Significance
Bacteria are protected from their surrounding environment by the peptidoglycan cell wall, which is a major target for antibiotics. Counterintuitively, cell wall assembly requires enzymes that cleave newly built peptidoglycan chains. Here, using nascent peptidoglycan we assembled in vitro, we characterized two membrane-bound glycosidases that are vital for proper cell division and elongation in Streptococcus pneumoniae. These enzymes were proposed to perform different chemical reactions. Instead, we show that they perform the same chemical reaction but cut the peptidoglycan backbone at different sites. We identify the mechanistic basis for cleavage site selection and also identify an amino acid switch that alters the cleavage chemistry. This work advances our understanding of how peptidoglycan glycosidases help build the cell wall.
Keywords: peptidoglycan, glycosidase, muramidase, lytic transglycosylase
Abstract
The peptidoglycan cell wall is a macromolecular structure that encases bacteria and is essential for their survival. Proper assembly of the cell wall requires peptidoglycan synthases as well as membrane-bound cleavage enzymes that control where new peptidoglycan is made and inserted. Previous studies have shown that two membrane-bound proteins in Streptococcus pneumoniae, here named MpgA and MpgB, are important in maintaining cell wall integrity. MpgA was predicted to be a lytic transglycosylase based on its homology to Escherichia coli MltG, while the enzymatic activity of MpgB was unclear. Using nascent peptidoglycan substrates synthesized in vitro from the peptidoglycan precursor Lipid II, we report that both MpgA and MpgB are muramidases. We show that replacing a single amino acid in E. coli MltG with the corresponding amino acid from MpgA results in muramidase activity, allowing us to predict from the presence of this amino acid that other putative lytic transglycosylases actually function as muramidases. Strikingly, we report that MpgA and MpgB cut nascent peptidoglycan at different positions along the sugar backbone relative to the reducing end, with MpgA producing much longer peptidoglycan oligomers. We show that the cleavage site selectivity of MpgA is controlled by the LysM-like subdomain, which is required for its full functionality in cells. We propose that MltG’s ability to complement the loss of MpgA in S. pneumoniae despite performing different cleavage chemistry is because it can cleave nascent peptidoglycan at the same distance from the lipid anchor.
The peptidoglycan cell wall is a major component of the bacterial cell envelope that defines the size and shape of bacteria (1). For the bacteria to constantly grow and divide while maintaining proper morphology, the activities of enzymes responsible for peptidoglycan assembly and modification need to be coordinated. Two major families of peptidoglycan synthases have been identified to date. The SEDS (shape, elongation, division, and sporulation) proteins are peptidoglycan glycosyltransferases that polymerize the lipid-linked peptidoglycan precursor Lipid II to make glycan strands (Fig. 1A) (2, 3). SEDS proteins form a complex with class B penicillin-binding proteins (bPBPs), which are monofunctional transpeptidases that cross-link the glycan strands into the peptidoglycan matrix (3, 4). The bifunctional class A PBPs (aPBPs) possess both glycosyltransferase and transpeptidase activities, and they work together with the SEDS-bPBP complexes to build the cell wall (5). Some peptidoglycan synthases are primarily devoted to synthesizing the cell wall along the cell periphery, while others are dedicated to constructing the cell wall partition between daughter cells during cell division (6). Many studies have focused on characterizing these enzymes because of their importance in bacterial physiology and attractiveness as targets for antibiotic development. Indeed, the β-lactam antibiotics that inhibit PBP transpeptidase activity have been one of the most clinically successful antibiotics to date (7).
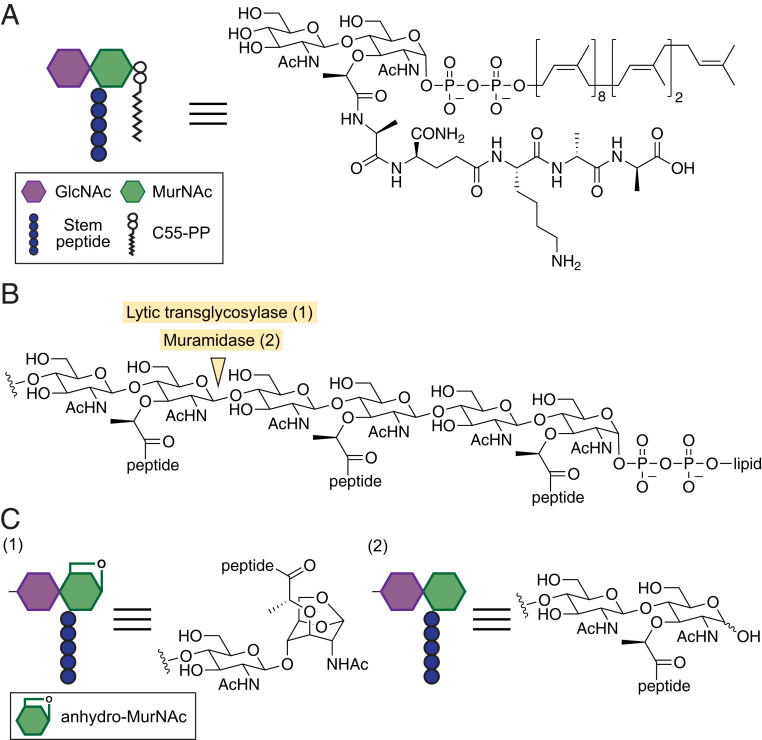
Fig. 1.
Lytic transglycosylases and muramidases cleave MurNAc(β-1,4)GlcNAc bonds in peptidoglycan. (A) Structure of the peptidoglycan precursor Lipid II used in this study to make nascent peptidoglycan. C55-PP: undecaprenyl pyrophosphate. (B) Structure of a peptidoglycan oligomer showing the chemical bond cleaved by lytic transglycosylases and muramidases. (C) Lytic transglycosylase and muramidase cleavage products have anhydro-MurNAc (1) and MurNAc (2) at the liberated reducing end, respectively.
Compared to peptidoglycan synthases, the proteins responsible for peptidoglycan maturation have received relatively little attention. The integration of the newly synthesized peptidoglycan into the cell wall requires liberating the glycan strand from the lipid anchor that tethers it to the cytoplasmic membrane. One class of enzymes that have been implicated in this process are membrane-bound glycosidases, which are capable of cleaving the peptidoglycan sugar backbone. MltG is a cytoplasmic membrane-anchored lytic transglycosylase that has been proposed to fulfill this role by catalyzing a nonhydrolytic cleavage of the β-1,4 glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) to yield muropeptide products containing an 1,6-anhydro-MurNAc (anhMurNAc) end (Fig. 1 B and C) (8, 9). MltG is a member of the YceG family proteins that are widely present in rod- and oval-shaped bacteria (8). Some bacteria, including Staphylococcus aureus, do not encode YceG family proteins, and other types of cell wall–cleaving enzymes must liberate the glycan strand from the lipid anchor. In S. aureus, the membrane-anchored N-acetylglucosaminidase SagB has been proposed to perform this role by forming a complex with a partner protein that controls cleavage site selection on the nascent peptidoglycan strand (10, 11).
Here, we have investigated two putative cell wall-cleaving enzymes in Streptococcus pneumoniae that were previously reported to play roles in cell wall integrity. S. pneumoniae is an opportunistic pathogen that colonizes mucosa in the upper respiratory tract and can lead to a wide range of sometime fatal infections, including pneumonia, meningitis, and sepsis (12). As S. pneumoniae is increasingly resistant to clinically used antibiotics, understanding its vulnerabilities is a priority (13). One protein studied here, SPD_1346 (formerly MltG; here renamed MpgA for membrane-bound peptidoglycan glycosidase A), contains an extracellular YceG domain that resembles the lytic transglycosylase MltG (8, 14). The loss of MpgA severely impairs growth in unencapsulated S. pneumoniae strains, and mpgA-null mutants can only be stably constructed in strains that also lack the bifunctional peptidoglycan synthase PBP1a (15). MpgA depletion causes spherical morphology, implying a role in cell shape (15). Based on these and other observations, it was proposed that MpgA’s putative lytic transglycosylase activity is crucial for peptidoglycan assembly along the cell periphery (15, 16). The other protein studied here, SPD_0912 (formerly PMP23; renamed MpgB), shares structural homology with the Escherichia coli lytic transglycosylase MltE and with a Bacillus subtilis muramidase that hydrolyzes the β-1,4 glycosidic bond between MurNAc and GlcNAc (Fig. 1 B and C) (17, 18). Although not essential, MpgB deletion causes the mislocalization of division proteins and septal defects, suggesting that MpgB’s putative enzymatic activity is important in cell wall assembly at the septum (19, 20). Here, we characterized the enzymatic functions of MpgA and MpgB and found surprising differences from what we expected. We also identified a domain in MpgA that regulates where cleavage occurs along the glycan backbone and is important for MpgA's cellular function. Our studies not only provide insights into S. pneumoniae but have broad implications for cell wall hydrolase function in other bacteria.
Results
S. pneumoniae Becomes More Sensitive to Divisome-Targeting β-Lactams upon mpgB Deletion.
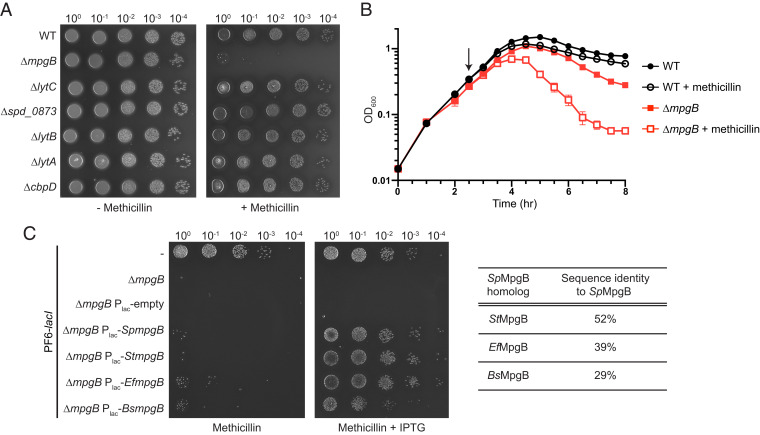
S. pneumoniae, like other bacteria, contains multiple genes that encode confirmed or putative cell wall hydrolases. Most cell wall hydrolases in S. pneumoniae are nonessential, so we needed a strategy to differentiate those involved in cell wall assembly during normal growth from those that play other roles. In E. coli and S. aureus, β-lactam hypersensitivity in deletion mutants has been used to identify cleavage enzymes that act early in the cell wall assembly pathway (10, 21–23). Hypersensitivity is notable because deleting cell wall hydrolases often makes cells more rather than less tolerant of β-lactam treatment, evidently because reducing cell wall degrading activity is advantageous when cell wall synthesis activity is inhibited (24–27). We individually deleted six known or putative cell wall hydrolase genes in S. pneumoniae and screened the mutants for hyper-susceptibility to methicillin and oxacillin. These β-lactams preferentially target PBP2x, which is an essential bPBP required for cell division (28). In a spot titer assay, only ∆mpgB cells displayed increased β-lactam susceptibility (Fig. 2A and SI Appendix, Fig. S1). Moreover, when grown in liquid culture, ∆mpgB cells lysed more rapidly than wild-type cells upon β-lactam treatment (Fig. 2B). The hypersensitivity phenotype was reversed by complementing ∆mpgB with ectopically expressed S. pneumoniae mpgB or with an mpgB homolog from Streptococcus thermophilus, B. subtilis, or Enterococcus faecalis (Fig. 2C). These results suggested that MpgB is involved in nascent cell wall assembly in S. pneumoniae. Unlike the majority of S. pneumoniae cell wall hydrolases, MpgB contains a transmembrane helix that anchors the extracellular catalytic domain to the membrane. This domain organization is also seen in the quasi-essential hydrolase MpgA, which was proposed to play an active role during peptidoglycan synthesis (15). Because previous reconstitution attempts for MpgA and MpgB were unsuccessful, we decided to biochemically examine the activities of these membrane-bound cell wall hydrolases (15, 20).
Fig. 2.
MpgB deletion renders S. pneumoniae hypersensitive to methicillin. (A) S. pneumoniae cells containing the indicated gene deletion were spotted on an agar plate with methicillin (64 ng/mL) to identify hypersensitivity phenotypes. A methicillin-dependent difference in colony morphology was observed for strains that were viable under methicillin treatment. (B) Representative growth curves of S. pneumoniae wild-type and ∆mpgB strains in THY broth showing effects of adding methicillin (black arrow; 16 ng/mL). Error bars represent mean ± SD from triplicates. (C) Methicillin sensitivity of ∆mpgB cells can be rescued by expressing MpgB homologs from S. pneumoniae, S. thermophilus, E. faecalis, or B. subtilis under an IPTG-inducible promoter (Plac). Cells were spotted on agar containing methicillin (64 ng/mL) and IPTG (1 mM). Strains used in this experiment express LacI that prevents leaky expression of Plac (51). Representative data from two independent experiments are shown in A–C.
MpgA and MpgB Cleave Nascent Peptidoglycan at Different Sites In Vitro.
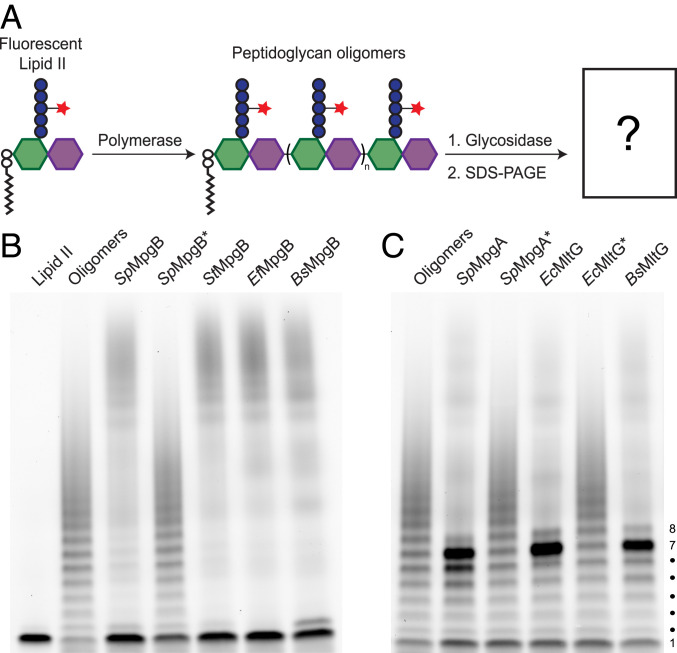
To demonstrate the enzymatic activities of MpgA and MpgB in vitro, we purified these proteins as well as several homologs from other organisms (SI Appendix, Fig. S2). As reported previously, neither MpgA nor MpgB displayed cleavage activity when purified sacculi were used as a substrate (SI Appendix, Fig. S3) (15, 20). To test whether these enzymes can cleave nascent peptidoglycan, we prepared substrates for our assays by polymerizing fluorescently labeled Lipid II, the peptidoglycan precursor, using a polymerase that was engineered to make short peptidoglycan strands (29). The labeled oligomers were incubated with the purified proteins and the reaction products were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to in-gel visualization (Fig. 3A). The untreated peptidoglycan oligomers run as a ladder in which each band differs by a monomer (i.e., disaccharide) unit. In lanes treated with either MpgB or MpgA, we observed clear changes in the banding pattern compared with the control (Fig. 3 B and C). For both proteins, mutating the proposed catalytic residue abolished the reaction, and we concluded that both proteins had glycosidase activity. However, there were striking differences in the products detected in the PAGE assay. Oligomers treated with MpgB were degraded to a predominant, rapidly migrating band with a mobility corresponding to Lipid II, the lipid-linked disaccharide–peptide starting material. In contrast, oligomers treated with MpgA were degraded to a predominant band with a mobility corresponding to a product having seven disaccharide units (i.e., Lipid 14). These distinct cleavage products were also observed for homologs of MpgB and MpgA. Therefore, these two classes of enzymes have an intrinsic ability to cleave nascent peptidoglycan at different sites relative to the lipid anchor on the reducing end of the polymer.
Fig. 3.
MpgA and MpgB cleave nascent peptidoglycan at different positions along the backbone. (A) Schematic of the assay used to detect peptidoglycan glycosidase activity. The fluorescent dye ATTO488 is attached to the stem peptide lysine for an in-gel visualization of peptidoglycan products. (B) MpgB cleaves nascent peptidoglycan to Lipid II in vitro (bottom band, no. 1). (C) MpgA and other YceG family proteins cleave nascent peptidoglycan to a major lipid-linked product with a mobility corresponding to seven disaccharide repeats. Representative blots from three independent experiments are shown in B and C. Asterisks indicate catalytically inactive variants. Diffuse signal high in the gel in reaction lanes are products released from the lipid anchor by cleavage (Fig. 4).
MpgA and MpgB Are Peptidoglycan Muramidases.
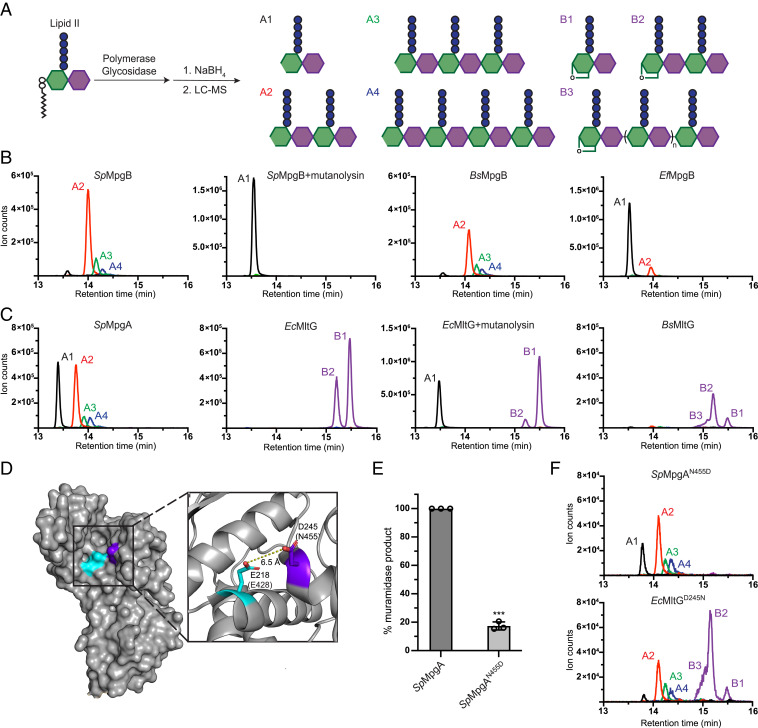
Before addressing mechanisms for product length control, we wanted to establish the nature of the bond cleavage reactions. We observed a diffuse signal in the upper half of the lanes for the enzyme-treated samples. This signal is due to cleavage products that lack the diphospholipid anchor (10). We used liquid chromatography–mass spectrometry (LC-MS) to characterize these products. MpgB and MpgA were each incubated with a polymerase and native Lipid II, and the reaction samples were treated with sodium borohydride (NaBH4) to reduce any hydrolysis products (Fig. 4A, products A1 to A4 and SI Appendix, Figs. S4 and S5). In the SpMpgB sample, the mass of the major peak corresponded to a tetrasaccharide hydrolysis product, but we also detected small peaks for larger products (Fig. 4 B, Left). To confirm the identities of these peaks, the hydrolase-treated peptidoglycan cleavage reactions were further treated with mutanolysin, a well-characterized muramidase that cleaves uncross-linked peptidoglycan oligomers to disaccharide–monopeptide monomer units. For muramidases, we would expect mutanolysin treatment to convert all products to the disaccharide A1. Indeed, the addition of mutanolysin to the SpMpgB cleavage reactions resulted in the disappearance of the oligomer peaks (A2 to A4) and the appearance of the monomer peak (A1) (Fig. 4 B, Middle Left). SpMpgB was previously speculated to have either muramidase activity or lytic transglycosylase activity, and our product analysis showed that it is a muramidase (15, 20). BsMpgB and EfMgpB were also shown to have muramidase activity (Fig. 4 B, Middle Right and Right).
Fig. 4.
MpgA is a muramidase rather than a lytic transglycosylase like MltG. (A) Schematic of the LC-MS assay used to assess peptidoglycan cleavage activity. Products released from the lipid anchor by cleavage activity are detected in this assay. (B) MpgB is a peptidoglycan muramidase. (C) MpgA is a muramidase unlike its lytic transglycosylase homologs in E. coli and B. subtilis. (D) Structure of the EcMltG active site with the catalytic residue, Glu218, and a highly conserved aspartate, Asp245, shown in cyan and purple, respectively (Protein Data Bank: 2R1F). The corresponding residues in SpMpgA are indicated in parentheses. (E) Normalized levels of muramidase products detected by LC-MS for SpMpgA and the SpMpgAN455D variant. Error bars represent mean ± SD; P < 0.001 (Welch's t test, GraphPad Prism 9.0). (F) LC-MS analysis of SpMpgAN455D and EcMltGD245N cleavage products shows muramidase activity for the latter. For B, C, and F, extracted ion chromatograms were generated by plotting the intensity of the signal corresponding to the mass-to-charge ratio (m/z) of the muramidase products (A1 to A4) and lytic transglycosylase products (B1 to B3). Lytic transglycosylase product lengths are distinguished by their charge states. B3 indicates unresolved lytic transglycosylase oligomer products having n > 0. Representative LC-MS data from three independent experiments are shown.
The results for MpgA were more surprising because bioinformatics predicted that this protein was a lytic transglycosylase (8, 15). Lytic transglycosylases release muropeptides with anhMurNAc ends that cannot be reduced by NaBH4 (Fig. 4A, products B1 to B3 and SI Appendix, Figs. S4 and S5). However, the products for MpgA had the same masses as those for MpgB and were all converted to product A1 upon mutanolysin treatment. Therefore, MpgA also has muramidase activity (Fig. 4 C, Left and SI Appendix, Fig. S6). In contrast, and as expected based on a previous study, EcMltG generated anhMurNAc products (Fig. 4 C, Middle Left) (8). When mutanolysin was added, some disaccharide (A1) was formed, evidently due to the partial cleavage of the anhMurNAc tetrasaccharide species (B2) plus cleavage of remaining unprocessed peptidoglycan polymer (Fig. 4 C, Middle Right). We also confirmed that the B. subtilis homolog, BsMltG, is a lytic transglycosylase (Fig. 4 C, Right).
Although surprising, our discovery that MpgA is a muramidase rather than a lytic transglycosylase is consistent with previous studies reporting that anhydromuropeptide species are undetectable in the pneumococcal cell wall (30). However, because the known lytic transglycosylase EcMltG was able to complement a ∆mpgA deletion, MpgA and EcMltG were speculated to have the same enzymatic activity in cells; the lack of detectable anhMurNAc in the wild-type S. pneumoniae cell wall was attributed to a low abundance of these modifications (15). To verify that it would be possible to detect anhMurNAc species due to the action of MltG, we constructed a S. pneumoniae ∆mpgA strain that ectopically expressed either EcMltG or SpMpgA. We analyzed the muropeptides released from the isolated cell wall from these strains after mutanolysin digestion (SI Appendix, Fig. S7). In line with our biochemical results, muropeptides containing anhMurNAc ends were observed in peptidoglycan isolated from EcMltG-expressing cells but not from peptidoglycan isolated from SpMpgA-expressing cells. These results support our conclusion that SpMpgA, unlike its homolog EcMltG, is a peptidoglycan muramidase.
EcMltG Gains Muramidase Activity When a Conserved Aspartate Is Replaced with an Asparagine.
The predicted structure of SpMpgA is nearly identical to the determined structures of the YceG domains of EcMltG and Listeria monocytogenes MltG, but our findings show that its bond-cleaving activity is different (15). To identify residues that might play a role in determining whether a YceG homolog functions as a lytic transglycosylase or a muramidase, we aligned sequences of >10,000 YceG domains to identify conserved residues (SI Appendix, Fig. S8). Several highly conserved residues are replaced in SpMpgA compared with verified YceG lytic transglycosylases, but SpMpgA residue Asn455 particularly caught our attention because of its predicted proximity to the putative catalytic glutamate (Fig. 4D) (8, 14). This residue is an aspartate in 95% of YceG family proteins, including EcMltG (SI Appendix, Fig. S8). We wondered if the presence of Asn or Asp at this position was sufficient to determine if a YceG family protein had muramidase or lytic transglycosylase activity. An N455D substitution of SpMpgA reduced enzymatic activity but did not result in anhMurNAc products (Fig. 4 E and F). However, replacing the corresponding aspartate with asparagine in EcMltG (EcMltGD245N) led to the appearance of muramidase products (Fig. 4F). Although other amino acids may also be involved in determining whether an MltG homolog is a lytic transglycosylase or a muramidase, we can conclude from the results on EcMltGD245N that the conserved active site Asn we identified in SpMpgA indeed plays a role in determining muramidase activity. We therefore suggest that other YceG family proteins with an asparagine at this position, which are mainly found in Streptococcus and Lactococcus species, will have muramidase activity (SI Appendix, Fig. S8). In showing that closely related cell wall–cleaving enzymes can make different products, our findings highlight the need to corroborate any predicted enzymatic functions. We note that recently developed methods to make peptidoglycan in vitro from readily obtained Lipid II now make this straightforward (31–33).
Cleavage Site Selection Is Regulated by the LysM-Like Subdomain.
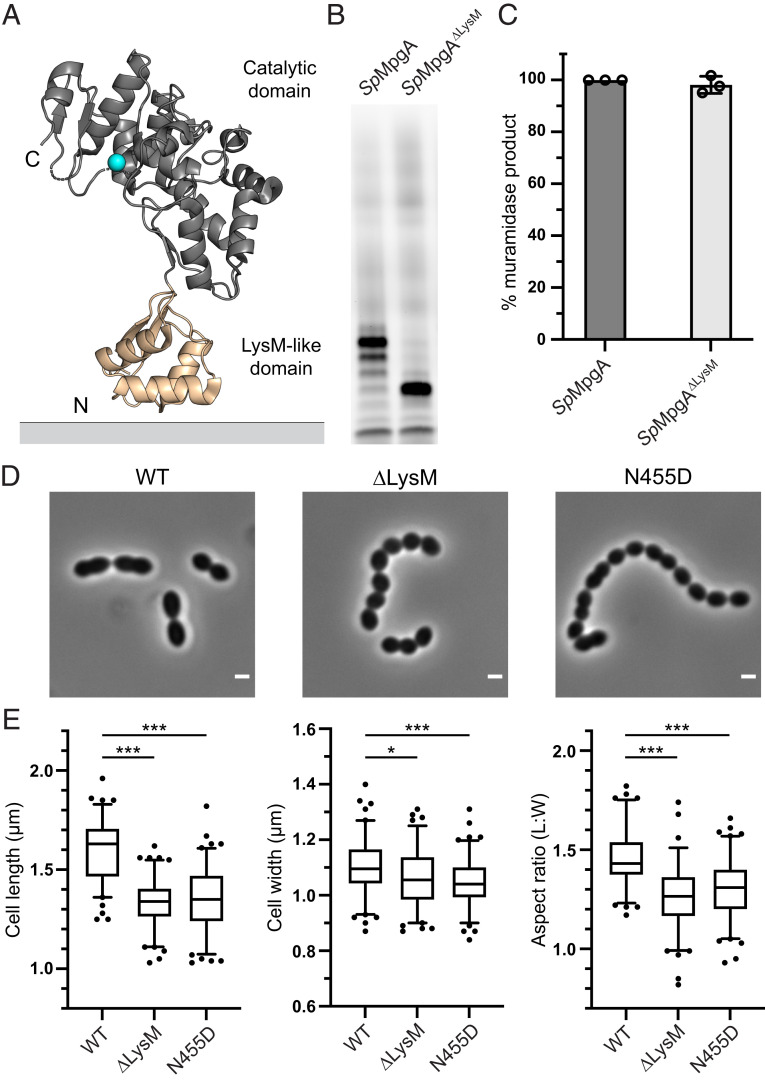
We next turned our attention to understanding length control of the lipid-linked cleavage products. Similar to mutanolysin, MpgB homologs cleave nascent peptidoglycan to Lipid II, but all the YceG proteins showed a predominant cleavage product with seven disaccharide repeats based on the gel mobility analysis. Because each disaccharide unit in peptidoglycan spans ∼10 Å, this would correspond to cleavage at a distance of ∼70 Å from the lipid anchor (34). A notable difference between MpgB and the YceG proteins is that the latter contain a membrane-proximal LysM-like subdomain between the transmembrane helix and the catalytic domain (Fig. 5A). LysM domains are widely distributed among peptidoglycan hydrolases and are proposed to modulate binding to peptidoglycan (35, 36). Although they are typically found in hydrolases that bind to mature (cross-linked) cell walls, it would be reasonable to speculate that the membrane-proximal LysM-like domain in YceG proteins binds nascent peptidoglycan near its membrane anchor to help direct the peptidoglycan strand into the active site for cleavage. Based on the crystal structure, the distance from the LysM-like subdomain to the catalytic residue is ∼50 Å, but there are additional residues between the LysM-like domain and the transmembrane helix anchoring the protein in the membrane that may extend the distance from the membrane to the active site (37). Overall, the dimensions of MpgA are sufficiently well matched with what we now know about its cleavage site preferences for the proposed model to be plausible. To test whether the LysM-like domain in SpMpgA and EcMltG does indeed affect product length, we expressed and purified a SpMpgA variant (SpMpgA∆LysM) and an EcMltG variant (EcMltG∆LysM) containing an internal deletion of this domain and tested their activity. SpMpgA∆LysM retained its muramidase activity but released a lipid-linked cleavage product that was approximately half as long as the product formed when the LysM-like domain was present (mobility consistent with Lipid 8; Fig. 5 B and C). Similarly, we observed a shorter cleavage product for EcMltG∆LysM (SI Appendix, Fig. S9). We conclude that the LysM-like domain is crucial for cleavage site selection in YceG proteins, supporting the hypothesis that it binds nascent peptidoglycan proximal to the membrane anchor.
Fig. 5.
The LysM-like domain of MpgA controls the length of lipid-linked cleavage product. (A) The crystal structure of the Listeria monocytogenes MltG homolog (Protein Data Bank: 4IIW) shows the N-terminal LysM-like subdomain (tan) and the catalytic subdomain (gray) with the catalytic glutamate in cyan. The LysM-like subdomain is connected to a transmembrane helix that anchors the protein to the cytoplasmic membrane. (B) Shorter lipid-linked cleavage products are generated when MpgA lacks the LysM-like domain (SpMpgA∆LysM). (C) Normalized levels of muramidase products detected by LC-MS for SpMpgA and the SpMpgA∆LysM variant. Error bars represent mean ± SD. (D) Phase-contrast images of S. pneumoniae cells containing wild-type mpgA, mpgA∆LysM, or mpgAN455D at the native locus. (Scale bar: 1 µm.) (E) Box and whisker plots (whiskers: 5 to 95 percentile) of cell length, cell width, and aspect ratio (cell length to width) of cells with wild-type mpgA, mpgA∆LysM, or mpgAN455D at the native locus. *P < 0.05, ***P < 0.001 (one-way ANOVA with Tukey's post hoc test, GraphPad Prism 9.0). See SI Appendix for details on the genetic background (rpsL1, ∆bgaA::Pzn-mpgA) of cells used in D and E. Representative data from three (B and C) or two (D and E) independent experiments are shown.
MpgA∆LysM Is Not Fully Functional in S. pneumoniae Cells.
Having demonstrated the role of the LysM-like domain in MpgA cleavage site selection, we wondered whether this domain is required for its function in cells. To address this question, we constructed an S. pneumoniae strain that contains a zinc-inducible mpgA at an ectopic locus and mpgA∆LysM at the native locus. No growth defect was observed for this strain in the absence of zinc (SI Appendix, Fig. S10); however, a morphological analysis revealed that cells containing mpgA∆LysM at the native locus are shorter and more spherical than their wild-type counterparts, similar to the phenotypes for cells expressing the MpgAN455D variant, which has defective catalytic activity (Figs. 4E and 5 D and E). We also found that PBP2b, an elongation-specific bPBP that is essential in the wild-type background but becomes dispensable in cells with defective MpgA, can readily be deleted in cells with MpgA∆LysM or MpgAN455D (SI Appendix, Fig. S11) (15). Taken together, these results indicate that MpgA∆LysM is sufficiently expressed and active to support growth but lacks the full functionality of wild-type MpgA in S. pneumoniae cells and instead behaves like cells expressing MpgA catalytic domain mutants.
Discussion
By reconstituting the activities of two S. pneumoniae membrane-bound peptidoglycan glycosidases and their homologs, we have made several discoveries that are also relevant to related glycosidases in other organisms. First, we have shown that not all YceG family proteins are lytic transglycosylases; despite its resemblance to EcMltG, MpgA is a muramidase. Second, we have shown that YceG family proteins cleave the glycan backbone of uncross-linked peptidoglycan at a distance of ∼70 Å from the lipid anchor. While this manuscript was in revision, Vollmer and coworkers also reported that EcMltG and BsMltG cleave peptidoglycan at a distance corresponding to seven disaccharides from the lipid anchor (38). Importantly, we have demonstrated that the LysM-like domain present in YceG family proteins regulates the length of the cleavage products. We have also shown that MpgB, speculated by some to be a muramidase and by others to be a lytic transglycosylase, has muramidase activity (15, 20). Unlike MpgA, MpgB does not contain another subdomain and it can cleave nascent peptidoglycan at the MurNAc-GlcNAc bond closest to the lipid anchor.
We were interested in that MpgA proved to be a muramidase given that it is so similar to MltG, a lytic transglycosylase. A single amino acid change in MltG caused it to become more promiscuous and to display muramidase activity in addition to lytic transglycosylase activity. The transition states for both lytic transglycosylases and muramidases are thought to have considerable oxocarbenium ion character (9). We presume that the EcMltGD245N mutant is still able to stabilize an oxocarbenium ion-like transition state, but a water molecule has gained the ability to compete with the intramolecular cleavage reaction resulting from an attack of the C6 hydroxyl. We speculate that it is much easier to convert a lytic transglycosylase to a muramidase by making a single amino acid change than it is to go in the other direction because converting a muramidase to a lytic transglycosylase would require positioning the C6 hydroxyl so its trajectory is well aligned for a productive attack on the anomeric carbon. If lytic transglycosylase activity appeared first in the YceG family, the switch of some of these enzymes to become muramidases might have started with a change of D to N in the active site. Directed evolution studies have taught us that an enzyme that performs a side reaction to even a small extent can often be rapidly optimized through a set of mutations to perform that reaction only (39). Perhaps something like that happened naturally with YceG family enzymes, but further sequence divergence among the family has obscured which amino acids hold the key to changing specificity completely and therefore which are crucial for going back in the other direction. This possibility can be addressed through a combination of sequence analysis, directed evolution, and perhaps structures of unsolved YceG family muramidases, including MpgA.
The studies reported here focus primarily on in vitro biochemistry of S. pneumoniae MpgA and MpgB, but it is worth asking what purpose these proteins serve in cells. Unlike MpgA and MpgB, many glycosidases do not localize in the cytoplasmic membrane; these other glycosidases tend to degrade mature peptidoglycan to enable daughter cell separation, facilitate cell wall recycling, or remodel the cell wall (40, 41). LysM domains are often found in these proteins and provide cell wall–targeting capability (35). Unlike proteins that degrade mature peptidoglycan, cell wall hydrolases anchored in the cytoplasmic membrane appear to act early in the peptidoglycan assembly pathway, cleaving uncross-linked regions of newly formed substrates that are not fully integrated into the cell wall matrix. For example, S. aureus LytH is a membrane-bound amidase that removes stem peptides only from uncross-linked peptidoglycan (23). In S. aureus, stem peptide removal from nascent peptidoglycan controls cell size by limiting substrate availability and regulating spatial localization of peptidoglycan synthases (23). E. coli MltG is a membrane-bound lytic transglycosylase that was proposed to terminate elongation by cleaving nascent polymer as it emerges from the peptidoglycan synthase active site (8). Supporting a role in terminating elongation, the presence of MltG reduces glycan strand lengths in the E. coli cell wall (8). S. aureus SagB, a membrane-bound glucosaminidase, also reduces glycan strand length (42). Because in vitro studies showed that SagB cleaves nascent peptidoglycan after it dissociates from a peptidoglycan synthase, SagB was proposed to function as a “release factor” that cleaves partially cross-linked peptidoglycan polymers from their lipid anchor. This allows a full integration of new strands into the mature cell wall while leaving a competent lipid-linked substrate in the membrane that can be extended once it rebinds to a peptidoglycan synthase (10, 43, 44).
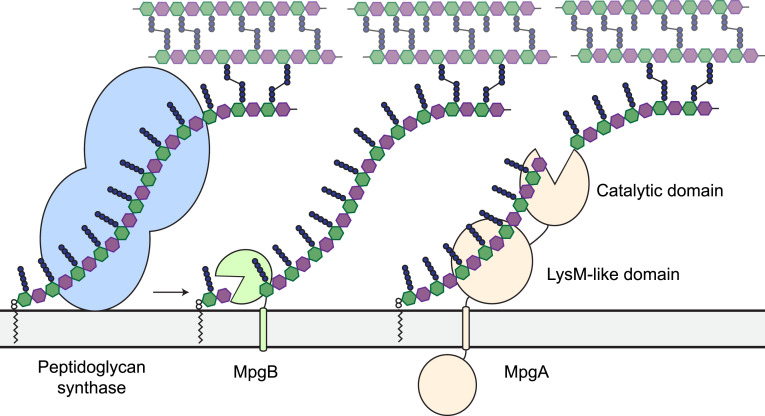
We favor the hypothesis that MpgA and MpgB, like SagB, serve as peptidoglycan release factors (Fig. 6). These proteins are anchored to the membrane, and they cleave nascent peptidoglycan bonds at a distance from the lipid anchor that is compatible with the estimated distance of their active sites from the membrane. In support of this hypothesis, we found that nascent peptidoglycan was an excellent substrate for both enzymes. In agreement with previous reconstitution attempts, no cleavage activity was detected when these enzymes were incubated with isolated cell wall, which consists of cross-linked peptidoglycan lacking the lipid anchor (15, 20).
Fig. 6.
Working model for MpgA and MpgB function in cells. Peptidoglycan synthases polymerize Lipid II and cross-link the nascent glycan strand into the existing cell wall. MpgA and MpgB cleave nascent peptidoglycan at different distances from the lipid anchor to permit full integration of the released polymer into the cell wall. The lipid-linked products are substrates for further extension by peptidoglycan synthases (10, 43, 44).
S. aureus SagB provides an interesting comparison to YceG family proteins. SagB forms a complex with SpdC, which is an integral membrane protein that shares homology with the CAAX protease family that processes polyprenylated proteins (10, 45). The SagB-SpdC complex has been shown to cleave nascent peptidoglycan at a defined distance from the lipid anchor. Based on biochemical data and the structure of the complex, SpdC was proposed to bind the lipid anchor on nascent peptidoglycan and guide the glycan strand up into the SagB active site (10). SagB without SpdC is functional as a glucosaminidase in vitro; however, without its partner protein to control cleavage site selectivity, SagB behaves like MpgB in that it cleaves nascent peptidoglycan to Lipid II. SagB also loses function in ∆spdC cells. This suggests that control over product length, and not simply enzymatic activity, is important (10). The LysM-like domain of MpgA mirrors SpdC in its ability to determine cleavage site selectivity with respect to the membrane, and the loss of this domain results in shorter lipid-linked cleavage products in vitro.
One question is what the primary cellular function of the LysM-like domain in MpgA is. A point mutation in the catalytic domain of MpgA was shown in a genetic selection to suppress lethality due to deletion of PBP2b, an essential bPBP that is part of the elongasome complex (15). This genetic link suggested that MpgA might be involved in peripheral peptidoglycan synthesis, and colocalization of MpgA with other elongasome proteins supports that idea, as does the morphology of MpgA deletion mutants. We have shown that the LysM-like domain in MpgA determines its cleavage site selection in vitro, and the deletion of this domain results in cellular phenotypes similar to those for defective catalytic domain mutants. MltG, which also contains a LysM-like domain and is connected to the elongasome, was proposed to interact with peptidoglycan synthases (8, 37, 38). One possibility is that the morphological defects in MpgA∆LysM are related to the loss of protein–protein interactions between this domain and components of the cell elongation machinery. However, the fact that E. coli MltG complements the loss of MpgA suggests that specific LysM-mediated protein–protein interactions are not crucial to MpgA’s activity (15). A more likely possibility is that the function of MpgA’s catalytic activity is compromised in cells when the LysM-like domain is deleted. The binding of nascent peptidoglycan to the LysM-like domain could be important for efficient cleavage in a cellular context. Alternatively, cleaving glycan strands to leave a long product may be important for reinitiating rapid cross-linking into the cell wall matrix because very few extension cycles would be necessary to bring the nonreducing end of the polymer into bonding distance.
A related question is why S. pneumoniae maintains two membrane-bound muramidases that form different lipid-linked cleavage products. In vitro studies have shown that the length of the lipid-linked substrate that is released upon cleavage affects the speed of polymerization. For aPBPs, Lipid IV and longer substrates initiate polymerization much faster than Lipid II (46). It is possible that where a peptidoglycan glycosidase cleaves can also influence the rate of polymerization. We presume that the differences in cleavage behavior for MpgA and MpgB reflect variations in peptidoglycan assembly and architecture along the side wall and the septum.
It remains to be answered how MpgA and MpgB coordinate their activities with peptidoglycan synthases and tailoring enzymes to build the S. pneumoniae cell wall. Models that posit direct physical interactions of MpgA and MpgB with peptidoglycan synthases provide intuitively appealing mechanisms for how cleavage and synthase activities are coordinated (8, 20, 38). However, the observation that MpgA and MpgB can be complemented with homologs sharing limited sequence identity strongly suggests that the enzymatic activities of these glycosidases determine their effects in cells. The enzymatic activities are conserved, but protein–protein interfaces undergo coevolution and often are not conserved between species. It is possible that activities of synthases and membrane-anchored hydrolases are “matched” through less elegant mechanisms, including relative expression levels of proteins, protein localization, and substrate availability. Peptidoglycan tailoring modifications installed by other enzymes may also modulate the availability of cleavage sites. With a clearer picture emerging for the biochemistry of membrane-bound cell wall hydrolases, we can begin to consider how their activities are coordinated with activities of synthetic and tailoring enzymes to build a new cell wall.
Methods
Materials.
Unless otherwise indicated, all chemicals and reagents were purchased from Sigma-Aldrich. Restriction enzymes were purchased from New England Biolabs. Oligonucleotide primers were purchased from Integrated DNA Technologies. Culture media were purchased from Becton Dickinson. S. thermophilus LMG 18311 genomic DNA was purchased from American Type Culture Collection (ATCC BAA-250D-5). S. pneumoniae ∆murMN Lipid II was isolated from cells as described previously (31, 32). S. aureus SgtB and SgtBY181D were expressed and purified as previously reported (29, 46).
Bacterial Strains, Plasmids, Oligonucleotide Primers, and Culture Conditions.
E. coli strains were grown with shaking at 37 °C in lysogeny broth (LB), Terrific Broth (TB), or on agarized LB plates with appropriate additives. S. pneumoniae strains were cultured statically in Todd Hewitt broth containing 0.5% yeast extract (THY) or Brain Heart Infusion broth (BHI) at 37 °C in an atmosphere containing 5% CO2. Prepoured Trypticase Soy Agar with 5% sheep blood (TSAII 5% SB) plates with a 5-mL overlay of 1% nutrient broth agar or TSA plates containing 5% defibrinated SB with appropriate additives were used for S. pneumoniae when growth on solid media was required. The following concentrations of antibiotics were used: carbenicillin, 50 µg/mL; chloramphenicol, 35 µg/mL; erythromycin, 0.2 µg/mL; gentamicin, 100 µg/mL; kanamycin, 50 µg/mL (E. coli) or 250 µg/mL (S. pneumoniae); spectinomycin, 200 µg/mL; streptomycin, 300 µg/mL; and tetracycline, 0.25 µg/mL The bacterial strains, plasmids, and oligonucleotide primers used in this study are summarized in SI Appendix. The protocol for plasmid construction can also be found in SI Appendix.
Protein Expression: General Procedure.
A previously described protocol was used for protein expression (3). E. coli C43(DE3) containing the expression plasmid(s) of interest was grown in 1 L TB supplemented with the appropriate antibiotics at 37 °C with shaking until the optical density at 600 nm (OD600) was ∼0.7. The culture was cooled to 20 °C, and protein expression was induced by adding 500 µM isopropyl β-D-1-thiogalactopyranoside (IPTG) (His6-tagged protein) or 500 µM IPTG and 0.1% arabinose (FLAG-tagged protein). Cells were harvested 18 h postinduction by centrifugation (4,200 × g, 15 min, 4 °C), and the pellet was stored at −80 °C.
Purification of His6-Tagged Proteins.
All steps after cell lysis were performed at 4 °C. Cells were resuspended in 50 mL lysis buffer (50 mM Hepes pH 7.5, 150 mM NaCl) and lysed by passaging the resuspended cells through a cell disruptor (EmulsiFlex-C5, Avestin) at 15,000 psi three times. Cell debris was removed by centrifugation (12,000 × g, 5 min, 4 °C), and the membrane fraction was collected by ultracentrifugation of the supernatant (100,000 × g, 1 h, 4 °C). The membrane pellet was resuspended in 30 mL solubilization buffer (50 mM Hepes pH 7.5, 500 mM NaCl, 1% n-dodecyl β-D-maltoside [DDM], and 10% glycerol) using a glass dounce tissue grinder (Wheaton). The resulting mixture was stirred for 1 h at 4 °C before ultracentrifugation (100,000 × g, 30 min, 4 °C). The resulting supernatant was supplemented with 0.75 mL pre-equilibrated Ni-NTA resin (Qiagen) and 20 mM imidazole and stirred for 30 min at 4 °C. The sample was then loaded onto a gravity column and washed with 30 mL buffer A (50 mM Hepes pH 7.5, 500 mM NaCl, 0.05% DDM, and 10% glycerol) containing 20 mM imidazole and 30 mL buffer A containing 40 mM imidazole. The protein was then eluted in 10 mL buffer A containing 300 mM imidazole. The eluate was further purified by size exclusion chromatography with a Superdex 200 10/300 GL column (MpgB) or a Superose 6 Increase 10/300 GL column (MpgA/MltG) equilibrated in buffer A. Fractions containing the target protein were concentrated by centrifugal filtration. The absorbance at 280 nm was measured using a NanoDrop One Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific), and the predicted extinction coefficient was used to calculate concentration. Protein samples were then aliquoted and stored at −80 °C.
Purification of FLAG-Tagged Proteins.
FLAG-tagged MpgBs were purified via the same protocol as His6-tagged proteins, above, with the following modifications. The supernatant containing the DDM-solubilized protein was supplemented with 2 mM CaCl2 and loaded onto a homemade 1 mL M1 α-Flag antibody resin. The resin was washed with 60 mL buffer A supplemented with 2 mM CaCl2, and the bound protein was eluted from the column with 10 mL buffer A supplemented with 5 mM ethylenediaminetetraacetic acid (EDTA) and 0.2 mg/mL FLAG peptide (Genscript).
Preparation of ATTO488-Labeled Lipid II.
S. pneumoniae ∆murMN Lipid II was fluorescently labeled with ATTO488 NHS-ester (ATTO-TEC) (NHS-ester:Lipid II = 8:1 mol ratio) in labeling buffer (0.1 M sodium borate pH 8.5) for 1 h at room temperature in the dark. The reaction mixture was then loaded onto a 1-mL Bakerbond C18 SPE column (J.T.Baker) by gravity flow. The column was sequentially washed with 1.5 mL 10, 20, 40 and 60% methanol to remove excess NHS-ester, and the labeled product was eluted with 100% methanol. The elution fraction was dried in vacuo and stored at −20 °C until further use.
In-Gel Detection of Peptidoglycan Glycosidase Activity.
Fluorescent peptidoglycan oligomers were prepared by incubating 0.25 µM ATTO488-labeled Lipid II with 1 µM SgtBY181D (monofunctional peptidoglycan glycosyltransferase with impaired processivity) in a 1× reaction buffer (50 mM Tris pH 7.0, 10 mM CaCl2 and 10% dimethyl sulfoxide [DMSO]) at 30 °C for 1 h (29). The polymerization reaction was heat quenched at 95 °C for 5 min. After cooling, the digestion reaction was set up by adding 1 µL 50 µM glycosidase to 9 µL polymerization reaction product (total volume 10 µL). After incubating this mixture at 30 °C for 3 h (MpgA/MltG) or 10 h (MpgB), the reaction was quenched by adding 10 µL 2× Laemmli sample buffer (Bio-Rad). The samples were then loaded onto a 4 to 20% Mini-PROTEAN TGX Precast Protein gel (Bio-Rad) and run at 180 V. The gels were imaged using a Typhoon FLA 7000 imager.
LC-MS Analysis of Peptidoglycan Glycosidase Products.
A previously published method was adapted to characterize peptidoglycan glycosidase products (3, 32). S. pneumoniae Lipid II (50 µM) and glycosidase (5 µM) were incubated with SgtB (2 µM: for reactions containing MpgA/MltG) or SgtBY181D (5 µM: for reactions containing MpgB) in 20 µL 1× reaction buffer (20 mM 2-(N-morpholino)ethanesulfonic acid [Mes] pH 6.5, 10 mM CaCl2, and 10% DMSO) at 30 °C for 16 h. This reaction was heat quenched at 95 °C for 5 min and cooled to room temperature. Several glycosidase-treated samples were further digested by adding 3 µL mutanolysin (from Streptomyces globisporus: 4,000 U/mL) and incubating at 37 °C for 3 h. To reduce the muropeptide products, 50 µL NaBH4 (10 mg/mL) was added, and the mixture was incubated at room temperature for 30 min. The pH was adjusted to ∼4 with 20% phosphoric acid, and the samples were lyophilized to dryness overnight. Dried samples were resuspended in 18 µL H2O and analyzed by LC-MS on an Agilent Technologies 1200 series HPLC system in line with an Agilent 6520 quadrupole time-of-flight (Q-TOF) mass spectrometer with electrospray ionization mass spectrometry (ESI-MS) operating in positive mode. Products were separated on a Waters Symmetry Shield RP18 column (5 µm; 3.9 × 150 mm) with a matching column guard using the following method: flow rate = 0.5 mL/min, 100% solvent A (H2O, 0.1% formic acid) for 5 min followed by a linear gradient of solvent B (acetonitrile, 0.1% formic acid) from 0 to 40% over 25 min. Agilent MassHunter Workstation Qualitative Analysis software was used for analyzing the MS data. We note that pilot experiments identified SgtB as optimal to use for maximizing the LC-MS signal for MpgA cleavage, whereas SgtBY181D was optimal for MpgB cleavage. We speculate that the short products released by SgtBY181D may be cleaved quickly by MpgB but would interfere with the MpgA reaction samples by binding to the LysM-like domain.
S. pneumoniae Strain Construction.
S. pneumoniae D39 ∆cps and its derivatives were transformed using a previously reported protocol (47). Strains containing antibiotic markers were constructed by transforming an antibiotic resistance cassette flanked by ∼1-kb upstream and downstream regions of the target gene. A markerless ∆mpgB strain was constructed by first transforming a DNA cassette that includes an antibiotic resistance marker and a B. subtilis sacB gene flanked by ∼1-kb upstream and downstream regions of mpgB. After antibiotic selection, a PCR fragment containing the upstream and downstream regions of mpgB was transformed, and transformants were selected with 10% sucrose (48). Strains containing markerless alleles in the native chromosomal mpgA locus were constructed by allelic replacement using the Janus cassette (49). Detailed protocols for strain construction can be found in SI Appendix.
Viability Assay of S. pneumoniae Strains.
Cells grown to the mid-log phase in THY were collected by centrifugation and normalized to OD600 = 1.0. The normalized cultures were serially diluted, and 3 µL of each dilution was spotted onto TSA 5% SB plates containing the indicated additives. To determine the antibiotic concentration that does not affect the viability of wild-type cells, the growth of wild-type cells in different concentrations of methicillin or oxacillin was assessed prior to the growth evaluation of mutant strains.
Growth Assessment and Microscopy Analysis of S. pneumoniae Strains.
For the growth assessment of ∆mpgB cells, cultures grown to the mid-log phase in THY were diluted to OD600 = 0.015, and growth was monitored by measuring OD600 at the indicated time points. For a growth assessment of mpgA variants, cells grown to the mid-log phase in BHI supplemented with 0.2 mM ZnCl2 and 0.02 mM MnSO4 were washed to remove Zn2+/Mn2+ and diluted to OD620 = 0.003 in BHI with no additional Zn2+/Mn2+. Growth was monitored by measuring OD620 at the indicated time points. Transformation assays using the pbp2b deletion cassette were performed as reported previously (15). For microscopy analysis, cultures were sampled at OD620 = 0.1 to 0.2, and the cell length and width of >100 cells from two independent experiments were measured as previously described (15).
Digestion and LC-MS Analysis of the S. pneumoniae Cell Wall.
A previously reported protocol was adapted for isolating S. pneumoniae peptidoglycan (50). S. pneumoniae cultures were prepared in 20 mL THY and grown until OD600 ∼0.7. Cells were pelleted and resuspended in 1 mL 100 mM Tris pH 6.8/0.25% SDS. After boiling the cells at 100 °C for 30 min, the suspension was pelleted and washed four times with 1 mL H2O. The washed pellet was resuspended in 1 mL 100 mM Tris pH 6.8 containing 10 mM CaCl2, 10 mM MgCl2 and 50 µg/mL trypsin and incubated at 37 °C for 1 h before heat inactivating the enzymes at 95 °C for 5 min. The suspension was centrifuged at 21,000 × g for 3 min, and the resulting pellet was washed once with 1 mL H2O. The pellet was then suspended in 500 µL 1 M HCl and incubated at 37 °C for 4 h. The suspension was pelleted and washed four times with 1 mL H2O. The resulting peptidoglycan pellet was suspended in 100 µL 12.5 mM NaHPO4 pH 5.5, and 10 µL mutanolysin (4,000 U/mL) was added to this suspension. The digestion reaction proceeded at 37 °C for 16 h. After heat inactivating mutanolysin at 95 °C for 5 min, the insoluble material was pelleted by centrifugation at 21,000 × g for 3 min. The supernatant was transferred to a new microcentrifuge tube, and muropeptide products were reduced by adding 50 µL NaBH4 (20 mg/mL) and incubating at room temperature for 30 min. The pH was adjusted to ∼4 with 20% phosphoric acid before LC-MS analysis.
LC-MS analysis of the cell wall muropeptide products was conducted with the same instrument setup as described above using the following method: flow rate = 0.5 mL/min, 100% solvent A (H2O, 0.1% formic acid) for 5 min followed by a linear gradient of solvent B (acetonitrile, 0.1% formic acid) from 0 to 20% over 120 min.
Detection of Sacculi Cleavage Activity.
Peptidoglycan was isolated from S. pneumoniae D39 ∆cps cells as described above (Digestion and LC-MS Analysis of the S. pneumoniae Cell Wall) but scaled proportionally for a 500-mL culture in THY. Peptidoglycan (19 mg) was then labeled in 4 mL 20 mM Remazol Brilliant Blue (RBB) in 250 mM NaOH at 37 °C overnight. After neutralizing with 1 M HCl, the labeled peptidoglycan was spun down at 21,000 × g for 5 min and washed with H2O until the supernatant was clear. The labeled peptidoglycan was then resuspended in 400 µL H2O and stored at 4 °C. For each cleavage reaction, 9 µL RBB-labeled peptidoglycan was treated with 5 µM enzyme in 60 µL 1× reaction buffer (20 mM Mes pH 6.5, 10 mM CaCl2, and 0.02% DDM). The reactions were incubated at 37 °C with shaking to 300 rpm. At 2-, 4-, and 20-h time points, the reactions were spun down at 21,000 × g for 5 min, and the absorbance of the supernatant was measured at 595 nm.
Supplementary Material
Acknowledgments
We thank Tyler Sisley for assistance with the initial characterization of SpMpgB and Shobnom Mustaree for help with bacterial strain constructions and growth experiments. We also thank Genevieve Dobihal and David Rudner for the kind gift of B. subtilis PY79 genomic DNA. LC-MS data were acquired on an Agilent 6520 Q-TOF mass spectrometer supported by the Taplin Funds for Discovery Program. Funding for this work was provided by NIH grants R01 AI139011 and R01 AI148752 to S.W., T32 GM007753 and F30 AI156972 to J.E.P., and R35 GM131767 to M.E.W. A.T. is supported in part by the Funai Overseas Scholarship.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2103740118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Silhavy T. J., Kahne D., Walker S., The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeske A. J., et al., SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 537, 634–638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taguchi A., et al., FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol. 4, 587–594 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjodt M., et al., Structural coordination of polymerization and crosslinking by a SEDS-bPBP peptidoglycan synthase complex. Nat. Microbiol. 5, 813–820 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H., et al., Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi-autonomously. Nat. Microbiol. 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massidda O., Nováková L., Vollmer W., From models to pathogens: How much have we learned about Streptococcus pneumoniae cell division? Environ. Microbiol. 15, 3133–3157 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Bush K., Bradford P. A., β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 6, a025247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yunck R., Cho H., Bernhardt T. G., Identification of MltG as a potential terminase for peptidoglycan polymerization in bacteria. Mol. Microbiol. 99, 700–718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dik D. A., Marous D. R., Fisher J. F., Mobashery S., Lytic transglycosylases: Concinnity in concision of the bacterial cell wall. Crit. Rev. Biochem. Mol. Biol. 52, 503–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer K., et al., Structure and reconstitution of a hydrolase complex that may release peptidoglycan from the membrane after polymerization. Nat. Microbiol. 6, 34–43 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willing S., Schneewind O., Missiakas D., Regulated cleavage of glycan strands by the murein hydrolase SagB in Staphylococcus aureus involves a direct interaction with LyrA (SpdC). J. Bacteriol. 203, e00014–e00021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henriques-Normark B., Tuomanen E. I., The pneumococcus: Epidemiology, microbiology, and pathogenesis. Cold Spring Harb. Perspect. Med. 3, 1–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim L., McGee L., Tomczyk S., Beall B., Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: A United States perspective. Clin. Microbiol. Rev. 29, 525–552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M., et al., From genome to proteome to elucidation of reactions for all eleven known lytic transglycosylases from Pseudomonas aeruginosa. Angew. Chem. Int. Ed. Engl. 56, 2735–2739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsui H. C. T., et al., Suppression of a deletion mutation in the gene encoding essential PBP2b reveals a new lytic transglycosylase involved in peripheral peptidoglycan synthesis in Streptococcus pneumoniae D39. Mol. Microbiol. 100, 1039–1065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamsås G. A., et al., Identification of EloR (Spr1851) as a regulator of cell elongation in Streptococcus pneumoniae. Mol. Microbiol. 105, 954–967 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Xu Q., et al., Structures of a bifunctional cell wall hydrolase CwlT containing a novel bacterial lysozyme and an NlpC/P60 DL-endopeptidase. J. Mol. Biol. 426, 169–184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukushima T., et al., Identification and characterization of novel cell wall hydrolase CwlT: A two-domain autolysin exhibiting n-acetylmuramidase and DL-endopeptidase activities. J. Biol. Chem. 283, 11117–11125 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Pagliero E., et al., The inactivation of a new peptidoglycan hydrolase Pmp23 leads to abnormal septum formation in Streptococcus pneumoniae. Open Microbiol. J. 2, 107–114 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacq M., et al., The cell wall hydrolase Pmp23 is important for assembly and stability of the division ring in Streptococcus pneumoniae. Sci. Rep. 8, 7591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templin M. F., Edwards D. H., Höltje J. V., A murein hydrolase is the specific target of bulgecin in Escherichia coli. J. Biol. Chem. 267, 20039–20043 (1992). [PubMed] [Google Scholar]
- 22.Cho H., Uehara T., Bernhardt T. G., Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 159, 1300–1311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Do T., et al., Staphylococcus aureus cell growth and division are regulated by an amidase that trims peptides from uncrosslinked peptidoglycan. Nat. Microbiol. 5, 291–303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomasz A., Albino A., Zanati E., Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227, 138–140 (1970). [DOI] [PubMed] [Google Scholar]
- 25.Heidrich C., Ursinus A., Berger J., Schwarz H., Höltje J. V., Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184, 6093–6099 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung H. S., et al., Rapid beta-lactam-induced lysis requires successful assembly of the cell division machinery. Proc. Natl. Acad. Sci. U.S.A. 106, 21872–21877 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores-Kim J., Dobihal G. S., Fenton A., Rudner D. Z., Bernhardt T. G., A switch in surface polymer biogenesis triggers growth-phase-dependent and antibiotic-induced bacteriolysis. eLife 8, 1–23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kocaoglu O., Tsui H. C. T., Winkler M. E., Carlson E. E., Profiling of β-lactam selectivity for penicillin-binding proteins in Streptococcus pneumoniae D39. Antimicrob. Agents Chemother. 59, 3548–3555 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebets Y., et al., Moenomycin resistance mutations in Staphylococcus aureus reduce peptidoglycan chain length and cause aberrant cell division. ACS Chem. Biol. 9, 459–467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bui N. K., et al., Isolation and analysis of cell wall components from Streptococcus pneumoniae. Anal. Biochem. 421, 657–666 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Qiao Y., et al., Lipid II overproduction allows direct assay of transpeptidase inhibition by β-lactams. Nat. Chem. Biol. 13, 793–798 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsh M. A., et al., Identification of a functionally unique family of penicillin-binding proteins. J. Am. Chem. Soc. 139, 17727–17730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer K., Owens T. W., Kahne D., Walker S., Substrate preferences establish the order of cell wall assembly in Staphylococcus aureus. J. Am. Chem. Soc. 140, 2442–2445 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlström D., The polysaccharide chain of chitin. Biochim. Biophys. Acta 59, 361–364 (1962). [DOI] [PubMed] [Google Scholar]
- 35.Buist G., Steen A., Kok J., Kuipers O. P., LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 68, 838–847 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Mesnage S., et al., Molecular basis for bacterial peptidoglycan recognition by LysM domains. Nat. Commun. 5, 4269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohrhunter J. L., Rohs P. D. A., Torres G., Yunck R., Bernhardt T. G., MltG activity antagonizes cell wall synthesis by both types of peptidoglycan polymerases in Escherichia coli. Mol. Microbiol. 115, 1170–1180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassine J., Pazos M., Breukink E., Vollmer W., Lytic transglycosylase MltG cleaves in nascent peptidoglycan and produces short glycan strands. Cell Surf. 7, 100053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeymer C., Hilvert D., Directed evolution of protein catalysts. Annu. Rev. Biochem. 87, 131–157 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Do T., Page J. E., Walker S., Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes. J. Biol. Chem. 295, 3347–3361 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vollmer W., Joris B., Charlier P., Foster S., Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 32, 259–286 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Chan Y. G. Y., Frankel M. B., Missiakas D., Schneewind O., SagB glucosaminidase is a determinant of Staphylococcus aureus glycan chain length, antibiotic susceptibility, and protein secretion. J. Bacteriol. 198, 1123–1136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlstein D. L., Zhang Y., Wang T. S., Kahne D. E., Walker S., The direction of glycan chain elongation by peptidoglycan glycosyltransferases. J. Am. Chem. Soc. 129, 12674–12675 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welsh M. A., Schaefer K., Taguchi A., Kahne D., Walker S., Direction of chain growth and substrate preferences of shape, elongation, division, and sporulation-family peptidoglycan glycosyltransferases. J. Am. Chem. Soc. 141, 12994–12997 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manolaridis I., et al., Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature 504, 301–305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T. S. A., et al., Primer preactivation of peptidoglycan polymerases. J. Am. Chem. Soc. 133, 8528–8530 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenton A. K., El Mortaji L., Lau D. T. C., Rudner D. Z., Bernhardt T. G., CozE is a member of the MreCD complex that directs cell elongation in Streptococcus pneumoniae. Nat. Microbiol. 2, 16237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Thompson C. M., Lipsitch M., A modified Janus cassette (Sweet Janus) to improve allelic replacement efficiency by high-stringency negative selection in Streptococcus pneumoniae. PLoS One 9, e100510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung C. K., Li H., Claverys J. P., Morrison D. A., An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67, 5190–5196 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kühner D., Stahl M., Demircioglu D. D., Bertsche U., From cells to muropeptide structures in 24 h: Peptidoglycan mapping by UPLC-MS. Sci. Rep. 4, 7494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller L. E., Rueff A.-S., Kurushima J., Veening J.-W., Three new integration vectors and fluorescent proteins for use in the opportunistic human pathogen Streptococcus pneumoniae. Genes (Basel) 10, 394 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.