSI-1 has been shown to interact with Jak2 and inhibit its activity (7, 17, 26), while in a yeast two-hybrid system SOCS-3 was shown to interact with Jak2-JH1 (12). However, whether SOCS proteins themselves are tyrosine phosphorylated has not been reported. We therefore examined whether SOCS-3 may be tyrosine phosphorylated in response to IL-2. Again Kit-225 cells were stimulated with IL-2 following 12 h in cytokine-free medium. Cells were lysed at various intervals, immunoprecipitated with antiserum to SOCS-3, and immunoblotted (for SOCS-3 expression and tyrosine phosphorylation). Tyrosine phosphorylation of SOCS-3 was observed at 30 min and was maximal between 1 and 2 h, in parallel with SOCS-3 protein levels (Fig. 2A). Phosphorylation decreased to undetectable levels within 4 h (data not shown). Of note, although IL-4 also signals through γc and activates the same Jak kinases, we did not observe induction of SOCS-3 protein expression in response to IL-4 at 1 h (Fig. 2A, lane 6). Interestingly, despite the high levels of SOCS-3 induced by IL-3 in BaF3 cells, no tyrosine phosphorylation was seen in four experiments. Consistent with previously published results (7), no tyrosine phosphorylation of CIS was seen following its induction by IL-2 or IL-3.
FIG. 2.
Tyrosine phosphorylation of SOCS-3 in lymphocytes treated with IL-2 and by Jak1 in 293 T cells. (A) Kit-225 cells were treated as described in the text, and lysates were immunoprecipitated (IP) with antiserum to SOCS-3 (αSOCS-3). Immunoblotting was performed with the same antibody or antiphosphotyrosine antibody (αPTyr). (B) 293 T cells were transfected with Jak1 or Jak3 in combination with SOCS-3 and IL-2Rβ as indicated. After 48 h, cells were harvested and a portion of the lysate was used for immunoprecipitation with antiserum to SOCS-3. Immunoprecipitates and whole-cell lysates were immunoblotted with antiphosphotyrosine antibody or antiserum to SOCS-3, Jak1, or Jak3. Expression levels of Jak kinases and IL-2Rβ were tested on whole-cell lysates. (C) 293 T cells were transfected with various combinations of plasmids containing DNA encoding Jak1, IL-2Rβ, SOCS-1, SOCS-2, and SOCS-3 as indicated. After 48 h, cells were harvested and whole-cell lysates were subjected to SDS-PAGE and immunoblotting with antiphosphotyrosine antibody as described above. Blots were reprobed to check expression levels. (D) 293 T cells were transfected with plasmids encoding Jak3, IL-2Rβ, SOCS-1, SOCS-2, and SOCS-3 as indicated. After 48 h, cells were harvested and analyzed as described above. (E) 293 T cells were transfected with a plasmid encoding Jak1 (all lanes), IL-2Rβ (lanes 1 and 4), SOCS-2 (lane 2), or SOCS-3 (lanes 3 and 4); the lysates were immunoprecipitated with MAb M2, and in vitro kinase (IVK) assays performed on FLAG-tagged Jak1 and SOCS proteins. Sizes are indicated in kilodaltons.
SOCS-3 associates with and is phosphorylated by Jak1.
The rapid tyrosine phosphorylation of SOCS-3 in response to IL-2 suggests that this protein is a substrate for an IL-2-activated tyrosine kinase. Therefore, we investigated its possible interaction with the IL-2-activated Jak kinases, Jak1 and Jak3. 293T cells were transfected with cDNA encoding SOCS-3, Jak1, Jak3, or IL-2Rβ, and the lysates were immunoprecipitated with antiserum directed against SOCS-3. Jak1 was detected in the SOCS-3 immunoprecipitates in cells expressing Jak1 or expressing Jak1 and IL-2Rβ (Fig. 2B, first and third panels, lanes 2 and 3). However, despite high levels of Jak3 expression in these cells, coprecipitation of Jak3 with SOCS-3 was not detected (lanes 5 and 6). SOCS-3 was strongly tyrosine phosphorylated in the presence of Jak1 (Fig. 2B, third panel, lanes 2 and 3) but only weakly in the presence of Jak3 (lane 5). Phosphorylation of SOCS-3 was also observed when coexpressed with Lck and Jak2 (data not shown). The strong interaction observed between Jak1 and SOCS-3 suggest that Jak1 could be responsible for the IL-2-induced phosphorylation of SOCS-3 observed in lymphocytes (Fig. 2A).
SOCS-3 inhibits Jak1 but not Jak3 phosphorylation, and this effect is maximal in the presence of IL-2Rβ.
As SOCS-1 inhibits tyrosine phosphorylation of Jak kinases, and in view of our findings, we wondered whether SOCS-3 would inhibit Jak1 or Jak3 phosphorylation. To explore this possibility, 293T cells were transfected with cDNA encoding Jak1 or Jak3 in combination with receptor component IL-2Rβ or IL-2Rγ and either SOCS-1, SOCS-2, or SOCS-3. Coexpression studies consistently revealed that SOCS-1 and SOCS-3 inhibited the phosphorylation of Jak1 (Fig. 2C, lanes 3 and 8), while SOCS-2 did not (lane 2). IL-2Rβ is known to associate (constitutively) with Jak1; interestingly, the SOCS-3-mediated inhibition of Jak1 phosphorylation was strongly enhanced in the presence of IL-2Rβ, with almost complete abolition of Jak1 tyrosine phosphorylation being observed (lane 3 versus lane 6). Furthermore, in the previous experiment where Jak1 and SOCS-3 were coimmunoprecipitated, the phosphorylation of both Jak1 and SOCS-3 was significantly reduced in the presence of IL-2Rβ (Fig. 2B, lane 3). Consistent with previously published data, we found that 293T cells expressing SOCS-1 showed reduced phosphorylation of Jak1 and Jak2; in addition, we observed a small reduction in expression of Jak1. However, the inhibitory effect of SOCS-1 on Jak1 tyrosine phosphorylation was not augmented by the presence of IL-2Rβ (data not shown).
Coexpression of SOCS-3 or SOCS-2 with Jak3 had no inhibitory effect on Jak3 tyrosine phosphorylation (Fig. 2D, lanes 1 to 3), and this was not influenced by the addition of either IL-2Rβ or IL-2Rγ (the latter which is known to associate with Jak3); interestingly, SOCS-1 also did not inhibit Jak3 autophosphorylation when coexpressed in 293 cells (data not shown). Once again, no tyrosine phosphorylation of SOCS-3 was seen in the presence of Jak3. Thus, the findings indicate that while SOCS-3 does not interact with Jak3, it can strongly associate with Jak1 and inhibit its phosphorylation, an effect that is enhanced in the presence of IL-2Rβ.
SOCS-3 inhibits Jak1 kinase activity.
Experiments from other groups have shown that SOCS-1 not only inhibits phosphorylation of Jak1, Jak2, and Tyk2 but also can inhibit Jak kinase activity, as measured by Jak1 autophosphorylation in in vitro kinase assays (20a). These studies failed to detect any inhibition of kinase activity by SOCS-3. In view of our findings demonstrating an enhanced inhibitory effect of SOCS-3 in the presence of IL-2Rβ, we performed in vitro kinase assays using 293 T cells transfected with FLAG-tagged Jak1 and SOCS proteins and immunoprecipitated with an anti-FLAG antibody in the presence and absence of IL-2Rβ. The addition of IL-2Rβ to Jak1 and SOCS-3 markedly reduced the kinase activity of Jak1, as measured by the ability to phosphorylate SOCS-3 (Fig. 2E, lanes 3 and 4). When SOCS-2 was expressed with Jak1, it was not phosphorylated in the in vitro kinase reaction (lane 2). This finding suggests that SOCS-3 can strongly inhibit Jak1 activity in the presence of IL-2Rβ.
SOCS-3 associates with tyrosine-phosphorylated Jak1 and IL-2Rβ following IL-2 stimulation.
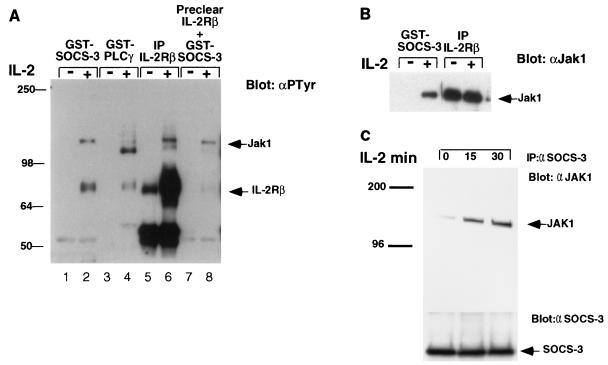
CIS has been shown to associate with EPOR and IL-3Rβc (29); in light of this finding and the interactions demonstrated above between SOCS-3, Jak1, and IL-2Rβ, we examined whether SOCS-3 might associate with the IL-2R complex in lymphocytes. We therefore generated a GST fusion protein containing full-length SOCS-3, and lysates from untreated or IL-2-stimulated Kit-225 cells were incubated with the SOCS-3–GST fusion protein or a fusion protein containing the SH2 domain of phospholipase Cγ (PLCγ). The GST pull-down products were resolved by SDS-PAGE and blotted with antiphosphotyrosine antibody. Following IL-2 treatment, GST–SOCS-3 coprecipitated a tyrosine-phosphorylated protein similar in molecular weight to IL-2Rβ and one similar in size to tyrosine-phosphorylated Jak1 (∼130 kDa) (Fig. 3A). The fusion protein containing the SH2 domain of PLCγ did not precipitate Jak1 but coprecipitated a protein of 120 kDa, perhaps tyrosine-phosphorylated PLCγ, also known to be activated in response to IL-2 (lanes 3 and 4). As a control, Kit-225 lysates were also immunoprecipitated with antibody specific for IL-2Rβ; in these lanes we could detect both tyrosine-phosphorylated IL-2Rβ chain and tyrosine phosphorylated Jak1 (lanes 5 and 6). To establish that the 75-kDa phosphoprotein with which the SOCS-3 fusion protein was observed to interact was indeed IL-2Rβ, lysates were initially precleared by using IL-2Rβ antibody before precipitation with SOCS-3 fusion protein. In fact, preclearing with antiserum to IL-2Rβ almost completely removed the 75-kDa phosphoprotein, indicating that this protein was IL-2Rβ (lanes 7 and 8). To confirm that SOCS-3 did associate with tyrosine-phosphorylated Jak1 following IL-2 treatment, lysates from IL-2-stimulated Kit-225 cells were precipitated with GST–SOCS-3 or antiserum to IL-2Rβ then and immunoblotted for Jak1. While the IL-2 receptor was constitutively associated with Jak1, coprecipitation of GST–SOCS-3 with Jak1 was observed only following IL-2 treatment (Fig. 3B). These findings suggest that in lymphocytes, SOCS-3 can physically interact with Jak1 and IL-2Rβ, but only following IL-2-induced tyrosine phosphorylation.
FIG. 3.
SOCS-3 associates with the IL-2R complex following IL-2 stimulation of lymphocytes. Kit-225 cells were prepared as described in the text and treated with IL-2 for 15 min. Cells lysates were incubated with a GST–SOCS-3 fusion protein, and the immunoprecipitates (IP) were subjected to SDS-PAGE and membrane transfer. Filters were immunoblotted with antiphosphotyrosine (αPTyr) (A) or anti-Jak1 (αJak1) (B). (C) BaF3 cells expressing both IL-2Rβ and SOCS-3 were treated with MG132 (20 mM) for 30 min and were stimulated with IL-2 for 15 or 30 min; lysates were immunoprecipitated (IP) with antiserum to SOCS3 and blotted for Jak1 (upper panel) or SOCS-3 (lower panel). Sizes are indicated in kilodaltons.
We next examined whether Jak1 could associate with SOCS-3 following IL-2 stimulation by using BaF3 cells expressing SOCS-3 (Materials and Methods). BaF3 cells expressing both IL-2Rβ and SOCS-3 that had been pretreated with MG132 (20 mM) for 30 min were stimulated with IL-2 for 15 or 30 min, and lysates were immunoprecipitated with antisera to SOCS-3 and immunoblotted with Jak1 MAb. In the presence of the proteosome inhibitor MG132, we observed an enhanced association of SOCS-3 with Jak1 following IL-2 stimulation (Fig. 3C). This result suggests that Jak1 can associate with SOCS-3 and implies that this complex may normally be short-lived.
SOCS-3 inhibits IL-2-induced STAT5 activation.
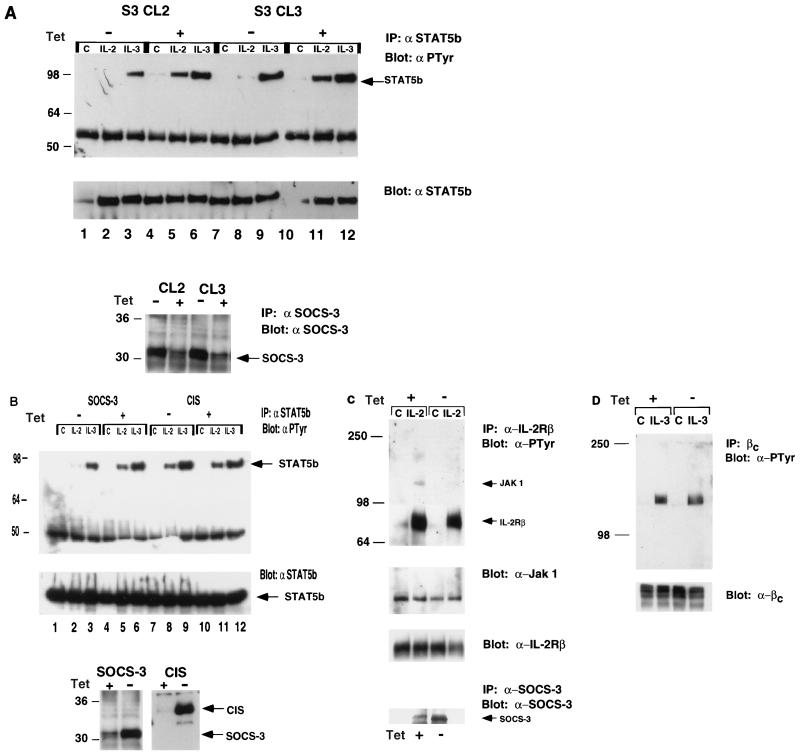
Both CIS and SOCS-1 have been shown to inhibit various cytokine responses. CIS was shown to associate with IL-3R and EPOR and to inhibit tyrosine phosphorylation of STAT5 (29). SOCS-1 was reported to reduce IL-6 and LIF responses by binding to Jak2 (7, 17, 26). We therefore examined the effect of SOCS-3 on IL-2- and IL-3-induced STAT5 phosphorylation. We established clones in which SOCS-3 expression is suppressed by the presence of tetracycline (8, 9, 23). BaF3 cells containing both tetracycline-responsive transcriptional activator and IL-2Rβ were created, following which SOCS-3 was introduced in the pUHD10-3 vector, such that expression of SOCS-3 was induced by removing the cells from tetracycline. At 36 h after removal from tetracycline, including a 12-h period in the absence of cytokine, the cells were stimulated with either IL-2 or IL-3 for 15 min, and levels of tyrosine-phosphorylated STAT5b and IL-2Rβ were compared with those of cells maintained in tetracycline. As shown in Fig. 4A, cells removed from tetracycline for 36 h express high levels of SOCS-3 in comparison to cells maintained in tetracycline. IL-2- and IL-3-induced tyrosine phosphorylation of STAT5b was seen in cells not expressing SOCS-3 (Fig. 4A, lanes 5, 6, 11, and 12); however, in cells removed from tetracycline (which express high levels of SOCS-3), a marked reduction in detectable STAT5b phosphorylation was observed in response to IL-2 (lane 2 compared to lane 5; lane 8 compared to lane 11), while there was only a marginal reduction in the IL-3-induced response (lane 3 compared to lane 6; lane 9 compared to lane 12).
FIG. 4.
SOCS-3 but not CIS inhibits IL-2-induced tyrosine phosphorylation of STAT5b and Jak1. (A) Two BaF3 clones (S3 CL2 and S3 CL3) expressing IL-2Rβ, in which SOCS-3 was absent due to the presence of tetracycline (tet) (+) or in which expression of SOCS-3 was induced by removal from tetracycline (−), were treated with IL-2 or IL-3 for 15 min, and lysates were subject to immunoblotting with antiphosphotyrosine (αPTyr) and immunoprecipitation (IP) with anti-STAT5b. SOCS-3 levels were checked by immunoblotting (lower panel). (B) BaF3 clones in which CIS or SOCS-3 expression was controlled by removal from tetracycline were treated as described above, and lysates were subjected to immunoblotting with antiphosphotyrosine and anti-STAT5b. CIS and SOCS-3 levels were checked by immunoblotting (lower panel). (C and D) SOCS-3-expressing cells were treated as described above with IL-2, immunoprecipitated anti-IL-2Rβ, and blotted for phosphotyrosine (C, top), Jak1 (C, middle), or anti-IL-2Rβ (C, bottom) or with IL-3, immunoprecipitated with anti-IL-3Rβc, and blotted for antiphosphotyrosine (D). Sizes are indicated in kilodaltons.
We next examined the effect of CIS on IL-2- and IL-3-induced STAT5b phosphorylation. Clones in which CIS expression is suppressed by the presence of tetracycline were established as described above. The cells were stimulated with either IL-2 or IL-3 for 15 min; as shown in Figure 4B (lower panel), cells removed from tetracycline for 36 h expressed high levels of CIS or SOCS-3 in comparison to cells maintained in tetracycline. There was no detectable inhibition of IL-2- and IL-3-induced tyrosine phosphorylation of STAT5b in cells expressing CIS (Fig. 4B, lanes 7 to 12), but again SOCS-3 expression induced a marked reduction in IL-2-mediated STAT5b phosphorylation (lane 2). These data suggest that CIS does not affect IL-2-mediated STAT5b activation as potently as can SOCS-3.
SOCS-3 partially inhibits IL-2R and Jak1 phosphorylation.
We performed experiments similar to those described above in which lysates were immunoprecipitated with an antibody to IL-2Rβ, in cells with and without SOCS-3. Induction of SOCS-3 (by removal from tetracycline) resulted in a small but consistent reduction of IL-2-mediated phosphorylation of the receptor complex (Fig. 4C). Following SOCS-3 induction, there was a marked reduction in the level of tyrosine-phosphorylated Jak1 associated with IL-2R (Fig. 4C, lane 2 versus lane 4), although there was no apparent reduction in the amount of Jak1 associated with the receptor (Fig. 4C, second panel). In parallel experiments no detectable reduction in IL-3-induced IL-3Rβc tyrosine phosphorylation was observed following removal of tetracycline (Fig. 4D).
SOCS-3 inhibits IL-2- and IL-3-mediated proliferation.
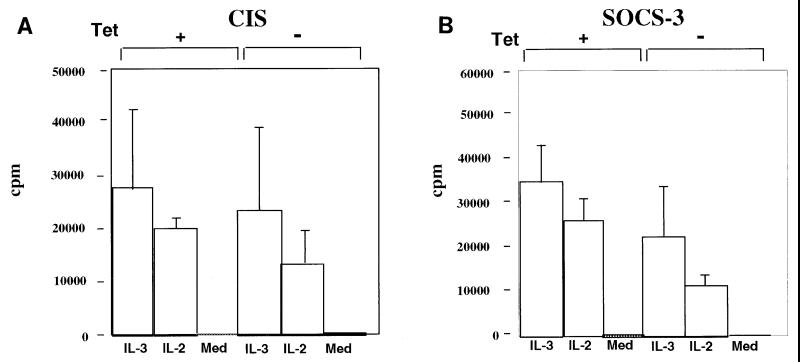
We next examined the effect of SOCS-3 and CIS expression on IL-2-mediated proliferation in BaF3 cells expressing IL-2Rβ. Again cells were incubated in the absence of tetracycline for 36 h, including 12 h free from cytokine. The cells were subsequently incubated in medium either with or without cytokine (IL-2 [100 U/ml] or IL-3 [50 U/ml]) for 24 h, and levels of DNA synthesis were measured by incorporation of [3H]thymidine. Cells expressing SOCS-3 had markedly reduced levels of thymidine incorporation in response to both IL-2 and IL-3 compared to clones that had been left in tetracycline and that did not express SOCS-3 (Fig. 5B). However, smaller reductions in thymidine incorporation were noted in cells that expressed CIS (Fig. 5A). These findings are representative of six individual experiments and demonstrate that induced expression of SOCS-3 can more strongly suppress IL-2 (and IL-3) proliferative responses than does expression of CIS.
FIG. 5.
SOCS-3 inhibits cytokine-induced lymphocyte proliferation. Lymphocyte proliferation was assessed by [3H]thymidine incorporation as described in Materials and Methods. The experiments were performed on BaF3 clones (expressing CIS [A] or SOCS-3 [B]), in which SOCS-3 was absent due to the presence of tetracycline (Tet) (+) or in which expression of SOCS-3 was induced by removal from tetracycline (−). SOCS-3 expression in the absence of tetracycline was confirmed by immunoblotting (not shown). Med, cytokine-free medium.
DISCUSSION
Signaling in response to IL-2 and to cytokines such as IL-4, IL-7, and IL-15 (which signal through γc, Jak1, and Jak3) is known to be essential for normal lymphocyte development and proliferation (22). Although much is understood with respect to how activating signals are transduced, little is known about their control. In this report, we describe the induction and tyrosine phosphorylation of SOCS-3 in lymphocytes, including in human PBLs, in response to IL-2 and report that it interacts with IL-2R and Jak1. We show that SOCS-3 is strongly phosphorylated by Jak1, that SOCS-3 can inhibit Jak1 tyrosine phosphorylation, and that this inhibition is enhanced by the presence of IL-2Rβ. Furthermore, we demonstrate that SOCS-3 can abrogate IL-2-induced phosphorylation and activation of STAT5 and IL-2-mediated proliferation, suggesting that in lymphocytes SOCS-3 can regulate IL-2 responses.
Cytokines induce SOCS/CIS/SSI family members as immediate-early genes (7, 17, 26, 29). Consistent with this, SOCS-3 protein is particularly rapidly induced and tyrosine phosphorylated in human PBLs in response to IL-2, being detected as early as 30 min following stimulation. This family of proteins are also rapidly degraded, suggesting that SOCS protein function is only transiently required. In Kit-225 cells, SOCS-3 levels fall within 4 h, while in PBLs they decline after 1 h; in comparison, CIS is strongly detected between 4 and 12 h. Interestingly, CIS has recently been shown to be subject to ubiquitination and to degradation by the proteasome pathway (28). In the experiments described, addition of proteasome inhibitors resulted in significant accumulation of both the ubiquitinated CIS and CIS-EPOR complexes, both of which are normally present only transiently; furthermore, proteasome inhibition also prolonged the phosphorylation of both the EPOR and STAT5. The proteasome inhibitors LLnL and LLM have also been shown to stabilize SOCS-1 expression in M1 cells (18). It had previously been suggested that the proteasome pathway contributes to regulation of the IL-2-stimulated Jak-STAT tyrosine phosphorylation (30), although neither STAT5 nor Jak kinase appears to be ubiquitinated (5, 30). Thus, ubiquitination of the SOCS proteins and their degradation by the proteasome might account for their rapid turnover and may be a means by which they modulate cytokine signaling pathways.
SOCS-3 is unusual among SOCS family members with regard to its tyrosine phosphorylation. Tyrosine phosphorylation was detectable in both Kit-225 cells and PBLs as early as protein was observed. SOCS-3 was strongly tyrosine phosphorylated in the presence of Jak1 in 293 T cells, and while tyrosine phosphorylation was also seen with Jak2 and Lck, it was significantly lower with Jak3. The presence of tyrosine residues in the region between the SH2 domain and the SOCS box may explain the propensity of SOCS-3 for tyrosine phosphorylation in comparison to other SOCS proteins. While the precise role and importance of this tyrosine phosphorylation is unknown, the strict regulation of SOCS-3 through both rapid changes in protein levels and tyrosine phosphorylation further point to an important role for SOCS-3 in the IL-2 response.
It is apparent that cytokine signaling can be controlled by a number of mechanisms. Phosphatases are known to regulate tyrosine phosphorylation in response to cytokines and growth factors, a classic example being the SH2-containing tyrosine phosphatase SHP-1, which is recruited to a number of activated cytokine receptors, including IL-4R, EPOR, IL-3R, and the IL-2R (19). IL-2 responses are also thought to be regulated by degradation of receptor components such as the calpain-mediated cleavage of γc (21) and by receptor internalization. A family of PIAS proteins that are thought to interact with STATs and inhibit their activity have also been identified (6). Clearly, many mechanisms may contribute to downregulation of the stimulatory response, and the SOCS proteins add yet another level of control.
The specificity of SOCS proteins for individual cytokine responses remains uncertain. In the case of CIS, the only established interactions are with EPOR and IL-3R signaling through which CIS can partially inhibit (13, 29). We also find that CIS can partially inhibit IL-2 responses, although the effects are less potent than those observed with SOCS-3. SOCS-1 has been shown to strongly inhibit IL-6-mediated signaling in M1 cells (7, 17, 26), and both SOCS-1 and SOCS-3 can inhibit LIF (12, 14)- and GH (1)-mediated signaling. LIF induces SOCS-3 mRNA expression in the hypothalamus, and more dramatically in the pituitary, of mice, and in AT-20 corticotroph cells, stable overexpression of SOCS-3 reduced LIF-mediated receptor tyrosine phosphorylation and activation and adenocorticotropin secretion (2). Leptin was shown to induce hypothalamic SOCS-3 mRNA, and overexpression of SOCS-3 was able to inhibit leptin-induced receptor tyrosine phosphorylation (3). Thus, it seems clear that SOCS-3 may have a role in response to a number of cytokines. SOCS-1-deficient mice have been generated and are reported to have small thymuses; though healthy at birth, they exhibit stunted growth and progressive lymphocytopenia and die before weaning in association with fatty degeneration and monocytic infiltration of the liver (16, 25). These observations suggest that at least SOCS-1 has an essential role in homeostasis.
In addition, the mechanisms by which SOCS family members inhibit signaling appear to differ. CIS can interact with tyrosine-phosphorylated forms of IL-3Rβc and the EPOR (29), associating with a known STAT5 binding site (28). Alternatively, following ubiquitination, CIS may chaperone the receptor into the proteasome pathway as alluded to earlier (28). While the effect of CIS seems to be mediated primarily through interaction with the receptor, SOCS-1 was cloned by its ability to interact with Jak2 (through its JH1 kinase domain) and has the capacity to interact with and inhibit members of the Jak family (7). A yeast two-hybrid screen showed that SOCS-3 could also interact with the JH1 domain of Jak2 and the lymphocyte-specific kinase Lck (12). Our findings suggest that SOCS-3 can interact with Jak1 and inhibit its activity, but the enhanced inhibition observed in the presence of IL-2Rβ suggests that the receptor plays an important role in facilitating SOCS-3 action. The SOCS-3 fusion protein was able to interact with Jak1 and IL-2Rβ only after the IL-2-induced tyrosine phosphorylation of the receptor complex; this is in keeping with the requirement for tyrosine phosphorylation of the targets of both SOCS-1 (18) and CIS (29). Interestingly, previous yeast two-hybrid studies failed to show an interaction between SOCS-3 and IL-2Rβ, possibly because of this dependence on tyrosine phosphorylation (12). However, it remains unclear whether the interaction of SOCS-3 with the receptor is direct or if it occurs solely through the binding of SOCS-3 to Jak1, which in turn is associated with the receptor. It is of note that SOCS-3 did not significantly inhibit IL-3-mediated STAT5 phosphorylation, even though IL-3R signals through Jak1 and Jak2. Also of note, CIS did not inhibit cytokine-mediated Stat5b phosphorylation, suggesting that SOCS-3 may specifically mediate this effect. Similarly, a recent report suggests that gamma interferon signaling (which uses both Jak1 and Jak2) is inhibited by SOCS-1 but not SOCS-3 (24). Therefore, while SOCS-3 has the ability to inhibit Jak activity, the specificity of the inhibition may be receptor dependent; with respect to the IL-2 response, the interaction with both IL-2R and Jak1 (rather than Jak3) seem pivotal.
In addition to the inhibitory effect SOCS-3 exerted on IL-2-induced STAT5 phosphorylation, we observed a significant inhibition of both IL-2- and IL-3-mediated lymphocyte proliferation. This is consistent with the ability of SOCS-1 to inhibit responses to various cytokines. However, despite the marked effect on STAT5 phosphorylation, SOCS-3 did not completely inhibit IL-2-induced receptor phosphorylation and proliferation, suggesting that not all responses to IL-2 are blocked by SOCS-3 expression. Additionally, the mechanism by which SOCS-3 inhibits IL-3-mediated proliferation is unclear but may be independent of Stat5b.
In conclusion, our findings indicate that in lymphocytes, SOCS-3 is strongly and rapidly expressed in response to IL-2 (and perhaps other cytokines) and can interact with IL-2R and Jak1, following which it inhibits further IL-2 responses. The detection of SOCS-3 in human PBLs in response to IL-2 is of particular importance in light of the continuing uncertainty of the precise physiological role(s) of SOCS proteins. This finding, coupled with its rapid induction, tyrosine phosphorylation, and ability to inhibit IL-2-mediated proliferation, suggest an important role in controlling lymphocyte function.
ACKNOWLEDGMENTS
We thank Ronald Herbst for critical comments and technical help, and we thank Maribel Andonian and Gary Burget for graphics.
DNAX Research Institute is supported by Schering Plough Corporation. S. Cohney is supported by a Jacquot Travelling Fellowship from the Royal Australasian College of Physicians and by the Australian & New Zealand Society of Nephrology.
REFERENCES
- 1.Adams T E, Hansen J A, Starr R, Nicola N A, Hilton D J, Billestrup N. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998;273:1285–1287. doi: 10.1074/jbc.273.3.1285. [DOI] [PubMed] [Google Scholar]
- 2.Auernhammer C J, Chesnokova V, Bousquet C, Melmed S. Pituitary corticotroph SOCS-3: novel intracellular regulation of leukemia-inhibitory factor-mediated proopiomelanocortin gene expression and adrenocorticotropin secretion. Mol Endocrinol. 1998;12:954–961. doi: 10.1210/mend.12.7.0140. [DOI] [PubMed] [Google Scholar]
- 3.Bjorbaek C, Elmquist J K, Frantz J D, Shoelson S E, Flier J S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 4.Cacalano N, Migone T, Bazan F, Hanson E, Chen M, Candotti F, O’Shea J J, Johnston J A. Autosomal SCID caused by a point mutation in the N-terminus of Jak3: mapping of the Jak3-receptor interaction domain. EMBO J. 1999;18:1549–1558. doi: 10.1093/emboj/18.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callus B A, Mathey-Prevot B. Interleukin-3-induced activation of the JAK/STAT pathway is prolonged by proteasome inhibitors. Blood. 1998;91:3182–3192. [PubMed] [Google Scholar]
- 6.Chung C D, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 7.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 8.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 10.Hilton D J, Richardson R T, Alexander W S, Viney E M, Willson T A, Sprigg N S, Starr R, Nicholson S E, Metcalf D, Nicola N A. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston J A, Kawamura M, Kirken R A, Chen Y Q, Blake T B, Shibuya K, Ortaldo J R, McVicar D W, O’Shea J J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 11a.Leonard W J, O’Shea J J. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 12.Masuhara M, Sakamoto H, Matsumoto A, Suzuki R, Yasukawa H, Mitsui K, Wakioka T, Tanimura S, Sasaki A, Misawa H, Yokouchi M, Ohtsubo M, Yoshimura A. Cloning and characterization of novel CIS family genes. Biochem Biophys Res Commun. 1997;239:439–446. doi: 10.1006/bbrc.1997.7484. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H, Miyajima A, Yoshimura A. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 14.Minamoto S, Ikegame K, Ueno K, Narazaki M, Naka T, Yamamoto H, Matsumoto T, Saito H, Hosoe S, Kishimoto T. Cloning and functional analysis of new members of STAT induced STAT inhibitor (SSI) family: SSI-2 and SSI-3. Biochem Biophys Res Commun. 1997;237:79–83. doi: 10.1006/bbrc.1997.7080. [DOI] [PubMed] [Google Scholar]
- 15.Mui A L, Wakao H, Kinoshita T, Kitamura T, Miyajima A. Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 16.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Accelerated apoptosis of lymphocytes by augmented induction of bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci USA. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 18.Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci USA. 1998;95:13130–13134. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neel B G. Role of phosphatases in lymphocyte activation. Curr Opin Immunol. 1997;9:405–420. doi: 10.1016/s0952-7915(97)80088-x. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson S E, Hilton D J. The SOCS proteins: a new family of negative regulators of signal transduction. J Leukoc Biol. 1998;63:665–668. doi: 10.1002/jlb.63.6.665. [DOI] [PubMed] [Google Scholar]
- 20a.Nicholson S E, Willson T A, Farley A, Starr R, Zhang J G, Baca M, Alexander W S, Metcalf D, Hilton D J, Nicola N A. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguchi M, Sarin A, Aman M J, Nakajima H, Shores E W, Henkart P A, Leonard W J. Functional cleavage of the common cytokine receptor gamma chain (gammac) by calpain. Proc Natl Acad Sci USA. 1997;94:11534–11539. doi: 10.1073/pnas.94.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Shea J J, Notrangelo L D, Johnston J A, Leonard W J, Candotti F. Advances in the understanding of cytokine signal transduction: the role of Jaks and STATs in immunoregulation and the pathogenesis of immunodeficiency. J Clin Immunol. 1997;17:431–447. doi: 10.1023/a:1027388508570. [DOI] [PubMed] [Google Scholar]
- 23.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto H, Yasukawa H, Masuhara M, Tanimura S, Sasaki A, Yuge K, Ohtsubo M, Ohtsuka A, Fujita T, Ohta T, Furukawa Y, Iwase S, Yamada H, Yoshimura A. A Janus kinase inhibitor, JAB, is an interferon-gamma-inducible gene and confers resistance to interferons. Blood. 1998;92:1668–1676. [PubMed] [Google Scholar]
- 25.Starr R, Metcalf D, Elefanty A G, Brysha M, Willson T A, Nicola N A, Hilton D J, Alexander W S. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 27.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdier F, Chretien S, Muller O, Varlet P, Yoshimura A, Gisselbrecht S, Lacombe C, Mayeux P. Proteasomes regulate erythropoietin receptor and signal transducer and activator of transcription 5 (STAT5) activation. Possible involvement of the ubiquitinated Cis protein. J Biol Chem. 1998;273:28185–28190. doi: 10.1074/jbc.273.43.28185. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins N A, Gilbert D J, Copeland N G, Hara T, Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu C L, Burakoff S J. Involvement of proteasomes in regulating Jak-STAT pathways upon interleukin-2 stimulation. J Biol Chem. 1997;272:14017–14020. doi: 10.1074/jbc.272.22.14017. [DOI] [PubMed] [Google Scholar]