Abstract
Background:
Evidence regarding lignan consumption in relation to coronary heart disease (CHD) risk remains limited and mixed.
Objective:
To prospectively examine associations between lignan intake and CHD risk in U.S. men and women.
Methods:
We prospectively followed 214,108 men and women in three cohorts who did not have cardiovascular disease or cancer at baseline. Diet was repeatedly assessed using a validated food frequency questionnaire every 2–4 years since baseline.
Results:
During 5,517,225 person-years of follow-up, we documented 10,244 CHD cases, including 6,283 non-fatal MI and 3,961 fatal CHD cases. In multivariable-adjusted analyses, comparing extreme quintiles, the pooled hazard ratios (HRs) of CHD (95% CIs) were 0.85 (0.79, 0.92) for total lignans, 0.76 (0.71, 0.82) for matairesinol, 0.87 (0.81, 0.93) for secoisolariciresinol, 0.89 (0.83, 0.95) for pinoresinol, and 0.89 (0.83, 0.95) for lariciresinol (All p values for trend≤ 0.003). Non-linear relationships were found for total lignan, matairesinol, and secoisolariciresinol: the risk reduction plateaued at intakes above approximately 300μg/d, 10μg/d, and 100μg/d, respectively (p<0.01 for all non-linearity). The inverse associations for total lignan intake appeared to be more apparent among participants with higher total fiber intake (p=0.04 for interaction). In addition, lignan intake was more strongly associated with plasma concentrations of enterolactone when fiber intake was higher.
Conclusions:
Increased long-term intake of lignans was associated with a significantly lower risk of total CHD in both men and women. Possible synergistic effects may exist between lignan and fiber intake in relation to CHD risk reduction, possibly through enhancing the production of enterolignans.
Keywords: Lignan, Coronary heart disease, Prospective cohorts
Condensed abstract:
The current study leveraged data from three large prospective U.S. cohort studies with updated diet information and over two decades of follow-up to examine associations of intake of total as well as four major individual lignans, including matairesinol, secoisolariciresinol, pinoresinol, and lariciresinol, in relation to CHD risk. Comparing participants in the extreme quintiles of consumption, 11% to 24% lower risks of CHD were observed for total and individual lignans. The inverse associations appeared to plateau at intake levels around approximately 300μg/d, 10μg/d, and 100μg/d for total lignan, matairesinol, and secoisolariciresinol, respectively. Other lignans showed more linear associations. Our findings support the notion that lignan can be a beneficial ingredient in healthy plant-based dietary patterns for the primary prevention of heart disease.
Lignans are polyphenolic substances that are produced by plant cells (1). Dietary lignans, including matairesinol, secoisolariciresinol, pinoresinol, and lariciresinol are primarily from intake of plant-based foods, especially seeds, whole grains, fruits, vegetables, wine, tea, and coffee. It is well-established that plant lignans can be efficiently processed by human gut microbiota to produce enterolignans, which are subsequently absorbed into human body (2). Experimental studies have shown that the enterolignans may improve cardiovascular health primarily through their estrogenic and anti-inflammatory effects (3,4). Through binding the estrogen receptors, enterolignans may inhibit inflammatory response to vascular injury and prevent atherosclerosis (5). In addition, enterolignans may also act as antioxidants and alleviate DNA damage and lipid peroxidation (6,7). In humans, higher circulating concentrations of enterolactone have been associated with lower risk of CHD in several prospective cohort studies, although the findings are not entirely consistent (8–10). Observational studies have also shown inverse association between plant lignan intake and ameliorated lipids profile, increased insulin sensitivity, higher flow-mediated dilation, reduced aortic stiffness, and lower metabolic syndrome score (11–14), although whether such improved cardiovascular disease (CVD) risk parameters could translate into lower coronary heart disease (CHD) risk is not established. One earlier prospective investigation in a European population did not observe overall beneficial associations between higher total lignan intake and CVD risk, despite a significantly lower risk of CHD found among ever smokers only (15). Moreover, given the various conversion efficiency to enterolignans and different food sources (2), it is unlikely that individual plant lignans have the same potency in improving cardiometabolic health. In the only previous prospective cohort study that examined individual plant lignan intake so far, only higher consumption of matairesinol was inversely associated with CVD and all-cause mortality while no associations were found for other individual lignans or total lignans (16). The mixed findings in these studies may be due to the lack of repeated measurements of lignan intake, relative short follow-up durations, and modest sample sizes.
In addition, the bioactive lignan metabolites, including enterolactone and enterodiol, are exclusively produced through gut microbiota fermentation. It is biologically plausible that prebiotics may potentially modulate the enterolignan production through modifying the gastrointestinal microbiota composition. Previous studies have shown an increased gut microbial diversity among people with higher fiber intake (17). However, evidence is lacking from large population-based studies regarding the interplay between lignan and fiber intake in relation to enterolignan production and whether such synergistic effects would enhance the cardioprotective effects of lignans has not been examined.
To provide a more comprehensive assessment of the associations between plant lignan intake and CHD risk and to explore the potential effect modification of dietary fiber, the current study has a two-fold aim: to examine intake of total lignans as well as the four individual lignans in relation to CHD risk and to investigate whether dietary fiber intake would enhance the associations between lignan intake and CHD risk in three large prospective cohorts of US men and women with dietary lignans repeatedly measured during over 30 years of follow-up.
Methods
Study population
The Nurses’ Health Study (NHS) was initiated in 1976, when 121,700 female registered nurses aged 30–55 years answered a mailed questionnaire on their medical history and lifestyle characteristics. A parallel cohort study of younger women, the Nurses’ Health Study II (NHSII), was established in 1989 and included 116,340 eligible female nurses aged 25–42 years. A questionnaire similar to that used in NHS was administered at baseline to assess medical history and lifestyle factors. In 1986, the Health Professionals Follow-up Study (HPFS) was started and recruited 51,529 U.S. male health professionals aged 40–75 years. The HPFS participants completed a baseline questionnaire that was similar to that used in the NHS and NHSII. In all three cohorts, participants were sent questionnaires biennially to update their demographic and lifestyle information and identify incident diseases. The cumulative response rates in three cohorts exceeded 90% (18,19). We also included data of enterolactone levels measured in plasma samples collected from 1,699 participants in two sub-studies in the cohorts (Supplemental methods).
In the primary analysis, the study baselines were set to be 1984 for NHS, 1991 for NHSII, and 1986 for HPFS when the major food sources of four lignans were assessed using a validated semi-quantitative food frequency questionnaire (FFQ). We excluded participants with prevalent cardiovascular disease or cancer at baseline, had unusual total energy intake (<500 or >3500 kcal/day for women and <800 or >4200 kcal/day for men), completed baseline questionnaire only, and had missing data on lignan intake. There were 77,354 participants from NHS, 93,504 from NHSII, and 43,250 HPFS included in the final analysis.
The study protocol was approved by the Human Research Committee of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health. Completion and return of study questionnaires implied informed consent of the participants.
Assessment of dietary lignan intake
In all three cohorts, diet was assessed using a validated FFQ at baseline and updated every 2–4 years during the follow-up. For each food item listed in the FFQ, participants were asked their average consumption frequency of a pre-specified portion size during the previous year. The average daily intake of individual lignans was calculated by multiplying the frequency of consumption of each lignan-containing food item by lignan content and then summing across from all foods. Total lignan intake was the sum of all four individual lignans. Because the intake as well as the major contributors of individual lignans changed during the follow-up, in Supplemental Figure 1 we listed top 10 food contributors of each individual lignan intake at baseline, middle of follow-up, and the end of follow-up. Of note, flaxseed intake was not included as an explicit item in the FFQ until 2006/2007 in the cohorts, which might explain the substantial increment of secoisolariciresinol intake in follow-up since 2006/2007. Based on data collected in 957 men participating in the Men’s Lifestyle Validation (MLVS) Study, Pearson correlation coefficient was 0.53 (p<0.0001) between total lignan intakes assessed by the FFQ and 7-day diet records (7DDR). We also observed a significant Pearson correlation of 0.30 (p<0.0001) between FFQ-assessed total dietary lignan intake and plasma enterolactone concentration.
Demographic and lifestyle factors assessment
Smoking status, vitamin supplements use, alcohol consumption, menopausal status (women only), and years of postmenopausal hormone use (women only), physician-diagnosed hypertension and hypercholesterinemia, and other time-varying variables were assessed at baseline and updated during follow-up. We also inquired about bowel movement frequency in 1982 in NHS and 2000 in HPFS. Height was reported at baseline, and body weight was updated biennially. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Recreational physical activity was measured using a validated questionnaire asking about the average time spent on 10 common activities. Based on this information, we calculated weekly energy expenditure in metabolic equivalents (METs) hours weighting each activity by its intensity level (20). Multiple validation studies demonstrated reasonable validity of these self-reported variables (21,22). Plant-based diet adherence was assessed by the healthy plant-based diet index (hPDI), a diet score positively rating healthy plant foods and inversely rating less healthy plant-based foods and animal foods (23).
Assessment of CHD
Total CHD including nonfatal myocardial infarction (MI) and fatal CHD was the primary disease outcome for the current analysis. Both definite and probable cases were included in the analysis because we found similar results in the definite case-only analysis. In all three cohorts, permission was sought to access medical records of participant who reported having a nonfatal MI on a follow-up questionnaire. Study physicians who were blinded to exposure status reviewed the medical records and confirmed/refuted reported MI cases according to the WHO criteria, which require the presence of symptoms, and either typical electrocardiographic changes or elevated cardiac enzyme levels (24,25). Deaths were identified through reports from the next of kin, the postal authorities, or by searching the National Death Index (NDI) (26). Fatal CHD was confirmed by a review of hospital records or autopsy reports if CHD was listed as the underlying cause of death and if evidence of previous CHD was available from medical records. Sudden deaths without cardiac causes were not considered as fatal CHD in the current analysis.
Statistical analysis
Due to the increasing trend of lignan intake during the follow-up, we presented participants characteristics at the median of the follow-up (2000 for NHS and HPFS, 2003 for NHS II). The total lignan intake as well as individual lignan intake were cumulatively averaged to reflect long-term usual intake. Person-years of follow-up for each participant were calculated from the return of the baseline questionnaires to the CHD diagnosis date, death date, date of last return of a valid follow-up questionnaire, or the end of follow-up (30 June 2014 in NHS, 30 June 2017 in NHSII, and 31 January 2016 in HPFS), whichever occurred first. To alleviate the potential reverse causality that participants with existing diseases might change their usual diet intake, we stopped updating diet once participants developed diabetes, stroke, coronary artery bypass graft, or cancer during follow-up (27). We replaced missing values with valid values in the preceding questionnaire for one follow-up cycle, and otherwise created missing indicators to handle remaining missing values.
An age- (months) and calendar time-stratified multivariable-adjusted Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between total lignans as well as individual lignan and risk of CHD. The proportional hazards assumption was evaluated by including an interaction term between categorical total lignan variable and the duration of follow-up, and we did not detect violations in the main analyses. Intakes of total and individual lignan intake were time-varying exposures and categorized into quintiles in the current analysis. Covariates considered in the multivariate models included ethnicity, time-varying BMI, smoking status, alcohol intake, multivitamin use, physical activity, hypertension, hypercholesterinemia, family history of myocardial infarction, postmenopausal hormone use, hPDI, and oral contraceptive use (women only). The median value of each individual lignan and total lignan consumption within each category was modeled as continuous variables to calculate HRs (95%CIs) and p value for trend. Data from each cohort were analyzed separately, combined estimates were calculated from a fixed-effect model. To explore the dose-response relationship between total as well as individual dietary lignan intake and CHD risk, we fitted cubic spline regressions with the same covariates adjusted in the primary analysis. Individual data from three cohorts were combined to increase statistical power and data were truncated at 1 and 99th percentiles of dietary lignan intake to limit the impact of extreme values. In the primary stratified analysis examining the interaction between dietary fiber intake and lignans on CHD risk, we calculated the HRs comparing higher and lower median of lignan intake by quintiles of fiber intake and adjusted for the same covariates as in the primary analysis. The p value for interaction was calculated by a product term between dichotomous lignan intake and continuous quintile ranks of fiber intake.
In light of the potential interactions between phytoestrogens and menopausal status and/or hormone use, in a secondary exploratory analysis, we also conducted stratified analysis by menopausal status and postmenopausal hormone use in the NHS and NHSII. In other exploratory analyses, we performed stratified analysis by baseline bowel movement frequency, as well as several other lifestyle factors including physical activity, BMI, smoking status, hPDI, and family history of MI. P values for interaction were calculated from the likelihood ratio tests comparing the full model including the product term between stratified variables and lignan intake (tertiles) with the reduced model without the product terms. Individual data were pooled from different cohorts in all stratified analyses and we used Bonferroni-corrected p value threshold 0.05/7=0.007 to account for potential multiple comparisons.
To examine whether the effect of individual lignans on CHD risk was independent of each other, we conducted a sensitivity analysis by mutually adjusting for four individual lignans in model. Due to the increase of total lignan intake in the middle of the follow-up attributed to the inclusion of flaxseed in lignan intake estimate, we repeated analysis after excluding participants who reported consuming flaxseed. All statistical tests were 2-sided with significant level of 0.05 and performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
At the median of follow-up across three cohorts, participants with higher total lignan intake were older and had more favorable health and lifestyle profiles including lower BMI, lower prevalence of hypertension and hypercholesterinemia, higher levels of physical activity, and better diet quality (Table 1). In all three cohorts, the intake levels of individual lignans were highly correlated with each other. During the entire follow-up, the age-adjusted Spearman correlations ranged from 0.37 (p<0.0001) between matairesinol and secoisolariciresinol in NHS to 0.78 (p<0.0001) between pinoresinol and lariciresinol in NHSII (Supplemental figure 1). Assessing the correlation between lignan intake with individual dietary factors, we found that matairesinol intake was particularly correlated with whole grain intake whereas secoisolariciresinol had the strongest correlations with wine intake. Higher pinoresinol and lariciresinol intake were predominantly correlated with higher fruit and vegetable intake and lower trans fat intake. Such correlation structure was consistent with the major food contributors for each individual lignan during the follow-up in three cohorts (Supplemental figure 2).
Table 1.
Age-standardized characteristics of study participants at median follow-up.
| Total lignans intake | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
|
|
||||||
| Nurses’ Health Study (2000) | ||||||
| No of participants | 14,153 | 14,154 | 14,153 | 14,154 | 14,154 | |
| Total lignans, μg/d1 | 173.4 (155.4,185.6) | 213.7 (205.1,221.8) | 246.4 (238.1,254.7) | 284.1 (273.5,296.4) | 352.6 (328.0,395.0) | |
| Matairesinol, μg/d1 | 5.2 (4.3,6.4) | 6.7 (5.5,8.3) | 8.0 (6.4,10.2) | 9.6 (7.4,12.8) | 13.5 (9.2,19.5) | |
| Secoisolariciresinol, μg/d1 | 60.0 (50.3,69.5) | 74.1 (65.2,83.4) | 82.9 (73.5,93.0) | 91.1 (80.7,102.3) | 103.9 (90.9,119.6) | |
| Pinoresinol, μg/d1 | 36.2 (30.6,42.1) | 46.6 (40.9,52.9) | 55.1 (48.8,62.2) | 65.7 (58.2,73.7) | 87.3 (75.5,103.6) | |
| Lariciresinol, μg/d1 | 66.1 (57.8,74.0) | 84.1 (76.5,91.6) | 98.4 (90.1,106.8) | 115.8 (105.5,126.3) | 149.2 (132.2,171.1) | |
| Age (years)* | 65.1 (7.3) | 66.0 (7.1) | 66.4 (7.1) | 66.8 (7.0) | 67.8 (6.9) | |
| Body mass index (kg/m2) | 25.6 (5.3) | 25.1 (4.6) | 25.0 (4.6) | 24.7 (4.4) | 24.2 (4.2) | |
| Race | ||||||
| - | White, % | 97.3 | 97.8 | 98.1 | 97.9 | 97.6 |
| - | African American, % | 1.7 | 1.3 | 1.0 | 1.1 | 1.2 |
| - | Asian, % | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 |
| - | Others, % | 0.7 | 0.7 | 0.6 | 0.7 | 0.8 |
| Physical activity(MET-h/wk) | 6.5 (1.7,16.0) | 8.4 (2.7,20.2) | 10.2 (3.2,22.4) | 11.7 (4.0,25.4) | 15.2 (5.2,30.7) | |
| Hypertension, % | 50.8 | 48.9 | 47.2 | 45.7 | 44.0 | |
| High cholesterol, % | 58.6 | 58.8 | 58.6 | 58.3 | 57.4 | |
| Smoking status | ||||||
| - | Never smokers, % | 48.9 | 46.5 | 45.1 | 43.0 | 41.7 |
| - | Past smokers, % | 40.0 | 43.9 | 46.3 | 48.5 | 50.3 |
| - | Current smokers, % | 11.0 | 9.6 | 8.6 | 8.5 | 8.0 |
| Family history of MI, % | 18.3 | 18.6 | 18.8 | 19.2 | 20.5 | |
| Multivitamin use, % | 53.9 | 58.9 | 62.0 | 63.7 | 65.2 | |
| Oral contraceptive use, % | 49.1 | 49.4 | 50.5 | 51.1 | 50.6 | |
| Hormone use | ||||||
| - | premenopausal, % | 2.7 | 2.5 | 2.1 | 2.5 | 2.0 |
| - | postmenopausal-never, % | 27.3 | 25.0 | 23.3 | 22.6 | 22.5 |
| - | postmenopausal-current, % | 41.9 | 44.5 | 46.4 | 47.3 | 46.0 |
| - | postmenopausal-past, % | 28.1 | 28.0 | 28.2 | 27.7 | 29.5 |
| Alcohol consumption (g/day) | 0.9 (0.0,4.6) | 1.8 (0.2,6.6) | 2.2 (0.4,7.7) | 2.9 (0.5,9.1) | 3.0 (0.5,9.6) | |
| Alternative healthy eating index | 43.3 (7.7) | 47.8 (7.4) | 50.7 (7.4) | 53.6 (7.6) | 58.4 (8.9) | |
| Total energy intake (Kcal/d) | 1726.0 (542.2) | 1743.1 (524.1) | 1755.4 (534.2) | 1737.6 (530.3) | 1740.9 (538.9) | |
|
| ||||||
| Nurses’ Health Study II (2003) | ||||||
| No of participants | 18,536 | 18,523 | 18,533 | 18,530 | 18,517 | |
| Total lignans, μg/d1 | 151.2 (131.5,165.8) | 198.4 (188.3,207.8) | 236.2 (226.4,246.4) | 281.3 (268.6,296.2) | 365.1 (334.8,416.8) | |
| Matairesinol, μg/d1 | 5.0 (3.9,6.6) | 7.0 (5.3,9.0) | 8.5 (6.4,10.9) | 10.4 (7.8,14.0) | 14.9 (10.5,22.6) | |
| Secoisolariciresinol, μg/d1 | 45.1 (36.2,55) | 62.7 (53.1,73) | 74.1 (63.5,85.5) | 85.1 (72.9,98) | 103.6 (87.7,121.1) | |
| Pinoresinol, μg/d1 | 33.8 (28.2,39.7) | 45.2 (39.3,51.5) | 54.3 (47.3,62) | 66.3 (57.6,75.3) | 90 (77,107.4) | |
| Lariciresinol, μg/d1 | 60.8 (51.8,69.3) | 80.9 (72.8,89.2) | 97.2 (87.5,107) | 117.8 (106.2,129.9) | 159.5 (139.9,187.4) | |
| Age (years)* | 41.5 (4.8) | 42.3 (4.6) | 42.8 (4.6) | 43.3 (4.5) | 43.8 (4.4) | |
| Body mass index (kg/m2) | 25.4 (6.2) | 24.8 (5.4) | 24.6 (5.2) | 24.5 (50) | 24.0 (4.7) | |
| Race | ||||||
| - | White, % | 95.3 | 96.5 | 96.8 | 96.9 | 96.4 |
| - | African American, % | 0.3 | 0.5 | 0.5 | 0.5 | 0.6 |
| - | Asian, % | 1.9 | 1.5 | 1.4 | 1.4 | 1.6 |
| - | Others, % | 2.5 | 1.5 | 1.2 | 1.2 | 1.3 |
| Physical activity(MET-h/wk) | 10.3 (3.4,30.9) | 11.9 (4.2,30.9) | 13.4 (5.1,34) | 15.7 (6.2,37.7) | 20.4 (8.3,47.4) | |
| Hypertension, % | 12.2 | 11.0 | 10.0 | 9.8 | 8.9 | |
| High cholesterol, % | 24.0 | 21.9 | 20.8 | 20.7 | 20.0 | |
| Smoking status | ||||||
| - | Never smokers, % | 72.8 | 67.5 | 64.7 | 61.7 | 59.8 |
| - | Past smokers, % | 17.3 | 22.0 | 24.9 | 27.3 | 29.8 |
| - | Current smokers, % | 9.9 | 10.5 | 10.5 | 11.0 | 10.4 |
| Family history of MI, % | 48.6 | 48.9 | 48.7 | 49.7 | 49.0 | |
| Multivitamin use, % | 39.0 | 43.2 | 45.6 | 48.2 | 51.7 | |
| Oral contraceptive use, % | 78.2 | 79.3 | 79.4 | 78.9 | 77.2 | |
| Hormone use | ||||||
| - | premenopausal, % | 89.2 | 89.2 | 89.4 | 89.8 | 89.1 |
| - | postmenopausal-never, % | 1.7 | 1.8 | 1.8 | 1.7 | 1.8 |
| - | postmenopausal-current, % | 8.1 | 8.0 | 8.0 | 7.4 | 8.0 |
| - | postmenopausal-past, % | 1.0 | 1.0 | 0.8 | 1.0 | 1.2 |
| Alcohol consumption (g/day) | 0 (0,1.8) | 0.9 (0,3) | 1.4 (0,4.2) | 1.7 (0,5.2) | 1.9 (0,6) | |
| Alternative healthy eating index | 51.1 (5.3) | 53.4 (5.4) | 54.7 (5.5) | 55.8 (5.6) | 57.5 (5.8) | |
| Total energy intake (Kcal/d) | 1789 (518.8) | 1811.9 (503.8) | 1810.4 (496.5) | 1803.1 (499.9) | 1782.2 (503.8) | |
|
| ||||||
| Health Professionals Follow-up Study (2000) | ||||||
| No of participants | 7,578 | 7,579 | 7,579 | 7,579 | 7,578 | |
| Total lignans, μg/d1 | 194.2 (170.2,211) | 249.8 (238.2,260.9) | 296.1 (284.1,308.7) | 351.6 (335.7,370.1) | 458.2 (419.9,518.8) | |
| Matairesinol, μg/d1 | 6 (4.9,7.7) | 7.8 (6.2,10.2) | 9.6 (7.2,13) | 11.9 (8.6,17.5) | 18.1 (10.7,28.8) | |
| Secoisolariciresinol, μg/d1 | 63.4 (52.5,75.5) | 81 (69.7,93.1) | 91.5 (79.4,104.6) | 101.9 (88.2,117.2) | 116.6 (98.7,138.9) | |
| Pinoresinol, μg/d1 | 41.5 (34,49.4) | 57.5 (49.7,66.3) | 70.8 (61.7,81) | 88 (76.2,101.6) | 124.8 (104.6,158.3) | |
| Lariciresinol, μg/d1 | 75.3 (64.5,85.5) | 99.9 (90.3,109.7) | 119.7 (109,131.2) | 144.5 (131,159.1) | 192.9 (168.5,224.7) | |
| Age (years)* | 64.9 (9.2) | 65.7 (9.1) | 65.6 (9) | 66.1 (8.9) | 66.8 (8.9) | |
| Body mass index (kg/m2) | 26.5 (4) | 26.4 (3.8) | 26.3 (3.8) | 26.1 (3.7) | 25.7 (3.7) | |
| Race | ||||||
| - | White, % | 94.2 | 95.5 | 94.9 | 95.4 | 94.9 |
| - | African American, % | 2.4 | 2.0 | 2.3 | 2.0 | 2.4 |
| - | Asian, % | 1.9 | 1.6 | 1.8 | 1.8 | 1.8 |
| - | Others, % | 1.5 | 0.9 | 0.9 | 0.7 | 0.9 |
| Physical activity(MET-h/wk) | 15.2 (4.1,35.6) | 18.9 (6.1,39.4) | 21.3 (7.4,42.2) | 24.2 (8.6,47.2) | 26.1 (9.9,50.5) | |
| Hypertension, % | 42.1 | 42.2 | 40.8 | 40.3 | 40.4 | |
| High cholesterol, % | 47.5 | 49.5 | 49.5 | 50.1 | 50.1 | |
| Smoking status | ||||||
| - | Never smokers, % | 50.6 | 45.2 | 44.7 | 43.9 | 43.0 |
| - | Past smokers, % | 42.3 | 49.2 | 50.1 | 51.9 | 52.6 |
| - | Current smokers, % | 7.0 | 5.6 | 5.2 | 4.2 | 4.4 |
| Family history of MI, % | 11.7 | 11.9 | 11.3 | 12.5 | 12.3 | |
| Multivitamin use, % | 43.9 | 50.3 | 53.8 | 56.1 | 57.2 | |
| Alcohol consumption (g/day) | 2.1 (0,8.1) | 5.2 (1.0,12.8) | 7.3 (1.8,15.2) | 8.7 (2.6,17.1) | 10.3 (2.8,23.2) | |
| Alternative healthy eating index | 49.5 (5.7) | 52.4 (5.4) | 54.4 (5.3) | 56.6 (5.5) | 60 (6) | |
| Total energy intake (Kcal/d) | 1980 (562) | 1987 (541) | 1990 (542) | 1979 (527) | 1971 (526) | |
Value is not age adjusted
Value is median (interquartile range).
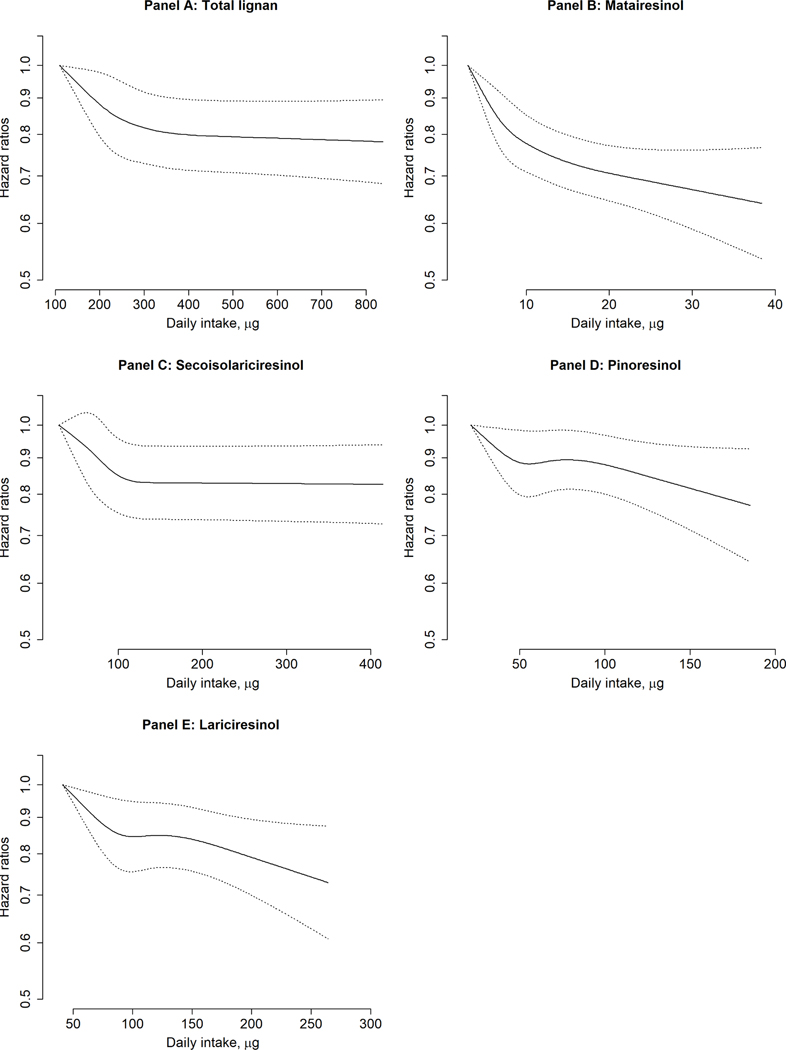
During 5,517,225 person-years of follow-up in three cohorts, we documented 10,244 CHD cases, of which 6,283 cases were nonfatal MI and 3,961 cases were fatal CHD. Higher total lignan intake as well as all individual lignan intake were associated with significantly lower risk of total CHD. In multivariable-adjusted model comparing extreme quintiles, the HRs (95% CIs) were 0.85 (0.89, 0.92) for total lignan (p trend <0.001), 0.76 (0.71, 0.82) for matairesinol (p trend <0.001), 0.87 (0.81, 0.93) for secoisolariciresinol (p trend=0.001), 0.89 (0.83, 0.95) for pinoresinol (p trend=0.002), and 0.89 (0.83, 0.95) for lariciresinol (p trend=0.003) (Table 2). The inverse associations were observed for total lignan, matairesinol, and secoisolariciresinol intake in relation to both nonfatal MI and fatal CHD risk, although pinoresinol and lariciresinol intake was not associated with fatal CHD risk. In the cubic spline regression, we found that the total CHD risk reduction flattened after ~300μg/d of total lignan intake (p=0.009 for non-linearity), ~10μg/d of matairesinol intake (p=0.004 for non-linearity) and ~100μg/d of secoisolariciresinol (p=0.002 for non-linearity) whereas the relationship between pinoresinol and lariciresinol and CHD risk appeared to be more linear (Central Illustration). One SD increment of pinoresinol and lariciresinol intake was associated with 3% [95% CI: (1%, 5%)] and 4% [95% CI: (2%, 6%)] lower risk of CHD. The estimates of HR were similar in men and women for total lignan whereas the inverse associations for matairesinol were significantly stronger in women than men (p for interaction 0.001; Supplemental table 1).
TABLE 2.
Pooled Associations Between Lignans Intake and CHD Risk in NHS (1984–2014), NHSII (1991–2017), HPFS (1986–2016)a
| Q1 | Q2 | Q3 | Q4 | Q5 | P for Trendb | |
|---|---|---|---|---|---|---|
| Total lignans | ||||||
| Median (IQR), μg/dc | 167.1 (146.1–182.9) | 215.3 (204.1–227.1) | 254.5 (242.6–267.6) | 302.7 (286.3–320.5) | 405.2 (362.0–491.8) | |
| Total CHD | ||||||
| Case/person-year | 2,344/1,099,137 | 2,128/1,101,960 | 2,002/1,104,119 | 1,977/1,105,423 | 1,793/1,106,586 | |
| Age-adjusted | 1 | 0.85 (0.80–0.90) | 0.79 (0.74–0.84) | 0.75 (0.71–0.80) | 0.65 (0.61–0.69) | <0.001 |
| Multivariable-adjustedd | 1 | 0.94 (0.89–1.00) | 0.93 (0.87–0.99) | 0.93 (0.87–1.00) | 0.85 (0.79–0.92) | <0.001 |
| Nonfatal MI | ||||||
| Case/person-year | 1,398/1,099,145 | 1,316/1,101,965 | 1,231/1,104,121 | 1,252/1,105,427 | 1,086/1,106,594 | |
| Age-adjusted | 1 | 0.89 (0.82–0.96) | 0.81 (0.75–0.88) | 0.80 (0.75–0.87) | 0.67 (0.62–0.72) | <0.001 |
| Multivariable-adjustedd | 1 | 0.96 (0.89–1.04) | 0.94 (0.86–1.02) | 0.98 (0.90–1.07) | 0.88 (0.80–0.97) | 0.07 |
| Fatal CHD | ||||||
| Case/person-year | 946/1,100,426 | 812/1,103,217 | 771/1,105,286 | 725/1,106,584 | 707/1,107,604 | |
| Age-adjusted | 1 | 0.80 (0.72–0.87) | 0.75 (0.68–0.82) | 0.68 (0.61–0.75) | 0.63 (0.57–0.69) | <0.001 |
| Multivariable-adjustedd | 1 | 0.91 (0.83–1.00) | 0.92 (0.83–1.02) | 0.87 (0.78–0.97) | 0.82 (0.73–0.92) | 0.002 |
| Matairesinol | ||||||
| Median (IQR), μg/dc | 4.5 (3.9–5.2) | 6.5 (5.7–7.2) | 8.5 (7.3–9.6) | 11.5 (9.9–13.0) | 18.9 (15.9–24.2) | |
| Total CHD | ||||||
| Case/person-year | 2,387/1,096,970 | 2,114/1,101,975 | 1,999/1,104,802 | 1,903/1,106,655 | 1,841/1,106,820 | |
| Age-adjusted | 1 | 0.86 (0.81–0.91) | 0.78 (0.74–0.83) | 0.70 (0.66–0.74) | 0.61 (0.57–0.64) | <0.001 |
| Multivariable-adjustedd | 1 | 0.93 (0.87–0.99) | 0.89 (0.84–0.94) | 0.83 (0.78–0.89) | 0.76 (0.71–0.82) | <0.001 |
| Nonfatal MI | ||||||
| Case/person-year | 1,419/1,096,978 | 1,259/1,101,981 | 1,273/1,104,807 | 1,214/1,106,660 | 1,118/1,106,826 | |
| Age-adjusted | 1 | 0.86 (0.80–0.93) | 0.85 (0.79–0.91) | 0.77 (0.71–0.83) | 0.66 (0.61–0.72) | <0.001 |
| Multivariable-adjustedd | 1 | 0.89 (0.82–0.96) | 0.90 (0.83–0.97) | 0.85 (0.78–0.92) | 0.77 (0.71–0.84) | <0.001 |
| Fatal CHD | ||||||
| Case/person-year | 968/1,098,295 | 855/1,103,132 | 726/1,105,989 | 689/1,107,811 | 723/1,107,888 | |
| Age-adjusted | 1 | 0.86 (0.78–0.94) | 0.69 (0.63–0.76) | 0.60 (0.54–0.66) | 0.53 (0.48–0.59) | <0.001 |
| Multivariable-adjustedd | 1 | 0.99 (0.90–1.09) | 0.86 (0.78–0.95) | 0.80 (0.72–0.89) | 0.76 (0.68–0.84) | <0.001 |
| Secoisolariciresinol | ||||||
| Median (IQR), μg/dc | 49.9 (42.0–55.7) | 67.9 (63.8–71.7) | 81.1 (77.2–84.9) | 95.6 (90.9–100.5) | 122.9 (111.6–149.8) | |
| Total CHD | ||||||
| Case/person-year | 2,197/1,099,679 | 2,186/1,102,049 | 2,010/1,103,139 | 2,006/1,105,836 | 1,845/1,106,520 | |
| Age-adjusted | 1 | 0.90 (0.85–0.96) | 0.81 (0.76–0.86) | 0.78 (0.74–0.83) | 0.70 (0.66–0.74) | <0.001 |
| Multivariable-adjustedd | 1 | 0.99 (0.93–1.05) | 0.92 (0.86–0.98) | 0.93 (0.87–1.00) | 0.87 (0.81–0.93) | 0.001 |
| Nonfatal MI | ||||||
| Case/person-year | 1,299/1,099,683 | 1,334/1,102,056 | 1,230/1,103,147 | 1,266/1,105,841 | 1,154/1,106,526 | |
| Age-adjusted | 1 | 0.95 (0.88–1.03) | 0.85 (0.79–0.92) | 0.85 (0.79–0.92) | 0.75 (0.69–0.81) | <0.001 |
| Multivariable-adjustedd | 1 | 1.01 (0.94–1.10) | 0.94 (0.87–1.02) | 0.98 (0.90–1.07) | 0.92 (0.84–1.00) | 0.19 |
| Fatal CHD | ||||||
| Case/person-year | 898/1,100,909 | 852/1,103,281 | 780/1,104,294 | 740/1,107,030 | 691/1,107,602 | |
| Age-adjusted | 1 | 0.82 (0.75–0.91) | 0.74 (0.67–0.82) | 0.69 (0.62–0.76) | 0.62 (0.56–0.69) | <0.001 |
| Multivariable-adjustedd | 1 | 0.95 (0.86–1.04) | 0.89 (0.80–0.98) | 0.87 (0.78–0.96) | 0.80 (0.72–0.90) | <0.001 |
| Pinoresinol | ||||||
| Median (IQR), μg/dc | 34.3 (29.3–38.6) | 47.3 (43.9–51.0) | 58.8 (54.9–62.8) | 73.3 (68.5–78.4) | 102.6 (91.6–122.4) | |
| Total CHD | ||||||
| Case/person-year | 2,454/1,097,715 | 2,064/1,102,943 | 1,945/1,104,651 | 1,973/1,105,866 | 1,808/1,106,048 | |
| Age-adjusted | 1 | 0.83 (0.78–0.88) | 0.77 (0.72–0.82) | 0.76 (0.71–0.80) | 0.67 (0.63–0.71) | <0.001 |
| Multivariable-adjustedd | 1 | 0.92 (0.87–0.98) | 0.91 (0.86–0.97) | 0.95 (0.89–1.02) | 0.89 (0.83–0.95) | 0.002 |
| Nonfatal MI | ||||||
| Case/person-year | 1,474/1,097,720 | 1,280/1,102,949 | 1,205/1,104,654 | 1,236/1,105,871 | 1,088/1,106,056 | |
| Age-adjusted | 1 | 0.85 (0.79–0.91) | 0.79 (0.73–0.85) | 0.79 (0.73–0.85) | 0.67 (0.62–0.73) | <0.001 |
| Multivariable-adjustedd | 1 | 0.92 (0.85–0.99) | 0.90 (0.83–0.98) | 0.95 (0.88–1.03) | 0.89 (0.81–0.97) | 0.03 |
| Fatal CHD | ||||||
| Case/person-year | 980/1,099,077 | 784/1,104,155 | 740/1,105,785 | 737/1,107,047 | 720/1,107,053 | |
| Age-adjusted | 1 | 0.80 (0.72–0.87) | 0.74 (0.68–0.82) | 0.71 (0.65–0.79) | 0.66 (0.60–0.73) | 0.0001 |
| Multivariable-adjustedd | 1 | 0.93 (0.85–1.03) | 0.93 (0.84–1.03) | 0.96 (0.86–1.06) | 0.89 (0.80–0.99) | 0.05 |
| Lariciresinol | ||||||
| Median (IQR), μg/dc | 63.7 (55.3–70.0) | 84.1 (79.6–88.9) | 101.9 (97.2–106.9) | 123.9 (117.5–131.2) | 166.7 (150.9–194.0) | |
| Total CHD | ||||||
| Case/person-year | 2,390/1,099,878 | 2,041/1,102,948 | 1,991/1,104,893 | 1,935/1,104,805 | 1,887/1,104,696 | |
| Age-adjusted | 1 | 0.82 (0.78–0.87) | 0.80 (0.75–0.85) | 0.76 (0.71–0.81) | 0.70 (0.66–0.74) | <0.001 |
| Multivariable-adjustedd | 1 | 0.92 (0.86–0.97) | 0.94 (0.88–1.00) | 0.92 (0.86–0.99) | 0.89 (0.83–0.95) | 0.003 |
| Nonfatal MI | ||||||
| Case/person-year | 1,444/1,099,886 | 1,248/1,102,954 | 1,266/1,104,898 | 1,225/1,104,811 | 1,100/1,104,701 | |
| Age-adjusted | 1 | 0.84 (0.77–0.90) | 0.83 (0.77–0.90) | 0.79 (0.73–0.85) | 0.68 (0.63–0.74) | <0.001 |
| Multivariable-adjustedd | 1 | 0.91 (0.84–0.99) | 0.96 (0.89–1.04) | 0.95 (0.88–1.04) | 0.88 (0.80–0.97) | 0.04 |
| Fatal CHD | ||||||
| Case/person-year | 946/1,101,229 | 793/1,104,119 | 725/1,106,115 | 710/1,105,937 | 787/1,105,716 | |
| Age-adjusted | 1 | 0.80 (0.73–0.88) | 0.74 (0.67–0.82) | 0.71 (0.64–0.78) | 0.73 (0.66–0.80) | <0.001 |
| Multivariable-adjustedd | 1 | 0.92 (0.83–1.01) | 0.90 (0.81–0.99) | 0.87 (0.79–0.97) | 0.89 (0.80–1.00) | 0.04 |
Values are hazard ratio (95% confidence interval) unless otherwise indicated.
Hazard ratios were meta-analyzed using fixed effect models.
Median value in each quintile category was used to calculate P value for trend.
Data were combined from 3 cohorts to calculate the median (IQR).
Models were age- (months) and calendar-time stratified and adjusted for ethnicity (White, Black, Asian, others), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes per day, 15–24 cigarettes per day, or $25 cigarettes per day), time-varying body mass index (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–32.9, 33.0–34.9, or $35.0 kg/m2), alcohol intake (0, 0.1–4.9, 5.0– 9.9, 10.0–14.9, 15.0–29.9, and $30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), healthy plant-based diet index (quintiles), and family history of myocardial infarction. For women, postmenopausal hormone use (premenopausal, never, former, current, or missing), and oral contraceptive use were further adjusted.
CHD = coronary heart disease; IQR = interquartile range; other abbreviations as in Table 1.
Central Illustration. Dose-response relationships between lignan intake and CHD risk.
Data were truncated between 1st −99th percentile value. The axis for hazard ratio is in natural log-scale. Models were age- (months) and calendar-time stratified and adjusted for ethnicity (white, African American, Asian, others), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes per day, 15–24 cigarettes per day, or ≥25 cigarettes per day), time-varying BMI (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–32.9, 33.0–34.9, or ≥35.0 kg/m2), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), healthy plant-based diet index (quintiles), and family history of myocardial infarction. Panel A: P value for non-linearity: 0.003.Panel B: P value for non-linearity: <0.001.Panel C: P value for non-linearity: <0.001.Panel D: P value for non-linearity: 0.17. Panel E: P value for non-linearity: 0.11.
The inverse associations between total lignan intake and CHD risk appeared to be more apparent among participants with higher total fiber intake (p=0.04 for interaction), although none of the associations achieved statistical significance (Table 3). Further analysis suggested that this potential effect modifications appeared to be somewhat more pronounced for insoluble fiber intake than soluble fiber intake in total fiber, although neither interaction tests achieved statistical significance. In secondary analyses for individual lignans, only intake of secoisolariciresinol was significantly more strongly associated with lower CHD risk among individuals who consumed higher levels of fiber (p for interaction=0.007, 0.004, and 0.008 for total fiber, soluble fiber, and insoluble fiber) (Supplemental Table 2).
Table 3.
| Nonfatal MI | ||||||
| Case/person-year | 1,444/1,099,886 | 1,248/1,102,954 | 1,266/1,104,898 | 1,225/1,104,811 | 1,100/1,104,701 | |
| Age-adjusted | 1 | 0.84 (0.77, 0.90) | 0.83 (0.77, 0.90) | 0.79 (0.73, 0.85) | 0.68 (0.63, 0.74) | <0.001 |
| Multivariable-adjusted4 | 1 | 0.91 (0.84, 0.99) | 0.96 (0.89, 1.04) | 0.95 (0.88, 1.04) | 0.88 (0.80, 0.97) | 0.04 |
| Fatal CHD | ||||||
| Case/person-year | 946/1,101,229 | 793/1,104,119 | 725/1,106,115 | 710/1,105,937 | 787/1,105,716 | |
| Age-adjusted | 1 | 0.80 (0.73, 0.88) | 0.74 (0.67, 0.82) | 0.71 (0.64, 0.78) | 0.73 (0.66, 0.80) | <0.001 |
| Multivariable-adjusted4 | 1 | 0.92 (0.83, 1.01) | 0.90 (0.81, 0.99) | 0.87 (0.79, 0.97) | 0.89 (0.80, 1.00) | 0.04 |
Hazard ratios were meta-analyzed using fixed effect models.
Median value in each quintile category was used to calculate p value for trend.
Data were combined from three cohorts to calculate the median (IQR).
Models were age- (months) and calendar-time stratified and adjusted for ethnicity (white, African American, Asian, others), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes per day, 15–24 cigarettes per day, or ≥25 cigarettes per day), time-varying BMI (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–32.9, 33.0–34.9, or ≥35.0 kg/m2), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), healthy plant-based diet index (quintiles), and family history of myocardial infarction. For women, postmenopausal hormone use (premenopausal, never, former, current, or missing), and oral contraceptive use were further adjusted.
Based on both FFQ and 7DDR-assessed lignan and fiber intake, we observed potential, synergistic effects between these two dietary components on enhancing the enterolactone production (Supplemental table 3). Using FFQ-assessed lignan intake in a nested case-control study, higher lignan intake was associated with significantly higher enterolactone levels when the intake of fiber was also higher (p=0.01 for interaction). Such interaction was primarily driven by insoluble fiber (p=0.03 for interaction) but less pronounced for soluble fiber (p=0.10 for interaction). For individual lignan, this pattern of interactions was primarily observed for secoisolariciresinol intake (p for interaction=0.01 for both total fiber and insoluble fiber). The corresponding estimates using 7DDR-assesed lignan intake in the MLVS showed similar direction of interactions, although the p values for interaction were not statistically significant.
The inverse associations of total lignan intake appeared to be slightly stronger among premenopausal women while similar associations were found for postmenopausal women with and without hormone use (Supplemental table 4). The associations were similar across subgroups defined by bowel movement frequency, body mass index, smoking status, healthy plant-based diet index or family history of MI (Supplemental table 5), except that we observed stronger inverse associations of total lignan intake among participants with higher physical activity level (p=0.001 for interaction).
In the sensitivity analysis that mutually adjusted for four individual lignans, the estimates for matairesinol and secoisolariciresinol did not substantially change while the associations for pinoresinol and lariciresinol were attenuated to null (Supplemental table 6). In the analysis excluding participants consuming flaxseeds, we found similar results for total lignans as well as four individual lignan intake in relation to total CHD risk (Supplemental table 7).
Discussion
In the current study, higher intake of total lignans, as well as four major individual lignans, was significantly associated with a lower risk of CHD. These inverse associations were independent of established and potential risk factors of CHD. Dose-response analyses showed a non-linear relationship for total lignan, matairesinol, and secoisolariciresinol in that the risk reduction plateaued at intakes above approximately 300μg/d, 10μg/d, and 100μg/d of intake, respectively. Potential synergistic interactions were found between lignans, especially secoisolariciresinol, and dietary fiber with higher circulating enterolactone levels and lower CHD risk. We found a stronger inverse association of total lignan intake for participants with higher physical activity level while the associations were largely similar in other subgroups such as adherence to healthy plant-based diet, family history of MI, BMI, menopausal status and hormone use or gastrointestinal physiology profile as reflected by bowel movement frequency.
It has been proposed that the cardio-protective effects of lignans may be mediated by the improvement of traditional CVD risk factors, although the findings from clinical studies were not entirely consistent. Some meta-analysis of dietary intervention studies have suggested that flaxseed supplementation may lower blood pressure (28), reduce circulating concentrations of inflammatory biomarkers and adhesion molecules (29,30), and improve blood lipids profiles (31), whereas a fiber-rich diet that might contain significant amount of lignans did not improve blood pressure and other CVD risk factors among diabetes patients (32). Only a couple prospective cohort studies have been conducted to specifically examine the associations of lignan intake with CHD risk. In the Dutch Prospect-EPIC cohort, no association was observed between higher total lignan intake and CHD risk among 16,165 individuals during ~6 years of follow-up (15). In the Zutphen Elderly Study, a prospective cohort study in which 570 Dutch men aged 64–84 years old were followed for 15 years, total lignan intake was not associated with mortality (16). In particular, this study also examined the associations for four individual lignans and only matairesinol intake was associated with lower CVD mortality. The current analysis addressed several limitations in these previous studies, including small sample size, relatively short duration of follow-up, and lack of repeated measurements of diet. In particular, through our repeated assessments of diet, we were able to depict and account for the dynamic time-trend of lignan intake. We found that long-term intake of both total lignans and all four individual lignans were associated with a lower risk of developing CHD, although the inverse associations may not be linear in that the dose-response relationship plateaued at higher intake levels for total lignans and certain individual lignans. It is worth noticing that in European populations the estimated median total lignan intake was nearly 1,000 μg/d (33), in contrast to around 200 μg/d in the current study. This difference in intake levels might be ascribed to the fact that European populations on average consumed more lignan-rich foods, such as whole grains, fruits, and vegetables than the US population (34,35). It is thus possible that the inverse association may be more likely to detect in populations with overall lower intake of lignans, although further studies are needed to substantiate these non-linear relationships.
It is well established that plant lignans are processed by human gut microbiota to produce more bioactive enterolignans, which are subsequently absorbed into human body (2). Higher circulating concentrations of enterolignans have been associated with lower risk of type 2 diabetes, cardiovascular mortality, and less weight gain (36–38). However, feeding studies among human participants observed significant differences of efficiency in enterolignan productions in participants consuming the same amount of plant lignans (39,40). Several incubation studies of human fecal samples suggested that such interindividual differences in lignan metabolisms were primarily attributed to the variability of microbiota compositions (41,42), suggesting that the gut microbiome may play an essential role in determining cardioprotective effects of lignan consumption. Although in the current analyses we were not able to directly assess the mediation effects of gut microbiota in the lignan-CHD associations, we observed stronger inverse associations of secoisolariciresinol among participants consuming more dietary fiber, the major prebiotics in our diet known to modulate microbial composition and functions. Moreover, we also observed synergistic interactions between intake of total as well as secoisolariciresinol and fiber intake on the circulating levels of enterolignans, and the effect modifications appeared to be stronger for insoluble fiber than soluble fiber. These findings are in line with an in vitro experimental study demonstrating that fecal suspensions incubated with insoluble fiber produced significantly more enterolignans comparing with soluble fibers because the insoluble fiber formed a more neutral pH range favoring enterolignan production (43). Clearly, more mechanistic studies and human microbiota research are warranted to further explore the potential interactions between lignans and fiber or other prebiotics.
The main strength of our current study is the comprehensive assessment of four individual lignans coupled with large sample size, long follow-up period, and repeated measurement of dietary intake as well as lifestyle factors. Several limitations merit discussion. First, the intake of flaxseeds, the single dominant food contributor to secoisolariciresinol intake, was not assessed in the FFQ until later stage of follow-up, and therefore, the total dietary intake lignan level might be underestimated at early follow-up. However, the flaxseed consumption was low in all three cohorts and the results were largely unchanged after removing data of participants who consumed flaxseeds. Second, the measurement error for dietary lignan assessment might affect the results. However, the measurement error is likely to be nondifferential with regard to the assessment of CHD and therefore would be more likely to attenuate the estimates towards null. Moreover, the use of cumulative average intake to represent long-term intake also helped to reduce the random measurement error and within-person variations. Third, we were unable to directly evaluate whether the inverse association between plant lignans and CHD risk might be mediated by the production of enterolignans. Fourth, because our analysis involved both total and individual lignans, the multiple comparisons issue may inflate the type 1 error, particularly for the subgroup analyses. However, the associations in main analysis between lignan intake and CHD were highly significant and remained robust even after the Bonferroni correction. Finally, our study population consisted of predominantly Caucasian health professions and so the generalizability of our findings may be limited.
Conclusions
In three large prospective cohorts of women and men, higher total lignan intake as well as individual lignan intake including matairesinol, secoisolariciresinol, pinoresinol, and lariciresinol were associated with significantly lower risk of total CHD. The inverse associations between secoisolariciresinol intake and CHD risk appeared be more pronounced among participants with higher fiber intake, which might also significantly enhance the circulating enterolactone levels at higher lignan intake levels. Our findings are in line with the recommendation of adhering to healthy plant-based dietary patterns that emphasize increased consumption of lignan-containing foods such as whole grains, fruits/vegetables, flax seed products, and coffee for the primary prevention of heart disease. The role of lignan intake, as well as the microbial processing of plant lignans, in the etiology of CHD deserves further investigation in future research.
Supplementary Material
| Fiber intake quintiles | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | P for interaction | |
| Total fiber intake | 1.01(0.89,1.16) | 1.09(0.98,1.22) | 1.06(0.96,1.17) | 0.90(0.82,1.00) | 0.93(0.82,1.05) | 0.04 |
| Soluble fiber intake | 0.98(0.86,1.12) | 1.04(0.92,1.07) | 0.98(0.88,1.08) | 0.92(0.83,1.01) | 0.98(0.88,1.09) | 0.19 |
| Insoluble fiber intake | 1.03(0.91,1.17) | 0.96(0.86,1.08) | 1.04(0.94,1.15) | 0.97(0.88,1.07) | 0.89(0.80,0.99) | 0.09 |
Comparing high vs low total lignan intake.
Data were combined from three cohorts.
Models were age- (months) and calendar-time stratified and adjusted for ethnicity (white, African American, Asian, others), smoking status (never smoked, past smoker, currently smoke 1–14 cigarettes per day, 15–24 cigarettes per day, or ≥25 cigarettes per day), time-varying BMI (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–32.9, 33.0–34.9, or ≥35.0 kg/m2), alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, and ≥30.0 g/d), multivitamin use (yes, no), physical activity (quintiles), healthy plant-based diet index (quintiles), and family history of myocardial infarction. For women, postmenopausal hormone use (premenopausal, never, former, current, or missing), and oral contraceptive use were further adjusted.
Acknowledgements:
We thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions.
Funding: The NHS, NHSII, HPFS, and the current analysis are supported by grants (UM1 CA186107, P01 CA87969, R01 CA49449, R01 HL034594, R01 HL088521, U01 CA176726, R01 CA67262, UM1 CA167552, R01 HL035464, R01 HL060712, R01 DK120870) from the National Institutes of Health. The funding sources did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations and Acronyms
- CVD
cardiovascular disease
- CHD
coronary heart disease
- MI
myocardial infarction
- FFQ
food frequency questionnaire
- 7DDR
7-day diet records
- BMI
body mass index
- METs
metabolic equivalents
- hPDI
healthy plant-based diet index
- HR
hazard ratio
- CI
confidence interval
Footnotes
Disclosure statement: All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Twitter handle:@hpfscohort.
Twitter text: This new study shows that diets rich in lignans, a group of beneficial phytoestrogens, may help to keep coronary heart disease risk at bay.
PERSPECTIVES
Competency in Medical Knowledge: Long-term intake of lignans is associated with a reduced risk of developing coronary artery disease in Americans, and dietary fiber may strengthen this association.
Translational Outlook: Additional studies are needed to confirm the interaction between dietary fiber and lignans and their impact on cardiovascular health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr Rev 2010;68(10):571–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinonen S, Nurmi T, Liukkonen K, et al. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem 2001;49(7):3178–86. [DOI] [PubMed] [Google Scholar]
- 3.Lignans Adlercreutz H. and human health. Crit Rev Clin Lab Sci 2007;44(5–6):483–525. [DOI] [PubMed] [Google Scholar]
- 4.Landete JM., Arqués J, Medina M, Gaya P, de Las Rivas BD, Muñoz R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit Rev Food Sci Nutr 2016;56(11):1826–43. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn ME., Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999;340(23):1801–11. [DOI] [PubMed] [Google Scholar]
- 6.Kitts DD., Yuan YV, Wijewickreme AN, Thompson LU. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem 1999;202(1–2):91–100. [DOI] [PubMed] [Google Scholar]
- 7.Lee B, Kim KH, Jung HJ, Kwon HJ. Matairesinol inhibits angiogenesis via suppression of mitochondrial reactive oxygen species. Biochem Biophys Res Commun 2012;421(1):76–80. [DOI] [PubMed] [Google Scholar]
- 8.Vanharanta M, Voutilainen S, Lakka TA, Van Der Lee M, Adlercreutz H, Salonen JT. Risk of acute coronary events according to serum concentrations of enterolactone: A prospective population-based case-control study. Lancet 1999;354(9146):2112–5. [DOI] [PubMed] [Google Scholar]
- 9.Kuijsten A, Bueno-de-Mesquita HB, Boer JMA, et al. Plasma enterolignans are not associated with nonfatal myocardial infarction risk. Atherosclerosis 2009;203(1):145–52. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Li J, Li Y, et al. Gut microbiota–derived metabolites and risk of coronary artery disease: a prospective study among US men and women. Am J Clin Nutr 2021;nqab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Schouw YT., Pijpe A, Lebrun CEI, et al. Higher usual dietary intake of phytoestrogens is associated with lower aortic stiffness in postmenopausal women. Arterioscler Thromb Vasc Biol 2002;22:1316–22. [DOI] [PubMed] [Google Scholar]
- 12.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Van Der Schouw YT. Dietary phytoestrogens and plasma lipids in Dutch postmenopausal women; a cross-sectional study. Atherosclerosis 2005;178:95–100. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini N, Valtueña S, Ardigò D, et al. Intake of the plant lignans matairesinol, secoisolariciresinol, pinoresinol, and lariciresinol in relation to vascular inflammation and endothelial dysfunction in middle age-elderly men and post-menopausal women living in Northern Italy. Nutr Metab Cardiovasc Dis 2010;20:64–71. [DOI] [PubMed] [Google Scholar]
- 14.de Kleijn MJJ., van der Schouw YT, Wilson PWF, Grobbee DE, Jacques PF. Dietary Intake of Phytoestrogens Is Associated with a Favorable Metabolic Cardiovascular Risk Profile in Postmenopausal U.S. Women: The Framingham Study. J Nutr 2002;132(2):276–82. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Schouw YT., Kreijkamp-Kaspers S, Peeters PHM, Keinan-Boker L, Rimm EB, Grobbee DE. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in Western women. Circulation 2005;111:465–71. [DOI] [PubMed] [Google Scholar]
- 16.Milder IEJ., Feskens EJM, Arts ICW, Bueno-de-Mesquita HB, Hollman PCH, Kromhout D. Intakes of 4 dietary lignans and cause-specific and all-cause mortality in the Zutphen Elderly Study. Am J Clin Nutr 2006;84:400–5. [DOI] [PubMed] [Google Scholar]
- 17.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulze MB., Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 19.Fung TT., Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89(4):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainsworth BE., Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA., Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 22.Wolf AM., Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 23.Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curb JD., Mctiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the women’s health initiative. Ann Epidemiol 2003;13(9 SUPPL.). [DOI] [PubMed] [Google Scholar]
- 25.Rose G, H. B. Cardiovascular survey methods.WHO monograph series no. 58. Geneva: World Health Organization. 1982. [PubMed] [Google Scholar]
- 26.Stampfer MJ., Willett WC, Speizer FE, et al. Test of the national death index. Am J Epidemiol 1984;119:837–9. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein AM., Rosner BA, Willett WC. Cereal fiber and coronary heart disease: A comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol 2011;26(11):877–86. [DOI] [PubMed] [Google Scholar]
- 28.Ursoniu S, Sahebkar A, Andrica F, Serban C, Banach M. Effects of flaxseed supplements on blood pressure: A systematic review and meta-analysis of controlled clinical trial. Clin Nutr 2016;35(3):615–25. [DOI] [PubMed] [Google Scholar]
- 29.Askarpour M, Karimi M, Hadi A, et al. Effect of flaxseed supplementation on markers of inflammation and endothelial function: A systematic review and meta-analysis. Cytokine 2020;126:154922. [DOI] [PubMed] [Google Scholar]
- 30.Ren GY., Chen CY, Chen GC, et al. Effect of flaxseed intervention on inflammatory marker C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016;8(136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr 2009;90(2):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins DJA., Kendall CWC, Augustin LSA, et al. Effect of Wheat Bran on Glycemic Control and Risk Factors for Cardiovascular Disease in Type 2 Diabetes. Diabetes Care 2002;25(9):1522–8. [DOI] [PubMed] [Google Scholar]
- 33.Zamora-Ros R, Jiménez C, Cleries R, et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology 2013;24:726–33. [DOI] [PubMed] [Google Scholar]
- 34.The European Commission’s science and knowledge service. Health Promotion & Disease Prevention, Nutrition. Available at: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition. [Google Scholar]
- 35.USDA. Food Consumption and Nutrient Intakes. Available at: https://www.ers.usda.gov/data-products/food-consumption-and-nutrient-intakes/. [Google Scholar]
- 36.Sun Q, Wedick NM, Pan A, et al. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of u.s. Women. Diabetes Care 2014;37(5):1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rienks J, Barbaresko J, Nöthlings U. Association of polyphenol biomarkers with cardiovascular disease and mortality risk: A systematic review and Meta-Analysis of observational studies. Nutrients 2017;9(4):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Song Y, Franke AA, Hu FB, Van Dam RM, Sun Q. A Prospective Investigation of the Association between Urinary Excretion of Dietary Lignan Metabolites and Weight Change in US Women. Am J Epidemiol 2015;182(6):503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowland IR., Wiseman H, Sanders TAB, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr Cancer 2000;36:27–32. [DOI] [PubMed] [Google Scholar]
- 40.Kuijsten A, Arts ICW, Vree TB, Hollman PCH. Pharmacokinetics of Enterolignans in Healthy Men and Women Consuming a Single Dose of Secoisolariciresinol Diglucoside. J Nutr 2005;135:795–801. [DOI] [PubMed] [Google Scholar]
- 41.Possemiers S, Bolca S, Eeckhaut E, Depypere H, Verstraete W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: Producer phenotyping and relation with intestinal community. FEMS Microbiol Ecol 2007;61:372–83. [DOI] [PubMed] [Google Scholar]
- 42.Clavel T, Henderson G, Alpert CA, et al. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol 2005;71:6077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aura AM., Myllymäki O, Bailey M, Penalvo JL, Adlercreutz H, Poutanen K. Interrelationships between carbohydrate type, phenolic acids and initial pH on in vitro conversion of enterolactone from rye lignans. Dietary Fibre Components and Functions. 2007. p. 235–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.