Significance
While numerous studies have focused on the ecological factors allowing for niche differentiation and thus species coexistence, the role of differentiation in species’ sensory abilities has received less attention. We measured auditory brainstem responses from 12 bat species that make their living gleaning prey off surfaces in the rainforest understory. In the lower-frequency ranges, auditory sensitivity reflected the foraging demands of each species, many of which find prey by attending to prey-produced noises such as prey mating calls and prey locomotion sounds. Our results suggest that interspecific differences in hearing abilities and thus the differential ability to hear prey-produced sounds could be an important mechanism contributing to niche differentiation.
Keywords: sensory niche partitioning, auditory brainstem response, echolocation, audiograms, Phyllostomidae
Abstract
Tropical ecosystems are known for high species diversity. Adaptations permitting niche differentiation enable species to coexist. Historically, research focused primarily on morphological and behavioral adaptations for foraging, roosting, and other basic ecological factors. Another important factor, however, is differences in sensory capabilities. So far, studies mainly have focused on the output of behavioral strategies of predators and their prey preference. Understanding the coexistence of different foraging strategies, however, requires understanding underlying cognitive and neural mechanisms. In this study, we investigate hearing in bats and how it shapes bat species coexistence. We present the hearing thresholds and echolocation calls of 12 different gleaning bats from the ecologically diverse Phyllostomid family. We measured their auditory brainstem responses to assess their hearing sensitivity. The audiograms of these species had similar overall shapes but differed substantially for frequencies below 9 kHz and in the frequency range of their echolocation calls. Our results suggest that differences among bats in hearing abilities contribute to the diversity in foraging strategies of gleaning bats. We argue that differences in auditory sensitivity could be important mechanisms shaping diversity in sensory niches and coexistence of species.
The tropics harbor stunningly rich species diversity (e.g., refs. 1–3). One of the principal questions underlying tropical biology is how so many species can coexist (e.g., refs. 4–6). Over decades, numerous studies have investigated niche partitioning and how it affects competition for food and other resources (e.g., refs. 7–10). It has been shown that species can coexist thanks to adaptations that have arisen in response to selection from a wide array of potential limiting factors (4, 11). For example, coexistence may reflect limitation by different foods when food is most scarce, different types of roost site, foraging in different places or times, using different foraging techniques, or limitation by different predators, pests, or diseases (10–16). All these differences can maintain the high species diversity we find in tropical regions. One important, often overlooked driver of niche differentiation is variation in sensory systems. Different species detect prey using different senses. Even within a sensory system, there can be considerable variation among species in sensory capability (17).
Bats in particular exhibit many modes of niche differentiation. Over 50 million y ago, the evolution of a key adaptation, the ability to echolocate and thus navigate and find food in the dark (18, 19), opened a whole suite of unoccupied niches to bats (20). Echolocation gave bats access to the foraging niches that birds occupied by day, allowing them to quickly radiate from simple insectivores to occupy the stunning diversity of foraging niches we find today (20). One family in particular, the Neotropical leaf-nosed bats (Phyllostomidae), is strikingly diverse. They show a wide range of diets, foraging strategies, and sensory abilities (e.g., ref. 21): from bats that feed on insects to fruit to nectar, blood, lizards, frogs, birds, and even other species of bats (22).
With each foraging strategy, we see a suite of associated sensory adaptations. Vampire bats, for example, use specialized thermosensory “leaf pit” organs near the nose that allow them to locate hot spots on their warm-blooded hosts, facilitating their search for a blood meal (23, 24). The neural circuitry vampire bats have evolved for this process—splicing TRPV1 transcripts in trigeminal ganglia—represents a unique molecular pathway, unknown in other taxa (25). Animalivorous phyllostomids rely primarily on acoustic cues to forage and have their own set of sensory adaptations to maximize their access to prey cues and increase foraging success. They have two main sensory strategies: active listening, in which they produce echolocation calls to find prey (e.g., ref. 26), and passive listening, in which they attend to prey-generated sounds (e.g., ref. 27). Phyllostomids have great variability in the external sensory structures they use to gather acoustic information (28). Such structures include intricate noseleaves, which they use to direct outgoing echolocation beams (29), and elaborate outer ears or pinnae, which they use to direct incoming sound (30).
In the predation process, bats must successfully tackle several different tasks: encountering, detecting, identifying, attacking, and eating their prey (31). Niche partitioning can take place in any of these tasks. For example, four tested animalivorous gleaners, bats that capture their prey from surfaces, differ little in echolocation call design, wing morphology, pinnae structure, or prey handling ability (32). We do, however, see evidence for sensory-based niche partitioning among these species due to differences in their acoustic preferences for prey cues, with each species preferring a different suite of acoustic properties in the mating calls of their katydid prey (32). Falk et al. (32) suggested that this divergence in predator acoustic preference could be contributing to the coexistence of gleaning bats.
While several studies have investigated the acoustic preferences of bats for their prey (e.g., refs. 27 and 32–36), to our knowledge, none have addressed the underlying physiological mechanisms driving differentiation in prey preference. So far, most studies have focused on the product of bat sensory abilities by investigating differences in prey preference and foraging behavior, with little attention to the underlying sensory mechanisms driving these differences. Given the variability in preference for the calls of different prey species, it is likely that how bats perceive sounds and their sensitivity to different acoustic signals varies greatly. Are the differences in auditory sensitivity and hearing abilities a key mechanism underlying the differences we see in bat ecological niches?
Here, we ask whether variation in hearing sensitivity reflects differences in foraging strategies of acoustically hunting animalivorous gleaning bats in the Neotropics and if it can contribute to their coexistence. One method for measuring the auditory or hearing sensitivity of animals is by monitoring the auditory brainstem response (ABR) to different acoustic stimuli. ABRs are acoustically evoked electrical potentials, which consist of stereotyped waveforms. These waveforms are generated by synchronous neural activity in the sound-processing centers along the auditory pathway (37, 38). While ABRs are sensitive to anesthesia depth and do not necessarily reflect absolute auditory sensitivity [i.e., they can lie more than 20 dB below behavioral thresholds (39–42)], this minimally invasive technique allows the assessment of audiograms of wild-caught, untrained individuals within a short time period, allowing the bats’ release right after the measurements. Comparisons between behavioral audiograms, ABRs, and neuronal hearing assessments (e.g., recorded from the inferior colliculus) in bats show major overlaps and are comparative in shape [but not necessarily in absolute threshold; see extensive discussion of this topic in the supplemental materials of Lattenkamp et al. (43)]. In this study, we measured audiograms across a wide range of frequencies in 12 sympatric Neotropical animalivorous gleaning bat species, which allowed us to compare hearing sensitivity and ability among and within species.
Results
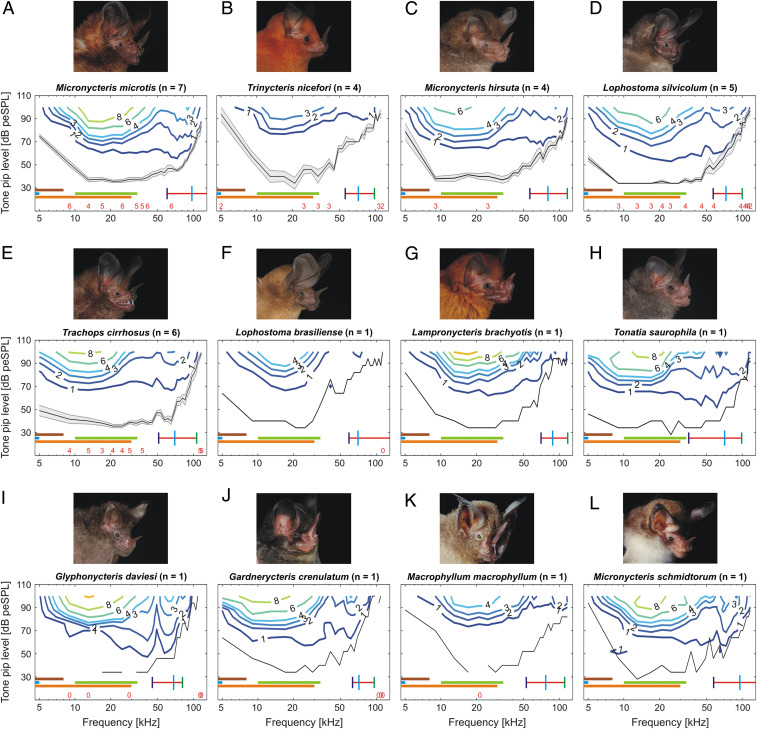
In total, we measured ABRs from 37 adult individuals of 12 different gleaning phyllostomid bat species. For five species, we were able to measure ABRs from three to eight individuals (Fig. 1 A–E and SI Appendix, Fig. S2 A–E and Table S1), whereas for the remaining seven species, we only got measurements from one individual per species (Fig. 1 F–L and SI Appendix, Fig. S2 F–L and Table S1). Thus, the audiograms for single individuals (Fig. 1 F–L and SI Appendix, Fig. S2 F–L) should be interpreted with care due to the low sample size. Still, we argue that the single audiograms can be indicative of a species’ hearing threshold. Especially as there has been no prior publication of the audiograms of these species in the literature, they provide invaluable baseline knowledge about their hearing characteristics. Although the shape and strength of the click-evoked ABR waveforms varied among the 12 species (SI Appendix, Fig. S4), the bootstrap thresholds for click-evoked ABRs were all detected at 60 (−10) dB peak-equivalent sound pressure level (peSPL). The peaks in an ABR waveform reflect the different neuronal processing centers along the auditory pathway.
Fig. 1.
Audiograms of 12 gleaning bat species. Graphs depict species-specific (mean) ABR thresholds (black lines; shading presents SEM for species with n ≥ 3), which were calculated via bootstrap analyses. Measurements were conducted for 29 tone pip frequencies linearly distributed between 5 and 117 kHz and 13 sound levels between 28 and 100 dB peSPL in 6-dB increments. The iso-response lines (colored lines) represent the different strengths of the measured ABR signal (black numbers indicate ABR strength in μV). Species names and the number of measured individuals (n) are given in the respective plot title (A–L). Audiograms for single individuals (F–L) should be interpreted with care due to the low sample size (n = 1). Red numbers above the x-axes indicate the number of individuals used for calculating the mean ± SEM if the n was lower than the total sample size. The numbers are positioned above the corresponding frequency. Horizontal bars represent different prey frequency ranges: 2 to 8 kHz (crickets; brown), 0.4 to 5 kHz (frogs; light blue), 10 to 34 kHz (katydids; light green), and 3 to 30 kHz (prey rustling sounds; orange). The red horizontal bar in the audiograms indicates the recorded frequency bandwidth of the species-specific echolocation calls. Vertical bars represent minimum frequency (dark blue), peak frequency (light blue), and maximum frequency (green) of the calls. For detailed echolocation call parameter measurements, refer to SI Appendix, Table S3. Bat portraits here and in Figs. 2 and 3 courtesy of Marco Tschapka.
Tone Pip–Evoked ABR Thresholds.
The audiograms or mean hearing thresholds (lowest sound levels evoking a significant ABR signal) in response to the tone pips between 5 and 117 kHz were shaped similarly for all 12 species (Figs. 1 A–L and 2 A and B and SI Appendix, Fig. 2 A–L). Overall, we recorded lower sensitivities to tone pips (≥50 dB peSPL) below 9 kHz and above 70 kHz (Fig. 2). However, despite a similar overall shape, the species differed in the slopes and sensitivity peaks of their audiograms.
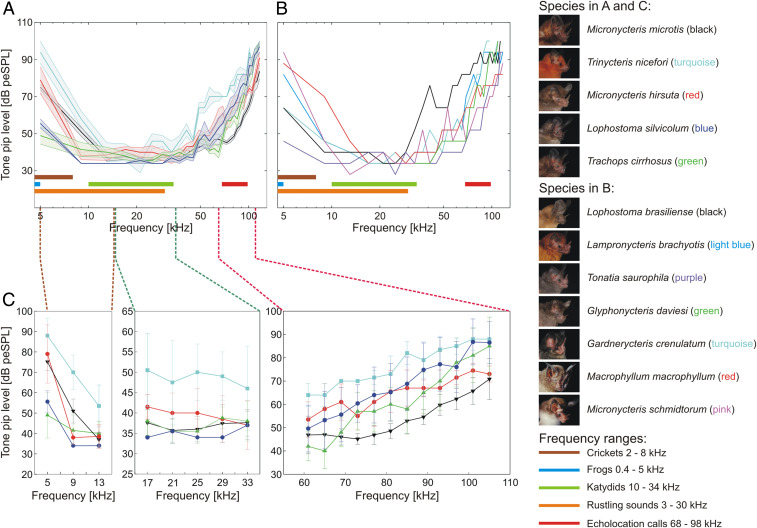
Fig. 2.
Comparison of audiograms of 12 gleaning bat species. (A and B) ABR thresholds (thick lines in different colors for each species) evoked by 29 tone pip frequencies linearly distributed between 5 and 117 kHz and 13 sound levels between 28 and 100 dB peSPL in 6-dB increments. (A) Mean thresholds and SEM (color shading around the lines) for five species with n > 1 individuals. (B) Thresholds for eight species with one individual. The different colored horizontal bars (in A and B) indicate the different frequency ranges of prey mating calls, prey rustling noises, or phyllostomid echolocation calls. (C) Detailed sections of the mean ± SEM. ABR thresholds of five species (M. microtis, M. hirsuta, L. silvicolum, T. cirrhosus, and T. nicefori) for three different frequency ranges. Left Panel: 5 to 13 kHz, Central Panel: 17 to 33 kHz, and Right Panel: 61 to 105 kHz.
For the five species with more than three individuals tested, hereafter “multi-individual species,” the differences between hearing thresholds was greatest in the low-frequency range around 9 kHz, where the audiograms did not show an overlap of the SEM (Fig. 2A). The audiograms for these five species overlapped greatly in a frequency range between 30 and 50 kHz. Above 80 kHz, the hearing threshold of Micronycteris microtis was visibly more sensitive than the thresholds of Lophostoma silvicolum, Trachops cirrhosus, and Micronycteris hirsuta, which strongly overlapped (Fig. 2A). In general, L. silvicolum had the highest sensitivity to tone pips for frequencies from 9 to 37 kHz (mean ≤37 dB peSPL; Fig. 2A), corresponding to the frequency range of katydid calls (10 to 34 kHz). With an around 2 to 6 dB peSPL difference to L. silvicolum, T. cirrhosus also showed high hearing sensitivity (mean ≤40 dB peSPL) covering the broad frequency range from 13 to 45 kHz. T. cirrhosus had by far the highest sensitivity in the low-frequency range in comparison to the other multi-individual species (3 to 6 kHz; Fig. 3E). L. silvicolum showed a drop-off above 49 kHz, and T. cirrhosus showed a sharp drop-off above 65 kHz (Fig. 1 D and E). M. microtis had a high sensitivity (mean ≤40 dB peSPL) between 13 and 41 kHz, with the sharpest drop-off in sensitivity by 14 dB from 37 ± 3.5 to 51 ± 5.9 dB peSPL at 13 to 9 kHz, respectively, compared to the other four multi-individual species. For M. microtis, we measured a high sensitivity in the higher frequency range (mean <50 dB peSPL: 45 to 81 kHz), with the highest sensitivity throughout above 69 kHz compared to the other four multi-individual species (Fig. 2C). M. hirsuta had its highest mean sensitivity between 9 and 37 kHz (<42 dB peSPL) and showed a sharp drop-off in sensitivity by 41 dB from 38 ± 3.5 to 79 ± 14.3 dB peSPL at 9 to 5 kHz, respectively. In the higher frequency range, M. hirsuta had sensitivities similar to L. silvicolum, except for frequencies above 85 kHz, where it was 6 to 14 dB peSPL more sensitive (Fig. 2C). Even though the overall shape of the mean tone pip–evoked ABR thresholds of Trinycteris nicefori was similar to the other multi-individual species, in comparison, we measured the lowest sensitivities for this species (Fig. 2 A and C).
Fig. 3.
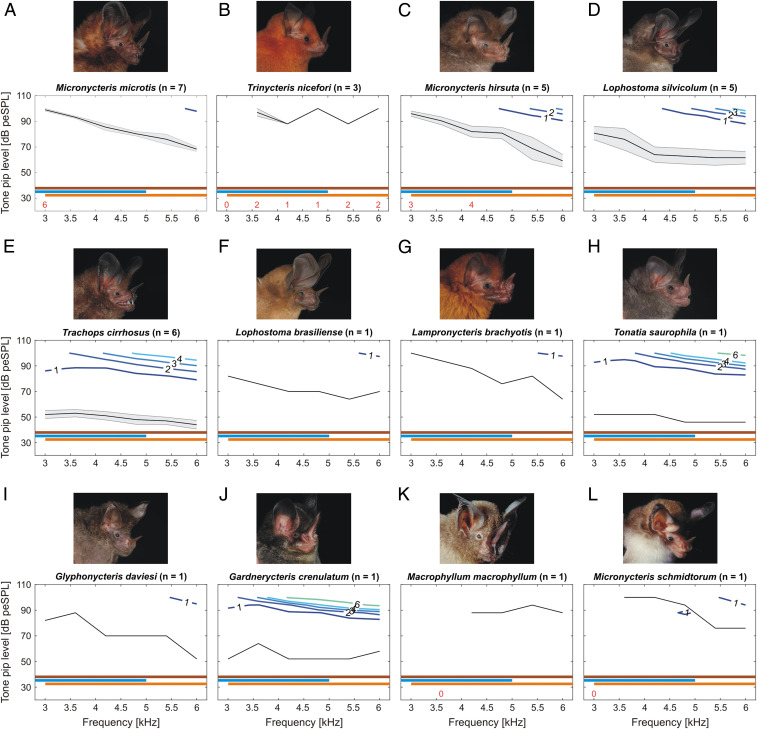
Audiograms of 12 gleaning bat species at a low-frequency range. Graphs depict species-specific (mean) ABR thresholds (black lines; shading for the SEM for species with a sample size of n > 1) assessed via bootstrap analyses at the low tone pip–frequency ranges of 3 to 6 kHz in linearly distributed 6-kHz increments and 13 sound levels between 28 and 100 dB peSPL. The iso-response lines (colored lines) represent the different strengths of the measured ABR signal (black numbers indicate ABR strength in μV). Species names and the number of measured individuals (n) are given in the respective plot title (A–L). Audiograms for single individuals (F–L) should be interpreted with care due to the low sample size (n = 1). Red numbers above the x-axes indicate the number of individuals used for calculating the mean ± SEM if the n was lower than the total sample size. The numbers are positioned above the corresponding frequency. Horizontal bars represent different prey frequency ranges: 2 to 8 kHz (crickets; brown), 0.4 to 5 kHz (frogs; light blue), and 3 to 30 kHz (prey rustling sounds; orange).
In addition to T. cirrhosus and L. silvicolum, we found low auditory thresholds in the low-frequency range (3 to 6 kHz) for Tonatia saurophila and Gardnerycteris crenulatum (Fig. 3 H and J). For these four species, we were even able to detect hearing thresholds below 3 kHz (SI Appendix, Fig. S3). Even though our very low–frequency measurements should be considered with caution due to the speaker not being calibrated below 2 kHz, we argue that it is likely that for these four species, sensitivities in the very low–frequency range are even higher than measured here. The other eight species showed only weak responses to frequencies between 3 to 6 kHz (Fig. 3). In species that showed a sensitivity maximum in the higher frequency range (50 to 120 kHz), it mostly corresponded to the echolocation call frequency range of the species (Fig. 1 A–L).
Dynamic Range of Hearing.
The strength of the ABR signal (in microvolts) is depicted as the differently colored iso-response lines in the contour plots (Figs. 1 A–L and 3 A–L and SI Appendix, Figs. S2 A–L and S3 A–D). A small distance between the iso-response lines represents a large increase in the RMS of the ABR waveform in response to a slight increase in level of the played stimulus. In other words, a small increase in stimulus amplitude leads to a large increase in ABR strength. Therefore, the distance between the iso-response lines can be an indicator of the dynamic range of hearing or the encoding of amplitude differences in the auditory pathway (44). A large distance between the iso-response lines can indicate a larger dynamic range and thus an improved amplitude resolution, allowing the detection of small amplitude differences. In general, the distance between the iso-response lines was smaller at lower frequencies (i.e., <20 kHz), corresponding to the prey-produced frequencies, and larger in high-frequency ranges (>40 kHz), mostly corresponding to the species’ echolocation calls (Fig. 1 A–L).
Echolocation Calls.
In total, we measured 1,700 calls from 34 adult individuals of the 12 different bat species and extracted different sound parameters. Like for the ABR measurements, for five species we were able to measure parameters for more than one individual, whereas for the remaining seven species, we only got measurements from one individual per species (SI Appendix, Tables S1 and S3). All of the recorded species emit short, multiharmonic, frequency-modulated echolocation calls typical of phyllostomids during flight (examples of single calls for each species in Fig. 4). The overall range of the measured peak frequencies for the 12 species was between 68 and 98 kHz (SI Appendix, Table S3). All species emitted fairly short calls, with Glyphonycteris daviesi having the shortest (0.55 ± 0.11 ms) and Macrophyllum macrophyllum having the longest (0.86 ± 0.14 ms) call duration. G. daviesi also had the lowest (68.1 ± 4.8 kHz) measured peak frequency, whereas we measured the highest peak frequency for M. microtis (97.6 ± 5.0 kHz). G. crenulatum emits calls with the smallest bandwidth (32.7 ± 4.8 kHz) and Micronycteris schmidtorum (78.5 ± 12.2 kHz) and M. microtis (76.1 ± 5.6 kHz) emit calls with the highest bandwidth compared to the other species. G. crenulatum also has the shallowest calls, with a sweep rate of 47.6 kHz/ms, and M. microtis emits the steepest frequency-modulated calls (132.6 kHz/ms sweep rate) of the 12 recorded phyllostomids.
Fig. 4.
Spectrograms of echolocation calls of 12 phyllostomid bat species. Spectrograms represent a single call emitted by each of the studied gleaners. Frequency and duration are scaled for comparison. Calls were filtered with a noise reduction filter (Methods). Spectrogram parameters: FFT size 1,024, FlatTop window, 96.43% overlap; frequency resolution: 488 Hz, temporal resolution: 0.064 ms.
Discussion
To better understand the role of sensory niche partitioning in species coexistence, we examined hearing abilities in 12 species of sympatric gleaning bat species. We derived species-specific audiograms by measuring click- and tone pip–evoked ABRs and related them to different frequency ranges of prey-produced sounds (e.g., crickets, katydids, frogs, and rustling noises) and our measured echolocation call ranges of the respective gleaners. Our study uses standardized testing of the hearing capacity of gleaning bat species in a large and consistent parameter space covering 29 frequencies (between 5 and 117 kHz) and 13 sound levels (between 28 and 100 dB peSPL). To our knowledge, with the exception of only one audiogram for T. cirrhosus (45), no previous audiogram has been published for the 12 studied species. Among the bat species examined, we found differences in the species-specific audiograms, suggesting that hearing abilities, specifically the sensitivity to different frequency ranges and a flexibility associated with auditory dynamic range, could undergird niche partitioning between bats in a feeding guild with similar foraging strategies.
Variation in Auditory Sensitivity.
The overall shape of the audiograms is similar across species in the medium-frequency range (between 9 kHz and 70 kHz). However, we find species-specific differences in hearing sensitivity at lower and higher frequencies.
The thresholds for the multi-individual species varied greatly for frequencies below 9 kHz, with T. cirrhosus and L. silvicolum having the highest sensitivity in the low-frequency range. For M. microtis, M. hirsuta, and T. nicefori, we found a drop in sensitivity for frequencies below 9 kHz. In addition to the multi-individual species, we found high sensitivity to low frequencies in T. saurophila and G. crenulatum. For four species (T. cirrhosus, L. silvicolum, T. saurophila, and G. crenulatum), we measured thresholds below 6 kHz, with hearing sensitivity even likely below 3 kHz.
Our results suggest that hearing abilities could be an underlying mechanism shaping foraging strategies and prey spectra, allowing multiple gleaners to coexist in a local bat assemblage. For example, our study and the behavioral audiogram from Ryan et al. (45) show that T. cirrhosus are sensitive to low-frequency sounds below 5 kHz, matching the main frequencies of the anuran mating calls this bat uses to localize its prey [0.2 to 5 kHz (45)], as well as rustling noises from moving prey such as beetles, another substantial part of their diet (46). Our results show a higher sensitivity in the frequency range below 15 kHz for six tested bats compared to the behavioral data previously recorded from a single bat (45). Behavioral studies offering only prey sounds demonstrate that this low-frequency sensitivity indeed allows T. cirrhosus to localize prey with high accuracy (47). In addition to T. cirrhosus, the low-frequency hearing we measured in the gleaning bats L. silvicolum, T. saurophila, and G. crenulatum could also be related to their foraging behavior. While more studies are needed, it is likely that these species attend to low-frequency prey-produced sounds. L. silvicolum mainly feeds on katydids (46) and is a passive listener (48, 49), with a preference for narrowband katydid calls (32). Even though L. silvicolum can hear low frequencies, it was not as sensitive to them as T. cirrhosus and so far has not been reported to feed on anurans (50), even though its size would permit it. Similarly, T. saurophila shows high sensitivity to low frequencies and is also considered a passive listener, attending to prey-produced sounds like katydid wing beats, rustling noises associated with landing (51), and calling song (32) to locate its prey. As no behavioral work has been done, little is known about the foraging behavior of G. crenulatum. The combination of its heavy reliance on beetles [76% of the diet (46)] and the low-frequency hearing we report in this study suggests that this bat is also likely a passively hunting gleaner using low-frequency prey-produced cues such as the rustling sounds beetles produce during locomotion (52). As such, results from measuring hearing sensitivities could orient behavioral studies to better understand the foraging and sensory strategies of these understudied bats.
In contrast to the four passive gleaners discussed above, for M. microtis, M. hirsuta, and T. nicefori, we found a drop in sensitivity for the low frequencies. These species might rely more on echolocation for prey detection than listening for prey-produced sounds. For example, more than one-half the katydids M. hirsuta consumes are silent females (49, 53). We infer from its high sensitivity to high frequencies (Fig. 1C) that in addition to listening for katydid sounds (49), M. hirsuta may be relying on echolocation to find silent prey, either by detecting the movements of the female katydids approaching males (54) or using a sophisticated echolocation strategy similar to the closely related M. microtis (55). The active gleaner M. microtis feeds on a large variety of insects, as well as spiders and lizards (56, 57), and gleans silent, stationary prey from the understory vegetation using echolocation alone (26) by exploiting an acoustic specular effect of the background (55).
In general, the acoustic parameters of the echolocation calls within a bat family vary substantially, reflecting the bats’ ecological niche (58, 59), to the point that bat species can often be distinguished from one another by their echolocation calls alone (e.g., refs. 60 and 61). The stereotypic short, broadband, frequency-modulated calls of phyllostomid species, however, are considered too similar to use to identify species (62). Upon closer examination, our study finds subtle differences in frequency parameters among the measured gleaners, phyllostomids that are generally considered to have highly similar calls (62). As one would predict, bats were most sensitive to the frequencies of their own echolocation calls. All 12 species had hearing sensitivity peaks in their echolocation frequency range, which generally reflected bat body mass (a general pattern found across bats; e.g., ref. 63). For example, in our study, larger species generally emitted lower-frequency echolocation calls and had correspondingly lower auditory sensitivity, enabling them to hear their own calls very well. However, the sensitivity peaks in the high-frequency range were not as distinct as in the low-frequency range.
In general, we found that the distance between the iso-response lines and thus the dynamic range for all species was greater at higher ultrasonic frequencies (above 40 kHz), mostly corresponding to the species’ echolocation calls and lower at frequencies below 20 kHz, corresponding to prey-produced sounds. Hearing sensitivity in the high-frequency range enhances echolocation performance, yielding higher target accuracy and resolution, thus aiding prey perception (64). We argue that a large dynamic range might be important in the echolocation call range for reliable amplitude coding, critical to accurately assess target distance and size with echolocation, should enable detection of minute changes in the amplitude of returning echoes. A larger dynamic range would result in a better encoding of small differences in sound levels and could lead to a finer processing resolution of echoes of different intensities, as they can vary substantially depending on the size and distance to an object (65). For M. microtis, we found a large dynamic range in high frequencies correlating to a large increase in responsiveness with only small increases in amplitude. We carefully argue that this responsiveness, measurable in ABR strength to small fluctuations in amplitude, might reflect higher sensitivity to minute changes in returning echoes, offering an increased resolution of prey echoes. Previous studies show that M. microtis are indeed echolocation experts, able to echolocate silent, motionless prey in the cluttered rainforest understory (26). However, our findings for the high-frequency range should be regarded with caution due to potential effects of recording in the near field of the loudspeaker.
Not all of the observed hearing sensitivities can be explained by the use of sound in the foraging context. In this study, we focused on bat responses in a foraging paradigm, but bats are using their auditory system for many different functions. For example, aside from the fine tuning of their auditory system to their own echolocation calls, it has been shown that some bat species have higher hearing sensitivities to frequencies of their social communication sounds (e.g., refs. 43 and 66–68). Unfortunately, to date social call recordings of the species studied here are lacking. It will be interesting to address the role of social communication in auditory sensitivity in gleaning bats in future studies.
Evaluating the phylogenetic relationships among the studied gleaners (SI Appendix, Fig. S5), we found that species with lower-frequency hearing were clumped in one clade. Of the six bat species in this clade, all but two (M. macrophyllum and Lophostoma brasiliense) were sensitive to low-frequency sounds. The species in this clade showing low-frequency hearing (T. cirrhosus, T. saurophila, L. silvicolum, and G. crenulatum) all exhibit similarities in foraging ecology. In contrast, M. macrophyllum, though sister species to T. cirrhosus, uses a completely different foraging behavior. Unlike the other gleaners, M. macrophyllum hunts for small insects, mainly Lepidoptera, on or just above the water surface (69). Unlike the other species in its clade, it does not use prey-generated sounds to find its prey; instead it employs an echolocation strategy more similar to that of aerial insectivores, including use of a terminal buzz (70). Very little is known about the foraging strategies of L. brasiliense and the degree to which it uses prey-generated sounds in hunting.
M. hirsuta, M. microtis, M. schmidtorum, and Lampronycteris brachyotis all fall within a single clade, and all show low sensitivity in frequencies below 10 kHz. Of all the bat species measured in this study, M. hirsuta, M. microtis, and M. schmidtorum had the best high-frequency hearing (>80 kHz). While M. hirsuta and M. microtis have been relatively well studied, very little is known about the foraging ecology of M. schmidtorum, but it is considered to be a highly cluttered space gleaner with an entirely insectivorous diet (71). Due to close relatedness and similarities in hearing characteristics to M. microtis and M. hirsuta, which are active listeners, we infer that M. schmidtorum also follows an active gleaning foraging strategy.
General Adaptations of the Auditory System in Bats.
Bats have evolved many intricate adaptations of their auditory system for detecting prey, often tailored to the species-specific requirements of different acoustic foraging strategies (e.g., ref. 72). Grinnell (44) showed that there is a tight correspondence between measured hearing sensitivities and emitted echolocation call frequencies, implying that the cochlea and the neural apparatus are very well adapted to the frequency bands of the emitted echolocation signals. For instance, it has previously been argued that a second sensitivity peak in audiograms in the lower frequency range can, besides corresponding to social communication signals (reviewed in ref. 68), also correspond to frequencies of faint prey-produced sounds, like flight noises of insects, rustling noises of moving arthropods on vegetation, or larger vertebrates that could even be moving under vegetation cover (72). Indeed, in his foundational review on bat auditory adaptations, Neuweiler (72) predicted the results of the current study. Referring to gleaning bat species that listen for prey sounds, Neuweiler states that “the audition of such bat species should be very sensitive in the low-frequency range,” allowing them “a more economical way to detect targets acoustically than echolocation in echo clutter.” With the few audiograms available for gleaning bats at that time, he indeed found preliminary evidence supporting this claim (72).
In addition to cochlear and neuronal mechanisms, the external ears or pinnae of bats and their sound-processing performance can also strongly influence hearing abilities (30, 73). In general, phyllostomid gleaning bats have large and conspicuous pinnae. The large pinnae in passive listening gleaning bats can contribute to low auditory thresholds by amplifying faint, low-frequency sounds of moving prey and allowing high directionality at low frequencies (30). From our study, we cannot predict how the pinnae of these bat species influence their hearing, but it is likely that the pinnae influence their hearing in different specific frequency regions for each species. An additional enhancement of the external ear would make certain frequency ranges even more important for the species concerned.
Sensory Niche Partitioning.
Adaptations of the auditory system for acoustically finding prey, such as those discussed, suggest that hearing sensitivity could be one underlying mechanism allowing the gleaners to coexist. In general, sensory systems are crucial for daily survival (e.g., as food only becomes available to a forager when its sensory system detects it). Varying sensory abilities determine which prey is available of the multiple resources present in the habitat (74, 75). Fine-grain differences in the sensory abilities of ecologically similar species with similar foraging strategies contribute to niche differentiation (17) and thereby further promote species coexistence.
Falk et al. (32) found substantial differences in preferences for different prey-generated sounds among four of our studied species and concluded that sensory biases toward different prey sounds contribute to niche partitioning among these gleaners. We argue that variation in hearing abilities could be an underlying mechanism for this sensory-based niche partitioning and influence foraging behavior and thus dietary spectrum. However, further mechanisms, such as horizontal information transfer, individual learning, innate search images, and prey availability, just to name a few, can further species-specific prey preferences and thus influence differentiation of prey niches. To date, detailed knowledge about diet and foraging behavior are still lacking for many of these species, especially the rarer ones. Thus, the extent to which each gleaning species attends to prey-produced sounds and whether the lower frequency range of these species is tuned to the sounds of certain prey sounds, largely remains unknown. Our measurements allow an overview of the variability in hearing abilities for 12 sympatric gleaning bat species. More detailed measurements, especially for the lower frequency range, and studies of nuances of different bats’ gleaning foraging strategies are needed for deeper insights into species-specific adaptations. We propose that fine-grained differences within a sensory system, here hearing abilities, between sympatric species are a key mechanism aiding resource partitioning in species-rich communities, thus facilitating species coexistence.
Methods
Study Species.
We conducted experiments from March to June 2019. We caught bats with mist nets (Ultrathin Mist Nets M-14/6 and Mist Net 716/6, Ecotone) at night and with hand nets (insect net, diameter 50 cm, Biologie-Bedarf Thorns) at their day roosts near Gamboa (9°07'11.5”N, 79°41'55.3”W), Old Gamboa Road, and Pipeline Road in Soberanía National Park in Panamá. We only tested adult bats. After the experiment, all individuals were released at their capture location. In total, we tested 37 individuals from 12 different Neotropical gleaning bat species of the family Phyllostomidae (refer to SI Appendix, Table S1 for further details on species, sex, ID, and method). Our experiments adhere to the guidelines of the Association for the Study of Animal Behaviour (ASAB) and the Animal Behavior Society (ABS) for ethical treatment of animals and were approved by the Government of Panamá (Ministerio de Ambiente permit SE/A-5-19) and the Smithsonian Tropical Research Institute Animal Care and Use Committee (STRI ACUC protocol 2019-0302-2022). The data are available in SI Appendix and Zenodo Data Repository (DOI: 10.5281/zenodo.4672839).
ABR Recording Setup.
To measure its ABR, each individual bat was anesthetized and placed in a small sound-attenuating box (37 × 26 × 15 cm; 1450 Protector Case, Pelican Products, Inc.; SI Appendix, Fig. S1). The box was electrically and acoustically insulated with a layer of copper mesh covered with sound-attenuation foam (SI Appendix, Fig. S1). The bats were positioned with their heads facing a loudspeaker (R2004/602000, ScanSpeak), which was embedded in one side wall of the box (SI Appendix, Fig. S1). The openings to the ear canals of the bats were always placed at a 4-cm distance to the center of the speaker. We used varying numbers of layers of soft foam pads to compensate for differences in body size and morphology among the species we measured to ensure that the bats’ heads were always placed in the center of the speaker (SI Appendix, Fig. S1). We very diligently checked the head positioning to avoid an off-center angle toward the speaker. The speaker was connected to an amplifier (12 W, M032N, Kemo Electronic), and both were powered by a four-battery pack (NCR18650GA batteries, Li-Ion, 3.6 V, 3,500 mAh, Panasonic/Sanyo). We calibrated the setup by positioning an ultrasonic 1/4” microphone (B&K4135, Brüel & Kjær) connected to an amplifier (B&K2610, Brüel & Kjær) at the bat’s head position in front of the speaker. We measured the acoustic impulse response of the speaker using broadband noise. From the measured acoustic impulse response, we calculated a compensation impulse response which was convolved with the acoustic stimuli. This allowed us to present the stimuli with a flat frequency response between 3 and 120 kHz.
Acoustic Stimuli.
We recorded ABRs to two different acoustic stimuli: tone pips and clicks. The tone pip stimuli consisted of 2.5-ms pure tones (Hanning windowed) with different carrier frequencies. We took four different measurements playing tone pips (SI Appendix, Table S1): 1) Tone pip frequencies were evenly spaced between 5 and 120 kHz in 11 steps on a logarithmic frequency axis and presented at sound levels between 0 and 110 dB peSPL in 12 equally distributed 10-dB steps; 2) The tone pip frequencies were also equally but linearly spaced along the frequency axis between 5 and 117 kHz in 29 steps. These stimuli were presented at 13 levels in 6-dB steps between 28 and 100 dB peSPL; 3) Tone pips were linearly spaced along the frequency axis between 3 and 6 kHz in six steps, also presented at 13 levels between 28 and 100 dB peSPL; and 4) Tone pips were linearly spaced along the frequency axis between 0 and 5 kHz in 11 steps, presented at 10 levels between 28 and 82 dB peSPL. The stimuli were generated at a sampling rate of 384 kHz and presented 256 times with a 44 Hz repetition rate. To cancel out electrical artifacts, every second stimulus was presented phase inverted. For all stimuli, the order of tested sound levels was randomized. For the tone pip measurements, all 256 repetitions of each carrier frequency were presented before the next frequency was randomly chosen within one sound level. After all frequencies were tested within one sound level step, the next sound level was randomly chosen and tested. The clicks were broadband impulses with a flat power spectrum between 3 and 120 kHz presented in 12 equally distributed 10-dB steps between 0 and 110 dB peSPL. A custom-written MATLAB script (Matlab, R2018b, MathWorks) was used to generate the stimuli and coordinate their presentation via an audio interface (ADI-2 PRO FS, RME).
ABR Recordings.
For the ABR recordings, we anesthetized the bats with a combination (hereafter MMF) of Medetomidine (67 µg/mL), Midazolam (667 µg/mL), and Fentanyl (6.7 µg/mL) injected subcutaneously at least 10 min before the beginning of the measurements. We applied diluted concentrations of the MMF combination (at least 1:3) based on concentrations that previously were used successfully in captive phyllostomid bats (Phyllostomus discolor; ref. 76). The reactions of the different bat species to the drug varied strongly. In some cases, additional MMF was needed to maintain the anesthetized state. The depth of anesthesia was tested via toe-pinch reflex before recording onset. For mean values of doses administered per bat species, refer to SI Appendix, Table S2. The anesthesia was not antagonized and thus varied in its duration between species (SI Appendix, Table S2). To prevent the bats’ eyes from drying, eye cream (Bepanthen, 5% Dexpanthenol, Bayer AG) was applied during anesthesia.
For ABR recordings of responses to the presented stimuli two electrodes (clipped syringe needles [0.5 × 16 mm]; NIPRO Medical Corporation) were placed subdermally at the caudal midline of the head (SI Appendix, Fig. S1), close to the brainstem (recording electrode), and at the dorsal midline of the head between the ears (reference electrode). A ground electrode was either placed on the wing or tail membrane (SI Appendix, Fig. S1). The electrodes were positioned after the fur was cut with scissors to enable their precise reproducible placement. Electrode placement was confirmed with an initial signal-to-noise ratio measurement, which allowed recording of comparable signal strengths between measurements. All recordings within one individual were done with the same electrode placement. The electrodes were connected via alligator clips (SI Appendix, Fig. S1) to a bioamplifier (BMA-200, CWE Inc.), which detected the brainstem responses and initially amplified the signal by 60 dB. The signal was converted to digital by the audio interface (ADI-2 PRO FS, RME) with a sampling rate of 384 kHz. The ABR signals were digitally filtered (0.1 to 3 kHz, second order butterworth) and down-sampled 20 times before each of the 256 repetitions to the same frequency-sound level combination were saved on a laptop computer (Latitude 5490, Dell). Averaged ABR signals for each combination were displayed to the experimenter for quality monitoring during the recording.
ABR Data Analysis.
The ABR recordings were analyzed following a protocol established in previous experiments (76). The amplitudes of the recorded ABRs were calculated as the RMS over the entire ABR waveform (i.e., over a 7-ms window starting directly (1 ms) after stimulus onset and ending at ms 8 of the recording). These averaged ABR signal strengths for each measured frequency–amplitude combination were presented as iso-response lines in contour plots for each species in μV (for tone pip stimuli). For the calculation of the characteristic ABR threshold, or audiogram, for each species, we used bootstrap analyses [n = 500; 95% confidence (77)], which tests the statistical likelihood that a recorded signal reflects random variation in the data rather than a physiological response. For this, we performed a repeated random resampling (with replacement) of the original data and evaluated it to determine whether the RMS of the resampled waveform exceeded the original RMS value. An ABR measurement was considered significant if 95% of the resampled waveforms had a lower RMS than the original waveform. An ABR threshold was only defined and accepted as the lowest sound level evoking a significant ABR signal at that specific frequency when significant responses were measured for all higher sound levels at the same frequency (depicted as a black line in contour plots for each species). This allows conservative and objective evaluations based on the significant evoked potentials. We calculated the mean and SEM for the ABR thresholds for species with more than three individuals (n ≥ 3) and omitted measurements for which the threshold could not be determined by the algorithm.
Echolocation Call Recordings and Analysis.
Prior to the ABR measurements, individuals were released into a small indoor flight cage (1.4 × 1.0 × 0.8 m) in which they were allowed to fly for up to 10 min. We recorded the echolocation calls of 34 individuals (see SI Appendix, Table S1 for further details on species, sex, and ID) during flight via an ultrasound condenser microphone (2 to 200 kHz frequency range, ±3 dB frequency response between 25 and 140 kHz; CM16, CMPA preamplifier unit, Avisoft Bioacoustics) and real time ultrasound acquisition board (6 dB gain, 500 kHz sampling rate, 16-bit resolution; UltraSoundGate 116Hm, Avisoft Bioacoustics) connected to a laptop (Think Pad X220, Lenovo), with a corresponding recording software (Avisoft RECORDER USGH, Avisoft Bioacoustics). For each individual, we recorded a minimum of five 30-s recordings. From these recordings, we picked five sequences of 10 continuous search calls with good signal-to-noise ratios, no clipping, and minimal background noise and echoes. If the series contained one clipped call, an additional call adjacent to the series was included, and the clipped call was excluded from the analysis. Prior to the analysis, first we applied a band pass filter with 20 kHz as the low cutoff frequency and 180 kHz as the high cutoff frequency for all species but M. macrophyllum, G. daviesi, and T. saurophila (for which we used a 10 kHz low cutoff frequency). We then applied a noise-reduction filter of 80 dB (−70 dB threshold) to all files.
For each echolocation call from the selected sequences, we extracted several signal parameters using the automatic measurements of the sound analysis software Avisoft SASLabPro (5.2.15, Avisoft Bioacoustics): call duration (milliseconds), peak frequency (kilohertz), minimum and maximum frequency (kilohertz), bandwidth (kilohertz), sweep rate (kilohertz/milliseconds), and entropy. We set the automated signal identification to a −20 dB threshold and verified it visually in the spectrograms (fast Fourier transformation [FFT] size 512, FlatTop window, 96.87% overlap, frequency resolution 977 Hz, and temporal resolution 0.032 ms). The call duration was based on the start and end times of the call. The peak frequency was the frequency with maximum amplitude of the respective call. The minimum frequency was defined as the lowest frequency below and maximum frequency as the highest frequency above the peak frequency with a threshold of −20 dB. We calculated call bandwidth (kilohertz) as the difference between the minimum and the maximum frequency of the multiharmonic calls.
In total, we analyzed 1,700 calls from a total of 170 echolocation sequences of 34 individuals of the 12 species (SI Appendix, Table S3). Per individual, we calculated the mean and SD for all described call parameters. In cases with more than one individual, we calculated the mean of the means.
Prey-Produced Frequency Ranges.
For reference, we marked five different frequency ranges on the plots of our results (Fig. 2 A–D). We included three ranges that corresponded to the main advertisement calls of the most common potential prey in the gleaners’ diet—two for the lower frequency prey (e.g., crickets: 2 to 8 kHz, Fig. 2 A–D, brown bar; frogs: 0.4 to 5 kHz, Fig. 2 A–D, blue bar) and one for the higher frequency prey (e.g., katydids: 10 to 34 kHz, Fig. 2 A–D, green bar). We included one range that corresponded to prey-generated rustling sounds (3 to 30 kHz, Fig. 2 A–D, orange bar) and one range that corresponded to the main echolocation call frequencies of the investigated gleaners (68 to 99 kHz, Fig. 2 A–D, red bar).
Crickets (Gryllidae) call mostly with a dominant or peak frequency ranging in the lower frequencies between 2 and 8 kHz (78, 79). The main frequency range of the mating calls of frogs known to be eaten by bats (50) lies between 0.4 and 5 kHz (80). Arthropods walking on different substrates generate rustling noises, which are a series of broadband clicks with the main energy ranging between 3 and 30 kHz (52). Male katydids (Tettigoniidae), likely one of the most substantial parts of the diet of gleaners, produce acoustic advertisement signals with the broadest range of song carrier frequencies, or peak frequencies, in insects. Their peak frequency ranges from 0.6 to 150 kHz (81, 82), with a median peak frequency at 22.15 kHz (lower quartile: 13.20 kHz, upper quartile: 34.10 kHz). On Barro Colorado Island (BCI, Panamá) the energy of 34 katydid species mostly ranges from 10 to 30 kHz (83). We assume that the katydid community on BCI is similar to the katydid community that the studied gleaners encounter in Gamboa. Except for the very rare species G. daviesi, all gleaners included in our study have been reported on BCI (84). The peak frequency of the echolocation calls of the 12 measured species ranges between 68 and 98 kHz (Results and , SI AppendixTable S3).
Supplementary Material
Acknowledgments
We thank Mirjam Knörnschild for generously supplying us with her ABR setup, Egbert Leigh and three anonymous reviewers for their insightful comments, and Thorin Jonsson for the calculation of the median peak frequencies of the katydids, his instant knowledge about all singing critters, and patient support solving software incompatibility problems. We thank Marco Tschapka for the gorgeous bat portraits, Damond Kyllo for redrawing of the bat phylogeny, and Raimund Specht for his excellent immediate and ongoing support with sound analysis software. We are grateful to STRI, especially to Gregg Cohen, for critical ongoing support and the Autoridad del Canal de Panamá for granting us access to their administered areas. We dedicate this study to the late Lutz Wiegrebe, without whose keen insight and kind guidance, this work and so much of our understanding of the perceptual abilities of echolocating bats, would not have been possible. I.G. was funded by a STRI Tupper Postdoctoral Fellowship. E.Z.L. was supported by a travelling fellowship of the Company of Biologists (JEBTF18113) and a short-term travel stipend of the German Academic Exchange Service (91710219). R.A.P. and M.M.D. were funded by STRI.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024943118/-/DCSupplemental.
Data Availability
ABR measurements and echolocation call parameter data have been deposited in the publicly accessible database Zenodo (https://doi.org/10.5281/zenodo.4672839) (85).
References
- 1.MacArthur R. H., Geographical Ecology: Patterns in the Distribution of Species (Princeton University Press, Princeton, NJ, 1972). [Google Scholar]
- 2.Gaston K. J., Global patterns in biodiversity. Nature 405, 220–227 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Dobzhansky T., Evolution in the tropics. Am. Sci. 38, 208–221 (1950). [Google Scholar]
- 4.Brown J. H., Why are there so many species in the tropics? J. Biogeogr. 41, 8–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson G. E., Homage to Santa Rosalia or why are there so many kinds of animals? Am. Nat. 93, 145–159 (1959). [Google Scholar]
- 6.Willig M. R., Kaufman D. M., Stevens R. D., Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (2003). [Google Scholar]
- 7.Chase J. M., Leibold M. A., Ecological Niches: Linking Classical and Contemporary Approaches (University of Chicago Press, Chicago, London, 2003). [Google Scholar]
- 8.Finke D. L., Snyder W. E., Niche partitioning increases resource exploitation by diverse communities. Science 321, 1488–1490 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Pittendrigh C. S., The ecoclimatic divergence of Anopheles bellator and A. homunculus. Evolution 4, 43–63 (1950). [Google Scholar]
- 10.Pittendrigh C. S., The ecotopic specialization of Anopheles homunculus; and its relation to competition with A. bellator. Evolution 4, 64–78 (1950). [Google Scholar]
- 11.Bonaccorso F. J., Foraging and reproductive ecology in a Panamanian bat community. Bull. Fla. State Mus. Biol. Sci. 24, 359–408 (1979). [Google Scholar]
- 12.Janzen D. H., Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 (1970). [Google Scholar]
- 13.Gillett J. B., Pest pressure, an underestimated factor in evolution. Systematics Association Pub. 4, 37–46 (1962). [Google Scholar]
- 14.De León L. F., Podos J., Gardezi T., Herrel A., Hendry A. P., Darwin’s finches and their diet niches: The sympatric coexistence of imperfect generalists. J. Evol. Biol. 27, 1093–1104 (2014). [DOI] [PubMed] [Google Scholar]
- 15.MacArthur R. H., Population ecology of some warblers of northeastern coniferous forests. Ecology 39, 599–619 (1958). [Google Scholar]
- 16.Paine R. T., Food web complexity and species diversity. Am. Nat. 100, 65–75 (1966). [Google Scholar]
- 17.Siemers B. M., Schnitzler H.-U., Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429, 657–661 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Jones G., Teeling E. C., The evolution of echolocation in bats. Trends Ecol. Evol. 21, 149–156 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Griffin D. R., Listening in the Dark: The Acoustic Orientation of Bats and Men (Yale University Press, New Haven: ), ed. 1, 1958), pp. 413. [Google Scholar]
- 20.Teeling E. C., et al., A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307, 580–584 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Kalko E. K. V., “Diversity in tropical bats” in Tropical Biodiversity and Systematics: Proceedings of the International Symposium on Biodiversity and Systematics in Tropical Ecosystems, Bonn, 2-7 May 1994, Ulrich H., Ed. (Zoologisches Forschungsinstitut und Museum Alexander Koenig, Bonn, Germany, 1997), pp. 13–43. [Google Scholar]
- 22.Fleming T. H., Dávalos L. M., Mello M. A. R., Phyllostomid Bats: A Unique Mammalian Radiation (University of Chicago Press, Chicago, 2020). [Google Scholar]
- 23.Kürten L., Schmidt U., Schäfer K., Warm and cold receptors in the nose of the vampire bat Desmodus rotundus. Naturwissenschaften 71, 327–328 (1984). [DOI] [PubMed] [Google Scholar]
- 24.Schäfer K., Braun H. A., Kürten L., Analysis of cold and warm receptor activity in vampire bats and mice. Pflügers Arch. 412, 188–194 (1988). [DOI] [PubMed] [Google Scholar]
- 25.Gracheva E. O., et al., Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 476, 88–91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geipel I., Jung K., Kalko E. K. V., Perception of silent and motionless prey on vegetation by echolocation in the gleaning bat Micronycteris microtis. Proc. Biol. Sci. 280, 20122830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuttle M. D., Ryan M. J., Bat predation and the evolution of frog vocalizations in the neotropics. Science 214, 677–678 (1981). [DOI] [PubMed] [Google Scholar]
- 28.Leiser-Miller L. B., Santana S. E., Morphological diversity in the sensory system of phyllostomid bats: Implications for acoustic and dietary ecology. Funct. Ecol. 34, 1416–1427 (2020). [Google Scholar]
- 29.Vanderelst D., et al., What noseleaves do for FM bats depends on their degree of sensorial specialization. PLoS One 5, e11893 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obrist M. K., Fenton M. B., Eger J. L., Schlegel P. A., What ears do for bats: A comparative study of pinna sound pressure transformation in chiroptera. J. Exp. Biol. 180, 119–152 (1993). [DOI] [PubMed] [Google Scholar]
- 31.Endler J. A., “Interactions between predators and prey. ” in Behavioural Ecology: An Evolutionary Approach, Krebs J. R., Davies N. B., Eds. (Blackwell Scientific Publications, Oxford, 1991), pp. 169–196. [Google Scholar]
- 32.Falk J. J., et al., Sensory-based niche partitioning in a multiple predator - Multiple prey community. Proc. Biol. Sci. 282, 20150520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page R. A., Bernal X. E., The challenge of detecting prey: Private and social information use in predatory bats. Funct. Ecol. 34, 344–363 (2020). [Google Scholar]
- 34.Jones P., Page R., Hartbauer M., Siemers B., Behavioral evidence for eavesdropping on prey song in two Palearctic sibling bat species. Behav. Ecol. Sociobiol. 65, 333–340 (2011). [Google Scholar]
- 35.Page R. A., Ryan M. J., Flexibility in assessment of prey cues: Frog-eating bats and frog calls. Proc. Biol. Sci. 272, 841–847 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghuram H., Deb R., Nandi D., Balakrishnan R., Silent katydid females are at higher risk of bat predation than acoustically signalling katydid males. Proc. Biol. Sci. 282, 20142319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smotherman M., Bakshi K., Forward masking enhances the auditory brainstem response in the free-tailed bat, Tadarida brasiliensis, during a critical time window for sonar reception. J. Acoust. Soc. Am. 145, EL19–EL24 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Corwin J. T., Bullock T. H., Schweitzer J., The auditory brain stem response in five vertebrate classes. Electroencephalogr. Clin. Neurophysiol. 54, 629–641 (1982). [DOI] [PubMed] [Google Scholar]
- 39.Belknap D. B., Suthers R. A., Brainstem auditory evoked responses to tone bursts in the echolocating bat. Rousettus. J. Comp. Physiol. A 146, 283–289 (1982). [Google Scholar]
- 40.Serpanos Y. C., O’Malley H., Gravel J. S., The relationship between loudness intensity functions and the click-ABR wave V latency. Ear Hear. 18, 409–419 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Wenstrup J. J., Auditory sensitivity in the fish-catching bat, Noctilio leporinus. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 155, 91–101 (1984). [Google Scholar]
- 42.Obrist M. K., Wenstrup J. J., Hearing and hunting in red bats (Lasiurus borealis, Vespertilionidae): Audiogram and ear properties. J. Exp. Biol. 201, 143–154 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Lattenkamp E. Z., et al., Hearing sensitivity and amplitude coding in bats are differentially shaped by echolocation calls and social calls. Proc. Biol. Sci. 288, 20202600 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinnell A. D., Comparative auditory neurophysiology of Neotropical bats employing different echolocation signals. Z. Vgl. Physiol. 68, 117–153 (1970). [Google Scholar]
- 45.Ryan M. J., Tuttle M. D., Barclay R. M. R., Behavioral responses of the frog-eating bat, Trachops cirrhosus, to sonic frequencies. J. Comp. Physiol. 150, 413–418 (1983). [Google Scholar]
- 46.Belwood J. J., “Foraging behavior, prey selection, and echolocation in phyllostomine bats (Phyllostomidae)” in Animal Sonar Processes and Performance, Nachtigall P. E., Moore P. W. B., Eds. (NATO Advanced Study Institute Series, Series A: Life Sciences, Plenum Press, New York, 1988), pp. 601–605. [Google Scholar]
- 47.Page R. A., Ryan M. J., The effect of signal complexity on localization performance in bats that localize frog calls. Anim. Behav. 76, 761–769 (2008). [Google Scholar]
- 48.Tuttle M. D., Ryan M. J., Belwood J. J., Acoustical resource partitioning by two species of phyllostomid bats (Trachops cirrhosus and Tonatia sylvicola). Anim. Behav. 33, 1369–1371 (1985). [Google Scholar]
- 49.Belwood J. J., Morris G. K., Bat predation and its influence on calling behavior in neotropical katydids. Science 238, 64–67 (1987). [DOI] [PubMed] [Google Scholar]
- 50.Jones P. L., et al., Sensory ecology of the frog-eating bat, Trachops cirrhosus, from DNA metabarcoding and behavior. Behav. Ecol. 31, 1420–1428 (2020). [Google Scholar]
- 51.Spehn S. E., "Etho-ecology and sensory physiology of two gleaning insectivorous bats, Tonatia saurophila and Micronycteris hirsuta in Panamá," Doctoral dissertation, University of Ulm, Ulm (2005).
- 52.Goerlitz H. R., Greif S., Siemers B. M., Cues for acoustic detection of prey: Insect rustling sounds and the influence of walking substrate. J. Exp. Biol. 211, 2799–2806 (2008). [DOI] [PubMed] [Google Scholar]
- 53.ter Hofstede H., et al., Revisiting adaptations of neotropical katydids (Orthoptera: Tettigoniidae) to gleaning bat predation. Neotrop. Biodivers. 3, 41–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geipel I., et al., Predation risks of signalling and searching: Bats prefer moving katydids. Biol. Lett. 16, 20190837 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geipel I., et al., Bats actively use leaves as specular reflectors to detect acoustically camouflaged prey. Curr. Biol. 29, 2731–2736.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Kalka M., Kalko E. K. V., Gleaning bats as underestimated predators of herbivorous insects: Diet of Micronycteris microtis (Phyllostomidae) in Panama. J. Trop. Ecol. 22, 1–10 (2006). [Google Scholar]
- 57.Santana S. E., Geipel I., Dumont E. R., Kalka M. B., Kalko E. K. V., All you can eat: High performance capacity and plasticity in the common big-eared bat, Micronycteris microtis (Chiroptera: Phyllostomidae). PLoS One 6, e28584 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones G., Holderied M. W., Bat echolocation calls: Adaptation and convergent evolution. Proc. Biol. Sci. 274, 905–912 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schnitzler H.-U., Kalko E. K. V., Echolocation by insect-eating bats. Bioscience 51, 557–569 (2001). [Google Scholar]
- 60.Jung K., Kalko E. K. V., von Helversen O., Echolocation calls in Central American emballonurid bats: Signal design and call frequency alternation. J. Zool. (Lond.) 272, 125–137 (2007). [Google Scholar]
- 61.Jung K., Molinari J., Kalko E. K. V., Driving factors for the evolution of species-specific echolocation call design in new world free-tailed bats (Molossidae). PLoS One 9, e85279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoh N., Syme P., Rocha R., Meyer C. F. J., López-Baucells A., Echolocation of Central Amazonian ‘whispering’ phyllostomid bats: Call design and interspecific variation. Mammal Res. 65, 583–597 (2020). [Google Scholar]
- 63.Thiagavel J., Santana S. E., Ratcliffe J. M., Body size predicts echolocation call peak frequency better than gape height in vespertilionid bats. Sci. Rep. 7, 828 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furuyama T., Hase K., Hiryu S., Kobayasi K. I., Hearing sensitivity evaluated by the auditory brainstem response in Miniopterus fuliginosus. J. Acoust. Soc. Am. 144, EL436–EL440 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Stilz W.-P., Schnitzler H.-U., Estimation of the acoustic range of bat echolocation for extended targets. J. Acoust. Soc. Am. 132, 1765–1775 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Boku S., Riquimaroux H., Simmons A. M., Simmons J. A., Auditory brainstem response of the Japanese house bat (Pipistrellus abramus). J. Acoust. Soc. Am. 137, 1063–1068 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Esser K. H., Daucher A., Hearing in the FM-bat Phyllostomus discolor: A behavioral audiogram. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 178, 779–785 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Bohn K. M., Moss C. F., Wilkinson G. S., Correlated evolution between hearing sensitivity and social calls in bats. Biol. Lett. 2, 561–564 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinbeer M., Meyer C. F. J., Kalko E. K. V., Activity pattern of the trawling phyllostomid bat, Macrophyllum macrophyllum, in Panamá. Biotropica 38, 69–76 (2006). [Google Scholar]
- 70.Weinbeer M., Kalko E. K. V., Ecological niche and phylogeny: The highly complex echolocation behavior of the trawling long-legged bat, Macrophyllum macrophyllum. Behav. Ecol. Sociobiol. 61, 1337–1348 (2007). [Google Scholar]
- 71.Kalko E. K. V., Handley C. O. Jr, Handley D., “Organization, diversity and long-term dynamics of a Neotropical bat community” in Long-Term Studies of Vertebrate Communities, Cody M. L., Smallwood J. A., Eds. (Academic Press, San Diego, 1996), pp. 597. [Google Scholar]
- 72.Neuweiler G., Auditory adaptations for prey capture in echolocating bats. Physiol. Rev. 70, 615–641 (1990). [DOI] [PubMed] [Google Scholar]
- 73.De Mey F., Reijniers J., Peremans H., Otani M., Firzlaff U., Simulated head related transfer function of the phyllostomid bat Phyllostomus discolor. J. Acoust. Soc. Am. 124, 2123–2132 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Faure P. A., Barclay R. M. R., The sensory basis of prey detection by the long-eared bat, Myotis evotis, and the consequences for prey selection. Anim. Behav. 44, 31–39 (1992). [Google Scholar]
- 75.Fenton M. B., The foraging behaviour and ecology of animal-eating bats. Can. J. Zool. 68, 411–422 (1990). [Google Scholar]
- 76.Linnenschmidt M., Wiegrebe L., Ontogeny of auditory brainstem responses in the bat, Phyllostomus discolor. Hear. Res. 373, 85–95 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Lv J., Simpson D. M., Bell S. L., Objective detection of evoked potentials using a bootstrap technique. Med. Eng. Phys. 29, 191–198 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Robillard T., Grandcolas P., Desutter-Grandcolas L., A shift toward harmonics for high-frequency calling shown with phylogenetic study of frequency spectra in Eneopterinae crickets (Orthoptera, Grylloidea, Eneopteridae). Can. J. Zool. 85, 1264–1275 (2007). [Google Scholar]
- 79.Robillard T., Desutter-Grandcolas L., High-frequency calling in Eneopterinae crickets (Orthoptera, Grylloidea, Eneopteridae): Adaptive radiation revealed by phylogenetic analysis. Biol. J. Linn. Soc. Lond. 83, 577–584 (2004). [Google Scholar]
- 80.Ibáñez R., Rand A. S., Jaramillo C. A., Los anfibios del Monumento Natural Barro Colorado, Parque Nacional Soberanía y áreas adyacentes/The amphibians of Barro Colorado Nature Monument, Soberania National Park and adjacent areas (Panamá, ed. 1, 1999), p. 192. [Google Scholar]
- 81.Montealegre-Z F., Ogden J., Jonsson T., Soulsbury C. D., Morphological determinants of signal carrier frequency in katydids (Orthoptera): A comparative analysis using biophysical evidence of wing vibration. J. Evol. Biol. 30, 2068–2078 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Chivers B. D., Jonsson T., Soulsbury C. D., Montealegre-Z F., Structural biomechanics determine spectral purity of bush-cricket calls. Biol. Lett. 13, 20170573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Symes L. B., Martinson S. J., Hoeger L.-O., Page R. A., ter Hofstede H. M., From understory to canopy: In situ behavior of Neotropical forest katydids in response to bat echolocation calls. Front. Ecol. Evol. 6, 227 (2018). [Google Scholar]
- 84.Kalko E. K. V., Estrada Villegas S., Schmidt M., Wegmann M., Meyer C. F. J., Flying high—Assessing the use of the aerosphere by bats. Integr. Comp. Biol. 48, 60–73 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Geipel I., Lattenkamp E. Z., Dixon M. M., Wiegrebe L., Page R. A., Data for "Hearing sensitivity: An underlying mechanism for niche differentiation in gleaning bats.” Zenodo. 10.5281/zenodo.4672839. Deposited 8 April 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
ABR measurements and echolocation call parameter data have been deposited in the publicly accessible database Zenodo (https://doi.org/10.5281/zenodo.4672839) (85).