Abstract
Introduction:
Opioid use disorder (OUD) co-occurring with depression and/or posttraumatic stress disorder (PTSD) is common and, if untreated, may lead to devastating consequences. Despite the availability of evidence-based treatments for these disorders, receipt of treatment is low. Even when treatment is provided, quality is variable. Primary care is an important and underutilized setting for treating co-occurring disorders (COD) because OUD, depression and PTSD are frequently co-morbid with medical conditions and most people visit a primary care provider at least once a year. With rising rates of OUD and opioid-related fatalities, this is a critical treatment and quality gap in a vulnerable and stigmatized population.
Methods:
CLARO (Collaboration Leading to Addiction Treatment and Recovery from Other Stresses) is a multi-site, randomized pragmatic trial of collaborative care (CC) for co-occurring disorders in 13 rural and urban primary care clinics in New Mexico to improve care for patients with OUD and co-occurring depression and/or PTSD. CC, a service delivery approach that uses multi-faceted interventions, has not been tested with COD. We will enroll and randomize 900 patients to either CC adapted for COD (CC-COD) or enhanced usual care (EUC) and will collect patient data at baseline, 3-, and 6-month follow-up. Our primary outcomes are medications for OUD (MOUD) access, MOUD continuity of care, depression symptoms, and PTSD symptoms.
Discussion:
Although CC is effective for improving outcomes in primary care among patients with mental health conditions, it has not been tested for COD. This article describes the CLARO CC-COD intervention and clinical trial.
Keywords: Opioid use disorder, depression, post-traumatic stress disorder, collaborative care, safety net Federally Qualified Health Centers (FQHCs), primary care, integrating primary care and mental health
1. Introduction
Untreated mental illness and substance use disorders are prevalent and can have devastating consequences for the individual, their families and the community.1–4 Co-occurring opioid use disorder (OUD) with either depression5 and/or posttraumatic stress disorder (PTSD)6–8 is of particular concern because depression and PTSD are prevalent in people with OUD, co-occurring mental illness is linked to an increased risk for overdose, and because of the high prevalence of the chronic use of prescription opioids in individuals with mental illness. Such use is a risk factor for transitioning to heroin use and/or the development of an OUD.9–16 Primary care is an important and underutilized setting in which to provide treatment for all three disorders.17 However, despite the effectiveness of treatments for all three disorders, many individuals never receive treatment; and, when treatment is provided, quality is low.18–26 With the rising number of opioid-related fatalities, this is a critical treatment and quality gap in a vulnerable and stigmatized population.
There are multiple reasons for this gap.27–30 Patients are often not ready for treatment, and there may be psychosocial barriers to engagement and retention. Timely treatment may be difficult to access, and primary care and behavioral health providers may not have needed expertise or certifications.31 Structural barriers hinder the delivery of integrated treatment. Collaborative care (CC) addresses these problems,32–34 and studies conducted by our team and others have shown that CC improves access, quality and outcomes in primary care patients with common mental health (MH) conditions. Some studies have shown more tempered results.35,36 However, CC has never been tested with co-occurring disorders (COD).37–42
CC consists of a team of providers that includes a care manager (CM), a primary care provider (PCP) and a behavioral health consultant (BHC), who provide evidence- and measurement-based care to a panel of patients using a clinical registry. In our CC model for COD (CC-COD), the CC team also includes a behavioral health psychotherapist (BHP). Additionally, the evidence-based treatments supported include medications for OUD (MOUD), pharmacotherapy for depression and PTSD, motivational interviewing (MI), problem solving therapy (PST) and Written Exposure Therapy (WET).
This randomized controlled trial (RCT) will adapt and test CC for co-occurring OUD and depressive disorder and/or PTSD (CC-COD). CLARO (Collaboration Leading to Addiction Treatment and Recovery from Other Stresses) is one of four studies funded by the National Institute of Mental Health’s (NIMH) HEAL initiative focused on collaborative care for co-occurring disorders.
2. Methods
2.1. Design overview
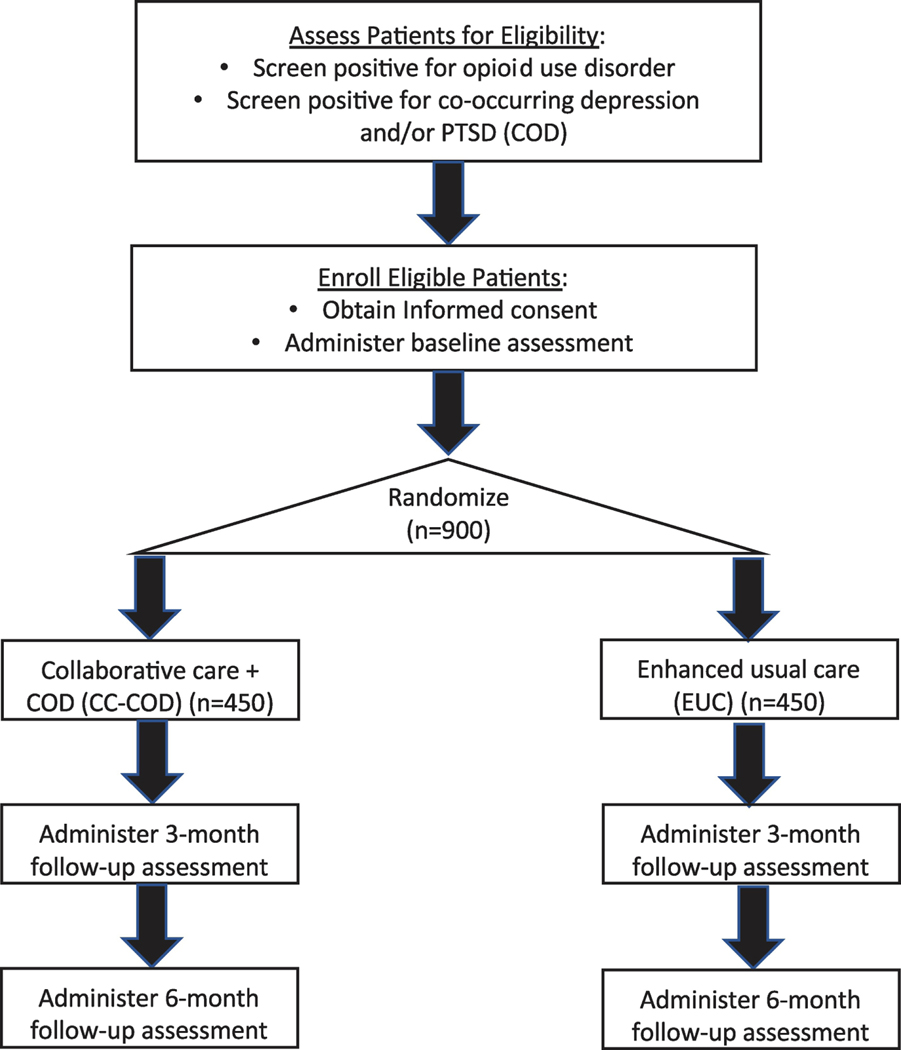
CLARO is a pragmatic, randomized controlled trial in 13 primary care clinics from three healthcare systems in New Mexico. Figure 1 illustrates the study design and anticipated patient flow from recruitment to enrollment and the evaluation. CLARO tests whether patients with OUD co-occurring with depression and/or PTSD who are randomized to CC-COD have improved access, quality and outcomes, as compared with those randomized to enhanced usual care (EUC). We adapt the CC model to the New Mexico setting and a COD patient population, and use findings on organizational readiness to inform implementation. Primary outcomes include MOUD access; MOUD continuity of care, depression symptoms, and PTSD symptoms. We will assess whether patient experiences of care and working alliance measured at 3 months mediate the impact of CC-COD, and explore whether mental health treatment improves OUD outcomes. Exploratory analyses will look at what factors mediate and moderate the effect of CC-COD. We will also assess contextual factors and implementation outcomes to inform future dissemination if the model is effective.
Figure 1.
Overview of study design
Under a classification proposed by Curran et al.,44 our study is a Type 1 effectiveness-implementation hybrid design because we will simultaneously conduct a multi-site trial to determine the effectiveness of CC-COD while also assessing context and implementation. Our trial design is pragmatic,45 in that it is designed to improve usual practice and inform clinical and policy decisions. These design characteristics include: collaborations with the health care system to adapt the intervention to local conditions, multiple heterogeneous settings, broad patient eligibility criteria that reflect how the intervention will be used in clinical practice, usual care practitioners, multiple outcomes important to decision-makers, intent-to-treat data analysis, and prospective controls.45–48
2.2. Study settings and target population.
New Mexico has one of the highest opioid-related overdose death rates,49 and the U.S. Centers for Disease Control and Prevention projects it will have the highest rates of death from drugs, alcohol and suicide in the nation by 2025.43 It is a primarily rural state with a Hispanic majority population.50 CLARO is a collaboration between researchers at the RAND Corporation, the University of New Mexico Health Sciences Center (UNM HSC), and Boston Medical Center (BMC) partnered with three health systems: First Choice Community Healthcare (FCCH, 7 clinics), UNM’s Health System (UNM-HS, 3 clinics), and Hidalgo Medical Services (HMS, 3 clinics).
These 13 clinics span three counties, include urban and rural clinics in the regions of New Mexico with the highest rates of opioid overdose (northeast and central) or are primarily rural (southwest). These organizations and clinics have varying capacity to deliver treatment; for example, the range of buprenorphine prescribers per clinic is 0 to 18. All clinics provide primary care for mostly low-income, predominantly Hispanic patients, and are in Health Professional Shortage Areas for primary care, mental healthcare, or both. Table 1 summarizes clinic and patient characteristics.
Table 1.
Characteristics of Clinics and Patient Populations Participating in CLARO
| Organization/Clinic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinic Characteristics | |||||||||||||
| First Choice Community Health Care (FCCH) | UNM | Hidalgo Med. Services | |||||||||||
| Alamosa | Belen | Edgewood | Los Lunas | N.Valley | S.Broadway | S.Valley | SE Heights | North Valley | Southwest Mesa | CHC | Lordsburg | Med Square | |
| Health | central | central | NE | central | central | central | central | central | central | Central | SW | SW | SW |
| region of NM | l | l | |||||||||||
| Rural per HRSA/HPSA | no | part | part | part | no | no | no | no | no | no | yes | yes | yes |
| County | Bernalillo | Valencia | Santa Fe | Valencia | Bernalillo | Bernalillo | Bernalillo | Bernalillo | Bernalillo | Bernalillo | Hidal go | Grant | Grant |
| HPSA primary care | no | yes | yes | yes | no | no | no | no | no | no | yes | yes | yes |
| HPSA mental health | yes | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| FQHC | yes | yes | yes | yes | yes | yes | yes | no | no | no | yes | yes | yes |
| # of PCPs (FTEs) | 5 | 3 | 5 | 5 | 5 | 6 | 11 | 6 | 3.5 | 3.25 | 2 | 4 | 7 |
| # of onsite BHPs* | 2 | 1 | 2 | 1 | 1 | 3 | 4 | 2 | 0.7 | 2 | 3* | 1 | 4* |
| Access to staff psychiatrist | limited | limited | limited | limited | limited | limited | yes | yes | Yes | limited | limited | limited | limited |
| # waivered providers* | 5 | 2 | 3 | 0 | 3 | 3 | 7 | 18 | 6 | 5 | 1 | 2 | 2 |
| # of Care Coordinators* | 2 | 2 | 2 | 2 | 2 | 1 | 4 | 3 | 1 | 2 | 0 | 0 | 0 |
| Screen for OUD | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Screen for depression | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Patient Demographics | |||||||||||||
| % Hispanic | 87 | 68 | 32 | 75 | 67 | 75 | 89 | 43 | 52 | 67 | 53 | 53 | 53 |
| % African American | 3 | 2 | <1 | <1 | 3 | 4 | 2 | 5 | <1 | <1 | 1 | 1 | 1 |
| % Asian | <1 | <1 | <1 | <1 | 2 | 1 | <1 | 23 | <1 | <1 | <1 | <1 | .<1 |
| % Native American/AK native | 1 | 2 | <1 | 1 | 1 | 1 | 1 | 2 | <1 | <1 | <1 | <1 | <1 |
| % Women | 57 | 57 | 57 | 63 | 55 | 59 | 58 | 63 | 63 | 63 | 55 | 55 | 55 |
| % Uninsured | 15 | 13 | 6 | 13 | 11 | 15.5 | 18 | 3 | 5 | 6 | 8 | 8 | 8 |
| % Medicaid | 69 | >50 | 40 | 66 | 66 | 63 | 65 | 72 | 29 | 46 | 28 | 28 | 28 |
| % Monolingual Spanish | 40 | 19 | 4 | 26 | 16 | 33 | 39 | 19 | 8 | 24 | 3 | 7 | 3 |
UNM=University of New Mexico; HRSA=Health Resources and Services; HPSA=Health Professional Shortage Area; NE=north east; SW=south west; AK=Alaskan.
May not be full-time as providers work across sites. limited=available by phone for consultation.
We will randomize 900 patients (within each of the 13 participating clinics) who have co-occurring OUD and depression and/or PTSD to receive either CC-COD or enhanced usual care (EUC). All interview data will be collected in REDCap,51 a secure, web-based Health Insurance Portability and Accountability Act-compliant electronic data capture system by a Statistics and Data Coordinating Center based at UNM.
2.3. Inclusion criteria
Patients will be eligible to participate in the study if they meet the following criteria: receive primary care at one of the 13 clinics, age 18 or older; have a probable OUD diagnosis, defined by scores ≥1 on the self-administered National Institute on Drug Abuse Tobacco, Alcohol, Prescription medication and other Substance use (myTAPS)53 screener; have probable co-occurring PTSD (having a score ≥3 on the Primary Care PTSD Screen for Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (PC-PTSD-5)54 or probable depression (a score ≥10 on the Patient Health Questionnaire-9);55 speak and understand English or Spanish; have capacity to give informed consent; and provide an informed consent electronically or in writing. Pregnant women will not be excluded.
2.4. Exclusion criteria
Patients will only be excluded if they require immediate medical (emergency procedure needed) or psychiatric intervention (i.e., self-injury, active psychosis).
2.5. Baseline assessment
We will include several recruitment strategies across sites. These include anonymous, clinic-population universal screening in clinic waiting areas where and when possible; we cannot currently use this approach due to COVID-19 precautions. Until the pandemic allows reopening, we will primarily rely on referrals from clinic staff, Institutional Review Board approved methods to identify potential participants by examining the electronic health record for individuals with qualifying diagnoses. We will also provide public information about the study through announcements in clinic newsletters, posters in waiting and exam rooms, and a pre-screening questionnaire on the CLARO website with the option of providing contact information for screening. Enrolled patients may refer people (e.g., friends or family) who are receiving primary care at one of the 13 clinics to the study. Some of these strategies are described in more detail in sub-sections below. We plan to take an adaptive approach to determining the optimal recruitment and retention strategies at each of these diverse clinic sites. We will do this by working closely with champions at each clinic and monitoring the enrollment data on a weekly basis to identify the most productive strategies to focus on. Using a variety of methods is key because some of the rural clinics are small, and it would not be cost efficient to station study personnel at them five days a week. It is important to note, due to the SARS-CoV-2/COVID-19 pandemic, there have been changes to service and patient flow at clinics. Therefore, we anticipate recruitment to vary by site and over time. Adaptations will be made on an as-needed basis, based on our observations, preliminary recruitment success, guidance from the clinical sites and the UNM Office of Research. Regardless of recruitment method, after screening and upon enrollment in the study, participants will complete a baseline assessment interview before being randomized to one of the treatment conditions.
Study subjects will be paid $50 for completing the screening and baseline assessments and will be re-interviewed by telephone three and six months following enrollment with a window of −2 weeks/+4 weeks to conduct each follow-up assessment. They will be offered $40 to complete the 3-month interview and $40 to complete the final interview at 6 months. We will contact patients via phone, text or email to schedule follow-up interviews. We will make multiple attempts at varied times of the day and day of the week during a window period of two weeks prior to the target interview date and four weeks following the target date to contact the participant for each of the two follow-up interviews, and will follow up with hard copy letters to their address, asking them to contact us. We estimate we will be unable to contact up to 20% of enrolled subjects for the follow-up, due to loss to follow-up, incarceration, or death. If all of these methods are unsuccessful, we will ask for help from the clinic staff to contact the patient, and if necessary will contact the patient at the time of one of their scheduled clinic visits.
2.6. Randomization
After a baseline assessment, study participants will be randomized 1:1 to either receive the collaborative care for co-occurring disorders (CC-COD) intervention (n=450), or to receive enhanced usual care (EUC) control (n=450). A stratified randomized block design will be used, with the strata determined by clinic and any prior MOUD exposure. A computer-generated random assignment sequence will be generated for each stratum and will include randomly permuted block sizes of 2 and 4. Research assistants will obtain the patient’s intervention allocation from REDCap following completion of the baseline interview. Research assistants, participants, clinic staff, and data coordinating center staff will not be blinded to the intervention assignment; however, all other members of the research team will be.
2.9. Follow-up assessments
Research staff, blinded to subject condition, will conduct an electronic health record review to assess study outcomes. We will abstract data on MOUD use, pharmacotherapy and psychotherapy treatment of depression and/or PTSD, and quality of care for OUD, depression, and PTSD. We will collect follow-up data via telephone interviews at 3 and 6 months after enrollment. The interviews will be approximately 45 minutes long.
We anticipate we will collect utilization data from the electronic health record from 100% of subjects. For the telephone survey, we plan for up to a 20% loss to attrition at follow-up. We recognize that the hardest subjects to reach might provide fundamentally different responses than members of the group who are relatively easier to find, introducing non-response bias and threatening the quality of statistical analyses and the validity and generalizability of research findings. We will use two categories of methods to increase retention: strategies to stay in touch and strategies for locating “lost” respondents. We will collect extensive contact and re-contact information at study enrollment, providing incentives including remuneration and reminder gift items.
3. Study conditions
There are two study conditions, CC-COD and EUC. Both approaches are hypothesized to be feasible to implement in primary care settings that deliver care with limited resources to diverse patients with complex needs.
3.1. EUC
We carefully considered the comparison condition in conversations with our clinical partners and determined that all patients and providers must have access to the basic elements necessary to provide the continuum of care for COD, including training in the supported treatments. A cluster RCT was not feasible because of the large sample size, and with patient-level randomization, it is not ethical to withhold these basic features of care. Additionally, our clinic partners told us that all treatments must be available to all patients at a given clinic. Thus, EUC includes availability to both the evidenced-based psychotherapy (i.e., Problem Solving Therapy for depression56 and Written Exposure Therapy for PTSD57) and pharmacotherapy (i.e., MOUD and psychotropic medications for depression/PTSD) provided in the CC-COD intervention. The primary difference is the absence of a care management team to coordinate care using a clinical registry for patients in EUC.
3.2. CC-COD
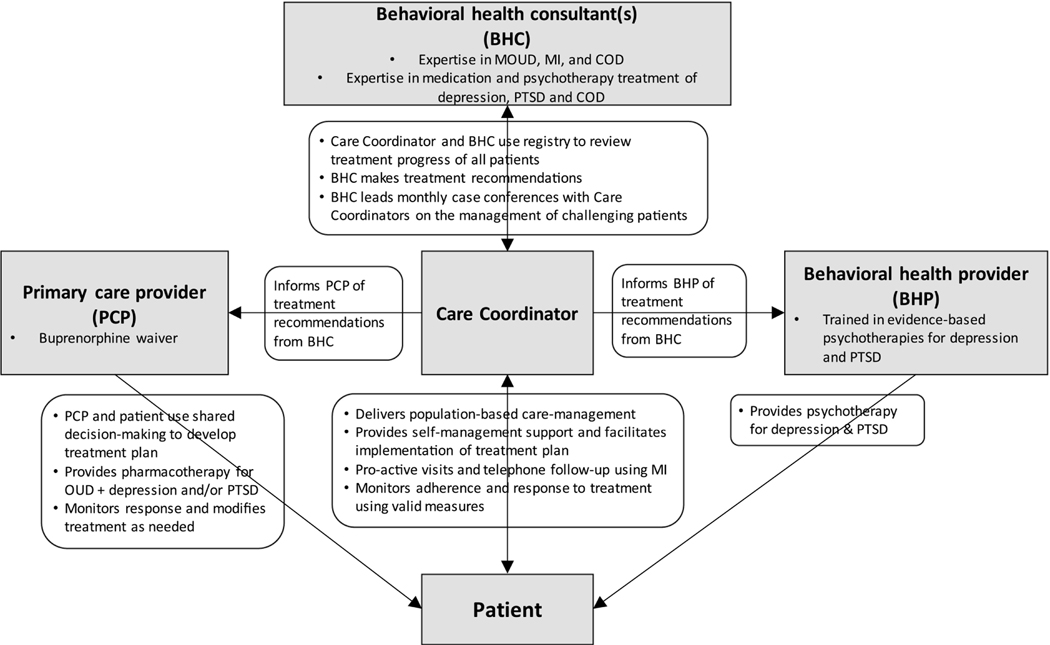
We adapted the traditional CC model to address COD (Table 2). The CC-COD intervention emphasizes the core principles of CC including: patient-centered care (shared decision-making with the patient), population-based care managed through a clinical registry, measurement-based treatment to target using thresholds on clinical outcome measures (e.g., administering symptom management questions at each patient encounter to assess program and adjust treatment plan as needed), and evidence-based psychotherapy and pharmacotherapy treatments58 (see Table 3 & Figure 2). The intervention is based on a service delivery approach that uses multi-faceted interventions to improve access and quality of care. It is based on Wagner’s Chronic Care Model59,60 and subsequent modifications.40,61 We hypothesize that the intervention will improve access to the supported evidence-based treatments including MOUD,62,63 medication treatment for depressive disorders and/or PTSD, motivational interviewing (MI), problem solving therapy (PST) for depression, and written exposure therapy (WET) for PTSD, and that receipt of these treatments will lead to improved clinical outcomes.
Table 2.
Traditional Collaborative Care (CC) and CC for Co-Occurring Disorders (CC-COD)
| Traditional CC | Characteristic of population, setting and organization that necessitate change to the traditional CC model | CC-COD Modification |
|---|---|---|
| Patients have only one disorder; Care Coordinator provides psychotherapy | Patients have at least 2 and possibly 3 complex disorders, making it difficult for a single Care Coordinator to provide BH treatment for all three conditions. | Add a psychotherapist as part of the CC-COD team (BHP); addresses clinical complexity of patients. |
| Nurses are in the Care Coordinator role | Setting is HPSA; nurses expensive and scarce, PCPs have large caseloads; Clinical population is impoverished and may prefer to work with a Care Coordinator who is “more like them”; may be ambivalent about treatment. | Care Coordinator is a “trained up” community health worker (addresses nursing shortage) who uses MI and is able to make home visits; leverages scarce professional resources; may address stigma experienced by population. |
| Psychiatrists are available to work with Care Coordinator in person and have the necessary expertise | Scarcity of professional resources with addiction and psychiatric expertise; Multiple co-morbidities make it difficult for single expert consultant to have all necessary expertise; Geographic remoteness. | Use of ECHO and monthly multi-disciplinary case conferences that include pharmacotherapy and psychotherapy experts in addiction and mental illness (BHCs); addresses geographic remoteness and need for interdisciplinary expertise; leverages scarce resources. |
BH=Behavioral Health; BHC=Behavioral Health Consultants; BHP=Behavioral Health Provider; EHCO=Extension for Community Healthcare Outcomes; HPSA=Health Professional Shortage Area; PCP=Primary Care Provider.
Table 3.
Description of How Core Principles are Integrated in the CC-COD Intervention
| Core-principles | CC-COD intervention |
|---|---|
| Patient-centered care117 | • Shared decision-making between the care management team and patients where problems to address are clearly defined, treatment plans are mutually agreed upon (e.g., initiation visit uses a menu of options to do this), and barriers are identified and addressed (e.g., social needs) |
| • Designation of a community health worker as Care Coordinator to engage the patient, actively monitor their progress, make necessary modifications to the treatment plan, and link them to care | |
| Measurement-based treatment to target | • Routine symptom monitoring through measurement-based care and goal attainment questions to assess progress |
| • Adjusting treatment plans by progress with the goal of symptom remission for all conditions | |
| Population-based care | • Use of registry to track a caseload of patients |
| • Use of registry flags that inform Care Coordinators/supervisors/consultants of patients in need of higher-level care (e.g., patients with missed visits, suicidality) | |
| • Use of registry to track patient progress by symptoms (e.g., MBC) and service receipt (e.g., MOUD, WET, PST) | |
| Evidence-based care | • Offering MOUD, WET, and PST to treat OUD and depression/PTSD |
| • Using MI to deliver care initiation and monitoring visits | |
| • Monitoring initiation, engagement, and retention of MOUD, WET, and PST |
CC-OUD=Collaborative Care for Opioid Use Disorder; MBC=Measurement-Based Care; MOUD=Medication for Opioid Use Disorder; PST=Problem-Solving Therapy; PTSD=Post-Traumatic Stress Disorder; WET=Written Exposure Therapy.
Figure 2.
Overview of CLARO Collaborative care intervention
In each session, Care Coordinators follow the acronym BALL that stands for build engagement, assess social needs and symptoms, link to care, and loop back with the care team. The Care Coordinator uses MI to assess treatment experiences and barriers to care, provides information about treatment options, and then coordinates next steps with the patient. After the care initiation visit, Care Coordinators will meet with the patient in monitoring visits for the remainder of the six-month intervention period. Care monitoring visits are similar to initiation visits but are shorter. Care Coordinators will meet with patients individually for at least 13 visits over six months. In the first two months, the Care Coordinator will meet weekly with the patient. In month three, they will meet biweekly. In months four through six, they will meet with patients once a month. Visits can be in-person or by phone, and ideally in-person prior to the PCP visit so that the Care Coordinator can relay information to the PCP before their visit (e.g., symptoms, insights regarding barriers to care). Visits can also be conducted more frequently or for longer than six months should the care team decide it is best for the patient.
The Care Coordinator is supported by a behavioral health consultant (BHC) and a Care Coordinator supervisor with expertise in supervising community health workers. The Care Coordinator meets with BHC and Care Coordinator supervisor, respectively, on a weekly basis to discuss the Care Coordinator’s patient caseload.
Finally, Care Coordinators enter patient information into a clinical registry on an ongoing basis. This registry documents a Care Coordinator’s patient caseload and has four main purposes:65 (1) tracks population-level outcomes and engagement (see Table 4), (2) prompts the Care Coordinator with reminders and alerts to ensure accountable outreach when patients have upcoming appointments or need a higher-level of care, (3) prompts treatment-to-target through ongoing symptom management scores that show trends over time in real-time and flags the Care Coordinator when to consult with the BHC, and (4) facilitates caseload review between the Care Coordinator, BHC, and Care Coordinator supervisor through caseload-level reports that display patient-level identification numbers of those who should be discussed.
Table 4.
CC-COD Intervention Visit Measures
| SCALES | NO. OF ITEMS | SYMPTOMS ASSESSED | WHEN TO ADMINISTER |
|---|---|---|---|
| WellRx64 | 11 | Social determinants of health | At 1st visit |
| OUD-5 | 5 | Opioid cravings, medication side effects, withdrawal, substance use | Every visit |
| PROMIS-7 | 7 | Cravings, interpersonal challenges related to substance use, severity of use | At 2nd visit and once per month |
| PHQ-9 | 9 | Depression symptoms | At 2nd visit and once per month (if applicable) |
| PCL-5 | 20 | PTSD symptoms | At 2nd visit and once per month (if applicable) |
| PEG | 3 | Pain severity and interference | As needed |
| ISI | Insomnia symptoms | As needed |
OUD=Opioid Use Disorder; PROMIS=Patient-Reported Outcomes Measurement Information System; PHQ=Patient Health Questionnaire; PCL-5=PTSD Checklist; PEG=Pain, Enjoyment, General Activity; ISI=Insomnia Severity Index; PTSD=Post-Traumatic Stress Disorder.
3.3. Training and supervision
Primary care providers (PCPs).
PCPs who do not have a Xwaiver were offered a 4-hour waiver training to qualify them as buprenorphine waivered practitioner to treat opioid use OUD. Subsequent to the initial training, Physicians then completed an additional 4 hours of online training to quality for a total of 8 hours. Physician Assistants, Nurse Practitioners, and Certified Nurse Midwives completed an additional 20 hours of online modules for a total of 24. The training was sponsored by University of New Mexico’s Department of Psychiatry & Behavioral Sciences, Division of Community Behavioral Health (CBH) as part of the Providers Clinical Support System (PCSS), a collaborative effort led by the American Academy of Addiction Psychiatry (AAAP) across the nation.
Behavioral health providers (BHPs).
BHPs are Master’s or Doctoral-level therapists who received training on Problem Solving Therapy (PST) to treat depression and Written Exposure Therapy (WET) to treat PTSD. BHPs were trained 2–3 months before the study launch to allow ample time to finish the full course of ongoing training prior to the start of the RCT. For both therapies, BHPs were trained through 6 hours of workshops delivered virtually across two days (3 hours each). Following the PST training workshop, there was an additional 4 hours of individual and group practice sessions for case review (including at least 2 audio recordings) and supervision by the PST developer. After the WET workshop, BHPs complete 2 cases and attend a minimum of 8 consultation meetings with the WET developer. In total, each BHP will devote at most 40 hours to the trainings; they were provided release time to complete them. Beyond the full course of each training, it is not planned for continued supervision once the trial starts though we will train new BHPs who come onboard during the intervention period.
Care Coordinators.
CHWs will be prepared for the role of Care Coordinators. They will receive at least 40 hours of training that correspond to the five core principles of collaborative care. Specifically, Care Coordinators will receive didactics on the symptoms underlying OUD, depression, and PTSD, and what evidence-based approaches will be offered in CC-COD to treat these illnesses. Care Coordinators will also receive a half-day MI training and will participate in several interactive exercises and role-plays to practice MI in the context of their rehearsal of CC-COD initiation and monitoring visits. Finally, Care Coordinators will receive training where they will learn to administer the measurement-based care questions, interpret findings to aid treatment planning, track patients in the registry (population-based care), and facilitate communication with the patient and members of the care team. Competency will be assessed while observing practice role-play sessions and through audio recordings of patient interactions upon starting the clinical trial.
In addition, Care Coordinators will receive weekly individual reflective supervision. Reflective supervision focuses on reflection between the Care Coordinator and the supervisor to builds on the Care Coordinator’s use of her thoughts, feelings, and values within a service encounter.66 Care Coordinators also participate in weekly group supervision with a BHC using the Extension for Community Healthcare Outcomes (ECHO) model,67–71 a group videoconference to conduct case conferences.
4. Measures
Our primary outcomes focus on use of MOUD and changes in PTSD and depression symptoms. We will also examine a variety of secondary/exploratory outcomes; mediators and moderators of treatment quality and outcomes; and costs of the CC-COD condition. We will collect measures through baseline/follow-up assessments, electronic health record (EHR) data extraction, and study records. All measures are summarized in Table 5.
Table 5.
CLARO Evaluation Measures, Data Collection Schedule, and Source of Data
| Measure | Pre 3M 6M Operational Definition | Source | |||
|---|---|---|---|---|---|
| Eligibility Criteria | |||||
| Probable OUD diagnosis | X | myTAPS score ≥ 153 | IAS | ||
| Probable depression | X | PHQ-9 score ≥ 10 55 | AS | ||
| Probable PTSD | X | PC-PTSD-5 score ≥ 3 54 | AS | ||
| Age 18 or older | X | n/a | AS | ||
| Patient at participating clinic | X | Receiving primary care at one of the 11 participating clinics | AS | ||
| Speak English or Spanish | X | n/a | AS | ||
| Primary Outcomes | |||||
| MOUD access*72 | X | X | X | Receipt of MOUD prescription within 30 days | MR |
| MOUD continuity of care73 | X | X | X | Max number of continuous days on MOUD (i.e., no breaks of more than 7 days) | MR |
| Depression symptoms | X | X | X | PHQ-9 (change in raw score) | IAS&TS |
| PTSD symptoms | X | X | X | PCL-5 74 (change in raw score) | IAS&TS |
| Secondary Outcomes | |||||
| Drug use frequency | X | X | X | Days of use in past 30 days for prescription opioids, heroin, cocaine/crack, methamphetamine/other stimulants, and tranquilizers/sedatives (NSDUH75 items) | IAS&TS |
| Opioid use severity | X | X | X | PROMIS Substance Use Short Form76 for past 30 days | IAS&TS |
| Alcohol use | X | X | X | AUDIT-C77 for past 3 months | IAS&TS |
| Urine drug screen | X | X | X | n/a | MR |
| Opioid overdose risk behaviors | X | X | X | Opioid Overdose Risk Assessment78 | IAS&TS |
| Opioid overdose events | X | X | X | Naloxone Overdose Baseline Questionnaire79 | IAS&TS |
| Depression remission | X | X | X | PHQ-9 score < 10 | TS |
| Depression response | X | X | X | PHQ-9 score < 50% of baseline score | TS |
| PTSD remission | X | X | X | PCL-5 score < 34 | TS |
| PTSD response | X | X | X | PCL-5 score < 50% of baseline score | TS |
| Severe suicidality | X | X | X | Columbia Suicide Severity Rating Scale,80 dichotomized based on presence vs. absence of a plan, intent, and/or attempt | IAS&TS |
| All-cause mortality | X | X | Death records81 | NDI | |
| MOUD initiation* | X | X | Receipt of MOUD prescription within 14 days of diagnosis82 | MR | |
| MOUD engagement* | X | X | Receipt of two or more MOUD prescriptions within 34 days of diagnosis82 | MR | |
| Access to treatment for depression and/or PTSD | X | X | Receipt of medication and/or behavioral treatment associated with diagnosis | MR | |
| Quality of care for depression* | X | X | 4 psychotherapy visits in the first 8 weeks or an adequate (12-week) medication trial | MR | |
| Quality of care for PTSD*83 | X | X | 4 psychotherapy visits in the first 8 weeks or an adequate (60-day) medication trial | MR | |
| General health functioning84 | X | X | X | Veterans RAND 12-item Health Survey (VR-12) | IAS&TS |
| Moderators/patient characteristics | |||||
| Demographics | X | (sex, race, ethnicity, education level, marital status) | IAS | ||
| Alcohol use severity | X | X | X | AUDIT77 for past 3 months | IAS |
| Pain levels | X | X | X | PEG Pain Monitor118 for past week | IAS&TS |
| History of MOUD treatment | X | items developed by the research team | IAS | ||
| Current depression/PTSD treatment | X | NSDUH75 items | IAS | ||
| Prior experiences with a Care Coordinator | X | items developed by the research team | IAS | ||
| Interpersonal support | X | Presence of a support person who does not have problematic opioid use | IAS | ||
| Homelessness | X | Homelessness Screening Clinical Reminder Tool;85 GRPA86 item | IAS | ||
| Legal involvement | X | X | X | NSDUH75 items; Addiction Severity Index119 items | IAS&TS |
| Disability and impairment | X | Sheehan Disability Scale120 | IAS | ||
| Rurality | X | X | X | Rural-Urban Commuting Area121 code associated with ZIP code | IAS&TS |
| Mediators | |||||
| Clinician (Care Coordinator ) communication | X | X | CAHPS | TS | |
| Ability to access treatment quickly | X | X | CAHPS | TS | |
| Satisfaction with treatment | X | X | CAHPS87 | TS | |
| Patient-Care Coordinator working alliance | X | X | Modified form of Working Alliance Inventory - General Practice88 | TS | |
myTAPS = self-administered Tobacco, Alcohol, Prescription medication, and other Substance use screener; PHQ = Patient Health Questionnaire; PC-PTSD-5 = Primary Care PTSD screener for DSM-5; PCL = PTSD checklist for DSM-5; NSDUH = National Survey on Drug Use and Health; PROMIS = Patient-Reported Outcomes Measurement Information System; AUDIT = Alcohol Use Disorder Consumption Test; AUDIT-C = AUDIT consumption questions; CAHPS = Consumer Assessment of Healthcare Providers and Systems; AS = anonymous screening; IAS = interview administered survey; MR = medical record; TS = telephone survey; NDI = National Death Index; MOUD = medication for opioid use disorder.
Outcome only assessed for patients with a new episode of care. A new episode of care for OUD is defined as no MOUD care in the previous 60 days. A new episode of care for depression or PTSD is defined as no visits associated with the respective diagnosis in the previous six months.
4.1. Primary outcomes
We focus on four primary patient outcomes in CLARO. The primary outcomes are: (1) MOUD access,72 defined as patients with a new episode of OUD care (i.e., no care for at least 60 days prior) receiving an MOUD prescription within the first 30 days of that care episode; (2) MOUD continuity of care,73 which refers to the maximum numbers of continuous (i.e., no breaks of more than 7 days) days the patient is prescribed MOUD in the 180 days after study enrollment; (3) depression symptoms, as measured by the PHQ-9);55 and (4) PTSD symptoms, as measured by the PTSD Checklist (PCL-5).74 MOUD continuity and access will be based on prescription (date and frequency) data from the EHR, supplemented by New Mexico Prescription Monitoring Program data if feasible; these measures are well-established metrics for evaluating MOUD quality of care72,73. Depression and PTSD symptoms will be collected in baseline and follow-up assessments, with primary outcome analyses based on change in raw scores across the six-month period (although we will analyze symptom change using other approaches as part of secondary outcomes).
4.2. Secondary/exploratory outcomes
Given the extensive scope and complexity of the CC-COD intervention, we will examine numerous additional outcomes of interest related to substance use and mental health symptoms, health service utilization and quality, and general patient functioning. These include measures of substance use, mental health symptoms, mortality, service utilization, access, quality, and functioning. Details about these measures are provided in Table 5.
4.3. Moderators/patient characteristics
A number of patient characteristics could moderate CC-COD outcomes. Some characteristics will be measured at baseline only; others will be measured in follow-ups as well, either because they could have a time-varying interaction with the intervention or because the most current information is needed for records retrieval (e. g., National Death Index). Moderator variables include (1) demographic characteristics (i.e., sex, and ethnicity) (2) alcohol use severity, measured for the past 3 months at baseline using the full 10-item AUDIT;77 (3) history of MOUD treatment; and (4) homelessness, measured using the Homelessness Screening Clinical Reminder Tool85 and an item from the Government Performance and Results Act86 clarifying where individuals who are homeless are currently living.
4.4. Mediators
During the 3-month follow-up assessment, we will measure key care processes thought to mediate the effects of CC-COD on patient outcomes at 6 months, including three measures from the Agency for Healthcare Research and Quality’s Consumer Assessment of Healthcare Providers and Systems87 survey items: (1) clinician (i.e., Care Coordinator) communication, (2) ability to quickly access treatment, and (3) satisfaction with treatment. We will also measure fourth mediator: patient-Care Coordinator working alliance using a modified Working Alliance Inventory.88
5. Data analysis
5.1. Overview
We will report descriptive statistics at the patient level by study arm. We will present categorical data as frequencies and percentages, and continuous data as means and standard deviations. We will conduct all primary statistical analyses using the intention-to-treat population based on randomization methods to compare CC-OUD with EUC (described in greater detail in Section 5.3).
5.2. Sample size and power
Our study was designed based on power calculations for the four primary outcomes described in Section 3.1. All calculations were for 80% power at a Type I error rate of 1.25%, which accounts for the multiple primary outcomes using a Bonferroni correction to control the family-wise error rate at 5% (0.05/4 = 0.0125). All calculations assumed 900 total study participants and, if relevant, 20% loss to follow up. Additional assumptions for the power calculations were: (1) at enrollment, 50% of study participants will have a new OUD episode of care, defined as no visits with an OUD diagnosis in the past 60 days; (2) at enrollment, one third of study participants will have probable depression, one third will have probable PTSD, and one third will have both probable depression and PTSD; (3) at enrollment, 25% of patients41 with a new OUD episode of care who receive enhanced usual care will initiate medication for OUD within 30 days of study enrollment; (4) among those who initiate medication for OUD who receive enhanced usual care, the mean number of days of continuous treatment for OUD is 80 with a standard deviation is 65 (personal communication, Asa Wilks); (5) among those with probable depression at enrollment (PHQ-9 ≥ 10), assume a mean depression symptoms score (PHQ-9) of 12 and standard deviation of 6; and among those with probable PTSD at enrollment (PCL-5 > 33), assume a mean PTSD symptoms score (PCL-5) of 50 and standard deviation of 11.
Based on the above assumptions, we will have 80% power to detect a 15 percentage point increase in MOUD access defined as initiation of medication for OUD within 30 days of study enrollment. A previous study of collaborative care for opioid and alcohol use disorders found a 22 percentage point increase over enhanced usual care.41 We will have 80% power to detect 14 additional days of continuous OUD treatment within the first 180 days. A growing body of evidence suggests that MOUD treatment is associated with decreases in mortality.89 For depression symptoms, we will be able to detect a 2-point reduction on the PHQ-9. This provides power to detect effects below the clinically important difference for individual change of 5 points.90 For PTSD symptoms, we will be able to detect a 3.5 point reduction the PCL-5. A previous study of delivering PTSD treatment in primary care setting to active duty military found a reduction in PTSD symptoms of 7 points.91
Dropout is of minimal concern in this study, as all analyses are intention-to-treat. For the outcomes based on self-reported symptoms, i.e., depression symptoms and PTSD symptoms, we expect a 20% loss to follow up. This loss to follow up has been accounted for in the power calculations. The sample size (n=900) also provides sufficient power for secondary outcomes.
5.3. Statistical analysis
The primary analyses will be performed for the intention-to-treat population, which consists of all randomized subjects. All statistical hypotheses will be tested using two-sided tests, with adjustment for multiple testing using a Bonferroni correction.
5.3.1. Primary outcomes
Primary outcomes will be analyzed at a Type I error rate of 1.25% to control the family-wise error rate at 5% (0.05/4=0.0125). All primary outcomes will be analyzed with a regression models that includes a clinic-level fixed effect, an indicator for any prior MOUD exposure, and an indicator for the random treatment assignment. Binary outcomes will be analyzed using logistic regression. All other outcomes will be analyzed using linear regression. Outcomes derived from the follow-up surveys are subject to nonresponse, and statistical hypotheses based on these outcomes will be tested using nonresponse weighted regression models. Specifically, a logistic regression model will be specified predicting response using baseline information, clinic, and the randomization assignment. Nonresponse weights derived from this model will then be used in subsequent analyses.
5.3.2. Secondary outcomes
We will group secondary outcomes into domains of conceptually related outcomes, and each will be analyzed adjusting for multiple comparison within domain. Planned domains are as follows: (1) MOUD treatment (MOUD initiation and MOUD engagement); (2) mental health (access to treatment for depression and/or PTSD; quality of care for depression; quality of care for PTSD and depression remission/response, PTSD remission/response, suicidality); (3) substance use (drug use frequency, alcohol use severity, opioid use severity, opioid overdose risk behaviors, opioid overdose events); and (4) overall health (all-cause mortality, physical health functioning, mental health functioning). We will analyze these secondary outcomes using similar approaches as described for the primary outcomes, but utilizing logistic regression for dichotomous outcomes where appropriate.
5.3.3. Moderators and mediators
In moderator analyses of subgroups, we will explore the data to understand if any of the baseline factors moderate the effect of the intervention. Statistical hypotheses testing whether the effect of the intervention varies by these factors will be tested by the inclusion of an interaction between the moderating factor and the treatment assignment into the previously described models.
In mediator analyses, we will assess whether patient experiences of care and working alliance with the care coordinator at 3 months mediate the impact of CC-COD on patient outcomes, and explore whether mental health treatment at 3 months improves OUD outcomes. To ensure a proper temporal ordering of the treatment, mediators, and outcomes, all outcomes for the mediation analyses will be measured at 6-months, while the mediators will be measured at the 3-month follow-up. All mediation analyses will follow the approach described in Imai, et al. (2010),92,93 including the technical assumptions necessary to identify causal mediation effects. This methodology requires the specification of a model predicting the mediator using only baseline information, and a model predicting the outcome using both baseline information and the mediator.
6. Discussion
OUD is prevalent, frequently co-occurs with depression and/or PTSD, and is associated with serious consequences. In 2015–16, there were over two million adults with a current OUD; 62% had a co-occurring mental illness and 24% had a co-occurring serious mental illness.20 While individuals with COD are more likely to receive MH treatment than OUD treatment, only 16–25% report receiving treatment for both conditions.20,24 Depression and PTSD are two of the most common MH co-morbidities in people with OUD, and when present, are associated with poorer outcomes.5–7,93–98 Both depression and PTSD are leading causes of disability, and mortality from OUD continues to rise.1,100
The CLARO study, one of four NIMH-funded research studies focused on collaborative care for OUD and co-occurring mental health disorders, will help address this problem by improving access, quality, and outcomes of care. These four studies were funded in October 2019 and will run for five years. We expect that findings from these studies will make a substantial contribution to furthering knowledge about the management of OUD co-occurring with mental health problems in primary care settings.
Primary care is an important and underutilized setting in which to identify and provide treatment, but utilization and quality of OUD and behavioral health care is at times low.100–102 Although specialty OUD care plays a critical role for individuals with severe disease, limited availability, the stigma associated with using specialty care, and a host of other barriers means that specialty care alone is unlikely to be able to address the unmet need for treatment.104,105 Recent federal legislation105,106 increased coverage for OUD treatment in primary care, and the prevalence of OUD is high among primary care patients.108,109 Primary care is also an important source of behavioral health care.110 Community health centers are of particular importance because they are the largest source of primary health care for underserved individuals and 1 in 12 people in the U.S. receives primary care in a community health center.111 Using a multi-faceted CC model within these primary care settings has potential to increase access and quality of care because CHWs will help coordinate care that is team-oriented to deliver evidence-based pharmacotherapy and psychotherapy.
It will be challenging to implement and deliver collaborative care for COD. The CLARO settings are diverse, with varying capacity and resources to address COD. There is a need for culturally and linguistically appropriate services, particularly in this minority-majority state. In addition, a host of barriers to care further complicate treatment initiation in rural and low-resource areas, including long distances between patients’ homes and clinical sites, over-burdened primary care systems, very limited availability of nursing care and psychiatric care, and the stigma of both substance use disorders and mental illness.
CLARO modifies traditional collaborative care to address some of the known barriers to implementation. CHWs, rather than nurses, are used to provide care management services, and may help increase access by being from the local community. CLARO incorporates telephonic patient follow-up, leverages the scarce resource of psychiatric specialists by using the ECHO model, and incorporates care for substance use disorder and mental illness into primary care in order to decrease stigma. For example, a systematic review identified 28 studies indicating that negative attitudes of health care providers towards individuals with substance use disorders contributes to worse health care provision for these patients.112
The undertreatment of OUD is arguably the most important public health problem related to the opioid crisis. In 2015, 11.5 million individuals reported misusing opioids and 1.9 million reported being addicted to opioids,113 yet fewer than 20% receive any treatment.114,115 Medication treatment for OUD saves lives, yet at least half of all rural counties in the United States lack a buprenorphine provider, and almost one third of rural Americans (compared with 2.2% of urban Americans) live in a county with no buprenorphine provider.116 Individuals with COD face similar problems, with fewer than 25% reporting receiving treatment for both conditions. By experimentally testing a new approach—CC-COD—and assessing implementation factors such as provider acceptability and feasibility, this study could improve public health by identifying an efficient and generalizable model to increase COD treatment delivery and decrease the downstream effects of untreated substance use disorder and mental illness. Our research will advance science by testing collaborative care in a new population and examining whether patient engagement mediates improved outcomes.
Acknowledgements
The CLARO Study Group includes the PIs and Co-Investigators from key staff (such as project directors and patient representatives), and key stakeholders. The authors appreciate the CLARO partnerships with First Choice Community Healthcare, Hidalgo Medical System, and the University of New Mexico Health System. We acknowledge the clinicians and staff who participated in the advisory group. We also thank key RAND Survey Research Group staff, including: Kirsten Becker, M.S. and Jennifer Parker, B.A. and University of New Mexico staff including Lina Tarhuni, B.S., Mary Carmody, M.P.H., Jessica Anderson, M.S., Fei Tang, M.S., and Katherine Wagner, M.P.H. for their assistance in screening, recruiting, interviewing, and translating materials for patient research participants. We thank Liisa Ecola, M.P.P. for her help with project management, and Tiffany Hruby for manuscript preparation and project administrative assistance. Finally, we thank Michael Schoenbaum, Ph.D., the study Science Officer from the National Institute of Mental Health.
Funding Source
This research was supported by a grant to Drs. Watkins and Komaromy (Multiple PIs) from the National Institute of Mental Health/NIMH (U01MH121954).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control. Drug overdose deaths. 2018; https://www.cdc.gov/drugoverdose/data/statedeaths.html.Accessed March 4, 2019.
- 2.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 3.Han B, Compton WM, Blanco C, Colpe LJ. Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Affairs. 2017;36(10):1739–1747. doi: 10.1377/hlthaff.2017.0584. [DOI] [PubMed] [Google Scholar]
- 4.National Drug Intelligence Center. The economic impact of illicit drug use on American society. Washington D.C.: U.S. Department of Justice; 2011. [Google Scholar]
- 5.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 6.Dore G, Mills K, Murray R, Teesson M, Farrugia P. Post-traumatic stress disorder, depression and suicidality in inpatients with substance use disorders. Drug and Alcohol Review. 2012;31(3):294–302. doi: 10.1111/j.1465-3362.2011.00314.x|. [DOI] [PubMed] [Google Scholar]
- 7.Meier A, Lambert-Harris C, McGovern MP, Xie H, An M, McLeman B. Co-occurring prescription opioid use problems and posttraumatic stress disorder symptom severity. The American Journal of Drug and Alcohol Abuse. 2014;40(4):304–311. doi: 10.3109/00952990.2014.910519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills KL, Teesson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: Findings from the Australian national survey of mental health and well-being. American Journal of Psychiatry. 2006;163(4):652–658. [DOI] [PubMed] [Google Scholar]
- 9.Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. Journal of Clinical Psychiatry. 2016;77(10):1413–1419. doi: 10.4088/JCP.15m09963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. New England Journal of Medicine. 2016;374(2):154–163. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug and Alcohol Dependence. 2013;132(1–2):95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Kern AM, Akerman SC, Nordstrom BR. Opiate dependence in schizophrenia: Case presentation and literature review. Journal of Dual Diagnosis. 2014;10(1):52–57. doi: 10.1080/15504263.2013.867199. [DOI] [PubMed] [Google Scholar]
- 13.Proctor SL, Estroff TW, Empting LD, Shearer-Williams S, Hoffmann NG. Prevalence of substance use and psychiatric disorders in a highly select chronic pain population. Journal of Addiction Medicine. 2013;7(1):17–24. doi: 10.1097/ADM.0b013e3182738655. [DOI] [PubMed] [Google Scholar]
- 14.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 15.Shei A, Rice JB, Kirson NY, Bodnar K, Enloe CJ, Birnbaum HG, Holly P, Ben-Joseph R. Characteristics of high-cost patients diagnosed with opioid abuse. Journal of Managed Care & Specialty Pharmacy. 2015;21(10):902–912. doi: 10.18553/jmcp.2015.21.10.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiner B, Leonard Westgate C, Bernardy NC, Schnurr PP, Watts BV. Trends in opioid use disorder diagnoses and medication treatment among veterans with posttraumatic stress disorder. Journal of Dual Diagnosis. 2017;13(3):201–212. doi: 10.1080/15504263.2017.1325033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):603–613. doi: 10.1001/archpsyc.62.6.603. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Otolaryngology - Head and Neck Surgery. Quality ID #468 (NQF 3175): Continuity of pharmacotherapy for opioid use disorder (OUD). 2018; https://www.entnet.org/sites/default/files/uploads/PracticeManagement/Resources/_files/2019_measure_468_mipscqm.pdf. Accessed March 19, 2019.
- 19.Jaycox LH, Miranda J, Meredith LS, Duan N, Benjamin B, Wells K. Impact of a primary care quality improvement intervention on use of psychotherapy for depression. Mental Health Services Research. 2003;5(2):109–120. doi: 10.1023/A:1023233612022. [DOI] [PubMed] [Google Scholar]
- 20.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug and Alcohol Dependence. 2019;197:78–82. doi: 10.1016/j.drugalcdep.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Katon WJ, Zatzick D, Bond G, Williams J Jr., Dissemination of evidence-based mental health interventions: Importance to the trauma field. Journal of Traumatic Stress. 2006;19(5):611–623. doi: 10.1002/jts.20147. [DOI] [PubMed] [Google Scholar]
- 22.Lecrubier Y. Posttraumatic stress disorder in primary care: A hidden diagnosis. Journal of Clinical Psychiatry. 2004;65 Suppl 1:49–54. [PubMed] [Google Scholar]
- 23.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of Substance Abuse Treatment. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak P, Feder KA, Ali MM, Chen J. Behavioral health treatment utilization among individuals with co-occurring opioid use disorder and mental illness: Evidence from a national survey. Journal of Substance Abuse Treatment. 2019;98:47–52. doi: 10.1016/j.jsat.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. Journal of Substance Abuse Treatment. 2018;95:9–17. doi: 10.1016/j.jsat.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unutzer J, Rubenstein L, Katon WJ, Tang L, Duan N, Lagomasino IT, Wells KB. Two-year effects of quality improvement programs on medication management for depression. Archives of General Psychiatry. 2001;58(10):935–942. doi: 10.1001/archpsyc.58.10.935. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine. Improving the quality of health care for mental and substance-use conditions: Quality chasm series. Appendix B, Constraints on sharing mental health and substance-use treatment information imposed by federal and state medical records privacy laws. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 28.Kopera M, Suszek H, Bonar E, Myszka M, Gmaj B, Ilgen M, Wojnar M. Evaluating explicit and implicit stigma of mental illness in mental health professionals and medical students. Community Mental Health Journal. 2015;51(5):628–634. doi: 10.1007/s10597-014-9796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raven MC, Carrier ER, Lee J, Billings JC, Marr M, Gourevitch MN. Substance use treatment barriers for patients with frequent hospital admissions. Journal of Substance Abuse Treatment. 2010;38(1):22–30. doi: 10.1016/j.jsat.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Stull LG, McGrew JH, Salyers MP, Ashburn-Nardo L. Implicit and explicit stigma of mental illness: Attitudes in an evidence-based practice. Journal of Nervous and Mental Disease. 2013;201(12):1072–1079. doi: 10.1097/nmd.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: Results from a survey of general internists. Substance Abuse. 2016;37(4):635–641. doi: 10.1080/08897077.2016.1187240. [DOI] [PubMed] [Google Scholar]
- 32.Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, Lovell K, Dickens C, Gask L, Waheed W, Bower P. Characteristics of effective collaborative care for treatment of depression: A systematic review and meta-regression of 74 randomised controlled trials. PloS One. 2014;9(9):e108114. doi: 10.1371/journal.pone.0108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroenke K, Unutzer J. Closing the false divide: Sustainable approaches to integrating mental health services into primary care. Journal of General Internal Medicine. 2017;32(4):404–410. doi: 10.1007/s11606-016-3967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woltmann E, Grogan-Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: Systematic review and meta-analysis. American Journal of Psychiatry. 2012;169(8):790–804. doi: 10.1176/appi.ajp.2012.11111616. [DOI] [PubMed] [Google Scholar]
- 35.Meredith LS, Eisenman DP, Han B, Green BL, Kaltman S, Wong EC, Sorbero M, Vaughan C, Cassells A, Zatzick D. Impact of collaborative care for underserved patients with PTSD in primary care: A randomized controlled trial. Journal of General Internal Medicine. 2016;31(5):509–517. doi: 10.1007/s11606-016-3588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnurr PP, Friedman MJ, Oxman TE, Dietrich AJ, Smith MW, Shiner B, Forshay E, Gui J, Thurston V. RESPECT-PTSD: Re-engineering systems for the primary care treatment of PTSD, a randomized controlled trial. Journal of General Internal Medicine. 2013;28(1):32–40. doi: 10.1007/s11606-012-2166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton I, Hurley B, Castillo E, Tang L, Gilmore J, Jones F, Watkins KE, Wells K. 6-month outcomes of two quality improvement depression care interventions in individuals with substance use problems. Community Partners in Care. Under review [Google Scholar]
- 38.Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. American Journal of Psychiatry. 2006;163(1):125–132. doi: 10.1176/appi.ajp.163.1.125. [DOI] [PubMed] [Google Scholar]
- 39.Fortney JC, Pyne JM, Kimbrell TA, Hudson TJ, Robinson DE, Schneider R, Moore WM, Custer PJ, Grubbs KM, Schnurr PP. Telemedicine-based collaborative care for posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry. 2015;72(1):58–67. doi: 10.1001/jamapsychiatry.2014.1575. [DOI] [PubMed] [Google Scholar]
- 40.Unützer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noël PH, Lin EH. Collaborative care management of late-life depression in the primary care setting: A randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 41.Watkins KE, Ober AJ, Lamp K, Lind M, Setodji C, Osilla KC, Hunter SB, McCullough CM, Becker K, Iyiewuare PO, Diamant A, Heinzerling K, Pincus HA. Collaborative care for opioid and alcohol use disorders in primary care: The SUMMIT randomized clinical trial. JAMA Internal Medicine. 2017;177(10):1480–1488. doi: 10.1001/jamainternmed.2017.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells KB, Sherbourne C, Schoenbaum M, Duan N, Meredith L, Unützer J, Miranda J, Carney MF, Rubenstein LV. Impact of disseminating quality improvement programs for depression in managed primary care: A randomized controlled trial. JAMA. 2000;283(2):212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 43.Trust for America’s Health. Pain in the nation: The drug, alcohol and suicide crises and the need for a national resilience strategy. Washington, D.C.: Trust for America’s Health; 2017. Available at: http://www.paininthenation.org/assets/pdfs/TFAH-2017-PainNationRpt.pdf. [Google Scholar]
- 44.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical Care. 2012;50(3):217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, Tunis S, Bergel E, Harvey I, Magid DJ, Chalkidou K. A Pragmatic-Explanatory Continuum Indicator Summary (PRECIS): A tool to help trial designers. Journal of Clinical Epidemiology. 2009;62(5):464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 46.NIH Collaboratory. Introduction to pragmatic clinical trials. undated; https://www.nihcollaboratory.org/Products/Introduction%20to%20pragmatic%20clinical%20trials.pdf. Accessed June 25, 2018.
- 47.NIH Collaboratory. Rethinking clinical trials: A living textbook of pragmatic clinical trials. undated; http://www.rethinkingclinicaltrials.org/.Accessed June 25, 2018.
- 48.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624 [DOI] [PubMed] [Google Scholar]
- 49.Henry J. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). 2017; https://www.kff.org/other/state-indicator/opioid-overdose-death-rates/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D.Accessed March 19, 2019.
- 50.United States Census Bureau. American community survey (ACS). 2017; https://www.census.gov/programs-surveys/acs/about.html.Accessed March 4, 2019.
- 51.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miranda J, Schoenbaum M, Sherbourne C, Duan N, Wells K. Effects of primary care depression treatment on minority patients’ clinical status and employment. Archives of General Psychiatry. 2004;61(8):827–834. doi: 10.1001/archpsyc.61.8.827. [DOI] [PubMed] [Google Scholar]
- 53.Adam A, Schwartz RP, Wu L-T, Subramaniam G, Laska E, Sharma G, Mili S, McNeely J. Electronic self-administered screening for substance use in adult primary care patients: Feasibility and acceptability of the tobacco, alcohol, prescription medication, and other substance use (myTAPS) screening tool. Addiction Science & Clinical Practice. 2019;14(1):39. doi: 10.1186/s13722-019-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, Kaloupek DG, Schnurr PP, Kaiser AP, Leyva YE. The primary care PTSD screen for DSM-5 (PC-PTSD-5): Development and evaluation within a veteran primary care sample. Journal of General Internal Medicine. 2016;31(10):1206–1211. doi: 10.1007/s11606-016-3703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 56.Renn BN, Mosser BA, Raue PJ. Problem-Solving Therapy. In: Tampi RR, Yarns BC, Zdanys KF, Tampi DJ, eds. Psychotherapy in later life. Cambridge, U.K.: Cambridge University Press; 2020:75–91. [Google Scholar]
- 57.Sloan DM, Marx BP. Written Exposure Therapy for PTSD: A brief treatment approach for mental health professionals. Washington, D.C.: American Psychological Association; 2019. [Google Scholar]
- 58.University of Washington, Psychiatry & Behavioral Sciences, Division of Population Health, AIMS Center. Patient-centered integrated behavioral health care: Principles & tasks checklist. 2014; https://aims.uw.edu/sites/default/files/CollaborativeCarePrinciplesAndComponents_2014-12-23.pdf.Accessed June 3, 2020.
- 59.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Affairs. 2009;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Quarterly. 1996;74(4):511–544. doi: 10.2307/3350391. [DOI] [PubMed] [Google Scholar]
- 61.Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, Dickens C, Coventry P. Collaborative care for depression and anxiety problems. Cochrane Database of Systematic Reviews. 2012;10:Cd006525. doi: 10.1002/14651858.CD006525.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leshner AI, Mancher M (Eds.). Medications for Opioid Use Disorder Save Lives. Washington, DC: National Academies of Sciences, Engineering, and Medicine; 2019. [PubMed] [Google Scholar]
- 63.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. New England Journal of Medicine. 2014;370(22):2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 64.Page-Reeves J, Bleecker M. WellRx Toolkit. Albuquerque, NM: Office for Community Health, University of New Mexico; 2014. [Google Scholar]
- 65.University of Washington, Psychiatry & Behavioral Sciences, AIMS Center. Behavioral health integration and collaborative care: Registry strategies in medical settings. 2018; https://aims.uw.edu/sites/default/files/Collaborative%20Care%20Registry%20Requirements%20Guide_2019.pdf.Accessed December 7, 2020.
- 66.Zero to Three. Reflective supervision essentials. 2020; https://www.zerotothree.org/resources/1020-reflective-supervision-essentials.Accessed December 7, 2020.
- 67.Komaromy M, Bartlett J, Manis K, Arora S. Enhanced primary care treatment of behavioral disorders with ECHO case-based learning. Psychiatric Services. 2017;68(9):873–875. doi: 10.1176/appi.ps.201600471. [DOI] [PubMed] [Google Scholar]
- 68.Komaromy M, Bartlett J, Zurawski A, Gonzales-van Horn S, Kalishman S, Ceballos V, Sun X, Jurado M, Arora S. ECHO Care: Providing multidisciplinary specialty expertise to support the care of complex patients. Journal of General Internal Medicine. 2020;35(1):326–330. doi: 10.1007/s11606-019-05205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Komaromy M, Bartlett J, Zurawski A, Gonzales-van Horn S, Kalishman S, Davis H, Ceballos V, Sun X, Jurado J, Page K, Hamblin A, Arora SA. Novel intervention for high-need, high-cost medicaid patients: A study of ECHO care. Journal of General Internal Medicine. 2020;35(1):21–27. doi: 10.1007/s11606-019-05206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komaromy M, Ceballos V, Zurawski A, Bodenheimer T, Thom DH, Arora S. Extension for Community Healthcare Outcomes (ECHO): A new model for community health worker training and support. Journal of Public Health Policy. 2018;39(2):203–216. doi: 10.1057/s41271-017-0114-8. [DOI] [PubMed] [Google Scholar]
- 71.Komaromy M, Duhigg D, Metcalf A, Carlson C, Kalishman S, Hayes L, Burke T, Thornton K, Arora S. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Substance Abuse. 2016;37(1):20–24. doi: 10.1080/08897077.2015.1129388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas CP, Garnick DW, Horgan CM, Miller K, Harris AH, Rosen MM. Establishing the feasibility of measuring performance in use of addiction pharmacotherapy. Journal of Substance Abuse Treatment. 2013;45(1):11–18. doi: 10.1016/j.jsat.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Medicare & Medicaid Services MIT. Continuity of pharmacotherapy for opioid use disorder (OUD). undated; https://cmit.cms.gov/CMIT_public/ViewMeasure?MeasureId=5881.Accessed June 9, 2020.
- 74.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 75.Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health (NSDUH), 2019 (Final CAI Specifications for Programming, English version). Rockville, MD: Substance Abuse and Mental Health Services Administration; 2019. [Google Scholar]
- 76.Pilkonis PA, Yu L, Dodds NE, Johnston KL, Lawrence SM, Hilton TF, Daley DC, Patkar AA, McCarty D. Item banks for substance use from the Patient-Reported Outcomes Measurement Information System (PROMIS(®)): Severity of use and positive appeal of use. Drug and Alcohol Dependence. 2015;156:184–192. doi: 10.1016/j.drugalcdep.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Babor TF, Higgins-Biddle JC, Robaina K. USAUDIT: The Alcohol Use Disorders Identification Test, Adapted for Use in the United States: A Guide for Primary Care Practitioners. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. Available at: https://www.dshs.wa.gov/sites/default/files/BHSIA/dbh/wasbirt/USAUDIT-Guide_2016.pdf. [Google Scholar]
- 78.Bohnert AS, Bonar EE, Cunningham R, Greenwald MK, Thomas L, Chermack S, Blow FC, Walton M. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug and Alcohol Dependence. 2016;163:40–47. doi: 10.1016/j.drugalcdep.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 79.Open Society Foundations. Overdose baseline questionnaire, Naloxone info. 2013; http://www.naloxoneinfo.org/sites/default/files/OD%20Baseline_Questionnaire%20ENG.pdf.Accessed March 18, 2019.
- 80.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S. The Columbia–Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. National death index. 2020; https://www.cdc.gov/nchs/ndi/index.htm.Accessed June 9, 2020.
- 82.National Committee for Quality Assurance (NCQA). Initiation and engagement of alcohol and other drug abuse or dependence treatment (IET). 2020; https://www.ncqa.org/hedis/measures/initiation-and-engagement-of-alcohol-and-other-drug-abuse-or-dependence-treatment/.Accessed June 9, 2020.
- 83.Hepner KA, Roth CP, Sloss EM, Paddock SM, Iyiewuare PI, Timmer M, Pincus HA. Quality of care for PTSD and depression in the Military Health System: Final report (RR-1542-OSD). Santa Monica, CA: RAND Corporation; 2017. [PMC free article] [PubMed] [Google Scholar]
- 84.Selim AJ, Rogers W, Fleishman JA, Qian SX, Fincke BG, Rothendler JA, Kazis LE. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Quality of Life Research. 2009;18(1):43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 85.Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. American Journal of Public Health. 2013;103(S2):S210–S211. doi: 10.2105/AJPH.2013.301398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Substance Abuse and Mental Health Services Administration. Government performance and results act (GPRA). Client outcome measures for discretionary programs. Question-by-question instruction guide, version 9.6. 2013; https://www.samhsa.gov/sites/default/files/GPRA/SAIS_GPRA_Services_Tool_QxQ_final.pdf.Accessed June 9, 2020.
- 87.Agency for Healthcare Research and Quality. CAHPS ECHO survey measures. 2018; http://www.ahrq.gov/cahps/surveys-guidance/echo/about/surveymeasures.html.Accessed March 18, 2019.
- 88.Sturgiss EA, Rieger E, Haesler E, Ridd MJ, Douglas K, Galvin SL. Adaption and validation of the Working Alliance Inventory for General Practice: Qualitative review and cross-sectional surveys. Family Practice. 2019;36(4):516–522. doi: 10.1093/fampra/cmy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Medical Care. 2004;42(12):1194–1201. https://www.jstor.org/stable/4640875. [DOI] [PubMed] [Google Scholar]
- 91.Cigrang JA, Rauch SA, Mintz J, Brundige AR, Mitchell JA, Najera E, Litz BT, Young-McCaughan S, Roache JD, Hembree EA. Moving effective treatment for posttraumatic stress disorder to primary care: A randomized controlled trial with active duty military. Families, Systems, & Health. 2017;35(4):450–462. doi: 10.1037/fsh0000315. [DOI] [PubMed] [Google Scholar]
- 92.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological Methods. 2010;15(4):309. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 93.Imai K, Keele L, Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Statistical science. 2010:51–71. doi: 10.1214/10-STS321. [DOI] [Google Scholar]
- 94.Brown PJ, Recupero PR, Stout R. PTSD substance abuse comorbidity and treatment utilization. Addictive Behaviors. 1995;20(2):251–254. doi: 10.1016/0306-4603(94)00060-3. [DOI] [PubMed] [Google Scholar]
- 95.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 96.Mills KL, Teesson M, Ross J, Darke S, Shanahan M. The costs and outcomes of treatment for opioid dependence associated with posttraumatic stress disorder. Psychiatric Services. 2005;56(8):940–945. doi: 10.1176/appi.ps.56.8.940. [DOI] [PubMed] [Google Scholar]
- 97.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biological Psychiatry. 2004;56(10):793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 98.Ouimette PC, Ahrens C, Moos RH, Finney JW. Posttraumatic stress disorder in substance abuse patients: Relationship to 1-year posttreatment outcomes. Psychology of Addictive Behaviors. 1997;11(1):34. doi: 10.1037/0893-164X.11.1.34. [DOI] [Google Scholar]
- 99.Ouimette PC, Brown PJ, Najavits LM. Course and treatment of patients with both substance use and posttraumatic stress disorders. Addictive Behaviors. 1998;23(6):785–795. doi: 10.1016/S0306-4603(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 100.National Institute of Mental Health. U.S. leading categories of diseases/disorders. undated; https://www.nimh.nih.gov/health/statistics/disability/us-leading-categories-of-diseases-disorders.shtml.Accessed March 19, 2019.
- 101.Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. New England Journal of Medicine. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 102.Saitz R, Daaleman TP. Now is the time to address substance use disorders in primary care. Annals of Family Medicine. 2017;15(4):306–308. doi: 10.1370/afm.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knudsen HK, Abraham AJ, Oser CB. Barriers to the implementation of medication-assisted treatment for substance use disorders: The importance of funding policies and medical infrastructure. Evaluation and Program Planning. 2011;34(4):375–381. doi: 10.1016/j.evalprogplan.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: Opinions of drug injecting street outreach clients and other system stakeholders. The American Journal of Drug and Alcohol Abuse. 2004;30(1):129–153. doi: 10.1081/ADA-120029870 [DOI] [PubMed] [Google Scholar]
- 105.Cunningham JA, Sobell LC, Sobell MB, Agrawal S, Toneatto T. Barriers to treatment: Why alcohol and drug abusers delay or never seek treatment. Addictive Behaviors. 1993;18(3):347–353. doi: 10.1016/0306-4603(93)90036-9. [DOI] [PubMed] [Google Scholar]
- 106.Beronio K, Po R, Skopec L. Affordable Care Act will expand mental health and substance use disorder benefits and parity protections for 62 million Americans. ASPE Research Brief. 2013; http://aspe.hhs.gov/health/reports/2013/mental/rb_mental.cfm.Accessed February 20, 2013. [Google Scholar]
- 107.Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Affairs. 2011;30(8):1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- 108.Cherpitel CJ, Ye Y. Trends in alcohol- and drug-related emergency department and primary care visits: Data from four U.S. national surveys (1995–2010). Journal of Studies on Alcohol and Drugs. 2012;73(3):454–458. doi: 10.15288/jsad.2012.73.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: A review. Journal of Substance Abuse and Rehabilitation. 2012;3(1):25–34. doi: 10.2147/SAR.S30057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander CL, Arnkoff DB, Glass CR. Bringing psychotherapy to primary care: Innovations and challenges. Clinical Psychology: Science and Practice. 2010;17(3):191–214. doi: 10.1111/j.1468-2850.2010.01211.x|. [DOI] [Google Scholar]
- 111.Rosenbaum S, Paradise J, Markus A, Sharac J, Tran C, Reynolds D, Shin P. Issue brief: Community health centers: Recent growth and the role of the ACA. Menlo Park, CA: The Henry J. Kaiser Foundation; 2017. [Google Scholar]
- 112.Van Boekel LC, Brouwers EPM, Van Weeghel J, Garretsen HFL. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: Systematic review. Drug and Alcohol Dependence. 2013;131(1–2):23–35. doi: 10.1016/j.drugalcdep.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 113.Davenport S, Matthews K. Milliman White Paper: Opioid use disorder in the United States: Diagnosed prevalence by payer, age, sex, and state. Seattle, WA: Milliman; 2018. Available at: http://www.milliman.com/uploadedFiles/insight/2018/Opioid_Use_Disorder_Prevalence.pdf. [Google Scholar]
- 114.Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed tables. 2016; http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUHDetTabs-2015/NSDUH-DetTabs-2015.pdf.Accessed December 7, 2020.
- 115.Wu L-T, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug and Alcohol Dependence. 2016;169:117–127. doi: 10.1016/j.drugalcdep.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrilla CHA, Moore TE, Patterson DG, Larson EH. Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: A 5-year update. The Journal of Rural Health. 2019;35(1):108–112. doi: 10.1111/jrh.12307|. [DOI] [PubMed] [Google Scholar]
- 117.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Annals of Internal Medicine. 1997;127(12):1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 118.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Asch SM, Kroenke K. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. Journal of General Internal Medicine. 2009;24(6):733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The Addiction Severity Index: A field study of internal consistency and validity. Journal of Substance Abuse Treatment. 2000;18(2):129–135. doi: 10.1016/S0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 120.Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. The International Journal of Psychiatry in Medicine. 1997;27(2):93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- 121.United States Department of Agriculture. Rural-urban commuting area codes. 2019; https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/.Accessed June 9, 2020.