Abstract
A bisthienylethene-dipyrimido[2,1-b][1,3]benzothiazole (BTE-2PBT) triad has been designed and synthesized based on our recent discovery of PBTs as atypical propeller-shaped novel AIEgens. The triad not only maintains the photochromic properties of BTE moiety in solution, film, and solid state but also exhibits remarkable AIE properties. Moreover, the fluorescence of BTE-2PBT PMMA film could be modulated with high contrast by alternate UV and visible light irradiation. Photoerasing, rewriting, and non-destructive readout of fluorescent images on BTE-2PBT PMMA film well demonstrate its potential application as optical memory media.
Keywords: AIE; photochromism; pyrimido[2,1-b][1,3]benzothiazole; diarylethene; photoswitch
1. Introduction
Photochromic diarylethenes have been acknowledged for their excellent photochemical reactivity, thermal stability, and fatigue resistance [1,2] and widely applied as photo-responsive materials in a variety of photonic devices [3]. Generally, the open-ring isomers of diarylethenes are weakly fluorescent depending on the nature of attached heteroaryl rings, but the fluorescence is typically quenched due to the formation of a larger conjugation system upon photo-induced ring closure. Therefore, diarylethene alone is not an ideal photo-switchable fluorophore. However, the huge absorption shift from 250–330 nm to 500–650 nm upon cyclization enables diarylethenes to function as photo-responsive energy acceptors in fluorescence resonance energy transfer (FRET) pairs. The photo-addressable fluorescence observed in diarylethene-fluorophore dyads [4,5] confirmed that the fluorescence modulation largely depends on the photoconversion ratio (PR) of diarylethene moiety. These photo-switchable fluorophores have been applied to labelling of biomolecules [6] and nanoparticles [7] to enable fluorescence switching with UV and visible light. However, most of these fluorescent photoswitches inevitably suffer from aggregation-caused quenching (ACQ), which greatly limits their applications as high-density optical materials [8].
The discovery of aggregation-induced emission (AIE) [9,10,11] has brought a new window to overcome the ACQ problem. It has been reported that the bisthienylethene-diquinolinemalononitrile triad [12] and bisthienylethene-dicoumarin triad [13] exhibited AIE properties. However, their photo-controlled modulation of solid-state fluorescence was not well realized. Currently, only a limited number of dyads and triads, which directly connect a bisthienylethene (BTE) with one or two AIEgens, such as trans-cyanostilbene [14,15], tetraphenylethene [16,17,18], and bispyridine salt [19], have been obtained as photo-switchable AIE materials [20,21].
Recently, our research group discovered pyrimido[2,1-b][1,3]benzothiazoles (PBTs) as atypical propeller-shaped novel AIEgens with full-color tunability, excellent solid-state fluorescence quantum yields, and high crystallizability [22]. Herein, we report the design and synthesis of a novel bisthienylethene-dipyrimido[2,1-b][1,3]benzothiazole (BTE-2PBT) triad. The novel triad not only well maintains its photochromic properties in solution, film, and solid state but also exhibits remarkable AIE properties. More importantly, the fluorescence of BTE-2PBT triad could be reversibly modulated with high contrast in solid state by UV and visible light irradiation, making BTE-2PBT triad a promising candidate for the application as photo-erasible optical memory media.
2. Results and Discussion
2.1. Synthesis of BTE-2PBT Triad
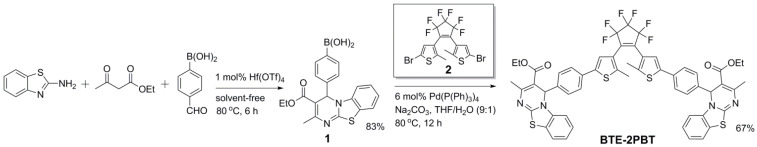
As shown in Scheme 1, boronic acid-modified PBT compound (1) was synthesized via the Hf(OTf)4-catalyzed three-component reaction (3CR) of 4-formylphenylboronic acid, 2-aminobenzothiazole, and ethyl acetoacetate under solvent-free conditions [23]. The presence of boronic acid was well tolerated by the Hf(OTf)4-catalyzed 3CR, and 1 was obtained in 83% yield over 6 h. The Suzuki coupling of 2.5 equiv of 1 with 1 equiv of dibromobisthienylethene (2) in THF/H2O mixture (9:1, v/v) at 80 °C for 12 h afforded the desired BTE-2PBT triad in 67% yield as a light greenish powder. The NMR and HRMS spectra are provided in the Supplementary Materials.
Scheme 1.
Synthetic route for BTE-2PBT triad.
2.2. Photochromism of BTE-2PBT in Solution, Film, and Solid State
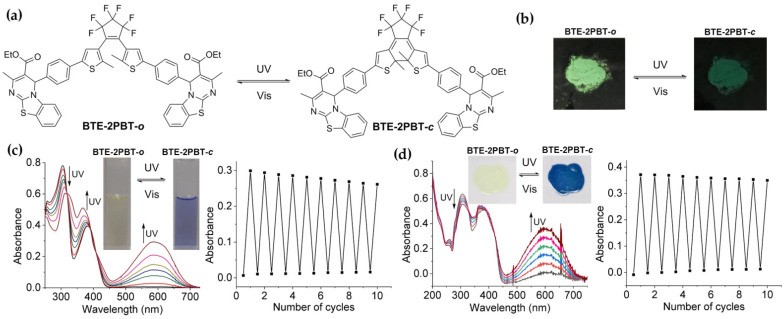
The absorption maxima of the open-ring form of the triad (BTE-2PBT-o) were observed at 305 nm and 380 nm corresponding to the BTE and PBT moieties, respectively. As shown in Figure 1a,c, irradiation of BTE-2PBT-o with UV light (254 nm) resulted in emergence and augmentation of a new absorption band at 584 nm with a color change from light yellow to blue due to the formation of closed-ring isomer (BTE-2PBT-c). The blue color faded to light yellow upon irradiation with visible light (>510 nm), and the absorption spectrum returned to the initial state of BTE-2PBT-o. The reversible color change along with isosbestic points at 319 nm, 392 nm, and 409 nm well demonstrated the bistable photochromism of the BTE-2PBT triad.
Figure 1.
Photochromic properties of BTE-2PBT triad. (a) Photochromic reactions of BTE-2PBT; (b) Photochromism of BTE-2PBT in solid state; (c) Absorption change and fatigue resistance of BTE-2PBT in hexane (2.0 × 10−5 M) upon photochromism; (d) Absorption change and fatigue resistance of BTE-2PBT in PMMA film (5 wt %) upon photochromism.
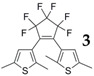
The key photochromic parameters including cyclization (Φo-c) and cycloreversion (Φc-o) quantum yields and photoconversion ratios (PRs) of BTE-2PBT triad in the photostationary state (PSS) in hexane (Table 1) were determined according to a known method [24,25]. Compared to unmodified perfluorobisthienylethene (3), BTE-2PBT exhibits bathochromically shifted λab,o and λab,c due to the extension of π-conjugation. Meanwhile, attachment of two PBT moieties also results in loweredcyclization and cycloreversion quantum yields. The PR of BTE-2PBT in PSS determined by analytical HPLC is 73%. In addition, BTE-2PBT could be reversibly switched between the two isomeric states without significant degradation for at least 10 times upon alternate irradiation with UV and visible light.
Table 1.
Photochromic Parameters of BTE-2PBT and a Reference Bisthienylethene (3) a.
| Compound | λab,o (ε = M−1·cm−1) |
λab,c (ε = M−1·cm−1) |
Φo-c (%) | Φc-o (%) | PR (%) |
|---|---|---|---|---|---|
| BTE-2PBT | 305 (3.91 × 104) |
584 (1.47 × 104) |
9.4 | 0.6 | 73 |
|
237 (2.15 × 104) |
503 (4.94 × 103) |
40 | 12 | 74 |
a Determined in hexane (2.0 × 10−5 M) at 25 °C.
The BTE-2PBT triad in PMMA film (Figure 1d) also exhibits similar photochromic properties. The light yellow PMMA film of BTE-2PBT-o turned into blue upon irradiation with UV. The blue BTE-2PBT-c film could be completely bleached to the original color when exposed to visible light (>510 nm). The photoreaction parameters, such as absorption maxima of BTE-2PBT-o (306 nm and 385 nm) and BTE-2PBT-c (602 nm) and isosbestic points (327 nm and 421 nm), all slightly redshift in PMMA. As expected, the PMMA film of BTE-2PBT showed better fatigue resistance than its solution by insulating the photochromic compound from external oxidizing agents. Moreover, amorphous BTE-2PBT powder could also be reversibly switched between light green and dark green via photo-induced cyclization and cycloreversion (Figure 1b). These results indicate that the photochromic properties of BTE could be maintained when two PBT appendages are installed.
2.3. Photophysical and AIE Properties of BTE-2PBT
The absorption and photoluminescence (PL) properties of BTE-2PBT in solution, solid state, and film are summarized in Table 2. The absorption peak of BTE-2PBT in hexane appearsat 388 nm and arises from π–π* transition. The absorption peak of film sample shows up at 385 nm, whereas that of solid sample redshifts to 413 nm.
Table 2.
Photophysical Data of BTE-2PBT and a Reference PBT AIEgen (4) a.
| Compound | State | λabs (nm) (ε = M−1cm−1) |
λem (nm) (Δν) |
ΦF (%) | αAIE (ΦF,solid/ΦF,soln) |
τ (ns) |
|---|---|---|---|---|---|---|
| BTE-2PBT | soln | 388 (2.91 × 104) | 477 (5560) | 0.1 | -- | <0.1 |
| solid b | 413 | 475(1219) | 1.2 | 12 | 0.78 | |
| film | 385 | 477(3307) | 2.6 | 26 | 1.62 | |
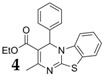
|
soln | 384 (1.26 × 104) | 474 (3903) | 0.5 | -- | <0.1 |
| solid c | 422 | 507 (2351) | 39.1 | 78.2 | 5.75 | |
| film | 385 | 471 (4410) | 4.2 | 8.4 | 2.05 |
a Abbreviations: soln = THF solution (5 × 10−5 M); ε = molar absorptivity (M−1cm−1); Δν = Stokes shift (cm−1); ΦF = fluorescence quantum yield determined using a calibrated integrating sphere; αAIE = value of AIE effect; τ = fluorescence lifetime measured at room temperature in air; b Determined on amorphous sample; c Determined on crystalline sample.
The data in Table 2 show that the triad is very dim in solution, but exhibits significant fluorescence in solid state and film when excited by 365 nm UV. The fluorescence lifetimes (τ) of BTE-2PBT in solid state and film show correlation with their fluorescence quantum yields. Comparison with a reference PBT AIEgen (4) shows that the excitation and emission wavelengths of PBT moieties in the triad are not affected by appending to BTE. However, the fluorescence quantum yields drop notably, possibly due to the enlarged molecular size, which may leave more space for intramolecular motion of the small ethyl ester rotor [22].
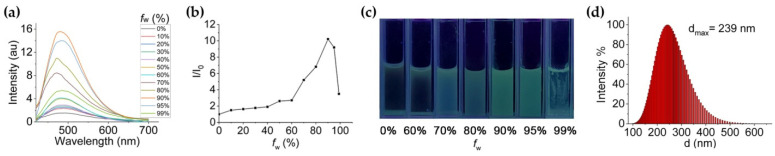
The more detailed aggregation-induced emission properties of BTE-2PBT was investigated by gradually increasing the water fraction in its THF solution. As shown in Figure 2, when water content was elevated to 70%, the solution of the triad began to exhibit strong green fluorescence due to the formation of nanoaggregates. The emission reached its maximum (11 fold) when fw was increased to 90%. Dynamic light scattering (DLS) method determined that the particle size upon aggregation in 90% water/THF mixture distributed between 100 nm and 500 nm with a maximum intensity at 239 nm. Further increase of water content led to declined fluorescence emission due to precipitation of BTE-2PBT. It is noteworthy that the maximum λem in 99% water only redshifts 10 nm compared to that in absolute THF. The insensitivity to solvent polarities indicates that locally excited (LE) state dominates the excitation of BTE-2PBT triad [22].
Figure 2.
AIE properties of BTE-2PBT triad. (a) PL spectra of BTE-2PBT in THF and water/THF mixtures with different water fractions (λex = 365 nm); (b) Plot of I/I0 value vs. water fraction; (c) Photographs of BTE-2PBT in different water fractions taken under 365 nm UV; (d) Dynamic light scattering (DLS) of BTE-2PBT in 90% water/THF mixture.
2.4. Photo-Switchable AIE Properties and Application of BTE-2PBT
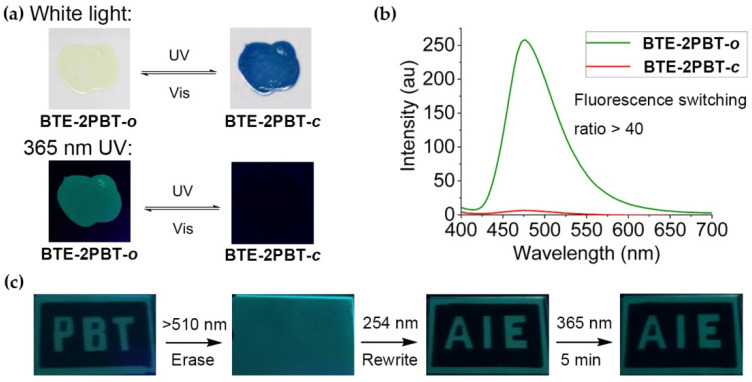
As BTE-2PBT triad maintains the reversible photochromic and AIE properties of the parent BTE and PBT moieties, we explored the possibility to modulate its solid-state fluorescence with photo-induced isomerizations. When the PMMA film containing 5 wt% BTE-2PBT was irradiated with 254 nm UV, its light yellow color turned into blue due to photochromism. Meanwhile, the green fluorescence of BTE-2PBT-o was almost completely quenched upon photocyclization (Figure 3a). The measurement of fluorescence intensity of BTE-2PBT PMMA film at “ON” and “OFF” states showed that 97.6% of open-ring state fluorescence was quenched upon photo-induced ring closure. The calculated fluorescence switching ratio is >40 (Figure 3b). The ultra high contrast could be ascribed to the combined quenching effects of both intra- and intermolecular energy transfer in the solid phase.
Figure 3.
Photo-switchable AIE properties and application of BTE-2PBT triad. (a) Photographs of BTE-2PBT PMMA film illuminated with alternate 254 nm UV and >510 nm visible light taken under white light or 365 nm UV; (b) PL spectra of BTE-2PBT PMMA film at PSS illuminated with alternate 254 nm UV and >510 nm visible light (λex = 365 nm); (c) Photoerasing, rewriting, and non-destructive reading of fluorescent images on BTE-2PBT PMMA film.
The application of BTE-2PBT PMMA film as erasible optical memory media was conducted by patterned UV illumination through photomasks (Figure 3c). The characters “PBT” were first recorded on the film with high contrast by 254 nm UV irradiation. After they were completely erased by >510 nm visible light, the second group of characters “AIE”were written on the film. The fluorescent image was continuously exposed to 365 nm UV for 5 min without notable decrease in fluorescence contrast, indicating that fluorescence readout of BTE-2PBT in PMMA film could be achieved in a non-destructive manner.
3. Materials and Methods
3.1. General Methods
3.1.1. Synthesis of BTE-2PBT Triad
All chemical reagents and solvents were obtained from a commercial supplier (Leyan-Shanghai Haohong Scientific Co. Ltd., Shanghai, China). All reactions were performed in AR grade solvents and monitored by thin layer chromatography on plates coated with 0.25 mm silica gel 60 F254 (Qingdao Haiyang Chemicals, Qingdao, China). TLC plates were visualized by UV irradiation (254 nm and 365 nm). Flash column chromatography employed silica gel (particle size 32–63 μm, Qingdao Haiyang Chemicals, Qingdao, China). NMR spectra were obtained with a Bruker AV-400 instrument (Bruker BioSpin, Faellanden, Switzerland) with chemical shifts reported in parts per million (ppm, δ) and referenced to CDCl3 or DMSO-d6. IR spectra were recorded on a Bruker Vertex-70 spectrometer. High-resolution mass spectra were obtained with a Bruker Dalton micrOTOF-Q II spectrometer (Bruker Optics, Billerica, MA, USA), and reported as m/z. Melting points were determined with a Thomas-Hoover melting point apparatus and uncorrected (Thomas Scientific, Swedesboro, NJ, USA).
3.1.2. UV-Vis and Fluorescent Spectrometry
Absorption spectra of solution samples of BTE-2PBT (2 × 10−5 M) was recorded on an Aglient 8453 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Absorption spectra of solid samples were recorded on a PerkinElmer Lambda 750 spectrophotometer (PerkinElmer, Waltham, MA, USA). Fluorescence spectra of BTE-2PBT solution (5 × 10−5 M) and solid samples were recorded on a Hitachi F-4600 fluorescence spectrophotometer (Hitachi, Yokohama, Kanagawa, Japan). Optical length of the quartz cell was 10 mm. Fluorescence quantum yields of BTE-2PBT solution (5 × 10−5 M) and solid samples were determined on a Hamamatsu absolute PL quantum yield spectrometer C11347 (Hamamatsu Photonics, Hamamatsu City, Shizuoka Pref, Japan). Fluorescence lifetimes were recorded on a Hamamatsu compact fluorescence lifetime spectrometer C11367 (Hamamatsu Photonics, Hamamatsu City, Shizuoka Pref, Japan).
3.1.3. DLS Measurement
Particle size distribution was measured on a Nano Brook 90 Plus instrument (Brookhaven Instruments, Holtsville, NY, USA) equipped with a diode laser as a light source (λ = 632.8 nm). The scattered light from the sample solution was detected using a fixed angle (90°).
3.1.4. Determination of Cyclization and Cycloreversion Quantum Yields
Φo-c and Φc-o values of BTE-2PBT in hexane [2 × 10−5 M] were measured according to a previously reported method with bis(2-methyl-1-benzothiophen-3-yl)hexafluorocyclopentene as the reference compound. A WFH-203B black-box type UV analyzer (Chitang Electronics, Shanghai, China) and an MVL-210M-visible light source (Mejiro Genossen, Akita, Japan) were employed for photo-induced cyclization and cycloreversion, respectively.
3.1.5. Determination of BTE-2PBT’s PR at PSS
HPLC traces of BTE-2PBT-o and BTE-2PBT-c were recorded on an Agilent 1220 instrument equipped with a ZORBAX SIL analytical column (4.6 × 250 mm, 5 μm; Agilent Technologies) (flow rate = 1.0 mL/min; 3.0% isopropanol in CH2Cl2 over 15 min; UV detection at 320 nm (isosbestic point)).
3.1.6. Photoerasing, Rewriting, and Nondestructive Reading of Fluorescent Images on BTE-2PBT PMMA Film
The PMMA film (MW = ca. 110,000) containing BTE-2PBT (5 wt %) was prepared by spin coating on a 1 cm × 2 cm quatz glass slide. During the writing process, the film was irradiated through a photomask under a 254 nmWFH-204B UV lamp (Qiwei Instrument, Hangzhou, China) at a distance of 5 cm for 5 min. For erasing, the film was directly subjected to an MVL-210M-visible light source (Mejiro Genossen, Akita, Japan) at a distance of 5 cm for 3 min. Continuous non-destructive reading was performed under a 365 nm WFH-204B UV lamp (Qiwei Instrument, Hangzhou, China) at a distance of 5 cm for 5 min.
3.2. Synthesis and Characterization of PBT Compound (1)
(4-(3-(Ethoxycarbonyl)-2-methyl-4H-benzo[4,5]thiazolo[3,2-a]pyrimidin-4-yl)phenyl)boronic acid (1). To a mixture of (4-formylphenyl)boronic acid (180 mg, 1.2 mmol), ethyl acetoacetate (195 mg, 1.5 mmol), 2-aminobenzothiazole (150 mg, 1.0 mmol) was added Hf(OTf)4 (8 mg, 0.01 mmol). The reaction was stirred in a sealed tube at 80 °C for 6 h. Flash column chromatography (DCM/MeOH = 20:1, v/v) afforded 1 as a yellow solid (327 mg, 83%); mp 241–242 °C. 1H-NMR (400 MHz, DMSO-d6): δ 8.00 (s, 2H), 7.74 (d, J = 7.8 Hz, 1 H), 7.66 (d, J = 7.8 Hz, 2 H), 7.42‒7.36 (m, 3H), 7.29 (dd, J1 = J2 = 7.5 Hz, 1H), 7.17 (dd, J1 = J2 = 7.5 Hz, 1H), 6.45 (s, 1H), 4.10‒4.00 (m, 2H), 3.34 (s, 3H), 1.20 (t, J = 7.1 Hz, 3H) ppm; 13C-NMR (100 MHz, DMSO-d6): δ 165.4, 162.6, 153.9, 142.9, 137.4, 134.1 (×2), 126.7, 125.9 (×2), 123.9, 122.7 (×2), 112.2, 102.6, 59.4, 56.6, 23.1, 14.0 ppm; IR (KBr): vmax 3346, 2975, 1714, 1608, 1519, 1470, 1427, 1414, 1368, 1342, 1323, 1272, 1241, 1212, 1165, 1085, 1046, 1018, 743 cm−1; HRMS (ESI+): m/z calcd for C20H20BN2O4S [M + H]+ 395.1231; found 395.1230.
3.3. Synthesis and Characterization of BTE-2PBT
Diethyl 4,4′-(((perfluorocyclopent-1-ene-1,2-diyl)bis(5-methylthiophene-4,2-diyl))bis(4,1-phenyl-ene))bis(2-methyl-4H-benzo[4,5]thiazolo[3,2-a]pyrimidine-3-carboxylate) (BTE-2PBT). To a solution of 1 (150 mg, 0.38 mmol) and dibromobisthienylethene 2 (80 mg, 0.15mmol) in THF/H2O (9:1, v/v, 5 mL) was added tetrakis(triphenylphosphine)palladium (11 mg, 0.009mmol) and Na2CO3 (97 mg, 0.92 mmol). The reaction was stirred at 80 °C for 12 h under nitrogen atmosphere. After the reaction was cooled to ambient temperature, THF was removed under reduced pressure. The residual aqueous solution was extracted with CH2Cl2 (20 mL) and washed with deionized H2O (10 mL × 2). The organic phase was combined, dried over Na2SO4, and concentrated in vacuo. Flash column chromatography on silica gel (CH2Cl2/MeOH = 30:1, v/v)afforded BTE-2PBT as a light greenish powder (108 mg, 67%); mp > 300 °C. 1H-NMR (400 MHz, CDCl3): δ 7.49‒7.39 (m, 10H), 7.26‒7.21 (m, 2H), 7.19 (s, 2H), 7.17‒7.07 (m, 4H), 6.41 (s, 2H), 4.25‒4.13 (m, 4H), 2.45 (s, 6H), 1.86 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H) ppm; 13C-NMR (100 MHz, CDCl3): δ 166.9 (×2), 163.5 (×2), 155.2 (×2), 141.7 (×3), 141.2 (×2), 138.2 (×2), 136.2, 133.5 (×2), 132.3 (×2), 128.7 (×2), 128.0 (×4), 126.8 (×2), 126.0 (×5), 124.2 (×2), 124.0 (×2), 122.8 (×2), 122.4 (×2), 111.8 (×2), 103.0 (×2), 60.2 (×2), 57.5 (×2), 29.8 (×2), 23.9 (×2), 14.6 (×2), 14.5 (×2) ppm; 19F NMR (470 MHz, CDCl3): δ −110.03 (s, 4F), −131.85 (s, 2F); IR (KBr): vmax 3442, 1700, 1674, 1597, 1508, 1371, 1331, 1272, 1241, 1199, 1113, 1093, 1076, 988, 742 cm−1; HRMS (ESI+): m/z calcd for C55H43F6N4O4S4 [M + H]+ 1065.2066; found 1065.2063.
4. Conclusions
In summary, a photo-switchable AIE-active BTE-2PBT triad has been designed and synthesized. The triad well combines the photochromic properties of BTE moiety and AIE properties of PBT moieties. More importantly, the solid-state fluorescence of BTE-2PBT could be reversibly switched by alternate UV and visible light irradiation with ultra-high contrast. Repeated writing and erasing of fluorescent images on BTE-2PBT PMMA film with non-destructive readout indicate that this novel triad has the potential to be applied as a practical solid-state luminescent material.
Acknowledgments
Not applicable.
Supplementary Materials
The following are available online, Figures S1 and S2: UV-Vis and fluorescence spectra of BTE-2PBT; Figure S3: HPLC traces of BTE-2PBT-o and BTE-2PBT-c; Figures S4–S8: NMR spectra of 1 and BTE-2PBT; Figure S9: HRMS of BTE-2PBT.
Author Contributions
S.-S.G., S.-Z.P. and Q.S. conceived and designed the experiments; S.-S.G., C.-H.Z., Z.-Z.C., D.-Z.Y. and M.C. performed the experiments and analyzed the data; S.-S.G. and Q.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (21961013 and 41867053).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1 and BTE-2PBT are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tian H., Yang S. Recent progresses on diarylethene based photochromic switches. Chem. Soc. Rev. 2004;33:85–97. doi: 10.1039/b302356g. [DOI] [PubMed] [Google Scholar]
- 2.Irie M., Fukaminato T., Matsuda K., Kobatake S. Photochromism of diarylethene molecules and crystals: Memories, switches, and actuators. Chem. Rev. 2014;114:12174–12277. doi: 10.1021/cr500249p. [DOI] [PubMed] [Google Scholar]
- 3.Pu S.-Z., Sun Q., Fan C.-B., Wang R.-J., Liu G. Recent advances in diarylethene-based multi-responsive molecular switches. J. Mater. Chem. C. 2016;4:3075–3093. doi: 10.1039/C6TC00110F. [DOI] [Google Scholar]
- 4.Giordano L., Jovin T.M., Irie M., Jares-Erijman E.A. Diheteroarylethenes as thermally stable photoswitchable acceptors in photochromic fluorescence resonance energy transfer (pcFRET) J. Am. Chem. Soc. 2002;124:7481–7489. doi: 10.1021/ja016969k. [DOI] [PubMed] [Google Scholar]
- 5.Ouhenia-Ouadahi K., Métivier R., Maisonneuve S., Jacquart A., Xie J., Léaustic A., Yu P., Nakatani K. Fluorescence photoswitching and photoreversible two-way energy transfer in a photochrome–fluorophore dyad. Photochem. Photobiol. Sci. 2012;11:1705–1714. doi: 10.1039/c2pp25129a. [DOI] [PubMed] [Google Scholar]
- 6.Soh N., Yoshida K., Nakajima H., Nakano K., Imato T., Fukaminato T., Irie M. A fluorescent photochromic compound for labeling biomolecules. Chem. Commun. 2007;48:5206–5208. doi: 10.1039/b713663c. [DOI] [PubMed] [Google Scholar]
- 7.Fölling J., Polyakova S., Belov V., Blaaderen A., Bossi M.L., Hell S.W. Synthesis and characterization of photoswitchable fluorescent silica nanoparticles. Small. 2008;4:134–142. doi: 10.1002/smll.200700440. [DOI] [PubMed] [Google Scholar]
- 8.Xie N.-H., Chen Y., Ye H., Li C., Zhu M.-Q. Progress on photochromic diarylethenes with aggregation-induced emission. Front. Optoelectron. 2018;11:317–332. doi: 10.1007/s12200-018-0839-4. [DOI] [Google Scholar]
- 9.Hong Y.N., Lam J.W.Y., Tang B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011;40:5361–5388. doi: 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- 10.Mei J., Leung N.L.C., Kwok R.T.K., Lam J.W.Y., Tang B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015;115:11718–11940. doi: 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]
- 11.Wang D., Tang B.Z. Aggregation-induced emission luminogens for activity-based sensing. Acc. Chem. Res. 2019;52:2559–2570. doi: 10.1021/acs.accounts.9b00305. [DOI] [PubMed] [Google Scholar]
- 12.Chen S., Li W., Li X., Zhu W.-H. Aggregation-controlled photochromism based on a dithienylethene derivative with aggregation-induced emission. J. Mater. Chem. C. 2017;5:2717–2722. doi: 10.1039/C7TC00023E. [DOI] [Google Scholar]
- 13.Chen L., Zhang J., Wang Q., Zou L. Photo-controllable and aggregation-induced emission based on photochromic bithienylethene. Dyes Pigments. 2015;123:112–115. doi: 10.1016/j.dyepig.2015.07.031. [DOI] [Google Scholar]
- 14.Lim S.-J., An B.-K., Jung S.D., Chung M.-A., Park S.Y. Photoswitchable organic nanoparticles and a polymer film employing multifunctional molecules with enhanced fluorescence emission and bistable photochromism. Angew. Chem. Int. Ed. 2004;43:6346–6350. doi: 10.1002/anie.200461172. [DOI] [PubMed] [Google Scholar]
- 15.Lim S.-J., An B.-K., Park S.Y. Bistable photoswitching in the film of fluorescent photochromic polymer: Enhanced fluorescence emissionand its high contrast switching. Macromolecules. 2005;38:6236–6239. doi: 10.1021/ma0504163. [DOI] [Google Scholar]
- 16.Li C., Gong W.-L., Hu Z., Aldred M.P., Zhang G.-F., Chen T., Huang Z.-L., Zhu M.-Q. Photoswitchable aggregation-induced emission of a dithienylethene-tetraphenylethene conjugate for optical memoryand super-resolution imaging. RSC Adv. 2013;3:8967–8972. doi: 10.1039/c3ra40674a. [DOI] [Google Scholar]
- 17.Dong H., Luo M., Wang S., Ma X. Synthesis and properties of tetraphenylethylene derivatived diarylethene with photochromism and aggregation-induced emission. Dyes Pigments. 2017;139:118–128. doi: 10.1016/j.dyepig.2016.11.054. [DOI] [Google Scholar]
- 18.Ma L., Li C., Yan Q., Wang S., Miao W., Cao D. Unsymmetrical photochromic bithienylethene-bridge tetraphenylethene molecular switches: Synthesis, aggregation-induced emission and information storage. Chin. Chem. Lett. 2020;31:361–364. doi: 10.1016/j.cclet.2019.07.040. [DOI] [Google Scholar]
- 19.Liu G., Zhang Y.-M., Zhang L., Wang C., Liu Y. Controlled photoerasable fluorescent behaviors with dithienylethene-based molecular turnstile. ACS Appl. Mater. Interfaces. 2018;10:12135–12140. doi: 10.1021/acsami.7b12822. [DOI] [PubMed] [Google Scholar]
- 20.Luo W., Wang G. Photo-responsive fluorescent materials with aggregation-induced emission characteristics. Adv. Opt. Mater. 2020;8:202001362. doi: 10.1002/adom.202001362. [DOI] [Google Scholar]
- 21.Yan Q., Wang S. Fusion of aggregation-induced emission and photochromics for promising photoresponsive smart materials. Mater. Chem. Front. 2020;4:3153–3175. doi: 10.1039/D0QM00522C. [DOI] [Google Scholar]
- 22.Gong S.-S., Kong R., Zheng C., Fan C., Wang C., Yang D.-Z., Chen Z.-Z., Duo S., Pu S., Sun Q., et al. Multicomponent reaction-based discovery of pyrimido[2,1-b][1,3]benzothiazole (PBT) as a novel core for full-color-tunable AIEgens. J. Mater. Chem. C. 2021;9:10029–10036. doi: 10.1039/D1TC02301B. [DOI] [Google Scholar]
- 23.Kong R., Han S.-B., Wei J.-Y., Peng X.-C., Xie Z.-B., Gong S.-S., Sun Q. Highly efficient synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones (DHPMs) catalyzed by Hf(OTf)4: Mechanistic insights into reaction pathways under metal Lewis acid catalysis and solvent-free conditions. Molecules. 2019;24:364. doi: 10.3390/molecules24020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irie M., Lifka T., Lifka S., Kato N. Photochromism of 1,2-bis(2-methyl-5-phenyl-3-thienyl)perfluorocyclopentene in a single-crystalline phase. J. Am. Chem. Soc. 2000;122:4871–4876. doi: 10.1021/ja993181h. [DOI] [Google Scholar]
- 25.Uchia K., Tsuchida E., Aoi Y., Nakamura S., Irie M. Substitution effect on the coloration quantum yield of a photochromic bisbenzothienylethene. Chem. Lett. 1999;28:63–64. doi: 10.1246/cl.1999.63. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Materials.