Abstract
The delivery of alkyl radicals through photocatalytic deoxygenation of primary alcohols under mild conditions is a so far unmet challenge. In this report, we present a one-pot strategy for deoxygenative Giese reaction of alcohols with electron-deficient alkenes, by using xanthate salts as alcohol-activating groups for radical generation under visible-light photoredox conditions in the presence of triphenylphosphine. The convenient generation of xanthate salts and high reactivity of sequential C–S/C–O bond homolytic cleavage enable efficient deoxygenation of primary, secondary and tertiary alcohols with diverse functionality and structure to generate the corresponding alkyl radicals, including methyl radical. Moreover, chemoselective radical monodeoxygenation of diols is achieved via selective formation of xanthate salts.
Subject terms: Reactive precursors, Synthetic chemistry methodology, Photocatalysis
The generation of alkyl radicals through deoxygenation of abundant alcohols via photoredox catalysis is of interest. In this study, the authors report a one-pot strategy for visible-light-promoted photoredox coupling of alcohols with electron-deficient alkenes, assisted by carbon disulfide and triphenylphosphine.
Introduction
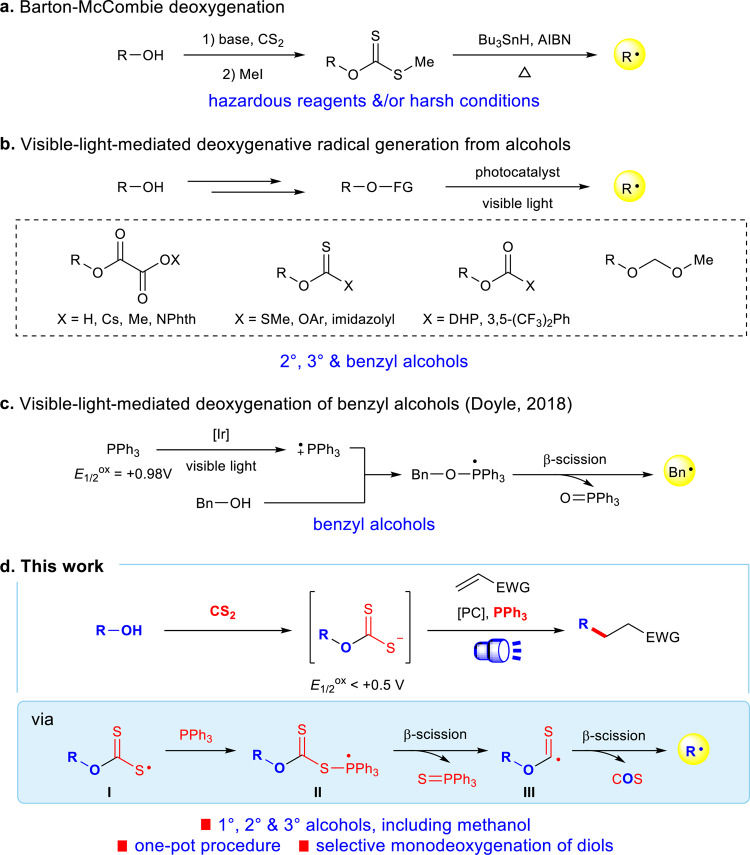
Over the past decades, visible-light photoredox catalysis has emerged as a pre-eminent handle for the generation of alkyl radicals via single electron transfer (SET)1–4. Avoiding the use of stoichiometric amounts of hazardous reagents and/or harsh reaction conditions in traditional methods such as Barton–McCombie deoxygenation (Fig. 1a)5–8, the delivery of alkyl radicals through deoxygenation of widely occurring, naturally abundant alcohols via photoredox catalysis is strategically appealing9–11. However, the strong bonding energy and the high redox potentials of C–O bonds have deterred the identification of general solutions in the field of photoredox catalysis12,13. To solve this problem, redox auxiliaries that include oxalates14–23, xanthates19,24–27, carboxylates28–30, and ethers31 have emerged to transform hydroxyl groups into activating groups for alkyl radical generation via C–O bond homolysis under visible-light photoredox conditions (Fig. 1b). Nevertheless, these approaches are generally not amenable to primary aliphatic alcohols. For example, oxalates derived from primary alcohols can only generate alkyl radicals at high temperatures, due to the relatively low reactivity of the intermediate alkoxycarbonyl radicals in losing carbon dioxide. Minisci-type reactions using primary alcohols, including methanol, as alkyl precursors have been achieved under photoredox conditions via α-H abstraction of alcohols and C–O bond cleavage by spin-center shift elimination of water; however, these approaches are limited to the alkylation of heteroarenes32–34. In 2018, Doyle and Rovis reported the elegant deoxygenative generation of alkyl radicals directly from alcohols under visible-light photoredox conditions without preactivation steps (Fig. 1c)35. In this catalytic system, triphenylphosphine is first oxidized to a radical cation by photoexcited *Ir(III) catalyst. The triphenylphosphine radical cation reacts ionically with an alcohol to form a phosphoranyl radical, which proceeds through β-scission to deliver an alkyl radical. However, due to the limited substrate scope of the β-scission of phosphoranyl radical36–38, this approach is only amenable to benzylic alcohols. To date, there is not a universally applicable photo-induced deoxygenation method for aliphatic alcohols with great selectivity and efficiency, especially one that is compatible with multiple free hydroxyl groups ubiquitously present in biological molecules39–42. Therefore, a more convenient and compatible protocol for deoxygenative generation of alkyl radicals from alcohols represents unmet challenge and urgent demand.
Fig. 1. Deoxygenative alkyl radical generation from alcohols under visible-light photoredox conditions.
a Barton–McCombie deoxygenation; b visible-light-mediated deoxygenative radical generation from alcohols; c visible-light-mediated deoxygenation of benzyl alcohols. d Our work. FG functional group, [Ir] [Ir(dFMeppy)2dtbbpy]PF6, PC photocatalyst, EWG electron-withdrawing group.
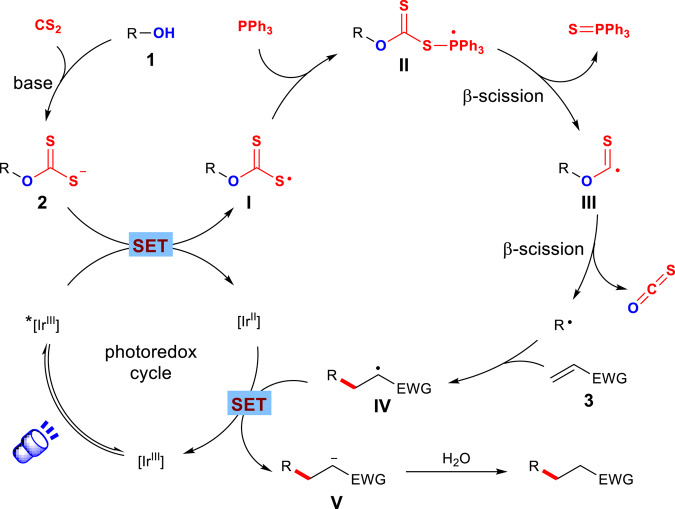
While Barton–McCombie deoxygenations with xanthate esters have been well established5–7,43,44, to the best of our knowledge, the direct use of xanthate salts for radical activations of alcohols has not been reported. Very recently, the strategy of visible-light photoredox-catalyzed phosphoranyl radical fragmentation45–49 provides a powerful platform for C–O and C–S bond homolysis of carboxylic acids50–56 and thiols57–59. Inspired by these discoveries, we proposed a masked strategy by switching the redox site from the C–O bond to the weaker and SET-amenable C–S bond (Fig. 1d). Treatment of an alcohol with carbon disulfide under basic conditions can readily generate a xanthate anion. We envisioned that the xanthate anion, with low oxidation potential, would undergo SET with an excited state photocatalyst to produce the xanthate radical I60,61. This sulfur-centered radical will subsequently couple with a phosphine source to afford the phosphoranyl radical II62–65, which then undergoes β-scission to form the alkoxythiocarbonyl radical III. Theoretical calculations have demonstrated that alkoxythiocarbonyl radicals III extrude carbonyl sulfide (COS) rapidly through β-scission to produce the corresponding alkyl radicals, including the methyl radical, in three orders of magnitude faster than the loss of carbon dioxide from alkoxycarbonyl radicals66. In comparison to the phosphine-mediated direct deoxygenation of alcohols developed by Doyle and co-workers (Fig. 1c)35, the high reactivity and low voltage gating of xanthate anion will advantage this strategy by energetically more accessible electron transfer and diverse substrate scope other than benzylic alcohols, providing a unique solution for the longstanding challenge on structure generality limitation of alcohols as alkyl radical precursors.
Here we show a visible-light-promoted photoredox coupling of alcohols with electron-deficient alkenes assisted by carbon disulfide and triphenylphosphine. This one-pot protocol enables the selective C–O bond homolysis of primary, secondary, and tertiary alcohols to form C–C bonds efficiently under mild conditions.
Results
Optimization of reaction conditions
To evaluate the reaction platform, we first explored the photoredox-catalyzed deoxygenative alkylation of N-Boc-4-hydroxy-piperidine 1aa with benzyl acrylate 3a by a carbon disulfide and triphenylphosphine promoted one-pot protocol. Through extensive condition optimization, we found that deprotonation of N-Boc-4-hydroxypiperidine 1aa using NaOtBu (1.0 equiv) as a base followed by treatment with carbon disulfide (1.5 equiv) in tetrahydrofuran (THF) readily formed the corresponding sodium xanthate 2aa (Table 1). After evaporating all volatiles, exposure of a solution of the residue with benzyl acrylate 3a, photocatalyst [Ir(ppy)2dtbbpy](PF6), and triphenylphosphine in acetonitrile containing water to a 30 W blue light-emitting diode (LED) lamp provided the desired alkylation product 4aa in 89% isolated yield, along with triphenylphosphine sulfide in 86% yield indicating a desulfurization process. Meanwhile, the carbonyl sulfur (COS) was detected by gas chromatography–mass spectrometry (Table 1, entry 1). Triethyl phosphite could also be used as the phosphorus source in place of triphenylphosphine, providing the product in 84% yield (entry 2). Use of [Ir(dFCF3ppy)2dtbbpy)](PF6) as the photocatalyst resulted in a slightly less efficient reaction (entry 3). Weak bases such as Na2CO3 were not suitable for the generation of the xanthate salt (entry 4). No reaction was observed without treatment with carbon disulfide, and this observation excluded the direct activation of alcohols by triphenylphosphine under the photoredox conditions (entry 5). Further control experiments conducted in the absence of phosphine, photocatalyst, or light resulted in no product formation, emphasizing the crucial role of all these components in the catalytic cycle (entries 6–8). Significantly lower yield was observed in the absence of water, perhaps due to its role as the proton source (entry 9). Reaction with 1.0 equiv of benzyl acrylate 3a provided the product in 66% yield (entry 10). Reducing the amount of triphenylphosphine to 0.20 equiv decreased the yield to 20%, correspondingly excluding a triphenylphosphine-initiated/catalyzed pathway (entry 11). In addition, our attempts on using 0.20 equiv of triphenylphosphine combined with stoichiometric reductants failed to increase the yield to exceed 20% (Supplementary Table 5).
Table 1.
Optimization and control studies.
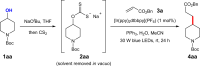 | ||
|---|---|---|
| Entry | Variation from optimized conditions | Yield (%)a |
| 1 | None | 92 (89)b |
| 2 | P(OEt)3 instead of PPh3 | 84 |
| 3 | [Ir(dFCF3ppy)2dtbbpy)](PF6) (1 mol %) | 83 |
| 4 | Na2CO3 instead of NaOtBu | 0 |
| 5 | No CS2 | 0 |
| 6 | No PPh3 | 0 |
| 7 | No photocatalyst | 0 |
| 8 | No light | 0 |
| 9 | Without H2O | 26 |
| 10 | 3a (1.0 equiv) | 66 |
| 11 | PPh3 (0.20 equiv) | 20 |
| 12 | PPh3 (0.20 equiv) + reductants (2.0 equiv) | <20 |
Standard procedure: 1aa (0.20 mmol), NaOtBu (1.0 equiv), THF (1.2 mL), CS2 (1.5 equiv), 3 h. After removing solvent in vacuo, then 3a (2.0 equiv), [Ir(ppy)2dtbbpy](PF6) (1 mol%), PPh3 (1.2 equiv), H2O (7.0 equiv), MeCN (2.0 mL), 24 h blue LED irradiation.
Boc t-butoxycarbonyl.
aDetermined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard.
bIsolated yield.
Substrate scope
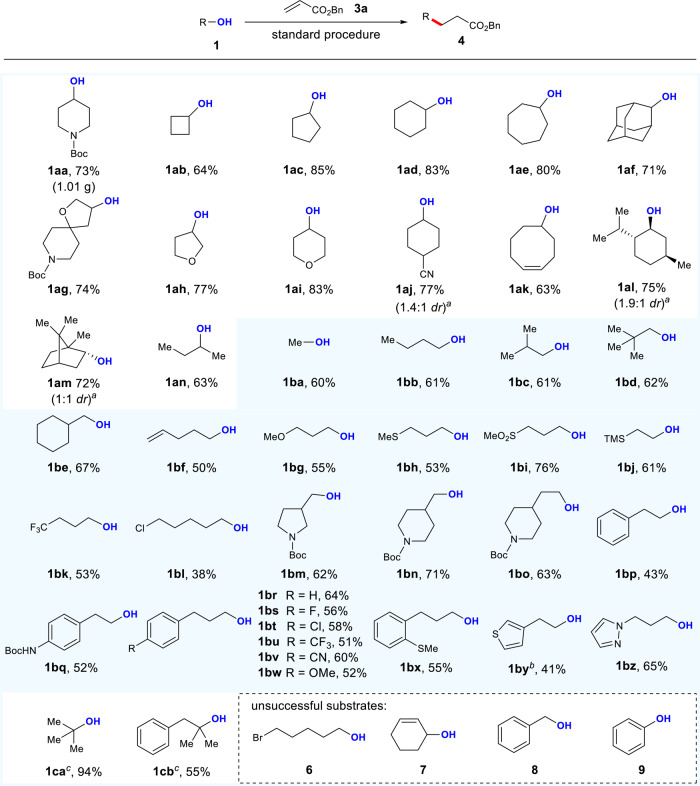
With the optimized conditions in hand, we investigated the scope of alcohols using this convenient one-pot protocol (Fig. 2). It is noteworthy that the reaction could be run on a 4.0 mmol scale in a Schlenk tube to afford 1.01 g of the desired product 4aa (73% yield). Cyclic alcohols (1ab–aj), ranging from four- to eight-membered rings, including spirocyclic or bridged motifs, adorned with nitrogen or oxygen atoms, successfully delivered the corresponding products in 64–85% yields upon Giese reaction with benzyl acrylate 3a. The alkene-retained product was obtained in 63% yield from cyclooct-4-en-1-ol 1ak, while intramolecular radical cyclization was not observed. This result suggests that the Giese addition of alkyl radical is faster than the 5-exo-trig cyclization (~1 × 105 s−1) under the reaction conditions67. It is noteworthy that natural products L-menthol 1al and (-)-borneol 1am reacted smoothly in this transformation, providing the deoxy-alkylated products in 75% and 72% yields, respectively. The linear secondary alcohol 1an also provided the desired adduct in 63% yield. Consistent with the expectation, we were pleased to find that a wide variety of primary alcohols (1ba–bz) including methanol 1ba were successfully applied in this protocol, furnishing the desired products in moderate-to-good yields. Functional groups such as terminal alkenes, ethers, thioethers, sulfones, silanes, trifluoromethyl, chlorides, fluorides, nitriles, Boc-protected amines, phenyls, and heteroarenes were well tolerated, with the deoxygenative alkylated products obtained in 38–76% yields. Furthermore, by using NaH as a base for sodium xanthate construction, tertiary alcohols (1ca, 1cb) could also be efficiently converted to the corresponding products. In terms of limitations, the current protocol is not applicable to alcohols containing highly electrophilic groups, like alkyl bromides 6. Unfortunately, neither allylic alcohols nor benzylic alcohols were competent substrates (7, 8). Deviating from radical conjugate addition, deoxygenation products cyclohexene and toluene were observed in these cases, respectively. Finally, using this protocol, the deoxygenative alkylation of phenol 9 via Csp2–O bond cleavage was not successful68–73.
Fig. 2. Scope of alcohols.
The standard procedure is as shown in Table 1. Isolated yields are reported. aThe dr was determined by 1H NMR analysis of the product after flash chromatography. b48 h. cNaH (1.0 equiv) as base. dr diastereoselectivity ratio.
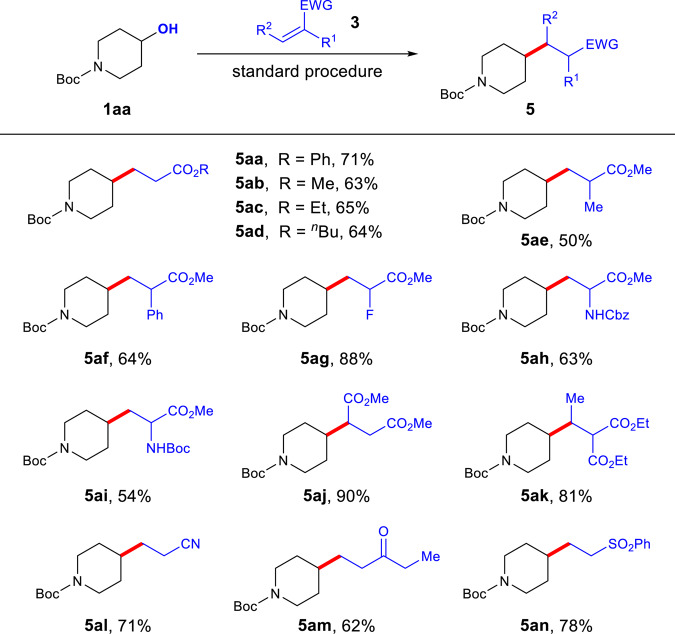
To further demonstrate the versatility of this method, a range of electron-deficient alkenes was tested (Fig. 3). As expected, various acrylates were capable acceptors in the reaction (5aa–ad). The reactions of α-aryl, α-fluoro, or α-alkyl substituted acrylates afforded desired products in 50–88% yields (5ae–ag). Notably, dehydroalanine derivatives participated in the reaction with good efficiency, providing convenient access to unnatural amino acids (5ah, 63%; 5ai, 54%). Substitution at the β-position was tolerated for more electron-deficient alkenes such as maleate and dimethyl ethylidenemalonate, furnishing the expected adducts with high efficiency (5aj, 90%; 5ak, 81%). Other electron-deficient alkenes, such as acrylonitrile, enone, and vinyl sulfone, all worked well (5al–an).
Fig. 3. Scope of alkenes.
The standard procedure is as shown in Table 1. Isolated yields are reported. Cbz benzyloxycarbonyl.
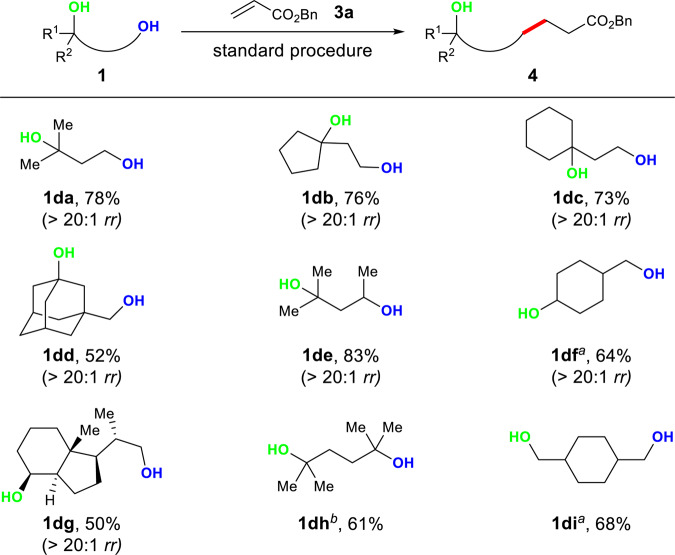
Polyols are a ubiquitous structural motif widely found in natural products and pharmaceuticals74. The selective transformation of hydroxyl groups in diols and polyols is a frequently encountered challenge42,75–80. We hypothesized that a proper base could identify primary, secondary, and tertiary alcohols in diols and polyols based on difference in acidity and steric hindrance, to form the corresponding xanthate salts selectively and realize the chemoselective radical O–H bond activation of diols and polyols. To our delight, by using NaOtBu as a base, high selectivity was observed when different diols were employed in the deoxygenative alkylation protocol, which can be summarized in decreasing order of reactivity as follows: primary alcohol > secondary alcohol > tertiary alcohol (Fig. 4). Selective deoxygenation of primary or secondary alcohol in diols (1da–de) over tertiary alcohol was achieved by the one-pot protocol to afford the corresponding products with free hydroxyl groups in 52–83% yields and >20:1 chemoselectivity. In addition, the deoxygenative transformation showed a predominant preference (>20:1 chemoselectivity) for primary alcohols over secondary ones (1df, 1dg). Mono-deoxylated products were obtained with the symmetrical diols (1dh, 1di). It is worth mentioning that no dialkylation products from these diols were observed under the standard conditions or with double the amount of other components in the protocol. Using 2.0 equiv of NaOtBu resulted in decreased yields of the desired products, while the deoxygenative alkylated compound from NaOtBu was formed.
Fig. 4. Selective deoxygenative alkylation of diols.
The standard procedure is as shown in Table 1. Isolated yields are reported. a48 h. bNaH as base. Unreacted hydroxyl groups are marked in green. rr regioselectivity ratio.
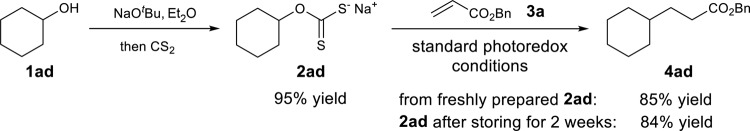
In addition to the one-pot procedure, the deoxygenative alkylation of alcohols can also be performed in steps (Fig. 5). Sodium cyclohexylxanthate 2ad was easily isolated as a pale yellow solid in 95% yield from the reaction mixture at the first step by filtration. Although xanthate salt is hygroscopic, it can be stored for extended periods in a capped container at room temperature without inert gas protection. Compared with freshly prepared salt (85%), the reaction of xanthate salt 2ad after storing for 2 weeks provided the deoxygenative alkylated product with no detriment to yield (84%).
Fig. 5. Preparation and reaction of xanthate salts.
Deoxygenative alkylation of alcohols performed in steps.
Synthetic applications
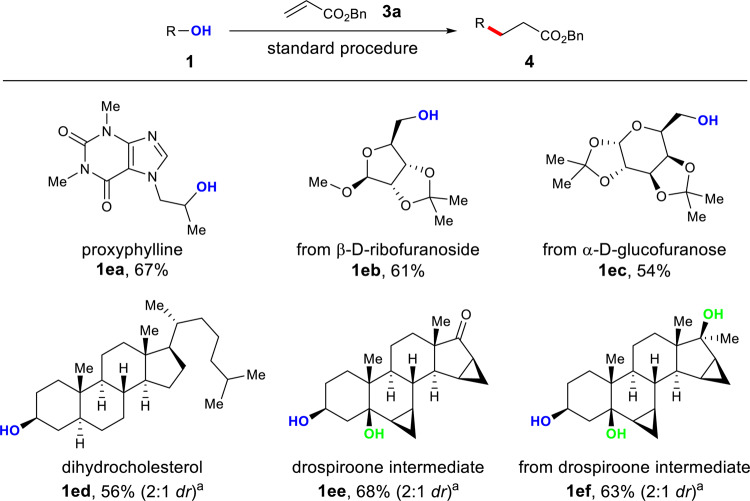
The generality of this method implies applications in the late-stage functionalization of pharmaceuticals and natural products (Fig. 6)81. Bronchodilator proxyphylline 1ea showed good reactivity to deliver the product in 67% yield. Acetonide D-ribofuranoside and D-glucofuranose derivatives (1eb, 1ec) were deoxygenated to afford the corresponding alkylated compounds in 61% and 54% yields, respectively. Naturally occurring steroids (1ed–ef) were successfully employed, furnishing the desired products in synthetically useful yields. The remarkable selectivity of the reaction was again showcased in alkaloids 1ee and 1ef with multiple hydroxyl groups by the selective deoxy-alkylation of secondary alcohol over tertiary alcohols.
Fig. 6. Synthetic applications of the methodology.
The standard procedure is as shown in Table 1. Isolated yields are reported. aThe dr was determined by 1H NMR analysis of the product after flash chromatography.
Mechanistic studies
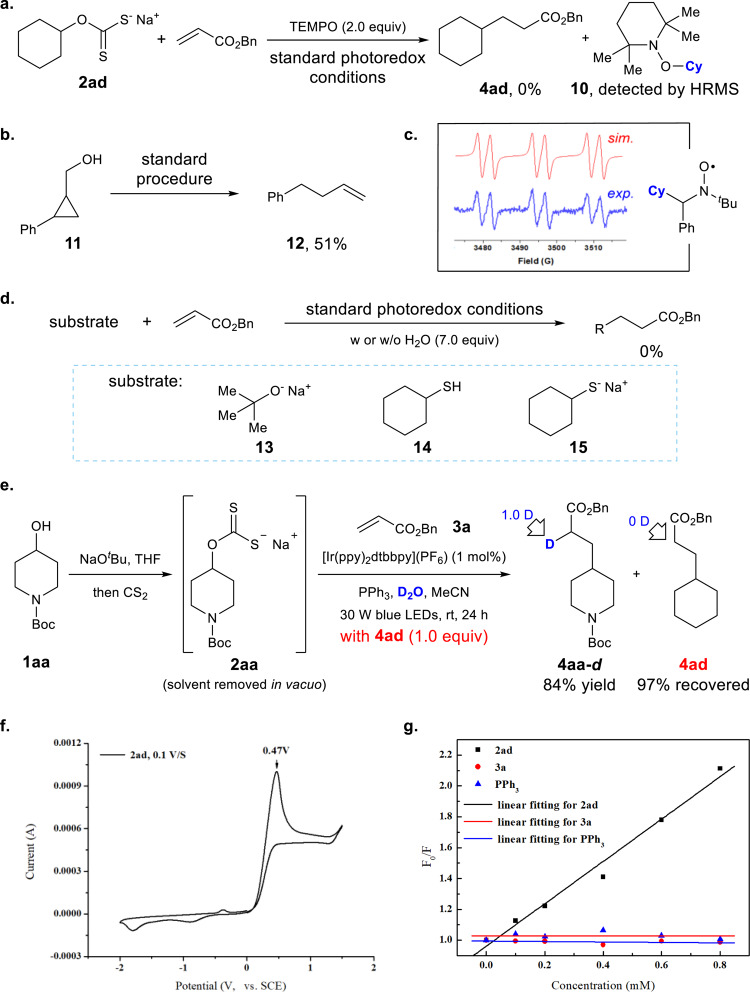
To gain insight into the possible reaction pathway, a set of preliminary mechanistic studies have been carried out82. The standard photoredox catalysis was completely inhibited in the presence of a stoichiometric amount of TEMPO, and the TEMPO-trapped cyclohexyl radical could be identified by high-resolution mass spectrometry (Fig. 7a). Ring opening was observed when cyclopropanemethanol 11 was employed, consistent with a radical intermediate (Fig. 7b). Next, electron paramagnetic resonance experiments were carried out with sodium cyclohexylxanthate 2ad by utilizing PBN as radical scavenger (Fig. 7c). It provided a signal that was consistent with that of cyclohexyl radical according to the literature data83. These results provide support for the radical nature of the deoxygenation process. When sodium alkoxide 13 was directly employed in the photoredox catalysis step, no reaction was observed, excluding the direct deoxygenation of alcohols with triphenylphosphine (Fig. 7d). No reaction was observed with thiol 14 or sodium thiol 15, indicating that O, S-rearrangement84 was not involved in the reaction pathway. When replacing water with deuterium oxide, the reaction of alcohol 1aa in the presence of ester 4ad (1.0 equiv) led to complete deuterium incorporation into the α-position of the corresponding product 4aa-d (Fig. 7e). Meanwhile, no deuterium incorporation was observed in recovered 4ad, indicating that no H/D exchange between the ester product and deuterium oxide via keto–enol tautomerism occurred under the reaction conditions. These results support the intermediacy of α-acyl carbon anion V in the catalytic cycle (Fig. 8). Oxidation potential of xanthate salt 2ad (E1/2ox = +0.47 V vs saturated calomel electrode (SCE) in MeCN) measured by cyclic voltammogram shows that it is significantly easier to oxidize compared with triphenylphosphine (E1/2ox = +0.98 V vs SCE)85 (Fig. 7f).
Fig. 7. Probing the mechanism.
a Radical trap experiment; b radical clock experiment; c EPR experiment (g = 2.00619, AN = 14.9123 G, AH = 3.41209 G); d probing the intermediate; e deuterium-labeling experiment; f cyclic voltammogram; g Stern–Volmer fluorescence quenching experiment.
Fig. 8. Proposed reaction mechanism.
SET single-electron transfer, [Ir] [Ir(ppy)2dtbbpy](PF6).
Stern–Volmer fluorescence quenching experiments verified that the excited state *Ir(III) was quenched effectively by xanthate anion but not by triphenylphosphine or the alkene (Fig. 7g). “Light–dark” experiments confirmed that the reaction required continuous light irradiation. This result and the quantum yield (Φ = 0.124) of the reaction suggest that a radical chain process, based on either a HAT process of an alkyl radical with a xanthate acid or an SET from xanthate anion to intermediate IV, is unlikely.
On the basis of the above experiments and precedent reports, a plausible mechanism was proposed (Fig. 8). Deprotonation of alcohols followed by the reaction with carbon disulfide generates the corresponding xanthate salt 2. The photoexcited iridium catalyst (E1/2ox[*Ir(III)/Ir(II)] = +0.66 V vs SCE in MeCN)86 oxidizes the xanthate anion 2 via SET to form the sulfur-centered radical I. The xanthate radical I couples with the phosphine to afford the phosphoranyl radical II and undergoes β-scission to form alkoxythiocarbonyl radical III. Fragmentation of the alkoxythiocarbonyl radical III extrudes carbonyl sulfide and produces the corresponding alkyl radical, which subsequently undergoes radical conjugate addition on the acceptor. Reduction of the resulting adduct radical IV (E1/2red = −0.59 to −0.73 V vs SCE in MeCN)87 by SET from the Ir(II) species (E1/2red[Ir(III)/Ir(II)] = −1.51 V vs SCE in MeCN)86 yields the product after protonation and regenerates the ground-state photocatalyst, completing the catalytic cycle.
Discussion
In conclusion, we have developed a visible-light photoredox-catalyzed method for the deoxygenative generation of alkyl radicals from various primary, secondary, and tertiary alcohols enabled by inexpensive carbon disulfide and triphenylphosphine-assisted C–O bond activation via xanthate salt intermediates. A broad range of alkyl groups from alcohols can be efficiently incorporated into various electron-deficient alkenes, and this easily handled one-pot protocol is scalable to the gram level. The two-step sequence is highly chemoselective, demonstrated by the monodeoxygenation of diols and steroids with multiple hydroxyl groups. This method features mild conditions, broad substrate scope, and good functional group tolerance, which can be further applied in the late-stage modification of biologically important complex molecules. The generality of this methodology, as well as the ready availability of the starting materials and reagents, will allow it to become a useful synthetic tool and enjoy extensive use in a range of chemical applications. Further synthetic applications of xanthate salts as alkyl radical precursors in photo-mediated radical processes are underway in our laboratory and will be reported in due course.
Methods
General procedure for the synthesis of products
In a nitrogen-filled glovebox, an oven-dried 5 mL quartz tube equipped with a magnetic stir bar was charged sequentially with alcohol 1 (0.20 mmol) and NaOtBu (19.2 mg, 0.20 mmol), followed by the addition of dry THF (1.2 mL). The quartz tube was sealed with a septum cap and transferred out of the glovebox, then stirred at room temperature for 30 min. The resulting solution was cooled to 0 °C followed by addition of CS2 (22.8 mg, 19.0 µL, 0.30 mmol) via microsyringe, then stirred at 0 °C for 3 h before removing the solvent in vacuo. The system was transferred into the glovebox, and PPh3 (63.0 mg, 0.24 mmol), [Ir(ppy)2dtbbpy](PF6) (1.8 mg, 1.0 mol%), alkene 3 (0.40 mmol), and H2O (25.2 mg, 1.4 mmol) in MeCN (2.0 mL) was added. Then the quartz tube was sealed with a septum cap and transferred out of the glovebox. The reaction mixture was irradiated with a 30 W blue LED lamp, maintained at a temperature of 26 °C, and stirred for 24 h. After removing the solvent in vacuo, the residue was purified by column chromatography to afford the product. The reaction could also be performed under nitrogen using a Schlenk tube, without the need of a glovebox (see Section 4 in Supplementary Information for details).
Supplementary information
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21801082), the Fundamental Research Funds for the Central Universities (2018KFYYXJJ122, 2021GCRC026), and the Innovation and Talent Recruitment Base of New Energy Chemistry and Device (B21003) for financial support. We thank Bin-Qing He (HUST) for checking the experimental procedures.
Author contributions
X.W. and H.-M.G. conceived and developed the project. H.-M.G. performed the experiments and collected the data. X.W. wrote the manuscript and both authors commented on the paper.
Data availability
The authors declare that the data supporting the findings of this study, including experimental details and compound characterization, are available within the article and its Supplementary Information file. All data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25702-4.
References
- 1.Romero NA, Nicewicz DA. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson, C. R. J., Yoon, T. P. & MacMillan, D. W. C. (eds) Visible Light Photocatalysis in Organic Chemistry (Wiley-VCH, 2018).
- 3.Marzo L, Pagire SK, Reiser O, Konig B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 2018;57:10034–10072. doi: 10.1002/anie.201709766. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Lu L-Q, Yu D-G, Zhu C-J, Xiao W-J. Visible light-driven organic photochemical synthesis in China. Sci. China Chem. 2019;62:24–57. doi: 10.1007/s11426-018-9399-2. [DOI] [Google Scholar]
- 5.Crich D, Quintero L. Radical chemistry associated with the thiocarbonyl group. Chem. Rev. 2002;89:1413–1432. doi: 10.1021/cr00097a001. [DOI] [Google Scholar]
- 6.McCombie, S. W., Motherwell, W. B. & Tozer, M. J. In Organic Reactions, Vol. 77 (eds Evans, A. et al.) 161−591 (John Wiley & Sons, 2012).
- 7.Zard, S. Z. In Encyclopedia of Radicals in Chemistry, Biology and Materials (eds Renaud, P. & Sibi, M. P.) 90–108 (John Wiley & Sons, 2012).
- 8.Herrmann JM, König B. Reductive deoxygenation of alcohols: catalytic methods beyond Barton−McCombie deoxygenation. Eur. J. Org. Chem. 2013;2013:7017–7027. doi: 10.1002/ejoc.201300657. [DOI] [Google Scholar]
- 9.Studer A, Curran DP. Catalysis of radical reactions: a radical chemistry perspective. Angew. Chem. Int. Ed. 2016;55:58–102. doi: 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- 10.Yan M, Lo JC, Edwards JT, Baran PS. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 2016;138:12692–12714. doi: 10.1021/jacs.6b08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespi S, Fagnoni M. Generation of alkyl radicals: from the tyranny of tin to the photon democracy. Chem. Rev. 2020;120:9790–9833. doi: 10.1021/acs.chemrev.0c00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr JA. Bond dissociation energies by kinetic methods. Chem. Rev. 2002;66:465–500. doi: 10.1021/cr60243a001. [DOI] [Google Scholar]
- 13.Nicewicz D, Roth H, Romero N. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett. 2015;27:714–723. doi: 10.1055/s-0035-1561297. [DOI] [Google Scholar]
- 14.Lackner GL, Quasdorf KW, Overman LE. Direct construction of quaternary carbons from tertiary alcohols via photoredox-catalyzed fragmentation of tert-alkyl N-phthalimidoyl oxalates. J. Am. Chem. Soc. 2013;135:15342–15345. doi: 10.1021/ja408971t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lackner GL, Quasdorf KW, Pratsch G, Overman LE. Fragment coupling and the construction of quaternary carbons using tertiary radicals generated from tert-alkyl N-phthalimidoyl oxalates by visible-light photocatalysis. J. Org. Chem. 2015;80:6012–6024. doi: 10.1021/acs.joc.5b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawrat CC, Jamison CR, Slutskyy Y, MacMillan DWC, Overman LE. Oxalates as activating groups for alcohols in visible light photoredox catalysis: formation of quaternary centers by redox-neutral fragment coupling. J. Am. Chem. Soc. 2015;137:11270–11273. doi: 10.1021/jacs.5b07678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, MacMillan DWC. Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3-sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc. 2016;138:13862–13865. doi: 10.1021/jacs.6b09533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L, Song F, Zhu S, Li H, Chu L. syn-Selective alkylarylation of terminal alkynes via the combination of photoredox and nickel catalysis. Nat. Commun. 2018;9:4543. doi: 10.1038/s41467-018-06904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friese FW, Studer A. Deoxygenative borylation of secondary and tertiary alcohols. Angew. Chem. Int. Ed. 2019;58:9561–9564. doi: 10.1002/anie.201904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitre SP, Muuronen M, Fishman DA, Overman LE. Tertiary alcohols as radical precursors for the introduction of tertiary substituents into heteroarenes. ACS Catal. 2019;9:3413–3418. doi: 10.1021/acscatal.9b00405. [DOI] [Google Scholar]
- 21.Weires NA, Slutskyy Y, Overman LE. Facile preparation of spirolactones by an alkoxycarbonyl radical cyclization-cross-coupling cascade. Angew. Chem. Int. Ed. 2019;58:8561–8565. doi: 10.1002/anie.201903353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye Y, Chen H, Sessler JL, Gong H. Zn-mediated fragmentation of tertiary alkyl oxalates enabling formation of alkylated and arylated quaternary carbon centers. J. Am. Chem. Soc. 2019;141:820–824. doi: 10.1021/jacs.8b12801. [DOI] [PubMed] [Google Scholar]
- 23.Li H, et al. Sequential C–O decarboxylative vinylation/C–H arylation of cyclic oxalates via a nickel-catalyzed multicomponent radical cascade. Chem. Sci. 2020;11:4904–4910. doi: 10.1039/D0SC01471K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chenneberg L, et al. Visible light photocatalytic reduction of O-thiocarbamates: development of a tin-free Barton−McCombie deoxygenation reaction. Adv. Synth. Catal. 2014;356:2756–2762. doi: 10.1002/adsc.201400729. [DOI] [Google Scholar]
- 25.Vara BA, Patel NR, Molander GA. O-Benzyl xanthate esters under Ni/photoredox dual catalysis: selective radical generation and Csp3-Csp2 cross-coupling. ACS Catal. 2017;7:3955–3959. doi: 10.1021/acscatal.7b00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Bar RM, Guo L, Noble A, Aggarwal VK. Photoinduced deoxygenative borylations of aliphatic alcohols. Angew. Chem. Int. Ed. 2019;58:18830–18834. doi: 10.1002/anie.201910051. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z-Y, Cook SP. Interrupting the Barton−McCombie reaction: aqueous deoxygenative trifluoromethylation of O-alkyl thiocarbonates. Org. Lett. 2021;23:808–813. doi: 10.1021/acs.orglett.0c04039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rackl D, Kais V, Kreitmeier P, Reiser O. Visible light photoredox-catalyzed deoxygenation of alcohols. Beilstein J. Org. Chem. 2014;10:2157–2165. doi: 10.3762/bjoc.10.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speckmeier E, Padie C, Zeitler K. Visible light mediated reductive cleavage of C–O bonds accessing α-substituted aryl ketones. Org. Lett. 2015;17:4818–4821. doi: 10.1021/acs.orglett.5b02378. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Ben-Zvi B, Diao T. Diastereoselective synthesis of aryl C-glycosides from glycosyl esters via C–O bond homolysis. Angew. Chem. Int. Ed. 2021;60:9433–9438. doi: 10.1002/anie.202014991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, et al. Synergistic catalysis for the umpolung trifluoromethylthiolation of tertiary ethers. Angew. Chem. Int. Ed. 2018;57:10357–10361. doi: 10.1002/anie.201805927. [DOI] [PubMed] [Google Scholar]
- 32.Jin J, MacMillan DW. Alcohols as alkylating agents in heteroarene C–H functionalization. Nature. 2015;525:87–90. doi: 10.1038/nature14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCallum T, Pitre SP, Morin M, Scaiano JC, Barriault L. The photochemical alkylation and reduction of heteroarenes. Chem. Sci. 2017;8:7412–7418. doi: 10.1039/C7SC03768F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Yang X, Zhou Z-Z, Li C-J. Simple and clean photo-induced methylation of heteroarenes with MeOH. Chem. 2017;2:688–702. doi: 10.1016/j.chempr.2017.03.009. [DOI] [Google Scholar]
- 35.Stache EE, Ertel AB, Tomislav R, Doyle AG. Generation of phosphoranyl radicals via photoredox catalysis enables voltage-independent activation of strong C–O bonds. ACS Catal. 2018;8:11134–11139. doi: 10.1021/acscatal.8b03592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentrude WG. Phosphoranyl radicals: their structure, formation, and reactions. Acc. Chem. Res. 2002;15:117–125. doi: 10.1021/ar00076a004. [DOI] [Google Scholar]
- 37.Walling C, Rabinowitz R. The reaction of trialkyl phosphites with thiyl and alkoxy radicals. J. Am. Chem. Soc. 2002;81:1243–1249. doi: 10.1021/ja01514a057. [DOI] [Google Scholar]
- 38.Han J-B, Guo A, Tang X-Y. Alkylation of allyl/alkenyl sulfones by deoxygenation of alkoxyl radicals. Chem. Eur. J. 2019;25:2989–2994. doi: 10.1002/chem.201806138. [DOI] [PubMed] [Google Scholar]
- 39.Dieguez HR, et al. Weakening C–O bonds: Ti(III), a new reagent for alcohol deoxygenation and carbonyl coupling olefination. J. Am. Chem. Soc. 2010;132:254–259. doi: 10.1021/ja906083c. [DOI] [PubMed] [Google Scholar]
- 40.Suga T, Shimazu S, Ukaji Y. Low-valent titanium-mediated radical conjugate addition using benzyl alcohols as benzyl radical sources. Org. Lett. 2018;20:5389–5392. doi: 10.1021/acs.orglett.8b02305. [DOI] [PubMed] [Google Scholar]
- 41.Xie H, et al. Radical dehydroxylative alkylation of tertiary alcohols by Ti catalysis. J. Am. Chem. Soc. 2020;142:16787–16794. doi: 10.1021/jacs.0c07492. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, et al. Electrochemically enabled, nickel-catalyzed dehydroxylative cross-coupling of alcohols with aryl halides. J. Am. Chem. Soc. 2021;143:3536–3543. doi: 10.1021/jacs.0c13093. [DOI] [PubMed] [Google Scholar]
- 43.Barton DHR, McCombie SW. A new method for the deoxygenation of secondary alcohols. J. Chem. Soc. Perkin Trans. 1975;1:1574–1585. doi: 10.1039/p19750001574. [DOI] [Google Scholar]
- 44.McCombie SW, Quiclet-Sire B, Zard SZ. Reflections on the mechanism of the Barton–McCombie deoxygenation and on its consequences. Tetrahedron. 2018;74:4969–4979. doi: 10.1016/j.tet.2018.03.042. [DOI] [Google Scholar]
- 45.Hu X-Q, Hou Y-X, Liu Z-K, Gao Y. Recent advances in phosphoranyl radical-mediated deoxygenative functionalisation. Org. Chem. Front. 2020;7:2319–2324. doi: 10.1039/D0QO00643B. [DOI] [Google Scholar]
- 46.Pan D, Nie G, Jiang S, Li T, Jin Z. Radical reactions promoted by trivalent tertiary phosphines. Org. Chem. Front. 2020;7:2349–2371. doi: 10.1039/D0QO00473A. [DOI] [Google Scholar]
- 47.Rossi-Ashton JA, Clarke AK, Unsworth WP, Taylor RJK. Phosphoranyl radical fragmentation reactions driven by photoredox catalysis. ACS Catal. 2020;10:7250–7261. doi: 10.1021/acscatal.0c01923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao X, Zheng Y, Ramadoss V, Tian L, Wang Y. Recent advances in P(III)-assisted deoxygenative reactions under photochemical or electrochemical conditions. Org. Biomol. Chem. 2020;18:5994–6005. doi: 10.1039/D0OB01083A. [DOI] [PubMed] [Google Scholar]
- 49.Maddigan‐Wyatt J, Hooper JF. Phosphorus compounds as precursors and catalysts for radical C−C bond-forming reactions. Adv. Synth. Catal. 2021;363:924–936. doi: 10.1002/adsc.202001397. [DOI] [Google Scholar]
- 50.Zhang M, Xie J, Zhu C. A general deoxygenation approach for synthesis of ketones from aromatic carboxylic acids and alkenes. Nat. Commun. 2018;9:3517. doi: 10.1038/s41467-018-06019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Yuan X-A, Zhu C, Xie J. Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 2019;58:312–316. doi: 10.1002/anie.201811522. [DOI] [PubMed] [Google Scholar]
- 52.Jiang H, et al. Synthesis of dibenzocycloketones by acyl radical cyclization from aromatic carboxylic acids using methylene blue as a photocatalyst. Green Chem. 2019;21:5368–5373. doi: 10.1039/C9GC02380A. [DOI] [Google Scholar]
- 53.Martinez Alvarado JI, Ertel AB, Stegner A, Stache EE, Doyle AG. Direct use of carboxylic acids in the photocatalytic hydroacylation of styrenes to generate dialkyl ketones. Org. Lett. 2019;21:9940–9944. doi: 10.1021/acs.orglett.9b03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruzi R, et al. Deoxygenative arylation of carboxylic acids by aryl migration. Chem. Eur. J. 2019;25:12724–12729. doi: 10.1002/chem.201903816. [DOI] [PubMed] [Google Scholar]
- 55.Guo Y-Q, Wang R, Song H, Liu Y, Wang Q. Visible-light-induced deoxygenation/defluorination protocol for synthesis of γ,γ-difluoroallylic ketones. Org. Lett. 2020;22:709–713. doi: 10.1021/acs.orglett.9b04504. [DOI] [PubMed] [Google Scholar]
- 56.Ruzi R, Liu K, Zhu C, Xie J. Upgrading ketone synthesis direct from carboxylic acids and organohalides. Nat. Commun. 2020;11:3312. doi: 10.1038/s41467-020-17224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, et al. Reductive C–C coupling by desulfurizing gold-catalyzed photoreactions. ACS Catal. 2019;9:6118–6123. doi: 10.1021/acscatal.9b01368. [DOI] [Google Scholar]
- 58.Shi S, Li R, Rao L, Sun Z. A mild, general, and metal-free method for site-specific deuteration induced by visible light using D2O as the source of deuterium atoms. Green. Chem. 2020;22:669–672. doi: 10.1039/C9GC04096J. [DOI] [Google Scholar]
- 59.Shi S, et al. Three-component radical homo Mannich reaction. Nat. Commun. 2021;12:1006. doi: 10.1038/s41467-021-21303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasanna R, Guha S, Sekar G. Proton-coupled electron transfer: transition-metal-free selective reduction of chalcones and alkynes using xanthate/formic acid. Org. Lett. 2019;21:2650–2653. doi: 10.1021/acs.orglett.9b00635. [DOI] [PubMed] [Google Scholar]
- 61.de Pedro Beato E, Mazzarella D, Balletti M, Melchiorre P. Photochemical generation of acyl and carbamoyl radicals using a nucleophilic organic catalyst: applications and mechanism thereof. Chem. Sci. 2020;11:6312–6324. doi: 10.1039/D0SC02313B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denes F, Pichowicz M, Povie G, Renaud P. Thiyl radicals in organic synthesis. Chem. Rev. 2014;114:2587–2693. doi: 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
- 63.Bentrude WG, Hansen ER, Khan WA, Min TB, Rogers PE. Free-radical chemistry of organophosphorus compounds. III. α vs. β scission in reactions of alkoxy and thiyl radicals with tivalent organophosphorus derivatives. J. Am. Chem. Soc. 2002;95:2286–2293. doi: 10.1021/ja00788a031. [DOI] [Google Scholar]
- 64.Bentrude WG, Hansen ER, Khan WA, Rogers PE. α vs. β Scission in rreactions of alkoxy and thiyl radicals with diethyl alkylphosphonites. J. Am. Chem. Soc. 2002;94:2867–2868. doi: 10.1021/ja00763a059. [DOI] [Google Scholar]
- 65.Walling C, Pearson MS. Some radical reactions of trivalent phosphorus derivatives with mercaptans, peroxides, and olefins. A new radical cyclization. J. Am. Chem. Soc. 2002;86:2262–2266. doi: 10.1021/ja01065a032. [DOI] [Google Scholar]
- 66.Coote ML, Easton CJ, Zard SZ. Factors affecting the relative and absolute rates of β-scission of alkoxythiocarbonyl radicals and alkoxycarbonyl radicals. J. Org. Chem. 2006;71:4996–4999. doi: 10.1021/jo0607313. [DOI] [PubMed] [Google Scholar]
- 67.Newcomb, M. In Radicals in Organic Synthesis, Vol. 1 (eds Renaud, P. & Sibi, M. P.) 327–329 (Wiley-VCH, 2001).
- 68.Yu D-G, Li B-J, Shi Z-J. Exploration of new C–O electrophiles in cross-coupling reactions. Acc. Chem. Res. 2010;43:1486–1495. doi: 10.1021/ar100082d. [DOI] [PubMed] [Google Scholar]
- 69.Li B-J, Yu D-G, Sun C-L, Shi Z-J. Activation of “inert” alkenyl/aryl C–O bond and its application in cross-coupling reactions. Chem. Eur. J. 2011;17:1728–1759. doi: 10.1002/chem.201002273. [DOI] [PubMed] [Google Scholar]
- 70.Cornella J, Zarate C, Martin R. Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity. Chem. Soc. Rev. 2014;43:8081–8097. doi: 10.1039/C4CS00206G. [DOI] [PubMed] [Google Scholar]
- 71.Tobisu M, Chatani N. Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysts. Acc. Chem. Res. 2015;48:1717–1726. doi: 10.1021/acs.accounts.5b00051. [DOI] [PubMed] [Google Scholar]
- 72.Zeng H, et al. An adventure in sustainable cross-coupling of phenols and derivatives via carbon–oxygen bond cleavage. ACS Catal. 2016;7:510–519. doi: 10.1021/acscatal.6b02964. [DOI] [Google Scholar]
- 73.Liu F, Jiang H-j, Zhou Y, Shi Z-j. Direct transformation of arenols based on C–O activation. Chin. J. Chem. 2020;38:855–863. doi: 10.1002/cjoc.201900506. [DOI] [Google Scholar]
- 74.Henkel T, Brunne RM, Müller H, Reichel F. Statistical investigation into the structural complementarity of natural products and synthetic compounds. Angew. Chem. Int. Ed. 1999;38:643–647. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643::AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 75.Shugrue CR, Miller SJ. Applications of nonenzymatic catalysts to the alteration of natural products. Chem. Rev. 2017;117:11894–11951. doi: 10.1021/acs.chemrev.7b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dimakos V, Taylor MS. Site-selective functionalization of hydroxyl groups in carbohydrate derivatives. Chem. Rev. 2018;118:11457–11517. doi: 10.1021/acs.chemrev.8b00442. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez-Rosello M, Puchlopek AL, Morgan AJ, Miller SJ. Site-selective catalysis of phenyl thionoformate transfer as a tool for regioselective deoxygenation of polyols. J. Org. Chem. 2008;73:1774–1782. doi: 10.1021/jo702334z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fowler BS, Laemmerhold KM, Miller SJ. Catalytic site-selective thiocarbonylations and deoxygenations of vancomycin reveal hydroxyl-dependent conformational effects. J. Am. Chem. Soc. 2012;134:9755–9761. doi: 10.1021/ja302692j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jordan PA, Miller SJ. An approach to the site-selective deoxygenation of hydroxy groups based on catalytic phosphoramidite transfer. Angew. Chem. Int. Ed. 2012;51:2907–2911. doi: 10.1002/anie.201109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai X-J, Li C-J. En route to a practical primary alcohol deoxygenation. J. Am. Chem. Soc. 2016;138:5433–5440. doi: 10.1021/jacs.6b02344. [DOI] [PubMed] [Google Scholar]
- 81.Cannalire R, et al. Visible light photocatalysis in the late-stage functionalization of pharmaceutically relevant compounds. Chem. Soc. Rev. 2021;50:766–897. doi: 10.1039/D0CS00493F. [DOI] [PubMed] [Google Scholar]
- 82.Buzzetti L, Crisenza GEM, Melchiorre P. Mechanistic studies in photocatalysis. Angew. Chem. Int. Ed. 2019;58:3730–3747. doi: 10.1002/anie.201809984. [DOI] [PubMed] [Google Scholar]
- 83.Panferova LI, Zubkov MO, Kokorekin VA, Levin VV, Dilman AD. Using the thiyl radical for aliphatic hydrogen-atom transfer: thiolation of unactivated C–H bonds. Angew. Chem. Int. Ed. 2021;60:2849–2854. doi: 10.1002/anie.202011400. [DOI] [PubMed] [Google Scholar]
- 84.Quiclet-Sire B, Zard SZ. A practical modification of the Barton–McCombie reaction and radical O- to S- rearrangement of xanthates. Tetrahedron Lett. 1998;39:9435–9438. doi: 10.1016/S0040-4039(98)02187-X. [DOI] [Google Scholar]
- 85.Pandey G, Pooranchand D, Bhalerao UT. Photoinduced single electron transfer activation of organophosphines: nucleophilic trapping of phosphine radical cation. Tetrahedron. 1991;47:1745–1752. doi: 10.1016/S0040-4020(01)96916-9. [DOI] [Google Scholar]
- 86.Slinker JD, et al. Efficient yellow electroluminescence from a single layer of a cyclometalated iridium complex. J. Am. Chem. Soc. 2004;126:2763–2767. doi: 10.1021/ja0345221. [DOI] [PubMed] [Google Scholar]
- 87.Bortolamei N, Isse AA, Gennaro A. Estimation of standard reduction potentials of alkyl radicals involved in atom transfer radical polymerization. Electrochim. Acta. 2010;55:8312–8318. doi: 10.1016/j.electacta.2010.02.099. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study, including experimental details and compound characterization, are available within the article and its Supplementary Information file. All data are available from the corresponding author upon reasonable request.